Abstract
In this research, a new triterpenoid, tirucalla-8,24-diene-3β,11β-diol-7-one (1), and eupha-8,24-diene-3β,11β-diol-7-one (2), which was isolated from Euphorbia kansui for the first time, together with twelve other known compounds (3–14), were isolated from the ethyl acetate extract of Euphorbia kansui. Their structures were elucidated based on High resolution electrospray ionization mass spectrometry (HR-ESI-MS), Infrared Spectroscopy (IR), 1D and 2D Nuclear Magnetic Resonance (NMR) data. Both constituents 1 and 2 exhibited moderate cytotoxicity against colon cancer HCT-116, gastric cancer MKN-45 and breast cancer MCF-7.
Keywords: Euphane and Tirucallane, triterpenes, cytotoxicity, Euphorbia kansui
1. Introduction
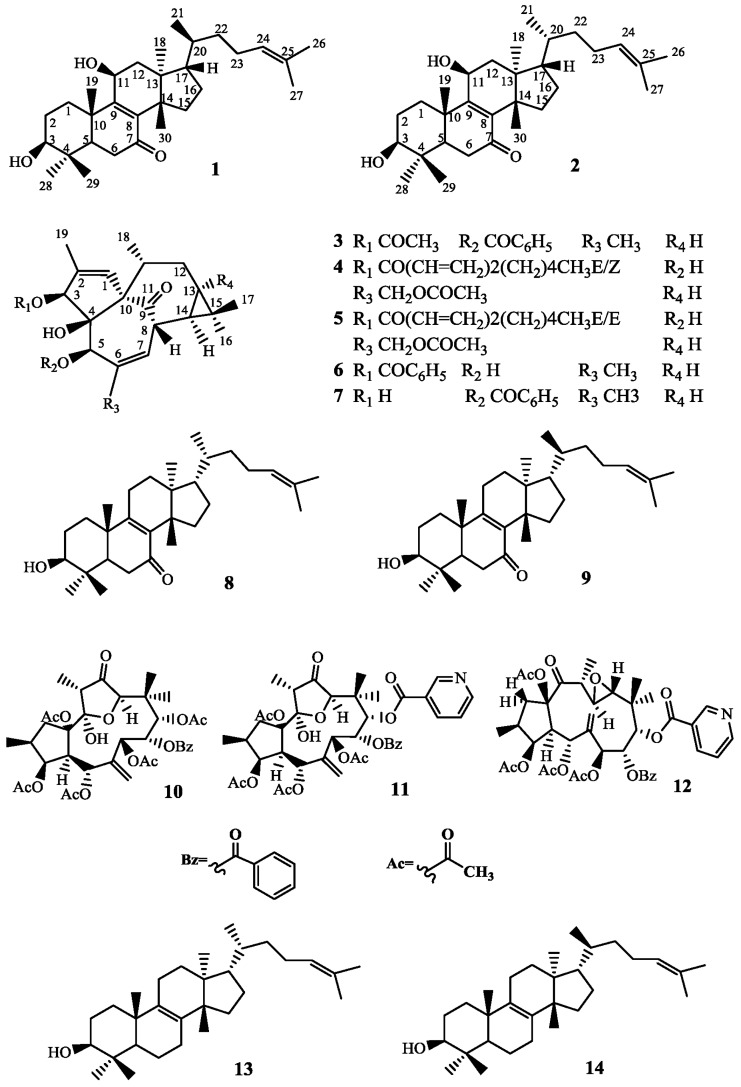
The plants of Euphorbia contain more than 2000 species spread all over the world, and about 80 species distribute in China [1,2]. The dried root of Euphorbia kansui has long been used for the treatment of asthma, edema and ascites in traditional Chinese medicine. The structure type of the compounds in Euphorbia kansui are diterpenes, triterpenes, flavonoids, phenolic and acids [3,4,5]. Among them, diterpenes and triterpenoids are the main compounds in Euphorbia kansui, which show a wide range of pharmacological activities, such as antiviral, skin irritating and modulation of multidrug resistance effects [6,7,8,9]. A new tirucallane-type triterpene named tirucalla-8,24-diene-3β,11β-diol-7-one (1) was first isolated from natural plants, and an euphane-type triterpene named eupha-8,24-diene-3β,11β-diol-7-one (2) (Figure 1) was isolated from Euphorbia kansui for the first time in our present study. The two compounds were identified by 1D and 2D NMR including Heteronuclear Single Quantum Coherence (HSQC), Heteronuclear Multiple-Bond Correlation (HMBC), COrrelation SpectroscopY (COSY), Nuclear Overhauser Effect Spectroscopy (NOESY) and HR-ESI-MS data.
Figure 1.
Chemical structures of Compounds 1–14.
