Abstract
Aim
Hormonal and nutritional disorders are the main causes of obesity and nonalcoholic fatty liver disease, especially in the elderly and postmenopausal women. Although physical activity may alleviate these disorders, the elderly may often have difficulty in conducting physical exercise. The purpose of this study was to investigate the therapeutic effect of knee loading, a new form of physical stimulation, on the symptom of obesity and fatty liver.
Methods
Using ovariectomized mice with high fat diet, we evaluated the effect of knee loading that applies gentle cyclic loads to the knee. Female C57BL/6 mice were divided into five groups: control (SCD), high fat diet (HF), HF with loading (HF+L), HF with ovariectomy (HF+OVX), and HF+OVX with loading (HF+OVX+L). Except for SCD, mice underwent sham operation or ovariectomy and maintained on high fat diet. After 6 weeks, the mice in HF+L and HF+OVX+L were treated with 6-week knee loading.
Results
Compared to the obesity groups (HF and HF+OVX), knee loading significantly decreased a gain in body weight, liver weight, and white adipose tissue (all P<0.01). It also reduced the lipid level in the serum (P<0.01) and histological severity of hepatic steatosis (P<0.01). Furthermore, knee loading downregulated biomarkers related to the endoplasmic reticulum stress (GRP78, p-eIF2α and ATF4) and altered biomarkers in autophagy (LC3 and p62).
Conclusions
Knee loading suppressed obesity-associated metabolic alterations and hepatic steatosis, the effect with knee loading might be associated with suppression of the ER stress and promotion of autophagy.
Keywords: Obesity, nonalcoholic fatty liver disease, endoplasmic reticulum stress, autophagy, knee loading
INTRODUCTION
According to the World Health Organization, obesity is a leading, preventable cause of death worldwide, with ~ 2 billion overweight adults and ~ 600 million adults suffering from obesity.1 It is generally accompanied by a series of systemic diseases, such as coronary heart disease, hypertension, type 2 diabetes, hyperlipidemia, and nonalcoholic fatty liver disease (NAFLD).2 In particular, hepatic steatosis, the early symptom of NAFLD,3 is characterized by excess accumulation of lipids in the liver and is the most common chronic liver disease.4, 5 Postmenopausal women are more susceptible than premenopausal women, especially in the intra-abdominal depot, and they are sensitive to obesity-related metabolic disorder.6 Estrogen is a crucial hormone in resistance to obesity and progression of NAFLD in women.7, 8 Animal experiments have also shown that ovariectomized (OVX) animals have high body weight and adipose tissue mass.9 In this study, we employed high fat diet (HFD)-induced obese model with and without estrogen depletion and investigated the role of physical stimulation in relieving pathological symptoms associated with obesity.
Physical activities are recognized as an adjuvant therapy to reduce body weight,10 improve the fitness of the heart, lung, and overall quality of life.11 It is important to note that the elderly or physically handicapped individuals may have difficulty in fully receiving benefits from physical activities, and excessive activities may lead to a risk of muscle damage and bone fracture. In this study, we examined the effect of knee loading, a joint loading modality, which applies dynamic lateral loads to the knee as a supportive substitute for physical activities.12, 13 In our previous studies, we have shown that knee loading is effective in preventing tissue degeneration in osteoarthritis, bone loss in femoral head necrosis, and enhancing healing of bone fracture.14, 15 We have also shown that knee loading is able to reduce the number of adipocytes in the bone marrow cavity in skeletal diseases. Hence, we addressed a question in this study whether gentle loads dynamically applied to the knee can ameliorate obesity and hepatic steatosis.
Skeletal muscle is one of the largest organs in the body and it has been recognized as an endocrine organ. Its contraction stimulates the production and secretion of cytokines, which are collectively known as myokines.16 Existing studies show that myokine is induced by physical activity,17, 18 and the most well-known exercise-induced myokine is interleukin (IL)-6 that was identified in the serum in response to muscle contraction.19 Contraction of skeletal muscle may stimulate molecular signaling, and it may directly or indirectly regulate whole-body glucose metabolism and lipolysis.20, 21 Herein, we hypothesized that the therapeutic effect of knee loading on obesity and fatty liver is induced by contraction of skeletal muscle that promotes IL-6 secretion and improves lipid metabolism.
It is reported that HFD provokes stress to the endoplasmic reticulum (ER).22 The ER is an organelle that plays an important role in protein folding, calcium homeostasis, and cell survival. Accumulation of lipids in the liver deteriorates ER’s actions, resulting in the chronic ER stress that interferes with a normal hepatocyte function.23 Particularly, an excess amount of free fatty acids in the liver contributes to the pathogenesis of NAFLD.24, 25 Furthermore, evidences suggest that autophagy is essential in regulating lipid metabolism in liver,26 and abnormal regulation of autophagy develops hepatic steatosis.27, 28 While HFD and OVX may induce obesity and NAFLD, the effect of physical stimulation on obesity and hepatic steatosis is largely unknown in connection with the ER stress and autophagy. In this study, we investigated whether application of knee loading to a mouse model of estrogen depletion and HFD-induced obesity improve fat metabolism, as well as cellular homeostasis involved in the ER stress and autophagy.
