Abstract
The salicylic acid (SA) plays a critical role during the establishment of systemic acquired resistance (SAR) in uninfected plant tissues after localised exposure to a pathogen. Here, we studied SA in Populus tomentosa infected by the plant pathogen Botryosphaeria dothidea. The accumulation of SA and methyl salicylate (MeSA) occurred in chronological order in P. tomentosa. The SA and MeSA contents were greater at infected than uninfected sites. Additionally, a gene expression analysis indicated that SA might be accumulated by phenylalanine ammonialyase (PAL) and converted to MeSA by SA carboxyl methyltransferase (SAMT), while MeSA might convert to SA by SA-binding protein 2 (SABP2). The expressions of SAMT at infected sites and SABP2 at uninfected sites, respectively, were significantly up-regulated. Thus, SA might be converted to MeSA at infected sites and transported as a signalling molecule to uninfected sites, where it is converted to SA for SAR. Moreover, the expressions of pathogenesis-related genes PR-1, PR-2 and PR-5 in P. tomentosa were up-regulated by the B. dothidea infection. Our study determined that variations in SA and MeSA contents occur at infected and uninfected sites in poplar after pathogen infection and contributed to the remote signals for poplar SAR.
Introduction
During evolution, plants evolved complex mechanisms to adapt to pathogen attack, wounds, heat and other environmental stresses1–4. These mechanisms include the rapid increase of signalling molecules, such as salicylic acid (SA), jasmonic acid and ethylene5. In recent years, SA has generally been considered a critical signal transduction factor in the defence against biotrophic pathogens6. Phenylalanine ammonialyase (PAL) is a key enzyme in the SA biosynthetic pathway7,8. The accumulation of endogenous SA in the healthy systemic tissue of infected plants is required for the expression of many defence genes, including pathogenesis-related (PR) genes, and the activation of systemic acquired resistance (SAR)9–11. SA-dependent SAR is a long-lasting, broad-spectrum resistance that is established in uninfected parts of plants after localised pathogen exposure12–14. However, SA itself is most probably not the mobile signal inducing SAR based on grafting experiments using transgenic tobacco expressing the SA-degrading salicylate hydroxylase encoded by NahG as rootstocks for primary infections15. In fact, methyl salicylate (MeSA), a derivative of SA, plays a pivotal role as a long-distance mobile signal for SAR in uninfected parts of plants16,17. MeSA is formed from SA by SA carboxyl methyltransferase (SAMT), and converted back to SA by SA-binding protein 2 (SABP2)18. SABP2’s enzymatic activity is required in systemic tissues to mount a successful SAR response to pathogen infection19. The reduction of MeSA in primary infected leaves by either the overexpression of a mutant SABP2 or the silencing of SAMT results in the loss of SAR and the suppression of PR gene expression levels20,21. Thus, MeSA is an essential part of the SAR signal in plants and it can coexist with SA, transmuting into each other, as needed, through the activities of SAMT and SABP2.
Thus, it is important to study SA as a universal signalling molecule in all plants. SA accumulation could induce SAR in relatively sensitive plants, such as tobacco (Nicotiana tabacum), cucumber (Cucumis sativus) and Arabidopsis (Arabidopsis thaliana). However, some plants, like rice (Oryza sativa) and potato (Solanum tuberosum), contain very high basal SA levels, which make it difficult to act as an effective signalling molecule to activate many defence genes and induce disease resistance22,23. Thus, SA’s functional mechanisms during immune responses in different plant–pathogen interaction systems may be completely different. The roles of the SA-dependent signalling pathway in a variety of annual herbs, such as Arabidopsis24,25, tobacco13 and rice26, have been well studied. However, very little is known about the role of the SA signalling pathway in response to pathogen challenge in perennial woody species, which have longer life spans and are more likely to experience pathogen attacks27.
Poplar (Populus species) is a widespread and economically important wood resource worldwide28. Botryosphaeria dothidea (Mougeot ex Fries) Cesati et de Notaris is a plant pathogen that causes the formation of cankers on a wide variety of tree and shrub species. This species infects several hundred plant hosts on all continents, except Antarctica29,30. In north-central China, B. dothidea causes stem cankers on poplar. It can reduce the growth of plants or even kill them, resulting in significant production losses, as large as 70%, for a plantation31. The development of a Populus species as a model for forest trees provides an unprecedented opportunity to expand our understanding of the interactions between fungi and woody perennial plants. SA treatments can induce the expression of defence genes in poplar32, suggesting that the SA signalling pathway may be very important for SAR in poplar.
Despite the importance of SA and MeSA in defence signalling in herbaceous plants, whether they play roles in poplar is unclear. There are limited reports on the effects of SA and MeSA on poplar stem canker33,34. To investigate the function of SA in poplar interactions with B. dothidea, we monitored SA and MeSA levels in infected and uninfected sites on P. tomentosa inoculated with B. dothidea, cloned SAMT and SABP2 from P. tomentosa, measured the expression levels of SAMT, SABP2 and PR genes, and analysed the relationships among SA, MeSA, SAMT and SABP2. Our aim was to clarify the role of SA and its signal transduction mechanism during P. tomentosa–B. dothidea interactions.
Results
SA and MeSA accumulated in P. tomentosa after B. dothidea inoculation
To investigate the functions SA in P. tomentosa infected with B. dothidea, we determined the SA and MeSA levels in P. tomentosa at 0, 6, 12, 24, 48, 72 and 96 h after inoculation with B. dothidea.
