Cytomegalovirus infection frequently causes blindness in untreated HIV/AIDS patients. This virus manipulates host cells to dysregulate immune functions and drive disease. Here, we use an animal model of this disease to demonstrate that cytomegalovirus infection within eyes during retinitis causes massive upregulation of immunosuppressive host proteins called SOCS. As viral overexpression of SOCS proteins exacerbates infection with other viruses, they may also enhance cytomegalovirus infection. Alternatively, the immunosuppressive effect of SOCS proteins may be protective against immunopathology during cytomegalovirus retinitis, and in such a case SOCS mimetics or overexpression treatment strategies might be used to combat this disease. The results of this work therefore provide crucial basic knowledge that contributes to our understanding of the mechanisms of AIDS-related cytomegalovirus retinitis and, together with future studies, may contribute to the development of novel therapeutic targets that could improve the treatment or management of this sight-threatening disease.
KEYWORDS: AIDS-related cytomegalovirus retinitis, suppressor of cytokine signaling (SOCS), murine cytomegalovirus (MCMV), retinitis, SOCS1, SOCS3, MAIDS
ABSTRACT
AIDS-related human cytomegalovirus retinitis remains the leading cause of blindness among untreated HIV/AIDS patients worldwide. To study mechanisms of this disease, we used a clinically relevant animal model of murine cytomegalovirus (MCMV) retinitis with retrovirus-induced murine AIDS (MAIDS) that mimics the progression of AIDS in humans. We found in this model that MCMV infection significantly stimulates ocular suppressor of cytokine signaling 1 (SOCS1) and SOCS3, host proteins which hinder immune-related signaling by cytokines, including antiviral type I and type II interferons. The present study demonstrates that in the absence of retinal disease, systemic MCMV infection of mice without MAIDS, but not in mice with MAIDS, leads to mild stimulation of splenic SOCS1 mRNA. In sharp contrast, when MCMV is directly inoculated into the eyes of retinitis-susceptible MAIDS mice, high levels of intraocular SOCS1 and SOCS3 mRNA and protein are produced which are associated with significant intraocular upregulation of gamma interferon (IFN-γ) and interleukin-6 (IL-6) mRNA expression. We also show that infiltrating macrophages, granulocytes, and resident retinal cells are sources of intraocular SOCS1 and SOCS3 protein production during development of MAIDS-related MCMV retinitis, and SOCS1 and SOCS3 mRNA transcripts are detected in retinal areas histologically characteristic of MCMV retinitis. Furthermore, SOCS1 and SOCS3 are found in both MCMV-infected cells and uninfected cells, suggesting that these SOCS proteins are stimulated via a bystander mechanism during MCMV retinitis. Taken together, our findings suggest a role for MCMV-related stimulation of SOCS1 and SOCS3 in the progression of retinal disease during ocular, but not systemic, MCMV infection.
IMPORTANCE Cytomegalovirus infection frequently causes blindness in untreated HIV/AIDS patients. This virus manipulates host cells to dysregulate immune functions and drive disease. Here, we use an animal model of this disease to demonstrate that cytomegalovirus infection within eyes during retinitis causes massive upregulation of immunosuppressive host proteins called SOCS. As viral overexpression of SOCS proteins exacerbates infection with other viruses, they may also enhance cytomegalovirus infection. Alternatively, the immunosuppressive effect of SOCS proteins may be protective against immunopathology during cytomegalovirus retinitis, and in such a case SOCS mimetics or overexpression treatment strategies might be used to combat this disease. The results of this work therefore provide crucial basic knowledge that contributes to our understanding of the mechanisms of AIDS-related cytomegalovirus retinitis and, together with future studies, may contribute to the development of novel therapeutic targets that could improve the treatment or management of this sight-threatening disease.
INTRODUCTION
Human cytomegalovirus (HCMV) is a betaherpesvirus that infects up to 80% of the adult population worldwide (1). Following primary infection that is usually asymptomatic (2), the virus establishes a lifelong latent infection in monocytes and bone marrow granulocyte-macrophage progenitors of the host (3) that is often without overt clinical consequences (2, 4). Upon immunosuppression during the progression of HIV/AIDS, however, a number of HCMV-associated pathologies may present themselves. Among these is AIDS-related HCMV retinitis, which remains the leading cause of vision loss and blindness among untreated HIV/AIDS patients worldwide (5–7). This slowly progressive, primarily immunopathologic, retinal disease of HCMV etiology initially resulted in vision loss and blindness in ∼30% of HIV/AIDS patients before the development of antiretroviral therapy (ART) (8, 9) that has significantly reduced the incidence of AIDS-related HCMV retinitis in the United States. Nonetheless, HCMV retinal disease remains a major ophthalmologic problem in HIV/AIDS patients worldwide who do not have access to ART or who fail to respond to ART (10–13).
While the clinical and histopathologic features of AIDS-related HCMV retinitis have been well characterized (14, 15), the precise virological, immunological, and pathological events that operate to allow onset and development of HCMV retinal disease during HIV-induced immunosuppression remain unclear. For this reason, for several years we have used a mouse model of experimental murine cytomegalovirus (MCMV) retinitis in mice with murine retrovirus-induced immunosuppression (murine AIDS, or MAIDS) to investigate the pathophysiology of AIDS-related HCMV retinitis (5, 16–18). Immunodeficiency in this model is accomplished by intraperitoneal injection of a mixture of retroviruses (LP-BM5) (19, 20) into 3-week-old C57BL/6 mice. The subsequent progression of MAIDS, with concomitant gradual decline of immune function and increasing susceptibility to opportunistic pathogens, follows a kinetically reproducible pattern that is measured in weeks after injection with the retrovirus mixture (21). MAIDS progresses through an early stage between the time of retrovirus injection at week 0 through week 3, a Th1/Th2 cytokine profile shift or transitional phase that takes place between week 3 and week 6, a mid-stage decline in cellular immunity that happens between week 6 and week 8, and a late-stage immunodeficiency that occurs between week 8 and week 12.
In our previous and current studies, we have observed various effects of subretinal MCMV injected during the transitional phase of MAIDS progression at 4 weeks (MAIDS-4) or injected during the late-stage phase at 10 weeks (MAIDS-10). Whereas MCMV-infected eyes of immunologically normal mice without MAIDS and MCMV-infected eyes of mice at MAIDS-4 are resistant to the development of necrotizing retinitis (22, 23), 80 to 100% of MCMV-infected eyes of mice at MAIDS-10 exhibit severe necrotizing retinitis (16, 22, 23) and other histopathologic features mimicking those observed in patients with AIDS-related HCMV retinitis. These include full-thickness retinal necrosis, hemorrhage, the appearance of cytomegalic cells and virus-specific inclusion bodies within various cells of the retinal tissue and transition zones between areas of relatively uninvolved retinal tissues and areas of severe retinal necrosis (16, 17). Importantly, MCMV delivered systemically by the intraperitoneal or intravenous route does not result in retinitis (24), regardless of the MAIDS status of the host (25). Therefore, achieving retinitis in this model requires both intraocular (subretinal) MCMV inoculation and the severe immunosuppression that occurs at MAIDS-10 but not MAIDS-4 (16, 23, 26). Our additional observation that MCMV-infected eyes of retinitis-resistant MAIDS-4 mice and MCMV-infected eyes of retinitis-sensitive MAIDS-10 mice contain equivalent and significantly large amounts of infectious virus within the ocular compartment (23, 26) suggest that virus replication alone does not determine the development or extent of MCMV retinitis, but other factors conspire to allow onset and development of MAIDS-related MCMV retinitis.
We have used the MAIDS model of MCMV retinitis as an experimental model for AIDS-related HCMV retinitis to investigate humoral immunity (27), cellular immunity (28, 29), cell death pathways (26), and a number of individual cytokines for their relative roles during retinal disease onset and development. Among the cytokines we have examined to date are tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), interleukin-2 (IL-2), IL-12, IL-4, IL-10, and IL-17 (22, 23, 30–33). The discovery by other investigators (34–37) of the suppressor of cytokine signaling (SOCS) family of host proteins, which negatively regulates signaling induced by antiviral IFNs and other cytokines, such as IL-6, prompted us to reassess our concepts of how cytokines mechanistically impact the pathogenesis of AIDS-related HCMV retinitis. SOCS proteins can be transcriptionally induced by many innate or adaptive immune factors, but the most prominent induction under normal physiologic conditions is through JAK/STAT pathways stimulated by cytokines such as type I IFNs (IFN-α and IFN-β), type II IFN (IFN-γ), and the IL-6 cytokine family (36, 38–43). These studies are focused on SOCS1 and SOCS3, two members of the SOCS family found by others to be stimulated by and to contribute to pathogenesis during infections with other viruses, such as HIV (44–47), hepatitis B virus (48), hepatitis C virus (49, 50), Semliki Forest virus (51), respiratory syncytial virus (52), herpes simplex virus 1 (53–55), varicella-zoster virus (56), and Kaposi's sarcoma-associated herpesvirus (57). In addition, SOCS1 and SOCS3 are stimulated in cell culture by HCMV (58) or MCMV (59), the latter depending on cell type and viral passage origin (60). Stimulation of host SOCS proteins is therefore a common survival strategy of many variant and distinct viruses, perhaps in a manner similar to the redundant, generalized means of cell entry by nonprofessional phagocytosis common to the diverse family of herpesviruses (61). The potential contributions of individual SOCS proteins toward the regulation of cytokines during onset and development of MAIDS-related MCMV retinitis are currently unknown. We therefore performed an in-depth in vivo investigation to test the hypothesis that MCMV stimulates SOCS1 and/or SOCS3 proteins within the ocular compartment during retrovirus-induced immunosuppression to induce immunologic events that favor onset and development of MCMV retinitis during MAIDS.
RESULTS
SOCS1, but not SOCS3, mRNA is moderately and transiently stimulated in whole splenic cells during acute, systemic MCMV infection in immunologically normal C57BL/6 mice without MAIDS.
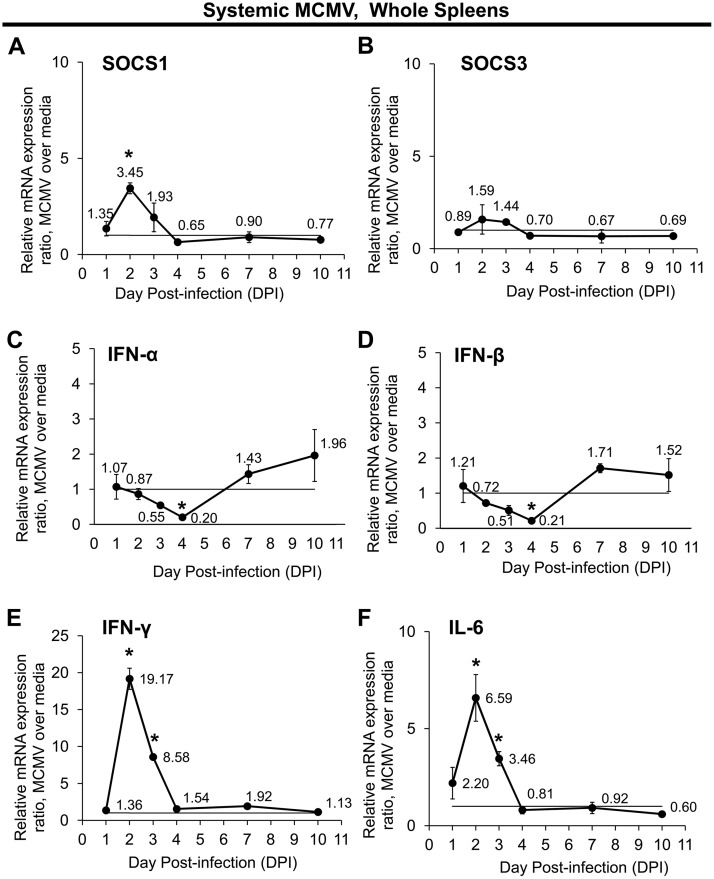
Intraperitoneal inoculation of mice with a nonlethal dose of MCMV results in a benign, self-limited, acute infection which disseminates systemically via the blood to many tissues and organs, including the spleen (62–64). Because SOCS1 and SOCS3 are most abundantly associated with cells of innate and adaptive immunity (43, 65) and because the spleen is a major site for these immune cells, we performed an initial study to determine the extent of SOCS1 and SOCS3 mRNA synthesis in whole splenic cells of immunologically normal C57BL/6 mice over time during acute, systemic MCMV infection. Splenic SOCS1 mRNA was significantly upregulated (P value of <0.05) 2 days after systemic MCMV inoculation but maintained control levels between 4 and 10 days after infection (Fig. 1A). Investigation of splenic SOCS3 mRNA expression revealed an apparent upregulation of splenic SOCS3 mRNA levels at 2 days after systemic MCMV inoculation, albeit at an average level that was not statistically significant (Fig. 1B).
FIG 1.