METHODS
Animals and study design
Fifty 8-week-old female C57BL/6 mice (body weight of ~18 g) were purchased from Animal Center of Academy of Military Medical Sciences (Beijing, China) and maintained in pathogen-free facilities. The room was maintained at 23–25°C, with 50–60% relative humidity and a 12-h light/dark cycle. Animals were given free access to water and food, and they were handled by one professional person. All procedures were approved by the Ethics Committee of Tianjin Medical University, and carried out according to the National Institutes of Health Guide for Care and Use of Laboratory Animals.
After 1 week of acclimation, mice were randomly assigned into five groups: age-matched control group fed with a standard chow diet (SCD; n=10), HFD group (HF; n=10, D12492, Beijing Huafu Kang Biological Co., Ltd. China), HFD with knee loading (HF+L; n=10), postmenopausal obesity group (HF+OVX; n=10), and postmenopausal obesity with knee loading group (HF+OVX+L; n=10). Two OVX groups (HF+OVX and HF+OVX+L) underwent bilateral ovariectomy, while three sham OVX groups (SCD, HF and HF+L) were subjected to sham surgery. All mice except for SCD were fed with HFD. After 6 weeks of HFD, two groups (HF+L and HF+OVX+L) received 6-week knee loading.
Knee loading
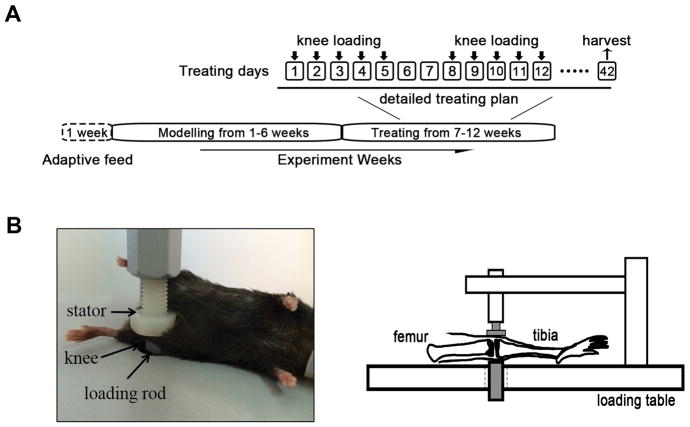
In the loaded groups, mice were mask-anesthetized using 1.5% isoflurane and received loads to both knees in the lateral-medial direction with a custom-made mechanical loader. Loads were 1N force at 10Hz and given for 6 min per day for 5 consecutive days every week (Fig. 1A). The lateral and medial sides of the tibia and femur were in contact with the loading rod and the stator, respectively (Fig. 1B). Three non-loaded groups (SCD, HF and HF+OVX) were given the sham loading, in which mice were placed on the loading station and given anesthesia, but did not receive any loading stimulus. Animals were sacrificed after 6-week loading.
Figure 1. Experimental timeline and setting of knee loading.
A) After 1-week adaption, ovariectomy was conducted and all mice except for the SCD control mice were fed with HFD for 6 weeks. Then, knee loading was performed for 6 weeks (5 days per week). B) Knee loading apparatus.
Body weight and analysis of body fat composition
The body weight and food intakes of each mouse were measured weekly and daily, respectively, by a person blinded to each group. Body fat content was measured by a whole body composition analyzer (ImpediVet, Australia).
Histological analysis
Liver and periuterine fat specimens were fixed in 10% formaldehyde for 48h and embedded in paraffin or used for frozen slices. Gonadal adipose tissue (periuterine fat) sections were stained with hematoxylin-eosin (HE) for quantifying adipocyte size, adipocyte area, diameter, and perimeter. To analyze hepatic steatosis, the paraffin sections and frozen sections of the liver were stained with HE and Oil Red O staining, respectively, and the fat vacuole area and quantity were measured.29 Liver steatosis was examined by histopathology and was classified as grade 0 (1–5% coverage), grade 1 (6–33%), grade 2 (34–66%), and grade 3 (67–100%).30 For determination of the adipocyte area, images were randomly taken from the fat and liver sections (five high power fields per section at 200×magnification). To analyze liver fibrosis, liver sections were processed with Masson’s trichrome staining. All graphics were performed using Cellsense Standard software in a blinded fashion.
