Abstract
Age and sex are two of the three major risk factors for Alzheimer’s disease (ApoE-e4 allele is the third), with women having a two-fold greater risk for Alzheimer’s disease after the age of 75 years. Sex differences have been shown across a wide range of cognitive skills in young and older adults, and evidence supports a role for sex steroids, especially estradiol, in protecting against the development of cognitive decline in women. Sleep may also be a protective factor against age-related cognitive decline, since specific electrophysiological sleep events (e.g. sleep spindle/slow oscillation coupling) are critical for offline memory consolidation. Furthermore, studies in young women have shown fluctuations in sleep events and sleep-dependent memory consolidation during different phases of the menstrual cycle that are associated with the levels of sex steroids. An under-appreciated possibility is that there may be an important interaction between these two protective factors (sex steroids and sleep) that may play a role in daily fluctuations in cognitive processing, in particular memory, across a woman’s lifespan. Here, we summarize the current knowledge of sex steroid-dependent influences on sleep and cognition across the lifespan in women, with special emphasis on sleep-dependent memory processing. We further indicate gaps in knowledge that require further experimental examination in order to fully appreciate the complex and changing landscape of sex steroids and cognition. Lastly, we propose a series of testable predictions for how sex steroids impact sleep events and sleep-dependent cognition across the three major reproductive stages in women (reproductive years, menopause transition, and post-menopause).
Key terms: Sleep spindles, Progesterone, Polysomnography, Menopause, Menstrual cycle
Introduction
Behavior and brain function are sexually dimorphic across a range of cognitive domains including memory, emotion, visual perception and spatial navigation (Voyer et al. 1995, Astur et al. 1998, Lewin et al. 2001, Canli et al. 2002, Cahill 2006, McDevitt et al. 2014, Diekelmann et al. 2016, Voyer et al. 2016). Generally, women outperform men in verbal memory tasks including word recall and recognition, name recognition, as well as memory for emotional stimuli, while men outperform women in visual-spatial tasks and with certain mathematical abilities (Voyer et al. 1995, Lewin et al. 2001, Voyer et al. 2016). There is also a marked sex difference in risk for neurodegenerative conditions associated with memory and learning problems, like Alzheimer’s disease. In fact, women have a 2-fold greater risk for Alzheimer’s disease after 75 years, a difference not fully accounted for by longevity or level of education (Hamson et al. 2016). Although the underlying mechanisms for these sex differences are still unclear, sex steroids likely play a role, particularly in the context of aging (Henderson 2009, Brinton et al. 2015, Engler-Chiurazzi et al. 2017, Merlo et al. 2017, Pike 2017).
Decades of preclinical and human research have established the substantial effects of sex steroids on hippocampal-dependent memory (Luine 2014, Duarte-Guterman et al. 2015, Hamson et al. 2016). Natural fluctuations in sex steroids across the menstrual cycle and the gradual diminishing levels in women approaching menopause (menopausal transition) provide two experimentally tractable windows into their impact on cognition. Studies in reproductive age women have shown changes in cognition across the difference phases of the menstrual cycle, yet the results are inconsistent and studies are frequently underpowered (See (Sundstrom Poromaa and Gingnell 2014) for review). On the other hand, the menopausal transition combines endocrine and chronological aging with unique mechanistic impacts on memory function that may make the female brain vulnerable to development of dementia (Yin et al. 2015). For example, the menopausal transition is associated with altered hippocampal activity and connectivity, poorer memory performance (Jacobs et al. 2016), and more memory complaints and difficulties (Gold et al. 2000). Thus, the menopausal transition has been introduced as an important critical window for the early detection of cognitive vulnerability, and for implementation of preventive interventions. Importantly, the influence of sex steroids on memory may be mediated by their effect on other modifiable, protective biological factors that influence memory, such as sleep. A growing body of evidence shows that sleep is critical for the long-term formation of memories, with several electrophysiological events, including slow oscillations (SO) and sleep spindles, linked with memory consolidation (Walker and Stickgold 2004, Diekelmann and Born 2010, Abel et al. 2013, Rasch and Born 2013).
The age-related diminishment in these sleep electrophysiological features is thought to contribute to age-related decline in memory (Mander et al. 2013, Helfrich et al. 2018), and new research points to the possibility of modifying sleep in older adults to mitigate age-related cognitive decline (Westerberg et al. 2015, Bubu et al. 2017). Independent lines of research show that the same electrophysiological features critical for sleep-dependent memory differ between men and women and are also influenced by sex steroids: women have more slow wave sleep (SWS) and more slow wave activity (SWA, 0–4Hz) (Dijk et al. 1989, Armitage and Hoffmann 2001, Carrier et al. 2001, Redline et al. 2004), higher sigma activity, reflecting activity in the spindle frequency range (Carrier et al. 2001), and more spindles (Gaillard and Blois 1981) during sleep, than men. Further, sleep spindles are modified by sex hormone changes across the menstrual cycle (Driver et al. 1996). These data raise the possibility that sleep-dependent memory consolidation may differ between men and women and also according to changes in sex steroid levels in women, such as across the menstrual cycle and the menopausal transition, when sex steroid levels decline. However, to date, only an handful of studies have investigated of the intersection of sex steroids, sleep, sleep-dependent memory (Genzel et al. 2012), and none have examined this intersection in the context of aging.
The purpose of this review is to focus attention on the impact of sex steroids on the formation of long-term memories in women across reproductive stages. Broadly, we discuss the effects of sex steroid hormones on sleep and memory and on sleep-dependent memory consolidation, in particular, considering the key role of electrophysiological events such as sleep spindles and slow oscillations. We also consider the potential effect of exogenous hormones, such as in the form of oral contraceptives or hormone therapy, on memory. We integrate the findings within the context of current understanding of the development of Alzheimer’s disease in women, positing that sleep is an important, modifiable factor that may protect against cognitive decline and progression to Alzheimer’s disease. Lastly, we call attention to the need for further research about sex steroids, sleep, and memory consolidation, integrating these diverse research fields (also see Gervais et al. (2017) for discussion), to work towards promoting understanding of, and protecting against, memory decline in aging women.
Although sex differences in sleep and sleep-dependent memory consolidation is not the main focus of the current work, given the stark sex difference in the prevalence of insomnia across the lifespan, we have discussed how sex differences in insomnia could contribute to sex differences in cognitive decline in the Sidebar.
Memory Overview: Focus on the Hippocampus
Memory has traditionally been divided into two distinct categories by the types of memories that are processed and the neural mechanisms underlying them, (although see alternative theoretical frameworks (Eichenbaum 2004, Henke 2010). Non-declarative memories comprise implicit memories such as sensorimotor skills, perceptual skills, habits, priming, and processing of these types of information are not dependent on the hippocampus (Squire 1992). Declarative memories comprise explicit memories of events, places, and general knowledge, and processing of these types of memories are initially dependent on the hippocampus (Squire 1992). A simple, yet revealing, distinction can be made between the retrieval performance profiles of these two memory systems. Non-declarative memories are enhanced after a period of consolidation such that performance increases from the end of training (Mednick et al. 2003). In contrast, the facilitation of declarative memories usually consists of decreased forgetting after a post-encoding consolidation period (Jenkins and Dallenbach 1924, Plihal and Born 1997, Wixted 2004, Payne et al. 2008). Importantly, sleep plays a role in both of these consolidation processes, such that for declarative memories, less forgetting is shown after a period of sleep, compared with an equal period of wake, while for non-declarative memories, performance increases are shown after a period of sleep, compared to a period of wake. These opposing performance outcomes (i.e. enhanced memory performance versus decreased forgetting) raise the possibility that the consolidation of declarative and non-declarative memories relies on distinct mechanisms. Interestingly, aging is associated with greater decrements in declarative memory, compared with non-declarative memory (Figure 1, top panel). Therefore, in the next section, we will cover recent discoveries that shed light on potential mechanisms in non-rapid eye movement sleep that have been strongly implicated in hippocampal-dependent, declarative memory consolidation.
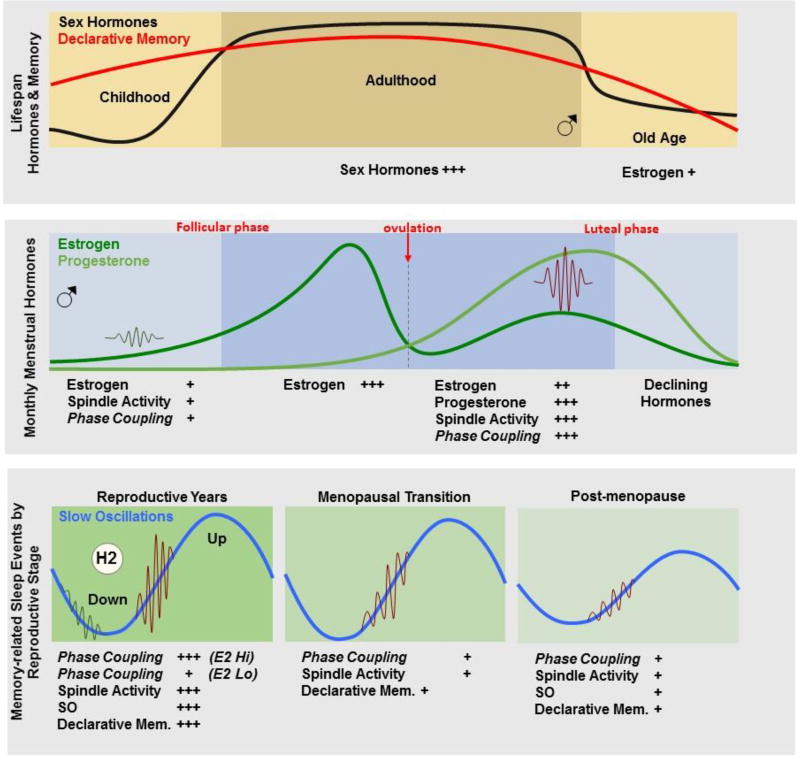
Figure 1.
The top panel depicts changes in sex hormones (black line) and declarative memory (red line) across the lifespan. The middle panel demonstrates fluctuations in ovarian hormones across the menstrual cycle, as well as changes in spindle activity (sigma EEG activity and spindle density) during the follicular and luteal phases of the menstrual cycle. We hypothesize that spindle/slow oscillation (SO) phase coupling may also fluctuate across the menstrual cycle. The bottom panel shows predictions for how memory-related sleep events (spindle activity and SO, and their phase coupling) may change across the reproductive stages. The left panel shows the reproductive years with monthly cycles of high hormone periods (luteal phase) containing high spindle activity, SO and spindle/SO phase coupling, as well as increased sleep-dependent declarative memory, compared with low hormone periods (follicular phase) characterized by less spindle activity and phase coupling and poorer sleep-dependent declarative memory. The middle panel depicts the menopause transition (midlife) when spindle activity begins to decline and there is the potential for impaired phase coupling and sleep-dependent declarative memory. During post-menopause (right panel, older age), women show lower spindle activity and SO, and potentially lower phase coupling, which may contribute to deficits in declarative memory. Overall, sleep is also more disturbed, associated with accumulation of Aβ in the brain, increasing risk for progression to Alzheimer’s disease. Links between sex hormone decline, less effective sleep-memory events and increased sleep disturbance, with Alzheimer disease risk in women remains to be determined.
Sleep-dependent memory consolidation
Sleep is not monolithic, but can be divided into stages of rapid eye movement (REM) and non-rapid eye movement (NREM) sleep, with NREM sleep further divided into Stages N1–3 (also termed Stages 1, 2 and slow wave sleep (SWS)) in which EEG brain activity becomes progressively slower and with greater synchrony of large amplitude slow waves throughout the cortex. Research has consistently shown that a period of NREM sleep yields greater memory retention in declarative memory than a comparable period of REM sleep or waking activity (Yaroush et al. 1971, Barrett and Ekstrand 1972, Plihal and Born 1997, Plihal and Born 1999). Several electrophysiological features of NREM sleep have been linked with memory consolidation, with the majority of these studies focusing on the role of Slow Oscillations (SOs, <1Hz) (Rasch and Born 2013, Genzel et al. 2014).
The breakthrough discovery in rodents that the spatio-temporal firing patterns of hippocampal place cells during waking got replayed during sleep, and that the replay was associated with hippocampal sharp wave ripples temporally coupled with NREM SOs (Buzsaki 2015), established a connection between cognitive processes during wake and specific offline electrophysiological NREM sleep events (Genzel et al. 2014). Although the exact nature of the NREM sleep mechanisms that contribute to memory consolidation are not understood, several theoretical models have been proposed, all of which posit the importance of coordination between some combination of cortical, hippocampal and thalamic brain areas via the coupling of neural oscillations associated with each area (Tononi and Cirelli 2006, Diekelmann and Born 2010, Mednick et al. 2011, Genzel et al. 2014). In particular, studies support a role of NREM SOs in memory consolidation (Diekelmann and Born 2010). SOs are cortically-driven <1Hz waveforms with high voltage up and down states, which reflect global periods of neuronal spiking and neuronal silence, respectively. Multiple different causal interventions have been used to enhance SOs during post-training sleep and subsequent memory, including transcranial direct current stimulation within the SO frequency range (Marshall et al. 2006) and closed-loop triggering of an auditory click during the down to up transition of an SO (Ngo et al. 2013).
