Abstract
The C/EBP-related proteins (C/EBPα, CRP1, C/EBPβ, and C/EBPδ) form a subfamily of bZIP (basic region/leucine zipper) transcription factors that display sequence homology within the bZIP domain. The conserved basic region contains two motifs that exhibit significant homology to the bipartite nuclear localization signal (NLS) first described in nucleoplasmin. CRP1 and C/EBPβ proteins bearing deletions of the basic region accumulate in the cytoplasm, in contrast to their normal nuclear location. Analysis of chimeric proteins consisting of CRP1 basic region sequences fused to β-galactosidase revealed that the CRP1 basic region contains a single NLS that differs from conventional bipartite signals in two ways. First, mutation of a pair of arginine residues at the N-terminus of the proposed NLS does not disrupt its function. Second, the CRP1 NLS requires additional nonbasic residues at its C-terminus. A basic residue within the CRP1 NLS that is not conserved within the C/EBP family is occupied instead by an uncharged residue in C/EBPα and C/EBPβ. When this nonconserved arginine residue was changed to alanine the CRP1 NLS behaved as a classical bipartite signal, suggesting that bipartite NLSs are present in all family members but that NLSs of the individual members differ slightly. Additionally, mutation of critical NLS residues in the intact CRP1 and C/EBPβ proteins showed that these elements exhibit more bipartite-like characteristics when present in their normal sequence context. Finally, we observed that a C/EBPβ protein lacking its NLS can be localized to the nucleus when coexpressed with C/EBPα, indicating that a single NLS is sufficient to promote nuclear transport of a bZIP dimer.
Keywords: C/EBP proteins, CRP1 (C/EBPε), C/EBPβ, Nuclear localization signals, Basic region, bZIP
C/EBPα, CRP1 (C/EBPε), C/EBPβ (CRP2, NF-IL6, IL-6DBP, LAP) and C/EBPδ (CRP3, NF-IL6β) comprise the C/EBP family of basic region/ leucine zipper (bZIP) transcription factors [reviewed in (20)]. The C/EBP proteins are most highly related in their DNA binding domains, including a perfectly conserved segment of 17 amino acids within the basic region and significant homology in the adjacent leucine zipper. Consequently, each C/EBP protein binds an identical DNA motif and can heterodimerize with the other family members (6,55). C/EBPα has been proposed to play a major role in the activation of tissue-specific gene expression during the differentiation of various cell types, including adipocytes, hepatocytes, and neutrophils (20,29,58). C/EBPβ and C/EBPδ are also important regulators of differentiation in adipocytes (29) and myeloid cells (36), but appear to be primarily involved in the regulation of inducible genes, such as the acute phase reactants in liver and inflammatory cytokine genes in macrophages (39). CRP1 is exclusively expressed in the hematopoietic cells although its function is currently unknown (2).
Structure-function studies have localized peptide domains in each of the C/EBP proteins that control sequence-specific DNA binding and dimerization (26) and transcriptional activation (15,35,37,54). In adition, two separate domains have been identified in C/EBPβ that regulate its DNA binding and transcriptional activities (24,54). The activity of a number of transcription factors may be regulated by modulation of their intracellular location [for reviews see (18,48)] and this mechanism has also been shown to regulate the activity of C/EBP proteins. C/EBPβ has been reported to undergo translocation from the cytoplasm to the nucleus in PC-12 cells exposed to forskolin (31), in macrophages stimulated with lipopolysaccharide (LPS) (23) and in hepatocytes treated with TNF-α (56). In addition, the nuclear/ cytoplasmic distribution of C/EBPα protein has been proposed to change during differentiation of intestinal enterocytes (3,7). Both C/EBPα and C/ EBPβ are phosphoproteins (30,31,34,46,53), and the subcellular distribution of C/EBPβ protein has been correlated with changes in phosphorylation (31), although the specific sites of phosphorylation have not been identified. As a first step towards understanding the mechanisms of regulated nuclear localization of C/EBP proteins, we have begun to identify the amino acid sequences that direct nuclear localization of this family of proteins.
Proteins targeted to the nucleus generally contain discrete sequences, termed nuclear localization signals (NLS), which are recognized and bound by a cytoplasmic receptor complex that transports the proteins to the nuclear pore. This receptor, known as the NLS binding protein (NBP), consists of a heterodimeric complex of importin α and β (16). Although a number of other proteins have been identified that are capable of binding to NLSs, the importin complex is the only functionally characterized NBP thus far (40). Importin α is the NLS binding component of this complex although the specific residues involved in NLS recognition are poorly characterized. The ability of proteins with divergent NLS sequences to enter the nucleus may be explained in higher eukaryotes by the existence of a number of related genes encoding importin α-like proteins (9). Recognition of the nuclear pore is mediated by importin β (17,33). Translocation across the nuclear pore is an energy-dependent step that is mediated by Ran, a guanosine triphosphatase, and ppl5 [reviewed in (16)].
A specific NLS was first defined in the large T-antigen protein of SV40 (21,27) and the sequence requirements for this signal have been extensively characterized. The minimal T-antigen NLS has been identified as the heptapeptide PKK-KRKV and this element is sufficient to direct a heterologous, nonnuclear protein to the nucleus. Several other proteins have been identified that contain SV40-like NLSs, and specific rules have been established to define the sequence of this element [reviewed in (4,25)]. A second type of NLS was revealed from studies of the Xenopus nucleoplasmin and N1 proteins (41). This element consists of two clusters of basic amino acids separated by a 10-amino acid spacer generally conforming to the 17 amino acid consensus sequence BB-(10-11aa)-B(3/5) or B(4/5), where B is either lysine or arginine. Although mutations in the spacer can affect NLS function, a spacer consensus sequence has not been demonstrated and the frequent presence of helix-destabilizing amino acids such as proline in the spacer sequence indicates that bipartite NLSs are unlikely to possess α-helical structure (12). A limited number of other NLSs that do not conform to either of the main types have also been identified (25). Nuclear localization signals often overlap nucleic acid binding domains in transcription factors (4,12,25). The colocalization of domains controlling two of the main functions of transcription factors might allow similar regulatory mechanisms to control two separate processes.
We have characterized sequences in the basic region of CRP1 and other members of the C/EBP family that are responsible for nuclear partitioning. Although two potential bipartite NLSs exist in the basic region, we show that only one functions to direct nuclear translocation. By using deletion and point mutagenesis, we identify residues critical for NLS function in CRP1 and C/EBPβ. We also demonstrate that C/EBPβ lacking a NLS may be localized to the nucleus by the coexpression of intact C/EBPα, indicating that C/EBP proteins may heterodimerize in the cytoplasm.