Systemic MCMV infection of immunologically normal mice without MAIDS differentially affects temporal mRNA expression of SOCS1, SOCS3, and SOCS-inducing cytokines in whole splenic cells. Groups of C57BL/6 mice without MAIDS were injected intraperitoneally with 104 PFU MCMV or an equal volume of medium (controls). Whole spleens collected at 1, 2, 3, 4, 7, and 10 days postinfection (dpi) were assessed for SOCS1 (A), SOCS3 (B), IFN-α (C), IFN-β (D), IFN-γ (E), and IL-6 (F) mRNA expression by quantitative RT-PCR assay. Mean mRNA expression ratios from MCMV samples are shown relative to their respective medium controls per day by the 2−ΔΔCT method. Values represent means from n = 8 mice per group from two independent experiments, and error bars indicate standard errors of the means (SEM). Statistical significance between groups per day (*, P < 0.05) was determined by Student's t test.
Signaling by type I or type II IFN as well as by IL-6 can induce SOCS mRNA expression (42, 43), and MCMV infection causes upregulation of IFN-γ (23, 66) and IL-6 (67). Because SOCS1 mRNA was moderately stimulated during acute, systemic MCMV infection of immunologically normal mice, we next sought to determine whether this could be explained by concurrent upregulation of representative cytokines previously shown to act as inducers of SOCS expression. On day 4 after MCMV infection, IFN-α (Fig. 1C) and IFN-β (Fig. 1D) mRNA expression levels were significantly reduced (0.2 expression ratio, or ∼5-fold decrease compared with the medium-only group; P < 0.05) in splenic cells from MCMV-infected mice compared with mRNA expression levels in splenic cells from control mice, although no significant differences in the type I IFNs (IFN-α and IFN-β) were observed at any other time points examined. This was not the case for splenic type II IFN (IFN-γ) (Fig. 1E) or IL-6 (Fig. 1F) mRNA expression levels that were found to be significantly stimulated (P < 0.05) between 2 and 3 days after MCMV infection, although they retained control levels between 4 and 10 days postinfection.
SOCS1, SOCS3, and type I IFN mRNA transcripts, but not type II IFN and IL-6 transcripts, are unaltered within whole splenic cells or whole eyes during acute, systemic MCMV infection of C57BL/6 mice with MAIDS.
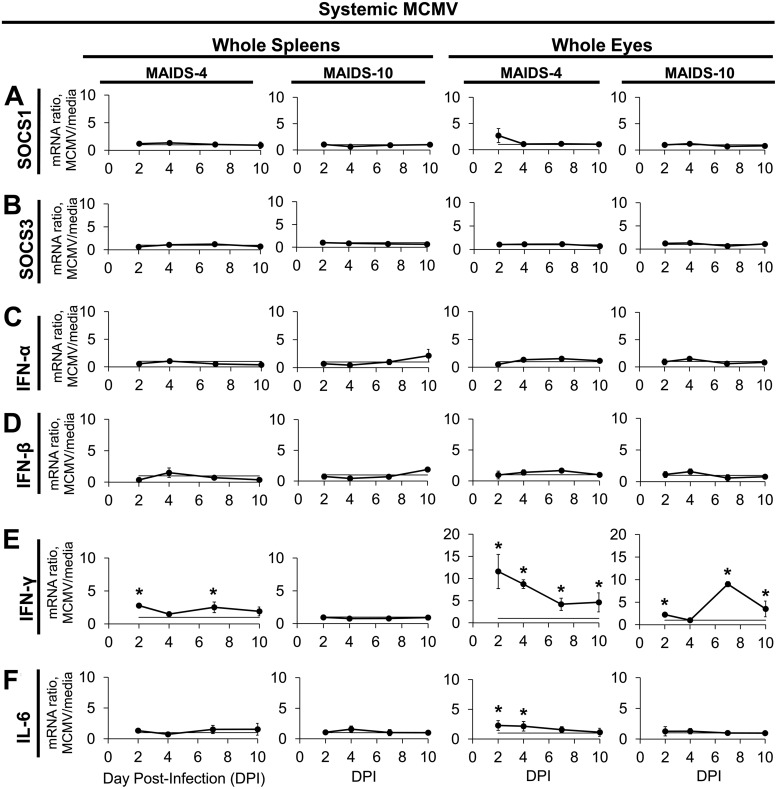
SOCS1 and SOCS3 are stimulated and modulate IFN immune responses during in vitro infection with HIV (44–47). Whereas subretinal MCMV injection into mice with MAIDS-10 reproducibly results in MCMV retinitis, intraperitoneal (systemic) MCMV injection into MAIDS mice leads to viral dissemination and ocular infection without retinitis (25). We therefore investigated whether acute, systemic MCMV infection of mice during retrovirus-induced immunosuppression with MAIDS significantly alters the mRNA expression levels of splenic and ocular SOCS1 or SOCS3 in the absence of overt retinal pathogenesis. Acute, systemic MCMV infection did not significantly alter SOCS1 or SOCS3 mRNA levels from whole spleens or whole eyes of either MAIDS-4 or MAIDS-10 animals (Fig. 2A and B). Although there was an upward trend for SOCS1 expression in the eyes of MAIDS-4 mice at day 2 following intraperitoneal MCMV infection, the standard deviation between individual mice was great enough to preclude statistical significance (P = 0.062) by two-tailed, unpaired Student's t test for this data point. Compared with unmanipulated age-matched healthy mice, in the absence of MCMV infection, neither MAIDS-4 nor MAIDS-10 mice exhibited significantly altered mRNA expression levels of SOCS1 in whole splenic cells (33). The difference in splenic SOCS1 expression at 2 days after systemic MCMV infection of immunologically normal mice without MAIDS (Fig. 1A) versus that in MAIDS mice (Fig. 2A) was therefore not attributable solely to MAIDS.
FIG 2.
Within the spleens and eyes of MAIDS-4 or MAIDS-10 mice, systemic MCMV infection stimulates IFN-γ and IL-6 mRNA expression but does not affect SOCS1, SOCS3, IFN-α, or IFN-β mRNA transcripts. Groups of C57BL/6 mice with MAIDS-4 or MAIDS-10 were injected intraperitoneally with 104 PFU MCMV or an equal volume of medium (controls). Whole spleens and whole eyes collected at 2, 4, 7, and 10 dpi were assessed for SOCS1 (A), SOCS3 (B), IFN-α (C), IFN-β (D), IFN-γ (E), and IL-6 (F) mRNA expression by real-time quantitative RT-PCR assay. Mean mRNA expression ratios (fold change) from MCMV samples are shown relative to their respective medium controls per day by the 2−ΔΔCT method. Values represent means ± standard deviations (SD) from n = 5 mice per group from two independent experiments. Statistical significance between groups per day (*, P < 0.05) was determined by Student's t test.
Splenic and ocular mRNA expression of type I IFN (IFN-α and IFN-β) was similarly unaffected following systemic MCMV infection of MAIDS-4 and MAIDS-10 mice at all times investigated (Fig. 2C and D). IFN-γ mRNA expression, however, was significantly stimulated in the eyes and spleens of MAIDS-4 mice with acute, systemic MCMV infection (Fig. 2E). Interestingly, IFN-γ mRNA was also significantly upregulated in the eyes, but not the spleens, of MAIDS-10 mice with systemic MCMV infection. By comparison, IL-6 mRNA was slightly but significantly elevated in the eyes, but not the spleens, of MAIDS-4 mice with systemic MCMV infection, but no change in IL-6 mRNA expression in eyes or spleens from MAIDS-10 mice was observed (Fig. 2F). Although the ocular mRNA expression ratios of SOCS1 and IL-6 during systemic MCMV infection of MAIDS-4 mice shared very similar means (∼2-fold increases over medium-injected MAIDS-4 mice), the IL-6 value achieved statistical significance (P < 0.005), whereas the SOCS1 mRNA value did not. This anomaly is mathematically attributable to the differences in the standard deviations of the groups.
SOCS1 and SOCS3 proteins are highly stimulated in MCMV-infected eyes of retinitis-susceptible MAIDS-10 mice.
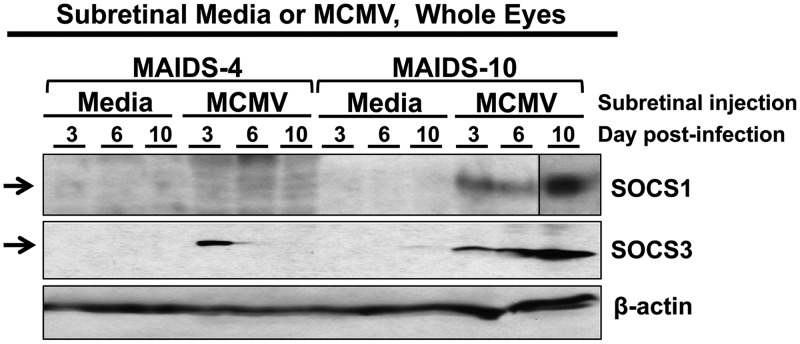
We reported previously in two separate studies that SOCS1 and SOCS3 mRNA levels are highly stimulated within MCMV-infected eyes of retinitis-susceptible MAIDS-10 mice compared with contralateral medium-injected control eyes but only slightly stimulated within MCMV-infected eyes of retinitis-resistant MAIDS-4 mice (33, 68). We therefore sought to test these eyes for the presence and distribution of SOCS1 and SOCS3 proteins by Western blot analysis or immunofluorescent staining.
SOCS3 protein was detected in the MCMV-infected eyes of MAIDS-4 mice that are resistant to retinitis, albeit only at day 3. Within the MCMV-infected eyes of MAIDS-10 retinitis-susceptible mice, however, both SOCS1 and SOCS3 proteins were present at all time points examined, with the highest levels at day 10 (Fig. 3), a time at which the greatest severity of retinitis has been observed in this model (23, 27). Neither SOCS1 nor SOCS3 protein was detected by Western blotting in medium-injected control eyes (Fig. 3).
FIG 3.
Ocular SOCS1 and SOCS3 proteins are stimulated in the MCMV-infected eyes of retinitis-susceptible mice with MAIDS-10. Groups of C57BL/6 mice with MAIDS-4 (retinitis resistant) or MAIDS-10 (retinitis susceptible) were injected subretinally with 104 PFU MCMV (left eyes) or an equal volume of medium (right eyes; controls). Whole eyes (n = 5 per group, pooled) were collected at 3, 6, and 10 days postinfection, and ocular protein was extracted and analyzed by Western blotting for SOCS1 and SOCS3 proteins. SOCS1 contains composite images from Western blots with identical parameters.
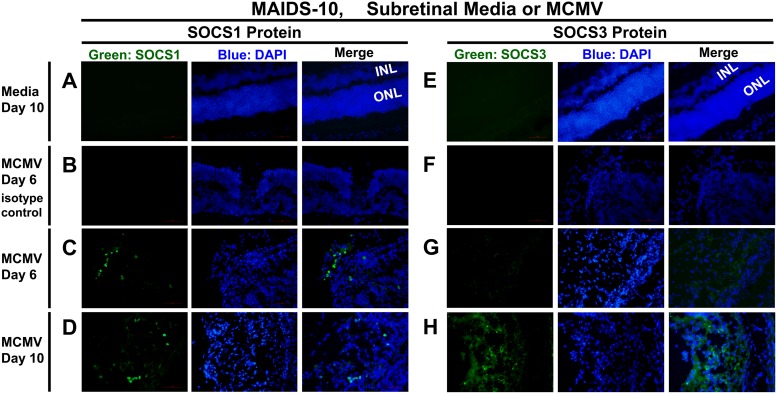
Of the many distinct and organized layers of cells and synapses stacked between the vitreous gel and the choroid of the eye, the inner nuclear layer (INL) and outer nuclear layer (ONL) are prominently revealed by 4′,6-diamidino-2-phenylindole (DAPI) staining of retinal nuclei. Cytomegalovirus retinitis disrupts the normal retinal architecture, often with complete destruction of the retinal layers (full-thickness retinal necrosis) and transition zones between areas of normal retina and areas of retinal necrosis. Immunofluorescent staining of sections of MCMV-infected MAIDS-10 eyes revealed detectable expression of SOCS1 protein (Fig. 4A to D) within individual cells of retinal tissues undergoing retinal necrosis at days 6 and 10 after subretinal MCMV infection. Similarly, SOCS3 protein (Fig. 4E to H) was found within individual retinal cells during retinitis at days 6 and 10, although expression at day 6 was less prominent than that observed for day 10 (Fig. 4G and H). As expected, no SOCS1 or SOCS3 protein was detected in immunostained, medium-injected control eyes of MAIDS-10 mice (medium day 10) (Fig. 4A and E) or in retinal tissue sections of MCMV-infected eyes of MAIDS-10 mice that were incubated with an IgG isotype control antibody rather than anti-SOCS1 or anti-SOCS3 primary antibody (MCMV day 6 isotype control) (Fig. 4B and F).
FIG 4.
