Abstract
Background
The aim of this observational case-control study was to compare the levels of plasma resistin between patients with acute aortic dissection and matched controls, and to use propensity score matching (PSM) to reduce case selection bias and clinical confounders.
Material/Methods
With the use of PSM, this study included 43 pairs of patients with acute aortic dissection (type-A and type-B dissection) and matched controls. Plasma resistin levels and other laboratory parameters were compared between the two groups, including white blood cell (WBC) count, glucose, high sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and D-dimer. The correlations between resistin and other laboratory parameters were evaluated in patients with acute aortic dissection.
Results
Following PSM adjustment for clinical variables, including age, sex, body mass index, smoking, alcohol drinking, hypertension, diabetes mellitus, coronary heart disease and stroke, plasma resistin levels were significantly increased in patients with acute aortic dissection when compared with controls (35.2±13.8 vs. 18.4±9.1 ng/ml) (p<0.001). WBC counts, and levels of glucose, hs-CRP, IL-6, TNF-α and D-dimer were also significantly increased in the patients with aortic dissection compared with the control group. After adjustment for these variables, the association between plasma resistin levels and acute aortic dissection remained significant (OR, 1.114; 95% CI, 1.036–1.224) (p<0.001). Plasma resistin levels was positively correlated with WBC count (r=0.368, p=0.015), hs-CRP (r=0.359, p=0.022), IL-6 (r=0.306, p=0.046) and TNF-α levels (r=0.315, p=0.040) in patients with acute aortic dissection.
Conclusions
Acute aortic dissection is associated with elevated levels of plasma resistin and other pro-inflammatory cytokines. Plasma resistin levels is positively associated with other pro-inflammatory cytokines in acute aortic dissection.
MeSH Keywords: Aneurysm, Dissecting; Case-Control Studies; Propensity Score; Resistin
Background
Acute aortic dissection has an estimated incidence of 6 cases per 100,000 persons per year and presents as a surgical emergency [1]. Acute aortic dissection has a high mortality rate, and the risk of death for patients with aortic dissection increases by 1% per hour before medical and surgical intervention [2]. Despite increasingly rapid diagnosis and surgical management, the in-hospital mortality rate for acute aortic dissection remains at more than 30% [3]. Also, the mechanisms that underly the development and progression of spontaneous cases of acute aortic dissection remain unclear, which means that a screening or prevention strategy for these patients have yet to be developed. Aortic dissection is believed to have multiple components involved in its etiology, including hypertension, aortic weakness, persistent inflammation, genetic predisposition, and connective tissue disorders, with recent interest in the role of inflammation [4].
Adipose tissue is now known not to be just an energy depot, but adipocytes are now known to secrete cytokine, known as adipokines [5]. Since the discovery of the first adipokine, leptin, in 1994 studies have shown that adipokines not only participate in metabolic processes, but also in inflammatory and immune responses [6]. Most adipokines are pro-inflammatory, including leptin, resistin, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), and promote the development of cardiovascular disease, as well as metabolic syndrome [7].
Resistin is an adipokine that has been shown to induce insulin resistance and glucose intolerance in mice [8]. In humans, resistin is also secreted by macrophages, targets vascular endothelial cells, and regulates other pro-inflammatory cytokines in response to inflammation [9]. Increased levels of plasma resistin have been shown to be associated with increased severity of diabetes, coronary artery atherosclerosis, and heart failure [10]. A previously published study showed that plasma levels of resistin were independently associated with non-dissecting atherosclerotic abdominal aortic aneurysm [11].
Aortic dissection occurs as a result of a genetic or acquired weakness in the aortic media and may be associated with inflammation and with aortic aneurysm [2]. Given the findings of previous studies on the pro-inflammatory and vascular associations with resistin and the lack of previous studies on plasma resistin levels in patients with acute aortic dissection, the present study was designed to investigate whether resistin might be involved in the pathogenesis of acute aortic dissection.
The aim of this observational case-control study was to compare the levels of plasma resistin and other pro-inflammatory cytokines in patients who presented with acute aortic dissection, with matched controls, and to use propensity score matching (PSM) to reduce case selection bias and clinical confounders.