MATERIALS AND METHODS
C/EBPβ and CRP1 Expression Vectors
pMEX-C/EBPβ has been described previously as pMEX-CRP2 (55). Basic region deletions were constructed in C/EBP/3 by removal of either a 27-bp FspI fragment to generate pMEX-C/EBPβ-ΔBR09 [resulting in the loss of amino acids 217-225, see Fig. 1; numbering as in (55)] or a 63-bp RsaI/FspI fragment to generate pMEX-C/EBPβ-ΔBR21 (resulting in the loss of amino acids 205–225). The CRP1 expression vector pMEX-CRPl was constructed by using PCR to insert a Ncol site at the methionine codon [amino acid 1 (55)] and a HindIII site immediately following the termination codon of the rat CRP1 gene. Oligonucleotide primers used for the introduction of restriction sites consisted of a 5′ “clamp” sequence followed by the restriction site and 12–15 bases of homology to the target sequence. The resulting 1314-bp PCR fragment was inserted into pMEX cut with NcoI and HindIII. These sequences contain a 259-amino acid open reading frame interrupted by a single intron, and a 29-kDa CRP1 protein was detected when this construct was introduced into HeLa cells (see Fig. 2). pMEX-CRP1ΔBR32 was generated by the removal of a 96-bp RsaI fragment encoding amino acids 176-207 (i.e., most of the CRP1 basic region). pMEX-C/EBPβδ-BR09 + BR and pMEX-C/EBPβΔBR21 +BR were constructed by inserting this 96-bp RsaI fragment in frame into an MscI site at the 5′ end of C/EBPβ. Point mutations were introduced into the basic regions of CRP1 and C/EBPβ using a previously described PCR-based method (54). Briefly, a conserved FspI site located between the two basic clusters of motif A (Fig. 1) was changed by PCR to a BspEI site. Mutations were introduced into codons within each basic cluster of motif A and the resulting fragments were reintroduced into the appropriate expression vector. Mutagenic oligonucleotides contained 12 bases of exact homology on either side of the codon(s) to be changed. Plasmids bearing mutations in codons in each half of the bipartite signal were generated by combining mutant fragments at the BspEI site in the same expression vector.
FIG. 1.

The basic regions of CRP1 and C/EBPβ contain two motifs with homology to a bipartite NLS. The bipartite NLSs from nucleoplasmin and N1 display basic residues that align with the bZIP basic region consensus. The conservation of these sequences in the four C/EBP proteins is shown by shading. The putative C/EBP NLS is designated motif A. Due to the extended basic region of C/EBP proteins, each contains a second element (motif B) that is homologous to the bipartite consensus. Deletion mutants that remove one or both motifs from C/EBPβ and CRP1 are shown beneath the wild-type sequences. Note that a substitution of a cysteine for a tyrosine residue is introduced in C/EBPβΔR21. Basic residues that are conserved among the four C/EBP proteins are indicated by plus (+) signs.
FIG. 2.
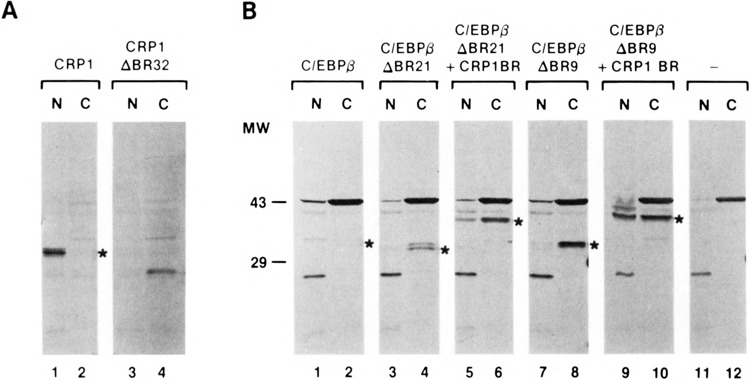
The basic regions of CRP1 and C/EBPβ direct nuclear localization. Wild-type CRP1 and C/EBPv and the deletion mutants described in Fig. 1, or C/EBPβ deletion mutants with the CRP1 basic region fused to their N-termini, were expressed in HeLa cells. The cells were harvested and nuclear (N) and cytoplasmic (C) fractions prepared and analyzed by immunoblotting using antisera specific to CRP1 (A) or C/EBPβ (B). Antigen-antibody complexes were detected using the ProtoBlot colorimetric detection kit (Promega) and are indicated with asterisks. Additional bands represent cross-reactive material recognized by the polyclonal rabbit antisera.
β-Gal Plasmids
pMβ was constructed by inserting the MSV LTR upstream of the bacterial lacZ in the plasmid pPD1.27 (14). pMβ(T) contains the SV40 T-antigen NLS inserted as a KpnI fragment close to the N-terminus of the lacZ gene in pM0. The 96-bp RsaI fragment containing the CRP1 basic region was inserted in frame at an adjacent SmaI site to generate pMβ(CRPl BR). A series of lacZ: CRP1 chimeric proteins was constructed that contained wild-type and mutant sequences derived from the CRP1 basic region. CRP1 sequences were generated by PCR using primers that carried a KpnI cloning site and the resultant fragments were inserted at the KpnI site of pMβ. The orientation and sequence of each insert was determined. In each hybrid protein the inserted segment is preceded by the sequence Pro-Arg-Val-Pro and followed by Val-Pro-Val-Gly. Expressed proteins used in these studies were named according to the following convention: 1) the C/EBP protein being studied (Cl for CRP1, β for C/ EBPβ); 2) whether the sequences are wild-type (WT) or mutant (M); 3) a number indicating the progression through a series of deletions; and 4) a G to indicate whether the protein is a β-gal fusion.
Protein Expression and Western Blot Analysis
HeLa cells were maintained in DMEM (4.5 g/1 glucose) (BioWhittaker) + 10% FetalClone (Hyclone, Inc.). Transfections were carried out by the calcium phosphate coprecipitation method as described previously (55), except that the DNA: CaPO4 coprecipitate was left on the cells for 24 h; the cells were then washed in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KC1, 4.3 mM Na2HPO4, 1.4 mM KH2PO4), and incubated in fresh medium for 18–36 h. After 2 days the cells were harvested and fractionated into nuclear and cytoplasmic fractions by a modified Dignam procedure (11,28). The cells were washed once with PBS, resuspended in 1 packed cell volume of buffer A [10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KC1, 0.5 mM DTT] and allowed to swell on ice for 20 min. The cells were lysed by passing eight times through a 26-gauge needle and crude nuclei were sedimented by centrifugation at 15,000 × g. The cytoplasmic fraction was transferred to a fresh tube and an equal volume of 2 × Laemmli sample buffer [LSB: 0.125 M Tris-HCl (pH 6.8), 4% SDS, 20% glycerol, 10% 2-mercaptoethanol] was added. The nuclear pellet was resuspended in 1 × LSB and sonicated. Equivalent amounts of nuclear and cytoplasmic factions were electrophoresed through 12% SDS-PAGE and transferred to Immobilon-P (Millipore) or NitroME nitrocellulose (Micron, Inc.) membranes. Two different rabbit antipeptide antisera were used to detect CRP1: the first was prepared in our laboratory and was directed against amino acids 234–247 of CRP1, and the second was a commercially available antiserum directed against amino acids 228–249 [Santa Cruz, Biotechnology, Inc.; numbering as in (55)]. The C/EBPβ antiserum was directed against amino acids 1–14 and has been described previously (55). Immune complexes were visualized using either the colorimetric ProtoBlot Western Blot system (Promega) or the SuperSignal CL-HRP chemiluminescent kit (Pierce).
