Abstract
Osteoarthritis is a painful joint disease characterized by progressive degeneration of the articular cartilage as well as associated changes to the subchondral bone, synovium, and surrounding joint tissues. While the effects of osteoarthritis on the cartilage extracellular matrix (ECM) have been well recognized, it is now becoming apparent that in many cases, the onset of the disease may be initially reflected in the matrix region immediately surrounding the chondrocytes, termed the pericellular matrix (PCM). Growing evidence suggests that the PCM – which along with the enclosed chondrocytes are termed the “chondron” – acts as a critical transducer or “filter” of biochemical and biomechanical signals for the chondrocyte, serving to help regulate the homeostatic balance of chondrocyte metabolic activity in response to environmental signals. Indeed, it appears that alterations in PCM properties and cell-matrix interactions, secondary to genetic, epigenetic, metabolic, or biomechanical stimuli, could in fact serve as initiating or progressive factors for osteoarthritis. Here, we discuss recent advances in the understanding of the role of the PCM, with an emphasis on the reciprocity of changes that occur in this matrix region with disease, as well as how alterations in PCM properties could serve as a driver of ECM-based diseases such as osteoarthritis. Further study of the structure, function, and composition of the PCM in normal and diseased conditions may provide new insights into the understanding of the pathogenesis of osteoarthritis, and presumably new therapeutic approaches for this disease.
Keywords: Chondron, chondrocyte, type VI collagen, perlecan, aggrecan, osteoarthritis, territorial matrix, decorin, mechanobiology, mechanotransduction, extracellular matrix, intervertebral disc, meniscus
Introduction
Under normal physiologic circumstances, articular cartilage functions as a nearly frictionless surface while exposed to loads of several times body weight. This remarkable function is attributed to the unique structure and composition that determine the mechanical properties of the cartilage extracellular matrix (ECM). The ECM of articular cartilage is primarily water (60–85% by wet weight). The remaining solid matrix is composed of a crosslinked network of type II collagen (15–22% by wet weight), proteoglycans (4–7% by wet weight), and lesser amounts of several important other collagens (e.g., VI, IX, X, XI) and non-collagenous proteins [1, 2]. The constituents of articular cartilage are organized in a complex porous and permeable ECM that provides the unique capabilities for fluid-pressurization that allow for the long-term load-bearing capabilities of the joint. Under pathologic conditions, such as osteoarthritis, however, the ECM exhibits a myriad of changes in its mechanical function that are associated with increased catabolic activity and inflammation in the joint. In this regard, the role of the ECM in osteoarthritis has been extensively studied and reported in several previous reviews [3–8].
The chondron: The chondrocyte and its pericellular matrix
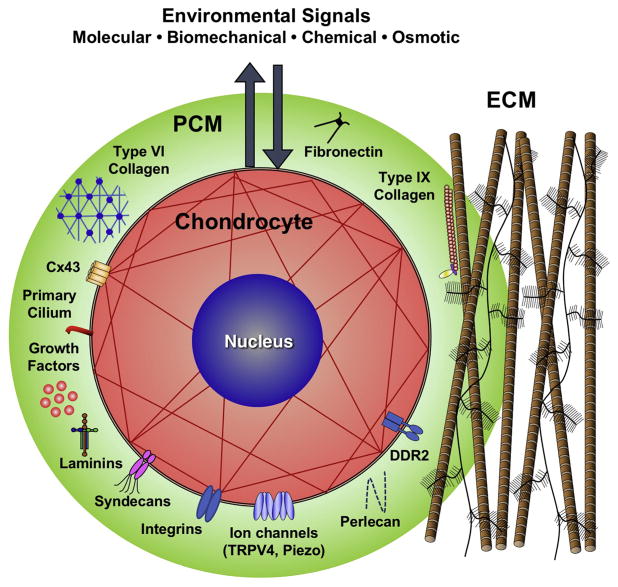
The ECM changes in osteoarthritis appear to be driven by an imbalance of anabolic and catabolic activities of the chondrocytes, the cell population within articular cartilage. Within the cartilage ECM, chondrocytes are surrounded by a narrow matrix region that is compositionally and structurally distinct from surrounding bulk ECM. This unique region is approximately 2 to 4 μm thick and is called the “pericellular matrix” (PCM) (Figure 1). The PCM then integrates with the surrounding tissue via the “territorial matrix,” connecting the PCM to the “interterritorial matrix” (i.e., the ECM). Together, the chondrocyte and its PCM have been termed the “chondron” [9–12]. This name was derived from “chondrone”, which was coined by Benninghoff in the early 19th century when he observed the altered matrix structure around chondrocytes via polarized light [13]. However, little direct research on the PCM was reported until it was discovered that intact chondrons could be retrieved as a by-product of cartilage homogenization [14]. Using this method, a number of seminal studies were performed by Poole and co-workers to provide the first reports on the composition, metabolic activity, and structure of the PCM [11, 15]. Chondrons were found to contain large amounts of proteoglycans and collagen types II, VI, and IX. Further examination showed that the PCM can be defined primarily by the presence of type VI collagen, fibronectin 1, and the proteoglycans perlecan and biglycan [11, 15–17].
Figure 1. Schematic of the chondrocyte, which together with its surrounding pericellular matrix (PCM) forms a chondron, embedded within the cartilage ECM.
The PCM is rich in proteoglycans such as perlecan and is characterized by the presence of collagens type VI and IX, as well as several other matrix macromolecules (fibronectin, laminin, etc.). Because the PCM surrounds the chondrocyte, it is believed to serve as a transducer, or “filter”, of the biomechanical milieu through regulation of the stress-strain, osmotic, and fluid-flow environments of the chondrocyte. In addition to this mechanobiologic role, the PCM can regulate cell-ECM ligand binding, growth factor and enzyme sequestration, transport, assembly, and activation, thus influencing major aspects of ECM turnover in cartilage
The function of the PCM and chondron
Significant evidence is now accumulating on the important role of the PCM (and chondron) in regulating the function of the chondrocyte (reviewed in [18, 19]). As every chondrocyte is surrounded by this tissue region, any chemical or physical signals that the chondrocyte perceives may be modulated by the PCM. Although the complete role of the PCM remains to be elucidated, it is apparent that the PCM can serve as a transducer, or “filter,” of both biomechanical and biochemical signals for the chondrocyte [20–23] (Figure 1). Data from a variety of theoretical models and experimental studies of the PCM and cell-matrix interactions indicate that the presence and properties of the PCM can regulate mechanical and physiochemical environments in cartilage, influencing chondrocyte physiology and cartilage ECM homeostasis [24–38]. By modulating the stress-strain, osmotic, and fluid-flow environments of the chondrocyte, the PCM is believed to serve as an additional regulator of the chondrocyte mechanotransduction process [39–41].
In addition to these biomechanical and mechanobiologic roles, the PCM can regulate cell-matrix ligand binding, growth factor and enzyme sequestration, transport, assembly, and activation, thus influencing major aspects of ECM turnover in cartilage [42, 43]. The presence of a PCM significantly influences chondrocyte gene expression and response to mechanical loading [44–46], while PCM retention and sequestration of various growth factors may play an important role in regulating chondrocyte activity [21, 47–49]. The critical role of the PCM has been recently highlighted in a review of the various protective effects that it may have against the development of osteoarthritis [19]. Thus, it is apparent that alterations in PCM properties, secondary to genetic, epigenetic, metabolic, or biomechanical stimuli, could in fact serve as initiating or progressive factors of osteoarthritis.
