Abstract
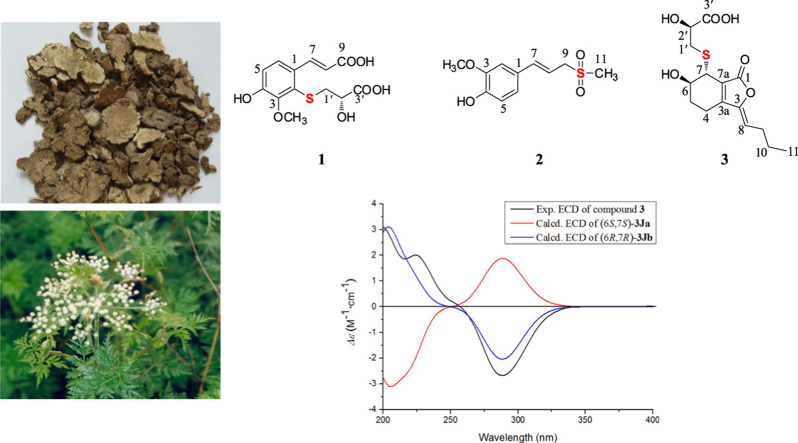
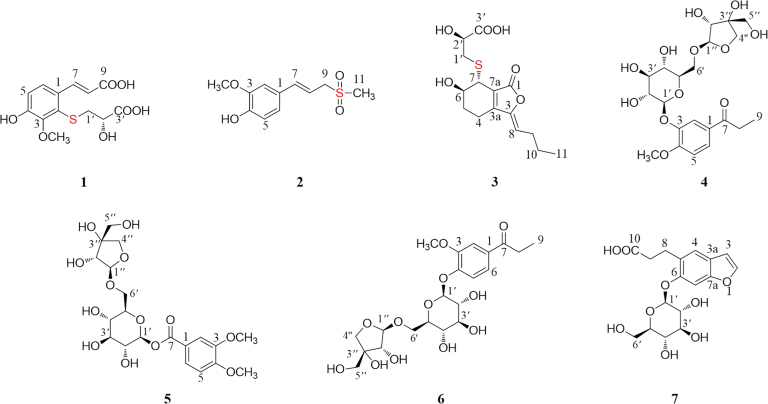
Three new thionic compounds, (S)-2-(2-carboxyl-2-hydroxyethylthio)-ferulic acid (1), (E)-2-methoxy-4-(3-(methylsulfonyl)prop-1-en-1-yl)phenol (2), and thiosenkyunolide C (3), together with two new aromatic glycosides (4 and 5) were isolated from the rhizome of Ligusticum chuanxiong Hort. Two known compounds (6 and 7) were also obtained. Their structures were elucidated based on extensive spectroscopic data (UV, IR, 1D and 2D NMR, and HR-ESI-MS). Furthermore the absolute configurations were established by comparison of their calculated and experimental circular dichroism spectra and by a dimolybdenum tetraacetate [Mo2(AcO)4]-induced circular dichroism procedure. All compounds were evaluated against lipopolysaccharide (LPS)-induced NO production in BV2 cells, and compounds 4 and 5 showed strong inhibitory activities with IC50 values of 2.03 and 3.09 µmol/L, respectively (positive control curcumin, IC50 = 6.17 µmol/L). In addition, compound 1 showed weak proteintyrosine phosphatase-1B (PTP1B) inhibitory activity.
KEY WORDS: Ligusticum chuanxiong Hort., Thionic compounds, Aromatic glycosides, Anti-inflammatory, Natural products
Graphical abstract
1. Introduction
Thionic compounds are common heteroatomic compounds in plants. They have also been detected and isolated from food, fruits and wine1, 2, 3, 4, 5, 6. Notably, they have been shown to possess various pharmacological activities, such as antifungal, anti-inflammatory, anti-helicobacter, antitumor, and antiparasitic activities, as well as being alternative carbonic anhydrase and neurosteroid-like inhibitors7, 8, 9. Chuanxiong, a famous traditional Chinese medicine, is derived from the rhizome of Ligusticum chuanxiong Hort. After years of research, Chuangxiong has been proven to possess excellent activities for the treatment of cardio-cerebral vascular diseases, inflammatory diseases, and tumor10, 11, 12, 13, 14, 15. Of its phytochemical constituents, its phenolic acids, alkaloids, and phthalides have been investigated15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30. In particular, the phthalides, which are present in a high concentration in Chuanxiong, have been reported to exhibit various bioactivities, such as neuroprotective, anti-inflammatory, antimicrobial, and anti-proliferative effects15, 16, 17, 18. In a recent report, several thionic phthalides were isolated from Chuanxiong29. These novel compounds increased the diversity of the known phthalides in Chuanxiong.
To identify additional novel bioactive thionic compounds, the rhizome of L. chuanxiong was investigated. Herein, three new thionic compounds (1–3, Fig. 1) were successfully isolated. In addition, two new aromatic glycosides (4 and 5, Fig. 1) and two known aromatic glycosides (6 and 7) were also isolated. Evaluation of their bioactivity showed that compounds 4 and 5 had strong anti-inflammatory activity against LPS-induced NO production in BV2 microglial cells, and compound 1 showed weak PTP1B inhibitory activity.
Figure 1.
The structures of the isolated compounds 1–7.
