Abstract
Breast cancer is the most common invasive cancer in women worldwide. Sirtuin 1 (SIRT1) has recently been shown to have implications in regulating cancer cell growth and apoptosis. SIRT1 regulates Forkhead box O3a (FOXO3a) by both inhibiting FOXO3-induced apoptosis and potentiating the ability of FOXO3a to resist oxidative stress. Matrix metalloproteinase 2 (MMP2) participates in tumor invasion and metastasis by degrading extracellular matrix. SIRT1 up regulates MMP2 expression by its deacetylation activity. This study aimed to investigate the expression of SIRT1, FOXO3a and MMP2 in breast tissues of women with breast cancer. In addition, the effect of SIRT1 inhibition on both FOXO3a and MMP2 expression in breast cancer (MCF-7) cells was assessed. The expression levels of SIRT1, FOXO3a and MMP2 in the breast tissues were determined by real-time PCR in 60 patients with malignant tumor and in 24 patients with benign tumors. After SIRT1 inhibition, protein levels of SIRT1 and FOXO3a were assessed by Western Blot and levels of MMP2 by ELISA in MCF-7 cells. The expression levels of SIRT1, FOXO3a and MMP2 were significantly higher in breast cancer tissues compared to in benign breast tumor and adjacent normal tissues. SIRT1, MMP2 and FOXO3a expression were associated directly with each other. SIRT1 inhibition suppresses MMP2 and FOXO3a expression compared to control MCF7. Sirtinol (SIRT1 inhibitor) effectively induced inhibition of MMP2 and FOXO3a expression in MCF-7 cells, indicating the promising therapeutic strategy of targeting SIRT1 for breast cancer.
Keywords: Breast cancer, FOXO3a, MMP2, SIRT1, Sirtinol
List of abbreviations
- CA 15.3
Cancer antigen 15.3
- CEA
Carcinoembryonic antigen
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
Dimethyl sulfoxide
- ELISA
Enzyme linked immunosorbent assay
- FOXO3a
Forkhead box O3a
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- IRB
Institutional Review Board
- MMP2
Matrix metalloproteinase 2
- NCI
National Cancer Institute
- PBS
Phosphate-buffered saline
- PCR
Polymerase chain reaction
- PVDF
Polyvinylidene difluoride
- RIPA
Radioimmunoprecipitation assay
- ROS
Reactive oxygen species
- SIRT1
Sirtuin 1
- TBS
Tris Buffer Saline
Introduction
Breast cancer represents the third most frequent cancer worldwide. It makes up to 21% of all new cancer diagnoses among women.1 Mortality from breast cancer is almost caused by carcinomic cells invasion and metastasis to distant organ sites; subsequently, identifying genes involved in metastasis of breast cancer is of great importance.2, 3
SIRT1 (Sirtuin 1, silent mating-type information regulation 2 homolog 1), also known as NAD-dependent deacetylase, is a nuclear enzyme that has a role in deacetylation of histones and several nonhistone proteins. Acetylation of key cell cycle and apoptosis regulatory proteins, including p53 and forkhead box O (FOXO), through stress-induced activation of cellular histone/protein acetyltransferases, promotes cell death. This effect is opposed by sirtuin-induced deacetylation of these protein targets with subsequent cell survival under stress.4
With regard to the growth of cancer cells, it has been found that SIRT1 regulates cell proliferation, survival, and death, and plays a pivotal role in tumorigenesis and longevity. In humans, numerous types of cancer; including prostate, breast, colon, glioblastoma, lymphoma and acute myeloid leukemia have been demonstrated to have a significantly elevated expression of SIRT1.5 Interestingly, Sirts seem to have a dual role in cancer. In fact, while protecting the organism against tumors by increasing genomic stability and limiting cellular replicative lifespan, they can also induce tumorigenesis by boosting cell survival under stress conditions and ameliorating the uncontrolled cell division.6 The possible explication of this double action of Sirts in cancer could be connected to their key role in cellular pathways such as cell cycle, cell growth, genome integrity, and cell death in response to stressor stimuli.7
The effects of SIRT1 on FOXO function are complex and vary depending upon the target genes of FOXO. It has been found that SIRT1 upregulated the transcription of FOXO target genes involved in cell survival under stress; however, it downregulated the transcription of genes associated with cell death. Thus, SIRT1 appeared to shift the FOXO-dependent response far from cell death toward stress resistance. This could be explicated by the concept that acetylation/deacetylation of FOXO protein may switch target specificity.8
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that have a pivotal role in promoting cancer cell invasion through the degradation of extracellular matrix. They have been found in greater amounts and higher activity inside and around malignant cancers than in normal, benign, or premalignant tissues.9 MMPs can present in both inactive pro-enzymes and active enzymes forms. The mechanism of activation in vivo is still unknown but may be mediated by proteolytic activity of other MMPs and/or serine proteases.10
One of the MMPs implicated in cancer invasion is MMP-2 which also known as gelatinase A. This MMP is thought to mediate invasion and metastasis through the degradation of type IV collagen, the main component of basement membranes which induces angiogenesis.11 MMP-2 expression and activity are regulated by SIRT1 at posttranslational level. It has been found that the up regulation of MMP-2 expression by SIRT1 is mediated by its deacetylation activity. However, SIRT1 knockdown reduces MMP-2 expression through decreasing its protein stability.12
The present study aimed to investigate SIRT1, FOXO3a and MMP2 gene expression in breast cancer, benign breast tumor and adjacent normal tissues as well as to study the correlations of SIRT1, FOXO3a and MMP2 with clinicopathological and biochemical parameters. Additionally, we aimed to assess the effect of SIRT1 inhibition on both FOXO3a and MMP2 gene expression in breast (MCF-7) human cancer cells.
