Abstract
This study estimates mean 2017 total and out-of-pocket costs for infliximab and its biosimilar infliximab-dyyb under Medicare Part D.
Biologic specialty drugs are effective for treating rheumatic diseases, cancer, and other conditions. They represent approximately 2% of prescriptions but accounted for 38% of US drug spending in 2015 and 70% of drug spending growth between 2010 and 2015.1 For rheumatoid arthritis (RA), biologics cost more than $14 000 annually and 3 RA biologics were among the top 15 drugs by Medicare expenditures in 2015.2
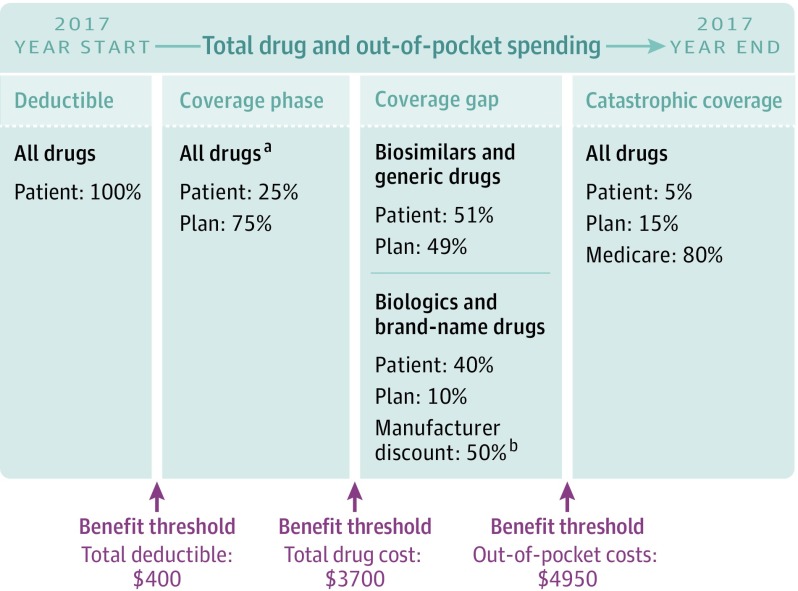
In 2010, Congress empowered the US Food and Drug Administration to approve “biosimilars” of biologics to increase competition and lower cost. However, for the more than 43 million beneficiaries with Medicare Part D drug benefits, it is unclear whether biosimilars lower out-of-pocket costs given Part D’s complex cost-sharing structure.3,4,5 Part D consists of a deductible, coverage phase, coverage gap, and catastrophic coverage (Figure).5 When beneficiaries’ total drug costs exceed a set threshold, they enter the coverage gap and cost-sharing increases. Currently, beneficiaries receive a 50% manufacturer discount during the gap for brand-name drugs and biologics, but not for biosimilars.5 Although the recent Bipartisan Budget Act requires gap discounts for biosimilars starting in 2019, patients’ out-of-pocket costs will continue to depend on whether biosimilars differ from biologics in drug pricing and plan cost-sharing requirements.6 We examined coverage and cost-sharing for the first RA biosimilar (infliximab-dyyb) released in 2016 compared with its biologic (infliximab).
Figure. Standard Cost-Sharing Structure of Medicare Part D Drug Benefits in 2017.
Adapted with permission from Kaiser Family Foundation Medicare Part D Prescription Drug Benefit.
aIn our analyses, patients’ out-of-pocket costs were based on average cost-sharing requirements for each drug across all Part D plans nationwide.
bManufacturer discount counts as out-of-pocket cost to reach catastrophic coverage threshold.
Methods
We analyzed nationwide benefit design data for all Part D plans from the June 2017 Medicare Prescription Drug Plan Formulary, Pharmacy Network, and Pricing Information Files to calculate mean total cost and out-of-pocket cost requirements for infliximab-dyyb and infliximab assuming a standard 8-week dosing regimen (6.5 prescriptions/y).3 We used these national means to project maximum annual out-of-pocket costs if beneficiaries used each drug and no other prescriptions under a standard 2017 Part D benefit, including a $400 deductible, coverage phase (out-of-pocket costs based on calculated national means), coverage gap starting at $3700 in total drug costs, and catastrophic coverage once out-of-pocket costs exceed $4950 (beneficiaries pay 5% for biologics or biosimilars thereafter). During the gap, beneficiaries pay 40% for biologics (plans pay 10%, manufacturer discounts 50%) and 51% for biosimilars (plans pay 49%).3,4 SAS (SAS Institute), version 9.4, was used to calculate means.
