Here we show that initiation of antiretroviral therapy in acute human immunodeficiency virus infection as early as Fiebig stage I/II, appears to limit CD163 shedding, which may halt a neuroinflammatory cascade associated with abnormal brain profiles.
Keywords: Soluble CD163, acute HIV infection, neurocognitive impairment, central nervous system, cerebrospinal fluid, plasma, combination antiretroviral therapy
Abstract
Background
Myeloid activation contributes to cognitive impairment in chronic human immunodeficiency virus (HIV) infection. We explored whether combination antiretroviral therapy (cART) initiation during acute HIV infection impacts CD163 shedding, a myeloid activation marker, and in turn, implications on the central nervous system (CNS).
Methods
We measured soluble CD163 (sCD163) levels in plasma and cerebrospinal fluid (CSF) by enzyme-linked immunosorbent assay in Thais who initiated cART during acute HIV infection (Fiebig stages I–IV). Examination of CNS involvement included neuropsychological testing and analysis of brain metabolites by magnetic resonance spectroscopy. Chronic HIV-infected or uninfected Thais served as controls.
Results
We examined 51 adults with acute HIV infection (Fiebig stages I–III; male sex, >90%; age, 31 years). sCD163 levels before and after cART in Fiebig stage I/II were comparable to those in uninfected controls (plasma levels, 97.9 and 93.6 ng/mL, respectively, vs 99.5 ng/mL; CSF levels, 6.7 and 6.4 ng/mL, respectively, vs 7.1 ng/mL). In Fiebig stage III, sCD163 levels were elevated before cART as compared to those in uninfected controls (plasma levels, 135 ng/mL; CSF levels, 10 ng/mL; P < .01 for both comparisons) before normalization after cART (plasma levels, 90.1 ng/mL; CSF levels, 6.5 ng/mL). Before cART, higher sCD163 levels during Fiebig stage III correlated with poor CNS measures (eg, decreased N-acetylaspartate levels), but paradoxically, during Fiebig stage I/II, this association was linked with favorable CNS outcomes (eg, higher neuropsychological test scores). After cART initiation, higher sCD163 levels during Fiebig stage III were associated with negative CNS indices (eg, worse neuropsychological test scores).
Conclusion
Initiation of cART early during acute HIV infection (ie, during Fiebig stage I/II) may decrease inflammation, preventing shedding of CD163, which in turn might lower the risk of brain injury.
Cognitive impairment persists in 30%–50% of individuals with chronic human immunodeficiency virus (HIV) infection despite access to combination antiretroviral therapy (cART) [1, 2]. In this cognitive impairment setting, indices of inflammation, such as plasma interferon α, interleukin 6, and soluble interleukin 2 receptor levels, are elevated in individuals with worsening HIV-associated neurocognitive disorder (HAND), compared with unimpaired persons or individuals with stable HAND diagnoses [3]. In Thais with chronic HIV infection, peripheral blood mononuclear cells (PBMCs) enriched for CD14+ cells from individuals with persistent HAND secrete greater levels of CXCL8 (also known as interleukin 8) and CCL2 (also known as monocyte chemoattractant protein 1) as compared to individuals with normal cognition [4]. These data highlight the crucial role of chronic inflammation in the context of HAND even during plasma viral suppression. Recent studies have highlighted the benefits of cART initiated during acute HIV infection in reducing select parameters of inflammation [5, 6]. Whether early cART initiation during acute HIV infection influences CD163 shedding is unknown.
Plasma sCD163, shed after monocyte/macrophage activation [7, 8], is an independent predictor of all-cause mortality during HIV infection [9] and a biomarker in the context of cognitive impairment. In cART-treated, chronically HIV-infected individuals with mild neurocognitive disorder, plasma sCD163 levels are elevated, compared to persons who are cognitively normal or who have asymptomatic neurocognitive impairment [10]. Similarly, in the Women’s Interagency HIV Study, higher plasma sCD163 levels are associated with worse overall cognitive performance and worse performance within multiple subdomains [11]. Plasma sCD163 levels also correlate with cerebrospinal fluid (CSF) levels of neopterin, an indicator of monocyte activation for which levels are elevated during HIV infection [12, 13], and to postmortem markers of synaptodendritic damage and microgliosis [14].