Materials and methods
Ethical approval
The study was approved by the Institutional Review Board (IRB) of the National Cancer Institute (NCI), Cairo University and was conducted according to the rules of Helsinki declaration for human studies. A Written informed consent was obtained from all patients.
Patients
The current study enrolled 84 female patients recruited from National Cancer Institute, Cairo, Egypt. They were divided into two groups; the first group included 60 patients who have newly diagnosed breast cancer while the second group included 24 patients who have newly diagnosed benign breast tumor. The classification of tumor and its stage were performed according to the international union against cancer (Tumor–Node–Metastasis) classification. The breast cancer histopathology was done using biopsy from tumor cancer tissues and from adjacent normal tissue. Also, the benign breast tumor histopathology was implemented using tissue biopsy of mammary tumor or after surgery. Furthermore, three tissue cores were taken from all breast lesions, one of them stored in RNA lysis solution at −80 °C for genetic processing of SIRT1, FOXO3a and MMP2 genes and the other two cores stored within formalin 10% for histopathological and hormonal receptors assessment.
Biochemical analysis
Three ml of peripheral blood sample were collected for assessment of serum cancer antigen 15.3 (CA 15.3) and serum carcinoembryonic antigen (CEA) following standard laboratory methods.
Real-time quantitative analysis for SIRT1, FOXO3a and MMP2 gene expression
Total RNA was extracted from tissue homogenate using SV Total RNA Isolation System (Promega, Madison, WI, USA) according to manufacturer's instruction. Complementary DNA (cDNA) was synthesized from 1 μg RNA using SuperScript III First-Strand Synthesis System as described in the manufacturer's protocol (#K1621, Fermentas, Waltham, MA, USA). Real-time quantitative PCR amplification and analysis were performed using an Applied Biosystem with software version 3.1 (StepOne™, USA). The reaction contained SYBR Green Master Mix (Applied Biosystems), gene-specific primer pairs designed with Gene Runner Software (Hasting Software, Inc., Hasting, NY) from RNA sequences from Gen Bank (SIRT1: Forward primer 5′-AGAGCCTCACATGCAAGCTCTAG-3′, Reverse primer 5′-GCCAATCATAAGATGTTGCTGAAC-3, FOXO3a: Forward primer 5′ CGACTATGCAGTGACAGGTTGTG 3′, Reverse primer 5′ CGACTATGCAGTGACAGGTTGTG 3′, MMP2: Forward primer 5′ GGCCCTGTCACTCCTGAGAT 3′, Reverse primer 5′ GGCAT CCAGGTTATCGGGGA 3′, GAPDH: Forward primer 5′ CCAGGTTGGTCTCCTCTGACTT 3′, Reverse primer 5′ GTTGCTGTAGCCAAATTCGTTGT 5′). All primer sets had a calculated annealing temperature of 60°. Real-time quantitative PCR was performed in a 25-μl reaction volume consisting of 2X SYBR Green PCR Master Mix, 900 nM of each primer and 2 μl of cDNA. Amplification conditions were: 2 min at 50°, 10 min at 95° and 40 cycles of denaturation for 15 s and annealing/extension at 60° for 10 min. Data from real-time assays were calculated using the v1·7 Sequence Detection Software from PE Biosystems (Foster City, CA). All values were normalized to the GAPDH which was used as the endogenous control (reference gene). Relative quantifications were calculated using the 2−ΔΔCt method.13
Cultures of human breast cancer (MCF7) cells
