Abstract
Hibernating mammals, like the arctic ground squirrel (AGS), exhibit robust resistance to myocardial ischemia/reperfusion (IR) injury. Regulated preference for lipid over glucose to fuel metabolism may play an important role. We tested whether providing lipid in an emulsion protects hearts from summer-active AGS better than hearts from Brown Norway (BN) rats against normothermic IR injury. Langendorff-prepared AGS and BN rat hearts were perfused with Krebs solution containing 7.5 mM glucose with or without 1% Intralipid™. After stabilization and cardioplegia, hearts underwent 45 minutes global ischemia and 60 minutes reperfusion. Coronary flow, isovolumetric left ventricular pressure and mitochondrial redox state were measured continuously; infarct size was measured at the end of the experiment. Glucose-only AGS hearts functioned significantly better on reperfusion than BN rat hearts. Intralipid™ administration resulted in additional functional improvement in AGS compared to glucose only and BN rat hearts. Infarct size was not different among groups. Even under non-hibernating conditions, AGS hearts performed better after IR than the best-protected rat strain. This, however, appears to strongly depend on metabolic fuel: Intralipid™ led to a significant improvement in return of function in AGS, but not in BN rat hearts, suggesting that year-round endogenous mechanisms are involved in myocardial lipid utilization that contributes to improved cardiac performance, independent of the metabolic rate decrease during hibernation. Comparative lipid analysis revealed four candidates as possible cardioprotective lipid groups. The improved function in Intralipid™-perfused AGS hearts also challenges the current paradigm that increased glucose and decreased lipid metabolism are favorable during myocardial IR.
Keywords: Arctic Ground Squirrel, Brown Norway Rat, Cardiac Performance, Intralipid, Langendorff, Myocardial Ischemia Reperfusion Injury
1. Introduction
Coronary artery disease and subsequent myocardial infarction continue to rank among the most common causes of mortality, accounting for nearly 40% of the deaths in the developed world (Gaziano et al. 2010; Roger et al. 2012) and one of every six deaths in the United States (Go et al. 2013). Health care costs in the United States associated with cardiovascular disease exceed $300 billion per year (Go et al. 2013), in addition to the enormous personal price of severely limited quality of life, loss of productivity, and premature death patients experience.
Myocardial ischemia/reperfusion (IR) and their resulting tissue injury are also an unavoidable consequence of cardiac surgery and heart transplantation, and remain a major source of perioperative morbidity and mortality (Bueno-Gonzalez et al. 2010; Ueki et al. 2016). Prognosis after myocardial IR injury primarily depends on the quantity of irreversibly damaged myocardium (Miller et al. 1995). Remarkable differences in individual myocardial IR tolerance, however, have been found among (Shen and Vatner 1996), and even within, different species. For example, the Brown Norway (BN) rat (Rattus norvegicus) exhibits a significantly lower susceptibility to myocardial IR injury than any other rat strain, including Dahl salt-sensitive (SS) rats (Baker et al. 2000) which can be exploited to study some of the phenotypical and genetic determinants of resistance to myocardial IR injury (Shi et al. 2005; Kwitek et al. 2006; Nabbi et al. 2014).
The physiological phenomenon of hibernation offers another intriguing example of natural adaptation to adverse conditions (Carey et al. 2003). During hibernation, regulatory mechanisms depress metabolism to as low as 2% of basal rates for days to weeks to support cell preservation and long-term viability in a fasting state (Heldmaier et al. 2004). Hibernating mammals have also been found to exhibit an extraordinary resistance to IR injury in numerous organs (Lindell et al. 2005; Kurtz et al. 2006; Dave et al. 2006; Dave et al. 2009; Bogren et al. 2014; Yan et al. 2015).
A recent in-vivo study of deep hypothermic myocardial IR (Quinones et al. 2016) compared hibernating and summer-active non-hibernating Arctic Ground Squirrels (Urocitellus parryii, AGS), the hibernating mammal with the lowest body temperature during hibernation (Barnes 1989; Andrews 2007), as well as the most (BN) and the least IR injury-resistant (SS) rat strain (Baker et al. 2000). Interestingly, AGS displayed increased resistance against myocardial IR injury even at euthermic body temperatures while under non-hibernating conditions when compared to BN and SS rats; as expected, hibernation further increased this resistance in AGS. A subsequent detailed proteomic analysis suggested that hibernator cardioprotection may be associated with a controlled fuel shift from myocardial carbohydrate to fatty acid metabolism (Quinones et al. 2016), which is in line with the notion that fatty acids may not just be preferred substrates during hibernation (Galster and Morrison 1975; Yan et al. 2008; Shao et al. 2010), but also during true myocardial IR (Quinones et al. 2014).
In this study, we used Langendorff-perfused isolated hearts from summer-active AGS and BN rats to test the hypotheses that a) non-hibernating AGS are better protected against normothermic myocardial IR injury than the best-protected rat strain, the BN rat (Baker et al. 2000); and b) that administration of lipids through perfusion with Intralipid™, a Food and Drug Administration-approved lipid emulsion routinely used for total parenteral nutrition (McNiff 1977), differentially improves resistance against IR injury in AGS more than in BN rat hearts.
2. Methods
2.1. Animals
All investigations conformed to the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, National Academy of Sciences, 8th edition, 2011) and were approved by the Institutional Animal Care and Use Committees of the Medical College of Wisconsin and the Clement J. Zablocki Veterans Affairs Medical Center (both in Milwaukee, WI). AGS were trapped by licensed members of the Institute of Arctic Biology at the University of Alaska Fairbanks along the Denali Highway in central Alaska in July 2012 and 2013, transported to the University of Alaska Fairbanks for quarantine and testing, and subsequently shipped to the Medical College of Wisconsin. Age- and gender-matched BN rats were obtained from the Department of Physiology at the Medical College of Wisconsin. The BN rat was chosen as it had been identified in several studies to be the rat strain most resistant against myocardial IR injury (Baker et al. 2000; Nabbi et al. 2014). A total of 11 AGS and 16 BN rats were used during the months of September and October for this study (Table 1).