2. Results and Discussion
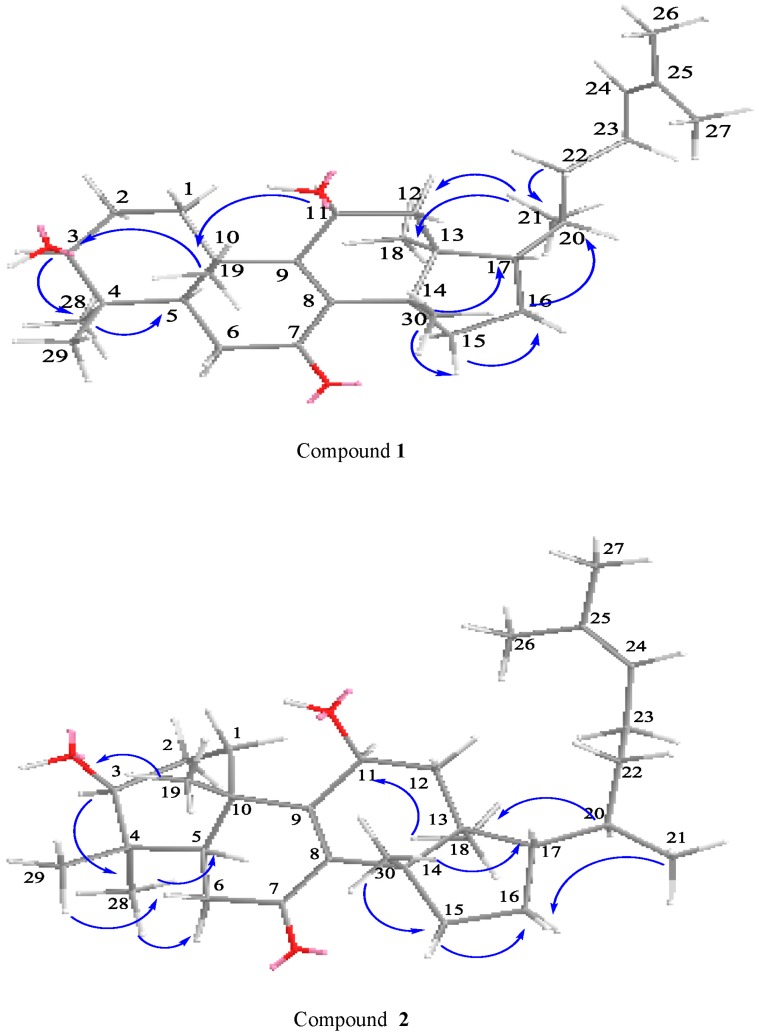
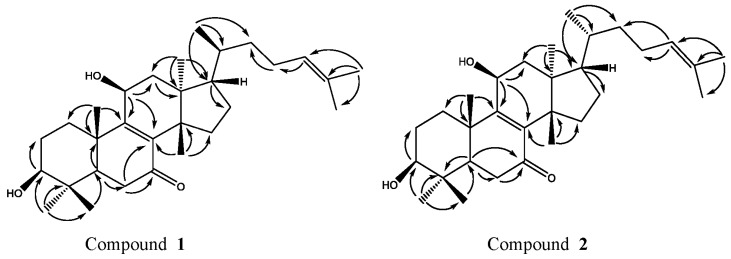
Compound 1 was obtained as a white powder. The molecular formula C30H48O3 was established by HR-ESI-MS (m/z 457.3770 [M + H]+, calcd. 457.3682) (Figure 1-1), IR (KBr) νmax 3371, 2977, 2861, 1635, 1456, 1376, 1036, 622 cm−1 (Figure 1-2), UV (MeOH) λmax 253 nm (Figure 1-3) (Figures 1-1, 1-2, 1-3, see the Supplementary Materials); The comparison of 1H-NMR, 13C-NMR (Table 1 and Table 2, Figures 1-4 and 1-5, see the Supplementary Materials) and NOESY data (Figure 2) showed that compound 1 and the known compound 2 [10] were semblable in structure, except that a Hydrogen at C-11 of compound 2 was superseded by a hydroxyl group in compound 1, which was verified by correlations of H-11 (δH 4.70, t, J = 8.2 Hz) with C-8 (δC 140.46), C-9 (δC 161.25) and C-12 (δC 42.79) in HMBC spectrum (Figure 3). Compared with 1H-NMR and 13C-NMR of 1 and 2, we can clearly see the differences between compound 2: H-C(21) δH 0.88, H-C(22) δH 1.08–1.13 (m), 1.56–1.62 (m), C-20 δC 35.61, C-22 δC 35.51) and compound 1: H-C(21) δH 0.94, H-C(22) δH 1.03–1.12 (m), C-20 δC 36.03, C-22 δC 36.24. In the HSQC plot (Figure 1-6, see the Supplementary Materials), δH 1.03–1.12 and 1.40–1.47 showed correlations with C(22), C(20) at δC 36.24, 36.03 respectively, which indicated compound 1, H-C(22) δH 1.03–1.12 (m). The relative configuration of 1 was determined by the 1H-NMR (Table 1) and NOESY data. 1H-NMR chemical shift of CH3-21 (δH 0.94, d, J = 6.6 Hz) confirmed that compound 1 was classified as the tirucallane series [11,12,13]. The large coupling constants H-C(3) (δH 3.31, J = 9.5, 6.4 Hz) obviously indicated that the 3-OH group was in equatorial β-position [10,14]. The NOESY correlations (Figure 2) H-C(3) (δH 3.31)/H-C(5) (δH 1.65–1.68), H-C(3) (δH 3.31)/CH3-28 (δH 1.00), H-C(11) (δH 4.70)/CH3-18 (δH 0.71), CH3-30 (δH 1.15)/H-C(17) (δH 1.54–1.61), CH3-29 (δH 0.92)/CH3-19 (δH 1.28), CH3-18 (δH 0.71)/CH3-19 (δH 1.28), showed that H-C(3), H-C(5), H-C(17), CH3-28, were all in α-orientation, whereas 11-OH, CH3-19, CH3-30 and CH3-29 were all in β-orientation. Furthermore, compound 1 showed NOESY correlations between CH3-18 and H-C(20) (δH 1.40–1.47) and CH3-21, between CH3-21 and H-12β (δH 2.38–2.46). These correlations were consistent with those of tirucallane-type triterpenes [14]. As a result, the structure of compound 1 was identified as tirucalla-8,24-diene-3β,11β-diol-7-one.
