Abstract
Introduction
The anterior cruciate ligament (ACL) consists of various components, such as collagen, elastin fibres, and fibroblasts. Because ACL has a poor regenerative ability, ACL reconstruction need require the use of autologous tendons. In recent years, tissue-resident stem cells have been studied to promote ACL regeneration as an alternatively method. However, the existence of stem cells in ligaments has not been clearly defined. Here, we prospectively isolated stem cells from ACLs and characterized their properties.
Methods
ACLs from 11 donors and bone marrows (BM) from 8 donors were obtained with total knee arthroplasty. We used flow cytometry to screen the cell surface markers on ACL cells. Frozen sections were prepared from patient ACL tissues and stained with specific antibodies. Cultured ACL-derived and BM-derived cells at passage 3 were differentiated into adipocytes, osteoblasts and tendon/ligament cells.
Results
ACL-derived mesenchymal stem/stromal cells (ACL-MSCs) expressed high levels of CD73 and CD90. Immunohistochemical analyses revealed that ACL-MSCs were located on the inner surface of ACL sinusoids. Furthermore, the expression of cell surface antigens was clearly different between ACL-MSCs and bone marrow (BM)-derived MSCs (BM-MSCs) at the time of isolation, but the two cell populations became indistinguishable after long-term culture. Interestingly, ACL-MSCs are markedly different from BM-MSCs in their differentiation ability and have a high propensity to differentiate into ligament-committed cells.
Conclusions
Our findings suggest that ACL-MSCs express CD90 and CD73 markers, and their differentiation capacity is maintained even through culture. The cell population having tissue-specific properties is an important research target for investigating the ligament therapies.
Keywords: Anterior cruciate ligament, Bone marrow, Mesenchymal stem/stromal cell, Cell surface marker, Differentiation, Regeneration therapy
Abbreviations: ACL, anterior cruciate ligament; BM, bone marrow; MSC, mesenchymal stem/stromal cell; FACS, fluorescence-activated cell sorting; BMP, bone morphogenic protein; MKX, transcription factor Mohawk
Highlights
-
•
CD73+/90+ cell population in ACL have the highest colony forming ability and can differentiate into mesenchymal lineages.
-
•
The expression pattern of cell surface antigen in CD73+/90+ ACL-MSCs become similar to that of BM-MSCs during culture.
-
•
CD73+/90+ ACL-MSCs may be important for ligament regeneration therapies.
-
•
CD73+/90+ ACL-MSCs may be important for ligament regeneration therapies.
1. Introduction
The anterior cruciate ligament (ACL), connecting the femur and tibia, is a key structure of the knee joint [1]. Injury to the ACL is a major clinical problem for athletes due to the limited self-repair capacity of the ACL. Although several treatment options are available for repairing the damaged ACL, including autografts, allografts, xenografts, and some prosthetic devices, the outcomes are variable [2], [3], [4], [5]. In addition, the malfunction of a reconstructed ACL is a risk factor for meniscus injury and the subsequent development of knee joint osteoarthritis [6]. Currently, ACL reconstruction using autologous tendons is the predominant method for ACL repair, with a fairly good recovery score [7]. Nevertheless, to avoid invasive procedures, cell-based therapies are being investigated [8]. Stem cells are an attractive cell source because of their high proliferation, self-renewal and multipotency, and they can be used as a therapeutic tool to repair the damaged ACL. Mesenchymal stem/stromal cells (MSCs) are widely used as a source of cells for regenerative applications, but the outcomes can be variable [9], [10]. One study in a rabbit model has been reported that the ACL could be regenerated after the transplantation of MSCs with a type I collagen scaffold [9]. However, there is also a report of the lack of significant difference in ACL regeneration between groups with or without transplantation of MSCs in a pig model [10]. Several reports indicate the presence of MSC-like cells in human ACL tissues [11], [12], but the specific markers and characteristics of these cells are still unknown. In this study, we investigated the existence of ACL-specific MSCs that could be used for the regeneration of an injured ACL.
The expression of cell surface antigens on MSCs changes during long-term culture; therefore, it is difficult to identify specific markers to isolate MSCs. To overcome this problem, research groups including ours had developed a method to prospectively isolate bone marrow-derived MSCs (BM-MSCs). In our previous report, the LNGFR+/THY-1+ (known as CD271 and CD90) population was enriched for BM-MSCs, which exhibited multilineage differentiation and self-renewal potential [13]. Somatic tissues, including adipose tissue [14], placenta [15], knee synovial membrane [16] and dental pulp [17], also contain MSC-like cells. Based on this knowledge, we sought to identify ACL stem cell-specific markers by screening for cell surface antigens and examine their differentiation into multiple lineages using a highly purified population.
In this study, we identified MSC-like cells in ACL tissues that express CD73 and CD90. This unique population of CD73+/CD90+ ACL-MSCs rapidly proliferated in vitro and had the potential to differentiate into mesenchymal lineages. Before being cultured, the ACL- and BM-MSCs were very different from each other with regard to their expression of cell surface antigen, however, the two populations became indistinguishable after being cultured in vitro. Bone morphogenic protein (BMP) signalling was significantly promoting their differentiation into the ligament lineage in ACL-MSCs. These results provide insights into endogenous ligament-derived MSCs and indicate a novel tissue regeneration strategy targeting endogenous tissue stem cells.
