Key Points
Question
Is there an association between cardiovascular health level in older age and risk of incident dementia?
Findings
In this French population-based cohort study involving 6626 individuals, an increased number of optimal cardiovascular health metrics (defined using a 7-item tool from the American Heart Association) were significantly associated with lower risk of incident dementia (hazard ratio, 0.90 for each additional metric at recommended optimal level).
Meaning
These findings may support the promotion of cardiovascular health to prevent development of risk factors associated with dementia.
Abstract
Importance
Evidence is limited regarding the relation between cardiovascular health level and dementia risk.
Objective
To investigate the association between cardiovascular health level, defined using the 7-item tool from the American Heart Association (AHA), and risk of dementia and cognitive decline in older persons.
Design, Setting, and Participants
Population-based cohort study of persons aged 65 years or older from Bordeaux, Dijon, and Montpellier, France, without history of cardiovascular diseases or dementia at baseline who underwent repeated in-person neuropsychological testing (January 1999–July 2016) and systematic detection of incident dementia (date of final follow-up, July 26, 2016).
Exposures
The number of the AHA’s Life’s Simple 7 metrics at recommended optimal level (nonsmoking, body mass index <25, regular physical activity, eating fish twice a week or more and fruits and vegetables at least 3 times a day, cholesterol <200 mg/dL [untreated], fasting glucose <100 mg/dL [untreated], and blood pressure <120/80 mm Hg [untreated]; score range, 0-7) and a global cardiovascular health score (range, 0-14; poor, intermediate, and optimal levels of each metric assigned a value of 0, 1, and 2, respectively).
Main Outcomes and Measures
Incident dementia validated by an expert committee and change in a composite score of global cognition (in standard units, with values indicating distance from population means, 0 equal to the mean, and +1 and −1 equal to 1 SD above and below the mean).
Results
Among 6626 participants (mean age, 73.7 years; 4200 women [63.4%]), 2412 (36.5%), 3781 (57.1%), and 433 (6.5%) had 0 to 2, 3 to 4, and 5 to 7 health metrics at optimal levels, respectively, at baseline. Over a mean follow-up duration of 8.5 (range, 0.6-16.6) years, 745 participants had incident adjudicated dementia. Compared with the incidence rate of dementia of 1.76 (95% CI, 1.38-2.15) per 100 person-years among those with 0 or 1 health metrics at optimal levels, the absolute differences in incident dementia rates for 2, 3, 4, 5, and 6 to 7 metrics were, respectively, −0.26 (95% CI, −0.48 to −0.04), −0.59 (95% CI, −0.80 to −0.38), −0.43 (95% CI, −0.65 to −0.21), −0.93 (95% CI, −1.18 to −0.68), and −0.96 (95% CI, −1.37 to −0.56) per 100 person-years. In multivariable models, the hazard ratios for dementia were 0.90 (95% CI, 0.84-0.97) per additional optimal metric and 0.92 (95% CI, 0.89-0.96) per additional point on the global score. Furthermore, the gain in global cognition associated with each additional optimal metric at baseline was 0.031 (95% CI, 0.009-0.053) standard units at inclusion, 0.068 (95% CI, 0.045-0.092) units at year 6, and 0.072 (95% CI, 0.042-0.102) units at year 12.
Conclusions and Relevance
In this cohort of older adults, increased numbers of optimal cardiovascular health metrics and a higher cardiovascular health score were associated with a lower risk of dementia and lower rates of cognitive decline. These findings may support the promotion of cardiovascular health to prevent risk factors associated with cognitive decline and dementia.
This cohort study assesses the association between cardiovascular (CV) health, defined by measures of healthy lifestyle and controlled CV risk factors, and risk of cognitive decline and dementia in community-dwelling people in France aged 65 years or older.
Introduction
High blood pressure, dyslipidemia, obesity, and diabetes in midlife have been associated with cardiovascular disease as well as increased risk of dementia and cognitive decline, whereas healthy behaviors, including nonsmoking, engaging in moderate-intensity exercise, and adopting diets rich in fruit, vegetables, and fish, have been related to lower risk of dementia outcomes.1,2,3 However, few studies have investigated the combined effect of these risk factors on the risk of dementia and on cognitive aging and have mostly focused on lifestyle risk factors.4,5
In 2010, the American Heart Association (AHA) emphasized the concept of primordial prevention, which aims to prevent the development of risk factors in first place, as a prevention strategy against cardiovascular disease.6 To this end, the AHA has developed a 7-item tool including 4 health behaviors (nonsmoking and maintaining body weight, physical activity, and diet at optimal levels) and 3 biological measures (untreated blood cholesterol, blood glucose, and blood pressure at optimal levels) to promote optimal cardiovascular health. Accordingly, higher cardiovascular health has been consistently associated with substantial lower risk of death, coronary heart disease, and stroke.7,8
Given that some risk factors and mechanisms are common to diseases affecting the heart and the brain, efforts guided by the AHA tool to prevent cardiovascular disease could have secondary benefits for the prevention of dementia. Recent evidence supports this hypothesis for cognitive decline9,10 and cognitive impairment in US middle-aged populations.11 Association with incident dementia is less clear, as the 2 studies published to date have provided inconsistent results.12,13
The objective of this study was to investigate the association between cardiovascular health level and risk of dementia and cognitive decline in a large French cohort of older persons.
Methods
The protocol of the Three-City (3C) Study was approved by the Consultative Committee for the Protection of Persons Participating in Biomedical Research at Kremlin-Bicêtre University Hospital, Paris, France, and the Committee for the Protection of Persons, Sud-Mediterranée III, Nîmes, France. All participants provided written informed consent.
