Abstract
Purpose
Most studies on neuroblastoma (NB) have been conducted in Western countries or Japan. The objective of our study was to analyze clinical and pathological features, MYCN status, surgical methods, and prognosis in Chinese NB patients.
Methods
A retrospective, single-center case series study of 275 NBs was implemented. Clinical manifestations, pathological features, MYCN status, and surgical treatment were analyzed. Log-rank test and Cox hazards models were used to assess overall survivals (OSs).
Results
The cohort consisted of 105 females and 170 males, with an age range of five days to 15 years. MYCN amplification was detected in 21.5% of all cases. The median OS was 15.0 months for MYCN amplified group. The five-year OS rates were 70.8% and 18.3% for MYCN unamplified and amplified groups, respectively, and the comparison of Kaplan–Meier curves for these two groups showed statistical significance (P < .001 by log-rank test). Gross total resection (GTR, n = 111) and subtotal resection (STR, n = 58) were administered in 169 patients at stages 3 and 4 who received chemotherapy and the comparison of Kaplan–Meier curves for different groups in these patients had statistical significance (STR vs. GTR, P = .009; MYCN unamplified vs. amplified, P < .001 by log-rank test, respectively).The multivariate survival analyses showed statistical significance (STR vs. GTR, P = .047; MYCN unamplified vs. amplified, P = .001 by Cox regression model).
Conclusions
MYCN amplification is an independently adverse prognostic factor in Chinese NB patients at stages 3 and 4 and GTR is associated with improved OS compared with STR in these patients.
Keywords: Neuroblastoma, MYCN status, Clinicopathological features, Prognosis, Surgery
Introduction
Neuroblastoma (NB) is the most common malignant extracranial solid tumor in children. More than half of pediatric NBs present with disseminated metastases at initial diagnosis, and most of them are in an advanced stage with poor prognoses. Risk assessment in NB depends on the comprehensive analysis of data regarding clinical and biologic characteristics. Children’s Oncology Group (COG) has established the risk stratification system relying on five characteristics: International Neuroblastoma Staging System (INSS) stage, patient age at diagnosis, MYCN gene status, DNA index, and International Neuroblastoma Pathology Classification (INPC) (London et al., 2005; Shimada et al., 1999; Peuchmaur et al., 2003). Specific chromosomal aberrations, such as the 11q loss, have been added to the International Neuroblastoma Risk Group (INRG) classification system (Cohn et al., 2009). According to the COG risk stratification system, three groups were established: low-risk, intermediate-risk, and high-risk groups. Most stage 1 and 2 NB patients (usually in the low-risk group) can be cured by surgery alone and have an excellent prognosis, which has been a consensus worldwide. However, the treatment for stage 3 and 4 NB patients, especially those in the high-risk group, is still tough, and the outcome is dismal. Whether gross total resection (GTR) of the primary tumor can render overall survival (OS) longer in these refractory patients than in those with subtotal tumor resection (STR) is still unclear. Several studies reported that GTR might bring benefits to clinical outcomes in these patients. However, others have drawn opposite conclusions (McGregor et al., 2005; Fischer et al., 2017; Simon et al., 2013; Englum et al., 2015). Studies about clinicopathological features and MYCN status in a cohort of NB patients and the prognosis diversity of different MYCN status and surgical methods (STR vs. GTR) in stage 3 and 4 NB patients in China have rarely been reported.
Herein, we collected 275 NBs between 2010 and 2015 in our center and made a retrospective study of the clinicopathological features, MYCN status, surgical management, follow-up, and prognosis. Our study highlights the prognostic values of MYCN status and different surgical methods in stage 3 and 4 patients in China.
Materials and Methods
Medical ethics
This retrospective cohort study was approved by the Ethical Committee of Children’s Hospital of Fudan University (no. 2017-217). The forms of consent were received from the patients’ parents.
Archive review
Two hundred and seventy-five NBs were retrieved from the medical records of Children’s Hospital of Fudan University between 2010 and 2015. We collected such data as sex, age, chief complaints, lesion site, isotope bone scan, ultrasound imaging, radiological finding, bone marrow biopsy, MYCN status, histopathological diagnosis, INPC, INSS stage, surgical treatment, and follow-up.
MYCN status was evaluated by fluorescence in situ hybridization study and analyzed according to the international consensus by INRG Biology Committee for neuroblastoma molecular diagnostics (Ambros et al., 2009). A >4-fold increase in MYCN signal compared with a reference probe was recognized as MYCN amplification. All the patients were assigned to high-, intermediate-, and low-risk categories according to the COG risk stratification criteria. For those who had finished the treatment course, one outpatient clinic visit for each year was requested. In these visits they were carefully evaluated by physical examination and imaging tests. Patients who failed to visit on time were contacted by phone, mail, and e-mail for collection of data concerning the children’s health and the test results in local hospitals.