Recent studies have shown that declarative memory performance in humans can be enhanced by reactivating memories during NREM, but not REM sleep. Specifically, during targeted memory reactivation (TMR), information is encoded with an associated cue (e.g., odor or sound) and then the cues are delivered during NREM, which enhances memory for the cued stimuli, suggesting a causal role of NREM in the memory consolidation process. A recent combined approach delivering the associated auditory cues during the down-to-up transition of the SO has also shown enhanced post-sleep, declarative memory, narrowing the effect of TMR to a specific phase of the NREM SO (Shimizu et al. 2018). Together, these developments demonstrate a critical role of NREM SOs for memory consolidation, as well as the potential to utilize these interventions to treat age-related deterioration in memory. However, further work is needed given that study outcomes using TMR are conflicting and small variations in the experimental manipulation may lead to different memory outcomes (e.g. enhanced, decreased or unaltered, see Schouten et al. (2017) for a review).
Sleep spindles are another electrophysiological feature of NREM sleep that play a role in memory consolidation. Sleep spindles are bursts of oscillatory activity generated by γ-amininobutyric acid (GABA) ergic neurons in the thalamus and the local circuit between thalamocortical and thalamic reticular nucleus cells. Repetitive spike-bursts cause rhythmic inhibitory post-synaptic potentials in thalamocortical pathways (Steriade et al. 1987, Wang and Rinzel 1993). These oscillations are then relayed to the cortex (Steriade et al. 1993, von Krosigk et al. 1993) and appear morphologically as spindles in the sleep EEG. Spindles are defined as groups of waves in the 9–16 Hz sigma range in NREM sleep, lasting at least 0.5 s (De Gennaro et al. 2005, Bodizs et al. 2009). Two kinds of spindles can be differentiated by distinct spatiotemporal dynamics. “Slow” spindles (<12 Hz, with a spectral peak ~ 10.2 Hz) predominate over frontal sites and are more pronounced during SWS than Stage 2 sleep, whereas “fast” spindles (> 12 Hz, with a peak spectral frequency ~ 13.4 Hz) are more densely distributed over parietal and central sites (Mölle, Bergmann, Marshall, & Born, 2011). However the functional difference between these spindle types is unclear (Andrillon et al., 2011; Timofeev & Bazhenov, 2005).
Regarding their role in memory consolidation, the number of sleep spindles: 1) increases following hippocampal-dependent learning (Eschenko et al. 2006); 2) is temporally coupled with hippocampal sharp wave ripples after spatial navigation in rodents (Siapas and Wilson 1998); 3) facilitates the integration of newly learned information with existing knowledge in humans (Tamminen et al. 2010); and, 4) correlates with better retention of declarative memories (Gais et al. 2002, Schabus et al. 2004, Clemens et al. 2005, Schmidt et al. 2006). In addition, pharmacologically enhancing sleep spindles in humans with zolpidem enhanced verbal memory performance compared with placebo or sodium oxybate (Mednick et al. 2013). Also, reactivation of hippocampal-neocortical representations during sleep is coupled with sleep spindles (Bergmann et al. 2012). As mentioned above, a potential mechanism for consolidation may involve temporal coupling between brain areas associated with replay, and SO up and down states may provide a temporal framework for synchronized communication between these areas (Molle et al. 2011). For example, following learning in a hippocampal-dependent task, sigma activity during the up-state of the slow oscillations was increased (Molle et al. 2009). Further, memory-boosting interventions such as auditory and electrical stimulation during sleep increased SO-coupled sigma activity (Marshall et al. 2006, Ngo et al. 2013). Also, pharmacologically increasing spindle density with zolpidem increased the temporal consistency of spindle occurrences during the down-to-up phase of SOs, with performance improvement correlating with this spindle/SO timing (Niknazar et al. 2015). Together, these findings suggest that declarative memory consolidation is facilitated when thalamic spindles coincide with the down-to-up phase of cortical SOs.
Replay studies in animals report consistent evidence of coupling between hippocampal sharp wave ripples and cortical SOs. Indeed, this temporal coupling is proposed to be a key mechanism underlying “the hippocampal-neocortical dialogue characteristic of systems consolidation, whereby SOs provide a top-down temporal frame for hippocampal oscillatory events (Crunelli and Hughes 2010, Lemieux et al. 2014). Further, it is hypothesized that temporal coupling between the thalamus, hippocampus and cortex occurs via the nesting of individual sharp wave ripple events in the trough of succeeding spindles (Timofeev and Bazhenov 2005, Staresina et al. 2015), and that these “spindle-ripple” events may represent a bottom-up mechanism where reactivated hippocampal memory information (coded in ripples) is passed to spindles, which then reach neocortical networks via the SO temporal framework (Latchoumane et al., 2017). In summary, both human and rodent studies demonstrate a relation between NREM sleep events (SOs, sharp wave ripples, and sleep spindles) and hippocampal memory consolidation, although further research is needed to elucidate exact mechanisms. In the next sections, we will investigate how these sleep features, and consequently memory consolidation, vary in concert with hormonal changes in women, such as across the menstrual cycle and in the menopausal transition.
The reproductive years: Sex steroids, sleep, and memory
Changes in sex steroids across the menstrual cycle and their effects on the brain
Across their lifespan, from puberty to post-menopause, women experience fluctuations in reproductive hormones (Figure 1, top panel). During the reproductive phase, hormones fluctuate across the menstrual cycle, which typically lasts 28 days (Rousseau 1998).In a normal ovulatory menstrual cycle there are cyclical changes in four principal reproductive hormones, namely luteinizing hormone (LH) and follicle-stimulating hormone (FSH), released from the anterior pituitary gland, and estrogen and progesterone, released from the ovaries and corpus luteum (Figure 1, middle panel) . Estrogens are synthesized from cholesterol in the ovary in fertile women; there are several forms of estrogen, including estradiol, estrone, and estriol (Farage et al. 2009). Estradiol (E2) is the most potent estrogen and is commonly measured in research studies. The first half of the cycle (i.e., follicular phase; days 1–14) begins with menstruation (menses, day 1 – 6) with low levels of E2 and progesterone, followed by a rise in E2 that peaks on days 12–14, stimulating a surge in LH, which triggers ovulation (~day 14). An oocyte is released from the follicle and the corpus luteum then evolves from the ruptured follicle and secretes progesterone and E2 during the second part of the cycle (i.e., luteal phase; days 15–28) If there is no implantation, hormone levels decline during the last week of the luteal phase and menstruation occurs. Sex steroids not only are critical for reproductive function but also impact biology in other ways through their effects on the brain.
Sex steroid hormones released from the gonads during development permanently organize the neural substrate (organizational effects). Later, during puberty and across adulthood, they exert activational effects in the brain, acting through nuclear estrogen and androgen receptors to activate functional sex differences in physiology and behavior and exert influence on many biological systems (McCarthy and Arnold 2011). Estrogen and progesterone receptors are present in sleep/wake-regulatory nuclei, including the basal forebrain, hypothalamus, dorsal raphe nucleus, and locus coeruleus (Shughrue et al. 1997, Curran-Rauhut and Petersen 2002), and E2 inhibits activation of sleep-promoting neurons in the ventrolateral preoptic nucleus (Hadjimarkou et al. 2008). Sex steroids can also induce rapid changes through membrane receptors to activate cell-signaling pathways and, adding further complexity, they influence brain function through their interactions with other hormonal systems and neurotransmitters. For example, estrogens increase the production rate and receptor concentration of neuroamines like serotonin and norepinephrine (Arpels 1996) and increase GABA levels in certain brain areas (Mortola, 1996). Therefore, there is the potential for sex steroids to influence sleep through many pathways. Indeed, rodent studies that have tracked sleep-wake behavior across the reproductive cycle or administered E2 and progesterone to ovariectomized rodents, have confirmed that sex steroids modulate sleep-wake patterns (reviewed in Mong et al. (2011) and Mong and Cusmano (2016)).
Effects of the menstrual cycle on sleep
Studies in humans have taken advantage of menstrual-cycle related variation in sex steroids to explore their effects on sleep (reviewed in (Baker et al. 2016)). Most studies have found that latency to sleep and sleep efficiency remain stable across the menstrual cycle in young women (Driver et al. 1996, Baker and Driver 2007, Driver et al. 2008, Shechter and Boivin 2010). There is a small effect on REM sleep (decreased in the luteal phase (Driver et al. 2008, Mong et al. 2011), but percentage of SWS and SWA in NREM sleep, averaged across the night, do not change in young women (Ishizuka et al. 1994, Driver et al. 1996, Baker and Driver 2007), although one study found that SWA is significantly higher in the first NREM sleep episode of the luteal phase compared with the follicular phase (Driver et al. 2008).
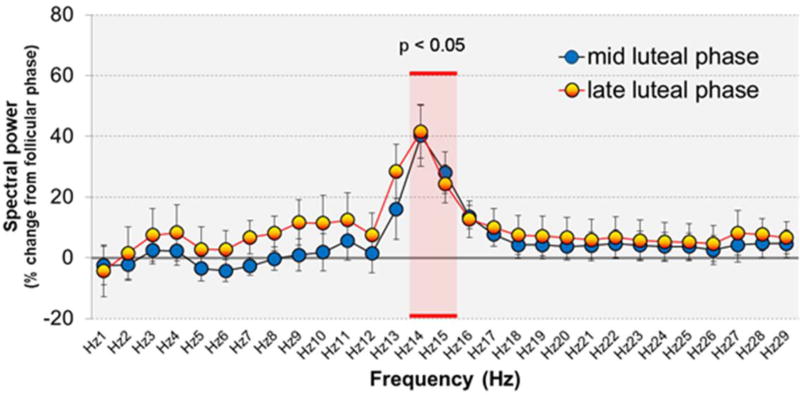
The most dramatic change in sleep in association with the menstrual cycle is an increase in EEG activity in sigma (spindle) frequency (Ishizuka et al. 1994, Driver et al. 1996, Baker et al. 2007, Baker et al. 2012) in the luteal phase, when progesterone and E2 are high, compared with the follicular phase (Figure 2). This increase in EEG activity in sigma activity likely reflects an increase in spindle density (Dijk 1995). While the mechanism for this increase in sleep spindles in the luteal phase is not fully understood, it is hypothesized to involve modulation of GABA-A receptors by progesterone metabolites (Driver et al. 1996), such as allopregnanolone, a potent positive allosteric modulator of the action of GABA at GABAA receptors (Harrison et al. 1987, Morrow et al. 1987). Allopregnanolone facilitates the occurrence of sleep spindles and exerts a similar effect on the high frequency EEG in rats, although it also attenuates power in the lower frequencies (Lancel et al. 1997, Damianisch et al. 2001), an effect not apparent in the luteal phase in young women. On the other hand, we previously failed to find a correlation between high progesterone and increased spindles in young women (Baker et al. 2012), and it remains to be determined what drives this menstrual phase-related increase in spindles. Given the dual presence of E2 and progesterone in the luteal phase, an effect of E2 and/or an interaction between progesterone and E2 on sleep spindles cannot be discounted. Further, other physiological changes that occur in the luteal phase, in association with the hormone milieu, such as raised body temperature (Deboer 1998) may be linked with increased spindling. In sum, the occurence and magnitude of memory-related sleep spindle activity (density and sigma) fluctuates with changes in sex steroids across the menstrual cycle in young, reproductive-age women. Given the importance of sleep spindles for memory consolidation, as described above, it is feasible that sleep-related memory consolidation may change across the menstrual cycle. The next section will review the effect on sleep of artificially altering the hormonal mileu with contraceptives.
Figure 2.
EEG spectral power during NREM sleep in 11 young women in the mid- and late-luteal phases (Progesterone >3 ng.ml1), relative to mid-follicular phase, showing the specific increase in sigma frequency activity (14–16 Hz), in the range of sleep spindles, in the luteal phase. Data taken from (Baker et al. 2012).