Biochemical analyses
Animals were fasted for 12h with free access to water and sacrificed. Blood samples were collected from the orbital vein. After fasting, the blood glucose, the serum levels of triglycerides (TG) and total cholesterol (TC) were determined with a colorimetric kit (CardioChek, USA). The concentrations of IL-6 and insulin were assessed by mouse IL-6 and insulin enzyme-linked immuno sorbent assays (CCC, USA).
Western blot analysis
For Western blot analysis, liver tissues were lysed in an ice-cold RIPA lysis buffer with protease inhibitors and phosphatase inhibitors (Roche Diagnostics GmbH, Mannheim, Germany), and rolled into a homogenate with a glass homogenizer. Membranes were incubated with primary antibodies of GRP78 (Affinity BioReagents, Suwanee, GA, USA), eIF2α, phospho-eIF2α, ATF4 (Cell Signaling, Danvers, MA, USA), CHOP (CCAAT/enhancer binding protein homologous protein) (Proteintech, Wuhan, China), p62 (MBL, Japan), LC3 (MBL, Japan) and β-actin (Sigma, St Louis, USA). After incubating with secondary IgG antibodies (1:20000) conjugated with horseradish peroxidase, blots were detected with enhanced chemiluminescence. Images were analyzed and quantified using Quantity One software.
Statistical analysis
Data were presented as mean±SEM. Unpaired two-tailed Student’s t test (GraphPad Prism 7.0) was conducted to determine significant difference. The differences among multiple groups were performed using one-way ANOVA. Statistical significance was set at P<0.05.
RESULTS
Knee loading reduced body weight
The body weight of the obese groups (HF and HF+OVX) was significantly higher than the control group in 6 weeks (Fig. 2A). Between the two obese groups, a significant weight increase was observed in HF+OVX. In response to knee loading, two loaded groups (HF+L and HF+OVX+L) exhibited a significant weight loss in comparison with two non-loaded groups (HF and HF+OVX), respectively (Fig. 2B). Of note, a food intake did not change significantly among all groups (Fig. 2C).
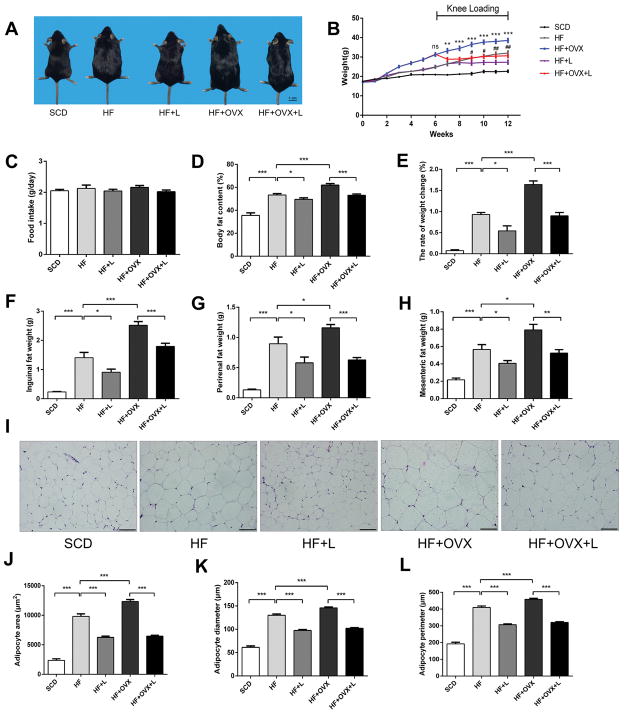
Figure 2. Reduction in body weight and fat mass in obese mice by knee loading.
A) Whole body images. B) Changes in body weight. C) Food intake per day among the five groups. D) Whole body fat content. E) Rate of weight changes. F) Inguinal fat pat weight. G) Perirenal fat pat weight. H) Mesenteric fat weight. I) Representative HE stained periuterine adipose tissue (magnification 200×; and scale bar=100μm). J-K) Measurement of periuterine adipocyte area, diameter, and perimeter. All values were expressed as mean±SEM (n=10 mice per group). Of note, #indicates P<0.05 compared with HF+L, *was used as comparison with HF+OVX+L. The asterisks (*, ** and ***) and pound signs (#, ##, and ###) represent P<0.05, P<0.01, and P<0.001, respectively, and “ns” indicates P>0.05.
We then evaluated the breakdown of weight alterations among tissues. Compared to the non-loaded groups (HF and HF+OVX), the loaded-groups (HF+L and HF+OVX+L) significantly reduced body fats. Because of OVX, fat content was higher in HF+OVX than HF (Fig. 2D). Compared to the non-loaded groups (HF and HF+OVX), knee loading reduced the rate of weight change by 5% in HF+L and 8% in HF+OVX+L, respectively (Fig. 2E).
