Abstract
Twelve new bis-Schiff bases of isatin, benzylisatin and 5-fluoroisatin 3a-3l were prepared by condensation of isatin, benzylisatin and 5-fluoroisatin with primary aromatic amines. The chemical structures of the products were confirmed by ¹H- and 13C-NMR, IR and mass spectral data. The compounds were screened for antiviral activity against a panel of DNA and RNA viruses. Minimum cytotoxic and minimum virus-inhibitory concentrations of these compounds were determined. Compounds 3c and 3i were the most cytotoxic in HEL cells. These newly synthesized bis-Schiff bases were also tested for their antibacterial and antifungal activities. They did not display activity against S. cerevisiae (ATCC 28383) or C. albicans (CIP 1180-79).
Keywords: Isatin, bis-Schiff base, antibacterial, antiviral, antifungal
Introduction
Isatin has been known for about 150 years and has been recently found, like oxindole and endogenous polyfunctional heterocyclic compounds, to exhibit biological activity in mammals [1]. Isatin also is a synthetically versatile substrate that can be used to prepare a large variety of heterocyclic compounds, such as indoles and quinolines, and as a raw material for drug synthesis [2]. Isatin is further known to be a color reagent for the amino acid proline, forming a blue derivative [3]. This property has been exploited for the determination of this amino acid in pollens [4] and other vegetable materials [5] using paper chromatography or for the detection of polymer-bound compounds possessing proline residues [6]. Some isatin derivatives exhibit antiplasmodial activity [7]. Schiff bases and Mannich bases of isatin are known to possess a wide range of pharmacological properties including antibacterial, [8,9,10] anticonvulsant, [11,12] anti-HIV, [13,14,15,16] antifungal [17,18,19,20] and antiviral activity [21]. Bis-Schiff bases are characterized by their capacity to completely co-ordinate a metal ion, forming chelate rings [22]. The Schiff bases of isatin have also been used as ligands for complexation of metals such as copper II [23]. These complexes catalyzed the oxidation of carbohydrates. Bis-Schiff bases can act as inhibitors of human α-thrombin [24]. Recently it has been reported that a bis-imine of isatin has antimicrobial properties [25] and affects cell viability [26]. Here we report the synthesis and characterization of new bis-Schiff bases of isatin, benzylisatin [27] and 5-fluoroisatin, which could be considered as potential biologically active compounds.
Results and Discussion
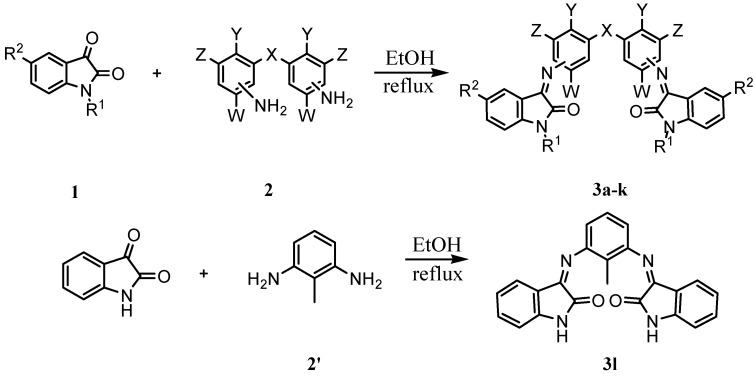
The desired bis-Schiff bases of isatin and its derivatives were prepared by the reactions of isatin, 5-fluoroisatin and benzylisatin with commercially available aromatic diamines in the presence of catalytic amounts of glacial acetic acid in EtOH under reflux condition (Scheme 1). The physico-chemical properties of bis-Schiff bases thus prepared are summarized in Table 1.
Scheme 1.
Table 1.