Effects of hormonal contraceptives on sleep
Combined oral contraceptives (OCs), used by millions of women around the world, typically contain ethinyl E2 and a synthetic progestin taken for 21 days and a placebo, taken for 7 days (when withdrawal bleeding occurs). Women taking OCs have high levels of synthetic hormones but low levels of endogenous E2 and progesterone, similar to levels in the follicular phase of non-users (Mordecai et al. 2008, Gogos et al. 2014). During the 7-day placebo interval, although the hypothalamic pituitary ovarian axis slowly regains activity, E2 levels remain suppressed until Day 7 (Mordecai et al. 2008). OCs are effective at preventing pregnancy through their multiple effects on the hypothalamic pituitary ovarian axis, leading to suppression of LH and FSH levels, a reduction in endogenous ovarian steroid levels, and thus inhibition of follicular development and ovulation (Cedars 2002). Ethinyl E2 has a high affinity for estrogen receptors and is more potent than endogenous E2 (Gogos et al. 2014). The type of progestin varies depending on the generation of OC, with second generation progestins (e.g. norgesterel) being more androgenic (the extent to which they can activate androgen receptors, a characteristic of progestins structurally related to testosterone), and third generation progestins being anti-androgenic (Gogos et al. 2014). OC brands also differ in the hormonal dose delivered in an attempt to optimize the balance between good cycle control and unwanted side effects like break through bleeding (Cedars 2002). Not only can the total hormonal dose differ across OC brands, with newer brands containing lower levels, but also the dose can vary across the 21 day period, with monphasic pills containing a constant dose of progestin and ethinyl E2, and triphasic pills containing a varying dose designed to mimic the production of progesterone across the natural menstrual cycle. Given the heterogeneous mix of OC combinations, even within studies, it is challenging to interpret different study effects.
Few studies have investigated the effects on sleep of OCs in women. Baker and colleagues (Baker et al. 2001) found that women had approximately 12% more Stage 2 (N2) sleep in the active phase (synthetic progestin + estrogen, monophasic OCs) versus the placebo phase of the oral contraceptive pack. Studies also show women taking OCs have more Stage 2 sleep and less SWS than naturally-cycling women in the luteal phase (Baker et al. 2001, Baker et al. 2001, Shine-Burdick et al. 2002). Given that Stage 2 sleep is a spindle-rich stage of sleep, it is tempting to speculate that OCs increase the number of sleep spindles, possibly to a greater extent than is evident in the natural luteal phase, however, none of these studies investigated the sleep EEG, and further studies are therefore needed to investigate whether OCs increase sleep spindles specifically. It should be noted that one study reported that use of medroxyprogesterone acetate (MPA), a synthetic form of human progesterone depot-injected and used as a contraceptive, was associated with greater activity in the sigma frequency band in addition to greater sleep spindle density and peak amplitude during NREM sleep in women (Plante and Goldstein 2013). This effect could be mediated through allopregnanolone acting on GABA-A receptors, since animal models have demonstrated that MPA increases central allopregnanolone (Bernardi et al. 2006).
Overall, few studies have investigated sleep architecture in women taking OCs, and only one has investigated the sleep EEG, finding increased spindle activity in users of MPA. However, MPA has negative effects on cognition (Braden et al. 2017), illustrating the complexity in interpreting how sex steroids could influence sleep on the one hand, and cognition, on the other, and this complexity extends to understanding effects of sex steroids on sleep-dependent memory consolidation.
Effects of sex steroids on hippocampus-dependent memory
Several studies have investigated the effects sex steroids (endogenous changes across the menstrual cycle, and exogenous in the form of OCs) on cognitive function, including memory, in women. As for sleep regulation, sex steroid hormones can influence cognitive function through nuclear estrogen and androgen receptors, distributed throughout the brain, including the prefrontal cortex and hippocampus. They can also act via membrane receptors to activate cell-signaling pathways, thought to be critical for mediating the cognitive effects of estradiol (Korol and Pisani 2015), and through their interactions with other hormonal systems and neurotransmitters (Barth et al. 2015, Nguyen et al. 2016). While sex steroids can cross the blood-brain barrier to exert their effects, they are also synthesized de novo in the brain, and specifically in the hippocampus (Frick et al. 2015). In fact, recent work in rodents suggests that the hippocampus is an important source of estrogen in both male and female animals, which may be necessary for hippocampal memory formation (Frick et al., 2015).
E2 affects a diverse range of cognitive processes, with the preponderance of studies reporting enhancements of learning and memory. The reader is referred to detailed reviews about the effects of E2 across a range of cognitive domains that are out of the scope of the current review (Luine 2014, Duarte-Guterman et al. 2015, Hamson et al. 2016). Regarding memory in particular, E2 exerts its effects at sites in the cerebral cortex, basal forebrain, hippocampus, and striatum, with the medial prefrontal cortex and hippocampus being the most studied in this context (Luine 2014). Several mechanisms have been proposed for E2’s effect on memory, including increasing hippocampal dendritic spine density (Wnuk et al. 2012), modifying cell morphology, signaling and excitability in the hippocampal formation (McEwen 2002, Romeo et al. 2004, Spencer et al. 2008), and protecting against oxidative damage to brain tissue (Pourganji et al. 2014). Less is known about the effects of progesterone on memory, although estradiol and progesterone appear to interact to influence performance on hippocampal-dependent memory tasks in female rodents (Hamson et al. 2016). Progesterone administered alone has positive effects on some tasks but, in combination with E2, it abolished the positive effects of E2 on a non-spatial memory task (Bimonte-Nelson et al. 2006). Progesterone may also have an indirect memory effect via allopregnanolone, a neurosteroid converted de novo in the brain from peripheral produced progesterone (Reddy and Kulkarni 1998) (Silvers et al. 2003).
Studies of variability in cognitive function, including memory, across the menstrual cycle have generally shown that women perform better on cognitive tests favoring females (e.g., verbal fluency) during the late-follicular (E2 surge) or mid-luteal (high E2/progesterone) phase of the cycle, compared with the early follicular (menstruation) phase (when E2 is low), and they perform better on cognitive skills favoring males (e.g., spatial ability) during the low-hormone early follicular phase (Hampson 1990, Sherwin 2012, Hampson and Morley 2013). E2 levels correlated positively with verbal fluency and negatively with scores on a test of spatial ability in young, cycling women (Maki et al. 2002). Recent work has examined whether strategies used by women to solve a spatial navigation task vary with menstrual cycle phase: women in the early luteal phase (progesterone + E2) showed greater engagement of hippocampal spatial strategies, whereas women in the early and late follicular phase (low E2 and high E2, respectively) relied on striatal response strategies (no group differences were found in overall navigation) (Hussain et al. 2016).
Overall, the picture of how sex hormones influence hippocampal processing is still emerging, and research in this area has been hampered by small sample sizes, underpowered to detect modest effect sizes across menstrual cycle phases, and a lack of thorough within-subject comparisons across all phases of the menstrual cycle (See (Sundstrom Poromaa and Gingnell 2014) for review). Studies have also investigated the effects of OCs on memory, although the same caveats described earlier, such as different brands and doses, apply here too. Several studies show improved hippocampal memory in women using OCs (reviewed in (Gogos et al. 2014)). For example, Mordecai and colleagues found that women taking OCs had better verbal memory during the 21-day active phase, compared to their placebo phase (Mordecai et al. 2008). Importantly, the majority of studies investigating the effects of sex steroid hormones on memory performance have only examined general ability in simplistic laboratory tasks such as dot memory or word pair learning. Examination of more complex cognitive processes such as hippocampal-dependent long-term memory formation are crucial next steps in full characterization of sex steroid effects on cognition.
Sex steroid effects on sleep-dependent memory consolidation
The effect of sex steroids on aspects of cognition susceptible to experience-dependent change, which primarily occur during sleep (such as memory consolidation) has received little attention in the literature. In a study of 15 young women (18–30 years old), recorded both in the early follicular and luteal phases of the menstrual cycle, Genzel and colleagues investigated changes in declarative memory and motor performance between a learning phase and retesting following either a nap or wake condition (Genzel et al. 2012). Women had a greater increase in performance on the declarative task after a nap compared to wake in the luteal phase but not in the follicular phase, suggesting that sleep-related memory consolidation depends on menstrual cycle phase in women. The authors ran correlational analyses to explore links between sex steroids, memory performance, and sleep spindles. In contrast to overnight studies (summarized above), women did not have a noticeable increase in spindle activity in the luteal phase relative to the follicular phase, possibly because of the short duration of stage 2 sleep in the nap (< 30 min, on average). However, they had more spindle activity (mean spindle amplitude × mean spindle duration) during a nap following learning compared with a control nap condition, in the luteal phase only, and E2 levels correlated with spindle density and frequency, suggesting that the menstrual cycle effect on declarative memory may be mediated by an interaction between E2 and sleep spindles (Genzel et al. 2012).
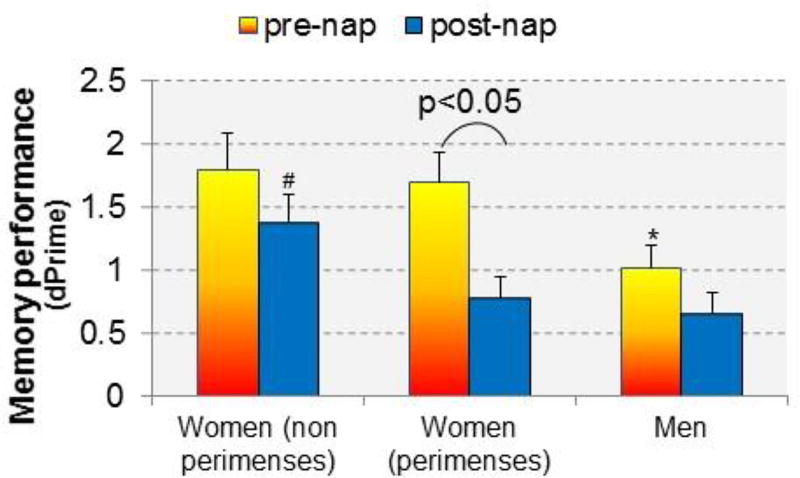
In our recent investigation, we considered the effect of self-reported menstrual cycle phase in addition to sex on memory consolidation of face-name associations following a daytime nap (Figure 3 (Sattari et al. 2017)). Replicating prior findings, women had superior memory at the pre-nap test compared with men. However, considering sleep-dependent improvement, we found that women had poorer post-nap memory consolidation during their menses phase compared with other times of the cycle and compared to men, similar to Genzel et al., (2012). Additionally, we found that men showed the typical associations between electrophysiological events during sleep (i.e., slow oscillations, spindles, and temporal coupling of spindles/SOs) and memory performance enhancements, whereas in women, the associations differed according to menstrual phase (Sattari et al. 2017). Specifically, sleep-dependent improvement correlated with slow oscillations when women were outside their menses, while sigma (spindle) activity correlated with improvement during menses.
Figure 3.
Memory performance before and after a nap in men and in women studied twice: (1) during perimenses (−5 days to + 6 days from first day of menstruation), when sex steroid hormones are expected to be low, and (2) at another time during the menstrual cycle, outside of perimenses (non-perimenses), when sex steroid hormones are expected to be higher. * significantly (p<0.05) poorer performance before the nap compared to women (non-perimenses). # significantly better performance after the nap compared to perimenses and men. Women in their perimenses recording showed significant forgetting after the nap. Data taken from (Sattari et al. 2017).
Together, these studies suggest that the effect of sex hormones on sleep-dependent memory consolidation may be mediated by modulation of electrophysiological events critical for thalamocortical replay (Figure 1, bottom left panel).
In a second study, Genzel and colleagues investigated the effect of exogenous sex steroids in the form of OCs on sleep-related memory consolidation in women (Genzel et al. 2014). Interestingly, they found that this group of women had a robust increase in declarative memory performance during retest compared to the learning phase but there was no additional benefit of a nap. The lack of benefit of a nap on memory consolidation in women taking OCs is similar to findings for women in the follicular phase (Genzel et al. 2012). Endogenous levels of E2 and progesterone were low in the women taking OCs (Genzel et al. 2014), a hormonal state that may not facilitate an extra boost to sleep-related memory consolidation that would otherwise occur from additional sleep (i.e. the nap), and suggesting that the exogenous hormones in OCs may not enhance sleep-dependent memory consolidation. However, comparison of data from both studies indicates that women taking OCs appeared to increase their performance during retest to a greater extent (~ 7 word pairs) than women in the follicular phase (~4 word pairs), regardless of a nap, suggesting that exogenous hormones might have an overall positive effect on memory consolidation (Genzel et al., 2014). Further studies are needed to investigate the effect of exogenous hormones on sleep-dependent memory consolidation, compared to endogenous hormones, and these studies need to carefully consider additional methodological issues, especially the heterogeneous mix of oral contraceptive combinations typically taken since different progestins may exert different effects (Gogos et al. 2014, Beltz et al. 2015).
As women progress from their reproductive years towards menopause, changes in the hormone environment occur, notably with a decline in E2. Given the above-mentioned influence of hormones on sleep, learning and memory, this change in hormonal environment could result in alterations in cognitive function, including poorer sleep-dependent memory consolidation, although this possibility has not yet been investigated, to our knowledge. In the following sections, we discuss literature that has considered changes in sleep as well as memory in the context of the menopausal transition to set the stage for future work.