β-Gal Staining
β-Gal expression plasmids were transfected into HeLa cells as described above in 60-mm dishes. Two days after transfection the cells were washed three times with PBS and fixed at room temperature in 2% paraformaldehyde, 0.1 M piperazine-N,N′ bis[2-ethanesulfonic acid] (PIPES), pH 6.8. After 30 min the cells were washed three times with PBS and β-gal staining solution (1 mg/ml X-Gal, 5 mM K-ferricyanide, 5 mM K-ferrocyanide, 2 mM MgCl2, 137 mM NaCl, 2.7 mM KC1, 4.3 mM Na2HPO4, 1.4 mM KH2PO4) was added. The cells were incubated at 37°C and the appearance of blue staining was monitored by light microscopy. Once the staining had become intense, the staining solution was removed and the cells were washed in PBS. Cells were photographed using a Nikon Microphot-FXA microscope.
RESULTS
The Basic Regions of C/EBP Proteins Contain Two Putative Nuclear Localization Signals
The basic regions from the C/EBP-related proteins were compared to the bipartite NLSs from nucleoplasmin and N1 (41). The basic region of each C/EBP protein contains two motifs, denoted motifs A and B, that exhibit similarity to the consensus bipartite NLS (Fig. 1). Motif A is encompassed within the consensus basic region sequence that is shared by all bZIP proteins. Due to the high degree of sequence conservation among bZIP proteins within the basic region, each contains a bipartite NLS-like sequence in this region (12). Unlike most bZIP proteins, the basic region of C/ EBP family members extends N-terminally and, consequently, each C/EBP family member contains a second bipartite NLS-like sequence (motif B) that partially overlaps motif A.
To determine whether nuclear localization of CRP1 and C/EBPβ is dependent on basic region sequences, deletion mutants were constructed that remove portions of their basic regions (Fig. 1). C/ EBPβ mutants lacking either 9 or 21 amino acids and a CRP1 mutant lacking 32 amino acids were transiently expressed in HeLa cells. Nuclear and cytoplasmic fractions were prepared and the cellular distribution of the overexpressed proteins was determined by Western blot analysis (Fig. 2). Wild-type C/EBPβ and CRP1 were found almost exclusively in the nucleus (Fig. 2A, lanes 1 and 2; Fig. 2B, lanes 1 and 2). Deletions that simultaneously affect motifs A and B (CRP1ABR32 and C/EBPβΔBR21) completely eliminated detectable nuclear localization of CRP1 and C/EBPβ (Fig. 2A, lanes 3 and 4; Fig. 2B, lanes 3 and 4). Removal of motif A alone also severely affected nuclear localization of C/EBPβ, although a small amount of this protein (C/EBPβΔBR9) was found in the nucleus (Fig. 2B, lanes 7 and 8). Thus, the CRP1 and C/EBPβ basic regions contain sequences necessary for nuclear localization.
Because the basic region is responsible for making sequence-specific interactions with DNA, one explanation for these results is that the basic region retains the proteins in the nucleus as a consequence of its DNA binding function. To test this possibility, a 96-bp RsaI fragment containing most of the CRP1 basic region was inserted near the N-termini of deletion mutants C/EBPβΔBR21 and C/EBPβΔBR9. In this location, distantly removed from the leucine zipper, the basic region should be unable to participate in DNA binding (1). The proteins were expressed in HeLa cells and assayed for subcellular distribution. As shown in Fig. 2B, both proteins exhibited partial restoration of nuclear localization, although in neither case was the hybrid localized to the nucleus as efficiently as the wild-type protein. This could be explained either by the fact that a complete NLS is not contained within this portion of the CRP1 basic region or that its context within the C/EBPβ chimera is not conducive to full NLS activity. In either case, these results indicate that the nuclear localization function of the CRP1 basic region is independent of its role in DNA binding.
The CRP1 Basic Region Can Direct a Heterologous Protein to the Nucleus
A restriction fragment containing the CRP1 basic region was fused to the E. coli LacZ gene to test its ability to direct a heterologous protein, β-galactosidase (β-gal), to the nucleus. The wild-type and chimeric β-gal proteins were expressed in HeLa cells and their subcellular localization was detected in situ by histochemical staining. In the absence of an NLS, β-gal is visualized as a diffusely staining protein throughout the cell (Fig. 3B). Apparent staining in the area of the nucleus is due to the fact that a small proportion of β-gal protein localizes to the nucleus in eukaryotic cells (22). A β-gal protein containing the SV40 T-antigen nuclear localization signal at its N-terminus was localized entirely to the nucleus, as expected for the potent SV40 NLS (Fig. 3A). Similarly, the CRP1-β-gal hybrid protein exhibited intense nuclear staining in HeLa cells (Fig. 3C), indicating that the basic region of CRP1 contains an NLS that functions as efficiently as the SV40 NLS in this assay.
FIG. 3.
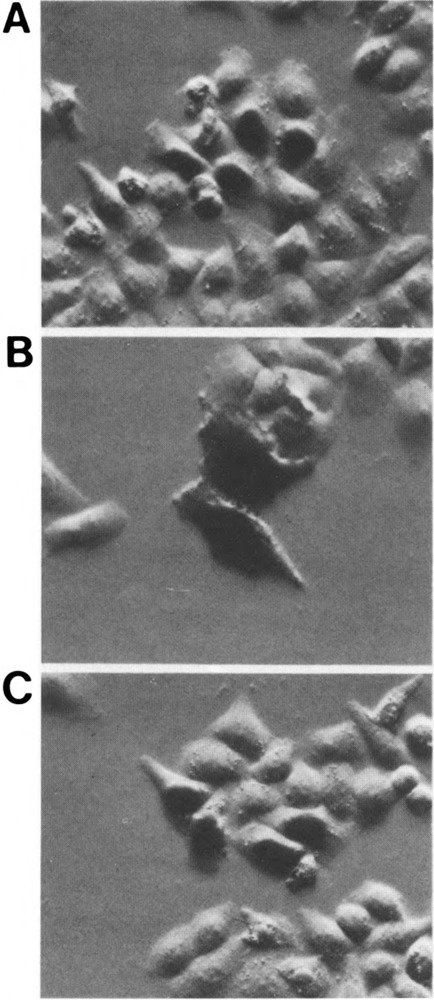
The CRP1 basic region can direct a heterologous protein to the nucleus. Expression vectors encoding the bacterial lacZ gene lacking a nuclear localization signal (B) or LacZ fused to the SV40 T-antigen NLS (A) or the 90-bp RsaI fragment from the CRP1 basic region (C) were introduced into HeLa cells. After 2 days, the cells were fixed and stained for β-gal activity. β-gal staining can be visualized either throughout the cytoplasm and nucleus (B) or specifically localized to the nucleus (A and C).
The segment of the basic region contained in the CRPl-LacZ chimera includes only motif A (see Fig. 1). However, the fact that this protein is nuclear does not eliminate the possibility that motif B could also function to direct nuclear localization. Moreover, the CRP1 sequence tested contains residues external to the core NLS that was predicted from comparisons with nucleoplasmin. Thus, we sought to map the minimal sequence required for CRP NLS function using the β-gal fusion assay. Initially, we tested the possibility that the CRP1 basic region indeed contains two functional NLSs. DNA fragments encoding different portions of the CRP1 basic region were synthesized by PCR and inserted at the 5′ end of the lacZ gene. The sequences and the subcellular location of the resulting hybrid proteins are shown in Fig. 4. To simplify the comparison of mapping studies in the different C/EBP family members we have used a numbering system for the basic regions of bZIP proteins established in Johnson (19). This numbering system facilitates the alignment of the basic regions of all bZIP proteins based on a conserved asparagine residue (residue 23; Fig. 4).
