Key Points
Question
How has platelet adenosine diphosphate P2Y12 receptor inhibitor use and adherence changed among patients receiving percutaneous coronary intervention with the introduction of prasugrel hydrochloride and ticagrelor hydrochloride?
Findings
In this national cohort study of 55 340 patients receiving percutaneous coronary intervention from 2008 to 2016 as the use of prasugrel and ticagrelor increased, the proportion of patients not filling any platelet adenosine diphosphate P2Y12 receptor inhibitor prescription within 30 days of discharge also significantly increased. Compared with clopidogrel bisulfate, prasugrel and ticagrelor adherence was lower and patient prescription costs were greater.
Meaning
Adherence to platelet adenosine diphosphate P2Y12 receptor inhibitor therapy after percutaneous coronary intervention decreased after the introduction of prasugrel and ticagrelor, disproportionately affecting the most economically disadvantaged patients.
Abstract
Importance
Current guidelines recommend prasugrel hydrochloride and ticagrelor hydrochloride as preferred therapies for patients with acute coronary syndrome (ACS) treated with percutaneous coronary intervention (PCI). However, it is not well known how frequently these newer agents are being used in clinical practice or how adherence varies among the platelet adenosine diphosphate P2Y12 receptor (P2Y12) inhibitors.
Objectives
To determine trends in use of the different P2Y12 inhibitors in patients who underwent PCI from 2008 to 2016 in a large cohort of commercially insured patients and differences in patient adherence and costs among the P2Y12 inhibitors.
Design, Setting, and Participants
A retrospective cohort study used administrative claims from a large US national insurer (ie, UnitedHealthcare) from January 1, 2008, to December 1, 2016, comprising patients aged 18 to 64 years hospitalized for PCI who had not received a P2Y12 inhibitor for 90 days preceding PCI. The P2Y12 inhibitor filled within 30 days of discharge was identified from pharmacy claims.
Main Outcomes and Measures
Proportion of patients filling prescriptions for P2Y12 inhibitors within 30 days of discharge by year, as well as medication possession ratios (MPRs) and total P2Y12 inhibitor copayments at 6 and 12 months for patients who received drug-eluting stents.
Results
A total of 55 340 patients (12 754 [23.0%] women; mean [SD] age, 54.4 [7.1] years) who underwent PCI were included in this study. In 2008, 7667 (93.6%) patients filled a prescription for clopidogrel bisulfate and 521 (6.4%) filled no P2Y12 inhibitor prescription within 30 days of hospitalization. In 2016, 2406 (44.0%) patients filled clopidogrel prescriptions, 2015 (36.9%) filled either prasugrel or ticagrelor prescriptions, and 1045 (19.1%) patients filled no P2Y12 inhibitor prescription within 30 days of hospitalization. At 6 months, mean MPRs for patients who received a drug-eluting stent filling clopidogrel, prasugrel, and ticagrelor prescriptions were 0.85 (interquartile range [IQR], 0.82-1.00), 0.79 (IQR, 0.66-1.00), and 0.76 (IQR, 0.66-0.98) (P < .001), respectively; mean copayments for a 6 months’ supply were $132 (IQR, $47-$203), $287 (IQR, $152-$389), and $265 (IQR, $53-$387) (P < .001), respectively. At 12 months, mean MPRs for clopidogrel, prasugrel, and ticagrelor were 0.76 (IQR, 0.58-0.99), 0.71 (IQR, 0.49-0.98), and 0.68 (IQR, 0.41-0.94) (P < .001), respectively; mean total copayments were $251 (IQR, $100-$371), $556 (IQR, $348-$730), and $557 (IQR, $233-$744) (P < .001), respectively.
Conclusions and Relevance
Between 2008 and 2016, increased use of prasugrel and ticagrelor was accompanied by increased nonfilling of prescriptions for P2Y12 inhibitors within 30 days of discharge. Prasugrel and ticagrelor had higher patient costs and lower adherence in the year following PCI compared with clopidogrel. The introduction of newer, more expensive P2Y12 inhibitors was associated with lower adherence to these therapies.
This population-based cohort study examines claims data in a large US insurer to assess changes in prescribing and adherence to platelet adenosine diphosphate P2Y12 receptor inhibitors by patients after percutaneous coronary intervention and after introduction of newer, more expensive agents.
