Abstract
Aconitum pseudolaeve Nakai and Aconitum longecassidatum Nakai, which belong to the Aconitum subgenus Lycoctonum, are distributed in East Asia and Korea. Aconitum species are used in herbal medicine and contain highly toxic components, including aconitine. A. pseudolaeve, an endemic species of Korea, is a commercially valuable material that has been used in the manufacture of cosmetics and perfumes. Although Aconitum species are important plant resources, they have not been extensively studied, and genomic information is limited. Within the subgenus Lycoctonum, which includes A. pseudolaeve and A. longecassidatum, a complete chloroplast (CP) genome is available for only one species, Aconitum barbatum Patrin ex Pers. Therefore, we sequenced the complete CP genomes of two Aconitum species, A. pseudolaeve and A. longecassidatum, which are 155,628 and 155,524 bp in length, respectively. Both genomes have a quadripartite structure consisting of a pair of inverted repeated regions (51,854 and 52,108 bp, respectively) separated by large single-copy (86,683 and 86,466 bp) and small single-copy (17,091 and 16,950 bp) regions similar to those in other Aconitum CP genomes. Both CP genomes consist of 112 unique genes, 78 protein-coding genes, 4 ribosomal RNA (rRNA) genes, and 30 transfer RNA (tRNA) genes. We identified 268 and 277 simple sequence repeats (SSRs) in A. pseudolaeve and A. longecassidatum, respectively. We also identified potential 36 species-specific SSRs, 53 indels, and 62 single-nucleotide polymorphisms (SNPs) between the two CP genomes. Furthermore, a comparison of the three Aconitum CP genomes from the subgenus Lycoctonum revealed highly divergent regions, including trnK-trnQ, ycf1-ndhF, and ycf4-cemA. Based on this finding, we developed indel markers using indel sequences in trnK-trnQ and ycf1-ndhF. A. pseudolaeve, A. longecassidatum, and A. barbatum could be clearly distinguished using the novel indel markers AcoTT (Aconitum trnK-trnQ) and AcoYN (Aconitum ycf1-ndhF). These two new complete CP genomes provide useful genomic information for species identification and evolutionary studies of the Aconitum subgenus Lycoctonum.
Keywords: Lycoctonum, plastid, Ranunculaceae, medicinal plant, species identification
1. Introduction
Chloroplasts (CPs) play important functional roles in photosynthesis, biosynthesis, and metabolism of starch and fatty acids throughout the plant life cycle [1]. The angiosperm CP genome is a circular molecule with a quadripartite structure consisting of large single-copy (LSC) and small single-copy (SSC) regions and two copies of an inverted repeat (IR) region. Typically, the CP genomes of higher plants contain 110–120 genes, encoding proteins, transfer RNAs (tRNAs), and ribosomal RNAs (rRNAs), and are 120–160 kb in length. The structure, gene content, and gene orientation is highly conserved at the genus level [2]. Even within genera, however, CP genomes have undergone size changes, structure rearrangement, contraction and expansion of IRs, and even pseudogenization due to adaptations to their host plants’ environments [1,3]. Since the first CP genome was reported (for tobacco), more than 9000 complete CP genomes (October 2017) have been deposited in GenBank [4]. These data contain valuable information that has been used in reconstruction of high-resolution phylogenic trees, development of markers for species identification, and for evolutionary genetic studies [3,5,6,7,8]. Universal DNA barcoding is useful tool for species identification. However, in the Aconitum species, it is difficult to distinguish using universal DNA barcode. The complete CP genome is useful tool to solve DNA barcode limitation.
Although the CP genome is more highly conserved than the nuclear genome, it frequently contains insertions/deletions (indels) and single-nucleotide polymorphisms (SNPs) [9,10]. These variations have been used to estimate divergence times among evolutionarily-related species [11,12,13]. Also, these mutations can be used as markers to rapidly distinguish species [14,15,16]. Several studies report the use of indels and SNP mutations from complete CP genomes for species identification in Panax ginseng, buckwheat, Aconitum, and other genera [7,14,15]. Specifically, the CP genomes of nine Panax ginseng cultivars were sequenced, and six markers consisting of three indels and three derived cleaved amplified polymorphic sequences (dCAPS) were developed to distinguish subspecies of P. ginseng through comparative analysis [14]. Three divergent coding regions (rpoC1, cpoC2 and ycf1) and three intergenic sequence (IGS) regions (rpl32-trnL, rpl16-trnQ and trnQ-trnT) were used for this purpose. Tartary buckwheat (Fagopyrum tataricum) and common buckwheat (Fagopyrum esculentum) were classified based on six indel markers from one coding region, ycf1, and four IGS regions (trnS-trnG, rpoB-trnC, psbM-trnD, and ndhC-trnV) [15]. Aconitum coreanum (H.Lév.) Rapaics also has a unique insertion mutation (in the ndhC-trnV region) not present in other Aconitum species, and this region was used to develop a sequence characterized amplified region marker specific for A. coreanum [7]. Thus, indel and SNP mutations provide useful information for identification of species, phylogenic tree analysis, and population studies. Such markers can also overcome the limits of universal DNA barcodes.
Aconitum species are largely classified into three subgenera, Aconitum, Lycoctonum, and Gymnaconitum, which together comprise approximately 400 species [17]. These plants, which belong to the family Ranunculaceae, are distributed in the Northern hemisphere. In particular, Aconitum pseudolaeve Nakai and Aconitum longecassidatum Nakai are widely distributed in East Asia [17], and A. pseudolaeve is a valuable plant endemic to Korea [18]. A. pseudolaeve and A. longecassidatum are 30–80 cm in height, with mean stem length of 65 cm, short branches, and retrorse yellowish pubescent and pentagonal-reniform leaf blades. Although these two species are very similar morphologically, their inflorescences differ slightly. First, the bracteoles of A. pseudolaeve are 2–3 times longer than the pedicel, whereas those of A. longecassidatum are as long as or slightly shorter than the pedicel. Second, the pistils of A. pseudolaeve have recurved hair. However, A. longecassidatum forms glabrous pistils [19].
Notwithstanding these morphological distinctions, the two species are used indiscriminately as herbal medicine as Radix Lycoctoni [20]. The roots of both plants are used to relieve neuralgia, reduce fever, and lower blood pressure [20], and extracts from A. pseudolaeve are purported to have anti-aging and anti-diabetes effects [21]. For these reasons, the extract of A. pseudolaeve is used in cosmetic compounds, as well as in herbal medicine [22]. In particular, perfume based on A. pseudolaeve is used in aromatherapy intended to improve emotional stability [23]. Although the two Aconitum species have medicinal and commercial value, they are often used without species identification. The ability to distinguish the two species would improve the medicinal potential, quality control, and stability of commercial products containing material from these plants.
In this study, we sequenced the complete CP genomes of A. pseudolaeve and A. longecassidatum. Comparative analysis of the two CP genomes revealed highly divergent regions and potential indels, simple sequence repeats (SSRs), and SNPs. In addition, based on comparative CP genome analysis of A. pseudolaeve, A. longecassidatum, and A. barbatum Patrin ex Pers., we developed indel markers to distinguish three species of the subgenus Lycoctonum based on divergent regions of the CP genome. These results will provide useful genetic tools for identification of Aconitum species of the subgenus Lycoctonum, and also will inform genomic resources for evolutionary studies of these plants.