Material and Methods
Patients studied
A hospital-based, cross-sectional, observational, case-control study was undertaken to compare the plasma resistin levels in patients with acute aortic dissection with matched control subjects in the Wuhan Asia Heart Hospital, Wuhan, China. The study protocol was approved by the Ethics Committee of the Wuhan Asia Heart Hospital. An informed written consent was signed by all individuals who participated in the study.
Forty-three patients (31 men and 12 women) with acute aortic dissection were enrolled in the study. The mean age of patients was 58.2±8.1 years. The diagnosis of aortic dissection was confirmed by computed tomography (CT) imaging using multidetector computed tomography (MDCT). Patients were excluded from the study who had recurrent, chronic, iatrogenic or traumatic aortic dissection, Marfan’s syndrome, and intramural aortic hematoma. Because a previous history of cardiac surgery, myocardial infarction, valvular heart disease, infection, or other inflammatory diseases are known to influence plasma levels of resistin, patients with these conditions were also excluded from the study. There were 43 patients who were identified as presenting with acute aortic dissection and who were suitable for inclusion in the study.
The control population was identified from an initial group of 826 healthy individuals who underwent a comprehensive routine outpatient health examination, of which, 43 subjects were confirmed to have normal findings on physical and echocardiographic examination and who were matched by age, gender with the study group.
Data collection and classification of acute aortic dissection
Clinical data were obtained from patient medical records on hospital admission. Demographic data and past medical history were recorded. The Stanford classification system was used to categorize aortic dissection into type A and type B, based on whether or not the ascending aorta was involved [3]. Acute aortic dissection was defined as patient admission to hospital within 14 days after the onset of symptoms [12]. For each patient, the height and body weight were used to calculate the body mass index (BMI), the estimated glomerular filtration rate (eGFR) was computed by using the abbreviated Modification of Diet in Renal Disease (MDRD) equation: 186.3×creatinine−1.154 ×age−0.203×(0.742 if female) [13].
Blood sampling and laboratory measurements
Blood samples were obtained when patients with aortic dissection were admitted to the emergency room before treatment. For the control group, fasting blood samples were obtained in the morning, before the physical examination. Plasma was separated by centrifuging the blood samples at 4,000 rpm for 10 min. Resistin was analyzed using an enzyme-linked immunosorbent assay (ELISA) (Biovendor, Brno, Czech Republic) with the between sample replicant coefficient of variability (CV) established as an intra-assay CV% of 5.9% and inter-assay CV% of 7.6%. All samples were analyzed according to the manufacturer’s instructions.
Other laboratory data recorded for each study participant included white blood cell (WBC) count, creatinine, glucose, hemoglobin A1c (HbA1c), triglyceride (TG), total cholesterol (TC), low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), high sensitivity C-reactive protein (hs-CRP), interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α) and D-dimer. Laboratory tests were performed in the central local laboratory according to established and validated clinical laboratory protocols.
Statistical analysis
This observational case-control study used propensity score matching (PSM) to reduce case-control selection bias and to reduce potential clinical confounders using a 1: 1 matching protocol. The model was evaluated by the Hosmer-Lemeshow goodness of fit test for logistic regression analysis, and the C-statistic test. Before PSM was performed, the clinical characteristics of the patient group and the control group were compared, using Student’s t-test for continuous variables and the chi-squared (χ2) test for categorical variables. After PSM was performed, the clinical characteristics and laboratory parameters were compared with a paired t-test for continuous variables and McNemar’s test for categorical variables. Paired logistic regression analysis was performed to assess the association between plasma resistin levels, laboratory parameters, and the presence of acute aortic dissection. The correlations between plasma resistin levels and other laboratory variables were determined by Pearson’s correlation for normally distributed data (WBC, creatinine, eGFR, glucose, HbA1c, TG, TC, LDL-C, and HDL-C) or Spearman’s correlation coefficient for skewed data (hs-CRP, IL-6, TNF-α, and D-dimer). A two-tailed p-value <0.05 was considered as statistically significant. All analysis was performed using SPSS version 21.0 (IBM, Armonk, NY, USA).
