Abstract
Multicellular organisms synthesize and renew components of their subcellular and scaffolding proteins, collectively known as the extracellular matrix molecules (ECMs). In the lung, ECMs maintain tensile strength, elasticity, and dictate the specialized function of multiple cell lineages. These functions are critical in lung homeostatic processes including cellular migration and proliferation during morphogenesis or in response to repair. Alterations in lung ECMs that expose cells to new cryptic fragments, generated in response to endogenous proteinases or exogenous toxins, are associated with the development of several common respiratory diseases. How lung ECMs provide or relay vital signals to epithelial and mesenchymal cells has shed new light on development and progression of several common chronic respiratory diseases. This review will consider how ECMs regulate lung homeostasis and their reorganization under pathological conditions that can modulate the inflammatory diseases asthma, chronic obstructive pulmonary disease (COPD), and idiopathic pulmonary fibrosis (IPF). Better understanding of changes in the distribution of lung ECM could provide novel therapeutic approaches to treat chronic lung diseases.
Introduction
Genomic and proteomic studies have identified over 300 different extracellular matrix molecules (hereafter referred to as ECM) that control the structure and function of multicellular organisms [1]. Although they were first recognized for their function as scaffolding and structural molecules that support of all living cells, the ECM are now known as critical, acellular components of cells because they orchestrate essential cellular processes, including morphogenesis, signal transduction, migration, proliferation and wound repair [2–4]. While some components of the ECM are highly preserved among different tissues, their unique qualities provide a window into organ-specific functions. For instance, the rigidity and pliability of the ECM is dependent on their macromolecular components, which are dictated by tissue-specific functions.
Predominately, proteins and glycoproteins comprise the largest portion of the ECM molecular niches [5, 6]. Two primary forms of matrices exist: 1) the basement membrane ECM that lie immediately below epithelial and endothelial cells, and 2) the acellular, interstitial ECM components embedded within the tissue that surrounds cells. Non-fibrillar collagens, laminins, glycoproteins, and proteoglycans make up most of the basement membrane, which compartmentalize the epithelium or endothelium from their surrounding stroma [7, 8]. The primary role of basement membrane ECM includes barrier function that is especially important for the mucosal tissues (e.g., respiratory, gastrointestinal, and genitourinary organs) [7, 8]. The tissue-specific acellular interstitial matrix is composed of connective tissue with large concentrations of fibrous proteins (e.g., collagen, elastin), fibronectin and proteoglycans that are assembled into intricate fibril networks [9–11]. The interstitial matrix provides the necessary scaffolding to enable structural stability and resistance among all tissue [9–11].
In addition to their major roles in maintaining structural stability and tissue homeostasis, ECM can also modulate cell signaling as intact or modified molecules. For example, ECMs can activate or repress cell-specific transcription factors, supporting their direct role in tissue morphogenesis and cell fate [12, 13], while secreted proteinases can cleave and modify ECMs, further diversifying their function [14]. The term ‘matrikine’ or ‘matricryptin’ has been attributed to the bioactive fragments of extracellular matrix that modulate multiple physiological processes [14–16]. A number of ECM-derived matrikines have been shown to be involved in inflammation and immune homeostasis, while others are implicated in tumor metabolism and invasion [14–16]. Here, we discuss several unique features of the lung ECM as they relate to disease pathogenesis driven by immune modulation and matrikine signaling functions. This review focuses on the role of ECM in the pathogenesis of idiopathic pulmonary fibrosis, asthma, and chronic obstructive pulmonary disease.
Lung ECM
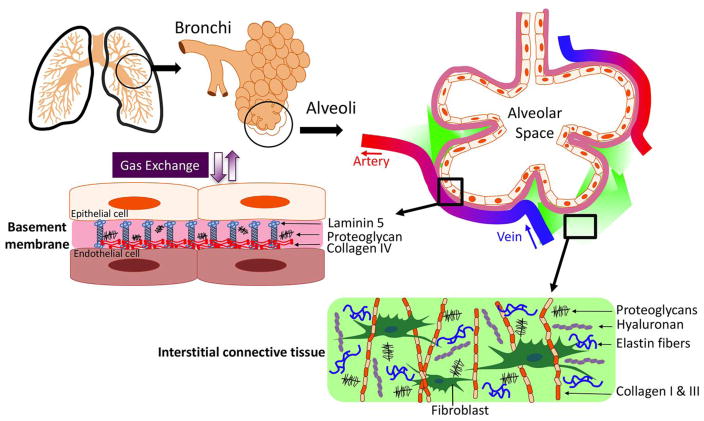
The lungs provide two vital physiological functions: passive gas exchange (alveolar respiration), and lung-specific innate immune defense against pathogenic insults that protect the airway epithelial barrier. Notably, the lung’s elastin-rich ECM is molecularly distinct from that of the kidney, which is a more rigid organ and is predominantly composed of several types of collagens (Type I, III, VI, VII,) that are less abundant in the lung [17]. Furthermore, although the intestine and lung share a common embryonic origin, the gut ECM has higher concentrations of laminins that are critical for tight cell-to-cell junctions that are necessary for interactions with the gut microbiota [18]. Lung ECM is often altered in response to different environmental insults that damage airway epithelia, such as chronic exposure to inhaled antigens, cigarette smoke, air pollution, viral or bacterial pathogens, and trauma [19]. Injured epithelial cells can promote specific ECM remodeling that in turn affects their local cellular function. Therefore, the unique composition of lung ECMs that support bronchial epithelial cells in the proximal conducting airway secrete more collagens, laminins, and proteoglycans [20], whereas, in the alveolar termini, type I epithelial cell walls nearly fuse with those of proximal capillary endothelial cells, creating an ultrathin elastin-predominant ECM to permit efficient gas exchange [20, 21] (Figure 1).
Figure 1. Composition and Distribution of lung ECM.
The ECM basement membrane matrix is composed of nonfibrillar collagens, laminin, and proteoglycans that support many vital physiological lung function. In the proximal airway, basement membrane ECM is dense, while the distal alveoli have an ultrathin basement membrane that aids in effective gas exchange. The lung’s interstitial connective tissue provides necessary elastic properties and tensile strength and is composed of complex networks of fibrous proteins (fibrillar collagen and elastin) as well as hyaluronan and proteoglycans. Excessive accumulation or degradation of ECM molecules within the interstitial matrix is thought to underlie the pathogenesis of chronic lung diseases like emphysema and idiopathic fibrosis.
A further unique aspect of the lung is the notable differences in the ECM basement membrane components and lung interstitial parenchyma. The airway basement membrane contains proteins (collagen IV & V, numerous proteoglycans, enactin, and laminins), which are specialized to promote airflow in the proximal airway and enhance appropriate gas exchange at the terminal alveoli [21].
Whilst specialized mesenchymal cells (e.g., myofibroblasts, fibrocytes, smooth muscle cells) within the lung interstitial space can secrete distinct sets of ECM molecules (e.g., collagens, laminin, elastin, proteoglycans, hyaluronans, etc.) [20, 22], they can also produce bioactive matrikines contributing to lung disease pathogenesis (Figure 2). For example, degradation of laminin by several MMPs (e.g. MMP3, MMP12, MMP14, MMP20) or neutrophil elastase, generates, laminin-332 and laminin-5, two matrikines that bind to EGFR to regulate epithelial proliferation (Figure 3) [23, 24]. In a preclinical model of asthma, overexpression of hyaluronan synthase 2 (HAS2) in myofibroblasts and smooth muscle cells resulted in increased airway fibrosis and reduced AHR. These findings suggest that HA accumulation in the airway wall may contribute to airway thickening in asthma via HAS2 [25].
