Abstract
Chemical evaluation of the semi-purified fraction from the seeds of guaraná, Paullinia cupana H.B.K. var. sorbilis (Mart.) Ducke, yielded the following compounds: caffeine, catechin, epicatechin, ent-epicatechin, and procyanidins B1, B2, B3, B4, A2, and C1. Measurement of the antioxidant activity by reduction of the DPPH radical confirmed the anti-radical properties of the aqueous (AqE) and crude (EBPC) extracts and semi-purified (EPA and EPB) fractions. The EPA fraction showed radical-scavenging activity (RSA) and protected DPPH from discoloration at 5.23±0.08 (RSD%=1.49) µg/mL, and for the phosphomolybdenum complex showed a higher Relative Antioxidant Capacity (RAC) at 0.75±0.01 (1.75). The EPA fraction had a total polyphenolics content of 65.80%±0.62 (RSD%=0.93). The plant drug showed 5.47% ± 0.19 (RSD%=3.51) and 6.19% ± 0.08 (RSD%=1.29) for total polyphenolics and methylxanthines, respectively. In vitro assessment of the antibacterial potential of the Paullinia cupana extracts against Streptococcus mutans showed that these could be used in the prevention of bacterial dental plaque.
Keywords: Paullinia cupana, methylxanthines, condensed tannins, antioxidant capacity, prevention of the formation of dental plaque
Introduction
Paullinia cupana H.B.K. var. sorbilis (Mart.) Ducke is a plant of the family Sapindaceae, native to the central Amazon basin and known as guaraná. Its seeds are used traditionally by the indigenous people as a stimulant drink, for festivals and hunting. They are used mainly as stimulants and contain high amounts of methylxanthines and tannins. Crude and semipurified extracts of guaraná seeds show an antidepressant action, including after a long period of treatment, differently from caffeine [1,2]. The tannin content can be influenced by the location where the plants were grown and the manner of drying the seeds [3,4]. One important characteristic of tannins is their antioxidant potential [3]. It has been shown that guaraná inhibits the lipid peroxidation process, an effect apparently associated with the high tannin content of the seeds [6,7]. In addition, enzymes such as glucosyltransferase, produced by Streptococcus mutans and S. sobrinus, are inhibited in vitro by tannins, preventing the formation of dental plaque and inflammations of the mouth and throat [8,9]. Aqueous extracts produced from guaraná seeds at 5 and 7.5%, applied via a mouthwash, were efficient in inhibiting the formation of bacterial dental plaque in vivo [10].
This article describes monthly monitoring of the tannin content of guaraná seeds, and the isolation of condensed tannins from this species, in addition to others isolated in a previous study [11]. In vitro biological assays were performed to determine the antioxidant capacity and analyze in preliminary fashion the antibacterial potential against Streptococcus mutans of the different extracts (crude-EBPC and aqueous-AqE), and semi-purified fractions (EPA and EPB).
Results and Discussion
The assessment of the quality of the P. cupana seeds established the minimum conditions for the plant drug, demostrating the quality and equivalence in contents of characteristic chemical substances (methylxanthines and tannins). The total content of tannins was 5.47% ± 0.19 (Relative Standard Deviation - RSD%=3.51), and of methylxanthines 6.19% ± 0.08 (RSD%=1.29). Monthly assessment of the total tannin content of guaraná seeds stored whole and ground immediately prior to analysis, and of seeds stored in ground form in cardboard barrels demonstrated that the method of storage did not statistically change the tannin content, which remained stable over a 24-month period (data not shown).
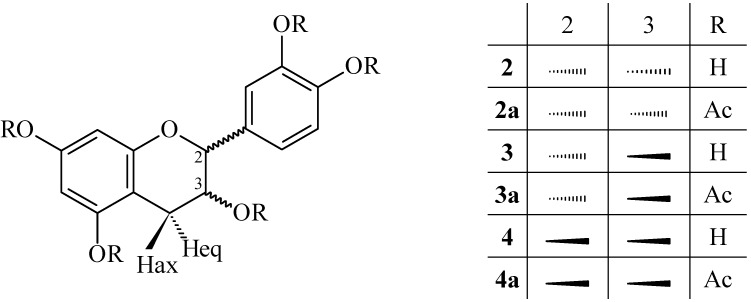
Chemical assay of the ethyl acetate fraction yielded compounds 1-10. Compounds 1, 2, and 3 were identified as caffeine, epicatechin, and catechin, respectively, by comparison of spectroscopic data (1H- and 13C NMR, COSY, mass spectrometry-ESI-MS, 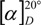 ) with authentic material and literature values [11]. Compound 4 (Figure 1) was obtained as a pale yellow, amorphous powder. The 1H-NMR spectrum of acetate 4a showed no difference from compound 3a (epicatechin pentaacetate). The heterocyclic protons displayed an ABMX-system characteristic for the spin pattern of 2,3-cis-flavan-3-ols (J2,3<2.0 Hz) [12,13]. An optical rotation
) with authentic material and literature values [11]. Compound 4 (Figure 1) was obtained as a pale yellow, amorphous powder. The 1H-NMR spectrum of acetate 4a showed no difference from compound 3a (epicatechin pentaacetate). The heterocyclic protons displayed an ABMX-system characteristic for the spin pattern of 2,3-cis-flavan-3-ols (J2,3<2.0 Hz) [12,13]. An optical rotation 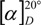 of +6° (methanol; c 0.5) verified the 2S,3S absolute configuration of 4a. The ESI mass spectrum showed a prominent peak [M+Na]+at m/z 523.4 and [M-H]- at m/z 499.4, indicating the molecular formula C25H24O11; therefore, 4 was identified as ent-epicatechin. A recent report described the analysis of samples of guaraná by capillary electrophoresis with a natural quiral selector (cyclodextrin), and demonstrated the presence of compounds 2R catechin and epicatechin and their chiral forms 2S (ent-catechin and ent-epicatechin) [14].
of +6° (methanol; c 0.5) verified the 2S,3S absolute configuration of 4a. The ESI mass spectrum showed a prominent peak [M+Na]+at m/z 523.4 and [M-H]- at m/z 499.4, indicating the molecular formula C25H24O11; therefore, 4 was identified as ent-epicatechin. A recent report described the analysis of samples of guaraná by capillary electrophoresis with a natural quiral selector (cyclodextrin), and demonstrated the presence of compounds 2R catechin and epicatechin and their chiral forms 2S (ent-catechin and ent-epicatechin) [14].
