Abstract
Background:
We model the epidemiological impact of providing isoniazid preventive therapy (IPT) to South African adolescents, among whom HIV prevalence is low, latent tuberculosis (TB) prevalence is high, and school-based programs may enable population-level coverage.
Methods:
We simulate a dynamic compartmental model of age-structured HIV and TB coepidemics in South Africa. HIV dynamics are modeled by infection status, CD4+ cell count, and antiretroviral therapy; TB dynamics are modeled by disease stage, diagnosis, treatment, and IPT status. We analyze the effects of continuous IPT coverage among adolescents from 5 (baseline) to 90%.
Results:
Our model is calibrated to WHO and the Joint United Nations Programme on HIV/AIDS epidemiological estimates. In simulations, increasing IPT coverage to 50% among adolescents reduced active TB incidence by 5–34%. Increasing coverage to 90% led to a 9–40% reduction in active TB incidence. Expanded IPT access causes TB incidence to decline in the general population of HIV-positive individuals, as well as in adult HIV-positive individuals.
Conclusion:
Targeting IPT to a secondary school population with high latent TB prevalence and low-HIV prevalence, in which risk of false-negative diagnosis of active TB is low and IPT benefits are more established, could have substantial benefits to adolescents and spillover benefits to the adult population.
Keywords: isoniazid preventive therapy, latent tuberculosis, South Africa, tuberculosis, tuberculosis/HIV coinfection
Introduction
Tuberculosis (TB) is the second greatest infectious cause of mortality worldwide [1]. Over two billion people are estimated to have latent infection, which does not cause disease symptoms, but increases the risk of reactivation and disease. For that reason, individuals diagnosed with latent TB in most countries with low prevalence of latent TB are treated with a regimen known as isoniazid preventive therapy (IPT), which presumably cures the latent TB infection, thus reducing the risk of future TB reactivation and disease.
The WHO is promoting the use of IPT in all contexts, and has been monitoring IPT coverage rates since 2003 [1]. However, in high-TB burden contexts, only a small fraction of individuals infected with latent TB is treated with IPT [2,3]. Developing countries typically are not able to screen for latent TB at the community level, as public health systems can be overwhelmed in treating cases of active TB. They are therefore largely unable to screen asymptomatic individuals for latent TB, leaving large numbers of latent TB cases untreated. In addition to competing resource needs, barriers include possible monitoring requirements for liver function for individuals on IPT [4], concerns about the development of drug resistance [5], and challenges of distinguishing between latent and active TB in areas of high HIV prevalence (inadvertently providing IPT to a person suffering from active TB could result in delay of appropriate treatment and development of resistance).
Individuals infected with latent TB who are, or who become, HIV-positive, have an elevated risk of TB activation [6,7]. Therefore, the WHO has recommended IPT for all HIV-positive individuals infected with latent TB [8]. This recommendation has led to debate surrounding its potential effectiveness, and the possibility of increasing the prevalence of drug resistance [5].
A recent study raised concerns over long-term benefits of IPT among miners in South Africa [9]. The study found significant rates of reinfection with TB following IPT discontinuation. This study echoes other epidemiological evidence suggesting high rates of reinfection in areas of high TB prevalence [9,10]. On balance, however, the evidence tends to favor IPT as an effective intervention for latent TB; a meta-analysis of trials among HIV-positive populations found IPT led to a 40% risk ratio reduction in cases of active TB in tuberculin skin-test positive individuals [11].
Although some modeling studies suggest that IPT does not create more selection for drug resistance at the individual level than would otherwise occur [12], others have cautioned that IPT will create selection pressure for drug resistant TB strains at the community level [5]. However, drug-resistant TB strains are often subject to significant negative selection pressure associated with maintaining resistance [13]; and, empirical evidence indicates that in the past, drug resistance at the community level has not increased as a result of broadly available IPT [14,15]. The development of some amount of resistance is indeed a potential risk whenever IPT is used in a population with ongoing TB transmission; furthermore, the complexity of monitoring TB treatment in a high-burden area should not be underestimated. However, a secondary school-based intervention such as the one we propose would minimize the possibility of emergence of resistance compared with other similar settings [5].
The intervention we propose also addresses concerns about inadvertently providing IPT to individuals infected with active TB who are misdiagnosed as having latent TB. A misdiagnosis is more likely to occur in HIV-positive individuals. By targeting adolescents, an age group in whom HIV prevalence is low, it is possible to reduce the likelihood of monotherapy for active TB.
