Abstract
Context:
Organophosphorus insecticides (OPs) have been used to control agricultural pests found in Washington state. Farmworkers (FW) have higher exposure to OP pesticides than non-farmworkers (NFW), and FW children may in turn have higher exposure than NFW children.
Objective:
To examine the association between the concentration in house dust of five OPs used commonly in pome fruit orchards and the concentration in urine of dialkylphosphate metabolites (DAP), in a cohort of Hispanic FW and NFW and their children.
Methods:
Parents and children participated in three data collection periods over the course of one year. Urine samples were evaluated for the DAPs characteristic of OP exposure, and dust from homes and vehicles was evaluated for intact OP residues.
Results:
Geometric mean (GM) concentrations of OPs in house and vehicle dust were higher in FW households than NFW households in all agricultural seasons. GM concentration of urinary DAPs was higher for children in FW households than NFW households.
Discussion:
Regression analysis found a positive association between OP residues in house dust and the children’s urinary DAPs.
Conclusions:
To our knowledge this study is the first to report an association between pesticides in house dust and their biological metabolites in urine.
INTRODUCTION
The University of Washington’s Center for Child Environmental Health Risks Research (CHC) has followed a community-based participatory research strategy in the lower Yakima Valley (Valley) of Washington state since 1999, in order to assess and reduce pesticide exposure among children of local farmworkers, most of whom are of Hispanic origin [1], [2]. CHC’s previous studies indicated that young children were exposed to pesticides through a take-home pathway and that farmworkers (FW) who worked in pome fruit crops, as well as their children, were more heavily exposed to pesticides than farmworkers working in non-pome crops [3], [4]. In response to that work, the Community Advisory Board (CAB) recommended a new project examining potential seasonal differences in exposure pathways important for children of agricultural workers compared to children of non-agricultural workers. Details on CAB members have been described elsewhere [5]. The CAB wanted to know if FW and their children had higher levels of OP exposure than nonfarmworkers (NFW) and their children living in the same communities. To do this, CHC and the CAB designed a cohort study to be conducted in the Valley [6], [7]. This longitudinal study, which took place between April 2005 and February 2006, collected samples of house dust, vehicle dust, urine, saliva, and blood from FW and NFW families at three points during the agricultural season.
Agricultural pesticides can cause a range of adverse effects on human health, and OPs carried in house dust can be a source of exposure to the inhabitants. Young children are exposed to OPs primarily in their homes, daycare settings, and schools, since they spend the majority of their day indoors [8]. Intact OPs can remain stable indoors for periods of time lasting from months to years [9]. Thus children can be exposed to pesticides through the take-home pathway, as the OP residues carried on parental clothing, shoes, skin, and hair to vehicles and homes are transferred to house dust. Because the developmental effects of OPs on young children have not been well characterized, it is important to investigate potential exposures in their homes.
The setting has been described elsewhere [1]. Briefly, the Valley covers both Yakima and Benton counties and includes not only the City of Yakima, but also manysmaller communities whose economies are centered around agricultural production. The Valley leads the state of Washington in apple production [10].
Organophosphate insecticides (OPs) have been applied seasonally to apple and pear (pome fruit) orchards in the Valley and elsewhere in Washington state in order to control damaging insects such as Cydia pomenella, the codling moth [11], [12]. In 2005, approved agricultural uses led to the application in the Valley of approximately 86 metric tons of azinphos-methyl (AZM; trade name Guthion; Chemical Abstracts Service (CAS) No. 86–50-0), 66 metric tons of chlorpyrifos (CP; trade name Dursban; CAS No. 2921–88-2), 39 metric tons of phosmet (PH; trade name Imidan; CAS No. 732–11-6), 5 metric tons of malathion (ML; CAS No. 121–75-5), and 1 metric ton of diazinon (DZ; CAS No. 333–41-5) [13], [14].
In this paper we examine the association between the concentration in house dust of five of the Valley’s most commonly used OPs and the concentration in urine of dialkylphosphate metabolites (DAP) from our cohort. We tested the hypothesis that adults and children living in homes with higher concentrations of OPs in house dust would have higher urinary metabolite concentrations of OPs, and that this relationship would differ significantly by occupation, agricultural season, and life stage.
METHODS
All study materials and sample collection protocols were reviewed and approved by the Human Subjects Division of the University of Washington and the Institutional Review Board of the Fred Hutchison Cancer Research Center of Seattle, Washington (File #5946). Informed consent was received from all participants. The CAB recruited 100 FW adults and 100 NFW adults, all of Hispanic origin and all with a referent child between the ages of 2 and 6 years living in the household at time of recruitment. Most of the agricultural work in the Valley is done by FW of Hispanic origin. Approximately 36% of Yakima County’s population was of Hispanic origin in 2000, and by 2010 that figure had risen to 45% [15], [16].
FW adults worked in pome fruit orchards in positions that included thinning, pruning, and harvesting, but not pesticide handling, while NFW adults worked in occupational settings such as schools, stores, daycare facilities, dairies, or factories. The same individuals were followed longitudinally across all three agricultural seasons. An expanded description of the demographic characteristics of the two cohorts can be found elsewhere [6].
CHC and the CAB designed a study protocol to assess urinary metabolites of OPs in Hispanic adults and children of FW and NFW families, as well as concentrations of OPs in house and vehicle dust. Adults and children participated in three data collection periods over the course of a year, which coincided with the three agricultural seasons typical of pome fruit cultivation. The first collection period (April through July 2005) coincided with the thinning season, when branches are pruned and buds are removed from the trees by hand to improve fruit development. The second collection period (August through October 2005) coincided with harvest season, when the fruit is picked. The third collection period (December 2005 through February 2006) coincided with the non-spray season, when the trees are dormant. Because pruning and thinning are tasks that bring farmworkers into direct contact with treated foliage, the thinning season is thought to coincide with the highest exposure to OPs.
Three independent first-morning urine voids, each separated by two days, were collected from each participant in each of the three agricultural seasons. Each sample was analyzed separately. Sampling protocols were based on standard procedures developed at the University of Washington which have been described elsewhere [17], [18]. Urine analysis was performed at the Centers for Disease Control and Prevention’s laboratory in Atlanta, Georgia using lyophilization with gas chromatography-mass spectrometry and isotope dilution quantification [19].
Urine was analyzed for the six DAP compounds that are produced by metabolism of most OPs [20]. Dimethyl OPs are metabolized into one or more of three non-specific compounds: dimethylphosphate (DMP), dimethylthiophosphate (DMTP), and dimethyldithiophosphate (DMDTP). Diethyl OPs are also metabolized into one or more of three non-specific compounds: diethylphosphate (DEP), diethylthiophosphate (DETP), and diethyldithiophosphate (DEDTP). The OPs analyzed in this report are metabolized into five different DAPs: DMP, DMTP, DMDTP, DEP, and DETP.
The laboratory limits of detection (LOD) for the six urinary DAPs were DMP (0.6 ng/mL), DMTP (0.2 ng/mL), DMDTP (0.01 ng/mL), DEP (0.2 ng/mL), DETP (0.01 ng/mL), and DEDTP (0.01 ng/mL). AZM, PH, and ML (dimethyl OPs) are all metabolized into DMP, DMTP, and DMDTP. CP and DZ (diethyl OPs) are metabolized into DEP and DETP but not DEDTP; therefore DEDTP results are not included in this analysis.
House dust samples were collected from the residences of the farmworkers and non-farmworkers with a Nilfisk vacuum cleaner. In each season, dust samples were collected on the same day that the household members provided their third urine samples. The residences were vacuumed once during each of the three collection periods described above and the vehicles were vacuumed once during each of the collection periods of thinning and non-spray seasons. Procedures for house dust sampling were developed by the University of Washington and have been described elsewhere [6], [18], [21]. Briefly, areas were vacuumed in a standardized manner. In the homes, vacuuming was done over the area where, according to the parent, “the child played most frequently.” In the household vehicles, the front footwell (and rear footwell, if present) were vacuumed thoroughly. Dust samples were analyzed at the University of Washington’s Environmental Health Laboratory and Trace Organics Analysis Center for residues of sixteen OPs, following the procedures described by Smith et al. [22]. The five OPs detected in the highest concentrations and their laboratory LODs were AZM (10.0 ng/g), ML (0.4 ng/g), PH (0.4 ng/g), CP (4.0 ng/g), and DZ (0.4 ng/g) [22]. The LODs were based on the analysis of one gram of dust; however in some cases less than one gram of dust was available, resulting in some LOD variability with smaller samples exhibiting a higher LOD.
Statistical Methods
We characterized OP concentrations in dust using a multivariate Normal distribution model with values below the LOD treated as censored values. An expanded description of the analytical techniques can be found elsewhere [22], [23]. Briefly, we estimated the GM molar concentration of each OP population, geometric standard deviation, and standard error. Separate multivariate Normal distributions were estimated for each season, and for FW and NFW households. Because of the large number of dust samples below the LOD we estimated distributions of the model parameters using a Bayesian Markov chain Monte Carlo method known as Gibbs sampling [23]. These model simulations were used for all subsequent analyses of dust.
Dust Samples
The molar concentration (nmol/g) of each OP was calculated by dividing the concentration in nanograms per gram by its associated molecular weight in grams per mole. Total concentrations of dimethyl OPs and diethyl OPs were calculated by combining the concentrations of the individual samples. The dimethyl OPs and diethyl OPs were then combined, resulting in total dialkyl OPs by season. We included only those home dust samples for which at least one member of the household had given at least one urine sample.
Urine Samples
Molar concentrations (nmol/mL) of DAPs were calculated by dividing the concentration of each urinary metabolite sample in nanograms per milliliter by its associated molecular weight in grams per mole. We included only those urine samples for which a house dust sample from the corresponding home had been analyzed. Results were not adjusted for urinary creatinine concentration [24], [25].
