Abstract
One new and six known flavone glycosides were isolated from the MeOH extract of Melilotus neapolitana Ten. The new compound, identified as 7-O-β-D-gluco-pyranosyloxy-4',5-dihydroxy-3-[O-α-L-rhamnopyranosyl-(1→6)-3-O-β-D-glucopyrano-syloxy]flavone (1) by 1D and 2D NMR techniques and mass spectra, was isolated along with kaempferol-3-O-rutinoside (2), kaempferol-3-O-glucoside (3), rutin (4), quercetin-3-O-glucoside (5), isorhamnetin-3-O-rutinoside (6), and isorhamnetin-3-O-glucoside (7). The antioxidant and radical scavenging activities of these compounds and the whole crude methanol extract were evaluated. The organic extract can inhibit MDA marker’s synthesis by 57%. All the metabolites displayed good reducing power, with the kaempferol (2,3) and isorhamnetin derivatives (6,7) being less active than the corresponding quercetin derivatives 4,5.
Keywords: Melilotus neapolitana Ten., flavone glycosides, ROSs, antioxidant activity
Introduction
Flavonoids are ubiquitous polyphenolic metabolites in plants that have diverse beneficial biochemical and antioxidant effects [1]. Their dietary intake is quite high, compared to other dietary antioxidants like vitamins C and E. The flavonoids have aroused considerable interest recently because of their potential beneficial effects on human health. They have been principally reported to have antioxidant activities [2]. Antioxidants are compounds that interact with harmful molecules in the body and may enhance the body's defence mechanisms against diseases like cancer and coronary heart disease [3]. Among potentially dangerous molecules, reactive oxygen species (ROSs) including oxygen ions, free radicals and peroxide are the main causes of many pathologies. They and are generally very small molecules. Their reactivity is due to the presence of unpaired valence shell electrons. ROSs are formed as a natural byproduct of the normal metabolism of oxygen and have important roles in cell signaling. However, during times of environmental stress ROS levels can increase dramatically, which can result in significant damage to cell structures.
The ROS scavenging activity of the flavonoids is imputable to the hydroxyl groups in the molecules. It has been observed that 2',3',4' hydroxyl substitution on the B ring plays a crucial role in radical scavenger activity in the DPPH radical assay and in the inhibitory effect on peroxydation of tissue lipids [4].
Melilotus spp. contain mainly aromatic compounds and many flavonoids have been isolated and characterized from plants belonging to this genus. Recently we have reported the isolation of new metabolites from plants having a strong antioxidant and radical scavenging activities [5,6]. In this study, one new and six known flavone glycosides were isolated from Melilotus neapolitana and the radical scavenging and antioxidant activities of these compounds were evaluated.
Results and Discussion
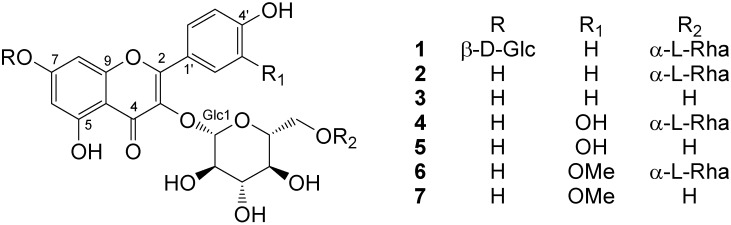
A new flavonoid glycoside, identified as 7-O-β-D-glucopyranosyloxy-4',5-dihydroxy-3-[O-α-L-rhamnopyranosyl-(1→6)-3-O-β-D-glucopyranosyloxy]flavone (1) was isolated, along with six known flavone glycosides, from the whole plant extract of M. neapolitana Ten. The known flavones were identified as kaempferol-3-O-rutinoside (2) [7], kaempferol-3-O-glucoside (3) [7], rutin (4) [8], quercetin-3-O-glucoside (5) [8], isorhamnetin-3-O-rutinoside (6) [9], and isorhamnetin-3-O-glucoside (7) [9], respectively (Figure 1). All the compounds were identified on the basis of their spectroscopic features obtained, mainly, by 1D and 2D NMR and mass spectroscopic techniques. The structures of known compounds were confirmed by comparison to literature data.
