Summary
In Drosophila, microbial association can promote development or extend life. We tested the impact of microbial association during malnutrition and show that microbial quantity is a predictor of fly longevity. Although all tested microbes, when abundantly provided, can rescue lifespan on low-protein diet, the effect of a single inoculation seems linked to the ability of that microbial strain to thrive under experimental conditions. Microbes, dead or alive, phenocopy dietary protein, and the calculated dependence on microbial protein content is similar to the protein requirements determined from fly feeding studies, suggesting that microbes enhance host protein nutrition by serving as protein-rich food. Microbes that enhance larval growth are also associated with the ability to better thrive on fly culture medium. Our results suggest an unanticipated range of microbial species that promote fly development and longevity and highlight microbial quantity as an important determinant of effects on physiology and lifespan during undernutrition.
Subject Areas: Nutrition in Life Cycle, Microbiology, Microbiome
Graphical Abstract
Highlights
-
•
Microbial association promotes fly longevity and development on low-protein diet
-
•
A wide range of microbes can serve as a source of protein during undernutrition
-
•
The extent of effects correlates with microbiota quantity and biomass
-
•
The most impactful microbial species simply thrive on fly culture medium
Nutrition in Life Cycle; Microbiology; Microbiome
Introduction
Host-microbe associations influence human health and disease. Altered microbiota can have a profound effect on host metabolism (Kau et al., 2011), and several studies have correlated imbalanced gut microbiota with cardiovascular disease, insulin resistance, diabetes, and aging (Claesson et al., 2012, Nicholson et al., 2012, Qin et al., 2012, Turnbaugh et al., 2009). Clearly, gut microbes are a promising target for metabolic disease intervention (Holmes et al., 2012), but further studies are needed to delineate the precise interplay between microbes and their hosts.
Studies in both humans and rodents have suggested that distinct microbial populations can act as a key signature of obesity (Jumpertz et al., 2011). Colonization of germ-free (axenic) mice with intestinal microbiota from obese donors induces a substantial increase in body weight (Turnbaugh et al., 2006). However, axenic rodents are protected against diet-induced obesity and display greater fatty acid oxidation (Bäckhed et al., 2007). It is unclear whether specific microbes or general traits such as microbial presence and number have the greatest effect on host metabolism—interestingly, microbial quantification has been useful in characterizing Crohn's disease-associated microbial changes (Vandeputte et al., 2017) and it has been suggested that microbial quantity is influential on Drosophila lifespan on undernutrition diets (Yamada et al., 2015). Together, these reports indicate that quantifying microbial abundance may be critical in future host-microbe studies.
Unfavorable microbial populations have also been implicated in the pathogenesis of kwashiorkor, the clinical manifestation of protein-specific malnutrition (Ahmed et al., 2009, Williams, 1963). Transfer of patient-derived microbiota into mice can induce symptoms of kwashiorkor, although only in the context of a low-protein diet (Smith et al., 2013). Although specific bacterial communities markedly impact the metabolic phenotype of the host, it remains uncertain whether such pathogenic microbial populations depend on the nutritional environment (Claus, 2013).
The interaction between host and microbe is multifaceted, involving host genetics (Spor et al., 2011), the immune system (Hooper et al., 2012), and diet (Faith et al., 2011, Hibberd et al., 2017). Identifying the functional basis of these interactions remains challenging owing to the complexity of mammalian microbial communities (Qin et al., 2010), high inter-individual variation (Turnbaugh et al., 2009), intra-individual fluctuations over time in response to disturbances such as antibiotics (Dethlefsen and Relman, 2011), and changes in diet over the course of life (Claesson et al., 2012). Defined models are beginning to reveal the impact of specific nutrients on microbial communities (Hibberd et al., 2017), yet further simplified models would greatly advance our understanding of nutrition-microbe-host interactions.
One such model, Drosophila, has already provided useful insights into host-microbe relationships, including microbial pathogenicity and host immunity (Chambers and Schneider, 2012, Lazzaro, 2008). Although similarities exist between mammalian- and Drosophila-associated microbes, flies typically harbor a considerably simpler microbiota with ∼200-fold fewer bacterial species (Broderick and Lemaitre, 2012, Chandler et al., 2011, Corby-Harris et al., 2007, Cox and Gilmore, 2007, Wong et al., 2011) that are distributed differently throughout the digestive tract (Keebaugh and Ja, 2016). Nonetheless, as in mammals, changes in the Drosophila diet can rapidly shift bacterial communities (Chandler et al., 2011) and the composition and quantity of gut microbes can shift with age (Claesson et al., 2011, Wong et al., 2011). These changes are associated with a decline in intestinal barrier function, the presence of microbes in non-canonical areas, and increased systemic inflammation (Clark et al., 2015, Guo et al., 2014, Li et al., 2016, Thevaranjan et al., 2017). These parallels highlight Drosophila, with its short lifespan and powerful genetic tools, as an ideal simplified model in which to determine the precise interplay between microbes, diet, metabolism, and lifelong health.
In nature, many host-microbe symbioses are characterized by nutritionally challenging or limited food substrates; microbes generally provide hosts with access to essential nutrients (Adams et al., 2011, Dillon and Dillon, 2004, Douglas, 2009, Douglas, 2011, Leitão-Gonçalves et al., 2017, Muhammad et al., 2017, Sannino et al., 2018). Drosophila-associated microbes promote larval development (Anagnostou et al., 2010, Bakula, 1969, Liu et al., 2017, Meshrif et al., 2016, Starmer, 1982)—an effect mimicked by dietary nutrients (Sang, 1956). More recently, host-associated microbes have been shown to modulate Tor and insulin signaling pathways and intestinal peptidase expression to promote growth and development (Erkosar et al., 2015, Shin et al., 2011, Storelli et al., 2011); similar effects were revealed in a mammalian model (Schwarzer et al., 2016). It remains unclear whether bacterial strains activate these pathways and enhance host nutrition by actively aiding host digestion/absorption or by serving directly as a source of micro- or macronutrients.
Transcriptomic, physiological, and nutritional studies indicate that microbes can alter Drosophila development, metabolism, and nutritional status in a manner consistent with the idea that microbes contribute to host nutrition (Broderick et al., 2014, Ridley et al., 2012, Wong et al., 2014). Larvae exposed to chronic nutritional stress for over 170 generations are less dependent on nutrition and microbial association (Erkosar et al., 2017), suggesting that microbes and nutrition might serve overlapping purposes. Recent studies also show that microbes are a source of protein for Drosophila suzukii, which consume fresh fruit (Bing et al., 2018), and that the abundance of Issatchenkia orientalis rescues D. melanogaster lifespan during undernutrition (Yamada et al., 2015). How other fly-associated microbes might contribute to nutrition is unclear. Here, we examine how individual microbial species influence Drosophila lifespan and larval development. Association with a single microbial species can significantly extend malnourished fly lifespan and promote larval development. Our results suggest that microbes act primarily as a critical source of protein regardless of the species to rescue metabolic health during malnutrition. Microbial abundance in the fly environment seems strongly tied to the impact on fly phenotypes—thus, it is the ability of a particular microbial species to thrive under experimental conditions that best predicts its impact on the fly host. This study reinforces the importance of considering both microbial quantity and quality in microbiota studies of aging and development and underscores the fly as a valuable model for understanding explicit nutrition-microbe-host relationships.