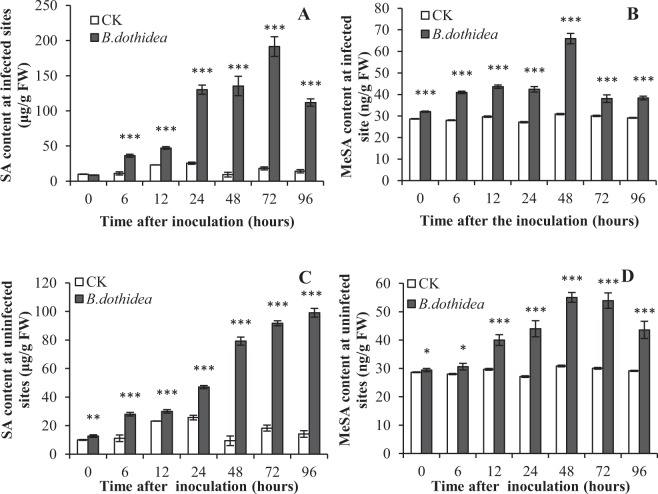
The SA content in P. tomentosa increased significantly near the infection sites 6 h after inoculation with B. dothidea and continued to accumulate to the peak 191.42 µg/g fresh weight (FW) by 72 h, after which the value gradually decreased (Fig. 1A). The SA levels in the control remained low (from 9.98 to 14.12 µg/g FW) throughout the sampling period. MeSA is a derivative of the SA, and they can transmute between forms. To further define the role of SA and its correlation to the MeSA content, we determined the MeSA content, which was significantly increased in P. tomentosa after inoculation with B. dothidea. The controls showed no significant changes (ranging from 28.65 to 29.13 ng/g FW). As the time after inoculation with B. dothidea increased, the MeSA content increased steadily from 6 h to 48 h, peaked at 65.93 ng/g FW at 48 h, and then decreased from 72 h to 96 h (Fig. 1B). After B. dothidea inoculation, the SA contents increased rapidly (8.67 ng/g FW at 0 h to 191.42 ng/g FW at 72 h). However, the MeSA content increased slowly (from 32 ng/g FW at 0 h to 66 ng/g FW at 48 h). In addition, the peak time for MeSA occurred at 48 h, which was earlier than that of SA at 72 h.
Figure 1.
Liquid chromatography of SA in P. tomentosa after B. dothidea inoculation. SA, Free salicylic acid. MeSA, methyl salicylate. Infected sites, 0.5 cm around inoculation sites. Uninfected sites, 10–15 cm away from inoculation sites. □, CK (control) inoculation with culture-medium; ■, inoculation with B. dothidea. Error bars represent SEM. P values for differences between inoculation with B. dothidea and control: *P < 0.05, **P < 0.01 and ***P < 0.001 (Independent-samples T test).
In addition to the SA and MeSA contents at infected sites, we measured their contents at uninfected sites far from the B. dothidea-inoculation point in the same poplars. They showed trends that were similar to those at the infected sites. In uninfected tissues, both the SA and MeSA contents were higher than in the controls. Compared with the MeSA contents, the SA contents increased rapidly. As the time after inoculation increased, the SA contents in poplar increased gradually to 99.01 ng/g FW at 96 h (Fig. 1C). The MeSA contents in poplar also increased from 6 h to 48 h, peaked at 55.04 ng/g FW at 48 h, and then decreased from 72 h to 96 h (Fig. 1D).
Thus, SA and MeSA contents increased after inoculation with B. dothidea in both the infected and uninfected sites.
Expression analyses of SAMT and SABP2 in P. tomentosa after inoculation with B. dothidea
SAMT and SABP2 are two key enzymes in the reciprocal transmutation between SA and MeSA. SA is converted to MeSA by SAMT, and SABP2 mediates the hydrolysis of MeSA to SA. To further determine whether a conversion exists between SA and MeSA, and to clarify their roles as regulatory factors in poplar, we cloned the SAMT and SABP2 genes. P. tomentosa first-strand cDNA was used as a template, and the SAMT and SABP2 genes were amplified with specific primers and checked by 1.5% agarose gel electrophoresis. Then, the fragments were recovered and sequenced by the Nomad Biological Company. The full-length coding sequence of PtoSAMT (GenBank No. JQ086572) was 1,092 bp, encoding a 364-amino acid protein. The full-length coding sequence of SABP2 was 787 bp, and PtoSABP2 (GenBank No. JQ086570) encoded a 262-amino acid protein.
