Abstract
Objective
This study aimed to investigate associations between maternal history of rotating night shift nursing work before pregnancy and number of night shifts worked during pregnancy with offspring weight outcomes from early life through adolescence.
Methods
More than 4,000 children, enrolled in the second phase of the Growing Up Today Study between 2004 and 2013, and their mothers participating in the Nurses' Health Study II were included in our analyses.
Results
Children of women with and without a history of rotating night shift work before pregnancy were similar in birth weight and body size at age 5. However, for mothers with night shift work before pregnancy, their children had a modestly elevated risk of having overweight or obesity (relative risk = 1.11; 95% CI: 1.02‐1.21), which was stronger for persistently having overweight or obesity during adolescence and early adulthood. Longer duration of rotating night shift work was not associated with any of these weight outcomes. Weight outcomes of children of women with versus without night shift work during pregnancy were similar, regardless of frequency of night shifts worked during pregnancy (all P > 0.09).
Conclusions
Overall, nurses' night shift work before or during pregnancy did not affect offspring weight outcomes. Future larger studies should explore these associations in more detail.
Introduction
Childhood and adolescent overweight and obesity have become a major public health concern in the United States and globally (1). Apart from childhood obesity's more immediate comorbidities, such as hyperinsulinemia, poor glucose tolerance, and increased risk for type 2 diabetes and hypertension, it can also lead to an increased risk of heart disease and certain cancers later in life (2). Although environmental and behavioral factors, such as parental socioeconomic status and children's sedentary behavior, have been established as major determinants of childhood obesity (3), growing evidence has suggested that several childhood and even adulthood health outcomes are, at least in part, programmed during pregnancy (4). Specifically, the maternal environment during pregnancy could influence fetal development and programming through alterations in the intrauterine environment (5).
Night shift work and the resulting disruption of social and biological rhythms have been linked to higher risk of several chronic diseases (6, 7, 8) as well as epigenetic alterations (9); in addition, night shift work during pregnancy has been linked to an increased risk of spontaneous abortions (10) and preterm delivery (11). Emerging evidence from animal models has suggested that maternal circadian disruption can also induce long‐term metabolic changes in the offspring (12, 13, 14). For example, exposing pregnant rats to reversals of the photoperiod twice every week throughout gestation and for the first week after birth showed no effect on litter size or birth weight; however, the progeny had an increased risk of adiposity, hyperleptinemia, and altered glucose metabolism in adulthood (13).
Despite the high prevalence of shift work among US women during pregnancy (15), little is known about the potential health implications of preconception shift work and night shift work during pregnancy on offspring later in life. We therefore aimed to provide insights regarding the association of preconception night shift work history and shift work during pregnancy with offspring weight outcomes during childhood and adolescence using existing data from mother‐child pairs participating in the Nurses' Health Study II (NHS II) and the second phase of the Growing Up Today Study (GUTS2).
Methods
Study population
The present study included mothers who were enrolled in NHS II and their children who participated in GUTS2. NHS II is an ongoing prospective cohort study of US female nurses, which was established in 1989 when 116,430 female nurses aged 25 to 42 years responded to mailed questionnaires regarding their lifestyle, reproductive factors, and medical history. Follow‐up questionnaires are mailed biennially to these nurses to update information on various risk factors and occurrence of major diseases. GUTS2 began in 2004 when NHS II participants who had previously reported to have children born between 1987 and 1995 were asked whether their children could participate in a follow‐up study. After receiving maternal consent, invitation letters and questionnaires were sent to 17,280 children; 10,918 children returned their completed questionnaire. Follow‐up questionnaires were sent to these children in 2006, 2008, 2011, and 2013 to update information on health, lifestyle factors, and growth indicators.
This study was approved by the Human Subjects Committees at the Brigham and Women's Hospital and the Harvard T.H. Chan School of Public Health (Boston, Massachusetts). Returning the self‐administered questionnaires was taken to imply informed consent in both cohorts.
Ascertainment of night shift work among mothers
Mother's history of rotating night shift before pregnancy
The NHS II baseline questionnaire queried number of years having worked rotating night shifts (defined as “at least three nights per month in addition to working days or evenings in the respective month”). This information was updated in 1991 and 1993 to capture changes since the previous questionnaire and then again in 2001 to capture changes between 1993 and 1995 (the latest possible year of conception). Based on the return date of the respective NHS II questionnaire and birth date of the GUTS2 participant, we calculated the cumulative number of years a mother had worked rotating night shifts before conception for children born in 1989 or later.
Mother's night shift work exposure during pregnancy
On the 2001 NHS II questionnaire, participants were asked whether they had experienced at least one pregnancy since 1993, had worked as a nurse during the most recent pregnancy, and would be willing to answer a supplemental questionnaire focused on occupational activities and exposures during this pregnancy. A supplement´l questionnaire that queried shift work information among other occupational exposures during the most recent pregnancy since 1993 was mailed to those participants who answered “yes” to all three questions. Women were asked to report usual working schedules and average number of night shifts per month for each trimester of pregnancy, with the following response options: none, one to two nights per month, three to four nights per month, two to three nights per week, and four or more nights per week.
Ascertainment of weight outcomes among offspring
Information on offspring birth weight was obtained from the 2009 GUTS2 mothers' questionnaire. Recall of offspring birth weight has been found to have excellent validity (16). On the baseline questionnaire in 2004, GUTS2 participants (age range 9‐15 years) were asked to recall their body size at age 5 by selecting one of eight pictograms (“somatotypes”) that would most accurately represent their body shape at that age (ranging from 1, most lean, to 8, most severe obesity). Correlations between recalled somatotypes and BMI measured at approximately the same ages have ranged from 0.53 to 0.75 among studies of adults (17). We created a binary outcome splitting at the median value of the distribution and defined larger than median body sizes as cases. Information on weight and height was self‐reported on each GUTS2 questionnaire. To classify the offspring as having normal weight, overweight, or obesity, we used age‐and sex‐specific cutoffs from the International Obesity Task Force (18) for participants aged 18 years or younger. Beyond age 18, overweight and obesity were defined using standard World Health Organization cutoffs (i.e., BMI [kilograms per meter squared] 25.0‐29.9 as overweight; BMI > 30 as obesity). We defined participants as cases if they were classified as having either overweight or obesity at any given time during follow‐up. Additionally, we defined participants as persistently having overweight or obesity if they were classified as having overweight or obesity at three consecutive questionnaire cycles. Figure 1 displays a timeline of exposure and outcomes assessments.