Knee loading attenuated obesity by reducing mass of adipose tissue
To further evaluate the effect of knee loading on lipid metabolism, we evaluated the adipose tissue (AT: inguinal, perirenal, and mesenteric fats). Compared to SCD, the adipose tissue of HF was increased after 6 weeks. Furthermore, the adipose tissue of HF+OVX was higher than that of HF. In sharp contrast, the adipose tissue was significantly decreased in the loaded groups (HF+L and HF+OVX+L) (Fig. 2F–H). To determine the hypertrophic changes, the adipocyte area, diameter and perimeter were evaluated using HE stained periuterine adipose tissues (Fig.2I). Mice in HF and HF+OVX exhibited larger adipocytes (area, diameter, and perimeter) than those in SCD. In response to knee loading, however, the hypertrophic change in the two non-loaded groups (HF and HF+OVX) was suppressed in the two loaded groups (HF+L and HF+OVX+L) (Fig. 2J–L).
Knee loading attenuated hepatic steatosis and fibrosis
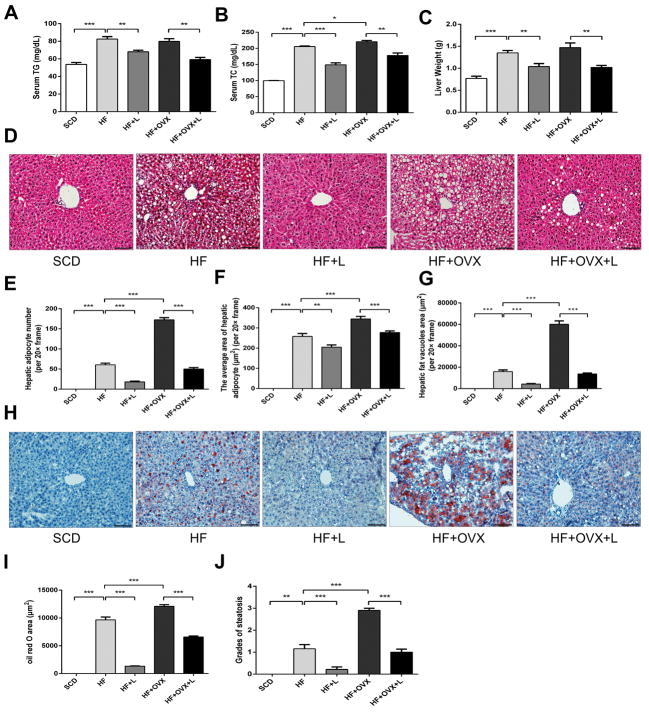
Since it has been known that lipids stored in the adipose tissue mostly originate from circulating triglycerides (TG), the serum levels of TG and total cholesterol (TC) were determined. While the levels of TG and TC in the serum were increased in HF, knee loading markedly reduced those levels in HF+L. The same loading effect was observed between HF+OVX and HF+OVX+L (Fig. 3A and B). Compared to SCD, liver weight was significantly increased in HF and it was further increased in HF+OVX. The increase was suppressed, however, in response to knee loading (Fig. 3C).
Figure 3. Effects of knee loading on hepatic steatosis.
A) Serum triglyceride levels. B) Serum cholesterol levels. C) Liver weight. D) Representative HE-stained sections of liver tissues (magnification 200×; and scale bar=100μm). E-G) Measurement of the hepatic adipocyte number, average area of a hepatic adipocyte, and area of hepatic fat vacuoles. H) Oil Red O stained sections of liver tissue (magnification 200×; and scale bar=100μm). I) Measurement of Oil Red O stained area. J) Grades of steatosis. All values were expressed as mean±SEM (n=10 mice per group). The asterisks (*, ** and ***) represent P<0.05, P<0.01, and P<0.001, respectively.
We next evaluated the effect of knee loading on hepatic steatosis, using histological specimens with HE and Oil Red O staining. Compared to SCD, the marked degeneration of microvesicular and macrovesicular regions was observed in HE-stained sections in HF, revealing the presence of excessive lipid droplets by HFD. While the steatosis was even severer in HF+OVX than HF, knee loading nearly abolished accumulation of intrahepatic fats in the loaded groups (HF+L and HF+OVX+L) (Fig. 3D). Consistent with those observations, mice fed with HFD exhibited more and larger adipocytes than SCD, and knee loading reversed this increase. The same trends were observed in HF+OVX and HF+OVX+L (Fig. 3E–G). While accumulation of lipid droplets was increased in HF and HF+OVX (Fig. 3H), it was significantly decreased in the samples with Oil Red O staining in the loaded groups (Fig. 3I and J).
To further evaluate the effect of knee loading on liver fibrosis, we analyzed liver sections with Masson’s trichrome staining. We detected mild fibrosis in the non-loaded group (HF and HF+OVX), but no fibrosis was detected in HF+L and HF+OVX+L (Fig. 4A).