The mechanical properties of the pericellular matrix
Over the past two decades, a variety of techniques have been pioneered to quantify the biomechanical and physical properties of the PCM, using either mechanically or enzymatically isolated chondrons, or in situ testing methods that combine experimental microscopy and computational modeling (reviewed in [18]). For example, physically extracted chondrons have been tested using osmotic swelling [25, 34], deformation within hydrogels [33, 34, 50], or individual chondron testing using compression [51–53] and micropipette aspiration [54–58]. More recently, methods have been developed for the direct quantification of PCM properties in situ through the application of osmotic swelling and 3D confocal microscopy [59] or atomic force microscopy (AFM)-based microindentation [60–67]. With this method, AFM-stiffness mapping showed comparable values for PCM properties as compared to other methods such micropipette aspiration [55] or combined computational modeling and 3D microscopy [68]. These studies have shown that the moduli of the PCM (Young’s moduli, EY ~ 40 – 100 kPa) are two orders of magnitude greater than those of chondrocytes (EY ~ 0.5 kPa) [54, 69] but as much as an order of magnitude lower than those of the surrounding ECM, depending on the zone of cartilage (aggregate modulus, HA ~ 0.1 – 2 MPa) [70]. With the growing prevalence of mouse models in cartilage and osteoarthritis research, recent studies have shown that the Young’s moduli of murine PCM (300 – 1000 kPa) is much higher than that of human PCM, while the surrounding ECM (0.5 – 2 MPa) is similar human values [59, 71]. These differences in mechanical moduli implicate the PCM as a crucial transducer or filter of mechanical signals to the chondrocyte.
The PCM in osteoarthritis
In healthy articular cartilage, the ECM is maintained in a slow, continuous state of turnover – often described as “homeostasis” – a balance of overall anabolic and catabolic activities of matrix synthesis and degradation. These activities are tightly controlled by the environmental signals (including both biochemical and biomechanical cues) through regulating genetic and epigenetic programming of the chondrocytes. As a transitional zone between the interterritorial matrix and chondrocytes, the PCM, which has a much higher proteoglycan turnover rate [72], can modulate these environmental signals before they reach the chondrocytes and thus play a key role in chondrocyte gene expression and epigenetic state. Consequently, in a pathologic state such as osteoarthritis, changes to PCM properties may not only reflect the disease state but may also influence the regulatory function of PCM and thus chondrocyte activity. Therefore, further study of the PCM remodeling with osteoarthritis may provide new insights into understanding the etiology and pathogenesis of the disease.
The first studies of osteoarthritic changes in PCM structure and composition reported on human and canine chondron morphology, viability, and metabolism. This work demonstrated that early changes in the collagen and proteoglycan distribution within the chondron precede chondrocyte proliferation and cell cluster formation [73]. Osteoarthritic cartilage also exhibited chondron swelling and chondrocyte cluster formation, with a loss of pericellular type IX collagen staining [74] and the prevalence of enlarged chondrons with “loosely-organized” PCM structures [73, 75, 76]. Osteoarthritic cartilage was also associated with the appearance of a sub-population of chondrocytes with multiple elongated cytoplasmic processes [77]. Indeed, using confocal microscopy, it has been observed that some of these cytoplasmic processes were longer than 8 μm, radiating beyond the PCM and extending into the territorial matrix [78, 79]. Together these seminal studies suggest that concomitant loss of PCM structure, composition, and mechanical function are present in osteoarthritis.
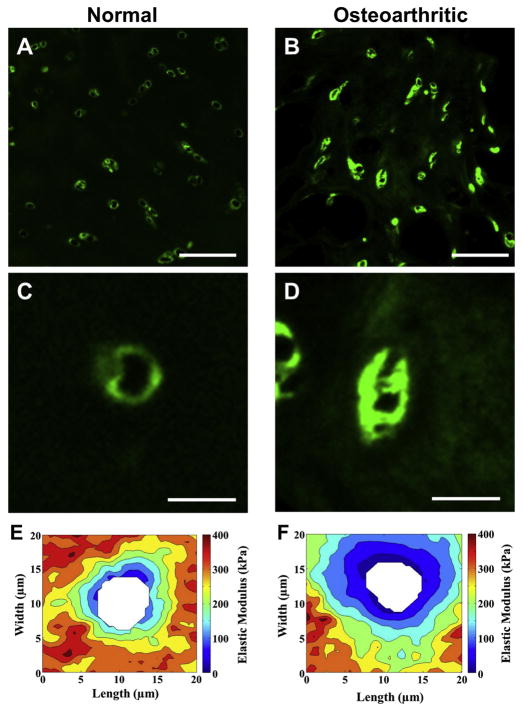
Interestingly, several other reports also identified early changes in PCM composition with osteoarthritis. In human cartilage with minor osteoarthritic lesions, focal pericellular deposition of collagens I and III was observed, while at more advanced stages of disease, extensive changes were seen in collagen expression in the PCM, with overlapping localization of collagens I, II and III [80]. More recent studies have also shown the presence of type I collagen in the PCM with osteoarthritis [81]. The protein collagen VI, as a primary component of the PCM, has also been shown to be increased with osteoarthritis [82, 83] (Figure 2), showing zone-dependent changes in expression and immunolabeling [84–86]. Moreover, differential mRNA expression analyses of preserved and lesioned articular cartilage of patients undergoing joint replacement surgery due to osteoarthritis show highly significant upregulated expression of collagen type VI with osteoarthritis pathophysiology [87].
Figure 2. Alterations in the morphology and mechanical properties of the PCM with osteoarthritis.
(A, B) Representative images of immunofluorescence labeling of type VI collagen in cartilage from (A) macroscopically normal and (B) osteoarthritic knee joints. Scale bar = 100 μm (C, D) Immunofluorescence labeling revealed altered structure and expanded regions that were positive for type VI collagen in the PCM of osteoarthritic cartilage. Scale bar = 25 μm. (E, F) Elastic mapping of the moduli of the ECM and PCM, from normal (E) and osteoarthritic (F) cartilage, showing a loss of mechanical properties with osteoarthritis. Modulus maps are presented on the same graded coloring scale, and cell-sized voids are depicted in white [Adapted from [65], with permission].
These arthritic changes in PCM composition can significantly affect mechanotransduction in chondrocytes, partly through chondrocyte’s primary cilium, a single cellular organelle that projects from the cell surface into the PCM. The primary cilium has recently been recognized as a potential mechanotransducer of the chondrocyte due to its capacity to interact with matrix proteins such as collagens type II and VI through the receptors including integrins and chondroitin sulfate proteoglycan 4 (also called neuron-glial antigen 2, NG2) [88, 89]. In addition to matrix protein receptors, several putative mechanosensors, including connexin 43 and a variety of ion channels such as the transient receptor potential vanilloid 4 (TRPV4) are expressed on the primary cilia of the chondrocytes [90]. While connexin 43 is a mechanosensitive adenosine 5′-triphosphate (ATP)-release hemichannel [91] found in chondrocytes [92], TRPV4 induces intracellular Ca2+ signaling cascades in response to osmotic or mechanical stimuli [90]. Furthermore, it has been reported that both cilia length and incidence (i.e., overall percentage of ciliated-chondrocytes in cartilage) increase with osteoarthritis severity, implying an altered cilia-mediated signaling in degenerated cartilage [93].
Other PCM molecules, such as nidogens and laminins, are also modulated with disease and appear to influence the calcification process of chondrocytes in osteoarthritis through the reciprocal regulation of RUNX2 and SOX9 [94]. Interestingly, the PCM-specific localization of laminins α5 and β1 was reported to be lost in aged, disrupted cartilage while laminin α1 and perlecan were robustly withheld within the PCM in old mice [16]. These points underpin the complex and delicate homeostatic balance maintained by the PCM in presenting biomechanical signals to the chondrocytes.