Breast (MCF-7) human cancer cells was obtained from the tissue culture unit of the Holding Company for Biological Products and Vaccines (VACSERA), Giza, Egypt and supplied through the American Type Culture Collection (ATCC; Virginia, USA) and were grown in a sterile 50 cm2 tissue culture flask in complete medium containing Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% phosphate-buffered saline (PBS) and 1% penicillin-streptomycin (100 units/mL penicillin and 100 μg/mL streptomycin). All cells were incubated in a humidified atmosphere incubator containing 5% CO2 at 37 °C. Cells were cultured to100% confluence. Cells were passaged using trypsin-EDTA. The cultured MCF7 cells were divided into 2 groups: 1st MCF7 cells as control cells, 2nd MCF7 cells were treated with sirtinol (SIRT1 inhibitor) which was purchased from Sigma; dissolved in dimethyl sulfoxide (DMSO) at a dose 120 μM for 24 h, then the media were collected and centrifuged at 10.000 rpm for 20 min, the supernatant was kept frozen at −80 °C till analysis of MMP2 by ELISA. MCF7 cells were harvested for assessment of protein expression of SIRT1 and FOXO3a by western blot.
Western blot analysis of SIRT1 and FOXO3a
(usingV3 Western Workflow™ Complete System, Bio-Rad® Hercules, CA, USA) Cells were washed with ice-cold PBS, trypsinized, and collected by centrifugation. Protein were extracted from cell lysates using ice-cold radioimmunoprecipitation assay (RIPA) lysis buffer PL005 was provided by Bio BASIC INC (Markham Ontario L3R 8T4 Canada) [50 mM Tris HCL, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS (sodium dodecyl sulphate)] supplemented with phosphatase and protease inhibitors, then centrifugation at 12,000 rpm for 20 min at 4 °C. The protein concentration for each sample was determined using Bradford assay. Equal amounts of protein (20–30 μg of total protein) and 2X Laemmli buffer were heated at 70 °C for 5–10 min and separated by SDS/polyacrylamide gel electrophoresis (10% acrylamide gel) using a Bio-Rad Mini-Protein II system. The protein was transferred to polyvinylidene difluoride (PVDF) membranes (Pierce, Rockford, IL, USA) with a Bio-Rad Trans-Blot system (TGX Stain-Free™ FastCast™ Acrylamide Kit which was provided by Bio-Rad Laboratories, TNC, USA). After transfer, the membranes were washed with Tris Buffer Saline (TBS) and blocked for 1 h at room temperature with 5% (w/v) skimmed milk powder in TBS. The manufacturer's instructions were followed for the primary antibody reactions. Following blocking, the blots were developed using antibodies for SIRT1, FOXO3a and β-actin supplied by (Thermoscientific, Rockford, Illinois, USA) incubated overnight at pH 7.6 at 4 °C with gentle shaking. After washing, peroxidase-labeled secondary antibodies were added, and the membranes were incubated at 37 °C for 1 h then washed with TBS 5 times for 5 min. Chemiluminescence substrate (Clarity Western ECL Substrate BIO-RAD, USA) was applied to the blot according to manufacturer's recommendation. Band intensity was analyzed by ChemiDocTM imaging system with Image LabTM software version 5.1 (Bio-Rad Laboratories Inc., Hercules, CA, USA). The results were expressed as arbitrary units after normalization for β-actin protein expression.
Assessment of MMP-2
(using ELISA Kit Catalog No: MBS2506130): Cell culture supernatant was centrifuged for 20 min to remove insoluble impurity and cell debris at 1000×g at 2–8 °C. The clear supernatant was used for assessment of MMP2 immediately.
Statistical analysis
Analysis of more than two variables was done by One-Way ANOVA followed by Tukey's comparison test. Two sample t test was used for comparison of two quantitative variables. Simple linear correlation (Pearson's correlation) was also carried out. Data are expressed as mean ± SD using Graph pad prism version 5.0. The statistical significance was set as P < 0.05.
