Abstract
Objective:
To determine the relationship between different forms of maternal diabetes and childhood obesity at different ages and to explore potential pathways.
Methods:
We analyzed prospective cohort data from the TEDDY study which comprised of 5,324 children examined during 0.25–6 years of age. Cross-sectional and longitudinal analyses were performed taking potential confounders and effect modifiers such as maternal pre-pregnancy BMI and birth weight z-scores into account.
Results:
Offspring of mothers with gestational diabetes mellitus (GDM) or type 1 diabetes (T1D) showed a higher BMI SDS and increased risk for overweight and obesity than offspring of non-diabetic mothers at 5.5 years of age. While these associations could be substantially explained by maternal pre-pregnancy BMI in offspring of GDM mothers, significant associations disappeared after adjustment for birth weight z-scores in offspring of T1D mothers. Further, overweight risk became stronger with increasing age in offspring of diabetic mothers than offspring of non-diabetic mothers.
Conclusion:
Maternal diabetes is associated with increased risk of offspring overweight, and the association appears to get stronger as children grow older. Indeed, intrauterine exposure to maternal T1D may predispose children to later obesity through increased birth weight, while maternal BMI is more important in children exposed to GDM.
Keywords: Maternal diabetes, offspring overweight, gestational diabetes, type 1 diabetes
Introduction:
The worldwide increase in the prevalence of childhood obesity in recent decades is alarming because it is also associated with other health consequences such as the metabolic syndrome, diabetes and cardiovascular disease in adulthood (1, 2). Previous research has indicated that overweight at 5–6 years of age is a strong predictor of overweight later in life (3), emphasizing the need to identify determinants of obesity in early life and even before birth (4). In particular, there is a growing body of literature that recognizes the role of maternal diabetes during pregnancy on the risk of offspring obesity (5–7). While a number of studies have shown that offspring of women with gestational diabetes mellitus (GDM), type 1 diabetes (T1D) or type 2 diabetes (T2D) have a higher risk for obesity during late childhood and adolescence (8–13), there is only weak and inconsistent evidence for an association between maternal diabetes and obesity during early childhood (14–18). Therefore, it is still not clear whether maternal diabetes has a delayed effect on offspring obesity.
In addition, most studies associating GDM with offspring obesity have shown that maternal obesity largely confounds this association (5, 9, 19, 20); only in one study a positive association between GDM and overweight in 6-year-old offspring remained significant after adjustment for maternal body mass index (BMI) (21); thus, it remains unclear whether this association is causal. Furthermore, high birth weight has been reported to be associated with maternal hyperglycemia in pregnancy regardless of the type of diabetes (22, 23), potentially via exposure to excess fetal glucose and insulin, and thus overgrowth of the fetus (4). However, the influence of birth weight on the pathway from maternal diabetes to childhood obesity has not been well investigated.
Therefore, this study aims to investigate 1) whether exposure to maternal diabetes during pregnancy (gestational, type 1 or type 2 diabetes) is associated with subsequent offspring growth during early childhood, 2) whether this association varies by offspring age or maternal diabetes status and 3) whether birth weight or maternal pre-pregnancy BMI are in the potential pathway.
Methods:
The Environmental Determinants of Diabetes in the Young (TEDDY) study is an ongoing international multicenter prospective cohort study that seeks to identify the environmental factors triggering islet autoimmunity and T1D. This large longitudinal cohort also offers the opportunity to investigate the factors influencing childhood overweight/obesity. The TEDDY study screened 424,788 newborns for T1D-associated human leukocyte antigen genotypes between 2004 and 2010, and of these children, 8,676 were enrolled and followed up in six clinical research centers located in the United States, Finland, Germany, and Sweden. Children’s study visits were scheduled every three months from birth until the age of 4 years and every 6 months thereafter. Further details on study design, eligibility, and data collection have been described elsewhere (24–26). Written informed consent was obtained separately for all participants from a parent or primary caretaker. The study is funded by the National Institutes of Health, approved by local institutional review boards and has been monitored by an external evaluation committee formed by the National Institutes of Health.
Maternal characteristics and offspring measurements
During each visit, children’s height and weight were measured by trained TEDDY personnel at TEDDY clinics. Each child’s height was measured as length before the age of 2 years and as standing height to the nearest 0.1 cm from the age of 2 years using a wall-mounted stadiometer (27). Body weight was measured in kilograms using regularly calibrated electronic scales. For subjects who missed their study visit, anthropometric data were taken from their pediatricians’ records collected near the TEDDY clinic visit date.
Information on maternal factors such as diabetes during pregnancy, age, pre-pregnancy BMI, gestational weight gain, gestational age at delivery, education, smoking or alcohol intake during pregnancy, as well as the child’s birth weight were obtained by self-administered questionnaires or structured interviews conducted during one of the follow-up visits in the first year of the study. Duration of both any and exclusive breastfeeding was assessed by giving a specific booklet to the parents at study entry, in which they recorded the age at weaning and age at introduction of all new foods.
Assessment of diet and physical activity:
Dietary intake was assessed using a 3-day food record every 3 months until 12 months of age and every 6 months thereafter. Participating families were instructed to keep a consecutive 3-day record of their child’s consumption of food and beverages, ideally for two weekdays and one weekend day, as described in detail elsewhere (27). To assess energy and nutrient intake, the food consumption data were entered and analyzed using country-specific food record databases which were harmonized for the TEDDY study (28). Average duration (in minutes) of moderate to vigorous physical activity (MVPA) per day was assessed using the Actigraph GT3X accelerometer (29), on an annual basis, beginning at 5 years of age. TEDDY staff provided demonstrations on how to wear and use the accelerometer for 7 consecutive days including two weekend days, during the study visit prior to the specific TEDDY visit targeted for activity data collection.
