Abstract
OBJECTIVE
Type 1 diabetes has been associated with high rates of urinary and sexual problems, but the cumulative burden and overlap of these complications are unknown. We sought to determine prevalence of urological complications in persons with type 1 diabetes, associations with clinical and diabetes-related factors, and rates of emergence, persistence, and remission.
RESEARCH DESIGN AND METHODS
This ancillary longitudinal study among participants in the Diabetes Control and Complications Trial (DCCT) and observational follow-up study Epidemiology of Diabetes Interventions and Complications (EDIC) (652 women and 713 men) was conducted in 2003 and 2010/2011. Urinary incontinence (UI), lower urinary tract symptoms, urinary tract infection, female sexual dysfunction, erectile dysfunction, low male sexual desire, and orgasmic dysfunction were measured with validated instruments. Logistic regression determined association of complications with demographics and clinical characteristics.
RESULTS
Of sexually active women completing the 2010/2011 survey, 35% reported no complications, 39% had one, 19% two, 5% three, and 2% four. In men, 31% had no complications, 36% had one, 22% two, 9% three, and 3% four. Sexual dysfunction was most prevalent (42% women and 45% men) followed by UI in women (31%) and low sexual desire in men (40%). Urological complications were associated with age, BMI, and HbA1c. Remission rates ranged from 4 to 12% over the 7-year interval between surveys.
CONCLUSIONS
Urological complications are prevalent and frequently co-occur in persons with type 1 diabetes. Remission rates in a minority subset indicate a rationale for future studies to mitigate the onset or impact of urological complications of diabetes.
Introduction
Type 1 diabetes is a significant public health problem because of its early onset, progressive course, and high rate of complications including retinopathy, nephropathy, neuropathy, and cardiovascular disease (1). Less studied are urological conditions associated with diabetes such as sexual dysfunction, urinary tract infection (UTI), poor bladder emptying, urinary incontinence (UI), and other lower urinary tract symptoms (LUTS) that often initiate care seeking and negatively impact quality of life (2,3). Studies have shown that a number of these specific urological complications are associated with more severe and less controlled type 1 diabetes; however, reports are based on singular complications and whether they co-occur with each other is unknown (4–9).
For better characterization of the burden of individual and cumulative urological complications in persons with type 1 diabetes, the Urologic EDIC, or UroEDIC, study was established as an ancillary study to the Epidemiology of Diabetes Interventions and Complications (EDIC) study, the observational follow-up to the Diabetes Control and Complications Trial (DCCT). The UroEDIC protocol included a series of well-validated urological symptom questionnaires self-administered to women and men in one of the largest and longest-running clinical studies of type 1 diabetes. We describe findings from two surveys obtained 7 years apart to document the prevalence and co-occurrence of sexual dysfunction, UI, LUTS, and UTI, as well as their rates of emergence, persistence, and remission. In addition, we identify associations between these urological complications and demographic and clinical diabetes-related variables. Findings from the UroEDIC ancillary study may provide accurate information to health care providers, patients, and families about traditionally embarrassing urological health problems and could be used to develop risk factor models for specific urological complications among persons with type 1 diabetes.
Research Design and Methods
A total of 1,441 subjects were recruited into the DCCT and randomized to two interventions: intensive insulin therapy or conventional therapy. Intensive therapy was aimed at achieving normal glycemic levels using three or more daily insulin injections or continuous subcutaneous insulin infusion with dose adjustments based on frequent self-monitoring of blood glucose, while conventional therapy was aimed at maintaining clinical well-being with one to two daily insulin injections with no specified glucose targets (10,11). Two parallel cohorts were recruited: a primary prevention cohort (with 1–5 years’ diabetes duration, no retinopathy, and urine albumin excretion rate [AER] of <40 mg/24 h) and a secondary intervention cohort (1–15 years’ duration, mild to moderate nonproliferative diabetic retinopathy, and AER ≤200 mg/24 h). After a mean follow-up of 6.5 years, the DCCT ended early in 1993 when the principal study question concerning treatment effects was answered. Intensive insulin treatment was found to dramatically delay the onset and slow the progression of retinopathy, nephropathy, and neuropathy by 35 to >70% (10). In 1994, 96% of the original DCCT cohort agreed to participate in EDIC, which includes annual examinations for complication status (11). The mean age of the participants at EDIC baseline was 33.6 years, with a mean duration of diabetes of 12.2 years.
All men and women enrolled in EDIC were invited to participate in UroEDIC at EDIC year 10 (2003 [UroEDIC I]) and again at EDIC year 17/18 (2010/2011 [UroEDIC II]) irrespective of participation at year 10. There was a net increase in participation from UroEDIC I to UroEDIC II. Consenting participants completed a confidential survey assessing sexual and urinary function, symptom impact, and history of UTI. The Human Subjects Division of the University of Washington approved a central study protocol, and the institutional review board of each participating clinical center approved the UroEDIC I and II protocols.
Measures of Urological Complications
Primary outcome variables were designated a priori for each urological complication based on established cut points for items derived from widely used, well-validated, and reliable instruments as outlined below.
Male Sexual Dysfunction
Erectile function, sexual desire, and orgasmic function were assessed by responses to items selected from the International Index of Erectile Function (12). Erectile function was dichotomized using a single-item proxy: “Over the past 4 weeks, how would you rate your confidence that you get and keep your erection?” If the participant answered “very low” (1) or “low” (2), they were considered to have erectile dysfunction (ED), and if “moderate” (3), “high” (4), or “very high” (5) they were considered to have no ED. This definition had a strong correlation with total erectile function domain scores and degree of bother related to ED (7). In addition, men reporting sildenafil citrate in the annual survey after EDIC year 10 or reporting any oral phosphodiesterase type 5 inhibitor (sildenafil, vardenafil, tadalafil) or other treatment (urethral suppositories, penile injections or implants, vacuum erection devices) at year 17/18 were considered to have ED. Low sexual desire (LD) and orgasmic dysfunction (OD) were defined as a score <7 on the respective 10-point Desire and Orgasm domains of the International Index of Erectile Function (12).
