Abstract
Mammography, as the primary screening modality, has facilitated a substantial decrease in breast cancer-related mortality in the general population. However, the sensitivity of mammography for breast cancer detection is decreased in women with higher breast densities, which is an independent risk factor for breast cancer. With increasing public awareness of the implications of a high breast density, there is an increasing demand for supplemental screening in these patients. Yet, improvements in breast cancer detection with supplemental screening methods come at the expense of increased false-positives, recall rates, patient anxiety, and costs. Therefore, breast cancer screening practice must change from a general one-size-fits-all approach to a more personalized, risk-based one that is tailored to the individual woman’s risk, personal beliefs, and preferences, while accounting for cost, potential harm, and benefits.
This overview will provide an overview of the available breast density assessment modalities, the current breast density screening recommendations for women at average risk of breast cancer, and supplemental methods for breast cancer screening. In addition, we will provide a look at the possibilities for a risk-adapted breast cancer screening.
Keywords: Screening, breast density, breast, mammography, ultrasound, MRI
Introduction
Breast cancer is the most common female cancer and the second leading cause of cancer death, with one in 37 women dying from the disease (1). According to the American Cancer Society, the incidence of breast cancer was estimated to be about 252,710 new cases of invasive breast cancer and 63,410 new cases of non-invasive (in situ) breast cancer in 2017 (1). Population-based screening programs using mammography have been implemented to detect breast cancer at an early stage and, consequently, have contributed to the reduction in mortality (2,3). Although there is agreement that full-field digital mammography (FFDM) is the current screening examination of choice, there is currently no broad consensus on the age at which to start screening or what screening intervals should be, with different recommendations being issued by different national breast cancer screening programs. In general, screening mammography has a good sensitivity and specificity of 81–87% and 98–92%, respectively, for breast cancer diagnosis in women aged 40–79 years, and a breast cancer detection rate of 4–5 breast cancers per 1000 examinations (4). However, it has been shown that screening performance is influenced by age at screening, as well as mammographic breast density. There is a strong body of evidence that screening mammography is less sensitive in women aged 40–49 years, and/ or with heterogeneous or extremely dense breast tissue, thus limiting the applicability and usefulness of screening in those groups (4). The decreased sensitivity is caused by the so-called “masking effect” of breast cancer, which is caused by an overlap with normal breast tissue and is most pronounced in extremely dense breast parenchyma (5–8). Although the “masking effect” is an important contributor to decreased screening performance, it should be noted that breast density has also been identified as a strong and independent risk factor for the subsequent development of breast cancer (9–13). The association between increased density and cancer risk opens new avenues for risk prediction and stratification, as well as the development of tailored breast-screening strategies. This overview will provide an overview of the available breast density assessment modalities, the current breast-density screening recommendations for women at average risk of breast cancer, and supplemental methods for a tailored breast cancer screening. In addition, we will provide a look at a risk-adapted breast cancer screening.
Breast density
Breast density, or the amount of fibroglandular tissue in the breast, is defined as the relationship of fat to epithelial and connective tissue by the total breast area. On mammography, fatty components appear radiolucent, whereas fibroglandular components, consisting of epithelial and stromal tissue, appear radiopaque. A wide variability of breast tissue composition exists among women, which is also subject to change during life and is influenced by hormonal fluctuations during the monthly cycle (14–17). According to the American College of Radiology (ACR), 50% of women in the United States belong to the high density group, with 40% being categorized as heterogeneously dense (ACR category C) and 10% as extremely dense (ACR category D) (6). Epidemiological studies have shown global disparity between different ethnic groups, with Caucasian and black women showing the highest incidence rates of breast cancer and an almost similar breast cancer occurrence (18). Asian women historically have had a lower incidence of the disease (19), but with increased adaptation to Western ways of living, the incidence has been rising constantly (20). In contrast to the lower breast cancer risk of the Asian population, studies have found an approximately 2–4% higher percentage grade of breast density compared to Caucasian women (21–23). Recent data from a comparative analysis showed that due to population differences in body height, weight, and parity, postmenopausal Asian women showed lower density volumes of 3.0 cm3 compared to postmenopausal Caucasian women (24). In addition to the age and hormonal changes during the menstrual cycle, breast density is also significantly influenced by body mass index (BMI) and number of childbirths. In a large twin study, Nguyen et al. showed that the number of childbirths was associated with a decreased mammographic breast density and corresponded to a breast cancer risk reduction of 4% per live birth (25). In another recently published study, childbirth status as well as elevated BMI showed a connection with lower breast density, regardless of age (26). The increase in weight and BMI through menopause due to fat storage in the breast and an associated decrease in breast density has also been observed by Wanders et al. (27). However, the decrease in breast density caused by weight gain seems to be contradictory to the observation of a higher breast cancer risk with a higher BMI in postmenopausal women (13,28). Hopper et al. demonstrated, in a longitudinal prospective study, a negative association between breast density at the ages of 47–50 years compared to BMI measured at the ages of 7–15 years and concluded that adolescent BMI is negatively associated with breast cancer risk (29), which is in line with other published data (30,31). In addition, lower BMI values or a moderate reduction of body weight resulted in postmenopausal breast cancer risk reduction up to 50% (32,33). Conclusive data have shown that an increased breast density is a strong independent imaging biomarker for increased breast cancer risk (10,12,34,35). After age and genetic factors, such as BRCA status, a linear increase in breast density, which means that women with a Breast Imaging Reporting and Data System (BI-RADS) category of D are associated with a four- to sixfold increase in the risk of breast cancer compared to women in the lowest density group, BI-RADS A (36). Health authorities have recognized the relevance and impact of breast density in screening and, in some countries, supplemental screening methods for women with dense breasts (ACR categories C and D) have been introduced (37–39). In this context, it also stands to reason that breast density may be used for individual risk assessment and tailored screening strategies (10,12,34,35).
