Key Points
Question
How is cardiovascular disease associated with hearing in elderly persons?
Findings
In this cohort study that reviewed the records of 433 patients aged 80 years or older, cardiovascular disease was primarily associated with worsened low-frequency hearing and with accelerated hearing loss. Of the conditions studied, coronary artery disease had the highest association with audiometric thresholds and was associated with hearing loss at all frequencies.
Meaning
Treating underlying cardiovascular disease may prevent or slow the progression of hearing loss, and conversely, hearing loss may suggest underlying cardiovascular disease.
This cohort study investigates the association of cardiovascular disease–related risk factors with auditory function among adults older than 80 years.
Abstract
Importance
In the United States, the population of individuals older than 80 years is expected to double in the next 40 years. Cardiovascular comorbidities are prevalent in this older old population, and their relationship with hearing loss has not been well characterized.
Objective
To investigate the association of cardiovascular disease (CVD)-related risk factors with auditory function among the older old (>80 years).
Design, Setting, and Participants
Audiological data and medical records from 2001 through 2014 of 433 patients aged 80 to 106 years at an academic medical center were analyzed in 2017.
Main Outcomes and Measures
The degree of low- and high-frequency hearing loss of participants with coronary artery disease, diabetes, hypertension, history of cerebrovascular accident, and smoking status was compared with that of disease-free individuals. Rate of hearing loss was also determined.
Results
Among the 433 patients (67% female; mean [SD] age, 89 [5.8] years), the presence of at least 1 cardiovascular morbidity was associated with elevated mean (SD) low-frequency pure-tone average (LFPTA) of 42.4 (1.6) vs 36.9 (3.5) decibels hearing loss (dB HL), a difference of 5.47 (95% CI, 4.15-9.49) dB HL. Among the 96 patients with 2 audiograms performed at age 80 years or older from which the rate of hearing loss could be calculated, 32 patients had CVD or related risk factors and 64 were healthy controls. Those with at least 1 disease had accelerated hearing loss. Patients with cardiovascular morbidity experienced a faster mean (SD) decline in LFPTA of 1.90 (0.27) vs 1.18 (0.42) dB HL/y, a difference of 0.72 (95% CI, 0.08-1.36) dB HL/y. Of the conditions studied, coronary artery disease had the highest association with audiometric thresholds and was associated with hearing loss at all frequencies tested and with poor word recognition score. Hearing loss was more strongly associated with CVD risk factors in men than in women.
Conclusions and Relevance
In this study of the older old, cardiovascular risk factors and disease were associated with worse hearing and a greater rate of hearing deterioration. Hearing loss in women was less associated with the presence of CVD, possibly owing to the cardioprotective effects of estrogen. The association of hearing with CVD severity and management remains to be determined.
Introduction
Hearing loss in elderly persons is a public health concern that is of increasing importance as the global population ages. Left untreated, hearing loss leads to diminished quality of life and has been associated with overall morbidity and mortality, as well as greater cognitive decline.1,2 It is estimated to affect more than half of the adults older than 75 years in the United States, a population that is expected to double over the next 40 years.3,4
Research in age-related hearing loss (ARHL) has commonly grouped together individuals older than 70 years. Recent studies of hearing loss in the older old, defined as individuals older than 80 years, noted that it differs in critical ways from the younger-old group.5 In addition to noting the ubiquitous nature of hearing loss in the older old, the investigators observed an increase in the rate of hearing loss in patients during the 10th decade of life compared with the ninth decade at all frequencies, representing a fundamental change that occurs along the course of ARHL.
Age-related hearing loss, which is hearing loss due to normal aging processes and likely genetically influenced,6 must be distinguished from sensorineural hearing loss that may accumulate with age but is not a result of normal aging. Other factors that may affect auditory function include medical comorbidities, nutritional status, and noise-induced hearing loss. While the individual effects of each cause of hearing loss are often difficult to distinguish, it is important to study non-ARHL etiologies because there may be modifiable risk factors to hearing loss that can relieve disease burden if addressed.
This study investigates the association between several cardiovascular comorbidities and hearing loss in the older old. The comorbidities—hypertension, type 2 diabetes, smoking, coronary artery disease (CAD), and history of cerebrovascular accident (CVA)—are vascular disease conditions that are all thought to ultimately impair hearing by compromising the blood supply to the cochlea, which is highly sensitive to disruptions of flow due to its high nutritive demand.4 Understanding the effect of these cardiovascular diseases on hearing would aid clinicians in preventing hearing loss by addressing its modifiable risk factors, better serving the needs of this rapidly growing segment of the elderly population.