2. Results and discussion
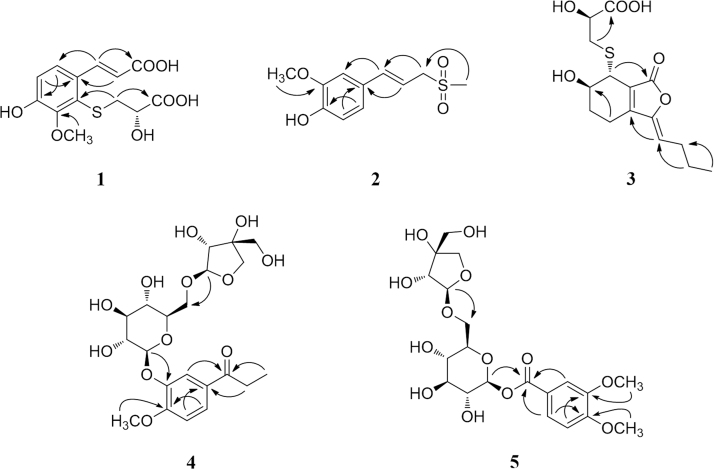
Compound 1 was obtained as a white, amorphous powder. The molecular formula was calculated to be C13H14O7S from the positive HR-ESI-MS [M + H]+ ion peak at m/z 315.0531 (Calcd. for 315.0533), suggesting the presence of 7 degrees of unsaturation. The 1H NMR resonances (Table 1) at δH 7.44 (1H, d, J = 8.5 Hz, H-6), 6.89 (1H, d, J = 8.5 Hz, H-5), 8.18 (1H, d, J = 16.0 Hz, H-7), and 6.25 (1H, d, J = 16.0 Hz, H-8) suggested a tetrasubstituted benzene ring and a trans double bond. In combination with the 13C NMR resonances (Table 1) at δC 128.7, 130.2, 148.7, 152.3, 117.4, 123.3, 142.4, 117.8 and 167.8, these data indicated the presence of a tetrasubstituted cinnamic acid group. Additionally, The HR-ESI-MS data indicated one S atom was present. In combination with the NMR data [δH 3.12 (1H, dd, J = 4.5, 13.0 Hz, H-1′a), 2.97 (1H, dd, J = 8.0, 13.0 Hz, H-1′b), and 3.93 (1H, dd, J = 4.5, 8.0 Hz, H-2′); δC 39.7, 69.7, and 173.9], a mercaptopropionic acid moiety was deduced, and it was attached at C-2 based on the correlation of H-1′/C-2 in the HMBC spectrum (Fig. 2). The correlation of H-OCH3/C-3 confirmed that the methoxy was attached at C-3. Therefore, the planar structure of 1 was determined. The absolute configuration of C-2′ was established by a dimolybdenum tetraacetate [Mo2(AcO)4]-induced circular dichroism procedure31. The diagnostic Cotton effect at approximately 342.5 nm was negative, which indicated the absolute configuration of C-2′ was S. Thus, the structure of 1 was confirmed as (S)-2-(2-carboxyl-2-hydroxyethylthio)-ferulic acid.
Table 1.
1H and 13C NMR data of compounds 1, 3 (in DMSO-d6) and 2 (in methanol-d4).
| Position |
1a |
2a |
3b |
|||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 128.7 | 129.6 | 168.1 | |||
| 2 | 130.2 | 7.05, d (1.5) | 110.6 | |||
| 3 | 148.7 | 149.3 | 147.9 | |||
| 3a | 151.6 | |||||
| 4 | 152.3 | 148.6 | 2.44, m | 16.1 | ||
| 5 | 6.89, d (8.5) | 117.4 | 6.76, d (8.0) | 116.4 | 1.82, m | 22.9 |
| 6 | 7.44, d (8.5) | 123.3 | 6.89, dd (1.5, 8.0) | 121.8 | 4.07, brs | 67.5 |
| 7 | 8.18, d (16.0) | 142.4 | 6.68, d (16.0) | 140.5 | 3.52, brs | 41.8 |
| 7a | 124.3 | |||||
| 8 | 6.25, d (16.0) | 117.8 | 6.11, dt (7.5, 16.0) | 113.7 | 5.46, t (8.0) | 112.4 |
| 9 | 167.8 | 3.95, d (7.5) | 59.6 | 2.27, q (8.0) | 27.6 | |
| 10 | 1.47, m | 21.8 | ||||
| 11 | 2.93, s | 39.6 | 0.91, t (7.5) | 13.7 | ||
| 1′ | 2.97, dd (8.0, 13.0); | 39.7 | 2.87, dd (7.0, 12.5); 3.05 dd (3.0, 12.5) | 37.4 | ||
| 3.12, dd (4.5, 13.0) | ||||||
| 2′ | 3.93, dd (4.5, 8.0) | 69.7 | 4.11, brs | 70.7 | ||
| 3′ | 173.9 | 174.0 | ||||
| -OCH3 | 3.76, s | 59.7 | 3.86, s | 56.5 | ||
500 MHz for 1H NMR, 125 MHz for 13C NMR. b600 MHz for 1H NMR, 150 MHz for 13C NMR.
Figure 2.
Key HMBC correlations of 1—5.