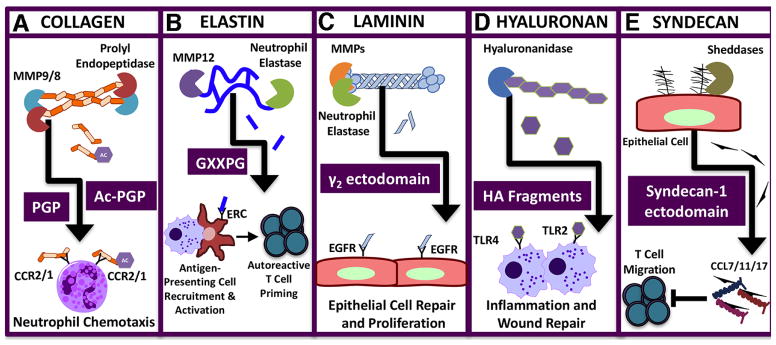
Figure 2. Matrix/Matrikine-derived signaling in the lung.
Lung proteinases generate a wide array of matrikines that initiate or dampen inflammatory events in response to diverse insults. A) Collagen fragments, PGP and Ac-PGP, promote neutrophil recruitment to the lung in inflammatory diseases such as COPD. B) The elastin matrikine GXXPG promotes chemotaxis of monocytes and antigen-presenting cells following chronic exposure to cigarette smoke; elastin fragments initiate the induction of elastin-reactive T cells that accelerate the pathogenesis of emphysema. C) Laminin matrikine γ2 ectodomain binds to the epithelial growth factor receptor (EGFR) to trigger repair mechanisms and promoting proliferation. D) Hyaluronan fragments initiate inflammation by binding to the pattern recognition receptors TLR2 and TLR4. E) Syndecan matrikine, generated from syndecan-1 ectodomains, neutralizes specific chemokines and prevents T cell migration in allergic airway disease.
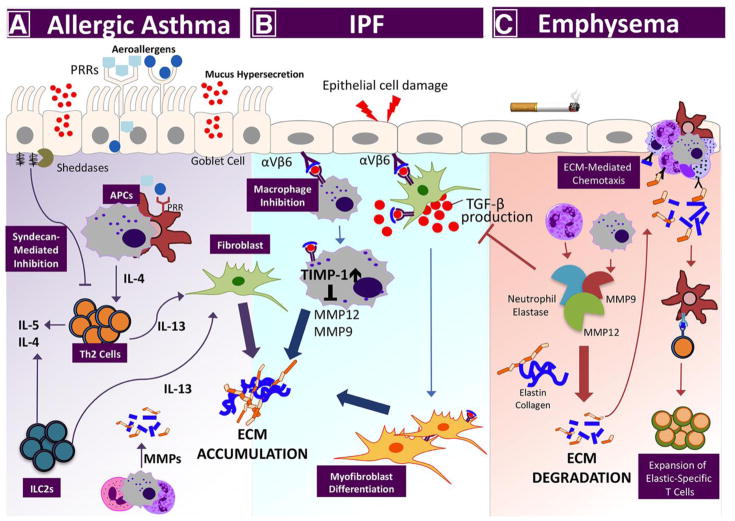
Figure 3. Lung disease pathogenesis by proteolytic enzyme/ECM imbalance.
Perturbations among the ECM networks of the lung are a hallmark feature of the most serious chronic lung diseases: asthma, IPF, and emphysema. A) In allergic asthma, aeroallergens bind pattern recognition receptors on airway epithelial cells and local antigen-presenting cells, initiating Th2-associated inflammation. Signature Th2 cytokines, (e.g., IL-13) from conventional T cells, and innate lymphoid cells (ILCs), signal through local fibroblasts to promote collagen synthesis, which leads to subepithelial fibrosis. Lung sheddases cleave proteoglycans, (e.g., syndecan-1) to release bioactive fragments that bind chemokines and impede Th2 cell recruitment. B) In response to cell injury, the immunosuppressive cytokine TGF-β promotes myofibroblast differentiation, inhibits macrophage MMP expression, and increases expression of tissue inhibitors of metalloproteinases (TIMPs) to promote idiopathic pulmonary fibrosis (IPF). (C) In emphysema, inflammatory cells secrete multiple proteinases, (e.g., MMP9, MMP12, and neutrophil elastase) that degrade collagen and elastin in the interstitial matrix. Collagen- and elastin-derived matrikines further promote lung inflammatory cell recruitment. Elastin fragments are also processed by antigen-presenting cells and presented to T cells in the regional lymph nodes to generate autoreactive, elastic-specific T cells that accelerate disease persistence and severity.
Although several scaffolding molecules in the lung are required for normal ECM assembly [26], their major components are collagens, and elastin molecules that constitute over approximately 60% [27], and 24% [28, 29] of dry lung weight, respectively. As such, dysregulation of collagen and elastin in the ECMs contribute to multiple pathological lung conditions. In this review, we highlight the primary components of lung ECM and describe how these proteins and their fragments are harnessed to promote health, and in instances of injury, encourage disease.
1. Collagen
Multiple isoforms of collagen provide structural stability and tensile strength in the lung [30]. Approximately 28 different types of collagen have been identified, all containing repetitive Glycine-Proline-X amino acid sequences that enable then to form triple helices that contribute to the intermolecular bonding and strength of collagen fibers [7, 30, 31]. Fibrillar collagens, or collagens that are post-translationally modified by enzymes such as lysyl oxidase, form polymerized fibers, and are the most abundant lung isoforms [21, 32, 33]. The triple helical structure of collagens I and III form a tight fibrous network throughout the large conducting airways, bronchi, and bronchioles, providing the strength and stability required for their proper function [32, 34, 35].
Non-fibrillar collagens, (e.g., collagen IV and V), that are found in the lung basement membrane, contain interruptions or non-Glycine-Proline-X sequences in their triple helical domains that result in kinks in the macromolecular structure that facilitate interactions with other ECM components [36, 37]. Collagens in the lung basement membrane and interstitial space are vital molecular scaffolds for necessary physiological processes such as fibroblast proliferation, migration, and adhesion. For instance, transmembrane cell adhesion molecules such as the integrin subunits α1, α2, α10, α11, recognize and bind to collagen (type I), facilitating cellular locomotion and cell migration during development [38–40]. Frequent lung collagen turnover of different isoforms during acute inflammation [41] further supports their functional role in essential biological processes, including cellular proliferation and migration. Collagens are implicated in the pathogenesis of lung diseases characterized by both matrix deposition and degradation. Here, we describe the roles of collagen and its associated fragments in the pathogenesis of idiopathic pulmonary fibrosis, COPD, and asthma.
1.1 Collagen in IPF Pathogenesis
Collagen deposition is a prominent feature of the fibrotic or scar-like lesions found in the lungs of patients with pulmonary fibrosis [42]. Accumulation of excessive fibrous tissue in the lungs physically impedes, and functionally restricts adequate gas exchange [43, 44]. Idiopathic Pulmonary Fibrosis (IPF), a prototypic and incurable form of fibrotic lung diseases, is characterized by increased numbers of myofibroblasts that aberrantly produce collagen I, that in turn expand fibrotic foci in the lungs [45]. The molecular mechanisms by which myofibroblast drive IPF are currently an area of active investigation; however, reduced expression of PTEN has been linked to the repression of FOXO3a, a transcriptional activator of collagen I, which is also a known apoptosis-inducing protein [46–48]. However, whether these mechanisms also control collagen expression in IPF remains unclear. Decellularization of IPF lungs has demonstrated abnormal expression of several collagens, particularly fibrillar collagen III [49]. Other groups have corroborated the role of collagens in the progressive lung remodeling associated with IPF whereby fibrillar collagen III is associated with early disease onset, but collagen I is associated with advanced disease [50].
Cytokines are thought to drive the progression of IPF by increasing deposition of collagens [51]. Specifically, TGF-β expression is upregulated in fibrotic lungs and has been shown to induce the differentiation of myofibroblasts, which orchestrate disease progression [52–54], enhance collagen gene transcription [55, 56], and alter the balance between matrix metalloproteinase and their associated tissue inhibitors of metalloproteinases (TIMPs) to favor accumulation of matrix components [57, 58].