Methods
Patients
A retrospective review of medical records was conducted for 433 patients between the ages of 80 and 106 years from cases encountered between 2001 and 2014. The institutional review board of Columbia University approved a search based on the criteria of (1) having an audiogram on file in the Department of Otolaryngology–Head & Neck Surgery and (2) being older than 80 years at the most recent audiogram. Due to the retrospective nature of the study, informed consent was waived. Participants with retrocochlear disease (eg, vestibular schwannoma) or any noncardiovascular diagnoses that may impair hearing such as connective tissue disease (eg, Alport syndrome, Paget disease of the bone) were excluded from the study.
Audiology
The hearing loss data collected from each audiogram included hearing thresholds measured in decibels hearing level (dB HL) at 0.25, 0.5, 1, 2, 3, 4, 6, and 8 kHz, as well as the word recognition score (WRS). The audiologist’s assessment of the type of hearing loss was also recorded. For each patient, the mean of the hearing thresholds at 0.5, 1, and 2 kHz was calculated and termed the low-frequency pure-tone average (LFPTA) to distinguish it from a high-frequency pure-tone average (HFPTA), similarly calculated from the thresholds at 3, 4, 6, and 8 kHz. Patients were represented by the better threshold or pure-tone average of 2 ears.
Comorbidities
The sex of each participant was noted, and the remainder of the medical record was examined for comorbidities related to hearing loss. The comorbidities investigated were all forms of cardiovascular disease or risk factors (hereafter referred to as cardiovascular disease [CVD]): (1) CAD, (2) diabetes, (3) history of CVA, (4) hypertension, and (5) smoking. Coronary artery disease was defined as having angina, a prior myocardial infarction, or angiographic evidence of significant coronary artery narrowing. Participants were categorized as current smokers or nonsmokers. Finally, CVA was defined as having any history of ischemic stroke, hemorrhagic stroke, or transient ischemic attack.
Statistical Analysis
Statistical analysis was conducted using Mathworks MATLAB 2015 and Microsoft Excel 2013. Comparison of mean thresholds between any 2 groups, such as those with and without particular comorbidities, was done using simple and multivariate regression analysis at a significance level of α = .05. Thresholds were also analyzed with respect to sex and sidedness. Simple linear regression was used to ascertain the Pearson correlation between age and pure-tone average thresholds. In participants with 2 audiograms, the rate of hearing loss was directly quantified by calculating the annual increase in audiometric thresholds at each frequency.
Results
CVD Comorbidity and Hearing Thresholds
The search identified 433 individuals. The mean and median age of the study sample was 89 years, with a standard deviation of 5.8 years about the mean. A total of 359 patients (83% of the population) exhibited sensorineural hearing loss. The remaining 74 (17%) had mixed hearing loss; their bone conduction levels were used in the analysis rather than air conduction levels. Hypertension was the most prevalent of the comorbidities (319 [74%]), while CVA was the least (52 [12%]) (Table 1). Of the 433 individuals, 96 had 2 audiograms performed at age 80 years or older from which the rate of hearing loss could be calculated.
Table 1. Overview of the Study Population.
| No. of Comorbidities | Patients, No. (N = 433) | No. (%) Female (n = 288 [67%]) | Patients With Comorbidity, No. | ||||
|---|---|---|---|---|---|---|---|
| Smoking (n = 64) | Hypertension (n = 319) | Diabetes (n = 102) | CVA (n = 52) | CAD (n = 135) | |||
| 0 | 64 | 32 (50) | 0 | 0 | 0 | 0 | 0 |
| 1 | 138 | 97 (70) | 5 | 100 | 12 | 3 | 18 |
| 2 | 165 | 116 (70) | 37 | 153 | 51 | 25 | 64 |
| 3 | 60 | 38 (63) | 18 | 60 | 34 | 20 | 48 |
| 4 | 6 | 5 (83) | 4 | 6 | 5 | 4 | 5 |
Abbreviations: CAD, coronary artery disease; CVA, cerebrovascular accident.
