Abstract
There is growing evidence that inflammatory responses may help to explain how emotions get “under the skin” to influence disease susceptibility. Moving beyond examination of individuals’ average level of emotion, this study examined how the breadth and relative abundance of emotions that individuals experience— emodiversity—is related to systemic inflammation. Using diary data from 175 adults aged 40 to 65 who provided end-of-day reports of their positive and negative emotions over 30 days, we found that greater diversity in day-to-day positive emotions was associated with lower circulating levels of inflammation (indicated by IL-6, CRP, fibrinogen), independent of mean levels of positive and negative emotions, body mass index, anti-inflammatory medications, medical conditions, personality, and demographics. No significant associations were observed between global or negative emodiversity and inflammation. These findings highlight the unique role daily positive emotions play in biological health.
Keywords: emodiversity, intraindividual variability, positive emotions, entropy, health
We differ in nothing more than in our capacity to feel…upon that degree the dignity and significance of each life depend.
(Hamilton, 1942, pp. 145–146)1
There is tremendous variety in the emotional states that constitute everyday life. Some people have emotional experiences that are differentiated, while others experience emotions in a global manner. In their influential work on mood variability, Wessman and Ricks (1966) coined the term “affective complexity” to characterize differences in the richness of emotional life. While conceptualizations and operationalizations of emotional complexity have differed across studies, an emerging literature suggests that indices of complexity may be broadly categorized according to the extent of covariation or differentiation in self-reported experiences of emotion (Grühn, Lumley, Diehl, & Labouvie-Vief, 2013; Hay & Diehl, 2011; Lindquist & Barrett, 2008).
Measures of emotional covariation typically assess individual differences in the extent of co-occurrence (i.e., mixed emotions) or correlation (i.e., emotional dialecticism) of positive and negative affect over time (Grossmann, Huynh, & Ellsworth, 2016; Larsen & McGraw, 2014; Ready, Carvalho, & Weinberger, 2008). Both greater dialectical and more mixed emotional experience are associated with higher well-being and greater resilience (Adler & Hershfield, 2012; Coifman, Bonanno, & Rafaeli, 2007; Hershfield, Scheiber, Sims, & Carstensen, 2013), particularly among East Asians (Miyamoto & Ryff, 2011; Miyamoto, Uchida, & Ellsworth, 2010) and older adults (Carstensen, Pasupathi, Mayr, & Nesselroade, 2000; Carstensen, Turan, Scheibe et al., 2011; Ong & Bergeman, 2004), with some evidence that these associations may be moderated by differences in ideal affect and interdependent self-construals (Grossmann, et al., 2016; Sims, Tsai, Jiang et al., 2015), the amount of intraindividual variability in positive and negative emotional states (Brose, de Roover, Ceulemans, & Kuppens, 2015; Grühn, et al., 2013), and cognitive ability (Hülür, Hoppmann, Ram, & Gerstorf, 2015).
Measures of emotional differentiation (also referred to as emotional granularity; Barrett, 2006; Lindquist & Barrett, 2008) assess individual differences in the propensity to categorize and label emotional experiences in discrete terms. Theoretically, individuals with more differentiated emotional experiences have greater ability to make subtle distinctions among the emotional states they experience (e.g., fear, sadness, anger; Barrett, Gross, Christensen, & Benvenuto, 2001). Between-person differences in emotional differentiation generated from diary and ecological momentary assessment data show that undifferentiated emotion (particularly of negative emotions) is associated with a range of psychopathologies, including borderline personality, social anxiety, and major depressive disorder (Demiralp, Thompson, Mata et al., 2012; Kashdan & Farmer, 2014; Tomko, Lane, Pronove et al., 2015). Other research has similarly established an association between greater differentiation in positive emotions and adaptive coping and adjustment (e.g., Tugade, Fredrickson, & Feldman-Barrett, 2004). To date, however, little is known about how—i.e., through what the biological processes—complex emotional experiences influence health outcomes. The current study examines the association between emodiversity—the breadth and relative abundance of different emotions that individuals experience—and biological inflammation.
Emotion and Inflammatory Processes
Inflammation is a key risk factor for early morbidity and mortality, and growing evidence links emotional processes with systemic inflammation. Across clinical and population-based samples, heightened systemic inflammation has been shown to contribute to poor health (e.g., atherosclerosis, Type II diabetes, rheumatoid disease, osteoporosis) and to elicit a number of pathogenic processes (e.g., oxidative stress, insulin resistance, plaque rupture, endothelial pathology) that play a major role in the risk of premature mortality (Cesari, Penninx, Newman et al., 2003; Epel & Lithgow, 2014; Miller, Chen, & Parker, 2011; Schneiderman, Ironson, & Siegal, 2008). Evidence from human laboratory research suggests that negative emotional states stimulate inflammatory responses (Duivis, de Jonge, Penninx et al., 2011; Howren, Lamkin, & Suls, 2009; Miller & Blackwell, 2006). For example, avoidance-oriented negative emotions, such as fear and shame, have been linked to greater inflammatory activity (Dickerson, Kemeny, Aziz, Kim, & Fahey, 2004; Moons, Eisenberger, & Taylor, 2010). Similarly, the onset and progression of particular negative moods and traits (e.g., depression, hostility, and anxiety) are often followed by elevated levels of inflammatory proteins, including the proinflammatory cytokine interleukin-6 (IL-6), the acute phase C-reactive protein (CRP), and the clotting factor fibrinogen (Al, Kronfol, Seymour, & Bolling, 2005; Duivis, et al., 2011; Miller, Rohleder, Stetler, & Kirschbaum, 2005; Moons & Shields, 2015; Pitsavos, Panagiotakos, Papageorgiou et al., 2006; Suarez, 2003).
Although the bulk of studies on affect and inflammation have focused on negative affect, there is growing evidence that positive affect has independent associations with inflammatory markers. In naturalistic studies of healthy adults, trait positive affect, but not negative affect, has been linked to lower levels of CRP and IL-6 (Deverts, Cohen, DiLillo et al., 2010; Stellar, John-Henderson, Anderson et al., 2015; Steptoe, O’Donnell, Badrick, Kumari, & Marmot, 2008). Similarly, evidence from laboratory viral challenge studies suggests that higher levels of trait positive affect are associated with lower production of proinflammatory cytokines (Cohen, Alper, Doyle, Treanor, & Turner, 2006; Janicki-Deverts, Cohen, Doyle, Turner, & Treanor, 2007; Prather, Marsland, Muldoon, & Manuck, 2007; Robles, Brooks, & Pressman, 2009). Finally, there is evidence from clinical populations that positive affect influences immune processes. For example, data from cancer patients undergoing radiation therapy suggests that positive affect enhances acute inflammatory responses to treatment (Blomberg, Alvarez, Diaz et al., 2009; Sepah & Bower, 2009) and prospectively predicts lower levels of CRP at treatment completion and through 6- and 12-month follow-ups (Moreno, Moskowitz, Ganz, & Bower, 2016). Taken together, experiences of negative and positive emotion in both trait and state form appear to influence the adaptive regulation of the core biological systems that maintain health.
Emodiversity and Health
Expanding beyond differences in level of negative and positive emotion, we consider how emodiversity—the relative breadth and abundance of different emotions (Benson, Ram, Almeida, Zautra, & Ong, in press; Quoidbach, Gruber, Mikolajczak et al., 2014)—may influence inflammation. Drawing on analytic approaches used to quantify the biodiversity of ecosystems (Magurran, 2004; Morin, 1999), measures of diversity have been used to assess a variety of social and psychological phenomena, including racial/ethnic diversity (Budescu & Budescu, 2012), behavioral flexibility (Ram, Conroy, Pincus, Hyde, & Molloy, 2012), population genetics (Sherwin, 2010), community social networks (Li, Zhang, Feng, & Wu, 2015), daily stressor diversity (Koffer, Ram, Conroy, Pincus, & Almeida, 2016) and activity diversity (Lee, Koffer, Sprague et al., in press).
Although, to date, no studies have directly investigated the link between emodiversity and inflammation, there are reasons to suspect having a rich and diverse emotional life may be beneficial to health. First, emotional experiences that are broad in range and differentiated may guide adaptation by prioritizing, organizing, and regulating behavior in ways that optimize an individual’s adjustment to situational demands (Barrett & Campos, 1987; Keltner & Gross, 1999). Additionally, representing emotions in discrete terms may have greater “informational value” than global affective states (Barrett, 1998; Barrett, Mesquita, Ochsner, & Gross, 2007). That is, the ability to characterize affective information with precision (i.e., in terms of qualitatively distinct events) may reduce the potential for individuals to make misattributions about their own affective reactions (Schwarz, 1990; Schwarz & Clore, 1983). Finally, experiencing a diversity of emotional states might reduce vulnerability to affective psychopathology by preventing an overabundance or prolonging of any one emotion from dominating an individuals’ emotional life (Benson, et al., in press; Gruber & Bekoff, 2017). Supporting this logic, Quoidbach and colleagues (2014) found that greater emodiversity was associated with better mental and physical health.
