Key Points
Question
What are the clinical features, impact on quality of life, and response to minoxidil of endocrine therapy-induced alopecia in patients with breast cancer?
Findings
In this cohort study of 112 patients, a pattern alopecia similar to androgenetic-type was confirmed by standardized clinical and trichoscopy images, and a significant negative emotional impact was reported by patients, despite most cases being mild. Alopecia improvement was observed in 37 patients (80%) with topical minoxidil.
Meaning
Patients with breast cancer developing alopecia from endocrine therapies may benefit from minoxidil.
Abstract
Importance
Endocrine therapy-induced alopecia (EIA) has been anecdotally reported but not systematically described.
Objective
To characterize EIA in patients with breast cancer.
Design, Setting, and Participants
Retrospective cohort study of 112 patients with breast cancer, diagnosed with EIA from January 1, 2009, to December 31, 2016, the patients were examined at the dermatology service in a large tertiary care hospital and comprehensive cancer center.
Main Outcomes and Measures
The clinical features, alopecia-related quality of life (QoL), and response to minoxidil of EIA in patients with breast cancer were assessed. Data from the Hairdex Questionnaire was used to assess the impact of the alopecia on patients QoL. Higher score indicates lower QoL (0-100 score). Efficacy of minoxidil was measured at 3 or 6 months by a single-blinded investigator through standardized clinical photographs of the scalp.
Results
A total of 112 female patients with breast cancer were included (median [range] age, 60 [34-90] years). A total of 104 patients (93%) had standardized clinical photographs; of these, 59 patients (53%) had trichoscopy images available at baseline, and 46 patients (41%) were assessed for response to minoxidil. Alopecia was attributed to aromatase inhibitors in 75 patients (67%) and tamoxifen in 37 (33%). Severity was grade 1 in 96 of 104 patients (92%), and the pattern was similar to androgenetic alopecia. The predominant trichoscopic feature at baseline was the presence of vellus hairs and intermediate- and thick-diameter terminal hair shafts. A negative impact on QoL was reported, with a higher effect in the emotion domain according to the Hairdex score (mean [SD], 41.8 [21.3]; P < .001). After treatment with topical minoxidil, moderate or significant improvement in alopecia was observed in 37 of 46 patients (80%).
Conclusions and Relevance
Endocrine therapies are associated with a pattern alopecia similar to androgenetic-type, consistent with the mechanism of action of causal agents. A significant negative impact on QoL was reported by patients, despite mostly mild alopecia severity.
This cohort study describes clinical features, quality of life, and response to minoxidil among female patients with breast cancer who have endocrine therapy–induced alopecia.
Introduction
Breast cancer is the most common malignant neoplasm among women in the United States, with approximately 250 000 new cases diagnosed in 2016.1 Despite the high numbers, the survival of patients with early breast cancer (stages I, IIA, IIB, and IIIA) is greater than 80%. Cytotoxic chemotherapy and endocrine therapy (ET) have been the cornerstone in the management of breast cancer. Endocrine therapy, including aromatase inhibitors and selective estrogen receptor modulators, have shown benefits in the adjuvant and therapeutic setting for premenopausal or postmenopausal women with early-stage or advanced breast cancer.2,3 Despite their benefit, ETs are known to have substantial adverse events (AEs) such as hot flashes, mood disorders, osteopenia, and arthralgias.4 Among 236 patients treated with aromatase inhibitors, 8% discontinued treatment owing to alopecia.5 An alopecia meta-analysis of 13 415 patients in 35 clinical trials revealed an overall incidence of alopecia of 4.4%, with the highest incidence in tamoxifen-treated patients (25.4%).6 Additionally, in a survey-based study including a total of 851 female patients with breast cancer receiving aromatase inhibitors, 34% reported hair loss or hair thinning during their last month of therapy, and these hair changes were independent of previous chemotherapy and age.7 To our knowledge, the clinical characteristics, impact on quality of life (QoL), and response to dermatologic therapy of the ET-induced alopecia (EIA) have not been systematically described. This study aims to characterize EIA in patients with breast cancer.