Female Sexual Dysfunction
Sexual function in women was assessed by means of an abbreviated version of the Female Sexual Function Index (FSFI-R), which included 7 of the 19 original FSFI items and scored as a total sum of all the items representing each domain of sexual functioning as well as the mean score of two items assessing satisfaction. The primary outcome variable defines the presence of female sexual dysfunction (FSD) as a score equal to or above the cutoff value of 22.75 on the total scale score. This has been shown to be equivalent to the full-scale measure (9). As with the full survey instrument, only sexually active women are included in the analyses.
UI
UI in women was assessed based on frequency and amount of urine lost per episode (drops, small splashes, more) using the Sandvik Severity Index (13). Calculated from frequency and amount of urine loss on a scale of 1–12 (mild 1–2, moderate 3–6, severe 8–9, very severe 10–12), the primary outcome was moderate (≥3) or more severe UI.
LUTS
LUTS, including nocturia, frequency, urgency, weak urinary stream, intermittency, straining, and the sensation of incomplete emptying, were assessed in men and women using the American Urological Association Symptom Index (14). Following widely accepted cut points of 0–7, 8–19, and 20–35 designated as none/mild, moderate, and severe LUTS, respectively, we dichotomized responses into no LUTS (0–7) versus LUTS (8–35).
UTI
UTI was assessed with questions asking “How many times in the last 12 months were you diagnosed with a bladder infection?” and, separately, “a kidney infection?” For the purpose of analysis, an answer of zero was considered a negative response, while an answer of one or more bladder or kidney infection(s) was considered a positive response.
Measures of Secondary Factors
Glycemic Control and Microvascular Complications
HbA1c was measured at baseline and quarterly during DCCT and annually in EDIC (11). The DCCT/EDIC time-weighted arithmetic mean HbA1c was calculated using quarterly DCCT and annual EDIC values weighted by 3 and 12 months, respectively (11,15). A time-weighted BMI was calculated in the same manner. Peripheral neuropathy was defined during EDIC by more than six positive responses on the Michigan Neuropathy Screening Instrument (MNSI) or a score >2 on the MNSI examination (16). Autonomic neuropathy was defined as follows: either R-R variation <15 or R-R variation between 15 and 19.9 plus either a Valsalva ratio ≤1.5 or a supine-to-standing drop in diastolic blood pressure of 10 mmHg. Retinopathy was assessed using fundus photographs that were centrally graded using the Early Treatment Diabetic Retinopathy Study (ETDRS) scale (17). AER was measured in half of the cohort annually during EDIC. Nephropathy was defined as microalbuminuria (AER 40–300 mg/24 h) or albuminuria (AER >300 mg/24 h).
Statistical Methods
Prevalence of urological complications was calculated at EDIC years 10 (UroEDIC I) and 17/18 (UroEDIC II). Emergence, persistence, and remission of urological complications were estimated as follows: emergence was defined as subjects being free of a complication at UroEDIC I but positive at UroEDIC II, persistence was defined as subjects having a complication at UroEDIC I and II, and remission was defined as subjects being positive at UroEDIC I but free of a complication at UroEDIC II. The classification of positive and negative for each complication was based on symptom scores crossing predefined thresholds. Logistic regression models were used to examine the associations between binary urological complication classifications and sociodemographic and diabetes-related characteristics. Associations were estimated using odds ratios and their respective 95% CIs. All analyses were performed using SAS, version 8.2 (SAS Institute, Cary, NC).
Results
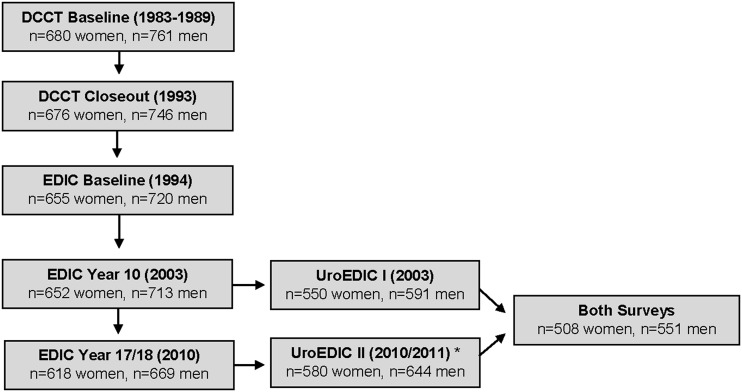
Participation rates of active EDIC participants in UroEDIC I and UroEDIC II surveys were 83.6 and 95.1%, respectively. Of the 1,141 participants who completed UroEDIC I, 1,059 (92.8%) participated in UroEDIC II. The number of study participants in DCCT, EDIC, and UroEDIC is shown in Fig. 1. Among UroEDIC study participants who completed either survey, specific item completion rates ranged from 92 to 99%, depending on complication type.
Figure 1.
Study cohort diagram. Flow of participants through the study from DCCT to EDIC and UroEDIC I and II. There were a greater number of participants (1,224) in UroEDIC II compared with UroEDIC I (1,141). *Enrollees were asked to take part in UroEDIC II irrespective of participation in UroEDIC I. There was a net increase in participation from UroEDIC I to UroEDIC II.