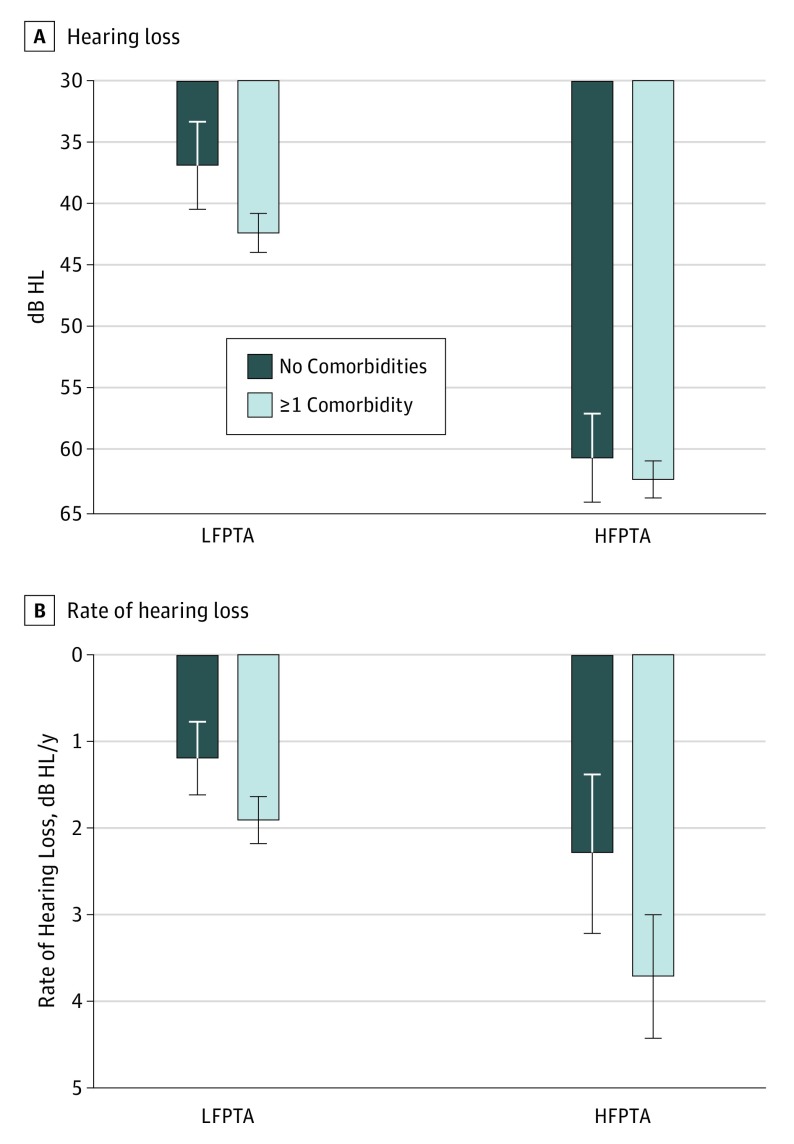
There were 64 healthy controls with no CVD comorbidities. Mean (SD) age was 85.8 (3.5) years for healthy controls and 89.6 (5.9) years for affected patients. When compared with patients with at least 1 CVD comorbidity, patients without disease demonstrated better mean LFPTA than those with a disease by a difference of 5.47 (95% CI, 4.15-9.49) dB HL (Figure 1A). There was a difference in mean HFPTA of 1.77 (95% CI, −2.02 to 5.56) dB HL between patients with no vs at least 1 CVD comorbidity. When patients with a low comorbidity load (1 or 2 diseases) were compared with patients with a high load (3 or 4 diseases), there was a difference in mean LFPTA of 1.79 (95% CI, −3.28 to 6.86) dB HL and a difference in mean HFPTA of 1.36 (95% CI, −2.45 to 5.17) dB HL.
Figure 1. Association of Cardiovascular Disease With Hearing Level and Rate of Decline.
A, The presence of at least 1 cardiovascular morbidity was associated with elevated mean (SD) low-frequency pure-tone average (LFPTA) of 42.4 (1.6) vs 36.9 (3.5) decibels hearing loss (dB HL), a difference of 5.47 (95% CI, 4.15-9.49) dB HL. Cardiovascular disease was also associated with elevated mean (SD) high-frequency pure-tone average (HFPTA) of 62.3 (1.5) vs 60.6 (3.5) dB HL, a difference of 1.8 (95% CI, −2.0 to 5.6) dB HL. B, Patients with cardiovascular morbidity experienced faster mean (SD) decline of LFPTA of 1.90 (0.27) vs 1.18 (0.42) dB HL/y, a difference of 0.72 (95% CI, 0.08-1.36) dB HL/y. High-frequency pure-tone average declined at a mean (SD) rate of 3.71 (0.72) dB HL/y in those with cardiovascular disease vs 2.29 (0.92) dB HL/y in those without, a difference of 1.43 (95% CI, −0.32 to 3.18) dB HL/y. Error bars indicate 95% confidence interval.
When the sample was stratified by sex, CAD was associated with an 8.81 dB HL elevation in mean LFPTA (95% CI, 3.90-13.72 dB HL) and 6.82 dB HL elevation in mean HFPTA (95% CI, 2.35-11.29 dB HL) in men. Men with diabetes and hypertension also demonstrated elevations in mean LFPTA of 9.75 (95% CI, 3.32-16.18) dB HL and 8.72 (95% CI, 3.16-14.28) dB HL, respectively. In women, CAD was associated with a 4.27 dB HL elevation in mean LFPTA (95% CI, 0.29-8.25 dB HL). No other comorbidities were associated with LFPTA or HFPTA elevations in women. On analysis of hearing in the left vs right ear, no comorbidity was associated with asymmetrically increased thresholds or decreased WRS.