Statistical analysis
All the data were collected and filed in a database. A descriptive analysis was carried out for clinical, pathological, and biological features, surgical management, and follow-up. Statistical analysis was processed by IBM SPSS version 22.0 (IBM Corporation. Armonk, NY, USA). OS was defined as the time from initial diagnosis to death or last follow-up if patients survived. The Kaplan–Meier survival curves were compared with log-rank test. Cox proportional hazards model was used for univariate and multivariate survival analyses. All statistical tests were two-sided and P values <0.05 were considered as statistically significant.
Results
Clinical characteristics
Clinical and pathological characteristics of 275 cases were summarized in Table 1. The cohort had a slightly male predominance with a male-to-female ratio of 1.6:1. The age range was five days to 15 years. There were 90 infantile NBs. The mean and median ages were 30 months and 23 months, respectively. The two leading primary tumor sites were the adrenal gland and mediastinum (Table 1), and others included retroperitoneal sympathetic chain (n = 5), pelvic cavity (n = 2), kidney (n = 2), neck (n = 1), oropharynx (n = 1), and orbit (n = 1). However, no primary tumor was detected in two cases with metastases in liver and bone marrow, respectively. Six patients had bilateral adrenal neuroblastomas. The most common chief complaints were abdominal mass (88/275), fever (38/275), abdominal pain (37/275), lower extremity pain (26/275), coughing (25/275), and abdominal distention (25/275). Less common symptoms included head and neck mass, skin mass, dyspnea, hepatomegaly, jaundice, and proptosis. The most common distant metastatic sites were bone and bone marrow (Table 1). Other rare metastatic locations included the brain, lung, pleura, orbit, paraspine, pancreas, spleen, and posterior eyeball.
Table 1. Clinicopathological features and MYCN status in 275 NBs.
| Features | Number | MYCN-amplified | MYCN-unamplified |
|---|---|---|---|
| Sex | |||
| Male | 170 | 39 | 131 |
| Female | 105 | 20 | 85 |
| Age(months) | |||
| <18 | 124 | 20 | 104 |
| 18–60 | 102 | 33 | 69 |
| ≥60 | 49 | 6 | 43 |
| Grade of differentiation | |||
| Undifferentiated | 89 | 37 | 52 |
| Poorly differentiated | 163 | 22 | 141 |
| Differentiating | 23 | 0 | 23 |
| Histology | |||
| Favorable | 183 | 9 | 174 |
| Unfavorable | 92 | 50 | 42 |
| INSS stage | |||
| 1 | 44 | 1 | 43 |
| 2 | 9 | 1 | 8 |
| 3 | 62 | 11 | 51 |
| 4 | 143 | 45 | 98 |
| 4S | 17 | 1 | 16 |
| Risk group | |||
| Low | 55 | 1 | 54 |
| Intermediate | 54 | 0 | 54 |
| High | 166 | 58 | 108 |
| Primary site | |||
| Adrenal gland | 221 | 54 | 167 |
| Mediastinum | 40 | 2 | 38 |
| Others | 14 | 3 | 11 |
| Distant metastatic site | |||
| Bone | 109 | 28 | 81 |
| Bone marrow | 84 | 23 | 61 |
| Lymph node | 35 | 8 | 27 |
| Liver | 36 | 11 | 25 |
| Skin | 21 | 6 | 15 |
| Others | 49 | 19 | 30 |
Pathological characteristics and MYCN status
Pathological diagnosis was determined according to the International Neuroblastoma Pathology Committee system (Peuchmaur et al., 2003; Cohn et al., 2009). The distribution of grade of differentiation and favorable/unfavorable group is listed in Table 1 and poorly differentiated NBs and favorable histology NBs made up the majority of the 275 cases. MYCN amplification accounted for 21.5% of the total cases. Non-MYCN-amplification included MYCN gain (n = 7) and wild-type (n = 209). MYCN amplification was relatively common for stage 3 and 4 patients, but very rare in stage 1, 2, and 4S patients. The majority of the MYCN-amplified cases appeared in high-risk group (58/59, 98.3%) and UH group (50/59, 84.7%). All the MYCN-amplified cases were either undifferentiated or poorly differentiated subtype. Non-MYCN-amplification was observed in the contemporaneous 77 ganglioneuroblastomas and 55 ganglioneuromas in our center.
