Abstract
The aim of this study was to explore the diversity of ectoparasitic fungi (Ascomycota, Laboulbeniales) that use bat flies (Diptera, Hippoboscoidea) as hosts. Bat flies themselves live as ectoparasites on the fur and wing membranes of bats (Mammalia, Chiroptera); hence this is a tripartite parasite system. Here, we collected bats, bat flies, and Laboulbeniales, and conducted phylogenetic analyses of Laboulbeniales to contrast morphology with ribosomal sequence data. Parasitism of bat flies by Laboulbeniales arose at least three times independently, once in the Eastern Hemisphere (Arthrorhynchus) and twice in the Western Hemisphere (Gloeandromyces, Nycteromyces). We hypothesize that the genera Arthrorhynchus and Nycteromyces evolved independently from lineages of ectoparasites of true bugs (Hemiptera). We assessed phylogenetic diversity of the genus Gloeandromyces by considering the LSU rDNA region. Phenotypic plasticity and position‐induced morphological adaptations go hand in hand. Different morphotypes belong to the same phylogenetic species. Two species, G. pageanus and G. streblae, show divergence by host utilization. In our assessment of coevolution, we only observe congruence between the Old World clades of bat flies and Laboulbeniales. The other associations are the result of the roosting ecology of the bat hosts. This study has considerably increased our knowledge about bats and their associated ectoparasites and shown the necessity of including molecular data in Laboulbeniales taxonomy.
Keywords: Ascomycota, ectoparasites, host specialization, phenotypic plasticity, ribosomal DNA, taxonomy
1. INTRODUCTION
Hyperparasitism, whereby parasites infect other parasites, is thought to be a common phenomenon in nature (Parratt & Laine, 2016). Few examples of obligate hyperparasites among fungi, however, have been well studied. Questions arise about what appears at first glance to be a risky lifestyle (Parratt, Barrès, Penczykowski, & Laine, 2017). How did such associations evolve? What population parameters are necessary to maintain these relationships? How strict are the species‐level relationships? The examples studied here involve bats, their blood‐sucking dipteran ectoparasites and the fungal ectoparasites of the blood‐sucking flies. An important question is whether this lifestyle could have arisen multiple times even though it seems tenuous. Another unexplored question is how diverse these fungal hyperparasites are, especially in the tropical regions (Arnold & Lutzoni, 2007).
Bats (Mammalia, Chiroptera) have received a great deal of attention due to their extraordinary morphological and ecological adaptations as well as their diversity in life history traits, qualities that make them ideal study organisms. Bats are parasitized by different groups of organisms, of which bat flies (Diptera, Hippoboscoidea, Nycteribiidae and Streblidae) are relatively well studied compared to other parasites. Published work has focused on host specificity, apparent male‐domination and population structure of bat flies (Dick & Patterson, 2007, 2008; Dittmar, Porter, Murray, & Whiting, 2006; Olival et al., 2013) and on associations between functional traits of bats and parasitism by bat flies (Patterson, Dick, & Dittmar, 2007). However, the addition of a second trophic level to the bat “microhabitat” is underexplored. Shockley and Murray (2006) reported two natural enemies of streblid bat flies (a hymenopteran parasitoid and a predaceous mirid bug). In addition, a handful of papers have discussed bacterial endosymbionts of bat flies in temperate and tropical regions (Duron et al., 2014; Hosokawa et al., 2012; Morse, Dick, Patterson, & Dittmar, 2012; Morse et al., 2013; Wilkinson et al., 2016).
In this study, we focus on the Laboulbeniales (Ascomycota, Laboulbeniomycetes), microscopic fungi that are obligate biotrophs on a wide range of arthropods, including bat flies. Prior to our current studies, the most recent papers dealing with Laboulbeniales on bat flies were published almost 40 years ago (Blackwell, 1980a, 1980b). Other papers on the same topic go back to the work of Harvard professor Roland Thaxter (1858–1932). Some of his publications presented species descriptions and new records for Arthrorhynchus, a genus apparently restricted to Old World bat flies (Thaxter, 1896, 1901, 1908, 1915, 1931), and two genera that thus far have only been reported on neotropical bat flies, Gloeandromyces and Nycteromyces (Thaxter, 1917, 1924, 1931). Until we initiated our studies on bat fly‐associated Laboulbeniales, five species were known from the type collections only (Haelewaters et al., 2017a, 2017b; Walker et al., 2018). This illustrates how underexplored these hyperparasites are. Windsor (1990, 1995) made the claim “Equal Rights for Parasites!” arguing that whereas parasites are generally either ignored or seen as a threat to conservation of endangered organisms, they should be recognized as a legitimate part of the earth's biodiversity. This applies as well to hyperparasites. All organisms are almost sure to acquire a parasite during their lifetime, even parasites themselves.
Laboulbeniales are one of three orders in the class Laboulbeniomycetes, the two others being Herpomycetales and Pyxidiophorales (Haelewaters et al., in review). All members of the class are obligately associated with arthropods for dispersal (Pyxidiophorales) or as biotrophs (Herpomycetales, Laboulbeniales). What sets the Laboulbeniales apart is its diversity, with 2,200 described species and many more awaiting discovery, and its wide variety of arthropod hosts. Representatives of three subphyla serve as hosts to Laboulbeniales: Chelicerata, with harvestmen (Opiliones) and mites (Acari); Myriapoda, with millipedes (Diplopoda); and Hexapoda, with cockroaches and termites (Blattodea), beetles (Coleoptera), earwigs (Dermaptera), flies (Diptera), true bugs (Hemiptera), ants (Hymenoptera, Formicidae), crickets and allies (Orthoptera), lice (Psocodea), and thrips (Thysanoptera). As ectoparasites, Laboulbeniales are attached to the exoskeleton of the host where they form multicellular units of determinate growth, or thalli. They are developmentally unique among the fungi that usually have mycelia of unlimited growth. Laboulbeniales thalli are the result of subsequent divisions of a single two‐celled ascospore. The ascospores are predominantly transmitted directly from infected to uninfected hosts (De Kesel, 1995).
Studying Laboulbeniales fungi has proven to be difficult for several reasons. The average size of Laboulbeniales thalli is around 200 μm, with extremes ranging from 35 μm (Rickia depauperata on mites of the genus Celaenopsis) to 4 mm (Laboulbenia kunkelii on Mormolyce phyllodes beetles). Because thalli are externally attached to a host, any study, morphological or molecular, requires micro‐manipulation with sterile techniques. Hosts may bear a large number of thalli, but often only few thalli are available for study. In some cases, thalli of a given species or morphotype may be restricted to a particular position on the host body (Goldmann & Weir, 2012; Goldmann, Weir, & Rossi, 2013). Unlike most fungi, Laboulbeniales have not been grown in culture to more than a few cells (never reaching maturity) (Whisler, 1968). The isolation of DNA has often been unsuccessful because of the often heavily pigmented cell walls (Weir & Blackwell, 2001b). This pigment, melanin, interferes during the PCR step by binding to the polymerase enzyme (Eckhart, Bach, Ban, & Tschachler, 2000). In addition, the cells are resilient in order to absorb impacts and friction on the host's integument. The combination of the melanized cell walls and resilient cells makes the thalli hard to break open.
Fungi of the order Laboulbeniales can display several types of specificity. Many species are host‐specific; they are associated with a single host species or species in the same genus. Based on experimental work, De Kesel (1996) showed that this specificity is driven by characteristics of the integument and living conditions of the arthropod host, but also by the habitat chosen by that host. For a number of species, such as Euzodiomyces lathrobii, Hesperomyces virescens, Laboulbenia flagellata and Rhachomyces lasiophorus, many host species are known, often in more than one host family (Santamaria, Balazuc, & Tavares, 1991). Our work with H. virescens has demonstrated that it is impossible to make accurate species‐level delimitations without molecular data (D. Haelewaters et al., unpublished data). It could be that more generalistic taxa are species complexes consisting of several species, whether cryptic or not, segregated by host. A different scenario is posed when hosts co‐occur in a single micro‐habitat. In this situation, opportunities exist for ascospores to be transmitted from a “typical” host to an “atypical” one. Such micro‐habitats might be ant nests (Pfliegler, Báthori, Haelewaters, & Tartally, 2016), subterranean caves (Reboleira, Fresneda, & Salgado, 2017), or seaweed and plant debris on beaches (De Kesel & Haelewaters, 2014). Another type of specificity is displayed when a given fungus shows “a remarkable tendency to grow on very restricted portions of the host integument” (Benjamin & Shanor, 1952). This phenomenon is referred to as position specificity. For example, 13 species of Chitonomyces can be observed on restricted positions of the aquatic diving beetle Laccophilus maculosus. Based on the combination of molecular and ecological data, Goldmann and Weir (2012) confirmed that sexual transmission is the mechanism behind the observed position specificity patterns, as suggested by Benjamin and Shanor (1952).
Around 10% of Laboulbeniales parasitize flies (Diptera). Species of Laboulbeniales on flies belong to eight genera: Arthrorhynchus, Dimeromyces, Gloeandromyces, Ilytheomyces, Laboulbenia, Nycteromyces, Rhizomyces, and Stigmatomyces. The genus Laboulbenia is by far the largest genus with over 800 species epithets listed in Index Fungorum (2018), but only 24 Laboulbenia species are known from flies (Rossi & Kirk‐Spriggs, 2011). Stigmatomyces is the second‐largest genus in the order, with 144 described species, all on flies (Rossi & Leonardi, 2013). The genera Arthrorhynchus, Gloeandromyces and Nycteromyces (Figure 1) are specific to bat flies, whereas none of the other genera have been recorded from bat flies.
Figure 1.
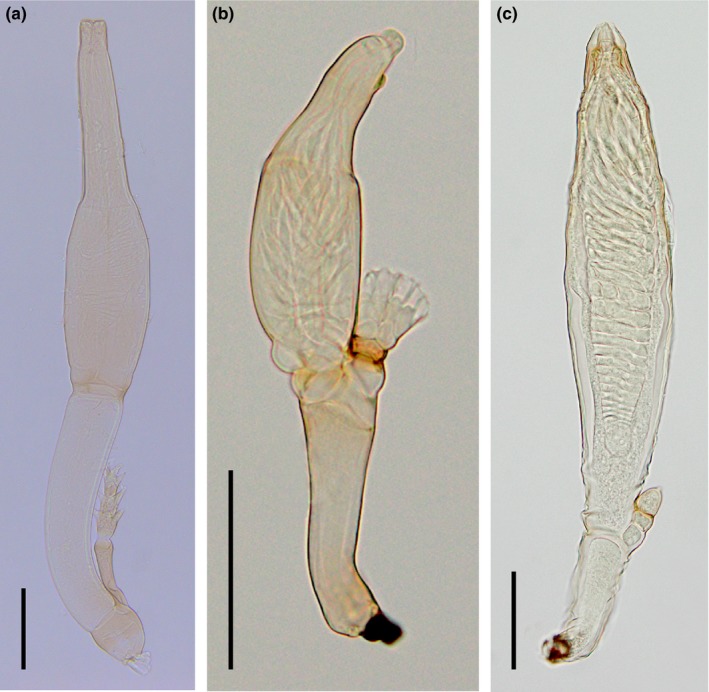
(a) Arthrorhynchus nycteribiae. (b) Gloeandromyces streblae. (c) Nycteromyces streblidinus, a female thallus
Arthrorhynchus is restricted to Old World species of Nycteribiidae. Kolenati (1857) was the first to report Laboulbeniales from bat flies; he described two species, Arthrorhynchus diesingii from Nycteribia vexata [as Acrocholidia montguei (vexata)] and A. westrumbii from Penicillidia conspicua [as Megistopoda westwoodii]. Peyritsch (1871) described Laboulbenia nycteribiae and suggested that Kolenati's species were synonyms of his newly described taxon. He later erected a new genus to accommodate his species: Helminthophana nycteribiae (Peyritsch, 1873). Thaxter (1896) followed Peyritsch's opinion but later he (Thaxter, 1901) retained Arthrorhynchus and described two additional species, A. cyclopodiae and A. eucampsipodae. Another species, A. acrandros, was described by Merola (1952) from the bat fly Phthiridium biarticulatum [as Nycteribia (Celepries) biarticulata]. The taxonomic status of all these species is unclear, because no sequence data exist for any of them (except A. nycteribiae). Arthrorhynchus nycteribiae has been reported from several host genera: Nycteribia, Penicillidia, Phthiridium (Blackwell, 1980b). Consequently, this taxon could be a complex of different species, each specialized to a single bat fly host or several hosts in a single genus—as is the situation in Hesperomyces virescens (D. Haelewaters et al., unpublished data).
The genera Gloeandromyces and Nycteromyces have hitherto only been found on streblid bat flies in the Americas (Haelewaters et al., 2017b; Thaxter, 1917, 1931; Walker et al., 2018). The diversity of both genera is thus far limited, as is knowledge of their distribution and biology. After their original description (Thaxter, 1917), G. nycteribiidarum, G. streblae [both described as Stigmatomyces] and Nycteromyces streblidinus were not reported again until a century later by Haelewaters et al. (2017b). Gloeandromyces nycteribiidarum was described on Megistopoda aranea [as Pterellipsis aranea] from Grenada, and G. streblae on Strebla wiedemanni [as S. vespertilionis] from Venezuela. Nycteromyces streblidinus was described on the same individual of S. wiedemanni from which G. streblae had been described (Thaxter, 1917). Haelewaters et al. (2017b) described a third species of Gloeandromyces, G. pageanus, from Trichobius dugesioides bat flies collected in Gamboa, Panama.
Except for a few disparate records of bat fly‐associated Laboulbeniales, virtually nothing is known about this triparatite system. Bat flies are dependent on their bat hosts (Ramasindrazana, Goodman, Gomard, Dick, & Tortosa, 2017) and it has been shown that habitat disturbance affects parasitism of bats by bat flies (Pilosof, Dick, Korine, Patterson, & Krasnov, 2012). The direction of the correlation (positive or negative) was reliant on the bat host species. Similarly, life history traits of both bats and bat flies may affect the ecology of Laboulbeniales species. If bat flies are affected by habitat disturbance, then Laboulbeniales species could be affected as well. For example, elevated population densities of bat flies would potentially increase transmission success of ascospores if they co‐occur on the same bat hosts or in the same roosts. However, for these sorts of data, hundreds or even thousands of bat flies need to be collected and screened for parasitic fungi. How life history traits and environmental factors such as habitat modification can shape species responses remains poorly understood and requires a large, non‐biased dataset. Toward this end, our main intentions were to collect and screen large numbers of bat flies, both through our own field collections and by expanding our network of collaborators who could provide us with bat flies.
2. MATERIALS AND METHODS
2.1. Capture of bats and collection of bat flies
Bats were captured and screened for ectoparasites by D.H. with the help of collaborators and field assistants during several field trips to Panama between 2015 and 2017. Field sites were located at Isla Barro Colorado (Panamá Oeste Province); in Gamboa and Parque Nacional Soberanía in the Canal Zone (Colón Province); Chilibre (Panamá Province); and Reserva Natural Chucantí (Darién Province) (Figure 2). Bats were captured using three to four 6 m‐wide 36 mm mesh ground‐level mistnets with four shelves (Avinet, Portland, Maine, USA). Mistnets were set over trails that were presumably used by bats as flight pathways (Palmeirim & Etherdige, 1985). Nets were usually examined every 10–20 min between sunset and ~11 p.m. Bats were disentangled and processed immediately or kept in clean cotton bags until processing. Bats were released at the capture site immediately after processing. Bats were identified on site using dichotomous keys (Handley, 1981; Timm & LaVal, 1998). Bat taxonomy follows Simmons (2005). In this study, Artibeus intermedius was considered a junior synonym of A. lituratus (Barquez, Perez, Miller, & Diaz, 2015; Guerrero et al., 2008).