Introduction
Prasugrel hydrochloride and ticagrelor hydrochloride are platelet adenosine diphosphate P2Y12 receptor (P2Y12) inhibitors that have been shown to significantly decrease cardiovascular death, myocardial infarction, and stroke in patients with acute coronary syndrome (ACS) after percutaneous coronary intervention (PCI) compared with clopidogrel bisulfate in large randomized clinical trials.1,2 These trials informed current guidelines that include a class IIA recommendation to use ticagrelor in preference to clopidogrel in patients with ACS who receive either PCI or medical therapy alone and to use prasugrel in preference to clopidogrel in nonelderly patients with ACS who undergo PCI who are not at increased risk of bleeding and who do not have a history of stroke or transient ischemic attack.3
Since the introduction of these newer antiplatelet agents, the proportion of patients discharged with prescriptions for prasugrel or ticagrelor after PCI has varied from 25% to 35% in US populations4,5,6 and from 45% to 70% in European populations.7,8,9 Antiplatelet agents have substantial rates of nonadherence in patients after acute myocardial infarction,10 and a review investigating long-term antiplatelet use found varying adherence, with 12-month adherence ranging from 30% to 90%.11 A recent study of patients with ACS treated with PCI found higher rates of early cessation of prasugrel compared with clopidogrel, but otherwise there has been little investigation into differences in adherence among the available P2Y12 inhibitors.12
To investigate whether the introduction of new agents affected patient adherence, we evaluated trends in prescribing patterns for P2Y12 inhibitors in post-PCI patients from 2008 to 2016, an interval spanning both the US Food and Drug Administration approval dates of prasugrel (July 2009) and ticagrelor (July 2011). We assessed primary nonadherence, defined as never filling an initial prescription for a P2Y12 inhibitor, and secondary nonadherence, defined as filling an initial prescription but not subsequently refilling prescriptions for P2Y12 inhibitors as prescribed, during this same period. We also measured rates of recurrent ACS and bleeding complications by P2Y12 inhibitor prescriptions filled. Last, to explore whether costs may have been associated with adherence, we compared patients’ out-of-pocket prescription costs across the different P2Y12 inhibitors.
Methods
Study Data
Data were obtained from the OptumInsight Clinformatics Data Mart database, an administrative claims database comprising members of a large, national health insurer (ie, UnitedHealthcare). The database consists of comprehensive inpatient, outpatient, and pharmacy claims for approximately 15 million patients annually throughout the United States and includes each patient’s dates of insurance coverage. Demographic and socioeconomic data are also available through zip code–linked enrollment data from the US Census Bureau. The study protocol was deemed exempt by the University of Pennsylvania Institutional Review Board.
Study Cohort
Using administrative claims from January 1, 2008, through December 1, 2016, we included patients aged 18 to 64 years who underwent PCI. Percutaneous coronary intervention was identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes (ie, 36.01, 36.02, 36.05, 36.06, 36.07, and 00.66), International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Procedural Coding System (ICD-10-PCS) codes (ie, 02703xx, 02713xx, 02723xx, and 02733xx except 027x3Tx and 027x3Zx) for procedures after September 30, 2015, and diagnosis related group codes (ie, 246, 247, 248, and 249).13,14,15 Patients who underwent major cardiothoracic surgery (ie, heart transplant, implanted ventricular assist device, surgical valve replacement, and coronary artery bypass grafting) during the index hospitalization were excluded from the analysis. Patients must have had continuous insurance enrollment for at least 90 days before and at least 30 days following index PCI hospitalization to be included.
Pharmacy claims including National Drug Codes, fill dates, days supplied, and copayment amounts were used to identify P2Y12 inhibitor use before and after each patient’s index hospitalization. The P2Y12 inhibitors (ie, clopidogrel, prasugrel, and ticagrelor) were identified using National Drug Codes.16 Patients were excluded if they had received a prescription for any P2Y12 inhibitor up to 90 days preceding the index hospitalization. Patients were also excluded if they had no pharmacy claims for any medication in the 365 days following discharge from the index hospitalization because these patients likely had pharmacy benefits from another insurer and thus their pharmacy data were unavailable.
Outcomes
The primary outcome was the number of patients identified by pharmacy claims as filling a prescription for a P2Y12 inhibitor following the index PCI during the study period of January 1, 2008, through December 1, 2016. We identified the P2Y12 inhibitor by the first P2Y12 inhibitor prescription filled between the day after the index hospital admission and 30 days after hospital discharge. When no P2Y12 inhibitor prescription was filled within 30 days of discharge, this was defined as primary nonadherence. Because prasugrel and ticagrelor were approved by the US Food and Drug Administration specifically for use in patients with ACS, a subgroup analysis of that cohort was also performed to assess P2Y12 inhibitor use and nonadherence for this patient group. The ICD-9-CM and ICD-10-CM (for events after September 30, 2015) diagnosis codes were used to identify ACS.17,18,19,20,21,22
Patients were then grouped by type of P2Y12 inhibitor filled after PCI, and baseline characteristics of these groups, including age, sex, race, US geographic region, and household net worth, were compared. Using ICD-9-CM and ICD-10-PCS procedure codes as well as diagnosis related group codes, receipt of either a bare metal stent or drug-eluting stent (DES) during the index hospitalization was also compared across these groups.13,14,15 Using zip code–linked socioeconomic data from the US Census Bureau, we also compared the distribution of approximate household net worth among patients by P2Y12 inhibitor at discharge and by year.
In addition to primary adherence, we measured rates of recurrent ACS and bleeding complications, secondary adherence, and prescription out-of-pocket costs. The proportion of patients with recurrent ACS and/or bleeding complications was defined by having a hospitalization for ACS and/or a bleeding event in the 6 months following the index PCI hospitalization. Recurrent ACS and bleeding events were identified using ICD-9-CM and ICD-10-CM diagnosis codes.17,18,19,20,21,22,23,24 Only patients with continuous insurance enrollment for 6 months after the index PCI were included in the analysis.