Figure 1.
Flavone glycosides from M. neapolitana.
Compound 1 showed a molecular formula C33H40O20, as deduced from the elemental analysis and the ESI mass spectrum, which showed the pseudomolecular peak at m/z 757 [M + H]+.. In the aromatic region of the 1H-NMR spectrum an AA'BB' system, appearing as two doublets at δ 8.12 and 6.89 and two meta coupled doublet protons at δ 6.75 and 6.51 were evident. In the saccharide region of the spectrum three anomeric proton signals were present, indicating the presence of three sugar moieties. Two protons were present as large doublets at δ 5.13 and 5.04, while the third signal resonated at δ 4.56 as a narrow doublet. In the upfield region of the 1H-NMR a methyl doublet at δ 1.17 was evident, indicating the presence of a rhamnose unit in the molecule. The 13C-NMR spectrum showed signals for 33 carbons identified, on the basis of a DEPT experiment, as 1 methyl, 2 methylenes, 19 methines, (including two signals of the four carbons of the AA'BB' system) and 9 tetrasubstituted carbons. These data were in accordance with the presence of kaempferol as aglycone and three sugar units. The acid hydrolysis gave an aglycone confirmed as kaempferol by comparison of its spectral data with those previously reported [7], and the sugar components which were isolated by HPLC and identified as D-glucose and L-rhamnose by GC analysis of the corresponding alditol acetates. The GC-mass spectral identification of 1,5-di-O-acethyl-2,3,4,6-tetramethylglucitol, 1,5,6-tri-O-acethyl-2,3,4-tri-O-methyl-glucitol and 1,5-di-O-acethyl-2,3,4-tri-O-methylrhamnitol indicate the presence of a disaccharide and a monosaccharide moieties in the molecule bonded to the aglycone by two different glycosylation sites. The downfield shift of the H-6 and H-8 proton, as well as the upfield shifts of the corresponding carbons at δ 94.6 and 92.0, respect to corresponding signals of the aglicone, suggested the linkage with the sugar moiety across the oxygen of the C(7)-OH group. In fact the HMBC experiment showed a correlation between the carbon signal at δ 171.1 and the anomeric proton at δ 5.04 of a glucose unit. This signal showed correlations with the carbon at δ 75.1, attributed to the C-5 carbon, which in turn showed correlations with the H-Glc6' methylene protons bonded to the carbon at δ 62.2. These data indicated the absence of further glycosylations on the glucose moiety. NOE observed in a NOESY experiment between the anomeric proton and the H-6 and H-8 aromatic protons confirmed these hypotheses. The disaccharide was thus identified as rutinose, which was positioned on the C-3 carbon on the basis of the two-dimensional data.
Reactive oxygen species scavenging activities
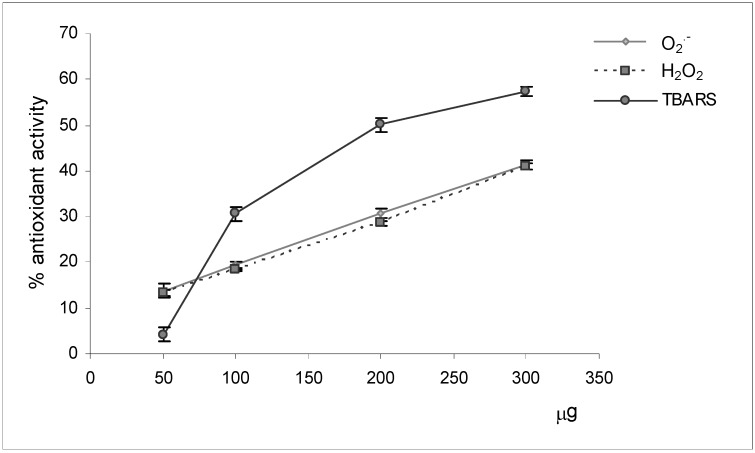
The methanolic extract of dried plants of M. neapolitana Ten. has been submitted to preliminary biological screening using suitable assays aiming at an evaluation of its potential antioxidant activity (Figure 2). Aliquots of the crude extract (50, 100, 200, 300 μg), dissolved in appropriate solvents, were added to specific assay media characterized to the presence of reactive radical species, the free radical O2.- and the prooxidant H2O2, or of markers of lipoperoxidation processes (TBARS).