For each of the five DAPs we calculated the geometric mean (GM) of the molar concentrations (nmol/mL) of each participant’s three urine samples within five days within an agricultural season. We summed the GM concentrations of each participant’s DMP, DMTP, and DMDTP results to determine total dimethyl alkylphosphate metabolites (DMAP), and similarly summed DEP and DETP to determine total DEP+DETP (DEP+DETP). We then summed the total GM concentrations of DMAP, DEP, and DETP (DMAP+DEP+DETP) to obtain total urinary DAPs.
The data were log-transformed to analyze the concentration of intact OPs in house and vehicle dust and OP metabolites in urine. We report GM concentrations with 95% confidence intervals. Regression and correlation analyses were utilized to examine the relationship between OPs in dust, urinary OP metabolites, and parental occupation. Significance was determined based on the p-value being less than 0.05.
The Gibbs sampling was performed using the WinBUGS 1.4.3 software program available at www.mrc-bsu.cam.ac.uk/bugs [26], while “R” statistical software was utilized for all other statistical analyses [27].
RESULTS
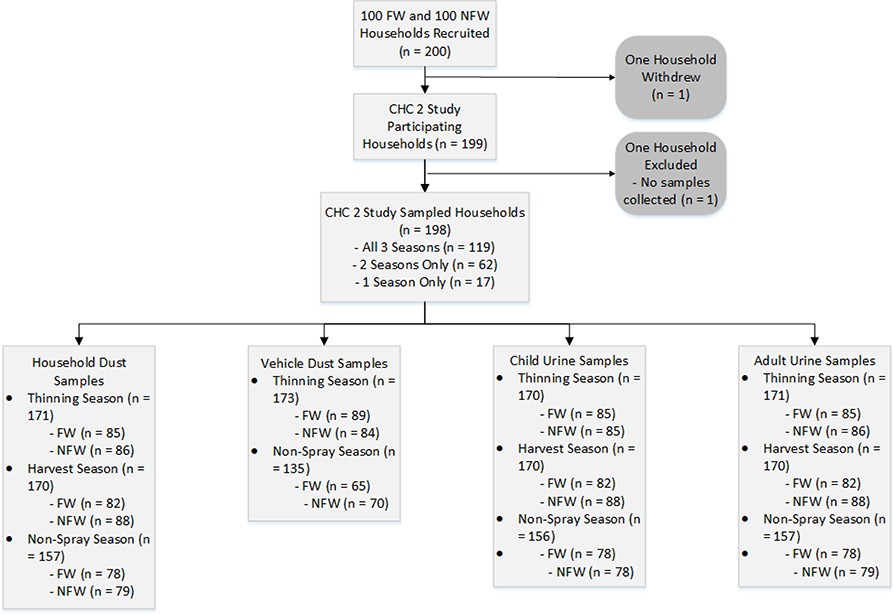
We analyzed house dust, vehicle dust, and urinary metabolites from 198 unique households. Of those 198 households, 119 households contributed urine and house dust samples for all three agricultural seasons, 62 households contributed samples for two seasons, and 17 households contributed samples for one season (Figure 1). The collected samples of house dust (n=498) and vehicle dust (n=270) were analyzed for sixteen OPs. The house dust samples were collected in thinning (n=171), harvest (n=170) and non-spray (n=157) seasons, while the vehicle dust samples were collected in thinning (n=150) and non-spray (n=120) seasons. The collected samples of urine were analyzed for six DAPs. The urine samples were collected in thinning (171 adults plus 170 children), harvest (170 adults plus 170 children), and non-spray (157 adults plus 156 children). Details of the detection rates and concentrations of OP residues in house and vehicle dust and OP metabolites in urine are shown in the Supplementary Material, indicating that there were occupational as well as seasonal trends in OPs found in house dust, vehicle dust, and urine.
Figure 1.
Flow of participating households through the study.
House Dust
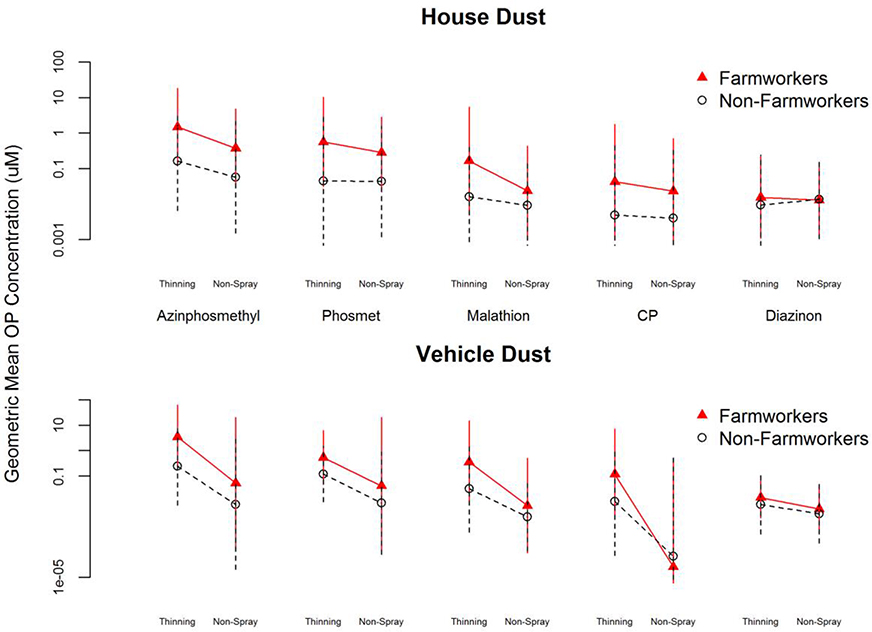
As reported previously by CHC [22], the five OPs detected in the house dust with the greatest frequency and in the highest concentrations were AZM, ML, PH, CP, and DZ (Figure 2). AZM exhibited the highest rate of detection and the highest GM concentration of any OP. Nine-fold more AZM was detected in FW than NFW house dust samples collected in thinning season (p<0.001). Dust samples from FW households contained almost four-fold more AZM in thinning season than in nonspray season (p<0.001), reflecting seasonal OP application patterns.
Figure 2.
Geometric mean concentration (nmol/g) and 95% confidence interval of the five most abundant OPs detected in dust samples from farmworker and nonfarmworker homes and vehicles, during thinning and non-spray seasons. (1a) Collected from residences, in the location where “the child played the most.” (1b) Collected from primary household vehicle.
CP exhibited the lowest rate of detection of the five OPs. Although CP was detected in comparatively low GM concentrations overall, FW house dust contained eight-fold more CP than NFW house dust in thinning season (p<0.001) and five-fold more in non-spray season (p<0.001). The GM concentration of CP fluctuated seasonally in FW but not NFW house dust samples.
Vehicle Dust
Similar to the house dust, the five OPs detected most frequently and in the highest concentrations in vehicle dust samples were AZM, ML, PH, CP, and DZ (Figure 2). In thinning season the most frequently detected OPs were AZM and PH, identified in 99% and 98%, respectively, of the dust samples taken from FW vehicles. The most frequently detected OP in non-spray season, CP, was identified in 65% of the FW vehicle dust samples.
AZM was detected in the highest GM concentration in vehicle dust samples. Sixteenfold more AZM was detected in FW than NFW samples from thinning season (p<0.001). A comparison of seasonal variation found that vehicle dust from FW households contained over fifty-fold more AZM in thinning season than in non-spray season (p<0.001) while NFW vehicle dust contained over twenty-fold more AZM in thinning than non-spray season (p<0.001).
Urine Samples
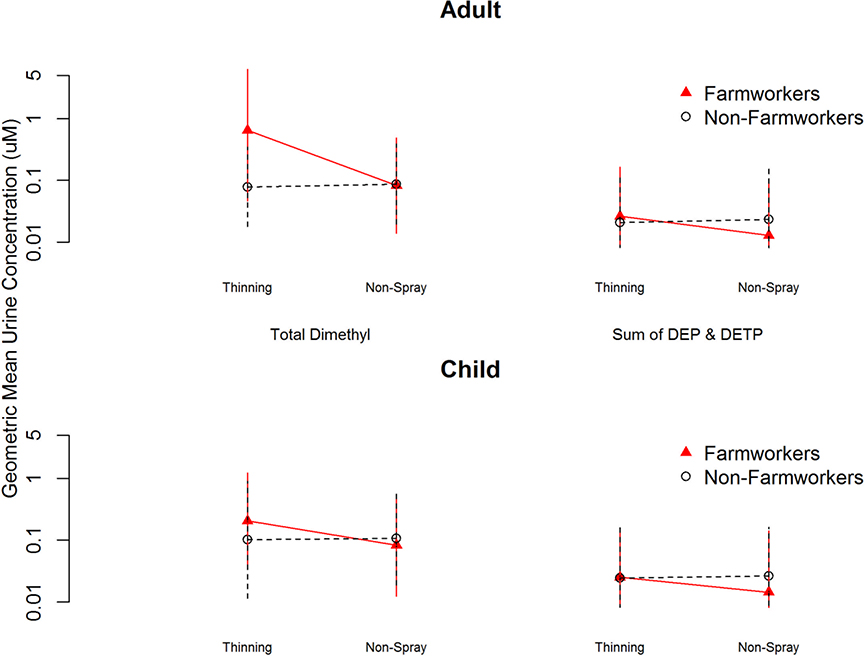
In the child urine samples, DMAP was detected in significantly higher GM concentrations than DEP+DETP (Figure 3). FW child urine showed eight-fold higher molar concentration of DMAP than DEP+DETP in thinning season and twenty-twofold higher DMAP than DEP+DETP in harvest season. FW children showed a twofold higher concentration of DMAP than NFW children in thinning season (p<0.001).
Figure 3.
Geometric mean concentration (nmol/mL) and 95% confidence interval of dimethylalkylphosphate (DMAP) and diethylphosphate plus diethylthiophosphate (DEP+DETP) urinary metabolites, collected from farmworker and non-farmworker adults and children during thinning and non-spray seasons. (2a) Adult DMAP. (2b) Adult DEP+DETP. (2c) Child DMAP. (2d) Child DEP+DETP.