The menopausal transition: Sex steroids, sleep, and memory
Changes in sex steroids across the menopausal transition
The menopausal transition, defined according to the Stages of Reproductive Aging Workshop (STRAW) criteria (Soules et al. 2001, Harlow et al. 2012), describes the years leading up to menopause, or final menstrual period (occurs at a median age of 51.4 years) (Burger et al. 2008). This transition period is a gradual process occurring over about 4 years, associated with changes in menstrual cycle lengths (early transition), progressing to increased variability in cycle lengths and skipping cycles (late transition), and finally, menopause. Overall, there is an increase in FSH and decline in E2 from early to late transition. But, within any ovulatory menstrual cycle, follicular phase E2 levels tend to be lower, luteal phase E2 levels higher, and progesterone levels lower, than in younger women (Hale et al. 2007). Early post-menopause (1–6 years after final menses) is characterized by a continued rise in FSH and decline in E2 particularly over the first two years (Harlow et al. 2012) after which levels gradually stabilize. Perimenopause is a common term to describe the menopausal transition and first year post-menopause.
For midlife women, the menopausal transition combines endocrine and chronological aging with unique mechanistic impacts on memory function that could make the female brain vulnerable to development of dementia (Yin et al. 2015). Perceived and objective memory problems emerge in the menopausal transition (Woods et al. 2000), with many women reporting increased forgetfulness and memory problems (Greendale et al. 2011). Thus, the menopausal transition may represent a critical window for the early detection of cognitive vulnerability, and for implementation of preventive interventions.
Changes in sleep across the menopausal transition
Midlife women transitioning to menopause and post-menopause are more likely to report sleep difficulties, with sleep difficulties due to frequent awakenings the most common problem (Joffe et al. 2010, Baker et al. 2018). Prevalence rates of self-report sleep difficulties range between 40–56%, compared to pre-menopausal women in the late-reproductive stage, who have rates of 31%. This greater prevalence of sleep difficulties in association with the menopausal transition, even after controlling for age, is evident across cross-sectional and longitudinal studies. PSG studies of women without severe sleep complaints (insomnia) and/or severe hot flashes have generally not found poorer PSG-defined sleep in peri-menopausal or post-menopausal women compared to premenopausal women (Young et al. 2003). However, in the presence of insomnia and hot flashes, PSG sleep disturbances, including increased wakefulness, are apparent and match the subjective experience of sleep (Baker et al. 2015).
A limited number of studies has investigated the association between sex steroids and EEG sleep features, particularly those implicated in sleep-dependent memory consolidation, in women in the menopausal transition. Higher levels of FSH correlated with more PSG-defined wakefulness in women in the menopausal transition similarly to findings in reproductive-age women (de Zambotti et al. 2015). On the other hand, another study reported that increasing FSH over time, reflecting more advancement through the menopausal transition, was associated with an increasing proportion of SWS (although not with slow wave EEG activity) (Lampio et al. 2017). In an analysis of PSG changes across the menstrual cycle in women in the menopausal transition who were still cycling, we found that the luteal phase was characterized by more awakenings and arousals and less N3 sleep percentage (de Zambotti et al., 2015). This finding is in contrast to findings described above for young women, who typically do not show PSG disturbances in the luteal phase, and suggests that sleep in midlife women may be more vulnerable to the physiological changes associated with the luteal phase.
Consistent with findings in young women, the luteal phase was associated with elevated EEG activity in the 14–17 Hz range, which encompasses the upper frequency range of spindles, as well as greater spindle density compared with the follicular phase (de Zambotti et al., 2015). These menstrual cycle phase effects were evident in women with and without insomnia disorder developed in the context of the menopause transition, although the increase in sigma frequency activity was blunted in women with insomnia (de Zambotti et al. 2015). Potentially, these weaker sleep spindles could impact memory consolidation in this group of women, although no studies have yet investigated this possibility (Figure 1, bottom middle panel).
A critical factor for sleep disruption in the context of the menopausal transition is the presence of hot flashes. Hot flashes are experienced by up to 80% of women, peaking in the late menopausal transition and first 2 years after menopause and declining in late menopause (> 6 years after final menstrual period) (Bacon 2017). Hot flashes are strongly associated with disturbed sleep architecture and quality (Ohayon 2006, Joffe et al. 2010, Baker et al. 2015), and are also linked with poor memory function (Drogos et al. 2013). Recent studies have reported an association between physiologically recorded hot flashes during sleep and altered brain functional connectivity (Thurston et al. 2015) and white matter hyperintensities (Thurston et al. 2016), but to date no study has thoroughly investigated the intersection between sleep, hot flashes, brain function and memory during menopause.
In summary, women report more sleep difficulties as they go through the menopausal transition. While not all women show PSG sleep disturbances, groups of women who are symptomatic (with insomnia and hot flashes) have more PSG-defined wakefulness, showing the importance of considering the presence and severity of menopausal symptoms. Importantly for the consideration of sleep-dependent memory consolidation, women in the menopausal transition with insomnia showed a less robust increase in spindle frequency activity during the luteal phase. In the context of the menopausal transition, multiple factors could disrupt sleep, including hormone changes and symptoms like hot flashes, and ultimately impact memory consolidation. Studies are needed to investigate this possibility.
Changes in memory across the menopausal transition
Complaints of memory difficulties are common during the menopausal transition (Mitchell and Woods 2001, Maki 2013). Women in the menopausal transition in clinical samples and in samples representative of the general population report increased forgetfulness (Gold et al. 2000). Subjective memory complaints are associated not only with menopausal stage, but also with advancing age, low education, lack of full-time employment, low physical activity, hot flashes, and concentration difficulties. In addition, considerable inter-individual variation in subjective complaints and objective memory outcomes exist, but subjective complaints nevertheless correlate significantly with memory performance (Drogos et al. 2013). Studies about memory performance have been inconsistent, with some studies finding a decline in verbal memory across the menopausal transition (Berent-Spillson et al. 2012, Epperson et al. 2013, Karlamangla et al. 2017), while others report no or only subtle effects of menopause on memory (Henderson and Popat (2011), (Henderson et al. 2003, Luetters et al. 2007, Greendale et al. 2010, Unkenstein et al. 2016). Investigations of relationships between memory and E2 levels also been mixed in cohort studies of women in the menopausal transition, with no relationship found between E2 levels and memory performance in adjusted models (Luetters et al. 2007, Ryan et al. 2012, Epperson et al. 2013) although higher levels of free E2 were related to better semantic memory (naming) in one cohort (Ryan et al. 2012) and with better recall in another (Rentz et al. 2017). As shown in rodent models (Koebele and Bimonte-Nelson 2017), task complexity may be a critical factor, and performance decrements may not be evident for simple tasks. However, even when using sensitive tasks of executive ability and incidental memory tasks, no difference in performance was found according to menopausal stage; although subjective perception of memory varied, with perimenopausal women feeling less content with their memory and reporting more memory lapses than pre- and post-menopausal women (Unkenstein et al. 2016). In summary, although memory complaints and memory difficulties are not universal, women in the menopausal transition who report memory problems do perform lower on memory tests (Maki 2015).
Given the potentially subtle behavioral changes in memory across the menopausal transition, recent studies have applied functional magnetic resonance imaging (fMRI) to investigate neural activity during cognitive processing - which could change in advance of measureable behavioral changes - in groups of women at different reproductive stages (Berent-Spillson et al. 2012, Jacobs et al. 2016). In a large study of women in a narrow age range (45–55) years, designed to minimize the potential confounding effect of age, Jacobs et al (2016) found that premenopausal and perimenopausal women recruited left hippocampus during a verbal encoding paradigm more strongly than postmenopausal women, whereas postmenopausal women had greater bilateral hippocampal connectivity relative to the other two groups, independent of chronological age. Better retrieval on the task was related to the magnitude of encoding-related activity within memory circuitry. Further, lower E2 was related to greater left-right hippocampal connectivity and less left hippocampal activity, implicating sex steroids in the regulation of this circuitry (Jacobs et al. 2016).
Taken together, studies show subtle declines in verbal learning and memory across the menopausal transition, with some of these changes correlating with declining E2 levels, and recent evidence relating differences in behavior with differences in neural activity in the hippocampus.
Effects of hormone therapy on memory
Menopausal hormone therapy (MHT) consists of estrogen alone (in women who have had a hysterectomy), or estrogen plus continuous or cyclical progestin. The formulations differ from those used for OCs and have lower potency (Gomes and Deitcher 2004). The two types of estrogen commonly used in MHT are conjugated equine estrogens and micronized E2 (Gomes and Deitcher 2004). MHT has shown mixed effects on cognitive function in post-menopausal women (Koebele and Bimonte-Nelson 2015, McCarrey and Resnick 2015). Notably, preclinical and clinical studies show that formulations containing medroxyprogesterone acetate have a negative effect on memory (Shumaker et al. 2003, Braden et al. 2016) and recent results from the KEEPS study found that even if initiated in recently postmenopausal women, according to the critical window hypothesis (Resnick and Henderson 2002, Sherwin 2012, Maki 2013, Singh et al. 2013) cognitive function was not improved with hormone therapy (E2/micronized progesterone (Gleason et al. 2015). Similarly, the Early vs Late Intervention Trial with Estradiol (ELITE), a randomized double-blind, placebo-controlled trial designed to test the critical window hypothesis, found no clinically-meaningful effect of oral 17β-estradiol on verbal memory, executive functions, or global cognition regardless of whether therapy was initiated within 6 years versus 10+ years after menopause (Henderson et al. 2016). Despite this evidence, results in animals continue to support the critical window for estrogen therapy, showing differential responses to E2 treatment depending on proximity to menopause onset and age (Galea et al. 2017).
Even though an effect on behavioral measures may not always be apparent in clinical studies, brain imaging studies have indicated that MHT influences the structure and function of regions important for memory such as the hippocampus (see Wnuk et al. (2012) and Comasco et al. (2014) for review). In a recent prospective study in post-menopausal women aged 52–60, posterior hippocampal gray matter volume was greater in women who received 2mg E2 for three months, compared to those who receive 1mg or placebo (Albert et al. 2017). However, other studies evoking placebo designs have not always found an association between E2 and greater hippocampal volumes (Resnick et al. 2009). These null results could be due to the typically short duration of such trials, relative to ‘real’ E2 therapy or due to the fact that E2 was administered outside of the potential ‘critical window’ for treatment (i.e. many years after the onset of menopause). Studies have also investigated how brain responses during cognitive tasks may be altered as a function of MHT. Overall, fMRI and PET studies have found that E2 therapy enhances fronto-cingulate brain regions during cognitive tasks (Comasco et al. (2014). Progestogen may counteract effects of E2, as although some studies identified increases in prefrontal cortical activation compared to placebo, effects were smaller compared to E2 only treatment (Smith et al. 2006, Persad et al. 2009). In addition, the majority of fMRI studies have revealed that menopausal women that began E2 therapy before or close to the onset of menopause had greater activation in the medial temporal lobe during verbal memory tasks, compared to those that did not undergo E2 therapy.
To date, only one study has attempted to include measures of sleep quality, hot flashes and brain function alongside E2 therapy. Joffe et al. (2006) identified greater inferior frontal and parietal response during verbal memory and working memory tasks for E2-treated participants compared to placebo-treated participants with marginally better memory performance for E2-treated participants. Performance or fMRI response were not correlated with self-reported sleep quality, however, those in the E2 group who reported hot flashes at baseline were more likely to show improvement in memory performance, suggesting that E2’s interaction with hot flashes, brain function and memory warrants further investigation.
In summary, studies of women in the menopausal transition show increased prevalence of memory complaints, which could be linked with declining E2 levels as well as with the emergence of symptoms such as hot flashes. While it would thus be expected that replacing E2 would improve memory, findings from clinical studies are controversial and conflict with the findings of preclinical studies in animal models. Brain imaging studies, however, show greater sensitivity to the effects of MHT on the brain and may reveal complex interactions between hormones, symptoms, and memory in the menopausal transition. To our knowledge, studies have not yet considered the intersection between the hormone changes of the menopausal transition and sleep-dependent memory consolidation.
Beyond menopause: sleep and cognitive decline in aging women
After menopause, FSH continues to increase and E2 continues to decrease until levels stabilize, over a period of about 5–8 years. At this point, further changes in reproductive endocrine function are limited and processes of somatic aging are more critical (Harlow et al. 2012). Given the co-occurrence of sleep and cognitive decline with aging, these factors may be linked (Nebes et al. 2009, Pace-Schott and Spencer 2011, Lim et al. 2012, Wilckens et al. 2012), although few studies have directly investigated whether sleep spindles and slow waves are as effective at consolidating memory in older men and women. Certainly, older adults do not appear to benefit from sleep-dependent consolidation as much as younger adults (Backhaus et al. 2007, Spencer et al. 2007, Siengsukon and Boyd 2008, Siengsukon and Boyd 2009). Another study found that in older women (61–74), declarative memory correlated with spindle density (Seeck-Hirschner et al. 2012). Additionally, Peters and colleagues reported that sleep spindles were enhanced after a motor learning task in young adults but not older adults (Peters et al. 2008). Helfrich and colleagues recently examined thalamocortical coupling of spindles and SO in older adults in relation to fMRI brain activity and memory performance (Helfrich et al. 2018). Compared with younger adults, older adults showed different spindle/SO alignment and lower coupling over the frontal pole, and alignment in both groups predicted post-sleep declarative memory improvement. In addition, grey matter atrophy within the medial prefrontal cortex predicted inter-individual differences in the temporal phase coupling between SOs and spindles.