To assess P2Y12 inhibitor secondary adherence, the subgroup of patients who underwent DES placement were analyzed to ensure that P2Y12 inhibitor use was indicated for at least 6 months after stent placement by consensus guidelines that were contemporaneous with our study period.3 We limited the subgroup to patients with continuous insurance enrollment for 6 months after the index hospitalization for PCI. Medication possession ratio (MPR) is defined by the sum of the days supplied of all prescriptions filled for a drug in a given time period divided by the number of days in the time period.25 The MPRs were calculated as the measure of secondary adherence at 6 months and 12 months following PCI. We also stratified our patients by household net worth and assessed secondary adherence for each net worth group. Last, patients’ mean copayments for P2Y12 inhibitors were calculated at 6 months and 12 months. Patients with more than 6 months but fewer than 12 months of continuous enrollment after PCI were not included in the 12-month calculations. The study periods used for measurements of cost and adherence at 6 months and 12 months were January 1, 2008, through June 30, 2016, and January 1, 2008, through December 31, 2015, respectively.
Statistical Analysis
Differences in characteristics and outcomes between groups were compared using χ2 tests for categorical variables and analysis of variance for continuous variables. All statistical tests were 2-sided, with P < .05 indicating statistical significance. Analyses were performed using Stata, version 14.1 (StataCorp).
Results
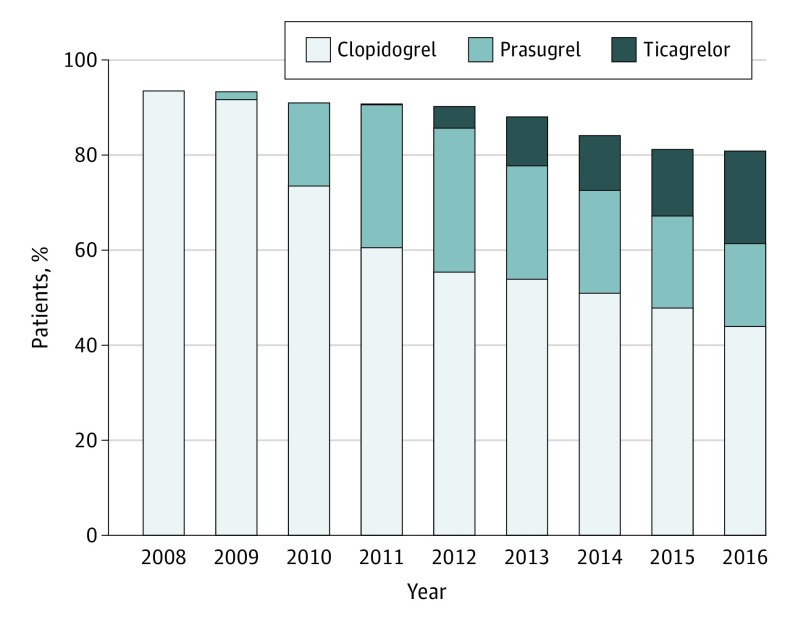
We identified 55 340 patients who underwent PCI during 2008 through 2016 (Figure 1). From 2008 to 2016, the proportion of patients filling a prescription for clopidogrel within 30 days of hospitalization decreased from 93.6% (n = 7667) to 44.0% (n = 2406) P < .001), while the proportion of patients filling a prescription for prasugrel or ticagrelor increased from zero to 36.9% (n = 2015) (P < .001). During the same period, the proportion of patients who did not fill any P2Y12 inhibitor prescription within 30 days of discharge increased from 6.4% (n = 521) to 19.1% (n = 1045) (P < .001). Among patients with ACS as an indication for PCI (n = 45 287), similar trends in P2Y12 inhibitor prescription filling after discharge occurred; from 2008 to 2016, the proportion of patients filling clopidogrel prescriptions decreased from 94.3% (n = 5626) to 42.2% (n = 1900) (P<.001), while filling of prasugrel or ticagrelor prescriptions increased from zero to 38.8% (n = 1746) (P<.001), and the proportion of patients not filling any P2Y12 inhibitor increased from 5.6% (n = 338) to 19.0% (n = 853) (P < .001).
Figure 1. Proportion of Patients With Percutaneous Coronary Intervention (PCI) by Discharge Platelet Adenosine Diphosphate P2Y12 Receptor Inhibitor, 2008-2016.
The bars represent the percentage of patients who underwent PCI and filled a prescription for clopidogrel, prasugrel, and ticagrelor within 30 days of discharge.
Most patients (n = 43 717) received a DES during the study period. In 2008, 94.3% (n = 5324) of patients with a DES filled a prescription for clopidogrel, while 5.7% (n = 322) did not fill a P2Y12 inhibitor prescription within 30 days of discharge. By 2016, 43.0% (n = 2159) of patients with a DES filled a prescription for clopidogrel, while 37.7% (n = 1892) filled a prescription for either prasugrel or ticagrelor, and 19.3% (n = 968) did not fill any P2Y12 inhibitor prescription within 30 days of discharge.
Compared with the patients who underwent PCI and filled a P2Y12 inhibitor prescription within 30 days of discharge, primary nonadherent patients who underwent PCI were younger (54.1 vs 54.4 years; P = .002), less likely to be white (4314 [69.7%] vs 36 867 [75.0%]; P < .001), less likely to be male (4626 [74.7%] vs 37 960 [77.2%]; P < .001), more likely to reside in the southern United States (3287 [53.1%] vs 23 483 [47.8%]; P < .001), and less likely to have ACS as an indication for PCI (4875 [78.7%] vs 40 412 [82.2%]; P < .001). Patients who filled a P2Y12 inhibitor prescription were most frequently living in zip codes with mean net worth between $250 000 to $499 999, while primary nonadherent patients most frequently lived in zip codes with mean net worth between $25 000 to $149 999 (Table 1).