Compound 2 was obtained as a white, amorphous powder. The positive HR-ESI-MS gave a [M + Na]+ ion peak at m/z 265.0504 (Calcd. for 265.0505), which was in accordance with an empirical molecular formula of C11H14O4S. The 1H NMR and 13C NMR spectra of 2 (Table 1) displayed an ABX system [δH 7.05 (1H, d, J = 1.5 Hz, H-2), 6.89 (1H, dd, J = 8.0, 1.5 Hz, H-6), and 6.76 (1H, d, J = 8.0 Hz, H-5); δC 110.6, 121.8, and 116.4]; a trans double bond [δH 6.68 (1H, d, J = 16.0 Hz, H-7) and 6.11 (1H, dt, J = 16.0, 7.5 Hz, H-8); δC 140.5 and 113.7]; a methylene [δH 3.95 (2H, d, J = 7.5 Hz, H-9); δC 59.6] and a methyl group [δH 2.93 (3H, s, H-11); δC 39.6]. Furthermore, a methoxy carbon was observed at δC 56.5 (3′-OCH3). A phenylpropanoid group was suggested by the correlations of H-5/C-1, H-6/C-4, H-7/C-2, H-8/C-1, and H-9/C-7 in the HMBC spectrum (Fig. 2). In conjunction with the key correlation of H-11/C-9, a methylsulfonyl moiety was confirmed, and the phenylpropanoid group was attached to the sulfur atom. Therefore, the structure of 2 was established as (E)-2-methoxy-4-(3-(methylsulfonyl)prop-1-en-1-yl)phenol.
Compound 3 was obtained as a white, amorphous powder, and its molecular formula was established as C15H20O6S from its HR-ESI-MS peak at m/z 329.1060 [M + H]+ (Calcd. 329.1053). The bands in the IR spectrum at 3364, 1747 and 1631 cm–1 suggested the presence of hydroxy, γ-lactone and diene groups, respectively. The 1H NMR spectrum (Table 1) show a double bond resonance at δH 5.46 (1H, t, J = 8.0 Hz, H-8), resonances for five methylene groups at δH 2.44 (2H, m, H-4), 1.82 (2H, m, H-5), 2.27 (2H, q, J = 8.0 Hz, H-9), 1.47 (2H, m, H-10), 2.87 (1H, dd, J = 7.0, 12.5 Hz, H-1′a), and 3.05 (1H, dd, J = 3.0, 12.5 Hz, H-1′b), one methyl group at δH 0.91 (3H, t, J = 7.5 Hz, H-11), one methine resonance at δH 3.52 (1H, brs, H-7), and two oxygenated methine resonances at δH 4.07 (1 H, brs, H-6) and 4.11 (1H, brs, H-2′). The 13C NMR spectrum (Table 1) showed fifteen carbon resonances, including two carboxyl carbons at δC 174.0 (C-3′) and 168.1 (C-1), four olefinic carbons, five methylene carbons, a methine carbon resonance at δC 41.8 (C-7), and two oxygenated methine resonances at δC 67.5 (C-6) and 70.7 (C-2′). A comparison of the NMR data of 3 with those of the known compound thiosenkyunolide A suggested that these two compounds shared a similar skeleton, except in 3 C-11 bears a methyl group, while in thiosenkyunolide A, that position has a hydroxymethyl moiety29. The trans configuration of C-6/7 was confirmed by comparing the chemical shifts and coupling constants seen in the 1H and 13C NMR with those of the known compounds thiosenkyunolide A (trans) and thiosenkyunolide B (cis). Further 2D NMR analysis, including HSQC and HMBC experiments (Fig. 2), confirmed the planar structure of compound 3.
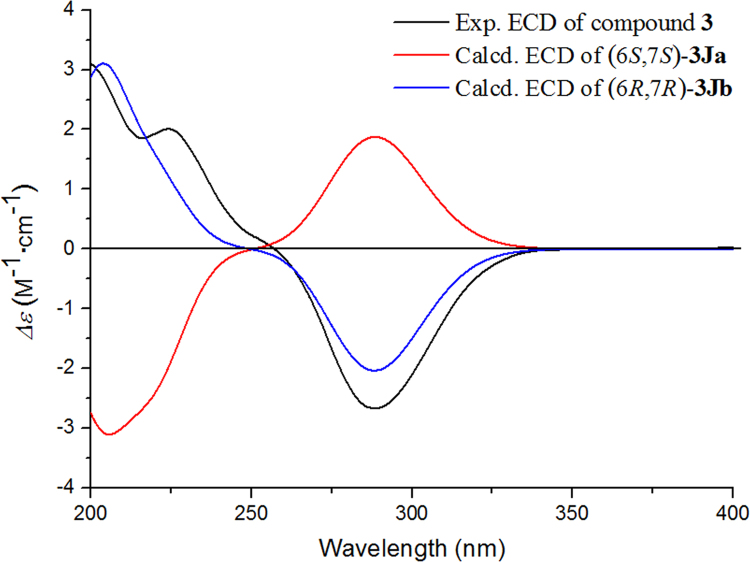
Using the same method as was used for compound 1, the absolute configuration of C-2′ in 3 was established by a dimolybdenum tetraacetate [Mo2(AcO)4]-induced circular dichroism procedure. The diagnostic Cotton effect at approximately 344.5 nm was negative, which indicated the absolute configuration of C-2′ in 3 was S31. The structure of 3 was simplified to 3Ja (6S,7S) and 3Jb (6R,7R) for convenience when calculating the ECD spectra (Fig. 3). By comparing the experimental ECD and ECD calculated based on the time-dependent density functional theory (TD-DFT) method at the B3LYP/6–31+G(d,p) level data (Fig. 4), the absolute configuration of 3 was confirmed as 6R,7R. Finally, the structure of 3 was determined to be (3Z,3aE)-(6R,7R,2′S)-6-hydroxy-7-(2-carboxyl-2-hydroxyethylthio)-3-butylidene-4,5,6,7-tetrahydrophthalide and was named thiosenkyunolide C.
Figure 3.