FIG. 4.

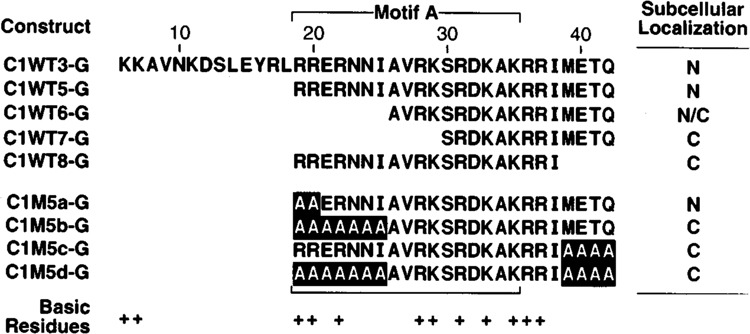
The CRP1 NLS requires additional residues C-terminal to the bipartite consensus. (Top) β-gal fusion proteins containing the indicated sequences from the CRP1 basic region were expressed in HeLa cells and analyzed as described in Fig. 3. The subcellular location of each fusion protein is indicated as cytoplasmic (C) or nuclear (N) in the right-hand column. Basic residues are indicated by plus (+) signs; note that the basic amino acid at position 36 is not conserved between CRP1 and C/EBPβ. Numbering of the basic region follows a published convention (19). Abbreviations and nomenclature: Cl, CRPl; WT, wild-type sequence; G, β-gal; mutants are numbered sequentially. (Bottom) Examples of β-gal staining for each of the constructs depicted in the top.
Unexpectedly, a CRPL:lacZ chimera that includes all of the predicted NLS sequences of motifs A and B (ClWT1-G) was localized to the cytoplasm (Fig. 4). This finding suggests that sequences in addition to those predicted from the bipartite consensus are required for NLS function. Hybrid proteins containing an additional two amino acids at the N-terminus of motif B (C1WT4-G), or three amino acids at the C-terminus of motif A (C1WT2-G) were also present in the cytoplasm. However, a hybrid protein bearing an additional seven amino acids at the C-terminus of motif A (C1WT3-G) was efficiently localized to the nucleus, indicating that flanking sequences are required for activity. Although two basic amino acids, Arg36 and Arg37, are located within the extended region, simply including these arginines was not sufficient for full NLS function because C1WT2-G was not efficiently transported to the nucleus. The inclusion of four additional nonbasic amino acids (METQ) was required for full NLS activity. Thus, the CRP1 NLS differs from a canonical nucleoplasmin-like NLS in its requirement for sequences external to the consensus element. Furthermore, the cytoplasmic location of C1 WT4-G indicates that motif B is incapable of functioning as an NLS in this assay.
Mapping the Minimal CRP1 NLS
To localize more precisely the sequences required for CRP1 NLS function, we first removed most of motif B (amino acids 6–18) from C1WT3-G to generate C1WT5-G (Fig. 5). This protein was localized to the nucleus, indicating that motif B sequences are dispensable for NLS function. Hybrid proteins lacking an additional seven N-terminal amino acids (ClWT6-G), including two residues (Arg19 and Arg20) that are part of the consensus bipartite sequence, exhibited reduced nuclear localization, whereas C1WT7-G, which lacks an additional four N-terminal residues, was localized entirely in the cytoplasm. In agreement with earlier results, nuclear localization was completely lost upon removal of the four C-terminal nonbasic amino acids (compare C1WT8-G to C1WT5-G).
FIG. 5.
Mapping the N- and C-terminal boundaries of the CRP1 NLS. The subcellular location of β-gal fusion proteins was analyzed as described in Figs. 3 and 4. These mutants were constructed in the background of C1WT5G and are therefore designated by the number 5 followed by a lower case letter. M, mutated CRP1 sequence. N/C, protein appearing primarily in the nucleus but displaying light staining in the cytoplasm.
Because flanking sequences from the β-gal fusion could potentially influence NLS activity, we next used alanine substitution mutagenesis to confirm the results from deletion mutants. Substituting alanine residues for Arg19 and Arg20 did not affect nuclear localization (compare ClM5a-G to C1WT5-G). However, replacement of five additional amino acids at the N-terminal end (ClM5b-G) completely eliminated nuclear localization, indicating that residues in this region are important for nuclear localization. The different results obtained from deletion and substitution of amino acids 19–25 (compare C1WT6-G to ClM5b-G) may be due to the effect of residues adjacent to the insertion site in the β-gal protein whose position changes relative to residues within the NLS. In this regard, we note that there is a single basic residue located N-terminal to the insertion site that could influence the function of the NLS in deletion mutants. As shown above, deletion of four nonbasic residues at the C-terminus of motif A significantly decreased NLS function. One possible explanation for this somewhat surprising result is that these amino acids simply act as a buffer to shield the NLS from surrounding residues that might negatively affect NLS function. However, mutating these four non-basic amino acids to alanine had the same effect as deleting them (compare C1WT2-G to ClM5c-G), demonstrating that one or more of these residues is critical for NLS function. As expected, combining the mutations from ClM5b-G and ClM5c-G to create ClM5d-G also abolished nuclear localization. Collectively, the data indicate that when fused to β-gal, the CRP1 NLS does not precisely conform to the bipartite consensus sequence. This conclusion is based on the fact that the pair of N-terminal arginine residues is dispensible for NLS function, as well as the requirement for a cluster of nonbasic C-terminal amino acids.
Mutation of a Single, Nonconserved Basic Residue Converts the CRP1 NLS to a True Bipartite Signal
Although the basic regions of the C/EBP proteins display a high degree of sequence identity, one of a triplet of basic residues within the CRP1 NLS (amino acids 35–37) is not conserved among all family members. As shown in Fig. 6B, position 36 is occupied by a nonbasic residue in C/EBPα and C/EBPβ and by an arginine residue in CRP1 and C/EBPδ. Because basic residues are important determinants of NLS function, we tested whether the presence of this additional basic residue in the CRP1 NLS might explain its divergence from the typical bipartite signal. In the context of construct C1WT5-G, which efficiently translocates to the nucleus, we changed single and multiple residues within the basic triplet (aa 35–37) to alanine and assessed the cellular localization of each β-gal hybrid protein. Proteins bearing a mutation in either of the conserved basic residues in this cluster (Lys35 in ClM5g-G or Arg37 in ClM5e-G) were localized in the cytoplasm. Conversely, a chimera carrying an alanine in place of the nonconserved Arg36 (ClM5f-G) was efficiently localized to the nucleus, indicating that residue 36 need not be basic to retain NLS function. However, the combination of alanine substitutions at positions 19, 20, and 36 (ClM5i-G) completely disrupted nuclear localization, revealing the bipartite structure of the CRP1 NLS. This result indicates that each C/EBP protein is likely to contain a bipartite NLS within its bask region, but the NLSs from different family members may differ slightly due to the presence or absence of a basic residue at position 36.
FIG. 6.