Results
Microbes Extend Fly Lifespan on Undernutrition Diet
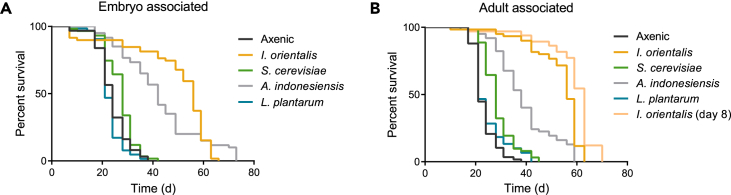
Previously, fly lifespan on a low-yeast, undernutrition diet was shown to be strongly influenced by I. orientalis compared with other tested microbes (Yamada et al., 2015). This effect is conserved in two commonly studied fly strains regardless of whether axenic animals were inoculated with microbes during development or adulthood (Figures 1 and S1). Flies maintained axenically until day 8 of adulthood and subsequently inoculated with I. orientalis were also long-lived (Figure 1B), excluding the first week of adult life as a critical period for microbial association in our paradigm (Brummel et al., 2004). Consistent with previous studies (Yamada et al., 2015), Saccharomyces cerevisiae, Acetobacter indonesiensis, and Lactobacillus plantarum influenced lifespan less than I. orientalis (Figures 1 and S1). Results from embryo or adult association of microbes were generally consistent (Figure 1), demonstrating that microbial association during adulthood is sufficient for extending fly life on a low-nutrient diet.
Figure 1.
Association with Microbes Modulates Adult Lifespan on Undernutrition Diet
(A) Survival of axenic or monoxenic Canton-S male flies on 0.1% yeast extract (YE) malnutrition diet (axenic versus L. plantarum, p = 0.75, all other comparisons to axenic, p ≤ 0.03, log rank test). Axenic flies were associated as embryos with specific microbes. N = 59–64 flies.
(B) Survival of axenic or monoxenic Canton-S male flies on 0.1% YE diet (axenic versus L. plantarum, p = 0.28, all other comparisons to axenic, p ≤ 1.0 × 10−6, log rank test). Axenic flies were associated with microbes 1 day after eclosion, except for the indicated trial with I. orientalis, which was inoculated 8 days after eclosion. N = 58–66 flies.
See also Figure S1.
Microbial Quantity and Biomass Correlate Positively with Fly Lifespan
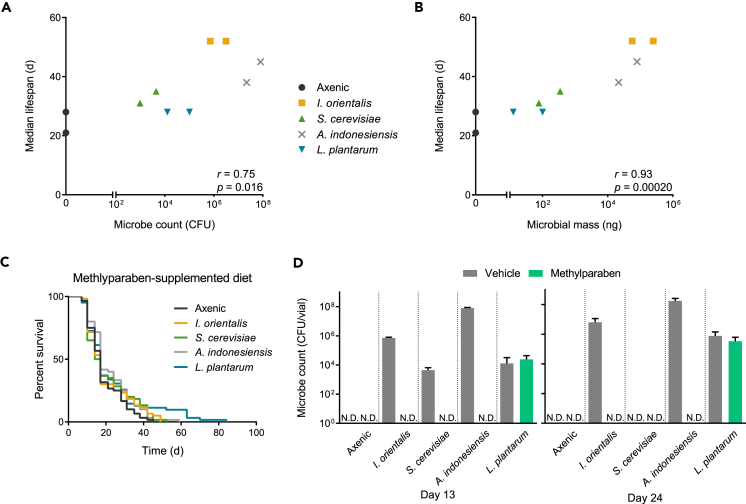
What are the differences between microbial species that drive lifespan extension? We anecdotally observed that I. orientalis and A. indonesiensis grow better on fly medium than other microbes. Given our previous studies suggesting that I. orientalis serves as a protein-rich food source for flies (Yamada et al., 2015), we hypothesized that a broad range of microbes might have the potential to extend life, but that only certain species proliferate well enough on fly media to maintain sufficient numbers to maximize fly longevity. Thus, microbial abundance available in the environment, rather than the transient gut microbiota (Blum et al., 2013), would be the key metric for predicting the impact on fly phenotypes. We quantified environmental microbes in spent vials and found that fly lifespan extension resulting from a single inoculation with live microbes shows a strong correlation with exogenous microbe counts (Figure 2A). The correlation is strengthened when accounting for the larger mass of yeasts compared with bacteria (Figure 2B).
Figure 2.
Microbial Growth in the Fly Environment Is Positively Correlated with Extended Life
(A) Microbe numbers in the fly environment correlate with median lifespan on malnutrition diet (r = 0.75, p = 0.016, Spearman's correlation coefficient). Average microbe counts from fly enclosures (N = 3), including the food surface, collected on day 8 or 13 from two independent survival studies with adult-associated live microbes.
(B) Microbial mass calculated from microbe counts (80 pg/cell for I. orientalis and S. cerevisiae, 1 pg/cell for A. indonesiensis and L. plantarum) correlates with median lifespan (r = 0.93, p = 0.00020, Spearman's correlation coefficient).
(C) Methylparaben abolishes the benefit of microbes on lifespan. None of the survival curves differ significantly from that of axenic flies (p > 0.05 for each comparison, log rank test). Median lifespan of microbe-associated flies also does not differ from that of axenic animals (p > 0.05 for each comparison, Fisher's exact test). N = 60–62 flies.
(D) Methylparaben eliminates I. orientalis, A. indonesiensis, and S. cerevisiae in the fly environment. Shown are average microbe counts (+ SD) from spent fly vials (N = 3) collected on days 13 and 24 (3–4 days after food change). N.D., not detected (in all replicates). Flies were maintained on 0.1% YE malnutrition diet with either our standard acid-based preservative or 0.3% methylparaben.
See also Figure S2.
If microbes serve as a direct source of nutrition for flies, then lifelong association with microbes is likely needed for their impact on fly lifespan. To test this hypothesis, we manipulated microbe growth with methylparaben (methyl 4-hydroxybenzoate, also known as Tegosept or Nipagin M), a common food preservative (Ashburner et al., 2005, Obadia et al., 2018). Methylparaben eliminated I. orientalis, A. indonesiensis, and S. cerevisiae in the fly environment and also abolished lifespan extension imparted by these microbes compared with our standard methylparaben-free diet (Figures 2C and 2D). Interestingly, L. plantarum had a small, positive effect on lifespan regardless of whether the food contained methylparaben—paralleling the lack of an effect of methylparaben on L. plantarum growth (Figures 2C, 2D, and S2).
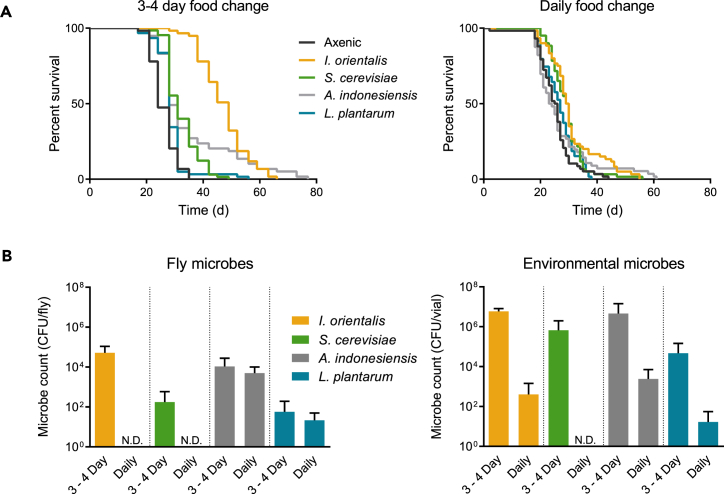
The number of microbes present in the fly environment results from multiple factors including microbial growth rate on the food surface, the presence of larvae, ingestion by flies, and spreading through fly excretion and external contact. These factors are likely further complicated by parameters such as the number of flies, enclosure size, and food surface area. Importantly, given that animals are provided with fresh, sterile food in an axenic environment every few days, microbial presence throughout life must be dependent on their passage using the fly as a vector. Since the transfer of flies to fresh food dramatically reduces the immediate microbial load (Blum et al., 2013), we next asked whether shorter intervals between food changes throughout life would affect microbe numbers and therefore attenuate the effects of microbial association on longevity. Axenic adult flies were inoculated once with microbial cultures, and fresh food was provided daily or every 3–4 days. The food change interval had no effect on axenic fly lifespan (Figure 3A). Association with I. orientalis, and to some extent A. indonesiensis, extended life when food was changed every 3–4 days, but this effect was severely diminished when transfers occurred daily (Figure 3A). These results correspond with the number of microbes found associated with flies and the spent vials. The abundance of I. orientalis and A. indonesiensis was largely maintained throughout life with the 3-4–day food change interval (Figure S3). Compared with counts from 3-4–day transfers, daily transfers generally diminished microbe numbers with the exception of A. indonesiensis and L. plantarum in fly samples (Figures 3B and S3). Our cumulative results demonstrate that continued microbial presence is required to impact fly lifespan on a low-yeast, malnutrition diet and that microbial persistence is maintained by flies inoculating their environment upon each passage to fresh food. The full benefit to fly lifespan is only conferred by microbes that are given sufficient time to grow on the new medium.
