Abstract
Background
Falls in care facilities and hospitals are common events that cause considerable morbidity and mortality for older people. This is an update of a review first published in 2010 and updated in 2012.
Objectives
To assess the effects of interventions designed to reduce the incidence of falls in older people in care facilities and hospitals.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (August 2017); Cochrane Central Register of Controlled Trials (2017, Issue 8); and MEDLINE, Embase, CINAHL and trial registers to August 2017.
Selection criteria
Randomised controlled trials of interventions for preventing falls in older people in residential or nursing care facilities, or hospitals.
Data collection and analysis
One review author screened abstracts; two review authors screened full‐text articles for inclusion. Two review authors independently performed study selection, 'Risk of bias' assessment and data extraction. We calculated rate ratios (RaR) with 95% confidence intervals (CIs) for rate of falls and risk ratios (RRs) and 95% CIs for outcomes such as risk of falling (number of people falling). We pooled results where appropriate. We used GRADE to assess the quality of evidence.
Main results
Thirty‐five new trials (77,869 participants) were included in this update. Overall, we included 95 trials (138,164 participants), 71 (40,374 participants; mean age 84 years; 75% women) in care facilities and 24 (97,790 participants; mean age 78 years; 52% women) in hospitals. The majority of trials were at high risk of bias in one or more domains, mostly relating to lack of blinding. With few exceptions, the quality of evidence for individual interventions in either setting was generally rated as low or very low. Risk of fracture and adverse events were generally poorly reported and, where reported, the evidence was very low‐quality, which means that we are uncertain of the estimates. Only the falls outcomes for the main comparisons are reported here.
Care facilities
Seventeen trials compared exercise with control (typically usual care alone). We are uncertain of the effect of exercise on rate of falls (RaR 0.93, 95% CI 0.72 to 1.20; 2002 participants, 10 studies; I² = 76%; very low‐quality evidence). Exercise may make little or no difference to the risk of falling (RR 1.02, 95% CI 0.88 to 1.18; 2090 participants, 10 studies; I² = 23%; low‐quality evidence).
There is low‐quality evidence that general medication review (tested in 12 trials) may make little or no difference to the rate of falls (RaR 0.93, 95% CI 0.64 to 1.35; 2409 participants, 6 studies; I² = 93%) or the risk of falling (RR 0.93, 95% CI 0.80 to 1.09; 5139 participants, 6 studies; I² = 48%).
There is moderate‐quality evidence that vitamin D supplementation (4512 participants, 4 studies) probably reduces the rate of falls (RaR 0.72, 95% CI 0.55 to 0.95; I² = 62%), but probably makes little or no difference to the risk of falling (RR 0.92, 95% CI 0.76 to 1.12; I² = 42%). The population included in these studies had low vitamin D levels.
Multifactorial interventions were tested in 13 trials. We are uncertain of the effect of multifactorial interventions on the rate of falls (RaR 0.88, 95% CI 0.66 to 1.18; 3439 participants, 10 studies; I² = 84%; very low‐quality evidence). They may make little or no difference to the risk of falling (RR 0.92, 95% CI 0.81 to 1.05; 3153 participants, 9 studies; I² = 42%; low‐quality evidence).
Hospitals
Three trials tested the effect of additional physiotherapy (supervised exercises) in rehabilitation wards (subacute setting). The very low‐quality evidence means we are uncertain of the effect of additional physiotherapy on the rate of falls (RaR 0.59, 95% CI 0.26 to 1.34; 215 participants, 2 studies; I² = 0%), or whether it reduces the risk of falling (RR 0.36, 95% CI 0.14 to 0.93; 83 participants, 2 studies; I² = 0%).
We are uncertain of the effects of bed and chair sensor alarms in hospitals, tested in two trials (28,649 participants) on rate of falls (RaR 0.60, 95% CI 0.27 to 1.34; I² = 0%; very low‐quality evidence) or risk of falling (RR 0.93, 95% CI 0.38 to 2.24; I² = 0%; very low‐quality evidence).
Multifactorial interventions in hospitals may reduce rate of falls in hospitals (RaR 0.80, 95% CI 0.64 to 1.01; 44,664 participants, 5 studies; I² = 52%). A subgroup analysis by setting suggests the reduction may be more likely in a subacute setting (RaR 0.67, 95% CI 0.54 to 0.83; 3747 participants, 2 studies; I² = 0%; low‐quality evidence). We are uncertain of the effect of multifactorial interventions on the risk of falling (RR 0.82, 95% CI 0.62 to 1.09; 39,889 participants; 3 studies; I² = 0%; very low‐quality evidence).
Authors' conclusions
In care facilities: we are uncertain of the effect of exercise on rate of falls and it may make little or no difference to the risk of falling. General medication review may make little or no difference to the rate of falls or risk of falling. Vitamin D supplementation probably reduces the rate of falls but not risk of falling. We are uncertain of the effect of multifactorial interventions on the rate of falls; they may make little or no difference to the risk of falling.
In hospitals: we are uncertain of the effect of additional physiotherapy on the rate of falls or whether it reduces the risk of falling. We are uncertain of the effect of providing bed sensor alarms on the rate of falls or risk of falling. Multifactorial interventions may reduce rate of falls, although subgroup analysis suggests this may apply mostly to a subacute setting; we are uncertain of the effect of these interventions on risk of falling.
Plain language summary
Interventions for preventing falls in older people in care facilities and hospitals
Review question How effective are interventions designed to reduce falls in older people in care facilities and hospitals?
Background Falls by older people in care facilities, such as nursing homes, and hospitals are common events that may cause loss of independence, injuries, and sometimes death as a result of injury. Effective interventions to prevent falls are therefore important. Many types of interventions are in use. These include exercise, medication interventions that include vitamin D supplementation and reviews of the drugs that people are taking, environment or assistive technologies including bed or chair alarms or the use of special (low/low) beds, social environment interventions that target staff members and changes in the organisational system, and knowledge interventions. A special type of intervention is the multifactorial intervention, where the selection of single interventions such as exercise and vitamin D supplementation is based on an assessment of a person's risk factors for falling. Falls are reported in two ways in our review. One outcome is rate of falls, which is the number of falls. The other outcome is risk of falling, which is the number of people who had one or more falls.
Search date
We searched the healthcare literature for reports of randomised controlled trials relevant to this review up to August 2017.
Study characteristics This review included 95 randomised controlled trials involving 138,164 participants. Seventy‐one trials (40,374 participants) were in care facilities, and 24 (97,790 participants) in hospitals. On average, participants were 84 years old in care facilities and 78 years old in hospitals. In care facilities, 75% were women and in hospitals, 52% were women.
Quality of the evidence The majority of trials were at high risk of bias, mostly relating to lack of blinding. With few exceptions, the quality of evidence for individual interventions in either setting was generally rated as low or very low. Risk of fracture and adverse events were generally poorly reported and, where reported, the evidence was very low quality, which means that we are uncertain of the estimates.
Key results
There was evidence, often from single studies, for a wide range of interventions used for preventing falls in both settings. However, in the following we summarise only the falls outcomes for four key interventions in care facilities and three key interventions in hospitals.
Care facilities We are uncertain of the effect of exercise on the rate of falls (very low‐quality evidence) and it may make little or no difference to the risk of falling (low‐quality evidence). General medication review may make little or no difference to the rate of falls (low‐quality evidence) or the risk of falling (low‐quality evidence). Prescription of vitamin D probably reduces the rate of falls (moderate‐quality evidence) but probably makes little or no difference to the risk of falling (moderate‐quality evidence). The population included in these studies appeared to have low vitamin D levels. We are uncertain of the effect of multifactorial interventions on the rate of falls (very low‐quality evidence). They may make little or no difference to the risk of falling (low‐quality evidence).
Hospitals We are uncertain whether physiotherapy aimed specifically at reducing falls in addition to usual rehabilitation in the ward has an effect on the rate of falls or reduces the risk of falling (very low‐quality evidence). We are uncertain of the effect of bed alarms on the rate of falls or risk of falling (very low‐quality evidence). Multifactorial interventions may reduce the rate of falls, although this is more likely in a rehabilitation or geriatric ward setting (low‐quality evidence). We are uncertain of the effect of these interventions on risk of falling.
Summary of findings
Background
Description of the condition
Studies of falls in nursing facilities show considerable variation in falls incidence rates but a “middle of the road” figure provided in a review of incidence rates is 1.7 falls per person‐year, compared with 0.65 falls per person‐year for older people living in the community (Rubenstein 2006). In a study conducted in 40 Canadian residential care facilities, 62% of participants fell over a one‐year period, with a falls rate of 2.51 falls per person per year (Kennedy 2015). It should be noted, however, that routine recording of falls incidents in standard reporting systems is likely to under‐estimate the incidence of falls (Hill 2010; Sutton 1994). In a prospective one‐year study in 528 nursing homes in Bavaria, Germany, about 75% of falls occurred in the residents' rooms or in bathrooms; 41% occurred during transfers and 36% when walking (Becker 2012). The fall rate was higher in men (2.8 falls per person year) than women (1.49 falls per person year), and falls were less common in people requiring the lowest and highest levels of care. Lord 2003 also found that fall rates were lower in frailer people who were unable to rise from a chair or stand unaided. In this group, increased age, male sex, higher care classifications, incontinence, psychoactive medication use, previous falls and slow reaction times were associated with increased falls. Systematic reviews have shown that in nursing homes, falls history, walking aid use, moderate disability, cognitive impairment, wandering, Parkinson's disease, dizziness, use of sedatives, antipsychotics, antidepressants and total number of medications used are associated with an increased risk of falling (Deandrea 2013; Muir 2012). In residents with dementia, age, use of psychotropic drugs, fair or poor general health, gait impairment and trunk restraint use are associated with an increased number of falls (Kropelin 2013).
In hospital settings, a falls incidence of 5.71 falls per 1000 bed days has been found in 16 US general medical surgical and speciality units (Shorr 2012), 6.45 falls per 1000 bed days in 24 Australian medical and surgical wards (Barker 2016), 10.9 falls per 1000 bed days in eight Australian rehabilitation/geriatric units (Hill 2015) and 17.1 falls per 1000 bed days in psychogeriatric wards (Nyberg 1997). In elderly care wards in an UK district general hospital in 2004, the reported rate was as high as 18.0 falls per 1000 bed days (Healey 2004). A similar rate has been reported in some high‐risk wards in Australia (Barker 2016).
Systematic reviews have shown that risk factors for falls in hospital inpatients are falls history, age, cognitive impairment, sedative and antidepressant use, gait instability, agitated confusion and urinary incontinence (Deandrea 2013; Oliver 2004). For older patients in rehabilitation hospital settings, risk factors include carpet flooring, vertigo, being an amputee, confusion, cognitive impairment, stroke, sleep disturbance, anticonvulsants, tranquillisers, antihypertensive medications, previous falls and need for transfer assistance (Vieira 2011).
There is considerable mortality and morbidity associated with falls in care facilities and hospitals. A study in 24 Australian medical and surgical wards reported a fall injury rate of 2.36 per 1000 bed days (Barker 2016). A study in both these settings reported an incidence of 533 per 1000 person years for all injuries, 20 per 1000 person years for hip fracture, and 270 per 1000 person years for head injuries, for which 13% (14/107) required medical attention (Nurmi 2002). Overall, men were 1.5 times more likely to be injured than women. Older people who sustain a hip fracture while in hospital have been shown to have poor outcomes compared with people sustaining similar fractures in the community (Murray 2007). Falls have been reported to be the most common cause of death from an external cause in residents of care facilities (Ibrahim 2015).
Description of the intervention
The majority of falls are caused by complex combinations of factors operating at the time of each fall event. Interventions may target risk factors in participants or target staff and clinicians with the aim of improving clinical practice or the organisation of care. In some studies, single interventions have been evaluated while in others, interventions with more than one component have been evaluated. Delivery of multiple‐component interventions may be based on individual assessment of risk (a multifactorial intervention) or the same components are provided to all participants (a multiple intervention). A taxonomy has been developed to describe and classify types of intervention (Lamb 2007; Lamb 2011). Key intervention categories include exercise, medication (drug target) interventions which include interventions targeting vitamin D and medication reviews, environment or assistive technologies including bed/chair alarms or the use of low/low beds, social environment interventions which target staff members and changes in the organisational system, knowledge interventions and multifactorial interventions.
The majority of randomised controlled trials considered within this review provide a comparison with ‘usual care’ in the care facilities and hospitals involved. Typically, 'usual care' will include standard practices for managing commonly known, potentially modifiable, risk factors for falls and, moreover, the components of usual care will vary both over time and between settings.
Why it is important to do this review
A systematic review is required to summarise evidence of the impact of purposeful interventions designed to prevent falls, in addition to the unknown impact of routine (and probably variable) care in care facilities and hospitals. Despite routine activities attempting to reduce falls, falls are common in these settings and they result in considerable mortality and morbidity. Results will inform healthcare professionals, researchers, policy makers, informal care givers and consumers. This review is an update of a Cochrane Review first published in 2010 (Cameron 2010), and previously updated in 2012 (Cameron 2012).
Objectives
To assess the effects of interventions designed to reduce the incidence of falls in older people in care facilities and hospitals.
Methods
Criteria for considering studies for this review
Types of studies
We considered for inclusion all randomised trials, including quasi‐randomised trials (for example, alternation), cluster‐randomised trials and trials in which treatment allocation was inadequately concealed.
Types of participants
We included trials of interventions to prevent falls in older people, of either sex, in care facilities or hospitals. We considered trials for inclusion if the majority of participants were over 65 years or the mean age was over 65 years, and the majority were living in care facilities or were patients in hospital. We excluded trials conducted in places of residence that do not provide residential health‐related care or rehabilitative services, for example retirement villages or sheltered housing. Trials with participants resident in the community and in care facilities were included either in this review or in the Cochrane Review of interventions for preventing falls in older people living in the community (Gillespie 2012), depending on the proportion of participants in each setting. Inclusion in either review was determined by discussion between the authors of both reviews. Trials recording falls in both settings may be included in both reviews.
We subdivided care facilities based on level of care provided. We defined high‐level care facilities as "establishments that are primarily engaged in providing inpatient nursing and rehabilitative services for long‐term care patients. The care is generally provided for an extended period of time to individuals requiring nursing care. These establishments have a permanent core staff of registered or licensed practical nurses that, along with other staff, provide nursing care in combination with personal care" (OECD 2011). We defined intermediate‐care facilities as "institutions which provide health‐related care and services to individuals who do not require the degree of care which hospitals or skilled nursing facilities provide, but because of their physical or mental condition require care and services above the level of room and board" (NLM 2012). Some facilities provided both these levels of care. For cluster‐randomised trials, the classification of the level of care was based on the description of the facility. For individually‐randomised trials where the level of care provided by the facility was clearly described, this description informed the classification. Where the inclusion/exclusion criteria of a trial selected patients who required high or intermediate level of care from a mixed‐care facility, the classification was based upon the care needs of the individual participants.
For trials in hospitals, participants included staff or in‐patients. We excluded interventions that took place in emergency departments, outpatient departments or where hospital services were provided in community settings. We subdivided hospitals into those providing acute, and those providing subacute care. We defined subacute care as "medical and skilled nursing services provided to patients who are not in an acute phase of an illness but who require a level of care higher than that provided in a long‐term care setting" (NLM 2012).
Studies recruiting participants post‐stroke were excluded as interventions to prevent falls in this population are reviewed in a separate Cochrane Review Interventions for preventing falls in people after stroke (Verheyden 2013).
Types of interventions
Any intervention designed to reduce falls in older people compared with any other intervention, usual care or placebo. We grouped interventions using the fall‐prevention classification system (taxonomy) developed by the Prevention of Falls Network Europe (ProFaNE) (Lamb 2011). Interventions have been grouped by combination (single, multiple, or multifactorial), and then by the type of intervention (descriptors). Full details are available in the ProFaNE taxonomy manual (Lamb 2007). The possible intervention descriptors are: exercises, medication (drug target, i.e. withdrawal, dose reduction or increase, substitution, provision), surgery, management of urinary incontinence, fluid or nutrition therapy, psychological interventions, environment/assistive technology, social environment, interventions to increase knowledge, other interventions.
Types of outcome measures
We included only trials that reported raw data or statistics relating to rate or number of falls, or number of participants sustaining at least one fall during follow‐up (fallers). Trials that reported only those participants who had more than one fall were included. Trials that reported only specific types of fall (e.g. injurious falls) were not included. Trials that focused on intermediate outcomes such as improved balance or strength, and did not report falls or falling as an outcome, were excluded.
Primary outcomes
Rate of falls (falls per unit of person time that falls were monitored)
Number of fallers (risk of falling)
Secondary outcomes
Number of participants sustaining fall‐related fractures
Complications of the interventions
Economic outcomes
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (to 3 August 2017), the Cochrane Central Register of Controlled Trials (CENTRAL) (2017, Issue 8), MEDLINE (including Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid MEDLINE and Versions) (1946 to 3 August 2017), Embase (1980 to 2017 Week 31), and CINAHL (1982 to 3 August 2017). We also searched ongoing trial registers via the World Health Organization's ICTRP Search Portal (3 August 2017) and ClinicalTrials.gov (3 August 2017). We did not apply any language restrictions.
For this update, the search results were limited from 2012 onwards. The search update process was run in two stages: the first search was run in February 2016 and a second top‐up search was run in August 2017. Details of the search strategies used for previous versions of the review are given in Cameron 2012.
In MEDLINE (OvidSP), subject‐specific search terms were combined with the sensitivity‐ and precision‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE (Lefebvre 2011). We modified this strategy for use in CENTRAL, Embase, and CINAHL (seeAppendix 1 for all strategies).
Searching other resources
We also checked reference lists of articles and further trials were identified by contact with researchers in the field. For the first version of this review, we identified trials in care facilities and hospitals included in Gillespie 2003.
Data collection and analysis
Data collection and analysis were carried out according to methods stated in the published protocol (Cameron 2005), and subsequently amended to concur with updated methods in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a) as described in Differences between protocol and review. Data collection and analysis were carried out according to methods stated in the published protocol (Cameron 2005), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Selection of studies
From the title, abstract, or descriptors, one review author screened all abstracts to identify potentially relevant trials for full review. Two review authors screened potentially relevant abstracts. From the full text, two review authors independently assessed potentially eligible trials for inclusion and resolved disagreement by discussion, or by adjudication with a third review author. Full‐text review was undertaken using Covidence. Disagreement was resolved by discussion and consensus or third party adjudication when necessary. We contacted trial authors for additional information if necessary to assess eligibility.
Data extraction and management
Pairs of review authors independently extracted data using a pre‐tested data extraction form for studies included to 2012. For this update,again pairs of review authors independently extracted data from the identified studies using Covidence. Multiple reports from the same study were linked as a single study in Covidence and evidence from all reports were reviewed in undertaking data extraction. Where data were unclear authors were contacted whenever possible for clarification. Disagreement was resolved by discussion and consensus or third party adjudication when necessary.
Assessment of risk of bias in included studies
Pairs of review authors independently assessed risk of bias for each included study based on recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Assessors were not blinded to author and source institution. Review authors did not assess their own trials. Disagreement was resolved by consensus, or by third party adjudication.
We assessed risk of bias for the following domains: sequence generation (selection bias); allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias). Since all the outcomes collected in our review are susceptible to the same risk of bias, we have not assessed outcomes for risk of detection bias or completeness of outcome data separately. Additionally, we assessed bias in the recall of falls due to less reliable methods of ascertainment (Hannan 2010), and bias resulting from major imbalances in key baseline characteristics (e.g. age, gender, previous falls, medical status, dependency, cognitive function). Assessors rated the risk of bias as low, high or unclear for each domain.
We established additional criteria within currently existing domains for assessing the additional risks of bias associated with cluster randomisation (Section 16.3.2; Higgins 2011b). Thus 'recruitment bias' was considered as a component of selection bias under allocation concealment; 'baseline imbalance' resulting from small numbers of clusters was considered in bias resulting from major imbalances in key characteristics; risk of bias resulting from 'loss of clusters' was considered under incomplete outcome data; and 'incorrect analysis' that failed to take into account the effect of clustering and that could not be satisfactorily remedied was considered under selective outcome reporting. We did not assess the risk of bias relating to the 'comparability with individually‐randomised trials' as a separate item as it is impossible to establish suitable criteria for an individual trial out of context. The potential for differences in effects between cluster‐ and individually‐randomised trials was considered in our assessment of the quality of the evidence and in our Discussion.
Our criteria for 'Risk of bias' assessments are shown in Appendix 2.
Measures of treatment effect
We have reported the treatment effect for rate of falls as a rate ratio (RaR) and 95% confidence interval (CI). For number of fallers and number of participants sustaining fall‐related fractures we have reported a risk ratio (RR) and 95% CI. We used results reported at discharge from hospital for trials that continued to monitor falls after discharge.
Rate of falls
The rate of falls is the total number of falls per unit of person time that falls were monitored (e.g. falls per person year). The rate ratio compares the rate of falls in any two groups during each trial.
We used a rate ratio (for example, incidence rate ratio or hazard ratio for all falls) and 95% CI if these were reported in the paper. If both adjusted and unadjusted rate ratios were reported, we used the unadjusted estimate, unless the adjustment was for clustering. If a rate ratio was not reported but appropriate raw data were available, we used Excel to calculate a rate ratio and 95% CI. We used the reported rate of falls (falls per person year) in each group and the total number of falls for participants contributing data, or we calculated the rate of falls in each group from the total number of falls and the actual total length of time falls were monitored (person years) for participants contributing data. In cases where data were only available for people who had completed the study, or where the trial authors had stated there were no losses to follow‐up, we assumed that these participants had been followed up for the maximum possible period. Where there were no falls in one arm of a study, and a low total number of falls and/or participants (e.g. Beck 2016; Cadore 2014), the rate of falls cannot be determined. Such data were therefore not pooled, however the omission of these data from the pooled analysis is considered unlikely to change any estimate of effect.
Risk of falling
For number of fallers, a dichotomous outcome, we used a risk ratio as the treatment effect. The risk ratio compares the number of people who fell once or more (fallers) in the intervention and control arms of each trial.
We used a reported estimate of risk (hazard ratio for first fall, risk ratio (relative risk), or odds ratio) and 95% CI if available. If both adjusted and unadjusted estimates were reported we used the unadjusted estimate, unless the adjustment was for clustering. If an odds ratio was reported, or there was no effect estimate and 95% CI, and appropriate data were available, we calculated a risk ratio and 95% CI using the csi command in Stata or in Review Manager. For the calculations, we used the number of participants contributing data in each group if this was known; if not reported, we used the number randomised to each group.
Secondary outcomes
For the number of participants sustaining one or more fall‐related fractures, we used a risk ratio as described in 'Risk of falling' above.
Unit of analysis issues
For trials that were cluster randomised, for example by care facility or ward, we performed adjustments for clustering (Higgins 2011c), if this was not done in the published report. We used intra‐cluster correlation coefficients reported by Dyer 2004 (falls per person year 0.100, number of residents falling 0.071, and residents sustaining a fracture 0.026).
For trials with multiple intervention groups, we either combined the groups or included only one pair‐wise comparison (intervention versus control) in any analysis in order to avoid the same group of participants being included twice.
For trials that excluded the intervention period from the falls outcomes, we did not pool the outcomes data with other studies.
Dealing with missing data
Only the available data were used in the analyses; we did not impute missing data.
Assessment of heterogeneity
We assessed heterogeneity within a pooled group of trials using a combination of visual inspection of the graph along with consideration of the Chi² test (with statistical significance set at P < 0.10), and the I² statistic (Higgins 2003). We based our interpretation of the I² results on that suggested by Higgins 2011a: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% may represent very substantial ('considerable') heterogeneity.
Assessment of reporting biases
To explore the possibility of publication and other reporting biases, we constructed funnel plots for analyses that contained more than 10 studies.
Data synthesis
We classified interventions into those taking place in care facilities and those taking place in hospitals, and pooled these separately because participant characteristics and the environment warrants different types of interventions in the different settings, possibly implemented by people with different skill mixes.
Within these categories, we grouped the results of trials with comparable interventions and participant characteristics, and compiled forest plots using the generic inverse variance method in Review Manager. This method enabled pooling of the adjusted and unadjusted treatment effect estimates (rate ratios or risk ratios) that were reported in the paper, or we calculated from data presented in the paper (seeMeasures of treatment effect). Where the total number of patients, rather than admissions, could not be determined, we did not pool these data with other studies. Where the reported trial outcomes did not include falls during the intervention period, we did not pool these data with those of other trials.
Where appropriate, we pooled results of comparable studies using both fixed‐effect and random‐effects models. We chose the model to report by careful consideration of the extent of heterogeneity and whether it can be explained by factors such as the number and size of included studies, or the level of care provided. We used 95% CIs throughout. We considered, on a case by case basis, not pooling data where there was considerable heterogeneity (I² statistic value of greater than 75%) that could not be explained by the diversity of methodological or clinical features among trials. Where it was inappropriate to pool data, we still presented trial data in the analyses or tables for illustrative purposes and reported these in the text.
Subgroup analysis and investigation of heterogeneity
We minimised heterogeneity as much as possible by grouping trials as described previously (using ProFaNE categories of interventions). We categorised broad interventions further by grouping subtypes of interventions according to ProFaNE (e.g. for exercise interventions). We explored heterogeneity by carrying out subgroup analyses based on level of care and level of cognition at enrolment in care facilities and hospitals where possible. We subdivided the care facilities into high, intermediate or mixed levels of care. The levels of care of the facilities reflect the levels of dependence of the participants. In hospitals, the level of care was subdivided by acute versus subacute or mixed levels of care. We also carried out subgroup analyses by stratification of intervention types according to ProFaNE (e.g. for exercise types, medication target interventions), and type of fracture. Subgroup analyses based upon the individual components of the multifactorial interventions was precluded by the study design and reporting. Data were inadequate for conducting a subgroup analysis by level of frailty of the participants in trials of exercise in care facilities.
We grouped trials by level of cognition into those that included only participants with cognitive impairment versus those with no cognitive impairment, or a mixed sample at enrolment.
We used the random‐effects model to pool data in all subgroup analyses testing for subgroup differences due to the high risk of false‐positive results when comparing subgroups in a fixed‐effect model (Higgins 2011d). We used the test for subgroup differences available in Review Manager to determine whether there was evidence for a difference in treatment effect between subgroups.
Sensitivity analysis
Where there was substantial statistical heterogeneity we carried out a post‐hoc sensitivity analysis to explore the effect of removing trials from the analysis if visual inspection of the graph showed poorly overlapping confidence intervals. Where there was considered to be significant statistical heterogeneity for rate of falls but not risk of falling, sensitivity analyses were carried out to determine the likely effects of using random‐effects versus fixed‐effect meta‐analyses for the risk of falling (e.g. for exercise versus usual care in care facilities and multifactorial interventions in care facilities). We conducted post‐hoc sensitivity analyses for exercise in care facilities, excluding trials with 20 participants or less in each arm of the trial to explore the possibility of small‐trial effects, due to the observed asymmetry in the Funnel plots. We conducted a sensitivity analysis for exercise compared to usual care in care facilities including Cadore 2014, which had zero falls in the intervention arm, using one fall in the intervention arm to examine the likely effect of omitting this trial from the analysis. We also conducted a sensitivity analysis excluding one trial with a known non‐normal distribution of falls in the intervention arm from the analysis of general medication review in care facilities for the rate of falls outcomes.
Sensitivity analyses according to study quality were not possible as most studies were at potential risk of bias.
Economic issues
We have noted the results from any economic evaluations (cost‐effectiveness analysis, cost‐utility analysis) incorporated in included studies. We also extracted from each trial reporting a cost analysis, cost description or analytic model, the type of resource use reported (e.g. delivering the intervention, hospital admissions, medication use) and the cost of the items for each group.
Assessing the quality of the evidence and 'Summary of findings' tables
For each comparison, we used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of the body of evidence (Schünemann 2011) for each outcome listed in Types of outcome measures. For all comparisons where there were two or more trials, GRADE assessment was performed independently by two review authors and disagreement was resolved by discussion, or by adjudication with a third review author. We adopted a different approach for single trial comparisons, where we started with the assumption that the quality of evidence was likely to be very low. This reflected assumptions of downgrading at a minimum for serious risk of bias (typically performance and detection bias), for serious indirectness (trial being conducted was a single trial or setting), and for serious imprecision (failure to meet the 200 to 300 events optimal size criteria) (Guyatt 2011). Where these assumptions did not hold, we performed GRADE assessment as above. The quality rating 'high' is reserved for a body of evidence based on randomised controlled trials. We ‘downgraded’ the quality rating to 'moderate', 'low' or 'very low' depending on the presence and extent of five factors: study limitations, inconsistency of effect, imprecision, indirectness or publication bias. We used the GRADE approach to assess quality of evidence related to the primary and secondary outcomes listed in the Types of outcome measures. We prepared a 'Summary of findings' table for each of the main categories of interventions, for listed outcomes.
We selected the following comparisons for presentation in 'Summary of findings' tables as these are the most common falls prevention activities considered and applied in clinical settings. In care facilities: exercise, vitamin D supplementation, medication review and multifactorial interventions; in hospitals: exercise, bed alarms and multifactorial interventions.
Results
Description of studies
Results of the search
For this update we screened a total of 3989 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (0 records); CENTRAL (127), MEDLINE (1104), Embase (1211), CINAHL (314) the WHO ICTRP (450) and Clinicaltrials.gov (783). We also found 29 potentially eligible studies from other sources. After removal of 503 duplicates, 3515 citations were screened for inclusion.
Screening of the search update identified a total of 413 records for potential inclusion, for which full‐text reports were obtained. Thirty‐five new trials were included in this update, 27 new ongoing trials identified and seven new studies await classification. In addition, a new subgroup analysis (Stenvall 2012) from the Stenvall 2007 trial and a cost‐effectiveness analysis (Haines 2013) of Haines 2011 have been added. A flow diagram summarising the study selection process is shown in Figure 1.
1.
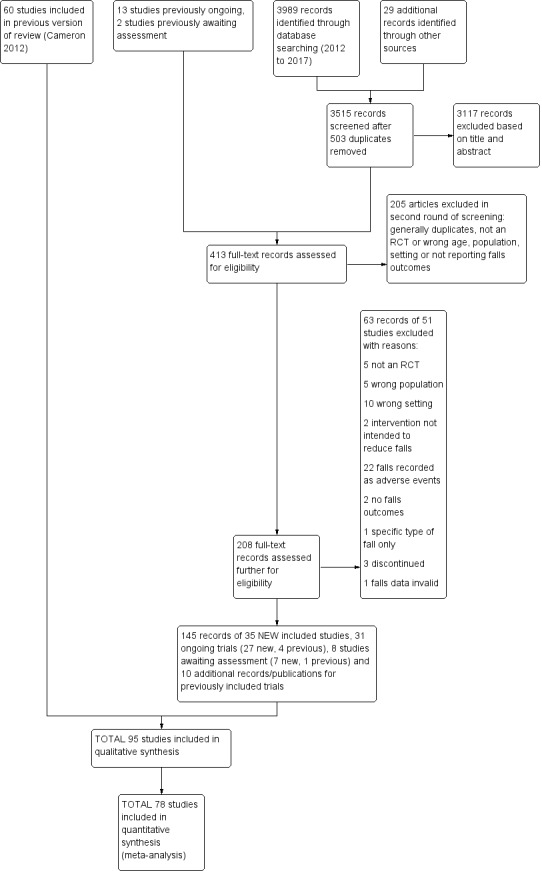
Study flow diagram
Overall, there are now 95 included trials, 105 excluded studies, eight studies awaiting classification and 31 ongoing trials.
Due to the review size, not all links to references have been inserted in the text but can be viewed in Table 8.
1. Description of included studies: reference links.
Included studies
Thirty‐five additional trials have been included in this update, 28 trials in care facilities and seven in hospitals (seeTable 8). This review now contains 95 trials with 138,164 participants. Details of individual trials are provided in the Characteristics of included studies, and are briefly outlined below.
Design
Participants were individually randomised in 53 studies, whereas 42 studies used a cluster‐randomised design (seeTable 8).
Settings
The included trials were carried out in 23 countries (seeTable 8).
Of the 71 studies (40,374 participants) in care facilities, 17 were in high‐level care facilities, 17 were in intermediate‐level care facilities and 37 were in facilities with mixed levels of care, or combinations of facilities that included both high and intermediate levels of care. Of the 24 studies (97,790 participants) in hospital settings, 10 were in an acute hospital setting, 12 were in subacute settings, and 2 were in both acute and subacute care settings (seeTable 8).
Van Gaal 2011a and Van Gaal 2011b have been included as two separate trials although reported in the same paper as the participants were randomised separately in two settings (nursing homes and hospitals) and results are reported by setting.
Participants
The mean age of participants was 83.5 years in care facilities and 77.6 years in hospitals. In care facilities, 75.3% were women and in hospitals, 51.6% were women.
All participants were women in seven trials (Bischoff 2003; Chapuy 2002; Faber 2006; Irez 2011; Jarvis 2007; Kovacs 2012; Sihvonen 2004). Ten studies specifically recruited participants with cognitive impairment (Buettner 2002; Chenoweth 2009; Klages 2011; Kovacs 2013; Mador 2004; Neyens 2009; Shaw 2003; Toulotte 2003; Van de Ven 2014; Whitney 2017). Exceptionally, Stenvall 2007 only recruited people with a proximal femoral (hip) fracture.
Interventions
Using ProFaNE taxonomy, all studies were categorised by intervention and grouped by combination (single, multiple, or multifactorial) (seeAppendix 3). The first column of Appendix 3 shows the intervention classification (single, multiple, or multifactorial) and setting type (care facility or hospital). The components of included 'Exercises' interventions, 'Environmental/assistive technology' and 'Medication (drug target)' interventions are shown in Appendix 4, Appendix 5 and Appendix 6 respectively.
In care facilities, 54 trials tested the effect of a single intervention only, three trials tested both single and multiple interventions (Huang 2016; Imaoka 2016; Sambrook 2012), one trial tested a multiple intervention only (Schnelle 2003), and 13 trials tested a multifactorial intervention. In hospitals, 18 trials tested the effect of a single intervention and six tested a multifactorial intervention.
Seven studies tested the effect of two interventions (Faber 2006; Haines 2011; Huang 2016; Nowalk 2001; Sambrook 2012; Saravanakumar 2014; Tuunainen 2013), and one tested three interventions (Imaoka 2016) in comparison with usual care. Donald 2000 was a 2 x 2 factorial study of supervised exercises and flooring types that has been classified as two single interventions.
In general, included studies compared an active falls prevention intervention with a control group comprising 'usual care', that typically would have included standard falls prevention activities. Often, however, standard practice in terms of falls prevention activities was not clearly described. Additional descriptions of the control groups provided for individual trials are provided in the Characteristics of included studies table, the 'Summary of findings' tables available for some comparisons, and the analyses headings and/or footnotes. A general description of the control arms for the main intervention categories is also given below.
In care facilities, 17 trials of exercise provided a comparison with usual care, defined as no exercise, no change in previous lifestyle or exercise type or level unlikely to change physical performance and nine trials provided a comparison of two different exercise programmes (see Table 9). Trials of medication target interventions in care facilities more often provided a comparison with placebo (see Table 10). Trials of vitamin D supplementation in care facilities provided estimates of effect compared with usual care or placebo. In hospitals, multifactorial interventions were generally compared with a control group consisting of standard falls prevention activities. Whether or not the control arm included some of the multifactorial intervention components was not always clearly reported. Additional detail is provided in the description of individual studies in the results text and within the Characteristics of included studies table.
2. Description of interventions in studies of exercise interventions in care facilities.
| Trial | Intervention | Control | Comment |
| Buckinx 2014 | Whole body vibration exercise programme | Usual care: no change to lifestyle | |
| Buettner 2002 | Supervised group exercises | Usual care | |
| Cadore 2014 | Multicomponent exercises. Twice‐weekly, 40‐minute duration | Usual care: “mobility” exercises (30 minutes per day at least 4 days per week), small active and passive movements applied as stretches in a rhythmic fashion | |
| Choi 2005 | Tai Chi | Usual care: routine activities, without participation in any regular exercise classes | |
| da Silva Borges 2014 | Ballroom dancing | Usual care: agreed not to engage in any regular physical activity | |
| Faber 2006 |
|
Usual care: no change usual pattern of activity | Both comparisons of interventions vs usual care considered under exercise vs usual care. Comparisons of interventions arms considered under comparisons of different exercise categories. |
| Fu 2015 | Wii balance training (1 hour 3 x week) | Different exercise: Balance training (Otago) (1 hour, 3 x week) | |
| Imaoka 2016 | Reduced exercise ‐ individualised exercise only. | Different exercise: groups plus individualised exercises (described by study authors as usual care) | |
| Irez 2011 | Combination exercises: Pilates | Usual care: no Pilates, instructed not to change current activity levels | |
| Kerse 2008 | Activity programme | Usual care | |
| Kovacs 2012 | Multimodal exercise – Otago Exercise programme | Different exercise: Osteoporosis exercise programme, includes balance and strengthening exercises | |
| Kovacs 2013 | Multimodal exercise – Otago Exercise programme | Usual care: social activities such as board games, listening to music | |
| Mulrow 1994 | Tailored exercises | Usual care: friendly visit, usually involved reading to participant, avoided physical activity | |
| Nowalk 2001 |
Plus control (basic enhanced programme) |
Usual care: basic enhanced programme including falls‐prevention programme with 3 education sessions and a walking programme | Results for interventions vs usual care as reported by study authors presented in Analysis 1.2 as data not suitable for calculation of RaR or RR. |
| Rosendahl 2008 | Functional exercise programme | Usual care: Seated activities, including watching films, reading, singing | |
| Sakamoto 2006 | Single leg practice 1 min / leg, 3 x daily | Usual care: no details | |
| Saravanakumar 2014 |
|
Different exercise: "staying active": includes games, group activities, a gym with bike and activities such as walking and gardening | All comparisons presented under comparisons of different exercise categories. |
| Schoenfelder 2000 | Ankle‐strengthening exercise | Usual care: little information | |
| Serra‐Rexach 2011 | Training sessions + usual care physiotherapy | Different exercise: usual care physiotherapy (40 to 45 minutes/day 5 x weekly)‐ stretches, aerobic exercise such as walking (though low intensity) | |
| Shimada 2004 | Gait exercises + usual exercises | Different exercise: physiotherapy for pain, stretches, low‐ and high‐intensity resistance training, gait training, stairs, lower limb function | |
| Sihvonen 2004 | Balance training (visual feedback) | Usual care: little information | |
| Sitja Rabert 2015 | Whole body vibration + exercise static and dynamic balance and strength exercise) | Different exercise: same exercise programme done on land | |
| Toulotte 2003 | Supervised exercises | Usual care: continued daily routine | |
| Tuunainen 2013 |
|
Different exercise: self‐administered training (1 hour, 2 x weekly): Stretching, crouching and rising administered by nurses written instructions from physiotherapist | All comparisons presented under comparisons of different exercise categories. |
| Yokoi 2015 | Group supervised seated stick exercises 25 minutes, 2 x weekly (included daily house‐keeping and hobbies for both exercise and control group) | Usual care: activities of daily living and 10‐minute group stretching exercises continued. No other exercises were conducted. |
3. Description of interventions in the medication review trials.
| Study | Medication review | Control | Comment |
| Crotty 2004a | Additional pharmacist | Usual care | |
| Crotty 2004b | Additional pharmacist | Usual care | |
| Frankenthal 2014 | Medication review | No interventional recommendations made by pharmacist to chief physician | |
| Garcia Gollarte 2014 | Physician education on drug use in older people, plus medication review in 10% | No intervention or information about an educational intervention | Falls data excludes the intervention period; not suitable for pooling |
| Houghton 2014 | Multiprofessional medication review | Usual care (support from the NHS) | |
| Juola 2015 | Nursing education to reduce medication use | Usual care | |
| Lapane 2011 | Clinical informatics tool for medication review: providing reports to pharmacists and nursing staff to assist identifying residents at risk for delirium and falls. Reports generated within 24 hours of admission, used during monthly medication review and at time of Minimum Data Set reporting or when falls or delirium triggered resident assessment protocols. | Usual care (includes monthly medication review by pharmacist) | |
| Patterson 2010 | Pharmacist review of psychoactive medications | Usual care | |
| Peyro Saint Paul 2013 | Ceasing medication to avoid hyponatraemia | Usual care | Unusual study, not pooled with others |
| Potter 2016 | Deprescribing | Medication review without deprescribing | |
| Streim 2012 | Deprescribing antidepressants | Continue taking antidepressants | Data not suitable for pooling. |
| Zermansky 2006 | Medication review by pharmacist | Usual care |
Outcomes
The source of data used for calculating outcomes for each trial for generic inverse variance analysis is shown in Appendix 7. Seventeen trials met our inclusion criteria but did not report data that could be included in pooled analyses. Reported results from these trials are presented in the text or additional tables. Raw data for rate of falls and number of fallers when reported or when they could be calculated are shown in Appendix 8. Twenty‐four trials reported data on fractures suitable for use in pooled analyses, other reported fractures data is presented in the text. Twenty‐nine trials clearly reported data on adverse events, but in many of these it was not clear if adverse‐event data were recorded systematically; for the majority of trials, this outcome was not reported.
Excluded studies
Overall there were 105 excluded studies (seeCharacteristics of excluded studies for details). Of the 51 newly excluded studies (see Figure 1): five were excluded as they were not randomised; five were conducted in the wrong population (e.g. including participants post stroke); 10 were conducted in the wrong setting (in most of these, the majority of participants were living in the community); two studies of flooring interventions were excluded as the intent was to reduce fall injuries, rather than falls (Drahota 2013; NCT01618786); 22 studies were excluded as they measured falls as a potential adverse outcome of the intervention; two did not report falls outcomes; one study was excluded as it reported a specific type of falls only (Sahota 2014); three trials were discontinued and one had invalid falls data (DeSure 2013).
Of the 54 studies excluded in the previous version of this review: 21 trials were excluded because the intervention they tested was not designed to reduce falls, rather falls were measured as a potential adverse outcome of an intervention with a different aim; in 11 trials the majority of participants were living in the community; eight excluded trials did not provide sufficient data on falls or fallers; seven included participants post stroke and seven were not randomised (Cameron 2012). Of note is that four trials that had been excluded in Cameron 2012 because they included participants with post‐stroke hemiplegia, have now either been retracted (Sato 2000; Sato 2005a; Sato 2005b; see Retraction Watch) or, for Sato 2011, likely to be retracted in future because of serious concerns about research misconduct as revealed in Bolland 2016.
Studies awaiting classification
Three studies await publication of full reports containing falls data (seeCharacteristics of studies awaiting classification). One of these is a study of whole body vibration in care facilities (Tallon 2013), another is likely to be an additional conference abstract of an already included study (Frohnhofen 2013), and the third is a thesis for which no study publication has been identified (MacRitchie 2001). Five newly published studies were identified in the top‐up search and await full assessment (Dever 2016; Hewitt 2014; Raymond 2017; Van der Linden 2017; Wylie 2017).
Ongoing studies
We are aware of 31 ongoing studies, 14 set in care facilities and 17 in hospitals (seeCharacteristics of ongoing studies for details). The ongoing studies in care facilities include five exercise trials in care facilities (two of whole body vibration), one trial of a multiple intervention of exercise and nutrition, one of nutrition, three of medication review, one of vitamin D supplementation, three of service model changes, and one of a telesurveillance system; two trials are likely to have been completed, one of whole body vibration (JPRN‐UMIN000000555) and one of vitamin D supplementation (JPRN‐UMIN000008361). The ongoing studies in hospitals include three trials of medication review, four of exercise, one of an education intervention, five social environment interventions including one of student training, one psychological intervention, one of a sensor technology, one educational intervention, and one multifactorial intervention; five trials are likely to be completed, three of medication review (ISRCTN42003273; NCT01876095; NCT02570945), one of exercise (Hassett 2016), and one of telesurveillance (NCT01561872).
Risk of bias in included studies
Details of 'Risk of bias' assessment for nine items for each trial are shown in the Characteristics of included studies. Summary results for these items are shown in Figure 2, Figure 3 and Table 11.
2.
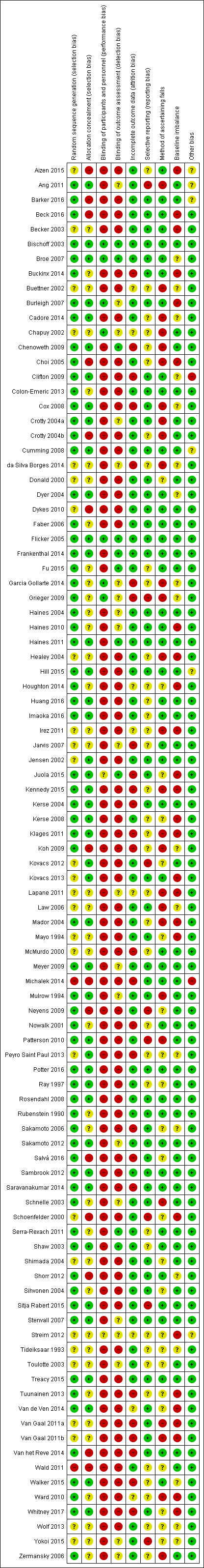
'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study.
3.
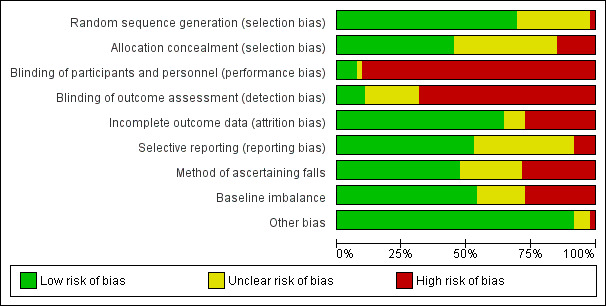
'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
4. Summary of 'Risk of bias' assessment of included studies.
| Risk of Bias | Low | High | Unclear |
| Sequence generation (selection bias) | 69% (66/95) | 2% (2/95) | 28% (27/95) |
| Allocation (selection bias) | 45% (43/95) | 15% (14/95) | 40% (38/95) |
| Blinding of participants and personnel (performance bias) | 7% (7/95) | 91% (86/95) | 2% (2/95) |
| Blinding of outcome assessors (detection bias) | 11% (10/95) | 68% (65/95) | 21% (20/95) |
| Incomplete outcome data (attrition bias) | 63% (60/95) | 27% (26/95) | 9% (9/95) |
| Selective reporting (reporting bias) | 53% (50/95) | 8% (8/95) | 39% (37/95) |
| Method of ascertaining falls | 47% (45/95) | 28% (27/95) | 24% (23/95) |
| Baseline imbalance | 54% (51/95) | 27% (26/95) | 19% (18/95) |
| Other bias | 92% (87/95) | 2% (2/95) | 6% (6/95) |
The majority of included studies were considered at high risk of bias for at least one domain. In particular, there was a high risk of performance bias for the majority of studies due to lack of blinding. Only three trials were considered at low risk of bias for all or the majority of domains (Bischoff 2003; Broe 2007; Flicker 2005), these all examined vitamin D supplementation in comparison with placebo. However, for many other types of interventions, blinding was generally not feasible (e.g. exercise, bed alarms). The risk of bias was often unclear, in particular for risk of selection bias due to allocation concealment. Potential bias varied within comparison groups and it is difficult to judge whether any bias would result in an over‐ or under‐estimation of treatment effect.
Allocation
Under half of included studies (39 in all) were considered at low risk of selection bias; this often reflected lack of clarity on the methods for allocation concealment. We assessed risk of bias in sequence generation as low in 66 trials, high in two trials that described inappropriate methods (Michalek 2014; Wald 2011), and unclear in the remaining 27 trials, usually because of a lack of reporting of methods. We judged methods for concealment of allocation prior to group assignment to carry low risk of bias in 43 trials, high in 14 trials and to be unclear in the remaining 38 trials, again typically due to lack of reporting. Barker 2016, a cluster‐randomised trial, is an example of a trial at high risk of selection bias due to lack of allocation concealment: although the initial cluster allocation was concealed, the subsequent recruitment of participants into the study (i.e. admission to the ward) was not.
Blinding
Blinding of participants and personnel was uncommon and indeed blinding of these was not feasible for many intervention types (e.g. exercise, multifactorial interventions). In all, 86 trials were at high risk of performance bias, with just seven trials being at low risk and the remaining two trials being judged at unclear risk of bias.
The likelihood of detection bias in relation to the ascertainment of falls by outcome assessors was also high in 65 trials, generally as falls were ascertained by staff who were not blinded (e.g. Barker 2016). Risk of bias was low in 10 trials, most commonly in vitamin D trials where administration of a placebo was possible (e.g. Flicker 2005) and unclear in 20 trials.
Incomplete outcome data
The risk of attrition bias due to incomplete outcome data was assessed as high in 26 trials (the high risk of attrition in some trials is likely to be related to longer periods of follow‐up; e.g. 12 months for Juola 2015 and 16 months for Kennedy 2015). Risk of bias was low in 61 trials, where there was no loss to follow‐up (this occurred more frequently in a hospital setting: e.g. Barker 2016; Hill 2015) or losses were balanced between groups (e.g. Cadore 2014; Kerse 2008). Risk of bias was unclear in eight trials, which generally reflected unclear reporting (e.g. Van de Ven 2014).
Selective reporting
Reporting bias was judged as unclear in 37 trials, generally as no protocol was identified (e.g. Healey 2004), and low risk in 50 trials where results were reported according to the protocol (e.g. Potter 2016), or all expected falls outcomes were reported (e.g. Law 2006). Eight trials were at high risk, usually where outcomes mentioned in the protocol or methods were not reported (e.g. Ang 2011).
Other potential sources of bias
The method of ascertaining falls was judged to be at a low risk of bias for 45 trials, at high risk of bias for 27 trials, generally where falls were poorly defined (e.g. Healey 2004), and at unclear risk for 23 trials when methods were not reported (e.g. Sakamoto 2006). The risk of bias relating to imbalance in baseline characteristics was considered to be low in 51 trials, high in 26 trials, and unclear in 18 trials. Risk of baseline imbalance usually occurred in small trials (e.g. Buckinx 2014) or cluster‐randomised trials (e.g. Becker 2003; Choi 2005; Van Gaal 2011a; Van Gaal 2011b; Whitney 2017). Two trials were considered to be a high risk of other bias, this was due to the author being employed by the company producing the intervention (Clifton 2009), or the individual randomisation being to one of two clusters, hence the trial was not truly individually randomised (Michalek 2014). There was a low risk of other bias in 87 trials and unclear risk in six trials due to unusual study design (stepped‐wedge trial in Aizen 2015; Hill 2015; and including a non‐randomised patient preference arm in Streim 2012) or ongoing falls prevention activities (Aizen 2015; Ang 2011; Barker 2016; Cumming 2008).
Cluster‐randomised trials
There were a large number of included cluster‐randomised trials (44%, 42/95), many of which had a large number of participants (e.g. Barker 2016; Shorr 2012). Risk of bias particular to cluster‐randomised trials were considered within other domains (seeAssessment of risk of bias in included studies). However, it is worth noting that some of these trials contained a small number of clusters and hence were more prone to baseline imbalance (e.g. Choi 2005; Van Gaal 2011a; Van Gaal 2011b), and in some cases prediction of allocation concealment (e.g. Choi 2005; Koh 2009). Loss of whole clusters could also lead to a high risk of attrition bias (e.g. Cox 2008).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Summary of findings for the main comparison. Summary of findings: Exercise compared with usual care in care facilities.
| Exercise compared with usual care for falls prevention in care facilities | ||||||
|
Population and setting: older (≥ 65 years) residents of care facilities Intervention: exercise Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
|
Assumed risk Usual care |
Corresponding risk Exercise |
|||||
| Rate of falls Length of follow‐up: 3 to 12 months |
Low‐risk population1 |
RaR 0.93 (0.72 to 1.20) |
2002 (10 studies) |
+ooo VERY LOW7 |
These results were heterogeneous: subgroup analysis by type of exercise did not explain the heterogeneity. Four additional trials (N = 130) with data not suitable for pooling reported a reduction in the rate of falls. |
|
| 1000 per 1000 py | 930 (720 to 1200) per 1000 py | |||||
| High‐risk population2 | ||||||
| 3500 per 1000 py | 3255 (2520 to 4200) per 1000 py | |||||
| Risk of falling Length of follow‐up: 3 to 12 months |
Low‐risk population3 |
RR 1.02 (0.88 to 1.18) |
2090 (10 studies) |
++oo LOW8 |
1 additional trial (2 comparisons, N = 110) reported no significant difference in the risk of falling. | |
| 250 per 1000 | 255 (220 to 295) per 1000 | |||||
| Moderate‐risk population4 | ||||||
| 500 per 1000 | 510 (440 to 590) per 1000 | |||||
| High‐risk population5 | ||||||
| 700 per 1000 | 714 (616 to 826) per 1000 | |||||
| Risk of fracture Length of follow‐up: 6 months |
Average risk population6 |
RR 0.88 (0.25 to 3.14) |
183 1 study |
+ooo VERY LOW9 |
This outcome poorly reported. | |
| 42 per 1000 | 37 (11 to 132) per 1000 | |||||
| Adverse events Length of follow‐up: 4 to 12 months |
See comment | See comment | Not estimable. |
1032 (4 studies) |
+ooo VERY LOW 10 |
1 serious adverse event reported (death due to a ruptured abdominal aortic aneurysm one week after the follow‐up tests, association could not definitely be ruled out) in 1 trial (183 participants). Three trials reported no differences in adverse events:
|
| *Illustrative risks for the control group were derived from all or subgroups of trials in care facilities reporting the outcome. The exact basis for the assumed risk for each outcome is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; py: person years; RaR: Rate Ratio; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Low risk was based on the mean control risk of the 17 (bottom third) trials with the lowest rate of falls. The mean rate of falls = 1.07, rounded to 1.0 per person year; thus 1000 per 1000 person years.
2 High risk was based on the mean control risk of the 18 (top third) trials with the highest rate of falls. The mean rate of falls = 3.69, rounded to 3.5 per person year; thus 3500 per 1000 person years.
3 Low risk was based on the mean control risk of the 20 trials with the lowest risk of falling. The mean risk of falling = 0.268, rounded to 0.25; thus 250 per 1000 people.
4 Moderate risk was based on the mean control risk of the 20 trials reporting a moderate risk of falling, not described as high‐risk populations. The mean risk of falling = 0.539, rounded to 0.5; thus 500 per 1000 people.
5 High risk was based on the mean control risk of the 13 trials reporting a high risk of falling, including populations with a description as a high‐risk population. The mean risk of falling = 0.680, rounded to 0.7; thus 700 per 1000 people.
6 Risk based on the median control risk of fracture of the trials reporting this outcome. Median risk = 0.042; thus 42 per 1000.
7 The quality of the evidence was downgraded one level for serious risk of bias (including high risk of bias for blinding (not feasible), baseline imbalance, attrition bias and high or unclear risk of bias in method of ascertaining falls), one level for inconsistency (considerable heterogeneity I² = 76%) and one level for publication bias (suspected based on asymmetry of funnel plots).
8 The quality of the evidence was downgraded one level for serious risk of bias (including high risk of bias based on blinding (not feasible), baseline imbalance and high or unclear risk of selection bias) and one level for publication bias (strongly suspected based on asymmetry of funnel plots).
9 The quality of the evidence was downgraded two levels for imprecision (extremely wide confidence intervals that include the possibility of both important benefit and harm) and one level for publication bias (strongly suspected based on asymmetry of funnel plots).
10 The quality of the evidence was downgraded one level for serious risk of bias (including high risk of bias for selection bias, baseline imbalance and selective reporting), two levels for imprecision (inadequate power to assess rare adverse events) and two levels for 'other reasons' (publication bias strongly suspected based on asymmetry of funnel plots and adverse events unlikely to have been recorded systematically).
Summary of findings 2. Summary of findings: General medication review compared with usual care in care facilities.
| General medication review compared with usual care for falls prevention in care facilities | ||||||
|
Population and setting: older (≥ 65 years) residents of care facilities Intervention: general medication review (NB: the primary aim of all medication review is to reduce psychoactive medications) Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | General medication review | |||||
| Rate of falls Length of follow‐up: 6 to 12 months |
Low‐risk population1 |
RaR 0.93 (0.64 to 1.35) |
2409 (6 studies) |
++oo LOW7 |
The approaches taken in the six pooled studies were:
2 additional RCTs found no strong evidence for an effect on the rate of falls (1 RCT of education of physicians on drug use in older people (716 participants, falls only reported following the intervention period); 1 trial of antidepressant deprescribing (36 participants randomised)). |
|
| 1000 per 1000 py | 930 (640 to 1350)per 1000 py | |||||
| High‐risk population2 | ||||||
| 3500 per 1000 py | 3255 (2240 to 4725)per 1000 py | |||||
| Risk of falling Length of follow‐up: 6 to 12 months |
Low‐risk population3 |
RR 0.93 (0.80 to 1.09) |
5139 (6 studies) |
++oo LOW8 |
The approaches taken in the six studies were:
1 additional RCT of education of physicians on drug use in older people (716 participants) found no strong evidence for an effect on the risk of falling following the intervention period. |
|
| 250 per 1000 | 233 (200 to 273) per 1000 | |||||
| Moderate‐risk population4 | ||||||
| 500 per 1000 | 465 (400 to 545) per 1000 | |||||
| High‐risk population5 | ||||||
| 700 per 1000 | 651 (560 to 763) per 1000 | |||||
| Risk of fracture Length of follow‐up: 12 months |
Average risk population6 |
RR 1.60 (0.28 to 9.16) |
93 (1 trial) |
+ooo VERY LOW9 |
Intervention was GP and a geriatrician/pharmacologist independently identifying deprescribing targets using a list of potentially inappropriate medicines vs medication review without deprescribing. | |
| 42 per 1000 | 67 (12 to 614) per 1000 | |||||
| Adverse events Length of follow‐up: 12 months |
Average risk population10 |
RR 1.07 (0.23 to 5.01) |
93 (1 trial) |
+ooo VERY LOW9 |
Serious vascular events in both trial arms and significant withdrawal reactions in 2 intervention participants (Potter 2016). | |
| 60 per 1000 |
64 (14 to 301) per 1000 |
|||||
| **Illustrative risks for the control group were derived from all or subgroups of trials in care facilities reporting the outcome. The exact basis for the assumed risk for each outcome is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; py: person years; RaR: Rate Ratio; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Low risk was based on the mean control risk of the 17 (bottom third) trials with the lowest rate of falls. The mean rate of falls = 1.07, rounded to 1.0 per person year; thus 1000 per 1000 person years.
2 High risk was based on the mean control risk of the 18 (top third) trials with the highest rate of falls. The mean rate of falls = 3.69, rounded to 3.5 per person year; thus 3500 per 1000 person years.
3 Low risk was based on the mean control risk of the 20 trials with the lowest risk of falling. The mean risk of falling = 0.268, rounded to 0.25; thus 250 per 1000 people.
4 Moderate risk was based on the mean control risk of the 20 trials reporting a moderate risk of falling, not described as high‐risk populations. The mean risk of falling = 0.539, rounded to 0.5; thus 500 per 1000 people.
5 High risk was based on the mean control risk of the 13 trials reporting a high risk of falling, including populations with a description as a high‐risk population. The mean risk of falling = 0.680, rounded to 0.7; thus 700 per 1000 people.
6 Risk based on the median control risk of fracture of the trials reporting this outcome. Median risk = 0.042; thus 42 per 1000.
7 The quality of the evidence was downgraded one level for serious risk of bias (including high risk of performance and detection bias, high or unclear risk of method of ascertaining falls, and high risk of baseline imbalance) and one level due to inconsistency (unexplained heterogeneity, I² = 93%).
8 The quality of the evidence was downgraded one level for serious risk of bias (including high risk of performance and detection bias, baseline imbalance, method of ascertaining falls and high or unclear risk of selection bias), and one level for inconsistency (I² = 48%, P > 0.05; inconsistency in point estimates between studies).
9The quality of the evidence was downgraded one level for serious risk of bias (including high risk of performance and detection bias), one level for indirectness (sIngle trial conducted in rural Western Australia (Potter 2016) that may have limited applicability), two levels for imprecision (extremely wide confidence intervals that include the possibility of both important benefit and harm) and one level for publication bias (few studies reported this outcome).
10 Determined from the control arm of Potter 2016.
Summary of findings 3. Summary of findings: Vitamin D supplementation in care facilities.
| Vitamin D supplementation compared with no vitamin D supplementation for falls prevention in care facilities | ||||||
|
Population and setting: older (≥ 65 years) residents of care facilities1 Intervention: vitamin D supplementation (vitamin D or vitamin D + calcium) Comparison: usual care (or calcium supplementation) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Vitamin D | |||||
| Rate of falls Length of follow‐up: 3 to 24 months |
Low‐risk population2 |
RaR 0.72 (0.55 to 0.95) |
4512 (4 studies) |
+++o MODERATE8 |
Studies included two studies of vitamin D3 + calcium versus calcium, and 2 studies of vitamin D2 versus usual care or placebo. | |
| 1000 per 1000 py | 720 (550 to 950)per 1000 py | |||||
| High‐risk population3 | ||||||
| 3500 per 1000 py | 2520 (1925 to 3325)per 1000 py | |||||
| Risk of falling Length of follow‐up: 3 to 24 months |
Low‐risk population4 |
RR 0.92 (0.76 to 1.12) |
4512 (4 studies) |
+++o MODERATE9 |
Studies included two studies of vitamin D3 + calcium versus calcium, and 2 studies of vitamin D2 versus usual care or placebo. | |
| 250 per 1000 | 230 (190 to 280)per 1000 | |||||
| Moderate‐risk population5 | ||||||
| 500 per 1000 | 460 (380 to 515)per 1000 | |||||
| High‐risk population6 | ||||||
| 700 per 1000 | 644 (532 to 784)per 1000 | |||||
| Risk of fracture Length of follow‐up: 3 to 24 months |
Average risk population7 |
RR 1.09 (0.58 to 2.03) |
4464 (3 studies) |
+ooo VERY LOW10 |
These studies represent only a subset of studies evaluating the effect of vitamin D on fractures. Included studies were two studies of vitamin D3 + calcium versus calcium, and 1 study of vitamin D2 versus usual care. | |
| 42 per 1000 | 46 (24 to 85) per 1000 | |||||
| Adverse events Length of follow‐up: 3 to 24 months |
ND12 | ND12 |
RR 4.84 (0.24 to 98.90) |
747 (2 studies) |
+ooo VERY LOW11 |
No serious events reported. Studies tested supplementation with 800 IU oral cholecalciferol (vitamin D3) and 1000 IU oral ergocalciferol (vitamin D2) daily. Data derived from just 2 cases of increased constipation in the intervention arm in 1 study (N = 122). No adverse events recorded in the other study (N = 625) |
| *Illustrative risks for the control group were derived from all or subgroups of trials in care facilities reporting the outcome. The exact basis for the assumed risk for each outcome is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; ND: not done; py: person years; RaR: Rate Ratio; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Studies confirmed the participants had low or very low serum vitamin D levels at baseline.
2 Low risk was based on the mean control risk of the 17 (bottom third) trials with the lowest rate of falls. The mean rate of falls = 1.07, rounded to 1.0 per person year; thus 1000 per 1000 person years.
3 High risk was based on the mean control risk of the 18 (top third) trials with the highest rate of falls. The mean rate of falls = 3.69, rounded to 3.5 per person year; thus 3500 per 1000 person years.
4 Low risk was based on the mean control risk of the 20 trials with the lowest risk of falling. The mean risk of falling = 0.268, rounded to 0.25; thus 250 per 1000 people.
5 Moderate risk was based on the mean control risk of the 20 trials reporting a moderate risk of falling, not described as high‐risk populations. The mean risk of falling = 0.539, rounded to 0.5; thus 500 per 1000 people.
6 High risk was based on the mean control risk of the 13 trials reporting a high risk of falling, including populations with a description as a high‐risk population. The mean risk of falling = 0.680, rounded to 0.7; thus 700 per 1000 people.
7 Risk based on the median control risk of fracture of the trials reporting this outcome. Median risk = 0.042; thus 42 per 1000.
8 The quality of the evidence was downgraded one level for serious risk of bias (including high risk of performance and detection bias and method of ascertaining falls for one trial contributing 49%).
9 The quality of the evidence was downgraded one level for serious risk of bias (including high risk of performance and detection bias and method of ascertaining falls for one trial contributing 56%).
10 The quality of the evidence was downgraded one level for serious risk of bias (including high risk of performance and detection bias and method of ascertaining falls for one trial contributing 49%), and two levels for imprecision (small number of fractures, confidence intervals cross the range of strong effect and significant harm).
11 The quality of the evidence was downgraded two levels for imprecision (low event rate, inadequate power to assess rare adverse events) and two levels for other reasons (concerns that adverse events were not recorded systematically and likely publication bias, few studies reported this outcome).
12 Not done. Illustrative comparative risks not presented as considered uninformative due to paucity of data available.
Summary of findings 4. Summary of findings: Multifactorial interventions compared with usual care in care facilities.
| Multifactorial interventions compared with usual care for falls prevention in care facilities | ||||||
|
Population and setting: older (≥ 65 years) residents of care facilities Intervention: multifactorial interventions (two or more categories of intervention given based on individual risk profile) Comparison: usual care (without intervention)1 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Vitamin D | |||||
| Rate of falls Length of follow‐up: 6 to 12 months |
Low‐risk population2 | RaR 0.88 (0.66 to 1.18) |
3439 (10 studies) |
+ooo VERY LOW8 |
One additional study (31 participants) of exercise plus nutritional support reported zero falls in the intervention arm and two in the control arm. | |
| 1000 per 1000 py | 720 (550 to 950)per 1000 py | |||||
| High‐risk population3 | ||||||
| 3500 per 1000 py | 2520 (1925 to 3325)per 1000 py | |||||
| Risk of falling Length of follow‐up: 6 to 12 months |
Low‐risk population4 | RR 0.92 (0.81 to 1.05) |
3153 (9 studies) |
++oo LOW9 |
One additional study (482 participants) reported a reduction in the proportion of recurrent fallers (difference 19%, 95% CI 2% to 36%: P = 0.03). | |
| 250 per 1000 | 230 (190 to 280)per 1000 | |||||
| Moderate‐risk population5 | ||||||
| 500 per 1000 | 460 (380 to 515)per 1000 | |||||
| High‐risk population6 | ||||||
| 700 per 1000 | 644 (532 to 784)per 1000 | |||||
| Risk of fracture Length of follow‐up: 6 to 12 months |
Average risk population7 | RR 0.79 (0.30 to 2.07) |
2160 (5 studies) |
+ooo VERY LOW10 |
||
| 42 per 1000 | 34 (13 to 87) per 1000 | |||||
| Adverse events Length of follow‐up: 11 weeks to 12 months |
See comment | See comment | Not estimable. | 312 (3 studies) |
+ooo VERY LOW11 |
One trial reported a case of a fall in the intervention arm; two studies reported no adverse events. |
| *Illustrative risks for the control group were derived from all or subgroups of trials in care facilities reporting the outcome. The exact basis for the assumed risk for each outcome is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; py: person years; RaR: Rate Ratio; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Nine of 11 trials described the control arm as usual care not receiving the intervention. In one trial contributing data to the risk of falling and fracture, the control arm received multidisciplinary assessment without the intervention in addition to usual care; in one trial contributing data to the rate of falls and risk of falling, the control included reminiscence therapy.
2 Low risk was based on the mean control risk of the 17 (bottom third) trials with the lowest rate of falls. The mean rate of falls = 1.07, rounded to 1.0 per person year; thus 1000 per 1000 person years.
3 High risk was based on the mean control risk of the 18 (top third) trials with the highest rate of falls. The mean rate of falls = 3.69, rounded to 3.5 per person year; thus 3500 per 1000 person years.
4 Low risk was based on the mean control risk of the 20 trials with the lowest risk of falling. The mean risk of falling = 0.268, rounded to 0.25; thus 250 per 1000 people.
5 Moderate risk was based on the mean control risk of the 20 trials reporting a moderate risk of falling, not described as high‐risk populations. The mean risk of falling = 0.539, rounded to 0.5; thus 500 per 1000 people.
6 High risk was based on the mean control risk of the 13 trials reporting a high risk of falling, including populations with a description as a high‐risk population. The mean risk of falling = 0.680, rounded to 0.7; thus 700 per 1000 people.
7 Risk based on the median control risk of fracture of the trials reporting this outcome. Median risk = 0.042; thus 42 per 1000.
8 The quality of the evidence was downgraded one level for serious risk of bias (including high risk of performance and attrition bias and baseline imbalance), one level for serious inconsistency (high heterogeneity I2 = 84%) and one level for imprecision (wide CIs despite large N).
9 The quality of the evidence was downgraded one level for serious risk of bias (including high risk of performance and attrition bias and some uncertainty in selection bias) and one level for inconsistency (inconsistency in point estimates between studies).
10 The quality of the evidence was downgraded one level for serious risk of bias (including high risk of performance and attrition bias and baseline imbalance), one level for inconsistency (moderate heterogeneity, I2 = 60%, P = 0.04) and two levels for imprecision (extremely wide confidence intervals)
11 The quality of the evidence was downgraded two levels for serious risk of bias (2 of 3 trials had a high risk of baseline imbalance or incomplete outcome data), two levels for imprecision (not powered for rare events) and two levels for other reasons (concerns that adverse events were not recorded systematically and few studies reported this outcome).
Summary of findings 5. Summary of findings: Additional exercise plus physiotherapy compared with usual physiotherapy in hospitals.
| Additional exercise plus physiotherapy compared with usual physiotherapy for falls prevention in hospitals | ||||||
|
Population and setting: older (≥ 65 years) patients in hospital settings Intervention: additional exercise plus physiotherapy Comparison: usual physiotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual physiotherapy | Additional Exercise | |||||
| Rate of falls Length of follow‐up: inpatient stay (mean 29 days) or 2 weeks |
Low‐risk population1 | RaR 0.59 (0.26 to 1.34) |
215 (2 studies) |
+ooo VERY LOW7 |
One study compared additional exercises versus conventional physiotherapy alone, and 1 study tested additional group standing balance circuit classes | |
| 1300 per 1000 py | 767 (338 to 1742) per 1000 py | |||||
| Moderate‐risk population2 | ||||||
| 3500 per 1000 py | 2065 (910 to 4690) per 1000 py | |||||
| High‐risk population3 | ||||||
| 6000 per 1000 py | 3540 (1560 to 8040) per 1000 py | |||||
| Risk of falling Length of follow‐up: inpatient stay (mean 29 days) or 8 weeks |
Low‐risk population4 |
RR 0.36 (0.14 to 0.93) |
83 (2 studies) |
+ooo VERY LOW8 |
One study compared additional exercises versus conventional physiotherapy alone, and 1 study tested additional daily physiotherapy sessions | |
| 30 per 1000 | 11 (4 to 28) per 1000 | |||||
| Moderate‐risk population5 | ||||||
| 150 per 1000 | 54 (21 to 140) per 1000 | |||||
| High‐risk population6 | ||||||
| 340 per 1000 | 122 (48 to 316) per 1000 | |||||
| Risk of fracture | See comment | See comment | See comment | No data available | ||
| Adverse events Length of follow‐up: 2 weeks |
0 events | 0 events | Not estimable |
161 (1 study) |
+ooo VERY LOW9 |
One study reported no adverse events, two studies did not report this outcome |
| *Illustrative risks for the control group were derived from all or subgroups of trials in hospitals reporting the outcome. The exact basis for the assumed risk for each outcome is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; py: person years; RaR: Rate Ratio; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Low risk was based on the mean control risk of the 7 (bottom third) trials with the lowest rate of falls. The mean rate of falls = 1.27, rounded to 1.3 per person year; thus 1300 per 1000 person years.
2 Moderate risk was based on the mean control risk of the 7 (middle third) trials with a moderate rate of falls. The mean rate of falls = 3.23, rounded to 3.5 per person year; thus 3500 per 1000 person years.
3 High risk was based on the mean control risk of the 7 (top third) trials with the highest rate of falls. The mean rate of falls = 6.33, rounded to 6.0 per person year; thus 6000 per 1000 person years.
4 Low risk was based on the mean control risk of 10 trials with the lowest risk of falling. The mean risk of falling = 0.034, rounded to 0.03; thus 30 per 1000 people.
5 Moderate risk was based on the mean control risk of 7 (middle third) trials reporting the risk of falling. The mean risk of falling = 0.156, rounded to 0.15; thus 150 per 1000 people.
6 High risk was based on the mean control risk of 6 (top third) trials reporting the risk of falling. The mean risk of falling = 0.340; thus 340 per 1000 people.
7The quality of the evidence was downgraded one level for risk of bias (including unclear risk of selection bias and method of ascertaining falls in one study) and two levels for very serious imprecision (the wide confidence intervals cross the range of estimates of harm and strong effect).
8The quality of the evidence was downgraded one level for risk of bias (including unclear risk of bias in both trials for selection bias and high risk of attrition bias for study contributing 69%), one level for indirectness (possibly limited applicability as both trials conducted in UK rehabilitation settings) and one level for imprecision (total N = 83, wide 95% confidence intervals).
9The quality of the evidence was downgraded one level for indirectness (single trial in Australian rehabilitation setting), two levels for imprecision (no events recorded, inadequate power to assess rare adverse events) and one level for other reasons (concerns that adverse events were not recorded systematically).
Summary of findings 6. Summary of findings: Bed alarms compared with usual care in hospitals.
| Bed alarms compared with usual care for falls prevention in hospitals | ||||||
|
Population and setting: older (≥ 65 years) patients in hospital settings Intervention: bed alarms Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Bed alarms | |||||
| Rate of falls Length of follow‐up: inpatient stay (mean 19 days; not known) |
Low‐risk population1 |
RaR 0.60 (0.27 to 1.34) |
28,649 (2 studies) |
++oo VERY LOW7 |
One cluster‐randomised study tested education and support on using bed/chair alarms; and one study tested sensor alarms fitted to patients' upper leg at rest time. A third study (n = 70) reported no difference in the number of falls (data not suitable for pooling). |
|
| 1300 per 1000 py | 780 (351 to 1742)per 1000 py | |||||
| Moderate‐risk population2 | ||||||
| 3500 per 1000 py | 2100 (945 to 4690) per 1000 py | |||||
| High‐risk population3 | ||||||
| 6000 per 1000 py | 3600 (1620 to 8040) per 1000 py | |||||
| Risk of falling Length of follow‐up: inpatient stay (mean 19 days; not known) |
Low‐risk population4 |
RR 0.93 (0.38 to 2.24) |
28,649 (2 studies) |
+ooo VERY LOW8 |
One cluster‐randomised study tested education and support on using bed/chair alarms; and one study tested sensor alarms fitted to patients' upper leg at rest time | |
| 30 per 1000 | 28 (11 to 67) per 1000 | |||||
| Moderate‐risk population5 | ||||||
| 150 per 1000 | 140 (57 to 336) per 1000 | |||||
| High‐risk population6 | ||||||
| 340 per 1000 | 316 (129 to 762) per 1000 | |||||
| Risk of fracture | See comment | See comment | See comment | No data available. | ||
| Adverse events Length of follow‐up: inpatient stay (mean 19 days; not known) |
0 events | 0 events | Not estimable. |
27,742 (2 studies) |
+ooo VERY LOW9 |
2 trials reported that there were no adverse events |
| *Illustrative risks for the control group were derived from all or subgroups of trials in hospitals reporting the outcome. The exact basis for the assumed risk for each outcome is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; py: person years; RaR: Rate Ratio; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Low risk was based on the mean control risk of the 7 (bottom third) trials with the lowest rate of falls. The mean rate of falls = 1.27, rounded to 1.3 per person year; thus 1300 per 1000 person years.
2 Moderate risk was based on the mean control risk of the 7 (middle third) trials with a moderate rate of falls. The mean rate of falls = 3.23, rounded to 3.5 per person year; thus 3500 per 1000 person years.
3 High risk was based on the mean control risk of the 7 (top third) trials with the highest rate of falls. The mean rate of falls = 6.33, rounded to 6.0 per person year; thus 6000 per 1000 person years.
4 Low risk was based on the mean control risk of 10 trials with the lowest risk of falling. The mean risk of falling = 0.034, rounded to 0.03; thus 30 per 1000 people.
5 Moderate risk was based on the mean control risk of 7 (middle third) trials reporting the risk of falling. The mean risk of falling = 0.156, rounded to 0.15; thus 150 per 1000 people.
6 High risk was based on the mean control risk of 6 (top third) trials reporting the risk of falling. The mean risk of falling = 0.340; thus 340 per 1000 people.
7The quality of the evidence was downgraded one level for risk of bias (including high risk of selection bias and unclear risk of bias for balance in baseline characteristics in the larger trial, a cluster RCT, Shorr 2012; unclear or high risk of bias for all domains for trial with greatest weighting; risk of performance and detection bias due to lack of blinding although this is not feasible); one level for imprecision (despite the large sample size, the wide confidence intervals cross the range of strong effect and significant harm) and one level for indirectness (the larger trial, Shorr 2012, is of education and support on using bed alarms, rather than directly implementing bed alarms).
8The quality of the evidence was downgraded one level for risk of bias (including high risk of selection bias and unclear risk of bias for balance of baseline characteristics in the larger trial, Shorr 2012), one level for indirectness (the larger trial, Shorr 2012, is of education and support on using bed alarms, directly implementing bed alarms) and one level for imprecision, despite the large sample size, the wide confidence intervals cross the range of strong effect and significant harm).
9The quality of the evidence was downgraded one level for risk of bias (including high risk of selection bias and unclear risk of bias for balance of baseline characteristics, one level for indirectness (trial is of education and support on using bed alarms, directly implementing bed alarms) and one level for imprecision (no events recorded, low power to assess rare adverse events) and one level for other reasons (concerns that adverse events were not recorded systematically).
Summary of findings 7. Summary of findings: Multifactorial interventions compared with usual care in hospitals.
| Multifactorial interventions compared with usual care for falls prevention in hospitals | ||||||
|
Population and setting: older (≥ 65 years) patients in hospital settings Intervention: multifactorial interventions (two or more categories of intervention given based on individual risk profile) Comparison: usual care 1 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Multifactorial | |||||
| Rate of falls Length of follow‐up: inpatient stay (median 4 days to mean 30 days) |
Low‐risk population2 |
RaR 0.80 (0.64 to 1.01) |
44,664 (5 studies) |
++oo LOW9 |
The 5 studies tested compared different multifactorial interventions versus usual care in acute, subacute or mixed care settings.
See footnote13 for comment on a post‐hoc subgroup analysis by setting. |
|
| 1300 per 1000 py | 1040 (832 to 1313) per 1000 py | |||||
| Moderate‐risk population3 | ||||||
| 3500 per 1000 py | 2,800 (2240 to 3535) per 1000 py | |||||
| High‐risk population4 | ||||||
| 6000 per 1000 py | 4800 (3840 to 6060) per 1000 py | |||||
| Risk of falling Length of follow‐up: inpatient stay (median 4 days to mean 30 days) |
Low‐risk population5 |
RR 0.82 (0.62 to 1.09) |
39,889 (3 studies) |
+ooo VERY LOW 10 |
The 3 studies tested compared different multifactorial interventions versus usual care in acute, subacute or mixed care settings.
One additional study analysed fallers by the number of admissions, and found a reduction in the risk of falling (adjusted OR 0.55, 95% CI 0.38 to 0.81). |
|
| 30 per 1000 | 25 (19 to 33) per 1000 | |||||
| Moderate‐risk population6 | ||||||
| 150 per 1000 | 123 (93 to 164) per 1000 | |||||
| High‐risk population7 | ||||||
| 340 per 1000 | 279 (211 to 371) per 1000 | |||||
| Risk of fracture Length of follow‐up: inpatient stay (mean in acute wards 8 days to mean 30 days) |
Average risk population8 |
RR 0.76 (0.14 to 4.10) |
4615 (2 studies) |
+ooo VERY LOW11 |
The 2 studies pooled tested compared different multifactorial interventions versus usual care in subacute or mixed care settings.
Two additional studies reported no difference in extremely low risk of fracture (1 study Intervention: 11/17698, 0.06% vs Control: 13/17566, 0.07%) or number of fractures (Intervention 4/1402 0.3% vs 6/1719, 0.3%). |
|
| 18 per 1000 | 14 (3 to 74) per 1000 | |||||
| Adverse events Length of follow‐up: inpatient stay |
0 events | 0 events | Not estimable. |
39,763 (4 studies) |
+ooo VERY LOW12 |
4 trials reported that there were no adverse events. |
| *Illustrative risks for the control group were derived from all or subgroups of trials in hospitals reporting the outcome. The exact basis for the assumed risk for each outcome is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MultiF: multifactorial; OR: Odds Ratio; OT: Occupational Therapist py: person years; RaR: Rate Ratio; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Usual care generally included some standard falls prevention activities. The degree to which this included components of the intervention was not always clear. Usual care falls prevention activities are likely to very over time and between settings.
2 Low risk was based on the mean control risk of the 7 (bottom third) trials with the lowest rate of falls. The mean rate of falls = 1.27, rounded to 1.3 per person year; thus 1300 per 1000 person years.
3 Moderate risk was based on the mean control risk of the 7 (middle third) trials with a moderate rate of falls. The mean rate of falls = 3.23, rounded to 3.5 per person year; thus 3500 per 1000 person years.
4 High risk was based on the mean control risk of the 7 (top third) trials with the highest rate of falls. The mean rate of falls = 6.33, rounded to 6.0 per person year; thus 6000 per 1000 person years.
5 Low risk was based on the mean control risk of 10 trials with the lowest risk of falling. The mean risk of falling = 0.034, rounded to 0.03; thus 30 per 1000 people.
6 Moderate risk was based on the mean control risk of 7 (middle third) trials reporting the risk of falling. The mean risk of falling = 0.156, rounded to 0.15; thus 150 per 1000 people.
7 High risk was based on the mean control risk of 6 (top third) trials reporting the risk of falling. The mean risk of falling = 0.340; thus 340 per 1000 people.
8 Risk based on the median risk of fracture in the control arm of trials reporting this outcome. Median risk = 0.018; thus 18 per 1000 people.
9 The quality of the evidence was downgraded one level for risk of bias (including high risk of selection bias, performance and detection bias) and one level for imprecision (confidence intervals overlap no effect but fail to exclude important benefit)
10 The quality of the evidence was downgraded one level for risk of bias (including high risk of selection bias, performance and detection bias), one level for imprecision (confidence intervals overlap no effect but fail to exclude important benefit) and one level for other bias (one study not included in pooled estimate creating uncertainty in overall point estimate).