A total of 508 women and 551 men completed surveys at both time points of the study. The prevalence and subsequent emergence, persistence, and remission of urological complications over the 7-year period were assessed (Table 1). The majority of participants with a urological complication at UroEDIC I had persistence of that same complication at UroEDIC II (range of 56–86%) with the exception of UTI in females, where only 29% of those reporting the complication at the first survey reported a bladder or kidney infection during the second survey. At UroEDIC II, the proportion of those reporting specific complications that were not present at year 10 ranged from 42 to 63% with the exception of UTI in women, where 73% reported the complication for the first time. In men, age was associated with emergence of LUTS (<0.01) and persistence of all four complications: LUTS (<0.001), ED (<0.0001), LD (<0.01), and OD (0.0063). HbA1c was associated with persistence of ED (<0.0001) and persistence of OD (<0.001). In women, age was associated with emergence of FSD (0.03) and persistence of FSD (<0.001) and UI (0.03). HbA1c was associated with emergence of LUTS (0.03) and persistence of UI (0.05). Treatment group was not associated with emergence or persistence of complications (data not shown).
Table 1.
Prevalence and change in urological complication status in women and men who completed both UroEDIC I and II surveys at EDIC years 10 and 17/18
| Respondents within entire cohort | Prevalence in UroEDIC I* | Prevalence in UroEDIC II* | Emergence at follow-up | Persistence at follow-up | Remission at follow-up | |
|---|---|---|---|---|---|---|
| Women | 508 | |||||
| LUTS | 506 (99) | 101 (20) | 113 (22) | 52 (10) | 61 (12) | 40 (8) |
| FSD† | 297 (58) | 98 (33) | 125 (42) | 53 (18) | 72 (24) | 26 (9) |
| UI | 493 (97) | 109 (22) | 151 (31) | 81 (16) | 70 (14) | 39 (8) |
| UTI | 468 (92) | 75 (16) | 80 (17) | 58 (12) | 22 (5) | 53 (11) |
| Men | 551 | |||||
| LUTS | 550 (99) | 100 (18) | 132 (24) | 70 (13) | 62 (11) | 38 (7) |
| ED‡ | 525 (95) | 129 (25) | 238 (45) | 126 (24) | 112 (21) | 17 (3) |
| LD | 506 (92) | 182 (36) | 203 (40) | 94 (19) | 109 (22) | 73 (14) |
| OD | 461 (84) | 41 (9) | 63 (14) | 40 (9) | 23 (5) | 18 (4) |
Data are N or N (% of respondents). Emergence defined as subjects being free of disorder at UroEDIC I but positive at UroEDIC II. Persistence defined as subjects being positive at UroEDIC I and positive at UroEDIC II. Remission defined as subjects being positive at UroEDIC I but free of disorder at UroEDIC II.
*UroEDIC I completed at EDIC year 10 and UroEDIC II completed at EDIC year 17/18.
†FSD only assessed in sexually active women per FSFI scoring algorithm.
‡ED at UroEDIC I includes men who were using sildenafil citrate based on self-report. ED at UroEDIC II includes men using any oral medications, urethral suppositories, intracavernosal injections, penile implants, or vacuum erection devices.
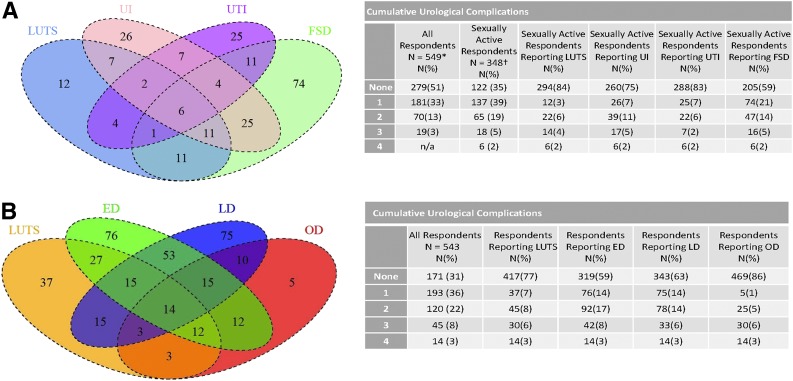
Overall, 65% of women and 68% of men reported at least one urological complication at UroEDIC II. The prevalence of the urological complications at UroEDIC II for women was LUTS 22%, FSD 42%, UI 31%, and UTI 17% and for men LUTS 24%, ED 45%, LD 40%, and OD 14%. The overlap and cumulative total of prevalent urological complications for respondents who had data for all urological complications at UroEDIC II are shown in Fig. 2A (women) and Fig. 2B (men). Of note, because FSD was assessed only in sexually active women, the proportion of patients with overlap between FSD and other urological complications was calculated based on the smaller denominator of sexually active women. Overall, in sexually active women, 35% reported no complications, 39% had one, and 26% had two or more other complications. The pattern of overlap in women differed across the specific urological complications: more commonly LUTS co-occurred with other complications and FSD occurred in isolation. In men, 31% had no complications, 36% had one, and 33% had two or more. Similarly, the pattern of overlap in men was specific to each complication, with LUTS occurring more commonly in isolation of other complications, while ED, LD, and OD co-occurred.
Figure 2.