Table 1.
Baseline data
| BN | AGS | |
|---|---|---|
| Male / Female (n) | 8 / 8 | 5 / 6 |
| Glucose / Intralipid (n) | 8 / 8 | 6 / 5 |
| Heart weight, g | 1.24 ± 0.07 | 2.97 ± 0.13† |
| Body weight, g | 257 ± 23 | 642 ± 35† |
| Heart-to-body weight ratio, % | 0.48 ± 0.03 | 0.51 ± 0.02 |
| Systolic LVP, mmHg | 116 ± 6 | 118 ± 7 |
| Diastolic LVP, mmHg | 9 ± 0 | 10 ± 0 |
| Developed LVP, mmHg | 107 ± 6 | 108 ± 7 |
| dLVP/dtmax, mmHg/s | 2,937 ± 221 | 2,733 ± 164 |
| dLVP/dtmin, mmHg/s | −1,913 ± 138 | −1,859 ± 132 |
| RPP, 1000 mmHg/min | 30.0 ± 2.4 | 33.3 ± 1.6 |
| HR, beats/min | 280 ± 12 | 319 ± 21 |
| Coronary flow, mlmin−1g−1 | 9.3 ± 0.6 | 6.1 ± 0.8† |
Shown are baseline data for the two species, the Brown Norway (BN) rat ( n = 16) and arctic ground squirrels (AGS, n = 11). Left ventricular pressure (LVP) in mmHg, spontaneous heart rate (HR) in beats/min, and coronary flow (in ml per min per g heart weight) were measured, and systolic, diastolic, and developed, i.e., systolic–diastolic, LVP, its positive and negative first derivatives dLVP/dtmax and dLVP/dtmin as indices of contractility and relaxation, respectively, and the rate-pressure-product (RPP) as the product of developed LVP and HR were calculated at baseline, i.e., after 20 min stabilization. All values are absolute numbers or means ± standard error.
P < 0.05 vs BN.
2.2. Langendorff Heart Preparation
Animals were anesthetized by inhaled isoflurane (1 to 5 Vol% titrated to loss of consciousness), followed by intraperitoneal injection of 3,000 U kg−1 heparin. After a negative response to a noxious stimulus 5 min later, animals were decapitated and hearts were excised through a thoracotomy. The aorta was cannulated distal to the aortic valve, and the heart perfused retrograde with 4°C cold oxygenated Krebs solution of the following composition (in mM): 148 Na+, 4.7 K+, 1.2 Mg2+, 1.6 Ca2+, 127 Cl−, 27.8 HCO3−, 1.2 H2PO4− , 1.2 SO42- , 7.5 glucose, 2 pyruvate, 0.026 ethylene diamine tetraacetic acid, and 5 U/L insulin. The venae cavae were ligated, and the heart was rapidly placed into the support system and perfused at 80 mmHg and 37°C. The perfusate was equilibrated with 95% O2 and 5% CO2 to maintain a constant pH of 7.40 (carbon dioxide partial pressure pCO2 40 mmHg; oxygen partial pressure pO2 570 mmHg). The perfusate was filtered (5 μm pore size) in-line. Left ventricular pressure (LVP) was measured isovolumetrically with a saline-filled latex balloon (Radnoti LLC, Monrovia, CA) inserted into the left ventricle. Its initial volume was titrated to achieve a diastolic LVP of 10 mmHg at baseline so that any subsequent increase reflected diastolic contracture. LVP-derived data were: systolic, diastolic, and developed (systolic minus diastolic) LVP, and its maximal and minimal first derivatives (dLVP/dtmax and dLVP/dtmin) as indices of ventricular contractility and relaxation, respectively. Spontaneous heart rate (HR) was monitored by an electrocardiogram via bipolar electrodes placed in the right atrial and ventricular walls. The rate pressure product (RPP) was calculated as developed LVP • HR to correct for potential HR-induced decreases in developed LVP due to HR-dependent decreases in calcium release from the sarcoplasmic reticulum. Changes in coronary flow, as a result of changes in coronary resistance at a constant perfusion pressure, were measured by an in-line ultrasonic flowmeter (T106X; Transonic Systems, Ithaca, NY).
2.3. Experimental Protocol
Hearts were randomized to perfusion with either 7.5 mM glucose only or with 7.5 mM glucose plus 1% Intralipid™. Intralipid™ was chosen because it is clinically used and contains a multitude of different lipids including saturated and unsaturated fatty acids (Morris et al. 1998). Prior experiments in in- and ex-vivo models of myocardial IR have identified 1% as an optimal concentration to elicit cardioprotection (Rahman et al. 2011; Li et al. 2012). Hearts from either species were allowed to stabilize for 20 minutes. Following baseline readings, all hearts were perfused for 5 minutes with Krebs solution that contained 18 mM K+ as cardioplegia; this was necessary to arrest the AGS hearts that would otherwise continue to beat despite subsequent ischemia. Hearts were then subjected to 45 minutes of global no-flow ischemia before 60 minutes of reperfusion with continuous monitoring of LVP, heart rate, coronary flow and nicotinamide adenine dinucleotide (NADH) fluorescence, followed by tissue harvest and ventricular infarct size assessment. If ventricular fibrillation occurred on reperfusion, a bolus of 250 μg lidocaine was immediately injected in the aortic cannula. All data were collected from hearts naturally in, or converted to, sinus rhythm.