Ocular SOCS1 and SOCS3 proteins are expressed in the retinas of MCMV-infected MAIDS-10 mice during MCMV retinitis. Whole eyes were collected from MAIDS-10 mice at days 6 and 10 after subretinal injection with 104 PFU MCMV (left eyes) (B to D and F to H) or an equal volume of medium (right eyes; control) (A and E). Formalin-fixed cryosections were analyzed by fluorescence microscopy for SOCS1 (A, C, and D) or SOCS3 (E, G, and H) proteins (green) or IgG isotype control antibody (B and F). Nuclei were counterstained with DAPI (blue). The inner nuclear layer (INL) and outer nuclear layer (ONL) of the retina are indicated for medium controls, where, in the absence of MCMV retinitis, distinct retinal layers are still intact. Original magnification, ×400.
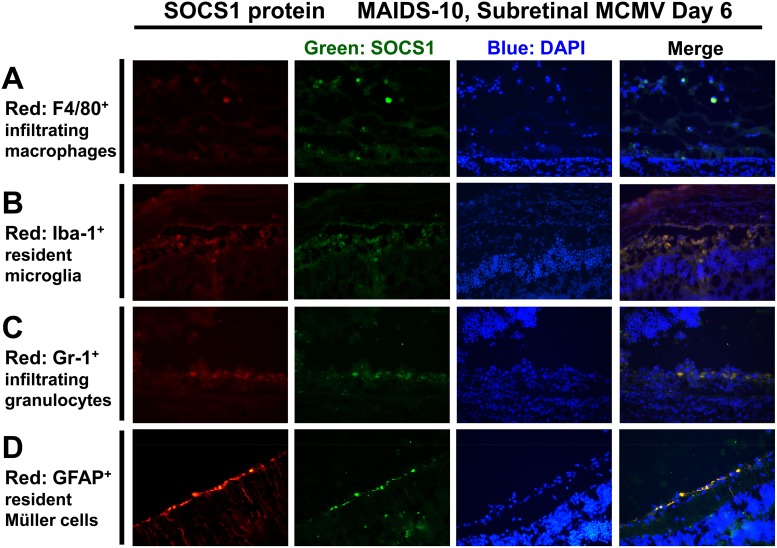
SOCS1 and SOCS3 proteins are stimulated in retinal tissue by resident retinal cells and infiltrating immune cells during MAIDS-related MCMV retinitis.
SOCS family proteins are expressed in many different organs (36) and cell types, but they are most abundantly associated with cells of hematopoietic origin (69) of the innate and adaptive immune systems (43, 65). Some of these SOCS-expressing cell types include monocytes (70), macrophages (35, 71), microglia (72), neutrophils (73), and Müller cells (74). Because SOCS family proteins typically execute their inhibitory functions within the cells that produce them and their multifaceted suppressive functions therefore are frequently cell type specific (43, 65, 75, 76), we sought to investigate whether specific types of infiltrating or resident retinal cells are the chief producers of SOCS1 or SOCS3 protein within the MCMV-infected eyes of MAIDS-10 mice during progression of retinitis. Compared with medium-injected control eyes, MCMV-infected eyes of MAIDS-10 mice that displayed retinitis on day 6 after subretinal MCMV infection exhibited expression of SOCS1 (Fig. 5) that colocalized with GFAP+ resident activated Müller cells, Iba-1+ microglial cells, F4/80+ infiltrating macrophages, and Gr-1+ (Ly-6G) infiltrating granulocytes (neutrophils). F4/80+ infiltrating macrophages (Fig. 5A) and Iba-1+ resident microglial cells (Fig. 5B) expressed only moderate amounts of SOCS1 protein, with more abundant SOCS1 protein expression colocalizing with Gr-1+-infiltrating granulocytes (Fig. 5C) and GFAP+ resident Müller cells (Fig. 5D).
FIG 5.
During MAIDS-related MCMV retinitis, SOCS1 protein is detectable in resident cells and infiltrating immune cells of the retinal tissue. Whole eyes were collected from MAIDS-10 mice 6 days after subretinal injection with 104 PFU MCMV. Formalin-fixed cryosections were immunofluorescently double stained as described in Materials and Methods for detection of SOCS1 protein (green) plus one of the following cell markers (red): F4/80 for infiltrating macrophages (A), Iba-1 for microglia (B), Gr-1 (Ly-6G) for granulocytes (neutrophils) (C), or GFAP for activated Müller cells (D). Nuclei were counterstained with DAPI (blue). Original magnification, ×400.
Retinal SOCS3 protein expression mirrored that found for SOCS1, with sparse SOCS3 protein colocalization found in F4/80+ infiltrating macrophages (Fig. 6A), moderate SOCS3 expression in Iba-1+ microglia (Fig. 6B), and prominent SOCS3 expression in Gr-1+ (Ly-6G) infiltrating granulocytes (Fig. 6C) and GFAP+-activated Müller cells (Fig. 6D). SOCS1 and SOCS3 proteins are therefore produced by infiltrating immune cells as well as resident cells of the retinal tissue, with infiltrating neutrophils and resident Müller cells being prominent cellular sources of these proteins during MAIDS-related MCMV retinitis.
FIG 6.
During MAIDS-related MCMV retinitis, SOCS3 protein is present in resident cells and infiltrating immune cells of the retina. As described for Fig. 5, whole eyes were collected from MAIDS-10 mice on day 6 following subretinal injection with 104 PFU MCMV. Formalin-fixed cryosections were immunofluorescently double stained for detection of SOCS3 protein (green) plus cell markers F4/80 (A), Iba-1 (B), Gr-1 (Ly-6G) (C), or GFAP (D) in the red channel. Nuclei were counterstained with DAPI (blue). Original magnification, ×400.
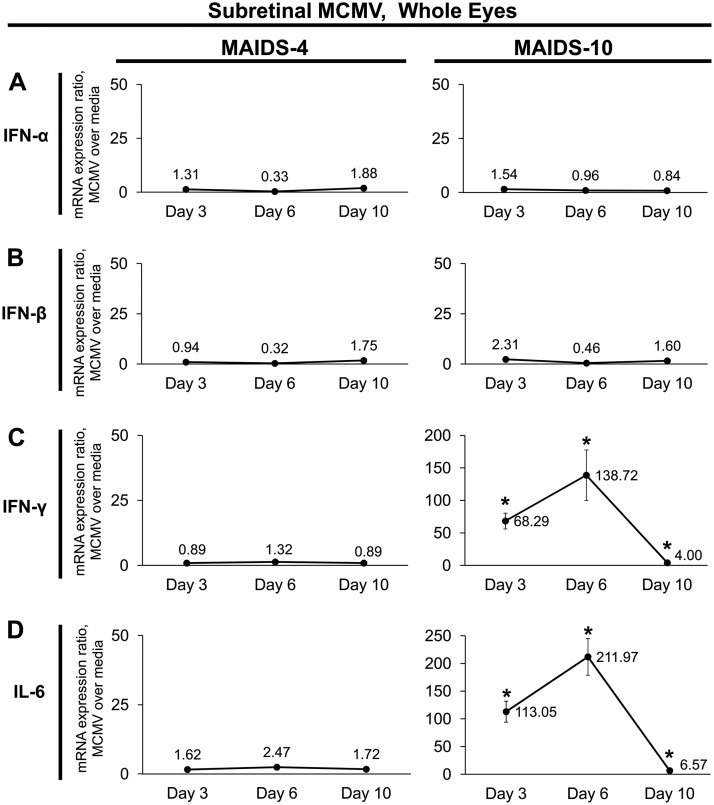
Transcripts of SOCS-inducing cytokines IFN-γ and IL-6, but not IFN-α or IFN-β, are concurrently upregulated with SOCS1 and SOCS3 in whole eyes during MAIDS-related MCMV retinitis.
Subretinal MCMV infection of MAIDS-10 mice results in stimulation of SOCS1 and SOCS3 mRNA transcripts (33, 68) and proteins (Fig. 3 and 4), but it is unclear whether this stimulation is the result of direct virus infection, an indirect immunologic consequence of virus infection, or a combination of both. We therefore probed MCMV-infected eyes of MAIDS-4 and MAIDS-10 mice for mRNA expression of known cytokine inducers of SOCS1 and SOCS3, which may facilitate an indirect immune mechanism. Neither IFN-α nor IFN-β mRNA expression was altered within the MCMV-infected eyes of MAIDS-4 or MAIDS-10 mice at any time sampled postinfection (Fig. 7A and B). Similarly, subretinal MCMV infection of MAIDS-4 mice did not affect IFN-γ or IL-6 mRNA expression levels (Fig. 7C and D, left). MCMV-infected eyes of MAIDS-10 mice, however, showed significant (P < 0.05) stimulation of IFN-γ and IL-6 mRNA transcripts, with expression levels peaking on day 6 following intraocular MCMV infection (Fig. 7C and D, right).
FIG 7.
SOCS-inducing cytokines IFN-γ and IL-6, but not IFN-α or IFN-β, are transcriptionally upregulated concurrently with SOCS1 and SOCS3 during MAIDS-related MCMV retinitis. Groups of C57BL/6 mice with MAIDS-4 (retinitis resistant) or MAIDS-10 (retinitis susceptible) were injected subretinally with 104 PFU MCMV (left eyes) or an equal volume of medium (right eyes, controls). Whole eyes were collected at 3, 6, and 10 days postinfection and analyzed for IFN-α (A), IFN-β (B), IFN-γ (C), and IL-6 (D) mRNA expression by real-time quantitative RT-PCR assay. Mean mRNA expression ratios (fold change) from MCMV-infected eyes are shown relative to their medium-injected control eyes by the 2−ΔΔCT method. Values represent means ± SD from n = 5 mice per group. (Note that the y axis scaling differs between gene targets.) Statistical significance between treatment with MCMV and subretinal medium per day (*, P < 0.05) was determined by Student's t test.
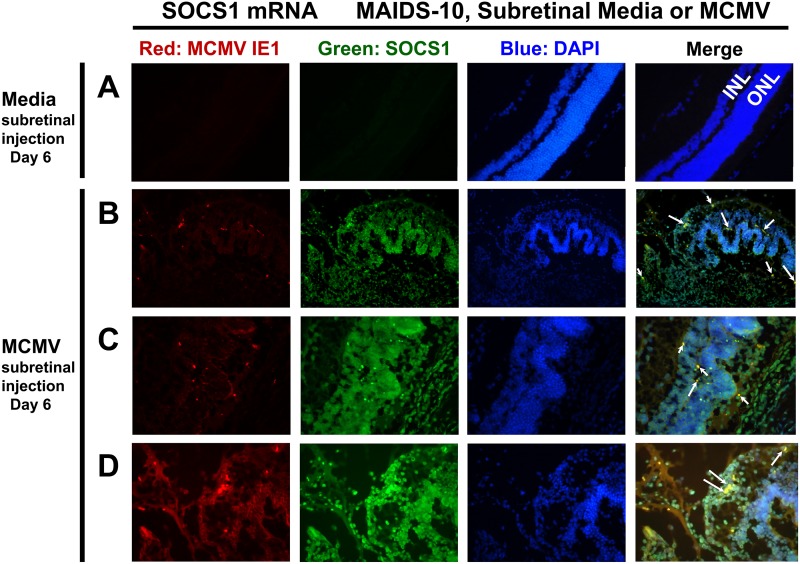
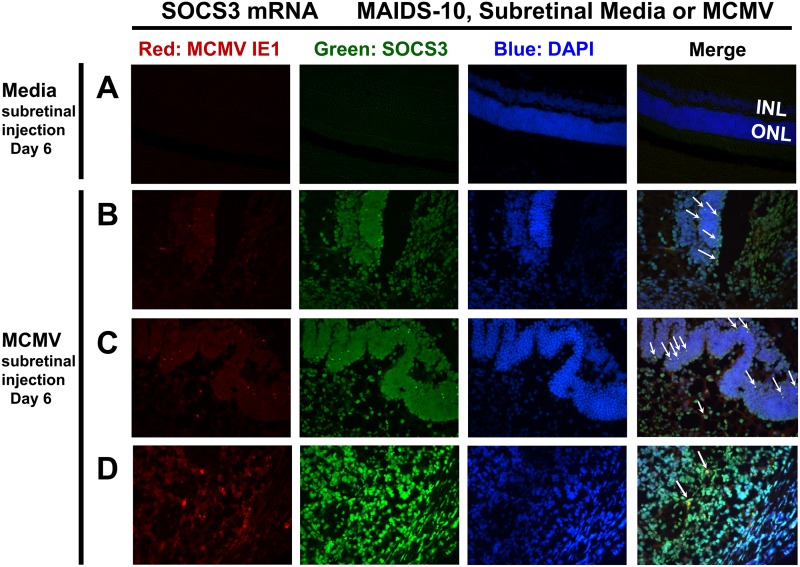
SOCS1 and SOCS3 mRNA transcripts are detected by fluorescent in situ hybridization (FISH) within retinal cells producing MCMV IE1 mRNA and in uninfected retinal cells.