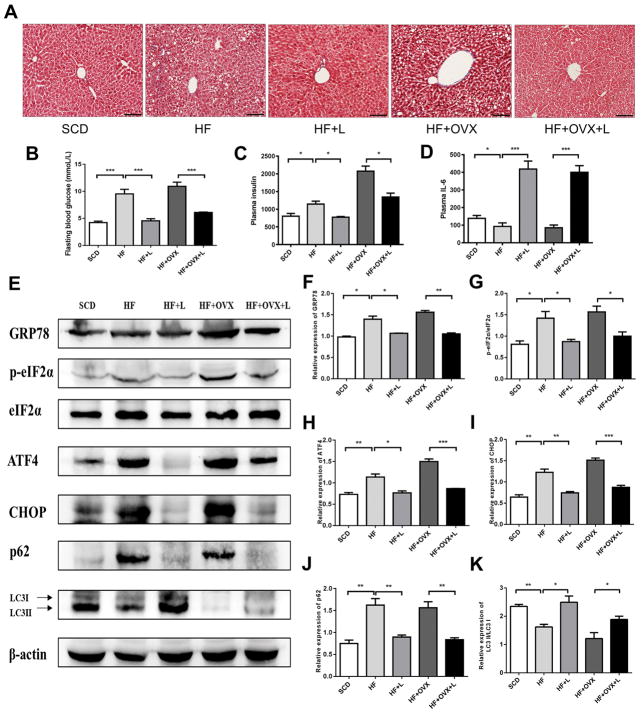
Figure 4. Knee loading-driven inhibition of ER stress and regulation of autophagy in the livers.
A) Masson trichrome stained sections of liver tissue (magnification 200×; and scale bar=100 μm). B) Plasma fasting blood glucose levels. C) Plasma insulin levels. D) Plasma IL-6 levels. E) Representative images of Western blotting in the five groups (n=3 mice per group), for the selected genes involved in the ER stress and autophagy. F-K) Quantified levels of GRP78, p-eIF2α, ATF4, CHOP, p62, LC3II/I. The asterisks (* and **) represent P<0.05, and P<0.01, respectively.
Knee loading improved insulin resistence
To investigate the effect of knee loading on insulin resistance, we determined the levels of plasma insulin and fasting blood glucose. The result showed that insulin and fasting blood glucose levels were markedly increased in the non-loaded groups (HF and HF+OVX), and insulin resistance occurred. After loading treatment, however, we observed that the insulin and blood glucose levels were restored to normal (Fig. 4B and C). Furthermore, the serum IL-6 level was decreased in the non-loaded groups but a significant increase was observed in the loading groups (Fig. 4D).
Knee loading inhibited hepatic ER stress and altered autophagy
To investigate the role of ER stress and autophagy in response to knee loading on hepatic steatosis, the levels of biomarkers involved in the ER stress and autophagy were evaluated (Fig. 4E). In the non-loaded groups (HF and HF+OVX), an increase in the levels of GRP78, p-eIF2α, ATF4 and CHOP was observed. These markers are linked to the stress to the hepatic ER. In contrast, the observed increase was significantly suppressed by knee loading (Fig. 4F–I). Furthermore, in obese mice the levels of two autophagy-linked proteins p62 and LC3II/I were increased and decreased, respectively. After 6 weeks of knee loading, however, those levels were reversed in which the level of p62 was decreased (Fig. 4J) and that of LC3II/I was increased (Fig. 4K).
Correlation analysis
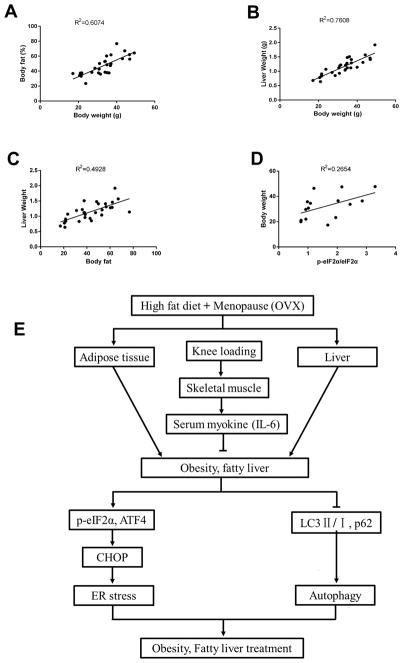
To summarize the effect of knee loading on obesity and hepatic steatosis, correlational analysis was conducted. The body weight was positively correlated with body fat and liver weight (R2=0.61, P<0.001; and R2=0.76, P<0.001, respectively). The body fat was positively correlated with liver weight (R2=0.49, P<0.001), while the level of p-eIF2α was positively correlated with body weight (R2=0.27, P<0.05) (Fig. 5A–D).
Figure 5. Correlation analysis.