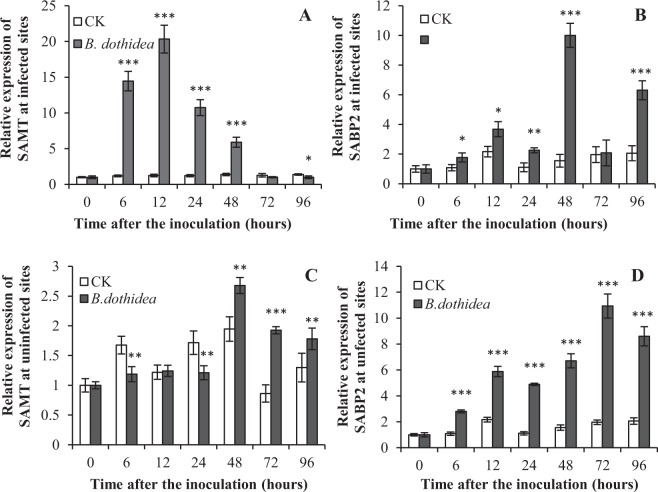
To determine the expression kinetics of the SAMT and SABP2 genes of P. tomentosa at different times after inoculation with B. dothidea, we measured the expression levels of the SAMT and SABP2 genes at infected sites. Both SAMT and SABP2 fluctuated significantly in P. tomentosa after inoculation with B. dothidea, while both SAMT and SABP2 showed lower expression levels and relatively smooth changes in the control after inoculation with culture medium (Fig. 2A,B). Moreover, the change patterns were strikingly different between SAMT and SABP2. The expression of SAMT increased and peaked at 12 h, decreased significantly by 72 h after inoculation, and reached its lowest level at 96 h after inoculation. In contrast, the expression of SABP2 increased rapidly, peaked at 48 h after infection, decreased by 72 h, and increased again at 96 h. SABP2’s peak expression time was later than that of SAMT. Importantly, the SAMT and SABP2 expression changes occurred prior to the changes in SA and MeSA contents, respectively.
Figure 2.
Expression of SAMT and SABP2 in P. tomentosa after inoculation with B. dothidea. SAMT, salicylic acid methyltransferases. SABP2, salicylic acid binding protein 2. Infected sites, 0.5 cm around inoculation sites. Uninfected sites, 10–15 cm away from inoculation sites. □, CK (control) inoculation with culture-medium; ■, inoculation with B. dothidea. Error bars represent SEM. P values for differences between inoculation with B. dothidea and control: *P < 0.05, **P < 0.01 and ***P < 0.001 (Independent-samples T test).
The expression levels of SAMT and SABP2 genes were also measured at uninfected sites. SABP2 gene expression was up-regulated in uninfected tissues after inoculation with B. dothidea compared with that of the control at almost all tested times (Fig. 2C,D). The expression level began to rise 6 h after infection, and the highest value occurred at 72 h. The SAMT gene’s expression level initially seemed un- or down-regulated compared with the control. Then, after 48 h, it was up-regulated. However, the range of the changes was smaller than that of SABP2. The SAMT gene’s expression in uninfected tissues was low relative to its level in infected tissues.
Thus, at infected sites, the expression level of SAMT was up-regulated earlier and to a greater extent, while the peak time of SABP2 occurred later than that of SAMT. However, at uninfected sites, SABP2 was up-regulated earlier, while the SAMT level initially remained low.
Up-regulation of PAL in SA synthesis
SA increased rapidly after inoculation with B. dothidea at both infected and uninfected sites. PAL activity leads to the accumulation of SA in plants (Pan et al., 2006). Therefore, we determined the expression and enzyme activity levels of PAL at infected sites.
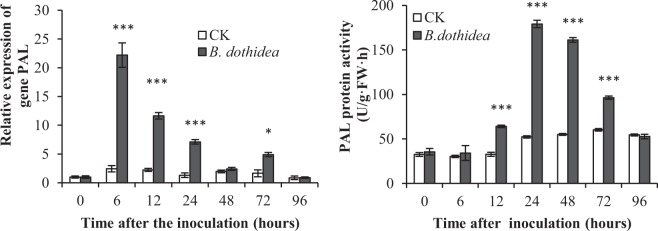
The expression of PAL was significantly up-regulated in P. tomentosa 6 h after B. dothidea inoculation and decreased significantly by 48 h after inoculation (Fig. 3). In the control, there was no significant change after inoculation. Thus, the expression of the PAL gene in P. tomentosa was affected by the B. dothidea infection and possibly caused the accumulation of SA (Fig. 1).
Figure 3.
Expression and protein activity of PAL in P. tomentosa after inoculation with B. dothidea. PAL, phenylalanine ammonia. □, CK (control) inoculation with culture-medium; ■, inoculation with B. dothidea. Infected sites were 5 mm around the inoculation sites and uninfected sites were 10 cm away from inoculation sites. Error bars represent SEM. P values for differences between inoculation with B. dothidea and control: *P < 0.05, **P < 0.01 and ***P < 0.001 (Independent-samples T test).
Moreover, the activity levels showed a similar variation trend as the expression in Poplar after inoculation with B. dothidea. PAL activity peak at 24 h after inoculation (up-regulated 3.4-fold) (P < 0.001), decreased gradually again from 72 h to 96 h after inoculation, and eventually returned to the control level (Fig. 3).
