Abstract
Developing a strategy for making the alginate base hydrogel components against burned wound infections could be promising for healing the mentioned wounds followed by elimination of the biofilm forming bacteria colonization. Construction of an alginate based hydrogel and evaluating healing activities of the mentioned component as local ointment were the main objectives of the current study. Following the collection of the honey from three different provinces of Iran, the components and structures of the collected materials were analyzed taking advantage of INSO-92 procedure subsequently, antibacterial effect of diluted three different kinds of honey against wild-type bacterial species got evaluated via agar well diffusion method. An alginate base hydrogel was prepared by the use of calcium chloride as a linker between the alginate and honey functional groups. Then, component was structurally analyzed by Fourier Transform Infrared spectroscopy (FTIR). Afterward, under in vivo conditions, the healing activities of prepared ointment were studied in infected burned rat models. According to the antibacterial effect of the honeys, 75% diluted thymol based honeys collected from Damavand province were the most efficient ones. Furthermore, it was the healing activity of mentioned ointment was proven in vivo studies. The difference between 1600-1800 wave numbers in constructed alginate-based hydrogel alginate and honey because of C = O bond variations structurally confirmed proper construction of hydrogel. The hydrogel was the better healing activity in rats burned wound too. In conclusion the promising efficiency of alginate-based hydrogel in an elimination of bacterial infections was confirmed as the main aim of the current survey.
Keywords: Alginate base honey, burn wound, bacterial infection, fourier transform infrared spectroscopy, burned patients, MDR bacteria
Introduction
Burn injury, one of the most common and devastating forms of trauma has been noticed a major problem worldwide [1]. Burned patients are prone to the bacterial and Fungal colonization followed by infections of mentioned microbes in situ [1,2]. In order to decrease the morbidity and mortality caused by bacterial colonization in the burned skins, intensive care for patients with thermal injuries is needed. According to National Center for Injury Prevention and Control in the United States, 1.2 million burn injuries occur due to 2 million fires each year [1,3,4]. Since the development of modern medical care, during the past decades, the survival rates of affected patients have been an increase [1].
Right after a burn injury happens to retract and blood vessels coagulation occurs to maintain the homeostasis [1]. Skin acts as an important barrier against microbial invasion. Surprisingly there is a correlation between the size of the injury and the risk of subsequent burn wound and systemic infections [1,3]. Following instant contacts with contaminated environments including external surfaces, water, fomites, endogenous bacteria and other sources, the wound will get colonized by either Gram-positive or Gram-negative biofilm-forming bacteria [1,3]. In one hand antibiotic-resistant bacteria such as biofilm producer ones in hospital niches have been assigned as the major concern in the worldwide [5,6]. In the other hands, burn wound bacterial infections have been noticed as the major problems in healing procedure as well. Biofilm-forming bacteria including Staphylococcus aureus, S. epidermidis, Acinetobacter baumannii, Klebsiella pneumoniae, enterococci species and pseudomonas aeruginosa owing to the high durability in hospital environments as well as multi-drug resistance phenotype are the major causes of the medical devices contamination and burn wound infections [6-10]. Antimicrobial agents such as modern antiseptics (super oxidizing solutions, polyhexanide, diluted sodium hypochlorite, chlorhexidine, silver and cadexomer iodine) and topical antibiotics are used to reduce the bacterial concentration and colonization of burn wounds and decrease the morbidity and mortality rates [1,3]. Nevertheless, there is a problem with the selective pressure and the emergence of resistant bacteria as well as the change in the normal flora of the gut [4]. It seems that the elimination of the colonizer bacteria is temporary and the colonization with MDR bacteria will be restored since the antimicrobials are withdrawn [4]. In vivo and in vitro assays have been shown that the use of antiseptic agents including povidone-iodine has the least effect on a reduction of bacterial count in burn wounds in animal models. Although, these agents were more efficient in human models since the chemical composition of these components interferes in the healing process of the injured skins [1,2,4]. Also, silver sulfadiazine, the most popular antimicrobial agent of choice for the treatment of burn wounds, has an enviable safety record, however, due to its side effects is not applicable on premature babies or newborns during the first 2 months of life and pregnancy [11].