2.4. Assessment of Mitochondrial Redox State by Spectrophotofluorometry
All experiments were conducted in a light-proof cage to enable continuous measurements of mitochondrial NADH. The distal end of a trifurcated fiberoptic cable (6.8 mm2 per bundle) was placed gently against the heart’s left ventricular anterior wall. A net was applied around the heart to insure optimal contact with the fiberoptic tip. The three proximal ends were connected to a customized spectrophotofluorometer (Photon Technology International [PTI], London, Canada). Fluorescence was excited with light at the appropriate wavelength from a xenon lamp filtered through a monochromator (Delta RAM; PTI). The beam was focused onto the in-going fibers of the optic bundle and the shutter was opened only for 3-sec recording intervals at select time points to prevent photo-bleaching. Light at the wavelength used in our studies penetrates transmurally, i.e., all cells from the epicardium to the endocardium contribute to the measured signal (Rhodes et al. 2003). Emissions were collected by fibers of the other two limbs and filtered before reaching the photomultiplier (PTI). Autofluorescence at 460 nm emission (350 nm excitation) was used to measure changes in mitochondrial NADH (Chance et al. 1965) as previously described (Riess et al. 2002a). Although autofluorescence may also arise from unknown intracellular constituents or cytosolic NADH, the majority is derived from mitochondrial NADH (Eng et al. 1989). NADH is given in arbitrary fluorescence units (afu).
2.5. Infarct Size Measurement
At the end of each experiment, hearts were removed, weighed and their atria discarded. Ventricles were cut into 2-mm transverse sections using a heart matrix. Incubation with 1% 2,3,5-triphenyltetrazolium chloride (TTC) in 0.1 M KH2PO4 buffer (pH 7.4, 38°C) for 10 minutes (Riess et al. 2009) stains viable tissue red by dehydrogenase enzymes present in viable cells, with infarcted areas remaining white. Both sides of each slice were digitally imaged on green background, and their infarcted areas measured automatically by planimetry using Image J 1.44i software (NIH, Bethesda, MD), its ColorThreshold plugin and a custom-developed, calibrated macro ensuring fast and operator-independent measurements (Shidham et al. 2011). Infarcted areas were averaged on the basis of their weight to calculate the total ventricular infarction of each heart.
2.6. Lipid Analysis
Blood samples were collected from all animals immediately after decapitation and centrifuged at 500g for 10 min at 4°C. The plasma supernatant was aspirated and immediately frozen at −80°C. Plasma samples from AGS and rats as well as 1% Intralipid™ were subsequently analyzed for their lipid composition. Over 1,800 lipid measurements by mass spectrometry produced a profile of 399 lipids. ESI-MS/MS lipid profiling of phospholipids and cholesterol esters was performed as described by Zhou and colleagues (Zhou et al. 2012); triacylglycerol analysis, resulting in 44 triacylglycerol species, was performed as described by Li and colleagues (Li et al. 2014).
2.7. Statistical Analysis
All analog signals were digitized (PowerLab/16 SP, ADInstruments North America, Colorado Springs, CO) and recorded at 200 Hz (Labchart, ADInstruments) for later analysis. All data are expressed as the mean ± standard error of the mean. Composite baseline data were compared by one-way analysis of variance (SigmaStat 3.5, Systat Software Inc, San Jose, CA), all other data were compared by two-way analysis of variance with species and substrate as two independent factors. If F values were significant, Student-Newman-Keuls post-hoc tests were conducted. Tests were considered statistically significant at P<0.05 (2-tailed): * vs glucose only, † vs BN.
3. Results
3.1. Baseline Data
AGS were significantly heavier than their BN counterparts as were their respective hearts (Table 1). The ratios of heart weight to body weight, however, were not different between the species or treatment groups. Baseline coronary flow per g heart weight was higher in AGS than in BN rat hearts; all other baseline functional data were not different between the species or treatment groups.
3.2. Myocardial and Coronary Function
There was no significant effect of species or treatment on the incidence of ventricular fibrillation upon reperfusion. Systolic LVP, expressed as % baseline, upon reperfusion (Figure 1, panel A) was the lowest in the BN Glucose group and not different to the BN Intralipid group. The AGS Glucose group had a higher systolic LVP than the two BN groups. The AGS Intralipid group exhibited an even higher systolic LVP than the other three groups throughout reperfusion.
Fig 1.
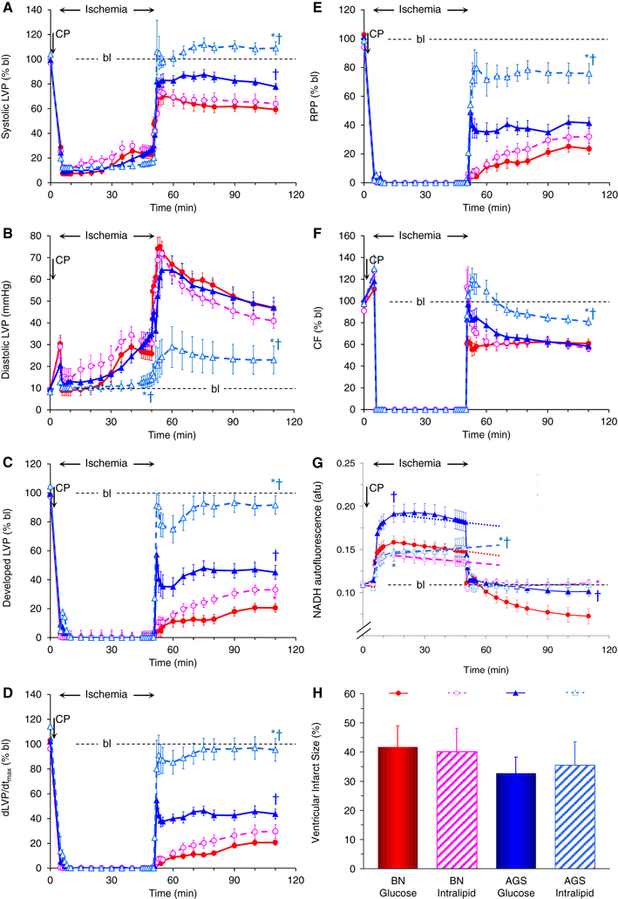
shows the time courses of A) systolic (in % baseline, bl), B) diastolic (in mmHg), and C) developed (systolic – diastolic, in % bl) left ventricular pressure (LVP), D) dLVP/dtmax (in % bl), E) the rate-pressure-product (RPP, in % bl), F) coronary flow (CF, in % bl), and G) NADH autofluorescence (in arbitrary fluorescence units, afu) before, during, and 45 minutes of global no-flow in hearts isolated from Brown Norway (BN) rats (blue triangles) compared with hearts isolated from arctic ground squirrels (AGS, red circles) perfused with either 7.5 mM glucose only (closed symbols, continuous lines) or 7.5 mM glucose and 1% Intralipid™ (open symbols, interrupted lines). Panel H shows ventricular infarct size (in %) for each of the four groups. CP: perfusion with cardioplegia for 5 minutes before ischemia. Dotted and interrupted straight lines in panel G represent average change in NADH fluorescence from 10 to 45 minutes of ischemia. Statistical symbols for P < 0.05 are * vs glucose only, and † vs BN
Diastolic contracture, expressed in mmHg (panel B), occurred in all hearts, starting during ischemia and lasting throughout reperfusion. While there was no difference among BN Glucose, BN Intralipid and AGS Glucose hearts, AGS Intralipid hearts exhibited a significantly later increase in diastolic LVP during ischemia, and a significantly lower contracture both during ischemia as well as throughout reperfusion compared to all other groups.