1.2. Collagen in COPD Pathogenesis
In response to cigarette smoke, innate and adaptive immune cells are recruited to the lungs and promote development of chronic obstructive pulmonary disease (COPD) [59, 60]. Collagen turnover is highly associated with COPD pathogenesis, which is in part mediated by activation and recruitment of innate immune cells (e.g., macrophages and neutrophils) to the lungs. Specifically, the lungs of smokers typically show increased concentrations of MMP2, MMP8, MMP9 and prolyl endopeptidases that can cleave collagen to form the matrikines Proline-Glycine-Proline (PGP) and acetylated-PGP (Ac-PGP) [61, 62]. These collagen-derived matrikines have chemotactic properties that can perpetuate lung inflammation because they utilize the chemokine receptors CXCR1 and CXCR2, to recruit neutrophils to the lungs [61, 62]. However, collagen-derived PGPs are degraded by the aminopeptidase activity of leukotriene A4 hydroxylase, which limits their pro-inflammatory function [63, 64]. Clinically PGP and N-a-PGP concentrations are elevated in the sputum and serum of smokers with COPD when compared to asthmatic and/or healthy controls; furthermore, fluctuations in PGP concentration correlate in disease exacerbations [65, 66]. The pathogenic roles for PGP and Ac-PGP in COPD are also corroborated by experimental animal models, which demonstrate that PGP enhances lung tissue destruction and emphysema (Figure 2) [67].
Small airways that are less than 2 mm in diameter show minimal airflow resistance under normal conditions but are highly abnormal in smokers with COPD. A comprehensive quantitative histology, computed tomography, and gene expression analysis of smokers with different levels of COPD severity showed that bronchiolar tissue is reduced in COPD, but while total tissue collagen is reduced, there was a relative increase in collagen-3 over collagen-1 [68]. The authors concluded that narrowing of the terminal bronchioles occurs early in COPD, involving small airway remodeling and abnormal collagen deposition [68]. Finally, in smokers, development of upper lobe emphysema and lower lobe fibrosis, or combined pulmonary fibrosis and emphysema (CPFE), is a defined endotype that portends a poor prognosis [69, 70]. Little is known about the pathophysiological responses to cigarette smoke that can promote emphysema and fibrosis, but in one study that compared smokers with IPF, emphysema, or CPFE, serum SP-A and SP-D levels were higher in patients with fibrosis irrespective of emphysema and which also correlated with disease severity [71].
1.3 Collagen in Asthma
The allergic airway disease asthma is defined by the presence of intermittent airway obstruction, termed hyper-responsiveness (AHR), that narrows bronchi and clinically manifests as wheezing and dyspnea. Exposure to allergens triggers recruitment of different innate inflammatory cells, including mast cells, eosinophils and neutrophils that release pre-formed vesicles that contain ECM remodeling collagenases (e.g., MMP2 and MMP9) in the lungs [72, 73]. Consequently, targeting collagenases to reduce collagen turnover and reduce lung inflammation has been considered a potential asthma treatment [74]. Notably however, using preclinical models of asthma, deficiency in MMP2, MMP9, or molecular inhibition of MMPs not only did not alleviate allergic lung inflammation, but resulted in reduced airway chemotactic gradient formation, culminating in exacerbated lung inflammation and asphyxiation [75–77]. These studies point to the diverse and non-redundant roles for collagenases that are acutely elevated in T helper type 2 (Th2) mediated lung inflammation.
Chronic allergic airway inflammation can also promote subepithelial fibrosis characterized by excessive deposition and/or reorganization of type I, III, and IV collagens [78–82]. Several associated proteins including fibronectin, periostin [83, 84], Fibulin-1 (Fbln1) [85] also play key roles in lung ECM formation and contribute to mucus hypersecretion and Th2-associated inflammation [86]. In preclinical models of asthma, inhibition or genetic ablation of Fbln1c, a Fbln1 isomer, reduced airway collagen deposition, mucin production, and protected against AHR. Fbln1c deficiency also attenuated responses to allergens, reduced interstitial contractile cells, and lowered Th2 inflammatory cytokines. These findings suggest that therapeutic targeting of proteins that destabilize collagen in asthma could reduce subepithelial fibrosis and inflammation [86].
Airway smooth muscle cells secrete collagen in response to Th2 inflammatory cytokines and can contribute to airway wall thickening in severe asthma [25]. Transgenic overexpression of IL-13, a canonical Th2 cytokine, in mouse lungs can induce allergic inflammation, characterized by mucus hypersecretion and subepithelial fibrosis [87]. Expression of IL-5 and IL-13 by conventional T cells or innate lymphoid type 2 cells (ILC2s) is also important in orchestrating chronic structural changes in the lungs. Furthermore, impeding IL-13-mediated signaling by neutralizing antibodies or decoy receptors can reduce airway remodeling and fibrosis associated with asthma [88–90]. Although much remains to be elucidated concerning the mechanisms by which IL-13 induces subepithelial fibrosis in asthma, studies using in vitro systems have demonstrated that IL-13-associated signaling modulates fibroblasts and myofibroblasts to enhance TGF-β-induced procollagen production and decrease collagenase (MMP1 and MMP3) activity via increases in TIMP expression (Figure 3) [89, 91, 92].
2. Elastin
Elastin fibers, arguably the most critical component of lung ECM, control respiratory compliance because of their ability to stretch up to 140%, in contrast to collagen, which provides tensile force, but can only stretch by 2% [93]. Lung elastin fibers are composed of approximately 90% tropoelastin and 10% microfibril proteins (e.g., fibulin, latent TGF-β binding proteins (LTBPs), fibrillin and microfibrin-associated glycoprotein (MAGP)) [29, 94]. After assembly, elastin fibers are highly insoluble because lysyl oxidase crosslinks tropoelastin, its soluble precursor, which contains multiple highly repetitive hydrophilic lysine rich domains as well as hydrophobic amino acids (e.g., valine, proline, alanine, and glycine) that confer elastin’s elasticity [95].
The unique structural features of elastin fibers provide it with stability and resistance against proteolytic degradation. Deposition of elastin fibers in the lung begin during the pseudoglandular, but is accelerated in the alveolarization stages of development. Maturation of lung elastin fibers requires cross-linking enzymes that assemble tropoelastin with microfibril proteins. Thus, it is not surprising that in response to injury, newly formed elastin fibrils are often less organized, and have reduced elasticity [96]. Under non-pathological conditions, the rate of elastin degradation in the lung, as estimated by radioimmunoassay with 14C labeling and desmosine quantitation showed a nearly 70-year half-life, further supporting the unique molecular stability of elastin. This finding contrasts notably with the approximate 10% per day turnover of lung collagen [97–100]. These biological insights into elastin longevity highlight the importance of this macromolecule in its structure, integrity, and proper function in the lungs.
Degradation of lung elastin requires activation of specific MMPs as well as the aspartic, cysteine, and serine families of proteinases. Under pathological conditions, elastases are released from mesenchymal cells (fibroblasts, myofibroblasts, etc.) and/or innate immune cells (e.g., neutrophils, eosinophils, mast cells, and macrophages) that cleave elastin, thereby exposing new antigenic sites that can be recognized by T and B cells. Elastin fragments have been found in the serum of smokers with COPD [101] and have been shown to induce auto-reactive immune responses [102, 103], (discussed below under Elastin in COPD). Elastin degradation also generates bioactive matrikines, called elastokines, such as the Val-Gly-Val-Ala-Pro-Gly (VGVAPG) hexamer that influences cell migration, mitogenesis, and other cellular signaling [104, 105]. Similarly, the elastokine consensus sequence GXXPG forms type VIII β-turn structures that maintain its stable binding with the elastin receptor (S-Gal complex) [106].