Data transformations:
Children were classified into different groups according to maternal diabetic status during pregnancy: 1) offspring of mothers with gestational diabetes (O-GDM), 2) offspring of mothers with type 1diabetes (O-T1DM), 3) offspring of mothers with type 2 diabetes (O-T2DM), and 4) offspring of mothers with no diabetes (O-nonDM). Body mass index (BMI) was calculated as weight (kg) / height² (m²). Prior to analysis, height, weight and BMI were transformed to standard deviation scores (SDS) using World Health Organization (WHO) reference values (30, 31). SDS values below −5 or greater than 5 were deemed implausible and excluded. BMI SDS values were also used to define overweight (including obesity; BMI SDS > 1) and obesity (BMI SDS > 2) according to WHO recommendations. Anthropometric outcomes at the age of 5.5 years were defined as those assessed at the 66-month visit, if available (as in 86% of the children), or at the next closest visit between the ages of 54 and 72 months. Similarly, diet and physical activity at the age of 5 years were defined as those assessed at the 60-month visit if available, or at the next closest visit of 66 or 72 months. Gestational weight gain was classified as inadequate, adequate, or excessive according to Institute of Medicine guidelines (32). Birth weight was transformed to a z-score adjusting for country, sex, gestational age, maternal height and birth type (singleton or multiplet) similar to previous analyses of the TEDDY data (27, 33).
Statistical analysis:
To assess our main hypothesis that maternal diabetes was associated with offspring anthropometric measures, we performed several analyses. Firstly, mean BMI, weight and height were visually compared in yearly time intervals between O-GDM, O-T1DM and O-nonDM. Secondly, cross-sectional associations between maternal diabetes and anthropometric outcomes (BMI, height, weight, overweight and obesity) measured in the children at 5.5 years of age were investigated through linear and logistic regression models. Thirdly, longitudinal analyses between maternal diabetes and anthropometric outcomes measured during 0.25–6 years of age were performed through mixed effects regression models with random intercept for each subject in order to account for the correlation between repeated observations within subjects. Associations in both the cross-sectional and the longitudinal setting were analyzed based on stepwise adjustment. In the first model, we adjusted for age (only longitudinal analysis), sex and country for all outcomes; then, we additionally adjusted for maternal pre-pregnancy BMI in the second model. Further, we included maternal age, gestational weight gain, maternal smoking during pregnancy (yes/no), maternal alcohol intake during pregnancy (any/none), maternal education (high school or lower/more than high school) and duration of any breastfeeding (less/more than 6 months) as potential confounders in the third model; and additionally birth weight z-scores in the fourth model to explore potential pathways. Furthermore, we explored interaction terms between maternal diabetes and child’s age (in years) in the fully adjusted longitudinal model to explore whether the association changed with an increase in age.
Sensitivity analyses:
We performed several sensitivity analyses. We added interaction terms between country and maternal diabetes in the cross-sectional and longitudinal models, to explore whether association between maternal diabetes and anthropometric outcomes differed by country. As, HLA-DQ2/2 genotype was reported to be associated with increased risk for obesity at 2–4 years of age in a previous TEDDY study (33), we additionally adjusted for HLA-DQ2/2 genotype in the cross-sectional and longitudinal models. We further re-calculated the cross-sectional analyses after exclusion of children who had developed persistent islet autoantibodies or T1D by 5.5 years of age. Furthermore, based on the subset of children with available energy intake and physical activity data at 5 years of age (54% of all children with available BMI measurements), we additionally adjusted for these two variables as potential confounders in cross-sectional models 3 and 4. We also assessed whether treatment with insulin compared to any other or no treatment during pregnancy was associated with anthropometric outcomes at 5.5 years of age in offspring of GDM and T2D women. All calculations were carried out with SAS 9.4 (SAS Institute Inc. Cary, North Carolina).
Results:
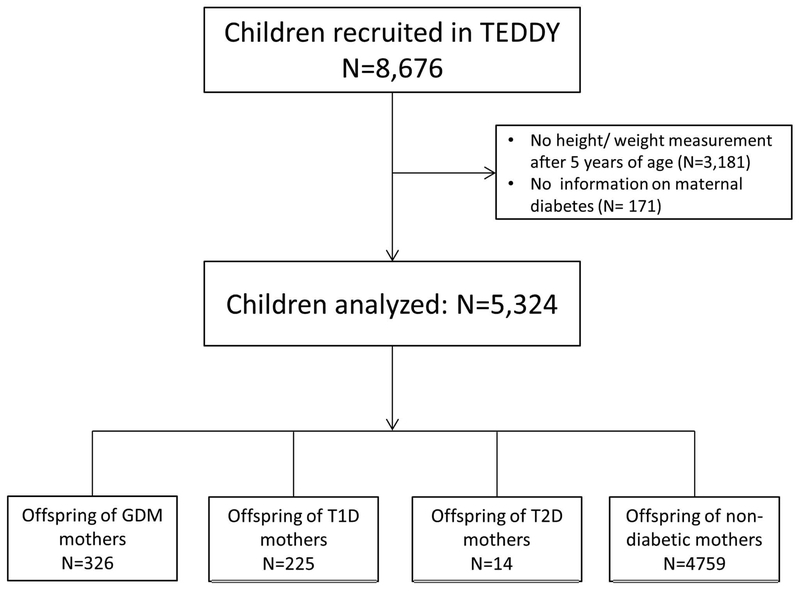
Of 8,676 children, 3,352 children with missing data on height/weight measurements after 5 years of age (N=3,181) or maternal diabetes status during pregnancy (N=171) were excluded (Fig. 1). Our final study sample comprised of 5,324 children, of which 2,746 (51.58%) were male; 326 (6.12%) and 225 (4.23%) were O-GDM and O-T1DM, respectively, while only 14 (0.26%) were O-T2DM (Table 1). Children who were excluded because of missing height/weight measurements were less likely to have a diabetic mother (GDM: 4.94%; T1D: 3.11%; Chi-Square test: p=0.02). However, children who were excluded because of missing maternal diabetes status did not differ significantly from those included with respect to BMI SDS at age 5.5 years (Mann-Whitney-U test: p=0.70). In total, children had a mean BMI SDS of 0.35, with 1154 (21.87%) and 303 (5.74%) children classified as having overweight and obesity, respectively, at the age of 5.5 years. O-nonDM had a mean birth weight z-score of −0.05, which was significantly lower than that in O-T1DM (0.87, p <0.0001) or O-GDM (0.13, p=0.004).
Fig. 1: Flowchart of children analyzed.