Honey contains more than 200 substances and as depicted by Stone Age paintings, it has been widely utilized for therapeutic goals since 8000 years ago [12]. Mentioned product primarily consists fructose, glucose, and frocto-oligosaccharides [12,13]. Honey composition mainly dependents on the sort of plants which the bee feeds on [12]. Flavonoids (such as apigenin, pinocembrin, kaempferol, quercetin, galangin, chrysin and hesperetin), phenolic acids (such as ellagic, caffeic, p-coumaric and ferulic acids), ascorbic acid, tocopherols, catalase (CAT), superoxide dismutase (SOD), reduced glutathione (GSH), Millard reaction products and peptides are the main components in almost all natural honey. A majority of mentioned compound work together providing a synergistic antioxidant effect [12]. One of the most studied and most effective uses of honey is found in the healing of wounds [14]. The Germans combined cod liver oil and honey to treat ulcers, burns, fistulas and boils [15]. Alginate, as a gel-forming material with hemostatic effect, has long been known as a rapidly wound healing agent due to a gel forming and dehydration preventing properties, since 1987 [16]. Calcium alginate dressing will absorb excess wound exudates and forms a non-adherent gel. This substance accelerates wound healing by providing a moist environment, facilitates debridement, helps to prevent trauma to the wound bed and the surrounding skin [17]. Regarding to the importance of accelerating the wound healing in burned patients and also prevention of antibiotic resistance in the of wound infections, the combination of honey and alginate for the production and evaluation of a topical ointment as an intervention possessing both therapeutic effects on the healing process and the ability to kill microbes are the main objectives of this study.
Materials and methods
Honey collection
Following the collection of three types of honey from different provinces in Iran including Damavand, Semnan, and Ardebill, samples got sterilized by gamma-ray exposing. The honeys were kept in dark bottles, away from sunlight. The age of the honey samples was ranged from 7 to 12 months.
Physicochemical properties of honeys
PH, moisture and sugar contents were determined according to the International Honey Commission [18].
Subsequently, determination of the honeys compartments was performed according to the INSO 92 standard in the Fellow of the Food and Drug Administration of the Health Ministry of Iran (Table 1).
Table 1.
Structural characteristics of honeys
| Kinds of Honey | Test | Results | Unit | Allowed range | Reference method |
|---|---|---|---|---|---|
| Semnan | pH | 3.59 | --- | < 3.5 | INSO-92 |
| Acidity | 8.23 | Milliequivalent/Kg | > 40 | INSO-92 | |
| Honey Sucrose | 1.71 | % | < 5 | INSO-92 | |
| Honey moisture | 17.9 | % | > 20 | INSO-92 | |
| Honey Diastasis Activity | Positive | --- | Active | INSO-92 | |
| Sugar before hydrolyzing honey | 87.5 | % | At least 65 | INSO-92 | |
| Damavand | pH | 4.15 | --- | < 3.5 | INSO-92 |
| Acidity | 19.6 | Milliequivalent/Kg | > 40 | INSO-92 | |
| Honey Sucrose | 2.7 | % | < 5 | INSO-92 | |
| Honey moisture | 14.4 | % | > 20 | INSO-92 | |
| Honey Diastasis Activity | Positive | --- | Active | INSO-92 | |
| Sugar before hydrolyzing honey | 71 | % | At least 65 | INSO-92 | |
| Ardabil | pH | 4.19 | --- | < 3.5 | INSO-92 |
| Acidity | 8.4 | Millieqivalan/Kg | > 40 | INSO-92 | |
| Honey Sucrose | 4.56 | % | < 5 | INSO-92 | |
| Honey moisture | 14 | % | > 20 | INSO-92 | |
| Honey Diastasis Activity | Negative | --- | Active | INSO-92 | |
| Sugar before hydrolyzing honey | 64.73 | % | At least 65 | INSO-92 |
Wild-type bacterial species isolation and identification
Majority of wild-type strains causing the wound infections after the trauma or burn injuries including S. aureus, Klebsiella pneumoniae, A. baumannii, P. aeruginosa were served as controls in this study. Mentioned bacteria got identified precisely taking advantage of conventional microbiological tests based on the Bergey’s Manual of Determinative Bacteriology recipes [19]. Briefly, the clinical specimens got cultured and suspected growth colonies were identified by gram staining. Furthermore, oxidase and catalase tests were done and other biochemical patterns got checked based upon the specific scheme for each bacterium [19].
All the bacterial cultures were diluted till the Optical Density (O.D) in range of 0.02-0.04 was obtained, which is equivalent to a bacterial concentration between 106 and 108 cells/ml.