2. Methods
2.1. Ethics statement and tissue preparation
ACL and bone fragments were obtained from donors undergoing total knee arthroplasty at Tokyo Medical and Dental University Hospital. Samples from patients with rheumatoid arthritis were not used for this study. In total, 14 samples were used for the experiments (male = 5, median age of 73 years, Supplemental Table S1). All experimental protocols were approved by the local Institutional Review Board of Tokyo Medical and Dental University (No. 1030), and oral and written informed consent was obtained from patients before experiments. All methods were conducted in strict accordance with the approved guidelines of the institutional committee. ACL and bone fragments were digested with 2 mg/mL collagenase (Wako) and 25 μg/mL DNase I (Sigma–Aldrich) with shaking at 37 °C for 1 h in Dulbecco's Modified Eagle's Medium (DMEM, Life Technologies). To remove debris, the cell suspensions (from ACL and bone fragments) were filtered through a cell strainer (BD Falcon, 70 μm). Following red blood cell lysis, the samples were used for flow cytometric analysis, cell sorting, and cell culture experiments.
2.2. Flow cytometry and cell sorting
Digested primary cells (1–5 × 107 cells/mL) or cultured cells (1–5 × 105 cells/mL) were suspended in Hank's balanced salt solution (HBSS, Wako) and stained for 30 min on ice with fluorescence-conjugated monoclonal antibodies (described in Supplementary Table S2) for sorting or analysis. Flow cytometric analysis and cell sorting were performed on the FACS Verse and FACS Aria II systems (BD), respectively, and the data were analysed using FlowJo software (Tree Star).
2.3. Colony-forming unit (CFU) assay
The CFU-F assay was performed by culturing 500 cells in a 35-mm dish for 21 days in culture medium (DMEM supplemented with 20% foetal bovine serum (Gibco), 1% penicillin/streptomycin (Gibco), and 5 ng/mL bFGF (Repro Cell)), and the medium was changed every 3–4 days. Clusters with more than 50 cells were counted as a colony.
2.4. Mesenchymal lineage differentiation assay
Cultured ACL- and BM-MSCs at passage 3 were harvested using trypsin–EDTA (Gibco), transferred to a 24-well plate and grown overnight in culture medium. The cell numbers used were as follows: 2.0 × 104 cells (for adipogenesis) and 1.5 × 104 cells (for osteogenesis). For adipogenic differentiation, adherent cells were cultured in adipogenic induction and maintenance medium (Lonza), which was changed every 3–4 days. After 14 days, Oil red O staining (Muto Pure Chemicals) was used to confirm the differentiation of the cells into adipocytes. For osteogenic differentiation, adherent cells were cultured in osteogenic induction medium (Lonza), which was changed every 3–4 days. After 14 days, the differentiation of the cells into osteoblasts was assessed by Alizarin Red staining (Millipore). For chondrogenic differentiation, 2.0 × 105 cells were transferred to a 1.5 mL tube and cultured in chondrogenic induction medium (Lonza) containing 10 ng/mL TGF-β3 (Lonza) and 500 ng/mL BMP-6 (R&D Systems), which was changed every 3–4 days.
2.5. Immunohistochemistry
Frozen ACL sections were prepared and immunostained according to the Kawamoto method [18]. ACL sections (12 μm) were fixed using dry ice/ethanol. The markers and antibodies used were as follows: Hoechst 33258 (Dojin), FITC-conjugated mouse anti-human CD90 antibody, PE-conjugated mouse anti-human CD73 antibody, APC-conjugated anti-CD31 antibody (BD Pharmingen), rabbit anti-human Mkx antibody (LSBio), mouse anti-human COL1A1 antibody (Millipore), Alexa 647-conjugated goat anti-rabbit, and goat anti-mouse antibodies (Life Technologies).
2.6. Ligament lineage differentiation assay
The ligament linage differentiation assay was based on previous method with minor changes [19]. Cultured MSCs at passage 3 were harvested using trypsin–EDTA. For tenogenic differentiation, 2.0 × 104 cells were transferred to a 24-well plate and cultured overnight in culture medium. Adherent cells were cultured in DMEM with BMP-12 (50 ng/mL, Sigma–Aldrich) and 10% FBS, which was changed every 3–4 days. After 6 days, the differentiation of cells into ligament-like cells was confirmed using real-time PCR.
2.7. Gene expression analysis
After the differentiation of MSCs into tenocyte/ligament lineage cells, total RNA was prepared using the TRI reagent (Sigma–Aldrich). Complementary DNA was amplified using the StepOne Real-Time PCR System (Life Technologies) and normalized against β-actin expression (20–35 cycles per gene). Experiments were performed with more than three independent biological samples, and triplicate values were averaged. The probes used to detect the expression of key markers were confirmed to be specific to humans (TaqMan gene expression assays: COL1A1, Hs00164004_m1; MKX, Hs00543190_m1; SCX, Hs03054634_g1; and ß-ACTIN (ACTB), Hs01060665_g1).
2.8. Statistical analysis
Quantitative data are presented as the mean ± standard deviation from at least three independent experiments. For statistical analysis, data were evaluated using Student's T-test or Mann–Whitney U-test with Bonferroni's correction. In all cases, p-values <0.05 were considered significant.