The impact of early cART on adverse non-AIDS events, such as cognitive function, in acute HIV infection is still unclear. Here, we evaluated plasma and CSF sCD163 levels and the relationship to central nervous system (CNS) outcomes in a prospective study of cART initiation during acute HIV infection.
METHODS
Cohort Descriptions
Acute HIV Infection (RV254/SEARCH010) Cohort
Walk-in clients seeking volunteer counseling and testing at the Thai Red Cross Anonymous Clinic were screened for acute HIV infection by pooled nucleic acid testing and a fourth-generation immunoassay (clinical trials registration NCT00796146) [15]. Participants were subsequently categorized as Fiebig stage I–IV [16, 17]. Participants were offered immediate cART via a local protocol (clinical trials registration NCT00796263). Our analyses included 9 individuals in Fiebig stage I, 10 in Fiebig stage II, and 32 in Fiebig stage III. Excluding the participant who did not begin cART, 86% of participants initiated cART within 3 days of study entry (median, 1.5 days; range, 0–5 days).
Control Cohorts
Fifty-three chronically HIV-infected participants who were cART naive and met Thai Ministry of Health guidelines to initiate therapy were enrolled in SEARCH011 (clinical trials registration NCT00782808), as previously described [13, 17]. Eighteen demographically matched uninfected individuals from Thailand were also evaluated as controls (SEARCH013; clinical trials registration NCT01397669).
Measurement of Soluble Levels of Monocyte/Macrophage Activation Markers by Enzyme-Linked Immunosorbent Assays (ELISAs)
sCD163 and neopterin levels were measured by single-plex ELISAs (Biorad [Hercules, CA] and Genway [San Diego, CA], respectively) in plasma and CSF samples collected before and 24 and 48 weeks after starting treatment, among individuals with acute HIV infection, or 48 weeks after treatment initiation, among those with chronic HIV infection.
Neurocognitive Assessment by Neuropsychological Testing
Acute HIV Infection and Uninfected Controls
Neuropsychological tests included Trail Making A, Color Trails 1 and 2, and Grooved Pegboard (nondominant hand) to compute a summary neuropsychological z-4 score. All raw scores were transformed to standard z scores using Thai normative data.
Chronic HIV Infection
Study participants completed a 1-hour neuropsychological testing battery, as previously described [18, 19]. All raw scores were transformed to standard z scores using Thai normative data. A measure of global performance (neuropsychological z global score) was calculated as the arithmetic mean of all tests with domain scores calculated from domain-specific components of the battery.
Assessment of Brain Metabolites by Magnetic Resonance Spectroscopy (MRS)
Single-voxel proton MRS scanning was performed for all HIV-infected participants using a 1.5 T GE scanner located at Chulalongkorn Hospital, as previously described [20, 21]. Assessment of alterations in the following brain metabolite levels during HIV infection were assessed [20, 22–26]: a combination of N-acetylaspartate and N-acetylaspartylglutamic acid, myoinositol, choline, a combination of glutamate and glutamine, and creatine, before and after cART initiation (24 weeks for acute HIV infection and 48 weeks for chronic HIV infection). Brain metabolite levels were measured in 4 brain regions reported to have abnormal brain chemical profiles in HIV: basal ganglia, frontal white matter, frontal gray matter, and posterior cingulate gyrus [20, 23, 24, 27–29].
