ABSTRACT
Therapeutic monoclonal antibodies and endogenous IgG antibodies show limited uptake into the central nervous system (CNS) due to the blood-brain barrier (BBB), which regulates and controls the selective and specific transport of both exogenous and endogenous materials to the brain. The use of natural transport mechanisms, such as receptor-mediated transcytosis (RMT), to deliver antibody therapeutics into the brain have been studied in rodents and monkeys. Recent successful examples include monovalent bispecific antibodies and mono- or bivalent fusion proteins; however, these formats do not have the capability to bind to both the CNS target and the BBB transport receptor in a bivalent fashion as a canonical antibody would. Dual-variable-domain immunoglobulin (DVD-Ig) proteins offer a bispecific format where monoclonal antibody-like bivalency to both the BBB receptor and the therapeutic target is preserved, enabling independent engineering of binding affinity, potency, valency, epitope and conformation, essential for successful generation of clinical candidates for CNS applications with desired drug-like properties. Each of these parameters can affect the binding and transcytosis ability mediated by different receptors on the brain endothelium differentially, allowing exploration of diverse properties. Here, we describe generation and characterization of several different DVD-Ig proteins, specific for four different CNS targets, capable of crossing the BBB through transcytosis mediated by the transferrin receptor 1 (TfR1). After systemic administration of each DVD-Ig, we used two independent methods in parallel to observe specific uptake into the brain. An electrochemiluminescent-based sensitive quantitative assay and a semi-quantitative immunohistochemistry technique were used for brain concentration determination and biodistribution/localization in brain, respectively. Significantly enhanced brain uptake and retention was observed for all TfR1 DVD-Ig proteins regardless of the CNS target or the systemic administration route selected.
KEYWORDS: bispecific antibodies, brain uptake of antibodies, blood-brain barrier (BBB), dual variable domain immunoglobulin, receptor-mediated transcytosis
Introduction
Biologic drugs, especially antibodies, are potentially highly attractive therapeutic regimens for central nervous system (CNS) disorders due to their high specificity, limited off-target toxicity and a relatively long serum half-life compared to small molecules. However, after systemic delivery, uptake of biotherapeutics across the blood-brain barrier (BBB), which is a dynamic, protective capillary endothelium cell system, 1 is minimal, posing a substantial challenge to drug development. Despite the recent developments in our understanding of the molecular physiology of the CNS environment and the biology of the diseases, many brain or CNS-associated disorders remain under-treated due to the relative inability of these molecules to cross the BBB, and the blood-cerebrospinal fluid barrier, to reach specific areas of brain. 2 , 3
It has been well established in the recent literature that the fraction of antibody that accumulates in brain is limited to about 0.2%, or less, of the blood concentration, 4 and occurs by a non-saturable, non-specific mechanism that, at least in mice, does not appear to involve FcRn or Fcγ receptor binding. 5 , 6 Therefore, current CNS biotherapeutics in the clinic have to rely on either this passive mechanism or the changes in the BBB permeability under pathological conditions 7 to reach to therapeutically relevant concentrations in the brain. Either scenario is likely inadequate; the former case may require high doses of the drug, which may cause serious side effects, and the latter may allow only non-homogeneous biodistribution of the drug within the brain for a limited duration.
Alternative routes of delivery for biopharmaceuticals bypassing the BBB are feasible, but have limitations. They are either considered invasive (intracerebroventricular, intraparenchymal or intrathecal) or have size restrictions and stability issues (intranasal), but most importantly, the brain uptake of proteins following such administration routes is still reported to be low, especially in the targeted regions of the brain. 8
Other drug delivery methods, such as nanoparticles and vector technologies, might be applicable for the delivery of biologic drugs, but have yet to prove useful for clinical applications.9-11
Given the challenges of these alternatives, there has been significant interest in engineering biologics with enhanced brain uptake properties following systemic administration. One of the endogenous active transport mechanisms of the BBB, receptor-mediated transcytosis (RMT), which exists for efficient transport of endogenous peptides such as insulin and transferrin, has been explored extensively as a promising non-invasive approach to enhance brain uptake of large molecules.12-15 More recently, Yu et al 16 reported the importance of low affinity for the efficient transcytosis of anti-transferrin receptor (TfR) antibodies, including bispecifics. Their bispecific antibody targeting TfR and enzyme beta-secretase (BACE) proved to be effective in delivering the antibody into the mouse and monkey brain at therapeutically relevant concentrations, resulting in a pharmacodynamic (PD) change. 17 As reviewed recently, 15 there are also reports claiming that bivalent binding of a fusion protein 18 , 19 or the high affinity binding of a bispecific antibody, 19 to the TfR may lead to lysosomal degradation of the drug rather than transcytosis.
In parallel with these ongoing investigations, we have studied TfR1-mediated BBB transport in a bivalent format, namely dual variable domain immunoglobulins (DVD-Igs). DVD-Igs are bivalent antibody-like molecules that have a second Ig variable domain engineered on the outside of the first, linked by peptide linkers. 20 , 21 We generated DVD-Ig molecules that can bind TfR1 and a CNS target in brain in a bivalent fashion while preserving the symmetry of the natural antibodies or antibody biotherapeutics. To ensure that the observed brain uptake is not restricted to a specific CNS target or pathway, or to a specific antibody sequence, we engineered multiple DVD-Ig molecules against four diverse targets (tumor necrosis factor (TNF), amyloid-beta (Aβ), repulsive guidance molecule A (RGMa) and human epidermal growth factor receptor 2 (HER2)) paired with four different humanized variants of a TfR1 antibody with varying affinities. All of the DVD-Ig proteins are capable of binding to TfR1 and a second target in a bivalent fashion. By harnessing orthogonal in vitro and in vivo methods, we show that DVD-Ig molecules with the best target potency and optimal receptor binding affinity, based on a sensitive cell-based binding assay, resulted in favorable brain uptake. Significant CNS uptake and retention for DVD-Ig proteins containing domains against different CNS targets were observed regardless of the different systemic routes of administration (i.e., intravenous (IV), subcutaneous (SC), or intraperitoneal (IP)).
Results
Brain uptake and exposure of anti-TfR1 antibodies
In order to study receptor-mediated uptake in mice, we selected AB221, a humanized antibody that specifically recognizes murine TfR1 with high affinity. In separate cohorts of animals, we administered (IV) 20 mg/kg of AB221 and an isotype control antibody to C57Bl/6N mice and perfused animals at 1 and 24 hours. Administered antibody was immunostained in brain tissue sections as described in materials and methods. Fig. 1(A-D) shows increased levels of TfR1 antibody AB221 in the brain compared to the human isotype IgG1 control at both the 1 hour and 24 hour time points. There was increased staining for AB221 on the endothelial cells/vasculature at both time points. At 24 hours, strong AB221 staining of the parenchyma was observed throughout the brain as compared to the isotype control. In addition, there was prominent cytoplasmic staining of Purkinje neurons (Fig. 1D) of the cerebellum as well as neurons of multiple brain stem nuclei, including the medial vestibular nucleus and neurons of the facial cranial nerve (Fig. 2C and I), the bed nucleus of the stria terminalis and occasional cerebral cortical neurons. There was also staining of neuronal processes and parenchyma of the glomerular and external plexiform layer of the olfactory bulb, the molecular layer of the dentate gyrus, the stratum lacunosum moleculare and stratum oriens of the hippocampus, and the processes of the anterior nucleus of the thalamus (data not shown). Similar enhanced positive staining pattern of anti-TfR1 antibody distribution was observed using two different staining protocols and detection antibodies (donkey anti-human (Fig. 1B and D) and rabbit anti-human (Fig. 1F)). Overall enhanced immunoreactive staining for AB221 (Fig. 1I) and AB405 (Fig. 1J, see below) compared to IgG controls (Fig. 1H and K) in many different regions of the brain was evident.
Figure 1.
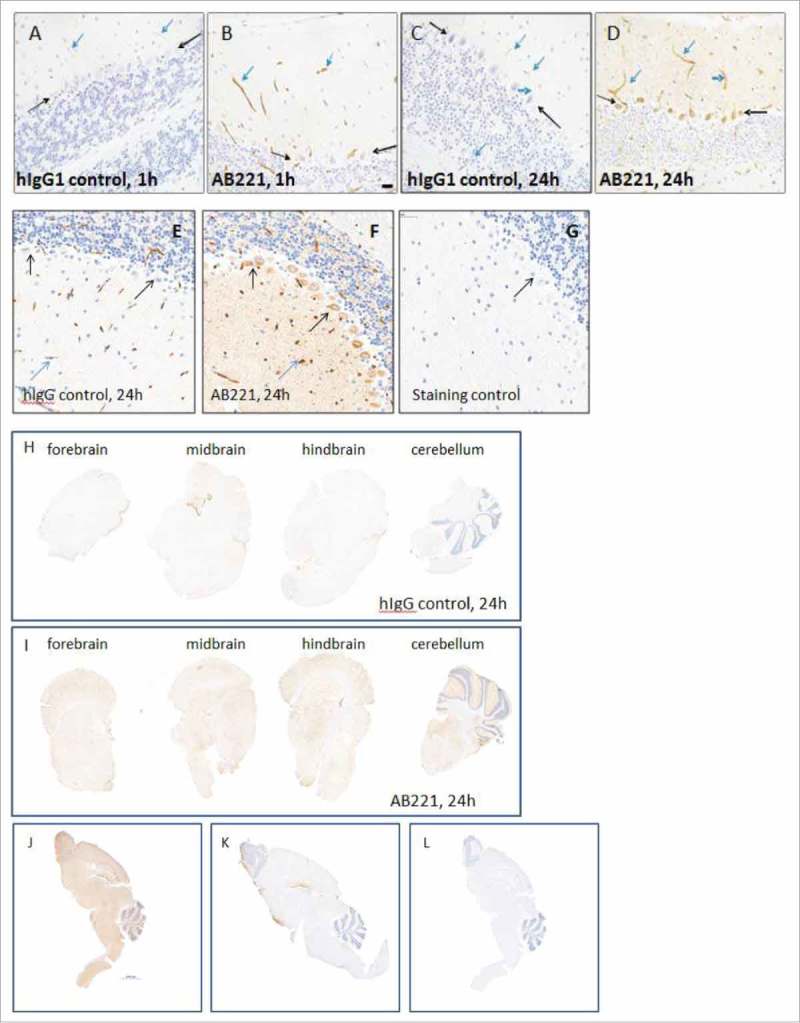
Brain uptake and distribution of anti-TfR1 antibodies. (A-I) Representative images of IHC study of anti-TfR, AB221 and hIgG control antibody. Positive IHC staining of endothelial cells/vasculature (blue arrows), Purkinje cells (black arrows) in coronal sections of mice cerebellum 1 or 24 hours (B,D) after intravenous injection of 20 mg/kg AB221 was observed by staining of brain sections with donkey anti-human IgG. Same staining pattern of antibody distribution with another staining protocol using rabbit anti-human detection is also shown (F). Positive IHC staining of endothelial cells/vasculature (F, blue arrows), in coronal sections of mice cerebellum 1 or 24 hours after intravenous injection of 20 mg/kg IgG control was observed by staining of brain sections with donkey anti-human IgG (A and C) or staining with rabbit anti- human antibody (E). Location of Purkinje cells, which shows negative staining, in IgG control is also indicated by black arrows (A and C). Rabbit anti-hamster was used as staining control (G). Representative images of coronal section of forebrain, midbrain, hindbrain and cerebellum regions show enhanced overall brain uptake of AB221 (I) compared with hIgG (H) using rabbit anti-human staining. (J-L) Representative images of IHC staining of sagittal section of whole brain with rabbit anti-human detection after an intravenous injection with either anti-TfR AB405 (J) or hIgG control antibody (K) at 20 mg/kg after 24 hours are shown. IHC staining of brain section with rabbit anti-hamster control indicates no non-specific staining (L). Scale bar equals 2000 μm.