Figure 3.
Food Change Interval Affects Lifespan and Microbial Abundance
(A) Survival of axenic or adult-associated, monoxenic flies on 0.1% YE malnutrition diet with fresh food provided every 3–4 days (left) or daily (right) (axenic versus microbes, 3-4–day transfers: p ≤ 0.01 for each comparison; daily transfers: p > 0.05 for axenic versus A. indonesiensis or L. plantarum, p ≤ 0.0029 for axenic versus I. orientalis or S. cerevisiae, log rank test). N = 56–65 flies.
(B) Average microbial counts (+SD) in adult flies (left) or spent vials (right) when flies were transferred to fresh vials every 3–4 days or daily (averaged over CFU counts from days 4, 8, 12, and 16 from the study shown in A). For each day sampled, CFU/fly was calculated from N = 6 flies each and CFU/vial was calculated from N = 3 enclosures each. N.D., not detected (in all replicates). CFU, colony-forming unit.
See also Figure S3.
Heat-Killed Microbes Have a Dose-Dependent Effect on Fly Longevity
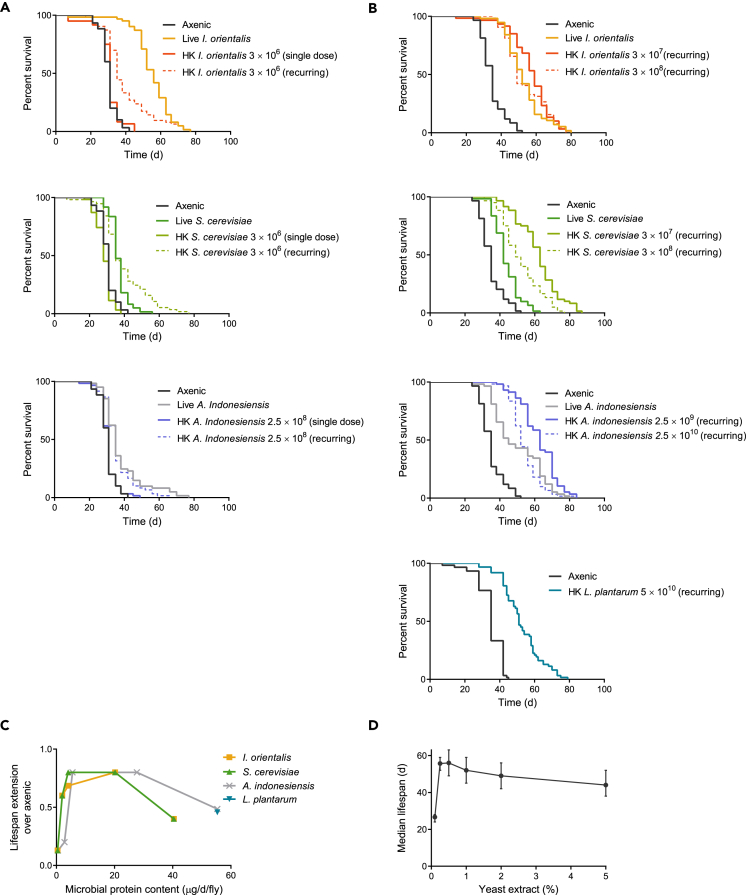
To more directly test the idea that microbial quantity broadly modulates fly lifespan, we assessed whether heat-killed microbes provided either one time in early adulthood or at every food change would promote longevity. A single and relatively low dose of heat-killed I. orientalis did not alter lifespan compared with axenic flies (Figure 4A). In contrast, a recurring supply of heat-killed I. orientalis extended life, although not to the extent of that from a single inoculation with live microbes (Figure 4A). Increasing the quantity of heat-killed I. orientalis given to flies throughout life revealed a dose-dependent effect on longevity that can match or exceed the lifespan extension observed with live microbe inoculation (Figure 4B). Surprisingly, providing an optimal dose of heat-killed S. cerevisiae, A. indonesiensis, or L. plantarum can also extend life beyond the effect of a single live inoculation. These results support the idea that microbes provide a broad benefit to flies and show that live, metabolically active microbes are not necessary for promoting fly longevity.
Figure 4.
Microbes Extend Fly Lifespan in a Dose-Dependent Manner
(A) Survival of axenic or microbe-associated flies on 0.1% YE malnutrition diet. For microbe-associated conditions, 5 × 105 CFU of live I. orientalis or S. cerevisiae or 5 × 107 CFU of live A. indonesiensis was supplied one time in early adulthood, or the indicated quantity of autoclaved (heat-killed [HK]) microbes were provided either one time (single dose) or at every food change (twice/week) throughout life (recurring). A single dose of HK microbes resulted in no change in survival (p > 0.05, log rank test). Both live and recurring doses of HK microbes resulted in significant lifespan extension compared with the axenic control (p ≤ 4.3 × 10−6 for each comparison, log rank test). N = 57–63 flies.
(B) HK microbes supplied throughout life can extend lifespan on a malnutrition diet (0.1% YE) (axenic versus live or HK microbes; p ≤ 1.5 × 10−8, log rank test). Axenic flies were associated once with live microbes or continuously with HK microbes throughout life. The indicated CFU of HK microbes was supplied at every food change. N = 57–64 flies.
(C) Relationship between lifespan extension and the estimated protein content of different doses of HK microbes. Lifespan extension is calculated as the change in median lifespan between flies receiving lifelong HK microbe supplementation and axenic controls. Estimated protein content from each dose of HK microbes is divided by the initial number of flies per enclosure and number of days between transfers to fresh food to show the protein content available per fly per day. N = 57–64 flies.
(D) Effect of yeast extract (YE) concentration on lifespan. As YE is reduced from the highest concentration tested (5%), dietary restriction results in lifespan extension. From the point of maximal longevity (0.25–0.5% YE), further reduction in YE results in malnutrition and drastically reduced lifespan. Median lifespan is plotted with whiskers representing the interquartile range (first and third quartiles). Diets were composed of the indicated YE (%, w/v) in a base medium containing 5% sucrose and 8.6% cornmeal (all w/v). N = 64–158 axenic flies. CFU, colony-forming unit.