All of the above contributed to better developed LVP (panel C), contractility (dLVP/dtmax, panel D) and relaxation (dLVP/dtmin, not shown), as well as a better RPP (panel E) for the AGS Intralipid group than the other three groups. Developed LVP and contractility were also higher in the AGS Glucose group compared to the two BN groups, RPP was not. Coronary flow (panel F) returned at a significantly higher rate in Intralipid™-treated AGS hearts than in glucose only AGS or BN hearts; Intralipid™ did not affect coronary flow in BN hearts.
3.3. Mitochondrial Redox State
With the onset of global ischemia, all hearts exhibited the previously described (Riess et al. 2002a; Riess et al. 2003; Riess et al. 2004) pattern of initially increased mitochondrial reduction (increased NADH fluorescence, panel G) with the AGS Glucose group exhibiting a significantly higher initial reduction than the other three groups. This was followed by an abrupt oxidation, i.e., return towards or below baseline levels upon reperfusion. While there was no difference in mitochondrial redox state among the other three groups, BN hearts treated with glucose only exhibited a profound and sustained oxidation throughout the entire reperfusion period. During ischemia, the AGS Intralipid group did not exhibit the same continuous rate of oxidation (decrease in NADH) (Riess et al. 2002a; Riess et al. 2003; Riess et al. 2004) as the other three groups.
3.4. Ventricular Infarct Size
There were no significant differences among the four groups comparing ventricular infarct size as assessed by TTC staining (panel H).
3.5. Gender
We did not find a significant effect of gender in either of the species on any of the tested parameters.
3.6. Lipid Analysis
Comparisons between BN, AGS and Intralipid™ revealed four different lipid groups that were commonly higher in 1% Intralipid™ and in AGS plasma compared to BN rat plasma (Table 2): 1) phosphatidyl-choline by 4.5-and 1.5-fold; 2) lyso-phosphatidyl-ethanolamine by 17.0- and 2.1-fold; 3) phosphatidyl-ethanolamine by 136.9- and 8.4-fold; and 4) ether-linked phosphatidyl-ethanolamine by 28.7- and 2.3-fold in 1% Intralipid™ and AGS plasma, respectively, compared to BN rat plasma.
Table 2.
Comparisons of significantly different lipid groups from 1% Intralipid™ and AGS plasma vs BN rat plasma
| Lipid Group | Fold-difference 1% Intralipid™ vs BN rat plasma | p-value | Fold-difference AGS plasma vs BN rat plasma | p-value |
|---|---|---|---|---|
| Phosphatidyl-choline | 4.5 | <1.0E-05 | 1.5 | 7.1E-09 |
| Lyso-phosphatidyl-ethanolamine | 17.0 | <1.0E-05 | 2.1 | 1.8E-07 |
| Phosphatidyl-ethanolamine | 136.9 | <1.0E-05 | 8.4 | 5.1E-17 |
| Ether-linked phosphatidyl-ethanolamine | 28.7 | <1.0E-05 | 2.3 | 2.3E-10 |
Shown are the four lipid groups found to be significantly higher in both 1% Intralipid™ and in plasma from arctic ground squirrels (AGS) compared to plasma from Brown Norway (BN) rats.
4. Discussion
This study, for the first time, characterizes and compares the effects of normothermic myocardial IR in hearts isolated from summer-active AGS with those from the best protected rat strain, the BN rat, and reports a differential effect of Intralipid™ on cardiac function after cardioplegic IR in both species. Our comparative analysis revealed a significantly improved systolic function under glucose-only conditions in AGS compared to BN rats. The additional administration of Intralipid™ as a source for lipids dramatically improved not only systolic, but also diastolic function both during ischemia and throughout reperfusion, as well as coronary flow during reperfusion, and mitochondrial redox state during ischemia in AGS, but not in BN rats. Mitochondrial NADH autofluorescence was significantly lower initially during ischemia in the AGS Intralipid compared to AGS Glucose hearts, possibly as a consequence of less glucose utilization and increased -oxidation. More importantly, and as consistently observed with other cardioprotective strategies (Riess et al. 2002a; Riess et al. 2003; Riess et al. 2004), redox state became more oxidized during reperfusion in BN rats treated with glucose only, while there was no difference between BN rats treated with Intralipid™ and either AGS group. In contrast, TTC-based infarct size analysis did not show significant differences between species or treatments. A comprehensive lipid analysis identified four major lipid groups to be more prevalent in both AGS plasma and in 1% Intralipid™ versus BN rat plasma.