Indeed, it has been suggested that degradation of PCM structure may be one of the earliest events during osteoarthritis onset due to the observation of elevated serine proteases, such as high-temperature requirement A serine peptidase 1 (HtrA1), in the synovial fluid from the osteoarthritis patients [95]. HtrA1 has the capacity to digest several major PCM components including aggrecan, decorin, fibromodulin, fibronectin, and biglycan, leading to the chondrocyte’s exposure to type II collagen fibrils, which is more highly expressed in ECM compared to the PCM. Increased interaction of type II collagen fibrils with cell surface receptors, potentially through discoidin domain receptor 2 (DDR2), may alter metabolic activity and intracellular signaling cascades in chondrocytes [19, 96–98]. For example, there is mounting evidence showing that binding of DDR2 to type II collagen up-regulates production of matrix metalloproteinase (MMP)-13 in chondrocytes [99], which in turn degrades type II collagen in cartilage matrix, suggesting a potential axis of HtrA1-DDR2-MMP13 degradative pathway in osteoarthritis development [100].
Moreover, because the PCM also serves as a repository for a variety of growth factors and regulatory molecules, the disruption of PCM structure, either due to mechanical injury or by proteolytic activity, may trigger the release of these modulatory proteins, which can function in an autocrine or paracrine manner. For instance, transforming growth factor (TGF)-β is normally sequestered by fibrillin and fibulin in the PCM; however, increased release and activation of TGF-β was observed in injured articular cartilage [41, 101, 102]. It has also been reported that both biglycan and syndecan, a family of transmembrane heparan sulfate proteoglycans, play a critical role in modulating Wnt signaling and phenotypic changes of chondrocyte in osteoarthritis [103–106]. Similarly, perlecan, a PCM-localized proteoglycan [107], modulates fibroblast growth factor (FGF) presentation and binding near chondrocytes through heparin sulfate substitutions [21], altering the proliferation and metabolism of chondrocytes in response to injury.
Abnormal cartilage matrix turnover in osteoarthritis is not only associated with elevated levels of MMPs but also with increased production of aggrecanases such as ADAMTS4 and 5 (a disintegrin and metalloproteinase with thrombospondin motifs) [108, 109], often occurring secondary to the action of pro-inflammatory cytokines such as interleukin 1 (IL-1) [110]. Furthermore, remodeling of the PCM in osteoarthritic cartilage appears to modify the response of chondrocytes to soluble mediators and matrix proteins [42, 111, 112]. For example, when articular cartilage is degraded following exposure to IL-1, hyaluronic acid (HA) penetrates the cartilage and accumulates in the chondrocyte PCM [113]. In collagen-induced arthritis mouse model, high levels of the aggrecan neo-epitopes, NITEGE (generated by aggrecanases [114]) and VDIPEN (generated by MMPs [115]), are present initially in the chondrocyte PCM, suggesting that stimulated chondrocytes can synthesize and/or activate both matrix-degrading enzymes [116]. Interestingly, it has been reported that NITEGE and VDIPEN are predominantly generated at different zonal regions in healthy tibial cartilage. However, once the spontaneous lesions develop in STR/ort mice, both neo-epitopes co-localize at the PCM and further extend to interterritorial matrices of chondrocytes adjacent to osteoarthritic lesions when the disease advances [117]. In human osteoarthritic cartilage, ADAMTS5 was present in association with cells throughout normal cartilage and was markedly increased in osteoarthritis, particularly in clonal groups in the superficial and transitional zones, where it was predominantly co-localized with HA in the PCM. HA-dependent sequestration of ADAMTS5 in the PCM may be a mechanism for regulating the activity of this proteinase in human osteoarthritis cartilage [118].
Interestingly, despite harboring several matrix-degrading enzymes in osteoarthritis, the PCM appears to possess a certain level of resistance to enzymatic degradation. For example, targeted digestion of articular cartilage with aggrecanase-1 (ADAMTS4), bacterial hyaluronidase, or chondroitinase ABC demonstrated that PCM mechanical properties exhibit high resistance to aggrecan-targeted digestion, despite significant degradative effects on the properties of the cartilage ECM [66]. This resistance to enzymatic digestion may provide a mechanism for enzyme transport from the chondrocyte to the surrounding ECM during normal matrix turnover without mechanical disruption of the PCM.
The mechanical properties of the PCM are strongly associated with its biochemical and structural changes. Using different micromechanical testing methods such as micropipette aspiration or AFM indentation, several studies have revealed that the elastic modulus of the PCM is reduced by 30–50% in osteoarthritic cartilage [55, 56, 65] (Figure 2). Conversely, alterations in PCM properties due to loss of type VI collagen in Col6a1−/− mice can accelerate the progression of osteoarthritis in the hip [57] in a joint-specific manner [119]. The loss of PCM properties is accompanied by altered calcium signaling in chondrocytes and increased cell swelling in response to osmotic stress [59]. Deletion of type IX collagen, which is more concentrated in the PCM [120, 121], has been shown to accelerate osteoarthritis progression [96] as well as intervertebral disc degeneration [122], potentially through the exposure and activation of DDR2 in chondrocytes [42, 96, 97]. Of particular interest is the discovery of a genome-wide association between polymorphisms in collagen type VI alpha 4 pseudogene 1 (COL6A4P1, also known as DVWA) with increased risk of knee osteoarthritis, indicating a potential role for alterations in pericellular collagen VI in the initiation of disease [123, 124]. These studies demonstrate the potential for a complex and interrelated role of the PCM as both an indicator as well as a potentiating factor in osteoarthritis and suggest additional studies of gene polymorphisms or mutations in PCM proteins as targets for osteoarthritis research.
In a similar manner to genetic predispositions to osteoarthritis, the PCM responds to injurious mechanical loading through both matrix turnover and mechanical softening [98, 125]. Proteomic analysis of an in vitro cartilage injury model demonstrates elevated loss of collagen type VI during mechanical injury [98]. Similarly, a recent study which used the murine destabilized medial meniscus (DMM) to model traumatic joint injury showed a dramatic decrease in mechanical modulus of the PCM [98, 125]. In addition to direct cellular mechanotransduction, growth factor sequestration in the PCM may serve to modulate chondrocyte response to injury. As noted earlier, perlecan, which is localized to the PCM, can modulate the activity of FGF on chondrocytes. Through deletion of the domain 1 heparan sulfate (HS) in perlecan, Shu and coworkers reported that the progressive degeneration of articular cartilage from DMM-injury could be partially rescued. They concluded that this chondroprotective could be attributed, at least in part, to the preservation of FGF signaling, providing further evidence of the complex interaction between cellular signaling and PCM mechanosensitivity [126]. Future studies to investigate whether mechanical loading either alters the PCM indirectly through chondrocyte-PCM interactions or directly remodels the PCM may establish the role of the PCM in trauma-induced osteoarthritis.
Conclusions
While the role of the cartilage ECM in osteoarthritis has been extensively studied, growing evidence suggests that many of the characteristics and influences of osteoarthritis are present – and possibly initiated – in the PCM. As the primary connection between the chondrocyte and the cartilage ECM, newly synthesized matrix components, enzymes, and growth factors will initially pass through the PCM. Furthermore, the important role that the PCM plays in modulating environmental signals makes it highly sensitive to changes that occur with degradation or osteoarthritis. Thus, further investigation of roles of individual PCM components, how they contribute to chondrocyte mechanotransduction, and how they may serve as potential biomarkers of disease could help to elucidate factors contributing to the progression of osteoarthritis, as well as degeneration changes in other connective tissues such as the meniscus or intervertebral disc that possess PCM-like structures rich in type VI collagen [127, 128].