What common factor from various microbial species might be mediating the effects on fly lifespan? We previously suggested that I. orientalis serves broadly as a protein-rich food source for flies (Yamada et al., 2015). Our heat-killed microbial studies show that many species are likely sufficient to serve as this food source, and that it is the ability of that species to grow or thrive on fly media that directly contributes to its ability to promote fly longevity. Numerous studies have emphasized the importance of protein as a determinant in modulating Drosophila lifespan (Bruce et al., 2013, Grandison et al., 2009b, Ja et al., 2009, Skorupa et al., 2008), and yeast (or yeast extract [YE]) is the primary source of protein in most laboratory fly diets. Interestingly, if we estimate the microbial protein content for all the heat-killed microbe doses used, maximum lifespan extension is achieved when flies are provided with sufficient microbial protein (4–20 μg/fly/day) to rescue nutrient-deficient conditions (Figure 4C). These values are consistent with the lifespan differences observed between flies on 0.1% and 0.5% YE diets (Figure 4D), where food consumption measurements reveal a difference in protein intake of ∼3 μg/fly/day (Bruce et al., 2013). Further increases in the dose of heat-killed microbes leads to shortened fly lifespan, reminiscent of the effect of high YE concentration diets (Figures 4C and 4D). These results are in strong agreement with previous studies demonstrating that longevity is maximized at a relatively low protein:carbohydrate ratio, and altering the ratio to cause protein deficiency or excess shortens life (Bruce et al., 2013, Galenza et al., 2016, Grandison et al., 2009a, Lee, 2015, Lee et al., 2008, Min and Tatar, 2006).
Microbes Can Serve as a Replacement for Dietary Protein
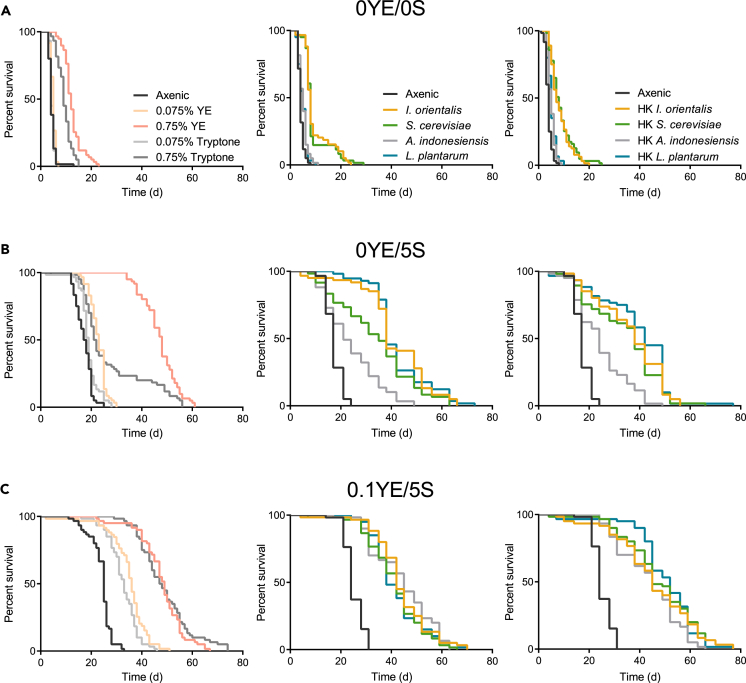
To further define the essential nutrients provided by microbes, we examined the effect of microbes on various minimal diets. We supplemented a simple agar medium lacking any nutritional value with either microbes or protein-rich ingredients. Live or heat-killed microbes were provided with every food change, ensuring an ad libitum supply of the additive regardless of microbial growth. Tryptone (digested casein) was used as a pure protein source. On the agar-only base medium, tryptone and YE at the highest concentration tested (0.75%) extended life, although median lifespan was still short (∼10 days). Live and heat-killed I. orientalis and S. cerevisiae mimicked the effect of tryptone and YE (Figure 5A and Table 1). These results suggest that microbes or equivalent bulk macronutrients cannot supply sufficient nutrition to act as the sole, optimal food source for fly survival.
Figure 5.
Microbes Provide Specific Nutritional Benefits under Different Malnutrition Diets
(A) On a starvation diet (0YE/0S = 0% yeast extract +0% sucrose), lifelong supplementation of live or autoclaved (heat-killed [HK]) microbes, YE, or tryptone has limited impact on longevity (left: axenic versus 0.075% tryptone, p > 0.05, all other comparisons, p ≤ 0.033; middle: all comparisons to axenic, p ≤ 0.015; right: all comparisons to axenic, p ≤ 0.0002, log rank test). At every food change, live or HK microbes (3 × 108 CFU/vial of I. orientalis or S. cerevisiae or 2.5 ×1010 CFU/vial of A. indonesiensis or L. plantarum), tryptone (equivalent to 0.075% or 0.75%, w/v, final), or YE (0.075% or 0.75%) were supplied. N = 59–64 flies.
(B) On a sucrose-only diet (0YE/5S), only lifelong supplementation of YE or certain microbes extends lifespan maximally (left: all comparisons to axenic, p ≤ 0.037; middle: all comparisons to axenic, p ≤ 5.0 × 10−6; right: all comparisons to axenic, p ≤ 5.4 × 10−8, log rank test). N = 56–66 flies.
(C) On a low yeast medium (0.1YE/5S), all additives supplied throughout life (tryptone, YE, or live or HK microbes) strongly extend lifespan (left: all comparisons, p < 1.0 × 10−10; middle: all comparisons, p < 1.0 × 10−10; right: all comparisons, p < 1.0 × 10−10, log rank test). N = 59–60 flies. All flies used were initially axenic. CFU, colony-forming unit.
Table 1.
The Impact of Yeast Extract (YE), Tryptone, and Microbes on Lifespan
| YE/S | Base Diet | YE | Tryptone | I. orientalis | S. cerevisiae | A. indonesiensis | L. plantarum |
|---|---|---|---|---|---|---|---|
| 0/0 | ● | + | + | + | + | ● | ● |
| 0/5 | ++ | +++ | ++ | +++ | +++ | ++ | +++ |
| 0.1/5 | ++ | +++ | +++ | +++ | +++ | +++ | +++ |
The effect of recurring supplementation with the indicated additive is categorized using median lifespan values from Figure 5. Categories were defined as: ●, 0–7.5 days; +, 7.5–15 days; ++, 15–30 days; +++, >30 days.
Next, we added only sucrose to the pure agar diet. On 5% sucrose medium (0YE/5S, containing 0% YE and 5% sucrose), YE supplementation greatly extended lifespan, whereas tryptone only partially rescued longevity (Figure 5B and Table 1). These results are consistent with the idea that on a pure sucrose diet, both additional protein and other nutrients—likely micronutrients such as vitamins and minerals—are required for optimal health. This aligns with previous findings in Queensland flies that show that the major contribution of yeast to a diet is protein, except in very low yeast diets, wherein the micronutrient contribution becomes important (Fanson and Taylor, 2012). Interestingly, live or heat-killed I. orientalis, S. cerevisiae, and L. plantarum are capable of mimicking the effects of YE, whereas the effect of A. indonesiensis was more similar to that of tryptone supplementation (Figure 5B and Table 1).
When a marginal level of YE is added to the base diet (0.1YE/5S), supplementation of tryptone or YE extended fly lifespan similarly (Figure 5C and Table 1). These results suggest that, on a 0.1YE/5S diet, the main contribution of YE is protein. All microbes tested—dead or alive—also fully rescue longevity (Figure 5C and Table 1). Given that microbes can mimic tryptone or YE, our findings are consistent with the idea that microbes extend fly lifespan on malnutrition diet by directly contributing to protein nutrition.