11 The quality of the evidence was downgraded one level for risk of bias (including high risk of selection bias, performance and detection bias) and two levels for imprecision (small number of fractures, the extremely wide confidence intervals include both possible benefit and possible harm).
12 The quality of the evidence was downgraded one level for risk of bias (including high risk of selection and performance bias and baseline imbalance), one level for imprecision (no events recorded, low power to assess rare adverse events) and one level for other reasons (concerns that adverse events were not recorded systematically).
13 A post‐hoc subgroup analysis by setting found a reduction in the rate of falls in 2 trials conducted in a subacute setting (RaR 0.67, 95% CI 0.54 to 0.83; 2 trials; 3747 participants; test for subgroup differences P = 0.04). These trials included including targeted patient education as a component of the multifactorial intervention.
We present results by setting (care facilities or hospitals), combination (single, multiple, or multifactorial) and intervention type (categorised according to ProFaNE, Lamb 2011) in Appendix 3.
Care facilities: single interventions
Single interventions consist of one major category of intervention only and are delivered to all participants in the group.
Exercise
Twenty‐five trials (2848 participants) investigated exercise as a single intervention (seeTable 9), four trials (986 participants) were cluster randomised (Choi 2005; Kerse 2008; Rosendahl 2008; Yokoi 2015), and the remaining 22 trials (1862 participants) were individually randomised. However, many of these trials were small (median 60 participants, range 16 to 682; seeTable 8). The types of exercise are shown in Table 9. The control arm of the different trials also varied. Four trials included three arms (Faber 2006; Nowalk 2001; Saravanakumar 2014; Tuunainen 2013). One was a cross‐over trial (Toulotte 2003). The trials are categorised below, both according to the ProFaNE exercise category (see Appendix 4) and the comparator arm of the trial. A summary of the evidence from exercise versus usual care for falls prevention in care facilities is provided in Table 1.
Only two trials reported on the impact of exercise interventions on fractures (Rosendahl 2008, Sitja Rabert 2015). Nine trials reported on adverse events, while 16 trials did not report adverse‐event data.
In seven trials, the reported data were incomplete and not suitable for pooling with other studies (Buettner 2002; Cadore 2014; da Silva Borges 2014; Imaoka 2016; Nowalk 2001; Serra‐Rexach 2011; Toulotte 2003); seeAnalysis 1.2 and Analysis 4.2). Falls data from Imaoka 2016 excluded the intervention period and thus are not presented in the forest plot.
1.2. Analysis.
Comparison 1 Care facilities: Exercise vs usual care, Outcome 2 Rate of falls and number of fallers: trials with incomplete data.
| Rate of falls and number of fallers: trials with incomplete data | ||||
|---|---|---|---|---|
| Study | Intervention | Comparator | Participants (N) | Study findings (NR = not reported) |
| Buettner 2002 | Exercise: Supervised group exercises, combination exercises. | Usual care | 27 | Rate of falls: Falls were reduced but the treatment effect estimate and confidence interval were not reported in the published study or research monograph. Risk of falling: NR |
| Cadore 2014 | Exercise: Multicomponent exercise programme including gait/balance and strength/resistance training | Usual care including mobility exercises | 24 | Rate of falls: Over 12 weeks there were no falls in the multicomponent arm in comparison to a rate of falls of 0.8 falls per patient per month in the mobility exercises arm of the study (P < 0.001). Participants were aged > 85 years. Risk of falling: NR |
| da Silva Borges 2014 | Exercise: Ballroom dancing (3D exercises; EG) | No regular physical activity (CG) | 59 | Rate of falls: The authors reported " fewer falls in the EG post‐test compared to the CG post‐test (p<0.0001)." Risk of falling: NR |
| Nowalk 2001 | Exercise: 1. "Fit NB Free" Individually tailored combination exercises. 2. "Living and Learning/Tai Chi" |
Usual routine activities | 110 | Rate of falls: NR Risk of falling: No significant difference in risk of falling (time to first fall) between either intervention group and the usual care group (P = 0.29). |
| Toulotte 2003 | Exercise: Supervised exercises, combination exercises. | Usual care | 20 | Rate of falls: The authors reported that falls were reduced but a falls rate could not be determined from the published data. Risk of falling: NR |
4.2. Analysis.
Comparison 4 Care facilities: Comparisons of different exercise programs (see Appendix 4 for details), Outcome 2 Rate of falls and number of fallers: trials with incomplete data.
| Rate of falls and number of fallers: trials with incomplete data | ||||
|---|---|---|---|---|
| Study | Intervention | Comparator | Participants (N) | Study findings |
| Imaoka 2016 | Exercise: Additional group exercise (described by author as "Usual care": combination group exercises plus individualised exercise) | Individualised exercise (described by author as "reduced exercise") | 39 | Rate of falls: Not reported Risk of falling: No strong evidence for a reduction in the risk of falling in the post‐intervention period with additional group exercise (RR 0.48, 95% CI 0.17 to 1.3). The falls data are not presented in the forest plot as they exclude the intervention period. |
| Serra‐Rexach 2011 | Exercise: Training sessions (combination exercises) plus usual care physiotherapy | Usual care physiotherapy (40‐45 min / day 5 x weekly) | 40 | Rate of falls: "The mean number of falls per participant recorded over the study period was 1.2 fewer in the intervention group than in the control group (95% CI = 0.0–3.0, P =.03)." Risk of falling: not reported |
Exercise versus usual care
Seventeen trials (2406 participants) compared an exercise intervention with usual care, defined as no exercise, no change in previous lifestyle or exercise type or level unlikely to change physical performance (e.g. seated flexibility exercise programme). Four trials (986 participants) of exercise in comparison with usual care were cluster randomised (Choi 2005; Kerse 2008; Rosendahl 2008; Yokoi 2015), the remaining 13 trials (1420 participants) were individually randomised. Faber 2006, included two exercise intervention arms, we combined the results from the two intervention groups in these analyses. As there is considerable clinical heterogeneity within these studies, we undertook analyses to explore heterogeneity, which are reported below.
Rate of falls
Ten trials (2002 participants) reporting on the impact of exercise in comparison with usual care in care facilities on the rate of falls had considerable statistical heterogeneity (I² = 76%, heterogeneity P < 0.0001). Nevertheless, as these trials were considered clinically similar in terms of the intervention, comparator, patient group and outcomes, these trials were pooled with a random effects meta‐analysis (Analysis 1.1: Rate ratio (RaR) = 0.93, 95% confidence interval (CI) 0.72 to 1.20). We are uncertain whether exercise reduces the rate of falls in care facilities as the quality of the evidence was assessed as very low (Table 1).
1.1. Analysis.
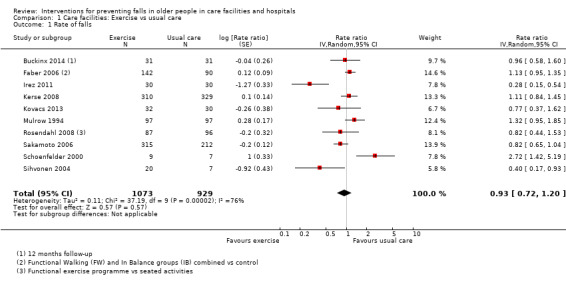
Comparison 1 Care facilities: Exercise vs usual care, Outcome 1 Rate of falls.
In a subgroup analysis by broad types of exercise, there was no evidence of a difference between subgroups (Analysis 2.1: test for subgroup differences P = 1.00).
2.1. Analysis.
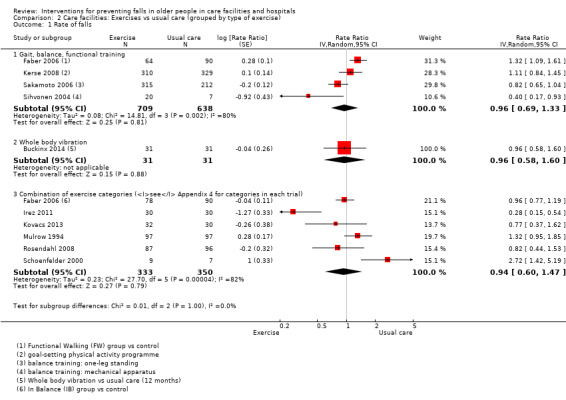
Comparison 2 Care facilities: Exercises vs usual care (grouped by type of exercise), Outcome 1 Rate of falls.
To explore further the heterogeneity in these findings, we carried out a post‐hoc subgroup analysis by level of care (high or intermediate levels of care, or mixed levels). There was evidence of a difference between these subgroups that partially explained the heterogeneity (Analysis 3.1: test for subgroup differences Chi² = 6.39, I² = 69%, 2 df, P = 0.04). In studies of facilities providing mixed levels of care, the heterogeneity was no longer evident (I² = 0%, P = 0.41) and there was no evidence of an effect (Analysis 3.1.3 RaR: 1.08, 95% CI 0.92 to 1.28, 3 trials, 477 participants: I² = 0%). However, heterogeneity remained considerable for trials in a high or intermediate level of care (I² = 78%, P = 0.001).
3.1. Analysis.
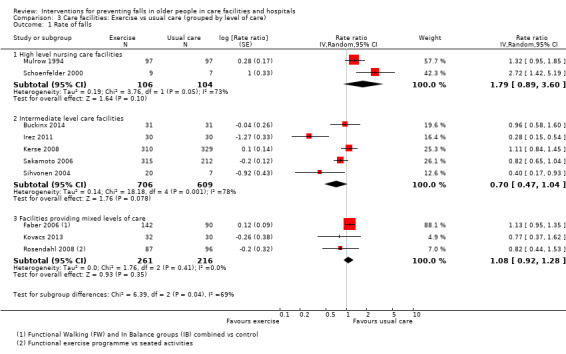
Comparison 3 Care facilities: Exercise vs usual care (grouped by level of care), Outcome 1 Rate of falls.
Four additional trials (130 participants) reported outcomes on rate of falls with data not suitable for pooling (Analysis 1.2); all reported a reduction in falls.
Risk of falling
Pooled data from 10 trials (2090 participants) indicated exercise may make little or no difference to the risk of falling (risk ratio (RR) with random‐effects RR 1.02, 95% CI 0.88 to 1.18: I² = 23%; Analysis 1.3; low‐quality evidence, Table 1).
1.3. Analysis.
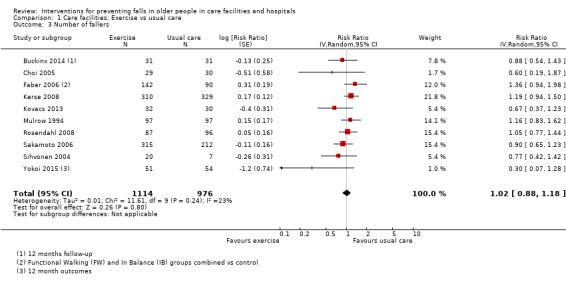
Comparison 1 Care facilities: Exercise vs usual care, Outcome 3 Number of fallers.
There were no subgroup differences in post‐hoc analyses for number of fallers between different levels of care (Analysis 3.2; test for subgroup differences P = 0.56) or types of exercise (Analysis 2.2; test for subgroup differences P = 0.71).
3.2. Analysis.
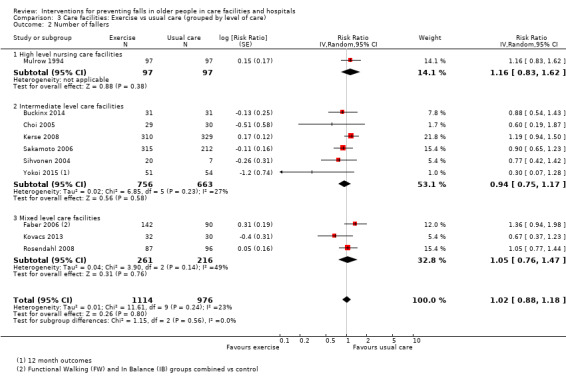
Comparison 3 Care facilities: Exercise vs usual care (grouped by level of care), Outcome 2 Number of fallers.
2.2. Analysis.
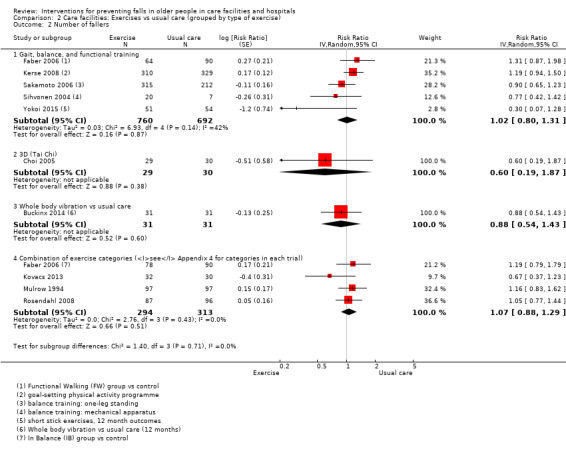
Comparison 2 Care facilities: Exercises vs usual care (grouped by type of exercise), Outcome 2 Number of fallers.
Faber 2006 carried out a post‐hoc subgroup analysis and found that the intervention in frail participants may increase risk of falling (hazard ratio (HR) 2.95, 95% CI 1.64 to 5.32; 115 participants), while in the pre‐frail subgroup there was no strong evidence for a reduction in the risk of falling (HR 0.62, 95% CI 0.29 to 1.33; 105 participants) (test for subgroup difference P ≤ 0.10). Other trials did not provide data suitable for a post‐hoc subgroup analysis of the effectiveness of the intervention according to the frailty of the participants.
Nowalk 2001 (N = 110) reported that there was no significant difference in the risk of falling between "Fit NB Free" individually‐tailored combination exercises, or the "Living and Learning/Tai Chi" in comparison with usual routine activities; data were not suitable for pooling (Analysis 1.2).
Risk of fracture
One trial of functional exercises (Rosendahl 2008, 183 participants) found no strong evidence for a reduction in the risk of hip fracture (Analysis 1.4.1: RR 0.16, 95% CI 0.01 to 2.81; 3 fractures) or total fractures (Analysis 1.4.2: RR 0.88, 95% CI 0.25 to 3.14; 10 fractures). We are uncertain whether exercise reduces the risk of fracture as the quality of the evidence was assessed as very low (Table 1).
1.4. Analysis.
Comparison 1 Care facilities: Exercise vs usual care, Outcome 4 Number of people sustaining a fracture.
Adverse events
Two trials (833 participants) of exercise compared with usual care reported the rates of adverse event outcomes including aches, pains, fatigue, soreness and bruises. Kerse 2008 (639 participants) reported no differences in the level of adverse outcomes on negative binomial regression adjusted for clustering (aches and pains at six months exercise 46.7, 95% CI 39.3 to 54.9 versus usual care 51.1, 95% CI 43.8 to 58.4, P = 0.75). Mulrow 1994 (194 participants) found no difference in the proportion of participants reporting severe soreness (Analysis 1.7.1: RR 0.91, 95% CI 0.40 to 2.04), severe bruises (Analysis 1.7.2: RR 2.00, 95% CI 0.18 to 21.69) or severe fatigue (Analysis 1.7.3: RR 4.00, 95% CI 0.46 to 35.14); there were no injuries during the therapy sessions. One trial (16 participants) reported that there were no adverse events (Schoenfelder 2000). One trial (183 participants) reported a death due to a ruptured abdominal aortic aneurysm one week after the follow‐up tests of the exercise intervention for which association could not definitely be excluded by geriatric review (Rosendahl 2008). We are uncertain of the effects of exercise on adverse events as the quality of the evidence has been assessed as very low; Table 1).
1.7. Analysis.
Comparison 1 Care facilities: Exercise vs usual care, Outcome 7 Adverse events: aches and pains.
Sensitivity analysis
As a sensitivity analysis, the pooled analysis of rate of falls was conducted with a fixed‐effect model. This made little difference to the estimate of effect (RaR 1.01, 95% CI 0.91 to 1.13). The pooled analysis of the risk of falling with a fixed‐effect model also made little difference to the estimate of effect (RR 1.04, 95% CI 0.92 to 1.18). We also conducted a sensitivity analysis including Cadore 2014, which had zero falls in the intervention arm, calculated using one fall in lieu of zero in this arm. This had little impact on the effect estimate (RaR 0.85, 95% CI 0.63 to 1.13; I² = 81%).
To further explore the heterogeneity in the results, outcomes for all trials excluding two trials (Schoenfelder 2000; Sihvonen 2004) with 20 participants or less in each arm of the trial were pooled (this chosen threshold was arbitrary but considered indicative of 'very small' trials). This did not reduce the heterogeneity for rate of falls (Analysis 1.5: I² = 70%), or change the overall pooled estimate of rate of falls (Analysis 1.5: RaR 0.91, 95% CI 0.72 to 1.15) or risk of falling (Analysis 1.6: RR 1.04, 95% CI 0.89 to 1.21; I² = 25%).
1.5. Analysis.
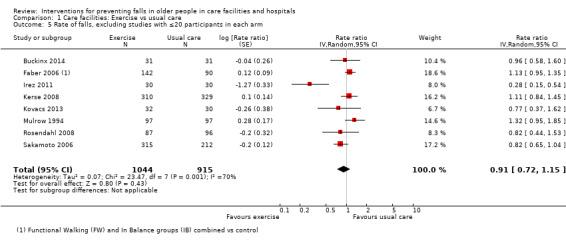
Comparison 1 Care facilities: Exercise vs usual care, Outcome 5 Rate of falls, excluding studies with ≤20 participants in each arm.
1.6. Analysis.
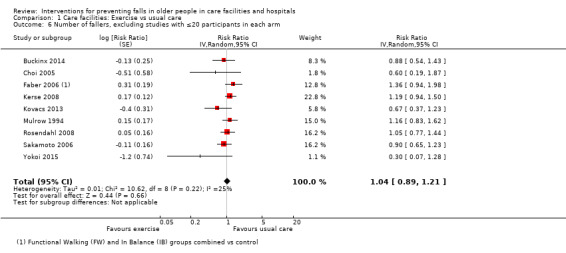
Comparison 1 Care facilities: Exercise vs usual care, Outcome 6 Number of fallers, excluding studies with ≤20 participants in each arm.
Funnel plots testing for publication bias
We constructed funnel plots of trials of exercise versus usual care for both the rate of falls and risk of falling outcomes. The funnel plots appeared asymmetrical for both rate of falls and risk of falling (Figure 4 and Figure 5), which may indicate publication bias or lower methodological quality leading to spuriously inflated effects in the smaller trials. In addition to the trials included in the funnel plots, there were four other trials reporting a reduction in the rate of falls.
4.
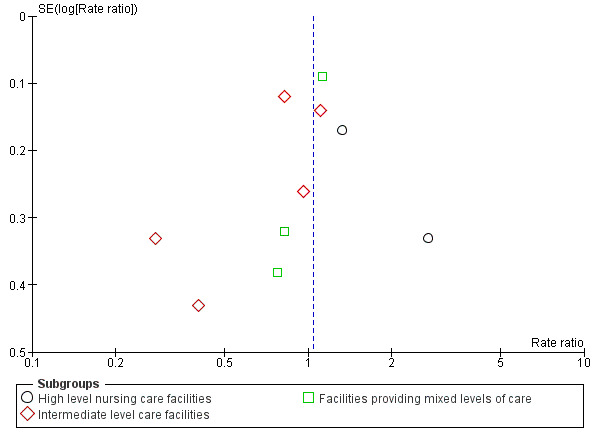
Funnel plot of comparison: 1 Care facilities: Exercise vs usual care (grouped by level of care), outcome: 1.1 Rate of falls. NB four additional trials with data unsuitable for pooling reported a reduction in the rate of falls.
5.
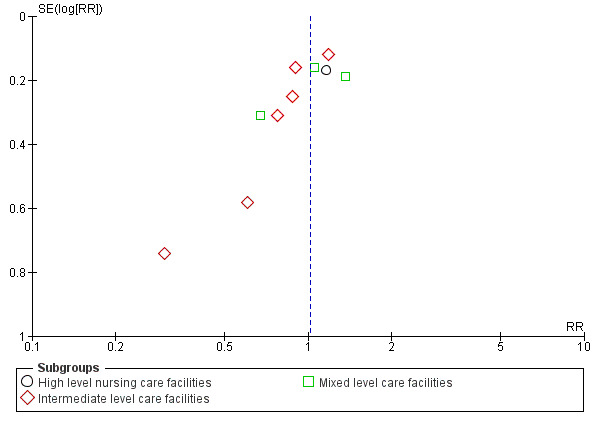
Funnel plot of comparison: 1 Care facilities: Exercise vs usual care (grouped by level of care), outcome: 1.2 Number of fallers. NB One additional trial with data not suitable for pooling reported no significant reduction in the risk of falling.
Comparisons of different exercise categories
Nine trials (584 participants) provided 12 comparisons of two different exercise programmes (Faber 2006; Fu 2015; Imaoka 2016; Kovacs 2012; Saravanakumar 2014; Shimada 2004; Serra‐Rexach 2011; Sitja Rabert 2015; Tuunainen 2013). All trials were individually randomised. Seven trials (nine comparisons; 505 participants) had data suitable for pooling (Faber 2006; Fu 2015; Kovacs 2012; Saravanakumar 2014; Shimada 2004; Sitja Rabert 2015; Tuunainen 2013). Two trials provided data on the effectiveness of additional balance exercises (Shimada 2004; Tuunainen 2013). All other comparisons included only single trials; the quality of evidence was considered very low for these comparisons.
Rate of falls
Five trials (Faber 2006; Fu 2015; Saravanakumar 2014; Shimada 2004; Tuunainen 2013; 305 participants) with data suitable for analysis reported the effect of nine comparisons of different exercise programmes on the rate of falls (Analysis 4.1). For eight of these comparisons there was only a single trial with less than 200 participants; the quality of the evidence was considered very low so the relative effectiveness of these exercise programmes on reducing the rate of falls remains uncertain.
4.1. Analysis.
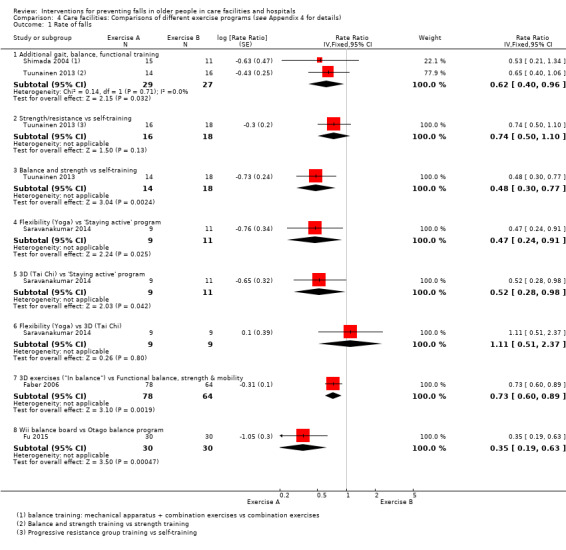
Comparison 4 Care facilities: Comparisons of different exercise programs (see Appendix 4 for details), Outcome 1 Rate of falls.
Pooled data from two trials (Shimada 2004; Tuunainen 2013) of additional balance exercises indicated a reduction in the rate of falls (Analysis 4.1.1: RaR 0.62, 95% CI 0.40 to 0.96; I² = 0%; 56 participants; 86 falls). We are uncertain of the effect of additional balance exercise on falls as the quality of the evidence has been assessed as very low (downgraded two levels due to serious risk of bias, and one level for imprecision).
Serra‐Rexach 2011 (40 participants) compared training sessions of a combination of exercises in addition to usual physiotherapy and reported fewer falls in the intervention group (Analysis 4.2).
Risk of falling
Six trials (Faber 2006; Imaoka 2016; Kovacs 2012; Shimada 2004; Sitja Rabert 2015; Tuunainen 2013; 327 participants) reported the effect of seven comparisons of different exercise categories on the risk of falling (Analysis 4.3). Six comparisons contained only a single trial and the quality of evidence for these comparisons was considered very low; the relative effectiveness of these exercise programmes on reducing the risk of falling remains uncertain.
4.3. Analysis.
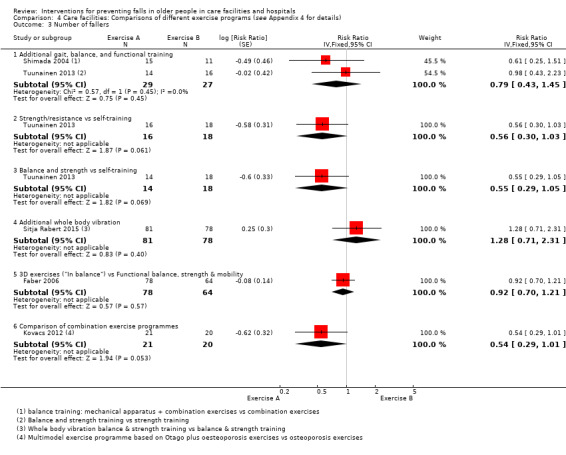
Comparison 4 Care facilities: Comparisons of different exercise programs (see Appendix 4 for details), Outcome 3 Number of fallers.
Pooled data from two trials (Shimada 2004; Tuunainen 2013) of additional balance exercises did not show evidence of a strong effect on reducing the risk of falling Analysis 4.3.1 (RR 0.79, 95% CI 0.43 to 1.45; I² = 0%; 56 participants; 24 fallers). We are uncertain of the effect of additional balance exercise on falls as the quality of the evidence has been assessed as very low (downgraded two levels for risk of bias, and one level for imprecision).
In Imaoka 2016, there was no strong evidence for a reduction in the risk of falling in the post‐intervention period with additional group exercise (RR 0.48, 95% CI 0.17 to 1.3).
Risk of fracture
Sitja Rabert 2015 (159 participants) compared exercise performed on a whole body vibration platform to the same land based exercises and reported one fracture in the intervention group and none in the control group (Analysis 4.4: RR 2.89, 95% CI 0.12 to 69.07; 1 fracture). We are uncertain whether or not whole body vibration reduces the risk of fracture.
4.4. Analysis.

Comparison 4 Care facilities: Comparisons of different exercise programs (see Appendix 4 for details), Outcome 4 Number of people sustaining a fracture.
Adverse events
Four trials (269 participants) comparing alternative exercise programmes reported on adverse events; no serious adverse events were reported. Saravanakumar 2014 (29 participants) reported an instance of a non‐injurious fall during a yoga intervention. Sitja Rabert 2015 (159 participants) comparing exercise on a whole body vibration platform with land‐based exercise reported that "statistical results showed no differences between groups (P=0.430)" and that "ten percent of participants in the exercise group and 16.3% in the whole body vibration plus exercise group presented a possible or probable relation of causality with the intervention, but this difference was not statistically significant (P =0.450)." The most commonly reported adverse events were pain (18%) and soreness (13%) but these data were not reported according to group allocation. Serra‐Rexach 2011 (40 participants), testing additional physiotherapy, reported a case of transient lumbalgia. Lastly, Kovacs 2012 (41 participants), which compared a multimodel exercise programme based on Otago plus osteoporosis exercises with osteoporosis exercises, reported that there were no adverse events.
Medication (drug target) interventions
Medication review
Twelve studies (7366 participants) examined the effect of medication review interventions in care facilities on falls (Crotty 2004a; Crotty 2004b; Frankenthal 2014; Garcia Gollarte 2014; Juola 2015; Frankenthal 2014; Houghton 2014; Lapane 2011; Patterson 2010; Potter 2016; Streim 2012; Zermansky 2006). Seven trials (4536 participants) were individually randomised (Crotty 2004a; Frankenthal 2014; Frankenthal 2014; Lapane 2011; Potter 2016; Streim 2012; Zermansky 2006), and five trials (2830 participants) were cluster randomised (Crotty 2004b; Garcia Gollarte 2014; Juola 2015; Houghton 2014; Patterson 2010). Two studies (1054 participants) did not report falls data suitable for pooling (Garcia Gollarte 2014; Streim 2012). The primary aim of all medication review is generally to reduce psychoactive medications. Therefore, all trials were considered clinically similar except for one study of medication review for hyponatraemia (Peyro Saint Paul 2013). Further details of the interventions and comparisons are provided in Table 10. A summary of the evidence for general medication review for falls prevention in care facilities is provided in Table 2.
Rate of falls
Six trials (2409 participants) reporting data on the rate of falls in trials of general medication review were considered clinically appropriate to pool, despite considerable statistical heterogeneity. General medication review may make little or no difference to the rate of falls (Analysis 5.1.1: RaR 0.93, 95% CI 0.64 to 1.35, 6 trials, 2409 participants; I² = 93%; low‐quality evidence). Subgroup analyses by level of care were not conducted as all trials were conducted in mixed settings.
5.1. Analysis.
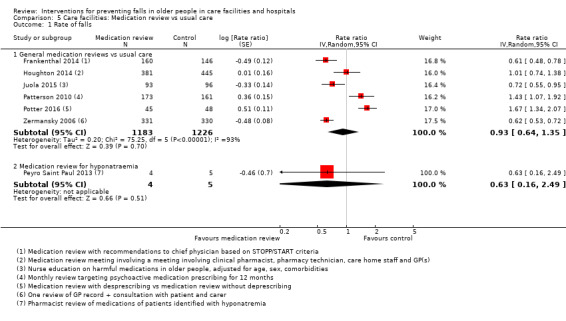
Comparison 5 Care facilities: Medication review vs usual care, Outcome 1 Rate of falls.
Garcia Gollarte 2014 (716 participants) conducted a cluster‐randomised trial of education of physicians on drug use in older people, plus medication review with feedback in 10% of patients. Data from this study were not pooled as falls during the six‐month intervention period were not reported. Over the three months following the intervention, after adjustment for clustering, the rate of falls (RaR 0.74, 95% CI 0.49 to 1.13) did not provide strong evidence for an effect.
A post‐hoc sensitivity analyses was conducted excluding Potter 2016 (93 participants), in which 3 participants in the intervention group had more than 30 falls. The heterogeneity in this analysis remained high (Analysis 5.4: I² = 87%) and there was no strong evidence of a reduction in the rate of falls.
5.4. Analysis.
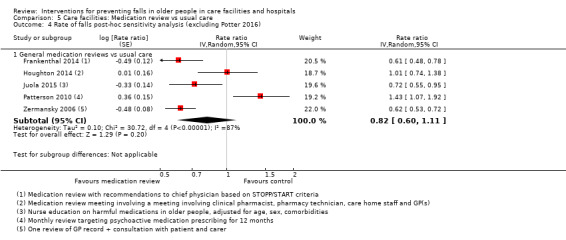
Comparison 5 Care facilities: Medication review vs usual care, Outcome 4 Rate of falls post‐hoc sensitivity analysis (excluding Potter 2016).
One additional small trial examined medication review to avoid hyponatraemia (Peyro Saint Paul 2013; Analysis 5.1.2: nine participants), we are uncertain whether medication review reduces falls in adults with chronic moderate hyponatraemia (serum sodium level 123 mEq/L to 134 mEq/L).
Streim 2012 conducted a trial that included both randomised and a non‐randomised patient‐preference arm. The randomised arms of the trial (36 participants), examined deprescribing of antidepressants. The authors reported that "the discontinuation and continuation groups exhibited similar non‐significant increases in the odds of fall per week with an increase in odds of falls of 1.38 per week (95% CI 4.07 to 0.47); Z=0.59; p=0.55) in the discontinuation group and 1.50 per week (95% CI 0.55 to 4.07); Z=0.80; p=0.43) in the continuation group. The similarity in odds ratios corresponds to discontinuation only reducing the odds ratio of falls relative to the continuation ratio by approximately 10% (ratio of ORs=0.92 (95% CI=(0.21, 4.01); Z=0.11; p=0.91)."
Risk of falling
Pooled data from six clinically similar trials (5139 participants) reporting falls risk data indicated that general medication review may make little or no difference to the risk of falling (Analysis 5.2.1: RR 0.93, 95% CI 0.80 to 1.09; 5139 participants: I² = 48%). The quality of the evidence was considered low (downgraded one level for risk of bias and one level for inconsistency).
5.2. Analysis.
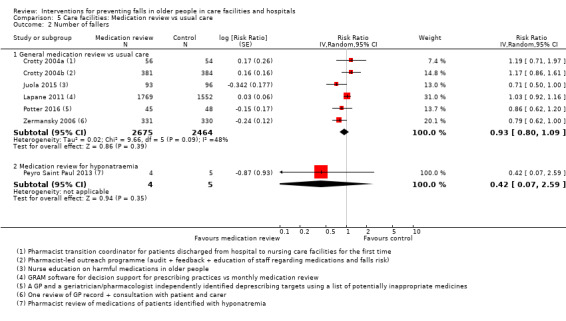
Comparison 5 Care facilities: Medication review vs usual care, Outcome 2 Number of fallers.
In Garcia Gollarte 2014 (716 participants), after adjustment for clustering, the risk of falling (RR 0.86, 95% CI 0.59 to 1.26) did not provide strong evidence for an effect over the three months following the intervention.
We are uncertain of whether medication review reduces falls in adults with chronic moderate hyponatraemia (Analysis 5.2.2: RR 0.42, 95% CI 0.07 to 2.59: 1 trial; 9 participants).
Risk of fracture
Potter 2016 (93 participants) reported the effect of medication review on the risk of fracture (Analysis 5.3: RR 1.60, 95%CI 0.28 to 9.16; 5 fractures), we are uncertain of the effect of medication review on risk of fracture as the quality of the evidence has been assessed as very low.
5.3. Analysis.
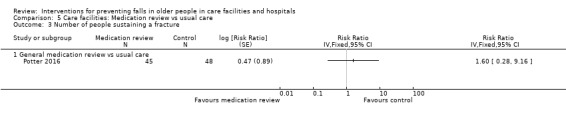
Comparison 5 Care facilities: Medication review vs usual care, Outcome 3 Number of people sustaining a fracture.
Subgroup analysis by cognitive status
Juola 2015 provided data for subgroups according to cognitive status. After adjustment for clustering, the rate of falls was reduced for those with an Mini Mental State Examination (MMSE) greater than 15 (RaR 0.23, 95% CI 0.12 to 0.44; 49 participants) or an MMSE of 10‐15 (RaR 0.27, 95%CI 0.17 to 0.44; 45 participants) but not for those with an MMSE <10 (RaR 1.27, 95% CI 0.95 to 1.69; 95 participants).
Adverse events
Two studies (102 participants) reported on adverse events; the remaining 10 studies did not clearly report on adverse events related to the intervention.
In a study of deprescribing (Potter 2016; 93 participants), serious vascular events occurred in three control participants and one intervention participant, and two intervention participants experienced significant adverse medicine withdrawal reactions (symptomatic rapid atrial fibrillation and agitation) (Analysis 5.5.1: RR 1.07, 95%CI 0.23 to 5.01; 1 trial).
5.5. Analysis.
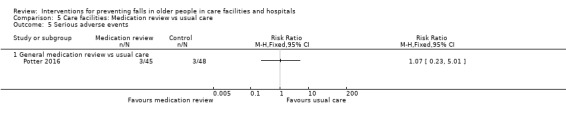
Comparison 5 Care facilities: Medication review vs usual care, Outcome 5 Serious adverse events.
Peyro Saint Paul 2013 (nine participants) reported one serious adverse event (a major gastrointestinal bleed) related to discontinuing a proton‐pump inhibitor in the intervention arm.
We are uncertain of the effects of medication review on adverse events as the quality of the evidence has been assessed as very low (Table 2).
Vitamin D supplementation
Eight studies (9278 participants) examined vitamin D supplementation administered in some form (Bischoff 2003; Broe 2007; Chapuy 2002; Flicker 2005; Grieger 2009; Imaoka 2016; Kennedy 2015; Law 2006). Six trials (5561 participants) were individually randomised (Bischoff 2003; Broe 2007; Chapuy 2002; Flicker 2005; Grieger 2009; Imaoka 2016) and two trials (3717 participants) were cluster randomised (Kennedy 2015; Law 2006). Four trials (4512 participants) tested the effect of vitamin D supplementation on falls (Bischoff 2003; Broe 2007; Flicker 2005; Law 2006), one trial (583 participants) tested the effect of vitamin D and calcium supplementation (Chapuy 2002), two trials (166 participants) tested multivitamin supplementation that included vitamin D plus calcium (Grieger 2009; Imaoka 2016), and one trial (4017 participants) tested an educational intervention aimed at increasing prescription of adequate levels of vitamin D, calcium and osteoporosis medications (Kennedy 2015). Seven of the eight studies reported serum vitamin D levels at baseline (Bischoff 2003; Broe 2007; Chapuy 2002; Flicker 2005; Grieger 2009; Imaoka 2016; Law 2006). Vitamin D levels were low or very low in these studies enrolling residents of care facilities. Baseline vitamin D levels for one trial (Kennedy 2015) were not reported. A summary of the evidence for vitamin D supplementation for falls prevention in care facilities is provided in Table 3.
For the specific comparison of multivitamin supplementation including vitamin D and calcium versus placebo (Grieger 2009; Imaoka 2016), the quality of the evidence was considered very low.
Rate of falls
Pooled data from four trials (4512 participants) indicated that vitamin D supplementation probably reduces the rate of falls (Analysis 6.1.1: RaR 0.72, 95% CI 0.55 to 0.95; I² = 62%: moderate‐quality evidence). The type of vitamin D administered is indicated in the footnotes.
6.1. Analysis.
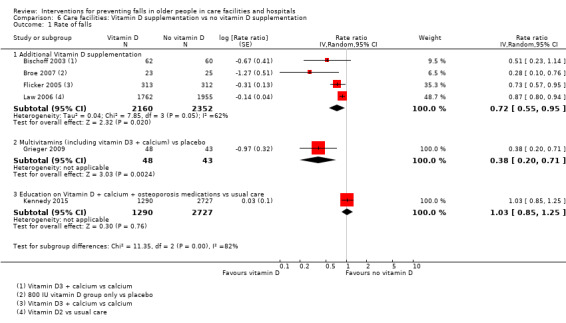
Comparison 6 Care facilities: Vitamin D supplementation vs no vitamin D supplementation, Outcome 1 Rate of falls.
We are uncertain whether multivitamin supplementation including vitamin D and calcium reduces the rate of falls as the quality of the evidence is very low (Analysis 6.1.2: RaR 0.38, 95% CI 0.20 to 0.71; 91 participants; 1 study).
An education intervention aimed at increasing the prescription of vitamin D, calcium and osteoporosis medication (Kennedy 2015) may make little or no difference to the rate of falls (Analysis 6.1.3: RaR 1.03, 95% CI 0.85 to 1.25; 4017 participants; 1 study; low‐quality evidence, downgraded two levels due to risk of bias).
Risk of falling
Pooled data from four trials (4512 participants) indicated that vitamin D supplementation probably makes little or no difference to the risk of falling (Analysis 6.2.1: RR 0.92, 95% CI 0.76 to 1.12; I² = 42%; moderate‐quality evidence, downgraded one level for risk of bias).
6.2. Analysis.
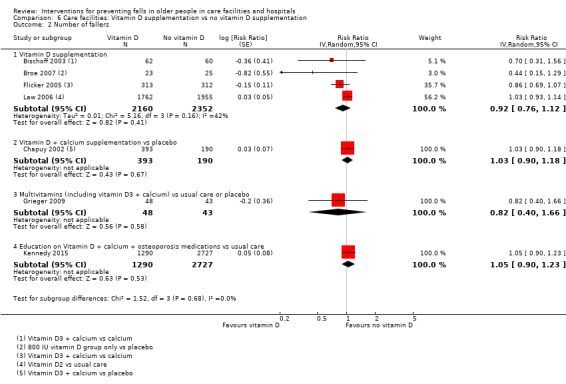
Comparison 6 Care facilities: Vitamin D supplementation vs no vitamin D supplementation, Outcome 2 Number of fallers.
Vitamin D plus calcium supplementation (Chapuy 2002), probably makes little or no difference to the risk of falling (Analysis 6.2.2: RR 1.03, 95% CI 0.90 to 1.18; 583 participants; 1 study; moderate‐quality evidence downgraded one level for risk of bias).
We are uncertain whether multivitamin supplementation including vitamin D and calcium reduces the risk of falling (Analysis 6.2.3: RR 0.82, 95% CI 0.40 to 1.66; 91 participants; 1 study). Imaoka 2016 (75 participants), conducted a four‐arm trial which found no strong evidence for an effect of daily nutritional supplementation including 900 IU vitamin D (including 400 IU vitamin D3 and 200mg calcium in a multivitamin supplement) in comparison with usual care over the six months following the three‐month intervention period (RR 0.58, 95%CI 0.20 to 1.68, N = 34). Outcomes data were not pooled with other studies as they excluded the intervention period; falls are for six months post‐intervention.
An education intervention aimed at increasing the prescription of vitamin D, calcium and osteoporosis medication (Kennedy 2015) may make little difference or no difference to the risk of falling (Analysis 6.2.4: RR 1.05, 95% CI 0.90 to 1.23; 4017 participants; 1 study; low‐quality evidence, downgraded two levels for risk of bias).
Risk of fracture
Pooled data from three trials of vitamin D supplementation showed little effect on fall related fractures (Analysis 6.3.1: RR 1.09, 95% CI 0.58 to 2.03; I² = 63%; 4464 participants; 178 fractures: very low‐quality evidence). Different trials reported different types of fractures; the type of fractures are shown in the footnotes to the analysis. We are uncertain whether vitamin D supplementation reduces the risk of fall related fractures as the evidence has been assessed as very low.
6.3. Analysis.
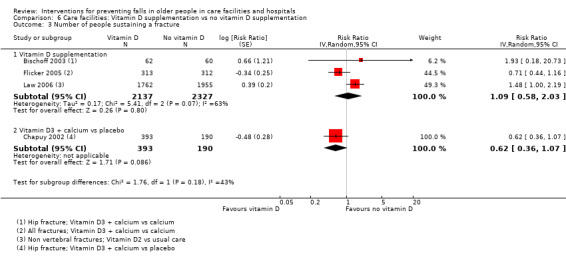
Comparison 6 Care facilities: Vitamin D supplementation vs no vitamin D supplementation, Outcome 3 Number of people sustaining a fracture.
We are uncertain whether vitamin D plus calcium supplementation reduces the risk of fall related fractures (Analysis 6.3.2: RR 0.62, 95% CI 0.36 to 1.07; 583 participants; 48 hip fractures; very low‐quality evidence, downgraded one level for risk of bias, one level for imprecision and one level as this review only includes a subset of the trials available reporting the effects of this intervention on fractures).
An education intervention aimed at increasing the prescription of vitamin D, calcium and osteoporosis medication (Kennedy 2015; 4017 participants) reported that 1.5% of falls in control participants and 1.6% of falls in intervention participants resulted in a fracture, the study was not powered to detect a difference in fall‐related fractures, we are uncertain of the effects of this intervention on fractures (very low‐quality evidence, downgraded two levels for risk of bias and two levels for imprecision).
Adverse events
Four trials (1365 participants) reported adverse‐event data.
Two of four trials (747 participants) of vitamin D supplementation reported on adverse events (Bischoff 2003, Flicker 2005); no serious adverse events were reported. Bischoff 2003 reported two cases of increased constipation in the intervention arm and no cases of hypercalcaemia (Analysis 6.4.1: constipation RR 4.84, 95%CI 0.24 to 98.80; 122 participants). Flicker 2005 reported that there were no adverse events. We are uncertain of the effects of Vitamin D supplementation (up to 1000 IU daily) on adverse events as the quality of the evidence has been assessed as very low (Table 3).
6.4. Analysis.
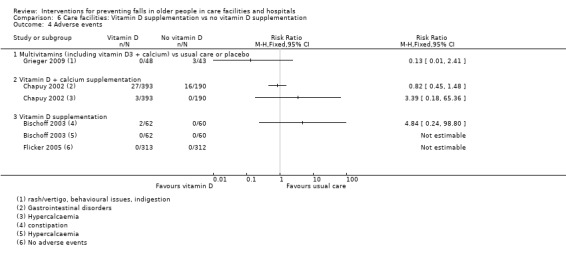
Comparison 6 Care facilities: Vitamin D supplementation vs no vitamin D supplementation, Outcome 4 Adverse events.
One trial of vitamin D and calcium supplementation (800 IU of vitamin D3 + 1200 mg calcium carbonate daily) reported a similar rate of gastrointestinal disorders in each arm of the study and three cases of hypercalcaemia in the intervention arm, we are uncertain of the effects on adverse events (Chapuy 2002; Analysis 6.4.2; gastrointestinal adverse events RR 0.82, 95% CI 0.45 to 1.48; 583 participants; very low‐quality, downgraded one level for risk of bias and two levels for imprecision).
Grieger 2009, which tested multivitamin supplementation including vitamin D and calcium, reported there were no serious adverse events; the three adverse events reported were in the control arm of the trial (rash/vertigo, behavioural issues, indigestion), we are uncertain of the effects on adverse events (Analysis 6.4.2: RR 0.13, 95% CI 0.01 to 2.41; 91 participants, 40 events; very low‐quality evidence).
Environment/assistive technology
In a cross‐over trial, Clifton 2009 (43 participants) tested a wireless position‐monitoring device and found no strong evidence for a reduction in the rate of falls (Analysis 7.1: RaR 0.65, 95% CI 0.33 to 1.27; no adjustments for cross‐over design made in the analysis). There were no serious adverse events. We are uncertain whether or not wireless position monitoring has an effect on the rate of falls in care facilities (very low‐quality evidence).
7.1. Analysis.
Comparison 7 Care facilities: Environmental interventions vs usual care, Outcome 1 Rate of falls.
Social environment
Seven cluster‐randomised trials examined service change interventions in care facilities (13,127 participants in six trials Cox 2008; Chenoweth 2009; Meyer 2009; Van de Ven 2014; Van Gaal 2011a; Ward 2010, plus 982 facility beds in Colon‐Emeric 2013). These included three trials of staff training interventions (Colon‐Emeric 2013; and 7029 participants from Cox 2008 and Van Gaal 2011a) and four of a service model change (6098 participants; Chenoweth 2009; Meyer 2009; Van de Ven 2014; Ward 2010). These interventions target staff or caregivers and changes in the organisational system in which an intervention is delivered, rather than targeting patients directly. The rate of falls for these interventions were not pooled due to high clinical and statistical heterogeneity (test for subgroup differences: P = 0.0001, I² = 85.6%). Two studies (6516 participants) reported data on risk of fracture (Meyer 2009, Ward 2010). No studies reported on adverse events. Although there were only single trials for the comparisons within this category, the generally larger size of these trials meant that optimal information size criteria may be met and GRADE assessments were conducted by two review authors.
Staff training
Cox 2008 (5637 participants) studied a half day education programme about fall and fracture prevention for managers, nurses and health care assistants, given by specialist osteoporosis nurses. There was no strong evidence for a reduction in the rate of falls, we are uncertain of the effects as the quality of the evidence was assessed as very low (Analysis 8.1.1: RaR 1.19, 95% CI 0.92 to 1.53; very low‐quality evidence, downgraded two levels for risk of bias and one level for imprecision). The intervention may make little or no difference to the rate of fracture (reported incidence rate ratio (IRR) for all fractures: IRR 0.94, 95% CI 0.71 to 1.26; for hip fractures: IRR 0.86, 95% CI 0.63 to 1.18; low‐quality evidence downgraded two levels for risk of bias).
8.1. Analysis.
Comparison 8 Care facilities: Social environment vs usual care, Outcome 1 Rate of falls.
The intervention in Van Gaal 2011a (392 participants) consisted of education to implement a patient‐safety programme directed at falls, urinary tract infection, and pressure ulcers based on available guidelines. There was no strong evidence for a reduction in rate of falls, we are uncertain of the effects on the rate of falls (Analysis 8.1.2: RaR 0.63, 95% CI 0.34 to 1.16; very low‐quality evidence, downgraded two levels for risk of bias, one level for indirectness and one level for imprecision).
Colon‐Emeric 2013 (number of resident participants not reported, 497 staff participants, 982 facility beds) conducted a pilot cluster‐randomised trial testing a programme to improve staff connections, communication, and problem solving compared to usual care during implementation of a falls quality improvement programme. There was no strong evidence for an effect on the change in falls rate from baseline to post intervention periods between the two arms of the study, we are uncertain of the effects in reducing falls (RaR of change in falls rate 0.81, 95% CI 0.55 to 1.20; very low‐quality evidence, downgraded one level for each of risk of bias, indirectness and imprecision).
Service model change
Meyer 2009 (1125 participants) found that use of a falls risk‐assessment tool in comparison with nurses' judgement alone probably makes little or no difference to the rate of falls or risk of falling (Analysis 8.1.3: RaR 0.96, 95% CI 0.84 to 1.10; Analysis 8.2: RR 0.99, 95% CI 0.85 to 1.16; both outcomes moderate‐quality evidence, downgraded one level for risk of bias). We are uncertain whether or not this intervention reduces the risk of fracture as the quality of the evidence was assessed as very low (Analysis 8.3.1: RR 0.96, 95% CI 0.57 to 1.63; 77 fractures in total; downgraded one level for risk of bias and two levels for imprecision).
8.2. Analysis.

Comparison 8 Care facilities: Social environment vs usual care, Outcome 2 Number of fallers.
8.3. Analysis.
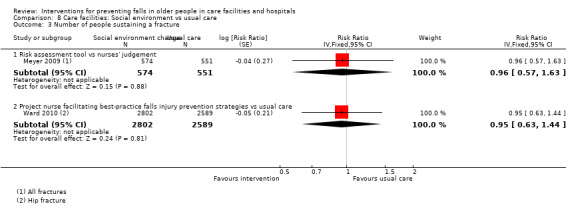
Comparison 8 Care facilities: Social environment vs usual care, Outcome 3 Number of people sustaining a fracture.
Two studies examined dementia care mapping, but data from Chenoweth 2009 were not suitable for pooling. Chenoweth 2009 (289 participants) reported that "... at follow‐up there were fewer falls with dementia‐care mapping than in usual care (p=0·02) and more falls in person‐centred care than in usual care (p=0·03)." Van de Ven 2014 (293 participants) delivered a four‐month dementia care mapping intervention twice during the 12‐month follow‐up period after baseline. The rate of falls at study endpoint was greater in the intervention arm of the study (Analysis 8.1.4: RaR 1.84, 95% CI 1.40 to 2.42). We are uncertain of the effects of dementia care mapping on the rate of falls as the quality of the evidence has been assessed as very low (downgraded two levels for risk of bias, one level for inconsistency and one level for imprecision).
Ward 2010 (5391 participants) employed a practice nurse to encourage the adoption of best practice strategies and reported "0.13 fewer falls per 100 beds per month; 95% CI, −0.36 to 0.10; P = 0.259" for the intervention period. There was no difference in risk of hip fracture between intervention and control groups during the 17 months of intervention (Analysis 8.3.2; RR 0.95, 95% CI 0.63 to 1.44; 215 hip fractures). We are uncertain of the effects of this intervention on fractures as the quality of the evidence has been assessed as very low (downgraded two levels for risk of bias, and two levels for imprecision).
Psychological interventions
Two studies (163 participants) examined the impact of psychological interventions on falls (Huang 2016; Van het Reve 2014). Both trials were individually randomised, Huang 2016 is a three‐arm trial for which falls excluded the intervention period; findings are also discussed under "Care facilities: multiple interventions". Neither trial reported data on the risk of fracture or adverse events.
In Van het Reve 2014 (114 participants) a computer‐based cognitive training programme focused on improving attention was combined with strength and balance training, and compared with strength and balance training alone. The intervention showed no strong evidence for an effect on falls rates (Analysis 9.1: RaR 1.22, 95% CI 0.78 to 1.92), risk of falling during the intervention period (Analysis 9.2.2; RR 1.35, 95% CI 0.23 to 7.88) or over 12 months post‐intervention (RR 1.38, 95% CI 0.76 to 2.51; data not shown).
9.1. Analysis.

Comparison 9 Care facilities: Psychological interventions vs control, Outcome 1 Rate of falls.
9.2. Analysis.

Comparison 9 Care facilities: Psychological interventions vs control, Outcome 2 Number of fallers.
In a three‐arm study, Huang 2016 tested the effects of a cognitive‐behavioural intervention conducted by a trained facilitator in comparison with usual care in 49 participants. Over the three months following the intervention, there were 1.67 falls per person year in the usual care arm of the study (10 falls in seven fallers), but no falls in the cognitive‐behavioural intervention arm. Data were not pooled as falls excluded the intervention period.
The quality of the evidence for both the rate and risk of falling was considered very low (downgraded one level for risk of bias, inconsistency and indirectness and two levels for imprecision), so we are uncertain of the effectiveness of psychological interventions in reducing falls.
Other single interventions
Three trials (564 participants) examined other single interventions of lavender olfactory stimulation (Sakamoto 2012), sunlight exposure (Sambrook 2012), and multisensory stimulation in a Snoezelen room (Klages 2011); two trials (169 participants) were individually randomised (Sakamoto 2012; Klages 2011) and one (Sambrook 2012; 395 participants) was cluster randomised. The quality of the evidence was considered very low for all of these single‐trial comparisons.
For one year, Sakamoto 2012 (145 participants) tested the effect of lavender olfactory stimulation by applying lavender patches or placebo patches to clothing near the neck daily. This intervention did not show strong evidence for a reduction in the rate of falls (Analysis 10.1: RaR 0.57, 95% CI 0.32 to 1.01) or risk of falling (Analysis 10.2: RR 0.67, 95% CI 0.40 to 1.12). The authors reported that there were no adverse events. We are uncertain of the effectiveness of lavender olfactory stimulation as the quality of the evidence is very low.
10.1. Analysis.
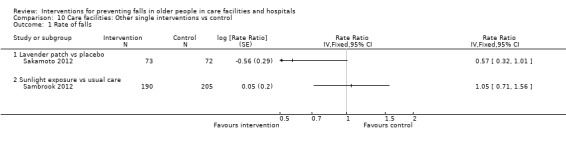
Comparison 10 Care facilities: Other single interventions vs control, Outcome 1 Rate of falls.
10.2. Analysis.
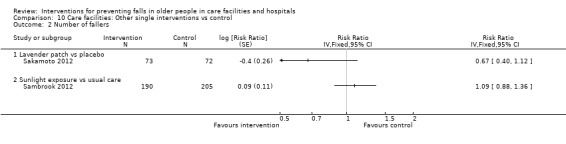
Comparison 10 Care facilities: Other single interventions vs control, Outcome 2 Number of fallers.
In Sambrook 2012 (395 participants), a trial of increased sunlight exposure had low adherence to the sunlight intervention (Durvasula 2012). We are uncertain of the effects on falls as the quality of the evidence has been assessed as very low for all outcomes (downgraded one level for each of risk of bias, indirectness and imprecision; Analysis 10.1.2: RaR 1.05, 95% CI 0.71 to 1.56; Analysis 10.2.2: RR 1.09, 95% CI 0.88 to 1.36; Analysis 10.3: risk of fracture: RR 1.07, 95% CI 0.53 to 2.17, total 32 fractures). The authors reported no difference in the incidence rates of new skin cancers between arms of the trial and one fall on the way to a sunlight session. Adverse‐event data for this three‐arm trial are also reported below under Multiple interventions.
10.3. Analysis.
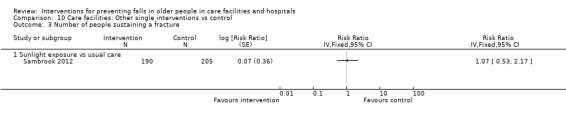
Comparison 10 Care facilities: Other single interventions vs control, Outcome 3 Number of people sustaining a fracture.
Klages 2011 (24 participants) compared the effect of multisensory stimulation in a Snoezelen room with control activities in people with dementia and reported, without providing data, that the "Group membership did not alter falls frequency". Adverse‐event data were not reported. We are uncertain of the effectiveness of multisensory stimulation as the quality of the evidence is very low.
Care facilities: multiple interventions
In multiple interventions, the same combination of single categories of intervention was delivered to all participants in the group. Three trials (652 participants) examined multiple interventions in care facilities (Sambrook 2012; Schnelle 2003; Huang 2016). One trial (412 participants) was cluster randomised (Sambrook 2012) and two trials (240 participants) were individually randomised. The quality of the evidence was considered very low for the single trial comparisons of exercise plus management of urinary incontinence and fluid therapy with usual care (Schnelle 2003), and cognitive‐behavioural therapy to address fear of falling with an exercise programme versus usual care (Huang 2016).
In Schnelle 2003 (190 participants), participants engaged in supervised exercises and were offered fluids and regular toileting. There was no strong evidence for an effect in reducing the rate of falls (Analysis 11.1.1: RaR 0.62, 95% CI 0.38 to 1.01), risk of falling (Analysis 11.2.1: RR 0.62, 95% CI 0.36 to 1.05) or risk of fracture (Analysis 11.3.1: RR 4.26, 95% CI 0.48 to 37.55; total five fractures). Adverse events were not reported. We are uncertain of the effectiveness of this intervention as the quality of the evidence is very low.
11.1. Analysis.
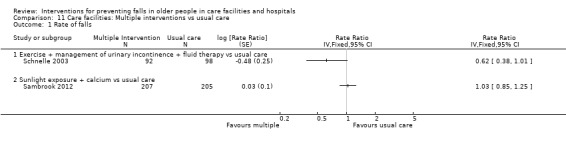
Comparison 11 Care facilities: Multiple interventions vs usual care, Outcome 1 Rate of falls.
11.2. Analysis.
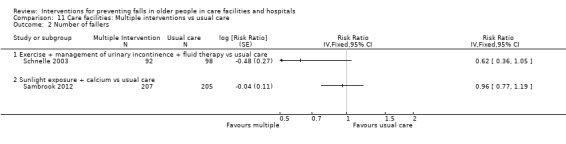
Comparison 11 Care facilities: Multiple interventions vs usual care, Outcome 2 Number of fallers.
11.3. Analysis.
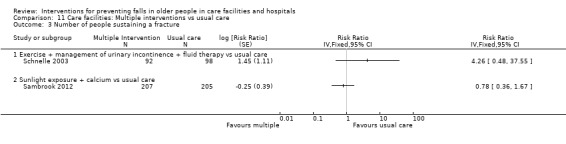
Comparison 11 Care facilities: Multiple interventions vs usual care, Outcome 3 Number of people sustaining a fracture.
One intervention group in Sambrook 2012 (412 participants), which was based in Australia, tested the effect of increased sunlight exposure plus calcium supplementation, with low adherence to the sunlight intervention (Durvasula 2012). We are uncertain of the effects on falls as the quality of the evidence has been assessed as very low for all outcomes (downgraded one level for each of risk of bias, indirectness and imprecision; Analysis 11.1.2: RaR 1.03, 95% CI 0.85 to 1.25; Analysis 11.2.2: RR 0.96, 95% CI 0.77 to 1.19; Analysis 11.3.2: risk of fracture RR 0.78, 95% CI 0.36 to 1.67; total 31 fractures). The authors reported no significant difference in the incidence rates of new skin cancers between arms of the trial (18 new cancers total) and an increase in the adjusted all‐cause mortality in the calcium‐treated group compared with the UV alone group (HR 1.23 versus 0.76, P = 0.03; 40 deaths; adjusted for age, sex and season). There was a lack of evidence for a strong effect on increased death rates from myocardial infarction (age‐adjusted HR 3.83, 95% CI 0.97 to 15.27, P = 0.06; sex‐adjusted HR 4.17, 95% CI 0.69 to 25.16, P = 0.12; the authors reported that they did not record cardiovascular events prospectively). We are uncertain of the effects on adverse events as the quality of the evidence is very low (downgraded one level for each of risk of bias, indirectness and imprecision).
In a three‐arm trial, Huang 2016 studied an intervention which combined cognitive‐behavioural therapy to address fear of falling with an exercise programme in comparison with usual care in 50 participants. In the three months following the eight‐week intervention the authors reported a reduction in falls in both the combined intervention and the cognitive‐behavioural intervention arm alone (reported Kruskal‐Wallis P < 0.001). There were 1.67 falls per person year in the usual care arm of the study (10 falls in seven fallers), and no falls in the cognitive behavioural plus exercise intervention arm; data were not pooled as falls excluded the intervention period. Adverse events were not reported. We are uncertain of cognitive‐behavioural therapy combined with an exercise programme as the quality of the evidence is very low.
Care facilities: multifactorial interventions
In multifactorial interventions, two or more categories of intervention are given, and these are linked to each individual's risk profile. An initial assessment is usually carried out by one or more health professionals and an intervention is then provided or recommendations given or referrals made for further action. A summary of the evidence for multifactorial interventions in comparison with usual care in care facilities is provided in Table 4.
Thirteen trials (4226 participants) in care facilities studied multifactorial interventions (Beck 2016; Becker 2003; Dyer 2004; Jensen 2002; Kerse 2004; McMurdo 2000; Neyens 2009; Ray 1997; Rubenstein 1990; Salvà 2016; Shaw 2003; Walker 2015; Whitney 2017). Eleven trials were cluster‐randomised trials (Beck 2016; Becker 2003; Dyer 2004; Jensen 2002; Kerse 2004; McMurdo 2000; Neyens 2009; Ray 1997; Salvà 2016; Walker 2015; Whitney 2017; 3470 participants), and two were individually randomised (Rubenstein 1990; Shaw 2003; 756 participants). Whitney 2017 was also a cross‐over trial. None of these trials were sufficiently similar to allow analysis of subgroups of specific combinations of interventions. Two studies did not report data suitable for use in the quantitative analysis (Beck 2016; Ray 1997). Three studies (2160 participants) reported data on hip fractures (Becker 2003; Jensen 2002; Shaw 2003), and one reported total fractures (Salvà 2016). Three studies (312 participants) reported adverse‐event data (Beck 2016; McMurdo 2000; Whitney 2017).
Rate of falls
Despite statistical heterogeneity between the trials for the rate of falls, trials were considered clinically similar enough for pooling to be meaningful. Pooled data from 10 trials (3439 participants) for rate of falls did not demonstrate strong evidence for a reduction in falls (Analysis 12.1: RaR random effects 0.88, 95% CI 0.66 to 1.18: I² = 84%). Beck 2016 (31 participants) reported falls outcomes in a cluster‐randomised trial of an exercise programme plus nutritional support. There were zero falls in the intervention arm and two in the control arm over an 11‐week period. Overall, we are uncertain of the effects of multifactorial interventions on the rate of falls in care facilities as the quality of evidence has been assessed as very low (Table 4).
12.1. Analysis.
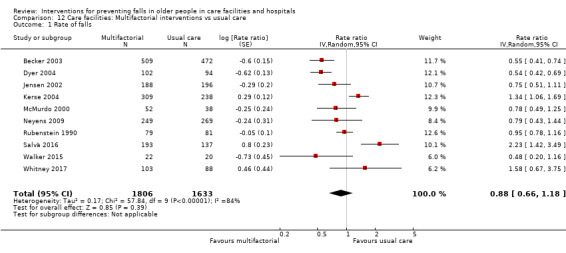
Comparison 12 Care facilities: Multifactorial interventions vs usual care, Outcome 1 Rate of falls.
Risk of falling
Pooled data from nine trials (3153 participants) for risk of falling (Analysis 12.2: RR random effects 0.92, 95% CI 0.81 to 1.05: I² = 42%) did not demonstrate strong evidence for a reduction in falls. Ray 1997 (482 participants) only recorded the number of people having two or more falls during follow‐up (recurrent fallers) and reported a reduction in the proportion of recurrent fallers (difference 19%, 95% CI 2% to 36%: P = 0.03). Overall, multifactorial interventions in care facilities may make little or no difference to the risk of falling (low‐quality evidence; Table 4).
12.2. Analysis.
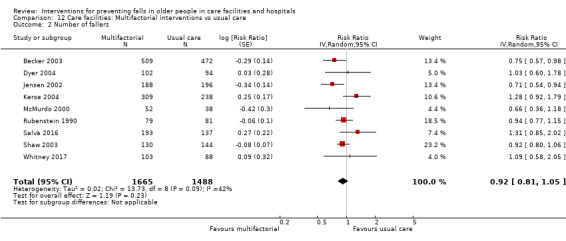
Comparison 12 Care facilities: Multifactorial interventions vs usual care, Outcome 2 Number of fallers.
Risk of fracture
Pooled results for five studies (2160 participants) reporting risk of fracture did not show strong evidence for an effect (Analysis 12.3: RR 0.79, 95% CI 0.30 to 2.07: I² = 44%; 76 fractures). Data from three of the five trials (1695 participants) were for hip fracture (Becker 2003; Jensen 2002; Salvà 2016) and two trials (465 participants) reported total fractures (Shaw 2003; Whitney 2017). Two trials (1255 participants) included hip protectors as an intervention (Becker 2003; Shaw 2003). We are uncertain of the effects of multifactorial interventions on the risk of fracture as the quality of evidence has been assessed as very low (Table 4).
12.3. Analysis.
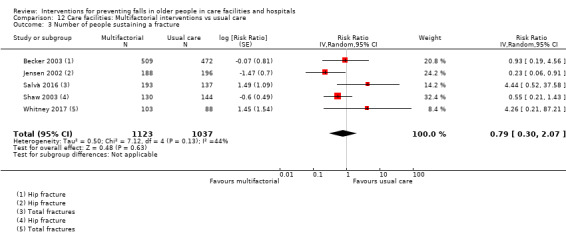
Comparison 12 Care facilities: Multifactorial interventions vs usual care, Outcome 3 Number of people sustaining a fracture.
Adverse events
Three studies (312 participants) reported adverse‐event data. One trial reported an instance of a fall in the intervention arm (Whitney 2017), two studies reported that there were no adverse events (Beck 2016; McMurdo 2000). We are uncertain of the effects of multifactorial interventions on adverse events as the quality of evidence has been assessed as very low (Table 4).
Subgroup analyses exploring heterogeneity
To explore the heterogeneity in these results, we carried out post‐hoc subgroup analysis by levels of care (high or intermediate or mixed levels of care). The test for subgroup differences showed a difference between subgroups for both the rate of falls (Analysis 13.1: P = 0.005, I² = 81%) and risk of falling (Analysis 13.2: P = 0.03, I² = 72%). Within care facilities providing either high or intermediate levels of care, statistical heterogeneity was not important and pooled data showed a reduction in both the rate of falls (Analysis 13.1.1: high‐level care: RaR 0.59, 95% CI 0.44 to 0.79; I² = 8%, P = 0.30; Analysis 13.1.2: intermediate‐level care: RaR 0.64, 95% CI 0.50 to 0.83; I² = 33%, P = 0.23), and the risk of falling (Analysis 13.2.1: high level care: RR 0.75, 95% CI 0.57 to 0.98; Analysis 13.2.2: intermediate level care: RR 0.75, 95% CI 0.60 to 0.94; I² = 0%, P = 0.44). However, heterogeneity remained high in studies of mixed levels of care (Analysis 13.1.3: RaR 1.23, 95% CI 0.85 to 1.77; I² = 77%, P = 0.001; Analysis 13.2.3: RR 1.01, 95% CI 0.88 to 1.15; I² = 24%, P = 0.26).
13.1. Analysis.
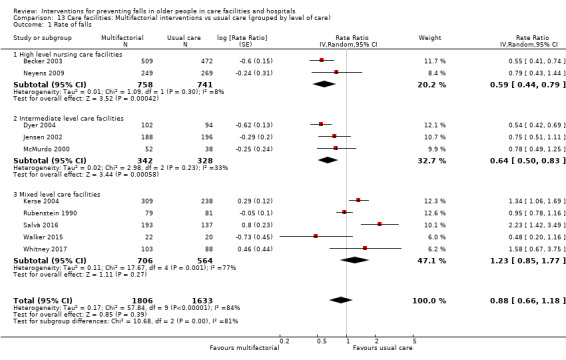
Comparison 13 Care facilities: Multifactorial interventions vs usual care (grouped by level of care), Outcome 1 Rate of falls.
13.2. Analysis.
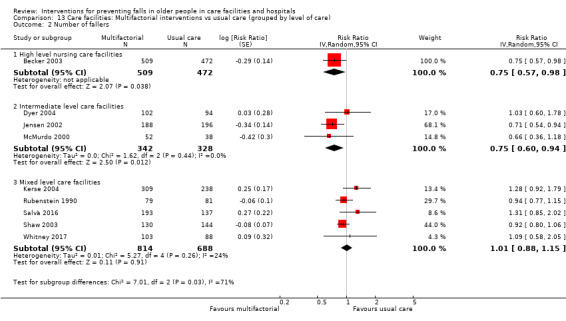
Comparison 13 Care facilities: Multifactorial interventions vs usual care (grouped by level of care), Outcome 2 Number of fallers.
We also carried out a subgroup analysis comparing trials recruiting people with cognitive impairment versus trials with participants with no cognitive impairment (based on inclusion/exclusion criteria) or a mixed sample. Two trials recruited residents with cognitive impairment only (Neyens 2009; Shaw 2003). In addition, two trials (Becker 2003; Jensen 2002) carried out pre‐planned subgroup analyses by levels of cognition, which are reported in Rapp 2008 and Jensen 2003, respectively. Cognitive impairment was defined differently in all four studies (see footnotes to Analysis 14.1 and Analysis 14.2). There was no evidence of subgroup differences between those with higher or mixed levels of cognition and those with lower cognition for both rate of falls (Analysis 14.1: test for subgroup differences P = 0.97, I² = 0%) and risk of falling (Analysis 14.2: test for subgroup differences P = 0.41, I² = 0%).
14.1. Analysis.
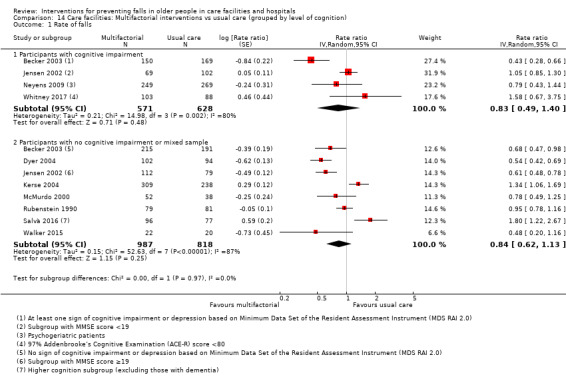
Comparison 14 Care facilities: Multifactorial interventions vs usual care (grouped by level of cognition), Outcome 1 Rate of falls.
14.2. Analysis.
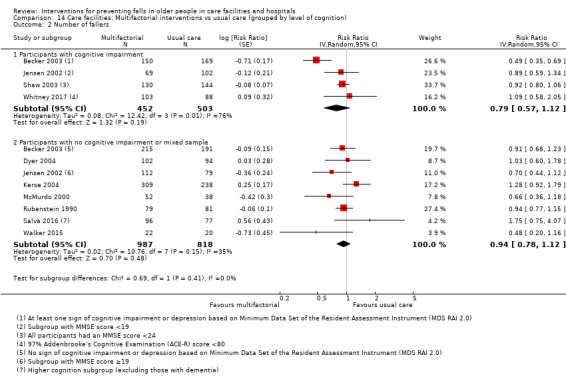
Comparison 14 Care facilities: Multifactorial interventions vs usual care (grouped by level of cognition), Outcome 2 Number of fallers.
Subgroup analysis based upon the individual components of the interventions was precluded by the study design.
Sensitivity analysis
Considering statistical heterogeneity in the rate of falls, meta‐analyses with a random‐effects model was considered the most appropriate. However, there was only moderate heterogeneity in the risk of falling data, therefore trials were pooled using the fixed‐effect model as a sensitivity analysis. Pooled data from 10 trials (3439 participants) using a fixed‐effect model for rate of falls showed an RaR 0.87, 95% CI 0.79 to 0.97 (compare with Analysis 12.1: I² = 84%) and from nine trials (3153 participants) for risk of falling showed an RR 0.92, 95% CI 0.84 to 1.00 (compare with Analysis 12.2: I² = 42%).
Funnel plots testing for publication bias
A funnel plot of trials of multifactorial interventions in care facilities was conducted for the outcome of rate of falls (Figure 6). There was no obvious asymmetry on visual inspection.
6.
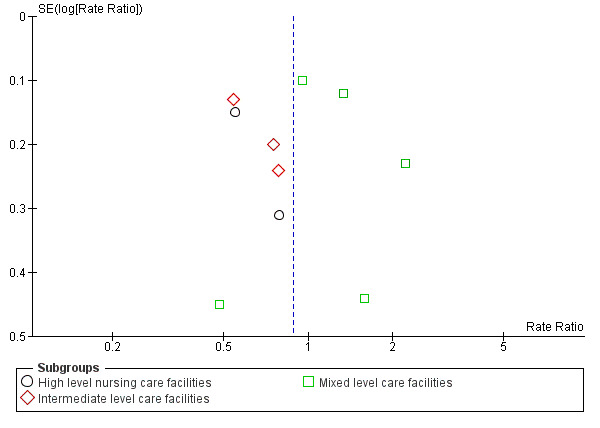
Funnel plot of comparison: 11 Multifactorial interventions vs usual care grouped by level of care (care facilities), outcome: 11.1 Rate of falls.
Hospitals: single interventions
Exercise
Three individually‐randomised trials (244 participants) tested the effect of additional physiotherapy in rehabilitation wards (Donald 2000; Jarvis 2007; Treacy 2015). One study tested additional strengthening exercises (Donald 2000), one additional balance training (Treacy 2015), and one additional physiotherapy (Jarvis 2007). A summary of the evidence for exercise for falls prevention in hospitals is provided in Table 5. No data on the risk of fractures were reported. One trial (161 participants) reported that there were no adverse events (Treacy 2015), two studies did not report adverse‐event data.
Pooled data did not provide evidence for a reduction in rate of falls (Analysis 15.1: RaR 0.59, 95% CI 0.26 to 1.34; 215 participants, 2 trials; I² = 0%; very low‐quality evidence). Pooled data from two trials (83 participants) showed a reduction in the risk of falling (Analysis 15.2: RR 0.36, 95% CI 0.14 to 0.93: I² = 0%: very low‐quality evidence). We are uncertain whether additional exercise reduces the rate or risk of falling or has adverse events as the evidence has been assessed as very low.
15.1. Analysis.
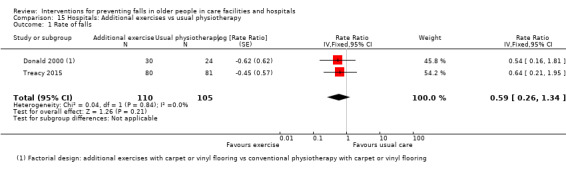
Comparison 15 Hospitals: Additional exercises vs usual physiotherapy, Outcome 1 Rate of falls.
15.2. Analysis.
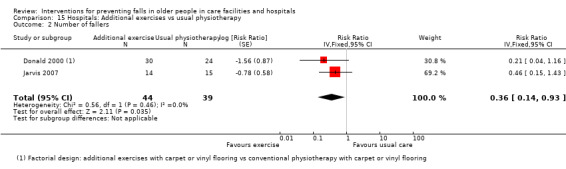
Comparison 15 Hospitals: Additional exercises vs usual physiotherapy, Outcome 2 Number of fallers.
Medication (drug target) interventions
Two trials (319 participants) examined medication target interventions, one examined medication review (Michalek 2014), and the other vitamin D supplementation (Burleigh 2007). These comparisons were from single trials only and the quality of evidence was considered very low.
Multiprofessional medication review
Michalek 2014 (114 participants) conducted a quasi cluster‐randomised trial that examined the effect of review of suitability of medications for aged patients in comparison with usual care. After adjustment for clustering there was no strong evidence for an effect on the rate of falls (Analysis 16.1: RaR 0.14, 95% CI 0.00 to 6.63) or risk of falling (Analysis 16.2: RR 0.18, 95% CI 0.01 to 3.47). Adverse‐event data were not reported. We are uncertain of the effectiveness of medication review in hospitals as the quality of the evidence is very low.
16.1. Analysis.
Comparison 16 Hospitals: Medication review vs usual care, Outcome 1 Rate of falls.
16.2. Analysis.
Comparison 16 Hospitals: Medication review vs usual care, Outcome 2 Number of fallers.
Vitamin D supplementation
Burleigh 2007 (205 participants) conducted an individually‐randomised trial that investigated whether 800 IU of vitamin D plus 1200 mg of calcium supplements reduced falls compared with 1200 mg calcium supplements alone in participants with a median length of stay of 30 days. There was no strong evidence for an effect on risk of falling (Analysis 17.1: RR 0.82, 95% CI 0.59 to 1.14) or fractures (Analysis 17.2: RR 0.34, 95% CI 0.04 to 3.05; total four fractures). The rates of gastrointestinal complaints were similar between the arms of the trial (Analysis 17.3: RR 1.37, 95% CI 0.32 to 5.98). We are uncertain of the effectiveness of vitamin D supplementation in hospitals as the quality of the evidence is very low.
17.1. Analysis.
Comparison 17 Hospitals: Vitamin D supplements vs no vitamin D supplements, Outcome 1 Number of fallers.
17.2. Analysis.
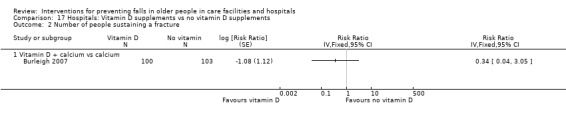
Comparison 17 Hospitals: Vitamin D supplements vs no vitamin D supplements, Outcome 2 Number of people sustaining a fracture.
17.3. Analysis.
Comparison 17 Hospitals: Vitamin D supplements vs no vitamin D supplements, Outcome 3 Adverse events.
Environment/assistive technology interventions
Six trials (39,127 participants) examined environment or assistive technology interventions, two trials (11,153 participants) were of furnishing adaptations (Donald 2000; Haines 2010), and four (27,974 participants) were of communication aids (Mayo 1994; Shorr 2012; Tideiksaar 1993; Wolf 2013). Four trials (356 participants) were individually randomised (Donald 2000; Mayo 1994; Tideiksaar 1993; Wolf 2013), and two (38, 771 participants) were cluster randomised (Haines 2010; Shorr 2012). Donald 2000 was a 2 x 2 factorial design. The quality of the evidence was considered very low for the single trial comparisons of carpet in comparison with vinyl floors (Donald 2000) and identification bracelets for high‐risk fallers (Mayo 1994).
Furnishing/adaptations
Donald 2000, in a factorial design with 54 participants, found that carpeted floors compared with existing vinyl floors in subacute hospital wards resulted in an increase in rate of falls (Analysis 18.1.1: RaR 14.73, 95% CI 1.88 to 115.35) and no strong evidence for an increase in the risk of falling (Analysis 18.2.1: RR 8.33, 95% CI 0.95 to 73.37). We are uncertain of the impact of carpeting on falls as the quality of the evidence is very low.
18.1. Analysis.
Comparison 18 Hospitals: Environmental interventions vs usual care, Outcome 1 Rate of falls.
18.2. Analysis.
Comparison 18 Hospitals: Environmental interventions vs usual care, Outcome 2 Number of fallers.
In a cluster‐randomised trial, Haines 2010 (11,099 participants) examined an intervention which consisted of providing one low‐low bed per 12 existing beds in acute and subacute wards. There was no strong evidence of an effect on the rate of falls; we are uncertain of the effectiveness of low‐low beds as the quality of the evidence is considered very low (Analysis 18.1.2: RaR 1.39, 95% CI 0.22 to 8.78; very low‐quality evidence downgraded two levels for risk of bias, one level for indirectness and two levels for imprecision).
Neither trial reported adverse event or fracture data.
Communication aids
Identification bracelet for high‐risk fallers
Mayo 1994 (134 participants) studied the effect of wearing a blue identification bracelet on falls in high‐risk patients in a subacute hospital setting. They found no reduction in rate of falls (Analysis 18.1.3: RaR 1.15, 95% CI 0.72 to 1.84) or risk of falling (Analysis 18.2.2: RR 1.34, 95% CI 0.76 to 2.36). In this study, there was no reduction in risk of falling in the subgroup with a Short Portable Mental Status Questionnaire (SPMSQ) score < 9 (low cognition) or the subgroup with SPMSQ score ≥ 9 (high cognition). Adverse events were not reported. We are uncertain of the effectiveness of identification bracelets for reducing falls in hospitals as the quality of the evidence is very low.
Bed exit alarms
Three trials (28,717 participants) examined bed exit alarms in hospital (Shorr 2012; Tideiksaar 1993; Wolf 2013). One large trial (Shorr 2012) was cluster randomised. A summary of the evidence for bed exit alarms for falls prevention in hospitals is provided in Table 6. Shorr 2012 (27,672 participants) examined an educational intervention to support clinical judgement on the use of bed or chair exit alarms. Wolf 2013 (98 participants) enrolled patients with an increased risk of falling that required assistance with mobilisation during rest time. Pooled data from these two studies did not show a strong reduction in the rate of falls (Analysis 18.1.4: RaR 0.60, 95% CI 0.27 to 1.34: very low‐quality evidence) or risk of falling (Analysis 18.2.3: RR 0.93, 95% CI 0.38 to 2.24: very low‐quality evidence). We are uncertain whether bed exit alarms reduce the rate of falls or risk of falling as the quality of the evidence has been assessed as very low
Tideiksaar 1993 (70 participants) studied bed exit alarms for preventing falls in hospital. During the nine‐month evaluation period, "There was no significant difference in the number of bed‐falls between the two groups (p = 1.00)."
Two trials of bed alarms (27,742 participants) indicated that there were no adverse events (Shorr 2012; Tideiksaar 1993); we are uncertain of the effects of bed alarms on adverse events as the quality of the evidence has been assessed as very low (Table 6).
Social environment
Social environment interventions target staff members and changes in the organisational system, rather than targeting patients directly. Six trials (9074 participants) examined service model change interventions (Dykes 2010; Koh 2009; Mador 2004; Stenvall 2007; Van Gaal 2011b; Wald 2011). Three trials (8587 participants) were cluster randomised (Dykes 2010; Koh 2009; Van Gaal 2011b), and three (487 participants) were individually randomised (Mador 2004; Stenvall 2007; Wald 2011). Studies were not pooled as they were considered to examine clinically heterogenous interventions. One study reported data on risk of fracture (Stenvall 2007). None of the studies reported adverse‐event data. We are uncertain of the effects of all social environment interventions in hospitals as the quality of the evidence was assessed as very low.