Highlights.
The PCM surrounds all chondrocytes in articular cartilage and regulates their interactions with the environment
Alterations in PCM properties and composition will influence their mechanobiologic response to loading
Some of the earliest biosynthetic and degradative changes in osteoarthritis may initially manifest in the PCM
Here we review the potential role of PCM pathology as a potential driver, as well as indicator, of osteoarthritis
Acknowledgments
This work was supported in part by the Arthritis Foundation, the Nancy Taylor Foundation for Chronic Diseases, the National Science Foundation (EAGER Award), Dutch Arthritis Association (DAA_10_1-402, DAF-16-1-405, DAF- 15-4-401), the Dutch Scientific Research Council (Grant 91816631/528), and National Institutes of Health grants (AG15768, AR48182, AR50245, AR48852, AG46927, T32 DK108742, T32 EB018266, and P30 AR057235).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30–5. doi: 10.1186/ar380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinegard D. Fell-Muir Lecture: Proteoglycans and more--from molecules to biology. Int J Exp Pathol. 2009;90(6):575–86. doi: 10.1111/j.1365-2613.2009.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eyre DR. Collagens and cartilage matrix homeostasis. Clinical orthopaedics and related research. 2004;(427 Suppl):S118–22. doi: 10.1097/01.blo.0000144855.48640.b9. [DOI] [PubMed] [Google Scholar]
- 4.Gouttenoire J, Valcourt U, Ronziere MC, Aubert-Foucher E, Mallein-Gerin F, Herbage D. Modulation of collagen synthesis in normal and osteoarthritic cartilage. Biorheology. 2004;41(3–4):535–42. [PubMed] [Google Scholar]
- 5.Heinegard D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nature reviews Rheumatology. 2011;7(1):50–6. doi: 10.1038/nrrheum.2010.198. [DOI] [PubMed] [Google Scholar]
- 6.Henrotin Y, Sanchez C, Bay-Jensen AC, Mobasheri A. Osteoarthritis biomarkers derived from cartilage extracellular matrix: Current status and future perspectives. Annals of physical and rehabilitation medicine. 2016;59(3):145–8. doi: 10.1016/j.rehab.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Maldonado M, Nam J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. BioMed research international. 2013;2013:284873. doi: 10.1155/2013/284873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahmati M, Nalesso G, Mobasheri A, Mozafari M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing research reviews. 2017;40:20–30. doi: 10.1016/j.arr.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Benninghoff A. Form und bau der Gelenkknorpel in ihren Beziehungen Zur Funktion. Zweiter Teil: der Aufbau des Gelenkknorpels in sienen Bezienhungen zur Funktion. 1925;2:783–862. [Google Scholar]
- 10.Szirmai JA. Structure of Cartilage. In: Engel A, Larsson T, editors. Aging of Connective and Skeletal Tissue. Nordiska Bokhandelns Forlag; Stockholm: 1968. pp. 163–184. [Google Scholar]
- 11.Poole CA, Flint MH, Beaumont BW. Chondrons in cartilage: ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 1987;5(4):509–22. doi: 10.1002/jor.1100050406. [DOI] [PubMed] [Google Scholar]
- 12.Vanden Berg-Foels WS, Scipioni L, Huynh C, Wen X. Helium ion microscopy for high-resolution visualization of the articular cartilage collagen network. J Microsc. 2012;246(2):168–76. doi: 10.1111/j.1365-2818.2012.03606.x. [DOI] [PubMed] [Google Scholar]
- 13.Benninghoff A. Form und bau der Gelenkknorpel in ihren Beziehungen Zur Funktion. In: Zellforsch Z, editor. Zweiter Teil: der Aufbau des Gelenkknorpels in sienen Bezienhungen zur Funktion. 1925. p. 783. [Google Scholar]
- 14.Szirmai JA. The concept of the chondron as a biomechanical unit. In: Hartmann F, editor. Biopolymer und Biomechanik von Bindegewebssystemen. Academic Press; Berlin: 1974. p. 87. [Google Scholar]
- 15.Poole CA, Ayad S, Schofield JR. Chondrons from articular cartilage: I. Immunolocalization of type VI collagen in the pericellular capsule of isolated canine tibial chondrons. Journal of Cell Science. 1988;90(Pt 4):635–43. doi: 10.1242/jcs.90.4.635. [DOI] [PubMed] [Google Scholar]
- 16.Kvist AJ, Nystrom A, Hultenby K, Sasaki T, Talts JF, Aspberg A. The major basement membrane components localize to the chondrocyte pericellular matrix--a cartilage basement membrane equivalent? Matrix biology: journal of the International Society for Matrix Biology. 2008;27(1):22–33. doi: 10.1016/j.matbio.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Miosge N, Flachsbart K, Goetz W, Schultz W, Kresse H, Herken R. Light and electron microscopical immunohistochemical localization of the small proteoglycan core proteins decorin and biglycan in human knee joint cartilage. Histochem J. 1994;26(12):939–45. [PubMed] [Google Scholar]
- 18.Wilusz RE, Sanchez-Adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix biology: journal of the International Society for Matrix Biology. 2014;39:25–32. doi: 10.1016/j.matbio.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C, Wang B, Xiao L, Li Y, Xu L, Zhao Z, Zhang L. Protective effects of the pericellular matrix of chondrocyte on articular cartilage against the development of osteoarthritis. Histology and histopathology. 2018:11967. doi: 10.14670/HH-11-967. [DOI] [PubMed] [Google Scholar]
- 20.Guilak F, Alexopoulos LG, Upton ML, Youn I, Choi JB, Cao L, Setton LA, Haider MA. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Annals of the New York Academy of Sciences. 2006;1068:498–512. doi: 10.1196/annals.1346.011. [DOI] [PubMed] [Google Scholar]
- 21.Vincent TL, McLean CJ, Full LE, Peston D, Saklatvala J. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthritis and cartilage. 2007;15(7):752–63. doi: 10.1016/j.joca.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Macri L, Silverstein D, Clark RA. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Advanced drug delivery reviews. 2007;59(13):1366–81. doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Tambe DT, Deng L, Yang L. Biomechanical properties and mechanobiology of the articular chondrocyte. American journal of physiology. Cell physiology. 2013;305(12):C1202–8. doi: 10.1152/ajpcell.00242.2013. [DOI] [PubMed] [Google Scholar]
- 24.Guilak F, Mow VC. The mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions in articular cartilage. Journal of biomechanics. 2000;33(12):1663–73. [PubMed] [Google Scholar]
- 25.Haider MA, Schugart RC, Setton LA, Guilak F. A mechano-chemical model for the passive swelling response of an isolated chondron under osmotic loading. Biomechanics and modeling in mechanobiology. 2006;5(2–3):160–71. doi: 10.1007/s10237-006-0026-1. [DOI] [PubMed] [Google Scholar]
- 26.Halloran JP, Sibole S, van Donkelaar CC, van Turnhout MC, Oomens CW, Weiss JA, Guilak F, Erdemir A. Multiscale mechanics of articular cartilage: potentials and challenges of coupling musculoskeletal, joint, and microscale computational models. Annals of biomedical engineering. 2012;40(11):2456–74. doi: 10.1007/s10439-012-0598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu JZ, Herzog W. Finite element simulation of location- and time-dependent mechanical behavior of chondrocytes in unconfined compression tests. Annals of biomedical engineering. 2000;28(3):318–30. doi: 10.1114/1.271. [DOI] [PubMed] [Google Scholar]