SOCS1 and SOCS3 are transcriptionally stimulated by cell signaling cascades initiated by IFNs and IL-6 (42, 43), and we found that IFN-γ and IL-6 mRNA transcripts are highly stimulated within the eyes of MAIDS-10 mice subretinally injected with MCMV (Fig. 7), consistent with the possibility that SOCS1 and SOCS3 stimulation could be the consequence of an immune response in uninfected bystander cells. We therefore tested whether direct viral infection in retinal cells is necessary for stimulation of SOCS1 or SOCS3 during MAIDS-related MCMV retinitis or whether uninfected bystander cells also produce SOCS1 or SOCS3 in this model. To this end, we designed RNA probes to detect SOCS1, SOCS3, or MCMV-specific immediate-early 1 (IE1) mRNA transcripts and used these RNA probes to perform FISH analysis of eye sections from MAIDS-10 mice subretinally injected with either MCMV or media (control).
As expected, medium-injected control eyes from MAIDS-10 mice retained histologically normal retinal strata, produced only baseline amounts of SOCS1 and SOCS3 mRNA transcripts, and had no detectable MCMV IE1 mRNA (Fig. 8A and 9A). Within the MCMV-infected eyes of these MAIDS-10 mice, however, ample expression of SOCS1 (Fig. 8B to D) and SOCS3 (Fig. 9B to D) mRNA transcripts spanned pathologically disrupted layers of the retinal architecture. Furthermore, SOCS1, SOCS3, and MCMV IE1 mRNA expression was detected within several distinct areas that are histopathologically characteristic of MCMV retinitis: transition zones (Fig. 8B and 9B), areas of retinal folding (Fig. 8C and 9C), and areas of retinal necrosis (Fig. 8D and 9D). Whereas SOCS1 mRNA was detected in cells negative for MCMV IE1 mRNA, almost all cells positive for MCMV IE1 mRNA showed colocalization with SOCS1 mRNA (Fig. 8, arrows). Similarly, SOCS3 mRNA staining appeared in many uninfected cells of the neurosensory retina, but nearly all cells positive for MCMV IE1 mRNA showed double staining with SOCS3 mRNA (Fig. 9, arrows). Therefore, SOCS1 and SOCS3 mRNA transcripts are produced not only by cells directly infected with MCMV but also by uninfected bystander cells during MAIDS-related MCMV retinitis.
FIG 8.
SOCS1 mRNA transcripts are detectable during MAIDS-related MCMV retinitis not only in retinal cells that are directly infected with MCMV but also in uninfected bystander retinal cells. Eyes from MAIDS-10 mice were collected 6 days following subretinal injection with medium (control) (A) or 104 PFU MCMV (B to D). Cryosections were double stained by fluorescent in situ hybridization (FISH) per Materials and Methods for mRNA transcripts of SOCS1 (green) and MCMV IE1 (red). Nuclei were counterstained with DAPI (blue). The inner nuclear layer (INL) and outer nuclear layer (ONL) of the retina are indicated for medium controls. Same-cell colocalization of SOCS1 and MCMV IE1 mRNA expression is indicated by white arrows. Ocular sections containing histopathologically characteristic areas of MCMV retinitis are shown: transition zones between retinal folding and retinal necrosis (B), retinal folding (C), and retinal necrosis (D). Original magnification, 200× (A and B) or 400× (C and D).
FIG 9.
SOCS3 mRNA transcripts are expressed during MAIDS-related MCMV retinitis in retinal cells directly infected with MCMV as well as in uninfected bystander retinal cells. As described for Fig. 8, eyes from MAIDS-10 mice were collected 6 days following subretinal injection with medium (control) (A) or 104 PFU MCMV (B to D). Cryosections were double stained by FISH for mRNA transcripts of SOCS3 (green) and MCMV IE1 (red). Nuclei were counterstained with DAPI (blue). The inner nuclear layer (INL) and outer nuclear layer (ONL) of the retina are indicated for medium controls. Same-cell colocalization of SOCS3 and MCMV IE1 mRNA transcripts is indicated by white arrows. Sections containing histopathologically characteristic areas of MCMV retinitis are shown: transition zones between retinal folding and retinal necrosis (B), retinal folding (C), and retinal necrosis (D). Original magnification, 200× (A) or 400× (B to D).
DISCUSSION
SOCS proteins function to help maintain a balance between inflammation and homeostasis in the host through control of immune cell differentiation and cytokine signaling. These intracellular proteins unfortunately also can become targets for pathogens, including several herpesviruses, which hijack SOCS proteins to cause enhanced virus replication and dissemination (53–57). Here, we provide evidence that substantial, prolonged SOCS1 and SOCS3 stimulation during MCMV infection is unique to the eye and is correlated with MCMV retinitis. We also show that infiltrating macrophages, infiltrating granulocytes, resident microglial cells, and resident activated Müller cells are all sources of intraocular SOCS1 and SOCS3 protein production during development of MAIDS-related MCMV retinitis. Furthermore, uninfected bystander cells of the retina are stimulated to produce these SOCS proteins during MCMV retinitis.
SOCS1 and SOCS3 expression is highly stimulated during the pathogenesis of MAIDS-related MCMV retinitis but only moderately during systemic MCMV infection or intraocular MCMV infection without overt retinal disease.
We examined whether systemic MCMV infection without concomitant retinal disease could affect splenic SOCS1 or SOCS3 expression and discovered in immunologically normal mice a slight but significant simulation of SOCS1 mRNA that was not observed in whole spleens or whole eyes following systemic MCMV infection during the progression of MAIDS. This suggests that the phenomenon responsible for moderate, transient stimulation of splenic SOCS1 during acute, systemic MCMV infection in immunologically normal mice is either abrogated or masked by the effects of retrovirus-induced immunosuppression as early as 4 weeks after retrovirus infection. Because the progression of MAIDS is associated with polyclonal B-cell activation (77) and splenomegaly due to macrophage and B cell infiltration (21), one possibility is that the splenic cell type(s) responsible for moderate SOCS1 expression during systemic MCMV infection in immunologically normal mice is/are simply outpopulated in spleens of MAIDS mice.
Our data further show that the route of MCMV infection (systemic versus subretinal) during the progression of MAIDS, along with the presence or absence of MCMV-related retinitis, dictates whether MCMV highly upregulates SOCS1 and SOCS3 expression in the eye. Intriguingly, ocular viral load is not the only factor in MCMV-related SOCS expression in the eye, because whereas systemic MCMV during MAIDS travels to the eye, where it is detectable in small amounts (∼265 PFU/eye) (23, 25), subretinally inoculated MCMV achieves equivalently high ocular titers (∼32,000 PFU/eye) in both retinitis-resistant MAIDS-4 eyes and retinitis-susceptible MAIDS-10 eyes (23, 26). Taken together, these observations suggest that MCMV induces and exploits SOCS protein expression in a fashion similar to those of other viruses (53–55) but perhaps only under specific conditions related to ocular disease.
Infiltrating immune cells and resident retinal cells produce SOCS1 and SOCS3 protein during MAIDS-related MCMV retinitis.
Immune-mediated pathogenesis of MCMV retinitis in mice with MAIDS includes infiltration of macrophages and neutrophils (26). We demonstrate here that infiltrating macrophages and granulocytes (neutrophils), as well as resident activated Müller cells and resident microglial cells, are cellular sources of intraocular SOCS1 and SOCS3 protein production in the eyes of MAIDS-10 mice with retinitis. These cell types are susceptible to direct MCMV infection and display complex roles in dissemination, latency, and/or immune control of the virus (78–81). As with other viruses (reviewed in reference 82), an abundance of SOCS1 and SOCS3 within these cells could render them less responsive to signaling by antiviral cytokines, thus putatively resulting in an environment more favorable for MCMV.
Macrophages are involved in this and other ocular diseases (83–85), in part due to their cytokine production repertoire following activation toward proinflammatory (M1) versus proangiogenic (M2) phenotypes (86, 87), and SOCS proteins affect this (88, 89). Indeed, we recently demonstrated that within the MCMV-infected eyes of mice with corticosteroid-induced immune suppression, which causes depletion of macrophages, the severity of retinitis as well as the stimulation of SOCS1 and SOCS3 are greatly decreased compared with those of MCMV-infected eyes of MAIDS mice, which retain macrophage populations (68). Taken together with the present study, these data suggest that SOCS1 and SOCS3 play multifaceted roles within macrophages or other SOCS-producing cells during MAIDS-related MCMV retinitis.
Although SOCS family proteins presumably function within the cells that produce them, the recent finding that alveolar macrophages are capable of transcellular movement of SOCS proteins via vesicular transport to epithelial cells (90) could have interesting implications for SOCS proteins during MCMV retinitis if this phenomenon occurs in the ocular compartment. It also remains possible that SOCS1 and/or SOCS3 expression in these or other cell types of the retina are protective against inflammation that occurs during MCMV retinitis, as has been described during experimental autoimmune uveitis (91–93). Whether SOCS1 and/or SOCS3 proteins themselves contribute to the pathogenesis of MCMV retinitis or are simply by-products of inflammation during immunopathogenesis remains unclear, but the fact remains that they so far have been detected in high, sustained amounts in retinal cells only when MCMV causes overt ocular disease.
Candidate cytokines are differentially expressed and may act as inducers of SOCS proteins during MAIDS-related MCMV retinitis.
SOCS proteins are typically induced through the JAK/STAT pathway by cytokine stimulation and establish a negative feedback loop in order to restrict excessive inflammation. Cytokines such as IFNs and IL-6 signal through specific JAKs and STATs in this pathway (36, 43, 94–97), and we have confirmed that these JAKs and STATs are functional during intraocular stimulation of SOCS1 and SOCS3 in the present study of MAIDS-related MCMV (data not shown). Many viruses are capable of directly and/or indirectly inducing SOCS proteins, exploiting their function to enhance virus replication by inhibiting the antiviral functions of the JAK/STAT pathway (82). MCMV infection of immunologically normal mice results in the induction of IL-6 as well as type I and type II IFNs (98–106). Our findings indicate that both IFN-γ and IL-6 are candidate inducers of SOCS expression during acute MCMV infection of healthy mice and during subretinal MCMV infection of retinitis-susceptible MAIDS-10 mice.
Although systemic MCMV infection initiates a very early type I IFN response (99, 100, 102, 106), the virus uses several mechanisms to impede this antiviral strategy. The significant downregulation of splenic IFN-α and IFN-β mRNA expression at day 4 after systemic MCMV infection is consistent with findings in other animal models (107) and in vitro in mouse fibroblast and macrophage cell lines (108). Because SOCS1 has demonstrated an ability to interfere with signaling pathways that transcriptionally regulate type I IFN (88, 109), it is not unreasonable to hypothesize that the modest induction of SOCS1 is sufficient to contribute to the downregulation of type I IFN during systemic MCMV infection in immunologically normal mice without MAIDS. Although MCMV also employs other virologic mechanisms that downregulate type I IFN in transcriptional or functional capacities (110), SOCS1 and/or SOCS3 may contribute to the arsenal that MCMV utilizes to counteract these antiviral cytokines. That the progression of MAIDS abrogated the ability of systemic MCMV to downregulate splenic type I IFN suggests that the mechanism requires fully functional immune cells, particularly lymphocytes, which are the first to become progressively aberrant during MAIDS.
During MAIDS-related MCMV retinitis (MAIDS-10, subretinal MCMV), concurrent stimulation of ocular IFN-γ and IL-6 with SOCS1 and SOCS3 suggests that these cytokines contribute to SOCS production in this model. Furthermore, previous studies by us have shown that other intraocular candidate cytokine inducers of SOCS1 and/or SOCS3 mirror the mRNA expression patterns found here for IFN-γ and IL-6, including IL-4 (22), TNF-α (26), and IL-10 (33). With the exception of IL-10 mRNA, which is moderately but significantly stimulated by subretinal MCMV infection in MAIDS-4 animals (33), none of these cytokines shows significant transcriptional stimulation during MAIDS-4 when SOCS1 and SOCS3 are mildly, transiently stimulated. During intraocular MCMV infection of MAIDS-10 mice, however, all of these cytokines are highly upregulated concurrently with strong, sustained SOCS1 and SOCS3 stimulation. Therefore, although they are not likely responsible for mild SOCS1 or SOCS3 stimulation during MAIDS-4 when MCMV-infected eyes are resistant to retinitis, one or more of these cytokines may contribute to the high levels of SOCS1 and SOCS3 stimulation occurring at MAIDS-10 during the development of MCMV retinitis. The possibility remains, however, that these cytokines and SOCS undergo simultaneous transcriptional induction by the same, as-yet undetermined, stimulant. Taken together, these data suggest that subretinal MCMV employs different virologic or immunologic induction mechanisms for mild intraocular SOCS1 and SOCS3 stimulation at MAIDS-4 without retinitis versus high intraocular SOCS1 and SOCS3 simulation during MAIDS-10 with retinitis.
