Abstract
Dioxins are a group of highly persistent chemicals that are generated as by-products of industrial and natural processes. Reduction in sperm counts is among the most sensitive endpoints of dioxin toxicity. The exact mechanism by which dioxins reduce sperm counts is not known. Recent data implicate the role of epididymal factors rather than disruption of spermatogenesis. Studies reviewed here demonstrate that dioxins induce the transfer of environmental conditions to the next generation via male germline following exposures during the window of epigenetic reprogramming of primordial germ cells. Increased incidence of birth defects in offspring of male veterans exposed to dioxin containing, Agent Orange, suggest that dioxins may induce epigenomic changes in male germ cells of adults during spermatogenesis. This is supported by recent animal data that show that environmental conditions can cause epigenetic dysregulation in sperm in the context of specific windows of epigenetic reprogramming during spermatogenesis.
Keywords: Dioxin, Sperm, Spermatogenesis, Epigenetic, Endocrine disruption, TCDD, Windows of susceptibility, DNA methylation
1. Introduction
Semen quality has been declining in some of developed countries during a period of half a century according to several large meta-analysis studies [1,2]. These results are supported by recent epidemiologic studies [3–5] showing that a significant proportion of young men has semen quality below what is considered to be compatible with good fecundity. A growing body of evidence links this deterioration of male reproductive health with chronic exposure to environmental endocrine disruptors (EDCs) [6–8]. One group of EDCs with potential deleterious effects on human reproductive system is dioxins and dioxin-like compounds (DLCs). Dioxins are a group of highly persistent chemical by-products of industrial process and by-products of combustion of organic material. Due to high lipophilicity and resistance to biological and environmental degradation, dioxins are able to bioaccumulate and biomagnify in food chains, which increases the potential burden of exposures to apex animals such as humans [9]. Despite significant decreases in the production of dioxin and DLCs, high persistence and bioaccumulation of these compounds results in omnipresence of dioxins [10]. All people have background exposure and more than 90% of exposure occurs through food, mainly meat and dairy products, fish and shellfish [10,11]. Additionally cases of accidental contamination of food and/or environment with DLCs have resulted in much higher acute and chronic exposures [12,13].
The name “dioxins” is used for the family of structurally and chemically related polychlorinated dibenzo para dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs). Certain dioxin-like polychlorinated biphenyls (PCBs) with similar toxic properties are also included under the term “dioxins” or dioxin-like compounds [14]. Among these, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic environmental contaminant in animal studies and is often referred to in scientific literature as dioxin. Based on similarity of toxic response induced by all DLCs, the toxic equivalency factor concept (TEF) has been developed [15] and reevaluated by World Health Organization (WHO) expert meetings [14,16] to facilitate risk assessment and regulatory control. In accordance with this concept, toxicity of dioxins, furans and dioxin-like PCBs is expressed as relative toxicity in comparison with TCDD. TEF allows for the expression of the toxicity of dioxin-like mixtures in a single number. Recently, more compounds with dioxin-like activity were proposed for inclusion in the TEF including polybrominated dibenzo-p-dioxins, dibenzofurans, biphenyls [17,18], and hydroxilated and methylated metabolites of polybrominated diphenyl ethers [19]. Studies of dioxin toxicity are thus of high significance as they a relevant for the understanding of mechanisms of action and health effects of a very broad range of chemical compounds.
TCDD and DLCs act as ligands for the aryl hydrocarbon receptor (AhR) – highly abundant, ligand-activated transcription factor. Upon entering the cell, TCDD binds to the cytosolic AhR and is then translocated to the nucleus where it forms another complex with the AhR nuclear translocator (ARNT) protein. Ligand/AhR/ARNT complex bind to dioxin response elements (DRE) on DNA, enhancing the transcription of specific genes [20–22] responsible for breakdown of toxic compounds [23]. While this mechanism is thought to confer protection from toxin exposure, TCDD-dependent AhR dysregulation of gene expression activates Phase I xenobiotic-metabolizing enzymes, which may be deleterious. Responsiveness to different doses of TCDDs and their analogs is different in different species putatively due to differences in AhR gene structure. Humans are more resistant to dioxins than many other animals, including laboratory rodents [24]. Traditionally, it is considered that the frequency of polymorphisms within AhR is low in humans resulting in small variations in susceptibility to DLCs across populations [24]; however, a recent study of a Greenland population reported that AhR variants significantly modify association between serum levels of DLCs and sperm characteristics, including chromatin integrity measured by TUNEL assay and concentration of zinc in seminal plasma [25].
TCDD has multiple effects on a diversity of health endpoints in mammalian species. In humans, high-dose acute exposures result in skin lesions, such as chloracne, and altered liver function; whereas chronic low-dose exposures are associated with impaired immune, endocrine and reproductive functions, as well as disruption of neurodevelopment. The most sensitive endpoints of TCDD toxicity in animal studies are reviewed elsewhere and include endometriosis and decreased sperm count, immune sup pression, increased genital malformations and neurobehavioral effects resulting from developmental exposures [26]. In laboratory animals, chronic exposure has resulted in several types of cancer, including tumors of the gastro-intestinal tract, liver, thyroid, lung, skin, and other sites [27]. Based on these data and limited human evidence, the International Agency for Research on Cancer classified dioxin as carcinogenic to humans (group 1) in 1997 [28]. However, accumulating body of population studies does not confirm the link between dioxins and cancer risk unequivocally – see recent reviews [29,30]. TCDD is known to be non-mutagenic or very weakly mutagenic substance [31,32] and carcinogenic effect of TCDD likely arise by receptor-mediated mechanisms [33]. Bacterial mutagenicity assays failed to clearly demonstrate mutagenic activity of TCDD [34]. Neither an increase in mutation frequency nor any change in mutation spectrum was observed in Big Blue rats after 6 weeks of exposure to 2 ug/kg TCDD [35].
2. Dioxins and male reproductive health
Epidemiologic data examining associations of DLCs with male pubertal onset and sexual maturity are summarized in Table 1. Environmental exposures to pollutants were associated with delay in pubertal development (genital stage and pubic hair stage) in Flemish boys living near dioxin-emitting waste incinerators [36]. Shorter penile length was reported in Yucheng boys exposed accidentally to high levels DLCs [37]. In the same cohort, increased abnormal sperm morphology, decreased sperm motility, and decreased hamster oocyte penetration by spermatozoa was found in men exposed to DLCs during prenatal period and lactation [37,38] and in men exposed at adulthood [39]. Higher peripubertal serum dioxins were also associated with delayed pubertal onset and sexual maturity in the Russian Children’s Study [40,41].
Table 1.
Summary of epidemiological studies analyzing effects of DLCs on male reproductive health.