A: Prevalence of urological complications in women at UroEDIC II. B: Prevalence of urological complications in men at UroEDIC II. Overlap of number of participants with specific complications and cumulative complication occurrence among women (A) and men (B) who completed UroEDIC II. In A, only women who were sexually active could be scored with the FSFI, reducing total number of participants included in the figure. Overlap of other complications was similar for women who were not sexually active (data not shown). Size of diagrams not proportional to number of participants with each complication. *FSD excluded in complication count. †Respondents had data available for all four complications.
We analyzed the cohort by sex to identify demographic, glycemic control, and microvascular complication variables associated with urological complications at UroEDIC II. In women (Table 2), increasing age was positively associated with FSD and UI. Women in the highest quartile of HbA1c (>8.54%) were two times more likely to report LUTS compared with those in the lowest quartile of HbA1c (≤7.38%). Additionally, BMI ≥30 kg/m2 was associated with a higher odds of reporting UI. Women with nephropathy had a higher odds of reporting UTI, those with peripheral neuropathy had a higher odds of LUTS, and those with autonomic neuropathy had higher odds of FSD and UTI. In men (Table 3), increasing age was positively associated with LUTS, ED, and OD. Participants in higher HbA1c quartiles had a higher odds of reporting ED and OD. BMI was not observed to be an independent risk factor for male urological complications. Men with any measured diabetes-related microvascular complications had a higher odds of reporting LUTS, ED, and OD. Complication prevalence was not associated with DCCT intensive insulin therapy versus conventional therapy.
Table 2.
Unadjusted odds of urological complications in women at UroEDIC II by demographic, clinical, and diabetes-related complication categories
| LUTS | FSD | UI | UTI | ||
|---|---|---|---|---|---|
| N total respondents | 580 | 579 | 371 | 571 | 555 |
| N (%) with complication | 128 (22) | 153 (41) | 172 (30) | 95 (17) | |
| Age quartiles (years) | |||||
| 30–39 | 35 (6) | Reference | Reference | Reference | Reference |
| 40–49 | 230 (40) | 1.95 (0.66–5.80) | 2.53 (0.92–6.98) | 4.67 (1.38–15.78) | 0.77 (0.31–1.92) |
| 50–59 | 238 (41) | 2.73 (0.93–8.05) | 4.27 (1.54–11.85) | 4.91 (1.46–16.56) | 0.63 (0.25–1.56) |
| 60–69 | 77 (13) | 2.03 (0.63–6.60) | 10.91 (3.30–36.08) | 4.48 (1.24–16.16) | 0.88 (0.32–2.41) |
| BMI (kg/m2) | |||||
| <25 | 153 (28) | Reference | Reference | Reference | Reference |
| 25–30 | 220 (40) | 1.08 (0.65–1.81) | 1.19 (0.71–1.99) | 1.26 (0.78–2.03) | 1.42 (0.81–2.50) |
| ≥30 | 177 (32) | 1.58 (0.94–2.66) | 0.91 (0.52–1.61) | 2.27 (1.40–3.68) | 1.00 (0.54–1.84) |
| Enrollment cohort | |||||
| Secondary intervention | 293 (51) | Reference | Reference | Reference | Reference |
| Primary prevention | 287 (49) | 1.10 (0.75–1.63) | 0.90 (0.59–1.36) | 0.83 (0.58–1.18) | 1.00 (0.64–1.55) |
| Treatment group | |||||
| Conventional | 303 (52) | Reference | Reference | Reference | Reference |
| Intensive | 277 (48) | 1.09 (0.73–1.61) | 1.22 (0.81–1.85) | 1.22 (0.85–1.75) | 0.79 (0.51–1.23) |
| HbA1c quartiles, % | |||||
| ≤7.38 | 138 (25) | Reference | Reference | Reference | Reference |
| >7.38–7.92 | 141 (25) | 1.71 (0.95–3.08) | 1.14 (0.63–2.06) | 1.00 (0.60–1.68) | 0.70 (0.34–1.45) |
| >7.92–8.54 | 138 (25) | 1.22 (0.66–2.25) | 1.09 (0.60–1.97) | 0.91 (0.54–1.53) | 1.52 (0.80–2.88) |
| >8.54 | 138 (25) | 2.06 (1.16–3.68) | 0.92 (0.50–1.68) | 1.15 (0.69–1.91) | 1.72 (0.91–3.25) |
| Retinopathy* | |||||
| No retinopathy | 51 (9) | Reference | Reference | Reference | Reference |
| Microaneurysms | 215 (37) | 0.89 (0.42–1.88) | 1.39 (0.65–2.99) | 1.26 (0.62–2.57) | 1.14 (0.47–2.75) |
| Mild/moderate NPDR | 216 (37) | 1.04 (0.50–2.18) | 1.42 (0.66–3.05) | 1.44 (0.71–2.94) | 1.24 (0.52–2.98) |
| PDR or worse | 98 (17) | 1.38 (0.62–3.08) | 2.23 (0.95–5.27) | 1.66 (0.77–3.60) | 1.24 (0.47–3.25) |
| Nephropathy (AER, mg/24 h)† | |||||
| None (<30) | 460 (84) | Reference | Reference | Reference | Reference |
| Microalbuminuria (30–300) | 79 (14) | 1.50 (0.87–2.58) | 0.68 (0.36–1.28) | 1.12 (0.67–1.89) | 2.08 (1.17–3.69) |
| Macroalbuminuria (≥300) | 11 (2) | 2.23 (0.64–7.76) | 1.05 (0.23–4.76) | 0.23 (0.03–1.85) | 2.86 (0.70–11.70) |
| Peripheral neuropathy‡ | |||||
| Normal MNSI | 324 (59) | Reference | Reference | Reference | Reference |
| Abnormal MNSI | 228 (41) | 1.80 (1.21–2.69) | 1.47 (0.94–2.29) | 1.00 (0.69–1.45) | 1.56 (0.99–2.46) |
| Autonomic neuropathy§ | |||||
| No | 333 (61) | Reference | Reference | Reference | Reference |
| Yes | 216 (39) | 1.28 (0.85–1.92) | 1.67 (1.07–2.60) | 1.28 (0.88–1.85) | 1.57 (1.00–2.47) |
Data are N (%) or unadjusted odds ratios (95% CI) unless otherwise indicated. Significant values are in boldface type. NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
*Retinopathy defined through EDIC year 14 using the ETDRS scale of 0–23.