Surgical management, follow-up, and prognosis
The general principles of treatment in our center were surgical resection alone for the low-risk group, surgical resection with chemotherapy for the intermediate-risk group, and multimodal therapy for the high-risk group. This included surgery, chemotherapy, radiation therapy, and autologous stem cell transplantation (ASCT). Anti-GD2 immunotherapy was not used at that time in our center because of its unavailability in China. The chemotherapeutic regimen was consistent with the COG chemotherapy protocols for intermediate-risk group (COG A3961) and high-risk group (COG A3973). Radiation therapy was administered to the high-risk group. ASCT was not widely used in this series because of economic overload and only performed in selectively 14 advanced-stage patients whose parents could afford the costs.
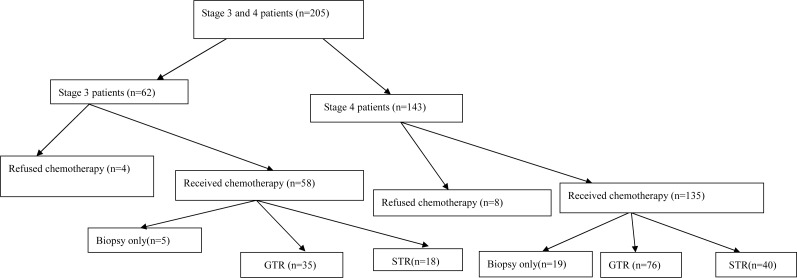
All the patients at stage 1 and 2 except for two with MYCN amplification (one at each stage) received GTR alone without any other therapy, and were all event-free in the follow-up. In those two patients with MYCN amplification, the tumor recurred a short time after GTR, so chemotherapy was administered and one of the patients also underwent radiation therapy in accordance with high-risk protocols. The GTR rate was 100% for those at stages 1 and 2 in our study. For stage 4S patients, 13 cases including two patients who had the mass enlargement in the wait-and-see approach received GTR of primary tumor and low-dose chemotherapy, three patients decided to wait and see and spontaneous tumor regression was recorded after biopsy, and one patient refused further therapy after biopsy and was lost in the follow-up. The GTR rate was 100% for those at stage 4S who chose to receive surgical removal of primary tumor. Comprehensive therapy was performed for stage 3 and 4 patients who were in the high-risk group. The general situation of stage 3 and 4 patients was frequently worse than that of stage 1 and 2 patients at initial presentation and the adjacent organs and main blood vessels were usually adherent to the tumor or encased by the tumor, which made GTR very difficult. Twenty-four patients at stages 3 and 4 underwent tumor biopsy and chemotherapy without surgical resection. Different surgical methods (GTR or STR) were administered in the rest 169 patients (Fig. 1): GTR (n = 111) including primary GTR and delayed GTR after biopsy and chemotherapy; STR (n = 58) including primary STR and delayed STR after biopsy and chemotherapy. The GTR rate was 66.0% for stage 3 patients and 65.5% for stage 4 patients whose parents preferred the surgical approach. No surgery-related death was noticed in all the patients. Very few surgical complications were observed.
Figure 1. Stage 3 and 4 patient flow diagram.
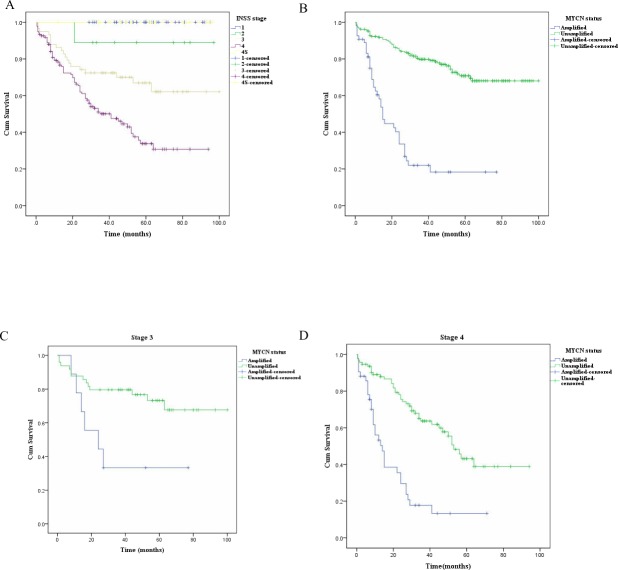
Thirteen patients were excluded from the analysis of follow-up because they had rejected further treatment after definite diagnosis by biopsy or GTR, so the analysis of follow-up was a summary of 262 patients. The follow-up periods ranged from half a month to 100 months (average 40.6 months and median 40.0 months). The three-year and five-year OS rates are listed in Table 2 and they were lower for stage 3 and 4 patients. The median OS estimate was 15.0 (±1.6) months (95% CI [11.8–18.2]) for amplified group. The differences among Kaplan–Meier survival curves for different stages or different MYCN status (unamplified vs. amplified) were statistically significant (both P < .001 by log-rank test) (Figs. 2A and 2B ). The median OS estimate for MYCN amplified group was 24.0 (±11.9) months (95% CI [0.6–47.4]) for stage 3 patients. The median OS estimates for amplified and unamplified groups at stage 4 were 14.0 (±2.3) months (95% CI [9.4–18.6]) and 53.0 (±3.7) months (95% CI [45.7–60.3]), respectively. The differences among Kaplan–Meier survival curves for different MYCN status groups at stages 3 or 4 had statistical significance (P = .012 and P<.001 by log-rank test, respectively) (Figs. 2C and 2D). As for the three-year and five-year OS rate estimates the stage 3 and 4 patients still had the worst performance. The three-year and five-year survival rate estimates were 79.8% and 70.8% for the MYCN unamplified group, and 22% and 18.3% for the amplified group, respectively.