3. Results
3.1. MSC-specific marker expression in ACL-derived cells
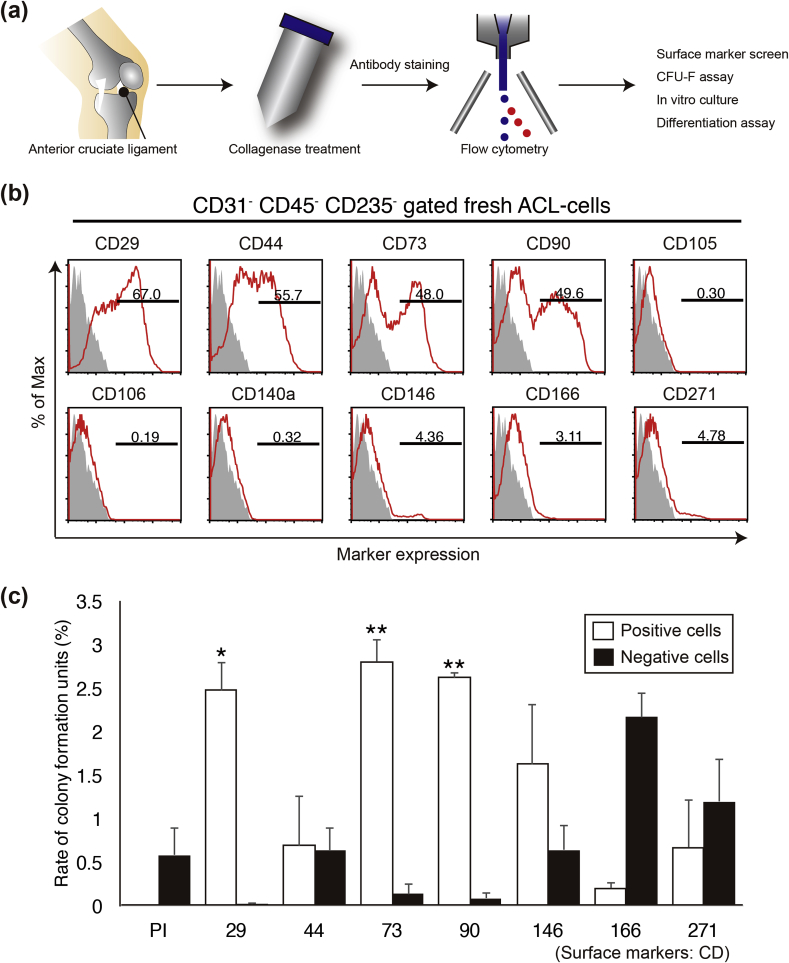
ACLs were dissected from human donors undergoing total knee arthroplasty with informed consent. In this study, samples from 14 patients (65–83 years old) were used (Supplementary Table S1). ACL tissues were treated with collagenase to obtain a cell suspension. To isolate ACL-resident stem cells, we screened for surface markers using flow cytometry. Suspended ACL-derived cells were stained for a series of MSC-specific cell surface antigens and analysed by FACS (Fig. 1a). ACL-derived cells expressed high levels of CD29, CD44, CD73, and CD90 and low or undetectable levels of CD105, CD106, CD140a (PDGFRα), CD146, CD166, and CD271 (LNGFR) (Fig. 1b). Next, to validate for MSCs, we performed a colony formation assay. The selected cell surface markers were used to separate the ACL-derived cell populations into positive/negative fractions, and the colony-forming ability of each population was investigated. On day 21 of in vitro culture, the CD29+, CD73+, and CD90+ populations displayed enhanced colony-forming ability (Fig. 1c). In contrast, the CD44+, CD146+, CD166+, and CD271+ fractions were not enriched in cells with colony-forming abilities (Fig. 1c). It is known that CD29, CD73, and CD90 are highly expressed in not only in BM-MSCs but also adipose tissue-derived and synovial MSCs; therefore, our data suggest that MSCs are contained in ACL tissues. In particular, the CD73+ cells exhibited a five-fold higher colony-forming ability than the Propdium Iodide- (PI-) cells (non-selected live cells) did. Although CD146 and CD271 are known as specific markers of MSCs from multiple organs [20], [21], they are not useful candidates for isolating ACL-derived MSCs.
Fig. 1.
Analysis of colony-forming cells in the anterior cruciate ligament (ACL). (a) Schema of cell isolation from the ACL. (b) Representative flow cytometric profiles of freshly isolated ACL-derived cells stained for CD29, CD44, CD73, CD90, CD105, CD106, CD140a, CD146, CD166, and CD271 (grey: isotype control; red: sample). (c) Colony formation rates during 3 weeks of culture after cell sorting.
3.2. Prospectively isolated ACL-MSCs are enriched in the CD73+CD90+ population
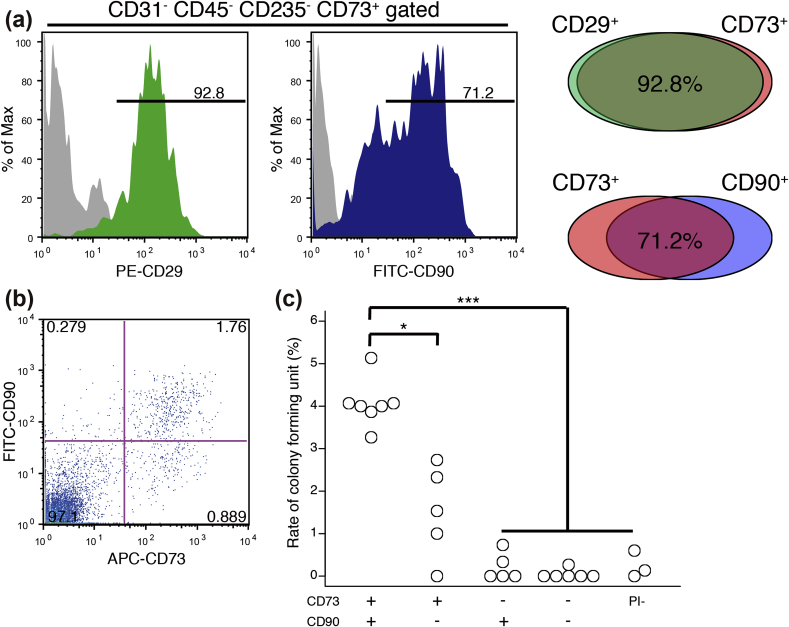
To investigate the relationships among the CD29+, CD73+, and CD90+ populations, multicolour staining was performed. Our group previously has reported that CD73 is a common marker of BM-MSCs in humans, mice, and rats [22]; thus we searched for a marker that is co-expressed with CD73. As a result, most of the CD73-positive cells were also positive for CD29 (92.8%) and CD90 (72.1%) (Fig. 2a, left). The CD29+ cells were almost always positive for CD73 (Fig. 2a, right); therefore, we focused on CD90 as a co-expressed marker and performed FACS to isolate populations of cells with or without CD73 and CD90. Using dual-colour staining, we confirmed the presence of 4 different fractions (CD90+/73+: 1.76%, +/−: 0.279%, −/+: 0.889%, and −/−: 97.1%) (Fig. 2b). Cells that express both CD73 and CD90 are an extremely rare population in ACL tissues. Colony-forming unit-fibroblast (CFU-F) assay using anti-CD73 and anti-CD90 antibodies showed that the CFUs were enriched in the CD73+ cell fraction (Fig. 2c). In particular, the CD73+/CD90+ fraction had the highest colony-forming ability among the ACL-derived cells (Fig. 2c) and differentiation potential into adipocytes, osteoblasts and chondrocytes (Supplementary Fig. S1). Next, the properties of “cultured” ACL-derived CD73+/CD90+ MSCs were investigated with regard to their cell surface antigens. Flow cytometric analyses showed that the expression of CD29, CD44, CD73, CD90, CD105, and CD166 increased in these cells after two passages (Supplementary Fig. S2), and the cell surface markers were maintained at a high level even after four passages (Supplementary Fig. S2). In contrast, the ACL-MSCs displayed low or negative expression of CD31 (endothelial cell-specific marker), CD45 (leukocyte marker), and CD235 (erythrocyte marker) (data not shown). Therefore, MSC-like cells were enriched in the CD73+/CD90+ population, and these cells maintained their properties after several passages.
Fig. 2.
Purification of ACL-derived mesenchymal stem/stromal cells (MSCs) using surface markers. (a, b) Representative flow cytometric profiles of fresh ACL-derived cells stained for CD29 and CD90 and gated for the CD73+ (a) and CD73+CD90+ fractions (b). (c) Rate of colony formation on day 21 in the following cells: CD73+/90+, +/−, −/+, −/−, and propidium iodide (PI)− (n = 3–7, p* < 0.05, p*** < 0.001, Student's t-test with Bonferroni's correction).
3.3. CD73+CD90+ ACL-MSCs reside on the inner surface of ligament sinusoids
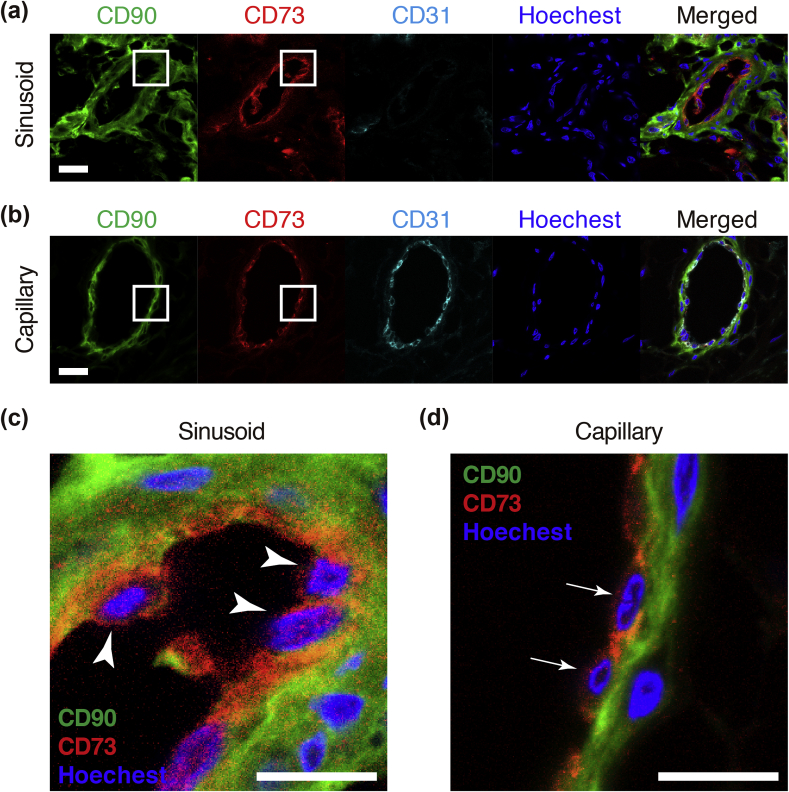
Next, we investigated the cellular localization of ACL-MSCs by isolating them using specific antibodies. The ACL consists of a bundle of ligaments that connects bones. Frozen sections were prepared from patient tissues and stained with antibodies against CD90 and CD73. Immunohistochemical analyses of transverse sections of ACL tissues showed that CD73 (red)-positive cells were also positive for CD90 (green), and CD73+/CD90+ cells were located in small ductal structures present near the surface of the ACL (Fig. 3a). Co-staining with CD31 (endothelial cell marker) showed that the CD73+/CD90+ sinusoids were not positive for CD31 (Fig. 3a, c: arrow head). In contrast, there were no CD73+/CD90+ cells in capillaries, but all the CD73+ cells were also positive for the endothelial marker CD31 (Fig. 3b, d: arrow). We further verified this cellular localization using antibodies against Mohawk (Mkx) (a marker of ligament lineage cells) and type I collagen (COL1A1). The CD73+/CD90+ cells were surrounded by collagen, and they expressed Mkx (Supplementary Fig. 2S). Therefore, the ACL-MSCs are located in the sinusoids of ligaments but not in the capillaries.
Fig. 3.