Results
Patient features
The study included 60 patients with primary malignant breast cancer with mean age of 52.18 ± 12.17 years and 24 patients with benign breast tumor with mean age of 45.55 ± 10.14 years. The clinical features of the patients, including histology, histopathological grade of the tumor, axillary lymph node status, tumor size and clinical grade (TNM stage) were shown in Table 1. The majority of the patients were invasive duct carcinoma (N = 49, 81.7%) and moderately differentiated in histologic grade (N = 46, 76.7%), and large number of patients had no lymph node metastasis (N = 35, 58.33%). Most of the cases were positive ER (N = 47, 78.33%), PR (N = 45, 75%) and negative HER2 (N = 42, 70%). The percentage of tumors at stage 2 and 3 at the time of diagnosis was 65% and 35%, respectively. Histology of benign breast tumor showed that 70.7% (N = 17) of patients were fibroadenoma, 12.5% (N = 3) were fibrocystic mastopathy, 4.2% (N = 1) were spindle cell tumor, 4.2% (N = 1) were granulomatous mastitis, 4.2% (N = 1) were fat necrosis and 4.2% (N = 1) were fibro-epithelial tumor.
Table 1.
Clinico-pathological features of malignant breast cancer group.
| Number of cases (%) | |
|---|---|
| Histology of malignant breast tumor | |
| IDC | 49 (81.7) |
| IDC-L | 5 (8.3) |
| Others | 6 (10) |
| Histological grade | |
| Grade I | 5 (8.3) |
| Grade II | 46 (76.7) |
| Grade III | 9 (15) |
| ER status | |
| Negative | 13 (21.66) |
| Positive | 47 (78.33) |
| PR status | |
| Negative | 15 (25) |
| Positive | 45 (75) |
| HER2 status | |
| Negative | 42 (70) |
| Positive | 18 (30) |
| Axillary lymph node: | |
| N0 | 35 (58.33) |
| N1 | 18 (30) |
| N2 | 5 (8.33) |
| N3 | 2 (3.33) |
| Tumor size (cm): | |
| T2 | 29 (48.3) |
| T3 | 25 (41.7) |
| ≥T4 | 6 (10) |
| TNM Stage: | |
| 2 | 39 (65) |
| 3 | 21 (35) |
IDC: invasive duct carcinoma, DC-L: invasive duct carcinoma with lobular features, ER: estrogen receptor, PR: progesterone receptor, HER2: human epidermal growth factor receptor 2.
Serum levels of tumor markers
The levels of CA 15.3 and CEA were significantly higher in malignant breast cancer group (96.7 ± 13.04, 12.2 ± 3.39, respectively) compared to benign tumor group (16.76 ± 4.9, 2.29 ± 0.7, respectively) at P < 0.001.
Tissue levels of SIRT1, MMP2 and FOXO3a
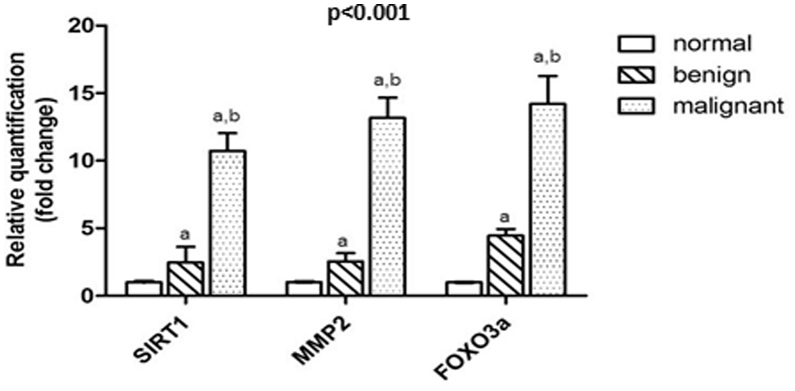
It has been shown that SIRT1, MMP2 and FOXO3a were significantly overexpressed in breast cancer tissues (10.73 ± 1.32, 13.18 ± 1.49, 14.21 ± 2.06, respectively) compared to in benign breast tumor (2.47 ± 1.15, 2.54 ± 0.6, 4.46 ± 0.49, respectively) and adjacent normal tissues (1.012 ± 0.044, 1.015 ± 0.033, 1.00 ± 0.013, respectively) at P < 0.001 (Fig. 1).
Figure 1.
Expression of SIRT1, MMP2 and FOXO3a in breast tissues of the studied groups. Parameters were presented on the charts as means ± SD. SIRT1: sirtuin 1, FOXO3a: forkhead box O3a, MMP2: matrix metalloproteinase 2. a: significant from normal tissues, b: significant from benign tissues.