Mammalian hibernation is a powerful physiological mechanism to drastically reduce energy expenditure seasonally by reducing metabolism at times of reduced to completely ceased food supply. It is characterized by phases of torpor lasting for days to weeks that go along with reduced physical activity, decreased metabolism and body temperature (Andrews 2007; Sheriff et al. 2011). These are interrupted by short episodes of interbout arousals during which metabolism and temperature return to normal for several hours (Andrews 2007; Karpovich et al. 2009). While going in and out of torpor with concomitant manifold changes in metabolism and hemodynamics may constitute repeated global IR injury by itself, organs of hibernating mammals are also protected against true IR injury as shown in models of global ischemia during cardiac arrest (Dave et al. 2006; Dave et al. 2009; Bogren et al. 2014), as well as liver (Lindell et al. 2005), intestinal (Kurtz et al. 2006), and most recently myocardial IR (Heinis et al. 2015; Quinones et al. 2016). Little is known, however, about year-long endogenous organ protective mechanisms in hibernating mammals that may persist during non-hibernating euthermic conditions and are independent of hibernation-induced reductions in metabolism and temperature (Dave et al. 2006).
Quinones et al. (Quinones et al. 2016) have used a technically demanding in-vivo model of deep hypothermic cardioplegic cardiac arrest, adapted from the rat (de Lange et al. 2008) to the AGS, and compared myocardial IR injury patterns between non-hibernating rodents (BN and SS rats) and AGS that were summer-active and those that hibernated. In their model, the phenotype of cardioprotection in summer-active AGS was found to be intermediate between the best IR protected (BN) rat and hibernating AGS.
The goal of the present study was to investigate if, and how, cardioprotection against IR injury can be further enhanced even under non-hibernating and normothermic conditions. Summer-active AGS exhibit a similar temperature, metabolism, hemodynamic patterns and behavior as rats, so that neither of the above would be associated with or identified as a cause for any observed differences in outcome. However, hearts from summer-active AGS displayed an already improved return of function compared to the best-protected rat under standard glucose-only conditions. When mimicking their naturally occurring hyperlipidemia (Galster and Morrison 1975) by addition of 1% Intralipid™ to the perfusate, AGS isolated hearts performed even better following myocardial IR, clearly suggesting a differential capacity and preference for fatty acid metabolism in AGS even under nonhibernating conditions compared to the rat. Thus, the presence of lipids to be metabolized may be necessary but by far not sufficient by itself for cardioprotection without a concomitant fuel switch present in the hibernator versus the non-hibernator heart.
In contrast to the studies by Rahman et al. (Rahman et al. 2011) and Li et al. (Li et al. 2012), we did not give Intralipid™ as a ‘post-conditioning’ agent only upon reperfusion, but rather before and after ischemia to mimic the AGS hearts’ natural hyperlipidemic environment. The ‘pre-conditioning’ effect of the Intralipid™ in the AGS hearts was clearly evidenced by the absent mitochondrial oxidation and the much delayed and attenuated diastolic contracture during late ischemia. Both are typically a result of better intracellular calcium handling and less calcium overload during ischemia and on reperfusion (Riess et al. 2002b; Riess et al. 2004), a phenomenon also reported in hibernating 13-line ground squirrels (Heinis et al. 2015). Also in contrast to Rahman et al. (Rahman et al. 2011) we did not see additional improvement in any of the functional indices, including RPP, with the administration of 1% Intralipid™ in the rat; this may be due to the maximal endogenous protection in the BN versus all other rat strains (Baker et al. 2000) that cannot be further improved by conditioning (Nabor et al. 2012) and has been reported to be nitric oxide-dependent (Shi et al. 2005; Stowe et al. 2012). In fact, we recently reported nitric oxide release from endothelial cells as a possible mechanism of cardioprotection by Intralipid™ (Douglas et al. 2016).
Our cross-comparison of lipid levels between 1% Intralipid™, AGS and BN rat plasma revealed a multitude of individual lipids whose respective concentrations were significantly and multifold higher or lower among the three tested groups. When narrowing them down from individual lipids to chemically related lipid groups and searching for common differences between 1% Intralipid™ and plasma from AGS (both associated with improved function after IR) on one and BN rat plasma on the other side, we identified 1) phosphatidyl-choline; 2) lyso-phosphatidyl-ethanolamine; 3) phosphatidyl-ethanolamine; and 4) ether-linked phosphatidyl-ethanolamine to be highly significantly increased in 1% Intralipid™ and AGS plasma, respectively, compared to BN rat plasma and, therefore, as possible candidates to convey improved myocardial function after IR in our model. Clearly, more research on their significance and possible individual effects in non-hibernators will have to be conducted. Nevertheless, our finding of enhanced post-IR performance by Intralipid™ in hibernators challenges the current paradigm of increased glucose utilization and inhibition of lipid metabolism as always favorable during myocardial IR (Lopaschuk et al. 1988; Quinones et al. 2014).
Adding to the uncertainty about possible mechanisms of Intralipid™-mediated cardioprotection are contradictory reports about the role of fatty acids during the different phases of IR. While an earlier study in rabbit isolated hearts could show a protective role for Intralipid™ only on reperfusion (Van de Velde et al. 2000), a later study in rat isolated hearts reported a benefit when given as a pre- or as a postconditioning agent (Liu et al. 2008). The latter is in line with the present findings in Langendorff-prepared hearts from AGS where 1% Intralipid™ clearly improved post-IR function when given before and after IR. The role of glucose versus fatty acid metabolization in attenuating IR injury has indeed been the subject of a number of investigations and reviews (Jani and Bergmann 2006; Barsotti et al. 2009; Jaswal et al. 2011). On one side, it is entirely conceivable that fatty acids can serve as a more efficient fuel during myocardial IR (Jaswal et al. 2011). On the other hand, metabolic modulators shifting metabolism preferentially to glucose utilization are a newer class of drugs to ameliorate myocardial IR injury (Jani and Bergmann 2006). Inhibition of fatty acid oxidation by etomoxir, for example, has been shown to be highly cardioprotective (Lopaschuk et al. 1988).