CD163 Cell Surface Expression by Flow Cytometry
In participants with available cryopreserved PBMCs, specimens were thawed and stained with viability dye (yellow Live/Dead Fixable Dead Cell Stain), followed by staining with monoclonal antibodies to exclude lymphocytes (CD3-V500), B cells (CD19-PE-Cy7 and CD20-PE-Cy7), and natural killer cells (CD56-PE-Cy7). Monocytes, positive for HLA-DR (APC-H7), were then classified into the following subsets on the basis of CD14 (Qdot605), CD16 (AlexaFluor700) expression: classical (CD14++CD16-), intermediate (CD14++CD16+), or nonclassical (CD14+CD16+) monocytes. Monocyte populations were further assessed for CD163 expression (Alexa Fluor 488). Cells were fixed with 1% paraformaldehyde and acquired on a custom 4-laser LSR Fortessa (BD Biosciences, San Jose, CA). Compensation and gating analyses were performed using FlowJo (Treestar, Ashland, OR). All monoclonal antibodies were purchased from BD Biosciences, except for CD14–Qdot 605 and Live/Dead Stain (Life Technologies, Grand Island, NY) and CD163–Alexa Fluor 488 (R&D Systems, Minneapolis, MN). The gating strategy for identification of monocytes, their subsets, and receptor expression are shown in Supplementary Figure 1.
Statistical Analyses
Demographic and clinical characteristics are presented using median and interquartile ranges, except for sex, which is given as a percentage. Comparisons between continuous variables were examined using Kruskal-Wallis tests, and those between categorical variables were examined using χ2 tests. For CD163 outcomes, Wilcoxon rank sum tests were conducted to compare between groups, and Friedman and Wilcoxon signed rank tests were used to compare within groups over time, without adjustment for multiple comparisons. Associations between 2 continuous variables were evaluated by Spearman correlation. Multiple linear regression analyses were performed to explore the relationship between circulating sCD163 and cell-surface CD163 levels and Fiebig stages with CNS measures. Statistical analyses were performed using R, version 3.2.2, or GraphPad Prism, version 7 (GraphPad Software, San Diego, CA).
RESULTS
Participant Characteristics
Clinical and demographic characteristics of the participants are shown in Table 1. The overall median age was 33 years for the uninfected group, 31 and 28 years for Fiebig stage I/II and III acute HIV infection, respectively, and 34 years for the chronically HIV-infected cohort. A total of 50% of uninfected controls, 95% and 91% of Fiebig stage I/II and III, respectively, and 45% of participants with chronic HIV infection were male. Acute HIV-infected participants were randomized to receive either a cART regimen, consisting of tenofovir, emtricitabine, and efavirenz, or the 3-drug cART regimen plus maraviroc and raltegravir (cART+) during the first 24 weeks. A total of 47% and 28% in Fiebig stage I/II and III, respectively, received cART, and 47% and 72%, respectively, received cART+. One participant in Fiebig stage I/II did not initiate cART. Chronically HIV-infected participants received a cART regimen that included lamivudine, nevirapine, and either stavudine, zidovudine, or tenofovir. Treatment for participants intolerant to this regimen was modified on the basis of clinical acumen [30].
Table 1.
Clinical and Demographic Characteristics
| Characteristic | HIV Uninfected (n = 18) |
HIV Infected | P a | ||
|---|---|---|---|---|---|
| Acute (Fiebig I/II) (n = 19) |
Acute (Fiebig III) (n = 32) |
Chronic (n = 53) |
|||
| Male sex, no. (%) | 9 (50) | 18 (95) | 29 (91) | 24 (45) | <.0001 |
| Age, y | 33 (28–39) | 31 (26–39) | 28 (23–29) | 34 (29–37) | <.001 |
| Education duration, y | 11 (7–14) | 18 (16–20) | 17 (14–18) | 11 (8–14) | <.0001 |
| NPZ global score | 0.23 (−0.13–0.86) | −0.09 (−0.44–0.17) | −0.22 (−0.49–0.07) | −0.06 (−0.58–0.31) | .863 |
| Log10 plasma viral load, copies/mL | … | 5.08 (4.28–5.54) | 5.92 (5.52–6.88) | 4.85 (4.48–5.50) | <.0001 |
| CD4+ T-cell count, cells/µL | … | 447 (307–567) | 370 (293–470) | 228 (114–353) | <.0001 |
| CD8+ T-cell count, cells/µL | … | 271 (228–396) | 560 (426–999) | 701 (559–1025) | <.0001 |
Data are presented as median (interquartile range), unless otherwise indicated.