Sleep disturbance is not just a symptom of Alzheimer’s disease (Mander et al. 2016, Cedernaes et al. 2017). Chronic sleep disruption seems to increase Alzheimer’s disease risk (Yaffe et al. 2014); sleep is disrupted in subjective cognitive decline, a preclinical condition that may led to mild cognitive impairment (MCI) and Alzheimer’s disease (Lauriola et al. 2016); better sleep quality in older adults is associated with lower Alzheimer’s disease risk (Lim et al. 2013); and treatment of sleep disturbance associated with sleep apnea delays MCI (Osorio et al. 2015). Other work links sleep deficits directly to Alzheimer’s disease pathology (Aβ and tau) and shows a bidirectional relationship: sleep disruption in rodents and drosophila leads to Aβ and tau accumulation in the brain (Kang et al. 2009, Roh et al. 2012, Xie et al. 2013, Tabuchi et al. 2015, Cedernaes et al. 2017); conversely, increasing cortical Aβ fragments sleep(Kang et al. 2009, Roh et al. 2012). In patients with Alzheimer’s disease, MCI, as well as in cognitively normal older adults, poor sleep correlates with severity of Aβ and tau pathology (Spira et al. 2013, Liguori et al. 2014, Mander et al. 2015, Sprecher et al. 2015, Branger et al. 2016), with recent studies showing selective associations between SOs and spindles and Alzheimer’s disease pathology (Mander et al. 2015, Sharma et al. 2017). Aβ clearance through the glymphatic system occurs during sleep (Xie et al. 2013), providing one mechanism linking sleep disturbance and accumulation of Aβ in the brain.
Sleep is a potentially modifiable factor that could be enhanced to counteract memory decline and progression to Alzheimer’s disease, however, it may be more effective before the onset of disease when increased Aβ and tau accumulation in the brain may lead to insurmountable damage to areas supporting SOs and spindles. Importantly, it is unknown if there are sex differences in sleep-dependent memory consolidation in older men and women. Furthermore, the gradual minimization of sex hormones and their cycles during the menopausal transition and post-menopause has not been investigated as a possible contributor to decreased spindles, spindle/SO coupling, and the associated memory deficits in older age (Figure 1, bottom right panel).
Conclusion
Sex hormones impact women’s sleep and cognitive function, including memory, across the lifespan. During reproductive years, consistent cognitive profiles have emerged that link fluctuations in sex hormones to specific changes in sleep features that are critical for memory consolidation. During midlife - which coincides with the transition to menopause - these same sleep features decline, along with memory. However, the link between changes in hormones, sleep, and memory has not been addressed, and there are many theoretical and practical research challenges that need to be considered (See Table 1). Furthermore, the cognitive hallmark of aging is a progressive deterioration of declarative memory, whilst considerable changes to sleep also occur. Understanding the implication of hormones in sleep-dependent memory processes may inform new line of treatments to prevent the most severe manifestation of cognitive decline, i.e. Alzheimer’s disease. Further, the menopause transition can offer a unique time-window in which relations among hormones, neurophysiological sleep process and sleep-dependent memory processes can be investigated by isolating single factors known to affect distinct domains.
Table 1.
Research challenges and proposed research agenda for understanding links between sex steroids, sleep, and memory in women
| Research challenges |
|---|
|
| Research agenda |
|
Highlights.
Sleep electrophysiological events are critical for memory consolidation.
Menstrual and menopausal variations in sex steroids modify sleep and memory.
Sleep-dependent memory consolidation is enhanced during high-hormone menstrual phases, compared with low-hormone phases.
Impact of menopausal decline in sex steroids on sleep-memory consolidation is unknown.
Sidebar: Sleep-dependent memory consolidation and insomnia disorder: a sex-specific path to cognitive decline?
Insomnia is the most common sleep disorder, affecting about 10% of the general population. Although studies about sex-age interaction effects on polysomnographic (PSG) measures of sleep are conflicting, there is a clear sex by age interaction in the development of insomnia disorder, which is diagnosed based on self-report rather than PSG. There is a 2.75-fold increase in the risk for insomnia after the onset of menses in girls, with no sex differences in the risk of insomnia in the pre-pubertal stage (Johnson et al. 2006, de Zambotti et al. 2017). These differences are evident even after accounting for depression and pubertal stage. Increased risk for insomnia in women compared with men persists across life (Zhang and Wing 2006), even after accounting for the higher prevalence of anxiety and depression in women, making female sex one of the strongest predictors of insomnia risk. Reproductive stages impact insomnia prevalence in women- new-onset insomnia may manifest in women transitioning to menopause, with menopause-related changes in the hormone environment and menopausal symptoms, like hot flashes, considered key factors implicated in the etiology of the disorder (Baker et al. 2015, Baker et al. 2018).
Insomnia, diagnosed based on self-report, also shows objective alterations in sleep macro (Baglioni et al. 2014) and micro (Feige et al. 2013) structure. Elevated activity in electroencephalographic (EEG) high frequencies (e.g., EEG beta power), considered a sign of cortical hyperarousal, is a common finding in insomnia patients (reviews: Buysse et al. (2008); Riemann et al. (2015)). Other alterations in insomnia are evident in EEG features traditionally thought to be implicated in sleep-dependent memory consolidation, such as a suppression of SWS (slow wave sleep) and SWA (slow wave activity). However, few studies have evaluated whether there are underlying sex differences in EEG markers of insomnia. Buysse et al. (2008) found that women but not men with insomnia disorder had increased high frequency EEG activity (along with increased low frequency EEG activity, reflecting increased homeostatic sleep drive, and thus a dual state of ‘tired but wired’) compared to controls, suggesting sex-specific differences in insomnia. Our group showed elevated high frequency EEG activity during rapid eye movement (REM) sleep in women who develop insomnia in the context of menopause (Baker et al. 2015), and interestingly, they also did not show a robust increase in EEG activity in the spindle frequency band in the luteal phase relative to the follicular phase of the menstrual cycle (de Zambotti et al. 2015), differently from women without insomnia, and young women (Baker and Driver 2007).
Regardless of sex, insomnia disorder is associated with alterations in memory consolidation processes (particularly for declarative memories) (for a review, see Cellini (2017)). Insomnia patients, compared to controls, lack an increase in number of correct word-pairs recalled at retrieval compared to the pre-sleep learning phase in a declarative memory word-pair association task (Backhaus et al. 2006); reduced sleep-dependent improvements (less procedural memory consolidation) in a mirror-tracing task (Nissen et al. 2006); reduced improvement in mirror tracing draw time on a mirror-tracing task and a non-significantly attenuated verbal retention rate on a declarative verbal memory task (Nissen et al. 2011); greater susceptibility to interference in a declarative word-pair association task (Griessenberger et al. 2013); and lack of a sleep-related enhancement in performance at a sequential finger tapping task (Cellini et al. 2014). In these studies, several sleep-specific features (e.g., amount of REM sleep, REM density, amount of SWS, activity in the spindle frequency band) were related to memory performance.
In summary, insomnia disorder and sleep-specific features characterizing insomnia seem to be implicated in the alteration of sleep-dependent memory processes. The long-term consequences of insomnia on sleep-dependent memory processes are still unknown, although it has been hypothesized that insomnia may be a factor implicated in the development of dementia. In an 8-year longitudinal study, normal older adults with insomnia showed a faster progression to Alzheimer’s disease compared to non-insomnia participants (Osorio et al. 2011). Whether changes in reproductive hormones (such as those occurring during menopause), and/or sex-specific factors can account for or interact with the link between insomnia disorder and dementia, needs to be determined.
Acknowledgments
Grants:
FCB: HL103688
SCM: R01AG046646
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abel T, Havekes R, Saletin JM, Walker MP. Sleep, plasticity and memory from molecules to whole-brain networks. Curr Biol. 2013;23(17):R774–788. doi: 10.1016/j.cub.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert K, Hiscox J, Boyd B, Dumas J, Taylor W, Newhouse P. Estrogen enhances hippocampal gray-matter volume in young and older postmenopausal women: a prospective dose-response study. Neurobiol Aging. 2017;56:1–6. doi: 10.1016/j.neurobiolaging.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armitage R, Hoffmann RF. Sleep EEG, depression and gender. Sleep Med Rev. 2001;5(3):237–246. doi: 10.1053/smrv.2000.0144. [DOI] [PubMed] [Google Scholar]
- 4.Arpels JC. The female brain hypoestrogenic continuum from the premenstrual syndrome to menopause. A hypothesis and review of supporting data. J Reprod Med. 1996;41(9):633–639. [PubMed] [Google Scholar]
- 5.Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav Brain Res. 1998;93(1–2):185–190. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 6.Backhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F, Junghanns K. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn Mem. 2007;14(5):336–341. doi: 10.1101/lm.470507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: Influence of sleep architecture and nocturnal cortisol release. Biol Psychiatry. 2006;60(12):1324–1330. doi: 10.1016/j.biopsych.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Bacon JL. The Menopausal Transition. Obstet Gynecol Clin North Am. 2017;44(2):285–296. doi: 10.1016/j.ogc.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Baglioni C, Regen W, Teghen A, Spiegelhalder K, Feige B, Nissen C, Riemann D. Sleep changes in the disorder of insomnia: a meta-analysis of polysomnographic studies. Sleep Med Rev. 2014;18(3):195–213. doi: 10.1016/j.smrv.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Baker FC, de Zambotti M, Colrain IM, Bei B. Sleep problems during the menopausal transition: prevalence, impact, and management challenges. Nature and Science of Sleep. 2018;10:1–23. doi: 10.2147/NSS.S125807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8(6):613–622. doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Baker FC, Joffe H, Lee K. In: Sleep and Menopause Principles and Practice of Sleep Medicine. Dement R Kryger., editor. Elsevier; 2016. [Google Scholar]
- 13.Baker FC, Kahan TL, Trinder J, Colrain IM. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep. 2007;30(10):1283–1291. doi: 10.1093/sleep/30.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker FC, Mitchell D, Driver HS. Oral contraceptives alter sleep and raise body temperature in young women. Pflugers Arch. 2001;442(5):729–737. doi: 10.1007/s004240100582. [DOI] [PubMed] [Google Scholar]
- 15.Baker FC, Sassoon SA, Kahan T, Palaniappan L, Nicholas CL, Trinder J, Colrain IM. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J Sleep Res. 2012;21(5):535–545. doi: 10.1111/j.1365-2869.2012.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker FC, Sattari N, de Zambotti M, Goldstone A, Alaynick W, Mednick S. Impact of sex steroids and reproductive stage on sleep-dependent memory consolidation in women. Neurobiology of Learning and Memory (under revision) 2018 doi: 10.1016/j.nlm.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker FC, Waner JI, Vieira EF, Taylor SR, Driver HS, Mitchell D. Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. J Physiol. 2001;530:565–574. doi: 10.1111/j.1469-7793.2001.0565k.x. (Pt 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker FC, Willoughby AR, Sassoon SA, Colrain IM, de Zambotti M. Insomnia in women approaching menopause: beyond perception. Psychoneuroendocrinology. 2015;60:96–104. doi: 10.1016/j.psyneuen.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett TR, Ekstrand BR. Effect of sleep on memory. 3. Controlling for time-of-day effects. J Exp Psychol. 1972;96(2):321–327. doi: 10.1037/h0033625. [DOI] [PubMed] [Google Scholar]
- 20.Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Frontiers in Neuroscience. 2015;9(37) doi: 10.3389/fnins.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beltz AM, Hampson E, Berenbaum SA. Oral contraceptives and cognition: A role for ethinyl estradiol. Horm Behav. 2015;74:209–217. doi: 10.1016/j.yhbeh.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Berent-Spillson A, Persad CC, Love T, Sowers M, Randolph JF, Zubieta JK, Smith YR. Hormonal environment affects cognition independent of age during the menopause transition. J Clin Endocrinol Metab. 2012;97(9):E1686–1694. doi: 10.1210/jc.2012-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergmann TO, Molle M, Diedrichs J, Born J, Siebner HR. Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage. 2012;59(3):2733–2742. doi: 10.1016/j.neuroimage.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 24.Bernardi F, Pluchino N, Pieri M, Begliuomini S, Lenzi E, Puccetti S, Casarosa E, Luisi M, Genazzani AR. Progesterone and medroxyprogesterone acetate effects on central and peripheral allopregnanolone and beta-endorphin levels. Neuroendocrinology. 2006;83(5–6):348–359. doi: 10.1159/000095400. [DOI] [PubMed] [Google Scholar]