Table 1.
1H-NMR data for compounds 1 and 2.
| Position | Compound 1 | Compound 2 |
|---|---|---|
| 1α | 1.56–1.62 (m) | 1.52–1.60 (m) |
| 1β | 2.41–2.47 (m) | 2.40–2.46 (m) |
| 2 | 1.70–1.79 (m) | 1.70–1.79 (m) |
| 3α | 3.31 (dd, J = 6.4, 9.6) | 3.31 (dd, J = 6.4, 9.6) |
| 5 | 1.65–1.68 (m) | 1.62–1.68 (m) |
| 6 | 2.40–2.47 (m) | 2.40–2.48 (m) |
| 11 | 4.70 (t, J = 8.2) | 4.69 (t, J = 8.2) |
| 12α | 1.76–1.81 (m) | 1.74–1.81 (m) |
| 12β | 2.38–2.46 (m) | 2.35–2.43 (m) |
| 15α | 2.10–2.12 (m) | 2.12–2.19 (m) |
| 15β | 1.42–1.46 (m) | 1.40–1.46 (m) |
| 16α | 1.93–1.99 (m) | 1.90–1.94 (m) |
| 16β | 1.28–1.33 (m) | 1.28–1.32 (m) |
| 17 | 1.54–1.61 (m) | 1.55–1.60 (m) |
| 18 | 0.71 (s) | 0.73 (s) |
| 19 | 1.28 (s) | 1.27 (s) |
| 20 | 1.40–1.47 (m) | 1.40–1.46 (m) |
| 21 | 0.94 (d, J = 6.6) | 0.88 (d, J = 6.4) |
| 22 | 1.03–1.12 (m) | 1.08–1.13 (m) 1.56–1.62 (m) |
| 23 | 1.83–1.91 (m) 2.01–2.08 (m) | 1.84–1.90 (m) 1.98–2.02 (m) |
| 24 | 5.09(t, J = 7.2) | 5.09 (t, J = 7.2) |
| 26 | 1.70 (s) | 1.70 (s) |
| 27 | 1.62 (s) | 1.63 (s) |
| 28 | 1.00 (s) | 1.01 (s) |
| 29 | 0.92 (s) | 0.93 (s) |
| 30 | 1.15 (s) | 1.16 (s) |
Record in CDCl3, 400 MHz for 1H, δ in ppm, J = Hz.
Table 2.
13C-NMR data of compounds 1 and 2.
| Position | Compound 1 | Compound 2 |
|---|---|---|
| 1 | 33.68 | 33.67 |
| 2 | 27.37 | 27.36 |
| 3 | 78.27 | 78.26 |
| 4 | 39.07 | 39.06 |
| 5 | 49.25 | 49.25 |
| 6 | 35.84 | 35.84 |
| 7 | 200.10 | 200.16 |
| 8 | 140.46 | 140.44 |
| 9 | 161.25 | 161.30 |
| 10 | 39.58 | 39.58 |
| 11 | 68.11 | 68.11 |
| 12 | 42.79 | 42.83 |
| 13 | 46.19 | 46.19 |
| 14 | 47.95 | 48.02 |
| 15 | 31.88 | 31.81 |
| 16 | 27.77 | 27.80 |
| 17 | 49.13 | 48.69 |
| 18 | 16.06 | 16.24 |
| 19 | 19.70 | 19.67 |
| 20 | 36.03 | 35.61 |
| 21 | 18.68 | 18.76 |
| 22 | 36.24 | 35.51 |
| 23 | 24.86 | 24.82 |
| 24 | 124.98 | 124.90 |
| 25 | 131.09 | 131.14 |
| 26 | 25.68 | 25.73 |
| 27 | 17.62 | 17.70 |
| 28 | 27.59 | 27.59 |
| 29 | 15.18 | 15.19 |
| 30 | 25.62 | 25.70 |
Record in CDCl3, 100 MHz for 13C, δ in ppm, J = Hz.