Physico-chemical properties of compounds 3a-3l.
| Compd. | X | Y | Z | W | R1 | R2 | Position of C=N relative to X | m.p. (˚C) | color | time(h) | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3a | CH2 | H | H | H | H | H | 3,3΄ | >260 | ochre | 17 | 77 |
| 3b | CH2 | H | H | H | H | H | 3,4΄ | 248-250 | light ochre | 20 | 70 |
| 3c | CH2 | H | H | H | H | F | 4,4΄ | >260 | ochre | 0.5 | 99 |
| 3d | CH2 | H | H | H | H | F | 3,3΄ | >260 | light brown | 5 | 68 |
| 3e | CH2 | Cl | Et | Et | H | H | 4,4΄ | >260 | orange-yellow | 15 | 74 |
| 3f | CH2 | H | H | H | Bn | H | 4,4΄ | 180-182 | orange | 8 | 98 |
| 3g | CH2 | H | H | H | Bn | H | 3,3΄ | 204-206 | oval-yellow | 3 | 82 |
| 3h | O | H | H | H | H | H | 3,4΄ | >260 | canary-yellow | 28 | 91 |
| 3i | O | H | H | H | H | F | 4,4΄ | >260 | brown-yellow | 0.5 | 87 |
| 3j | O | H | H | H | Bn | H | 4,4΄ | 204-206 | dark orange | 1 | 88 |
| 3k | CO | H | H | H | H | H | 4,4΄ | 242-244 | yellow | 0.5 | 70 |
| 3l | - | - | - | - | - | - | - | >260 | brick-red | 19 | 80 |
Derivatives 3a-3l were evaluated for their in vitro biological properties against several human pathogens [28] (Table 2).
Table 2.
Antimicrobial activities of derivatives 3a-3l.
| Sample CIP | Antimicrobial activity (MIC) (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| S. cerevisiae (28383)a | S. aureus (4.83) | C. albicans (1180-79) | E. coli (54127) | |||||
| 3a | <50 | <50 | <50 | <50 | ||||
| 3b | <50 | <50 | <50 | <50 | ||||
| 3c | <50 | <50 | <50 | <50 | ||||
| 3d | <50 | <50 | <50 | <50 | ||||
| 3e | <50 | <50 | <50 | <50 | ||||
| 3f | <50 | <50 | <50 | <50 | ||||
| 3g | <50 | <50 | <50 | <50 | ||||
| 3h | <50 | <50 | <50 | <50 | ||||
| 3i | <50 | <50 | <50 | <50 | ||||
| 3j | <50 | <50 | <50 | <50 | ||||
| 3k | <50 | <50 | <50 | <50 | ||||
| 3l | <100 | <100 | <100 | <100 | ||||
a ATCC Number
The compounds were also evaluated for their cytotoxicity (Table 3) and antiviral activity (Table 4) in human embryonic lung (HEL) and human epithelial (HeLa) cells and African green monkey kidney (Vero) cells, according to well-established procedures [29,30,31]. Derivatives 3a-3l were found to possess no antifungal activities against S. cerevisiae (ATCC 28383) and C. albicans (CIP 1180-79). Moreover, no antibacterial activities against Gram-positive and Gram-negative bacteria were noted, as is shown in Table 2. The minimum cytotoxic concentration of the compounds varied from 8 µg/mL to ≥ 400 µg/mL, compounds 3c and 3i being the most cytotoxic (at 8 and 16 µg/mL) in HEL cells (Table 3).
Table 3.
The minimum cytotoxic concentration of compounds 3a-3l.
| compound | Minimum cytotoxic concentration(µg/mL)* | ||
|---|---|---|---|
| HEL | Vero | HeLa | |
| 3a | 80 | 80 | ≥16 |
| 3b | 80 | 80 | ≥16 |
| 3c | 16 | ≥16 | 80 |
| 3d | 80 | 80 | 80 |
| 3e | 80 | 16 | 16 |
| 3f | 40 | 40 | 200 |
| 3g | 200 | 200 | 200 |
| 3h | 400 | 400 | ≥16 |
| 3i | 8 | 40 | ≥16 |
| 3j | 400 | ≥80 | 80 |
| 3k | 80 | ≥16 | 80 |
| 3l | 400 | 400 | ≥400 |
| Brivudin | >500 | 500 | >500 |
| Ribavirin | >500 | >500 | >500 |
| Acyclovir | >500 | - | - |
| Ganciclovir | >100 | - | - |
| (S)-DHPA | - | 500 | >500 |
Table 4.