Figure 2.
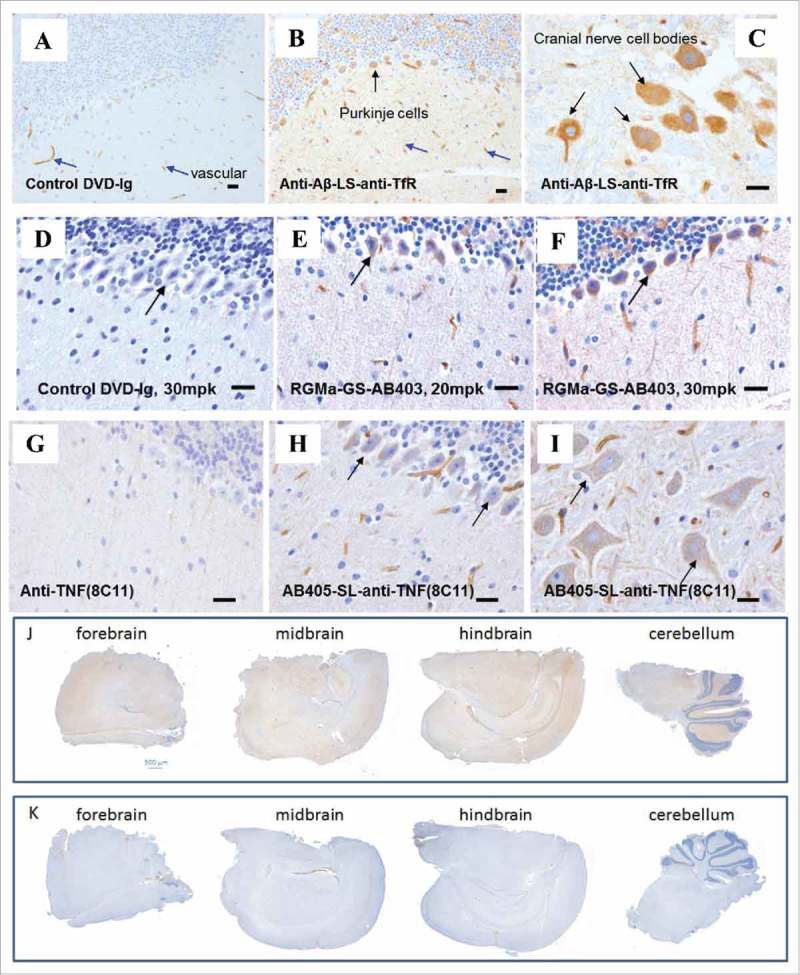
Brain uptake and distribution of multiple DVD-Igs. (A-C) Representative images of IHC staining of brain sections from mice 24 hours after an intravenous injection with either control DVD-Ig or anti-Aβ-LS-anti-TfR1(AB221) DVD-Ig (20 mg/kg). Images show broad overall staining in the brain parenchyma for anti-TfR1/Aβ DVD-Ig (B) compared with DVD-Ig control (A). Significant amounts of brown neuronal staining of Purkinje cells (B, black arrow) and cranial nerve cells (C, black arrow) were detected for anti-Aβ-LS-anti-TfR1(AB221) DVD-Ig. Occasional endothelial cell/vasculature staining (blue arrow) was observed with control DVD-Ig (A). (D-F) IHC staining of brain sections from mice after an intravenous injection with either anti-RGMa-GS-anti-TfR1(AB403) DVD-Ig or DVD-Ig control at indicated doses after 24 hours. Images show broad staining in the brain parenchyma for anti-RGMa-GS-anti-TfR1(AB403) DVD-Ig (E,F) which was lacking in DVD-Ig control (D). Neuronal staining of Purkinje cells (black arrow) by anti-RGMa-GS-anti-TfR1(AB403) DVD-Ig was observed (E,F). (G-I) IHC staining of brain cerebellum sections from mice after an intravenous injection with either anti-TNF (8C11) antibody, or anti-TfR1(AB405)-SL-anti-TNF(8C11) DVD-Ig at 20 mg/kg at 24 hours is shown. Images show broad staining in the brain parenchyma for anti-TfR1(AB405)-SL-anti-TNF(8C11) DVD-Ig (H,I). Neuronal staining of Purkinje cells (H, black arrows) and cranial nerves (I, black arrows) by anti-TfR1(AB405)-SL-anti-TNF(8C11) DVD-Ig was observed. Parenchyma or neuronal staining was not detected for anti-TNF antibody (G). Images shown are representative of data from cohorts of animals n = 4. Scale bar equals 20 μm. (J, K) Representative images of coronal section of forebrain, midbrain, hindbrain and cerebellum regions from mice after an intravenous injection with either anti-RGMa-GS-anti-TfR1(AB403) DVD-Ig or DVD-Ig control after 24 hours show enhanced overall brown staining of anti-RGMa-GS-anti-TfR1(AB403) DVD-Ig (J) compared with DVD-Ig control (K). Images shown are representative of data from cohorts of animals n = 4. Scale bar equals 500 μm.
We generated a series of heavy and light chain sequence variants of AB221 with a view to modulating the affinity to TfR1; the comparison of variable domain sequences from selected variants is shown in Fig. 3. Two heavy chain sequences and two light chain sequences were selected for antibody production, resulting in four antibodies. AB402 and AB403, which differ from AB221 only in light chain sequence, show similar TfR1 binding affinity compared to AB221. However, AB404 and AB405, which have differences in the heavy chain sequence, show reduced affinity to TfR1. Brain uptake of these affinity variants was studied using a modified in vivo paradigm where, after harvesting and perfusion, brains were bifurcated, one half being processed for immunostaining as before, the other half being subject to extraction and measurement of human antibody levels by electrochemiluminescence-Meso Scale Discovery (ECL-MSD). Two of the anti-TfR1 antibodies (AB404 and AB405) with binding affinities ranging from 3 to 13.55 nM based on binding to TfR1 over-expressing cells showed significantly improved uptake. Broad parenchymal distribution of anti-TfR1 antibodies across different brain regions is shown in low magnification pictures from immunohistochemistry (IHC) staining of sagittal section (Fig. 1I and J). In some cases, greater than six-fold increase in brain antibody concentration over control IgG was measured by the ECL-MSD assay 24 hours after injection (Table 1). Strong parenchymal and neuronal IHC staining reflecting this increased brain uptake was also observed (data shown as IHC quantification, Table 1). In addition, AB404 and AB405 displayed elevated serum levels relative to controls over 48 hours, likely reflecting a longer serum half-life.
Figure 3.
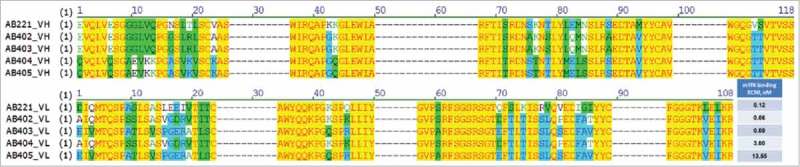
Humanized variants of anti-TfR1 antibody with different TfR1 binding affinities. Sequence alignment of four humanized variants (AB402, AB403, AB404 and AB405) compared to the parent AB221 (anti-mTfR1) is shown and modifications made are highlighted. CDRs (dash lines) of both heavy and light chains remain the same. Cell-based binding assay shows reduced TfR1 binding affinity for AB404 and AB405 compared to AB221.
Table 1.