Antimicrobial susceptibility testing of the bacterial isolates
Susceptibility testing for the bacteria isolated from burn wound infections was performed using disk agar diffusion method according to the CLSI standard guidelines [20] by the use of several disks including, ceftriaxone 30 µg, ceftazidime 10 µg, ciprofloxacin 5 µg, ceftazidime 30 µg, amikacin 30 µg, cefotaxime 30 µg, imipenem 10 µg, cefepime 30 µg, meropenem 10 µg and gentamycin 10 µg (BD BBLTM Sensi DiscTM). Evaluation of the results was also performed considering that proposed by the manufacturer’s recommendation breakpoint set for Pseudomonas aeruginosa (Table 2).
Table 2.
Antibiotic susceptibility pattern of clinical isolated strains
| Antibiotics | S. aureus | K. pneumoniae | P. aeruginosa | A. baumannii | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | Amikacin | 24 mm | S | 20 mm | S | - | R | - | R |
| 2 | Ceftazidime | - | R | 20 mm | I | 15 mm | R | - | R |
| 3 | Cefotaxime | 20 mm | - | 30 mm | S | - | R | - | R |
| 4 | Ceftriaxone | 25 mm | - | 30 mm | S | - | R | - | R |
| 5 | Cefepime | 17 mm | - | 25 mm | S | - | R | - | R |
| 6 | Ciprofloxacin | 26 mm | S | - | R | - | R | - | R |
| 7 | Gentamycin | 23 mm | S | - | R | - | R | - | R |
| 8 | Imipenem | 28 mm | - | 25 mm | S | - | R | - | R |
| 9 | Meropenem | 24 mm | - | 25 mm | S | - | R | - | R |
In brief, bacteria were suspended in normal saline in a ratio of one-tenth so the turbidity of the 0.5 McFarland standard was reached, then they directly got inoculated onto Mueller-Hinton agar. After 16 to 20 hours of incubation, the effects of each antibiotic was determined. E. coli ATCC 25922 was used as the quality control strain.
Qualitative assay of biofilm-formation ability
Biofilm formation assay was performed using wrinkled colony development according to the previous studies [21]. Briefly, the clinical isolates were cultured overnight on LB agar. The next day, a single colony was inoculated in LB broth and shaken at 180 rpm at 37°C until OD600 = 0.2. LB broth containing bacteria (5 μl) was spotted on LB agar and incubated at 37°C for 24 h. The morphologies of wrinkled colonies got assessed by light microscopy. Then the clinical isolates were classified according to the background of the colonies in the presence of light, the ground state of the bacteria involved in the molecular matrix as well as the thickness of the surrounding colonies (Figure 1).
Figure 1.
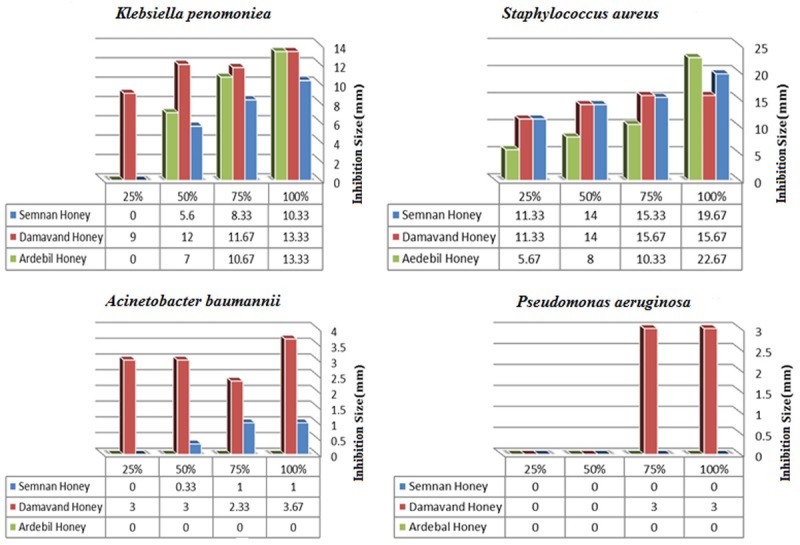
Antibacterial effects of Honeys against clinical isolates of tested bacteria. X: dilutions of tested Honeys, Y: zone diameter.