- 28.Alexopoulos LG, Setton LA, Guilak F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta biomaterialia. 2005;1(3):317–25. doi: 10.1016/j.actbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Michalek AJ, Iatridis JC. A numerical study to determine pericellular matrix modulus and evaluate its effects on the micromechanical environment of chondrocytes. Journal of biomechanics. 2007;40(6):1405–9. doi: 10.1016/j.jbiomech.2006.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korhonen RK, Herzog W. Depth-dependent analysis of the role of collagen fibrils, fixed charges and fluid in the pericellular matrix of articular cartilage on chondrocyte mechanics. Journal of biomechanics. 2008;41(2):480–5. doi: 10.1016/j.jbiomech.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Mow VC, Bachrach N, Setton LA, Guilak F. Stress, strain, pressure, and flow fields in articular cartilage. In: Mow VC, Guilak F, Tran-Son-Tay R, Hochmuth R, editors. Cell Mechanics and Cellular Engineering. Springer Verlag; New York: 1994. pp. 345–79. [Google Scholar]
- 32.Khoshgoftar M, Torzilli PA, Maher SA. Influence of the pericellular and extracellular matrix structural properties on chondrocyte mechanics. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2018;36(2):721–729. doi: 10.1002/jor.23774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knight MM, Lee DA, Bader DL. The influence of elaborated pericellular matrix on the deformation of isolated articular chondrocytes cultured in agarose. Biochimica et biophysica acta. 1998;1405(1–3):67–77. doi: 10.1016/s0167-4889(98)00102-5. [DOI] [PubMed] [Google Scholar]
- 34.Hing WA, Sherwin AF, Poole CA. The influence of the pericellular microenvironment on the chondrocyte response to osmotic challenge. Osteoarthritis and cartilage. 2002;10(4):297–307. doi: 10.1053/joca.2002.0517. [DOI] [PubMed] [Google Scholar]
- 35.Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108(Pt 4):1497–508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 36.Choi JB, Youn I, Cao L, Leddy HA, Gilchrist CL, Setton LA, Guilak F. Zonal changes in the three-dimensional morphology of the chondron under compression: the relationship among cellular, pericellular, and extracellular deformation in articular cartilage. Journal of biomechanics. 2007;40(12):2596–603. doi: 10.1016/j.jbiomech.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sibole SC, Erdemir A. Chondrocyte deformations as a function of tibiofemoral joint loading predicted by a generalized high-throughput pipeline of multi-scale simulations. PloS one. 2012;7(5):e37538. doi: 10.1371/journal.pone.0037538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. Journal of biomechanical engineering. 2000;122(3):252–60. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 39.Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, McNulty AL, Wu J, Beicker KN, Coles J, Zauscher S, Grandl J, Sachs F, Guilak F, Liedtke WB. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(47):E5114–22. doi: 10.1073/pnas.1414298111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark AL, Votta BJ, Kumar S, Liedtke W, Guilak F. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis and rheumatism. 2010;62(10):2973–83. doi: 10.1002/art.27624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vincent TL. Targeting mechanotransduction pathways in osteoarthritis: a focus on the pericellular matrix. Current opinion in pharmacology. 2013;13(3):449–54. doi: 10.1016/j.coph.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Xu L, Polur I, Servais JM, Hsieh S, Lee PL, Goldring MB, Li Y. Intact pericellular matrix of articular cartilage is required for unactivated discoidin domain receptor 2 in the mouse model. The American journal of pathology. 2011;179(3):1338–46. doi: 10.1016/j.ajpath.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leddy HA, Christensen SE, Guilak F. Microscale diffusion properties of the cartilage pericellular matrix measured using 3D scanning microphotolysis. Journal of biomechanical engineering. 2008;130(6):061002. doi: 10.1115/1.2979876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Conor C, Leddy HA, Benefield H, Liedtke W, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proceedings of the National Academy of Sciences USA. 2014;111(4):1316–21. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, Fan J, Becker KG, Graff RD, Lee GM, Francomano CA. Comparison of gene expression profile between human chondrons and chondrocytes: a cDNA microarray study. Osteoarthritis and cartilage. 2006;14(5):449–59. doi: 10.1016/j.joca.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Bomer N, Cornelis FM, Ramos YF, den Hollander W, Storms L, van der Breggen R, Lakenberg N, Slagboom PE, Meulenbelt I, Lories RJ. The effect of forced exercise on knee joints in Dio2(−/−) mice: type II iodothyronine deiodinase-deficient mice are less prone to develop OA-like cartilage damage upon excessive mechanical stress. Annals of the rheumatic diseases. 2016;75(3):571–7. doi: 10.1136/annrheumdis-2014-206608. [DOI] [PubMed] [Google Scholar]
- 47.Vincent TL, Hermansson MA, Hansen UN, Amis AA, Saklatvala J. Basic fibroblast growth factor mediates transduction of mechanical signals when articular cartilage is loaded. Arthritis and rheumatism. 2004;50(2):526–33. doi: 10.1002/art.20047. [DOI] [PubMed] [Google Scholar]
- 48.Andhare RA, Takahashi N, Knudson W, Knudson CB. Hyaluronan promotes the chondrocyte response to BMP-7. Osteoarthritis and cartilage. 2009;17(7):906–16. doi: 10.1016/j.joca.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chia SL, Sawaji Y, Burleigh A, McLean C, Inglis J, Saklatvala J, Vincent T. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis and rheumatism. 2009;60(7):2019–27. doi: 10.1002/art.24654. [DOI] [PubMed] [Google Scholar]
- 50.Knight MM, Ross JM, Sherwin AF, Lee DA, Bader DL, Poole CA. Chondrocyte deformation within mechanically and enzymatically extracted chondrons compressed in agarose. Biochimica et biophysica acta. 2001;1526(2):141–6. doi: 10.1016/s0304-4165(01)00118-0. [DOI] [PubMed] [Google Scholar]
- 51.Ng L, Hung HH, Sprunt A, Chubinskaya S, Ortiz C, Grodzinsky A. Nanomechanical properties of individual chondrocytes and their developing growth factor-stimulated pericellular matrix. Journal of biomechanics. 2007;40(5):1011–23. doi: 10.1016/j.jbiomech.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen BV, Wang Q, Kuiper NJ, El Haj AJ, Thomas CR, Zhang Z. Strain-dependent viscoelastic behaviour and rupture force of single chondrocytes and chondrons under compression. Biotechnology letters. 2009;31(6):803–9. doi: 10.1007/s10529-009-9939-y. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen BV, Wang QG, Kuiper NJ, El Haj AJ, Thomas CR, Zhang Z. Biomechanical properties of single chondrocytes, chondrons determined by micromanipulation, finite-element modelling. Journal of the Royal Society, Interface/the Royal Society. 2010;7(53):1723–33. doi: 10.1098/rsif.2010.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guilak F, Jones WR, Ting-Beall HP, Lee GM. The deformation behavior and mechanical properties of chondrocytes in articular cartilage. Osteoarthritis and cartilage. 1999;7(1):59–70. doi: 10.1053/joca.1998.0162. [DOI] [PubMed] [Google Scholar]
