Abstract
Mesenchymal stem cells (MSCs) are multipotent cells that can differentiate into various mesodermal lines forming fat, muscle, bone, and other lineages of connective tissue. MSCs possess plasticity and under special metabolic conditions may transform into cells of unusual phenotypes originating from ecto- and endoderm. After transplantation, MSCs release the humoral factors promoting regeneration of the damaged tissue. During last five years, the numbers of registered clinical trials of MSCs have increased about 10 folds. This gives evidence that MSCs present a new promising resource for cell therapy of the most dangerous diseases. The efficacy of the MSCs therapy is limited by low possibilities to regulate their conversion into cells of damaged tissues that is implemented by the pRb-E2F signaling. The widely accepted viewpoint addresses pRb as ubiquitous regulator of cell cycle and tumor suppressor. However, current publications suggest that basic function of the pRb-E2F signaling in development is to regulate cell fate and differentiation. Through facultative and constitutive chromatin modifications, pRb-E2F signaling promotes transient and stable cells quiescence, cell fate choice to differentiate, to senesce, or to die. Loss of pRb is associated with cancer cell fate. pRb regulates cell fate by retaining quiescence of one cell population in favor of commitment of another or by suppression of genes of different cell phenotype. pRb is the founder member of the “pocket protein” family possessing functional redundancy. Critical increase in the efficacy of the MSCs based cell therapy will depend on precise understanding of various aspects of the pRb-E2F signaling.
Keywords: Cell cycle, Cell differentiation, Cell fate, Mesenchymal stem cells, pRb-E2F signaling
Introduction
MSCs are a type of somatic stem cells (SSCs) for nonhematopoietic tissues of mesodermal origin possessing self-renewal and capable to differentiate into bone, fat, and other lineages of connective tissue.1, 2, 3, 4 Under special metabolic conditions MSCs may transform into cells of unusual ecto- or endoderm phenotypes including neurons or epithelium.5 During last 5 years, the number of registered MSCs clinical trials have increased by about 10 folds. This reflects general viewpoint that MSCs present a new promising resource for cell therapy. MSCs produce a variety of humoral factors promoting efficacy of the regenerative therapy.6 Effective tissue reconstitution is based on the replacement of damaged cells by MSCs that are capable to the tissue specific differentiation. Epigenetic reprogramming of MSCs underlies their differentiation and plasticity, both of which include fate determination and terminal differentiation. Currently, the mechanisms of terminal differentiation have been well documented for bone, fat, and muscle cell lineages,1, 4 whereas the cell fate determination is still remained to be investigated.
The key role of signaling pathways in altering cell fate has recently been demonstrated for Wnt/β-catenin signaling. The Wnt3a ligand immobilized to beads and attached to single dividing embryonic stem cell (ESC) induced asymmetric distribution of centrosome, mitotic spindle and downstream components of the Wnt/β-catenin signal pathway (Lrp6, Apc, β-catenin) to the daughter cell proximal to the ligand location. The ligand attached cell retained self-renewal potential while its distal sister became committed. Under the same condition, the Wnt5a ligand transmitting noncanonical Wnt signals did not change the symmetry of division.7 These results show that Wnt/β-catenin pathway plays key role in the fate cell choice of ESCs, however, do not provide evidence for the signals initiating asymmetric division under normal conditions.
In making decision to divide or not, the cell accumulates external and internal signals helping to overcome the negative barrier imposed by the protein of pRb family, collectively named as “pocket proteins”.8 Pocket proteins are deprived of DNA binding site and regulate cell cycle progression through binding and suppression of E2f transcription factors.9, 10 Mitogen signals promote phosphorylation of pocket proteins and liberation of E2fs which induce synthesis of proteins required for cell cycle progression.11 Orthologs of the pRb-E2F pathway present in some unicellular and all multicellular organisms and seems to play key role in multicellular development due to their central position in regulation of cell cycle, cell fate and differentiation.12 Currently, basic pRb-E2F function is considered to be associated with cell cycle regulation and tumor suppression.8, 13 However, the structures of the ancestral pRb-E2fs molecules are more similar to p107/p130-E2f4,5 that play role of quiescence gate keepers in complex self-renewing organisms.14 RB1 and E2F1-3 functions in development were related to diversification of cell cycle, regulation of apoptosis, metabolism and tumor suppression.12 Lin35, the only ortholog of pocket proteins in C. elegans, is more related to p130/p107 than to pRb and does not contribute to G1/S transition.15 Lin35 interacts with Efl-1, an ortholog of E2fs, to form the core of DRM complex regulating vulva cells differentiation in C. elegans.16 The ortholog of E2fs in Drosophila, dE2f1, similar to E2f1-3 in mammals, activates cell proliferation, while dE2f2 forms repressive complexes with pRb orthologs, Rbf1 or Rbf2.17 In plants, pRb ortholog, RBR, determines cell fate of meristem stem cells committed into different tissue specific cells in embryonic and postnatal life.18 In contrast to animals, organs in plants develop post-embryonically, the meristem cells change their fate after germination when seedling switches from heterotrophic to autotrophic growth and later, when the vegetative apical meristem began to produce flowers.19 Various turns in cell fate regulated by RBR in plants, possibly, correspond to similar mechanisms in animals. In mammals, the homolog of DRM, DREAM, suppresses expression of more than 800 E2fs responsive genes at G0 phase of cell cycle that associates with combined regulation of cell cycle and differentiation.20 Roughly, in plants and animals pRb-E2F pathway regulates cell cycle, cell fate, and cell differentiation.
Mesenchymal stem cells as a type of tissue specific adult stem cells
The honor for discovery of MSCs belongs to Russian scientist A. Friedenstein and his coworkers. In search for osteogenic precursors, they found that bone marrow cells in culture form colonies of fibroblast like cells possessing a key feature of stem cells – clonogenicity.21 When introduced in cell impermeable chamber into abdominal cavity of syngeneic recipients, these cells retain clonogenic ability and form bone in the course of repeated transplantation.22 These experiments demonstrated that bone marrow derived MSCs have self-renewal and differentiation capacities and thus may be addressed as a type of SSCs. Later, results of Dr. A. Friedenstein were confirmed and developed. It was found that MSCs from human bone marrow possess multipotency and are inducible to differentiation into fat, bone and chondrocyte lineages under definite conditions.4 MSCs were found in all studied tissues (including peripheral blood) of adult animals belonging to various species.23, 24, 25 Due to ability to accept unusual phenotype termed plasticity,5 immunomodulating ability26, 27 and secretion of humoral factors activating endogenous mechanisms of regeneration, MSCs became a valuable new source for cell therapy.28, 29 MSCs show efficacy in cell therapy of variety of degenerative, inflammatory, traumatic, and immune diseases of various organs.30 This suggests that MSCs contribute to different mechanisms of regenerative therapy, the biological basis of which needs to be studied in future.
There are still no specific markers for MSCs. International Society for Cellular Therapy defined MSCs as being positive for CD73, CD90, CD105, negative for CD45, CD34, CD14 or CD11b and differentiate into at least three mesodermal cell lines: adipocytes, osteocytes and chondrocytes.31 There are also a number of other positive markers for MSCs the expression levels of which depend on various conditions that corresponds to their intrinsic heterogeneity29 and variability in culture.32 MSCs reside in connective tissue of all postnatal organs,24, 25 however, their developmental origin is still undefined. By default, it is widely accepted that postnatal MSCs have mesodermal origin.33 MSCs from distinct tissues reveal different functional and marker abilities.34, 35, 36, 37 It is unclear if these differences are linked to the MSCs origin or result from action of specific tissue environment. To find out whether tissue specific MSCs originate from one or several cell types, Sagi and colleagues38 performed comparative study of expression of 177 genes in MSCs cell lines established from adult adipose tissue (AAD), adult bone marrow (ABM), juvenile spleen (JSpl), juvenile aorta (JAo), and juvenile thymus (JThy). The authors found that MSCs from any source do not express markers of pluripotency (Oct4, Rex-1, Nanog), do express typical stromal markers and are characterized by distinct patterns of the HOX gene expression corresponding to their anatomical location: JThy express TBX5 and PITX2, JSpl – TLX1 and NKX2.5, femoral ABM – PITX1, and JAo – EN2. These MSCs features are stable in long-term culture. The authors concluded that tissue specific MSCs descent from mesodermal precursors developing in the course of body segmentation.38
The difference in molecular imprinting of MSCs from various tissues may directly associates with their distinct regenerative potential that was demonstrated by repair of damaged myocardium,39 differentiation into myocytes of distrophyc mice,40 and modulation of immune response.41 Functional interplay between tissue specific stem cells and surrounding mesenchyme was found in various organs. Thymic stroma produces factors that induce generation of mature T-cells.42 Regulation of proliferative activity in the bladder urothelium of adult animals occurs via Shh and Wnt/β-catenin signals exchange between mesenchyme and parenchyma.43 MSCs from murine fetal hearts express the precursor cell markers, Isl1 and c-kit, that indicates relationship between mesenchyme and parenchyma in the same organs.44 MSCs from murine adult bladder do not possess clonogenic and differentiation capacities in contrast to embryonic bladder MSCs and adult bone marrow MSCs.45 In correspondence with these data, cardiac fibroblasts can be reprogrammed into cardiomyocytes more effectively than the tail skin fibroblasts.44 Molecular imprinting and corresponding differences in marker profiles, ability to proliferate and differentiate into distinct lines in MSCs from various tissues may be termed for short as “tissue imprinting”.
MSCs in culture represent a heterogenous population consisting of multi-, bi- or unipotent lineage restricted progenitors and fibroblasts lacking differentiation potential.28, 46 The serial analysis of gene expression showed that MSCs transcriptome contains a variety of transcripts that play a role in the specification of mesoderm, lineage specific mesodermal derivatives and regulation of the MSCs induced engraftment.28, 47 Currently it is widely accepted, that efficacy of MSCs mediated cell therapy is mostly based on their humoral effects. Inversion of MSCs into tissue specific cells of damaged tissues may greatly enhance the clinical significance of this recourse in treatment of widely distributed diseases. The condition which critically limits the MSCs therapeutic efficacy is misunderstanding of the mechanisms regulating cell fate choice. The origin of lineage restricted progenitors and determination of cell fate occur in G1 phase of cell cycle via interaction of several signal pathways including the pRb-E2F.
