Abstract
Post-traumatic stress disorder (PTSD) is a prevalent, debilitating and sometimes deadly consequence of exposure to severe psychological trauma. Although effective treatments exist for some individuals, they are limited. New approaches to intervention, treatment and prevention are therefore much needed. In the past few years, the field has rapidly developed a greater understanding of the dysfunctional brain circuits underlying PTSD, a shift in understanding that has been made possible by technological revolutions that have allowed the observation and perturbation of the macrocircuits and microcircuits thought to underlie PTSD-related symptoms. These advances have allowed us to gain a more translational knowledge of PTSD, have provided further insights into the mechanisms of risk and resilience and offer promising avenues for therapeutic discovery.
Post-traumatic stress disorder (PTSD) is, from a historical point of view, a recent diagnosis. There are loose strands of the idea that trauma may inflict psychological distress throughout ancient Western literature; however, more recent works have been mostly silent on the topic of the syndrome we have come to call PTSD, a situation that may have arisen as a result of cultural norms and social stigma surrounding trauma survival1. This silence is especially true for the suffering of women after trauma2. The establishment of PTSD as a medical diagnosis in 1980 (REF3) has allowed for its acceptance by the wider culture and has facilitated research into this previously neglected psychiatric disorder.
It is now widely accepted that there is considerable genetic heritability in the development of PTSD (Box 1). Recently, the groundwork has been laid for the establishment of large-scale genome-wide association studies (GWAS) that aim to identify the genes that contribute to PTSD risk4. However, when compared with other psychiatric disorders (such as schizophrenia and autism), there has been relatively little progress made in our understanding of the definitive molecular changes involved in determining vulnerability to PTSD. As a result, there are few identified therapeutic targets for pharmacological intervention in this disorder.
Box 1|. Genomic influences on post-traumatic stress disorder circuits
Although a large proportion of people (estimates range from 50% to 85%) are exposed to major traumatic events during their lifetimes, only a small fraction subsequently develop persistent symptoms of post-traumatic stress disorder (PTsD)168. This observation has led to investigations of the mechanisms underlying PTsD risk and resilience. Twin studies, which allow for an estimate of the heritability of disorders by comparing their prevalence in monozygotic and dizygotic twins, suggest heritability in the range of 40–50% for PTSD (although this number varies depending on the type of trauma and the sample in the survey)169. The remaining risk is thought to arise owing to environmental factors, possibly mediated through epigenetic mechanisms (which we here define in the broadest sense to include mechanisms of persistent cellular memory and reprogramming)170.
The investigation of the genetic basis for PTSD remains in its early stages, in part owing to the highly polygenic nature of the genetic risk, the heterogeneity of trauma exposures and the heterogeneity among methodologies employed, which has made meta-analysis difficult4, 171. The basic design of genetic studies within PTSD is to apply a case–control approach, in which alleles of interest are tested for their association with individuals in which PTsD has developed compared with trauma-exposed controls. Initially, such studies relied upon a candidate gene approach, in which hypotheses generated from existing knowledge of genes involved in stress and threat processing were tested for association with PTSD risk. Unfortunately, the genetic hits from gene candidate studies largely failed to replicate robustly across cohorts172.
However, there may be some exceptions to this rule. For example, FKBP5, encoding a molecular chaperone and regulator of the glucocorticoid receptor, has been shown across multiple studies to mediate gene by environmental risk of both depression and PTSD173. FKBP5 risk alleles have been associated with increased amygdala activation, altered hippocampal function, decreased hippocampal size and decreased cingulum bundle white matter integrity174. Similarly, the genes encoding pituitary adenylyl cyclase-activating polypeptide (PACAP) and its receptor, PAC1, showed a modest significant association with PTSD risk in a recent meta-analysis175 and have also been associated with increased amygdala activation in humans and rodent studies43, 176. Ongoing larger scale genetic studies are clearly required to know with certainty the genetic underpinnings of PTSD.
Thus, studies investigating the genetics of PTSD risk have largely moved to an unbiased genome-wide association study (GWAS) model, which seeks to determine whether associations exist between single-nucleotide polymorphisms (SNPs) distributed across the entire genome and PTSD risk. There have been a number of small GWAS studies published on PTSD risk177–180; however, genes surviving tests for multiple-testing correction have not yet replicated across studies. Promising gene candidates that have emerged from these studies include those encoding the nuclear receptor ROR-a (RORA), neuroligin 1 (NLGN1), tolloid-like protein 1 (TLL1), protein cordon-bleu (COBL), ankyrin repeat domain-containing protein 55 (ANKRD55) and cGMP-dependent protein kinase 1 (PRKG1)4,177–187. COBL risk alleles were associated with decreased white matter integrity in the uncinate cortex, part of the medial prefrontal cortex, suggesting that decreased connectivity between this region and the amygdala contributes to PTSD risk185. NLGN1 is strongly expressed in the cortex and hippocampus and plays an important role in the proper development and maintenance of excitatory synapses188. PRKG1 has been shown to regulate nitric oxide signalling and to be necessary for the encoding of auditory cue fear memory in the mouse189. Results from GWAS approaches for other psychiatric disorders suggest that, with increasing sample sizes, SNPs that have a small effect with genome-wide significance will be found; however, these SNPs may reside in either coding or non-coding regions of the genome, and the underlying biological function of the association may take some time to elucidate.
Increasingly, psychiatric disorders — including PTSD — are becoming understood as disorders of circuits5. Indeed, most of the recent advances in PTSD research have come from the elucidation of the brain circuits implicated in the disorder. Functional neuroimaging of human patients has identified brain regions with altered activity and connectivity6. In parallel, the expansion of the use of optogenetics, chemogenetics, fibre photometry and other circuit-perturbing and circuit-monitoring tools in animal models has led to terrific progress in our understanding of the microcircuitry that underlies the normal behavioural processes that become per turbed in PTSD. These include those involved in fear and threat detection7, reward processing8,9 and valence representation10, among others.
Alongside the growing emphasis on circuits, there has also been a move away from a categorical under standing of psychiatric illness towards a dimensional approach, as embodied within the Research Domain Criteria (RDoC) issued by the National Institute of Mental Health5. If circuits control behaviours and there is a dimensional continuum between normal and mala daptive processes, then it becomes important to under stand how normal circuits function in both humans and animal models. This understanding will allow us to develop better hypotheses about how circuits are altered to produce maladaptive behaviours and will also provide avenues for future therapeutic discovery by suggesting how circuits can be targeted to restore normal function.
In this Review, we will examine the brain circuit mechanisms underlying each of the main clusters of symptoms identified in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5)11 classification of PTSD by considering evidence from human neurobiological studies and animal model approaches (Figs 1,2). Although there have been recent reviews of PTSD that have included a discussion of the functional neuroimaging literature12 and several recent reviews of the microcircuitry involved in brain regions implicated in PTSD-related circuits7,8,10, there has not yet been an attempt to bridge human and animal literatures in the discussion of PTSD pathophysiology. Because the DSM-5 remains dominant in clinical academic medicine, we have chosen to structure this Review according to its criteria rather than the categories outlined in the RDoC; however, we provide an attempt to align the DSM-5 criteria with the current RDoC matrix (TABLE 1). We also offer a brief summary of progress in our under standing of the genetics underlying PTSD risk and suggest future strategies for research. We hope to convey our belief that the intersection of PTSD biology and neuro science is driving these fields towards interesting and potentially feasible new neurobiology-driven approaches to treatment and prevention.
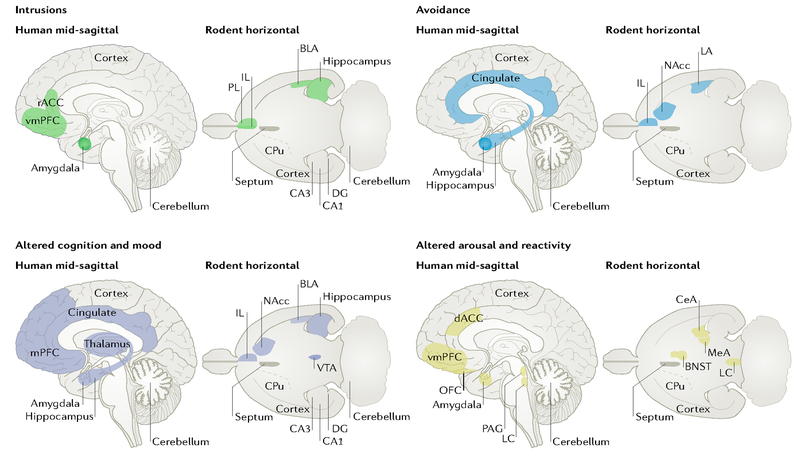
Fig. 1|. An expanded neurocircuitry of post-traumatic stress disorder.
Recent work suggests that an expanded brain network is implicated in post-traumatic stress disorder (PTSD) symptoms. This figure highlights brain regions that have been implicated in either human imaging studies of PTSD (mid-sagittal section) or in rodent models of related behaviours (horizontal section). Each quadrant illustrates brain regions that have evidence linking them to symptoms in the labelled cluster. Rodent behaviours were chosen that map aspects of the corresponding symptom cluster: fear extinction (intrusions); active and passive avoidance (avoidance); spatial memory, emotional valence and anhedonia (altered cognition and mood); and aggression and arousal (altered reactivity and arousal). BLA, basolateral amygdala; BNST, bed nucleus of the stria terminals; CeA, central amygdala; CPu, caudate and putamen; dACC, dorsal anterior cingulate cortex; DG, dentate gyrus; IL, infralimbic cortex; LA, lateral amygdala; LC, locus coeruleus; MeA, medial amygdala; mPFC, medial prefrontal cortex; NAcc, nucleus accumbens; OFC, orbitofrontal cortex; PAG, periaqueductal grey; PL, prelimbic cortex; rACC, rostral anterior cingulate cortex; vmPFC, ventromedial prefrontal cortex; VTA, ventral tegmental area.
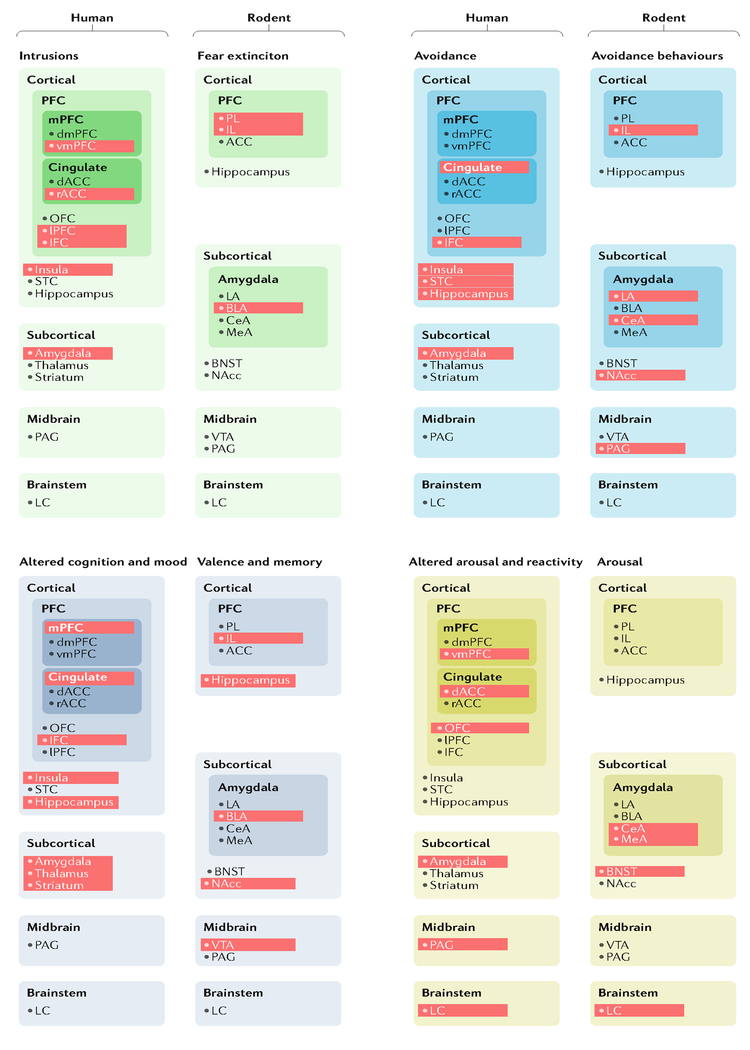
Fig. 2|. A map of post-traumatic stress disorder neurocircuits in humans and rodent models.