Table 1. Baseline Characteristics of Patients Who Underwent Percutaneous Coronary Intervention by P2Y12 Inhibitor.
| Characteristic | P2Y12 Inhibitor, No. (%) | P Value | |||
|---|---|---|---|---|---|
| Clopidogrel (n = 36 584) | Prasugrel (n = 9395) | Ticagrelor (n = 3168) | None (n = 6193) | ||
| Age, mean (SD), y | 54.6 (7.0) | 53.9 (7.1) | 54.3 (7.2) | 54.1 (7.7) | <.001 |
| Women | 8613 (23.5) | 1861 (19.8) | 713 (22.5) | 1567 (25.3) | <.001 |
| Region | |||||
| Northeast | 3620 (9.9) | 791 (8.4) | 372 (11.7) | 492 (7.9) | <.001 |
| Midwest | 11 190 (30.6) | 2449 (26.1) | 924 (29.2) | 1679 (27.1) | |
| South | 17 143 (46.9) | 4873 (51.9) | 1467 (46.3) | 3287 (53.1) | |
| West | 4597 (12.6) | 1276 (13.6) | 402 (12.7) | 729 (11.8) | |
| Unknown | 34 (0.1) | 6 (0.1) | 3 (0.1) | 6 (0.1) | |
| Race | |||||
| White | 27 432 (75.0) | 7132 (75.9) | 2303 (72.7) | 4314 (69.7) | <.001 |
| Black | 3733 (10.2) | 836 (8.9) | 276 (8.7) | 801 (12.9) | |
| Hispanic | 2635 (7.2) | 641 (6.8) | 207 (6.5) | 486 (7.8) | |
| Asian | 765 (2.1) | 263 (2.8) | 86 (2.7) | 132 (2.1) | |
| Unknown | 2019 (5.5) | 523 (5.6) | 296 (9.3) | 460 (7.4) | |
| Household net worth, $ | |||||
| <25 000 | 3965 (10.8) | 925 (9.8) | 359 (11.3) | 855 (13.8) | <.001 |
| 25 000-149 999 | 7844 (21.4) | 1943 (20.7) | 637 (20.1) | 1476 (23.8) | |
| 150 000-249 999 | 5571 (15.2) | 1362 (14.5) | 470 (14.9) | 910 (14.7) | |
| 250 000-499 999 | 8642 (23.6) | 2288 (24.4) | 678 (21.4) | 1231 (19.9) | |
| ≥500 000 | 6129 (16.8) | 1772 (18.9) | 500 (15.8) | 700 (11.3) | |
| Unknown | 4433 (12.1) | 1105 (11.8) | 524 (16.5) | 1021 (16.5) | |
| Stent type | |||||
| Drug-eluting | 28 013 (76.6) | 8170 (87.0) | 2818 (89.0) | 4716 (76.2) | <.001 |
| Bare metal | 7219 (19.7) | 1017 (10.8) | 283 (8.9) | 894 (14.4) | |
| Unspecified | 1352 (3.7) | 208 (2.2) | 67 (2.1) | 583 (9.4) | |
| ACS as indication for PCI | 29 317 (80.1) | 8236 (87.7) | 2859 (90.2) | 4875 (78.7) | <.001 |
| Copayment of prescription per 30-d supply, mean (SD), $ | 21.4 (17.9) | 48.1 (34.4) | 48.6 (46.9) | NA | <.001 |
| Medication days supplied of prescription, mean (SD) | 32.9 (13.4) | 32.2 (11.7) | 32.4 (13.4) | NA | <.001 |
Abbreviations: ACS, acute coronary syndrome; NA, not applicable; P2Y12, platelet adenosine diphosphate P2Y12 receptor; PCI, percutaneous coronary intervention.
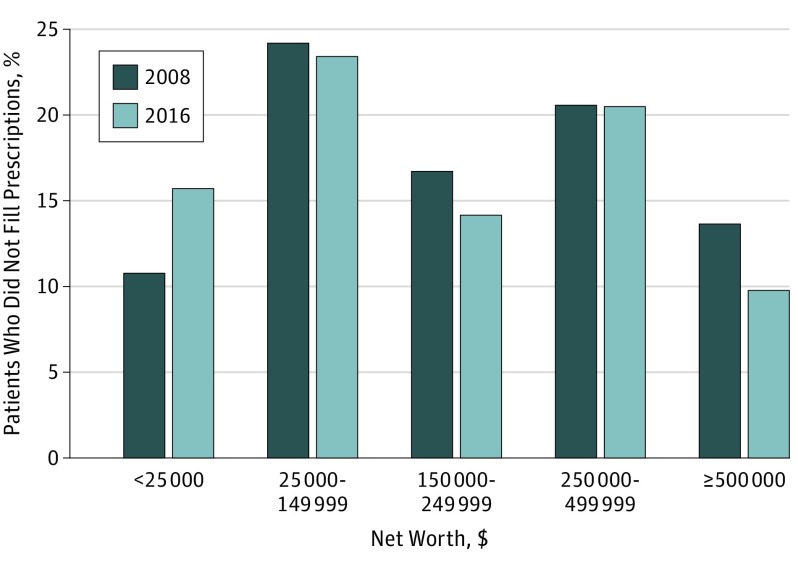
Among patients with primary nonadherence to P2Y12 inhibitors (n = 6193), the proportion of patients from the lowest net worth communities significantly increased from 2008 to 2016 (10.8% to 15.7%; P = .01), while patients from the highest net worth communities decreased from 13.6% to 9.8% (P = .03) during the study period (Figure 2).