General view of pRb-E2F signaling
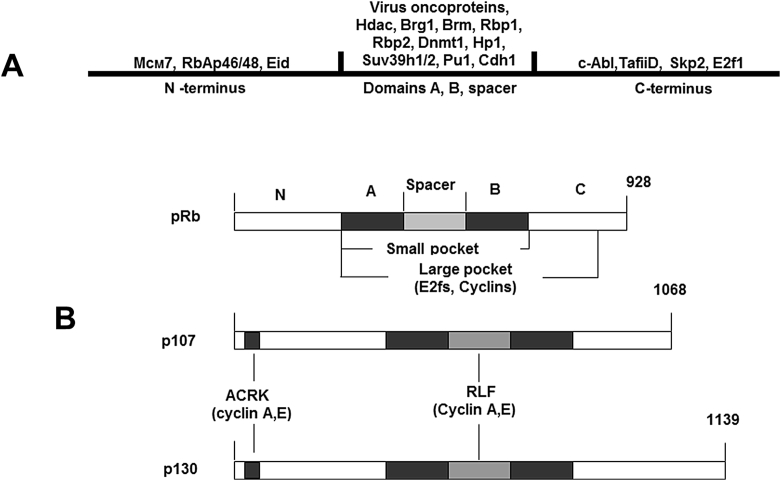
pRb was the first tumor suppressor to be cloned.48, 49 The pRb loss causes retinoblastoma – rare form of eye children's cancer that occurs in high or low penetrant forms depends on type of the RB mutation.50, 51 pRb is an ubiquitous negative regulator of cell cycle progression in all tissues of multicellular organism and the founder member of the pocket protein family which includes two other proteins: p107 and p130 (Fig. 1).52, 53 Structurally, p130 and p107 are more related to one another than to pRb, are expressed, accordingly, in quiescent and proliferating cells, while pRb activity is determined at all cell cycle stages.54, 55 In contrast to pRb, p107 and p130 are able to bind and inhibit cyclin E/A-Cdk2 in regulation of S phase entry and M phase exit.56, 57, 58 Pocket proteins do not possess DNA binding domain and regulate cell cycle progression via interaction with E2f transcription factors.9, 59 E2f family includes 9 proteins. E2f1-5 possessing the ability to bind pocket proteins are divided into activators (E2f1-3) and suppressors (E2f4,5). Е2f4-5 accumulate in quiescent, while Е2f1-3 – in proliferating cells. Activator and suppressor E2fs bind distinct pocket proteins: Е2f1-3 – pRb, while Е2f4,5 – ⤳107/⤳130, that allow pocket proteins to regulate different E2f-responsive genes.54, 60 E2fs bound to a pocket protein lose the ability to activate transcription because pRb binding site is located inside E2fs transactivation domain.59 E2fs activate transcription when form dimers with Dp proteins.8, 10, 52 When unbound to pocket proteins E2fs activate transcription of many genes the products of which are required for G1/S transition, replication and mitosis. Inhibition of E2fs transcriptional activity induces cell cycle arrest.8, 10
Figure 1.
Comparative structures of pocket proteins. А. Domain structure of pRb and list of pRb-binding proteins. B. Comparative structures of human pRb, p107, и p130. The pocket proteins structures reveal high levels of homology in A and B domains composed of the cyclin folds. The similar tandem cyclin folds were found in the N-terminus of all pocket proteins. The B-domain of small pocket contains binding site for the LxCxE motif detected in many functionally different molecules including oncoproteins. The small pocket and proximal part of the C-terminus form the large pocket mediating interactions of pRb with E2fs and cyclins. The p107 and p130 do not show homology with pRb outside the A, B and N-domains and are more similar to each other than to pRb.
Whichever signal impinges on cell of multicellular organism it is processed at cell cycle check points to make decision what to do: commit to another round of division, exit the cell cycle or change the cell fate. The key regulator of the cell cycle control system making this decision is pRb-E2F pathway which is highly conserved in development. The orthologs of pocket and E2fs proteins are present in unicellular plants and animals.12 RB and E2F ancestral genes divided, correspondingly, into RB1 (including RB1), RBL (including RBL1/p107 and RBL2/p130) subgroups, while E2F – into E2F4/5 (including E2F4 and E2F5) and E2F1/2/3 (including E2F1, E2F2, E2F3) subgroups before placozoans and bilaterians diverged. Members of RBL and E2F4/5 subgroups show more sequence similarity, correspondingly, with RB and E2F ancestral sequences, than members of RB1 and E2F1/2/3 subgroups. These results suggest that ancestral of p130/p107 and E2f4/E2f5 proteins might represent more ancient function of pRb-E2F signaling associated with control of quiescence and cell fate choice.12 In C. elegans, Drosophila, and mammals the DREAM complex was identified which includes as core element the p130/E2f4/Dp proteins and functionally directed to keep quiescence.16, 20, 61 The outlined data suggest that pRb-E2F signaling creates mechanism for cell fate choice to shift from proliferation to transient or constitutive quiescence including long last G0 state, differentiation or cell senescence. On the other hand, the pRb in complex with activator E2fs might contribute to functional diversification in pRb-E2F signaling, for example, tumor suppression via control on all aspects of cell cycle and coupling cell cycle with differentiation, cell senescence and apoptosis.12, 17
Among all pocket proteins only pRb owns function of tumor suppressor and is functionally inactivated in all types of human cancer.62, 63 However, pocket proteins show features of functional redundancy the physiological relevance of which is currently not completely clear.64 DNA microarray analysis showed that pRb deficiency targets genes encoding cell cycle regulatory proteins.65 These genes were previously shown to be regulated by E2F1-3.66, 67 In contrast, loss of p107/p130 alters expression of genes regulating quiescent state in response to growth or differentiation signals.65 Some genes showed overlapping pattern of regulation by pRb and p107/p130. This may reflect the consistency of regulation of the same genes by pRb blocking their activity through interaction with E2f1-3 followed by stable repression of these genes with p130/E2f4.65, 68 Evidence of functional redundancy among pocket proteins in interaction with separate genes and regulation of separate functions were supported by the demonstrations of immortalization, loss of differentiation ability and sensitivity to cell senescence signals in fibroblast lacking all pocket proteins. In contrast, none of other knockout combinations induced these functional defects.69, 70
Cell cycle regulation
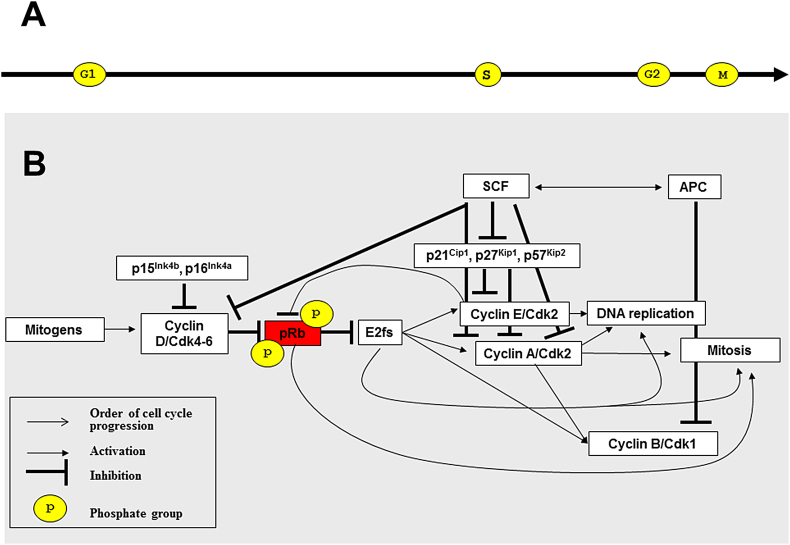
pRb/E2f4 and p130/E2f4,5 complexes induce transient cell cycle arrest in G1 by suppression of transcription of genes required for replication and mitosis.71, 72 Similar growth arrest is induced in response to serum deprivation, DNA damage or action of TGFb growth factors,73, 74, 75 while permanent cell cycle exit occurs during cell differentiation and senescence.76, 77 Mitogens activate cyclins D/Cdk4-6 complexes leading to eventual phosphorylation and inactivation of pRb from early G1 phase to mitosis (Fig. 2).78, 79, 80 pRb phosphorylations by Cdks on multiple phosphorylation sites induce unique conformations of pRb altering its ability for different protein interactions81 and releasing of E2fs which promote cell cycle progression.82, 83 Similar mechanism underlies the transforming effect of the oncogenic viruses the products of which bind pocket proteins and convert E2fs into constitutive activators of transcription.10, 84 In G1/S transition pRb changes its partner from E2f4 to E2f1, while p130 is downregulated and degraded.85 The rest of p130 in complex with E2f4 is converted into p130/E2f4/cyclinE/A-Cdk271, 72 and loses its suppressor activity. DREAM complex after G1/S dissociates from p130 and changes it to Myb.20 G1/S transition and followed DNA replication are initiated by expression of cyclin E which downregulates pocket proteins and upregulates E2f1-3.8 The activator E2fs induce expression of cyclin A, Pcna, Mcm2-7, Cdc6 and other proteins of replication machinery whose production are negatively regulated by pocket proteins.86 pRb negatively regulates expression of the mitotic checkpoint protein Mad2 which in its turn downregulates the anaphase promoting complex (Apc). Loss of pRb causes overexpression of Mad2, premature chromosome segregation, aneuploidy and tumor formation.87, 88 In Drosophila, a pRb ortholog, RBF, interacts with the CAP-D3 condensin complex subunit promoting chromosome condensation during prophase.89 Under pRb deficiency, mammalian cells show decreased interaction of condensin II with chromatin, hypocondensation of chromosomes and delayed progression to metaphase.90
Figure 2.