Breast density assessment
Subjective qualitative assessment
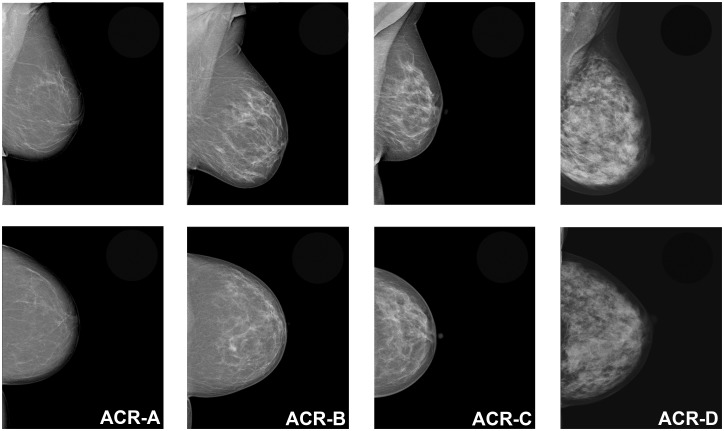
The assessment of breast density is usually performed based on the mammographic appearance of the amount of fibroglandular tissue relative to fatty tissue on mammography. There is, at the moment, no recommendation or criteria for standardized breast density assessment (40,41). Various methods of breast density classification exist, ranging from the early classification systems of Wolfe (42) and Tabár (43) to the most commonly used BI-RADS classification of the ACR (6). The BI-RADS lexicon classifies breast density on mammography according to four categories, which are mainly assessed qualitatively by subjective visual estimation of the reporting radiologist. The current fifth edition of the BI-RADS atlas, which was updated in 2013, defines the four breast density categories as: ACR-A = the breasts are almost entirely fatty; ACR-B = there are scattered areas of fibroglandular density; ACR-C = the breasts are heterogeneously dense, which may obscure small masses; and ACR-D = the breasts are extremely dense, which lowers the sensitivity of mammography (6). Women classified as either ACR-A or -B are considered to have non-dense breasts, whereas women with ACR-C or -D are considered to have dense breasts. The fifth edition of the BI-RADS lexicon saw a change from a percentage categorization of total breast density to descriptive categories and identification of coalescent areas on the mammogram, acknowledging the possible masking of underlying breast masses (Fig. 1) and the potential benefit of supplemental screening (44). Several studies showed that this subjective visual estimation of breast density on mammography is prone to error, with great inter- and intra-observer variability (45–48). Although it has been demonstrated that training and experience can improve inter- and intra-observer variability (47,49,50), it is evident that subjective qualitative breast density assessment is not equipped to provide a reliable and reproducible objective assessment of breast density as a risk factor.
Fig. 1.
Image example of the four descriptive breast density categories for mammography defined by the fifth edition of the ACR BI-RADS atlas: ACR-A = the breasts are almost entirely fatty; ACR-B = there are scattered areas of fibroglandular density; ACR-C = the breasts are heterogeneously dense, which may obscure small masses; and ACR-D = the breasts are extremely dense, which lowers the sensitivity of mammography.
Objective automated quantitative assessment
To overcome this limitation, efforts have been made to develop automated quantitative technologies for breast density measurement. There are computer-aided semi-automated and fully automated measurement approaches that allow either a two-dimensional (2D) or three-dimensional (3D) assessment of breast tissue structures. Cumulus™, the so-called gold standard of mammographic assessment of breast density, which has been validated by epidemiological studies, allows the estimation of the percentage area of dense breast tissue from mammographic images (51)—with higher reproducibility—compared to BI-RADS visual assessment (36). The limitation of Cumulus™ is that breast density measurements are derived from 2D mammography and this method still requires some user interaction, which renders it prone to bias. Recently, other 3D mammography-based breast density measurement systems have become available. Highnam (52) and van Engeland (53) introduced fully automated approaches, Quantra (46,54,55) and Volpara (46,48,54,55), which allow mammography-based, volumetric, quantitative breast density measurements. However, although these approaches are fully automated, breast density calculations based on mammography may vary due to differences in tissue compression and breast positioning (56). What all these assessment methods have in common is a positively association between breast cancer risk and breast density (57,58). However, data from Kontos et al. (59) discussed how the differences in quantitative breast density measurements are influenced by processed or raw mammographic images, as well as specific features of image acquisition, physical properties, and vendors.
Breast cancer screening
Breast cancer screening using mammography takes a central position in early breast cancer detection and mortality reduction (60). An effective breast cancer mortality reduction, as much as 20%, has been found during follow-up periods of up to 20 years in large, randomized controlled trials (RCTs) (61,62). However, it is not clear, as yet, to what extend mammography screening or improvements in treatment have provided a major contribution that would have helped to significantly reduce breast cancer-related mortality. Different screening recommendations, with respect to age at screening start and appropriate intervals for screening, have led to confusion for patients, as well as physicians, and have increased the demand for a tailored, risk-adapted screening strategy. The ACR and the Society of Breast Imaging (SBI) recommends annual mammographic screening starting at the age of 40 years (63). This is in good agreement with the latest guidelines of the American Cancer Society (ACS) from 2015, with a reaffirmation of mammography as a life-saving measure (64). According to the ACS, women at an average risk of breast cancer may start screening with annual mammography at the age of 40 years. For women aged 45–54 years, an annual mammography is recommended, and for women aged > 54 years, screening mammography could be switched to a biennial interval, but with the opportunity to continue annually (64). In contrast, the updated guidelines of the U.S. Preventive Service Task Force (USPSTF) recommend screening women aged 50–75 years with biennial mammography, but also with an individual choice of screening before the age of 50 years (63). However, the Executive Board and the Scientific Committee of the European Society of Breast Imaging (EUSOBI) issued a statement that was approved by 30 national breast radiology bodies, which supports biennial screening mammography for average-risk women aged 50–69 years. An extension to 73–75 years and from 40–45 years to 49 years is also encouraged but should be evaluated on a country-by-country basis (65). Tables 1 and 2 summarize the current recommendation guidelines for breast cancer screening for women at an average-risk and high-risk of breast cancer.
Table 1.