Figure 2. Distribution of Household Net Worth of Patients With Percutaneous Coronary Intervention Not Filling a Platelet Adenosine Diphosphate P2Y12 Receptor (P2Y12) Inhibitor Within 30 Days of Discharge, 2008 and 2016.
Histograms of zip code–linked (ie, community) mean household net worth in patients who underwent PCI and did not fill any prescription for any P2Y12 inhibitor within 30 days of discharge.
Among patients who underwent PCI with 6 months of continuous enrollment (n = 45 857), rates of recurrent ACS within 6 months of PCI for patients who filled prescriptions for clopidogrel, prasugrel, ticagrelor, or no P2Y12 inhibitor within 30 days were 6.8%, 5.5%, 5.3%, and 8.0% (P < .001), respectively. Rates of bleeding complications within 6 months for clopidogrel, prasugrel, ticagrelor, and no filled P2Y12 inhibitor were 0.9%, 0.7%, 0.8%, and 1.3% (P = .003), respectively.
Of patients who received a DES during their hospitalization, 32 547 (74.4%) and 25 882 (59.2%) had continuous enrollment for 6 months and 12 months after discharge, respectively (Table 2). Patients who filled clopidogrel prescriptions after discharge had a greater mean MPR at 6 months (0.85; interquartile range [IQR], 0.82-1.00) compared with prasugrel (0.79; IQR, 0.66-1.00) and ticagrelor (0.76; IQR, 0.66-0.98) (P < .001). At 12 months, MPR had decreased across all groups, but patients who filled clopidogrel prescriptions continued to have the highest MPR (0.76; IQR, 0.58-0.99), followed by prasugrel (0.71; IQR, 0.49-0.98) and ticagrelor (0.68; IQR, 0.41-0.94) (P < .001). Mean copayment per 6 months’ supply was greatest for patients prescribed prasugrel ($287; IQR, $152-$389), followed by ticagrelor ($265; IQR, $53-$387) and clopidogrel ($132; IQR, $47-$203) (P < .001), and at 12 months, ticagrelor had the highest cost ($557; IQR, $233-$744), followed by prasugrel ($556; IQR, $348-$730) and clopidogrel ($251; IQR, $100-$371) (P < .001). Adherence to clopidogrel was higher than for prasugrel or ticagrelor in all community net worth subgroups (Table 3).
Table 2. Adherence and Copayment for Patients Who Underwent Drug-Eluting Stent Placement by P2Y12 Inhibitor.
| Outcome | Discharge P2Y12 Inhibitor | ||
|---|---|---|---|
| Clopidogrel | Prasugrel | Ticagrelor | |
| MPR at 6 mo | |||
| No. | 23 654 | 6850 | 2043 |
| Mean (IQR)a | 0.85 (0.82-1) | 0.79 (0.66-1) | 0.76 (0.66-0.98) |
| MPR at 12 mo | |||
| No. | 19 047 | 5467 | 1368 |
| Mean (IQR)a | 0.76 (0.58-0.99) | 0.71 (0.49-0.98) | 0.68 (0.41-0.94) |
| Copayment per 6-mo supplyb | |||
| No. | 23 654 | 6850 | 2043 |
| Mean (IQR), $a | 132 (47-203) | 287 (152-389) | 265 (53-387) |
| Copayment per 12-mo supplyb | |||
| No. | 19 047 | 5467 | 1368 |
| Mean (IQR), $a | 251 (100-371) | 556 (348-730) | 557 (233-744) |
Abbreviations: IQR, interquartile range; MPR, medication possession ratio; P2Y12, platelet adenosine diphosphate P2Y12 receptor.
P < .001 for all comparisons.
Copayments represent the out-of-pocket costs patients incurred if they were 100% adherent to a P2Y12 inhibitor during the given time period.
Table 3. Adherence for Patients Who Underwent DES Placement by P2Y12 Inhibitor and Zip Code–Linked Mean Household Net Worth.