BMI: Body mass index; GDM: Gestational diabetes; T1D: Type 1 diabetes; T2D: Type 2 diabetes
Table 1:
Characteristics of the study population stratified according to maternal diabetes
| Variable | Available N | Category | N (%) in each category | O-nonDM (N=4759) |
O-GDM (N=326) |
O-T1DM (N=225) |
O-T2DM (N=14) |
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||||
| Sex | 5324 | Males | 2746 (51.6) | 2457 (51.6) | 174 (53.4) | 109 (48.4) | 6 (42.9) |
| Country | USA | 2013 (37.8) | 1807 (38.0) | 117 (35.9) | 78 (34.7) | 11 (78.6) | |
| 5324 | Finland | 1237 (23.2) | 1052 (22.1) | 138 (42.3) | 47 (20.9) | 0 | |
| Germany | 274 (5.2) | 192 (4.0) | 17 (5.2) | 65 (28.9) | 0 | ||
| Sweden | 1800 (33.8) | 1708 (35.9) | 54 (16.6) | 35 (15.6) | 3 (21.4) | ||
| Maternal smoking during pregnancy | 5320 | Yes | 497 (9.3) | 426 (9.0) | 40 (12.3) | 30 (13.3) | 1 (7.1) |
|
Maternal alcohol drinking during pregnancy |
5322 | Yes | 1831 (34.4) | 1623 (34.1) | 109 (33.4) | 95 (42.2) | 4 (28.6) |
| Maternal education | 5251 | High school |
4371 (83.2) | 3877 (82.6) | 288 (89.2) | 194 (87.4) | 12 (85.7) |
|
Gestational weight gain (according to Institute of Medicine guidelines) |
5241 | Inadequate | 909 (17.3) | 754 (16.1) | 117 (36.7) | 34 (15.2) | 4 (28.6) |
| Adequate | 1899 (36.2) | 1725 (36.8) | 92 (28.8) | 78 (34.9) | 4 (28.6) | ||
| Excessive | 2433 (46.4) | 2205 (47.1) | 110 (34.5) | 112 (50.0) | 6 (42.9) | ||
| Breastfed ≥ 6 months | 5324 | Yes | 3469 (65.2) | 3150 (66.2) | 193 (59.2) | 121 (53.8) | 5 (35.7) |
| Overweight at age 5.5 y | 5277 | Yes | 1154 (21.9) | 998 (21.2) | 89 (27.6) | 58 (25.9) | 9 (64.3) |
| Obesity at age 5.5 y | 5277 | Yes | 303 (5.7) | 252 (5.3) | 32 (9.9) | 17 (7.6) | 2 (14.3) |
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | |||
| Maternal pre-pregnancy BMI (kg/m2) | 5276 | 24.8±5.2 | 24.5±5.0 | 28.3±6.4 | 25.3±4.7 | 35.0±7.5 | |
| Maternal age at delivery (y) | 5324 | 31.0±4.9 | 30.9±4.9 | 32.2±5.3 | 30.8±4.9 | 34.0±5.6 | |
| Gestational age (weeks) | 5318 | 39.5±1.6 | 39.6±1.6 | 39.2±1.7 | 37.7±1.8 | 38.1±2.1 | |
| Birth weight z-scores | 5186 | 0.0±1.0 | −0.1±1.0 | 0.1±1.1 | 0.9±1.3 | 0.2±1.0 | |
| BMI SDS at age 5.5 y | 5277 | 0.4±1.0 | 0.3±1.0 | 0.5±1.1 | 0.4±1.1 | 1.1±1.3 | |
| Height SDS at age 5.5 y | 5291 | 0.4±1.0 | 0.4±1.0 | 0.3±1.0 | 0.3±0.9 | 0.2±0.7 | |
| Weight SDS at age 5.5 y | 5304 | 0.5±1.0 | 0.5±1.0 | 0.5±1.0 | 0.5±0.9 | 0.9±1.2 | |
| Mean energy intake (kcal/day) at age 5 y | 4263 | 1442.7±362.2 | 1443.6±359.7 | 1461.7±428.5 | 1402.1±316.4 | 1353.2±317.3 | |
| MVPA (min/day) at age 5 y | 3276 | 68.0±34.4 | 68.3±34.4 | 63.0±33.8 | 69.4±36.8 | 54.4±29.8 | |
N: Number; O-nonDM: Offspring of non-diabetic mothers; O-GDM: Offspring of gestational diabetic mothers; O-T1DM: Offspring of type 1 diabetic mothers; O-T2DM: Offspring of type 2 diabetic mothers; SD: Standard deviation; SDS: Standard deviation score; BMI: Body mass index; MVPA: moderate to vigorous physical activity
O-GDM had a similar SDS of both height and weight compared to O-nonDM from 3 months to 2–3 years of age, while O-T1DM showed clearly lower values at this age, but caught up with O-GDM until age 5–6 years (Fig. 2). O-nonDM had similar mean BMI SDS as O-GDM at age 2 years but gradually declined afterwards and had considerably lower values than O-GDM/O-T1DM at age 6 years. Accordingly, maternal diabetes was associated with higher BMI SDS (O-GDM: +0.19 (95% confidence interval (CI): 0.07; 0.29); O-T1DM: +0.22 (95% CI: 0.08; 0.35)) and increased risk for overweight (O-GDM odds ratio (OR): 1.48 (95% CI: 1.14; 1.92); O-T1DM OR: 1.60 (95% CI: 1.16; 2.20) and obesity (O-GDM OR: 1.98 (95% CI: 1.34; 2.93); O-T1DM OR: 1.84 (95% CI: 1.09; 3.10)) at 5.5 years of age compared to O-nonDM when adjusted for sex and country (Table 2). After additional adjustment for maternal pre-pregnancy BMI, the respective associations for O-GDM attenuated and became non-significant (for example, OR for overweight: 1.05 (95% CI: 0.80; 1.38)). In contrast, the O-T1DM estimates remained largely unaffected by adjustment for maternal BMI and also for further confounders such as breastfeeding, but attenuated considerably after adjustment for birthweight z-scores (OR for overweight: 1.15 (95% CI: 0.81; 1.62)). O-T2DM had a largely increased risk for overweight despite the small sample size (9 of the 14 O-T2DM children had overweight) and independently of birth weight z-scores (OR in the full model: 4.92 (95% CI: 1.40; 17.30)). No significant differences between offspring of diabetic mothers and O-nonDM were observed for height SDS and weight SDS, with the exception of lower height and weight SDS in O-T1DM subjects after adjustment for birth weight z-scores. The observed associations between maternal diabetes and offspring anthropometric outcomes remained similar even after adjusting for HLA-DQ2/2 genotype or excluding children with islet autoantibodies or T1D (data not shown). Sensitivity analyses on the reduced subset where physical activity and energy intake were available did not indicate a major confounding role for these two variables (Table S1).