Population
The 3C Study is a prospective cohort initiated in 1999-2000 among 9294 noninstitutionalized community dwellers aged at least 65 years selected from the electoral rolls of 3 French cities (Bordeaux [n = 2104], Dijon [n = 4931], and Montpellier [n = 2259]).14 Data collected at baseline during face-to-face interviews included sociodemographic, lifestyle, and health information, medication use, a brief food frequency questionnaire,15 leisure activities (from a self-administered questionnaire in Bordeaux), neuropsychological testing, blood pressure and anthropometric measurements, and blood samples for assessment of standard biology (blood lipids and glucose) and APOE genotyping. Participants have been followed every 2 to 3 years during face-to-face interviews conducted at home and at the study center, with repeated cognitive evaluations and ascertainment of incident dementia cases until 2012 in Dijon and until 2016 in Bordeaux and Montpellier (date of final follow-up, July 26, 2016). Participants in the overall cohort were excluded from this study if the results of a baseline blood draw or data from the questionnaire were not available, if they had prevalent dementia or history of cardiovascular diseases at baseline, or if they were not followed up for dementia or cognitive performance.
Ascertainment of Cardiovascular Health Level
Each level of the 7 AHA metrics was categorized as poor, intermediate, and optimal according to the AHA cutoffs and criteria (except physical activity and diet, which needed adaptations) (Table 1).6 For physical activity, owing to slight variations in questionnaires, we used 2 site-specific definitions of optimal status. The diet metric was based on intakes of fruit, vegetables, and fish,6 which were available in the whole cohort (and ignored fibers, sodium, and sweetened beverages) and which have shown robust associations with dementia in previous studies.6,16,17
Table 1. Definition of Cardiovascular Health Metrics for Ascertainment of Cardiovascular Health Statusa.
| Metric | Recommended Optimal Level | Intermediate Level | Poor Level |
|---|---|---|---|
| Smoking | Never or quit ≥12 mo prior | Quit <12 mo | Current smokers |
| Physical activity | |||
| Montpellier and Dijon sites | Recreational walking ≥2 h per d or practicing sport ≥2 times per wk | Recreational walking 1 to 2 h per d or practicing sport monthly to weekly | Recreational walking <1 h per d or no sport practice |
| Bordeaux site | Recreational walking ≥8 h per wk or having ≥4 h of sport or intensive leisure activity per wk | Recreational walking 2 to 8 h per wk or having 1 to 3 h of sport or intensive leisure activity per wk | Recreational walking ≤1 h per wk or having <1 h of sport or intensive leisure activity per wk |
| Healthy diet | ≥1 Portion per d of each of fresh fruit, raw vegetables, cooked fruit/vegetables and ≥2 portions per wk of fish | ≥1 Portion per d of fresh fruit, raw vegetables, cooked fruit/vegetables or ≥2 portions per wk of fish | <1 portion per d of fresh fruit, raw vegetables, cooked fruit/vegetables and <2 portions per wk of fish |
| Body mass indexb | <25 | 25-29.9 | ≥30 |
| Total cholesterol | <200 mg/dL untreated | 200-240 mg/dL or <200 mg/dL treated | >240 mmol/L |
| Blood pressure | <120/80 mm Hg untreated | <120/80 mm Hg treated or 120-139/80-89 mm Hg | ≥140/90 mm Hg |
| Fasting plasma glucose | <100 mg/dL untreated | 100-126 mg/dL or <100 mg/dL treated | >126 mg/dL |
SI conversions: To convert cholesterol to millimoles per liter, multiply by 0.0259; to convert glucose to millimoles per liter, multiply by 0.0555.
Adapted from the American Heart Association.6
Calculated as weight in kilograms divided by height in meters squared.
Cardiovascular health level was ascertained through the number of cardiovascular health metrics at recommended optimal level (0-7) and a global cardiovascular health score, calculated by assigning 0 point for each metric at poor level, 1 point for each metric at intermediate level, and 2 points for each metric at recommended optimal level (total score range, 0-14).
Outcomes
The diagnosis of dementia was based on a 3-step procedure. First, trained psychologists administered a battery of neuropsychological tests at baseline and at each follow-up visit. Second, a neurologist examined all participants in Bordeaux and Montpellier at baseline. In Dijon, because of the large number of participants, only those who had positive screening results for dementia based on their neuropsychological performance underwent further clinical examination. At each follow-up visit, all participants who had suspected dementia were secondarily examined by a neurologist in the 3 study centers to establish a provisional diagnosis. Third, an independent committee of neurologists (blinded to the cardiovascular health level and risk factors of participants) examined all potential cases of dementia to obtain a consensus on its diagnosis based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition); ie, having (1) impairment in memory and in at least 1 of aphasia, apraxia, agnosia, or disturbance in executive functioning; (2) impairment and decline in social or occupational function; and (3) cognitive deficits that do not occur exclusively during the course of a delirium episode.18
Analyses of cognitive decline used composite scores of global cognition and memory. A global cognitive score (with higher scores indicating better cognition) was computed as the mean of z scores of 4 cognitive tests assessing (1) global cognition (using the Mini-Mental State Examination19; range of possible values, 0-30 points); (2) verbal semantic fluency (the Isaacs’ Set Test20; range, 0-66 points); (3) working memory and attention (the Benton Visual Retention Test21; range, 0-12 points); and (4) executive functioning (the Trail Making Test Part A,22 assessed as the number of correct displacements divided by time to perform the test; range, 0-96) (see eAppendix in the Supplement for details on the cognitive tests included). In addition, memory tests were combined in a composite memory score (with higher scores indicating better memory). This memory score was calculated as the mean of z scores of the Benton Visual Retention Test and a subset of the Mini-Mental State Examination (range, 0-8 points), defined as the sum of items related to orientation to time and the 3-word recall task. In a validation study,23 this subscore correlated reasonably well (ρ>0.40) with scores obtained on the Free and Cued Selective Reminding Test, a validated test of episodic memory,24 and was equivalent to that test in predicting incident Alzheimer disease in this cohort,23 demonstrating its validity for use as a proxy of episodic memory in our cohort.
