Abstract
Natural killer (NK) cell function is regulated by a fine balance between numerous activating and inhibitory receptors, of which killer-cell immunoglobulin-like receptors (KIRs) are among the most polymorphic and comprehensively studied. KIRs allow NK cells to recognize downregulation or the absence of HLA class I molecules on target cells (known as missing-self), a phenomenon that is commonly observed in virally infected cells or cancer cells. Because KIR and HLA genes are located on different chromosomes, in an allogeneic environment such as after hematopoietic stem cell transplantation, donor NK cells that express an inhibitory KIR for an HLA class I molecule that is absent on recipient targets (KIR/KIR-ligand mismatch), can recognize and react to this missing self and mediate cytotoxicity. Accumulating data indicate that epistatic interactions between KIR and HLA influence outcomes in several clinical conditions. Herein, we discuss the genetic and functional features of KIR/KIR-ligand interactions in hematopoietic stem cell transplantation and how these data can guide donor selection. We will also review clinical studies of adoptive NK cell therapy in leukemia and emerging data on the use of genetically modified NK cells that could broaden the scope of cancer immunotherapy.
Learning Objectives
To understand the influence of various inhibitory and activating KIR receptors on HSCT outcomes
To understand how these effects may vary by donor source and underlying disease
Introduction
Natural killer (NK) cells characterized by a CD3–CD56+ immunophenotype are bone marrow–derived lymphocytes capable of mediating early innate immune responses against virally infected cells or malignant cells.1-7 Because they are the first lymphocytes to reconstitute after hematopoietic stem cell transplantation (HSCT),8-17 NK cells play an important role in mediating the graft-versus-tumor effect.18-28 One of the earliest observations of NK cell alloreactivity was reported in the hybrid resistance model,29,30 which noted that lethally irradiated heterozygous F1 hybrid mice derived from a cross of 2 inbred mouse strains (parent A × parent B) rejected hematopoietic grafts donated by either parent A or parent B.31 Rejection of the parental graft was later shown to be mediated by a subset of recipient NK cells that lacks the appropriate inhibitory receptors to recognize major histocompatibility complex class I (MHC-I) molecules on the donor cells. This observation led to the ingenious “missing self” concept of NK recognition, which postulates that the absence or reduced expression of “self” MHC-I allows a cell to be killed by NK cells.1,2
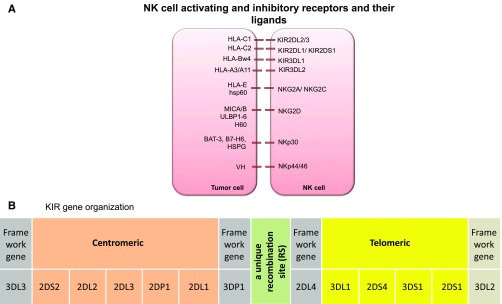
Subsequently, the receptors that recognize MHC-I were identified on NK cells (reviewed by Parham32 and Moretta33). Briefly, each mature NK cell expresses a wide array of germ-line–encoded activating and inhibitory receptors.32-49 Inhibitory NK receptors mediate two important functions. They recognize self-HLA class I alleles and contribute to the acquisition of NK function by a dynamic process known as NK cell education or licensing.50 When inhibitory receptors engage with their cognate class I ligands, they deliver inhibitory signals to suppress NK cell activity. If HLA class I antigen expression is sufficiently reduced or modified, as is often observed in virally infected cells or tumor cells (as an immune escape mechanism from T-cell recognition), NK cells can eliminate the abnormal cells.2,51 However, missing-self alone is insufficient to trigger NK cell effector function, because many recipient cells with no ligands (eg, red blood cells) are not lysed. Some level of expression of a stressed ligand is also required. Activating receptors recognize stress proteins expressed on the surface of transformed or abnormal cells33-49 and provide signals for NK cells to kill. Ultimately, NK effector function is dictated by integration of signals received through these activating and inhibitory receptors (Figure 1A).
Figure 1.
(A) NK cell activating and inhibitory receptors and their ligands. (B) KIR gene organization. KIR haplotype illustrating centromeric and telomeric KIR gene motifs. BAT-3, HLA-B–associated transcript 3; H60, histocompatibility 60; hsp60, heat shock protein 60; HSPG, heparin sulfate proteoglycans; MIC, MHC class I chain-related gene; VH, viral hemagglutinin; ULBP, UL16 binding protein.83
Killer-cell immunoglobulin-like receptors and their ligands
Among the most comprehensively studied NK cell receptors are the killer-cell immunoglobulin-like receptors (KIRs). KIRs are clonally expressed on the surface of NK cells in a stochastic fashion. Each NK cell can in turn express any possible combination of receptors, leading to the generation of complex NK cell repertoires.52-57
All KIRs are named 2D or 3D, which denotes the number of immunoglobulin-like domains in the molecule. The alphabet following 2D or 3D signifies the length of the cytoplasmic tail, which is either short (S) in activating KIRs or long (L) in inhibitory KIRs.58 The ligands for KIRs are HLA-A, -B, or -C molecules.59 KIR2DL1 recognizes group 2 HLA-C molecules (HLA-C2; alleles with Lys80 residue [eg, Cw2, 4, 5, 6]), KIR2DL2 recognizes group 1 HLA-C molecules (HLA-C1; alleles with an Asn80 residue [eg, Cw1, 3, 7, 8]), and KIR3DL1 recognizes HLA-Bw4 alleles20,55,60-62 (Figure 1A). In vivo63 and in vitro64 studies suggest that KIR3DL2 recognizes HLA-A3 and A11 but this binding occurs only in the presence of the Epstein-Barr virus EBNA3A peptide. In contrast to the inhibitory KIRs, the ligands for many activating KIRs are largely unknown. KIR2DS1 has been shown to interact with HLA-C2 alleles,65-67 whereas KIR2DS2 was recently shown to recognize HLA-A*11.68 The frequencies of KIR alleles vary from population to population, but most individuals have inhibitory KIRs specific for HLA-C1, -C2 or -Bw4 alleles. For instance, in English and white Americans, inhibitory KIR2DL1 (95% to 100%), 2DL2 (43% to 53%), and 2DL3 (85% to 95%) are present in a majority, whereas the genes for the activating KIR2DS1 (35% to 45%) and 2DS2 (45% to 55%) are less commonly present.69
Because KIRs are encoded by a family of genes in the leukocyte receptor complex on chromosome 19q13.4 and segregate independently from the HLA genes,70 in the setting of HSCT, a donor-recipient pair can be HLA-matched and KIR-ligand mismatched at the same time. This generates a situation in which alloreactive donor NK cells can elicit a graft-versus-tumor effect without increasing the risk of graft-versus-host disease (GVHD). A KIR ligand calculator to predict NK cell alloreactivity based on donor and recipient HLA-B and -C typing is available at the Immuno Polymorphism Database Web site (https://www.ebi.ac.uk/ipd/kir/ligand.html).
KIR-ligand mismatch leads to superior outcomes after T-cell deplete haploidentical HSCT in patients with acute myeloid leukemia
In HSCT, KIR-ligand mismatch in the graft-versus-host (GvH) direction occurs when the recipient lacks 1 or more major KIR-ligands (eg, C1, C2, or Bw4). Valiante and Parham56 were the first to predict NK cell alloreactivity in the clinical transplant setting on the basis of the donor and recipient KIR and HLA repertoire. The Perugia group was then the first to report that KIR-ligand mismatch in the GvH direction was associated with a significant reduction in the risk of relapse and improved survival.19,20 In that study, patients with acute myeloid leukemia (AML; n = 57) or acute lymphoblastic leukemia (ALL; n = 35) received granulocyte colony-stimulating factor mobilized, CD34+ selected peripheral blood progenitor cell haploidentical HSCT after conditioning with total body irradiation, thiotepa, fludarabine, and anti-thymocyte globulin. Because the graft was T-cell depleted, no post-HSCT immunosuppression was given. KIR-ligand mismatch correlated positively with NK cell alloreactivity in vitro. Patients who received KIR-ligand mismatched HSCT in the GvH direction had significantly reduced risk of relapse compared with those in the KIR-ligand matched group (probability of relapse at 5 years, 0% vs 75%).20 Furthermore, despite the presence of donor-versus-recipient alloreactive NK cells, no patient in the KIR-ligand mismatched group experienced GVHD or graft rejection compared with 14% and 16%, respectively, in the KIR-ligand matched group.20 Overall survival (OS) was also improved in the KIR-ligand mismatch group (60%) compared with the KIR-ligand match group (5%). Interestingly, these favorable outcomes were observed only in AML patients and not in ALL patients. These findings were later corroborated in a larger cohort of 112 patients with AML who underwent T-cell deplete haploidentical HSCT.21 This study also reported a 52% reduction in the risk of relapse or death in the KIR-ligand mismatched group compared with the KIR-ligand matched group (relative risk [RR], 0.48; 95% confidence interval [CI], 0.29-0.78; P < .001). Moreover, even patients who were transplanted with active disease experienced improved disease-free survival (DFS) if they received a graft from a KIR-ligand mismatched compared with a KIR-ligand matched donor.21 It is noteworthy that the myeloablative regimen used in these studies was highly immunosuppressive, which in conjunction with a T-cell–deplete graft, created a state of intense lymphodepletion in the host, providing an ideal scenario for in vivo persistence and expansion of NK cells.71 Consequently, alloreactive NK cells were detected for up to 7 months in one-third of patients and for up to a year in ∼10% of patients.20 These data led to several retrospective analyses on the effects of KIR-ligand mismatch on outcomes in the HLA-matched unrelated and matched sibling transplant settings (Table 1).23-27,72,73 Although some studies showed improvements in 1 or all aspects of disease outcome, including relapse, DFS, or OS,23-27 others reported a detrimental impact of KIR-ligand mismatch,27,74,75 including greater risk of GVHD and death.76 The conflicting data in the various studies may be explained by a number of factors, most importantly, differences in the degree of T-cell depletion in the graft and the resultant T-cell alloreactivity, which may in turn overcome any potential benefit of NK cell alloreactivity.26,72 Indeed, T cells in the graft have been shown to negatively affect donor-derived NK cell reconstitution and expression of KIRs, thereby impairing NK effector function in vivo.26,72,77 This is exemplified by studies that showed improved outcomes after KIR-ligand mismatched T-cell deplete22,78,79 but not with T-cell replete HSCTs.72,75,80,81
Table 1.