Figure 1.
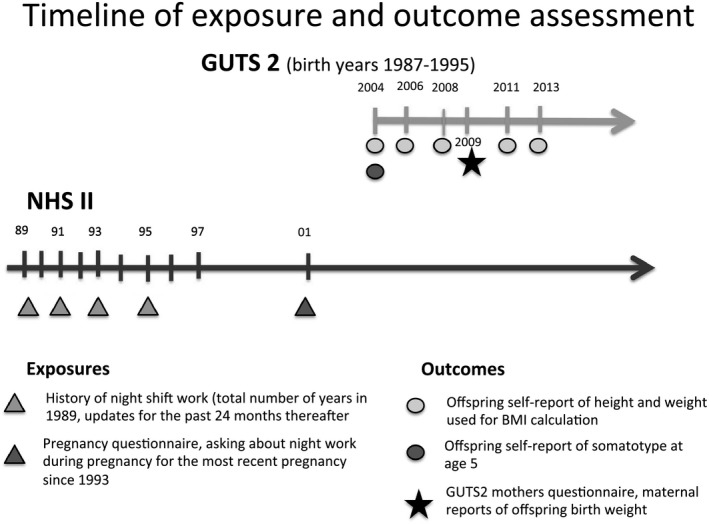
Timeline of exposure and outcome assessments in NHSII and GUTS2 cohorts.
Ascertainment of covariates
Maternal age at delivery was derived calculating the difference between a mother's birth date and that of her child. We utilized maternal dietary information assessed by the well‐validated Willett food frequency questionnaire (19), using information from the 1991 NHS II questionnaire to calculate the Alternative Healthy Eating Index (AHEI) (20). Physical activity was assessed in 1989 and derived as metabolic equivalent hours per week (21). Smoking status and BMI were queried biannually, and we selected the most recent value prior to conception of the first included child. Maternal chronotype was assessed in 2009. As a proxy for socioeconomic status, we used husband's education, which was assessed in 1999.
Information on the number of previous pregnancies, mode of delivery, pregnancy‐related hypertension, preeclampsia, gestational age at delivery, and pregnancy multiplicity was extracted from the lifetime pregnancy assessment in 2009. We approximated pregnancy‐related weight gain by taking the difference of the first BMI reported after delivery and the last recorded BMI before conception.
From the occupational supplemental questionnaire, we had trimester‐specific information on smoking behavior, alcohol consumption, coffee consumption, and frequency of activities involving lifting or moving a physical load of 25 lb or more during pregnancy.
Offspring Tanner stage of pubertal development was determined in 2004 on the GUTS2 baseline questionnaire, using a validated scale of pubic hair illustrations (22). Tanner stage ranges from 1 to 5, with stage 1 indicating prepubescence and stage 5 indicating maturity. Offspring sedentary behavior was summarized by the sum of the hours per week spent watching television, using the computer, surfing on the Internet, and reading or doing homework over the past year, as reported in 2004. Detailed cutoffs used in all analyses are presented in the footnotes of Tables 1 and 2.
Table 1.
Adjusted mean differences and relative risks for offspring weight outcomes through childhood and adolescence according to maternal rotating night shift work history before pregnancy using data from GUTS2 from 2004 to 2013, restricted to singleton full‐term births
| History of rotating night shift work | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Never worked rotating night shifts | <3 years | 3‐5 years | ≥6 years | P trend | Ever worked rotating night shifts | |||||
| MD in offspring birth weight (g) | ||||||||||
| Participants | 1,458 | 1,305 | 964 | 416 | 2,685 | |||||
| MD | 95% CI | MD | 95% CI | MD | 95% CI | MD | 95% CI | |||
| Basic modela | 0 (reference) | 10.68 | −26.33 to 47.69 | 0.98 | −39.06 to 41.03 | −7.80 | −65.11 to 49.51 | 0.79 | 4.41 | −27.48 to 36.30 |
| MV model 1b | 0 (reference) | 12.16 | −24.54 to 48.87 | 4.73 | −34.55 to 44.02 | −1.43 | −59.29 to 56.43 | 0.99 | 7.51 | −23.89 to 38.92 |
| MV model 2c | 0 (reference) | 10.33 | −26.26 to 49.91 | 2.39 | −36.85 to 41.64 | −6.28 | −63.84 to 51.27 | 0.85 | 5.06 | −26.32 to 36.44 |
| RR of offspring's somatotype at age 5 being larger than the median | ||||||||||
| Cases/participants | 628/1,631 | 584/1,451 | 437/1,103 | 186/474 | 1,207/3,028 | |||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |||
| Basic modela | 1 (reference) | 1.04 | 0.95 to 1.14 | 1.02 | 0.93 to 1.13 | 1.01 | 0.89 to 1.15 | 0.82 | 1.03 | 0.95 to 1.11 |
| MV model 1b | 1 (reference) | 1.03 | 0.94 to 1.12 | 1.02 | 0.92 to 1.12 | 1.01 | 0.88 to 1.15 | 0.87 | 1.02 | 0.95 to 1.10 |
| MV model 2c | 1 (reference) | 1.02 | 0.94 to 1.12 | 1.01 | 0.92 to 1.12 | 1.00 | 0.88 to 1.14 | 0.97 | 1.02 | 0.94 to 1.10 |
| RR of offspring having overweight or obesity at any time between 2004 and 2013 d | ||||||||||
| Cases/participants | 523/1,667 | 523/1,494 | 394/1,125 | 168/487 | 1,085/3,106 | |||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |||
| Basic modela | 1 (reference) | 1.10 | 1.00 to 1.22 | 1.11 | 1.00 to 1.24 | 1.08 | 0.94 to 1.25 | 0.11 | 1.10 | 1.01 to 1.20 |
| MV model 1b | 1 (reference) | 1.11 | 1.01 to 1.23 | 1.12 | 1.01 to 1.25 | 1.07 | 0.92 to 1.23 | 0.14 | 1.11 | 1.02 to 1.21 |
| MV model 2c | 1 (reference) | 1.11 | 1.00 to 1.22 | 1.10 | 0.99 to 1.23 | 1.03 | 0.89 to 1.18 | 0.31 | 1.09 | 1.00 to 1.19 |
| RR of offspring persistently having overweight or obesity e | ||||||||||
| Cases/participants | 114/1,667 | 124/1,494 | 103/1,125 | 37/487 | 264/3,106 | |||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |||
| Basic modela | 1 (reference) | 1.19 | 0.92 to 1.54 | 1.35 | 1.04 to 1.76 | 1.07 | 0.74 to 1.55 | 0.13 | 1.23 | 0.99 to 1.53 |