Results
The clinical characteristics of the 43 patients with aortic dissection and the 43 control subjects are presented in Table 1. Before propensity score matching (PSM) was used to reduce selection bias and potential clinical confounders, there were major significant differences between the two groups for several clinical variables, including age, gender, smoking, alcohol intake, hypertension, and diabetes. With the use of PSM, the comparison between the 43 matched pairs of patients with aortic dissection and the control subjects, using the Hosmer–Lemeshow goodness of fit test for logistic regression analysis was p=0.68, and with the C-statistic test, it was p=0.81. Following PSM, the differences in clinical characteristics were eliminated between the two groups. In the aortic dissection group, there were 15 cases of type A aortic dissection, and 28 cases of type B aortic dissection, based on the Stanford classification [3].
Table 1.
Clinical characteristics in AD patients and control subjects before and after PSM.
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| AD | Control | P | AD | Control | P | |
| Patients number | 43 | 826 | 43 | 43 | ||
| Age, years | 58.2±8.1 | 55.1±12.4 | 0.021 | 58.2±8.1 | 58.2±8.1 | 0.786 |
| Men | 31 (72.1) | 437 (52.9) | 0.014 | 31 (72.1) | 31 (72.1) | 1.000 |
| BMI, kg/m2 | 25.3±3.3 | 24.9±3.4 | 0.452 | 25.3±3.3 | 25.3±3.3 | 0.937 |
| Smoking | 24 (55.8) | 332 (40.2) | 0.042 | 24 (55.8) | 24 (55.8) | 1.000 |
| Alcohol drinking | 23 (53.5) | 303 (36.7) | 0.027 | 23 (53.5) | 23 (53.5) | 1.000 |
| Hypertension | 31 (72.1) | 382 (46.2) | 0.001 | 31 (72.1) | 31 (72.1) | 1.000 |
| Diabetes mellitus | 2 (4.7) | 132 (16.0) | 0.045 | 2 (4.7) | 2 (4.7) | 1.000 |
| Coronary heart disease | 7 (16.3) | 159 (19.2) | 0.629 | 7 (16.3) | 7 (16.3) | 1.000 |
| Stroke | 3 (7.0) | 71 (8.6) | 0.928 | 3 (7.0) | 3 (7.0) | 1.000 |
| Stanford classification | ||||||
| Type-A | 15 (34.9) | 15 (34.9) | ||||
| Type-B | 28 (65.1) | 28 (65.1) | ||||
Values are mean ±SD or n (%). AD – aortic dissection; BMI – body mass index; PSM – propensity score matching.
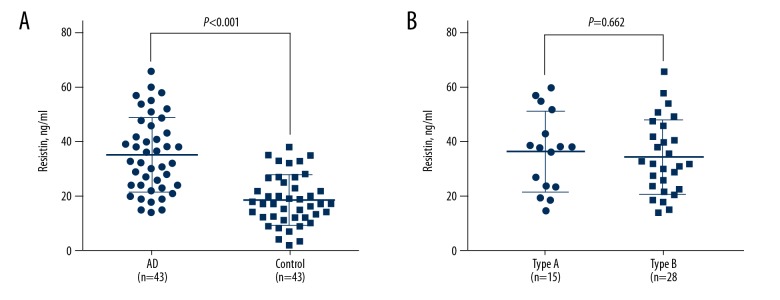
The level of plasma resistin was significantly increased in patients with aortic dissection when compared with the control subjects (35.2±13.8 vs. 18.4±9.1 ng/ml) (p<0.001) (Figure 1A). There was no significant difference in plasma resistin level between type A and type B aortic dissection (36.5±14.8 vs. 34.5±13.5 ng/ml) (p=0.662) (Figure 1B). The laboratory findings are summarized in Table 2 and show that the white blood cell (WBC) count, glucose, high sensitivity C-reactive protein (hs-CRP), interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α) and D-dimer were all increased in the patients with aortic dissection compared with the control group. No significant differences were found between the two groups in the levels of creatinine, the estimated glomerular filtration rate (eGFR), hemoglobin A1c (HbA1c), triglyceride (TG), total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), and high-density lipoprotein-cholesterol (HDL-C).
Figure 1.
Plasma resistin levels comparison between acute aortic dissection and control (A) and between type-A and type-B acute aortic dissection (B).
Table 2.