Microbes Promote Larval Growth on Malnutrition Diet
Association with certain strains of L. plantarum and Acetobacter pomorum was previously shown to enhance larval growth on low-yeast or low-protein diets (Shin et al., 2011, Storelli et al., 2011), mimicking the effect of increasing dietary yeast concentration to shorten time to pupariation (Anagnostou et al., 2010). We hypothesized that enhanced larval growth on an undernutrition diet might be associated with higher microbial quantity of the growth-promoting strains of Lactobacillus and Acetobacter over non–growth-promoting controls. Our experimental 0.1% YE diet lacks sufficient nutrition for axenic larval development, so we increased the YE to 0.5%. Axenic larvae do not reach the pupal stage on this diet, but tryptone and YE supplementation can rescue pupariation (Figure S4), suggesting that this diet is undernutritious but suitable for determining if microbes can enhance larval development.
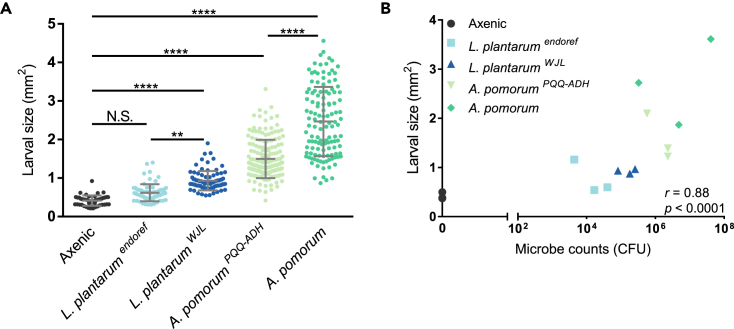
We next monocolonized embryos with Lactobacillus plantarumWJL or A. pomorum, which promote larval growth over two conspecific control strains, Lactobacillus plantarumendoref or Acetobacter pomorumPQQ-ADH, respectively (Shin et al., 2011, Storelli et al., 2011). Similar to previous reports (Shin et al., 2011, Storelli et al., 2011), microbes generally promoted growth over the axenic state, and the two growth-promoting strains produced larger larvae compared with their respective controls after 6 days of association on the 0.5% YE diet (Figure 6A). There was a significant correlation between larval size and microbial counts washed from food surfaces (Figure 6B). Microbial counts on spent larval diets varied from larval-free conditions and also across diets, suggesting that a multitude of context-dependent factors can influence microbial growth (Figure S5)—this interpretation is consistent with recent studies showing that larval presence helps sustain L. plantarumWJL abundance in the environment (Storelli et al., 2018). Overall, microbial effects on monoxenic larval development are consistent with previous reports on Lactobacillus (Storelli et al., 2011) and Acetobacter (Shin et al., 2011), and the effects of microbial association on larval development mirror the apparent nutritional benefits in adult flies. These results suggest that the growth of larvae on a protein-poor diet is influenced by the abundance of associated bacteria.
Figure 6.
Microbial Quantity Is Associated with Enhanced Larval Development
(A) Larval size (mm2) on 0.5% YE diet 6 days after axenic treatment or monoxenic exposure to approximately 5.4 × 106 CFU/well of L. plantarumendoref, L. plantarumWJL, A. pomorumPQQ-ADH, or A. pomorum. Live microbes were provided one time immediately following axenic processing of embryos (**p ≤ 0.01, ****p ≤ 0.0001, Dunn's multiple comparisons test). Average of all larvae from N = 3 arenas per group is represented by a horizontal line placed between error bars representing ± SD; individual arena averages are shown in (B). N = 46–121 measured larvae per strain.
(B) Average larval size (mm2) compared with CFU from larval food surfaces in N = 3 arenas containing the cohorts of larvae displayed in A (r = 0.88, p < 0.0001, Spearman's correlation coefficient). CFU, colony-forming unit.
See also Figures S4 and S5.
Discussion
As with mammals, insects are associated with important microbial symbionts (Douglas, 2011, Vega and Dowd, 2005) and can form stable intestinal symbioses with microbes (Inamine et al., 2018, Obadia et al., 2017). Drosophila melanogaster has an important relationship with microbes—flies consume microbes in rotting vegetation and microbes benefit from fly transmission to new environments. The capacity for Drosophila to vector microbes has been demonstrated in the wild (Ganter, 1988, Gilbert, 1980, Morais et al., 1994) and detailed in the laboratory (Stamps et al., 2012). Larvae and adult flies influence the density and composition of yeast growing on the nutritional substrate (Stamps et al., 2012) to the extent that microbial communities within the fly are similar to those in the nutritional environment (Tefit et al., 2018, Wong et al., 2015). Microbes support larval development on some minimal substrates (Anagnostou et al., 2010, Becher et al., 2012, Liu et al., 2017, Meshrif et al., 2016, Starmer, 1982), indicating that the quality of the nutritional environment itself is an integral factor in fly-microbe symbioses (Shin et al., 2011, Storelli et al., 2011, Tefit et al., 2018, Yamada et al., 2015). The interplay between diet, microbe, and host translates into vertebrates as well (Dalby et al., 2017). Recent studies of kwashiorkor—protein-specific malnutrition coupled with dysbiosis—have demonstrated that the interaction between diet and gut microbiota should be considered when designing interventions for maintaining long-term health following malnutrition (Smith et al., 2013).
Host microbiota can affect nutrition by aiding the digestion of food or altering assimilation of nutrients (Bäckhed et al., 2005). Microbial metabolism can provide essential nutrients such as vitamins and lipids, increase the bioavailability of dietary components, help process nutritional substrates (Adams et al., 2011, Dillon and Dillon, 2004, Douglas, 2009, Kesnerova et al., 2017, Muhammad et al., 2017, Suh et al., 2003), and promote D. melanogaster nutrition by modifying the culture medium (Huang and Douglas, 2015, Wong et al., 2014). Interestingly, some changes in host nutritional indices that are attributed to microbe-mediated dietary modifications can also be achieved by placing axenic flies on diets with higher protein content (Yamada et al., 2015), suggesting that microbes passively mimic a protein-rich diet for axenic flies. In fact, microbes can serve as a direct source of protein for some detritivorous animals (Steffan et al., 2017). Here, we show that in D. melanogaster, multiple bacterial and yeast species phenocopy the effect of tryptone or YE supplementation during malnutrition to restore lifespan and development. Our results are consistent with the idea that microbes serve directly as a protein-rich food source during undernutrition—thereby modifying the effective protein:carbohydrate ratio of the culture medium—to affect host physiology. The impact of microbes is observed even with a single, early-life inoculation, thereby linking the effects of specific microbial strains to their ability to thrive under experimental conditions associated with flies. The fly gut microbiota is generally transient under laboratory conditions (Blum et al., 2013), and the number of live microbes in the fly gut is likely reflective of the quantity in the fly environment—thus, it is a sufficient supply of environmental microbes that seems to be the critical determinant for fly lifespan during malnutrition. Although we also associate microbial abundance—and therefore increased nutrient availability—with enhanced larval growth, further studies are needed to quantify the nutritional contribution to larval development.