†Nephropathy defined at EDIC year 15/16.
‡Peripheral neuropathy defined at EDIC year 17 by the MNSI: >6 responses on the questionnaire or a score of >2 on exam.
§Autonomic neuropathy defined at EDIC year 16/17 as an R-R variation <15 or R-R variation <20 in combination with a Valsalva ratio ≤1.5 or a decrease of >10 mmHg in diastolic blood pressure upon standing.
Table 3.
Unadjusted odds of urological complications in men at UroEDIC II by demographic, clinical, and diabetes-related complication categories
| LUTS | ED | LD | OD | ||
|---|---|---|---|---|---|
| N total respondents | 644 | 643 | 635 | 598 | 564 |
| N (%) with complication | 158 (25) | 290 (46) | 243 (41) | 83 (15) | |
| Age quartiles (years) | |||||
| 30–39 | 30 (5) | Reference | Reference | Reference | — |
| 40–49 | 201 (31) | 1.28 (0.42–3.92) | 4.30 (1.26–14.73) | 1.16 (0.50–2.71) | Reference |
| 50–59 | 336 (52) | 2.20 (0.75–6.49) | 8.31 (2.47–27.98) | 1.51 (0.66–3.44) | 1.52 (0.88–2.64) |
| 60–69 | 77 (12) | 5.71 (1.82–17.92) | 30.08 (8.11–111.61) | 2.46 (0.97–6.25) | 2.49 (1.17–5.28) |
| BMI (kg/m2) | |||||
| <25 | 132 (21) | Reference | Reference | Reference | Reference |
| 25–30 | 268 (43) | 0.81 (0.51–1.30) | 0.91 (0.60–1.39) | 0.58 (0.38–0.90) | 0.82 (0.43–1.55) |
| ≥30 | 228 (36) | 0.72 (0.44–1.18) | 1.35 (0.87–2.07) | 0.80 (0.51–1.25) | 1.12 (0.59–2.13) |
| Enrollment cohort | |||||
| Secondary intervention | 320 (50) | Reference | Reference | Reference | Reference |
| Primary prevention | 324 (50) | 1.01 (0.71–1.45) | 0.69 (0.50–0.94) | 0.96 (0.69–1.33) | 0.93 (0.58–1.28) |
| Treatment group | |||||
| Conventional | 320 (50) | Reference | Reference | Reference | Reference |
| Intensive | 324 (50) | 0.89 (0.62–1.28) | 0.95 (0.95–0.70) | 0.97 (0.70–1.34) | 0.91 (0.57–1.45) |
| HbA1c quartiles, % | |||||
| ≤7.25 | 157 (25) | Reference | Reference | Reference | Reference |
| >7.25–7.85 | 158 (25) | 0.99 (0.59–1.68) | 1.33 (0.84–2.10) | 1.06 (0.67–1.69) | 1.62 (0.79–3.34) |
| >7.85–8.58 | 160 (25) | 1.20 (0.72–2.00) | 1.60 (1.02–2.51) | 0.91 (0.57–1.45) | 1.39 (0.66–2.92) |
| >8.58 | 157 (25) | 1.08 (0.64–1.82) | 2.74 (1.73–4.35) | 1.10 (0.69–1.75) | 2.69 (1.35–5.35) |
| Retinopathy* | |||||
| No retinopathy | 43 (7) | Reference | Reference | Reference | Reference |
| Microaneurysms | 204 (32) | 0.53 (0.26–1.07) | 0.96 (0.49–1.90) | 0.67 (0.34–1.32) | 0.83 (0.29–2.36) |
| Mild/moderate NPDR | 267 (41) | 0.55 (0.28–1.10) | 1.66 (0.86–3.23) | 0.81 (0.42–1.59) | 1.13 (0.41–3.11) |
| PDR or worse | 130 (20) | 0.75 (0.36–1.56) | 2.11 (1.04–4.30) | 0.72 (0.35–1.49) | 1.39 (0.48–4.03) |
| Nephropathy (AER, mg/24 h)† | |||||
| None (<30) | 475 (77) | Reference | Reference | Reference | Reference |
| Microalbuminuria (30–300) | 104 (17) | 1.86 (1.17–2.97) | 2.83 (1.80–4.43) | 1.28 (0.81–2.04) | 2.44 (1.35–4.42) |
| Macroalbuminuria (≥300) | 40 (6) | 2.56 (1.31–5.00) | 1.63 (0.85–3.12) | 0.82 (0.41–1.65) | 3.50 (1.62–7.56) |
| Peripheral neuropathy‡ | |||||
| Normal MNSI | 360 (57) | Reference | Reference | Reference | Reference |
| Abnormal MNSI | 270 (43) | 1.97 (1.36–2.84) | 2.15 (1.55–2.97) | 1.21 (0.87–1.69) | 2.25 (1.39–3.64) |
| Autonomic neuropathy§ | |||||
| No | 391 (63) | Reference | Reference | Reference | Reference |
| Yes | 234 (37) | 2.07 (1.42–3.01) | 2.82 (2.01–3.94) | 1.28 (0.91–1.80) | 2.40 (1.49–3.88) |
Data are N (%) or unadjusted odds ratios (95% CI) unless otherwise indicated. Significant values are in boldface type. NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
*Retinopathy defined through EDIC year 14 using the ETDRS scale of 0–23.