In vivo biodistribution characteristics of anti-TfR1 antibodies with intravenous administration. Anti-TfR1 antibodies were intravenous dosed at 20 mg/kg. Mouse serum concentration and brain uptake of two lower affinity variants (AB404 and AB405) of AB221 and isotype human IgG control after 1, 24 and 48 hours of injection. AB404 and AB405 showed longer serum half-life and better brain uptake, as shown by antibody concentration measurements and parenchymal and neuronal immunohistochemical staining-based evaluation. Data was expressed as means ± SD, n = 4 mice per group.
| IHC scoring, range 0–4 |
||||||
|---|---|---|---|---|---|---|
| Name of Ab | Collection Time (hr) | Serum conc. (nM) | Brain conc. (nM) | Fold increased over IgG | Parenchymal staining | Neuronal staining |
| IgG | 24 | 1693 +/−189 | 1.1 +/− 0.2 | 1 | 0.0 | 0.0 |
| AB221 | 1 | 2266 +/− 415 | 2.1 +/− 0.1 | n.d. | 0.0 | 0.0 |
| 24 | 466 +/− 406 | 1.5 +/− 0.6 | 1.5 | 1.3 | 1.3 | |
| 48 | 46 +/− 42 | 1.7 +/− 0.4 | n.d. | 2.0 | 2.0 | |
| AB404 | 1 | 2580 +/− 571 | 2.8 +/− 0.6 | n.d. | 0.0 | 0.0 |
| 24 | 1606 +/− 161 | 6.5 +/− 1.1 | 6.6 | 3.0 | 2.3 | |
| 48 | 993 +/− 89 | 4.8 +/− 0.3 | n.d. | 2.5 | 1.5 | |
| AB405 | 1 | 2566 +/− 239 | 3.7 +/− 1.0 | n.d. | 0.0 | 0.0 |
| 24 | 1966 +/− 767 | 7.0 +/− 0.4 | 6.6 | 2.0 | 1.5 | |
| 48 | 933 +/− 157 | 6.0 +/− 0.4 | n.d. | 2.0 | 2.3 | |
Generation and characterization of anti-Amyloid Beta (Aβ)/TfR1 DVD-Igs and anti-HER2/TfR1 DVD-Ig proteins
Aβ is an early biomarker of Alzheimer's disease and other neurodegenerative pathologies. 22 , 23 Murine antibody 3D6 is the parent of the humanized monoclonal antibody bapineuzumab, which targets the neurotoxic amyloid beta (Aβ) peptides. 24 Anti-Aβ-LS-anti-TfR1(AB221) DVD-Ig was generated with AB221 in the inner domain and 3D6 anti-Aβ antibody sequence in the outer domain. This DVD-Ig has a 10-fold lower (EC50 = 1.2 nM) TfR1 binding affinity compared to the parent AB221 monoclonal antibody. When administered at a therapeutic dose (20 mg/kg), similar distribution behavior to AB221 antibodies was observed, with broad distribution of anti-Aβ-LS-anti-TfR1(AB221) DVD-Ig in brain parenchyma 24 hours post IV injection compared with control (Fig. 2A-C). Neuronal staining was also observed, for example in Purkinje cells in cerebellum and cranial nerve nuclei in pons / medulla. The cellular staining is mainly observed in cell bodies, and in some cases, neuronal processes were also labeled. The staining appears to be in the cytoplasm and diffuse. To quantify the amount visualized in the brain parenchyma and cells, anti-Aβ-LS-anti-TfR1(AB221) DVD-Ig levels in brain homogenates were measured by ECL-MSD method. Following a 20 mg/kg or a 50 mg/kg IV injection, a dose-dependent accumulation of anti-Aβ-LS-anti-TfR1(AB221) DVD-Ig in the brain was observed. At both doses, brain antibody concentration reached plateau at 24 hours, which was sustained at a comparable level for at least 48 hours after dosing (Fig. 4). Serum levels were also tested and were lower compared to the control DVD-Ig (at 20 mg/kg) at 24 hours, suggesting target-mediated clearance. As expected, by 48 hours, anti-Aβ-LS-anti-TfR1(AB221) DVD-Ig serum levels were lower than at 24 hours for both doses.
Figure 4.
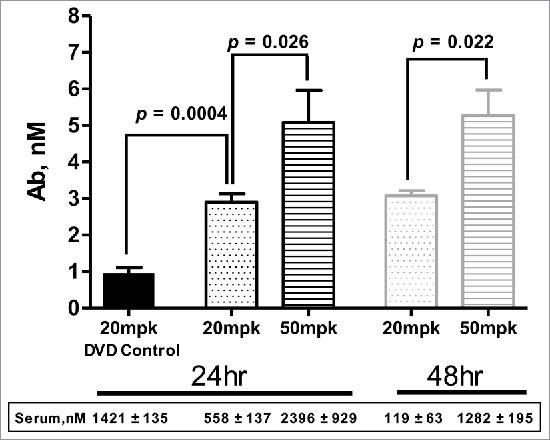
Brain uptake of anti-Aβ(3D6)-LS-anti-TfR1(AB221) DVD-Ig. Mouse brain concentration of anti-Aβ-LS-anti-TfR1(AB221) DVD-Ig increased compared to control DVD-Ig at 24 hours and remained at the similar level up to 48 hours post intravenous dosing. Higher brain concentration was observed with 50 mg/kg dosing compared to 20 mg/kg. Unpaired two-tailed t-test of anti-Aβ-LS-anti-TfR1(AB221) DVD-Ig concentration at 24 and 48 hours post single intravenous injection of 20 mg/kg or 50 mg/kg showed significantly enhanced brain uptake with higher dose of injection. Data was expressed as means ±SD, n = 4 mice per group.
We next characterized the brain uptake properties of the anti-Aβ-LS-anti-TfR1(AB221) DVD-Ig with an alternative route of systemic administration. Mice were injected subcutaneously at various therapeutic doses (20 or 50 mg/kg) either once or twice (at 0 and 48 hours) before harvesting the brains 96 hours after the first injection. Brain concentration of 1.6 +/− 0.20 nM was retained at 96 hours after single 20 mg/kg SC administration. Brain concentration of 6.9 nM and 9.0 nM were reached with 50 mg/kg single or multiple dosing, respectively (Table 2). It should be noted that similar brain levels were measured post 24 hours injection of this DVD-Ig (20 mg/kg) by IV (3.15+/−0.26 nM), IP (3.72+/−0.82 nM) or SC (2.27+/−1.04 nM) administration routes.
Table 2.
Retention of anti-Aβ-LS-anti-TfR1(AB221) DVD-Ig in brain for 96 hours after subcutaneous dosing. Mice injected subcutaneously with anti-Aβ-LS-anti-TfR1(AB221) DVD-Ig at 20 mg/kg or 50 mg/kg either once (at 0 hour) or at 40 mg/kg twice (0 and 48 hours) before processing at 96 hours. Serum anti-Aβ-LS-anti-TfR1(AB221) DVD-Ig concentrations were measured at indicated times post injection, except for 40 mg/kg dose second injection at 48 h was performed right after serum collection at 48 h. Note an anti-Aβ-LS-anti-TfR1(AB221) DVD-Ig brain concentration of 1.6 ± 0.20 nM was retained at 96 hours after a single injection. Data was expressed as means ± SD, n = 4 mice per group, except where indicated with *n = 3.
| Treatment | Collection Time. (hr) | Serum conc. (nM) | Brain conc. (nM) |
|---|---|---|---|
| 20 mpk | 1 | 3.7 +/− 3.1 | |
| 24 | 142.5 +/− 3.2 | ||
| 48 | 33.7 +/− 12.4 | ||
| 72 | 8.0 +/− 2.8 | ||
| 96 | 1.7 +/− 0.2 | 1.6 +/− 0.2 | |
| 50mpk | 1 | 11.2 +/− 7.9 | |
| 24 | 679.8 +/− 142.0 | ||
| 48 | 187.3 +/− 67.2 | ||
| 72 | 94.6 +/− 36.4 | ||
| 96 | 6.1 +/− 1.7 | 6.9 +/− 2.6 | |
| 40 mpk (at 0 and48 hr) | 1 | 3.2 +/− 2.5 | |
| 24 | 109.9 +/− 16.7* | ||
| 48 | 20.0 +/− 1.6 | ||
| 72 | 365.0 +/− 189.1 | ||
| 96 | 95.6 +/− 70.2 | 9.0 +/− 2.9 |
Upon obtaining favorable brain uptake with anti-Aβ-LS-anti-TfR1(AB221) DVD-Ig, other DVD-Ig proteins were designed and constructed using multiple TfR1 affinity variant variable domains and alternative linkers. DVD-Ig proteins showing optimal affinity to TfR1 while retaining the highest binding to Aβ were selected for brain uptake and distribution studies (Table 3). Immunohistochemistry staining and serum and brain concentrations were assessed 24 hours after administration of 20 mg/kg of these DVD-Igs. Anti-TfR1(AB405)-SL-anti-Aβ DVD-Ig, anti-Aβ-GS-anti-TfR1(AB403) DVD-Ig and anti-Aβ-SS-anti-TfR1(AB402) DVD-Ig (see Material and Methods section for descriptions of the DVD-Igs) were present in higher amounts in the brain extracts as measured by ECL-MSD and were mostly localized to parenchyma and neurons compared to the control IgG (Table 3). Unlike the other DVD constructs described here, in this instance, lowest affinity did not result in the highest brain penetration.
Table 3.
Brain uptake and localization of anti-Aβ/anti-TfR1 DVD-Ig proteins. Anti-Aβ/anti-TfR1 DVD-Igs, capable of binding simultaneously both to the TfR1 (mTfR1 cell-based binding, EC50) and Aβ molecules (Aβ binding, EC50), with different orientation, linker and paired anti-TfR1 variable domain diversity were generated. EC50 for binding of Anti-Aβ Ab to Aβ was 34 ng/mL. Serum and brain concentrations were determined at 24 hour after 20 mg/kg intravenous administration into C57Bl/6N mice. Parenchyma and neuronal staining quantifications by immunohistochemistry analyses are shown. Data was expressed as means ± SD, n = 4 mice per group.
| IHC scoring, range 0–4 |
||||||
|---|---|---|---|---|---|---|
| Name of Ab / DVD | mTfR binding EC50 (nM) | Aβ binding (ng/ml) | Serum conc. (nM) | Brain conc. (nM) | Parenchymal staining | Neuronal staining |
| Anti-TfR1(AB405)-SL-anti-Aβ | 4.7 | 73.9 | 421.4 +/− 41.6 | 10.4 +/− 1.3 | 1.8 | 2.0 |
| Anti-Aβ-GS-anti-TfR1(AB403) | 2.0 | 29.6 | 448.1 +/− 54.5 | 4.7 +/− 0.4 | 1.4 | 1.5 |
| Anti-Aβ-SS-anti-TfR1(AB402) | 12.9 | n.d. | 1,298.8 +/− 362.9 | 6.7 +/− 1.7 | 1.0 | 1.0 |
| control huIgG | No binding | No binding | 2527.4 +/− 51.6 | 2.6 +/− 0.7 | 0.2 | 0.0 |
Without a compromised BBB, anti-HER2 antibodies are thought to have limited access to the brain. 25 Several approaches are being developed to enhance the delivery of anti-HER2 antibodies to the brain, which include physical or pharmacologic disruption of the BBB, direct intracerebral drug delivery, drug manipulation, and coupling to transport vectors. 25 Here, a non-invasive RMT delivery method of anti-HER2 coupled with anti-TfR1 in DVD-Ig format was explored. Enhanced brain uptake of multiple anti-HER2/TfR1 DVD-Ig proteins was observed, evident by broad brain parenchymal and neuronal IHC staining, which was absent in mice brain injected with DVD-Ig control (Table 4). Furthermore, when anti-TfR1 was in the inner domain orientation, a lower TfR1 binding activity was observed, which resulted in significant brain penetration relative to the outer domain orientation (expressed as fold increase over DVD-Ig).