Methods
Patients
Patients referred to the oncodermatology clinic at Memorial Sloan Kettering Cancer Center for EIA between January 1, 2009, and December 31, 2016, were retrospectively identified using a hospital wide medical record search and a medical imaging software archive (Mirror; Canfield Scientific). Relevant data were abstracted from each patient’s electronic medical record including demographics, primary cancer diagnosis, attributed ET, and therapy received to treat the alopecia. Female patients who received ET for breast cancer with a diagnosis of alopecia attributed to their current therapy were included. Those who either previously received cytotoxic chemotherapy or had a previous diagnosis of alopecia or any scalp condition were excluded from the study. This study was approved by the Memorial Sloan Kettering Cancer Center institutional review board. Written informed consent was obtained from each participant who answered the Hairdex Questionnaire. For the clinical assessment, consent was waived by the Memorial Sloan Kettering Cancer Center institutional review board. All data were deidentified.
Alopecia Grading and Pattern
Standardized clinical photographs of vertex, frontal, and lateral views of the scalp with hair parted and combed in the center were examined. Clinical photographs of each patient were evaluated to obtain an alopecia score using the Common Terminology Criteria for Adverse Events, version 4.0,8 and the basic and specific classification system,9 which is based on observed patterns of alopecia including the predominance on the frontoparietal hairline (basic type C, M, or U), and on the crown and vertex areas (specific type F or V).
Trichometric Measurements
Hair Density and Diameter, and Trichoscopy Features
Trichoscopy images were obtained using the Folliscope 2.8 (LeadM Corp), a camera-based trichoscope that through an interface trichoscope uses a magnification lens of ×50. All the trichoscopy images were obtained mid-scalp in a standardized manner (12 cm from the interciliary space). The total hair shaft count was determined in each trichoscopy image, and the measurement of hair density per square centimeter was obtained. Hair shaft diameter was measured at ×50 magnification, in direct proximity to follicular ostia. Hairs were further classified as vellus hairs (≤30 μm), intermediate (31-50 μm), or terminal hairs (>50 μm). The trichoscopic features were assessed by a dermatologist (A.F.M.).
Impact on QoL
Data from the Hairdex Questionnaire were used to assess the impact of the alopecia on their QoL. The self-administered modified-Hairdex Questionnaire includes 48 items and was adapted and translated from the validated German Hairdex,10 which is an alopecia-specific QoL tool (eAppendix in the Supplement). This tool was used in the oncodermatology clinic at the Memorial Sloan Kettering Cancer Center. Each question is categorized to 1 of 5 domains to assess different aspects of QoL. These domains include emotions, functioning, symptoms, stigmatization, and self-confidence. Patients responded to each question on a scale from 0 to 4 (higher score indicates lower QoL). Domain scores and total scores were then linearly transformed to represent a 0 to 100 point scale, on which 0 is the lowest possible score and 100 is the maximum possible score.
Response to Therapy
Efficacy of topical minoxidil 5%, was measured at 3 or 6 months by a single-blinded investigator (A.F.M.), through standardized clinical photographs of the scalp obtained at baseline and 3 or 6 months. Clinical response to therapy was assessed using the following 3-point scale: 0 indicates no improvement; 1, moderate improvement; and 2, significant improvement.11
Statistical Analysis
Descriptive statistics and graphical methods were used to describe the study participants, the degree of alopecia at baseline and follow-up, and the responses to the Hairdex QoL Questionnaire. A 2-sided P value <.05 was considered statistically significant. Analysis was performed with Microsoft Excel and Stata version 14.2 (StataCorp).