Laboratory findings in AD patients and control subjects.
| AD | Control | P | |
|---|---|---|---|
| Patients number | 43 | 43 | |
| WBC count, 109/L | 12.1±3.5 | 6.3±2.1 | <0.001 |
| Creatinine, umol/L | 80.1±24.9 | 78.9±11.2 | 0.776 |
| eGFR, ml/min/1.73 m2 | 88.1±20.1 | 90.9±11.9 | 0.285 |
| Glucose, mmol/L | 7.44±1.85 | 5.99±1.29 | <0.001 |
| HbA1c,% | 5.55±0.34 | 5.58±0.35 | 0.688 |
| TG, mmol/L | 1.31±0.83 | 1.56±0.64 | 0.125 |
| TC, mmol/L | 4.26±0.90 | 4.08±0.57 | 0.266 |
| LDL-C, mmol/L | 2.39±0.53 | 2.37±0.39 | 0.235 |
| HDL-C, mmol/L | 1.25±0.34 | 1.17±0.20 | 0.213 |
| hs-CRP, mg/L | 6.6 (2.3–41.6) | 2.4 (0.7–8.0) | <0.001 |
| IL-6, pg/ml | 29.3 (13.7–52.1) | 6.8 (5.3–21.1) | <0.001 |
| TNF-α, pg/ml | 7.0 (4.4–9.4) | 4.8 (3.6–6.1) | <0.001 |
| D-dimer, ug/ml | 1.02 (0.54–2.53) | 0.08 (0.06–0.13) | <0.001 |
Values are mean ±SD or median (interquartile range). AD – aortic dissection; eGFR – evaluated glomerular filtration rate; hs-CRP – high-sensitivity C-reactive protein; HDL-C – high density lipoprotein cholesterol; HbA1c – hemoglobin A1c; IL-6 – interleukin 6; LDL-C – low density lipoprotein cholesterol; TG – triglyceride; TC – total cholesterol; TNF – tumor necrosis factor; WBC – white blood cell.
Table 3 shows the results of the paired logistic regression analysis. The association between resistin and aortic dissection remained significant after adjustment for WBC, hs-CRP, IL-6, TNF-α, glucose, and D-dimer, which are shown in Table 2. Also, the correlations between resistin and other laboratory parameters were analyzed in patients with aortic dissection (Table 4). Although the correlation factors were small, the plasma resistin level was positively correlated with WBC, creatinine, hs-CRP, IL-6, and TNF-α, but inversely correlated with eGFR.
Table 3.
Association between resistin and laboratory findings and AD.
| Unadjusted model | Adjusted model* | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Resistin | 1.140 | 1.076–1.207 | <0.001 | 1.114 | 1.036–1.224 | <0.001 |
| WBC | 2.064 | 1.538–2.769 | <0.001 | 1.753 | 1.255–2.450 | <0.001 |
| Glucose | 2.101 | 1.396–3.162 | <0.001 | 2.037 | 0.726–5.716 | 0.177 |
| hs-CRP | 1.052 | 1.015–1.093 | <0.001 | 1.119 | 1.014–1.233 | 0.025 |
| IL-6 | 1.080 | 1.038–1.123 | <0.001 | 1.002 | 0.926–1.173 | 0.820 |
| TNF-α | 1.137 | 1.089–1.307 | <0.001 | 1.038 | 0.671–1.604 | 0.868 |
| D-dimer | 6.971 | 2.765–31.125 | <0.001 | 5.388 | 2.134–22.768 | <0.001 |
Adjustment for resistin, WBC, glucose, hs-CRP, IL-6, TNF-α and D-dimer.
AD – aortic dissection; hs-CRP – high-sensitivity C-reactive protein; IL-6 – interleukin 6; TNF – tumor necrosis factor; WBC – white blood cell; OR – odds ratio; CI – confidence interval.
Table 4.
Correlation coefficient between resistin and laboratory findings in patients with AD.
| Resistin | P | ||
|---|---|---|---|
| Pearson | Spearman | ||
| WBC | 0.368 | – | 0.015 |
| Creatinine | 0.481 | – | 0.001 |
| eGFR | −0.437 | – | 0.003 |
| Glucose | −0.006 | – | 0.969 |
| HbA1c | −0.155 | – | 0.327 |
| TG | −0.053 | – | 0.736 |
| TC | −0.159 | – | 0.307 |
| LDL-C | −0.247 | – | 0.111 |
| HDL-C | −0.132 | – | 0.397 |
| hs-CRP | – | 0.359 | 0.022 |
| IL-6 | – | 0.306 | 0.046 |
| TNF-α | – | 0.315 | 0.040 |
| D-dimer | – | 0.276 | 0.073 |
AD – aortic dissection; eGFR – evaluated glomerular filtration rate; hs-CRP – high-sensitivity C-reactive protein; HDL-C – high density lipoprotein cholesterol; HbA1c – hemoglobin A1c; IL-6 – interleukin 6; LDL-C – low density lipoprotein cholesterol; TG – triglyceride; TC – total cholesterol; TNF – tumor necrosis factor; WBC – white blood cell.