Several limitations to our study need to be acknowledged. Due to the current inability to breed AGS in captivity they have to be trapped in the wild, which limits their availability to us for research, and therefore, their respective research group sizes. Thus, the study was not designed or powered to exclude a type 2 error in further subgroup analyses. This research project did not include hearts from hibernating AGS or other hibernating mammals which may have added further important findings to this study. Instead of studying the specific effects of individual lipids, we used Intralipid™, a water soluble fat emulsion approved for human use as a part of total parenteral nutrition (McNiff 1977), made of soy bean oil, egg phospholipids and glycerin and consisting of a multitude of different lipids (Morris et al. 1998). The fact that only functional parameters were found to be improved in AGS with and without Intralipid™, but not infarct size, points more towards improved stunning rather than reduced infarction following myocardial IR. Furthermore, we cannot exclude with certainty that our findings were not influenced, in part, by a possible species- or strain-dependent differential preconditioning effect (Nabor et al. 2012; Shi et al. 2005; Stowe et al. 2012), e.g., by exposure to a volatile anesthetic in vivo before the isolation of the heart. This, however, would not be expected to affect our Intralipid-related findings. Finally, we used an isolated heart model with a blood- and plasma-free perfusate, devoid of autonomic and hormonal input, all of which may have influenced our findings.
In summary, even under non-hibernating euthermic conditions, AGS hearts are better protected against stunning following IR than the best-protected rat strain. Furthermore, Intralipid™ perfusion leads to a remarkable improvement in return of function in AGS, but not in BN rats, suggesting that year-round endogenous mechanisms involved in myocardial lipid utilization without the production of harmful metabolites contribute to improved outcome, independent of decreased metabolism during hibernation. The concept of metabolic fuel switching in the AGS heart, with increased fatty acid oxidation, challenges the current paradigm that increased glucose and decreased lipid metabolism are always favorable during myocardial IR.
5. Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. In addition, all procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Acknowledgments:
The authors would like to thank James S. Heisner, John G. Krolikowski, Mary Roth, Ruth Welti, Franziska Kohl, Jeanette Moore and Øivind Tøien for their valuable contributions. This work was supported in part by the Department of Veterans Affairs (IK2BX001278), the National Science Foundation (IOS-1147187), the National Institutes of Health (5R01 HL123227), and departmental funds from the Department of Anesthesiology at the Medical College of Wisconsin, Milwaukee, WI. The lipid analyses described in this work were performed at the Kansas Lipidomics Research Center Analytical Laboratory, where instrument acquisition and lipidomics method development was supported by the National Science Foundation (EPS 0236913, MCB 1413036, DBI 0521587, DBI1228622), the Kansas Technology Enterprise Corporation, the K-IDeA Networks of Biomedical Research Excellence (INBRE) of the National Institutes of Health (P20GM103418), and Kansas State University.
Abbreviations in the text:
- AGS
Arctic Ground Squirrel
- BN
Brown Norway rat
- HR
Heart rate
- IR
Ischemia-reperfusion
- LE
Lipid emulsion
- LVP
Left ventricular pressure
- NADH
Nicotinamide adenine dinucleotide
- RPP
Rate pressure product
- SS
Dahl salt-sensitive rat
6. References
- Andrews MT (2007) Advances in molecular biology of hibernation in mammals. Bioessays 29 (5):431–440. doi: 10.1002/bies.20560 [DOI] [PubMed] [Google Scholar]
- Baker JE, Konorev EA, Gross GJ, Chilian WM, Jacob HJ (2000) Resistance to myocardial ischemia in five rat strains: is there a genetic component of cardioprotection? Am J Physiol 278 (4):H1395–1400 [DOI] [PubMed] [Google Scholar]
- Barnes BM (1989) Freeze avoidance in a mammal: body temperatures below 0 degree C in an Arctic hibernator. Science 244 (4912):1593–1595 [DOI] [PubMed] [Google Scholar]
- Barsotti A, Giannoni A, Di Napoli P, Emdin M (2009) Energy metabolism in the normal and in the diabetic heart. Curr Pharm Des 15 (8):836–840 [DOI] [PubMed] [Google Scholar]
- Bogren LK, Olson JM, Carpluk J, Moore JM, Drew KL (2014) Resistance to systemic inflammation and multi organ damage after global ischemia/reperfusion in the arctic ground squirrel. PLoS One 9 (4):e94225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno-Gonzalez AM, Perez-Vela JL, Hernandez F, Renes E, Arribas P, Corres MA, Gutierrez J, Perales N (2010) [Diagnostic and therapeutic alternatives in perioperative acute myocardial ischemia in heart surgery]. Med Intensiva 34 (1):64–73 [DOI] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL (2003) Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83 (4):1153–1181 [DOI] [PubMed] [Google Scholar]
- Chance B, Williamson JR, Jamieson D, Schoenner B (1965) Properties and kinetics of reduced pyridine nucleotide fluorescence of the isolated and in vivo rat heart. Biochem Z 341:357–377 [Google Scholar]