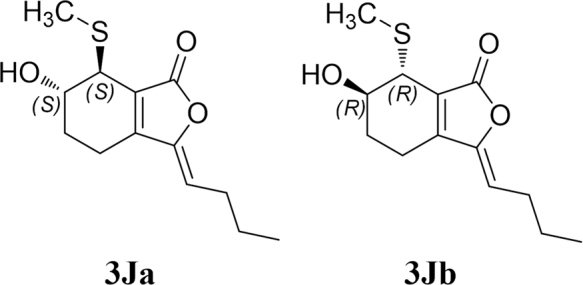
The structures of 3Ja and 3Jb.
Figure 4.
Experimental ECD and calculated ECD spectrum of 3 in MeOH.
The molecular formula of compound 4 was confirmed as C21H30O12 by analysis of its HR-ESI-MS signal at m/z 497.1637 [M + Na]+ (Calcd. 497.1629). A comparison of the NMR data (Table 2) of 4 with those of the known compound 4-[β-d-apiofuranosyl-(1→6)-β-d-glucopyranosyloxy]-3-methoxypropiophenone suggested that these two compounds shared a similar skeleton except for the location of their sugar moieties32. In the HMBC spectrum of 4 (Fig. 2), the correlations of H-1′′/C-6′ and H-1′/C-3 suggested that an apiose moiety was attached at C-6′ of the glucose moiety, and the glucose moiety was attached at C-3 of the aglycone. The configurations of the apiose and glucose were determined to both be d by GC analysis after acidic hydrolysis and chiral derivatization. The β-anomeric configurations were deduced based on the coupling constants of the anomeric protons (Glc: J = 7.5 Hz; Api: J = 3.0 Hz). Finally, compound 4 was confirmed to be 3-hydroxy-4-methoxypropiophenone 3-O-β-d-apiofuranosyl-(1→6)-β-d-glucopyranoside.
Table 2.
1H NMR (500 MHz) and 13C NMR (125 MHz) data of compounds 4 and 5 in DMSO-d6.
| Position |
4 |
5 |
||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 129.5 | 121.2 | ||
| 2 | 7.60, d (2.0) | 114.8 | 7.49, d (2.0) | 112.0 |
| 3 | 146.0 | 148.4 | ||
| 4 | 153.0 | 153.3 | ||
| 5 | 7.08, d (8.5) | 111.7 | 7.09, d (8.5) | 111.1 |
| 6 | 7.63, dd (2.0, 8.5) | 123.0 | 7.65, dd (2.0, 8.5) | 123.8 |
| 7 | 198.8 | 164.3 | ||
| 8 | 2.97, q (7.0) | 30.7 | ||
| 9 | 1.06, t (7.0) | 8.2 | ||
| 1′ | 4.99, d (7.5) | 100.0 | 5.53, d (8.0) | 94.7 |
| 2′ | 3.26, overlap | 73.1 | 3.29, overlap | 72.4 |
| 3′ | 3.27, overlap | 76.6 | 3.29, overlap | 76.3 |
| 4′ | 3.10, m | 69.8 | 3.11, m | 69.6 |
| 5′ | 3.50, m | 75.5 | 3.44, overlap | 76.2 |
| 6′ | 3.39, dd (7.0, 11.0); | 67.4 | 3.42, overlap; | 67.3 |
| 3.85, dd (1.5, 11.0) | 3.85, overlap | |||
| 1′′ | 4.76, d (3.0) | 109.1 | 4.79, d (3.5) | 109.1 |
| 2′′ | 3.66, d (3.0) | 75.9 | 3.74, d (3.0) | 75.6 |
| 3′′ | 78.8 | 78.7 | ||
| 4′′ | 3.56, d (9.0); | 73.3 | 3.56, d (9.5); | 73.2 |
| 3.82, d (9.0); | 3.83, overlap | |||
| 5′′ | 3.31, overlap | 63.4 | 3.30, overlap | 62.9 |
| 3-OCH3 | 3.82, s | 55.6 | ||
| 4-OCH3 | 3.84, s | 55.8 | 3.84, s | 55.7 |
Compound 5 was obtained as a white, amorphous powder, which was found to have a molecular formula of C20H28O13 based on its HR-ESI-MS signal at m/z 477.1611 [M + H]+. Its 1H NMR (Table 2) spectrum indicated an ABX system involving the benzene ring at δH 7.65 (1H, dd, J = 8.5, 2.0 Hz, H-6), 7.49 (1H, d, J = 2.0 Hz, H-2) and 7.09 (1H, d, J = 8.5 Hz, H-5). In addition, the presence of multiple protons between δH 3.11 and δH 3.85 and two doublets at δH 5.53 (1H, d, J = 8.0 Hz, H-1′) and 4.79 (1H, d, J = 3.5 Hz, H-1′′) suggested the presence of two sugar moieties. The 13C NMR spectrum (Table 2) showed six aromatic carbons, an ester carbonyl carbon (δC 164.3), and two methoxy carbons. The remaining eleven carbons were indicative of an apiose moiety (δC 109.1, 78.7, 75.6, 73.2, and 62.9) and a glucose moiety (δC 94.7, 76.3, 76.2, 72.4, 69.6, and 67.3). The HMBC correlations of H-1′/C-7 and H-1′′/C-6′ confirmed that the glucose moiety was attached at C-7 and the apiose was attached at C-6′ of the glucose moiety (Fig. 2). The d-configurations of the glucose and apiose were confirmed through the same method as was used for 4. The β-anomeric configurations were deduced based on the coupling constants of the anomeric protons (Glc: J = 8.0 Hz; Api: J = 3.5 Hz). In addition, the correlations of H-2/C-7, H-2/C-4, H-5/C-3, H-6/C-2, H-OCH3 (δH 3.82)/C-3, and H-OCH3 (δH 3.84)/C-4 proved the structure of the aglycone moiety as shown. Therefore, the structure of 5 was confirmed as β-d-apiofuranosyl-(1→6)-β-d-glucopyranosyl-3,4-dimethoxy-benzoate.