2.1 Elastin in IPF pathogenesis
In response to lung injury, mesenchymal cells increase the expression of tropoelastin mRNA; however, newly synthesized elastin remains highly disorganized, i.e., amorphous [94]. Following injury, TGF-β, the prototypic pro-fibrotic cytokine, promotes transcriptional expression and stability of the elastin mRNA mediated by activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway [107–112]. Increased elastin expression has been shown to promote lung fibrosis in part by increasing collagen deposition. For example, in a bleomycin-model of lung fibrosis, elastin expression positively correlated with deposition of newly synthesized collagen [113]. Furthermore, in response to TGF-β, elastin but not collagen can increase differentiation of fibroblasts to myofibroblasts, indicating a pathogenic role for elastin in IPF [114]. Lung elastin is further stabilized by the epithelial integrin αvβ6 that bind to the latency-associated peptide (LAP), facilitating activation of TGF-β, suppression of MMP12, and increases in TIMP-1 expression in macrophages [114–116]. Furthermore, decellularized lung ECM from IPF patients, but not controls, can induce a specific set of genes in fibroblasts that are targets of microRNA(miR)-29, indicating that abnormal ECM can program new cellular responses [117]. Whether amorphous elastin or TGF-β play direct roles in these aberrant signaling remains an area of active investigation.
2.2. Elastin in Emphysema pathogenesis
Targeted disruption of proteins required for elastin fiber assembly (fibulin, latent transforming growth factor-β-binding protein-4 (Ltbp4), and lysyl oxidase (Lox)) as well as genetic deficiencies of the elastin gene (Eln+/−) result in abnormal lung development [118–121] and increased susceptibility to smoke-induced emphysema [110]. Genome-wide gene expression profiling using lung tissue samples from current and former smokers showed upregulation of fibulin-5 (FBLN5), elastin (ELN), and LTBP2 [122], indicating a dynamic process that is activated to repair lung elastin fibers in response to chronic smoke exposure. Furthermore, spatio-temporal expression of FBLN5, ELN, LTBP2 and microfibril associated protein 4 in response to cigarette smoke further provides strong support for their critical roles in lung elastin fiber repair in response to chronic inflammation in emphysema [122].
Three of the most important elastolytic enzymes: MMP9, MMP12, and neutrophil elastase, are produced by innate immune cells [77]. In response to elastolysis, newly formed elastin fragments or elastokines, recruit monocytes to the lungs, whereby they can differentiate into macrophages and further release MMP12 that inactivates alpha 1 anti-trypsin, deficiency of which is associated with early onset emphysema [123]. Elastokines also induce expression of NADPH oxidase (NOX2) in macrophages. This enzyme in turn can inhibit SIRT1, negative regulator of MMP9 expression in neutrophils and macrophages [124]. Neutrophil elastase can increase the availability of epidermal growth factor (EGF) for lung fibroblasts, which consequently stabilizes the Smad transcriptional corepressor, TG-interacting factor (TGIF), inhibit TGF-β production, and reduce new tropoelastin synthesis [125–127]. These findings suggest that elastolytic enzymes may have dual functions: 1) direct cleavage of elastin fibers, and 2) reduction in new elastin fiber biogenesis that together aggravate the lung repair process in response to cigarette smoke exposure.
Detection of desmosine, a biomarker of emphysema, signifies active breakdown of elastin in active and former smokers [128]. Several lines of evidence strongly support a role for the induction of acquired immunity to degraded elastin fragments in a subset of smokers who develop emphysema [129]. Specifically, T and B lymphocytes that recognize and respond to elastin fragments in the context of major histocompatibility complex class II (MHC II), are associated with emphysema severity [103]. Furthermore, elastin-specific T cells have been cloned from the peripheral blood of active and former smokers [102], and former smokers have been shown to harbor interferon gamma (IFN-γ) expressing T helper type 1 (Th1) and Th17 cells in their lungs [130]. The clinical significance of anti-elastin immunity is underscored by the positive correlation between the loss of lung function and Th1-specific immune responses to lung elastin fragments [131]. The mechanism for development of elastin-specific acquired immunity is due in part to cigarette smoke-induced activation of lung myeloid dendritic cells (mDC) [130]. Identification of specific regions within the elastin molecule that promote autoimmune responses could provide novel T cell-based therapies in COPD and emphysema.
Final considerations and new advances
Recent advances targeting ECM production and repair have provided novel approaches that could be used to treat chronic lung diseases. For example, doxycycline (DOX), a broad-spectrum MMP inhibitor, has been used to inhibit elastin breakdown and allow regeneration and repair of elastin in models of aortic aneurysm [132, 133]. DOX has also been shown to inhibit c-Jun-N-terminal kinase 2 (JNK 2), which promotes TGF-β1 expression and elastogenesis mediated by the lysyl oxidase crosslinking of elastin [134]. Because systemic MMP inhibition can prevent normal ECM turnover in healthy tissues [135–137], efforts are now being directed towards local delivery of MMP inhibitors. For instance, applied magnetic fields have been used to deliver DOX via incorporation of super-paramagnetic iron oxide polymer nanoparticles (SPIONs) to localize elastin restoration in the aortic wall [133]. Similarly, SPIONs could be applied to emphysematous lung to inhibit MMPs, an intervention that could lead to regeneration of normal lung.
Another novel bioengineering approach is the utilization of photo cross-linkable, elastin-like polypeptide (ELP) hydrogels. These matrices have highly extensible properties and have been used to generate scaffolds to seal rents in the lung parenchyma [138, 139]. A futuristic approach to generate functional alveoli could include seeding these synthetic, elastin scaffold structures with mesenchymal stem cells (MSCs), induced pluripotent stem cells (iPSCs), or the newly identified alveolar epithelial stem cells expressing Lgr5 [140] to generate new lung tissue. Finally, mesenchymal alveolar niche cells (MANCs), a functionally unique lung mesenchymal population, have been shown to secrete necessary molecular cues, (e.g., IL-6 and Fgf7), to form ECM and promote proliferation and regeneration of the alveolar epithelium [141]. Together, these novel, synthetic biotechnologies and lung stem cell discoveries provide exciting advances in medicine that could lead to restoring lung tissue structure and function in instances of chronic lung disease.
Highlights.
The extracellular matrix plays key roles in lung structural integrity, and function.
Proteinases generate cryptic extracellular matrix fragments or ‘matrikines’ that further diversify their function.
Fragments of collagen and elastin play pathogenic roles in several common chronic inflammatory lung diseases including asthma, idiopathic pulmonary fibrosis, and emphysema.
Novel bioengineering approaches utilize photo-crosslinkable elastin-like polypeptides (ELPs) hydrogel to repair or seal injured lung.
Acknowledgments
Funding: This work was supported by the R01HL117181-01, (FK, DBC), CX000104 (FK) VA Merit Award, DAMD W81XWH-16-1-0361 (FK).