| Study | Population | Analyzed compounds | Levels | Design and timing of exposure | Exposure assessment | Timing of outcomes | Reproductive outcomes |
|---|---|---|---|---|---|---|---|
| Burns et al., 2016 [41] | 473 Russian boys (Chapaevsk) | Dioxins, furans, dioxin-like PCBs | Wide range ΣDLC, median 362 pg/g lipid ΣTEQ, median 21.1 pg TEQ/g lipid |
Longitudinal, 8–9 years, 2003–2005 | Serum concentrations and TEQ | 8–18 years, 2003–2015 | Delayed pubertal onset and sexual maturity by testicular volume and genitalia staging. |
| Den Hond et al., 2002 [36] | 80 Flemish boys in 3 areas, 2 exposed and one control | DLCs | CALUX assay, geometric means 0.15–0.20 in 3 areas | Cross-sectional, 15.8–19.6 Years | CALUX assay | 15.8–19.6 years | No effect on pubertal staging. |
| Faure et al., 2014 [51] | 251 French men-patients of infertility clinic | Dioxins | - | Longitudinal, adulthood, 1971–2007 | Atmospheric diffusion model around the municipal waste incinerator in two periods, high and low emission | Adulthood, 2001–2003 and 2004–2007 | Increased abnormal spermatozoa. |
| Guo et al., 2000 [38] | 35 Taiwanese young adults (Yu-Cheng and unexposed) | PCBs, PCDFs | - | Longitudinal, prenatal and lactational, 1978–1998 | Serum concentrations in mothers and children | 16–18 years, 1998 | Increased abnormal sperm morphology, decreased motility, decreased hamster oocyte penetration. |
| Guo et al., 2004 [37] | 110 Taiwanese boys (Yu-Cheng and unexposed) | PCBs, PCDFs | Mothers: 2,3,4,7,8-Pe CDF, 6940 pg/g lipid; Children: PnCDF, 89pg/g/lipid |
Longitudinal, prenatal and lactational, 1978–1991 | Estimated (in mothers) and measured (in children) serum concentrations | 11–14 years, 1991–1993 | Decreased penile length. |
| Henriksen et al., 1996 [59] | 1006 U.S. men (veterans of Operation Ranch Hand and unexposed) | Agent Orange, TCDD | TCDD median 130ppt | Longitudinal, adulthood, 1962–1971 | Serum concentrations | 37–74 years, 1982, 1985, 1987, 1992 | No effect on testosterone, follicle stimulating hormone (FSH), luteinizing hormone (LH), testicular abnormalities, sperm count, sperm abnormalities. |
| Hsu et al., 2003 [39] | 68 Taiwanese young men (Yu-Cheng and unexposed) | PCBs, PCDFs | - | Adulthood | Serum concentration | 37–50 years | Increased abnormal sperm morphology, decreased motility, decreased hamster oocyte penetration. |
| Korrick et al., 2011 [40] | 499 Russian boys (Chapaevsk) | Dioxins | Wide range Medians, pg/TEQ/g lipid for TCDD - 2.8; PCDDTEQ-8.2 |
Longitudinal, 8–9 years; 2003–2005 | Serum concentrations and TEQ | 8–12 years, 2003–2006 | Delayed pubertal onsetby testicular volume |
| Minguez-Alarcon et al.,2016 [55] | 133 Russian young adults (Chapaevsk) | TCDD, dioxins | Wide range Medians, pg/TEQ/g lipid for TCDD-2.9; PCDDTEQ-8.7 |
Longitudinal, 8–9 years; 2003–2005 | Serum concentrations and TEQ | 18–19 years, 2012–2015 | Decreased sperm count, total sperm count and total motile sperm count. |
| Mocarelli et al., 2008 [13] | 319 Italian men (Seveso accident and unexposed) | TCDD | Medians, ppt 1–9 years - 210; 10–17 years - 164: 18–26 years - 123 |
Longitudinal, three age groups: 1–9 years 10–17 years 18–26 years; 1976 |
Serum concentration | Three age groups: 22–31 years 32–39 years 40–47 years |
Exposed at 1–9 years - decreased sperm count, motility, estradiol, increased
FSH Exposed at 10–17 years - increased total sperm count, total motile sperm count, FSH, reduced estradiol Exposed at 18–26 years - no effects. |
| Mocarelli et al., 2011 [50] | 97 Italian men (Seveso accident breast-fed, formula-fed and un xposed) | TCDD | Median, ppt at conception -breast-fed - 19.0: formula-fed - 27.9 |
Longitudinal, prenatal and lactational, 1976–1983 | Maternal serum concentrations | 18–26 years, 2002–2003 | In breast-fed group - decreased sperm count, total sperm count and progressive motility, increased FSH, decreased inhibin B. |
| Paoli et al., 2015 [53] | 125 testicular cancer patients and 103 controls | PCBs | - | Adulthood | Serum concentrations | Adulthood | Decreased sperm concentration count and motility, increased abnormalspermatozoa. |
Decrease in sperm count is among the most sensitive outcomes of dioxin toxicity in both human and experimental studies. Tolerable daily intake of DLCs established by WHO was derived from an exposure dose 0.064 μg TCDD/kg on gestational day 15 that resulted in a significant decrease of epididymal sperm count in rats [42]. Adverse effects of dioxins on Leydig cells were observed at higher doses in marmosets [43] and rodents [44]. Decrease in epididymal sperm count was demonstrated in many other experimental studies [45–49]. Recent longitudinal epidemiological studies also have shown associations between serum concentrations of DLCs and decreased semen parameters [13,50–53] (Table 1). The Mocarelli group investigated acutely exposed men to high level of TCDD in Seveso, Italy, during different periods of onto-genies: perinatal, infancy/prepuberty (1–9 years), puberty (10–17 years), and adulthood (18–26 years). They have found that exposure to TCDD in utero and infancy/prepuberty resulted in reduced sperm concentration and motility, while exposure during puberty had the opposite effect [13]. In their study perinatal and lactational exposure to relatively low TCDD doses was associated with reduction of sperm quality [50]. The sensitivity of reproductive function from dioxins toxicity during peripubertal developmental window was recently confirmed by the Russian Children’s Study [54,55]. This prospective cohort enrolled 516 boys at 8–9 years old, residing in Chapaevsk, Russia, and followed annually till young adulthood, at which time semen quality parameters were evaluated [54,55]. Results demonstrated that higher peripubertal serum TCDD concentrations and PCDD toxic equivalents were associated with decrease in sperm concentration, total sperm count, and total motile sperm count [55]. In the Russian Children’s Study, the median serum TCDD was 2.9 pg TEQ/g lipid [55] – about seventy-fold lower than Seveso cohort [13].
In the most recent review of existing bodies of literature on the effect of dioxins on male reproductive health, Foster and others [56] found no convincing evidence of treatment-related effect of environmentally-relevant doses of dioxins on weight and/or morphology of testis, changes in Sertoli cell structure and count, and functioning of hypothalamic-pituitary-testicular axis. Thus, the authors concluded that effects of dioxins on sperm count can be due to induced changes in epididymal structure and function rather than changes in spermatogenesis or the testis itself.
3. Intergenerational effects of dioxins in human studies
The widespread use of Agent Orange – defoliant containing TCDD used by the U.S. military in herbicidal warfare program, Operation Ranch Hand has provided opportunities to examine the long-term effects of TCDD exposures among Vietnam War veterans and civilians. The first study, published by the U.S. Centers of Disease Control in 1984, found increases in the incidence of birth defects incidence in offspring of male veterans exposed to Agent Orange including increased rates of neural tube defects (NTDs), especially spina bifida, and to a lesser degree anencephaly [57]. The study suggested that the Agent Orange-associated increase in NTDs of offspring altered genetic or epigenetic information in spermatozoa, thus directly implicating spermatogenesis disruption. Although these findings were highly debated, a recent meta-analysis consisting of nine publications from the United States and thirteen from Vietnamese sources on Agent Orange exposure and birth defects [58] concluded a causal relationship between Agent Orange exposure and stillbirth, cleft palate, and neural tube defects. To our knowledge, there is only one study which analyzed sperm parameters in veterans of Operation Ranch Hand in relation of Agent Orange [59], of which no associations were observed for testicular abnormalities, sperm count, and percentage abnormal sperm.
Given that TCDD is known to be likely non-mutagenic, it is unlikely that TCDD-induced mutagenesis in germ cells is responsible for increased incidence of birth defects in the offspring of Vietnam Veterans, leaving epigenetic errors in spermatozoa as the most likely candidate mechanism linking paternal exposure to dioxins and birth defects in offspring. The potential possibility of the transfer of a legacy of environmental conditions to future generations via sperm epigenetics have been demonstrated in several recent studies of animal models [60].