Effect of SIRT1 inhibition on MMP2 and FOXO3a expression
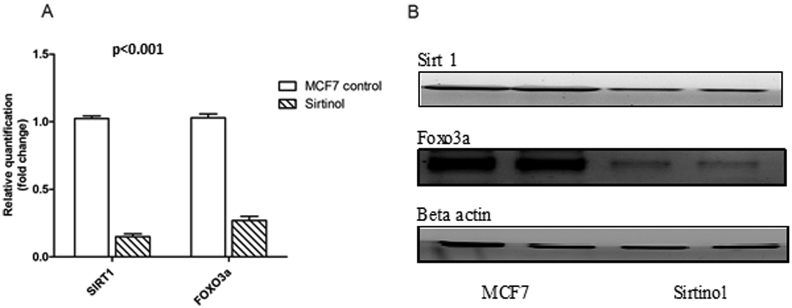
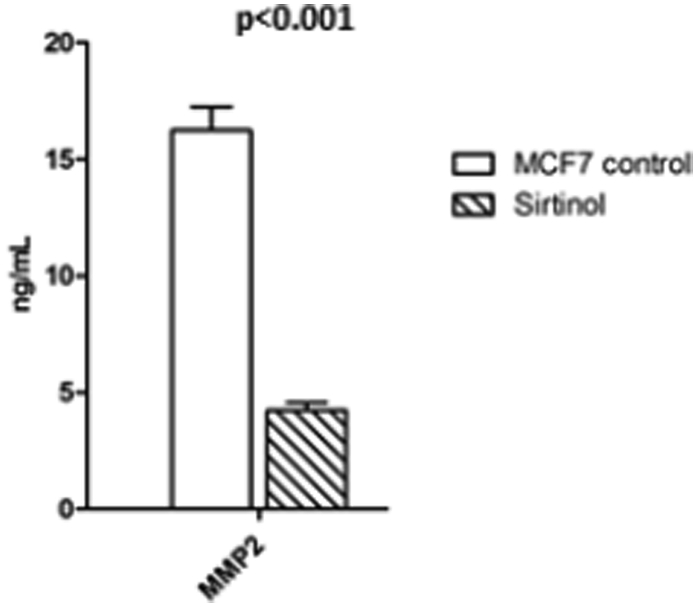
In order to study the regulation of SIRT1 on MMP2 and FOXO3a expression, we treated breast cancer cell line (MCF7) with sirtinol (SIRT1 inhibitor), and found that SIRT1 inhibition suppresses FOXO3a expression (0.27 ± 0.029) and decrease MMP2 level (4.22 ± 0.334) compared to control MCF7 (1.03 ± 0.028, 16.23 ± 1.01, respectively) at P < 0.001 as shown in Figure 2, Figure 3.
Figure 2.
Effect of sirtinol on SIRT1 and FOXO3a protein levels. Parameters were presented on the charts as means ± SD. SIRT1: sirtuin 1, FOXO3a: forkhead box O3a.
Figure 3.
Effect of sirtinol on MMP2 protein levels. Parameters were presented on the charts as means ± SD, MMP2: matrix metalloproteinase 2.
Pearson's correlation analysis
As shown in Table 2, there was a significant inverse correlation between MMP2, FOXO3a expression with age and CEA (P < 0.001, 0.01 and 0.02, 0.001, respectively). SIRT1 was associated directly with tumor grade and stage (P: 0.005 and 0.02, respectively). Also, there was a significant direct correlation between MMP2, FOXO3a expression and tumor stage (P: 0.01 and 0.03, respectively). There was a significant inverse correlation between SIRT1, FOXO3a expression with CA 15.3 (P: 0.03 and 0.002, respectively). SIRT1, MMP2 and FOXO3a were associated directly with lymph node status (P: <0.001, <0.001 and 0.01, respectively). Regarding correlation between SIRT1, MMP2 and FOXO3a expression, there was a significant direct correlation between them (P < 0.001).
Table 2.