Table 2. The three-year and five-year OS rates of 262 NBs in different INSS stages.
| INSS Stage | No | 3-year OS rate | 5-year OS rate |
|---|---|---|---|
| 1 | 44 | 100% | 100% |
| 2 | 9 | 88.9% | 88.9% |
| 3 | 58 | 72.4% | 66.9% |
| 4 | 135 | 50.1% | 33.8% |
| 4S | 16 | 100% | 100% |
Notes.
Abbreviations
- OS
- overall survival
- NB
- neuroblastoma
- INSS
- International Neuroblastoma Staging System
Figure 2. The Kaplan–Meier survival curves for NB patients (n = 262) with different stages and different MYCN status, stage 3 patients (n = 58) with different MYCN status, and stage 4 patients (n = 135) with different MYCN status.
(A–B) The differences in Kaplan–Meier survival curves for NB patients (n = 262) with different stages (A) and different MYCN status (B) showed statistical significance (both P < .001 by log-rank test). (C–D) The comparison of Kaplan-Meier survival curves for different MYCN status groups at stage 3 (C, n = 58) and 4 (D, n = 135) had statistical significance (P = .012 and P < .001 by log-rank test, respectively).
The median OS estimates for STR group and MYCN amplified group for 169 stage 3 and 4 patients were 27.0 (±11.6) months (95% CI [4.2–49.8]) and 15.0 (±4.9) months (95% CI [5.4–24.6]), respectively. The differences between Kaplan–Meier survival curves for different surgery groups or different MYCN status groups had statistical significance (P = .009 and P < .001 by log-rank test, respectively) in those patients (Figs. 3A and 3B). The univariate and multivariate analyses by Cox regression analyses were depicted in Table 3. The former analysis revealed statistically significant variables included INSS stage, risk group, grade of differentiation, FH/UH, MYCN status, and sugical methods. In the multivariate Cox model different surgical methods (STR vs. GTR) and different MYCN status (unamplified vs. amplified) still showed statistically significant differences (P = .047 and P = .001, respectively) and they were the independent variables influencing the OSs. STR had a high relative risk compared with GTR (HR = 1.590,95% CI [1.006–2.513]).
Figure 3. The Kaplan-Meier survival curves in the 169 stage 3 and 4 NB patients with different surgical methods (STR vs. GTR) and different MYCN status.
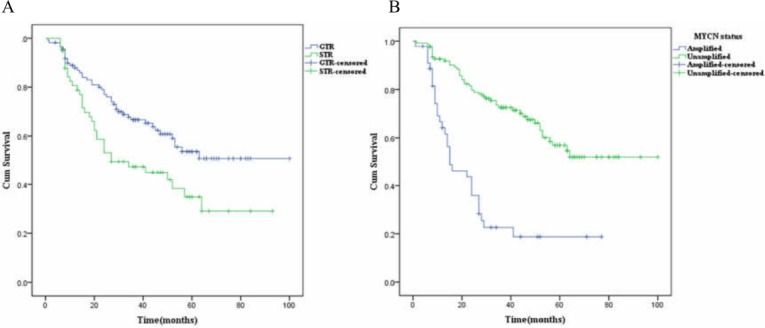
(A–B) The differences in Kaplan–Meier survival curves for (A) different surgery groups and (B) different MYCN status showed statistical significance (P = .009 and P < .001 by log-rank test, respectively) in the 169 stage 3 and 4 patients.
Table 3. Univariate and multivariate analyses of OS rates in the 169 stage 3 and 4 patients by Cox regression model.