Location of CD73+CD90+ cells in the ACL. (a, b) Immunofluorescence staining of transverse sections of human ACL tissue with anti-human CD73 (red), anti-human CD90 (green), and anti-CD31 (cyan) antibodies and Hoechst (blue) in a representative sinusoid (a) and capillary (b) (Scale bars = 20 μm). (c, d) The location of CD73+CD90+ cells in ACL tissues at high-power fields in the sinusoid (c) and capillary (d) (Scale bars = 10 μm).
3.4. Cell surface markers of ACL-MSCs are distinct from those of BM-MSCs
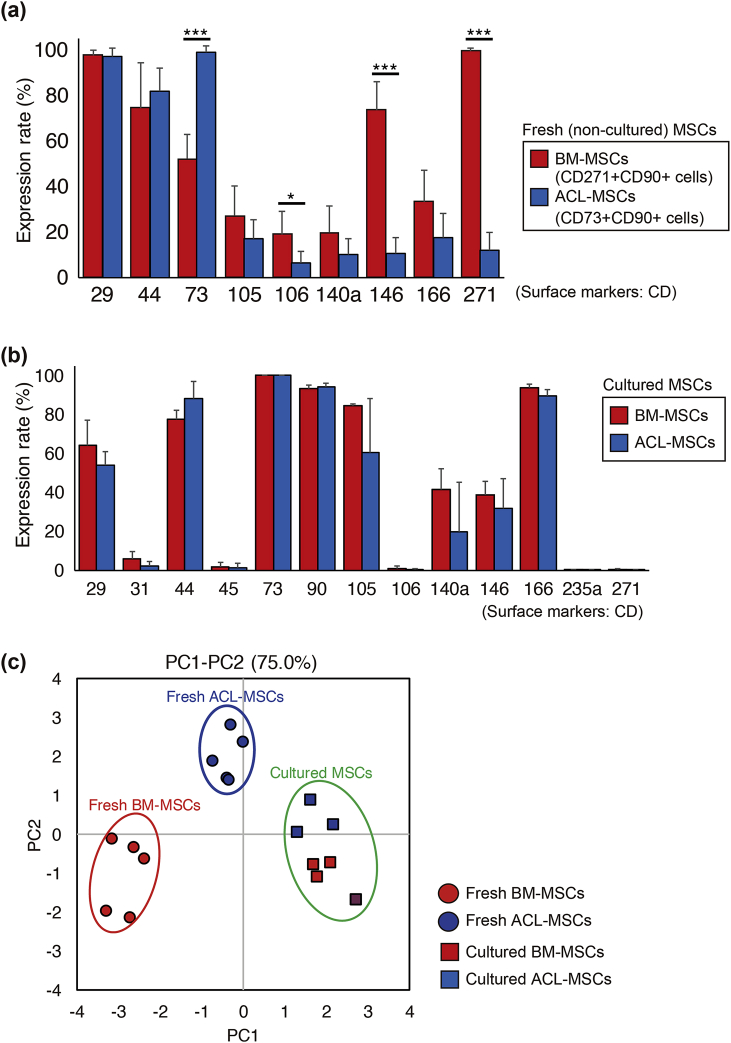
To dissect the differences in cell surface antigen expression in detail, ACL- and BM-MSCs were analysed before and after long-term culture. BM-MSCs were identified as the CD271+/CD90+ (LNGFR+/THY-1+) population. The marker CD73 was highly expressed in ACL-MSCs, whereas CD106 and CD146, and CD271 were highly expressed in BM-MSCs (Fig. 4a). After the culture period, the expression of CD73, CD146, and CD271 was comparable between ACL-MSCs and BM-MSCs (Fig. 4b). Principal component analysis (PCA) based on cell surface antigen expression showed no overlap between ACL-MSCs and BM-MSCs when the cells were freshly isolated, indicating that two populations are distinct regarding the stem cell markers (Fig. 4c, fresh MSCs). However, prolonged culture altered the cell surface marker expression, resulting in similar cell populations (Fig. 4c, cultured MSCs).
Fig. 4.
Comparison of cell surface proteins between ACL- and bone marrow (BM)-derived MSCs. Cell surface protein expression in ACL-MSCs (CD73+/90+) and BM-MSCs (CD90+/271+) before (a) and after culture (b). (c) Principal component analysis of four types of cells. ACL-derived CD73+/90+ MSCs: fresh (blue, circle) and cultured (blue, square); BM-derived CD90+/271+ MSCs: fresh (red, circle) and cultured (red, square).
3.5. CD73+CD90+ MSCs tend to differentiate into ligament-like cells
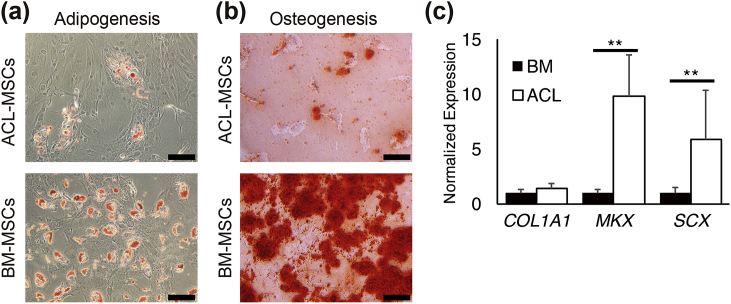
We analysed the potential of ACL- and BM-MSCs to differentiate into mesenchymal lineages. Both BM- and ACL-MSCs differentiated into mesenchymal lineages, whereas low adipogenesis was observed in ACL-MSCs (Fig. 5a and b). Next, we compared the differentiation potential into ligament cells to ACL-MSCs with BM-MSCs. The transcription factors MKX and Scleraxis (SCX) are highly expressed in human ACL tissue [23]. Moreover, Mkx promotes ligament differentiation [24]. Thus, these markers were used to measure the ligament differentiation potential of ACL- and BM-MSCs. Quantitative PCR (q-PCR) data showed that the potential of ACL-MSCs to differentiate into ligament-like cells was significantly higher than that of BM-MSCs (MKX: 10-fold, SCX: 6-fold) (Fig. 5c). The expression of COL1A1 is also important for differentiation into ligament lineage; however, no significant differences were observed via q-PCR and immunohistochemical analyses for the expression of COL1A1 in BM- and ACL-MSCs (Fig. 5c and Supplementary Fig. S3). Therefore, the potential of MSCs to differentiate into ligament-like cells depends on their tissue of origin.