Results
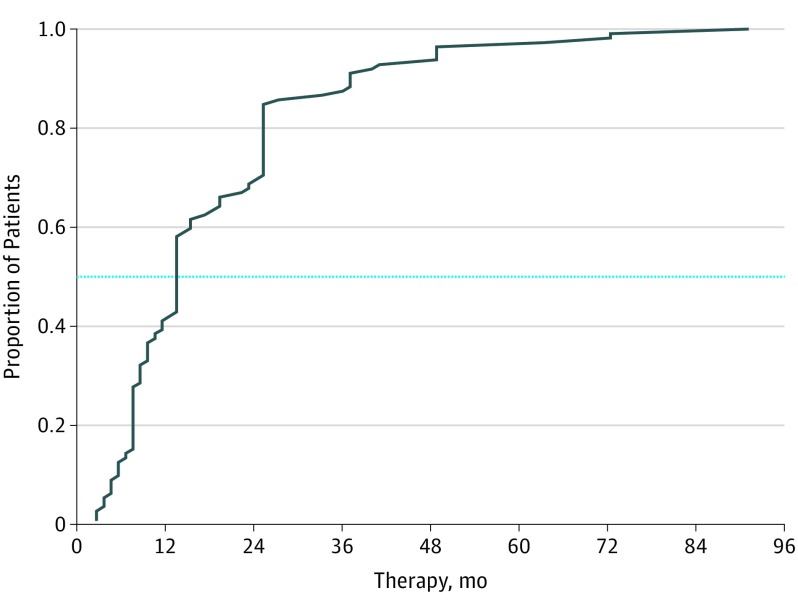
A total of 112 female patients with breast cancer were included (median [range] age, 60 [34-90] years). Alopecia was attributed to aromatase inhibitors (anastrozole, letrozole, leuprolide with letrozole, and exemestane) in 75 patients (67%) and tamoxifen in 37 (33%). Severity was grade 1 in 97 of 104 patients (93%) patients, and the pattern was similar to androgenetic alopecia (Table). The mean time to development of alopecia from ET initiation was 16.8 months (range, 1-91 months). Sixty-five patients (58%) reported alopecia within the first 12 months of ET (Figure 1).
Table. Patient Demographic Characteristics.
| Variable | No. (%) (n = 112) |
|---|---|
| Age, y | |
| Mean (SD) | 59.8 (11.6) |
| Median (range) | 60 (34-90) |
| Race | |
| White | 85 (76) |
| Asian | 10 (9) |
| Black | 7 (6) |
| Other | 10 (9) |
| Endocrine therapy | |
| Tamoxifen | 37 (33) |
| Letrozole | 38 (34) |
| Anastrozole | 24 (21) |
| Exemestane | 8 (7) |
| Leuprolide and letrozole | 5 (4) |
| Breast cancer stage | |
| 0-I | 97 (87) |
| II-III | 12 (11) |
| IV | 3 (3) |
| Prior endocrine therapy received | |
| None | 81 (72) |
| Tamoxifen | 8 (7) |
| Letrozole | 7 (6) |
| Anastrozole | 5 (4) |
| Exemestane | 2 (2) |
| ≥2 Endocrine drugs prior to attributed drug | 9 (8) |
Figure 1. Empirical Cumulative Distribution Function of Noticing Alopecia After the Initiation of Endocrine Therapy.
The dotted line represents the point at which 50% of the sample indicated the initiation of hair loss.
Clinical features and alopecia scores were assessed using standardized clinical photographs in 104 patients (93%). The basic type of alopecia using the basic and specific classification system showed that in 79 patients (76%), there was a more prominent recession of the frontotemporal area than of the mid-anterior hairline (type M). Also, the specific type of alopecia seen in 86 patients (83%) was mild to moderate alopecia on the crown area of the scalp (type F). The severity of alopecia using Common Terminology Criteria for Adverse Events, version 4.0 was grade 1 in 96 patients (92%) and grade 2 in 8 (8%). One patient developed clinical and histologic findings of lichen planus pilaris (1%) and another 3 patients developed frontal fibrosing alopecia (3%).