2. Results and Discussion
2.1. CP Genome Organization of A. pseudolaeve and A. longecassidatum
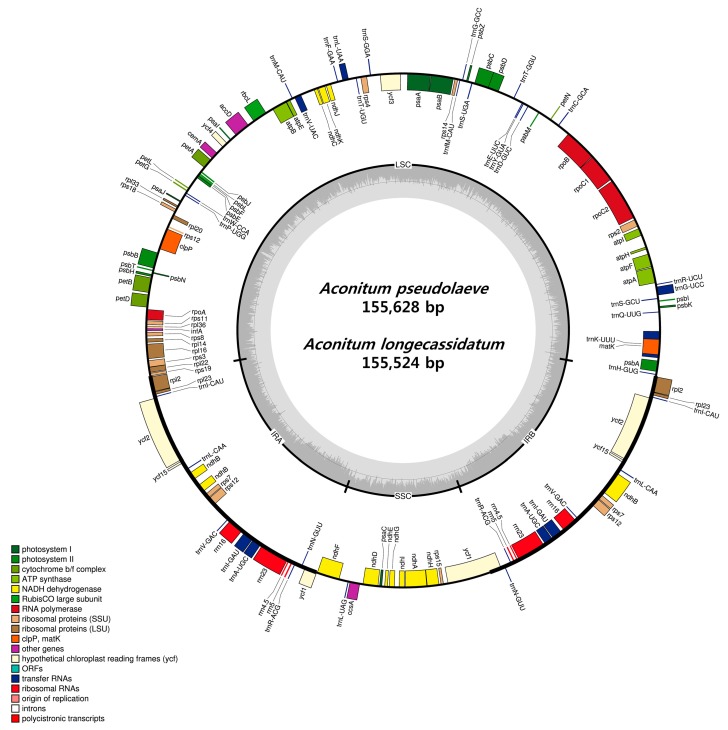
We obtained trimmed reads (approximately 2.7 Gb) from A. pseudolaeve Nakai and A. longecassidatum Nakai using the MiSeq platform. Seven and six initial CP contigs of A. pseudolaeve and A. longecassidatum, respectively, were de novo assembled from low-coverage whole-genome sequence. The complete CP genomes of A. pseudolaeve and A. longecassidatum are 155,628 and 155,524 bp in length, with approximately 345× and 222× coverage, respectively (Table S1). The complete CP genomes of both Aconitum species have the quadripartite structure characteristic of most angiosperms (Figure 1): a pair of IRs (51,854 bp and 52,108 bp in A. pseudolaeve and A. longecassidatum, respectively) and two single-copy regions (LSC, 86,683 bp and 86,466 bp; SSC, 17,091 and 16,950 bp in A. pseudolaeve and A. longecassidatum, respectively) (Figure 1 and Table 1). The guanine-cytosine (GC) contents of the two Aconitum CP genomes are 38.0% and 38.1%, with IR regions having higher GC content (43.1% and 43.0% in A. pseudolaeve and A. longecassidatum, respectively) than LSC (36.1% in both species) and SSC regions (32.6% and 32.7% in A. pseudolaeve and A. longecassidatum, respectively) (Table S2). Thus, the A. pseudolaeve and A. longecassidatum CP genomes are AT-rich, similar to those of other land plants [7,24,25].
Figure 1.
Circular gene map of A. pseudolaeve and A. longecassidatum. Genes drawn inside the circle are transcribed clockwise, and those outside the circle counterclockwise. The darker gray in the inner circle represents (GC) content. The gene map corresponds to A. pseudolaeve. LSC: large single copy; IR: inverted repeat; SSC: small single copy; GC: guanine-cytosine; ORF: open reading frame.
Table 1.
Size comparison of two Aconitum species’ CP genomic regions.
| Species | Aconitum pseudolaeve | Aconitum longecassidatum |
|---|---|---|
| Total CP genome size (bp) | 155,628 | 155,524 |
| LSC region (bp) | 86,683 | 86,466 |
| IR region (bp) | 51,854 | 52,108 |
| SSC region (bp) | 17,091 | 16,950 |
| GC content (%) | 38.0 | 38.1 |
| LSC (%) | 36.1 | 36.1 |
| IR (%) | 43.1 | 43.0 |
| SSC (%) | 32.6 | 32.7 |
| Number of genes | 112 | 112 |
| Protein-coding genes | 78 | 78 |
| rRNAs | 4 | 4 |
| tRNAs | 30 | 30 |
CP: chloroplast; LSC: large single copy; IR: inverted repeat; SSC: small single copy; tRNAs: transfer RNAs; rRNAs: ribosomal RNAs. GC: guanine-cytosine.
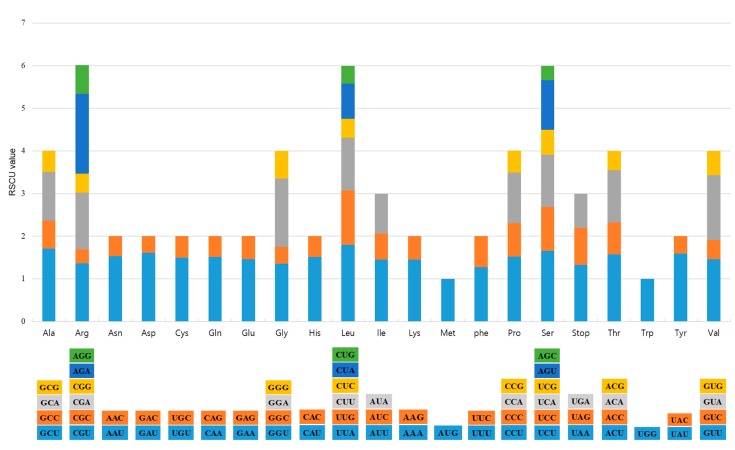
Gene content and gene order were similar to those in other Aconitum CP genomes [7]. Both the A. pseudolaeve and A. longecassidatum CP genomes have 112 unique genes, including 78 protein-coding genes, 30 tRNAs, and 4 rRNAs (Table 2). Of these, 18 genes are present as duplicates: seven tRNAs, four rRNAs, and seven protein-coding genes (ndhB, rpl2, rpl23, rps7, rps12, ycf1 and ycf2). The two Aconitum CP genomes each have 17 intron-containing genes, including 15 genes with a single intron and two with two introns; rps12 is trans-spliced, as in other Aconitum species. The trnK-UUU gene (2526 bp in A. pseudolaeve and 2525 bp in A. longecassidatum) has the longest intron region with matK (Table 3). The ndhD and rpl2 genes use the alternative start codon ACG; rps19 and ycf2 use GTG; and rpl2 and rps19 use ACG or GTG, as previously reported [26]. rps16 contains one exon deletion in both A. pseudolaeve and A. longecassidatum. ycf1 is present in two copies; one ycf1 copy was located in the boundary region between IRa and SSC in A. pseudolaeve. The 78 protein-coding sequences comprise 26,459 codons in A. pseudolaeve and 26,487 codons in A. longecassidatum (Table S3). Leucine and isoleucine are abundant in both CP genomes. (Figure S1). Two or more synonymous codons are used for all amino acids except methionine and tryptophan (Figure 2). The most preferred synonymous codons (relative synonymous codon usage; RSCU > 1) contain A or T in the third position, contributing to the AT bias, as in other Aconitum CP genomes [7,27]. Arginine, leucine, and serine are each represented by six synonymous codons with higher RSCU values [26,28]. This may be for protecting protein mutations due to important amino acids in biosynthesis. The sequence of both Aconitum CP genomes consists of 58% protein-coding genes, 1.8% tRNA genes, and 5.8% rRNA; the remaining 41.4% is comprised of non-coding regions, including pseudogenes and introns. ycf1 and rps16 are pseudogenes, as in other Aconitum species [27,29]. Both CP genomes are very similar in terms of gene order, content, and structure, and are highly conserved relative to the CP genomes of other Aconitum species [7,27,29].