Studies evaluating the impact of donor-recipient KIR-ligand mismatch in related or unrelated donor HSCT
| Reference | Davies 200225 | Giebel 200322 | Bornhäuser 200423 | Schaffer 200475 | Hsu 200578 | Beelen 200580 | Verheyden 200579 | Farag 200674 | Chen 200624 | Kröger 200673 | Sun 200727 | Miller 200776 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | 175 | 130 | 118 | 190 | 178 | 374 | 65 | 1571 | 131 | 142 | 378 | 2062 |
| Disease | CML, AML, ALL, others, (other leukemias, MDS, metabolic disease, immune deficiencies, SAA) | ALL, AML, MDS, CML, NHL, HL, MM | AML, CML, MDS | ALL, AML, CML, MDS, NHL, HL, MM | AML, ALL, CML, MDS | AML, CML, MDS | AML, CML, ALL | AML, MDS, CML | AML, CML, ALL, MDS, NHL, other | AML, MDS, CMML, ALL | ALL, AML, MDS, CML | AML, CML, MDS |
| Donor | URD | URD | URD | URD | HLA-matched sibling | Related or URD | HLA-matched sibling | URD | HLA-matched sibling | URD | HLA-matched sibling/ URD | URD |
| TCD (%) | 37 in KIR-ligand compatible group; 29 in KIR-ligand incompatible group | ATG (100) | TCD (20) + ATG (100) | TCD (17) + ATG (100) | 100 | 0 | TCD (52) | 20 | No | ATG | No | — |
| KIR-ligand mismatched (GvH direction) (%) | 35 | 15 | 13 | 12 | 63 | 13 | 68 | 9 | 63 | 70 | 16 | — |
| Proportion of patients with aGVHD (grade 2-4) in KIR-ligand mismatched vs matched groups | NS | NS | NS | NS | NS | NS | NS | NS; aGVHD III to IV higher in KIR-ligand mismatch | NS | NS | NS; aGVHD III to IV higher in KIR-ligand mismatch (CML patients >1 year from diagnosis only) | |
| Risk | HR, 1.64 | RR, 1.58 | ||||||||||
| 95% CI | 1.11–2.42 | 1.13-2.22 | ||||||||||
| P | .001 | .008 | ||||||||||
| Relapse | NS | NS | Higher in KIR-ligand mismatch | NS | Lower in KIR-ligand mismatch (myeloid only) | Lower in KIR-ligand mismatch | NS | Higher in KIR-ligand mismatch | NS | NS | Higher in KIR-ligand mismatch | Lower in KIR-ligand mismatch (myeloid early disease only) |
| Risk | HR, 0.41 | RR, 0.24 | HR, 1.56 | RR, 2.98 | RR, 0.54 | |||||||
| 95% CI | 0.18-0.97 | 0.08-0.69 | 0.88–2.75 | 1.17-7.59 | 0.30-0.95 | |||||||
| P | .02 | .04 | < .008 | .04 | .02 | .03 | ||||||
| OS | Lower in KIR-ligand mismatch (myeloid only) | Higher in KIR-ligand mismatch | NS | Lower in KIR-ligand mismatch | Higher in KIR-ligand mismatch (myeloid only) | NS | NS | Lower in KIR-ligand mismatch | NS | Lower in KIR-ligand mismatch | NS | NS |
| Risk | 13% vs 38% at 5 years | 87% vs 48% at 4.5 years | HR, 2.13 | HR, 0.53 | HR, 1.94 | HR, 2.015 | ||||||
| 95% CI | 0%-26% vs 24%-52% | 1.17-3.90 | 0.3-0.93 | 1.47-2.57 | ||||||||
| P | < .01 | .006 | .01 | .026 | < .001 | .02 |
aGVHD, acute graft-versus-host disease; ATG, anti-thymocyte globulin; CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; HL, Hodgkin lymphoma; HR, hazard ratio; MDS, myelodysplastic syndrome; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; NS, nonsignificant; RR, relative risk; SAA, severe aplastic anemia; TCD, T-cell depletion; URD, unrelated donor.
Activating KIRs in donor NK cells are protective against leukemia relapse
In contrast to the well-studied biology of inhibitory KIRs, the function of activating KIRs and their respective ligands has remained largely unexplored. Activating KIRs recognize stress molecules and possibly also HLA molecules or modified HLA molecules, but only the specificity of KIR2DS1 for HLA-C2 alleles has been firmly established.65-67 Although all individuals carry the genes for inhibitory KIR receptors—which indicates that they are necessary for NK cell function—there is striking heterogeneity in the number of inherited activating KIR genes in the normal population.69 Based on the number and distribution of activating KIR genes, individuals can be considered to have 2 broad KIR haplotypes. KIR haplotype A comprises 5 inhibitory genes and the single activating gene KIR2DS4, whereas KIR haplotype B incorporates various combinations of activating genes (KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS5, KIR3DS1)82 and the inhibitory KIR gene KIR2DL5. The KIR haplotype model proposes that the more activating KIR genes the donor carries, the higher the potential for alloreactivity and the lower the risk of relapse. Indeed, recent analyses by Cooley et al83 in 448 AML patients who received a T-cell-replete unrelated donor HSCT reported that patients transplanted from a donor carrying a higher number of activating KIR genes (haplotype B) have a survival advantage over patients receiving a graft from a haplotype A donor (3-year DFS, 28% vs 17%; P = .003; 3-year OS, 31% vs 20%; P = .007).3 This protection was not observed in adult ALL, suggesting that myeloid leukemia is more susceptible to NK killing, although a recent report showed a benefit of donor haplotype B in relapse protection and survival for pediatric patients with ALL undergoing T-cell-deplete haploidentical HSCT.84
Moreover, KIR haplotype B donors with a higher number of B-specific genes (KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS5, KIR2DL2, and KIR2DL5), especially if also homozygous for those in the centromeric part of the KIR locus (Cen-B/B), conferred greater protection against relapse after HSCT85,86 (Figure 1B). The risk of relapse was significantly lower if the donor had 2 or more KIR B gene-content motifs (KIR B content score) compared with those with KIR B content score of less than 2, both in the HLA-matched and -mismatched settings,85 supporting the notion that the more activating KIR genes the donor carries, the higher the potential for alloreactivity and protection against relapse.
The influence of specific activating KIR genes on outcomes after HSCT has also been investigated by a number of groups.87-89 A large retrospective study from the Center for International Blood and Marrow Transplant Research included more than 1700 patients with AML (75%) or ALL (25%) who received a 9/10 HLA-matched (48%) or 10/10 HLA-matched (52%) HSCT from unrelated donors.88 In this study, AML (but not ALL) patients whose donors were KIR2DS1 positive had significantly lower risk of relapse (hazard ratio, 0.76; 95% CI, 0.61-0.96; P = .02) and reduced overall mortality (hazard ratio, 0.85; 95% CI, 0.73-1.00; P = .04) compared with those whose donors were KIR2DS1 negative. Similar results have also been reported in the setting of matched sibling donor transplants; patients with AML who received allografts from KIR2DS1-positive donors had a risk of relapse 4 times lower than those who received a graft from KIR2DS1-negative donors.87 Taken together, these studies suggest that selecting donors with KIR haplotype B may have a beneficial effect on outcomes after HSCT for AML and childhood ALL.