Antiviral activity of compounds 3a-3l.
| Compound | Minimum virus-inhibitory concentration* (µg/mL) | ||||||
| HEL | |||||||
| Herpes simplex virus-1(KOS) | Herpes simplex virus-2(G) | Vaccinia virus | Vesicular stomatitis virus | Herpes simplex virus-1 KOS ACVr (TK-) | |||
| 3a | >16 | >16 | >16 | >16 | >16 | ||
| 3b | >16 | >16 | >16 | >16 | >16 | ||
| 3c | >3.2 | >3.2 | >3.2 | >3.2 | >3.2 | ||
| 3d | >16 | >16 | >16 | >16 | >16 | ||
| 3e | >16 | >16 | 9.6 | >16 | >16 | ||
| 3f | >8 | >8 | >8 | >8 | >8 | ||
| 3g | >40 | >40 | >40 | >40 | >40 | ||
| 3h | >80 | >80 | >80 | >80 | >80 | ||
| 3i | >1.6 | >1.6 | >1.6 | >1.6 | >1.6 | ||
| 3j | >80 | >80 | >80 | >80 | >80 | ||
| 3k | >16 | >16 | >16 | >16 | >16 | ||
| 3l | >80 | >80 | >80 | >80 | 16 | ||
| Brivudin | 0.16 | >500 | 60 | >500 | 500 | ||
| Ribavirin | 500 | >500 | 300 | >500 | >500 | ||
| Acyclovir | 2.4 | 20 | >500 | >500 | 300 | ||
| Ganciclovir | 0.48 | 4 | >100 | >100 | 12 | ||
| (S)-DHPA | - | - | - | - | - | ||
| Compound | Vero | ||||||
| Para-influenza-3 virus | Reovirus-1 | Sindbis virus | Coxsackie virus B4 | PuntaToro virus | |||
| 3a | >16 | >16 | >16 | >16 | >16 | ||
| 3b | >16 | >16 | >16 | >16 | >16 | ||
| 3c | ≥16 | >16 | >16 | >16 | >16 | ||
| 3d | >16 | >16 | >16 | >16 | >16 | ||
| 3e | >3.2 | >3.2 | >3.2 | >3.2 | >3.2 | ||
| 3f | >8 | >8 | >8 | >8 | >8 | ||
| 3g | >40 | >40 | >40 | >40 | >40 | ||
| 3h | >80 | >80 | >80 | >80 | >80 | ||
| 3i | >8 | >8 | >8 | >8 | >8 | ||
| 3j | >80 | >80 | >80 | >80 | >80 | ||
| 3k | >16 | >16 | >16 | >16 | >16(80) | ||
| 3l | >80 | >80 | >80 | >80 | >80 | ||
| Brivudin | >100 | >100 | >100 | >100 | >100 | ||
| Ribavirin | 300 | 300 | 300 | >500 | 60 | ||
| Acyclovir | - | - | - | - | - | ||
| Ganciclovir | - | - | - | - | - | ||
| (S)-DHPA | >100 | 300 | >100 | >100 | |||
| Compound | Minimum virus-inhibitory concentration* (µg/mL) | ||||||
| HeLa | |||||||
| Vesicular stomatitis virus | Coxsackie virus B4 | Respiratory syncytial virus | |||||
| 3a | 9.6 | >16 | >16 | ||||
| 3b | 3.2 | >16 | >16 | ||||
| 3c | >16 | >16 | >16 | ||||
| 3d | 9.6 | >16 | >16 | ||||
| 3e | >3.2 | >3.2 | >3.2 | ||||
| 3f | >40 | >40 | >40 | ||||
| 3g | >40 | >40 | >40 | ||||
| 3h | >16 | >16 | >16 | ||||
| 3i | >16 | >16 | >16 | ||||
| 3j | >16 | >16 | >16 | ||||
| 3k | 48 | >16 | >16 | ||||
| 3l | >400 | >400 | >400 | ||||
| Brivudin | >500 | >500 | >500 | ||||
| Ribavirin | 60 | >500 | 60 | ||||
| Acyclovir | - | - | - | ||||
| Ganciclovir | - | - | - | ||||
| (S)-DHPA | 500 | >500 | >500 | ||||
*Required to reduce virus- induced cytopathogenicity by 50%.