The schematic illustrates the brain regions currently implicated in each of the four major post-traumatic stress disorder (PTSD) symptom clusters by experiments involving human neuroimaging or rodent models. Red shading indicates areas for which there is evidence linking that particular brain region to the symptom cluster or related rodent behaviour. ACC, anterior cingulate cortex; BLA, basolateral amygdala; BNST, bed nucleus of the stria terminals; CeA, central amygdala; dACC, dorsal anterior cingulate cortex; dmPFC, dorsomedial prefrontal cortex; IFC, inferior frontal cortex; IL, infralimbic cortex; LA, lateral amygdala; LC, locus coeruleus; LPFC, lateral prefrontal cortex; MeA, medial amygdala; mPFC, medial prefrontal cortex; NAcc, nucleus accumbens; OFC, orbitofrontal cortex; PAG, periaqueductal grey; PFC, prefrontal cortex; PL, prelimbic cortex; rACC, rostral anterior cingulate cortex; STC, superior temporal cortex; vmPFC, ventromedial prefrontal cortex; VTA, ventral tegmental area.
Table 1|.
Translation of DSM-5 diagnostic criteria to RDoC domains
| DSM-5 H diagnostic criteria | RDoC domains | ||||
|---|---|---|---|---|---|
| Negative valence systems | Positive valence systems | Cognitive systems | Social processes | Arousal and regulatory systems | |
| Criterion B: intrusions | |||||
| Intrusive memories | Loss | None | Attention, declarative memory, perception, cognitive control and working memory | None | Arousal |
| Distressing dreams | Sustained threat | None | None | None | Arousal and sleep-wakefulness |
| Dissociative reactions | Loss | None | Attention, perception, declarative memory and cognitive control | Perception and understanding of self | None |
| Psychological distress to trauma cues | Potential threat and sustained threat | None | Cognitive control | None | None |
| Marked physiological reactions | Acute threat | None | None | None | Arousal |
| Criterion C: avoidance | |||||
| Avoidance of internal reminders | Acute threat, potential threat and sustained threat | None | Attention and cognitive control | None | None |
| Avoidance of external reminders | Acute threat, potential threat and sustained threat | Approach motivation | Attention and cognitive control | None | None |
| Criterion D: negative cognitions and mood | |||||
| Inability to remember traumatic event |
Loss | None | Attention, declarative memory and cognitive control | None | None |
| Persistent exaggerated negative beliefs | Acute threat, potential threat, sustained threat and loss | None | Attention, declarative memory and cognitive control | Perception and understanding of self and of others | None |
| Distorted cognitions | Loss | None | None | Perception and understanding of self and of others | None |
| Persistent negative emotional state | Acute threat, potential threat and sustained threat | None | Cognitive control | None | None |
| Markedly diminished interest | Loss | Approach motivation, initial responsiveness to reward attainment, sustained and/or longer-term responsiveness to reward attainment and reward learning | None | None | None |
| Feelings of detachment or estrangement | None | None | None | Affiliation and attachment | None |
| Inability to experience positive emotions | Loss | Initial responsiveness to reward attainment and sustained and/or longer-term responsiveness to reward attainment | None | None | None |
| Criterion E: arousal and reactivity | |||||
| Irritable behaviour and angry outbursts | Acute threat, potential threat, sustained threat and frustrative non-reward | None | Cognitive control | None | Arousal |
| Reckless or self-destructive Dehaviour | None | Approach motivation, initial responsiveness to reward attainment, sustained and/or longer-term responsiveness to reward attainment and reward learning | Cognitive control | Perception and understanding of self | None |
| Hypervigilance | Sustained threat | None | Attention and cognitive control | None | Arousal |
| Exaggerated startle response | Acute threat, potential threat and sustained threat | None | None | None | Arousal |
| Problems with concentration | Sustained threat | None | Attention, cognitive control and working memory | None | Arousal |
| Sleep disturbance | None | None | None | None | Arousal and sleep-wakefulness |
| Dissociative symptoms | |||||
| Depersonalization | Acute threat | None | Perception and cognitive control | Perception and understanding of self | None |
| Derealization | Acute threat | None | Perception and cognitive control | Perception and understanding of others | None |
DSM-5, Diagnostic and Statistical Manual of Mental Disorders, fifth edition; RDoC, Research Domain Criteria, issued by the National Institutes of Mental Health.
Symptoms and diagnosis
Epidemiological studies show that PTSD is quite prevalent, with lifetime estimates ranging from 1.3% to 12.2% depending on the population studied13. PTSD is also very costly to our society in terms of both treatment cost and loss of productivity14. Furthermore, PTSD is highly comorbid with depression, other anxiety disorders and substance abuse and is a leading cause of suicide. Women are at much greater risk of developing PTSD than men and suffer from more debilitating symptoms after trauma15.
A diagnosis of PTSD according to DSM-5 relies upon several key criteria: an individual must have been exposed to a death, threatened death, actual or threatened serious injury, or actual or threatened sexual violence (criterion A) and this must have been followed by ongoing symptoms of intrusive re-experiencing (criterion B, at least one such symptom required), avoidance (criterion C, at least one such symptom required), negative cognitions and mood (criterion D, at least two such symptoms required) and arousal and reactivity (criterion E, at least two such symptoms required), resulting in functional impairment and not being caused by other medical or psychiatric illness11. However, evidence suggests that considerable heterogeneity exists within the syndrome and that the set of symptoms present is dependent on factors including the timing of the traumatizing events (in childhood versus adulthood, for example) and the type of exposure. For example, early-onset chronic interpersonal trauma and adult sexual assault are associated with more severe PTSD symptoms and more emotional dysregulation than late-onset or single-event traumas16–17. Recent neurobiological evidence of a dissociative subtype of PTSD and its subsequent addition to DSM-5 confirms that considerable heterogeneity remains within the syn drome18 (Box 2). Within the RDoC, these DSM-5-defined symptoms of PTSD can be seen to be distributed across multiple domains, including systems related to negative valence, positive valence, cognition, social processing and arousal19 (TABLE 1).
Box 2|. Post-traumatic stress disorder dissociative subtype
Traumatic dissociation encompasses a range of distinct, yet clinically interrelated, symptoms, including depersonalization, derealization, amnesia, numbing, intrusive flashbacks, passive influence phenomena and identity disturbances190,191. The Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DsM-5), recognized a dissociative subtype of post-traumatic stress disorder (PTsD), characterizing individuals who, in addition to the criteria explicated in this article, experience dissociative feelings of detachment from their body, thoughts and surroundings. It is estimated that 13–30% of individuals with PTSD meet the criteria for the dissociative subtype18.
Evidence from studies that have used symptom provocation paradigms in individuals with PTSD has implicated differential patterns of brain activity and bodily arousal in those with high versus low levels of dissociative symptoms192–196. In such paradigms, PTSD symptoms are elicited by exposing participants to reminders of their past traumatic events197,198. During this exposure, participants with low levels of dissociation reported classic re-experiencing symptoms associated with hyperarousal, whereas those with high levels of dissociation reported entering into a state characterized predominantly by feelings of detachment and numbness. These symptoms were reflected in differential patterns of neural activity in regions related to emotion regulation and inhibition of limbic regions (ventromedial prefrontal cortex (vmPFC)), emotional expression, conflict monitoring, integration of threat information (dorsal anterior mid-cingulate cortex (daMCC)195,199,200) and salience detection (amygdala201). Specifically, the low-dissociation group exhibited the ‘classic’ PTSD neural signatures of decreased vmPFC activity, increased amygdala and insula activity and increased heart rate in response to trauma cues (see the figure). By contrast, the high-dissociation group exhibited the opposite pattern — increased vmPFC and daMCC activity, decreased amygdala and insula activity and no change in, or decrease in, heart rate. Resting-state functional connectivity analyses also supported this pattern: both the basolateral amygdala (BLA) and centromedial amygdala subregions demonstrated increased connectivity with prefrontal structures in the dissociative subtype of PTSD compared with classic PTSD202. The BLA also exhibited increased connectivity with subregions of the insula203, and the ventrolateral periaqueductal grey exhibited increased functional connectivity with brain regions linked to passive responses to threat in individuals with the dissociative subtype, when compared with those with classic PTSD204.
Subsequent studies in individuals with dissociative identity disorder (DID) have built on these PTSD studies. According to predominant theories, DID is a type of developmental post-traumatic adaptation typically associated with chronic childhood trauma205. In addition to depersonalization and derealization symptoms, individuals with DID experience amnesia and identity disturbances in which their own thoughts, emotions, bodily sensations and sense of self sometimes feel like they are not their own, despite intact reality testing, in which individuals with DID know these experiences must be their own190,205. In a symptom provocation paradigm, individuals with DID exhibited either classic PTSD neural signatures or dissociative PTSD neural signatures to trauma cues when they were feeling activated and hyperaroused or numb and detached, respectively206,207.
These findings are consistent with a ‘top-down’ overmodulation hypothesis of the dissociative subtype of PTSD. This theory suggests that, in individuals with high levels of dissociation, top-down cortical activation overmodulates limbic activity, inhibiting sympathetic bodily arousal and upregulating parasympathetic activity192,199. The daMCC in particular may also be highly involved in the appraisal and expression of negative emotion in these paradigms and may drive the increased top-down modulation of the amygdala by the vmPFC199,208. The existence of the dissociative subtype may have implications for treatment. Given evidence that dissociation affects emotional learning209, individuals with this type of PTSD may have a differential response to current cognitive behavioural therapies; evidence is currently mixed on this front210–219.
| Classic PTSD | Dissociative identity disorder | PTSD dissociative subtype |
| Responses to tra urn a-related cues characterized by | Responses to trauma-related cues fluctuate between classic and dissociative subtype neurobiological patterns | Responses to trauma-related cues characterized by |
| • intrusive re-experiencing symptoms | • symptoms of detachment and numbness | |
| • behavioural activation | ||
| • increased physiological arousal | • behavioural deactivation | |
| • increased amygdala and insula activation | • decreased and/or maintained physiological arousal | |
| • decreased vmPFC activation | • decreased amygdala and insula activation | |
| • increased vmPFC activation |
Intrusion symptoms
Criterion B of the DSM-5 criteria for PTSD diagnosis focuses on intrusion symptoms, in which the traumatic event is persistently re-experienced11. This re-experiencing can occur in a number of ways, including recurring, involuntary intrusive memories, dissociative reactions (such as flashbacks), distressing dreams and physiological reactivity11. It has been suggested that these intrusive symptoms are a product of emotional under modulation — that is, a failure of the cortex to inhibit the limbic system20. In support of this theory, individuals with PTSD often demonstrate increased activity in the amygdala and decreased medial prefrontal cortex (mPFC) activity during symptom provocation studies when compared with individuals without PTSD21–30. The amygdala is a key limbic structure involved in emotional reactivity, over which regions of the mPFC exert an inhibitory effect31. Furthermore, reports of in-the-moment re-experiencing symptoms in individuals with PTSD have shown some association with increased insula activity and decreased rostral anterior cingulate cortex (rACC) and inferior frontal cortex activity32. The insula is thought to be involved in interoception and bodily awareness33, whereas the rACC and lateral prefrontal cortex are often involved in attention, emotion and arousal regulation34. Together, these results suggest that, in PTSD, there is a failure of top-down cortical inhibition (from the mPFC or rACC, for example) of the reactivation of memory traces associated with trauma-related thoughts and feelings, many of which may be centred around the visceral experience of one’s body (in which the amygdala and insula have a role).