Figure 1.
Flavan-3-ols identified from seeds of Paullinia cupana. Epicatechin (2), catechin (3), and ent-epicatechin (4).
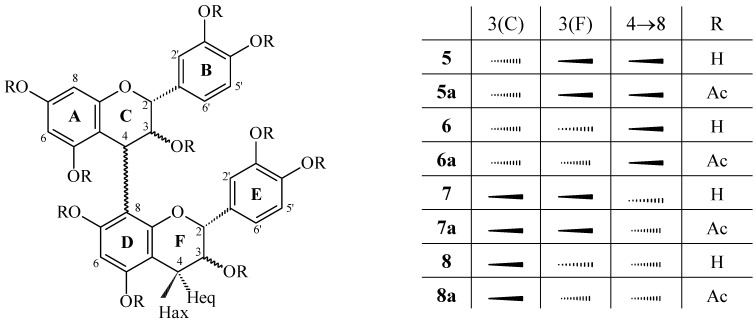
Procyanidins 6, 7, and 8 were identified as epicatechin-(4β→8)-epicatechin (procyanidin B2), catechin-(4α→8)-catechin (procyanidin B3), and catechin-(4α→8)-epicatechin (procyanidin B4), respectively, by comparison of the physical and spectroscopic data (1H-NMR, 1H-1H COSY, 13C-, HMQC, HMBC, mass spectrometry-ESI-MS, 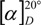 , CD) of their corresponding peracetates 6a, 7a, and 8a with recently published data [11].
, CD) of their corresponding peracetates 6a, 7a, and 8a with recently published data [11].
Identification of epicatechin-(4β→8)-catechin (5), characterized several times in the literature in the underived phenolic form [15,16,17,18], was effected by comparison of the 1H-NMR spectral data of its peracetate 5a with those of the analogous derivative 6a. The heterocyclic coupling constant (J2,3 < 2.0) confirmed a relative 2,3-cis configuration of the ‘upper’ constituent unit [12,13], whereas the 2,3-trans stereochemistry of the ‘lower’ flavan unit was indicated by the large coupling constant (J2,3 = 9,6 Hz) of the corresponding resonances. The aromatic region (δ 6.87 and 7.30 ppm) showed two AMX-systems, confirming dihydroxylation on the B- and E-rings. The proton signals of H-6(A), H-8(A) and H-6(D) at δ 5.99, 6.29 and 6.68 ppm, respectively, as well as the position of the H-2(F) resonance at δ 4.33 ppm indicated a (4→8)-linked dimeric peracetate proanthocyanidin [19,20,21,22,23,24], and this was confirmed by HMBC. The absolute configuration was assigned by optical rotation 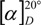 +2° (methanol; c 1.0), and in combination with the positive Cotton effect in the 220-240 nm region on the CD spectrum of 5a, the identity of 5 (Figure 2) was established as epicatechin-(4β→8)-catechin (procyanidin B1). The proposed structure was supported by ESI mass spectrum data, which showed a prominent [M+Na+]+ peak at m/z 1021.5 and [M-H+]-
m/z 997.5.
+2° (methanol; c 1.0), and in combination with the positive Cotton effect in the 220-240 nm region on the CD spectrum of 5a, the identity of 5 (Figure 2) was established as epicatechin-(4β→8)-catechin (procyanidin B1). The proposed structure was supported by ESI mass spectrum data, which showed a prominent [M+Na+]+ peak at m/z 1021.5 and [M-H+]-
m/z 997.5.
Figure 2.
Dimeric procyanidins isolated from Paullinia cupana. Procyanidins B1 (5), B2 (6), B3 (7), and B4 (8).
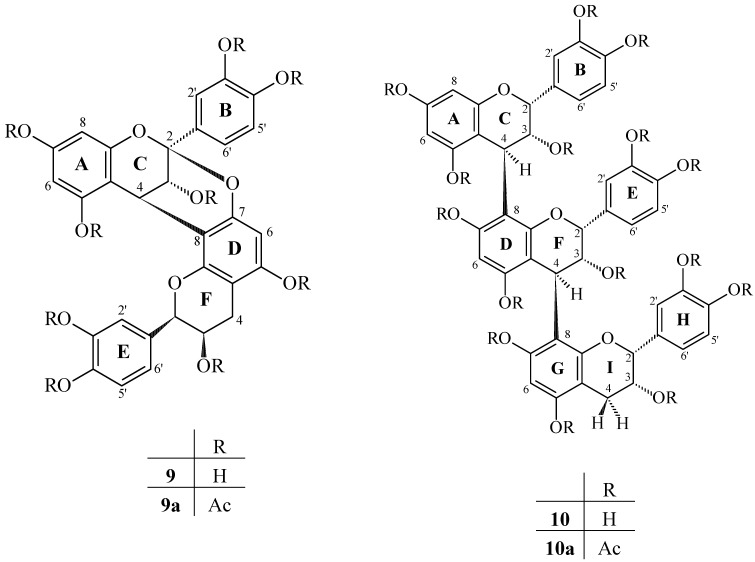
Compound 9 was obtained as a pale yellow, amorphous powder. The positive and negative mass spectral mode (ESI-MS) of peracetate compound 9 showed a [M+Na]+ ion at m/z 977.4 and a [M-H+]- at m/z 953.5, corresponding to a biflavonoid structure (C48H42O21). These spectra showed 42 and 1 mass units fewer than procyanidin B2, respectively, providing initial evidence that it was a doubly linked procyanidin dimer of the A-type. The integration of the 1H-NMR spectrum showed the presence of 15 protons, all magnetically nonequivalent (excluding the protons of hydroxyl groups), confirming the above observation. A diagnostic feature of the 1H-NMR spectrum of compound 9a was the presence of an isolated AB system in the heterocyclic region [δ 5.05 and 4.60, H-3(C) and H-4(C), J3,4=4.2 Hz, respectively], characteristic of the C ring protons of an A-type proanthocyanidin [25]. This suggested the existence of a double linkage between the C- and D-rings (4β→8;2β→O→7) [25]. Insignificant coupling for J2,3(F) established that the “lower” flavan-3-ol unit has a 2,3-cis relative configuration [19,23,24]. The aromatic region protons demonstrated a two-AMX spin system. This is in accordance with dihydroxylated B- and E-rings. Compound 9 was, also in comparison with the literature, thus determined to be epicatechin-(4β→8;2β→O→7)-epicatechin (procyanidin A2, Figure 3). In addition, a solid-state 13C-NMR spectrum analysis of the EPB fraction indicated chemical shifts in the region consistent with C5 and C7 of the phloroglucinol A-ring involved in the double linkage. The chemical shift of the ketal carbon (C2) formed as a result of this additional bond observed at 105.66 ppm provided further support for A-type procyanidin [26].