- 55.Alexopoulos LG, Haider MA, Vail TP, Guilak F. Alterations in the mechanical properties of the human chondrocyte pericellular matrix with osteoarthritis. Journal of biomechanical engineering. 2003;125(3):323–33. doi: 10.1115/1.1579047. [DOI] [PubMed] [Google Scholar]
- 56.Alexopoulos LG, Williams GM, Upton ML, Setton LA, Guilak F. Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. Journal of biomechanics. 2005;38(3):509–17. doi: 10.1016/j.jbiomech.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 57.Alexopoulos LG, Youn I, Bonaldo P, Guilak F. Developmental and osteoarthritic changes in Col6a1-knockout mice: biomechanics of type VI collagen in the cartilage pericellular matrix. Arthritis and rheumatism. 2009;60(3):771–9. doi: 10.1002/art.24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guilak F, Alexopoulos LG, Haider MA, Ting-Beall HP, Setton LA. Zonal uniformity in mechanical properties of the chondrocyte pericellular matrix: micropipette aspiration of canine chondrons isolated by cartilage homogenization. Annals of biomedical engineering. 2005;33(10):1312–8. doi: 10.1007/s10439-005-4479-7. [DOI] [PubMed] [Google Scholar]
- 59.Zelenski NA, Leddy HA, Sanchez-Adams J, Zhang J, Bonaldo P, Liedtke W, Guilak F. Type VI Collagen Regulates Pericellular Matrix Properties, Chondrocyte Swelling, and Mechanotransduction in Mouse Articular Cartilage. Arthritis & rheumatology (Hoboken NJ) 2015;67(5):1286–94. doi: 10.1002/art.39034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen DM, Mao JJ. Heterogeneous nanostructural and nanoelastic properties of pericellular and interterritorial matrices of chondrocytes by atomic force microscopy. J Struct Biol. 2004;145(3):196–204. doi: 10.1016/j.jsb.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Darling EM, Wilusz RE, Bolognesi MP, Zauscher S, Guilak F. Spatial mapping of the biomechanical properties of the pericellular matrix of articular cartilage measured in situ via atomic force microscopy. Biophysical journal. 2010;98(12):2848–56. doi: 10.1016/j.bpj.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLeod MA, Wilusz RE, Guilak F. Depth-dependent anisotropy of the micromechanical properties of the extracellular and pericellular matrices of articular cartilage evaluated via atomic force microscopy. Journal of biomechanics. 2013;46(3):586–592. doi: 10.1016/j.jbiomech.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilusz RE, DeFrate LE, Guilak F. Immunofluorescence-guided atomic force microscopy to measure the micromechanical properties of the pericellular matrix of porcine articular cartilage. Journal of the Royal Society, Interface/the Royal Society. 2012;9(76):2997–3007. doi: 10.1098/rsif.2012.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilusz RE, Defrate LE, Guilak F. A biomechanical role for perlecan in the pericellular matrix of articular cartilage. Matrix biology: journal of the International Society for Matrix Biology. 2012;31(6):320–7. doi: 10.1016/j.matbio.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilusz RE, Zauscher S, Guilak F. Micromechanical mapping of early osteoarthritic changes in the pericellular matrix of human articular cartilage. Osteoarthritis and cartilage. 2013;21(12):1895–903. doi: 10.1016/j.joca.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilusz RE, Guilak F. High resistance of the mechanical properties of the chondrocyte pericellular matrix to proteoglycan digestion by chondroitinase, aggrecanase, or hyaluronidase. Journal of the mechanical behavior of biomedical materials. 2014 doi: 10.1016/j.jmbbm.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prein C, Warmbold N, Farkas Z, Schieker M, Aszodi A, Clausen-Schaumann H. Structural and mechanical properties of the proliferative zone of the developing murine growth plate cartilage assessed by atomic force microscopy. Matrix biology: journal of the International Society for Matrix Biology. 2016;50:1–15. doi: 10.1016/j.matbio.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Kim E, Guilak F, Haider MA. An axisymmetric boundary element model for determination of articular cartilage pericellular matrix properties in situ via inverse analysis of chondron deformation. Journal of biomechanical engineering. 2010;132(3):031011. doi: 10.1115/1.4000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Darling EM, Zauscher S, Guilak F. Viscoelastic properties of zonal articular chondrocytes measured by atomic force microscopy. Osteoarthritis and cartilage. 2006;14(6):571–9. doi: 10.1016/j.joca.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 70.Schinagl RM, Gurskis D, Chen AC, Sah RL. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 1997;15(4):499–506. doi: 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]
- 71.Chery DR, Rozans SJ, Han B, Qin L, Birk DE, Iozzo RV, Enomoto-Iwamoto M, Han L. Direct investigation of the roles of decorin in cartilage pericellular matrix via immunofluorescence-guided AFM. Trans Orthop Res Soc. 2017;42:165. [Google Scholar]
- 72.Quinn TM, Maung AA, Grodzinsky AJ, Hunziker EB, Sandy JD. Physical and biological regulation of proteoglycan turnover around chondrocytes in cartilage explants. Implications for tissue degradation and repair. Annals of the New York Academy of Sciences. 1999;878:420–41. doi: 10.1111/j.1749-6632.1999.tb07700.x. [DOI] [PubMed] [Google Scholar]
- 73.Poole CA, Matsuoka A, Schofield JR. Chondrons from articular cartilage. III. Morphologic changes in the cellular microenvironment of chondrons isolated from osteoarthritic cartilage. Arthritis and rheumatism. 1991;34(1):22–35. doi: 10.1002/art.1780340105. [DOI] [PubMed] [Google Scholar]
- 74.Poole CA, Gilbert RT, Herbage D, Hartmann DJ. Immunolocalization of type IX collagen in normal and spontaneously osteoarthritic canine tibial cartilage and isolated chondrons. Osteoarthritis and cartilage. 1997;5(3):191–204. doi: 10.1016/s1063-4584(97)80014-3. [DOI] [PubMed] [Google Scholar]
- 75.Lee GM, Paul TA, Slabaugh M, Kelley SS. The incidence of enlarged chondrons in normal and osteoarthritic human cartilage and their relative matrix density. Osteoarthritis and cartilage. 2000;8(1):44–52. doi: 10.1053/joca.1999.0269. [DOI] [PubMed] [Google Scholar]
- 76.Ross JM, Sherwin AF, Poole CA. In vitro culture of enzymatically isolated chondrons: a possible model for the initiation of osteoarthritis. Journal of anatomy. 2006;209(6):793–806. doi: 10.1111/j.1469-7580.2006.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holloway I, Kayser M, Lee DA, Bader DL, Bentley G, Knight MM. Increased presence of cells with multiple elongated processes in osteoarthritic femoral head cartilage. Osteoarthritis and cartilage. 2004;12(1):17–24. doi: 10.1016/j.joca.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 78.Bush PG, Hall AC. The volume and morphology of chondrocytes within non-degenerate and degenerate human articular cartilage. Osteoarthritis and cartilage. 2003;11(4):242–51. doi: 10.1016/s1063-4584(02)00369-2. [DOI] [PubMed] [Google Scholar]