NK cell effects in cord blood HSCT
Similarly, several studies have explored the role of KIR-ligand mismatch on outcomes after umbilical cord blood transplantation (CBT) (Table 2). In a study of 218 patients who received a single-unit CBT, Willemze et al28,90 reported that KIR-ligand mismatch between the cord blood unit and the patient was associated with significantly improved DFS (40% vs 55%; P = .005) and OS (31% vs 57%; P = .02). In contrast, 3 studies91-93 failed to show a beneficial effect of KIR-ligand mismatch, whereas 1 study from Minnesota reported a detrimental impact of KIR-ligand mismatch in the setting of double-unit CBT or reduced-intensity conditioned CBT.94 A limitation of those studies is that they take into account only KIR-ligand mismatch without considering the role of activating KIRs or NK licensing. Moreover, they are exclusively based on genetic studies, and they lack functional correlates and a plausible mechanistic basis for the observed effects of KIR genotype on outcome.
Table 2.
Studies assessing the role of KIR-ligand mismatch in cord blood transplantation
| Reference | Willemze 200928 | Brunstein 200994 | Tanaka 201392 | Garfall 201391 | Rocha 201693 | Sekine 201697 | |
|---|---|---|---|---|---|---|---|
| No. of patients | 218 | 155 | 102 | 357 AML) | 80 | 199 | 204 |
| Conditioning (%) | MA (83) | MA (100) | RIC (100) | MA (62) | MA (27) | MA (100) | MA (72) |
| ATG/ALG (%) | 82 | 40 | 28 | 0 | 100 | 70 | 100 |
| Single-CBT (%) | 100 | 61 | 100 | 100 | 0 | 0 | — |
| KIR-ligand mismatched (GvH direction) (%) | 32 | 26 | 32 | 81 | 44 | 43 | HLA C1/x patients who receive CB unit with HLA-C1-KIR2DL2, KIR2DL3, or KIR2DS2 vs all other |
| aGVHD (2-4) | NS | NS | Higher in KIR-ligand mismatch | NS | NS | NS | NS |
| Risk | 79% vs 57% | ||||||
| 95% CI | 59%-99% vs 44%-70% | ||||||
| P | .01 | ||||||
| TRM | NS | NS | Higher in KIR-ligand mismatch | NS | — | NS | NS |
| Risk | 27% vs 12% at 2 years |
||||||
| 95% CI | 12%-42% vs 5%-19% | ||||||
| P | .03 | ||||||
| Relapse | Lower in KIR-ligand mismatch | NS | NS | NS | NS | NS | Lower in HLA-C1/x recipients who received an HLA-C1-KIR2DL2, KIR2DL3, or KIR2DS2 CB unit |
| Risk | RR, 0.53 | HR, 0.04 | |||||
| 95% CI | 0.28-0.99 | 1.57-31.47 | |||||
| P | .05 | .002 | |||||
| OS | Higher in KIR-ligand mismatch | NS | Lower in KIR-ligand mismatch | NS | NS | NS | Higher in HLA-C1/x recipients who received an HLA-C1-KIR2DL2, KIR2DL3, or KIR2DS2 CB unit |
| Risk | RR, 2 | 32% vs 52% at 3 years | HR, 3.46 | ||||
| 95% CI | 1.24-3.22 | 15%-59% vs 47%-67% | 1.46-8.20 | ||||
| P | .004 | .03 | .005 | ||||
ALG, anti-lymphocyte globulin; CB, cord blood; MA, myeloablative; RIC, reduced-intensity conditioning; TRM, treatment-related mortality.
Our group recently studied the impact of NK reconstitution on outcome after CBT. We showed that patients homozygous for HLA-C2 group alleles had a higher 1-year relapse rate and worse survival after CBT than did HLA-C1/C1 or HLA-C1/C2 (HLA-C1/x) patients: 67.8% vs 26.0% (P < .001) and 15.0% vs 52.9% (P < .001), respectively. This inferior outcome was associated with delayed posttransplant recovery of NK cells expressing the HLA-C2–specific KIR2DL1/S1 receptors. Our data support previous studies that show a statistically significant increase in relapse rate in HLA-C2/C2 patients in other transplant settings22,95,96 and suggest that HLA-C2/C2 CBT recipients constitute a high-risk group that may benefit from NK cell-based intervention to accelerate NK cell reconstitution. We also observed that HLA-C1/x patients receiving a CB graft that was licensed (HLA-C1/x positive) and was positive for the activating receptor KIR2DS2 had a lower 1-year relapse rate (6.7% vs 40.1%; P = .002) and superior survival (74.2% vs 41.3%; P = .003) compared with recipients of grafts lacking KIR2DS2 or HLA-C1.98 Thus, we have initiated a clinical trial of CB selection for HLA-C1/x recipients on the basis of HLA-KIR typing and adoptive therapy with CB-derived NK cells for HLA-C2/C2 patients at our center.
Can we select the most appropriate donor based on HLA-KIR genotype?
The degree of HLA match is the single most important factor that determines outcomes after unrelated donor HSCT.99-110 In cytomegalovirus (CMV) –seronegative recipients, the best survival outcomes are seen with a CMV-seronegative donor; however, donor CMV serostatus has no prognostic impact in CMV-seropositive recipients.111 Among several other donor characteristics, including age, sex, parity, and ABO compatibility, increasing donor age is the only factor consistently shown to have a negative impact on survival after transplantation.99-103,112,113 Whereas some studies have reported a lower risk of relapse in female-donor to male-recipient HSCT, this benefit was offset by higher treatment-related mortality in secondary to severe acute or chronic GVHD.115-120 Several maternal and paternal noninherited antigens have also been shown to influence outcomes after HSCT. For instance, using the mother as donor is associated with a lower risk of relapse and treatment-related mortality compared with a paternal graft in the setting of T-cell-deplete haploidentical HSCT,121 but not in the T-cell-replete setting.122 Disparity in some minor histocompatibility antigens (miHAs) has also been reported to influence risk of GVHD and/or relapse.123-126 However, choosing a donor on the basis of miHAs (mismatch except for sex) is not yet practical; more than 50 autosomally encoded miHAs have been identified to date, which when combined with the large extent of variability in HLA genes, makes it likely that one or more immunological disparities will be present in at least 80% of all HSCTs.127
Because many clinical studies support a role for KIR-HLA interactions in HSCT (Table 1), it is likely that KIR immunogenetics will also be included in algorithms of donor selection in the future. KIR genotyping can be performed easily and cheaply along with HLA genotyping at the time of donor screening. Indeed, a number of clinical trials are prospectively investigating the incorporation of KIR genotyping as part of the donor selection criteria. These studies are either observational or interventional, and their approach is dependent on the underlying disease and whether the transplant procedure is performed with or without T-cell depletion. The majority of these studies are based on the following models:
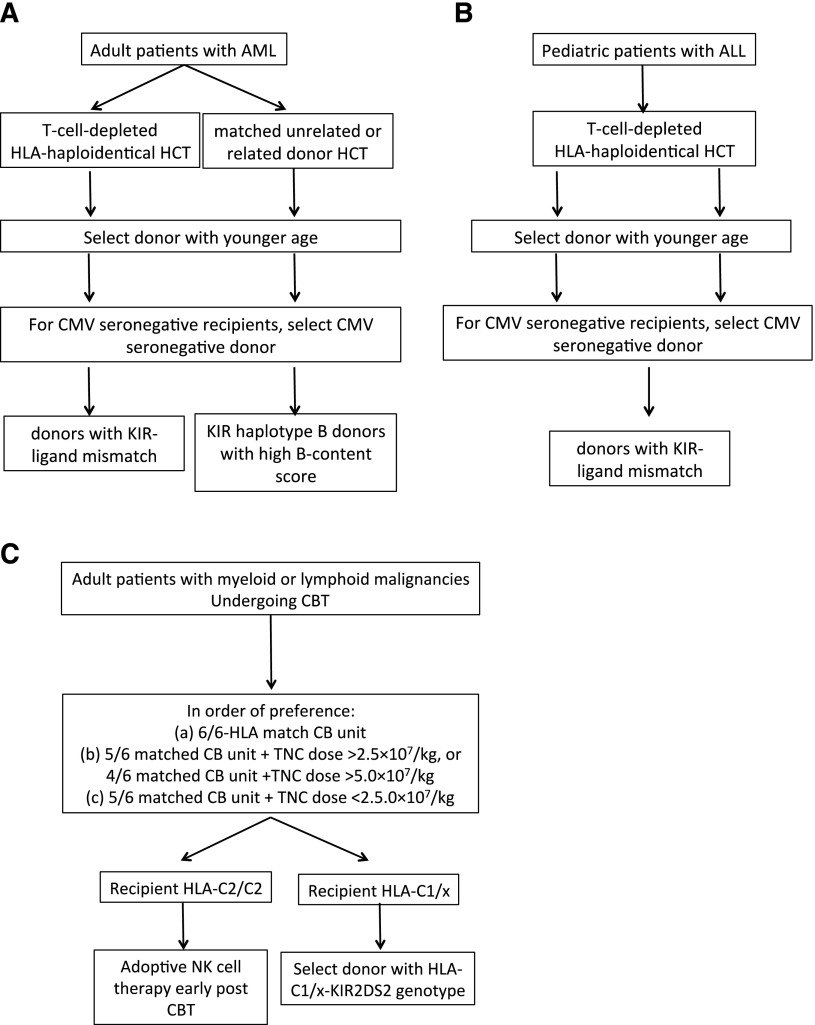
The KIR-ligand model: The data on KIR-ligand mismatch and outcome after HSCT are conflicting (Table 1). Although the majority of studies support a beneficial effect for KIR-ligand mismatch on the risk of relapse in the setting of T-cell-depleted haploidentical HSCT for adult AML and childhood ALL,20,84 a beneficial role for KIR-ligand mismatch on outcomes in other hematologic malignancies has not been convincingly shown. Thus, in adults with AML or children with ALL undergoing a T-cell-deplete haploidentical HSCT, we propose that the selection of a donor with KIR-ligand mismatch may improve outcomes (Figure 2A-B). Several clinical trials (NCT01787474, NCT02646839, NCT01807611, NCT02519114, NCT02508038) are prospectively studying selection of donors on the basis of KIR-ligand mismatch for adult patients with AML or multiple myeloma and children with ALL, AML, myelodysplastic syndrome, or lymphoma in both the T-cell-deplete and T-cell-replete settings.