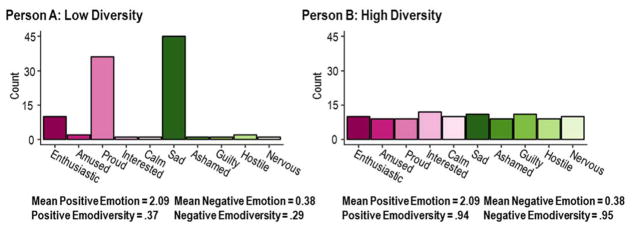
Individual differences in emodiversity are illustrated in Figure 1. The figure depicts two individuals who have identical mean levels of positive and negative emotion but differ in diversity of day-to-day emotional experiences. For conceptual display, emotions are ordered along the x-axis in accordance with a circumplex perspective (Russell, 1980), wherein emotions range from high arousal positive to low arousal positive (e.g., enthusiastic to calm), and from high arousal negative to low arousal negative (e.g., nervous to sad). Positive valence emotions are depicted in pink and negative emotions in green, with the darker hues corresponding to higher arousal emotions and lighter hues to lower arousal emotions. The height of each bar indicates the number of occasions on which each emotion was experienced. Person A’s (left panel) emotions are relatively low in diversity in that they are concentrated in a few emotion categories. In contrast, Person B’s (right panel) emotions are relatively high in diversity in that they are distributed more evenly across categories. Importantly, these differences are distinct from mean levels of emotion. Our interest here is whether individual differences in diversity of emotion (emodiversity) may be associated with systemic inflammation.
Figure 1.
Individual differences in emodiversity – the breadth (the number of discrete emotions experienced) and evenness (the distribution of experiences across discrete emotions) of emotional experience. Left panel: Person A has low emodiversity, with emotion experiences that are relatively homogenous and concentrated in a few emotion categories. Right panel: Person B has high emodiversity, with emotion experiences that are relatively diverse and distributed more evenly across categories.
The Present Investigation
The current investigation sought to examine the associations between emodiversity and systemic inflammation in a community-based sample of middle-aged adults. Given prior work suggesting that greater diversity in positive and negative emotions is associated with better health (Benson, et al., in press; Quoidbach, et al., 2014), we hypothesized that greater global emodiversity would be associated with decreased circulating levels of inflammatory markers (IL-6, CRP, fibrinogen). Furthermore, given previously documented associations between differentiated positive and negative emotions and adjustment (Barrett, et al., 2001; Tugade, et al., 2004), and evidence that positive and negative affect independently predict inflammation (Stellar, et al., 2015; Steptoe, et al., 2008), we tested the hypothesis that positive and negative emodiversity contribute uniquely to inflammation.
Our analyses were designed to extend conceptual understanding of emodiversity in four important ways. First, we consider within-person variation in emotions using time-intensive study designs that minimize retrospection bias and allow researchers to simultaneously account for within- and between-person sources of variation in data (cf. Ram & Gerstorf, 2009; West & Hepworth, 1991). This approach is in line with recent demonstrations that the intensive study of individuals over time enables researchers to move from static to more dynamic conceptual and methodological frameworks that observe peoples’ emotional lives as they unfold day to day (Ram, et al., 2012; Zautra, Affleck, Tennen, Reich, & Davis, 2005). Second, building on prior cross-sectional work examining links between emodiversity and mental and physical health (Quoidbach, et al., 2014), the present study investigated how diversity in day-to-day emotions is related to inflammation. To account for overlap in the putative measures of inflammation (Friedman & Herd, 2010), we fit structural equation models in which IL-6, CRP, and fibrinogen scores were used as indicators of a latent inflammation construct. Third, we tested whether emodiversity was associated with inflammation above and beyond mean levels of emotion (Gruber, Kogan, Quoidbach, & Mauss, 2013). Finally, drawing on functionalist and core affect theories of emotion (Keltner & Gross, 1999; Russell, 1980; Shiota, Campos, Oveis et al., in press; Shiota, Neufeld, Danvers et al., 2014) and following prior research on emodiversity (Quoidbach et al. (2014), we explored differential effects of positive and negative emodiversity, as well as global emodiversity across positive and negative emotions.
Methods
Participants
Data were drawn from a larger study of community-dwelling adults (40–65 years, N = 688) conducted in the Phoenix, Arizona metropolitan area between 2007 and 2012 (Sturgeon, Arewasikporn, Okun et al., 2016). The analytic sample for the current study consisted of 175 participants (46% male), age 40 to 65 (M = 53.42, SD = 7.57), who provided a minimum of 6 of 30 daily diary records and completed a 6-month follow-up interview. The median household income in the current study was between $50,000 and $65,000 per year. Participants self-identified as White (67%), Hispanic/Latino (8%), African American (3%), Asian (2%), and Native American or American Indian (1%), with 19% identifying with more than one ethnic group.
Procedure
After providing informed consent, participants completed a demographic questionnaire and training session where they were introduced to the study procedures and instructed on how to use a study-provided tablet computer. Participants used the tablet computer to complete daily diaries each night for 30 days. Participants underwent a blood draw to assess levels of IL-6 (pg/ml), CRP (mg/L), and fibrinogen (mg/dL). Blood samples were drawn by a research phlebotomist during a six-month follow-up visit to participants’ homes (samples obtained between 7:30 AM and 8:00 PM, with participants asked to fast for at least 8 hours). All procedures were approved by the Institutional Review Board at Arizona State University.
Measures and Materials
Daily emotion reports
Daily emotions were assessed as part of the daily tablet computer-based questionnaires using 32 items from the Positive Affect-Negative Affect Schedule (PANAS and PANAS-X; Watson, Clark, & Tellegen, 1988). At the end of each day, participants rated the extent to which they had experienced 16 positive valence emotions (enthusiastic, interested, determined, excited, amused, inspired, alert, active, strong, proud, attentive, happy, relaxed, cheerful, at ease, calm) and 16 negative valence emotions (scared, afraid, upset, distressed, jittery, nervous, ashamed, guilty, irritable, hostile, tired, sluggish, sleepy, blue, sad, drowsy) on a 1 = “very slightly or not at all” to 5 = “extremely” Likert-type rating scale. Daily emotion reports were summarized with respect to mean level and diversity of emotion.
Mean emotion
Mean positive and negative emotion scores were calculated using the continuous Likert scale ratings (0–4). Within each occasion (i.e., day), positive and negative emotion items were averaged separately, and then used to calculate an across-day average for each individual.
Emodiversity
Individual differences in the diversity of emotions were quantified in terms of emodiversity. Specifically, after recoding into a binary variable that indicated the absence or presence of each emotion on a given day, scores for global emodiversity (m = 32 items), positive emodiversity (m = 16), and negative emodiversity (m = 16) were each indexed using the Gini (1912) coefficient,
where cij is the count of individual i’s emotion experiences within j = 1 to m emotion types, indexed in ascending order (cij ≤ cij+1) for each participant. Using this index, scores for global, positive, and negative emodiversity can each range from 0 to 1, with higher values indicating more diversity, and in particular, evenness across the j emotion types. To illustrate the calculation, the vector of observed counts for Person A’s positive emotions in Figure 1 is [10, 2, 36, 1, 1]. Ordered [Proud, Enthusiastic, Amused, Interest, Calm] and weighted by relative order [1*1, 2*1, 3*2, 4*10, 5*36], the Gini coefficient for this individual . In contrast, the vector of observed counts for Person B is [10, 9, 9, 12, 10]. Ordered [Interest, Enthusiastic, Calm, Amused, Proud] and weighted by relative order [1*9, 2*9, 3*10, 4*10, 5*12], the Gini coefficient for this individual . The differences in Gini diversity thus quantify the relative unevenness/evenness of the heights of the bars evident in the visual representations. This emphasis on differences in evenness is useful in study designs like the current one, where a fixed-length list of emotion items are presented at all occasions (for a discussion, see Benson, et al., in press). Of note, Gini diversity can also be calculated using counts weighted by the original 1 to 5 Likert scale by recoding values to be on a 0 to 4 Likert scale so that a true zero point is present. In these data, the pattern of results reported below for the binary counts is substantively the same as results obtained with Likert-weighted counts.