Summary of recommendation guidelines for breast cancer screening in average-risk women.
| Average-risk women | American Cancer Society (ACS) | American College of Obstetricians and Gynecologists (ACOG) | U.S. Preventive Service Task Force (USPSTF) | National Comprehensive Cancer Network (NCCN) | European Society of Breast Imaging (EUSOBI) |
|---|---|---|---|---|---|
| Clinical breast examination | Not recommended at any age | Not recommended at any age | Insufficient evidence to recommend for or against clinical breast examination | Women aged 25–39 years every 1–3 years Women aged > 40 years annually | No recommendation |
| Mammography lower age limit | At the age of 45 years Opportunity to start at 40–45 years | At the age of 40 years No later than age 50 years if not initiated in the 40s | At the age of 50 years Start biennial screening before age 50 years should be an individual decision based on patient beliefs with regard to benefits and harm | At the age of 40 years | At the age of 50 years Opportunity to start at 40–45 years by country-specific priority |
| Mammography screening interval | Annual for women aged 40–45 years Biennial for women aged ≥ 45 years with the opportunity to continue annually | Annual or biennial based on an informed and shared decision-making process, including benefits and harms of screening, and patients’ beliefs and preferences Biennial screening particularly after age 55 years | Biennial | Annual screening | Biennial Annual screening at 40–49 years |
| Mammography upper age limit | Continue until life expectancy is <10 years | Continue until age 75 years Beyond age 75 years, the decision to discontinue screening mammography should be based on a shared decision-making process based on health status and longevity | Insufficient evidence to recommend for or against screening beyond age 75 years | Continue until severe co-morbidities limit life expectancy to 10 years or less | Extend screening up to 73 or 75 years |
Table 2.
Summary of recommendation guidelines for breast cancer screening in high-risk women.
| High-risk women | American Cancer Society (ACS) | National Comprehensive Cancer Network (NCCN) |
|---|---|---|
| Clinical breast examination | – | Clinical encounter every 6–12 months from the point of risk identification with additional genetic counseling Breast awareness |
| Screening initiation age | Start screening with MRI at age 25 years Continue screening with MRI and MG at age 30 years | Start screening MRI ten years before the youngest affected family member but not < 25 years Start screening MG ten years before the youngest affected family member but not < 30 years Consideration of tomosynthesis rather than MG |
| Screening interval | Annual | Annual |
| Screening upper age limit | Continue for as long as a woman is in good health | Upper age limit for screening is not yet established |
The term “clinical encounter” is defined as any physical or virtual contact between an individual/patient and a healthcare provider, during which an evaluation or diagnostic activity is performed.
Frequent over-diagnosis, false-positive results, increased costs, and patient anxiety are major points of criticism of any mammography screening program. Nelson et al. (66) performed a data analysis of screening FFDM in women aged 40–89 years and showed that the highest rate of false-positive diagnoses (121.2/1000) and recommendations for additional breast imaging (124.9/1000) were found in women aged 40–49 years. In a systematic review, they reported higher cumulative false-positive rates for mammography and biopsies with annual screening, compared to a two-year interval, for women aged 40–49 years, women with dense breasts, and for women under combined hormone substitution (67). Estimated over-diagnosis rates of up to 54% were reported in a series of 29 studies and were in the range of 11–22% in RCTs (67). Further, they found higher levels of anxiety, distress, and breast cancer-specific worry in women with false-positive diagnoses. In contrast, Pitman et al. (68) demonstrated a benefit of screening mammography for women aged 40–49 years, supporting the current ACS screening recommendations. Women with almost exclusively dense breasts in this age group had 18.8% of all screening-detected breast cancers and > 60% were invasive at the time of detection.
Another issue fueling the debate about the benefits of screening mammography is interval cancers. Interval cancers are defined as symptomatic or palpable breast cancers that present less than two years after a normal screening mammography (69). Interval cancers are often related to high breast densities, with a greater than 17-times risk compared to women with non-dense breasts (9,70,71). In a study by Webb et al. (69), the investigators showed greater interval cancer rates with significant breast cancer mortality, especially in younger women aged < 40 years (60%) or in the age range of 40–49 years (47%), who more often presented with dense breasts, but who had not participated in a screening program. However, for older women under screening, interval cancer rates do not exceed 28% (69). These findings highlight that a one-size-fits-all screening approach is not appropriate and that risk-adapted breast screening strategies that use supplemental screening, when indicated, are warranted.
Supplemental screening modalities
The aim of supplemental screening is to improve early breast cancer detection in women where traditional mammography screening has limitations. With increasing public awareness of the implications of increased breast density as a risk factor for breast cancer and its impact on the sensitivity of screening mammography, more women demand supplemental screening (72). However, there is great uncertainty and controversy about the potential beneficial effect of supplemental screening with regard to further reducing breast cancer mortality, since there is no evidence and it is unclear whether it is cost-effective. In the following sections, supplemental screening modalities that can may be offered to women, including digital breast tomosynthesis (DBT), breast ultrasound (US), and magnetic resonance imaging (MRI) of the breast, will be described and discussed.