Figure 3.
– Procyanidins A2 (9) and C1 (10) from the seeds of Paullinia cupana.
Structure elucidation and assignment of proton resonances of the trimer epicatechin-(4β→8)-epicatechin-(4β→8)-epicatechin (10) were achieved by 1H-NMR (1H-1H COSY) of its peracetate 10a. The three proton resonances of the AMX-type rings at 6.65 to 7.30 (3x 3H) were assigned to the B-, E- and H-rings, by comparison with the spectra of procyanidin C1 [27]. The signals due to the heterocyclic protons of the flavanyl units were subsequently identified by the long-range couplings in the 1H-1H COSY spectra between the C-2 protons of the flavanyl units and the corresponding three proton resonances of the B-rings. All heterocyclic protons showed a small coupling constant (J2,3<2 Hz), confirming a 2,3-cis relative configuration. The high-amplitude negative Cotton effects in the 220-300 nm region of the CD spectrum indicated the configuration at both interflavan linkages to be 4S. ESI mass spectral ([M-H]- m/z 865.5 and [M+Na]+ m/z 889.4) analysis and chemical degradation studies suggested that all three units were epicatechins. These results confirm the structure of 10 to be identical to that previously described from Tilia sp. (Tiliaceae) [27].
The compounds ent-epicatechin (4) and the procyanidins B1 (5), A2 (9), and C1 (10) are reported here as natural constituents of guaraná (genus Paullinia) for the first time.
Biological activity
The results indicate that the antioxidant capacity was directly proportional to the content of polyphenols (Table 1). The semi-purified fraction (EPA) showed the highest content of total polyphenols, reflecting the antioxidant analysis, with a low IC50 and a higher RAC in relation to the other extracts. Inverse relationships were observed for the semi-purified fraction (EPB). All the samples were significantly different from vitamin C in the relative antioxidant capacity assay (RAC) (P<0.001) and in the IC50 (P<0.05 for EPA, and P<0.001 for all other samples); EPA also showed a significant difference in the EBPC (RAC, P<0.01; IC50, P<0.001). The results from the DPPH (IC50) method for the substances epicatechin-(4β→8)-catechin (PB1), epigallocatechin, catechin, and gallocatechin isolated from this and from another plant species by our research group showed values of 3.29, 8.56, 9.93, and 15.26 µM, respectively. These data show that the larger the amount of phenolic hydroxyls, the more the free-radical scavenging. Additionally, the stereochemistry of catechin and gallocatechin (2R,3S) in relation to epigallocatechin (2R,3R) may be responsible for the decrease in free-radical scavenging activity. This last condition demonstrates that trihydroxylation at the B-ring increases the power of scavenging DPPH radicals. Substances of the cinchonain Ia and Ib type, obtained from Trichilia catigua A. Juss., show that the phenylpropanoid unit significantly increases the antioxidant activity of the condensed tannins [28]. Thus, it is possible to establish correlations between the antioxidant capacity in plant extracts and the antioxidant activity of substances isolated from plant drugs containing tannins, and demonstrates the efficiency of these substances in capturing free radicals.
Table 1.
Assessment of contents of total polyphenols (TP) and antioxidant capacity by the methods of the phosphomolybdenum complex (RAC) and DPPH-radical scavenging (IC50).
| Sample | TP (%)±s.d. (RSD%) | RAC±s.d. (RSD%) | IC50 (µg/ml)±s.d. (RSD%) |
|---|---|---|---|
| AqE | 36.47±0.18 (0.51) | 0.46±0.01 (0.96) | 9.59±0.04 (0.41) |
| EBPC | 55.95±1.12 (2.01) | 0.69±0.03 (4.15)* | 6.67±0.05 (0.82) |
| EPA | 65.80±0.62 (0.93) | 0.75±0.01 (1.75)* | 5.23±0.08 (1.49)** |
| EPB | 22.1±0.37 (1.64) | 0.36±0.02 (5.20) | 14.97±0.15 (1.01) |
| Vitamin C | 1.00 | 4.93±0.05 (1.07) |
RAC = relative antioxidant capacity; IC50= amount of antioxidant necessary to decrease the concentration of the DPPH radical by 50%; s.d.= standard deviation; RSD= relative standard deviation. ANOVA, *P<0.01; **P<0.05 in relation to vitamin C.