Statistical Analyses
In this epidemiological longitudinal cohort study, analyses were considered exploratory and were all prespecified (except supplementary analyses addressing residual confounding and missing values, which were established post hoc). Because of small numbers of participants in extreme categories, descriptive analyses are presented by increasing number of optimal cardiovascular health metrics grouped into 3 classically used categories (ie, 0-2, 3-4, and 5-7). In multivariate analyses of incident dementia, the hazard ratios (HRs) of dementia per each additional metric at recommended optimal level and per 1-point increase in the cardiovascular health score were estimated using Cox proportional hazard models with delayed entry and taking age as a time scale. The date of dementia was imputed as the mid-point between the last visit without dementia and the visit at which the diagnosis was made.25 Models were stratified by study site and were adjusted for sex, highest attained educational level (no or primary school, secondary school, high school, university) and apolipoprotein E ε4 (APOEε4) carrier status. The dose-response relationship between each exposure and dementia risk was investigated in multivariable Cox models including penalized splines,26 and log linearity was formally tested using Wald tests. The proportional hazard assumption was assessed through the interaction between log(time) and Schoenfeld residuals. Interactions between cardiovascular health level and both sex and APOEε4 carrier status on the risk of dementia were evaluated.
Analyses of cardiovascular health level and trajectories of cognitive composite scores were conducted using linear mixed models.27 The composite scores were primarily normalized using latent process mixed models28 and standardized before being entered as dependent variables in the models. Change in cognition was modeled using natural cubic splines with 2 internal knots (at tertiles of measurement times); the nonlinear trajectory approximated by splines provided a better fit than a linear trajectory for both global score (Akaike information criteria = 53 650 and 53 939, respectively) and memory score (Akaike information criteria = 83 007 and 83 073). The models included an intercept that represented the cognitive score at baseline, the splines functions of time, and corresponding random effects to account for interindividual variability. Covariates (both as a simple effect and in interaction with splines functions of time) included the cardiovascular health level exposure variable, study center, age at baseline, sex, educational level, and APOEε4 carrier status. As in the analysis of incident dementia, the assumption of a linear relationship between cardiovascular health level exposures and cognitive trajectory constituents was investigated using splines.
Supplementary Analyses
These exploratory analyses were conducted for the dementia outcome only. Residual confounding by socioeconomic status was evaluated by further adjusting models for monthly income (<€750, €750-<€1500, €1500-€2250, and >€2250) and occupational attainment (in 5 classes of socioprofessional categories) from the baseline questionnaire. The possible influence of intercurrent stroke was evaluated in secondary analyses restricted to incident events until the year 2010 (the last visit with validation of stroke events in the cohort) by (1) further adjusting the Cox model for time-dependent incident stroke and (2) repeating the analysis after excluding the participants with incident stroke events. Moreover, competing risk by death was evaluated using an illness-death model for interval-censored time-to-event data.29,30 To help address bias related to complete case analysis, missing values for covariates and dementia outcome were imputed by multiple imputation (multiple imputation by chained equations with fully conditional specification method; M = 10 imputations).
Analyses were performed using SAS, version 9.3 (SAS Institute Inc), and R, version 3.3.4 (R Foundation). Two-sided P values were used with an α = .05 threshold for statistical significance.