Table 2.
Genes present in the CP genomes of A. pseudolaeve Nakai and A. longecassidatum Nakai.
| Gene Products of Aconitum Species | |
|---|---|
| Photosystem I | psaA, B, C, I, J |
| Photosystem II | psbA, B, C, D, E, F, H, I, J, K, L, M, N, T, Z |
| Cytochrome b6_f | petA, B (1), D (1), G, L, N |
| ATP synthase | atpA, B, E, F (1), H, I |
| Rubisco | rbcL |
| NADH oxidoreductase | ndhA (1), B (1),(3), C, D, E, F, G, H, I, J, K |
| Large subunit ribosomal proteins | rpl2 (1),(3), 14, 16 (1), 20, 22, 23 (3), 32, 33, 36 |
| Small subunit ribosomal proteins | rps2, 3, 4, 7 (3), 8, 11, 12 (2)–(4), 14, 15, 18, 19 |
| RNA polymerase | rpoA, B, C1 (1), C2 |
| Unknown function protein-coding gene | ycf1 (3), 2 (3), 3 (2), 4 |
| Other genes | accD, ccsA, cemA, clpP (2), infA, matK |
| Ribosomal RNAs | rrn16 (3), rrn23 (3), rrn4.5 (3), rrn5 (3) |
| Transfer RNAs | trnA-UGC (1),(3), trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnG-UCC (1), trnG-GCC, trnH-GUG, trnI-CAU (3), trnI-GAU (1),(3) trnK-UUU (1), trnL-UAA (1), trnL-UAG, trnL-CAA (3), trnM-CAU, trnfM-CAU, trnN-GUU (3), trnP-UGG, trnQ-UUG, trnR-ACG (3), trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-UAC (1), trnV-GAC (3), trnW-CCA, trnY-GUA |
(1) Gene containing a single intron; (2) gene containing two introns; (3) two gene copies in inverted repeats; (4) trans-spliced gene. CP: chloroplast.
Table 3.
Genes with introns in the A. pseudolaeve Nakai CP genome, and lengths of exons and introns.
| Gene | Region | Exon I | Intron I | Exon II | Intron II | Exon III |
|---|---|---|---|---|---|---|
| trnk-UUU | LSC | 37 | 2526 (2525) | 35 | ||
| trnG-UCC | LSC | 23 | 744 (747) | 48 | ||
| atpF | LSC | 145 | 733 (735) | 410 | ||
| rpoC1 | LSC | 432 | 757 | 1635 (1611) | ||
| ycf3 | LSC | 124 | 730 (729) | 230 | 761 (762) | 153 |
| trnL-UAA | LSC | 35 | 501 (495) | 50 | ||
| trnV-UAC | LSC | 39 | 597 | 37 | ||
| rps12 * | LSC | 114 | - | 232 | - | 26 |
| clpP | LSC | 71 | 833 (830) | 292 | 651 (661) | 246 |
| petB | LSC | 6 | 801 | 642 | ||
| petD | LSC | 8 | 704 | 496 | ||
| rpl16 | LSC | 9 | 1045 (1054) | 399 | ||
| rpl2 | IR | 391 | 667 | 434 | ||
| ndhB | IR | 777 | 702 | 756 | ||
| trnI-GAU | IR | 42 | 937 | 35 | ||
| trnA-UGC | IR | 38 | 802 | 35 | ||
| ndhA | SSC | 553 | 1006 (1004) | 539 |
* rps12 gene is a trans-spliced gene. Gene length in A. longecassidatum Nakai is shown in parentheses. CP: chloroplast; LSC: large single copy; IR: inverted repeat; SSC: small single copy.
Figure 2.
Codon content for the 20 amino acids and stop codon in 78 protein-coding genes in the A. pseudolaeve Nakai and A. longecassidatum Nakai CP genomes. Colors in the column graph reflect codons in the same colors shown in the lower part of the figure. RSCU: relative synonymous codon usage; Ala: alanine; Arg: arginine; Asn: asparagine; Asp: asparagine; Cys: cysteine; Gln: glutamine; Glu: glutamic acid; Gly: glycine; His: histidine; Leu: leucine; Ile: isoleucine; Lys: lysine; Met: methionine; Phe: phenylalanine; Pro: proline; Ser: serine; Thr: threonine; Trp: tryptophan; Tyr: tyrosine; Val: valine.
2.2. Repeat Analysis in Two Aconitum Chloroplast Genomes
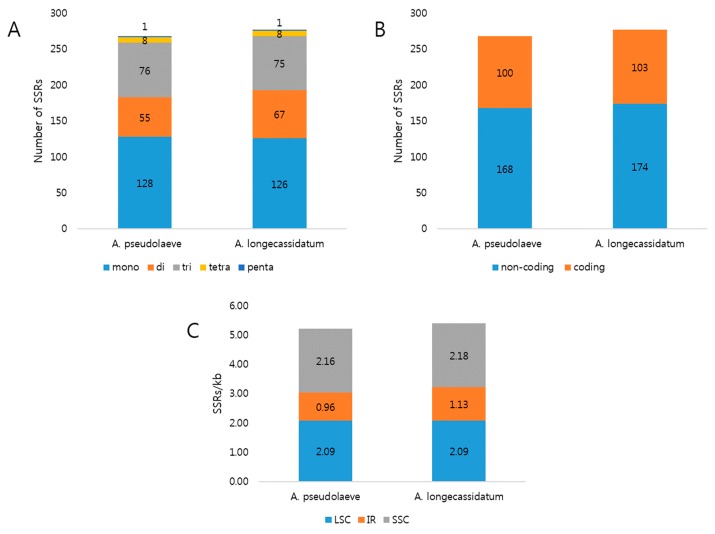
Microsatellites or simple sequence repeats (SSRs) are made up of abundant tandem repeat sequences consisting of 1–6-nt motifs. These elements are useful markers due to their high degree of polymorphism. In addition, SSRs are used for phylogenic analysis in population genetics [30,31]. We identified SSR loci, revealing 268 and 277 SSRs in the CP genomes of A. pseudolaeve Nakai and A. longecassidatum Nakai, respectively (Figure 3). Mononucleotides were the most abundant motifs, constituting 128 (47.8%) and 126 (45.5%) of the SSRs in A. pseudolaeve and A. longecassidatum, respectively. Approximately 37% of SSRs were distributed in coding regions. More SSRs were present in single-copy regions than in IR regions. To detect potential SSR loci for development of markers to distinguish the two Aconitum species, we identified 36 SSR indels consisting of A or T motifs, ranging in length from 1 to 6 bp (Table 4). The region exhibiting the greatest difference between species was a 6-bp SSR in psbM-trnD (IGS). One SSR is present in an exon of ndhG (Table 4).
Figure 3.
Distribution of simple sequence repeats (SSRs) in A. pseudolaeve Nakai and A. longecassidatum Nakai CP genomes. (A) Distribution of SSR types in the two Aconitum CP genomes. (B) Distribution of SSRs between coding and non-coding regions. (C) Number of SSRs per unit length in the indicated genomic regions of Aconitum CP genomes. CP: chloroplast; LSC: large single copy; IR: inverted repeat; SSC: small single copy.
Table 4.