Figure 2.
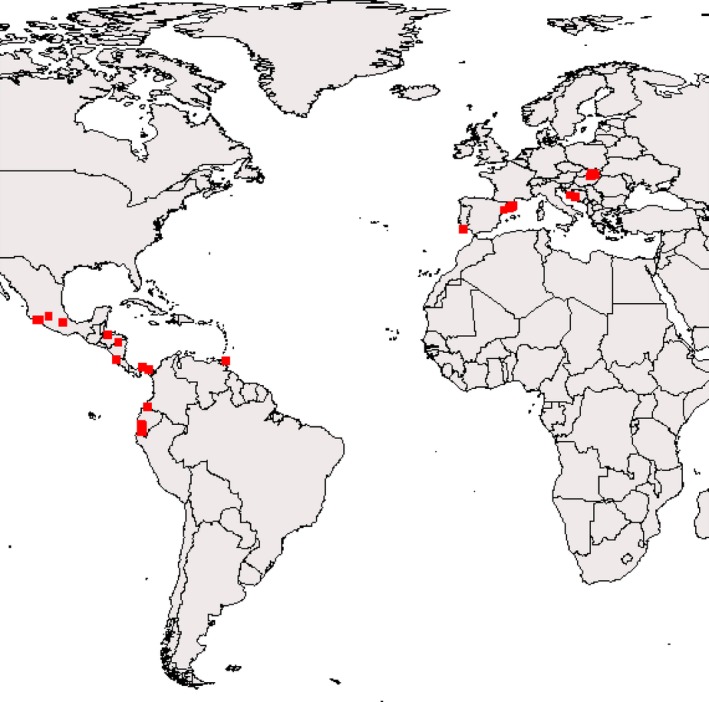
Field sites where bat flies for this project have been collected. Field sites are located in North and Central America (Costa Rica, Honduras, Mexico, Nicaragua, Panama), South America (Ecuador, Trinidad), and Europe (Croatia, Hungary, Slovakia, Portugal, Spain)
To remove bat flies from their bat hosts, 99% ethanol was applied using a paintbrush to reduce their activity. Subsequently, the bat flies were carefully removed using a rigid Swiss Style Forceps #5 with superfine tip (BioQuip #4535, Rancho Dominguez, California) or a Featherweight Forceps with narrow tip (BioQuip #4748). Some bat flies were collected using forceps alone or simply by hand. Preservation and long‐term storage of bat flies was in 99% ethanol in separate vials (one vial per bat host). Identification of bat flies to species level was based on published keys (Guerrero, 1993, 1994a, 1994b, 1995a, 1995b, 1996, 1997, 1998a, 1998b; Wenzel, 1976; Wenzel & Tipton, 1966) and complementary publications (Dick, 2013; Miller & Tschapka, 2001). Voucher specimens will be deposited at the following locations: Museo de Peces de Agua e Invertebrados, David, Panamá (MUPADI) and Naturalis Biodiversity Center, Leiden, Netherlands (RMNH). Labels for infected bat flies can be found in the Supporting Information.
Additional bat fly specimens preserved in 70%–99% ethanol were available from fieldwork by collaborators. Included in this study were bat flies from Latin America [Costa Rica (T. Hiller, unpublished data), Ecuador, Mexico, Nicaragua (C.W. Dick, unpublished data), Honduras (Dick, 2013), Panama (Walker et al., 2018) and Trinidad (J.J. Camacho, unpublished data)] and Europe [Croatia, Hungary, Portugal, Slovakia, and Spain in Europe (Haelewaters et al., 2017a; Szentiványi et al., 2018)].
2.2. Collection and identification of Laboulbeniales
Bat flies were screened for the presence of Laboulbeniales thalli under a Zeiss Stemi 508 stereomicroscope (Thornwood, New York). Thalli were removed from the host at the point of attachment (foot or haustorium) using Minuten Pins (BioQuip #1208SA, Rancho Dominguez, California) inserted onto wooden rods. Following Benjamin's (1971) procedure, thalli or groups of thalli were removed and mounted in Amann's medium, a liquid solution. Before applying Amann's medium and to facilitate microscopic observations, thalli first had to be arranged and fixed onto the microscope slide. To make thalli a bit sticky, they were first placed in a droplet of Hoyer's medium (30 g arabic gum, 200 g chloral hydrate, 16 ml glycerol, 50 ml ddH2O). Next, thalli were individually picked up and arranged in one or two rows. After a brief period of drying, the slide was closed using a cover slip with a drop of Amann's medium (drop facing downward) and subsequently sealed with nail polish or B‐72 in acetone (Gaylord #AB72, Syracuse, New York). Mounted specimens were viewed at 400× to 1,000× magnification under an Olympus BX53 compound microscope equipped with an Olympus DP73 digital camera (Waltham, Massachusetts). For detailed morphological study and descriptions at the Farlow Herbarium an Olympus BX40 microscope with XC50 camera was used. Fungal specimens were identified using Thaxter (1917, 1924, 1931) and Haelewaters et al. (2017b). Voucher slides are deposited at Farlow Herbarium (FH; Harvard University, Cambridge, Massachusetts) and Herbario de la Universidad Autónoma de Chiriquí (UCH; David, Panamá).
2.3. DNA extraction, PCR amplification, sequencing
DNA was extracted from 1–14 Laboulbeniales thalli using the Extract‐N‐Amp Plant PCR Kit (Sigma‐Aldrich, St. Louis, Missouri) (Haelewaters et al., 2015) or the REPLI‐g Single Cell Kit (Qiagen, Valencia, California) (Haelewaters et al., in review). Pretreatments employed with the Extract‐N‐Amp method included a prolonged incubation period at 56°C in 20 μl Extraction Solution up to 24‐hr in a Shake ‘N Bake Hybridization Oven (Boekel Scientific model #136400‐2, Feasterville, Pennsylvania) and mechanically crushing fungal material in a FastPrep FP120 Cell Disrupter (Thermo Fisher Scientific, Waltham, Massachusetts) at 5.5 m/s for 20 s. For about two thirds of our extractions, and as a rule for later extractions, thalli were manually cut in 2 or 3 parts (usually through the perithecium) using a #10 surgical blade on disposable Bard‐Parker handle (Aspen Surgical, Caledonia, Michigan) to ensure successful lysis.
The nuclear small and large ribosomal subunits of the ribosomal DNA (SSU and LSU rDNA) were amplified. Primer pairs for SSU were NSL1 (5′‐GTAGTGTCCTCrCATGCTTTTGAC‐3′) and NSL2 (5′‐AATCyAAGAATTTCACCTCTGAC‐3′) or NSL1 and R (5′‐TGATCCTTCTGCAGGTTCACCTACG‐3′) (Haelewaters et al., 2015; Wrzosek, 2000). Primer pairs for LSU were LR0R (5′‐ACCCGCTGAACTTAAGC‐3′) and LR5 (5′‐ATCCTGAGGGAAACTTC‐3′) or LIC24R (5′‐GAAACCAACAGGGATTG‐3′) and LR3 (5′‐GGTCCGTGTTTCAAGAC‐3′) (Miadlikowska & Lutzoni, 2000, Vilgalys & Hester, 1990; R. Vilgalys, unpublished data). PCR reactions consisted of 13.3 μl of RedExtract Taq polymerase (Sigma‐Aldrich), 2.5 μl of each 10 μM primer, 5.7 μl of H2O and 1.0 μl of template DNA. All amplifications were done in a 2720 Thermal Cycler (Applied Biosystems, Foster City, California) with initial denaturation at 94°C for 3:00 min; followed by 35 cycles of denaturation at 94°C for 1:00 min, annealing at 50°C for 0:45 min and extension at 72°C for 1:30 min; and final extension at 72°C for 10:00 min.
Unsuccessful PCR reactions were re‐run using the Q5 Host Start High‐Fidelity DNA Polymerase (New England BioLabs, Ipswich, Massachusetts). PCR was done in 25 μl consisting of 5.0 μl of 5× Q5 Reaction Buffer, 0.5 μl of 10 mM dNTP Mix (Quantabio, Beverly, Massachusetts), 1.25 μl of each 10 μM primer, 0.25 μl of Q5 High‐Fidelity DNA Polymerase, 12.75 μl of H2O and 4.0 μl of template DNA. Thermal cycling conditions were as follows: initial denaturation at 98°C for 30 s; 35 cycles of denaturation at 98°C for 10 s, annealing at 58–61.5°C for 30 s and extension at 72°C for 30 + 5/cycle s; followed by final extension at 72°C for 2 min. The optimal annealing temperature (Ta) was calculated for every primer combination using the New England BioLabs online Tm Calculator tool (tmcalculator.neb.com/) selecting “Q5” as product group and “Q5 Hot Start High‐Fidelity DNA Polymerase” as polymerase/kit, and with 500 mM for primer concentration. When smears or weak bands were observed on gel, conditions were optimized to include multiple annealing temperatures: 98°C for 3 min; 30 cycles at 98°C for 10 s, 65–68.5°C for 30 s (decreasing 1°C every three cycles) and 72°C for 1:30 min; then 30 cycles at 98°C for 10 s, 58–61.5°C for 30 s and 72°C for 1:30 min; and a final extension step of 72°C for 2 min.
Molecular work was done both at the Molecular Multi‐User's Lab at the Naos Marine Laboratories (Smithsonian Tropical Research Institute, Panama) and at the Harvard University Herbaria (Cambridge, Massachusetts). The protocol was identical except for purification and sequencing. In Panama, PCR products were purified using the QIAquick PCR Purification Kit (Qiagen). Subsequently, 10 μl reactions were prepared with the same primers and 3.0 μl of purified PCR product. Sequencing reactions were performed using the Big Dye® Terminator v3.1 Cycle Sequencing Kit (Life Technologies, Carlsbad, California). In Cambridge, purification and sequencing steps were outsourced to Genewiz (South Plainfield, New Jersey). Generated sequences were assembled and edited in Sequencher 4.10.1 (Gene Codes Corporation, Ann Arbor, Michigan). All sequences have been deposited in GenBank (accession numbers in Table 1).
Table 1.
Overview of Laboulbeniomycetes sequences used in this study. Species names are listed for all isolates, with their hosts and country
| Genus | Species | Host | Country | Isolate | Extraction protocol | # thalli used | SSU | LSU |
|---|---|---|---|---|---|---|---|---|
| Arthrorhynchus | nycteribiae | Penicillidia conspicua | Hungary | Edeleny_ 13.xi.2014 | Heat extraction | 4–5 | KY094496 | KY094497 |
| Arthrorhynchus | nycteribiae | Penicillidia conspicua | Hungary | D. Haelew. 1015d | ExNA | 7 | MG438336 | MG438363 |
| Camptomyces | sp. nov. | Astenus sp. | Tanzania | D. Haelew. 1222d | REPLI‐g | 1 | MF314140 | MF314141 |
| Gloeandromyces | sp. nov. 3 | Trichobius joblingi | Panama | D. Haelew. 1312b | REPLI‐g, crushed | 2 | MH040546 | MH040580 |
| Gloeandromyces | sp. nov. 3 | Trichobius joblingi | Panama | D. Haelew. 1312c | REPLI‐g | 2 | MH040547 | MH040581 |
| Gloeandromyces | sp. nov. 3 | Trichobius joblingi | Panama | D. Haelew. 1323b | REPLI‐g, crushed | 4 | MG958011 | MH040582 |
| Gloeandromyces | sp. nov. 3 | Trichobius joblingi | Panama | D. Haelew. 1323c | REPLI‐g, crushed | 4 | MH040548 | MH040583 |
| Gloeandromyces | nycteribiidarum | Megistopoda aranea | Panama | D. Haelew. 1319b | REPLI‐g | 2 | MH040533 | MH040566 |
| Gloeandromyces | nycteribiidarum | Megistopoda aranea | Panama | D. Haelew. 1334c | REPLI‐g, crushed | 3 | MH040534 | MH040567 |
| Gloeandromyces | sp. nov. 1 | Trichobius joblingi | Panama | D. Haelew. 1306b | REPLI‐g | 2 | MH040541 | MH040574 |
| Gloeandromyces | sp. nov. 1 | Trichobius joblingi | Panama | D. Haelew. 1322a | REPLI‐g, crushed | 1 | MH040543 | MH040577 |
| Gloeandromyces | sp. nov. 1 | Trichobius joblingi | Panama | D. Haelew. 1327a | REPLI‐g, crushed | 1 | MH040544 | MH040578 |
| Gloeandromyces | sp. nov. 4 | Trichobius joblingi | Trinidad | D. Haelew. 619a | ExNA | 12 | MH040537 | KT800008 |
| Gloeandromyces | sp. nov. 4 | Trichobius joblingi | Panama | D. Haelew. 1073b | ExNA, prolonged, crushed | 3 | MH040538 | MH040570 |
| Gloeandromyces | sp. nov. 4 | Trichobius dugesioides | Panama | D. Haelew. 1089a | ExNA, prolonged, crushed | 4 | MH040539 | MH040571 |
| Gloeandromyces | sp. nov. 4 | Trichobius joblingi | Panama | D. Haelew. 1100b | ExNA, prolonged, crushed | 7 | MH040307 | MH040572 |
| Gloeandromyces | sp. nov. 3 | Trichobius dugesioides | Panama | D. Haelew. 1272a | REPLI‐g, crushed | 2 | MH040540 | MH040573 |
| Gloeandromyces | sp. nov. 4 | Trichobius joblingi | Panama | D. Haelew. 1315a | REPLI‐g, crushed | 1 | — | MH040575 |
| Gloeandromyces | sp. nov. 4 | Trichobius joblingi | Panama | D. Haelew. 1315b | REPLI‐g | 2 | MH040542 | MH040576 |
| Gloeandromyces | pageanus | Trichobius dugesioides | Panama | D. Haelew. 1091b | ExNA, prolonged, crushed | 6 | MH040535 | MG906798 |
| Gloeandromyces | pageanus | Trichobius dugesioides | Panama | D. Haelew. 1367b | EXNA, crushed, FastPrep | 6 | — | MH040568 |
| Gloeandromyces | pageanus | Trichobius dugesioides | Panama | D. Haelew. 1425a | REPLI‐g, crushed | 4 | MH040536 | MH040569 |
| Gloeandromyces | streblae | Trichobius dugesioides | Panama | D. Haelew. 1090a | ExNA, prolonged, crushed | 7 | — | MH040584 |
| Gloeandromyces | streblae | Trichobius joblingi | Panama | D. Haelew. 1306c | REPLI‐g | 4 | MG958012 | MH040585 |
| Gloeandromyces | streblae | Trichobius dugesioides | Panama | D. Haelew. 1308b | REPLI‐g | 2 | MH040549 | MH040586 |
| Gloeandromyces | streblae | Trichobius dugesioides | Panama | D. Haelew. 1309a | REPLI‐g | 1 | MH040550 | MH040587 |
| Gloeandromyces | streblae | Trichobius joblingi | Panama | D. Haelew. 1317a | REPLI‐g | 1 | MH040551 | MH040588 |
| Gloeandromyces | streblae | Trichobius joblingi | Panama | D. Haelew. 1335c | REPLI‐g, crushed | 2 | MH040552 | MH040589 |
| Gloeandromyces | sp. nov. 2 | Trichobius joblingi | Panama | D. Haelew. 1320b | REPLI‐g, crushed | 1 | MH040545 | MH040579 |
| Herpomyces | chaetophilus | Periplaneta americana | USA | D. Haelew. 483b | ExNA | 11 fem | MG438319 | MG438350 |
| Herpomyces | chaetophilus | Periplaneta americana | USA | D. Haelew. 602b | ExNA | 10 fem | KT800023 | KT800009 |
| Herpomyces | periplanetae | Periplaneta americana | USA | D. Haelew. 602d | ExNA | 8 fem | MG438327 | MG438357 |
| Herpomyces | periplanetae | Periplaneta americana | USA | D. Haelew. 1187d | REPLI‐g | 1 fem | MG438331 | MG438359 |
| Herpomyces | shelfordellae | Shelfordella lateralis | Hungary | DE_HerpBL1 | Heat extraction | ±30 | KT800026 | KT800011 |
| Herpomyces | shelfordellae | Shelfordella lateralis | Hungary | Bud_Slat | Heat extraction | 10–20 | MG438333 | MG438361 |
| Herpomyces | stylopygae | Blatta orientalis | Hungary | Bud_Bori | Heat extraction | 10–20 | MG438332 | MG438360 |
| Hesperomyces | coleomegillae | Coleomegilla maculata | Ecuador | 631C | 0.1 × TE buffer + dry ice | 3–15 | KF266882 | — |