Among the individual DAPs, DMTP was detected in the highest GM concentration in child urine in all three seasons. DMTP also showed the highest frequency of detection in child urine. In both thinning and harvest seasons, 100% of the children showed DMTP in at least one of their urine samples, while in non-spray season, 100% of FW children and 99% of NFW children showed DMTP in at least one sample. DEP and DETP were detected in the lowest GM concentrations and with the lowest frequency of detection in child urine.
In the adult urine samples, DMAP was detected in significantly higher GM concentrations than DEP+DETP, in all three agricultural seasons (Figure 3). FW adult urine showed a twenty-five-fold higher concentration of DMAP than DEP+DETP in thinning season, over forty-eight-fold higher DMAP in harvest season, and six-fold higher DMAP in non-spray season. The GM concentration of DMAP was eight-fold higher in FW than NFW adults in thinning season (p<0.001).
DMTP was detected in the highest GM concentration among the individual DAPs in adult urine samples in all three seasons. DMTP also showed the highest frequency of detection: 100% of adults had DMTP in at least one urine sample in each season. DETP showed the lowest rate of detection of the five OPs analyzed, in both FW and NFW adults, in all three seasons.
Regression Analysis of Urine DMAP+DEP+DETP vs. House Dust OP Residues
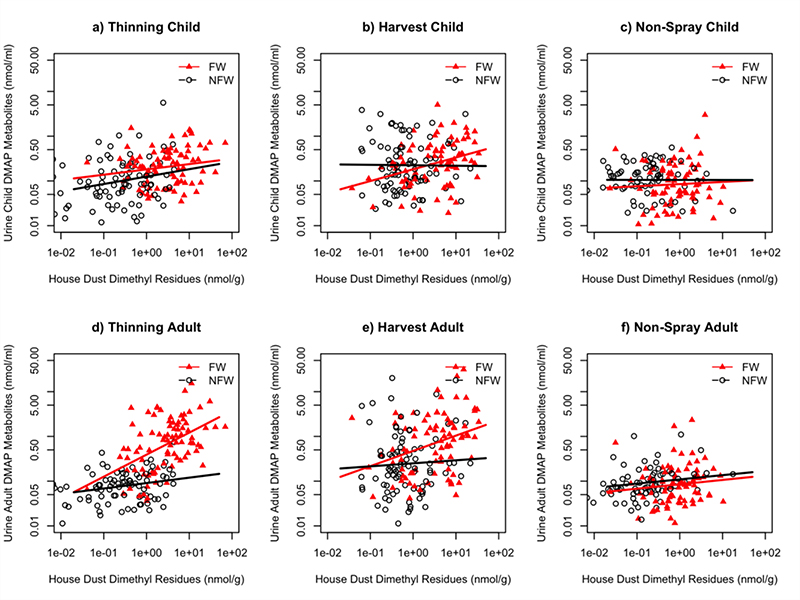
Regression analysis on log-transformed values was conducted to evaluate the relationship between the measured biologic levels of urinary OP metabolites in the study participants and the concentration of OP pesticide residues in household dust. Separate models were developed for the adults and the children, and for dimethyl and diethyl OPs. Initially a simple regression model of urine DMAP+DEP+DETP vs. residues of AZM, ML, PH, CP, and DZ in house dust was generated (Figure 4). This model indicated that, for the children, a significant relationship existed in thinning season (p<0.001) and harvest season (p<0.05). The independent categorical variable of parental occupation was then added to the regression model in order to examine whether an interaction existed between parental occupation and the concentration of OPs in house dust. Results indicated that, for the children, there was not an interaction between parental occupation and OPs in house dust in any season.
Figure 4:
Regression of log of DMAP urinary metabolites (nmol/mL) vs. log of dimethyl OP residues in house dust (nmol/g) by agricultural season, with regression lines calculated separately for the two occupational groups. All figures include samples with residue levels below LOD. (3a) Difference between FW and NFW children in thinning season: p<0.001. (3b) Difference between FW and NFW children in harvest season: p<0.05. (3c) Difference between FW and NFW children in non-spray season: p=0.62. (3d) Difference between FW and NFW adults in thinning season: p<0.001. (3e) Difference between FW and NFW adults in harvest season: p<0.001. (3f) Difference between FW and NFW adults in non-spray season: p=0.33.
We observed a significant positive relationship between adult urinary DMAP+DEP+DETP vs. residues of AZM, ML, PH, CP, and DZ in thinning (p<0.001) and harvest (p<0.001) seasons. An interaction between occupation and OP residues in house dust existed in thinning (p<0.001) and harvest (p<0.001) seasons.
We then restricted the regression model to dimethyl OPs, and found that a significant positive correlation existed between child DMAP and dimethyl OP house dust residues during thinning (p<0.001, r2=0.13) and harvest (p<0.05, r2=0.02) seasons. We also found that an interaction existed between parental occupation and GM concentration of dimethyl OPs in house dust in harvest season (p<0.05).
Urine DMAP concentration differed significantly between FW and NFW children in thinning (p<0.001) and harvest (p<0.05) seasons (Figures 3a, 3b, and 3c).
Results for the adults were similar to those of the children. A significant positive relationship between adult DMAP and dimethyl OP house dust residues was observed during thinning (p<0.001, r2=0.40) and harvest (p<0.001, r2 = 0.04) seasons, and an interaction existed between occupation and dimethyl OPs in house dust in thinning (p<0.001) and harvest (p<0.001) seasons. DMAP concentration in the two occupational groups differed significantly from one another in thinning (p<0.001) and harvest (p<0.001) seasons (Figures 3d, 3e, and 3f.)
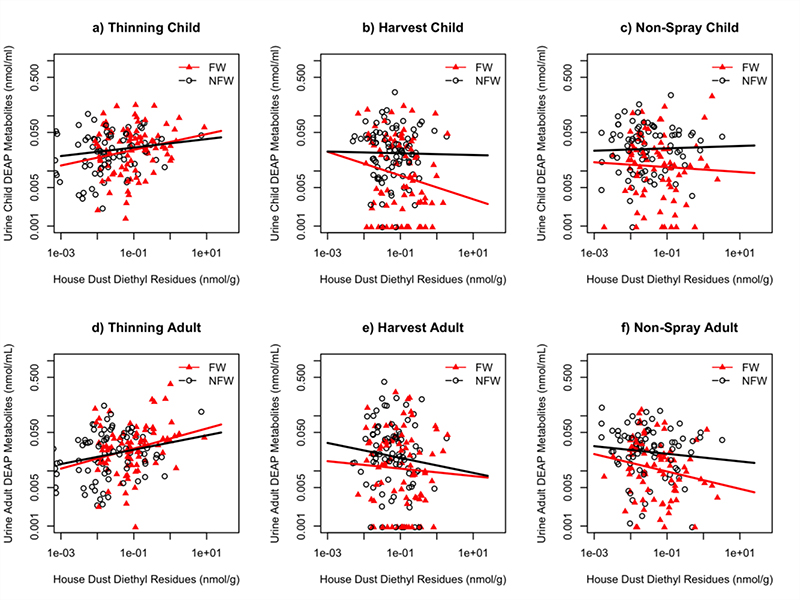
Diethyl OPs were used less frequently than dimethyl OPs during the agricultural seasons when urine and dust samples were collected [28], [29]. A regression model restricted to diethyl OPs (Figure 5) found a significant positive relationship existed between diethyl OP residues in house dust and DEP+DETP in child urine during thinning (p<0.05, r2=0.02) and harvest (p<0.05, r2=0.02) seasons. An interaction between parental occupation and GM concentration of diethyl OPs in house dust was observed in non-spray season (p<0.05). Urine DEP+DETP concentration differed significantly between FW and NFW children in harvest (p<0.001) and nonspray (p<0.05) seasons (Figures 4a, 4b, and 4c).
Figure 5:
Regression of log of DEP+DETP urinary metabolites (nmol/mL) vs. log of diethyl OP residues in house dust (nmol/g) by agricultural season, with regression lines calculated separately for the two occupational groups. All figures include samples with residue levels below LOD. (4a) Difference between FW and NFW children in thinning season: p<.05. (4b) Difference between FW and NFW children in non-spray season: p<.001. (4c) Difference between FW and NFW children in nonspray season: p<.05. (4d) Difference between FW and NFW adults in thinning season: p<0.05. (4e) Difference between FW and NFW adults in harvest season: p=0.06. (4f) Difference between FW and NFW adults in non-spray season: p<0.05.
A significant positive relationship between adult DEP+DETP and diethyl OP residues in house dust was observed during thinning (p<0.001, r2=0.07) and non-spray (p<0.05, r2 = 0.03) seasons. When occupation was added to the regression model as an independent categorical variable, results indicated that occupation was a significant factor affecting adults’ urinary DEP+DETP in non-spray season (p<0.001). The DEP+DETP concentration in the two occupational groups (FW adults vs. NFW adults) differed significantly from one another in harvest (p<0.05) and non-spray (p<0.001) seasons (Figures 4d, 4e, and 4f).
DISCUSSION
In this paper we evaluated the association between intact OP residues in the household dust of families of Hispanic farmworkers and non-farmworkers living in agricultural communities in the Valley, and urinary metabolites of those pesticides. Our data demonstrate that a positive correlation exists between the concentrations of adult urinary DMAP+DEP+DETP vs. OP residues in house dust in thinning season, when OPs are being applied regularly, and also during harvest season, when OPs are applied sporadically, but not during non-spray season. Children exhibit the same pattern of a positive correlation between child DMAP+DEP+DETP and intact OP residues in house dust during thinning and harvest seasons, but not in non-spray season. The detection of DAPs in urine indicates recent OP exposures, since OPs are metabolized within 24–48 hours [30]–[33]. The FW adults would have been exposed in the course of their ordinary occupational duties, but the presence of urinary DAPs in NFW adults and in children indicates exposure from nonoccupational sources.
