Abstract
Importance
Nigella sativa oil (NSO) is widely used for cosmetic and culinary purposes. Cases of severe acute contact dermatitis due to NSO are poorly described, with no histologic description.
Objectives
To describe the clinical and histologic features of severe acute contact dermatitis due to NSO and investigate the components responsible for such eruptions.
Design, Setting, and Participants
A case series study of 3 patients with contact dermatitis admitted to the dermatology department between August 21, 2009, and February 19, 2017, was conducted. All patients had been referred to the dermatology department for acute contact dermatitis due to NSO and had patch tests performed.
Main Outcomes and Measures
Clinical and histologic features of the cutaneous eruptions, length of hospital stay, chemical analysis of NSO, and results of patch tests.
Results
Three patients (3 women; median age, 27 years [range, 20-47 years]) were included in the case series. All patients had polymorphic skin lesions spreading beyond the area of NSO application: typical and atypical targets, patches with central blisters, erythematous or purpuric plaques with a positive Nikolsky sign mimicking Stevens-Johnson syndrome, or toxic epidermal necrolysis. Two patients had pustules. They had severe impairment, with more than 15% skin detachment and fever. The results of skin biopsies showed epidermal apoptosis characterized by vacuolar alteration of the basal layer, keratinocyte apoptosis, and a moderate perivascular infiltrate of lymphocytes in the dermis. The results of patch tests using the patients’ NSO were all positive. The results of gas chromatography combined with mass spectrometry performed on the NSO of 1 patient identified several constituent substances, mainly terpenes, thymoquinone, linoleic acid, and fatty acids.
Conclusions and Relevance
These cases suggest that acute contact dermatitis due to NSO may induce topically triggered epidermal apoptosis, previously described as the concept of acute syndrome of apoptotic pan epidermolysis. Thymoquinone and p-cymene may be the main agents involved in the pathophysiologic characteristics of this acute contact dermatitis. Clinicians should be aware of such severe reactions to NSO and report these cases to pharmacovigilance authorities.
This case series describes the clinical and histologic features of severe acute contact dermatitis due to Nigella sativa oil and investigates the components responsible for such eruptions.
Key Points
Question
What are the histologic findings of severe acute contact dermatitis due to Nigella sativa oil?
Findings
This case series describes 3 patients who, after topical use of N sativa oil, displayed extensive lesions mimicking Stevens-Johnson syndrome or toxic epidermal necrolysis, bullous fixed drug eruption, and/or erythema multiforme and histologic features of epidermal apoptosis. Analysis of the N sativa oil showed thymoquinone and p-cymene to be major components; results of patch tests using the patients N sativa oil were positive.
Meaning
Acute contact dermatitis due to N sativa oil is severe, polymorphic, and histologically characterized by epidermal apoptosis.
Introduction
Nigella sativa oil (NSO), extracted from the seeds of N sativa or black caraway (one of the plants also called black cumin), found in Southern Europe, North Africa, and Southwest Asia, is traditionally used for its cosmetic and culinary properties.1 Its main components are thymoquinone and terpenes,1 but the exact composition of commercial NSO and the proportion of its constituents are highly variable. N sativa oil and essential oils, in general, are known to be responsible for benign eczematous contact dermatitis, including occupational contact dermatitis, but sometimes severe reactions, which have been poorly described, can occur.2,3 We report 3 cases of severe acute contact dermatitis (ACD) due to NSO, showing severe polymorphic lesions that mimicked erythema multiforme, bullous fixed drug eruption, Stevens-Johnson syndrome, or toxic epidermal necrolysis (TEN) and histologically showing epidermal apoptosis.
Methods
We performed a retrospective single-center study for consecutive patients referred to our dermatology department between August 21, 2009, and February 19, 2017, for dermatitis after NSO application who had patch tests performed. Cases were extracted from the database of our department, which is a reference center for toxic bullous dermatoses. According to the Public Health French Law (art L 1121-1-1, art L 1121-1-2), Institutional Review Board approval and written consent are not required for human noninterventional studies; oral consent was provided by the patients.