Inflammation
To quantify levels of IL-6 and CRP, 10 ml of blood was collected into EDTA tubes (Becton-Dickinson, Franklin Lakes, NJ), held on ice, and centrifuged within 2 hours of collection for 15 minutes at 1500g. Plasma was then aspirated, aliquoted, and frozen at −80°C until assay. Plasma levels of IL-6 were quantified using Quantikine High Sensitivity human IL-6 kits (R&D Systems, Inc, Minneapolis, MN), an enzyme-linked immunosorbent assay (33) with an intra-assay coefficient of variation of 4% and interassay coefficient of variation of 10%. The minimal detectable level of IL-6 was 0.156 pg/ml. CRP was measured using the Dade Behring N High Sensitivity CRP turbidimetric immunoassay (Dade Behring Diagnostics, Marburg, Germany) on the BN ProSpec. Fibrinogen levels (mg/dL) were determined by a commercial laboratory (Quest Diagnostics, Los Angeles, CA) through use of a clotting assay. Data from 8 participants with IL-6 values greater than 10 pg/ml and CRP values greater than 10 mg/L, suggesting the presence of acute illness, were excluded (McCaffery, Marsland, Strohacker, Muldoon, & Manuck, 2012).
Covariates
Body mass index (BMI), anti-inflammatory and steroid medication use, medical conditions, personality facets of neuroticism and extraversion, and demographics including age and gender were used as covariates. Medication use was coded using separate binary variables representing use of at least one anti-inflammatory medication or at least one steroid medication versus those who did not use any of these medications. A full list of the anti-inflammatory and steroid medications assessed in the current study can be found in an online appendix (Supplemental Digital Content 1, http://links.lww.com/PSYMED/A259). History of medical conditions included a series of yes/no questions pertaining to any occurrence of hypertension or high blood pressure, angina pectoris or coronary artery disease, congestive heart failure, myocardial infarction or heart attack, other heart conditions, stroke, emphysema or asthma or chronic obstructive pulmonary disease (COPD), arthritis of the hip or knee, arthritis of the hand or wrist, sciatica, diabetes or high blood sugar or sugar in the urine, and cancer (other than skin cancer). An overall medical conditions score was calculated for each participant as none, one, two, or 3 or more of the above (Petrov, Davis, Belyea, & Zautra, 2016). Personality facets of neuroticism and extraversion were assessed using 16 items from the Big 5 Inventory (John & Srivastava, 1990), with composite scores calculated as the sum of 8 items for each facet.
Data Analysis
A series of structural equation models were used to examine relations between emodiversity (global, positive, negative) and inflammation (latent variable indicated by IL-6, CRP, and fibrinogen). Four models were fit to the data. In Model 1, the latent inflammation factor was constructed and regressed on global emodiversity. In Model 2, age, gender, anti-inflammatory medications, BMI, medical conditions, and personality were added as covariates. In Model 3, the global diversity predictor was replaced by the positive emodiversity and negative emodiversity variables. In Model 4, mean positive emotion and mean negative emotion variables were added as covariates. Models were estimated using the lavaan package in R (Rosseel, 2012) with all predictor variables centered at sample means, and incomplete data was accommodated using Full Information Maximum Likelihood (Enders, 2010).
As a general framework, SEM has been successfully used to examine associations between psychological predictors and inflammation (characterized as a latent construct with multiple indicators) (e.g., Hostinar, Ross, Chen, & Miller, 2015; Petrov, et al., 2016). While this approach conceptualizes each measure of inflammation as driven by a common factor (and thereby reduces measure-specific measurement error), the individual measures may also provide unique information about more specific inflammation processes. Making use of both of these perspectives, we also conducted follow-up regression analyses wherein each indicator was examined separately in three single-outcome regression models. Adequacy of fit of the SEM was determined using standard measures of fit as the discrepancy between the observed means and variance-covariance matrix (i.e., observed data) and the means and variance-covariance implied by the model (Bentler, 1990; Hu & Bentler, 1999). Specifically, we examined the χ2 (overall measure of misfit), and a variety of metrics derived from that misfit, including the comparative fit index (comparison to a saturated model, good fit evaluated as CFI > .96), the root mean square error of approximation (penalizes for model complexity, good fit as RMSEA < .05), and the standardized root-mean-square residual (no penalty for complexity, good fit as SRMR < .05).
Results
Descriptives
Descriptive statistics and intercorrelations among the study variables are provided in Table 1. As expected, IL-6 was positively correlated with both CRP (r = .51) and fibrinogen (r = .33), which were also positively correlated with each other (r = .52). CRP levels ranged from 0.10 to 75.00 (M = 3.59, SD = 7.82) with 94% of sample within normal range of 0 to 10 mg/L; IL-6 levels ranged from 0.23 to 72.64 (M = 2.56, SD = 6.08) with 95% of sample within normal range of 0 to 7 pg/ml; and fibrinogen levels ranged from 195.00 to 712.00 (M = 333.05, SD = 75.52) with 87% of sample within normal range of 150 to 400 mg/dL. Distributions for IL-6, CRP, and fibrinogen values were positively skewed and therefore log-transformed for statistical analyses. Follow-up analyses with outlier cases (e.g., more than ±3 standard deviations away from the sample mean) removed provided the same pattern of results.
Table 1.
Descriptives and Correlations among Emotion, Inflammation, and Demographic Variables
| Construct | Min | Max | Mean | SD | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Global Emodiversity | 0.27 | 0.93 | 0.61 | 0.10 | - | |||||||||||||
| 2. Positive Emodiversity | 0.39 | 1.00 | 0.92 | 0.11 | .36 | - | ||||||||||||
| 3. Negative Emodiversity | 0.12 | 0.95 | 0.49 | 0.18 | .79 | .02 | - | |||||||||||
| 4. Mean Positive Emotion | 0.20 | 3.96 | 2.09 | 0.77 | −.03 | .72 | −.23 | - | ||||||||||
| 5. Mean Negative Emotion | 0.01 | 2.51 | 0.38 | 0.38 | .66 | −.23 | .64 | . −.36 | - | |||||||||
| 6. CRP* | 0.10 | 75.00 | 3.59 | 7.82 | −.09 | −.30 | .02 | −.13 | .01 | - | ||||||||
| 7. IL-6* | 0.23 | 72.64 | 2.56 | 6.08 | −.10 | −.11 | −.12 | −.03 | −.01 | .51 | - | |||||||
| 8. Fibrinogen* | 195.00 | 712.00 | 333.05 | 75.52 | −.15 | −.19 | −.10 | −.10 | −.01 | .52 | .33 | - | ||||||
| 9. BMI | 16.38 | 65.16 | 27.76 | 6.52 | .07 | −.16 | .14 | −.09 | .15 | .42 | .29 | .25 | - | |||||
| 10. Anti-Inflammatory Medication | No=0 | Yes=1 | 29%Y | - | .04 | .01 | .03 | −.04 | .10 | .05 | −.03 | .02 | .18 | - | ||||
| 11. Medical Conditions | 0 | 3+ | 1.39 | 1.12 | .03 | −.08 | .00 | −.09 | .17 | .16 | .19 | .12 | .17 | .33 | - | |||
| 12. Neuroticism | 7 | 38 | 21.72 | 6.21 | .31 | −.17 | .31 | −.26 | .29 | .02 | .01 | −.05 | .15 | .06 | .20 | - | ||
| 13. Extraversion | 8 | 39 | 25.97 | 6.78 | .04 | .32 | .03 | .31 | −.15 | .03 | .03 | −.10 | .06 | −.08 | −.05 | −.23 | - | |
| 14. Age | 40.00 | 65.00 | 53.42 | 7.57 | −.16 | .12 | −.19 | .19 | −.06 | .13 | .26 | .16 | .03 | .18 | .27 | −.07 | −.05 | - |
| 15. Gender (% male) | F = 0 | M = 1 | 46%M | - | .06 | .11 | .03 | .12 | .03 | −.01 | .04 | −.07 | .10 | .01 | −.02 | −.22 | .07 | .07 |
Note: N = 175; SD = standard deviation; CRP = C-reactive protein; IL-6 = interleukin-6; Summary statistics for the biomarkers of inflammation denoted with a * are given in raw units, whereas log transformed versions were used for the correlations and structural equation model analyses. BMI = body mass index.