| Hesperomyces | coleomegillae | Coleomegilla maculata | Ecuador | 632A | 0.1 × TE buffer + dry ice | 3–15 | KF266880 | — |
| Hesperomyces | palustris | Coleomegilla maculata | Ecuador | 631K | 0.1 × TE buffer + dry ice | 3–15 | KF266902 | — |
| Hesperomyces | palustris | Coleomegilla maculata | Ecuador | 632B | 0.1 × TE buffer + dry ice | 3–15 | KF266891 | — |
| Hesperomyces | virescens | Harmonia axyridis | USA | D. Haelew. 316a | ExNA | 10–12 | MG438339 | KJ842339 |
| Hesperomyces | virescens | Harmonia axyridis | Netherlands | D. Haelew. 334b | ExNA | 10 | MG438340 | MG438364 |
| Hesperomyces | virescens | Olla v‐nigrum | USA | JP352b | ExNA | 11 | MG760581 | MG745337 |
| Hesperomyces | virescens | Olla v‐nigrum | USA | JP353a | QIAamp Micro | 10 | KT800028 | KT800013 |
| Hesperomyces | virescens | Olla v‐nigrum | USA | JP354b | ExNA | 10 | MG760583 | MG745339 |
| Hesperomyces | virescens | Harmonia axyridis | South Africa | D. Haelew. 648c | ExNA | 8–10 | KU574863 | KU574865 |
| Hesperomyces | virescens | Cheilomenes propinqua | South Africa | D. Haelew. 655c | ExNA | 11 | KU574866 | KU574867 |
| Hesperomyces | virescens | Cheilomenes propinqua | South Africa | D. Haelew. 659a/b | ExNA | 20 | MG760590 | MG745342 |
| Hesperomyces | virescens | Harmonia axyridis | Netherlands | D. Haelew. 1174a | ExNA, crushed, prolonged | 12 | MG760598 | MG745345 |
| Hesperomyces | virescens | Adalia bipunctata | Denmark | D. Haelew. 1193g | REPLI‐g, crushed | 1 | MG760599 | MG745346 |
| Hesperomyces | virescens | Adalia bipunctata | Sweden | D. Haelew. 1199h | REPLI‐g, crushed | 1 | MG760600 | MG745347 |
| Hesperomyces | virescens | Olla v‐nigrum | USA | D. Haelew. 1200i | REPLI‐g, crushed | 4 | MG760602 | MG745349 |
| Hesperomyces | virescens | Adalia bipunctata | Italy | D. Haelew. 1231a | REPLI‐g | 2 | MG760603 | MG745350 |
| Hesperomyces | virescens | Psyllobora vigintimaculata | USA | D. Haelew. 1250b | REPLI‐g | 5 | MG760607 | MG745354 |
| Hesperomyces | virescens | Psyllobora vigintimaculata | USA | D. Haelew. 1250c | REPLI‐g, crushed | 2 | MG760608 | MG745355 |
| Hesperomyces | virescens | Psyllobora vigintimaculata | USA | D. Haelew. 1251b | REPLI‐g, crushed | 1 | MG760609 | MG745356 |
| Hesperomyces | virescens | Harmonia axyridis | Japan | D. Haelew. 1268b | REPLI‐g, crushed | 3 | MG760610 | MG745357 |
| Nycteromyces | streblidinus | Trichobius parasiticus | Honduras | D. Haelew. 956a | ExNA | 8 fem | MH040553 | — |
| Nycteromyces | streblidinus | Trichobius joblingi | Panama | D. Haelew. 1324b | REPLI‐g, crushed | 4 m | MH040554 | MH040590 |
| Nycteromyces | streblidinus | Trichobius joblingi | Panama | D. Haelew. 1324c | REPLI‐g, crushed | 1 fem | MH040555 | — |
| Nycteromyces | streblidinus | Trichobius joblingi | Panama | D. Haelew. 1324d | REPLI‐g | 1 fem | MH040556 | MH040591 |
| Nycteromyces | streblidinus | Trichobius joblingi | Panama | D. Haelew. 1324e | REPLI‐g, crushed | 1 m | MH040557 | MH040592 |
| Polyandromyces | coptosomalis | Phoeacia sp. nov. | Ecuador | D. Haelew. 313f | ExNA | 7 fem, 2 m | KT800035 | KT800020 |
| Polyandromyces | coptosomalis | Acrosternum sp. | Canary Islands | HM499a | ExNA | 15 fem, 3 m | MG438347 | — |
| Prolixandromyces | triandrus | Velia (Plesiovelia) saulii | Hungary | Nagyvisnyo1 | Heat extraction | 5 | LT158294 | LT158295 |
| Rickia | laboulbenioides | Cylindroiulus punctatus | Denmark | SR4s | ExNA, crushed | 5 | MH040558 | MH040593 |
| Rickia | pachyiuli | Pachyiulus hungaricus | Serbia | SR1s | ExNA, crushed | 10–12 | MH040559 | MH040594 |
| Rickia | wasmannii | Myrmica scabrinodis | Hungary | DE_Rak4 | Heat extraction | 30 | KT800037 | KT800021 |
| Rickia | wasmannii | Myrmica sabuleti | Netherlands | D. Haelew. 1234a | REPLI‐g | 3 | MH040560 | MH040595 |
| Stigmatomyces | borealis | Parydra breviceps | USA | AW‐797 | JN835186 | — | ||
| Stigmatomyces | ceratophorus | Fannia canicularis | USA | D. Haelew. 1136h | REPLI‐g, crushed | 8 | MG958013 | MH145384 |
| Stigmatomyces | entomophilus | Drosophila funebris | Netherlands | D. Haelew. 1062c | ExNA, prolonged, crushed | 6 | MG958014 | — |
| Stigmatomyces | entomophilus | Drosophila funebris | Netherlands | D. Haelew. 1063a | ExNA, prolonged, crushed | 14 | MH040561 | — |
| Stigmatomyces | gregarius | Diopsidae sp. | Sierra Leone | D. Haelew. 1008a | ExNA | 5 | MG438348 | — |
| Stigmatomyces | gregarius | Diopsidae sp. | Sierra Leone | D. Haelew. 1008b | ExNA | ±10 | MH040562 | — |
| Stigmatomyces | hydrelliae | Hydrellia sp. | 0.1 × TE buffer + dry ice | 4–10 | AF431757 | — | ||
| Stigmatomyces | limnophorae | Muscidae sp. | USA | AW‐785 | 1% Triton 100 | 4–10 | AF407576 | — |
| Stigmatomyces | protrudens | Ephydridae sp. | USA | AW‐793 | 0.1 × TE buffer + dry ice | 4–10 | AF298232 | AF298234 |
| Stigmatomyces | rugosus | Psilopa sp. | 0.1 × TE buffer + dry ice | 4–10 | AF431759 | — | ||
| Stigmatomyces | rugosus | Psilopa sp. | Portugal | D. Haelew. 1138a | ExNA, prolonged, crushed | 6 | MH040563 | — |
| Stigmatomyces | scaptomyzae | Scaptomyza sp. | 0.1 × TE buffer + dry ice | 4–10 | AF431758 | — | ||
| Stigmatomyces | sp. nov. | cf. Chamaemyia | Portugal | D. Haelew. 1137a | ExNA, prolonged, crushed | 8 | MH040564 | — |
| Stigmatomyces | sp. nov. | cf. Chamaemyia | Portugal | D. Haelew. 1137c | ExNA, prolonged, crushed | 1 | MH040565 | — |
Notes. Also included are extraction protocols and numbers of thalli used per extraction for all isolates: 1% Triton 100‐based protocol from Weir and Blackwell (2001a); 0.1 × TE buffer + dry ice protocol from Weir and Blackwell (2001b); heat extraction protocol, Extract‐N‐Amp Plant PCR Kit (ExNA) and QIAamp DNA Micro Kit (QIAamp Micro) from Haelewaters et al. (2015); REPLI‐g Single Cell Kit (REPLI‐g) from Haelewaters et al. (in review). GenBank accession numbers are provided (newly generated sequences in bold).
2.4. Sequence alignment and phylogenetic analyses
SSU and LSU rDNA datasets were constructed of newly generated sequences and sequences downloaded from GenBank, in order to assess (a) the position of bat fly‐associated genera among Laboulbeniales from other hosts and (b) phylogenetic diversity in the genus Gloeandromyces. Alignments were done using Muscle v3.7 (Edgar, 2004) on the Cipres Science Gateway version 3.3 (Miller, Pfeiffer, & Schwartz, 2010) and manually edited in BioEdit v7.2.6 (Hall, 1999). The SSU and LSU aligned data matrices were concatenated in MEGA v7.0.21 (Kumar, Stecher, & Tamura, 2016). Maximum likelihood (ML) analysis of the SSU + LSU dataset was run using PAUP on XSEDE 4.0b (Swofford, 1991), which is available on Cipres. The appropriate nucleotide substitution model was selected by considering the Akaike Information Criterion (AIC) in jModelTest 2.1 (Darriba, Taboada, Doallo, & Posada, 2012). The general time reversible model (GTR) with the assumption of a gamma distribution (+G) gave the best scoring tree (−lnL = 15262.1769). ML was inferred under this model and bootstrap (BS) values were calculated with 200 replicates.
Bayesian analyses were run using the BEAST on XSEDE tool in Cipres with a Markov chain Monte Carlo (MCMC) coalescent approach, under an uncorrelated lognormal relaxed molecular clock model allowing rates of evolution to vary across the tree. The Birth‐Death Incomplete Sampling speciation model (Stadler, 2009) was selected as tree prior with the GTR+G nucleotide substitution model (considering the Bayesian Information Criterion, jModelTest 2.1) and a lognormal ucld.mean (mean = 5.0, SD = 1.0). Four independent runs were performed from a random starting tree for 80 million generations, with a sampling frequency of 8,000. Resulting log files of the individual runs were imported in Tracer v1.6 (Rambaut, Suchard, Xie, & Drummond, 2014) to check trace plots for convergence (= straight hairy‐caterpillar profile; Drummond, Ho, Rawlence, & Rambaut, 2007) and effective sample size (ESS). ESS values were well above 200 and so a minimum burn‐in of 10% was selected for all three runs. Log files and trees files were combined in LogCombiner v.1.8.4 (Drummond, Suchard, Xie, & Rambaut, 2012) after removal of burn‐in. TreeAnnotator v1.8.4 was used to generate consensus trees (0% burn‐in) and to infer the Maximum Clade Credibility (MCC) tree, presenting the highest product of individual clade posterior probabilities. Final trees with bootstrap values (BS) and posterior probabilities (pp) were visualized in FigTree v1.4.3 (tree.bio.ed.ac.uk/software/figtree/).
2.5. Diversity in Gloeandromyces
To assess phylogenetic diversity within the genus Gloeandromyces, the LSU rDNA dataset was used. This region was put forward by previous studies to replace ITS as barcode for species delimitation in Laboulbeniomycetes (D. Haelewaters et al., unpublished data; Walker et al., 2018). Maximum likelihood (ML) analysis was run using the PAUP on XSEDE 4.0b tool (Swofford, 1991). The appropriate nucleotide substitution model was selected statistically with the help of jModelTest 2.1 (Darriba et al., 2012) by considering the Akaike Information Criterion (AIC). A transitional substation model (TIM2) with the assumption of a gamma distribution (+G) gave the best scoring tree (− lnL = 2,114.8480). Rapid bootstrapping (BS) was implemented with 500 replicates. Next, for our Bayesian inference approach, two independent MCMC chains were conducted under a strict molecular clock, with a Yule speciation tree prior (Gernhard, 2008; Yule, 1925) and the TPM2uf+G model of nucleotide substitution as selected by the Bayesian Information Criterion from jModelTest 2.1. The runs were performed from a random starting tree for 40 million generations, with sampling of parameters and trees every 4,000 generations. The two resulting log files were combined in LogCombiner v1.8.4 with 10% burn‐in. Consensus trees with 0% burn‐in were generated and the MCC tree was constructed in TreeAnnotator v.1.8.4.
2.6. Comparison of host and Laboulbeniales phylogenies
Sequence data for analyses were obtained by taking a single isolate per species for both the hosts and Laboulbeniales. For bat flies, mitochondrial cytochrome oxidase gene subunit I (COI) sequences were used. The bat fly dataset included: Brachytarsina alluaudi (outgroup); Exastinion clovisi, Megistopoda aranea, Nycteribia schmidlii, Penicillidia conspicua, P. monoceros, Speiseria ambigua, Trichobius costalimai, Tri. dugesioides, Tri. joblingi, Tri. parasiticus, Tri. yunkeri (hosts); Mastoptera guimaraesi, Paratrichobius longicrus, Strebla wiedemanni (to add structure and provide support to the tree). Penicillidia monoceros is not a host to Laboulbeniales, but this bat fly species was selected as a substitute for P. dufourii, for which no sequences exist. For Laboulbeniales, large subunit ribosomal DNA (LSU rDNA) sequences were used. The dataset of Laboulbeniales included the following species: Herpomyces periplanetae (outgroup); Arthrorhynchus nycteribiae, G. nycteribiidarum, G. pageanus, G. spp. nov. 1–4, G. streblae Clade A, G. streblae Clade B, Nycteromyces streblidinus (species associated with bat flies); Hesperomyces virescens, Polyandromyces coptosomalis, Stigmatomyces protrudens (to add structure and provide support to the tree). Sequences were aligned in Muscle v3.7 (Edgar, 2004) on Cipres. Alignments were visually inspected in BioEdit v7.2.6 (Hall, 1999). Maximum likelihood (ML) phylogenetic trees were generated using RAxML v8.2.X (Stamatakis, 2014) available on Cipres. ML was inferred under a GTRCAT model, with 1,000 bootstrapping replicates. To visualize host–Laboulbeniales interactions, cladograms were generated from the best ML trees in FigTree v1.4.3 and saved as NEXUS files. The co‐phylogeny plot was constructed in R (R Core Team, 2013) using the package “ape” (Paradis, Claude, & Strimmer, 2004).
2.7. Associations network
All presence/absence data of Laboulbeniales on bat flies and bat flies on bats were entered in a database. Data were partitioned to represent distinct climatic zones (temperate, neotropical). The bat–bat fly–Laboulbeniales associations were visualized with the help of the R package “bipartite” (Dormann, Gruber, & Fründ, 2008). Weighted data and the function plotweb were used to build a network showing host‐dependencies and prevalence. Bats and bat flies that were not identified to genus level, bats without specimen label and infected bat flies with unidentified Laboulbeniales were excluded from the analysis. Bats and bat flies for which n < 10 were also excluded.