| MV model 1b | 1 (reference) | 1.22 | 0.95 to 1.58 | 1.39 | 1.06 to 1.80 | 1.04 | 0.71 to 1.52 | 0.17 | 1.25 | 1.00 to 1.56 |
| MV model 2c | 1 (reference) | 1.21 | 0.94 to 1.55 | 1.33 | 1.02 to 1.73 | 0.96 | 0.67 to 1.40 | 0.31 | 1.21 | 0.97 to 1.51 |
| MD in offspring BMI (kg/m2) using n = 15,008 repeated measurements, 2004‐2013 | ||||||||||
| Observations | 5,309 | 4,674 | 3,528 | 1,487 | 9,689 | |||||
| MD | 95% CI | MD | 95% CI | MD | 95% CI | MD | 95% CI | |||
| Basic modela,f | 0 (reference) | 0.04 | −0.20 to 0.29 | 0.22 | −0.04 to 0.49 | 0.53 | 0.17 to 0.89 | <0.01 | 0.18 | −0.03 to 0.39 |
| MV model 1b | 0 (reference) | 0.07 | −0.18 to 0.31 | 0.26 | −0.01 to 0.52 | 0.49 | 0.13 to 0.85 | <0.01 | 0.20 | −0.01 to 0.41 |
| MV model 2c | 0 (reference) | 0.03 | −0.21 to 0.26 | 0.20 | −0.06 to 0.45 | 0.33 | −0.02 to 0.67 | 0.03 | 0.13 | −0.01 to 0.41 |
Abbreviations: CI, confidence interval; MD, mean difference; MV, multivariable model; RR, relative risk.
Adjusted for offspring gender (boy/girl) and gestational age ≥ 42 weeks (yes/no).
Additionally adjusted for maternal age at pregnancy, smoking status before pregnancy (never, current, past), alternative healthy eating score (quintiles), physical activity (MET‐hours/week; quintiles), husband's education (less than 2 years of college, 4 years of college, graduate school), parity (nulliparity, 1, 2, or 3+ previous pregnancies).
Additionally adjusted for BMI before pregnancy (<25, 25‐29, ≥30 kg/m2).
Overweight and obesity status defined according to age‐ and sex‐specific cutoffs from International Obesity Task Force.
Persistently having overweight or obesity was defined as falling into that category in three consecutive follow‐up cycles.
All models are additionally adjusted for age at BMI measurement.
Table 2.
Adjusted mean differences and relative risks for offspring weight outcomes through childhood and adolescence by categories of average number of night shifts worked per month during pregnancy using data from GUTS2 from 2004 to 2013 restricted to singleton, full‐term births (n = 545)
| Average number of night shifts/ month | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None | <3 nights/month | 3‐9 nights/month | ≥10 nights/month | P trend | Any number nights/month | |||||
| MD in offspring birth weight (g) | ||||||||||
| Participants | 352 | 39 | 17 | 35 | 91 | |||||
| MD | 95% CI | MD | 95% CI | MD | 95% CI | MD | 95% CI | |||
| Basic modela | 0 (reference) | −54.19 | −209.96 to 101.58 | −203.53 | −432.97 to 25.92 | −46.27 | −210.25 to 117.70 | 0.23 | −79.04 | −187.53 to 29.45 |
| MV model 1b | 0 (reference) | −54.48 | −215.12 to 106.17 | −231.85 | −463.36 to −0.33 | 18.94 | −146.85 to 184.73 | 0.58 | −57.76 | −170.10 to 54.58 |
| MV model 2c | 0 (reference) | −64.28 | −225.75 to 97.19 | −223.23 | −456.26 to 9.80 | 19.51 | −146.50 to 185.51 | 0.60 | −59.64 | −172.11 to 52.82 |
| RR of offspring's somatotype at age 5 being larger than the median | ||||||||||
| Cases/participants | 159/417 | 16/43 | 8/21 | 10/45 | 34/109 | |||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |||
| Basic modela | 1 (reference) | 0.95 | 0.64 to 1.42 | 1.01 | 0.57 to 1.78 | 0.59 | 0.34 to 1.04 | 0.08 | 0.82 | 0.60 to 1.11 |
| MV model 1b | 1 (reference) | 0.96 | 0.62 to 1.47 | 1.03 | 0.60 to 1.78 | 0.59 | 0.33 to 1.04 | 0.09 | 0.82 | 0.60 to 1.12 |
| MV model 2c | 1 (reference) | 0.96 | 0.63 to 1.48 | 1.06 | 0.61 to 1.84 | 0.59 | 0.33 to 1.05 | 0.10 | 0.82 | 0.60 to 1.13 |
| RR of offspring having overweight or obesity at any time between 2004 and 2013 d | ||||||||||
| Cases/participants | 156/431 | 12/43 | 4/21 | 13/45 | 29/109 | |||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |||
| Basic modela | 1 (reference) | 0.75 | 0.46 to 1.21 | 0.54 | 0.22 to 1.33 | 0.82 | 0.51 to 1.31 | 0.19 | 0.74 | 0.53 to 1.03 |
| MV model 1b | 1 (reference) | 0.76 | 0.45 to 1.29 | 0.51 | 0.20 to 1.27 | 0.80 | 0.49 to 1.29 | 0.16 | 0.73 | 0.51 to 1.03 |
| MV model 2c | 1 (reference) | 0.80 | 0.48 to 1.35 | 0.53 | 0.21 to 1.37 | 0.78 | 0.48 to 1.25 | 0.14 | 0.74 | 0.52 to 1.05 |
| MD in offspring BMI (kg/m2) using n = 1,632 repeated measurements, 2004‐2013 | ||||||||||
| Observations | 1,306 | 120 | 64 | 142 | 326 | |||||
| MD | 95% CI | MD | 95% CI | MD | 95% CI | MD | 95% CI | |||
| Basic modela,e | 0 (reference) | 0.24 | −0.85 to 1.32 | −0.27 | −1.78 to 1.23 | −0.02 | −1.07 to 1.03 | 0.88 | 0.03 | −0.69 to 0.75 |
| MV model 1b | 0 (reference) | 0.21 | −0.91 to 1.34 | −0.54 | −2.08 to 1.00 | −0.21 | −1.29 to 0.87 | 0.56 | −0.11 | −0.86 to 0.64 |
| MV model 2c | 0 (reference) | 0.45 | −0.64 to 1.54 | −0.26 | −1.75 to 1.24 | −0.31 | −1.35 to 0.73 | 0.53 | −0.01 | −0.74 to 0.72 |
Abbreviations: CI, confidence interval; MD, mean difference; MV, multivariable model; RR, relative risk.