Discussion
The findings of this observational case-control study used propensity score matching (PSM) to reduce case selection bias and clinical confounders, to investigate the relationship between resistin, one of the pro-inflammatory adipokines, and aortic dissection by measuring the plasma resistin levels in 43 pairs of patients with acute aortic dissection and control subjects. After PSM for age, gender, body mass index (BMI), a history of smoking, alcohol intake, hypertension, diabetes mellitus, coronary heart disease, and stroke, the plasma resistin levels were significantly increased in patients with acute aortic dissection compared with control subjects. After further adjustment for white blood cell (WBC) count, high sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), glucose and D-dimer, which were also increased in the aortic dissection group, the association between resistin and acute aortic dissection remained significant. Also, plasma resistin levels were positively correlated with pro-inflammatory cytokines, including hs-CRP, IL-6, and TNF-α in patients with aortic dissection.
Resistin was first described in 2001 as an adipokine involved in the development of insulin resistance [8]. There is only about 60% homology in the mRNA or amino acid structure between rodent and human resistin [14]. Macrophages are the major resource of resistin in humans [9]. Resistin is a pro-inflammatory cytokine, which is involved in the NF-κB signaling pathway, and resistin has been shown to induce IL-6, IL-1β, and TNF-α as part of the inflammatory response [15]. All these reported characteristics for resistin support its possible role in diseases other than metabolic disease. Although the specific mechanisms remain unknown, resistin may be involved in the progression of cardiovascular disease by stimulating inflammation [16]. Previously published clinical studies have shown that plasma resistin levels were significantly associated with risk factors and adverse clinical outcomes in cardiovascular disease, which further supports a role for resistin and its associated signaling pathways in cardiovascular disease [17].
Acute aortic dissection is rare, but can be fatal, and requires rapid diagnosis and surgical treatment. The overall survival (OS) rate following acute aortic dissection remains low, despite improvements in diagnosis and surgery. The mechanisms that promote the rupture of the aortic wall remain unclear. Apoptosis of smooth muscle cells in the aortic media may contribute to the weakness of aortic wall that may not be able to tolerate normal or increased blood pressure [18]. Atherosclerosis may also damage the aortic endothelium and the inflammation associated with the atherosclerotic plaque may become the focus for future aortic wall rupture or dissection [19].
Adipokines, such as resistin, are now believed to have a role in the development of cardiovascular disease by promoting inflammation, vascular endothelial cell dysfunction, and apoptosis in smooth muscle cells [20]. However, there have been few studies that have investigated the possible role of resistin in aortic dissection, despite the fact that a previous study has shown that plasma resistin levels were significantly elevated and independently associated with the development of aortic aneurysm [11]. The findings of the present study showed that plasma resistin levels were significantly increased in patients with acute aortic dissection, suggesting that resistin might be a diagnostic biomarker for this condition.
This study had several limitations. Although an attempt was made to eliminate the effects of age, sex, previous medical history, and other clinical confounders by using PSM, the relationship between resistin and aortic dissection may have been confounded by other unknown or unmeasured parameters. The second main limitation of this study was the small study sample size, obtained from a single center, which may have introduced study bias. The third main limitation of this study was that the patients included in the study presented with acute, sporadic aortic dissection with strict study exclusion criteria chosen to eliminate the possible confounding factors in the analysis of the association between aortic dissection and plasma levels of resistin. Therefore, in future studies, the relationship between plasma resistin levels and aortic dissection due to known causes, such as Marfan’s syndrome, should be investigated.
Conclusions
The aim of the present case-control study was to compare the levels of plasma resistin between patients with acute aortic dissection and matched controls, and to use propensity score matching (PSM) to reduce case selection bias and confounders. The findings showed that plasma resistin levels were significantly increased and associated with pro-inflammatory cytokines in patients with acute aortic dissection. Future studies should be undertaken to investigate the mechanisms and signaling pathways that might explain the role for resistin in acute aortic dissection, and future large-scale controlled clinical studies are needed to determine the potential diagnostic or predictive role of measurement of plasma resistin in this condition.