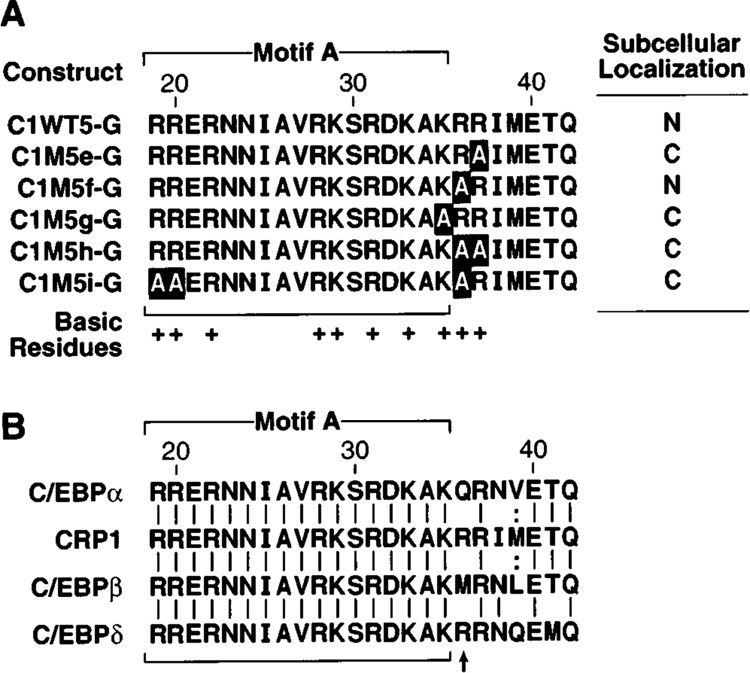
Identification of critical basic residues within the CRP1 NLS. (A) The subcellular location of β-gal fusion proteins carrying the indicated wild-type and mutated CRP1 sequences was analyzed as described in Figs. 3 and 4. B. Comparison of basic region sequences of the C/EBP family members in the putative NLS region. Sequence identities and conservative substitutions are indicated by vertical lines and colons, respectively. The position of the nonconserved basic residue at position 36 that controls the requirement for a bipartite signal is indicated by an arrow. The sequences shown represent amino acids 288–311 of rat C/EBPα, 178–201 of rat CRP1, 208–231 of rat C/ EBPβ, and 197–220 of mouse C/EBPδ [amino acid numbering as described in (55)].
NLS Determinants in Intact CRP1 and C/EBPβ Proteins
We next tested whether residues required for nuclear localization in the β-gal hybrids were also essential for nuclear localization of the intact CRP1 and C/EBPβ proteins. PCR was used to replace selected residues in CRP1 and C/EBPβ with alanine, and the subcellular location of the resulting proteins was determined by Western blotting following expression in HeLa cells (Fig. 7). Again, wild-type CRP1 was entirely localized to the nucleus. However, mutation of Arg19 and Arg20 to alanine (ClM5a) significantly decreased nuclear localization (Fig. 7A), indicating that in their normal context these residues are required for NLS function. Mutation of single or multiple residues in the basic cluster at amino acids 35–37 had little effect on nuclear transport (ClM5e, f, g, and efg). By contrast, combining mutations at both ends of the basic region of CRP1 almost completely abolished nuclear transport (ClM5a + f, a + e, a + g, and a + efg). Similar results were obtained for C/EBPβ, except that C/EBPβ proteins carrying mutations at both ends of the basic region were equally partitioned between the nucleus and cytoplasm as opposed to primarily cytoplasmic. Finally, a C/EBPβ mutant carrying alanines in place of the four nonbasic amino acids located at positions 39–42 (βM5c) was partially localized to the cytoplasm. This result confirms the observation that, in addition to basic residues predicted from the consensus bipartite NLS, the NLSs of C/EBP proteins require additional basic and nonbasic residues for optimal function.
FIG. 7.

Mutation of critical NLS residues in native CRP1 and C/EBPβ proteins. Subcellular localization of C/EBPβ and CRP1 proteins bearing mutations in residues defined as critical for nuclear localization in β-gal fusion proteins. The indicated mutations were introduced into the coding sequence of C/EBPβ and CRPl by PCR and each protein was expressed in HeLa cells. Subcellular location of each protein was determined by Western blotting of nuclear (N) and cytoplasmic (C) fractions as described in Fig. 2, except that immune complexes were detected using the Amersham ECL kit.
C/EBPα Can Transport an NLS-Deficient C/EBPβ Protein to the Nucleus
C/EBPβΔBR21, which lacks the entire NLS, was localized exclusively to the cytoplasm when expressed in HeLa cells (Fig. 2). We sought to test whether coexpression of wild-type C/EBPα might facilitate the nuclear transport of C/EBPβΔBR21 by heterodimerization in the cytoplasm and subsequent localization of the complex to the nucleus. A constant amount of C/EBPβΔBR21 was expressed either alone or in the presence of increasing amounts of wild-type C/EBPα. The subcellular location of each protein was analyzed by cellular fractionation followed by Western blotting. When expressed alone, C/EBPβΔBR21 was found only in the cytoplasm (Fig. 8, lane 1) and C/EBPα was localized entirely to the nucleus (data not shown). However, C/EBPβΔBR21 was partially nuclear in cells cotransfected with a C/EBPα expression vector (Fig. 8, lanes 2 and 3). Nuclear targeting of C/EBPβΔBR21 occurred in a C/EBPα dose-dependent manner, consistent with the idea that C/EBPα:C/EBPβΔBR21 heterodimers are transported to the nucleus. Thus, nuclear localization is achieved even when only one subunit of the dimer contains an NLS.
FIG. 8.
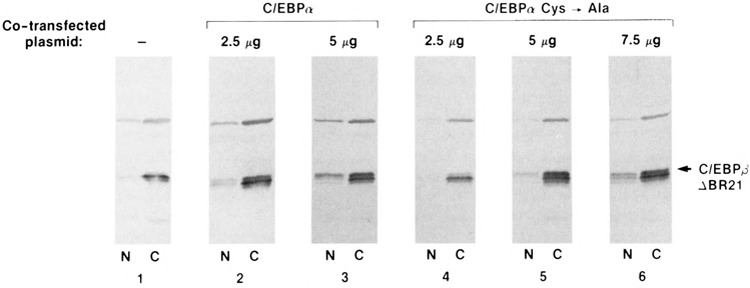
C/EBPα can transport an NLS-deficient C/EBPβ protein to the nucleus. Coexpression of wild-type C/EBPα or C/EBPα C →A with C/EBPβΔBR21 in HeLa cells. C/EBPβΔBR21 was expressed alone (panel 1) or with increasing amounts of wild-type C/EBPα (panels 2–3) or C/EBPα C →A (panels 4–6). The subcellular location of C/EBPβΔBR21was analyzed by Western blotting as described in Fig. 2. The upper band is a protein that cross-reacts with the antiserum.
We have previously shown that a conserved cysteine residue at the C-terminus of each C/EBP protein may participate in the formation of disulfide-linked dimers in vitro (55) and in vivo (S. C. Williams and P. F. Johnson, unpublished data). Therefore, we tested whether a mutant form of C/EBPα in which the C-terminal cysteine was changed to alanine (C/EBPα C→A) could function in the C/EBPα:C/EBPβΔBR21 coexpression assay. The C/EBPα C→A mutant was only slightly less efficient than wild-type C/EBPα at transporting C/EBPβΔBR21 to the nucleus (Fig. 8, lanes 4–6). These findings indicate that disulfide cross-linking is not essential for the formation of C/EBPα:C/EBPβΔBR21 heterodimers in the cytoplasm and their subsequent transport to the nucleus, although the cysteine residue may contribute to the stability of the dimer complex.