| Community Mean Household Net Wortha | Discharge P2Y12 Inhibitor | ||
|---|---|---|---|
| Clopidogrel (n = 23 654) | Prasugrel (n = 6850) | Ticagrelor (n = 2043) | |
| <$25 000 | |||
| No. (%) | 2343 (9.9) | 658 (9.6) | 220 (10.8) |
| MPR at 6 mo, mean (IQR)b | 0.80 (0.66-1) | 0.74 (0.55-0.98) | 0.71 (0.49-0.98) |
| $25 000-149 999 | |||
| No. (%) | 4921 (20.8) | 1393 (20.3) | 449 (22.0) |
| MPR at 6 mo, mean (IQR)b | 0.83 (0.73-1) | 0.76 (0.66-0.98) | 0.74 (0.65-0.97) |
| $150 000-249 999 | |||
| No. (%) | 3635 (15.4) | 998 (14.6) | 311 (15.2) |
| MPR at 6 mo, mean (IQR)b | 0.86 (0.82-1) | 0.79 (0.67-1) | 0.79 (0.70-0.98) |
| $250 000-499 999 | |||
| No. (%) | 5786 (24.5) | 1730 (25.3) | 433 (21.2) |
| MPR at 6 mo, mean (IQR)b | 0.87 (0.83-1) | 0.81 (0.75-1) | 0.76 (0.66-0.98) |
| ≥$500 000 | |||
| No. (%) | 4323 (18.3) | 1372 (20.0) | 357 (17.5) |
| MPR at 6 mo, mean (IQR)b | 0.89 (0.91-1) | 0.83 (0.80-1) | 0.80 (0.73-0.99) |
Abbreviations: DES, drug-eluting stent; IQR, interquartile range; MPR, medication possession ratio; P2Y12, platelet adenosine diphosphate P2Y12 receptor.
Approximately 11% of patients who received a DES did not have available community household net worth data and are not reported in the table; hence, percentages do not total 100% for each of the P2Y12 inhibitor cohorts.
P < .001 for all comparisons.
Discussion
In a large national cohort of P2Y12 inhibitor–naive patients who underwent PCI, we found the proportion of patients who did not fill any P2Y12 inhibitor prescription at 30 days significantly increased from 6% in 2008 to 19% in 2016. Furthermore, we found that patients from communities with low household net worth had the largest increase in primary nonadherence during the study period. In our cohort, patients with primary nonadherence had higher incidences of recurrent ACS and hospitalizations for bleeding. We also found that prasugrel and ticagrelor secondary nonadherence at 6 and 12 months was greater than clopidogrel secondary nonadherence, with higher out-of-pocket prescription costs for the 2 newer medications. These findings suggest that the higher out-of-pocket costs of prasugrel and ticagrelor may be contributing to increasing nonadherence to P2Y12 inhibitors after PCI.
While an alternative possible explanation for the lower rates of P2Y12 inhibitor prescription fills immediately following PCI would be lower rates of physicians prescribing P2Y12 inhibitors, this explanation appears unlikely. The National Cardiovascular Data Registry recently reported an average P2Y12 inhibitor prescribing rate at discharge for patients who underwent stenting of greater than 99%.26,27 This prescribing rate suggests that not filling P2Y12 inhibitors at discharge was owing to primary nonadherence rather than lack of prescribing.
Primary nonadherence is common. A Canadian study revealed that more than 30% of prescriptions for all newly prescribed medications are never filled and that prescriptions for medications used for ischemic heart disease had the highest rate of primary nonadherence (51%).28 Moreover, studies conducted prior to the multiagent P2Y12 inhibitor era assessing primary nonadherence after hospital discharge for myocardial infarction found nonadherence at 30 days ranging from 14% to 50% for antiplatelet agents.10,29 Patients who were older, had preexisting cardiovascular disease at presentation, and had lower educational attainment were most likely to stop thienopyridine therapy within 30 days of DES placement.29 Rates of primary nonadherence were lower in our study, which may be partly explained by our population including younger, privately insured patients with a higher mean socioeconomic status compared with the general US population.
A systematic review found that patients’ most common reason for primary nonadherence was medication affordability.30 Our study showed the most financially disadvantaged patients in our population had higher rates of primary nonadherence, and nonadherence rates among those patients increased from 2008 to 2016. While it was impossible to know the specific P2Y12 inhibitor that primary nonadherent patients failed to fill, the rising proportion of patients who were given prescriptions for prasugrel and ticagrelor during the study period as well as the sizeable difference in mean copayments between clopidogrel and the newer agents suggest that increased prescribing of the newer, more expensive P2Y12 inhibitors may be contributing to increasing primary nonadherence.
An important policy ramification of our findings is that the introduction of new pharmacotherapies may have exacerbated socioeconomic health disparities. This phenomenon has been reported previously; Goldman and Lakdawalla31 showed that the availability of highly active antiretroviral therapy increased disparities in favor of individuals in high socioeconomic categories who were more likely to successfully follow complex medication regimens and subsequently had greater improvements in immune function with the introduction of a new, effective therapeutic class. In cardiovascular medicine, the introduction of high-potency statins also disproportionately benefited higher income patients who were more likely to afford these medications as well as the obligatory laboratory services and physician visits.32 In the case of P2Y12 inhibitors, increased prescribing of newer, more expensive agents may be similarly affecting health outcomes disparities by worsening adherence among patients with low socioeconomic status and placing them at increased risk for downstream adverse cardiovascular events.
Analyses of secondary nonadherence largely mirrored those of our primary outcome with patients less likely to continue filling prescriptions for the newer and more expensive P2Y12 inhibitors. While differences in medication costs may affect secondary adherence, it is appropriate to acknowledge that clinical factors, such as drug tolerability, adverse events, treatment complexity (eg, initiation of multiple medications after ACS, multiple daily dosing of certain drugs), and various other patient and clinician factors may also play a role.33 In the case of ticagrelor, dyspnea and increased bleeding were shown to be the most common causes for discontinuing the agent in the PEGASUS-TIMI 54 trial.34,35 Hence, more frequent adverse effects, along with the challenge of adhering to twice-daily dosing, may be additional factors explaining lower adherence to ticagrelor at 12 months compared with the other P2Y12 inhibitors in our cohort.