CVD Comorbidity and Rate of Hearing Loss
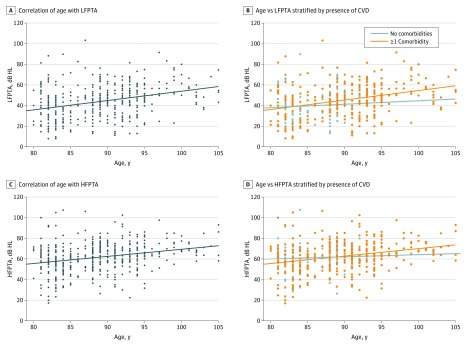
The presence of cardiovascular morbidity was associated with faster decline of low-frequency hearing. Low-frequency pure-tone average had a positive correlation with age in the group of patients with at least 1 comorbidity (Pearson r = 0.34; 95% CI, 0.25-0.43). The correlation between LFPTA and age was weak in the group free of CVD (Pearson r = 0.07; 95% CI, −0.18 to 0.38) (Figure 2). Rate of hearing loss was quantified directly by comparing 2 audiograms. Patients with at least 1 comorbidity experienced accelerated low-frequency hearing loss. Low-frequency pure-tone average increased by 1.90 (95% CI, 1.63 to 2.17) dB HL/y in patients with CVD comorbidity, compared with 1.18 (95% CI, 0.76 to 1.60) dB HL/y in healthy controls, a difference 0.72 (95% CI, 0.08 to 1.36) dB HL/y (Figure 1B).
Figure 2. Association of Age and Presence of Comorbidities With Low- and High-Frequency Hearing.
A, Age was correlated with low-frequency pure-tone average (LFPTA) (r = 0.33; 95% CI, 0.25-0.42). B, When the sample was stratified by presence or absence of cardiovascular disease (CVD), age was only correlated with thresholds in those with disease (r = 0.34; 95% CI, 0.25-0.43) and not in healthy individuals (r = 0.07; 95% CI, −0.18 to 0.38). C, Age was correlated with high-frequency pure-tone average (HFPTA) (r = 0.27; 95% CI, 0.18-0.36). D, When the sample was stratified by presence or absence of CVD, age was only correlated with thresholds in those with disease (r = 0.30; 95% CI, 0.21-0.39) and not in healthy individuals (r = 0.04; 95% CI, −0.20 to 0.29). dB HL indicates decibels hearing loss; dots indicate individual patients.
High-frequency pure-tone average similarly demonstrated a positive correlation with age in the group with CVD comorbidity (r = 0.30; 95% CI, 0.21-0.39) but not in those without comorbidities (r = 0.04; 95% CI, −0.20 to 0.29) (Figure 2). However, on direct quantification of hearing decline, there was no difference in the rate of high-frequency loss between participants with CVD and healthy controls. High-frequency pure-tone average worsened faster in patients with CVD than in healthy patients by 1.43 dB (95% CI, −0.32 to 3.18) HL/y.
Strength of Association of Individual CVD Comorbidity
In general, the study sample had high-frequency hearing loss. There was no difference in HFPTA between healthy controls and patients with comorbidities. However, the presence of comorbidities was associated with low-frequency hearing loss. Simple and multiple linear regressions were calculated to predict HFPTA based on smoking, hypertension, diabetes, CVA, and CAD. Coronary artery disease was the only comorbidity predictive for HFPTA on simple regression, with an effect of 4.39 (95% CI, 1.23-7.55) dB HL (Table 2). No risk factor predicted HFPTA on multiple regression. Simple and multivariate regressions were also calculated for LFPTA. Coronary artery disease, diabetes, and CVA were predictive on simple regressions, in order of greatest to least association with hearing loss, with respective effect sizes of 6.07 (95% CI, 2.81-9.34), 5.57 (95% CI, 1.84-9.31), and 5.06 (95% CI, 0.46-9.66) dB HL (Table 2). Multiple regression showed no predictive risk factors.
Table 2. Linear Regression Models for Cardiovascular Disease and Hearing Thresholdsa.