Fig.2. Comparison of mean BMI, weight and height standard deviations scores (SDS) with 95% confidence interval (CI) between offspring of mothers with gestational diabetes (GDM), type 1 diabetes (T1D) and no diabetes at different ages in the TEDDY study.

*This figure does not include trends for offspring of type 2 diabetic mothers due to low numbers (N=14) and wide confidence intervals.
Table 2:
Cross-sectional analysis of anthropometric outcomes between 5.5 year old offspring of mothers with and without diabetes of different types during pregnancy (reference: no diabetes; N: number of subjects). Significant associations (p<0.05) are shown in bold font.
| Outcomes | Exposure (Maternal diabetes) |
Model 1 (N=5277) |
Model 2 (N=5232) |
Model 3 (N=5119) |
Model 4 (N=4994) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) |
p | Estimate (95% CI) |
p | Estimate (95% CI) |
p | Estimate (95% CI) |
p | ||
|
Absolute change in SD scores | |||||||||
| BMI SDS | O-GDM | 0.19 (0.07; 0.29) | 0.002 | −0.02 (−0.13; 0.10) | 0.78 | 0.03 (−0.08; 0.14) | 0.61 | 0.001 (−0.11; 0.11) | 0.99 |
| O-T1DM | 0.22 (0.08; 0.35) | 0.002 | 0.18 (0.04; 0.31) | 0.009 | 0.17 (0.04; 0.30) | 0.01 | −0.007 (−0.14; 0.13) | 0.91 | |
| O-T2DM | 0.75 (0.23; 1.27) | 0.005 | 0.24 (−0.27; 0.74) | 0.36 | 0.28 (−0.22; 0.78) | 0.27 | 0.32 (−0.18; 0.83) | 0.21 | |
| Height SDS |
O-GDM | −0.02 (−0.13; 0.09) | 0.67 | −0.07 (−0.18; 0.04) | 0.24 | −0.04 (−0.16; 0.07) | 0.44 | −0.08 (−0.19; 0.04) | 0.20 |
| O-T1DM | −0.07 (−0.20; 0.07) | 0.34 | −0.08 (−0.21; 0.06) | 0.26 | −0.08 (−0.22; 0.05) | 0.24 | −0.27 (−0.40; −0.13) | 0.0001 | |
| O-T2DM | −0.06 (−0.57; 0.45) | 0.82 | −0.20 (−0.71; 0.32) | 0.45 | −0.20 (−0.71; 0.31) | 0.44 | −0.21 (−0.73; 0.31) | 0.44 | |
| Weight SDS |
O-GDM | 0.10 (−0.01; 0.21) | 0.08 | −0.06 (−0.17; 0.05) | 0.32 | −0.01 (−0.12; 0.10) | 0.84 | −0.05 (−0.16; 0.06) | 0.36 |
| O-T1DM | 0.12 (−0.02; 0.25) | 0.08 | 0.08 (−0.05; 0.21) | 0.23 | 0.07 (−0.06; 0.20) | 0.27 | −0.16 (−0.29; −0.03) | 0.02 | |
| O-T2DM | 0.51 (−0.01; 1.02) | 0.05 | 0.08 (−0.42; 0.58) | 0.31 | 0.11 (−0.39; 0.61) | 0.67 | 0.13 (−0.37; 0.63) | 0.60 | |
|
Odds ratios | |||||||||
| Overweight | O-GDM | 1.48 (1.14; 1.92) | 0.003 | 1.05 (0.80; 1.38) | 0.75 | 1.14 (0.86; 1.51) | 0.38 | 1.10 (0.82; 1.46) | 0.52 |
| O-T1DM | 1.60 (1.16; 2.20) | 0.004 | 1.52 (1.10; 2.11) | 0.01 | 1.50 (1.08; 2.09) | 0.02 | 1.15 (0.81; 1.62) | 0.44 | |
| O-T2DM | 7.39 (2.46; 22.23) | 0.0004 | 3.36 (1.06; 10.70) | 0.04 | 3.68 (1.14; 11.81) | 0.03 | 4.92 (1.40; 17.30) | 0.01 | |
| Obesity | O-GDM | 1.98 (1.34; 2.93) | 0.0007 | 1.23 (0.81; 1.86) | 0.34 | 1.33 (0.87; 2.04) | 0.19 | 1.31 (0.85; 2.01) | 0.23 |
| O-T1DM | 1.84 (1.09; 3.10) | 0.02 | 1.79 (1.05; 3.06) | 0.03 | 1.75 (1.02; 3.00) | 0.04 | 1.48 (0.85; 2.59) | 0.17 | |
| O-T2DM | 2.93 (0.65; 13.22) | 0.16 | 0.95 (0.20; 4.57) | 0.95 | 0.94 (0.19; 4.60) | 0.94 | 1.02 (0.20; 5.09) | 0.98 | |
O-GDM: Offspring of gestational diabetic mothers; O-T1DM: Offspring of type 1 diabetic mothers; O-T2DM: Offspring of type 2 diabetic mothers; BMI: Body mass index; SDS: Standard deviation scores.
Model 1: Adjusted for sex, country; Model 2: Model 1 + maternal pre-pregnancy BMI; Model 3: Model 2 + breastfeeding, maternal smoking and drinking during pregnancy, gestational weight gain, maternal age and education ; Model 4: Model 3 + birth weight
In the longitudinal analysis, O-GDM was again not significantly associated with any outcome when adjusted for maternal pre-pregnancy BMI (Table 3). Similarly, O-T1DM showed no significant differences in any outcome except height SDS compared to O-nonDM in longitudinal models without birth weight z-scores. After inclusion of birth weight z-scores, maternal T1D was associated with lower BMI, overweight and obesity risk as well as lower height and weight SDS in the offspring.