The results of antiviral activity of the new Schiff bases of isatin and 5-flouroisatin are shown in Table 4. None of the compounds exhibited specific antiviral activity, which means that they did not inhibit the replication (induction of viral cytopathogenicity) of any of the viruses tested at a concentration that was ≥ 5-fold lower than the minimum cytotoxic concentration.
Experimental
General
Chemical materials and solvents were obtained from Merck, Fluka and Aldrich chemical companies. Melting points were determined in open capillary tubes in Buchi 530 circulating oil apparatus and are not corrected. FT-IR spectra (KBr) were run on a Shimadzu FTIR-8300 spectrophotometer. NMR spectra were recorded on a Bruker Avance DPX-250 spectrometer (1H-NMR 250 MHz, 13C-NMR 62.9 MHz) in CDCl3 or DMSO-d6 solvents using TMS as an internal standard. Mass spectra were obtained on a Shimadzu GCMS-QP 1000 EX instrument at 70 ev. The determination of the prepared products and reaction monitoring were carried out on silica gel 254 analytical sheets obtained from Fluka. Column chromatography was carried out by silica gel 60 Merck (230-270).
General procedure for preparation of bis-Schiff bases of isatin and its derivatives
Isatin, benzylisatin [27] or 5-fluoroisatin (13.6 mmol) and the aromatic diamines (6.8 mmol) were dissolved in warm ethanol (20 mL) containing glacial acetic acid (0.45 mL). The reaction mixture was refluxed for several hours. After standing at room temperature, the resulting solid was separated by filtration and vacuum dried.
3,3´-[Methylenebis(3,1-phenylenenitrilo))bis[1,3-dihydro)-2H-indol-2-one (3a): IR (cm-1): 1652 (C=N), 1726 (C=O), 3168 (N-H); 1H-NMR (DMSO-d6) δ (ppm): 4.07 (2H, s, CH2), 6.29-7.43 (16H, m, ArH), 10.96 (2H, s, N-H); 13C-NMR (DMSO-d6) δ (ppm): 44.40 (CH2), 115.63-155.53 (aromatic carbons), 159.76 (C=N), 168.29 (C=O); MS (m/z, %): 458 (12.5), 457 (34.9), 456 (100.0), 327 (64.2), 312 (39.2), 299 (53.5), 284 (29.9), 283 (27.1), 200 (18.5), 180 (29.9), 165 (48.5), 44 (15.7).
3,3´-[Methylenebis[(3,1)-(4´,1´)phenylenenitrilo))bis[1,3-dihydro)-2H-indol-2-one (3b): IR (cm-1): 1612 (C=N), 1732 (C=O), 3213 (N-H); 1H-NMR (DMSO-d6) δ (ppm): 4.02 (2H, s, CH2), 6.37-7.56 (16H, m, ArH), 10.97 (2H, s, N-H); 13 C-NMR (DMSO-d6) δ (ppm): 40.74 (CH2), 111.84-151.01 (aromatic carbons), 155.31(C=N), 163.85 (C=O); MS (m/z, %): 456 (23.9), 326 (6.0), 312 (28.5), 299 (100.0), 198 (20.2), 182 (11.4), 180 (50.9), 166 (21.7), 44 (27.5).
3,3´-[Methylenebis(4,1-phenylenenitrilo))bis[1,4-dihydro)-5-fluoro-2H-indol-2-one (3c): IR (cm-1): 1618 (C=N), 1739 (C=O), 3261 (N-H); 1H NMR (DMSO-d6) δ (ppm): 4.03 (2H, s, CH2), 6.09-7.41 (14H, m, ArH), 11.01 (2H, s, N-H); 13C-NMR (DMSO-d6) δ (ppm): 44.30 (CH2), 113.23-153.68 (aromatic carbons), 159.16 (C=N), 164.38 (C=O); MS (m/z, %): 492 (1.4), 467 (2.8), 439 (2.1), 368 (52.8), 339 (10.0), 313 (22.8), 236 (28.5), 83 (50.7), 57 (100.0).