Service model change
Two studies examined implementation of guidelines in acute care settings in hospitals. Koh 2009 (1122 participants) compared multifaceted fall‐prevention guideline implementation with routine dissemination. There was no strong evidence for an effect on the rate of falls (Analysis 19.1.1: RaR 1.82, 95% CI 0.23 to 14.55; very low‐quality evidence, downgraded two levels for risk of bias, one level for indirectness and two levels for imprecision). Van Gaal 2011b (2201 participants) studied the implementation of three guidelines (falls, urinary tract infection, pressure ulcers) targeting nursing staff in comparison with usual care. There was no strong evidence for an effect on the rate of falls (Analysis 19.1.2: RaR 0.67, 95% CI 0.17 to 2.59; very low‐quality evidence, downgraded two levels for risk of bias, and two levels for imprecision). We are uncertain of the effects of guideline implementation on falls as the quality of the evidence is considered very low.
19.1. Analysis.
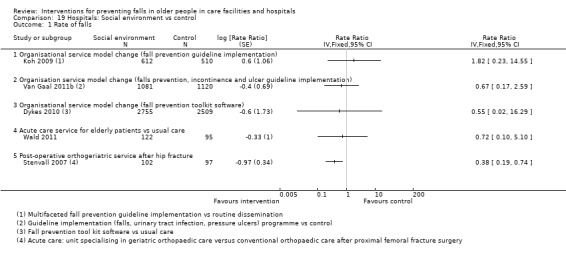
Comparison 19 Hospitals: Social environment vs control, Outcome 1 Rate of falls.
Dykes 2010 (5264 participants) tested the effect of a computer‐based fall‐prevention tool kit in comparison with usual care. There was no strong evidence for an effect on the rate of falls (Analysis 19.1.3: RaR 0.55, 95% CI 0.02 to 16.29) or risk of falling (Analysis 19.2.1 RR 0.91, 95% CI 0.06 to 14.21). We are uncertain of the effectiveness of this intervention (very low‐quality evidence, downgraded two levels for risk of bias, and two levels for imprecision).
19.2. Analysis.
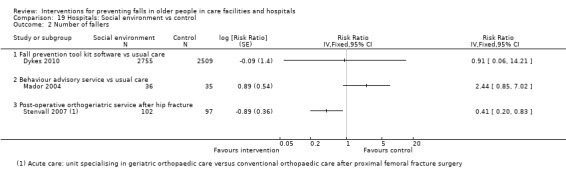
Comparison 19 Hospitals: Social environment vs control, Outcome 2 Number of fallers.
Wald 2011 (217 participants) compared providing care in an acute ward for the elderly with care in general medical wards to usual care. There was no strong evidence for an effect on the rate of falls (Analysis 19.1.4: RaR 0.72, 95% CI 0.10 to 5.10).
Mador 2004 (71 participants) examined a new behavioural advisory service for people with confusion in comparison with usual care. There was no strong evidence for an effect on the risk of falling (Analysis 19.2.2: RR 2.44, 95% CI 0.85 to 7.02).
Stenvall 2007 (199 participants) compared post‐operative care in a ward providing a comprehensive ortho‐geriatric service with usual care in an orthopaedic ward following surgery for hip fracture. This intervention achieved a reduction in the rate of falls (Analysis 19.1.5: RaR 0.38, 95% CI 0.19 to 0.74) and the risk of falling (Analysis 19.2.3: RR 0.41, 95% CI 0.20 to 0.83) at discharge. There were four new fractures in the control group but none in the intervention group (Analysis 19.3.1: RR 0.11, 95% CI 0.01 to 1.52). These findings also applied to the subgroup analysis of patients with dementia (64 participants), i.e. the rate of falls and risk of falling was reduced (RaR 0.07, 95% CI 0.01 to 0.57; RR 0.12, 95% CI 0.02 to 0.85).
19.3. Analysis.
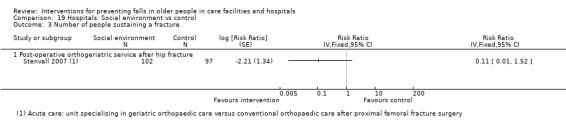
Comparison 19 Hospitals: Social environment vs control, Outcome 3 Number of people sustaining a fracture.
Knowledge interventions
Two trials (3028 participants) examined knowledge interventions in hospitals in individually‐randomised trials. Neither trial reported data on the risk of fracture. Haines 2011 reported that there were no adverse events from interaction with the education materials; Ang 2011 did not report on adverse events.
Ang 2011 (1822 participants), testing an educational session by a trained research nurse targeting individual fall risk factors in patients at high risk of falling in an acute setting and achieved a reduction in risk of falling (Analysis 20.2: RR 0.29, 95% CI 0.11 to 0.74); however, we are uncertain of the effects of this intervention as the quality of the evidence has been assessed as very low (downgraded two levels for risk of bias, one level for indirectness and one level for imprecision).
20.2. Analysis.
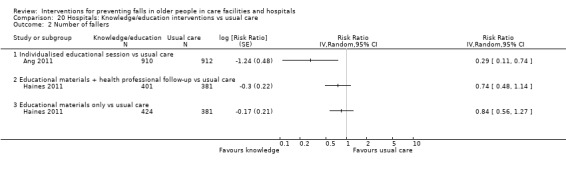
Comparison 20 Hospitals: Knowledge/education interventions vs usual care, Outcome 2 Number of fallers.
Haines 2011 (1206 participants) evaluated two forms of multimedia patient education compared with usual care in a mixture of acute and subacute wards. One intervention consisted of written and video‐based materials plus one‐on‐one bedside follow‐up from a physiotherapist (complete programme) and the other intervention group received educational materials only. Neither intervention showed strong evidence of a reduction in the rate of falls (Analysis 20.1.1 complete programme RaR 0.83, 95%CI 0.54 to 1.27; very low‐quality evidence, downgraded one level for indirectness, one level for inconsistency and one level for imprecision; Analysis 20.1.2 educational materials only RaR 0.91, 95%CI 0.62 to 1.35; low‐quality evidence, downgraded one level for indirectness and one level for imprecision) or risk of falling (Analysis 20.2.2 complete programme RR 0.74, 95%CI 0.48 to 1.14; very low‐quality evidence, downgraded one level for indirectness, one level for inconsistency and one level for imprecision; Analysis 20.2.3 educational materials only RR 0.84, 95% CI 0.56 to 1.27; low‐quality evidence, downgraded one level for indirectness and one level for imprecision). In a post‐hoc subgroup analysis, in participants who were cognitively intact the authors reported that falls were less frequent in those receiving the complete programme, compared with those in the materials only group (adjusted hazard ratio (HR) for rate of falls 0.51, 95% CI 0.28 to 0.93; risk of falling 0.65, 95% CI 0.36 to 1.18; 626 participants) and the control group (adjusted HR for rate of falls 0.43, 95% CI 0.24 to 0.78; risk of falling 0.51, 95%CI 0.28 to 0.94; 590 participants) (test for subgroup differences P < 0.05). There was a higher risk of injurious falls in those with cognitive impairment with the complete programme (7.49 falls per 1000 patient days compared with 2.89 falls per 1000 patient days in the control group; 192 participants). We are uncertain of the effects of the complete educational programme with follow‐up on falls (very low‐quality evidence) but providing educational materials only may make little or no difference to the rate of falls or risk of falling (low‐quality evidence).
20.1. Analysis.
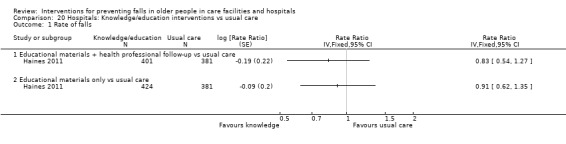
Comparison 20 Hospitals: Knowledge/education interventions vs usual care, Outcome 1 Rate of falls.
Other single interventions
No included studies examined other single interventions in a hospital setting.
Hospitals: multiple interventions
No included studies examined multiple interventions in a hospital setting.
Hospitals: multifactorial interventions
Six trials (45,416 participants) tested the effect of multifactorial interventions in comparison with usual care in a hospital setting (Aizen 2015; Barker 2016; Cumming 2008; Haines 2004; Healey 2004; Hill 2015). Five trials (44,790 participants) were cluster randomised (Aizen 2015; Barker 2016; Cumming 2008; Healey 2004; Hill 2015), and one (626 participants) was individually randomised (Haines 2004). Two trials used a stepped‐wedge design (Aizen 2015; Hill 2015). The categories of interventions for each trial are shown in Appendix 3 and further details are provided in the Characteristics of included studies. A summary of the evidence for multifactorial interventions for falls prevention in hospitals is provided in Table 7. Two studies (4625 participants) reported data on risk of fracture (Cumming 2008; Haines 2004). Four of six trials (39,763 participants) reported on adverse events (Aizen 2015; Barker 2016; Haines 2004; Hill 2015). We have shown whether the settings were acute or subacute in the footnotes of the analyses. Given most of these trials were large with important differences such as in the setting and in the format and delivery of their multifactorial intervention, we present some details of the individual trials first before reporting the pooled analyses.
Aizen 2015 (752 participants) conducted a two‐stage (stepped‐wedge) cluster randomised trial in five geriatric rehabilitation wards. The multifactorial intervention included medical, behavioural, cognitive and environmental modifications with additional orientation guidance and mobility restriction for moderate‐risk patients and permanent personal supervision for high‐risk patients. The usual care arm included any activities undertaken by the participants recommended or administered by their treating team. The authors reported that "No significant difference was found in fall rates during follow‐up between intervention and control wards". The findings of this study were not pooled as some aspects of the study methodology and data collection could not be confirmed.
Barker 2016 (35,264 participants, 46,245 admissions) investigated a “6‐PACK” intervention in comparison with usual care (which included standard falls prevention activities) with a cluster‐randomised trial in 24 acute medical or surgical wards and found no change in rate of falls or risk of falling. There was no evidence of effect on the rate of injurious falls (RaR 0.96, 95% CI 0.72 to 1.27). Data were determined based on admissions, some patients were admitted more than once.
Cumming 2008 (3999 participants) examined an intervention in both acute and subacute wards in which a nurse and physiotherapist each worked for 25 hours per week for three months in all intervention wards. No trial interventions were delivered in the usual care arm. This trial also found no change in the rate of falls or risk of falling. The review authors consider both Barker 2016 and Cumming 2008 to be well‐conducted trials. The interventions they studied would be regarded as sound falls prevention practice including use of falls risk‐assessment tools and supervision for patients at risk but no effect on falls was observed.
The multidisciplinary intervention in Haines 2004 (626 participants) took place in three subacute wards. The programme included a falls risk alert card with an information brochure, exercise, education programme, and hip protectors, in addition to usual care. In the control arm, patients received usual care but none of the interventions from the falls prevention programme; the study staff completed the risk assessment and generated recommendations but none of these recommendations were instituted. The authors reported that the difference in falls between the two groups was "most obvious after 45 days of observation", suggesting that this programme benefited people staying longer in hospital but it could also be explained by long staying frequent fallers in the control group.
Healey 2004 (1654 participants) examined a risk‐factor reduction care plan for patients with a history of falls in a cluster‐randomised trial in eight acute and subacute wards. Interventions included assessment and interventions targeted at eyesight, medications, blood pressure management, mobility, urine testing, bed rail use, bed height, footwear, ward positioning, environmental causes and call bells. In the usual care arm, the care plan was not introduced and no changes to practice or environment relevant to falls prevention were made during the study.
Hill 2015 conducted a stepped‐wedge cluster‐randomised controlled trial in eight hospital rehabilitation and geriatric wards (3121 participants, 3606 admissions), which tested the effect of an individualised multimedia education intervention (also tested in Haines 2011) provided to eligible patients with basic cognition, and staff, aiming to educate patients about falls prevention strategies and to motivate engagement in falls‐prevention strategies (ProFaNE categories of social environment and knowledge). Usual care included patient’s screening, assessment and implementation of individualised falls‐prevention strategies, ongoing staff training and environmental strategies. There was a reduction in the rate of falls (Analysis 21.1: RaR 0.60, 95% CI 0.42 to 0.94). There was also a reduction in the rate of injurious falls (adjusted RaR 0.65, 95% CI 0.42 to 0.88; data analysed by number of admissions rather than participants).
21.1. Analysis.
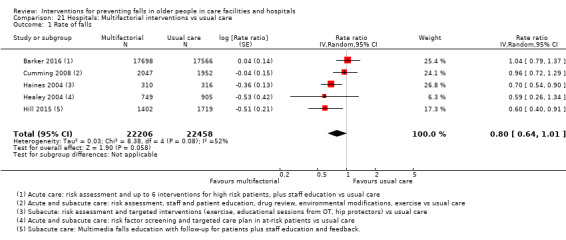
Comparison 21 Hospitals: Multifactorial interventions vs usual care, Outcome 1 Rate of falls.
In a pre‐specified subgroup analysis, Hill 2015 reported that the rate of falls was reduced in people without significant cognitive impairment who received the educational intervention (MMSE > 23/30; adjusted RaR 0.53, 95%CI 0.36 to 0.77, P < 0.001; 1930 participants), but there was no strong evidence for an effect in the subgroup of patients who were cognitively impaired (who did not receive the patient intervention, but may have benefited from the staff training intervention component; adjusted RaR 0.65, 95% CI 0.40 to 1.05; 1676 participants).
Rate of falls
Pooled results from five trials (44,664 participants) of multifactorial interventions showed a borderline reduction in the rate of falls, with a reduction overall of 20%; the 95% confidence intervals indicated this estimate of effect may range as high as a reduction of 36% or result in an increase in falls rates of 1%; (Analysis 21.1: RaR random‐effects 0.80, 95% CI 0.64 to 1.01; 5 trials: I² = 52%; low‐quality evidence, downgraded one level for risk of bias and one level for imprecision; Table 7). These findings were further explored in a subgroup analysis by setting (see below).
Risk of falling
Pooled data from three trials (39,889 participants) of the five trials pooled for the rate of falls outcome were generally consistent with the effect estimate for the rate of falls with a reduction in the risk of falling that did not reach statistical significance (Analysis 21.2: RR random‐effects 0.82, 95% CI 0.62 to 1.09; 3 trials: I² = 0%; very low‐quality evidence; Table 7). Notably Hill 2015 reported a reduction in the risk of falling (adjusted odds ratio (OR) 0.55, 95% CI 0.38 to 0.81) in a subacute setting; however, these data were analysed by number of admissions, rather than participants, so these data were not pooled. The choice of model for the pooled analysis did not affect the estimate of effect as the statistical heterogeneity was 0%. We are uncertain of the effects of multifactorial interventions on risk of falling in hospitals (very low‐quality evidence).
21.2. Analysis.
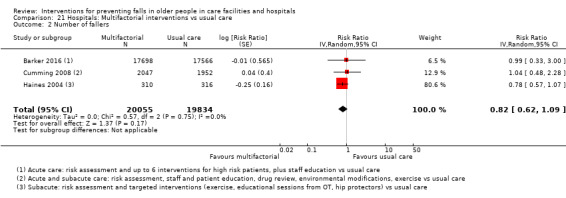
Comparison 21 Hospitals: Multifactorial interventions vs usual care, Outcome 2 Number of fallers.
Risk of fracture
Two trials (4625 participants; Cumming 2008; Haines 2004) reported fracture data suitable for pooling. There was no strong evidence for a reduction in the number of people sustaining a fracture (Analysis 21.3: RR 0.76, 95% CI 0.14 to 4.10: I² = 0%; nine fractures; very low‐quality evidence; Table 7).
21.3. Analysis.
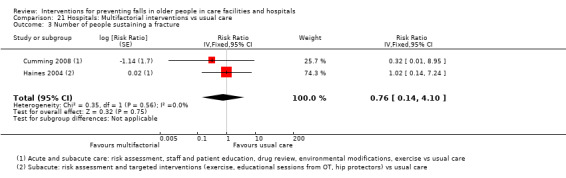
Comparison 21 Hospitals: Multifactorial interventions vs usual care, Outcome 3 Number of people sustaining a fracture.
In Barker 2016, there were very few fractures in an acute setting, with 11 (0.06%) people experiencing a fall‐related fracture in the intervention arm and 13 (0.07%) in the control arm. In Hill 2015, there were six fractures in the control group (three hip fractures) and four in the intervention group (not hip) in a subacute setting; these data represent number of fractures and admissions rather than patients. The data from these two studies are not pooled; however, the results are consistent with the pooled estimate showing no strong effect on the risk of fracture.
We are uncertain whether multifactorial interventions reduce the risk of fracture as the quality of the evidence has been assessed as very low.
Adverse events
No adverse events were reported in the four trials (39,763 participants; Aizen 2015; Barker 2016; Haines 2004; Hill 2015) that reported this outcome. We are uncertain of the effects of multifactorial interventions on adverse events as the quality of the evidence has been assessed as very low (Table 7).
Subgroup analysis by type of care (acute, subacute or mixed settings)
A post‐hoc subgroup analysis was conducted for multifactorial interventions conducted in hospitals for acute care settings, subacute settings or mixed (both subacute and acute) settings. The test for subgroup differences indicated a possible difference between the settings (types of care) for rate of falls (Analysis 22.1, P = 0.04). Pooled data indicate a reduction in the falls rate in trials conducted in the subacute setting (Analysis 22.1.3: RaR 0.67, 95% CI 0.54 to 0.83), but not in the acute (Analysis 22.1.1: RaR 1.04, 95% CI 0.79 to 1.37) or mixed settings (Analysis 22.1.2: RaR 0.88, 95% CI 0.61 to 1.27). There were no differences between subgroups for pooled data by setting for risk of falling (Analysis 22.2, test for subgroup differences P = 0.75) or risk of fracture (Analysis 22.3, test for subgroup differences P = 0.56). One additional study reporting data for the risk of falling and fracture that were not pooled was conducted in a subacute setting (Hill 2015).
22.1. Analysis.
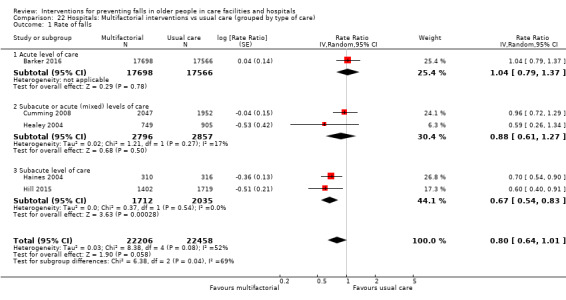
Comparison 22 Hospitals: Multifactorial interventions vs usual care (grouped by type of care), Outcome 1 Rate of falls.
22.2. Analysis.
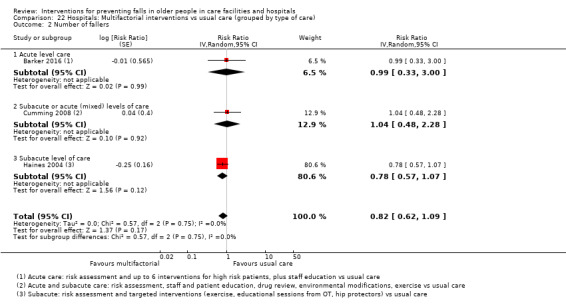
Comparison 22 Hospitals: Multifactorial interventions vs usual care (grouped by type of care), Outcome 2 Number of fallers.
22.3. Analysis.
Comparison 22 Hospitals: Multifactorial interventions vs usual care (grouped by type of care), Outcome 3 Number of people sustaining a fracture.
Multifactorial interventions including targeted patient education may reduce the rate of falls in a subacute setting (low‐quality evidence, downgraded one level for risk of bias and one level for inconsistency due to some uncertainty in the subgroup analysis).
Studies in participants with cognitive impairment
Eleven trials reported findings specifically for patients with dementia or cognitive impairment.
Care facilities
In care facilities, Juola 2015 (227 participants) included 93% of participants with a dementia diagnosis in a trial of nurse education on harmful medications. The intervention showed a reduction in the rate of falls in those with an MMSE score of 10 or greater, but no strong evidence of an effect in those with an MMSE of less than 10. In a trial of a multifactorial intervention (Whitney 2017; 191 participants), 97% of participants were cognitively impaired but the intervention did not show any strong evidence for an effect on the rate of falls or risk of falling.The effects of combination exercise, a multimodal exercise programme, a behaviour advisory service for people with confusion, dementia care mapping, and multisensory stimulation in a Snoezelen room have been examined in people with dementia in several studies (Chenoweth 2009; Klages 2011; Kovacs 2013; Mador 2004; Toulotte 2003; Van de Ven 2014). However, these interventions were tested in single small studies or the studies did not report data suitable for further analysis.Chenoweth 2009 and Buettner 2002 reported costs associated with interventions for participants with dementia in care facilities.
Hospitals
In hospitals, a knowledge‐based intervention that did not show strong evidence for a reduction in the rate of falls overall showed a reduction in falls in those who were cognitively intact, but not in those with cognitive impairment in a post‐hoc analysis (Haines 2011). When the intervention was applied as a multifactorial intervention, only delivered to those with basic cognition, a reduction in both the rate of falls and risk of falling was observed (Hill 2015). In an acute hospital setting, Stenvall 2007 found that a multifactorial intervention including comprehensive geriatric assessment and rehabilitation for people with femoral neck fractures reduced falls in a subgroup with dementia, however the number of participants was low and the evidence assessed as very low quality, so we are uncertain of the effectiveness of this intervention.
Economic evaluations
The 11 studies reporting economic outcomes (nine in care facilities and two in a hospital setting) are summarised in Appendix 10. Only one study (Haines 2013), reported an economic evaluation in terms of the cost to prevent falls.
In a subgroup of hospital inpatients who were cognitively intact, a falls patient education programme in a hospital setting had a cost of AUD 294 to prevent one fall and AUD 526 to prevent one faller (Haines 2013).
Discussion
Summary of main results
This review now includes 95 trials (138,164 participants) of which 71 trials (40,374 participants; mean age 84 years; 75% women) were in care facilities and 24 trials (97,790 participants; mean age 78 years; 52% women) were in hospitals. Despite the addition of 35 trials (77,869 participants) to the previous review, many of the results from the pooled analyses remain inconsistent and inconclusive. Although 24 trials reported data on fractures suitable for use in the analyses, all fracture data were very low‐quality evidence and thus we are uncertain of the effects of any intervention on risk of fracture. Twenty‐nine trials clearly reported data on adverse events, although in several it was to report an absence of adverse events. There were very few serious adverse events and minor complications, where reported, were usually similar in the intervention and control groups. Overall, we are uncertain of the effects on adverse events as the quality of the evidence has been assessed as very low.
Care facilities
Exercise
Twenty‐five trials in care facilities investigated exercise as a single intervention. Despite the large number of trials, many were small (< 100 participants). Only two trials reported the effects of exercise on risk of fracture and nine on adverse events.
Seventeen trials compared an exercise intervention with usual care. A summary of the evidence for exercise in comparison with usual care in care facilities is provided in Table 1. Funnel plots of the pooled trials (10 trials each for rate of falls and risk of falling; plus positive findings in an additional four trials reporting rate of falls that could not be pooled) indicated potential publication bias for this comparison.
In the 10 trials of exercise compared with usual care that were pooled reporting rate of falls, there was considerable heterogeneity in the results, which was only partially explained by a subgroup analysis grouping trials according to level of nursing care provided. We are uncertain whether exercise had an effect on the rate of falls in care facilities as the quality of the evidence has been assessed as very low. Subgroup analyses by type of exercise did not explain the heterogeneity.
There was less statistical heterogeneity in the data on risk of falling for trials of exercise compared with usual care. Pooled data indicated exercise may make little or no difference to the risk of falling (low‐quality evidence).
There was limited evidence for exercise types other than gait, balance and functional training or trials testing a combination of exercise categories in comparison with usual care. Whilst three trials tested Tai Chi programmes (which have been demonstrated to be effective at reducing the risk of falling in a community setting), data were not suitable for pooling.
We are uncertain of the impact of exercise on the risk of fracture or adverse events (very low‐quality evidence).
Nine trials provided 12 comparisons of two different exercise programmes. Comparisons of different types of exercise were all considered of very low quality so we are uncertain of the relative effectiveness of different types of exercise.
While no clear effect on reduction in falls from exercise was identified within the current review, either overall or by subgroups according to level of care or type of exercise, there was a high degree of heterogeneity between the studies. The range of different types of exercise, populations and settings investigated plus the small size of many trials has resulted in only limited evidence being available for any particular combination of these factors. Importantly, the limited evidence does not represent convincing evidence of a lack of effect and the possibility of some types, intensity or duration of exercise being effective for specific populations remains.
Medication (drug target)
Medication review
Twelve studies examined medication review in care facilities. One study reported on the risk of fracture. Two studies reported instances of adverse events.
A summary of the evidence for general medication review in care facilities is provided in Table 2. Pooled results from five trials of general medication review indicated that this intervention may make little or no difference to the rate of falls or risk of falling (low‐quality evidence). We are uncertain of the effect of general medication review on risk of fracture or adverse events as the quality of the evidence has been assessed as very low.
Vitamin D supplementation
Eight trials examined vitamin D interventions in care facilities. Five trials examined the effect of vitamin D supplementation, two trials investigated the effect of daily multivitamin supplementation which included vitamin D and calcium and one tested an education intervention aimed at increasing prescription of adequate levels of vitamin D, calcium and osteoporosis medications. Only three trials reported data on the risk of fracture and five on adverse events.
A summary of the evidence for vitamin D supplementation in care facilities is provided in Table 3. Vitamin D supplementation probably reduces the rate of falls (moderate‐quality evidence) but vitamin D supplementation (with or without calcium) probably makes little or no difference to the risk of falling (moderate‐quality evidence). The 28% reduction in falls rate observed (RaR 0.72, 95% CI 0.55 to 0.95) is substantial. Average serum vitamin D levels at baseline were reported to be low or very low in seven of eight studies (including the five studies of vitamin D with or without calcium supplementation), indicating that these results are applicable to residents of care facilities with low vitamin D levels. Based on other studies, the reduction in the rate of falls may be related to improvement in muscle function (De Spiegeleer 2018).
We are uncertain of the effect of vitamin D supplementation (up to 1000 IU daily) on the risk of fall‐related fractures or adverse events as the quality of the evidence has been assessed as very low. These studies represent only a subset of the studies evaluating the effect of vitamin D on fractures.
We are uncertain whether multivitamin supplementation including vitamin D and calcium reduces the rate or risk of falling based on two studies as the quality of the evidence is very low.
One study of an education intervention aimed at increasing the prescription of vitamin D, calcium and osteoporosis medication may make little or no difference to the rate of falls or risk of falling (low‐quality evidence).
Environment/assistive technology
There were no large trials of this type in care facilities. We are uncertain of the effect on rate of falls of wireless position monitoring in care facilities (very low‐quality evidence).
Social environment
Seven trials in care facilities targeted staff training or implemented service model changes. Two studies reported data on the risk of fracture and no studies reported adverse‐event data. None of the interventions showed strong evidence for a reduction in falls. These interventions included staff education on fall and fracture prevention, a project nurse facilitating best‐practice falls injury prevention strategies, guideline implementation (falls, urinary tract infection, and pressure ulcers), dementia care mapping, a risk‐assessment tool versus nurses' judgement and a programme to improve staff connections, communication, and problem‐solving. Results were inconsistent in two trials of dementia care mapping. Use of a falls risk‐assessment tool in comparison with nurses' judgement alone probably makes little or no difference to the rate of falls or risk of falling (moderate‐quality evidence). We are uncertain of the effect on falls of a half‐day education programme about fall and fracture prevention for staff (very low‐quality evidence). We are uncertain of the impact of the other social environment interventions on falls.
Knowledge/education
There were no trials of knowledge interventions in care facilities.
Psychological interventions
Two studies in care facilities evaluated the effect of psychological interventions on falls. Neither trial reported data on the risk of fracture and adverse‐event data were not reported.
One trial examined a cognitive‐behavioural intervention with a focus on falls‐risk reduction, the other examined a computer‐based cognitive training programme focused on improving attention combined with strength and balance training, compared with strength and balance training alone. We are uncertain of the effects of psychological interventions on rate of falls or risk of falling as the quality of the evidence is very low.
Other single interventions
Three trials (564 participants) examined other single interventions. We are uncertain whether lavender olfactory stimulation, multisensory stimulation in a Snoezelen room or sunlight exposure reduces falls as the quality of the evidence has been assessed as very low.
Multiple interventions
An intervention for incontinent residents in high‐level nursing care facilities that included exercise, offering regular fluids and toileting, showed no strong evidence for an effect and we are uncertain of the effectiveness as the quality of the evidence is very low (Schnelle 2003).
Increased sunlight exposure plus calcium supplementation had low adherence to sunlight exposure; we are uncertain of the effects on falls or adverse events as the quality of the evidence is very low (Sambrook 2012). There was no difference in the incidence rates of new skin cancers, but an increase in the adjusted all‐cause mortality in the calcium‐treated group compared with the UV alone group (hazard ratio (HR) 1.23 versus 0.76, P = 0.03). Despite documented concerns about increased risk of cardiovascular events, in particular myocardial infarction, with calcium supplementation (Bolland 2010), there was a lack of evidence for a strong effect on increased death rates from myocardial infarction, so the biological reason for the observed increase in all‐cause mortality is uncertain. We are uncertain of the effects on adverse events as the quality of evidence is very low.
Multifactorial interventions
In multifactorial interventions, two or more categories of intervention are given, and these are linked to each individual's risk profile. An initial assessment is usually carried out by one or more health professionals and an intervention is then provided or recommendations given or referrals made for further action. All trials compared a multifactorial intervention with 'usual care', which in many cases included some falls‐prevention activities. These standard care practices may have changed over time; however, the degree to which the comparator arm does or does not include components of the intervention activities is not clear enough to base any additional analysis on. A summary of the evidence for multifactorial interventions in comparison with usual care in care facilities is provided in Table 4.
This review included 13 multifactorial trials in care facilities. Five studies reported data on risk of fractures. One study reported an instance of a fall as an adverse event, two studies reported that there were no adverse events, and the remaining studies did not report on adverse events. The interpretation of pooled data from multifactorial interventions is problematic because of variation in components between trials, and variation of combinations of components delivered to individuals in the trials.
Pooled results did not show strong evidence for a reduction in the risk of falling or risk of fracture; however, there was considerable statistical heterogeneity. Multifactorial interventions may make little or no difference to the risk of falling in care facilities (low‐quality evidence). We are uncertain of the effects of multifactorial interventions in care facilities on the rate of falls or risk of fractures as the quality of evidence has been assessed as very low. A post‐hoc subgroup analysis based on high, intermediate or mixed levels of nursing care showed a statistical difference between subgroups, with a reduction in falls in high‐ and intermediate‐level care facilities, but not in studies or facilities with a mixed level of care. As there is no clear external evidence that could explain these subgroup results, and the finding is not completely consistent across studies, the finding is not considered credible (Guyatt 2011a), and no conclusion based on these subgroups is made. Subgroup analysis by level of cognition did not explain the heterogeneity.
Hospitals
Exercise
Three trials in hospitals (244 participants) investigated exercise as a single intervention. Two of these were small, including less than 60 participants. Only one trial reported on adverse events.
The three trials tested the effect of additional physiotherapy in rehabilitation wards (Table 5); however, we are uncertain of the effect of this intervention on rate of falls or whether it reduces risk of falling as the quality of the evidence has been assessed as very low. There were no data available on fractures and the one study reporting on adverse events found none.
Medication (drug target)
Medication review
In hospitals, we are uncertain of the effects of medication review on either rate of falls or risk of falling; this was tested in only one trial (very low‐quality evidence).
Vitamin D supplementation
One trial in an acute geriatric unit found no strong evidence of an effect of vitamin D supplementation on risk of falling, despite the low levels of vitamin D at baseline. The median length of stay was only 30 days. We are uncertain of the effects of vitamin D in hospitals on rate of falls or risk of falling, rate of fracture or adverse events as the quality of the evidence has been assessed as very low.
Environment/assistive technology
Six trials in hospitals investigated environment/assistive technology interventions.
Pooled data from two trials (28,649 participants) were available on the use of bed alarms in hospitals (Table 6). The larger trial, which was a cluster‐randomised trial with 28,551 participants, of bed/chair alarms was an education intervention to support judgement on their use. We are uncertain of the effects of bed alarms on the rate of falls, risk of falling or adverse events as the quality of the evidence has been assessed as very low.
We are uncertain whether carpet flooring, tested in one small trial, increases the rate of falls and risk of falling compared with vinyl flooring (very low‐quality evidence). We are uncertain of the effects on rate of falls or risk of falling of using identification bracelets for patients at high risk.
A large trial of the use of one low‐low bed per 12 existing beds in hospitals had no effect on rate of falls. However, large confidence intervals indicate a lack of precision in the estimate and we are uncertain of the effect of providing low‐low beds on the rate of falls (very low‐quality evidence).
Social environment
Six trials in hospitals targeted staff training or implemented service model changes. One trial in a hospital setting reported data on the risk of fracture. No studies reported adverse‐event data. Trials tested a comprehensive post‐operative ortho‐geriatric service in a geriatric ward for patients with proximal femoral fracture surgery compared with usual care in an orthopaedic ward, guideline implementation, fall‐prevention toolkit software, a new acute care service for elderly patients, and a new behavioural advisory service for people with confusion. We are uncertain of the effects of these interventions on falls as the quality of the evidence has been assessed as very low.
Knowledge/education
Two trials examined knowledge interventions in hospitals. Neither trial reported data on the risk of fracture and one study reported that there were no adverse events.
We are uncertain of the effects of an educational session based on identified risk factors and usual fall‐prevention care in acute medical wards as the quality of the evidence was assessed as very low.
In a mixture of acute and subacute wards, a trial providing patients with educational materials alone and educational materials with professional follow‐up did not show strong evidence for a reduction in the rate of falls (Haines 2011). Providing patients with educational materials alone may make little or no difference to the rate of falls or risk of falling (low‐quality evidence).
In a post‐hoc subgroup analysis, educational materials with professional follow‐up showed a reduction in falls in participants with no cognitive impairment in comparison with usual care. There is moderate credibility for this post‐hoc subgroup analysis (Guyatt 2011a); however, we are uncertain of the effectiveness of this intervention in reducing the rate of falls as the quality of the evidence has been assessed as very low. Due to the contrast between the effectiveness of providing this intervention as a single intervention and its effectiveness when provided as a multifactorial intervention targeted at cognitively intact participants (Hill 2015; which further supports the credibility of the result found in the subgroup analysis within Haines 2011), no conclusion on the effectiveness of this intervention when delivered as a single intervention is made as this is likely to result in difficulty in interpretation.
Psychological interventions
There were no trials of psychological interventions in hospitals.
Other single interventions
There were no trials of other single interventions in hospitals.
Multiple interventions
There were no trials of multiple interventions in hospitals.
Multifactorial interventions
In multifactorial interventions, two or more categories of intervention are given, and these are linked to each individual's risk profile. An initial assessment is usually carried out by one or more health professionals and an intervention is then provided or recommendations given or referrals made for further action. All trials included a comparison with 'usual care' that in many cases included some falls prevention activities. These standard care practices may have changed over time; however, the degree to which the comparator arm does or does not include components of the intervention activities was not clear enough to explore this.
This review included six multifactorial trials in hospitals. Five trials provided data suitable for pooling for the rate of falls, three for the risk of falling. Two studies reported data on risk of fractures. Four studies reported adverse‐event data, there were no adverse events.
The evidence for multifactorial interventions in hospitals is summarised in Table 7. Pooled results showed a borderline reduction in the rate of falls with a point estimate of a reduction of 20%; the 95% confidence intervals indicated this estimate of effect may range as high as a reduction of 36% or result in an increase in falls rates of 1% (Analysis 21.1: RaR random effects 0.80, 95% CI 0.64 to 1.01; 5 trials: I² = 52%); however, there was moderate heterogeneity. The interpretation of pooled data from multifactorial interventions is problematic because of variation in components between trials, and variation of combinations of components delivered to individuals in the trials. A subgroup analysis based on the setting demonstrated a likely significant difference between subgroups. Pooled data from two trials in a subacute setting showed that multifactorial interventions, both included targeted patient education, may reduce the rate of falls (RaR 0.67, 95%CI 0.54 to 0.83; low‐quality evidence).
Pooled results on the risk of falling included only three of the five trials that were pooled for the rates of falls, but the overall effect estimate was generally consistent with the rate of falls, giving a point estimate of a 18% reduction in the risk of falling, with wider 95% confidence intervals indicating this may range between a 38% reduction and a 9% increase (Analysis 21.2: RR random‐effects 0.82, 95% CI 0.62 to 1.09; 3 trials: I² = 0%). This did not achieve statistical significance, but one of the additional trials that was not pooled also reported a reduction in the risk of falling based on admissions in a subacute setting (Hill 2015; 3121 participants). No difference between subgroups by setting was observed. We are uncertain of the effects on risk of falling as the quality of the evidence was assessed as very low.
We are uncertain of the effect of multifactorial interventions on the risk of fracture or adverse events as the quality of the evidence has been assessed as very low.
Subgroup analyses by level of care partly explained the heterogeneity, but due to variations in study design there is some uncertainty if findings are due to the setting or other factors, including the specific combination of interventions provided. Multifactorial interventions that include targeted patient education may reduce the rate of falls in a subacute setting (low‐quality evidence).
A cost‐effectiveness analysis from one trial of multifactorial interventions is to be published (Hill 2014 protocol for Hill 2015).
Studies in participants with cognitive impairment
There is limited evidence for interventions to reduce falls in people with cognitive impairment where these people are a clearly defined group. Although only 11 trials reported findings specifically for patients with dementia or cognitive impairment, many participants in care facilities trials, including those testing interventions that probably or may reduce falls (e.g. vitamin D supplementation), had cognitive impairment.
Economic evaluations
A cost‐effectiveness analysis of a patient education programme reduced falls in a subgroup of hospital patients who were cognitively intact (Haines 2011). In this subgroup the intervention, which consisted of written and video‐based materials plus one‐on‐one bedside follow‐up from a trained health professional, cost AUD 294 to prevent one fall and AUD 526 to prevent one person falling (2008 dollars; reported in Haines 2013).
No conclusions can be drawn from the other 10 trials reporting economic outcomes.
Overall completeness and applicability of evidence
Although we have included 95 trials in this review, these have tested a very wide variety of interventions, sometimes with different comparators rather than control or usual care, in various types of facility. Approximately three quarters of included trials were conducted in care facilities, however many of these were small.
In this review, we have reported results from care facilities and hospitals separately to improve applicability of the interventions to each setting. Careful consideration of the context of effective interventions is required. As Becker 2010 points out, the type of care provided in care facilities differs between countries and healthcare systems. Also, consideration needs to be taken of cultural and organisational contexts when generalising the results from this review. Unfortunately, the level of care and case mix in each facility in this review was often not clearly defined. In addition there is striking variability in type, targeting, intensity and duration of the falls prevention programmes that were studied. Reports of trials in hospitals are also unlikely to adequately describe the complex interaction that is likely to occur between the intervention and the usual falls‐prevention practices occurring within hospitals.
Twenty‐five trials of exercise in care facilities were included, 17 of which tested exercise with usual care. However, many of these were small and whilst there were a number of trials examining balance, gait or functional training exercise programmes, there were few trials on flexibility, strength/resistance training and 3D exercise (including Tai Chi). There were several comparisons of different exercise programs; however, there was generally only one small trial for each comparison so the data were too few to be informative.
The quality of available evidence for vitamin D supplementation was reasonable (moderate‐quality evidence). However, there were few studies of vitamin D supplementation taken in the form of a multivitamin. Trials of environmental/assistive technologies and social environment (e.g. staff training, service model changes) generally studied clinically different interventions, precluding pooling of trial results. Whilst there was a very large trial of bed alarms conducted in hospitals, this trial was of education, training and support for their use and there were no trials of bed alarms in care facilities. Medication review is generally aimed at reducing psychoactive medications. There were a number of trials of medication review in care facilities considered clinically similar enough to justify pooling. However, there was a large degree of inconsistency in the trial findings.
The interpretation of the multifactorial interventions is complex because of the variation in components, duration and intensity of the intervention, and how the interventions were implemented. The study design does not allow evaluation of individual components of the interventions in either care facilities or hospitals.
Only one trial specifically assessed the benefit of using a validated falls risk‐assessment tool in comparison with clinical judgement in a care facility (Meyer 2009) and none did in hospital, although this approach is widely used in both settings. Some multifactorial trials (e.g. Barker 2016) used validated falls risk‐assessment tools to determine the application of appropriate interventions, but the effects of the falls risk‐assessment tool cannot be separated from that of the interventions. This lack of evidence calls into question the wide use of these tools internationally and further trials examining the effectiveness of the tools are warranted.
Few trials have incorporated interventions relating to the circumstances of falls, e.g. assistance with toileting, rather than targeting individual risk factors, as in the continuous quality improvement model used to develop a falls‐prevention programme in Lohse 2012.
The comparator in many trials is 'usual care'. Frequently, what falls‐prevention activities are included as a component of usual care is not clearly reported. This hinders interpretation of how 'usual' care may change over time and any potentially useful subgroup analyses based on this.
In terms of outcomes, 30 of the included trials did not report usable data for calculating rate of falls and 36 trials for risk of falling (seeAppendix 7). Many studies reporting data suitable for pooling reported data for one but not both of these outcomes. This may explain some of the inconsistency between the findings. Even fewer studies reported the impact of the interventions on fractures or adverse events. Within those studies that did report on adverse events, it was often unclear if these data were recorded systematically. Studies that reported data on fractures reported outcomes for different types of fractures (e.g. hip fractures only versus total fractures). Other studies not eligible for inclusion in this review may provide additional evidence for the impact of the interventions on fractures. In particular, whilst a larger proportion of included studies reported data on the risk of fracture following vitamin D supplementation, it is important to consider that these trials represent only a subset of the studies evaluating the effect of vitamin D on fractures available. In addition, some trials of interventions that may increase falls during the intervention period (exercise, medication review) only reported falls during the post‐intervention period. Other studies report only a subset of falls (e.g. bedside falls in Sahota 2014), and therefore do not meet the inclusion criteria for this review. Many cluster‐randomised trials did not adjust for clustering, therefore this was performed post‐hoc by the review authors (as indicated by a "c" in Appendix 7, for details see Unit of analysis issues).
Vitamin D supplementation in care facilities reduced the rate of falls but not the risk of falling. This discrepancy might be explained by differential effects on multiple fallers (i.e. those falling more than once over the study period). However, too few of these trials reported data on multiple fallers to enable meaningful analysis of this outcome.
Only Haines 2011 included a cost‐effectiveness evaluation of their hospital patient education programme in terms of falls prevented to inform the value for money for the intervention tested. An economic evaluation of the intervention tested in Hill 2015 is still to be published.
Many of the interventions studied would be difficult to sustain in usual clinical practice due to competing factors in the clinical environment. In aged‐care settings, vitamin D supplementation is relatively cheap, and once it commences as part of a person’s regular medication regimen it can be continued indefinitely. In hospital settings, educating staff and patients regarding falls prevention would be regarded as good clinical practice and is sustainable in the long term provided the necessary resources are available.
There is scope for realigning clinical practice with less emphasis on use of scales to assess falls risk (because there is no convincing research evidence of their effectiveness) and encouraging clinical staff to focus on factors that may be more effective, for example educating patients and families about falls and how to avoid them.
Quality of the evidence
This review containing 95 trials (138,164 participants) does not provide robust evidence regarding effective interventions for reducing falls in the settings considered. We assessed the quality of the evidence using the GRADE approach which considers the risk of bias, inconsistency, indirectness, imprecision and other biases (including publication bias) for the evidence for each outcome of the main comparisons. The GRADE assessments are reported in Table 1 to Table 7 and the findings are cross‐referenced in the relevant results sections. The GRADE quality of evidence for many outcomes was low or very low. This largely reflects the risk of bias in the individual studies and also the significant heterogeneity and imprecision in many of the pooled study estimates.
Despite the addition of 35 trials in this update, this has generally not improved the robustness of the results compared with the previous version of this review (Cameron 2012). Although there are now a number of trials conducted for some interventions types (e.g. exercise, medication review and vitamin D supplementation in care facilities and multifactorial interventions in hospitals), the overall quality of the evidence was low to very low for all outcomes and comparisons except for rate and risk of falling for vitamin D supplementation, and use of a falls risk‐assessment tool, all in care facilities. There was also evidence indicating potential publication bias in trials of exercise conducted in care facilities.
Studies in this review varied widely in their risk of bias (seeTable 11). The majority of included studies all contained some risk of bias. The included studies illustrated the wider problems of variation in the methods of ascertaining, recording, analysing, and reporting falls described in Hauer 2006. Many trials have used a single approach for ascertaining the number of falls, the limitations of this have been demonstrated in a study of falls data derived from a large hospital based randomised controlled trial (Hill 2010). For some aspects of study design, minimisation of bias is difficult. For example, it is not possible to blind participants and treatment providers for exercise, bed alarms and other types of interventions. Falls were generally recorded by nursing or care home staff who were frequently not blinded to the intervention. In addition, not all studies met the contemporary standards of the extended CONSORT statement (Schulz 2010), including the extensions for cluster‐randomised trials (Campbell 2004), non‐pharmacological trials (Boutron 2008), and pragmatic randomised trials (Zwarenstein 2008), so reporting was unclear in many instances, particularly for allocation concealment or selective outcome reporting when no protocol could be identified.
There is a potential for differences between individually‐ and cluster‐randomised trials. This review included a large proportion of cluster‐randomised trials (44%). Within this review, in general trials were more likely to be cluster randomised or not depending on the intervention being investigated and the setting. Thus, whilst five of six trials of multifactorial interventions in hospitals (enrolling 99% of participants), and 85% of those conducted in care facilities (82% of participants) were cluster randomised, in contrast for trials of exercise in care facilities, 88% of trials with 65% of participants were individually randomised. Similarly, for trials of vitamin D supplementation in care facilities, 75% of trials (with 60% of participants) were individually randomised. Although it has been reported that contamination, or 'herd effects' in individually‐randomised trials conducted in facilities may result in decreasing the estimate of effect (Hahn 2005), this is considered unlikely to have had a major impact on the estimates of effect or conclusions for this review. The reasons for this according to the major categories of intervention are described below.
For trials of exercise in care facilities, the estimates of effect of the three cluster‐randomised trials that contributed to pooling (Kerse 2008; Rosendahl 2008; Yokoi 2015), did not appear to differ to the range of estimates for the individually‐randomised trials. For vitamin D in care facilities, as the single cluster‐randomised trial contributing to the pooled result (Law 2006) had a smaller estimate of effect compared to the individually‐randomised trials, this indicates that contamination of the control group was unlikely to have played a role in the estimate of effect, which increases the confidence in the effect estimate. For medication review in care facilities, there was a more even balance of individually‐ and cluster‐randomised trials; 58% of trials (62% of participants) were individually randomised. The estimates of effect from the trials were inconsistent within both the cluster‐ and individually‐randomised trials, thus the high inconsistency of findings between trials for this intervention cannot be explained by the type of randomisation used. Two cluster‐randomised trials contributed only 18% of the participants for the evidence for multifactorial interventions in care facilities, the estimates of effect in these two trials were similar to that for the pooled overall effect estimates. All trials of additional exercise in care facilities were individually randomised. In trials of bed exit alarms in hospitals, only two trials contributed to pooled data; 96% of participants were enrolled in one trial that was cluster randomised, thus consideration of the findings of trials that were individually in comparison with cluster randomised is uninformative. Similarly, comparisons of individually‐ and cluster‐randomised trials within multifactorial interventions in hospitals are not feasible given 99% of participants were enrolled in cluster‐randomised trials.
There was significant unexplained heterogeneity in the findings for the rate of falls for several comparisons (exercise, medication review and multifactorial in care facilities), which limited the confidence in the results (seeTable 1, Table 2 and Table 4), and was reflected in the generally low quality of evidence. The heterogeneity may be due to variations in intervention components, duration, intensity and settings as well as variations in the populations.
The evidence for some ProFaNE categories of interventions contained a degree of indirectness, where the intervention was a recommendation for, or education on, use of the intervention, rather than implementing the intervention for all participants (e.g. Kennedy 2015 for vitamin D, Shorr 2012 for bed alarms). In addition, where evidence was from a single trial or setting, it was likely to be considered to have a degree of limited applicability, or indirectness to other settings, (e.g. Sambrook 2012 which examined sunlight exposure in Australia).
There was also imprecision in some estimates, where the number and size of trials was small (seeTable 5) or in particular for the risk of fracture where few trials reported this outcome and events were infrequent (e.g. vitamin D Table 3).
There was some evidence for likely publication bias for trials in exercise, where the included studies appeared to include a disproportionate number of small studies with positive findings (seeFigure 4, Figure 5) .
Potential biases in the review process
We attempted to minimise publication bias in the review by searching multiple databases, and drew on the handsearch results published in the Cochrane Library in the Cochrane Central Register of Controlled Trials (CENTRAL). We also contacted authors of studies identified in trials registers that were completed, but for which full reports had not been identified, studies where only conference abstracts were identified, and many studies where it was unclear whether or not they met the inclusion criteria. We placed no foreign language restrictions in our search strategy; two studies were published in languages other than English (Peyro Saint Paul 2013; Salvà 2016), correspondence with authors provided information on study methods and results. However, despite these efforts, evidence of likely publication bias in trials of exercise conducted in care facilities remained.
Although the majority of screening of search citations for potentially eligible studies in this update was performed by only one author, we suggest this was not a source of bias given that the screening was over‐inclusive with the onus being given to obtaining full‐text reports for all potentially eligible studies. We observe also that where screening was undertaken by two review authors, the progression to full‐text review was reduced.
Five newly published studies that were identified in the top‐up search in August 2017 await classification (Dever 2016; Hewitt 2014; Raymond 2017; Van der Linden 2017; Wylie 2017). This was a pragmatic decision taken in view of the delay that would have resulted from their likely inclusion and after consideration of the potential impact of these trials on review findings. We concluded that our decision to postpone the inclusion of these five trials was not an important source of bias.
Whilst we strictly applied a priori inclusion and exclusion criteria to the selection of studies for this review, which should minimise bias, this does result in the inclusion of a subset of the available evidence and this applies in particular to risk of fracture outcome. All included studies were required to present data on the overall rate of falls or risk of falling, those reporting only a subset of falls (e.g. injurious falls, bedside falls) were excluded. We also excluded 22 trials reporting falls as adverse effects, although in some instances the intervention might plausibly have reduced falls. For a more comprehensive systematic review of the effect of vitamin D supplementation on fractures, see Avenell 2014.
For single‐trial comparisons, we took a different approach to GRADE assessment where a single rater checked whether the trial findings for each outcome met pre‐specified criteria for downgrading the evidence. The criteria were established before this alternative assessment took place. For 26 single‐trial comparisons these criteria were met. For 18 comparisons in 16 trials these criteria did not apply, generally because of a large trial size, and GRADE assessment was conducted in duplicate. For these assessments, in two trials (three outcomes), the quality of the evidence was considered moderate (Chapuy 2002; Meyer 2009), and in three trials (five outcomes) the quality of the evidence was considered low (Cox 2008; Haines 2011; Kennedy 2015); for all other comparisons and outcomes the quality of the evidence was considered very low.
There are potential biases within the data included in the review in terms of non‐normal distribution of falls rates in the included studies (as seen in Potter 2016), missing data including the loss of clusters within some trials, selective outcome reporting (seeTable 11), decisions regarding pooling of studies where there is high heterogeneity and selection of models used for meta‐analyses where there is heterogeneity for one falls outcome, but not another (e.g. high heterogeneity for rate of falls but not risk of falling). The potential biases due to these factors are captured by the GRADE assessments of the overall quality of evidence (Table 1 to Table 7). There are also potential biases in decisions to conduct post‐hoc subgroup and sensitivity analyses (e.g. Analysis 5.4; seeSubgroup analysis and investigation of heterogeneity and Sensitivity analysis). This has been taken into account in conducting GRADE assessments (e.g. confidence in the credibility of subgroup analysis is considered in the inconsistency rating for the subgroup analysis by setting for multifactorial interventions in hospitals), making cautious interpretations of the findings (e.g. considering findings based on subgroup analysis by setting for multifactorial interventions in care facilities of low credibility) and transparently reporting these analyses under Differences between protocol and review.
We explored the possibility of publication bias by constructing funnel plots of trials of exercise in care facilities and multifactorial interventions in care facilities (Figure 4, Figure 5, Figure 6). There was some asymmetry in the falls outcomes for trials of exercise in care facilities indicating potential publication bias.
Using the generic inverse variance method in this review enabled us to pool results as reported by trial authors with our own calculated from raw data, and results adjusted for clustering.
The ProFaNE falls prevention taxonomy enabled us to pool similar interventions in the analyses using a systematic approach. However, classification of some interventions according to this taxonomy was unclear and required judgement in some cases. We consulted with the ProFaNE authors when necessary.
Agreements and disagreements with other studies or reviews
We searched for other systematic reviews of falls prevention initiatives in care facilities and hospitals published since 2012 within our search described in Appendix 1. We compared our review results with the Cochrane Review 'Interventions for preventing falls in older people living in the community' (Gillespie 2012), and identified six other systematic reviews incorporating meta‐analyses (Chan 2015; Le Blanc 2015; Sherrington 2017; Silva 2013; Stubbs 2015; Vlaeyen 2015).
Comparison with trials in community‐living older people
In contrast to the findings in this review for residents of care facilities and hospital inpatients, the evidence is clear that falls can be prevented using exercise in older people living in the community (Gillespie 2012). The effectiveness of group‐based and home‐based exercise programmes and Tai Chi in particular is well established in the community setting. There is the potential for falls to be reduced in care facilities using the same multiple‐component exercise programmes, but despite 25 trials in this review testing exercise programmes in care facilities, the results were inconsistent. Only three trials examined exercises in hospitals; the quality of the evidence was considered very low.
Vitamin D supplementation may reduce falls in community‐living people with lower vitamin D levels (Gillespie 2012). This is consistent with the finding in this review that vitamin D is effective in reducing falls in care facilities as most residents have low vitamin D levels (Pilz 2012).
The effects of multifactorial approaches are inconsistent between trials and settings. In the community setting, multifactorial interventions, including falls‐risk assessment, reduced the rate of falls but not the risk of falling (Gillespie 2012). Similarly, multifactorial interventions overall may make little or no difference to the risk of falling in care facilities. However, findings on the rate of falls were inconsistent. In hospitals, multifactorial interventions (that include targeted patient education) may reduce the rate of falls in a subacute hospital setting.
There is some evidence that falls prevention strategies in the community can be cost saving (Gillespie 2012), but there were no economic evaluations conducted within the care facilities and only one in hospital trials (Haines 2011) to provide information on value for money for effective interventions.
Supplementary review
Nyman 2011 conducted a supplementary review of the 41 trials included in Cameron 2010 with specific reference to people’s recruitment, retention in the trial, and adherence to intervention components. Adherence was high for individually‐targeted and group‐based exercise (72% to 89%) and for medication interventions (68% to 88%). The authors reported that adherence was related to treatment effectiveness in three studies testing medication and multifactorial interventions in care facilities. They estimated that by 12 months, on average, only a third of care‐facility residents are likely to be adhering to falls prevention interventions. The current review was not able to comment on adherence or retention. Nyman 2011 provides an important perspective giving context to interpretation of the research.
Exercise
Chan 2015 conducted a systematic review of exercise interventions for older adults with cognitive impairment, only three of seven trials in a pooled analysis enrolled participants living in a care setting. Two of these studies were included in this review (Toulotte 2003 and Rosendahl 2008), but Chan 2015 included unpublished subgroup data for Rosendahl 2008, and Rolland 2007 and was excluded from this review as falls were monitored as adverse events.
Sherrington 2017 conducted a systematic review and meta‐analysis of exercise interventions to prevent falls in older adults. This review included 14 RCTs (15 comparisons) of exercise interventions in care settings and found no significant effect on the rate of falls. These authors observed possible asymmetry in the funnel plot, which was not statistically significant on Egger's test. Three of the trials included in Sherrington 2017 were excluded from this review (DeSure 2013; Resnick 2002; Rolland 2007; seeCharacteristics of excluded studies). Two of the trials included in the pooled estimate in Sherrington 2017 were considered as multiple interventions under the ProFaNE classification system in this review (Huang 2016, ; seeAppendix 3). Data reported for one study were considered not suitable for pooling in this review (Toulotte 2003). All other trials were included.
Silva 2013 included 12 studies of exercise in care facilities. This review pooled studies of exercise as a single intervention with studies of exercise as a component of a multifactorial intervention. The authors found a significant reduction in the risk of falling (RR 0.71, 95% CI 0.64 to 0.92, I² = 72%). There was no significant effect on the risk of fracture (RR 0.57, 95% CI 0.21 to 1.57). All of the included trials were included in our review.
Lee 2017 included 21 studies of exercise in care facilities, 15 with exercise as a single intervention, six with exercise combined with one or more interventions. Data were pooled from studies comparing exercise with other interventions, usual care or placebo. In the current review, comparisons of alternate exercise programs were not pooled with trials of exercise in comparison with usual care (for details see Table 9). Three of the trials included in Lee 2017 were excluded from this review (DeSure 2013; Lord 2003b; Wolf 2003); two of these were considered to be conducted in a community setting. Data from one trial were not pooled in our review as there were zero falls in the intervention arm (Cadore 2014); this study has a weighting of 0.4% in the meta‐analysis in Lee 2017. Pooled data of trials of exercise as a single intervention in Lee 2017 found no difference in the rate of falls or risk of falling, consistent with the findings of our review.
The current review found inconsistent effects for exercise in care facilities and is broadly consistent with Silva 2013 and Sherrington 2017 although pooling combinations differed. Our review contrasts with Chan 2015 as Chan 2015 pooled trials across both community and care facility settings and much of the impact observed in their meta‐analysis may have been from trials conducted in the community.
Vitamin D supplementation
A systematic review conducted for the US Preventative Services Task Force (Le Blanc 2015), examining trials conducted in both institutionalised or community settings, found that vitamin D significantly reduced the number of falls per person but did not significantly reduce the risk of falling, consistent with the findings in care facilities in this review. The authors reported that sensitivity analysis based on institutionalised status "resulted in similar estimates". The two included studies conducted in institutionalised settings are included in this Cochrane Review. The authors concluded that "Treatment of vitamin D deficiency in asymptomatic persons might reduce mortality risk in institutionalised elderly persons and risk for falls but not fractures."
Bolland 2014 pooled outcomes from six randomised trials conducted in care facilities or hospitals and found no significant reduction in falls with vitamin D supplementation with or without calcium supplementation (RR 0.96, 95% CI 0.88 to 1.05). The authors concluded that supplementation with vitamin D does not reduce risk of falling by a 'clinically relevant' threshold of 15% or more and that future trials are unlikely to alter this conclusion. One study included as institutional in the Bolland 2014 review was excluded from this review as 51% of participants were residing in the community (Graafmans 1996); all other studies were included in this review. This Cochrane Review has analysed studies conducted in care facilities or hospitals separately and found that whilst vitamin D supplementation did not reduce the risk of falling, it did reduce the rate of falls in care facilities. Our analysis included data on the rate of falls in care facilities from the same four studies pooled for the risk of falling and whilst there was heterogeneity for the pooled rate of falls outcome (I² = 62%), it was lower than observed in Bolland 2014 when pooling studies in either setting (I² = 92%).
Other recent systematic reviews
Vlaeyen 2015 included 13 randomised controlled trials of fall‐prevention programmes conducted in nursing homes. The authors found no significant effect of the interventions overall on the number of falls (10 studies) or risk of falling (six studies). They reported that multifactorial interventions significantly reduced the number of falls (four studies) and the number of recurrent fallers (four studies), but not the risk of falling (four studies). They reported that staff training and education had a significant harmful effect on the number of falls (two studies). All trials were included in our review.
Stubbs 2015 conducted an umbrella review of meta‐analyses in care facilities and hospitals and concluded that there was consistent evidence that multifactorial interventions reduce falls in care facilities and hospitals and reported that there was consistent evidence that exercise and vitamin D reduces falls in care facilities, based on the inclusion of nine individual meta‐analyses including Cameron 2012, Bolland 2014 and Sherrington 2011 (Sherrington 2017 is discussed above). Other meta‐analyses included in Stubbs 2015 and published since 2012 were Choi 2012, Guo 2014 and Santesso 2014. Choi 2012 pooled three studies conducted in care settings, all of which were included in this review: a vitamin D trial (Broe 2007), a multifactorial trial (Neyens 2009), and Rapp 2008, which is included as a subgroup analysis of Becker 2003 in our review. Guo 2014 conducted an 'exploratory meta‐analysis' examining fall‐prevention interventions for those with or without cognitive impairment in institutionalised and non‐institutionalised settings. Eight trials included in Guo 2014 were not considered for our review as they had been assessed as being conducted in the community setting: all eight trials were considered in Gillespie 2012, seven of which were included (Conroy 2010, Davison 2005, Haines 2009, Hendriks 2008, Latham 2003, Lightbody 2002, Lord 2005) and one of which was excluded because falls were reported as adverse events (Vogler 2009). Santesso 2014 conducted a meta‐analysis of hip protectors; as we consider hip protectors are intended to reduce fractures rather than falls, this intervention is not included in our review.
Authors' conclusions
Implications for practice.
We found evidence of effectiveness for some fall‐prevention interventions in care facilities and hospitals, although for many the quality of the evidence was considered low or very low. For all interventions, we are uncertain of their effects on fractures and on adverse events as the quality of the evidence for both outcomes was assessed as very low. For each setting, the summary is structured by the main categories of interventions evaluated in at least one setting in the review: exercise, medication (medication review; vitamin D supplementation); psychological interventions, environment/assistive technology, social environment, interventions to increase knowledge, other interventions, multiple interventions and multifactorial interventions. There was a lack of evidence on surgery, management of urinary incontinence, or fluid or nutrition therapy in both settings.
Care facilities
-
Exercise
We are uncertain of the effect of exercise on the rate of falls as the quality of the evidence was assessed as very low. Exercise may make little or no difference to the risk of falling (low‐quality evidence; Table 1).
-
Medication
General medication review may make little or no difference to the rate of falls or risk of falling (low‐quality evidence); Table 2.
The prescription of vitamin D in care facilities probably reduces rate of falls (moderate‐quality evidence), but prescription of vitamin D (with or without calcium) probably makes little or no difference to the risk of falling (moderate‐quality evidence); Table 3.
An education intervention aimed at increasing the prescription of vitamin D, calcium and osteoporosis medication may make little or no difference to the rate of falls or risk of falling (low‐quality evidence).
-
Environment/assistive technology
There is a general lack of evidence on these interventions in care facilities.
We are uncertain of the effect on rate of falls of wireless position monitoring in care facilities (very low‐quality evidence).
-
Social environment
Use of a falls risk‐assessment tool in comparison with nurses' judgement alone probably makes little or no difference to the rate of falls or risk of falling (moderate‐quality evidence).
We are uncertain of the effects on falls of a half‐day education programme about fall and fracture prevention for staff given by specialist osteoporosis nurses in care facilities (very low‐quality evidence).
We are uncertain of the effects on falls of other interventions targeting staff and the organisation of care on falls, including guideline implementation and dementia care mapping (very low‐quality evidence).
-
Knowledge/education
There is a lack of evidence on these interventions in care facilities.
-
Psychological interventions
We are uncertain of the effects on falls of a cognitive‐behavioural intervention with a focus on falls risk reduction (very low‐quality evidence).
We are uncertain of the effects on falls of a computer‐based cognitive training programme focused on improving attention (very low‐quality evidence).
-
Other single interventions
We are uncertain whether lavender olfactory stimulation, multisensory stimulation in a Snoezelen room or sunlight exposure reduces falls (very low‐quality evidence).
-
Multiple interventions
We are uncertain about the effect on falls of a multiple intervention for incontinent residents that included exercise, offering regular fluids and toileting (very low‐quality evidence).
We are uncertain about the effect on falls of a multiple intervention comprising increased sunlight exposure plus calcium supplementation (very low‐quality evidence).
-
Multifactorial
We are uncertain of the effects of multifactorial interventions on the rate of falls (very low‐quality evidence). Multifactorial interventions may make little or no difference to the risk of falling (low‐quality evidence); Table 4.
Hospitals
-
Exercise.
We are uncertain whether providing additional physiotherapy in subacute wards has an effect on the rate of falls or whether it reduces the risk of falling (very low‐quality evidence); Table 5.
-
Medication
We are uncertain of the effect of medication review on either rate of falls or risk of falling (very low‐quality evidence).
We are uncertain of the effect of vitamin D supplementation on either rate of falls or risk of falling (very low‐quality evidence).
-
Environment/assistive technology
We are uncertain of the effect of bed sensor alarms on the rate of falls or risk of falling (very low‐quality evidence); Table 6.
We are uncertain whether carpet flooring, tested in one small trial, increases the rate of falls and risk of falling compared with vinyl flooring (very low‐quality evidence).
We are uncertain of the effects on rate of falls or risk of falling of using identification bracelets for patients at high risk of falling (very low‐quality evidence).
We are uncertain of the effect of providing low‐low beds on the rate of falls (very low‐quality evidence).
-
Social environment
We are uncertain of the effects of interventions targeting staff and the organisation of care (including guideline implementation) on rate of falls or risk of falling (very low‐quality evidence).
-
Knowledge or education
We are uncertain of the effects on falls of an educational session based on identified risk factors and usual fall‐prevention care in acute medical wards (very low‐quality evidence).
Providing patients with educational materials alone may make little or no difference to the rate of falls or risk of falling (low‐quality evidence).
-
Psychological interventions
There is a lack of evidence on these interventions in hospitals.
-
Other single interventions
There is a lack of evidence on whether or not falls risk‐assessment tools and associated interventions reduce falls.
-
Multiple interventions
There is a lack of evidence on these interventions in hospitals.
-
Multifactorial intervention
Multifactorial interventions may reduce the rate of falls, although subgroup analysis suggest this may apply mostly to a subacute setting (low‐quality evidence). We are uncertain of the effects of multifactorial interventions on the risk of falling (very low‐quality evidence); Table 7.
Implications for research.
Further research, primarily randomised controlled trials, is warranted to help inform decisions in this key area. We suggest the following guide to help discussions on future priorities.
Further research into supervised exercise programmes in both settings. There is a particular need for larger trials in care facilities and trials that clearly describe the care needs of the participants.
Further research to strengthen the evidence for multifactorial interventions in both settings. Of note is that there are some substantial individual trials that have shown an important effect in reducing the rate of falls. A key feature of these multifactorial interventions is the individualised nature of the interventions delivered. This implies that further research with emphasis on an individualised, standardised approach to delivery of interventions with consistent description and application within further trials is warranted, including as a clear description of existing falls prevention practices in the control arm of any trials and the interaction of the intervention arm of the trial with usual care. A mixed methods approach may be necessary to achieve this.
Further trials of patient‐directed interventions, especially in care facilities; for example, with a psychological and educational focus.
Trials with interventions incorporating approaches based on the circumstances of falls in addition to individual risk factors, e.g. regular assisted toileting in both care facilities and hospitals (Lohse 2012; Schnelle 2003).
Further trials testing the routine use of validated falls risk‐assessment tools.
Further research is required testing interventions targeting staff, and changes to the organisational system in which an intervention is delivered or the introduction of new healthcare models.
In care facilities, additional trials on medication review, vitamin D plus calcium supplementation, environmental/assistive technologies and social environment interventions are required. There should be an emphasis on large trials.
In hospitals, more trials of additional exercise, social environment and knowledge interventions are needed.
Further research focusing on participants with dementia.
Other aspects, including research methods, that need to be adopted in all future studies are as follows.
Classification of the components of the fall‐prevention intervention using the taxonomy developed by the Prevention of Falls Network Europe (ProFaNE) (Lamb 2007; Lamb 2011). This will produce consistency between trials allowing for more effective pooling of data.
Consideration is needed of the nature of 'usual care' and its potential interaction with the intervention group.
For multifactorial trials, clear descriptions are needed of the components and the proportion of the participants receiving the different interventions.
Falls data should be collated by a researcher blind to group allocation.
Fall events should be reported by group as total number of falls, fallers, and people sustaining a fall‐related fracture or brain injury; rate of falls (falls per person year or per 1000 patient days); multiple fallers and number in each analysis.
Results should be analysed using appropriate, pre‐specified methodology (e.g. negative binomial regression, survival analysis) (Robertson 2005). Group comparisons should be expressed as incidence rate ratios and risk ratios with 95% confidence intervals.
Authors of trials not excluding people with cognitive impairment should plan to report the results by level of cognitive impairment to indicate whether degree of impairment is an effect modifier.
Design and reporting of trials should meet the contemporary standards of the extended CONSORT statement including those relating to randomised sequence generation and allocation concealment prior to randomisation (Schulz 2010). Pragmatic trials and those testing non‐pharmacological interventions should incorporate the requirements defined in Zwarenstein 2008 and Boutron 2008.
Clear description of usual care in the control arms of trials and discussion of the interaction of the intervention with this is needed.
Design and reporting of cluster randomised trials should follow contemporary guidance (Campbell 2004) including the reporting of intra‐class correlation coefficients.
Where factorial designs are employed, data for each treatment cell should be reported to allow interpretation of possible interactions between different intervention components (McAlister 2003).
There is a clear need for further research clearly reporting on the cognitive status of the included participants and including those with cognitive impairment.
Economic evaluations should be conducted alongside randomised controlled trials to establish the cost‐effectiveness of each intervention being tested. This involves measuring health‐related quality of life as an outcome, defining the perspective and timeframe for costs, collecting data on healthcare use, costing healthcare resources, calculating cost‐effectiveness ratios (if the intervention is effective in reducing falls), and evaluating uncertainty. Guidelines for carrying out and reporting economic evaluations in falls prevention trials have been published (Davis 2011).
Feedback
Feedback: "inaccurate assumptions and errors in calculations", 8 November 2019
Summary
According to the Cochrane Handbook for Systematic Reviews of Interventions (1), systematic reviews summarize the results of controlled healthcare trials to provide a high level of evidence on the effectiveness of healthcare interventions. This evidence is then used to enlighten judgments about the evidence and to inform practice recommendations. While fall prevention in hospitals has been studied for several decades, much of the evidence has been inconclusive. Our team read this review with great interest hoping that it would inform practice recommendations (2).
Unfortunately, this most recent fall prevention systematic review is again inconclusive and furthermore adds to the confusion related to the benefits of fall prevention interventions in hospital settings. However, the reported limitations relate to inaccurate assumptions and errors in calculations made by the systematic review authors, rather than the quality of the studies and associated evidence. For example, in relation to our team’s study, Cameron et al reported the following: "Dykes 2010 (5264 participants) tested the effect of a computer‐based fall prevention tool kit in comparison with usual care. There was no strong evidence for an effect on the rate of falls (Analysis 19.1.3: RaR 0.55, 95%CI 0.02 to 16.29) or risk of falling (Analysis 19.2.1 RR 0.91, 95% CI 0.06 to 14.21)." To calculate the confidence interval for rate of falls, Cameron et al used a formula that did not consider the effect of matching in their analyses. As noted by Imai (2009), matching greatly reduces the standard error in cluster‐randomised experiments (3). Based on our data, the RaR 0.55 has 95% CI 0.36 to 0.83 and the p‐value for that test that pRaR=1 was p =.005 (this is the same p‐value for the test that the rate difference = 0 as published in our results paper (4)). In addition, Cameron et al incorrectly reported the relative risk of falling for patients in our study as .91, rather than .61 (see Table 3 of our results paper [(34/2755)/(51/2509) = .61]). Perhaps this was a typographical error or a miscalculation but given the difference in results found by the systematic review authors compared to those reported by our team in Journal of the American Medical Association (4), Cameron and colleagues should have contacted us to clarify their results, rather than risk including inaccurate data in their systematic review and meta‐analysis. The inaccurate assumptions and miscalculations associated with our clinical trial call into question the rigor and accuracy of Cameron et al’s systematic review since the authors calculated rate ratios, risk ratios, and 95% confidence intervals for many of the included studies and therefore they may have made similar errors in other calculations that they made for other studies they evaluated.
The inaccurate assumptions and calculation errors in this systematic review further perpetuate the myth that patient falls in hospitals are not preventable. It is our hope that Cochrane will refine its systematic review methodology to include a process for systematic review authors to check their assumptions with study authors to ensure that accurate results are reported that can be used to enlighten judgments and to inform practice recommendations. Cochrane reviews carry substantial weight; they should have high methodological standards.
1. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
2. Cameron ID, Dyer SM, Panagoda CE, Murray GR, Hill KD, Cumming RG, Kerse N. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No.: CD005465. DOI: 10.1002/14651858.CD005465.pub4.
3. Imai K, King G, and Nall, C. The essential role of pair matching in cluster‐randomized experiments, with application to the Mexican universal health insurance evaluation. Statistical Science 2009;24(1):29‐53.
4. Dykes PC, Carroll DL, Hurley A, Lipsitz S, Benoit A, Chang F, et al. Fall prevention in acute care hospitals: A randomized trial. JAMA 2010;304(17):1912‐8
Reply
Thank you for your feedback. We agree that Cochrane reviews hold to a high standard for evidence quality to inform formal recommendations. The authors of this review share your frustration that the evidence is, on the whole, insufficient to be certain of the effects of the various and often complex interventions used for falls prevention in the settings covered in our review.
Cochrane reviews are undertaken using standard methods that need to be applied to all studies. For cluster randomised trials, these methods also involve adjustment for clustering. The approach taken in our review is detailed in the methods section under Unit of analysis issues. Of note is that these methods do not include consideration of matching of clusters in the results.
Your trial, Dykes 2010, was added to the review in the previous 2012 update. We can confirm that the analysis of the trial was done in an appropriate manner that was consistent with all other cluster randomised trials within the review. As the results for number of fallers was not reported as an adjusted risk ratio for falling within the trial report, adjustment was necessary and performed. The adjustment for clustering requires rounding to whole numbers of participants for determination of the risk ratio, and thus the reported 0.91 rather than 0.61 is not an error but a consequence of rounding following adjustment for a relatively small number of clusters (8 clusters, adjusted values intervention 1/58 versus control 1/53; RR 0.91). For all versions of this review, the authors have approached trial investigators for missing data and clarification where necessary; your trial report was considered sufficiently reported not to need this action.
We hope this explanation will restore your faith in our review.
Contributors
Feedback from: Patricia C Dykes1,2, Jason S Adelman3, Michael Bogaisky4, Ann C Hurley1, David W Bates1,2 and Stuart R Lipsitz1,2. (1Center for Patient Safety, Research and Practice, Brigham and Women’s Hospital, Boston, MA; 2Harvard Medical School, Boston, MA; 3Columbia University Irving Medical Center/New York‐Presbyterian, New York, NY; 4Albert Einstein College of Medicine/Montefiore Medical Center, Bronx, NY) Reply from: Suzanne Dyer and Ian Cameron (review authors) Editors: Cathie Sherrington (Feedback Editor; Cochrane Bone, Joint and Muscle Trauma Group) and Helen Handoll (Co‐ordinating Editor, Cochrane Bone, Joint and Muscle Trauma Group)
What's new
| Date | Event | Description |
|---|---|---|
| 27 January 2020 | Amended | Feedback and response added |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 1, 2010
| Date | Event | Description |
|---|---|---|
| 7 September 2018 | Amended | NIHR acknowledgement added |
| 10 August 2018 | New search has been performed | For this update, the following changes were made.
|
| 10 August 2018 | New citation required and conclusions have changed | New evidence, the reclassification of some intervention categories and the implementation of new methods, including assessment of the quality of the evidence using GRADE, has resulted in some changed conclusions. Changes made to authorship, including addition of new authors. |
| 27 February 2013 | Feedback has been incorporated | Changes relate to two pieces of feedback, received 19 December 2013 and 12 February 2013. Two Summary [of feedback] and Reply entries were added to the Feedback section. There were no changes to the review in relation to the second piece of feedback. Changes in relation to the first piece included: 1. Appendix 6 was revised and Appendices 7 and 8 were deleted. 2. A new Appendix 7, containing raw data, was added. 3. Sections of the review (principally, the 'Description of studies') were revised to reflect these changes. |
| 9 November 2012 | New search has been performed | For this update, published in Issue 12, 2012, the following changes were made: 1. Search updated to March 2012 2. Twenty additional trials (35,270 participants) included in this update 3. One previously included trial recruiting people post stroke (Barreca 2004) excluded, as no longer within the scope of this version of the review 4. Kerse 2008 reclassified as an exercise intervention (formerly multifactorial) 5. Additional trials testing multifactorial interventions with results for subgroups with and without cognitive impairment 6. Evidence relating to additional interventions, these include: patient education in hospital (Ang 2011; Haines 2011), dementia care mapping (Chenoweth 2009), motion sensors (Clifton 2009), decision‐support software (Dykes 2010; Lapane 2011), multivitamin supplementation (Grieger 2009), low‐low beds (Haines 2010), multisensory stimulation (Klages 2011), guideline implementation (Koh 2009; Van Gaal 2011a; Van Gaal 2011b), a fall risk assessment tool (Meyer 2009), increased sunlight exposure (Sambrook 2012), lavender oil stimulation (Sakamoto 2012), an acute care service for elderly people (Wald 2011) 7. One newly included trial included a cost‐effectiveness analysis (Chenoweth 2009) 8. Background section revised and citations updated 9. 'Risk of bias' item relating to 'Allocation concealment' split into two: 'Sequence generation' and 'Allocation concealment' and applied to all included studies 10. Subgroup analyses revised |
| 9 November 2012 | New citation required and conclusions have changed | 1. In response to the external referee's comments, the title of this review has been changed to reflect the fact that facilities which do not include nursing care are also included in this review. 2. Change in conclusion for multifactorial interventions in care facilities from no evidence of effect to a suggestion of possible benefits. Evidence from one trial for the effectiveness of an educational session targeting identified risk factors in acute hospital setting. |
| 30 November 2009 | Amended | Correction of two minor errors |
| 23 September 2009 | Amended | The published review 'Interventions for preventing falls in elderly people' (Gillespie 2003) is not being updated. Due to its size and complexity it was split into two reviews: 'Interventions for preventing falls in older people living in the community' and 'Interventions for preventing falls in older people in nursing care facilities and hospitals' |
| 1 April 2009 | Amended | Converted to new review format |
Acknowledgements
The authors would like to acknowledge the considerable contributions of Leslie Gillespie and Clare Robertson to earlier versions of the review, and Clare Robertson for support on data management and statistical calculations. The authors would like to thank Lindsey Elstub and Joanne Elliott for their support at the editorial base.
We thank the following for their useful and constructive comments on this version of the review: Dr Joanne Elliott, Dr Helen Handoll, Prof Finbarr Martin, and Prof Cameron Swift.
We thank the following for their useful and constructive comments on earlier versions of the protocol and/or review: Assoc Prof Jacqueline Close, Dr Simon Gates, Dr Helen Handoll, Prof Peter Herbison, Prof Finbarr Martin, Prof Cathie Sherrington, and Dr Janet Wale. We are grateful to Prof Sarah Lamb, Prof Clemens Becker and Dr Klaus Pfeiffer for their assistance with use of the ProFaNE taxonomy, and to Prof Peter Herbison for his advice on statistical issues in previous versions of the review. We are also grateful to Prof William Gillespie and Dr Mohit Arora for assistance in assessing the risk of bias for some previously included studies and Dr Mohit Arora for screening trial registry records in the top‐up search. We thank Geraldine Wallbank of the George Institute for Global Health, Sydney for her assistance in completing Appendix 7 for previously included studies.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the National Health Service (NHS) or the Department of Health. Funding for immediate open access was received from the NIHR Cochrane review gold open access scheme.
Appendices
Appendix 1. Search strategies (2012 to February 2016)
For this update the searches were modified to broaden sensitivity and bring them in line with current Cochrane guidelines. Previous search strategies are given in Cameron 2012.
The search process was run in two stages: the first search was run in February 2016 and a second top‐up search was run in August 2017.