Table 4.
Brain uptake and localization of anti-HER2/anti-TfR1 DVD-Ig proteins. Two anti-HER2/anti-TfR1 DVD-Igs were engineered comprising both the domain orientations in a DVD-Ig format. Serum and brain concentrations were determined at 24 hour after 20 mg/kg intravenous administration into C57Bl/6N mice. Brain concentrations are expressed as fold increase over control DVD-Ig Immunohistochemistry staining in the parenchyma and neurons indicated as present (yes) or absent (no) for 2 DVD-Ig proteins with TfR1 domains compared to the control DVD-Ig. Data was expressed as means ± SD, n = 4 mice per group.
| IHC |
|||||
|---|---|---|---|---|---|
| Name of Ab / DVD | mTfR binding EC50 (nM) | Serum conc. (nM) | Fold increased over DVD-IgG | Parenchymal staining | Neuronal staining |
| Control DVD-Ig | No binding | 497 +/− 358 | 1.0 | No | No |
| Anti-TfR1(AB221)-SS-anti-HER2) | 0.1 | 188 +/− 64 | 5.4 | Yes | Yes |
| Anti-HER2-SS-anti-TfR1(AB221) | 3.4 | 432 +/− 132 | 10.8 | Yes | Yes |
Generation and characterization of anti-RGMa/TfR1 DVD-Ig proteins
The early stages of many neurodegenerative diseases are characterized by neurite damage and compromised synaptic function. Neurite degeneration is also a pathological indicator of the autoimmune disease multiple sclerosis (MS). RGMa, which is a GPI-anchored glycoprotein, was first described as a neurite growth repellent or neurite growth inhibitor during development of topographic neuronal projections. 26 RGMa is a potent inhibitor of neurite outgrowth and emerged as an important factor inhibiting neuronal regeneration and functional recovery after CNS trauma or inflammation and identified as a potential factor contributing to MS-associated neurodegeneration. RGMa exists in membrane-bound and soluble forms, both of which can be inhibitory for neurite growth. 27 , 28 Antibodies that block RGMa function have the potential for therapeutic benefit in MS-associated neurodegeneration; elevated exposure with TfR1 DVD-Igs may provide improved efficacy. A series of anti-RGMa/anti-TfR1 DVD-Igs were generated using three parent TfR1 antibodies with different orientations and linkers (Table 5). All DVD-Igs with varying affinities to mTfR1 expressed well and were purified as >90% monomer. In general, lower affinity to mTfR1 was observed when anti-TfR1 was at the inner domain. In addition, affinity of both variable domains to their targets varied based on the linker used. The criteria for selection of DVD-Ig proteins for subsequent detailed uptake/distribution and future PD/efficacy studies included: 1) having an optimal TfR1 binding affinity; 2) with highest penetration through the BBB, resulting in prolonged brain and serum exposure; and 3) retention of high anti-RGMa potency. Anti-RGMa-GS-anti-TfR1(AB403)DVD-Ig and anti-TfR1(AB405)-SL-anti-RGMa DVD-Ig, with 14.8 nM and 13.1 nM brain exposures, respectively (Table 5), which showed corresponding elevated parenchymal (Fig. 2J) and neuronal staining (Fig. 2E and F) by IHC at 24 hr post IV injection, were selected for further studies. A more detailed brain uptake and distribution study with anti-TfR1(AB405)-SL-anti-RGMa (Table 6) at 20 mg/kg or 40 mg/kg (multiple doses at 0, 24 and 48 hours) was carried out. With repeat dosing, the brain concentration of the DVD-Ig reached 30.4 nM at 49.5 hours after the last dose at 48 hr. The brain concentration of anti-TfR1(AB405)-SL-anti-RGMa DVD-Ig was significantly higher compared to the control antibodies (Fig. 5 and Table 6). Additionally, anti-TfR1(AB405)-SL-anti-RGMa DVD-Ig concentration in spinal cord increased in a dose-dependent manner and reached to 13.5 nM at 24 hours post 40 mg/kg IV injection compared to the 8.3 nM post 20 mg/kg dosing.
Table 5.
Evaluation and Selection of anti-RGMa/anti-TfR1 DVD-Igs. Diverse anti-RGMa/anti-TfR1 DVD-Ig proteins incorporating different domain orientations, linkers and paired variable domains were generated using recombinant methods and % monomer was determined by SEC. They were screened using a cell-based mTfR1 binding assay and RGMa BMP potency assay for functional activity. DVD-Ig concentrations in mouse serum and brain at 24 hour post intravenous injection at the indicated doses were determined using MSD-ECL assay. Anti-TfR1(AB405)-SL-anti-RGMa DVD-Ig and anti-RGMa-GS-anti-TfR1(AB403) showed favorable brain penetration and target potency. Data was expressed as means ± SD, n = 4 mice per group.
| Ab/DVD name | % Monomer | mTFR binding EC50 (nM) | BMP Assay IC50 (nM) | Serum conc. (nM) | Brain conc. (nM) |
|---|---|---|---|---|---|
| Anti-RGMa-LS-anti-TfR1(AB402)** | 99.3 | 127.4 | 0.21 | 1,184 +/− 279 | 10.7 +/− 1.3 |
| Anti-RGMa-SL- anti-TfR1(AB402)** | 90.5 | 2.1 | 0.37 | 819 +/− 206 | 6.5 +/− 0.9 |
| Anti-RGMa-LL-anti-TfR1(AB402)** | 91.8 | 1.7 | 1.78 | 534 +/− 101 | 6.3 +/− 0.9 |
| Anti-RGMa-SS-anti-TfR1(AB402)* | 96.4 | 30.9 | 0.44 | 670 +/− 108 | 8.0 +/− 1.0 |
| Anti-RGMa-GS- anti-TfR1(AB402)* | 91.4 | 5.4 | 0.01 | 187 +/− 40 | 10.0 +/− 1.2 |
| Anti-RGMa-LS- anti-TfR1(AB403)* | 99.1 | 25.7 | <0.03 | 481 +/− 74 | 18.5 +/− 5.1 |
| Anti-RGMa-SS- anti-TfR1(AB403)* | 98.2 | 71.0 | 1.18 | 487 +/− 92 | 9.3 +/− 1.1 |
| Anti-RGMa-GS- anti-TfR1(AB403)** | 98.8 | 4.3 | <0.03 | 397 +/− 99 | 14.8 +/− 0.7 |
| Anti-TfR1(AB405)-SL-anti-RGMa** | 95.2 | 4.4 | 0.73 | 643 +/− 72 | 13.1 +/− 2.8 |
| Anti-TfR1(AB405)-LS-anti-RGMa* | 99.2 | 3.9 | No inhibition | 462 +/− 32 | 18.4 +/− 1.8 |
| Anti-TfR1(AB405)-GS-anti-RGMa* | 90.1 | 28.1 | 3.12 | 612 +/− 221 | 19.2 +/− 1.9 |
| DVD-Ig control*** | 97.9 | NA | NA | 2,287+/−188 | 2.5+/−0.6 |
| anti-RGMa hFc**** | 100% | NA | 0.21 | 4,512 +/− 77 | 5.9 +/− 2.1 |
10mpk;
20mpk,
30mpk,
40mpk.
Table 6.
Brain Concentration and localization of anti-TfR1(AB405)-SL-anti-RGMa DVD-Ig in an exposure study. Mouse serum and brain concentrations of anti-TfR1(AB405)-SL-anti-RGMa DVD-Igs were compared to anti-RGMa human IgG and isotype human IgG control. Decreasing serum concentration with respect to elevated brain concentrations of anti-TfR1(AB405)-SL-anti-RGMa DVD-Ig at 1, 24 and 48 hours after single (20 mg/kg or 40 mg/kg, (also shown in Fig. 5)) or at 49.5 hours after multiple (20 mg/kg at 0, 24, 48 hours) intravenous injections is shown. Corresponding immunohistochemistry staining showed localization to brain parenchyma and neuron cells. 40 mg/kg intravenous injection of either anti-RGMa human IgG or isotype human IgG control yielded higher serum concentrations but about 4-fold lower brain concentrations at 24 hours with minimal parenchyma or neuronal staining. Data was expressed as means ±SD, n = 4 mice per group.
| IHC scoring, range 0–4 |
||||||
|---|---|---|---|---|---|---|
| Name of Ab | Treatment | Collection Time (hrs) | Serum conc. (nM) | Brain conc. (nM) | Parenchymal staining | Neuronal staining |
| Anti- TfR1(AB405)- SL-anti-RGMa | IV single 20mpk | 1 | 577 +/− 128 | |||
| 24 | 602 +/− 419 | 21.6 +/− 4.6 | 0.8 | 0.8 | ||
| 48 | 205 +/− 31 | 18.0 +/− 1.7 | 1.1 | 1.1 | ||
| IV single 40mpk | 1 | 1,446 +/− 167 | ||||
| 24 | 1,036 +/− 109 | 25.7 +/− 4.1 | 1.5 | 0.9 | ||
| 48 | 398 +/− 79 | 23.3 +/− 2.1 | 1.8 | 0.9 | ||
| IV multiple 20mpk, at 0, 24, 48 h | 49.5 | 2,274 +/− 849 | 30.4 +/− 4.7 | |||
| anti-RGMa hFc | IV single 40mpk | 24 | 4,354 +/− 1,011 | 5.3 +/− 1.9 | 0.3 | 0.0 |
| Control IgG | IV single 40mpk | 24 | 2,999 +/− 516 | 5.4 +/− 1.1 | 0.0 | 0.1 |
Figure 5.