Figure 2.
Antioxidant activity of M. neapolitana Ten. methanolic extract.
The results of radical scavenging activity assays showed that the extract exercised a good and comparable reducing power towards the radical target species. In fact it scavenged both radicals in a dose-response manner, causing an inhibition of 41% at the higher tested concentration. The results of the assay evaluating the capacity to inhibit the formation of TBARS species confirmed the valid antioxidant capacity of the extract. In fact, the results of this assay allowed us to observe an exponential increase of the activity with the amount of sample tested. The addition of aliquots of extract equal to 300 μg determined an inhibition of 57% of MDA marker’s synthesis.
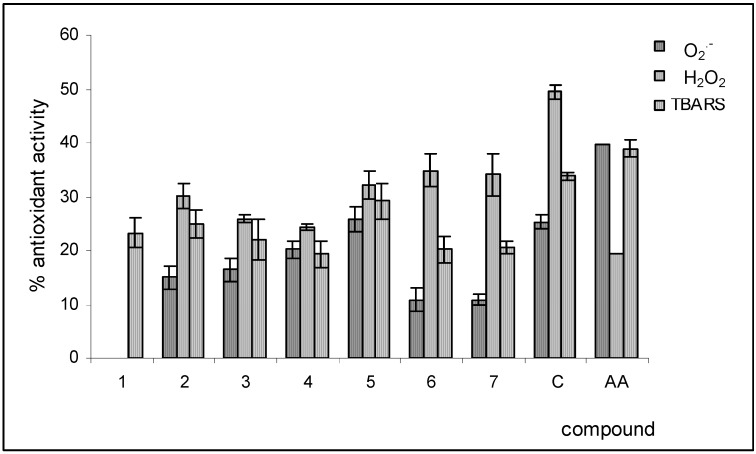
Pure metabolites were the object of an analogous antioxidant property investigation. The metabolites were assayed at a concentration of 0.3 mM and their activities were compared with that of catechin (C) and ascorbic acid (AA), two known natural antioxidant molecules. The results are reported in Figure 3. The isolated substances, except for the untested metabolite 1, showed scavenging activity towards the free radical O2.- and the prooxidant hydrogen peroxide. The strongest activities were exercised by metabolite 5, inhibiting the reduction of two target species by 26% and 32%, respectively. The kaempferol (2, 3) and isorhamnetin (6, 7) derivatives resulted less active compared to the corresponding quercetin derivatives 4, 5. All the metabolites caused inhibition of the formation of TBARS species, but the activities registered were less than of those dispalyed by standard compounds.
Figure 3.
Antioxidant activity of M. neapolitana Ten. flavones glycosides. C = catechin; AA = ascorbic acid.
Conclusions
In the present study we reported the isolation from M. neapolitana of six known flavonoid glycosides and a new metabolite, identified as 7-O-β;-D-glucopyranosyloxy-4’,5-dihydroxy-3-[O-α-L-rhamnopyranosyl-(1→6)-3-O-β-D-glucopyranosyloxy]flavone (1). The antioxidant activities of the crude methanol extract of the studied plant and the pure compounds have been reported. The data obtained confirmed the role of the hydroxylation pattern on the flavone B-ring in determining antioxidant activity and demonstrated that the presence of glycone moieties resulted in a decrease of antioxidative capacity.