- 25.Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24(1):229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- 26.Bodizs R, Kormendi J, Rigo P, Lazar AS. The individual adjustment method of sleep spindle analysis: methodological improvements and roots in the fingerprint paradigm. J Neurosci Methods. 2009;178(1):205–213. doi: 10.1016/j.jneumeth.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Braden BB, Andrews MG, Acosta JI, Mennenga SE, Lavery C, Bimonte-Nelson HA. A comparison of progestins within three classes: Differential effects on learning and memory in the aging surgically menopausal rat. Behav Brain Res. 2016 doi: 10.1016/j.bbr.2016.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braden BB, Andrews MG, Acosta JI, Mennenga SE, Lavery C, Bimonte-Nelson HA. A comparison of progestins within three classes: Differential effects on learning and memory in the aging surgically menopausal rat. Behav Brain Res. 2017;322:258–268. doi: 10.1016/j.bbr.2016.06.053. (Pt B) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Branger P, Arenaza-Urquijo EM, Tomadesso C, Mezenge F, Andre C, de Flores R, Mutlu J, de La Sayette V, Eustache F, Chetelat G, Rauchs G. Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiol Aging. 2016;41:107–114. doi: 10.1016/j.neurobiolaging.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11(7):393–405. doi: 10.1038/nrendo.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastiao YV, Wen Y, Schwartz S, Borenstein AR, Wu Y, Morgan D, Anderson WM. Sleep, Cognitive impairment, and Alzheimer’s disease: A Systematic Review and Meta-Analysis. Sleep. 2017;40(1) doi: 10.1093/sleep/zsw032. [DOI] [PubMed] [Google Scholar]
- 32.Burger HG, Hale GE, Dennerstein L, Robertson DM. Cycle and hormone changes during perimenopause: the key role of ovarian function. Menopause. 2008;15:603–612. doi: 10.1097/gme.0b013e318174ea4d. (4 Pt 1) [DOI] [PubMed] [Google Scholar]
- 33.Buysse DJ, Germain A, Hall ML, Moul DE, Nofzinger EA, Begley A, Ehlers CL, Thompson W, Kupfer DJ. EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep. 2008;31(12):1673–1682. doi: 10.1093/sleep/31.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buzsaki G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25(10):1073–1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7(6):477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 36.Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci U S A. 2002;99(16):10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old) Psychophysiology. 2001;38(2):232–242. [PubMed] [Google Scholar]
- 38.Cedars MI. Triphasic oral contraceptives: review and comparison of various regimens. Fertil Steril. 2002;77(1):1–14. doi: 10.1016/s0015-0282(01)02927-2. [DOI] [PubMed] [Google Scholar]
- 39.Cedernaes J, Osorio RS, Varga AW, Kam K, Schioth HB, Benedict C. Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer’s disease. Sleep Med Rev. 2017;31:102–111. doi: 10.1016/j.smrv.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cellini N. Memory consolidation in sleep disorders. Sleep Med Rev. 2017;35:101–112. doi: 10.1016/j.smrv.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Cellini N, de Zambotti M, Covassin N, Sarlo M, Stegagno L. Impaired off-line motor skills consolidation in young primary insomniacs. Neurobiol Learn Mem. 2014;114:141–147. doi: 10.1016/j.nlm.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132(2):529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Comasco E, Frokjaer VG, Sundstrom-Poromaa I. Functional and molecular neuroimaging of menopause and hormone replacement therapy. Front Neurosci. 2014;8:388. doi: 10.3389/fnins.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci. 2010;13(1):9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curran-Rauhut MA, Petersen SL. The distribution of progestin receptor mRNA in rat brainstem. Brain Res Gene Expr Patterns. 2002;1(3–4):151–157. doi: 10.1016/s1567-133x(02)00011-x. [DOI] [PubMed] [Google Scholar]
- 46.Damianisch K, Rupprecht R, Lancel M. The influence of subchronic administration of the neurosteroid allopregnanolone on sleep in the rat. Neuropsychopharmacology. 2001;25(4):576–584. doi: 10.1016/S0893-133X(01)00242-1. [DOI] [PubMed] [Google Scholar]
- 47.De Gennaro L, Ferrara M, Vecchio F, Curcio G, Bertini M. An electroencephalographic fingerprint of human sleep. Neuroimage. 2005;26(1):114–122. doi: 10.1016/j.neuroimage.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 48.de Zambotti M, Goldstone A, Colrain IM, Baker FC. Insomnia disorder in adolescence: Diagnosis, impact, and treatment. Sleep Med Rev. 2017 doi: 10.1016/j.smrv.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Zambotti M, Willoughby AR, Sassoon SA, Colrain IM, Baker FC. Menstrual Cycle-Related Variation in Physiological Sleep in Women in the Early Menopausal Transition. J Clin Endocrinol Metab. 2015;100(8):2918–2926. doi: 10.1210/jc.2015-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deboer T. Brain temperature dependent changes in the electroencephalogram power spectrum of humans and animals. J Sleep Res. 1998;7(4):254–262. doi: 10.1046/j.1365-2869.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- 51.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 52.Diekelmann S, Born J, Rasch B. Increasing Explicit Sequence Knowledge by Odor Cueing during Sleep in Men but not Women. Front Behav Neurosci. 2016;10:74. doi: 10.3389/fnbeh.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dijk DJ. EEG slow waves and sleep spindles: windows on the sleeping brain. Behav Brain Res. 1995;69(1–2):109–116. doi: 10.1016/0166-4328(95)00007-g. [DOI] [PubMed] [Google Scholar]
- 54.Dijk DJ, Beersma DG, Bloem GM. Sex differences in the sleep EEG of young adults: visual scoring and spectral analysis. Sleep. 1989;12(6):500–507. doi: 10.1093/sleep/12.6.500. [DOI] [PubMed] [Google Scholar]
- 55.Driver HS, Dijk DJ, Werth E, Biedermann K, Borbely AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81(2):728–735. doi: 10.1210/jcem.81.2.8636295. [DOI] [PubMed] [Google Scholar]
- 56.Driver HS, Werth E, Dijk D-J, Borbely A. The menstrual cycle effects on sleep. Sleep Med Clin. 2008;3:1–11. [Google Scholar]
- 57.Driver HS, Werth E, Dijk D, Borbely AA. The menstrual cycle effects on sleep. Sleep medicine clinics. 2008;3:1–11. [Google Scholar]
- 58.Drogos LL, Rubin LH, Geller SE, Banuvar S, Shulman LP, Maki PM. Objective cognitive performance is related to subjective memory complaints in midlife women with moderate to severe vasomotor symptoms. Menopause. 2013;20(12):1236–1242. doi: 10.1097/GME.0b013e318291f5a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duarte-Guterman P, Yagi S, Chow C, Galea LA. Hippocampal learning, memory, and neurogenesis: Effects of sex and estrogens across the lifespan in adults. Horm Behav. 2015;74:37–52. doi: 10.1016/j.yhbeh.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 60.Eichenbaum H. Hippocampus: Cognitive Processes and Neural Representations that Underlie Declarative Memory. Neuron. 2004;44(1):109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 61.Engler-Chiurazzi EB, Brown CM, Povroznik JM, Simpkins JW. Estrogens as neuroprotectants: Estrogenic actions in the context of cognitive aging and brain injury. Prog Neurobiol. 2017;157:188–211. doi: 10.1016/j.pneurobio.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Epperson CN, Sammel MD, Freeman EW. Menopause effects on verbal memory: findings from a longitudinal community cohort. J Clin Endocrinol Metab. 2013;98(9):3829–3838. doi: 10.1210/jc.2013-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eschenko O, Molle M, Born J, Sara SJ. Elevated sleep spindle density after learning or after retrieval in rats. J Neurosci. 2006;26(50):12914–12920. doi: 10.1523/JNEUROSCI.3175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Farage MA, Neill S, MacLean AB. Physiological changes associated with the menstrual cycle: a review. Obstet Gynecol Surv. 2009;64(1):58–72. doi: 10.1097/OGX.0b013e3181932a37. [DOI] [PubMed] [Google Scholar]
- 65.Feige B, Baglioni C, Spiegelhalder K, Hirscher V, Nissen C, Riemann D. The microstructure of sleep in primary insomnia: an overview and extension. Int J Psychophysiol. 2013;89(2):171–180. doi: 10.1016/j.ijpsycho.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Frick KM, Kim J, Tuscher JJ, Fortress AM. Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn Mem. 2015;22(9):472–493. doi: 10.1101/lm.037267.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaillard JM, Blois R. Spindle density in sleep of normal subjects. Sleep. 1981;4(4):385–391. doi: 10.1093/sleep/4.4.385. [DOI] [PubMed] [Google Scholar]
- 68.Gais S, Molle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22(15):6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galea LAM, Frick KM, Hampson E, Sohrabji F, Choleris E. Why estrogens matter for behavior and brain health. Neurosci Biobehav Rev. 2017;76:363–379. doi: 10.1016/j.neubiorev.2016.03.024. (Pt B) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Genzel L, Baurle A, Potyka A, Wehrle R, Adamczyk M, Friess E, Steiger A, Dresler M. Diminished nap effects on memory consolidation are seen under oral contraceptive use. Neuropsychobiology. 2014;70(4):253–261. doi: 10.1159/000369022. [DOI] [PubMed] [Google Scholar]
- 71.Genzel L, Kiefer T, Renner L, Wehrle R, Kluge M, Grozinger M, Steiger A, Dresler M. Sex and modulatory menstrual cycle effects on sleep related memory consolidation. Psychoneuroendocrinology. 2012;37(7):987–998. doi: 10.1016/j.psyneuen.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 72.Genzel L, Kroes MC, Dresler M, Battaglia FP. Light sleep versus slow wave sleep in memory consolidation: a question of global versus local processes? Trends Neurosci. 2014;37(1):10–19. doi: 10.1016/j.tins.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 73.Gervais NJ, Mong JA, Lacreuse A. Ovarian hormones, sleep and cognition across the adult female lifespan: An integrated perspective. Front Neuroendocrinol. 2017;47:134–153. doi: 10.1016/j.yfrne.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, Brinton EA, Cedars MI, Lobo RA, Merriam GR, Neal-Perry G, Santoro NF, Taylor HS, Black DM, Budoff MJ, Hodis HN, Naftolin F, Harman SM, Asthana S. Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833. doi: 10.1371/journal.pmed.1001833. discussion e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gogos A, Wu YC, Williams AS, Byrne LK. The effects of ethinylestradiol and progestins (the pill) on cognitive function in pre-menopausal women. Neurochem Res. 2014;39(12):2288–2300. doi: 10.1007/s11064-014-1444-6. [DOI] [PubMed] [Google Scholar]
- 76.Gold EB, Sternfeld B, Kelsey JL, Brown C, Mouton C, Reame N, Salamone L, Stellato R. Relation of demographic and lifestyle factors to symptoms in a multiracial/ethnic population of women 40–55 years of age. Am J Epidemiol. 2000;152(5):463–473. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 77.Gomes MP, Deitcher SR. Risk of venous thromboembolic disease associated with hormonal contraceptives and hormone replacement therapy: a clinical review. Arch Intern Med. 2004;164(18):1965–1976. doi: 10.1001/archinte.164.18.1965. [DOI] [PubMed] [Google Scholar]
- 78.Greendale GA, Derby CA, Maki PM. Perimenopause and cognition. Obstet Gynecol Clin North Am. 2011;38(3):519–535. doi: 10.1016/j.ogc.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greendale GA, Wight RG, Huang MH, Avis N, Gold EB, Joffe H, Seeman T, Vuge M, Karlamangla AS. Menopause-associated symptoms and cognitive performance: results from the study of women’s health across the nation. Am J Epidemiol. 2010;171(11):1214–1224. doi: 10.1093/aje/kwq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Griessenberger H, Heib DP, Lechinger J, Luketina N, Petzka M, Moeckel T, Hoedlmoser K, Schabus M. Susceptibility to declarative memory interference is pronounced in primary insomnia. PLoS One. 2013;8(2):e57394. doi: 10.1371/journal.pone.0057394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hadjimarkou MM, Benham R, Schwarz JM, Holder MK, Mong JA. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur J Neurosci. 2008;27(7):1780–1792. doi: 10.1111/j.1460-9568.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- 82.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab. 2007;92(8):3060–3067. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 83.Hampson E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology. 1990;15(2):97–111. doi: 10.1016/0306-4530(90)90018-5. [DOI] [PubMed] [Google Scholar]
- 84.Hampson E, Morley EE. Estradiol concentrations and working memory performance in women of reproductive age. Psychoneuroendocrinology. 2013;38(12):2897–2904. doi: 10.1016/j.psyneuen.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 85.Hamson DK, Roes MM, Galea LA. Sex Hormones and Cognition: Neuroendocrine Influences on Memory and Learning. Compr Physiol. 2016;6(3):1295–1337. doi: 10.1002/cphy.c150031. [DOI] [PubMed] [Google Scholar]
- 86.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure-activity relationships for steroid interaction with the gamma-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1987;241(1):346–353. [PubMed] [Google Scholar]