3,3´-[Methylenebis(3,1-phenylenenitrilo))bis[1,3-dihydro)-5-fluoro-2H-indol-2-one (3d): IR (cm-1): 1622 (C=N), 1733 (C=O), 3290 (N-H); 1H-NMR (DMSO-d6) δ (ppm): 4.06 (2H, s, CH2), 6.78-7.48 (14H, m, ArH), 10.86 (2H, s, N-H); 13C-NMR (DMSO-d6) δ (ppm): 40.50 (CH2), 112.02-150.67 (aromatic carbons), 150.67 (C=N), 163.73 (C=O); MS (m/z, %): 493 (1.4), 492 (0.7), 368 (7.1), 313 (10.7), 178 (22.1), 147 (22.1), 91 (24.2), 43 (100.0).
3,3´-[Methylenebis(2-chloro-3,5-diethyl-4,1-phenylenenitrilo))bis[1,3-dihydro)-2H-indol-2-one (3e): IR (cm-1): 1614 (C=N), 1735 (C=O), 3247 (N-H); 1H-NMR (DMSO-d6) δ (ppm): 0.86-1.05 (12H, t, 4CH3), 4.19 (CH2), 6.66-7.70 (10H, m, ArH), 10.98 (2H, s, N-H); 13C-NMR (DMSO-d6) δ (ppm): 13.21-13.73 (2CH3), 23.02-23.77 (2CH2), 37.50 (CH2), 112.10- 147.13 (aromatic carbons), 156.83 (C=N), 163.42 (C=O); MS (m/z, %): 637 (0.7), 603 (2.1), 577 (4.2), 555 (9.2), 368 (34.2), 339 (7.8), 313 (15.0), 236 (15.0), 97 (34.2), 96 (56.4), 43 (100.0).
3,3´-[Methylenebis(4,1-phenylenenitrilo))bis [1,3-dihydro)-1-phenylmethylene-2H-indol-2-one (3f): IR (cm-1): 1604 (C=N), 1732 (C=O); 1H-NMR (CDCl3) δ (ppm): 4.06 (2H, s, CH2), 5.00 (4H, s, CH2), 6.41-7.70 (26H, m, ArH); 13C-NMR (DMSO-d6) δ (ppm): 44.40 (CH2), 110.04-148.83 (aromatic carbons), 154.57 (C=N), 163.78 (C=O); MS (m/z, %): 636 (3.5), 442 (2.8), 417 (100.0), 388 (12.8), 326 (27.8), 207 (20.0), 180 (17.8), 106 (37.8), 91 (35.7), 43 (13.5).
3,3´-[Methylenebis(3,1-phenylenenitrilo))bis[1,3-dihydro)-1-phenylmethylene-2H-indol-2-one (3g): IR (cm-1): 1604 (C=N), 1732 (C=O); 1H-NMR (CDCl3) δ (ppm): 4.01 (2H, s, CH2), 4.96 (4H, s, CH2), 6.53-7.67 (26H, m, ArH); 13C-NMR (CDCl3) δ (ppm): 44.36 (CH2), 110.06-150.90 (aromatic carbons), 154.58 (C=N), 163.67 (C=O); MS (m/z, %): 636 (1.4), 417 (100.0), 326 (32.1), 285 (14.2), 198 (40.0), 180 (43.5), 165 (27.1), 106 (17.1), 91 (35.0), 44 (5.0).
3,3´-[Oxybis[(3,1)-(4´,1´)phenylenenitrilo))bis[1,3-dihydro)-2H-indol-2-one (3h): IR (cm-1): 1620 (C=N), 1733 (C=O), 3195 (N-H). 1H NMR (DMSO-d6) δ (ppm): 6.16-7.77 (16H, m, ArH), 10.94 (2H, s, N-H); 13C NMR (DMSO-d6) δ (ppm): 111.27-155.49 (aromatic carbons), 158.91 (C=N), 163.69 (C=O); MS (m/z, %): 458 (3.8), 330 (14.6), 329 (56.3), 302 (17.9), 301 (48.8), 234 (10.8), 200 (44.4), 171 (14.3), 128 (18.3), 108 (53.2), 92 (33.8), 66 (100.0), 44 (53.9).