Correlation of SIRT1, MMP2, and FOXO3a with other parameters in the malignant group.
| SIRT1 |
MMP2 |
FOXO3a |
||||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| Age | −0.39 | 0.05 | −0.77 | <0.001 | -05 | 0.01 |
| Grade | 0.55 | 0.005 | 0.12 | 0.57 | 0.005 | 0.98 |
| Stage | 0.48 | 0.02 | 0.52 | 0.01 | 0.435 | 0.03 |
| Tumor size | 0.36 | 0.08 | 0.03 | 0.87 | 0.103 | 0.63 |
| CA15.3 | −0.44 | 0.03 | −0.4 | 0.05 | -0.6 | 0.002 |
| CEA | −0.34 | 0.10 | −0.45 | 0.02 | −0.65 | 0.001 |
| Lymph node | 0.67 | <0.001 | 0.71 | <0.001 | 0.51 | 0.01 |
| SIRT1 | – | – | 0.75 | <0.001 | 0.8 | <0.001 |
| MMP2 | 0.75 | <0.001 | – | – | 0.7 | <0.001 |
| FOXO3a | 0.8 | <0.001 | 0.7 | <0.001 | – | – |
CA: cancer antigen, CEA: carcinoembryonic antigen, SIRT1: sirtuin 1, MMP2: matrix metalloproteinases, FOXO3a: forkhead box O3a. Significance was considered at P < 0.05.
Discussion
Breast cancer is one of the most frequent cancers and it is the leading cause of death related to cancer among women worldwide. Death from breast cancer results from distance metastasis rather than primary cancer.14
It has been suggested that SIRT1 has a promoting function in tumor development and progression through the deacetylation of some key cell cycle and apoptosis regulatory proteins like p53 and FOXO leading to suppression of their function.4
The present study showed that there was an extremely high frequency of overexpressed SIRT1 in breast malignant tumor tissues compared to their paired normal tissues and to benign tumor tissues. The expression of SIRT1 in breast cancer tissues is contentious. In accordance to our results, Derr et al,15 Kuo et al16 and Sung et al17 demonstrated an overexpression of the SIRT1 in breast cancer tissue than in normal tissue. In addition, several reports have demonstrated that overexpression of SITR1 is not exclusively present in breast cancer tissue but also was observed in other cancer tissue, including prostate cancer,12 lung cancer,18, 19 gastric cancer,20 and hepatocellular carcinoma.21
On contrary to our results, Cao et al22 and Wang et al23 found a significant lower expression of SIRT1 in breast cancer tissue than in normal tissue. They reported that SIRT1 has an antitumor potential. Thus, SIRT1 may act as a tumor suppressor through its role in DNA damage repair and maintaining genome integrity. Until now, the definite role of SIRT1 is controversial.
Regarding MMP2 and FOXO3a, our results revealed a significant overexpression in breast cancer tissues compared to in benign breast tumor and adjacent normal tissues. In agreement with these results, other studies by Mahmood et al,24 Radenkovic et al,10 Sil et al25 and Sullu et al26 found that MMP2 expression was increased in breast cancer tissues as compared to that of benign tumor and adjacent normal tissues. On the other hand, Jiang et al27 found that only 37% of breast cancer tissue samples expressing high level of FOXO3a. Furthermore, Chen et al28 and Lam et al29 revealed that FOXO3a has been shown to be deregulated in breast cancer.
In the present study, SIRT1 was associated directly with tumor grade, stage and lymph node status. These finding came in agreement with Wu et al5 who found that the expression of SIRT1 was significantly correlated with tumor stage and lymph node status.
In this study, both of MMP2 and FOXO3a were significantly correlated directly with tumor stage and lymph node status which came in accordance with other studies that found a significant correlation between MMP2 and both tumor stage and lymph node metastasis.10, 24 Additionally, Jiang et al27 found that FOXO3a expression was strongly associated with axillary lymph node status and TNM stage. Furthermore, it has been revealed that nuclear FOXO3a was associated with lymph node metastasis and poor survival in invasive ductal breast carcinoma.28
Regarding correlation between SIRT1, MMP2 and FOXO3a expression, there was a significant direct correlation between them.
To the best of our knowledge this is the first study assessing the effect of SIRT1 inhibition on both MMP2 and FOXO3a in breast cancer. In order to study the effect of SIRT1 on MMP2 and FOXO3a expression, the breast cancer cell line (MCF7) was treated by sirtinol (SIRT1 inhibitor). We found that SIRT1 inhibition suppresses MMP2 and FOXO3a expression compared to control MCF7. These finding came in agreement with Lovaas et al12 who found that SIRT1 inhibition using sirtinol suppressed MMP2 expression in both LNCaP and PC3 prostate cancer cells. Furthermore, Kuo et al16 revealed that the cell viability was alleviated by sirtinol in a time- and dose-dependent manner in MCF-7. In addition, Grbesa et al18 found that SIRT1 inhibition via siRNA suppressed cell growth in lung cancer cell lines. These results suggested an association of SIRT1 expression with breast cancer development and pointed out the SIRT1 role in cancer cell growth.