| Comorbidity | β (95% CI) dB Hearing Level (N = 433) | |
|---|---|---|
| Simple Linear Regression | Multiple Linear Regression | |
| Smoking | ||
| LFPTA | 0.99 (−4.14 to 6.12) | −2.73 (−10.24 to 4.78) |
| HFPTA | −0.09 (−4.44 to 4.26) | −4.54 (−12.57 to 3.48) |
| Hypertension | ||
| LFPTA | 2.94 (−0.84 to 6.71) | −0.40 (−9.75 to 8.94) |
| HFPTA | 0.21 (−3.23 to 3.66) | −3.46 (−13.43 to 6.52) |
| Diabetes | ||
| LFPTA | 5.57 (1.84 to 9.31) | 3.69 (−4.82 to 12.20) |
| HFPTA | 2.79 (−0.63 to 6.21) | 3.24 (−5.84 to 12.33) |
| Cerebrovascular accident | ||
| LFPTA | 5.06 (0.46 to 9.66) | 6.92 (−3.55 to 17.39) |
| HFPTA | 2.05 (−2.34 to 6.44) | 0.61 (−10.56 to 11.80) |
| Coronary artery disease | ||
| LFPTA | 6.07 (2.81 to 9.34) | 2.22 (−6.12 to 10.57) |
| HFPTA | 4.39 (1.23 to 7.55) | 0.98 (−5.92 to 11.89) |
Abbreviations: HFPTA, high-frequency pure-tone average; LFPTA, low-frequency pure-tone average.
Coronary artery disease was predictive for both LFPTA and HFPTA on simple regression. Diabetes and cerebrovascular accident were predictive only for LFPTA on simple regression. No comorbidities were predictive for either LFPTA or HFPTA on multiple regression.
Hearing thresholds at individual frequencies were also analyzed with respect to each comorbidity (eFigures 1-5 in the Supplement). Coronary artery disease was associated with threshold elevations at all frequencies (eFigure 1 in the Supplement). In addition, CAD was also the only condition associated with worsened WRS, showing a reduction of 3.70% (95% CI, 0.38%-7.02%). Smoking was not associated with elevated thresholds at any of the 8 frequencies (eFigure 5 in the Supplement).
Discussion
This study investigated the association between hearing loss and CVD comorbidities in the older old—individuals 80 years and older. In general, we found that the presence of CVD, and its associated comorbidities, was associated with poorer hearing and a greater rate of hearing loss. The associated hearing loss was greater in the low frequencies than in high frequencies, although this may reflect a ceiling effect. On average, individuals with CVD demonstrated a 5.5 dB HL elevation in mean LFPTA and minimal difference in HFPTA. The association of CVD morbidity with low-frequency hearing decline is consistent with prior reports.4,6 A 5.5 dB HL elevation in isolation is not clinically significant because it is less than the naturally occurring interaural threshold difference in this age group.5 However, in this situation, this elevation qualified the mean LFPTA to represent moderate hearing loss, as opposed to mild hearing loss in patients without CVD. Overall, patients with CVD displayed generally downsloping audiograms with a 15–dB HL decline from 0.25 to 2 kHz and less than a 10–dB HL decline from 4 to 8 kHz. This fits a strial pattern based on parameters described by Friedland et al7 and is consistent with previous findings. A variety of mechanisms have been postulated to affect auditory function. Cardiovascular disease is commonly thought to cause hearing loss due to compromised blood flow to the cochlea. The reduction may be due to microvascular changes in the stria vascularis or to macrovascular changes of the internal auditory artery. Decreased blood flow in the cochlea may directly cause stereocilia cell death. Insufficient nutritive supply to the inner ear may disrupt the electrolyte balance in the endolymph, causing electrical dysfunction of stereocilia.4 Interestingly, there was no difference in LFPTA or HFPTA between groups with low (1 or 2 diseases) and high comorbidity load (3 or 4 diseases). This lack of difference may be explained by a binary rather than graduated effect of CVD on hearing. One disease alone may cause enough of an insult such that additional disease processes do not further worsen hearing. The lack of difference may be further attributed to inherently protective genes that have allowed the group with high comorbidity load to survive to such elderly age in the first place.
Rate of Hearing Loss
In the presence of CVD comorbidity, there was progression of ARHL in both low and high frequencies that was not observed in the healthy group with absence of CVD cofactors. Direct comparisons of the rate of hearing decline, based on patients who had multiple audiograms, confirmed faster deterioration of mean LFPTA among patients with CVD compared with healthy controls by 0.72 (95% CI, 0.08-1.36) dB HL/y. This finding suggests that in addition to causing low-frequency hearing loss, CVD may also have a permissive or accelerating effect on both low- and high-frequency ARHL.
However, HFPTA did not demonstrate faster deterioration in patients with CVD. We postulate that the lack of an accelerated high-frequency loss reflects a ceiling effect. This phenomenon was first described by Glorig et al,8 who hypothesized that a diminished hearing reserve at higher frequencies results in slower rates of decline. Despite no acceleration of high-frequency loss in those with CVD, the strengthened correlation of HFPTA with age in this group prompts further investigation into the modulation of ARHL by CVD.