Experimental Section
General
NMR spectra were recorded at 300 (1H) and 75 MHz (13C) on a Varian 300 FT-NMR spectrometer in CD3OD, at 25 °C. Proton-detected heteronuclear correlations were measured by gradient heteronuclear single-quantum coherence (HSQC) optimized for 1J(H,C) = 140 Hz, and by gradient heteronuclear multiple-bond coherence (HMBC) optimized for nJ(H,C) = 8 Hz. Electrospray mass spectra were recorded using a Waters ZQTM mass spectrometer equipped with an electrospray ionization (ESI) probe operating in positive or negative ion mode. The scan range was m/z 80-2,000. Analytical TLC was performed on Kieselgel 60 F254 (Merck) or RP-8 F254 (Merck) plates with 0.2 mm layer thickness. Spots were visualized by UV light or by spraying with H2SO4/AcOH/H2O (1:20:4). The plates were then heated for 5 min at 110 °C. Preparative TLC was performed on Kieselgel 60 F254 (Merck) plates, with 0.5 or 1 mm film thickness. Column chromatography (CC) was performed on Kieselgel 60 (70-240 mesh, Merck), RP-8 (Baker) and RP-18 (Baker). The preparative HPLC apparatus consisted of a pump (Shimadzu LC-10AD), a refractive index detector (Shimadzu RID-10A) and a Shimadzu Chromatopac C-R6A recorder. Preparative HPLC was performed using a RP-8 column (Luna 10 μm, 250 x 10 mm i.d., Phenomenex).
Plant material
Plants of Melilotus neapolitana (Leguminosae) were collected in the Spring of 2004 in the vegetative state, in the Natural Reserve of Castel Volturno (Caserta, Italy), and identified by Dr. Assunta Esposito of the Second University of Naples. A voucher specimen (CE240) has been deposited at the Herbarium of the Dipartimento di Scienze della Vita of the Second University of Naples.
Extraction and isolation
Dried M. neapolitana plants (425 g) were extracted in methanol for 5 days at 4°C in a dark refrigerated room. The organic solution, distilled under reduced pressure on a Rotavapor® to obtain 36.1 g of crude extract, was dissolved in water (1.0 L) and shaken with EtOAc (1.5 L) to obtain an organic (9.1 g) and aqueous (23.0 g) fractions. The EtOAc fraction was chromatographed on SiO2 with MeOH-CHCl3 solutions as eluents to give two fractions I-II.
Fraction I (896.0 mg), eluted with MeOH-CHCl3 (1:4), was chromatographed on SiO2 under N2 pressure eluting with the lower organic phase of a CHCl3/MeOH/H2O (13:7:3) biphasic solution to obtain three fractions A-C. Fraction A (40 mg), re-chromatographed on TLC (1.0 mm) eluting EtOAc/EtOH/H2O (24:4:1), furnished pure metabolite 7 (6.8 mg). Fraction B (299.0 mg) was purified by column chromatography (RP-8) by eluting with MeOH/MeCN/H2O (1:2:7) to obtain a fraction (20.5 mg) which, purified by TLC (0.5 mm) eluting with EtOAc/EtOH/H2O (24:4:1), supplied pure compounds 3 (6.0 mg), 5 (7.0 mg) and 6 (5.0 mg). Fraction C (71.4 mg) was rechromatographed on an RP-8 column eluting with MeOH/MeCN/H2O (1:1:3). One of the obtained fractions (12.0 mg), purified by RP-8 HPLC eluting with MeCN/H2O (1:4), gave pure metabolite 2 (3.0 mg).