- 88.Helfrich RF, Mander BA, Jagust WJ, Knight RT, Walker MT. Old Brains Come Uncoupled in Sleep: Slow Wave-Spindle Synchrony, Brain Atrophy, and Forgetting. Neuron. 2018;97(1):221–230. doi: 10.1016/j.neuron.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Henderson VW. Aging, estrogens, and episodic memory in women. Cogn Behav Neurol. 2009;22(4):205–214. doi: 10.1097/WNN.0b013e3181a74ce7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henderson VW, Guthrie JR, Dudley EC, Burger HG, Dennerstein L. Estrogen exposures and memory at midlife: a population-based study of women. Neurology. 2003;60(8):1369–1371. doi: 10.1212/01.wnl.0000059413.75888.be. [DOI] [PubMed] [Google Scholar]
- 91.Henderson VW, Popat RA. Effects of endogenous and exogenous estrogen exposures in midlife and late-life women on episodic memory and executive functions. Neuroscience. 2011;191:129–138. doi: 10.1016/j.neuroscience.2011.05.059. [DOI] [PubMed] [Google Scholar]
- 92.Henderson VW, St John JA, Hodis HN, McCleary CA, Stanczyk FZ, Shoupe D, Kono N, Dustin L, Allayee H, Mack WJ. Cognitive effects of estradiol after menopause: A randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708. doi: 10.1212/WNL.0000000000002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Henke K. A model for memory systems based on processing modes rather than consciousness. Nat Rev Neurosci. 2010;11(7):523–532. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- 94.Hussain D, Hanafi S, Konishi K, Brake WG, Bohbot VD. Modulation of spatial and response strategies by phase of the menstrual cycle in women tested in a virtual navigation task. Psychoneuroendocrinology. 2016;70:108–117. doi: 10.1016/j.psyneuen.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 95.Ishizuka Y, Pollak CP, Shirakawa S, Kakuma T, Azumi K, Usui A, Shiraishi K, Fukuzawa H, Kariya T. Sleep spindle frequency changes during the menstrual cycle. J Sleep Res. 1994;3(1):26–29. doi: 10.1111/j.1365-2869.1994.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 96.Jacobs EG, Weiss BK, Makris N, Whitfield-Gabrieli S, Buka SL, Klibanski A, Goldstein JM. Impact of Sex and Menopausal Status on Episodic Memory Circuitry in Early Midlife. J Neurosci. 2016;36(39):10163–10173. doi: 10.1523/JNEUROSCI.0951-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jenkins JG, Dallenbach KM. Obliviscence during sleep and waking. The American Journal of Psychology. 1924;35(4):605–612. [Google Scholar]
- 98.Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13(3):411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- 99.Joffe H, Massler A, Sharkey KM. Evaluation and management of sleep disturbance during the menopause transition. Semin Reprod Med. 2010;28(5):404–421. doi: 10.1055/s-0030-1262900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johnson E, Roth T, Schultz L, Breslau N. Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics. 2006;117(2):e247–e256. doi: 10.1542/peds.2004-2629. [DOI] [PubMed] [Google Scholar]
- 101.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karlamangla AS, Lachman ME, Han W, Huang M, Greendale GA. Evidence for Cognitive Aging in Midlife Women: Study of Women’s Health Across the Nation. PLoS One. 2017;12(1):e0169008. doi: 10.1371/journal.pone.0169008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koebele SV, Bimonte-Nelson HA. Trajectories and phenotypes with estrogen exposures across the lifespan: What does Goldilocks have to do with it? Horm Behav. 2015;74:86–104. doi: 10.1016/j.yhbeh.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koebele SV, Bimonte-Nelson HA. Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas. 2016;87:5–17. doi: 10.1016/j.maturitas.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koebele SV, Bimonte-Nelson HA. The endocrine-brain-aging triad where many paths meet: female reproductive hormone changes at midlife and their influence on circuits important for learning and memory. Exp Gerontol. 2017;94:14–23. doi: 10.1016/j.exger.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koebele SV, Mennenga SE, Hiroi R, Quihuis AM, Hewitt LT, Poisson ML, George C, Mayer LP, Dyer CA, Aiken LS, Demers LM, Carson C, Bimonte-Nelson HA. Cognitive changes across the menopause transition: A longitudinal evaluation of the impact of age and ovarian status on spatial memory. Horm Behav. 2017;87:96–114. doi: 10.1016/j.yhbeh.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Korol DL, Pisani SL. Estrogens and cognition: Friends or foes?: An evaluation of the opposing effects of estrogens on learning and memory. Horm Behav. 2015;74:105–115. doi: 10.1016/j.yhbeh.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lampio L, Polo-Kantola P, L Himanen S, Kurki S, Huupponen E, Engblom J, Heinonen OJ, Polo O, Saaresranta T. Sleep During Menopausal Transition: A 6-Year Follow-Up. Sleep. 2017;40(7) doi: 10.1093/sleep/zsx090. [DOI] [PubMed] [Google Scholar]
- 109.Lancel M, Faulhaber J, Schiffelholz T, Romeo E, Di Michele F, Holsboer F, Rupprecht R. Allopregnanolone affects sleep in a benzodiazepine-like fashion. J Pharmacol Exp Ther. 1997;282(3):1213–1218. [PubMed] [Google Scholar]
- 110.Lauriola M, Esposito R, Delli Pizzi S, de Zambotti M, Londrillo F, Kramer JH, Rabinovici GD, Tartaro A. Sleep changes without medial temporal lobe or brain cortical changes in community-dwelling individuals with subjective cognitive decline. Alzheimers Dement. 2016 doi: 10.1016/j.jalz.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lemieux M, Chen JY, Lonjers P, Bazhenov M, Timofeev I. The impact of cortical deafferentation on the neocortical slow oscillation. J Neurosci. 2014;34(16):5689–5703. doi: 10.1523/JNEUROSCI.1156-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lewin C, Wolgers G, Herlitz A. Sex differences favoring women in verbal but not in visuospatial episodic memory. Neuropsychology. 2001;15(2):165–173. doi: 10.1037//0894-4105.15.2.165. [DOI] [PubMed] [Google Scholar]
- 113.Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, Albanese M, Mercuri NB, Izzi F, Bernardini S, Nitti A, Sancesario GM, Sica F, Marciani MG, Placidi F. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 2014;71(12):1498–1505. doi: 10.1001/jamaneurol.2014.2510. [DOI] [PubMed] [Google Scholar]
- 114.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep. 2013;36(7):1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lim AS, Yu L, Costa MD, Leurgans SE, Buchman AS, Bennett DA, Saper CB. Increased fragmentation of rest-activity patterns is associated with a characteristic pattern of cognitive impairment in older individuals. Sleep. 2012;35(5):633–640B. doi: 10.5665/sleep.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Luetters C, Huang MH, Seeman T, Buckwalter G, Meyer PM, Avis NE, Sternfeld B, Johnston JM, Greendale GA. Menopause transition stage and endogenous estradiol and follicle-stimulating hormone levels are not related to cognitive performance: cross-sectional results from the study of women’s health across the nation (SWAN) J Womens Health (Larchmt) 2007;16(3):331–344. doi: 10.1089/jwh.2006.0057. [DOI] [PubMed] [Google Scholar]
- 117.Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav. 2014;66(4):602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maki PM. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause. 2013;20(6):695–709. doi: 10.1097/GME.0b013e3182960cf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maki PM. Verbal memory and menopause. Maturitas. 2015;82(3):288–290. doi: 10.1016/j.maturitas.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 120.Maki PM, Rich JB, Rosenbaum RS. Implicit memory varies across the menstrual cycle: estrogen effects in young women. Neuropsychologia. 2002;40(5):518–529. doi: 10.1016/s0028-3932(01)00126-9. [DOI] [PubMed] [Google Scholar]
- 121.Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ, Walker MP. beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18(7):1051–1057. doi: 10.1038/nn.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16(3):357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer’s Disease? Trends Neurosci. 2016;39(8):552–566. doi: 10.1016/j.tins.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 125.McCarrey AC, Resnick SM. Postmenopausal hormone therapy and cognition. Horm Behav. 2015;74:167–172. doi: 10.1016/j.yhbeh.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nature neuroscience. 2011;14(6):677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McDevitt EA, Rokem A, Silver MA, Mednick SC. Sex differences in sleep-dependent perceptual learning. Vision Res. 2014;99:172–179. doi: 10.1016/j.visres.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 129.Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nat Neurosci. 2003;6(7):697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- 130.Mednick SC, Cai DJ, Shuman T, Anagnostaras S, Wixted JT. An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 2011;34(10):504–514. doi: 10.1016/j.tins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mednick SC, McDevitt EA, Walsh JK, Wamsley E, Paulus M, Kanady JC, Drummond SP. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci. 2013;33(10):4494–4504. doi: 10.1523/JNEUROSCI.3127-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Merlo S, Spampinato SF, Sortino MA. Estrogen and Alzheimer’s disease: Still an attractive topic despite disappointment from early clinical results. Eur J Pharmacol. 2017;817:51–58. doi: 10.1016/j.ejphar.2017.05.059. [DOI] [PubMed] [Google Scholar]
- 133.Mitchell ES, Woods NF. Midlife women’s attributions about perceived memory changes: observations from the Seattle Midlife Women’s Health Study. J Womens Health Gend Based Med. 2001;10(4):351–362. doi: 10.1089/152460901750269670. [DOI] [PubMed] [Google Scholar]
- 134.Molle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011;34(10):1411–1421. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Molle M, Eschenko O, Gais S, Sara SJ, Born J. The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur J Neurosci. 2009;29(5):1071–1081. doi: 10.1111/j.1460-9568.2009.06654.x. [DOI] [PubMed] [Google Scholar]
- 136.Mong JA, Baker FC, Mahoney MM, Paul KN, Schwartz MD, Semba K, Silver R. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31(45):16107–16116. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mong JA, Cusmano DM. Sex differences in sleep: impact of biological sex and sex steroids. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150110. doi: 10.1098/rstb.2015.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mordecai KL, Rubin LH, Maki PM. Effects of menstrual cycle phase and oral contraceptive use on verbal memory. Horm Behav. 2008;54(2):286–293. doi: 10.1016/j.yhbeh.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 139.Morrow AL, Suzdak PD, Paul SM. Benzodiazepine, barbiturate, ethanol and hypnotic steroid hormone modulation of GABA-mediated chloride ion transport in rat brain synaptoneurosomes. Advances in biochemical psychopharmacology. 1987;(45):247–261. [PubMed] [Google Scholar]
- 140.Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64(2):180–187. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ngo HV, Martinetz T, Born J, Molle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78(3):545–553. doi: 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 142.Nguyen TV, Ducharme S, Karama S. Effects of Sex Steroids in the Human Brain. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0198-3. [DOI] [PubMed] [Google Scholar]
- 143.Niknazar M, Krishnan GP, Bazhenov M, Mednick SC. Coupling of Thalamocortical Sleep Oscillations Are Important for Memory Consolidation in Humans. PLoS One. 2015;10(12):e0144720. doi: 10.1371/journal.pone.0144720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nissen C, Kloepfer C, Feige B, Piosczyk H, Spiegelhalder K, Voderholzer U, Riemann D. Sleep-related memory consolidation in primary insomnia. J Sleep Res. 2011;20:129–136. doi: 10.1111/j.1365-2869.2010.00872.x. (1 Pt 2) [DOI] [PubMed] [Google Scholar]
- 145.Nissen C, Kloepfer C, Nofzinger EA, Feige B, Voderholzer U, Riemann D. Impaired sleep-related memory consolidation in primary insomnia--a pilot study. Sleep. 2006;29(8):1068–1073. doi: 10.1093/sleep/29.8.1068. [DOI] [PubMed] [Google Scholar]
- 146.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166(12):1262–1268. doi: 10.1001/archinte.166.12.1262. [DOI] [PubMed] [Google Scholar]
- 147.Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu SE, Lim J, Wohlleber ME, Ducca EL, Koushyk V, Glodzik L, Mosconi L, Ayappa I, Rapoport DM, de Leon MJ, I. Alzheimer’s Disease Neuroimaging Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964–1971. doi: 10.1212/WNL.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Osorio RS, Pirraglia E, Aguera-Ortiz LF, During EH, Sacks H, Ayappa I, Walsleben J, Mooney A, Hussain A, Glodzik L, Frangione B, Martinez-Martin P, de Leon MJ. Greater risk of Alzheimer’s disease in older adults with insomnia. J Am Geriatr Soc. 2011;59(3):559–562. doi: 10.1111/j.1532-5415.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pace-Schott EF, Spencer RM. Age-related changes in the cognitive function of sleep. Prog Brain Res. 2011;191:75–89. doi: 10.1016/B978-0-444-53752-2.00012-6. [DOI] [PubMed] [Google Scholar]