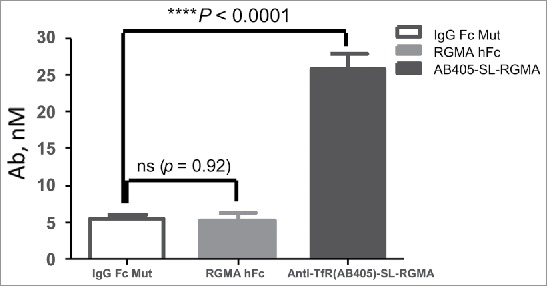
Significantly enhanced brain uptake of anti-TfR1(AB405)-SL-anti-RGMa DVD-Ig in comparison to control antibodies. Unpaired two-tailed t-test of brain antibody or DVD-Ig concentration at 24 hours post intravenous injection showed significantly enhanced brain uptake of 40 mg/kg anti-TfR1(AB405)-SL-anti-RGMa DVD-Ig in comparison to control antibodies anti-RGMa-huIgG and human IgG control (shown also in Table 6).
Generation and characterization of anti-TNF/TfR1 DVD-Ig proteins
TNF is a potent pro-inflammatory cytokine, with both soluble and membrane-associated forms. In addition to its well-established role in inflammatory diseases such as arthritis and psoriasis, soluble TNF is implicated in neuro-degenerative diseases such as Parkinson's disease, Alzheimer's disease as well as multiple sclerosis, 29 , 30 and may have a role in chronic neuropathic pain. 31 Minimal brain exposure of anti-TNF antibodies (e.g., Fig. 2G) has been a hurdle to evaluate their therapeutic potential.
A number of anti-TNF/TfR1 DVD-Ig proteins were generated using variable domains from anti-murine TNF (8C11) and anti-TfR1 (AB221 or humanized variants). Anti-TNF/TfR1 DVD-Ig proteins that had the best TNF neutralization potency and optimal TfR1 binding affinity were selected. Multiple DVD-Igs were tested in mice at 20 mg/kg; a representative DVD-Ig, anti-TfR1(AB405)-SL-anti-TNF(8C11) DVD-Ig showed increased brain uptake, which resulted in overall broad staining in the brain parenchyma and neurons at 24 hours post IV injection (Fig. 2H and I). Similar observations were made with anti-TNF-GS-anti-TfR1(AB221) DVD-Ig, a DVD-Ig with the anti-TfR1 located as the inner domain, a further example demonstrating elevated BBB penetration for DVD-Igs in both orientations (data not shown). The overall pattern of IHC staining was similar to that of anti-TfR1 antibodies and other anti-TfR1 domain containing DVD-Igs.
In further serum and brain exposure studies, anti-TfR1(AB405)-SL-anti-TNF (8C11) DVD-Ig was administered to mice at various doses using different routes of administration (IV, IP or SC) (Table 7). Similar brain and spinal cord uptake of anti-TfR1(AB405)-SL-anti-TNF DVD-Ig was observed with IV or IP single administration (20 mg/kg) in 24 hours. SC administration gave lower serum levels at the 24 hour time point, consequently yielding lower brain and spinal cord concentrations (Table 7).
Table 7.
Delivery of anti-TfR1 (AB405)-SL-anti-TNF DVD-Ig into brain by systemic administration via intravenous, subcutaneous or intraperitoneal injections. Anti-TfR1 (AB405)-SL-anti-TNF DVD-Ig was administrated at different doses (single or multiple dose of 20 or 40 mg/kg) via either subcutaneous (SC), intravenous (IV) or intraperitoneal (IP) routes for indicated durations. Antibody / DVD-Ig concentrations in serum, brain tissue and spinal cord tissue were measured using MSD-ECL method. Immunohistochemical staining of brain sections was done to evaluate parenchymal and neuronal staining. Comparable brain penetration and distribution of anti-TfR1 (AB405)-SL-anti-TNF DVD-Ig were reached at 24h post either IV or IP dosing. SC dosing showed relatively reduced but similar brain penetration and distribution. Data was expressed as means ± SD, n = 4 mice per group.
| IHC scoring, range 0–4 |
||||||
|---|---|---|---|---|---|---|
| Treatment | Collection Time (hrs) | Serum conc. (nM) | Brain conc. (nM) | Spinal Cord (nM) | Parenchymal staining | Neuronal staining |
| SC – single dose 20mpk | 1 | 3.1 +/− 1.2 | n.d. | n.d. | n.d. | n.d. |
| 24 | 194.9 +/− 57.6 | 8.4 +/− 1.6 | 5.8 +/− 1.6 | 0.9 | 1.0 | |
| IP – single dose 20mpk | 1 | 173.6 +/− 43.2 | n.d. | n.d. | n.d. | n.d. |
| 24 | 547.2 +/− 81.3 | 16.2 +/− 1.3 | 10.0 +/− 0.6 | 1.5 | 1.9 | |
| IV – single dose 20mpk | 1 | 419.5 +/− 105.9 | n.d. | n.d. | n.d. | n.d. |
| 24 | 545.0 +/− 104.7 | 16.8 +/− 1.9 | 9.4 +/− 1.5 | 1.9 | 1.4 | |
| SC – single dose 40mpk | 1 | 5.4 +/− 3.4 | n.d. | n.d. | n.d. | n.d. |
| 24 | 440.0 +/− 37.0 | 16.2 +/− 1.8 | 8.1 +/− 1.4 | 1.1 | 0.9 | |
| IP – single dose 40mpk | 1 | 225.5 +/− 57.8 | n.d. | n.d. | n.d. | n.d. |
| 24 | 1,092.4 +/− 75.5 | 22.4 +/− 4.4 | 9.7 +/− 2.3 | 1.5 | 0.9 | |
| IV – single dose 40mpk | 1 | 843.3 +/− 223.4 | n.d. | n.d. | n.d. | n.d. |
| 24 | 863.2 +/− 50.0 | 20.7 +/− 2.6 | 10.2 +/− 2.7 | 2.2 | 1.7 | |
| IV – multiple dose 20mpk | 48 | 4,301.8 +/− 326.9 | 23.3 +/− 2.7 | n.d. | n.d. | n.d. |
Discussion
Specific, non-invasive uptake of proteins across the blood-brain barrier through RMT has been established for over a decade, with many studies focused on the transferrin receptor. However, it has only more recently emerged that the expression and efficiency of the chosen receptor and binding characteristics of the biotherapeutic to the receptor, such as affinity, 16 pH-dependence, 32 valency, 15 , 18 , 19 epitope and conformation, are critical determinant factors for optimal CNS exposure. As noted by Yi X et al., 33 agile protein engineering technologies and sensitive in vitro / in vivo characterization methods are needed to select the optimal molecule.
In this study, we have investigated the RMT properties of TfR-specific DVD-Ig molecules. Engineering different binding affinities of DVD-Ig proteins carrying an anti-TfR1 domain was essential to identify optimal properties for all the DVD-Ig proteins against diverse therapeutic targets. Humanization by complementarity-determining region (CDR) grafting provided a quick and effective way to obtain affinity variants of anti-TfR1 antibodies, and enabled efficient application of the technology to various CNS targets and indications: RGMa for MS; TNF for Parkinson's Disease; Aβ for Alzheimer's Disease; and HER2 for brain cancer. It should be emphasized that all the DVD-Ig molecules generated in this study contained the affinity variants of a single parent antibody that recognizes one particular epitope on TfR1. Therefore, the range of affinities, which yielded significant brain penetration in this study, may not be applicable to other antibodies or DVD-Ig proteins recognizing a different epitope on TfR1. For example, in at least one instance in this study, the lowest affinity did not result in the highest brain penetration (anti-Aβ-SS-anti-TfR1(AB402) DVD-Ig, in Table 3) indicating that other factors, such as binding conformation, may play additional role in transport into the brain. The data reported here, which are consistent with other reports in the literature, indicate that for TfR-specific antibodies and bispecifics, both monovalent, 15 , 16 , 34 and bivalent formats, such as anti-TfR antibodies ( 16 , and this study) and bispecific fusion proteins,12-15 exhibit transcytosis across the BBB in vitro and/or in vivo. So far, in only one report, 34 one specific bivalent format (anti-TfR Fab fusion) resulted in lysosomal degradation rather than transcytosis. As discussed in detail in a recent study by Stanimirovic et al., 15 other determinants such as affinity, epitope and pH rather than just bivalency may play a role in the fate of this one particular fusion protein described by Niewoehner et al. 34 Here, lower binding affinity of bivalent DVD-Igs to the TfR1 mostly favored the entry of the molecule by transcytosis more efficiently; it remains to be seen if this observation is applicable to other TfR1-binding epitopes or other BBB receptors generally and indeed to all bispecific formats as well.
The DVD-Ig format has proven to be a robust platform for the investigation of the molecular determinants of BBB transcytosis through utilization of different combinations of inner or outer domains of each DVD-Ig arm and linkers with different length and sequences. Engineering flexibility of this platform enabled the incorporation of CNS target domains with high affinity or potency, while allowing exploration of the optimal affinity for the anti-TfR1 domain. This contrasts with other reported bispecifics (e.g., anti-TfR/BACE) that are asymmetric monovalent antibodies in which each arm binds to a different antigen, which had reduced affinity / avidity to CNS disease targets. 6 As Goulatis and Shusta 35 reviewed, knowledge in this field is just emerging, and making generalizations about parameters (e.g., binding conformation, affinity and valency) that dictate receptor binding, internalization, sorting and transcytosis based on one particular receptor or one antibody epitope on a given receptor or one particular bispecific format would be premature, potentially hindering further progress.