Adjusted for offspring gender (boy/girl) and gestational age ≥ 42 weeks (yes/no).
Additionally adjusted for maternal age at pregnancy, smoking status before pregnancy (never, current, past), alternative healthy eating score (quintiles), physical activity (MET‐hours /week; quintiles), husband's education (less than 2 years of college, 4 years of college, graduate school), parity (nulliparity, 1, 2, or 3+ previous pregnancies), history of rotating night shift work (never, <3 years, 3‐5 years, ≥6 years).
Additionally adjusted for BMI before pregnancy (<25, 25‐29, ≥30 kg/m2).
Overweight and obesity status defined according to age‐ and sex‐specific cutoffs from International Obesity Task Force.
All models are additionally adjusted for age at BMI measurement.
Statistical analysis
Mother's history of rotating night shift exposure before conception
As preconception history of rotating night shift work could be assessed only after 1989, we excluded the 4,721 children conceived before 1989 from the original cohort of 10,918 children. Among the remainder born after 1989, we further excluded twins and triplets (195 children, 87 mothers), children who were not born full term (less than 37 weeks) (1,182 children, 1,001 mothers), and mother‐child pairs with missing exposure information (7 children, 6 mothers), leaving 4,813 children born to 4,044 mothers that made up our analytic sample. Out of these, 91% had a least two measurements of BMI at different time points.
We used generalized estimating equation regression models specifying an exchangeable correlation structure with appropriate link functions (to account for within‐sibling correlation in outcomes) to estimate mean differences and 95% confidence intervals (CIs) in offspring birth weight, comparing none versus any number of years of rotating night shift work as well as across four data‐driven categories of cumulative years of rotating night shift work before conception (none, < 3 years, 3‐5 years, and ≥ 6 years). We calculated relative risks (RR) and 95% CIs of offspring overweight and/or obesity and larger than median somatotype at age 5. We considered women without a history of rotating night shift work as the reference group. If the log‐binomial model did not converge, we approximated RRs using Poisson models with robust variance estimators (23).
To utilize all available repeated measurements of offspring BMI, we applied linear mixed effects models with random intercepts and an unstructured covariance structure, which permit description of individual BMI trajectories across age and provide explicit tests for changes in BMI with age (24). In these analyses, we included terms for the exposure, covariates, offspring age, and the interaction of BMI and offspring age to evaluate differences in change in BMI over time across exposure groups.
In basic models, we adjusted for offspring sex, gestational age, and offspring age at baseline where appropriate (basic model). We then considered the inclusion of several sets of potential confounding variables. First, we added maternal lifestyle characteristics (multivariate [MV] model 1), including smoking status before pregnancy, AHEI score, physical activity, husband's education, and parity. The addition of pregnancy‐related factors, including preeclampsia gestational hypertension or diabetes, type of delivery, or change in maternal BMI before and after pregnancy, did not markedly change effect estimates for shift work; therefore, these variables were not retained in the model. Lastly, we additionally adjusted for maternal BMI before pregnancy (MV model 2).
Mother's night shift work exposure during pregnancy
For analyses assessing associations between night shift work during pregnancy and weight outcomes in the offspring, we identified all GUTS2 participants whose mothers had completed the supplemental pregnancy questionnaire between 2001 and 2003, resulting in 621 mother‐child pairs with available information on pregnancy behaviors coupled with outcome information on the matching GUTS2 child. Of these, only full‐term pregnancies were considered eligible (n = 549). After further removal of mother‐child pairs with missing exposure information, 545 matching pairs were left for these analyses, of which 89% had at least two repeated BMI measurements.
Using trimester‐specific information on shift work frequency from the supplemental questionnaire, we calculated the average number of night shifts per month throughout the entire pregnancy and created four categories (none, ≤ 2, 3‐9, and ≥ 10 night shifts per month); we also created the category of any number of night shifts per month.
We used standard generalized linear models with appropriate link functions (linear or log‐binomial/Poisson) and added covariates following a similar approach as described above; we added history of night shift work to MV model 1. We also considered maternal pregnancy‐related lifestyle factors (smoking behavior, alcohol consumption, coffee consumption, heavy lifting during first trimester), but we did not retain these variables in our main models because they did not alter our estimates (data not shown).
Because previous studies have found maternal BMI before pregnancy (25) to be one of the strongest predictors of offspring obesity during adolescence, in addition to adjustment for maternal BMI, we assessed effect modification for offspring weight outcomes by maternal BMI before pregnancy. Maternal chronotype has also been found to potentially interact with work schedules in determining a person's disease risk (26, 27, 28); therefore, we examined whether associations were different in children born to mothers with reportedly different chronotypes. In all analyses, missing indicators were created for missing covariate values. P values for interactions were obtained by testing the significance of multiplicative interaction terms in multivariable regression models. All statistical tests were two‐sided and were considered statistically significant at P < 0.05. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
Mother's history of rotating night shift work before pregnancy
The 4,044 mothers were on average 33.4 (SD = 3.6) years old when they gave birth to the children included in this analysis, and 65% of them reported ever having worked night shifts. At enrollment in GUTS2 in 2004, children were on average 11.7 (SD = 1.2) years old. Overall, there were only modest differences in terms of maternal and offspring characteristics according to history of night shift work before pregnancy (Table 3). Mothers who had worked night shifts for 6 years or more prior to conception of the first considered child were older, more likely to be past or current smokers, more adherent to a healthy diet (AHEI), and more physically active compared with women with no history of working night shifts. Also, offspring born to mothers with longer shift work history were more likely to be delivered by Cesarean section compared with those born to mothers without a history of shift work.
Table 3.