Children are more susceptible to the health effects of pesticide exposures than are adults, due to their rapid growth and development [34], and exposure in early childhood may lead to adverse health outcomes in later childhood and adulthood [35]. The existence of the take-home pathway [1], [6], [18], in which pesticide residues are introduced into the home by adults exposed in the workplace, creates an exposure route for children that is cause for concern. Pesticide exposure in childhood may affect neurodevelopment [36]–[40], and emerging evidence suggests that pesticides may affect children’s pulmonary development [41], [42].
Analyzing dimethyl OPs and diethyl OPs and their corresponding urinary metabolites separately gave further insight into the relationship between pesticides in house dust and pesticide metabolites in urine. A positive correlation existed between urinary DMAP and dimethyl OP residues in house dust in thinning and harvest seasons, but not in non-spray season, for both the adults and the children. When parental occupation (FW vs. NFW) was included in the regression model, a significant interaction between occupation and concentration of dimethyl OPs in house dust was observed in thinning season and harvest season for the adults.
CP, a diethyl OP utilized regularly in the Valley during 2005 and 2006, was applied on a different seasonal schedule than the dimethyl OPs, so it is not surprising that a somewhat different pattern was seen in the relationship between urinary DEP+DETP and diethyl OP residues in house dust. A positive correlation between adult DEP+DETP and diethyl OP residues existed in thinning and non-spray seasons, but not in harvest season, while a positive correlation between child DEP+DETP and diethyl OP residues was seen in thinning and harvest seasons but not in non-spray season. When parental occupation was added to the regression model, the results showed that FW status influenced the concentration of diethyl OPs in house dust significantly in harvest and non-spray seasons, for both adults and children.
These results support our hypothesis that in certain agricultural seasons a takehome pathway exists, by which adult FWs exposed occupationally to OPs carry residues into their vehicles and then into their homes, increasing the likelihood that children living in those homes will be exposed to OPs. Among our cohort, we found that the GM concentrations of AZM, CP, ML, and PH in house dust in FW homes exceeded those in NFW homes in all three seasons.
Our paper is not the first to investigate OP residues in house dust and OP metabolites in the urine of adults and children living in those homes [43], but to our knowledge it is the first to report on an association between pesticides in house dust and their biological metabolites in urine. Our study is unique in that it collected urine and dust data across three agricultural seasons, from a cohort of farmworker and non-farmworker adults and their children living in agricultural communities in the Lower Yakima Valley of Washington state. All of the FW adults were employed with the same agricultural crop (pome fruits) during the sample collection periods, thereby ensuring that their occupational OP exposure profiles were similar.
AZM is considered to be one of the most effective pesticides against the codling moth and was applied in significant quantities in the Valley during 2005 and 2006, primarily during thinning season. AZM is a possible source of the DMAPs detected in the participants’ urine. AZM was the most commonly detected OP in house dust, while DMTP was the most commonly detected urinary metabolite in adults and children. CP, a diethyl OP, is also effective against the codling moth and was also applied in significant quantities in the Valley during those same years. However, CP’s application period of February-March overlapped only partially with the urine sampling intervals, which may explain the low levels of CP detected in this study. CP and DZ were the least commonly detected OPs in house dust, and DEP+DETP were the least commonly detected urinary metabolites in adults and children. The patterns and timing of OP presence in house dust and urinary metabolites reflect those of orchard applications, giving support to the hypothesis that adults who work in agricultural settings carry pesticides into their homes and thereby expose family members to workplace contaminants.
Only a few studies have been conducted examining the relationship between postnatal OP exposure and the neurodevelopment of school-age children, and the results are inconsistent. Cross-sectional data from the U.S. National Health and Nutrition Examination Survey were analyzed, and 119 of 1,139 children ages 8–15 met the diagnostic criteria for attention deficit hyperactivity disorder (ADHD). Children with higher urinary DMAP concentrations were more likely be diagnosed as having ADHD, while diethyl alkylphosphate (DEAP) levels were not significantly associated with the odds of ADHD [36]. Researchers assessed the development of 79 children from Ecuador: the urinary DAP levels of the 7-year-old children were associated with an increase in simple reaction time [37]. Analysis of data on 1,081 children ages 6–11 years from the Canadian Health Measures Survey found urinary DAPs in 91% of the children, but urinary DAPs were not associated significantly with a high level of parent-reported behavioral problems [38]. In a group of 48 children from the Children Pesticide Survey of southern Arizona, 100% of the children had detectable levels of the OP metabolite DMP. Higher urinary DAPs were correlated with poorer performance on some subsets of the Wisconsin Card Sorting Test, although results were dependent upon the inclusion of two samples with significantly higher DAPs [39]. In a cohort of 323 five-year-old Mexican-American children in California, researchers found no association between total DAPs and measures of attention, but there was a doubling of the odds of ADHD for every 10-fold increase in urinary DEAP [40]. The effect of long-term, low-level exposures to OP pesticides, especially among children who live in agricultural areas where pesticides are applied continuously, remains open to investigation.
Recent evidence suggests that prenatal and early childhood OP exposure may affect pulmonary development [41], [42]. In a cohort of 359 California children, urinary DAP, DMAP, and DEAP concentrations measured during ages 0.5–5 years were associated with respiratory symptoms and coughing at 5 or 7 years of age and with significant decreases in lung function at 7 years.
There is general acknowledgement that DAPs are available from food. A portion of the urinary DAPs measured in this study may well have originated from ingestion of DAPs found in food or the environment rather than from metabolism of the intact OP compounds. OPs on food commodities degrade to DAPs at levels which may approach that of the parent OP [44]. At this time there is little understanding of whether ingested DAPs are further metabolized; several studies done in rats suggest that at least some DAPs are further metabolized after oral consumption [31], [32]. The house and vehicle dust samples from this study were only analyzed for intact OPs and not for DAPs resulting from environmental breakdown of OP compounds. It is possible that some proportion of the DAPS measured in the urine samples resulted from oral consumption of DAPs rather than metabolism of ingested OPs. However, the purpose of this report was to examine whether there was an association between intact OPs in house dust and the urinary DAPs detected in FW and NFW families living in an agricultural area.
Our study has many strengths. Its design was longitudinal, and the same participants were followed over three agricultural seasons encompassing a full year. All urine samples were first morning voids, which have been found to be the best predictor of weighted-average daily metabolite concentration [45]. The participants contributed three urine samples over a five-day period (days 1, 3 and 5); our analysis used the geometric means of the DAP metabolites detected in the three samples, thereby reducing the day-to-day variability of the subjects’ measurements. House dust and urine samples were collected during three different intervals in a single agricultural year and therefore reflect the annual cycle of OP use in the orchards. The urine and dust samples reflect the seasonal application patterns of dimethyl OPs particularly well, as the collection intervals coincided with thinning season, when dimethyl OPs are applied regularly; harvest season, when OP application tapers off, and non-spray season, when dimethyl OPs are rarely used on pome fruits. All of the adult farmworkers were employed in pome fruit orchards and thus they were exposed to similar types of OPs.
A limitation to this study is that exact OP usage during the harvest season can be difficult to determine, as application times vary according to the specific fruit variety. Different apple varieties ripen at different times during the harvest season, and late-ripening orchards may receive OP applications on a different schedule than early-ripening orchards. Another limitation is that seasonal CP application did not coincide with any of the three sample collection intervals. Therefore the urine and dust samples are less reflective of CP exposure than they are of AZM, DZ, ML, and PH exposures, since AZM, DZ, ML, and PH were applied concurrent with the sample collection intervals. This may explain why CP and its urinary metabolites DEP+DETP were detected at low levels even though CP was applied in the Valley in significant quantities in 2005–2006. A third limitation is that DAPs were not measured in the house dust samples. A more complete picture of the relationship between pesticide-contaminated house dust and urinary pesticide metabolites might be obtained if concentrations of both intact OPs and OP breakdown products were known.
A potential confounding factor in the dust observations could include home pesticide use. Of the five OP pesticides found most abundantly in the house dust samples, only one of the five – DZ – was available to consumers for home use at the time the samples were taken [46]. Although this may have increased the variability of DZ detection in the dust samples, we do not believe it detracts from our conclusion. Moreover, regression analysis of the total DEP+DETP urinary metabolites vs. DZ residues in house dust showed no significant association for either adults or children.
CONCLUSIONS
We found significant seasonal and occupational associations between urinary DAPs and intact OP residues in house dust among this cohort of FW and NFW adults and children. We found both higher concentrations of intact OP residues in house dust, and higher concentrations of OP metabolites in urine, in the FW families. Results of our regression analysis show a significant positive association between the concentration of dimethyl OPs in household dust and the concentration of dimethyl OP metabolites in urine, most notably during thinning season, when dimethyl OPs are applied regularly, but also during harvest season when OPs are applied less frequently. When parental occupation is included in the model, results indicate that both parental occupation and concentration of OP residues in household dust influence the concentration of urinary dimethyl OP metabolites in certain agricultural seasons. Occupationally exposed farmworkers transport OPs into their homes, creating a take-home pathway by which young children are exposed to agricultural OPs.
Table 1.