It is interesting that such strong induction of several cytokines with various functions occurs without stimulation of type I IFN during MAIDS-related MCMV retinitis (MAIDS-10, subretinal MCMV). MCMV infection normally initiates a very early type I IFN response (99, 100, 102, 106), produced primarily from dendritic cells and monocytes/macrophages as a result of innate signaling pathways triggered by various pattern recognition receptors (PRR) which bind to viral components (111, 112). Among its other antiviral functions, this early type I IFN stimulation, with IL-12 and IL-15, drives type II IFN (IFN-γ) production from natural killer (NK) cells, a crucial step in controlling acute MCMV infection in otherwise healthy mice (106, 113). Like type I IFN, IL-6 can also be upregulated by PRR signaling as well as by other pathways, including IL-1 and TNF-α (114), and its induction and effects are diverse and cell type specific (115). Despite the lack of stimulation found for type I IFN in the eye during MAIDS-related MCMV retinitis, however, the virus retains its ability to stimulate IFN-γ and IL-6, as well as IL-1 (26) and TNF-α (23). This suggests that during this disease, MCMV either orchestrates very specific activation or inhibition of precise components of the shared innate pathway that induces type I IFN, IL-1, TNF-α, and IL-6, or it stimulates alternative, redundant pathways, perhaps in different cell types. It is possible, however, that type I IFNs are induced at earlier time points not investigated here. Nevertheless, it is clear that during MAIDS-related MCMV retinitis, ample production of proinflammatory cytokines (e.g., IFN-γ and TNF-α) remains insufficient to control viral pathogenesis, while upregulation of prototypically anti-inflammatory cytokines (e.g., IL-10 and IL-4) fails to protect the retina from immune pathogenesis. Because of their divergent, multivariate, and cell type-specific inhibitory functions, SOCS1 and SOCS3 are attractive putative candidates for this phenomenon.
During MAIDS-related MCMV retinitis, SOCS1 and SOCS3 are stimulated in uninfected bystander cells of the retina by a mechanism that does not require target cells to be directly infected with MCMV.
Evidence suggests that MCMV infection can manipulate cellular protein expression in uninfected cells via a bystander mechanism, as we have shown previously for production of vascular endothelial growth factor (VEGF) protein (83) as well as SOCS1 and SOCS3 proteins (60) during in vitro MCMV infection of IC-21 mouse macrophages. Upregulation of the SOCS-inducing cytokines IFN-γ and IL-6 is consistent with an immune-driven mechanism of SOCS stimulation in target cells. Indeed, we found that direct MCMV infection is not required for retinal cells to produce SOCS1 and SOCS3 during MCMV retinitis, although nearly all MCMV-infected cells express SOCS1 and SOCS3. Taken together, these findings suggest that the mechanism driving SOCS1 and SOCS3 production in retinal cells during MAIDS-related MCMV retinitis is chiefly due to a bystander effect, where MCMV-infected cells modulate their surrounding uninfected bystander cells by shifting their microenvironment, possibly through stimulation and secretion of candidate SOCS-inducing cytokines.
Conclusions.
Our findings suggest a role for MCMV-related stimulation of SOCS1 and SOCS3 in the progression of retinal disease during ocular, but not systemic, MCMV infection. Whether MCMV takes advantage of SOCS as a strategy to evade immune surveillance or protect host cells remains to be seen, but it is clear from the present investigation that intraocular MCMV infection of mice with MAIDS results in massive stimulation of host-encoded SOCS1 and SOCS3 mRNA and protein that (i) appears to be unique to ocular rather than systemic infection, (ii) requires a progression of retrovirus-induced immune suppression that renders the eye susceptible to MCMV retinitis in MAIDS-10 mice, (iii) involves several types of infiltrating immune cells and resident retinal cells, and (iv) employs a mechanism involving indirect viral manipulation of uninfected bystander cells. This investigation therefore establishes the crucial framework for future studies to elucidate with greater precision the role of SOCS proteins on the progression of retinal disease during MAIDS-related MCMV retinitis. As complete knockdown of either SOCS1 or SOCS3 protein is lethal in mice (116–119), studies with SOCS1 or SOCS3 conditional knockout mice may prove useful in uncovering the detailed mechanism(s) by which MCMV engages SOCS proteins during infection.
MATERIALS AND METHODS
Animals.
Adult female BALB/c mice (8 to 12 weeks old) used for MCMV propagation were purchased from Taconic Farms (Germantown, NY, USA). Wild-type female C57BL/6 mice were used for all in vivo experiments and were purchased from Charles River Laboratories (Raleigh, NC, USA). Mice used in MAIDS studies were 3 weeks old upon MAIDS induction, and 6- to 10-week-old mice were used for studies in immunologically normal mice (without MAIDS). All animal procedures were conducted in compliance with Georgia State University Institutional Animal Care and Use Committee (IACUC) protocols.
Virus and cell lines.
Stocks of MCMV (Smith strain; ATCC, Manassas, VA, USA) prepared from salivary gland homogenates of BALB/c mice (Taconic Farms, Germantown, NY) as previously described (16) were aliquoted and stored in liquid nitrogen. Fresh aliquots were thawed and used for each experiment. Virus titers were determined by plaque assay in monolayers of murine embryonic fibroblast (MEF) cells (SCRC-1008; ATCC, Manassas, VA) without centrifugation enhancement. The SC-1 mouse fibroblast cell line infected with the murine retrovirus cocktail LP-BM5 (SC-1/MuLV LP-BM5) (20) was kindly provided by the AIDS Research and Reference Reagent Program, Division of AIDS, NAIAD, NIH, Germantown, MD. LP-BM5 stocks used to induce MAIDS were propagated as previously described (16), with modifications. In brief, after SC-1/MuLV LP-BM5 cells were seeded in a 1:1 ratio with uninfected SC-1 cells (CRL-1404; ATCC, Manassas, VA) and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) for 6 days, cells were scraped into their own medium, pooled, aliquoted, and frozen at −80°C. Fresh aliquots thawed for each experiment were clarified by centrifugation to remove cell debris before injection.
Induction of MAIDS.
MAIDS was induced in 3-week-old female C57BL/6 mice by intraperitoneal injection of 1.0 ml of MuLV LP-BM5 stock preparation (20, 120). Over the course of several weeks, this retrovirus mixture progresses into kinetically reproducible stages of MAIDS (21). After 4 weeks (MAIDS-4), mice are 7 weeks old, undergo a cytokine profile shift, and show partial immune dysfunction but remain resistant to MCMV retinitis. After 10 weeks (MAIDS-10), mice are 13 weeks old and show complete immune dysfunction, including susceptibility to MCMV retinitis, as previously described by us (16, 32, 33).
Systemic MCMV infection.
Groups of adult C57BL/6 mice at MAIDS-4 or MAIDS-10 or age-matched immunologically normal mice without MAIDS were systemically infected by intraperitoneal injection with either 104 PFU of MCMV (Smith strain) or an equal volume of DMEM. At the indicated days following infection, groups of medium-injected or MCMV-infected mice were euthanized, and whole spleens and/or whole eyes were collected and stored appropriately or processed immediately. Tissues from MCMV-infected mice were compared between subjects with their respective medium-injected control mice from the same day postinfection.
Subretinal MCMV inoculation.
For subretinal injections, MAIDS-4 or MAIDS-10 mice were anesthetized by intramuscular injection of 0.1 ml xylazine (1.72 mg/ml) and 0.1 ml acepromazine (0.28 mg/ml), followed by intraperitoneal injection of 0.1 to 0.2 ml ketamine (8.58 mg/ml). Following dilation with atropine and tropicamide ophthalmic drops, approximately 104 PFU of MCMV contained within 2 μl was injected subretinally into the left eyes of all mice. The right (contralateral) eyes were injected subretinally with the same volume of DMEM. Cohorts were euthanized and both eyes harvested at 3, 6, and 10 days following subretinal injection. Because subretinally injected MCMV does not travel to the contralateral eye within this time (16, 121), the right eyes served as baseline expression level controls.
Quantitative real-time RT-PCR assay.
Whole MCMV-infected or medium-injected eyes or spleens were stored in RNAlater solution (Ambion, Austin, TX) per the manufacturer's instructions. For RNA extraction and cDNA synthesis, tissues were homogenized with 1 ml TRIzol Reagent (Invitrogen, Carlsbad, CA) in a 2-ml Tenbroeck tissue grinder (Wheaton, Millville, NJ), and total RNA was extracted using the PureLink RNA minikit (Invitrogen) per the manufacturer's instructions. RNA concentrations for each sample were determined with the SmartSpec 3000 spectrometer (Bio-Rad Laboratories, Hercules, CA), and cDNA synthesis was performed using a SuperScript III first-strand synthesis system (Invitrogen) per the manufacturer's instructions. Detection and quantification of gene expression for each gene of interest was performed with specific primers and Power SYBR green PCR MasterMix reagent (Applied Biosystems, Foster City, CA, USA), and transcription levels and average cycles to threshold (CT) were detected using an ABI Prism 7500 real-time PCR instrument and sequence detection system software (Applied Biosystems). Primer sets of SOCS1, SOCS3, IFN-α, IFN-β, IFN-γ, IL-6, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for detection and quantification of transcript were purchased from Qiagen (Valencia, CA). The parameters for quantitative real-time reverse transcription-PCR (RT-PCR) assay cycles were 10 min at 95°C, followed by 35 cycles of 15 s at 94°C, 31 s at 55°C, and 35 s at 70°C. Data analysis was performed with ABI Prism 7500 real-time PCR software using the comparative 2−ΔΔCT method (relative mRNA expression ratio, or fold change), with GAPDH acting as the endogenous housekeeping gene (ΔCT) for each sample. Medium-injected eyes were used as control comparison groups for subretinal injection studies, and spleens or eyes from medium-injected mice were the control comparison groups for systemic injection experiments.
Western blot analysis.
Whole eyes and spleens collected from all animal groups were stored separately in liquid nitrogen. Individual samples were thawed and homogenized in 1 ml of phosphate-buffered saline (PBS) containing a protease inhibitor cocktail (Sigma, St. Louis, MO). Western blot analysis was performed following SDS-PAGE and transfer of protein samples to nitrocellulose membranes (Bio-Rad, Hercules, CA) for detection of SOCS1 and SOCS3 proteins using rabbit anti-mouse SOCS1 antibody (1:1,000; Cell Signaling Technology, Danvers, MA) or rabbit anti-mouse SOCS3 primary antibody (1:1,500; Cell Signaling Technology) with ImmunoPure goat anti-rabbit IgG secondary antibody (heavy plus light chains [H+L]; Thermo Scientific, Pittsburgh, PA) and enhanced chemiluminescence (ECL) Western blot detection reagents (GE Healthcare, Piscataway, NJ) for exposure with BioMax light film (Kodak, Rochester, NY).
Immunofluorescent staining.
Whole eyes were carefully removed from all animal groups and immediately fixed in 10% buffered formalin (Electron Microscopy Sciences, Hatfield, PA) at 4°C for at least 5 days. Formalin-fixed eyes were then frozen in OCT compound (TissueTek, Torrance, CA) and serially cut into 8-μm sections with a Shandon Cryotome SME cryostat (Thermo Electron Corporation, Pittsburgh, PA). Immunofluorescent staining was completed as described previously (26). All eye sections were rehydrated in PBS (Cellgro, Manassas, VA) for 3 min at room temperature, incubated with 10 mM sodium citrate (Sigma) for 10 min at room temperature, rinsed for 5 min with PBS, and blocked with 5% normal donkey serum containing 0.2% Triton X-100 (Sigma) for 30 min at room temperature. Fluorescent immunohistochemical staining for SOCS1 or SOCS3 in retinal tissues was accomplished by incubating rehydrated slides with polyclonal rabbit anti-mouse SOCS1 antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), polyclonal rabbit anti-mouse SOCS3 antibody (1:100; Santa Cruz Biotechnology), or normal rabbit IgG antibody (control; Santa Cruz Biotechnology) at 4°C overnight in a humidified atmosphere. Following incubation, slides were rinsed with PBS for 10 min at room temperature and incubated with goat anti-rabbit DyLight 488-labeled antibody (1:100; Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature in a humidified atmosphere. Double staining for identification of SOCS1- or SOCS3-producing retinal cells was accomplished by incubating slides with the rabbit anti-mouse SOCS1 or SOCS3 antibody together with either goat anti-mouse ionized calcium-binding adapter molecule 1 (Iba-1) antibody (1:100; Santa Cruz Biotechnology) as a marker for retinal microglial cells (122), goat-anti-mouse F4/80 IgG (1:100; Santa Cruz Biotechnology) as a marker for infiltrating macrophages, or chicken anti-mouse glial fibrillary acidic protein (GFAP) antibody (1:500; Abcam, Cambridge, MA) as a marker for activated retinal Müller cells (123, 124). For visualization of SOCS1 and SOCS3 protein expression in infiltrating granulocytes (neutrophils), slides were incubated with goat anti-mouse SOCS1 and SOCS3 antibody (Santa Cruz Biotechnology) and rabbit anti-mouse Ly-6G antibody (1:100; Santa Cruz Biotechnology). Secondary antibodies for all double-stained slides were donkey anti-goat DyLight 488-labeled antibody (1:100; Jackson ImmunoResearch) and goat anti-rabbit DyLight 594-labeled antibody (1:100; Jackson ImmunoResearch). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) in Vectashield mounting solution (Vector Laboratories, Burlingame, CA), and cover slides fixed to microscope slides were observed under a Nikon Eclipse fluorescence microscope.