The failure of such top-down cortical inhibition has also been suggested to be related to impairments of fear extinction35. In classical conditioning theory, fear conditioning occurs when a neutral cue (such as a tone or an image) is paired with an intrinsically aversive cue (such as an electric shock). Subsequent presentations of the neutral cue provoke a fear response. Fear extinction refers to the gradual decrement of the fear response to a conditioned stimulus when it fails to be reinforced (by the repeated presentation of the conditioned stimulus in the absence of the aversive cue, for example)36. There is strong evidence that fear extinction involves the formation of a competing new memory that inhibits the fear response, rather than an erasure of the original memory36,37; however, fear memories may also weaken during recall through a process called reconsolidation38. There have been studies showing that individuals with PTSD are able to encode such new fear extinction memories but have difficulty retaining them35,39–41, suggesting that deficits in fear extinction retention underlie PTSD. Furthermore, there is evidence that the size and activity of the ventromedial prefrontal cortex (vmPFC) is associated with the extent of fear extinction deficits in individuals with PTSD42 as well as evidence for changes to the functional connectivity between the left vmPFC and the amygdala in individuals with PTSD43. Given that vmPFC-mediated inhibition of the amygdala is thought to be necessary for fear extinction31, these changes could offer a mechanistic basis for the decrements in extinction retention observed in PTSD subjects. There is also some evidence to support the idea that patients with PTSD might exhibit an increased capacity for fear conditioning itself44 or a greater propensity for increased fear generalization45–47; however, there have been no longitudinal studies of these traits in patients at risk of developing PTSD, making it unclear whether these characteristics are a cause or an effect of developing PTSD.
Animal models of fear conditioning and fear extinction have highlighted the importance of the relation ship between the vmPFC and the amygdala in both the expression and extinction of fear behaviours (BOX 3). Although there is some debate among comparative neuroanatomists regarding the precise homologies of the human and rodent prefrontal cortex (PFC), projection patterns and cytoarchitecture suggest that the vmPFC in rodents is composed of the prelimbic and infralimbic cortices48,49. Pharmacological or electrolytic inhibition of the prelimbic cortex prevents expression of conditioned fear behaviours, whereas inhibition of the infralimbic cortex impairs retrieval of fear extinction50,51.
Box 3|. Animal models of post-traumatic stress disorder
The sprawling set of symptoms, many of which can be defined only in a subjective manner, that characterize post-traumatic stress disorder (PTsD) in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DsM-5), make it challenging to develop suitable animal models of the disorder. Moreover, the lack of identified alleles with high genetic penetrance for increasing PTsD risk makes it difficult to create PTsD animal models with high construct validity — that is, models that translate a known biological cause in humans into a disorder in animals220. Lacking known causative agents, the PTsD research community has therefore historically relied upon animal models with high face validity — that is, models that recapitulate certain behavioural features of human PTsD.
Commonly used behavioural paradigms that have been used to assess PTsD-like symptoms in animal models include chronic social defeat stress, inescapable foot shock, early-life stress and stress-enhanced fear learning221,222. These tests have different advantages and disadvantages, which have recently been reviewed221; however, they all suffer from a lack of robustness and from low construct validity. One criticism that has been levelled against PTsD animal models is that responses to stress are typically measured in all animals, rather than in a select group of vulnerable animals, as happens with human PTsD. However, it has been slowly recognized that there is still variability in many animal models, with some animals being stress-susceptible and others stress-resilient. For instance, one group demonstrated a predator-based psychosocial stress paradigm that can generate groups of rats with either extreme (PTsD-like) or minimal (resilient) responses to stress223.
Given the current limitations in our understanding of the genetic basis of PTsD and our rapidly growing understanding of the neural circuitry of PTsD, a promising future avenue for developing animal models of PTsD could be to use optogenetic manipulations of selected neuronal populations to recapitulate the dysfunctional circuit features of the disorder. This method would effectively model the specific circuit disruption directly and there would be less focus on interpretation of disparate and, at times, evolutionarily non-conserved behaviours across species and more focus on the conserved neural circuits.
Experiments using optogenetics have confirmed and refined this dichotomy. These studies have shown that, in mice, optogenetic inhibition of prelimbic cortex projection neurons inhibits the expression of fear behaviours52, whereas the release of prelimbic cortex projection neuron firing through optogenetic inhibition of parvalbumin-expressing interneurons drives expression of fear behaviours53. Silencing infralimbic projection neurons during extinction training prevents the retention of fear extinction memories, and protein synthesis within the infralimbic cortex is necessary for retention of fear extinction54,55. Interestingly, silencing infralimbic projection neurons after extinction has occurred has no impact on extinction retrieval, suggesting that these neurons are crucial for formation, but not retention, of extinction memories54. A recent study may explain this phenomenon by suggesting that it is the activation of basolateral amygdala (BLA) inputs within the infralimbic cortex that facilitates fear extinction retention rather than the activation of infralimbic neuronal somata56. It is not currently known whether prelimbic and infralimbic projection neuron cell types are molecularly distinct or whether there is heterogeneity among these neurons. The identification of genetic markers for neurons that project from the infralimbic cortex to the BLA could lead to pharmacological targets for increasing the encoding of fear extinction memories.
Avoidance
Criterion C of the DSM-5 framework encapsulates persistent, effortful avoidance of distressing trauma-related stimuli11, which can include thoughts, feelings and environmental cues (including people, places, conversations, activities, objects and situations). Theoretical commentary has suggested that an imbalance in approach versus avoidance behavioural responses is at the centre of PTSD dysfunction57; however, the neurobiological underpinnings of trauma-cue avoidance are surprisingly understudied in human-based PTSD research.
A study using a symptom provocation paradigm found that the experience of actively avoiding trauma-related thoughts or feelings was associated with decreased activity in multiple regions of the anterior cingulate cortex (ACC) and inferior frontal cortex and with increased activity in the superior temporal cortex32. Avoidance has also been examined in the context of fear conditioning. In a recent fear conditioning study, the severity of interview-assessed PTSD avoidance symptoms within the past month was shown to be positively associated with hippocampal responses to context cues and conditioned stimuli in the acquisition phase of a fear conditioning paradigm and to amygdala, hippocampus and insula activity during the extinction phase58. These findings suggest that avoidance symptoms and fear circuit activation are closely linked and that avoidance is central to the fear extinction deficits reported in PTSD (see above). By definition, a cue or context that is avoided cannot be extinguished in a normal fashion; thus, many behavioural therapy approaches to the treatment of PTSD focus on decreasing such avoidance behaviours.
A growing literature describes the brain circuits involved in determining whether an animal will respond to a threat-associated stimulus with passive avoidance (freezing; see also the dissociative subtype of PTSD, Box 2) or more active avoidance strategies (such as running towards a safe chamber)59. Both strategies can be maladaptive if used excessively or in a fashion in which the individual has no control; however, there is evidence to suggest that active avoidance strategies can dampen responses to subsequent stressors60. For example, a recent human study with a clever yoked-subject design showed a decreased skin conductance response in non-psychiatric control participants who were given access to an active avoidance strategy to prevent electrical shock compared with those who were not61. This decreased skin conductance was observed both for subsequent presentations of the extinguished conditioned stimulus and for presentation of a novel conditioned stimulus. In this case, active avoidance was shown to be associated with changes in striatal blood-oxygen-level-dependent (BoLD) signalling.
In rodents, avoidance behaviours are often studied using a shuttlebox learning paradigm. In this test, rodents first undergo Pavlovian threat conditioning, in which a previously innocuous conditioned stimulus becomes paired with an aversive unconditioned stimulus. In an inescapable chamber, rodents will respond by freezing when the conditioned stimulus is subsequently presented. In a second phase of the task, the rodents learn that if they run into a second compartment when presented with the conditioned stimulus within the testing chamber, they can avoid receiving the unconditioned stimulus. Rodents will thus learn to run from the context in which the conditioned and unconditioned stimuli were paired (active avoidance) instead of passively freezing. The propensity to pursue active avoidance strategies is heterogeneous in rodent populations and as such is thought to be a possible model for resilience62.
Several lesion studies in rodents have shown that passive freezing responses are mediated by signals transmitted from neurons within the lateral amygdala to the central amygdala and then to neurons within the periaqueductal grey (PAG)63,64 (FIG. 3). Active avoidance strategies appear to require signalling from the lateral amygdala to the basal amygdala and then to the shell of the nucleus accumbens65. The infralimbic cortex is also critical for active avoidance66. Active avoidance and freezing appear to be mutually inhibitory, as lesions of the central amygdala increase active avoidance, whereas lesions of the infralimbic cortex, which releases central amygdala inhibition, create increases in freezing66. Furthermore, recent experiments have shown that, within the central amygdala, there are microcircuits of mutually inhibitory corticotropin-releasing hormone (CRH)-expressing and somatostatin-expressing neuronal subpopulations that mediate active avoidance and freezing, respectively67.
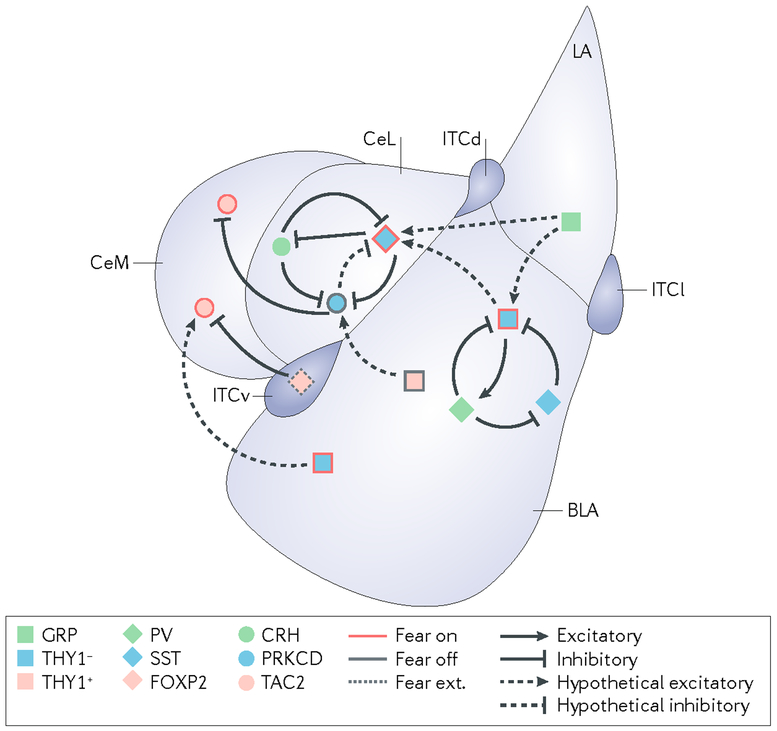
Fig. 3|. Amygdala microcircuits implicated in fear conditioning.
The schematic offers a simplified representation of the mouse amygdala microcircuits that are currently implicated in fear conditioning. During fear conditioning, output neurons from the central amygdala increase their responsiveness to a conditioned stimulus. This increased responsiveness is likely to occur through the mutual interaction of two parallel ‘fear on’ and ‘fear off’ pathways that project to these neurons from the basolateral amygdala (BLA) and lateral amygdala (LA). There is an additional pathway for the establishment of competing fear extinction memories that is likely to originate from neurons in the infralimbic cortex that activate intercalated cells in the amygdala (‘fear ext.’ neurons). Although our understanding of these pathways remains incomplete, several cell types have been demonstrated to modify the expression of fear behaviours. Excitatory connections are indicated with arrows. Inhibitory connections are indicated with blunt arrows. Solid lines designate proven connections, whereas dashed lines illustrate hypothetical connections. CeL, lateral division of the central amygdala; CeM, medial division of the central amygdala; CRH, corticotropin-releasing hormone; FOXP2, forkhead box protein P2; GRP, gastrin-releasing peptide; ITCd, ITCv and ITCl, intercalated cell masses dorsal, ventral and lateral, respectively; PRKCD, protein kinase C delta type; PV, parvalbumin; SST, somatostatin; TAC2, protachykinin 1 (also known as TAC1); THY1, Thy-1 membrane glycoprotein.