Estimated annual agricultural pesticide use in metric tons for Benton and Yakima counties following the methods described in Thelin and Stone (2013). U.S. Department of Agriculture countylevel data for harvested-crop acreage were used in conjunction with proprietary Crop Reporting District-level pesticide-use data to estimate county-level pesticide use. Estimated pesticide use (EPest) values were calculated with both the EPest-high and EPest-low methods.
| Estimate (1,000 kg) | ||||||
|---|---|---|---|---|---|---|
| 2006 | 2005 | |||||
| Benton | Yakima | Total | Benton | Yakima | Total | |
| Azinphos-methyl | 14.0 | 68.5 | 82.5 | 15.5 | 70.4 | 85.9 |
| Malathion | 0.5–1.2 | 1.0−1.2 | 1.5−2.4 | 1.5−1.6 | 3.3 | 4.8−4.9 |
| Phosmet | 0.7 | 5.4 | 6.2 | 5.9 | 33.3 | 39.4 |
| Chlorpyrifos | 11.9−12.3 | 49.3−49.5 | 61.2−61.8 | 13.8−14.6 | 52.4−52.6 | 66.2−67.2 |
| Diazinon | 0.7−1.7 | 2.1−2.2 | 2.8−3.9 | 0.4−0.5 | 0.8−0.9 | 1.2−1.4 |
Thelin, G.P., and Stone, W.W., 2013, Estimation of annual agricultural pesticide use for counties of the conterminous United States, 1992–2009: U.S. Geological Survey. Scientific Investigations Report 20135009, 54 p.
Table 2.
Concentrations (Geometric Mean) and Detection Frequencies of Five Organophosphate Pesticides Most Commonly Detected in House Dust in Lower Yakima Valley, WA. Farmworker (FW) and non-farmworker (NFW) household dust concentrations are in units of nmol OP/gram house dust.
| OP Pesticide | Thinning Season | Harvest Season | Non-spray Season | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geometric | Geometric | Geometric | ||||||||||
| Azinphosmethyl | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 99% | 1.482** | (0.119–18.441) | 82 | 98% | 1.351** | (0.071–25.734) | 78 | 95% | 0.372** | (0.029–4.805) |
| NFW | 86 | 79% | 0.161 | (0.007–3.992) | 88 | 95% | 0.301 | (0.022–4.113) | 79 | 65% | 0.057 | (0.002–2.124) |
| Geometric | Geometric | Geometric | ||||||||||
| Malathion | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 88% | 0.163** | (0.005–5.442) | 82 | 80% | 0.054* | (0.002–1.216) | 78 | 62% | 0.023** | (0.001–0.429) |
| NFW | 86 | 72% | 0.016 | (0.006–0.538) | 88 | 81% | 0.031 | (0.003–0.337) | 79 | 54% | 0.009 | (0.001–0.161) |
| Geometric | Geometric | Geometric | ||||||||||
| Phosmet | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 98% | 0.556** | (0.031–10.116) | 82 | 96% | 1.057** | (0.032–29.972) | 78 | 94% | 0.282** | (0.029–2.783) |
| NFW | 86 | 73% | 0.046 | (0.001–3.393) | 88 | 97% | 0.267 | (0.019–3.733) | 79 | 73% | 0.043 | (0.001–1.549) |
| Geometric | Geometric | Geometric | ||||||||||
| Chlorpyrifos | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 60% | 0.042** | (0.001–1.762) | 82 | 49% | 0.023** | (0.001–1.076) | 78 | 47% | 0.023** | (0.001–0.702) |
| NFW | 86 | 29% | 0.005 | (0.001–0.460) | 88 | 35% | 0.007 | (0.001–0.323) | 79 | 27% | 0.004 | (<0.001–0.425) |
| Geometric | Geometric | Geometric | ||||||||||
| Diazinon | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 73% | 0.015* | (0.001–0.215) | 82 | 93% | 0.038 | (0.003–0.478) | 78 | 65% | 0.013 | (0.001–0.144) |
| NFW | 86 | 72% | 0.009 | (0.001–0.237) | 88 | 91% | 0.034 | (0.004–0.295) | 79 | 66% | 0.014 | (0.001–0.174) |
indicates significant difference at p<0.001 and
at p<0.05 between FW and NFW, based on Welch two-sample t-tests.
Table 3.
Concentrations (Geometric Mean) and Detection Frequencies of Five Organophosphate Pesticides Most Commonly Detected in Vehicle Dust in Lower Yakima Valley, WA. Farmworker (FW) and nonfarmworker (NFW) vehicle dust concentrations are in units of nmol OP/ gram house dust.
| OP Pesticide | Thinning Season | Non-spray Season | ||||||
|---|---|---|---|---|---|---|---|---|
| Geometric | Geometric | |||||||
| Azinphos-methyl | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 89 | 99% | 3.304** | (0.181–60.222) | 65 | 60% | 0.064** | (0.001–27.459) |
| NFW | 84 | 79% | 0.206 | (0.007–6.995) | 70 | 40% | 0.009 | (0.001–4.553) |
| Geometric | Geometric | |||||||
| Malathion | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 89 | 91% | 0.306** | (0.008–11.378) | 65 | 15% | 0.008** | (0.001–0.650) |
| NFW | 84 | 56% | 0.025 | (0.001–1.332) | 70 | 14% | 0.003 | (0.001–1.055) |
| Geometric | Geometric | |||||||
| Phosmet | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 89 | 98% | 0.523** | (0.047–5.861) | 65 | 38% | 0.053* | (0.001–33.634) |
| NFW | 84 | 80% | 0.101 | (0.007–1.541) | 70 | 14% | 0.010 | (0.001–1.279) |
| Geometric | Geometric | |||||||
| Chlorpyrifos | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 89 | 70% | 0.099** | (0.001–6.885) | 65 | 65% | 0.001 | (0.001–0.519) |
| NFW | 84 | 36% | 0.009 | (0.001–1.179) | 70 | 41% | 0.001 | (0.001–0.584) |
| Geometric | Geometric | |||||||
| Diazinon | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 89 | 55% | 0.014** | (0.002–0.081) | 65 | 34% | 0.005* | (0.001–0.054) |
| NFW | 84 | 36% | 0.007 | (0.001–0.103) | 70 | 27% | 0.003 | (0.001–0.047) |
indicates significant difference at p<0.001 and
at p<0.05 between FW and NFW, based on Welch two-sample ttests.
Table 4A.
Concentrations (Geometric Mean) and Detection Frequencies of Six Dialkylphosphate (DAP) Metabolites Detected in Child Urine in Lower Yakima Valley, WA. Farmworker (FW) and non-farmworker (NFW) urinary metabolite concentrations are in units of nmol DAP/milliliter urine.
| Urinary Metabolite | Thinning Season | Harvest Season | Non-Spray Season | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geometric | Geometric | Geometric | ||||||||||
| DMAP | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 100% | 0.205** | (0.031–1.341) | 82 | 100% | 0.231 | (0.027–1.987) | 78 | 100% | 0.089 | (0.011–0.709) |
| NFW | 85 | 100% | 0.100 | (0.012–0.866) | 88 | 100% | 0.216 | (0.018–2.597) | 78 | 100% | 0.113 | (0.017–0.754) |
| Geometric | Geometric | Geometric | ||||||||||
| DMP | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 96% | 0.045* | (0.005–0.434) | 82 | 94% | 0.030 | (0.002–0.455) | 78 | 83% | 0.016 | (0.001–0.188) |
| NFW | 85 | 98% | 0.030 | (0.004–0.241) | 88 | 92% | 0.044 | (0.003–0.705) | 78 | 94% | 0.031** | (0.003–0.309) |
| Geometric | Geometric | Geometric | ||||||||||
| DMTP | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 100% | 0.120** | (0.017–0.861) | 82 | 100% | 0.155 | (0.016–1.480) | 78 | 100% | 0.044 | (0.005–0.352) |
| NFW | 85 | 100% | 0.050 | (0.004–0.591) | 88 | 100% | 0.118 | (0.010–1.416) | 78 | 99% | 0.050 | (0.005–0.485) |
| Geometric | Geometric | Geometric | ||||||||||
| DMDTP | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 100% | 0.015** | (0.001–0.157) | 82 | 89% | 0.008 | (0.002-0.217) | 78 | 90% | 0.004 | (0.001–0.047) |
| NFW | 85 | 98% | 0.005 | (0.001–0.052) | 88 | 98% | 0.017* | (0.001–0.349) | 78 | 97% | 0.006 | (0.001–0.043) |
| Geometric | Geometric | Geometric | ||||||||||
| DEP+DETP | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 100% | 0.023 | (0.003–0.164) | 85 | 78% | 0.008 | (<0.001–0.193) | 78 | 91% | 0.013 | (0.001–0.162) |
| NFW | 85 | 100% | 0.024 | (0.012–0.866) | 85 | 98% | 0.020** | (0.003–0.160) | 78 | 99% | 0.025** | (0.003–0.185) |
| Geometric | Geometric | Geometric | ||||||||||
| DEP | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 94% | 0.013 | (0.001–0.199) | 82 | 57% | 0.004 | (<0.001–0.121) | 78 | 64% | 0.004 | (0.001–0.112) |
| NFW | 85 | 98% | 0.012 | (0.001–0.200) | 88 | 81% | 0.008* | (0.001–0.156) | 78 | 92% | 0.014** | (0.001–0.212) |
| Geometric | Geometric | Geometric | ||||||||||
| DETP | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 98% | 0.004 | (0.001–0.024) | 82 | 66% | 0.002 | (<0.001–0.037) | 78 | 91% | 0.004 | (0.001–0.004) |
| NFW | 85 | 96% | 0.004 | (0.001–0.025) | 88 | 97% | 0.006** | (0.001–0.048) | 78 | 99% | 0.005 | (0.001–0.031) |
| DEDTP | Geometric | Geometric | Geometric | |||||||||
| (not included in calculations) | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 79% | 0.001 | (0.001–0.007) | 82 | 39% | 0.001 | (<0.001–0.004) | 78 | 49% | 0.001 | (<0.001–0.005) |
| NFW | 85 | 92% | 0.001 | (0.001–0.004) | 88 | 73% | 0.001 | (0.001–0.008) | 78 | 86% | 0.001 | (0.001–0.007) |
indicates significant difference at p<0.001 and
at p<0.05 between FW and NFW, based on Welch two-sample t-tests.
Table 4B.