The following data were collected from the patients’ medical records: age, sex, nature of the NSO (essential or vegetable), previous application of NSO, oral intake of NSO, duration and localization of the application, time between applications and onset of the lesions, localization and clinical aspect of skin lesions, mucous involvement, number of cutaneous flares, length of hospital stay, histologic analysis, direct immunofluorescence, treatment and evolution, and results of patch tests.
All patients had patch tests performed with their own NSO diluted at 1% in petrolatum and the European Baseline series with 2 readings at 48 and 96 hours, according to the International Contact Dermatitis Research Group guidelines.4 N sativa oil diluted in water, open tests (10% in petrolatum and water), or other essential oils could be added. We investigated the composition of the NSO of 1 patient using gas chromatography (Trace GC Ultra Chromatograph; Thermo Fisher Scientific) combined with mass spectrometry (TSQ Quantum Spectrometer; Thermo Fisher).
Results
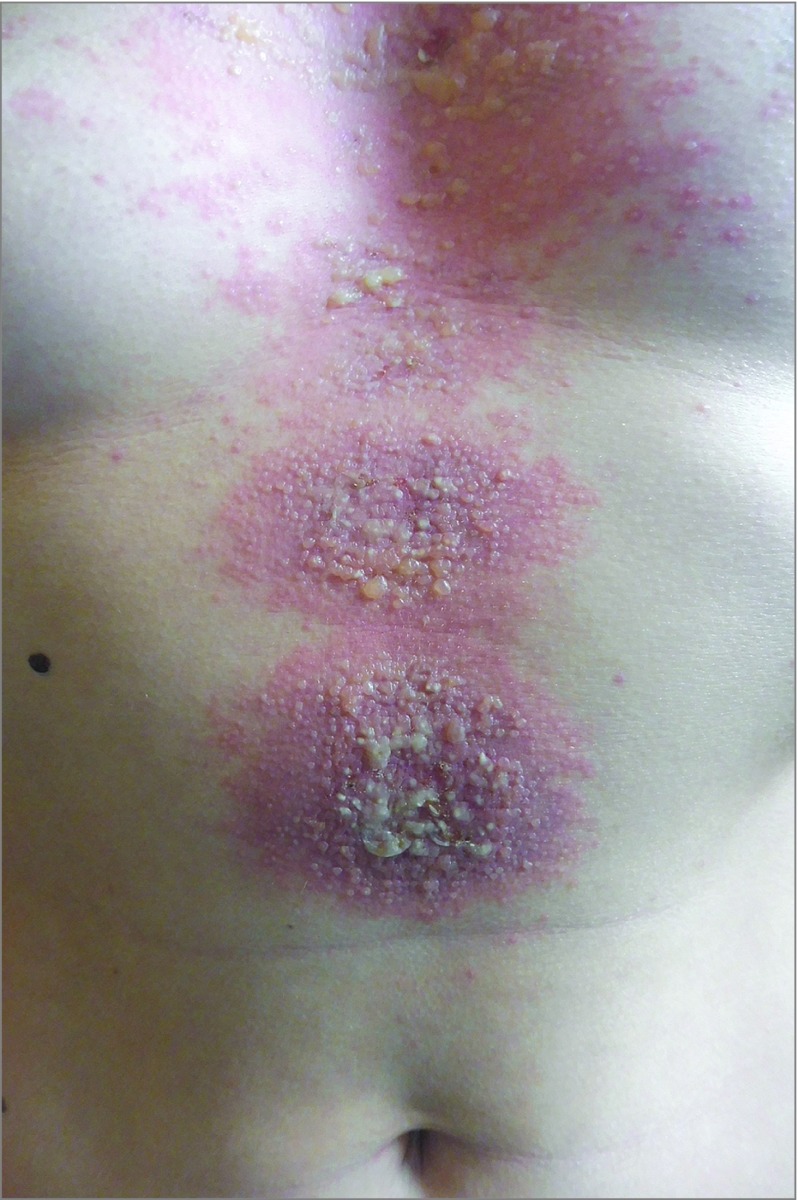
During the study period, 7 patients were hospitalized in our dermatology department for ACD due to NSO, but 4 of the 7 patients did not have patch tests performed. Three patients who met the inclusion criteria were thus included (3 women; median age, 27 years [range, 20-47 years]) (Table). They all had used vegetable oil extracted from N sativa seeds, which they applied to various areas of the skin or scalp for cosmetic purposes. The median time between application of NSO and the onset of disease was 2 days (range, 1-2 days). Two patients had previously used NSO, with no cutaneous reaction. The results of clinical examination showed polymorphic skin lesions spreading over the area of application: typical and atypical targets (3 patients), patches with central vesicles and blisters (3 patients) (Figure 1), erythematous and purpuric macules and plaques with the Nikolsky sign (3 patients), and pustules (2 patients). All 3 patients had severe impairment with more than 15% of the body surface area involved, with Nikolsky sign and fever, and had a hospital length of stay of more than 10 days. One patient had a second cutaneous flare of the same lesions during her hospital stay, after washing her hair where she had initially applied NSO.