Avoidance behaviour may also be conceptualized more generally. When interacting with an uncertain environment, an organism must make decisions about whether to approach or avoid external stimuli. A recent hypothesis proposed by several groups reconceptualizes amygdala function as the gating of decisions about ‘behavioural engagement’; that is, the decisions that an organism makes when approaching its environment68,69. This hypothesis stems from work that has shown that amygdala lesions can affect risk-taking behaviour in rats in a food foraging paradigm70 as well as the identification of populations of neurons within the BLA that respond to movement during a risk-taking behavioural task rather than to the presence of a threat per se71. This finding brings the rodent literature more in line with the widespread changes in activation patterns seen in the human neuroimaging literature. Deficits in a ‘behavioural engagement’ circuit may align more closely with the functional impairments seen in individuals with PTSD than do the deficits pre dicted by the more traditional model that theorizes that the amygdala acts only as a locus for conditioned fear72.
Altered cognition and mood
Criterion D of the DSM-5 PTSD diagnosis describes negative alterations in cognition and mood that begin or worsen after a traumatic event11. This criterion includes memory deficits, distorted beliefs, persistent negative emotions, diminished interest in previously rewarding activities, constricted emotional experience and social detachment symptoms11. Many of these symptoms are nonspecific and highly overlapping with the symptoms of depression. For the most part, the neurobiological understanding of these symptoms is still preliminary; however, emerging evidence again points towards potential aberrations in regions that are part of limbic circuits (particularly the hippocampus and amygdala) and their relationship with top-down prefrontal cortical control.
Dissociative amnesia and memory deficits.
In addition to the maladaptive enhancement of emotional memories described above, some forms of memory can be impaired in PTSD. A marked inability to recall key features of the traumatic event is a common occurrence in PTSD and is frequently referred to as dissociative or psychogenic amnesia. This amnesia often occurs in the realm of declarative memory — that is, memory that can be explicitly recalled, such as semantic or episodic memory73 — whereas nondeclarative and/or implicit memory is preserved. Individuals with PTSD also report the experience of memory fragmentation11. These declarative memory deficits have been proposed to be the result of impaired encoding or retrieval mechanisms, or a combination thereof.
A PTSD-specific neurobiological model focusing on the hippocampus has been proposed to account for these memory deficits74. The hippocampus is crucial for memory and learning processes and in particular for declarative memory73. The hippocampus is involved in the initial storage of this type of memory and in integrating aspects of memory during retrieval. A sizeable literature demonstrates that there is reduced hippocampal volume in individuals with PTSD75. This observation has been established in studies of different types of trauma and different sample populations and was supported by the recent findings of the largest brain imaging study of PTSD to date76. However, whether trauma leads to hippocampal atrophy in individuals who develop PTSD or whether having small hippocampi predisposes an individual to PTSD remains controversial77. In positron emission tomography and functional MRI (fMRI) studies, individuals with PTSD have been demonstrated to exhibit decreased hippocampal activity while taking part in a declarative memory task, when compared with trauma-exposed controls without PTSD78, as well as decreased hippocampal activity and a failure to recall extinction learning when taking part in a fear conditioning paradigm35. It remains to be determined whether reduced hippocampal activity is caused by the reduced size or vice versa and whether reduced activity and/or size affects the capacity to encode or store memories.
Extensive research also documents deficits in a number of executive function tasks in individuals with PTSD when compared with trauma-exposed controls79. However, there is also evidence to suggest that there are subtle deficits in these functions that exist before the trauma exposure in PTSD cohorts, suggesting that individuals with executive function deficits have a heightened risk of developing PTSD after trauma exposure80. It is thought that these deficits would then be further exacerbated after trauma exposure and may contribute to lack of recovery80. Evidence of changes to neural circuitry that may explain this finding is varied; however, most evidence suggests that there are changes in PFC engagement81–83. Individuals with PTSD have increased prefrontal activity during sustained attention tasks, and decreased prefrontal involvement when participating in more taxing inhibition-related tasks, when compared with those without PTSD81–83. This pattern could be a by-product of attentional hypervigilance associated with PTSD and an inability to inhibit this pattern of activation during more demanding tasks. Alternatively, it could reflect a compensatory mechanism for maintaining focus during sustained attention tasks that stops working with more difficult inhibition tasks80. Another interpretation is that this pattern of activity reflects a decreased ability to regulate midline cortical self-referential processing activity during attentional tasks84. It is yet to be determined how these changes in PFC engagement might also alter the hippocampal-related declarative memory processes discussed above.
Studies that have used animal models have given us more information on the cellular-level changes in the hippocampus that may drive PTSD memory deficits. In rodent models, both acute and chronic corticosterone secretion occur in response to traumatic experiences85 and can induce profound molecular changes in the hippocampus. These changes include decreased brain-derived neurotrophic factor (BDNF) and cAMP-responsive element-binding protein 1 (CREB1) expression in the dentate gyrus, impaired neurogenesis in the dentate gyrus, decreased long-term potentiation in the CA1 region and dendritic retraction in CA3 (REFs86–88). The loss of dendritic complexity observed in rodents after stress suggests a mechanism for the hippocampal atrophy observed in patients with PTSD. There are likely to be many more molecular pathways that are involved in adaptations to both acute and chronic stress. Indeed, a recent study showed that there are hundreds of gene expression changes within CA3 neurons after rodents are exposed to either acute forced swim stress or chronic restraint stress; however, the gene expression signatures observed in these two conditions were almost completely non-overlapping89.
Research with rodents and nonhuman primates also demonstrates that exposure to an acute, uncontrollable stress impairs performance in cognitive and memory tasks that depend heavily on the PFC90. For example, restraint-stressed rats display impaired performance in a delayed spatial alternation task, a rodent behavioural test of spatial working memory91. The stressed animals made more perseverative errors on the task, suggesting a lack of attention and short-term memory as well as cognitive and/or behavioural inflexibility91. Interestingly, correlated neural activity in the PFC and hippocampus is believed to be important for successful performance on this task: hippocampal theta oscillations have been shown to be phase-locked with both theta and single-unit activity in the PFC during the performance of a similar task92. Furthermore, a genetic mouse model of schizophrenia in which this hippocampus-PFC synchrony is disrupted also shows impairments in the acquisition of spatial working memory, and a recent study in which optogenetic manipulation was combined with a spatial working memory paradigm confirmed that gamma activity in the pathway from the ventral hippocampus to the PFC is crucial for encoding task-relevant cues93. These findings suggest that deficits in memory processes in patients with PTSD are caused by atypical coordination of neural synchronization in large-scale networks including the PFC.
Persistent negative emotions.
Criterion D of the DSM-5 PTSD diagnosis also includes the experience of enduring negative trauma-related emotions such as fear, horror, anger, guilt or shame11. Constructivist views of emotion suggest that our emotional experience is generated through a dynamic interaction of more basic processes in memory, perception and attention94,95. Consistent with this idea, a broad network of cortical and subcortical regions is associated with our experience of emotion96. In particular, recent neuroimaging evidence has associated fluctuating valence experience with activity in the orbitofrontal cortex and arousal with amygdala activation across different emotions (such as fear, sadness and happiness)97.
A key characteristic of the negative emotional experiences of individuals with PTSD is that they seem to persist despite interfering with the day-to-day functioning of the individual. The persistence of these negative emotions is often described as a failure of emotion regulation — that is, the ability to exert ‘voluntary control’ over one’s emotional responses98. For example, individuals with PTSD were shown to have poorer ability to down-regulate emotional reactions to negative pictures than trauma-exposed and non-trauma-exposed controls, and this was associated with decreased PFC activity99. Similarly, the experience of negatively valenced (but not traumatic) memories elicited emotions of sadness and anxiety in individuals with PTSD and was associated with decreased ACC and thalamus activity — patterns of activity analogous to those seen when personally triggering trauma cues are presented100. Likewise, viewing of negatively valenced pictures was associated with lower vmPFC activity in individuals with PTSD than it was in trauma and non-trauma-exposed controls34. The vmPFC and ACC are regions that are often associated with emotion regulation101.
There is some evidence to suggest that processing of emotional valence itself is altered in PTSD, in particular for individuals who have suffered childhood maltreatment102–103. Given our desire to understand the basic neuroscience of negative emotions, there has been considerable recent effort made to use modern optogenetic and chemogenetic tools to understand the coding of valence in the mouse brain subpopulations of neurons in the BLA104, central amygdala105, nucleus accumbens106 and ventral tegmental area (VTA)107, and other regions appear to respond to the appetitive or aversive qualities of a stimulus, independent of its sensory features10. However, it is not yet known whether these neurons can be defined by their molecular properties, projection targets or some combination of these characteristics10. It is also not yet known to what extent these valence-encoding neurons are fixed in the nature of their response (that is, whether they always respond to either positive or negative cues) or exhibit plasticity. There is some evidence to suggest that such plasticity depends on brain region: an experiment that attempted to ‘flip’ the responses of appetitive and aversive neuronal populations was successful in the dentate gyrus but not in the BLA108. There is also evidence to suggest that the ‘tunable range’ of valences to which these neurons respond depends on the emotional state of the animal: the valence tuning of neurons in the nucleus accumbens changes during stress109. Given that these valence-coding neurons are likely to influence affective behaviours, these findings suggest that a stressful context can influence the range of such behaviours available to an organism. A similar mechanism may help to explain how trauma can alter the range of affective experience available to individuals with PTSD.
Constricted affect and interest.
Individuals with PTSD frequently exhibit markedly diminished interest in activities that were important to them before the trauma, demonstrate constricted affect and have a persistent inability to experience positive emotions (anhedonia11). Dysfunction in reward processing is thought to be one of the mechanisms underlying these experiences110,111. Reward processing can be divided into different components: an approach phase, in which rewarding stimuli are sought out, and a consumption phase, in which hedonic pleasure is experienced once the reward is acquired111. Evidence points towards potential dysfunction in both components of this process in PTSD112. The brain’s reward circuitry includes regions responsible for evaluating a stimulus as rewarding and regions involved in acting to acquire the reward (including the VTA, amygdala, orbitofrontal cortex, anterior insula, ACC, dorsomedial prefrontal cortex (dmPFC), striatum and motor cortex)110,113. There is evidence of hypoactivity in both the striatum and PFC in response to rewarding stimuli for individuals with PTSD in comparison to controls114,115. Furthermore, one study found that less activity in the striatum in the reward paradigm was associated with motivational and social deficits per self-report114. These studies imply that reduced activity in these regions leads to altered reward processing in PTSD.
One human neuroimaging study has focused on the experience of a restricted range of affect — also termed ‘emotional numbing’116 — in individuals with PTSD. In this study, decreased dmPFC activity was associated with experiences of being emotionally numb in both positive and negative scenarios designed to elicit emotional imagery117. Alexithymia describes the condition of having difficulty recognizing and naming emotional states and is considered to be closely related to (or perhaps a result of) anhedonia and emotional numbing. Specifically, in participants with PTSD, alexithymia has been associated with decreased vmPFC, anterior insula and inferior frontal gyrus activity during paradigms designed to trigger PTSD symptoms116. These are areas associated with a visceral sense of emotion, self and emotion regulation.
In rodents, chronic stress protocols are commonly used to induce anhedonia-like states, which are then assayed with behavioural paradigms such as the sucrose preference test. In this paradigm, the loss of a rodent’s natural preference to seek out a rewarding sweet solution is used as a surrogate for anhedonia symptoms. These models are typically discussed with respect to depression; however, they may also be applicable to our understanding of PTSD given that the inability to experience positive emotions can also occur in this disorder. Studies in which optogenetic manipulations have been focused on dopaminergic neurons in the VTA have provided valuable insight into dysfunctions in subcortical circuits that are related to anhedonia-like symptoms. Specifically, phasic activation of VTA dopaminergic neurons can promote sucrose preference following subthreshold social defeat stress118. Interestingly, in a parallel study, optogenetic activation of nucleus accumbens-projecting VTA dopaminergic neurons following longer chronic mild stress also alleviated sucrose preference deficits119. This finding suggests that there are more complex long-term neuronal adaptations within the dopaminergic systems after stress exposure that may also occur in more chronic forms of PTSD.