3,3´-[Oxybis(4,1-phenylenenitrilo))bis[1,3-dihydro)-5-fluoro-2H-indol-2-one (3i): IR (cm-1): 1618 (C=N), 1739 (C=O), 3261 (N-H); 1H-NMR (DMSO-d6) δ (ppm): 6.25-7.43 (14H, m, ArH), 11.07 (2H, s, N-H); 13C-NMR (DMSO-d6) δ (ppm): 111.27-154.00 (aromatic carbons), 155.41 (C=N), 163.66 (C=O); MS (m/z, %): 495 (2.8), 494 (2.1), 426 (22.1), 396 (9.2), 368 (14.2), 313 (31.4), 264 (22.1), 236 (28.5), 168 (30.0), 97 (43.5), 69 (65.0), 43 (100.0).
3,3´-[Oxybis(4,1-phenylenenitrilo))bis[1,4-dihydro)-1-phenylmethylene-2H-indol-2-one (3j): IR (cm-1): 1604 (C=N), 1732 (C=O); 1H-NMR (CDCl3) δ (ppm): 5.01 (4H, s, CH2), 6.72-7.71 (26H, m, ArH); 13C-NMR (CDCl3) δ (ppm): 44.42 (CH2), 110.03-154.65 (aromatic carbons), 155.33 (C=N), 163.76 (C=O); MS (m/z, %): 638 (0.7), 577 (3.5), 551 (5.0), 419 (100.0), 328 (20.0), 288 (6.4), 237 (12.8), 207 (20.7), 180 (7.1), 146 (7.1), 91 (27.1), 43 (26.4).
3,3´-[Methanonebis(4,1-phenylenenitrilo))bis[1,4-dihydro)-2H-indol-2-one (3k): IR (cm-1): 1593 (C=N), 1735 (C=O, amide), 1660 (C=O, ketone), 3222 (N-H); 1H-NMR (DMSO-d6) δ (ppm): 6.25-7.58 (16H, m, ArH), 10.99 (2H, s, N-H); 13C-NMR (DMSO-d6) δ (ppm): 119.74-134.76 (aromatic carbons), 164.20 (C=N), 197.53 (C=O); MS (m/z, %): 470 (5.3), 341 (27.7), 313 (35.4), 221 (14.6), 212 (7.8), 180 (4.3), 120 (98.5), 104 (14.6), 92 (36.1), 76 (30.8), 44 (100.0), 43 (99.0).
3,3΄-[(2-Methyl-1,3-phenylene)dinitrilo)bis[1,3-dihydro)-2H-indole-2-one (3l): IR (cm-1): 1616 (C=N), 1716 (C=O), 3209 (N-H); 1H-NMR (DMSO-d6) δ (ppm): 2.50 (3H, s, CH3), 6.57-7.06(11H, m, ArH), 10.42 (1H, s, N-H); 13C-NMR (DMSO-d6) δ (ppm): 18.88 (CH3), 117.84-152.52 (aromatic carbons), 163.35 (C=N), 167.51 (C=O); MS (m/z, %):380 (0.7), 368 (13.5), 339 (2.8), 313 (8.5), 285 (5.0), 264 (5.0), 236 (11.4), 129 (9.2), 97 (28.5), 69 (42.8), 43 (100.0).
Acknowledgements
We gratefully acknowledge the financial support of this study by Shiraz University Research Council (85-GR-SC-23).
Footnotes
Sample availability: Contact the authors.