Antibacterial effects of honeys
Because of differences in feeding process among bees, determination of the best antibacterial activity was conducted by the collection of the three different honey samples including (Damavand honey, Semnan honey, and Sabalan honey). The antibacterial activity of diluted honeys was assessed using agar well diffusion method [22]. The minimum inhibitory concentration (MIC) of each honey sample on clinically isolated bacteria was carried out by the agar-well diffusion method [20]. The MIC was estimated as the minimum honey concentration which showed a measurable inhibition zone. In essence, the cleaned glass plates with UV lamp in 45 min was filled by 150 ml of prepared culture media (one milliliter of growth bacteria/165 ml of 45°C culture media) and then it was kept at 4°C for 1 h. Four millimeters holes in diameter were drilled on the culture media and 50 microliters of the diluted honeys were added to each column. Each glass plate contained three columns (one column per dilution) and four rows (three repetitions), so that the four holes in the first column held honey at 100%, the four holes of the second column contained honey at 75%, and so on. The plates got incubated at 37°C for 24 h. The diameters of the circular inhibition zones were measured with a caliper [22]. Data was shown in Figure 1.
Construction of alginate base hydrogel
Preparation of alginate-based hydrogel was performed according to the previously published study with slight modifications [23,24]. In short, the hydrogel was prepared with a ratio of 50-50. The materials were overshadowed and mixed, as the viscose hydrogel was obtained after about 5 minutes. The hydrogel was placed in a sonication bath in order to remove airborne bubbles from the hydrogel. In addition, hydrogel was combined to either CaCl2 or the crosslinking agents. The crosslink factors, time and also the pH of the environment could play an important role in cross-linking, which provides the optimal test for inflation. In this study we used CaCl2 with 2.5% w/w, and 60 minutes were given to complete the cross-link at pH = 7 [23,24]. The hydrogel was prepared from alginate salts in honey with a suitable concentration on the mentioned bacteria. Sodium chloride was utilized as the coupling agent in this procedure. The purity of alginate was checked via its components using FTIR procedure. In brief, diluted honey (75%) in normal saline was linked to alginate using CaCl2 (2.5 gram in 97.5 milliliters distilled water) at pH = 7, as the coupling agent. The mixture was stirred for 2 h at 25°C resulting cocktail assessed by Fourier Transform Infrared spectroscopy (FTIR) following the removal of bubbles during the hydrogel preparation process by the use of sonication bath system [23].
Fourier transform infrared spectroscopy (FTIR) of hydrogel
Total components of prepared hydrogel were determined by Fourier Transform Infrared spectroscopy (FTIR) (TENSOR 27 Burker instrumental averaging of 256 scans on the FTIR spectrometer) according to established methods [10,25]. Moreover, mentioned spectrum was compared with the alginate and CaCl2 IR spectrum results. The structural formula of prepared alginate from lactobacillus was used as a reference for FTIR patterns analysis [26] (Figure 3).
Figure 3.

IR spectra of calcium alginate (Hydrogel) in the 3000-3600 cm-1 range and the result of this deconvolution.
In vivo rat burned model assay
In vivo healing assay was accomplished by the method described in the previous study with slight modifications [27]. For this experiments, thirty 6-8 weeks Wistar female inbred rats (purchased from the Research Institution of Pasteur Karaj, IR Iran) were divided into control and test groups of one rat in each group. Before the experiment, the rats were housed in standard stainless cages at 23-25°C, 60-70% humidity, and a 12-hours light/dark cycle for a week. The rats were given free access to a standard diet and water. Tested animals were anesthetized with intra-peritoneal Ketamine, Ketamine Injection 100 mg/mL (Parnell Laboratories, Auckland, New Zealand) and Xylazine, Ilium-Xylazine-20 20 mg/mL (Troy Laboratories, Sydney, Australia). 7.5 mL of Ketamine and 5.0 mL of Xylazine were diluted with 7.5 mL of MilliQ water (Millipore, Billerica, MA, USA). A dosage of 0.2 mL/100 g body-weight was used for the induction of anesthesia. One rat from the specific group was burned in back shaved skins with 2 cm heated steel disks in 9-11 seconds. Afterward, 100 microliters of prepared 0.5 McFarland (1.5 × 105 CFU/ml) suspension of each mentioned bacteria was injected in the burned site was given a one-day interval for bacterial colonization and also infected burned wounds were utilized as the negative and positive control [27] (Figures 4, 5).
Figure 4.