Protein metabolism is a key mechanism underlying microbial benefits on development and lifespan on low-protein diets, although additional nutrients or factors may contribute in other conditions. For instance, A. indonesiensis and tryptone showed limited effects on a carbohydrate-only diet, suggesting that A. indonesiensis and tryptone do not contribute micronutrients that YE, I. orientalis, S. cerevisiae, and L. plantarum provide. Interestingly, previous studies examining bacterial contributions to fly growth and development have implicated the roles of Tor and insulin signaling in the context of low-nutrient diets (Shin et al., 2011, Storelli et al., 2011). Although similar research identified an acid modification on bacterial cell walls that is important in L. plantarum-induced larval growth on low-protein diet (Matos et al., 2017), the involvement of key nutrient-sensing pathways and the requirement of low-nutrient conditions for bacteria-derived phenotypes is consistent with the hypothesis that microbes act as a critical macronutrient source to influence fundamental metabolic pathways during malnourished conditions. Microbial supplementation on more nutritious diets, however, can be neutral or detrimental (Brummel et al., 2004, Clark et al., 2015, Li et al., 2016, Yamada et al., 2015). Thus, it is under conditions in which dietary nutrients are scarce that microbial quantity may be more critical than microbial quality; indeed, we find that growth-promoting bacterial strains do grow better or are sustained at higher levels on an undernutrition diet.
More recent studies show that heat-killed L. plantarumWJL can also promote larval growth, but only in repeated and high doses—maximal growth is achieved with a constant supply of live microbes (Storelli et al., 2018). Further measurements of microbial growth kinetics in the fly environment or the use of ad libitum heat-killed microbe supplementation may help fully describe microbial influences in future research (i.e., to identify a direct nutritional contribution of microbes). Studies detailing microbial cell size or physiological characteristics across different nutritional environments may also reveal how these parameters affect the nutritional value of microbes (Bren et al., 2013, Sezonov et al., 2007, Storelli et al., 2018). It may be interesting to determine if microbial quantity impacts fly behavior, physiology, or longevity in nature, particularly under multi-microbial experimental conditions.
Although yeast is a common food source for wild and laboratory flies, the nutritional contribution of yeast and bacteria in the context of microbiota research seems largely ignored. It should be important to decouple the effects of microbivory or nutrition from specialized host-microbe interactions in future studies, especially in organisms that consume microbes for sustenance (Virk et al., 2016). Non-specific effects might impact a range of physiological, metabolic, and behavioral phenotypes (Obadia et al., 2018), and recent studies on microbe communities reveal that some microbe-induced host phenotypes are general across a multitude of microbial strains (Faith et al., 2014). Measurement of bacterial growth rates or abundance in experimentally relevant conditions (Storelli et al., 2018), identifying stable bacterial colonizers (Inamine et al., 2018, Obadia et al., 2017), and carefully choosing diets to control for diet-induced changes in microbial composition or quantity (Dalby et al., 2017) will support studies on specialized interactions.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by grants from the NIH (R00AG030493 and R01AG045036 to W.W.J.; DP5OD017851 to W.B.L.), The Ellison Medical Foundation, the Glenn Foundation for Medical Research, and the U.C. Berkeley MCB Department Bowes Research Fellows Program. E.S.K. is an O'Keeffe Neuroscience Scholar of the Esther B. O'Keeffe Charitable Foundation. We thank M. Piper and L. Partridge (University College London) for the Dahomey fly line, T. Brummel (Long Island University) for comments on the manuscript, and Chenchen Su for technical support and graphics.
Author Contributions
Conceptualization, E.S.K., R.Y., and W.W.J.; Investigation, E.S.K., R.Y., B.O., and W.B.L.; Writing – Original Draft, E.S.K., R.Y., and W.W.J.; Writing – Reviewing & Editing, E.S.K., R.Y., B.O., W.B.L., and W.W.J.; Supervision, W.B.L. and W.W.J.; Funding Acquisition, W.B.L. and W.W.J.
Declaration of Interests
W.B.L. was a co-founder of and is a shareholder in uBiome, Inc.
Published: June 29, 2018
Footnotes
Supplemental Information includes Transparent Methods and five figures and can be found with this article online at https://doi.org/10.1016/j.isci.2018.06.004.
Supplemental Information
References
- Adams A.S., Jordan M.S., Adams S.M., Suen G., Goodwin L.A., Davenport K.W., Currie C.R., Raffa K.F. Cellulose-degrading bacteria associated with the invasive woodwasp Sirex noctilio. ISME J. 2011;5:1323–1331. doi: 10.1038/ismej.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed T., Rahman S., Cravioto A. Oedematous malnutrition. Indian J. Med. Res. 2009;130:651–654. [PubMed] [Google Scholar]
- Anagnostou C., Dorsch M., Rohlfs M. Influence of dietary yeasts on Drosophila melanogaster life-history traits. Entomol. Exp. Appl. 2010;136:1–11. [Google Scholar]
- Ashburner M., Golic K.G., Hawley S.R. Parasites, pests, and diseases. In: Ashburner M., Golic K.G., Hawley S.R., editors. Drosophila: A Laboratory Handbook. CSHL Press; 2005. pp. 1285–1332. [Google Scholar]
- Bäckhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Bäckhed F., Manchester J.K., Semenkovich C.F., Gordon J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakula M. Persistence of a microbial flora during postembryogenesis of Drosophila melanogaster. J. Invertebr. Pathol. 1969;14:365–374. doi: 10.1016/0022-2011(69)90163-3. [DOI] [PubMed] [Google Scholar]
- Becher P.G., Flick G., Rozpedowska E., Schmidt A., Hagman A., Lebreton S., Larsson M.C., Hansson B.S., Piskur J., Witzgall P. Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Funct. Ecol. 2012;26:822–828. [Google Scholar]
- Bing X., Gerlach J., Loeb G., Buchon N. Nutrient-dependent impact of microbes on Drosophila suzukii development. MBio. 2018;9 doi: 10.1128/mBio.02199-17. e02199–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J.E., Fischer C.N., Miles J., Handelsman J. Frequent replenishment sustains the beneficial microbiome of Drosophila melanogaster. MBio. 2013;4 doi: 10.1128/mBio.00860-13. e00860–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bren A., Hart Y., Dekel E., Koster D., Alon U. The last generation of bacterial growth in limiting nutrient. BMC Syst. Biol. 2013;7:27. doi: 10.1186/1752-0509-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick N.A., Lemaitre B. Gut-associated microbes of Drosophila melanogaster. Gut Microbes. 2012;3:307–321. doi: 10.4161/gmic.19896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick N.A., Buchon N., Lemaitre B. Microbiota-induced changes in Drosophila melanogaster host gene expression and gut morphology. MBio. 2014;5 doi: 10.1128/mBio.01117-14. e01117–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce K.D., Hoxha S., Carvalho G.B., Yamada R., Wang H.D., Karayan P., He S., Brummel T., Kapahi P., Ja W.W. High carbohydrate-low protein consumption maximizes Drosophila lifespan. Exp. Gerontol. 2013;48:1129–1135. doi: 10.1016/j.exger.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummel T., Ching A., Seroude L., Simon A.F., Benzer S. Drosophila lifespan enhancement by exogenous bacteria. Proc. Natl. Acad. Sci. USA. 2004;101:12974–12979. doi: 10.1073/pnas.0405207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers M.C., Schneider D.S. Pioneering immunology: insect style. Curr. Opin. Immunol. 2012;24:10–14. doi: 10.1016/j.coi.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Chandler J.A., Lang J.M., Bhatnagar S., Eisen J.A., Kopp A. Bacterial communities of diverse Drosophila species: ecological context of a host-microbe model system. PLoS Genet. 