Concentrations (Geometric Mean) and Detection Frequencies of Six Dialkylphosphate (DAP) Metabolites Detected in Adult Urine in Lower Yakima Valley, WA. Farmworker (FW) and non-farmworker (NFW) urinary metabolite concentrations are in units of nmol DAP/milliliter urine.
| Urinary Metabolite | Thinning Season | Harvest Season | Non-Spray Season | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geometric | Geometric | Geometric | ||||||||||
| DMAP | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 100% | 0.656** | (0.050–8.718) | 82 | 100% | 0.690** | (0.037–12.824) | 78 | 100% | 0.082 | (0.012–0.552) |
| NFW | 86 | 100% | 0.076 | (0.016–0.355) | 88 | 100% | 0.246 | (0.012–4.976) | 79 | 100% | 0.092 | (0.019–0.456) |
| Geometric | Geometric | Geometric | ||||||||||
| DMP | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 98% | 0.107** | (0.008–1.492) | 82 | 98% | 0.075* | (0.002–2.511) | 78 | 73% | 0.012 | (0.001–0.166) |
| NFW | 86 | 97% | 0.019 | (0.003–0.135) | 88 | 91% | 0.047 | (0.002–0.927) | 79 | 95% | 0.024** | (0.003–0.215) |
| Geometric | Geometric | Geometric | ||||||||||
| DMTP | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 100% | 0.433** | (0.027–7.548) | 82 | 100% | 0.472** | (0.023–9.873) | 78 | 100% | 0.048 | (0.008–0.289) |
| NFW | 86 | 100% | 0.037 | (0.006–0.220) | 88 | 100% | 0.131 | (0.005–3.504) | 79 | 100% | 0.043 | (0.008–0.289) |
| Geometric | Geometric | Geometric | ||||||||||
| DMDTP | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 100% | 0.038** | (0.003–0.570) | 82 | 90% | 0.021 | (0.001–1.561) | 78 | 95% | 0.003 | (0.001–0.040) |
| NFW | 86 | 100% | 0.005 | (0.001–0.031) | 88 | 98% | 0.020 | (0.001–0.602) | 79 | 97% | 0.004 | (0.001–0.026) |
| Geometric | Geometric | Geometric | ||||||||||
| DEP+DETP | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 99% | 0.025 | (0.003–0.127) | 82 | 82% | 0.011* | (0.001–0.240) | 78 | 95% | 0.011 | (0.001–0.112) |
| NFW | 86 | 100% | 0.020 | (0.003–0.133) | 88 | 92% | 0.018 | (0.001–0.265) | 79 | 99% | 0.022** | (0.003–0.177) |
| Geometric | Geometric | Geometric | ||||||||||
| DEP | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 93% | 0.012 | (0.001–0.212) | 82 | 63% | 0.004 | (<0.001–0.163) | 78 | 64% | 0.003 | (<0.001–0.071) |
| NFW | 86 | 93% | 0.011 | (0.001–0.173) | 88 | 74% | 0.007 | (0.001–0.226) | 79 | 86% | 0.011** | (0.001–0.201) |
| Geometric | Geometric | Geometric | ||||||||||
| DETP | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 95% | 0.005* | (0.001–0.045) | 82 | 71% | 0.002 | (<0.001–0.055) | 78 | 94% | 0.004 | (0.001–0.041) |
| NFW | 86 | 99% | 0.004 | (0.001–0.022) | 88 | 91% | 0.005** | (0.001–0.075) | 79 | 99% | 0.004 | (0.001–0.029) |
| DEDTP | Geometric | Geometric | Geometric | |||||||||
| (not included in calculations) | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 84% | 0.001 | (0.001–0.007) | 82 | 49% | 0.001 | (<0.001–0.003) | 78 | 55% | 0.001 | (<0.001 –0.005) |
| NFW | 86 | 91% | 0.001 | (0.001–0.006) | 88 | 80% | 0.001 | (0.001–0.010) | 79 | 84% | 0.001 | (0.001–0.008) |
indicates significant difference at p<0.001 and
at p<0.05 between FW and NFW, based on Welch two-sample t-tests.
Table 5A.
Proportions (Geometric Mean) and Detection Frequencies of Three Dimethylalkylphosphate (DMAP) Metabolites Detected in Child Urine in Lower Yakima Valley, WA. Farmworker (FW) and non-farmworker (NFW) urinary metabolite concentrations are in units of nmol DAP/milliliter urine.
| Urinary Metabolite | Thinning Season | Harvest Season | Non-Spray Season | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geometric | Geometric | Geometric | ||||||||||
| DMP | n | % Detect | Mean (%) | 95% CI | n | % Detect | Mean (%) | 95% CI | n | % Detect | Mean (%) | 95% CI |
| FW | 85 | 96% | 22.7** | 5.9–86.9 | 82 | 94% | 13.9** | 2.5–75.5 | 78 | 83% | 22.2* | 5.1–96.2 |
| NFW | 85 | 98% | 32.4 | 12.2–85.8 | 88 | 92% | 22.2 | 5.1–97.5 | 78 | 94% | 31.3 | 8.1–120.7 |
| Geometric | Geometric | Geometric | ||||||||||
| DMTP | n | % Detect | Mean (%) | 95% CI | n | % Detect | Mean (%) | 95% CI | n | % Detect | Mean (%) | 95% CI |
| FW | 85 | 100% | 61.1 | 37.2–100.6 | 82 | 100% | 73.6 | 50.6–107.1 | 78 | 100% | 62.1 | 35.2–109.8 |
| NFW | 85 | 100% | 53.6* | 27.7–103.6 | 88 | 100% | 60.5** | 38.2–95.7 | 78 | 99% | 51.3* | 18.4–142.6 |
| Geometric | Geometric | Geometric | ||||||||||
| DMDTP | n | % Detect | Mean (%) | 95% CI | n | % Detect | Mean (%) | 95% CI | n | % Detect | Mean (%) | 95% CI |
| FW | 85 | 100% | 7.9 | 2.3–27.5 | 82 | 89% | 3.6** | 0.3–48.7 | 78 | 90% | 5.7 | 1.3–25.8 |
| NFW | 85 | 98% | 5.9* | 1.6–25.9 | 88 | 98% | 8.7 | 2.2–34.2 | 78 | 97% | 5.6 | 1.4–22.5 |
indicates significant difference at p<0.001 and
at p<0.05 between FW and NFW, based on Welch twosample t-tests.
Table 5B.
Proportions (Geometric Mean) and Detection Frequencies of Three Dimethylalkylphosphate (DMAP) Metabolites Detected in Adult Urine in Lower Yakima Valley, WA. Farmworker (FW) and non-farmworker (NFW) urinary metabolite concentrations are in units of nmol DAP/milliliter urine.
| Urinary Metabolite | Thinning Season | Harvest Season | Non-Spray Season | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geometric | Geometric | Geometric | ||||||||||
| DMP | n | % Detect | Mean (%) | 95% CI | n | % Detect | Mean (%) | 95% CI | n | % Detect | Mean (%) | 95% CI |
| FW | 85 | 98% | 17.0** | 4.0–72.2 | 82 | 98% | 11.9** | 1.4–99.2 | 78 | 73% | 17.2** | 2.9–102.7 |
| NFW | 86 | 97% | 27.8 | 6.8–112.9 | 88 | 91% | 21.3 | 5.4–84.4 | 79 | 95% | 29.1 | 6.5–130.4 |
| Geometric | Geometric | Geometric | ||||||||||
| DMTP | n | % Detect | Mean (%) | 95% CI | n | % Detect | Mean (%) | 95% CI | n | % Detect | Mean (%) | 95% CI |
| FW | 85 | 100% | 69.2 | 44.0–108.6 | 82 | 100% | 74.9 | 51.7–108.5 | 78 | 100% | 67.0 | 38.3–117.1 |
| NFW | 86 | 100% | 54.7** | 27.6–108.2 | 88 | 100% | 59.4** | 30.3–116.8 | 79 | 100% | 52.7** | 20.1–138.1 |
| Geometric | Geometric | Geometric | ||||||||||
| DMDTP | n | % Detect | Mean (%) | 95% CI | n | % Detect | Mean (%) | 95% CI | n | % Detect | Mean | 95% CI |
| FW | 85 | 100% | 6.0 | 1.5–31.7 | 82 | 90% | 3.3** | 0.2–68.4 | 78 | 95% | 4.8 | 0.9–24.9 |
| NFW | 86 | 100% | 7.1 | 2.0–25.6 | 88 | 98% | 9.0 | 2.4–34.1 | 79 | 97% | 5.1 | 1.4–18.0 |
indicates significant difference at p<0.001 and
“*” at p<0.05 between FW and NFW, based on Welch twosample t-tests.
Table 6A.