Regarding the effect of SIRT1 on MMP2, SIRT1 has been reported as a positive regulator of MMP2 activity by promoting its expression, stability and activity.30 MMP2 has been found to have potential roles in intravasation and metastasis sustaining neovasculature not solely by stimulating induction of angiogenic factors like vascular endothelial growth factor, but also through proteolytic remodeling activity of the tumor matrix.31 Taken together, SIRT1 inhibition suppresses MMP2 expression and as a result can decrease cancer invasion. Thus, SIRT1 inhibition may represent a potential therapeutic strategy for breast cancer.
Regarding the effect of SIRT1 on FOXO3a, it has been demonstrated that SIRT1 regulates FOXO3 by both inhibiting FOXO3-induced apoptosis and potentiating the ability of FOXO3 to resist oxidative stress.32 The effects of SIRT1 on FOXO vary depending on the FOXO target genes. SIRT1 potentiates the expression of FOXO target genes involved in stress resistance, but on the other hand decreases the transcription of genes involved in apoptosis leading to shift the FOXOs-dependent response away from apoptosis and toward stress resistance. Moreover, it has been shown that the inhibition of SIRT1 enhances the apoptosis triggered by oxidative stress. Thus, SIRT1 inhibition may contribute to the treatment of cancer combined with reactive oxygen species (ROS)-generating anti-cancer drugs.33
Conclusion
SIRT1 inhibition decreased MMP2 and FOXO3a expression in MCF-7 cells, so it may attenuate or dampen cancer invasion and increase tumor cell apoptosis, pointing out the potential role of SIRT1 as promising therapeutic target for breast cancer.
Conflict of interest
No potential conflict of interest was reported by the authors.
Acknowledgment
We sincerely thank Dr. M. Kamel (Clinical Pathology department, National Cancer Institute, Cairo University) for help in collection and pathological analysis of the samples.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Taylor S., Lam M., Pararasa C., Brown J.E., Carmichael A.R., Griffiths H.R. Evaluating the evidence for targeting FOXO3a in breast cancer: a systematic review. Cancer Cell Int. 2015;15(1):1. doi: 10.1186/s12935-015-0156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis C., Siegel R., Bandi P., Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61(6):409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 3.Zani S., Clary B.M. Role for hepatic metastasectomy in stage IV melanoma and breast cancer: re-establishing the surgical modality. Oncology. 2011;25(12):1158–1164. (Williston Park) [PubMed] [Google Scholar]
- 4.Lin Z., Fang D. The roles of SIRT1 in cancer. Genes Cancer. 2013;4(3–4):97–104. doi: 10.1177/1947601912475079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu M., Wei W., Xiao X. Expression of SIRT1 is associated with lymph node metastasis and poor prognosis in both operable triple-negative and non-triple-negative breast cancer. Med Oncol. 2012;29(5):3240–3249. doi: 10.1007/s12032-012-0260-6. [DOI] [PubMed] [Google Scholar]
- 6.Taylor D.M., Maxwell M.M., Luthi-Carter R., Kazantsev A.G. Biological and potential therapeutic roles of sirtuin deacetylases. Cell Mol Life Sci. 2008;65(24):4000–4018. doi: 10.1007/s00018-008-8357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmirotta R., Cives M., Della-Morte D. Sirtuins and cancer: role in the epithelial-mesenchymal transition. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/3031459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nho R.S., Hergert P. FoxO3a and disease progression. World J Biol Chem. 2014;5(3):346–354. doi: 10.4331/wjbc.v5.i3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noel A., Jost M., Maquoi E. Matrix metalloproteinases at cancer tumor-host interface. Semin Cell Dev Biol. 2008;19(1):52–60. doi: 10.1016/j.semcdb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Radenkovic S., Konjevic G., Jurisic V., Karadzic K., Nikitovic M., Gopcevic K. Values of MMP-2 and MMP-9 in tumor tissue of basal-like breast cancer patients. Cell Biochem Biophys. 2014;68(1):143–152. doi: 10.1007/s12013-013-9701-x. [DOI] [PubMed] [Google Scholar]