Table. Characteristics of 3 Patients With Acute Contact Dermatitis Following the Application of NSO.
| Patient No. | Sex/Age | NSO Use | Time to Onset, d | Fever | Clinical Aspects | No. of Flares | Length of Hospital Stay, d | Patch-Tested Product (Result)a | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area of Application | Previous Use/Reaction | Body Surface, % | Area Involved | Skin Lesions | Extension Beyond Area of Application | Mucous Involvement | |||||||
| 1 | F/40s | Left hand and stomach | No previous use | 2 | Yes | ND | Scalp, stomach, forearms, hands, and thighs | Patches, blisters, and atypical targets | Yes | None | 1 | 10 | Patient’s NSO (++) |
| 2 | F/20s | Face | Yes/none | 2 | Yes | 40 | Face, torso, and forearms | Blisters, atypical targets, and pustules | Yes | None | 1 | 10 | Patient’s NSO (+++); limonene hydroperoxide (++); linalool hydroperoxide (++); Chamomilla recutita extract (++); Achillea millefolium extract (++); laurel leaf oil (+) |
| 3 | F/20s | Hair and scalp | Yes/none | 1 | Yes | 30 | Scalp, face, trunk, forearms, hands, elbows, and pubis | Patches, blisters, atypical targets, and pustules | Yes | Conjunctivitis | 2 | 10 | Patient’s NSO (++) |
Abbreviations: ND, not determined; NSO, Nigella sativa oil; +, weak positive reaction; ++, strong positive reaction; +++, extreme positive reaction.
According to the International Contact Dermatitis Research Group Score for epicutaneous tests.
Figure 1. Clinical Findings.
Well-delineated patches with central vesicles and blisters on the trunk of patient 3.
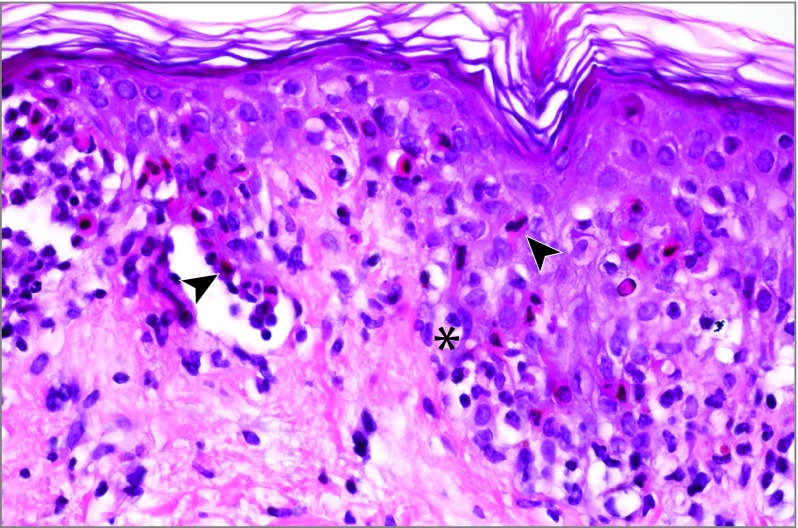
Histologic examination of a skin biopsy was performed in all cases and revealed epidermal apoptosis characterized by confluent nests of apoptotic keratinocytes, with subepidermal detachment or epidermal regenerative changes, and a slight to moderate perivascular infiltrate of lymphocytes in the superficial dermis (Figure 2). All patients had negative results from direct immunofluorescence.