CENTRAL 2016, Issue 2 (Cochrane Central Register of Studies Online)
Initial search (February 2016)
#1 MESH DESCRIPTOR Accidental Falls (945) #2 MESH DESCRIPTOR Hip Fractures EXPLODE ALL TREES WITH QUALIFIERS PC (122) #3 (falls or faller*):TI,AB,KY (2980) #4 #1 OR #2 OR #3 (3051) #5 MESH DESCRIPTOR Aged EXPLODE ALL TREES (863) #6 (older or senior* or elderly):TI,AB,KY (35860) #7 #5 OR #6 (36186) #8 #4 AND #7 (1491) #9 MESH DESCRIPTOR Residential Facilities EXPLODE ALL TREES (1269) #10 MESH DESCRIPTOR Long‐Term Care (989) #11 MESH DESCRIPTOR Institutionalization (159) #12 MESH DESCRIPTOR Hospitalization (3772) #13 MESH DESCRIPTOR Subacute Care (9) #14 MESH DESCRIPTOR Hospitals EXPLODE ALL TREES (2630) #15 MESH DESCRIPTOR Hospital Units (173) #16 MESH DESCRIPTOR Rehabilitation Centers (233) #17 MESH DESCRIPTOR Inpatients (631) #18 MESH DESCRIPTOR Geriatric Assessment (1117) #19 ((long stay or long term or acute or sub‐acute or subacute or residential or hospital) adj3 (care or ward* or hospital)):TI,AB,KY (9444) #20 ((rehabilitation or geriatric) adj (ward* or hospital* or unit* or department*)):TI,AB,KY (2261) #21 (hostel* or nursing home*):TI,AB,KY (2109) #22 inpatient*:TI,AB,KY (7336) #23 residen*:TI,AB,KY (7244) #24 institution*:TI,AB,KY (8275) #25 #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 (36817) #26 #8 AND #25 (495) #27 18/04/2012 TO 29/02/2016:DL (261267) #28 #26 AND #2 (7214) #29 * NOT INMEDLINE NOT INEMBASE AND 18/04/2012 TO 29/02/2016:DL (61657) #30 #28 AND #29 (7)
Top‐up search (August 2017)
#27 29/02/2016 TO 31/08/2017:DL (146249) #28 #26 AND #27 (120)
MEDLINE (OvidSP)
Initial search (February 2016)
1 Accidental Falls/ or exp Hip Fractures/pc [Prevention & Control] (18380) 2 (falls or faller$).tw. (33218) 3 or/1‐2 (42468) 4 exp Aged/ or Middle Aged/ (4118285) 5 (older or senior$ or elderly).tw. (473795) 6 or/4‐5 (4287430) 7 and/3,6 (21348) 8 exp Residential Facilities/ (45187) 9 Long‐Term Care/ (22760) 10 Institutionalization/ or Hospitalization/ (84278) 11 Subacute Care/ (757) 12 exp Hospitals/ (230464) 13 Hospital Units/ (9255) 14 Rehabilitation Centers/ (7271) 15 Inpatient/ (14941) 16 Geriatric Assessment/ (20228) 17 ((long stay or long term or acute or sub‐acute or subacute or residential or hospital) adj3 (care or ward$1 or hospital)).tw. (744645) 18 ((rehabilitation or geriatric) adj (ward$1 or hospital$1 or unit$1 or department$1)).tw. (7183) 19 (hostel$1 or nursing home$).tw. (24258) 20 inpatient.tw. (51064) 21 residen$.tw. (170300) 22 institution$.tw. (191229) 23 or/8‐22 (1281719) 24 and/7,23 (6980) 25 Randomized controlled trial.pt. (406953) 26 Controlled clinical trial.pt. (90108) 27 randomized.ab. (336211) 28 placebo.ab. (166425) 29 Drug therapy.fs. (1819658) 30 randomly.ab. (242642) 31 trial.ab. (347439) 32 groups.ab. (1517503) 33 or/25‐32 (3659217) 34 exp Animals/ not Humans/ (4187037) 35 33 not 34 (3146945) 36 24 and 35 (1918) 37 (2012* or 2013* or 2014* or 2015* or 2016*).ed,dc. (4739332) 38 36 and 37 (660)
Top‐up search (August 2017)
37 (2016* or 2017*).ed,dc,yr. (2902640) 38 36 and 37 (444)
Embase (OvidSP)
Initial search (February 2016)
1 Falling/ or exp Hip fracture/pc (30681) 2 (falls or faller$).tw. (42331) 3 or/1‐2 (60124) 4 Aged/ or Middle Aged/ (2951209) 5 (older or senior$ or elderly).tw. (623077) 6 or/3‐4 (2990799) 7 and/3,6 (60124) 8 Residential Home/ or Nursing Home/ or Assisted Living Facility/ (48670) 9 Halfway House/ or Long Term Care/ (102560) 10 Hospitalization/ (243942) 11 Institutional Care/ or Residential Care/ or Home For The Aged/ or Institutionalization/ (29979) 12 exp Hospital/ or Hospital Patient/ (893392) 13 Rehabilitation Center/ (10566) 14 ((long stay or long term or acute or sub‐acute or subacute or residential or hospital) adj3 (care or ward$1 or hospital)).tw. (1054527) 15 ((rehabilitation or geriatric) adj (ward$1 or hospital$1 or unit$1 or department$1)).tw. (11032) 16 (hostel$1 or nursing home$).tw. (30080) 17 inpatient.tw. (78633) 18 residen$.tw. (208729) 19 institution$.tw. (287669) 20 or/8‐19 (2160272) 21 and/7,20 (15557) 22 exp Randomized Controlled Trial/ or exp Single Blind Procedure/ or exp Double Blind Procedure/ or Crossover Procedure/ (443586) 23 (random* or RCT or placebo or allocat* or crossover* or 'cross over' or trial or (doubl* adj1 blind*) or (singl* adj1 blind*)).ti,ab. (1472662) 24 22 or 23 (1551624) 25 (exp Animal/ or animal.hw. or Nonhuman/) not (exp Human/ or Human cell/ or (human or humans).ti.) (5440113) 26 24 not 25 (1369711) 27 21 and 26 (1849) 28 (2012* or 2013* or 2014* or 2015* or 2016*).em,dd. (6468106) 29 27 and 28 (849)
Top‐up search (August 2017)
28 (2016* or 2017*).dd,yr. (2947022) 29 27 and 28 (362)
CINAHL (EBSCOhost)
Initial search (February 2016)
S1 (MH "Accidental Falls") (14,702) S2 TI ( (falls or faller or fallers) ) OR AB ( (falls or faller or fallers) ) (18,518) S3 S1 or S2 (25,905) S4 (MH "Aged+") (554,747) S5 TI ( (senior or seniors or elderly or older) ) OR AB ( (senior or seniors or elderly or older) ) (154,950) S6 S4 or S5 (606,645) S7 S3 and S6 (12,500) S8 (MH "Residential Facilities+") (24,586) S9 (MH "Long Term Care") (20,495) S10 MH Hospitalization OR MH institutionalisation (22,416) S11 (MH "Subacute Care") (1,163) S12 (MH "Hospitals+") (82,740) S13 (MH "Hospital Units") (5,365) S14 (MH "Rehabilitation Centers") (6,003) S15 TX (long stay or acute or sub‐acute or subacute or residential) N3 (care or ward or wards or hospital*) (42,572) S16 TX (rehabilitation or geriatric) N1 (ward* or hospital* or unit* or department*) (27,626) S17 TX hostel OR TX hostels (342) S18 TI inpatient OR AB inpatient (23,497) S19 TI residen* OR AB residen* (44,727) S20 TI institution* OR AB institution* (42,946) S21 TX nursing home (49,403) S22 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 (306,197) S23 S7 AND S22 (3,504) S24 PT Clinical Trial (79,124) S25 (MH "Clinical Trials+") (196,188) S26 TI clinical trial* OR AB clinical trial* (51,126) S27 TI ( (single blind* or double blind*) ) OR AB ( (single blind* or double blind*) ) (23,585) S28 TI random* OR AB random* (166,482) S29 S24 OR S25 OR S26 OR S27 OR S28 (302,149) S30 S23 AND S29 (496) S31 EM 2012 OR EM 2013 OR EM 2014 OR EM 2015 OR EM 2016 (1,482,299) S32 S30 AND S31 (145)
Top‐up search (August 2017)
S31 EM 2016 OR EM 2017 (1,830,054) S32 S30 AND S31 (169)
WHO ICTRP
Initial search (February 2016)
fall* AND prevent* OR fall AND reduc* (368 records for 361 trials)
Top‐up search (August 2017)
89 additional records identified
ClinicalTrials.gov
Initial search (March 2016)
(fall OR falls OR falling) AND (prevention OR prevent OR reduce OR reduction)
Interventional Studies
received from 01/01/2012 to 22/03/2016
551 records
Top‐up search (August 2017)
232 additional records identified
Appendix 2. 'Risk of bias' assessment criteria
| Bias | Judgement of risk of bias: LOW, HIGH, or UNCLEAR |
|
Random sequence generation Relating to selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
According to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions. |
|
Allocation concealment Relating to selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
According to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions. In cluster randomised trials, if patients were recruited following allocation of the cluster, this was considered as high risk. The timing of recruitment of individuals to clusters was considered within this domain. |
| Blinding of participants and personnel Relating to performance bias due to knowledge of the allocated interventions by participants and personnel carrying out the interventions | According to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions. |
|
Blinding of outcome assessment Relating to detection bias due to knowledge of the allocated interventions by outcome assessors |
According to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions. |
|
Incomplete outcome data Relating to attrition bias due to amount, nature or handling of incomplete outcome data |
According to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions. For cluster‐randomised trials, potential bias due to loss of clusters was considered within this domain. |
|
Selective outcome reporting Relating to bias due to the selective reporting or non reporting of findings |
According to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions. Where no protocol was identified, but all expected falls outcomes were reported and appropriate adjustments for clustering were performed, a ‘low risk’ rather than unclear judgement was made. |
| Method of ascertaining falls Relating to bias in the recall of falls due to unreliable methods of ascertainment | All studies were assessed as follows. Judgement of ’Low risk’ if the study used a clear definition of falls plus some form of concurrent collection of data about falling, e.g. staff recorded falls daily on a hospital register. Judgement of ’High risk’ if ascertainment relied on participant recall at longer intervals than one month during the study or at its conclusion, or if there were important differences in the methods of ascertainment of falls between study arms, or falls were poorly defined. Judgement of ’Unclear’ if there was retrospective recall over a short period only, or a definition of falls was not described, or details of ascertainment were not described, i.e. insufficient information was provided to allow a judgement of ’Low risk’ or ’High risk’. |
|
Bias resulting from major baseline imbalances Relating to bias resulting from major imbalances in key baseline characteristics |
Judgement of 'Low risk' if good comparability of groups, or confounding adjusted for in analysis. Judgement of ‘High risk’ if imbalance in characteristics likely to impact on falls rate (particularly age, previous falls/falls risk, medical status, dependency, cognitive function) and confounding not adjusted for in analysis. Judgement of ‘Unclear’ if not discussed. |
Appendix 3. Settings, combinations and categories of interventions (ProFaNE) for each included study
|
Setting/ Combination |
Study ID | Exercises | Medication (drug target) | Management of urinary incontinence | Fluid or nutritional therapy | Environment/ assistive technology | Social environment | Knowledge | Other |
| CARE FACILITIES | |||||||||
| Single | Bischoff 2003 | **** | |||||||
| Broe 2007 | **** | ||||||||
| Buckinx 2014 | **** | ||||||||
| Buettner 2002 | **** | ||||||||
| Cadore 2014 | **** | ||||||||
| Chapuy 2002 | **** | ||||||||
| Chenoweth 2009 | **** | ||||||||
| Choi 2005 | **** | ||||||||
| Clifton 2009 | **** | ||||||||
| Colon‐Emeric 2013 | **** | ||||||||
| Cox 2008 | **** | ||||||||
| Crotty 2004a | **** | ||||||||
| Crotty 2004b | **** | ||||||||
| da Silva Borges 2014 | **** | ||||||||
| Houghton 2014 | **** | ||||||||
| Faber 2006 | **** | ||||||||
| Flicker 2005 | **** | ||||||||
| Frankenthal 2014 | **** | ||||||||
| Fu 2015 | **** | ||||||||
| Garcia Gollarte 2014 | **** | ||||||||
| Grieger 2009 | **** | ||||||||
| Huang 2016 (CB) | **** Psychological |
||||||||
| Imaoka 2016 (RED EX) | **** | ||||||||
| Imaoka 2016 (Vit D) | **** | ||||||||
| Irez 2011 | **** | ||||||||
| Juola 2015 | **** | ||||||||
| Kennedy 2015 | **** | ||||||||
| Kerse 2008 | **** | ||||||||
| Klages 2011 | **** Multisensory stimulation | ||||||||
| Kovacs 2012 | **** | ||||||||
| Kovacs 2013 | **** | ||||||||
| Lapane 2011 | **** | **** | |||||||
| Law 2006 | **** | ||||||||
| Meyer 2009 | **** | ||||||||
| Mulrow 1994 | **** | ||||||||
| Nowalk 2001 | **** | ||||||||
| Patterson 2010 | **** | ||||||||
| Peyro Saint Paul 2013 | **** | ||||||||
| Potter 2016 | **** | ||||||||
| Rosendahl 2008 | **** | ||||||||
| Sakamoto 2006 | **** | ||||||||
| Sakamoto 2012 | **** Lavender patches |
||||||||
| Sambrook 2012 (UV) | **** Sunlight |
||||||||
| Saravanakumar 2014 | **** | ||||||||
| Schoenfelder 2000 | **** | ||||||||
| Serra‐Rexach 2011 | **** | ||||||||
| Shimada 2004 | **** | ||||||||
| Sihvonen 2004 | **** | ||||||||
| Sitja Rabert 2015 | **** | ||||||||
| Streim 2012 | **** | ||||||||
| Toulotte 2003 | **** | ||||||||
| Tuunainen 2013 | **** | **** | |||||||
| Van de Ven 2014 | |||||||||
| Van Gaal 2011a | **** | ||||||||
| Van het Reve 2014 | **** Psychological |
||||||||
| Ward 2010 | **** | ||||||||
| Yokoi 2015 | **** | ||||||||
| Zermansky 2006 | **** | ||||||||
| Multiple | Huang 2016 | **** | **** | ||||||
| Imaoka 2016 | **** | **** | |||||||
| Schnelle 2003 | **** | **** | **** | ||||||
| Sambrook 2012 (UV+) | **** | **** Sunlight |
|||||||
| Multifactorial | Beck 2016 | **** | **** | ||||||
| Becker 2003 | **** | **** | **** | **** | |||||
| Dyer 2004 | **** | **** | **** | **** | **** Podiatry referral |
||||
| Jensen 2002 | **** | **** | **** | **** | |||||
| Kerse 2004 | **** | **** | **** | **** | |||||
| McMurdo 2000 | **** | **** | **** | **** | |||||
| Neyens 2009 | **** | **** | **** | **** | |||||
| Ray 1997 | **** | **** | **** | **** | |||||
| Rubenstein 1990 | **** | **** | |||||||
| Salvà 2016a | **** | **** | **** | **** | |||||
| Shaw 2003 | **** | **** | **** | ||||||
| Walker 2015 | **** | **** | **** | **** | **** | **** | |||
| Whitney 2017 | **** | **** | **** | **** | |||||
| HOSPITALS | |||||||||
| Single | Ang 2011 | **** | |||||||
| Burleigh 2007 | **** | ||||||||
| Donald 2000 (2 x 2 factorial) | **** | **** | |||||||
| Dykes 2010 | **** | ||||||||
| Haines 2010 | **** | ||||||||
| Haines 2011 | **** | ||||||||
| Jarvis 2007 | **** | ||||||||
| Koh 2009 | **** | ||||||||
| Mador 2004 | **** | ||||||||
| Mayo 1994 | **** | ||||||||
| Michalek 2014 | **** | ||||||||
| Shorr 2012 | **** | ||||||||
| Stenvall 2007 | **** | ||||||||
| Tideiksaar 1993 | **** | ||||||||
| Treacy 2015 | **** | ||||||||
| Van Gaal 2011b | **** | ||||||||
| Wald 2011 | **** | ||||||||
| Wolf 2013 | **** | ||||||||
| Multifactorial | Aizen 2015 | **** | **** | **** | **** | Psychological | |||
| Barker 2016 | **** | **** | **** | ||||||
| Cumming 2008 | **** | **** | **** | **** | **** | ||||
| Haines 2004 | **** | **** | **** | ||||||
| Healey 2004 | **** | **** | **** Opthalmology referral | ||||||
| Hill 2015 | **** | **** |
aLikely types of interventions based on falls risk factors assessed, actual interventions instigated unclear
Abbreviations
CB: cognitive behavioural RED EX: reduced exercise UV: increased sunlight exposure group. UV+: increased sunlight exposure + calcium supplementation group
Appendix 4. Categories of exercise (ProFaNE) by study setting and combination
| Study setting/type | Study ID | Gait/balance/ functional training | Strength/ resistance training | Flexibility | 3D (Tai Chi, dance etc) | General physical activity | Endurance | Other |
| CARE FACILITIES | ||||||||
| Single | Buckinx 2014 | **** | **** | **** (WBV) | ||||
| Buettner 2002 | **** | **** | **** | **** | **** | |||
| Cadore 2014 | **** | **** | **** | |||||
| Choi 2005 | **** | |||||||
| da Silva Borges 2014 | **** | **** | ||||||
| Faber 2006 (FW) | **** | |||||||
| Faber 2006 (IB) | **** | **** | **** | **** | ||||
| Fu 2015 | **** | |||||||
| Imaoka 2016 | **** | |||||||
| Irez 2011 | **** | **** | **** | |||||
| Kerse 2008 | **** | |||||||
| Kovacs 2012 | **** | **** | **** | **** | ||||
| Kovacs 2013 | **** | **** | **** | **** | ||||
| Mulrow 1994 | **** | **** | **** | |||||
| Nowalk 2001 (FNBF) | **** | **** | ||||||
| Nowalk 2001 (LL/TC) | **** | |||||||
| Rosendahl 2008 | **** | **** | ||||||
| Sakamoto 2006 | **** | |||||||
| Saravanakumar 2014 (Tai Chi) | **** | |||||||
| Saravanakumar 2014 (Yoga) | **** | |||||||
| Schoenfelder 2000 | **** | **** | ||||||
| Serra‐Rexach 2011 | **** | **** | **** | |||||
| Shimada 2004 | **** | |||||||
| Sihvonen 2004 | **** | |||||||
| Sitja Rabert 2015 | **** | **** | **** (WBV) | |||||
| Toulotte 2003 | **** | **** | **** | |||||
| Tuunainen 2013 (MF) | **** | |||||||
| Tuunainen 2013(MFB) | **** | **** | ||||||
| Yokoi 2015 | **** | **** | ||||||
| Multiple | Huang 2016 | **** | **** | |||||
| Imaoka 2016 | **** | |||||||
| Schnelle 2003 | **** | **** | ||||||
| Multifactorial | Beck 2016 | **** | **** | |||||
| Becker 2003 | **** | **** | ||||||
| Dyer 2004 | **** | **** | **** | **** | ||||
| Jensen 2002 | **** | **** | ||||||
| McMurdo 2000 | **** | **** | **** | |||||
| Neyens 2009a | ||||||||
| Salvà 2016 | **** | **** | **** | **** | ||||
| Shaw 2003 | **** | **** | **** | |||||
| Walker 2015a | ||||||||
| Whitney 2017 | **** | |||||||
| HOSPITALS | ||||||||
| Single | Donald 2000 (EX) | **** | ||||||
| Jarvis 2007 | **** | **** | **** | |||||
| Treacy 2015 | **** | |||||||
| Multifactorial | Cumming 2008 | **** | ||||||
| Haines 2004 | **** | **** | **** |
a No description of the exercise components
Abbreviations
EX: supplementary exercises FNBF: 'Fit NB Free' group FW: 'Functional Walking' group IB: 'In Balance' group LL/TC: 'Living and learning/Tai Chi' group MF: muscle force MFB: muscle force & balance WBV: whole body vibration
Appendix 5. Categories of environment/assistive technology interventions (ProFaNE) by study setting and combination
| Study setting/type | Study ID | Furnishing/adaptations | Personal mobility aids | Communication/signalling aids | Body worn care/protection aids | Other environmental |
| CARE FACILITIES | ||||||
| Single | Clifton 2009 | **** | ||||
| Multifactorial | Becker 2003 | **** | **** | **** | ||
| Dyer 2004 | **** | |||||
| Jensen 2002 | **** | **** | **** | **** | ||
| Kerse 2004 | **** | **** | **** | |||
| McMurdo 2000 | **** | |||||
| Neyens 2009 | **** | **** | ||||
| Ray 1997 | **** | **** | ||||
| Rubenstein 1990 | **** | |||||
| Salvà 2016a | ||||||
| Shaw 2003 | **** | **** | **** | |||
| Walker 2015 | **** | **** | **** | |||
| Whitney 2017 | **** | **** | **** | |||
| HOSPITALS | ||||||
| Single | Donald 2000 (FL) | **** | ||||
| Mayo 1994 | **** | |||||
| Haines 2010 | **** | |||||
| Shorr 2012 | **** | |||||
| Tideiksaar 1993 | **** | |||||
| Wolf 2013 | **** | |||||
| Multifactorial | Aizen 2015 | **** | **** | **** | ||
| Barker 2016 | **** | **** | ||||
| Cumming 2008 | **** | **** | **** | |||
| Haines 2004 | **** | **** | ||||
| Healey 2004 | **** | **** | **** | **** | ||
| Stenvall 2007 | **** Home visit by OT and/or PT |
aNo clear description of types of environment/assistive technology
Abbreviations
FL: carpet flooring group OT: occupational therapist PT: physiotherapist
Appendix 6. Categories of medication (drug target, ProFaNE) interventions by study setting and combination
|
Setting/ Combination |
Study ID | Vitamin D | Calcium |
Other bone health medication |
Antidepressants |
Antipsychotics/ neuroleptics |
Medication review | Other |
| CARE FACILITIES | ||||||||
| Single | Bischoff 2003 | **** | ||||||
| Broe 2007 | **** | |||||||
| Chapuy 2002 | **** | **** | ||||||
| Crotty 2004a | **** Pharm | |||||||
| Crotty 2004b | **** Pharm | |||||||
| Houghton 2014 | **** MultiP | |||||||
| Flicker 2005 | **** | |||||||
| Frankenthal 2014 | **** Pharm | |||||||
| Garcia Gollarte 2014 | **** Educ | |||||||
| Grieger 2009a | **** | **** | ||||||
| Imaoka 2016a | **** | **** | ||||||
| Juola 2015b | **** | |||||||
| Kennedy 2015c | **** | **** | **** | |||||
| Law 2006 | **** | |||||||
| Patterson 2010d | **** | |||||||
| Peyro Saint Paul 2013e | **** | |||||||
| Potter 2016 | ||||||||
| Sambrook 2012 | **** (UV) | |||||||
| Streim 2012 | **** Depresc | |||||||
| Zermansky 2006 | **** Pharm | |||||||
| Multiple | Imaoka 2016a | |||||||
| Sambrook 2012 | **** (UV) | **** | ||||||
| Multifactorial | Dyer 2004 | **** | ||||||
| Jensen 2002 | **** | |||||||
| McMurdo 2000 | **** | |||||||
| Neyens 2009 | **** | |||||||
| Ray 1997 | **** | |||||||
| Rubenstein 1990 | **** | |||||||
| Salvà 2016 | **** | |||||||
| Shaw 2003 | **** | |||||||
| Walker 2015 | ||||||||
| Whitney 2017 | **** | |||||||
| HOSPITALS | ||||||||
| Burleigh 2007 | **** | |||||||
| Michalek 2014 | **** | |||||||
| Multifactorial | Aizen 2015 | ?? | ||||||
| Cumming 2008 | **** | |||||||
| Healey 2004 | **** | |||||||
| Stenvall 2007 | **** | **** | **** |
a Multivitamin b Nurse education on harmful medications cTraining to increase appropriate prescription of vitamin D, calcium and osteoporosis medications d Medication review of antipsychotics e Review by a pharmacologist for patients with hyponatraemia
Abbreviations
Depresc: deprescribing Educ: education on medication review Multi P: multiprofessional review by clinical pharmacist, pharmacy technician, care home staff and GP Pharm: pharmacist UV: increased sunlight exposure group
Appendix 7. Source of data for generic inverse variance analysis (see footnotes for explanation of codes)
| Study ID | Source for rate ratio (falls) | Source for risk ratio (fallers) | Source of risk ratio (number with fractures) |
| Aizen 2015 | ND | ND | NA |
| Ang 2011 | NA | 4 | NA |
| Barker 2016 | 1b | 7c | ND |
| Beck 2016 | ND | NA | NA |
| Becker 2003 | 1b | 5b | 7c |
| Becker 2003 (Cognitively impaired/not impaired subgroup analysis) | 1 | 5 | NA |
| Bischoff 2003 | 1a | 5a | 7 |
| Broe 2007 (800 IU) | 1a | 4a | NA |
| Buckinx 2014 | 3 | 7 | NA |
| Buettner 2002 | ND | NA | NA |
| Burleigh 2007 | ND | 5 | 7 |
| Cadore 2014 | ND | NA | NA |
| Chapuy 2002 | NA | 7 | 7 |
| Chenoweth 2009 | NA | ND | NA |
| Choi 2005 | NA | 7c | NA |
| Clifton 2009 | 3 | NA | NA |
| Colon‐Emeric 2013 | ND | NA | NA |
| Cox 2008 | 1ab | NA | ND |
| Crotty 2004a | NA | 5 | NA |
| Crotty 2004b | NA | 5ab | NA |
| Cumming 2008 | 1ab | 7c | 7c |
| da Silva Borges 2014 | ND | NA | NA |
| Donald 2000 | 3 | 5 | NA |
| Dyer 2004 | 3c | 6b | NA |
| Dykes 2010 | 3c | 7c | NA |
| Faber 2006 | 3 | 4 (FW vs control and IB vs control) 4a (FW + IB vs control) |
NA |
| Flicker 2005 | 1 | 4 | 7 |
| Frankenthal 2014 | 3 | NA | NA |
| Fu 2015 | 1a | NA | NA |
| Garcia Gollarte 2014 | ND | ND | NA |
| Grieger 2009 | 3 | 7 | NA |
| Haines 2004 | 3 | 5 | 7 |
| Haines 2010 | 3c | NA | NA |
| Haines 2011 | 2a | 6a | NA |
| Healey 2004 | 3c | NA | NA |
| Hill 2015 | 1ab | NDa | NDa |
| Houghton 2014 | 1b | NA | NA |
| Huang 2016 | ND | ND | NA |
| Imaoka 2016 | ND | ND | NA |
| Irez 2011 | 3 | NA | NA |
| Jarvis 2007 | ND | 7 | NA |
| Jensen 2002 | 1b | 4b | 6a |
| Jensen 2002 (MMSE < 19/ ≥ 19 subgroup analysis) | 1b | 7c | NA |
| Juola 2015 | 1ac | 7c | NA |
| Juola 2015 (MMSE >15, 10‐15, <10 subgroups) | 3ac | NA | NA |
| Kennedy 2015 | 3c | 7c | ND |
| Kerse 2004 | 1ab | 7c | NA |
| Kerse 2008 | 2b | 7c | NA |
| Klages 2011 | ND | NA | NA |
| Koh 2009 | 3c | NA | NA |
| Kovacs 2012 | NA | 5 | NA |
| Kovacs 2013 | 1 | 5 | NA |
| Lapane 2011 | NA | 4b | NA |
| Law 2006 | 3c | 7c | 5ab |
| Mador 2004 | NA | 7 | NA |
| Mayo 1994 | 3 | 4 | NA |
| McMurdo 2000 | 3c | 7c | 7c |
| Meyer 2009 | 3c | 7c | 7c |
| Michalek 2014 | 3c | 7c | NA |
| Mulrow 1994 | 3 | 7 | NA |
| Neyens 2009 | 1b | NA | NA |
| Nowalk 2001 | NA | ND | NA |
| Patterson 2010 | 3c | NA | NA |
| Peyro Saint Paul 2013 | 3 | 7 | NA |
| Potter 2016 | 3 | 7 | 7 |
| Ray 1997 | NA | ND | NA |
| Rosendahl 2008 | 1c | 7c | 7c |
| Rubenstein 1990 | 3 | 7 | 7 |
| Sakamoto 2006 | 3 | 7 | 7 |
| Sakamoto 2012 | 1 | 4 | NA |
| Salvà 2016 | 1ab | 7c | 7c |
| Salvà 2016 (subgroup excluding dementia) | 1ab | 6ab | NA |
| Sambrook 2012 | 1c | 7c | 7c |
| Saravanakumar 2014 | 3 | NA | NA |
| Schnelle 2003 | 3 | 7 | 7 |
| Schoenfelder 2000 | 3 | NA | NA |
| Serra‐Rexach 2011 | ND | NA | NA |
| Shaw 2003 | ND | 5 | 5 |
| Shimada 2004 | 3 | 7 | NA |
| Shorr 2012 | 3ab | 7c | NA |
| Sihvonen 2004 | 1a | 7 | NA |
| Sitja Rabert 2015 | ND | 7 | 7 |
| Stenvall 2007 | 1 | 4 | ND |
| Stenvall 2007 (dementia subgroup in Stenvall 2012) | 1 | 7 | 7 |
| Streim 2012 | ND | NA | NA |
| Tideiksaar 1993 | ND | NA | NA |
| Toulotte 2003 | ND | NA | NA |
| Treacy 2015 | 1 | NA | NA |
| Tuunainen 2013 | 3 | 7 | NA |
| Van de Ven 2014 | 3c | NA | NA |
| Van Gaal 2011a | 1c | NA | NA |
| Van Gaal 2011b | 1c | NA | NA |
| Van het Reve 2014 | 3 | 7 | NA |
| Wald 2011 | 3 | NA | NA |
| Walker 2015 | 3c | NA | NA |
| Ward 2010 | ND | NA | 7c |
| Whitney 2017 | 1b | 5a | 7c |
| Wolf 2013 | 3 | 7 | NA |
| Yokoi 2015 | NA | 7c | NA |
| Zermansky 2006 | 3 | 7 | NA |
|
aData reported as admissions not patients Abbreviations FW: 'Functional Walking' group IB: 'In Balance' group MMSE: Mini Mental State Examination 800 IU: 800 International Units vitamin D group Codes for source of rate ratio: 1: incidence rate ratio reported by trial authors 2: hazard ratio/relative hazard (multiple events) reported by trial authors 3: incidence rate ratio calculated by review authors a: adjusted for confounders by trial authors b: adjusted for clustering by trial authors c: adjusted for clustering by review authors Codes for source of risk ratio: 4: hazard ratio/relative hazard (first fall only) reported by trial authors 5: relative risk reported by trial authors 6: odds ratio reported by trial authors 7: relative risk calculated by review authors a: adjusted for confounders by trial authors b: adjusted for clustering by trial authors c: adjusted for clustering by review authors NA: not applicable. Falls (for rate ratio) or fallers (for risk ratio) or number of people sustaining a fracture (for risk ratio) not reported as an outcome in the trial ND: outcomes relating to falls or fallers or fractures were reported, but there were no useable data; results from the paper reported in the text of the review | |||
Appendix 8. Raw data for rate of falls and number of fallers when available
| Study ID | Intervention group: falls per person year | Control group: falls per person year | Intervention group: number of fallers | Intervention group: number in analysis | Intervention group: proportion of fallers | Control group: number of fallers | Control group: number in analysis | Control group: proportion of fallers |
| CARE FACILITIES | ||||||||
| Beck 2016 | 0 | 0.43 | ‐‐‐ | 9 | ‐‐‐ | ‐‐‐ | 22 | |
| Becker 2003 | 1.40 | 2.56 | 188 | 509 | 0.37 | 247 | 472 | 0.52 |
| Becker 2003 (Cognitively impaired) | 1.10 | 2.71 | 50 | 150 | 0.33 | 98 | 169 | 0.58 |
| Becker 2003 (Not cognitively impaired) | 1.42 | 2.04 | 93 | 215 | 0.43 | 91 | 191 | 0.48 |
| Bischoff 2003 | ‐‐‐ | ‐‐‐ | 14 | 62 | 0.23 | 18 | 60 | 0.30 |
| Broe 2007 (800 IU) | 0.28 | 1.00 | 5 | 23 | 0.22 | 11 | 25 | 0.44 |
| Buckinx 2014 | 1.16 | 1.21 | 15 | 31 | 0.48 | 17 | 31 | 0.55 |
| Buettner 2002 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Cadore 2014 | 0 | 9.6 | ‐‐‐ | 11 | ‐‐‐ | ‐‐‐ | 13 | ‐‐‐ |
| Chapuy 2002 | ‐‐‐ | ‐‐‐ | 251 | 393 | 0.64 | 118 | 190 | 0.62 |
| Chenoweth 2009 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Choi 2005 | ‐‐‐ | ‐‐‐ | 9 | 29 | 0.31 | 15 | 30 | 0.50 |
| Clifton 2009 | 2.45 | 3.79 | ‐‐‐ | 43 | ‐‐‐ | ‐‐‐ | 43 | ‐‐‐ |
| Colon‐Emeric 2013 | 2.06a | 2.64a | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Cox 2008b | ‐‐‐ | ‐‐‐ | ‐‐‐ | 3315 | ‐‐‐ | ‐‐‐ | 2322 | ‐‐‐ |
| Crotty 2004a | ‐‐‐ | ‐‐‐ | 19 | 44 | 0.43 | 16 | 44 | 0.36 |
| Crotty 2004b | ‐‐‐ | ‐‐‐ | 97 | 381 | 0.26 | 73 | 334 | 0.22 |
| da Silva Borges 2014 | ‐‐‐ | ‐‐‐ | ‐‐‐ | 30 | ‐‐‐ | ‐‐‐ | 29 | ‐‐‐ |
| Houghton 2014 | 3.32 | 3.0 | ‐‐‐ | 381 | ‐‐‐ | ‐‐‐ | 445 | ‐‐‐ |
| Dyer 2004 | 2.17 | 4.02 | 56 | 102 | 0.55 | 51 | 94 | 0.54 |
| Faber 2006 (FW) | 3.3 | 2.5 | 40 | 64 | 0.63 | 48 | 90 | 0.53 |
| Faber 2006 (IB) | 2.4 | 2.5 | 45 | 78 | 0.58 | 48 | 90 | 0.53 |
| Faber 2006 (FW + IB) | 2.8 | 2.5 | 85 | 142 | 0.60 | 48 | 90 | 0.53 |
| Flicker 2005 | 1.26 | 1.90 | 170 | 313 | 0.54 | 185 | 312 | 0.59 |
| Frankenthal 2014 | 0.80 | 1.30 | ‐‐‐ | 160 | ‐‐‐ | ‐‐‐ | 146 | ‐‐‐ |
| Fu 2015 | 0.54 | 1.52 | ‐‐‐ | 30 | ‐‐‐ | ‐‐‐ | 30 | ‐‐‐ |
| Garcia Gollarte 2014 | 1.28 | 1.72 | 82 | 344 | 0.24 | 104 | 372 | 0.28 |
| Grieger 2009 | 0.60 | 1.60 | 11 | 48 | 0.23 | 12 | 43 | 0.28 |
| Huang 2016 (CB) | 0.00 | 1.67 | 0 | 25 | 0.00 | 7 | 24 | 0.29 |
| Huang 2016 (CB + EX) | 0.00 | 1.67 | 0 | 24 | 0.00 | 7 | 24 | 0.29 |
| Imaoka 2016 (RED EX) | ‐‐‐ | ‐‐‐ | 7 | 22 | 0.32 | 9 | 17 | 0.53 |
| Imaoka 2016 (Vit D) | ‐‐‐ | ‐‐‐ | 6 | 17 | 0.35 | 9 | 17 | 0.53 |
| Imaoka 2016 (multiple) | ‐‐‐ | ‐‐‐ | 4 | 19 | 0.21 | 9 | 17 | 0.53 |
| Irez 2011 | 1.60 | 5.63 | ‐‐‐ | 30 | ‐‐‐ | ‐‐‐ | 30 | ‐‐‐ |
| Jensen 2002 | 2.45 | 3.03 | 82 | 188 | 0.44 | 109 | 196 | 0.56 |
| Jensen 2002 (MMSE < 19) | 3.50 | 3.34 | 37 | 69 | 0.54 | 62 | 102 | 0.61 |
| Jensen 2002 (MMSE ≥ 19) | 1.77 | 2.90 | 42 | 112 | 0.38 | 43 | 79 | 0.54 |
| Juola 2015 | 2.25 | 3.25 | 42 | 93 | 0.45 | 60 | 96 | 0.63 |
| Juola 2015 (MMSE >15) | 3.90 | 3.08 | ‐‐‐ | 45 | ‐‐‐ | ‐‐‐ | 50 | ‐‐‐ |
| Juola 2015 (MMSE 10‐15) | 1.12 | 4.22 | ‐‐‐ | 23 | ‐‐‐ | ‐‐‐ | 22 | ‐‐‐ |
| Juola 2015 (MMSE <10) | 0.61 | 2.70 | ‐‐‐ | 25 | ‐‐‐ | ‐‐‐ | 24 | ‐‐‐ |
| Kennedy 2015 | 2.57 | 2.51 | 853 | 1290 | 0.66 | 1712 | 2727 | 0.63 |
| Kerse 2004 | 4.1 | 2.3 | 173 | 309 | 0.56 | 103 | 238 | 0.43 |
| Kerse 2008 | ‐‐‐ | ‐‐‐ | 162 | 310 | 0.52 | 146 | 329 | 0.44 |
| Klages 2011 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Koh 2009 | 0.40 | 0.22 | ‐‐‐ | 612 | ‐‐‐ | ‐‐‐ | 510 | ‐‐‐ |
| Kovacs 2012 | ‐‐‐ | ‐‐‐ | 8 | 21 | 0.38 | 14 | 20 | 0.70 |
| Kovacs 2013 | 0.69 | 0.97 | 16 | 32 | 0.50 | 20 | 30 | 0.67 |
| Lapane 2011 | ‐‐‐ | ‐‐‐ | ‐‐‐ | 1769 | ‐‐‐ | ‐‐‐ | 1552 | ‐‐‐ |
| Law 2006 | 2.01 | 2.31 | 770 | 1762 | 0.44 | 833 | 1955 | 0.43 |
| McMurdo 2000 | 3.02 | 3.85 | 20 | 52 | 0.38 | 22 | 38 | 0.58 |
| Meyer 2009 | 1.97 | 2.04 | 299 | 574 | 0.52 | 291 | 551 | 0.53 |
| Mulrow 1994 | 1.86 | 2.44 | 44 | 97 | 0.45 | 38 | 97 | 0.39 |
| Neyens 2009 | 2.09 | 2.54 | ‐‐‐ | 249 | ‐‐‐ | ‐‐‐ | 269 | ‐‐‐ |
| Nowalk 2001 (LL/TC) | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Nowalk 2001 (FNBF) | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Patterson 2010 | 1.96 | 1.37 | ‐‐‐ | 173 | ‐‐‐ | ‐‐‐ | 161 | ‐‐‐ |
| Peyro Saint Paul 2013 | 3.00 | 4.80 | 1 | 4 | 0.25 | 3 | 5 | 0.60 |
| Potter 2016 | 4.91 | 2.96 | 25 | 45 | 0.56 | 31 | 48 | 0.65 |
| Ray 1997 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Rosendahl 2008 | 3.6 | 4.6 | 46 | 87 | 0.53 | 49 | 96 | 0.51 |
| Rubenstein 1990 | 2.49 | 2.63 | 56 | 79 | 0.71 | 61 | 81 | 0.75 |
| Sakamoto 2006 | 0.93 | 1.14 | 68 | 315 | 0.22 | 51 | 212 | 0.24 |
| Sakamoto 2012 | 1.04 | 1.40 | 26 | 73 | 0.36 | 36 | 72 | 0.50 |
| Salvà 2016 | 1.93 | 0.89 | 94 | 193 | 0.49 | 52 | 137 | 0.38 |
|
Salvà 2016 (excluding dementia) |
‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Sambrook 2012 (UV) | ‐‐‐ | ‐‐‐ | 111 | 190 | 0.58 | 111 | 205 | 0.54 |
| Sambrook 2012 (UV+) | ‐‐‐ | ‐‐‐ | 108 | 207 | 0.52 | 111 | 205 | 0.54 |
| Saravanakumar 2014 (Tai Chi) | 2.02 | 3.90 | ‐‐‐ | 9 | ‐‐‐ | ‐‐‐ | 11 | ‐‐‐ |
| Saravanakumar 2014 (Yoga) | 2.87 | 3.90 | ‐‐‐ | 9 | ‐‐‐ | ‐‐‐ | 11 | ‐‐‐ |
| Schnelle 2003 | 0.68 | 1.09 | 17 | 92 | 0.18 | 29 | 98 | 0.30 |
| Schoenfelder 2000 | 9.33 | 3.43 | ‐‐‐ | 9 | ‐‐‐ | ‐‐‐ | 7 | ‐‐‐ |
| Serra‐Rexach 2011 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Shaw 2003 | ‐‐‐ | ‐‐‐ | 96 | 130 | 0.74 | 115 | 144 | 0.80 |
| Shimada 2004 | 1.07 | 2.00 | 5 | 15 | 0.33 | 6 | 11 | 0.55 |
| Sihvonen 2004 | ‐‐‐ | ‐‐‐ | 11 | 20 | 0.55 | 5 | 7 | 0.71 |
| Sitja Rabert 2015 | ‐‐‐ | ‐‐‐ | 20 | 81 | 0.25 | 15 | 78 | 0.19 |
| Streim 2012 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Toulotte 2003 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Tuunainen 2013 (MF) | 0.88 | 1.19 | 7 | 16 | 0.44 | 14 | 18 | 0.78 |
| Tuunainen 2013 (MFB) | 0.57 | 1.19 | 6 | 14 | 0.43 | 14 | 18 | 0.78 |
| Van de Ven 2014 | 1.81 | 3.33 | ‐‐‐ | 137 | ‐‐‐ | ‐‐‐ | 156 | ‐‐‐ |
| Van Gaal 2011a | 1.56 | 2.08 | ‐‐‐ | 196 | ‐‐‐ | ‐‐‐ | 196 | ‐‐‐ |
| Van het Reve 2014 | 0.50 | 0.80 | 3 | 54 | 0.06 | 2 | 60 | 0.03 |
| Walker 2015 | 4.00 | 1.90 | 22 | 20 | ||||
| Ward 2010 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Whitney 2017 | 1.51 | 0.93 | 31 | 103 | 0.30 | 25 | 88 | 0.28 |
| Yokoi 2015 | ‐‐‐ | ‐‐‐ | 6 | 51 | 0.12 | 16 | 54 | 0.30 |
| Zermansky 2006 | 1.60 | 2.60 | 84 | 331 | 0.25 | 106 | 330 | 0.32 |
| HOSPITALS | ||||||||
| Aizen 2015 | 0.67 | 0.48 | 13.00 | 200 | 0.065 | 8.00 | 308 | 0.026 |
| Ang 2011 | ‐‐‐ | ‐‐‐ | 4 | 910 | 0.004 | 14 | 912 | 0.02 |
| Barker 2016 | 2.72 | 2.57 | 623 | 17698 | 0.035 | 646 | 17566 | 0.04 |
| Burleigh 2007 | ‐‐‐ | ‐‐‐ | 36 | 100 | 0.36 | 45 | 103 | 0.44 |
| Cumming 2008 | 3.36 | 3.39 | 157 | 2047 | 0.08 | 143 | 1952 | 0.07 |
| Donald 2000 (FL) | 5.75 | 0.39 | 7 | 28 | 0.25 | 1 | 26 | 0.04 |
| Donald 2000 (EX) | 2.22 | 2.10 | 2 | 30 | 0.07 | 6 | 24 | 0.25 |
| Dykes 2010 | 1.01 | 1.84 | 34 | 2755 | 0.01 | 51 | 2509 | 0.02 |
| Haines 2004 | 4.12 | 5.94 | 54 | 310 | 0.17 | 71 | 316 | 0.22 |
| Haines 2010 | 1.91 | 1.37 | ‐‐‐ | 6113 | ‐‐‐ | ‐‐‐ | 4986 | ‐‐‐ |
| Haines 2011 (ED) | 3.14 | 3.39 | 56 | 424 | 0.13 | 54 | 381 | 0.14 |
| Haines 2011 (ED+) | 2.79 | 3.39 | 44 | 401 | 0.11 | 54 | 381 | 0.14 |
| Healey 2004 | 4.12 | 7.03 | ‐‐‐ | 749 | ‐‐‐ | ‐‐‐ | 905 | ‐‐‐ |
| Hill 2015 | 2.85 | 5.03 | 136 | 1623 | 0.08 | 248 | 1983 | 0.13 |
| Jarvis 2007 | ‐‐‐ | ‐‐‐ | 3 | 14 | 0.21 | 7 | 15 | 0.47 |
| Mador 2004 | ‐‐‐ | ‐‐‐ | 10 | 36 | 0.28 | 4 | 35 | 0.11 |
| Mayo 1994 | 4.62 | 4.01 | 27 | 65 | 0.42 | 21 | 69 | 0.30 |
| Michalek 2014 | 0.55 | 3.87 | 2 | 58 | 0.03 | 12 | 56 | 0.21 |
| Shorr 2012 | 2.05 | 1.66 | 282 | 11115 | 0.03 | 359 | 17436 | 0.02 |
| Stenvall 2007 | 2.30 | 5.95 | 12 | 102 | 0.12 | 26 | 97 | 0.27 |
| Stenvall 2007 dementia subgroup (Stenvall, 2012) | 0.65 | 10.67 | 1 | 28 | 0.04 | 11 | 36 | 0.31 |
| Tideiksaar 1993 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Treacy 2015 | 2.28 | 3.53 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Van Gaal 2011b | 1.04 | 1.04 | ‐‐‐ | 1081 | ‐‐‐ | ‐‐‐ | 1120 | ‐‐‐ |
| Wald 2011 | 1.75 | 2.45 | ‐‐‐ | 122 | ‐‐‐ | ‐‐‐ | 95 | ‐‐‐ |
| Wolf 2013 | 3.00 | 5.66 | 6 | 48 | 0.13 | 7 | 50 | 0.14 |
|
aFalls per bed year. bRaw data not available, data reported by authors as rate ratios. Abbreviations ED: educational materials only group ED+: educational materials plus physiotherapist follow‐up EX: supplementary exercises group FL: carpet flooring group FNBF: 'Fit NB Free' group FW: 'Functional Walking' group (a functional balance, strength & mobility programme) IB: 'In Balance' group MF: muscle force MFB: muscle force & balance MMSE: Mini Mental State Examination LL/TC: 'Living and learning/Tai Chi' group RED EX: reduced exercise UV: increased sunlight exposure group. UV+: increased sunlight exposure + calcium supplementation group Vit D: Vitamin D3 & calcium in multivitamin supplement 800 IU: 800 International Units vitamin D group | ||||||||
Appendix 9. Raw data for number of fractures when available
| Study ID | Intervention group: number of people with fractures | Intervention group: number in analysis | Intervention group: proportion of fracture fallers | Control group: number of people with fractures | Control group: number in analysis | Control group: proportion of fracture fallers |
| CARE FACILITIES | ||||||
| Beck 2016 | ‐‐‐ | 9 | ‐‐‐ | ‐‐‐ | 22 | ‐‐‐ |
| Becker 2003: hip | 17 | 509 | 0.033 | 15 | 472 | 0.032 |
| Bischoff 2003: hip | 2 | 62 | 0.032 | 1 | 60 | 0.017 |
| Broe 2007 (800 IU) | ‐‐‐ | 23 | ‐‐‐ | ‐‐‐ | 25 | |
| Buckinx 2014 | ‐‐‐ | 31 | ‐‐‐ | ‐‐‐ | 31 | |
| Buettner 2002 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | |
| Cadore 2014 | ‐‐‐ | 11 | ‐‐‐ | ‐‐‐ | 13 | |
| Chapuy 2002: NV | 70 | 393 | 0.178 | 34 | 190 | 0.179 |
| Chapuy 2002: hip | 27 | 393 | 0.069 | 21 | 190 | 0.111 |
| Chenoweth 2009 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | |
| Choi 2005 | ‐‐‐ | 29 | ‐‐‐ | ‐‐‐ | 30 | |
| Clifton 2009 | ‐‐‐ | 43 | ‐‐‐ | ‐‐‐ | 43 | |
| Colon‐Emeric 2013 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | |
| Cox 2008a | ‐‐‐ | 3315 | ‐‐‐ | ‐‐‐ | 2322 | |
| Crotty 2004a | ‐‐‐ | 44 | ‐‐‐ | ‐‐‐ | 44 | |
| Crotty 2004b | ‐‐‐ | 381 | ‐‐‐ | ‐‐‐ | 334 | |
| da Silva Borges 2014 | ‐‐‐ | 30 | ‐‐‐ | ‐‐‐ | 29 | |
| Houghton 2014 | ‐‐‐ | 381 | ‐‐‐ | ‐‐‐ | 445 | |
| Dyer 2004 | 4 | 102 | 0.039 | 3 | 94 | 0.032 |
| Faber 2006 (FW) | ‐‐‐ | 64 | ‐‐‐ | 90 | ||
| Faber 2006 (IB) | ‐‐‐ | 78 | ‐‐‐ | 90 | ||
| Faber 2006 (FW + IB) | ‐‐‐ | 142 | ‐‐‐ | 90 | ||
| Flicker 2005 | 25 | 313 | 0.080 | 35 | 312 | 0.112 |
| Frankenthal 2014 | ‐‐‐ | 160 | ‐‐‐ | 146 | ||
| Fu 2015 | ‐‐‐ | 30 | ‐‐‐ | 30 | ||
| Garcia Gollarte 2014 | ‐‐‐ | 344 | ‐‐‐ | 372 | ||
| Grieger 2009 | ‐‐‐ | 48 | ‐‐‐ | 43 | ||
| Huang 2016 | ‐‐‐ | 51 | ‐‐‐ | 24 | ||
| Imaoka 2016 (RED EX) | ‐‐‐ | 22 | ‐‐‐ | 17 | ||
| Imaoka 2016 (Vit D) | ‐‐‐ | 17 | ‐‐‐ | 17 | ||
| Imaoka 2016 (multiple) | ‐‐‐ | 19 | ‐‐‐ | 17 | ||
| Irez 2011 | ‐‐‐ | 30 | ‐‐‐ | 30 | ||
| Jensen 2002 | 3 | 188 | 0.016 | 12 | 196 | 0.061 |
| Juola 2015 | ‐‐‐ | 93 | ‐‐‐ | 96 | ||
| Kennedy 2015 | ‐‐‐ | 1290 | ‐‐‐ | 2727 | ||
| Kerse 2004 | ‐‐‐ | 309 | ‐‐‐ | 238 | ||
| Kerse 2008 | ‐‐‐ | 310 | ‐‐‐ | 329 | ||
| Klages 2011 | ‐‐‐ | ‐‐‐ | ‐‐‐ | ‐‐‐ | ||
| Koh 2009 | ‐‐‐ | 612 | ‐‐‐ | 510 | ||
| Kovacs 2012 | ‐‐‐ | 21 | ‐‐‐ | 20 | ||
| Kovacs 2013 | ‐‐‐ | 32 | ‐‐‐ | 30 | ||
| Lapane 2011 | ‐‐‐ | 1769 | ‐‐‐ | 1552 | ||
| Law 2006: NV | 64 | 1762 | 0.036 | 51 | 1955 | 0.026 |
| Law 2006: hip | 24 | 1762 | 0.014 | 20 | 1955 | 0.010 |
| McMurdo 2000 | 1 | 52 | ‐‐‐ | 3 | 38 | |
| Meyer 2009 | 39 | 574 | 0.068 | 38 | 551 | 0.069 |
| Mulrow 1994 | ‐‐‐ | 97 | 97 | |||
| Neyens 2009 | ‐‐‐ | 249 | 269 | |||
| Nowalk 2001 (LL/TC) | ‐‐‐ | ‐‐‐ | ‐‐‐ | |||
| Nowalk 2001 (FNBF) | ‐‐‐ | ‐‐‐ | ‐‐‐ | |||
| Patterson 2010 | ‐‐‐ | 173 | 161 | |||
| Peyro Saint Paul 2013 | ‐‐‐ | 4 | 5 | |||
| Potter 2016 | 3 | 45 | 0.067 | 2 | 48 | 0.042 |
| Ray 1997 | ‐‐‐ | ‐‐‐ | ‐‐‐ | |||
| Rosendahl 2008 | 4 | 87 | 0.046 | 6 | 96 | 0.063 |
| Rubenstein 1990 | 7 | 79 | 0.089 | 5 | 81 | 0.062 |
| Sakamoto 2006: hip | 1 | 315 | 0.003 | 1 | 212 | 0.005 |
| Sakamoto 2012 | ‐‐‐ | 73 | 72 | |||
| Salvà 2016 | 10 | 193 | 0.052 | 1 | 137 | 0.007 |
|
Salvà 2016 (excluding dementia) |
‐‐‐ | ‐‐‐ | ‐‐‐ | |||
| Sambrook 2012 (UV) | 17 | 190 | 0.089 | 17 | 205 | 0.083 |
| Sambrook 2012 (UV+) | 13 | 207 | 0.063 | 17 | 205 | 0.083 |
| Saravanakumar 2014 | ‐‐‐ | |||||
| Schnelle 2003 | 4 | 92 | 0.043 | 1 | 98 | 0.010 |
| Schoenfelder 2000 | ‐‐‐ | 9 | 7 | |||
| Serra‐Rexach 2011 | ‐‐‐ | ‐‐‐ | ‐‐‐ | |||
| Shaw 2003 | 6 | 130 | 0.046 | 12 | 144 | 0.083 |
| Shimada 2004 | ‐‐‐ | 15 | 11 | |||
| Sihvonen 2004 | ‐‐‐ | 20 | 7 | |||
| Sitja Rabert 2015 | 1 | 81 | 0.012 | 0 | 78 | 0 |
| Streim 2012 | ‐‐‐ | ‐‐‐ | ‐‐‐ | |||
| Toulotte 2003 | ‐‐‐ | ‐‐‐ | ‐‐‐ | |||
| Tuunainen 2013 (MF) | ‐‐‐ | 16 | 18 | |||
| Tuunainen 2013 (MFB) | ‐‐‐ | 14 | 18 | |||
| Van de Ven 2014 | ‐‐‐ | 137 | 156 | |||
| Van Gaal 2011a | ‐‐‐ | 196 | 196 | |||
| Van het Reve 2014 | ‐‐‐ | 54 | 60 | |||
| Walker 2015 | ‐‐‐ | 22 | 20 | |||
| Ward 2010 | 109 | 2802 | 0.039 | 106 | 2589 | 0.041 |
| Whitney 2017 | 3 | 103 | 0.029 | 0 | 88 | 0 |
| Yokoi 2015 | ‐‐‐ | 51 | 54 | |||
| Zermansky 2006 | ‐‐‐ | 331 | 330 | |||
| HOSPITALS | ||||||
| Aizen 2015 | ‐‐‐ | 200 | 308 | |||
| Ang 2011 | ‐‐‐ | 910 | 912 | |||
| Barker 2016 | 11 | 17698 | 0.0006 | 13 | 17566 | 0.0007 |
| Burleigh 2007 | 1 | 100 | 0.010 | 3 | 103 | 0.029 |
| Cumming 2008 | 2 | 2047 | 0.001 | 3 | 1952 | 0.002 |
| Donald 2000 (FL) | ‐‐‐ | 28 | 26 | |||
| Donald 2000 (EX) | ‐‐‐ | 30 | 24 | |||
| Dykes 2010 | ‐‐‐ | 2755 | 2509 | |||
| Haines 2004 | 2 | 310 | 0.006 | 2 | 316 | 0.006 |
| Haines 2010 | ‐‐‐ | 6113 | 4986 | |||
| Haines 2011 (ED) | ‐‐‐ | 424 | 381 | |||
| Haines 2011 (ED+) | ‐‐‐ | 401 | 381 | |||
| Healey 2004 | ‐‐‐ | 749 | 905 | |||
| Hill 2015 | 4b | 1623 | ‐‐‐ | 6b | 1983 | ‐‐‐ |
| Jarvis 2007 | ‐‐‐ | 14 | 15 | |||
| Mador 2004 | ‐‐‐ | 36 | 35 | |||
| Mayo 1994 | ‐‐‐ | 65 | 69 | |||
| Michalek 2014 | ‐‐‐ | 58 | 56 | |||
| Shorr 2012 | ‐‐‐ | 11115 | 17436 | |||
| Stenvall 2007 | 0 | 102 | 0 | 4 | 97 | 0.041 |
| Stenvall 2007 dementia subgroup (Stenvall 2012) | 0 | 28 | 0 | 3 | 36 | 0.083 |
| Tideiksaar 1993 | ‐‐‐ | ‐‐‐ | ‐‐‐ | |||
| Treacy 2015 | ‐‐‐ | ‐‐‐ | ‐‐‐ | |||
| Van Gaal 2011b | ‐‐‐ | 1081 | 1120 | |||
| Wald 2011 | ‐‐‐ | 122 | 95 | |||
| Wolf 2013 | ‐‐‐ | 48 | 50 | |||
|
aRaw data not available, data reported by authors as rate ratios. badmissions Abbreviations ED: educational materials only group ED+: educational materials plus physiotherapist follow‐up EX: supplementary exercises group FL: carpet flooring group FNBF: 'Fit NB Free' group FW: 'Functional Walking' group (a functional balance, strength & mobility programme) IB: 'In Balance' group LL/TC: 'Living and learning/Tai Chi' group MF: muscle force MFB: muscle force & balance NV: non‐vertebral RED EX: reduced exercise UV: increased sunlight exposure group. UV+: increased sunlight exposure + calcium supplementation group Vit D: Vitamin D3 & calcium in multivitamin supplement 800 IU: 800 International Units vitamin D group | ||||||
Appendix 10. Studies reporting cost‐effectiveness or costs of the intervention and/or healthcare resource use
| Study ID (source if not primary reference), sample, efficacy analyses, type of evaluation | Intervention(s) and comparator (N in analysis) | Perspective(s), type of currency, price year, time horizon | Cost items measured | Mean (SD) intervention cost per person | Healthcare service costs | Incremental cost per fall prevented/per QALY gained |
| •Buettner 2002 •Residents of 3 dementia care units (Oxford, Boston, and Palo Alto, USA) ≥ 2 falls in 1 month, mean age 83 (range 60 to 98) years •No effectiveness data available for analysis •Cost analysis |
•Daily "graded" walking, "exercise for function" programme 3 x week, sensory air mat 2 x week (evenings) for 2 months vs usual care, number allocated to each group not reported (total N = 27) |
•Not stated •US dollar •Not stated •2 months |
•Therapist time (intervention only) •Cost of falls and injuries ("based on research data on falls") |
•Treatment group USD 30,031, control group USD 79,535 | ||
| •Chenoweth 2009 (Norman 2008) •Residents from 15 dementia care sites across Sydney, Australia, category 1 to 3 on Australian Resident Classification Scale (high level of care), mean age 84 (SD 7) years •No effectiveness data available for analysis •Cost‐effectiveness analysis |
•Dementia care mapping (DCM) (N = 109, 5 sites) vs person centred care (PCC) (N = 98, 5 sites) vs usual care (N = 82, 5 sites) for 4 months |
•Health service •Australian dollar •2008 •8 months |
•Trainer time, post‐training support, staff replacement (DCM, PCC) •Pharmaceutical use |
•Not reported (annual total cost per residential care setting DCM AUD 10,034, PCC AUD 2250) | •Annual pharmaceutical cost per resident AUD 545.55 | •Not reported •Incremental cost per behaviour (CMAI point) averted DCM vs usual care AUD 46.89, PCC vs usual care AUD 6.43 |
| •Clifton 2009 •Skilled nursing care‐facility residents, Eastern Washington State, USA, mean age 82 (SD 7) years •Analysis 7.1 •Analytic model |
•Wear FallSaver monitor for 60 days (N = 33) vs no device for 60 days (N = 39), cross‐over trial |
•Not stated •US dollar •2004 •1 year |
•Annual intervention implementation for 100 residents (direct costs only) •Mean hospitalisation cost for injurious fall (from the literature) |
•USD 2 per resident per day (annual cost for 100 resident facility USD 73,000) | •Assuming 35 injurious falls per 100 residents per year, annual cost savings for 100 resident facility if 12% fewer injurious falls USD 429, USD 232,953 if 50% fewer injurious falls |
|
|
Houghton 2014 (Sach 2015) •Residents from care homes with average age > 65, registered with GP in local area and registered with Care Quality Commission for at least 6 months. •Analysis 5.1 •Cost analysis, detailed micro‐costing |
• Multiprofessional medication review (N = 826) | •NHS and care homes •Pound sterling •2012 •1 year |
•Intervention costs: personnel and resources, Staff costs for time spent on reviews, travel time and costs •Medication costs •Healthcare resource use •Hospitalisations |
•GBP 104.80 (SD 50.91) per resident | ||
| •Haines 2013 (analysis of Haines 2011) •Acute and Rehabilitation hospital inpatients age ≥ 60, Brisbane and Perth, Australia, mean age 75 (SD 11) years •Analysis 20.1, Analysis 20.2 •Cost‐effectiveness analysis |
•Multimedia patient education programme with physiotherapist follow up (total N = 1,206) | •Health service provider •Australian dollar •2008 •Period of hospitalisation |
•Acute care costs •Rehabilitation costs •Direct falls related costs: radiological investigations, medical costs, nursing costs, medication costs, on‐call payment costs, suture procedure costs, orthoses costs, and other tests costs. |
Intervention/control group costs post consent per participant (mean (SD) AUD) Subgroup cognitively intact: Intervention cost (complete programme) •Acute care AUD 10,774 (18,344) •Rehabilitation AUD 11,197 (18,906) •Direct falls related costs AUD 1 (7) Control cost •Acute care AUD 8,481 (12,856) •Rehabilitation AUD 10,964 (19,972) •Direct falls related costs intact AUD 8 (47) Subgroup cognitively impaired: Intervention cost (complete programme) •Acute care AUD 11,128 (28,570) •Rehabilitation AUD 21,740 (37,130) •Direct falls related costs AUD 187 (1,602) Control group •Acute care AUD 5,140 (8,142) •Rehabilitation AUD 26,050 (36,776) •Direct falls related costs AUD 15 (85) |
For subgroup who were cognitively intact: • AUD 294 per fall prevented •AUD 526 per faller prevented |
|
| •Meyer 2009 •Nursing home residents in Hamburg, Germany, mean age 86 (SD 6) years •Analysis 8.1, Analysis 8.2, Analysis 8.3 •Cost description |
•Administer standardised risk assessment tool (Downton Index) monthly (N = 574, 29 nursing homes) vs usual care (N = 551, 29 nursing homes) |
•Nursing care facility •Euro •2006 •1 year |
•Nurse time for training and assessing using the Downton Index | •Not reported (total during the study EUR 10,500 (USD 16,170, GBP 8160) |
||
| •Mulrow 1994 •Residents from 9 nursing homes in San Antonio, Texas, USA, dependent in ≥ 2 activities of daily living, mean age 80 (SD 8) years •Analysis 3.1, Analysis 3.2, Analysis 2.1, Analysis 2.2 •Cost analysis |
•One‐on‐one physical therapy sessions (N = 97) vs friendly visits (N = 97) 3 x week for 4 months |
•Not stated •US dollar •Not stated •4 months |
•Intervention delivery (wages, travel expenses, equipment, overheads) •Nursing home, hospitalisation, physician and other health professional visits, emergency department visits, procedures, and medication charges |
•USD 1220 (95% CI 412 to 1832) for physical therapy programme, USD 189 (95% CI 80 to 298) control group | •Healthcare charges (81% nursing home, 15% hospitalisation) USD 11,398 (95% CI 10,929 to 11,849) per participant (NS) | |
| •Schnelle 2003 •Residents of 4 nursing homes, incontinence of urine, US, mean age 88 (SD 8) years •Analysis 11.1, Analysis 11.2, Analysis 11.3 •Cost analysis |
•Low‐intensity functionally orientated exercise and incontinence care 5 days a week every 2 hours between 8:00 am and 4:00 pm for 8 months (N = 92) vs usual care (N = 98) |
•Not stated •US dollar •1997/98 •8 months |
•Diagnostic tests, treatment related to each acute condition (dermatological, genitourinary, gastrointestinal, respiratory and cardiovascular systems; falls; pain; psychiatric and nutritional disturbances) | •USD 24.42 per resident per week to evaluate and treat the selected conditions intervention group, USD 38.36 control group (NS) | ||
| •Van de Ven 2014 •Dementia special care units, diagnosed with dementia, ≥1 neuropsychiatric symptom, mean age 84.7 years •Analysis 8.1 •Cost analysis |
•Dementia care mapping (DCM), 4 months intervention delivered twice during the study (N = 154) vs usual care (N=164) | •Healthcare perspective •US dollar •Not stated •18 months |
•Intervention costs: DCM basic and advanced training, mapping exercise, inter‐rater reliability test, observation, preparing the DCM reports, feedback sessions •Hospital costs: outpatient, inpatient, emergency department & ambulance •Psychotropic drugs •Nursing home healthcare professional costs |
•Intervention cost per resident per day USD 0.63 (SD 0.23) | •Healthcare consumption and drug use per resident per day at 18 months (mean(SD): intervention group USD 4.25 (0.59) vs usual care USD 4.4 (0.57) |
|
| •Wald 2011 •Medical inpatients at University of Colorado Hospital, USA, aged ≥ 70 years, mean age 81 (SD 7) years •Analysis 19.1 •Cost analysis |
•Hospitalist run acute care service for elderly people (N = 122) vs usual hospital inpatient care (N = 95) | •Not stated •US dollar •2007 •6 months |
•"Hospital charges" | •Mean “hospital charges” USD 24,617 (SD 15,828) intervention vs USD 21,488 (SD 13,407) usual care, P = 0.12 | ||
| •Zermansky 2006 •Residents of 65 nursing care facilities in Leeds, UK taking ≥ 1 medicines, mean age 85 (interquartile range 80 to 91) years •Analysis 5.1, Analysis 5.2 •Cost analysis |
•Clinical medication review by pharmacist (N = 331) vs usual general practitioner care (N = 330) |
•Not stated •Pound sterling •2003 •6 months |
• Pharmaceutical use | •Mean medication cost per patient per 28 days medication review group GBP 42.24 (SD 38.33) vs GBP 42.95 (SD 41.01) control group, mean difference GBP ‐0.70 (95% CI ‐7.28 to 5.71) |
CMAI: Cohen‐Mansfield agitation inventory NS: difference between groups not statistically significant QALY: quality adjusted life year SD: standard deviation
Appendix 11. Additional detail for other identified systematic reviews including meta‐analyses
Additional detailed discussion of comparisons of the current review with other identified systematic reviews is provided.
Exercise
Lee 2017 included 21 studies of exercise in care facilities, 15 with exercise as a single intervention, six with exercise combined with one or more interventions. Data were pooled from studies comparing exercise with other interventions, usual care or placebo. In the current review, comparisons of alternate exercise programmes were not pooled with trials of exercise in comparison with usual care. Lee 2017 reported that pooled data from all trials showed a decrease in the rate of falls (RaR 0.81, 95% CI 0.68 to 0.97) but not risk of falling (RR 0.93, 95% CI to 0.86 to 1.01). When exercise was combined with other falls interventions (which were considered as multifactorial interventions in our review) the effect on the rate of falls was greater (RR 0.61, 95% CI 0.52–0.72) and there was a reduction in the risk of falling (RR 0.85, 95% CI 0.77 to 0.95). Post‐hoc analysis in Lee 2017 indicated that gait, balance, and functional training with mechanical devices (two studies, Shimada 2004; Sihvonen 2004) reduced the rate of falls. The current review has pooled gait, balance, and functional training with mechanical devices in Sihvonen 2004 with the functional walking arm of Faber 2006, Kerse 2008 and the Sakamoto 2006 one‐leg standing arm as interventions of gait, balance, and functional training compared to usual care and found no change in the rate of falls. A post‐hoc analysis of balance and strength training in Lee 2017 that shows a reduction in the rate of falls also considers different studies within this category to the current review.
Vitamin D supplementation
Le Blanc 2015, in a systematic review examining trials conducted in both institutionalised or community settings, found that vitamin D significantly reduced the number of falls per person (5 trials, RR 0.66, 95%CI 0.50 to 0.88) but did not significantly reduce the risk of falling (5 trials, RR 0.84, 95%CI 0.69 to 1.02, I² = 70%), consistent with the findings in care facilities in this review. The authors found subgroup analyses based on institutionalisation, baseline 25‐hydroxyvitamin D level, vitamin D dosage study duration and age did not explain the heterogeneity in the risk of falling outcome. Heterogeneity was reduced to zero when two studies treating with a combination of vitamin D and calcium were excluded; vitamin D treatment alone decreased the risk of falling (3 studies, RR 0.65, 95%CI 0.51 to 0.81, I² = 0%). The two included studies conducted in institutionalised settings are included in this review. The other trials included patients of an older age (>70 years), with mobility problems or multiple co‐morbidities. Pooled analysis of four trials and one nested case‐control study did not find a significant effect on the risk of any fracture (RR 0.98 95%CI, 0.82 to 1.16, I² = 32%) or hip fracture (4 trials; RR, 0.96 95%CI, 0.72 to 1.29, I² = 46%).
Bolland 2014, pooled outcomes from six randomised trials conducted in care facilities or hospitals and found no significant reduction in falls with vitamin D supplementation, with or without calcium supplementation (RR 0.96, 95%CI 0.88 to 1.05). These authors conducted a sequential analysis of trials in any setting and considered that a risk reduction of less than 15% was not clinically relevant for an individual, but also considered a threshold of 10% as a sensitivity analysis. It was proposed that smaller treatment benefits are unlikely to be considered attractive to an individual. It was concluded that supplementation with vitamin D does not reduce risk of falling by 15% or more and that future trials are unlikely to alter this conclusion. One study included as institutional in the Bolland 2014 review was excluded from this review as 51% of participants were residing in the community (Graafmans 1996); all other studies were included in this review. The Bolland 2014 review focused on analysis of falls risk but also acknowledges that it is useful to consider the rate of falls from a public health perspective due to plausible effects on multiple fallers. The authors conducted a secondary analysis of rate of falls of studies conducted in any setting, and did not consider pooling to be appropriate due to high heterogeneity (I² = 92%). This Cochrane Review has focused on studies conducted in care facilities or hospitals and found that whilst vitamin D supplementation did not reduce the risk of falling, it did reduce the rate of falls in care facilities. Our analysis included data on the rate of falls from the same four studies pooled for the risk of falling and whilst there was heterogeneity for the pooled rate of falls outcome (I² = 62%), it was lower than observed in Bolland 2014 for studies overall. Consideration of the acceptability of the intervention should be explored in a cost‐effectiveness analysis and/or discrete choice experiments to gain insight into consumer preferences.
Appendix 12. Contribution of authors for the first version of this review
Contribution of authors for the first version of this review
Ian Cameron and Lesley Gillespie initiated splitting the previous review, entitled 'Interventions for preventing falls in elderly people', into separate reviews for older people living in the community and for older people in nursing care facilities and hospitals. The protocol was adapted by Geoffrey Murray from the previous review with guidance from Lesley Gillespie and Ian Cameron. All authors then met to finalise the protocol before preparation by Geoffrey Murray. Geoffrey Murray was primarily responsible for locating studies, and both he and Ian Cameron decided independently and then by consensus which studies met inclusion criteria. All seven authors assessed quality and extracted data from included studies. Keith Hill adjudicated differences in quality assessments and data in most studies and Geoffrey Murray adjudicated the others. Geoffrey Murray prepared the drafts and did the primary data entry and analysis into RevMan. Lesley Gillespie and Clare Robertson provided guidance with this process. Clare Robertson prepared the generic inverse data for entry into RevMan. All authors commented on re‐analyses and revisions at all stages. Ian Cameron is the guarantor of the review.
Contribution of authors for the 2012 update of this review
Ian Cameron, the guarantor of the review, conceived and designed the review and for this update carried out ’Risk of bias’ assessment and data extraction, assisted with categorisation of trial interventions using the ProFaNE taxonomy, and commented on drafts of the review. Lesley Gillespie conceived the review and for this update co‐ordinated the review, modified the search strategies, carried out the searches, screened search results and obtained papers, screened retrieved papers against inclusion criteria, carried out ’Risk of bias’ assessment and data extraction, entered data into RevMan, and wrote the review. Clare Robertson carried out ’Risk of bias’ assessment and data extraction for all newly included trials, managed data and carried out statistical calculations, wrote the economic evaluation section and Appendix 9, and wrote the review. Geoff Murray conceived and designed the review, and for this update screened retrieved papers against inclusion criteria, updated the Characteristics of included studies table, Appendix 3, Appendix 4 and Appendix 5, assisted with categorisation of trial interventions using the ProFaNE taxonomy, and commented on drafts of the review. Keith Hill carried out ’Risk of bias’ assessment and data extraction, and commented on drafts of the review. Robert Cumming carried out ’Risk of bias’ assessment and data extraction, and commented on drafts of the review. Ngaire Kerse carried out ’Risk of bias’ assessment and data extraction, and commented on drafts of the review.