Methods
Model structure
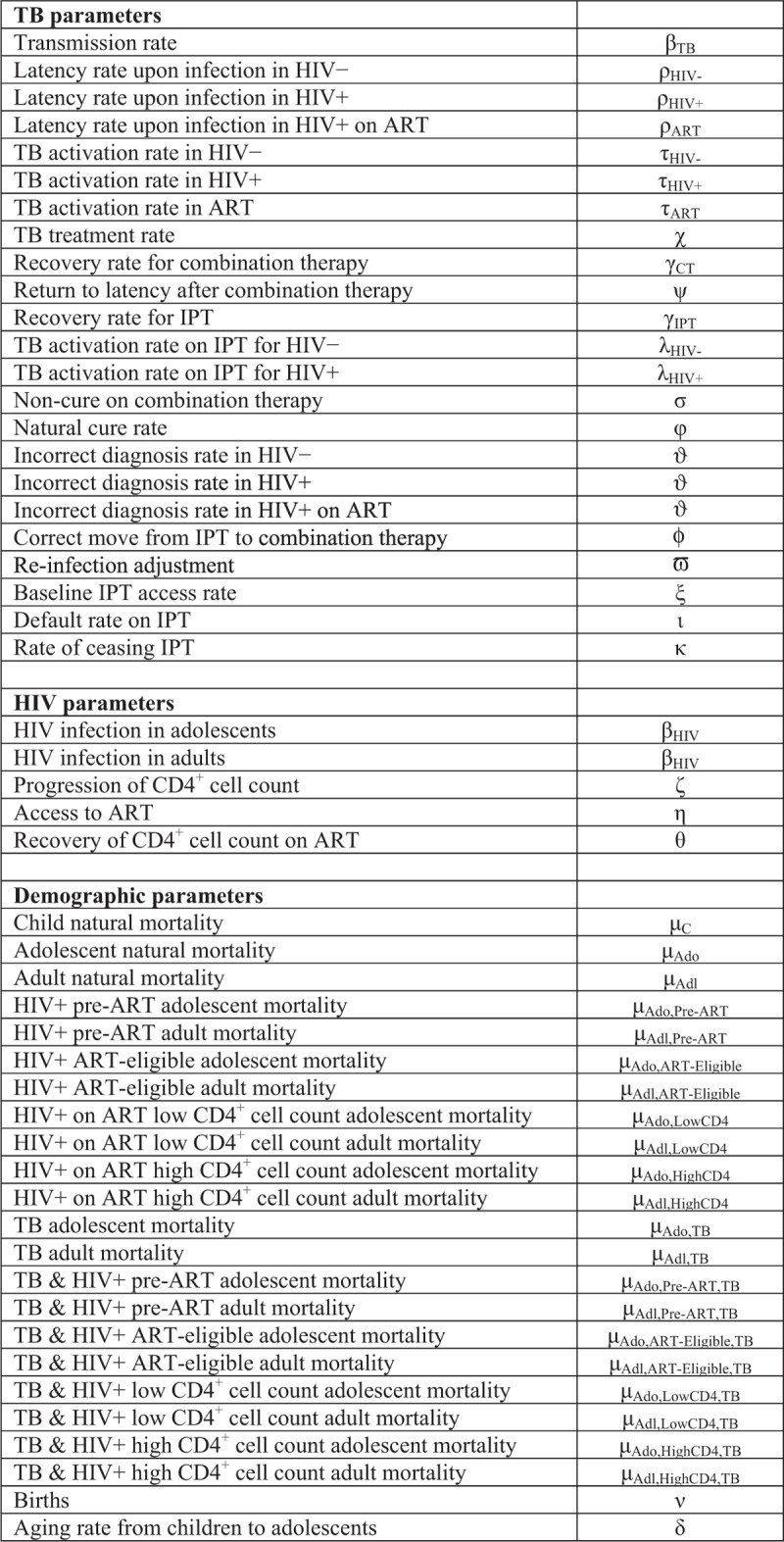
We employ a dynamic, deterministic, compartmental model to simulate the introduction of continuous IPT by age group in South Africa. Our ordinary differential equation-based model includes three sets of dynamics relevant to disease transmission: aging, HIV infection, and TB infection (Fig. 1 ). The model is calibrated as described below according to overall HIV prevalence, antiretroviral therapy (ART) scale-up in the region, HIV prevalence in TB cases, and percent of all TB deaths that are HIV positive, based on the Global TB Report [16] and the Joint United Nations Programme on HIV/AIDS (UNAIDS) estimates [17] (Table S2).
Fig. 1.
Tuberculosis, HIV, and demographic dynamics of the model, followed by a parameter key.
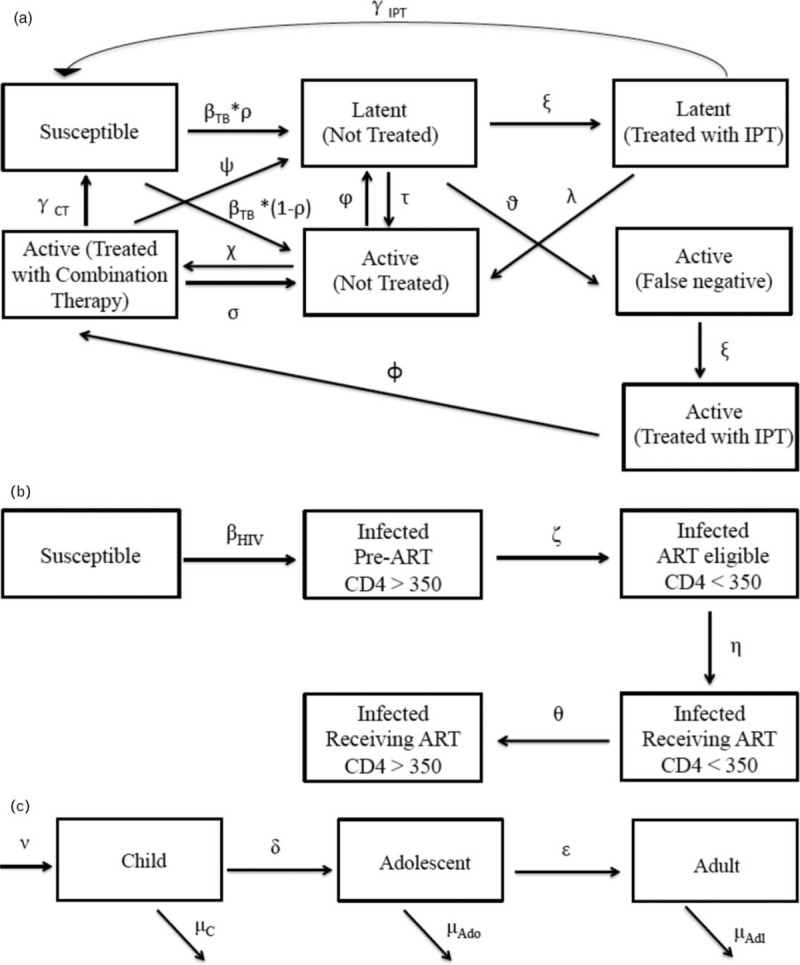
(a) Tuberculosis dynamics in adolescents and adults, (b) HIV dynamics, (c) aging dynamics.
Demographic dynamics
Individuals age through the life stages as children, adolescents, and adults. We calibrate the population size based on United Nations estimates [18] (Table 1). Estimates of latent TB prevalence among adolescents as compared with adults are based on a local study by Middelkoop et al.[19].
Table 1.