Footnotes
Competing interests: The authors do not have any conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hynes RO, Naba A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2012;4(1):a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendrix AY, Kheradmand F. The Role of Matrix Metalloproteinases in Development, Repair, and Destruction of the Lungs. Prog Mol Biol Transl Sci. 2017;148:1–29. doi: 10.1016/bs.pmbts.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Couchman JR. Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol. 2010;26:89–114. doi: 10.1146/annurev-cellbio-100109-104126. [DOI] [PubMed] [Google Scholar]
- 4.Raghow R. The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J. 1994;8(11):823–31. doi: 10.1096/fasebj.8.11.8070631. [DOI] [PubMed] [Google Scholar]
- 5.Mecham RP. Overview of extracellular matrix. Curr Protoc Cell Biol. 2012;Chapter 10(Unit 10):1. doi: 10.1002/0471143030.cb1001s00. [DOI] [PubMed] [Google Scholar]
- 6.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341(1):126–40. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol. 2014;15(12):771–85. doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timpl R. Macromolecular organization of basement membranes. Curr Opin Cell Biol. 1996;8(5):618–24. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- 9.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3(6):422–33. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 10.Timpl R. Structure and biological activity of basement membrane proteins. Eur J Biochem. 1989;180(3):487–502. doi: 10.1111/j.1432-1033.1989.tb14673.x. [DOI] [PubMed] [Google Scholar]
- 11.Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974;77(2):314–46. [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt-Ney M, Doppler W, Ball RK, Groner B. Beta-casein gene promoter activity is regulated by the hormone-mediated relief of transcriptional repression and a mammary-gland-specific nuclear factor. Mol Cell Biol. 1991;11(7):3745–55. doi: 10.1128/mcb.11.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells JM, Gaggar A, Blalock JE. MMP generated matrikines. Matrix Biol. 2015;44–46:122–9. doi: 10.1016/j.matbio.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricard-Blum S, Salza R. Matricryptins and matrikines: biologically active fragments of the extracellular matrix. Exp Dermatol. 2014;23(7):457–63. doi: 10.1111/exd.12435. [DOI] [PubMed] [Google Scholar]
- 16.Ricard-Blum S, Vallet SD. Proteases decode the extracellular matrix cryptome. Biochimie. 2016;122:300–13. doi: 10.1016/j.biochi.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Genovese F, Manresa AA, Leeming DJ, Karsdal MA, Boor P. The extracellular matrix in the kidney: a source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair. 2014;7(1):4. doi: 10.1186/1755-1536-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li AC, Thompson RP. Basement membrane components. J Clin Pathol. 2003;56(12):885–7. doi: 10.1136/jcp.56.12.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess JK, Mauad T, Tjin G, Karlsson JC, Westergren-Thorsson G. The extracellular matrix - the under-recognized element in lung disease? J Pathol. 2016;240(4):397–409. doi: 10.1002/path.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White ES. Lung extracellular matrix and fibroblast function. Ann Am Thorac Soc. 2015;12(Suppl 1):S30–3. doi: 10.1513/AnnalsATS.201406-240MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunsmore SE, Rannels DE. Extracellular matrix biology in the lung. Am J Physiol. 1996;270(1 Pt 1):L3–27. doi: 10.1152/ajplung.1996.270.1.L3. [DOI] [PubMed] [Google Scholar]
- 22.Davidson JM. Biochemistry and turnover of lung interstitium. Eur Respir J. 1990;3(9):1048–63. [PubMed] [Google Scholar]
- 23.Mydel P, Shipley JM, Adair-Kirk TL, Kelley DG, Broekelmann TJ, Mecham RP, Senior RM. Neutrophil elastase cleaves laminin-332 (laminin-5) generating peptides that are chemotactic for neutrophils. J Biol Chem. 2008;283(15):9513–22. doi: 10.1074/jbc.M706239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schenk S, Hintermann E, Bilban M, Koshikawa N, Hojilla C, Khokha R, Quaranta V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161(1):197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker JKL, Theriot BS, Ghio M, Trempus CS, Wong JE, McQuade VL, Liang J, Jiang D, Noble PW, Garantziotis S, Kraft M, Ingram JL. Targeted HAS2 Expression Lessens Airway Responsiveness in Chronic Murine Allergic Airway Disease. Am J Respir Cell Mol Biol. 2017 doi: 10.1165/rcmb.2017-0095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill RC, Calle EA, Dzieciatkowska M, Niklason LE, Hansen KC. Quantification of extracellular matrix proteins from a rat lung scaffold to provide a molecular readout for tissue engineering. Mol Cell Proteomics. 2015;14(4):961–73. doi: 10.1074/mcp.M114.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang MR, Fiaux GW, Gillooly M, Stewart JA, Hulmes DJ, Lamb D. Collagen content of alveolar wall tissue in emphysematous and non-emphysematous lungs. Thorax. 1994;49(4):319–26. doi: 10.1136/thx.49.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starcher BC, Galione MJ. Purification and comparison of elastins from different animal species. Anal Biochem. 1976;74(2):441–7. doi: 10.1016/0003-2697(76)90224-4. [DOI] [PubMed] [Google Scholar]
- 29.Rucker RB, Dubick MA. Elastin metabolism and chemistry: potential roles in lung development and structure. Environ Health Perspect. 1984;55:179–91. doi: 10.1289/ehp.8455179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricard-Blum S, Ruggiero F. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol (Paris) 2005;53(7):430–42. doi: 10.1016/j.patbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 31.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20(1):33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Hulmes DJ. Building collagen molecules, fibrils, and suprafibrillar structures. J Struct Biol. 2002;137(1–2):2–10. doi: 10.1006/jsbi.2002.4450. [DOI] [PubMed] [Google Scholar]
- 33.Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3(1):a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balestrini JL, Niklason LE. Extracellular matrix as a driver for lung regeneration. Ann Biomed Eng. 2015;43(3):568–76. doi: 10.1007/s10439-014-1167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hance AJ, Bradley K, Crystal RG. Lung collagen heterogeneity. Synthesis of type I and type III collagen by rabbit and human lung cells in culture. J Clin Invest. 1976;57(1):102–11. doi: 10.1172/JCI108250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konomi H, Hayashi T, Nakayasu K, Arima M. Localization of type V collagen and type IV collagen in human cornea, lung, and skin. Immunohistochemical evidence by anti-collagen antibodies characterized by immunoelectroblotting. Am J Pathol. 1984;116(3):417–26. [PMC free article] [PubMed] [Google Scholar]