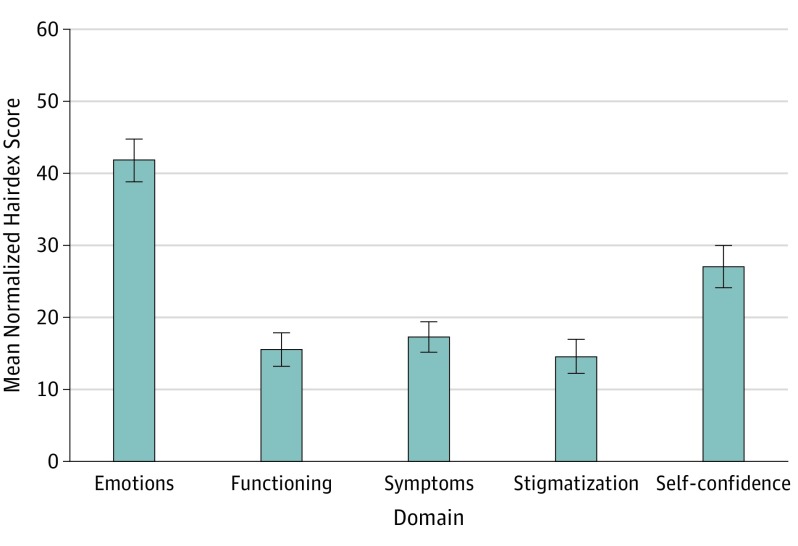
Alopecia-related QoL was analyzed in 52 of 112 patients (46%). The mean (SD) score of the Hairdex Questionnaire was 25.6 (14.5), which included emotions (41.8 [21.3]), functioning (15.6 [16.8]), symptoms (17.3 [15.3]), stigmatization (14.6 [17.0]), and self-confidence (27.0 [21.1]) domains. A higher negative impact on emotions was found when compared with other domains (P < .001) (Figure 2).
Figure 2. Mean Normalized Hairdex Score Among 52 Patients by Domain.
The highest negative score for the emotions domain (mean [SD], 41.8 [21.3]) compared with other domains (P < .001). A higher score indicates lower quality of life. Error bars indicate standard deviation.
Response to topical minoxidil was assessed using standardized clinical photographs in 46 of 112 patients (41%). Overall, 37 of 46 patients (80%) showed a moderate to significant improvement in alopecia.
A total of 59 patients (53%) had trichoscopy images available at baseline. The predominant trichoscopic feature was the presence of vellus hairs, with a mean (SD) density of 17.3 (8.6) hairs/cm2 (13% of total hairs)and intermediate- and thick-density terminal hair shafts, with a mean (SD) of 118.7 (39.6) hairs/cm2 (87% of total hairs). The mean (SD) hair shaft diameter was 60.8 (11.6) μm.