DISCUSSION
The existence of a nuclear localization signal within the basic region/leucine zipper domain of C/EBP proteins had previously been implied from the nuclear localization of a truncated C/EBPα protein containing only the DNA binding domain (15). The C/EBP proteins contain an extended basic region that is generally not conserved with other bZIP proteins. The additional basic residues in C/EBPα are not required for sequence-specific DNA binding (26) but have been implicated in regulating the DNA binding activity of C/EBPβ (5). Based on comparisons between the basic region of C/EBP proteins and consensus bipartite and SV40-like NLSs, it has been suggested that nuclear localization of C/EBP proteins may be controlled either by a bipartite-like sequence (12) or the “more common” SV40-like NLS, which is characterized by the presence of at least four basic amino acids within an eight-amino acid segment (4). Due to the high degree of conservation among basic regions of bZIP proteins, each can theoretically contain at least one bipartite-like sequence (12). This prediction has been supported by experimental identification of bipartite signals in a number of bZIP proteins, including c-Jun and the Epstein-Barr virus EB1 protein (32) and the plant proteins Opaque2 (50,51) and GT-2 (10), as well as other transcription factors and nuclear proteins such as retinoblastoma protein (57), the androgen receptor (59), and poly(ADP-ribose) polymerase (43).
A second potential NLS (motif B) in the C/EBP proteins, located within the N-terminal extension of the basic region, is not conserved among all bZIP proteins. In this report we have mapped the sequences that function as an NLS in two members of the C/EBP family, CRP1 and C/ EBPβ, and have directly tested whether multiple NLSs are present in these proteins. Our studies demonstrate that nuclear localization of both CRP1 and C/EBPβ is entirely controlled by sequences within their basic regions. However, only one of the two putative bipartite NLS-like sequences (motif A) appears to be capable of functioning as an NLS. Multiple NLSs identified in nuclear proteins such as the glucocorticoid receptor (38) have been proposed to increase the efficiency of nuclear transport (13). Although our data do not indicate a role for motif B in the C/EBP family, we cannot rule out the possibility that the additional basic residues in motif B increase the efficiency of nuclear localization.
Despite the high degree of similarity between motif A and the consensus bipartite NLS, motif A was not sufficient to direct a heterologous protein to the nucleus. An additional seven amino acids at the C-terminus were needed for efficient nuclear localization, and four nonbasic amino acids (aa 39–42) were among the critical residues in this region. Previous studies have demonstrated that the activity of an NLS can be significantly affected by surrounding residues, especially hydrophobic amino acids (49). However, these nonbasic residues do not function merely by shielding NLSs of C/EBP proteins from surrounding residues, because replacing the four residues with alanine had a similar deleterious effect as removing them. Although all NLSs described thus far are characterized by a predominance of basic residues, there are a number of examples where the presence of nonbasic residues at the C-terminus of the signal has functional significance, including the SV40 T-antigen (21), cAMP response element binding protein (CREB) (52), and Fos (45). In the case of the C/EBP proteins, the apparent requirement for additional C-terminal residues may explain why the putative NLS contained in motif B is nonfunctional, as related nonbasic residues are not present in motif B. Currently, we do not know precisely which additional C-terminal residues are required for full NLS functionality, and further mutagenesis will be required to address this point.
The NLS of SV40 T-antigen may be a more efficient form of the bipartite signal which, by virtue of the higher density of basic amino acids, no longer requires the second basic cluster for activity. Analysis of the sequence requirements of the CRP1 NLS has provided indirect evidence for this hypothesis. The downstream cluster of basic amino acids in CRP1 differs from those in C/EBPα and C/EBPβ by the presence of one additional basic residue (Arg36). NLS sequence requirements are relaxed in constructs in which this residue is unchanged, in that the upstream basic doublet (Arg19 and Arg20) is not required. However, changing this residue to a nonbasic amino acid converted the CRP1 NLS to a true bipartite signal (i.e., displaying an absolute requirement for the basic doublet). Therefore, the C/EBPα and C/EBPβ NLSs may be slightly less active than those in CRP1 and C/EBPδ, perhaps by virtue of decreased affinity for the NLS receptor molecule.
Nuclear transport of native CRP1 and C/EBPβ proteins was more sensitive to mutations affecting motif A than was observed using β-gal hybrid proteins. In particular, alanine substitutions of Arg19 and Arg20 caused a significant decrease in nuclear localization, especially for CRP1. There are several possible explanations for why the NLSs behave differently in the two protein contexts. For example, because C/EBP proteins are dimers whereas β-gal is reported to form a tetramer, the presence of four NLS elements in a multimeric protein may decrease the threshold at which an NLS can direct nuclear localization, thereby allowing weaker signals to exhibit NLS function. Also, the folded structures of NLS regions may differ depending on whether they occur naturally or as part of a heterologous protein. However, both the chimeric protein approach and the analysis of native proteins yield useful information about potential NLS elements.
We have shown that a C/EBPβ protein lacking a NLS can be transported to the nucleus by coexpression of wild-type C/EBPα, presumably via the formation of heterodimers that can enter the nucleus by virtue of the functional NLS in C/EBPα. Although we cannot rule out the possibility that C/EBPα is acting to upregulate the expression of an unidentified protein that causes the mutant C/EBPβ protein to enter the nucleus, the correlation between the amount of input C/EBPα plasmid and the proportion of C/EBPβ in the nucleus supports the heterodimerization model. We previously showed that C/EBP family members may be covalently cross-linked in vitro via a conserved C-terminal cysteine residue (55). Mutation of this residue to alanine in C/EBPα had no observable effect on the colocalization of the mutant C/EBPβ protein to the nucleus (Fig. 8), suggesting that cross-linking via this residue is not required. However, these studies leave open the possibility that disulphide bridges may form following trans-location to the nucleus and stabilize specific homo- and heterodimeric complexes.
Nuclear localization is one of the steps at which transcription factor activity may be regulated. For example, cell cycle-dependent nuclear localization of v-Jun is controlled by phosphorylation of a serine residue adjacent to the NLS (8,44) and tyrosine phosphorylation of Stat proteins is required for nuclear translocation [reviewed in (42)]. The C/EBP proteins are targeted by a number of signaling pathways, and the regulation of C/EBPβ activity, in particular, has been attributed to phosphorylation events [reviewed in (20)]. C/EBPβ is reportedly phosphorylated by MAP kinases (34), a calcium/calmodulin-dependent kinase (53), protein kinase C (46), and a number of kinase oncogenes (47). In each case, increased phosphorylation of C/EBPβ was correlated with increased transcriptional activity. As mentioned previously, nuclear localization of C/EBP proteins has also been reported to be regulated by phosphorylation in multiple cell types, although the sites of phosphorylation have not been identified (25,30,44). The description of sequences controlling nuclear localization of C/EBP proteins should aid in the identification of these sites and in determining whether phosphorylation of specific residues in C/ EBPβ and other C/EBP family members directly affects NLS function.