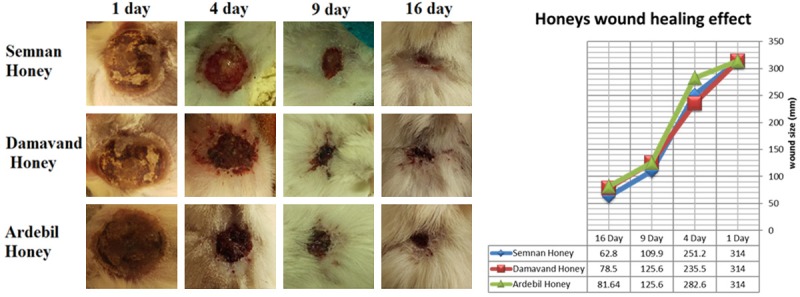
Wound healing effect of pure honeys in the burned rats. Five grams of each pure honey has been applicate in two times for a day, between 1-16 days after wound infection.
Figure 5.
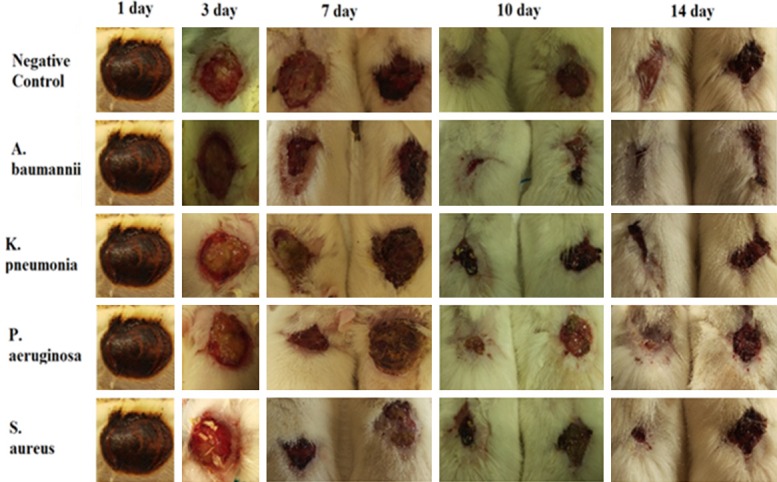
Wound healing activity of constructed hydrogel on the infected wounds. Five grams of Hydrogel was used two times in a day, between 1-16 days after wound infection.
Results
Honey analysis
The following tests for honey analysis were performed according to the INSO 92 standard in the Fellow of the Food and Drug Administration of the Health Ministry of Iran, and the results are verified. Detailed data were listed in Table 1.
Antibiotic susceptibility testing for clinical isolates
Susceptibility testing by disk agar diffusion method showed that the two main causative agents in burn wound infections (P. aeruginosa and A. baumannii) were resistant to almost all tested antibiotics (Table 2).
Honeys antibacterial pattern
Different kinds of honeys possess various efficacies and mechanisms against the same type of bacteria, while the Thymol based Damavand honey showed the best activity. Antibiotic susceptibility of the isolated bacteria to antibiotics depicted that the honey resistant isolates were also more resistant to all tested antibiotics (Table 2 and Figure 1). Assessment the antibacterial effect of honeys indicated that tested honeys had promising activity against biofilm producer S. aureus and K. pneumonia. However, only thymol based Damavand honey showed a more tremendously effect on all tested clinical isolates confirming the efficiency of thymol in this specific honey.
Biofilm formation capability of the clinical isolates showed that all of the tested bacteria were biofilm producer. The wrinkled colony morphology of the clinical isolates was similar to a categorization enlarged reported by a previous study [21], and also mostly belonged to type C wrinkled colony morphology. The background of the colonies in the presence of light, the ground state of the bacteria involved in the molecular matrix and the thickness of the surrounding colonies differed amongst the clinical isolates (Figure 2). The characteristics of biofilms were a dark context with dense a surrounding caused by colonies. The morphology of this colony was similar to the pellicle form.
Figure 2.

Qualitative assay of biofilm-formation ability in clinical isolates.
Biofilm formation ability of isolated strains was checked by S. epidermidis ATCC 12228 as the non-biofilm forming control strain.
Construction of hydrogel
The Fourier transform infrared (FTIR) spectra of the calcium alginate (Hydrogel) were recorded and compared with CaCl2 and Alginate (Figure 3). The spectrum of calcium alginate indicated important absorption bands regarding hydroxyl, ether and carboxylic functional groups. Stretching vibrations of O-H bonds of alginate appeared in the range of 3000-3600 cm-1. Stretching vibrations of aliphatic C-H were observed at 2920-2850 cm-1. Observed bands in 1649 and 1460 cm-1 were attributed to asymmetric and symmetric stretching vibrations of carboxylate salt ion, respectively. Later bands were very significant and can be used for characterization of alginate structure from its derivatives and ingredients. The bands at 1107 and 935 cm-1 were attributed to the C-O stretching vibration of the pyranosyl ring and the C-O stretching with contributions from C-C-H and C-O-H deformation. The shift to lower wavenumbers in comparison with the calcium alginate samples may reveal an interaction of the regular homopolymeric chain with the calcium ions.