3. RESULTS
3.1. Nucleotide alignment datasets
We generated 54 sequences of bat fly‐associated Laboulbeniales during this study, of which 26 SSU and 28 LSU sequences. Our SSU + LSU concatenated dataset comprised 3,969 characters, of which 2,962 were constant and 789 were parsimony‐informative. A total of 84 isolates were included (Table 1): Arthrorhynchus (2), Camptomyces (1), Fanniomyces (1), Gloeandromyces (26), Herpomyces (7, outgroup), Hesperomyces (22), Nycteromyces (5), Polyandromyces (2), Prolixandromyces (1), Rickia (4) and Stigmatomyces (13). Our LSU dataset consisted of 27 isolates (including 1 Stigmatomyces as outgroup) and 955 characters, of which 817 were constant and 110 were parsimony‐informative.
3.2. Phylogenetic inferences
The three genera of bat fly‐associated Laboulbeniales occur in three disparate places of our phylogenetic reconstruction of the SSU + LSU dataset (Figure 3): Arthrorhynchus nycteribiae is placed in a sister relationship to Prolixandromyces triandrus with pp = 0.8; Nycteromyces streblidinus is placed in a sister relationship to Polyandromyces coptosomalis with maximum support; and the genus Gloeandromyces is placed sister to the genus Stigmatomyces, with very strong support (ML BS = 99, pp = 1.0). The subtribe Stigmatomycetinae, which holds several genera included in our dataset (Table 2), is a polyphyletic taxon.
Figure 3.
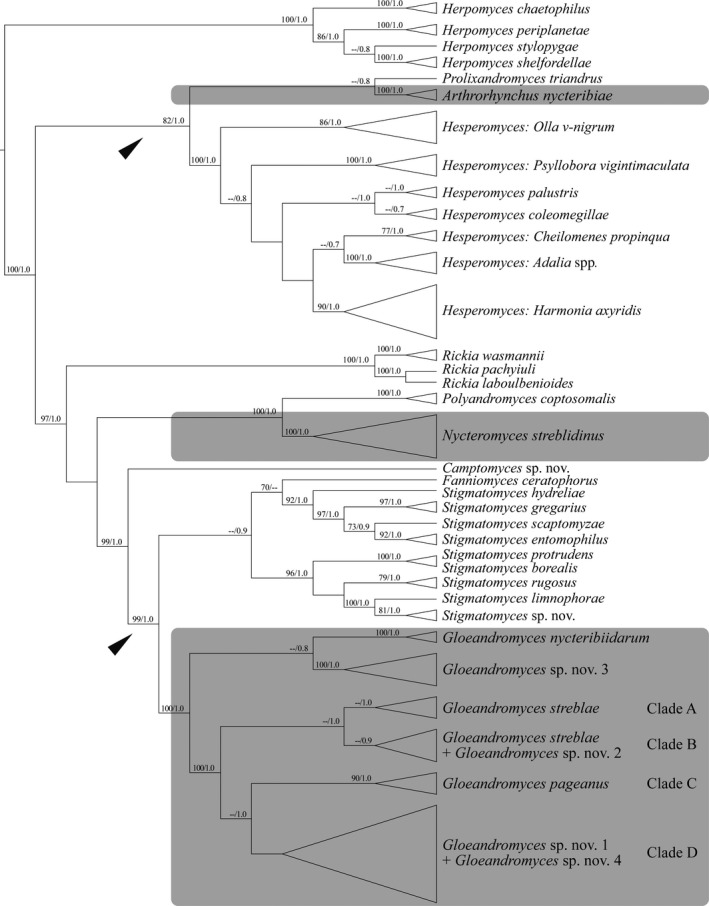
Maximum clade credibility tree, reconstructed from the concatenated SSU + LSU dataset. The tree is the result of a Bayesian analysis performed in BEAST. For each node, ML BS (if ≥70)/Bayesian pp (if ≥0.7) are presented above to the branch leading to that node. The arrowheads denote the Stigmatomycetinae subtribe sensu Tavares (1985)
Table 2.
Genera included in the concatenated SSU + LSU dataset, with classification up to ordinal level
| Order | Genus | Subtribus | Tribus | Subfamily |
|---|---|---|---|---|
| Herpomycetales | Herpomyces | Herpomycetaceae | ||
| Laboulbeniales | Arthrorhynchus | Stigmatomycetinae | Laboulbenieae | Laboulbenioideae |
| Laboulbeniales | Camptomyces | Haplomycetinae | Haplomyceteae | Peyritschielloideae |
| Laboulbeniales | Fanniomyces | Stigmatomycetinae | Laboulbenieae | Laboulbenioideae |
| Laboulbeniales | Gloeandromyces | Stigmatomycetinae | Laboulbenieae | Laboulbenioideae |
| Laboulbeniales | Hesperomyces | Stigmatomycetinae | Laboulbenieae | Laboulbenioideae |
| Laboulbeniales | Nycteromyces | N/A | Dimorphomyceteae | Peyritschielloideae |
| Laboulbeniales | Polyandromyces | N/A | Dimorphomyceteae | Peyritschielloideae |
| Laboulbeniales | Prolixandromyces | Stigmatomycetinae | Laboulbenieae | Laboulbenioideae |
| Laboulbeniales | Rickia | Peyritschiellinae | Peyritschielleae | Peyritschielloideae |
| Laboulbeniales | Stigmatomyces | Stigmatomycetinae | Laboulbenieae | Laboulbenioideae |
In the LSU dataset, Gloeandromyces forms six distinct clades (Figure 4). Gloeandromyces nycteribiidarum and G. sp. nov. 3 (sensu Walker et al., 2018) are sister taxa and have high support. Gloeandromyces streblae falls apart into two clades A and B, each lacking ML BS support but with moderate to high pp support. Gloeandromyces sp. nov. 2 (sensu Walker et al., 2018) falls in Clade B, among isolates of G. streblae. Clade C includes isolates of the recently described G. pageanus. Support for clade C is high (BS = 96, pp = 1.00) whereas support is lacking for its sister clade D, which includes isolates of G. sp. nov. 1 (sensu Walker et al., 2018) and another undescribed form, Gloeandromyces sp. nov. 4. All isolates included in clade D are identical in their LSU. Out of the 955 nucleotides, three are different between the isolates in clade C and those in clade D.
Figure 4.
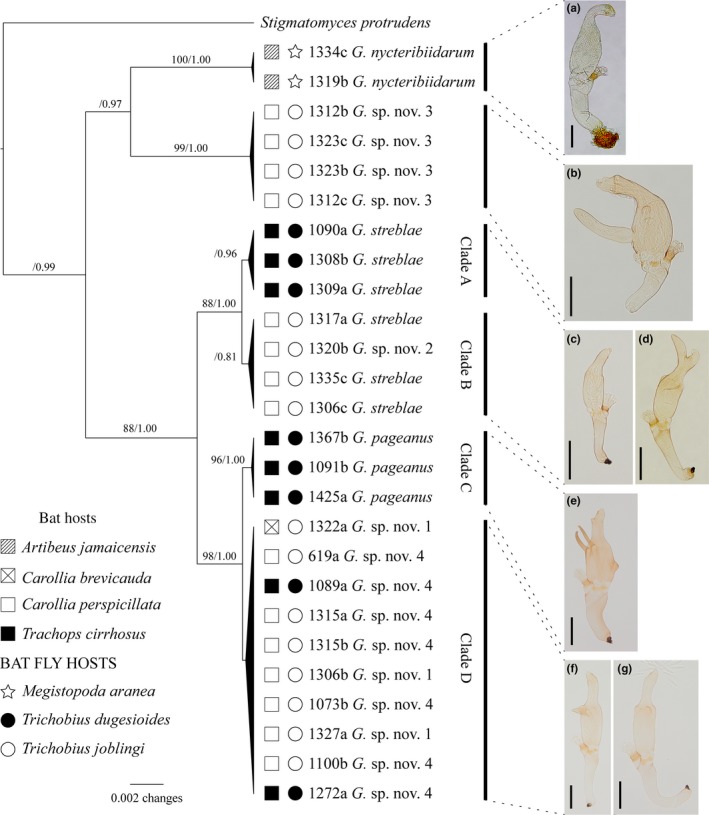
Maximum clade credibility tree showing species in the genus Gloeandromyces, with Stigmatomyces protrudens as outgroup. The tree is the result of a Bayesian analysis of the LSU dataset performed in BEAST. For each node, ML BS (if ≥70)/Bayesian pp (if ≥0.7) are presented above the branch leading to that node. At the right, thalli are shown of the different morphologies observed. From top to bottom: Gloeandromyces nycteribiidarum, G. sp. nov. 3, G. streblae (left) and G. sp. nov. 2 (right), G. pageanus, G. sp. nov. 1 (left), and G. sp. nov. 4 (right)
3.3. Bats, bat flies and Laboulbeniales
Our complete dataset, prior to excluding specimens and partitioning (Supporting Information), was composed of 2,599 bats and 7,949 bat flies, of which 363 (= 4.6%) were infected by Laboulbeniales. Seven bat species were included in our final temperate dataset (Haelewaters et al., 2017a; Szentiványi et al., 2018). The most abundantly parasitized bat species was Miniopterus schreibersii (n = 414), followed by Myotis daubentonii (n = 206). Eight species of bat flies were removed from bats: Basilia natali (n = 10), Nycteribia kolenatii (n = 899), N. pedicularia (n = 24), N. schmidlii (n = 607), N. vexata (n = 13), Penicillidia conspicua (n = 278), P. dufourii (n = 134) and Phthiridium biarticulatum (n = 36). The highest number of bat flies was found on M. schreibersii bats (n = 942 bat flies altogether), closely followed by M. daubentonii (n = 896 bat flies). On the other bat species, less than 100 bat flies per species were found altogether. Laboulbeniales infection was found on three bat fly species: Nycteribia schmidlii (n = 26 + 1), Penicillidia conspicua (n = 59), and P. dufourii (n = 6). The overall parasite prevalence of Laboulbeniales on temperate bat flies was 4.6%. Nycteribia schmidlii was host for two species of Laboulbeniales, A. eucampsipodae (n = 26) and A. nycteribiae (n = 1). Both Penicillidia host species only carried A. nycteribiae thalli. Associations are shown in Figure 5.
Figure 5.
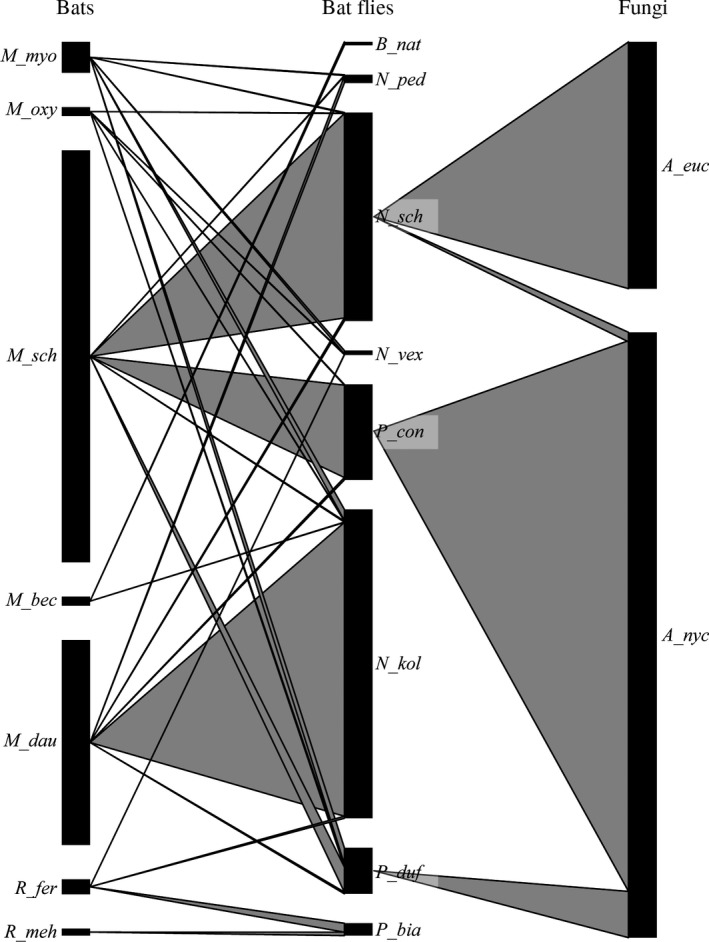
Host–parasite–parasite network of the final temperate dataset. Shown is the association of bat flies with their bat hosts (left) as well as the association of Laboulbeniales (right) and their bat fly hosts. Bar width represents the relative abundance of a species within each network level
In our neotropical dataset (Figure 6) 1,703 bats were present, Artibeus jamaicensis (n = 660), Carollia perspicillata (n = 333) and Pteronotus parnellii (n = 114) being the most abundant in addition to 19 other species (with each <70 individuals). The highest number of bat flies was found on A. jamaicensis bats (n = 1,309 bat flies altogether), followed by C. perspicillata (n = 1,102), P. parnellii (n = 755), and Trachops cirrhosus (n = 334). Of 39 sampled species of bat fly species, nine carried Laboulbeniales thalli (in decreasing order): Trichobius joblingi (n = 50 infected specimens), Tri. dugesioides (n = 19), Tri. yunkeri (n = 4), Megistopoda aranea, Tri. sphaeronotus (n = 3), Tri. parasiticus (n = 2), Exastinion clovisi, Speiseria ambigua, and Tri. costalimai (n = 1). The most frequently encountered species of Laboulbeniales was Gloeandromyces streblae (on 33 bat flies of three species), followed by Nycteromyces streblidinus (on 21 bat flies of four species). Trichobius joblingi was not only most often infected with Laboulbeniales, it also bore the highest number of Laboulbeniales taxa: Gloeandromyces sp. nov. 1, G. sp. nov. 2, G. sp. nov. 3, G. sp. nov. 4, G. streblae, and N. streblidinus. Gloeandromyces nycteribiidarum had the highest number of host species: E. clovisi, Megistopoda aranea, Tri. costalimai, Tri. sphaeronotus and Tri. yunkeri.
Figure 6.
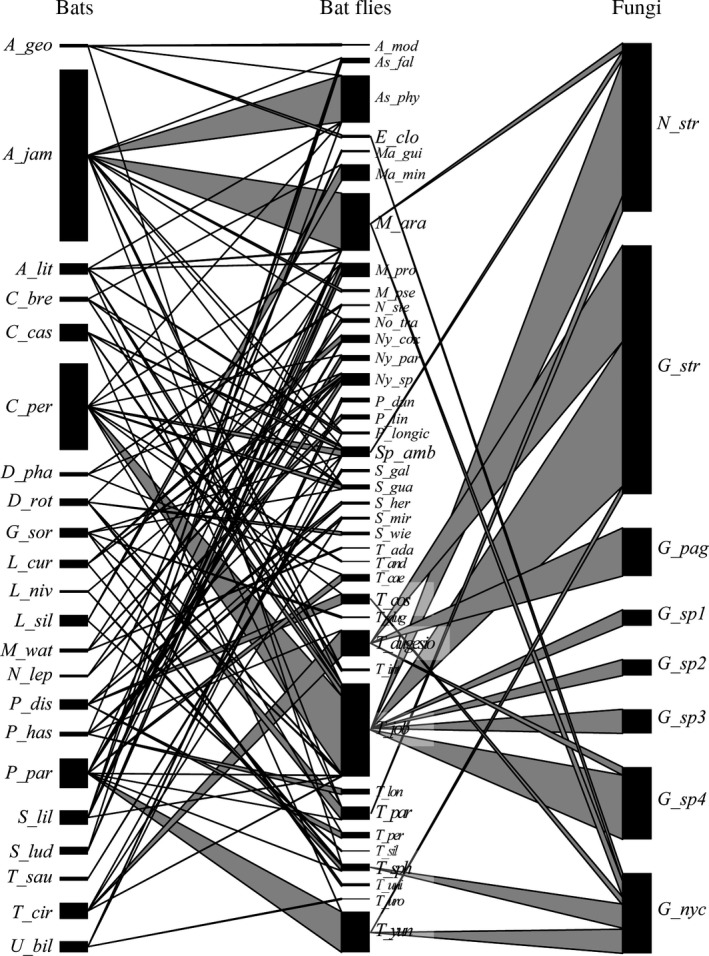
Host–parasite–parasite network of the final neotropical dataset. Shown is the association of bat flies with their bat hosts (left) as well as the association of Laboulbeniales (right) and their bat fly hosts. Bar width represents the relative abundance of a species within each network level
3.4. Co‐phylogenetic relationships between bat flies and Laboulbeniales
Our COI dataset of bat flies consisted of 15 taxa (one outgroup) and 677 characters, of which 410 were constant and 177 were parsimony‐informative. Our LSU dataset of Laboulbeniales consisted of 14 taxa (1 outgroup) and 998 characters, of which 610 were constant and 217 were parsimony‐informative. The co‐phylogeny plot is shown in Figure 7. There is congruence between the (basal‐most) Old World clades, otherwise the evidence for coevolution is lacking.