Figure 2.
Key NOESY correlations for 1 and 2.
Figure 3.
Key HMBC correlations for 1 and 2.
The comparison of 1H-NMR, 13C-NMR (Table 1 and Table 2) and NOESY data (Figure 2) showed that compound 2 and the known Compound 1 [10] were semblable in structure, except that a Hydrogen at C-11 of Compound 1 was superseded by a hydroxyl group in compound 2, which was proven by correlations of H-11 (δH 4.69, t, J = 8.2 Hz) with C-8 (δC 140.44), C-9 (δC 161.30) and C-12 (δC 42.83) in HMBC spectrum (Figure 3). The large coupling constants H-C(3) (δH 3.31, J = 9.5, 6.4 Hz) obviously indicated that the 3-OH group was in equatorial β-position [11,12]. The NOESY correlations (Figure 2) H-C(3) (δH 3.31)/H-C(5) (δH 1.62–1.68), H-C(3) (δH 3.31)/CH3-28 (δH 1.01), H-C(11) (δH 4.69)/CH3-18 (δH 0.73), CH3-30 (δH 1.16)/H-C(17) (δH 1.55–1.60), CH3-29 (δH 0.93)/CH3-19 (δH 1.27), CH3-18 (δH 0.73)/CH3-19 (δH 1.27), showed that H-C(3), H-C(5), H-C(17), and CH3-28 were all in α-orientation, whereas 11-OH, CH3-19, CH3-30 and CH3-29 were in β-orientation, and no correlations between CH3-21/CH3-18 [10,15] indicated that it belonged to the euphane-type triterpenes. The 1H-NMR chemical shift of CH3-21 (δH 0.88, d, J = 6.4 Hz) further confirmed that compound 2 pertained to the euphane rather than the tirucallane series [12,13]. As a result, compound 2 was elucidated to be eupha-8,24-diene-3β,11β-diol-7-one.
All 12 of the known compounds (3–14) were identified according to the spectroscopic data (1H-NMR, 13C-NMR, see the Supplementary Materials) together with the comparsion of those reported, kansuiphorin C (3) [16], 3-O-(2′E,4′Z-decadienoyl)-20-O-acetylingenol (4) [4], 3-O-(2′E,4′E-decadienoyl)-20-O-acetylingenol (5) [4], 3-O-benzoyl-20-deoxyingenol (6) [4], 5-O-benzoyl-20-deoxyingenol (7) [4], kansenone (8) [10], epi-kansenone (9) [10], kansuinin A (10) [4,17], kansuinin D (11) [4], kansuinin E (12) [4], euphol (13) [4,18], and tirucallol (14) [4,19].
Compounds 1 and 2 were assessed for their inhibitory effects on HCT-116, MKN-45 and MCF-7 cell lines (Table 3), as well as L-O2 and GES-1 cell lines (Table 4). The results show that compounds 1 and 2 inhibit normal cells (L-O2 and GES-1) less than cancer cells (HCT-116, MKN-45 and MCF-7), and compounds 1 and 2 presented definite anticancer activities with IC50 values of 20.84 ± 1.28 and 33.97 ± 2.15 µΜ against HCT-116 cells, 10.18 ± 1.36 and 14.95 ± 1.82 µΜ against MKN-45 cells, and 10.82 ± 1.18 and 20.11 ± 2.16 µΜ against MCF-7 cells, respectively. kansenone induces apoptosis through both the death receptor and mitochondrial pathways [5], and compounds 1 and 2 were similar with kansenone in structure, excluding that a Hydrogen at C-11 of kansenone was superseded by a hydroxyl group in compounds 1 and 2, thus compounds 1 and 2 may induce apoptosis in the same way as kansenone. Further research will be conducted on the anticancer mechanism of compounds 1 and 2.
Table 3.
Cytotoxicity of compounds 1 and 2 against three human cancer cell lines.
| Compound | IC50 (μΜ) | ||
|---|---|---|---|
| HCT-116 | MKN-45 | MCF-7 | |
| 1 | 20.84 ± 1.28 | 10.18 ± 1.36 | 10.82 ± 1.18 |
| 2 | 33.97 ± 2.15 | 14.95 ± 1.82 | 20.11 ± 2.16 |
| Cisplatin | 8.465 ± 0.84 | 6.142 ± 1.12 | 9.035 ± 0.92 |
| 5-Fu | 6.172 ± 2.03 | 2.624 ± 2.06 | 1.629 ± 1.42 |
Table 4.