References
- 1.Somogyi L. Transformation of Isatin 3-acylhydrazones under acetylating conditions: Synthesis and structure elucidation of 1,5′-disubstituted 3′-acetylspiro[oxindole-3,2′-[1,3,4]oxadiazolines] Bull. Chem. Soc. Jpn. 2001;74:873–881. [Google Scholar]
- 2.Da Silva J. F. M., Garden S. J., Pinto A. C. The Chemistry of Isatins: a Review from 1975 to 1999. J. Braz. Chem. Soc. 2001;12(3):273–324. [Google Scholar]
- 3.Elliott J., Gardner D. L. Proline determination with isatin, in the presence of amino acids. Anal. Biochem. 1976;70:268–273. doi: 10.1016/S0003-2697(76)80068-1. [DOI] [PubMed] [Google Scholar]
- 4.Palfi G., Palfi Z. Determination of viality of pollen on the basis of its amino acids content. Mydica. 1982;27:107. [Google Scholar]
- 5.Eriksen A. B. Quantitative determination of the amino acid proline by glass fiber paper chromatography. Medd. Nor. Inst. Skogforsk. 1976;32:389–404. [Google Scholar]
- 6.Shah A., Rahman S. S., de Biasi V., Camilleri P. Development of Colorimetric Method for the Detection of Amines Bound to Solid Support. Anal. Commun. 1997;34(11):325–328. [Google Scholar]
- 7.Chiyanzu I., Clarkson C., Smith P. J., Lehman J., Gut J., Rosenthal P. J., Chibale K. Design, synthesis and anti-plasmodial evaluation in vitro of new 4-aminoquinoline isatin derivatives. Bioorg. Med. Chem. 2005;13:3249–3261. doi: 10.1016/j.bmc.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 8.Pandeya S. N., Sriram D. Synthesis and screening for antibacterial activity of Schiff’s and Mannich bases of Isatin and its derivatives. Acta. Pharm. Turc. 1998;40:33–38. [Google Scholar]
- 9.Sarangapani M., Reddy V. M. Synthesis and antimicrobial activity of 1-[(N,N-disubstituted amino)methyl]-3-[(2-phenyl-3,4-dihydro-4-oxoquinazoline-3-yl]indole-2-one. Indian J. Hetero-cycl. Chem. 1994;3:257–260. [Google Scholar]
- 10.Varma R. S., Nobles W. L. Antiviral, antibacterial, and antifungal activities of isatin N-Mannich bases. J. Pharm. Sci. 1975;64:881–882. doi: 10.1002/jps.2600640539. [DOI] [PubMed] [Google Scholar]
- 11.Sridhar S. K., Pandeya S. N., Stables J. P., Ramesh A. The Wide Pharmacological Versatility of Semicarbazones, Thiosemicarbazones and Their Metal Complexes. Eur. J. Med. Chem. 2002;16:129–132. [Google Scholar]
- 12.Varma M., Pandeya S, N., Singh K. N., Stables J. P. Anticonvulsant activity of Schiff bases of isatin derivatives. Acta Pharm. 2004;54:49–56. [PubMed] [Google Scholar]
- 13.Pandeya S. N., Yogeeswari P., Sriram D., De Clercq E., Pannecouque C., Witvrouw M. Synthesis and Screening for Anti-HIV Activity of Some N-Mannich Bases of Isatin Derivatives. Chemotherapy. 1999;45:192–196. doi: 10.1159/000007182. [DOI] [PubMed] [Google Scholar]
- 14.Pandeya S. N., Sriram D., Nath G., De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV activities of Norfloxacin Mannich bases. Eur. J. Med. Chem. 2000;35:249–255. doi: 10.1016/s0223-5234(00)00125-2. [DOI] [PubMed] [Google Scholar]
- 15.Pandeya S. N., Sriram D., Nath G., De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin and its derivatives with triazole. Arzneim Forsch./Drug Res. 2000;50:55–59. doi: 10.1055/s-0031-1300164. [DOI] [PubMed] [Google Scholar]
- 16.Pandeya S. N., Yogeeswari P., Sriram D., Nath G. Synthesis and antimicrobial activity of N-Mannich bases of 3-[N′-sulphadooximino]isatin and its methyl derivatives. Bull. Chim. Farm. 1998;137:321–324. [PubMed] [Google Scholar]