4. Epigenetic response to dioxins
Many experimental studies report changes in DNA methylation in response to TCDD using a variety of models, doses and target tissues/cells. Mouse preimplantation embryos exposed to 10 nM TCDD from the 1-cell stage to the blastocyst stage and then transferred to unexposed recipient mice weighed less on embryonic day 14, and had decreased expression levels of the imprinted genes H19 and Igf2, increased methylation of the H19/Igf2 imprint control region and increased methyltransferase activity [61]. Thus, it is likely that TCDD can interfere with the process of erasure and reestablishment of DNA methylation profiles that occurs in preimplantation embryos (see Fig. 1) [62]. This same window of epigenetic remodeling was targeted by in utero exposure to TCDD [63], which resulted in reduced BRCA-1 expression in mammary tissue of rat offspring, induced occupancy of the BRCA-1 promoter by DNA methyltransferase-1 (DNMT-1) and increased CpG methylation of the BRCA-1 promoter [63]. Some studies report cell-specific epigenetic effects of dioxins. For example, a modest decrease in global DNA methylation was observed in murine N2A neuroblastoma cells exposed to 10 μM TCDD but not in the human SK-N-AS neuroblastoma cells [64]. Changes in DNA methylation induced by TCDD are likely mediated by AhR. Response of splenocytes to TCDD was associated with AhR-dependent changes in DNA methylation in multiple genomic regions [65]. Methylation of CpG islands was decreased in Foxp3 promoter and increased in IL-17 promoter in lamina propria and mesenteric lymph nodes of mouse colon following TCDD treatment and this effect was also AhR dependent [66]. Dioxins have also been reported to affect size and shape of space occupied by each chromosome within the interphase nucleus in human preadipocyte cells via AhR dependent mechanism [67], indicating the potential of dioxins to remodel chromatin. Exposure of zebrafish embryos to 5 nM TCDD for 1 h altered expression of DNA methyl transferase genes: expression of dnmt1 and dnmt3b2 was upregulated, whereas dnmt3a1, 3b1, and 3b4 were downregulated several hours after exposure was ceased [68]. The same exposure regimen resulted in differential expression of several microRNAs in zebrafish embryos [69]. While no TCDD-induced differences in global methylation or hydroxymethylation levels was observed in this study, the promoter methylation of AhR target genes was altered: decreased in the c-fos promoter and increased in the ahrra promoter.
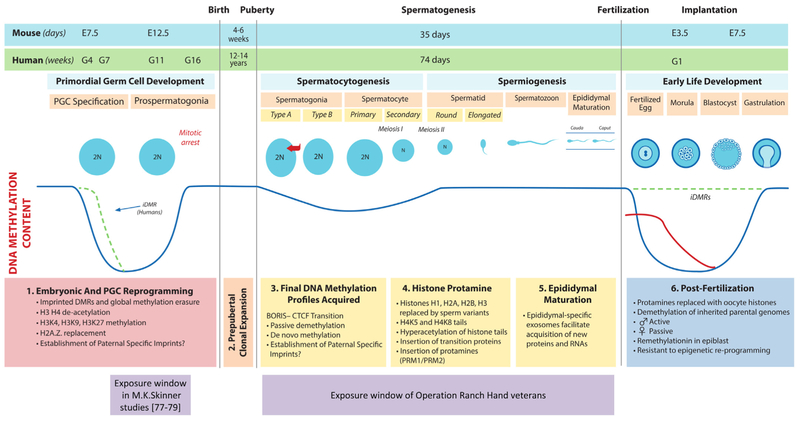
Fig. 1.
Scheme of events of epigenetic reprograming in male germ line (from [90] with modifications). Several animal transgenerational studies target the window of primordial germ cell epigenetic remodeling which includes significant erasure and reestablishment of DNA methylation profile. Spermatogenesis is another window during which epigenetic remodeling occurs throughout the reproductive life of the adult male and includes changes in DNA methylation, histone–protamine exchange and exosomal shuttling of ncRNA to mature spermatozoa in the epididymis. We hypothesize that disruption of epigenetic reprograming by dioxin and DLCs during this window may provide an epigenetic legacy of paternal exposures that are transferred to the next generation. See [90] for detailed figure legend.
5. Mechanisms of epigenetic reprograming by dioxins
The mechanisms by which epigenetic landscape in spermatozoa and other tissues responds to dioxins have not been fully clarified. One possibility is that epigenetic changes are AhR dependent (Fig. 2). Activation of AhR by its agonist 3-methylcholanthrene (3MC) increases expression of histone deacetylate, HDAC1, resulting in decreased cell proliferation and cell cycle arrest due to epigenetic modification of cell cycle genes [70]. The AhR/ARNT interact with histone modification cofactors such as CREBBP and the protein arginine methyltransferases (PRMTs) enzymes, such as PRMT1 and PRMT4 (CARM1) [71], which regulate gene expression through methylation of histone and non-histone proteins. PRMT1 methylates arginine 3 of histone H4 (H4R3) and is a major methyltransferase in mammalian cells playing an important role in development and pathophysiological processes [72,73]. It has been shown recently that CARM1 positively regulates the expression of pluripotency-related genes through the alteration of the chromatin structure and upregulation of this protein results in delayed spontaneous differentiation in embryonic stem cells [74]. H4R3 methylation by PRMT1 is an initiation step necessary for the establishment or maintenance of a wide range of “active” chromatin modifications [75]. Other mechanisms may involve altered hormonal signaling as developmental exposure to dioxins inhibit sex steroid biosynthesis by suppressing activity of testicular STAR protein [76]. Male mice with AhR knockout (AhR(−/−)) have impaired testosterone synthesis in Leydig cells and low sperm counts [77]. Furthermore, dioxin-activated AhR/ARNT can recruit estrogen receptor and co-activator p300 to estrogen-responsive elements (EREs), leading to transactivation and estrogenic effects in the absence of estrogenic ligand [78]. Sex steroid signaling is also a likely regulator of the epigenome; however, it is beyond the scope of current review.
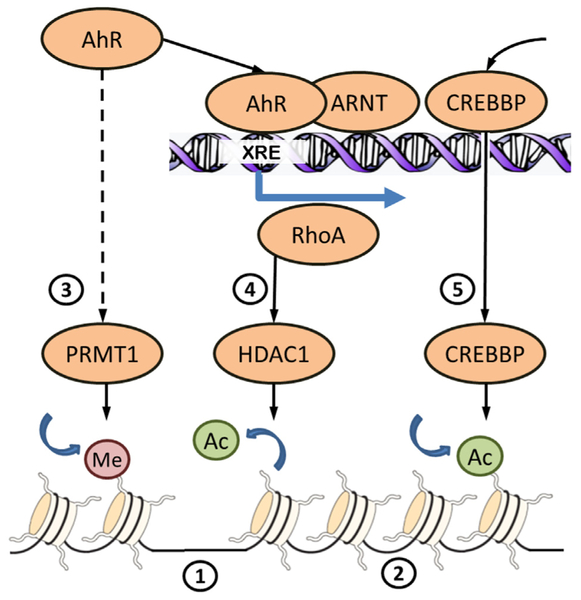
Fig. 2.