Fig. 5.
Ability to differentiate into adipocytes, osteoblasts, and ligament cells (a, b) Representative phase contrast micrographs of ACL- and BM-MSCs differentiated into adipocytes (a) and osteoblasts (b) (Scale bars = 100 μm). (c) Expression levels of mRNA (MKX, SCX, and COL1A1) following ligament differentiation (n = 5, p** < 0.01, Mann–Whitney U-test).
4. Discussion
MSCs are multipotent stem cells capable of self-renewal and differentiation into mesodermal lineages such as chondrocytes, adipocytes and osteocytes [25]. These cells have been identified in multiple tissues such as BM, umbilical cord blood, amniotic fluid, adipose tissue and dental pulp [26]. MSCs are considered a novel modality of therapy for a wide variety of degenerative and immunological disorders. In this study, we prospectively isolated MSCs residing in ACL tissues using a combination of CD73 and CD90 as positive selection markers. In vitro analyses showed that these cells had the ability to differentiate into the ligament lineage as well as mesenchymal lineages.
We show here that CD73+/CD90+ cells are located on the inner surface of small ducts that are negative for CD31, suggesting that these ducts are not capillaries but rather sinusoids within the ligament (Fig. 3d). The sinusoidal structures form a network that covers the surface of the ligament. Although the function of ACL-MSCs remains unclear, we observed that the CD73+/CD90+ MSCs have a high preference to differentiate into ligament-like cells. According to our data, ACL-MSCs express the Mkx gene (Supplementary Fig. S3). ACL-MSCs are known to exist in a progenitor state. Although a majority of ACL-MSCs are quiescent during homeostasis, they can be activated by stimuli such as injury. It is important for tissue stem cells to reside near network structures under resting conditions so that they can contribute to tissue repair in response to injuries.
CD271, a widely used BM-MSC marker [27], [28], was not useful for the detection of ACL-MSCs (Fig. 1). CD271 (also known as p75 NTR) is a neural crest marker [28]. A fraction of MSCs are derived from the neural crest lineage during developmental stages [29], [30], [31]. During embryonic development, BM cells are derived from neural crest lineages; however, the ACL is mainly derived from the mesoderm. The difference in origin between the BM and ligaments may explain the divergence of the surface markers and differentiation preference. On the other hand, CD73 is commonly expressed on MSCs derived from various organs. Our group had confirmed CD73 as a universal MSC marker in mice, humans, and rats [22]. CD73 dephosphorylates ATP and ADP into AMP. Thus, CD73 suppresses inflammatory reactions [32]. Inflammation is frequently observed in ligaments and synovial tissues in association with ageing. MSCs play an important role in suppressing inflammation [33]. The ability to suppress inflammation is critical for MSCs derived from various organs.
Our data shows that there was no significant difference in COL1A1 expression between ACL- and BM-MSCs. In a previous report, ACLs from patients with osteoarthritis exhibited lower COL1A1 expression than the ACLs from healthy controls did [23]. We used samples from 65 to 83-year-old patients suffering from osteoarthritis. Thus, a limitation of our study is the lack of analysis of tissue from younger individuals. However, in a previous report, the properties of ACL-MSCs were very similar in both old and young tissues [34]. Thus, the CD73+/CD90+ ACL-MSCs from young and healthy individuals should be studied in the future.
Because a number of isolation techniques and culture conditions have been described in the literature, it is difficult to identify suitable criteria to compare MSC sub-populations. The minimal criteria of MSCs are that these cells should adhere to plastic dishes without expressing haematopoietic markers and that they should be able to differentiate into mesenchymal lineages [35]. Most studies in the field have used classical methods of MSC isolation that require long-term culture and have validated MSCs based on cell surface markers after culturing them in vitro [36]. Therapies utilizing cells that have been expanded in long-term culture not only elicit safety concerns due to the risk of transformation but also tend to be expensive. Therefore, prospectively isolated MSCs have an advantage for clinical application. Although the prospectively isolated tissue-specific MSCs were separated into unique populations at the time of isolation, long-term cultured MSCs were categorized into the same cluster as the cells isolated from different tissue origins (Fig. 4c). Therefore, we strongly suggest that two aspects of MSCs should be taken into the consideration, namely, the timing of isolation, i.e., whether the cells were freshly isolated or cultured before use, and the tissue of origin. Our previous report indicated that synovial MSCs have a stronger propensity to differentiate into cartilage than do BM-MSCs [16]. These properties of cells are also dependent on the environment in which the MSCs reside [37]. Recently, it has been reported that the properties of MSCs are regulated by the expression of the lamin A gene [38]. Moreover, the differentiation preference of cells is maintained for a certain period of time during long-term culture. Additionally, the cytoskeleton and epigenetic mechanisms have been thought to influence differentiation propensity; nevertheless, this is an important issue that needs to be elucidated in the future.