Digital breast tomosynthesis
To overcome the inherent limitations of FFDM, with respect to coalescent parenchymal areas and superimpositions at higher density grades, DBT, or 3D mammography, has been implemented in breast imaging. Meanwhile, there are several prospective population-based trials available that have shown improved cancer detection and reduced recall rates when tomosynthesis is added to mammography. In the STORM-1 trial that evaluated the efficacy of tomosynthesis in combination with FFDM, Ciatto et al. showed an improved cancer detection rate from 5.3 to 8.1 cancers per 1000 screening examinations as well as a reduction in false-positive recalls by 17.2% (73). In addition, Bernardi et al. demonstrated similar results in the STORM-2 trial, with a cancer detection rate of up to 8.5 cancers per 1000 screens, when FFDM is combined with DBT, and up to 8.8 cancers per 1000 screens, when a synthesized 2D mammographic image is reconstructed and then combined with DBT. However, the results also showed an increase in the percentage of false-positive readings with 3.97%, 4.45%, and 2.42%, respectively (74). Other prospective population-based studies reported a significantly higher cancer detection rate with the possibility to detect more invasive cancers, when tomosynthesis was combined with FFDM (75). In this study, a detection rate for invasive and in situ cancers of 8.0 per 1000 screening examinations was reported when tomosynthesis was added to FFDM, compared to 6.1 per 1000 for mammography alone. Skaane et al. reported also a slight reduction in false-positives for the combined use, 53.1% vs. 61.1% (75). The Malmö Breast Tomosynthesis Screening Trial (MBTST), another prospective, population-based study revealed on the other side an increase of false-positive rates when breast cancer screening was carried out with tomosynthesis alone (1.7%), when tomosynthesis was combined with mammography (1.5%), and for mammography alone (1.1%), the latter of which was attributed to the appearance of stellate distortions (76). In addition, none of the studies investigated the cost-effectiveness of DBT as a supplemental screening modality to a great extent or evaluate the impact on a population-based screening program. However, with the improvements in the image-processing techniques of DBT and the possibility to synthesize 2D image projections from 3D acquisitions, DBT is likely to replace FFDM as a primary screening tool in the near future (77).
Breast ultrasound
US is a ubiquitous, available, cost-effective, and reliable imaging modality that does not use ionizing radiation. The reported cancer detection rate of US, in combination with FFDM, yields 3–4 additional cancers per 1000 screening examinations (78–80). However, the increased cancer detection rate of supplemental US screening comes at the expense of increased false-positive recall rates, costs, and patient anxiety (81). In a multicenter RCT that investigated screening with FFDM and US, Berg et al. (78) reported an increased cancer detection rate of 11.8 cancers per 1000 women, compared to FFDM alone (7.6 detected cancers per 1000 women) at the expense of a false-positive rate of up to 8.1% with US alone, and of up to 10.4% for combined FFDM and US. Other data by Berg et al. (80), using a RCT with a triennial observation of women with higher-density breast categories, yielded a cancer detection of 32 cases by US only, nearly as much as with FFDM only (33 cancers). Supplemental US after mammography yielded an additional cancer detection of 5.3 per 1000 women in the first year and 3.7 cancers per 1000 women in each of the next two years, with an average additional cancer detection rate of 4.3 per 1000 screened women (80). Similar results with respect to additional cancer yield—3.2 (79), 3.4 (82), and 7.1 (83)—and false-positive rates of 4.7% (79) and 3.3% (83) were reported by retro- and prospective studies. The Japan Strategic Anti-Cancer Randomized Trial (J-STAT) investigated the efficacy of additional US in a large nationwide screening program for breast cancer, with a significantly higher breast cancer detection rate, a lower number of interval cancers, and the detection of additional cancers, compared to mammography alone, in women aged 40–49 years (84). In addition, Austria started a nationwide biannual mammography screening program offering additional US for those with density categories C and D. Initial results are expected to be presented soon (39). To overcome the observer-dependency of hand-held supplemental screening US, automated, 3D, whole-breast US (ABUS) has been introduced (85–87). A recently published study by Wilczek et al., where ABUS was used in addition to mammography, yielded an increased cancer detection rate of 6.6 per 1000 screening exams in contrast to 4.2 cancers detected with mammography alone. Initial results suggest that ABUS can provide 3D volumetric imaging (86,87), and thus, may be a valid option in this setting to enable the detection of additional breast cancers that are invisible on mammography (88).
Breast magnetic resonance imaging
Another supplemental screening modality that can be offered is MRI. MRI is a radiation- and compression-free 3D imaging modality and, currently, is the most sensitive test for breast cancer detection (89–92). According to international guidelines, annual screening with dynamic contrast-enhanced MRI is recommended for women with an estimated lifetime risk of breast cancer of >20% (38,80,93,94). Per ACS screening, breast MRI may be recommended for women with heterogeneous or extremely dense tissue as a supplement to mammography (94). In a multicenter trial of women at high genetic risk for inherited breast cancer, the reported diagnostic performance of MRI showed an overall sensitivity of 91% and a specificity of 97%. Sensitivity increased up to 93% if MRI was a supplement to mammography or sonography (93). Data from Berg et al. (80), on an asymptomatic screening collective of women with at least one other risk factor in addition to heterogeneous or extremely dense breasts, showed a sensitivity of 100% if mammography was combined with US and MRI; however, the specificity in this study for the combined use of all three modalities was reportedly at least 65%. In a triple-modality screening study of high-risk women, Riedl et al. (38) showed the superiority of MRI over mammography and US for early breast cancer detection, with no influence of age, breast density, or breast cancer risk status. The reported MRI sensitivity and specificity were 90% and 89%, respectively. Sensitivity was further increased when MRI was combined with mammography or if all three modalities were used together (95%) (95). However, specificities and positive predictive values were lower in younger women and in higher density categories (38).
Currently, there are no data available on supplemental screening with MRI in women with higher breast density categories. The Dutch DENSE trial is investigating the effectiveness and cost-effectiveness of screening with mammography and MRI compared with those of screening with mammography alone in women with extremely dense breasts (95). The study has finished enrollment and results are eagerly anticipated to better define the role of MRI in this patient population. At this time, the value of supplemental breast MRI screening in women with higher breast density categories remains unclear and the ACS guidelines do not recommend for or against screening MRI in women with the risk factor of a higher breast density (94,96,97).
Although MRI of the breast is the most sensitive test for breast cancer detection, its application as a screening tool for women at an average risk of breast cancer is limited by costs, availability, as well as reading and examination time. However, the introduction of abbreviated imaging protocols, with scan times of approximately 3 min has sparked the discussion about whether to also offer MRI screening also to women at average risk for breast cancer (98,99). Although initial results for abbreviated MRI as a screening tool are promising, there is currently not enough data to recommend breast MRI screening for the average population.