Key outcomes.
| Incidence | |||
| Year | 5% IPT coverage | 50% IPT coverage | 90% IPT coverage |
| 2012 | 1113 | 1113 | 1113 |
| 2013 | 1076 | 1076 | 1076 |
| 2014 | 1041 | 1041 | 1041 |
| 2015 | 1008 | 1008 | 1008 |
| 2016 | 978 | 978 | 978 |
| 2017 | 951 | 951 | 951 |
| 2018 | 927 | 877 | 847 |
| 2019 | 906 | 814 | 772 |
| 2020 | 887 | 761 | 716 |
| 2021 | 871 | 718 | 671 |
| 2022 | 857 | 682 | 634 |
| 2023 | 845 | 651 | 603 |
| 2024 | 835 | 626 | 576 |
| 2025 | 827 | 605 | 554 |
| 2026 | 821 | 587 | 535 |
| 2027 | 816 | 572 | 520 |
| 2028 | 813 | 560 | 508 |
| 2029 | 811 | 551 | 498 |
| 2030 | 811 | 545 | 492 |
| 2031 | 813 | 541 | 487 |
| 2032 | 815 | 539 | 486 |
| Mortality | |||
| Year | 5% IPT coverage | 50% IPT coverage | 90% IPT coverage |
| 2012 | 268 | 268 | 268 |
| 2013 | 257 | 257 | 257 |
| 2014 | 245 | 245 | 245 |
| 2015 | 234 | 234 | 234 |
| 2016 | 224 | 224 | 224 |
| 2017 | 214 | 214 | 214 |
| 2018 | 206 | 204 | 203 |
| 2019 | 198 | 192 | 188 |
| 2020 | 192 | 179 | 173 |
| 2021 | 186 | 167 | 161 |
| 2022 | 181 | 157 | 150 |
| 2023 | 176 | 149 | 141 |
| 2024 | 172 | 141 | 133 |
| 2025 | 169 | 135 | 126 |
| 2026 | 166 | 129 | 121 |
| 2027 | 163 | 125 | 116 |
| 2028 | 161 | 121 | 112 |
| 2029 | 160 | 118 | 109 |
| 2030 | 159 | 115 | 106 |
| 2031 | 158 | 113 | 104 |
| 2032 | 158 | 112 | 103 |
Calibration targets used in the model. IPT, isoniazid preventive therapy.
Tuberculosis dynamics
In our model, both slow and rapid TB disease progression pathways are considered [20]. We do not model active TB in children because, for the purpose of this analysis, we consider their contribution to population level TB transmission and mortality to be less relevant; although they can be infected with latent TB in our model, they are not affected by active TB and their mortality is not influenced by TB. Adolescents and adults move between the following disease states: susceptible to TB, latently infected and not treated with IPT, latently infected and treated with IPT, diagnosed accurately with active TB but not yet treated, having active TB and treated with combination drug therapy, having active TB but incorrectly diagnosed as latently infected without IPT, and having active TB but incorrectly diagnosed as latently infected with IPT. TB activation can occur through either slow or rapid pathways, respectively; that is individuals infected with TB can remain in the latent phase before progressing to active TB, or TB activation can occur immediately upon infection. Infected individuals can return to susceptibility, that is, achieve cure, following IPT in the case of latent TB, or following combination therapy in the case of active TB. We also adjust for reinfection [21].
The benefits of IPT include a return to TB susceptibility (a cure for existing infection) among 47% of the population. They also include a TB activation rate while taking IPT that is five to 90 times slower than among those not taking IPT. The imperfect cure rate of IPT, resulting from both efficacy and treatment interruption is reflected in the partial cure rate of IPT used in the model. Individuals remain on IPT as they age until they achieve cure. The model was dynamically calibrated according to declining TB incidence in 2012 [16], as well as to TB mortality [16]. All parameter values can be found in Table 2.
Table 2.
Parameters.
| TB parametersa | |||
| Parameter | Description | Calibrated value | Source |
| Transmission rate | Rate of TB transmission | 0.0000004 | Sensitivity analysis |
| Latency rate upon infection in HIV− | Rate at which HIV− individuals infected with TB remain latently, rather than actively, infected | 0.9 | Abu-Raddad et al. [22]b |
| Latency rate upon infection in HIV+ | Rate at which HIV+ individuals infected with TB remain latently, rather than actively, infected | 0.5 | Lawn et al. [7] |
| Latency rate upon infection in HIV+ on ART | Rate at which HIV+ individuals on ART infected with TB remain latently, rather than actively, infected | 0.9 | Abu-Raddad et al. [22]b |
| TB activation rate in HIV− | Rate at which HIV− individuals experience latent TB activation | 0.005 | Horsburgh [23] |
| TB activation rate in HIV+ | Rate at which HIV+ individuals experience latent TB activation | 0.09 | Sensitivity analysis |
| TB activation rate in ART | Rate at which HIV+ individuals on ART experience latent TB activation | 0.005 | Horsburgh [23] |
| TB treatment rate | Rate at which TB is treated with combination therapy | 0.9 | WHO [1] |
| Recovery rate for combination therapy | Rate at which individuals with TB recover when treated with combination therapy | 0.9 | WHO [1], Bacaër et al. [24]b |
| Return to latency after combination therapy | Rate at which individuals receiving combination therapy for active TB return to latent TB | 0.08 | Suen et al. [25]b |
| Recovery rate for IPT | Rate at which individuals recover on IPT | 0.47 | Zelner et al. [26] |
| TB activation rate on IPT for HIV− | Rate at which HIV− individuals latently infected with TB experience TB activation | 0.0001 | Houben et al. [27]b |
| TB activation rate on IPT for HIV+ | Rate at which HIV+ individuals latently infected with TB experience TB activation | 0.0001 | Houben et al. [27]b, Selwyn et al. [28], Lin and Flynn [29] |
| Non-cure on combination therapy | Rate at which individuals who have received combination therapy are not cured | 0.02 | Suen et al. [25]b |
| Natural cure rate | Rate at which individuals with active TB experience spontaneous resolution to latent TB | 0.1 | Tiemersma et al. [30] |
| Incorrect diagnosis rate in HIV− | Rate at which HIV− individuals who are actively infected with TB are incorrectly diagnosed as latently infected | 0.00001 | Boehme et al. [31] |
| Incorrect diagnosis rate in HIV+ | Rate at which HIV+ individuals not on ART who are actively infected with TB are incorrectly diagnosed as latently infected | 0.0001 | Boehme et al. [31] |
| Incorrect diagnosis rate in HIV+ on ART | Rate at which HIV+ individuals on ART who are actively infected with TB are incorrectly diagnosed as latently infected | 0.00001 | Boehme et al. [31] |
| Correct move from IPT to combination therapy | Rate at which individuals who had incorrectly been placed on combination therapy are redirected to IPT | 0.9 | Boehme et al. [31] |
| Reinfection adjustment | Factor to adjust for the possibility of reinfection with TB following treatment and cure | 0.21 | Andrews et al. [21]b |
| IPT access rate | Rate at which individuals latently infected with TB are placed on IPT | 0.05/0.5/0.9 | Sensitivity analysis |