ACKNOWLEDGEMENTS
We thank Andrew Fire for providing LacZ vectors, Jeffrey Zsohar for technical assistance, and Carla Weinstock and Hilda Marusiodis for help with preparation of the manuscript. The research was sponsored in part by the National Cancer Institute, DHHS, under contract with ABL, and by a Grant-in-Aid from the American Heart Association, Texas Affiliate, to S.C.W. The contents of this publication do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
REFERENCES
- 1. Agre P.; Johnson P. F.; McKnight S. L. Cognate DNA binding specificity retained after leucine zipper exchange between GCN4 and C/EBP. Science 246:922–926; 1989. [DOI] [PubMed] [Google Scholar]
- 2. Antonson P.; Stellan B.; Yamanaka R.; Xanthopoulos K. G. A novel human CCAAT/enhancer binding protein gene, C/EBPE, is expressed in cells of lymphoid and myeloid lineages and is localized on chromosome 14q11.2 close to the T-cell receptor alpha/delta locus. Genomics 35:30–38; 1996. [DOI] [PubMed] [Google Scholar]
- 3. Boudreau F.; Blais S.; Asselin C. Regulation of CCAAT/enhancer binding protein isoforms by serum and glucocorticoids in the rat intestinal epithelial crypt cell line IEC-6. Exp. Cell Res. 222:1–9; 1996. [DOI] [PubMed] [Google Scholar]
- 4. Boulikas T. Putative nuclear localization signals (NLS) in protein transcription factors. J. Cell. Bio-chem. 55:32–58; 1994. [DOI] [PubMed] [Google Scholar]
- 5. Brasier A. R.; Kumar A. Identification of a novel determinant for basic domain-leucine zipper DNA binding activity in the acute-phase inducible nuclear factor-interleukin-6 transcription factor. J. Biol. Chem. 269:10341–10351; 1994. [PubMed] [Google Scholar]
- 6. Cao Z.; Umek R. M.; McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 5:1538–1552; 1991. [DOI] [PubMed] [Google Scholar]
- 7. Chandrasekaran, C; Gordon J. I. Cell lineage-specific and differentiation-dependent patterns of CCAAT/enhancer binding protein alpha expression in the gut epithelium of normal and transgenic mice. Proc. Natl. Acad. Sci. USA 90:8871–8875; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chida K.; Vogt P. K. Nuclear translocation of viral Jun but not of cellular Jun is cell cycle dependent. Proc. Natl. Acad. Sci. USA 89:4290–4294; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cortes Z.; Ye S.; Baltimore D. RAG-1 interacts with the repeated amino acid motif of the human homologue of the yeast protein SRP1. Proc. Natl. Acad. Sci. USA 91:7633–7637; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dehesh K.; Smith L. G.; Tepperman J. M.; Quail P. H. Twin autonomous bipartite nuclear localization signals direct nuclear import of GT-2. Plant J. 8:25–36; 1995. [DOI] [PubMed] [Google Scholar]
- 11. Dignam J. D.; Lebovitz R. M.; Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475–1488; 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dingwall C. ; Laskey R. A. Nuclear targeting sequences—a consensus? Trends Biochem. Sci. 16:478–481; 1991. [DOI] [PubMed] [Google Scholar]
- 13. Dworetzky S. I.; Lanford R. E.; Feldherr C. M. The effects of variations in the number and sequence of targeting signals on nuclear import. J. Cell Biol. 107:1279–1287; 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fire A.; Harrison S. W.; Dixon D. A modular set of lacZ fusion vectors for studying gene expression in Caenorhabditis elegans. Gene 93:189–198; 1983. [DOI] [PubMed] [Google Scholar]
- 15. Friedman A. D.; McKnight S. L. Identification of two polypeptide segments of CCAAT/enhancer-binding protein required for transcriptional activation of the serum albumin gene. Genes Dev. 4:1416–1426; 1990. [DOI] [PubMed] [Google Scholar]
- 16. Gorlich D.; Mattaj I. W. Nucleocytoplasmic transport. Science 271:1513–1518; 1996. [DOI] [PubMed] [Google Scholar]
- 17. Gorlich D.; Vogel F.; Mills A. D.; Hartmann E.; Laskey R. A. Distinct function for the two importin subunits in nuclear protein import. Nature 377:246–248; 1995. [DOI] [PubMed] [Google Scholar]
- 18. Hunter T.; Karin M. The regulation of transcription by phosphorylation. Cell 70:375–387; 1992. [DOI] [PubMed] [Google Scholar]
- 19. Johnson P. F. Identification of C/EBP basic region residues involved in DNA sequence recognition and half-site spacing preference. Mol. Cell. Biol. 13:6919–6930; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson P. F.; Williams S. C. CCAAT/enhancer binding (C/EBP) proteins. In: Tronche F.; Yaniv M. , eds. Liver gene expression. Austin: R. G. Landes Company; 1994:231–258. [Google Scholar]
- 21. Kalderon D.; Richardson W. D.; Markham A. F.; Smith A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature 311:33–38; 1984. [DOI] [PubMed] [Google Scholar]
- 22. Kalderon D.; Roberts B. L.; Richardson W. D.; Smith A. E. A short amino acid sequence able to specify nuclear location. Cell 39:499–509; 1984. [DOI] [PubMed] [Google Scholar]
- 23. Katz S.; Kowenz-Leutz E.; Muller C. ; Meese K.; Ness S. A.; Leutz A. The NF-M transcription factor is related to C/EBP-beta and plays a role in signal transduction, differentiation and leukemogenesis of avian myelomonocytic cells. EMBO J. 12:1321–1332; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kowenz-Leutz E.; Twamley G.; Ansieau S.; Leutz A. Novel mechanism of C/EBP beta (NF-M) transcriptional control: Activation through derepression. Genes Dev. 8:2781–2791; 1994. [DOI] [PubMed] [Google Scholar]
- 25. LaCasse E. C.; Lefebvre Y. A. Nuclear localization signals overlap DNA- or RNA-binding domains in nucleic acid-binding proteins. Nucleic Acids Res. 23:1647–1656; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Landschulz W. H.; Johnson P. F.; McKnight S. L. The DNA binding domain of the rat liver nuclear protein C/EBP is bipartite. Science 243:1681–1688; 1989. [DOI] [PubMed] [Google Scholar]
- 27. Lanford R. E.; Butel J. S. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell 37:801–813; 1984. [DOI] [PubMed] [Google Scholar]
- 28. Lee K. A.; Bindereif A.; Green M. R. A small-scale procedure for preparation of nuclear extracts that support efficient transcription and pre-mRNA splicing. Gene Anal. Tech. 5:22–31; 1988. [DOI] [PubMed] [Google Scholar]
- 29. MacDougald O. A.; Lane M. D. Transcriptional regulation of gene expression during adipocyte differentiation. Annu. Rev. Biochem. 64:345–373; 1995. [DOI] [PubMed] [Google Scholar]
- 30. Mahoney C. W.; Shuman J.; McKnight S. L.; Chen H. C.; Huang K. P. Phosphorylation of CCAAT-enhancer binding protein by protein kinase C attenuates site-selective DNA binding. J. Biol. Chem. 267:19396–19403; 1992. [PubMed] [Google Scholar]