Polymorphic SSRs between the A. pseudolaeve Nakai and A. longecassidatum Nakai CP genomes. CP: chloroplast; IGS: intergenic sequence.
| No. | Location | Region | Motif | Repeat Number | |
|---|---|---|---|---|---|
| A. pseudolaeve | A. longecassidatum | ||||
| 1 | trnH-psbA | IGS | A | 9 | 8 |
| 2 | trnK-matK | IGS | T | 13 | 12 |
| 3 | trnK-trnQ | IGS | A | 12 | 10 |
| 4 | trnK-trnQ | IGS | T | 9 | 10 |
| 5 | trnG | intron | T | 15 | 18 |
| 6 | atpF | intron | T | 16 | 14 |
| 7 | psbM-trnD | IGS | T | 16 | 11 |
| 8 | psbM-trnD | IGS | AT | 18 | 12 |
| 9 | trnS-psbZ | IGS | A | 11 | 10 |
| 10 | trnG-trnfM | IGS | A | 18 | 19 |
| 11 | psaA-ycf3 | IGS | T | 9 | 10 |
| 12 | ycf3 | intron | T | 11 | 12 |
| 13 | ycf3 | intron | A | 9 | 8 |
| 14 | rps4-trnT | IGS | A | 12 | 11 |
| 15 | trnF-ndhJ | IGS | T | 8 | 10 |
| 16 | ndhC-trnV | IGS | A | 9 | 11 |
| 17 | ndhC-trnV | IGS | A | 14 | 9 |
| 18 | accD-psaI | IGS | A | 9 | 8 |
| 19 | accD-psaI | IGS | A | 13 | 12 |
| 20 | psaI-ycf4 | IGS | T | 10 | 11 |
| 21 | ycf4-cemA | IGS | T | 10 | 9 |
| 22 | petA-psbJ | IGS | T | 10 | 9 |
| 23 | psbE-petL | IGS | T | 9 | 8 |
| 24 | psbE-petL | IGS | A | 9 | 8 |
| 25 | psbE-petL | IGS | T | 11 | 10 |
| 26 | rps12-clpP | IGS | ATT | 12 | 9 |
| 27 | clpP | intron | A | 13 | 11 |
| 28 | clpP | intron | A | 14 | 11 |
| 29 | rpl16 | intron | A | 12 | 15 |
| 30 | rpl16-rps3 | IGS | T | 11 | 14 |
| 31 | ndhF-trnL | IGS | A | 12 | 11 |
| 32 | ndhF-trnL | IGS | T | 12 | 11 |
| 33 | ccsA-ndhD | IGS | T | 13 | 10 |
| 34 | ndhD-psaC | IGS | A | 8 | 9 |
| 35 | ndhG | Exon | T | 11 | 10 |
| 36 | ndhA | intron | A | 11 | 9 |
Repeat sequences play important evolutionary roles, influencing changes in genome structure such as duplication and rearrangement [32]. We detected tandem repeats of 20 or 21 bp in A. pseudolaeve and 19 bp in A. longecassidatum (Table S4). Most tandem repeats were located in IGS regions, and were present in both the ycf1 and ycf2 genes. Fourteen repeats were shared between the two Aconitum species. Three tandem repeats were located in ycf2, and two in trnK-rps16. In both species, six palindromic repeats were present, ranging in size from 21 to 33 bp (Table 5). In particular, the ycf2 gene contained short tandem repeats as well as a palindromic repeat.
Table 5.
Distribution of palindromic repeats in the CP genomes of A. pseudolaeve Nakai and A. longecassidatum Nakai.
| Species | Position | Repeat Unit Length (bp) | Repeat Units Sequences | Region |
|---|---|---|---|---|
| A. pseudolaeve | IGS (trnE-trnT) | 31 | TCTATTTCTTATTTCTATATATTCTAATGAT | LSC |
| IGS (petA-psbJ) | 33 | GTAAGAATAAGAACTCAATGGACCTTGCCCCTC | LSC | |
| IGS (psbT-psbN) | 28 | TTGAAGTAAAGTAATGAGCCTCCCATAT | LSC | |
| IGS (petD-rpoA) | 24 | ATGTATCTAGGGACTAGTCCCTTC | LSC | |
| Exon (ycf2) | 24 | AGATCCATTAGATAATGAACTATT | IR | |
| Exon (ycf15) | 21 | TGGTTGTTCGCCGTTCAAGAA | IR | |
| A. longecassidatum | IGS (trnE-trnT) | 31 | TCTATTTCTTATTTCTATATATTCTAATGAT | LSC |
| IGS (petA-psbJ) | 33 | GTAAGAATAAGAACTCAATGGACCTTGCCCCTC | LSC | |
| IGS (psbT-psbN) | 28 | TTGAAGTAAAGTAATGAGCCTCCCATAT | LSC | |
| IGS (petD-rpoA) | 24 | ATGTATCTAGGGACTAGTCCCTTC | LSC | |
| Exon (ycf2) | 24 | AGATCCATTAGATAATGAACTATT | IR | |
| Exon (ycf15) | 21 | TGGTTGTTCGCCGTTCAAGAA | IR |
CP: chloroplast; IGS: intergenic sequence; LSC: large single copy; IR: inverted repeat region.
2.3. Comparison of the Chloroplast Genomes of A. pseudolaeve Nakai, A. longecassidatum Nakai and Aconitum barbatum Patrin ex Pers.
Based on a phylogenetic analysis of the CP genome sequence, A. pseudolaeve and A. longecassidatum have been clustered within the Aconitum subgenus Lycoctonum, genetically closest to A. barbatum [27]. Consistent with this, the CP genomes of A. pseudolaeve and A. longecassidatum are 99.7% similar, with nearly identical genome structure, gene content, and gene order, although the single-copy (LSC and SSC) and IR regions differ slightly. The LSC and IR regions of the A. barbatum CP genome are slightly longer than those of A. pseudolaeve and A. longecassidatum, whereas the SSC regions are shorter. Thus, overall, the CP genomes of the three Aconitum species are very similar.
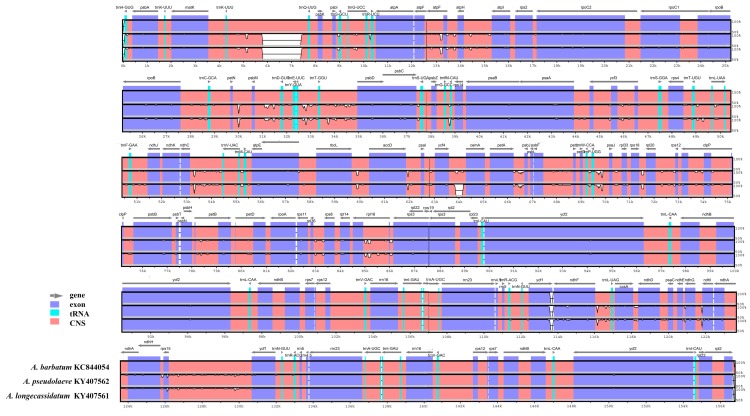
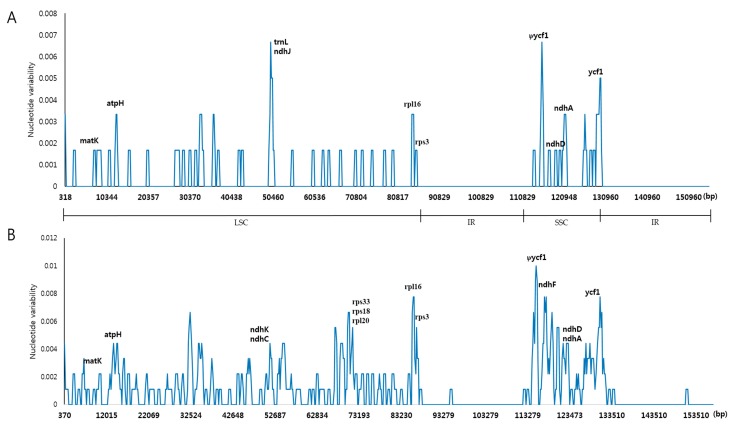
To identify divergent regions among the three species, we performed sequence alignment against the A. barbatum CP genome (Figure 4). The greatest divergence was observed in non-coding regions. In particular, A. barbatum contains a large insertion in trnK-trnQ that is not present in the other two species. Smaller divergent regions are present in petN-psbM, trnT-trnL, ndhC-trnV, rbcL-accD, and other loci; A. barbatum has more divergent regions than the other two species. Almost all divergent regions are located in non-coding regions such as trnR-atpA, trnT-psbD, ycf4-cemA, ndhC-trnV, and ycf1-ndhF. As noted above, coding regions are highly conserved between A. pseudolaeve and A. longecassidatum (Figure S2). The most divergent regions were found in non-coding regions such as trnR-atpA, trnT-psbD, ycf4-cemA, ndhC-trnV, and ycf1-ndhF (Figure S2). In addition, to analyze divergence at the sequence level among the three Aconitum CP genomes, we also calculated the nucleotide variability (Pi) value (Figure 5). As expected, IR regions are dramatically conserved among the three species. In other words, single-copy (LSC and SSC) regions are more variable than IR regions. The divergence among the three Aconitum species is greater than that between A. pseudolaeve and A. longecassidatum. As shown in Figure 5, a few regions exhibited divergence (atpH, trnL, ndhJ, rpl16, ycf1, and ndhA), with a maximal Pi value of 0.7%.