| Default rate on IPT | Rate at which individuals on IPT default | 0 | Sensitivity analysis |
| Rate of ceasing IPT | Rate at which individuals on IPT stop treatment | 0 | Sensitivity analysis |
| HIV parametersc | |||
| Parameter | Description | Value | Source |
| HIV infection in adolescents | Rate at which adolescents are infected with HIV | 0.0000000039 | Auvert et al. [32] |
| HIV infection in adults | Rate at which adults are infected with HIV | 0.0000000039 | Calibration |
| Progression of CD4+ cell count | Rate at which individuals infected with HIV progress to ART eligibility (experience CD4+ cell count decline) | 0.13 | Mellors et al. [33] |
| Access to ART | Rate at which eligible HIV+ individuals are placed on ART | 0.02/ 0.1 | Calibration |
| Recovery of CD4+ cell count on ART | Rate at which HIV+ individuals on ART experience CD4+ cell count recovery | 0.01 | Calibration |
| Demographic parametersd | ||
| Parameter | Description | Value |
| Child natural mortality | Mortality rate among children not infected with TB or HIV | 0.001 |
| Adolescent natural mortality | Mortality rate among adolescents not infected with TB or HIV | 0.009 |
| Adult natural mortality | Mortality rate among adults not infected with TB or HIV | 0.02 |
| HIV+ pre-ART adolescent mortality | Mortality rate among adolescents who are HIV+ and not yet eligible for ART | 0.02 |
| HIV+ pre-ART adult mortality | Mortality rate among adults who are HIV+ and not yet eligible for ART | 0.03 |
| HIV+ ART-eligible adolescent mortality | Mortality rate among adolescents who are HIV+ and eligible for ART | 0.05 |
| HIV+ ART-eligible adult mortality | Mortality rate among adults who are HIV+ and eligible for ART | 0.05 |
| HIV+ on ART low CD4+ cell count adolescent mortality | Mortality rate among adolescents who are HIV+ and on ART with low CD4+ cell count | 0.09 |
| HIV+ on ART low CD4+ cell count Adult Mortality | Mortality rate among adults who are HIV+ and on ART with low CD4+ cell count | 0.02 |
| HIV+ on ART High CD4+ cell count adolescent mortality | Mortality rate among adolescents who are HIV+ and on ART with high CD4+ cell count | 0.09 |
| HIV+ on ART high CD4+ cell count adult mortality | Mortality rate among adults who are HIV+ and on ART with high CD4+ cell count | 0.02 |
| TB adolescent mortality | Mortality rate among adolescents who are infected with TB, and are HIV− | 0.058 |
| TB adult mortality | Mortality rate among adults who are infected with TB, and are HIV− | 0.068 |
| TB & HIV+ pre-ART adolescent mortality | Mortality rate among adolescents who are infected with TB, and are HIV+, not yet eligible for ART | 0.12 |
| TB & HIV+ pre-ART adult mortality | Mortality rate among adults who are infected with TB, and are HIV+, not yet eligible for ART | 0.23 |
| TB & HIV+ ART-eligible adolescent mortality | Mortality rate among adolescents who are infected with TB, and are HIV+, and eligible for ART | 0.17 |
| TB & HIV+ ART-eligible adult mortality | Mortality rate among adults who are infected with TB, and are HIV+, and eligible for ART | 0.3 |
| TB & HIV+ low CD4+ cell count adolescent mortality | Mortality rate among adolescents who are infected with TB, and are HIV+, on ART with low CD4+ cell count | 0.07 |
| TB & HIV+ low CD4+ cell count adult mortality | Mortality rate among adults who are infected with TB, and are HIV+, on ART with low CD4+ cell count | 0.1 |
| TB & HIV+ high CD4+ cell count adolescent mortality | Mortality rate among adolescents who are infected with TB, and are HIV+, on ART with high CD4+ cell count | 0.062 |
| TB & HIV+ high CD4+ cell count adult mortality | Mortality rate among adults who are infected with TB, and are HIV+, on ART with high CD4+ cell count | 0.072 |
| Births | Birth rate | 0.03 |
| Aging rate from children to adolescents | Rate at which children age into the adolescent group | 0.0769 |
| Aging rate from adolescents to adults | Rate at which adolescents age into the adult group | 0.2 |
Parameters used in the model. All parameters subject to adjustment for calibration. ART, antiretroviral therapy; IPT, isoniazid preventive therapy; TB, tuberculosis.
aWith regard to the units of the TB and HIV parameters, each of these parameters is reported as the fraction of the population within a given compartment flowing out of that compartment into another specified by the model diagram per unit time.
bParameter taken as either an input or an output from a modeling publication.
cWith regard to the units of the TB and HIV parameters, each parameter is reported as the fraction of the population within a given compartment flowing out of that compartment into another specified by the model diagram per unit time.
dAll demographic parameters were generated via calibration to the population structure.
HIV dynamics
HIV is represented by progression through the stages of being uninfected, infected with HIV prior to ART eligibility, ART-eligible prior to initiating treatment on ART with a low CD4+ cell count, and on ART with high CD4+ cell count. Effective ART is associated with lowered rate of TB activation by a factor of 18, and with lowered TB related mortality compared with HIV-positive without ART. The model is dynamically calibrated to reflect the recent scale up of ART coverage in South Africa, a driver of recent declines in TB incidence.
Modeling strategies
The primary purpose of our model is to investigate unexplored aspects of the potential effectiveness of IPT at the population level. Our model addresses concerns surrounding reinfection with latent TB following IPT in a high TB prevalence, high HIV prevalence population that has limited resources, by exploring the potential effects of a public health intervention in which IPT is provided to adolescents through the secondary school system. It is strategic to examine such an intervention in adolescents in a high HIV prevalence area, as HIV infection is a major predictive factor in the activation of latent TB, and adolescence precedes peak transmission of HIV. Given that HIV-negative individuals are a reservoir for transmitting TB to HIV-positive individuals, treating HIV-negative individuals with IPT could have a substantial impact on TB in both HIV-positive and HIV-negative individuals. The school system also provides a logistically feasible pathway to target a population that can be easily followed, screened for latent TB, and if infected, offered IPT and monitored for reinfection via the secondary school system. Following calibration of the model, sensitivity analysis to key parameters is performed.