In conclusion, ACL-derived MSC-like cells isolated using CD73 and CD90 as positive markers exhibited stem cell-like characteristics. The purification of ACL-MSCs according to the expression of cell surface antigen enabled the identification of stem cells in the ligament. ACL-derived MSCs have a high propensity to differentiate into MKX+ and SCX+ ligament cells and may thus contribute to ligament regeneration. These findings provide important insights into potential stem cell therapies that can be used to repair ACL tissues.
5. Conclusion
We demonstrated that cell surface markers of cultured MSCs became similar among different source. However, the property for differentiation remained different, and CD73+/CD90+-positive cell population in ACL may be important for ligament regeneration therapies.
Author contributions
Y.O.: conception and design of the study, collection, assembly, analysis and interpretation of data, and writing of the manuscript; Y.M.: conception and design of the study, financial support, collection, assembly, analysis and interpretation of data, and writing of the manuscript; K.S. and Y.H.: collection, assembly, analysis and interpretation of data; E.G.S. and N.S.: conception and design of the study and analysis and interpretation of data; M.M., K.O. and I.S.: data analysis and interpretation and provision of study materials or patient data; and C.A.: conception and design of the study, financial support, analysis and interpretation of data, and writing and final approval of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
We thank Mr. Keiichiro Komori and Ms. Shizuka Fujii (Tokyo Medical and Dental University, Tokyo, Japan) for sample preparation and Dr. Hiroshi Asahara (Tokyo Medical and Dental University, Tokyo, Japan) for discussion on ACL histology. This work was supported by the Research Project for Practical Applications of Regenerative Medicine from Japan Agency for Medical Research and Development (AMED) (Grant no. 15bk0104037h0002). Additional support was obtained from the Grant-in-Aid for Scientific Research (C) (C.A. (Grant no. 17K01753), Y.M. (Grant no. 15K06853) and for young scientists (B) (E.G.S. (Grant no. 16K19191)) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) in Japan.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.reth.2017.12.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Asahara H., Inui M., Lotz M.K. Tendons and ligaments: connecting developmental biology to musculoskeletal disease pathogenesis. J Bone Miner Res. 2017;32(9):1773–1782. doi: 10.1002/jbmr.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grassi A., Nitri M., Moulton S.G., Marcheggiani Muccioli G.M., Bondi A., Romagnoli A. Does the type of graft affect the outcome of revision anterior cruciate ligament reconstruction? Bone Joint J. 2017;99-B:714–723. doi: 10.1302/0301-620X.99B6.BJJ-2016-0929.R2. [DOI] [PubMed] [Google Scholar]
- 3.Zaffagnini S., Grassi A., Marcheggiani Muccioli G.M., Roberti Di Sarsina T., Raggi F., Benzi A. Anterior cruciate ligament reconstruction with a novel porcine xenograft: the initial Italian experience. Joints. 2015;3(2):85–90. doi: 10.11138/jts/2015.3.2.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray M.M., Flutie B.M., Kalish L.A., Ecklund K., Fleming B.C., Proffen B.L. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure. Orthop J Sports Med. 2016;4(11) doi: 10.1177/2325967116672176. 232596711667217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu A., Xue G.H., Sun M., Shao H.F., Ma C.Y., Gao Q. 3D printing surgical implants at the clinic: a experimental study on anterior cruciate ligament reconstruction. Sci Rep. 2016;6:21704. doi: 10.1038/srep21704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson F., Billinghurst R.C., Pidoux I., Reiner A., Langworthy M., McDermott M. Early post-traumatic osteoarthritis-like changes in human articular cartilage following rupture of the anterior cruciate ligament. Osteoarthritis Cartilage. 2006;14(2):114–119. doi: 10.1016/j.joca.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Rahr-Wagner L., Thillemann T.M., Pedersen A.B., Lind M. Comparison of hamstring tendon and patellar tendon grafts in anterior cruciate ligament reconstruction in a nationwide population-based cohort study: results from the Danish registry of knee ligament reconstruction. Am J Sports Med. 2014;42(2):278–284. doi: 10.1177/0363546513509220. [DOI] [PubMed] [Google Scholar]
- 8.Liu H., Fan H., Wang Y., Toh S.L., Goh J.C. The interaction between a combined knitted silk scaffold and microporous silk sponge with human mesenchymal stem cells for ligament tissue engineering. Biomaterials. 2008;29(6):662–674. doi: 10.1016/j.biomaterials.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa D., Espinosa M., Calvo R., Scheu M., Vaisman A., Gallegos M. Anterior cruciate ligament regeneration using mesenchymal stem cells and collagen type I scaffold in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1196–1202. doi: 10.1007/s00167-013-2471-6. [DOI] [PubMed] [Google Scholar]
- 10.Proffen B.L., Vavken P., Haslauer C.M., Fleming B.C., Harris C.E., Machan J.T. Addition of autologous mesenchymal stem cells to whole blood for bioenhanced ACL repair has no benefit in the porcine model. Am J Sports Med. 2015;43(2):320–330. doi: 10.1177/0363546514559826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu W., Li Q., Tang X., Chen G., Zhang C., Li J. Mesenchymal stem cells reside in anterior cruciate ligament remnants in situ. Int Orthop. 2016;40(7):1523–1530. doi: 10.1007/s00264-015-2925-1. [DOI] [PubMed] [Google Scholar]
- 12.Ghebes C.A., Kelder C., Schot T., Renard A.J., Pakvis D.F., Fernandes H. Anterior cruciate ligament- and hamstring tendon-derived cells: in vitro differential properties of cells involved in ACL reconstruction. J Tissue Eng Regen Med. 2017;11(4):1077–1088. doi: 10.1002/term.2009. [DOI] [PubMed] [Google Scholar]