- Dave KR, Anthony Defazio R, Raval AP, Dashkin O, Saul I, Iceman KE, Perez-Pinzon MA, Drew KL (2009) Protein kinase C epsilon activation delays neuronal depolarization during cardiac arrest in the euthermic arctic ground squirrel. J Neurochem 110 (4):1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave KR, Prado R, Raval AP, Drew KL, Perez-Pinzon MA (2006) The arctic ground squirrel brain is resistant to injury from cardiac arrest during euthermia. Stroke 37 (5):1261–1265 [DOI] [PubMed] [Google Scholar]
- de Lange F, Yoshitani K, Podgoreanu MV, Grocott HP, Mackensen GB (2008) A novel survival model of cardioplegic arrest and cardiopulmonary bypass in rats: a methodology paper. J Cardiothorac Surg 3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas HF, Salzman MM, Riess ML (2016) Cardioprotection by Intralipid is Mediated by Nitric Oxide. Anästh Intensivmed 57:S218 [Google Scholar]
- Eng J, Lynch RM, Balaban RS (1989) Nicotinamide adenine dinucleotide fluorescence spectroscopy and imaging of isolated cardiac myocytes. Biophys J 55 (4):621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galster W, Morrison PR (1975) Gluconeogenesis in arctic ground squirrels between periods of hibernation. Am J Physiol 228 (1):325–330 [DOI] [PubMed] [Google Scholar]
- Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A (2010) Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol 35 (2):72–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS,Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C, Stroke Statistics S (2013) Heart disease and stroke statistics−−2013 update: a report from the American Heart Association. Circulation 127 (1):e6–e245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinis FI, Vermillion KL, Andrews MT, Metzger JM (2015) Myocardial performance and adaptive energy pathways in a torpid mammalian hibernator. Am J Physiol Regul Integr Comp Physiol 309 (4):R368–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldmaier G, Ortmann S, Elvert R (2004) Natural hypometabolism during hibernation and daily torpor in mammals.Respir Physiol Neurobiol 141 (3):317–329 [DOI] [PubMed] [Google Scholar]
- Jani S, Bergmann SR (2006) Metabolic modulation of myocardial ischemia. Curr Cardiol Rep 8 (2):123–130 [DOI] [PubMed] [Google Scholar]
- Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD (2011) Targeting fatty acid and carbohydrate oxidation--a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta 1813 (7):1333–1350 [DOI] [PubMed] [Google Scholar]
- Karpovich SA, Toien O, Buck CL, Barnes BM (2009) Energetics of arousal episodes in hibernating arctic ground squirrels. J Comp Physiol B 179 (6):691–700 [DOI] [PubMed] [Google Scholar]
- Kurtz CC, Lindell SL, Mangino MJ, Carey HV (2006) Hibernation confers resistance to intestinal ischemiareperfusion injury. Am J Physiol Gastrointest Liver Physiol. 291 (5):G895–901 [DOI] [PubMed] [Google Scholar]
- Kwitek AE, Jacob HJ, Baker JE, Dwinell MR, Forster HV, Greene AS, Kunert MP, Lombard JH, Mattson DL,Pritchard KA Jr., Roman RJ, Tonellato PJ, Cowley AW Jr. (2006) BN phenome: detailed characterization of the cardiovascular, renal, and pulmonary systems of the sequenced rat. Physiol Genomics 25 (2):303–313 [DOI] [PubMed] [Google Scholar]
- Li J, Iorga A, Sharma S, Youn JY, Partow-Navid R, Umar S, Cai H, Rahman S, Eghbali M (2012) Intralipid, a clinically safe compound, protects the heart against ischemia-reperfusion injury more efficiently than cyclosporine-a. Anesthesiology 117 (4):836–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Baughman E, Roth MR, Han X, Welti R, Wang X (2014) Quantitative profiling and pattern analysis of triacylglycerol species in Arabidopsis seeds by electrospray ionization mass spectrometry. Plant J 77 (1):160–172 [DOI] [PubMed] [Google Scholar]
- Lindell SL, Klahn SL, Piazza TM, Mangino MJ, Torrealba JR, Southard JH, Carey HV (2005) Natural resistance to liver cold ischemia-reperfusion injury associated with the hibernation phenotype. Am J Physiol Gastrointest Liver Physiol 288 (3):G473–480 [DOI] [PubMed] [Google Scholar]
- Liu SL, Wang Y, Wang RR, Chai YF, Wu W, Huang H, Liu J (2008) Protective effect of intralipid on myocardial ischemia/reperfusion injury in isolated rat heart. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 20 (4):227–230 [PubMed] [Google Scholar]
- Lopaschuk GD, Wall SR, Olley PM, Davies NJ (1988) Etomoxir, a carnitine palmitoyltransferase I inhibitor, protects hearts from fatty acid-induced ischemic injury independent of changes in long chain acylcarnitine. Circ Res 63 (6):1036–1043 [DOI] [PubMed] [Google Scholar]
- McNiff BL (1977) Clinical use of 10% soybean oil emulsion. Am J Hosp Pharm 34 (10):1080–1086 [PubMed] [Google Scholar]
- Miller TD, Christian TF, Hopfenspirger MR, Hodge DO, Gersh BJ, Gibbons RJ (1995) Infarct size after acute myocardial infarction measured by quantitative tomographic 99mTc sestamibi imaging predicts subsequent mortality. Circulation 92 (3):334–341 [DOI] [PubMed] [Google Scholar]
- Morris S, Simmer K, Gibson R (1998) Characterization of fatty acid clearance in premature neonates during intralipid infusion. Pediatr Res 43 (2):245–249 [DOI] [PubMed] [Google Scholar]
- Nabbi R, Gadicherla AK, Kersten JR, Stowe DF, Lazar J, Riess ML (2014) Genetically determined mitochondrial preservation and cardioprotection against myocardial ischemia-reperfusion injury in a consomic rat model. Physiol Genomics 46 (5):169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabor D, Cheng Q, Kersten J, Stowe D, Lazar J, Riess M (2012) Differential Cardioprotection by Chromosomal Substitution in a Consomic Rat Model: Role of Nitric Oxide in Anesthetic Preconditioning. Anesth Analg 114 (5):S38 [Google Scholar]