Strength of Association of Each Comorbidity
Of the conditions studied, CAD had the strongest association with hearing thresholds in the older old, with worsened hearing at all frequencies. These results in individuals older than 80 years are consistent with previous investigations reporting an association between CAD and low-frequency hearing loss.4,7,9 More recently, Erkan et al10 found a positive correlation between the extent of coronary atherosclerosis and degree of hearing loss, noting an effect that extended to 4 kHz. Traditionally, CAD has been associated with a strial pattern of hearing loss, which is hypothesized to be vascular and is associated with only mild loss of word discrimination.7 In the present study, CAD was the only condition to be associated with a negative effect on WRS, a 3.70% reduction (95% CI, 0.38%-7.02%). Although likely limited, this reduction may have clinical significance because it is greater than the test-retest variability for WRS and is the equivalent of 3 years of age-related decline in WRS in this age group.5,11 While this may reflect strial loss or suggest a component of neural loss, which is associated with worsened speech discrimination, it cannot be entirely attributed to CAD alone because the comparison did not control for other comorbidities. Coronary artery disease is an indicator of systemic vascular disease; similarly, it is likely an indicator of cochlear microvascular disease.
Diabetes is thought to damage the stria vascularis through nonenzymatic glycosylation, generation of reactive oxygen species, basement membrane disruption, and hair cell excitotoxicity.12 In the present study, diabetes had the second strongest association with change in hearing thresholds. Despite a logical mechanism and circumstantial evidence highly suggestive of an association with hearing loss, prior studies have yielded inconsistent conclusions.6,13,14 These findings in elderly patients support a previously published National Health and Nutrition Examination Survey study on individuals 40 to 69 years of age, in which the prevalence of low- to mid-frequency hearing loss was 21.3% among diabetics, compared with 9.4% among nondiabetics.15
Prior CVA can compromise blood flow to the cochlea, vestibulocochlear nerve, or the auditory processing centers. While hearing loss due to vertebrobasilar CVA has been thoroughly studied, there have been limited investigations into the association between CVA in general and sensorineural hearing loss. A retrospective study on 1168 patients determined CVA and transient ischemic attack to have the highest independent association with low-frequency hearing loss, more than other forms of CVD.7 In a 2009 cross-sectional study, middle-aged adults with moderate to severe hearing loss were more likely to report prior CVA (odds ratio, 2.04; 95% CI, 1.20-3.49).16 In our population of individuals 80 years and older, prior CVA was associated with low-frequency hearing loss.17 Hearing loss is likely underdiagnosed in patients with CVA, precipitating important implications on rehabilitation and cognitive health. The identification of hearing loss among the elderly with prior CVA and its subsequent treatment is especially important in the light of recent findings that hearing aid use is associated with improved cognitive function and quality of life.2,5
Hypertension is both a microvascular and macrovascular disease. Through the processes of microvascular rarefaction and hypertrophic remodeling of small arteries, it may compromise organ function over time.18 The present study found hypertension to be associated with poorer low-frequency hearing. A retrospective study examining self-reported hearing loss in female nurses demonstrated a modest association between hypertension and hearing loss (multivariable adjusted relative risk, 1.04; 95% CI, 1.01-1.07).19 Similarly, a prospective cohort study comparing 32 hypertensive patients with age- and sex-matched controls demonstrated impaired hearing in hypertensive patients at all frequencies (0.125-12.5 kHz).20 Further investigations into hypertension’s effect on hearing are warranted given the extensive prevalence of hypertension and presence of effective treatment.
While smoking is a well-established cause of CVD, there has been inconsistent evidence linking it to hearing loss. Recent cross-sectional studies have associated active and second-hand smoking with sensorineural hearing loss in adolescents and middle-aged adults.21,22 A meta-analysis conducted by Nomura et al,23 including studies focused mostly on middle-aged adults, reported an overall risk ratio of 1.33 (95% CI, 1.24-1.44). Longitudinal data that examined smoking and hearing loss in elderly adults, however, found no association.6,24 The present study examines a population much older than previous works and found no significant association with hearing loss. It is possible that while smoking may cause sensorineural hearing loss from adolescence to middle adulthood, the effect of ARHL on overall hearing masks any effects from smoking in old age. This is particularly likely if the effects of smoking are small and predominantly at high frequencies, as demonstrated by Engdahl et al25 in a 2015 cross-sectional study. The analysis of smoking in the present study is limited by the inability to stratify smokers by lifetime dose. Stratification by pack-year history may have revealed a threshold after which smoking becomes associated with hearing loss.
Sex and Sidedness
Hearing loss is more profound in elderly men than women. While the cause is unclear, it may be related to the otoprotective effects of estrogen.26 In the present study, elderly men with hypertension, diabetes, or CAD demonstrated worse hearing than their healthy counterparts, while elderly women with these conditions generally did not. It is possible that due to influences from estrogen, vitamin B12, and folate, which are otoprotective and generally present at higher levels in women, CVD has a stronger effect on hearing in men than in women.27,28 Additionally, the women in this cohort may have had these conditions for a shorter duration or to a less advanced extent, as they benefited from the cardioprotective effects of estrogen premenopausally.