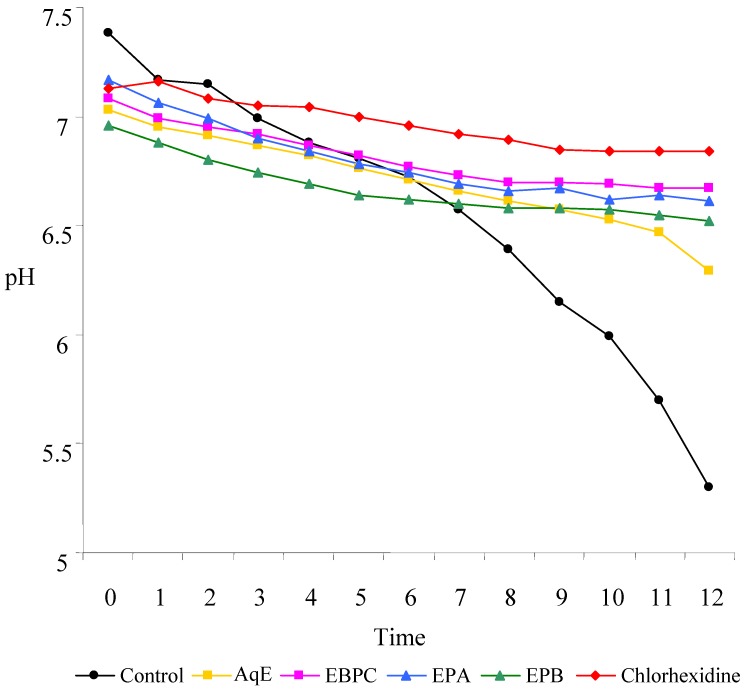
Previous results obtained by in vivo evaluation of the antibacterial potential against Streptococcus mutans suggested that extracts from P. cupana could be used to prevent the formation of dental bacterial plaque [10]. All the extracts evaluated inhibited the adherence of S. mutans on a smooth glass surface, and also reduced the production of acids, compared to a solution of chlorhexidine gluconate. In the test of adherence to glass, we used extracts with a tannin concentration of 750 μg/mL (Table 2) and a solution of chlorhexidine gluconate at 1.2 µg/mL. The EBPC gave the best result; even at a lower concentration, it showed the best action on the adherence of S. mutans, with 79.69% inhibition. The AqE extract, and semi-purified fractions (EPA and EPB) showed adherence inhibitions of 62.18%, 52.69%, and 69.95%, respectively. As these fractions did not affect the growth of S. mutans, as evaluated through determination of the minimum inhibitory concentration [29], with a MIC>5,000 μg/mL, the extracts affected the adherent action of S. mutans rather than its growth. All the samples were efficient in reducing the production of acids, with an effect comparable to the chlorhexidine gluconate solution (Figure 4). The decrease of pH was significantly inhibited in the presence of AqE, EBPC, EPA, EPB, and the positive control (chlorhexidine), showing at the onset pH 7.0, 7.1, 7.2, 7.0, and 7.1 and after 12 h of incubation pH 6.3, 6.7, 6.6, 6.5, and 6.8, respectively. On the other hand, untreated cells (negative control) showed pH 7.3 at the onset, and pH 5.3 after 12 h of incubation. This is significant, because to the extent that the process of acidification of the medium between the bacterial plaque and the outer surface of the tooth is decreased, the possibility of demineralization of this surface, an essential factor in the formation of dental caries, is also decreased.
Table 2.
Total tannins content, concentration of sample evaluated, and inhibition of bacterial adherence on a smooth surface.
| Sample | Total Tannins g (%)±sd (RSD%) |
Concentration of the sample (mg/ml) | Adherence Inhibition (%) |
|---|---|---|---|
| AqE | 16.16±0.44 (2.75) | 4.64 | 62.18±2.09 |
| EBPC | 31.15±1.46 (4.68) | 2.41 | 79.69±5.23 |
| EPA | 30.05±0.54 (1.80) | 2.50 | 52.69±17.95 |
| EPB | 17.09±0.52 (3.03) | 4.39 | 69.95±2.90 |
| Chlorhexidine | 82.88±4.28 |
Figure 4.
– Effect of the samples in reducing production of acids by Streptococcus mutans.
Experimental Section
General
The 1H-NMR spectra were obtained on a 300 MHz NMR Varian model Mercury plus BB spectrometer, using CDCl3 and TMS as references. Solid-state 13C-NMR spectroscopy was carried out on a Varian NMR spectrometer operating at a frequency of 75 MHz. The sample was packed into a 7 mm diameter silicon nitrite rotor, retained with Kel-F end caps, and spun at 5 kHz. Each proton preparation pulse was 4.9 µs at 33.9º, followed by 50 ms of data acquisition and recovery delay of 10 s. Transients from 5120 such sequences were averaged. The mass spectra (MS) were obtained on an ESI-MS Quattro LCZ (Micromass, Manchester, UK). The optical rotation measurments (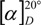 ) were performed in methanol on a Perkin-Elmer 241 spectropolarimeter. Column chromatography (CC; Sephadex℘ LH-20) was carried out using ethanol-water in increasing volumetric proportions. Multi-layer counter-current chromatography (MLCCC): PC ITO Multi-layer Coil Separator-Extractor, Model #1; 800 rpm; eluent system: ethyl acetate-n-propanol-water (140:8:80; v/v); upper layer=mobile phase; flow: 1.0 mL/min. The CC and MLCCC were monitored by TLC (aluminum sheets: silica gel 60 F254; Merck℘; 0.200 mm), under UV254 light and with a solution of 1% FeCl3 in ethanol. DPPH (Fluka) and ammonium molybdate (Merck®) were used as the antioxidant solution.