Figure 2. Histologic Analysis of Skin Biopsy.
Vacuolar alteration of the basal layer (asterisk) and dispersed or confluent nests of apoptotic keratinocytes with subepidermal detachment (arrowheads) in patient 3 (hematoxylin-eosin saffron, original magnification ×400).
The patients were successfully treated with clobetasol propionate 0.05% cream and white petroleum jelly. Their lesions healed, but their skin exhibited postinflammatory hypopigmentation or hyperpigmentation.
Results of patch tests were positive at 48 and 96 hours in the 3 cases. Gas chromatography and mass spectrometry performed on the NSO of patient 3 showed the chemical composition of the oil to be a complex mixture including p-cymene, thymoquinone, longifolene, linoleic acid, and fatty acids as the main components (eFigure in the Supplement).
Discussion
We report 3 cases of severe and extensive ACD after topical use of NSO clinically mimicking erythema multiforme, bullous fixed drug eruption, Stevens-Johnson syndrome, or TEN and histologically showing epidermal apoptosis, as previously described in erythema multiforme and TEN.5 The severity of these cases suggests a systemic effect of NSO, inducing an extension of the lesions away from the area of application, even after topical use alone. Furthermore, in the third case, a second flare during the hospital stay suggested a remanence phenomenon from the hair where NSO had been initially applied. Such severe eruptions, which are similar to erythema multiforme and TEN eruptions, have been described after contact with NSO,6,7 as well as with other essential oils (tea tree oil, iron wood trees, and poison ivy).8
Acute syndrome of apoptotic panepidermolysis (ASAP), a syndrome characterized by hyperacute apoptotic injury of the epidermis, comprises various severe dermatoses, such as TEN,9 TEN-like acute graft-vs-host disease, TEN-like cutaneous lupus erythematous, and severe eruptions associated with Mycoplasma pneumoniae infections.10,11 Histologically, ASAP is characterized by epidermal apoptosis. Acute syndrome of apoptotic panepidermolysis probably results from the expression of various activation effectors, leading to the massive death of keratinocytes.10 Our series suggests that ASAP can also be triggered by topical medications, such as NSO, possibly followed by systemic diffusion, explaining the extension of the lesions.
The market (often via online sales) of these oils is not controlled. They are often impure, and the concentration of their components is uncertain. The constituents of NSO have been previously described, showing the presence of thymoquinone and p-cymene.1,12 Gas chromatography performed on the NSO belonging to 1 of the patients confirms this chemical composition. Thymoquinone is investigated for therapeutic uses in neurology and oncology owing to its antioxidant, anti-inflammatory, and supposed antineoplastic and proapoptotic properties.13,14 p-Cymene is a monoterpene that acts as a penetration enhancer by disrupting the stratum corneum lipid structure, thus facilitating the transport of drugs through the skin.15 N sativa oil contains thymoquinone and p-cymene in often variable unknown proportions. The pathophysiologic characteristics of ACD from NSO remain poorly understood. Synergy of p-cymene and thymoquinone may promote epidermal apoptosis by combining a topical toxic effect with systemic diffusion and/or a hypersensitivity.
Limitations
The limitations of this study are its retrospective nature and the small number of patients included. More patients could have been included if patch tests had been performed every time. Furthermore, thymoquinone and p-cymene should be tested separately and together to better understand the pathophysiologic characteristics of this ACD.