Discussion
Endocrine therapy in breast cancer modulates endocrine receptor–mediated pathways. Selective estrogen receptor modulators include tamoxifen, raloxifene, and toremifene,12 which are competitive inhibitors of estrogen binding to endocrine receptors. From this group, tamoxifen has been the historical standard of care for adjuvant ET and remains the gold standard for premenopausal patients at risk of recurrence.13 By contrast, aromatase inhibitors, including anastrozole, letrozole, and exemestane, suppress plasma estrogen levels by inhibition of aromatase and are now considered the preferred option for adjuvant ET in postmenopausal patients. They all appear to be comparable in efficacy and have similar AE profiles that include hot flashes, mood disorders, osteopenia, and arthralgias.14 Additionally, for premenopausal women with hormone receptor–positive breast cancer, leuprolide can be used to suppress estrogen production through ovarian function suppression in combination with other ET or chemotherapy,15,16 with similar AEs observed in patients receiving aromatase inhibitors and selective estrogen receptor modulators.15
Estrogens and androgens also play a critical role as hair growth modulators.17 When endocrine receptor activation and pathway signaling are blocked, dihydrotestosterone levels increase,18 and this action may contribute to the induction of alopecia in susceptible women receiving ET. Therefore, EIA may be physiologically similar to androgenetic alopecia.19 In our study, 79 of 104 patients (76%) with basic-type alopecia presented with recession of frontal and parietal hairlines, mimicking an androgenetic pattern of alopecia that has anecdotally been described in 15 patients receiving aromatase inhibitors.20 Additionally, at baseline, the predominant trichoscopic features were the presence of vellus hairs (13% of total hairs) and intermediate- and thick-density terminal hairs (87% of total hairs). This is thought to be the result of unsynchronized miniaturization of hair follicles, also described as one of the trichoscopy hallmarks of androgenetic alopecia.21
In 37 patients (80%) treated with topical minoxidil 5%, there was a moderate or significant clinical improvement, as assessed by investigator and blinded reviewer. No AEs were observed with the use of minoxidil in the patient population described herein.
Currently, topical minoxidil 2% and 5%, is the only US Food and Drug Administration–cleared agent for the treatment of androgenetic alopecia in women. The mechanism of action of minoxidil on human hair follicles and hair growth is still not fully understood. Minoxidil has been reported to open adenosine triphosphate–sensitive potassium channels through regulatory sulfonylurea receptors, which induce vasodilatation and increased local blood flow.22 In addition, minoxidil causes entry and prolongs the hair follicle cycle into the anagen phase23 and suppresses androgen activity in the hair follicle.24 These local mechanisms of action support the use of minoxidil as a safe therapy for alopecia in patients with hormone-sensitive tumors. Based on the previously described relative hyperandrogenism as a result of the ET, we hypothesized that patients with EIA may also benefit from therapies used for androgenetic alopecia in women, including spironolactone. No significant changes in serum levels of estradiol, testosterone, or progesterone have been reported with the use of spironolactone,25,26,27 and a previous epidemiologic study showed that there is no evidence of increased risk of breast cancer with the use of spironolactone.28 However, further randomized clinical trials using spironolactone for androgenetic alopecia are needed.29
Alopecia is often cited as one of the most negative effects on QoL in patients with cancer,30 and it has been reported that 8% of patients with cancer would rescind life-saving therapy had they known they were going to have alopecia.31 In 96 of 104 patients with standardized clinical photographs (92%) included in this study, alopecia was Common Terminology Criteria for Adverse Events grade 1 (hair loss of <50%, does not require camouflage). Despite this result, a high negative emotion score was obtained in the QoL questionnaire. This may suggest that even with alopecia grade 1, patients are greatly emotionally affected. Previous studies showed that the clinical severity or physician’s assessment of alopecia was not necessarily associated with higher negative impact on QoL.32,33 Therefore, it should be important for oncology health care professionals treating patients with breast cancer to consider the distress that grade 1 alopecia may have on patients’ QoL. These events are likely to be compounded given the long duration of administration of ET in the adjuvant setting, ranging from 5 to 10 years.3
Limitations
Limitations of this study include the retrospective design, patient cohort origination from a single center, and the limited number of patients with objective follow-up (due to availability of standardized clinical photographs and appropriate trichoscopy follow-up). However, the main purpose of this study was to characterize the EIA in patients with breast cancer and response to dermatologic therapy, when follow-up data were available. Despite the limited cohort, to our knowledge, this is the largest number of patients with EIA described in the literature with clinical features, trichoscopy findings, and response to dermatologic therapy. Future studies should be aimed at conducting large-scale randomized clinical trials to better understand the efficacy of topical and systemic therapies used for androgenetic alopecia in patients with EIA. We also believe that a long-term follow-up, at least 6 months after ET is interrupted, may be needed to determine whether this EIA is reversible or permanent after the therapy. The Hairdex Questionnaire used in the study was an in-house translation adapted from the original German Hairdex.10 Additional work needs to be completed to further assess the validity of this instrument in English-speaking patients.