- Quinones QJ, Ma Q, Zhang Z, Barnes BM, Podgoreanu MV (2014) Organ protective mechanisms common to extremes of physiology: a window through hibernation biology. Integr Comp Biol 54 (3):497–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones QJ, Zhang Z, Ma Q, Smith MP, Soderblom E, Moseley MA, Bain J, Newgard CB, Muehlbauer MJ Hirschey M, Drew KL, Barnes BM, Podgoreanu MV (2016) Proteomic Profiling Reveals Adaptive Responses to Surgical Myocardial Ischemia-Reperfusion in Hibernating Arctic Ground Squirrels Compared to Rats. Anesthesiology 124 (6):1296–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Li J, Bopassa JC, Umar S, Iorga A, Partownavid P, Eghbali M (2011) Phosphorylation of GSK-3 mediates intralipid-induced cardioprotection against ischemia/reperfusion injury. Anesthesiology 115 (2):242–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SS, Ropella KM, Camara AK, Chen Q, Riess ML, Stowe DF (2003) How inotropic drugs alter dynamic and static indices of cyclic myoplasmic [Ca2+] to contractility relationships in intact hearts. J Cardiovasc Pharmacol 42 (4):539–553 [DOI] [PubMed] [Google Scholar]
- Riess ML, Camara AKS, Chen Q, Novalija E, Rhodes SS, Stowe DF (2002a) Altered NADH and improved function by anesthetic and ischemic preconditioning in guinea pig intact hearts. Am J Physiol Heart Circ Physiol 283 (1):H53–60 [DOI] [PubMed] [Google Scholar]
- Riess ML, Camara AKS, Kevin LG, An J, Stowe DF (2004) Reduced reactive O2 species formation and preserved mitochondrial NADH and [Ca2+] levels during short-term 17°C ischemia in intact hearts. Cardiovasc Res 61 (3):580–590 [DOI] [PubMed] [Google Scholar]
- Riess ML, Camara AKS, Novalija E, Chen Q, Rhodes SS, Stowe DF (2002b) Anesthetic preconditioning attenuates mitochondrial Ca2+ overload during ischemia in guinea pig intact hearts: reversal by 5-hydroxydecanoic acid. Anesth Analg 95 (6):1540–1546 [DOI] [PubMed] [Google Scholar]
- Riess ML, Novalija E, Camara AK, Eells JT, Chen Q, Stowe DF (2003) Preconditioning with sevoflurane reduces changes in nicotinamide adenine dinucleotide during ischemia-reperfusion in isolated hearts: reversal by 5-hydroxydecanoic acid. Anesthesiology 98 (2):387–395 [DOI] [PubMed] [Google Scholar]
- Riess ML, Rhodes SS, Stowe DF, Aldakkak M, Camara AK (2009) Comparison of cumulative planimetry versus manual dissection to assess experimental infarct size in isolated hearts. J Pharmacol Toxicol Methods 60 (3):275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS,Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB (2012) Heart disease and stroke statistics−−2012 update: a report from the American Heart Association. Circulation 125 (1):e2–e220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C, Liu Y, Ruan H, Li Y, Wang H, Kohl F, Goropashnaya AV, Fedorov VB, Zeng R, Barnes BM, Yan J (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotgun proteomics analysis of hibernating arctic ground squirrels. Mol Cell Proteomics 9 (2):313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YT, Vatner SF (1996) Differences in myocardial stunning following coronary artery occlusion in conscious dogs, pigs, and baboons. Am J Physiol 270 (4 Pt 2):H1312–1322 [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Kenagy GJ, Richter M, Lee T, Toien O, Kohl F, Buck CL, Barnes BM (2011) Phenological variation in annual timing of hibernation and breeding in nearby populations of Arctic ground squirrels. Proc Biol Sci. 278 (1716):2369–2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Hutchins W, Ogawa H, Chang CC, Pritchard KA Jr., Zhang C, Khampang P, Lazar J, Jacob HJ, Rafiee P, Baker JE (2005) Increased resistance to myocardial ischemia in the Brown Norway vs. Dahl S rat: role of nitric oxide synthase and Hsp90. J Mol Cell Cardiol 38 (4):625–635 [DOI] [PubMed] [Google Scholar]
- Shidham SV, Nabbi R, Camara AKS, Riess ML (2011) Development of automated infarct size measurement in TTC stained rat isolated hearts after global ischemia/reperfusion. FASEB J 25:1130.2 [Google Scholar]
- Stowe DF, Nabor DS, Cheng Q, Lazar J, Riess ML (2012) Differential Cardioprotection by Ischemic Preconditioning Mediated by Rat Chromosome 6 Is Nitric Oxide-Dependent. FASEB J 26:1135.5 [Google Scholar]
- Ueki C, Sakaguchi G, Akimoto T, Ohashi Y, Sato H (2016) On-pump beating-heart technique is associated with lower morbidity and mortality following coronary artery bypass grafting: a meta-analysis. Eur J Cardiothorac Surg 50(5):813–821 [DOI] [PubMed] [Google Scholar]
- Van de Velde M, DeWolff M, Leather HA, Wouters PF (2000) Effects of lipids on the functional and metabolic recovery from global myocardial stunning in isolated rabbit hearts. Cardiovasc Res 48 (1):129–137 [DOI] [PubMed] [Google Scholar]
- Yan J, Barnes BM, Kohl F, Marr TG (2008) Modulation of gene expression in hibernating arctic ground squirrels.Physiol Genomics 32 (2):170–181 [DOI] [PubMed] [Google Scholar]
- Yan L, Kudej RK, Vatner DE, Vatner SF (2015) Myocardial ischemic protection in natural mammalian hibernation. Basic Res Cardiol 110 (2):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Mao J, Ai J, Deng Y, Roth MR, Pound C, Henegar J, Welti R, Bigler SA (2012) Identification of plasma lipid biomarkers for prostate cancer by lipidomics and bioinformatics. PLoS One 7 (11):e48889. [DOI] [PMC free article] [PubMed] [Google Scholar]