- 79.Murray DH, Bush PG, Brenkel IJ, Hall AC. Abnormal human chondrocyte morphology is related to increased levels of cell-associated IL-1beta and disruption to pericellular collagen type VI. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2010;28(11):1507–14. doi: 10.1002/jor.21155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nerlich AG, Wiest I, von der Mark K. Immunohistochemical analysis of interstitial collagens in cartilage of different stages of osteoarthrosis. Virchows Archiv B, Cell pathology including molecular pathology. 1993;63(4):249–55. doi: 10.1007/BF02899269. [DOI] [PubMed] [Google Scholar]
- 81.Mrosek EH, Lahm A, Erggelet C, Uhl M, Kurz H, Eissner B, Schagemann JC. Subchondral bone trauma causes cartilage matrix degeneration: an immunohistochemical analysis in a canine model. Osteoarthritis and cartilage. 2006;14(2):171–8. doi: 10.1016/j.joca.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Chang J, Poole CA. Sequestration of type VI collagen in the pericellular microenvironment of adult chrondrocytes cultured in agarose. Osteoarthritis and cartilage. 1996;4(4):275–85. doi: 10.1016/s1063-4584(05)80105-0. [DOI] [PubMed] [Google Scholar]
- 83.Arican M, Carter SD, Bennett D, Ross G, Ayad S. Increased metabolism of collagen VI in canine osteoarthritis. Journal of comparative pathology. 1996;114(3):249–56. doi: 10.1016/s0021-9975(96)80046-6. [DOI] [PubMed] [Google Scholar]
- 84.Hambach L, Neureiter D, Zeiler G, Kirchner T, Aigner T. Severe disturbance of the distribution and expression of type VI collagen chains in osteoarthritic articular cartilage. Arthritis and rheumatism. 1998;41(6):986–96. doi: 10.1002/1529-0131(199806)41:6<986::AID-ART5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 85.Soder S, Hambach L, Lissner R, Kirchner T, Aigner T. Ultrastructural localization of type VI collagen in normal adult and osteoarthritic human articular cartilage. Osteoarthritis and cartilage. 2002;10(6):464–70. doi: 10.1053/joca.2002.0512. [DOI] [PubMed] [Google Scholar]
- 86.Horikawa O, Nakajima H, Kikuchi T, Ichimura S, Yamada H, Fujikawa K, Toyama Y. Distribution of type VI collagen in chondrocyte microenvironment: study of chondrons isolated from human normal and degenerative articular cartilage and cultured chondrocytes. Journal of orthopaedic science: official journal of the Japanese Orthopaedic Association. 2004;9(1):29–36. doi: 10.1007/s00776-003-0737-4. [DOI] [PubMed] [Google Scholar]
- 87.Ramos YF, den Hollander W, Bovee JV, Bomer N, van der Breggen R, Lakenberg N, Keurentjes JC, Goeman JJ, Slagboom PE, Nelissen RG, Bos SD, Meulenbelt I. Genes involved in the osteoarthritis process identified through genome wide expression analysis in articular cartilage; the RAAK study. PloS one. 2014;9(7):e103056. doi: 10.1371/journal.pone.0103056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2006;54(9):1005–14. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- 89.Ruhlen R, Marberry K. The chondrocyte primary cilium. Osteoarthritis and cartilage. 2014;22(8):1071–6. doi: 10.1016/j.joca.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 90.Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, Lee SH, Liedtke W, Guilak F. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis and rheumatism. 2009;60(10):3028–37. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Knight MM, McGlashan SR, Garcia M, Jensen CG, Poole CA. Articular chondrocytes express connexin 43 hemichannels and P2 receptors - a putative mechanoreceptor complex involving the primary cilium? Journal of anatomy. 2009;214(2):275–83. doi: 10.1111/j.1469-7580.2008.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Donahue HJ, Guilak F, Vander Molen MA, McLeod KJ, Rubin CT, Grande DA, Brink PR. Chondrocytes isolated from mature articular cartilage retain the capacity to form functional gap junctions. J Bone Miner Res. 1995;10(9):1359–64. doi: 10.1002/jbmr.5650100913. [DOI] [PubMed] [Google Scholar]
- 93.McGlashan SR, Cluett EC, Jensen CG, Poole CA. Primary cilia in osteoarthritic chondrocytes: from chondrons to clusters. Developmental dynamics: an official publication of the American Association of Anatomists. 2008;237(8):2013–20. doi: 10.1002/dvdy.21501. [DOI] [PubMed] [Google Scholar]
- 94.Schminke B, Frese J, Bode C, Goldring MB, Miosge N. Laminins and Nidogens in the Pericellular Matrix of Chondrocytes: Their Role in Osteoarthritis and Chondrogenic Differentiation. The American journal of pathology. 2016;186(2):410–8. doi: 10.1016/j.ajpath.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 95.Wu J, Liu W, Bemis A, Wang E, Qiu Y, Morris EA, Flannery CR, Yang Z. Comparative proteomic characterization of articular cartilage tissue from normal donors and patients with osteoarthritis. Arthritis and rheumatism. 2007;56(11):3675–84. doi: 10.1002/art.22876. [DOI] [PubMed] [Google Scholar]
- 96.Hu K, Xu L, Cao L, Flahiff CM, Brussiau J, Ho K, Setton LA, Youn I, Guilak F, Olsen BR, Li Y. Pathogenesis of osteoarthritis-like changes in the joints of mice deficient in type IX collagen. Arthritis and rheumatism. 2006;54(9):2891–900. doi: 10.1002/art.22040. [DOI] [PubMed] [Google Scholar]
- 97.Polur I, Lee PL, Servais JM, Xu L, Li Y. Role of HTRA1, a serine protease, in the progression of articular cartilage degeneration. Histology and histopathology. 2010;25(5):599–608. doi: 10.14670/hh-25.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stevens AL, Wishnok JS, White FM, Grodzinsky AJ, Tannenbaum SR. Mechanical injury and cytokines cause loss of cartilage integrity and upregulate proteins associated with catabolism, immunity, inflammation, and repair. Mol Cell Proteomics. 2009;8(7):1475–89. doi: 10.1074/mcp.M800181-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu L, Peng H, Wu D, Hu K, Goldring MB, Olsen BR, Li Y. Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. The Journal of biological chemistry. 2005;280(1):548–55. doi: 10.1074/jbc.M411036200. [DOI] [PubMed] [Google Scholar]
- 100.Holt DW, Henderson ML, Stockdale CE, Farrell JT, Kooyman DL, Bridgewater LC, Seegmiller RE. Osteoarthritis-like changes in the heterozygous sedc mouse associated with the HtrA1-Ddr2-Mmp-13 degradative pathway: a new model of osteoarthritis. Osteoarthritis and cartilage. 2012;20(5):430–9. doi: 10.1016/j.joca.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 101.Berendsen AD, Fisher LW, Kilts TM, Owens RT, Robey PG, Gutkind JS, Young MF. Modulation of canonical Wnt signaling by the extracellular matrix component biglycan. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(41):17022–7. doi: 10.1073/pnas.1110629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neu CP, Khalafi A, Komvopoulos K, Schmid TM, Reddi AH. Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor beta signaling. Arthritis and rheumatism. 2007;56(11):3706–14. doi: 10.1002/art.23024. [DOI] [PubMed] [Google Scholar]
- 103.Knudson CB, Knudson W. Cartilage proteoglycans. Semin Cell Dev Biol. 2001;12(2):69–78. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 104.Pap T, Bertrand J. Syndecans in cartilage breakdown and synovial inflammation. Nature reviews. Rheumatology. 2013;9(1):43–55. doi: 10.1038/nrrheum.2012.178. [DOI] [PubMed] [Google Scholar]
- 105.Pataki CA, Couchman JR, Brabek J. Wnt Signaling Cascades and the Roles of Syndecan Proteoglycans. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2015;63(7):465–80. doi: 10.1369/0022155415586961. [DOI] [PubMed] [Google Scholar]