- 13.Mabuchi Y., Morikawa S., Harada S., Niibe K., Suzuki S., Renault-Mihara F. LNGFR(+)THY-1(+)VCAM-1(hi+) cells reveal functionally distinct subpopulations in mesenchymal stem cells. Stem Cell Reports. 2013;1(2):152–165. doi: 10.1016/j.stemcr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong W.K., Tan C.S., Chan K.L., Goesantoso G.G., Chan X.H., Chan E. Identification of specific cell-surface markers of adipose-derived stem cells from subcutaneous and visceral fat depots. Stem Cell Reports. 2014;2(2):171–179. doi: 10.1016/j.stemcr.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Battula V.L., Treml S., Abele H., Buhring H.J. Prospective isolation and characterization of mesenchymal stem cells from human placenta using a frizzled-9-specific monoclonal antibody. Differentiation. 2008;76(4):326–336. doi: 10.1111/j.1432-0436.2007.00225.x. [DOI] [PubMed] [Google Scholar]
- 16.Ogata Y., Mabuchi Y., Yoshida M., Suto E.G., Suzuki N., Muneta T. Purified human synovium mesenchymal stem cells as a good resource for cartilage regeneration. PLoS One. 2015;10(6):e0129096. doi: 10.1371/journal.pone.0129096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasui T., Mabuchi Y., Toriumi H., Ebine T., Niibe K., Houlihan D.D. Purified human dental pulp stem cells promote osteogenic regeneration. J Dent Res. 2015;95(2):206–214. doi: 10.1177/0022034515610748. [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch Histol Cytol. 2003;66(2):123–143. doi: 10.1679/aohc.66.123. [DOI] [PubMed] [Google Scholar]
- 19.Otabe K., Nakahara H., Hasegawa A., Matsukawa T., Ayabe F., Onizuka N. Transcription factor Mohawk controls tenogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo. J Orthop Res. 2015;33(1):1–8. doi: 10.1002/jor.22750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 21.Li H., Ghazanfari R., Zacharaki D., Ditzel N., Isern J., Ekblom M. Low/negative expression of PDGFR-alpha identifies the candidate primary mesenchymal stromal cells in adult human bone marrow. Stem Cell Reports. 2014;3(6):965–974. doi: 10.1016/j.stemcr.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suto E.G., Mabuchi Y., Suzuki N., Suzuki K., Ogata Y., Taguchi M. Prospectively isolated mesenchymal stem/stromal cells are enriched in the CD73+ population and exhibit efficacy after transplantation. Sci Rep. 2017;7(1):4838. doi: 10.1038/s41598-017-05099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakahara H., Hasegawa A., Otabe K., Ayabe F., Matsukawa T., Onizuka N. Transcription factor Mohawk and the pathogenesis of human anterior cruciate ligament degradation. Arthritis Rheum. 2013;65(8):2081–2089. doi: 10.1002/art.38020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamichi R., Ito Y., Inui M., Onizuka N., Kayama T., Kataoka K. Mohawk promotes the maintenance and regeneration of the outer annulus fibrosus of intervertebral discs. Nat Commun. 2016;7:12503. doi: 10.1038/ncomms12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 26.Mabuchi Y., Matsuzaki Y. Prospective isolation of resident adult human mesenchymal stem cell population from multiple organs. Int J Hematol. 2016;103(2):138–144. doi: 10.1007/s12185-015-1921-y. [DOI] [PubMed] [Google Scholar]
- 27.Mabuchi Y., Houlihan D.D., Akazawa C., Okano H., Matsuzaki Y. Prospective isolation of murine and human bone marrow mesenchymal stem cells based on surface markers. Stem Cells Int. 2013;2013 doi: 10.1155/2013/507301. 507301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quirici N., Soligo D., Bossolasco P., Servida F., Lumini C., Deliliers G.L. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30(7):783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 29.Morikawa S., Mabuchi Y., Niibe K., Suzuki S., Nagoshi N., Sunabori T. Development of mesenchymal stem cells partially originate from the neural crest. Biochem Biophys Res Commun. 2009;379(4):1114–1119. doi: 10.1016/j.bbrc.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 30.Takashima Y., Era T., Nakao K., Kondo S., Kasuga M., Smith A.G. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129(7):1377–1388. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 31.Nagoshi N., Shibata S., Kubota Y., Nakamura M., Nagai Y., Satoh E. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell. 2008;2(4):392–403. doi: 10.1016/j.stem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Kerkela E., Laitinen A., Rabina J., Valkonen S., Takatalo M., Larjo A. Adenosinergic immunosuppression by human mesenchymal stromal cells requires co-operation with T cells. Stem Cells. 2016;34(3):781–790. doi: 10.1002/stem.2280. [DOI] [PubMed] [Google Scholar]
- 33.Ozeki N., Muneta T., Koga H., Nakagawa Y., Mizuno M., Tsuji K. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthritis Cartilage. 2016;24(6):1061–1070. doi: 10.1016/j.joca.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Lee D.H., Ng J., Kim S.B., Sonn C.H., Lee K.M., Han S.B. Effect of donor age on the proportion of mesenchymal stem cells derived from anterior cruciate ligaments. PLoS One. 2015;10(3):e0117224. doi: 10.1371/journal.pone.0117224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedenstein A.J., Petrakova K.V., Kurolesova A.I., Frolova G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230–247. [PubMed] [Google Scholar]
- 36.Gullo F., De Bari C. Prospective purification of a subpopulation of human synovial mesenchymal stem cells with enhanced chondro-osteogenic potency. Rheumatology (Oxford) 2013;52(10):1758–1768. doi: 10.1093/rheumatology/ket205. [DOI] [PubMed] [Google Scholar]
- 37.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 38.Swift J., Ivanovska I.L., Buxboim A., Harada T., Dingal P.C., Pinter J. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341(6149) doi: 10.1126/science.1240104. 1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.