Consistent with previous work (Quoidbach, et al., 2014), global emodiversity scores were correlated with negative emodiversity (r = .79) and positive emodiversity (r = .36), while positive emodiversity and negative emodiversity scores were uncorrelated (r = .02).
Associations between emodiversity and inflammation
Global emodiversity
Models 1 and 2 examined the relation between global emodiversity and inflammation. Results are shown in Table 2. Although the overall fit of Model 1 to the data was good (e.g., RMSEA < .05) and the inflammation factor was well defined (standardized factor loadings .88, .58, and .60), global emodiversity was, contrary to predictions, not significantly related to latent inflammation (B = -.14, p = .13). Although Model 2 also fit the data well (e.g., RMSEA < .05), inclusion of age, gender, BMI, medication, medical conditions, and personality as covariates did not reveal any association between global emodiversity and inflammation (B = −.14, p = .09).
Table 2.
Results from Structural Equation Models Examining Associations between Global Emodiversity and Latent Inflammation
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Unstd | SE | Std | p | Unstd | SE | Std | p | |
| Common Factor | ||||||||
| Inflammation → CRP | =1.00 | - | 0.88 | - | =1.00 | - | 0.84 | - |
| Inflammation → IL-6 | 0.39 | 0.08 | 0.58 | <.001 | 0.43 | 0.07 | 0.62 | <.001 |
| Inflammation → Fibrinogen | 0.11 | 0.02 | 0.60 | <.001 | 0.12 | 0.02 | 0.61 | <.001 |
| Regression(s) | ||||||||
| Global Emodiversity → Inflammation | −1.47 | 0.96 | −0.14 | .126 | −1.24 | 0.88 | −0.12 | .160 |
| BMI → Inflammation | - | - | - | - | 0.09 | 0.01 | 0.50 | <.001 |
| Anti-inflammatory Medication → Inflammation | - | - | - | - | −0.30 | 0.21 | −0.13 | .149 |
| Medical Conditions → Inflammation | - | - | - | - | 0.14 | 0.08 | 0.15 | .093 |
| Neuroticism → Inflammation | - | - | - | - | −0.01 | 0.02 | −0.07 | .423 |
| Extraversion → Inflammation | - | - | - | - | −0.002 | 0.01 | −0.01 | .902 |
| Age → Inflammation | - | - | - | - | 0.03 | 0.01 | 0.18 | .027 |
| Gender → Inflammation | - | - | - | - | −0.17 | 0.18 | −0.08 | .324 |
| Intercepts | ||||||||
| CRP | 0.37 | 0.10 | 0.28 | <.001 | 0.38 | 0.09 | 0.29 | <.001 |
| IL6 | 0.46 | 0.06 | 0.61 | <.001 | 0.47 | 0.06 | 0.61 | <.001 |
| Fibrinogen | 5.79 | 0.02 | 28.10 | <.001 | 5.79 | 0.02 | 28.06 | <.001 |
| Inflammation | 0.00 | - | 0.00 | - | 0.00 | - | 0.00 | - |
| Variances | ||||||||
| CRP | 0.39 | 0.22 | 0.23 | .08 | 0.51 | 0.15 | 0.30 | .001 |
| IL6 | 0.38 | 0.05 | 0.66 | <.001 | 0.36 | 0.05 | 0.62 | <.001 |
| Fibrinogen | 0.03 | 0.004 | 0.65 | <.001 | 0.03 | 0.004 | 0.63 | <.001 |
| Inflammation | 1.28 | 0.28 | 0.98 | <.001 | 0.79 | 0.18 | 0.67 | <.001 |
|
| ||||||||
| Model Fit Statistics | ||||||||
| χ2 | (df = 2) = 1.920, p = .383 | (df = 10) = 17.22, p =.372 | ||||||
| CFI | 1.00 | .991 | ||||||
| RMSEA | .000 [.000, .148] | .021 [.000, .074] | ||||||
| SRMR | .022 | .027 | ||||||
Note: N = 175; Unstd = unstandardized coefficient; SE = standard error; Std = standardized path coefficient; p = p-value; CRP = c-reactive protein; IL-6 = interleukin-6; BMI = body mass index; CFI comparative fit index; RMSEA = root-mean-square error of approximation; SRMR = standardized root-mean-square residual. Bracketed values represent 95% confidence intervals.
Positive and negative emodiversity
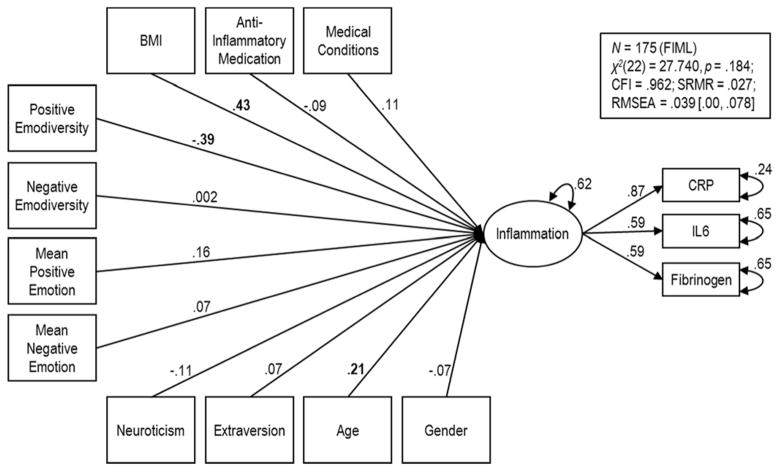
We next examined the extent to which positive and negative emodiversity were uniquely associated with inflammation. As seen in Table 3, Model 3 fit the data well (e.g., RMSEA < .05). In accordance with hypotheses, positive emodiversity was related to inflammation (B = −.26, p = .001). In particular, greater positive emodiversity was associated with lower inflammation, independent of age, gender, anti-inflammatory medications, BMI, medical conditions, and personality. Contrary to hypotheses, negative emodiversity was not significantly related to latent inflammation (B = −.03, p = .71). We then explored whether the association between positive emodiversity and inflammation held, over and above mean levels of positive and negative affect. Results from this comprehensive model are shown in Figure 2. As seen in the figure and in Table 3, positive emodiversity was associated with inflammation, even after controlling for mean levels of positive and negative emotion (B = −.38, p = .002), while negative emodiversity was not associated with inflammation (B = −.02, p = .86).
Table 3.
Results from Structural Equation Models Examining Associations between Positive and Negative Emodiversity and Latent Inflammation
| Model 3 | Model 4 | |||||||
|---|---|---|---|---|---|---|---|---|
| Unstd | SE | Std | p | Unstd | SE | Std | p | |
| Measurement Model | ||||||||
| Inflammation → CRP | =1.00 | - | 0.86 | - | =1.00 | - | 0.87 | - |
| Inflammation → IL-6 | 0.40 | 0.07 | 0.60 | <.001 | 0.40 | 0.07 | 0.59 | <.001 |
| Inflammation → Fibrinogen | 0.11 | 0.02 | 0.60 | <.001 | 0.11 | 0.02 | 0.59 | <.001 |
| Structural Model | ||||||||
| Positive Emodiversity → Inflammation | −2.90 | 0.81 | −0.29 | <.001 | −3.88 | 1.20 | −0.39 | .001 |
| Negative Emodiversity → Inflammation | 0.02 | 0.54 | 0.003 | .976 | 0.02 | 0.71 | 0.002 | .982 |
| Mean Positive Emotion → Inflammation | - | - | - | - | 0.23 | 0.18 | 0.16 | .186 |
| Mean Negative Emotion → Inflammation | - | - | - | - | 0.20 | 0.33 | 0.07 | .546 |
| BMI → Inflammation | 0.08 | 0.01 | 0.45 | <.001 | 0.08 | 0.01 | 0.43 | <.001 |
| Anti-inflammatory Medication → Inflammation | −0.24 | 0.21 | −0.10 | .244 | −0.23 | 0.21 | −0.09 | .279 |
| Medical Conditions → Inflammation | 0.12 | 0.08 | 0.11 | .169 | 0.11 | 0.08 | 0.11 | .179 |
| Neuroticism → Inflammation | −0.02 | 0.02 | −0.11 | .188 | −0.02 | 0.02 | −0.11 | .202 |
| Extraversion → Inflammation | 0.01 | 0.01 | 0.08 | .325 | 0.01 | 0.01 | 0.07 | .368 |
| Age → Inflammation | 0.03 | 0.01 | 0.23 | .004 | 0.03 | 0.01 | 0.21 | .009 |
| Gender → Inflammation | −0.15 | 0.17 | −0.07 | .394 | −0.15 | 0.17 | −0.07 | .393 |
| Intercepts | ||||||||
| CRP | 0.39 | 0.09 | 0.30 | <.001 | 0.39 | 0.09 | 0.30 | <.001 |
| IL6 | 0.47 | 0.06 | 0.62 | <.001 | 0.47 | 0.06 | 0.61 | <.001 |
| Fibrinogen | 5.79 | 0.02 | 28.09 | <.001 | 5.79 | 0.02 | 28.08 | <.001 |
| Inflammation | 0.00 | - | 0.00 | - | 0.00 | - | 0.00 | - |
| Residual Variances | ||||||||
| CRP | 0.43 | 0.16 | 0.25 | .006 | 0.40 | 0.16 | 0.24 | .013 |
| IL6 | 0.37 | 0.05 | 0.64 | <.001 | 0.38 | 0.05 | 0.65 | <.001 |
| Fibrinogen | 0.03 | 0.004 | 0.65 | <.001 | 0.03 | 0.004 | 0.65 | <.001 |
| Inflammation | 0.79 | 0.18 | 0.62 | <.001 | 0.80 | 0.18 | 0.62 | <.001 |
|
| ||||||||
| Model Fit Statistics | ||||||||
| χ2 | (df = 18) = 22.07, p = .229 | (df = 22) = 27.740, p = .184 | ||||||
| CFI | .973 | .962 | ||||||
| RMSEA | .036 [.000, .080] | .039 [.000, .078] | ||||||
| SRMR | .030 | .027 | ||||||
Note: N = 175; Unstd = unstandardized coefficient; SE = standard error; Std = standardized coefficient; p = p-value; CRP = c-reactive protein; IL-6 = interleukin-6; BMI = body mass index. CFI comparative fit index; RMSEA = root-mean-square error of approximation; SRMR = standardized root-mean-square residual. Bracketed values represent 95% confidence intervals.