Defence-related gene expression levels in P. tomentosa after inoculation with B. dothidea
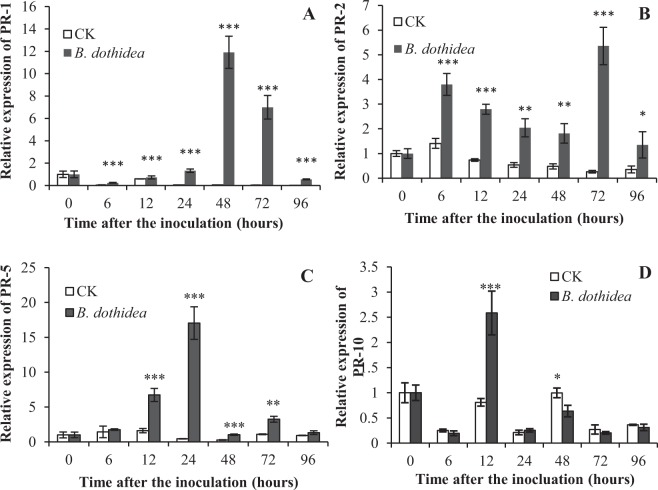
Commonly, during pathogen attacks, SA signalling molecules induce the expression of PR genes for plant defence. To study variations in PR gene expression in P. tomentosa after inoculation with B. dothidea, the expression levels of four defence-related genes (PR-1, PR-2, PR-5 and PR-10) were measured at infected sites of poplar. Following the stem inoculation with B. dothidea, the PR-1 expression level in P. tomentosa was not elevated prior to 48 h but it significantly increased at 48 h compared with the control and decreased significantly by 96 h (Fig. 4A). The level of PR-2 expression was much greater at 6 h and peaked at 72 h (Fig. 4 B), while that of PR-5 peaked at 24 h (Fig. 4C). However, for PR-10, the expression was up-regulated at 12 h, while the other time points were almost stable. In the control, there was no significant change in the expression levels of the four genes after pathogen inoculation. Thus, the expression levels of PR-1, PR-2 and PR-5 in P. tomentosa were affected by the B. dothidea infection.
Figure 4.
Expression of PR genes in P. tomentosa after inoculation with B. dothidea. PR genes, pathogenesis related genes. □, CK (control) inoculation with culture-medium; ■, inoculation with B. dothidea. Infected sites were 5 mm around the inoculation sites and uninfected sites were 10 cm away from inoculation sites. Error bars represent SEM. P values for differences between inoculation with B. dothidea and control: *P < 0.05, **P < 0.01 and ***P < 0.001 (Independent-samples T test).
Discussion
SA is necessary for activating plant resistance and plays an important role in plant defence mechanisms35–37. Free SA and SA glucoside (SAG, a storage form of SA) are two forms of SA in plants. Large amounts of SA accumulated in P. tomentosa tissues after inoculation with B. dothidea, while the control did not induce SA accumulation under the same conditions, indicating that the changes in SA were closely correlated with the B. dothidea infection. This was consistent with plants accumulating large amounts of SA to activate defence mechanisms under stress conditions1. The SAG content increased rather than decreased (Fig. S2), which indicated that free SA played a key role in the interaction between the P. tomentosa and B. dothidea and that SA was synthesised by other pathways. Thus, in addition to SA from SAG, in plants, increases in SA are derived from the biosynthetic pathway in which PAL is the key enzyme7. In our study, the SA content increased according as the PAL enzymes activity and expression levels were up-regulated. The accumulation of endogenous SA in infected plants is essential for PR gene expression9–11, and this was corroborated by the up-regulated expression levels of the four PR genes in P. tomentosa after infection with B. dothidea.
Plants accumulate a large amount of SA to activate the defence reaction mechanisms under stress conditions12, and some of the SA can be converted into MeSA near the site of infection. MeSA then acts as a signalling molecule to communicate with uninfected plant parts. MeSA is transformed into SA again by the SABP2 protein at uninfected sites, after which the plant defence mechanisms are activated by SA38. Our study demonstrated that large amounts of SA and MeSA accumulated in P. tomentosa tissues after inoculation with B. dothidea, indicating that the changes in SA and MeSA contents were closely correlated with the B. dothidea infection (Fig. 1). Importantly, our data indicated a chronological order for the accumulation of the SA and MeSA contents in P. tomentosa tissues after inoculation with B. dothidea. The SA levels were slightly higher in P. tomentosa at 6 h after inoculation with B. dothidea, and increased gradually between 12 h and 72 h, while the MeSA levels increased at 48 h after inoculation. Thus, there might be a conversion from SA to MeSA. After 48 h, the MeSA levels decreased rapidly, while the SA content continued to increase, suggesting that MeSA might have been converted back to SA. Thus, MeSA might act as a signalling molecule and play a key role in the induced defence reaction of SA.
In plants, SA is synthesised through two routes. One route involves isochorismate synthase, which is believed to be responsible for >90% of SA synthesised during the activation of stress responses. The other route uses the PAL-mediated pathway18. PAL is also essential for SA accumulation and SAR39,40. To date, the contributions of the two synthesis pathways to SA levels were not clear41. In our studies, the expression and enzyme activity of PAL were significantly up-regulated in P. tomentosa 6 h after B. dothidea inoculation and decreased significantly 48 h after inoculation (Fig. 3). Thus, the expression level of the PAL gene in P. tomentosa was affected by the B. dothidea infection and related to the SA accumulation (Fig. 1).
SAMT and SABP2 are required for SAR18,42. A large amount of SA accumulated at the site of infection and activated defence responses in the plant. Some of the SA can be converted into MeSA catalysed by SAMT, and MeSA is transported to the uninfected site through trachea, and it re-forms SA catalysed by SABP2 to activate the defence responses of uninfected plant tissues19. Our results showed the same timeline. At infected sites the expression levels of SAMT were up-regulated earlier and to a greater extant, which might contribute to MeSA synthesis for long-distance transportation. The peak time of SABP2 expression was later than that of SAMT. However, at uninfected sites, SABP2 was up-regulated first, which might elevate SA levels through the hydrolysis of MeSA, while SAMT levels initially remained low. Thus, we hypothesise that SA and MeSA had important roles in the signal transduction necessary for the defence response of poplar against pathogen attack. The conversion between MeSA and SA might be regulated by SAMT and SABP2 in both infected and uninfected tissues12,19. Our findings suggested that SABP2 mediates SA signalling through the demethylation of MeSA and that the reverse reaction (SA to MeSA catalysed by SAMT) was also necessary for disease defence in poplar. These results were consistent with previous observations that SA and MeSA were crucial signals for the activation of defence responses, suggesting that SAMT and SABP2 might be induced for proper signalling in poplar.