The right-ear advantage, referring to better vowel-consonant syllable perception in the right ear than the left, is a well-documented phenomenon.29 Because the language-processing center is lateralized to the left temporal lobe, decussating auditory fibers result in improved WRS in the right ear. Ren et al14 found that middle-aged patients with type 2 diabetes lose their right-ear advantage. In our elderly study population, we found no evidence of the right-ear advantage. This supports previous findings that peripheral right-ear advantage is lost with age.12,30 Therefore, it is likely that the right-ear advantage is lost as ARHL worsens and possible that cardiovascular comorbidities accelerate this loss.
Limitations
By selecting participants at the elderly extreme of age, the present study was able to characterize the effects of CVD over time on hearing level and rate of hearing loss. While the sample size was large enough to demonstrate that these effects are low frequency predominant, the study was not sufficiently powered for multivariate analysis to distinguish the isolated effect of each comorbidity. In a practical sense, the study implies that the average patient with CAD may have worse hearing than healthy counterparts, but cannot theoretically distinguish whether this is due to CAD itself or the naturally occurring comorbidities that patients with CAD have.
The study population was composed of mostly women, given the inclusion criterion that participants be older than 80 years and the longer life expectancy of women. The study did not take into account the severity of each disease. For instance, there was no differentiation between patients with well-controlled and poorly controlled hypertension or stratification of diabetics by hemoglobin A1c level. In future studies, considering the degrees of each disease and accounting for other risk factors, such as hyperlipidemia, race, socioeconomic status, and prior noise exposure, may better detail the relationship between CVD and hearing loss. It is possible that including these variables in the analysis would expose confounders or reveal underlying effect modification.
Conclusions
In the older old, CVD was associated with low-frequency hearing loss, increasing not only hearing thresholds, but also the rate at which hearing worsened. Low-frequency pure-tone average deteriorated at a faster rate in patients with CVD, and both LFPTA and HFPTA had a stronger correlation with age in the disease group than the healthy group. These findings suggest that CVD may accelerate ARHL in individuals 80 years and older. Of the diseases studied, CAD had the strongest association with hearing and was associated with low, middle, and high-frequency losses. Diabetes, hypertension, and history of CVA were associated with low-frequency loss. Smoking was not associated with hearing loss in this population, likely because smoking-related losses were overtaken by ARHL. In general, men were more susceptible than women to the effects of CVD on hearing, possibly owing to the cardioprotective effects of estrogen. Future studies are needed to investigate the relationships between severity of CVD and its treatment and hearing impairment.
eFigure 1. Effect of CAD on hearing
eFigure 2. Effect of DM on hearing
eFigure 3. Effect of CVA on hearing
eFigure 4. Effect of hypertension on hearing
eFigure 5. Effect of smoking on hearing
References
- 1.Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43(5):623-629. [DOI] [PubMed] [Google Scholar]
- 2.Qian ZJ, Wattamwar K, Caruana FF, et al. Hearing aid use is associated with better Mini-Mental State Exam performance. Am J Geriatr Psychiatry. 2016;24(9):694-702. [DOI] [PubMed] [Google Scholar]
- 3.US Census Bureau The next four decades: the older population in the United States: 2010 to 2050. https://www.census.gov/prod/2010pubs/p25-1138.pdf. Published 2010. Accessed August 2017.