†Nephropathy defined at EDIC year 15/16.
‡Peripheral neuropathy defined at EDIC year 17 by the MNSI: >6 responses on the questionnaire or a score of >2 on exam.
§Autonomic neuropathy defined at EDIC year 16/17 as an R-R variation <15 or R-R variation <20 in combination with a Valsalva ratio ≤1.5 or a decrease of >10 mmHg in diastolic blood pressure upon standing.
Conclusions
Urological morbidity associated with diabetes results from metabolic effects on genitourinary tissues and the neural, vascular, and hormonal input to these organs. With a reported 65.5% prevalence of at least one urological complication, these complications pose a significant burden on this cohort of middle-aged patients with type 1 diabetes compared with population-based surveys with considerably lower reported prevalence (18–21). In addition, observed urological complications co-occurred significantly more than would be expected were these independent complications (e.g., tests for concordance of independence [data not shown]). Presumably, the overlap of symptom complexes reflects shared mechanisms, conditioning of responses across complications, or other factors. The impact of both singular and co-occurring sexual and urinary dysfunctions on quality of life, health perception, and depression, although beyond the scope of this report, has been shown to be negatively associated independent of diabetes complications and similar in magnitude to the impact of hypertension or diabetes itself (22). Thus, as improvements in diabetes management reduce the severity of retinopathy, nephropathy, and neuropathy, urological complications may become important drivers of quality of life (23,24).
Findings in Women
Prevalence of FSD in this cohort is similar to that in women in the Boston Area Community Health (BACH) survey (41 and 36%, respectively) (25). FSD was strongly associated with age but not glycemic control or diabetes complications, confirming earlier findings in this and other cohorts (9). Reporting of FSD as a single complication was more common compared with the other urological complications, supporting a separate etiological mechanism from UI, LUTS, and UTI. Prior publications including findings from UroEDIC I show depression to be the major factor associated with FSD (9).
The second most prevalent urological complication reported in women participants of UroEDIC was UI, which can lead to significant distress, social isolation, and poorer quality of life (25,26). In the current study, 31% of women reported weekly or greater UI. The comparable rates in women in their forties are 15% in BACH and 17% in the National Health and Nutrition Examination Survey (NHANES) (26,27). For LUTS, differences were less striking (22% in UroEDIC vs. 17% in BACH) (22). The association of UI with BMI replicates findings in populations without diabetes and women with type 2 diabetes (28–30). Further studies in the UroEDIC cohort offer the opportunity to investigate the effects of aging, glycemia, and other diabetes complications such as peripheral or autonomic neuropathy on the onset of UI as this population ages. Of note, a third of women with UI also reported LUTS (Fig. 2)—a subset that requires further investigation.
UTI prevalence did not differ substantially from previously reported population-based estimates such as NHANES (5). It is notable that women with autonomic neuropathy exhibited increased odds of UTI, which could reflect poor bladder emptying due to diabetic cystopathy. In a recent more detailed analysis, we demonstrated an association between UTI and higher HbA1c levels (31). Forty percent of the participants reporting UTI also reported either UI or LUTS, suggesting potential overlap in etiological mechanisms for these complications.
Findings in Men
Urological complications were highly prevalent in the male participants, with 62% of men having at least one complication. ED was the most common and demonstrated predictable associations with age, poorer diabetes control, and other diabetes complications including autonomic neuropathy. The proportion of men reporting ED was much higher than in a comparable group in NHANES (e.g., 42% in UroEDIC II vs. 8% in the NHANES 40- to 50-year-old age-group) (32). LD was highly prevalent as well, despite only 9.5% of the cohort having low serum testosterone levels at UroEDIC II (data not shown). Similar to FSD, LD has a higher rate of men reporting the one complication alone and may have etiological pathways, such as depression, that are independent of metabolic, neural, and vascular mechanisms. Disorders of orgasm, including retrograde ejaculation and anejaculation, which may be signals of impaired autonomic innervation of the bladder neck or ejaculatory system, overlapped closely with ED. A potential subcohort for future investigation of mechanisms could be the two-thirds of the men reporting ED who also reported LD or OD.
One quarter of men reported moderate to severe LUTS. LUTS in males with diabetes may be partly attributed to benign prostatic hyperplasia, other related comorbidities, and relevant demographics (33,34). Incident cases identified in UroEDIC II offer the potential for investigations of the interaction between benign prostatic enlargement, diabetes control, and other diabetes complications.
Remission of Urological Complications
Few longitudinal studies of urological complication temporal patterns exist in persons with diabetes and none in women. A notable finding of the current study is remission rates in all urological complications. For incontinent episodes specifically, a small percentage of women showed reduction in occurrence between EDIC years 10 and 17. Improvements in UI were seen in a small randomized controlled trial of weight loss and exercise in obese women (35), and weight loss was the factor most highly associated with reductions in incident or prevalent UI in the Look AHEAD (Action for Health in Diabetes) and Diabetes Prevention Program (DPP) cohorts (29,35–38). Remission of ED in the current study mirrors what has been reported in some population-based studies despite the fact that ED has traditionally been considered an irreversible consequence of prolonged hyperglycemia in males with diabetes (39,40). LD had the highest rate of remission, reinforcing the notion that a separate mechanistic pathway may exist. Identifying factors that predict improvement in urological complications in men and women with type 1 diabetes is an important future goal for UroEDIC.