The regulation of PR gene expression is a biological effect and a sign of SAR43. In particular, PR-1, PR-2 and PR-5 are markers of SAR44,45. An increased SA content could induce the expression of PR genes in plants after pathogen infection46–48. Here, the expression levels of the defence-related genes PR-1, PR-2 and PR-5 were significantly up-regulated in P. tomentosa after inoculation, which indicated that P. tomentosa initiated the SA signalling pathways upon B. dothidea infection. Thus, poplar may activate the SA signalling pathway as a remote signal for SAR and the defence reactions after B. dothidea infection.
Methods
Materials, inoculations and samples
One-year-old plants of P. tomentosa (tolerant to B. dothidea; Disease Index of Canker in nature: 0–6.0) were cultured in pots (24-cm diameter) containing compost in a greenhouse with a day/night cycle of 10 h at 25 °C/14 h at 20 °C, with a constant relative humidity of 70%.
The B. dothidea used in this study was isolated from the canker lesions of P. × beijingensis in the Haidian District of Beijing, China and was preserved in the Forest Pathology Laboratory of the Research Institute of Forest Ecology, Environment and Protection, Beijing, China. Before inoculation, B. dothidea was activated on potato dextrose agar for 6 d at 25 °C in the dark. For inoculation, 5-mm wounds were created in the bark of each stem with a dissecting needle. Then, 5-mm plugs of potato dextrose agar without (control) or with B. dothidea were placed in the wound, packed in with sterile cotton and sterile water, and sealed with plastic wrap to retain moisture.
After inoculation, samples were taken at 0, 6, 12, 24, 48, 72 and 96 h at the infected sites (5 mm around the inoculation site) and uninfected sites (10 cm away from the inoculation site). The samples (containing cambiums) were immediately frozen in liquid nitrogen and stored at −80 °C for biochemical analysis, and 10 samples from each tree were mixed in equal volumes and used as one sample. Each treatment had three replicates (three trees).
Analysis of SA and MeSA contents in P. tomentosa after inoculation with B. dothidea
The SA extraction was based on a previous method49. Phloem samples were collected and frozen in liquid nitrogen. Prior to extraction, all of the materials were pulverised in liquid nitrogen using a mortar and pestle. Samples of 1.0 g were further homogenised using liquid nitrogen and transferred to 1.5-ml Eppendorf tubes. An aliquot (1 ml) of 80% methanol was added to the homogenate, and the samples were centrifuged for 5 min at 10,000 g in a centrifuge. The supernatant was collected in a 2-ml Eppendorf tube, and the centrifugation steps were repeated. The supernatants were combined and placed in a −20 °C refrigerator for 1 h. The supernatants were divided into two parts, one for determining the free SA and the other the SAG contents. Then, 2 mM HCl was added to the each of the supernatants, mixed and moved to sealed tubes. They were incubated in an 80 °C bath for 1 h, to transform SAG to free SA, to measure total SA. Then, 2 ml of 5% trichloroacetic acid (TCA; 5% solution in water) was added to the supernatants, which were partitioned with 3 ml of ethyl acetate:cyclohexane (1:1, v/v). This partitioning was carried out three times. The combined upper layers were evaporated to dryness in a SpeedVac concentrator. The samples were dissolved in 1 ml of 70% methanol. The C18 column was washed with 5 ml of 70% methanol, the sample was passed through the column, and the column was eluted with the addition of 3 ml of 70% methanol. Then, the wash eluates were combined and evaporated to dryness in a SpeedVac concentrator. After removal from the concentrator, 250 μl of the HPLC eluent was added to each tube, and the SA contents were analysed by HPLC. The SA analysis was performed on an HPLC (Waters 2695, USA) column, sunfire C18 (4.6 mm × 250 mm, i.d.: 0.45 μm). The eluent was 70% methanol, pH 3, at a flow rate of 0.80 ml/min, retention time of 10.943 min, and injection volume of 20 μl.
The MeSA extraction protocol was based on a previous method16. Samples were flash-frozen in liquid nitrogen and stored at −80 °C until analysis. After grinding to a fine powder in liquid nitrogen, 1 g of phloem tissue (fresh weight) was extracted twice with 5 ml of 80% ethanol, placed in a −4 °C refrigerator overnight and centrifuged for 30 min at 12,000 rpm. The supernatant was collected and condensed in a vacuum. Then, 4 ml of ethyl acetate was added to the supernatant and mixed by vortexing until the ethyl acetate phase was colourless. The sample was generated by collecting the ethyl acetate phase and distilling to remove ethyl acetate. The sample was detected by gas chromatography, in which the chromatogram peaks were evaluated using their retention times. The injection port and detector temperatures were set to 250 °C and 270 °C, respectively. The initial oven temperature was held at 50 °C for 1 min and was programmed to increase at 10 °C per min to 200 °C, and held for 30 min, before cycling to the initial conditions. Each treatment had three replicates.