Figure 4.
Comparison of A. pseudolaeve Nakai, A. longecassidatum Nakai and Aconitum barbatum Patrin ex Pers. chloroplast (CP) genomes using mVISTA. Complete CP genomes of A. pseudolaeve and A. longecassidatum were compared with A. barbatum as a reference. Blue block: conserved gene; sky-blue block: tRNA and rRNA; red block: intergenic region. White peaks are sequence variation regions between A. barbatum and A. pseudolaeve, and A. barbatum and A. longecassidatum. CP: chloroplast; tRNA; transfer RNA; CNS: conserved non-coding sequence.
Figure 5.
Sliding-window analysis of CP genomes. (A) Comparison of the nucleotide variability (Pi) between A. pseudolaeve Nakai and A. longecassidatum Nakai CP genomes; (B) Comparison of the nucleotide variability (Pi) among Aconitum subgenus Lycoctonum CP genomes, including A. barbatum Patrin ex Pers., A. pseudolaeve, and A. longecassidatum. CP: chloroplast
2.4. Indel and SNP Mutation between A. pseudolaeve and A. longecassidatum
Indels and SNPs are common events in the evolution of higher plant CP genomes [9,33,34,35]. These mutations provide information that is useful for resolving evolutionary relationships in phylogenetic analyses of related taxa [36]. We detected 61 indels between A. pseudolaeve Nakai and A. longecassidatum Nakai (Table S5), of which 53 are located in IGS regions and the remaining eight are in coding regions. Most indels range from 1 to 6 bp, and eight indels are longer than 10 bp; the longest indel, in ycf4-cemA, has a length of 256 bp. No indels were found in IR regions. Comparison of the Aconitum species revealed a large insertion (1582 bp) in A. barbatum Patrin ex Pers. not present in A. pseudolaeve or A. longecassidatum. trnK-trnQ is highly conserved between A. pseudolaeve and A. longecassidatum.
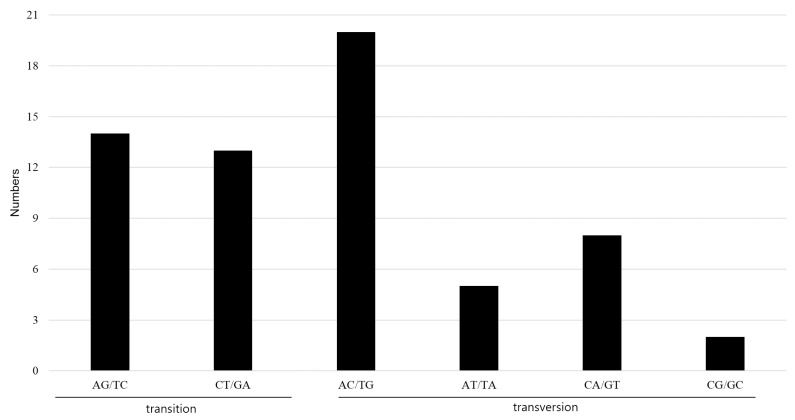
We also detected 62 SNPs consisting of 27 transitions (Ts) and 35 transversions (Tv) between two CP genomes (Figure 6 and Table S6). The ratio of Ts/Tv was 1:0.77, similar to that of other CP genomes [9,37]. Some nucleotides were substituted A-to-C and T-to-G (32%). Substitution of C-to-G and G-to-C showed the lowest frequency (3%). Of these 62 SNPs, 26 are located in coding regions. In particular, the ycf1 gene contains nine SNPs (three Ts, six Tv), and thus represents a hotspot region containing clustered variation [9,38]. We detected no non-synonymous SNPs between A. pseudolaeve and A. longecassidatum.
Figure 6.
The nucleotide substitution pattern in A. pseudolaeve Nakai and A. longecassidatum Nakai CP genomes. The patterns were divided into six types, as indicated by the six non-strand-specific base-substitution types (i.e., numbers of considered G to A and C to T site sites for each respective set of associated mutation types). The A. pseudolaeve chloroplast genome was used as a reference. CP: chloroplast
2.5. Development and Validation of the Indel Marker for Authentication of Three Species in the Aconitum Subgenus Lycoctonum
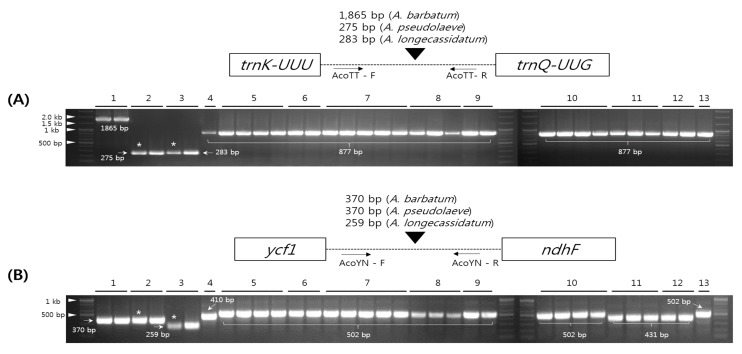
Indel regions are commonly used for development of markers because they are easy to detect, and it is straightforward to design suitable primers for them [14,15,39]. We developed indel markers using the sequence variability of the large indel regions in A. pseudolaeve Nakai, A. longecassidatum Nakai, and A. barbatum Patrin ex Pers. (Figure 4). Specifically, we designed indel primers based on the conserved regions of trnK-trnQ and ycf1-ndhF. AcoTT (Aconitum trnK-trnQ) and AcoYN (Aconitum ycf1-ndhF) primers successfully amplified the predicted products in all three Aconitum species (Figure 7 and Data S1). A. pseudolaeve and A. longecassidatum exhibit a small length difference in AcoTT, whereas A. barbatum exhibits a longer PCR product than the other two species, as expected. As shown in Figure 7, A. barbatum, A. pseudolaeve, and A. longecassidatum yielded amplicons of 1865 bp, 275 bp, and 283 bp, respectively. Furthermore, A. longecassidatum has a 6-bp insertion relative to A. pseudolaeve. In AcoYN, only A. longecassidatum (259 bp) exhibits a difference to A. pseudolaeve and A. barbatum (370 bp). In the previous study analyzing molecular phylogeny based on the CP genome sequences of Aconitum species, we found that two Aconitum subgenera, Aconitum and Lycoctonum, were clearly classified [7]. To confirm the variability of indel regions between Aconitum species and subgenera, we conducted analysis of PCR amplification profiles using the indel markers AcoTT and AcoYN, and a total 27 samples of Aconitum species (nine species and one variety) consisting of Aconitum subgenera Aconitum and Lycoctonum (Figure 7). Interestingly, all 27 other Aconitum samples yielded only the 877-bp amplicon for AcoTT, but three band patterns for AcoYN (Figure 7): the PCR products for A. monanthum Nakai and A. kirinense Nakai were 431 bp; that of A. coreanum was 410 bp; and those of the other species were 502 bp. However, A. longecassidatum was clearly distinguished from the other Aconitum species. Taken together, these findings confirm that the three Aconitum species each have specific sequences, and that it is possible to distinguish them from other Aconitum species.