Pocket proteins connect outside signals with the cell cycle control system regulating cell proliferation. А. Direct sequence of the cell cycle phases. B. Basic components of the cell cycle control system. Mitogens induce synthesis of cyclin D which forms active kinase complexes with Cdk4-6. Cyclin D is under negative control of p15Ink4b/p16Ink4a inhibitors. Cyclin D/Cdk4-6 phosphorylate pRb and liberate E2fs which promote synthesis of cyclins E/A/B required for initiation and progression of DNA replication and mitosis. Cyclins eventually phosphorylate pRb until end of mitosis. p21Cip1, p27Kip1 and p57Kip2 are CdkI inhibiting Cyclin E/A; SCF and APC are ubiquitin ligases promoting periodical inactivation and degradation of the cell cycle regulatory proteins.
The rate of cancer progression in patients with retinoblastomas is mostly related to epigenetic, but not to genetic changes.91 This may be related to pRb phosphorylations on multiple sites during cell cycle progression which induce diverse conformational changes in its structure.92 There are few known RB mutations that cause retinoblastoma, however, they do not induce discrete loss of its functions.93 Some low penetrance forms of retinoblastoma have been analyzed to study pRb role in cell cycle signaling.94, 95 The product of a native pRb mutation R661W that causes formation of the low penetrance retinoblastoma, does not bind activator E2fs but retains the ability to interact with repressor E2fs. In vitro the low penetrance mutants show some activity in proliferation control and induce differentiation in the pRb deficient Saos-2 cell line.51 In experiments in vivo with the knock in R661W were obtained similar results.96 Using a panel of synthetic pRb pocket mutants it was shown that cell cycle and differentiation capacity of pRb are genetically and mechanistically separated.97 There are two mechanisms by which the low penetrance pRb mutants may retain partial functional capacity. First, they retain the ability to bind suppressor E2fs. A pRb mutant with small deletion at the end of T antigen-binding site showed higher affinity for E2f4 compared to E2f1, formed complex with E2f4, retained tumor suppressor activity and induced early muscle commitment more effectively than the wild type pRb.98 Low pRb penetrance mutants may also control cell cycle progression through their capacity to inhibit Skp2 mediating ubiquitination and degradation of p27 CdkI.99 C-domain of pRb binds Skp2 while small pocket simultaneously interacts with Cdh1 component of Apc making conditions for ubiquitination and degradation of Skp2.100 This allows p27 CdkI to escape degradation and to block the activity of Cdks through pRb phosphorylation.
Regulation of cell fate via chromatin modifications
pRb can alter cell fate via different types of chromatin modifications: 1) via recruiting of co-repressors when bound to a gene promoter by E2fs, 2) through interaction with proteins of Polycomb (PcG) family; 3) by regulation of genome wide formation of heterochromatin domains, pericentric heterochromatin, telomeres, and senescence-associated heterochromatin foci (SAHF).101 When bound to E2fs, pRb can induce active suppression of transcription at local chromatin areas by recruiting functionally distinct molecules to the gene promoters encompassing E2fs binding sites. The list of the bound proteins contains functionally different molecules: DNA methyltransferases (Dnmt1),102 histone deacetylases, (Hdac1,2),103 histone methyltransferases (Suv39h1/2, Suv4-20h1/2),104 histone demethylases (Rbp2),105 chromatin-associated proteins of Hp-1 family,106 key components of chromatin remodeling complexes.107 pRb regulates stability of Dnmt1 methylating promoters of some regulatory genes. Inactivation of pRb results in abnormal DNA methylation and malignant progression.108 One of the well studied chromatin modification is histone deacetylation followed with histone methylation.109, 110 Interaction of pRb proteins with Brg1 and Brm – the ATPase components of the SWI/SNF nucleosome remodeling complexes, may regulate translocation of nucleosomes along the DNA strand, exchange of histone variants to repress or activate transcription.111, 112 The mechanism of interaction of Brg1 and Brm with pocket proteins is still undefined.113 At the same time, its functional significance is evident, since genetic inactivation of these proteins in mouse model in vivo results in hyperproliferation and tumor formation.114
Generation of facultative heterochromatin by Polycomb (PcG) proteins is initiated via trimethylation of H3K27 on promoters of regulatory genes followed by the establishment of stable repressive complexes on this histone mark.115 This chromatin modification is induced by Ezh2 methyltransferase – a component of the PcG repressive complex 2 (PRC2),116, 117 the expression of which is under control of pRb.118 Inactivation of pocket proteins abolishes H3K27 trimethylation on promoters of many genes including the p16 CdkI,119 which functionally associated with regulation of cell cycle, cell senescence and cancer.120 The mechanism for PRC2 recruitment to target genes in vertebrates is still unknown. At the same time, it was established that it may be mediated by the RbAp46/48, a component of PRC2, which indirectly binds pRb.121
Although role of pRb family in formation of the PcG mediating gene silencing is commonly recognized, its functional significance much more exemplified in plants than in animals. In Arabidopsis the germ line evolves from uncommitted cells in floral meristems.122 Loss of RBR allele alters cell determination, induces activation of nuclear division and misexpression of specific markers in female and male gender cells.123 Specification of gametes in Arabidopsis depends on appropriate interplay between RBR and PRC2. RBR is required for cell differentiation of male and female gametophytes. Loss of RBR perturbs expression levels of the DNA methyltransferase 1 (MET1), a subunit of PRC2. Additionally, RBR binds MET1 which regulates maintenance of heterochromatin. PRC2-specific H3K27-trimethylation activity represses paternal RBR, suggesting reciprocal RBR-PRC2 regulatory circuit that is important for the reproductive cells development.124 The RBR-PRC2 interaction may present an established net to control gametogenesis and expression of imprinted genes evolved prior to the separation of animal and plants.125, 126 Loss of RBR results in hyperproliferation in Arabidopsis late embryogenesis, while after germination the seedlings are unable to shift from heterotrophic to autotrophic growth that associates with inappropriate expression of late embryonic genes controlled by PRC2 through H3K27 trimethylation.127, 128 Plants in contrast to animals maintain pools of totipotent cells in meristems during all life to form new organs and promote sporophytic development.129 Conditional loss of RBR in Arabidopsis prevents differentiation of stem cells and increase in their pool,18 while transient expression of RBR in the tobacco shoot apical meristem induces opposite result by activating stem cells differentiation.130
pRb can alter cell fate by supporting cell senescence through formation of SAHF,131, 132 pericentric chromatin and telomeres.104 pRb is able to bind Suv39h1/2 methyltransferases, which trimethylate H3K9 and constitute binding site for chromo domains of HP-1 proteins.133 Embryonic knockout of RB causes sharp decrease in H3K9 methylation and HP-1 enrichment in cyclin E promoter.106 When bound to histones the Suv39h1/2 create new binding sites for HP-1 proteins on newly synthesized DNA promoting the spread of heterochromatin and formation of SAHF. H4K20me3 is another genome-wide modification of chromatin which is composed under control of pocket proteins. In triple knockout mice H4K20me3 levels decrease in all heterochromatin domains: telomeres, long interspersed nuclear elements and pericentric heterochromatin.134, 135 pRb as well as p130 and p107 physically bind Suv4-20h1/h2 methytransferases which trimethylate H4K20.104
pRb proteins in fate determination and differentiation of MSCs
Mechanism of asymmetric division includes unequal distribution of cell polarity factors and cell fate determinants between daughter cells. Well studied players of this mechanism in C. elegance and Drosophila are Par complex and Numb protein.136, 137 However, triggers of asymmetric cell division and their connection to pocket proteins are still waiting to be discovered. pRb-E2f signaling acts as the switch altering functional status of the cell and thereby changing its fate.138 Additionally to the switch from proliferation to quiescence associated with differentiation, pRb regulates E2f1 mediated apoptosis67 and cell senescence.131, 132 In C. elegans, pRb ortholog Lin35 determines cell fate during larval development. Combined inactivation of LIN35 and a synthetic multivulva B (synMuv B) genes causes hyperproduction of vulva cells during larval development. Under normal conditions the products of LIN35, EFL1 (an E2F ortholog in C. elegans) and synMuv B genes form DRM complex providing transcriptional repression of the LIN-3/EGF (epidermal growth factor) gene regulating proliferation of vulva precursor cells.139, 140 Additionally, Lin35 maintains repressive status of chromatin at germline specific genes in somatic cells and its mutation causes transformation of somatic into germline cells.141, 142
pRb may induce differentiation by sequestering its inhibitors Eid-1 and Id2. Eid-1 mediates degradation of the p300 histone acetylase, a co-activator of MyoD transcription.143, 144 Id2 inhibits myogenic differentiation by binding and sequestering the E2 factors which form heterodimers with proteins of MyoD family to activate the tissue specific transcription.145, 146 pRb also binds and inactivates Rbp2/Kdma5 H3K4 demethylase.105 H3K4me3 mark associates with active status of chromatin and its demethylation shifts the balance to differentiation. It was found more recently that in terminally differentiated cells common Kdm5a and E2f4 targets are bound not by pRb but p130 and DREAM complex.147
pRb loss in progenitors of various tissues causes their expansion, blockage of differentiation and initiation of tumors.70 Genome wide analysis in mammalian fibroblasts and C. elegance showed that Utx/Kdm6A (Utx after) activity promotes pRb signaling. Inactivation of Utx changes the cell fate and initiates malignant transformation.148 The question raises what function of pRb is primarily associated with tumor formation? Because retinoblastoma cells express markers of multiple cell lines, one may suggest that retina cells lacking RB lose the ability to control fate determination and establish or maintain the tissue specific differentiation profile.138 There is a new viewpoint that Rb family members promote general organization of chromatin.149 Presumably, all effects of pocket proteins previously addressed in the context of regulation of separate genes, should be reevaluated as results of activity of protein complexes at specific locations in the genome.
pRb influence on differentiation includes its direct interactions with variety of tissue specific transcription factors beyond pRb-E2F pathway. List of these factors regulating specification of MSCs into osteoblasts includes Runx2,150 adipocytes – C/Ebps and Pparγ,151, 152 myocytes – MyoD.153, 154 In these cases pRb acts as transcriptional activator of terminal differentiation by promoting induction of tissue specific master genes. The pRb specific role in early stages of differentiation is still unclear.