Conclusions
Clinicians and patients should be aware of the possibility of severe cutaneous adverse reactions to topical NSO. The components of NSO should be labeled and their proportions better defined. Sales of NSO online need to be better regulated. Severe cases of ACD due to NSO should be reported to the pharmacovigilance authorities.
eFigure. Gas Chromatography and Mass Spectrometry of the Nigella sativa Oil of Patient Number 6
References
- 1.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17(4):299-305. doi: 10.1002/ptr.1309 [DOI] [PubMed] [Google Scholar]
- 2.de Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27(4):170-175. doi: 10.1097/DER.0000000000000197 [DOI] [PubMed] [Google Scholar]
- 3.Trattner A, David M, Lazarov A. Occupational contact dermatitis due to essential oils. Contact Dermatitis. 2008;58(5):282-284. doi: 10.1111/j.1600-0536.2007.01275.x [DOI] [PubMed] [Google Scholar]
- 4.Johansen JD, Aalto-Korte K, Agner T, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—recommendations on best practice. Contact Dermatitis. 2015;73(4):195-221. doi: 10.1111/cod.12432 [DOI] [PubMed] [Google Scholar]
- 5.Côté B, Wechsler J, Bastuji-Garin S, Assier H, Revuz J, Roujeau JC. Clinicopathologic correlation in erythema multiforme and Stevens-Johnson syndrome. Arch Dermatol. 1995;131(11):1268-1272. doi: 10.1001/archderm.1995.01690230046008 [DOI] [PubMed] [Google Scholar]
- 6.Bonhomme A, Poreaux C, Jouen F, Schmutz JL, Gillet P, Barbaud A. Bullous drug eruption to Nigella sativa oil: consideration of the use of a herbal medicine—clinical report and review of the literature. J Eur Acad Dermatol Venereol. 2017;31(4):e217-e219. doi: 10.1111/jdv.13982 [DOI] [PubMed] [Google Scholar]
- 7.Nosbaum A, Ben Said B, Halpern S-J, Nicolas J-F, Bérard F. Systemic allergic contact dermatitis to black cumin essential oil expressing as generalized erythema multiforme. Eur J Dermatol. 2011;21(3):447-448. [DOI] [PubMed] [Google Scholar]
- 8.Cohen LM, Cohen JL. Erythema multiforme associated with contact dermatitis to poison ivy: three cases and a review of the literature. Cutis. 1998;62(3):139-142. [PubMed] [Google Scholar]
- 9.Duong TA, Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet. 2017;390(10106):1996-2011. doi: 10.1016/S0140-6736(16)30378-6 [DOI] [PubMed] [Google Scholar]
- 10.Ting W, Stone MS, Racila D, Scofield RH, Sontheimer RD. Toxic epidermal necrolysis–like acute cutaneous lupus erythematosus and the spectrum of the acute syndrome of apoptotic pan-epidermolysis (ASAP): a case report, concept review and proposal for new classification of lupus erythematosus vesiculobullous skin lesions. Lupus. 2004;13(12):941-950. doi: 10.1191/0961203304lu2037sa [DOI] [PubMed] [Google Scholar]
- 11.Fournier S, Bastuji-Garin S, Mentec H, Revuz J, Roujeau JC. Toxic epidermal necrolysis associated with Mycoplasma pneumoniae infection. Eur J Clin Microbiol Infect Dis. 1995;14(6):558-559. doi: 10.1007/BF02113442 [DOI] [PubMed] [Google Scholar]
- 12.Ramadan MF, Mörsel J-T. Characterization of phospholipid composition of black cumin (Nigella sativa L.) seed oil. Nahrung. 2002;46(4):240-244. doi: [DOI] [PubMed] [Google Scholar]
- 13.Majdalawieh AF, Fayyad MW. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: a comprehensive review. Int Immunopharmacol. 2015;28(1):295-304. doi: 10.1016/j.intimp.2015.06.023 [DOI] [PubMed] [Google Scholar]
- 14.Gali-Muhtasib HU, Abou Kheir WG, Kheir LA, Darwiche N, Crooks PA. Molecular pathway for thymoquinone-induced cell-cycle arrest and apoptosis in neoplastic keratinocytes. Anticancer Drugs. 2004;15(4):389-399. doi: 10.1097/00001813-200404000-00012 [DOI] [PubMed] [Google Scholar]
- 15.Godwin DA, Michniak BB. Influence of drug lipophilicity on terpenes as transdermal penetration enhancers. Drug Dev Ind Pharm. 1999;25(8):905-915. doi: 10.1081/DDC-100102251 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Gas Chromatography and Mass Spectrometry of the Nigella sativa Oil of Patient Number 6