Some mechanisms of chromatin rearrangement downstream AhR signaling. DNA in somatic cells is wrapped around nucleosomes consisting of histone proteins. Sparse position of nucleosomes (1) insures accessibility of DNA for transcription machinery and is associated with highly expressed genes. DNA tightly packed on nucleosomes (2) is associated with inactive genes. Compaction and relaxation of chromatin is regulated by covalent modification of histone tails. Activation of AhR by ligand-binding activates histone methyltransferase PRMT1 (3) by unknown mechanisms. PRMT1 methylates arginine 3 of histone 4 what favors further acetylation of histone 4. Acetylation neutralizes the positive charges on the histone, decreases histone–DNA binding and results in transcriptional activation. Binding of AhR/ARNT heterodimer to xenobiotic response element (XRE) initiates transcription of genes, including RhoA, resulting in increased expression and nuclear translocation of histone deacetylase HDAC1 (4). Histone deacetylation condenses the chromatin structure resulting in the downregulation of target genes. HDAC1 participates in repression of cell cycle genes. Binding of AhR/ARNT to XRE recruits histone acetyltransferase CREBBP (5). Aacetylation of histone tails by CREBBP participates in transcription initiation
6. Transgenerational effects in animal experiments
Animal experiments examining transgenerational effects of TCDD are summarized in Table 2. In a series of studies performed in M.K. Skinner’s group [79–81] pregnant F0 rats were exposed to 100 ng/kg BW/day TCDD by intraperitoneal injections during gestational days 8 through 14, period that covers the erasure and de-novo methylation of male primordial germ cells [62]. TCDD promoted early-onset female puberty transgenerationally (F3 generation) and several adult-onset diseases were increased in F1 and F3 generations. In F3 descendants of dioxin-exposed animals, the incidence of kidney disease in males, and ovarian abnormalities in females were increased. Interestingly, spermatogenic cell apoptosis was also affected transgenerationally. Analysis of sperm epigenome from F3 generation identified 50 differentially methylated regions in gene promoters. The dose of TCDD used in these studies [79–81] was in nanogram ranges while human exposures via food basket were estimated to be in a picogram range in U.S. and Europe [11,82]. Several ten-fold uncertainty and modifying factors are applied to transfer dose-response data from animal experiments to human regulatory procedures to account for intraspecies sensitivity, inter-species sensitivity, use of other than chronic exposures, and use of low observed adverse effect level (LOAEL) rather than no observed adverse effect level (NOAEL). Due to these uncertainty factors, a safe dose for humans is typically determined as a dose 1000 times lower than NOAEL. Thus, experiments conducted by M.K. Skinner’s group have moderate relevance for the general population. More important is that these experiments provide proof of principle and demonstrate that TCDD-induced epimutations can persist across many generations due to abnormal DNA methylation in sperm.
Table 2.
Summary of animal studies of transgenerational effects of dioxin
| Study | Species | TCDD dose | Exposure window | Effects in F1 | Effects in F2 | Effects in F3 |
|---|---|---|---|---|---|---|
| Manikkam et al., 2012 [79] | Rat | 100 ng/kg BW/d | G8-G14 | Early puberty onset | Increased ano-genital distance, decreased testosterone, 50 differentially methylated regions in sperm DNA | |
| Nilsson et al., 2012 [80] | Rat | 100 ng/kg BW/d | G8-G14 | Reduction of primordial follicles | Reduction of primordial follicles, increased ovarian cyst | |
| Manikkam et al., 2012 [81] | Rat | 100 ng/kg BW/d | G8-G14 | Early puberty onset, atrophic prostatic duct epithelium | Increased serum testosterone, kidney cysts in males, reduction of primordial follicles, increased ovarian cyst | |
| Bruner-Tran et al., 2014 [83] | Mouse | 10 μg/kg/BW | G15.5 | Reduced male fertility, increased AhR expression in spermatocytes, increased apoptosis and inflammatory markers in testis | Reduced male fertility, decreased sperm count, increased AhR expression in spermatocytes, increased apoptosis and inflammatory markers in testis | Reduced male fertility, increased AhR expression in spermatocytes, increased apoptosis and inflammatory markers in testis |
| Sanabria et al., 2016 [84] | Rat | 0.1, 0.5 and 1.0 μg/kg/BW | G15 | Decreased testosterone (1.0 group), reduced implants per corpus luteum, increased abnormal spermatozoa (0.5, 1 groups) | Reduced implants per corpus luteum (0.1 group) | Reduced implants per corpus luteum (all groups) |
| Olsvik et al., 2014 [85] | Zebrafish | 20 μg/kg diet | 47 days adult exposure | No effect on global DNA methylation in liver, increase in Cyp1A1 expression | No effect on global DNA methylation in liver | Not analyzed |
| Baker et al., 2014 [87] | Zebrafish | 50 pg/ml in water | Juveniles exposed on 4th and 7th week post-fertilization, for 1 h each time | Increased spinal kinks, increased ratio of females to males, ovarian disorganization, male and female dependent decrease in egg release | Increased spinal kinks, increased ratio of females to males, male dependent decrease in egg release | Not analyzed |
In another transgenerational study performed by another research group, exposure of pregnant mice to 10 [H9262]g/kg TCDD by gavage on gestation day 15.5 resulted in decrease in fertility and bias to preterm birth [83]: about 50% of F1–F3 males were sterile, 33–38% that were able to impregnate their mating female showed spontaneous delivery prior to E19.0. In all three generations of treated male mice there were signs of testicular inflammation and increased apoptosis of germ cells. In a recent rodent study, pregnant Wistar rats were exposed to a single dose (0.1; 0.5 and 1.0 μg/kg body weight) of TCDD on gestational day 15 and reproductive health of male offspring was analyzed in 3 generations of progeny [84]. The fertility of male offspring assessed by the number of implants per corpus luteum after intrauterine artificial insemination with sperm of exposed and control animals was significantly decreased in F1 animals exposed to two higher doses, in F2 animals exposed only to lowest dose and in F3 animals exposed to all three doses. Transgenerational effects of TCDD on global DNA methylation were not found in a zebrafish study in which adult females were fed diets added 20 [H9262]g/kg 2,3,7,8 TCDD for 47 days and bred with unexposed males in clean water to produce F1 and F2 off-spring [85]. Juvenile zebrafish exposed to 50 pg/ml TCDD in water produced a significantly higher female:male ratio in F0, F1 and F2 generations. F1 and F2 generations had increased incidence of scoliosis-like axial skeleton abnormalities, reduced egg release and fertilization success [86,87]. Thus, evidence from both human studies and animal experiments suggest that dioxins have the potential to change epigenetic profiles in cells and such changes in spermatozoa can deliver perturbed epigenetic information to future generations.
7. Spermatogenesis: a window of epigenetic susceptibility during the preconception period of males
Although human male germ cells do not reach reproductive capacity until the second decade of life, their development begins in utero shortly after sex determination. Derived from the epiblast, primordial germ cells require extensive epigenetic remodeling events to establish totipotency to allow for sex-specific programming [62]. These include genome-wide loss of methylation including imprinted regions as well as histone remodeling. It must be noted that although demethylation is thought to be complete, certain sequences, such as intracisternal A particle elements (IAPs) and their proximal genes, are resistant to erasure, which may provide a platform for epigenetic inheritance [88]. Owing to the plasticity of the epigenome and the extensive epigenetic reprogramming during PGC development, it is not surprising that environmental exposures during this period have been shown to sculpt the epigenetic landscape of male germ cells resulting in inter- and transgenerational epigenetic inheritance (as discussed above). However, in regard to Agent Orange, phenotypical changes were observed in the offspring of males who were exposed in adulthood, suggesting that epigenetic changes in male germ cells may also occur during this window of male germ cell development.
The preconception period is now recognized one of the earliest susceptible window of human development [89]. In adult males, spermatogenesis occurs over 74 days in which spermatogonia differentiate through mitotic and meiosis divisions in the testis followed by epididymis maturation to produce spermatozoa capable of fertilization. During this process, three distinct epigenetic reprogramming events occur during spermatogenesis [90]. First, final DNA methylation patterns are obtained during mitotic divisions of spermatogonia in which both passive loss of methylation and de novo methylation has been shown to occur in animal models [91,92]. In light of this, recent data demonstrate that environmental exposures in adult mice may influence offspring phenotype via sperm epigenetics. For example, nutritional manipulation in adult males, such as low-protein diet [93] and pre-diabetic conditions [94], induced metabolic disorders in offspring through changes in sperm epigenetics. Moreover, low paternal dietary folate in mice resulted in an increase in birth defects in offspring and changes in sperm methylation in genes related to development, cancer and autism [95]. Interestingly, over 300 genes were differentially expressed in the placenta of fetuses produced using sperm of fathers fed folate deficient diet, suggesting that sperm epigenetic changes may also affect offspring development through changes in placental function [95].