Results
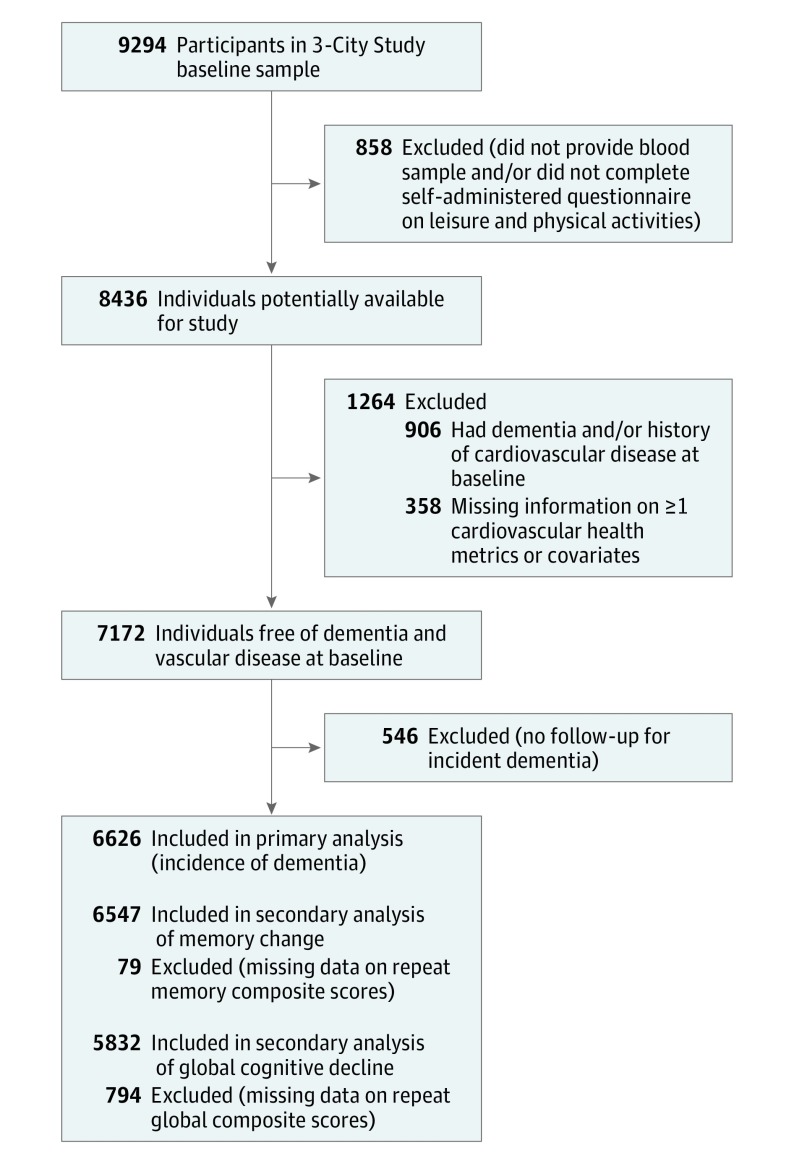
Among the 9294 cohort participants available for inclusion, 8436 individuals (91% of the initial sample) provided blood samples and answered both the general and self-administered baseline questionnaires; among these individuals, 7530 were free of a history of cardiovascular disease or prevalent dementia at baseline. After further excluding participants with missing information for cardiovascular health metrics or covariates (n = 358) and those with no follow-up for dementia (n = 546), the study sample size was composed of 6626 individuals with at least 1 repeated cognitive test and evaluation for dementia (Figure 1). Participants excluded were generally in worse health (eTable 1 in the Supplement). Overall, more than 90% of participants had at least 1 follow-up examination for dementia (with a dropout rate ranging from 3.7% to 12.2% at follow-up visits), and 77% had at least 3 repeated cognitive assessments.
Figure 1. Flow of Participants Included in the Three-City Study Population.
The mean age of participants was 73.7 (SD, 5.2) years at baseline; 4200 participants (63.4%) were women. At inclusion, 36.4%, 57.1%, and 6.5% had 0 to 2, 3 to 4, and 5 to 7 metrics at optimal level, respectively. The baseline characteristics of the participants by number of metrics at optimal level are reported in Table 2 and eTable 2 in the Supplement).
Table 2. Baseline Characteristics by Increasing Number of Cardiovascular Health Metrics at Recommended Optimal Level (N = 6626)a.
| Characteristics | 0-2 Metrics at Optimal Level (n = 2412) | 3-4 Metrics at Optimal Level (n = 3781) | 5-7 Metrics at Optimal Level (n = 433) |
|---|---|---|---|
| Study center, No. (%) | |||
| Bordeaux | 514 (21.3) | 691 (18.3) | 79 (18.2) |
| Dijon | 1403 (58.2) | 2089 (55.3) | 210 (48.5) |
| Montpellier | 495 (20.5) | 1001 (26.5) | 144 (33.3) |
| Age, mean (SD), y | 73.8 (5.1) | 73.7 (5.3) | 72.6 (4.9) |
| Female, No. (%) | 1342 (55.6) | 2560 (67.7) | 298 (68.8) |
| Education, No. (%) | |||
| None or primary | 655 (27.2) | 844 (22.3) | 67 (15.5) |
| Secondary | 905 (37.5) | 1406 (37.2) | 139 (32.1) |
| High school | 442 (18.3) | 807 (21.3) | 102 (23.6) |
| University | 410 (17.0) | 724 (19.2) | 125 (28.9) |
| APOEε4 carrier, No. (%) | 484 (20.1) | 724 (19.2) | 96 (22.2) |
| Current smoker, No. (%) | 254 (10.5) | 112 (3.0) | 2 (0.5) |
| Body mass index, mean (SD)b | 28.1 (3.6) | 24.3 (3.5) | 22.6 (2.4) |
| Fish, ≥2 portions per wk, No. (%) | 947 (39.3) | 2052 (54.3) | 350 (80.8) |
| Fruit and vegetables, ≥3 portions per d, No. (%) | 537 (22.3) | 1486 (39.3) | 320 (73.9) |
| Regular exercise, No. (%)c | 151 (6.3) | 1179 (31.2) | 341 (78.8) |
| Total cholesterol, mean (SD), mg/dL | 228.2 (36.3) | 226.2 (37.9) | 213.1 (36.3) |
| Triglycerides, mean (SD), mg/dL | 124.9 (58.5) | 101.9 (47.8) | 86.8 (34.5) |
| Systolic blood pressure, mean (SD), mm Hg | 151.1 (20.4) | 145.1 (21.2) | 133.6 (23.5) |
| Lipid-lowering medication, No. (%) | 865 (35.9) | 1003 (26.5) | 89 (20.6) |
| Antihypertensive medication, No. (%) | 1392 (57.7) | 1569 (41.5) | 103 (23.8) |
| Diabetes, No. (%)d | 420 (17.4) | 126 (3.3) | 6 (1.4) |
| MMSE score (range, 0-30), mean (SD)e | 27.4 (1.9) | 27.4 (1.9) | 27.5 (1.8) |
| Episodic memory MMSE subscore (range, 0-8), mean (SD)e | 6.6 (0.9) | 6.7 (0.9) | 6.7 (0.9) |
| BVRT score (range, 0-12), mean (SD)e | 11.4 (2.0) | 11.6 (2.0) | 11.8 (1.9) |
| IST score, mean (SD)f | 31.9 (6.7) | 32.6 (6.8) | 33.1 (7.1) |
| TMT-A score, mean (SD)f | 29.2 (10.2) | 29.7 (10.3) | 31.9 (10.3) |
Abbreviations: APOEε4, ε4 allele of the apolipoprotein E gene; BVRT, Benton Visual Retention Test; IST, Isaacs Set Test; MMSE, Mini-Mental State Examination; TMT-A, Trail Making Test part A.