Table 3:
Longitudinal analysis of anthropometric outcomes assessed during study visits from 0.25 to 6 years of age in offspring of mothers with or without diabetes during pregnancy (reference: no diabetes; N: number of observations). Significant associations (p<0.05) are shown in bold font.
| Outcomes | Exposure (Maternal diabetes) |
Model 1 N=95162 |
Model 2 N=94330 |
Model 3 N=92782 |
Model 4 N=90569 |
||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) |
p | Estimate (95% CI) |
p | Estimate (95% CI) |
p | Estimate (95% CI) |
p | ||
|
Absolute change in SD scores | |||||||||
| BMI SDS | O-GDM | 0.10 (0.01; 0.19) | 0.03 | −0.02 (−0.11; 0.07) | 0.70 | 0.03 (−0.06; 0.12) | 0.52 | −0.003 (−0.09; 0.09) | 0.94 |
| O-T1DM | 0.09 (−0.02; 0.20) | 0.12 | 0.06 (−0.05; 0.17) | 0.27 | 0.04 (−0.06; 0.15) | 0.42 | −0.15 (−0.26; −0.04) | 0.006 | |
| O-T2DM | 0.46 (0.04; 0.87) | 0.03 | 0.15 (−0.26; 0.56) | 0.48 | 0.19 (−0.22; 0.60) | 0.36 | 0.19 (−0.22; 0.60) | 0.37 | |
| Height SDS | O-GDM | −0.002 (−0.10; 0.10) | 0.97 | −0.03 (−0.14; 0.07) | 0.53 | −0.001 (−0.11; 0.11) | 0.98 | −0.04 (−0.15; 0.06) | 0.40 |
| O-T1DM | −0.14 (−0.26; −0.01) | 0.03 | −0.15 (−0.27; −0.02) | 0.02 | −0.16 (−0.28; −0.03) | 0.01 | −0.41 (−0.54; −0.29) | <0.001 | |
| O-T2DM | −0.18 (−0.65; 0.29) | 0.46 | −0.28 (−0.75; 0.20) | 0.25 | −0.27 (−0.75; 0.20) | 0.26 | −0.30 (−0.78; 0.17) | 0.21 | |
| Weight SDS |
O-GDM | 0.07 (−0.03; 0.17) | 0.15 | −0.03 (−0.12; 0.07) | 0.56 | 0.02 (−0.07; 0.12) | 0.64 | −0.02 (−0.12; 0.07) | 0.60 |
| O-T1DM | −0.02 (−0.14; 0.09) | 0.72 | −0.05 (−0.16; 0.07) | 0.44 | −0.06 (−0.18; 0.05) | 0.28 | −0.35 (−0.46; −0.24) | <0.001 | |
| O-T2DM | 0.23 (−0.21; 0.67) | 0.31 | −0.05 (−0.48; 0.39) | 0.85 | −0.01 (−0.44; 0.42) | 0.96 | −0.03 (−0.45; 0.39) | 0.88 | |
|
Odds ratios | |||||||||
| Overweight | O-GDM | 1.29 (0.99; 1.68) | 0.06 | 0.94 (0.72; 1.23) | 0.66 | 1.06 (0.76; 1.30) | 0.69 | 0.98 (0.75; 1.28) | 0.89 |
| O-T1DM | 1.27 (0.92; 1.75) | 0.14 | 1.18 (0.86; 1.62) | 0.30 | 1.14 (0.83; 1.57) | 0.41 | 0.69 (0.50; 0.96) | 0.03 | |
| O-T2DM | 3.84 (1.19; 12.83) | 0.03 | 1.72 (0.54; 5.48) | 0.36 | 1.93 (0.61; 6.11) | 0.27 | 1.95 (0.60; 6.34) | 0.27 | |
| Obesity | O-GDM | 1.47 (1.11; 1.95) | 0.01 | 1.08 (0.81; 1.45) | 0.60 | 1.19 (0.89; 1.61) | 0.24 | 1.14 (0.85; 1.54) | 0.38 |
| O-T1DM | 0.99 (0.69; 1.44) | 0.97 | 0.94 (0.65; 1.35) | 0.72 | 0.91 (0.63; 1.31) | 0.61 | 0.62 (0.42; 0.91) | 0.01 | |
| O-T2DM | 2.53 (0.79; 8.11) | 0.12 | 1.14 (0.35; 3.65) | 0.83 | 1.19 (0.37; 3.85) | 0.77 | 1.39 (0.42; 4.59) | 0.59 | |
O-GDM: Offspring of gestational diabetic mothers; O-T1DM: Offspring of type 1 diabetic mothers; O-T2DM: Offspring of type 2 diabetic mothers; BMI: Body mass index; SDS: Standard deviation scores.