Figure 7.
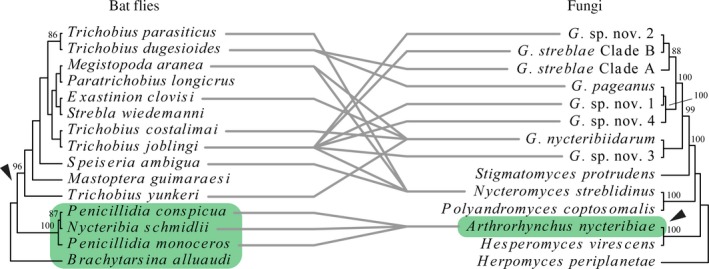
Co‐phylogenetic relationships between bat flies and Laboulbeniales. Maximum likelihood phylogenies for bat flies (left) and their Laboulbeniales parasites (right). For each node, ML BS (if ≥70) are presented above the branch leading to that node. All associations are shown as gray connecting lines. Old World bat flies and Laboulbeniales are highlighted in green. Penicillidia monoceros substituted for Penicillidia dufourii
4. DISCUSSION
4.1. Bats and bat flies in Panama
Bats are the most diverse mammal group in Panama, with a total of 119 documented species (Moras, Gregorin, Sattler, & Tavares, 2018; Samudio & Pino, 2014). Although species reports are numerous, many come from lowland research (Handley, 1966; Samudio, 2002). This implies that mammal inventories have not been conducted in many highland Panamanian regions, such as Chiriquí and the unexplored Darién Gap. We chose to conduct intensive fieldwork in one such area, a private cloud‐forested nature reserve in Darién, Reserva Natural Chucantí, managed by the NGO Adopt a Panama Rainforest (ADOPTA). Most of the bat flies infected by species of Laboulbeniales used in this study were collected in this reserve. With a team of six, we captured bats at Chucantí for seven nights, investing 68 mnh (mistnet hours, 1 mnh = a single 6 m‐wide mistnet open for 1 hr). We captured 227 bats representing 17 species. We captured Micronycteris schmidtorum, a species reported previously only from the Los Santos Province (Handley, 1966). In addition, we encountered the rarely collected Platyrrhinus dorsalis, representing the westernmost report of this species (Velazco, 2005). Of the captured bats, 148 carried bat flies (65%). The number of sampled bat flies was 437, representing 16 species. One species was a new country record (Trichobius anducei) and five species represented first reports for Darién (Basilia anceps, Anatrichobius scorzai, Nycterophilia parnelli, Tri. johnsonae, Tri. parasiticus) (Guerrero, 1998a; Lourenço, Almeida, & Famadas, 2016; Stamper, 2012; Table 3). Of all screened bat flies, 30 bore species of Laboulbeniales (6.86%). The results of the tripartite survey at Chucantí were published by Walker et al. (2018).
Table 3.
All species of bat flies reported in Panama to date. Bat flies reported as host to Laboulbeniales fungi are bolded, details are provided in the last column
| Bat fly species | Reference(s) | Reported Laboulbeniales taxa |
|---|---|---|
| Nycteribiidae | ||
| Basilia anceps | Guimarães (1966), Walker et al. (2018) | |
| Basilia dunni | Guimarães (1966) | |
| Basilia ferruginea | Guimarães (1966) | |
| Basilia handleyi | Guimarães (1966) | |
| Basilia myotis | Guimarães (1966) | |
| Basilia tiptonii | Guimarães (1966) | |
| Basilia wenzeli | Guimarães (1966) | |
| Streblidae | ||
| Anastrebla mattadeni | Wenzel and Tipton (1966) | |
| Anastrebla modestini | Wenzel and Tipton (1966) | |
| Anastrebla nycteridis | Wenzel and Tipton (1966) | |
| Anatrichobius scorzai | Wenzel and Tipton (1966), Walker et al. (2018) | |
| Aspidoptera phyllostomatis | González et al. (2004), Wenzel and Tipton (1966), Walker et al. (2018) | |
| Aspidoptera delatorrei | González et al. (2004), Wenzel and Tipton (1966) | |
| Eldunnia breviceps | Wenzel and Tipton (1966) | |
| Exastinion clovisi | Wenzel and Tipton (1966) | Gloeandromyces nycteribiidarum, Mexico (this study); Laboulbeniales gen. & sp. indet., Brazil (Bertola et al., 2005) |
| Joblingia schmidti | Wenzel and Tipton (1966) | |
| Mastoptera guimaraesi | González et al. (2004), Wenzel and Tipton (1966) | |
| Mastoptera minuta | Wenzel and Tipton (1966) | |
| Megistopoda aranea | González et al. (2004), Wenzel and Tipton (1966), Walker et al. (2018) | Gloeandromyces nycteribiidarum, Grenada (Thaxter, 1917), Panama (Walker et al., 2018); G. streblae & Nycteromyces streblidinus, Panama (Walker et al., 2018); Laboulbeniales gen. & sp. indet., Brazil (Bertola et al., 2005) |
| Megistopoda proxima | Wenzel and Tipton (1966), Walker et al. (2018) | Laboulbeniales gen. & sp. indet., Brazil (Bertola et al., 2005) |
| Megistopoda theodori | Wenzel and Tipton (1966) | |
| Metelasmus pseudopterus | González et al. (2004), Wenzel and Tipton (1966) | |
| Neotrichobius stenopterus | González et al. (2004), Wenzel and Tipton (1966) | |
| Noctiliostrebla maai | Wenzel and Tipton (1966) | |
| Noctiliostrebla traubi | Wenzel and Tipton (1966) | |
| Nycterophilia fairchildi | Wenzel and Tipton (1966) | |
| Nycterophilia natali | Wenzel and Tipton (1966) | |
| Nycterophilia parnelli | Wenzel and Tipton (1966), Walker et al. (2018) | |
| Paradyschiria lineata | Wenzel and Tipton (1966) | |
| Paradyschiria parvuloides | Wenzel and Tipton (1966) | |
| Parastrebla handleyi | Wenzel and Tipton (1966) | |
| Paratrichobius dunni | González et al. (2004), Wenzel and Tipton (1966) | |
| Paratrichobius longicrus | Wenzel and Tipton (1966), Walker et al. (2018) | Laboulbeniales gen. & sp. indet., Brazil (Bertola et al., 2005) |
| Paratrichobius lowei | Wenzel and Tipton (1966) | |
| Paratrichobius salvini | González et al. (2004), Wenzel and Tipton (1966) | |
| Paratrichobius sanchezi | Wenzel and Tipton (1966) | |
| Paratrichobius sp. (longicrus complex) | Wenzel and Tipton (1966) | |
| Pseudostrebla greenwelli | Wenzel and Tipton (1966) | |
| Pseudostrebla ribeiroi | Wenzel and Tipton (1966) | |
| Speiseria ambigua | González et al. (2004), Wenzel and Tipton (1966), Walker et al. (2018) | Gloeandromyces streblae, Ecuador; Nycteromyces streblidinus, Honduras (this study); Laboulbeniales gen. & sp. indet., Costa Rica (Fritz, 1983) |
| Strebla altmani | Wenzel and Tipton (1966) | |
| Strebla alvarezi | Wenzel and Tipton (1966) | |
| Strebla guajiro | González et al. (2004), Wenzel and Tipton (1966), Walker et al. (2018) | Laboulbeniales gen. & sp. indet., Costa Rica (Fritz, 1983) |
| Strebla christinae | Wenzel and Tipton (1966) | |
| Strebla diaemi | Wenzel and Tipton (1966) | |
| Strebla galindoi | Wenzel and Tipton (1966) | |
| Strebla hertigi | Wenzel and Tipton (1966) | |
| Strebla hoogstraali | Wenzel and Tipton (1966) | |
| Strebla kohlsi | Wenzel and Tipton (1966) | |
| Strebla mirabilis | González et al. (2004), Wenzel and Tipton (1966) | |
| Strebla wiedemanni | Wenzel and Tipton (1966) | Gloeandromyces streblae & Nycteromyces streblidinus, Venezuela (Thaxter, 1917) |
| Trichobioides perspicillatus | Wenzel and Tipton (1966) | |
| Trichobius anducei | Walker et al. (2018) | |
| Trichobius bequarti | Wenzel and Tipton (1966) | |
| Trichobius brennani | Wenzel and Tipton (1966) | |
| Trichobius costalimai | Wenzel and Tipton (1966) | Gloeandromyces nycteribiidarum, Panama (this study) |
| Trichobius dugesii | Wenzel and Tipton (1966) | Laboulbeniales gen. & sp. indet., Brazil (Bertola et al., 2005) |
| Trichobius dugesioides | Wenzel and Tipton (1966), Walker et al. (2018) | Gloeandromyces pageanus & G. streblae, Panama (Haelewaters et al., 2017b; Walker et al., 2018) |
| Trichobius dunni | Wenzel and Tipton (1966) | |
| Trichobius galei | Wenzel and Tipton (1966) | |
| Trichobius joblingi | González et al. (2004), Wenzel and Tipton (1966), Walker et al. (2018) | Gloeandromyces spp. nov. 1–4 & G. streblae, Panama (Haelewaters et al., 2017b; Walker et al., 2018); Laboulbeniales gen. & sp. indet., Costa Rica (Fritz, 1983) |
| Trichobius johnsonae | Wenzel and Tipton (1966), Walker et al. (2018) | |
| Trichobius keenani | Wenzel and Tipton (1966) | |
| Trichobius lionycteridis | Wenzel and Tipton (1966) | |
| Trichobius lonchophyllae | Wenzel and Tipton (1966) | |
| Trichobius longipes | González et al. (2004), Wenzel and Tipton (1966) | |
| Trichobius macrophylli | Wenzel and Tipton (1966) | |
| Trichobius mendezi | Wenzel and Tipton (1966) | |
| Trichobius parasiticus | Wenzel and Tipton (1966), Walker et al. (2018) | Nycteromyces streblidinus, Honduras (this study) |
| Trichobius sparsus | González et al. (2004), Wenzel and Tipton (1966) | |
| Trichobius uniformis | Wenzel and Tipton (1966) | Laboulbeniales gen. & sp. indet., Brazil (Bertola et al., 2005) |
| Trichobius urodermae | Wenzel and Tipton (1966) | |
| Trichobius vampyropis | Wenzel and Tipton (1966) | |
| Trichobius yunkeri | Wenzel and Tipton (1966), Walker et al. (2018) | Gloeandromyces nycteribiidarum, Costa Rica; G. streblae, Panama (Haelewaters et al., 2017b) |
4.2. Prevalences
A comprehensive study of nycteribiid bat fly‐associated Laboulbeniales was conducted by Blackwell (1980b). She screened 2,517 bat flies, of which 56 were infected with Arthrorhynchus eucampsipodae or A. nycteribiae, denoting a parasite prevalence of 2.2%. In our larger study, we screened 7,949 bat flies of which 363 were infected by Laboulbeniales (4.6%). This includes both temperate and neotropical material. Taking only temperate flies into consideration (n = 2,001), parasite prevalence was again 4.6%. These low percentages can be explained by life history traits of the bat flies. Deposition of third instar larvae occurs on roosting substrates. Therein lies some risk, because flies need to return to their host within 25 hr. Since the flies are so closely tied to their bat host, we assume that transmission of ascospores of the fungi only occurs on the bat itself, most likely through direct contact (De Kesel, 1995). Host grooming is the main cause of death for bat flies (Marshall, 1981). Apparently, this behavior is an important selective factor driving evolution of host specific and even position‐specific parasites (ter Hofstede, Fenton, & Whitaker, 2004) and may to some extent be an explanatory factor in the observed patterns of Laboulbeniales.
Several studies confirm that bats are often infected by several bat fly species (Dick & Gettinger, 2005; Wenzel, 1976; Wenzel, Tipton, & Kiewlicz, 1966). At the same time, the average number of (nycteribiid) bat flies on their bat hosts is only 1.79 (Haelewaters et al., 2017a). This number depends on bat host species and is much higher for Myotis daubentonii (up to 21) and Miniopterus schreibersii (up to 13). A majority of Laboulbeniales species are strictly host specific. For those taxa occurring on several host species, such as Arthrorhynchus nycteribiae, caution is required in the assessment of their ecology—it is possible that these represent more than a single species. All in all, the number of times an infected bat fly comes into contact with new potential hosts (of the same species) may be very low.
4.3. Independent lineages of bat fly‐associated Laboulbeniales
Parasitism of bat flies by Laboulbeniales arose at least three times independently, once in the Eastern Hemisphere and twice in the Western Hemisphere. The genus Gloeandromyces is placed sister to the speciose genus Stigmatomyces, species of which infect only flies. The other two bat fly‐associated genera form two separate clades, both sisters to a genus of Laboulbeniales that is associated with true bugs (Hemiptera). Arthrorhynchus and Prolixandromyces form a clade with moderate Bayesian support. The genus Prolixandromyces consists of eight species parasitizing taxa in the semi‐aquatic family Veliidae (Weir, 2008). Nycteromyces forms a clade with Polyandromyces; the basal node of this clade received maximum support. Polyandromyces is a monotypic genus; its sole representative, P. coptosomalis, occurs on terrestrial species in the families Pentatomidae and Plataspidae. In other words, using the phylogenetic reconstruction of the SSU + LSU dataset, for the first time including molecular data from the rarely sampled bat fly‐associated Laboulbeniales, we identified two interordinal host shifts (true bugs to bat flies). We hypothesize that two bat fly‐associated lineages, Arthrorhynchus and Nycteromyces, have independently evolved from lineages of true bug ectoparasites. Tavares (1985) noted that bugs are secondary hosts to Laboulbeniales, and that their fungus parasites arose from taxa occurring on beetles (Coleoptera). We cannot confirm this suggestion because our phylogenetic reconstruction is far from complete and does not encompass many taxa with beetle hosts. However, it is clear that Laboulbeniales on beetle hosts are evolutionary very successful; 80% of known species are reported from beetles (Weir & Hammond, 1997). In contrast, the numbers of known species from bugs is 4%, whereas the number from bat flies is less than 1%.