Next, spermatids undergo global reorganization of chromatin in which approximately 90% of histones in humans (99% in mice) are replaced by protamines, which restricts transcriptional activity [96,97]. This histone–protamine exchange condenses the nucleus to enhance the motility of spermatozoa and to protect the genome from the harsh environment encountered in the female reproductive tract [98]. In humans, it has been reported that histone retention is not random but is enriched in regulatory regions of genes known to be important for development [96,99–101]. Two other studies have found histone retention in gene-poor regions [102,103]. Subsequent bioinformatic reanalysis of raw data from one of these studies [102] did not confirm histone retention in gene poor regions [104]. Other possible causes of controversial results on histone retention in different functional genomic elements are discussed elsewhere [105]. Interestingly, nutritional manipulation of sperm chromatin has been shown in Drosophila, where high sugar diet in adult males altered methylation of H3K9/K27me3 within chromatin-bound regions of mature sperm, which subsequently conferred metabolic programming of offspring [106].
Lastly, upon exiting the testes, human sperm undergo maturation during the 1–2 week transit through the epididymis [107,108]. Here, extracellular vesicles (EV), known as epididymosomes, have been shown to shuttle somatic proteins and RNA to sperm [109–111]. For example a gain of 115 miRNAs was observed between mouse sperm collected from the proximal and distal epididymal segments [112]. Thus, it has been proposed that EV shuttling provides the final opportunity for sperm to epigenetically match their environment prior to fertilization [90]. Indeed, recent work from Rando and colleagues have shown that protein-restriction in adult male mice altered small RNA profiles in EVs that matched changes observed in mature sperm and subsequently affected preimplantation embryo development [106]. Similarly, high fat diets in adult mice resulted in altered sperm miRNA content and resulted in metabolic abnormalities in across two generations [113]. Thus spermatogenesis is accompanied by diverse and fundamental epigenetic changes and may represent a sensitive window for epigenetic reprograming by environmental stressors like dioxins.
8. Conclusions
According to accumulating body of evidence from both experiments with laboratory animals and studies of human population exposed to high doses of dioxins, exposures result in altered information transferred with sperm to next generations. Given TCDD is non mutagenic or only mildly mutagenic substance, it is very likely that changes in transferred information are epigenetic in nature. A growing body of evidence demonstrate responsiveness of epigenome to dioxins in a variety of cells/tissues and animal models. Although molecular pathway(s) involved in the alteration of epigenetic landscape in response to dioxins are largely unknown, several mechanisms of AhR dependent histone modification were described. Epigenetic effects may also be linked with sex steroid signaling affected by dioxins due to their effect on Leydig cells. Several animal experiments showed that exposure of fetuses during the window when primordial germ cells undergo global erasure and reestablishment of DNA methylation landscapes may result in multigenerational transfer of defective epigenome via male germline. In male subjects exposed to Agent Orange at adulthood toxic effects were found in F1. Thus, we hypothesize that epigenetic reprogramming during spermatogenesis represent another window of sensitivity susceptible to environmentally-induced epigenetic errors [90]. To test this hypothesis, future research in humans and animal models should be directed at examining the effect of preconception DLC exposures on epigenetic reprogramming during spermatogenesis including DNA methylation, overall histone retention, covalent modifications of retained histone tails, and epididymal miRNA. Such research will advance our understanding of DLC-induced male reproductive toxicity as well as the mechanisms of inter- and transgenerational transfer of exposure legacies via the paternal germ line.
Acknowledgments
Funding
This work was supported by the Russian Science Foundation (grant #14–45-00065). For JRP, work was also supported by K22-ES023085–02 and R21-E5026778.
Footnotes
Transparency document
The http://dx.doi.org/10.1016/j.reprotox.2017.03.002 associated with this article can be found in the online version.
References
- [1].Carlsen E, Giwercman A, Keiding N, Skakkebaek NE, Evidence for decreasing quality of semen during past 50 years, BMJ 305 (1992) 609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Swan SH, Elkin EP, Fenster L, The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996, Environ. Health Perspect 108 (2000) 961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jorgensen N, Vierula M, Jacobsen R, Pukkala E, Perheentupa A, Virtanen HE, et al. , Recent adverse trends in semen quality and testis cancer incidence among Finnish men, Int. J. Androl 34 (2011) e37–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jorgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, Olesen IA, et al. , Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men, BMJ Open 2 (2012), 10.1136/bmjopen-2012-000990, Print 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rolland M, Le Moal J, Wagner V, Royere D, De Mouzon J, Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France, Hum. Reprod 28 (2013) 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bergman A, Heindel JJ, Jobling S, Kidd KA, Zoeller RT. State of the Science of Endocrine Disrupting Chemicals – 2012. UNEP and the WHO; 2013:260. [Google Scholar]
- [7].Le Moal J, Rolland M, Goria S, Wagner V, De Crouy-Chanel P, Rigou A, et al. , Semen quality trends in French regions are consistent with a global change in environmental exposure, Reproduction 147 (2014) 567–574. [DOI] [PubMed] [Google Scholar]
- [8].Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. , EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals, Endocr. Rev 36 (2015) E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fernandez-Gonzalez R, Yebra-Pimentel I, Martinez-Carballo E, Simal-Gandara J, A critical review about human exposure to polychlorinated dibenzo-p-dioxins (PCDDs) polychlorinated dibenzofurans (PCDFs) and polychlorinated biphenyls (PCBs) through foods, Crit. Rev. Food Sci. Nutr 55 (2015) 1590–1617. [DOI] [PubMed] [Google Scholar]
- [10].WHO, Dioxins and Their Effects on Human Health, vol. 2016, 2016. [Google Scholar]
- [11].Schecter A, Cramer P, Boggess K, Stanley J, Papke O, Olson J, et al. , Intake of dioxins and related compounds from food in the U.S. population, J. Toxicol. Environ. Health A 63 (2001) 1–18. [DOI] [PubMed] [Google Scholar]
- [12].Rose M, Incidents involving contamination of food with dioxins, in: 14th Fera JIFSAN Annual Symposium, FDA, College Park, MD, USA, 2013. [Google Scholar]
- [13].Mocarelli P, Gerthoux PM, Patterson DG Jr., Milani S, Limonta G, Bertona M, et al. , Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality, Environ. Health Perspect 116 (2008) 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, et al. , The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds, Toxicol. Sci 93 (2006) 223–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ahlborg UG, Hanberg A, Toxic equivalency factors for dioxin-like PCBs, Environ. Sci. Pollut. Res. Int 1 (1994) 67–68. [DOI] [PubMed] [Google Scholar]