The KIR haplotype model: This model proposes selection of KIR haplotype B donors with high B-content score in adults with AML undergoing matched unrelated or related donor transplant83,85,87 or children with ALL undergoing T-cell-depleted haploidentical HSCT.84 In situations in which multiple potential donors of similar ages and CMV serostatus are available, we suggest that a donor with high KIR B content and Cen B/B genotype may improve outcomes in adult patients with AML undergoing an HLA-matched unrelated HSCT (Figure 2A). Clinical trial NCT01288222 is prospectively studying this approach.
Selection of cord blood donors based on the combination of licensing and activating KIR genes: After considering HLA match and the total nucleated cell content of the CB unit,129 we propose that the selection of a donor with an HLA-C1-KIR2DS2 genotype may improve outcomes for HLA-C1/x recipients (Figure 2C). The NCT02727803 trial is exploring this approach in adults with myeloid or lymphoid malignancies undergoing CBT.
Figure 2.
Proposed algorithm for donor selection based on KIR genotyping in (A) adult AML, (B) childhood ALL, and (C) CBT.
NK cell adoptive immunotherapy
Given the potent antitumor efficacy of NK cells, adoptive transfer of NK cells to treat a variety of malignancies has also been explored by several groups.71,130-144 Results of the initial trials using ex vivo activated autologous NK cells were mostly unsatisfactory,130-138 likely because of the inhibition of autologous NK cells by self-HLA molecules. As the impact of KIR-ligand mismatch in the transplant setting became evident, the focus of the trials shifted toward the use of allogeneic NK cells either in combination with HSCT or in a non-HSCT setting (Table 3).71,141-144 Allogeneic NK cells are less likely to be subject to the inhibitory response resulting from NK cell recognition of self-MHC molecules, as seen with autologous NK cells. Moreover, several studies have shown that infusion of haploidentical NK cells to exploit the missing-self concept is safe and can mediate impressive clinical activity in some patients with AML.71,139-144 In those studies, NK cells are activated and expanded ex vivo with a variety of cytokines such as interleukin-2 (IL-2), IL-15, or IL-21 to increase their persistence, enhance their proliferation, and augment their in vivo effector function (Table 3). In a landmark trial of NK cell adoptive therapy in patients with relapsed or refractory AML, Miller et al71 were among the first to show that adoptive transfer of ex vivo–activated haploidentical NK cells after lymphodepleting chemotherapy followed by IL-2 administration to support their in vivo expansion is safe and can result in NK cell persistence for up to 4 weeks without inducing GVHD. Although the clinical responses were modest, a subsequent study that used recombinant IL-2 diphtheria toxin to deplete regulatory T cells (Tregs) (which can impair NK effector function145), resulted in enhanced in vivo NK cell expansion with improved leukemia clearance.141
Table 3.
Studies evaluating the impact of donor KIR haplotype or activating KIRs in related or unrelated donor HSCT
| Study | Kröger 200673 | Cooley 200983 | Cooley 201085 | Stringaris 201087 | Cooley 201486 | Oevermann 201484 |
|---|---|---|---|---|---|---|
| No. of patients | 142 | 448 | 1409 | 246 | 1532 | 85 |
| Disease | AML, ALL, CML, MDS | AML | AML, ALL | AML, ALL, CML, CLL, MDS, NHL | AML | Childhood ALL |
| Donor | URD | URD | URD | HLA-identical sibling | URD | Haploidentical |
| TCD (%) | In vivo 100 (ATG) | 0 | 0 | 100 | 0 | 100 |
| Study group | Donor activating KIRs | Donor genotype B/x | Centromeric B/B donors vs others | Donor KIR B haplotype genes (2DS1, 3DS1, and 2DL5A) | C1/x patient receiving an HSCT from a donor with ≥2 KIR B genes vs other donor | Donor genotype B/x |
| Proportion of patients with aGVHD (grade 2-4) | NS | NS | NS | — | NS | — |
| Relapse | Higher with increasing number of donor activating KIRs | NS | Lower with centromeric B/B donors AML only) | Lower in study group (AML only) | Lower in study group | Lower with genotype B/x donor |
| Risk | HR, 1.37 | RR, 0.34 | HR, 0.24 | RR, 0.70 | RR, 2.82 | |
| 95% CI | 1.1-1.7 | 0.20-0.57 | 0.56-0.87 | 1.37-5.77 | ||
| P | .005 | .001 | .02 | .0018 | .005 | |
| DFS | Lower with increasing number of donor activating KIRs | Higher with genotype B/x donor | Higher with centromeric B/B donors (AML only) | — | Higher in study group | Higher with genotype B/x donor |
| Risk | HR, 1.16 | RR, 0.70 | RR, 0.72 | RR, 0.78 | 50.6% vs 29.5% at 5 years | |
| 95% CI | 0.55-0.88 | 0.55-0.93 | 0.67-0.91 | |||
| P | .04 | .002 | .01 | .0015 | .033 | |
| OS | NS | Higher with genotype B/x donor | Higher with centromeric B/B donors AML only) | NS; (AML only); higher in study group (all patients) | — | — |
| Risk | 31% vs 20% | NR | HR, 0.64 | |||
| 95% CI | 26%-36% vs 13%-27% | 0.43-0.94 | ||||
| P | .007 | .024 |
CLL, chronic lymphoid leukemia; NR, not reported.
IL-15, in contrast to IL-2, does not support the expansion of Tregs.146 Thus, IL-15 administration holds promise for specifically boosting NK cell alloreactivity without the undesired stimulation of Tregs. Several groups are evaluating the efficacy of highly potent recombinant cytokines such as an IL-15 superagonist to induce in vivo NK cell activation and expansion (without adoptive therapy) in various malignancies (NCT01885897, NCT01946789, NCT02099539, NCT02384954).
Future directions
Chimeric antigen receptors (CARs) have been used extensively to redirect the specificity of T cells against leukemia with dramatic clinical responses in patients with lymphoid malignancies.147-153 These infusions have been primarily restricted to the autologous setting because activated T cells from an allogeneic source are likely to increase the risk of GVHD. Given their shorter lifespan and potent cytolytic function, mature NK cells provide attractive candidate effector cells to express CARs and provide an excellent source of off-the-shelf cellular therapy for patients with cancer. First, allogeneic NK cells should not cause GVHD, as predicted by observations in murine models,154,155 as well as in patients with leukemia and solid malignancies treated with haploidentical or CB-derived NK cells.19,20,71,141 Second, mature NK cells have a limited life span of a few weeks, allowing for antitumor activity while reducing the probability of long-term adverse events, such as prolonged cytopenias caused by on-target/off-tumor toxicity to normal tissues, or the risk of malignant transformation. Third, unlike T cells, NK cells will also have activity through their native receptors to kill antigen-negative target cells, potentially preventing a mechanism of immune escape. The feasibility of genetically engineering NK cells to express CARs against a number of targets has been shown in the preclinical setting,156-164 and 2 clinical studies (both targeting CD19+ malignancies using a retroviral transduced anti-CD19-BB-ζ NK-CAR) are testing the safety and efficacy of this approach in the clinic (NCT00995137 and NCT01974479).
An alternative strategy under investigation to redirect NK cytotoxicity toward tumor cells is to create either bispecific or trispecific antibodies (BiKE, TriKE).165-171 BiKEs are constructed by joining a single-chain variable fragment (Fv) against CD16 and a single-chain Fv against a tumor-associated antigen (BiKE), or 2 tumor-associated antigens (TriKE), such as CD19, CD22, CD33, CD30, or EpCAM.165-170 To enhance NK cell expansion and survival in vivo, investigators have developed a novel TriKE that also includes a modified recombinant human IL-15 cross-linker sandwiched between single-chain Fv against CD16 and the target antigen of interest.171 It will be interesting to observe whether these strategies alone or in combination with HSCT can provide durable clinical responses and immediate access to treatment with the development of off-the-shelf products.