2011;7:e1002272. doi: 10.1371/journal.pgen.1002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson M.J., Cusack S., O'Sullivan O., Greene-Diniz R., de Weerd H., Flannery E., Marchesi J.R., Falush D., Dinan T., Fitzgerald G. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA. 2011;108(Suppl 1):4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson M.J., Jeffery I.B., Conde S., Power S.E., O'Connor E.M., Cusack S., Harris H.M.B., Coakley M., Lakshminarayanan B., O'Sullivan O. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- Clark R.I., Salazar A., Yamada R., Fitz-Gibbon S., Morselli M., Alcaraz J., Rana A., Rera M., Pellegrini M., Ja W.W. Distinct shifts in microbiota composition during Drosophila aging impair intestinal function and drive mortality. Cell Rep. 2015;12:1656–1667. doi: 10.1016/j.celrep.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus S.P. Fighting undernutrition: don't forget the bugs. Cell Host Microbe. 2013;13:239–240. doi: 10.1016/j.chom.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Corby-Harris V., Pontaroli A.C., Shimkets L.J., Bennetzen J.L., Habel K.E., Promislow D.E. Geographical distribution and diversity of bacteria associated with natural populations of Drosophila melanogaster. Appl. Environ. Microbiol. 2007;73:3470–3479. doi: 10.1128/AEM.02120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C.R., Gilmore M.S. Native microbial colonization of Drosophila melanogaster and its use as a model of Enterococcus faecalis pathogenesis. Infect. Immun. 2007;75:1565–1576. doi: 10.1128/IAI.01496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby M.J., Ross A.W., Walker A.W., Morgan P.J. Dietary uncoupling of gut microbiota and energy harvesting from obesity and glucose tolerance in mice. Cell Rep. 2017;21:1521–1533. doi: 10.1016/j.celrep.2017.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L., Relman D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA. 2011;108:4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon R.J., Dillon V.M. The gut bacteria of insects: nonpathogenic interactions. Annu. Rev. Entomol. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- Douglas A.E. The microbial dimension in insect nutritional ecology. Funct. Ecol. 2009;23:38–47. [Google Scholar]
- Douglas A.E. Lessons from studying insect symbioses. Cell Host Microbe. 2011;10:359–367. doi: 10.1016/j.chom.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkosar B., Kolly S., van der Meer J.R., Kawecki T.J. Adaptation to chronic nutritional stress leads to reduced dependence on microbiota in Drosophila melanogaster. MBio. 2017;8 doi: 10.1128/mBio.01496-17. e01496–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkosar B., Storelli G., Mitchell M., Bozonnet L., Bozonnet N., Leulier F. Pathogen virulence impedes mutualist-mediated enhancement of host juvenile growth via inhibition of protein digestion. Cell Host Microbe. 2015;18:445–455. doi: 10.1016/j.chom.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith J.J., Ahern P.P., Ridaura V.K., Cheng J., Gordon J.I. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci. Transl. Med. 2014;6:220ra211. doi: 10.1126/scitranslmed.3008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith J.J., McNulty N.P., Rey F.E., Gordon J.I. Predicting a human gut microbiota's response to diet in gnotobiotic mice. Science. 2011;333:101–104. doi: 10.1126/science.1206025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanson B.G., Taylor P.W. Additive and interactive effects of nutrient classes on longevity, reproduction, and diet consumption in the Queensland fruit fly (Bactrocera tryoni) J. Insect Physiol. 2012;58:327–334. doi: 10.1016/j.jinsphys.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Galenza A., Hutchinson J., Campbell S.D., Hazes B., Foley E. Glucose modulates Drosophila longevity and immunity independent of the microbiota. Biol. Open. 2016;5:165–173. doi: 10.1242/bio.015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganter P.F. The vectoring of cactophilic yeasts by Drosophila. Oecologia. 1988;75:400–404. doi: 10.1007/BF00376943. [DOI] [PubMed] [Google Scholar]
- Gilbert D.G. Dispersal of yeasts and bacteria by Drosophila in a temperate forest. Oecologia. 1980;46:135–137. doi: 10.1007/BF00346979. [DOI] [PubMed] [Google Scholar]
- Grandison R.C., Piper M.D., Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandison R.C., Wong R., Bass T.M., Partridge L., Piper M.D. Effect of a standardised dietary restriction protocol on multiple laboratory strains of Drosophila melanogaster. PLoS One. 2009;4:e4067. doi: 10.1371/journal.pone.0004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Karpac J., Tran S.L., Jasper H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell. 2014;156:109–122. doi: 10.1016/j.cell.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd M.C., Wu M., Rodionov D.A., Li X., Cheng J., Griffin N.W., Barratt M.J., Giannone R.J., Hettich R.L., Osterman A.L. The effects of micronutrient deficiencies on bacterial species from the human gut microbiota. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E., Li J.V., Marchesi J.R., Nicholson J.K. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab. 2012;16:559–564. doi: 10.1016/j.cmet.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Hooper L.V., Littman D.R., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.H., Douglas A.E. Consumption of dietary sugar by gut bacteria determines Drosophila lipid content. Biol. Lett. 2015;11:20150469. doi: 10.1098/rsbl.2015.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamine H., Ellner S.P., Newell P.D., Luo Y., Buchon N., Douglas A.E. Spatiotemporally heterogeneous population dynamics of gut bacteria inferred from fecal time series data. MBio. 2018;9 doi: 10.1128/mBio.01453-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ja W.W., Carvalho G.B., Zid B.M., Mak E.M., Brummel T., Benzer S. Water- and nutrient-dependent effects of dietary restriction on Drosophila lifespan. Proc. Natl. Acad. Sci. USA. 2009;106:18633–18637. doi: 10.1073/pnas.0908016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumpertz R., Le D.S., Turnbaugh P.J., Trinidad C., Bogardus C., Gordon J.I., Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau A.L., Ahern P.P., Griffin N.W., Goodman A.L., Gordon J.I. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh E.S., Ja W.W. Microbes without borders: decompartmentalization of the aging gut. Cell Host Microbe. 2016;19:133–135. doi: 10.1016/j.chom.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Kesnerova L., Mars R.A.T., Ellegaard K.M., Troilo M., Sauer U., Engel P. Disentangling metabolic functions of bacteria in the honey bee gut. PLoS Biol. 2017;15:e2003467. doi: 10.1371/journal.pbio.2003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro B.P. Natural selection on the Drosophila antimicrobial immune system. Curr.Opin.Microbiol. 2008;11:284–289. doi: 10.1016/j.mib.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.P. Dietary protein:carbohydrate balance is a critical modulator of lifespan and reproduction in Drosophila melanogaster: a test using a chemically defined diet. J. Insect Physiol. 2015;75:12–19. doi: 10.1016/j.jinsphys.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Lee K.P., Simpson S.J., Clissold F.J., Brooks R., Ballard J.W., Taylor P.W., Soran N., Raubenheimer D. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl. Acad. Sci. USA. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitão-Gonçalves R., Carvalho-Santos Z., Francisco A.P., Fioreze G.T., Anjos M., Baltazar C., Elias A.P., Itskov P.M., Piper M.D.W., Ribeiro C. Commensal bacteria and essential amino acids control food choice behavior and reproduction. PLoS Biol. 2017;15:e2000862. doi: 10.1371/journal.pbio.2000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Qi Y., Jasper H. Preventing age-related decline of gut compartmentalization limits microbiota dysbiosis and extends lifespan. Cell Host Microbe. 2016;19:240–253. doi: 10.1016/j.chom.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Zhang K., Li Y., Su W., Hu K., Jin S. Enterococci mediate the oviposition preference of Drosophila melanogaster through sucrose catabolism. Sci. Rep. 2017;7:13420. doi: 10.1038/s41598-017-13705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos R.C., Schwarzer M., Gervais H., Courtin P., Joncour P., Gillet B., Ma D., Bulteau A.L., Martino M.E., Hughes S. D-alanylation of teichoic acids contributes to Lactobacillus plantarum-mediated Drosophila growth during chronic undernutrition. Nat. Microbiol. 2017;2:1635–1647. doi: 10.1038/s41564-017-0038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshrif W.S., Rohlfs M., Roeder T. The effect of nutritive yeasts on the fitness of the fruit fly Drosophila melanogaster (Diptera: Drosophilidae) Afr. Entomol. 2016;24:90–99. [Google Scholar]