The structures of two known compounds (6 and 7) were identified by comparison of their spectroscopic data with those of the known compounds. They were elucidated as 4-[β-d-apiofuranosyl-(1→6)-β-d-glucopyranosyloxy]-3-methoxypropiophenone32 and cnidioside A33.
All compounds were tested for their anti-inflammatory activity against LPS-induced NO production in BV2 cells and for PTP1B inhibitory activity. The results showed that 4 and 5 have strong anti-inflammatory activities with IC50 values of 2.03 and 3.09 µmol/L, respectively (positive control curcumin, IC50 = 6.17 µmol/L), and compound 1 exhibited a weak PTP1B inhibitory activity with an inhibitory rate of 19.6% at a concentration of 10 µmol/L.
3. Experimental
3.1. General experimental procedures
The optical rotations, UV spectra and ECD spectra were recorded with JASCO P-2000, V650 and J-815 spectrometers (JASCO, Easton, MD, USA), respectively. The infrared spectra were measured on Nicolet 5700 spectrometer (Thermo Scientific, FL, USA). The NMR spectra were recorded with Bruker 500 MHz (Bruker-Biospin, Billerica, MA, USA) and 600 MHz NMR spectrometers (Varian, Inc., Palo Alto, CA, USA). HR-ESI-MS spectra were obtained using an Agilent 6520 HPLC-Q-TOF instrument (Agilent Technologies, Waldbronn, Germany). Preparative HPLC separations were performed using a Shimadzu LC-10AT with an ODS-A column (250 mm × 20 mm, 5 μm; YMC Corp., Kyoto, Japan). An Agilent 1200 series system with an Apollo C18 column (250 mm × 4.6 mm, 5 μm; Alltech Corp., KY, USA) was used for HPLC-DAD analysis. An Agilent 7890 A system with a capillary column (HP-5, 60 m × 0.32 mm, with a 1 μm film; Agilent Technologies Inc., CA, USA) was used for GC analysis. Macroporous resin (Diaion HP-20, Mitsubishi Chemical Corp., Tokyo, Japan), RP-C18 (50 μm, YMC Corp., Kyoto, Japan), and Sephadex LH-20 (Pharmacia Fine Chemicals, Uppsala, Sweden) were used for column chromatography.
3.2. Plant material
The roots of Ligusticum chuanxiong Hort. were collected from Pengzhou Town, Sichuan Province, PRC, in June 2013 and identified by professor Lin Ma A voucher specimen (ID-S-2594) was deposited at the Institute of Materia Medica, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China.
3.3. Extraction and isolation
The powdered rhizome of L. chuanxiong Hort. (100.0 kg) was exhaustively extracted with 80% EtOH at reflux. The solvent was evaporated under reduced pressure, and the residue (23.1 kg) was then successively partitioned with EtOAc and n-BuOH. The n-BuOH-soluble portion (1300 g) was separated on a macroporous resin (Diaion HP-20) column to give five fractions, A–E, using a gradient elution with H2O, 15% ethanol, 30% ethanol, 50% ethanol, and 95% ethanol. Fraction C (103.0 g) was chromatographed on an RP-C18 column eluting with H2O/MeOH (from 100:0 to 0:100) to give 16 fractions (C1–C16) based on HPLC analyses. Fraction C5 was separated by column chromatography over Sephadex LH-20 using H2O as the eluent and was further purified by preparative HPLC (MeOH/H2O, 20:80, v/v, HOAc, 0.2%) to give 4 (67 mg), 5 (5 mg), 6 (17 mg) and 7 (64 mg). Fraction C8 was separated by column chromatography over Sephadex LH-20 using H2O as the eluent and was further purified by preparative HPLC (MeOH/H2O, 30:70, v/v, HOAc, 0.2%) to give 1 (17 mg). Fraction D (48.0 g) was chromatographed on an RP-C18 column eluting with H2O/MeOH (from 95:5 to 0:100) to give 24 fractions (D1–D24) based on HPLC and TLC analyses. Fraction D9 was chromatographed over Sephadex LH-20 with a gradient of increasing MeOH (30%–100%) in H2O and was further purified by preparative HPLC (MeCN/H2O, 30:70, v/v, HOAc, 0.2%) to give 3 (2 mg).
The EtOAc-soluble portion (5.0 kg) was chromatographed on a silica gel column eluting with petroleum ether/ethyl acetate (from 100:0 to 50:50) to give 10 fractions (Ea–Ej) based on HPLC and TLC analyses. Fraction Ej (179.3 g) was separated on a silica gel column eluting with petroleum ether/ethyl acetate (from 100:1 to 1:10) to give 10 fractions (Ej1–Ej10) based on HPLC and TLC analyses. Fraction Ej5 was chromatographed over Sephadex LH-20 eluting with dichloromethane/MeOH (2:1) and was further purified by preparative HPLC (MeCN/H2O, 18:82, v/v, HOAc, 0.2%) to give 2 (18 mg).
3.3.1. (S)-2-(2-Carboxyl-2-hydroxyethylthio)-ferulic acid (1)
White, amorphous powder; [α]20D−7.8 (c 0.10, MeOH); UV (MeOH) λmax (logε) 203 (4.22), 308 (4.07) nm; IR (KBr) νmax 3282, 2975, 2939, 1692, 1626, 1583, 1477, 1415, 1282, 1193, 1095, 948 cm−1; 1H and 13C NMR data, see Table 1; HR-ESI-MS m/z 315.0531 [M + H]+ (Calcd. for C13H15O7S, 315.0533).