- [16].Van den Berg M, Birnbaum L, Bosveld AT, Brunstrom B, Cook P, Feeley M, et al. , Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife, Environ. Health Perspect 106 (1998) 775–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].van den Berg M, Denison MS, Birnbaum LS, Devito MJ, Fiedler H, Falandysz J, et al. , Polybrominated dibenzo-p-dioxins, dibenzofurans, and biphenyls: inclusion in the toxicity equivalency factor concept for dioxin-like compounds, Toxicol. Sci 133 (2013) 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Venkatesan AK, Halden RU, Contribution of polybrominated dibenzo-p-dioxins and dibenzofurans (PBDD/Fs) to the toxic equivalency of dioxin-like compounds in archived biosolids from the U.S. EPA’s 2001 national sewage sludge survey, Environ. Sci. Technol 48 (2014) 10843–10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Su G, Xia J, Liu H, Lam MH, Yu H, Giesy JP, et al. , Dioxin-like potency of HO- and MeO-analogues of PBDEs’ the potential risk through consumption of fish from eastern China, Environ. Sci. Technol 46 (2012) 10781–10788. [DOI] [PubMed] [Google Scholar]
- [20].Dere E, Lo R, Celius T, Matthews J, Zacharewski TR, Integration of genome-wide computation DRE search AhR ChIP-chip and gene expression analyses of TCDD-elicited responses in the mouse liver, BMC Genomics 12 (2011) 365, 10.1186/1471-2164-12-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hankinson O, The aryl hydrocarbon receptor complex, Annu. Rev. Pharmacol. Toxicol 35 (1995) 307–340. [DOI] [PubMed] [Google Scholar]
- [22].Mulero-Navarro S, Fernandez-Salguero PM, New trends in aryl hydrocarbon receptor biology, Front. Cell Dev. Biol 4 (2016) 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tijet N, Boutros PC, Moffat ID, Okey AB, Tuomisto J, Pohjanvirta R, Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries, Mol. Pharmacol 69 (2006) 140–153. [DOI] [PubMed] [Google Scholar]
- [24].Okey AB, An aryl hydrocarbon receptor odyssey to the shores of toxicology: the Deichmann Lecture, International Congress of Toxicology-XI, Toxicol. Sci 98 (2007) 5–38. [DOI] [PubMed] [Google Scholar]
- [25].Brokken LJ, Lundberg PJ, Spano M, Manicardi GC, Pedersen HS, Strucinski P, et al. , Interactions between polymorphisms in the aryl hydrocarbon receptor signalling pathway and exposure to persistent organochlorine pollutants affect human semen quality, Reprod. Toxicol 49 (2014) 65–73. [DOI] [PubMed] [Google Scholar]
- [26].van Leeuwen FX, Feeley M, Schrenk D, Larsen JC, Farland W, Younes M, Dioxins: WHO’s tolerable daily intake (TDI) revisited, Chemosphere 40 (2000) 1095–1101. [DOI] [PubMed] [Google Scholar]
- [27].Knerr S, Schrenk D, Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models, Mol. Nutr. Food Res 50 (2006) 897–907. [DOI] [PubMed] [Google Scholar]
- [28].Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans. IARC Monographs on the Evaluation of Carcinogenic risks to humans. [PMC free article] [PubMed] [Google Scholar]
- [29].Tuomisto J, Tuomisto JT, Is the fear of dioxin cancer more harmful than dioxin? Toxicol. Lett 210 (2012) 338–344. [DOI] [PubMed] [Google Scholar]
- [30].Cole P, Trichopoulos D, Pastides H, Starr T, Mandel JS, Dioxin and cancer: a critical review, Regul. Toxicol. Pharmacol 38 (2003) 378–388. [DOI] [PubMed] [Google Scholar]
- [31].WHO. Dioxins and their effects on human health. World Health Organization, Fact Sheet #225 2014; 2015. [Google Scholar]
- [32].IPCS INCHEM, Safety Evaluation of Certain Food Additives and Contaminants. Polychlorinated Dibenzodioxins, Polychlorinated Dibenzofurans, and Coplanar Polychlorinated Biphenyls, Chemical Safety Information from Intergovernmental Organizations, WHO Food Additives Series: 48, 2015, pp. 2015. [Google Scholar]
- [33].Safe S, Lee SO, Jin UH, Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target, Toxicol. Sci 135 (2013) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Huff JE, Salmon AG, Hooper NK, Zeise L, Long-term carcinogenesis studies on 2,3,7,8-tetrachlorodibenzo-p-dioxin and hexachlorodibenzo-p-dioxins, Cell Biol. Toxicol 7 (1991) 67–94. [DOI] [PubMed] [Google Scholar]
- [35].Thornton AS, Oda Y, Stuart GR, Glickman BW, de Boer JG, Mutagenicity of TCDD in big blue transgenic rats, Mutat. Res 478 (2001) 45–50. [DOI] [PubMed] [Google Scholar]
- [36].Den Hond E, Roels HA, Hoppenbrouwers K, Nawrot T, Thijs L, Vandermeulen C, et al. , Sexual maturation in relation to polychlorinated aromatic hydrocarbons: Sharpe and Skakkebaek’s hypothesis revisited, Environ. Health Perspect 110 (2002) 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Guo YL, Lambert GH, Hsu CC, Hsu MM, Yucheng: health effects of prenatal exposure to polychlorinated biphenyls and dibenzofurans, Int. Arch. Occup. Environ. Health 77 (2004) 153–158. [DOI] [PubMed] [Google Scholar]
- [38].Guo YL, Hsu PC, Hsu CC, Lambert GH, Semen quality after prenatal exposure to polychlorinated biphenyls and dibenzofurans, Lancet 356 (2000) 1240–1241. [DOI] [PubMed] [Google Scholar]
- [39].Hsu PC, Huang W, Yao WJ, Wu MH, Guo YL, Lambert GH, Sperm changes in men exposed to polychlorinated biphenyls and dibenzofurans, JAMA 289 (2003) 2943–2944. [DOI] [PubMed] [Google Scholar]
- [40].Korrick SA, Lee MM, Williams PL, Sergeyev O, Burns JS, Patterson DG, et al. , Dioxin exposure and age of pubertal onset among Russian boys, Environ. Health Perspect 119 (2011) 1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Burns JS, Lee MM, Williams PL, Korrick SA, Sergeyev O, Lam T, et al. , Associations of peripubertal serum dioxin and polychlorinated biphenyl concentrations with pubertal timing among Russian boys, Environ. Health Perspect (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mably TA, Bjerke DL, Moore RW, Gendron-Fitzpatrick A, Peterson RE, In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin 3 Effects on spermatogenesis and reproductive capability, Toxicol. Appl. Pharmacol 114 (1992) 118–126. [DOI] [PubMed] [Google Scholar]
- [43].Rune GM, de Souza P, Krowke R, Merker HJ, Neubert D, Morphological and histochemical pattern of response in rat testes after administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), Histol. Histopathol 6 (1991) 459–467. [PubMed] [Google Scholar]
- [44].Johnson L, Dickerson R, Safe SH, Nyberg CL, Lewis RP, Welsh TH Jr., Reduced Leydig cell volume and function in adult rats exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin without a significant effect on spermatogenesis, Toxicology 76 (1992) 103–118. [DOI] [PubMed] [Google Scholar]
- [45].Bjerke DL, Peterson RE, Reproductive toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male rats: different effects of in utero versus lactational exposure, Toxicol. Appl. Pharmacol 127 (1994) 241–249. [DOI] [PubMed] [Google Scholar]
- [46].Gray LE, Wolf C, Mann P, Ostby JS, In utero exposure to low doses of 2,3,7,8-tetrachlorodibenzo-p-dioxin alters reproductive development of female Long Evans hooded rat offspring, Toxicol. Appl. Pharmacol 146 (1997) 237–244. [DOI] [PubMed] [Google Scholar]
- [47].Ohsako S, Miyabara Y, Sakaue M, Ishimura R, Kakeyama M, Izumi H, et al. , Developmental stage-specific effects of perinatal 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on reproductive organs of male rat offspring, Toxicol. Sci 66 (2002) 283–292. [DOI] [PubMed] [Google Scholar]