Candidate RMT receptors as a class are membrane proteins, often with complicated molecular structures where it will likely be necessary to characterize binding properties in the context of cell lines rather than with recombinant protein, as native biochemical and physiocochemical properties may be challenging to recover. Consistent with this generalizable approach, we developed a cell-based binding assay where native TfR1 is expressed on the cell surface, facilitating the characterization and selection of optimal DVD-Ig molecules with respect to the receptor binding. The utilization of the recombinant receptors used in a binding ELISA or surface plasmon resonance (SPR) measurement was abandoned due to artificially low or lack of binding to low affinity TfR1 antibodies owing to the difficulty of expressing any recombinant receptor protein with a native conformation and properties. For example, AB221, which binds to the mTfR1-expressing cells with an EC50 of 0.12 nM, showed about 50-fold lower binding affinity for recombinant mTfR1 (aa123-763). More importantly, AB405, which has an EC50 of 13.55 nM, failed to bind to recombinant mTfR1 (aa123-763) using the SPR method (data not shown).
The use of sensitive quantitative techniques that do not rely on radiolabel 13 , 14 or immunofluorescence-based signal amplification imaging 34 is critically important in the accurate assessment of brain uptake consistent with prior findings. 6 , 36 In this study, two orthogonal methods have been used, namely a sensitive quantitative electrochemiluminescence-based assay (ECL-MSD) to measure the brain extract tissue concentration (in nM) enabling characterization of brain versus serum exposure properties of each DVD-Ig protein, complemented by semi-quantitative IHC techniques to determine the corresponding location of changed protein levels in the same brain for each animal. It should be emphasized that neither of these methods would be sufficient alone because ECL-MSD quantification of the brain extracts is subject to extraction efficiency and includes residual endothelial cell binding by all IgGs even after perfusion, and IHC is only semi-quantitative scoring, but clearly shows endothelial, parenchymal and cellular localization of antibody/DVD-Igs in the brain. Having this type of drug level information will be invaluable for interpretation of future efficacy studies with respect to brain pharmacokinetic/PD relationships.
Our results indicate that control IgG or control DVD-Ig without a TfR1 binding domain, and other target-specific antibodies (including anti-TNF, anti-RGMa and anti-Aβ) used throughout this study, localized mainly to the BBB endothelial cells/vasculature with minimal parenchymal or neuronal staining. Measured brain extract concentration ranged between about 1 to 6 nM at 2–48 hours following single or multiple injections (20-50 mg/kg). At the microscopic resolution of this study, it is not possible to distinguish endothelial cell surface vs transcytosed IgG, so it is possible that some proportion of levels measured in extracts includes antibody that has not crossed the BBB. Under comparable conditions, all the anti-TfR1 DVD-Ig molecules carrying an active TfR1 binding domain localized additionally to parenchyma and neuronal cells, as well as BBB vasculature, with total brain concentrations elevated to about 4-11-fold higher levels (about 6 to 30 nM) compared to control IgG antibodies. Although concentration differences measured in extracts are sometimes modest, the apparent levels measured in controls are likely an overestimate of brain exposure due to endothelial cell surface binding.
The absolute brain concentrations achieved here for TfR1-specific DVD-Igs are commonly in the double-digit nM range, which is very much within an anticipated therapeutically active range for many biologics. This is supported by reports that bispecifics with observed PD responses accumulated at similar brain concentrations of bispecific antibodies (15-20 nM anti-TfR/BACE1 at 24 h) 16 or fusion proteins (about 2–3 nM in whole wild type mouse brain in 8 h), 34 following systemic injections at therapeutic levels (20-50 mg/kg), albeit measured using different quantitative assays. We have preliminary data showing that intrathecal injection of anti-TNF-GS-anti-TfR1(AB221)DVD-Ig (55 μg), which in mouse resulted in 17 nM of the DVD-Ig in the brain and 52 nM in the spinal cord after 5 days, showed efficacy in the Bennett pain model (data not shown). The amount of anti-TfR1(AB405)-SL-anti-TNFDVD-Ig present in the brain following an IV injection (20 mg/kg) was observed to be similar (16.8 nM, Table 7), indicating that physiologically relevant amounts could be delivered to the brain by systemic injection of DVD-Igs containing domains with RMT function.
IV administration has been the most commonly reported route for systemic injection of molecules capable of penetrating brain by RMT. As very little is known about the determinants of the biodistribution of biotherapeutics within the CNS, we also tested different routes of administration using two DVD-Ig molecules with 2 different CNS targets (Aβ and RGMa) and found that systemic injections of TfR1 DVD-Ig molecules by IV, IP and SC resulted in similar brain concentrations, albeit at lower levels with SC injections. Also, following a single IV injection, a DVD-Ig protein could be detected even after 96 hours (Table 2), and, through multiple injections, higher brain concentrations (e.g., 30.4 nM at 49.5 hours, Table 6) can be achieved. Data in Table 6 show that increasing the dose from 20 to 50 mg/kg resulted in significant increase in brain concentration of DVD-Ig, therefore demonstrating that at 20 mg/kg RMT capacity was not saturated. In addition, multiple (3X) 20 mg/kg dosing also gave higher brain concentration compared to the single 20 mg/kg dose, again indicating that receptor kinetics or capacity is amenable to reach higher brain exposures with repeat dosing.
In this study, we observed that distribution of the anti-TfR1 DVD-Ig molecules in brain appeared to be dictated mostly by location or expression of TfR as Purkinje cells in cerebellum showed darker staining regardless of the nature of the therapeutic targeting domain used. Purkinje cell transferrin receptor distribution in particular has been shown by IHC, 37 and TfR density has been shown to be normally 2–3 times higher in the cerebellum than in the cerebral cortex, in accordance with the Purkinje cell distribution. 38 , 39 It is possible that in diseased tissue, elevated expression of the CNS target might affect the distribution of the DVD-Ig, particularly targets that can be expressed on the cell membrane such as RGMa. As previously reported, 40 anti-TfR antibodies, which show transcytosis capability into brain, may also distribute to other organs due to wide-spread expression of TfR in almost all tissues.
In the past decades, substantial advancements have been made in non-invasive BBB technologies as antibody engineering capabilities have advanced. 41 Here, DVD-Ig engineering 42 provided us with the flexibility to harness different binding affinities/orientations as well as valency to obtain elevated CNS exposure in the context of a bispecific platform whose drug-like properties are superior to other fusion protein formats that have been studied. 34 , 43 We have demonstrated the ability to enhance exposure of multiple CNS targeting domains, providing a robust basis for future engineering of therapeutic DVD-Ig molecules for CNS diseases.
Materials and methods
Antibody and DVD-Ig generation
Chimeric variants of an anti-TfR1 antibody were generated by CDR grafting of mouse CDRs into human framework sequences. DVD-Ig binding proteins using parent antibodies with known amino acid sequences were generated by synthesizing polynucleotide fragments encoding DVD binding protein variable heavy and light chain sequences and cloning the fragments into a pHybE-D2 vector. 42 The linker sequences were N-terminal 5–6 amino acid residues (short, or “S”), or 11–12 amino acid residues (long or “L”), of C kappa or CH1 in the light chain and heavy chain, respectively. The following heavy chain linkers were used: HG-long (amino acid sequence – ASTKGPSVFPLAP), HG-short (ASTKGP) and GS-H10 (GGGGSGGGGS; or “GS”). Light chain linkers were LK-long (TVAAPSVFIFPP); LK-short (TVAAP) and GS-L10 (GGSGGGGSG or “GS”). In DVD names, the first letter indicates a heavy chain linker, the second letter stands for a light chain, e.g., SS means HG-short and LK-short linkers; SL means HG-short and LK-long linkers and GS stands for the case when both heavy and light chain linkers were GS. In DVD nomenclature, the first target/Ab name indicates the DVD outer domain location followed by linker name and the second target/Ab name, which indicates the inner domain of the DVD-Ig structure. The DVD binding protein constructs were cloned into and expressed in HEK293 cells and purified according to established methods. 42 Production quantity was determined by absorbance measured with Nanodrop. Percentage of monomer (200 kDa species) was determined by size-exclusion chromatography.
Cell-based transferrin receptor binding assay
For the cell-based ECL-MSD binding assay, HEK293 cells overexpressing mouse transferrin receptor were added onto MSD 96-well plates (MSD Cat# L15XB-3 / L11XB-3) and incubated at 37°C for 1 hour. Cells were blocked using 15% fetal bovine serum (Hyclone, Thermo Scientific Cat# SH300700.03) at room temperature for 30 min with mild agitation; plates were then washed with Dulbecco's phosphate-buffered saline (DPBS) 3 times and antibody / DVD-Igs were added. After 1 hour incubation at room temperature (incubating on ice for 1 h gave similar results), plates were washed with DPBS and goat anti-human Sulfo-TAG (MSD Cat# R32AJ-1) was added. Plates were incubated at RT for 1 hour, washed with DPBS and immersed in MSD read buffer T surfactant free (MSD Cat# R92TD-2) before reading on MSD SECTOR Imager 6000. EC50 values were obtained using GraphPad Prism 6 software package (GraphPad Software, Inc., La Jolla, CA).
Aβ binding assay
High binding MSD plates (MSD Cat# L15XB-3 / L11XB-3) were coated with 10 μg/ml of antibody/DVD-Ig overnight at 4°C. Next day, each plate was blocked with 3% MSD blocking buffer (MSD Cat# R93AA-01) for 1 hour at room temperature. Then, plates were washed with TTBS buffer (20mM Tris; 0.5% Tween, 150 mM sodium chloride; pH 7.5) 4 times and human β-amyloid peptide (1-42) (Calbiochem; Cat # PP69) was added. After incubating for 2 hours at room temperature, each plate was washed and anti-Aβ (4G8) Sulfo-AG (Meso Scale Diagnostics, LLC, Cat# D20RQ-3) was added. Plates were incubated at room temperature for 1 hour, washed and MSD read buffer T with surfactant (MSD Cat# R92TC-1) was added. MSD signal reading was performed with MSD SECTOR Imager 6000. Antibody/DVD-Ig EC50 values were calculated using GraphPad Prism 6 software package (GraphPad Software, Inc., La Jolla, CA).