Additional analyses (data not shown) indicate that some patients had varying degrees of change in urological and sexual function complications without crossing predefined thresholds for a specific complication. Thus, some level of remission may not be accounted for in these analyses. The study was also limited by use of questionnaires to define subjective complications, missing data owing to nonparticipation, and use of unvalidated UTI questions. We were not able to assess impact of treatment for these complications on persistence and emergence. Future work is needed to determine whether the magnitudes of change are associated with increased symptom impact on quality of life. Lastly, the UroEDIC cohort represents a motivated and highly selected group of individuals; thus, findings may not be generalizable to the whole population.
Conclusion
Urological complications are prevalent and frequently coexist in men and women with type 1 diabetes. The majority of participants in the UroEDIC study demonstrate persistence of urological complications over a 7-year period, although remission in a subset offers the potential for future studies to investigate mechanisms of symptom improvement. As people with diabetes live longer and avoid other diabetes-related complications, urological complications may represent a more significant burden and impact on quality of life, as well as present an opportunity for increased symptom mitigation efforts.
Supplementary Material
Acknowledgments
Funding. Support for this DCCT/EDIC collaborative study (UroEDIC) was provided by grant 5R01-DK-083927. The DCCT/EDIC has been supported by U01 Cooperative Agreement grants (1982–1993 and 2011–2016) and contracts (1982–2011) with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (current grant numbers U01-DK-094176 and U01-DK-094157) and through support by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the Genetic Clinical Research Centers Program (1993–2007), and the Clinical Translational Science Center Program (2006-present). Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), CanAm Care (Atlanta, GA), Eli Lilly (Indianapolis, IN), LifeScan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MI), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), OmniPod Insulin Management System (Bedford, MA), Roche Diabetes Care (Indianapolis, IN), and Sanofi (Bridgewater, NJ).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. H.W., B.H.B., S.K.H., A.M.J., J.W.K., C.C., R.L.D., and A.V.S. critically reviewed the manuscript. H.W., B.H.B., S.K.H., A.M.J., R.L.D., and A.V.S. participated in data analysis and interpretation. H.W., S.K.H., and A.V.S. drafted the manuscript. H.W., J.W.K., C.C., and A.V.S. participated in the study conception and design. B.H.B. contributed to data acquistion. B.H.B., S.K.H., and R.L.D. performed statistical analysis. H.W. and A.V.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. nos. NCT00360815 and NCT00360893, clinicaltrials.gov.
A complete list of the DCCT/EDIC Study Group can be found in the Supplementary Data online.
References
- 1.Centers for Disease Control and Prevention National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA, U.S. Department of Health and Human Services, 2014 [Google Scholar]
- 2.Brown JS, Wessells H, Chancellor MB, et al. . Urologic complications of diabetes. Diabetes Care 2005;28:177–185 [DOI] [PubMed] [Google Scholar]
- 3.Kupelian V, Rosen RC, Link CL, et al. . Association of urological symptoms and chronic illness in men and women: contributions of symptom severity and duration--results from the BACH Survey. J Urol 2009;181:694–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Den Eeden SK, Sarma AV, Rutledge BN, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Research Group . Effect of intensive glycemic control and diabetes complications on lower urinary tract symptoms in men with type 1 diabetes: Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care 2009;32:664–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czaja CA, Rutledge BN, Cleary PA, Chan K, Stapleton AE, Stamm WE; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Urinary tract infections in women with type 1 diabetes mellitus: survey of female participants in the Epidemiology of Diabetes Interventions and Complications study cohort. J Urol 2009;181:1129–1134; discussion 1134–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarma AV, Kanaya A, Nyberg LM, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Risk factors for urinary incontinence among women with type 1 diabetes: findings from the Epidemiology of Diabetes Interventions and Complications study. Urology 2009;73:1203–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wessells H, Penson DF, Cleary P, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Effect of intensive glycemic therapy on erectile function in men with type 1 diabetes. J Urol 2011;185:1828–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimitropoulos K, Bargiota A, Mouzas O, Melekos M, Tzortzis V, Koukoulis G. Sexual functioning and distress among premenopausal women with uncomplicated type 1 diabetes. J Sex Med 2012;9:1374–1381 [DOI] [PubMed] [Google Scholar]
- 9.Enzlin P, Rosen R, Wiegel M, et al.; DCCT/EDIC Research Group . Sexual dysfunction in women with type 1 diabetes: long-term findings from the DCCT/EDIC study cohort. Diabetes Care 2009;32:780–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 11.Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822–830 [DOI] [PubMed] [Google Scholar]
- 13.Sandvik H, Seim A, Vanvik A, Hunskaar S. A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourol Urodyn 2000;19:137–145 [DOI] [PubMed] [Google Scholar]
- 14.Barry MJ, Fowler FJ Jr., O’Leary MP, et al.; Measurement Committee of the American Urological Association . The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 1992;148:1549–1557; discussion 1564 [DOI] [PubMed] [Google Scholar]