PAL enzyme activity determinations in P. tomentosa after inoculation with B. dothidea
The PAL enzyme activity was determined using the methods described in The Modern Plant Physiology Experiment edited by the Chinese Academy of Sciences, Shanghai Institute of Plant Physiology50. Samples of 1.0 g from infected sites were ground to homogenates, then centrifuged for 20 min at 12,000 rpm at 4 °C. The supernatant fluid is required for the enzyme analysis. Then, 2 ml 0.1 M borate buffer (pH 8.8) and 1 ml 0.02 M L-phenylalanine were added to 1 ml enzyme liquid, and the OD value was immediately determined at 290 nm using an ultraviolet spectrophotometer. At a constant temperature of 37 °C, 5 M HCl was added to terminate the reaction after 1 h, and a second of OD290 value was determined. The enzyme activity unit is 0.01 of the OD change. Each treatment had three replicates.
Identification of the SAMT and SABP2 genes, which are putatively associated with resistance in P. tomentosa
The total RNA was isolated using an RNA Isolation Kit (BioTeke, Beijing, China) from stems that were or were not infected with B. dothidea according to the manufacturer’s instructions. The total RNA (2 µg) was reverse-transcribed into first-strand cDNA in a 20 µl reaction volume using a First-Strand cDNA Synthesis Kit (BioTeke). PtoSABP2 full-length cDNA was amplified from stem cDNA using the forward primer 5′-ATGGTAGAGACCAAGAATCAGGA-3′ and the reverse primer 5′-AGCATGTTTATTTGCTATCTCTGA-3′ from the sequences of the closely related species Populus trichocarpa. For PtoSAMT, the full-length cDNA was amplified from stem cDNA using the forward primer 5′-ATGGAGGTTGCTCAAGTGCTTCA-3′ and the reverse primer 5′-TCCCTTTCTAGTCACGGAAACAG-3′. The PCR proceeded as follows: 94 °C for 4 min, followed by 30 cycles of 94 °C for 30 s, 58 °C for 30 s and 72 °C for 1 min, and a final extension at 72 °C for 4 min. The PCR reaction was performed using hot start EX Taq polymerase (TaKaRa Bio, Japan), after which the product was ligated to the pEASY-T3 Cloning Vector and transformed into Trans1-T1 Phage Resistant Chemically Competent Cells (TransGen Biotech, Beijing, China). Clones were obtained and sequenced by Invitrogen. Phylogenetic trees of SAMT and SABP2 were constructed with homologous proteins from other species using MEGA51.
The expression analysis of poplar defence-related genes using RT-qPCR
The total RNA extractions from the infected stems of one-year-old poplar trees that were treated with B. dothidea and subsequent first-strand cDNA synthesis were performed essentially as for full-length cDNA cloning. The sequences of the five poplar defence-related genes, PR-1, -2, -5 and -10, and PAL, were submitted to the NCBI database by other researchers, and the cDNA samples were analysed by RT-qPCR using gene-specific primers (Table S1), The endogenous β-tubulin gene was used as a control because its expression remains relatively constant. Phylogenetic trees for PR-1, PR-2, PR-5, PR-10 and PAL were constructed with homologous genes of other species using MEGA (Fig. S1).
RT-qPCR amplification was performed using the SYBR Premix EX Taq Kit (TaKaRa) in a 20 µl reaction volume, using 2 µl of 20-fold diluted cDNA as a template, 200 nM universal RT-qPCR primer and 200 nM miRNA-specific forward primers. The reactions were conducted on a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using the TaKaRa-recommended cycling conditions. Each test had three experimental and two technical replicates.
Statistical analyses
Excel 2007 was used to statistically analyse the SA and MeSA contents, as well as the expression levels of SABP2, SAMT, PR and PAL. All of the data are presented as the means ± SD, and were evaluated using the unpaired two-tailed Student’s t test or Mann-Whitney U test, depending on the results of the normality and homogeneity tests conducted using SPSS 13.0 software52.
Electronic supplementary material
Acknowledgements
This project was financially supported by Fundamental Research Fund for Central Public Welfare Research Institute (CAFYBB2016SY037) and the National Key Research and Development Program (2017YFD0600102).
Author Contributions
Y.X.L., W.Z. and X.Y.Z. designed the article; Y.X.L. and W.Z. performed and analyzed the biological experiments; H.X.D. and J.M. analyzed the transcriptome of genes. Z.Y.L. and J.M. measured the S.A. and M.e.S.A., Y.X.L. and W.Z. wrote the article.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yong-xia Li and Wei Zhang contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32204-9.
References
- 1.Vlot AC, Klessig DF, Park S-W. Systemic acquired resistance: the elusive signal (s) Curr. Opin. Plant Biol. 2008;11:436–442. doi: 10.1016/j.pbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Ondrasek, G., Rengel, Z. & Veres, S. In Abiotic stress in plants-mechanisms and adaptations (InTech, 2011).
- 3.Jayakannan M, et al. The NPR1-dependent salicylic acid signalling pathway is pivotal for enhanced salt and oxidative stress tolerance in. Arabidopsis. J. Exp. Bot. 2015;66:1865–1875. doi: 10.1093/jxb/eru528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mithöfer, A. & Maffei, M. E. General mechanisms of plant defense and plant toxins. Plant Toxins, 3–24 (2017).