3.3.2. (E)-2-Methoxy-4-(3-(methylsulfonyl)prop-1-en-1-yl)phenol (2)
White, amorphous powder; UV (MeOH) λmax (logε) 211 (3.94), 272 (3.79), 299 (3.56) nm; IR (KBr) νmax 3453, 3005, 2943, 1594, 1515, 1465, 1426, 1273, 1238, 1177, 1126, 1031, 974, 880, 822, 798, 777 cm−1; 1H and 13C NMR data, see Table 1; HR-ESI-MS m/z 265.0504 [M + Na]+ (Calcd. for C11H14O4SNa, 265.0505).
3.3.3. Thiosenkyunolide C (3)
White, amorphous powder; [α]20D —32.0 (c 0.10, MeOH); UV (MeOH) λmax (logε) 277 (3.90) nm; IR (KBr) νmax 3364, 2961, 1747, 1414, 1242, 1093, 1047, 952 cm−1; CD (MeOH) 224 (Δε +2.01), 289 (Δε —2.67) nm; 1H and 13C NMR data, see Table 1; HR-ESI-MS m/z 329.1060 [M + H]+ (Calcd. for C15H21O6S, 329.1053).
3.3.4. 3-Hydroxy-4-methoxypropiophenone 3-O-β-d-apiofuranosyl-(1→6)-β-d-glucopyranoside (4)
White, amorphous powder; [α]20D —68.7 (c 0.10, MeOH); UV (MeOH) λmax (logε) 201 (4.09), 224 (3.99), 271 (3.80) nm; IR (KBr) νmax 3409, 2934, 2881, 1735, 1669, 1599, 1515, 1459, 1269, 1174, 930 cm−1; 1H and 13C NMR data, see Table 2; HR-ESI-MS m/z 497.1637 [M + Na]+ (Calcd. for C21H30O12Na, 497.1629).
3.3.5. β-d-Apiofuranosyl-(1→6)-β-d-glucopyranosyl-3,4-dimethoxy-benzoate (5)
White, amorphous powder; [α]20D —41.7 (c 0.10, MeOH); UV (MeOH) λmax (logε) 216 (4.17), 275 (3.79) nm; IR (KBr) νmax 3417, 2974, 2924, 1717, 1697, 1609, 1410, 1380, 1240, 1194, 975 cm−1; 1H and 13C NMR data, see Table 2; HR-ESI-MS m/z 477.1611 [M + H]+ (Calcd. for C20H29O13, 477.1603).
3.4. ECD calculations
Briefly, conformational analyses of 3Ja and 3Jb were carried out via systematic searching in Discovery Studio (version 16.1.0.15350) using the MMFF94 force field. Conformers with Boltzmann distributions over 1% were chosen for ECD calculations. Ground-state geometries were optimized at the B3LYP/6–31+G(d,p) level in the gas phase using the Gaussian 09 program (Gaussian Inc., Wallingford, CT, USA). All quantum computations were performed on an IBM cluster machine located at the High Performance Computing Center of Peking Union Medical College. The energies, oscillator strengths, and rotational strengths (velocity) of the electronic excitations were calculated using TD-DFT methodology at the B3LYP/6–31+G(d,p) level in methanol. The calculated ECD spectra were simulated by the overlapping Gaussian function (half the bandwidth at 1/e peak height, 0.25 eV), and their lowest energy conformers were generated by Boltzmann weighting.
3.5. Anti-inflammatory activity assay
The anti-inflammatory activity was evaluated in microglial BV2 cell, which were purchased from the Cell Culture Center at the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. Curcumin was used as a positive control drug. The BV2 microglial cells were cultured at 37 °C (5% CO2, 100% relative humidity) and maintained in DMEM containing 10% FCS (fetal calf serum). The cells were transferred to 96-well plates and treated with different samples (each compound was tested at three concentrations) after incubation for 24 h. Then, the cells in the 96-well plates were stimulated with LPS (Sigma—Aldrich) for another 24 h. Then, 100 μL aliquots of the supernatants were added to 100 μL of Griess reagent [0.1% (naphthyl)ethylenediamine and 1% sulfanilamide in a 5% H3PO4 solution], and the mixture was left to stand at room temperature for 20 min. NO production was measured by recording the concentration of nitrite in the supernatant. The absorbances were measured at 540 nm. Curcumin was used as the positive control drug.
3.6. PTP1B inhibitory activity assay
The pellets of recombinant GST-hPTP1B bacteria were purified on a GST bead column. The dephosphorylation of para-nitrophenyl phosphate (p-NPP) to para-nitrophenol was catalyzed by PTP1B. Enzyme activity using an end-point assay, which intensifies the yellow color, was measured at a wavelength of 405 nm. Compounds were dissolved in 100% dimethyl sulfoxide (DMSO). The DMSO solutions were diluted to 10% DMSO prior to use in the assays. Selected compounds were first evaluated for their ability to inhibit PTPase at a concentration of 10 µmol/L at 30 °C for 10 min in a reaction system with 3 mmol/L p-NPP in HEPES assay buffer (pH 7.0). The reaction was initiated by the addition of the enzyme. The reaction was quenched by the addition of 1 mol/L NaOH. A microplate spectrophotometer was used to determine the amount of p-nitrophenol present.