- 4.Helzner EP, Patel AS, Pratt S, et al. Hearing sensitivity in older adults: associations with cardiovascular risk factors in the health, aging and body composition study. J Am Geriatr Soc. 2011;59(6):972-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wattamwar K, Qian ZJ, Otter J, et al. Increases in the rate of age-related hearing loss in the older old. JAMA Otolaryngol Head Neck Surg. 2017;143(1):41-45. [DOI] [PubMed] [Google Scholar]
- 6.Gates GA, Cobb JL, D’Agostino RB, Wolf PA. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch Otolaryngol Head Neck Surg. 1993;119(2):156-161. [DOI] [PubMed] [Google Scholar]
- 7.Friedland DR, Cederberg C, Tarima S. Audiometric pattern as a predictor of cardiovascular status: development of a model for assessment of risk. Laryngoscope. 2009;119(3):473-486. [DOI] [PubMed] [Google Scholar]
- 8.Glorig A, Ward WD, Nixon J. Damage risk criteria and noise-induced hearing loss. Arch Otolaryngol. 1961;74:413-423. [DOI] [PubMed] [Google Scholar]
- 9.Torre P III, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. The association between cardiovascular disease and cochlear function in older adults. J Speech Lang Hear Res. 2005;48(2):473-481. [DOI] [PubMed] [Google Scholar]
- 10.Erkan AF, Beriat GK, Ekici B, Doğan C, Kocatürk S, Töre HF. Link between angiographic extent and severity of coronary artery disease and degree of sensorineural hearing loss. Herz. 2015;40(3):481-486. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Lee J, Lee KW, et al. Test-retest reliability of word recognition score using Korean standard monosyllabic word lists for adults as a function of the number of test words. J Audiol Otol. 2015;19(2):68-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frisina ST, Mapes F, Kim S, Frisina DR, Frisina RD. Characterization of hearing loss in aged type II diabetics. Hear Res. 2006;211(1-2):103-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalton DS, Cruickshanks KJ, Klein R, Klein BE, Wiley TL. Association of NIDDM and hearing loss. Diabetes Care. 1998;21(9):1540-1544. [DOI] [PubMed] [Google Scholar]
- 14.Ren J, Zhao P, Chen L, Xu A, Brown SN, Xiao X. Hearing loss in middle-aged subjects with type 2 diabetes mellitus. Arch Med Res. 2009;40(1):18-23. [DOI] [PubMed] [Google Scholar]
- 15.Bainbridge KE, Hoffman HJ, Cowie CC. Diabetes and hearing impairment in the United States: audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann Intern Med. 2008;149(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gopinath B, Schneider J, Rochtchina E, Leeder SR, Mitchell P. Association between age-related hearing loss and stroke in an older population. Stroke. 2009;40(4):1496-1498. [DOI] [PubMed] [Google Scholar]
- 17.Bamiou DE. Hearing disorders in stroke. Handb Clin Neurol. 2015;129:633-647. [DOI] [PubMed] [Google Scholar]
- 18.Feihl F, Liaudet L, Levy BI, Waeber B. Hypertension and microvascular remodelling. Cardiovasc Res. 2008;78(2):274-285. [DOI] [PubMed] [Google Scholar]
- 19.Lin BM, Curhan SG, Wang M, Eavey R, Stankovic KM, Curhan GC. Hypertension, diuretic use, and risk of hearing loss. Am J Med. 2016;129(4):416-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Przewoźny T, Gójska-Grymajło A, Kwarciany M, et al. Hypertension is associated with dysfunction of both peripheral and central auditory system. J Hypertens. 2016;34(4):736-744. [DOI] [PubMed] [Google Scholar]
- 21.Lalwani AK, Liu YH, Weitzman M. Secondhand smoke and sensorineural hearing loss in adolescents. Arch Otolaryngol Head Neck Surg. 2011;137(7):655-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawes P, Cruickshanks KJ, Moore DR, et al. Cigarette smoking, passive smoking, alcohol consumption, and hearing loss. J Assoc Res Otolaryngol. 2014;15(4):663-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nomura K, Nakao M, Morimoto T. Effect of smoking on hearing loss: quality assessment and meta-analysis. Prev Med. 2005;40(2):138-144. [DOI] [PubMed] [Google Scholar]
- 24.Brant LJ, Fozard JL. Age changes in pure-tone hearing thresholds in a longitudinal study of normal human aging. J Acoust Soc Am. 1990;88(2):813-820. [DOI] [PubMed] [Google Scholar]
- 25.Engdahl B, Aarhus L, Lie A, Tambs K. Cardiovascular risk factors and hearing loss: the HUNT study. Int J Audiol. 2015;54(12):958-966. [DOI] [PubMed] [Google Scholar]
- 26.Hultcrantz M, Simonoska R, Stenberg AE. Estrogen and hearing: a summary of recent investigations. Acta Otolaryngol. 2006;126(1):10-14. [DOI] [PubMed] [Google Scholar]
- 27.Houston DK, Johnson MA, Nozza RJ, et al. Age-related hearing loss, vitamin B-12, and folate in elderly women. Am J Clin Nutr. 1999;69(3):564-571. [DOI] [PubMed] [Google Scholar]
- 28.Hinds HE, Johnson AA, Webb MC, Graham AP. Iron, folate, and vitamin B12 status in the elderly by gender and ethnicity. J Natl Med Assoc. 2011;103(9-10):870-877. [DOI] [PubMed] [Google Scholar]
- 29.Sætrevik B. The right ear advantage revisited: speech lateralisation in dichotic listening using consonant-vowel and vowel-consonant syllables. Laterality. 2012;17(1):119-127. [DOI] [PubMed] [Google Scholar]
- 30.Tadros SF, Frisina ST, Mapes F, Kim S, Frisina DR, Frisina RD. Loss of peripheral right-ear advantage in age-related hearing loss. Audiol Neurootol. 2005;10(1):44-52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Effect of CAD on hearing
eFigure 2. Effect of DM on hearing
eFigure 3. Effect of CVA on hearing
eFigure 4. Effect of hypertension on hearing
eFigure 5. Effect of smoking on hearing