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | ||
| Age | 12 months | 1 | 1 | ||||
| 12–60 months | .022 | 2.729 | 1.159–6.426 | .544 | .674 | .188–2.410 | |
| ≥60 months | .039 | 2.594 | 1.049–6.415 | .599 | .691 | .174–2.743 | |
| Sex (Female vs. male) | .709 | .914 | .570–1.466 | .968 | 1.010 | .616–1.657 | |
| Site | Adrenal gland | 1 | |||||
| Mediastinum | .130 | .524 | .227–1.210 | ||||
| Others | .868 | .918 | .334–2.524 | ||||
| INSS stage (4 vs. 3) | .002 | 2.451 | 1.408-4.266 | .262 | 1.398 | .778-2.512 | |
| Risk group(High-risk vs. intermediate-risk) | .000 | 10.005 | 3.140–31.873 | .007 | 7.829 | 1.752–34.983 | |
| Grade of differentiation | Undifferentiated subtye | 1 | 1 | ||||
| Poorly differentiated subtype | .005 | .519 | .330–.817 | .794 | .931 | .546–1.587 | |
| Differentiating subtype | .035 | .118 | .016–.863 | .807 | .735 | .062–8.695 | |
| FH/UH (FH vs UH) | .003 | .313 | .143–.682 | .874 | 1.110 | .308–3.996 | |
| MYCN status (Unamplificaction vs. amplification) | .000 | .247 | .154–.394 | .001 | .357 | .196-.651 | |
| ASCT (No vs. yes) | .193 | 1.557 | .799–3.032 | ||||
| Radiation (No vs. yes) | .105 | .678 | .423–1.085 | ||||
| Surgical methods(STR vs. GTR) | .011 | 1.794 | 1.143–2.817 | .047 | 1.590 | 1.006–2.513 | |
Notes.
Abbreviations
- HR
- hazard ratio
- CI
- confidence interval
- FH
- favorable histology
- UH
- unfavorable histology
- ASCT
- autologous stem cell transplantation
- STR
- subtotal resection
- GTR
- gross total resection
Discussion
Most clinical studies into NBs have been conducted in Western countries or Japan, and the criterion on diagnosis, risk stratification, and treatment protocols was established by COG and INRG based on these studies. There is no specialized and collaborative research institute majoring in NB in China, and studies on a large series of Chinese NBs were uncommon. The three leading primary tumor sites were the adrenal gland (47%–50%), other abdominal sites (27%–29%), and thoracic sites (15%–16%) verified by two large series studies (Vo et al., 2014; Berthold et al., 2017). The main tumor original sites in our study were the adrenal gland (80.4%), thoracic sites (14.5%), and other abdominal sites (3.3%). The proportion of NBs at stage 4 in our center was 52% slightly higher than about 40% reported by the INRG database (Cohn et al., 2009). Most distant metastatic sites were bone and bone marrow consistent with other studies (Berthold et al., 2017; Morgenstern et al., 2016). This series supported most of the clinicopathological features published by previous studies, and we speculate that subtle differences may be partially attributable to geographical and racial factors.
MYCN status includes amplification, gain, and wild-type. MYCN amplification is a powerful prognostic factor for NBs regardless of other factors, such as age, staging, INPC, and genetic aberrations of 1p and 11q, and it has appeared in approximately 20% of NBs and about 38% of all stage 4 patients (Berthold et al., 2017; Maris, 2010). Two recent large series studies from COG and Germany showed the incidence of MYCN amplification in NBs was about 18% (Berthold et al., 2017; Campbell et al., 2017). The incidence of MYCN amplification in our series was about 21.5%, very close to that in the preceding studies. Its strong relationship with unfavorable histology, high-risk group, advanced stage, and poor OS rates in our study confirmed its significance in risk stratification and outcome prediction. However, Wang et al. (2013) reported that the proportion of MYCN amplification was 13.5% in another regional center in northern China, which suggested it might have regional diversity in Chinese NB patients. The two primary renal NBs in our study all had MYCN amplification. MYCN amplification in stage 1, 2, and 4S NBs is rare. Only three cases were observed in our series, and they often had a greater likelihood of relapse compared with the counterparts without MYCN amplification. Bagatell et al. (2009) reported 87 (3%) of the total 2660 NBs at stages 1 and 2 had MYCN amplified tumors, and they had less favorable OS than those with non-amplified tumors. No consensus has been reached regarding the significance of MYCN gain. Campbell et al. (2017) reported 133 (2.8%) of 4672 NBs had MYCN gain, and MYCN gain was associated with an inferior prognosis. However, different results were reported by Wang et al. (2013), Souzaki et al. (2011) and Spitz et al. (2004). The amount of MYCN gain in our series was so small that we could not derive any useful information.
Surgery is the mainstay for stage 1 and stage 2 NBs, and GTR has shown satisfactory outcome in these types of patients. This has been demonstrated in many studies, including this one (Pinto et al., 2015). For stage 4S patients, primary tumor resection is not obligatory because other treatment strategies such as wait-and-see and low-dose chemotherapy alone after biopsy can also be effective. There is no clear gap in the OS rates at all stages between our study and previous reports. However, the five-year OS rate in patients with MYCN amplification (most at stages 3 and 4) was only 18.3% in our study, far lower than that in previous studies (33%–34% for Japan and Germany). This is the core subgroup which should be dealt with to improve the OS in NBs (Berthold et al., 2017). The quick introduction of novel drugs and treatment methods may help to solve this problem.