) were performed in methanol on a Perkin-Elmer 241 spectropolarimeter. Column chromatography (CC; Sephadex℘ LH-20) was carried out using ethanol-water in increasing volumetric proportions. Multi-layer counter-current chromatography (MLCCC): PC ITO Multi-layer Coil Separator-Extractor, Model #1; 800 rpm; eluent system: ethyl acetate-n-propanol-water (140:8:80; v/v); upper layer=mobile phase; flow: 1.0 mL/min. The CC and MLCCC were monitored by TLC (aluminum sheets: silica gel 60 F254; Merck℘; 0.200 mm), under UV254 light and with a solution of 1% FeCl3 in ethanol. DPPH (Fluka) and ammonium molybdate (Merck®) were used as the antioxidant solution.
Plant Material
Seeds of Paullinia cupana H.B.K. var. sorbilis (Mart.) Ducke were acquired from Mr. José Augusto de Souza, in the region of Alta Floresta, Mato Grosso, Brazil. A dried specimen was deposited in the Herbarium of the Department of Biology of the State University of Maringá under number HUEM 9.065. It was identified by Prof. Dr. Cássia Mônica Sakuragui. The methylxanthine content was determined according to [30].
Extraction and isolation of compounds
The seeds were ground in a hammer mill (<180 µm) (Tiger ASN-5). The aqueous extract (AqE) from the 5% (w/v) guaraná seeds used in the biological assays was prepared by Ultra-Turrax® (UTC115KT) processing for 15 min. The AqE was then concentrated and lyophilized.
The crude extract from the guaraná seeds (EBPC) (100 g) was prepared by Ultra-Turrax® (UTC-115KT) processing for 15 min, using acetone-water, giving a 15.76% yield. From the EBPC, two semi-purified fractions were obtained: EPB (29.11 g) and EPA (43.93 g) (Patent No. PI 0006638-9, Brazil) [31]. The EPA fraction was used for multi-layer counter-current chromatography (MLCCC) and column chromatography (CC), to isolate the compounds.
Compounds 2 (16.3 mg), 3 (15.1 mg), 4 (4.0 mg), 5 (10.5 mg), 6 (80.1 mg), 8 (4.5 mg), 9 (4.3 mg), and 10 (5.9 mg) were obtained directly by MLCCC [22]. Compounds 1 (40.6 mg) and 7 (10.2 mg) were obtained sequentially by CC in Sephadex℘ LH-20 [19,22]. All compounds were obtained from the semi-purified EPA fraction.
Total polyphenolics and tannins in EBPC, EPA, EPB, and AqE samples
The total polyphenolics (TP) and tannins (TT) contents in the EBPC, EPA, EPB, and AqE samples were estimated by a colorimetric assay based on procedures described in [32], with slight modifications. Briefly, the EBPC (0.187 mg), EPA (0.082 mg), EPB (0.054 mg) or AqE (0.135 mg) was dissolved in water (250 mL; MS), respectively. An aliquot (5 mL) was diluted in water to 25 mL. A aliquot of the later solution (2 mL) was transferred to a 25-ml vial with Folin-Ciocalteu phenol reagent (1 mL) and water (10 mL), and made up to volume with a solution of 14.06 % sodium carbonate. After 15 min the absorbance was measured at 691 nm (TP). Water was used as the blank. For determination of the nonadsorbent polyphenols (NAP), MS (10 mL) was transferred with of Hide powder (100 mg) and vortexed for 60 min. Next, the solution was filtered, and an aliquot (5 mL) was diluted in water to 25 mL. An aliquot of this solution (2 mL) was transferred to a 25-ml vial with of the Folin-Ciocalteu phenol reagent (1 mL) and water (10 mL), and made up to volume with a 14.06 % sodium carbonate solution. After 15 min the absorbance was measured at 691 nm (NAP). Water was used as the blank. The percentages of total phenolics and tannins were determined (in triplicate) as follows:
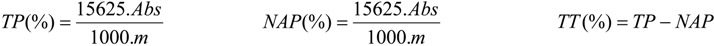 |
where TP = total polyphenolics (%); NAP = non-adsorbent phenolics (%); TT = total tannins (%); Abs = absorbance; m= mass (g) of EBPC, EPA, EPB, or AqE.
Relative Antioxidant Capacity (RAC)
An aliquot of sample solution (0.3 mL) containing a reducing species (in water) was combined in a test tube with reagent solution (3 mL, 0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The test tubes were capped and incubated in a water bath at 95 °C for 90 min. After the samples had cooled to room temperature, the absorbance of the aqueous solution of each was measured at 695 nm against a blank. A typical blank solution contained reagent solution (1 mL) and the appropriate volume of the same solvent used for the sample, and was incubated under the same conditions as the rest of the samples. For samples of unknown composition, the water-soluble antioxidant capacity was expressed as equivalents of ascorbic acid. The antioxidant capacity of the extracts was expressed in relation to ascorbic acid (0.3 ml of a 2 mM solution) used as the standard, the antioxidant activity of which was considered as 1 (100%) [33].