New avenues for risk-adapted screening
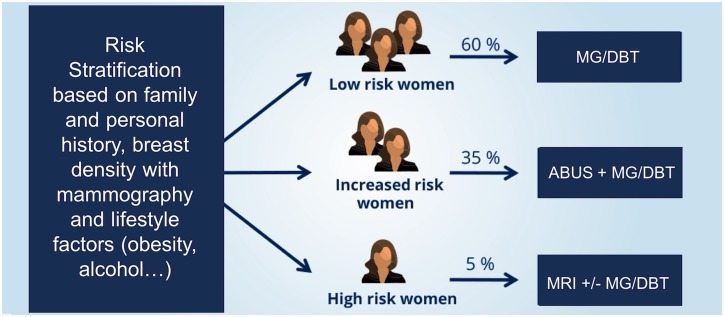
In addition to breast density as an individual risk factor for breast cancer, there are several other risk factors that increase the risk, such as genetic predisposition, number of affected first-degree relatives, chest radiation therapy at a young age, ethnicity, lifestyle factors, and previous personal history of breast malignancies exist (18). To date, there are no recommendations for risk-adapted screening available. Implemented population-based screening programs aimed at detection breast cancer detection at an early stage using mammography, as a cornerstone technique, have resulted in an effective decrease in breast cancer mortality of up to 49% (2,3). Breast cancer risk estimation tools, such as the Gail and Tyrer-Cuzick models, have been introduced with the purpose of identifying women at risk of developing a future breast cancer (100–102). The Gail model from the National Cancer Institute, which is based on the general population, is an eight-question tool using age, hormonal factors, benign disease, and number of first-degree relatives who have already been diagnosed with breast cancer to estimate the relative risk of developing invasive breast cancer (103). In the Tyrer-Cuzick model, similar risk factors from the Gail approach are used in conjunction with personal and genetic factors, including the BRCA1/2 genes for risk assessment of invasive breast cancer (102). However, it has been demonstrated that mammographic density seems to be a stronger risk factor, and the combination of breast density with either the Gail or the Tyrer-Cuzick model resulted in a better breast cancer risk assessment (104). Moreover, the process of screening for breast cancer remains controversial, with a number of recommendations from various national Breast Cancer Screening Programs concerning the starting points and the intervals for screening. A potential model for risk-adapted screening could include an initial risk stratification that incorporates family and personal history, breast density assessed with mammography, and, potentially, also lifestyle risk factors, such as obesity (105) and alcohol (106). Thereafter, women would be classified into three categories—low risk, intermediate risk, and high risk—and would undergo screening tailored to their individual risk.
Based on risk stratification, patients would then be offered risk-adapted screening with different imaging modalities, as depicted in Fig. 2. Low-risk women could continue to be screened with FFDM, or, when available, DBT with synthesized mammography annually, bi-annually, or triennially based on their national recommendations. Intermediate-risk women could undergo additional supplemental screening with US. High-risk women, who constitute a minority of women, would be offered MRI and MG only when a benefit has been demonstrated (e.g. BRCA 2 carrier) (107).
Fig. 2.
Model for risk-adapted screening.
The use of mammography screening in female populations has helped to lower the rates of breast cancer-related mortality in the general population. However, the sensitivity of mammography for breast cancer detection is decreased in women aged 40–49 years and in women with higher breast densities. In addition, there is evidence that breast density is also a strong independent risk factor for breast cancer. With increasing public awareness of the implications of a high breast density, there is an increasing demand for supplemental screening in these women (72). Currently potential supplemental screening modalities include DBT, US, and MRI of the breast, among which MRI is the most sensitive method available. However, the improvements in breast cancer detection with supplemental screening methods come at the expense of increased false-positives, recall rates, patient anxiety, and costs. Therefore, the approach to breast cancer screening will soon change, while we need further evidence to demonstrate the show of cost-effectiveness of supplemental screening. Such screening strategies would be tailored to the individual woman’s risk, personal beliefs, and preferences, while accounting for cost, potential harm, and patient-relevant outcomes. New strategies are warranted, and it can be expected that breast density and its assessment will play a huge role in the development of these strategies.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding was provided by the Austrian Nationalbank “Jubilaeumsfond” Project No. 16219 and the 2020 – Research and Innovation Framework Programme PHC-11-2015 No. 667211-2.
References
- 1.American Cancer Society. About breast cancer. Atlanta, GA: American Cancer Society, 2017. Available at: https://www.cancer.org/cancer/breast-cancer/about.html.