Fluorescent in situ hybridization (FISH). (i) Cloning of MCMV IE1, mouse SOCS1, and mouse SOCS3 mRNA sequences.
MCMV immediate-early gene 1 (IE1) cDNA was generated by RT-PCR using mRNA from MCMV-infected mouse embryonic fibroblasts. Total RNA was extracted using TRIzol Reagent (Invitrogen) coupled with the PureLink RNA minikit (Invitrogen) and reverse transcribed using a SuperScript III first-strand synthesis system (Invitrogen) according to the manufacturer's instructions. Resulting cDNA was PCR purified using the following MCMV IE1-specific primers: IE1 sense, 5′-ATGGAGCCCGCCGCACC; IE1 antisense, 5′-CTTCTTGCTCTTCTTCTTGGGC (125). Mouse SOCS1-specific and SOCS3-specific mRNA sequences were each cloned from mouse salivary gland extracts. Primers used for the amplification of mouse SOCS1 and SOCS3 partial sequences were the following: SOCS1 sense, 5′-ATGGTAGCACGCAACCAGGTGGC; SOCS1 antisense, 5′-GTGAGTGTCGCCAGGGGCTG; SOCS3 sense, 5′-ATGGTCACCCACAGCAAGTTTC; SOCS3 antisense, 5′-GCGCAGGCTGGTGTCCAGGG. Standard conditions for PCR amplification included an annealing temperature of 55°C for 40 cycles. PCR products were obtained, applied to a 1% agarose gel, purified using a QIAquick gel extraction kit (Qiagen), and cloned into pGEM-T Easy vector (Promega, Madison, WI, USA). All clones were sequenced in Georgia State University's Core Facility.
(ii) RNA probe preparation.
RNA was labeled with digoxigenin-UTP or biotin-16-UTP by in vitro transcription with Sp6 and T7 RNA polymerase per the supplier's instructions (Roche Diagnostics, Indianapolis, IN). Mouse SOCS1 and mouse SOCS3 vectors were subcloned from the pGEM-T Easy vector into the pSPT18 vector through the SphI and SacI restriction enzyme sites. For preparation of the MCMV IE1 RNA probe, the MCMV IE1 sequence containing pGEM-T Easy vector was used as the template. In a 0.5-ml tube, 1 μg of this purified template DNA or 4 μl control DNA was added to sterile, RNase-free diethylpyrocarbonate (DEPC) water for a total sample volume of 13 μl. For each sample, 2 μl 10× deoxynucleoside triphosphate mixture, 2 μl 10× transcription buffer, 1 μl Protector RNase inhibitor, and 2 μl either RNA polymerase SP6 or RNA polymerase T7 were added. The socs1 and socs3 RNA probes were labeled with digoxigenin-UTP (Roche diagnostics), and the MCMV IE1 RNA probe was labeled with biotin-16-UTP (Roche diagnostics) per the manufacturer's instructions. Following incubation for 2 h at 37°C, 2 ml of RNase-free-DNase I was added to each tube to remove template DNA, and tubes were incubated for 10 min at 37°C. The DNase I reaction was stopped by adding 2 ml 0.2 M EDTA (pH 8.0) to each tube. RNA probes were isolated via lithium chloride precipitation (Life Technologies, Grand Island, NY) and were resuspended in 25 μl DEPC water.
(iii) Hybridization.
Fluorescent in situ hybridization was performed per the Cold Spring Harbor Laboratories Press classic protocol (126), with modifications. Ocular tissue sections were prepared as described above and then air dried for 10 min and incubated in PBS (pH 7.4) for 3 min, followed by washes with proteinase K in Tris-EDTA (TE) buffer at 37°C for 12 min, PBS for 3 min, 0.2 M HCl at room temperature for 10 min, PBS for 3 min, 0.1 M triethanolamine-HCl (pH 8.0) for 1 min with stirring, 0.25% acetic anhydrate with 0.1 M triethanolamine-HCl (pH 8.0) for 10 min with stirring, PBS at room temperature for 3 min, and 60%, 80%, 95%, and 100% ethanol, each for 90 s. Samples were air dried and placed in a humidified chamber containing 50% formamide in distilled water. Fresh hybridization solution (50% formamide in distilled H2O, 10 mM Tris-Cl [pH 8.0], 200 μg/ml yeast tRNA, 10% dextran sulfate, 1× Denhardt's solution, 600 mM NaCl, 0.25% SDS, 1 mM EDTA [pH 8.0], and DEPC water) was heated to 85°C for 10 min. Approximately 0.5 μg of digoxigenin-RNA probe and/or biotin-RNA probe was added to 200 ml heated hybridization solution, mixed, and incubated at 85°C for an additional 10 min before being added to each slide and incubated overnight at 55°C. Slides were then washed with gentle shaking with the following procedure: 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) buffer with 50% formamide at 65°C for 30 min, TNE buffer (10 mM Tris-HCl [pH 7.5], 0.5 M NaCl, 1 mM EDTA) at 37°C for 10 min, 20 μg/ml RNase in TNE buffer at 37°C for 30 min, TNE buffer at 37°C for 10 min, 2× SSC at 65°C for 25 min, 0.2× SSC at 65°C for 20 min, and 0.1× SSC at 65°C for 20 min.
(iv) Detection.
Sections were processed by treatment with 0.1 M PBS at room temperature for 5 min, blocking buffer (5% bovine serum albumin in 150 mM NaCl, 100 mM Tris-Cl [pH 7.5], 0.5% Triton X-100) at room temperature for 60 min, and 1:300 Alexa Fluor 488-conjugated IgG fraction monoclonal mouse anti-digoxin antibody (Jackson ImmunoResearch) and 1:500 Dylight 594-conjugated IgG fraction monoclonal mouse anti-biotin antibody (Jackson ImmunoResearch) in blocking buffer at room temperature for 60 min. All antibody-treated retinal sections ultimately were washed three times for 5 min each with PBS, mounted with DAPI (Vectashield; Vector Laboratories), and examined and photographed under a fluorescence microscope.
Statistical analysis.
All quantitative data from real-time RT-PCR assays are expressed as means from n = 5 to 8 mice per group per time point from at least two independent experiments, ± standard errors of the means (SEM) or standard deviations (SD), as indicated. Statistical analyses were performed using Student's t test, with P values of <0.05 considered significant.
ACKNOWLEDGMENTS
We thank the Biology Core Facilities, Department of Biology, Georgia State University, for technical assistance with real-time RT-PCR analysis and DNA sequencing.
This work was supported in part by NIH/NEI grant EY010568, NIH/NEI core grant P30EY006360, Emory Eye Center vision training grant NIH/NEI T32-EY007092, and Fight for Sight, Inc.
This article is dedicated to the memory of Richard J. Courtney, mentor and friend.
REFERENCES
- 1.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. 2006. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis 43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 2.Mocarski ES Jr, Shenk T, Pass RF. 2007. Cytomegalovirus, p 2702–2772. In Knipe DM, Howley PM (ed), Fields virology, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 3.Hahn G, Jores R, Mocarski ES. 1998. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc Natl Acad Sci U S A 95:3937–3942. doi: 10.1073/pnas.95.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mocarski ES Jr, Shenk T, Griffiths PD, Pass RF. 2013. Cytomegaloviruses, p 1960–2014. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 5.Dix RD, Cousins SW. 2004. AIDS-related cytomegalovirus retinitis: lessons from the laboratory. Curr Eye Res 29:91–101. doi: 10.1080/02713680490504641. [DOI] [PubMed] [Google Scholar]
- 6.Holland GN, Tufail A, Jordan MC. 1996. Cytomegalovirus diseases, p 1088–1120. In Pepose JS, Holland GN, Wilhelmus KR (ed), Ocular infection and immunity. Mosby Year Book, St. Louis, MO. [Google Scholar]
- 7.Jabs DA. 1995. Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc 93:623–683. [PMC free article] [PubMed] [Google Scholar]
- 8.Hoover DR, Peng Y, Saah A, Semba R, Detels RR, Rinaldo CR Jr, Phair JP. 1996. Occurrence of cytomegalovirus retinitis after human immunodeficiency virus immunosuppression. Arch Ophthalmol 114:821–827. doi: 10.1001/archopht.1996.01100140035004. [DOI] [PubMed] [Google Scholar]
- 9.Jabs DA, Enger C, Bartlett JG. 1989. Cytomegalovirus retinitis and acquired immunodeficiency syndrome. Arch Ophthalmol 107:75–80. doi: 10.1001/archopht.1989.01070010077031. [DOI] [PubMed] [Google Scholar]
- 10.Heiden D, Ford N, Wilson D, Rodriguez WR, Margolis T, Janssens B, Bedelu M, Tun N, Goemaere E, Saranchuk P, Sabapathy K, Smithuis F, Luyirika E, Drew WL. 2007. Cytomegalovirus retinitis: the neglected disease of the AIDS pandemic. PLoS Med 4:e334. doi: 10.1371/journal.pmed.0040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart MW. 2010. Optimal management of cytomegalovirus retinitis in patients with AIDS. Clin Ophthalmol 4:285–299. doi: 10.2147/OPTH.S6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugar EA, Jabs DA, Ahuja A, Thorne JE, Danis RP, Meinert CL, Studies of the Ocular Complications of AIDS Research Group. 2012. Incidence of cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol 153:1016–1024 e1015. doi: 10.1016/j.ajo.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabs DA, Ahuja A, Van Natta ML, Lyon AT, Yeh S, Danis R. 2015. Long-term outcomes of cytomegalovirus retinitis in the era of modern antiretroviral therapy: results from a United States cohort. Ophthalmology 122:1452–1463. doi: 10.1016/j.ophtha.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holland GN, Vaudaux JD, Shiramizu KM, Yu F, Goldenberg DT, Gupta A, Carlson M, Read RW, Novack RD, Kuppermann BD, Southern California HIV/Eye Consortium. 2008. Characteristics of untreated AIDS-related cytomegalovirus retinitis. II. Findings in the era of highly active antiretroviral therapy (1997 to 2000). Am J Ophthalmol 145:12–22. [DOI] [PubMed] [Google Scholar]
- 15.Holland GN, Vaudaux JD, Jeng SM, Yu F, Goldenberg DT, Folz IC, Cumberland WG, McCannel CA, Helm CJ, Hardy WD, UCLA CMV Retinitis Study Group. 2008. Characteristics of untreated AIDS-related cytomegalovirus retinitis. I. Findings before the era of highly active antiretroviral therapy (1988 to 1994). Am J Ophthalmol 145:5–11. [DOI] [PubMed] [Google Scholar]
- 16.Dix RD, Cray C, Cousins SW. 1994. Mice immunosuppressed by murine retrovirus infection (MAIDS) are susceptible to cytomegalovirus retinitis. Curr Eye Res 13:587–595. doi: 10.3109/02713689408999892. [DOI] [PubMed] [Google Scholar]
- 17.Crough T, Khanna R. 2009. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev 22:76–98. doi: 10.1128/CMR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jolicoeur P. 1991. Murine acquired immunodeficiency syndrome (MAIDS): an animal model to study the AIDS pathogenesis. FASEB J 5:2398–2405. doi: 10.1096/fasebj.5.10.2065888. [DOI] [PubMed] [Google Scholar]
- 19.Haas M, Meshorer A. 1979. Reticulum cell neoplasms induced in C57BL/6 mice by cultured virus grown in stromal hematopoietic cell lines. J Natl Cancer Inst 63:427–439. [PubMed] [Google Scholar]
- 20.Mosier DE, Yetter RA, Morse HC III. 1985. Retroviral induction of acute lymphoproliferative disease and profound immunosuppression in adult C57BL/6 mice. J Exp Med 161:766–784. doi: 10.1084/jem.161.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thacore HR, Cunningham RK, Zhou P, Nakeeb S, Terzian R, Zaleski MB. 1994. Acquired immunodeficiency in murine lymphoproliferative disease: considerations on pathogenesis. Immunobiology 190:195–211. doi: 10.1016/S0171-2985(11)80269-X. [DOI] [PubMed] [Google Scholar]
- 22.Dix RD, Cousins SW. 2003. Murine cytomegalovirus retinitis during MAIDS: susceptibility correlates with elevated intraocular levels of interleukin-4 mRNA. Curr Eye Res 26:211–217. doi: 10.1076/ceyr.26.3.211.14902. [DOI] [PubMed] [Google Scholar]
- 23.Dix RD, Cousins SW. 2004. Susceptibility to murine cytomegalovirus retinitis during progression of MAIDS: correlation with intraocular levels of tumor necrosis factor-alpha and interferon-gamma. Curr Eye Res 29:173–180. doi: 10.1080/02713680490504876. [DOI] [PubMed] [Google Scholar]
- 24.Bale JF Jr, O'Neil ME, Hogan RN, Kern ER. 1984. Experimental murine cytomegalovirus infection of ocular structures. Arch Ophthalmol 102:1214–1219. doi: 10.1001/archopht.1984.01040030984032. [DOI] [PubMed] [Google Scholar]
- 25.Dix RD. 1998. Systemic murine cytomegalovirus infection of mice with retrovirus-induced immunodeficiency results in ocular infection but not retinitis. Ophthalmic Res 30:295–301. doi: 10.1159/000055488. [DOI] [PubMed] [Google Scholar]
- 26.Chien H, Dix RD. 2012. Evidence for multiple cell death pathways during development of experimental cytomegalovirus retinitis in mice with retrovirus-induced immunosuppression: apoptosis, necroptosis, and pyroptosis. J Virol 86:10961–10978. doi: 10.1128/JVI.01275-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dix RD, Cray C, Cousins SW. 1997. Antibody alone does not prevent experimental cytomegalovirus retinitis in mice with retrovirus-induced immunodeficiency (MAIDS). Ophthalmic Res 29:381–392. doi: 10.1159/000268039. [DOI] [PubMed] [Google Scholar]