Determination of antioxidant capacity by the DPPH• (2,2-diphenyl-1-picrylhydrazyl) method
The antioxidant capacity with the DPPH• radical (1 mM, 0.5 mL) was determined with the sample (4 mL) previously dissolved in methanol in concentrations of 2.0 to 20.0 μg/mL. The solutions were homogenized, and the reaction occurred in ambient temperature for 30 min, protected from light. A solution of DPPH• (0.5 mL) in methanol (4 mL) was used as a control, and another solution of BHT (2 mg) in methanol (4 mL) with DPPH• solution (0.5 mL) was used as the blank(as described in [34], with certain modifications). The absorbance values were measured at 517 nm and converted to a percentage of antioxidant activity or percentage of DPPH• consumed (AA%), using the following formula:
| AA% = 100 - [(Abssample - Absblank).100]/Abscontrol, where: Abs = absorbance |
Cover slip adherence test
The method of Hamada [35] was used, partially modified. Streptococcus mutans ATCC 25175 cultured for 24 h in BHI broth at 37 °C and in microaerophilia (10% CO2) were centrifuged for 20 min and resuspended with buffer (3.0 mL) adjusted by the McFarland scale (1.5x108 cells/mL). From this bacterial suspension, a sample (1.5 mL) was added to buffer (control, 1.5 mL) or to extract (1.5 ml). This was incubated at 37 °C in microaerophilia for 4 h, centrifuged, and resuspended with BHI + saccharose (3.0 mL). Serial dilutions were made in BHI medium: 10-1, 10-2. The 10-2 dilution (500 μL) was placed on a 24-well plate and a cover slip added. The plate was incubated at 37 °C in microaerophilia for 2 h. Next, the suspension was removed from each well, and the cover slip was removed and washed in buffer, and placed on another 24-well plate. 10% sheep-blood agar in BHI (500 μL) was added on the cover slip, and the plate was incubated. The colony-forming units (CFU) were counted after 24 h of incubation at 37 °C in microaerophilia in triplicate. Viable colonies in dishes with 10% sheep-blood agar were also counted.
Determination of the minimum inhibitory concentration (MIC) by the plate-dilution method
The minimum inhibitory concentration was determined according to the NCCLS [29], using a sample of Streptococcus mutans ATCC 25175 kindly provided by the Instituto Oswaldo Cruz, Rio de Janeiro, Brazil.
Effect of the extract on the production of acids
Streptococcus mutans was seeded (1 mL in 100 mL red phenol broth containing 1% glucose and the extract). This was incubated at 37 °C in microaerophilia, and at regular intervals a sample (4 mL) of the culture was removed and its pH measured in a pH meter [36], with certain modifications.
Caffeine, epicatechin and catechin (1, 2, and 3). The 1H-NMR (CDCl3, 300 MHz) data and the ESI-MS are identical with those recently published in [11].
ent-Epicatechin (4). C15H14O6; ESI-MS [M+Na+]+
m/z 523.4; [M-H+]-
m/z 499.4; 1H-NMR [CDCl3, δ (ppm), multiplicity and J (Hz)]: δ 2.88 [dd, H-4ax (C), J=18.0, 2.1], 2.98 [dd, H-4eq (C), J=17.7, 4.5], 5.12 [s, H-2 (C)], 5.39 [m, H-3 (C)], 6.57 [d, H-6 (A), J=2.1], 6.67 [d, H-8 (A), J=2.4], 7.20 [d, H-5’ (B), J=8.4], 7.27 [dd, partially hidden by the CDCl3, H-6’ (B), J=1.8], 7.36 [d, H-2’ (B), J=2.1 Hz]; 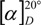 +6° (methanol; c 0.5).
+6° (methanol; c 0.5).
Epicatechin-(4β→8)-catechin (Procyanidin B1) (5). C50H46O22; ESI-MS [M+Na+]+
m/z 1021.5; [M-H+]-
m/z 997.5; 1H-NMR [CDCl3, δ (ppm), multiplicity and J (Hz)]: δ 2.56 [dd, H-4ax (F), J=16.5, 9.0], 3.21 [dd, H-4eq (F), J=16.5, 6.6], 4.33 [d, H-2 (F), J=9.6], 4.42 [d, H-4 (C), J=2.1], 5.05 [ddd, H-3 (F), J=9.6, 9.3, 6.6], 5.15 [m, H-3 (C), J=1.8], 5.47 [s, H-2 (C)], 5.99 [d, H-6 (A), J=2.1], 6.29 [d, H-8 (A), J=2.1], 6.68 [s, H-6 (D)], 6.88 [d, H-2’ (E), J=1.8], 6.94 [dd, H-6’ (E), J=8.4, 2.1], 7.09 [d, H-5’ (E), J=8.1], 7.16 [d, H-5’(B), J=8.7], 7.24 [dd, H-6’ (B), J=8.7, 2.1], 7.29 [d, H-2’ (B), J=1.8]; 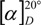 +2° (methanol; c 1.0).
+2° (methanol; c 1.0).
Epicatechin-(4β→8)-epicatechin (Procyanidin B2) (6). C50H46O22; ESI-MS [M+Na+]+
m/z 1021.5; [M-H+]-
m/z 997.5. The spectral data are identical with those recently published [11]; 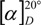 –4.5° (methanol; c 2.0).
–4.5° (methanol; c 2.0).
Catechin-(4α→8)-catechin (Procyanidin B3) (7). C50H46O22; ESI-MS [M+Na+]+
m/z 1021.6; [M-H+]-
m/z 997.7; The spectral data are identical with those recently published [11]; 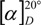 –31.5° (methanol; c 1.43).
–31.5° (methanol; c 1.43).
Catechin-(4α→8)-epicatechin (Procyanidin B4) (8). C50H46O22; ESI-MS [M+Na+]+
m/z 1021.5; [M-H+]-
m/z 997.6. The spectral data are identical with those recently published [11]; 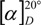 –16.8° (methanol; c 1.43).
–16.8° (methanol; c 1.43).
Epicatechin-(4β→8;2β→O→7)-epicatechin (Procyanidin A2) (9). C48H42O21; ESI-MS [M+Na+]+
m/z 977.4; [M-H+]-
m/z 953.5; 1H-NMR [CDCl3, δ (ppm), multiplicity and J (Hz)]: δ 2 x 2.88-2.90 [m, H-4 (F)], 4.60 [d, H-4 (C), J=4.2], 4.85 [s, H-2 (F)], 5.05 [d, H-3 (C), J=4.2], 5.35 [m, H-3 (F)], 6.38 [d, H-8 (A), J=2.1], 6.58 [s, H-6 (D)], 6.69 [d, H-6 (A), J=2.4], 2 x 7.21-7.24 [m, H-5’ (B and E)], 7.38 [m, H-6’ (E)], 7.41 [d, H-2’ (B and E), J=2.1], 7.51 [dd, H-6’ (B), J=8.7, 2.1]; 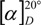 +5.3° (methanol; c 1.5).