Concentrations (Geometric Mean) of Six Dialkylphosphate (DAP) Metabolites Detected in Child Urine in United States and Lower Yakima Valley, WA, compared to data from the National Health and Nutrition Examination Survey (NHANES), conducted by the U.S. Center for Disease Control's National Center for Health Statistics. Urinary metabolite concentrations are in units of nmol DAP/milliliter urine, without creatinine correction.
| NHANES 2005–06 | UW Center for Child Environmental Health Risks Research | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50th Percentile | Sample | Thinning | Harvest | Non-Spray | |||||||||||||||||
| OP Metabolite | GM | 95% Cl | GM | 95% Cl | Size | GM | 95% Cl | GM | 95% Cl | GM | 95% Cl | ||||||||||
| DMP | |||||||||||||||||||||
| Children 6–11 | # | <LOD | 350 | ||||||||||||||||||
| FW | |||||||||||||||||||||
| Children 2–6 | 0.045 | (0.005–0.434) | 0.03 | (0.002–0.455) | 0.016 | (0.001–0.188) | |||||||||||||||
| NFW | |||||||||||||||||||||
| Children 2–6 | 0.030 | (0.004–0.241) | 0.044 | (0.003–0.705) | 0.031 | (0.003–0.309) | |||||||||||||||
| DMTP | 1 | ||||||||||||||||||||
| Children 6–11 | 0.019 | (0.016–0.023) | 0.017 | (0.012–0.024) | 349 | ||||||||||||||||
| FW | |||||||||||||||||||||
| Children 2–6 | (0.017–0.861) | 0.155 | (0.016–1.480) | 0.044 | (0.005–0.352) | ||||||||||||||||
| NFW | |||||||||||||||||||||
| Children 2–6 | 0.050 | (0.004–0.591) | 0.118 | (0.010–1.416) | 0.050 | (0.005–0.485) | |||||||||||||||
| DMDTP | |||||||||||||||||||||
| Children 6–11 | # | <LOD | 350 | ||||||||||||||||||
| FW | |||||||||||||||||||||
| Children 2–6 | 0.015 | (0.001–0.157) | 0.008 | (0.002–0.217) | 0.004 | (0.001–0.047) | |||||||||||||||
| NFW | |||||||||||||||||||||
| Children 2–6 | 0.005 | (0.001–0.052) | 0.017 | (0.001–0.349) | 0.006 | (0.001–0.043) | |||||||||||||||
| DEP | |||||||||||||||||||||
| Children 6–11 | # | <LOD | 350 | ||||||||||||||||||
| FW | |||||||||||||||||||||
| Children 2–6 | 0.013 | (0.001–0.199) | 0.004 | (C0.001–0.121) | 0.004 | (0.001–0.112) | |||||||||||||||
| NFW Children 2–6 | 0.012 | (0.001–0.200) | 0.008 | (0.001–0.156) | 0.014 | (0.001–0.212) | |||||||||||||||
| DETP | |||||||||||||||||||||
| Children 6–11 | # | <LOD | 350 | ||||||||||||||||||
| FW | |||||||||||||||||||||
| Children 2–6 | 0.004 | (0.001–0.024) | 0.002 | (<0.001–0.0.7) | 0.004 | (0.001-0.004) | |||||||||||||||
| NFW | |||||||||||||||||||||
| Childre n 2–6 | 0.004 | (0.001–0.025) | 0.006 | (0.001–0.048) | 0.005 | (0.001-0.031) | |||||||||||||||
| DE DTP | |||||||||||||||||||||
| Childre n 6–11 | # | <LOD | 317 | ||||||||||||||||||
| FW | |||||||||||||||||||||
| Childre n 2–6 | 0.001 | (0.001-0.00^. | o | (CO.OOI-0.004) | 0.001 | (cO.OO1-0.005) | |||||||||||||||
| NFW | |||||||||||||||||||||
| Children 2–6 | 0.001^1 | (0.001-0.004) | 0.001 | (0.001-0.008) | 0.001 | (0.001-0.007) | |||||||||||||||
# Geometric Mean was not calculated if the proportion of results below the limit of detection was greater than 40%.
"<LOD" means less than the limit of detection, which may vary by year and by individual sample.
Table 6B.
Concentrations (Geometric Mean) of Six Dialkylphosphate (DAP) Metabolites Detected in Adult Urine in United States and Lower Yakima Valley, WA, compared to data from the National Health and Nutrition Examination Survey (NHANES), conducted by the U.S. Center for Disease Control's National Center for Health Statistics. Urinary metabolite concentrations are in units of nmol DAP/milliliter urine, without creatinine correction.
| NHANES 2005–06 | UW Center for Child Environmental Health Risks Research | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50th Percentile | Sampie | Thinning | Harvest | Non-Spray | |||||||||
| OP | |||||||||||||
| Metabolite | GM | 95% Cl | GM | 95% Cl | Size | GM | 95% Cl | GM | 95% Cl | GM | 95% Cl | ||
| DMP | A, | ||||||||||||
| Adults20–59 | # | <LOD | 1,092 | > | <5 | ||||||||
| MexicanAmericans | <LOD | ||||||||||||
| FWAdults | 0.107 | (0.008-1.492) | ^©.075 | (0.002-2.511) | 0.012 | (0.001-0.166) | |||||||
| NFWAdults | 0.019. | (0.003- | 0.047 | (0.002-0.927) | 0.024 | (0.003-0.215) | |||||||
| DMTP | |||||||||||||
| Adults20–59 | 0.012 | (0.010-0.014) | 0.010 | (0.009-0.012) | 1/09 | r | |||||||
| MexicanAmericans | 0.017 | (0.015-0.019) | 1 | À | |||||||||
| FWAdultsNFWAdults | 0.4330.037 | (0.027-7.548)(0.006-0.220) | 0.4720.131 | (0.024-9.873)(0.005-3.504) | 0.0480.043 | (0.008-0.289)(0.008-0.289) | |||||||
| DMDTP | (S | t | |||||||||||
| Adults | XL) | > | 1,09 | ||||||||||
| 20–59 | # | <LOD | 2 | ||||||||||
| MexicanAmericans | <LOD | rV | J | ||||||||||
| FWAdults | 0.038 | (0.003-0.127) | 0.021 | (0.001-0.240) | 0.003 | (0.001-0.112) | |||||||
| NFWAdults | \ | 0.005 | (0.003-0.133) | 0.020 | (0.001-0.265) | 0.004 | (0.003-0.177) | ||||||
| DEP | |||||||||||||
| Adults20–59 | # | <LOD | 1,092 | ||||||||||
| MexicanAmericans | <LOD | ||||||||||||
| FWAdults | 0.012 | (0.001-0.212) | 0.004 | (C0.001-0.163) | 0.003 | (co.001-0.071) | |||||||
| NFWAdults | 0.011 | (0.001-0.173) | 0.007 | (0.001-0.226) | 0.011 | (0.001–0.201) | |||||||
| DETP | |||||||||||||
| Adults20–59 | # | <LOD | 1,092 | ||||||||||
| MexicanAmericans | <LOD | ||||||||||||
| FWAdults | 0.005 | (0.001-0.045) | 0.002 | (<0.001-0.055) | 0.004 | (0.001–0.041) | |||||||
| NFWAdults | 0.004 | (0.001-0.022) | 0.005JL | (0.001-0.075)vv | 0.004 | (0.001–0.029) | |||||||
| DEDTP | |||||||||||||
| Adults20–59 | # | <LOD | 1,006 | ||||||||||
| MexicanAmericans | <LOD | ||||||||||||
| FWAdults | 0.001 | (0.001-0.007) | 0.001 | (<0.001–0.003) | 0.001 | (<0.001–0.005) | |||||||
| NFWAdults | K Vi | (0.001-0.006) | 0.001 | (0.001-0.010) | 0.001 | (0.001–0.008) | |||||||
# Geometric Mean was not calculated if the proportion of results below the limit of detection was greater than 40%.
"<LOD" means less than the limit of detection, which may vary by year and by individual sample.
CLINICAL SIGNIFICANCE.
Exposure to OPs, which are used to control agricultural pests, have been shown to cause adverse health outcomes in adults and children. Thus this paper looks at how they are exposed is significant.
FWs have higher exposure to OPs than NFWs living in the same community.
Adult FWs may carry OPs into their homes, creating an exposure pathway for young children residing in the household.
Evaluation of exposure biomarkers helps in understanding mechanisms underlying the association between adult’s workplace exposure to OPs and children’s home exposure to OPs via house dust.
This biomarker information on exposure pathways for pesticides provides new options for interventions to reduce exposure of both adults and children.
Acknowledgments
FUNDING DETAILS
This project was made possible by The University of Washington Center for Child Environmental Health Risks Research, supported by the National Institute of Environmental Health Sciences (grant PO1 ES009601), the USEPA (grants RD826886, RD83451401, and RD-83273301), and the National Institutes of Health (contract HHSN267200700023C). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences, the National Institutes of Health, or the USEPA.
Footnotes
DISCLOSURE STATEMENT
The authors have no conflicts of interest to disclose.
References
- [1].Thompson B, Coronado GD, Grossman JE, Puschel K, Solomon CC, Islas I, Curl CL, Shirai JH, Kissel JC, and Fenske RA, “Pesticide take-home pathway among children of agricultural workers: Study design, methods, and baseline findings,” J. Occup. Environ. Med, vol. 45, no. 1, pp. 42–53, 2003. [DOI] [PubMed] [Google Scholar]
- [2].Thompson B, Coronado GD, Vigoren EM, Griffith WC, Fenske RA, Kissel JC, Shirai JH, and Faustman EM, “Para ninos saludables: a community intervention trial to reduce organophosphate pesticide exposure in children of farmworkers,” Environ. Health Perspect, vol. 116, no. 5, pp. 687–694, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Coronado GD, Vigoren EM, Thompson B, Griffith WC, and Faustman EM, “Organophosphate pesticide exposure and work in pome fruit: Evidence for the take-home pesticide pathway,” Environ. Health Perspect, vol. 114, no. 7, pp. 999–1006, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Coronado GD, Vigoren EM, Griffith WC, Faustman EM, and Thompson B, “Organophosphate Pesticide Exposure Among Pome and Non-Pome Farmworkers: A Subgroup Analysis of a Community Randomized Trial,” J. Occup. Environ. Med, pp. 500–509, 2009. [DOI] [PubMed] [Google Scholar]
- [5].Thompson B, Coronado G, Puschel K, and Allen E, “Identifying constituents to participate in a project to control pesticide exposure in children of farmworkers,” Environ. Health Perspect, vol. 109, no. Supplement 3 June, pp. 443–8, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Thompson B, Griffith WC, Barr DB, Coronado GD, Vigoren EM, and Faustman EM, “Variability in the take-home pathway: farmworkers and nonfarmworkers and their children,” J. Expo. Sci. Environ. Epidemiol, vol. 24, no. 5, pp. 522–31, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Coronado GD, Holte S, Vigoren E, Griffith WC, Barr DB, Faustman E, and Thompson B, “Organophosphate pesticide exposure and residential proximity to nearby fields: evidence for the drift pathway,” J. Occup. Environ. Med, vol. 53, no. 8, pp. 884–91, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].United States Environmental Protection Agency, Exposure Factors Handbook, 2011 Edition (Final). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-09/052F, 2011. [Google Scholar]
- [9].Fenske RA, Kissel JC, Lu C, Kalman DA, Simcox NJ, Allen EH, and Keifer MC, “Biologically based pesticide dose estimates for children in an agricultural community,” Environ. Health Perspect, vol. 108, no. 6, pp. 515–20, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].United States Department of Agriculture, “Washington Tree Fruit Survey 2006.” [Google Scholar]
- [11].United States Department of Agriculture Washington Field Office, “2006 Washington Annual Agriculture Bulletin,” 2006. [Google Scholar]
- [12].United States Department of Agriculture, “Department of Agricuture National Agricultural Statistics Service, Agricultural Statistics 2006,” 2006. [Google Scholar]
- [13].Thelin GP and Stone WW, “Estimation of annual agricultural pesticide use for counties of the conterminous United States, 1992–2009: Scientific investigation report 2013–5009, U.S. Geological Survey Scientific Investigations Report,” 2013. [Google Scholar]
- [14].Stone WW, “Estimated Annual Agricultural Pesticide Use for Counties of the Conterminous United States, 1992–2009,” U.S. Geol. Surv. Data Ser. 752, p. 1–pamphlet, 14 tables, 2013. [Google Scholar]
- [15].United States Department of Commerce, “Profiles of General Demographic Characteristics: 2000 Census of Population and Housing,” 2001.