Is it possible bat fly‐associated lineages have evolved from bug‐associated lineages? Representatives of both host groups make use of the bat microhabitat and roost environment. Two families of terrestrial bugs are known as obligatory hematophagous ectoparasites: Cimicidae and Polyctenidae (Schuh & S̆tys, 1991). Both families belong to the superfamily Cimicoidea, along with Anthocoridae, Lasiochilidae, Lyctocoridae, and Plokiophilidae (Jung, Kim, Yamada, & Lee, 2010; Schuh & S̆tys, 1991). One lasiochilid, Lasiochilus pallidulus, has been found as a host to Cupulomyces lasiochili in Grenada, a member of the Stigmatomycetinae subtribe (Benjamin, 1992a). Benjamin (1992a) used the family name Anthocoridae for the host but he probably used this in the broad sense, whereas Schuh and S̆tys (1991) proposed to split up this non‐monophyletic family into three, Anthocoridae sensu stricto, Lasiochilidae, and Lyctocoridae. Lasiochilids live on the ground, under bark and in vegetation (Schuh & Slater, 1995). It is probable that transmission of ascospores occurs now and then between bugs and bat flies and that this at some point in time may have led to segregation of populations, microevolutionary changes and ultimately speciation.
Only C. lasiochili has been found on either cimicid or polyctenid bugs, but the limitation with Laboulbeniales reports is that the absence of reports on certain host groups is due to a lack of sampling and screening efforts. We recommend that future studies focus on screening bugs for Laboulbeniales parasites and on generating molecular data for taxa found on bugs. The phylogenetic placement of these taxa, including C. lasiochili, will be a crucial data point in evaluating our hypothesis. Cupulomyces and Prolixandromyces, which is represented in our phylogeny by P. triandrus, have a similar receptacle structure (Figure 8): cell II is positioned posterior and next to cell I, separated by an oblique septum, and cell II carries cells III obliquely and VI distally (Benjamin, 1981, 1992a). In Cupulomyces, the perithecial wall cells are arranged in five tiers (Benjamin, 1992a). The situation has been described differently for Prolixandromyces, where in each vertical row of outer wall cells there are four tiers. However, Tavares (1985) mentioned that the fourth tier “may divide by maturity” even though the septa are extremely thin. Five tiers can be observed in drawings of mature thalli by Benjamin (1981: figure 13, reproduced here) and Weir (2008: figure 10). Consequently, also the perithecial outer wall structure is similar between both genera. Incorporating sequence data for Cupulomyces into our phylogenetic reconstruction will help elucidate whether contact between insects in the bat roost environment may have mediated host jumps to and subsequent speciation of Laboulbeniales on bat flies.
Figure 8.
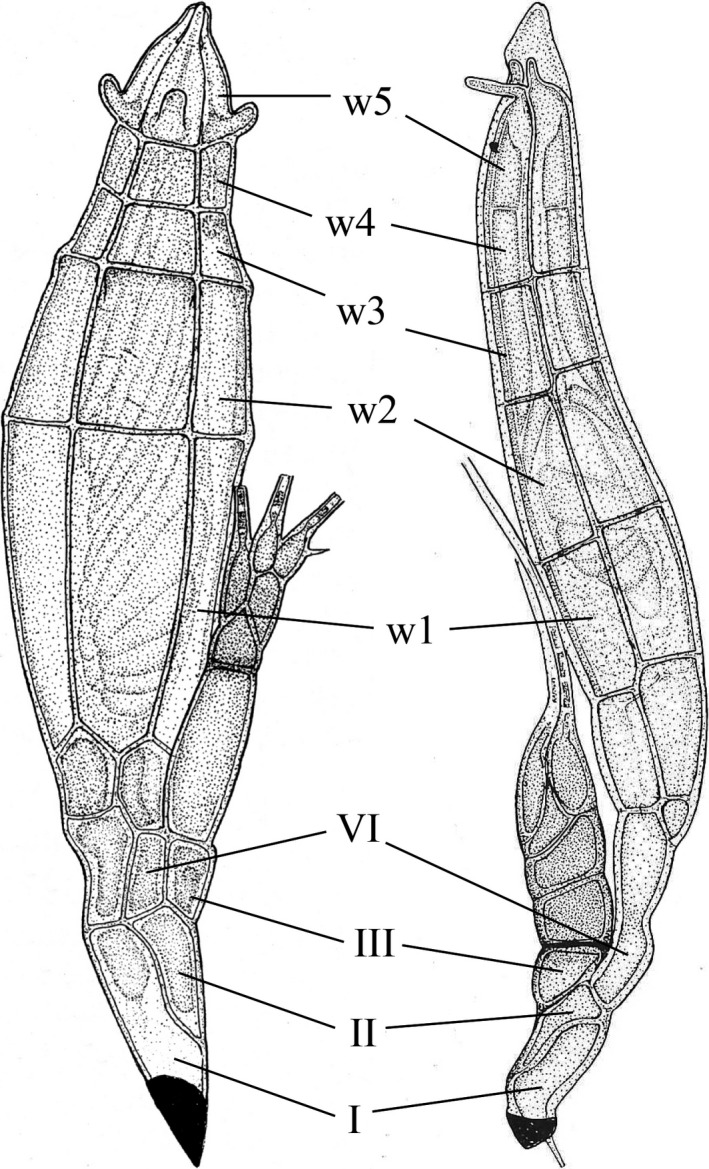
Comparison of two species of Laboulbeniales. Left. Mature thallus of Cupulomyces lasiochili, reproduced from Benjamin (1992a). Right. Mature thallus of Prolixandromyces rhinoceralis, reproduced from Benjamin (1981). Annotated are cells I, II, III, and VI, and tiers of perithecial outer wall cells (w1 to w5)
4.4. Polyphyly of subtribe Stigmatomycetinae
The subtribe Stigmatomycetinae is characterized by a simple receptacle consisting of three superposed cells, of which cell II carries the stalk cell of the perithecium (cell VI) and cell III carries the appendage. Taking synonymies and recent additions into consideration, Stigmatomycetinae now holds 40 genera (Benjamin, 1992a, 1992b, 2001; Santamaria, 1995; Tavares, 1985; Tavares & Balazuc, 1989). Our phylogenetic analysis shows that this subtribe is polyphyletic. We found two well‐supported clades. One clade consists of Gloeandromyces and Stigmatomyces (including its synonym, Fanniomyces), the second clade includes Arthrorhynchus, Hesperomyces, and Prolixandromyces. Even Thaxter's (1908) original circumscription of what he called the “Stigmatomyceteae” tribe, including only five genera, Acallomyces, Acompsomyces, Arthrorhynchus, Polyascomyces, and Stigmatomyces, is polyphyletic. These findings undermine classification systems of both Thaxter (1908) and Tavares (1985) and are in line with Goldmann and Weir (2018), who retrieved 12 genera of Stigmatomycetinae in three unrelated clades.
4.5. Associations between bat flies and Laboulbeniales
Both the temperate bat flies and Laboulbeniales are geographically separated from their neotropical counterparts, so it is no surprise that we observe congruence of the Old World‐clades. The other relationships are difficult to disentangle from an evolutionary point of view. Nycteromyces streblidinus is a plurivorous species, with hosts in the genera Megistopoda, Speiseria, and Trichobius. All these are parasitic on phyllostomid bats that commonly roost in hollow trees (Kunz & Lumsdem, 2003; Overal, 1980; Wenzel et al., 1966). Also G. nycteribiidarum is plurivorous, with hosts in the genera Exastinion, Megistopoda, and Trichobius. The ecology of the bat hosts of these bat flies is similar. Mormoopidae (Pteronotus parnellii, host of Tri. yunkeri) almost always roost in caves or mines. Anoura geoffroyi (host of Exastinion clovisi) and Phyllostomus discolor (host of Tri. costalimai) preferably roost in caves. The morphospecies within G. pageanus and G. streblae are restricted to a single host species. We cannot provide an evolutionary explanation for the observed Neotropical patterns in the co‐phylogeny plot, instead we think the patterns can be linked to the roosting ecology of the bat hosts.
Artibeus and Sturnira are two genera of bats (Phyllostomidae, Stenodermatinae) that use hollow trees as main roosting sites, whereas most other stenodermatine bats roost in foliage or leaf tents (Evelyn & Stiles, 2003; Garbino & Tavares, 2018; Patterson et al., 2007). As a consequence, species of three genera of bat flies parasitize these two host genera. Megistopoda proxima, Metelasmus wenzeli, Aspidoptera delatorrei, and A. falcata parasitize species of Sturnira; and Megistopoda aranea, Metelasmus pseudopterus and Aspidoptera phyllostomatis parasitize species of Artibeus (Graciolli & Dick, 2004). This pattern can be generalized: bats with similar roosting behaviors share similar parasite species. Upon adding another parasite level, it is not hard to imagine that these fungi can be on several, even distantly related species of bat flies, when their bat hosts share the same roosts.
4.6. Morphological diversity versus phylogenetic diversity
Based on morphological study, we identified seven species of Gloeandromyces. These are G. nycteribiidarum, G. pageanus, G. streblae and four undescribed, putative species (Figure 4a–g). However, this morphological diversity is not reflected in molecular structuring based on the LSU rDNA region. Gloeandromyces sp. nov. 1 and G. sp. nov. 4 (names consistent with Walker et al., 2018) are identical based on sequence data but show morphological differences. In other words, these isolates are not independent species; instead they represent morphotypes as part of the phenotypic plasticity exhibited by a single phylogenetic species (sensu Goldmann & Weir, 2012; Goldmann et al., 2013). In the case of G. pageanus and G. streblae, we reveal specialization to host species. For G. streblae, no obvious morphological features are observed to distinguish between thalli from Tri. dugesioides (Clade A) and Tri. joblingi (Clade B). In fact, G. streblae exhibits high phenotypic plasticity (West‐Eberhard, 1989). In the case of G. streblae, this plasticity makes it hard to make morphologically based identifications. Some thalli are morphologically so similar to G. sp. nov. 4 that it is difficult to impossible to separate these taxa without sequence data. We have observed and included in our molecular work a range of G. streblae thalli, from short, stout, and curved to elongate, some with conspicuous bumps at the distal end of the perithecial venter. Even so, two clades were retrieved that are only segregated by host species. There is one exception: isolate D. Haelew. 1320b represents Gloeandromyces sp. nov. 2, which in reality is a morphotype. This morphotype was removed from the last sternite/tergite. We believe the sigmoid habitus of this morphotype is a consequence of morphological adaptions induced by growing on that specific portion of the insect integument.
5. CONCLUSIONS
This study has not only substantially increased our knowledge about bats and their ectoparasitic associates, but also shown the need to include molecular data in Laboulbeniales taxonomy. Several phenomena come into play in the morphological and phylogenetic diversity of these parasites. Phenotypic plasticity and position‐induced morphological adaptations go hand in hand. Position‐induced morphotypes belong to the same phylogenetic species. In Chitonomyces, transmission of ascospores during mating between hosts seems to be the mechanism leading to position specific morphotypes (Goldmann & Weir, 2012). For bat fly‐associated Laboulbeniales, it is unclear what is driving morphological divergence within phylogenetic species. Another important contributor to diversity, whether or not ephemeral or incipient (Rosenblum et al., 2012), is host specialization. Segregation by host species is observed for at least two bat fly‐associated species. Concerning studies in diversity and taxonomy of Laboulbeniales, our main recommendation is to always include molecular data. The examples discussed in this study have made it clear that it has become impossible to assess diversity by morphology alone.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest with regard to this article.
DATA ACCESSIBILITY
The complete dataset of bats, batflies and Laboulbeniales is available as Supporting Information (in Excel format). All sequence data used in this study have been submitted to GenBank under accession numbers MH040307 & MH040533–MH040595. Sequence alignments generated during this study (in NEXUS format), input XML and output log and trees files from Bayesian analyses may be accessed from the figshare online repository with the URL https://doi.org/10.6084/m9.figshare.c.4154336.v1.
AUTHOR CONTRIBUTIONS
D.H. initiated the project, generated all sequence data, performed molecular phylogenetic analyses, prepared all figures, described the new species and morphotypes, and wrote the manuscript with input from R.A.P. and D.H.P. R.A.P. contributed to fieldwork and facilitated research in Panama. D.H.P. provided expertise at all stages of research.
Supporting information
ACKNOWLEDGMENTS
All capturing and sampling procedures were licensed and approved by the Smithsonian Tropical Research Institute (IACUC protocol: 2017‐0102‐2020‐A5) and the Government of Panama (Ministerio de Ambiente de Panamá: SE/AH‐2‐16, SC/AH‐1‐17, SE/P‐13‐17). We would like to thank Carl W. Dick (Western Kentucky University) for providing thousands of well‐curated bat fly specimens for this study; Thomas Hiller (University of Ulm, Germany) for bat fly identifications; Jasmin J. Camacho, Annabel Dorrestein, Lauren A. Meckler, Kirk A. Silas and Melissa J. Walker for assistance in the field; Guido C. Berguido (Asociación Adopta el Bosque Panamá) and the Gamboa Bat Lab (Smithsonian Tropical Research Institute, Panama) for generous logistical assistance during fieldwork; and W. Owen McMillan (Smithsonian Tropical Research Institute, Panama) and Edilma Gomez (Molecular Multi‐User's Lab, Panama) for facilitating molecular work in Panama. Funding for fieldwork was received from the following sources (to D.H.): Harvard University Herbaria (Fernald Fund), David Rockefeller Center for Latin American Studies (Summer Research Travel Grant), Smithsonian Tropical Research Institute (Short‐Term Research Fellowship) and Mycological Society of America (Graduate Fellowship, Robert W. Lichtwardt Student Research Award). The publication of this manuscript was supported by the Harvard Open‐Access Publishing Equity (HOPE) fund.
Haelewaters D, Page RA, Pfister DH. Laboulbeniales hyperparasites (Fungi, Ascomycota) of bat flies: Independent origins and host associations. Ecol Evol. 2018;8:8396–8418. 10.1002/ece3.4359
REFERENCES
- Arnold, A. E. , & Lutzoni, F. (2007). Diversity and host range of foliar fungal endophytes: Are tropical leaves biodiversity hotspots? Ecology, 88, 541–549. 10.1890/05-1459 [DOI] [PubMed] [Google Scholar]
- Barquez, R. M. , Perez, S. , Miller, B. , & Diaz, M. (2015). Artibeus lituratus. The IUCN red list of threatened species 2015. Available at http://www.iucnredlist.org/details/2136/0 (accessed 06 October 2017).