Model 1: Adjusted for age, sex, country; Model 2 : Model 1 + maternal pre-pregnancy BMI; Model 3: Model 2 + breastfeeding, maternal smoking and drinking during pregnancy, gestational weight gain, maternal age and education ; Model 4: Model 3 + birth weight
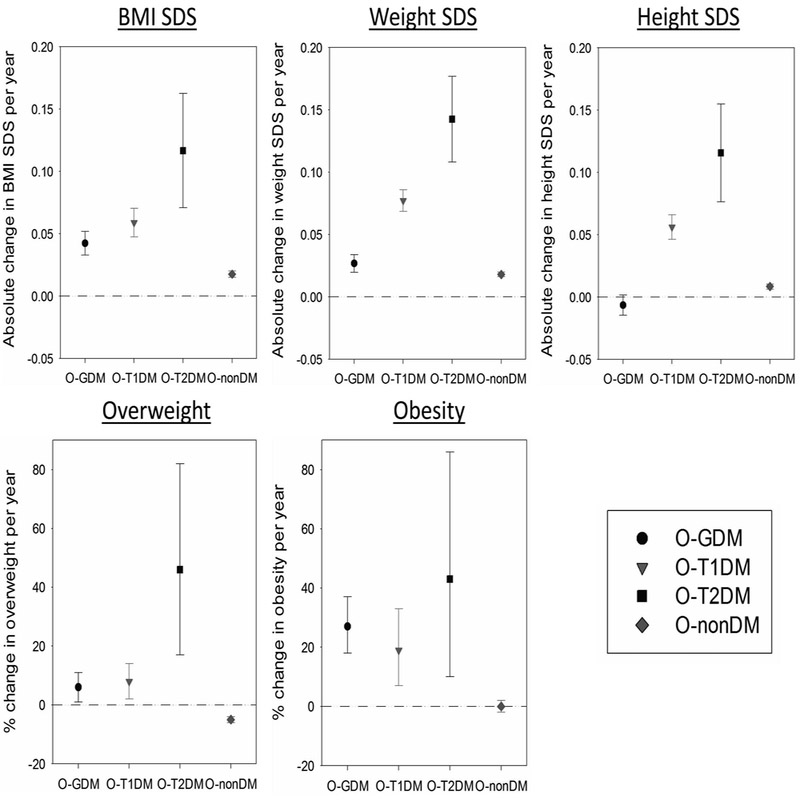
After including an interaction term between child’s age and maternal diabetes in the fully adjusted model, we observed that O-GDM, O-T1DM and O-T2DM showed comparatively higher increase in BMI SDS per year compared to O-nonDM (Fig. 3), indicating that the potential impact of maternal diabetes on childhood BMI becomes stronger with increasing age. For example, the average increase in BMI SDS per year increase in age was 0.06 (95% CI: 0.05; 0.07) in O-T1DM compared to 0.02 (95% CI: 0.01; 0.02) in O-nonDM. Hence, a child with a BMI SDS of 0.00 at age 2 years would be expected to have a BMI SDS of 0.08 at age 6 years if it was O-nonDM, compared to 0.24 at age 6 years if it was O-T1DM. Similarly, a one year increase in age was associated with a higher risk for overweight or obesity in O-GDM, O-T1DM and O-T2DM groups, while null or negative effects were found in O-nonDM. For example, OR for overweight risk per year increase in age was 1.08 (95% CI: 1.02; 1.14) in O-T1DM compared to 0.95 (95% CI: 0.94; 0.96) in O-nonDM, implying a relative increase in risk of +13% per year in O-T1DM compared to O-nonDM subjects. Further, we observed no significant interaction terms between country and maternal diabetes in any of the cross-sectional and longitudinal models (data not shown). In addition, treatment with insulin (N=72) compared to diet (N=243), pills only (N=1) or no treatment (N=24) during pregnancy in GDM and T2D women was not associated with any of the anthropometric outcomes in offspring at 5.5 years of age (e.g. difference in BMI SDS of insulin compared to no insulin treatment: −0.05 (95% CI: −0.34; 0.25)).
Fig. 3: Modifications of association between child’s age (per year) and anthropometric outcomes by maternal diabetes status presented as estimates (symbols) with 95% confidence intervals (lines).
O-GDM: Offspring of gestational diabetic mothers; O-T1DM: Offspring of type 1 diabetic mothers; O-T2DM: Offspring of type 2 diabetic mothers; O-nonDM: Offspring of non-diabetic mothers; BMI: Body mass index; SDS: Standard deviation scores
Discussion:
In this large prospective multicenter cohort study, we observed that children with intrauterine exposure to diabetes had an increased risk for overweight and obesity at 5.5 years of age. This association was not clearly evident when the whole time span of 0.25 to 6 years of age was investigated in a longitudinal analysis. However, we observed that as children grew older, their overweight or obesity risk tended to increase when born to diabetic mothers compared to non-diabetic mothers, implying that the association may not be evident in the first years of life. Furthermore, the observed associations attenuated significantly after adjustment for pre-pregnancy BMI in O-GDM and for birth weight z-scores in O-T1DM, indicating possible mediating effects by these two factors.
Our findings for exposure to maternal T1D or GDM were generally in line with other studies indicating a positive association with offspring overweight/obesity. These positive associations were predominantly seen in studies examining offspring > 5 years of age (8–12, 21, 34). However, studies on early childhood offspring have shown inconsistent results. Silverman et al (35) observed an increased weight in offspring of diabetic mothers at birth and progressively after the ages of 4 years but not between 1–3 years of age. Similarly, Baptiste-Roberts et al (36) reported a significantly increased BMI at age 7 years in offspring of gestational diabetic mothers, but not at ages 3 and 4 years. A recent meta-analysis, which pooled studies according to different age subgroups, reported a higher risk for overweight/obesity in O-GDM or O-T1DM only during late childhood and adolescence (7). Accordingly, our study showed stronger effects as children grew older. Therefore, it may be possible that maternal diabetes has a delayed influence on offspring obesity that increases with age (37, 38). However, two recent studies, of which one examined 3-year olds (15) and the other predominantly 3–6 year-olds (16), showed positive associations of GDM with offspring adiposity measured by sum of skinfolds or fat mass but not by BMI SDS. Therefore, it could be speculated that the differences may be subtle in early ages and become evident with respect to BMI only after a certain age. Moreover, evidence suggests that early catch-up growth may lead to obesity in later life (39). Accordingly, the associations between maternal diabetes and offspring obesity at 5.5 years may be partly attributable to early catch up growth, as Figure 2 indicates that O-T1DM seemed to have an accelerated growth during early childhood compared to O-nonDM. These findings may further indicate that environmental factors may contribute to the association between maternal diabetes and offspring overweight. However, the associations in our data remained stable after adjustment for a number of those variables such as breastfeeding, parental education or maternal age.
In addition, we found that the positive association of maternal GDM with offspring overweight/obesity attenuated significantly after adjustment for maternal pre-pregnancy BMI. Several GDM studies have shown similar findings of maternal BMI playing a major confounding role in their analyses (5, 9, 37, 40, 41). Indeed, maternal obesity is clearly a risk factor for and often precedes GDM; thus, it may be difficult to clearly separate the effects of GDM and maternal BMI on offspring obesity. Further, birth weight seemed to substantially explain the positive association between maternal T1D and offspring overweight/obesity in our data. Moreover, we found no considerable mediating effect of birth weight on the association between GDM and offspring obesity, in accordance with other studies (8, 16, 19, 37). Indeed, rates of macrosomia as well as of other adverse outcomes have been reported to be higher in offspring of mothers with pre-gestational diabetes than with GDM (42, 43). High birth weight may therefore be a proxy of poor glycemic control, which is possibly of greater importance in O-T1DM, as they are exposed to hyperglycemia during the whole pregnancy period, than O-GDM. In that case, adding birth weight to the model might even lead to an over-adjustment of the O-T1DM association, which might help to explain why we observed protective associations with respect to overweight in O-T1DM compared to O-nonDM in longitudinal analyses.