Bone differentiation
Bone and fat unipotent precursors evolve from bi-potent ancestral MSCs on alternative basis (Fig. 3) by an epigenetic switch regulated by histone H3K27 methyltransferase, Ezh2, and demethylase, Utx.155 Ezh2 and Utx exhibit an inverse expression pattern during MSCs osteogenic and adipogenic differentiation. Ezh2 acts as the negative regulator of osteogenesis and positive regulator of adipogenesis of human MSCs, whereas Utx induces opposite effects.156 The master osteogenic regulator, Runx2,150 is repressed during adipogenic differentiation due to strong increase in H3K27me3 on the Runx2 transcription start site. Ezh2 represses transcription and increases histone H3K27me3 level for the downstream Runx2 targets osteopontin, and osteocalcin.155 Conversely, Runx2, osteopontin and osteocalcin transcripts are upregulated by Utx that coincides with downregulation of H3K27me3. Presumably, Ezh2 trimethylates H3K27 on the promoters of Runx2 and its downstream targets causing inhibition of these genes expression. Utx acts in opposite direction by removing H3K27me3 and promoting osteogenic differentiation.155 Active status of Utx in MSCs is supported by pRb pathway. The genome wide Gene Ontology analysis found that in fibroblasts Utx occupies 49 genes associated with the pRb pathway.148 Loss of Utx ortholog (dKdm6a) in Drosophila results in increased proliferation due to suppression of Notch and pRb pathway.157 On the protein levels, pRb binds Runx2 through small pocket and form complex which is detected at the promoters of its targets.150 pRb maintains differentiation status of bone tissue. The bone specific pRb inactivation in mice causes dedifferentiation of the osteoclasts.158 Additionally, pRb promotes Runx2 mediated activation of p27 CdkI that turns on feedback signals and keeps pRb in active hypophosphorylated form.150 Patients with retinoblastoma are predisposed to growth of osteosarcoma in teenager's age that gives evidence of specific role of pRb in proliferation of osteoblast cell line compare to all other MSCs derived lines.64
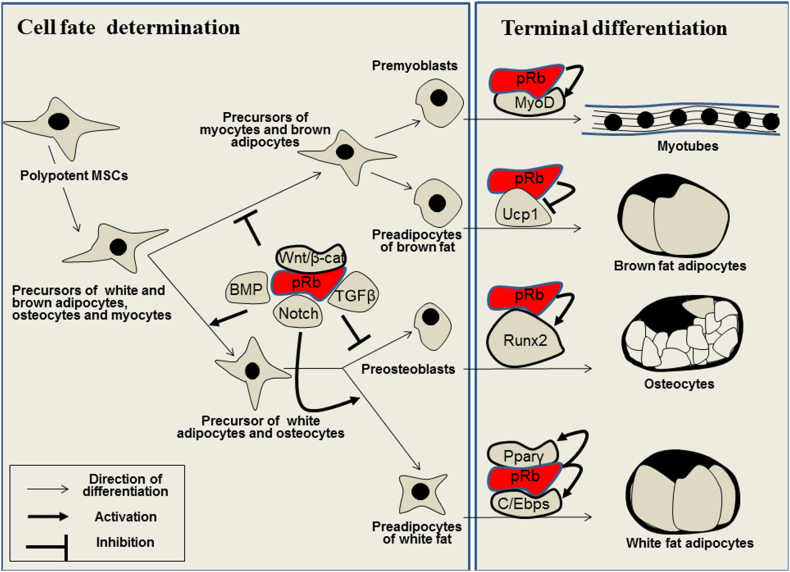
Figure 3.
Role of the pRb signaling in differentiation of MSCs into adipocytes, osteocytes, and myocytes. MSCs differentiation includes the cell fate determination and terminal differentiation phases. In the course of cell fate determination MSCs differentiation potential is eventually limited and the cells form three-, bi-, and unipotent precursors. This process is regulated by pRb interacting with distinct signal pathways including Wnt/β-catenin, BMP, TGFβ, Notch and others. Terminal differentiation is directed by the tissue specific transcription factors the nature of which has been determined.
Fat differentiation
Ezh2 shows negative regulation of osteogenesis while Utx – negative regulation of adipogenesis for murine and human MSCs.156 Inhibition of the methyltransferase activity using siRNA mediated knockdown or chemical reagents demonstrated existence of epigenetic Utx switch enhancing fat differentiation when level of Ezh2 elevated while level of Utx dropped. In freshly harvested human MSCs the promoters for adipogenic genes PPARγ2, leptin, fatty acid-binding protein 4, lipoprotein lipase are hypermethylated,159 but became hypomethylated after induction of fat differentiation by overexpression of Ezh2.160 In contrast, under conditions of Ezh2 hyperexpression, promoters of RUNX2, osteocalcin, and osteopontin became hypermethylated and expression of these genes was suppressed.155 The possible mechanism of activation of the Ezh2 mediated fat differentiation may include the alternative repression of WNT genes that are negative targets of Ezh2.161 It is possible that Ezh2 represses osteogenesis at multiple levels by direct affecting WNT genes that upregulate Runx2 and its downstream targets.162 Simultaneously, Ezh2 activates adipogenesis which is active by default in the case of suppression of osteogenesis. In the course of determination of fat differentiation MSCs are triggered by appropriate stimuli to make cell fate choice, then they become restricted to the adipocyte lineage and generate preadipocytes. After that, induced preadipocytes undergo multiple rounds of proliferation (mitotic clonal expansion) followed by terminal differentiation.163
Wnt/β-catenin signaling activates commitment and inhibits terminal fat differentiation.164 Forced expression of Wnt10b maintains undifferentiated status of preadipocytes that is mediated by inhibition of activity of the master fat differentiation factors, C/Ebpα and Pparγ. Wnt10a,b and Wnt6 through β-catenin attenuate adipogenesis and activate osteogenesis of committed cells. Inactivation of β-catenin prevents inhibition of adipogenesis and activation of osteogenesis by these factors. Transgenic mice constitutively expressing high levels of Wnt10b produce less fat tissue than normal animals.165 Mutation of C256Y in structure of WNT10b abolishes its ability to activate β-catenin and leads to obesity.166 Inhibition of β-catenin signaling by expression of dominant-negative form of Tcf4 enhances fat differentiation and promotes reversion of myoblasts into adipocytes. The expression of some proteins transmitting Wnt signals, such as R-spondins2,3, Wnt1, transcription factors Tcf1,3,4 is significantly elevated in the А33 preadipocytes, compared to maternal cells. β-catenin accumulates in A33 cells nuclei and causes elevation of the levels of Lef/Tcf.167 Possibly, Wnt signals promote production of Bmp4, which in its turn induces appearance of preadipocytes by inducing transcription factors C/Ebpβ and Pparγ. These tissue specific master factors induce terminal stage of fat differentiation (Fig. 3).168 On the other hand, Wnt/β-catenin signals inhibit terminal stage of fat differentiation. Wnt10b attenuates formation of fat cells by decreasing activity of Pparγ, whereas reduction of Wnt10b production, in opposite, results in activation of adipogenesis.169 Obviously, Wnt/β-catenin signals support generation of proliferating preadipocytes. However, positive role of these signals turns into negative when preadipocytes become quiescent during initial step of terminal differentiation.163
There are two types of fat tissues: white (WAT) and brown (BAT). Brown fat is found only in mammals and its color depends of big number of mitochondria. Function of BAT cells is linked to Ucp1 (uncoupling protein-1) promoting energy expenditure at the expense of its intake in WAT.170 pRb signaling promotes the MSCs fate choice to WAT commitment. Embryonic fibroblasts derived from mice (MEFs) with RB embryonic knockout, are not sensitive to induction of fat differentiation in vitro.151 This defect may be eliminated by forced Pparγ expression.171 These results support the idea that pRb promotes differentiation of MSCs into preadipocytes that eventually generate WAT. Opposite, pRb loss facilitates generation of BAT (Fig. 3).152 Elimination of pRb results in elevation of the U≿⤳1 levels. MEFs derived from RB-/- mice express U≿⤳1 on the level similar to that in BAT adipocytes. This suggests that under normal conditions pRb plays role of the differentiation switch promoting formation of WAT at the expense of ВАТ.172 The WAT adipocytes with low expression of pRb show increase in number of mitochondria, elevate the ВАТ-specific expression and decrease in the WAT-specific one.173 Loss of pRb in MEFs results in elevation of the levels of myogenin and heavy chain of muscle myosin. Possibly, pRb inhibits commitment of MSCs into common precursors of myocytes/ВАТ.174 On the other hand, ВАT phenotype recapitulates after pRb loss in mature WAT cells according to energy expenditure, oxygen intake, elevation of thermogenesis and increase in the number of mitochondria.175
Muscle differentiation
Myocytes are generated from ancestral mesodermal cells in the course of early and terminal commitment. Myoblasts which are formed during early commitment express tissue specific master factor MyoD and an early muscle marker desmin, but still proliferate.176, 177 Under serum deprivation, MyoD turns on full program of striated muscle differentiation (Fig. 3) which includes eventual expression of Myf-5, myogenin, and MRF4. These factors induce formation of nondividing myotubes and production of terminal muscle markers like myosin heavy chain.178, 179, 180, 181 The functional status of pocket proteins in regulation of MSCs differentiation is epigenetically regulated by Ezh2/Utx switch.148 Ezh2 trimethylates Н3К27 at RB and RB-associated genes promoters supporting their suppression by the Polycomb repressive complex 1 (PRC1), while Utx demethylates Н3К27me3, enhances active status of RB gene set and prevents the cells proliferation.148 Being active, the pocket proteins activate differentiation regulating the Ezh2/Utx switch on promoters of master tissue specific factors. Determination the cell fate occurs in dividing cells in which pRb interplays with proteins transmitting the Wnt/β-catenin signals. pRb loss in these cells promotes proliferation of satellite cells and increases in population of postnatal myoblasts.182 The satellite cells in postnatal life present stem cells for striated muscle but retain the capacity to differentiate into adipocytes.183 Possibly, the pRb ability to regulate cell fate choice between muscle and fat cell is mediated via its interaction with Wnt/β-catenin pathway. Hyperproduction of R-spondins activates the Wnt/β-catenin signal pathway, while injection of recombinant R-spondins enhances expression of mRNA of the tissue specific muscle factor Myf5 in myoblasts С2С12 and primary satellite cells.184 R-spondins promote myogenic differentiation and induce formation of hypertrophic myotubes in C2C12 cell line. Inversely, somatic knockdown of R-spondin genes downregulates Myf5 expression and myotubes formation. In MSCs β-catenin binds the p130/E2f4 complex and alters its ability to inhibit proliferation.185
The described results provide evidence that pRb and Wnt/β-catenin pathways may mutually interact each other to regulate fat and muscle differentiation. Fine details of this interaction and its inductive influence on fat determination are to be studied in future. Our results suggest that constitutive expression of functional pRb in polypotent 10T1/2 cell line enhances fat differentiation in contrast to pRb functional mutant which acts in opposite direction suppressing fat but activating muscle differentiation.98, 186 These results correspond to recent published data that activation of one type of mesodermal differentiation, for example, differentiation into WAT inhibits the alternative MSCs commitment into bone or BAT.152, 172 In the experiments in vivo performed 6 months after tissue specific inactivation of RB, the number of satellite cells in murine muscle tissue increased by 5 folds and the number of myoblasts – by 3 folds.182 These results suggest that pRb inhibits determination of ancestral cells to muscle lineage. Presumably, myoblasts and BAT cells are derived from common ancestral precursor for muscle and BAT cells, the formation of which is suppressed by pRb (Fig. 3).