Cytotoxicity of compounds 1 and 2 against two human normal cell lines.
| Compound | IC50 (μΜ) | |
|---|---|---|
| L-O2 | GES-1 | |
| 1 | 56.98 ± 1.74 | 40.99 ± 0.85 |
| 2 | 49.89 ± 2.12 | 40.27 ± 1.28 |
3. Materials and Methods
3.1. General Experimental Procedures
HPLC: Hanbon NP 7000 (Jiangsu Hanbang Technology Companies, Huaian, China) Serials pump with an NU 3000 Serials UV-Vis detector (Jiangsu Hanbang Technology Companies, Huaian, China), Phecda Si (20 × 250 mm, 5 μm); Waters 1525 with a 2996 Diode Array Detector (DAD) (Waters, Milford, CT, USA), XBrige-Prep C18 (150 × 19 mm, 5 μm), (Ultimate XB-C8, 30 × 150 mm, 5 μm). IR spectra were gained on a Nicole Is5 of Thermo Fisher spectrophotometer (Nicolet Instrument Corporation, Madison, WI, USA). The NMR spectra were measured on Avance 400 spectrometers (Bruker, Karlsruhe, Germany), with TMS as an internal standard. The UV spectra were measured on a Shimadzu UV-2401 UV-Vis spectrophotometer (Shimadzu, Kyoto, Japan). The HR-ESI-MS data were obtained by using a LTQ Orbitrap MS (Thermo Fisher Scientific, San Jose, CA, USA). Column chromatography (CC): silica gel (200–300 mesh, Qingdao Marine Chemical Industry, Qingdao, China).
3.2. Plant Materials
The dried root of Euphorbia kansui was collected from Red River valley of Baoji, Shaanxi Province of China, in October 2015 and was identified by Prof. Yu Ping Tang (college of pharmacy, Nanjing University of Chinese Medicine, Nanjing, China). The voucher specimen (20151020) has been deposited in the Herbarium of college of pharmacy, Nanjing University of Chinese Medicine (Nanjing, China).
3.3. Extraction and Isolation
The dried roots of Euphorbia kansui (12.2 kg) were extracted twice (each time for 2 h) with 95% EtOH under reflux to give the 95% EtOH extract (871.9 g) by evaporation of the solvent under reduced pressure, and then the 95% EtOH extract was extracted with ethyl acetate (EtOAc) to obtain ethyl acetate extract (530.8 g). Finally, the fraction of EtOAc was subjected to silica gel column chromatography (14 × 59 cm) with a gradient elution (Pet and ethyl acetate, 100:1–1:1) to get fractions A-T. Fr. G (2 g) was eluted with Pet:EtOAc (100:20). Compound 1 (40.8 mg) was isolated by HPLC (Pet:EtOAc, 100:38), and further purified by reversed-phase HPLC (MeCN:H2O, 70:30) flow rate 16 mL/min (tR 15.342 min). Compound 2 (70.3 mg) was isolated by HPLC (Pet:EtOAc, 100:30), and further purified by reversed-phase HPLC (MeCN:H2O, 70:30) flow rate 16 mL/min (tR 16.060 min). Fr. A (18 g) was eluted with Pet:EtOAc (100:3). Compound 13 (6.082 g) was isolated by HPLC (MeCN:H2O, 95:5) with (Ultimate XB-C8, 30 × 150 mm, 5 μm) flow rate 16 mL/min (tR 35.452 min). Compound 14 (782.5 mg) was isolated by HPLC (MeCN:H2O, 95:5) with (Ultimate XB-C8, 30 × 150 mm, 5 μm) flow rate 16 mL/min (tR 33.645 min). Fr. B (3 g) was eluted with Pet:EtOAc (100:5). Compound 6 (320 mg) was isolated by HPLC (Pet:EtOAc, 100:10) flow rate 16 mL/min (tR 24.003 min). Compound 7 (46 mg) was isolated by HPLC (Pet:EtOAc, 100:10) flow rate 16 mL/min (tR 22.209 min). Fr. C (5 g) was eluted with Pet:EtOAc (100:6). Compound 3 (920 mg) was isolated by HPLC (Pet:EtOAc, 100:11) flow rate 16 mL/min (tR 24.128 min). Fr. D (2 g) was eluted with Pet:EtOAc (100:8). Compound 8 (161 mg) was isolated by HPLC (Pet:EtOAc, 100:15) flow rate 16 mL/min (tR 23.218 min). Compound 9 (28 mg) was isolated by HPLC (Pet:EtOAc, 100:15) flow rate 16 mL/min (tR 20.674 min). Fr. E (2 g) was eluted with Pet:EtOAc (100:13). Compound 4 (80 mg) was isolated by HPLC (Pet:EtOAc, 100:20) flow rate 16 mL/min (tR 21.097 min). Compound 5 (40 mg) was isolated by HPLC (Pet:EtOAc, 100:20) flow rate 16 mL/min (tR 24.298 min). Fr. H (10 g) was eluted with Pet:EtOAc (100:55). Compound 10 (186 mg) was isolated by HPLC (Pet:EtOAc, 100:40), and further purified by reversed-phase HPLC (MeCN:H2O, 70:30) flow rate 16 mL/min (tR 10.502 min). Compound 11 (60 mg) was isolated by HPLC (Pet:EtOAc, 100:40), and further purified by reversed-phase HPLC (MeCN:H2O, 70:30) flow rate 16 mL/min (tR 9.702 min). Compound 12 (80 mg) was isolated by HPLC (Pet:EtOAc, 100:40), and further purified by reversed-phase HPLC (MeCN:H2O, 70:30) flow rate 16 mL/min (tR 11.302 min).