Regarding surgery for stage 3 and 4 patients, there are no guidelines covering the choice of different surgical methods (STR vs. GTR), and the role of surgery on the improvement of OS in these patients is still debatable. GTR may be a challenge in some stage 3 and 4 patients because of the poor general situation, vascular encasement by the tumor, and invasion of adjacent organs. Aggressive operations are usually accompanied with potential high risk and may be life-threatening. There are many difficulties in estimating the role of surgery in stage 3 and 4 NBs, such as lack of available prospective studies, non-standardized assessment on the extent of surgical resection, differences in surgeons’ technical skills, and differences among hospital situations. The single-center study usually has a relatively stable surgical team and steady description of surgical approach but a small case number. However, multicenter studies may have the opposite advantages and disadvantages. Several early studies have shown that GTR could improve the prognosis, but they were often single-center studies and had a small case number, and miscellaneous factors were usually undiscussed (McGregor et al., 2005; La Quaglia et al., 2004; Escobar et al., 2006). However, recent studies have drawn the opposite conclusion. Simon et al. (2013) made a study of 278 stage 4 NBs aged 18 months or older from a German clinical trial and found primary tumor resection had no impact on local control rate and outcome. The radicality of surgery was not found to be significantly associated with better OS in stage 3 and 4 patients in Adeline’s study (Salim et al., 2011). What should be noticed is that new and effective chemotherapy/radiation regimens and the application of novel treatment protocols may conceal the real effects of GTR in stage 3 and 4 patients. The median OS estimate of the GTR group was longer than that of the STR group, the statistical significance of the Kaplan–Meier survival curves was determined by the log-rank test in our study, and multivariate survival analyses showed different surgical methods (STR vs. GTR) still had statistically significant difference under the Cox regression model with the adjustment of other variables, such as INSS stage, risk group, and MYCN status. Our study provides evidence supporting the positive conclusion and skilled surgeons should make great efforts to achieve GTR in stage 3 and 4 NB patients. More standardized studies from both single and multiple centers are needed to verify the effect of GTR in stage 3 and 4 patients and provide surgical guidelines for the treatment strategies.
Our study has some limitations. First, this is a single-center retrospective study with a relatively small number of NB patients, so a large-scale multicenter study is required to verify our findings. Second, there may be selective bias in the percentage of MYCN amplification in our NB patients. Last, the number of patients lost to follow-up may have some effects on the mean survival estimates and the comparison of survival rates for the GTR group and STR group in stage 3 and 4 patients.
Conclusions
In summary, we report a large series of NBs in Eastern China and make a comprehensive study about the clinicopathological features, MYCN status, surgical management, and follow-up. We find that MYCN amplification is an independently adverse prognostic factor in Chinese NB patients at stages 3 and 4. GTR is closely associated with improved OS compared with STR in these types of patients, which may guide the optimal choice of surgical methods and a balance of risk and resection extent in these advanced-stage patients. The OS of stage 4 NBs with MYCN amplification is extremely poor, which may merit more detailed risk stratification and more effective treatment strategies.
Supplemental Information
Outcome: 1, dead of disease, 0, alive or lost follow-up.
MYCN status: N, unamplified, A, amplified;
Outcome:1, dead of disease, 0, alive or lost follow-up.
Outcome:1, dead of disease, 0, alive or lost follow-up;
MYCN status;N, unamplified, A, amplified.
Outcome:1, dead of disease, 0, alive or lost follow-up; MYCN status:U, unamplified, A, amplified.
Age: 0, <12 months, 1, 12–60 months, 2, ≥60 months;
Sex:0, male, 1, female;
Site:0, adrenal gland, 1, mediastinum, 2, others;
Stage:0, 3, 1, 4
; Risk:0, intermediate-risk, 1, high-risk;
Outcome: 1, dead of disease, 0, alive or lost follow-up;
Autologous stem cell transplantation:0, no, 1, yes;
Radiation: 0, no, 1, yes;
Degree of differentiation:0, undifferentiated subtype, 1, poorly differentiated subtype, 2, differentiating subtype;
UH or FH: 0, UH,1, FH;
MYCN status: 0, amplified, 1, unamplified;
Surgical methods: 0, gross total resection, 1, subtotal resection.
Acknowledgments
We thank Peng Shi for the statistical support in this study.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Yangyang Ma conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Jicui Zheng performed the experiments, analyzed the data, approved the final draft.
Jiayan Feng, Lian Chen and Kuiran Dong contributed reagents/materials/analysis tools, approved the final draft.