- 37.Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131(7):1619–28. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 38.Gullberg D, Gehlsen KR, Turner DC, Ahlen K, Zijenah LS, Barnes MJ, Rubin K. Analysis of alpha 1 beta 1, alpha 2 beta 1 and alpha 3 beta 1 integrins in cell--collagen interactions: identification of conformation dependent alpha 1 beta 1 binding sites in collagen type I. EMBO J. 1992;11(11):3865–73. doi: 10.1002/j.1460-2075.1992.tb05479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb Perspect Biol. 2011;3(9):a005074. doi: 10.1101/cshperspect.a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 41.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3(12) doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378(9807):1949–61. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 43.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2(2):103–21. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208(7):1339–50. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132(4):1311–21. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 46.Im J, Hergert P, Nho RS. Reduced FoxO3a expression causes low autophagy in idiopathic pulmonary fibrosis fibroblasts on collagen matrices. Am J Physiol Lung Cell Mol Physiol. 2015;309(6):L552–61. doi: 10.1152/ajplung.00079.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nho RS, Hergert P. IPF fibroblasts are desensitized to type I collagen matrix-induced cell death by suppressing low autophagy via aberrant Akt/mTOR kinases. PLoS One. 2014;9(4):e94616. doi: 10.1371/journal.pone.0094616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nho RS, Peterson M, Hergert P, Henke CA. FoxO3a (Forkhead Box O3a) deficiency protects Idiopathic Pulmonary Fibrosis (IPF) fibroblasts from type I polymerized collagen matrix-induced apoptosis via caveolin-1 (cav-1) and Fas. PLoS One. 2013;8(4):e61017. doi: 10.1371/journal.pone.0061017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186(9):866–76. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuhn C, 3rd, Boldt J, King TE, Jr, Crouch E, Vartio T, McDonald JA. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis. 1989;140(6):1693–703. doi: 10.1164/ajrccm/140.6.1693. [DOI] [PubMed] [Google Scholar]
- 51.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors. 2011;29(5):196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Timpl R, Brown JC. Supramolecular assembly of basement membranes. Bioessays. 1996;18(2):123–32. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- 53.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156–72. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 55.Sato M, Shegogue D, Gore EA, Smith EA, McDermott PJ, Trojanowska M. Role of p38 MAPK in transforming growth factor beta stimulation of collagen production by scleroderma and healthy dermal fibroblasts. J Invest Dermatol. 2002;118(4):704–11. doi: 10.1046/j.1523-1747.2002.01719.x. [DOI] [PubMed] [Google Scholar]
- 56.Varela-Rey M, Montiel-Duarte C, Oses-Prieto JA, Lopez-Zabalza MJ, Jaffrezou JP, Rojkind M, Iraburu MJ. p38 MAPK mediates the regulation of alpha1(I) procollagen mRNA levels by TNF-alpha and TGF-beta in a cell line of rat hepatic stellate cells(1) FEBS Lett. 2002;528(1–3):133–8. doi: 10.1016/s0014-5793(02)03276-3. [DOI] [PubMed] [Google Scholar]
- 57.Mauviel A. Transforming growth factor-beta: a key mediator of fibrosis. Methods Mol Med. 2005;117:69–80. doi: 10.1385/1-59259-940-0:069. [DOI] [PubMed] [Google Scholar]
- 58.Schiller M, Javelaud D, Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci. 2004;35(2):83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Grumelli S, Corry DB, Song LZ, Song L, Green L, Huh J, Hacken J, Espada R, Bag R, Lewis DE, Kheradmand F. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 2004;1(1):e8. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shan M, You R, Yuan X, Frazier MV, Porter P, Seryshev A, Hong JS, Song LZ, Zhang Y, Hilsenbeck S, Whitehead L, Zarinkamar N, Perusich S, Corry DB, Kheradmand F. Agonistic induction of PPARgamma reverses cigarette smoke-induced emphysema. J Clin Invest. 2014;124(3):1371–81. doi: 10.1172/JCI70587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaggar A, Jackson PL, Noerager BD, O’Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180(8):5662–9. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Reilly PJ, Hardison MT, Jackson PL, Xu X, Snelgrove RJ, Gaggar A, Galin FS, Blalock JE. Neutrophils contain prolyl endopeptidase and generate the chemotactic peptide, PGP, from collagen. J Neuroimmunol. 2009;217(1–2):51–4. doi: 10.1016/j.jneuroim.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snelgrove RJ. Leukotriene A4 hydrolase: an anti-inflammatory role for a proinflammatory enzyme. Thorax. 2011;66(6):550–1. doi: 10.1136/thoraxjnl-2011-200234. [DOI] [PubMed] [Google Scholar]
- 64.Snelgrove RJ, Jackson PL, Hardison MT, Noerager BD, Kinloch A, Gaggar A, Shastry S, Rowe SM, Shim YM, Hussell T, Blalock JE. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science. 2010;330(6000):90–4. doi: 10.1126/science.1190594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Reilly P, Jackson PL, Noerager B, Parker S, Dransfield M, Gaggar A, Blalock JE. N-alpha-PGP and PGP, potential biomarkers and therapeutic targets for COPD. Respir Res. 2009;10:38. doi: 10.1186/1465-9921-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Reilly PJ, Jackson PL, Wells JM, Dransfield MT, Scanlon PD, Blalock JE. Sputum PGP is reduced by azithromycin treatment in patients with COPD and correlates with exacerbations. BMJ Open. 2013;3(12):e004140. doi: 10.1136/bmjopen-2013-004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12(3):317–23. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 68.Hogg JC, McDonough JE, Gosselink JV, Hayashi S. What drives the peripheral lung-remodeling process in chronic obstructive pulmonary disease? Proc Am Thorac Soc. 2009;6(8):668–72. doi: 10.1513/pats.200907-079DP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Awano N, Inomata M, Ikushima S, Yamada D, Hotta M, Tsukuda S, Kumasaka T, Takemura T, Eishi Y. Histological analysis of vasculopathy associated with pulmonary hypertension in combined pulmonary fibrosis and emphysema: comparison with idiopathic pulmonary fibrosis or emphysema alone. Histopathology. 2017;70(6):896–905. doi: 10.1111/his.13153. [DOI] [PubMed] [Google Scholar]
- 70.Kitaguchi Y, Fujimoto K, Hanaoka M, Kawakami S, Honda T, Kubo K. Clinical characteristics of combined pulmonary fibrosis and emphysema. Respirology. 2010;15(2):265–71. doi: 10.1111/j.1440-1843.2009.01676.x. [DOI] [PubMed] [Google Scholar]
- 71.Papaioannou AI, Kostikas K, Manali ED, Papadaki G, Roussou A, Spathis A, Mazioti A, Tomos I, Papanikolaou I, Loukides S, Chainis K, Karakitsos P, Griese M, Papiris S. Serum Levels of Surfactant Proteins in Patients with Combined Pulmonary Fibrosis and Emphysema (CPFE) PLoS One. 2016;11(6):e0157789. doi: 10.1371/journal.pone.0157789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corry DB, Rishi K, Kanellis J, Kiss A, Song Lz LZ, Xu J, Feng L, Werb Z, Kheradmand F. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol. 2002;3(4):347–53. doi: 10.1038/ni773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cundall M, Sun Y, Miranda C, Trudeau JB, Barnes S, Wenzel SE. Neutrophil-derived matrix metalloproteinase-9 is increased in severe asthma and poorly inhibited by glucocorticoids. J Allergy Clin Immunol. 2003;112(6):1064–71. doi: 10.1016/j.jaci.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 74.Vartak DG, Gemeinhart RA. Matrix metalloproteases: underutilized targets for drug delivery. J Drug Target. 2007;15(1):1–20. doi: 10.1080/10611860600968967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Corry DB, Kiss A, Song LZ, Song L, Xu J, Lee SH, Werb Z, Kheradmand F. Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. Faseb J. 2004;18(9):995–997. doi: 10.1096/fj.03-1412fje. Epub 2004 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greenlee KJ, Corry DB, Engler DA, Matsunami RK, Tessier P, Cook RG, Werb Z, Kheradmand F. Proteomic identification of in vivo substrates for matrix metalloproteinases 2 and 9 reveals a mechanism for resolution of inflammation. J Immunol. 2006;177(10):7312–21. doi: 10.4049/jimmunol.177.10.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87(1):69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boser SR, Mauad T, Araujo-Paulino BB, Mitchell I, Shrestha G, Chiu A, Butt J, Kelly MM, Caldini E, James A, Green FHY. Myofibroblasts are increased in the lung parenchyma in asthma. PLoS One. 2017;12(8):e0182378. doi: 10.1371/journal.pone.0182378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brewster CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1990;3(5):507–11. doi: 10.1165/ajrcmb/3.5.507. [DOI] [PubMed] [Google Scholar]
- 80.Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1(8637):520–4. doi: 10.1016/s0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- 81.Wilson JW, Li X. The measurement of reticular basement membrane and submucosal collagen in the asthmatic airway. Clin Exp Allergy. 1997;27(4):363–71. [PubMed] [Google Scholar]