3.4. Cytotoxicity Test
Cytotoxicity of two compounds against HCT-116, MKN-45 and MCF-7 cancer cell lines (American Type Culture Collection, ATCC, Manassas, VA, USA), normal liver cell L-O2 (Zhongqiaoxinzhou Biotech, Shanghai, China) and gastric epithelial cell GES-1 (Nanjingkebai Biotech, Nanjing, China) were evaluated with the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylt-etrazolium bromide) method as described in the literature [20,21]. All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, three cancer cells were seeded in 96-well plate at a concentration of 8 × 104 cells/mL, two normal cells L-O2 and GES-1 were seeded in 96-well plate at a concentration of 1 × 104 cells/mL and 1 × 105 cells/mL respectively [21], and incubated for 24 h in humidifyed atmosphere with 5% CO2 at 37 °C. One hundred microliters of cells were treated with compound 1 and 2 at different doses of 1.25, 2.5, 5, 10, 20 and 40 μg/mL in dimethyl sulfoxide (DMSO) in triplicate for 48 h at 37 °C with 5% CO2. Then, each of them were added with 20 μL of MTT (5.0 mg/mL) and incubated for further 4 h, the growth medium was removed from all the wells. Finally, 150 μL DMSO were added to every sample. Cisplatin (Qilu pharmaceutical, Jinan, China) and 5-fluorouracil (5-Fu) (Sichuan Kangyi, pharmaceutical, Chengdu, China) served as positive control. Absorbance was determined by a microplate spectrophotometer at 490 nm.
4. Conclusions
The article reported two compounds, 1 and 2, which are triterpenes, as well as twelve other known compounds (3–14). Compound 1 is a new tirucallane-type triterpene named tirucalla-8,24-diene-3β,11β-diol-7-one. Compound 2 was isolated from Euphorbia kansui for the first timeand named eupha-8,24-diene-3β,11β-diol-7-one. They also display effective anticancer activities against HCT-116, MKN-45 and MCF-7 cells. As we all know, Euphorbia kansui has pharmacological activities including tumor inhibition and antiviral effects [22,23]; this study further confirmed kansui may be a potential candidate for anticancer including colon cancer, gastric cancer and breast cancer and inferred that compounds 1 and 2 may be the main material basis of anticancer for Euphorbia kansui.
Some features about compounds 1, 2, 8, 9, 13 and 14 may be drawn based on their chemical structures. Above all, the CH3-21 of compounds 9 and 14 were all in β-orientation and their 1H-NMR chemical shift of CH3-21 at (δH 0.94, d, J = 6.6 Hz), whereas the CH3-21 of compounds 8 and 13 were all in α-orientation and their 1H-NMR chemical shift of CH3-21 at (δH 0.88, d, J = 6.4 Hz) [4,18,19]. The C-20 and C-22 of compound 9 had a chemical shift greater than compound 8. The C-20 and C-22 of compound 14 was larger than compound 13 (Table 5), which were identical with the 1H-NMR and 13C-NMR of compounds 1 and 2, respectively. Then, the polarity order of compound 9 was larger than compound 8. Compound 14 is also greater than 13 in the same way, which were also the same as the polarity order of compounds 1 and 2. Thus, the different positions of CH3-21 of compounds 1 and 2, 8 and 9, and 13 and 14 may have a good correlation with their polarity order.
Table 5.
13C-NMR data of compounds 8, 9, 13 and 14.