A) Correlations between the body weight and body fat. B) Correlations between the body weight and liver weight. C) Correlations between the body fat and liver weight. D) Correlations between the level of p-eIF2α and body weight. E) Proposed mechanism of knee loading for obesity and NAFLD.
DISCUSSION
The main causes of obesity in the elderly include high calorie intake, lack of physical activity, and postmenopausal hormonal changes. In this study, two obese groups (HF and OVX) presented varying degrees of symptoms associated with obesity and hepatic steatosis, and those pathological features were severer in HF+OVX (combined high fat diet and ovariectomy) than HF (high fat diet alone). While depletion of estrogen in postmenopausal women increases a risk of visceral obesity and metabolic disease, current pharmacological options are not sufficient.22 In this study, we demonstrated that knee loading markedly suppressed development of HF/OVX-induced obesity, with a significant reduction in body weight, fat content, and adipocyte size. These results suggest that physical stimulation such as knee loading may provide an adjuvant therapy for alleviating obesity-associated metabolic disorders.
NAFLD is the most common liver disease worldwide and one of the most serious complications of obesity.31, 32 Although hepatic steatosis is generally considered benign, it can have serious consequences, such as fibrosis, which is likely to progress to cirrhosis and liver failure, or even hepatocellular carcinoma. As an important metabolic organ, the liver is sensitively responsive to and damaged by overweight. NAFLD is caused by an increase in de novo synthetic fatty acids, followed by esterification of fatty acids to TG.33, 34 A high concentration of TG generally leads to the accumulation of TC,35, 36 and this linkage is consistent with our result, showing that HF- and OVX-induced obesity increased liver weight as well as the serum levels of TG and TC. Histological analysis revealed that HF led to the accumulation of lipid droplets and OVX further aggravated the pathological manifestations. By contrast, knee loading significantly suppressed development of NAFLD and reduced deposition of intrahepatic fats. We observed that HF and HF+OVX led to hypertriglyceridemia, hepatic steatosis and mild fibrosis, and the pathological changes were pronounced in HF+OVX. Knee loading prevented hepatic steatosis from HFD and estrogen deficiency, suggesting that this physical stimulation may potentially provide a novel strategy to alleviate abnormal lipid metabolism in postmenopausal women.
Many lines of evidence have shown that the ER stress played a crucial role in obesity, as well as in NAFLD progression.37 Sustained induction of chronic stress in the fatty liver resulted in an increase in de novo lipogenesis and fat accumulation.22 To examine whether knee loading provided a curative effect via the ER stress pathway in the liver, we evaluated the levels of ER stress-linked biomarkers in a PERK (protein kinase RNA-like ER kinase)-ATF4 axis. In the non-loaded groups (HF and OVX), the level of CHOP was significantly increased, together with GRP78, p-eIF2α, and ATF4, suggesting that a long-term high fat diet may result in chronic ER stress. However, the levels of these proteins were significantly reduced by knee loading. We also analyzed correlation among the body weight, body fat, liver weight, and the ER stress. The relationship presented a strong positive correlation among those values. These results indicate that ER stress is involved in the progression of obesity and hepatic steatosis as well as the suppression by knee loading. Our observation is consistent with the notion that knee loading suppresses the ER stress in liver and alleviates postmenopausal obesity and hepatic steatosis.
Prolonged ER stress induces autophagy, since the unfolded protein responses (UPR) with the ER stress may facilitate formation of an autophagosome by promoting the expression of autophagy-related proteins.38 Some studies have shown, however, that expression of an autophagy-associated protein, ATG7, is suppressed in the liver in the late stage of obesity.39 It is thus worthwhile to examine whether autophagy is involved in the response to UPR caused by the ER stress. It is reported that inhibition of ER stress and promotion of autophagy may improve obesity and hepatic steatosis.26, 40, 41 We have shown that expression of autophagy markers was significantly altered in the non-loaded obese groups (HF and OVX), while knee loading partially reversed this obesity-induced alteration of autophagy. Autophagy is a dynamic process and depending on the timing of protein detection its markers can be positively or negatively regulated. The result indicates that knee loading alleviates obesity and protects the liver from excessive lipids by suppression of the ER stress and alteration of autophagy (Fig. 5E). Further studies are needed to evaluate the molecular mechanism of knee loading’s action on lipid metabolism.
The therapeutic effects of knee loading on obesity and NAFLD might be caused by contraction of skeletal muscle followed by secretion of myokines such as IL-6.42 Liver is a main insulin-sensitive organ, which is responsible for glucose production, and an impaired insulin function may promote fat accumulation, leading to obesity.43 Muscle-derived IL-6 might improve glucose and lipid metabolism as well as insulin signaling.20, 21 In fact, our data showed that knee loading elevated muscle-derived IL-6 levels and improved insulin resistance, suggesting that IL-6 acts as a mediator of communication between skeletal muscle and the liver.44, 45 Furthermore, the beneficial effects of IL-6 elevation might be linked to AMPK activity.46 Activation of AMPK enhances glucose uptake and fatty acid oxidation in skeletal muscle, and it inhibits glucose production in the liver.47 AMPK activation reportedly reduces ER stress and rescues beta cells.48 Further studies might clarify the crosstalk between bone/muscle and liver at the molecular level.