- 82.Yamauchi K. Airway remodeling in asthma and its influence on clinical pathophysiology. Tohoku J Exp Med. 2006;209(2):75–87. doi: 10.1620/tjem.209.75. [DOI] [PubMed] [Google Scholar]
- 83.Agrawal S, Townley RG. Role of periostin, FENO, IL-13, lebrikzumab, other IL-13 antagonist and dual IL-4/IL-13 antagonist in asthma. Expert Opin Biol Ther. 2014;14(2):165–81. doi: 10.1517/14712598.2014.859673. [DOI] [PubMed] [Google Scholar]
- 84.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118(1):98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 85.Liu G, Cooley MA, Jarnicki AG, Hsu AC, Nair PM, Haw TJ, Fricker M, Gellatly SL, Kim RY, Inman MD, Tjin G, Wark PA, Walker MM, Horvat JC, Oliver BG, Argraves WS, Knight DA, Burgess JK, Hansbro PM. Fibulin-1 regulates the pathogenesis of tissue remodeling in respiratory diseases. JCI Insight. 2016;1(9) doi: 10.1172/jci.insight.86380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu G, Cooley MA, Nair PM, Donovan C, Hsu AC, Jarnicki AG, Haw TJ, Hansbro NG, Ge Q, Brown AC, Tay H, Foster PS, Wark PA, Horvat JC, Bourke JE, Grainge CL, Argraves WS, Oliver BG, Knight DA, Burgess JK, Hansbro PM. Airway remodelling and inflammation in asthma are dependent on the extracellular matrix protein fibulin-1c. J Pathol. 2017 doi: 10.1002/path.4979. [DOI] [PubMed] [Google Scholar]
- 87.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103(6):779–88. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lumsden RV, Worrell JC, Boylan D, Walsh SM, Cramton J, Counihan I, O’Beirne S, Medina MF, Gauldie J, Fabre A, Donnelly SC, Kane R, Keane MP. Modulation of pulmonary fibrosis by IL-13Ralpha2. Am J Physiol Lung Cell Mol Physiol. 2015;308(7):L710–8. doi: 10.1152/ajplung.00120.2014. [DOI] [PubMed] [Google Scholar]
- 89.Oriente A, Fedarko NS, Pacocha SE, Huang SK, Lichtenstein LM, Essayan DM. Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J Pharmacol Exp Ther. 2000;292(3):988–94. [PubMed] [Google Scholar]
- 90.Yang G, Volk A, Petley T, Emmell E, Giles-Komar J, Shang X, Li J, Das AM, Shealy D, Griswold DE, Li L. Anti-IL-13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine. 2004;28(6):224–32. doi: 10.1016/j.cyto.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 91.Richter A, Puddicombe SM, Lordan JL, Bucchieri F, Wilson SJ, Djukanovic R, Dent G, Holgate ST, Davies DE. The contribution of interleukin (IL)-4 and IL-13 to the epithelial-mesenchymal trophic unit in asthma. Am J Respir Cell Mol Biol. 2001;25(3):385–91. doi: 10.1165/ajrcmb.25.3.4437. [DOI] [PubMed] [Google Scholar]
- 92.Zhou X, Trudeau JB, Schoonover KJ, Lundin JI, Barnes SM, Cundall MJ, Wenzel SE. Interleukin-13 augments transforming growth factor-beta1-induced tissue inhibitor of metalloproteinase-1 expression in primary human airway fibroblasts. Am J Physiol Cell Physiol. 2005;288(2):C435–42. doi: 10.1152/ajpcell.00035.2004. [DOI] [PubMed] [Google Scholar]
- 93.Starcher BC. Elastin and the lung. Thorax. 1986;41(8):577–85. doi: 10.1136/thx.41.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shifren A, Mecham RP. The stumbling block in lung repair of emphysema: elastic fiber assembly. Proc Am Thorac Soc. 2006;3(5):428–33. doi: 10.1513/pats.200601-009AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Indik Z, Yeh H, Ornstein-Goldstein N, Sheppard P, Anderson N, Rosenbloom JC, Peltonen L, Rosenbloom J. Alternative splicing of human elastin mRNA indicated by sequence analysis of cloned genomic and complementary DNA. Proc Natl Acad Sci U S A. 1987;84(16):5680–4. doi: 10.1073/pnas.84.16.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mizikova I, Morty RE. The Extracellular Matrix in Bronchopulmonary Dysplasia: Target and Source. Front Med (Lausanne) 2015;2:91. doi: 10.3389/fmed.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dubick MA, Rucker RB, Cross CE, Last JA. Elastin metabolism in rodent lung. Biochim Biophys Acta. 1981;672(3):303–6. doi: 10.1016/0304-4165(81)90297-x. [DOI] [PubMed] [Google Scholar]
- 98.Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87(5):1828–34. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harel S, Janoff A, Yu SY, Hurewitz A, Bergofsky EH. Desmosine radioimmunoassay for measuring elastin degradation in vivo. Am Rev Respir Dis. 1980;122(5):769–73. doi: 10.1164/arrd.1980.122.5.769. [DOI] [PubMed] [Google Scholar]
- 100.Laurent GJ. Rates of collagen synthesis in lung, skin and muscle obtained in vivo by a simplified method using [3H]proline. Biochem J. 1982;206(3):535–44. doi: 10.1042/bj2060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shinohara T, Suzuki K, Okada M, Shiigai M, Shimizu M, Maehara T, Ohsuzu F. Soluble elastin fragments in serum are elevated in acute aortic dissection. Arterioscler Thromb Vasc Biol. 2003;23(10):1839–44. doi: 10.1161/01.ATV.0000085016.02363.80. [DOI] [PubMed] [Google Scholar]
- 102.Xu C, Hesselbacher S, Tsai CL, Shan M, Spitz M, Scheurer M, Roberts L, Perusich S, Zarinkamar N, Coxson H, Krowchuk N, Corry DB, Kheradmand F. Autoreactive T Cells in Human Smokers is Predictive of Clinical Outcome. Front Immunol. 2012;3:267. doi: 10.3389/fimmu.2012.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Cogswell S, Storness-Bliss C, Corry DB, Kheradmand F. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13(5):567–9. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 104.Gaggar A, Weathington N. Bioactive extracellular matrix fragments in lung health and disease. J Clin Invest. 2016;126(9):3176–84. doi: 10.1172/JCI83147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Senior RM, Griffin GL, Mecham RP, Wrenn DS, Prasad KU, Urry DW. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J Cell Biol. 1984;99(3):870–4. doi: 10.1083/jcb.99.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brassart B, Fuchs P, Huet E, Alix AJ, Wallach J, Tamburro AM, Delacoux F, Haye B, Emonard H, Hornebeck W, Debelle L. Conformational dependence of collagenase (matrix metalloproteinase-1) up-regulation by elastin peptides in cultured fibroblasts. J Biol Chem. 2001;276(7):5222–7. doi: 10.1074/jbc.M003642200. [DOI] [PubMed] [Google Scholar]
- 107.Cha SI, Groshong SD, Frankel SK, Edelman BL, Cosgrove GP, Terry-Powers JL, Remigio LK, Curran-Everett D, Brown KK, Cool CD, Riches DW. Compartmentalized expression of c-FLIP in lung tissues of patients with idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;42(2):140–8. doi: 10.1165/rcmb.2008-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaminski N, Allard JD, Pittet JF, Zuo F, Griffiths MJ, Morris D, Huang X, Sheppard D, Heller RA. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc Natl Acad Sci U S A. 2000;97(4):1778–83. doi: 10.1073/pnas.97.4.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoff CR, Perkins DR, Davidson JM. Elastin gene expression is upregulated during pulmonary fibrosis. Connect Tissue Res. 1999;40(2):145–53. doi: 10.3109/03008209909029110. [DOI] [PubMed] [Google Scholar]
- 110.Lucey EC, Ngo HQ, Agarwal A, Smith BD, Snider GL, Goldstein RH. Differential expression of elastin and alpha 1(I) collagen mRNA in mice with bleomycin-induced pulmonary fibrosis. Lab Invest. 1996;74(1):12–20. [PubMed] [Google Scholar]
- 111.Shifren A, Woods JC, Rosenbluth DB, Officer S, Cooper JD, Pierce RA. Upregulation of elastin expression in constrictive bronchiolitis obliterans. Int J Chron Obstruct Pulmon Dis. 2007;2(4):593–8. [PMC free article] [PubMed] [Google Scholar]
- 112.Kuang PP, Zhang XH, Rich CB, Foster JA, Subramanian M, Goldstein RH. Activation of elastin transcription by transforming growth factor-beta in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2007;292(4):L944–52. doi: 10.1152/ajplung.00184.2006. [DOI] [PubMed] [Google Scholar]