Conclusions
During last decade, the cell therapy based on transplantation of MSCs became promising in treatment of various widely distributed and dramatic diseases. Further expansion of these clinical trials is limited due to low engraftment efficacy of MSCs and non-availability of methods for directed regulation of their differentiation and plasticity. Cell differentiation of MSCs is initiated at G1 phase of cell cycle via interaction of mediators of different signal pathways with ubiquitous pocket proteins. Together with E2f transcription factors, pocket proteins form pRb-E2F pathway regulating cell cycle progression. Functional inactivation of pRb leads to deviations in cell cycle and underlies cancer formation. Recent evidence suggests that ancient function of the pRb-E2F signaling was to regulate cell quiescence, cell fate choice and differentiation. The ancestral molecules transmitting pRb-E2F signals were closely related to suppressor E2f4,5 and p107/p130, while pRb and E2F1-3 played roles in diversification of cell cycle control and tumor suppression. Eventually, p130/E2f4 has served as core for DREAM complex which controls quiescence in mammals and connects it to cell proliferation in development.20 Pocket proteins reveal features of functional redundancy. Inactivation of all pocket proteins is the only condition for cells immortalization, loss of differentiation and apoptosis. Presumably, pocket proteins regulate transcription of overlapping targets via initial blockage of their activity by pRb/E2F1-3 followed with the deep transcription suppression by p130/E2f4. The stress-induced senescence in Saos pRb-/- cells may be initiated by exogenous pRb which later delegates its role to p130 detected at the E2f targeted genes promoters.187 pRb may initiate differentiation via sequestration of Kdm5a H3K4 demethylase, however, in terminally differentiated cells the Kdm5a targets are bound by p130 but not pRb.147 Recent publications support the suggestion, that pRb regulates activity of enzymes generated key chromatin marks like H3K4me3, H3K27me3, H4K20me3. Through this ability, pRb functions as a local chromatin modifier and regulates cell fate choice by suppressing proliferation of one cell population in favor of another, inducing differentiation, cell senescence while loss pRb leads to malignant transformation and cancer.
Disclosures
Nothing to disclose.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We would like to thank Dr. O. Anatskaya, Institute of Cytology RAS, for critical reading of the manuscript.
This work was supported by the Russian Foundation for Basic Research (projects Nos. 12-04-00252 and 14-04-31115).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Friedenstein A.J., Petrakova K.V., Kurolesova A.I., Frolova G.P. Precursor cells for osteogenic and hemopoietic tissues. Analysis of heterotopic transplants of bone marrow. Tsitologia. 1968;10:557–567. [PubMed] [Google Scholar]
- 2.Caplan A.I. The mesengenic process. Clin Plast Surg. 1994;21:429–435. [PubMed] [Google Scholar]
- 3.Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger M.F., Mackay A.M., Beck S.C. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Herzog E.L., Chai L., Krause D.S. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- 6.Prockop D.J., Kota D.J., Bazhanov N., Reger R.L. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010;14:2190–2219. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habib S.J., Chen B.C., Tsai F.C. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339:1445–1448. doi: 10.1126/science.1231077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 9.Nevins J.R. Transcriptional regulation. A closer look at E2F. Nature. 1992;358:375–376. doi: 10.1038/358375a0. [DOI] [PubMed] [Google Scholar]
- 10.Nevins J.R. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998;9:585–593. [PubMed] [Google Scholar]
- 11.Sherr C.J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 12.Cao L., Peng B., Yao L. The ancient function of RB-E2F pathway: insights from its evolutionary history. Biol Direct. 2010;5:55. doi: 10.1186/1745-6150-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sage J. The retinoblastoma tumor suppressor and stem cell biology. Genes Dev. 2012;26:1409–1420. doi: 10.1101/gad.193730.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campisi J. Cancer and ageing: rival demons? Nat Rev Cancer. 2003;3:339–349. doi: 10.1038/nrc1073. [DOI] [PubMed] [Google Scholar]
- 15.Boxem M., van den Heuvel S. lin-35 Rb and cki-1 Cip/Kip cooperate in developmental regulation of G1 progression in C. elegans. Development. 2001;128:4349–4359. doi: 10.1242/dev.128.21.4349. [DOI] [PubMed] [Google Scholar]
- 16.Harrison M.M., Ceol C.J., Lu X., Horvitz H.R. Some C. elegans class B synthetic multivulva proteins encode a conserved LIN-35 Rb-containing complex distinct from a NuRD-like complex. Proc Natl Acad Sci U S A. 2006;103:16782–16787. doi: 10.1073/pnas.0608461103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Heuvel S., Dyson N.J. Conserved functions of the pRB and E2F families. Nat Rev Mol Cell Biol. 2008;9:713–724. doi: 10.1038/nrm2469. [DOI] [PubMed] [Google Scholar]
- 18.Wildwater M., Campilho A., Perez-Perez J.M. The RETINOBLASTOMA-RELATED gene regulates stem cell maintenance in Arabidopsis roots. Cell. 2005;123:1337–1349. doi: 10.1016/j.cell.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 19.Gutzat R., Borghi L., Gruissem W. Emerging roles of RETINOBLASTOMA-RELATED proteins in evolution and plant development. Trends Plant Sci. 2012;17:139–148. doi: 10.1016/j.tplants.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Litovchick L., Sadasivam S., Florens L. Evolutionarily conserved multisubunit RBL2/p130 and E2F4 protein complex represses human cell cycle-dependent genes in quiescence. Mol Cell. 2007;26:539–551. doi: 10.1016/j.molcel.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Friedenstein A.J., Chailakhjan R.K., Lalykina K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 22.Miskarova E.D., Lalykina K.S., Kokorin I.N., Fridenshteĭn A.J. Osteogenic properties of repeatedly-passaged diploid cultures of bone marrow cells. Biull Eksp Biol Med. 1970;70:78–81. [PubMed] [Google Scholar]
- 23.Panasyuk A.F., Luria E.A. The formation of fibroblast colonies in cultures of peripheral blood cells. Biull Eksp Biol Med. 1970;70:96–98. [PubMed] [Google Scholar]
- 24.Meirelles Lda S., Chagastelles P.C., Nardi N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 25.Meirelles Lda S., Nardi N.B. Methodology, biology and clinical applications of mesenchymal stem cells. Front Biosci (Landmark Ed) 2009;14:4281–4298. doi: 10.2741/3528. [DOI] [PubMed] [Google Scholar]
- 26.Le Blanc K., Tammik C., Rosendahl K., Zetterberg E., Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 27.Le Blanc K., Rasmusson I., Sundberg B. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 28.Phinney D.G. Biochemical heterogeneity of mesenchymal stem cell populations: clues to their therapeutic efficacy. Cell Cycle. 2007;6:2884–2889. doi: 10.4161/cc.6.23.5095. [DOI] [PubMed] [Google Scholar]
- 29.Phinney D.G. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem. 2012;113:2806–2812. doi: 10.1002/jcb.24166. [DOI] [PubMed] [Google Scholar]
- 30.Trounson A., Thakar R.G., Lomax G., Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011;9:52. doi: 10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dominici M., Le Blanc K., Mueller I. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 32.Bara J.J., Richards R.G., Alini M., Stoddart M.J. Concise review: bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem Cells. 2014;32:1713–1723. doi: 10.1002/stem.1649. [DOI] [PubMed] [Google Scholar]
- 33.Bianco P., Robey P.G., Simmons P.J. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedenstein A.J., Lalykina K.S. Bone induction and osteogenic precursor cells. Moscow: Medicine. 1973 [Google Scholar]
- 35.Wagner W., Wein F., Seckinger A. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Chateauvieux S., Ichanté J.L., Delorme B. Molecular profile of mouse stromal mesenchymal stem cells. Physiol Genomics. 2007;29:128–138. doi: 10.1152/physiolgenomics.00197.2006. [DOI] [PubMed] [Google Scholar]
- 37.Jansen B.J., Gilissen C., Roelofs H. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells Dev. 2010;19:481–490. doi: 10.1089/scd.2009.0288. [DOI] [PubMed] [Google Scholar]
- 38.Sági B., Maraghechi P., Urbán V.S. Positional identity of murine mesenchymal stem cells resident in different organs is determined in the postsegmentation mesoderm. Stem Cells Dev. 2012;21:814–828. doi: 10.1089/scd.2011.0551. [DOI] [PubMed] [Google Scholar]
- 39.Gaebel R., Furlani D., Sorg H. Cell origin of human mesenchymal stem cells determines a different healing performance in cardiac regeneration. PLoS One. 2011;6:e15652. doi: 10.1371/journal.pone.0015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vieira N.M., Zucconi E., Bueno C.R., Jr. Human multipotent mesenchymal stromal cells from distinct sources show different in vivo potential to differentiate into muscle cells when injected in dystrophic mice. Stem Cell Rev. 2010;6:560–566. doi: 10.1007/s12015-010-9187-5. [DOI] [PubMed] [Google Scholar]
- 41.Jung Y., Bauer G., Nolta J.A. Concise review: Induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. Stem Cells. 2012;30:42–47. doi: 10.1002/stem.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bodey B. Thymic reticulo-epithelial cells: key cells of neuroendocrine regulation. Expert Opin Biol Ther. 2007;7:939–949. doi: 10.1517/14712598.7.7.939. [DOI] [PubMed] [Google Scholar]
- 43.Shin K., Lee J., Guo N. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110–114. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ieda M., Fu J.D., Delgado-Olguin P. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhidkova O.V., Petrov N.S., Popov B.V. Production and characteristics of the growth and marker properties of mesenchymal stem cells of urinary bladder. Zh Evol Biokhim Fiziol. 2013;49:67–77. [PubMed] [Google Scholar]