Maternal and offspring characteristics by rotating night shift work history before pregnancy for 4,044 mothers of a total of 4,813 children born between 1989 and 1995 enrolled in GUTS2
| History of rotating night shift work | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Never worked rotating night shifts (n = 1,424) | <3 y (n = 1,254) | 3‐5 y (n = 946) | ≥6 y (n = 420) | Ever worked rotating night shifts (n = 2,620) | |||||
| Mean (SD) | % a | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | |
| Maternal age at deliveryb | 33.1 (3.5) | 33.2 (3.6) | 33.4 (3.5) | 35.0 (3.2) | 33.6 (3.5) | |||||
| BMI before pregnancy | 22.7 (3.9) | 22.7 (3.8) | 23.0 (3.9) | 23.3 (4.2) | 22.9 (3.9) | |||||
| AHEIc | 42.6 (10.4) | 43.5 (9.9) | 43.7 (10.2) | 44.3 (10.2) | 43.7 (10.0) | |||||
| Physical activity, METs/wkd | 18.9 (21.4) | 23.2 (31.4) | 22.4 (28.7) | 22.6 (31.1) | 22.8 (30.4) | |||||
| Smoking history before pregnancyb | ||||||||||
| Never | 75.3 | 74.0 | 73.1 | 63.2 | 71.9 | |||||
| Past | 18.2 | 19.6 | 20.2 | 26.3 | 20.9 | |||||
| Current | 6.5 | 6.4 | 6.7 | 10.5 | 7.2 | |||||
| Husband holds graduate degree | 31.9 | 36.5 | 37.1 | 31.1 | 35.9 | |||||
| Mom's chronotype | ||||||||||
| Definite morning type | 31.9 | 36.0 | 34.4 | 37.4 | 35.6 | |||||
| Intermediate type | 56.9 | 55.0 | 55.6 | 52.1 | 54.8 | |||||
| Definite evening type | 11.2 | 9.0 | 10.0 | 10.5 | 9.6 | |||||
| Parity before first included pregnancy | ||||||||||
| Nulliparity | 20.3 | 21.8 | 22.0 | 20.5 | 21.6 | |||||
| One previous pregnancy | 32.4 | 29.0 | 30.0 | 30.7 | 29.7 | |||||
| Two previous pregnancies | 24.0 | 25.8 | 26.0 | 25.2 | 25.8 | |||||
| Three previous pregnancies | 23.3 | 23.4 | 22.0 | 23.6 | 22.9 | |||||
| Number of pregnancies (n) | 1,683 | 1,507 | 1,132 | 491 | 3,130 | |||||
| Gestational diabetes | 4.9 | 5.9 | 5.1 | 6.5 | 5.7 | |||||
| Preeclampsia | 2.3 | 2.4 | 3.3 | 2.7 | 2.8 | |||||
| Gestational hypertension | 4.1 | 3.7 | 5.0 | 5.3 | 4.4 | |||||
| Cesarean delivery | 21.6 | 21.6 | 23.7 | 27.1 | 23.2 | |||||
| Gestational age ≥ 42 weeks | 4.7 | 4.8 | 4.3 | 4.9 | 4.7 | |||||
| Maternal change in BMI from before to after current pregnancy | 1.0 (1.7) | 1.0 (1.6) | 1.1 (1.7) | 1.0 (1.8) | 1.1 (1.7) | |||||
| Offspring sex | ||||||||||
| Male | 46.0 | 45.0 | 48.2 | 49.3 | 46.8 | |||||
| Female | 54.0 | 55.0 | 51.8 | 50.7 | 53.2 | |||||
| Offspring age at GUTS2 baseline, 2004 | 11.8 (1.2) | 11.6 (1.2) | 11.6 (1.2) | 11.6 (1.3) | 11.6 (1.2) | |||||
| Offspring Tanner stagee | 2.8 (1.3) | 2.6 (1.3) | 2.6 (1.3) | 2.7 (1.3) | 2.6 (1.3) | |||||
| Offspring sedentary behavior, h/wke | 4.0 (2.8) | 3.9 (2.7) | 3.9 (2.5) | 4.1 (2.4) | 3.9 (2.6) | |||||
METs, metabolic equivalent hours.
Percentages are of nonmissing values.
Recorded on the most recent questionnaire prior to conception of first included offspring.
Recorded in 1991.
One MET is proportional to the amount of energy spent sitting quietly for 1 hour.
At GUTS2 baseline (2004).
Overall, when we evaluated the associations between night shift work history before pregnancy with different weight outcomes across childhood and adolescence, we observed few differences in offspring weight when comparing women with and without night shift work history (Table 4). Because differences between basic and multivariable models were small, we describe results of fully adjusted models only (i.e., MV model 1 including the most important confounding variables).
Table 4.