- 113.Blaauboer ME, Emson CL, Verschuren L, van Erk M, Turner SM, Everts V, Hanemaaijer R, Stoop R. Novel combination of collagen dynamics analysis and transcriptional profiling reveals fibrosis-relevant genes and pathways. Matrix Biol. 2013;32(7–8):424–31. doi: 10.1016/j.matbio.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 114.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature. 2003;422(6928):169–73. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- 115.Feinberg MW, Jain MK, Werner F, Sibinga NE, Wiesel P, Wang H, Topper JN, Perrella MA, Lee ME. Transforming growth factor-beta 1 inhibits cytokine-mediated induction of human metalloelastase in macrophages. J Biol Chem. 2000;275(33):25766–73. doi: 10.1074/jbc.M002664200. [DOI] [PubMed] [Google Scholar]
- 116.Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12(1):22–9. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 117.Parker MW, Rossi D, Peterson M, Smith K, Sikstrom K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest. 2014;124(4):1622–35. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shifren A, Durmowicz AG, Knutsen RH, Hirano E, Mecham RP. Elastin protein levels are a vital modifier affecting normal lung development and susceptibility to emphysema. Am J Physiol Lung Cell Mol Physiol. 2007;292(3):L778–87. doi: 10.1152/ajplung.00352.2006. [DOI] [PubMed] [Google Scholar]
- 119.Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J, Jr, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415(6868):171–5. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 120.Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415(6868):168–71. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 121.Bultmann-Mellin I, Dinger K, Debuschewitz C, Loewe KMA, Melcher Y, Plum MTW, Appel S, Rappl G, Willenborg S, Schauss AC, Jungst C, Kruger M, Dressler S, Nakamura T, Wempe F, Alejandre Alcazar MA, Sterner-Kock A. Role of LTBP4 in alveolarization, angiogenesis, and fibrosis in lungs. Am J Physiol Lung Cell Mol Physiol. 2017;313(4):L687–L698. doi: 10.1152/ajplung.00031.2017. [DOI] [PubMed] [Google Scholar]
- 122.Brandsma CA, van den Berge M, Postma DS, Jonker MR, Brouwer S, Pare PD, Sin DD, Bosse Y, Laviolette M, Karjalainen J, Fehrmann RS, Nickle DC, Hao K, Spanjer AI, Timens W, Franke L. A large lung gene expression study identifying fibulin-5 as a novel player in tissue repair in COPD. Thorax. 2015;70(1):21–32. doi: 10.1136/thoraxjnl-2014-205091. [DOI] [PubMed] [Google Scholar]
- 123.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277(5334):2002–4. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 124.Trocme C, Deffert C, Cachat J, Donati Y, Tissot C, Papacatzis S, Braunersreuther V, Pache JC, Krause KH, Holmdahl R, Barazzone-Argiroffo C, Carnesecchi S. Macrophage-specific NOX2 contributes to the development of lung emphysema through modulation of SIRT1/MMP-9 pathways. J Pathol. 235(1):65–78. doi: 10.1002/path.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.DiCamillo SJ, Carreras I, Panchenko MV, Stone PJ, Nugent MA, Foster JA, Panchenko MP. Elastase-released epidermal growth factor recruits epidermal growth factor receptor and extracellular signal-regulated kinases to down-regulate tropoelastin mRNA in lung fibroblasts. J Biol Chem. 2002;277(21):18938–46. doi: 10.1074/jbc.M200243200. [DOI] [PubMed] [Google Scholar]
- 126.DiCamillo SJ, Yang S, Panchenko MV, Toselli PA, Naggar EF, Rich CB, Stone PJ, Nugent MA, Panchenko MP. Neutrophil elastase-initiated EGFR/MEK/ERK signaling counteracts stabilizing effect of autocrine TGF-beta on tropoelastin mRNA in lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2006;291(2):L232–43. doi: 10.1152/ajplung.00530.2005. [DOI] [PubMed] [Google Scholar]
- 127.Yang S, Nugent MA, Panchenko MP. EGF antagonizes TGF-beta-induced tropoelastin expression in lung fibroblasts via stabilization of Smad corepressor TGIF. Am J Physiol Lung Cell Mol Physiol. 2008;295(1):L143–51. doi: 10.1152/ajplung.00289.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Luisetti M, Ma S, Iadarola P, Stone PJ, Viglio S, Casado B, Lin YY, Snider GL, Turino GM. Desmosine as a biomarker of elastin degradation in COPD: current status and future directions. Eur Respir J. 2008;32(5):1146–57. doi: 10.1183/09031936.00174807. [DOI] [PubMed] [Google Scholar]
- 129.Kheradmand F, Shan M, Xu C, Corry DB. Autoimmunity in chronic obstructive pulmonary disease: clinical and experimental evidence. Expert Rev Clin Immunol. 2012;8(3):285–92. doi: 10.1586/eci.12.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shan M, Cheng HF, Song LZ, Roberts L, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Storness-Bliss C, Ramchandani M, Lee SH, Corry DB, Kheradmand F. Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci Transl Med. 2009;1(4):4ra10. doi: 10.1126/scitranlsmed.3000154. [DOI] [PubMed] [Google Scholar]
- 131.Bhavani S, Tsai CL, Perusich S, Hesselbacher S, Coxson H, Pandit L, Corry DB, Kheradmand F. Clinical and Immunological Factors in Emphysema Progression. Five-Year Prospective Longitudinal Exacerbation Study of Chronic Obstructive Pulmonary Disease (LES-COPD) Am J Respir Crit Care Med. 2015;192(10):1171–8. doi: 10.1164/rccm.201504-0736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jennewine B, Fox J, Ramamurthi A. Cathepsin K-targeted sub-micron particles for regenerative repair of vascular elastic matrix. Acta Biomater. 2017;52:60–73. doi: 10.1016/j.actbio.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sivaraman B, Swaminathan G, Moore L, Fox J, Seshadri D, Dahal S, Stoilov I, Zborowski M, Mecham R, Ramamurthi A. Magnetically-responsive, multifunctional drug delivery nanoparticles for elastic matrix regenerative repair. Acta Biomater. 2017;52:171–186. doi: 10.1016/j.actbio.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 134.Camardo A, Seshadri D, Broekelmann T, Mecham R, Ramamurthi A. Multifunctional, JNK-inhibiting nanotherapeutics for augmented elastic matrix regenerative repair in aortic aneurysms. Drug Deliv Transl Res. 2017 doi: 10.1007/s13346-017-0419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett JW, Jr, Kent KC, Upchurch GR, Jr, Chaikof EL, Mills JL, Fleckten B, Longo GM, Lee JK, Thompson RW. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (Phase II) multicenter study. J Vasc Surg. 2002;36(1):1–12. doi: 10.1067/mva.2002.125018. [DOI] [PubMed] [Google Scholar]
- 136.Chang WY, Clements D, Johnson SR. Effect of doxycycline on proliferation, MMP production, and adhesion in LAM-related cells. Am J Physiol Lung Cell Mol Physiol. 2010;299(3):L393–400. doi: 10.1152/ajplung.00437.2009. [DOI] [PubMed] [Google Scholar]
- 137.Franco C, Ho B, Mulholland D, Hou G, Islam M, Donaldson K, Bendeck MP. Doxycycline alters vascular smooth muscle cell adhesion, migration, and reorganization of fibrillar collagen matrices. Am J Pathol. 2006;168(5):1697–709. doi: 10.2353/ajpath.2006.050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Annabi N, Zhang YN, Assmann A, Sani ES, Cheng G, Lassaletta AD, Vegh A, Dehghani B, Ruiz-Esparza GU, Wang X, Gangadharan S, Weiss AS, Khademhosseini A. Engineering a highly elastic human protein-based sealant for surgical applications. Sci Transl Med. 2017;9(410) doi: 10.1126/scitranslmed.aai7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang YN, Avery RK, Vallmajo-Martin Q, Assmann A, Vegh A, Memic A, Olsen BD, Annabi N, Khademhosseini A. A Highly Elastic and Rapidly Crosslinkable Elastin-Like Polypeptide-Based Hydrogel for Biomedical Applications. Adv Funct Mater. 2015;25(30):4814–4826. doi: 10.1002/adfm.201501489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee JH, Tammela T, Hofree M, Choi J, Marjanovic ND, Han S, Canner D, Wu K, Paschini M, Bhang DH, Jacks T, Regev A, Kim CF. Anatomically and Functionally Distinct Lung Mesenchymal Populations Marked by Lgr5 and Lgr6. Cell. 2017;170(6):1149–1163 e12. doi: 10.1016/j.cell.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zepp JA, Zacharias WJ, Frank DB, Cavanaugh CA, Zhou S, Morley MP, Morrisey EE. Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell. 2017;170(6):1134–1148 e10. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]