Our models for HIV and TB dynamics are shown in Fig. 1 a and b, respectively. For each population group, mortality is composed of HIV-specific mortality, TB-specific mortality, and background mortality, as shown in Fig. 1 c. Increasing IPT coverage among adolescents from the 5% baseline to 50 or 90% is analyzed. We model the introduction of IPT beginning in 2016.
The analytic code is available from the authors upon request; all analyses were performed using R (R language software, initial contributors: Robert Gentleman and Ross Ihaka, 1996, University of Aukland, New Zealand). No human participants or animals were involved in the conduct of this research.
Calibration
Calibration is based on estimates from the Global TB Report 2014 [16], the UNAIDS Report on the Global AIDS Epidemic [17], and the United Nations Population Division [18]. In 2012, the first year of our model, calibration yields an overall adolescent and adult population TB incidence of 1113 per 100 000 person years, adolescent and adult TB mortality of 268 per 100 000, and active TB prevalence of 1849 per 100 000. The total population is approximately 51 million. HIV prevalence is 15%, with ART coverage scaled up from 25% of all HIV-infected individuals in 2007, to 31% in 2012, to 55% in 2022. In addition to the static calibration of TB incidence to its absolute value in 2012, TB incidence is dynamically calibrated to approximate a rate of decline of 3.3% per year in 2012, as driven by scale-up of ART access. Forty-nine percent of new TB cases and 41% of TB mortality occur in HIV+ individuals in 2012. The calibration targets are shown in Table 1.
Sensitivity analyses
We perform sensitivity analysis for key parameters. We explore the sensitivity of TB incidence to variation in some of the most uncertain and influential parameters: TB transmission rate (Fig. S2), recovery rate on IPT (Fig. S3), TB activation rate in HIV-positive individuals infected with latent TB (Fig. S4), and the rate at which individuals discontinue IPT treatment (Fig. S5). The calibration resulting from the original baseline values is applied consistently across all sensitivity analyses.
Results
Isoniazid preventive therapy program in schools
We used the model to explore the effects of continuing at current baseline levels of IPT (5%), moderate scale up (50%), and significant scale up (90%). Results are represented graphically in Fig. 2, and key numerical results are shown in Table 3. At the baseline rate of 5% IPT coverage, we estimate that TB incidence will decline in the general population aged 14 and above between 0 and 3.3% per year by the end of the simulation in the year 2032.
Fig. 1 (Continued).
Tuberculosis, HIV, and demographic dynamics of the model, followed by a parameter key.
(a) Tuberculosis dynamics in adolescents and adults, (b) HIV dynamics, (c) aging dynamics.
Table 3.
Key calibration targets and calibration values achieved.
| Calibration indicator | Target value | Calibration value achieved | Source |
| Incidence | 1117 | 1113.13 | Global TB Report |
| Mortality | 220 | 268.33 | Global TB Report |
| HIV prevalence in adult population | 15 | 14.99 | South African National HIV Incidence, Prevalence and Behavior Survey |
| ART coverage among HIV+ | 31 | 30.91 | South African National HIV Incidence, Prevalence and Behavior Survey |
| HIV-positivity in TB deaths | 74 | 40.82 | Global TB Report |
Key numerical outcomes for TB incidence and mortality over time, at varying IPT coverage levels. ART, antiretroviral therapy; IPT, isoniazid preventive therapy; TB, tuberculosis.
Increasing IPT coverage among adolescents is expected to further reduce TB incidence. Beginning in the year following IPT introduction in 2016, the reduction in TB incidence in the 50% IPT access scenario each year, compared with baseline, is between 5 and 34%. Although the fastest decrease in incidence rates occurs in the beginning of the simulation period, gains in incident cases averted relative to baseline tended to increase with time, indicating that averted ongoing transmission may further multiply the benefits of expanded IPT access. In our model, as a result of 50% IPT access in adolescents, TB incidence decreases in both adolescents and adults; in adolescents by as much as 47% and in adults by as much as 30%, in some years, relative to baseline (Fig. S6).
A scale up of IPT to 90% among adolescents further increases the anticipated benefits of IPT. At the end of the 20-year period, as a result of 90% IPT access in adolescents, TB incidence in adolescents decreases by 55% and in adults by 36% relative to baseline. We show the impact of IPT on additional variables in Fig. 3.
Fig. 2.
Effects of isoniazid preventive therapy on key tuberculosis disease indicators.