In summary, we demonstrated herein that knee loading suppressed the progression of obesity and hepatic steatosis in the obese mouse model, by reducing fat weight and inhibiting adipocyte hypertrophy. Furthermore, the present study indicates a possible mechanism with knee loading in which induction of hepatic steatosis from the HFD and estrogen deficiency can be suppressed by load-driven inhibition of the ER stress and alteration of autophagy in the liver.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81572100 and 81772405 to PZ; 81601863 to XL), Tianjin Binhai New Area Health and Family Planning Commission (2016BWKL002 to PZ), and NIH (AR052144 to HY).
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
NT, XL and PZ designed research; NT, LZ, DL and JL conducted research; XL, HY and PZ analyzed the data; NT, XL and PZ wrote the manuscript. NT, XL, HY and PZ approved the final manuscript as submitted. PZ accepted responsibility for integrity of data analysis.
References
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang CC, Tseng TL, Huang WC, Chung YH, Chuang HL, Wu JH. Whole-body vibration training effect on physical performance and obesity in mice. Int J Med Sci. 2014;11:1218–27. doi: 10.7150/ijms.9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moscatiello S, Di Luzio R, Sasdelli AS, Marchesini G. Managing the combination of nonalcoholic fatty liver disease and metabolic syndrome. Expert Opin Pharmacother. 2011;12:2657–72. doi: 10.1517/14656566.2011.629188. [DOI] [PubMed] [Google Scholar]
- 4.Rinella M, Charlton M. The globalization of nonalcoholic fatty liver disease: Prevalence and impact on world health. Hepatology. 2016;64:19–22. doi: 10.1002/hep.28524. [DOI] [PubMed] [Google Scholar]
- 5.Setiawan VW, Stram DO, Porcel J, Lu SC, Le Marchand L, Noureddin M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: The multiethnic cohort. Hepatology. 2016;64:1969–77. doi: 10.1002/hep.28677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiol Behav. 2009;97:199–204. doi: 10.1016/j.physbeh.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med (Maywood) 2004;229:1127–35. doi: 10.1177/153537020422901107. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki A, Abdelmalek MF. Nonalcoholic fatty liver disease in women. Womens Health (Lond) 2009;5:191–203. doi: 10.2217/17455057.5.2.191. [DOI] [PubMed] [Google Scholar]
- 9.Lee H, Choi J, Shin SS, Yoon M. Effects of Korean red ginseng (Panax ginseng) on obesity and adipose inflammation in ovariectomized mice. J Ethnopharmacol. 2016;178:229–37. doi: 10.1016/j.jep.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Trottier SK, MacPherson REK, Knuth CM, et al. Dairy attenuates weight gain to a similar extent as exercise in rats fed a high-fat, high-sugar diet. Obesity (Silver Spring) 2017;25:1707–15. doi: 10.1002/oby.21941. [DOI] [PubMed] [Google Scholar]
- 11.Wolfarth B, Rankinen T, Hagberg JM, et al. Advances in exercise, fitness, and performance genomics in 2013. Med Sci Sports Exerc. 2014;46:851–9. doi: 10.1249/MSS.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 12.Zhang P, Yokota H. Knee loading stimulates healing of mouse bone wounds in a femur neck. Bone. 2011;49:867–72. doi: 10.1016/j.bone.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang P, Tanaka SM, Jiang H, Su M, Yokota H. Diaphyseal bone formation in murine tibiae in response to knee loading. J Appl Physiol (1985) 2006;100:1452–9. doi: 10.1152/japplphysiol.00997.2005. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Yang J, Liu D, et al. Knee loading inhibits osteoclast lineage in a mouse model of osteoarthritis. Sci Rep. 2016;6:24668. doi: 10.1038/srep24668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D, Li X, Li J, Yang J, Yokota H, Zhang P. Knee loading protects against osteonecrosis of the femoral head by enhancing vessel remodeling and bone healing. Bone. 2015;81:620–31. doi: 10.1016/j.bone.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev. 2005;33:114–9. doi: 10.1097/00003677-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Yoon JH, Yea K, Kim J, et al. Comparative proteomic analysis of the insulin-induced L6 myotube secretome. Proteomics. 2009;9:51–60. doi: 10.1002/pmic.200800187. [DOI] [PubMed] [Google Scholar]
- 18.Henningsen J, Rigbolt KT, Blagoev B, Pedersen BK, Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteomics. 2010;9:2482–96. doi: 10.1074/mcp.M110.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 20.Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes. 2004;53:1643–8. doi: 10.2337/diabetes.53.7.1643. [DOI] [PubMed] [Google Scholar]