Fraction II (530.0 mg), eluted with MeOH-CHCl3 (1:4), was chromatographed on SiO2 under N2 pressure eluting with a EtOAc/EtOH/H2O (24:4:1) solution to obtain a fraction (130.0 mg) which was re-chromatographed on RP-18 by column chromatography eluting with MeOH/MeCN/H2O (1:2:7). One of the obtained fractions (7.0 mg), purified by TLC (0.2 mm) eluting with EtOAc/EtOH/H2O (24:4:1), gave pure compounds 1 (1.0 mg) and 4 (2.5 mg). 7-O-β-D-glucopyranosyloxy-4’,5-dihydroxy-3-[O-a-L-rhamnopyranosyl-(1→6)-3-O-β-D-glucopyranosyloxy]flavone (1): ESI m/z 757 [M + H]+., 594, 448; 1H-NMR δ ppm: 8.12 (2H, d, J = 8.3 Hz, H-4' and H-6'), 6.89 (2H, d, J = 8.3 Hz, H-3' and H-5'), 6.75 (1H, d, J = 2.4 Hz, H-8), 6.51 (1H, d, J = 2.4 Hz, H-6), 5.13 (1H, d, J = 7.8 Hz, H-Glc1), 5.04 (1H, d, J = 7.9 Hz, H-Glc1'), 4.56 (1H, d, J = 1.8 Hz, H-Rha1), 4.2-3.1 (16H, overlapped, saccharide protons), 1.17 (3H, d, J = 6.3 Hz, H-Rha6); 13C-NMR δ ppm: 179.0 (C-4), 171.1 (C-7), 162.5 (C-5), 160.0 (C-4'), 158.8 (C-2), 158.1 (C-9), 135.3 (C-3), 130.9 (C-2'/C-6'), 120.9 (C-1'), 115.1 (C-3'/C-5'), 105.8 (C-10), 105.0 (C-Glc1), 104.5 (C-Glc1'), 101.4 (C-Rha1), 94.6 (C-6), 92.0 (C-8), 77.3 (C-Glc3'), 75.5 (C-Glc3), 75.1 (C-Glc5), 75.0 (C-Glc5'), 73.8 (C-Rha4), 73.2 (C-Glc2'), 73.1 (C-Glc2), 72.3 (C-Rha3), 72.1 (C-Rha2), 70.0 (C-Glc4/C-Glc4'), 69.7 (C-Rha5), 66.8 (C-Glc6), 62.2 (C-Glc6'), 18.0 (C-Rha6); Anal. Calcd. for C33H40O20 C, 52.38; H, 5.33; Found C, 52.22; H, 4.98.
O2.- scavenging activity
The assay of superoxide anion radical scavenging activity was based on the capacity of aliquots of investigated extract (50, 100, 200 and 300 μg) and isolated metabolites (0.3 mM) to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) in the riboflavin-light-NBT system [10]. Each 3 mL of reaction mixture contained 50 mM sodium phosphate buffer (pH 7.8), 13 mM methionine, 2 μM riboflavin, 100 μM EDTA, 75 μM NBT and 100 μL sample solution. The production was followed by monitoring the increase in absorbance at 560 nm after 10 min illumination from a fluorescent lamp.
H2O2 scavenging activity
The hydrogen peroxide scavenging activity assay was performed by the method of Pick and Keisari, as reported by Sroka and Cisowski [11]. 0.003% hydrogen peroxide (100 μL), 0.1 M phosphate buffer (700 μL) and 0.1 M sodium chloride (100 μL) were added to aliquots of investigated extract (50, 100, 200 and 300 μg) and isolated metabolites (0.3 mM), previously dissolved in H2O (100 μL). The reaction mixture was incubated for 20 min at 37°C. Then phenol red dye (0.2 mg/mL, 1 mL) with 0.1 mg/mL horseradish peroxidase in 0.1 M phosphate buffer was added. After 15 min, 0.5 M NaOH (100 μL) was added and absorbance was measured at 610 nm using a Shimadzu UV-1601 spectrophotometer. The results were expressed as percentage of reduction of H2O2 adsorption by test samples.
Determination of TBARS
Determination of thiobarbituric acid reactive substances (TBARS) was carried out by the method of Kulisic [12]. Thiobarbituric acid (TBA) reagent was prepared as follows: For reagent A, TBA (Fluka, 375 mg) and tannic acid (Riedel, 30 mg) were dissolved in hot water (30 mL); for reagent B, trichloracetic acid (Riedel, 15 g) was dissolved in 0.3 M aqueous hydrogen chloride solution (70 mL). Then, reagent A (30 mL) was mixed with reagent B (70 mL). Next, rapeseed oil (5.2 μL) was emulsified with Tween-40 (Fluka, 15.6 mg) initially dissolved in 0.2 M Tris-HCl buffer (2 mL, pH 7.4). The emulsion was irradiated with UV light at 254 nm at 25 °C for 60 min. Then, a water solution (100 µL) of test aliquots of extract sample (50, 100, 200 and 300 μg in 300 μL of solvent) and isolated metabolites (0.3 mM) were added to the reaction mixture (1 ml). The samples were irradiated with UV radiation for 30 min again. After addition of the TBA reagent (2 mL), all test tubes were placed in a boiling water bath for 15 min and then centrifuged using a Beckman GS-15R centrifuge for 3 min at 1500 g, and the absorbance of the supernatant was measured at 532 nm. Inhibition of lipid peroxidation was measured as a percentage vs blank containing no test sample.