Results
Among 2547 plans, fewer plans covered infliximab-dyyb (10%) than infliximab (96%). Infliximab-dyyb had modestly lower mean total cost per 8-week prescription ($2185 vs $2667) and annually ($14 202 vs $17 335). However, plans universally required coinsurance cost-sharing for infliximab-dyyb, and set coinsurance rates similar to infliximab (26.6% vs 28.4% of drug cost, respectively) (Table). Without gap discounts, projected annual out-of-pocket costs were higher for infliximab-dyyb than infliximab ($5118 vs $3432).
Table. 2017 Medicare Part D Plan Coverage and Cost-Sharing for Infliximab-dyyb (Biosimilar) and Infliximab (Biologic) (N = 2547 Plans).
| Drug | Medicare Part D Plan Coveragea | Total Drug Cost, $b | Projected Sum of Out-of-Pocket Costs in Each Benefit Phase, $c | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Covered, % of Plans | Required Beneficiary Pay Coinsurance in Coverage Phase, % of Plans | Mean Coinsurance Rate, % of Total Drug Cost | Single Prescription (8-wk Prescription) | Annual (6.5 Prescriptions/y) | Deductible | Coverage Phase | Coverage Gap | Catastrophic Coverage | Annual | |
| Infliximab-dyyb (biosimilar) | 10 | 100 | 26.6 | 2185 | 14 202 | 400 | 908 | 3642 | 168 | 5118 |
| Infliximab (biologic) | 96 | 100 | 28.4 | 2667 | 17 335 | 400 | 950 | 1600 | 482 | 3432 |
Coverage calculations are based on 2017 Medicare Part D plan formularies and drug benefit data nationwide unweighted by plan enrollment and not based on patient claims.
Total drug cost is calculated from 2017 Medicare Part D plans' drug benefit data nationwide based on plans’ cost-sharing requirements per standard 8-week prescription and averaging across all plans by counties and states.
Projected out-of-pocket costs are estimated based on beneficiaries using only the biosimilar or biologic drug and no other prescriptions under a standard 2017 Part D plan. This includes an initial $400 deductible, followed by a coverage phase (out-of-pocket cost estimated based on mean cost-sharing requirements nationwide), and a coverage gap after reaching $3700 in total drug cost. During the gap, beneficiaries pay 51% of biosimilar drug cost and plans pay 49%; for biologics, beneficiaries pay 40% of drug cost, plans pay 10%, and 50% is paid through the manufacturer gap discount. For biologics, the 50% manufacturer discount counts toward reaching the catastrophic threshold. Beneficiaries enter catastrophic coverage once out-of-pocket costs reach $4950 and pay 5% of biosimilar or biologic drug cost the remainder of the year.
Discussion
Nationwide under Medicare Part D, RA biosimilar infliximab-dyyb was only moderately less expensive (18% less) than biologic infliximab and exceeded $14000 annually. Without biosimilar gap discounts in 2017, beneficiaries would have paid more than $5100 for infliximab-dyyb or nearly $1700 more in projected out-of-pocket costs than infliximab. Study limitations include calculating mean costs unweighted by plan enrollment, and projecting annual out-of-pocket costs assuming use of a biologic or biosimilar and no other prescriptions. Although biosimilar gap discounts begin in 2019, infliximab-dyyb may still not significantly reduce Part D beneficiaries’ out-of-pocket costs given its high price and coinsurance cost-sharing similar to infliximab.6 Further policies are needed to address affordability and access to specialty drugs.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: initial experience and future potential. https://www.rand.org/pubs/perspectives/PE264.html. Accessed April 9, 2018. [PMC free article] [PubMed]
- 2.Centers for Medicare & Medicaid Services 2015 Medicare drug spending dashboard. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Information-on-Prescription-Drugs/2015Medicare.html. Accessed April 9, 2018.
- 3.Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474-1480. doi: 10.1002/art.39079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hakim A, Ross JS. Obstacles to the adoption of biosimilars for chronic diseases. JAMA. 2017;317(21):2163-2164. doi: 10.1001/jama.2017.5202 [DOI] [PubMed] [Google Scholar]
- 5.Kaiser Family Foundation The Medicare Part D prescription drug benefit. https://www.kff.org/medicare/fact-sheet/the-medicare-prescription-drug-benefit-fact-sheet/. Accessed April 9, 2018.
- 6.Cubanski J. Summary of recent and proposed changes to Medicare prescription drug coverage and reimbursement. https://www.kff.org/medicare/issue-brief/summary-of-recent-and-proposed-changes-to-medicare-prescription-drug-coverage-and-reimbursement/. Accessed April 9, 2018.