Figure 2.
Structural and measurement models depicting results from Model 4. Values are standardized path coefficients and variances. Bolded coefficients are significant at p < .05.
Supplemental analyses
To assess the unique associations among the predictors and individual markers of inflammation, we supplemented the 4 SEMs with 12 regression models wherein the three markers of inflammation were examined as unique outcome variables. The associations were generally consistent with the common factor approach, although some of the associations did not reach statistical significance. Parallel to the SEMs, regression models indicated that global emodiversity was negatively, but non-significantly associated with CRP, IL6, or fibrinogen in base models (Table 4), or after accounting for covariates (Table 5). Regression models indicated that higher positive emodiversity was significantly associated with lower CRP and lower fibrinogen after adjusting for covariates (Table 6). Additionally, the inverse relation between positive emodiversity and CRP remained significant after mean levels of positive and negative emotions had been included as additional covariates (Table 7). Regression models indicated that positive emodiversity was not significantly associated with IL6, but the sign of the coefficient was in the hypothesized direction. Finally, in none of the regression models was negative emodiversity significantly associated with individual markers of inflammation.
Table 4.
Results from Unadjusted Regression Models Examining Associations between Global Emodiversity and Markers of Inflammation
| CRP (N = 162) | IL6 (N = 162) | Fibrinogen (N = 158) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Estimate | SE | p | Estimate | SE | p | Estimate | SE | p | |
| Intercept | 0.37 | 0.10 | <.001 | 0.46 | 0.06 | <.001 | 5.79 | 0.02 | <.001 |
| Global Emodiversity | −1.18 | 0.99 | .234 | −0.76 | 0.58 | .189 | −0.31 | 0.16 | .052 |
|
| |||||||||
| Residual SE | 1.30 | 0.76 | 0.20 | ||||||
| Adjusted R2 | .003 | .005 | .02 | ||||||
Note: CRP = C-Reactive Protein; IL6 = Interleukin-6; SE = standard error; p = p-value
Table 5.
Results from Adjusted Regression Models Examining Associations between Global Emodiversity and Markers of Inflammation
| CRP (N = 153) | IL6 (N = 153) | Fibrinogen (N = 150) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Estimate | SE | p | Estimate | SE | p | Estimate | SE | p | |
| Intercept | 0.37 | 0.10 | <.001 | 0.48 | 0.06 | <.001 | 5.78 | 0.02 | <.001 |
| Global Emodiversity | −1.37 | 1.02 | .181 | −0.49 | 0.61 | .419 | −0.29 | 0.17 | .089 |
| BMI | 0.09 | 0.02 | <.001 | 0.03 | 0.01 | <.001 | 0.01 | 0.002 | <.001 |
| Anti-inflammatory Medication | −0.22 | 0.23 | .330 | −0.32 | 0.14 | .021 | −0.04 | 0.04 | .326 |
| Medical Conditions | 0.11 | 0.10 | .251 | 0.10 | 0.06 | .080 | 0.02 | 0.02 | .270 |
| Neuroticism | −0.01 | 0.02 | .777 | −0.002 | 0.01 | .886 | −0.004 | 0.003 | .153 |
| Extraversion | 0.01 | 0.01 | .668 | 0.005 | 0.01 | .568 | −0.004 | 0.002 | .133 |
| Age | 0.02 | 0.01 | .098 | 0.03 | 0.01 | <.001 | 0.003 | 0.002 | .118 |
| Gender | −0.17 | 0.20 | .405 | −0.02 | 0.12 | .886 | −0.04 | 0.03 | .185 |
|
| |||||||||
| Residual SE | 1.19 | 0.71 | 0.19 | ||||||
| Adjusted R2 | .19 | .16 | .11 | ||||||
Note: CRP = C-Reactive Protein; IL6 = Interleukin-6; SE = standard error; p = p-value
Table 6.
Results from Adjusted Regression Models Examining Associations between Positive and Negative Emodiversity and Markers of Inflammation
| CRP (N = 153) | IL6 (N = 153) | Fibrinogen (N = 150) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Estimate | SE | p | Estimate | SE | p | Estimate | SE | p | |
| Intercept | 0.37 | 0.09 | <.001 | 0.47 | 0.06 | <.001 | 5.78 | 0.02 | <.001 |
| Positive Emodiversity | −3.40 | 0.85 | <.001 | −0.73 | 0.53 | .167 | −0.30 | 0.15 | .045 |
| Negative Emodiversity | 0.27 | 0.59 | .652 | −0.37 | 0.36 | .311 | −0.08 | 0.10 | .432 |
| BMI | 0.08 | 0.01 | <.001 | 0.03 | 0.01 | <.001 | 0.01 | 0.003 | .002 |
| Anti-inflammatory Medication | −0.16 | 0.22 | .465 | −0.28 | 0.14 | .044 | −0.03 | 0.04 | .376 |
| Medical Conditions | 0.09 | 0.09 | .352 | 0.10 | 0.06 | .100 | 0.02 | 0.02 | .320 |
| Neuroticism | −0.01 | 0.02 | .424 | −0.0003 | 0.01 | .979 | −0.005 | 0.003 | .099 |
| Extraversion | 0.02 | 0.01 | .103 | 0.01 | 0.01 | .265 | −0.002 | 0.003 | .404 |
| Age | 0.03 | 0.01 | .015 | 0.03 | 0.01 | .001 | 0.004 | 0.002 | .063 |
| Gender | −0.15 | 0.19 | .440 | −0.01 | 0.12 | .945 | −0.05 | 0.03 | .167 |
|
| |||||||||
| Residual SE | 1.14 | 0.70 | 0.19 | ||||||
| Adjusted R2 | .26 | .17 | .12 | ||||||
Note: CRP = C-Reactive Protein; IL6 = Interleukin-6; SE = standard error; p = p-value
Table 7.