Footnotes
Sample Availability: Samples of compounds 2–7 are available from the authors.
References
- 1.Dajas F., Arredondo F., Echeverry C., Ferreira M., Morquio A., Rivera F. Flavonoids and the brain: Evidences and putative mechanisms for a protective capacity. Curr. Neuropharmacol. 2005;3:193–205. doi: 10.2174/1570159054368303. [DOI] [Google Scholar]
- 2.Jovanovic S.V., Steenken S., Tosic M., Marjanovitz B., Simic M.G. Flavonoids as antioxidants. J. Am.Chem. Soc. 1994;116:4846–4851. doi: 10.1021/ja00090a032. [DOI] [Google Scholar]
- 3.Hertog M.G., Feskens E.J., Kromhout D. Antioxidant flavonols and coronary heart disease risk. Lancet. 1997;349:699. doi: 10.1016/S0140-6736(05)60135-3. [DOI] [PubMed] [Google Scholar]
- 4.Cotelle N., Bernier J.L., Catteau J.P., Pommery J., Wallet J.C., Gaydou E.M. Antioxidant properties of hydroxy-flavones. Free Radic. Biol. Med. 1996;20:35–43. doi: 10.1016/0891-5849(95)02014-4. [DOI] [PubMed] [Google Scholar]
- 5.D’Abrosca B., Fiorentino A., Oriano P., Monaco P., Pacifico S. Annurcoic acid: a new antioxidant ursane triterpene from fruits of cv. Annurca apple. Food Chem. 2006;98:285–290. doi: 10.1016/j.foodchem.2005.05.072. [DOI] [Google Scholar]
- 6.Cefarelli G., D’Abrosca B., Fiorentino A., Izzo A., Mastellone C., Pacifico S., Piscopo V. Free radical scavenging and antioxidant activities of secondary metabolites from reddened cv. Annurca apple fruits. J. Agric. Food Chem. 2006;54:803–809. doi: 10.1021/jf052632g. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y., Foo L.Y. The polyphenol constituents of grape pomace. Food Chem. 1999;65:1–8. doi: 10.1016/S0308-8146(98)00245-3. [DOI] [Google Scholar]
- 8.D'Abrosca B., DellaGreca M., Fiorentino A., Monaco P., Previtera L., Simonet A. M., Zarrelli A. Potential allelochemicals from Sambucus nigra. Phytochemistry. 2001;58:1073–1081. doi: 10.1016/S0031-9422(01)00401-0. [DOI] [PubMed] [Google Scholar]
- 9.Yeskaliyeva B., Mesaik M.A., Abbaskhan A., Kulsoom A., Burasheva G.Sh., Abilov Zh.A., Choudhary M.I., Atta-ur-Rahman Bioactive flavonoids and saponins from Climacoptera obtusifolia. Phytochemistry. 2006;67:2392–2397. doi: 10.1016/j.phytochem.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta N., De B. Antioxidant activity of Piper betle L. leaf extract in vitro. Food Chem. 2004;88:219–224. doi: 10.1016/j.foodchem.2004.01.036. [DOI] [Google Scholar]
- 11.Sroka Z., Cisowski W. Hydrogen peroxide scavenging, antioxidant and antiradical activity of some phenolics acids. Food Chem. Toxicol. 2003;41:753–758. doi: 10.1016/S0278-6915(02)00329-0. [DOI] [PubMed] [Google Scholar]
- 12.Kulisic T., Radonic A., Katalinic V., Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004;85:633–640. doi: 10.1016/j.foodchem.2003.07.024. [DOI] [Google Scholar]