Conclusions
Our cohort study supports the fact that patients with breast cancer receiving ET may develop pattern alopecia similar to androgenetic type, with a significant negative impact on their QoL. Despite these observations, these patients may benefit from topical minoxidil, suggesting that supportive care for EIA is also an important component in the care of patients with breast cancer and survivors.
eAppendix. Hairdex Questionnaire (modified English version)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. [DOI] [PubMed] [Google Scholar]
- 2.Poggio F, Ceppi M, Lambertini M, et al. Concurrent versus sequential adjuvant chemo-endocrine therapy in hormone-receptor positive early stage breast cancer patients: a systematic review and meta-analysis. Breast. 2017;33:104-108. [DOI] [PubMed] [Google Scholar]
- 3.Pan HGR, Davies C, Peto R, Bergh J. Predictors of recurrence during years 5-14 in 46,138 women with ER+ breast cancer allocated 5 years only of endocrine therapy (ET). J Clin Oncol. 2016;34(15)(suppl):505. doi: 10.1200/JCO.2016.34.15 [DOI] [Google Scholar]
- 4.Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348(24):2431-2442. [DOI] [PubMed] [Google Scholar]
- 5.Moscetti L, Agnese Fabbri M, Sperduti I, et al. Adjuvant aromatase inhibitor therapy in early breast cancer: what factors lead patients to discontinue treatment? Tumori. 2015;101(5):469-473. [DOI] [PubMed] [Google Scholar]
- 6.Saggar V, Wu S, Dickler MN, Lacouture ME. Alopecia with endocrine therapies in patients with cancer. Oncologist. 2013;18(10):1126-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallicchio L, Calhoun C, Helzlsouer KJ. Aromatase inhibitor therapy and hair loss among breast cancer survivors. Breast Cancer Res Treat. 2013;142(2):435-443. [DOI] [PubMed] [Google Scholar]
- 8.Chen AP, Setser A, Anadkat MJ, et al. Grading dermatologic adverse events of cancer treatments: the common terminology criteria for adverse events version 4.0. J Am Acad Dermatol. 2012;67(5):1025-1039. [DOI] [PubMed] [Google Scholar]
- 9.Lee WS, Ro BI, Hong SP, et al. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57(1):37-46. [DOI] [PubMed] [Google Scholar]
- 10.Fischer TW, Schmidt S, Strauss B, Elsner P. Hairdex: a tool for evaluation of disease-specific quality of life in patients with hair diseases[in German]. Hautarzt. 2001;52(3):219-227. [DOI] [PubMed] [Google Scholar]
- 11.Dhurat R, Sukesh M, Avhad G, Dandale A, Pal A, Pund P. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5(1):6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosman F, Lindsay R. Selective estrogen receptor modulators: clinical spectrum. Endocr Rev. 1999;20(3):418-434. [DOI] [PubMed] [Google Scholar]
- 13.Davies C, Godwin J, Gray R, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burstein HJ, Prestrud AA, Seidenfeld J, et al. ; American Society of Clinical Oncology . American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28(23):3784-3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmid P, Untch M, Kossé V, et al. Leuprorelin acetate every-3-months depot versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant treatment in premenopausal patients with node-positive breast cancer: the TABLE study. J Clin Oncol. 2007;25(18):2509-2515. [DOI] [PubMed] [Google Scholar]
- 16.Klijn JG, Blamey RW, Boccardo F, Tominaga T, Duchateau L, Sylvester R; Combined Hormone Agents Trialists’ Group and the European Organization for Research and Treatment of Cancer . Combined tamoxifen and luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist alone in premenopausal advanced breast cancer: a meta-analysis of four randomized trials. J Clin Oncol. 2001;19(2):343-353. [DOI] [PubMed] [Google Scholar]