Maternal and offspring characteristics by categories of average number of night shifts per month during pregnancy for 545 mothers and 545 children born between 1993 and 1995 enrolled in GUTS2 with whom they were pregnant during reported night work
| Average number of night shifts per month during pregnancy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
None
( n = 435) |
<3 nights/month
( n = 44) |
3‐9 nights/month
( n = 21) |
≥10 nights/month
( n = 45) |
Any number nights/month
( n = 110) |
||||||
| Mean (SD) | % a | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | |
| Maternal age at deliveryb | 34.9 (3.4) | 33.8 (2.5) | 34.4 (2.8) | 34.0 (3.1) | 34.0 (2.8) | |||||
| BMI before pregnancy | 23.9 (4.5) | 23.1 (3.6) | 22.8 (3.4) | 24.1 (4.1) | 23.5 (3.8) | |||||
| AHEIc | 42.7 (9.9) | 44.4 (11.6) | 40.3 (8.9) | 38.4 (9.4) | 41.0 (10.5) | |||||
| Physical activity, METs/wkd | 23.2 (34.0) | 23.9 (27.2) | 13.3 (13.1) | 17.7 (19.6) | 19.3 (22.2) | |||||
| Smoking before pregnancyb | ||||||||||
| Never | 76.0 | 72.7 | 76.2 | 71.1 | 72.7 | |||||
| Past | 18.7 | 25.0 | 14.3 | 22.2 | 21.8 | |||||
| Current | 5.3 | 2.3 | 9.5 | 6.7 | 5.5 | |||||
| Husband holding graduate degree | 29.4 | 22.7 | 33.3 | 24.4 | 25.5 | |||||
| Mom's chronotype | ||||||||||
| Definite morning type | 29.6 | 43.6 | 10.0 | 20.5 | 27.6 | |||||
| Intermediate type | 60.6 | 41.0 | 70.0 | 51.3 | 51.0 | |||||
| Definite evening type | 9.8 | 15.4 | 20.0 | 28.2 | 21.4 | |||||
| Parity | ||||||||||
| Nulliparity | 6.0 | 5.4 | 0 | 15.4 | 8.5 | |||||
| One previous pregnancy | 28.3 | 29.7 | 33.3 | 28.2 | 29.8 | |||||
| Two previous pregnancies | 32.7 | 37.9 | 33.3 | 23.1 | 30.8 | |||||
| Three previous pregnancies | 33.0 | 27.0 | 33.3 | 33.3 | 30.9 | |||||
| History of rotating night shift work | ||||||||||
| None | 34.7 | 6.8 | 14.3 | 35.6 | 20.0 | |||||
| <3 y | 36.6 | 40.9 | 33.3 | 24.4 | 32.7 | |||||
| 3‐5 y | 21.6 | 31.8 | 38.1 | 24.4 | 30.0 | |||||
| ≥6 y | 7.1 | 20.5 | 14.3 | 15.6 | 17.3 | |||||
| Number of pregnancies (n) | 435 | 44 | 21 | 45 | 110 | |||||
| Gestational diabetes | 6.4 | 2.3 | 4.8 | 4.4 | 3.6 | |||||
| Preeclampsia | 0.9 | 0.0 | 0.0 | 2.2 | 0.9 | |||||
| Gestational hypertension | 3.0 | 2.3 | 0.0 | 6.7 | 3.6 | |||||
| Cesarean delivery | 22.3 | 18.2 | 14.3 | 20.0 | 18.2 | |||||
| Gestational age ≥42 weeks | 5.8 | 9.1 | 0.0 | 2.2 | 4.6 | |||||
| Maternal change in BMI from before to after current pregnancy | 0.8 (1.7) | 0.6 (1.9) | 0.8 (1.7) | 1.2 (2.0) | 0.9 (1.9) | |||||
| Offspring gender | ||||||||||
| Male | 45.3 | 52.3 | 47.6 | 42.2 | 47.3 | |||||
| Female | 54.7 | 47.7 | 52.4 | 57.8 | 52.7 | |||||
| Offspring age at GUTS2 baseline, 2004 | 10.0 (0.6) | 9.9 (0.6) | 10.0 (0.6) | 10.0 (0.6) | 10.0 (0.6) | |||||
| Offspring Tanner stagee | 1.5 (0.7) | 1.6 (0.8) | 1.5 (0.8) | 1.5 (0.8) | 1.5 (0.8) | |||||
|
Offspring sedentary behavior (h/wk)e |
3.5 (2.3) | 3.1 (1.5) | 3.9 (2.5) | 4.1 (2.4) | 3.7 (2.1) | |||||
Percentages are of nonmissing values.
Recorded on the most recent questionnaire prior to conception of first included offspring.
Recorded in 1991.
One MET is proportional to amount of energy spent sitting quietly for 1 hour.
At GUTS2 baseline (2004).
METs, metabolic equivalent hours.
There were no associations of history of shift work with birth weight or somatotype at age 5 (Table 4). We did find a significantly higher risk of the offspring having overweight or obesity at any time during follow‐up between 2004 and 2013 for mothers with any history of night shift work before pregnancy (MV RRany 1.11; 95% CI: 1.02‐1.21) when compared with mothers who never worked any night shifts (Table 4). The association was slightly more pronounced for the risk of persistently having overweight or obesity (MV RRany 1.25; 95% CI: 1.00‐1.56). However, there was no evidence of a dose‐response association with increasing number of years of night shift work history (overweight/obesity MV RRextreme 1.07; 95% CI: 0.92‐1.23; P trend = 0.14; persistently overweight/obesity MV RRextreme 1.04; 95% CI: 0.71‐1.52; P trend = 0.17). Longer duration of night shift work was not associated with changes in BMI with age (P trend > 0.34) but was associated with greater differences in mean BMI (P trend < 0.01) at baseline age (Table 4).
Because maternal diet was first assessed in 1991, we conducted a sensitivity analysis including only children who were born after 1991, and the results remained largely unchanged. In addition to adjustment for maternal BMI, we examined whether associations were different in children born to mothers with normal weight versus mothers with overweight or obesity. Formal tests for interactions revealed no significant effect modification (P interaction for all outcomes > 0.43). When examining whether associations were different in children born to mothers with reportedly different chronotypes (evening versus morning chronotypes), tests for interaction were not significant (all P interaction > 0.44).
Maternal night shift work exposure during pregnancy
Mothers included in the analyses exploring associations between night shift work during pregnancy and weight outcomes in their offspring were, on average, 34.7 (SD = 3.3) years old when they gave birth to the children considered in this analysis, while these children were, on average, 10.0 (SD = 0.6) years old at enrollment in GUTS2 (Table 1). Of the 545 women total, 110 women (20.2%) had worked night shifts during their pregnancy; they worked a mean of 6.0 (SD = 4.8) night shifts per month (range from 0.5 to 16 night shifts per month). Mothers reporting a higher number of night shifts were more likely to be either current or past smokers and more likely to describe themselves as definite evening chronotypes compared with women who did not work night shifts during pregnancy. They also exercised less and adhered to a less healthy diet than mothers with no night shift work during pregnancy.
Because estimates comparing results from basic and multivariable‐adjusted models were similar, we report results from the fully adjusted models (MV model 1) only. We found no associations of night shift work during pregnancy with offspring birth weight, offspring somatotype at age 5, or RR of having overweight or obesity (Table 2); we were unable to examine the risk for persistently having overweight and/or obesity because of small numbers. We observed no differences in the change in BMI at baseline age or over time across exposure groups.
When investigating potential effect modification by maternal BMI before pregnancy and mother's chronotype using collapsed exposure categories (no night shifts, 1‐5 nights per month and ≥6 nights per month), we did not find evidence for effect modification by maternal BMI (P interaction for all outcomes > 0.13) or mother's chronotype (all P interaction > 0.28).