- 46.Muraglia A., Cancedda R., Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113:1161–1166. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- 47.Phinney D.G., Hill K., Michelson C. Biological activities encoded by the murine mesenchymal stem cell transcriptome provide a basis for their developmental potential and broad therapeutic efficacy. Stem Cells. 2006;24:186–198. doi: 10.1634/stemcells.2004-0236. [DOI] [PubMed] [Google Scholar]
- 48.Friend S.H., Bernards R., Rogelj S. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323:643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- 49.Lee W.H., Shew J.Y., Hong F.D. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987;329:642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- 50.Knudson A.G., Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otterson G.A., Chen Wd., Coxon A.B., Khleif S.N., Kaye F.J. Incomplete penetrance of familial retinoblastoma linked to germ-line mutations that result in partial loss of RB function. Proc Natl Acad Sci U S A. 1997;94:12036–12040. doi: 10.1073/pnas.94.22.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cobrinik D. Regulatory interactions among E2Fs and cell cycle control proteins. Curr Top Microbiol Immunol. 1996;208:31–61. doi: 10.1007/978-3-642-79910-5_2. [DOI] [PubMed] [Google Scholar]
- 53.Mulligan G., Jacks T. The retinoblastoma gene family: cousins with overlapping interests. Trends Genet. 1998;14:223–229. doi: 10.1016/s0168-9525(98)01470-x. [DOI] [PubMed] [Google Scholar]
- 54.Classon M., Dyson N. p107 and p130: versatile proteins with interesting pockets. Exp Cell Res. 2001;264:135–147. doi: 10.1006/excr.2000.5135. [DOI] [PubMed] [Google Scholar]
- 55.Hassler M., Singh S., Yue W.W. Crystal structure of the retinoblastoma protein N domain provides insight into tumor suppression, ligand interaction, and holoprotein architecture. Mol Cell. 2007;28:371–385. doi: 10.1016/j.molcel.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu L., Enders G., Lees J.A., Beijersbergen R.L., Bernards R., Harlow E. The pRB-related protein p107 contains two growth suppression domains: independent interactions with E2F and cyclin/cdk complexes. EMBO J. 1995;14:1904–1913. doi: 10.1002/j.1460-2075.1995.tb07182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hansen K., Farkas T., Lukas J., Holm K., Rönnstrand L., Bartek J. Phosphorylation-dependent and -independent functions of p130 cooperate to evoke a sustained G1 block. EMBO J. 2001;20:422–432. doi: 10.1093/emboj/20.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chibazakura T., McGrew S.G., Cooper J.A., Yoshikawa H., Roberts J.M. Regulation of cyclin-dependent kinase activity during mitotic exit and maintenance of genome stability by p21, p27, and p107. Proc Natl Acad Sci U S A. 2004;101:4465–4470. doi: 10.1073/pnas.0400655101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Helin K., Harlow E., Fattaey A. Inhibition of E2F-1 transactivation by direct binding of the retinoblastoma protein. Mol Cell Biol. 1993;13:6501–6508. doi: 10.1128/mcb.13.10.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hurford R.K., Jr., Cobrinik D., Lee M.H., Dyson N. pRB and p107/p130 are required for the regulated expression of different sets of E2F responsive genes. Genes Dev. 1997;11:1447–1463. doi: 10.1101/gad.11.11.1447. [DOI] [PubMed] [Google Scholar]
- 61.Korenjak M., Taylor-Harding B., Binné U.K. Native E2F/RBF complexes contain Myb-interacting proteins and repress transcription of developmentally controlled E2F target genes. Cell. 2004;119:181–193. doi: 10.1016/j.cell.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 62.Weinberg R.A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 63.Knudsen E.S., Knudsen K.E. Retinoblastoma tumor suppressor: where cancer meets the cell cycle. Exp Biol Med (Maywood) 2006;231:1271–1281. doi: 10.1177/153537020623100713. [DOI] [PubMed] [Google Scholar]
- 64.Burkhart D.L., Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer. 2008;8:671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Black E.P., Huang E., Dressman H. Distinct gene expression phenotypes of cells lacking Rb and Rb family members. Cancer Res. 2003;63:3716–3723. [PubMed] [Google Scholar]
- 66.Ishida S., Huang E., Zuzan H. Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol Cell Biol. 2001;21:4684–4699. doi: 10.1128/MCB.21.14.4684-4699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moroni M.C., Hickman E.S., Lazzerini Denchi E. Apaf-1 is a transcriptional target for E2F and p53. Nat Cell Biol. 2001;3:552–558. doi: 10.1038/35078527. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi Y., Rayman J.B., Dynlacht B.D. Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev. 2000;14:804–816. [PMC free article] [PubMed] [Google Scholar]
- 69.Dannenberg J.H., van Rossum A., Schuijff L., te Riele H. Ablation of the retinoblastoma gene family deregulates G(1) control causing immortalization and increased cell turnover under growth-restricting conditions. Genes Dev. 2000;14:3051–3064. doi: 10.1101/gad.847700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sage J., Mulligan G.J., Attardi L.D. Targeted disruption of the three Rb-related genes leads to loss of G(1) control and immortalization. Genes Dev. 2000;14:3037–3050. doi: 10.1101/gad.843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moberg K., Starz M.A., Lees J.A. E2F-4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol Cell Biol. 1996;16:1436–1449. doi: 10.1128/mcb.16.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Popov B., Chang L.S., Serikov V. Cell cycle-related transformation of the E2F4-p130 repressor complex. Biochem Biophys Res Commun. 2005;336:762–769. doi: 10.1016/j.bbrc.2005.08.163. [DOI] [PubMed] [Google Scholar]
- 73.Herrera R.E., Mäkelä T.P., Weinberg R.A. TGF beta-induced growth inhibition in primary fibroblasts requires the retinoblastoma protein. Mol Biol Cell. 1996;7:1335–1342. doi: 10.1091/mbc.7.9.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harrington E.A., Bruce J.L., Harlow E., Dyson N. pRB plays an essential role in cell cycle arrest induced by DNA damage. Proc Natl Acad Sci U S A. 1998;95:11945–11950. doi: 10.1073/pnas.95.20.11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Classon M., Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nat Rev Cancer. 2002;2:910–917. doi: 10.1038/nrc950. [DOI] [PubMed] [Google Scholar]
- 76.Lipinski M.M., Jacks T. The retinoblastoma gene family in differentiation and development. Oncogene. 1999;18:7873–7882. doi: 10.1038/sj.onc.1203244. [DOI] [PubMed] [Google Scholar]
- 77.Goodrich D.W. The retinoblastoma tumor-suppressor gene, the exception that proves the rule. Oncogene. 2006;25:5233–5243. doi: 10.1038/sj.onc.1209616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buchkovich K., Duffy L.A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989;58:1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- 79.Chen P.L., Scully P., Shew J.Y., Wang J.Y., Lee W.H. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989;58:1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- 80.DeCaprio J.A., Ludlow J.W., Lynch D. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989;58:1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- 81.Rubin S.M. Deciphering the retinoblastoma protein phosphorylation code. Trends Biochem Sci. 2013;38:12–19. doi: 10.1016/j.tibs.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ewen M.E., Sluss H.K., Sherr C.J., Matsushime H., Kato J., Livingston D.M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 83.Kato J., Matsushime H., Hiebert S.W., Ewen M.E., Sherr C.J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 84.Nevins J.R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 85.Tedesco D., Lukas J., Reed S.I. The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCF(Skp2) Genes Dev. 2002;16:2946–2957. doi: 10.1101/gad.1011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ren B., Cam H., Takahashi Y. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. 2002;16:245–256. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hernando E., Nahlé Z., Juan G. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature. 2004;430:797–802. doi: 10.1038/nature02820. [DOI] [PubMed] [Google Scholar]
- 88.Sotillo R., Hernando E., Díaz-Rodríguez E. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11:9–23. doi: 10.1016/j.ccr.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Longworth M.S., Herr A., Ji J.Y., Dyson N.J. RBF1 promotes chromatin condensation through a conserved interaction with the Condensin II protein dCAP-D3. Genes Dev. 2008;22:1011–1024. doi: 10.1101/gad.1631508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Coschi C.H., Martens A.L., Ritchie K. Mitotic chromosome condensation mediated by the retinoblastoma protein is tumor-suppressive. Genes Dev. 2010;24:1351–1363. doi: 10.1101/gad.1917610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang J., Benavente C.A., McEvoy J. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature. 2012;481:329–334. doi: 10.1038/nature10733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burke J.R., Hura G.L., Rubin S.M. Structures of inactive retinoblastoma protein reveal multiple mechanisms for cell cycle control. Genes Dev. 2012;26:1156–1166. doi: 10.1101/gad.189837.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dick F.A. Structure-function analysis of the retinoblastoma tumor suppressor protein - is the whole a sum of its parts? Cell Div. 2007;2:26. doi: 10.1186/1747-1028-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bremner R., Du D.C., Connolly-Wilson M.J. Deletion of RB exons 24 and 25 causes low-penetrance retinoblastoma. Am J Hum Genet. 1997;61:556–570. doi: 10.1086/515499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harbour J.W. Molecular basis of low-penetrance retinoblastoma. Arch Ophthalmol. 2001;119:1699–1704. doi: 10.1001/archopht.119.11.1699. [DOI] [PubMed] [Google Scholar]