- 28.Dix RD, Cousins SW. 2005. Cell-mediated cytotoxicity of murine cytomegalovirus-infected target cells allows for release of residual infectious virus. Arch Virol 150:797–803. doi: 10.1007/s00705-004-0459-8. [DOI] [PubMed] [Google Scholar]
- 29.Dix RD, Ekworomadu CO, Hernandez E, Cousins SW. 2004. Perforin knockout mice, but not mice with MAIDS, show protection against experimental cytomegalovirus retinitis after adoptive transfer of immune cells with a functional perforin cytotoxic pathway. Arch Virol 149:2235–2244. doi: 10.1007/s00705-004-0370-3. [DOI] [PubMed] [Google Scholar]
- 30.Dix RD, Cousins SW. 2003. Interleukin-2 immunotherapy of murine cytomegalovirus retinitis during MAIDS correlates with increased intraocular CD8+ T-cell infiltration. Ophthalmic Res 35:154–159. doi: 10.1159/000070051. [DOI] [PubMed] [Google Scholar]
- 31.Dix RD, Cousins SW. 2004. Interleukin-2 immunotherapy and AIDS-related cytomegalovirus retinitis. Curr HIV Res 2:333–342. doi: 10.2174/1570162043351066. [DOI] [PubMed] [Google Scholar]
- 32.Blalock EL, Chien H, Dix RD. 2012. Systemic reduction of interleukin-4 or interleukin-10 fails to reduce the frequency or severity of experimental cytomegalovirus retinitis in mice with retrovirus-induced immunosuppression. Ophthalmol Eye Dis 4:79–90. doi: 10.4137/OED.S10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blalock EL, Chien H, Dix RD. 2013. Murine cytomegalovirus downregulates interleukin-17 in mice with retrovirus-induced immunosuppression that are susceptible to experimental cytomegalovirus retinitis. Cytokine 61:862–875. doi: 10.1016/j.cyto.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Leonor N, Taniguchi T, Fujita T, Kanakura Y, Komiya S, Yoshimura A. 1997. A new protein containing an SH2 domain that inhibits JAK kinases. Nature 387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 35.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T. 1997. Structure and function of a new STAT-induced STAT inhibitor. Nature 387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 36.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. 1997. A family of cytokine-inducible inhibitors of signalling. Nature 387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimura A, Ohkubo T, Kiguchi T, Jenkins NA, Gilbert DJ, Copeland NG, Hara T, Miyajima A. 1995. A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. EMBO J 14:2816–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honda K, Takaoka A, Taniguchi T. 2006. Type I interferon gene induction by the interferon regulatory factor family of transcription factors. Immunity 25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Navajas JM, Lee J, David M, Raz E. 2012. Immunomodulatory functions of type I interferons. Nat Rev Immunol 12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gil MP, Bohn E, O'Guin AK, Ramana CV, Levine B, Stark GR, Virgin HW, Schreiber RD. 2001. Biologic consequences of Stat1-independent IFN signaling. Proc Natl Acad Sci U S A 98:6680–6685. doi: 10.1073/pnas.111163898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczarek CM, Kay TW, Nicola NA, Hertzog PJ, Metcalf D, Hilton DJ. 1999. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell 98:597–608. doi: 10.1016/S0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 42.Kubo M, Hanada T, Yoshimura A. 2003. Suppressors of cytokine signaling and immunity. Nat Immunol 4:1169–1176. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- 43.Baker BJ, Akhtar LN, Benveniste EN. 2009. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol 30:392–400. doi: 10.1016/j.it.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akhtar LN, Qin H, Muldowney MT, Yanagisawa LL, Kutsch O, Clements JE, Benveniste EN. 2010. Suppressor of cytokine signaling 3 inhibits antiviral IFN-beta signaling to enhance HIV-1 replication in macrophages. J Immunol 185:2393–2404. doi: 10.4049/jimmunol.0903563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller RC, Schlaepfer E, Baenziger S, Crameri R, Zeller S, Byland R, Audige A, Nadal D, Speck RF. 2011. HIV interferes with SOCS-1 and -3 expression levels driving immune activation. Eur J Immunol 41:1058–1069. doi: 10.1002/eji.201041198. [DOI] [PubMed] [Google Scholar]
- 46.Song XT, Evel-Kabler K, Rollins L, Aldrich M, Gao F, Huang XF, Chen SY. 2006. An alternative and effective HIV vaccination approach based on inhibition of antigen presentation attenuators in dendritic cells. PLoS Med 3:e11. doi: 10.1371/journal.pmed.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryo A, Tsurutani N, Ohba K, Kimura R, Komano J, Nishi M, Soeda H, Hattori S, Perrem K, Yamamoto M, Chiba J, Mimaya J, Yoshimura K, Matsushita S, Honda M, Yoshimura A, Sawasaki T, Aoki I, Morikawa Y, Yamamoto N. 2008. SOCS1 is an inducible host factor during HIV-1 infection and regulates the intracellular trafficking and stability of HIV-1 Gag. Proc Natl Acad Sci U S A 105:294–299. doi: 10.1073/pnas.0704831105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koeberlein B, zur Hausen A, Bektas N, Zentgraf H, Chin R, Nguyen LT, Kandolf R, Torresi J, Bock CT. 2010. Hepatitis B virus overexpresses suppressor of cytokine signaling-3 (SOCS3) thereby contributing to severity of inflammation in the liver. Virus Res 148:51–59. doi: 10.1016/j.virusres.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Ma CJ, Ni L, Zhang CL, Wu XY, Kumaraguru U, Li CF, Moorman JP, Yao ZQ. 2011. Cross-talk between programmed death-1 and suppressor of cytokine signaling-1 in inhibition of IL-12 production by monocytes/macrophages in hepatitis C virus infection. J Immunol 186:3093–3103. doi: 10.4049/jimmunol.1002006. [DOI] [PubMed] [Google Scholar]
- 50.Bode JG, Ludwig S, Ehrhardt C, Albrecht U, Erhardt A, Schaper F, Heinrich PC, Haussinger D. 2003. IFN-alpha antagonistic activity of HCV core protein involves induction of suppressor of cytokine signaling-3. FASEB J 17:488–490. doi: 10.1096/fj.02-0664fje. [DOI] [PubMed] [Google Scholar]
- 51.Fenner JE, Starr R, Cornish AL, Zhang JG, Metcalf D, Schreiber RD, Sheehan K, Hilton DJ, Alexander WS, Hertzog PJ. 2006. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nat Immunol 7:33–39. doi: 10.1038/ni1287. [DOI] [PubMed] [Google Scholar]
- 52.Zhao DC, Yan T, Li L, You S, Zhang C. 2007. Respiratory syncytial virus inhibits interferon-alpha-inducible signaling in macrophage-like U937 cells. J Infect 54:393–398. doi: 10.1016/j.jinf.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Frey KG, Ahmed CM, Dabelic R, Jager LD, Noon-Song EN, Haider SM, Johnson HM, Bigley NJ. 2009. HSV-1-induced SOCS-1 expression in keratinocytes: use of a SOCS-1 antagonist to block a novel mechanism of viral immune evasion. J Immunol 183:1253–1262. doi: 10.4049/jimmunol.0900570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yokota S, Yokosawa N, Okabayashi T, Suzutani T, Fujii N. 2005. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 confers efficient viral replication. Virology 338:173–181. doi: 10.1016/j.virol.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 55.Yokota S, Yokosawa N, Okabayashi T, Suzutani T, Miura S, Jimbow K, Fujii N. 2004. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 contributes to inhibition of the interferon signaling pathway. J Virol 78:6282–6286. doi: 10.1128/JVI.78.12.6282-6286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi EJ, Lee CH, Shin OS. 2015. Suppressor of cytokine signaling 3 expression induced by varicella-zoster virus infection results in the modulation of virus replication. Scand J Immunol 82:337–344. doi: 10.1111/sji.12323. [DOI] [PubMed] [Google Scholar]
- 57.Butler LM, Jeffery HC, Wheat RL, Rae PC, Townsend K, Alkharsah KR, Schulz TF, Nash GB, Blackbourn DJ. 2011. Kaposi's sarcoma-associated herpesvirus infection of endothelial cells inhibits neutrophil recruitment through an interleukin-6-dependent mechanism: a new paradigm for viral immune evasion. J Virol 85:7321–7332. doi: 10.1128/JVI.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carlier J, Martin H, Mariame B, Rauwel B, Mengelle C, Weclawiak H, Coaquette A, Vauchy C, Rohrlich P, Kamar N, Rostaing L, Herbein G, Davrinche C. 2011. Paracrine inhibition of GM-CSF signaling by human cytomegalovirus in monocytes differentiating to dendritic cells. Blood 118:6783–6792. doi: 10.1182/blood-2011-02-337956. [DOI] [PubMed] [Google Scholar]
- 59.Strobl B, Bubic I, Bruns U, Steinborn R, Lajko R, Kolbe T, Karaghiosoff M, Kalinke U, Jonjic S, Muller M. 2005. Novel functions of tyrosine kinase 2 in the antiviral defense against murine cytomegalovirus. J Immunol 175:4000–4008. doi: 10.4049/jimmunol.175.6.4000. [DOI] [PubMed] [Google Scholar]
- 60.Alston CI, Dix RD. 2017. Murine cytomegalovirus infection of mouse macrophages stimulates early expression of suppressor of cytokine signaling (SOCS)1 and SOCS3. PLoS One 12:e0171812. doi: 10.1371/journal.pone.0171812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tiwari V, Shukla D. 2012. Nonprofessional phagocytosis can facilitate herpesvirus entry into ocular cells. Clin Dev Immunol 2012:651691. doi: 10.1155/2012/651691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hudson JB. 1979. The murine cytomegalovirus as a model for the study of viral pathogenesis and persistent infections. Arch Virol 62:1–29. doi: 10.1007/BF01314900. [DOI] [PubMed] [Google Scholar]
- 63.Loh L, Hudson JB. 1979. Interaction of murine cytomegalovirus with separated populations of spleen cells. Infect Immun 26:853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mercer JA, Wiley CA, Spector DH. 1988. Pathogenesis of murine cytomegalovirus infection: identification of infected cells in the spleen during acute and latent infections. J Virol 62:987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshimura A, Naka T, Kubo M. 2007. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol 7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 66.Pomeroy C, Delong D, Clabots C, Riciputi P, Filice GA. 1998. Role of interferon-gamma in murine cytomegalovirus infection. J Lab Clin Med 132:124–133. doi: 10.1016/S0022-2143(98)90007-5. [DOI] [PubMed] [Google Scholar]
- 67.Ruzek MC, Miller AH, Opal SM, Pearce BD, Biron CA. 1997. Characterization of early cytokine responses and an interleukin (IL)-6-dependent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J Exp Med 185:1185–1192. doi: 10.1084/jem.185.7.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alston CI, Dix RD. 2017. Reduced frequency of murine cytomegalovirus retinitis in C57BL/6 mice correlates with low levels of suppressor of cytokine signaling (SOCS)1 and SOCS3 expression within the eye during corticosteroid-induced immunosuppression. Cytokine 97:38–41. doi: 10.1016/j.cyto.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Metcalf D, Alexander WS, Elefanty AG, Nicola NA, Hilton DJ, Starr R, Mifsud S, Di Rago L. 1999. Aberrant hematopoiesis in mice with inactivation of the gene encoding SOCS-1. Leukemia 13:926–934. [DOI] [PubMed] [Google Scholar]
- 70.Dickensheets HL, Venkataraman C, Schindler U, Donnelly RP. 1999. Interferons inhibit activation of STAT6 by interleukin 4 in human monocytes by inducing SOCS-1 gene expression. Proc Natl Acad Sci U S A 96:10800–10805. doi: 10.1073/pnas.96.19.10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stoiber D, Kovarik P, Cohney S, Johnston JA, Steinlein P, Decker T. 1999. Lipopolysaccharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activating factor IFN-gamma. J Immunol 163:2640–2647. [PubMed] [Google Scholar]
- 72.O'Keefe GM, Nguyen VT, Ping Tang LL, Benveniste EN. 2001. IFN-gamma regulation of class II transactivator promoter IV in macrophages and microglia: involvement of the suppressors of cytokine signaling-1 protein. J Immunol 166:2260–2269. doi: 10.4049/jimmunol.166.4.2260. [DOI] [PubMed] [Google Scholar]
- 73.Ratthé C, Pelletier M, Chiasson S, Girard D. 2007. Molecular mechanisms involved in interleukin-4-induced human neutrophils: expression and regulation of suppressor of cytokine signaling. J Leukoc Biol 81:1287–1296. doi: 10.1189/jlb.0306209. [DOI] [PubMed] [Google Scholar]