Data and analyses
Comparison 1. Care facilities: Exercise vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 10 | 2002 | Rate ratio (Random, 95% CI) | 0.93 [0.72, 1.20] |
| 2 Rate of falls and number of fallers: trials with incomplete data | Other data | No numeric data | ||
| 3 Number of fallers | 10 | 2090 | Risk Ratio (Random, 95% CI) | 1.02 [0.88, 1.18] |
| 4 Number of people sustaining a fracture | 1 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 4.1 Hip fractures | 1 | 183 | Risk Ratio (Fixed, 95% CI) | 0.16 [0.01, 2.81] |
| 4.2 All fractures | 1 | 183 | Risk Ratio (Fixed, 95% CI) | 0.88 [0.25, 3.14] |
| 5 Rate of falls, excluding studies with ≤20 participants in each arm | 8 | 1959 | Rate ratio (Random, 95% CI) | 0.91 [0.72, 1.15] |
| 6 Number of fallers, excluding studies with ≤20 participants in each arm | 9 | Risk Ratio (Random, 95% CI) | 1.04 [0.89, 1.21] | |
| 7 Adverse events: aches and pains | 1 | 582 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.61, 2.48] |
| 7.1 Severe soreness | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.40, 2.04] |
| 7.2 Severe bruises | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.69] |
| 7.3 Severe fatigue | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.46, 35.14] |
Comparison 2. Care facilities: Exercises vs usual care (grouped by type of exercise).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 10 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Gait, balance, functional training | 4 | 1347 | Rate Ratio (Random, 95% CI) | 0.96 [0.69, 1.33] |
| 1.2 Whole body vibration | 1 | 62 | Rate Ratio (Random, 95% CI) | 0.96 [0.58, 1.60] |
| 1.3 Combination of exercise categories (see Appendix 4 for categories in each trial) | 6 | 683 | Rate Ratio (Random, 95% CI) | 0.94 [0.60, 1.47] |
| 2 Number of fallers | 10 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Gait, balance, and functional training | 5 | 1452 | Risk Ratio (Random, 95% CI) | 1.02 [0.80, 1.31] |
| 2.2 3D (Tai Chi) | 1 | 59 | Risk Ratio (Random, 95% CI) | 0.60 [0.19, 1.87] |
| 2.3 Whole body vibration vs usual care | 1 | 62 | Risk Ratio (Random, 95% CI) | 0.88 [0.54, 1.43] |
| 2.4 Combination of exercise categories (see Appendix 4 for categories in each trial) | 4 | 607 | Risk Ratio (Random, 95% CI) | 1.07 [0.88, 1.29] |
Comparison 3. Care facilities: Exercise vs usual care (grouped by level of care).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 10 | Rate ratio (Random, 95% CI) | Subtotals only | |
| 1.1 High level nursing care facilities | 2 | 210 | Rate ratio (Random, 95% CI) | 1.79 [0.89, 3.60] |
| 1.2 Intermediate level care facilities | 5 | 1315 | Rate ratio (Random, 95% CI) | 0.70 [0.47, 1.04] |
| 1.3 Facilities providing mixed levels of care | 3 | 477 | Rate ratio (Random, 95% CI) | 1.08 [0.92, 1.28] |
| 2 Number of fallers | 10 | 2090 | Risk Ratio (Random, 95% CI) | 1.02 [0.88, 1.18] |
| 2.1 High level nursing care facilities | 1 | 194 | Risk Ratio (Random, 95% CI) | 1.16 [0.83, 1.62] |
| 2.2 Intermediate level care facilities | 6 | 1419 | Risk Ratio (Random, 95% CI) | 0.94 [0.75, 1.17] |
| 2.3 Mixed level care facilities | 3 | 477 | Risk Ratio (Random, 95% CI) | 1.05 [0.76, 1.47] |
Comparison 4. Care facilities: Comparisons of different exercise programs (see Appendix 4 for details).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 5 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Additional gait, balance, functional training | 2 | 56 | Rate Ratio (Fixed, 95% CI) | 0.62 [0.40, 0.96] |
| 1.2 Strength/resistance vs self‐training | 1 | 34 | Rate Ratio (Fixed, 95% CI) | 0.74 [0.50, 1.10] |
| 1.3 Balance and strength vs self‐training | 1 | 32 | Rate Ratio (Fixed, 95% CI) | 0.48 [0.30, 0.77] |
| 1.4 Flexibility (Yoga) vs 'Staying active' program | 1 | 20 | Rate Ratio (Fixed, 95% CI) | 0.47 [0.24, 0.91] |
| 1.5 3D (Tai Chi) vs 'Staying active' program | 1 | 20 | Rate Ratio (Fixed, 95% CI) | 0.52 [0.28, 0.98] |
| 1.6 Flexibility (Yoga) vs 3D (Tai Chi) | 1 | 18 | Rate Ratio (Fixed, 95% CI) | 1.11 [0.51, 2.37] |
| 1.7 3D exercises ("In balance") vs Functional balance, strength & mobility | 1 | 142 | Rate Ratio (Fixed, 95% CI) | 0.73 [0.60, 0.89] |
| 1.8 Wii balance board vs Otago balance program | 1 | 60 | Rate Ratio (Fixed, 95% CI) | 0.35 [0.19, 0.63] |
| 2 Rate of falls and number of fallers: trials with incomplete data | Other data | No numeric data | ||
| 3 Number of fallers | 5 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Additional gait, balance, and functional training | 2 | 56 | Risk Ratio (Fixed, 95% CI) | 0.79 [0.43, 1.45] |
| 3.2 Strength/resistance vs self‐training | 1 | 34 | Risk Ratio (Fixed, 95% CI) | 0.56 [0.30, 1.03] |
| 3.3 Balance and strength vs self‐training | 1 | 32 | Risk Ratio (Fixed, 95% CI) | 0.55 [0.29, 1.05] |
| 3.4 Additional whole body vibration | 1 | 159 | Risk Ratio (Fixed, 95% CI) | 1.28 [0.71, 2.31] |
| 3.5 3D exercises ("In balance") vs Functional balance, strength & mobility | 1 | 142 | Risk Ratio (Fixed, 95% CI) | 0.92 [0.70, 1.21] |
| 3.6 Comparison of combination exercise programmes | 1 | 41 | Risk Ratio (Fixed, 95% CI) | 0.54 [0.29, 1.01] |
| 4 Number of people sustaining a fracture | 1 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 4.1 Total fractures | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Care facilities: Medication review vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 7 | Rate ratio (Random, 95% CI) | Subtotals only | |
| 1.1 General medication reviews vs usual care | 6 | 2409 | Rate ratio (Random, 95% CI) | 0.93 [0.64, 1.35] |
| 1.2 Medication review for hyponatraemia | 1 | 9 | Rate ratio (Random, 95% CI) | 0.63 [0.16, 2.49] |
| 2 Number of fallers | 7 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 General medication review vs usual care | 6 | 5139 | Risk Ratio (Random, 95% CI) | 0.93 [0.80, 1.09] |
| 2.2 Medication review for hyponatraemia | 1 | 9 | Risk Ratio (Random, 95% CI) | 0.42 [0.07, 2.59] |
| 3 Number of people sustaining a fracture | 1 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 3.1 General medication review vs usual care | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Rate of falls post‐hoc sensitivity analysis (excluding Potter 2016) | 5 | Rate ratio (Random, 95% CI) | Subtotals only | |
| 4.1 General medication reviews vs usual care | 5 | Rate ratio (Random, 95% CI) | 0.82 [0.60, 1.11] | |
| 5 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 General medication review vs usual care | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Care facilities: Vitamin D supplementation vs no vitamin D supplementation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 6 | Rate ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Additional Vitamin D supplementation | 4 | 4512 | Rate ratio (Random, 95% CI) | 0.72 [0.55, 0.95] |
| 1.2 Multivitamins (including vitamin D3 + calcium) vs placebo | 1 | 91 | Rate ratio (Random, 95% CI) | 0.38 [0.20, 0.71] |
| 1.3 Education on Vitamin D + calcium + osteoporosis medications vs usual care | 1 | 4017 | Rate ratio (Random, 95% CI) | 1.03 [0.85, 1.25] |
| 2 Number of fallers | 7 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Vitamin D supplementation | 4 | 4512 | Risk Ratio (Random, 95% CI) | 0.92 [0.76, 1.12] |
| 2.2 Vitamin D + calcium supplementation vs placebo | 1 | 583 | Risk Ratio (Random, 95% CI) | 1.03 [0.90, 1.18] |
| 2.3 Multivitamins (including vitamin D3 + calcium) vs usual care or placebo | 1 | 91 | Risk Ratio (Random, 95% CI) | 0.82 [0.40, 1.66] |
| 2.4 Education on Vitamin D + calcium + osteoporosis medications vs usual care | 1 | 4017 | Risk Ratio (Random, 95% CI) | 1.05 [0.90, 1.23] |
| 3 Number of people sustaining a fracture | 4 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Vitamin D supplementation | 3 | 4464 | Risk Ratio (Random, 95% CI) | 1.09 [0.58, 2.03] |
| 3.2 Vitamin D3 + calcium vs placebo | 1 | 583 | Risk Ratio (Random, 95% CI) | 0.62 [0.36, 1.07] |
| 4 Adverse events | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Multivitamins (including vitamin D3 + calcium) vs usual care or placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Vitamin D + calcium supplementation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Vitamin D supplementation | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Care facilities: Environmental interventions vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Wireless position‐monitoring patch vs usual care | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Care facilities: Social environment vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 4 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Staff education on fracture prevention vs usual care | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Guideline implementation programme vs control | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Risk assessment tool vs nurses' judgement | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Dementia care mapping vs usual care | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of fallers | 1 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 Risk assessment tool vs nurses' judgement | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of people sustaining a fracture | 2 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Risk assessment tool vs nurses' judgement | 1 | 1125 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.57, 1.63] |
| 3.2 Project nurse facilitating best‐practice falls injury prevention strategies vs usual care | 1 | 5391 | Risk Ratio (Fixed, 95% CI) | 0.95 [0.63, 1.44] |
Comparison 9. Care facilities: Psychological interventions vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Exercise + cognitive training vs exercise | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of fallers | 1 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 Exercise + cognitive training vs exercise | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Care facilities: Other single interventions vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 2 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Lavender patch vs placebo | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sunlight exposure vs usual care | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of fallers | 2 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 Lavender patch vs placebo | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Sunlight exposure vs usual care | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of people sustaining a fracture | 1 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 3.1 Sunlight exposure vs usual care | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 11. Care facilities: Multiple interventions vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 2 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Exercise + management of urinary incontinence + fluid therapy vs usual care | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sunlight exposure + calcium vs usual care | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of fallers | 2 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 Exercise + management of urinary incontinence + fluid therapy vs usual care | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Sunlight exposure + calcium vs usual care | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of people sustaining a fracture | 2 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 3.1 Exercise + management of urinary incontinence + fluid therapy vs usual care | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Sunlight exposure + calcium vs usual care | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. Care facilities: Multifactorial interventions vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 10 | 3439 | Rate ratio (Random, 95% CI) | 0.88 [0.66, 1.18] |
| 2 Number of fallers | 9 | 3153 | Risk Ratio (Random, 95% CI) | 0.92 [0.81, 1.05] |
| 3 Number of people sustaining a fracture | 5 | 2160 | Risk Ratio (Random, 95% CI) | 0.79 [0.30, 2.07] |
Comparison 13. Care facilities: Multifactorial interventions vs usual care (grouped by level of care).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 10 | 3439 | Rate Ratio (Random, 95% CI) | 0.88 [0.66, 1.18] |
| 1.1 High level nursing care facilities | 2 | 1499 | Rate Ratio (Random, 95% CI) | 0.59 [0.44, 0.79] |
| 1.2 Intermediate level care facilities | 3 | 670 | Rate Ratio (Random, 95% CI) | 0.64 [0.50, 0.83] |
| 1.3 Mixed level care facilities | 5 | 1270 | Rate Ratio (Random, 95% CI) | 1.23 [0.85, 1.77] |
| 2 Number of fallers | 9 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 High level nursing care facilities | 1 | 981 | Risk Ratio (Random, 95% CI) | 0.75 [0.57, 0.98] |
| 2.2 Intermediate level care facilities | 3 | 670 | Risk Ratio (Random, 95% CI) | 0.75 [0.60, 0.94] |
| 2.3 Mixed level care facilities | 5 | 1502 | Risk Ratio (Random, 95% CI) | 1.01 [0.88, 1.15] |
Comparison 14. Care facilities: Multifactorial interventions vs usual care (grouped by level of cognition).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 10 | Rate ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Participants with cognitive impairment | 4 | 1199 | Rate ratio (Random, 95% CI) | 0.83 [0.49, 1.40] |
| 1.2 Participants with no cognitive impairment or mixed sample | 8 | 1805 | Rate ratio (Random, 95% CI) | 0.84 [0.62, 1.13] |
| 2 Number of fallers | 10 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Participants with cognitive impairment | 4 | 955 | Risk Ratio (Random, 95% CI) | 0.79 [0.57, 1.12] |
| 2.2 Participants with no cognitive impairment or mixed sample | 8 | 1805 | Risk Ratio (Random, 95% CI) | 0.94 [0.78, 1.12] |
Comparison 15. Hospitals: Additional exercises vs usual physiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 2 | 215 | Rate Ratio (Fixed, 95% CI) | 0.59 [0.26, 1.34] |
| 2 Number of fallers | 2 | 83 | Risk Ratio (Fixed, 95% CI) | 0.36 [0.14, 0.93] |
Comparison 16. Hospitals: Medication review vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 2 Number of fallers | 1 | Risk Ratio (Fixed, 95% CI) | Totals not selected |
Comparison 17. Hospitals: Vitamin D supplements vs no vitamin D supplements.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of fallers | 1 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Vitamin D + calcium vs calcium | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of people sustaining a fracture | 1 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 Vitamin D + calcium vs calcium | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Gastrointestinal complaints (nausea, vomiting, diarrhoea) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 18. Hospitals: Environmental interventions vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 5 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Carpet flooring vs vinyl flooring | 1 | 54 | Rate Ratio (Random, 95% CI) | 14.73 [1.88, 115.35] |
| 1.2 Low‐low beds vs usual care | 1 | 11099 | Rate Ratio (Random, 95% CI) | 1.39 [0.22, 8.78] |
| 1.3 Blue identification bracelet vs usual care (no bracelet) | 1 | 134 | Rate Ratio (Random, 95% CI) | 1.15 [0.72, 1.84] |
| 1.4 Bed alarms vs usual care | 2 | 28649 | Rate Ratio (Random, 95% CI) | 0.60 [0.27, 1.34] |
| 2 Number of fallers | 4 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 Carpet flooring vs vinyl flooring | 1 | 54 | Risk Ratio (Fixed, 95% CI) | 8.33 [0.95, 73.37] |
| 2.2 Blue identification bracelet vs usual care (no bracelet) | 1 | 134 | Risk Ratio (Fixed, 95% CI) | 1.34 [0.76, 2.36] |
| 2.3 Bed alarms vs usual care | 2 | 28649 | Risk Ratio (Fixed, 95% CI) | 0.93 [0.38, 2.24] |
Comparison 19. Hospitals: Social environment vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 5 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Organisational service model change (fall prevention guideline implementation) | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Organisation service model change (falls prevention, incontinence and ulcer guideline implementation) | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Organisational service model change (fall prevention toolkit software) | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Acute care service for elderly patients vs usual care | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Post‐operative orthogeriatric service after hip fracture | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of fallers | 3 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 Fall prevention tool kit software vs usual care | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Behaviour advisory service vs usual care | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Post‐operative orthogeriatric service after hip fracture | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of people sustaining a fracture | 1 | Risk Ratio (Fixed, 95% CI) | Totals not selected | |
| 3.1 Post‐operative orthogeriatric service after hip fracture | 1 | Risk Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 20. Hospitals: Knowledge/education interventions vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Educational materials + health professional follow‐up vs usual care | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Educational materials only vs usual care | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of fallers | 2 | Risk Ratio (Random, 95% CI) | Totals not selected | |
| 2.1 Individualised educational session vs usual care | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Educational materials + health professional follow‐up vs usual care | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Educational materials only vs usual care | 1 | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 21. Hospitals: Multifactorial interventions vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 5 | 44664 | Rate ratio (Random, 95% CI) | 0.80 [0.64, 1.01] |
| 2 Number of fallers | 3 | 39889 | Risk Ratio (Random, 95% CI) | 0.82 [0.62, 1.09] |
| 3 Number of people sustaining a fracture | 2 | Risk Ratio (Fixed, 95% CI) | 0.76 [0.14, 4.10] |
Comparison 22. Hospitals: Multifactorial interventions vs usual care (grouped by type of care).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of falls | 5 | 44664 | Rate Ratio (Random, 95% CI) | 0.80 [0.64, 1.01] |
| 1.1 Acute level of care | 1 | 35264 | Rate Ratio (Random, 95% CI) | 1.04 [0.79, 1.37] |
| 1.2 Subacute or acute (mixed) levels of care | 2 | 5653 | Rate Ratio (Random, 95% CI) | 0.88 [0.61, 1.27] |
| 1.3 Subacute level of care | 2 | 3747 | Rate Ratio (Random, 95% CI) | 0.67 [0.54, 0.83] |
| 2 Number of fallers | 3 | Risk Ratio (Random, 95% CI) | 0.82 [0.62, 1.09] | |
| 2.1 Acute level care | 1 | Risk Ratio (Random, 95% CI) | 0.99 [0.33, 3.00] | |
| 2.2 Subacute or acute (mixed) levels of care | 1 | Risk Ratio (Random, 95% CI) | 1.04 [0.48, 2.28] | |
| 2.3 Subacute level of care | 1 | Risk Ratio (Random, 95% CI) | 0.78 [0.57, 1.07] | |
| 3 Number of people sustaining a fracture | 2 | Risk Ratio (Fixed, 95% CI) | 0.76 [0.14, 4.10] | |
| 3.1 Subacute or acute (mixed) levels of care | 1 | Risk Ratio (Fixed, 95% CI) | 0.32 [0.01, 8.95] | |
| 3.2 Subacute level of care | 1 | Risk Ratio (Fixed, 95% CI) | 1.02 [0.14, 7.24] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aizen 2015.
| Methods | Stepped‐wedge, cluster‐randomised controlled trial. | |
| Participants |
Setting: hospital, subacute, 5 geriatric rehabilitation wards, Israel. N = 508 participants; 5 clusters Sample: 52% women Age (years): mean 83.2 Baseline characteristics Individualised fall prevention programme
Usual care
Inclusion criteria: Over 65 years; admitted to rehabilitation ward Exclusion criteria: Restricted to bed; refused to participate Pretreatment differences: Phase 1: Longer stay in the control group patients (P < 0.001); higher percentage of females in the control group (P = 0.03) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | Period of inpatient admission | |
| Notes | Outcomes of phase one used only. Outcomes data for phase one and two only reported separately, attempts to contact authors unsuccessful. Excluded from pooling as group allocation of clusters unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information for judgement. |
| Allocation concealment (selection bias) | High risk | Allocation not concealed as consent only required for those receiving the intervention. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Senior nursing staff in control wards were aware of the study because the researchers were collecting study data. Researchers were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition balanced across groups and missing outcomes not great enough to have a clinically relevant impact on observed effect size. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Method of ascertaining falls | Low risk | Quote: "from notes in medical records themselves, and by asking a senior nurse each day about any falls on the ward in the past 24 h." Quote: "Information on falls was collected by the researchers from incident reports filed in patients’ medical records," |
| Baseline imbalance | High risk | Longer length of stay in control group at baseline suggests greater dependency in this group at baseline and not adjusted for in analysis |
| Other bias | Unclear risk | Quote: "some falls prevention activities were already occurring in control (and intervention) wards before the start of our study. These activities continued during the study period, making it more difficult to show any effect of our interventions." Impact of other falls intervention approaches unclear. Stepped‐wedge trial but only data from phase 1 used as falls data not reported for both phases in combination. |
Ang 2011.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: acute care hospital, Singapore
N = 1822 participants
Sample: newly admitted patients from 8 medical wards (50% women)
Age (years): mean (SD) intervention group 70.3 (14.2), control group 69.7 (14.7)
Inclusion criteria: aged ≥ 21; Hendrich II Fall Risk Model score ≥ 5 Exclusion criteria: admitted before start of study; fallen prior to falls risk assessment |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 8 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation of the participants to control or intervention groups was determined using block randomisation with the aid of a computer program and stratified by ward to ensure an even mix in the ward." |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed, opaque, serially numbered envelopes were produced from the randomizations sequence separately for each stratum." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The research investigator scanned the electronic hospital occurrence report (eHOR) daily during weekday for entries of fall incidences reported by the nurses from the wards and ascertained if the entries were on participants involved in the study." Nursing staff recording falls described as blind to group allocation. Not clear if the research investigator was blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: all data analysed according to ITT. |
| Selective reporting (reporting bias) | High risk | Judgement comment: methods mention incidence of falls but only data on risk of falling reported. |
| Method of ascertaining falls | High risk | Judgement comment: falls not clearly defined. |
| Baseline imbalance | Low risk | No important differences at baseline. |
| Other bias | Unclear risk | Unclear impact of standard falls prevention activities. |
Barker 2016.
| Methods | Cluster‐randomised controlled trial | |
| Participants |
Setting: 24 acute medical and surgical wards from 6 hospitals, Australia N = 31,411 unique participants, including 3853 admitted to both intervention and control wards at different times; 24 clusters. Sample: 48.5% women Age (years): median 67 (interquartile range 51‐79) Baseline characteristics: 6‐PACK programme
Usual care
Inclusion criteria: Wards: where fall‐related injuries have been identified as a problem, acute medical or surgical wards; average patient length of stay <10 days; wards to have one or less low‐low beds to each six standard beds on medical wards and one or less low‐low beds to each, 29 standard beds on surgical wards; a fall risk assessment and/or prevention strategy checklist is not already included in the daily patient care plan documentation. Wards that have a fall risk assessment and/or prevention strategy checklist included on admission documentation but do not have a policy that this must be updated daily will not be excluded from participating in the study. Exclusion criteria: No patient level exclusion criteria. Pretreatment differences: Nil |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months intervention period plus 3 month pre‐randomisation baseline period | |
| Notes | ACTRN12611000332921 "The use of all 6‐PACK programme components (fall risk tool and six interventions) was threefold higher on intervention wards than on control wards (incidence rate ratio 3.05, 95% confidence interval 2.14 to 4.34; P<0.001)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "used the RALLOC command in Stata to develop the randomisation schedule, using a random sequence in blocks of two generated by the study statistician." Judgement comment: random sequence allocation done. |
| Allocation concealment (selection bias) | High risk | Quote: "Concealment of allocation was ensured, as the schedule was accessible only by the study statistician, who was not involved in ward recruitment or data collection." Judgement comment: although allocation sequence initially concealed, subjects were enrolled after cluster randomisation, and sequence would have been known at this time. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "It will not be feasible to blind ward nurses or patients to the intervention." Judgement comment: not done. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Blinding of the assessors collecting the fall and falls prevention practice data was also not possible. Assessors blinded to group allocation did the secondary coding of characteristics of falls and injuries, and the primary assessor completed the coding. A statistician blinded to group allocation (RW) did the data analysis." Judgement comment: not done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all falls outcomes reported as per trial registry record. |
| Method of ascertaining falls | Low risk | Quote: "by daily auditing of patient medical records and verbal report of the nurse unit managers. These data will then be triangulated with hospital incident reporting and administrative patient episode datasets. Concurrent to this will be hospital‐wide education and reminders of the fall definition and incident reporting best practice, facilitated by use of an existing training package. 23 Patient" Judgement comment: multiple methods of concurrent recording of falls data used. |
| Baseline imbalance | Low risk | Quote: "Characteristics of admitted patients and length of stay were similar for intervention and control groups and across baseline and randomised controlled trial periods" Judgement comment: no imbalance across groups. |
| Other bias | Unclear risk | Unclear impact of any ongoing falls prevention activities. |
Beck 2016.
| Methods | RCT (cluster randomised), nursing home subgroup data. | |
| Participants |
Setting: 3 residential care homes, high‐level care, Denmark N = 31 participants; 3 clusters. Sample: 65% women Age (years): mean 88 Baseline Characteristics Multidisciplinary nutritional support
Control
Inclusion criteria: 65+ years, at nursing home or receiving home care (assistance with meals) with 2 points according to Eating Validation Scheme (EVS) completed by nursing staff caregivers (would benefit from intervention) able to completed planned tests Exclusion criteria: not able or willing to give informed consent Pretreatment differences: living in a nursing home: intervention 16%, control 55% (P < 0.001); 30‐seconds chair‐stand modified, mean (SD) 4.9 (3.3) intervention, 2.5 (2.7) control (P = 0.004); cognitive problem 56% intervention versus 78% control (P = 0.03) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 11 weeks | |
| Notes | A trial of nutritional support using a structured and multidisciplinary approach, focusing on nutritional risk factors, in undernourished older adults in both home care and nursing home settings, with results reported separately. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: sequence generation by drawing a lot for an opaque envelope. |
| Allocation concealment (selection bias) | High risk | Judgement comment: randomisation by researcher not involved in the study (2016 p200). Author correspondence quote: "participants were invited by means of the staff who did not know about the result of the group allocation". and "we did not include new admissions". However: "Due to the limited knowledge about the benefit of nutritional support among home‐care clients, the aim was to randomly assign 2 of the 3 home‐care clusters to the intervention group", this is likely to enable the randomisation sequence to be predicted, concealment not possible for the final cluster. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The researchers for this study included the research assistants (AGC, BSH, SD‐S, and TKSM) and the primary investigator (AB), who were not blinded for the intervention." Judgement comment: blinding not done. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The researchers for this study included the research assistants (AGC, BSH, SD‐S, and TKSM) and the primary investigator (AB), who were not blinded for the intervention. Before starting the analysis the primary investigator (AB) was reblinded for participants’ group assignment." Judgement comment: not done, falls data collected by unblinded research nurse. Although primary investigator "reblinded" before analysis no details were reported on the method for this and it is considered likely to include a risk of residual unblinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: author correspondence clarified data and indicated one withdrawal, no other loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: trial protocol available and falls outcomes consistently reported. |
| Method of ascertaining falls | Low risk | Quote: "The information was gathered by means of data from the RAI‐NH version 2.0 and RAI‐HC version 2.0 assessments and the municipality care register system. For each participant, the same trained nurse collected" Judgement comment: concurrent falls data collection with clear definition. |
| Baseline imbalance | High risk | Judgement comment: baseline imbalance in nursing home subgroup for cognition, no adjustment performed |
| Other bias | Low risk | None detected. |
Becker 2003.
| Methods | RCT (cluster randomised by facility). | |
| Participants | Setting: 6 long‐term care facilities (high‐level nursing care), Germany N = 981 participants; 6 clusters. Sample: 79% women Age (years): mean (SD) intervention group 83.5 (7.5), control group 84.3 (6.9) Inclusion criteria: resident of facility. Inclusion criteria for exercise programme: able to stand while holding a chair, able to lift one foot Exclusion criteria: none stated | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster randomisation of 6 facilities using sealed envelopes selected by an independent person. Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Allocation in sealed envelopes, but individuals admitted after group allocation by a person who may have been unblinded and may have had knowledge of participant characteristics |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff at facilities who recorded falls were likely to be aware of their facility's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All residents included in analysis. |
| Selective reporting (reporting bias) | Low risk | All expected falls outcomes completely and thoroughly reported. Adjustment for clustering conducted |
| Method of ascertaining falls | Low risk | Fall definition provided and concurrent recording of falls |
| Baseline imbalance | High risk | Greater proportion of intervention group were taking 4 or more medications |
| Other bias | Low risk | None identified. |
Bischoff 2003.
| Methods | RCT (individually randomised) | |
| Participants | Setting: 2 hospitals with long‐stay geriatric care units, Basel, Switzerland N = 122 participants Sample: 100% women Age (years): mean (SD) intervention group 85.4 (5.9), control group 84.9 (7.7) Inclusion criteria: female; aged ≥ 60; able to walk 3 metres Exclusion criteria: primary hyperparathyroidism; hypercalcaemia; hypercalcuria; renal insufficiency; fracture or stroke in last 3 months | |
| Interventions | 1. 800 IU oral cholecalciferol (vitamin D3) plus 1200 mg calcium daily for 12 weeks 2. Control: 1200 mg calcium daily for 12 weeks | |
| Outcomes |
|
|
| Duration of the study | 12 weeks | |
| Notes | 50% of participants had a baseline serum vitamin level < 30 nmol/L | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was performed by an independent statistician." |
| Allocation concealment (selection bias) | Low risk | Participants randomised in groups of four by an independent statistician |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patients, nurses, and all investigators were blinded to the treatment assignment throughout the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: high loss to follow‐up (31% in vit D and 25% in control group); however, analysed as ITT with rate ratio accounting for days of follow‐up and balanced between groups |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no study protocol identified, but data on falls, fallers, multiple falls as adjusted and adjusted outcomes reported. |
| Method of ascertaining falls | Low risk | Quote: "Falls were recorded by the nurses on the inpatient units who had received training in the use of the fall protocol (date, time, circumstances, injuries). Falls were defined as “unintentionally coming to rest on the ground, floor, or other lower level.” Coming to rest against furniture or a wall was not counted as a fall. (24) Nurses completed the fall protocol if they observed or received a report of a fall." |
| Baseline imbalance | Low risk | Judgement comment: characteristics and number of falls balanced at baseline |
| Other bias | Low risk | Judgement comment: none identified. Small groups randomised however given trial is double‐blinded randomisation unlikely to be predictable |
Broe 2007.
| Methods | RCT (individually randomised) | |
| Participants | Setting: 1 long‐term care facility (high‐level care), USA N = 48 participants included in review (total of 124 in the study) Sample: 73% women Age (years): mean 89 (SD 6) Inclusion criteria: life expectancy > 6 months; able to swallow medications; resident for > 3 months Exclusion criteria: taking glucocorticoids; anti‐seizure medications; pharmacological doses of vitamin D; calcium metabolism disorders; severe mobility restriction; fracture within previous 6 months | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 5 months | |
| Notes | Mean baseline serum vitamin D level for 800 IU group and control group combined was 53 nmol/L | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... computer‐generated randomisation list." |
| Allocation concealment (selection bias) | Low risk | Pharmacy conducted randomisation and supplied medication in blister packs with name and patient identification number only |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nursing staff completing incident forms blinded to treatment status because blister packs and tablets identical in appearance. Also, quote: "a programmer, not involved with this study and not aware of participant study group assignments, created the falls dataset linking the participant identification number with falls reported during the study period" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: low loss to follow‐up and ITT analysis performed. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no protocol identified, however all expected outcomes (falls, rate of falls and fallers) reported. |
| Method of ascertaining falls | Low risk | Judgement comment: falls concurrently recorded and clearly defined |
| Baseline imbalance | Unclear risk | Judgement comment: few differences at baseline; however baseline cognition, medical comorbidities and function not reported |
| Other bias | Low risk | None identified. |
Buckinx 2014.
| Methods | RCT. Individually randomised. | |
| Participants |
Setting: 2 residential care facilities, intermediate‐level care, Belgium N = 62 participants Sample: 76% women Age (years): mean 83.2 (SD 7.9) Baseline characteristics Whole body vibration
Control
Inclusion criteria: residents from two nursing homes; able to remain standing; able to move with or without technical assistance Exclusion criteria: weight greater than 150 kg; electronic implants; knee or hip prostheses; epilepsy; bleeding disorders; inflammatory abdominal disorders; high risk of thromboembolism; malignancy; unconsolidated fracture; refusal of doctor or family Pretreatment differences: gender (more women in control group) P = 0.04; lower body mass in control group P < 0.01; lower MMSE in control group, P = 0.04 |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 6 months intervention, follow‐up to 12 months. | |
| Notes | Compliance: 91.9% of exercise sessions performed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We performed the randomisation by blocks of four with a computer‐generated randomisation procedure." Judgement comment: computer‐generated randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "An identification number and a randomisation number were created for each participant." Judgement comment: method of concealment of allocation sequence from those enrolling participants was unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not done. Blinding not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: not done. Nurses recorded falls, they were not blinded. Blinded assessment unlikely to include falls outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: numbers and reasons balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: number of falls not defined as outcome in trial registry. Trials registry indicates 3 months outcomes. Reporting of falls data appears complete although not predefined. |
| Method of ascertaining falls | Low risk | Quote: "Nurses completed the fall record with the date, time, and circumstances of the falls." Judgement comment: likely that falls were recorded at time of event. |
| Baseline imbalance | High risk | Baseline differences in weight, gender, MMSE may impact on falls rates. |
| Other bias | Low risk | Judgement comment: none identified |
Buettner 2002.
| Methods | RCT (individually randomised) | |
| Participants | Setting: 3 nursing care facilities, USA (1 high‐level nursing facility, 1 skilled nursing facility, 1 intermediate‐level facility) N = 27 participants Sample: 44% women Age (years): mean 83.3 (range 60 to 98) Inclusion criteria: ≥ 2 falls in past 2 months between 7.00 am to 9 am; MMSE score < 23; aged > 60; walking independently, or with 1 assistant or assistive device Exclusion criteria: not resident for ≥ 60 days; a healing fracture; attending physiotherapy | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 2 months | |
| Notes | Published data incomplete. Further data provided by authors could not be analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff collecting falls data do not appear to have been blinded to allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: insufficient detail on which patients are included in data analysis for judgement. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified. Number of falls analysed as t‐test of absolute numbers without rate, considering pre‐test falls. |
| Method of ascertaining falls | High risk | Judgement comment: falls not clearly defined |
| Baseline imbalance | Unclear risk | Judgement comment: Baseline characteristics not reported by allocation group |
| Other bias | Low risk | Judgement comment: None detected |
Burleigh 2007.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: general assessment and rehabilitation wards in an acute geriatric unit, Glasgow, Scotland
N = 205 participants
Sample: 59% women, median serum vitamin D (25 OHD) = 22.00 nmol/L, IQR 15.00 to 30.50 at baseline.
Age (years): mean (SD) intervention 82.3 (7.6), control 83.7 (7.6)
Inclusion criteria: admitted to a ward in the acute geriatric unit; aged ≥ 65 Exclusion criteria: hypercalcaemia; urolithiasis; renal dialysis; terminal illness; bed bound; reduced Glasgow Coma Score; already prescribed vitamin D and calcium; 'nil by mouth' on admission |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | Aproximately 9 months. Median length of stay 30 days | |
| Notes | Baseline serum vitamin D (25 OHD) = median 22.00 nmol/L, IQR 15.00 to 30.50 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... randomised using a random numbers table" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was known only to the statistician and pharmacist who subsequently issued an appropriate uniquely numbered drug blister pack to each patient’s ward." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Staff completing falls data may have been aware of treatment status as there was no placebo in place of vitamin D. Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: ITT analysis and losses balanced between groups |
| Selective reporting (reporting bias) | Low risk | Judgement comment: falls and fallers outcomes reported as per trial registration |
| Method of ascertaining falls | Low risk | Judgement comment: falls defined and recorded concurrently |
| Baseline imbalance | High risk | Judgement comment: 2x2 indicates significant difference in proportion with Zimmer frame between groups (P = 0.02). |
| Other bias | Low risk | Judgement comment: falls and fallers outcomes reported as per trial registration |
Cadore 2014.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: residential care facility, mixed‐level care, Spain N = 24 participants Sample: 70% women Age (years): mean 91.9 (SD 4.1) Baseline Characteristics Multicomponent exercises
Control
Inclusion criteria: nursing home residents from Pamplona, Spain; 85 years or older; frail (as per Fried's criteria): 3 or more of slowness, weakness, weight loss, exhaustion, and low physical activity Exclusion criteria: the absence of frailty or pre‐frailty syndrome; dementia; disability (defined as a Barthel Index (BI) lower than 60 and inability to walk independently without help of another person); recent cardiac arrest; unstable coronary syndrome; active cardiac failure; cardiac block; any unstable medical condition Pretreatment differences: baseline demographic data not reported |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation sequence was generated by http://www.randomization.com and concealed until interventions were assigned." |
| Allocation concealment (selection bias) | Low risk | Quote: "and concealed until interventions were assigned." Judgement comment: author correspondence. Quote: "The group allocation was concealed. A researcher with no previous contact with subjects as well as not involved with assessment and training made the allocation of subjects." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding of participants not possible due to active involvement in intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: blinding mentioned is not for falls outcomes. Residents who were not blinded recorded falls. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: loss to follow‐up low and balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol or trials registry records identified |
| Method of ascertaining falls | High risk | Quote: "Data on the incidence of falls were assessed retrospectively using questionnaires to residents." Judgement comment: based on recall of participants. |
| Baseline imbalance | Unclear risk | Judgement comment: baseline demographic data not reported |
| Other bias | Low risk | Judgement comment: none identified |
Chapuy 2002.
| Methods | RCT (individually randomised) | |
| Participants | Setting: 55 intermediate nursing care facilities, France N = 610 participants Sample: 100% women Age (years): mean 85.2 (SD 7.1) Inclusion criteria: ambulatory; life expectancy > 2 years Exclusion criteria: malabsorption; serum calcium > 2.63 mmol/L; chronic renal failure (serum creatinine >150 μmol/L), taking bone metabolism altering medications within the past year, e.g. corticosteroids, anticonvulsants or high doses of thyroxine; fluoride salts (43 months), bisphosphonates, calcitonin (41 month), calcium (4500 mg/day) and vitamin D (4100 IU/day) during the last 12 months | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 24 months | |
| Notes | Described as "apartment houses for elderly people" in Chapuy 2002 but provision of drugs supervised by nursing staff "to ensure compliance". Mean baseline serum vitamin D level 22 nmol/L | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Although described as multicentre, randomised, double‐masked, placebo‐controlled, the method of concealment prior to allocation is not described in sufficient detail to allow a definite judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of treatment status to outcome assessors not mentioned. Participants were asked if they had an adverse event (including falls) in last 3 months. Not clear if the person asking would have known allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: loss to follow‐up over 2‐year period unclear. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified. |
| Method of ascertaining falls | High risk | Falls events poorly defined. |
| Baseline imbalance | Low risk | Judgement comment: groups balanced at baseline |
| Other bias | Low risk | Judgement comment: none detected |
Chenoweth 2009.
| Methods | RCT (cluster randomised by unit). | |
| Participants |
Setting: 15 residential dementia care units (high‐level nursing care), Sydney, Australia
N = 289 residents; 15 clusters
Sample: people with dementia (78% women) Age (years): mean (SD) person‐centred care group 83 (7.6), dementia‐care mapping group 84 (6.4), usual care group 83 (7.6) Inclusion criteria (facilities): task‐focused (not person‐centred) care systems. Inclusion criteria (residents): dementia and low cognitive function; aged >60; high dependency needs; persistent need‐driven dementia compromised behaviours Exclusion criteria (residents): serious co‐morbidities complicating or masking dementia; palliative care; unremitting pain; distressing physical symptoms; respite placement |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 8 months | |
| Notes | Person‐centred care emphasised social interactions at affective level based on life histories; aimed to preserve personal identity and foster meaningful relationships. Dementia‐care mapping: "mapping" consisted of observation of each participant for 6 hours per day for 2 days to identify factors related to well‐being |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation was done by the study statistician (MTK), who was unaware of the identity of sites, using an SAS20 program." |
| Allocation concealment (selection bias) | Low risk | Eligible residents were selected by facility managers or directors before randomisation of sites |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Treatment allocation was masked to assessors." Three separate research assistants collected outcome data from each cluster of five facilities. Staff of facilities instructed not to inform assessors of interventions |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: >20% loss from person‐centred care and usual care arms. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: falls stated as outcome ‐ Incidents, quote: "and subsequent admissions to hospital were discerned from official records of incidents including residents’ falls, fractures, lacerations, bruises, medication errors, and behavioural incidents" (p320, column 1, para 2). However, falls not stated as outcome in initial trial registry record (added retrospectively) |
| Method of ascertaining falls | High risk | Judgement comment: falls poorly defined and multiple sites enrolled. |
| Baseline imbalance | Low risk | Judgement comment: differences at baseline adjusted for in analysis. |
| Other bias | Low risk | Judgement comment: none identified. |
Choi 2005.
| Methods | RCT (cluster randomised). | |
| Participants | Setting: 2 residential care facilities (intermediate‐level care), Korea N = 68 participants; 2 clusters. Sample: 75% women Age (years): mean 77.9 (range 61 to 91) Inclusion criteria: ambulatory; age > 60; at least one fall risk factor (impaired gait, impaired balance; a fall in the last year; postural hypotension; four or more medications affecting balance) Exclusion criteria: severe dementia; physical illness that may prevent completion of 12‐week course of exercise; involvement in any other exercise | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 3 months | |
| Notes | Cluster randomised, described as quasi‐experimental design with a non‐equivalent control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... two facilities with similar characteristics were selected and randomly assigned to either the experimental or control group by coin tossing." |
| Allocation concealment (selection bias) | High risk | After first toss the allocation of the second facility would be known. No description of whether individual participant recruitment was undertaken after group allocation by a person who was unblinded and may have had knowledge of participant characteristics |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff at facilities who recorded falls were likely to be aware of their facility's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: loss similar between groups. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified. |
| Method of ascertaining falls | High risk | Judgement comment: falls defined but only recorded weekly. |
| Baseline imbalance | High risk | Judgement comment: significant difference between groups in muscle strength and balance measures ‐ addressed for balance and strength scores by using difference scores ‐ but no adjustment apparent for falls data. |
| Other bias | Low risk | Judgement comment: assignment predicable as cluster randomised and only 2 facilities, however this accounted for under allocation concealment. No other sources of bias identified. |
Clifton 2009.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: 1 veterans skilled nursing facility (high‐level nursing care), Washingon state, USA
N = 43 participants
Sample: 5% women
Age (years): mean 82.2 (SD 7.1)
Inclusion criteria: expected length of stay > 120 days; high risk of falling (Morse Scale score ≥ 50); unable to ambulate or transfer without assistance Exclusion criteria: history of adverse reaction to medical adhesives; mechanobullous disease; skin breakdown on the legs > 10 cm; skin eruption on the legs |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | Cross‐over after 60 days for second 60‐day period | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence generated using a web‐based programme |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation of sequence, performed by the study coordinator, was masked until informed consent was obtained from each respective subject." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Caregivers recorded falls. Not blind to FallSaver use |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: higher loss to follow‐up in intervention arm due to discontinuing intervention. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: falls outcomes reported as per trial registration |
| Method of ascertaining falls | Low risk | Judgement comment: falls defined and recorded concurrently. |
| Baseline imbalance | Unclear risk | Judgement comment: characteristics not reported by group allocation. |
| Other bias | High risk | Judgement comment: author employed by company making FallSaver devices |
Colon‐Emeric 2013.
| Methods | RCT (cluster randomised), pilot study | |
| Participants |
Setting: 8 residential care facilities, 4 veterans affair, 4 community, USA. N = Not Reported (NR). 8 clusters, 982 facility beds. Sample: NR Age (years): NR Baseline Characteristics CONNECT & FALLS
FALLS only
Inclusion criteria: residents: aged 50 years or over; experienced one or more falls during the study period, and remained in the NH at least 72 hours after the fall. Staff: all NH employees aged 18 and older who had direct resident contact were eligible for participation. Emloyees from nursing, rehabilitation, social work, dietary services, environmental services, activities, medical services and administration. Exclusion criteria: Staff: temporary agency staff and staff working only as needed Pretreatment differences: more patients who fell had visual impairment in intervention nursing homes, more Caucasian staff in intervention nursing homes |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 24 weeks intervention (12 weeks CONNECT/control plus 12 weeks FALLS), 6 months post‐intervention follow‐up. | |
| Notes | NCT00836433. Baseline data and N for all residents not known, confirmed by author correspondence. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: sequence by random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: person assigning treatment groups was blinded to nursing home identity, but unclear if individual participant recruitment (staff) was completed prior to assignment of the cluster. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: unable to blind personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: as staff would have recorded falls and staff were the subject of the intervention, it is unlikely that blinding would have been possible for those recording falls data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: there were no missing data for fall rates. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: data on falls are reported as per trial record and includes the main expected falls outcomes. |
| Method of ascertaining falls | Low risk | Quote: "Falls were ascertained from facility fall logs, incident reports, and the Minimum Data Set; occupied bed days were calculated from daily census data that each facility provided." Judgement comment: falls were clearly defined and likely to be recorded concurrently in facility fall logs used as the data source. |
| Baseline imbalance | Low risk | Judgement comment: the analysis accounted for clustering and potential confounders |
| Other bias | Low risk | Judgement comment: none identified. |
Cox 2008.
| Methods | RCT (cluster randomised by Primary Care Organisation (PCO) each containing nursing care facilities). | |
| Participants | Setting: 209 care homes (high and intermediate level care), England and Wales N = 5637 participants. 29 clusters Sample: 77% women Age (years): not stated Inclusion criteria (facilities): if local ethics and research governance procedures were swift enough to enable enrolment Exclusion criteria (facilities): if demographic information was not provided | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | 5 of 29 clusters lost to follow‐up in intervention group compared with 16 of 29 clusters in control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The PCOs were stratified into two groups, larger PCOs and smaller PCOs based on the median number of care homes. Within each stratum, a single block of allocations was undertaken using a computer package to ensure equivalent numbers of PCOs in each group." |
| Allocation concealment (selection bias) | Low risk | Quote: "All PCO demographic data were forwarded to the Department of Health Science at the University of York for randomisation and allocation." "The allocation was undertaken by an independent researcher." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no statement re blinding. Facilities and staff (including manager reporting outcome data) knew of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff at facilities who recorded falls were likely to be aware of their facility's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: 16% loss to follow‐up for control group |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no protocol, but all expected outcomes reported (number of falls, risk of falls and fractures) and as described in methods. |
| Method of ascertaining falls | High risk | Judgement comment: no fall definition reported. Fall and fracture data collected via questionnaire to each facility manager ‐ likely variability |
| Baseline imbalance | Unclear risk | Judgement comment: baseline data on cognition, comorbidities, function not reported. |
| Other bias | Low risk | Judgement comment: none identified. |
Crotty 2004a.
| Methods | RCT (individually randomised) | |
| Participants | Setting: patients awaiting transfer from a hospital to a long‐term care facility, Australia N = 110 participants Sample: 61% women Age (years): mean 82.7 (SD 6.4) Inclusion criteria: acute and subacute hospital patients being transferred to nursing care facility; life expectancy greater than a month Exclusion criteria: none stated | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months. Participants followed up for 8 weeks post discharge | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study biostatistician provided a computer‐generated allocation sequence that used block randomization and was stratified by hospital.” |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was coordinated by a centralized hospital pharmacy service." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear whether staff recording falls were aware of existence of transfer summaries and case conferences |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: reasons for loss to follow‐up similar between groups. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: falls were a secondary outcome measure. |
| Method of ascertaining falls | High risk | Judgement comment: no clear definition or staff training described |
| Baseline imbalance | Low risk | Judgement comment: no significant difference between groups at baseline |
| Other bias | Low risk | None identified. |
Crotty 2004b.
| Methods | RCT (cluster randomised) Cluster randomisation of regions such that each metropolitan health area allocated to intervention or control. Facility in an intervention region selected at random and matched to a facility in a control region. Matching facilities not randomised | |
| Participants | Setting: 20 residential care facilities (10 high‐ and 10 low‐level care), Adelaide, Australia N = 715 participants. 20 clusters. Sample: 84% women Age (years): mean 84.1 (SD 7.8) Inclusion: none stated Exclusion criteria: none stated | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 7 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All randomisation was conducted using a computer‐generated random allocation program by a person external to the project." |
| Allocation concealment (selection bias) | High risk | Cluster randomisation of regions. Facility in an intervention region selected at random and matched to a facility in a control region |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: outcome was assessed blind to group allocation but intervention facilities would have been aware of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff at facilities who recorded falls were likely to be aware of their facility's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: losses to follow‐up even between groups |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: injurious falls included as outcome measure but not reported |
| Method of ascertaining falls | High risk | Judgement comment: no clear definition of falls, no staff training. |
| Baseline imbalance | Low risk | Judgement comment: adjusted for baseline differences. |
| Other bias | Low risk | None identified. |
Cumming 2008.
| Methods | RCT (cluster randomised) Cluster randomisation of 12 matched pairs of wards | |
| Participants | Setting: 24 acute and subacute wards in 12 hospitals, Sydney, Australia N = 24 wards, 3999 patients. 24 clusters. Sample: 59% women Age (years): mean 79.0 (SD 12.8) Inclusion criteria: all admitted patients Exclusion criteria: none stated | |
| Interventions |
NB. Continuation of existing pre‐trial falls prevention activities in control and intervention wards during the study. |
|
| Outcomes |
|
|
| Duration of the study | 3 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation of each matched pair of wards was usually done during the week before the study started for that pair of wards. Randomisation involved sealed, opaque envelopes and was supervised by a study investigator ... unaware of ward characteristics." |
| Allocation concealment (selection bias) | Low risk | Quote: "We included all patients in study wards during each three month study period." "Randomisation of each matched pair of wards was usually done during the week before the study started for that pair of wards. Randomisation involved sealed, opaque envelopes and was supervised by a study investigator ... unaware of ward characteristics." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff at the wards who recorded falls were likely to be aware of their ward's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: outcomes reported as per trial registration |
| Method of ascertaining falls | Low risk | Judgement comment: falls clearly defined and collected concurrently. |
| Baseline imbalance | Low risk | Judgement comment: groups well‐balanced at baseline. |
| Other bias | Unclear risk | Quote: "Another limitation is that some falls prevention activities were already occurring in control (and intervention) wards before the start of our study. These activities would have continued during the study period, making it more difficult to show any effect of our interventions." Judgement comment: some other falls prevention activities ongoing ‐ impact of this unclear. |
da Silva Borges 2014.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: residential care facilities, intermediate‐level care, Brazil N = 59 Sample: NR Age (years): 68 Baseline Characteristics Ballroom dancing programme
Control
Inclusion criteria: resident of long‐stay institution in Rio de Janeiro state, Brazil, functionally autonomous in ADL, had not engaged in any regular physical activity for at least three months Exclusion criteria: any condition that could prevent a participant from undergoing tests or interventions (such as cardiopathy, hypertension, uncontrolled asthmatic bronchitis, osteoarthritis, recent fracture, tendinitis, neurological problems and severe obesity, as well as the use of a prosthesis or medication that could cause attention disorders); cognitive impairment, especially memory function Pretreatment differences: unclear, baseline characteristics not reported |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated by simple draw" Judgement comment: unclear how the draw was conducted and whether or not this would result in a truly random sequence. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: it is unclear who reported the falls data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: to exclude patients due to falls may have a significant impact on falls data if these patients were multiple fallers or at high risk. Group allocation is not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available.Falls data not published due to error in article. |
| Method of ascertaining falls | High risk | Judgement comment: falls data were not recorded concurrently, it is unclear what type of medical records were accessed to confirm falls, this may not include records of non‐injurious falls. |
| Baseline imbalance | Unclear risk | Judgement comment: baseline characteristics of participants not reported. |
| Other bias | Low risk | Judgement comment: none detected |
Donald 2000.
| Methods | RCT (2 x 2 factorial design) | |
| Participants | Setting: 1 elderly care rehabilitation (subacute) ward, Gloucester, UK N = 54 Sample: individuals admitted to one elderly care rehabilitation ward over an 8‐month period (81% women) Age (years): mean 83 Inclusion criteria: patients admitted for rehabilitation Exclusion criteria: none stated | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 9 months. Follow‐up of individual patients was duration of admission (mean length of stay 29 days) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. Quote: "Using randomized envelopes for each risk group, patients were assigned a floor group (carpet or vinyl) and a physiotherapy group (conventional physiotherapy or additional exercise)." |
| Allocation concealment (selection bias) | Unclear risk | Randomised achieved by randomising envelopes. Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors do not appear to have been blinded to treatment status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: high loss to follow‐up but ITT analysis for falls outcomes. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no protocol identified, but falls and fallers data reported completely. |
| Method of ascertaining falls | Unclear risk | Judgement comment: falls clearly defined, but insufficient information on frequency of recording of falls data for judgement. |
| Baseline imbalance | Low risk | Judgement comment: groups balanced at baseline. |
| Other bias | Low risk | Judgement comment: none identified. |
Dyer 2004.
| Methods | RCT (cluster randomised) | |
| Participants |
Setting: 20 residential care homes (intermediate‐level care), UK
N = 196 participants. 20 clusters.
Sample: 78% women
Age (years): mean (SD) intervention group 87.4 (6.9), control group 87.2 (6.9)
Inclusion criteria (facilities): ≥ 5 residents; not specializing in mental illness; without nursing services. Inclusion criteria (residents): aged ≥ 60 Exclusion criteria: temporary residents or terminal illness |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence used computer‐generated random number tables |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence was performed and kept secure by a researcher independent of the study, and blinded to baseline assessment results." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff at the facilities who recorded falls were likely to be aware of their facility's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses balanced between groups. |
| Selective reporting (reporting bias) | Low risk | No protocol identified but all expected falls data comprehensively reported as falls, fallers, multiple fallers and fractures reported. |
| Method of ascertaining falls | Low risk | Data collected concurrently and clear definition. |
| Baseline imbalance | Unclear risk | Differences in cognition and medications at baseline, unclear if adjusted for in analysis. |
| Other bias | Low risk | None identified. |
Dykes 2010.
| Methods | RCT (cluster randomised) randomised 2 units matched on fall rates and patient days within each of 4 hospitals | |
| Participants |
Setting: 8 acute medical units, Boston, Massachusetts, USA
N = 5264 patients aged ≥ 65. 8 clusters.
Sample used in this review: patients aged ≥ 65 (% women not available) Age (years): mean 78.8 (SD 8.4) in patients aged ≥ 65 Inclusion criteria (units): fall rates higher than institution's mean rate for previous year; had a match within the institution (unit with similar fall rate and length of stay). Inclusion criteria (patients): all patients admitted to randomised units during study Exclusion criteria (units): involved in other performance improvement efforts relating to fall prevention |
|
| Interventions |
Both groups used Morse Falls Scale to assess risk of falls on admission, daily and with change in status |
|
| Outcomes |
|
|
| Duration of the study | 6 months | |
| Notes | Data for participants aged < 65 and ≥ 65 reported separately in Dykes 2010. Only data for participants aged ≥ 65 included in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "Matched units were randomised" Insufficient information to permit judgement |
| Allocation concealment (selection bias) | High risk | At each hospital pairs of wards were allocated to intervention and control, then patients admitted to these wards were recruited |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “the intervention was not blinded and falls were reported by unit‐based caregivers who implemented fall prevention interventions.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: all patients included in ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: falls outcomes reported consistent with trial registration |
| Method of ascertaining falls | Low risk | Judgement comment: falls recorded concurrently and would be defined in hospital system. |
| Baseline imbalance | Low risk | Judgement comment: no significant differences at baseline, potential confounders adjusted for. |
| Other bias | Low risk | Judgement comment: none identified. |
Faber 2006.
| Methods | RCT (individually randomised) Facilities randomised to one of two interventions, then residents individually randomised to intervention or control group within facilities | |
| Participants |
Setting: 15 long‐term care residences (combined high‐ and intermediate‐level care within each), the Netherlands
N = 238
Sample: 79% women
Age (years): mean 84.9 (range 63 to 98) Inclusion criteria: resident of facility Exclusion criteria: unable to walk 6 metres unaided; poor cognition as judged by staff; GP contraindication |
|
| Interventions |
Usual care (same 15 residences as above) |
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | Only data for combined control groups reported in Faber 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 15 centres cluster randomised to one of two exercise regimens using "sealed envelopes". Individuals then randomised into intervention and control within each participating centre using computer generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether initial randomisation to clusters used envelopes which were sequentially numbered, opaque and sealed. Insufficient information to permit judgement in relation to randomisation of individuals after cluster allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff who recorded falls were likely to be aware of individual's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: withdrawals balanced across interventions |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no protocol identified but all expected outcomes ‐ falls and fallers thoroughly reported. |
| Method of ascertaining falls | Low risk | Judgement comment: falls defined and recorded concurrently. |
| Baseline imbalance | Low risk | Judgement comment: no differences at baseline. |
| Other bias | Low risk | Judgement comment: none detected |
Flicker 2005.
| Methods | RCT (individually randomised) | |
| Participants | Setting: 60 assisted living facilities and 89 nursing homes (intermediate‐ and high‐level nursing care facilities), urban and rural Australia N = 693 Sample: 95% women Age (years): mean 83.4 Inclusion criteria: serum 25‐hydroxyvitamin D between 25 nmol/L and 90 nmol/L Exclusion criteria: use of medications affecting bone and mineral metabolism; thyrotoxicosis within 3 years; primary hyperparathyroidism treated within 3 years; multiple myeloma; Paget's disease of bone, history of malabsorption, intercurrent active malignancy, other disorders affecting bone and mineral metabolism | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 24 months | |
| Notes | 58% of participants had a serum vitamin D between 25 nmol/L and 40 nmol/L at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomized via computer‐generated lists," "Within each institution … in blocks of eight." |
| Allocation concealment (selection bias) | Low risk | Quote: "Subjects were randomized to receive sequentially numbered bottles containing vitamin D supplementation or placebo." Individual not involved in contact with subjects or facilities performed randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Residential staff recording falls events blinded to whether participants were receiving vitamin D or placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: ITT analyses performed. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no protocol identified but falls reported extensively as number of falls, fallers, fracture and ITT, raw and adjusted and additional analyses |
| Method of ascertaining falls | Low risk | Judgement comment: falls clearly defined and recorded concurrently |
| Baseline imbalance | Low risk | Judgement comment: groups balanced at baseline |
| Other bias | Low risk | Judgement comment: none identified |
Frankenthal 2014.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: 1 residential care facility, mixed level of care, Israel
N = 359 residents Sample: 67% female, 46.8% 84 or over Age (years): mean 82.7 (SD 8.7) Baseline Characteristics Medication intervention (STOP/START)
Control
Inclusion criteria: all residents aged 65 and older in a chronic care geriatric facility in Israel, prescribed at least one daily medicine Exclusion criteria: terminally ill residents, those whose stay in the facility was shorter than 3 months Pretreatment differences: no significant differences |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | 24 month follow‐up data reported as retrospective cohort data for those alive at 24 months. These data not considered eligible for inclusion in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: simple list generation. Fixed stratified randomisation ‐ level of independence and cognition levels |
| Allocation concealment (selection bias) | Low risk | Judgement comment: physician who were not involved in the study did randomisation. Use of sealed envelopes. Study pharmacist (main person determining intervention recommendations) not involved in allocation, but aware of group allocation after randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: pharmacist was aware of group allocation when making recommendations and implementing intervention group recommendations. Was also aware of control group medication use as well, as recommendations were made but not implemented for this group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Nurses who were unaware of participants’ group assignments assessed the outcome measures in the study population. The chief nurses routinely report falls, hospitalizations, and FIM in residents’ records." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: similar loss to follow‐up between groups |
| Selective reporting (reporting bias) | Low risk | Judgement comment: protocol registered and outcome measures are reported as per protocol. |
| Method of ascertaining falls | Low risk | Judgement comment: clear definition, concurrent reporting by nurses |
| Baseline imbalance | Low risk | Judgement comment: no significant difference on main reported baseline measures. |
| Other bias | Low risk | Judgement comment: none detected |
Fu 2015.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: 1 residential care facility, China N = 60 Sample: 65% women Age (years): 82 Baseline Characteristics Wii Exercise
Conventional exercise
Inclusion criteria: 65 years and older, living in a nursing home, Functional Ambulation Category (FAC) grade 2 or 3, alert, medically stable and able to follow instructions, history of falls in the previous year. Exclusion criteria: visual problems that might affect their training, unable to follow instructions, history of seizure, stroke, parkinsonism, or uncontrolled cardiovascular disease Pretreatment differences: no important differences between groups on a wide range of potential confounders |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 6 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned to the conventional or Wii Fit balance training group by using a random number produced by the computerized method of minimization" |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not possible given nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Nurses at the nursing home who documented falls were unaware of participants’ group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: less than 10% missing from each group. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available |
| Method of ascertaining falls | Low risk | Judgement comment: falls were recorded by the nursing staff according to a clear definition and reported to the investigator for each participants monthly over the 12‐month period after randomisation. |
| Baseline imbalance | Low risk | Quote: "There was no statistically significant difference in age, sex, height, weight, body mass index, FAC distribution, or number of falls in the previous year between the 2 groups." |
| Other bias | Low risk | Judgement comment: none identified |
Garcia Gollarte 2014.
| Methods | RCT (cluster randomised) | |
| Participants |
Setting: residential care facilities, mixed‐level care, 60 physicians, Spain N = 1018 residents. 59 physicians, 37 nursing home clusters. Sample: 73% women. Age (years): 84.4 (SD 12.7) Baseline Characteristics Educational intervention
Control
Inclusion criteria: facilities: owned by the same private company in Spain; Physicians: at included nursing homes Residents: older than 65 years; living in nursing home for at least 3 months; expected to stay for 6 months or longer; clinically stable (no changes in prescription in the last 2 months); accepted that their clinical data were used for the study Exclusion criteria: residents: receiving palliative care; usually cared by other primary care providers outside the nursing home Pretreatment differences: significant difference in Barthel index at baseline P = 0.003, indicated made no difference to results but methods of adjustment not reported |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months total, 6 months intervention period. Baseline recorded following 3 months pre‐intervention. Endpoint at 12 months, for 3 months post‐intervention. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done using random number tables and" Judgement comment: random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details of timing of individual participant recruitment/person recruiting not reported (i.e. whether completed before cluster randomisation or not) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: physicians were blinded to purpose of trial. Unclear if participants were blinded but unlikely to be aware of educational interventions of physicians. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: do not know who did outcome assessment or how |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: loss of one nursing home cluster after randomisation |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available. Falls only reported for pre and post‐intervention periods |
| Method of ascertaining falls | High risk | Quote: "We did not use a daily systematic registry of falls and delirium, therefore, some episodes may have gone unnoticed, as is suggested by our lower rates of both syndromes compared with similar studies." |
| Baseline imbalance | Unclear risk | Judgement comment: significant difference in Barthel index at baseline. Results indicate that adjusting for this imbalance made no difference in results, however no details of how adjustment was performed are provided. |
| Other bias | Low risk | Judgement comment: none identified |
Grieger 2009.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: 1 aged care facility (high and intermediate‐level care), Victoria, Australia
N = 115
Sample: 65% women in analysis Age (years): not stated Inclusion criteria: able to consume food orally Exclusion criteria: residents in the dementia, rehabilitation and palliative care wards |
|
| Interventions |
|
|
| Outcomes |
Other outcomes not included in this review |
|
| Duration of the study | 6 months | |
| Notes | Mean baseline serum vitamin D level 36 nmol/L | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator used in Excel |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement comment: double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind administration of tablets but no mention of maintaining blinding of researchers when falls were extracted from medical histories at the end of the 6‐month trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: large loss from groups as randomised. 25% loss as randomised from placebo group, 16% from intervention group. |
| Selective reporting (reporting bias) | High risk | Judgement comment: excluded multiple faller from number of falls data as outlier |
| Method of ascertaining falls | High risk | Judgement comment: falls not clearly defined |
| Baseline imbalance | Unclear risk | Judgement comment: baseline age, cognition, medical comorbidities not reported |
| Other bias | Low risk | Judgement comment: none identified |
Haines 2004.
| Methods | RCT (individually randomised) | |
| Participants | Setting: one hospital (three subacute wards), specialising in rehabilitation and care of elderly patient, sMelbourne, Australia, N = 626 Sample: 67% women Age (years): mean 80 (SD 9) Inclusion criteria: all patients admitted to three subacute wards Exclusion criteria: none stated | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 10 months recruitment. Follow‐up time was until participants were discharged from hospital | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomly allocated participants by using a random number table held at the centre by one investigator (TPH) who revealed allocation on receipt of written consent." |
| Allocation concealment (selection bias) | Unclear risk | See above. Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: unblinding likely |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Staff recorded falls on incident report forms likely to be aware of individual's allocation status. Survey of staff indicated they were relatively unaware of participant group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: ITT analysis |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all outcome measures reported |
| Method of ascertaining falls | Low risk | Judgement comment: falls clearly defined and recorded concurrently. |
| Baseline imbalance | Low risk | Judgement comment: groups similar at baseline |
| Other bias | Low risk | Judgement comment: none identified. |
Haines 2010.
| Methods | RCT (cluster randomisation of pairs of hospital wards matched on rate of falls in preceding 6 months) | |
| Participants |
Setting: 18 publicly funded hospital wards (acute and subacute), Queensland, Australia N = 11,099 patients. 18 clusters. Sample: patients admitted to study wards after October 2007 when beds provided to intervention wards (% women not stated) Age (years): not stated Inclusion criteria: no previous access to or provision of low‐low beds Exclusion criteria: none described |
|
| Interventions |
Staff on intervention and control wards received falls incident reporting training video |
|
| Outcomes |
|
|
| Duration of the study | 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...18 wards were then matched into pairs ... and ordered alphabetically within pairs. A research assistant in a separate location and blinded to this ordering flipped a coin to determine whether the first or second listed ward in the pair was to be allocated to the intervention group." |
| Allocation concealment (selection bias) | Unclear risk | See above, but patients could have been allocated to a specific ward with the knowledge that it was an intervention or control ward |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Falls recorded by ward staff using routine computer‐based incident reporting scheme. Would not be blind to allocation. No mention of blinding in relation to the person extracting data from centrally held database |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: ITT analysis performed. |
| Selective reporting (reporting bias) | Low risk | Quote: "(ANZCTR registration number: 12609000243213)." Judgement comment: all outcome measures reported |
| Method of ascertaining falls | Low risk | Judgement comment: falls clearly defined and recorded concurrently. |
| Baseline imbalance | High risk | Judgement comment: patient level characteristics at baseline not reported. Intervention wards included 2 stand‐alone acute medicine wards, no standalone acute medicine wards in control arm. |
| Other bias | Low risk | Judgement comment: none identified. |
Haines 2011.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: 6 acute and subacute wards in 2 hospitals, Brisbane and Perth, Australia
N = 1206
Sample: patients admitted to acute (orthopedic and acute‐respiratory medicine) and subacute (geriatric assessment and rehabilitation) wards of one hospital, and to the acute (medical‐surgical) and subacute (restorative–stroke rehabilitation) wards of a second hospital (53% women) Age (years): mean (SD) intervention group (complete programme) 75.3 (11.0), intervention group (materials only programme) 74.7 (11.7), control group 75.3 (10.1) Inclusion criteria: aged > 60; expected to stay at least 3 days (acute wards only) Exclusion criteria: medically too unwell; previously participated in the trial |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 22 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer‐generated random allocation sequence" |
| Allocation concealment (selection bias) | Low risk | Quote: "opaque, consecutively numbered envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: participants not blinded, blinded assessment but treatment providers not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "research assistants ... completed weekly falls reviews ... were blind to group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: ITT analysis, no loss. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: falls reported as per publication, To check ACTRN12608000015347 |
| Method of ascertaining falls | Low risk | Judgement comment: falls clearly defined and recorded concurrently. |
| Baseline imbalance | Low risk | Judgement comment: baseline characteristics similar. |
| Other bias | Low risk | Judgement comment: none identified |
Healey 2004.
| Methods | RCT (cluster randomised by ward in matched pairs) | |
| Participants | Setting: 8 elderly care wards (acute and subacute) in 1 hospital, York, UK N = 1654 participants, 32,528 bed days during intervention. 8 clusters. Sample: approximately 60% women Age (years): mean 81.3 (range 63 to 102) Inclusion criteria: all patients admitted to target wards Exclusion criteria: none specified | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. Quote: "The study wards were divided into matched pairs. In each pair, one ward was randomly allocated to control or intervention by lottery ..." |
| Allocation concealment (selection bias) | Unclear risk | Individual study wards aware of their allocation from beginning of study. It is unclear whether knowledge of group status could have influenced admission of new patients during the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff at the wards who recorded falls were likely to be aware of their ward's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: ITT analysis. all occupied bed days and falls analysed, unlikely to be loss in hospital. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified. |
| Method of ascertaining falls | High risk | Judgement comment: no definition of falls used. Used accident and incidence reporting forms |
| Baseline imbalance | High risk | Judgement comment: imbalance in length of stay and dementia diagnoses. |
| Other bias | Low risk | Judgement comment: none detected. |
Hill 2015.
| Methods | RCT (cluster‐randomised, stepped‐wedge) | |
| Participants |
Setting: 24 wards in 8 rehabilitation or geriatric evaluation and management units in Australian hospitals, Western Australia. N = 3606 admissions; 3121 unique patients. 24 clusters. Sample: 62% women Age (years): 82 Baseline Characteristics Individualized fall education programme
Usual care
Inclusion criteria: for individuals on units to receive intervention: aged more than 60 years, projected length of stay of at least 3 days, basic cognitive functioning (MMSE > 23/30 and AMTS > 7/10), when the treating clinical team judged that the patient had a high enough level of cognition to benefit from the education Exclusion criteria: for individuals on units not to receive intervention: diagnosis of delirium, patients with moderate or severe cognitive impairment (MMSE of less than 24/30 or AMTS of less than 8/10), permanently unable to mobilise and remain bed‐bound or are receiving palliative care Pretreatment differences: significant difference in comorbidities at baseline (more comorbidities in intervention period), but confounding adjusted for in analysis. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 50 weeks. After a 10‐week control period, two units started the intervention—this procedure continued at 10‐week intervals until all eight units had crossed over into the intervention period. | |
| Notes | Outcomes reported for subgroups by level of cognition. Stable median site control falls rate and absence of interaction effect of time and falls outcomes indicates confounding by seasonal effects unlikely. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated, random allocation sequences." Judgement comment: computer generated. |
| Allocation concealment (selection bias) | Low risk | Judgement comment: allocation concealed, no individual participant recruitment required. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: falls collected by staff who are blinded, but entered into hospital report systems by unit staff who were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: protocol available, outcome measures consistent with final report |
| Method of ascertaining falls | Low risk | Judgement comment: falls clearly defined and collected concurrently. |
| Baseline imbalance | Low risk | Judgement comment: imbalances at baseline adjusted for in analyses. |
| Other bias | Unclear risk | Judgement comment: stepped‐wedge design means there is a potential for bias due to systematic influence of other external factors during the conduct of the trial. Possible influence of seasonal trends addressed by pre‐specified statistical analysis. |
Houghton 2014.
| Methods | RCT (cluster randomised) | |
| Participants |
Setting: 31 residential care facilities, mixed‐level care, UK
N = 953 residents. 31 clusters. Sample: 76% women Age (years): 87 Baseline Characteristics Medication review
Control
Inclusion criteria: care homes: average age > 65, registered with GP in local area; registered with Care Quality Commission for at least 6 months. Exclusion criteria: care homes specifically for people (of all ages) with learning disability, sensory impairment, mental health problems, physical disabilities and alcohol dependence; if have received a medication review service from the Primary Care Trust in the last 6 months; if they receive the services of a community geriatrician; or if they are subject to investigation of the safeguarding of vulnerable adults.Residents: those who self‐medicate; those in respite care. Pretreatment differences: nil significant |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 6 months intervention, follow‐up to 12 months. | |
| Notes | ISRCTN90761620 CAREMED trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For practical (i.e. workload) reasons, consenting homes will be allocated to intervention or control sequentially after consent is obtained using minimisation." Judgement comment: Sequential allocation by minimisation is equivalent to being random. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: insufficient information for judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: staff were involved in medication review meetings so were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: falls recorded by staff who were not blinded as they were involved in medication review meetings. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: 1 care home lost from intervention group, reason unclear, unclear if accounted for in analysis. |
| Selective reporting (reporting bias) | Unclear risk | Method of analysis of falls data as provided by author unclear. Unsure if falls analysed using a linear mixed model as per published abstract, impact unclear. |
| Method of ascertaining falls | Unclear risk | Insufficient information for judgement. |
| Baseline imbalance | High risk | Judgement comment: higher number of participants requiring nursing care in control group |
| Other bias | Low risk | Judgement comment: n0ne detected. |
Huang 2016.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: 6 residential care facilities, mixed‐level care, Taiwan N = 80 Sample: 50% women Age (years): 79.4 Baseline Characteristics: Cognitive behavioural alone
Cognitive behavioural plus exercise
Usual care
Inclusion criteria: 65 years or over; MMSE 13 or over; ability to communicate in Mandarin or Taiwanese; Ability to ambulate independently or with an assistive device; CB group needed to complete all 8 sessions Exclusion criteria: unstable physical condition or evidence of end stage terminal disease Pretreatment differences: no significant group differences |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 8‐month trial: 8 weeks intervention, falls over monitored over 3 months pre‐intervention and 3 months post‐intervention. | |
| Notes | 80 participants randomised, 5 withdrew during the study, final sample =75 participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "used a computer‐developed table to randomise patient assignment to each of the three groups in each nursing home." |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was concealed from the recruiting RA." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: unable to blind participants/personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote "To achieve greater accuracy in the number of falls during the study period, we collected data from chart record, accident report, in charge staff, and participants." Judgement comment: falls were recorded by participants and staff who were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: little missing data from randomisation, and are balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified |
| Method of ascertaining falls | Low risk | Quote: "we collected data from chart record, accident report, in charge staff, and participants." Judgement comment: Quote " The number of falls was recorded using the Falls Record Checklist (Huang & Acton, 2004)" ‐ this is a checklist for concurrent recording of falls by participants. |
| Baseline imbalance | Low risk | Judgement comment: no imbalance at baseline |
| Other bias | Low risk | Judgement comment: none detected |
Imaoka 2016.
| Methods | RCT (individually randomised.) | |
| Participants |
Setting: residential care facility, high‐level care, Japan N = 91 Sample: 76% women Age (years): 84.8 (SD 8.8) Baseline Characteristics Usual care group
Reduced exercise group
Nutrition group
Multifactorial group
Inclusion criteria: residents of long‐term health facility, not received any regular supplementation of vitamin D during the previous 12 months Exclusion criteria: receiving terminal care; with renal failure (chronic kidney disease [CDK] stage 3 or an estimated glomerular filtration rate [eGFR] of G2 or poorer); poor glycaemic control; a pacemaker Pretreatment differences: nil significant |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 3 months intervention, follow‐up to 9 months. Outcomes data exclude the intervention period. | |
| Notes | Effect of group exercise presented by comparing 'usual care' to 'reduced exercise' group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: envelope drawn |
| Allocation concealment (selection bias) | Low risk | Judgement comment: opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: outcome assessors not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: loss generally balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified. |
| Method of ascertaining falls | Low risk | Quote: "Falls were carefully recorded by the staff who found a resident falling down." Quote: "Falls were defined according to the International Classification of Diseases." |
| Baseline imbalance | Low risk | Judgement comment: no significant differences at baseline. |
| Other bias | Low risk | Judgement comment: none identified |
Irez 2011.
| Methods | RCT | |
| Participants |
Setting: 1 residential care facility in Ankara, Turkey, intermediate‐level care. N = 60 Sample: 100% women Age (years): 75.4 Baseline Characteristics Exercise ‐ Pilates
Usual care
Inclusion criteria: female, healthy, over 65 years of age, and have been relatively sedentary (undertaking no leisure time physical activity or less than 30 minutes of physical activity per day) for at least a year Exclusion criteria: male, significant general health problem or orthopaedic problem that would keep them from fully participating in the intervention protocol and/or the inability to attend at least 80% of the training sessions. Pretreatment differences: intervention group younger. Falls risk factors not reported. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: falls recorded by study participants who could not be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: loss to follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified. |
| Method of ascertaining falls | High risk | Judgement comment: patient reported falls, calendars collected monthly |
| Baseline imbalance | High risk | |
| Other bias | Low risk | Judgement comment:nNone identified |
Jarvis 2007.
| Methods | RCT (individually randomised) | |
| Participants | Setting: 1 elderly care rehabilitation ward (subacute), Leicester, UK N = 29 Sample: 100% women Age (years): not stated Inclusion criteria: female patients admitted for rehabilitation Exclusion criteria: acute stroke; Parkinson's disease; Abbreviated Mental Test Score ≤ 5; severe cardiac, lung or kidney disease; severe osteoarthritis or rheumatoid arthritis | |
| Interventions |
Physiotherapy consisted of stretches, lower limb exercises, and balance and gait activities in both groups |
|
| Outcomes |
|
|
| Duration of the study | 8 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... randomly assigned, using sealed envelopes ..." Insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The elderly women fallers were randomly assigned, using sealed envelopes, to either a control group or intervention group." Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Physiotherapy team responsible for measurement of outcomes reported to be blinded of intervention. Some chance of unblinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: large loss to follow‐up; 28.6% dropout in intervention arm |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified |
| Method of ascertaining falls | Low risk | Judgement comment: falls clearly defined and recorded concurrently. |
| Baseline imbalance | Low risk | Judgement comment: groups balanced at baseline |
| Other bias | Low risk | Judgement comment: none identified |
Jensen 2002.
| Methods | RCT (cluster randomised) | |
| Participants | Setting: 9 residential care facilities (intermediate care), Umeå, Sweden N = 402. 9 clusters. Sample: 72% women Age (years): mean (range) intervention group 83 (65 to 97), control group 84 (65 to100) Inclusion criteria: facilities with ≥ 25 residents; residents aged ≥ 65 Exclusion criteria: none stated | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 34‐week follow‐up | |
| Notes | Eight extra physiotherapists employed for intervention period (a total of 200 hours/week) and three during the follow‐up period (total of 10 hours/week) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomised study in nine facilities, divided into groups A and B (control or intervention). Quote: "Two sealed, dark envelopes" were used. Carried out by a person not connected with the study. Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Randomisation achieved by using by sealed dark envelopes by a person with no knowledge of study. Particiating individuals underwent baseline assessment prior to the randomisation of facilities. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff at the facilities who recorded falls were likely to be aware of their facility's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Large loss to follow‐up but loss is balanced and all patients included in fall analysis until lost. |
| Selective reporting (reporting bias) | Low risk | No protocol identified but all expected falls outcomes reported: Falls, fallers, IRR and injuries reported and adjusted for clustering. |
| Method of ascertaining falls | Low risk | Falls recorded concurrently with clear definition |
| Baseline imbalance | Low risk | Baseline differences adjusted for in analysis |
| Other bias | Low risk | None identified |
Juola 2015.
| Methods | RCT (cluster randomised). | |
| Participants |
Setting: 20 wards of assisted living facilities in Helsinki, residential care, mixed‐level care, Finland.
N = 227 residents. 20 clusters. Sample: 71% women Age (years): 83 Baseline Characteristics 93% of population had dementia diagnosis. Nursing educational intervention
Usual care
Inclusion criteria: age 65 years or older; living permanently in an assisted living facility; Finnish speaking; using at least one medication; having an estimated life expectancy of > 6 months; being able to provide written informed consent (or have a proxy who is able to provide written informed consent in the case of cognitive impairment) Exclusion criteria: none provided Pretreatment differences: significant baseline differences in Chalsons comorbidity index,dependence in mobility, prior stroke or transient ischaemic attack (TIA), 15D quality of life score; PRN dug use; proportion of sample using harmful medications; and borderline significant difference between groups in gender (P = 0.05). NOTE ‐ some of these reported in Pitkala paper, some in Joula paper |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | ACTRN12611001078943. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: dyads of matched facilities, then random number generator |
| Allocation concealment (selection bias) | Low risk | Judgement comment: person independent of assessment procedures telephoned another person not familiar with wards or residents to receive allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: nursing staff were not aware that falls data was being analysed as part of the study, however, there is no explanation of whether attempts were made to keep participants and personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: nursing staff recorded falls as part of routine care ‐ not aware that data was being analysed (main study outcome / focus was change in medications) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: imbalance in group losses, in addition to 3 intervention and 5 control participants not accounted for |
| Selective reporting (reporting bias) | Low risk | Falls outcomes additional in secondary analysis. Describes all outcomes reported as per methods in the paper. |
| Method of ascertaining falls | Unclear risk | Judgement comment: no definition of falls provided |
| Baseline imbalance | High risk | Judgement comment: significant baseline differences on mobility and Charlson comorbidity index, no adjustments reported |
| Other bias | Low risk | None detected |
Kennedy 2015.
| Methods | RCT (cluster‐randomised, pilot study) | |
| Participants |
Setting: 40 residential care facilities, mixed‐level care, Canada. Mean 137 beds. N = 5478. 40 clusters. Sample: 71% women Age (years): 84.4 (SD 10.9) Baseline Characteristics ViDOS multifaceted KT intervention
Control
Inclusion criteria: facilities: long‐term care facilities ‐ serviced by a particular pharmacy provider; have more than one prescribing physician; residents: none Exclusion criteria: residents: none Pretreatment differences: mean facility size was larger in control (157 beds, SD 80.2) versus intervention homes (115 beds, SD 67.9); however, both study arms had a similar proportion of small (< 100 beds) and large (> 250 beds) homes.In the control arm, there was a higher prevalence of hip fractures; osteoporosis diagnoses; and baseline use of vitamin D≥ 800 IU/day, calcium≥ 500 mg/day, and osteoporosis medications |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12.2 months; final follow‐up 16 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: computer‐generated random allocation sequence |
| Allocation concealment (selection bias) | Low risk | Judgement comment: allocation adequately concealed at unit level and individual residents not recruited |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: staff recording falls were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reports ITT for all participating facilities and also separately for the facilities in the intervention group who were "active". Large loss of intervention facilities from recruitment to active participation (7 of the 19 intervention facilities recruited did not proceed to implement the intervention). Baseline data are reported for all intervention and control facilities (i.e. all 19 intervention facilities), but there is no comparison between those who participated (n = 12) and those who were recruited but did not participate in the intervention (n = 7 facilities) to ensure the remaining sample were not biased in any way relative to the recruited and randomised intervention sample. |
| Selective reporting (reporting bias) | Unclear risk | Protocol indicated outcomes as per adjusted analyses would be reported but absolute number of falls and fractures only reported. Impact of this unclear |
| Method of ascertaining falls | High risk | Quote: "Researchers provided the homes with a standardized data collection sheet and homes completed the information using various sources including electronic/paper‐based charts, internal monitoring systems, Resident Assessment Instrument ‐ Minimum Data Set 2.0 (RAI‐MDS 2.0), and critical incident reports." Judgement comment: falls data collected for 3 month blocks from various data sources ‐ different homes had different reporting systems. This is acknowledged as a limitation. |
| Baseline imbalance | High risk | Judgement comment: there were imbalances in baseline characteristics that may impact on falls rates (e.g. hip fractures), the protocol indicated adjustment in analyses (with generalised estimating equations) but adjusted analyses not reported for falls outcomes. P = 0.002 for hip fracture |
| Other bias | Low risk | Judgement comment: none detected |
Kerse 2004.
| Methods | RCT (cluster randomised) | |
| Participants | Setting: 14 mixed‐level dependency residential care homes (intermediate‐ and high‐level care), New Zealand N = 617 residents. 14 clusters. Sample: 72% women Age (years): mean 83.2 (SD 10.6) Inclusion criteria: resident in one of the included residential care homes Exclusion criteria: none stated but data excluded if enrolled in the study for < 2 days and had > 2 falls in one of those days | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... homes were stratified by type, and an independent researcher, not involved in the study, block randomized them into intervention or control group using computer‐generated random numbers." |
| Allocation concealment (selection bias) | Low risk | See above, and allocation of all cluster units performed at the start of the study AND individual participant recruitment was completed prior to assignment of the cluster, and the same participants were followed up over time |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff at the facilities who recorded falls were likely to be aware of their facility's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All falls data included in analysis, but large imbalance in those transferred or discharged (15 vs 35 |
| Selective reporting (reporting bias) | Low risk | Falls, fallers, injurious falls and rates of falls reported and appropriately adjusted. |
| Method of ascertaining falls | Low risk | Falls clearly defined and recorded concurrently |
| Baseline imbalance | Low risk | Baseline differences accounted for in analysis |
| Other bias | Low risk | None identified. |
Kerse 2008.
| Methods | RCT (cluster randomised) | |
| Participants | Setting: 41 low‐level dependency residential care homes (intermediate‐level care), New Zealand. N = 682 residents. 41 clusters. Sample: 74% women Age (years): mean 84.3 (SD 7.2) Inclusion criteria: able to engage in conversation about a goal; remember the goal; participate in a programme to achieve the goal Exclusion criteria: unable to communicate to complete the study measures; anxiety as main diagnosis; acutely unwell; terminally ill | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After recruitment of all homes and residents and collection of baseline data, a biostatistician not involved in recruitment randomised homes to the intervention or control group by using computer generated random numbers." |
| Allocation concealment (selection bias) | Low risk | Allocation of all cluster units performed at the start of the study. Individual participant recruitment was completed prior to assignment of the cluster, and the same participants were followed up over time |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff at the facilities who recorded falls were likely to be aware of their facility's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: losses balanced between groups. Falls data for 310/330 and 329/352 |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: details of falls outcomes not reported in trials registration |
| Method of ascertaining falls | Unclear risk | Judgement comment: falls clearly defined, but method of ascertainment unclear |
| Baseline imbalance | High risk | Judgement comment: difference in antidepressants at baseline between groups |
| Other bias | Low risk | Judgement comment: none identified |
Klages 2011.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: 1 long‐term care home (appears to be high‐ and intermediate‐level care), Ontario, Canada N = 24 Sample: 68% women in the analysis Age (years): mean (SD) intervention group 84 (6.6), control group 89 (3.2) Inclusion criteria: cognitively impaired (MMSE score < 25); able to follow simple walking instructions; able to walk with minimal assistance; no Snoezelen room attendance in 3 months prior to study Exclusion criteria: history of seizures; legal blindness; profound hearing loss; history of limb fractures; extrapyramidal system disruptions (inability to remain motionless or to initiate movement) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 3 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A total of 24 eligible residents were recruited. Prior to the commencement of the study a computer‐based random number generator was used to randomly select 12 numbers out of 24. These numbers were assigned to the intervention group. The remaining 12 numbers were allotted to participants in the control group." |
| Allocation concealment (selection bias) | Low risk | Quote: "As multiple recruitment packages were sent out simultaneously, and the participants were assigned a number in chronological order when a signed consent document was received, recruitment order and group allocation were unpredictable." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Nursing staff recording falls were not blind to group allocation and "The investigator [reviewing charts] ... was not blind to group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: one frequent faller excluded from the analysis |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified |
| Method of ascertaining falls | High risk | Judgement comment: falls recorded concurrently, but falls definition not reported |
| Baseline imbalance | High risk | Judgement comment: significant difference in age between groups |
| Other bias | Low risk | Judgement comment: none detected |
Koh 2009.
| Methods | RCT (cluster randomised) | |
| Participants |
Setting: two acute care hospitals, Singapore N = 1122 patients. 2 clusters. Sample: 641 nurses in medical, surgical and geriatric units in the two hospitals (% female patients not stated) Age (years) patients: mean 68 Inclusion criteria: all patients Exclusion criteria: none stated |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 6 months | |
| Notes | Intervention targeted nursing staff. Age of patients not stated in Koh 2009. Obtained by personal communication with author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The two study hospitals were randomly allocated either to the "intervention" site... or the "control” site". Author states carried out by supervised coin toss; heads gets the intervention |
| Allocation concealment (selection bias) | High risk | No concealment. After first site randomised, second site automatically becomes the control group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Fall incidence and fall‐associated injury rates were obtained from the hospitals’ fall incidence database" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: falls data for a random sample of medical records used. How representative these are of all patients and what proportion unknown |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified |
| Method of ascertaining falls | High risk | Judgement comment: falls determined through audits of hospital records. Definitions and practices may vary between hospitals. |
| Baseline imbalance | Unclear risk | Judgement comment: baseline characteristics of patients not reported |
| Other bias | Low risk | Judgement comment: none detected |
Kovacs 2012.
| Methods | Pilot RCT (individually randomised). | |
| Participants |
Setting: One residential care facility, intermediate‐level care, Hungary N = 41 Sample: 100% women Age (years): 69.2 Baseline Characteristics Multimodal exercise plus osteoporosis exercise
Osteoporosis exercise programme
Inclusion criteria: living in the National Institution for Blind People (partial sightedness or blindness); aged 60 years or over; being female. Exclusion criteria: being totally blind; had lived in the nursing home for less than 2 months; being unable to walk around their own residence; having progressive neurological, and unstable cardiovascular diseases that would limit participation in exercise programme; planned moving away from the nursing home during the study period and; participated in an exercise programme including balance exercise within 6 months. Pretreatment differences: nil |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 6 months | |
| Notes | Visually impaired participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: details of sequence generation not reported |
| Allocation concealment (selection bias) | Low risk | Judgement comment: numbered opaque identical sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: falls extracted from staff records (medical and nursing documentation), blinding of staff not feasible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Judgement comment: number of falls in the follow‐up period not reported |
| Method of ascertaining falls | Unclear risk | Judgement comment: falls clearly defined but details of documentation inadequate for judgement |
| Baseline imbalance | Low risk | Quote: "There were no significant differences between groups on any baseline characteristics." |
| Other bias | Low risk | Judgement comment: none detected |
Kovacs 2013.
| Methods | Study design: RCT (individually randomised) | |
| Participants |
Setting: One residential care facility, mixed‐level care, Hungary. N = 86 Sample: 81% women Age (years): 77.9 Baseline Characteristics Multimodal exercises programme
Control
Inclusion criteria: cognitive impairment (MMSE < 24), residents of nursing home, 60 years or over Exclusion criteria: living in nursing home < 2 months, < 60 years of age, unable to walk 6 metres with or without walking aid, unable to follow simple verbal exercise instructions, unstable cardiovascular or pulmonary diseases that would limit participation in exercise programme, terminal illness, planned moving from the nursing home during the study, no consent Pretreatment differences: using a frame (20.9% int, 41.9% con) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | Compliance reported. Cognitively impaired participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: insufficient detail for judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Consecutively numbered opaque identical sealed envelopes were used for allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: carers recorded falls not blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: attrition numbers and reasons balanced between groups |
| Selective reporting (reporting bias) | Low risk | Judgement comment: although no protocol was identified, falls outcomes were reported clearly and as multiple measures (fallers, falls, recurrent fallers, as n and RR) |
| Method of ascertaining falls | Low risk | Judgement comment: falls recorded concurrently on calendar using clear definition |
| Baseline imbalance | High risk | Judgement comment: significant difference between groups in the proportion using a frame, not adjusted for in analysis |
| Other bias | Low risk | Judgement comment: none detected |
Lapane 2011.
| Methods | RCT (cluster randomised) | |
| Participants |
Setting: 25 nursing homes (appear to be high‐ and intermediate‐level care), Ohio, USA N = 3321 residents. 25 clusters. Sample: 73% women Age (years): no overall age available Inclusion criteria (facilities): facilities serviced by one of two Omincare pharmacies and with stable contracts; Medicare and Medicaid certified; ≥ 50 geriatric beds; few short‐stay residents Exclusion criteria: none stated |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Homes were randomised ..." Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement, although clinical staff recording falls would have been aware of allocation of the nursing home |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up not clearly reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified. Data not suitable for use of rate of falls or injurious falls in meta‐analysis as per review Appendix 6 |
| Method of ascertaining falls | High risk | Judgement comment: no definition of falls provided, only states "MDS data", may vary between sites |
| Baseline imbalance | High risk | Judgement comment: number of falls in past 30 days was much higher in intervention group |
| Other bias | Low risk | Judgement comment: none detected |
Law 2006.
| Methods | RCT (cluster randomised by unit). | |
| Participants | Setting: 118 homes for elderly people, 223 units (intermediate‐ and high‐level care), throughout the UK N = 3717 residents. 223 clusters. Sample: 76% women Age (years): mean 85 Inclusion criteria: facility resident; aged ≥ 60 Exclusion criteria: temporary residents; taking vitamin D or calcium supplements or medications to increase bone density; sarcoidosis; malignancy; life threatening illness | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | Median length of follow‐up 10 months (interquartile range 7 to 14) | |
| Notes | Mean baseline serum vitamin D level collected from 1% of the intervention group; mean 59 nmol/L | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster randomisation by computer. No further information provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not possible to blind participants but personnel recorded the fall data were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff at the facilities who recorded falls were likely to be aware of their facility's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: losses balanced between groups. 2.8% left care homes in intervention group, 3.3% control group, other losses due to death (p484 first para, text) |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no protocol identified, but fractures, fallers and falls reported, adjusted for clustering |
| Method of ascertaining falls | High risk | Judgement comment: falls not clearly defined. Study conducted across 118 homes which may have variations in reporting practice and definitions. Falls recorded daily |
| Baseline imbalance | Unclear risk | Judgement comment: similar at baseline for demographic characteristics (e.g. age, gender), but did not discuss prognostic factors e.g. falls rate/ medical status |
| Other bias | Low risk | Judgement comment: none identified |
Mador 2004.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: two metropolitan acute hospitals, South Australia N = 71 Sample: 48% women Age (years): mean 82.5 Inclusion criteria: inpatients on medical and surgical wards; aged ≥ 60; confusion due to either dementia or delirium; problematic behaviour Exclusion criteria: primary psychiatric illness; no next of kin available to give consent |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 11 months. Median length of stay 12 days for intervention group and 9 days for control group | |
| Notes | Potential contamination as staff receiving training were also caring for controls | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Sequential sealed opaque envelopes were prepared by a person who was external to the study in blocks of ten stratified for the two hospitals, using a computer‐generated table of random numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequential sealed opaque envelopes were prepared by a person who was external to the study..." Randomised by the Repatriation Hospital Pharmacy Department |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: little loss and ITT analysis conducted |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified |
| Method of ascertaining falls | High risk | Quote: "Number of falls for each patient was extracted from the hospital’s database of critical incidents." Judgement comment: no falls definition reported |
| Baseline imbalance | High risk | Quote: "There was a significant difference for prior residence, with more control participants entering hospital from home com‐ pared with the intervention group ( p ¼ 0.035). The number of participants under the care of a geriatrician was greater in the intervention than in the control group ( p ¼ 0.006)." |
| Other bias | Low risk | Judgement comment: none identified |
Mayo 1994.
| Methods | RCT (individually randomised) | |
| Participants | Setting: rehabilitation (subacute) hospital, Canada N = 134 Sample: 46% women Age (years): mean (SD) intervention 70.9 (12.6), control 72.9 (11.8) Inclusion criteria: one or more of the following: admission diagnosis of stroke or ataxia; an episode of incontinence; a history of multiple falls; aged ≥ 80; using topical eye medication, anticonvulsants, vitamin supplements or anti‐ulcer medications Exclusion criteria: unable to understand what was being asked of them; participated in this study during a previous admission | |
| Interventions | All participants selected as being high risk of falling
|
|
| Outcomes |
|
|
| Duration of the study | 12 months. Median lengths of stay 75 days (intervention group), 65 days (control group) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were interviewed to obtain baseline information ... and were then randomly assigned to either the intervention group or the control group." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on process of allocation to permit judgement of 'Low risk' or 'High risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Falls ascertained through incident reports. Staff completing incident reports would have been aware of whether or not participant was wearing a blue bracelet |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: ITT analysis, rate of falls, all patients appear to have been included |
| Selective reporting (reporting bias) | Low risk | Judgement comment: data presented for number of falls, fallers and rate of falls as per methods |
| Method of ascertaining falls | Unclear risk | Judgement comment: falls clearly defined and recorded concurrently |
| Baseline imbalance | High risk | Judgement comment: some imbalance in ability to walk independently at baseline |
| Other bias | Low risk | Judgement comment: none identified |
McMurdo 2000.
| Methods | RCT (cluster randomised) | |
| Participants | Setting: 9 residential care facilities (intermediate‐level care), Dundee, Scotland, UK N = 133 residents. 9 clusters. Sample: 81% women Age (years): mean 84 (SD 7) Inclusion criteria: aged ≥ 70 Exclusion criteria: MMSE score < 12 | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months. 6 month intervention + 6 months follow‐up | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... allocated at random ..." Insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on process of allocation to permit judgement of 'Low risk' or 'High risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff at the facilities recording falls in calendar were likely to be aware of their facility's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large difference in dropout rates between arms |
| Selective reporting (reporting bias) | Unclear risk | Number of falls, fallers, multiple fallers and fracture falls reported. No adjustment for clustering. NIHR link broken |
| Method of ascertaining falls | Low risk | Falls clearly defined and recorded daily on a falls calendar by staff |
| Baseline imbalance | Low risk | Groups balanced at baseline |
| Other bias | Low risk | None identified |
Meyer 2009.
| Methods | RCT (cluster randomised) | |
| Participants |
Setting: 58 nursing homes (high‐level nursing care), Hamburg, Germany
N = 1125 residents. 58 clusters.