Xianmin Xiao conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
Children’s Hospital of Fudan University granted Ethical approval to carry out the study within its facilities (no. 2017-217).
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.
References
- Ambros et al. (2009).Ambros PF, Ambros IM, Brodeur GM, Haber M, Khan J, Nakagawara A, Schleiermacher G, Speleman F, Spitz R, London WB, Cohn SL, Pearson AD, Maris JM. International consensus for neuroblastoma molecular diagnostics: report from the International Neuroblastoma Risk Group (INRG) biology committee. British Journal of Cancer. 2009;100(9):1471–1482. doi: 10.1038/sj.bjc.6605014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagatell et al. (2009).Bagatell R, Beck-Popovic M, London WB, Zhang Y, Pearson AD, Matthay KK, Monclair T, Ambros PF, Cohn SL, International Neuroblastoma Risk Group Significance of MYCN amplification in international neuroblastoma staging system stage 1 and 2 neuroblastoma: a report from the International Neuroblastoma Risk Group database. Journal of Clinical Oncology. 2009;27(3):365–370. doi: 10.1200/JCO.2008.17.9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold et al. (2017).Berthold F, Spix C, Kaatsch P, Lampert F. Incidence, survival, and treatment of localized and metastatic neuroblastoma in Germany 1979–2015. Pediatric Drugs. 2017;19(6):577–593. doi: 10.1007/s40272-017-0251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell et al. (2017).Campbell K, Gastier-Foster JM, Mann M, Naranjo AH, Van Ryn C, Bagatell R, Matthay KK, London WB, Irwin MS, Shimada H, Granger MM, Hogarty MD, Park JR, DuBois SG. Association of MYCN copy number with clinical features, tumor biology, and outcomes in neuroblastoma: a report from the Children’s Oncology Group. Cancer. 2017;123(21):4224–4235. doi: 10.1002/cncr.30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn et al. (2009).Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D, Mosseri V, Simon T, Garaventa A, Castel V, Matthay KK, INRG Task Force The International Neuroblastoma Risk Group (INRG) classification system: an INRG task force report. Journal of Clinical Oncology. 2009;27(2):289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englum et al. (2015).Englum BR, Rialon KL, Speicher PJ, Gulack B, Driscoll TA, Kreissman SG, Rice HE. Value of surgical resection in children with high-risk neuroblastoma. Pediatric Blood & Cancer. 2015;62(9):1529–1535. doi: 10.1002/pbc.25504. [DOI] [PubMed] [Google Scholar]
- Escobar et al. (2006).Escobar MA, Grosfeld JL, Powell RL, West KW, Scherer 3rd LR, Fallon RJ, Rescorla FJ. Long-term outcomes in patients with stage IV neuroblastoma. Journal of Pediatric Surgery. 2006;41(2):377–381. doi: 10.1016/j.jpedsurg.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Fischer et al. (2017).Fischer J, Pohl A, Volland R, Dübbers M, Cernaianu G, Berthold F, Von Schweinitz D, Simon T. Complete surgical resection improves outcome in INRG high-risk patients with localized neuroblastoma older than 18 months. BMC Cancer. 2017;17(1):520. doi: 10.1186/s12885-017-3493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Quaglia et al. (2004).La Quaglia MP, Kushner BH, Su W, Heller G, Kramer K, Abramson S, Rosen N, Wolden S, Cheung NK. The impact of gross total resection on local control and survival in high-risk neuroblastoma. Journal of Pediatric Surgery. 2004;39(3):412–417. doi: 10.1016/j.jpedsurg.2003.11.028. [DOI] [PubMed] [Google Scholar]
- London et al. (2005).London WB, Castleberry RP, Matthay KK, Look AT, Seeger RC, Shimada H, Thorner P, Brodeur G, Maris JM, Reynolds CP, Cohn SL. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s Oncology Group. Journal of Clinical Oncology. 2005;23(27):6459–6465. doi: 10.1200/JCO.2005.05.571. [DOI] [PubMed] [Google Scholar]
- Maris (2010).Maris JM. Recent advances in neuroblastoma. New England Journal of Medicine. 2010;362(23):2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor et al. (2005).McGregor LM, Rao BN, Davidoff AM, Billups CA, Hongeng S, Santana VM, Hill DA, Fuller C, Furman WL. The impact of early resection of primary neuroblastoma on the survival of children older than 1 year of age with stage 4 disease : the St. Jude Children’s Research Hospital experience. Cancer. 2005;104(12):2837–2846. doi: 10.1002/cncr.21566. [DOI] [PubMed] [Google Scholar]