Footnotes
Conflict of interest
None
Source of support: The study was supported by Hubei Provincial Natural Science Foundation of China (No. 2017CFB381), Hubei Province Health and Family Planning Scientific Research Project (No.WJ2018H0050) and Innovational Funding in Wuhan Asia Heart Hospital (No. 2017CX4-A03)
References
- 1.Howard DP, Banerjee A, Fairhead JF, et al. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation. 2013;127:2031–37. doi: 10.1161/CIRCULATIONAHA.112.000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeMaire SA, Russell L. Epidemiology of thoracic aortic dissection. Nat Rev Cardiol. 2011;8:103–13. doi: 10.1038/nrcardio.2010.187. [DOI] [PubMed] [Google Scholar]
- 3.Golledge J, Eagle KA. Acute aortic dissection. Lancet. 2008;372:55–66. doi: 10.1016/S0140-6736(08)60994-0. [DOI] [PubMed] [Google Scholar]
- 4.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2873–926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 5.Shuldiner AR, Yang R, Gong DW. Resistin, obesity, and insulin resistance – the emerging role of the adipocyte as an endocrine organ. N Engl J Med. 2001;345:1345–46. doi: 10.1056/NEJM200111013451814. [DOI] [PubMed] [Google Scholar]
- 6.Tilg H, Moschen AR. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 7.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 9.Patel L, Buckels AC, Kinghorn IJ, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472–76. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 10.Jamaluddin MS, Weakley SM, Yao Q, Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2012;165:622–32. doi: 10.1111/j.1476-5381.2011.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golledge J, Clancy P, Jamrozik K, Norman PE. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116:2275–79. doi: 10.1161/CIRCULATIONAHA.107.717926. [DOI] [PubMed] [Google Scholar]
- 12.Pape LA, Awais M, Woznicki EM, et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the international registry of acute aortic dissection. J Am Coll Cardiol. 2015;66:350–58. doi: 10.1016/j.jacc.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 13.Zuo L, Ma YC, Zhou YH, et al. Application of GFR-estimating equations in Chinese patients with chronic kidney disease. Am J Kidney Dis. 2005;45:463–72. doi: 10.1053/j.ajkd.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Steppan CM, Lazar MA. The current biology of resistin. J Intern Med. 2004;255:439–47. doi: 10.1111/j.1365-2796.2004.01306.x. [DOI] [PubMed] [Google Scholar]
- 15.Jiang CY, Wang W, Tang JX, Yuan ZR. The adipocytokine resistin stimulates the production of proinflammatory cytokines TNF-alpha and IL-6 in pancreatic acinar cells via NF-kappaB activation. J Endocrinol Invest. 2013;36:986–92. doi: 10.3275/9002. [DOI] [PubMed] [Google Scholar]
- 16.Park HK, Kwak MK, Kim HJ, Ahima RS. Linking resistin, inflammation, and cardiometabolic diseases. Korean J Intern Med. 2017;32:239–47. doi: 10.3904/kjim.2016.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marouga A, Dalamaga M, Kastania AN, et al. Circulating resistin is a significant predictor of mortality independently from cardiovascular comorbidities in elderly, non-diabetic subjects with chronic kidney disease. Biomarkers. 2016;21:73–79. doi: 10.3109/1354750X.2015.1118536. [DOI] [PubMed] [Google Scholar]
- 18.Wu D, Shen YH, Russell L, et al. Molecular mechanisms of thoracic aortic dissection. J Surg Res. 2013;184:907–24. doi: 10.1016/j.jss.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbetseas J, Alexopoulos N, Brili S, et al. Atherosclerosis of the aorta in patients with acute thoracic aortic dissection. Circ J. 2008;72:1773–76. doi: 10.1253/circj.cj-08-0433. [DOI] [PubMed] [Google Scholar]
- 20.Zuniga MC, Raghuraman G, Zhou W. Physiologic levels of resistin induce a shift from proliferation to apoptosis in macrophage and VSMC co-culture. Surgery. 2018;164:906–11. doi: 10.1016/j.surg.2017.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]