Limitations
This investigation has limitations inherent to retrospective studies using administrative claims. Our study data lacked detailed clinical information, such as the results of coronary angiography, which would have more accurately confirmed the clinical indications for PCI as well as the choice of stent type. However, we used well-validated administrative codes for ACS, bare metal stents, and DES to optimally identify patients and procedures with the data available. The nature of pharmacy claims precluded us from determining which P2Y12 inhibitor was prescribed for each patient at discharge, so when identifying patients with primary nonadherence, we do not know which P2Y12 inhibitor was prescribed at discharge and the proportions of each P2Y12 inhibitor prescription that went unfilled.
Furthermore, it was possible for patients in our cohort to have a second pharmacy benefits plan from a different insurer that would not be included in our study data. However, we limited the numbers of such patients in our cohort by requiring all cohort members to have filled at least 1 prescription for a medication covered by the primary insurer during the study period. Also, if patients had secondary prescription drug insurance, it likely would have been used for all long-term medications after an initial fill immediately following hospitalization, but we observed that 93% filled at least 1 prescription between days 31 to 90 after PCI, and there was minimal change in this percentage over time. It is also possible that patients were able to obtain prescriptions outside their drug insurance through other means, such as paying in cash or receiving free samples, which we would not be able to account for in our analysis.
Our socioeconomic measures were derived from zip code–linked US Census data rather than direct patient measurement, and therefore these measures may not accurately reflect individual patients’ socioeconomic status; nevertheless, similar measures have been used extensively in the literature as a reasonable proxy for group-level socioeconomic status.36 Our measure of adherence (MPR) is a commonly used, although indirect, measure of adherence; but it does not indicate how many days a patient took a medication; hence, MPR tends to overestimate medication adherence.25,37 Finally, because our data excluded patients older than 64 years, our results may not be generalizable to older populations.
Conclusions
We observed increased rates of nonfilling of P2Y12 inhibitor prescriptions within 30 days of hospital discharge after PCI from 2008 to 2016. Prasugrel and ticagrelor had higher out-of-pocket patient costs and lower rates of adherence compared with clopidogrel. Our findings suggest that adherence to P2Y12 inhibitors after PCI has decreased during the period when newer, more expensive therapies were introduced.
References
- 1.Wiviott SD, Braunwald E, McCabe CH, et al. ; TRITON-TIMI 38 Investigators . Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015. [DOI] [PubMed] [Google Scholar]
- 2.Wallentin L, Becker RC, Budaj A, et al. ; PLATO Investigators . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057. [DOI] [PubMed] [Google Scholar]
- 3.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134(10):e123-e155. [DOI] [PubMed] [Google Scholar]
- 4.Karve AM, Seth M, Sharma M, et al. Contemporary use of ticagrelor in interventional practice (from Blue Cross Blue Shield of Michigan Cardiovascular Consortium). Am J Cardiol. 2015;115(11):1502-1506. [DOI] [PubMed] [Google Scholar]
- 5.Kim K, Lee TA, Touchette DR, DiDomenico RJ, Ardati AK, Walton SM. Contemporary trends in oral antiplatelet agent use in patients treated with percutaneous coronary intervention for acute coronary syndrome. J Manag Care Spec Pharm. 2017;23(1):57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudaravalli M, Althouse AD, Marroquin OC, et al. Assessment of P2Y12 inhibitor usage and switching in acute coronary syndrome patients undergoing percutaneous coronary revascularization. Int J Cardiol. 2016;223:854-859. [DOI] [PubMed] [Google Scholar]
- 7.Tscharre M, Egger F, Machata M, et al. Contemporary use of P2Y12-inhibitors in patients with acute coronary syndrome undergoing percutaneous coronary intervention in Austria: a prospective, multi-centre registry. PLoS One. 2017;12(6):e0179349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexopoulos D, Goudevenos JA, Xanthopoulou I, et al. ; GRAPE Investigators . Implementation of contemporary oral antiplatelet treatment guidelines in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a report from the GReek AntiPlatelet rEgistry (GRAPE). Int J Cardiol. 2013;168(6):5329-5335. [DOI] [PubMed] [Google Scholar]
- 9.Angerås O, Hasvold P, Thuresson M, Deleskog A, ÖBraun O. Treatment pattern of contemporary dual antiplatelet therapies after acute coronary syndrome: a Swedish nationwide population-based cohort study. Scand Cardiovasc J. 2016;50(2):99-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackevicius CA, Li P, Tu JV. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117(8):1028-1036. [DOI] [PubMed] [Google Scholar]
- 11.Czarny MJ, Nathan AS, Yeh RW, Mauri L. Adherence to dual antiplatelet therapy after coronary stenting: a systematic review. Clin Cardiol. 2014;37(8):505-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fosbøl EL, Ju C, Anstrom KJ, et al. Early cessation of adenosine diphosphate receptor inhibitors among acute myocardial infarction patients treated with percutaneous coronary intervention: insights from the TRANSLATE-ACS Study (Treatment With Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events After Acute Coronary Syndrome). Circ Cardiovasc Interv. 2016;9(11):e003602. [DOI] [PubMed] [Google Scholar]
- 13.Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001-2008. JAMA. 2011;305(17):1769-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brennan JM, Peterson ED, Messenger JC, et al. ; Duke Clinical Research Institute DEcIDE Team . Linking the National Cardiovascular Data Registry CathPCI Registry with Medicare claims data: validation of a longitudinal cohort of elderly patients undergoing cardiac catheterization. Circ Cardiovasc Qual Outcomes. 2012;5(1):134-140. [DOI] [PubMed] [Google Scholar]
- 15.Agency for Healthcare Research and Quality Inpatient quality indicators technical specifications updates—version 6.0 (ICD 10), July 2016. https://www.qualityindicators.ahrq.gov/Modules/IQI_TechSpec_ICD10_v60.aspx. Accessed September 23, 2017.