- 96.Sun H., Chang Y., Schweers B. An E2F binding-deficient Rb1 protein partially rescues developmental defects associated with Rb1 nullizygosity. Mol Cell Biol. 2006;26:1527–1537. doi: 10.1128/MCB.26.4.1527-1537.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sellers W.R., Novitch B.G., Miyake S. Stable binding to E2F is not required for the retinoblastoma protein to activate transcription, promote differentiation, and suppress tumor cell growth. Genes Dev. 1998;12:95–106. doi: 10.1101/gad.12.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Popov B.V., Watt S.M., Rozanov Iu.M., Chang L.-S. A pocket pRb mutation induces the increase in its affinity to E2F4 coupled with activation of muscle differentiation. Mol Biol (Mosk) 2010;44:323–334. [PubMed] [Google Scholar]
- 99.Ji P., Jiang H., Rekhtman K. An Rb-Skp2-p27 pathway mediates acute cell cycle inhibition by Rb and is retained in a partial-penetrance Rb mutant. Mol Cell. 2004;16:47–58. doi: 10.1016/j.molcel.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 100.Binné U.K., Classon M.K., Dick F.A. Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nat Cell Biol. 2007;9:225–232. doi: 10.1038/ncb1532. [DOI] [PubMed] [Google Scholar]
- 101.Talluri S., Dick F.A. Regulation of transcription and chromatin structure by pRB: here, there and everywhere. Cell Cycle. 2012;11:3189–3198. doi: 10.4161/cc.21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Robertson K.D., Ait-Si-Ali S., Yokochi T., Wade P.A., Jones P.L., Wolffe A.P. DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nat Genet. 2000;25:338–342. doi: 10.1038/77124. [DOI] [PubMed] [Google Scholar]
- 103.Brehm A., Miska E.A., McCance D.J., Reid J.L., Bannister A.J., Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- 104.Gonzalo S., García-Cao M., Fraga M.F. Role of the RB1 family in stabilizing histone methylation at constitutive heterochromatin. Nat Cell Biol. 2005;7:420–428. doi: 10.1038/ncb1235. [DOI] [PubMed] [Google Scholar]
- 105.Benevolenskaya E.V., Murray H.L., Branton P., Young R.A., Kaelin W.G., Jr. Binding of pRB to the PHD protein RBP2 promotes cellular differentiation. Mol Cell. 2005;18:623–635. doi: 10.1016/j.molcel.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 106.Nielsen S.J., Schneider R., Bauer U.M. Rb targets histone H3 methylation and HP1 to promoters. Nature. 2001;412:561–565. doi: 10.1038/35087620. [DOI] [PubMed] [Google Scholar]
- 107.Dunaief J.L., Strober B.E., Guha S. The retinoblastoma protein and BRG1 form a complex and cooperate to induce cell cycle arrest. Cell. 1994;79:119–130. doi: 10.1016/0092-8674(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 108.Shamma A., Suzuki M., Hayashi N. ATM mediates pRB function to control DNMT1 protein stability and DNA methylation. Mol Cell Biol. 2013;33:3113–3124. doi: 10.1128/MCB.01597-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weintraub S.J., Prater C.A., Dean D.C. Retinoblastoma protein switches the E2F site from positive to negative element. Nature. 1992;358:259–261. doi: 10.1038/358259a0. [DOI] [PubMed] [Google Scholar]
- 110.Nicolas E., Roumillac C., Trouche D. Balance between acetylation and methylation of histone H3 lysine 9 on the E2F-responsive dihydrofolate reductase promoter. Mol Cell Biol. 2003;23:1614–1622. doi: 10.1128/MCB.23.5.1614-1622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Strober B.E., Dunaief J.L., Guha, Goff S.P. Functional interactions between the hBRM/hBRG1 transcriptional activators and the pRB family of proteins. Mol Cell Biol. 1996;16:1576–1583. doi: 10.1128/mcb.16.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Strobeck M.W., Knudsen K.E., Fribourg A.F. BRG-1 is required for RB-mediated cell cycle arrest. Proc Natl Acad Sci U S A. 2000;97:7748–7753. doi: 10.1073/pnas.97.14.7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Singh M., Krajewski M., Mikolajka A., Holak T.A. Molecular determinants for the complex formation between the retinoblastoma protein and LXCXE sequences. J Biol Chem. 2005;280:37868–37876. doi: 10.1074/jbc.M504877200. [DOI] [PubMed] [Google Scholar]
- 114.Reyes J.C., Barra J., Muchardt C., Camus A., Babinet C., Yaniv M. Altered control of cellular proliferation in the absence of mammalian brahma (SNF2alpha) EMBO J. 1998;17:6979–6991. doi: 10.1093/emboj/17.23.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 116.Kuzmichev A., Nishioka K., Erdjument-Bromage H., Tempst P., Reinberg D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev. 2002;16:2893–2905. doi: 10.1101/gad.1035902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beck D.B., Bonasio R., Kaneko S. Chromatin in the nuclear landscape. Cold Spring Harb Symp Quant Biol. 2010;75:11–22. doi: 10.1101/sqb.2010.75.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bracken A.P., Pasini D., Capra M., Prosperini E., Colli E., Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22:5323–5335. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kotake Y., Cao R., Viatour P., Sage J., Zhang Y., Xiong Y. pRB family proteins are required for H3K27 trimethylation and Polycomb repression complexes binding to and silencing p16INK4alpha tumor suppressor gene. Genes Dev. 2007;21:49–54. doi: 10.1101/gad.1499407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li J., Poi M.J., Tsai M.D. Regulatory mechanisms of tumor suppressor P16(INK4A) and their relevance to cancer. Biochemistry. 2011;50:5566–5582. doi: 10.1021/bi200642e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qian Y.W., Wang Y.C., Hollingsworth R.E., Jr., Jones D., Ling N., Lee E.Y. A retinoblastoma-binding protein related to a negative regulator of Ras in yeast. Nature. 1993;364:648–652. doi: 10.1038/364648a0. [DOI] [PubMed] [Google Scholar]
- 122.Berger F., Twell D. Germline specification and function in plants. Annu Rev Plant Biol. 2011;62:461–484. doi: 10.1146/annurev-arplant-042110-103824. [DOI] [PubMed] [Google Scholar]
- 123.Ebel C., Mariconti L., Gruissem W. Plant retinoblastoma homologues control nuclear proliferation in the female gametophyte. Nature. 2004;429:776–780. doi: 10.1038/nature02637. [DOI] [PubMed] [Google Scholar]
- 124.Johnston A.J., Matveeva E., Kirioukhova O., Grossniklaus U., Gruissem W. A dynamic reciprocal RBR-PRC2 regulatory circuit controls Arabidopsis gametophyte development. Curr Biol. 2008;18:1680–1686. doi: 10.1016/j.cub.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 125.Johnston A.J., Gruissem W. Gametophyte differentiation and imprinting control in plants: Crosstalk between RBR and chromatin. Commun Integr Biol. 2009;2:144–146. doi: 10.4161/cib.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen Z., Higgins J.D., Hui J.T., Li J., Franklin F.C., Berger F. Retinoblastoma protein is essential for early meiotic events in Arabidopsis. EMBO J. 2011;30:744–755. doi: 10.1038/emboj.2010.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gutzat R., Borghi L., Fütterer J. RETINOBLASTOMA-RELATED PROTEIN controls the transition to autotrophic plant development. Development. 2011;138:2977–2986. doi: 10.1242/dev.060830. [DOI] [PubMed] [Google Scholar]
- 128.Kuwabara A., Gruissem W. Arabidopsis RETINOBLASTOMA-RELATED and Polycomb group proteins: cooperation during plant cell differentiation and development. J Exp Bot. 2014;65:2667–2676. doi: 10.1093/jxb/eru069. [DOI] [PubMed] [Google Scholar]
- 129.Byrne M.E., Kidner C.A., Martienssen R.A. Plant stem cells: divergent pathways and common themes in shoots and roots. Curr Opin Genet Dev. 2003;13:551–557. doi: 10.1016/j.gde.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 130.Wyrzykowska J., Schorderet M., Pien S., Gruissem W., Fleming A.J. Induction of differentiation in the shoot apical meristem by transient overexpression of a retinoblastoma-related protein. Plant Physiol. 2006;141:1338–1348. doi: 10.1104/pp.106.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Narita M., Nũnez S., Heard E. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 132.Chicas A., Wang X., Zhang C. Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell. 2010;17:376–387. doi: 10.1016/j.ccr.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bannister A.J., Zegerman P., Partridge J.F. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 134.García-Cao M., Gonzalo S., Dean D., Blasco M.A. A role for the Rb family of proteins in controlling telomere length. Nat Genet. 2002;32:415–419. doi: 10.1038/ng1011. [DOI] [PubMed] [Google Scholar]
- 135.Montoya-Durango D.E., Liu Y., Teneng I. Epigenetic control of mammalian LINE-1 retrotransposon by retinoblastoma proteins. Mutat Res. 2009;665:20–28. doi: 10.1016/j.mrfmmm.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Morrison S.J., Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 137.Goulas S., Conder R., Knoblich J.A. The Par complex and integrins direct asymmetric cell division in adult intestinal stem cells. Cell Stem Cell. 2012;11:529–540. doi: 10.1016/j.stem.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chinnam M., Goodrich D.W. RB1, development, and cancer. Curr Top Dev Biol. 2011;94:129–169. doi: 10.1016/B978-0-12-380916-2.00005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lu X., Horvitz H.R. lin-35 and lin-53, two genes that antagonize a C. elegans Ras pathway, encode proteins similar to Rb and its binding protein RbAp48. Cell. 1998;95:981–991. doi: 10.1016/s0092-8674(00)81722-5. [DOI] [PubMed] [Google Scholar]
- 140.Kirienko N.V., Fay D.S. Transcriptome profiling of the C. elegans Rb ortholog reveals diverse developmental roles. Dev Biol. 2007;305:674–684. doi: 10.1016/j.ydbio.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang D., Kennedy S., Conte D., Jr. Somatic misexpression of germline P granules and enhanced RNA interference in retinoblastoma pathway mutants. Nature. 2005;436:593–597. doi: 10.1038/nature04010. [DOI] [PubMed] [Google Scholar]