- 2.Broeders M, Moss S, Nystrom L, et al. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen 2012; 19: 14–25. [DOI] [PubMed] [Google Scholar]
- 3.Nickson C, Mason KE, English DR, et al. Mammographic screening and breast cancer mortality: a case-control study and meta-analysis. Cancer Epidemiol Biomarkers Prev 2012; 21: 1479–1488. [DOI] [PubMed] [Google Scholar]
- 4.Kerlikowske K, Hubbard RA, Miglioretti DL, et al. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: a cohort study. Ann Intern Med 2011; 155: 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price ER, Hargreaves J, Lipson JA, et al. The California breast density information group: a collaborative response to the issues of breast density, breast cancer risk, and breast density notification legislation. Radiology 2013; 269: 887–892. [DOI] [PubMed] [Google Scholar]
- 6.D’Orsi CJ, Sickles EA, Mendelson EB, et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System, Reston, VA: American College of Radiology, 2013. [Google Scholar]
- 7.Jackson VP, Hendrick RE, Feig SA, et al. Imaging of the radiographically dense breast. Radiology 1993; 188: 297–301. [DOI] [PubMed] [Google Scholar]
- 8.Rhodes DJ, Radecki Breitkopf C, Ziegenfuss JY, et al. Awareness of breast density and its impact on breast cancer detection and risk. J Clin Oncol 2015; 33: 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med 2007; 356: 227–236. [DOI] [PubMed] [Google Scholar]
- 10.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiology Biomarkers Prev 2006; 15: 1159–1169. [DOI] [PubMed] [Google Scholar]
- 11.Kerlikowske K, Ichikawa L, Miglioretti DL, et al. Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst 2007; 99: 386–395. [DOI] [PubMed] [Google Scholar]
- 12.Boyd NF, Martin LJ, Bronskill M, et al. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst 2010; 102: 1224–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huo CW, Chew GL, Britt KL, et al. Mammographic density-a review on the current understanding of its association with breast cancer. Breast Cancer Res Treat 2014; 144: 479–502. [DOI] [PubMed] [Google Scholar]
- 14.Boyd NF, Martin LJ, Yaffe MJ, et al. Mammographic density: a hormonally responsive risk factor for breast cancer. J Br Menopause Soc 2006; 12: 186–193. [DOI] [PubMed] [Google Scholar]
- 15.Sterns EE, Zee B. Mammographic density changes in perimenopausal and postmenopausal women: is effect of hormone replacement therapy predictable? Breast Cancer Res Treat 2000; 59: 125–132. [DOI] [PubMed] [Google Scholar]
- 16.van Duijnhoven FJ, Peeters PH, Warren RM, et al. Postmenopausal hormone therapy and changes in mammographic density. J Clin Oncol 2007; 25: 1323–1328. [DOI] [PubMed] [Google Scholar]
- 17.Byrne C, Ursin G, Martin CF, et al. Mammographic density change with estrogen and progestin therapy and breast cancer risk. J Natl Cancer Inst 2017; 109: 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monticciolo DL, Newell MS, Moy L, et al. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol 2018; 15: 408–414. [DOI] [PubMed] [Google Scholar]
- 19.Chia KS, Reilly M, Tan CS, et al. Profound changes in breast cancer incidence may reflect changes into a Westernized lifestyle: a comparative population-based study in Singapore and Sweden. Int J Cancer 2005; 113: 302–306. [DOI] [PubMed] [Google Scholar]
- 20.Sung H, Rosenberg PS, Chen WQ, et al. Female breast cancer incidence among Asian and Western populations: more similar than expected. J Natl Cancer Inst 2015; 107: 107–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habel LA, Capra AM, Oestreicher N, et al. Mammographic density in a multiethnic cohort. Menopause 2007; 14: 891–899. [DOI] [PubMed] [Google Scholar]
- 22.Heller SL, Hudson S, Wilkinson LS. Breast density across a regional screening population: effects of age, ethnicity and deprivation. Br J Radiol 2015; 88: 20150242–20150242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maskarinec G, Pagano I, Lurie G, et al. Mammographic density and breast cancer risk: the multiethnic cohort study. Am J Epidemiol 2005; 162: 743–752. [DOI] [PubMed] [Google Scholar]
- 24.Rajaram N, Mariapun S, Eriksson M, et al. Differences in mammographic density between Asian and Caucasian populations: a comparative analysis. Breast Cancer Res Treat 2017; 161: 353–362. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen TL, Schmidt DF, Makalic E, et al. Explaining variance in the cumulus mammographic measures that predict breast cancer risk: a twins and sisters study. Cancer Epidemiol Biomarkers Prev 2013; 22: 2395–2403. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan K, Baglietto L, Stone J, et al. Longitudinal study of mammographic density measures that predict breast cancer risk. Cancer Epidemiol Biomarkers Prev 2017; 26: 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wanders JO, Bakker MF, Veldhuis WB, et al. The effect of weight change on changes in breast density measures over menopause in a breast cancer screening cohort. Breast Cancer Res 2015; 17: 74–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keum N, Greenwood DC, Lee DH, et al. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst 2015; 107. [DOI] [PubMed] [Google Scholar]
- 29.Hopper JL, Nguyen TL, Stone J, et al. Childhood body mass index and adult mammographic density measures that predict breast cancer risk. Breast Cancer Res Treat 2016; 156: 163–170. [DOI] [PubMed] [Google Scholar]
- 30.Harris HR, Tamimi RM, Willett WC, et al. Body size across the life course, mammographic density, and risk of breast cancer. Am J Epidemiol 2011; 174: 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersen ZJ, Baker JL, Bihrmann K, et al. Birth weight, childhood body mass index, and height in relation to mammographic density and breast cancer: a register-based cohort study. Breast Cancer Res 2014; 16: R4–R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvie M, Howell A, Vierkant RA, et al. Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women's health study. Cancer Epidemiol Biomarkers Prev 2005; 14: 656–661. [DOI] [PubMed] [Google Scholar]
- 33.Eliassen AH, Colditz GA, Rosner B, et al. Adult weight change and risk of postmenopausal breast cancer. JAMA 2006; 296: 193–201. [DOI] [PubMed] [Google Scholar]
- 34.Checka CM, Chun JE, Schnabel FR, et al. The relationship of mammographic density and age: implications for breast cancer screening. Am J Roentgenol 2012; 198: W292–295. [DOI] [PubMed] [Google Scholar]
- 35.Vachon CM, Pankratz VS, Scott CG, et al. Longitudinal trends in mammographic percent density and breast cancer risk. Cancer Epidemiol Biomarkers Prev 2007; 16: 921–928. [DOI] [PubMed] [Google Scholar]