- 16.US Food and Drug Administration National Drug Code Directory. https://www.accessdata.fda.gov/scripts/cder/ndc/. Updated March 12, 2018.Accessed March 27, 2017.
- 17.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148(1):99-104. [DOI] [PubMed] [Google Scholar]
- 18.Arciero TJ, Jacobsen SJ, Reeder GS, et al. Temporal trends in the incidence of coronary disease. Am J Med. 2004;117(4):228-233. [DOI] [PubMed] [Google Scholar]
- 19.Varas-Lorenzo C, Castellsague J, Stang MR, Tomas L, Aguado J, Perez-Gutthann S. Positive predictive value of ICD-9 codes 410 and 411 in the identification of cases of acute coronary syndromes in the Saskatchewan Hospital automated database. Pharmacoepidemiol Drug Saf. 2008;17(8):842-852. [DOI] [PubMed] [Google Scholar]
- 20.GRACE Investigators Rationale and design of the GRACE (Global Registry of Acute Coronary Events) Project: a multinational registry of patients hospitalized with acute coronary syndromes. Am Heart J. 2001;141(2):190-199. [DOI] [PubMed] [Google Scholar]
- 21.Bezin J, Girodet PO, Rambelomanana S, et al. Choice of ICD-10 codes for the identification of acute coronary syndrome in the French hospitalization database. Fundam Clin Pharmacol. 2015;29(6):586-591. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd-Jones D, Adams RJ, Brown TM, et al. ; Writing Group Members; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46-e215. [DOI] [PubMed] [Google Scholar]
- 23.Buresly K, Eisenberg MJ, Zhang X, Pilote L. Bleeding complications associated with combinations of aspirin, thienopyridine derivatives, and warfarin in elderly patients following acute myocardial infarction. Arch Intern Med. 2005;165(7):784-789. [DOI] [PubMed] [Google Scholar]
- 24.Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007;167(13):1414-1419. [DOI] [PubMed] [Google Scholar]
- 25.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028-3035. [DOI] [PubMed] [Google Scholar]
- 26.Dehmer GJ, Jennings J, Madden RA, et al. The National Cardiovascular Data Registry Voluntary Public Reporting Program: an interim report from the NCDR Public Reporting Advisory Group. J Am Coll Cardiol. 2016;67(2):205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardiosmart, American College of Cardiology. American College of Cardiology (ACC) National Cardiovascular Data Registry (NCDR)—public reporting metric list. https://services.ncdr.com/PublicReportingApi/api/HospitalMetricsAndMeasuresDataDownload. Accessed August 25, 2017.
- 28.Tamblyn R, Eguale T, Huang A, Winslade N, Doran P. The incidence and determinants of primary nonadherence with prescribed medication in primary care: a cohort study. Ann Intern Med. 2014;160(7):441-450. [DOI] [PubMed] [Google Scholar]
- 29.Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006;113(24):2803-2809. [DOI] [PubMed] [Google Scholar]
- 30.McHorney C. Patient-centered reasons for primary non-adherence as derived from the peer-reviewed literature. Value Health. 2015;18(7):A737. [Google Scholar]
- 31.Goldman DP, Lakdawalla D. A theory of health disparities and medical technology. Contrib Econ Analysis Policy. 2005;4(1):1-30. [Google Scholar]
- 32.Chang VW, Lauderdale DS. Fundamental cause theory, technological innovation, and health disparities: the case of cholesterol in the era of statins. J Health Soc Behav. 2009;50(3):245-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolandaivelu K, Leiden BB, O’Gara PT, Bhatt DL. Non-adherence to cardiovascular medications. Eur Heart J. 2014;35(46):3267-3276. [DOI] [PubMed] [Google Scholar]
- 34.Bonaca MP, Bhatt DL, Oude Ophuis T, et al. Long-term tolerability of ticagrelor for the secondary prevention of major adverse cardiovascular events: a secondary analysis of the PEGASUS-TIMI 54 Trial. JAMA Cardiol. 2016;1(4):425-432. [DOI] [PubMed] [Google Scholar]
- 35.Granger CB, Berger PB. Understanding the adverse effects of ticagrelor in practice. JAMA Cardiol. 2016;1(4):381-383. [DOI] [PubMed] [Google Scholar]
- 36.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lam WY, Fresco P. Medication adherence measures: an overview [published online October 11, 2015]. Biomed Res Int. 2015;2015:217047. doi: 10.1155/2015/217047 [DOI] [PMC free article] [PubMed] [Google Scholar]