In addition to the subcortical dopaminergic circuits, the PFC and its projections have also been targeted using optogenetics. High-frequency stimulation of PFC terminals in the nucleus accumbens was found to reduce sucrose preference in mice following chronic social defeat stress120. Moreover, a recent study that used a combination of local optogenetic stimulation and global brain-wide fMRI in rats highlighted an inhibitory influence of the PFC on reward-related behaviour and anhedonia121. This study demonstrated that hyperexcitability in the PFC led to a reduction in sucrose preference through top-down suppression of striatal responses to dopamine release and by driving dynamic interactions in corticolimbic areas. This study is of particular interest for translational work because it demonstrates how microcircuit-based manipulations can be related to larger changes in brain activity and offers a template for ways in which we may move forward in our understanding of how larger changes in brain BOLD activity may relate to the firing patterns of artificially activated neuronal populations.
Altered arousal and reactivity
PTSD criterion E in DSM-5 includes alterations in arousal and reactivity that started or were exacerbated after the traumatic event11. Arousal and reactivity changes can include any combination of hypervigilant, irritable, aggressive, self-destructive or reckless behaviours, exaggerated startle responses, problems with concentration or sleep disturbances11. This cluster has traditionally been the most well studied of the PTSD symptoms and is in some ways the most easily translatable to nonhuman animal subjects. Evidence from these studies suggests that both heightened salience detection and dysfunctional emotion or arousal regulation underlie symptoms in this cluster6,122. The overarching theory emerging from human and animal subject experiments is that symptoms from this cluster are generally likely to arise owing to decreased activity in the mPFC and ventral hippocampus and hyperactivity in the amygdala and the bed nucleus of the stria terminalis (BNST)122, although investigation into the microcircuitry that produces these global regional effects is ongoing.
Hypervigilance.
For a long time, neuroimaging stud ies of PTSD focused upon the role of the amygdala in mediating symptoms of hypervigilance. Across paradigms and sample populations, individuals with PTSD typically display increased amygdala activity123. Even at rest, there is evidence that the salience network, which includes the amygdala, ‘dominates’ processing in individuals with PTSD rather than the more typical default-mode network activity observed in controls124. Interestingly, however, individuals with Urbach-Wiethe disease, a rare genetic disorder that presents with focal damage to the BLA, are hypervigilant to fear cues, suggesting that the BLA plays a role in inhibiting hypervigilant monitoring125. The discrepancies between these findings may relate to differences in the activity of amygdala subnuclei, and literature from rodent studies has begun to elucidate many layers of complexity within amygdala microcircuitry.
In mice, there have been a number of studies that suggest that different subpopulations of neurons in the BLA can either trigger or inhibit anxiogenic responses in mice. Optogenetic activation of all cell bodies within the BLA leads to anxiogenic responses, whereas selective activation of BLA terminals in the central amygdala triggers anxiolysis126. The projection-specific modulation of threat responses by the BLA seems to be mediated by distinct molecularly defined sub populations of ‘fear on’ and ‘fear off’ neurons within the BLA127,128 and the central amygdala129–130. Given the functional heterogeneity of neurons within these regions, it may be difficult to interpret human neuroimaging studies that lack the resolution to observe activity at a cellular level.
Both human and animal data implicate the BNST in the production of hypervigilant states, although the BNST is difficult to precisely and reliably delineate with current human neuroimaging methods, and results should be interpreted cautiously. That said, human neuroimaging studies of healthy participants show that the BNST, along with the insula, is activated during threat monitoring tasks131,132. To our knowledge, the BNST has not emerged as a key area of differential activation in individuals with PTSD. However, research in rodent models suggests that it is a key structure for future investigations.
Studies using optogenetics have renewed interest in the role of the BNST in producing rodent anxiety-like behaviours133. Recent work suggests that the complicated subnuclear structure of the BNST can be roughly divided into two functional subregions: the oval BNST, which has been shown to control anxiogenic features of mouse behaviour, and the anterodorsal BNST, which mediates anxiolysis134. Another group found that a behavioural rodent model of PTSD, in which mice were exposed to a trauma (sustained, inescapable foot shock) followed the next day by a trigger (shorter duration foot shocks), demonstrated features of hypervigilant behaviour135. They observed decreased risk assessment in a light-dark paradigm, lower pre-pulse inhibition and higher light-phase locomotion, some of which were blocked by the optogenetic inhibition of a subpopulation of BNST neurons that express CRH receptor 2 (CRHR2)135,136. These data, although incomplete, implicate alterations in both amygdala and BNST circuits in the production of behaviours associated with hyperarousal and hypervigilance.
Aggressive behaviour.
Individuals with PTSD will frequently demonstrate reactive aggression to perceived threat. Our understanding of the neural circuits mediating aggression is still preliminary. Neuroimaging studies implicate the amygdala and PAG in threat detection, and activation in some of these regions is altered in individuals with PTSD137. Other studies have found that activation of the locus coeruleus, an important structure in the hormone cascade associated with the autonomic stress response, is linked specifically to aggression138,139. It is hypothesized that locus coeruleus activity helps to orient attention to salient information and that changes to activity within this region may alter threat reactivity140.
Perhaps more importantly, aggressive and/or impulsive behaviour is also related to a failure of top-down control of these circuits. Some studies have found that medial PFC structures regulate threat detection and help us to select an appropriate behavioural response given the broader context141,142. For example, the vmPFC is implicated in the regulation of emotion, including aggression143,144, and angry rumination is associated with dorsal ACC activity and individual differences in aggression145. Orbitofrontal cortex and BLA circuitry are also important in the regulation and maintenance of emotional responses142. Orbitofrontal cortex dysfunction in particular has been linked to aggressive and/or impulsive behaviour146–148. Specifically, in PTSD there is empirical evidence of differential activity in the orbitofrontal cortex, vmPFC and locus coeruleus in anger-related paradigms140,142.
In animal models, we currently have limited understanding at a cellular level of how circuits related to fear conditioning may influence subsequent development of aggressive behaviours: there have been some studies implicating the medial amygdala and ventromedial hypothalamus in rodent aggression149–151. A population of neurons within the central amygdala has recently been shown to mediate mouse predatory behaviour152. However, as in the other limbic regions discussed above, parallel, opposing pathways that mediate antithetical behavioural responses are likely to exist within both of these regions. Optogenetic and chemogenetic manipulations have demonstrated the existence of these intermingled pathways150,153, but we have limited understanding of the extent of these circuits and how they change their firing patterns in response to stress.
Unifying themes and future directions
A few overarching theoretical ideas have emerged from the recent advances in PTSD research outlined above. The studies discussed above demonstrate the existence of parallel, mutually inhibitory pathways within the larger brain regions that are implicated in PTSD. These ‘push-and-pull’ circuits, which have a long history in our understanding of the functioning of the striatum154, have now been identified in the rodent BLA127,128, central amygdala129 and BNST134 in addition to the midline mPFC51. The existence of these anatomically intermixed opposing pathways complicates our interpretation of the human PTSD neuroimaging literature, which lacks the spatial resolution to parse these opposing pathways and necessitates a deeper understanding of the molecular fingerprints of the neuronal cell types that define these pathways to more directly target potential therapeutics.
At the same time that our knowledge of microcircuitry has deepened, our understanding of the functional roles of larger brain regions has evolved, both implicating novel brain regions in the pathogenesis of PTSD (such as the BNST) and challenging traditional dogma about regions long understood to contribute to PTSD pathogenesis (such as the amygdala). The reconceptualization of amygdala function, from playing a restricted role in conditioned fear learning to a model in which it helps to detect salience and coordinate behavioural engagement, helps to explain how a deficit in amygdala circuitry might affect multiple domains of PTSD symptoms, including avoidance, re-experiencing and the altered perception of valence.
As we illustrate above, preclinical and clinical research has begun to elucidate the neurocircuitry of PTSD, and there are areas of convergence between the two approaches. The stunning growth in our understanding of the microcircuits governing threat processing, avoidance, reward and arousal have been made possible by circuit-altering technological breakthroughs. However, there remains a need within animal model research for improved technologies to allow monitoring of neuronal activity with cellular resolution throughout the entire brain rather than in only certain regions of interest. The production of brain-wide quantitative data sets will offer unbiased insights into how neural dynamics change during different cognitive demands and how these demands are affected by stress and by brain disorders.
Within human neuroimaging research, the need exists for the development of imaging technologies that can offer spatial resolution at the cellular level and temporal resolution at the millisecond level. However, until such a technology becomes available, we need to invest further in strategies to translate regional changes in BOLD signal activation into putative models of microcircuit alteration. The technique of optogenetic fMRI offers one possible path forward in this endeavour155,156. By mapping BOLD changes observed from optogenetic manipulations, it may enable the development of a library of BOLD signal changes created by manipulation of certain neuronal populations and the ability to more closely target the circuits creating dysfunction in PTSD and other neuropsychiatric disorders.
Given the advances in our understanding of the neurocircuits in PTSD, it is natural to ask whether these discoveries offer therapeutic hope. There is currently some effort underway to develop transcranial magnetic stimulation (TMS) and deep brain stimulation (DBS) interventions for PTSD157–161. Although the invasiveness of DBS is likely to mean that such treatments would be reserved for only the most intractable of cases, the use of TMS has been steadily increasing as a therapeutic tool in psychiatry. The mechanisms by which TMS might exert its effects to mediate symptom improvement in PTSD remain unclear; however, the concurrent combination of TMS with electroencephalography (EEG) or fMRI is becoming a powerful technology for identifying pathological changes in brain functional network connectivity and predicting the efficacy of the treatment161,162. The hope of using more targeted methodologies to modulate circuits remains. Nevertheless, these ideas are currently largely theoretical and are limited by our understanding of the neural circuitry, which lends urgency to the development of technological innovation in this area163.
Understanding whether the differences in neural activity related to PTSD are risk factors for developing PTSD or whether they are a product of the trauma exposure and/or the disorder itself is key to developing targeted methodologies to modulate circuits. Current evidence from PTSD samples and twin studies suggests that increased amygdala and dorsal ACC activity in fear learning contexts is a vulnerability that pre disposes an individual to the development of PTSD, whereas brain changes related to reduced capacities to extinguish fear (such as the reduced functional connectivity between the mPFC and hippocampus) are an acquired dysfunction164,165. More research, in particular from neuroimaging genetic twin studies and prospective longitudinal work, is needed to better understand the relationship between risk-related and acquired differences in PTSD.
Molecular neuroscience has lagged behind in the study of PTSD. There has been some dissection of the molecular properties of subtypes of neurons within the amygdala, but our understanding of the molecular fingerprints of the neuronal subpopulations in the mPFC, BNST, hippocampus and PAG and the transcriptional changes that occur within these cell types after stress and fear conditioning remains underdeveloped. These studies may be ‘low-hanging fruit’ in the identification of novel therapeutic drug targets for PTSD. The availability of genetic, projection-specific and activity-dependent means of cell-type-specific translational profiling166 in mouse models and the application of single-cell sequencing technologies such as Drop-seq167 to human post-mortem brain samples will allow this work to proceed in the near future. These approaches offer the promise of identifying unique receptor profiles for cell types of specific valence and function within the complex microcircuitry described. Such an approach could lead to targeted multidrug pharmacotherapy, based on understanding specific clusters of quantitative symptoms, with drugs targeting known symptom-related circuits.
Conclusion
Although our treatments for PTSD have yet to change, our understanding of the genetics, neural circuitry and behaviour related to the disorder and its component symptoms and intermediate phenotypes has advanced considerably in recent years. Furthermore, technological progress in preclinical models has moved rapidly in advancing our understanding of the neural circuitry underlying basic behaviours such as fear and threat processing, fear extinction, avoidance behaviour and appetitive and anhedonic behaviour as well as the effects of stress on memory and cognition. Thus, the translation of these behaviours to human trauma and stress-related and anxiety-related disorders, such as PTSD, is progressing rapidly and promises to soon lead to novel treatments and interventions based upon the underlying neurobiology.