- 36.Boyd NF, Martin LJ, Yaffe MJ, et al. Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res 2011; 13: 223–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2016; 164: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riedl CC, Luft N, Bernhart C, et al. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol 2015; 33: 1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiller-Fruehwirth I, Jahn B, Einzinger P, et al. The long-term effectiveness and cost effectiveness of organized versus opportunistic screening for breast cancer in Austria. Value Health 2017; 20: 1048–1057. [DOI] [PubMed] [Google Scholar]
- 40.Winkler NS, Raza S, Mackesy M, et al. Breast density: clinical implications and assessment methods. Radiographics 2015; 35: 316–324. [DOI] [PubMed] [Google Scholar]
- 41.Colin C, Schott AM, Valette PJ. Mammographic density is not a worthwhile examination to distinguish high cancer risk women in screening. Eur Radiol 2014; 24: 2412–2426. [DOI] [PubMed] [Google Scholar]
- 42.Wolfe JN. Breast patterns as an index of risk for developing breast cancer. Am J Roentgenol 1976; 126: 1130–1137. [DOI] [PubMed] [Google Scholar]
- 43.He W, Hogg P, Juette A, et al. Breast image pre-processing for mammographic tissue segmentation. Comput Biol Med 2015; 67: 61–73. [DOI] [PubMed] [Google Scholar]
- 44.van der Waal D, Ripping TM, Verbeek AL, et al. Breast cancer screening effect across breast density strata: A case-control study. Int J Cancer 2017; 140: 41–49. [DOI] [PubMed] [Google Scholar]
- 45.Ciatto S, Houssami N, Apruzzese A, et al. Categorizing breast mammographic density: intra- and interobserver reproducibility of BI-RADS density categories. Breast 2005; 14: 269–275. [DOI] [PubMed] [Google Scholar]
- 46.Morrish OW, Tucker L, Black R, et al. Mammographic breast density: comparison of methods for quantitative evaluation. Radiology 2015; 275: 356–365. [DOI] [PubMed] [Google Scholar]
- 47.Wengert GJ, Helbich TH, Woitek R, et al. Inter- and intra-observer agreement of BI-RADS-based subjective visual estimation of amount of fibroglandular breast tissue with magnetic resonance imaging: comparison to automated quantitative assessment. Eur Radiology 2016; 26: 3917–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee HN, Sohn YM, Han KH. Comparison of mammographic density estimation by Volpara software with radiologists’ visual assessment: analysis of clinical-radiologic factors affecting discrepancy between them. Acta Radiol 2015; 56: 1061–1068. [DOI] [PubMed] [Google Scholar]
- 49.Raza S, Mackesy MM, Winkler NS, et al. Effect of training on qualitative mammographic density assessment. J Am Coll Radiol 2016; 13: 310–315. [DOI] [PubMed] [Google Scholar]
- 50.Gao J, Warren R, Warren-Forward H, et al. Reproducibility of visual assessment on mammographic density. Breast Cancer Res Treat 2008; 108: 121–127. [DOI] [PubMed] [Google Scholar]
- 51.Byng JW, Boyd NF, Fishell E, et al. The quantitative analysis of mammographic densities. Phys Med Biol 1994; 39: 1629–1638. [DOI] [PubMed] [Google Scholar]
- 52.Highnam R, Jeffreys M, McCormack V, et al. Comparing measurements of breast density. Phys Med Biol 2007; 52: 5881–5895. [DOI] [PubMed] [Google Scholar]
- 53.van Engeland S, Snoeren PR, Huisman H, et al. Volumetric breast density estimation from full-field digital mammograms. IEEE Trans Med Imaging 2006; 25: 273–282. [DOI] [PubMed] [Google Scholar]
- 54.Brandt KR, Scott CG, Ma L, Mahmoudzadeh AP, et al. Comparison of clinical and automated breast density measurements: implications for risk prediction and supplemental screening. Radiology 2015; 279: 710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Azziz A, Fan B, et al. Agreement of mammographic measures of volumetric breast density to MRI. PloS One 2013; 8: e81653–e81653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kopans DB. Basic physics and doubts about relationship between mammographically determined tissue density and breast cancer risk. Radiology 2008; 246: 348–353. [DOI] [PubMed] [Google Scholar]
- 57.Eng A, Gallant Z, Shepherd J, et al. Digital mammographic density and breast cancer risk: a case-control study of six alternative density assessment methods. Breast Cancer Res 2014; 16: 439–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeffers AM, Sieh W, Lipson JA, et al. Breast cancer risk and mammographic density assessed with semiautomated and fully automated methods and BI-RADS. Radiology 2017; 282: 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gastounioti A, Oustimov A, Keller BM, et al. Breast parenchymal patterns in processed versus raw digital mammograms: A large population study toward assessing differences in quantitative measures across image representations. Med Phys 2016; 43: 5862–5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith RA, Duffy SW, Gabe R, et al. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am 2004; 42: 793–806. [DOI] [PubMed] [Google Scholar]
- 61.Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012; 380: 1778–1786. [DOI] [PubMed] [Google Scholar]
- 62.Lauby-Secretan B, Loomis D, Straif K. Breast-cancer screening–viewpoint of the IARC Working Group. N Engl J Med 2015; 373: 1479–1479. [DOI] [PubMed] [Google Scholar]
- 63.Siu AL, Force USPST. Screening for breast cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016; 164: 279–296. [DOI] [PubMed] [Google Scholar]
- 64.Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015; 314: 1599–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sardanelli F, Aase HS, Alvarez M, et al. Position paper on screening for breast cancer by the European Society of Breast Imaging (EUSOBI) and 30 national breast radiology bodies from Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Israel, Lithuania, Moldova, The Netherlands, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Spain, Sweden, Switzerland and Turkey. Eur Radiol 2017; 27: 2737–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nelson HD, O'Meara ES, Kerlikowske K, et al. Factors associated with rates of false-positive and false-negative results from digital mammography screening: an analysis of registry data. Ann Intern Med 2016; 164: 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: systematic review to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med 2016; 164: 256–267. [DOI] [PubMed] [Google Scholar]
- 68.Pitman JA, McGinty GB, Soman RR, et al. Screening mammography for women in their 40s: the potential impact of the American Cancer Society and U.S. Preventive Services Task Force breast cancer screening recommendations. Am J Roentgenol 2017; 209: 697–702. [DOI] [PubMed] [Google Scholar]
- 69.Webb ML, Cady B, Michaelson JS, et al. A failure analysis of invasive breast cancer: most deaths from disease occur in women not regularly screened. Cancer 2014; 120: 2839–2846. [DOI] [PubMed] [Google Scholar]