+5.3° (methanol; c 1.5).
Epicatechin-(4β→8)-epicatechin-(4β→8)-epicatechin (Procyanidin C1) (10). C45H38O18; ESI-MS [M+Na+]+
m/z 889.4; [M-H+]-
m/z 865.5; 1H-NMR [CDCl3, δ (ppm), multiplicity and J (Hz)]: δ 2 x 2.93-3.11 [m, H-4 (I)], 4.65 [s, H-4 (C)], 4.70 [s, H-4 (F)], 4.78 [s, H-2 (F)], 5.12 [m, H-3 (C)], 5.21 [s, H-2 (I)], 5.36 [s, H-2 (C)], 5.40 [m, H-3 (F)], 5.48 [m, H-3 (I)], 5.94 [d, H-6 (A), J=2.4], 6.26 [d, H-8 (A), J=2.4], 6.65 [s, H-6 (D)], 6.71 [s, H-6 (G)], 9 x 6.65-7.30 [m, H- (B, E, and H)]; 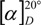 +5.81° (methanol; c 3.1).
+5.81° (methanol; c 3.1).
Acknowledgements
We thank CNPq, CAPES, the Programa de Pós-Graduação em Ciências Farmacêuticas, the Institut für Pharmazeutische Biologie und Phytochemie der Universität Münster for MLCCC, and Admir Arantes and Cláudio Roberto Novello for support of this work. The valuable observations of two anonymous reviewers contributed significantly to improvements in the manuscript.
Footnotes
Sample Availability: Available from the authors.
References
- 1.Otobone F.J., Sanches A.C., Nagae R.L., Martins J.V.C., Obici S., Mello J.C.P., Audi E.A. Effect of Crude Extract and its Semi Purified Constituents from Guaraná Seeds [Paullinia cupana var. sorbilis (Mart.) Ducke on Cognitive Performance in Morris Water Maze in Rats. Braz. Arch. Biol. Technol. 2005;48:723–728. [Google Scholar]
- 2.Otobone F.J., Sanches A.C., Nagae R.L., Martins J.V.C., Sela V.L., Mello J.C.P., Audi E.A. Effect of lyophilized extracts from guaraná seeds [Paullinia cupana var. sorbilis (Mart.) Ducke] on behavorial profiles in rats. Phytother. Res. 2007 doi: 10.1002/ptr2089. [DOI] [PubMed] [Google Scholar]
- 3.Antonelli-Ushirobira T.M., Yamaguti E., Uhemura L.M., Mello J.C.P. Controle de qualidade de amostras de Paullinia cupana H.B.K. var. sorbilis (Mart.) Ducke. Acta Farm. Bonaerense. 2004;23:383–386. [Google Scholar]
- 4.Ushirobira T.M.A., Yamaguti E., Uemura L.M., Audi E.A., Mello J.C.P. de. Avaliação físico-química de sementes de guaraná secas por diferentes métodos. Rev. Bras. Farmacogn. 2004;14:15–20. doi: 10.1590/S0102-695X2004000100003. [DOI] [Google Scholar]
- 5.Santos S.C., Mello J.C.P. In: Farmacognosia: da Planta ao Medicamento. 5th ed. Simões C.M.O., Schenkel E.P., Gosmann G., Mello J.C.P., Mentz L.A., Petrovick P.R., editors. UFSC; Florianópolis: 2004. pp. 517–544. Chapter 24. [Google Scholar]
- 6.Mattei R., Dias R.F., Espínola E.B., Carlini E.A., Barros S.B.M. Guaraná (Paullinia cupana): toxic behavioral effects in laboratory animals and antioxidant activity in vitro. J. Ethnopharmacol. 1998;60:111–116. doi: 10.1016/S0378-8741(97)00141-4. [DOI] [PubMed] [Google Scholar]
- 7.Basile A., Ferrara L., Del Pezzo M., Mele G., Sorbo S., Bassi P., Montesano D. Antibacterial and antioxidant activities of ethanol extract from Paullinia cupana Mart. J. Ethnopharmacol. 2005;102:32–36. doi: 10.1016/j.jep.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 8.Nakahara K., Kawabata S., Ono H., Ogura K., Tanaka T., Ooshima T., Hamada S. Inhibitory effect of oolong tea polyphenols on glucosyltransferases of mutans streptococci. Appl. Environ. Microbiol. 1993;59:968–973. doi: 10.1128/aem.59.4.968-973.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholz E. Pflanzliche Gerbstoffe: Pharmakologie und Toxikologie. Deutsch. Apoth. Ztg. 1994;134:3167–3179. [Google Scholar]
- 10.Barbosa G.D.A.F., Mello J.C.P. Avaliação clínica do extrato de guaraná no controle da placa bacteriana dentária. Rev. Paulista. Odontol. 2004;4:28–30. [Google Scholar]
- 11.Antonelli-Ushirobira T.M., Yamaguti E., Uemura L.M., Nakamura C.V., Dias Filho B.P., Mello J.C.P. Chemical and microbiological study of extract from seeds of guaraná (Paullinia cupana var. sorbilis) Lat. Am. J. Pharm. 2007;26:5–9. [Google Scholar]
- 12.Weinges K., Göritz K., Nader F., Perner J. Zur Kenntnis der Proanthocyanidine, XI Konfigurationberstimmung von C30H26O12 – Procyanidine und Strukturaufklärung eines neuen Procyanidins. Liebigs Ann. Chem. 1968;715:164–171. doi: 10.1002/jlac.19687150118. [DOI] [Google Scholar]
- 13.Weinges K., Bähr W., Ebert W., Göritz K., Marx H.D. Konstitution, Entstehung und Bedeutung der Flavonoid-Gerbstoffe. Fortschr. Chem. Org. Naturst. 1969;27:158–260. [Google Scholar]
- 14.Kofink M., Galensa R. Enantiomerentrennung von Catechinen mittels Kapillarelektrophorese. Lebensmittelchem. 2005;59:111–112. [Google Scholar]