- [48].Sommer RJ, Ippolito DL, Peterson RE, In utero and lactational exposure of the male Holtzman rat to 2,3,7,8-tetrachlorodibenzo-p-dioxin: decreased epididymal and ejaculated sperm numbers without alterations in sperm transit rate, Toxicol. Appl. Pharmacol 140 (1996) 146–153. [DOI] [PubMed] [Google Scholar]
- [49].Wilker C, Johnson L, Safe S, Effects of developmental exposure to indole-3-carbinol or 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive potential of male rat offspring, Toxicol. Appl. Pharmacol 141 (1996) 68–75. [DOI] [PubMed] [Google Scholar]
- [50].Mocarelli P, Gerthoux PM, Needham LL, Patterson DG Jr., Limonta G, Falbo R, et al. , Perinatal exposure to low doses of dioxin can permanently impair human semen quality, Environ. Health Perspect 119 (2011) 713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Faure AC, Viel JF, Bailly A, Blagosklonov O, Amiot C, Roux C, Evolution of sperm quality in men living in the vicinity of a municipal solid waste incinerator possibly correlated with decreasing dioxins emission levels, Andrologia 46 (2014) 744–752. [DOI] [PubMed] [Google Scholar]
- [52].Meeker JD, Hauser R, Exposure to polychlorinated biphenyls (PCBs) and male reproduction, Syst. Biol. Reprod. Med 56 (2010) 122–131. [DOI] [PubMed] [Google Scholar]
- [53].Paoli D, Giannandrea F, Gallo M, Turci R, Cattaruzza MS, Lombardo F, et al. , Exposure to polychlorinated biphenyls and hexachlorobenzene, semen quality and testicular cancer risk, J. Endocrinol. Invest 38 (2015) 745–752. [DOI] [PubMed] [Google Scholar]
- [54].Burns JS, Williams PL, Sergeyev O, Korrick S, Lee MM, Revich B, et al. , Predictors of serum dioxins and PCBs among peripubertal Russian boys, Environ. Health Perspect 117 (2009) 1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Minguez-Alarcon L, Sergeyev O, Burns JS, Williams PL, Lee MM, Korrick SA, et al. , A longitudinal study of peripubertal serum organochlorine concentrations and semen parameters in young men: the Russian Children’s Study, Environ. Health Perspect (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Foster WG, Maharaj-Briceno S, Cyr DG, Dioxin-induced changes in epididymal sperm count and spermatogenesis, Cien. Saude Colet 16 (2011) 2893–2905. [DOI] [PubMed] [Google Scholar]
- [57].Erickson JD, Mulinare J, McClain PW, Fitch TG, James LM, McClearn AB, et al. , Vietnam veterans’ risks for fathering babies with birth defects, JAMA 252 (1984) 903–912. [PubMed] [Google Scholar]
- [58].Ngo AD, Taylor R, Roberts CL, Nguyen TV, Association between Agent Orange and birth defects: systematic review and meta-analysis, Int. J. Epidemiol 35 (2006) 1220–1230. [DOI] [PubMed] [Google Scholar]
- [59].Henriksen GL, Michalek JE, Swaby JA, Rahe AJ, Serum dioxin, testosterone, and gonadotropins in veterans of Operation Ranch Hand, Epidemiology 7 (1996) 352–357. [PubMed] [Google Scholar]
- [60].Estill MS, Krawetz SA, The epigenetic consequences of paternal exposure to environmental contaminants and reproductive toxicants, Curr. Environ. Health Rep 3 (2016) 202–213. [DOI] [PubMed] [Google Scholar]
- [61].Wu Q, Ohsako S, Ishimura R, Suzuki JS, Tohyama C, Exposure of mouse preimplantation embryos to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters the methylation status of imprinted genes H19 and Igf2, Biol. Reprod 70 (2004) 1790–1797. [DOI] [PubMed] [Google Scholar]
- [62].Smallwood SA, Kelsey G, De novo DNA methylation: a germ cell perspective, Trends Genet 28 (2012) 33–42. [DOI] [PubMed] [Google Scholar]
- [63].Papoutsis AJ, Selmin OI, Borg JL, Romagnolo DF, Gestational exposure to the AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin induces BRCA-1 promoter hypermethylation and reduces BRCA-1 expression in mammary tissue of rat offspring: preventive effects of resveratrol, Mol. Carcinog 54 (2015) 261–269. [DOI] [PubMed] [Google Scholar]
- [64].Bastos Sales L, Kamstra JH, Cenijn PH, van Rijt LS, Hamers T, Legler J, Effects of endocrine disrupting chemicals on in vitro global DNA methylation and adipocyte differentiation, Toxicol In Vitro 27 (2013) 1634–1643. [DOI] [PubMed] [Google Scholar]
- [65].McClure EA, North CM, Kaminski NE, Goodman JI, Changes in DNA methylation and gene expression during 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced suppression of the lipopolysaccharide-stimulated IgM response in splenocytes, Toxicol. Sci 120 (2011) 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS, Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis, PLoS ONE 6 (2011) e23522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Oikawa K, Yoshida K, Takanashi M, Tanabe H, Kiyuna T, Ogura M, et al. , Dioxin interferes in chromosomal positioning through the aryl hydrocarbon receptor, Biochem. Biophys. Res. Commun 374 (2008) 361–364. [DOI] [PubMed] [Google Scholar]
- [68].Aluru N, Kuo E, Helfrich LW, Karchner SI, Linney EA, Pais JE, et al. , Developmental exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin alters DNA methyltransferase (dnmt) expression in zebrafish (Danio rerio), Toxicol. Appl. Pharmacol 284 (2015) 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jenny MJ, Aluru N, Hahn ME, Effects of short-term exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on microRNA expression in zebrafish embryos, Toxicol. Appl. Pharmacol 264 (2012) 262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chang CC, Sue YM, Yang NJ, Lee YH, Juan SH, 3-Methylcholanthrene, an AhR agonist, caused cell-cycle arrest by histone deacetylation through a RhoA-dependent recruitment of HDAC1 and pRb2 to E2F1 complex, PLOS ONE 9 (2014) e39279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tian Y, Ah receptor and NF-kappaB interplay on the stage of epigenome, Biochem. Pharmacol 77 (2009) 670–680. [DOI] [PubMed] [Google Scholar]
- [72].Barrero MJ, Malik S, Two functional modes of a nuclear receptor-recruited arginine methyltransferase in transcriptional activation, Mol. Cell 24 (2006) 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Cheung N, Chan LC, Thompson A, Cleary ML, So CW, Protein arginine-methyltransferase-dependent oncogenesis, Nat. Cell Biol 9 (2007) 1208–1215. [DOI] [PubMed] [Google Scholar]
- [74].Choi S, Jo J, Seol DW, Cha SK, Lee JE, Lee DR, Regulation of pluripotency-related genes and differentiation in mouse embryonic stem cells by direct delivery of cell-penetrating peptide-conjugated CARM1 recombinant protein, Balsaenggwa Saengsig 17 (2013) 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Huang S, Litt M, Felsenfeld G, Methylation of histone H4 by arginine methyltransferase PRMT1 is essential in vivo for many subsequent histone modifications, Genes Dev. 19 (2005) 1885–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mutoh J, Ishida T, Ishii Y, Yamada H, Effect on the expression of testicular steroidogenic enzymes in fetal mouse by maternal exposure to TCDD, Fukuoka Igaku Zasshi 98 (2007) 203–207. [PubMed] [Google Scholar]
- [77].Baba T, Shima Y, Owaki A, Mimura J, Oshima M, Fujii-Kuriyama Y, et al. , Disruption of aryl hydrocarbon receptor (AhR) induces regression of the seminal vesicle in aged male mice, Sex. Dev 2 (2008) 1–11. [DOI] [PubMed] [Google Scholar]
- [78].Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, et al. , Modulation of oestrogen receptor signalling by association with the activated dioxin receptor, Nature 423 (2003) 545–550. [DOI] [PubMed] [Google Scholar]