Figure 7.
Schematic diagram of development of indel markers (AcoTT and AcoYN) in A. pseudolaeve Nakai, A. longecassidatum Nakai, and A. barbatum Patrin ex Pers. (A) Primers for AcoTT were tested in 13 Aconitum species. AcoTT length of A. barbatum was measured in the CP sequence; (B) Primers for AcoYN were validated in 13 Aconitum species: (1) A. barbatum, (2) A. pseudolaeve, (3) A. longecassidatum, (4) A. coreanum (H.Lév.) Rapaics, (5) A. carmichaelii Debeaux, (6) A. voluvile var. pubescens Regel, (7) A. jaluense var. triphyllum (Nakai) U.C.La, (8) A. kusnezoffii Rchb., (9) A. jaluense Kom., (10) A. austrokoreense Koidz., (11) A. monanthum Nakai, (12) A. kirinense Nakai, (13) A. chiisanense Nakai. * The CP genomes of A. pseudolaeve and A. longecassidatum were completed in this study. AcoYN: Aconitum ycf1-ndhF; AcoTT: Aconitum trnK-trnQ. CP: chloroplast.
Because A. pseudolaeve and A. longecassidatum have highly conserved CP genome structures, it is difficult to develop markers for the Aconitum genus that can distinguish at the species level. Furthermore, A. pseudolaeve and A. longecassidatum had consistent sequences in the universal DNA barcode regions such as internal transcribed spacer (ITS), matK. By comparative analysis, however, we detected genetic variants and used them to develop indel markers. Specifically, the trnK-trnQ region could distinguish A. pseudolaeve, A. longecassidatum, and A. barbatum. These three species of the Aconitum subgenus Lycoctonum contain specific indel regions not present in the subgenus Aconitum. In this study, we overcame the limitations of universal DNA barcodes for inter-species identification. Thus, our indel markers (AcoTT and AcoYN) will be useful in identification of A. pseudolaeve, A. longecassidatum, and A. barbatum (Table 6). Furthermore, we confirmed that these markers can be used to distinguish Aconitum at the subgenus level. It is likely that the subgenus Aconitum exhibits greater conservation (i.e., less variation) than the subgenus Lycoctonum. Although only a few Aconitum species were used in this study, our findings will contribute to species classification in Aconitum subgenus Lycoctonum.
Table 6.
Primer information for insertion and selection (indel) marker development in this study.
| Primer Name | Primer Sequence (5′ > 3′) | Position |
|---|---|---|
| AcoTT-F | TGC TTA CGA AGT TGT TCC GGC T | trnK-trnQ |
| AcoTT-R | CAC AAA CCA AAT CCG AGT ACC GA | |
| AcoYN-F | GAT GGA ATC GTC CAT CGC GT | ycf1-ndhF |
| AcoYN-R | TGT AAG TGG AGG ACG GAT CTC T |
3. Materials and Methods
3.1. Plant Materials and Genome Sequencing
We collected fresh leaves of A. pseudolaeve Nakai (KIOM201401010986) and A. longecassidatum Nakai (KIOM201401010506) from medicinal plantations in Korea, and subjected the samples to CP genome sequencing. A. pseudolaeve and A. longecassidatum were given identification numbers, and specimens were registered in the Korean Herbarium of Standard Herbal Resources (Index-Herbarium code KIOM) at the Korea Institute of Oriental Medicine (KIOM) [20]. DNA was extracted using the DNeasy Plant Maxi kit (Qiagen, Valencia, CA, USA). Illumina paired-end sequencing libraries were constructed and generated using MiSeq platform (Illumina, San Diego, Valencia, CA, USA).
3.2. Assembly and Annotation of Two Aconitum Species
CP genomes were obtained by de novo assembly from low-coverage whole-genome sequence data. Trimmed paired-end reads (Phred scores ≥ 20) were assembled using CLC Genome Assembler (ver. 4.06 beta, CLC Inc, Aarhus, Denmark) with default parameters. The principal contigs representing the CP genome were retrieved from total contigs using Nucmer [40] using the CP genome sequence of Aconitum barbatum var. puberulum (KC844054) as the reference sequence. Gene annotation was performed using DOGMA [41] and manual curation using BLAST. The circular maps of A. pseudolaeve and A. longecassidatum were obtained using OGDRAW [42]. Codon usage and base composition analysis of CP genomes were performed using MEGA6 [43]. NCBI accession numbers of CP genome sequences are KY407562 and KY407561 for A. pseudolaeve and A. longecassidatum, respectively.
3.3. SSR, Tandem, and Palindromic Repeat Analysis in Two Aconitum CP Genomes
Tandem repeats were ≥20 bp with minimum alignment score and maximum period size set at 50 and 500, respectively, and identity of repeats was set at ≥90% [44]. SSRs were detected using MISA [45] with the minimum repeat numbers set to 10, 5, 4, 3, 3 and 3 for mono-, di- tri- tetra-, penta-, and hexanucleotides, respectively. IRs were detected using the Inverted Repeats Finder [46] with default parameters. IRs were required to be ≥20 bp in length with 90% similarity.
3.4. Comparative Analysis of CP Genomes of A. pseudolaeve and A. longecassidatum
The mVISTA program [47] was used to compare the CP genomes of Aconitum barbatum var. puberulum (KC844054), A. pseudolaeve, and A. longecassidatum. To calculate nucleotide variability (Pi) between CP genomes, we performed sliding-window analysis using DnaSP version 5.1 [48] with a window length of 600 bp and step size of 200 bp. Indels and SNPs were analyzed based on sequence alignments using MAFFT [49].
3.5. Development and Validation of Indel Markers (AcoTT and AcoYN) Among Aconitum Species
We selected indel regions based on mVISTA similarities and designed primers using Primer-BLAST (NCBI). Indel regions were amplified from 20 ng of genomic DNA in a 20-µL PCR mixture (SolgTM 2X Taq PCR smart mix 1, Solgent, Daegeon, Korea) with 10 pmol of each primer (Bioneer, Daejeon, Korea). Amplification was performed on a Pro Flex PCR system (Applied Biosystems, Waltham, MA, USA) according to the following program: (1) AcoTT primer: initial denaturation at 95 °C for 2 min; 35 cycles at 95 °C for 1 min, 61 °C for 1 min, and 72 °C for 1.5 min; and final extension at 72 °C for 5 min; and (2) AcoYN primer: initial denaturation at 95 °C for 2 min; 35 cycles at 95 °C for 50 s, 60 °C for 50 s, and 72 °C for 50 s; and final extension at 72 °C for 5 min. PCR products were separated on 2% agarose gels at 150 V for 40 min. To validate the specificity of indel markers and confirm the variability of indel regions between Aconitum species and subgenera Aconitum and Lycoctonum, we checked PCR amplification profiles using 27 additional samples from nine species and one variety of Aconitum consisting of both Aconitum subgenus Aconitum and Lycoctonum, which were provided from the KIOM herbarium. In addition, to confirm that the sizes of the PCR products were accurate, two samples per species were sequenced. Each PCR product was rescued from the agarose gel, subcloned into the pGEM-T Easy vector (Promega, Madison, WI, USA), and sequenced on a DNA sequence analyzer (ABI 3730, Applied Biosystems Inc., Foster City, CA, USA) to estimate sizes and verify the sequences of amplicons.