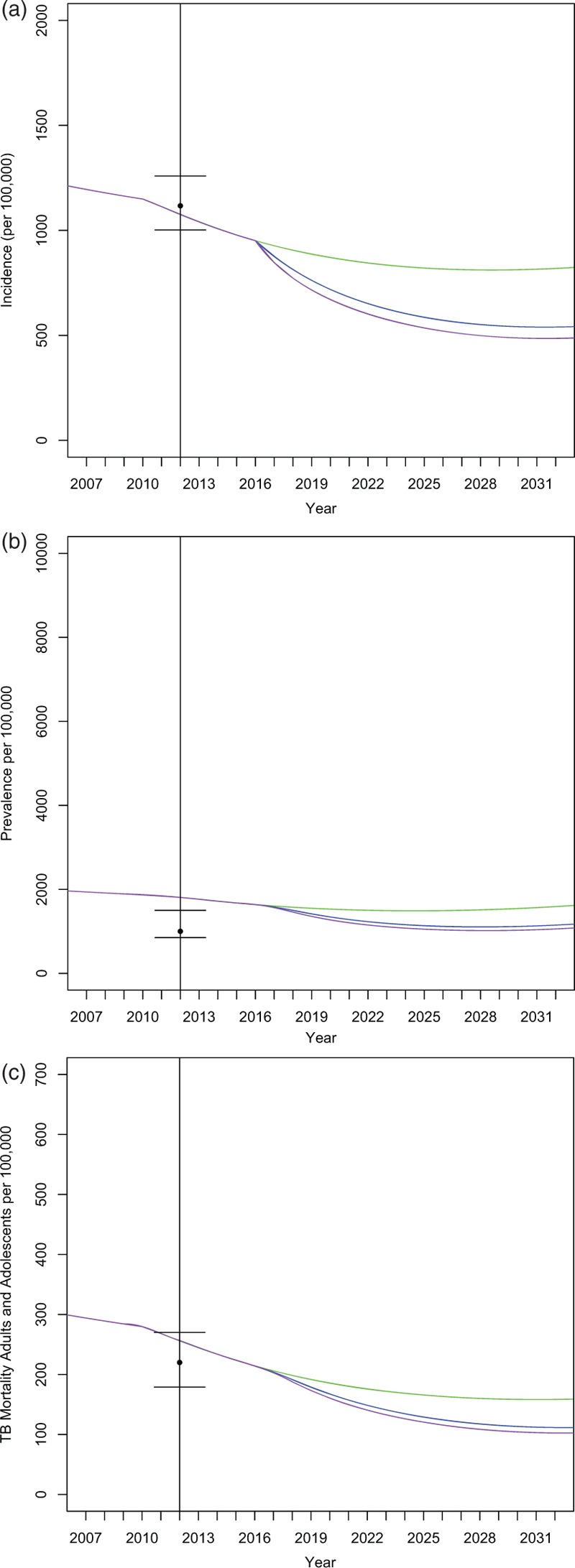
Bars indicate calibration target ranges. The baseline isoniazid preventive therapy case is shown in green, 50% isoniazid preventive therapy coverage in the blue intervention line, and 90% isoniazid preventive therapy coverage in the purple intervention line. (a) Tuberculosis Incidence in general population aged 14 and older, (b) tuberculosis prevalence in the general population aged 14 and older, (c) tuberculosis mortality in the general population aged 14 and older.
Fig. 3.
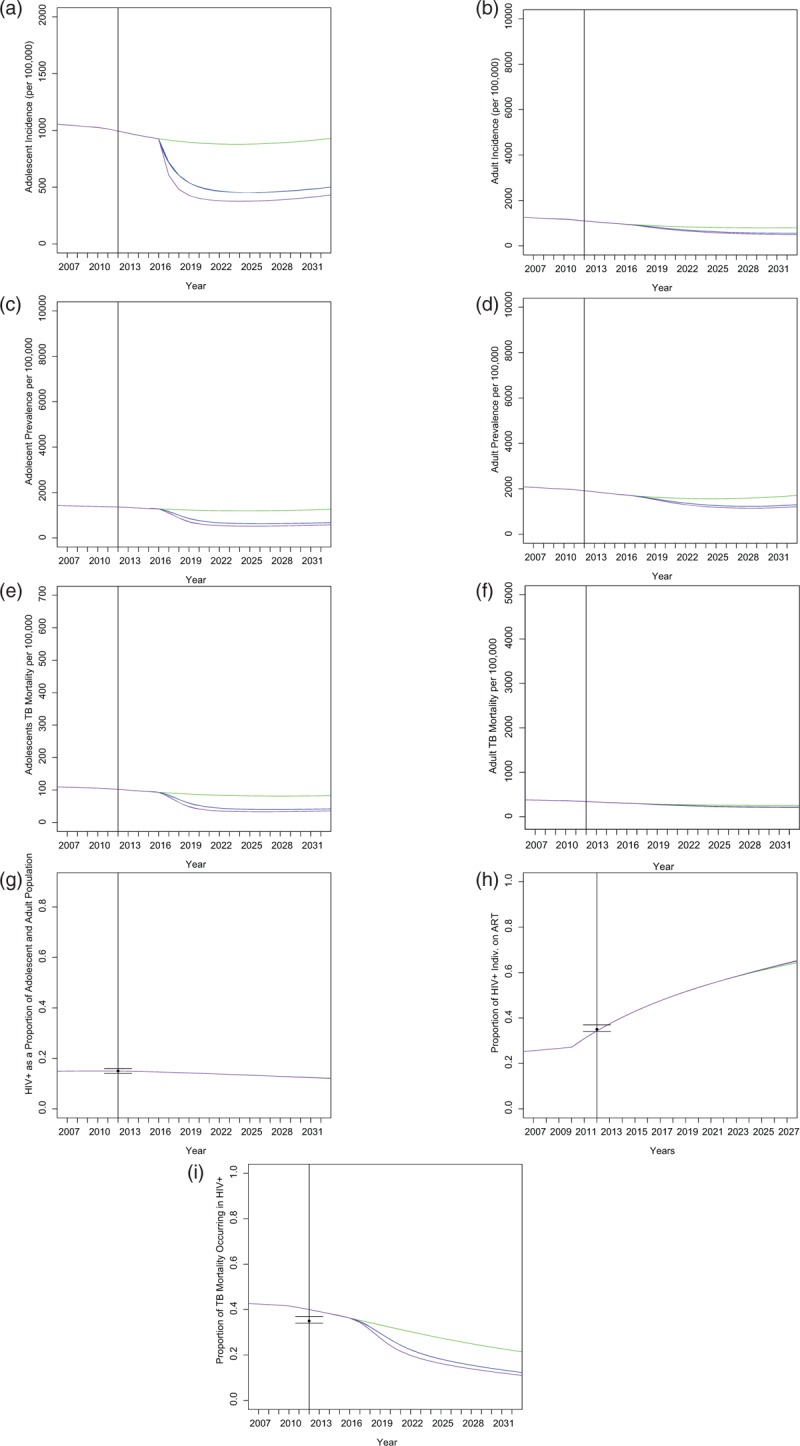
Effects of varying isoniazid preventive therapy access on additional variables.
Sensitivity to transmission parameters and role of reinfection
We show sensitivity of TB incidence to two of the most uncertain parameters under baseline IPT coverage: TB transmission rate (Fig. S2) and the activation among HIV-positive individuals (Fig. S4). TB incidence is significantly more sensitive to rate of transmission than to the relative activation rate among HIV+ individuals.
Sensitivity of tuberculosis incidence to recovery rate on isoniazid preventive therapy
We explore the sensitivity of TB incidence to the recovery rate (return to susceptibility) of latently infected individuals taking IPT; this parameter is subject to some uncertainty as there is not currently a diagnostic available that can detect cure of latent TB following treatment with IPT (Fig. S3). In our simulations, the effects of IPT recovery rate appear to be less important than the proportion of the population on IPT in influencing TB incidence.