Genome-wide association studies
(GWAS). Studies in which statistical associations between genetic variants and a disease or trait of interest are identified by genotyping individuals with disease and healthy controls for a set of single-nucleotide polymorphisms that capture variation across the entire genome.
Optogenetics
The use of genetically encoded light-activated proteins (for example, ion channels) to control the functional parameters (for example, membrane potential) of targeted neuronal populations
Chemogenetics
The use of exogenous macromolecules to manipulate activity of genetically encoded receptors with no endogenous ligands (that is, designer receptors exclusively activated by designer drugs).
Fibre photometry
Technology that utilizes an optical fibre for monitoring of activity of neuronal ensembles through genetically encoded activity indicators.
Valence
The appetitive or aversive nature of a stimulus.
Gene by environmental risk
The interaction between a genotype and environmental variation.
Symptom provocation studies
Studies designed to elicit PTsD symptoms by exposing participants to their own trauma narratives.
Fear generalization
Describes a situation in which conditioned fear responses are elicited in response to stimuli related to the conditioned stimulus.
Blood-oxygen-level-dependent (BOLD) signaling
An index of brain activation based on detecting changes in blood oxygenation with fMRi.
Memory fragmentation
Trauma memory retrieval that is experienced as only portions of various sensory and emotional representations and that lacks an integrative personal narrative.
Executive function
A set of top-down cognitive control processes including inhibition (resisting habits, temptations or distractions), working memory (mentally holding and using information) and cognitive flexibility (adjusting to change).
Long-term potentiation
A long-lasting (hours or days) increase in the response of neurons to stimulation of their afferents following a brief patterned stimulus (for example, a 100 Hz stimulus).
Salience detection
The detection of information relevant to basic biological drives and psychological needs (for example, potential threats).
Default-mode network
A large-scale brain network that is more active when individuals are not directing attention to the external environment.
Acknowledgements
The work was supported by US National Institutes of Health (NIH) grants R01MH108665, R01MH094757 and R21MH112956 to K.J.R., NIH fellowship grant F32MH109274 to L.A.M.L. and the Frazier Foundation Grant for Mood and Anxiety Research to K.J.R. K.J.R. has received research funding from the US National Institute of Mental Health, the Howard Hughes Medical Institute, the National Alliance for Research on Schizophrenia & Depression and the Burroughs Wellcome Foundation.
Footnotes
Competing interests
K.J.R. is on the scientific advisory boards for Resilience Therapeutics, the Sheppard Pratt-Lieber Research Institute, the Laureate Institute for Brain Research, the Army Study to Assess Risk and Resilience in Servicemembers (STARRS) project, the University of California-San Diego VA Center of Excellence for Stress and Mental Health (CESAMH) and the Anxiety and Depression Association of America; provides fee-for-service consultation for Biogen and Resilience Therapeutics; and holds patents for the use of d-cycloserine and psychotherapy, targeting the pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor for extinction, targeting tachykinin 2 for prevention of fear and targeting angiotensin to improve extinction of fear. R.J.F., L.A.M.L. and J.S. declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morris DJ The Evil Hours: A Biography of Post-Traumatic Stress Disorder. (Houghton Mifflin Harcourt, 2015). [Google Scholar]
- 2.van der Kolk B Interview: what is PTSD really? Surprises, twists of history, and the politics of diagnosis and treatment. Interview by Lisa M Najavits. J. Clin. Psychol 69, 516–522 (2013). [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-3). (APA Publishing, 1980). [Google Scholar]
- 4.Logue MW et al. The Psychiatric Genomics Consortium Posttraumatic Stress Disorder Workgroup: posttraumatic stress disorder enters the age of large-scale genomic collaboration. Neuropsychopharmacology 40, 2287–2297 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Insel T et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Rauch SL, Shin LM & Phelps EA Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol. Psychiatry 60, 376–382 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Tovote P, Fadok JP & Luthi A Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci 16, 317–331 (2015).This review offers an excellent overview of current literature on fear circuitry.
- 8.Janak PH & Tye KM From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu H Reward and aversion. Annu. Rev. Neurosci 39, 297–324 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Namburi P, Al-Hasani R, Calhoon GG, Bruchas MR & Tye KM Architectural representation of valence in the limbic system. Neuropsychopharmacology 41, 1697–1715 (2016).This review takes a comprehensive, forward-looking view of the basic neuroscience of valence representation in the brain.
- 11.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5). (APA Publishing, 2013). [Google Scholar]
- 12.Yehuda R et al. Post-traumatic stress disorder. Nat. Rev. Dis. Primers 1, 15057 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Shalev A, Liberzon I & Marmar C Post-traumatic stress disorder. N. Engl. J. Med 376, 2459–2469 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Kessler RC Posttraumatic stress disorder: the burden to the individual and to society. J. Clin. Psychiatry 61, (Suppl. 5), 4–12; discussion 13–14 (2000). [PubMed] [Google Scholar]
- 15.Holbrook TL, Hoyt DB, Stein MB & Sieber WJ Gender differences in long-term posttraumatic stress disorder outcomes after major trauma: women are at higher risk of adverse outcomes than men. J. Trauma 53, 882–888 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Ehring T & Quack D Emotion regulation difficulties in trauma survivors: the role of trauma type and PTSD symptom severity. Behav. Ther. 41, 587–598 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Kelley LP, Weathers FW, McDevitt-Murphy ME, Eakin DE & Flood AM A comparison of PTSD symptom patterns in three types of civilian trauma. J. Trauma Stress 22, 227–235 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Wolf EJ et al. A latent class analysis of dissociation and posttraumatic stress disorder: evidence for a dissociative subtype. Arch. Gen. Psychiatry 69, 698–705 (2012).This paper offers pivotal psychometric evidence in favour of a dissociative subtype of PTSD with critical clinical implications.
- 19.Cuthbert BN & Insel TR Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med 11, 126 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanius RA et al. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am. J. Psychiatry 167, 640–647 (2010).This paper provides important biological evidence in favour of a dissociative subtype of PTSD.
- 21.Bremner JD et al. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol. Psychiatry 45, 806–816 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bremner JD et al. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am. J. Psychiatry 156, 1787–1795 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanius RA et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am. J. Psychiatry 158, 1920–1922 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Liberzon I et al. Brain activation in PTSD in response to trauma-related stimuli. Biol. Psychiatry 45, 817–826 (1999). [DOI] [PubMed] [Google Scholar]
- 25.Osuch EA et al. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biol. Psychiatry 50, 246–253 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Pissiota A et al. Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. Eur. Arch. Psychiatry Clin. Neurosci 252, 68–75 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Rauch SL et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script driven imagery. Arch. Gen. Psychiatry 53, 380–387 (1996).This study is one of the first to demonstrate increased limbic activation in a symptom provocation study paradigm for individuals with PTSD.
- 28.Shin LM et al. Regional cerebral blood flow during scriptdriven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am. J. Psychiatry 156, 575–584 (1999). [DOI] [PubMed] [Google Scholar]
- 29.Shin LM et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch. Gen. Psychiatry 61, 168–176 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Zubieta J-K et al. Medial frontal cortex involvement in PTSD symptoms: a SPECT study. J. Psychiatr. Res 33, 259–264 (1999). [DOI] [PubMed] [Google Scholar]
- 31.Phelps EA, D. M., Nearing, K. I. & LeDoux, J. E. Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43, 897–905 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Hopper JW, Frewen PA, van der Kolk BA & Lanius RA Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J. Trauma Stress 20, 713–725 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Craig AD How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci 3, 655–666 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Phan KL, Britton JC, Taylor SF, Fig LM & Liberzon I Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch. Gen. Psychiatry 63, 184–192 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Milad MR et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry 66, 1075–1082 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milad MR & Quirk GJ Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol 63, 129–151 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rescorla RA & Heth CD Reinstatement of fear to an extinguished conditioned stimulus. J. Exp. Psychol. Anim. Behav. Process 1, 88–96 (1975). [PubMed] [Google Scholar]
- 38.Kroes MC, Schiller D, LeDoux JE & Phelps EA Translational approaches targeting reconsolidation. Curr. Top. Behav. Neurosci 28, 197–230 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garfinkel SN et al. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J. Neurosci 34, 13435–13443 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norrholm SD et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol. Psychiatry 69, 556–563 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wicking M et al. Deficient fear extinction memory in posttraumatic stress disorder. Neurobiol. Learn. Mem 136, 116–126 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Rauch SL et al. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport 14, 913–916 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Stevens JS et al. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J. Psychiatr. Res 47, 1469–1478 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orr SP et al. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J. Abnorm Psychol 109, 290–298 (2000). [PubMed] [Google Scholar]
- 45.Kaczkurkin AN et al. Neural substrates of overgeneralized conditioned fear in PTSD. Am. J. Psychiatry 174, 125–134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thome J et al. Generalisation of fear in PTSD related to prolonged childhood maltreatment: an experimental study. Psychol. Med 28, 1–12 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Morey RA et al. Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Transl Psychiatry 5, e700 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogt BA & Paxinos G Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct. Funct 219, 185–192 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ & Haber SN Circuit-based corticostriatal homologies between rat and primate. Biol. Psychiatry 80, 509–521 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sierra-Mercado D, Padilla-Coreano N & Quirk GJ Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36, 529–538 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gourley SL & Taylor JR Going and stopping: dichotomies in behavioral control by the prefrontal cortex. Nat. Neurosci 19, 656–664 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Do-Monte FH, Quinones-Laracuente K & Quirk GJ A temporal shift in the circuits mediating retrieval of fear memory. Nature 519, 460–463 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Courtin J et al. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature 505, 92–96 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Do-Monte FH, Manzano-Nieves G, Quinones-Laracuente K, Ramos-Medina L & Quirk GJ Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J. Neurosci 35, 3607–3615 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santini E, Ge H, Ren K, Pena de Ortiz S & Quirk GJ Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J. Neurosci 24, 5704–5710 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hong J & Kim D Freezing response-independent facilitation of fear extinction memory in the prefrontal cortex. Sci. Rep 7, 5363 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stein MB & Paulus MP Imbalance of approach and avoidance: the yin and yang of anxiety disorders. Biol. Psychiatry 66, 1072–1074 (2009).This review contains a concise theoretical discussion suggesting that PTSD is characterized by an imbalance of approach-avoidance systems.
- 58.Sripada RK, Garfinkel SN & Liberzon I Avoidant symptoms in PTSD predict fear circuit activation during multimodal fear extinction. Front. Hum. Neurosci 7, 672 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.LeDoux JE, Moscarello J, Sears R & Campese V The birth, death and resurrection of avoidance: a reconceptualization of a troubled paradigm. Mol. Psychiatry 22, 24–36 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maier SF Behavioral control blunts reactions to contemporaneous and future adverse events: medial prefrontal cortex plasticity and a corticostriatal network. Neurobiol. Stress 1, 12–22 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boeke EA, Moscarello JM, LeDoux JE, Phelps EA & Hartley CA Active avoidance: neural mechanisms and attenuation of pavlovian conditioned responding. J. Neurosci 37, 4808–4818 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Galatzer-Levy IR et al. Heterogeneity in signaled active avoidance learning: substantive and methodological relevance of diversity in instrumental defensive responses to threat cues. Front. Syst. Neurosci 8, 179 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi JS, Cain CK & LeDoux JE The role of amygdala nuclei in the expression of auditory signaled two-way active avoidance in rats. Learn. Mem 17, 139–147 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lazaro-Munoz G, LeDoux JE & Cain CK Sidman instrumental avoidance initially depends on lateral and basal amygdala and is constrained by central amygdala-mediated Pavlovian processes. Biol. Psychiatry 67, 1120–1127 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramirez F, Moscarello JM, LeDoux JE & Sears RM Active avoidance requires a serial basal amygdala to nucleus accumbens shell circuit. J. Neurosci 35, 3470–3477 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moscarello JM & LeDoux JE Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions. J. Neurosci 33, 3815–3823 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fadok JP et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542, 96–100 (2017).This paper begins to elucidate the divergent neurocircuitries for active and passive fear responses.