| Position | Compound 8 | Compound 9 | Compound 13 | Compound 14 |
|---|---|---|---|---|
| 1 | 34.61 | 34.61 | 35.26 | 35.26 |
| 2 | 27.40 | 27.41 | 27.95 | 27.92 |
| 3 | 78.07 | 78.06 | 79.00 | 79.03 |
| 4 | 38.82 | 38.83 | 38.94 | 38.94 |
| 5 | 48.19 | 48.24 | 50.97 | 50.97 |
| 6 | 35.77 | 35.78 | 18.95 | 18.95 |
| 7 | 198.37 | 198.35 | 27.68 | 27.67 |
| 8 | 138.94 | 138.93 | 134.03 | 134.08 |
| 9 | 165.46 | 165.48 | 133.55 | 133.51 |
| 10 | 39.27 | 39.27 | 37.24 | 37.27 |
| 11 | 23.73 | 23.67 | 21.53 | 21.45 |
| 12 | 29.95 | 29.87 | 30.90 | 30.80 |
| 13 | 44.62 | 44.61 | 44.12 | 44.11 |
| 14 | 47.68 | 47.61 | 50.03 | 50.12 |
| 15 | 31.39 | 31.45 | 29.77 | 28.83 |
| 16 | 28.67 | 28.65 | 28.14 | 28.05 |
| 17 | 48.24 | 48.76 | 49.64 | 49.96 |
| 18 | 15.73 | 15.54 | 15.63 | 15.52 |
| 19 | 18.60 | 18.61 | 20.15 | 20.14 |
| 20 | 35.65 | 36.16 | 35.88 | 36.33 |
| 21 | 18.90 | 18.75 | 18.92 | 18.69 |
| 22 | 35.52 | 36.35 | 35.43 | 36.40 |
| 23 | 24.74 | 24.91 | 24.77 | 24.94 |
| 24 | 125.07 | 125.12 | 125.22 | 125.26 |
| 25 | 131.04 | 131.01 | 130.08 | 130.90 |
| 26 | 25.76 | 25.73 | 17.69 | 17.62 |
| 27 | 17.71 | 17.65 | 25.74 | 25.71 |
| 28 | 27.28 | 27.29 | 27.92 | 27.92 |
| 29 | 15.07 | 15.07 | 15.53 | 15.43 |
| 30 | 24.42 | 24.31 | 24.47 | 24.36 |
Record in CDCl3, 100 MHz for 13C, δ in ppm, J = Hz.
Acknowledgments
The authors gratefully acknowledge the National Natural Science Foundation of China (81373972, 81673599 and 30973940), the National Basic Research Program of China (973 Program) (2011CB-505300 and 2011CB505303) for financially supporting this work. This research was also supported by a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD-2014), Six Talent Peaks Project in Jiangsu Province (2010-YY-026 and 2016-YY-009) and Innovative Project of College Students in Jiangsu Province (201610315023Z). In addition, this work was completed in Class III Laboratory of Chemistry of Chinese Materia Medica of State Administration of Traditional Chinese Medicine of China.
Supplementary Materials
Supplementary materials are available online.
Author Contributions
Q.Z., Q.-R.Z., J.-W.L. and L.Z. participated in research design. Q.Z., P.-D.C., W.-F.Y. and W.-W.T. performed the experiments and analyzed data. Q.Z., K.W. and G.-C.D. wrote this manuscript. Y.-P.T. and L.Z. reviewed and edited this manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Geng T., Huang H.Y., Ding A.W., Zhang L. Irritation and diarrhea effect of different polar parts of Euphorbia kansui T. and vinegar-preparing Euphorbia kansui T. Cent. South Pharm. 2008;6:385–388. [Google Scholar]
- 2.Wang L., Ma Y.T., Sun Q.Y., Li X.N., Yan Y., Yang J., Yang F.-M., Liu F.-Y., Zang Z., Wu X.H., et al. Structurally diversified diterpenoids from Euphorbia dracunculoides. Tetrahedron. 2015;71:5484–5493. doi: 10.1016/j.tet.2015.06.078. [DOI] [Google Scholar]
- 3.Wang H., Wang J., Luo J., Kong L. Isolation of ingenol-type diterpenoids from Euphorbia kansui by offline coupling of HPLC-ESI-MSn and HSCCC. Sep. Sci. Technol. 2013;48:1745–1751. doi: 10.1080/01496395.2012.753632. [DOI] [Google Scholar]
- 4.Wang L.Y. Ph.D. Thesis. Shenyang Pharmaceutical University; Shenyang, China: 2003. The Bioactive Study of Constituents in Kansui. [Google Scholar]
- 5.Cheng F., Yang Y., Zhang L., Cao Y., Yao W., Tang Y., Ding A. A natural triterpene derivative from Euphorbia kansui inhibits cell proliferation and induces apoptosis against rat intestinal epithelioid cell line in vitro. Int. J. Mol. Sci. 2015;16:18956–18975. doi: 10.3390/ijms160818956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Q.W., Su X.H., Kiyota H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008;108:4295–4327. doi: 10.1021/cr078350s. [DOI] [PubMed] [Google Scholar]
- 7.Zheng W.F., Cui Z., Zhu Q. Cytotoxicity and antiviral activity of the compounds from Euphorbia kansui. Planta Med. 1998;64:754–756. doi: 10.1055/s-2006-957574. [DOI] [PubMed] [Google Scholar]
- 8.Li X.R., Zhang Y.D., Tang H.H. Study of auxiliary therapeutic effect of kansui root on patients with severe acute pancreatitis. China J. Mod. Med. 2002;12:8. [Google Scholar]