Results from Adjusted Regression Models Examining Associations between Positive and Negative Emodiversity, Mean Positive and Negative Emotion, and Markers of Inflammation
| CRP (N = 153) | IL6 (N = 153) | Fibrinogen (N = 150) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Estimate | SE | p | Estimate | SE | p | Estimate | SE | p | |
| Intercept | 0.36 | 0.09 | <.001 | 0.47 | 0.06 | <.001 | 5.78 | 0.02 | <.001 |
| Positive Emodiversity | −5.03 | 1.25 | <.001 | −0.75 | 0.77 | .329 | −0.27 | 0.22 | .213 |
| Negative Emodiversity | 0.45 | 0.74 | .546 | −0.83 | 0.46 | .071 | −0.05 | 0.13 | .698 |
| Mean Positive Emotion | 0.36 | 0.19 | .060 | 0.07 | 0.12 | .571 | −0.01 | 0.03 | .768 |
| Mean Negative Emotion | 0.09 | 0.36 | .801 | 0.42 | 0.22 | .056 | −0.03 | 0.06 | .628 |
| BMI | 0.07 | 0.01 | <.001 | 0.03 | 0.01 | <.001 | 0.01 | 0.003 | .002 |
| Anti-inflammatory Medication | −0.12 | 0.22 | .600 | −0.30 | 0.14 | .032 | −0.03 | 0.04 | .400 |
| Medical Conditions | 0.09 | 0.09 | .328 | 0.08 | 0.06 | .149 | 0.02 | 0.02 | .304 |
| Neuroticism | −0.01 | 0.02 | .507 | −0.002 | 0.01 | .843 | −0.005 | 0.003 | .109 |
| Extraversion | 0.02 | 0.01 | .137 | 0.01 | 0.01 | .231 | −0.002 | 0.003 | .409 |
| Age | 0.03 | 0.01 | .034 | 0.03 | 0.01 | .002 | 0.004 | 0.002 | .060 |
| Gender | −0.15 | 0.19 | .425 | −0.02 | 0.12 | .898 | −0.05 | 0.03 | .173 |
|
| |||||||||
| Residual SE | 1.13 | 0.70 | 0.19 | ||||||
| Adjusted R2 | .26 | .18 | .11 | ||||||
Note: CRP = C-Reactive Protein; IL6 = Interleukin-6; SE = standard error; p = p-value
Discussion
This study had two principal goals. The first was to test the hypothesis that diversity in day-to-day positive and negative emotions would be associated with lower inflammatory activity. In SEM analyses adjusting for demographic and health covariates, we did not find an association between global emodiversity and latent inflammation (characterized by IL-6, CRP, and fibrinogen). These results differ from those of a prior study documenting better mental and physical health among adults reporting greater global emodiversity (Quoidbach, et al., 2014). The discrepancies may reflect the different measurement approaches and populations sampled. For example, Quoidbach et al. (2014) used a single-occasion measure to derive emodiversity scores, whereas the current study used repeated measures of daily emotional experience obtained over 30 days. Thus, it may be that the associations between global emodiversity and health are limited to across-person (nomothetic) responses that are not captured by our within-person (idiographic) measure of emodiversity (see Kenny, Kashy, & Bolger, 1998; Tennen & Affleck, 1996). A more systematic investigation of the relations between global emodiversity—assessed at multiple time scales—and inflammation is warranted to better understand the nature and health implications of individual differences in emodiversity. The study samples also differed in terms of cultural background. The sample in the Quoidbach et al. study was European (i.e., French and Belgian), whereas the sample in the current study was from the southwestern U.S. Potential cultural differences in the links between global emodiversity and inflammation should be examined more closely in future work.
A second goal of the current study was to examine unique associations of positive and negative emodiversity with inflammation. As predicted, greater diversity in day-to-day positive emotions was related to lower systemic inflammation. This association remained significant after accounting for differences in demographic characteristics, BMI, medication use, medical conditions, personality, and mean levels of emotion. The finding is consistent with other studies examining links between positive affect and inflammation using conventional, single-occasion indices (Stellar, et al., 2015; Steptoe, et al., 2008). Importantly, the results are in line with prior work suggesting that intraindividual variability in positive emotions is important to psychological and physical health above and beyond mean levels (Gruber, et al., 2013; Ong, Exner-Cortens, Riffin et al., 2013). Overall, these findings align with a functional account of “discrete” positive emotions that suggests biopsychosocial environments encountered in daily life can activate a diversity of positive emotions (e.g., pride, amusement, contentment), each serving a specific adaptive purpose (Shiota, et al., in press; Shiota, et al., 2014). In contrast, there was no association between negative emodiversity and inflammation. Prior research demonstrates that older adults show less intraindividual variability in negative emotions than younger adults (Brose, et al., 2015; Grühn, et al., 2013; Röcke, Li, & Smith, 2009). It is possible that the lack of association between emodiversity and inflammation in this study may reflect reduced intraindividual variability in participants’ negative emotions. Future studies should attempt to replicate these findings in more age-heterogeneous samples.
This investigation also showed that higher positive emodiversity was associated with lower levels of CRP and fibrinogen. Further, the single-outcome regression models revealed that the association between positive emodiversity and CRP was unchanged when age, gender, anti-inflammatory medications, BMI, medical conditions, personality, and mean levels of positive and negative emotions were included as covariates. While not all associations were significant, it is worth noting that the substantive pattern of findings across all three markers of inflammation was in the predicted direction (i.e., higher positive emodiversity associated with lower inflammation). Mirroring the findings from the SEM models, negative emodiversity was not associated with any of the biomarkers of inflammation in the separate regression models.
Limitations
Our conclusions are limited by some features of our methods and analyses. First, our sample consisted of a cross-section of relatively healthy middle-aged adults. Both the restricted age range (age 45 to 60 years) and sample size (N = 175) limit the generalizability of results. Although we attempted to examine the extent to which associations between emodiversity and inflammatory markers (i.e., IL-6, CRP, fibrinogen) were independent of potential confounding variables (e.g., age, gender, anti-inflammatory medications, BMI, medical conditions, personality, mean level of emotions), future research should explore whether the associations hold when accounting for a variety of other personal characteristics that may drive emodiversity (e.g., cognitive control). Second, our analyses of emodiversity relied heavily on emotion reports that were completed at the end of each day. It is well established the emotions vary both within day and across days (Clark, Watson, & Leeka, 1989; Watson, Wiese, Vaidya, & Tellegen, 1999). Thus, future research should include more intensive experience sampling approaches (Steptoe & Wardle, 2011) that allow for modeling of diurnal and circadian patterns in emotion. Third, our data do not speak to the underlying mechanisms of emodiversity. Emodiversity may act to reduce negative appraisals of stress and facilitate adaptive coping. Alternatively, emodiversity may impact behaviors relevant to health in general, irrespective of its influence on stress responses. It may be that systemic inflammation is among the key mediating factors linking emodiversity to subsequent psychological morbidity. These hypothesized processes have yet to be empirically investigated. Finally, because our study was observational in nature, the directionality of the observed associations cannot be determined. For example, it is possible that a lack of diversity of both positive and negative emotional experience may result from heightened inflammatory responses. This issue highlights the need for longitudinal assessments to better characterize the temporal relationships between emodiversity and inflammation.
Conclusions
Despite these limitations, the findings add to the evidence that positive affective states are related to favorable profiles of biological functioning that may contribute to reduced risk of chronic disease, while suggesting that diversity in day-to-day positive emotions is related to reduced levels of systemic inflammation.
Acknowledgments
This research was supported by grants from the National Institutes of Health (R01 HD076994, R01 AG 026006, P2C HD041025, UL TR000127) and the Penn State Social Science Research Institute, and a Pennsylvania State University Graduate Fellowship.
Footnotes
As cited in Wessman and Ricks (1966), p. 251.
This document has benefitted from helpful comments from Felix Thoemmes and Adam Anderson. We are indebted to our friend and colleague Alex Zautra who passed away unexpectedly during the writing of this manuscript. He was a dedicated scientist who was admired and respected by us all.