R-C = O groups of the native alginate and modification of the mentioned group position in FTIR pattern and also the presence of an R-NO bond in the hydrogel were determined. Moreover, the composition of the hydrogel was confirmed in comparison with the native alginate pattern.
In vivo wound healing effect
Since better antibacterial efficiency had been shown by Thymol based honey from Damavand, it was chosen for Hydrogel construction. Although all collected honeys had wound healing activity, the healing period will be diminished from 16 days to 14 days by using the constructed Hydrogel (Figures 4, 5).
Discussion
One of the most important concerns among scientists and medical specialists is the bacterial resistance among human beings [28]. Burn wound infections in hospitalized individuals have been assigned as the main health treat worldwide [29]. Usually, burn wound infections caused by multi-drug resistant bacterial pathogens have a direct bearing on the duration of hospitalization. During the first days of the post-burn hospitalization, more susceptible, Gram-positive organisms are predominate, while later more resistant Gram-negative organisms are found, which have an impact on the election of empiric alginate-based hydrogel therapy in critically burned patients as well as bacterial elimination and wound healing [29,30]. The widespread existence of unhealed wounds and burns have a great influence on public health and economy [28,31]. New medication’s processes are being used as new interventions to achieve a better wound healing and to eliminate the infections. Therefore, to find an intervention owing both therapeutic effects on the healing process and also the ability to kill microbes will have a great value [31].
Honey is a natural acidic substance, which its pH has been ranged from 5.5 to 6 and pH value of honey should not be less than 3.5. The honey diastase test should be positive, showing that, honey has not been heated. Diastatic activity is a qualitative factor that is different between heated (fresh) and unheated honey. Measuring the percentage of sucrose is one of the most commonly used parameters in the honey test. If sucrose percentage in honey is less than 2%, it can be claimed that the honey has a very high quality. If sugar is added to honey, honey sucrose is much higher than that mentioned by the Fahling test and can be found by the sacrament sucrose in the laboratory [12]. According to the results obtained from the honey samples tested and also with regard to important parameters such as sucrose, the activity of diastase and sugar reduction, it can be said that Semnan honey has a better grade, then Damavand honey and Ardabil honey will come after. Furthermore, by the use of agar well diffusion method, the impact of honeys on multi-drug resistant (MDR) bacterial strains such as (P. aeruginosa, S. aureus, K. pneumoniae and A. baumannii) were studied [22,30]. Thymol as natural monoterpene phenol derivative of cymene plays as a proper antibacterial agent [32]. Our results indicated that tested honeys have promising activity against biofilm forming S. aureus and K. pneumoniae but only thymol based Damavand honey showed a more tremendous effect on all tested clinical isolates, which may confirm the effect of thymol in this honey. Susceptibility patterns indicated that all clinical isolates were resistant to applied antibiotics. Our findings were similar to other previously published studies [30,33]. The antimicrobial susceptibility pattern of the different Gram-negative strains isolated from the burned patients revealed that Pseudomonas sp. were resistant to amikacin, ceftriaxone, ciprofloxacin, and gentamicin. Findings from this study were similar to those of Shahzad et al. which reported the range of 35%, 85%, 70% and 97% resistance to amikacin, ceftriaxone, ciprofloxacin, and gentamicin, respectively [33].
Because of better antibacterial effect for Damavand honey compared to the others, this sample was used for alginate-based hydrogel preparation. The results can be attributed to the type of bee’s feeding. The antibacterial properties of thymol have been proven as well [32]. Thyme plant, as a source of nutrition in Damavand bees, possesses a thymol active ingredient which enhances the antibacterial activity of Damavand honey. The healing effect of honeys (without alginate) was investigated in comparison with a control group. Data revealed that healing activity of thymol-based Damavand and Semnan honey was better than Ardebil honey in the first few days meaning the healing related process started sooner in the presence of these honeys (Figure 4). Although healing process had been observed in pure honey samples, this was postponed compared with those rats which were treated by prepared hydrogel as well. According to the results obtained about the healing activity of hydrogel in infected wounds in this study, its promising effect compared with the control group was clear. All of the infected wounds were healed after a fourteen-day interval following hydrogels usage. Hydrogel-honey could accelerate the process of healing during a period of fourteen days (Figure 5). Ardabil honey showed less healing activity during the first days. The results of the structural analysis of honeys indicated that other two kinds of honey not only had diastolic activity, but also a better quality. Ardabil honey possesses both high levels of sucrose and also inactivity of diastase, which is evidence of the use of sugar in the preparation of Ardabil honey and the loss of honey and loss of diastasis. These results were fully confirmed during healing test. Based on the results of this study the combination of alginate and honey could be used as a natural product for biological wounding, as they are resistant to antibiotics and have an impact on bacterial growth inhibition.