- [79].Manikkam M, Guerrero-Bosagna C, Tracey R, Haque MM, Skinner MK, Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures, PLoS ONE 7 (2012) e31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Nilsson E, Larsen G, Manikkam M, Guerrero-Bosagna C, Savenkova MI, Skinner MK, Environmentally induced epigenetic transgenerational inheritance of ovarian disease, PLoS ONE 7 (2012) e36129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK, Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations, PLoS ONE 7 (2012) e46249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Darnerud PO, Atuma S, Aune M, Bjerselius R, Glynn A, Grawe KP, et al. , Dietary intake estimations of organohalogen contaminants (dioxins, PCB PBDE and chlorinated pesticides, e.g. DDT) based on Swedish market basket data, Food Chem. Toxicol 44 (2006) 1597–1606. [DOI] [PubMed] [Google Scholar]
- [83].Bruner-Tran KL, Ding T, Yeoman KB, Archibong A, Arosh JA, Osteen KG, Developmental exposure of mice to dioxin promotes transgenerational testicular inflammation and an increased risk of preterm birth in unexposed mating partners, PLOS ONE 9 (2014) e105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sanabria M, Cucielo MS, Guerra MT, Dos Santos Borges C, Banzato TP, Perobelli JE, et al. , Sperm quality and fertility in rats after prenatal exposure to low doses of TCDD: A three-generation study, Reprod. Toxicol 65 (2016) 29–38. [DOI] [PubMed] [Google Scholar]
- [85].Olsvik PA, Williams TD, Tung HS, Mirbahai L, Sanden M, Skjaerven KH, et al. , Impacts of TCDD and MeHg on DNA methylation in zebrafish (Danio rerio) across two generations, Comp. Biochem. Physiol. C: Toxicol. Pharmacol 165 (2014) 17–27. [DOI] [PubMed] [Google Scholar]
- [86].Baker TR, King-Heiden TC, Peterson RE, Heideman W, Dioxin induction of transgenerational inheritance of disease in zebrafish, Mol. Cell. Endocrinol (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Baker TR, Peterson RE, Heideman W, Using zebrafish as a model system for studying the transgenerational effects of dioxin, Toxicol. Sci 138 (2014) 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lane N, Dean W, Erhardt S, Hajkova P, Surani A, Walter J, et al. , Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse, Genesis 35 (2003) 88–93. [DOI] [PubMed] [Google Scholar]
- [89].Chapin RE, Robbins WA, Schieve LA, Sweeney AM, Tabacova SA, Tomashek KM, Off to a good start: the influence of pre- and periconceptional exposures, parental fertility, and nutrition on children’s health, Environ. Health Perspect 112 (2004) 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wu H, Hauser R, Krawetz SA, Pilsner JR, Environmental susceptibility of the sperm epigenome during windows of male germ cell development, Curr. Environ. Health Rep (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Oakes CC, La Salle S, Smiraglia DJ, Robaire B, Trasler JM, Developmental acquisition of genome-wide DNA methylation occurs prior to meiosis in male germ cells, Dev. Biol 307 (2007) 368–379. [DOI] [PubMed] [Google Scholar]
- [92].Loukinov DI, Pugacheva E, Vatolin S, Pack SD, Moon H, Chernukhin I, et al. , BORIS, a novel male germ-line-specific protein associated with epigenetic reprogramming events, shares the same 11-zinc-finger domain with CTCF, the insulator protein involved in reading imprinting marks in the soma, Proc. Natl. Acad. Sci. U. S. A 99 (2002) 6806–6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, et al. , Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals, Cell 143 (2010) 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wei Y, Yang CR, Wei YP, Zhao ZA, Hou Y, Schatten H, et al. , Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 1873–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lambrot R, Xu C, Saint-Phar S, Chountalos G, Cohen T, Paquet M, et al. , Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes, Nat. Commun 4 (2013) 2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR, Distinctive chromatin in human sperm packages genes for embryo development, Nature 460 (2009) 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Brykczynska U, Hisano M, Erkek S, Ramos L, Oakeley EJ, Roloff TC, et al. , Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa, Nat. Struct. Mol. Biol 17 (2010) 679–687. [DOI] [PubMed] [Google Scholar]
- [98].Oliva R, Protamines and male infertility, Hum. Reprod. Update 12 (2006) 417–435. [DOI] [PubMed] [Google Scholar]
- [99].Hammoud SS, Nix DA, Hammoud AO, Gibson M, Cairns BR, Carrell DT, Genome-wide analysis identifies changes in histone retention and epigenetic modifications at developmental and imprinted gene loci in the sperm of infertile men, Hum. Reprod 26 (2011) 2558–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Arpanahi A, Brinkworth M, Iles D, Krawetz SA, Paradowska A, Platts AE, et al. , Endonuclease-sensitive regions of human spermatozoal chromatin are highly enriched in promoter and CTCF binding sequences, Genome Res 19 (2009) 1338–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wykes SM, Krawetz SA, The structural organization of sperm chromatin, J. Biol. Chem 278 (2003) 29471–29477. [DOI] [PubMed] [Google Scholar]
- [102].Samans B, Yang Y, Krebs S, Sarode GV, Blum H, Reichenbach M, et al. , Uniformity of nucleosome preservation pattern in Mammalian sperm and its connection to repetitive DNA elements, Dev. Cell 30 (2014) 23–35. [DOI] [PubMed] [Google Scholar]
- [103].Carone BR, Hung JH, Hainer SJ, Chou MT, Carone DM, Weng Z, et al. , High-resolution mapping of chromatin packaging in mouse embryonic stem cells and sperm, Dev. Cell 30 (2014) 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Royo H, Stadler MB, Peters AH, Alternative computational analysis shows no evidence for nucleosome enrichment at repetitive sequences in mammalian spermatozoa, Dev. Cell 37 (2016) 98–104. [DOI] [PubMed] [Google Scholar]
- [105].Saitou M, Kurimoto K, Paternal nucleosomes: are they retained in developmental promoters or gene deserts? Dev. Cell 30 (2014) 6–8. [DOI] [PubMed] [Google Scholar]
- [106].Ost A, Lempradl A, Casas E, Weigert M, Tiko T, Deniz M, et al. , Paternal diet defines offspring chromatin state and intergenerational obesity, Cell 159 (2014) 1352–1364. [DOI] [PubMed] [Google Scholar]
- [107].Cornwall GA, Role of posttranslational protein modifications in epididymal sperm maturation and extracellular quality control, Adv. Exp. Med. Biol 759 (2014) 159–180. [DOI] [PubMed] [Google Scholar]
- [108].Dacheux JL, Dacheux F, New insights into epididymal function in relation to sperm maturation, Reproduction 147 (2013) R27–R42. [DOI] [PubMed] [Google Scholar]
- [109].Sullivan R, Frenette G, Girouard J, Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit, Asian J. Androl 9 (2007) 483–491. [DOI] [PubMed] [Google Scholar]
- [110].Cossetti C, Lugini L, Astrologo L, Saggio I, Fais S, Spadafora C, Soma-to-germline transmission of RNA in mice xenografted with human tumour cells: possible transport by exosomes, PLoS One 9 (2014) e101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Johnson GD, Mackie P, Jodar M, Moskovtsev S, Krawetz SA, Chromatin and extracellular vesicle associated sperm RNAs, Nucleic Acids Res. 43 (2015) 6847–6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Nixon B, Stanger SJ, Mihalas BP, Reilly JN, Anderson AL, Tyagi S, et al. , The microRNA signature of mouse spermatozoa is substantially modified during epididymal maturation, Biol. Reprod 93 (2015) 91. [DOI] [PubMed] [Google Scholar]
- [113].Fullston T, Ohlsson Teague EM, Palmer NO, DeBlasio MJ, Mitchell M, Corbett M, et al. , Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content, FASEB J. 27 (2013) 4226–4243. [DOI] [PubMed] [Google Scholar]