- 70.Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst 2000; 92: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 71.Hooley RJ. Breast density legislation and clinical evidence. Radiol Clin North Am 2017; 55: 513–526. [DOI] [PubMed] [Google Scholar]
- 72.Evers K. Are you dense? Acad Radiol 2015; 22: 677–678. [DOI] [PubMed] [Google Scholar]
- 73.Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14: 583–589. [DOI] [PubMed] [Google Scholar]
- 74.Bernardi D, Macaskill P, Pellegrini M, et al. Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): a population-based prospective study. Lancet Oncol 2016; 17: 1105–1113. [DOI] [PubMed] [Google Scholar]
- 75.Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267: 47–56. [DOI] [PubMed] [Google Scholar]
- 76.Lang K, Nergarden M, Andersson I, et al. False positives in breast cancer screening with one-view breast tomosynthesis: An analysis of findings leading to recall, work-up and biopsy rates in the Malmo Breast Tomosynthesis Screening Trial. Eur Radiol 2016; 26: 3899–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gilbert FJ, Tucker L, Gillan MG, et al. Accuracy of digital breast tomosynthesis for depicting breast cancer subgroups in a UK retrospective reading study (TOMMY Trial). Radiology 2015; 277: 697–706. [DOI] [PubMed] [Google Scholar]
- 78.Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008; 299: 2151–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hooley RJ, Greenberg KL, Stackhouse RM, et al. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology 2012; 265: 59–69. [DOI] [PubMed] [Google Scholar]
- 80.Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012; 307: 1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sprague BL, Stout NK, Schechter C, et al. Benefits, harms, and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med 2015; 162: 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bae MS, Moon WK, Chang JM, et al. Breast cancer detected with screening US: reasons for nondetection at mammography. Radiology 2014; 270: 369–377. [DOI] [PubMed] [Google Scholar]
- 83.Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial. J Clin Oncol 2016. 34:1882–1888. Doi: 10.1200/JCO.2015.63.4147. [DOI] [PubMed] [Google Scholar]
- 84.Ohuchi N, Suzuki A, Sobue T, et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet 2016; 387: 341–348. [DOI] [PubMed] [Google Scholar]
- 85.Chae EY, Shin HJ, Kim HJ, et al. Diagnostic performance of automated breast ultrasound as a replacement for a hand-held second-look ultrasound for breast lesions detected initially on magnetic resonance imaging. Ultrasound Med Biol 2013; 39: 2246–2254. [DOI] [PubMed] [Google Scholar]
- 86.Chen JH, Lee YW, Chan SW, et al. Breast density analysis with automated whole-breast ultrasound: comparison with 3-D magnetic resonance imaging. Ultrasound Med Biol 2016; 42: 1211–1220. [DOI] [PubMed] [Google Scholar]
- 87.Moon WK, Shen YW, Huang CS, et al. Comparative study of density analysis using automated whole breast ultrasound and MRI. Med Phys 2011; 38: 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Houssami N, Ciatto S. The evolving role of new imaging methods in breast screening. Prev Med 2011; 53: 123–126. [DOI] [PubMed] [Google Scholar]
- 89.Pinker K, Bogner W, Baltzer P, et al. Improved diagnostic accuracy with multiparametric magnetic resonance imaging of the breast using dynamic contrast-enhanced magnetic resonance imaging, diffusion-weighted imaging, and 3-dimensional proton magnetic resonance spectroscopic imaging. Invest Radiol 2014; 49: 421–430. [DOI] [PubMed] [Google Scholar]
- 90.Pinker K, Grabner G, Bogner W, et al. A combined high temporal and high spatial resolution 3 Tesla MR imaging protocol for the assessment of breast lesions: initial results. Invest Radiol 2009; 44: 553–558. [DOI] [PubMed] [Google Scholar]
- 91.Sardanelli F, Boetes C, Borisch B, et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer 2010; 46: 1296–1316. [DOI] [PubMed] [Google Scholar]
- 92.Dietzel M, Baltzer PA, Schon K, et al. MR-mammography: high sensitivity but low specificity? New thoughts and fresh data on an old mantra. Eur J Radiol 2012; 81: 30–32. [DOI] [PubMed] [Google Scholar]
- 93.Sardanelli F, Podo F, Santoro F, et al. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the high breast cancer risk italian 1 study): final results. Invest Radiol 2011; 46: 94–105. [DOI] [PubMed] [Google Scholar]
- 94.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57: 75–89. [DOI] [PubMed] [Google Scholar]
- 95.Emaus MJ, Bakker MF, Peeters PH, et al. MR imaging as an additional screening modality for the detection of breast cancer in women aged 50-75 years with extremely dense breasts: The DENSE Trial study design. Radiology 2015; 277: 527–537. [DOI] [PubMed] [Google Scholar]
- 96.Sardanelli F. Considerations on the application of EUSOMA criteria for preoperative MRI. Breast 2013; 22: 368–369. [DOI] [PubMed] [Google Scholar]
- 97.Mann RM, Balleyguier C, Baltzer PA, et al. Breast MRI: EUSOBI recommendations for women’s information. Eur Radiol 2015; 25: 3669–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuhl CK, Schrading S, Strobel K, et al. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol 2014; 32: 2304–2310. [DOI] [PubMed] [Google Scholar]
- 99.Schrading S, Strobel K, Kuhl CK. MRI screening of women at average risk of breast cancer. San Antonio Breast Cancer Symposium 2013; Dec 10–14; San Antonio, TX, USA.
- 100.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989; 81: 1879–1886. [DOI] [PubMed] [Google Scholar]
- 101.Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2014; 64: 30–51. [DOI] [PubMed] [Google Scholar]
- 102.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004; 23: 1111–1130. [DOI] [PubMed] [Google Scholar]
- 103.Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst 1999; 91: 1541–1548. [DOI] [PubMed] [Google Scholar]
- 104.Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res 2015; 17: 147–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mahoney MC, Bevers T, Linos E, et al. Opportunities and strategies for breast cancer prevention through risk reduction. CA Cancer J Clin 2008; 58: 347–371. [DOI] [PubMed] [Google Scholar]
- 106.Zhang SM, Lee IM, Manson JE, et al. Alcohol consumption and breast cancer risk in the Women’s Health Study. Am J Epidemiol 2007; 165: 667–676. [DOI] [PubMed] [Google Scholar]
- 107.Phi XA, Saadatmand S, De Bock GH, et al. Contribution of mammography to MRI screening in BRCA mutation carriers by BRCA status and age: individual patient data meta-analysis. Br J Cancer 2016; 114: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]