Sample: 85% women Age (years): mean (SD) intervention group 86 (6), control group 87 (6) Inclusion criteria (facilities): ≥ 30 residents; not using a fall risk assessment tool or willing to stop using a tool. Inclusion criteria (residents): ≥ 70 years; able to walk with or without assistance; living in the nursing home for > 3 months Exclusion criteria: none stated |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomisation lists were prepared by the biostatistician for concealed allocation of clusters by external central telephone." |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Nursing staff recorded falls (presumably not blind). External investigator verified completeness of falls data – not clear if blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: fall data reported for all participants for time in study |
| Selective reporting (reporting bias) | Low risk | Judgement comment: outcomes consistent with protocol and adjusted for clustering |
| Method of ascertaining falls | Low risk | Judgement comment: falls recorded concurrently and clearly defined |
| Baseline imbalance | Low risk | Judgement comment: balanced at baseline |
| Other bias | Low risk | Judgement comment: none detected |
Michalek 2014.
| Methods | Pilot RCT (pseudo‐randomised to one of two clusters) | |
| Participants |
Setting: Subacute hospital setting. Median length of stay 20 days. Germany N = 114. 2 clusters. Sample: 79% women Age (years): Mean NR Baseline Characteristics FORTA
Usual care
Inclusion criteria: aged >70 years; stable health condition defined as no need for intermediate or intensive care unit treatment; at least three diseases in need for drug treatment; at least three medical prescriptions; admitted during the first 3 days of the week because of staff availability; patients or proxies had to give written informed consent. Exclusion criteria: critical or terminal illness; dementia (MMSE <25); refusal to participate. Pretreatment differences: nil significant reported at baseline, BMI Borderline (P = 0.052) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | Until discharge (median hospital stay 20 days) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement comment: patients were assigned randomly by number of entrance to one of two wards |
| Allocation concealment (selection bias) | High risk | Judgement comment: quasi randomised to one of two wards ‐ high risk of bias. Individuals randomised by number of entrance, sequence predictable |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible, patients admitted to intervention or control wards |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: falls recorded in hospital recording system by staff who will know ward allocation of patients |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: no reported loss to follow‐up during study period, attrition after enrolment unlikely in acute hospital setting, however falls data reported for 178 patients in Frohnhofen 2013 abstract |
| Selective reporting (reporting bias) | Low risk | Judgement comment: falls not recorded as outcome on trials registry but falls outcomes seems to be completely reported in multiple ways (fallers, falls rate) |
| Method of ascertaining falls | Low risk | Judgement comment: falls clearly defined and likely concurrent through established hospital reporting system |
| Baseline imbalance | Low risk | Judgement comment: groups balanced at baseline |
| Other bias | High risk | Judgement comment: analysis was by individual but quasi randomisation and it was to one of two clusters (although not specifically cluster randomised), which should have been addressed in the analysis |
Mulrow 1994.
| Methods | RCT (individually randomised) | |
| Participants | Setting: 9 nursing homes (high‐level nursing care), USA N = 194 Sample: 71% women Age (years): mean (SD) intervention group 79.7 (8.5), control group 81.4 (7.9) Inclusion criteria: aged > 60; resident in nursing home for ≥ 3 months; dependant in ≥ 2 ADLs Exclusion criteria: terminal illness; acute medical condition; MMSE score < 50%, unable to follow two‐step command; assaultive behaviour; received physiotherapy within last 2 months | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 4 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed after baseline assessments by calling a central number. Randomization was blocked in groups of four and stratified by nursing home site." |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed after baseline assessments by calling a central number. No further description |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Falls recorded in charts and incident reports. Staff recording falls likely to be aware of allocation status. Research assistants examining charts and incident reports were reported to be blinded to allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: 14 dropouts, 12 due to death, other 2 unexplained but unlikely to be related to outcome |
| Selective reporting (reporting bias) | Low risk | Judgement comment: falls and fallers outcomes reported |
| Method of ascertaining falls | High risk | Judgement comment: no falls definition reported and may vary between sites |
| Baseline imbalance | Low risk | Judgement comment: groups balanced at baseline |
| Other bias | Low risk | Judgement comment: none identified |
Neyens 2009.
| Methods | RCT (cluster randomised by ward) | |
| Participants |
Setting: 12 nursing homes, psychogeriatric wards (high‐level nursing care), the Netherlands (6 wards in intervention group and 6 in control group).
N = 518 residents. 12 clusters.
Sample: 68% women Age (years): mean (SD) intervention group 82.1 (7.7), control group 83.3 (7.7) Inclusion criteria (wards): ≥ 25 beds; not using a fall prevention protocol; having the largest number of mobile patients Exclusion criteria: none stated |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "At random, using computer techniques, two intervention homes and two control homes were selected from each group [groups based on the mean fall incidence rate of psychogeriatric patients per psychogeriatric bed], resulting in a total of six intervention homes and six control homes." |
| Allocation concealment (selection bias) | High risk | One ward per home was chosen after randomisation, based on inclusion criteria |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study was cluster randomised and nursing staff recorded falls |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data analysed by ITT |
| Selective reporting (reporting bias) | High risk | No protocol identified, fallers not reported |
| Method of ascertaining falls | Unclear risk | Not stated whether falls clearly defined |
| Baseline imbalance | Low risk | Reasonable comparability. More falls pre‐trial in intervention arm, but adjusted for in analyses |
| Other bias | Low risk | None identified |
Nowalk 2001.
| Methods | RCT (individually randomised) | |
| Participants | Setting: 2 long‐term care facilities (combined high‐level nursing care and independent living), USA N = 110 participants Sample: 86% women Age (years): mean 84 Inclusion criteria: aged ≥ 65; cognitively able to be tested; able to ambulate with or without assistive device; able to follow simple directions; co‐operative; capable of participating in group sessions Exclusion criteria: unwilling or unable to complete baseline assessments | |
| Interventions |
Note: all groups also exposed to educational activities |
|
| Outcomes |
|
|
| Duration of the study | 24 months | |
| Notes | True N for each group unknown and data discrepancies within published manuscript. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Following completion of all assessments, participants were randomly assigned to one of three groups ... using permuted blocks ..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on process of allocation to permit judgement of 'Low risk' or 'High risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff who recorded falls on incident report forms were likely to be aware of individual's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: attrition by allocation group unclear, but overall 41/112 lost, died or not followed for full time period |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified. Number of falls not reported |
| Method of ascertaining falls | Low risk | Judgement comment: falls defined and reliant on facility incident reports |
| Baseline imbalance | Low risk | Judgement comment: no important differences at baseline |
| Other bias | Low risk | Judgement comment: none identified |
Patterson 2010.
| Methods | RCT (cluster‐randomised matched pairs of nursing homes) | |
| Participants |
Setting: 22 nursing homes (high‐ and intermediate‐level care), Northern Ireland
N = 334 residents. 22 clusters.
Sample: 73% women Age (years): mean 82.7 (SD 8.4) Inclusion criteria (facilities): > 30 resident beds (including homes for general nursing category residents and for elderly mentally infirm people). Inclusion criteria (residents): aged ≥ 65 Exclusion criteria (facilities): caring exclusively for terminally ill people. Exclusion criteria (residents): terminally ill; attending day care only |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned ... using a computer generated table of random numbers" |
| Allocation concealment (selection bias) | Low risk | An independent researcher blind to the identity of the homes carried out the randomisation (after consent obtained from the homes) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Routinely collected falls data were used. Staff not blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: loss to follow‐up entirely due to death, with similar percentages of deaths in each group. ITT analysis |
| Selective reporting (reporting bias) | High risk | Judgement comment: trial registry record indicates outcome as number of people falling, but only rate of falls reported |
| Method of ascertaining falls | High risk | Judgement comment: falls definition not reported and reliant on falls reporting within each home which may vary |
| Baseline imbalance | Low risk | Judgement comment: no major differences at baseline. Similar for falls risk factors. Main difference is more urban nursing homes in control group than in intervention group |
| Other bias | Low risk | Judgement comment: none identified |
Peyro Saint Paul 2013.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: hospital acute and residential care facility setting (92% residential care), France.
N = 19 residents Sample: 58% women Age (years): 89.9 Baseline Characteristics Changing drug therapy
Usual care
Inclusion criteria: aged 65 and over; chronic moderate hyponatraemia (serum sodium 123 mEq/L to 134 mEq/L) detected using a biological control routine; in acute care unit or retirement home Exclusion criteria: Pretreatment differences: age and sex same, Nz level same, renal clearance worse in control |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 3 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: Author correspondence, quote: "Random sequence was managed as a single randomization list managed by the sponsor". It is unclear how the randomisation sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Judgement comment: Author correspondence, quote: "Random sequence was managed as a single randomization list managed by the sponsor. Allocation was concealed using masking envelope." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: author correspondence: "Staff were not blind to group allocation. Residents were not blind to group allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: author correspondence, quote: "The fall was recorded as soon in the patient file by the first caregiver who noted: carer, nurse or doctor. Caregiver were not blind to group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: the proportion of missing data is considered high enough to potentially have a relevant effect on the effect estimate: falls data only available for 9/19 randomised patients. Response to enquiry received 19/7 from Peyro Saint Paul ‐ participant flow chart still unclear |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified |
| Method of ascertaining falls | Unclear risk | Judgement comment: author correspondence, quote: "the software allows to record falls in the patient file." Methods of ascertaining falls not reported |
| Baseline imbalance | Unclear risk | Judgement comment: characteristics for key baseline factors (falls risk, medical status, dependency, cognitive status) relevant to falls are not reported. |
| Other bias | Low risk | Judgement comment: none detected. Main publication not in English |
Potter 2016.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: 4 care facilities, mixed level of care, rural Australia.
N = 95 participants randomised; 93 in analysis Sample: 52% women Age (years): mean 84.3 (SD 6.9) Baseline Characteristics Deprescribing intervention
Usual care
Inclusion criteria: residents of residential aged care facilities aged 65 years or older Exclusion criteria: taking no regular medicines; were in the final terminal stages of an illness; or if their usual general practitioner (GP) or the RACF nurse manager did not agree to their participation Pretreatment differences: control participants had lower mean blood pressure. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | After 12 months, 59% of targeted medicines were deprescribed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: using a digital random number generator |
| Allocation concealment (selection bias) | Low risk | Judgement comment: sealed opaque envelopes opened after the medication review, withdrawal plan and baseline assessments |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding with reference to falls outcome assessment possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: falls outcomes were assessed by persons who would know the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no evidence of significant incompleteness of falls outcome data |
| Selective reporting (reporting bias) | Low risk | Judgement comment: protocol available, outcomes reported as per protocol |
| Method of ascertaining falls | Low risk | Judgement comment: used routine data collection plus recall from relatives. Clear definition used. |
| Baseline imbalance | Low risk | Judgement comment: difference in systolic blood pressure, however, deemed unlikely to significantly affect outcome |
| Other bias | Low risk | None detected |
Ray 1997.
| Methods | RCT (cluster randomised) | |
| Participants | Setting: 14 nursing homes (high‐level nursing care), USA N = 499 participants. 14 clusters. Sample: 78% women Age (years): mean 83 Inclusion criteria: high risk of falls with potential problem in a safety domain; likely to remain in nursing home Exclusion criteria: age < 65; anticipated stay < 6 months; bed bound; no fall in previous year | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | No published data on numbers of falls or fallers who had a single fall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Seven "matched" pairs of facilities participated. Quote: "The statistician ... generated sealed‐envelope random assignments for each pair from the SAS function RANUNI (using the clock for the seed)." |
| Allocation concealment (selection bias) | Low risk | Study author (statistician) generated sealed envelope random number assignments for each pair using the SAS function from RANUNI using the clock for the seed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff at the facilities who recorded falls were likely to be aware of their facility's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis, little loss to follow‐up, reasons balanced |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
| Method of ascertaining falls | Unclear risk | Falls clearly defined, relies on incidence reports, trial guidance on concurrent reporting unclear |
| Baseline imbalance | Low risk | No major differences. Difference in BMI, life space diameter, multivariate regression conducted, no differences in main falls risk factors |
| Other bias | Low risk | None identified |
Rosendahl 2008.
| Methods | RCT (cluster randomised) | |
| Participants |
Setting: 9 residential care facilities (intermediate‐ and high‐level nursing care), Sweden
N = 191. 34 clusters.
Sample: 73% women in 34 clusters (cluster equals 3 to 9 participants living on the same floor, wing, or unit) Age (years): mean 84.7 (SD 6.5) Inclusion criteria: aged ≥ 65; dependent in ≥ 1 personal ADLs; able to stand from armchair with help from 1 person; MMSE score ≥ 10; physician approval Exclusion criteria: none stated |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Researchers not involved in the study performed the randomization by using lots in sealed non‐transparent envelopes." |
| Allocation concealment (selection bias) | Low risk | Randomisation by cluster was performed after the inclusion of participants and baseline assessments using sealed nontransparent envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff who recorded falls were likely to be aware of individual's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: losses balanced and unlikely to affect outcome |
| Selective reporting (reporting bias) | Low risk | Judgement comment: outcomes reported consistently with trial registration. All expected outcomes reported |
| Method of ascertaining falls | Low risk | Judgement comment: falls clearly defined and recorded concurrently |
| Baseline imbalance | Low risk | Judgement comment: no major differences at baseline. Difference in self‐perceived health but no differences in diagnoses, functional assessments, falls or drugs. |
| Other bias | Low risk | Judgement comment: none identified. |
Rubenstein 1990.
| Methods | RCT (individually randomised) | |
| Participants | Setting: long‐term care facility (intermediate‐ and high‐level nursing care), Los Angeles, USA N = 160 Sample: 85% women Age (years): mean (SD) intervention group 86.8 (0.6), control group 87.9 (0.7) Inclusion criteria: fall within 7 days of nurse receiving fall incident report Exclusion criteria: unable to walk; unable to be evaluated within 7 days of fall due to acute illness or hospitalisation; unable to understand English | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 24 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible fallers were ... randomly assigned to either the intervention or control group, using computer generated, randomly sequenced cards in sealed envelopes." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on process of allocation to permit judgement of 'Low risk' or 'High risk'. It is unclear who conducted the randomisation and envelopes not described as opaque and sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff who recorded falls after intervention were likely to be aware of individual's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data balanced between arms |
| Selective reporting (reporting bias) | Low risk | No protocol identified (1990 study) but expected falls outcomes reported as number of falls and fallers reported |
| Method of ascertaining falls | Low risk | Falls clearly defined and recorded concurrently |
| Baseline imbalance | Low risk | No major differences between groups at baseline |
| Other bias | Low risk | None identified |
Sakamoto 2006.
| Methods | RCT (individually randomised) | |
| Participants | Setting: nursing care facilities and rehabilitation outpatient departments (intermediate care), Japan N = 553 Sample: 74% women Age (years): mean 81.6 (SD 9.0) Inclusion criteria: able to stand on their own while holding on to a bar Exclusion criteria: severe dementia | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization of the subjects into an exercise group or a control group was performed by the Department of Information Science of our university." using a "table of random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Randomisation by Department of Information Science. Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff who recorded falls were likely to be aware of individual's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: greater loss from intervention group, 22 vs 4 |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no protocol identified, but number of falls, and fallers reported |
| Method of ascertaining falls | Unclear risk | Judgement comment: no definition of falls. Method of ascertaining falls not described |
| Baseline imbalance | Unclear risk | Judgement comment: baseline characteristics by group allocation unclear |
| Other bias | Low risk | Judgement comment: none identified |
Sakamoto 2012.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: 3 nursing homes (intermediate‐level care), Aomori, Japan N = 145 Sample: 81% women Age (years): mean (SD) intervention group 84.2 (7.8), control group 84.1 (7.7) Inclusion criteria: aged ≥ 65; able to transfer independently with or without assistive devices Exclusion criteria: non consenting; pica disorder (the desire to eat “unnatural” things) in case they ate the patches |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent statistician performed resident allocations using computer‐generated randomization of numbers at each nursing home." |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent statistician performed resident allocations ... at each nursing home. Treatment allocation status was delivered to the head nurse at each nursing home, and patches were prepared accordingly." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: placebo patch used but as was olfactory stimulation is a reasonable chance of unblinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although the staff recording falls were blind to group allocation, the head nurse who "supervised the recording of falls regularly", was not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: approx 30% withdrawal due to death and discharge, balanced between study arms. ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Judgement comment: falls, falls rate, fallers and recurrent falls reported unadjusted and adjusted. Falls outcomes thoroughly and completely reported |
| Method of ascertaining falls | Low risk | Judgement comment: falls clearly defined and recorded concurrently |
| Baseline imbalance | Low risk | Judgement comment: no differences between groups at baseline |
| Other bias | Low risk | Judgement comment: none identified |
Salvà 2016.
| Methods | RCT (cluster randomised). | |
| Participants |
Setting: 16 residential care facilities, mixed‐level care, Spain. N = 16 clusters randomised, 12 clusters in analysis. Sample: 72% women Age (years): 84.4 Baseline Characteristics Multifactorial falls prevention programme
Control
Inclusion criteria: 65 years or more; People with or without cognitive impairment living indefinitely in a nursing home place; Able to walk with or without any kind of help or able to self transfer (as defined in category d420 of the WHO International Classification of Functioning, Disability and Health) without help; Give their consent (or the legal guardian in case of cognitive impairment) Exclusion criteria: terminal illness; occupying temporarily a nursing home place (convalescence period) or another kind of place (day centre, long‐term care, etc). Pretreatment differences: nil |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | Additional information provided by author correspondence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: random draw with opaque envelopes |
| Allocation concealment (selection bias) | High risk | Judgement comment: allocation not concealed from the person performing recruitment, as per author correspondence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: falls recorded by staff who were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: large loss to follow‐up after randomisation which is greater in the control arm (41%); 2 centres in control arm left the study (65 participants); 1 centre in each arm provided no falls data (14 participants in intervention group, 32 participants in control group) |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all outcomes reported as specified in trial record, some by author correspondence |
| Method of ascertaining falls | Unclear risk | Judgement comment: falls recording concurrent, unclear if a definition of falls was provided |
| Baseline imbalance | Low risk | Judgement comment: no major imbalances. Imbalance in those with depression in dementia, however numbers are small |
| Other bias | Low risk | Judgement comment: none identified |
Sambrook 2012.
| Methods | RCT (cluster randomised by facility). | |
| Participants |
Setting: 51 aged care facilities (intermediate care), North Sydney, Australia N = 602 residents. 51 clusters. Sample: 71% women Age (years): mean 86.4 (SD 6.6) Inclusion criteria: aged ≥ 70; ambulant; likely to survive for ≥ 12 months Exclusion criteria: taking vitamin D or calcium supplements; history of skin cancer in previous 3 years |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random allocation sequence ... was generated by a statistician who was not involved in the recruitment" |
| Allocation concealment (selection bias) | Low risk | Quote: "... it was concealed from the study coordinators until after randomisation." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study was cluster randomised and nursing staff reported falls. Researchers visited each home every two months to record falls |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: low loss to follow‐up and ITT analysis |
| Selective reporting (reporting bias) | Low risk | Judgement comment: falls, fallers, risk ratio and rate ratio reported, adjusted for clustering |
| Method of ascertaining falls | Low risk | Judgement comment: clear definition, falls documented concurrently (in nursing notes and incident reports) and recorded by research staff monthly |
| Baseline imbalance | Low risk | Judgement comment: only significant difference in cognition at baseline adjusted for in analysis |
| Other bias | Low risk | Judgement comment: none identified |
Saravanakumar 2014.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: residential care facilities, mixed‐level care, Australia Baseline Characteristics Tai chi group
Yoga group
Usual care
Inclusion criteria: aged 60 and over; able to stand with support; able to understand English; able to understand and follow simple instructions and demonstrations Exclusion criteria: severe debilitating illness; severe cognitive impairment; severe hearing or visual impairment (as determined by the RCF staff) Pretreatment differences: nil significant |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 14 weeks | |
| Notes | ACTRN12612000103864 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Permuted block randomisation with a block size of 6 was generated using MS office Excel." |
| Allocation concealment (selection bias) | Low risk | Quote: "After baseline assessments, participants were randomly allocated to tai chi, yoga or usual care groups by a researcher not involved in recruitment who prepared the randomised list in sealed envelopes that were given to the facility staff a day before the commencement of the interventions." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: falls would recorded by care home staff in RCF records, who would not be blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: loss from groups Control 9%, Tai Chi 18%, Yoga 27%. Given small trial numbers losses may have impacted on outcomes |
| Selective reporting (reporting bias) | Low risk | Judgement comment: falls outcomes reported as per trial registration |
| Method of ascertaining falls | Low risk | Quote: "Falls were defined as ‘events that resulted in a per‐ son coming to rest inadvertently on the ground or floor or other lower level, excluding inten‐ tional change in position to rest in furniture, wall or other objects’ (WHO, 2007). Fall incidence information was collected from the records main‐tained at the RCF. The data was collected for the period of 6 months pre‐intervention, intervention period and 6 months post‐intervention period." |
| Baseline imbalance | Low risk | Judgement comment: no significant differences at baseline |
| Other bias | Low risk | Judgement comment: none identified |
Schnelle 2003.
| Methods | RCT (individually randomised) | |
| Participants | Setting: 4 nursing homes (high‐level nursing care), USA N = 190 Sample: 85% women Age (years): mean (SD) intervention group 87.3 (8.0), control group 88.6 (6.7) Inclusion criteria: incontinent; no in‐dwelling catheter; follows one stage commands; not Medicare Part A for post acute care or terminal; occupying long stay bed Exclusion criteria: none stated | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 8 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... subjects were randomized within NHs by computerized programs into intervention and control groups." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information on process of allocation to permit judgement of 'Low risk' or 'High risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Falls recorded in medical records. Staff recording falls were likely to be aware of allocation status. Researchers examining records were blinded to allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: ITT analysis |
| Selective reporting (reporting bias) | Low risk | Judgement comment: falls, fallers, injurious falls, fracture falls and falls incidence reported |
| Method of ascertaining falls | High risk | Judgement comment: no falls definition reported |
| Baseline imbalance | Low risk | Judgement comment: groups balanced at baseline |
| Other bias | Low risk | Judgement comment: none identified |
Schoenfelder 2000.
| Methods | RCT (individually randomised) | |
| Participants | Setting: 2 nursing homes (high‐level nursing care), USA N = 16 Sample: 75% women Age (years): mean 82.8 (range 66 to 95) Inclusion criteria: aged ≥ 65; ambulating independently with or without assistive device; understand English; MMSE score > 20 Exclusion criteria: unstable physical condition; terminal illness; history of acting out or abusive behaviour | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk'. Quote: "... subjects were matched in pairs and assigned randomly within each pair to the intervention or control group." |
| Allocation concealment (selection bias) | High risk | Allocation concealment not described and researchers changed group allocation of one participant after randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff who recorded falls after intervention were likely to be aware of individual's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Judgement comment: no fallers data reported |
| Method of ascertaining falls | Unclear risk | Judgement comment: methods of collecting falls data unclear, no definition provided |
| Baseline imbalance | High risk | Judgement comment: differences in gender and falls efficacy at baseline |
| Other bias | Low risk | Judgement comment: none identified |
Serra‐Rexach 2011.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: 1 geriatric nursing home (intermediate‐level care), Madrid, Spain
N = 40
Sample: 80% women Age (years): mean 92 (SD 2) Inclusion criteria: aged ≥ 90; planning to stay in the same nursing home during the study; able to ambulate with or without cane, walker, or parallel bars); able to communicate; able and willing to consent Exclusion criteria: acute or terminal illness; myocardial infarction in previous 3 months; unstable medical condition; upper or lower extremity fracture in previous 3 months; severe dementia; neuromuscular disease; using drugs affecting neuromuscular function |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 weeks (8 weeks intervention and further 4 weeks follow‐up) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated randomization sequence" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The assessment staff was blinded to participant randomization assignment. Participants were... reminded not to discuss their randomization assignment with assessment staff." "An independent researcher was in charge of auditing all nursing and medical records to record the number of falls in each participant over the study period" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: loss to follow‐up low and reasons balanced. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: falls a secondary outcome. Falls defined as adverse event in published protocol but not final publication |
| Method of ascertaining falls | Low risk | Quote: "In our study, we will define falls as "unexpected event in which the participants come to rest on the ground, floor, or other lower level" [61,62]. An independent researcher will be in charge of auditing all nursing and medical records to record all falls in the participants over the study period." Judgement comment: falls defined and recorded concurrently |
| Baseline imbalance | Low risk | Judgement comment: groups similar at baseline |
| Other bias | Low risk | Judgement comment: none identified |
Shaw 2003.
| Methods | RCT (individually randomised) | |
| Participants | Setting: 2 accident and emergency (A&E) departments, Newcastle, UK N = 308 Sample: 79% of participants lived in high and intermediate nursing care facilities (personal communication), (80% women) Age (years): mean 84 (range 71 to 97) Inclusion criteria: presenting to A&E after a fall; age ≥ 65; MMSE score < 24; consent from patient; immediate carer and next of kin Exclusion criteria: unable to walk; medical diagnosis likely to have caused index fall, e.g. stroke; unfit for investigation within 4 months; unable to communicate for reasons other than dementia; living outside of a 15‐mile radius of recruitment site; no major informant | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomised patients by block randomisation using computer generated random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "Group allocation was performed by a researcher who was independent of the recruitment process and blind to baseline interview data" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data from postcards processed and coded off site by researcher blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most losses due to death, withdrawals low and balanced |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
| Method of ascertaining falls | Low risk | Falls clearly defined and recorded concurrently |
| Baseline imbalance | Low risk | Balanced at baseline |
| Other bias | Low risk | None identified |
Shimada 2004.
| Methods | RCT (individually randomised) | |
| Participants | Setting: 1 long‐term care facility (intermediate‐level care), Japan N = 32 Sample: 78% women Age (years): mean (SD) intervention group 81.8 (5.9), control group 83.1 (6.4) Inclusion criteria: none stated Exclusion criteria: not able to walk more than 3 minutes on treadmill at greater than 0.5 km/hour; unable to participate because of recognisable dementia; unspecified health problems | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 32 subjects were randomly divided into two groups ..." Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible for participants |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Collection of falls data not described but states "This study ... was carried out without blinding." Staff who recorded falls were likely to be aware of individual's allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: losses similar between groups |
| Selective reporting (reporting bias) | Low risk | Judgement comment: fall rates, number of falls and time to first fall reported |
| Method of ascertaining falls | Unclear risk | Judgement comment: falls ascertainment not reported |
| Baseline imbalance | Low risk | Judgement comment: groups similar at baseline |
| Other bias | Low risk | Judgement comment: one detected |
Shorr 2012.
| Methods | RCT (cluster randomised). | |
| Participants |
Setting: 16 nursing units in an urban community hospital, acute care, USA N = 27,672 participants. 16 clusters. Sample: not stated. Age (years): not stated. Baseline Characteristics Automated tele‐vigilance system
Usual care
Inclusion criteria: admission to one of 16 general medical‐surgical nursing units in Methodist Healthcare‐University Hospital, Memphis, Tennessee, during period 1 May 2006 to 30 Oct 2007 Exclusion criteria: nil. Pretreatment differences: baseline characteristics of patients unknown. Staffing hours significantly differ between groups, but controlled for in analysis. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | Admission period. Trials recruitment over 18 months. | |
| Notes | Additional data provided by author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation on the basis of baseline fall rates |
| Allocation concealment (selection bias) | High risk | Allocation of clusters unblinded and recruitment of participants in acute hospital wards occurred over May 2006 ‐ Oct 2007 after cluster allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of staff not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were likely to be unblinded due to the cluster randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of two clusters due to closure of the units, but reason for loss not related to outcome and appropriately accounted for in analysis |
| Selective reporting (reporting bias) | Low risk | Falls, fallers and injurious falls reported |
| Method of ascertaining falls | Low risk | Falls clearly defined and recorded concurrently |
| Baseline imbalance | Unclear risk | Staffing hour for all 3 staff types significantly differ between groups, but controlled for in analysis. However baseline characteristics at patient level not known |
| Other bias | Low risk | Allocation of clusters occurred in pairs of units with similar falls rates within one hospital which may allow the randomisation sequence to be predicted. However this issue already considered under allocation concealment. No other risk identified. |
Sihvonen 2004.
| Methods | RCT (individually randomised) | |
| Participants | Setting: 2 residential care homes (intermediate‐level care), Finland N = 28 Sample: 100% women Age (years): mean (SD) intervention group 80.7 (6.1), control group 82.9 (4.2) Inclusion criteria: aged ≥ 70; able to stand without walking aid; able to visualise feedback from a computer; able to follow instructions Exclusion criteria: acute illness; dementia; impending hip surgery | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects … were randomly assigned to an exercise group or a control group ... Since the study was carried out in two separate places, the randomization was done in blocks." "Randomisation was carried out by drawing lots." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Falls recorded by participants who were aware of group allocation. No mention of blinding of researchers contacting participants for details or if no diary returned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: low loss to follow‐up, unlikely to affect outcome |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no protocol identified. Falls and fallers reported, falls rate calculable |
| Method of ascertaining falls | Unclear risk | Judgement comment: falls recorded by participants |
| Baseline imbalance | Low risk | Judgement comment: groups balanced at baseline |
| Other bias | Low risk | Judgement comment: none identified |
Sitja Rabert 2015.
| Methods | RCT (individually‐randomised, multicentre trial) | |
| Participants |
Setting: 10 residential care facilities, mixed‐level care, Spain N = Sample: Age (years): Baseline Characteristics WBV + exercise
Exercise (control)
Inclusion criteria: volunteers of either sex aged older than 65 years; resident in a nursing home; and able to adopt a squat position on a vibrating platform Exclusion criteria: acute illness (not resolved within 10 days); epilepsy; severe heart disease; use of a pacemaker; high risk of thromboembolism; a hip or knee replacement; musculoskeletal disorders; cognitive or physical disorders that could interfere with training methods Pretreatment differences: nil significant |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 6 weeks, total follow‐up 6 months | |
| Notes | NCT01375790 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated randomisation list will be generated for participants at each nursing‐home using the statistical software SPSS17." |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation to treatment will be centralized by telephone. All the researchers will be blinded to the randomisation sequence list." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: falls collected from nursing home staff or relatives who were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: losses to follow‐up balanced between groups, reasons balanced |
| Selective reporting (reporting bias) | High risk | Judgement comment: number of falls not reported by group allocation |
| Method of ascertaining falls | Low risk | Judgement comment: fall definition provided in Clinical Trial Registry (NCT01375790).Quote: "Fall: an unexpected event in which the participants come to rest on the ground, floor, or lower level". Concurrently recorded. Additional information from author 11/7: Report calendar: During the study, every falls was registered in a register falls specially created by the study and data concerning falls were regularly collected from each nursing home or from relatives if a participant had moved to a different address. During the follow‐up period, systematically every week the two blinded physiotherapists registered the falls occurred |
| Baseline imbalance | Low risk | Judgement comment: groups well balanced at baseline |
| Other bias | Low risk | Judgement comment: none identified |
Stenvall 2007.
| Methods | RCT (individually randomised) | |
| Participants | Setting: acute hospital wards (geriatric and orthopaedic), Umeå, Sweden N = 199 Sample: 74% women Age (years): mean 82.2 (SD 6.3) Inclusion criteria: admitted to hospital with femoral neck fracture; aged ≥ 70 Exclusion criteria: severe rheumatoid arthritis; severe hip osteoarthritis; pathological fracture of the femoral neck; severe renal failure; bedridden prior to the fracture | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 32 months. Follow‐up time was until participants were discharged from hospital | |
| Notes | Dementia subgroup analysis published in Stenvall 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized ... in opaque sealed envelopes. The lots in the envelopes were sequentially numbered ... Persons not involved in the study performed these procedures." |
| Allocation concealment (selection bias) | Low risk | Used sequentially numbered, opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The staffs on the intervention and control wards were not aware of the nature of the present study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: all patients included in analysis (ITT) |
| Selective reporting (reporting bias) | Low risk | Judgement comment: no protocol identified but falls outcomes thoroughly reported. Falls, fallers, falls incidence and fracture falls reported, plus data by dementia subgroup |
| Method of ascertaining falls | Low risk | Judgement comment: falls clearly defined and recorded concurrently |
| Baseline imbalance | Low risk | Judgement comment: significant imbalance in depression and non‐significant imbalance in dementia at baseline adjusted for in analyses |
| Other bias | Low risk | Judgement comment: none detected |
Streim 2012.
| Methods | RCT | |
| Participants |
Setting: residents in nursing homes and assisted living facilities within 30 miles of Philadelphia, USA. Mixed levels of care. N = 94 (36 randomised, 56 in a non‐randomised patient preference arm) Sample: NR Age (years): NR Baseline Characteristics Age (years): range 60 to 95. Baseline characteristics not provided. Inclusion criteria: 65 years and older; ambulatory; cognitively intact or with mild‐moderate impairment but capable of self‐reporting depression symptoms; receiving antidepressant treatment for a single episode of depression; in full remission for at least six months Exclusion criteria: bedridden; severe cognitive impairment Pretreatment differences: no differences in race and gender. Differences in medication use at baseline (benzodiazepines P = 0.034, serotonin norepinephrine reuptake inhibitors P = 0.0004, Lexapro P < 0.0001). |
|
| Interventions |
A third non‐randomised arm of people choosing to discontinue antidepressants |
|
| Outcomes |
Other outcomes not included in this review, e.g. depression and cognition |
|
| Duration of the study |
|
|
| Notes | Trial identified as an abstract only, with no falls results reported. Excerpt from unpublished manuscript provided by author correspondence. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: insufficient information for judgement |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: insufficient information for judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: insufficient information for judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: insufficient information for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: insufficient information for judgement |
| Selective reporting (reporting bias) | Unclear risk | No details of falls outcomes provided in trial registry |
| Method of ascertaining falls | Unclear risk | Judgement comment: insufficient information for judgement |
| Baseline imbalance | High risk | Judgement comment: differences in medication use between randomised groups at baseline |
| Other bias | Unclear risk | Judgement comment: imbalance in randomisation due to high number of patients choosing third 'preference' arm of study |
Tideiksaar 1993.
| Methods | RCT (individually randomised) | |
| Participants | Setting: acute geriatric care hospital ward, New York city, USA N = 70 Sample: 86% women Age (years): mean 84 (range 67 to 97) Inclusion criteria: one or more abnormal factors on a 9 point performance orientated environmental mobility screen (indicating impaired bed mobility) Exclusion criteria: none stated | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 9 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients … were randomly assigned to either the experimental group … or the control group". Insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff who recorded falls not blinded to individual participants' allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no loss to follow‐up, acute setting |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified |
| Method of ascertaining falls | Unclear risk | Judgement comment: definition of falls provided but not clearly. Falls recorded concurrently |
| Baseline imbalance | Unclear risk | Judgement comment: baseline characteristics not reported |
| Other bias | Low risk | Judgement comment: none identified |
Toulotte 2003.
| Methods | RCT (individually randomised) | |
| Participants | Setting: nursing care facility, France. Published data implies residents receiving mixed high and intermediate levels of care N = 20 Sample: % women not stated Age (years): mean 81.4 (SD 4.7) Inclusion criteria: dementia (MMSE score < 21); history of ≥ 2 falls (not involving an environmental hazard) in previous 3 months; able to walk 10 metres without human assistance Exclusion criteria: none stated | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 4 months follow‐up | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomised cross‐over design was used." Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Physician conducting tests was blinded to allocation status. Unlikely that these tests included recording of falls. Staff who recorded falls likely to be aware of individual participants' allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: loss to follow‐up unclear |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified |
| Method of ascertaining falls | Unclear risk | Judgement comment: falls clearly defined but method of recording falls unclear |
| Baseline imbalance | Low risk | Judgement comment: groups balanced at baseline |
| Other bias | Low risk | Judgement comment: none identified |
Treacy 2015.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: general rehabilitation ward (subacute) at one hospital, Australia N = Sample: Age (years): Baseline Characteristics Standing balance circuit classes
Usual care
Inclusion criteria: 18 years or over; admission to the general rehabilitation ward a Bankstown‐Lidcombe Hospital, NSW; no medical contraindications to exercise; able to: fully weight bear; stand unaided independently for at least 30 seconds; and participate in group therapy sessions with minimal supervision Exclusion criteria: 1. unable to fully weight bear as ordered by a medical officer (i.e. non, partial or touch weight bearing status through one or both legs).2. Have a medical condition precluding exercise, e.g. unstable cardiac disease, uncontrolled hypertension, uncontrolled metabolic diseases, large abdominal aortic aneurysm. 3. Have an identified multi‐resistant organism infection or other infection that would pose a significant risk to others in a group setting. Pretreatment differences: no imbalances. See online appendix. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 2 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation schedule was computer generated using randomly ordered blocks of four and six." |
| Allocation concealment (selection bias) | Low risk | Quote: "A concealed allocation procedure (numbered sealed opaque envelopes)" Quote: "Randomisation schedule and envelopes were prepared and held by a staff member not involved in study recruitment or intervention. Participants and therapists were made aware of group allocation once the envelopes had been opened." Judgement comment: allocation adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: falls reliant on self‐report, person responsible and hospital incident reporting system. Not possible to blind staff |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: little loss to follow‐up at 2 weeks |
| Selective reporting (reporting bias) | Low risk | Judgement comment: protocol available and outcomes reported as planned |
| Method of ascertaining falls | Low risk | Judgement comment: Trial registry: "Fall incidence will be measured by participant and/or 'person responsible' self‐report. In‐patient fall data will also be collected via the hospital Incident Information Management System (incident reporting system)."Hospital system will have clear definition and concurrent recording of falls" |
| Baseline imbalance | Low risk | Judgement comment: groups balanced on range of demographic variables at baseline |
| Other bias | Low risk | Judgement comment: none detected |
Tuunainen 2013.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: residential care facility, mixed‐level care, Finland N = Sample: Age (years): Baseline Characteristics Strength training
Balance and strength training
Self‐administered training
Inclusion criteria: participant's ability to raise himself/herself from a chair without using hands or arms for support.Willingness to participate. Prof Pyykko stated in email 31/8/16 additional inclusion criteria: could move independently, arise from a chair 5 times in a row, follow instructions. Exclusion criteria: nil stated. Pretreatment differences: the Strength training group had 33% male, The Balance and Muscle Training had 11% male, The self‐administered group had 26% male.In the Strength training group, 39% were prescribed sleeping medications. In the balance and muscle training group, 56% were prescribed sleeping medications. In the self‐administered group, 68% were prescribed sleeping medications. |
|
| Interventions |
|
|
| Outcomes |
Other outcomes not included in this review |
|
| Duration of the study | 13 weeks. Follow‐up 3 years. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: drawing of envelopes |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: details of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: falls recorded by ward nurses who are unlikely to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: it appears that the residents in the intervention groups who stopped training were not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: study protocol not available. In correspondence, author states data on fracture falls data was collected but not included |
| Method of ascertaining falls | Unclear risk | Judgement comment: insufficient information to enable judgement |
| Baseline imbalance | High risk | Judgement comment: larger proportion of prescribing of sleeping medications in the Self administered group may have contributed to that group's higher falls rate |
| Other bias | Low risk | Judgement comment: none identified |
Van de Ven 2014.
| Methods | RCT (cluster randomised). | |
| Participants |
Setting: 34 units from 11 residential care facilities, high‐level care, the Netherlands. N = 318. 11 clusters. Sample: 75% women Age (years): 84.7 Baseline Characteristics Dementia care mapping
Usual care
Inclusion criteria (facilities): those with Dementia‐Special Care Units (DSCUs).Residents: Age of 65 years or more;Dementia diagnosed by an elderly care physician according to the Diagnostic and statistical manual of mental disorders‐IV criteria for dementia; Approval of the elderly care physician for inclusion; At least one of the following neuropsychiatric symptoms: aggression, motor or verbal agitation,psychosis, depression, and apathy; Informed consent given by the residents themselves, their families, or their legal guardians; The resident must use the common areas, such as the shared living room, at least 4 hours a day. Exclusion criteria: residents: an estimated life expectancy of 6 weeks; those who are physically unable to spend time in common areas of the facility; withdrawal of consent Pretreatment differences: the intervention and control groups differed in terms of the proportions of staff in permanent positions. There were no other statistically significant differences at baseline between the intervention and control groups |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 18 months | |
| Notes | Author contact: Geertje van de Ven, Radboud University, G.vandeVen@elg.umcn.nl. Author clari fied study details by email. Dutch Trials Registry NTR2314http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2314 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: computer‐generated sequence "soft‐ware" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation will take place after the study sample has been recruited and informed consent has been given," |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: falls recorded by staff who are not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: attrition rates due to no medical file higher in the intervention group (44% 68/154) vs control group (21% 35/164). (Fig 1). Unclear if medical file is source of falls data |
| Selective reporting (reporting bias) | Low risk | Judgement comment: outcomes reported as per protocol |
| Method of ascertaining falls | Unclear risk | Judgement comment: insufficient information for judgement |
| Baseline imbalance | High risk | Judgement comment: large difference in baseline fall rates. Baseline data for many potential confounders for falls outcomes not recorded. |
| Other bias | Low risk | Judgement comment: none identified |
Van Gaal 2011a.
| Methods | RCT (cluster randomised by ward) | |
| Participants | Setting: 6 nursing homes, 10 wards (high‐level nursing care), the Netherlands N = 392 participants included in study. 10 clusters. Sample: 66% women Age (years): mean (SD) intervention group 78 (9.9), control group 78 (11.7) Inclusion criteria (facilities): 2 or 4 more or less comparable wards. Inclusion criteria (residents): none stated Exclusion criteria: none stated | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 23 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised after stratification. Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding of staff not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff recording falls would be aware of allocation. Cluster randomised trial so likely the person collecting data from patient files would be aware also |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: high loss to follow‐up for Van Gaal 2011a (nursing home setting) |
| Selective reporting (reporting bias) | Low risk | Judgement comment: falls reported as per protocol and adjusted for clustering |
| Method of ascertaining falls | High risk | Judgement comment: falls clearly defined but reliant on existing reporting systems in patient records which may vary between sites |
| Baseline imbalance | High risk | Judgement comment: 2011a (NH): nurse characteristics balanced at baseline but significant difference in physically impaired patients (reviewer P < 0.001 Chi2), rehabilitation patients (reviewer Chi‐2 P < 0.001) |
| Other bias | Low risk | Judgement comment: none detected |
Van Gaal 2011b.
| Methods | RCT (cluster randomised). | |
| Participants |
Sample: 4 hospitals (acute care), 10 wards, the Netherlands N = 2201 participants included in study. 10 clusters. Sample: 55% women Age (years): mean (SD) intervention group 66 (14.5), control group 64 (16.9) Inclusion criteria (hospitals): 2 or 4 more or less comparable wards. Inclusion criteria (patients): expected length of stay of ≥ 5 days Exclusion criteria: none stated |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 23 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised after stratification. Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding of staff not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff recording falls would be aware of allocation. Cluster‐randomised trial so likely the person collecting data from patient files would be aware also |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: high loss to follow‐up for van Gaal 2011b (hospitals) |
| Selective reporting (reporting bias) | Low risk | Judgement comment: falls reported as per protocol and adjusted for clustering |
| Method of ascertaining falls | High risk | Judgement comment: falls clearly defined but reliant on existing reporting systems in patient records which may vary between sites |
| Baseline imbalance | High risk | Judgement comment: 2011a (NH): nurse characteristics balanced at baseline but significant difference in physically impaired patients (reviewer P < 0.001 Chi‐2), rehabilitation patients (reviewer Chi‐2 P < 0.001). |
| Other bias | Low risk | Judgement comment: none detected |
Van het Reve 2014.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: residential care, intermediate‐level care, Switzerland (13 care facilities) and Germany (1 facility). N = 182 Sample: 55% women Age (years): 81.5 (SD 7.3) Baseline Characteristics Strength‐balance‐cognitive training
Strength‐balance training
Inclusion criteria: older than 65 years; able to walk 20 meters with or without aids; signed informed consent statement Exclusion criteria: severe cognitive impairment (Mini‐Mental State Examination below 22 points); rapidly progressive or terminal illness, acute illness or unstable chronic illness Pretreatment differences: nil significant |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 15 months comprising 12 weeks intervention and 12 months post‐intervention follow‐up period. | |
| Notes | ISRCTN75134517 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using simple (unrestricted) randomisation [70] based on a table of random numbers." |
| Allocation concealment (selection bias) | High risk | Judgement comment: an "assessor" performed the randomisation and group allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: unable to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: falls calendars filled in by staff. "Blinding of investigator was not possible." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: approximately 25% missing data for falls |
| Selective reporting (reporting bias) | Low risk | Judgement comment: protocol available (ISRCTN75134517) with falls reported as per protocol. |
| Method of ascertaining falls | Low risk | Quote: "Falls, defined as ‘unexpected events in which the participant comes to rest on the ground, floor or lower level, were assessed from 6 months retrospectively to 12 months prospectively using a fall calendar." |
| Baseline imbalance | Low risk | Judgement comment: nil significant |
| Other bias | Low risk | Judgement comment: four participants with vision impairment reallocated to control group, however, this number is small relative to intervention group sizes |
Wald 2011.
| Methods | CCT (odd vs even medical record number) | |
| Participants |
Setting: acute medical units in 1 hospital, Colorado, USA
N = 217
Sample: 55% women Age (years): mean (SD) intervention group 80.5 (6.5), control group 80.7 (7.0) Inclusion criteria: aged ≥ 70 Exclusion criteria: patients admitted to medical subspecialty service (cardiology, pulmonary, oncology) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 22 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | A systematic non‐random method was used (odd /even case record number) |
| Allocation concealment (selection bias) | High risk | Not possible to blind prior to allocation (see above) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Falls from hospital event reports. Last digit of medical record number was used for group allocation. Allocation not concealed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: all patients included in analyses of other outcomes. Falls incidence per patient days reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified |
| Method of ascertaining falls | Unclear risk | Judgement comment: falls definition not reported. Falls determined from standard reporting system which will be concurrent |
| Baseline imbalance | Low risk | Judgement comment: groups balanced at baseline |
| Other bias | Low risk | Judgement comment: none identified. |
Walker 2015.
| Methods | RCT (cluster randomised). | |
| Participants |
Setting: 6 residential care facilities, mixed‐level care, UK N = 52 residents. 6 clusters. Sample: 67% women Age (years): 83 Baseline Characteristics Implementation of the Guide to Action Care Home tool
Usual care
Inclusion criteria: care homes: listed on the Care Quality Commission database, long stay, old age, dementia or learning disability registration, nursing/residential registration, over 10 residents, no prior experience of Guide to Action Care Home. Care homes were purposively selected from those who replied expressing interest, to reflect a range of ownership, size and registration. Residents: (high risk): aged over 50 years, fallen at least once in the past year Exclusion criteria: bed‐bound, hoist‐dependent or terminally ill Pretreatment differences: nil |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: randomisation done |
| Allocation concealment (selection bias) | Low risk | Judgement comment: allocation concealed according to standard operating procedure |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: 26% missing data (7/27) from control arm vs 12% intervention arm |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: falls would have been recorded by staff who would not be blinded to the intervention (staff training) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: 26% missing data (7/27) from control arm vs 12% intervention arm |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified |
| Method of ascertaining falls | Low risk | Judgement comment: no information on most potential confounders (e.g. medical status, dependency) |
| Baseline imbalance | Unclear risk | Judgement comment: none detected |
| Other bias | Low risk | Judgement comment: blinding not feasible |
Ward 2010.
| Methods | RCT (cluster randomised by facility). | |
| Participants |
Setting: 88 residential aged care facilities (high‐care, low‐care and dementia‐specific), New South Wales, Australia
N = 5391 residents. 88 clusters.
Sample: 73% women Age (years): median age 86 Inclusion criteria (facilities): ≥ 20 beds Exclusion criteria: none stated |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 17 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly allocated within strata into intervention or control groups by the statistician ... using the procedure "surveyselect" in SAS statistical software" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff recording falls and carrying out monthly record audit were aware of group allocation. Failure to produce monthly data followed up by project nurse (also aware of group allocation) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: losses balanced but large loss of 3 facilities/arm of study. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified, fallers data not reported |
| Method of ascertaining falls | High risk | Judgement comment: no definition of falls. Fall data retrieved by facility through record audit ‐ likely to be variable reporting between facilities |
| Baseline imbalance | High risk | Judgement comment: although data in table 1 (limited participant variables) show reasonable balance between groups, there was moderate difference (2 falls /month) between groups in the 7‐month pre‐intervention falls data |
| Other bias | Low risk | Judgement comment: none detected |
Whitney 2017.
| Methods | Cluster RCT (pilot, cross‐over study). | |
| Participants |
Setting: Four nursing homes and five residential homes in London, UK, mixed‐level care, 97% cognitively impaired. 9 clusters: 5 intervention, 4 usual care. N = 191 participants. 9 clusters. Sample: 69% women Age (years): mean 83.5 (SD 8.8) Baseline Characteristics Individualised fall prevention programme
Usual care
Inclusion criteria: over 65 years; admitted to rehabilitation ward Exclusion criteria: restricted to bed; refused to participate Pretreatment differences (phase 1): longer stay in the control group patients (P <0.001); higher percentage of females in the control group (P =0.03) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 6 months | |
| Notes | Costs of the programme to be reported. Other outcomes not included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: computer‐generated randomisation, stratified by nursing home beds |
| Allocation concealment (selection bias) | Low risk | Judgement comment: randomisation conducted by separate clinical trials unit. Allocation concealed and no recruitment after allocation revealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: falls recorded by care home staff who were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: 17.8% loss to follow‐up. Large amounts of missing data on many outcomes (up to 60%). Not clear what loss to follow‐up/missing data for falls outcome |
| Selective reporting (reporting bias) | Low risk | Judgement comment: falls, fallers, injury and fracture falls data reported |
| Method of ascertaining falls | Unclear risk | Judgement comment: falls definition used.Facilities used their "usual reporting mechanisms" for falls ‐ no detail of what these mechanisms were or if they varied substantially between facilities |
| Baseline imbalance | High risk | Judgement comment: significant baseline differences in number of medical conditions, time to complete Timed Up and Go, and likelihood on being in nursing home bed. Although analysis involved some adjustments (for the baseline score on the outcome being investigated) it does not appear these baseline differences were adjusted for across the outcome measures. |
| Other bias | Low risk | None detected |
Wolf 2013.
| Methods | RCT (individually randomised) | |
| Participants |
Setting: Subacute hospital setting, single geriatric ward, Germany N = 98 Sample: 65% women Age (years): 76.1 Baseline Characteristics Bed‐exit alarm
Usual care
Inclusion criteria: patients at high risk of falls defined by a score of 3 or more in STRATIFY; requirement for assistance with mobilization during resting time Exclusion criteria: immobility; participation in another trial Pretreatment differences: NR |
|
| Interventions |
Intervention Characteristics
|
|
| Outcomes |
|
|
| Duration of the study | During admission period, total trial period 13 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: method of sequence generation not described in adequate detail |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no information provided about allocation methods |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: falls recorded by nurses who were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: author correspondence indicated no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol identified |
| Method of ascertaining falls | Unclear risk | Judgement comment: no falls definition provided, standard reporting mechanisms used |
| Baseline imbalance | Unclear risk | Judgement comment: inadequate details on baseline characteristics of patients to make a judgement |
| Other bias | Low risk | Judgement comment: none detected |
Yokoi 2015.
| Methods | RCT (cluster randomised). | |
| Participants |
Setting: 5 residential care facilities, intermediate‐level care, Japan N = 105 participants. 5 clusters. Sample: 60% women Age (years): 79.4 Baseline Characteristics Short stick exercises
Usual care
Inclusion criteria (facilities): with 50 beds in the Kinki area in Japan; where no intervention for fall prevention was conducted. Residents: able to walk without assistive devices and take care of themselves without assistance; had sufficient cognition to follow directions; had never performed an SSE before; were allowed by their chief physician to exercise Exclusion criteria: residents: with dementia or severe cardiac, pulmonary or musculoskeletal disorders that are associated with a higher fall risk Pretreatment differences: BMI significantly less in the Intervention group, but as both groups were in normal range, probably would not have had impact on outcome. |
|
| Interventions |
Intervention Characteristics
|
|
| Outcomes |
|
|
| Duration of the study | 12 months, 6 months intervention period. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: randomisation of the 5 facilities was by lottery using envelopes by a researcher not involved with study. Insufficient information but reason for not using sequence generation not really valid despite only 5 facilities, so some risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation of facilities probably adequate, assuming envelopes were sealed and opaque. It does not appear that individual participant recruitment was completed prior to cluster allocation. The study states that research assistants were not informed of the results of randomisation, but it appears that the research assistants were involved with falls data collection, not with recruitment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not possible to blind participants. Highly unlikely that personnel could be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Research assistants were not informed of the results of the randomization. The staff was asked not to tell the research assistants about which group was undergoing the intervention." Judgement comment: unblinding is likely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: losses to follow‐up balanced between groups with similar reasons |
| Selective reporting (reporting bias) | High risk | Judgement comment: the number of falls were not reported |
| Method of ascertaining falls | Unclear risk | Falls determined by interviews with staff and medical records. Not clear whether staff were asked to recall for periods longer than one month. Unclear whether the method and reliability of staff recording falls in patient records were the same in all the facilities. |
| Baseline imbalance | Unclear risk | Judgement comment: baseline characteristics of individuals in the facilities appear to be reasonably balanced although BMI significantly different but both groups within normal range for BMI so not likely to be important. Baseline characteristics of the facilities were not compared ‐ in particular the rates of falling in each of the facilities prior to the intervention. |
| Other bias | Low risk | None detected |
Zermansky 2006.
| Methods | RCT (individually randomised) | |
| Participants | Setting: 65 care homes for the elderly (high, intermediate and mixed levels of care), UK N = 661 Sample: 77% women Age (years): mean 85 (interquartile range 80 to 90) Inclusion criteria: aged ≥ 65; resident in a care home with ≥ 6 residents Exclusion criteria: participating in another trial; terminally ill; already receiving clinical medication review; at GP request | |
| Interventions |
|
|
| Outcomes |
|
|
| Duration of the study | 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After collection of baseline data, patients were randomised in randomly sized blocks of two to eight patients using an algorithm written in Visual Basic in Microsoft Access." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not possible to blind the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Falls data collected from accident book. Unclear whether staff recording falls in accident book would have been aware of allocation status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: loss to follow‐up similar in both groups, as was main reason for loss (death) |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: falls not reported as an outcome in trial registration |
| Method of ascertaining falls | High risk | Judgement comment: no falls definition reported |
| Baseline imbalance | Low risk | Judgement comment: groups balanced at baseline |
| Other bias | Low risk | Judgement comment: none detected |
A&E: emergency department ADLs: activities of daily living AMTS: Abbreviated Mental Test Score BMI: body mass index CPG: clinical practice guideline DCM: dementia care mapping GCS: Glasgow Coma Score GP: general practitioner IQR: interquartile range ITT: intention‐to‐treat IU: international unit MMSE: Mini Mental State Examination N: No NR: not reported OT: occupational therapist RCT: randomised controlled trial SD: standard deviation STOPP/START: Screening Tool of Older Persons potentially inappropriate Prescriptions/Screening Tool to Alert doctors to Right Treatment vs: versus Y: Yes
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barreca 2004 | RCT. Falls outcomes. Supervised exercises in older people post stroke |
| Bernhardt 2008 | RCT. Falls recorded as adverse events. Early rehabilitation post stroke |
| Bosner 2012 | Not randomised. Five nursing homes agreed to participate; three were assigned sequentially for the intervention and two for the control group |
| Bouwen 2008 | RCT (cluster randomised). Nursing homes. Outcome of the study was a subgroup of falls only (falls with medical consequences) |
| Capezuti 1998 | RCT (cluster randomised). Nursing homes. The intervention was designed to minimise restraints, not to reduce falls. Falls reported as adverse events |
| Crotty 2002 | RCT. Accelerated discharge after hip fracture and home based rehabilitation in the community. Not designed to reduce falls. Falls recorded as adverse events |
| Cucca 2017 | Falls recorded as adverse events |
| Cummings 2015 | Falls recorded as adverse events |
| Dattalo 2015 | Wrong setting, participants likely to be in the community. Attempts to contact authors unsuccessful. |
| Davison 2005 | RCT. Post‐fall intervention with falls outcomes. Only one participant in residential/nursing care |
| de Morton 2007 | CCT. The primary outcome was discharge destination. Falls were recorded as adverse events |
| de Souto 2016 | Falls recorded as adverse events |
| de Souto 2017 | Falls recorded as adverse events |
| DeSure 2013 | RCT, cross‐over trial. Exercise Program to Prevent Falls in Institutionalized Elderly with Cognitive Deficits. Falls data for phase 1 not clearly reported, falls data for phase 2 has contamination of intervention group. Attempts to contact author unsuccessful. Available falls data considered invalid. |
| Donat 2007 | RCT. Exercise interventions in nursing homes. No falls outcomes |
| Drahota 2013 | Pilot RCT. Intervention was intended to reduce fall injuries, rather than falls. |
| Fiatarone 1994 | RCT. Boston FICSIT study in nursing home residents. No falls outcomes |
| Forster 2017 | Falls recorded as adverse events |
| Fossey 2006 | RCT. Nursing homes. Intervention to reduce antipsychotics in people with severe dementia. Falls were recorded as adverse events |
| Galik 2014 | Falls reported as adverse events |
| Galik 2015 | Falls recorded as adverse events |
| Gianturco 2013 | Wrong setting, RCT conducted at a geriatric day service with community‐dwelling participants |
| Ginde 2017 | Falls recorded as adverse events |
| Graafmans 1996 | Wrong setting. 49% residing in homes for the elderly, included in community review. |
| Grant 2005 | RCT. Participants recruited in hospital after a hip fracture. Preventing falls in older people living in the community |
| Greenspan 2013 | Wrong study design, not an RCT. |
| Greenspan 2015 | Falls recorded as adverse events |
| Gruber‐Baldini 2011 | RCT. Intervention to motivate nursing assistants to actively engage nursing home residents in functional and physical activities. Falls recorded as adverse events |
| Gu 2006 | Non‐randomised controlled trial of exercise intervention in nursing homes. Experimental group was a convenience sample from two nursing homes; matched control group selected from another nursing home [personal communication] |
| Hardin 2013 | Wrong patient population. Hospital setting. Author confirmed age of participants unknown |
| Harwood 2004 | RCT. Participants recruited at the end of ward rehabilitation post hip fracture. Preventing falls in older people living in the community |
| Hauer 2001 | RCT. Exercise intervention. Recruited at the end of ward rehabilitation. Majority were community‐dwelling (4% living in nursing homes) |
| Heiberg 2017 | Falls recorded as adverse events |
| Herrmann 2016 | Falls reported as adverse events |
| Hopman‐Rock 1999 | RCT. Participants with dementia in homes for the elderly. Falls recorded as safety issue, i.e. as adverse events |
| Huang 2005 | RCT. Discharge planning intervention to prevent falls in older people living in the community |
| Il'nitskii 2014 | Wrong study design, not an RCT |
| Ilfeld 2010 | Falls recorded as adverse events |
| Jarret 2015 | RCT. Intervention delivered in a rehabilitation setting, patients admitted from community, no falls in hospitals, falls outcomes recorded post‐discharge. Included in community review. |
| Jeon 2015 | Only injurious falls reported |
| JPRN‐UMIN0000167 | Wrong setting: likely community. Attempts to contact author unsuccessful. Trial discontinued. |
| Kato 2006 | Not RCT. "Prospective clinical trial" of an exercise programme in a long‐term care facility with falls outcomes. Nurses volunteered their ward to be an intervention ward (personal communication from authors) |
| Katz 2004 | RCT in residential care population. Intervention: three doses of risperidone in people with dementia and psychosis or agitation. Post hoc subgroup analysis of falls based on 85.9% of those randomised. Falls reported as adverse events |
| Katz 2005 | This study was not primarily a falls prevention intervention. Falls reported as adverse events |
| Kenny 2001 | RCT. Follow‐up of falls outcomes appears to be primarily in the community |
| Koczy 2011 | The intervention was designed to minimise restraints, not to reduce falls. Falls reported as adverse events |
| Kopke 2012 | RCT (cluster randomised). Nursing homes. The intervention was designed to minimise restraints, not to reduce falls. Falls reported as adverse events |
| Kwok 2006 | RCT. Intervention to determine whether bed‐chair pressure sensors reduced physical restraint use. Falls reported as adverse events |
| Lackner 2008 | RCT in cognitively impaired nursing home residents with urge urinary incontinence. Falls reported as adverse events |
| Li 2017 | Falls recorded as adverse events |
| Lord 2003b | RCT. Majority of participants community‐dwelling. Only 121/551 participants were residents of an intermediate level nursing care facility |
| Mailhot 2012 | Falls recorded as adverse events |
| Mailhot 2014 | Falls reported as adverse events |
| Mak 2016 | Wrong setting. Intervention delivered in hospital, falls recorded in the community |
| Mansfield 2015 | Falls recorded as adverse events |
| McRae 1996 | Not RCT. Falls and fallers were not primary outcomes but were monitored as possible adverse events |
| Mudge 2008 | Non‐randomised controlled study. Patients admitted to an intervention ward or control ward |
| NCT00973297 | Wrong population: Patients post‐stroke |
| NCT01054287 | Author correspondence confirmed that study unpublished and unlikely to be published as primary author has left the institution. Trial discontinued (results unavailable) |
| NCT01523600 | Trial discontinued due to lack of funding. |
| NCT01618786 | Intervention intended to reduce injuries not falls. |
| NCT02686515 | Wrong population: Patients post‐stroke |
| Nyaruhirira 2013 | Wrong setting. Setting unclear, attempts to contact author unsuccessful. |
| Ouslander 2005 | RCT testing 'Functional Incidental Training' in nursing homes. Not designed to reduce falls. Falls recorded as adverse events |
| Parasurum 2011 | Wrong patient population. Hospital mental health setting, patient age unknown, attempt to contact authors unsuccessful, participants unlikely to be elderly. |
| Pedreira 2014 | Wrong population: Patients post‐stroke |
| Peng 2014 | Falls recorded as adverse events |
| Peri 2008 | RCT (cluster). Pilot for Kerse 2008 (same intervention). Excluded because falls were recorded as possible adverse effects of the intervention |
| Rantz 2001 | RCT. Quality improvement intervention in nursing care facilities targeting 29 quality indicators, of which falls was one. Only included 87/113 homes in the analysis (23% loss). Insufficient information provided on falls outcomes to use in this review |
| Ray 2005 | RCT. Study of falls related injuries. No data provided on falls or fallers |
| Reinhardt 2014 | Falls reported as adverse events |
| Resnick 2002 | Participants resident in continuing care retirement community but all living independently |
| Resnick 2012 | RCT in assisted living facilities. Testing changing model of care to function‐focused care. Falls monitored as a safety issue, i.e. adverse events. Hypothesised that the intervention might increase the likelihood of falling |
| Richter 2015 | Falls recorded as adverse events |
| Rolland 2007 | RCT. Exercise programme to improve ability to perform ADL for people with Alzheimer's disease in nursing homes. Falls monitored as a safety issue, i.e. adverse events |
| Sackley 2009 | RCT. Falls described as an outcome at trial registration but not mentioned as an outcome in the published paper |
| Sahota 2014 | Specific type of falls only, reported bedside and injurious falls, not total falls. |
| Said 2012 | Falls recorded as adverse events |
| Said 2015 | RCT. Falls recorded as adverse events |
| Sato 2000 | RCT. Etidronate versus placebo in older people with post stroke hemiplegia. Falls outcomes. Wrong population; article subsequently retracted |
| Sato 2005a | RCT. Vitamin D vs placebo in older people with post stroke hemiplegia. Falls outcomes. Wrong population; article subsequently retracted |
| Sato 2005b | RCT. Folate and mecobalamine (vitamin B12) vs placebo in older people with post stroke hemiplegia. Falls outcomes. Wrong population; article subsequently retracted |
| Sato 2011 | RCT. Aledronate versus alphacalcidol in older people post‐stroke. Falls outcomes. Wrong population; scientific misconduct also likely |
| Schneider 2006 | The objective of this study was to determine the effectiveness of atypical antipsychotic medications. Falls were monitored as a potential adverse effect |
| Schwendimann 2006 | Not RCT. Described as quasi‐randomised in abstract but author confirmed that all consecutively admitted patients were allocated at non‐random order either to nursing unit A or B whenever a free hospital bed was available (1 to 5 admissions/discharges per day). Nurse‐led fall prevention programme |
| Sherrington 2016a | Wrong setting, correspondence with the author indicated 3% participants were in care ‐ excluded as majority living in a community setting |
| Shimada 2003 | RCT. Majority of participants community‐dwelling (62%) |
| Shimada 2009 | Not RCT. Exercise intervention versus control in a residential‐care facility. Falls outcomes. Intervention on 2 days per week and 2 other days randomly selected to be control days |
| Siddiqi 2016 | No falls outcomes |
| Sjoberg 2013 | Wrong setting. Intervention partly in hospital and partly in community. Author confirmed that < 50% residing in nursing homes at 6 and 12 months |
| Smith 2017 | Falls data not reported separately to slips and trips. Not an RCT |
| Sola 2014 | RCT. Setting unclear, likely to be in the community. Attempts to contact author unsuccessful |
| Southard 2006 | RCT with no falls outcomes. Balance and confidence were the primary outcomes of this study |
| Steadman 2003 | RCT. Participants were attendees of a hospital‐based falls clinic. "Prevously living in the community" [personal communication]. Not preventing falls in hospital or nursing care facility |
| Tanikawa 2014 | Falls recorded as adverse events |
| Tariot 2004 | RCT. Trial testing effectiveness of memantine in people with Alzheimer's disease already receiving donepezil. Falls were monitored as a potential adverse effect of the intervention |
| Tariot 2005 | RCT. Trial testing effectiveness of divalproex sodium in nursing home residents with possible or probable Alzheimer disease. Falls were monitored as a potential adverse effect of the intervention |
| Teresi 2013 | Wrong study design. Not an RCT, random selection for data collection, rather than allocation |
| Underwood 2011 | Ongoing RCT (cluster randomised). Exercise intervention in residential and nursing homes Primary outcome depression. No falls outcomes. Recording peripheral fractures and fear of falling |
| van Ooijen 2013 | Wrong setting. Intervention delivered in hospital, author confirmed falls recorded post dischage and the majority of participants were in the community. |
| Vassallo 2004 | Non‐randomised controlled trial of a multidisciplinary fall‐prevention programme in hospital. Falls outcomes |
| Visvanathan 2015 | Not an RCT |
| Von Koch 2001 | RCT. Intervention: rehabilitation at home after a stroke. Not intervention to prevent falls; falls recorded as adverse events |
| Wolf 2003 | RCT. Participants in independent living facilities or congregate living facilities, i.e. not nursing care facilities. Community‐dwelling |
| Zhong 2007 | RCT. Institutionalised participants with dementia randomised to quetiapine 200 mg per day, 100 mg per day, or placebo. Falls monitored as a potential adverse effect of the intervention |
ADL: activities of daily living CCT: controlled clinical trial RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Dever 2016.
| Methods | RCT |
| Participants | Setting and sample: 26 care facilities, N = 150, Canada Age (years): mean 86 |
| Interventions |
|
| Outcomes |
|
| Notes | Article located in search update (August 2017); pending processing Falls reported as medians with range |
Frohnhofen 2013.
| Methods | RCT |
| Participants | Setting and sample: Hospital setting, N = 178 geriatric patients during rehabilitation |
| Interventions | FORTA (Fit‐for‐the‐Aged) medication review |
| Outcomes |
|
| Notes | Conference abstract only. Publication likely to be same study as Michalek 2014, attempts to contact author unsuccessful 6/7/16 and 26/10/16. |
Hewitt 2014.
| Methods | RCT (cluster randomised) |
| Participants | Inclusion criteria: residential‐aged care facilities: have a mix of high‐care residents and/or low‐care residents; likely to have 15 residents willing to participate; the facility manager consents to participation in the trial and to the allocation of staff time.Participants: permanently reside in residential‐aged care Exclusion criteria: terminal or unstable illness; significant advanced cognitive decline (Mini Mental State Examination </= 15); physical symptoms that preclude the safe use of exercise equipment in a group setting (e.g.. Parkinson’s disease or hemiplegia); permanently wheelchair‐ or bed‐bound; performed a similar balance and/or resistance training programme within the previous 12 months. |
| Interventions | SUNBEAM program (Strength and Balance Exercise in Aged Care) conducted in group settings; comprising progressive resistance training and balance exercises from 0‐6 months; then maintenance exercises for 7‐12 months Usual care |
| Outcomes |
|
| Notes | ACTRN12613000179730 |
MacRitchie 2001.
| Methods | RCT |
| Participants | Setting and sample: two nursing homes, Connecticut, USA N = 88 Age (years): mean 84 (SD 6.9), range 65 to 98 Inclusion criteria: none stated |
| Interventions |
|
| Outcomes |
|
| Notes | Thesis identified in the Cochrane Library (CENTRAL). No usable falls data in abstract. No published papers identified. |
Raymond 2017.
| Methods | RCT |
| Participants | Setting and sample: Hospital setting, sub‐acute, N = 468, Australia. ≥ 65 years. |
| Interventions |
|
| Outcomes |
|
| Notes | Article located in search update (August 2017); pending processing. Few falls (total 12), not reported by group allocation. |
Tallon 2013.
| Methods | RCT |
| Participants | Setting and sample: residential care Inclusion criteria: living in nursing home, able to walk, no contra‐indication to whole body vibration |
| Interventions |
|
| Outcomes |
|
| Notes | Study completed. Conference abstract available. Author indicated study completed but analysis ongoing, study unpublished [email 11/7/16]. No response received to follow‐up email 31/1/2017. |
Van der Linden 2017.
| Methods | Consecutive allocation, prospective controlled trial. |
| Participants | Setting and sample: hospital setting, sub‐acute, N = 172, Belgium. |
| Interventions |
|
| Outcomes |
|
| Notes | Article located in search update (August 2017); pending processing. NCT01513265 |
Wylie 2017.
| Methods | Pilot RCT |
| Participants | Setting and sample: 6 care facilities, N = 468, UK, East Scotland. |
| Interventions |
|
| Outcomes |
|
| Notes | Article located in search update (August 2017); pending processing. NCT02178527 |
RCT: randomised controlled trial SD: standard deviation
Characteristics of ongoing studies [ordered by study ID]
ACTRN12613000228785.
| Trial name or title | Preventing falls and fractures in low‐level aged‐care residents by increasing dairy food intake by two serves per day |
| Methods | RCT |
| Participants | Low‐level aged care residents with dietary calcium intake below 600 mg/day |
| Interventions |
|
| Outcomes |
|
| Starting date | Not commenced. |
| Contact information | Dr Sandra Iuliano Endocrinology, Level 2 Centaur Building Heidelberg Repatriation Hospital Waterdale Rd, West Heidelberg, VIC, 3081 Australia +61394963216 sandraib@unimelb.edu.au |
| Notes |
ACTRN12615000817549.
| Trial name or title | Establishing the effectiveness, cost‐effectiveness and student experience of simulation training for the prevention of falls amongst hospitalised inpatients |
| Methods | RCT |
| Participants |
Inclusion criteria: patients admitted to intervention wards within a public hospital. Group 1
Group 2 Patients admitted to intervention wards within PH |
| Interventions |
|
| Outcomes |
|
| Starting date | 17/08/2015 |
| Contact information | Dr Cylie Williams Peninsula Health 2 Hastings Rd Frankston, VIC, 3199, Australia cyliewilliams@phcn.vic.gov.au +61 3 97848125 |
| Notes |
ACTRN12617000314325.
| Trial name or title | Does abbreviating patient falls risk screening in documentation impact on falls in hospital inpatients: a stepped wedge cluster randomised control trial |
| Methods | RCT (stepped wedge) |
| Participants | All patients who are admitted to intervention wards at Peninsula Health, Non inclusion of paediatric and maternity wards. |
| Interventions |
|
| Outcomes |
|
| Starting date | March 2017. |
| Contact information | Dr Cylie Williams Peninsula Health Level 3 ‐ Office for Research 2 Hastings Rd, Frankston VIC 3199 Australia cyliewilliams@monash.edu |
| Notes | Trial may be eligible depending on mean age of patients on trial completion. |
Dal Bello‐Haas 2012.
| Trial name or title | The effects of a long‐term care walking programme on balance, falls and well‐being |
| Methods | RCT |
| Participants |
Inclusion criteria: 60 years or older; living in long‐term care facility; able to follow simple instructions; able to ambulate with or without an aid for at least 10 m; available Monday to Friday; willing to participate in a 5 days per week walking programme over a 4‐month period. Exclusion criteria: recent cardiovascular event; uncontrolled hypertension; uncontrolled epilepsy; recent fracture; unable to satisfactorily comply with the protocol requirements; recent admission into an acute care facility (past 4 months); scheduled for surgery or hospitalisation in the next 6 months; participating in another regular exercise programme (half an hour or more, three or more times per week ) aimed at improving balance or strength |
| Interventions |
|
| Outcomes | 1. Falls incidence |
| Starting date | December 2010 Estimated completion December 2016 |
| Contact information | Vanina PM Dal Bello‐Haas School of Rehabilitation Sciences, McMaster University, 1400 Main Street West, 403/E, Hamilton, Ontario L8S 1C7, Canada vdalbel@mcmaster.ca |
| Notes | CT.gov NCT01277809 |
Hassett 2016.
| Trial name or title | Activity and MObility UsiNg Technology (AMOUNT) rehabilitation trial |
| Methods | RCT |
| Participants |
Inclusion criteria: admitted for rehabilitation or assessment at one of the 3 study sites with: reduced mobility (Short Physical Performance Battery score of less than 12); clinician‐assessed capacity for improvement in mobility; likely life expectancy of more than 12 months; anticipated length of stay of greater than or equal to 10 days; ability to maintain a standing position with 1 person assist as a minimum standard Exclusion criteria: marked cognitive impairment; insufficient English language skills to participate in rehabilitation and no available interpreter; inadequate vision to use the devices; medical condition precluding exercise (unstable cardiac disease, uncontrolled hypertension, uncontrolled metabolic diseases, large abdominal aortic aneurysm or a weight‐bearing restriction); lack of interest in the use of the technologies; anticipated discharge to nursing home; discharge location too far from study site to complete home visits and follow‐up assessments. |
| Interventions |
|
| Outcomes | 1. Number of falls. |
| Starting date | September 2014. Data collection completed. |
| Contact information | Prof Cathie Sherrington The George Institute for Global Health PO Box M201, Missenden Road Sydney NSW 2050 Australia Phone: +61280524300 Email: csherrington@georgeinstitute.org.au |
| Notes | ANZCTR. ACTRN12614000936628 |
ISRCTN34353836.
| Trial name or title | Finch: Falls in care homes study |
| Methods | RCT (cluster randomised) |
| Participants |
Inclusion criteria: Care Home inclusion criteria
Resident inclusion criteria
Staff Inclusion Criteria (Process Evaluation Only)
Exclusion criteria: Care Home exclusion criteria
Resident exclusion criteria
Staff Exclusion Criteria (Process Evaluation Only)
|
| Interventions |
|
| Outcomes |
|
| Starting date | 1 November 2016 No longer recruiting |
| Contact information | Pip Logan B108a Div Rehab and Ageing Medical School Queen's Medical Centre NG7 2UH Nottingham United Kingdom pip.logan@nottingham.ac.uk |
| Notes |
ISRCTN42003273.
| Trial name or title | Polypharmacy reduction in patients treated for chronic diseases (POLITE‐RCT) |
| Methods | RCT (cluster randomised) |
| Participants | Hospital (acute care) inpatients aged 65 and over |
| Interventions |
|
| Outcomes |
|
| Starting date | 1 November 2013. Completed October 2016. |
| Contact information | Prof Attila Altiner Rostock University Medical Center Institute of General Practice (Universitätsmedizin Rostock Institut für Allgemeinmedizin) POB 100888 Rostock 18055 Germany +49 (0)381 4942481 altiner@med.uni‐rostock.de |
| Notes |
JPRN‐UMIN000000555.
| Trial name or title | The effects of whole body vibration for the prevention of falls in elderly |
| Methods | RCT |
| Participants | ≥ 65 years, resident of senior citizen institution Excluded criteria: bedridden |
| Interventions |
|
| Outcomes |
|
| Starting date | Study registered 25/12/2006 Study completed. Analysis completed 1/6/2009. |
| Contact information | Tatsuya Koike, Osaka City University Medical School, Abenoku Asahimachi 1‐4‐3, Osaka, 545‐8585, Japan |
| Notes | Trials registry page last updated on 28/11/2012 .Attempt to contact author 3/7/16 unsuccessful. |
JPRN‐UMIN000008361.
| Trial name or title | Multicenter, randomised, double‐blind, placebo controlled, parallel group trial to evaluate the effect of Vitamin D supplementation for fall prevention |
| Methods | RCT (double‐blind) |
| Participants | Residents in the social welfare corporation kensyokai associated facilities |
| Interventions |
|
| Outcomes |
|
| Starting date | Start: 20 Jan 2013. Data analysis completed 31/12/2014. No publication identified |
| Contact information | Tetsuya Enishi Division of Rehabilitation, Tokushima University Hospital, Tokushima University enishi.tetsuya@tokushima‐u.ac.jp |
| Notes | Authors contacted 16/5/16, no response received. Last modified 17/8/2017, status indicates unpublished. |
McCullagh 2016.
| Trial name or title | A twice‐daily individual targeted exercise program in frail hospitalised older medical in‐patients (APEP) |
| Methods | RCT |
| Participants |
Inclusion criteria: ≥65 years, medical patients, anticipated length of stay greater than 2 days, planned for discharge home, mobility aid and /or assistance required on admission. Exclusion criteria: contraindications to exercise, unable to follow commands in the English language, unable to exercise with the assistance of one person only, when active palliative care is required, when full isolation for containment of a contagious infection is required |
| Interventions |
|
| Outcomes |
|
| Starting date | March 2015. Estimated completion May 2017. |
| Contact information | Dr Suzanne Timmons, Senior Lecturer in Gerontology and Rehabilitation, University College Cork |
| Notes | NCT02463864 |
Mestres 2017.
| Trial name or title | Supporting Clinical Rules Engine in the Adjustment of Medication (SCREAM) |
| Methods | RCT (cluster randomised) |
| Participants | Inclusion criteria: residents living in a nursing home in the Netherlands. The nursing homes are able to deliver the medication and lab data electronically |
| Interventions |
|
| Outcomes |
|
| Starting date | June 2013. Planned completion June 2016. |
| Contact information | Dr. PHM van der Kuy |
| Notes | NTR5165 |
Mudge 2017.
| Trial name or title | CHERISH (Collaborative for Hospitalised Elders: Reducing the Impact of Stays in Hospital) |
| Methods | RCT (cluster randomised) |
| Participants |
Inclusion criteria: ≥ 65 years, admitted to hospital for 3 or more days, with admission to nominated intervention or control ward. Exclusion criteria: discharged from hospital within 2 days; palliative intent of care. |
| Interventions |
|
| Outcomes |
|
| Starting date | October 2015. |
| Contact information | Prof Alison Mudge Building C28 Level 1 Royal Brisbane and Women's Hospitals Herston Queensland 4029 Australia Email Alison.Mudge@health.qld.gov.au |
| Notes | ACTRN12615000879561 |
NCT00636675.
| Trial name or title | CONNECT |
| Methods | RCT (cluster randomised by nursing home) |
| Participants | 16 nursing homes (560 residents and 576 staff members) |
| Interventions |
|
| Outcomes |
|
| Starting date | September 2009. Estimated completion September 2016. |
| Contact information | Ruth Anderson, RN, PhD Duke University School of Nursing Durhan, North Carolina, USA, 27710 Email: ruth.anderson@duke.edu |
| Notes | Included study (Colon‐Emeric 2013) is a pilot study including 8 care facilities, this study includes 16 sites. |
NCT01483456.
| Trial name or title | Impact of multidisciplinary program on falls in elderly inpatients (IPR) |
| Methods | RCT (stepped wedge) |
| Participants |
Setting: hospitals (rehabilitation wards and geriatric acute wards), France N = 1680 (target sample size) Inclusion Criteria: aged ≥ 65; admitted during study; consenting Exclusion Criteria: cognitively impaired (MMSE < 10); psychiatric pathology; bedridden |
| Interventions |
|
| Outcomes |
|
| Starting date | July 2011 |
| Contact information | P Krolak‐Salmon Hospices Civils de Lyon Email: pierre.krolak‐salmon@chu‐lyon.fr |
| Notes | IPR (in French "Identifier, Prévenir, Relever"). Study design described as "Intervention model: single group assignment" no mention of a control group. Contact person has confirmed that this is an RCT. Author correspondence confirmed trial design. Enquired about study completion 13 Jan 2017, no response received. |
NCT01551121.
| Trial name or title | Assessment of an automated telesurveillance system on the incidence of serious falls in nursing homes (TELEHPAD) |
| Methods | RCT (individually randomised) |
| Participants | Settting: 3 Nursing homes in the Limousin region Target sample size: N = 216 Sample: people admitted to Limoges or Gueret nursing homes Inclusion criteria: aged 75; consenting; able to understand the study and complete evaluations; able to stand up from the bed; covered by French health insurance Exclusion criteria: short‐term prognosis; in multiple bed room and one co‐occupant does consent to participate |
| Interventions |
|
| Outcomes | Duration: 1 year
|
| Starting date | March 2012. |
| Contact information | Thierry Dantoine, MD University Hospital Limoges Email: thierry.dantoine@chu‐limoges.fr |
| Notes | Correspondence with T Dantoine confirmed study ongoing 10 August 2016. Study listed as recruiting as at 10 November 2017. |
NCT01561872.
| Trial name or title | Assessment of an automated telesurveillance system on serious falls prevention in an elderly suffering from dementia specialized care unit: the URCC (GET‐BETTER) |
| Methods | RCT (individually randomised) |
| Participants |
Setting: Limoges and Brive's URCC Target sample size = 350 Inclusion Criteria: men and women aged > 65; admitted to Limoges or Brive's URCC (dementia care unit); consenting; covered by French health insurance Exclusion Criteria: short‐term prognosis |
| Interventions |
|
| Outcomes | Duration of study: 6 months
|
| Starting date | April 2012.Completed 2016 |
| Contact information | Dr T Dantoine University Hospital Limoges France Email: thierry.dantoine@chu‐limoges.fr |
| Notes | URCC: Unité de Réadaptation Cogintico‐Comportementale (Unit for demented patients’ rehabilitation) (Dantoine T, personal communication Oct 20 2012). Correspondence with T Dantoine confirmed study completed, analysis ongoing as at 10 August 2016 |
NCT01735682.
| Trial name or title | Whole body vibration exercise training for institutionalized elderly |
| Methods | RCT (single blind) |
| Participants | Inclusion Criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | Estimted study completion October 2015. Last verified May 2014 |
| Contact information | The Hong Kong Polytechnic, University Shatin Hospital, Hong Kong |
| Notes | Enquiry sent to author about study completion 3 July 2016. No response received |
NCT01876095.
| Trial name or title | Discontinuing Inappropriate Medication in Nursing Home Residents (DIM‐NHR) |
| Methods | RCT |
| Participants |
Inclusion criteria Wards
Participants
|
| Interventions |
|
| Outcomes | 1. Falling |
| Starting date | Study completed April 2016 |
| Contact information | Dr Katja Taxis University of Groningen ZonMw: The Netherlands Organisation for Health, Research Development, |
| Notes | Author enquiry sent 3 July 2016, 14 Oct 2016, no response received |
NCT02295462.
| Trial name or title | Effect of person‐centred‐care on antipsychotic drug use in nursing homes: a cluster‐randomised trial |
| Methods | RCT |
| Participants |
Inclusion Criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | Start date December 2014. Estimated completion March 2017 |
| Contact information | Prof. Dr.Gabriele Meyer, Martin‐Luther‐Universität Halle‐Wittenberg Halle (Saale), Sachsen‐Anhalt, Germany, 06110 +49 ext 3455574498gabriele.meyer@medizin.uni‐halle.de |
| Notes |
NCT02570945.
| Trial name or title | Trial of a pharmacist‐physician intervention model to reduce high‐risk drug use by hospitalised elderly patients |
| Methods | RCT |
| Participants |
Inclusion Criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | Study completed. Completion December 2015 |
| Contact information | Benoit Cossette, Principal investigator, Centre de recherche du Centre hospitalier universitaire de Sherbrooke |
| Notes |
NCT02604056.
| Trial name or title | Pragmatic cluster trial for nursing home antipsychotic prescribing |
| Methods | RCT (cluster randomised) |
| Participants |
Inclusion Criteria
Exclusion Criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | September 2015. Estimated completion December 2017 |
| Contact information | Women's College Hospital, Ontario Ministry of Health, Long Term Care, Ontario Medical Association, Health Quality Ontario, Centre for Effective Practice |
| Notes |
NCT02702037.
| Trial name or title | Older Person's Exercise and Nutrition study (OPEN): a simple physical exercise combined with protein supplement ‐ effects on functional status and independence among older people: a cluster randomised controlled trial |
| Methods | RCT (individually randomised) |
| Participants |
Inclusion Criteria
Exclusion Criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | March 2016 |
| Contact information | Karolinska Institute, Nutricia Foundation |
| Notes | Anne‐Marie Bostrom, PhD Karolinska Institutet Stockholm, Sweden anne‐marie.bostrom@ki.se |
NCT02714257.
| Trial name or title | Seniors avoiding falls through exercise study |
| Methods | RCT |
| Participants |
Inclusion Criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | September 2016 Estimated study completion August 2020 |
| Contact information | Sol M Rodriguez‐Colon Penn State Hershey Medical Center Hershey, Pennsylvania, USA, 17033 smr359@psu.edu |
| Notes | The intervention will be held in churches, community centres, and senior residential facilities. Study may be eligible depending on proportion of participants in aged‐care facilities. |
NCT02714582.
| Trial name or title | Feasibility, appropriateness, meaningfulness and effectiveness of bedside shift reporting |
| Methods | RCT |
| Participants |
Inclusion Criteria
Exclusion Criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | March 2016 Estimated completion February 2018 |
| Contact information | Ann Van Hecke, MSc, PhD Ghent University/Ghent University Hospital |
| Notes |
NCT02757131.
| Trial name or title | Dedicated ambulator‐assisted physical activity to improve hospital outcome measures in elderly patients |
| Methods | RCT |
| Participants | Hospital setting. Inclusion Criteria
Exclusion Criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | July 2016. Completed July 2017. |
| Contact information | Aaron Hamilton, MD Cleveland Clinic Foundation Cleveland, Ohio, USA, 44195 |
| Notes |
NCT02969343.
| Trial name or title | Patient safety learning laboratory: making acute care more patient‐centered |
| Methods | RCT (stepped wedge) |
| Participants |
Hospital setting Estimated enrolment 21,000 participants. Inclusion Criteria: patients 18‐99 years of age on hospital care units where the PSLL patient safety health information technology tools are implemented. |
| Interventions |
|
| Outcomes |
|
| Starting date | April 2015. Estimated completion September 2018. |
| Contact information | Principal Investigator: David W. Bates, MD, MSc, Brigham and Women's Hospital Contact: Alexandra C Businger 617‐732‐7063 abusinger@partners.org Contact: Patricia Dykes, RN PhD 617‐732‐8925 pdykes@partners.org Boston, Massachusetts, USA, 02115 |
| Notes | Trial may be eligible depending on age of patients on trial completion. |
NCT03014570.
| Trial name or title | Testing iImplementation of EIT‐4‐BPSD. |
| Methods | RCT |
| Participants |
Inclusion Criteria
Exclusion Criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | April 2016. |
| Contact information | Barbara Resnick, Professor, University of Maryland Baltimore, Maryland, USA, 21201 |
| Notes |
NCT03019211.
| Trial name or title | Feasibility aquatic physical exercise to reduce falls in institutionalized elderly (PrePhysFalls) |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | Started April 2016. Completed March 2017. |
| Contact information | Mercè Sitjà Rabert Universitat Ramon Llull, Barcelona, Spain |
| Notes |
NCT03192384.
| Trial name or title | A service intervention to reduce falls in hospital |
| Methods | RCT (stepped wedge, cluster randomised) |
| Participants |
Inclusion Criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | May 2017 |
| Contact information | Professor Richard Lilford, University of Warwick Coventry, Warwickshire, United Kingdom, CV2 2DX |
| Notes |
NTR5015.
| Trial name or title | Randomized controlled intervention trial on falling and functional decline in the hospitalised elderly |
| Methods | RCT (cross‐over trial) |
| Participants |
Inclusion criteria: patients >70 years; stay in hospital > 3 days; agreement by the attending doctor; informed consent; ability to read and write Dutch Exclusion criteria: patients in isolation precautions; patients who can not go to the room where the activity programme is given; patients participating in another study |
| Interventions |
|
| Outcomes |
|
| Starting date | 5 January 2015 |
| Contact information | Sandra Koster s.koster@mst.nl |
| Notes | Author correspondence indicated that quote: "we can inform you that the main group of participants can be defined as elderly patients (> 65 year)" |
Scheffers‐Barnhoorn 2017.
| Trial name or title | FIT‐HIP. Fear of falling intervention in hip fracture geriatric rehabilitation: a cluster randomised controlled trial |
| Methods | RCT (cluster randomised) |
| Participants |
Inclusion criteria
Exclusion criteria The patient has a condition interfering with learning ability, such as:
|
| Interventions |
|
| Outcomes |
|
| Starting date | March 2016. |
| Contact information | Maaike Scheffers‐Barnhoorn Leiden University Medical Center (LUMC), Department of Public Health and Primary Care The Netherlands. |
| Notes | NTR5695 |
ADL: activities of daily living BMI: body mass index IC: informed consent ICU: intensive care unit IU: international unit RCT: randomised controlled trial
Differences between protocol and review
Latest update
'Risk of bias' assessment
In this version of the review, we now exclusively assess risk of bias of each included study based on the recommended tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We also assessed bias in the recall of falls due to less reliable methods of ascertainment (Hannan 2010).