The main strengths of our study include the large sample size, the prospective study design with standardized protocols, multiple follow-up visits and availability of many important covariates like maternal pre-pregnancy BMI, gestational weight gain, birth weight, breastfeeding, and other postnatal influences like children’s diet and physical activity at 5 years of age. These data allowed us to investigate the effects of different types of diabetes during pregnancy on offspring’s BMI / overweight at different ages from shortly after birth until 6 years. It should be mentioned, however, that the number of children exposed to maternal type 2 diabetes during pregnancy was quite limited (n=14) and therefore all associations for this subgroup showed large variability and have to interpreted with great caution. Further, we were not able to assess such associations beyond 6 years of age because most subjects did not have sufficient follow-up after 6 years of age at the time these analyses were performed. GDM was defined based on maternal reports only and could thus neither be confirmed by medical records, lab values or similar, nor be harmonized between countries, unfortunately. This issue might have somewhat contributed to different prevalences of GDM between countries, but we do not expect that it has substantially biased our main results, however. A note of caution is due here with regard to generalizability of our results, as these TEDDY cohort participants are all at increased genetic or familial risk to develop T1D. We can therefore not exclude that the associations were slightly overestimated, as all the children may generally have a higher background prevalence of overweight regardless of maternal diabetes status. We investigated a number of outcomes using different statistical models without formal adjustment for multiple testing. Although we can therefore not exclude that this approach yielded some false-positive results, we would not expect this to be a major limitation, as the main findings were relatively consistent between the different models. Further, exclusion due to missing height/weight measurements after 5 years of age was significantly associated with maternal diabetes status, indicating that families with diabetic mothers were slightly less likely to drop out of the TEDDY follow-up. However, these differences were small and we do not expect that they have biased our findings considerably. In summary, maternal hyperglycemia seems to be associated with increased risk for childhood overweight/obesity. The strength of this association appears to increase as children grow older. Moreover, the association of maternal GDM with offspring obesity can be largely explained by confounding through maternal BMI, whereas the association of maternal T1D with offspring overweight is substantially mediated by birth weight, suggesting possibly different pathways. Nevertheless, our study indicates that children exposed to maternal diabetes during pregnancy may need closer attention with respect to obesity and its consequences, beyond early childhood.
Supplementary Material
What is already known about this subject?
Exposure to intrauterine hyperglycemia has been suggested to influence offspring obesity, but it is unclear whether the associations are evident during early childhood.
What does your study add?
Children exposed to maternal diabetes during pregnancy show a higher risk for overweight/obesity, which gets stronger as they grow older.
This association seems to be largely explained by maternal pre-pregnancy BMI in children exposed to gestational diabetes and by birth weight in children exposed to maternal type 1 diabetes.
Acknowledgments
Funding: Funded by U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, UC4 DK95300, UC4 DK100238, UC4 DK106955, and Contract No. HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Human Development (NICHD), National Institute of Environmental Health Sciences (NIEHS), Juvenile Diabetes Research Foundation (JDRF), and Centers for Disease Control and Prevention (CDC). This work was supported in part by the NIH/NCATS Clinical and Translational Science Awards to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR001082). The funders had no impact on the design, implementation, analysis and interpretation of the data.
Footnotes
shared last authorship
A complete list of the TEDDY study group can be found in the supplementary material.
Disclosure: The authors declared no conflict of interest.
References:
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet.384(9945):766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss R, Kaufman FR. Metabolic Complications of Childhood Obesity. Diabetes Care. 2008;31(Supplement 2):S310. [DOI] [PubMed] [Google Scholar]
- 3.von Kries R, Müller MJ, Heinrich J. Early Prevention of Childhood Obesity: Another Promise or a Reliable Path for Battling Childhood Obesity? Obesity facts. 2014;7(2):77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freinkel N Banting Lecture 1980: of Pregnancy and Progeny. Diabetes. 1980;29(12):1023. [DOI] [PubMed] [Google Scholar]
- 5.Kim SY, Sharma AJ, Callaghan WM. Gestational diabetes and childhood obesity: what is the link? Current opinion in obstetrics & gynecology. 2012;24(6):376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008;31(2):340–6. [DOI] [PubMed] [Google Scholar]
- 7.Kawasaki M, Arata N, Miyazaki C, Mori R, Kikuchi T, Ogawa Y, et al. Obesity and abnormal glucose tolerance in offspring of diabetic mothers: A systematic review and meta-analysis. PLOS ONE. 2018;13(1):e0190676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Zhu Y, Yeung E, Chavarro JE, Yuan C, Field AE, et al. Offspring risk of obesity in childhood, adolescence and adulthood in relation to gestational diabetes mellitus: a sex-specific association. International Journal of Epidemiology. 2017;46(5):1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawlor DA, Fraser A, Lindsay RS, Ness A, Dabelea D, Catalano P, et al. Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia. 2010;53(1):89–97. [DOI] [PubMed] [Google Scholar]
- 10.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. The Journal of clinical endocrinology and metabolism. 2009;94(7):2464–70. [DOI] [PubMed] [Google Scholar]
- 11.Vlachova Z, Bytoft B, Knorr S, Clausen TD, Jensen RB, Mathiesen ER, et al. Increased metabolic risk in adolescent offspring of mothers with type 1 diabetes: the EPICOM study. Diabetologia. 2015;58(7):1454–63. [DOI] [PubMed] [Google Scholar]
- 12.Lindsay RS, Nelson SM, Walker JD, Greene SA, Milne G, Sattar N, et al. Programming of Adiposity in Offspring of Mothers With Type 1 Diabetes at Age 7 Years. Diabetes Care. 2010;33(5):1080–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dabelea D The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care. 2007;30 Suppl 2:S169–74. [DOI] [PubMed] [Google Scholar]