Effects of HIV and demographic parameters
We observe that HIV infection rate (Fig. S7) and population birth rate (Fig. S8) were major drivers of the impact of IPT. TB incidence increases with both birth rate and HIV incidence; the impact of IPT increases with TB incidence. At birth rates of 0.04 and greater, baseline TB incidence increases, rather than decreases, over time.
Sensitivity to stopping isoniazid preventive therapy treatment
We explore the impact of stopping IPT treatment once individuals return to susceptibility following cessation of IPT treatment (Fig. S5). As expected, TB incidence increases consistently with the rate of IPT discontinuation. The duration of IPT treatment in areas with ongoing TB transmission is likely to affect of the impact of the IPT program.
Expanding isoniazid preventive therapy beyond schools
We also explore the effects of expanding IPT coverage not only in schools, but in the adult population as well. Although large-scale coverage expansion among adults may not be feasible, we find the effects of IPT to be even more substantial (Fig. S9). In sum, IPT coverage of 50% in the general population aged 14 and older results in a reduction of TB incidence by 96% compared with baseline after 20 years; 90% coverage leads to a reduction of 98% compared with baseline after 20 years.
Effects of expanded isoniazid preventive therapy on tuberculosis incidence in HIV-positive population
Expanded IPT access causes TB incidence to decline in both the general population of HIV-positive individuals (Fig. S10), as well as in adult HIV-positive individuals specifically (Fig. S11). At 50% IPT access, gains of 29% were observed in the general population, and gains of 41% were observed in adult HIV-positive individuals specifically. At 90% IPT access, gains of 36 and 48% were observed for these populations, respectively.
Discussion
Our model departs from previous work on latent TB in South Africa in that it considers the complexity associated with HIV and demographic dynamics in effectively implementing IPT. By designing a program that strategically targets a segment of the population that has yet to experience peak HIV infection, we are able to address latent TB prior to increase in one of the most important determinants of TB activation.
We show that expanding IPT delivery is associated with lowered TB prevalence and mortality (Fig. 2), including among HIV-infected individuals (Figs. S10 and S11). IPT is also associated with lowered TB incidence at lower transmission rates (Fig. S2).
We also show that an IPT intervention targeted at adolescents has an impact on key TB epidemiological indicators in the general population, including adults outside the intervention target age group (Fig. S6). Although the results indicate the superiority of full population IPT in an ideal scenario, the benefits to those outside the target group generated by the intervention we propose indicate that this intervention would be strategic in a resource-constrained area.
Our model has some limitations. First, our calibrated prevalence value is at the higher end of the probable range for the region, as is the fraction of TB deaths occurring in HIV-positive individuals. However, we are confident in our model as TB incidence, total TB mortality, and HIV prevalence are all well within expected ranges, especially considering that prevalence is often vulnerable to under-reporting. Second, though we show simulated results for incidence and mortality over a 20-year period, in the last 3 years of our predicted range, across the base case and all IPT levels, we observe a very slight increase in TB incidence toward the end of the simulation period. We do not consider this minor departure from the otherwise consistent trend to be significant, due to both its extremely low magnitude, and its presence only at the tail end of the simulation period, as simulations approaching 20 years necessarily feature some uncertainty. We also observe some oscillation in the relationship between transmission rate and incidence at low values of the transmission rate. However, the values at which this phenomenon is observed are low enough that they are likely outside of the realistic epidemiological range of values for this parameter (Table 1). Finally, the HIV model employed is somewhat simplified, particularly with regard to aspects of HIV treatment such as ART treatment interruption. Nonetheless, we believe that the essence of this relationship between HIV infection and TB incidence is captured.
Accurate diagnosis of TB will always be an issue, especially in the case of smear-negative TB, which presents a challenge in implementing the program we propose. For a program such as the one we propose to be effective, individuals must be correctly diagnosed and effectively monitored for adverse events that may occur as a result of IPT; the school-based nature of this program would facilitate such monitoring for adverse events. Although a diagnostic that detects recovery from latent TB is not currently available, we are optimistic about the possibility of improvements in this space. Given the small absolute number of individuals who will develop active TB over the course of their lifetimes, under such a program, there will likely be individuals who undergo treatment with IPT, who never would have developed TB. Implementation of such a program will likely be challenging, and require collaboration at multiple levels among community organizations, local and national governments, and potentially, international donors.
The concern has been raised that IPT could paradoxically cause TB incidence to increase as the result of rapid reinfection following cure. We show the results of simulation using a theoretical parameter under which such reinfection could occur (Fig. S12). However, the parameter range required for this outcome is far from the biologically probable parameter range, suggesting that such an outcome would be unlikely.
Although there is no empirical evidence to support concerns about selection for TB strains resistant to isoniazid as a result of the potential increase in selective pressure for resistance in the presence of IPT, any preventative use of antibiotics should proceed with appropriate caution, thorough analysis of the potential risks and benefits at the population level, and regular evaluation of resistance levels. If new data were to emerge that challenged the findings of the Thibela study, there would be cause to re-evaluate the implementation of such an intervention. However, in the absence of such data, these concerns do not provide cause to delay mass IPT.
The issue of drug-resistant latent TB could also be a concern. Although it appears that IPT actually has some effectiveness against IPT-resistant bacteria when infection is latent, depending on the mechanism of resistance [34], if an increase in prevalence of IPT resistance among individuals with latent TB were to occur, it could limit the effectiveness of this intervention.
Although whole population IPT would of course be ideal, our model suggests that a targeted program of community level IPT in adolescents could be of great help in reducing TB transmission and activation among both adolescents and adults.
Acknowledgements
The material is based upon work supported by a National Science Foundation Graduate Research Fellowship under grant no. DGE-114747, and under a Stanford University Gerald J. Lieberman Fellowship (A.S.R). E.B. is supported by funding from the National Institute of Drug Abuse (R01DA15612). M.W.F. was supported by funding from Morrison Institute for Population and Resource Studies at Stanford University. The funding institutions did not contribute to the content or direction of this work.
Author contributions: A.S.R. and E.B. designed the study. A.S.R., M.W.F., and E.B. iterated the model. A.S.R., E.B., and M.W.F. analyzed data. A.S.R. coded the model and wrote the article. A.S.R., M.W.F, and E.B. reviewed and revised the article.