- 68.Pellman BA & Kim JJ What can ethobehavioral studies tell us about the brain’s fear system? Trends Neurosci 39, 420–431 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pare D & Quirk GJ When scientific paradigms lead to tunnel vision: lessons from the study of fear. Sci. Learn 2, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi J-S & Kim JJ Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proc. Natl Acad. Sci. USA 107, 21773–21777 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amir A, Lee SC, Headley DB, Herzallah MM & Pare D Amygdala signaling during foraging in a hazardous environment. J. Neurosci 35, 12994–13005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davis M & Whalen PJ The amygdala: vigilance and emotion. Mol. Psychiatry 6, 13–34 (2001). [DOI] [PubMed] [Google Scholar]
- 73.Squire LR & Zola-Morgan S The medial temporal lobe memory system. Science 253, 1380–1386 (1991). [DOI] [PubMed] [Google Scholar]
- 74.Elzinga BM & Bremner JD Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J. Affect Disord 70, 1–17 (2002).This excellent review synthesizes the memory-related symptoms in PTSD into a neurobiological model.
- 75.Pitman RK et al. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci 13, 769–787 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Logue MW et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from Posttraumatic Stress Disorder Consortia. Biol. Psychiatry 83, 244–253 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kremen WS, Koenen KC, Afari N & Lyons MJ Twin studies of posttraumatic stress disorder: differentiating vulnerability factors from sequelae. Neuropharmacology 62, 647–653 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bremner JD et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am. J. Psychiatry 160, 924–932 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Polak AR, Witteveen AB, Reitsma JB & Olff M The role of executive function in posttraumatic stress disorder: a systematic review. J. Affect Disord 141, 11–21 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Aupperle RL, Melrose AJ, Stein MB & Paulus MP Executive function and PTSD: disengaging from trauma. Neuropharmacology 62, 686–694 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bryant RA et al. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biol. Psychiatry 58, 111–118 (2005). [DOI] [PubMed] [Google Scholar]
- 82.Falconer E et al. The neural networks of inhibitory control in posttraumatic stress disorder. J. Psychiatry Neurosci 33, 413–422 (2008). [PMC free article] [PubMed] [Google Scholar]
- 83.Moores KA et al. Abnormal recruitment of working memory updating networks during maintenance of trauma-neutral information in post-traumatic stress disorder. Psychiatry Res 163, 156–170 (2008). [DOI] [PubMed] [Google Scholar]
- 84.Clausen AN et al. PTSD and cognitive symptoms relate to inhibition-related prefrontal activation and functional connectivity. Depress. Anxiety 34, 427–436 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McEwen BS, Nasca C & Gray JD Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41 , 3–23 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gould E & Tanapat P Stress and hippocampal neurogenesis. Biol. Psychiatry 46, 1472–1479 (1999). [DOI] [PubMed] [Google Scholar]
- 87.Gronli J et al. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol. Biochem. Behav 85, 842–849 (2006). [DOI] [PubMed] [Google Scholar]
- 88.Segal M, Richter-Levin G & Maggio N Stress- induced dynamic routing of hippocampal connectivity: a hypothesis. Hippocampus 20, 1332–1338 (2010). [DOI] [PubMed] [Google Scholar]
- 89.Gray JD et al. Translational profiling of stress- induced neuroplasticity in the CA3 pyramidal neurons of BDNF Val66Met mice. Mol. Psychiatry 23, 904–913 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tomar A, Polygalov D, Chattarji S & McHugh TJ The dynamic impact of repeated stress on the hippocampal spatial map. Hippocampus 25, 38–50 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Shansky RM, Rubinow K, Brennan A & Arnsten AF The effects of sex and hormonal status on restraint- stress-induced working memory impairment. Behav. Brain Funct 2, 8 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Benchenane K et al. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron 66, 921–936 (2010). [DOI] [PubMed] [Google Scholar]
- 93.Spellman T et al. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature 522, 309–314 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barrett LF Are emotions natural kinds? Perspect. Psychol. Sci 1, 28–58 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Russell JA Core affect and the psychological construction of emotion. Psychol. Rev 110, 145–172 (2003). [DOI] [PubMed] [Google Scholar]
- 96.Kober H et al. Functional grouping and cortical- subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage 42, 998–1031 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilson-Mendenhall CD, Barrett LF & Barsalou LW Neural evidence that human emotions share core affective properties. Psychol. Sci 24, 947–956 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ochsner KN, The GJ cognitive control of emotion. Trends Cogn. Sci 9, 242–249 (2005). [DOI] [PubMed] [Google Scholar]
- 99.New AS et al. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol. Psychiatry 66, 656–664 (2009). [DOI] [PubMed] [Google Scholar]
- 100.Lanius RA et al. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol. Psychiatry 53, 204–210 (2003). [DOI] [PubMed] [Google Scholar]
- 101.Goldin PR, M. K., Ramel, W. & Gross, J. J. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol. Psychiatry 63, 577–586 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Keding TJ & Herringa RJ Paradoxical prefrontal- amygdala recruitment to angry and happy expressions in pediatric posttraumatic stress disorder. Neuropsychopharmacology 41, 2903–2912 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fonzo GA, Huemer J & Etkin A History of childhood maltreatment augments dorsolateral prefrontal processing of emotional valence in PTSD. J. Psychiatr. Res 74, 45–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stuber GD et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim J, Zhang X, Muralidhar S, LeBlanc SA & Tonegawa S Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron 93, 1464–1479.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Airan RD, Thompson KR, Fenno LE, Bernstein H & Deisseroth K Temporally precise in vivo control of intracellular signalling. Nature 458, 1025–1029 (2009). [DOI] [PubMed] [Google Scholar]
- 107.Tsai HC et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Redondo RL et al. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513, 426–430 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reynolds SM & Berridge KC Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat. Neurosci 11, 423–425 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Der-Avakian A & Markou A The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci 35, 68–77 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Treadway MT & Zald DH Parsing anhedonia: translational models of reward-processing deficits in psychopathology. Curr. Dir. Psychol. Sci 22, 244–249 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nawijn L et al. Reward functioning in PTSD: a systematic review exploring the mechanisms underlying anhedonia. Neurosci. Biobehav Rev 51, 189–204 (2015).This paper is a meta-analytic study of reward functioning in PTSD that finds evidence for both decreased reward anticipation and approach and reduced hedonic responses.
- 113.Liu X, Hairston J, Schrier M & Fan J Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav Rev 35, 1219–1236 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Elman I et al. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biol. Psychiatry 66, 1083–1090 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sailer U et al. Altered reward processing in the nucleus accumbens and mesial prefrontal cortex of patients with posttraumatic stress disorder. Neuropsychologia 46, 2836–2844 (2008). [DOI] [PubMed] [Google Scholar]
- 116.Litz B Emotional numbing in combat-related post- traumatic stress disorder: a critical review and reformulation. Clin. Psychol. Rev 12, 417–432 (1992). [Google Scholar]
- 117.Frewen PA et al. Emotional numbing in posttraumatic stress disorder: a functional magnetic resonance imaging study. J. Clin. Psychiatry 73, 431–436 (2012). [DOI] [PubMed] [Google Scholar]
- 118.Chaudhury D et al. Rapid regulation of depression- related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tye KM et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Covington HE 3rd et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci 30, 16082–16090 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ferenczi EA et al. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 351, aac9698 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liberzon I & Sripada CS The functional neuroanatomy of PTSD: a critical review. Prog. Brain Res 167, 151–169 (2008). [DOI] [PubMed] [Google Scholar]
- 123.Shin LM & Liberzon I The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35, 169–191 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sripada RK et al. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom. Med 74, 904–911 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Terburg D et al. Hypervigilance for fear after basolateral amygdala damage in humans. Transl Psychiatry 2, e115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tye KM et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jasnow AM et al. Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition. J. Neurosci 33, 10396–10404 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McCullough KM et al. Molecular characterization of Thy1 expressing fear-inhibiting neurons within the basolateral amygdala. Nat. Commun 7, 13149 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ciocchi S et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282 (2010).This study draws attention to the existence of opposing microcircuits within the extended amygdala.
- 130.Li H et al. Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci 16, 332–339 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Somerville LH, Whalen PJ & Kelley WM Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol. Psychiatry 68, 416–424 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Avery SN, Clauss JA & Blackford JU The Human BNST: functional role in anxiety and addiction. Neuropsychopharmacology 41, 126–141 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lebow MA & Chen A Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 21, 450–463 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim SY et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496, 219–223 (2013).This paper begins the dissection of microcircuits within the BNST.
- 135.Rau V, DeCola JP & Fanselow MS Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci. Biobehav Rev 29, 1207–1223 (2005). [DOI] [PubMed] [Google Scholar]
- 136.Lebow M et al. Susceptibility to PTSD-like behavior is mediated by corticotropin-releasing factor receptor type 2 levels in the bed nucleus of the stria terminalis. J. Neurosci 32, 6906–6916 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Blair RJ Psychopathy, frustration, and reactive aggression: the role of ventromedial prefrontal cortex. Br. J. Psychol 101, 383–399 (2010). [DOI] [PubMed] [Google Scholar]
- 138.Haden SC & Scarpa A The noradrenergic system and its involvement in aggressive behaviors. Aggression Violent Behav 12, 1–15 (2007). [Google Scholar]
- 139.Haller J, Makara G & Kruk M Catecholaminergic involvement in the control of aggression: hormones, the peripheral sympathetic, and central noradrenergic systems. Neurosci. BiobehavRev 22, 85–97 (1997). [DOI] [PubMed] [Google Scholar]
- 140.Gilam G, Lin T, Fruchter E & Hendler T Neural indicators of interpersonal anger as cause and consequence of combat training stress symptoms. Psychol. Med 47, 1561–1572 (2017). [DOI] [PubMed] [Google Scholar]
- 141.Davidson RJ, Putnam KM & Larson CL Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science 289, 591–594 (2000). [DOI] [PubMed] [Google Scholar]
- 142.Dileo JF, Brewer WJ, Hopwood M, Anderson V & Creamer M Olfactory identification dysfunction, aggression and impulsivity in war veterans with post-traumatic stress disorder. Psychol. Med 38, 523–531 (2008). [DOI] [PubMed] [Google Scholar]
- 143.Siever LJ Neurobiology of aggression and violence. Am. J. Psychiatry 165, 429–442 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gilam G et al. Neural substrates underlying the tendency to accept anger-infused ultimatum offers during dynamic social interactions. Neuroimage 120, 400–411 (2015). [DOI] [PubMed] [Google Scholar]
- 145.Denson TF, Pedersen WC, Ronquillo J & Nandy AS The angry brain: neural correlates of anger, angry rumination, and aggressive personality. J. Cogn. Neurosci 21, 734–744 (2009). [DOI] [PubMed] [Google Scholar]
- 146.Pietrini P, Guazzelli M, Basso G, Jaffe K & Grafman J Neural correlates of imaginal aggressive behavior assessed by positron emission tomography in healthy subjects. Am. J. Psychiatry 157, 1772–1781 (2000). [DOI] [PubMed] [Google Scholar]
- 147.Best M, Williams JM & Coccaro EF Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proc. Natl Acad. Sci. USA 99, 8448–8453 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Blair RJ The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn 55, 198–208 (2004). [DOI] [PubMed] [Google Scholar]