- Min K.J., Tatar M. Restriction of amino acids extends lifespan in Drosophila melanogaster. Mech. Ageing Dev. 2006;127:643–646. doi: 10.1016/j.mad.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Morais P.B., Rosa C.A., Hagler A.N., Mendonca-Hagler L.C. Yeast communities of the cactus Pilosocereus arrabidae as resources for larval and adult stages of Drosophila serido. Antonie Van Leeuwenhoek. 1994;66:313–317. doi: 10.1007/BF00882766. [DOI] [PubMed] [Google Scholar]
- Muhammad A., Fang Y., Hou Y.M., Shi Z.H. The gut entomotype of red palm weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae) and their effect on host nutrition metabolism. Front. Microbiol. 2017;8:2291. doi: 10.3389/fmicb.2017.02291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J.K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W., Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- Obadia B., Guvener Z.T., Zhang V., Ceja-Navarro J.A., Brodie E.L., Ja W.W., Ludington W.B. Probabilistic invasion underlies natural gut microbiome stability. Curr. Biol. 2017;27:1999–2006 e8. doi: 10.1016/j.cub.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obadia B., Keebaugh E.S., Yamada R., Ludington W.B., Ja W.W. Diet influences host-microbiota associations in Drosophila. Proc. Natl. Acad. Sci. USA. 2018;115:E4547–E4548. doi: 10.1073/pnas.1804948115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J.J., Li R.Q., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J.J., Li Y.R., Cai Z.M., Li S.H., Zhu J.F., Zhang F., Liang S.S., Zhang W.W., Guan Y.L., Shen D.Q. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- Ridley E.V., Wong A.C., Westmiller S., Douglas A.E. Impact of the resident microbiota on the nutritional phenotype of Drosophila melanogaster. PLoS One. 2012;7:e36765. doi: 10.1371/journal.pone.0036765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang J.H. The quantitative nutritional requirements of Drosophila melanogaster. J. Exp. Biol. 1956;33:45–72. [Google Scholar]
- Sannino D.R., Dobson A.J., Edwards K., Angert E.R., Buchon N. The Drosophila melanogaster gut microbiota provisions thiamine to its host. MBio. 2018;9 doi: 10.1128/mBio.00155-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer M., Makki K., Storelli G., Machuca-Gayet I., Srutkova D., Hermanova P., Martino M.E., Balmand S., Hudcovic T., Heddi A. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351:854–857. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- Sezonov G., Joseleau-Petit D., D'Ari R. Escherichia coli physiology in Luria-Bertani broth. J. Bacteriol. 2007;189:8746–8749. doi: 10.1128/JB.01368-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S.C., Kim S.H., You H., Kim B., Kim A.C., Lee K.A., Yoon J.H., Ryu J.H., Lee W.J. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science. 2011;334:670–674. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- Skorupa D.A., Dervisefendic A., Zwiener J., Pletcher S.D. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.I., Yatsunenko T., Manary M.J., Trehan I., Mkakosya R., Cheng J.Y., Kau A.L., Rich S.S., Concannon P., Mychaleckyj J.C. Gut microbiomes of malawian twin pairs discordant for kwashiorkor. Science. 2013;339:548–554. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spor A., Koren O., Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat. Rev. Microbiol. 2011;9:279–290. doi: 10.1038/nrmicro2540. [DOI] [PubMed] [Google Scholar]
- Stamps J.A., Yang L.H., Morales V.M., Boundy-Mills K.L. Drosophila regulate yeast density and increase yeast community similarity in a natural substrate. PLoS One. 2012;7:e42238. doi: 10.1371/journal.pone.0042238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer W.T. Associations and interactions among yeasts, Drosophila and their habitats. In: Barker J.S.F., Starmer W.T., editors. Ecological Genetics and Evolution: The Cactus-Yeast-Drosophila Model System. Academic Press; 1982. pp. 159–174. [Google Scholar]
- Steffan S.A., Chikaraishi Y., Dharampal P.S., Pauli J.N., Guedot C., Ohkouchi N. Unpacking brown food-webs: animal trophic identity reflects rampant microbivory. Ecol. Evol. 2017;7:3532–3541. doi: 10.1002/ece3.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storelli G., Defaye A., Erkosar B., Hols P., Royet J., Leulier F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 2011;14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Storelli G., Strigini M., Grenier T., Bozonnet L., Schwarzer M., Daniel C., Matos R., Leulier F. Drosophila perpetuates nutritional mutualism by promoting the fitness of its intestinal symbiont Lactobacillus plantarum. Cell Metab. 2018;27:362–377. doi: 10.1016/j.cmet.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh S.O., Marshall C.J., McHugh J.V., Blackwell M. Wood ingestion by passalid beetles in the presence of xylose-fermenting gut yeasts. Mol. Ecol. 2003;12:3137–3145. doi: 10.1046/j.1365-294x.2003.01973.x. [DOI] [PubMed] [Google Scholar]
- Tefit M.A., Gillet B., Joncour P., Hughes S., Leulier F. Stable association of a Drosophila-derived microbiota with its animal partner and the nutritional environment throughout a fly population's life cycle. J. Insect Physiol. 2018;106:2–12. doi: 10.1016/j.jinsphys.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Thevaranjan N., Puchta A., Schulz C., Naidoo A., Szamosi J.C., Verschoor C.P., Loukov D., Schenck L.P., Jury J., Foley K.P. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21:455–466 e454. doi: 10.1016/j.chom.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E., Sogin M.L., Jones W.J., Roe B.A., Affourtit J.P. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Vandeputte D., Kathagen G., D'Hoe K., Vieira-Silva S., Valles-Colomer M., Sabino J., Wang J., Tito R.Y., De Commer L., Darzi Y. Quantitative microbiome profiling links gut community variation to microbial load. Nature. 2017;551:507–511. doi: 10.1038/nature24460. [DOI] [PubMed] [Google Scholar]
- Vega F.E., Dowd P.F. The role of yeasts as insect endosymbionts. In: Vega F.E., Blackwell M., editors. Insect-Fungal Associations: Ecology and Evolution. Oxford University Press; 2005. pp. 211–243. [Google Scholar]
- Virk B., Jia J., Maynard C.A., Raimundo A., Lefebvre J., Richards S.A., Chetina N., Liang Y., Helliwell N., Cipinska M. Folate acts in E. coli to accelerate C. elegans aging independently of bacterial biosynthesis. Cell Rep. 2016;14:1611–1620. doi: 10.1016/j.celrep.2016.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C.D. The story of kwashiorkor. Courrier. 1963;13:361–367. [PubMed] [Google Scholar]
- Wong A.C., Luo Y., Jing X., Franzenburg S., Bost A., Douglas A.E. The host as the driver of the microbiota in the gut and external environment of Drosophila melanogaster. Appl. Environ. Microbiol. 2015;81:6232–6240. doi: 10.1128/AEM.01442-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A.C., Dobson A.J., Douglas A.E. Gut microbiota dictates the metabolic response of Drosophila to diet. J. Exp. Biol. 2014;217:1894–1901. doi: 10.1242/jeb.101725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C.N.A., Ng P., Douglas A.E. Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ. Microbiol. 2011;13:1889–1900. doi: 10.1111/j.1462-2920.2011.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada R., Deshpande S.A., Bruce K.D., Mak E.M., Ja W.W. Microbes promote amino acid harvest to rescue undernutrition in Drosophila. Cell Rep. 2015;10:865–872. doi: 10.1016/j.celrep.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.