Summary
Significant advances have been made in understanding the role of NK cell activity after HSCT. Although it is well recognized that the antitumor activity of NK cells is intensified in the setting of HLA-mismatched HSCT, the benefit of selecting a donor on the basis of KIR genotyping has not yet been established. A number of institutions have established local guidelines to assist in the selection of donors with NK cell alloreactivity predicted by HLA or KIR genotype; however, the definition and models of alloreactivity used by these centers are not standardized, underscoring the importance of well-designed, prospective studies of donor selection based on KIR-HLA immunogenetics.
We suggest that NK cells and KIR immunogenetics are likely to gain increasing importance to clinicians in the selection of the best donors for transplant, along with HLA matching, CMV status, blood group, age, and sex. Furthermore, the identification of optimal donors on the basis of KIR genotype may be an important next step for the design of successful NK cell-based interventions and the development of the next generation of engineered NK cells for immunotherapy of cancer.
References
- 1.Ljunggren HG, Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162(6):1745-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ljunggren HG, Kärre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11(7):237-244. [DOI] [PubMed] [Google Scholar]
- 3.Tong L, Assenmacher M, Zänker KS, Jähn P. Virus-specific peptide dependent NK cell cytotoxicity. Inflamm Allergy Drug Targets. 2014;13(2):128-133. [DOI] [PubMed] [Google Scholar]
- 4.Vidal SM, Khakoo SI, Biron CA. Natural killer cell responses during viral infections: flexibility and conditioning of innate immunity by experience. Curr Opin Virol. 2011;1(6):497-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189-220. [DOI] [PubMed] [Google Scholar]
- 6.Haller O, Kiessling R, Orn A, Kärre K, Nilsson K, Wigzell H. Natural cytotoxicity to human leukemia mediated by mouse non-T cells. Int J Cancer. 1977;20(1):93-103. [DOI] [PubMed] [Google Scholar]
- 7.Kiessling R, Petranyi G, Kärre K, Jondal M, Tracey D, Wigzell H. Killer cells: a functional comparison between natural, immune T-cell and antibody-dependent in vitro systems. J Exp Med. 1976;143(4):772-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Small TN, Papadopoulos EB, Boulad F, et al. . Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93(2):467-480. [PubMed] [Google Scholar]
- 9.Thomson BG, Robertson KA, Gowan D, et al. . Analysis of engraftment, graft-versus-host disease, and immune recovery following unrelated donor cord blood transplantation. Blood. 2000;96(8):2703-2711. [PubMed] [Google Scholar]
- 10.Ruggeri A, Peffault de Latour R, Carmagnat M, et al. . Outcomes, infections, and immune reconstitution after double cord blood transplantation in patients with high-risk hematological diseases. Transpl Infect Dis. 2011;13(5):456-465. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson CA, Turki AT, McDonough SM, et al. . Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(4):565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somers JA, Brand A, van Hensbergen Y, et al. . Double umbilical cord blood transplantation: a study of early engraftment kinetics in leukocyte subsets using HLA-specific monoclonal antibodies. Biol Blood Marrow Transplant. 2013;19(2):266-273. [DOI] [PubMed] [Google Scholar]
- 13.Saliba RM, Rezvani K, Leen A, et al. . General and Virus-Specific Immune Cell Reconstitution after Double Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015;21(7):1284-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahmi Z, Hommel-Berrey G, Smith F, Thomson B. NK cells recover early and mediate cytotoxicity via perforin/granzyme and Fas/FasL pathways in umbilical cord blood recipients. Hum Immunol. 2001;62(8):782-790. [DOI] [PubMed] [Google Scholar]
- 15.Hercend T, Takvorian T, Nowill A, et al. . Characterization of natural killer cells with antileukemia activity following allogeneic bone marrow transplantation. Blood. 1986;67(3):722-728. [PubMed] [Google Scholar]
- 16.Hokland M, Jacobsen N, Ellegaard J, Hokland P. Natural killer function following allogeneic bone marrow transplantation. Very early reemergence but strong dependence of cytomegalovirus infection. Transplantation. 1988;45(6):1080-1084. [DOI] [PubMed] [Google Scholar]
- 17.Petersen SL, Ryder LP, Björk P, et al. . A comparison of T-, B- and NK-cell reconstitution following conventional or nonmyeloablative conditioning and transplantation with bone marrow or peripheral blood stem cells from human leucocyte antigen identical sibling donors. Bone Marrow Transplant. 2003;32(1):65-72. [DOI] [PubMed] [Google Scholar]
- 18.Igarashi T, Wynberg J, Srinivasan R, et al. . Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood. 2004;104(1):170-177. [DOI] [PubMed] [Google Scholar]
- 19.Ruggeri L, Capanni M, Casucci M, et al. . Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94(1):333-339. [PubMed] [Google Scholar]
- 20.Ruggeri L, Capanni M, Urbani E, et al. . Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097-2100. [DOI] [PubMed] [Google Scholar]
- 21.Ruggeri L, Mancusi A, Capanni M, et al. . Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110(1):433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giebel S, Locatelli F, Lamparelli T, et al. . Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102(3):814-819. [DOI] [PubMed] [Google Scholar]
- 23.Bornhäuser M, Schwerdtfeger R, Martin H, Frank KH, Theuser C, Ehninger G. Role of KIR ligand incompatibility in hematopoietic stem cell transplantation using unrelated donors. Blood. 2004;103(7):2860-2861. [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Busson M, Rocha V, et al. . Activating KIR genes are associated with CMV reactivation and survival after non-T-cell depleted HLA-identical sibling bone marrow transplantation for malignant disorders. Bone Marrow Transplant. 2006;38(6):437-444. [DOI] [PubMed] [Google Scholar]
- 25.Davies SM, Ruggieri L, DeFor T, et al. . Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood. 2002;100(10):3825-3827. [DOI] [PubMed] [Google Scholar]
- 26.Lowe EJ, Turner V, Handgretinger R, et al. . T-cell alloreactivity dominates natural killer cell alloreactivity in minimally T-cell-depleted HLA-non-identical paediatric bone marrow transplantation. Br J Haematol. 2003;123(2):323-326. [DOI] [PubMed] [Google Scholar]
- 27.Sun JY, Dagis A, Gaidulis L, et al. . Detrimental effect of natural killer cell alloreactivity in T-replete hematopoietic cell transplantation (HCT) for leukemia patients. Biol Blood Marrow Transplant. 2007;13(2):197-205. [DOI] [PubMed] [Google Scholar]
- 28.Willemze R, Rodrigues CA, Labopin M, et al. ; Eurocord-Netcord and Acute Leukaemia Working Party of the EBMT. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia. 2009;23(3):492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cudkowicz G, Bennett M. Peculiar immunobiology of bone marrow allografts. II. Rejection of parental grafts by resistant F 1 hybrid mice. J Exp Med. 1971;134(6):1513-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snell GD, Jackson RB. Histocompatibility genes of the mouse. II. Production and analysis of isogenic resistant lines. J Natl Cancer Inst. 1958;21(5):843-877. [PubMed] [Google Scholar]
- 31.Bennett M. Biology and genetics of hybrid resistance. Adv Immunol. 1987;41:333-445. [DOI] [PubMed] [Google Scholar]
- 32.Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol. 2013;13(2):133-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moretta L, Moretta A. Killer immunoglobulin-like receptors. Curr Opin Immunol. 2004;16(5):626-633. [DOI] [PubMed] [Google Scholar]
- 34.Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76(12):2421-2438. [PubMed] [Google Scholar]
- 35.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633-640. [DOI] [PubMed] [Google Scholar]
- 36.Fehniger TA, Cooper MA, Nuovo GJ, et al. . CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101(8):3052-3057. [DOI] [PubMed] [Google Scholar]
- 37.Yokoyama WM. Natural killer cell receptors. Curr Opin Immunol. 1995;7(1):110-120. [DOI] [PubMed] [Google Scholar]
- 38.Brandt CS, Baratin M, Yi EC, et al. . The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206(7):1495-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pogge von Strandmann E, Simhadri VR, von Tresckow B, et al. . Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27(6):965-974. [DOI] [PubMed] [Google Scholar]
- 40.Bloushtain N, Qimron U, Bar-Ilan A, et al. . Membrane-associated heparan sulfate proteoglycans are involved in the recognition of cellular targets by NKp30 and NKp46. J Immunol. 2004;173(4):2392-2401. [DOI] [PubMed] [Google Scholar]