- 150.Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychol Sci. 2008;19(8):781–788. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Persad CC, Zubieta JK, Love T, Wang H, Tkaczyk A, Smith YR. Enhanced neuroactivation during verbal memory processing in postmenopausal women receiving short-term hormone therapy. Fertil Steril. 2009;92(1):197–204. doi: 10.1016/j.fertnstert.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Peters KR, Ray L, Smith V, Smith C. Changes in the density of stage 2 sleep spindles following motor learning in young and older adults. J Sleep Res. 2008;17(1):23–33. doi: 10.1111/j.1365-2869.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 153.Pike CJ. Sex and the development of Alzheimer’s disease. J Neurosci Res. 2017;95(1–2):671–680. doi: 10.1002/jnr.23827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Plante DT, Goldstein MR. Medroxyprogesterone acetate is associated with increased sleep spindles during non-rapid eye movement sleep in women referred for polysomnography. Psychoneuroendocrinology. 2013;38(12):3160–3166. doi: 10.1016/j.psyneuen.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9(4):534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 156.Plihal W, Born J. Effects of early and late nocturnal sleep on priming and spatial memory. Psychophysiology. 1999;36(5):571–582. [PubMed] [Google Scholar]
- 157.Pourganji M, Hosseini M, Soukhtanloo M, Zabihi H, Hadjzadeh MA. Protective role of endogenous ovarian hormones against learning and memory impairments and brain tissues oxidative damage induced by lipopolysaccharide. Iran Red Crescent Med J. 2014;16(3):e13954. doi: 10.5812/ircmj.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013;93(2):681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reddy DS, Kulkarni SK. The effects of neurosteroids on acquisition and retention of a modified passive-avoidance learning task in mice. Brain research. 1998;791(1–2):108–116. doi: 10.1016/s0006-8993(98)00085-7. [DOI] [PubMed] [Google Scholar]
- 160.Redline S, L Kirchner H, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164(4):406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 161.Rentz DM, Weiss BK, Jacobs EG, Cherkerzian S, Klibanski A, Remington A, Aizley H, Goldstein JM. Sex differences in episodic memory in early midlife: impact of reproductive aging. Menopause. 2017;24(4):400–408. doi: 10.1097/GME.0000000000000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Resnick SM, Espeland MA, Jaramillo SA, Hirsch C, Stefanick ML, Murray AM, Ockene J, Davatzikos C. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology. 2009;72(2):135–142. doi: 10.1212/01.wnl.0000339037.76336.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: a critical time. JAMA. 2002;288(17):2170–2172. doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- 164.Riemann D, Nissen C, Palagini L, Otte A, L Perlis M, Spiegelhalder K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 2015;14(5):547–558. doi: 10.1016/S1474-4422(15)00021-6. [DOI] [PubMed] [Google Scholar]
- 165.Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, Holtzman DM. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012;4(150):150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Romeo RD, Waters EM, McEwen BS. Steroid-induced hippocampal synaptic plasticity: sex differences and similarities. Neuron Glia Biol. 2004;1(3):219–229. doi: 10.1017/S1740925X05000086. [DOI] [PubMed] [Google Scholar]
- 167.Rousseau ME. Women’s midlife health. Reframing menopause. J Nurse Midwifery. 1998;43(3):208–223. doi: 10.1016/s0091-2182(98)00005-6. [DOI] [PubMed] [Google Scholar]
- 168.Ryan J, Stanczyk FZ, Dennerstein L, Mack WJ, Clark MS, Szoeke C, Kildea D, Henderson VW. Hormone levels and cognitive function in postmenopausal midlife women. Neurobiol Aging. 2012;33(7):1138–1147. doi: 10.1016/j.neurobiolaging.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 169.Sattari N, McDevitt EA, Panas D, Niknazar M, Ahmadi M, Naji M, Baker FC, Mednick SC. The effect of sex and menstrual phase on memory formation during a nap. Neurobiol Learn Mem. 2017;145:119–128. doi: 10.1016/j.nlm.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 170.Schabus M, Gruber G, Parapatics S, Sauter C, Klosch G, Anderer P, Klimesch W, Saletu B, Zeitlhofer J. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27(8):1479–1485. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 171.Schmidt C, Peigneux P, Muto V, Schenkel M, Knoblauch V, Munch M, de Quervain DJ, Wirz-Justice A, Cajochen C. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci. 2006;26(35):8976–8982. doi: 10.1523/JNEUROSCI.2464-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schouten DI, Pereira SI, Tops M, Louzada FM. State of the art on targeted memory reactivation: Sleep your way to enhanced cognition. Sleep Med Rev. 2017;32:123–131. doi: 10.1016/j.smrv.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 173.Seeck-Hirschner M, Baier PC, Weinhold SL, Dittmar M, Heiermann S, Aldenhoff JB, Goder R. Declarative memory performance is associated with the number of sleep spindles in elderly women. Am J Geriatr Psychiatry. 2012;20(9):782–788. doi: 10.1097/JGP.0b013e31823033da. [DOI] [PubMed] [Google Scholar]
- 174.Sharma RA, Kam K, Parekh A, Uribe-Cano S, Tweardy S, Bubu OM, Ayappa I, Rapoport DM, Varga AW, Osorio RS. Reduced spindle frequency and density in stage 2 NREM sleep in association with increased CSF P-TAU in cognitively normal elderly. Sleep. 2017;40:A281. [Google Scholar]
- 175.Shechter A, Boivin DB. Sleep, Hormones, and Circadian Rhythms throughout the Menstrual Cycle in Healthy Women and Women with Premenstrual Dysphoric Disorder. Int J Endocrinol. 2010;2010:259345. doi: 10.1155/2010/259345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sherwin BB. Estrogen and cognitive functioning in women: lessons we have learned. Behav Neurosci. 2012;126(1):123–127. doi: 10.1037/a0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shimizu RE, Connolly PM, Cellini N, Armstrong D, Hernandez L, Estrada R, Aguilar M, Weisend MP, Mednick SC, Simons SB. Closed-loop targeted memory reactivation during sleep improves spatial navigation. Fontiers in Human Neuroscience. 2018 doi: 10.3389/fnhum.2018.00028. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shine-Burdick R, Hoffmann R, Armitage R. Short note: oral contraceptives and sleep in depressed and healthy women. Sleep. 2002;25(3):347–349. [PubMed] [Google Scholar]
- 179.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 180.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J, Investigators W. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 181.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21(5):1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 182.Siengsukon C, Boyd LA. Sleep enhances off-line spatial and temporal motor learning after stroke. Neurorehabil Neural Repair. 2009;23(4):327–335. doi: 10.1177/1545968308326631. [DOI] [PubMed] [Google Scholar]
- 183.Siengsukon CF, Boyd LA. Sleep enhances implicit motor skill learning in individuals poststroke. Top Stroke Rehabil. 2008;15(1):1–12. doi: 10.1310/tsr1501-1. [DOI] [PubMed] [Google Scholar]
- 184.Silvers JM, Tokunaga S, Berry RB, White AM, Matthews DB. Impairments in spatial learning and memory: ethanol, allopregnanolone, and the hippocampus. Brain Res Brain Res Rev. 2003;43(3):275–284. doi: 10.1016/j.brainresrev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 185.Singh M, Simpkins JW, Simpkins JW, Bimonte-Nelson HA, Bimonte-Nelson HA, Brinton RD, Brinton RD. Window of opportunity for estrogen and progestin intervention in brain aging and Alzheimer’s disease. Brain Res. 2013;1514:1–2. doi: 10.1016/j.brainres.2013.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Smith YR, Love T, Persad CC, Tkaczyk A, Nichols TE, Zubieta JK. Impact of combined estradiol and norethindrone therapy on visuospatial working memory assessed by functional magnetic resonance imaging. J Clin Endocrinol Metab. 2006;91(11):4476–4481. doi: 10.1210/jc.2006-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Fertil Steril. 2001;76(5):874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 188.Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29(2):219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Spencer RM, Gouw AM, Ivry RB. Age-related decline of sleep-dependent consolidation. Learn Mem. 2007;14(7):480–484. doi: 10.1101/lm.569407. [DOI] [PubMed] [Google Scholar]
- 190.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70(12):1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, Sager MA, Asthana S, Johnson SC, Benca RM. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. 2015;36(9):2568–2576. doi: 10.1016/j.neurobiolaging.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Squire LR. Declarative and nondeclarative memory: Multiple brain systems supporting learning and memory. Journal of Cognitive Neuroscience. 1992;4:231–243. doi: 10.1162/jocn.1992.4.3.232. [DOI] [PubMed] [Google Scholar]
- 193.Staresina BP, Bergmann TO, Bonnefond M, van der Meij R, Jensen O, Deuker L, Elger CE, Axmacher N, Fell J. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci. 2015;18(11):1679–1686. doi: 10.1038/nn.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Steriade M, Domich L, Oakson G, Deschenes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57(1):260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- 195.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 196.Sundstrom Poromaa I, Gingnell M. Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front Neurosci. 2014;8:380. doi: 10.3389/fnins.2014.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tabuchi M, Lone SR, Liu S, Liu Q, Zhang J, Spira AP, Wu MN. Sleep interacts with abeta to modulate intrinsic neuronal excitability. Curr Biol. 2015;25(6):702–712. doi: 10.1016/j.cub.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. J Neurosci. 2010;30(43):14356–14360. doi: 10.1523/JNEUROSCI.3028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Thurston RC, Aizenstein HJ, Derby CA, Sejdic E, Maki PM. Menopausal hot flashes and white matter hyperintensities. Menopause. 2016;23(1):27–32. doi: 10.1097/GME.0000000000000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Thurston RC, Maki PM, Derby CA, Sejdic E, Aizenstein HJ. Menopausal hot flashes and the default mode network. Fertil Steril. 2015;103(6):1572–1578. doi: 10.1016/j.fertnstert.2015.03.008. e1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Timofeev I, Bazhenov M. Trends in Chronobiology Research. F. Columbus: 2005. Mechanisms and biological role of thalamocortical oscillations; pp. 1–47. [Google Scholar]
- 202.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 203.Unkenstein AE, Bryant CA, Judd FK, Ong B, Kinsella GJ. Understanding women’s experience of memory over the menopausal transition: subjective and objective memory in pre-, peri-, and postmenopausal women. Menopause. 2016;23(12):1319–1329. doi: 10.1097/GME.0000000000000705. [DOI] [PubMed] [Google Scholar]
- 204.von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261(5119):361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- 205.Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol Bull. 1995;117(2):250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- 206.Voyer D, Voyer SD, Saint-Aubin J. Sex differences in visual-spatial working memory: A meta-analysis. Psychon Bull Rev. 2016 doi: 10.3758/s13423-016-1085-7. [DOI] [PubMed] [Google Scholar]
- 207.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44(1):121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 208.Wang XJ, Rinzel J. Spindle rhythmicity in the reticularis thalami nucleus: synchronization among mutually inhibitory neurons. Neuroscience. 1993;53(4):899–904. doi: 10.1016/0306-4522(93)90474-t. [DOI] [PubMed] [Google Scholar]
- 209.Westerberg CE, Florczak SM, Weintraub S, Mesulam MM, Marshall L, Zee PC, Paller KA. Memory improvement via slow-oscillatory stimulation during sleep in older adults. Neurobiology of Aging. 2015;36(9):2577–2586. doi: 10.1016/j.neurobiolaging.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wilckens KA, Erickson KI, Wheeler ME. Age-related decline in controlled retrieval: the role of the PFC and sleep. Neural Plast. 2012;2012:624795. doi: 10.1155/2012/624795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wixted JT. The psychology and neuroscience of forgetting. Annu Rev Psychol. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]
- 212.Wnuk A, Korol DL, Erickson KI. Estrogens, hormone therapy, and hippocampal volume in postmenopausal women. Maturitas. 2012;73(3):186–190. doi: 10.1016/j.maturitas.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Woods NF, Mitchell ES, Adams C. Memory functioning among midlife women: observations from the Seattle Midlife Women’s Health Study. Menopause. 2000;7(4):257–265. [PubMed] [Google Scholar]
- 214.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–1028. doi: 10.1016/S1474-4422(14)70172-3. [DOI] [PubMed] [Google Scholar]
- 216.Yaroush R, Sullivan MJ, Ekstrand BR. Effect of sleep on memory. II. Differential effect of the first and second half of the night. J Exp Psychol. 1971;88(3):361–366. doi: 10.1037/h0030914. [DOI] [PubMed] [Google Scholar]
- 217.Yin F, Yao J, Sancheti H, Feng T, Melcangi RC, Morgan TE, Finch CE, Pike CJ, Mack WJ, Cadenas E, Brinton RD. The perimenopausal aging transition in the female rat brain: decline in bioenergetic systems and synaptic plasticity. Neurobiol Aging. 2015;36(7):2282–2295. doi: 10.1016/j.neurobiolaging.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Young T, Rabago D, Zgierska A, Austin D, Laurel F. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26(6):667–672. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
- 219.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]