Conclusions
In conclusion, using an alginate-based honey hydrogel, we designed a topical ointment that could protect burned wounds of rats from biofilm-forming bacterial infections and it could also shorten the hospitalization period for burned patients. It seems that this hydrogel may protect individuals from the bacterial colonization and enhance the healing process in burn wounds.
Acknowledgements
The authors wish to acknowledge the Microbiology and Virology department of Mazandaran University of Medical Sciences for their kind support.
Disclosure of conflict of interest
None.
References
- 1.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19:403–34. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Association AB. Burn incidence and treatment in the US: 2000 fact sheet. 2000. Back to cited text. 2008 [Google Scholar]
- 3.Deng Y, Bao X, Ji L, Chen L, Liu J, Miao J, Chen D, Bian H, Li Y, Yu G. Resistance integrons: class 1, 2 and 3 integrons. Ann Clin Microbiol Antimicrob. 2015;14:45. doi: 10.1186/s12941-015-0100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebner W, Kropec-Hübner A, Daschner F. Bacterial resistance and overgrowth due to selective decontamination of the digestive tract. Eur J Clin Microbiol Infect Dis. 2000;19:243–7. doi: 10.1007/s100960050470. [DOI] [PubMed] [Google Scholar]
- 5.Mirzaei B, Bameri Z, Babaei R, Shahcheraghi F. Isolation of high level macrolide resistant bordetella pertussis without transition mutation at domain V in iran. Jundishapur J Microbiol. 2015;8:e18190. doi: 10.5812/jjm.8(5)2015.18190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mousavi SF, Mirzaei B, Shaghaghi B, Jalali P, Setayesh T, Moosavi SH. Phenotypic and genotypic features of first biofilm forming nasopharyngeal colonized Streptococcus pneumoniae isolates. Iran J Microbiol. 2017;9:200–207. [PMC free article] [PubMed] [Google Scholar]
- 7.Goli HR, Nahaei MR, Rezaee MA, Hasani A, Kafil HS, Aghazadeh M, Sheikhalizadeh V. Prevalence and molecular characterization of class 1 integrons among clinical isolates of pseudomonas aeruginosa in Northwest of Iran. Molecular Genetics, Microbiology and Virology. 2017;32:109–115. [Google Scholar]
- 8.Hasani A, Sheikhalizadeh V, Ahangarzadeh Rezaee M, Rahmati-Yamchi M, Hasani A, Ghotaslou R, Goli HR. Frequency of aminoglycoside-modifying enzymes and ArmA among different sequence groups of acinetobacter baumannii in Iran. Microb Drug Resist. 2016;22:347–53. doi: 10.1089/mdr.2015.0254. [DOI] [PubMed] [Google Scholar]
- 9.Mirzaei B, Babaei R, Asiabar AP, Bameri Z. Detection of both vanA & vanBgenes in vanA phenotypes of enterococci by Taq Man RT-PCR. Braz J Microbiol. 2015;46:161–5. doi: 10.1590/S1517-838246120131234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirzaei B, Moosavi SF, Babaei R, Siadat SD, Vaziri F, Shahrooei M. Purification and evaluation of polysaccharide intercellular adhesion (PIA) antigen from staphylococcus epidermidis. Curr Microbiol. 2016;73:611–617. doi: 10.1007/s00284-016-1098-5. [DOI] [PubMed] [Google Scholar]
- 11.Fuller FW. The side effects of silver sulfadiazine. J Burn Care Res. 2009;30:464–70. doi: 10.1097/BCR.0b013e3181a28c9b. [DOI] [PubMed] [Google Scholar]
- 12.Eteraf-Oskouei T, Najafi M. Traditional and modern uses of natural honey in human diseases: a review. Iran J Basic Med Sci. 2013;16:731–42. [PMC free article] [PubMed] [Google Scholar]
- 13.Chow J. Probiotics and prebiotics: a brief overview. J Ren Nutr. 2002;12:76–86. doi: 10.1053/jren.2002.31759. [DOI] [PubMed] [Google Scholar]
- 14.Medhi B, et al. Topical application of honey in the treatment of wound healing: a metaanalysis. 2008 [Google Scholar]