- 106.Xie Z, Khair M, Shaukat I, Netter P, Mainard D, Barre L, Ouzzine M. Non-canonical Wnt induces chondrocyte de-differentiation through Frizzled 6 and DVL-2/B-raf/CaMKIIalpha/syndecan 4 axis. Cell Death Differ. 2018 doi: 10.1038/s41418-017-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Melrose J, Hayes AJ, Whitelock JM, Little CB. Perlecan the “jack of all trades” proteoglycan of cartilaginous weight-bearing connective tissues. BioEssays: news and reviews in molecular, cellular and developmental biology. 2008;30(5):457–69. doi: 10.1002/bies.20748. [DOI] [PubMed] [Google Scholar]
- 108.Aigner T, Zien A, Hanisch D, Zimmer R. Gene expression in chondrocytes assessed with use of microarrays. The Journal of bone and joint surgery. 2003;85-A(Suppl 2):117–23. doi: 10.2106/00004623-200300002-00016. [DOI] [PubMed] [Google Scholar]
- 109.Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, Griggs DW. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis and rheumatism. 2007;56(2):575–85. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 110.Goldring MB, Otero M. Inflammation in osteoarthritis. Current opinion in rheumatology. 2011;23(5):471–8. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peters HC, Otto TJ, Enders JT, Jin W, Moed BR, Zhang Z. The protective role of the pericellular matrix in chondrocyte apoptosis. Tissue engineering. Part A. 2011;17(15–16):2017–24. doi: 10.1089/ten.TEA.2010.0601. [DOI] [PubMed] [Google Scholar]
- 112.Vonk LA, Doulabi BZ, Huang C, Helder MN, Everts V, Bank RA. Collagen-induced expression of collagenase-3 by primary chondrocytes is mediated by integrin α1 and discoidin domain receptor 2: a protein kinase C-dependent pathway. Rheumatology (Oxford) 2011;50(3):463–72. doi: 10.1093/rheumatology/keq305. [DOI] [PubMed] [Google Scholar]
- 113.Fukuda K, Dan H, Takayama M, Kumano F, Saitoh M, Tanaka S. Hyaluronic acid increases proteoglycan synthesis in bovine articular cartilage in the presence of interleukin-1. The Journal of pharmacology and experimental therapeutics. 1996;277(3):1672–5. [PubMed] [Google Scholar]
- 114.Lark MW, Gordy JT, Weidner JR, Ayala J, Kimura JH, Williams HR, Mumford RA, Flannery CR, Carlson SS, Iwata M, et al. Cell-mediated catabolism of aggrecan. Evidence that cleavage at the “aggrecanase” site (Glu373-Ala374) is a primary event in proteolysis of the interglobular domain. The Journal of biological chemistry. 1995;270(6):2550–6. doi: 10.1074/jbc.270.6.2550. [DOI] [PubMed] [Google Scholar]
- 115.Singer II, Kawka DW, Bayne EK, Donatelli SA, Weidner JR, Williams HR, Ayala JM, Mumford RA, Lark MW, Glant TT, et al. VDIPEN, a metalloproteinase-generated neoepitope is induced and immunolocalized in articular cartilage during inflammatory arthritis. The Journal of clinical investigation. 1995;95(5):2178–86. doi: 10.1172/JCI117907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Singer II, Scott S, Kawka DW, Bayne EK, Weidner JR, Williams HR, Mumford RA, Lark MW, McDonnell J, Christen AJ, Moore VL, Mudgett JS, Visco DM. Aggrecanase and metalloproteinase-specific aggrecan neo-epitopes are induced in the articular cartilage of mice with collagen II-induced arthritis. Osteoarthritis and cartilage. 1997;5(6):407–18. doi: 10.1016/s1063-4584(97)80045-3. [DOI] [PubMed] [Google Scholar]
- 117.Chambers MG, Cox L, Chong L, Suri N, Cover P, Bayliss MT, Mason RM. Matrix metalloproteinases and aggrecanases cleave aggrecan in different zones of normal cartilage but colocalize in the development of osteoarthritic lesions in STR/ort mice. Arthritis and rheumatism. 2001;44(6):1455–65. doi: 10.1002/1529-0131(200106)44:6<1455::AID-ART241>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 118.Plaas A, Osborn B, Yoshihara Y, Bai Y, Bloom T, Nelson F, Mikecz K, Sandy JD. Aggrecanolysis in human osteoarthritis: confocal localization and biochemical characterization of ADAMTS5-hyaluronan complexes in articular cartilages. Osteoarthritis and cartilage. 2007;15(7):719–34. doi: 10.1016/j.joca.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 119.Christensen SE, Coles JM, Zelenski NA, Furman BD, Leddy HA, Zauscher S, Bonaldo P, Guilak F. Altered trabecular bone structure and delayed cartilage degeneration in the knees of collagen VI null mice. PloS one. 2012;7(3):e33397. doi: 10.1371/journal.pone.0033397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Poole CA. Articular cartilage chondrons: form, function and failure. Journal of anatomy. 1997;191(Pt 1):1–13. doi: 10.1046/j.1469-7580.1997.19110001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hagg R, Bruckner P, Hedbom E. Cartilage fibrils of mammals are biochemically heterogeneous: differential distribution of decorin and collagen IX. The Journal of cell biology. 1998;142(1):285–94. doi: 10.1083/jcb.142.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kamper M, Hamann N, Prein C, Clausen-Schaumann H, Farkas Z, Aszodi A, Niehoff A, Paulsson M, Zaucke F. Early changes in morphology, bone mineral density and matrix composition of vertebrae lead to disc degeneration in aged collagen IX −/− mice. Matrix biology: journal of the International Society for Matrix Biology. 2016;49:132–143. doi: 10.1016/j.matbio.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 123.Wagener R, Gara SK, Kobbe B, Paulsson M, Zaucke F. The knee osteoarthritis susceptibility locus DVWA on chromosome 3p24.3 is the 5′ part of the split COL6A4 gene. Matrix biology: journal of the International Society for Matrix Biology. 2009;28(6):307–10. doi: 10.1016/j.matbio.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 124.Meulenbelt I, Chapman K, Dieguez-Gonzalez R, Shi D, Tsezou A, Dai J, Malizos KN, Kloppenburg M, Carr A, Nakajima M, van der Breggen R, Lakenberg N, Gomez-Reino JJ, Jiang Q, Ikegawa S, Gonzalez A, Loughlin J, Slagboom EP. Large replication study and meta-analyses of DVWA as an osteoarthritis susceptibility locus in European and Asian populations. Human molecular genetics. 2009;18(8):1518–23. doi: 10.1093/hmg/ddp053. [DOI] [PubMed] [Google Scholar]
- 125.Chery DR, Li Q, Lu J, Han B, Qin L, Lu XL, Enomoto-Iwamoto M, Han L. OR Society, editor. Pericellular Matrix is Highly Sensitive to Cartilage Degeneration in Early Post-Traumatic Osteoarthritis. 2018 Orthopedic Research Society Annual Meeting; New Orleans, LA. 2018. [Google Scholar]
- 126.Shu CC, Jackson MT, Smith MM, Smith SM, Penm S, Lord MS, Whitelock JM, Little CB, Melrose J. Ablation of Perlecan Domain 1 Heparan Sulfate Reduces Progressive Cartilage Degradation, Synovitis, Osteophyte Size in a Preclinical Model of Posttraumatic Osteoarthritis. Arthritis & rheumatology (Hoboken, NJ) 2016;68(4):868–79. doi: 10.1002/art.39529. [DOI] [PubMed] [Google Scholar]
- 127.Cao L, Guilak F, Setton LA. Three-dimensional morphology of the pericellular matrix of intervertebral disc cells in the rat. Journal of anatomy. 2007;211(4):444–52. doi: 10.1111/j.1469-7580.2007.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sanchez-Adams J, Wilusz RE, Guilak F. Atomic force microscopy reveals regional variations in the micromechanical properties of the pericellular and extracellular matrices of the meniscus. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2013;31(8):1218–25. doi: 10.1002/jor.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]