- 15.Foo L.Y. Polymeric proanthocyanidins of Photinia glabrescens, modification of molecular weight and nature of products from hydrogenolysis. Phytochemistry. 1982;21:1741–1746. doi: 10.1016/S0031-9422(82)85051-6. [DOI] [Google Scholar]
- 16.Romeyer F.M., Macheix J.J., Sapis J.C. Changes and importance of oligomeric procyanidins during maturation of grape seeds. Phytochemistry. 1985;25:219–221. doi: 10.1016/S0031-9422(00)94532-1. [DOI] [Google Scholar]
- 17.Lu Y., Foo L.Y. The polyphenol constituents of grape pomace. Food Chem. 1999;65:1–8. doi: 10.1016/S0308-8146(98)00245-3. [DOI] [Google Scholar]
- 18.Khallouki F., Haubner R., Hull W.E., Erben G., Spiegelhalder B., Bartsch H., Owen R.W. Isolation, purification and identification of ellagic acid derivatives, catechins, and procyanidins from the root bark of Anisophyllea dichostyla R. Br. Food Chem. Toxicol. 2007;45:472–485. doi: 10.1016/j.fct.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Petereit F., Kolodziej H., Nahrstedt A. Flavan-3-ols and proanthocyanidins from Cistus incanus. Phytochemistry. 1991;30:981–985. doi: 10.1016/0031-9422(91)85291-7. [DOI] [Google Scholar]
- 20.Hemingway R.W., Foo L.Y., Porter L.J. Linkage isomerism in trimeric and polymeric 2,3-cis-procyanidins. J. Chem. Soc., Perkin Trans. 1. 1982:1209–1216. doi: 10.1039/p19820001209. [DOI] [Google Scholar]
- 21.Kolodziej H. Tannins of medicinal plants: application of 1H NMR parameters to the analysis of procyanidins. Farmaceut. Tijdschr. Belg. 1989;66e:44. [Google Scholar]
- 22.Mello J.P., Petereit F., Nahrstedt A. Flavan-3-ols and prodelphinidins from Stryphnodendron adstringens. Phytochemistry. 1996;41:807–813. doi: 10.1016/0031-9422(95)00686-9. [DOI] [Google Scholar]
- 23.Kolodziej H. In: Plant Polyphenols: Synthesis, Properties, Significance. Hemingway R.W., Laks P.E., editors. Plenum Press; New York: 1992. pp. 295–320. [Google Scholar]
- 24.Hör M., Rimpler H., Heinrich M. Inibition of intestinal chloride secretion by proanthocyanidins from Guazuma ulmifolia. Planta Med. 1995;61:208–212. doi: 10.1055/s-2006-958057. [DOI] [PubMed] [Google Scholar]
- 25.Jacques D., Haslam E., Bedford G.R., Greatbanks D. Plant proanthocyanidins. Part II. Proanthocyanidin-A2 and its derivatives. J. Chem. Soc., Perkin Trans. 1. 1974:2663–2671. [Google Scholar]
- 26.Foo L.Y., Lu Y., Howell A.B., Vorsa N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry. 2000;54:173–181. doi: 10.1016/S0031-9422(99)00573-7. [DOI] [PubMed] [Google Scholar]
- 27.Ueffing I. PhD. Thesis. Münster, Germany: 1988. Untersuchung von Procyanidinen in Tilia spec.-ein Beitrag zur qualitativen und quantitativen HPLC-Analytik von Flavanolen. [Google Scholar]
- 28.Resende F.O. M.Sc. Thesis. Maringá, Brazil: 2007. Trichilia catigua: Avaliação farmacognóstica, fitoquímica e biológica in vitro; p. 164p. [Google Scholar]
- 29.National Committee For Clinical Laboratory Standards . Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; NCCLS Approved Standard M7-A5. 2000. Wayne, PA. Vol. 20, n. 2. [Google Scholar]
- 30.Andrade L., Schenkel E.P., Bergold A.M. Estudo da metodologia de análise de cafeína em sementes de guaraná (Paullinia cupana) Rev. Bras. Farmacogn. 1999;80:7–9. [Google Scholar]
- 31.Patent No. PI 0006638-9. Efeito antidepressivo do extrato da droga vegetal guaraná (Paullinia cupana var. sorbilis (Martius) Ducke) [Accessed at 31 july 2007]. Brazil. Available online: http://www.inpi.gov.br.
- 32.Glasl H. Zur Photometrie in der Drogenstandardisierung. 3. Gehaltsbestimmung von Gerbstoffdrogen. Deutsch. Apoth. Ztg. 1983;123:1979–1983. [Google Scholar]
- 33.Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 34.Amarowicz R., Pegg R.B., Rahimi-Moghaddam P., Barl B., Weil J.A. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84:551–562. doi: 10.1016/S0308-8146(03)00278-4. [DOI] [Google Scholar]
- 35.Hamada S., Torii M., Kotani S., Tsuchitani Y. Adherence of Streptococcus sanguis clinical isolates to smooth surfaces and interaction of the isolates with Streptococcus mutans glucosyltransferase. Infect. Immun. 1981;32:364–372. doi: 10.1128/iai.32.1.364-372.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ooshima T., Osaka Y., Sasaki H., Osawa K., Yasuda H., Matsumura M., Sobue S., Matsumoto M. Caries inhibitory activity of cacao bean husk extract in in-vitro and animal experiments. Arch. Oral Biol. 2000;45:639–645. doi: 10.1016/S0003-9969(00)00042-X. [DOI] [PubMed] [Google Scholar]