- Benjamin, R. K. (1971). Introduction and supplement to Roland Thaxter's contribution towards a monograph of the Laboulbeniaceae. Bibliotheca Mycologica, 30, 1–155. [Google Scholar]
- Benjamin, R. K. (1981). Laboulbeniales on semiaquatic Hemiptera. IV. Addenda to Prolixandromyces. Aliso, 10, 1–17. 10.5642/aliso [DOI] [Google Scholar]
- Benjamin, R. K. (1992a). Cupulomyces, a new genus of Laboulbeniales (Ascomycetes) based on Stigmatomyces lasiochili . Aliso, 13, 355–364. 10.5642/aliso [DOI] [Google Scholar]
- Benjamin, R. K. (1992b). A new genus of Laboulbeniales (Ascomycetes) on a species of Phalacrichus (Coleoptera: Dryopoidea; Limnichidae), with a note on mirror‐image asymmetry in the order. Aliso, 13, 427–446. 10.5642/aliso [DOI] [Google Scholar]
- Benjamin, R. K. (2001). Autophagomycetes, Bordea, and a new genus, Rossiomyces (Laboulbeniales). Aliso, 19, 99–136. [Google Scholar]
- Benjamin, R. K. , & Shanor, L. (1952). Sex of host specificity and position specificity of certain species of Laboulbenia on Bembidion picipes . American Journal of Botany, 39, 125–131. 10.1002/j.1537-2197.1952.tb14255.x [DOI] [Google Scholar]
- Bertola, P. B. , Aires, C. C. , Favorito, S. E. , Graciolli, G. , Amaku, M. , & Pinto‐da‐Rocha, R. (2005). Bat flies (Diptera: Streblidae, Nycteribiidae) parasitic on bats (Mammalia: Chiroptera) at Parque Estadual da Cantareira, São Paulo, Brazil: Parasitism rates and host‐parasite associations. Memorias do Instituto Oswaldo Cruz, 100, 25–32. 10.1590/S0074-02762005000100005 [DOI] [PubMed] [Google Scholar]
- Blackwell, M. (1980a). Developmental morphology and taxonomic characters of Arthrorhynchus nycteribiae and A. eucampsipodae (Laboulbeniomycetes). Mycologia, 72, 159–168. 10.2307/3759428 [DOI] [Google Scholar]
- Blackwell, M. (1980b). Incidence, host specificity, distribution, and morphological variation in Arthrorhynchus nycteribiae and A. eucampsipodae (Laboulbeniomycetes). Mycologia, 72, 143–158. 10.2307/3759427 [DOI] [Google Scholar]
- Darriba, D. , Taboada, G. L. , Doallo, R. , & Posada, D. (2012). jModelTest 2: More models, new heuristics and parallel computing. Nature Methods, 9, 772 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kesel, A. (1995). Relative importance of direct and indirect infection in the transmission of Laboulbenia slackensis (Ascomycetes, Laboulbeniales). Belgian Journal of Botany, 128, 124–130. [Google Scholar]
- De Kesel, A. (1996). Host specificity and habitat preference of Laboulbenia slackensis . Mycologia, 88, 565–573. 10.2307/3761150 [DOI] [Google Scholar]
- De Kesel, A. , & Haelewaters, D. (2014). Laboulbenia slackensis and L. littoralis sp. nov. (Ascomycota, Laboulbeniales), two sibling species as a result of ecological speciation. Mycologia, 106, 407–414. 10.3852/13-348 [DOI] [PubMed] [Google Scholar]
- Dick, C. W. (2013). Review of the bat flies of Honduras, Central America (Diptera: Streblidae). Journal of Parasitology Research, 2013, 437696 10.1155/2013/437696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick, C. W. , & Gettinger, D. (2005). A faunal survey of streblid flies (Diptera: Streblidae) associated with bats in Paraguay. Journal of Parasitology, 91, 1015–1024. 10.1645/GE-536R.1 [DOI] [PubMed] [Google Scholar]
- Dick, C. W. , & Patterson, B. D. (2007). Against all odds: Explaining high host specificity in dispersal‐prone parasites. International Journal for Parasitology, 37, 871–876. 10.1016/j.ijpara.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Dick, C. W. , & Patterson, B. D. (2008). An excess of males: Skewed sex ratios in bat flies (Diptera: Streblidae). Evolutionary Ecology, 22, 757–769. 10.1007/s10682-007-9201-9 [DOI] [Google Scholar]
- Dittmar, K. , Porter, M. L. , Murray, S. , & Whiting, M. F. (2006). Molecular phylogenetic analysis of nycteribiid and streblid bat flies (Diptera: Brachycera, Calyptratae): Implications for host associations and phylogeographic origins. Molecular Phylogenetics and Evolution, 38, 155–170. 10.1016/j.ympev.2005.06.008 [DOI] [PubMed] [Google Scholar]
- Dormann, C. F. , Gruber, B. , & Fründ, J. (2008). Introducing the bipartite package: Analysing ecological networks. R News, 8, 8–11. [Google Scholar]
- Drummond, A. J. , Ho, S. Y. W. , Rawlence, N. , & Rambaut, A. (2007) A rough guide to BEAST 1.4. University Auckland, New Zealand. Available at http://beast.bio.ed.ac.uk (accessed 05 March 2018).
- Drummond, A. J. , Suchard, M. A. , Xie, D. , & Rambaut, A. (2012). Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution, 29, 1969–1973. 10.1093/molbev/mss075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron, O. , Schneppat, U. E. , Berthomieu, A. , Goodman, S. M. , Droz, B. , Paupy, C. , … Tortosa, P. (2014). Origin, acquisition and diversification of heritable bacterial endosymbionts in louse flies and bat flies. Molecular Ecology, 23, 2105–2117. 10.1111/mec.12704 [DOI] [PubMed] [Google Scholar]
- Eckhart, L. , Bach, J. , Ban, J. , & Tschachler, E. (2000). Melanin binds reversibly to thermostable DNA polymerase and inhibits its activity. Biochemical and Biophysical Research Communications, 271, 726–730. 10.1006/bbrc.2000.2716 [DOI] [PubMed] [Google Scholar]
- Edgar, R. C. (2004). MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evelyn, M. J. , & Stiles, D. A. (2003). Roosting requirements of two frugivorous bats (Sturnira lilium and Arbiteus intermedius) in fragmented Neotropical forest. Biotropica, 35, 405–418. 10.1111/j.1744-7429.2003.tb00594.x [DOI] [Google Scholar]
- Fritz, G. N. (1983). Biology and ecology of bat flies (diptera: streblidae) on bats in the genus Carollia . Journal of Medical Entomology, 20, 1–10. 10.1093/jmedent/20.1.1 [DOI] [PubMed] [Google Scholar]
- Garbino, G. S. T. , & Tavares, V. C. (2018). 2018. Roosting ecology of Stenodermatinae bats (Phyllostomidae): Evolution of foliage roosting and correlated phenotypes. Mammal Review, 48, 75–89. 10.1111/mam.12114 [DOI] [Google Scholar]
- Gernhard, T. (2008). The conditioned reconstructed process. Journal of Theoretical Biology, 253, 769–778. 10.1016/j.jtbi.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Goldmann, L. , & Weir, A. (2012). Position specificity in Chitonomyces (Ascomycota, Laboulbeniomycetes) on Laccophilus (Coleoptera, Dytiscidae): A molecular approach resolves a century‐old debate. Mycologia, 104, 1143–1158. 10.3852/11-358 [DOI] [PubMed] [Google Scholar]
- Goldmann, L. , & Weir, A. (2018). Molecular phylogeny of the Laboulbeniomycetes (Ascomycota). Fungal Biology, 122, 87–100. 10.1016/j.funbio.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Goldmann, L. , Weir, A. , & Rossi, W. (2013). Molecular analysis reveals two new dimorphic species of Hesperomyces (Ascomycota, Laboulbeniomycetes) parasitic on the ladybird Coleomegilla maculata (Coleoptera, Coccinellidae). Fungal Biology, 117, 807–813. 10.1016/j.funbio.2013.10.004 [DOI] [PubMed] [Google Scholar]
- González, D. P. , Santos, A. M. , & Miranda, J. R. (2004). Streblidae (Diptera: Pupipara) ectoparásitos de murciélagos, en las tierras bajas del Parque Nacional Darien, provincia de Darién, Panamá. Tecnociencias, 6, 1–12. [Google Scholar]
- Graciolli, G. , & Dick, C. W. (2004). A new species of Metelasmus (Diptera: Streblidae: Streblinae) from southern South America. Zootaxa, 509, 1–8. https://doi.org/10.11646/zootaxa.509.1.1 [Google Scholar]
- Guerrero, R. (1993). Catalogo de los Streblidae (Diptera: Pupipara) parasitos de murcielagos (Mammalia: Chiroptera) del Nuevo Mundo I. Clave para los géneros y Nycterophiliinae. Acta Biologica Venezuelica, 14, 61–75. [Google Scholar]
- Guerrero, R. (1994a). Catalogo de los Streblidae (Diptera: Pupipara) parasitos de murcielagos (Mammalia: Chiroptera) del Nuevo Mundo II. Los grupos: pallidus, caecus, major, uniformis y longipes del género Trichobius (Gervais, 1844). Acta Biologica Venezuelica, 15, 1–18. [Google Scholar]
- Guerrero, R. (1994b). Catalogo de los Streblidae (Diptera: Pupipara) parasitos de murcielagos (Mammalia: Chiroptera) del Nuevo Mundo IV. Trichobiinae con alas desarrolladas. Boletín de Entomología Venezolana, 9, 161–192. [Google Scholar]
- Guerrero, R. (1995a). Catalogo de los Streblidae (Diptera: Pupipara) parasitos de murcielagos (Mammalia: Chiroptera) del Nuevo Mundo III. Los grupos: dugesii, dunni y phyllostomae del género Trichobius (Gervais, 1844). Acta Biologica Venezuelica, 15, 1–27. [Google Scholar]
- Guerrero, R. (1995b). Catalogo de los Streblidae (Diptera: Pupipara) parasitos de murcielagos (Mammalia: Chiroptera) del Nuevo Mundo V. Trichobiinae con alas reducidas o ausentes y miscelaneos. Boletín de Entomología Venezolana, 10, 135–160. [Google Scholar]
- Guerrero, R. (1996). Catálogo de los Streblidae (Diptera: Pupipara) parásitos de murciélagos (Mammalia: Chiroptera) del Nuevo Mundo VI. Streblinae. Acta Biologica Venezuelica, 16, 1–26. [Google Scholar]
- Guerrero, R. (1997). Catálogo de los Streblidae (Diptera: Pupipara) parásitos de murciélagos (Mammalia: Chiroptera) del Nuevo Mundo VII. Lista de especies, hospedadores y países. Acta Biologica Venezuelica, 17, 9–24. [Google Scholar]
- Guerrero, R. (1998a). Notes on Neotropical batflies (Diptera, Streblidae). I. The genus Trichobius, with description of two new species and one new subspecies from Venezuela. Acta Parasitologica, 43, 86–93. [Google Scholar]
- Guerrero, R. (1998b). Notes on Neotropical bat flies (Diptera: Streblidae): II. Review of the genus Xenotrichobius . Acta Parasitologica, 43, 142–147. [Google Scholar]
- Guerrero, J. A. , Ortega, J. , Gonzalez, D. , Maldonado, J. E. , Lorenzo, C. , & Espinoza, E. (2008). Molecular phylogenetics and taxonomy of the fruit‐eating bats of the genus Artibeus (Chiroptera: Phyllostomidae). Avances en el Estudio de los Mamiferos de México. Publicaciones Especiales, 2, 125–146. [Google Scholar]
- Guimarães, L. R. (1966). Nycteribiid batflies from Panama (Diptera: Nycteribiidae) In Wenzel R. L., & Tipton V. J. (Eds.), Ectoparasites of Panama (pp. 393–404). Chicago: Field Museum of Natural History. [Google Scholar]
- Haelewaters, D. , Gorczak, M. , Pfliegler, W. P. , Tartally, A. , Tischer, M. , Wrzosek, M. , & Pfister, D. H. (2015). Bringing Laboulbeniales into the 21st century: Enhanced techniques for extraction and PCR amplification of DNA from minute ectoparasitic fungi. IMA Fungus, 6, 363–372. 10.5598/imafungus.2015.06.02.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haelewaters, D. , Pfliegler, W. P. , Gorczak, M. , & Pfister, D. H. (in review). Birth of an order: Comprehensive phylogenetic study excludes Herpomyces (Fungi, Laboulbeniomycetes) from Laboulbeniales. Molecular Phylogenetics and Evolution. [DOI] [PubMed] [Google Scholar]
- Haelewaters, D. , Pfliegler, W. P. , Szentiványi, T. , Földvári, M. , Sándor, A. D. , Barti, L. , … Pfister, D. H. (2017a). Parasites of parasites of bats: Laboulbeniales (Fungi: Ascomycota) on bat flies (Diptera: Nycteribiidae) in Central Europe. Parasites & Vectors, 10, 96 10.1186/s13071-017-2022-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haelewaters, D. , Verhaeghen, S. J. C. , Rios Gonzalez, T. A. , Bernal Vega, J. A. , & Villarreal Saucedo, R. V. (2017b). New and interesting Laboulbeniales from Panama and neighboring areas. Nova Hedwigia, 105, 267–299. 10.1127/nova_hedwigia/2017/0410 [DOI] [Google Scholar]
- Hall, T. A. (1999). BioEdit: A user‐friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98. [Google Scholar]
- Handley, C. O. Jr. (1966). Checklist of the mammals of Panama In Wenzel R. L., & Tipton V. J. (Eds.), Ectoparasites of Panama (pp. 753–795). Chicago: Field Museum of Natural History. [Google Scholar]
- Handley, C. O. Jr. (1981). Key to the bats of the lowlands of Panama. Washington DC: National Museum of Natural History. [Google Scholar]
- Hosokawa, T. , Nikoh, N. , Koga, R. , Satô, M. , Tanahashi, M. , Meng, X. Y. , & Fukatsu, T. (2012). Reductive genome evolution, host‐symbiont co‐speciation and uterine transmission of endosymbiotic bacteria in bat flies. The ISME Journal, 6, 577–587. 10.1038/ismej.2011.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Index Fungorum . (2018). Search Index Fungorum. Available at http://www.indexfungorum.org/names/Names.asp (accessed 15 May 2018).
- Jung, S. , Kim, H. , Yamada, K. , & Lee, S. (2010). Molecular phylogeny and evolutionary habitat transition of the flower bugs (Heteroptera: Anthocoridae). Molecular Phylogenetics and Evolution, 57, 1173–1183. 10.1016/j.ympev.2010.09.013 [DOI] [PubMed] [Google Scholar]
- Kolenati, F. A. (1857). Epizoa der Nycteribien. Wiener Entomologische Monatsschrift, 1, 66–69. [Google Scholar]
- Kumar, S. , Stecher, G. , & Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz, T. H. , & Lumsdem, L. F. (2003). Ecology of cavity and foliage roosting bats In Kunz T. H., & Fenton M. B. (Eds.), Bat ecology (pp. 3–90). Chicago: The University of Chicago Press. [Google Scholar]
- Lourenço, E. C. , Almeida, J. C. , & Famadas, K. M. (2016). Richness of ectoparasitic flies (Diptera: Streblidae) of bats (Chiroptera)—a systematic review and meta‐analysis of studies in Brazil. Parasitology Research, 115, 4379–4388. 10.1007/s00436-016-5223-y [DOI] [PubMed] [Google Scholar]
- Marshall, A. G. (1981). The ecology of ectoparasitic insects. New York: Academic Press. [Google Scholar]
- Merola, A. (1952). Interessante ritrovamento di labulbeniologia cavernicola: Arthrorhynchus acrandros n. sp. (con considerazioni sul gen. Arthrorhynchus). Bollettino della Società dei naturalisti in Napoli, 60, 1–30. [Google Scholar]
- Miadlikowska, J. , & Lutzoni, F. (2000). Phylogenetic revision of the genus Peltigera (lichen‐forming Ascomycota) based on morphological, chemical, and large subunit nuclear ribosomal DNA data. International Journal of Plant Sciences, 161, 925–958. 10.1086/317568 [DOI] [Google Scholar]
- Miller, M. A. , Pfeiffer, W. , & Schwartz, T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees In Proceedings of the Gateway Computing Environments Workshop (GCE) 14 Nov. 2010, pp. 1–8. New Orleans, Louisiana. [Google Scholar]
- Miller, J. , & Tschapka, M. (2001). The bat flies of La Selva (Diptera: Nycteribiidae, Streblidae). Available at http://www.biologie.uni-ulm.de/bio3/Batfly/index.html (accessed 04 November 2017).