- 17.Pandeya S. N., Sriram D., Nath G., De Clercq E. Synthesis and antimicrobial activity of Schiff and Mannich bases of isatin and its derivatives with pyrimidine. Farmaco. 1999;54:624–628. doi: 10.1016/s0014-827x(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 18.Pandeya S. N., Sriram D., Nath G., De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV activity of Schiff and Mannich bases of isatin with N-[6-chlorobenzthiazole-2-yl] thiosemicarbazid. Indian J. Pharm. Sci. 1999;61:358–361. [Google Scholar]
- 19.Pandeya S. N., Sriram D., Nath G., De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methylmercapto quinazolin-4(3H)-one. Pharm. Acta Helv. 1999;74:11–17. doi: 10.1016/S0031-6865(99)00010-2. [DOI] [PubMed] [Google Scholar]
- 20.Pandeya S. N., Sriram D., Nath G., De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(4′-chlorophenyl)thiazol-2-yl] thiosemicarbazide. Eur. J. Pharm. Sci. 1999;9:25–31. doi: 10.1016/s0928-0987(99)00038-x. [DOI] [PubMed] [Google Scholar]
- 21.Singh S. P., Shukla S. K., Awasthi L. P. Synthesis of some 3-(4′-nitrobenzoylhydrazono)-2-indolinones as a potential antiviral agents. Curr. Sci. 1983;52:766–769. [Google Scholar]
- 22.Marcu G. Chimica complecsilor coordinative. Ed. Academiei Bucuresti; Bucarest: 1984. pp. 44–73. [Google Scholar]
- 23.Cerchiaro G., Micke G. A., Tavares M. F. M., Ferriera A. M. D. C. Kinetic studies of carbohydrate oxidation catalyzed by novel isatin–Schiff base copper(II) complexes. J. Mol. Catal. A: Chem. 2004;221:29–39. doi: 10.1016/j.molcata.2004.06.017. [DOI] [Google Scholar]
- 24.Takeuchi T., Bottcher A., Quezada C. M., Simon M. I., Meade T. J., Gray H. B. Selective Inhibition of Human -Thrombin by Cobalt(III) Schiff Base Complexes. J. Am. Chem. Soc. 1998;120:8555–8556. [Google Scholar]
- 25.Bacchi A., Carcelli M., Pelagatti P., Pelizzi G., Rodriguez-Arguelles M. C., Rogolino D., Solinas C., Zani F. Antimicrobial and mutagenic properties of organotin(IV) complexes with isatin and N-alkylisatin bisthiocarbonohydrazones. J. Inorg. Biochem. 2005;99:397–408. doi: 10.1016/j.jinorgbio.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Cerchiaro G., Aquilano K., Filomeni G., Rotilio G., Ciriolo M. R., Ferriera A. M. D. C. Isatin-Schiff base copper(II) complexes and their influence on cellular viability. J. Inorg. Biochem. 2005;99:1433–1440. doi: 10.1016/j.jinorgbio.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Garden S. J., Torres J. C., da Silva L. E., Pinto A. C. A convenient methodlogy for the N-alkylation of isatin compounds. Synth. Commun. 1998;28:1679–1689. [Google Scholar]
- 28.Dei Cas E., Dujardin L., Ribeiro Pinto M. E., Ajana F., Fruit J., Poulain D., Camus D., Vernes A., Francois N. Growth was measured in vitro using a liquid-phase method according to NCCLS guidelines from the American Society of Microbiology for 24 hours using various concentrations of drugs. Mycoses. 1991;34:167–172. [Google Scholar]
- 29.De Clercq E., Descamps J., Verhelst G., Walker R.T., Jones A.S., Torrence P. F., Shugar D. Relative potencies of different anti-herpes agents in the topical treatment of cutaneous herpes simples virus infection of athymic nude mice. J. Infect. Dis. 1980;141:563–574. [Google Scholar]
- 30.De Clercq E. Antiviral and antimetabolic activities of neplanocins. Antimicrob. Agents Chemother. 1985;28:84–89. doi: 10.1128/aac.28.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Clercq E., Holý A., Rosenberg I., Sakuma T., Balzarini J., Maudgal P.C. A novel selective broad-spectrum anti-DNA virus agent. Nature. 1986;323:464–467. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]