- 15.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–1289 [DOI] [PubMed] [Google Scholar]
- 17.Early Treatment Diabetic Retinopathy Study Research Group Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology 1991;98(5 Suppl.):741–756 [DOI] [PubMed] [Google Scholar]
- 18.Kupelian V, Wei JT, O’Leary MP, et al.; BACH Survery Investigators . Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med 2006;166:2381–2387 [DOI] [PubMed] [Google Scholar]
- 19.Rohrmann S, Smit E, Giovannucci E, Platz EA. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III). Int J Obes 2005;29:310–316 [DOI] [PubMed] [Google Scholar]
- 20.Kupelian V, Araujo AB, Chiu GR, Rosen RC, McKinlay JB. Relative contributions of modifiable risk factors to erectile dysfunction: results from the Boston Area Community Health (BACH) Survey. Prev Med 2010;50:19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minassian VA, Stewart WF, Wood GC. Urinary incontinence in women: variation in prevalence estimates and risk factors. Obstet Gynecol 2008;111:324–331 [DOI] [PubMed] [Google Scholar]
- 22.Litman HJ, McKinlay JB. The future magnitude of urological symptoms in the USA: projections using the Boston Area Community Health survey. BJU Int 2007;100:820–825 [DOI] [PubMed] [Google Scholar]
- 23.Jacobson AM, Braffett BH, Cleary PA, Gubitosi-Klug RA, Larkin ME; DCCT/EDIC Research Group . The long-term effects of type 1 diabetes treatment and complications on health-related quality of life: a 23-year follow-up of the Diabetes Control and Complications/Epidemiology of Diabetes Interventions and Complications cohort. Diabetes Care 2013;36:3131–3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson AM, Braffett BH, Cleary PA, et al.; DCCT/EDIC Research Group . Relationship of urologic complications with health-related quality of life and perceived value of health in men and women with type 1 diabetes: the Diabetes Control and Complications Trial/Epidemiology of Interventions and Complications (DCCT/EDIC) cohort. Diabetes Care 2015;38:1904–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutfey KE, Link CL, Rosen RC, Wiegel M, McKinlay JB. Prevalence and correlates of sexual activity and function in women: results from the Boston Area Community Health (BACH) Survey. Arch Sex Behav 2009;38:514–527 [DOI] [PubMed] [Google Scholar]
- 26.Tennstedt SL, Link CL, Steers WD, McKinlay JB. Prevalence of and risk factors for urine leakage in a racially and ethnically diverse population of adults: the Boston Area Community Health (BACH) Survey. Am J Epidemiol 2008;167:390–399 [DOI] [PubMed] [Google Scholar]
- 27.Nygaard I, Barber MD, Burgio KL, et al.; Pelvic Floor Disorders Network . Prevalence of symptomatic pelvic floor disorders in US women. JAMA 2008;300:1311–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson SL, Scholes D, Boyko EJ, Abraham L, Fihn SD. Urinary incontinence and diabetes in postmenopausal women. Diabetes Care 2005;28:1730–1738 [DOI] [PubMed] [Google Scholar]
- 29.Phelan S, Kanaya AM, Subak LL, et al.; Look AHEAD Research Group . Weight loss prevents urinary incontinence in women with type 2 diabetes: results from the Look AHEAD trial. J Urol 2012;187:939–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown JS, Grady D, Ouslander JG, Herzog AR, Varner RE, Posner SF; Heart & Estrogen/Progestin Replacement Study (HERS) Research Group . Prevalence of urinary incontinence and associated risk factors in postmenopausal women. Obstet Gynecol 1999;94:66–70 [DOI] [PubMed] [Google Scholar]
- 31.Lenherr SM, Clemens JQ, Braffett BH, et al.; DCCT/EDIC Research Group . Glycemic control and urinary tract infections in women with type 1 diabetes: results from the DCCT/EDIC. J Urol 2016;196:1129–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saigal CS, Wessells H, Pace J, Schonlau M, Wilt TJ; Urologic Diseases in America Project . Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med 2006;166:207–212 [DOI] [PubMed] [Google Scholar]
- 33.Gacci M, Corona G, Vignozzi L, et al. . Metabolic syndrome and benign prostatic enlargement: a systematic review and meta-analysis. BJU Int 2015;115:24–31 [DOI] [PubMed] [Google Scholar]
- 34.Bavendam TG, Norton JM, Kirkali Z, et al. . Advancing a comprehensive approach to the study of lower urinary tract symptoms. J Urol 2016;196:1342–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subak LL, Johnson C, Whitcomb E, Boban D, Saxton J, Brown JS. Does weight loss improve incontinence in moderately obese women? Int Urogynecol J Pelvic Floor Dysfunct 2002;13:40–43 [DOI] [PubMed] [Google Scholar]
- 36.Brown JS, Wing R, Barrett-Connor E, et al.; Diabetes Prevention Program Research Group . Lifestyle intervention is associated with lower prevalence of urinary incontinence: the Diabetes Prevention Program. Diabetes Care 2006;29:385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breyer BN, Phelan S, Hogan PE, et al.; Look AHEAD Research Group . Intensive lifestyle intervention reduces urinary incontinence in overweight/obese men with type 2 diabetes: results from the Look AHEAD trial. J Urol 2014;192:144–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phelan S, Kanaya AM, Ma Y, et al.; Diabetes Prevention Program Research Group . Long-term prevalence and predictors of urinary incontinence among women in the Diabetes Prevention Program Outcomes Study. Int J Urol 2015;22:206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Travison TG, Shabsigh R, Araujo AB, Kupelian V, O’Donnell AB, McKinlay JB. The natural progression and remission of erectile dysfunction: results from the Massachusetts Male Aging Study. J Urol 2007;177:241–246; discussion 246 [DOI] [PubMed] [Google Scholar]
- 40.Martin SA, Atlantis E, Lange K, Taylor AW, O’Loughlin P, Wittert GA; Florey Adelaide Male Ageing Study . Predictors of sexual dysfunction incidence and remission in men. J Sex Med 2014;11:1136–1147 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.