Acknowledgments
This work was supported by a grant of the Development of Foundational Techniques for the Domestic Production of Authentic Herbal Medicines, based on the Establishment of Molecular Authentication System (K17403) from the Korea Institute of Oriental Medicine (KIOM). The grant was funded by the Ministry of Science, ICT, and Future Planning (MSIP) of the Republic of Korea.
Abbreviations
| CP | Chloroplast |
| LSC | Large single copy |
| SSC | Small single copy |
| IR | Inverted repeat |
| tRNA | Transfer RNA |
| rRNA | Ribosomal RNA |
| KIOM | Korea Institute of Oriental Medicine |
| SSRs | Simple Sequence Repeats |
| SNPs | Single-Nucleotide Polymorphisms |
| Indel | Insertion and Deletion |
Supplementary Materials
Supplementary Materials are available online.
Author Contributions
I.P. designed the experiment framework and drafted and revised the manuscript. S.Y., G.C. and B.C.M. collected and identified plant materials. G.C. provided Chinese medicine information. W.J.K. performed experiments. B.C.M. revised the manuscript. All authors contributed to the experiments and approved the final manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Samples of the A. pseudolaeve, A. longecassidatum, A. voluvile var. pubescens, A. jaluense var. triphyllum, A. kusnezoffii, A. jaluense, A. austrokoreense and A. chiisanense are available from the authors and the herbarium of KIOM.
References
- 1.Wicke S., Schneeweiss G.M., dePamphilis C.W., Muller K.F., Quandt D. The evolution of the plastid chromosome in land plants: Gene content, gene order, gene function. Plant Mol. Biol. 2011;76:273–297. doi: 10.1007/s11103-011-9762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi K.S., Park S. The complete chloroplast genome sequence of Aster spathulifolius (Asteraceae); genomic features and relationship with Asteraceae. Gene. 2015;572:214–221. doi: 10.1016/j.gene.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Daniell H., Lin C.S., Yu M., Chang W.J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016;17:134. doi: 10.1186/s13059-016-1004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson D.A., Karsch-Mizrachi I., Lipman D.J., Ostell J., Rapp B.A., Wheeler D.L. Genbank. Nucleic Acids Res. 2000;28:15–18. doi: 10.1093/nar/28.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Provan J., Powell W., Hollingsworth P.M. Chloroplast microsatellites: New tools for studies in plant ecology and evolution. Trends Ecol. Evol. 2001;16:142–147. doi: 10.1016/S0169-5347(00)02097-8. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y., Li X., Yang Z., Yang C., Yang J., Ji Y. Analysis of complete chloroplast genome sequences improves phylogenetic resolution in paris (Melanthiaceae) Front. Plant Sci. 2016;7:1797. doi: 10.3389/fpls.2016.01797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park I., Kim W.J., Yang S., Yeo S.M., Li H., Moon B.C. The complete chloroplast genome sequence of Aconitum coreanum and Aconitum carmichaelii and comparative analysis with other Aconitum species. PLoS ONE. 2017;12:e0184257. doi: 10.1371/journal.pone.0184257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park I., Kim W.J., Yeo S.M., Choi G., Kang Y.M., Piao R., Moon B.C. The complete chloroplast genome sequences of Fritillaria ussuriensis maxim. and Fritillaria cirrhosa D. Don, and comparative analysis with other Fritillaria species. Molecules. 2017;22 doi: 10.3390/molecules22060982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song Y., Dong W., Liu B., Xu C., Yao X., Gao J., Corlett R.T. Comparative analysis of complete chloroplast genome sequences of two tropical trees Machilus yunnanensis and Machilus balansae in the family Lauraceae. Front. Plant Sci. 2015;6:662. doi: 10.3389/fpls.2015.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang H., Shi C., Liu Y., Mao S.Y., Gao L.Z. Thirteen Camellia chloroplast genome sequences determined by high-throughput sequencing: Genome structure and phylogenetic relationships. BMC Evol. Biol. 2014;14:151. doi: 10.1186/1471-2148-14-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bielsa B., Jiwan D., i Marti A.F., Dhingra A., Rubio-Cabetas M. Detection of SNP and validation of a SFP indel (deletion) in inverted repeat region of the prunus species chloroplast genome. Sci. Hortic. 2014;168:108–112. doi: 10.1016/j.scienta.2014.01.028. [DOI] [Google Scholar]
- 12.Cai Z., Guisinger M., Kim H.-G., Ruck E., Blazier J.C., McMurtry V., Kuehl J.V., Boore J., Jansen R.K. Extensive reorganization of the plastid genome of Trifolium subterraneum (Fabaceae) is associated with numerous repeated sequences and novel DNA insertions. J. Mol. Evol. 2008;67:696–704. doi: 10.1007/s00239-008-9180-7. [DOI] [PubMed] [Google Scholar]
- 13.Yin P., Kang J., He F., Qu L.-J., Gu H. The origin of populations of Arabidopsis thaliana in china, based on the chloroplast DNA sequences. BMC Plant Biol. 2010;10:22. doi: 10.1186/1471-2229-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K., Lee S.C., Lee J., Lee H.O., Joh H.J., Kim N.H., Park H.S., Yang T.J. Comprehensive survey of genetic diversity in chloroplast genomes and 45 s nrDNAs within Panax ginseng species. PLoS ONE. 2015;10:e0117159. doi: 10.1371/journal.pone.0117159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho K.S., Yun B.K., Yoon Y.H., Hong S.Y., Mekapogu M., Kim K.H., Yang T.J. Complete chloroplast genome sequence of tartary buckwheat (Fagopyrum tataricum) and comparative analysis with common buckwheat (F. esculentum) PLoS ONE. 2015;10:e0125332. doi: 10.1371/journal.pone.0125332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho K.S., Cheon K.S., Hong S.Y., Cho J.H., Im J.S., Mekapogu M., Yu Y.S., Park T.H. Complete chloroplast genome sequences of Solanum commersonii and its application to chloroplast genotype in somatic hybrids with Solanum tuberosum. Plant Cell Rep. 2016;35:2113–2123. doi: 10.1007/s00299-016-2022-y. [DOI] [PubMed] [Google Scholar]
- 17.Wang W., Fu D., Li L.Q., Bartholomew B., Brach A.R., Dutton B.E., Gilbert M.G., Kadota Y., Robinson O.R., Tamura M., et al. Ranunculaceae. In: Wu Z.Y., Raven P.H., editors. Flora of China. Volume 6. Science Press; Beijing, China: Missouri Botanical Garden Press; St. Louis, MO, USA: 2001. pp. 149–222. [Google Scholar]
- 18.National Institute of Biological Resources (NIBR) Endemic Species of Korea. [(accessed on 15 May 2017)];2013 Available online: https://species.nibr.go.kr/index.do.