SI conversions: To convert cholesterol to millimoles per liter, multiply by 0.0259; to convert triglycerides to millimoles per liter, multiply by 0.0113.
Baseline values were missing for 0.3% for the MMSE, 1.2% for both the BVRT and the IST, and 2.5% for the TMT-A.
Calculated as weight in kilograms divided by height in meters squared.
Defined as recreational walking ≥2 hours per day or practicing sports ≥2 times per week in Montpellier and Dijon study centers and as recreational walking ≥8 hours per week or having ≥4 hours of sports or intensive leisure activity per week in Bordeaux center.
Fasting plasma glucose level ≥7 mmol/L or taking antidiabetic medication.
Higher scores indicate better performance.
No theoretical superior limit; higher scores indicate better performance.
After a mean follow-up of 8.5 years (SD, 4.2 years; range, 0.6-16.6 years), a total of 745 incident dementia cases were adjudicated. The incidence rates of dementia decreased with increasing number of metrics at recommended optimal level (Table 3). Compared with the incident rate of dementia of 1.76 (95% CI, 1.38-2.15) per 100 person-years among those with 0 or 1 health metrics at optimal levels, the absolute rate differences per 100 person-years for each additional metric at optimal level were −0.26 (95% CI, −0.48 to −0.04) for 2 metrics, −0.59 (95% CI, −0.80 to −0.38) for 3 metrics, −0.43 (95% CI, −0.65 to −0.21) for 4 metrics, −0.93 (95% CI, −1.18 to −0.68) for 5 metrics, and −0.96 (95% CI, −1.37 to −0.56) for 6 or 7 metrics. In multivariable models, the risk of dementia decreased significantly and linearly with both increasing number of metrics at recommended optimal level (HR, 0.90 [95% CI, 0.84-0.97] per each additional metric) and increasing global cardiovascular health score (HR, 0.92 [95% CI, 0.89-0.96] per 1-point increase) (eFigure 1 in the Supplement). The proportional hazard assumption was met in all models. No significant interactions between the cardiovascular health level exposures and either sex or APOEε4 allele on dementia risk were found (P ≥ .23 for interaction terms).
Table 3. Incidence Rates of Dementia Across Increasing Number of Cardiovascular Health Metrics at Recommended Optimal Level (N = 6626).
| Overall Population | Metrics at Optimal Level | |||
|---|---|---|---|---|
| 0-2 | 3-4 | 5-7 | ||
| No. of incident cases/total No. (%) | 745/6626 (11.2) | 307/2412 (12.7) | 404/3781 (10.7) | 34/433 (7.9) |
| Incidence rate per 100 person-years (95% CI) | 1.32 (1.22-1.41) | 1.56 (1.39-1.74) | 1.23 (1.11-1.35) | 0.83 (0.55-1.11) |
| Absolute rate difference per 100 person-years (95% CI) | 1 [Reference] | −0.33 (−0.44 to −0.22) | −0.73 (−0.90 to −0.56) | |
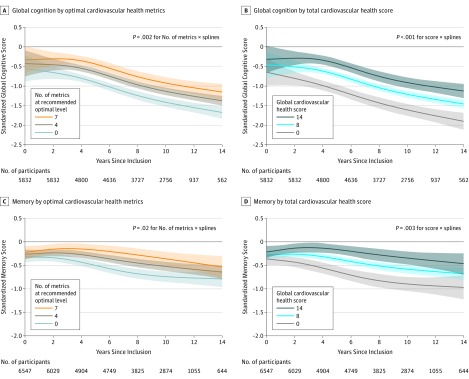
Secondary analyses of cognitive change were consistent with the analysis on dementia. Both increasing number of metrics at optimal level and higher global cardiovascular health score were significantly related to a lower rate of decline in global cognition (Figure 2, A and B; P = .002 and P < .001, respectively) and in memory (Figure 2, C and D; P = .02 and P = .003, respectively). For example, for global cognition, the estimated change in cognitive score for each additional metric at optimal level was 0.031 (95% CI, 0.009-0.053) standard units at inclusion, 0.068 (95% CI, 0.045-0.092) standard units at year 6, and 0.072 (95% CI, 0.042-0.102) standard units at year 12. Thus, a male participant with low education who is aged 73 years, is not an APOEɛ4 carrier (ie, the profile chosen as an example in Figure 2), and has no metrics at the recommended level would reach a global cognitive level of −1 standard unit approximately 6 years after inclusion, whereas an individual with a similar profile but with 7 metrics at the optimal level would reach a global cognitive level of −1 approximately 12 years after baseline. Similarly, the estimated change in cognitive score for each 1 point on the cardiovascular health score at inclusion, year 6, and year 12 was 0.024 (95% CI, 0.011-0.037), 0.049 (95% CI, 0.035-0.625), and 0.052 (95% CI, 0.034-0.070) standard units, respectively.