Discussion
In this study conducted among NHS II participants and their children enrolled in GUTS2, we found little evidence to support the hypothesis that a history of night shift work before pregnancy or a higher frequency of night shift work during pregnancy increases the risk of adverse weight outcomes in offspring during childhood and adolescence. Although we observed a modest association between a history of night shift work before pregnancy and the offspring's risk of having overweight or obesity, there was no dose‐response association with longer duration of night shift work before pregnancy, and other weight outcomes were not related to the mother's shift work history before or during pregnancy.
Animal studies have suggested that perturbations at critical windows of development can cause lifelong alterations in adiposity (29). Particularly, studies in rats have demonstrated that disruptions of the maternal photoperiod (i.e., exposing pregnant rats to chronic phase shifts, thereby mimicking shift work) negatively influence several metabolic parameters in the subsequent adult offspring, such as increased adiposity, hyperleptinemia, hyperinsulinemia, and reduced glucose tolerance and insulin sensitivity (12, 13, 30). A recent study modeling the impact of circadian disruption through mice with knockout clock genes did not find any of these associations. However, the authors suggested that previous findings might be explained by mediation through other pathways activated by circadian disruption that may not have been activated in the knockout gene experiment (14).
Previous meta‐analyses have consistently suggested no association between shift work exposure during pregnancy and preterm birth (11, 31, 32) or low birth weight (31, 32), which is in line with our findings. We also did not observe any associations between shift work during pregnancy and birth weight even after adjusting for maternal history of prepregnancy night shift work.
There are virtually no data regarding the potential long‐term impacts of night shift work on weight outcomes after birth on children born to night shift workers.
In our study, we observed no association between night shift work before or during pregnancy and offspring childhood somatotype. While a recent study showed associations between nighttime feeding postpartum and risk for overweight in early childhood (33), our study is the first to examine the relationship between circadian disruption before and during pregnancy and offspring early life weight outcomes. We did find a modestly increased risk for overweight or obesity among offspring born to mothers who worked night shifts at any time before pregnancy, but the risk did not increase with longer duration of shift work. Night work during pregnancy did not further alter this association.
To the best of our knowledge, there is no intergenerational study thus far relating preconception shift work exposure and shift work during pregnancy to offspring outcomes during childhood and adolescence. Strengths of the current study include the longitudinal follow‐up of mothers and children and detailed information on multiple potential confounders, including shift work information during pregnancy. However, our study also has several limitations. We had only limited information on paternal factors. Nevertheless, in all our multivariable models, we adjusted for husband's education, a proxy for socioeconomic status and important confounding variable possibly affecting both maternal shift work exposure and offspring weight outcomes. Most mothers gave birth to their respective child included in our study relatively late, i.e., in their early to mid‐30s. Therefore, our results might not be generalizable to women giving birth at a younger age. Furthermore, power was limited for some secondary analyses, including for mothers who continued to work night shifts throughout their pregnancies and in analyses differentiating having overweight or obesity. Other limitations include the retrospective assessment of body size at age 5 and the self‐reported nature of maternal and offspring weight measures. Previous validation studies within the Harvard cohorts (34, 35, 36, 37, 38) have shown reasonable accuracy of self‐reported maternal physical characteristics. Studies comparing measured versus self‐reported weight and height in US adolescents (39, 40, 41) have concluded that, on average, self‐reports are a useful proxy. Self‐reported history of rotating night shift work may have been misclassified, though likely randomly, biasing our results only toward the null. Furthermore, this exposure has previously been linked to several chronic disease outcomes (42).
Conclusion
Though women's night shift work before pregnancy was associated with a modestly increased risk of having overweight or obesity in their offspring through adolescence and young adulthood, there was no overall evidence that the duration of night shift work before pregnancy or night shift work during pregnancy was associated with offspring adiposity in this study. Further studies are needed with larger sample sizes and more detailed information on shift work exposure and outcomes into adulthood as well as more specific markers of metabolic alterations during adulthood.
Acknowledgments
We thank the thousands of participants in the Growing Up Today Study as well as their mothers.
Strohmaier S, Devore EE, Vetter C, et al. Night Shift Work Before and During Pregnancy and Offspring Weight Outcomes Through Adolescence. Obesity. 2018;00:000–000. 10.1002/oby.22267
Funding agencies: This study was supported by the Centers for Disease Control and Prevention/The National Institute for Occupational Safety and Health grant 5R01OH009803 (PI: Schernhammer E) as well as grant UM1 CA176726 from the National Cancer Institute. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosure: EED has received consulting fees from Epi Excellence and Bohn Epidemiology. The other authors declared no conflict of interest.
Author contributions: SS and EED had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. ESS, SS, SM, and AEF: study concept and design; all authors: acquisition, analysis, or interpretation of data; SS: drafting of the manuscript; all authors: critical revision of the manuscript for important intellectual content; SS and BR: statistical analysis; ESS: obtaining funding; SS: administrative, technical, or material support; and ESS: study supervision.