TNF and RGMa neutralization potency assays
Neutralizing activities of DVD-Ig against human TNF were measured on the murine fibroblast L929 cells (ATCC Cat. No. CCL-1) treated with actinomycin D according to the method described previously. 44 Briefly, L929 cells were seeded in triplicate at 3 × 105 cells/well into a 96-well plate and cultured in RPMI 1640 medium supplemented with 10% (v/v) fetal bovine serum for 16 hours. Then, several dilutions of the antibodies were prepared in medium containing actinomycin D (2 µg/ml) and TNF (100 ng/ml) and incubated at 37°C for 16 hours. After the supernatant were removed, 3–4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazoliumbromide (5 mg/ml) (Sigma-Aldrich) was added and incubated in 37°C for 4 hours. SDS solution (10%) was then added to the well. After 24 hours of incubation at room temperature, color in each well was recorded by colorimeter at 570 nm. Blank control (culture alone), TNF control (TNF alone) and antibody control (antibody alone) were also designed in the experiment. The EC50 value was calculated by complex sigmoid non-linear regression analysis using SigmaPlot software (Systat software, Inc. Richmond, CA). RGMa potency assay was performed as described. 45
In vivo studies
Wild type female C57Bl/6N mice 6–8 weeks were purchased from Taconic Bioscience, Inc. (Germantown, NY). Mice were maintained and used following Institutional Animal Care and User Committee-approved protocol. Animals were injected with various amounts (5–50 mg/kg) of antibodies / DVD-Igs via IV, IP or SC route. Groups consisted of four animals per group unless otherwise indicated. After the indicated time (1-96 hours), animals were euthanized with an overdose of ketamine-xylazine (Fort Dodge, Anased) administered via IP injection. The right atrium was incised and animals were transcardially perfused with cold Dulbeccos's phosphate-buffered saline (PBS) containing heparin (1000 units/L) at a rate of 2 ml/min for 10 min via programmable peristaltic pump (NE-1000). Serum and tissue were collected.
Measuring antibody concentration in mouse brain, spinal cord and serum
Brain was dissected from each perfused mouse, vertically divided into equal halves; one half was saved for IHC (see below), and the other half was homogenized using Bullet Blender Blue (NextAdvance, BBX24B) and zirconium beads (NextAdvance, ZROB05/ZROB10) in 1% NP-40 (Thermo Scientific Cat# 28324) in PBS containing protease inhibitors (Roche Diagnostics Complete Mini, EDTA-Free Ref# 11836170001). Homogenized brain samples were rotated at 4°C for 1 hour before spinning at 14,000 rpm for 20 min. Supernatant was isolated and antibody measurement in brain was made using an ECL-MSD assay.
Spinal cord was collected in some experiments. After an incision was made post axis and prior to hips, spinal cord was displaced from the spinal cavity using syringe containing PBS. A segment of spinal cord (0.06-0.08 g) was cut, placed in a cryogenic test tube and snap-frozen. A second segment of spinal cord (0.06-0.08 g) was cut and placed flat on an IHC cassette and fixed in 4% PFA. The same homogenization and antibody detection methods for spinal cords were used as stated for brain tissue.
Whole blood was collected from tail nick or cardiac puncture (terminal). Whole blood from tail nick was diluted 1:5 in assay buffer with EDTA and was snap-frozen. Whole blood from cardiac puncture was collected in serum separator BD microtainer™ tubes (BD Diagnostics, Ref# 365956), allowed to clot for 30 min and spun down at 13,000 rpm at room temperature for 8 min. Supernatant was isolated and antibody measurement in serum was made using an ECL-MSD assay.
Antibody or DVD-Ig concentrations in mouse serum and tissue samples were measured with an ECL-MSD assay. MSD 96-well plates (MSD Cat# L15XB-3 / L11XB-3) were coated with an F(ab’)2 fragment of donkey anti-human IgG Fc-specific polyclonal antibody (Jackson ImmunoResearch Code# 709-006- 098) at 2 ug/mL overnight at 4°C. Plates were blocked with 3% MSD blocking buffer (MSD Cat# R93BA-04) for 1 hour at 25°C. Plates were washed three times with 1X Tween-Tris buffered saline with a microplate washer (ELx45 Bio-Tek Instruments Inc.). Standards were made by serial dilution in 1% MSD assay buffer or 1% MSD assay buffer containing 0.1% serum. Tissue samples were diluted to 1:2 or 1:4 in 1% MSD assay buffer and serum samples were serially diluted starting at 1:10 in 1% MSD assay buffer and 25 uL (in duplicates) were added per well. Each antibody or DVD-Ig was used as an internal standard to quantify the respective antibody or DVD-Ig concentrations. Plates were incubated for 2 hours at 25°C and bound antibody was detected with goat anti-human Sulfo- TAG (MSD Cat# R32AJ-1). Plates were read on an MSD SECTOR Imager 6000. Concentration was determined from the standard curve with a Four-Parameter Logistic (4PL) nonlinear regression program from IDBS XLfit® an add-in of Microsoft® software. The ECL-MSD assay lower limit of quantitation values range from 0.05-0.46 ng/mL in serum and tissue samples. Molar concentration was calculated by the MSD quantification value (in ng/mL) that best fit its corresponding standard curve with a coefficient of variance ≤20% and within acceptable recovery of 80–120% divided by the respective antibody or DVD-Ig molecular weight. For tissue samples, multiplication of tissue homogenate dilution factor was considered. Data was expressed as means +/− SD and statistics were assessed by unpaired, two-tailed t-tests. Total protein concentrations in brain extracts measured using the BCA protein assay kit (Thermo Scientific, Cat#23225) were consistently found to be within 15% of the coefficient of variation. Samples that did not meet these criteria were not used for analysis.
Immunohistochemistry methods and analysis
Half brains from perfused antibody / DVD-Ig -treated mice were fixed in 4% paraformaldehyde for 6 hours. Following fixation, tissues were processed through a graded series RUSH protocol (Leica TP1050 Tissue Processor) of alcohol to xylene and then embedded in paraffin (Leica EG1150H). Brain sections (5 μM) were cut with a microtome (Microm, HM355S). Sections were de-paraffinized and rehydrated with water and placed into Tris with Tween-20 buffer (Teknova Cat# T5155). Staining was then performed on a Dako autostainer links 48 system or a Leica Biosystems BOND Rx stainer. Briefly, the sections were blocked with 3% hydrogen peroxide plus methanol for 30 min, washed with 10x Tris with Tween-20 buffer (Teknova Cat# T5155) then incubated for 8 minutes with protease I (Ventana Ref# 760–2018). For Dako autostainer, sections were blocked with a streptavidin and biotin blocking kit (Vector Laboratories Cat# SP-2002) for 8 minutes each, followed by Dako protein block for 30 minutes. Next, the sections were incubated for 1 hour at room temperature with a biotinylated donkey anti-human IgG (H+L) F(ab’) (Jackson ImmunoResearch Code# 709-066-149) at 15 μg/mL followed by an incubation with peroxidase-conjugated avidin for 30 minutes at room temperature (R.T.U ABC Kit (Vector PK-7100)). For Leica Bond, sections were blocked with DAKO protein block for 20 minutes and incubated for 1 hour at room temperature with rabbit anti-human (Southern Biotech 6145-01) or rabbit anti-hamster (Southern Biotech 6215-01). Then, sections were incubated for 10 min with Bond polymer refine detection (Leica DS9800) which uses polymeric horseradish peroxidase (HRP)-linker antibody conjugation system. The sections were then reacted with DAB chromogen (Dako Ref# K3468) for 3 minutes to form a brown precipitate, washed with water, counterstained with Gill Modified Hematoxylin (EMD Harleco Ref# 65065) for 30 seconds and bluing reagent by dipping slides 5–6 times in a reservoir (Richard-Allan Scientific Ref#7301), dehydrated and mounted for microscopy observation.
Sections from four different brain regions (forebrain, midbrain, hindbrain, cerebellum) from the four animals per group were stained. Representative staining images were captured by Olympus BX43 or slides were scanned with Pannoramic 250 Slide Scanner. All settings (filters and light levels) for each image were kept constant throughout the experiment. Brown DAB staining intensity of vasculature, parenchyma and neurons were visually scored under microscope using 0 to 4 scale, where 0 is no staining; 1 is light staining at small area of tissue; 2 is light staining at most area of the tissue, 3 is moderate staining at most area of tissue; 4 is strong staining at most area of tissue. Evaluation was blinded. Average score of each group (3 to 4) animals was reported.
Disclosures
Denise Karaoglu Hanzatian, Annette Schwartz, Jamie Erickson, Kangwen Deng, Ruth Villanueva, Christopher Stedman, Cristina Harris, Tariq Ghayur and Andrew Goodearl are employees of AbbVie. Farid Gizatullin was an employee of AbbVie at the time of the study. The design, study conduct, and financial support for this research were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication.
Acknowledgments
Authors would like to thank Jiyong Zhang for his help with IHC as well as helpful comments on the manuscript. We are also thankful to Tammy Dellovade and Jochen Salfeld for critical review of the manuscript and Susanne Scesney for data cross checking.