- 41.Jinushi M, Takehara T, Tatsumi T, et al. . Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. Int J Cancer. 2003;104(3):354-361. [DOI] [PubMed] [Google Scholar]
- 42.Boyington JC, Sun PD. A structural perspective on MHC class I recognition by killer cell immunoglobulin-like receptors. Mol Immunol. 2002;38(14):1007-1021. [DOI] [PubMed] [Google Scholar]
- 43.Boyington JC, Brooks AG, Sun PD. Structure of killer cell immunoglobulin-like receptors and their recognition of the class I MHC molecules. Immunol Rev. 2001;181:66-78. [DOI] [PubMed] [Google Scholar]
- 44.Steinle A, Li P, Morris DL, et al. . Interactions of human NKG2D with its ligands MICA, MICB, and homologs of the mouse RAE-1 protein family. Immunogenetics. 2001;53(4):279-287. [DOI] [PubMed] [Google Scholar]
- 45.Das H, Groh V, Kuijl C, et al. . MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity. 2001;15(1):83-93. [DOI] [PubMed] [Google Scholar]
- 46.Cosman D, Müllberg J, Sutherland CL, et al. . ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14(2):123-133. [DOI] [PubMed] [Google Scholar]
- 47.Bauer S, Groh V, Wu J, et al. . Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727-729. [DOI] [PubMed] [Google Scholar]
- 48.Gumperz JE, Barber LD, Valiante NM, et al. . Conserved and variable residues within the Bw4 motif of HLA-B make separable contributions to recognition by the NKB1 killer cell-inhibitory receptor. J Immunol. 1997;158(11):5237-5241. [PubMed] [Google Scholar]
- 49.Mandelboim O, Reyburn HT, Sheu EG, et al. . The binding site of NK receptors on HLA-C molecules. Immunity. 1997;6(3):341-350. [DOI] [PubMed] [Google Scholar]
- 50.Kim S, Poursine-Laurent J, Truscott SM, et al. . Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709-713. [DOI] [PubMed] [Google Scholar]
- 51.Harel-Bellan A, Quillet A, Marchiol C, DeMars R, Tursz T, Fradelizi D. Natural killer susceptibility of human cells may be regulated by genes in the HLA region on chromosome 6. Proc Natl Acad Sci USA. 1986;83(15):5688-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andersson S, Malmberg JA, Malmberg KJ. Tolerant and diverse natural killer cell repertoires in the absence of selection. Exp Cell Res. 2010;316(8):1309-1315. [DOI] [PubMed] [Google Scholar]
- 53.Raulet DH, Vance RE, McMahon CW. Regulation of the natural killer cell receptor repertoire. Annu Rev Immunol. 2001;19:291-330. [DOI] [PubMed] [Google Scholar]
- 54.Schönberg K, Sribar M, Enczmann J, Fischer JC, Uhrberg M. Analyses of HLA-C-specific KIR repertoires in donors with group A and B haplotypes suggest a ligand-instructed model of NK cell receptor acquisition. Blood. 2011;117(1):98-107. [DOI] [PubMed] [Google Scholar]
- 55.Valiante NM, Lienert K, Shilling HG, Smits BJ, Parham P. Killer cell receptors: keeping pace with MHC class I evolution. Immunol Rev. 1997;155:155-164. [DOI] [PubMed] [Google Scholar]
- 56.Valiante NM, Parham P. Natural killer cells, HLA class I molecules, and marrow transplantation. Biol Blood Marrow Transplant. 1997;3(5):229-235. [PubMed] [Google Scholar]
- 57.Yawata M, Yawata N, Draghi M, Little AM, Partheniou F, Parham P. Roles for HLA and KIR polymorphisms in natural killer cell repertoire selection and modulation of effector function. J Exp Med. 2006;203(3):633-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marsh SG, Parham P, Dupont B, et al. . Killer-cell immunoglobulin-like receptor (KIR) nomenclature report, 2002. Immunogenetics. 2003;55(4):220-226. [DOI] [PubMed] [Google Scholar]
- 59.Ohlén C, Kling G, Höglund P, et al. . Prevention of allogeneic bone marrow graft rejection by H-2 transgene in donor mice. Science. 1989;246(4930):666-668. [DOI] [PubMed] [Google Scholar]
- 60.Moretta A, Moretta L. HLA class I specific inhibitory receptors. Curr Opin Immunol. 1997;9(5):694-701. [DOI] [PubMed] [Google Scholar]
- 61.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359-393. [DOI] [PubMed] [Google Scholar]
- 62.Khakoo SI, Thio CL, Martin MP, et al. . HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305(5685):872-874. [DOI] [PubMed] [Google Scholar]
- 63.Augusto DG, O’Connor GM, Lobo-Alves SC, et al. . Pemphigus is associated with KIR3DL2 expression levels and provides evidence that KIR3DL2 may bind HLA-A3 and A11 in vivo. Eur J Immunol. 2015;45(7):2052-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hansasuta P, Dong T, Thananchai H, et al. . Recognition of HLA-A3 and HLA-A11 by KIR3DL2 is peptide-specific. Eur J Immunol. 2004;34(6):1673-1679. [DOI] [PubMed] [Google Scholar]
- 65.Pittari G, Liu XR, Selvakumar A, et al. . NK cell tolerance of self-specific activating receptor KIR2DS1 in individuals with cognate HLA-C2 ligand. J Immunol. 2013;190(9):4650-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chewning JH, Gudme CN, Hsu KC, Selvakumar A, Dupont B. KIR2DS1-positive NK cells mediate alloresponse against the C2 HLA-KIR ligand group in vitro. J Immunol. 2007;179(2):854-868. [DOI] [PubMed] [Google Scholar]
- 67.Stewart CA, Laugier-Anfossi F, Vély F, et al. . Recognition of peptide-MHC class I complexes by activating killer immunoglobulin-like receptors. Proc Natl Acad Sci USA. 2005;102(37):13224-13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu J, Xiao Z, Ko HL, Shen M, Ren EC. Activating killer cell immunoglobulin-like receptor 2DS2 binds to HLA-A*11. Proc Natl Acad Sci USA. 2014;111(7):2662-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.González-Galarza FF, Takeshita LY, Santos EJ, et al. . Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Res. 2015;43(Database issue):D784-D788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bashirova AA, Martin MP, McVicar DW, Carrington M. The killer immunoglobulin-like receptor gene cluster: tuning the genome for defense. Annu Rev Genomics Hum Genet. 2006;7:277-300. [DOI] [PubMed] [Google Scholar]
- 71.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. . Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051-3057. [DOI] [PubMed] [Google Scholar]
- 72.Bishara A, De Santis D, Witt CC, et al. . The beneficial role of inhibitory KIR genes of HLA class I NK epitopes in haploidentically mismatched stem cell allografts may be masked by residual donor-alloreactive T cells causing GVHD. Tissue Antigens. 2004;63(3):204-211. [DOI] [PubMed] [Google Scholar]
- 73.Kröger N, Binder T, Zabelina T, et al. . Low number of donor activating killer immunoglobulin-like receptors (KIR) genes but not KIR-ligand mismatch prevents relapse and improves disease-free survival in leukemia patients after in vivo T-cell depleted unrelated stem cell transplantation. Transplantation. 2006;82(8):1024-1030. [DOI] [PubMed] [Google Scholar]
- 74.Farag SS, Bacigalupo A, Eapen M, et al. ; KIR Study Group, Center for International Blood and Marrow Transplantation Research. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12(8):876-884. [DOI] [PubMed] [Google Scholar]
- 75.Schaffer M, Malmberg KJ, Ringdén O, Ljunggren HG, Remberger M. Increased infection-related mortality in KIR-ligand-mismatched unrelated allogeneic hematopoietic stem-cell transplantation. Transplantation. 2004;78(7):1081-1085. [DOI] [PubMed] [Google Scholar]
- 76.Miller JS, Cooley S, Parham P, et al. . Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109(11):5058-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cooley S, McCullar V, Wangen R, et al. . KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood. 2005;106(13):4370-4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hsu KC, Keever-Taylor CA, Wilton A, et al. . Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105(12):4878-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Verheyden S, Schots R, Duquet W, Demanet C. A defined donor activating natural killer cell receptor genotype protects against leukemic relapse after related HLA-identical hematopoietic stem cell transplantation. Leukemia. 2005;19(8):1446-1451. [DOI] [PubMed] [Google Scholar]
- 80.Beelen DW, Ottinger HD, Ferencik S, et al. . Genotypic inhibitory killer immunoglobulin-like receptor ligand incompatibility enhances the long-term antileukemic effect of unmodified allogeneic hematopoietic stem cell transplantation in patients with myeloid leukemias. Blood. 2005;105(6):2594-2600. [DOI] [PubMed] [Google Scholar]
- 81.Schellekens J, Rozemuller EH, Petersen EJ, van den Tweel JG, Verdonck LF, Tilanus MG. Activating KIRs exert a crucial role on relapse and overall survival after HLA-identical sibling transplantation. Mol Immunol. 2008;45(8):2255-2261. [DOI] [PubMed] [Google Scholar]
- 82.Immuno Polymorphism Database (IPD). KIR Haplotypes. https://www.ebi.ac.uk/ipd/kir/haplotypes.html.