References
- 1. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lobstein T, Baur L, Uauy R; IASO International Obesity Task Force. Obesity in children and young people: a crisis in public health. Obes Rev 2004;5(suppl 1):4‐104. [DOI] [PubMed] [Google Scholar]
- 3. Vos MB, Welsh J. Childhood obesity: update on predisposing factors and prevention strategies. Curr Gastroenterol Rep 2010;12:280‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol Rev 2016;96:1509‐1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gaillard R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur J Epidemiol 2015;30:1141‐1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vyas MV, Garg AX, Iansavichus AV, et al. Shift work and vascular events: systematic review and meta‐analysis. BMJ 2012;345:e4800. doi:10.1136/bmj.e4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Straif K, Baan R, Grosse Y, et al. Carcinogenicity of shift‐work, painting, and fire‐fighting. Lancet Oncol 2007;8:1065‐1066. [DOI] [PubMed] [Google Scholar]
- 8. Knutsson A, Kempe A. Shift work and diabetes–a systematic review. Chronobiol Int 2014;31:1146‐1151. [DOI] [PubMed] [Google Scholar]
- 9. Zhu YSR, Hoffman AE, Tjonneland A, Vogel UB, Zheng T, Hansen J. Epigenetic impact of long‐term shiftwork: pilot evidence from circadian genes and whole‐genome methylation analysis. Chronobiol Int 2011;28:852‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonde JP, Jorgensen KT, Bonzini M, Palmer KT. Miscarriage and occupational activity: a systematic review and meta‐analysis regarding shift work, working hours, lifting, standing, and physical workload. Scand J Work Environ Health 2013;39:325‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Melick MJ, van Beukering MD, Mol BW, Frings‐Dresen MH, Hulshof CT. Shift work, long working hours and preterm birth: a systematic review and meta‐analysis. Int Arch Occup Environ Health 2014;87:835‐849. [DOI] [PubMed] [Google Scholar]
- 12. Mendez N, Halabi D, Spichiger C, et al. Gestational chronodisruption impairs circadian physiology in rat male offspring, increasing the risk of chronic disease. Endocrinology 2016;157:4654‐4668. [DOI] [PubMed] [Google Scholar]
- 13. Varcoe TJ, Wight N, Voultsios A, Salkeld MD, Kennaway DJ. Chronic phase shifts of the photoperiod throughout pregnancy programs glucose intolerance and insulin resistance in the rat. PloS One 2011;6:e18504. doi:10.1371/journal.pone.0018504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Varcoe TJ, Voultsios A, Gatford KL, Kennaway DJ. The impact of prenatal circadian rhythm disruption on pregnancy outcomes and long‐term metabolic health of mice progeny. Chronobiol Int 2016;33:1171‐1181. [DOI] [PubMed] [Google Scholar]
- 15. Whelan EA, Lawson CC, Grajewski B, Hibert EN, Spiegelman D, Rich‐Edwards JW. Work schedule during pregnancy and spontaneous abortion. Epidemiology 2007;18:350‐355. [DOI] [PubMed] [Google Scholar]
- 16. Tomeo CA, Rich‐Edwards JW, Michels KB, et al. Reproducibility and validity of maternal recall of pregnancy‐related events. Epidemiology 1999;10:774‐777. [PubMed] [Google Scholar]
- 17. Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol 1993;138:56‐64. [DOI] [PubMed] [Google Scholar]
- 18. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51‐65. [DOI] [PubMed] [Google Scholar]
- 20. McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76:1261‐1271. [DOI] [PubMed] [Google Scholar]
- 21. Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25:71‐80. [DOI] [PubMed] [Google Scholar]
- 22. Morris NM, Udry JR. Validation of a self‐administered instrument to assess stage of adolescent development. J Youth Adolesc 1980;9:271‐280. [DOI] [PubMed] [Google Scholar]
- 23. Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005;162:199‐200. [DOI] [PubMed] [Google Scholar]
- 24. Berkey CS, Colditz GA. Adiposity in adolescents: change in actual BMI works better than change in BMI z score for longitudinal studies. Ann Epidemiol 2007;17:44‐50. [DOI] [PubMed] [Google Scholar]
- 25. Dhana K, Zong G, Yuan C, et al. Lifestyle of women before pregnancy and the risk of offspring obesity during early childhood through early adulthood. Int J Obes 2018;42:1275‐1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vetter C, Devore EE, Ramin CA, Speizer FE, Willett WC, Schernhammer ES. Mismatch of sleep and work timing and risk of type 2 diabetes. Diabetes Care 2015;38:1707‐1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hansen J, Lassen CF. Nested case‐control study of night shift work and breast cancer risk among women in the Danish military. Occup Environ Med 2012;69:551‐556. [DOI] [PubMed] [Google Scholar]
- 28. Papantoniou K, Castano‐Vinyals G, Espinosa A, et al. Night shift work, chronotype and prostate cancer risk in the MCC‐Spain case‐control study. Int J Cancer 2015;137:1147‐1157. [DOI] [PubMed] [Google Scholar]
- 29. Tibu F, Hill J, Sharp H, Marshall K, Glover V, Pickles A. Evidence for sex differences in fetal programming of physiological stress reactivity in infancy. Dev Psychopathol 2014;26:879‐888. [DOI] [PubMed] [Google Scholar]
- 30. Varcoe TJ, Boden MJ, Voultsios A, Salkeld MD, Rattanatray L, Kennaway DJ. Characterisation of the maternal response to chronic phase shifts during gestation in the rat: implications for fetal metabolic programming. PloS One 2013;8:e53800. doi:10.1371/journal.pone.0053800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bonzini M, Palmer KT, Coggon D, Carugno M, Cromi A, Ferrario MM. Shift work and pregnancy outcomes: a systematic review with meta‐analysis of currently available epidemiological studies. BJOG 2011;118:1429‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palmer KT, Bonzini M, Harris EC, Linaker C, Bonde JP. Work activities and risk of prematurity, low birth weight and pre‐eclampsia: an updated review with meta‐analysis. Occup Environ Med 2013;70:213‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheng TSLS, Toh JY, Cheung YB, et al. Predominantly nighttime feeding and weight outcomes in infants. Am J Clin Nutr 2016;104:380‐388. [DOI] [PubMed] [Google Scholar]
- 34. Colditz GAMP, Stampfer MJ, Willett WC, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol 1986;123:894‐900. [DOI] [PubMed] [Google Scholar]
- 35. Colditz GASM, Willett WC, Stason WB, Rosner B, Hennekens CH, Speizer FE. Reproducibility and validity of self‐reported menopausal status in a prospective cohort study. Am J Epidemiol 1987;126:319‐325. [DOI] [PubMed] [Google Scholar]
- 36. Rich‐Edwards JWGM, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, Manson JE. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol 1994;171:171‐177. [DOI] [PubMed] [Google Scholar]
- 37. Rimm EBSM, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self‐reported waist and hip circumferences in men and women. Epidemiology 1990;1:466‐473. [DOI] [PubMed] [Google Scholar]
- 38. Troy LMHD, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord 1995;19:570‐572. [PubMed] [Google Scholar]
- 39. Field AE, Aneja P, Rosner B. The validity of self‐reported weight change among adolescents and young adults. Obes Rev 2007;15:2357‐2364. [DOI] [PubMed] [Google Scholar]
- 40. Straus R. Comparision of measured and self‐reported weight and height in a cross‐sectional sample of young adolescents. Int J Obes Relat Metab Disord 1999;23:904‐908. [DOI] [PubMed] [Google Scholar]
- 41. Himes J, Faricy A. Validity and reliability on self‐reported stature and weight of US adolescents. Am J Hum Biol 2001;13:255‐260. [DOI] [PubMed] [Google Scholar]
- 42. Vetter C, Devore EE, Wegrzyn LR, et al. Association between rotating night shift work and risk of coronary heart disease among women. JAMA 2016;315:1726‐1734. [DOI] [PMC free article] [PubMed] [Google Scholar]