3.7. Determination of the absolute configurations of the sugars
Compound 4 (2 mg) was dissolved in 1 mol/L CF3COOH (14 mL), and then the mixture was heated at 70 °C for 1 h. The mixture was then extracted three times with EtOAc, and the aqueous layer was freeze-dried to obtain the residue. Using a method reported in the literature34, the residue was dissolved in anhydrous pyridine (2 mL), l-cysteine methyl ester hydrochloride (4 mg) was added, and then the mixture was heated in a water bath (60 °C) for 1 h. After the reaction, the solution was dried under vacuum, N-trimethylsilylimidazole (1 mL) was added, and the solution was heated in a water bath (60 °C) for 1 h. The reaction mixture was extracted three times with H2O/n-hexane, and the n-hexane layer was analyzed using GC under the following conditions: injection temperature, 300 °C; detector temperature (FID), 300 °C; capillary column, HP-5 (60 m × 0.32 mm, Dikma); initial oven temperature of 200 °C, increased to 260 °C at a rate of 10 °C/min, and the final temperature was maintained for 30 min; and carrier gas, N2. Compound 5 was also evaluated by the same procedure, and all its sugars were determined to be in the d-configuration.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81773588).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.apsb.2018.04.002.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Thomas C., Mercier F., Tournayre P., Martin J.L., Berdagué J.L. Identification and origin of odorous sulfur compounds in cooked ham. Food Chem. 2014;155:207–213. doi: 10.1016/j.foodchem.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Conde-Martínez N., Sinuco D.C., Osorio C. Chemical studies on curuba (Passiflora mollissima (Kunth) L. H. Bailey) fruit flavour. Food Chem. 2014;157:356–363. doi: 10.1016/j.foodchem.2014.02.056. [DOI] [PubMed] [Google Scholar]
- 3.Moreira N., De Pinho P.G., Santos C., Vasconcelos I. Relationship between nitrogen content in grapes and volatiles, namely heavy sulphur compounds, in wines. Food Chem. 2011;126:1599–1607. doi: 10.1016/j.foodchem.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 4.Schmidberger P.C., Schieberle P. Characterization of the key aroma compounds in White Alba Truffle (Tuber magnatum pico) and Burgundy Truffle (Tuber uncinatum) by means of the sensomics approach. J Agric Food Chem. 2017;65:9287–9296. doi: 10.1021/acs.jafc.7b04073. [DOI] [PubMed] [Google Scholar]
- 5.Nohara T., Fujiwara Y., Kudo R., Yamaguchi K., Ikeda T., Murakami K. Isolation and characterization of new onionins A2 and A3 from Allium cepa, and of onionins A1, A2, and A3 from Allium fistulosum. Chem Pharm Bull. 2014;62:1141–1145. doi: 10.1248/cpb.c14-00461. [DOI] [PubMed] [Google Scholar]
- 6.Xu K., Yang P.F., Yang Y.N., Feng Z.M., Jiang J.S., Zhang P.C. Direct assignment of the Threo and Erythro configurations in polyacetylene glycosides by 1H NMR spectroscopy. Org Lett. 2017;19:686–689. doi: 10.1021/acs.orglett.6b03855. [DOI] [PubMed] [Google Scholar]
- 7.Siddique Y.H., Beg T., Ara G., Gupta J., Afzal M. Antigenotoxic effect of allicin against estradiol-17β-induced genotoxic damage in cultured mammalian cells. Nat Prod Res. 2010;24:1087–1094. doi: 10.1080/14786410802263568. [DOI] [PubMed] [Google Scholar]
- 8.Nikitina L.E., Artemova N.P., Startseva V.A., Fedyunina I.V., Klochkov V.V. Biological activity of S-containing monoterpenoids. Chem Nat Compd. 2017;53:811–819. [Google Scholar]
- 9.Slavikova B., Chodounska H., Nekardova M., Vyklicky V., Ladislav M., Hubalkova P. Neurosteroid-like inhibitors of N-methyl-d-aspartate receptor: substituted 2-sulfates and 2-hemisuccinates of perhydrophenanthrene. J Med Chem. 2016;59:4724–4739. doi: 10.1021/acs.jmedchem.6b00079. [DOI] [PubMed] [Google Scholar]
- 10.Li C.M., Guo Y.Q., Dong X.L., Li H., Wang B., Wu J.H. Ethanolic extract of rhizome of Ligusticum chuanxiong Hort. (chuanxiong) enhances endothelium-dependent vascular reactivity in ovariectomized rats fed with high-fat diet. Food Funct. 2014;5:2475–2485. doi: 10.1039/c4fo00211c. [DOI] [PubMed] [Google Scholar]
- 11.Li B.H., Xu X., Wang X., Yu H., Li X.X., Tao W.Y. A systems biology approach to understanding the mechanisms of action of Chinese herbs for treatment of cardiovascular disease. Int J Mol Sci. 2012;13:13501–13520. doi: 10.3390/ijms131013501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L., Ning Z.Q., Shan S., Zhang K., Deng T., Lu X.P. Phthalide lactones from Ligusticum chuanxiong inhibit lipopolysaccharide-induced TNF-α production and TNF-α-mediated NF-ĸB activation. Planta Med. 2005;71:808–813. doi: 10.1055/s-2005-871231. [DOI] [PubMed] [Google Scholar]