Figure 2. Mean Trajectories of Change in Global Cognition and Memory Predicted by a Multivariable Linear Mixed Model for a Specific Profile of Covariates, by Increasing Number of Recommended Optimal Cardiovascular Health Metrics and by Higher Total Cardiovascular Health Score.
Trajectories of change in global cognition and memory were estimated using linear mixed models among individuals with at least 1 complete battery of cognitive tests across repeated visits for computation of the composite score of global cognition (panels A and B) and of memory (panels C and D). Models considered a nonlinear trajectory with time approximated by natural cubic splines (with corresponding random effects); they also included a cardiovascular health level variable (number of recommended cardiovascular health metrics at optimal level [panels A and C] or higher total cardiovascular health score [panels B and D]) and its interactions with splines functions of time as well as age, study center, educational level, and apolipoprotein E ε4 (APOEε4) carrier status and their interactions with time. Composite scores for global cognition and memory were normalized using latent process mixed modeling and standardized before being entered as dependent variables in mixed models. Data were plotted for a chosen profile of covariates (eg, a man aged 73 years [median baseline age in the sample] from the Bordeaux study center with no higher than primary educational level who does not carry the APOEε4 allele). “Metrics × splines” and “score × splines” refer to the interactions of each cardiovascular health level exposure variable with splines functions of time in the linear mixed models. See Methods section of text for further explanation. Numbers of participants followed up at least to each time point are shown under the x-axes.
The relationships with trajectory constituents (intercept and splines functions) appeared to be generally linear (eFigure 2 and eFigure 3 in the Supplement).
Supplementary Analyses
After further controlling for other indicators of socioeconomic status (ie, income and occupational attainment), the results were similar (for each additional optimal metric, HR, 0.92 [95% CI, 0.85-0.98; P = .02] and 0.91 [95% CI, 0.85-0.98; P = .008] controlling for income and occupational attainment, respectively). Moreover, analyses adjusting for the 212 incident stroke events (eTable 3 in the Supplement) or excluding stroke events (eTable 4 in the Supplement) and analyses accounting for the competing risk of death (eTable 5 in the Supplement) yielded results consistent with the main analysis. Similarly, imputations of missing covariates and dementia outcomes yielded consistent results (eTable 6 in the Supplement).
Discussion
In this cohort of older persons, increased numbers of cardiovascular health metrics at recommended optimal levels and higher cardiovascular health scores based on the AHA Life’s Simple 7 tool were associated with a lower risk of dementia and lower rate of cognitive decline.
The association between better cardiovascular health and lower risk of dementia and less cognitive decline reported herein complement previous findings on mortality, coronary heart disease, and stroke.7 Although data are limited, prior results on cardiovascular health and cognition have been consistent. Greater cardiovascular health in early adulthood (number of metrics at optimal level) has been related to better cognitive performance in adulthood in the Coronary Artery Risk Development in Young Adults study.31 Moreover, greater cardiovascular health has been associated with lower cognitive decline in the Northern Manhattan Study (number of metrics at optimal level)9 and the Atherosclerosis Risk in Communities Study (global cardiovascular health score)9,10 and with lower risk of cognitive impairment in the Reasons for Geographic and Racial Differences in Stroke study (global cardiovascular health score).11 Still, these 4 prior studies were conducted in young and middle-aged participants and in US populations. Therefore, the present study indicates that the relationship of greater cardiovascular health to attenuated cognitive decline also applies to elderly and non-US populations.
To our knowledge, only 2 community studies have investigated the association of cardiovascular health with incident dementia and have reported inconsistent results. A German study found no association between cardiovascular health level and dementia risk in primary care practice. However, this study was limited by the nonsystematic detection of dementia cases (health insurance claims data) and by incomplete definitions of several metrics.13 In the Framingham Heart Study Offspring cohort, remote (ie, 7 years prior to baseline) but not recent (ie, baseline examination) recommended optimal cardiovascular health status (number of metrics at optimal level) was related to significant lower global cognitive decline and lower risk of dementia. However, the study was of modest sample size (<1500 participants), had few incident dementia cases (<100), and adjusted for age and sex only.12
The present study extends the results of these 2 prior studies with a larger sample size and higher number of incident events, a longer follow-up for dementia and cognition, and systematic detection of dementia cases with validation by an independent committee of neurologists. Additionally, the present study considered confounding factors, including education and APOEε4 genotype. The study results support the recent recommendations of the AHA and the American Stroke Association for the promotion of the Life’s Simple 7 tool.32 From a pragmatic and public health perspective, promoting change in cardiovascular health metrics from poor to intermediate levels with increasing cardiovascular health scores may be more achievable and is likely to have a greater population-level effect for preventing risk factors associated with dementia and cognitive decline than the much more challenging change from poor to optimal level.
Limitations
This study has several limitations. First, both diet and physical activity assessments may be prone to measurement error, although this is not specific to this study. Second, cardiovascular metrics were obtained only at baseline, and changes over time were not accounted for in this study. Third, the scores of global cognition and memory used in this study were not formally validated in an external study, and there is no established minimal clinically important difference for such composite outcomes. Fourth, loss to follow-up occurred, and participants included were in better health than those excluded owing to missing data; although the effect of selection and attrition on the results cannot be formally evaluated, selection of healthier participants generally leads to underestimate associations. Fifth, evaluation of differential weighting of metrics and of potential interaction between metrics would be important in future studies; furthermore, the AHA approach was developed to prevent cardiovascular disease and thus should not be interpreted as the best summary of existing epidemiologic knowledge for preventing dementia. Sixth, study participants were mostly urban and primarily white, and findings may not be generalizable to populations from different sociogeographic and ethnic origins.