- 142.Wu X., Shi Z., Cui M., Han M., Ruvkun G. Repression of germline RNAi pathways in somatic cells by retinoblastoma pathway chromatin complexes. PLoS Genet. 2012;8:e1002542. doi: 10.1371/journal.pgen.1002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.MacLellan W.R., Xiao G., Abdellatif M., Schneider M.D. A novel Rb- and p300-binding protein inhibits transactivation by MyoD. Mol Cell Biol. 2000;20:8903–8915. doi: 10.1128/mcb.20.23.8903-8915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Miyake S., Sellers W.R., Safran M. Cells degrade a novel inhibitor of differentiation with E1A-like properties upon exiting the cell cycle. Mol Cell Biol. 2000;20:8889–8902. doi: 10.1128/mcb.20.23.8889-8902.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Iavarone A., Garg P., Lasorella A., Hsu J., Israel M.A. The helix-loop-helix protein Id-2 enhances cell proliferation and binds to the retinoblastoma protein. Genes Dev. 1994;8:1270–1284. doi: 10.1101/gad.8.11.1270. [DOI] [PubMed] [Google Scholar]
- 146.Iavarone A., King E.R., Dai X.M., Leone G., Stanley E.R., Lasorella A. Retinoblastoma promotes definitive erythropoiesis by repressing Id2 in fetal liver macrophages. Nature. 2004;432:1040–1045. doi: 10.1038/nature03068. [DOI] [PubMed] [Google Scholar]
- 147.Beshiri M.L., Holmes K.B., Richter W.F. Coordinated repression of cell cycle genes by KDM5A and E2F4 during differentiation. Proc Natl Acad Sci U S A. 2012;109:18499–18504. doi: 10.1073/pnas.1216724109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang J.K., Tsai M.C., Poulin G. The histone demethylase UTX enables RB dependent cell fate control. Genes Dev. 2010;24:327–332. doi: 10.1101/gad.1882610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Longworth M.S., Dyson N.J. pRb, a local chromatin organizer with global possibilities. Chromosoma. 2010;119:1–11. doi: 10.1007/s00412-009-0238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thomas D.M., Carty S.A., Piscopo D.M. The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol Cell. 2001;8:303–316. doi: 10.1016/s1097-2765(01)00327-6. [DOI] [PubMed] [Google Scholar]
- 151.Chen P.L., Riley D.J., Chen Y., Lee W.H. Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes Dev. 1996;10:2794–2804. doi: 10.1101/gad.10.21.2794. [DOI] [PubMed] [Google Scholar]
- 152.Calo E., Quintero-Estades J.A., Danielian P.S., Nedelcu S., Berman S.D., Lees J.A. Rb regulates fate choice and lineage commitment in vivo. Nature. 2010;466:1110–1114. doi: 10.1038/nature09264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gu W., Schneider J.W., Condorelli G., Kaushal S., Mahdavi V., Nadal-Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell. 1993;72:309–324. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- 154.Novitch B.G., Mulligan G.J., Jacks T., Lassar A.B. Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J Cell Biol. 1996;135:441–456. doi: 10.1083/jcb.135.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hemming S., Cakouros D., Isenmann S. EZH2 and KDM6A act as an epigenetic switch to regulate mesenchymal stem cell lineage specification. Stem Cells. 2014;32:802–815. doi: 10.1002/stem.1573. [DOI] [PubMed] [Google Scholar]
- 156.Wang L., Jin Q., Lee J.E., Su I.H., Ge K. Histone H3K27 methyltransferase Ezh2 represses Wnt genes to facilitate adipogenesis. Proc Natl Acad Sci U S A. 2010;107:7317–7322. doi: 10.1073/pnas.1000031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Herz H.M., Madden L.D., Chen Z. The H3K27me3 demethylase dUTX is a suppressor of Notch- and Rb-dependent tumors in Drosophila. Mol Cell Biol. 2010;30:2485–2497. doi: 10.1128/MCB.01633-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gutierrez G.M., Kong E., Sabbagh Y. Impaired bone development and increased mesenchymal progenitor cells in calvaria of RB1-/- mice. Proc Natl Acad Sci U S A. 2008;105:18402–18407. doi: 10.1073/pnas.0805925105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Noer A., Lindeman L.C., Collas P. Histone H3 modifications associated with differentiation and long-term culture of mesenchymal adipose stem cells. Stem Cells Dev. 2009;18:725–736. doi: 10.1089/scd.2008.0189. [DOI] [PubMed] [Google Scholar]
- 160.Fujiki K., Kano F., Shiota K., Murata M. Expression of the peroxisome proliferator activated receptor gamma gene is repressed by DNA methylation in visceral adipose tissue of mouse models of diabetes. BMC Biol. 2009;7:38. doi: 10.1186/1741-7007-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wei Y., Chen Y.H., Li L.Y. CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat Cell Biol. 2011;13:87–94. doi: 10.1038/ncb2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Komori T. Signaling networks in RUNX2-dependent bone development. J Cell Biochem. 2011;112:750–755. doi: 10.1002/jcb.22994. [DOI] [PubMed] [Google Scholar]
- 163.Tang Q.Q., Lane M.D. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012;81:715–736. doi: 10.1146/annurev-biochem-052110-115718. [DOI] [PubMed] [Google Scholar]
- 164.Laudes M. Role of WNT signalling in the determination of human mesenchymal stem cells into preadipocytes. J Mol Endocrinol. 2011;46:R65–R72. doi: 10.1530/JME-10-0169. [DOI] [PubMed] [Google Scholar]
- 165.Wright W.S., Longo K.A., Dolinsky V.W. Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes. 2007;56:295–303. doi: 10.2337/db06-1339. [DOI] [PubMed] [Google Scholar]
- 166.Christodoulides C., Scarda A., Granzotto M. WNT10B mutations in human obesity. Diabetologia. 2006;49:678–684. doi: 10.1007/s00125-006-0144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bowers R.R., Lane M.D. Wnt signaling and adipocyte lineage commitment. Cell Cycle. 2008;7:1191–1196. doi: 10.4161/cc.7.9.5815. [DOI] [PubMed] [Google Scholar]
- 168.Otto T.C., Lane M.D. Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol. 2005;40:229–242. doi: 10.1080/10409230591008189. [DOI] [PubMed] [Google Scholar]
- 169.Kang S., Bennett C.N., Gerin I., Rapp L.A., Hankenson K.D., Macdougald O.A. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- 170.Rosen E.D., Spiegelman B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hansen J.B., Petersen R.K., Larsen B.M., Bartkova J., Alsner J., Kristiansen K. Activation of peroxisome proliferator-activated receptor gamma bypasses the function of the retinoblastoma protein in adipocyte differentiation. J Biol Chem. 1999;274:2386–2393. doi: 10.1074/jbc.274.4.2386. [DOI] [PubMed] [Google Scholar]
- 172.Hansen J.B., Jørgensen C., Petersen R.K. Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc Natl Acad Sci U S A. 2004;101:4112–4117. doi: 10.1073/pnas.0301964101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hallenborg P., Feddersen S., Madsen L., Kristiansen K. The tumor suppressors pRB and p53 as regulators of adipocyte differentiation and function. Expert Opin Ther Targets. 2009;13:235–246. doi: 10.1517/14712590802680141. [DOI] [PubMed] [Google Scholar]
- 174.Schneider J.W., Gu W., Zhu L., Mahdavi V., Nadal-Ginard B. Reversal of terminal differentiation mediated by p107 in Rb-/- muscle cells. Science. 1994;264:1467–1471. doi: 10.1126/science.8197461. [DOI] [PubMed] [Google Scholar]
- 175.Dali-Youcef N., Mataki C., Coste A. Adipose tissue-specific inactivation of the retinoblastoma protein protects against diabesity because of increased energy expenditure. Proc Natl Acad Sci USA. 2007;104:10703–10708. doi: 10.1073/pnas.0611568104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Olson E.N., Klein W.H. bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 1994;8:1–8. doi: 10.1101/gad.8.1.1. [DOI] [PubMed] [Google Scholar]
- 177.Constantinides P.G., Jones P.A., Gevers W. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature. 1977;267:364–366. doi: 10.1038/267364a0. [DOI] [PubMed] [Google Scholar]
- 178.Davis R.L., Weintraub H., Lassar A.B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 179.Braun T., Bober E., Buschhausen-Denker G., Kohtz S., Grzeschik K.H., Arnold H.H. Differential expression of myogenic determination genes in muscle cells: possible autoactivation by the Myf gene products. EMBO J. 1989;8:3617–3625. doi: 10.1002/j.1460-2075.1989.tb08535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Edmondson D.G., Olson E.N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989;3:628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- 181.Rhodes S.J., Konieczny S.F. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 1989;3:2050–2061. doi: 10.1101/gad.3.12b.2050. [DOI] [PubMed] [Google Scholar]
- 182.Hosoyama T., Nishijo K., Prajapati S.I., Li G., Keller C. Rb1 gene inactivation expands satellite cell and postnatal myoblast pools. J Biol Chem. 2011;286:19556–19564. doi: 10.1074/jbc.M111.229542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dodson M.V., Hausman G.J., Guan L. Skeletal muscle stem cells from animals I. Basic cell biology. Int J Biol Sci. 2010;6:465–474. doi: 10.7150/ijbs.6.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Han X.H., Jin Y.R., Seto M., Yoon J.K. A WNT/beta-catenin signaling activator, R-spondin, plays positive regulatory roles during skeletal myogenesis. J Biol Chem. 2011;286:10649–10659. doi: 10.1074/jbc.M110.169391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Petrov N., Zhidkova O., Serikov V., Zenin V., Popov B. Induction of Wnt/β-catenin signaling in mouse mesenchymal stem cells is associated with activation of the p130 and E2f4 and formation of the p130/Gsk3β/β-catenin complex. Stem Cells Dev. 2012;21:589–597. doi: 10.1089/scd.2011.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Popov B.V., Shilo P.S., Zhidkova O.V., Zaichik F.V., Petrov N.S. Experimental model to study the role of pRb in determination of adipocyte differentiation. Biull Eksp Biol Med. 2014 doi: 10.1007/s10517-015-2944-3. [in press] [DOI] [PubMed] [Google Scholar]
- 187.Tschöp K., Conery A.R., Litovchick L. A kinase shRNA screen links LATS2 and the pRB tumor suppressor. Genes Dev. 2011;25:814–830. doi: 10.1101/gad.2000211. [DOI] [PMC free article] [PubMed] [Google Scholar]