- 13.Xie X., Tian Y., Yin S., Lin Y., Tan G. Anticancer effects of Ligusticum chuanxiong Hort. alcohol extracts on HS766T cell. Afr J Tradit Complement Altern Med. 2013;10:542–546. doi: 10.4314/ajtcam.v10i6.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun K., Fan J.Y., Han J.Y. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm Sin B. 2015;5:8–24. doi: 10.1016/j.apsb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J., Lu X.Q., Zhang C., Lu J., Li G.Y., Lin R.C. Anti-inflammatory ligustilides from Ligusticum chuanxiong Hort. Fitoterapia. 2013;91:21–27. doi: 10.1016/j.fitote.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Miao C.P., Wu S.H., Luo B.Z., Wang J., Chen Y.W. A new sesquiterpenoid from Ligusticum chuanxiong Hort. Fitoterapia. 2010;81:1088–1090. doi: 10.1016/j.fitote.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Lim L.S., Shen P., Gong Y.H., Yong E.L. Dimeric progestins from rhizomes of Ligusticum chuanxiong. Phytochemistry. 2006;67:728–734. doi: 10.1016/j.phytochem.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Huang J., Lu X.Q., Lu J., Li G.Y., Wang H.Y., Li L.H. Two new phthalides with BuChE inhibitory activity from Ligusticum chuanxiong. J Asian Nat Prod Res. 2013;15:1237–1242. doi: 10.1080/10286020.2013.825610. [DOI] [PubMed] [Google Scholar]
- 19.Yang N.Y., Ren D.C., Duan J.A., Xu X.H., Xie N., Tian L.J. Ceramides and cerebrosides from Ligusticum chuanxiong Hort. Helv Chim Acta. 2009;92:291–297. [Google Scholar]
- 20.Kim M., Kim S.O., Lee M., Lee J.H., Jung W.S., Moon S.W. Tetramethylpyrazine, a natural alkaloid, attenuates pro-inflammatory mediators induced by amyloid β and interferon-γ in rat brain microglia. Eur J Pharmacol. 2014;740:504–511. doi: 10.1016/j.ejphar.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 21.Li S.L., Chan S.S.K., Lin G., Ling L., Yan R., Chung H.S. Simultaneous analysis of seventeen chemical ingredients of Ligusticum chuanxiong by on-line high performance liquid chromatography-diode array detector-mass spectrometry. Planta Med. 2003;69:445–451. doi: 10.1055/s-2003-39709. [DOI] [PubMed] [Google Scholar]
- 22.Li Y.H., Peng S.L., Zhou Y., Yu K.B., Ding L.S. Two new phthalides from Ligusticum chuanxiong. Planta Med. 2006;72:652–656. doi: 10.1055/s-2006-931560. [DOI] [PubMed] [Google Scholar]
- 23.Naito T., Katsuhara T., Niitsu K., Ikeya Y., Okada M., Mitsuhashi H. Two phthalides from Ligusticum chuangxiong. Phytochemistry. 1992;31:639–642. [Google Scholar]
- 24.Naito T., Niitsu K., Ikeya Y., Okada M., Mitsuhashi H. A phthalide and 2-farnesyl-6-methyl benzoquinone from Ligusticum chuangxiong. Phytochemistry. 1992;31:1787–1789. [Google Scholar]
- 25.Naito T., Ikeya Y., Okada M., Mistuhashi H., Maruno M. Two phthalides from Ligusticum chuanxiong. Phytochemistry. 1996;41:233–236. [Google Scholar]
- 26.Yang J., Feng X.L., Yu Y., Wang Q., Zou J., Wang C.X. Novel phthalide derivatives identified from Ligusticum chuanxiong (Chuanxiong) Chin Med. 2016;11:10. doi: 10.1186/s13020-016-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei W., Xu W., Yang X.W. Two new phthalide dimers from the rhizomes of Ligusticum chuanxiong. J Asian Nat Prod Res. 2017;19:704–711. doi: 10.1080/10286020.2016.1275584. [DOI] [PubMed] [Google Scholar]
- 28.Wei W., Wu X.W., Yang X.W. Novel phthalide derivatives from the rhizomes of Ligusticum chuanxiong and their inhibitory effect against lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophage cells. RSC Adv. 2016;6:61037–61046. [Google Scholar]
- 29.Zhang X., Han B., Feng Z.M., Yang Y.N., Jiang J.S., Zhang P.C. Phthalide derivatives from Ligusticum chuanxiong. RSC Adv. 2017;7:37478–37486. [Google Scholar]
- 30.Chang X.L., Jiang Z.Y., Ma Y.B., Zhang X.M., Tism K.W.K., Chen J.J. Two new compounds from the roots of Ligusticum chuanxiong. J Asian Nat Prod Res. 2009;11:805–810. doi: 10.1080/10286020903071068. [DOI] [PubMed] [Google Scholar]
- 31.Snatzke G., Wagner U., Wolff H.P. Circulardichroism—LXXV1: cottonogenic derivatives of chiral bidentate ligands with the complex [Mo2(O2CCH3)4] Tetrahedron. 1981;37:349–361. [Google Scholar]
- 32.Yuan Z., Tezuka Y., Fan W., Kadota S., Li X. Constituents of the underground parts of Glehnia littoralis. Chem Pharm Bull. 2002;50:73–77. doi: 10.1248/cpb.50.73. [DOI] [PubMed] [Google Scholar]
- 33.Chem C.C., Huang Y.L., Huang F.I., Wang C.W., Ou J.C. Water-soluble glycosides from Ruta graveolens. J Nat Prod. 2001;64:990–992. doi: 10.1021/np000582y. [DOI] [PubMed] [Google Scholar]
- 34.Gan M.L., Liu M.T., Gan L.S., Lin S., Liu B., Zhang Y.L. Dammarane glycosides from the root of Machilus yaoshansis. J Nat Prod. 2012;75:1373–1382. doi: 10.1021/np300310a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material