- 149.Falkner AL, Dollar P, Perona P, Anderson DJ & Lin D Decoding ventromedial hypothalamic neural activity during male mouse aggression. J. Neurosci 34, 5971–5984 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee H et al. Scalable control of mounting and attack by Esr1 + neurons in the ventromedial hypothalamus. Nature 509, 627–632 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lin D et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Han W et al. Integrated control of predatory hunting by the central nucleus of the amygdala. Cell 168, 311–324.e318 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hong W, Kim DW & Anderson DJ Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell 158, 1348–1361 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gerfen CR & Surmeier DJ Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci 34, 441–466 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee JH et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788–792 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bernal-Casas D, Lee HJ, Weitz AJ & Lee JH Studying brain circuit function with dynamic causal modeling for optogenetic fMRI. Neuron 93, 522–532.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Reznikov R & Hamani C Posttraumatic stress disorder: perspectives for the use of deep brain stimulation. Neuromodulation 20, 7–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boggio PS et al. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. J. Clin. Psychiatry 71, 992–999 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cohen H et al. Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled study. Am. J. Psychiatry 161, 515–524 (2004). [DOI] [PubMed] [Google Scholar]
- 160.Watts BV, Landon B, Groft A & Young-Xu Y A sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain Stimul 5, 38–43 (2012). [DOI] [PubMed] [Google Scholar]
- 161.Taghva A et al. Magnetic resonance therapy improves clinical phenotype and EEG alpha power in posttraumatic stress disorder. Trauma Mon 20, e27360 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fonzo GA et al. PTSD psychotherapy outcome predicted by brain activation during emotional reactivity and regulation. Am. J. Psychiatry 174, 1163–1174 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rajasethupathy P, Ferenczi E & Deisseroth K Targeting neural circuits. Cell 165, 524–534 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Admon R, Milad MR & Hendler T A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn. Sci 17, 337–347 (2013). [DOI] [PubMed] [Google Scholar]
- 165.Shin LM et al. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. Am. J. Psychiatry 168, 979–985 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Heiman M, Kulicke R, Fenster RJ, Greengard P & Heintz N Cell type-specific mRNA purification by translating ribosome affinity purification (TRAP). Nat. Protoc 9, 1282–1291 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Macosko EZ et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kessler RC, Sonnega A, Bromet E, Hughes M & Nelson CB Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 52, 1048–1060 (1995). [DOI] [PubMed] [Google Scholar]
- 169.Afifi TO, Asmundson GJ, Taylor S & Jang KL The role of genes and environment on trauma exposure and posttraumatic stress disorder symptoms: a review of twin studies. Clin. Psychol. Rev 30, 101–112 (2010). [DOI] [PubMed] [Google Scholar]
- 170.Lappalainen T & Greally JM Associating cellular epigenetic models with human phenotypes. Nat. Rev. Genet 18, 441–451 (2017). [DOI] [PubMed] [Google Scholar]
- 171.Daskalakis NP, Rijal CM, King C, Huckins LM & Ressler KJ Recent genetics and epigenetics approaches to PTSD. Curr. Psychiatry Rep 20, 30 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cornelis MC, Nugent NR, Amstadter AB & Koenen KC Genetics of post-traumatic stress disorder: review and recommendations for genome- wide association studies. Curr. Psychiatry Rep 12, 313–326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tabery J Biometric and developmental gene- environment interactions: looking back, moving forward. Dev. Psychopathol 19, 961–976 (2007). [DOI] [PubMed] [Google Scholar]
- 174.Fani N et al. FKBP5 genotype and structural integrity of the posterior cingulum. Neuropsychopharmacology 39, 1206–1213 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lind MJ et al. Association of posttraumatic stress disorder with rs2267735 in the ADCYAP1R1 gene: a meta-analysis. J. Trauma Stress 30, 389–398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ressler KJ et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470, 492–497 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Almli LM et al. A genome-wide identified risk variant for PTSD is a methylation quantitative trait locus and confers decreased cortical activation to fearful faces. Am. J. Med. Genet. B Neuropsychiatr. Genet 168B, 327–336 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Duncan LE et al. Largest GWAS of PTSD (N = 20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol. Psychiatry 23, 666–673 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Guffanti G et al. Genome-wide association study implicates a novel RNA gene, the lincRNA AC068718.1, as a risk factor for post-traumatic stress disorder in women. Psychoneuroendocrinology 38, 3029–3038 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kilaru V et al. Genome-wide gene-based analysis suggests an association between Neuroligin 1 (NLGN1) and post-traumatic stress disorder. Transl Psychiatry 6, e820 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stein MB et al. Genome-wide association studies of posttraumatic stress disorder in 2 cohorts of US Army soldiers. JAMA Psychiatry 73, 695–704 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ashley-Koch AE et al. Genome-wide association study of posttraumatic stress disorder in a cohort of Iraq-Afghanistan era veterans. J. Affect Disord 184, 225–234 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nievergelt CM et al. Genomic predictors of combat stress vulnerability and resilience in U. S. Marines: a genome-wide association study across multiple ancestries implicates PRTFDC1 as a potential PTSD gene. Psychoneuroendocrinology 51, 459–471 (2015). [DOI] [PubMed] [Google Scholar]
- 184.Liberzon I et al. Interaction of the ADRB2 gene polymorphism with childhood trauma in predicting adult symptoms of posttraumatic stress disorder. JAMA Psychiatry 71, 1174–1182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Almli LM et al. Follow-up and extension of a prior genome-wide association study of posttraumatic stress disorder: gene x environment associations and structural magnetic resonance imaging in a highly traumatized African-American civilian population. Biol. Psychiatry 76, e3–4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xie P et al. Genome-wide association study identifies new susceptibility loci for posttraumatic stress disorder. Biol. Psychiatry 74, 656–663 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Logue MW et al. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Mol. Psychiatry 18, 937–942 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sudhof TC Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Paul C et al. Signaling through cGMP-dependent protein kinase I in the amygdala is critical for auditory-cued fear memory and long-term potentiation. J. Neurosci 28, 14202–14212 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dell PF The multidimensional inventory of dissociation (MID): a comprehensive measure of pathological dissociation. J. Trauma Dissociation 7, 77–106 (2006). [DOI] [PubMed] [Google Scholar]
- 191.Holmes EA et al. Are there two qualitatively distinct forms of dissociation? A review and some clinical implications. Clin. Psychol. Rev 25, 1–23 (2005). [DOI] [PubMed] [Google Scholar]
- 192.Lanius RA, Bluhm R, Lanius U & Pain C A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. J. Psychiatr. Res 40, 709–729 (2006). [DOI] [PubMed] [Google Scholar]
- 193.Lanius RA, Hopper JW & Menon RS Individual differences in a husband and wife who developed PTSD after a motor vehicle accident: a functional MRI case study. Am. J. Psychiatry 160, 667–669 (2003). [DOI] [PubMed] [Google Scholar]
- 194.Felmingham K et al. Dissociative responses to conscious and non-conscious fear impact underlying brain function in post-traumatic stress disorder. Psychol. med 38, 1771–1780 (2008). [DOI] [PubMed] [Google Scholar]
- 195.Lanius RA et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol. Psychiatry 52, 305–311 (2002). [DOI] [PubMed] [Google Scholar]
- 196.Lanius RA et al. Functional connectivity of dissociative responses in posttraumatic stress disorder: a functional magnetic resonance imaging investigation. Biol. Psychiatry 57, 873–884 (2005). [DOI] [PubMed] [Google Scholar]
- 197.Pitman RK, Orr SP, Forgue DF, de Jong JB & Claiborn JM Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch. Gen. Psychiatry 44, 970–975 (1987). [DOI] [PubMed] [Google Scholar]
- 198.Orr SP, Metzger LJ & Kaloupek DG Psychophysiological Assessment of PTSD. Assessing psychological trauma and PTSD, 289 (2004). [Google Scholar]
- 199.Etkin A, Egner T & Kalisch R Emotional processing in anterior cingulate and medial prefrontal cortex. Trends sci 15, 85–93 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Robinson OJ et al. Towards a mechanistic understanding of pathological anxiety: the dorsal medial prefrontal-amygdala ‘aversive amplification’circuit in unmedicated generalized and social anxiety disorders. Lancet. Psychiatry 1, 294 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shin LM in Neurobiology of PTSD (eds Shiromani PJ, Keane TM & LeDoux JE) (Humana Press, 2009). [Google Scholar]
- 202.Nicholson AA et al. The dissociative subtype of posttraumatic stress disorder: unique resting-state functional connectivity of basolateral and centromedial amygdala complexes. Neuropsychopharmacology 40, 2317–2326 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nicholson AA et al. Unique insula subregion resting-state functional connectivity with amygdala complexes in posttraumatic stress disorder and its dissociative subtype. Psychiatry Res 250, 61–72 (2016). [DOI] [PubMed] [Google Scholar]
- 204.Harricharan S et al. fMRI functional connectivity of the periaqueductal gray in PTSD and its dissociative subtype. Brain Behav 6, e00579 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dalenberg CJ et al. Evaluation of the evidence for the trauma and fantasy models of dissociation. Psychol. Bull 138, 550–588 (2012). [DOI] [PubMed] [Google Scholar]
- 206.Reinders AATS et al. One brain, two selves. Neuroimage 20, 2119–2125 (2003). [DOI] [PubMed] [Google Scholar]
- 207.Reinders AS et al. Psychobiological characteristics of dissociative identity disorder: a symptom provocation study. Biol. Psychiatry 60, 730–740 (2006). [DOI] [PubMed] [Google Scholar]
- 208.Schiller D & Delgado MR Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn sci 14, 268–276 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ebner-Priemer UW et al. Emotional learning during dissociative states in borderline personality disorder. J. Psychiatry Neurosci 34, 214–222 (2009). [PMC free article] [PubMed] [Google Scholar]
- 210.Bae H, Kim D & Park YC Dissociation predicts treatment response in eye-movement desensitization and reprocessing for posttraumatic stress disorder. J. TraumaDissoci 17, 112–130 (2016). [DOI] [PubMed] [Google Scholar]
- 211.Kleindienst N et al. State dissociation moderates response to dialectical behavior therapy for posttraumatic stress disorder in women with and without borderline personality disorder. Eur. J. Psychotraumatol 7, 30375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kleindienst N et al. Dissociation predicts poor response to dialectial behavioral therapy in female patients with borderline personality disorder. J. Pers Disord 25, 432–447 (2011). [DOI] [PubMed] [Google Scholar]
- 213.Price M, Kearns M, Houry D & Rothbaum BO Emergency department predictors of posttraumatic stress reduction for trauma-exposed individuals with and without an early intervention. J. Consult Clin. Psychol 82, 336–341 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wolf EJ, Lunney CA & Schnurr PP The influence of the dissociative subtype of posttraumatic stress disorder on treatment efficacy in female veterans and active duty service members. J. Consult Clin. Psychol 84, 95–100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cloitre M, Petkova E, Wang J & Lu Lassell F An examination of the influence of a sequential treatment on the course and impact of dissociation among women with PTSD related to childhood abuse. Depress. Anxiety 29, 709–717 (2012). [DOI] [PubMed] [Google Scholar]
- 216.Resick PA, Suvak MK, Johnides BD, Mitchell KS & Iverson KM The impact of dissociation on PTSD treatment with cognitive processing therapy. Depress. Anxiety 29, 718–730 (2012). [DOI] [PubMed] [Google Scholar]
- 217.Hagenaars MA, van Minnen A & Hoogduin KA The impact of dissociation and depression on the efficacy of prolonged exposure treatment for PTSD. Behav. Res. Ther 48, 19–27 (2010). [DOI] [PubMed] [Google Scholar]
- 218.Halvorsen JO, Stenmark H, Neuner F & Nordahl HM Does dissociation moderate treatment outcomes of narrative exposure therapy for PTSD? A secondary analysis from a randomized controlled clinical trial. Behav. Res. Ther 57, 21–28 (2014). [DOI] [PubMed] [Google Scholar]
- 219.Jaycox LH, Foa EB & Morral AR Influence of emotional engagement and habituation on exposure therapy for PTSD. J. Consult Clin. Psychol 66, 185–192 (1998). [DOI] [PubMed] [Google Scholar]
- 220.Nestler EJ & Hyman SE Animal models of neuropsychiatric disorders. Nat. Neurosci 13, 1161–1169 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hendriksen H, Olivier B & Oosting RS From non-pharmacological treatments for post-traumatic stress disorder to novel therapeutic targets. Eur. J. Pharmacol 732, 139–158 (2014). [DOI] [PubMed] [Google Scholar]
- 222.Sillivan SE et al. Susceptibility and resilience to posttraumatic stress disorder-like behaviors in inbred mice. Biol. Psychiatry 82, 924–933 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cohen H & Zohar J An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria. Ann. NY Acad. Sci 1032, 167–178 (2004). [DOI] [PubMed] [Google Scholar]