- 83.Cooley S, Trachtenberg E, Bergemann TL, et al. . Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113(3):726-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oevermann L, Michaelis SU, Mezger M, et al. . KIR B haplotype donors confer a reduced risk for relapse after haploidentical transplantation in children with ALL. Blood. 2014;124(17):2744-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cooley S, Weisdorf DJ, Guethlein LA, et al. . Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116(14):2411-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cooley S, Weisdorf DJ, Guethlein LA, et al. . Donor killer cell Ig-like receptor B haplotypes, recipient HLA-C1, and HLA-C mismatch enhance the clinical benefit of unrelated transplantation for acute myelogenous leukemia. J Immunol. 2014;192(10):4592-4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stringaris K, Adams S, Uribe M, et al. . Donor KIR Genes 2DL5A, 2DS1 and 3DS1 are associated with a reduced rate of leukemia relapse after HLA-identical sibling stem cell transplantation for acute myeloid leukemia but not other hematologic malignancies. Biol Blood Marrow Transplant. 2010;16(9):1257-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Venstrom JM, Pittari G, Gooley TA, et al. . HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367(9):805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Venstrom JM, Dupont B, Hsu KC, et al. . Donor activating KIR2DS1 in leukemia. N Engl J Med. 2014;371(21):2042. [DOI] [PubMed] [Google Scholar]
- 90.Willemze R, Ruggeri A, Purtill D, Rodrigues CA, Gluckman E, Rocha V; Eurocord and of the European Group of Blood and Marrow Transplantation. Is there an impact of killer cell immunoglobulin-like receptors and KIR-ligand incompatibilities on outcomes after unrelated cord blood stem cell transplantation? Best Pract Res Clin Haematol. 2010;23(2):283-290. [DOI] [PubMed] [Google Scholar]
- 91.Garfall A, Kim HT, Sun L, et al. . KIR ligand incompatibility is not associated with relapse reduction after double umbilical cord blood transplantation. Bone Marrow Transplant. 2013;48(7):1000-1002. [DOI] [PubMed] [Google Scholar]
- 92.Tanaka J, Morishima Y, Takahashi Y, et al. . Effects of KIR ligand incompatibility on clinical outcomes of umbilical cord blood transplantation without ATG for acute leukemia in complete remission. Blood Cancer J. 2013;3:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rocha V, Ruggeri A, Spellman S, et al. ; Eurocord, Cord Blood Committee Cellular Therapy Immunobiology Working Party of the European Group for Blood and Marrow Transplantation, Netcord, and the Center for International Blood and Marrow Transplant Research. Killer Cell Immunoglobulin-Like Receptor-Ligand Matching and Outcomes after Unrelated Cord Blood Transplantation in Acute Myeloid Leukemia. Biol Blood Marrow Transplant. 2016;22(7):1284-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brunstein CG, Wagner JE, Weisdorf DJ, et al. . Negative effect of KIR alloreactivity in recipients of umbilical cord blood transplant depends on transplantation conditioning intensity. Blood. 2009;113(22):5628-5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fischer JC, Kobbe G, Enczmann J, Haas R, Uhrberg M. The impact of HLA-C matching depends on the C1/C2 KIR ligand status in unrelated hematopoietic stem cell transplantation. Immunogenetics. 2012;64(12):879-885. [DOI] [PubMed] [Google Scholar]
- 96.Cook MA, Milligan DW, Fegan CD, et al. . The impact of donor KIR and patient HLA-C genotypes on outcome following HLA-identical sibling hematopoietic stem cell transplantation for myeloid leukemia. Blood. 2004;103(4):1521-1526. [DOI] [PubMed] [Google Scholar]
- 97.Sekine T, Marin D, Cao K, et al. . Specific combinations of donor and recipient KIR-HLA genotypes predict for large differences in outcome after cord blood transplantation. Blood 2016;128(2)297-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stringaris K, Sekine T, Khoder A, et al. . Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica. 2014;99(5):836-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kollman C, Howe CW, Anasetti C, et al. . Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043-2051. [DOI] [PubMed] [Google Scholar]
- 100.Ringdén O, Labopin M, Bacigalupo A, et al. . Transplantation of peripheral blood stem cells as compared with bone marrow from HLA-identical siblings in adult patients with acute myeloid leukemia and acute lymphoblastic leukemia. J Clin Oncol. 2002;20(24):4655-4664. [DOI] [PubMed] [Google Scholar]
- 101.Finke J, Schmoor C, Bethge WA, et al. ; ATG-Fresenius Trial Group. Prognostic factors affecting outcome after allogeneic transplantation for hematological malignancies from unrelated donors: results from a randomized trial. Biol Blood Marrow Transplant. 2012;18(11):1716-1726. [DOI] [PubMed] [Google Scholar]
- 102.Castro-Malaspina H, Harris RE, Gajewski J, et al. . Unrelated donor marrow transplantation for myelodysplastic syndromes: outcome analysis in 510 transplants facilitated by the National Marrow Donor Program. Blood. 2002;99(6):1943-1951. [DOI] [PubMed] [Google Scholar]
- 103.Carreras E, Jiménez M, Gómez-García V, et al. . Donor age and degree of HLA matching have a major impact on the outcome of unrelated donor haematopoietic cell transplantation for chronic myeloid leukaemia. Bone Marrow Transplant. 2006;37(1):33-40. [DOI] [PubMed] [Google Scholar]
- 104.Lee SJ, Klein J, Haagenson M, et al. . High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576-4583. [DOI] [PubMed] [Google Scholar]
- 105.Morishima Y, Sasazuki T, Inoko H, et al. . The clinical significance of human leukocyte antigen (HLA) allele compatibility in patients receiving a marrow transplant from serologically HLA-A, HLA-B, and HLA-DR matched unrelated donors. Blood. 2002;99(11):4200-4206. [DOI] [PubMed] [Google Scholar]
- 106.Petersdorf EW, Gooley TA, Anasetti C, et al. . Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998;92(10):3515-3520. [PubMed] [Google Scholar]
- 107.Sasazuki T, Juji T, Morishima Y, et al. . Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. Japan Marrow Donor Program. N Engl J Med. 1998;339(17):1177-1185. [DOI] [PubMed] [Google Scholar]
- 108.Fernández-Viña MA, Klein JP, Haagenson M, et al. . Multiple mismatches at the low expression HLA loci DP, DQ, and DRB3/4/5 associate with adverse outcomes in hematopoietic stem cell transplantation. Blood. 2013;121(22):4603-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Flomenberg N, Baxter-Lowe LA, Confer D, et al. . Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923-1930. [DOI] [PubMed] [Google Scholar]
- 110.Petersdorf EW, Gooley T, Malkki M, et al. . The biological significance of HLA-DP gene variation in haematopoietic cell transplantation. Br J Haematol. 2001;112(4):988-994. [DOI] [PubMed] [Google Scholar]
- 111.Schmidt-Hieber M, Labopin M, Beelen D, et al. . CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013;122(19):3359-3364. [DOI] [PubMed] [Google Scholar]
- 112.Servais S, Porcher R, Xhaard A, et al. . Pre-transplant prognostic factors of long-term survival after allogeneic peripheral blood stem cell transplantation with matched related/unrelated donors. Haematologica. 2014;99(3):519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fabre C, Koscielny S, Mohty M, et al. . Younger donor’s age and upfront tandem are two independent prognostic factors for survival in multiple myeloma patients treated by tandem autologous-allogeneic stem cell transplantation: a retrospective study from the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). Haematologica. 2012;97(4):482-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mehta J, Gordon LI, Tallman MS, et al. . Does younger donor age affect the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies beneficially? Bone Marrow Transplant. 2006;38(2):95-100. [DOI] [PubMed] [Google Scholar]
- 115.Kongtim P, Di Stasi A, Rondon G, et al. . Can a female donor for a male recipient decrease the relapse rate for patients with acute myeloid leukemia treated with allogeneic hematopoietic stem cell transplantation? Biol Blood Marrow Transplant. 2015;21(4):713-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Frassoni F, Labopin M, Gluckman E, et al. . Results of allogeneic bone marrow transplantation for acute leukemia have improved in Europe with time--a report of the acute leukemia working party of the European group for blood and marrow transplantation (EBMT). Bone Marrow Transplant. 1996;17(1):13-18. [PubMed] [Google Scholar]
- 117.Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood. 2004;103(1):347-352. [DOI] [PubMed] [Google Scholar]
- 118.Stern M, Passweg JR, Locasciulli A, et al. ; Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation. Influence of donor/recipient sex matching on outcome of allogeneic hematopoietic stem cell transplantation for aplastic anemia. Transplantation. 2006;82(2):218-226. [DOI] [PubMed] [Google Scholar]
- 119.Stern M, Brand R, de Witte T, et al. . Female-versus-male alloreactivity as a model for minor histocompatibility antigens in hematopoietic stem cell transplantation. Am J Transplant. 2008;8(10):2149-2157. [DOI] [PubMed] [Google Scholar]
- 120.Loren AW, Bunin GR, Boudreau C, et al. . Impact of donor and recipient sex and parity on outcomes of HLA-identical sibling allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(7):758-769. [DOI] [PubMed] [Google Scholar]
- 121.Stern M, Ruggeri L, Mancusi A, et al. . Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood. 2008;112(7):2990-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Y, Chang YJ, Xu LP, et al. . Who is the best donor for a related HLA haplotype-mismatched transplant? Blood. 2014;124(6):843-850. [DOI] [PubMed] [Google Scholar]