- Moras, L. M. , Gregorin, R. , Sattler, T. , & Tavares, V. C. (2018). Uncovering the diversity of dog‐faced bats of the genus Cynomops (Chiroptera: Molossidae), with the redescription of C. milleri and the description of two new species. Mammalian Biology, 89, 37–51. 10.1016/j.mambio.2017.12.005 [DOI] [Google Scholar]
- Morse, S. F. , Bush, S. E. , Patterson, B. D. , Dick, C. W. , Gruwell, M. E. , & Dittmar, K. (2013). Evolution, multiple acquisition, and localization of endosymbionts in bat flies (Diptera: Hippoboscoidea: Streblidae and Nycteribiidae). Applied and Environmental Microbiology, 79, 2952–2961. 10.1128/AEM.03814-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse, S. F. , Dick, C. W. , Patterson, B. D. , & Dittmar, K. (2012). Some like it hot–Evolution and ecology of novel endosymbionts in bat flies of cave‐roosting bats (Hippoboscoidea, Nycterophiliinae). Applied and Environmental Microbiology, 78, 8639–8649. 10.1128/AEM.02455-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olival, K. J. , Dick, C. W. , Simmons, N. B. , Morales, J. C. , Melnick, D. J. , Dittmar, K. , … DeSalle, R. (2013). Lack of population genetic structure and host specificity in the bat fly, Cyclopodia horsfieldi, across species of Pteropus bats in Southeast Asia. Parasites & Vectors, 6, 231 10.1186/1756-3305-6-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overal, W. L. (1980). Host‐relations of the batfly Megistopoda aranea (Diptera: Streblidae) in Panama. University of Kansas Science Bulletin, 52, 1–20. [Google Scholar]
- Palmeirim, J. M. , & Etherdige, K. (1985). The influence of man‐made trails on foraging by tropical frugivorous bats. Biotropica, 17, 82–83. 10.2307/2388385 [DOI] [Google Scholar]
- Paradis, E. , Claude, J. , & Strimmer, K. (2004). APE: Analyses of phylogenetics and evolution in R language. Bioinformatics, 20, 289–290. 10.1093/bioinformatics/btg412 [DOI] [PubMed] [Google Scholar]
- Parratt, S. R. , Barrès, B. , Penczykowski, R. M. , & Laine, A. L. (2017). Local adaptation at higher trophic levels: Contrasting hyperparasite–pathogen infection dynamics in the field and laboratory. Molecular Ecology, 26, 1964–1979. 10.1111/mec.13928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parratt, S. R. , & Laine, A. L. (2016). The role of hyperparasitism in microbial pathogen ecology and evolution. The ISME Journal, 10, 1815–1822. 10.1038/ismej.2015.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, B. D. , Dick, C. W. , & Dittmar, K. (2007). Roosting habits of bats affect their parasitism by bat flies (Diptera: Streblidae). Journal of Tropical Ecology, 23, 177–189. 10.1017/S0266467406003816 [DOI] [Google Scholar]
- Peyritsch, J. (1871). Über einige Pilze aus der Familie der Laboulbenien. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. Mathematisch‐naturwissenschaftliche Classe, 64, 441–458. [Google Scholar]
- Peyritsch, J. (1873). Beiträge zur Kenntniss der Laboulbenien. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. Mathematisch‐naturwissenschaftliche Classe, 68, 227–254. [Google Scholar]
- Pfliegler, W. P. , Báthori, F. , Haelewaters, D. , & Tartally, A. (2016). Studies of Laboulbeniales on Myrmica ants (III): Myrmecophilous arthropods as alternative hosts of Rickia wasmannii . Parasite, 23, 50 10.1051/parasite/2016060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilosof, S. , Dick, C. W. , Korine, C. , Patterson, B. D. , & Krasnov, B. R. (2012). Effects of anthropogenic disturbance and climate on patterns of bat fly parasitism. PLoS ONE, 7, e41487 10.1371/journal.pone.0041487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2013). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Available at http://www.R-project.org (accessed 06 March 2018). [Google Scholar]
- Ramasindrazana, B. , Goodman, S. M. , Gomard, Y. , Dick, C. W. , & Tortosa, P. (2017). Hidden diversity of Nycteribiidae (Diptera) bat flies from the Malagasy region and insights on host‐parasite interactions. Parasites & Vectors, 10, 630 10.1186/s13071-017-2582-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut, A. , Suchard, M. A. , Xie, D. , & Drummond, A. J. (2014). Tracer v1.6. Available at http://tree.bio.ed.ac.uk/software/tracer/ (accessed 02 November 2017).
- Reboleira, A. S. P. S. , Fresneda, J. , & Salgado, J. M. (2017). A new species of Speonemadus from Portugal, with the revision of the escalerai‐group (Coleoptera, Leiodidae). European Journal of Taxonomy, 261, 1–23. [Google Scholar]
- Rosenblum, E. B. , Sarver, B. A. J. , Brown, J. W. , Des Roches, S. , Hardwick, K. M. , Hether, T. D. , … Harmon, L. J. (2012). Goldilocks meets Santa Rosalia: An ephemeral speciation model explains patterns of diversification across time scales. Evolutionary Biology, 39, 255–261. 10.1007/s11692-012-9171-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, W. , & Kirk‐Spriggs, A. H. (2011). A new species of Laboulbenia (Ascomycota) parasitic on an African fly (Diptera: Curtonotidae), with a brief review of Diptera‐associated species of the genus. African Invertebrates, 52, 211–216. 10.5733/afin.052.0111 [DOI] [Google Scholar]
- Rossi, W. , & Leonardi, M. (2013). New species of Stigmatomyces (Laboulbeniomycetes) from Sierra Leone. Plant Biosystems, 147, 79–83. 10.1080/11263504.2012.695297 [DOI] [Google Scholar]
- Saitou, N. , & Nei, M. (1987). The neighbor‐joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4, 406–425. [DOI] [PubMed] [Google Scholar]
- Samudio Jr, R. (2002). Patterns of diversity and ecology in Panamanian bats at two elevations. PhD dissertation, University of Florida, Gainesville.
- Samudio, R. Jr. , & Pino, J. L. (2014). Historia de la Mastozoología en Panamá In Ortega J., Martínez J. L., & Tirira D. G. (Eds.), Historia de La Mastozoología En Latinoamérica, Las Guayanas Y El Caribe (pp. 329–344). Quito, Ecuador: Editorial Murciélago Blanco y Asociación Ecuatoriana de Mastozoología. [Google Scholar]
- Santamaria, S. (1995). Parvomyces, a new genus of Laboulbeniales from Spain. Mycological Research, 99, 1071–1077. 10.1016/S0953-7562(09)80775-2 [DOI] [PubMed] [Google Scholar]
- Santamaria, S. , Balazuc, J. , & Tavares, I. I. (1991). Distribution of the European Laboulbeniales (Fungi, Ascomycotina). An annotated list of species. Treballs de l'Institut Botanic de Barcelona, 14, 1–123. [Google Scholar]
- Schuh, R. T. , & Slater, J. A. (1995). True bugs of the world (Hemiptera: Heteroptera): Classification and natural history. Ithaca, New York: Cornell University Press. [Google Scholar]
- Schuh, R. T. , & S̆tys, P. (1991). Phylogenetic analysis of cimicomorphan family relationships (Heteroptera). Journal of the New York Entomological Society, 99, 298–350. [Google Scholar]
- Shockley, F. W. , & Murray, K. L. (2006). Discovery of a parasitoid and a predator of bat flies (Diptera: Streblidae) at La Selva, Costa Rica. Biotropica, 38, 789–790. 10.1111/j.1744-7429.2006.00207.x [DOI] [Google Scholar]
- Simmons, N. B. (2005). Order Chiroptera In Wilson D. E., & Reeder D. A. M. (Eds.), Mammal species of the world: A taxonomic and geographic reference, 3rd ed. (pp. 312–529). Baltimore, Maryland: Johns Hopkins University Press. [Google Scholar]
- Stadler, T. (2009). On incomplete sampling under birth–death models and connections to the sampling‐based coalescent. Journal of Theoretical Biology, 261, 58–66. 10.1016/j.jtbi.2009.07.018 [DOI] [PubMed] [Google Scholar]
- Stamatakis, A. (2014). RAxML Version 8: A tool for phylogenetic analysis and post‐analysis of large phylogenies. Bioinformatics, 30, 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper, E. (2012). Host Specificity of Ecuadorian bat flies (Diptera: Streblidae). Honors College Capstone Experience/Thesis Projects. Paper 358. Available at http://digitalcommons.wku.edu/stu_hon_theses/358 (accessed 17 October 2017).
- Swofford, D. L. (1991). PAUP: Phylogenetic Analysis Using Parsimony, Version 3.1. Computer program distributed by the Illinois Natural History Survey, Champaign, Illinois.
- Szentiványi, T. , Haelewaters, D. , Pfliegler, W. P. , Clément, L. , Christe, P. , & Glaizot, O. (2018). Laboulbeniales (Fungi: Ascomycota) infection of bat flies (Diptera: Nycteribiidae) from Miniopterus schreibersii across Europe. Parasites & Vectors, 11, 395 10.1186/s13071-018-2921-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares, I. I. (1985). Laboulbeniales (Fungi, Ascomycetes). Mycol Memoir, 9, 1–627. [Google Scholar]
- Tavares, I. I. , & Balazuc, J. (1989). Sugiyamaemyces, a new genus of Laboulbeniales (Ascomycetes) on Clidicus (Scydmaenidae). Mycotaxon, 34, 565–576. [Google Scholar]
- ter Hofstede, H. M. , Fenton, M. B. , & Whitaker, J. O. Jr. (2004). Host and host‐site specificity of bat flies (Diptera: Streblidae and Nycteribiidae) on Neotropical bats (Chiroptera). Canadian Journal of Zoology, 82, 616–626. 10.1139/z04-030 [DOI] [Google Scholar]
- Thaxter, R. (1896). Contribution towards a monograph of the Laboulbeniaceae. Memoirs of the American Academy of Arts and Sciences, 12, 187–429. [Google Scholar]
- Thaxter, R. (1901). Preliminary diagnosis of new species of Laboulbeniaceae. III. Proceedings of the American Academy of Arts and Sciences, 36, 397–414. 10.2307/20021044 [DOI] [Google Scholar]
- Thaxter, R. (1908). Contribution toward a monograph of the Laboulbeniaceae. Part II. Memoirs of the American Academy of Arts and Sciences, 13, 217–469. [Google Scholar]
- Thaxter, R. (1915). New Indo‐Malayan Laboulbeniales. Proceedings of the American Academy of Arts and Sciences, 51, 3–51. 10.2307/20025560 [DOI] [Google Scholar]
- Thaxter, R. (1917). New Laboulbeniales, chiefly dipterophilous American species. Proceedings of the American Academy of Arts and Sciences, 52, 649–721. 10.2307/20025703 [DOI] [Google Scholar]
- Thaxter, R. (1924). Contribution toward a monograph of the Laboulbeniaceae III. Memoirs of the American Academy of Arts and Sciences, 14, 309–426. [Google Scholar]
- Thaxter, R. (1931). Contribution toward a monograph of the Laboulbeniaceae V. Memoirs of the American Academy of Arts and Sciences, 16, 1–435. 10.2307/25058136 [DOI] [Google Scholar]
- Timm, R. M. , & LaVal, R. K. (1998). A field key to the bats of Costa Rica. Occasional Publication Series, University of Kansas Center of Latin American Studies, 22, 1–30. [Google Scholar]
- Velazco, P. M. (2005). Morphological phylogeny of the bat genus Platyrrhinus Saussure, 1860 (Chiroptera: Phyllostomidae) with the description of four new species. Fieldiana: Zoology, 105, 1–53. [Google Scholar]
- Vilgalys, R. , & Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology, 172, 4238–4246. 10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, M. J. , Dorrestein, A. , Camacho, J. J. , Meckler, L. A. , Silas, K. A. , Hiller, T. , & Haelewaters, D. (2018). A tripartite survey of hyperparasitic fungi associated with ectoparasitic flies on bats (Mammalia: Chiroptera) in a neotropical cloud forest in Panama. Parasite, 25, 19 10.1051/parasite/2018017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir, A. (2008). The genus Prolixandromyces (Laboulbeniales) in the old world. Aliso, 26, 29–35. 10.5642/aliso [DOI] [Google Scholar]
- Weir, A. , & Blackwell, M. (2001a). Molecular data support the Laboulbeniales as a separate class of Ascomycota, Laboulbeniomycetes. Mycological Research, 105, 1182–1190. 10.1016/S0953-7562(08)61989-9 [DOI] [Google Scholar]
- Weir, A. , & Blackwell, M. (2001b). Extraction and PCR amplification of DNA from minute ectoparasitic fungi. Mycologia, 93, 802–806. 10.2307/3761835 [DOI] [Google Scholar]
- Weir, A. , & Hammond, P. M. (1997). Laboulbeniales on beetles: Host utilization patterns and species richness of the parasites. Biodiversity and Conservation, 6, 701–719. 10.1023/A:1018318320019 [DOI] [Google Scholar]
- Wenzel, R. L. (1976). The streblid batflies of Venezuela (Diptera: Streblidae). Brigham Young University Science Bulletin, 20, 1–177. [Google Scholar]
- Wenzel, R. L. , & Tipton, V. J. (1966). Ectoparasites of Panama. Chicago: Field Museum of Natural History. [Google Scholar]
- Wenzel, R. L. , Tipton, V. J. , & Kiewlicz, A. (1966). The streblid batflies of Panama (Diptera Calypterae: Streblidae) In Wenzel R. L., & Tipton V. J. (Eds.), Ectoparasites of Panama (pp. 405–676). Chicago: Field Museum of Natural History. [Google Scholar]
- West‐Eberhard, M. J. (1989). Phenotypic plasticity and the origins of diversity. Annual Review of Ecology, Evolution, and Systematics, 20, 249–278. 10.1146/annurev.es.20.110189.001341 [DOI] [Google Scholar]
- Whisler, H. C. (1968). Experimental studies with a new species of Stigmatomyces (Laboulbeniales). Mycologia, 60, 65–75. 10.2307/3757314 [DOI] [PubMed] [Google Scholar]
- Wilkinson, D. A. , Duron, O. , Cordonin, C. , Gomard, Y. , Ramasindrazana, B. , Mavingui, P. , … Tortosa, P. (2016). The bacteriome of bat flies (Nycteribiidae) from the Malagasy region: A community shaped by host ecology, bacterial transmission mode, and host‐vector specificity. Applied and Environmental Microbiology, 82, 1778–1788. 10.1128/AEM.03505-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor, D. A. (1990). Heavenly hosts. Nature, 348, 104 10.1038/348104c0 2234071 [DOI] [Google Scholar]
- Windsor, D. A. (1995). Equal rights for parasites. Conservation Biology, 9, 1–2. 10.1046/j.1523-1739.1995.09010001.x [DOI] [PubMed] [Google Scholar]
- Wrzosek, M. (2000). Taksonomia i filogeneza Mucorales (Zygomycetes) w świetle analiz morfometrycznych oraz wybranych markerów molekularnych. [Taxonomy and phylogeny of Mucorales (Zygomycetes) in the light of morphometrical and selected molecular markers analyses]. PhD dissertation, University of Warsaw.
- Yule, G. U. (1925). A mathematical theory of evolution based on the conclusions of Dr. J. C. Willis. Philosophical Transactions of the Royal Society B, 213, 21–87. 10.1098/rstb.1925.0002 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete dataset of bats, batflies and Laboulbeniales is available as Supporting Information (in Excel format). All sequence data used in this study have been submitted to GenBank under accession numbers MH040307 & MH040533–MH040595. Sequence alignments generated during this study (in NEXUS format), input XML and output log and trees files from Bayesian analyses may be accessed from the figshare online repository with the URL https://doi.org/10.6084/m9.figshare.c.4154336.v1.