- 5.Di X, Gomila J, Takken FL. Involvement of salicylic acid, ethylene and jasmonic acid signalling pathways in the susceptibility of tomato to Fusarium oxysporum. Mol. Plant Pathol. 2017;18:1024–1035. doi: 10.1111/mpp.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan Y, et al. PtrWRKY73, a salicylic acid-inducible poplar WRKY transcription factor, is involved in disease resistance in. Arabidopsis thaliana. Plant Cell Rep. 2015;34:831–841. doi: 10.1007/s00299-015-1745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shadle GL, et al. Phenylpropanoid compounds and disease resistance in transgenic tobacco with altered expression of L-phenylalanine ammonia-lyase. Phytochemistry. 2003;64:153–161. doi: 10.1016/S0031-9422(03)00151-1. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, et al. Molecular cloning and promoter analysis of the specific salicylic acid biosynthetic pathway gene phenylalanine ammonia-lyase (AaPAL1) from Artemisia annua. Biotechnol. Appl. Biochem. 2016;63:514–524. doi: 10.1002/bab.1403. [DOI] [PubMed] [Google Scholar]
- 9.Métraux J, Signer H, Ryals J, Ward E, Wyss-Benz M. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science. 1990;250:1004. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- 10.Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu. Rev. Plant Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- 11.Tateda C, et al. Salicylic acid regulates Arabidopsis microbial pattern receptor kinase levels and signaling. The Plant Cell. 2014;26:4171–4187. doi: 10.1105/tpc.114.131938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlot AC, Dempsey DMA, Klessig DF. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- 13.Yan S, Dong X. Perception of the plant immune signal salicylic acid. Curr. Opin. Plant Biol. 2014;20:64–68. doi: 10.1016/j.pbi.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stahl E, et al. Regulatory and functional aspects of indolic metabolism in plant systemic acquired resistance. Molecular Plant. 2016;9:662–681. doi: 10.1016/j.molp.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Vernooij B, et al. Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance but is required in signal transduction. The Plant Cell. 1994;6:959–965. doi: 10.1105/tpc.6.7.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park S-W, Kaimoyo E, Kumar D, Mosher S, Klessig DF. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science. 2007;318:113–116. doi: 10.1126/science.1147113. [DOI] [PubMed] [Google Scholar]
- 17.Hilfiker O, et al. Insect eggs induce a systemic acquired resistance in Arabidopsis. The Plant Journal. 2014;80:1085–1094. doi: 10.1111/tpj.12707. [DOI] [PubMed] [Google Scholar]
- 18.Kumar D. Salicylic acid signaling in disease resistance. Plant Sci. 2014;228:127–134. doi: 10.1016/j.plantsci.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Forouhar F, et al. Structural and functional evidence for Bacillus subtilis PaiA as a novel N1-spermidine/spermine acetyltransferase. J. Biol. Chem. 2005;280:40328–40336. doi: 10.1074/jbc.M505332200. [DOI] [PubMed] [Google Scholar]
- 20.Kumar D, Klessig DF. High-affinity salicylic acid-binding protein 2 is required for plant innate immunity and has salicylic acid-stimulated lipase activity. Proceedings of the National Academy of Sciences. 2003;100:16101–16106. doi: 10.1073/pnas.0307162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar D, Gustafsson C, Klessig DF. Validation of RNAi silencing specificity using synthetic genes: salicylic acid‐binding protein 2 is required for innate immunity in plants. The Plant Journal. 2006;45:863–868. doi: 10.1111/j.1365-313X.2005.02645.x. [DOI] [PubMed] [Google Scholar]
- 22.Yu D, Liu Y, Fan B, Klessig DF, Chen Z. Is the high basal level of salicylic acid important for disease resistance in potato? Plant Physiol. 1997;115:343–349. doi: 10.1104/pp.115.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y, Qi M, Mei C. Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. The Plant Journal. 2004;40:909–919. doi: 10.1111/j.1365-313X.2004.02267.x. [DOI] [PubMed] [Google Scholar]
- 24.Clarke JD, Volko SM, Ledford H, Ausubel FM, Dong X. Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. The Plant Cell. 2000;12:2175–2190. doi: 10.1105/tpc.12.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wittek F, et al. Folic acid induces salicylic acid‐dependent immunity in Arabidopsis and enhances susceptibility to Alternaria brassicicola. Mol. Plant Pathol. 2015;16:616–622. doi: 10.1111/mpp.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chern M, Fitzgerald HA, Canlas PE, Navarre DA, Ronald PC. Overexpression of a rice NPR1 homolog leads to constitutive activation of defense response and hypersensitivity to light. Mol. Plant. Microbe Interact. 2005;18:511–520. doi: 10.1094/MPMI-18-0511. [DOI] [PubMed] [Google Scholar]
- 27.Iwai T, Seo S, Mitsuhara I, Ohashi Y. Probenazole-induced accumulation of salicylic acid confers resistance to Magnaporthe grisea in adult rice plants. Plant Cell Physiol. 2007;48:915–924. doi: 10.1093/pcp/pcm062. [DOI] [PubMed] [Google Scholar]
- 28.Zhao N, et al. Two poplar methyl salicylate esterases display comparable biochemical properties but divergent expression patterns. Phytochemistry. 2009;70:32–39. doi: 10.1016/j.phytochem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Crous PW, et al. Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology. 2006;55:235–253. doi: 10.3114/sim.55.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farr D, Rossman A, Palm M, McCray E. Fungal databases, systematic mycology and microbiology laboratory, ARS, USDA. Retrieved August. 2013;26:2013. [Google Scholar]
- 31.Zhang, X. & Luo, Y. Major forest diseases and insect pests in China. China Forestry Publication House, Beijing (in Chinese) (2003).