- 74.Liu X, Mameza MG, Lee YS, Eseonu CI, Yu C-R, Kang Derwent JJ, Egwuagu CE. 2008. Suppressors of cytokine-signaling proteins induce insulin resistance in the retina and promote survival of retinal cells. Diabetes 57:1651–1658. doi: 10.2337/db07-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alexander WS, Hilton DJ. 2004. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol 22:503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- 76.Dimitriou ID, Clemenza L, Scotter AJ, Chen G, Guerra FM, Rottapel R. 2008. Putting out the fire: coordinated suppression of the innate and adaptive immune systems by SOCS1 and SOCS3 proteins. Immunol Rev 224:265–283. doi: 10.1111/j.1600-065X.2008.00659.x. [DOI] [PubMed] [Google Scholar]
- 77.Klinman DM, Morse HC III. 1989. Characteristics of B cell proliferation and activation in murine AIDS. J Immunol 142:1144–1149. [PubMed] [Google Scholar]
- 78.Zhang M, Xin H, Roon P, Atherton SS. 2005. Infection of retinal neurons during murine cytomegalovirus retinitis. Investig Ophthalmol Vis Sci 46:2047–2055. doi: 10.1167/iovs.05-0005. [DOI] [PubMed] [Google Scholar]
- 79.Hanson LK, Slater JS, Karabekian Z, Virgin HWT, Biron CA, Ruzek MC, van Rooijen N, Ciavarra RP, Stenberg RM, Campbell AE. 1999. Replication of murine cytomegalovirus in differentiated macrophages as a determinant of viral pathogenesis. J Virol 73:5970–5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bale JF, Kern ER, Overall JC, Baringer JR. 1983. Impaired Migratory and chemotactic activity of neutrophils during murine cytomegalovirus infection. J Infect Dis 148:518–525. doi: 10.1093/infdis/148.3.518. [DOI] [PubMed] [Google Scholar]
- 81.Schut RL, Gekker G, Hu S, Chao CC, Pomeroy C, Jordan MC, Peterson PK. 1994. Cytomegalovirus replication in murine microglial cell cultures: suppression of permissive infection by interferon-gamma. J Infect Dis 169:1092–1096. doi: 10.1093/infdis/169.5.1092. [DOI] [PubMed] [Google Scholar]
- 82.Akhtar LN, Benveniste EN. 2011. Viral exploitation of host SOCS protein functions. J Virol 85:1912–1921. doi: 10.1128/JVI.01857-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cousins SW, Espinosa-Heidmann DG, Miller DM, Pereira-Simon S, Hernandez EP, Chien H, Meier-Jewett C, Dix RD. 2012. Macrophage activation associated with chronic murine cytomegalovirus infection results in more severe experimental choroidal neovascularization. PLoS Pathog 8:e1002671. doi: 10.1371/journal.ppat.1002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grossniklaus HE, Ling JX, Wallace TM, Dithmar S, Lawson DH, Cohen C, Elner VM, Elner SG, Sternberg P Jr. 2002. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis 8:119–126. [PubMed] [Google Scholar]
- 85.Hofman FM, Hinton DR. 1992. Tumor necrosis factor-alpha in the retina in acquired immune deficiency syndrome. Investig Ophthalmol Vis Sci 33:1829–1835. [PubMed] [Google Scholar]
- 86.Mantovani A, Sica A, Locati M. 2005. Macrophage polarization comes of age. Immunity 23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 87.Mosser DM. 2003. The many faces of macrophage activation. J Leukoc Biol 73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 88.Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, Ohishi M, Yoshida H, Kubo M, Yoshimura A. 2002. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity 17:583–591. doi: 10.1016/S1074-7613(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 89.Liang YB, Tang H, Chen ZB, Zeng LJ, Wu JG, Yang W, Li ZY, Ma ZF. 2017. Downregulated SOCS1 expression activates the JAK1/STAT1 pathway and promotes polarization of macrophages into M1 type. Mol Med Rep 16:6405–6411. doi: 10.3892/mmr.2017.7384. [DOI] [PubMed] [Google Scholar]
- 90.Bourdonnay E, Zasłona Z, Penke LRK, Speth JM, Schneider DJ, Przybranowski S, Swanson JA, Mancuso P, Freeman CM, Curtis JL, Peters-Golden M. 2015. Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling. J Exp Med 212:729–742. doi: 10.1084/jem.20141675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.He C, Yu CR, Sun L, Mahdi RM, Larkin J III, Egwuagu CE. 2015. Topical administration of a suppressor of cytokine signaling-1 (SOCS1) mimetic peptide inhibits ocular inflammation and mitigates ocular pathology during mouse uveitis. J Autoimmun 62:31–38. doi: 10.1016/j.jaut.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu CR, Mahdi RR, Oh HM, Amadi-Obi A, Levy-Clarke G, Burton J, Eseonu A, Lee Y, Chan CC, Egwuagu CE. 2011. Suppressor of cytokine signaling-1 (SOCS1) inhibits lymphocyte recruitment into the retina and protects SOCS1 transgenic rats and mice from ocular inflammation. Investig Ophthalmol Vis Sci 52:6978–6986. doi: 10.1167/iovs.11-7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu CR, Kim SH, Mahdi RM, Egwuagu CE. 2013. SOCS3 deletion in T lymphocytes suppresses development of chronic ocular inflammation via upregulation of CTLA-4 and expansion of regulatory T cells. J Immunol 191:5036–5043. doi: 10.4049/jimmunol.1301132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leonard WJ. 2008. Type I cytokines and interferons and their receptors, p 706–748. In Paul WE. (ed), Fundamental immunology, 6th ed Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 95.Platanias LC. 2005. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 96.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. 2003. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol 4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 97.Seki Y, Inoue H, Nagata N, Hayashi K, Fukuyama S, Matsumoto K, Komine O, Hamano S, Himeno K, Inagaki-Ohara K, Cacalano N, O'Garra A, Oshida T, Saito H, Johnston JA, Yoshimura A, Kubo M. 2003. SOCS-3 regulates onset and maintenance of T(H)2-mediated allergic responses. Nat Med 9:1047–1054. doi: 10.1038/nm896. [DOI] [PubMed] [Google Scholar]
- 98.Banks TA, Rickert S, Benedict CA, Ma L, Ko M, Meier J, Ha W, Schneider K, Granger SW, Turovskaya O, Elewaut D, Otero D, French AR, Henry SC, Hamilton JD, Scheu S, Pfeffer K, Ware CF. 2005. A lymphotoxin-IFN-beta axis essential for lymphocyte survival revealed during cytomegalovirus infection. J Immunol 174:7217–7225. doi: 10.4049/jimmunol.174.11.7217. [DOI] [PubMed] [Google Scholar]
- 99.Garcia-Sastre A, Biron CA. 2006. Type 1 interferons and the virus-host relationship: a lesson in detente. Science 312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- 100.Loewendorf A, Benedict CA. 2010. Modulation of host innate and adaptive immune defenses by cytomegalovirus: timing is everything. J Intern Med 267:483–501. doi: 10.1111/j.1365-2796.2010.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Orange JS, Wang B, Terhorst C, Biron CA. 1995. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med 182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schneider K, Loewendorf A, De Trez C, Fulton J, Rhode A, Shumway H, Ha S, Patterson G, Pfeffer K, Nedospasov SA, Ware CF, Benedict CA. 2008. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe 3:67–76. doi: 10.1016/j.chom.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shanley JD, Shanley JA, Albert G, Biegel D. 2001. Characterization of virus-induced interferon-gamma responses in mice previously infected with murine cytomegalovirus. J Infect Dis 183:697–706. doi: 10.1086/318830. [DOI] [PubMed] [Google Scholar]
- 104.Tang-Feldman YJ, Wojtowicz A, Lochhead GR, Hale MA, Li Y, Pomeroy C. 2006. Use of quantitative real-time PCR (qRT-PCR) to measure cytokine transcription and viral load in murine cytomegalovirus infection. J Virol Methods 131:122–129. [DOI] [PubMed] [Google Scholar]
- 105.Presti RM, Pollock JL, Dal Canto AJ, O'Guin AK, Virgin HWT. 1998. Interferon gamma regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J Exp Med 188:577–588. doi: 10.1084/jem.188.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Orange JS, Biron CA. 1996. Characterization of early IL-12, IFN-alphabeta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol 156:4746–4756. [PubMed] [Google Scholar]
- 107.Kelsey DK, Olsen GA, Overall JC Jr, Glasgow LA. 1977. Alteration of host defense mechanisms by murine cytomegalovirus infection. Infect Immun 18:754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Le VT, Trilling M, Zimmermann A, Hengel H. 2008. Mouse cytomegalovirus inhibits beta interferon (IFN-beta) gene expression and controls activation pathways of the IFN-beta enhanceosome. J Gen Virol 89:1131–1141. doi: 10.1099/vir.0.83538-0. [DOI] [PubMed] [Google Scholar]
- 109.Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, Crack PJ, Nicholson SE, Hilton DJ, O'Neill LA, Hertzog PJ. 2006. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol 7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 110.Marshall EE, Geballe AP. 2009. Multifaceted evasion of the interferon response by cytomegalovirus. J Interferon Cytokine Res 29:609–619. doi: 10.1089/jir.2009.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Delale T, Paquin A, Asselin-Paturel C, Dalod M, Brizard G, Bates EE, Kastner P, Chan S, Akira S, Vicari A, Biron CA, Trinchieri G, Briere F. 2005. MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-alpha release and initiation of immune responses in vivo. J Immunol 175:6723–6732. [DOI] [PubMed] [Google Scholar]
- 112.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A 101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Biron CA, Tarrio ML. 2015. Immunoregulatory cytokine networks: 60 years of learning from murine cytomegalovirus. Med Microbiol Immunol 204:345–354. doi: 10.1007/s00430-015-0412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tosato G, Jones KD. 1990. Interleukin-1 induces interleukin-6 production in peripheral blood monocytes. Blood 75:1305–1310. [PubMed] [Google Scholar]
- 115.Kishimoto T. 2010. IL-6: from its discovery to clinical applications. Int Immunol 22:347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 116.Marine JC, Topham DJ, McKay C, Wang D, Parganas E, Stravopodis D, Yoshimura A, Ihle JN. 1999. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell 98:609–616. [DOI] [PubMed] [Google Scholar]
- 117.Naka T, Matsumoto T, Narazaki M, Fujimoto M, Morita Y, Ohsawa Y, Saito H, Nagasawa T, Uchiyama Y, Kishimoto T. 1998. Accelerated apoptosis of lymphocytes by augmented induction of Bax in SSI-1 (STAT-induced STAT inhibitor-1) deficient mice. Proc Natl Acad Sci U S A 95:15577–15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Starr R, Metcalf D, Elefanty AG, Brysha M, Willson TA, Nicola NA, Hilton DJ, Alexander WS. 1998. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc Natl Acad Sci U S A 95:14395–14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marine JC, McKay C, Wang D, Topham DJ, Parganas E, Nakajima H, Pendeville H, Yasukawa H, Sasaki A, Yoshimura A, Ihle JN. 1999. SOCS3 is essential in the regulation of fetal liver erythropoiesis. Cell 98:617–627. [DOI] [PubMed] [Google Scholar]
- 120.Hartley JW, Fredrickson TN, Yetter RA, Makino M, Morse HC III. 1989. Retrovirus-induced murine acquired immunodeficiency syndrome: natural history of infection and differing susceptibility of inbred mouse strains. J Virol 63:1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kercher L, Mitchell BM. 2002. Persisting murine cytomegalovirus can reactivate and has unique transcriptional activity in ocular tissue. J Virol 76:9165–9175. doi: 10.1128/JVI.76.18.9165-9175.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW. 2007. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem 55:687–700. doi: 10.1369/jhc.6A7156.2007. [DOI] [PubMed] [Google Scholar]
- 123.Kuzmanovic M, Dudley VJ, Sarthy VP. 2003. GFAP promoter drives Muller cell-specific expression in transgenic mice. Investig Ophthalmol Vis Sci 44:3606–3613. doi: 10.1167/iovs.02-1265. [DOI] [PubMed] [Google Scholar]
- 124.Sarthy PV, Fu M, Huang J. 1991. Developmental expression of the glial fibrillary acidic protein (GFAP) gene in the mouse retina. Cell Mol Neurobiol 11:623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rawlinson WD, Farrell HE, Barrell BG. 1996. Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol 70:8833–8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Anonymous. 2005. Fluorescence in situ hybridization. Nat Methods 2:237–238. doi: 10.1038/nmeth0305-237. [DOI] [Google Scholar]