- 17.Alonso LC, Rosenfield RL. Molecular genetic and endocrine mechanisms of hair growth. Horm Res. 2003;60(1):1-13. [DOI] [PubMed] [Google Scholar]
- 18.Trüeb RM. Molecular mechanisms of androgenetic alopecia. Exp Gerontol. 2002;37(8-9):981-990. [DOI] [PubMed] [Google Scholar]
- 19.Gan DC, Sinclair RD. Prevalence of male and female pattern hair loss in Maryborough. J Investig Dermatol Symp Proc. 2005;10(3):184-189. [DOI] [PubMed] [Google Scholar]
- 20.Rossi A, Iorio A, Scali E, et al. Aromatase inhibitors induce ‘male pattern hair loss’ in women? Ann Oncol. 2013;24(6):1710-1711. [DOI] [PubMed] [Google Scholar]
- 21.Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Rudnicka L. Dermoscopy in female androgenic alopecia: method standardization and diagnostic criteria. Int J Trichology. 2009;1(2):123-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shorter K, Farjo NP, Picksley SM, Randall VA. Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. FASEB J. 2008;22(6):1725-1736. [DOI] [PubMed] [Google Scholar]
- 23.Uno H, Cappas A, Brigham P. Action of topical minoxidil in the bald stump-tailed macaque. J Am Acad Dermatol. 1987;16(3 Pt 2):657-668. [DOI] [PubMed] [Google Scholar]
- 24.Hsu CL, Liu JS, Lin AC, Yang CH, Chung WH, Wu WG. Minoxidil may suppress androgen receptor-related functions. Oncotarget. 2014;5(8):2187-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sert M, Tetiker T, Kirim S. Comparison of the efficiency of anti-androgenic regimens consisting of spironolactone, Diane 35, and cyproterone acetate in hirsutism. Acta Med Okayama. 2003;57(2):73-76. [DOI] [PubMed] [Google Scholar]
- 26.Keleştimur F, Everest H, Unlühizarci K, Bayram F, Sahin Y. A comparison between spironolactone and spironolactone plus finasteride in the treatment of hirsutism. Eur J Endocrinol. 2004;150(3):351-354. [DOI] [PubMed] [Google Scholar]
- 27.Siegberg R, Ylöstalo P, Laatikainen T, Pelkonen R, Stenman UH. Endocrine and clinical effects of spironolactone in female hyperandrogenism. Arch Gynecol. 1987;240(2):67-73. [DOI] [PubMed] [Google Scholar]
- 28.Biggar RJ, Andersen EW, Wohlfahrt J, Melbye M. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37(6):870-875. [DOI] [PubMed] [Google Scholar]
- 29.van Zuuren EJ, Fedorowicz Z, Schoones J. Interventions for female pattern hair loss. Cochrane Database Syst Rev. 2016;(5):CD007628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gandhi M, Oishi K, Zubal B, Lacouture ME. Unanticipated toxicities from anticancer therapies: survivors’ perspectives. Support Care Cancer. 2010;18(11):1461-1468. [DOI] [PubMed] [Google Scholar]
- 31.Trüeb RM. Chemotherapy-induced hair loss. Skin Therapy Lett. 2010;15(7):5-7. [PubMed] [Google Scholar]
- 32.Reid EE, Haley AC, Borovicka JH, et al. Clinical severity does not reliably predict quality of life in women with alopecia areata, telogen effluvium, or androgenic alopecia. J Am Acad Dermatol. 2012;66(3):e97-e102. [DOI] [PubMed] [Google Scholar]
- 33.Kluger N, Jacot W, Frouin E, et al. Permanent scalp alopecia related to breast cancer chemotherapy by sequential fluorouracil/epirubicin/cyclophosphamide (FEC) and docetaxel: a prospective study of 20 patients. Ann Oncol. 2012;23(11):2879-2884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Hairdex Questionnaire (modified English version)