- 11.Köhrmann A., Kammerer U., Kapp M., Dietl J., Anacker J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: new findings and review of the literature. BMC Cancer. 2009;9:188. doi: 10.1186/1471-2407-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovaas J.D., Zhu L., Chiao C.Y., Byles V., Faller D.V., Dai Y. SIRT1 enhances matrix metalloproteinase-2 expression and tumor cell invasion in prostate cancer cells. Prostate. 2013;73(5) doi: 10.1002/pros.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 15.Derr R.S., van Hoesel A.Q., Benard A. High nuclear expression levels of histone-modifying enzymes LSD1, HDAC2 and SIRT1 in tumor cells correlate with decreased survival and increased relapse in breast cancer patients. BMC Cancer. 2014;14:604. doi: 10.1186/1471-2407-14-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo S.-J., Lin H.-Y., Chien S.-Y., Chen D.-R. SIRT1 suppresses breast cancer growth through downregulation of the Bcl-2 protein. Oncol Rep. 2013;30(1):125–130. doi: 10.3892/or.2013.2470. [DOI] [PubMed] [Google Scholar]
- 17.Sung J.Y., Kim R., Kim J.E., Lee J. Balance between SIRT1 and DBC1 expression is lost in breast cancer. Cancer Sci. 2010;101(7):1738–1744. doi: 10.1111/j.1349-7006.2010.01573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grbesa I., Pajares M.J., Martínez-Terroba E. Expression of sirtuin 1 and 2 is associated with poor prognosis in non-small cell lung cancer patients. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0124670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C., Wang L., Zheng L. SIRT1 expression is associated with poor prognosis of lung adenocarcinoma. Onco Targets Ther. 2015;8:977–984. doi: 10.2147/OTT.S82378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cha E.J., Noh S.J., Kwon K.S. Expression of DBC1 and SIRT1 is associated with poor prognosis of gastric carcinoma. Clin Cancer Res. 2009;15(13):4453–4459. doi: 10.1158/1078-0432.CCR-08-3329. [DOI] [PubMed] [Google Scholar]
- 21.Choi H.N., Bae J.S., Jamiyandorj U. Expression and role of SIRT1 in hepatocellular carcinoma. Oncol Rep. 2011;26(2):503–510. doi: 10.3892/or.2011.1301. [DOI] [PubMed] [Google Scholar]
- 22.Cao Y.-W., Li W.-Q., Wan G.-X. Correlation and prognostic value of SIRT1 and Notch1 signaling in breast cancer. J Exp Clin Cancer Res. 2014;33:97. doi: 10.1186/s13046-014-0097-2. http://www.jeccr.com/content/33/1/97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang R.H., Sengupta K., Li C. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14(4):312–323. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahmood N.A., Fakhoury R.M., Yaseen N.Y., Moustafa M.E. Matrix metalloproteinases MMP2 and MMP9 expression in stages II-III breast cancer in Iraqi women. J Med Biol Sci Res. 2015;1(3):30–37. [Google Scholar]
- 25.Sil H., Moulik S., Singha I., Mandal S.S., Biswas J., Chatterjee A. Activation of matrix metalloproteinase 2(MMP-2) in breast cancer progression. J Tumor. 2015;3(1):292–304. [Google Scholar]
- 26.Sullu Y., Demirag G.G., Yildirim A., Karagoz F., Kandemir B. Matrix metalloproteinase-2 (MMP-2) and MMP-9 expression in invasive ductal carcinoma of the breast. Pathol Res Pract. 2011;207(12):747–753. doi: 10.1016/j.prp.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Y., Zou L., Lu W.-Q., Zhang Y., Shen A.-G. Foxo3a expression is a prognostic marker in breast cancer. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0070746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J., Gomes A.R., Monteiro L.J. Constitutively nuclear FOXO3a localization predicts poor survival and promotes Akt phosphorylation in breast cancer. PLoS One. 2010;5(8) doi: 10.1371/journal.pone.0012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam M., Carmichael A.R., Griffiths H.R. An aqueous extract of Fagonia cretica induces DNA damage, cell cycle arrest and apoptosis in breast cancer cells via FOXO3a and p53 expression. PLoS One. 2012;7:e40152. doi: 10.1371/journal.pone.0040152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong Y., Chippada-Venkata U.D., Oh W.K. Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancers. 2014;6:1298–1327. doi: 10.3390/cancers6031298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deryugina E.I., Quigley J.P. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015;44–46:94–112. doi: 10.1016/j.matbio.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nogueiras R., Habegger K.M., Chaudhary N. Sirtuin 1 and Sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92(3):1479–1514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hori Y.S., Kuno A., Hosoda R., Horio Y. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0073875. [DOI] [PMC free article] [PubMed] [Google Scholar]