Assessing the quality of the evidence and 'Summary of findings' tables
We now use the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of the body of evidence. We prepared 'Summary of findings' tables for each of the main categories of interventions, for the listed outcomes. The risk of bias has been assessed according to the Cochrane tool for assessing risk of bias, plus two items relating to method of ascertaining falls and baseline imbalance.
Data synthesis
Where the reported trial outcomes did not include falls during the intervention period, we did not pool these data with those of other trials.
Subgroup analysis and investigation of heterogeneity
In addition to subgroup analyses by intervention types according to the Prevention of Falls Network Europe (ProFaNE) fall‐prevention taxonomy (Lamb 2007; Lamb 2011), we conducted sensitivity analyses of exercise trials excluding those with 20 participants or less in each arm of the trial. We also conducted a sensitivity analysis of medication review excluding one trial with three participants with more than 30 falls in the intervention arm of the trial. In the previous version of this review, subgroup analyses were conducted according to level of cognition and level of care in care facilities. In this update, we have added subgroup analysis by level of care (setting) in hospitals. We have conducted a sensitivity analysis for the rate of falls analysis for exercise versus usual care in care facilities to test the exclusion of one trial with zero falls recorded in the intervention arm of the trial.
Reconsideration of categorisation of some interventions according to ProFaNE
Upon further consideration, we have re‐categorised some interventions across different ProFaNE categories that fall within the social environment classification. Stenvall 2007 has been reclassified as a social environment intervention (previously multifactorial). Koh 2009 and Van Gaal 2011b remain classified within the social environment ProFaNE category but are considered as organisational service model change rather than staff training as these interventions are primarily to introduce new guidelines and staff training was secondary.
Update in 2012
Criteria for considering studies for this review
Trials including only participants after stroke were excluded as a protocol for a Cochrane Review on interventions for preventing falls in people after stroke has been published (Verheyden 2010).
Separation of analyses by setting
We reported the results for care facilities and hospitals separately as the primary analyses because this is likely to be more useful to the users of this review. Interventions will be organised differently in these two types of settings and there may be different effectiveness of similar interventions between the two settings.
'Risk of bias' assessment
The protocol was completed and submitted for publication prior to the general release of RevMan 5 and the supporting version of the Cochrane Handbook for Systematic Reviews of Interventions (version 5.0) in February 2008. In the protocol, we stated that we would assess methodological quality using the 11‐item tool used in Gillespie 2003.
For this version of the review, we used three criteria from the Cochrane tool for assessing risk of bias: 'Random sequence generation', 'Allocation concealment', and 'Blinding of outcome assessment', and eight items from the 11‐item tool (seeAppendix 2). The items relating to allocation concealment and blinding of outcome assessors have not been used (now redundant). Also, the item relating to appropriateness of duration of clinical surveillance was not used due to very poor agreement between assessors during preparation of the first version of this review.
Other changes
Interventions were classified using the Prevention of Falls Network Europe (ProFaNE) fall‐prevention taxonomy (Lamb 2007; Lamb 2011). Subgroup analyses were conducted to explore heterogeneity where appropriate.
Contributions of authors
ID Cameron, the guarantor for this review, conceived and designed the review and for this update contributed to assessment of retrieved studies against inclusion criteria, carried out 'Risk of bias' assessment, data extraction and assessment of GRADE quality of the evidence, assisted with categorisation of trial interventions using the ProFaNE taxonomy, and commented on drafts of the review.
SM Dyer for this update co‐ordinated the review, carried out trial registry searches, screened search results and obtained papers, screened retrieved papers against inclusion criteria, carried out 'Risk of bias' assessment, data extraction and assessment of GRADE quality of the evidence, managed data and carried out statistical calculations, entered data into Review Manager, and drafted the review.
CE Panagoda screened search results and obtained papers, screened retrieved papers against inclusion criteria, carried out 'Risk of bias' assessment and data extraction, and commented on drafts of the review.
GR Murray carried out 'Risk of bias' assessment, data extraction and assessment of GRADE quality of the evidence, assisted with categorisation of trial interventions using the ProFaNE taxonomy, and commented on drafts of the review.
KD Hill carried out 'Risk of bias' assessment and data extraction, and commented on drafts of the review.
RG Cumming carried out 'Risk of bias' assessment and data extraction, and commented on drafts of the review.
N Kerse carried out 'Risk of bias' assessment and data extraction, and commented on drafts of the review.
See Appendix 12 for 'Contribution of authors' for the previous version of this review.
Sources of support
Internal sources
-
John Walsh Centre for Rehabilitation Research, Kolling Institute, The University of Sydney, Australia.
Salary, administration, computing, and library services (IDC, RGC)
-
Illawarra Shoalhaven Local Health Network, Warrawong, Australia.
Computing and library services (GM)
-
Curtin University, Perth, Australia.
Salary, administration, computing, and library services (KDH)
-
University of Auckland, New Zealand.
Salary, administration, computing and library services (NK)
External sources
-
National Health and Medical Research Council, Practitioner Fellowship, Australia.
Salary contribution (IDC)
Declarations of interest
Four review authors were investigators for seven included studies: ID Cameron (Cumming 2008; Sambrook 2012) and RG Cumming (Barker 2016; Cumming 2008; Sambrook 2012); KD Hill (Barker 2016; Haines 2004; Haines 2011); N Kerse (Kerse 2004; Kerse 2008). Authors did not assess risk of bias in their own trials.
Edited (no change to conclusions)
References
References to studies included in this review
Aizen 2015 {published data only}
- Aizen E, Lutsyk G, Wainer L, Carmeli S. Effectiveness of individualized fall prevention program in geriatric rehabilitation hospital setting: a cluster randomized trial. Aging Clinical and Experimental Research 2015;27(5):681‐8. [DOI] [PubMed] [Google Scholar]
Ang 2011 {published data only}
- Ang E, Mordiffi SZ, Wong HB. Evaluating the use of a targeted multiple intervention strategy in reducing patient falls in an acute care hospital: a randomized controlled trial. Journal of Advanced Nursing 2011;67(9):1984‐92. [DOI] [PubMed] [Google Scholar]
Barker 2016 {published and unpublished data}
- Ayton DR, Barker AL, Morello RT, Brand CA, Talevski J, Landgren FS, et al. Barriers and enablers to the implementation of the 6‐PACK falls prevention program: A pre‐implementation study in hospitals participating in a cluster randomised controlled trial. PLOS One [Electronic Resource] 2017; Vol. 12, issue 2:e0171932. [DOI] [PMC free article] [PubMed]
- Barker A. RE: Queries re: your 6‐pack trial. [personal communication] Email to: S. Dyer 24 October 2016.
- Barker A, Brand C, Haines T, Hill K, Brauer S, Jolley D, et al. The 6‐PACK programme to decrease fall‐related injuries in acute hospitals: protocol for a cluster randomised controlled trial. Injury Prevention 2011;17(4):e5. [DOI: 10.1136/injuryprev-2011-040074] [DOI] [PubMed] [Google Scholar]
- Barker AL, Morello RT, Ayton DR, Hill KD, Brand CA, Livingston PM, et al. Acceptability of the 6‐PACK falls prevention program: A pre‐implementation study in hospitals participating in a cluster randomized controlled trial. PLOS One 2017; Vol. 12, issue 2) (no pagination. [DOI] [PMC free article] [PubMed]
- Barker AL, Morello RT, Ayton DR, Hill KD, Landgren FS, Brand CA. Development of an implementation plan for the 6‐PACK falls prevention programme as part of a randomised controlled trial: protocol for a series of preimplementation studies. Injury Prevention 2016; Vol. 22, issue 6:446‐52. [DOI] [PubMed]
- Barker AL, Morello RT, Wolfe R, Brand CA, Haines TP, Hill KD, et al. 6‐PACK programme to decrease fall injuries in acute hospitals: cluster randomised controlled trial. BMJ 2016;352:h6781. [DOI: 10.1136/bmj.h6781] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello R, Barker A, Zavarsek S, Watts JJ, Haines T, Hil K, et al. The 6‐PACK programme to decrease falls and fall‐related injuries in acute hospitals: protocol for an economic evaluation alongside a cluster randomised controlled trial. Injury Prevention 2012;18(2):e2. [DOI] [PubMed] [Google Scholar]
- Morello RT, Barker AL, Haines T, Zavarsek S, Watts JJ, Hill K, et al. In‐hospital falls and fall‐related injuries: A protocol for a cost of fall study. Injury Prevention 2013;19(5):363. [DOI] [PubMed] [Google Scholar]
Beck 2016 {published and unpublished data}
- Beck AM. Re: NCT01873456: Trial of multidisciplinary nutrition in nursing home and home‐care. Email to: S Dyer 4 July 2016.
- Beck AM, Christensen AG, Hansen BS, Damsbo‐Svendsen S, Kreinfeldt Skovgaard Moller T. Multidisciplinary nutritional support for undernutrition in nursing home and home‐care: A cluster randomized controlled trial. Nutrition 2016;32(2):199‐205. [DOI: 10.1016/j.nut.2015.08.009] [DOI] [PubMed] [Google Scholar]
- Beck AM, Gogsig CA, Stenbaek HB, Damsbo‐Svendsen S, Kreinfeldt SM, Boll HE, et al. Study protocol: Cost‐effectiveness of multidisciplinary nutritional support for undernutrition in older adults in nursing home and home‐care: Cluster randomized controlled trial. Nutrition Journal 2014;13(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck Docent AM, Christensen AG, Hansen BS, Damsbo‐Svendsen S, Moller TK. Author response re. "Rehabilitation nutrition for undernourished participants in nursing home and home care: Cluster randomized controlled study". Nutrition 2016; Vol. 32, issue 4:504. [PUBMED: 26803332] [DOI] [PubMed]
- Nishioka S, Wakabayashi H, Maeda K. Rehabilitation nutrition for undernourished participants in nursing home and home care: Cluster randomized controlled study. Nutrition (Burbank, Los Angeles County, Calif.) 2016; Vol. 32, issue 4:503. [PUBMED: 26732831] [DOI] [PubMed]
Becker 2003 {published data only}
- Becker C, Kron M, Lindemann U, Sturm E, Eichner B, Walter‐Jung B, et al. Effectiveness of a multifaceted intervention on falls in nursing home residents. Journal of the American Geriatrics Society 2003;51(3):306‐13. [DOI] [PubMed] [Google Scholar]
- Becker C, Lindemann U, Nikolaus T. Multifactorial intervention on falls and fractures in nursing homes (abstract). Age and Ageing 2000;29(Suppl 2):18. [Google Scholar]
- Becker C, Walter‐Jung B, Nikolaus T. The other side of hip protectors [letter]. Age and Ageing 2000;29(2):186. [DOI] [PubMed] [Google Scholar]
- Becker C, Walter‐Jung B, Scapan K, Kron M, Nikolaus T. Effectiveness of multi‐factorial intervention for reducing falls with proximal femoral fractures in homes for the aged and nursing homes. Goals and study design of a population‐based study [Effektivitat einer multifaktoriellen Intervention zur Reduktion von Sturzen mit proximalen Femurfrakturen in Alten‐ und Pflegeheimen. Ziele und Studiendesign einer populationsbasierten Untersuchung]. Zeitschrift fur Gerontologie und Geriatrie 1997;30(4):293‐7. [PubMed] [Google Scholar]
- Rapp K, Lamb SE, Buchele G, Lall R, Lindemann U, Becker C. Prevention of falls in nursing homes: subgroup analyses of a randomized fall prevention trial. Journal of the American Geriatrics Society 2008;56(6):1092‐7. [DOI] [PubMed] [Google Scholar]
Bischoff 2003 {published data only}
- Bischoff HA, Hannes BS, Dick W, Akos R, Knecht M, Salis C, et al. Effects of vitamin D supplementation on falls: a randomized controlled trial. Journal of Bone and Mineral Research 2003;18(2):343‐51. [DOI] [PubMed] [Google Scholar]
Broe 2007 {published data only}
- Broe KE, Chen TC, Weinberg J, Bischoff‐Ferrari HA, Holick MF, Kiel DP. A higher dose of vitamin D reduces the risk of falls in nursing home residents: A randomized, multiple‐dose study. Journal of the American Geriatrics Society 2007;55(2):234‐9. [DOI] [PubMed] [Google Scholar]
Buckinx 2014 {published and unpublished data}
- Beaudart C, Buckinx F, Demonceau M, Maquet D, Crielaard JM, Reginster JY, et al. Evaluation of the impact of a 6‐month training by whole body vibration on the risk of falls among nursing home residents. Osteoporosis International. 2013;24(1 Suppl):S243. [Conference Abstract P435] [DOI] [PubMed] [Google Scholar]
- Beaudart C, Buckinx F, Maquet D, Crielaard JM, Reginster JY, Bruyere O. What are the clinical characteristics of patients improving their gait and body balance with whole body vibration? Results of a 3‐month randomized controlled trial. 9th Congress of the European Union Geriatric Medicine Society, EUGMS13; 2013; Venice, Italy. 2013.
- Beaudart C, Maquet D, Mannarino M, Buckinx F, Demonceau M, Crielaard JM, et al. Effects of 3 months of short sessions of controlled whole body vibrations on the risk of falls among nursing home residents. BMC Geriatrics 2013;13:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckinx F. Re: Trial NCT 01759680: 6 month whole body vibration training in nursing home residents [personal communication]. Email to: S Dyer. 14 July 2016.
- Buckinx F, Beaudart C, Demonceau M, Maquet D, Crielaard JM, Reginster JY, et al. Impact of a 6‐month training by whole body vibration on functional and motor abilities among nursing home residents observed over a 12‐months period. 9th Congress of the European Union Geriatric Medicine Society, EUGMS13; 2013; Venice, Italy. 2013:S55.
- Buckinx F, Beaudart C, Maquet D, Demonceau M, Crielaard JM, Reginster JY, et al. Evaluation of the impact of 6‐month training by whole body vibration on the risk of falls among nursing home residents, observed over a 12‐month period: a single blind, randomized controlled trial. Aging‐Clinical & Experimental Research 2014;26(4):369‐76. [DOI] [PubMed] [Google Scholar]
Buettner 2002 {published data only}
- Buettner LL. Efficacy of prescribed therapeutic recreation protocols on falls and injuries in nursing home residents with dementia (Research monograph). Fort Myers (FL): Florida Gulf Coast University, 2001. [ISBN‐13: 978‐1889435190] [Google Scholar]
- Buettner LL. Focus on caregiving. Falls prevention in dementia populations. Provider 2002;28(2):41‐3. [PubMed] [Google Scholar]
Burleigh 2007 {published data only}
- Burleigh E, McColl J, Potter J. Does vitamin D stop inpatients falling? A randomised controlled trial. Age and Ageing 2007;36(5):507‐13. [DOI] [PubMed] [Google Scholar]
- Burleigh E, Potter J, McColl J. Does vitamin D stop hospital inpatients falling? ‐ a randomised controlled trial [abstract]. Age and Ageing 2006;35(Suppl 3):i40. [DOI] [PubMed] [Google Scholar]
- Burleigh E, Potter J, McColl J. Does vitamin D stop hospital inpatients falling? A randomized controlled trial [abstract]. Internal Medicine Journal 2006;36(Suppl 5):A165. [Google Scholar]
- ISRCTN18282824. Does vitamin D stop inpatients falling? ‐ a randomised control trial. controlled‐trials.com/ISRCTN18282824 (first received 25 August 2005).
Cadore 2014 {published and unpublished data}
- Cadore EL, Casas‐Herrero A, Zambom‐Ferraresi F, Idoate F, Millor N, Gomez M, et al. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age 2014;36(2):773‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo M. Re: Your trial of multicomponent exercises in institutionalised frail elderly [personal communication]. Email to: S Dyer. 5 October 2016.
Chapuy 2002 {published data only}
- Chapuy MC, Pamphile R, Paris E, Kempf C, Schlichting M, Arnaud S, et al. Combined calcium and vitamin D3 supplementation in elderly women: Confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: The Decalyos II study. Osteoporosis International 2002;13(3):257‐64. [DOI] [PubMed] [Google Scholar]
Chenoweth 2009 {published data only}
- ACTRN12608000084381. Dementia Care Mapping in residential aged care. www.anzctr.org.au/trial_view.aspx?ID=82599 (first received 14 February 2008).
- Chenoweth L, King MT, Jeon YH, Brodaty H, Stein‐Parbury J, Norman R, et al. Caring for Aged Dementia Care Resident Study (CADRES) of person‐centred care, dementia‐care mapping, and usual care in dementia: a cluster‐randomised trial. [Erratum appears in Lancet Neurology 09;8(5):419]. Lancet Neurology 2009;8(4):317‐25. [DOI] [PubMed] [Google Scholar]
- Norman R, Haas M, Chenoweth L, Jeon Y‐H, King M, Brodaty H, et al. Dementia care mapping and patient‐centred care in Australian residential homes: an economic evaluation of the CARE Study. Sydney: Centre for Health Economics Research and Evaluation, 2008. [Google Scholar]
Choi 2005 {published data only}
- Choi JH, Moon JS, Song R. Effects of Sun‐style Tai Chi exercise on physical fitness and fall prevention in fall‐prone older adults. Journal of Advanced Nursing 2005;51(2):150‐7. [DOI] [PubMed] [Google Scholar]
Clifton 2009 {unpublished data only}
- Clifton GD, Shonkwiler JS, Kelly KE. Report of a randomized, controlled trial to assess reduction in falls and related injuries using the FallSaver™ position monitor. Unpublished report 2009.
- NCT00249743. Clinical evaluation of a wireless monitoring device to reduce falls in the elderly and others at high risk of falling. clinicaltrials.gov/ct2/show/NCT00249743 (first received 03 November 2005).
Colon‐Emeric 2013 {published data only}
- Colon‐Emeric C. RE: CONNECT for better fall prevention in nursing homes: Results from a pilot intervention study. Email to: C Panagoda. 12 July 2016. [DOI] [PMC free article] [PubMed]
- Colon‐Emeric C, Pinheiro SM, Simpson K, Porter K, Corazzini K, Anderson RA. Improving uptake of a falls educational program by focusing on staff interactions. Journal of the American Geriatrics Society Conference 2012 May 3‐5; Seattle (WA) 2012;60(Suppl s4):S157. [C74] [Google Scholar]
- Colon‐Emeric CS, McConnell E, Pinheiro S, Corazzini K, Porter K, Anderson R. CONNECT for fall prevention: A randomized controlled pilot study. Journal of the American Geriatrics Society Conference 2013 May 3‐5; Grapevine (TX) 2013;61(Suppl s1):S1. [P2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon‐Emeric CS, McConnell E, Pinheiro SO, Corazzini K, Porter K, Earp KM, et al. CONNECT for better fall prevention in nursing homes: results from a pilot intervention study. Journal of the American Geriatrics Society 2013;61(12):2150‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon‐Emeric CS, Pinheiro SO, Anderson RA, Porter K, McConnell E, Corazzini K, et al. Connecting the learners: improving uptake of a nursing home educational program by focusing on staff interactions. Gerontologist 2014;54(3):446‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00836433. CONNECT for better falls prevention in VA Community Living Centers. clinicaltrials.gov/show/NCT00836433 (first received 02 February 2009).
Cox 2008 {published data only}
- Cox H, Puffer S, Morton V, Cooper C, Hodson J, Masud T, et al. Educating nursing home staff on fracture prevention: a cluster randomised trial. Age and Ageing 2008;37(2):167‐72. [DOI] [PubMed] [Google Scholar]
Crotty 2004a {published data only}
- Crotty M, Rowett D, Spurling L, Giles LC, Phillips PA. Does the addition of a pharmacist transition coordinator improve evidence‐based medication management and health outcomes in older adults moving from the hospital to a long‐term care facility? Results of a randomized, controlled trial. American Journal of Geriatric Pharmacotherapy 2004;2(4):257‐64. [DOI] [PubMed] [Google Scholar]
Crotty 2004b {published data only}
- Crotty M, Whitehead C, Rowett D, Halbert J, Weller D, Finucane P, et al. An outreach intervention to implement evidence based practice in residential care: A randomized controlled trial [ISRCTN67855475]. BMC Health Services Research 2004;4(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cumming 2008 {published data only}
- Cumming RG, Sherington C, Lord SR, Simpson JM, Vogler C, Cameron ID, et al. Cluster randomised trial of a targeted multifactorial intervention to prevent falls among older people in hospital. BMJ 2008;336(7647):758‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
da Silva Borges 2014 {published data only}
- Silva Borges E, Souza Vale RG, Cader SA, Leal S, Miguel F, Pernambuco CS, et al. Postural balance and falls in elderly nursing home residents enrolled in a ballroom dancing program. Archives of Gerontology and Geriatrics 2014;59(2):312‐6. [DOI] [PubMed] [Google Scholar]
Donald 2000 {published data only}
- Donald IP, Pitt K, Armstrong E, Shuttleworth H. Preventing falls on an elderly care rehabilitation ward. Clinical Rehabilitation 2000;14(2):178‐85. [DOI] [PubMed] [Google Scholar]
Dyer 2004 {published data only}
- Dyer CA, Taylor GJ, Halpin M, Dyer CA, Robertson DR, Harrington R. Falls prevention in residential homes: a randomised controlled trial (abstract). Age and Ageing 2003;32(Suppl 1):16. [DOI] [PubMed] [Google Scholar]
- Dyer CA, Taylor GJ, Reed M, Dyer CA, Robertson DR, Harrington R. Falls prevention in residential care homes: a randomised controlled trial. Age and Ageing 2004;33(6):596‐602. [DOI] [PubMed] [Google Scholar]
- N0037081503. Preventing falls in residential homes: a multi‐agency pilot study. www.nihr.ac.uk/Profiles/NRR.aspx?Publication_ID=N0037081503 (accessed 04 March 2012).
Dykes 2010 {published data only}
- Dykes PC, Carroll DL, Hurley A, Lipsitz S, Benoit A, Chang F, et al. Fall prevention in acute care hospitals: A randomized trial. JAMA ‐ Journal of the American Medical Association 2010;304(17):1912‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Faber 2006 {published data only}
- Faber MJ, Bosscher RJ, Chin A, Paw MJ, Wieringen PC. Effects of exercise programs on falls and mobility in frail and pre‐frail older adults: A multicenter randomized controlled trial. Archives of Physical Medicine & Rehabilitation 2006;87(7):885‐96. [DOI] [PubMed] [Google Scholar]
Flicker 2005 {published data only}
- Flicker L, MacInnis R, Stein M, Scherer S, Mead K, Nowson C, et al. Erratum: Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial (Journal of the American Geriatrics Society (2005) 53 (1881‐1888)). Journal of the American Geriatrics Society 2012;60(8):1599. [DOI] [PubMed] [Google Scholar]
- Flicker L, MacInnis R, Stein M, Scherer S, Mead K, Nowson C, et al. Should all older people in residential care be supplemented with vitamin D to prevent falls? Results of a randomised trial [abstract]. 14th National conference on falls and postural instability 2003 Sept 8; London.
- Flicker L, MacInnis R, Stein M, Scherer S, Mead K, Nowson C, et al. Should all older people in residential care receive vitamin D to prevent falls? Results of a randomised trial [abstract]. Journal of Bone and Mineral Research 2004;19(Suppl 1):S99. [DOI] [PubMed] [Google Scholar]
- Flicker L, MacInnis R, Stein M, Scherer S, Mead K, Nowson C, et al. Should older people in residential care receive Vitamin D to prevent falls? Results of a randomized trial. Journal of the American Geriatrics Society 2005;53(11):1881‐8. [DOI] [PubMed] [Google Scholar]
- Flicker L, MacInnis R, Stein M, Scherer S, Mead K, Nowson C, et al. Vitamin D to prevent falls in older people in residential care. Asia Pacific Journal of Clinical Nutrition 2005;14 Suppl:S18. [Google Scholar]
Frankenthal 2014 {published data only}
- Frankenthal D, Israeli A, Caraco Y, Kalendaryev E, Zandman‐Goddard G, Lerman Y. Long‐term outcomes of medication intervention using the screening tool of older persons potentially inappropriate prescriptions screening tool to alert doctors to right treatment criteria. Journal of the American Geriatrics Society 2017; Vol. 65, issue 2:e33‐8. [DOI] [PubMed]
- Frankenthal D, Kalendaryev E, Lerman Y. Intervention with the STOPP/START criteria among elderly residents of a chronic geriatric facility: A randomized clinical trial. 10th International Congress of the European Union Geriatric Medicine Society ‐ Geriatric Medicine Crossing Borders, EUGMS14; 2014; Rotterdam, Netherlands. 2014:S69.
- Frankenthal D, Lerman Y, Kalendaryev E, Lerman Y. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. Journal of the American Geriatrics Society 2014;62(9):1658‐65. [DOI] [PubMed] [Google Scholar]
- Frankenthal D, Lerman Y, Lerman Y, Kalendaryev E. Response to Lavan and colleagues. Journal of the American Geriatrics Society 2015; Vol. 63, issue 5:1044‐5. [PUBMED: 25989578] [DOI] [PubMed]
- Lavan AH, O'Mahony D, Gallagher P. Comments on "intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial". Journal of the American Geriatrics Society 2015; Vol. 63, issue 5:1043‐4. [PUBMED: 25989577] [DOI] [PubMed]
- NCT01602744. Wolfson Medical Center. The use of STOPP/START criteria for medication intervention among elderly population living in a geriatric hospital. ClinicalTrials.gov/show/NCT01602744 (first received 1 May 2012).
Fu 2015 {published data only}
- Fu AS, Gao KL, Tung AK, Tsang WW, Kwan MM. Effectiveness of exergaming training in reducing risk and incidence of falls in frail older adults with a history of falls. Archives of Physical Medicine & Rehabilitation 2015;96(12):2096‐102. [DOI] [PubMed] [Google Scholar]
- Tsang WW, Fong SS, Tung KK, Fu AS. Is virtual reality exercise effective in reducing falls among older adults with a history of falls?. Physiotherapy (United Kingdom). World Confederation for Physical Therapy Congress 2015;101:Suppl 1. [Conference Abstract 201552] [Google Scholar]
Garcia Gollarte 2014 {published data only}
- Garcia‐Gollarte F, Baleriola‐Julvez J, Ferrero‐Lopez I, Cuenllas‐Diaz A, Cruz‐Jentoft AJ. An educational intervention on drug use in nursing homes improves health outcomes resource utilization and reduces inappropriate drug prescription. Journal of the American Medical Directors Association 2014;15(12):885‐91. [DOI] [PubMed] [Google Scholar]
Grieger 2009 {published data only}
- Grieger JA, Nowson CA, Jarman HF, Malon R, Ackland LM. Multivitamin supplementation improves nutritional status and bone quality in aged care residents. European Journal of Clinical Nutrition 2009;63(4):558‐65. [DOI] [PubMed] [Google Scholar]
Haines 2004 {published data only}
- Haines TP, Bennell KL, Osborne RH, Hill KD. Effectiveness of targeted falls prevention programme in subacute hospital setting: randomised controlled trial. BMJ 2004;328(7441):676‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines TP, Hill KD, Bennell KL, Osborne RH. Additional exercise for older subacute hospital inpatients to prevent falls: benefits and barriers to implementation and evaluation. Clinical Rehabilitation 2007;21(8):742‐53. [DOI] [PubMed] [Google Scholar]
- Haines TP, Hill KD, Bennell KL, Osborne RH. Patient education to prevent falls in subacute care. Clinical Rehabilitation 2006;20(11):970‐9. [DOI] [PubMed] [Google Scholar]
Haines 2010 {published data only}
- ACTRN12609000243213. Cluster randomized trial to evaluate the effectiveness of low‐low beds for the prevention of in‐hospital falls. www.anzctr.org.au/trial_view.aspx?ID=83489 (first received 12 May 2009).
- Haines TP, Bell RA, Varghese PN. Pragmatic, cluster randomized trial of a policy to introduce low‐low beds to hospital wards for the prevention of falls and fall injuries. Journal of the American Geriatrics Society 2010;58(3):435‐41. [DOI] [PubMed] [Google Scholar]
Haines 2011 {published data only}
- Haines TP, Hill AM, Hill KD, Brauer SG, Hoffmann T, Etherton‐Beer C, et al. Cost effectiveness of patient education for the prevention of falls in hospital: economic evaluation from a randomized controlled trial. BMC Medicine 2013;11:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines TP, Hill AM, Hill KD, McPhail S, Oliver D, Brauer S, et al. Patient education to prevent falls among older hospital inpatients: a randomized controlled trial. Archives of Internal Medicine 2011;171(6):516‐24. [DOI] [PubMed] [Google Scholar]
- Hill AM, Hill K, Brauer S, Oliver D, Hoffmann T, Beer C, et al. Evaluation of the effect of patient education on rates of falls in older hospital patients: Description of a randomised controlled trial. BMC Geriatrics 2009;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AM, Hoffmann T, Beer C, McPhail S, Hill KD, Oliver D, et al. Falls after discharge from hospital: is there a gap between older peoples' knowledge about falls prevention strategies and the research evidence?. Gerontologist 2011;51(5):653‐62. [DOI] [PubMed] [Google Scholar]
- Hill AM, Hoffmann T, Haines TP. Circumstances of falls and falls‐related injuries in a cohort of older patients following hospital discharge. Clinical Interventions In Aging 2013;8:765‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AM, Hoffmann T, McPhail S, Beer C, Hill KD, Oliver D, et al. Evaluation of the sustained effect of inpatient falls prevention education and predictors of falls after hospital discharge‐‐follow‐up to a randomized controlled trial. Journals of Gerontology Series A‐Biological Sciences & Medical Sciences 2011;66(9):1001‐12. [DOI] [PubMed] [Google Scholar]
Healey 2004 {published data only}
- Healey F, Monro A, Cockram A, Adams V, Heseltine D. Using targeted risk factor reduction to prevent falls in older in‐patients: a randomised controlled trial. Age and Ageing 2004;33(4):390‐5. [DOI] [PubMed] [Google Scholar]
Hill 2015 {published and unpublished data}
- ACTRN12612000877886. Improving older patients' safety in Western Australian hospitals ‐ reducing falls in rehabilitation units. www.anzctr.org.au/ACTRN12612000877886.aspx (first received 16 August 2012).
- Hill AM, McPhail S, Waldron N, Etherton‐Beer C, Flicker L, Ingram K, et al. Reducing falls in hospital through education to change patient and staff behaviour: A stepped wedge cluster randomised controlled effectiveness trial. Physiotherapy (United Kingdom) 2015;101(Suppl 1):e984. [Google Scholar]
- Hill AM, McPhail SM, Waldron N, Etherton‐Beer C, Ingram K, Flicker L, et al. Fall rates in hospital rehabilitation units after individualised patient and staff education programmes: a pragmatic, stepped‐wedge, cluster‐randomised controlled trial. Lancet 2015;385(9987):2592‐9. [DOI] [PubMed] [Google Scholar]
- Hill, AM, Waldron N, Etherton‐Beer C, McPhail SM, Ingram K, Flicker L, et al. A stepped‐wedge cluster randomised controlled trial for evaluating rates of falls among inpatients in aged care rehabilitation units receiving tailored multimedia education in addition to usual care: a trial protocol. BMJ Open 2014;4(1):e004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Houghton 2014 {published and unpublished data}
- Desborough J. Re: Multi‐professional clinical medication reviews in care homes for the elderly: study protocol for a randomised controlled trial with cost effectiveness analysis [personal communication]. Email to: S. Dyer. 26 July 2016. [DOI] [PMC free article] [PubMed]
- Desborough J, Houghton J, Wood J, Wright D, Holland R, Sach T, et al. Multi‐professional clinical medication reviews in care homes for the elderly: study protocol for a randomised controlled trial with cost effectiveness analysis. Trials 2011;12:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desborough J, Somally D. Multi‐professional medication reviews in care homes for older people: What were the interventions in the CAREMED study?. International Journal of Pharmacy Practice 2014;22(Suppl S2):10. [Abstract 0008] [Google Scholar]
- Houghton J, Kirthisinga V, Desborough JA. Multi‐professional medication reviews in care homes for older people: Results from the CARE MED randomised controlled trial. Age and Ageing 2014;43(suppl 2):ii19‐20. [DOI: 10.1093/ageing/afu131.5] [DOI] [Google Scholar]
- ISRCTN90761620. Multi‐professional clinical medication reviews in care homes for the elderly. controlled‐trials.com/ISRCTN90761620 (first received 21 October 2010).
- Sach TH, Desborough J, Houghton J, Holland R, CAREMED study team. Applying micro‐costing methods to estimate the costs of pharmacy interventions: an illustration using multi‐professional clinical medication reviews in care homes for older people. International Journal of Pharmacy Practice 2015;23:237‐47. [DOI: 10.1111/ijpp.12162] [DOI] [PubMed] [Google Scholar]
Huang 2016 {published data only}
- Huang TT, Chung ML, Chen FR, Chin YF, Wang BH. Evaluation of a combined cognitive‐behavioural and exercise intervention to manage fear of falling among elderly residents in nursing homes. Aging & Mental Health 2016;20(1):2‐12. [DOI: 10.1080/13607863.2015.1020411] [DOI] [PubMed] [Google Scholar]
Imaoka 2016 {published and unpublished data}
- Imaoka M. RE: "Intervention for falls: Reduced exercise and vitamin D supplementation among the institutionalized frail elderly" [personal communication]. Email to: S Dyer. 12 September 2016.
- Imaoka M, Higuchi Y, Todo E, Hirasima K, Kitagawa T, Ueda T, et al. Intervention for falls: Reduced exercise and vitamin D supplementation among the institutionalized frail elderly. Physiotherapy (United Kingdom) 2015;101(Suppl 1):e641. [Conference Abstract 201552] [Google Scholar]
- Imaoka M, Higuchi Y, Todu E, Kitagwa T, Ueda T. Low‐frequency exercise and vitamin D supplementation reduce falls among institutionalized frail elderly. International Journal of Gerontology 2016;10(4):202‐6. [Google Scholar]
Irez 2011 {published data only}
- Irez GB, Ozdemir RA, Evin R, Irez SG, Korkusuz F. Integrating pilates exercise into an exercise program for 65+ year‐old women to reduce falls. Journal of Sports Science & Medicine 2011;10(1):105‐11. [PMC free article] [PubMed] [Google Scholar]
Jarvis 2007 {published data only}
- Clague N, Kerr KM, Mockett SP. A pilot randomised trial to assess the effects of inpatient physiotherapy on falls in the elderly (poster 49). Proceedings of the Chartered Society of Physiotherapy Annual Congress and Exhibition; 2003 Oct 17‐19; Birmingham (UK). London: Chartered Society of Physiotherapy, 2003:81.
- Jarvis N, Kerr K, Mockett S. Pilot study to explore the feasibility of a randomised controlled trial to determine the dose effect of physiotherapy on patients admitted to hospital following a fall. Practical Evidence 2007;2(2):4‐12. [Google Scholar]
Jensen 2002 {published data only}
- Jensen J, Lundin‐Olsson L, Nyberg L, Gustafson Y. Falls and injury prevention in older people living in residential care facilities. A cluster randomized trial. Annals of Internal Medicine 2002;136(10):733‐41. [DOI] [PubMed] [Google Scholar]
- Jensen J, Nyberg L, Gustafson Y, Lundin‐Olsson L. Fall and injury prevention in residential care‐effects in residents with higher and lower levels of cognition. Journal of the American Geriatrics Society 2003;51(5):627‐35. [DOI] [PubMed] [Google Scholar]
- Jensen J, Nyberg L, Rosendahl E, Gustafson Y, Lundin‐Olsson L. Effects of a fall prevention program including exercise on mobility and falls in frail older people living in residential care facilities. Aging‐Clinical & Experimental Research 2004;16(4):283‐92. [DOI] [PubMed] [Google Scholar]
Juola 2015 {published data only}
- Juola AL, Bjorkman MP, Kautiainen H, Pylkkanen S, Finne‐Soveri UH, Soini H, et al. Nursing staff education to reduce potentially harmful medication use among older people in assisted living facilities: Effects of randomized controlled trial on cognition and falls. 10th International Congress of the European Union Geriatric Medicine Society ‐ Geriatric Medicine Crossing Borders, EUGMS14; Rotterdam, Netherlands. 2014. [S51]
- Juola AL, Bjorkman MP, Pylkkanen S, Finne‐Soveri H, Soini H, Kautiainen H, et al. Nurse education to reduce harmful medication use in assisted living facilities: effects of a randomized controlled trial on falls and cognition. Drugs and Aging 2015;32(11):947‐55. [DOI] [PubMed] [Google Scholar]
- Pitkälä KH, Juola AL, Kautiainen H, Soini H, Finne‐Soveri UH, Bell JS, et al. Education to reduce potentially harmful medication use among residents of assisted living facilities: a randomized controlled trial. Journal of the American Medical Directors Association 2014;15(12):892‐8. [DOI: 10.1016/j.jamda.2014.04.002] [DOI] [PubMed] [Google Scholar]
Kennedy 2015 {published data only}
- Ioannidis G, Papaioannou A, Kennedy C, Giangregorio L, Pickard L, Johnson J, et al. Vitamin D and calcium supplementation in women and men living in long term care (LTC) homes: The vitamin D osteoporosis study (VIDOS). Journal of Bone and Mineral Research 2010;25:S1. [Abstract SU0416] [Google Scholar]
- Kennedy C, Papaioannou A, Ioannidis G, Giangregorio L, Pickard L, Johnson J, et al. The vitamin D in osteoporosis study (VIDOS): A novel knowledge translation initiative in Canadian long‐term care homes. Journal of Bone and Mineral Research 2010;25:S1. [Abstract S344] [Google Scholar]
- Kennedy CC, Ioannidis G, Giangregorio LM, Adachi JD, Thabane L, Morin SN, et al. An interdisciplinary knowledge translation intervention in long‐term care: Study protocol for the vitamin D and osteoporosis study (ViDOS) pilot cluster randomized controlled trial. Implementation Science 2012;7(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CC, Ioannidis G, Thabane L, Adachi JD, Marr S, Giangregorio LM, et al. Successful knowledge translation intervention in long‐term care: final results from the vitamin D and osteoporosis study (ViDOS) pilot cluster randomized controlled trial. Trials [Electronic Resource] 2015;16:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou A, Kennedy C, Ioannidis G, Giangregorio L, Thabane L, Morin S, et al. A successful knowledge translation intervention in long‐term care: Results from the vitamin D and osteoporosis (ViDOS) cluster randomized trial. Journal of Bone and Mineral Research 2013;28:Suppl 1. [Abstract SU0394] [Google Scholar]
- Papaioannou A, Marr S, Ioannidis G, Kennedy C, Giangregorio L, Pickard L, et al. Bisphosphonate use in women and men who are at high risk for new fractures and living in long‐term care homes: The vitamin D osteoporosis study (ViDOS). Journal of Bone and Mineral Research 2010;25:S1. [Abstract SA0385] [Google Scholar]
Kerse 2004 {published data only}
- Kerse N, Butler M, Robinson E, Todd M. Fall prevention in residential care: a cluster, randomized, controlled trial. Journal of the American Geriatrics Society 2004;52(4):524‐31. [DOI] [PubMed] [Google Scholar]
Kerse 2008 {published data only}
- ACTRN12605000667617. Promoting Independence in residential care. www.anzctr.org.au/trial_view.aspx?id=735 (first received 20 October 2005).
- Kerse N, Peri K, Robinson E, Wilkinson T, Randow M, Kiata L, et al. Does a functional activity programme improve function, quality of life, and falls for residents in long term care? Cluster randomised controlled trial. BMJ 2008;337(7675):a1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri K, Kerse N, Kiata L, Wilkinson T, Robinson E, Parsons J, et al. Promoting independence in residential care: successful recruitment for a randomized controlled trial. Journal of the American Medical Directors Association 2008;9(4):251‐6. [DOI] [PubMed] [Google Scholar]
Klages 2011 {published data only}
- Klages K, Zecevic A, Orange JB, Hobson S. Potential of Snoezelen room multisensory stimulation to improve balance in individuals with dementia: a feasibility randomized controlled trial. Clinical Rehabilitation 2011;25(7):607‐16. [DOI] [PubMed] [Google Scholar]
Koh 2009 {published and unpublished data}
- Koh S. personal communication June 8 2012.
- Koh SL, Hafizah N, Lee JY, Loo YL, Muthu R. Impact of a fall prevention programme in acute hospital settings in Singapore. Singapore Medical Journal 2009;50(4):425‐32. [PubMed] [Google Scholar]
Kovacs 2012 {published data only}
- Kovacs E, Toth K, Denes L, Valasek T, Hazafi K, Molnar G, et al. Effects of exercise programs on balance in older women with age‐related visual problems: a pilot study. Archives of Gerontology & Geriatrics 2012;55(2):446‐52. [DOI] [PubMed] [Google Scholar]
Kovacs 2013 {published data only}
- Kovacs E, Sztruhar JI, Karoczi CK, Korpos A, Gondos T. Effects of a multimodal exercise program on balance, functional mobility and fall risk in older adults with cognitive impairment: a randomized controlled single‐blind study. European Journal of Physical & Rehabilitation Medicine 2013;49(5):639‐48. [PubMed] [Google Scholar]
Lapane 2011 {published data only}
- Lapane KL, Hughes CM, Daiello LA, Cameron KA, Feinberg J. Effect of a pharmacist‐led multicomponent intervention focusing on the medication monitoring phase to prevent potential adverse drug events in nursing homes. Journal of the American Geriatrics Society 2011;59(7):1238‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Law 2006 {published data only}
- ISRCTN47348080. A trial of vitamin D in preventing hip fracture. controlled‐trials.com/ISRCTN47348080 (first received 19 December 2002).
- Law M, Withers H, Morris J. Vitamin D supplementation and the prevention of fractures and falls [reply]. Age and Ageing 2007;36(2):233. [DOI: 10.1093/ageing/afl176] [DOI] [PubMed] [Google Scholar]
- Law M, Withers H, Morris J, Anderson F. Vitamin D supplementation and the prevention of fractures and falls: results of a randomised trial in elderly people in residential accommodation. Age and Ageing 2006;35(5):482‐6. [DOI] [PubMed] [Google Scholar]
Mador 2004 {published data only}
- Mador JE, Giles L, Whitehead C, Crotty M. A randomized controlled trial of a behavior advisory service for hospitalized older patients with confusion. International Journal of Geriatric Psychiatry 2004;19(9):858‐63. [DOI] [PubMed] [Google Scholar]
Mayo 1994 {published data only}
- Mayo NE, Gloutney L, Levy AR. A randomized trial of identification bracelets to prevent falls among patients in a rehabilitation hospital. Archives of Physical Medicine & Rehabilitation 1994;75(12):1302‐8. [PubMed] [Google Scholar]
McMurdo 2000 {published data only}
- McMurdo ME, Millar AM, Daly F. A randomized controlled trial of fall prevention strategies in old peoples' homes. Gerontology 2000;46(2):83‐7. [DOI] [PubMed] [Google Scholar]
- Millar AM, McMurdo MET. A trial of falls prevention [abstract]. Age and Ageing 1999;28(Suppl 1):15. [Google Scholar]
- N0405062690. Preventing falls in residents of old peoples homes. www.nihr.ac.uk/Profiles/NRR.aspx?Publication_ID=N0405062690 (accessed 13 March 2012).
Meyer 2009 {published data only}
- ISRCTN37794278. Predicting the risk of falling ‐ efficacy of a risk assessment tool compared to nurses' judgement: a cluster‐randomised controlled trial. www.controlled‐trials.com/ISRCTN37794278 (first received 11 August 2005). [DOI] [PMC free article] [PubMed]
- Meyer G, Kopke S, Bender R, Muhlhauser I. Predicting the risk of falling‐‐efficacy of a risk assessment tool compared to nurses' judgement: a cluster‐randomised controlled trial [ISRCTN37794278]. BMC Geriatrics 2005;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Kopke S, Haastert B, Muhlhauser I. Comparison of a fall risk assessment tool with nurses' judgement alone: a cluster‐randomised controlled trial. Age and Ageing 2009;38(4):417‐23. [DOI] [PubMed] [Google Scholar]
Michalek 2014 {published data only}
- Michalek C, Wehling M, Schlitzer J, Frohnhofen H. Effects of "Fit fOR The Aged" (FORTA) on pharmacotherapy and clinical endpoints‐‐a pilot randomized controlled study. European Journal of Clinical Pharmacology 2014;70(10):1261‐7. [DOI] [PubMed] [Google Scholar]
- Wehling M, Kuhn‐Thiel A, Throm C, Burkhardt H, Frohnhofen H, Pazan F, et al. Clinical validation of the FORTA (Fit fOR The Aged) List in a prospective randomized controlled clinical study. 11th International Congress of the European Union Geriatric Medicine Society, EUGMS15; 2015 September 16‐18; Oslo Norway. 2015:S146. [Abstract P‐426; Available from: www.europeangeriaticmedicine.com/]
Mulrow 1994 {published data only}
- Mulrow CD, Gerety MB, Kanten DN. Effects of physical therapy on functional status of nursing home residents. Journal of the American Geriatrics Society 1993;41(3):326‐8. [DOI] [PubMed] [Google Scholar]
- Mulrow CD, Meghan BG, Kanten D, Cornell JE, DeNino LA, Chioda L, et al. A randomized trial of physical rehabilitation for very frail nursing home residents. JAMA 1994;271(7):519‐24. [PubMed] [Google Scholar]
Neyens 2009 {published data only}
- Neyens JC, Dijcks BP, Twisk J, Schols JM, Haastregt JC, Heuvel WJ, et al. A multifactorial intervention for the prevention of falls in psychogeriatric nursing home patients, a randomised controlled trial (RCT). Age and Ageing 2009;38(2):194‐9. [DOI] [PubMed] [Google Scholar]
- Neyens JC, Schols JM, Dijcks BP, Haastregt JC, Heuvel WJ, Crebolder HF, et al. Development and implementation of a multifactorial intervention for psychogeriatric nursing home patients targeted on the prevention of falls and fall‐related injuries [Ontwikkeling en implementatie van een multifactoriele interventie gericht op de preventie van valincidenten en de gevolgen daarvan bij psychogeriatrische verpleeghuispatienten]. Tijdschrift voor Verpleeghuisgeneeskunde 2002;26(Special Issue):24‐8. [Google Scholar]
Nowalk 2001 {published data only}
- Nowalk MP, Prendergast JM, Bayles CM, D'Amico FJ, Colvin GC. A randomized trial of exercise programs among older individuals living in two long‐term care facilities: the FallsFREE program. Journal of the American Geriatrics Society 2001;49(7):859‐65. [DOI] [PubMed] [Google Scholar]
Patterson 2010 {published data only}
- ISRCTN18113807. An evaluation of an adapted United States model of pharmaceutical care to improve psychoactive prescribing for nursing home residents in Northern Ireland. controlled‐trials.com/ISRCTN18113807 (accessed 07 March 2012).
- Patterson SM, Hughes CM, Cardwell C, Lapane KL, Murray AM, Crealey GE. A cluster randomized controlled trial of an adapted U.S. model of pharmaceutical care for nursing home residents in Northern Ireland (Fleetwood Northern Ireland study): a cost‐effectiveness analysis. Journal of the American Geriatrics Society 2011;59(4):586‐93. [DOI] [PubMed] [Google Scholar]
- Patterson SM, Hughes CM, Crealey G, Cardwell C, Lapane KL. An evaluation of an adapted U.S. model of pharmaceutical care to improve psychoactive prescribing for nursing home residents in Northern Ireland (Fleetwood Northern Ireland study). Journal of the American Geriatrics Society 2010;58(1):44‐53. [DOI] [PubMed] [Google Scholar]
- Patterson SM, Hughes CM, Lapane KL. Assessment of a United States pharmaceutical care model for nursing homes in the United Kingdom. Pharmacy World and Science 2007;29(5):517‐25. [DOI] [PubMed] [Google Scholar]
Peyro Saint Paul 2013 {published and unpublished data}
- Peyro Saint Paul L, Martin J, Gaillard C, Mosquet B, Coquerel A, Gastine B. Moderate potentially drug‐induced hyponatremia in older adults: benefit in drug reduction. [L'hyponatrémie modérée potentiellement médicamenteuse du sujet âgé : bénéfice de la réduction des medicaments]. Therapie 2013;68(6):341‐6. [DOI] [PubMed] [Google Scholar]
- Peyro Saint Paul L, Martin J, Gaillard C, Mosquet B, Coquerel A, Gastine B, et al. Moderate, potentially drug‐induced hyponatremia in older adults: is there a benefit in drug reduction?. Journal of the American Geriatrics Society 2012;60(10):1991‐3. [DOI: 10.1111/j.1532-5415.2012.04186.x] [DOI] [PubMed] [Google Scholar]
- Peyro Saint Paul L, Martin J, Mosquet B, Gaillard C, Gastine B. Benefit of pharmacological intervention on drug‐induced mild hyponatremia in elderly: A prospective randomised trial. Fundamental and Clinical Pharmacology 2012;26(Suppl s1):74. [Abstract P229] [Google Scholar]
Potter 2016 {published and unpublished data}
- ACTRN12611000370909. Deprescribing in frail older people: a randomised controlled trial [A randomised controlled trial in frail older people living in residential aged care facilities in Western Australia designed to test the effect of deprescribing on medication burden at one year]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336757&isReview=true (first received 4 April 2011).
- Potter K. Re: Falls data from deprescribing study [personal communication]. Email to: S Dyer. 4 March 2017. [ACTRN12611000370909]
- Potter K, Flicker L, Page A, Etherton‐Beer C. Deprescribing in frail older people: a randomised controlled trial. PLOS One 2016;11(3):e0149984. [DOI: 10.1371/journal.pone.0149984] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter KB, Flicker L, Beer C. Deprescribing in frail older people: Protocol for a randomised controlled trial. Basic and Clinical Pharmacology and Toxicology 2011;109(Suppl s1):1‐176. [Abstract P335] [Google Scholar]
Ray 1997 {published data only}
- Ray WA, Taylor JA, Meador KG, Thapa PB, Brown AK, Kajihara HK, et al. A randomized trial of a consultation service to reduce falls in nursing homes. JAMA 1997;278(7):557‐62. [PubMed] [Google Scholar]
Rosendahl 2008 {published data only}
- ISRCTN31631302. The Frail Older People‐Activity and Nutrition [FOPANU] study in Umeå: a cluster‐randomised controlled trial. controlled‐trials.com/ISRCTN31631302 (accessed 09 March 2012).
- Littbrand H, Carlsson M, Lundin Olsson L, Lindelöf N, Håglin L, Gustafson Y, et al. Effect of a high‐intensity functional exercise program on functional balance: preplanned subgroup analyses of a randomized controlled trial in residential care facilities. Journal of the American Geriatrics Society 2011;59(7):1274‐82. [DOI] [PubMed] [Google Scholar]
- Littbrand H, Lundin Olsson L, Gustafson Y, Rosendahl E. The effect of a high‐intensity functional exercise program on activities of daily living: a randomized controlled trial in residential care facilities. Journal of the American Geriatrics Society 2009;57(10):1741‐9. [DOI] [PubMed] [Google Scholar]
- Littbrand H, Rosendahl E, Lindelof N, Lundin‐Olsson L, Gustafson Y, Nyberg L. A high‐intensity functional weight‐bearing exercise program for older people dependent in activities of daily living and living in residential care facilities: evaluation of the applicability with focus on cognitive function. Physical Therapy 2006;86(4):489‐98. [PubMed] [Google Scholar]
- Rosendahl E. Fall prediction and a high‐intensity functional exercise programme to improve physical functions and to prevent falls among older people living in residential care facilities [thesis]. Umeå, Sweden: Umeå University, 2009. [Google Scholar]
- Rosendahl E, Gustafson Y, Nordin E, Lundin‐Olsson L, Nyberg L. A randomized controlled trial of fall prevention by a high‐intensity functional exercise program for older people living in residential care facilities. Aging Clinical and Experimental Research 2008;20(1):67‐75. [DOI] [PubMed] [Google Scholar]
- Rosendahl E, Lindelof N, Littbrand H, Yifter Lindgren E, Nordin E, Lundin Olssen L, et al. High‐intensity functional exercise program for older people dependent in ADL: an RCT evaluating the effects on physical functions and falls [abstract]. Physiotherapy 2007;93(Suppl 1):S377. [Google Scholar]
- Rosendahl E, Lindelof N, Littbrand H, Yifter‐Lindgren E, Lundin‐Olsson L, Haglin L, et al. High‐intensity functional exercise program and protein‐enriched energy supplement for older persons dependent in activities of daily living: a randomised controlled trial. Australian Journal of Physiotherapy 2006;52(2):105‐13. [DOI] [PubMed] [Google Scholar]
Rubenstein 1990 {published data only}
- Rubenstein LZ, Robbins AS, Josephson KR, Schulman BL, Osterweil D. The value of assessing falls in an elderly population. A randomized clinical trial. Annals of Internal Medicine 1990;113(4):308‐16. [DOI] [PubMed] [Google Scholar]
Sakamoto 2006 {published data only}
- Sakamoto K, Nakamura T, Hagino H, Endo N, Mori S, Muto Y, et al. Effects of unipedal standing balance exercise on the prevention of falls and hip fracture among clinically defined high‐risk elderly individuals: A randomized controlled trial. Journal of Orthopaedic Science 2006;11(5):467‐72. [DOI] [PubMed] [Google Scholar]
Sakamoto 2012 {published data only}
- Ebihara S, Shannon F, Sakamoto Y, Ebihara T, Kohzuki M. Response Letter to Lakin and Doe...Lakin JR, Doe EW. Something just doesn’t smell quite right. J Am Geriatr Soc. 2013;61:313. Journal of the American Geriatrics Society 2013; Vol. 61, issue 2:313‐4. [DOI] [PubMed]
- JPRN‐UMIN000004222. Prevention of fall by lavender oil olfactory stimulation. apps.who.int/trialsearch/trial.aspx?trialid=JPRN‐UMIN000004222 (accessed 12 June 2012).
- Lakin JR, Doe EW. Something just doesn't smell quite right...Sakamoto Y, Ebihara S, Ebihara T, et al. Fall prevention using olfactory stimulation with lavender odor in elderly nursing home residents: a randomized controlled trial. J Am Geriatr Soc. 2012;60:1005–1011... [corrected][published erratum appears in J AM GERIATR SOC 2013 Apr; 61(4): 673]. Journal of the American Geriatrics Society 2013; Vol. 61, issue 2:313. [DOI] [PubMed]
- Sakamoto Y, Ebihara S, Ebihara T, Tomita N, Toba K, Freeman S, et al. Erratum. Fall prevention using olfactory stimulation with lavender odor in elderly nursing home residents: a randomized controlled trial. Journal of the American Geriatrics Society 2012; Vol. 60, issue 11:2193. [DOI] [PubMed]
- Sakamoto Y, Ebihara S, Ebihara T, Tomita N, Toba K, Freeman S, et al. Fall prevention using olfactory stimulation with lavender odor in elderly nursing home residents: a randomized controlled trial. Journal of the American Geriatrics Society 2012;60(6):1005‐11. [DOI] [PubMed] [Google Scholar]
Salvà 2016 {published and unpublished data}
- Figuls, M.R. Randomized clinical trial of a fall‐prevention strategy for institutionalized elderly based on the Mini Falls Assessment Instrument [personal communication]. Email to: S Dyer. 11 October 2016. [NCT00888953]
- Salvà A, Rojano X, Coll‐Planas L, Domenèch S, Roqué I Figuls M. [Randomized clinical trial of a fall‐prevention strategy for institutionalized elderly based on the Mini Falls Assessment Instrument]. [Spanish]. Revista Espanola de Geriatria y Gerontologia 2016;51(1):18‐24. [DOI] [PubMed] [Google Scholar]
Sambrook 2012 {published data only}
- Durvasula S, Gies P, Mason RS, Chen JS, Henderson S, Seibel MJ, et al. Vitamin D response of older people in residential aged care to sunlight‐derived ultraviolet radiation. Archives of Osteoporosis 2014;9(1):1‐7. [DOI] [PubMed] [Google Scholar]
- Durvasula S, Kok C, Sambrook PN, Cumming RG, Lord SR, March LM, et al. Sunlight and health: attitudes of older people living in intermediate care facilities in southern Australia. Archives of Gerontology & Geriatrics 2010;51(3):e94‐9. [DOI] [PubMed] [Google Scholar]
- Durvasula S, Sambrook PN, Cameron ID. Factors influencing adherence with therapeutic sunlight exposure in older people in intermediate care facilities. Archives of Gerontology & Geriatrics 2012;54(2):e234‐41. [DOI] [PubMed] [Google Scholar]
- March LM, Seibell MJ, Simpson JM, Sambrook P, Cameron ID, Durvasula S, et al. A randomised controlled trial of increased sunlight exposure to reduce vitamin D deficiency and falls risk in the elderly [abstract]. Journal of Bone and Mineral Research 2009;24(Suppl 1):S73. [Google Scholar]
- NCT00322166. The FREEDOM study: a randomised controlled trial of sunlight and calcium in older people. clinicaltrials.gov/ct2/show/NCT00322166 (first received 4 May 2006).
- Sambrook PN, Cameron ID, Chen JS, Cumming RG, Durvasula S, Herrmann M, et al. Does increased sunlight exposure work as a strategy to improve vitamin D status in the elderly: a cluster randomised controlled trial. Osteoporosis International 2012;23(2):615‐24. [DOI] [PubMed] [Google Scholar]
- Wilson N, Hilmer S, March L, Cameron I, Lord S, Mason R, et al. Physical functioning measures and risk of falling in older people living in residential aged care facilities. Therapeutic Advances in Musculoskeletal Disease 2011;3(1):9‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NM, Hilmer SN, March LM, Cameron ID, Lord SR, Seibel MJ, et al. Associations between drug burden index and falls in older people in residential aged care. Journal of the American Geriatrics Society. 2011;59(5):875‐80. [DOI] [PubMed] [Google Scholar]
- Wilson NM, Hilmer SN, March LM, Cameron ID, Lord SR, Seibel MJ, et al. Associations between drug burden index and physical function in older people in residential aged care facilities. Age and Ageing 2010;39(4):503‐7. [DOI] [PubMed] [Google Scholar]
Saravanakumar 2014 {published data only}
- ACTRN12612000103864, University of Newcastle. Preventing falls in older people in residential care settings: improving balance through Tai Chi and Yoga ‐ a randomised controlled study. www.anzctr.org.au/ACTRN12612000103864.aspx (first received 8 January 2012).
- Saravanakumar P, Higgins IJ, Riet PJ, Marquez J, Sibbritt D. The influence of tai chi and yoga on balance and falls in a residential care setting: A randomised controlled trial. Contemporary Nurse 2014;48(1):76‐87. [DOI] [PubMed] [Google Scholar]
Schnelle 2003 {published data only}
- Bates‐Jensen BM, Alessi CA, Al‐Samarrai NR, Schnelle JF. The effects of an exercise and incontinence intervention on skin health outcomes in nursing home residents. Journal of the American Geriatrics Society 2003;51(3):348‐55. [DOI] [PubMed] [Google Scholar]
- Schnelle JF, Alessi CA, Simmons SF. Translating clinical records into practice. A randomized controlled trial of exercise and incontinence care with nursing home residents. Journal of the American Geriatrics Society 2002;50(9):1476‐83. [DOI] [PubMed] [Google Scholar]
- Schnelle JF, Kanika K, Alessi C, Osterweil D, Beck JG, Al‐Samarrai N, et al. Does an exercise and incontinence intervention save healthcare costs in a nursing home population?. Journal of the American Geriatrics Society 2003;51(2):161‐8. [DOI] [PubMed] [Google Scholar]
Schoenfelder 2000 {published data only}
- Schoenfelder DP. A fall prevention program for elderly individuals. Exercise in long‐term care settings. Journal of Gerontological Nursing 2000;26(3):43‐51. [DOI] [PubMed] [Google Scholar]
- Schoenfelder DP, Rubenstein LM. An exercise program to improve fall‐related outcomes in elderly nursing home residents. Applied Nursing Research 2004;17(1):21‐31. [DOI] [PubMed] [Google Scholar]
Serra‐Rexach 2011 {published data only}
- NCT00848978. Strength training in nonagenarians (STRONG). clinicaltrials.gov/ct2/show/NCT00848978 (accessed 04 June 2012).
- Serra Rexach JA, Ruiz JR, Bustamante‐Ara N, Villaran MH, Gil PG, Sanz Ibanez MJ, et al. Health enhancing strength training in nonagenarians (STRONG): rationale, design and methods. BMC Public Health 2009;9:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra‐Rexach JA, Bustamante‐Ara N, Hierro Villaran M, Gonzalez Gil P, Sanz Ibanez M, Blanco Sanz N, et al. Short‐term, light‐ to moderate‐intensity exercise training improves leg muscle strength in the oldest old: A randomized controlled trial. Journal of the American Geriatrics Society 2011;59(4):594‐602. [DOI] [PubMed] [Google Scholar]
Shaw 2003 {published data only}
- Chapman KL, Dawson P, Shaw FE, Kenny RA. Physiotherapy intervention for cognitively impaired elderly fallers attending casualty [abstract]. Age and Ageing 1997;26(Suppl 1):13. [Google Scholar]
- Dawson P, Chapman KL, Shaw FE, Kenny RA. Measuring the outcome of physiotherapy in cognitively impaired elderly patients who fall. Physiotherapy 1997;83(7):352. [Google Scholar]
- Shaw FE. Risk modification of falls in older patients with cognitive impaired and dementia attending a casualty department [thesis]. Newcastle upon Tyne (UK): Univ. of Newcastle upon Tyne, 2001. [Google Scholar]
- Shaw FE, Bond J, Richardson DA, Dawson P, Steen IN, McKeith IG, et al. Multifactorial intervention after a fall in older people with cognitive impairment and dementia presenting to the accident and emergency department: randomised controlled trial. BMJ 2003;326(7380):73‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw FE, Richardson DA, Dawson P, Steen IN, McKeith IG, Bond J, et al. Can multidisciplinary intervention prevent falls in patients with cognitive impairment and dementia attending a casualty department (abstract). Age and Ageing 2000;29(Suppl 1):47. [Google Scholar]
Shimada 2004 {published data only}
- Shimada H, Obuchi S, Furuna T, Suzuki T. New intervention program for preventing falls among frail elderly people: The effects of perturbed walking exercise using a bilateral separated treadmill. American Journal of Physical Medicine and Rehabilitation 2004;83(7):493‐9. [DOI] [PubMed] [Google Scholar]
Shorr 2012 {published and unpublished data}
- Shorr RI. RE: Information on number of patients. Email to: S. Dyer. 28 November 2016.
- Shorr RI, Chandler AM, Kessler LA, Miller ST, Waters TM, Daniels MJ, et al. Trial of proximity alarms to prevent patient falls in hospitals. Journal of the American Geriatrics Society 2010;58(Suppl S1):S103. [Abstract B80] [Google Scholar]
- Shorr RI, Chandler AM, Mion LC, Waters TM, Liu M, Daniels MJ, et al. Effects of an intervention to increase bed alarm use to prevent falls in hospitalized patients: a cluster randomized trial. Annals of Internal Medicine 2012;157(10):692‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sihvonen 2004 {published data only}
- Sihvonen S, Sipila S, Taskinen S, Era P. Fall incidence in frail older women after individualized visual feedback‐based balance training. Gerontology 2004;50(6):411‐6. [DOI] [PubMed] [Google Scholar]
- Sihvonen SE, Sipila S, Era PA. Changes in postural balance in frail elderly women during a 4‐week visual feedback training: a randomized controlled trial. Gerontology 2004;50(2):87‐95. [DOI] [PubMed] [Google Scholar]
Sitja Rabert 2015 {published and unpublished data}
- NCT01375790. Whole‐body vibration training in older people (GERIAPLAT). clinicaltrials.gov/ct2/show/NCT01375790 (first received 16 June 2011).
- Sitja‐Rabert M, Martinez‐Zapata MJ, Fort‐Vanmeerhaeghe A, Rey‐Abella F, Romero‐Rodriguez D, Bonfill X. Whole body vibration for older persons: an open randomized, multicentre, parallel, clinical trial. BMC Geriatrics 2011;11:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitja‐Rabert M, Martinez‐Zapata MJ, Vanmeerhaeghe AF, Abella FR, Romero‐Rodriguez D, Bonfill X. Effects of a whole body vibration (WBV) exercise intervention for institutionalized older people: a randomized, multicentre, parallel, clinical trial. Journal of the American Medical Directors Association 2015;16(2):125‐31. [DOI] [PubMed] [Google Scholar]
Stenvall 2007 {published data only}
- Berggren M, Stenvall M, Olofsson B, Gustafson Y. Evaluation of a fall‐prevention program in older people after femoral neck fracture: A one‐year follow‐up. Osteoporosis International 2008;19(6):801‐9. [DOI] [PubMed] [Google Scholar]
- Gustafson Y. Outcomes of hip fractures: Rehabilitation programmes: Comprehensive Geriatric Assessment and Rehabilitation‐a prerequisite for successful treatment of people who have suffered a hip‐fracture. European Geriatric Medicine 2012;3(Suppl S1):S19. [Google Scholar]
- Lundström M, Olofsson B, Stenvall M, Karlsson S, Nyberg L, Englund U, et al. Postoperative delirium in old patients with femoral neck fracture: A randomized intervention study. Aging Clinical and Experimental Research. 2007;19(3):178‐86. [DOI] [PubMed] [Google Scholar]
- Stenvall M, Berggren M, Lundstrom M, Gustafson Y, Olofsson B. A multidisciplinary intervention program improved the outcome after hip fracture for people with dementia‐‐subgroup analyses of a randomized controlled trial. Archives of Gerontology & Geriatrics 2012;54(3):e284‐e289. [DOI] [PubMed] [Google Scholar]
- Stenvall M, Olofsson B, Lundstrom M, Englund U, Borssen B, Svensson O, et al. A multidisciplinary, multifactorial intervention program reduces postoperative falls and injuries after femoral neck fracture. Osteoporosis International 2007;18(2):167‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvall M, Olofsson B, Lundstrom M, Svensson O, Nyberg L, Gustafson Y. Inpatient falls and injuries in older patients treated for femoral neck fracture. Archives of Gerontology and Geriatrics 2006;43(3):389‐99. [DOI] [PubMed] [Google Scholar]
- Stenvall M, Olofsson B, Nyberg L, Lundstrom M, Gustafson Y. Improved performance in activities of daily living and mobility after a multidisciplinary postoperative rehabilitation in older people with femoral neck fracture: a randomized controlled trial with 1‐year follow‐up. Journal of Rehabilitation Medicine 2007;39(3):232‐8. [DOI] [PubMed] [Google Scholar]
Streim 2012 {published data only}
- NCT00076622. Medication treatment for depression in nursing home residents. https://clinicaltrials.gov/ct2/show/NCT00076622 (first received 28 January 2004).
- Streim 2016. NCT00076622 ‐ Trial of antidepressant discontinuation in older residents of long term care facilities [personal communication]. Email to: S Dyer. 3 November 2016.
- Streim JE, Di FilippoS, Ten HaveT, Mavandadi S, Weintraub D, Oslin D. Antidepressant discontinuation associated with cognitive decline in older adult residents of long‐term care facilities. American Journal of Geriatric Psychiatry 2012;20(Suppl 1):S148‐9. [Google Scholar]
Tideiksaar 1993 {published data only}
- Tideiksaar R, Feiner CF, Maby J. Falls prevention: the efficacy of a bed alarm system in an acute‐care setting. Mount Sinai Journal of Medicine 1993;60(6):522‐7. [PubMed] [Google Scholar]
Toulotte 2003 {published data only}
- Toulotte C, Fabre C, Dangremont B, Lensel G, Thevenon A. Effects of physical training on the physical capacity of frail, demented patients with a history of falling: a randomised controlled trial. Age and Ageing 2003;32(1):67‐73. [DOI] [PubMed] [Google Scholar]
- Toulotte C, Fabre C, Dangremont B, Thevenon A. Prevention of falls by physical training in frail demented and faller elderly subjects [Prevention des chutes par l'entrainement chez des seniors dements et chuteurs]. Revue de Geriatrie 2003;28(3):221‐6. [Google Scholar]
Treacy 2015 {published and unpublished data}
- Treacy 2016. Your trial ACTRN12611000412932 of balance circuit classes in hospital [personal communication]. Email to: S Dyer. 14 October 2016. [ACTRN12611000412932]
- Treacy D, Schurr K, Lloyd B, Sherrington C. Additional standing balance circuit classes during inpatient rehabilitation improved balance outcomes: An assessor blinded randomised controlled trial. Physiotherapy (United Kingdom) 2015;101(Suppl 1):e1533‐e1534. [DOI] [PubMed] [Google Scholar]
- Treacy D, Schurr K, Lloyd B, Sherrington C. Additional standing balance circuit classes during inpatient rehabilitation improved balance outcomes: An assessor‐blinded randomised controlled trial. Age and Ageing 2015;44(4):580‐6. [DOI] [PubMed] [Google Scholar]
- Treacy D, Schurr K, Sherrington C. Balance circuit classes to improve balance among rehabilitation inpatients: a protocol for a randomised controlled trial. BMC Geriatrics 2013;13:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tuunainen 2013 {published and unpublished data}
- Tuunainen E, Jantti P, Pyykko I, Rasku J, Moisio‐Vilenius P, Makinen E, et al. Intervention to prevent falls in elderly adults living in a residential home. Journal of the American Geriatrics Society 2013;61(8):1426‐7. [DOI] [PubMed] [Google Scholar]
- Tuunainen E, Rasku J, Jantti P, Moisio‐Vilenius P, Makinen E, Toppila E, et al. Postural stability and quality of life after guided and self‐training among older adults residing in an institutional setting. Clinical Interventions In Aging 2013;8:1237‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van de Ven 2014 {published and unpublished data}
- Ven G. RE: NTR 2314: Dementia‐care mapping in nursing homes: a cluster‐randomised controlled trial [personal communication]. Email to: S Dyer. 7 March 2017. [NTR 2314]
- Ven G, Draskovic I, Adang EM, Donders R, Zuidema SU, Koopmans RT, et al. Effects of dementia‐care mapping on residents and staff of care homes: a pragmatic cluster‐randomised controlled trial. PLOS One 2013;8(7):e67325. [PUBMED: 23844003] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ven G, Draskovic I, Adang EM, Donders RA, Post A, Zuidema SU, et al. Improving person‐centred care in nursing homes through dementia‐care mapping: design of a cluster‐randomised controlled trial. BMC Geriatrics 2012;12:1. [DOI: 10.1186/1471-2318-12-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ven G, Draskovic I, Herpen E, Koopmans RT, Donders R, Zuidema SU, et al. The economics of dementia‐care mapping in nursing homes: a cluster‐randomised controlled trial. PLOS One 2014;9(1):e86662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Gaal 2011a {published data only}
- NCT00365430. SAFE or SORRY? Patient safety study of the prevention of adverse patient outcomes. clinicaltrials.gov/ct/show/NCT00365430 (accessed 11 March 2012).
- Gaal BG, Schoonhoven L, Hulscher ME, Mintjes JA, Borm GF, Koopmans RT, et al. The design of the SAFE or SORRY? study: a cluster randomised trial on the development and testing of an evidence based inpatient safety program for the prevention of adverse events. BMC Health Services Research 2009;9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal BG, Schoonhoven L, Mintjes JA, Borm GF, Hulscher ME, Defloor T, et al. Fewer adverse events as a result of the SAFE or SORRY? programme in hospitals and nursing homes. part i: primary outcome of a cluster randomised trial. International Journal of Nursing Studies 2011;48(9):1040‐8. [DOI] [PubMed] [Google Scholar]
- Gaal BG, Schoonhoven L, Mintjes JA, Borm GF, Koopmans RT, Achterberg T. The SAFE or SORRY? programme. part II: effect on preventive care. International Journal of Nursing Studies 2011;48(9):1049‐57. [DOI] [PubMed] [Google Scholar]
- Gaal BG, Schoonhoven L, Vloet LC, Mintjes JA, Borm GF, Koopmans RT, et al. The effect of the SAFE or SORRY? programme on patient safety knowledge of nurses in hospitals and nursing homes: a cluster randomised trial. International Journal of Nursing Studies 2010;47(9):1117‐25. [DOI] [PubMed] [Google Scholar]
Van Gaal 2011b {published data only}
- NCT00365430. SAFE or SORRY? Patient safety study of the prevention of adverse patient outcomes. clinicaltrials.gov/ct/show/NCT00365430 (accessed 11 March 2012).