References
- Adler JM, Hershfield HE. Mixed emotional experience is associated with and precedes improvements in psychological well-being. PLoSONE. 2012;7:1–10. doi: 10.1371/journal.pone.0035633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al AL, Kronfol Z, Seymour E, Bolling SF. Effects of mood state and psychosocial functioning on plasma interleukin-6 in adult patients before cardiac surgery. International Journal of Psychiatry in Medicine. 2005;35:363–376. doi: 10.2190/2ELG-RDUN-X6TU-FGC8. [DOI] [PubMed] [Google Scholar]
- Barrett K, Campos J. Perspectives on emotional development: II. A functionalist approach to emotion. In: Osofsky J, editor. Handbook of infant development. Vol. 2. New York: Wiley; 1987. pp. 555–578. [Google Scholar]
- Barrett LF. Discrete emotions or dimensions? The role of valence focus and arousal focus. Cognition and Emotion. 1998;12:579–599. [Google Scholar]
- Barrett LF. Solving the emotion paradox: Categorization and the experience of emotion. Personality and Social Psychology Review. 2006;10:20–46. doi: 10.1207/s15327957pspr1001_2. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Gross J, Christensen TC, Benvenuto M. Knowing what you’re feeling and knowing what to do about it: Mapping the relation between emotion differentiation and emotion regulation. Cognition and Emotion. 2001;15:713–724. doi: 10.1080/02699930143000239. [DOI] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annual Review of Psychology. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson L, Ram N, Almeida D, Zautra A, Ong AD. Fusing biodiversity metrics into investigations of daily life and healthy aging: Recommendations and illustrations with emodiversity. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. doi: 10.1093/geronb/gbx025. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler P. Comparative fix indexes in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Blomberg BB, Alvarez JP, Diaz A, Romero MG, Lechner SC, Carver CS, … Antoni MH. Psychosocial adaptation and cellular immunity in breast cancer patients in the weeks after surgery: An exploratory study. Journal of Psychosomatic Research. 2009;67:369–376. doi: 10.1016/j.jpsychores.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose A, de Roover K, Ceulemans E, Kuppens P. Older adults’ affective experiences across 100 days are less variable and less complex than younger adults’. Psychology & Aging. 2015;30:194–208. doi: 10.1037/a0038690. [DOI] [PubMed] [Google Scholar]
- Budescu DV, Budescu M. How to measure diversity when you must. Psychological Methods. 2012;17:215–227. doi: 10.1037/a0027129. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology. 2000;79:644–655. doi: 10.1037/0022-3514.79.4.644. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Turan B, Scheibe S, Ram N, Ersner-Hershfield H, Samanez-Larkin R. Emotional experience improves with age: Evidence based on over 10 years of experience sampling. Psychology and Aging. 2011;26:21–33. doi: 10.1037/a0021285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, … Pahor M. Inflammatory markers and cardiovascular disease. American Journal of Cardiology. 2003;92:522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Leeka J. Diurnal variation in the positive affects. Motivation and Emotion. 1989;13:205–234. [Google Scholar]
- Cohen S, Alper M, Doyle WJ, Treanor JJ, Turner RB. Positive emotional style predicts resistance to illness after experimental exposure to rhinovirus or influenza A virus. Psychosomatic Medicine. 2006;68:809–815. doi: 10.1097/01.psy.0000245867.92364.3c. [DOI] [PubMed] [Google Scholar]
- Coifman KG, Bonanno GA, Rafaeli E. Affect dynamics, bereavement and resilience to loss. Journal of Happiness Studies. 2007;8:371–392. doi: 10.1007/s10902-006-9014-5. [DOI] [Google Scholar]
- Demiralp E, Thompson RJ, Mata J, Jaeggi SM, Buschkuehl M, Barrett LF, … Jonides J. Feeling blue or turquoise? Emotional differentiation in major depressive disorder. Psychological Science. 2012;23:1410–1416. doi: 10.1177/0956797612444903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverts DJ, Cohen S, DiLillo VG, Lewis CE, Kiefe C, Whooley M. Depressive symptoms, race, and circulating C-reactive protein: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychosomatic Medicine. 2010;72:734–741. doi: 10.1097/PSY.0b013e3181ec4b98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, Aziz N, Kim KH, Fahey JL. Immunological effects of induced shame and guilt. Psychosomatic Medicine. 2004;66:124–131. doi: 10.1097/01.psy.0000097338.75454.29. [DOI] [PubMed] [Google Scholar]
- Duivis HE, de Jonge P, Penninx BW, Na BY, Cohen BE, Whooley MA. Depressive symptoms, health behaviors, and subsequent inflammation in patients with coronary heart disease: prospective findings from the heart and soul study. American Journal of Psychiatry. 2011;168:913–920. doi: 10.1176/appi.ajp.2011.10081163. [DOI] [PubMed] [Google Scholar]
- Enders CK. Applied missing data analysis. New York: Guilford Press; 2010. [Google Scholar]
- Epel ES, Lithgow GJ. Stress biology and aging mechanisms: Toward understanding the deep connection between adaptation to stress and longevity. Journal of Gerontology: Biological Sciences. 2014;69:S10–S16. doi: 10.1093/gerona/glu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Herd P. Income, education, and inflammation: Differential associations in a national probability sample (The MIDUS Study) Psychosomatic Medicine. 2010;72:290–300. doi: 10.1097/PSY.0b013e3181cfe4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann I, Huynh AC, Ellsworth PC. Emotional complexity: Clarifying definitions and cultural correlates. Journal of Personality and Social Psychology. 2016;111:895–916. doi: 10.1037/pspp0000084. [DOI] [PubMed] [Google Scholar]
- Gruber J, Bekoff M. A cross-species comparative approach to positive emotion disturbance. Emotion Review. 2017;9:72–78. [Google Scholar]
- Gruber J, Kogan A, Quoidbach J, Mauss IB. Happiness is best kept stable: Positive emotion variability is associated with poorer psychological health. Emotion. 2013;13:1–6. doi: 10.1037/a0030262. [DOI] [PubMed] [Google Scholar]
- Grühn D, Lumley MA, Diehl M, Labouvie-Vief G. Time-based indicators of emotional complexity: Interelations and correlates. Emotion. 2013;13:226–237. doi: 10.1037/a0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton E. The Greek way. New York: Norton; 1942. [Google Scholar]
- Hay EL, Diehl M. Emotion complexity and emotion regulation across adulthood. European Journal of Ageing. 2011;8:157–168. doi: 10.1007/s10433-011-0191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield HE, Scheiber S, Sims TL, Carstensen LL. When feeling bad can be good: Mixed emotions benefit physical health across adulthood. Social Psychological and Personality Science. 2013;4:54–61. doi: 10.1177/1948550612444616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Ross KM, Chen E, Miller GE. Modeling the association between lifecourse socioeconomic disadvantage and systemic inflammation in healthy adults: The role of self-control. Health Psychology. 2015;34:580–590. doi: 10.1037/hea0000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Hülür G, Hoppmann CA, Ram N, Gerstorf D. Developmental associations between short-term variability and long-term changes: Intraindividual correlation of positive and negative affect in daily life and cognitive aging. Developmental Psychology. 2015;51:987–997. doi: 10.1037/a0039341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicki-Deverts D, Cohen S, Doyle WJ, Turner RB, Treanor JJ. Infection-induced proinflammatory cytokines are associated with decreases in positive affect, but not increases in negative affect. Brain, Behavior, and Immunity. 2007;21:301–307. doi: 10.1016/j.bbi.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashdan TB, Farmer AS. Differentiating emotions across contexts: Comparing adults with and without social anxiety disorder using random, social interaction, and daily experience sampling. Emotion. 2014;14:629–638. doi: 10.1037/a0035796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner D, Gross JJ. Functional accounts of emotions. Cognition and Emotion. 1999;13:467–480. [Google Scholar]
- Kenny DA, Kashy DA, Bolger N. Data analysis in social psychology. In: Fiske ST, Gilbert DT, editors. The handbook of social psychology. 4. New York: McGraw Hill; 1998. pp. 233–265. [Google Scholar]
- Koffer RE, Ram N, Conroy DE, Pincus AL, Almeida DM. Stressor diversity: Introduction and empirical integration into the daily stress model. Psychology and Aging. 2016;31:301–320. doi: 10.1037/pag0000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JT, McGraw AP. The case for mixed emotions. Social and Personality Psychology Compass. 2014;8:263–274. [Google Scholar]
- Lee S, Koffer RE, Sprague BN, Charles ST, Ram N, Almeida DM. Activity Diversity and Its Associations With Psychological Well-Being Across Adulthood. Journal of Gerontology: Psychological Sciences. doi: 10.1093/geronb/gbw118. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang G, Feng Y, Wu C. An entropy-based social network community detecting method and its application to scientometrics. Scientometrics. 2015;102:1003–1017. [Google Scholar]
- Lindquist K, Barrett LF. Emotional complexity. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. The handbook of emotion. 3. New York: Guilford Press; 2008. pp. 513–530. [Google Scholar]
- Magurran AE. Measuring biology diversity. Malden: Blackwell Publishing; 2004. [Google Scholar]