- 32.Koch JR, et al. Ozone sensitivity in hybrid poplar correlates with insensitivity to both salicylic acid and jasmonic acid. The role of programmed cell death in lesion formation. Plant Physiol. 2000;123:487–496. doi: 10.1104/pp.123.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han, X.-M. et al. Evolution and function of the Populus SABATH family reveal that a single amino acid change results in a substrate switch. Plant Cell Physiol. (2017). [DOI] [PubMed]
- 34.Dong H, Li Y, Lv Q, Jia X, Zhang X. Construction of salicylic acid methyltransferase gene over-expression vectors and genetic transformation in poplar (Populus alba × P. glandulosa) Journal of Agricultural Biotechnology. 2016;24:1709–1717. [Google Scholar]
- 35.Chen Z, Silva H, Klessig DF. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science-AAAS-Weekly Paper Edition-including Guide to Scientific Information. 1993;262:1883–1885. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- 36.Casano LM, Martın M, Sabater B. Hydrogen peroxide mediates the induction of chloroplastic Ndh complex under photooxidative stress in barley. Plant Physiol. 2001;125:1450–1458. doi: 10.1104/pp.125.3.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W-P, et al. Impact of salicylic acid on the antioxidant enzyme system and hydrogen peroxide production in Cucumis sativus under chilling stress. Zeitschrift für Naturforschung C. 2011;66:413–422. doi: 10.1515/znc-2011-7-814. [DOI] [PubMed] [Google Scholar]
- 38.Manosalva PM, et al. Methyl esterase 1 (StMES1) is required for systemic acquired resistance in potato. Mol. Plant. Microbe Interact. 2010;23:1151–1163. doi: 10.1094/MPMI-23-9-1151. [DOI] [PubMed] [Google Scholar]
- 39.Elkind Y, et al. Abnormal plant development and down-regulation of phenylpropanoid biosynthesis in transgenic tobacco containing a heterologous phenylalanine ammonia-lyase gene. Proceedings of the National Academy of Sciences. 1990;87:9057–9061. doi: 10.1073/pnas.87.22.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dehghan, S. et al. Differential inductions of phenylalanine ammonia-lyase and chalcone synthase during wounding, salicylic acid treatment, and salinity stress in safflower, Carthamus tinctorius. Biosci. Rep. 34, 10.1042/bsr20140026 (2014). [DOI] [PMC free article] [PubMed]
- 41.Liu X, Rockett KS, Kørner CJ, Pajerowska-Mukhtar KM. Salicylic acid signalling: new insights and prospects at a quarter-century milestone. Essays Biochem. 2015;58:101–113. doi: 10.1042/bse0580101. [DOI] [PubMed] [Google Scholar]
- 42.Tripathi D, Jiang Y, Kumar D. SABP2, a methyl salicylate esterase is required for the systemic acquired resistance induced by acibenzolar-S-methyl in plants. FEBS Lett. 2010;584:3458–3463. doi: 10.1016/j.febslet.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 43.Sels J, Mathys J, De Coninck BM, Cammue BP, De Bolle MF. Plant pathogenesis-related (PR) proteins: a focus on PR peptides. Plant Physiol. Biochem. 2008;46:941–950. doi: 10.1016/j.plaphy.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Park CJ, et al. Pathogenesis-related protein 10 isolated from hot pepper functions as a ribonuclease in an antiviral pathway. The Plant Journal. 2004;37:186–198. doi: 10.1046/j.1365-313X.2003.01951.x. [DOI] [PubMed] [Google Scholar]
- 45.Bagnoli F, et al. Molecular cloning, characterisation and expression of a manganese superoxide dismutase gene from peach (Prunus persica [L.] Batsch) Mol. Genet. Genomics. 2002;267:321–328. doi: 10.1007/s00438-002-0664-7. [DOI] [PubMed] [Google Scholar]
- 46.Smirnov S, Shulaev V, Tumer NE. Expression of pokeweed antiviral protein in transgenic plants induces virus resistance in grafted wild-type plants independently of salicylic acid accumulation and pathogenesis-related protein synthesis. Plant Physiol. 1997;114:1113–1121. doi: 10.1104/pp.114.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Obara N, Hasegawa M, Kodama O. Induced volatiles in elicitor-treated and rice blast fungus-inoculated rice leaves. Biosci. Biotechnol. Biochem. 2002;66:2549–2559. doi: 10.1271/bbb.66.2549. [DOI] [PubMed] [Google Scholar]
- 48.Huang J, et al. Differential volatile emissions and salicylic acid levels from tobacco plants in response to different strains of Pseudomonas syringae. Planta. 2003;217:767–775. doi: 10.1007/s00425-003-1039-y. [DOI] [PubMed] [Google Scholar]
- 49.Verberne MC, et al. Method for the extraction of the volatile compound salicylic acid from tobacco leaf material. Phytochem. Anal. 2002;13:45–50. doi: 10.1002/pca.615. [DOI] [PubMed] [Google Scholar]
- 50.Ren S-C, Sun J-T. Changes in phenolic content, phenylalanine ammonia-lyase (PAL) activity, and antioxidant capacity of two buckwheat sprouts in relation to germination. Journal of Functional Foods. 2014;7:298–304. doi: 10.1016/j.jff.2014.01.031. [DOI] [Google Scholar]
- 51.Tamura K, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green, S. B. & Salkind, N. J. Using SPSS for Windows and Macintosh: Analyzing and understanding data. (Prentice Hall Press, 2010).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.