- 15.Bansal V, Medhi B, Pandhi P. Honey--a remedy rediscovered and its therapeutic utility. Kathmandu Univ Med J (KUMJ) 2005;3:305–9. [PubMed] [Google Scholar]
- 16.Barnett S, Varley S. The effects of calcium alginate on wound healing. Ann R Coll Surg Engl. 1987;69:153–5. [PMC free article] [PubMed] [Google Scholar]
- 17.Fanucci D, Seese J. Multi-faceted use of calcium alginates. A painless, cost-effective alternative for wound care management. Ostomy Wound Manage. 1991;37:16–22. [PubMed] [Google Scholar]
- 18.Bogdanov S. Characterisation of antibacterial substances in honey. Lebensm.-Wiss. Technol. 1984;17:74–76. [Google Scholar]
- 19.Brown JH. Bergey’s manual of determinative bacteriology. American Public Health Association; 1939. [Google Scholar]
- 20.Wayne P. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; approved guideline. 2010 Document M45-A2. [Google Scholar]
- 21.Badmasti F, Siadat SD, Bouzari S, Ajdary S, Shahcheraghi F. Molecular detection of genes related to biofilm formation in multidrug-resistant acinetobacter baumannii isolated from clinical settings. J Med Microbiol. 2015;64:538–543. doi: 10.1099/jmm.0.000058. [DOI] [PubMed] [Google Scholar]
- 22.Basualdo C, Sgroy V, Finola MS, Marioli JM. Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds. Vet Microbiol. 2007;124:375–81. doi: 10.1016/j.vetmic.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 23.Balakrishnan B, Mohanty M, Umashankar PR, Jayakrishnan A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials. 2005;26:6335–6342. doi: 10.1016/j.biomaterials.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Saarai A, et al. A comparative study of crosslinked sodium alginate/gelatin hydrogels for wound dressing. In recent researches in geography, geology, energy, environment and biomedicine-proc of the 4th WSEAS Int Conf on EMESEG. 2011 [Google Scholar]
- 25.Buslov DK, Nikonenko NA. A priori estimation of the parameters of the method of spectral curve deconvolution. Applied Spectroscopy. 1998;52:613–620. [Google Scholar]
- 26.Farjah A, Owlia P, Siadat SD, Mousavi SF, Ardestani MS, Mohammadpour HK. Immunological evaluation of an alginate-based conjugate as a vaccine candidate against pseudomonas aeruginosa. Apmis. 2015;123:175–183. doi: 10.1111/apm.12337. [DOI] [PubMed] [Google Scholar]
- 27.Cai EZ, Ang CH, Raju A, Tan KB, Hing EC, Loo Y, Wong YC, Lee H, Lim J, Moochhala SM, Hauser CA, Lim TC. Creation of consistent burn wounds: a rat model. Arch Plast Surg. 2014;41:317–24. doi: 10.5999/aps.2014.41.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40:277–83. [PMC free article] [PubMed] [Google Scholar]
- 29.Lachiewicz AM, Hauck CG, Weber DJ, Cairns BA, van Duin D. Bacterial infections after burn injuries: impact of multidrug resistance. Clin Infect Dis. 2017;65:2130–2136. doi: 10.1093/cid/cix682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forson OA, Ayanka E, Olu-Taiwo M, Pappoe-Ashong PJ, Ayeh-Kumi PJ. Bacterial infections in burn wound patients at a tertiary teaching hospital in Accra, Ghana. Ann Burns Fire Disasters. 2017;30:116–120. [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Waili N, Salom K, Al-Ghamdi AA. Honey for wound healing, ulcers, and burns; data supporting its use in clinical practice. ScientificWorldJournal. 2011;11:766–87. doi: 10.1100/tsw.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du E, Gan L, Li Z, Wang W, Liu D, Guo Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with clostridium perfringens. J Anim Sci Biotechnol. 2015;6:58. doi: 10.1186/s40104-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahzad MN, et al. Bacterial profile of burn wound infections in burn patients. Ann Pak Inst Med Sci. 2012;8:54–57. [Google Scholar]