- 123.Martin PJ. Increased disparity for minor histocompatibility antigens as a potential cause of increased GVHD risk in marrow transplantation from unrelated donors compared with related donors. Bone Marrow Transplant. 1991;8(3):217-223. [PubMed] [Google Scholar]
- 124.Spierings E, Kim YH, Hendriks M, et al. . Multicenter analyses demonstrate significant clinical effects of minor histocompatibility antigens on GvHD and GvL after HLA-matched related and unrelated hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(8):1244-1253. [DOI] [PubMed] [Google Scholar]
- 125.Gallardo D, Aróstegui JI, Balas A, et al. ; GvHD Subcommittee of the Grupo Español de Trasplante Hemapoyético (GETH). Disparity for the minor histocompatibility antigen HA-1 is associated with an increased risk of acute graft-versus-host disease (GvHD) but it does not affect chronic GvHD incidence, disease-free survival or overall survival after allogeneic human leucocyte antigen-identical sibling donor transplantation. Br J Haematol. 2001;114(4):931-936. [DOI] [PubMed] [Google Scholar]
- 126.Katagiri T, Shiobara S, Nakao S, et al. . Mismatch of minor histocompatibility antigen contributes to a graft-versus-leukemia effect rather than to acute GVHD, resulting in long-term survival after HLA-identical stem cell transplantation in Japan. Bone Marrow Transplant. 2006;38(10):681-686. [DOI] [PubMed] [Google Scholar]
- 127.Spierings E. Minor histocompatibility antigens: past, present, and future. Tissue Antigens. 2014;84(4):374-460. [DOI] [PubMed] [Google Scholar]
- 128.Tucunduva L, Ruggeri A, Sanz G, et al. . Impact of Myeloablative and Reduced Intensity Conditioning on Outcomes After Unrelated Cord Blood Transplantation for Adults with Acute Lymphoblastic Leukemia. Biol Blood Marrow Transplant. 2013;19(2):S127. [Google Scholar]
- 129.Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood. 2010;115(9):1843-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burns LJ, Weisdorf DJ, DeFor TE, et al. . IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant. 2003;32(2):177-186. [DOI] [PubMed] [Google Scholar]
- 131.Miller JS, Tessmer-Tuck J, Pierson BA, et al. . Low dose subcutaneous interleukin-2 after autologous transplantation generates sustained in vivo natural killer cell activity. Biol Blood Marrow Transplant. 1997;3(1):34-44. [PubMed] [Google Scholar]
- 132.Caligiuri MA, Murray C, Robertson MJ, et al. . Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Invest. 1993;91(1):123-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Soiffer RJ, Murray C, Cochran K, et al. . Clinical and immunologic effects of prolonged infusion of low-dose recombinant interleukin-2 after autologous and T-cell-depleted allogeneic bone marrow transplantation. Blood. 1992;79(2):517-526. [PubMed] [Google Scholar]
- 134.Weisdorf DJ, Anderson PM, Blazar BR, Uckun FM, Kersey JH, Ramsay NK. Interleukin 2 immediately after autologous bone marrow transplantation for acute lymphoblastic leukemia--a phase I study. Transplantation. 1993;55(1):61-66. [DOI] [PubMed] [Google Scholar]
- 135.Lister J, Rybka WB, Donnenberg AD, et al. . Autologous peripheral blood stem cell transplantation and adoptive immunotherapy with activated natural killer cells in the immediate posttransplant period. Clin Cancer Res. 1995;1(6):607-614. [PubMed] [Google Scholar]
- 136.Blaise D, Attal M, Pico JL, et al. . The use of a sequential high dose recombinant interleukin 2 regimen after autologous bone marrow transplantation does not improve the disease free survival of patients with acute leukemia transplanted in first complete remission. Leuk Lymphoma. 1997;25(5-6):469-478. [DOI] [PubMed] [Google Scholar]
- 137.deMagalhaes-Silverman M, Donnenberg A, Lembersky B, et al. . Posttransplant adoptive immunotherapy with activated natural killer cells in patients with metastatic breast cancer. J Immunother. 2000;23(1):154-160. [DOI] [PubMed] [Google Scholar]
- 138.Meropol NJ, Porter M, Blumenson LE, et al. . Daily subcutaneous injection of low-dose interleukin 2 expands natural killer cells in vivo without significant toxicity. Clin Cancer Res. 1996;2(4):669-677. [PubMed] [Google Scholar]
- 139.Curti A, Ruggeri L, D’Addio A, et al. . Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118(12):3273-3279. [DOI] [PubMed] [Google Scholar]
- 140.Rubnitz JE, Inaba H, Ribeiro RC, et al. . NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28(6):955-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bachanova V, Cooley S, Defor TE, et al. . Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shaffer BC, Le Luduec JB, Forlenza C, et al. . Phase II Study of Haploidentical Natural Killer Cell Infusion for Treatment of Relapsed or Persistent Myeloid Malignancies Following Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016;22(4):705-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Geller MA, Cooley S, Judson PL, et al. . A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13(1):98-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Iliopoulou EG, Kountourakis P, Karamouzis MV, et al. . A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother. 2010;59(12):1781-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ghiringhelli F, Ménard C, Terme M, et al. . CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202(8):1075-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6(8):595-601. [DOI] [PubMed] [Google Scholar]
- 147.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. . T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maude SL, Frey N, Shaw PA, et al. . Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Davila ML, Riviere I, Wang X, et al. . Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kochenderfer JN, Dudley ME, Carpenter RO, et al. . Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122(25):4129-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kochenderfer JN, Dudley ME, Feldman SA, et al. . B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brentjens RJ, Rivière I, Park JH, et al. . Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118(18):4817-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Asai O, Longo DL, Tian ZG, et al. . Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest. 1998;101(9):1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115(21):4293-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Han J, Chu J, Keung Chan W, et al. . CAR-Engineered NK Cells Targeting Wild-Type EGFR and EGFRvIII Enhance Killing of Glioblastoma and Patient-Derived Glioblastoma Stem Cells. Sci Rep. 2015;5:11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hermanson DL, Ni Z, Knorr DA, et al. . Functional Chimeric Antigen Receptor-Expressing Natural Killer Cells Derived From Human Pluripotent Stem Cells [abstract]. Blood. 2013;122(21). Abstract 896. [Google Scholar]
- 158.Boissel L, Betancur-Boissel M, Lu W, et al. . Retargeting NK-92 cells by means of CD19- and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. OncoImmunology. 2013;2(10):e26527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jiang H, Zhang W, Shang P, et al. . Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol. 2014;8(2):297-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chu J, Deng Y, Benson DM, et al. . CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28(4):917-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Esser R, Müller T, Stefes D, et al. . NK cells engineered to express a GD2 -specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cell Mol Med. 2012;16(3):569-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Boissel L, Betancur M, Wels WS, Tuncer H, Klingemann H. Transfection with mRNA for CD19 specific chimeric antigen receptor restores NK cell mediated killing of CLL cells. Leuk Res. 2009;33(9):1255-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Müller T, Uherek C, Maki G, et al. . Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol Immunother. 2008;57(3):411-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Uherek C, Tonn T, Uherek B, et al. . Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood. 2002;100(4):1265-1273. [PubMed] [Google Scholar]
- 165.Gleason MK, Ross JA, Warlick ED, et al. . CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123(19):3016-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wiernik A, Foley B, Zhang B, et al. . Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 x 33 bispecific killer cell engager and ADAM17 inhibition. Clin Cancer Res. 2013;19(14):3844-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vallera DA, Zhang B, Gleason MK, et al. . Heterodimeric bispecific single-chain variable-fragment antibodies against EpCAM and CD16 induce effective antibody-dependent cellular cytotoxicity against human carcinoma cells. Cancer Biother Radiopharm. 2013;28(4):274-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Reiners KS, Kessler J, Sauer M, et al. . Rescue of impaired NK cell activity in hodgkin lymphoma with bispecific antibodies in vitro and in patients. Mol Ther. 2013;21(4):895-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gleason MK, Verneris MR, Todhunter DA, et al. . Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther. 2012;11(12):2674-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Singer H, Kellner C, Lanig H, et al. . Effective elimination of acute myeloid leukemic cells by recombinant bispecific antibody derivatives directed against CD33 and CD16. J Immunother. 2010;33(6):599-608. [DOI] [PubMed] [Google Scholar]
- 171.Vallera DA, Felices M, McElmurry R, et al. . IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced Function. Clin Cancer Res. 2016;22(14):3440-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]