- McCaffery JM, Marsland AL, Strohacker K, Muldoon MF, Manuck SB. Factor structure underlying components of allostatic load. PLoS One. 2012;7:e47246. doi: 10.1371/journal.pone.0047246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Blackwell E. Turning up the heat: Inflammation as a mechanism linking chronic stress, depression, and heart disease. Current Directions in Psychological Science. 2006;15:269–272. [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, Stetler CA, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosomatic Medicine. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Ryff CD. Cultural differences in the dialectical and non-dialectical emotional styles and their implications for health. Cognition and Emotion. 2011;25:22–39. doi: 10.1080/02699931003612114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Uchida Y, Ellsworth PC. Culture and mixed emotions: Co-occurrence of positive and negative emotions in Japan and the United States. Emotion. 2010;10:404–415. doi: 10.1037/a0018430. [DOI] [PubMed] [Google Scholar]
- Moons WG, Eisenberger NI, Taylor SE. Anger and fear responses to stress have different biological profiles. Brain, Behavior, and Immunity. 2010;24:215–219. doi: 10.1016/j.bbi.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Moons WG, Shields GS. Anxiety, not anger, induces inflammatory activity: An avoidance/approach model of immune system activation. Emotion. 2015;15:463–476. doi: 10.1037/emo0000055. [DOI] [PubMed] [Google Scholar]
- Moreno P, Moskowitz AL, Ganz PA, Bower JE. Positive affect and inflammatory activity in breast cancer survivors: Examining the role of affective arousal. Psychosomatic Medicine. 2016;78:532–541. doi: 10.1097/PSY.0000000000000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin PJ. Community ecology. Malden: Blackwell Publishing; 1999. [Google Scholar]
- Ong AD, Bergeman CS. The complexity of emotions in later life. The Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2004;59B:P117–P122. doi: 10.1093/geronb/59.3.P117. [DOI] [PubMed] [Google Scholar]
- Ong AD, Exner-Cortens D, Riffin C, Steptoe A, Zautra A, Almeida DM. Linking stable and dynamic features of positive affect to sleep. Ann Behav Med. 2013;46:52–61. doi: 10.1007/s12160-013-9484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov ME, Davis MC, Belyea MJ, Zautra A. Linking childhood abuse and hypertension: sleep disturbance and inflammation as mediators. Journal of Behavioral Medicine. 2016;39:716–726. doi: 10.1007/s10865-016-9742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsavos C, Panagiotakos DB, Papageorgiou C, Tsetsekou E, Soldatos C, Stefanadis C. Anxiety in relation to inflammation and coagulation markers, among healthy adults: The ATTICA Study. Atherosclerosis. 2006;185:320–326. doi: 10.1016/j.atherosclerosis.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Prather AA, Marsland AL, Muldoon MF, Manuck SB. Positive affective style covaries with stimulated IL-6 and IL-10 production in a middle-aged community sample. Brain Behavior and Immunity. 2007;21:1033–1037. doi: 10.1016/j.bbi.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoidbach J, Gruber J, Mikolajczak M, Kogan A, Kotsou I, Norton M. Emodiversity and the emotional ecosystem. Journal of Experimental Psychology: General. 2014;143:2057–2066. doi: 10.1037/a0038025. [DOI] [PubMed] [Google Scholar]
- Ram N, Conroy DE, Pincus AL, Hyde AL, Molloy L. Tethering theory to method: Using measures of intraindividual variability to operationalize individuals’ dynamic characteristics. In: Hancock G, Harring J, editors. Advances in longitudinal modeling in the social and behavioral sciences. Charlotte: Information Age Publishing; 2012. pp. 81–110. [Google Scholar]
- Ram N, Gerstorf D. Time-structured and net intraindividual variability: Tools for examining the development of dynamic characteristics and processes. Psychology and Aging. 2009;24:778–791. doi: 10.1037/a0017915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready RE, Carvalho JO, Weinberger MI. Emotional complexity in younger, midlife, and older adults. Psychology & Aging. 2008;23:928–933. doi: 10.1037/a0014003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF, Brooks KP, Pressman SD. Trait positive affect buffers the effects of acute stress on skin barrier recovery. Health Psychology. 2009;28:373–378. doi: 10.1037/a0014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röcke C, Li SC, Smith J. Intraindividual variability in positive and negative affect over 45 days: Do older adults fluctuate less than young adults? Psychology and Aging. 2009;24:863–878. doi: 10.1037/a0016276. [DOI] [PubMed] [Google Scholar]
- Rosseel Y. lavaan: An R package for structural equation modeling. Journal of Statistical Software. 2012;48:1–36. [Google Scholar]
- Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39:1161–1178. doi: 10.1037//0022-3514.79.2.286. [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Ironson G, Siegal SD. Stress and health: Psychological, behavioral, and biological determinants. Annual Review of Clinical Psychology. 2008;1:607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz N. Feelings as information: Informational and motivational functions of affective states. In: Sorrentino RM, Higgins ET, editors. Handbook of motivation and cognition: Foundations of social behavior. Vol. 2. New York: Guilford Press; 1990. pp. 527–561. [Google Scholar]
- Schwarz N, Clore GL. Mood, misattribution, and judgments of well-being: Informative and directive functions of affective states. Journal of Personality and Social Psychology. 1983;45:513–523. doi: 10.1037/0022-3514.45.3.513. [DOI] [Google Scholar]
- Sepah SC, Bower JE. Positive affect and inflammation during radiation treatment for breast and prostate cancer. Brain, Behavior, and Immunity. 2009;23:1068–1072. doi: 10.1016/j.bbi.2009.06.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin WB. Entropy and information approaches to genetic diversity and its expression: Genomic geography. Entropy. 2010;12:1765–1798. [Google Scholar]
- Shiota MN, Campos B, Oveis C, Hertenstein MJ, Simon-Thomas E, Keltner D. Beyond happiness: Building a science of discrete positive emotions. American Psychologist. doi: 10.1037/a0040456. (in press) [DOI] [PubMed] [Google Scholar]
- Shiota MN, Neufeld SL, Danvers AF, Osborne EA, Sng O, Yee CI. Positive emotion differentiation: A functional approach. Social and Personality Psychology Compass. 2014;8:104–117. [Google Scholar]
- Sims TL, Tsai JL, Jiang D, Wang Y, HFH, Zhang X. Wanting to maximize the positive and minimize the negative: implications for mixed affective experience in American and Chinese contexts. Journal of Personality and Social Psychology. 2015;109:292–315. doi: 10.1037/a0039276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellar JE, John-Henderson N, Anderson CL, Gordon AM, McNeil G, Keltner D. Positive affect and markers of inflammation: discrete positive emotions predict lower levels of inflammatory cytokines. Emotion. 2015;16:129–133. doi: 10.1037/emo0000033. [DOI] [PubMed] [Google Scholar]
- Steptoe A, O’Donnell K, Badrick E, Kumari M, Marmot M. Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women: the Whitehall II study. American Journal of Epidemiology. 2008;167:96–102. doi: 10.1093/aje/kwm252. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Wardle J. Positive affect measured using ecological momentary assessment and survival in older men and women. Proceedings of the National Academy of Sciences. 2011;108:18244–18248. doi: 10.1073/pnas.1110892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgeon JA, Arewasikporn A, Okun MA, Davis MC, Ong AD, Zautra AJ. The psychosocial context of financial stress: Implications for inflammation and psychological health. Psychosomatic Medicine. 2016;78:134–143. doi: 10.1097/PSY.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez EC. Joint effect of hostility and severity of depressive symptoms on plasma interleukin-6 concentration. Psychosomatic Medicine. 2003;65:523–527. doi: 10.1097/01.psy.0000062530.94551.ea. [DOI] [PubMed] [Google Scholar]
- Tennen H, Affleck G. Daily processes in coping with chronic pain: Methods and analytic strategies. In: Endler NS, Zeidner M, editors. Handbook of coping: Theory, research, applications. Oxford: John Wiley & Sons; 1996. pp. 151–177. [Google Scholar]
- Tomko RL, Lane SP, Pronove LM, Treloar HR, Brown WC, Solhan MB, … Trull TJ. Undifferentiated negative affect and impulsivity in borderline personality and depressive disorders: A momentary perspective. Journal of Abnormal Psychology. 2015;124:740–753. doi: 10.1037/abn0000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugade MM, Fredrickson BL, Feldman-Barrett L. Psychological resilience and positive emotional granularity: Examining the benefits of positive emotions on coping and health. Journal of Personality and Social Psychology. 2004;72:1161–1190. doi: 10.1111/j.1467-6494.2004.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson D, Wiese D, Vaidya J, Tellegen A. The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology. 1999;76:820–838. [Google Scholar]
- Wessman AE, Ricks DF. Mood and personality. Oxford: Holt, Rinehart, and Winston; 1966. [Google Scholar]
- West SG, Hepworth JT. Statistical issues in the study of temporal data: Daily experiences. Journal of Personality. 1991;59:609–662. doi: 10.1111/j.1467-6494.1991.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Affleck GG, Tennen H, Reich JW, Davis MC. Dynamic approaches to emotions and stress in everyday life: Bolger and Zuckerman reloaded with positive as well as negative affects. Journal of Personality. 2005;76:1511–1538. doi: 10.1111/j.0022-3506.2005.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]