References
- 1.Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherited Metabolic Dis. 2013;36(3):437–49. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 2.Neuwelt E, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis T, Engelhardt B, Grammas P, Nedergaard M, Nutt J, et al.. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7(1):84–96. doi: 10.1016/S1474-4422(07)70326-5. PMID:18093565. [DOI] [PubMed] [Google Scholar]
- 3.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discovery. 2014;13(9):655–72. doi: 10.1038/nrd4363. PMID:25103255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardridge WM. Drug transport across the blood-brain barrier. J Cerebral Blood Flow Metab. 2012;32(11):1959–72. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abuqayyas L, Balthasar JP. Investigation of the role of FcgammaR and FcRn in mAb distribution to the brain. Mol Pharmaceutics. 2013;10(5):1505–13. doi: 10.1021/mp300214k. [DOI] [PubMed] [Google Scholar]
- 6.Watts RJ, Dennis MS. Bispecific antibodies for delivery into the brain. Curr Opin Chem Biol. 2013;17(3):393–9. doi: 10.1016/j.cbpa.2013.03.023. PMID:23570979. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv Drug Delivery Rev. 2012;64(7):640–65. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Roy S. Strategic drug delivery targeted to the brain: A review. Der Pharmacia Sinica. 2012;3(1):76–92. [Google Scholar]
- 9.Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14(2):282–95. doi: 10.1208/s12248-012-9339-4. PMID:22407288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh S. Nanomaterials as Non-viral siRNA delivery agents for cancer therapy. BioImpacts: BI. 2013;3(2):53–65. PMID:23878788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel M, Souto EB, Singh KK. Advances in brain drug targeting and delivery: limitations and challenges of solid lipid nanoparticles. Exp Opin Drug Delivery. 2013;10(7):889–905. doi: 10.1517/17425247.2013.784742. [DOI] [PubMed] [Google Scholar]
- 12.Pardridge WM. Drug targeting to the brain. Pharm Res. 2007;24(9):1733–44. doi: 10.1007/s11095-007-9324-2. PMID:17554607. [DOI] [PubMed] [Google Scholar]
- 13.Pardridge WM, Buciak JL, Friden PM. Selective transport of an anti-transferrin receptor antibody through the blood-brain barrier in vivo. J Pharmacol Exp Ther. 1991;259(1):66–70. PMID:1920136. [PubMed] [Google Scholar]
- 14.Pardridge WM, Kang YS, Buciak JL, Yang J. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. Pharmaceutical Res. 1995;12(6):807–16. doi: 10.1023/A:1016244500596. [DOI] [PubMed] [Google Scholar]
- 15.Stanimirovic D, Kemmerich K, Haqqani AS, Farrington GK. Engineering and pharmacology of blood-brain barrier-permeable bispecific antibodies. Adv Pharmacol. 2014;71:301–35. doi: 10.1016/bs.apha.2014.06.005. PMID:25307221. [DOI] [PubMed] [Google Scholar]
- 16.Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, Lu Y, Atwal J, Elliott JM, Prabhu S, Watts RJ, et al.. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. 2011;3(84):84ra44. doi: 10.1126/scitranslmed.3002230. PMID:21613623. [DOI] [PubMed] [Google Scholar]
- 17.Yu YJ, Atwal JK, Zhang Y, Tong RK, Wildsmith KR, Tan C, Bien-Ly N, Hersom M, Maloney JA, Meilandt WJ, et al.. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Science Transl Med. 2014;6(261):261ra154. doi: 10.1126/scitranslmed.3009835. [DOI] [PubMed] [Google Scholar]
- 18.Hackel BJ, Bohrmann B, Collin L, Urich E, Sade H, Maier P, Rueger P, Stracke JO, Lau W, Tissot AC, et al.. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. 2014;81(1):49–60. doi: 10.1016/j.neuron.2013.10.061. PMID:24411731. [DOI] [PubMed] [Google Scholar]
- 19.Bien-Ly N, Yu YJ, Bumbaca D, Elstrott J, Boswell CA, Zhang Y, Luk W, Lu Y, Dennis MS, Weimer RM, et al.. Transferrin receptor (TfR) trafficking determines brain uptake of TfR antibody affinity variants. J Exp Med. 2014;211(2):233–44. doi: 10.1084/jem.20131660. PMID:24470444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C, Ying H, Grinnell C, Bryant S, Miller R, Clabbers A, Bose S, McCarthy D, Zhu RR, Santora L, et al.. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol. 2007;25(11):1290–7. doi: 10.1038/nbt1345. PMID:17934452. [DOI] [PubMed] [Google Scholar]
- 21.Gu J, Ghayur T. Generation of dual-variable-domain immunoglobulin molecules for dual-specific targeting. Methods Enzymol. 2012;502:25–41. doi: 10.1016/B978-0-12-416039-2.00002-1. PMID:22208980. [DOI] [PubMed] [Google Scholar]
- 22.Shaw LM, Korecka M, Clark CM, Lee VM, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discovery. 2007;6(4):295–303. doi: 10.1038/nrd2176. PMID:17347655. [DOI] [PubMed] [Google Scholar]
- 23.Blennow K, Vanmechelen E, Hampel H. CSF total tau, Abeta42 and phosphorylated tau protein as biomarkers for Alzheimer's disease. Mol Neurobiol. 2001;24(1-3):87–97. doi: 10.1385/MN:24:1-3:087. PMID:11831556. [DOI] [PubMed] [Google Scholar]
- 24.Miles LA, Crespi GA, Doughty L, Parker MW. Bapineuzumab captures the N-terminus of the Alzheimer's disease amyloid-beta peptide in a helical conformation. Scientific Reports. 2013;3:1302. doi: 10.1038/srep01302. PMID:23416764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta AI, Brufsky AM, Sampson JH. Therapeutic approaches for HER2-positive brain metastases: circumventing the blood-brain barrier. Cancer Treat Rev. 2013;39(3):261–9. doi: 10.1016/j.ctrv.2012.05.006. PMID:22727691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller BK, Yamashita T, Schaffar G, Mueller R. The role of repulsive guidance molecules in the embryonic and adult vertebrate central nervous system. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1513–29. doi: 10.1098/rstb.2006.1888. PMID:16939972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwab JM, Monnier PP, Schluesener HJ, Conrad S, Beschorner R, Chen L, Meyermann R, Mueller BK. Central nervous system injury-induced repulsive guidance molecule expression in the adult human brain. Arch Neurol. 2005;62(10):1561–8. doi: 10.1001/archneur.62.10.1561. PMID:16216939. [DOI] [PubMed] [Google Scholar]
- 28.Marques F, Sousa JC, Coppola G, Geschwind DH, Sousa N, Palha JA, Correia-Neves M. The choroid plexus response to a repeated peripheral inflammatory stimulus. BMC Neurosci. 2009;10:135. doi: 10.1186/1471-2202-10-135. PMID:19922669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. PMID:18925972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leal MC, Casabona JC, Puntel M, Pitossi FJ. Interleukin-1beta and tumor necrosis factor-alpha: reliable targets for protective therapies in Parkinson's Disease? Front Cell Neurosci. 2013;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung L, Cahill CM. TNF-alpha and neuropathic pain–a review. J Neuroinflammation. 2010;7:27. doi: 10.1186/1742-2094-7-27. PMID:20398373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sade H, Baumgartner C, Hugenmatter A, Moessner E, Freskgard PO, Niewoehner J. A human blood-brain barrier transcytosis assay reveals antibody transcytosis influenced by pH-dependent receptor binding. PloS One. 2014;9(4):e96340. doi: 10.1371/journal.pone.0096340. PMID:24788759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi X, Manickam DS, Brynskikh A, Kabanov AV. Agile delivery of protein therapeutics to CNS. J Controll Rel. 2014;190:637–63. doi: 10.1016/j.jconrel.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niewoehner J, Bohrmann B, Collin L, Urich E, Sade H, Maier P, Rueger P, Stracke JO, Lau W, Tissot AC, et al.. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. 2014;81(1):49–60. doi: 10.1016/j.neuron.2013.10.061. PMID:24411731. [DOI] [PubMed] [Google Scholar]
- 35.Goulatis LI, Shusta EV. Protein engineering approaches for regulating blood-brain barrier transcytosis. Curr Opin Struct Biol. 2016;45:109–15. doi: 10.1016/j.sbi.2016.12.005. PMID:28040636. [DOI] [PubMed] [Google Scholar]
- 36.Bell RD, Ehlers MD. Breaching the blood-brain barrier for drug delivery. Neuron. 2014;81(1):1–3. doi: 10.1016/j.neuron.2013.12.023. PMID:24411725. [DOI] [PubMed] [Google Scholar]
- 37.Moos T. Immunohistochemical localization of intraneuronal transferrin receptor immunoreactivity in the adult mouse central nervous system. J Comp Neurol. 1996;375(4):675–92. doi: 10.1002/(SICI)1096-9861(19961125)375:4%3c675::AID-CNE8%3e3.0.CO;2-Z. PMID:8930792. [DOI] [PubMed] [Google Scholar]
- 38.Roskams AJ, Connor JR. Transferrin receptor expression in myelin deficient (md) rats. J Neurosci Res. 1992;31(3):421–7. doi: 10.1002/jnr.490310304. PMID:1640494. [DOI] [PubMed] [Google Scholar]
- 39.Roskams AJ, Connor JR. Iron, transferrin, and ferritin in the rat brain during development and aging. J Neurochem. 1994;63(2):709–16. doi: 10.1046/j.1471-4159.1994.63020709.x. PMID:8035195. [DOI] [PubMed] [Google Scholar]
- 40.Boado RJ, Zhou QH, Lu JZ, Hui EK, Pardridge WM. Pharmacokinetics and brain uptake of a genetically engineered bifunctional fusion antibody targeting the mouse transferrin receptor. Mol Pharm. 2010;7(1):237–44. doi: 10.1021/mp900235k. PMID:19921848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byrne H, Conroy PJ, Whisstock JC, O'Kennedy RJ. A tale of two specificities: bispecific antibodies for therapeutic and diagnostic applications. Trends Biotechnol. 2013;31(11):621–32. doi: 10.1016/j.tibtech.2013.08.007. PMID:24094861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DiGiammarino E, Ghayur T, Liu J. Design and generation of DVD-Ig molecules for dual-specific targeting. Methods Mol Biol. 2012;899:145–56. doi: 10.1007/978-1-61779-921-1_9. PMID:22735951. [DOI] [PubMed] [Google Scholar]
- 43.Pardridge WM, Boado RJ. Reengineering biopharmaceuticals for targeted delivery across the blood-brain barrier. Methods Enzymol. 2012;503:269–92. doi: 10.1016/B978-0-12-396962-0.00011-2. PMID:22230573. [DOI] [PubMed] [Google Scholar]
- 44.Fendly BM, Toy KJ, Creasey AA, Vitt CR, Larrick JW, Yamamoto R, Lin LS. Murine monoclonal antibodies defining neutralizing epitopes on tumor necrosis factor. Hybridoma. 1987;6(4):359–70. doi: 10.1089/hyb.1987.6.359. PMID:2442093. [DOI] [PubMed] [Google Scholar]
- 45.Severyn CJ, Shinde U, Rotwein P. Molecular biology, genetics and biochemistry of the repulsive guidance molecule family. Biochem J. 2009;422(3):393–403. doi: 10.1042/BJ20090978. PMID:19698085. [DOI] [PMC free article] [PubMed] [Google Scholar]