- 19.Floral of Korea Editorial Committee (FKEC) The Genera of Vascular Plants of Korea. Academy Publishing Co.; Seoul, Korea: 2007. pp. 176–182. [Google Scholar]
- 20.Korea Institute of Oriental Medicine (KIOM) Defining Dictionary for Medicinal Herbs. [(accessed on 15 May 2017)];2016 Available online: http://boncho.kiom.re.kr/codex/
- 21.Lee S.-Y., Kwon O.-J., Park J.-H., Lee J.-Y. Anti-aging and anti-diabetes effects of Aconitum pesudo-laeve var. Erectum extracts. J. Life Sci. 2013;23:616–621. [Google Scholar]
- 22.Lee J.-Y., Lee S.Y., Jeoun H., Youn J.Y. Cosmetic Composition Comprising the Extract of Aconitum pseudolaeve var. erectum Nakai as Active Ingredient. No. KR20130040413A. Patent. 2013 Apr 24;
- 23.Lee J.-Y., Choi Y.-H., Lee S.-M., Lee S.-Y., Jun H.-J. Physiological activity of Aconitum pseudolaeve var. erectum Nakai and its effect on perfume on an electroencephalogram (EEG) J. Life Sci. 2012;22:1214–1223. doi: 10.5352/JLS.2012.22.9.1214. [DOI] [Google Scholar]
- 24.Qian J., Song J., Gao H., Zhu Y., Xu J., Pang X., Yao H., Sun C., Li X., Li C., et al. The complete chloroplast genome sequence of the medicinal plant Salvia miltiorrhiza. PLoS ONE. 2013;8:e57607. doi: 10.1371/journal.pone.0057607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang M., Zhang X., Liu G., Yin Y., Chen K., Yun Q., Zhao D., Al-Mssallem I.S., Yu J. The complete chloroplast genome sequence of date palm (Phoenix dactylifera l.) PLoS ONE. 2010;5:e12762. doi: 10.1371/journal.pone.0012762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Zhan D.F., Jia X., Mei W.L., Dai H.F., Chen X.T., Peng S.Q. Complete chloroplast genome sequence of Aquilaria sinensis (Lour.) Gilg and evolution analysis within the Malvales order. Front. Plant Sci. 2016;7:280. doi: 10.3389/fpls.2016.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X., Li Q., Li Y., Qian J., Han J. Chloroplast genome of Aconitum barbatum var. puberulum (Ranunculaceae) derived from CCS reads using the pacbio RS platform. Front. Plant Sci. 2015;6:42. doi: 10.3389/fpls.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuo L.H., Shang A.Q., Zhang S., Yu X.Y., Ren Y.C., Yang M.S., Wang J.M. The first complete chloroplast genome sequences of Ulmus species by de novo sequencing: Genome comparative and taxonomic position analysis. PLoS ONE. 2017;12:e0171264. doi: 10.1371/journal.pone.0171264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim C.E., Kim G.B., Baek S., Han S.M., Yu H.J., Mun J.H. The complete chloroplast genome of Aconitum chiisanense Nakai (Ranunculaceae) Mitochondrial DNA. 2015:1–2. doi: 10.3109/19401736.2015.1110805. [DOI] [PubMed] [Google Scholar]
- 30.Echt C.S., DeVerno L., Anzidei M., Vendramin G. Chloroplast microsatellites reveal population genetic diversity in red pine, Pinus resinosa Ait. Mol. Ecol. 1998;7:307–316. doi: 10.1046/j.1365-294X.1998.00350.x. [DOI] [Google Scholar]
- 31.Nishikawa T., Vaughan D.A., Kadowaki K.-I. Phylogenetic analysis of Oryza species, based on simple sequence repeats and their flanking nucleotide sequences from the mitochondrial and chloroplast genomes. Theor. Appl. Genet. 2005;110:696–705. doi: 10.1007/s00122-004-1895-2. [DOI] [PubMed] [Google Scholar]
- 32.Nie X., Lv S., Zhang Y., Du X., Wang L., Biradar S.S., Tan X., Wan F., Weining S. Complete chloroplast genome sequence of a major invasive species, crofton weed (Ageratina adenophora) PLoS ONE. 2012;7:e36869. doi: 10.1371/journal.pone.0036869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho K.-S., Hong S.-Y., Yun B.-K., Won H.-S., Yoon Y.-H., Kwon K.-B., Mekapogu M. Application of indel markers based on the chloroplast genome sequences for authentication and traceability of tartary and common buckwheat. Czech J. Food Sci. 2017;35:122–130. [Google Scholar]
- 34.Tang J., Xia H.A., Cao M., Zhang X., Zeng W., Hu S., Tong W., Wang J., Wang J., Yu J. A comparison of rice chloroplast genomes. Plant Physiol. 2004;135:412–420. doi: 10.1104/pp.103.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suo Z., Zhang C., Zheng Y., He L., Jin X., Hou B., Li J. Revealing genetic diversity of tree peonies at micro-evolution level with hyper-variable chloroplast markers and floral traits. Plant Cell Rep. 2012;31:2199–2213. doi: 10.1007/s00299-012-1330-0. [DOI] [PubMed] [Google Scholar]
- 36.Dong W., Xu C., Cheng T., Lin K., Zhou S. Sequencing angiosperm plastid genomes made easy: A complete set of universal primers and a case study on the phylogeny of saxifragales. Genome Biol. Evol. 2013;5:989–997. doi: 10.1093/gbe/evt063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong W., Xu C., Li D., Jin X., Li R., Lu Q., Suo Z. Comparative analysis of the complete chloroplast genome sequences in Psammophytic haloxylon species (Amaranthaceae) PeerJ. 2016;4:e2699. doi: 10.7717/peerj.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redwan R.M., Saidin A., Kumar S.V. Complete chloroplast genome sequence of MD-2 pineapple and its comparative analysis among nine other plants from the subclass Commelinidae. BMC Plant Biol. 2015;15:196. doi: 10.1186/s12870-015-0587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suo Z., Jia Z., Lu Q., Pan B., Jin X., Xu G., Peng X., Sun H., Tao Y. Distinguishing Haloxylon persicum and H. Ammodendron (Haloxylon Bunge, Amaranthaceae) using DNA marker. AASRI Procedia. 2012;1:305–310. doi: 10.1016/j.aasri.2012.06.047. [DOI] [Google Scholar]
- 40.Delcher A.L., Salzberg S.L., Phillippy A.M. Using mummer to identify similar regions in large sequence sets. Curr. Protoc. Bioinform. :2003. doi: 10.1002/0471250953.bi1003s00. [DOI] [PubMed] [Google Scholar]
- 41.Wyman S.K., Jansen R.K., Boore J.L. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004;20:3252–3255. doi: 10.1093/bioinformatics/bth352. [DOI] [PubMed] [Google Scholar]
- 42.Lohse M., Drechsel O., Bock R. OrganellarGenomeDRAW (OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2007;52:267–274. doi: 10.1007/s00294-007-0161-y. [DOI] [PubMed] [Google Scholar]
- 43.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. Mega6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benson G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999;27:573–580. doi: 10.1093/nar/27.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thiel T. Misa—Microsatellite Identification Tool. [(accessed on 10 June 2017)];2003 Available online: http://pgrc.ipk-gatersleben.de/misa/
- 46.Warburton P.E., Giordano J., Cheung F., Gelfand Y., Benson G. Inverted repeat structure of the human genome: The X-chromosome contains a preponderance of large, highly homologous inverted repeats that contain testes genes. Genome Res. 2004;14:1861–1869. doi: 10.1101/gr.2542904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frazer K.A., Pachter L., Poliakov A., Rubin E.M., Dubchak I. Vista: Computational tools for comparative genomics. Nucleic Acids Res. 2004;32:W273–W279. doi: 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Librado P., Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 49.Katoh K., Misawa K., Kuma K., Miyata T. Mafft: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.