- Morgenstern et al. (2016).Morgenstern DA, London WB, Stephens D, Volchenboum SL, Simon T, Nakagawara A, Shimada H, Schleiermacher G, Matthay KK, Cohn SL, Pearson AD, Irwin MS. Prognostic significance of pattern and burden of metastatic disease in patients with stage 4 neuroblastoma: a study from the International Neuroblastoma Risk Group database. European Journal of Cancer. 2016;65:1–10. doi: 10.1016/j.ejca.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Peuchmaur et al. (2003).Peuchmaur M, d’Amore ES, Joshi VV, Hata J, Roald B, Dehner LP, Gerbing RB, Stram DO, Lukens JN, Matthay KK, Shimada H. Revision of the international neuroblastoma pathology classification. Cancer. 2003;98(10):2274–2281. doi: 10.1002/cncr.11773. [DOI] [PubMed] [Google Scholar]
- Pinto et al. (2015).Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, Nakagawara A, Berthold F, Schleiermacher G, Park JR, Valteau-Couanet D, Pearson AD, Cohn SL. Advances in risk classification and treatment strategies for neuroblastoma. Journal of Clinical Oncology. 2015;33(27):3008–3017. doi: 10.1200/JCO.2014.59.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim et al. (2011).Salim A, Mullassery D, Pizer B, McDowell HP, Losty PD. Neuroblastoma: a 20-year experience in a UK regional centre. Pediatric Blood & Cancer. 2011;57(7):1254–1260. doi: 10.1002/pbc.23149. [DOI] [PubMed] [Google Scholar]
- Shimada et al. (1999).Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B, Stram DO, Gerbing RB, Lukens JN, Matthay KK, Castleberry RP. The international neuroblastoma pathology classification (the Shimada system) Cancer. 1999;86(2):364–372. doi: 10.1002/(SICI)1097-0142(19990715)86:2<364::AID-CNCR21>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Simon et al. (2013).Simon T, Häberle B, Hero B, Von Schweinitz D, Berthold F. Role of surgery in the treatment of patients with stage 4 neuroblastoma age 18 months or older at diagnosis. Journal of Clinical Oncology. 2013;31(6):752–758. doi: 10.1200/JCO.2012.45.9339. [DOI] [PubMed] [Google Scholar]
- Souzaki et al. (2011).Souzaki R, Tajiri T, Teshiba R, Higashi M, Kinoshita Y, Tanaka S, Taguchi T. The genetic and clinical significance of MYCN gain as detected by FISH in neuroblastoma. Pediatric Surgery International. 2011;27(3):231–236. doi: 10.1007/s00383-010-2781-4. [DOI] [PubMed] [Google Scholar]
- Spitz et al. (2004).Spitz R, Hero B, Skowron M, Ernestus K, Berthold F. MYCN-status in neuroblastoma: characteristics of tumours showing amplification, gain, and non-amplification. European Journal of Cancer. 2004;40(18):2753–2759. doi: 10.1016/j.ejca.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Vo et al. (2014).Vo KT, Matthay KK, Neuhaus J, London WB, Hero B, Ambros PF, Nakagawara A, Miniati D, Wheeler K, Pearson AD, Cohn SL, DuBois SG. Clinical, biologic, and prognostic differences on the basis of primary tumor site in neuroblastoma: a report from the international neuroblastoma risk group project. Journal of Clinical Oncology. 2014;32(28):3169–3176. doi: 10.1200/JCO.2014.56.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2013).Wang M, Zhou C, Cai R, Li Y, Gong L. Copy number gain of MYCN gene is a recurrent genetic aberration and favorable prognostic factor in Chinese pediatric neuroblastoma patients. Diagnostic Pathology. 2013;8(1):5. doi: 10.1186/1746-1596-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Outcome: 1, dead of disease, 0, alive or lost follow-up.
MYCN status: N, unamplified, A, amplified;
Outcome:1, dead of disease, 0, alive or lost follow-up.
Outcome:1, dead of disease, 0, alive or lost follow-up;
MYCN status;N, unamplified, A, amplified.
Outcome:1, dead of disease, 0, alive or lost follow-up; MYCN status:U, unamplified, A, amplified.
Age: 0, <12 months, 1, 12–60 months, 2, ≥60 months;
Sex:0, male, 1, female;
Site:0, adrenal gland, 1, mediastinum, 2, others;
Stage:0, 3, 1, 4
; Risk:0, intermediate-risk, 1, high-risk;
Outcome: 1, dead of disease, 0, alive or lost follow-up;
Autologous stem cell transplantation:0, no, 1, yes;
Radiation: 0, no, 1, yes;
Degree of differentiation:0, undifferentiated subtype, 1, poorly differentiated subtype, 2, differentiating subtype;
UH or FH: 0, UH,1, FH;
MYCN status: 0, amplified, 1, unamplified;
Surgical methods: 0, gross total resection, 1, subtotal resection.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.