Conclusions
In this cohort of older adults without history of cardiovascular disease, increased numbers of cardiovascular health metrics at optimal levels were associated with a lower risk of dementia and lower rates of cognitive decline. These findings may support the promotion of cardiovascular health to prevent risk factors associated with cognitive decline and dementia.
eAppendix. Supplemental Methods and Results
eFigure 1. Dose-Response Relationships Between Number of Recommended Optimal Metrics (Panel A), Global Cardiovascular Health Score (Panel B), and Relative Risk (RR) of Dementia Evaluated Using Penalized Splines With 4 Degrees of Freedom in a Cox Proportional Hazard Model
eFigure 2. Dose-Response Relationships Between Number of Recommended Optimal Metrics (Panel A), Global Cardiovascular Health Score (Panel B), and the Trajectory of Global Cognitive Change Evaluated Using Natural Cubic Splines in a Linear Mixed Model With Splines Functions of Time
eFigure 3. Dose-Response Relationships Between Number of Recommended Optimal Metrics (Panel A), Global Cardiovascular Health Score (Panel B), and the Trajectory of Memory Change Evaluated Using Natural Cubic Splines in a Linear Mixed Model With Splines Functions of Time
eTable 1. Comparison of Baseline Characteristics Between Included and Excluded Participants
eTable 2. Baseline Characteristics Across Increasing Number of Cardiovascular Health Metrics at Recommended Optimal Level (n=6,626)
eTable 3. Multivariable Associations Between Cardiovascular Health Level and the Incidence of Dementia Over 10 Years Adjusting for Incident Stroke (N=6,620)
eTable 4. Multivariable Associations Between Cardiovascular Health Level and the Incidence of Dementia Over 10 Years Excluding Incident Stroke Events From Study Sample (N=6,408)
eTable 5. Multivariable Associations Between Cardiovascular Health Level and the Incidence of Dementia Over 10 Years Taking Into Account Age-Specific Mortality Rates Using Illness-Death Models (n=6,620)
eTable 6. Multivariable Associations Between Cardiovascular Health Level and the Incidence of Dementia With Imputation of Missing Values for Covariates and the Outcome Under Various Scenarios
References
- 1.Winblad B, Amouyel P, Andrieu S, et al. . Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15(5):455-532. doi: 10.1016/S1474-4422(16)00062-4 [DOI] [PubMed] [Google Scholar]
- 2.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794. doi: 10.1016/S1474-4422(14)70136-X [DOI] [PubMed] [Google Scholar]
- 3.Livingston G, Sommerlad A, Orgeta V, et al. . Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673-2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 4.Sabia S, Nabi H, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Health behaviors from early to late midlife as predictors of cognitive function: the Whitehall II Study. Am J Epidemiol. 2009;170(4):428-437. doi: 10.1093/aje/kwp161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelber RP, Petrovitch H, Masaki KH, et al. . Lifestyle and the risk of dementia in Japanese-American men. J Am Geriatr Soc. 2012;60(1):118-123. doi: 10.1111/j.1532-5415.2011.03768.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Hong Y, Labarthe D, et al. ; American Heart Association Strategic Planning Task Force and Statistics Committee . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121(4):586-613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 7.Guo L, Zhang S. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality: a meta-analysis of prospective studies. Clin Cardiol. 2017;40(12):1339-1346. doi: 10.1002/clc.22836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaye B, Canonico M, Perier MC, et al. . Ideal cardiovascular health, mortality, and vascular events in elderly subjects: the Three-City Study. J Am Coll Cardiol. 2017;69(25):3015-3026. doi: 10.1016/j.jacc.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 9.Gardener H, Wright CB, Dong C, et al. . Ideal cardiovascular health and cognitive aging in the Northern Manhattan Study. J Am Heart Assoc. 2016;5(3):e002731. doi: 10.1161/JAHA.115.002731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.González HM, Tarraf W, Harrison K, et al. . Midlife cardiovascular health and 20-year cognitive decline: Atherosclerosis Risk in Communities Study results. Alzheimers Dement. 2018;14(5):579-589. doi: 10.1016/j.jalz.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thacker EL, Gillett SR, Wadley VG, et al. . The American Heart Association Life’s Simple 7 and incident cognitive impairment: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2014;3(3):e000635. doi: 10.1161/JAHA.113.000635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pase MP, Beiser A, Enserro D, et al. . Association of ideal cardiovascular health with vascular brain injury and incident dementia. Stroke. 2016;47(5):1201-1206. doi: 10.1161/STROKEAHA.115.012608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hessler JB, Ander KH, Brönner M, et al. . Predicting dementia in primary care patients with a cardiovascular health metric: a prospective population-based study. BMC Neurol. 2016;16:116. doi: 10.1186/s12883-016-0646-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.3C Study Group Vascular factors and risk of dementia: design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology. 2003;22(6):316-325. doi: 10.1159/000072920 [DOI] [PubMed] [Google Scholar]