- Gaal BG, Schoonhoven L, Hulscher ME, Mintjes JA, Borm GF, Koopmans RT, et al. The design of the SAFE or SORRY? study: a cluster randomised trial on the development and testing of an evidence based inpatient safety program for the prevention of adverse events. BMC Health Services Research 2009;9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal BG, Schoonhoven L, Mintjes JA, Borm GF, Hulscher ME, Defloor T, et al. Fewer adverse events as a result of the SAFE or SORRY? programme in hospitals and nursing homes. part i: primary outcome of a cluster randomised trial. International Journal of Nursing Studies 2011;48(9):1040‐8. [DOI] [PubMed] [Google Scholar]
- Gaal BG, Schoonhoven L, Mintjes JA, Borm GF, Koopmans RT, Achterberg T. The SAFE or SORRY? programme. part II: effect on preventive care. International Journal of Nursing Studies 2011;48(9):1049‐57. [DOI] [PubMed] [Google Scholar]
- Gaal BG, Schoonhoven L, Vloet LC, Mintjes JA, Borm GF, Koopmans RT, et al. The effect of the SAFE or SORRY? programme on patient safety knowledge of nurses in hospitals and nursing homes: a cluster randomised trial. International Journal of Nursing Studies 2010;47(9):1117‐25. [DOI] [PubMed] [Google Scholar]
Van het Reve 2014 {published and unpublished data}
- het Reve E. AW: Strength‐balance supplemented with computerized cognitive training to improve dual task gait and divided attention in older adults: a multicenter randomized‐controlled trial [personal communication]. Email to: C Panagoda 11 September 2016. [DOI] [PMC free article] [PubMed]
- het Reve E, Bruin ED. Strength‐balance supplemented with computerized cognitive training to improve dual task gait and divided attention in older adults: a multicenter randomized‐controlled trial. BMC Geriatrics 2014;14:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wald 2011 {published data only}
- Wald HL, Glasheen JJ, Guerrasio J, Youngwerth JM, Cumbler EU. Evaluation of a hospitalist‐run acute care for the elderly service. Journal of Hospital Medicine (Online) 2011;6(6):313‐21. [DOI] [PubMed] [Google Scholar]
Walker 2015 {published data only}
- Logan PA, Walker G, Gladman JR, Robertson K, Ward M, Armstrong S, et al. A feasibility study of a cluster randomised controlled trial to evaluate a falls prevention intervention in care homes for older people. European Geriatric Medicine 2014;5(Suppl 1):s240. [Abstract P497] [Google Scholar]
- Walker GM, Armstrong S, Gordon AL, Gladman J, Robertson K, Ward M, et al. The Falls In Care Home study: A feasibility randomized controlled trial of the use of a risk assessment and decision support tool to prevent falls in care homes. Clinical Rehabilitation 2016;30(10):972‐83. Epub 2015 Sep 18. [DOI: 10.1177/0269215515604672] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2010 {published data only}
- Ward JA, Harden M, Gibson RE, Byles JE. A cluster randomised controlled trial to prevent injury due to falls in a residential aged care population. Medical Journal of Australia 2010;192(6):319‐22. [DOI] [PubMed] [Google Scholar]
Whitney 2017 {published and unpublished data}
- ISRCTN00695885, King's College, Hospital. PROF‐COG prevention of falls in older people with cognitive impairment. isrctn.com/ISRCTN00695885 (first received 26 March 2013).
- Whitney J. RE: PROF‐COG trial ISRCTN00695885 [personal communication]. Email to: S Dyer 10 October 2016.
- Whitney J, Jackson SH, Martin FC. Feasibility and efficacy of a multi‐factorial intervention to prevent falls in older adults with cognitive impairment living in residential care (ProF‐Cog). A feasibility and pilot cluster randomised controlled trial. BMC Geriatrics 2017;17(1):115. [PUBMED: 28558714] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolf 2013 {published and unpublished data}
- Wolf K‐H. Re: Trial "Development and pilot study of a bed‐exit alarm based on a body‐worn accelerometer". Email to: S Dyer 9 November 2016. [DOI] [PubMed]
- Wolf, K‐H, Hetzer K, zu Schwabedissen HM, Wiese B, Marschollek M. Development and pilot study of a bed‐exit alarm based on a body‐worn accelerometer. Zeitschrift fur Gerontologie und Geriatrie 2013;46(8):727‐33. [DOI] [PubMed] [Google Scholar]
Yokoi 2015 {published and unpublished data}
- Yokoi H. Re: "short stick exercises for fall prevention among older adults: a cluster randomised trial" [personal communication]. Email to: C Panagoda 19 July 2016.
- Yokoi K, Yoshimasu K, Takemura S, Fukumoto J, Kurasawa S, Miyashita K. Short stick exercises for fall prevention among older adults: a cluster randomized trial. Disability & Rehabilitation 2015;37(14):1268‐76. [DOI] [PubMed] [Google Scholar]
Zermansky 2006 {published data only}
- ISRCTN45416155. Can a review of the medication of elderly nursing and residential home patients improve the quality of prescribing and residents' outcomes?. controlled‐trials.com/ISRCTN45416155 (accessed 04 April 2012).
- Zermansky AG, Alldred DP, Petty DR, Raynor DK, Freemantle NE, Eastaugh J, et al. Clinical medication review by a pharmacist of elderly people living in care homes‐randomised controlled trial. Age and Ageing 2006;35(6):586‐91. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barreca 2004 {published data only}
- Barreca S, Sigouin CS, Lambert C, Ansley BA. Effects of extra training on the ability of stroke survivors to perform an independent sit‐to‐stand: A randomized controlled trial. Journal of Geriatric Physical Therapy 2004;27(2):59‐68. [Google Scholar]
Bernhardt 2008 {published data only}
- Bernhardt J, Dewey H, Thrift A, Collier J, Donnan G. A very early rehabilitation trial for stroke (AVERT): phase II safety and feasibility. Stroke 2008;39(2):390‐6. [DOI] [PubMed] [Google Scholar]
Bosner 2012 {published data only}
- Bosner S, Keller H, Wohner A, Wohner C, Sonnichsen A, Baum E, et al. Prevention of falls by outdoor‐walking in elderly persons at risk ("power") ‐ A pilot study. European Geriatric Medicine 2012;3(1):28‐32. [Google Scholar]
Bouwen 2008 {published data only}
- Bouwen A, Lepeleire J, Buntinx F. Rate of accidental falls in institutionalised older people with and without cognitive impairment halved as a result of a staff‐orientated intervention. Age and Ageing 2008;37:306‐10. [DOI] [PubMed] [Google Scholar]
Capezuti 1998 {published data only}
- Capezuti E. The relationship between physical restraint removal and fall‐related incidents and injuries among nursing home residents [thesis]. Pittsburgh (PA): Univ. of Pennsylvania, 1995. [Google Scholar]
- Capezuti E, Evans L, Strumpf N, Maislin G. Physical restraint use and falls in nursing home residents. Journal of the American Geriatrics Society 1996;44(6):627‐33. [DOI] [PubMed] [Google Scholar]
- Capezuti E, Maislin G, Strumpf N, Evans LK. Side rail use and bed‐related fall outcomes among nursing home residents. Journal of the American Geriatrics Society 2002;50(1):90‐6. [DOI] [PubMed] [Google Scholar]
- Capezuti E, Strumpf N, Evans L, Maislin G. Outcomes of nighttime physical restraint removal for severely impaired nursing home residents. American Journal of Alzheimer's Disease 1999;14(3):157‐64. [Google Scholar]
- Capezuti E, Strumpf NE, Evans LK, Grisso JA, Maislin G. The relationship between physical restraint removal and falls and injuries among nursing home residents. Journals of Gerontology Series A‐Biological Sciences and Medical Sciences 1998;53(1):M47‐52. [DOI] [PubMed] [Google Scholar]
- Evans LK, Strumpf NE, Allen‐Taylor SL, Capezuti E, Maislin G, Jacobsen B. A clinical trial to reduce restraints in nursing homes. Journal of the American Geriatrics Society 1997;45:675‐81. [DOI] [PubMed] [Google Scholar]
- Siegler EL, Capezuti E, Maislin G, Baumgarten M, Evans L, Strumpf N. Effects of a restraint reduction intervention and OBRA '87 regulations on psychoactive drug use in nursing homes. Journal of the American Geriatrics Society 1997;45:791‐6. [DOI] [PubMed] [Google Scholar]
Crotty 2002 {published data only}
- Crotty M, Whitehead CH, Gray S, Finucane PM. Early discharge and home rehabilitation after hip fracture achieves functional improvements: a randomized controlled trial. Clinical Rehabilitation 2002;16(4):406‐13. [DOI] [PubMed] [Google Scholar]
Cucca 2017 {published data only}
- Cucca A, Migdadi H, Son AY, Gallo EC, Fisher SJ, Agarwal S, et al. Feasibility and safety of combining rtms with physical therapy: Preliminary data in Parkinson's disease. Brain Stimulation 2017; Vol. 10, issue 4:e63‐4. [1876‐4754]
Cummings 2015 {published data only}
- Cummings J, Lyketsos CG, Tariot P, Peskind E, Nguyen U, Knowles N, et al. Dextromethorphan/quinidine (AVP‐923) efficacy and safety for treatment of agitation in persons with Alzheimer's disease: Results from a phase 2 study (NCT01584440). American Journal of Geriatric Psychiatry 2015;23(Supplement 3):S165. [Conference Abstract 201512] [Google Scholar]
Dattalo 2015 {published data only}
- Dattalo M, Ford J, Kedrowski K, Mahoney J. Bringing healthy aging to scale: Will quality improvement coaching facilitate the dissemination of evidence‐based health promotion programs to rural communities?. Journal of the American Geriatrics Society 2015;63(Suppl 1):S134. [Google Scholar]
Davison 2005 {published data only}
- Davison J, Bond J, Dawson P, Steen IN, Kenny RA. Patients with recurrent falls attending Accident & Emergency benefit from multifactorial intervention ‐ a randomised controlled trial. Age and Ageing 2005;34(2):162‐8. [DOI] [PubMed] [Google Scholar]
de Morton 2007 {published data only}
- Morton NA, Keating JL, Berlowitz DJ, Jackson B, Lim WK. Additional exercise does not change hospital or patient outcomes in older medical patients: a controlled clinical trial. Australian Journal of Physiotherapy 2007;53(2):105‐11. [DOI] [PubMed] [Google Scholar]
de Souto 2016 {published data only}
- Souto Barreto P, Denormandie P, Lepage B, Armaingaud D, Rapp T, Chauvin P, et al. Effects of a long‐term exercise programme on functional ability in people with dementia living in nursing homes: Research protocol of the LEDEN study, a cluster randomised controlled trial. Contemporary Clinical Trials 2016; Vol. 47:289‐95. [1559‐2030] [DOI] [PubMed]
de Souto 2017 {published data only}
- Souto Barreto P, Cesari M, Denormandie P, Armaingaud D, Vellas B, Rolland Y. Exercise or social intervention for nursing home residents with dementia: a pilot randomized, controlled trial. Journal of the American Geriatrics Society. 2017; Vol. 65, issue 9:E123‐9. [0002‐8614: 1532‐5415] [DOI] [PubMed]
DeSure 2013 {published data only}
- DeSure AR, Peterson K, Gianan FV, Pang L. An exercise program to prevent falls in institutionalized elderly with cognitive deficits: a crossover pilot study. Hawaii Journal of Medicine & Public Health : A Journal of Asia Pacific Medicine & Public Health 2013;72(11):391‐5. [PMC free article] [PubMed] [Google Scholar]
Donat 2007 {published data only}
- Donat H, Ozcan A. Comparison of the effectiveness of two programmes on older adults at risk of falling: unsupervised home exercise and supervised group exercise. Clinical Rehabilitation 2007;21(3):273‐83. [DOI] [PubMed] [Google Scholar]
Drahota 2013 {published data only}
- Drahota A, Gal D, Windsor J, Dixon S, Udell J, Ward D, et al. Pilot cluster randomised controlled trial of flooring to reduce injuries from falls in elderly care units: study protocol. Injury Prevention 2011;17(6):e7. [DOI] [PubMed] [Google Scholar]
- Drahota AK, Ward D, Udell JE, Soilemezi D, Ogollah R, Higgins B, et al. Pilot cluster randomised controlled trial of flooring to reduce injuries from falls in wards for older people. Age & Ageing 2013;42(5):633‐40. [DOI] [PubMed] [Google Scholar]
- NCT00817869. The HIP‐HOP flooring study: helping injury prevention in hospitalised older people. clinicaltrials.gov/ct2/show/NCT00817869 (first received 6 January 2009).
Fiatarone 1994 {published data only}
- Fiatarone MA, O'Neill EF, Doyle N, Clements KM, Roberts SB, Kehayias JJ, et al. The Boston FICSIT study: the effects of resistance training and nutritional supplementation on physical frailty in the oldest old. Journal of the American Geriatrics Society 1993;41(3):333‐7. [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. New England Journal of Medicine 1994;330(25):1769‐75. [DOI] [PubMed] [Google Scholar]
Forster 2017 {published data only}
- Forster A, Airlie J, Birch K, Cicero R, Cundill B, Ellwood A, et al. Research Exploring Physical Activity in Care Homes (REACH): study protocol for a randomised controlled trial. Trials 2017; Vol. 18, issue 1:182. [1745‐6215] [DOI] [PMC free article] [PubMed]
Fossey 2006 {published data only}
- Fossey J, Ballard C, Juszczak E, James I, Alder N, Jacoby R, et al. Effect of enhanced psychosocial care on antipsychotic use in nursing home residents with severe dementia: cluster randomised trial. BMJ 2006;332(7554):756‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galik 2014 {published data only}
- Galik E, Resnick B, Hammersla M, Brightwater J. Optimizing function and physical activity among nursing home residents with dementia: testing the impact of function‐focused care. Gerontologist 2014;54(6):930‐43. [DOI] [PubMed] [Google Scholar]
Galik 2015 {published data only}
- Galik E, Resnick B, Lerner N, Hammersla M, Gruber‐Baldini AL. Function focused care for assisted living residents with dementia. Gerontologist 2015; Vol. 55:S13‐26. [DOI] [PMC free article] [PubMed]
Gianturco 2013 {published data only}
- Gianturco V, Troisi G, Ripani M, Marigliano V. Impact of exoskeleton Human Body Posturizer on the fall risk in the elderly: A randomized controlled trial. European Geriatric Medicine 2013;4(Suppl 1):S46. [Google Scholar]
Ginde 2017 {published data only}
- Ginde AA, Blatchford P, Breese K, Zarrabi L, Linnebur SA, Wallace JI, et al. High‐dose monthly vitamin D for prevention of acute respiratory infection in older long‐term care residents: a randomized clinical trial. Journal of the American Geriatrics Society 2017; Vol. 65, issue 3:496‐503. [DOI] [PMC free article] [PubMed]
Graafmans 1996 {published data only}
- Graafmans WC, Ooms ME, Hofstee HM, Bezemer PD, Bouter LM, Lips P. Falls in the elderly: a prospective study of risk factors and risk profiles. American Journal of Epidemiology 1996;143(11):1129‐36. [PUBMED: 8633602] [DOI] [PubMed] [Google Scholar]
Grant 2005 {published data only}
- Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, et al. Oral vitamin D3 and calcium for secondary prevention of low‐trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo‐controlled trial. Lancet 2005;365(9471):1621‐8. [DOI] [PubMed] [Google Scholar]
Greenspan 2013 {published data only}
- Greenspan S, Ferchak M, Lee C, Nace D, Perera S, Resnick NM. Are the iom vitamin d guidelines sufficient for long term care residents?. Journal of the American Geriatrics Society 2013;61(Suppl s1):S99. [Google Scholar]
Greenspan 2015 {published data only}
- Greenspan SL, Perera S, Ferchak MA, Nace DA, Resnick NM. Efficacy and safety of single‐dose zoledronic acid for osteoporosis in frail elderly women: a randomized clinical trial. JAMA Internal Medicine 2015;175(6):913‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gruber‐Baldini 2011 {published data only}
- Gruber‐Baldini AL, Resnick B, Hebel JR, Galik E, Zimmerman S. Adverse events associated with the Res‐Care Intervention. Journal of the American Medical Directors Association 2011;12(8):584‐9. [DOI] [PubMed] [Google Scholar]
Gu 2006 {published data only}
- Gu MO. personal communication May 22 2012.
- Gu MO, Jeon MY, Eun Y. The development & effect of a tailored falls prevention exercise for older adults [Korean]. Taehan Kanho Hakhoe Chi 2006;36(2):341‐52. [DOI] [PubMed] [Google Scholar]
Hardin 2013 {published and unpublished data}
- Hardin SR. RE: FW: "Inpatient Fall Prevention: Use of In‐room Webcams" [personal communication]. Email to: S Dyer. 28 June 2016.
- Hardin SR, Dienemann J, Rudisill P, Mills KK. Inpatient fall prevention: use of in‐room Webcams. Journal of Patient Safety 2013;9(1):29‐35. [DOI] [PubMed] [Google Scholar]
Harwood 2004 {published data only}
- Harwood RH, Sahota O, Gaynor K, Masud T, Hosking DJ. A randomised, controlled comparison of different calcium and vitamin D supplementation regimens in elderly women after hip fracture: The Nottingham Neck of Femur (NONOF) Study. Age and Ageing 2004;33(1):45‐51. [DOI] [PubMed] [Google Scholar]
Hauer 2001 {published data only}
- Hauer K, Pfisterer M, Schuler M, Bartsch P, Oster P. Two years later: a prospective long‐term follow‐up of a training intervention in geriatric patients with a history of severe falls. Archives of Physical Medicine and Rehabilitation 2003;84(10):1426‐32. [DOI] [PubMed] [Google Scholar]
- Hauer K, Rost B, Rutschle K, Opitz H, Specht N, Bartsch P, et al. Exercise training for rehabilitation and secondary prevention of falls in geriatric patients with a history of injurious falls. Journal of the American Geriatrics Society 2001;49(1):10‐20. [DOI] [PubMed] [Google Scholar]
Heiberg 2017 {published data only}
- Heiberg KE, Bruun‐Olsen V, Bergland A. The effects of habitual functional training on physical functioning in patients after hip fracture: the protocol of the HIPFRAC study. BMC Geriatrics 2017; Vol. 17, issue 1:23. [1471‐2318] [DOI] [PMC free article] [PubMed]
Herrmann 2016 {published data only}
- Herrmann N, O'Regan J, Ruthirakuhan M, Kiss A, Eryavec G, Williams E, et al. A randomized placebo‐controlled discontinuation study of cholinesterase inhibitors in institutionalized patients with moderate to severe Alzheimer disease. Journal of the American Medical Directors Association 2016;17(2):142‐7. [DOI: 10.1016/j.jamda.2015.08.019] [DOI] [PubMed] [Google Scholar]
- Lanctot KL, Ruthirakuhan M, O'Regan JN, Eryavec G, Williams E, Black SE, et al. Cholinesterase inhibitor discontinuation in institutionalized persons with moderate to severe AD: Results of a double‐blind, placebo controlled trial. American Journal of Geriatric Psychiatry 2015;23(3 Suppl):S172‐3. [Google Scholar]
Hopman‐Rock 1999 {published data only}
- Hopman‐Rock M, Staats PG, Tak EC, Droes R. The effects of a psychomotor activation programme for use in groups of cognitively impaired people in homes for the elderly. International Journal of Geriatric Psychiatry 1999;14:633‐42. [DOI] [PubMed] [Google Scholar]
Huang 2005 {published data only}
- Huang TT, Liang SH. A randomized clinical trial of the effectiveness of a discharge planning intervention in hospitalized elders with hip fracture due to falling. Journal of Clinical Nursing 2005;14(10):1193‐201. [DOI] [PubMed] [Google Scholar]
Il'nitskii 2014 {published data only}
- Il'nitskii AN, Proshchayev KI, Schwartsman GI, Bahmutova IuV, Pozdnyakova NM, Krivetskiy VV, et al. The use of piribedil for the prevention of falls in elderly patients with metabolic syndrome. [Russian]. Klinicheskaia Meditsina 2014;92(5):46‐50. [PubMed] [Google Scholar]
Ilfeld 2010 {published data only}
- Ilfeld BM, Loland VJ, Donovan JF, Le LT, Mariano ER. A multicenter, randomized, triple‐masked, placebo‐controlled trial of the effect of ambulatory continuous femoral nerve blocks on discharge‐readiness following total knee arthroplasty in patients on general orthopaedic wards. Regional Anesthesia and Pain MedicineConference: 2010;35th Annual Regional Anesthesia Meeting and Workshops, ASRA10 Toronto, ON Canada(var.pagings):Conference‐October. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarret 2015 {published data only}
- Jarret G, Orpanna A, Helbostad J. Can a three weeks program in a rehabilitation center improve balance in elderly people? A randomized clinical controlled trial. Physiotherapy (United Kingdom) 2015;101(Suppl 1):eS671‐2. [Google Scholar]
Jeon 2015 {published data only}
- Jeon YH, Simpson JM, Li Z, Cunich MM, Thomas TH, Chenoweth L, et al. Cluster randomized controlled trial of an aged care specific leadership and management program to improve work environment, staff turnover, and care quality. Journal of the American Medical Directors Association 2015;16(7):629‐e28. [DOI] [PubMed] [Google Scholar]
JPRN‐UMIN0000167 {published data only}
- JPRN‐UMIN000016716. Effect of fall prevention education programs for patients in convalescent phase after stroke: a randomized controlled trial. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000019395 (first received 5 May 2015).
Kato 2006 {published data only}
- Kato M, Izumi K, Hiramatsu T, Shogenji M. Development of an exercise program for fall prevention for elderly persons in a long‐term care facility. Japan Journal of Nursing Science 2006;3(2):107‐17. [Google Scholar]
Katz 2004 {published data only}
- Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double‐blind trial. Risperidone Study Group. Journal of Clinical Psychiatry 1999;60(2):107‐15. [DOI] [PubMed] [Google Scholar]
- Katz IR, Rupnow M, Kozma C, Schneider L. Risperidone and falls in ambulatory nursing home residents with dementia and psychosis or agitation: secondary analysis of a double‐blind, placebo‐controlled trial. American Journal of Geriatric Psychiatry 2004;12(5):499‐508. [DOI] [PubMed] [Google Scholar]
Katz 2005 {published data only}
- Katz IR. Atypical antipsychotics and falls in the elderly [abstract]. 158th Annual Meeting of the American Psychiatric Association; 2005 May 21‐26; Atlanta (GA).
Kenny 2001 {published data only}
- Kenny RA, Richardson DA, Steen N, Bexton RS, Shaw FE, Bond J. Carotid sinus syndrome: a modifiable risk factor for nonaccidental falls in older adults (SAFE PACE). Journal of the American College of Cardiology 2001;38(5):1491‐6. [DOI] [PubMed] [Google Scholar]
Koczy 2011 {published data only}
- Branitzki S, Koczy P. ReduFix ‐ a study of reducing physical restraint: preventing risk of injury [ReduFix ‐ Eine Studie zur Reduktion von körpernaher Fixierung: Heimbewohner vor Schaden bewahren]. Pflege Zeitschrift 2005;58(5):310‐3. [PubMed] [Google Scholar]
- Koczy P, Becker C, Rapp K, Klie T, Beische D, Buchele G, et al. Effectiveness of a multifactorial intervention to reduce physical restraints in nursing home residents. Journal of the American Geriatrics Society 2011;59(2):333‐9. [DOI] [PubMed] [Google Scholar]
- Koczy P, Klie T, Kron M, Bredthauer D, Rissmann U, Branitzki S, et al. Effectiveness of a multifactorial intervention to reduce physical restraints in nursing home residents with dementia [Effektivität einer multifaktoriellen Intervention zur Reduktion von körpernaher Fixierung bei demenzerkrankten Heimbewohnern: Ziele und Studiendesign einer prospektiven clusterrandomisierten Interventionsstudie]. Zeitschrift fur Gerontologie und Geriatrie 2005;38(1):33‐9. [DOI] [PubMed] [Google Scholar]
Kopke 2012 {published data only}
- Haut A, Kopke S, Gerlach A, Muhlhauser I, Haastert B, Meyer G. Evaluation of an evidence‐based guidance on the reduction of physical restraints in nursing homes: a cluster‐randomised controlled trial [ISRCTN34974819]. BMC Geriatrics 2009;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN34974819. Evaluation of an evidence‐based guidance on the reduction of physical restraints in nursing homes EBAGRAP. controlled‐trials.com/ISRCTN34974819 (first received 29 April 2009). [DOI] [PMC free article] [PubMed]
- Kopke S, Muhlhauser I, Gerlach A, Haut A, Haastert B, Mohler R, et al. Effect of a guideline‐based multicomponent intervention on use of physical restraints in nursing homes: a randomized controlled trial. JAMA 2012;307(20):2177‐84. [DOI] [PubMed] [Google Scholar]
Kwok 2006 {published data only}
- Kwok T, Mok F, Chien WT, Tam E. Does access to bed‐chair pressure sensors reduce physical restraint use in the rehabilitative care setting?. Journal of Clinical Nursing 2006;15(5):581‐7. [DOI] [PubMed] [Google Scholar]
Lackner 2008 {published data only}
- Lackner TE, Wyman JF, McCarthy TC, Monigold M, Davey C. Randomized, placebo‐controlled trial of the cognitive effect, safety, and tolerability of oral extended‐release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. Journal of the American Geriatrics Society 2008;56:862‐70. [DOI] [PubMed] [Google Scholar]
Li 2017 {published data only}
Lord 2003b {published data only}
- Lord SR, Castell S, Corcoran J, Dayhew J, Matters B, Shan A, et al. The effect of group exercise on physical functioning and falls in frail older people living in retirement villages: a randomized, controlled trial. Journal of the American Geriatrics Society 2003;51(12):1685‐92. [DOI] [PubMed] [Google Scholar]
Mailhot 2012 {published data only}
- Mailhot T, Cossette S, Van T. Nursing intervention to support self‐efficacy of family caregivers to improve delirium management in cardiac surgery patients: A randomized study protocol. Canadian Journal of CardiologyConference 2012;65th Annual Meeting of the Canadian Cardiovascular Society Toronto, ON Canada. Conference Start(var.pagings):121027‐October. [Google Scholar]
Mailhot 2014 {published and unpublished data}
- Mailhot T. RE: MENTOR_D trial [personal communication]. Email to: S Dyer. 20 April 2016.
- Mailhot T, Cossette S, Denault AY, Lamarche Y, Cote MC, Carbonneau MH, et al. Nursing intervention involving family caregiver to improve the management of post‐cardiac surgery delirium: Results from a randomized pilot study. Canadian Journal of Cardiology. 2014;67th Annual Meeting of the Canadian Cardiovascular Society Vancouver, BC Canada.:141025. [Google Scholar]
- Mailhot T, Cossette S, Van, Tassel J. Nursing intervention to support self‐efficacy of family caregivers to improve delirium management in cardiac surgery patients: A randomized study protocol. Canadian Journal of Cardiology. 2012;65th Annual Meeting of the Canadian Cardiovascular Society Toronto, ON Canada.:121027‐October. [Google Scholar]
Mak 2016 {published data only}
- Mak JC, Klein LA, Finnegan T, Mason RS, Cameron ID. An initial loading‐dose vitamin D versus placebo after hip fracture surgery: baseline characteristics of a randomized controlled trial (REVITAHIP). BMC Geriatrics 2014;14:101. [DOI: 10.1186/1471-2318-14-101] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak JC, Mason R, Klein L, Cameron ID. Improving mobility and reducing disability in older people through early high‐dose vitamin d replacement following hip fracture: a protocol for a randomized controlled trial and economic evaluation. Geriatric Orthopaedic Surgery & Rehabilitation 2011;2(3):94‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak JC, Mason RS, Klein L, Cameron ID. An initial loading‐dose vitamin D versus placebo after hip fracture surgery: randomized trial. BMC Musculoskeletal Disorders 2016;17:336. [DOI: 10.1186/s12891-016-1174-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak JCS, Cameron ID, Mason RS, Klein L, Soong M, Ohn K. Improving mobility and reducing disability in older people through early high‐dose vitamin D replacement following hip fracture (the revitahip trial): Preliminary results. Osteoporosis International 2011;22(4 Suppl):S587. [Google Scholar]
Mansfield 2015 {published data only}
- Mansfield A, Wong JS, Bryce J, Brunton K, Inness EL, Knorr S, et al. Use of accelerometer‐based feedback of walking activity for appraising progress with walking‐related goals in inpatient stroke rehabilitation: A randomized controlled trial. Neurorehabilitation and Neural Repair 2015;29(9):847‐57. [DOI] [PubMed] [Google Scholar]
McRae 1996 {published data only}
- MacRae PG, Asplund LA, Schnelle JF, Ouslander JG, Abrahamse A, Morris C. A walking program for nursing home residents: effects on walk endurance, physical activity, mobility, and quality of life. Journal of the American Geriatrics Society 1996;44(2):175‐80. [DOI] [PubMed] [Google Scholar]
Mudge 2008 {published data only}
- Mudge AM, Giebel AJ, Cutler AJ. Exercising body and mind: an integrated approach to functional independence in hospitalized older people. Journal of the American Geriatrics Society 2008;56(4):630‐5. [DOI] [PubMed] [Google Scholar]
NCT00973297 {published data only}
- NCT00973297. A targeted falls prevention program in rehabilitation in‐patients. clinicaltrials.gov/show/NCT00973297 (first received 25 August 2009).
NCT01054287 {published and unpublished data}
- NCT01054287. Falls prevention in acute care hospital PRECEPT. clinicaltrials.gov/show/NCT01054287 (first received 21 January 2010).
- Waeber G. RE:NCT01054287 (2010). Falls Prevention in Acute Care Hospital [personal communication]. Email to: S Dyer. 1 August 2016.
NCT01523600 {published data only}
- NCT01523600. Whole body vibration training among older people using sheltered housing. ClinicalTrials.gov/show/NCT01523600 (first received 27 January 2012).
NCT01618786 {published data only}
- NCT01618786. Flooring for injury prevention trial (FLIP). clinicaltrials.gov/show/NCT01618786 (first received 11 June 2012).
NCT02686515 {published data only}
- Chang Gung University, Mackay Memorial Hospital. Comparative effectiveness research of dual‐task and single‐task balance training in people with stroke [NCT02686515]. ClinicalTrials.gov/show/NCT02686515 (first received 16 February 2016). [NCT02686515]
Nyaruhirira 2013 {published data only}
- Nyaruhirira I, Romana S, Peretz A, Baillon R, Rozenberg S. Management of hip fracture patients in a network of public hospitals in Brussels II: Protocol of a randomized controlled trial, added value of coordinator. Osteoporosis International. 2013;24(1 Suppl):S370. [Abstract P729] [Google Scholar]
Ouslander 2005 {published data only}
- Ouslander JG, Griffiths P, McConnell E, Riolo L, Schnelle J. Functional Incidental Training: applicability and feasibility in the Veterans Affairs nursing home patient population. Journal of the American Medical Directors Association 2005;6(2):121‐7. [DOI] [PubMed] [Google Scholar]
- Ouslander JG, Griffiths PC, McConnell E, Riolo L, Kutner M, Schnelle J. Functional incidental training: a randomized, controlled, crossover trial in Veterans Affairs nursing homes. Journal of the American Geriatrics Society 2005;53(7):1091‐100. [DOI] [PubMed] [Google Scholar]
Parasurum 2011 {published data only}
- Parasurum R, Chua PS, Kannusamy P. A randomised controlled study examining the impact of a staffing model and nursing care delivery system on patient, nurse and organisational outcomes. Annals of the Academy of Medicine Singapore.Conference: Singapore Health and Biomedical Congress, SHBC 2011;40(11 Suppl):S8. [Abstract SG‐NA‐03] [Google Scholar]
Pedreira 2014 {published data only}
- Pedreira ÉMD. Use of virtual reality games for the treatment of balance and reducing the occurrence of falls in patients after stroke. ClinicalTrials.gov/show/NCT02475083 (first received 12 June 2015).
Peng 2014 {published data only}
- Peng L, Ren L, Qin P, Chen J, Feng P, Lin H, et al. Continuous femoral nerve block versus intravenous patient controlled analgesia for knee mobility and long‐term pain in patients receiving total knee replacement: A randomized controlled trial. Evidence‐based Complementary and Alternative Medicine 2014;2014:Article ID 569107. [DOI: 10.1155/2014/569107] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peri 2008 {published data only}
- Peri K, Kerse N, Robinson E, Parsons M, Parsons J, Latham N. Does functionally based activity make a difference to health status and mobility? A randomised controlled trial in residential care facilities (The Promoting Independent Living Study; PILS). Age and Ageing 2008;37(1):57‐63. [DOI] [PubMed] [Google Scholar]
Rantz 2001 {published data only}
- Rantz MJ, Popejoy L, Petroski GF, Madsen RW, Mehr DR, Zwygart‐Stauffacher M, et al. Randomized clinical trial of a quality improvement intervention in nursing homes. Gerontologist 2001;41(4):525‐38. [DOI] [PubMed] [Google Scholar]
Ray 2005 {published data only}
- Ray WA, Taylor JA, Brown AK, Gideon P, Hall K, Arbogast P, et al. Prevention of fall‐related injuries in long‐term care: a randomized controlled trial of staff education. Archives of Internal Medicine 2005;165(19):2293‐8. [DOI] [PubMed] [Google Scholar]
Reinhardt 2014 {published data only}
- Reinhardt KR, Duggal S, Umunna BP, Reinhardt GA, Nam D, Alexiades M, et al. Intraarticular analgesia versus epidural plus femoral nerve block after TKA: a randomized, double‐blind trial. Clinical Orthopaedics & Related Research 2014;472(5):1400‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Resnick 2002 {published data only}
- Resnick B. Testing the effect of the WALC intervention on exercise adherence in older adults. Journal of Gerontological Nursing 2002;28(6):40‐9. [DOI] [PubMed] [Google Scholar]
Resnick 2012 {published data only}
- Resnick B, Galik E, Gruber‐Baldini A, Zimmerman S. Testing the effect of function‐focused care in assisted living. Journal of the American Geriatrics Society 2011;59(12):2233‐40. [DOI] [PubMed] [Google Scholar]
- Resnick B, Galik E, Gruber‐Baldini AL, Zimmerman S. Falls and fall‐related injuries associated with function‐focused care. Clinical Nursing Research 2012;21(1):43‐63. [DOI] [PubMed] [Google Scholar]
Richter 2015 {published data only}
- Richter C, Berg A, Fleischer S, Kopke S, Balzer K, Fick EM, et al. Effect of person‐centred care on antipsychotic drug use in nursing homes (EPCentCare): study protocol for a cluster‐randomised controlled trial. Implementation Science 2015; Vol. 10:82. [1748‐5908] [DOI] [PMC free article] [PubMed]
Rolland 2007 {published data only}
- Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, et al. Exercise program for nursing home residents with Alzheimer's disease: a 1‐year randomized, controlled trial. Journal of the American Geriatrics Society 2007;55(2):158‐65. [DOI] [PubMed] [Google Scholar]
Sackley 2009 {published data only}
- ISRCTN79859980. A randomised trial of an occupational therapy and physiotherapy intervention to enhance mobility and activity in a nursing or residential home setting after stroke. controlled‐trials.com/ISRCTN79859980 (accessed 11 March 2012).
- Sackley CM, Berg ME, Lett K, Patel S, Hollands K, Wright CC, et al. Effects of a physiotherapy and occupational therapy intervention on mobility and activity in care home residents: a cluster randomised controlled trial. BMJ 2009;339:b3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sahota 2014 {published data only}
- ISRCTN44972300. REducing Falls in IN‐patient Elderly: a randomised controlled trial. www.controlled‐trials.com/ISRCTN44972300 (first received 17 August 2009).
- Sahota O, Drummond A, Kendrick D, Grainge MJ, Vass C, Sach T, et al. REFINE (REducing Falls in In‐patieNt Elderly) using bed and bedside chair pressure sensors linked to radio‐pagers in acute hospital care: a randomised controlled trial. Age & Ageing 2014;43(2):247‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass CD, Sahota O. REFINE ‐ reducing falls in inpatient elderly using bed and bedside pressure sensors linked to radio‐pagers in acute hospital care: a randomized controlled trial. Clinical Rehabilitation 2014;28(4):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass CD, Sahota O, Drummond A, Kendrick D, Gladman J, Sach T, et al. REFINE (Reducing Falls in In‐patient Elderly)‐‐a randomised controlled trial. Trials [Electronic Resource] 2009;10:Article number 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass CD, Sahota O, Drummond A, Kendrick D, Grainge M, Gladman J, et al. Refine‐reducing falls in in‐patient elderly using bed and chair pressure sensors in acute hospital care: A randomised controlled trial. Age and Ageing 2012;41:Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Said 2012 {published data only}
- Said CM, Morris ME, Woodward M, Churilov L, Bernhardt J. Enhancing physical activity in older adults receiving hospital based rehabilitation: a phase II feasibility study. BMC Geriatrics 2012;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Said 2015 {published data only}
- Said CM, Morris ME, McGinley JL, Szoeke C, Workman B, Liew D, et al. Evaluating the effects of increasing physical activity to optimize rehabilitation outcomes in hospitalized older adults (MOVE Trial): study protocol for a randomized controlled trial. Trials 2015;16:13. [PUBMED: 25588907] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sato 2000 {published data only}
- Sato Y, Asoh T, Kaji M, Oizumi K. Beneficial effect of intermittent cyclical etidronate therapy in hemiplegic patients following an acute stroke. Journal of Bone and Mineral Research 2000;15(12):2487‐94. [DOI] [PubMed] [Google Scholar]
Sato 2005a {published data only}
- Sato Y, Iwamoto J, Kanoko T, Satoh K. Low‐dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovascular Diseases 2005;20:187‐92. [DOI] [PubMed] [Google Scholar]
Sato 2005b {published data only}
- Sato Y, Honda Y, Iwamoto J, Kanoko T, Satoh K. Effect of folate and mecobalamin on hip fractures in patients with stroke: a randomized controlled trial. JAMA 2005;293(9):1082‐8. [DOI] [PubMed] [Google Scholar]
- Sato Y, Honda Y, Iwamoto J, Kanoko T, Satoh K. Inaccurate description of collaborating hospitals in a study of the effect of folate and mecobalamin on hip fractures after stroke. JAMA 2006;296(4):396. [DOI] [PubMed] [Google Scholar]
Sato 2011 {published data only}
- Sato Y, Iwamoto J, Honda Y. An open‐label trial comparing alendronate and alphacalcidol in reducing falls and hip fractures in disabled stroke patients. Journal of Stroke and Cerebrovascular Diseases 2011;20(1):41‐6. [DOI] [PubMed] [Google Scholar]
Schneider 2006 {published data only}
- Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. New England Journal of Medicine 2006;355(15):1525‐38. [DOI] [PubMed] [Google Scholar]
- Tariot PN. Efficacy and tolerability of atypical antipsychotics in agitation and psychosis: Research results [abstract]. 158th Annual Meeting of the American Psychiatric Association; 2005 May 21‐26; Atlanta (GA) 31A.
- Tariot PN, Schneider L, Katz IR, Mintzer JE, Street J, Copenhaver M, et al. Quetiapine treatment of psychosis associated with dementia: A double‐blind, randomized, placebo‐controlled clinical trial. American Journal of Geriatric Psychiatry 2006;14(9):767‐76. [DOI] [PubMed] [Google Scholar]
- Zhong KX, Tariot PN, Mintzer J, Minkwitz MC, Devine NA. Quetiapine to treat agitation in dementia: a randomized, double‐blind, placebo‐controlled study. Current Alzheimer Research 2007;4(1):81‐93. [DOI] [PubMed] [Google Scholar]
Schwendimann 2006 {published data only}
- Schwendimann R. personal communication 22 April 2005.
- Schwendimann R, Buhler H, Geest S, Milisen K. Falls and consequent injuries in hospitalized patients: effects of an interdisciplinary falls prevention program. BMC Health Services Research 2006;6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendimann R, Milisen K, Buhler H, Geest S. Fall prevention in a Swiss acute care hospital setting Reducing multiple falls. Journal of Gerontological Nursing 2006;32(3):13‐22. [DOI] [PubMed] [Google Scholar]
Sherrington 2016a {published data only}
- Sherrington C, Fairhall N, Kirkham C, Clemson L, Howard K, Vogler C, et al. Exercise and fall prevention self‐management to reduce mobility‐related disability and falls after fall‐related lower limb fracture in older people: protocol for the RESTORE (Recovery Exercises and STepping On afteR fracturE) randomised controlled trial. BMC Geriatrics 2016;16(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shimada 2003 {published data only}
- Shimada H, Uchiyama Y, Kakurai S. Specific effects of balance and gait exercises on physical function among the frail elderly. Clinical Rehabilitation 2003;17(5):472‐9. [DOI] [PubMed] [Google Scholar]
Shimada 2009 {published data only}
- Shimada H, Tiedemann A, Lord S, Suzuki T. The effect of enhanced supervision on fall rates in residential aged care. American Journal of Physical Medicine and Rehabilitation 2009;88(10):823‐8. [DOI] [PubMed] [Google Scholar]
Siddiqi 2016 {published data only}
- Heaven, A, Cheater F, Clegg A, Collinson M, Farrin A, Forster A, et al. Pilot trial of Stop Delirium! (PiTStop)‐‐a complex intervention to prevent delirium in care homes for older people: study protocol for a cluster randomised controlled trial. Trials [Electronic Resource] 2014;15:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi N, Cheater F, Collinson M, Farrin A, Forster A, George D, et al. The PiTSTOP study: a feasibility cluster randomized trial of delirium prevention in care homes for older people. Age and Ageing 2016;45(5):652‐61. [DOI: 10.1093/ageing/afw091] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sjoberg 2013 {published data only}
- Sjoberg C. SV: Effects of medication reviews performed by a physician on treatment with fracture‐preventing and fall‐risk‐increasing drugs in older adults with hip fracture‐a randomized controlled study [personal communication]. Email to: S Dyer. 19 September 2016. [DOI] [PubMed]
- Sjoberg C, Wallerstedt SM. Effects of medication reviews performed by a physician on treatment with fracture‐preventing and fall‐risk‐increasing drugs in older adults with hip fracture‐a randomized controlled study. Journal of the American Geriatrics Society 2013;61(9):1464‐72. [DOI] [PubMed] [Google Scholar]
Smith 2017 {published data only}
- Smith T, Clark A, Dodd E, Khoo ME, Heneker S, Cross J, et al. Feasibility study suggests no impact from protected engagement time on adverse events in mental health wards for older adults. International Journal of Mental Health Nursing 2017 Jul 6 [Epub ahead of print]. [DOI: 10.1111/inm.12362] [DOI] [PubMed]
Sola 2014 {published data only}
- Sola SM, Lopez del Amo JL, Valero O. The effect of 24 weeks of moderate‐to‐high intensity strength training on the elderly [Spanish]. Revista Espanola de Geriatria y Gerontologia 2014;49(3):115‐20. [DOI] [PubMed] [Google Scholar]
Southard 2006 {published data only}
- Southard V. A randomized control trial of the application of efficacy training to balance assessment. Physical and Occupational Therapy in Geriatrics 2006;25(2):51‐66. [Google Scholar]
Steadman 2003 {published data only}
- Steadman J, Donaldson N, Kalra L. A randomized controlled trial of an enhanced balance training program to improve mobility and reduce falls in elderly patients. Journal of the American Geriatrics Society 2003;51(6):847‐52. [DOI] [PubMed] [Google Scholar]
Tanikawa 2014 {published data only}
- Tanikawa H, Sato T, Nagafuchi M, Takeda K, Oshida J, Okuma K. Comparison of local infiltration of analgesia and sciatic nerve block in addition to femoral nerve block for total knee arthroplasty. Journal of Arthroplasty 2014;29(12):2462‐7. [DOI] [PubMed] [Google Scholar]
Tariot 2004 {published data only}
- Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA 2004;291(3):317. [DOI] [PubMed] [Google Scholar]
- Dyck CH, Tariot PN, Meyers B, Malca Resnick E. A 24‐week randomized, controlled trial of memantine in patients with moderate‐to‐severe Alzheimer disease. Alzheimer Disease and Associated Disorders 2007;21(2):136‐43. [DOI] [PubMed] [Google Scholar]
Tariot 2005 {published data only}
- Profenno LA, Jakimovich L, Holt CJ, Porsteinsson A, Tariot PN. A randomized, double‐blind, placebo‐controlled pilot trial of safety and tolerability of two doses of divalproex sodium in outpatients with probable Alzheimer's disease. Current Alzheimer Research 2005;2(5):553‐8. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Raman R, Jakimovich L, Schneider L, Porsteinsson A, Thomas R, et al. Divalproex sodium in nursing home residents with possible or probable Alzheimer disease complicated by agitation: A randomized, controlled trial. American Journal of Geriatric Psychiatry 2005;13(11):942‐9. [DOI] [PubMed] [Google Scholar]
Teresi 2013 {published data only}
- Teresi JA, Ramirez M, Remler D, Ellis J, Boratgis G, Silver S, et al. Comparative effectiveness of implementing evidence‐based education and best practices in nursing homes: Effects on falls, quality‐of‐life and societal costs. International Journal of Nursing Studies. 2013;50(4):448‐63. [DOI] [PubMed] [Google Scholar]
Underwood 2011 {published data only}
- Ellard DR, Taylor SJ, Parsons S, Thorogood M. The OPERA trial: a protocol for the process evaluation of a randomised trial of an exercise intervention for older people in residential and nursing accommodation. Trials [Electronic Resource] 2011;12:Article number 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN43769277. Older people's exercise intervention in residential and nursing accommodation. www.controlled‐trials.com/ISRCTN43769277 (accessed 11 March 2012).
- Underwood M, Eldridge S, Lamb S, Potter R, Sheehan B, Slowther AM, et al. The OPERA trial: protocol for a randomised trial of an exercise intervention for older people in residential and nursing accommodation. Trials [Electronic Resource] 2011;12:Article number 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Ooijen 2013 {published and unpublished data}
- Ooijen KMV. RE: C‐mill gait‐adaptability training following hip fracture study [personal communication]. Email to: S Dyer. 6 May 2016.
- Ooijen MW, Roerdink M, Trekop M, Visschedijk J, Janssen TW, Beek PJ. Functional gait rehabilitation in elderly people following a fall‐related hip fracture using a treadmill with visual context: design of a randomized controlled trial. BMC Geriatrics 2013;13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vassallo 2004 {published data only}
- Vassallo M, Vignaraja R, Sharma JC, Briggs RS, Allen SC. Can intervention prevent falls and injury in geriatric wards? Hospital injury prevention (HIP) study [abstract]. Age and Ageing 2001;30(Suppl 2):15. [Google Scholar]
- Vassallo M, Vignaraja R, Sharma JC, Hallam H, Binns K, Briggs R, et al. The effect of changing practice on fall prevention in a rehabilitative hospital: the Hospital Injury Prevention Study. Journal of the American Geriatrics Society 2004;52(3):335‐9. [DOI] [PubMed] [Google Scholar]
Visvanathan 2015 {published data only}
Von Koch 2001 {published data only}
- Thorsen AM, Widen Holmqvist L, Pedro‐Cuesta J, Koch L. A randomized controlled trial of early supported discharge and continued rehabilitation at home after stroke: five‐year follow‐up of patient outcome. Stroke 2005;36(2):297‐303. [DOI] [PubMed] [Google Scholar]
- Thorsen AM, Widen Holmqvist L, Koch L. Early supported discharge and continued rehabilitation at home after stroke: 5‐year follow‐up of resource use. Journal of Stroke and Cerebrovascular Diseases 2006;15(4):139‐43. [DOI] [PubMed] [Google Scholar]
- Widen Holmqvist L, Koch L, Kostulas V, Holm M, Widsell G, Tegler H, et al. A randomized controlled trial of rehabilitation at home after stroke in southwest Stockholm. Stroke 1998;29:591‐7. [DOI] [PubMed] [Google Scholar]
- Koch L, Pedro‐Cuesta J, Kostulas V, Almazan J, Widen Holmqvist L. Randomized controlled trial of rehabilitation at home after stroke: one‐year follow‐up of patient outcome, resource use and cost. Cerebrovascular Disease 2001;12(2):131‐8. [DOI] [PubMed] [Google Scholar]
- Koch L, Widen Holmqvist L, Kostulas V, Almazan J, Pedro‐Cuesta J. A randomized controlled trial of rehabilitation at home after stroke in Southwest Stockholm: outcome at six months. Scandinavian Journal of Rehabilitation Medicine 2000;32(2):80‐6. [DOI] [PubMed] [Google Scholar]
Wolf 2003 {published data only}
- Sattin RW, Easley KA, Wolf SL, Chen Y, Kutner MH. Reduction in fear of falling through intense tai chi exercise training in older, transitionally frail adults. Journal of the American Geriatrics Society 2005;53(7):1168‐78. [DOI] [PubMed] [Google Scholar]
- Wolf SL, O'Grady M. The influence of intense Tai Chi training on physical performance and hemodynamic outcomes in transitionally frail, older adults. Journals of Gerontology Series A‐Biological Sciences and Medical Sciences 2006;61(2):184‐9. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Sattin RW, Kutner M, O'Grady M, Greenspan AI, Gregor RJ. Intense Tai Chi exercise training and falls occurrences in older, transitionally frail adults: A randomized controlled trial. Journal of the American Geriatrics Society 2003;51:1693‐1701. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Sattin RW, O'Grady M, Freret N, Ricci L, Greenspan AI, et al. A study design to investigate the effect of intense Tai Chi in reducing falls among older adults transitioning to frailty. Controlled Clinical Trials 2001;22(6):689‐704. [DOI] [PubMed] [Google Scholar]
Zhong 2007 {published data only}
- Zhong KX, Tariot PN, Mintzer J, Minkwitz MC, Devine NA. Quetiapine to treat agitation in dementia: a randomized, double blind, placebo‐controlled study. Current Alzheimers Research 2007;4(1):81‐93. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Dever 2016 {published data only}
Frohnhofen 2013 {published data only (unpublished sought but not used)}
- Frohnhofen H, Schlitzer J, Wehling M. Pharmacotherapy and frequency of inhospital falls among geriatric patients. Osteoporosis International.Conference: European Congress on Osteoporosis and Osteoarthritis, ESCEO13‐IOF Rome Italy.Conference Start: 2013;24(1 Suppl):P590. [Google Scholar]
Hewitt 2014 {published data only}
- Hewitt J, Goodall S, Clemson L, Henwood T, Refshauge K. Progressive resistance and balance training for falls Prevention in long‐term residential aged care: A cluster randomized trial of the SUNBEAM program. Journal of the American Medical Directors Association 2018;19(4):361‐9. [PUBMED: 29402651] [DOI] [PubMed] [Google Scholar]
- Hewitt J, Refshauge KM, Goodall S, Henwood T, Clemson L. Does progressive resistance and balance exercise reduce falls in residential aged care? Randomized controlled trial protocol for the SUNBEAM program. Clinical Interventions In Aging 2014;9:369‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacRitchie 2001 {published data only}
- MacRitchie RF. Reducing the incidence of falls among elderly nursing home residents: An evaluation of an ameliorative pilot program [thesis]. Dublin, USA: Southern Connecticut State Univ, 2001. [CENTRAL: CN‐00691333] [Google Scholar]
Raymond 2017 {published data only}
Tallon 2013 {published data only}
- Tallon G, Ramdani S, Jaussent A, Decker L, Bernard PL, Blain H. Effect of whole‐body‐vibration training in institutionalized older adults. European Geriatric Medicine 2013;4(Suppl 1):S55. [Google Scholar]
Van der Linden 2017 {published data only}
- Linden L. Reduction of inappropriate prescribing in older persons using the RASP list: A cluster‐randomised controlled trial. European Journal of Hospital Pharmacy 2014; Vol. 21:A212.
- Linden L, Decoutere L, Walgraeve K, Milisen K, Flamaing J, Spriet I, et al. combined use of the rationalization of home medication by an adjusted STOPP in older patients (RASP) list and a pharmacist‐led medication review in very old inpatients: impact on quality of prescribing and clinical outcome. Drugs and Aging 2017; Vol. 34, issue 2:123‐33. [DOI] [PubMed]
Wylie 2017 {published data only}
- NCT02178527. Podiatry Intervention to Reduce Falls in Elderly Care Trial (PIRFECT). ClinicalTrials.gov/show/NCT02178527 (first received 17 June 2014). [N. H.S.Tayside; University of Stirling; La Trobe University; University of Toronto; Uniersity of Dundee]
- Wylie G, Menz HB, McFarlane S, Ogston S, Sullivan F, Williams B, et al. Podiatry intervention versus usual care to prevent falls in care homes: pilot randomised controlled trial (the PIRFECT study). BMC Geriatrics 2017; Vol. 17, issue 1:143. [DOI] [PMC free article] [PubMed]
References to ongoing studies
ACTRN12613000228785 {published data only}
- ACTRN12613000228785. Preventing falls and fractures in low‐level aged‐care residents by increasing dairy food intake by two serves per day. www.anzctr.org.au/ACTRN12613000228785.aspx (first received 20 February 2013).
ACTRN12615000817549 {published data only}
- ACTRN12615000817549. Establishing the effectiveness, cost‐effectiveness and student experience of simulation training for the prevention of falls amongst hospitalised inpatients. www.anzctr.org.au/ACTRN12615000817549.aspx (first received 27 July 2015).
- Williams C, Bowles KA, Kiegaldie D, Maloney S, Nestel D, Kaplonyi J, et al. Establishing the effectiveness, cost‐effectiveness and student experience of a Simulation‐based education Training program On the Prevention of Falls (STOP‐Falls) among hospitalised inpatients: A protocol for a randomised controlled trial. BMJ Open 2016; Vol. 6, issue 6:e010192. [DOI: 10.1136/bmjopen-2015-010192.] [DOI] [PMC free article] [PubMed]
ACTRN12617000314325 {published data only}
- ACTRN12617000314325. Does abbreviating patient falls risk screening in documentation impact on falls in hospital inpatients: A stepped wedge cluster randomised control trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12617000314325 (first received 21 February 2017).
Dal Bello‐Haas 2012 {published data only}
- Dal Bello‐Haas VP, Thorpe LU, Lix LM, Scudds R, Hadjistavropoulos T. The effects of a long‐term care walking program on balance, falls and well‐being. BMC Geriatrics 2012;12:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hassett 2016 {published data only}
- Hassett L, Berg M, Lindley RI, Crotty M, McCluskey A, Ploeg HP, et al. Effect of affordable technology on physical activity levels and mobility outcomes in rehabilitation: A protocol for the Activity and MObility UsiNg Technology (AMOUNT) rehabilitation trial. BMJ Open 2016; Vol. 6, issue 6:e012074. [DOI] [PMC free article] [PubMed]
ISRCTN34353836 {published data only}
- ISRCTN34353836. Finch: Falls in care homes study. http://www.isrctn.com/ISRCTN34353836 (first received 22 March 2016).
ISRCTN42003273 {published data only}
- Loffler C, Drewelow E, Paschka SD, Frankenstein M, Eger J, Jatsch L, et al. Optimizing polypharmacy among elderly hospital patients with chronic diseases‐‐study protocol of the cluster randomized controlled POLITE‐RCT trial. Implementation Science 2014;9:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
JPRN‐UMIN000000555 {published data only}
- JPRN‐UMIN000000555. The effects of whole body vibration for the prevention of falls in elderly. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000000555 (first received 25 December 2006).
JPRN‐UMIN000008361 {published data only}
- JPRN‐UMIN000008361. Multicenter, randomized, double‐blind, placebo controlled, parallel group trial to evaluate the effect of Vitamin D supplementation for fall prevention. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000008361 (first received 6 July 2012).
McCullagh 2016 {published data only}
- McCullagh R, O'Connell E, O'Meara S, Perry I, Fitzgerald A, O'Connor K, et al. A study protocol of a randomised controlled trial to measure the effects of an augmented prescribed exercise programme (APEP) for frail older medical patients in the acute setting. BMC Geriatrics 2016; Vol. 16:79. [DOI] [PMC free article] [PubMed]
Mestres 2017 {published data only}
- Mestres Gonzalvo C, Wit HA, Oijen BP, Hurkens KP, Janknegt R, Schols JM, et al. Supporting clinical rules engine in the adjustment of medication (SCREAM): protocol of a multicentre, prospective, randomised study. BMC Geriatrics 2017; Vol. 17, issue 1:35. [DOI] [PMC free article] [PubMed]
Mudge 2017 {published data only}
- Mudge AM, Banks MD, Barnett AG, Blackberry I, Graves N, Green T, et al. CHERISH (collaboration for hospitalised elders reducing the impact of stays in hospital): protocol for a multi‐site improvement program to reduce geriatric syndromes in older inpatients. BMC Geriatrics 2017; Vol. 17, issue 1:11. [DOI] [PMC free article] [PubMed]
NCT00636675 {published data only}
- Anderson RA, Corazzini K, Porter K, Daily K, McDaniel RR, Colón‐Emeric C. CONNECT for quality: protocol of a cluster randomized controlled trial to improve fall prevention in nursing homes. Implementation Science 2012;7(1):11. [DOI: 10.1186/1748-5908-7-11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00636675. CONNECT for Quality: A Study to Reduce Falls in Nursing Homes (CONNECT). clinicaltrials.gov/ct2/show/NCT00636675 (first received 14 March 2008).
NCT01483456 {published and unpublished data}
- Grangier C, Mouchoux C, Pogam M, Toulouze B, Colin C, Krolak‐Salmon P. A multidisciplinary program for preventing falls "identify, prevent and get up": Impact on falls in elderly inpatients. European Geriatric Medicine. 7th Congress of the European Union Geriatric Society (EUGMS); 2011 Sept 28‐30; Malaga, Spain. 2011. [Conference abstract 201200]
- Mouchoux C. NCT01483456 "Impact of multidisciplinary program on falls in elderly inpatients (IPR)" [personal communication]. Email to: S Dyer. 29 June 2016.
- NCT01483456. Impact of multidisciplinary program on falls in elderly inpatients (IPR). clinicaltrials.gov/show/NCT01483456 (first received 21 July 2007).
NCT01551121 {published and unpublished data}
- NCT01551121. Assessment of an automated telesurveillance system on the incidence of serious falls in nursing homes (TELEHPAD). clinicaltrials.gov/show/NCT01551121 (first received 12 March 2012).
NCT01561872 {published data only}
- NCT01561872. Assessment of an automated telesurveillance system on serious falls prevention in an elderly suffering from dementia specialized care unit: the URCC (GET‐BETTER). clinicaltrials.gov/show/NCT01561872 (first received 21 March 2012).
- Saulnier I, Lachal F, Tchalla A, Trimouillas J, Gourdeau‐Nauche F, Bernard‐Bourzeix L, et al. Assessment of an automated tele vigilance system on serious falls prevention in a dementia specialized care unit: The URCC. Journal of Nutrition, Health and Aging 2012;16(9):865. [Abstract P80] [Google Scholar]
NCT01735682 {published data only}
- NCT01735682. Whole body vibration exercise training for institutionalized elderly. ClinicalTrials.gov/show/NCT01735682 (first received 20 November 2012). [The Hong Kong Polytechnic University, Shatin Hospital Hong Kong]
NCT01876095 {published data only}
- NCT01876095. Discontinuing inappropriate medication in nursing home residents. ClinicalTrials.gov/show/NCT01876095 (first received 10 June 2013).
- Wouters H, Quik EH, Boersma F, Nygard P, Bosman J, Bottger WM, et al. Discontinuing inappropriate medication in nursing home residents (DIM‐NHR Study): protocol of a cluster randomised controlled trial. BMJ Open 2014;4(10):e006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02295462 {published data only}
- NCT02295462. Effect of person‐centred‐care on antipsychotic drug use in nursing homes: a cluster‐randomised trial. ClinicalTrials.gov/show/NCT02295462 (first received 12 November 2014).
NCT02570945 {published data only}
- NCT02570945. Trial of a pharmacist‐physician intervention model to reduce high‐risk drug use by hospitalised elderly patients. ClinicalTrials.gov/show/NCT02570945 (first received 25 September 2015).
NCT02604056 {published data only}
- NCT02604056. Pragmatic cluster trial for nursing home antipsychotic prescribing. ClinicalTrials.gov/show/NCT02604056 (first received 5 November 2015).
NCT02702037 {published data only}
- NCT02702037. Older person's exercise and nutrition study. ClinicalTrials.gov/show/NCT02702037 (first received 26 February 2016).
NCT02714257 {published data only}
- NCT02714257. Seniors avoiding falls through exercise study. https://ClinicalTrials.gov/show/NCT02714257 (first received 22 February 2016).
NCT02714582 {published data only}
- NCT02714582. Feasibility, appropriateness, meaningfulness and effectiveness of bedside shift reporting. ClinicalTrials.gov/show/NCT02714582 (first received 29 February 2016).
NCT02757131 {published data only}
- NCT02757131. Dedicated ambulator‐assisted physical activity to improve hospital outcome measures in elderly patients. https://clinicaltrials.gov/show/NCT02757131 (first received 29 April 2016).
NCT02969343 {published data only}
- NCT02969343. Patient safety learning laboratory: making acute care more patient‐centered. https://clinicaltrials.gov/show/NCT02969343 (first received 21 November 2016).
NCT03014570 {published data only}
- NCT03014570. Testing implementation of EIT‐4‐BPSD. https://clinicaltrials.gov/show/NCT03014570 (first received 9 January 2017).
NCT03019211 {published data only}
- NCT03019211. Feasibility aquatic physical exercise to reduce falls in institutionalized elderly. https://clinicaltrials.gov/show/NCT03019211 (first received 12 January 2017).
NCT03192384 {published data only}
- NCT03192384. A service intervention to reduce falls in hospital. https://clinicaltrials.gov/show/NCT03192384 (first received 20 June 2017).
NTR5015 {published data only}
- NTR5015. Prevention of falling. www.trialregister.nl/trialreg/admin/rctview.asp?TC=5015 (first received 22 December 2014).
Scheffers‐Barnhoorn 2017 {published data only}
- NTR5695. A randomised controlled trial to improve outcomes of hip fracture patients with fear of falling in geriatric rehabilitation. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=5695 (first received 7 March 2016).
- Scheffers‐Barnhoorn MN, Haastregt JC, Schols JM, Kempen GI, Balen R, Visschedijk JH, et al. A multi‐component cognitive behavioural intervention for the treatment of fear of falling after hip fracture (FIT‐HIP): protocol of a randomised controlled trial. BMC Geriatrics 2017; Vol. 17, issue 1:71. [DOI] [PMC free article] [PubMed]
Additional references
Avenell 2014
- Avenell A, Mak JC, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures in post‐menopausal women and older men. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD000227.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 2010
- Becker C, Rapp K. Fall prevention in nursing homes. [Review]. Clinics In Geriatric Medicine 2010;26(4):693‐704. [DOI] [PubMed] [Google Scholar]
Becker 2012
- Rapp K, Becker C, Cameron ID, Konig HH, Buchele G. Epidemiology of falls in residential aged care: analysis of more than 70,000 falls from residents of Bavarian nursing homes. Journal of the American Medical Directors Association 2012;13(2):187.e1‐6. [DOI] [PubMed] [Google Scholar]
Bolland 2010
- Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta‐analysis. BMJ 2010;341:c3691. [PUBMED: 20671013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolland 2014
- Bolland MJ, Grey A, Gamble GD, Reid IR. Vitamin D supplementation and falls: a trial sequential meta‐analysis. Lancet. Diabetes and Endocrinology 2014;2(7):573‐80. [PUBMED: 24768505] [DOI] [PubMed] [Google Scholar]
Bolland 2016
- Bolland MJ, Avenell A Gamble GD, Grey A. Systematic review and statistical analysis of the integrity of 33 randomized controlled trials. Neurology 2016;87(23):2391‐402. [DOI] [PubMed] [Google Scholar]
Boutron 2008
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P, CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Annals of Internal Medicine 2008;148(4):295‐309. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Campbell 2004
- Campbell MK, Elbourne DR, Altman DG, CONSORT group. CONSORT statement: extension to cluster randomised trials. BMJ 2004;328(7441):702‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2015
- Chan WC, Yeung JW, Wong CS, Lam LC, Chung KF, Luk JK, et al. Efficacy of physical exercise in preventing falls in older adults with cognitive impairment: a systematic review and meta‐analysis. Journal of the American Medical Directors Association 2015;16(2):149‐54. [DOI] [PubMed] [Google Scholar]
Choi 2012
- Choi M, Hector M. Effectiveness of intervention programs in preventing falls: a systematic review of recent 10 years and meta‐analysis. Journal of the American Medical Directors Association 2012;13(2):188.e13‐21. [PUBMED: 21680249] [DOI] [PubMed] [Google Scholar]
Conroy 2010
- Conroy S, Kendrick D, Harwood R, Gladman J, Coupland C, Sach T, et al. A multicentre randomised controlled trial of day hospital‐based falls prevention programme for a screened population of community‐dwelling older people at high risk of falls. Age and Ageing 2010;39(6):704‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, Accessed 3 March 2016.
Davis 2011
- Davis JC, Robertson MC, Comans T, Scuffham PA. Guidelines for conducting and reporting economic evaluation of fall prevention strategies. Osteoporosis International 2011;22(9):2449‐59. [DOI] [PubMed] [Google Scholar]
De Spiegeleer 2018
- Spiegeleer A, Beckwée D, Bautmans I, Petrovic M. Pharmacological interventions to improve muscle mass, muscle strength and physical performance in older people: an umbrella review of systematic reviews and meta‐analyses. Drugs & Aging 2018;35(8):719‐34. [DOI] [PubMed] [Google Scholar]
Deandrea 2013
- Deandrea S, Bravi F, Turati F, Lucenteforte E, Vecchia C, Negri E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta‐analysis. Archives of Gerontology and Geriatrics 2013;56(3):407‐15. [PUBMED: 23294998] [DOI] [PubMed] [Google Scholar]
Durvasula 2012
- Durvasula S, Sambrook PN, Cameron ID. Factors influencing adherence with therapeutic sunlight exposure in older people in intermediate care facilities. Archives of Gerontology and Geriatrics 2012;54(2):e234‐41. [DOI] [PubMed] [Google Scholar]
Excel [Computer program]
- Microsoft. Excel X for Mac. Version 8. Microsoft, 2001.
Gillespie 2003
- Gillespie LD, Gillespie WJ, Robertson MC, Lamb SE, Cumming RG, Rowe BH. Interventions for preventing falls in elderly people. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000340.pub2] [DOI] [PubMed] [Google Scholar]
Gillespie 2012
- Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, Lamb SE. Interventions for preventing falls in older people living in the community. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD007146.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guo 2014
- Guo JL, Tsai YY, Liao JY, Tu HM, Huang CM. Interventions to reduce the number of falls among older adults with/without cognitive impairment: an exploratory meta‐analysis. International Journal of Geriatric Psychiatry 2014;29(7):661‐9. [PUBMED: 24318959] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence‐‐inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Hahn 2005
- Hahn S, Puffer S, Torgerson DJ, Watson J. Methodological bias in cluster randomised trials. BMC Medical Research Methodology 2005;5:10. [PUBMED: 15743523] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haines 2009
- Haines TP, Russell T, Brauer SG, Erwin S, Lane P, Urry S, et al. Effectiveness of a video‐based exercise programme to reduce falls and improve health‐related quality of life among older adults discharged from hospital: a pilot randomized controlled trial. Clinical Rehabilitation 2009;23(11):973‐85. [DOI] [PubMed] [Google Scholar]
Haines 2013
- Haines TP, Hill AM, Hill KD, Brauer SG, Hoffmann T, Etherton‐Beer C, et al. Cost effectiveness of patient education for the prevention of falls in hospital: economic evaluation from a randomized controlled trial. BMC Medicine 2013;11:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hannan 2010
- Hannan MT, Gagnon MM, Aneja J, Jones RN, Cupples LA, Lipsitz LA, et al. Optimizing the tracking of falls in studies of older participants: comparison of quarterly telephone recall with monthly falls calendars in the MOBILIZE Boston Study. American Journal of Epidemiology 2010;171(9):1031‐6. [PUBMED: 20360242] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hauer 2006
- Hauer K, Lamb SE, Jorstad EC, Todd C, Becker C, ProFaNE‐Group. Systematic review of definitions and methods of measuring falls in randomised controlled fall prevention trials. Age and Ageing 2006;35(1):5‐10. [DOI] [PubMed] [Google Scholar]
Hendriks 2008
- Hendriks MR, Evers SM, Bleijlevens MH, Haastregt JC, Crebolder HF, Eijk JT. Cost‐effectiveness of a multidisciplinary fall prevention program in community‐dwelling elderly people: A randomized controlled trial (ISRCTN 64716113). International Journal of Technology Assessment in Health Care 2008;24(2):193‐202. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8.5: The Cochrane Collaboration’s tool for assessing risk of bias. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16.3.4: Approximate analyses of cluster‐randomized trials for meta‐analysis: effective sample sizes. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011d
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9.6.3.1 Is the effect different in different subgroups? In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hill 2010
- Hill AM, Hoffmann T, Hill K, Oliver D, Beer C, McPhail S, et al. Measuring falls events in acute hospitals‐a comparison of three reporting methods to identify missing data in the hospital reporting system. Journal of the American Geriatrics Society 2010;58(7):1347‐52. [PUBMED: 20487077] [DOI] [PubMed] [Google Scholar]
Hill 2014
- Hill, AM, Waldron N, Etherton‐Beer C, McPhail SM, Ingram K, Flicker L, et al. A stepped‐wedge cluster randomised controlled trial for evaluating rates of falls among inpatients in aged care rehabilitation units receiving tailored multimedia education in addition to usual care: a trial protocol. BMJ Open 2014;4(1):e004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibrahim 2015
- Ibrahim JE, Murphy BJ, Bugeja L, Ranson D. Nature and extent of external‐cause deaths of nursing home residents in Victoria, Australia. Journal of the American Geriatrics Society 2015;63(5):954‐62. [PUBMED: 25940003] [DOI] [PubMed] [Google Scholar]
Jensen 2003
- Jensen J, Nyberg L, Gustafson Y, Lundin‐Olsson L. Fall and injury prevention in residential care‐effects in residents with higher and lower levels of cognition. Journal of the American Geriatrics Society 2003;51(5):627‐35. [DOI] [PubMed] [Google Scholar]
Kropelin 2013
- Kropelin TF, Neyens JC, Halfens RJ, Kempen GI, Hamers JP. Fall determinants in older long‐term care residents with dementia: a systematic review. International Psychogeriatrics 2013;25(4):549‐63. [PUBMED: 23253253] [DOI] [PubMed] [Google Scholar]
Lamb 2007
- Lamb SE, Hauer K, Becker C. Manual for the fall prevention classification system. www.profane.eu.org/documents/Falls_Taxonomy.pdf (accessed 18 July 2012).
Lamb 2011
- Lamb SE, Becker C, Gillespie LD, Smith JL, Finnegan S, Potter R, et al. Reporting of complex interventions in clinical trials: development of a taxonomy to classify and describe fall‐prevention interventions. Trials [Electronic Resource] 2011;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Latham 2003
- Latham NK, Anderson CS, Lee A, Bennett DA, Moseley A, Cameron ID. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: The Frailty Interventions Trial in Elderly Subjects (FITNESS). Journal of the American Geriatrics Society 2003;51:291‐9. [DOI] [PubMed] [Google Scholar]
Le Blanc 2015
- Blanc ES, Zakher B, Daeges M, Pappas M, Chou R. Screening for vitamin D deficiency: a systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2015;162(2):109‐22. [DOI] [PubMed] [Google Scholar]
Lee 2017
- Lee SH, Kim HS. Exercise interventions for preventing falls among older people in care facilities: a meta‐analysis. Worldviews on Evidence‐Based Nursing 2017;14(1):74‐80. [PUBMED: 27984675] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6.4.11.1 The Cochrane Highly Sensitive Search Strategies for identifying randomized trials in MEDLINE. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lightbody 2002
- Lightbody E, Watkins C, Leathley M, Sharma A, Lye M. Evaluation of a nurse‐led falls prevention programme versus usual care: a randomized controlled trial. Age and Ageing 2002;31(3):203‐10. [DOI] [PubMed] [Google Scholar]
Lohse 2012
- Lohse GR, Leopold SS, Theiler S, Sayre C, Cizik A, Lee MJ. Systems‐based safety intervention: Reducing falls with injury and total falls on an orthopaedic ward. Journal of Bone and Joint Surgery. American Volume 2012;94(13):1212‐22. [DOI] [PubMed] [Google Scholar]
Lord 2003
- Lord SR, March LM, Cameron ID, Cumming RG, Schwarz J, Zochling J, et al. Differing risk factors for falls in nursing home and intermediate‐care residents who can and cannot stand unaided. Journal of the American Geriatrics Society 2003;51(11):1645‐50. [DOI] [PubMed] [Google Scholar]
Lord 2005
- Lord SR, Tiedemann A, Chapman K, Munro B, Murray SM, Gerontology M, et al. The effect of an individualized fall prevention program on fall risk and falls in older people: a randomized, controlled trial. Journal of the American Geriatrics Society 2005;53(8):1296‐304. [DOI] [PubMed] [Google Scholar]
McAlister 2003
- McAlister FA, Straus SE, Sackett DL, Altman DG. Analysis and reporting of factorial trials: a systematic review. JAMA 2003;289(19):2545‐53. [DOI] [PubMed] [Google Scholar]
Muir 2012
- Muir SW, Gopaul K, Montero Odasso MM. The role of cognitive impairment in fall risk among older adults: a systematic review and meta‐analysis. Age and Ageing 2012;41(3):299‐308. [PUBMED: 22374645] [DOI] [PubMed] [Google Scholar]
Murray 2007
- Murray GR, Cameron ID, Cumming RG. The consequences of falls in acute and subacute hospitals in Australia that cause proximal femoral fractures. Journal of the American Geriatrics Society 2007;55:577‐82. [DOI] [PubMed] [Google Scholar]
NLM 2012
- National Library of Medicine. Medical Subject Headings: MeSH Browser (2012 MeSH). www.nlm.nih.gov/mesh/2012/mesh_browser/MBrowser.html (accessed 21 August 2012).
Norman 2008
- Norman R, Haas M, Chenoweth L, Jeon Y‐H, King M, Brodaty H, et al. Dementia Care Mapping and Patient‐centred Care in Australian Residential Homes: an Economic Evaluation of the CARE Study. Sydney: Centre for Health Economics Research and Evaluation, 2008. [Google Scholar]
Nurmi 2002
- Nurmi I, Luthje P. Incidence and costs of falls and falls injuries among elderly in institutional care. Scandinavian Journal of Primary Health Care 2002;20(2):118‐22. [PubMed] [Google Scholar]
Nyberg 1997
- Nyberg L, Gustafson Y, Janson A, Sandman PO, Eriksson S. Incidence of falls in three different types of geriatric care. A Swedish prospective study. Scandinavian Journal of Social Medicine 1997;25(1):8‐13. [DOI] [PubMed] [Google Scholar]
Nyman 2011
- Nyman SR, Victor CR. Older people's recruitment, sustained participation, and adherence to falls prevention interventions in institutional settings: a supplement to the Cochrane systematic review. [Review]. Age and Ageing 2011;40(4):430‐6. [DOI] [PubMed] [Google Scholar]
OECD 2011
- OECD, Eurostat, WHO. Part 1, Chapter 6: ICHA‐HP Classification of Health Care Providers. In: A System of Health Accounts. 2011 edition. OECD Publishing. 2011:133‐4. Available from http://dx.doi.org/10.1787/9789264116016‐en (accessed 22 August 2012).
Oliver 2004
- Oliver D, Daly F, Martin FC, McMurdo ME. Risk factors and risk assessment tools for falls in hospital in‐patients: a systematic review. Age and Ageing 2004;33(2):122‐30. [DOI] [PubMed] [Google Scholar]
Pilz 2012
- Pilz S, Dobnig H, Tomaschitz A, Kienreich K, Meinitzer A, Friedl C, et al. Low 25‐hydroxyvitamin D is associated with increased mortality in female nursing home residents. Journal of Clinical Endocrinology and Metabolism 2012;97(4):E653–7. [DOI] [PubMed] [Google Scholar]
Rapp 2008
- Rapp K, Lamb SE, Buchele G, Lall R, Lindemann U, Becker C. Prevention of falls in nursing homes: subgroup analyses of a randomized fall prevention trial. Journal of the American Geriatrics Society 2008;56(6):1092‐7. [DOI] [PubMed] [Google Scholar]
Retraction Watch
- Retraction Watch. JAMA journals pull 3 papers by same authors for misconduct. retractionwatch.com/2016/06/03/jama‐journals‐pull‐3‐papers‐by‐same‐authors‐for‐misconduct/ (accessed 1 July 2017).
Review Manager [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Robertson 2005
- Robertson MC, Campbell AJ, Herbison P. Statistical analysis of efficacy in falls prevention trials. Journals of Gerontology Series A‐Biological Sciences and Medical Sciences 2005;60(4):530‐4. [DOI] [PubMed] [Google Scholar]
Rubenstein 2006
- Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age and Ageing 2006;35 Suppl 2:ii37‐41. [PUBMED: 16926202] [DOI] [PubMed] [Google Scholar]
Sach 2015
- Sach TH, Desborough J, Houghton J, Holland R, CAREMED study team. Applying micro‐costing methods to estimate the costs of pharmacy interventions: an illustration using multi‐professional clinical medication reviews in care homes for older people. International Journal of Pharmacy Practice 2015;23:237‐47. [DOI: 10.1111/ijpp.12162] [DOI] [PubMed] [Google Scholar]
Santesso 2014
- Santesso N, Carrasco‐Labra A, Brignardello‐Petersen R. Hip protectors for preventing hip fractures in older people. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD001255.pub5; PUBMED: 24687239] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Journal of Clinical Epidemiology 2010;63(8):834‐40. [PUBMED: 20346629] [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sherrington 2011
- Sherrington C, Tiedemann A, Fairhall N, Close JC, Lord SR. Exercise to prevent falls in older adults: an updated meta‐analysis and best practice recommendations. New South Wales Public Health Bulletin 2011;22(3‐4):78‐83. [PUBMED: 21632004] [DOI] [PubMed] [Google Scholar]
Sherrington 2017
- Sherrington C, Michaleff ZA, Fairhall N, Paul SS, Tiedemann A, Whitney J, et al. Exercise to prevent falls in older adults: an updated systematic review and meta‐analysis. British Journal of Sports Medicine 2017;51(24):1750‐8. [PUBMED: 27707740] [DOI] [PubMed] [Google Scholar]
Silva 2013
- Silva RB, Eslick GD, Duque G. Exercise for falls and fracture prevention in long term care facilities: a systematic review and meta‐analysis. Journal of the American Medical Directors Association 2013;14(9):685‐9.e2. [PUBMED: 23860265] [DOI] [PubMed] [Google Scholar]
Stata [Computer program]
- StataCorp LP. Stata Statistical Software. Version 8. StataCorp LP, 2003.
Stenvall 2012
- Stenvall M, Berggren M, Lundstrom M, Gustafson Y, Olofsson B. A multidisciplinary intervention program improved the outcome after hip fracture for people with dementia‐‐subgroup analyses of a randomized controlled trial. Archives of Gerontology and Geriatrics 2012;54(3):e284‐9. [DOI] [PubMed] [Google Scholar]
Stubbs 2015
- Stubbs B, Denkinger MD, Brefka S, Dallmeier D. What works to prevent falls in older adults dwelling in long term care facilities and hospitals? An umbrella review of meta‐analyses of randomised controlled trials. Maturitas 2015;81(3):335‐42. [PUBMED: 25935294] [DOI] [PubMed] [Google Scholar]
Sutton 1994
- Sutton JC, Standen PJ, Wallace WA. Patient accidents in hospital: incidence, documentation and significance. British Journal of Clinical Practice 1994;48(2):63‐6. [PUBMED: 8024991] [PubMed] [Google Scholar]
Verheyden 2013
- Verheyden GS, Weerdesteyn V, Pickering RM, Kunkel D, Lennon S, Geurts AC, et al. Interventions for preventing falls in people after stroke. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD008728.pub2; CD008728] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vieira 2011
- Vieira ER, Freund‐Heritage R, Costa BR. Risk factors for geriatric patient falls in rehabilitation hospital settings: a systematic review. Clinical Rehabilitation 2011;25(9):788‐99. [DOI] [PubMed] [Google Scholar]
Vlaeyen 2015
- Vlaeyen E, Coussement J, Leysens G, Elst E, Delbaere K, Cambier D, et al. Characteristics and effectiveness of fall prevention programs in nursing homes: a systematic review and meta‐analysis of randomized controlled trials. Journal of the American Geriatrics Society 2015;63(2):211‐21. [PUBMED: 25641225] [DOI] [PubMed] [Google Scholar]
Vogler 2009
- Vogler CM, Sherrington C, Ogle SJ, Lord SR. Reducing risk of falling in older people discharged from hospital: a randomized controlled trial comparing seated exercises, weight‐bearing exercises, and social visits. Archives of Physical Medicine & Rehabilitation 2009;90(8):1317‐24. [DOI] [PubMed] [Google Scholar]
Zwarenstein 2008
- Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337:a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Cameron 2005
- Cameron I, Murray GR, Gillespie LD, Cumming RG, Robertson MC, Hill KD, et al. Interventions for preventing falls in older people in residential care facilities and hospitals. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005465] [DOI] [Google Scholar]
Cameron 2010
- Cameron ID, Murray GR, Gillespie LD, Robertson MC, Hill KD, Cumming RG, et al. Interventions for preventing falls in older people in nursing care facilities and hospitals. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD005465.pub2] [DOI] [PubMed] [Google Scholar]
Cameron 2012
- Cameron ID, Gillespie LD, Robertson MC, Murray GR, Hill KD, Cumming RG, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD005465.pub3] [DOI] [PubMed] [Google Scholar]