Conflicts of interest
During a portion of the time this work was in progress, A.S.R. was employed as an intern by the Bill and Melinda Gates Foundation. She was subsequently employed as an intern, a consultant, and a postdoctoral fellow by the Global Public Health division of Johnson & Johnson, on work unrelated to this project. She is not currently employed by either organization.
Material from this article was presented in an oral presentation at the International Union against Tuberculosis and Lung Disease meeting, October 2016.
Supplementary Material
References
- 1.WHO. Global tuberculosis report 2013. Geneva: WHO; 2013. [Google Scholar]
- 2.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons (review). Cochrane Database Syst Rev 2010. CD000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dye C, Williams BG. The population dynamics and control of tuberculosis. Science 2010; 328:856–861. [DOI] [PubMed] [Google Scholar]
- 4.Byrd RB, Horn BR, Solomon DA, Griggs GA. Toxic effects of isoniazid in tuberculosis chemoprophylaxis. Role of biochemical monitoring in 1,000 patients. JAMA 1979; 241:1239–1241. [PubMed] [Google Scholar]
- 5.Mills HL, Cohen T, Colijn C. Community-wide isoniazid preventive therapy drives drug-resistant tuberculosis: a model-based analysis. Sci Transl Med 2013; 5:180ra49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harries AD, Zachariah R, Corbett EL, Lawn SD, Santos-Filho ET, Chimzizi R, et al. The HIV-associated tuberculosis epidemic-when will we act?. Lancet 2010; 375:1906–1919. [DOI] [PubMed] [Google Scholar]
- 7.Suthar AB, Lawn SD, del Amo J, Getahun H, Dye C, Sculier D, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med 2012; 9:e1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO report 2011: global tuberculosis control. 2011. http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf. [Google Scholar]
- 9.Churchyard GJ, Fielding KL, Lewis JJ, Coetzee L, Corbett EL, Godfrey-Faussett P, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med 2014; 370:301–310. [DOI] [PubMed] [Google Scholar]
- 10.Wood R, Lawn SD, Caldwell J, Kaplan R, Middelkoop K, Bekker LG. Burden of new and recurrent tuberculosis in a major South African city stratified by age and HIV-status. PLoS One 2011; 6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bucher HC, Griffith LE, Guyatt GH, Sudre P, Naef M, Sendi P, Battegay M. Isoniazid prophylaxis for tuberculosis in HIV infection: a meta-analysis of randomized controlled trials. AIDS 1999; 13:501–507. [DOI] [PubMed] [Google Scholar]
- 12.Basu S, Maru D, Poolman E, Galvani A. Primary and secondary tuberculosis preventive treatment in HIV clinics: simulating alternative strategies. Int J Tuberc Lung Dis 2009; 13:652–658. [PubMed] [Google Scholar]
- 13.Gagneux S, Long CD, Small PM, Van T, Schoolnik GK, Bohannan BJM. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science 2006; 312:1944–1946. [DOI] [PubMed] [Google Scholar]
- 14.Balcells ME, Thomas SL, Godfrey-Faussett P, Grant AD. Isoniazid preventive therapy and risk for resistant tuberculosis. Emerg Infect Dis 2006; 12:744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Halsema CL, Fielding KL, Chihota VN, Russell EC, Lewis JJ, Churchyard GJ, Grant AD. Tuberculosis outcomes and drug susceptibility in individuals exposed to isoniazid preventive therapy in a high HIV prevalence setting. AIDS 2010; 24:1051–1055. [DOI] [PubMed] [Google Scholar]
- 16.WHO. Global tuberculosis report 2014. Geneva: WHO; 2014. [Google Scholar]
- 17.UNAIDS. UNAIDS report on the global AIDS epidemic 2013. Geneva: UNAIDS; 2013. [Google Scholar]
- 18.United Nations. World population prospects: 2012 revision. United Nations; 2012, http://esa.un.org/wpp/. [Google Scholar]
- 19.Middelkoop K, Bekker L-G, Myer L, Dawson R, Wood R. Rates of tuberculosis transmission to children and adolescents in a community with a high prevalence of HIV infection among adults. Clin Infect Dis 2008; 47:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowdy DW, Dye C, Cohen T. Data needs for evidence-based decisions: a TB modeler's ‘Wish List’. Int J Tuberc Lung Dis 2013; 17:866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis 2012; 54:784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu-Raddad LJ, Sabatelli L, Achterberg JT, Sugimoto JD, Longini IM. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci U S A 2009; 106:13980–13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horsburgh CR. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med 2004; 350:2060–2067. [DOI] [PubMed] [Google Scholar]
- 24.Bacaër N, Ouifki R, Pretorius C, Wood R, Williams B. Modeling the joint epidemics of TB and HIV in a South African township. J Math Biol 2008; 57:557–593. [DOI] [PubMed] [Google Scholar]
- 25.Suen S-C, Bendavid E, Goldhaber-Fiebert JD. Disease control implications of India's changing multi-drug resistant tuberculosis epidemic. PLoS One 2014; 9:e89822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zelner JL, Murray MB, Becerra MC, Galea J, Lecca L, Calderon R, et al. Bacillus Calmette-Guerin and isoniazid preventative therapy protect contacts of tuberculosis patients. Am J Respir Crit Care Med 2014; 189:853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houben RMGJ, Sumner T, Grant AD, White RG. Ability of preventive therapy to cure latent Mycobacterium tuberculosis infection in HIV-infected individuals in high-burden settings. Proc Natl Acad Sci U S A 2014; 111:5325–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selwyn PA, Alcabes P, Hartel D, Buono D, Schoenbaum EE, Klein RS, et al. Clinical manifestations and predictors of disease progression in drug users with human immunodeficiency virus infection. N Engl J Med 1992; 327:1697–1703. [DOI] [PubMed] [Google Scholar]
- 29.Lin PL, Flynn JL. Understanding latent tuberculosis: a moving target. J Immunol 2010; 185:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJD. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS One 2011; 6:e17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 2011; 377:1495–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med 2005; 2:e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mellors JW, Muñoz a, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 1997; 126:946–954. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Yew W. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2009; 13:1320–1330. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