- 15.Larrieu S, Letenneur L, Berr C, et al. . Sociodemographic differences in dietary habits in a population-based sample of elderly subjects: the 3C Study. J Nutr Health Aging. 2004;8(6):497-502. [PubMed] [Google Scholar]
- 16.Barberger-Gateau P, Raffaitin C, Letenneur L, et al. . Dietary patterns and risk of dementia: the Three-City Cohort Study. Neurology. 2007;69(20):1921-1930. doi: 10.1212/01.wnl.0000278116.37320.52 [DOI] [PubMed] [Google Scholar]
- 17.Samieri C, Morris MC, Bennett DA, et al. . Fish intake, genetic predisposition to Alzheimer disease, and decline in global cognition and memory in 5 cohorts of older persons. Am J Epidemiol. 2018;187(5):933-940. doi: 10.1093/aje/kwx330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 20.Isaacs B, Kennie AT. The Set Test as an aid to the detection of dementia in old people. Br J Psychiatry. 1973;123(575):467-470. doi: 10.1192/bjp.123.4.467 [DOI] [PubMed] [Google Scholar]
- 21.Benton A. Manuel pour l’application du Test de Rétention Visuelle: Applications cliniques et expérimentales. Paris, France: Centre de Psychologie appliquée; 1965. [Google Scholar]
- 22.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19(5):393-394. doi: 10.1037/h0044509 [DOI] [PubMed] [Google Scholar]
- 23.Carcaillon L, Amieva H, Auriacombe S, Helmer C, Dartigues JF. A subtest of the MMSE as a valid test of episodic memory? comparison with the Free and Cued Reminding Test. Dement Geriatr Cogn Disord. 2009;27(5):429-438. doi: 10.1159/000214632 [DOI] [PubMed] [Google Scholar]
- 24.Grober EBH. Genuine memory deficits 1 in dementia. Dev Neuropsychol. 1987;3:13-36. doi: 10.1080/87565648709540361 [DOI] [Google Scholar]
- 25.Law CG, Brookmeyer R. Effects of mid-point imputation on the analysis of doubly censored data. Stat Med. 1992;11(12):1569-1578. doi: 10.1002/sim.4780111204 [DOI] [PubMed] [Google Scholar]
- 26.Eilers PHC, Marx BD. Flexible smoothing with B-splines and penalties. Stat Sci. 1996;11(2):89-121. doi: 10.1214/ss/1038425655 [DOI] [Google Scholar]
- 27.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963-974. doi: 10.2307/2529876 [DOI] [PubMed] [Google Scholar]
- 28.Proust-Lima C, Philipps V, Dartigues JF, et al. . Are latent variable models preferable to composite score approaches when assessing risk factors of change? evaluation of type-I error and statistical power in longitudinal cognitive studies [published online January 1, 2017]. Stat Methods Med Res. doi: 10.1177/0962280217739658 [DOI] [PubMed] [Google Scholar]
- 29.Touraine C, Helmer C, Joly P. Predictions in an illness-death model. Stat Methods Med Res. 2016;25(4):1452-1470. doi: 10.1177/0962280213489234 [DOI] [PubMed] [Google Scholar]
- 30.Leffondré K, Touraine C, Helmer C, Joly P. Interval-censored time-to-event and competing risk with death: is the illness-death model more accurate than the Cox model? Int J Epidemiol. 2013;42(4):1177-1186. doi: 10.1093/ije/dyt126 [DOI] [PubMed] [Google Scholar]
- 31.Reis JP, Loria CM, Launer LJ, et al. . Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol. 2013;73(2):170-179. doi: 10.1002/ana.23836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorelick PB, Furie KL, Iadecola C, et al. ; American Heart Association/American Stroke Association . Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke. 2017;48(10):e284-e303. doi: 10.1161/STR.0000000000000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplemental Methods and Results
eFigure 1. Dose-Response Relationships Between Number of Recommended Optimal Metrics (Panel A), Global Cardiovascular Health Score (Panel B), and Relative Risk (RR) of Dementia Evaluated Using Penalized Splines With 4 Degrees of Freedom in a Cox Proportional Hazard Model
eFigure 2. Dose-Response Relationships Between Number of Recommended Optimal Metrics (Panel A), Global Cardiovascular Health Score (Panel B), and the Trajectory of Global Cognitive Change Evaluated Using Natural Cubic Splines in a Linear Mixed Model With Splines Functions of Time
eFigure 3. Dose-Response Relationships Between Number of Recommended Optimal Metrics (Panel A), Global Cardiovascular Health Score (Panel B), and the Trajectory of Memory Change Evaluated Using Natural Cubic Splines in a Linear Mixed Model With Splines Functions of Time
eTable 1. Comparison of Baseline Characteristics Between Included and Excluded Participants
eTable 2. Baseline Characteristics Across Increasing Number of Cardiovascular Health Metrics at Recommended Optimal Level (n=6,626)
eTable 3. Multivariable Associations Between Cardiovascular Health Level and the Incidence of Dementia Over 10 Years Adjusting for Incident Stroke (N=6,620)
eTable 4. Multivariable Associations Between Cardiovascular Health Level and the Incidence of Dementia Over 10 Years Excluding Incident Stroke Events From Study Sample (N=6,408)
eTable 5. Multivariable Associations Between Cardiovascular Health Level and the Incidence of Dementia Over 10 Years Taking Into Account Age-Specific Mortality Rates Using Illness-Death Models (n=6,620)
eTable 6. Multivariable Associations Between Cardiovascular Health Level and the Incidence of Dementia With Imputation of Missing Values for Covariates and the Outcome Under Various Scenarios