- 14.Rijpert M, Evers IM, de Vroede MA, de Valk HW, Heijnen CJ, Visser GH. Risk factors for childhood overweight in offspring of type 1 diabetic women with adequate glycemic control during pregnancy: Nationwide follow-up study in the Netherlands. Diabetes Care. 2009;32(11):2099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, Oken E. Intrauterine Exposure to Gestational Diabetes, Child Adiposity, and Blood Pressure. American journal of hypertension. 2009;22(2):215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kearney M, Perron J, Marc I, Weisnagel SJ, Tchernof A, Robitaille J. Association of prenatal exposure to gestational diabetes with offspring body composition and regional body fat distribution. Clinical Obesity. 2017. [DOI] [PubMed] [Google Scholar]
- 17.Hummel S, Pfluger M, Kreichauf S, Hummel M, Ziegler AG. Predictors of overweight during childhood in offspring of parents with type 1 diabetes. Diabetes Care. 2009;32(5):921–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pirkola J, Vaarasmaki M, Leinonen E, Bloigu A, Veijola R, Tossavainen P, et al. Maternal type 1 and gestational diabetes: postnatal differences in insulin secretion in offspring at preschool age. Pediatric diabetes. 2008;9(6):583–9. [DOI] [PubMed] [Google Scholar]
- 19.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal Gestational Diabetes, Birth Weight, and Adolescent Obesity. Pediatrics. 2003;111(3):e221. [DOI] [PubMed] [Google Scholar]
- 20.Beyerlein A, Nehring I, Rosario AS, von Kries R. Gestational diabetes and cardiovascular risk factors in the offspring: results from a cross-sectional study. Diabetic medicine : a journal of the British Diabetic Association. 2012;29(3):378–84. [DOI] [PubMed] [Google Scholar]
- 21.Nehring I, Chmitorz A, Reulen H, von Kries R, Ensenauer R. Gestational diabetes predicts the risk of childhood overweight and abdominal circumference independent of maternal obesity. Diabetic medicine : a journal of the British Diabetic Association. 2013;30(12):1449–56. [DOI] [PubMed] [Google Scholar]
- 22.Sletner L, Jenum AK, Yajnik CS, Mørkrid K, Nakstad B, Rognerud-Jensen OH, et al. Fetal growth trajectories in pregnancies of European and South Asian mothers with and without gestational diabetes, a population-based cohort study. PLoS ONE. 2017;12(3):e0172946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackin ST, Nelson SM, Kerssens JJ, Wood R, Wild S, Colhoun HM, et al. Diabetes and pregnancy: national trends over a 15 year period. Diabetologia. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teddy Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatric diabetes. 2007;8(5):286–98. [DOI] [PubMed] [Google Scholar]
- 25.Teddy Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Annals of the New York Academy of Sciences. 2008;1150:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagopian WA, Erlich H, Lernmark A, Rewers M, Ziegler AG, Simell O, et al. The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatric diabetes. 2011;12(8):733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beyerlein A, Uusitalo UM, Virtanen SM, Vehik K, Yang J, Winkler C, et al. Intake of energy and protein is associated with overweight risk at age 5.5 years: Results from the prospective TEDDY study. Obesity (Silver Spring). 2017;25(8):1435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uusitalo U, Kronberg-Kippila C, Aronsson CA, Schakel S, Schoen S, Mattisson I, et al. Food composition database harmonization for between-country comparisons of nutrient data in the TEDDY Study. Journal of food composition and analysis. 2011;24(4–5):494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos-Lozano A, Santin-Medeiros F, Cardon G, Torres-Luque G, Bailon R, Bergmeir C, et al. Actigraph GT3X: validation and determination of physical activity intensity cut points. International journal of sports medicine. 2013;34(11):975–82. [DOI] [PubMed] [Google Scholar]
- 30.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta paediatrica (Oslo, Norway : 1992) Supplement. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva: World Health Organization; 2006. [Google Scholar]
- 32.Institute of Medicine. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 33.Yang J, Lernmark Å, Uusitalo UM, Lynch KF, Veijola R, Winkler C, et al. Prevalence of Obesity was Related to HLA-DQ in 2–4 Year Old Children at Genetic Risk for Type 1 Diabetes. International journal of obesity (2005). 2014;38(12):1491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss PA, Scholz HS, Haas J, Tamussino KF, Seissler J, Borkenstein MH. Long-term follow-up of infants of mothers with type 1 diabetes: evidence for hereditary and nonhereditary transmission of diabetes and precursors. Diabetes Care. 2000;23(7):905–11. [DOI] [PubMed] [Google Scholar]
- 35.Silverman BL, Rizzo T, Green OC, Cho NH, Winter RJ, Ogata ES, et al. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes. 1991;40 Suppl 2:121–5. [DOI] [PubMed] [Google Scholar]
- 36.Baptiste-Roberts K, Nicholson WK, Wang N-Y, Brancati FL. Gestational Diabetes and Subsequent Growth Patterns of Offspring: The National Collaborative Perinatal Project. Maternal and child health journal. 2012;16(1):125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crume TL, Ogden L, West NA, Vehik KS, Scherzinger A, Daniels S, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54(1):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vohr BR, Boney CM. Gestational diabetes: The forerunner for the development of maternal and childhood obesity and metabolic syndrome? The Journal of Maternal-Fetal & Neonatal Medicine. 2008;21(3):149–57. [DOI] [PubMed] [Google Scholar]
- 39.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life--a systematic review. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2005;6(2):143–54. [DOI] [PubMed] [Google Scholar]
- 40.Zhao P, Liu E, Qiao Y, Katzmarzyk PT, Chaput J-P, Fogelholm M, et al. Maternal gestational diabetes and childhood obesity at age 9–11: results of a multinational study. Diabetologia. 2016;59(11):2339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boerschmann H, Pfluger M, Henneberger L, Ziegler AG, Hummel S. Prevalence and predictors of overweight and insulin resistance in offspring of mothers with gestational diabetes mellitus. Diabetes Care. 2010;33(8):1845–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ray JG, Vermeulen MJ, Shapiro JL, Kenshole AB. Maternal and neonatal outcomes in pregestational and gestational diabetes mellitus, and the influence of maternal obesity and weight gain: the DEPOSIT* study. QJM: An International Journal of Medicine. 2001;94(7):347–56. [DOI] [PubMed] [Google Scholar]
- 43.Feig DS, Hwee J, Shah BR, Booth GL, Bierman AS, Lipscombe LL. Trends in incidence of diabetes in pregnancy and serious perinatal outcomes: a large, population-based study in Ontario, Canada, 1996–2010. Diabetes Care. 2014;37(6):1590–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.