- 31. Metz R.; Ziff E. cAMP stimulates the C/EBP-related transcription factor rNFIL-6 to translocate to the nucleus and induce c-fos transcription. Genes Dev. 5:1754–1766; 1991. [DOI] [PubMed] [Google Scholar]
- 32. Mikaelian I.; Drouet E.; Marechal V.; Denoyel G.; Nicolas J. C.; Sergeant A. The DNA-binding domain of 2 bZIP transcription factors, the Epstein-Barr virus switch gene product EB1 and Jun, is a bipartite nuclear targeting sequence. J. Virol. 67:734–742; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moroianu J.; Hijikata M.; Blobel G.; Radu A. Mammalian karyopherin a1 β and a2 β heterodimers: αl or α2 subunit binds nuclear localization signal and 0 subunit interacts with peptide repeat-containing nucleoporins. Proc. Natl. Acad. Sci. USA 92:6532–6536; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakajima T.; Kinoshita S.; Sasagawa T.; Sasaki K.; Naruto M.; Kishimoto T.; Akira S. Phosphorylation at threonine-235 by a ras-dependent mitogen-activated protein kinase cascade is essential for transcription factor NF-IL6. Proc. Natl. Acad. Sci. USA 90:2207–2211; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nerlov C. ; Ziff E. B. Three levels of functional interaction determine the activity of CCAAT/enhancer binding protein-alpha on the serum albumin promoter. Genes Dev. 8:350–362; 1994. [DOI] [PubMed] [Google Scholar]
- 36. Ness S. A.; Kowenz-Leutz E.; Casini T.; Graf T.; Leutz A. Myb and NF-M-combinatorial activators of myeloid genes in heterologous cell types. Genes Dev. 7:749–759; 1993. [DOI] [PubMed] [Google Scholar]
- 37. Pei D. Q.; Shih C. H. An “attenuator domain” s sandwiched by two distinct transactivation domains in the transcription factor C/EBP. Mol. Cell. Biol. 11:1480–1487; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Picard D.; Yamamoto K. R. Two signals mediate hormone-dependent nuclear localization of the glu-cocorticoid receptor. EMBO J. 6:3333–3340; 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poli V.; Ciliberto G. Transcriptional regulation of acute phase genes by IL-6 an related cytokines. In: Tronche F.; Yaniv M. , eds. Liver gene expression. Austin:R. G. Landes Company; 1994:131–151. [Google Scholar]
- 40. Powers M. A.; Forbes D. J. Cytosolic factors in nuclear transport: What’s importin? Cell 79:931–934; 1994. [DOI] [PubMed] [Google Scholar]
- 41. Robbins J.; Dilworth S. M.; Laskey R. A.; Ding-wall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: Identification of a class of bipartite nuclear targeting sequence. Cell 64:615–623; 1991. [DOI] [PubMed] [Google Scholar]
- 42. Schindler C. ; Darnell J. E. J. Transcriptional responses to polypeptide ligands: The JAK-STAT pathway. Annu. Rev. Biochem. 64:621–651; 1995. [DOI] [PubMed] [Google Scholar]
- 43. Schreiber V.; Molinete M.; Boeuf H.; de Murcia G.; Menissier-de Murcia J. The human poly(ADP-ribose) polymerase nuclear localization signal is a bipartite element functionally separate from DNA binding and catalytic activity. EMBO J. 11:3263–3269; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tagawa T.; Kuroki T.; Vogt P. K.; Chida K. The cell cycle-dependent nuclear import of v-Jun is regulated by phosphorylation of a serine adjacent to the nuclear localization signal. J. Cell Biol. 130:255–263; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tratner I.; Verma I. M. Identification of a nuclear targeting sequence in the Fos protein. Oncogene 6:2049–2053; 1991. [PubMed] [Google Scholar]
- 46. Trautwein C. ; Caelles C. ; van der Geer P.; Hunter T.; Karin M.; Chojkier M. Transactivation by NF-IL6/LAP is enhanced by phosphorylation of its activation domain. Nature 364:544–547; 1993. [DOI] [PubMed] [Google Scholar]
- 47. Twamley-Stein G.; Kowenz-Leutz E.; Ansieau S.; Leutz A. Regulation of C/EBP beta/NF-M activity by kinase oncogenes. Curr. Top. Microbiol. Immunol. 211:129–136; 1996. [PubMed] [Google Scholar]
- 48. Vandromme M.; Gauthier-Rouviere C. ; Lamb N.; Fernandez A. Regulation of transcription factor localization: Fine-tuning of gene expression. Trends Biochem. Sci. 21:59–64; 1996. [PubMed] [Google Scholar]
- 49. van Zee K.; Appel F.; Fanning E. A hydrophobic protein sequence can override a nuclear localization signal independently of protein context. Mol. Cell. Biol. 11:5137–5146; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Varagona M. J.; Raikhel N. V. The basic domain in the bZIP regulatory protein Opaque2 serves two independent functions: DNA binding and nuclear localization. Plant J. 5:207–214; 1994. [DOI] [PubMed] [Google Scholar]
- 51. Varagona M. J.; Schmidt R. J.; Raikhel N. V. Nuclear localization signal(s) required for nuclear targeting of the maize regulatory protein opaque-2. Plant Cell 4:1213–1227; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Waeber G.; Habener J. F. Nuclear translocation and DNA recognition signals colocalized within the bZIP domain of cyclic adenosine 3′,5′-monophosphate response element-binding protein CREB. Mol. Endocrinol. 5:1431–1438; 1991. [DOI] [PubMed] [Google Scholar]
- 53. Wegner M.; Cao Z.; Rosenfeld M. G. Calcium-regulated phosphorylation within the leucine zipper of C/EBP beta. Science 256:370–373; 1992. [DOI] [PubMed] [Google Scholar]
- 54. Williams S. C.; Baer M.; Dillner A. J.; Johnson P. F. CRP2 (C/EBPβ) contains a bipartite regulatory domain that controls transcriptional activation, DNA-binding and cell specificity. EMBO J. 14:3170–3183; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Williams S. C.; Cantwell C. A.; Johnson P. F. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 5:1553–1567; 1991. [DOI] [PubMed] [Google Scholar]
- 56. Yin M.; Yang S. Q.; Lin H. Z.; Lane M. D.; Chatterjee S.; Diehl A. M. Tumor necrosis factor α promotes nuclear localization of cytokine-inducible CCAAT/enhancer binding protein isoforms in hepatocytes. J. Biol. Chem. 271:17974–17978; 1996. [DOI] [PubMed] [Google Scholar]
- 57. Zacksenhaus E.; Bremner R.; Phillips R. A.; Gallie B. L. A bipartite nuclear localization signal in the retinoblastoma gene product and its importance for biological activity. Mol. Cell. Biol. 13:4588–4599; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang D.-E.; Zhang P.; Wang N.; Hetherington C. J.; Darlington G. J.; Tenen D. G. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc. Natl. Acad. Sci. USA 94:569–574; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhou Z. X.; Sar M.; Simental J. A.; Lane M. V.; Wilson E. M. A ligand-dependent bipartite nuclear targeting signal in the human androgen receptor. Requirement for the DNA-binding domain and modulation by NH2-terminal and carboxy-terminal sequences. J. Biol. Chem. 269:13115–13123; 1994. [PubMed] [Google Scholar]