- 21.Wallenius V, Wallenius K, Ahren B, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–9. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 22.Baiceanu A, Mesdom P, Lagouge M, Foufelle F. Endoplasmic reticulum proteostasis in hepatic steatosis. Nat Rev Endocrinol. 2016;12:710–22. doi: 10.1038/nrendo.2016.124. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki T, Gao J, Ishigaki Y, et al. ER stress protein CHOP mediates insulin resistance by modulating adipose tissue macrophage polarity. Cell Rep. 2017;18:2045–57. doi: 10.1016/j.celrep.2017.01.076. [DOI] [PubMed] [Google Scholar]
- 24.Zheng X, Xu F, Liang H, et al. SIRT1/HSF1/HSP pathway is essential for exenatide-alleviated, lipid-induced hepatic endoplasmic reticulum stress. Hepatology. 2017;66:809–24. doi: 10.1002/hep.29238. [DOI] [PubMed] [Google Scholar]
- 25.Leamy AK, Egnatchik RA, Young JD. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res. 2013;52:165–74. doi: 10.1016/j.plipres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li R, Guo E, Yang J, et al. 1,25(OH)2D3 attenuates hepatic steatosis by inducing autophagy in mice. Obesity (Silver Spring) 2017;25:561–71. doi: 10.1002/oby.21757. [DOI] [PubMed] [Google Scholar]
- 27.Rautou PE, Mansouri A, Lebrec D, Durand F, Valla D, Moreau R. Autophagy in liver diseases. J Hepatol. 2010;53:1123–34. doi: 10.1016/j.jhep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Singh R, Cuervo AM. Lipophagy: connecting autophagy and lipid metabolism. Int J Cell Biol. 2012;2012:282041. doi: 10.1155/2012/282041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel VS, Chan ME, Pagnotti GM, Frechette DM, Rubin J, Rubin CT. Incorporating refractory period in mechanical stimulation mitigates obesity-induced adipose tissue dysfunction in adult mice. Obesity (Silver Spring) 2017;25:1745–53. doi: 10.1002/oby.21958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chitturi S, Farrell GC, Hashimoto E, et al. Non-alcoholic fatty liver disease in the Asia-Pacific region: definitions and overview of proposed guidelines. J Gastroenterol Hepatol. 2007;22:778–87. doi: 10.1111/j.1440-1746.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- 31.Brunt EM. Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:195–203. doi: 10.1038/nrgastro.2010.21. [DOI] [PubMed] [Google Scholar]
- 32.Rutkowski DT, Wu J, Back SH, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–40. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–85. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 34.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 36.Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30:734–43. doi: 10.2337/dc06-1539. [DOI] [PubMed] [Google Scholar]
- 37.Amir M, Czaja MJ. Autophagy in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. 2011;5:159–66. doi: 10.1586/egh.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deegan S, Koryga I, Glynn SA, Gupta S, Gorman AM, Samali A. A close connection between the PERK and IRE arms of the UPR and the transcriptional regulation of autophagy. Biochem Biophys Res Commun. 2015;456:305–11. doi: 10.1016/j.bbrc.2014.11.076. [DOI] [PubMed] [Google Scholar]
- 39.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–78. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong K, Li H, Zhang M, et al. Endoplasmic reticulum stress induces up-regulation of hepatic beta-Klotho expression through ATF4 signaling pathway. Biochem Biophys Res Commun. 2015;459:300–5. doi: 10.1016/j.bbrc.2015.02.104. [DOI] [PubMed] [Google Scholar]
- 41.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–32. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol (1985) 2007;103:1093–8. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 43.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–81. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–65. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 45.Weigert C, Lehmann R, Hartwig S, Lehr S. The secretome of the working human skeletal muscle--a promising opportunity to combat the metabolic disaster? Proteomics Clin Appl. 2014;8:5–18. doi: 10.1002/prca.201300094. [DOI] [PubMed] [Google Scholar]
- 46.Knudsen JG, Bertholdt L, Joensen E, Lassen SB, Hidalgo J, Pilegaard H. Skeletal muscle interleukin-6 regulates metabolic factors in iWAT during HFD and exercise training. Obesity (Silver Spring) 2015;23:1616–24. doi: 10.1002/oby.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomas E, Tsao TS, Saha AK, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–13. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nyblom HK, Sargsyan E, Bergsten P. AMP-activated protein kinase agonist dose dependently improves function and reduces apoptosis in glucotoxic beta-cells without changing triglyceride levels. J Mol Endocrinol. 2008;41:187–94. doi: 10.1677/JME-08-0006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.