- 9.Yan X., Zhang L., Guo J., Cao Y., Shang E., Tang Y., Ding A., Duan J.A. Processing of kansui roots stir-baked with vinegar reduces kansui-induced hepatocyte cytotoxicity by decreasing the contents of toxic terpenoids and regulating the cell apoptosis pathway. Molecules. 2014;19:7237–7254. doi: 10.3390/molecules19067237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L.Y., Wang N.L., Yao X.S., Miyata S., Kitanaka S. Euphane and tirucallane triterpenes from the roots of Euphorbia kansui and their in vitro effects on the cell division of Xenopus. J. Nat. Prod. 2003;66:630–633. doi: 10.1021/np0205396. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed E., Malik A., Ferheen S., Afza N., Lodhi M.A., Choudhary M.I. Chymotrypsin inhibitory triterpenoids from Silybum marianum. Chem. Pharm. Bull. 2006;54:103–106. doi: 10.1248/cpb.54.103. [DOI] [PubMed] [Google Scholar]
- 12.Abe I., Rohmer M. Enzymic cyclization of 2,3-dihydrosqualene and squalene 2,3-epoxide by squalene cyclases: From pentacyclic to tetracyclic triterpenes. J. Chem. Soc. Perkin Trans. 1. 1994;7:783–791. doi: 10.1039/p19940000783. [DOI] [Google Scholar]
- 13.Mishra M., Shukla Y.N., Kumar S. Euphane triterpenoid and lipid constituents from Butea monosperma. Phytochemistry. 2000;54:835–838. doi: 10.1016/S0031-9422(00)00136-9. [DOI] [PubMed] [Google Scholar]
- 14.Wang S., Liang H., Zhao Y., Wang G., Yao H., Kasimu R., Wu Z., Li Y., Huang J., Wang J. New triterpenoids from the latex of Euphorbia resinifera Berg. Fitoterapia. 2016;108:33–40. doi: 10.1016/j.fitote.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Akihisa T., Yasukawa K., Kimura Y., Takase S.I., Yamanouchi S., Tamura T. Triterpene alcohols from camellia and sasanqua oils and their anti-inflammatory effects. Chem. Pharm. Bull. 1997;45:2016–2023. doi: 10.1248/cpb.45.2016. [DOI] [PubMed] [Google Scholar]
- 16.Pan D.-J., Hu C.-Q., Chang J.-J., Lee T.T.-Y., Chen Y.-P., Hsu H.-Y., Mcphail D.R., Mcphail A.T., Lee K.-H. Kansuiphorin-C and-D, cytotoxic diterpenes from Euphorbia kansui. Phytochemistry. 1991;30:1018–1020. [Google Scholar]
- 17.Uemura D., Hirata Y., Chen Y.P., Hsu H.Y. The structure of kansuinine A, a new multi-oxygenated diterpene. Tetrahedron Lett. 1975;16:1697–1700. doi: 10.1016/S0040-4039(00)72236-2. [DOI] [Google Scholar]
- 18.Gewali M.B., Hattori M., Tezuka Y., Kikuchi T., Namba T. Constituents of the latex of Euphorbia antiquorum. Phytochemistry. 1990;29:1625–1628. doi: 10.1016/0031-9422(90)80134-3. [DOI] [Google Scholar]
- 19.Itoh T., Tamura T., Matsumoto T. Tirucalla-7, 24-dienol: A new triterpene alcohol from tea seed oil. Lipids. 1976;11:434–441. doi: 10.1007/BF02532833. [DOI] [Google Scholar]
- 20.Gao J., Gao L., Zhang L., Yao W., Cao Y., Bao B., Ding A. 3-O-(2′E,4′Z-decadienoyl)-20-O-acetylingenol induces apoptosis in intestinal epithelial cells of rats via mitochondrial pathway. J. Ethnopharmacol. 2015;174:331–338. doi: 10.1016/j.jep.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L., Gao L., Li Z., Yan X., Yang Y., Tang Y., Cao Y., Ding A. Bio-guided isolation of the cytotoxic terpenoids from the roots of Euphorbia kansui against human normal cell lines L-O2 and GES-1. Int. J. Mol. Sci. 2012;13:11247–11259. doi: 10.3390/ijms130911247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen J., Wang J., Shang E.X., Tang Y.P., Kai J., Cao Y.J., Zhou G.S., Tao W.W., Kang A., Su S.L., et al. The dosage-toxicity-efficacy relationship of kansui and licorice in malignant pleural effusion rats based on factor analysis. J. Ethnopharmacol. 2016;186:251–256. doi: 10.1016/j.jep.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Yan X., Zhang L., Cao Y., Yao W., Tang Y., Ding A. An Ingenol Derived from Euphorbia kansui Induces Hepatocyte Cytotoxicity by Triggering G0/G1 Cell Cycle Arrest and Regulating the Mitochondrial Apoptosis Pathway in Vitro. Molecules. 2016;21:813. doi: 10.3390/molecules21060813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.