- [16].United States Department of Commerce, “Profiles of General Demographic Characteristics: 2010 Census of Population and Housing,” 2012.
- [17].Moate TF, Lu C, a Fenske R, Hahne RM, and a Kalman D, “Improved cleanup and determination of dialkyl phosphates in the urine of children exposed to organophosphorus insecticides,” J. Anal. Toxicol, vol. 23, no. August, pp. 230–236, 1999. [DOI] [PubMed] [Google Scholar]
- [18].Curl CL, Fenske RA, Kissel JC, Shirai JH, Moate TF, Griffith W, Coronado G, and Thompson B, “Evaluation of take-home organophosphorus pesticide exposure among agricultural workers and their children,” Environ. Health Perspect, vol. 110, no. 12, pp. 787–792, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bravo R, Caltabiano LM, Weerasekera G, Whitehead RD, Fernandez C, Needham LL, Bradman A, and Barr DB, “Measurement of dialkyl phosphate metabolites of organophosphorus pesticides in human urine using lyophilization with gas chromatography-tandem mass spectrometry and isotope dilution quantification,” J. Expo. Anal. Environ. Epidemiol, vol. 14, pp. 249–259, 2004. [DOI] [PubMed] [Google Scholar]
- [20].Coye MJ, Lowe JA, and Maddy KJ, “Biological monitoring of agricultural workers exposed to pesticides II. Monitoring of Intact Pesticides and Their Metabolites,” J. Occup. Med, vol. 28, no. 8, pp. 628–636, 1986. [DOI] [PubMed] [Google Scholar]
- [21].Coronado GD, Griffith WC, Vigoren EM, and Faustman EM, “Where’s the Dust? Characterizing Locations of Azinphos-Methyl Residues in House and Vehicle Dust Among Farmworkers with Young Children,” J. Environ. Occup. Hyg, vol. 7, no. 12, pp. 663–671, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Smith MN, Workman T, McDonald KM, Vredevoogd MA, Vigoren EM, Griffith WC, Thompson B, Coronado GD, Barr D, and Faustman EM, “Seasonal and occupational trends of five organophosphate pesticides in house dust,” J. Expo. Sci. Environ. Epidemiol, vol. 2016, no. 0, pp. 1–7, 2016. [DOI] [PubMed] [Google Scholar]
- [23].Griffith W, Curl CL, Fenske RA, Lu CA, Vigoren EM, and Faustman EM, “Organophosphate pesticide metabolite levels in pre-school children in an agricultural community: within- and between-child variability in a longitudinal study,” Environ. Res, vol. 111, no. 6, pp. 751–6, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, and Pirkle JL, “Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements,” Environ. Health Perspect, vol. 113, no. 2, pp. 192–200, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Barr DB, Bravo R, Weerasekera G, Caltabiano LM, Whitehead RD, Olsson AO, Caudill SP, Schober SE, Pirkle JL, Sampson EJ, Jackson RJ, and Needham LL, “Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U.S. population,” Environ. Health Perspect, vol. 112, no. 2, pp. 186–200, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lunn DJ, Thomas A, Best N, and Spiegelhalter D, “WinBUGS – A Bayesian modelling framework: Concepts, structure, and extensibility,” Stat. Comput, vol. 10, pp. 325–337, 2000. [Google Scholar]
- [27].R Core Team 2016, “R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.” [Google Scholar]
- [28].Washington State University Extension, 2008 Crop Protection Guide for Tree Fruits in Washington. 2008.
- [29].Brunner JF, Jones W, Beers E, Gerald V, Dunley J, Xiao C, and Grove GG, “A Decade of Pesticide Use and IPM Practices in Washington’s Apple Orchards,” WSU Tree Fruit Res. Ext. Cent, no. 205, 2003. [Google Scholar]
- [30].World Health Organization (WHO), Organophosphorus Insecticides: A General Introduction. World Health Organization, New York, NY: 1986. [Google Scholar]
- [31].Forsberg ND, Rodriguez-Proteau R, Ma L, Christensen JM, Maier CS, Jenkings JJ, and Anderson KA, “Organophosphorus pesticide degradation product in vitro metabolic stability and time-course uptake and elimination in rats following oral and intravenous dosing,” Xenobiotica, vol. 41, no. 5, pp. 422–429, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Timchalk C, Busby A, Campbell JA, Needham LL, and Barr DB, “Comparative pharmacokinetics of the organophosphorus insecticide chlorpyrifos and its major metabolites diethylphosphate, diethylthiophosphate and 3,5,6-trichloro-2-pyridinol in the rat,” Toxicology, vol. 237, no. 1–3, pp. 145–157, 2007. [DOI] [PubMed] [Google Scholar]
- [33].Barr DB, “Biomonitoring of exposure to pesticides,” J. Chem. Heal. Saf, vol. 15, no. 6, pp. 20–29, 2008. [Google Scholar]
- [34].Faustman EM, Silbernagel SM, Fenske RA, Burbacher TM, and Ponce RA, “Mechanisms Underlying Children’s Susceptibility to Environmental Toxicants,” Environ. Heal, vol. 108, no. March, pp. 13–21, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].National Academy of Sciences (NAS), Pesticides in the Diets of Infants and Children. 1993. [PubMed] [Google Scholar]
- [36].Bouchard MF, Bellinger DC, Wright RO, and Weisskopf MG, “Attention-Deficit/Hyperactivity Disorder and Urinary Metabolites of Organophosphate Pesticides,” Pediatrics, vol. 125, no. 6, pp. e1270–e1277, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Grandjean P, “Pesticide Exposure and Stunting as Independent Predictors of Neurobehavioral Deficits in Ecuadorian School Children,” Pediatrics, vol. 117, no. 3, pp. e546–e556, 2006. [DOI] [PubMed] [Google Scholar]
- [38].Oulhote Y and Bouchard MF, “Urinary metabolites of organophosphate and pyrethroid pesticides and behavioral problems in Canadian children,” Environ. Health Perspect, vol. 121, no. 11–12, pp. 1378–1384, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sánchez Lizardi P, O’Rourke MK, and Morris RJ, “The effects of organophosphate pesticide exposure on Hispanic children’s cognitive and behavioral functioning,” J. Pediatr. Psychol, vol. 33, no. 1, pp. 91–101, 2008. [DOI] [PubMed] [Google Scholar]
- [40].Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, Calderon N, and Eskenazi B, “Organophosphate Pesticide Exposure and Attention in Young Mexican-American Children: The CHAMACOS Study,” Environ. Health Perspect, vol. 118, no. 12, pp. 1768–1774, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Raanan R, Balmes JR, Harley KG, Gunier RB, Magzamen S, Bradman A, and Eskenazi B, “Decreased lung function in 7-year-old children with earlylife organophosphate exposure.,” Thorax, vol. 71, no. 2, pp. 148–153, 2015. [DOI] [PubMed] [Google Scholar]
- [42].Raanan R, Harley KG, Balmes JR, Bradman A, Lipsett M, and Eskenazi B, “Early-life exposure to organophosphate pesticides and pediatric respiratory symptoms in the CHAMACOS cohort,” Environ. Health Perspect, vol. 123, no. 2, pp. 179–185, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Quiros-Alcala L, Bradman A, Smith K, Weerasekera G, Odetodun M, Barr DB, Nishioka M, Castorina R, Hubbard AE, Nicas M, Katherine HS, McKone TE, and Eskenazi BB, “Organophosphorus pesticide breakdown products in house dust and children’s urine,” J. Expo. Sci. Environ. Epidemiol, vol. 22, no. 6, pp. 559–568, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen L, Zhao TF, Pan CP, Ross JH, and Krieger RI, “Preformed biomarkers including dialkylphosphates (DAPs) in produce may confound biomonitoring in pesticide exposure and risk assessment,” J. Agric. Food Chem, vol. 60, no. 36, pp. 9342–9351, 2012. [DOI] [PubMed] [Google Scholar]
- [45].Kissel JC, Curl CL, Kedan G, Lu C, Griffith W, Barr DB, Needham LL, and Fenske RA, “Comparison of organophosphorus pesticide metabolite levels in single and multiple daily urine samples collected from preschool children in Washington State,” J. Expo. Anal. Environ. Epidemiol, vol. 15, no. 2, pp. 164–171, 2005. [DOI] [PubMed] [Google Scholar]
- [46].United States Environmental Protection Agency, “Interim Reregistration Eligibility Decision for Diazinon,” 2004. [Google Scholar]