Abbreviation: NPZ, neuropsychological z test.
aBy the χ2 test (for categorical variables) and the Kruskal-Wallis test (for continuous variables), for comparison of the 3 HIV-positive groups.
Comparison of Plasma and CSF sCD163 Levels in Acute and Chronic HIV Infection
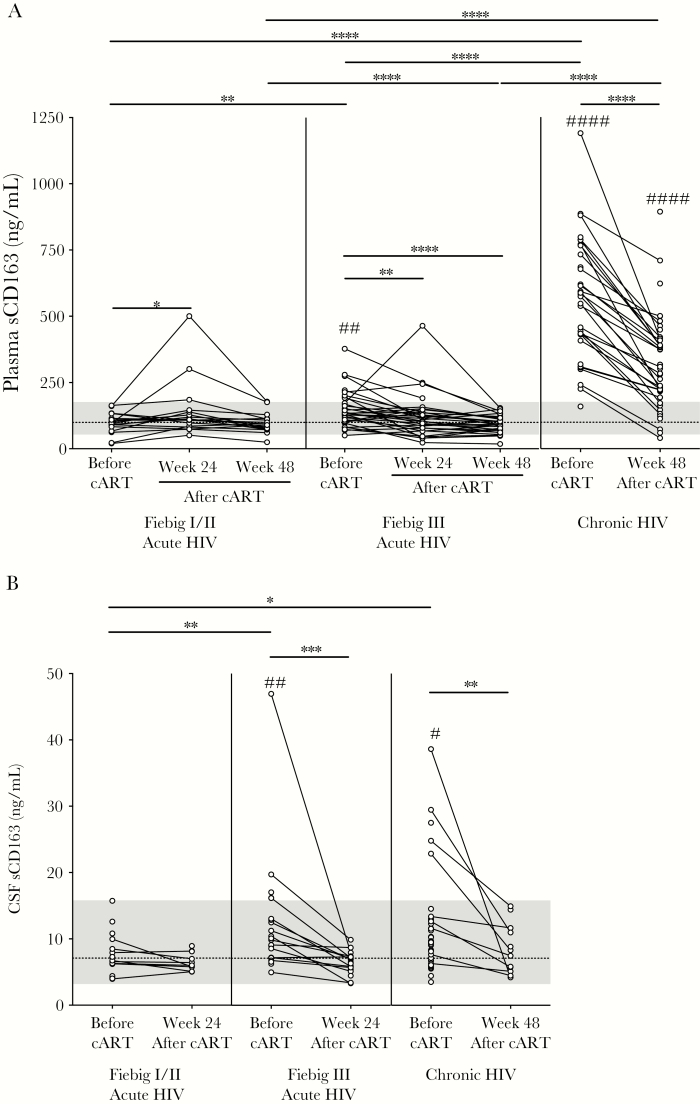
Before cART, median plasma sCD163 levels in participants in Fiebig stage I/II (97.9 ng/mL) were lower than in participants in Fiebig stage III (134.8 ng/mL; P = .002) or chronic HIV infection (582.9 ng/mL; P < .0001) and were similar to uninfected controls (99.5 ng/mL; Figure 1A). After cART initiation in participants in Fiebig stage I/II, plasma sCD163 levels transiently increased at week 24 (to 109.0 ng/mL; P = .034) and by week 48 decreased to pre-cART levels (93.6 ng/mL; Figure 1A). After cART initiation in participants in Fiebig stage III, plasma sCD163 levels continuously declined (to 109.9 ng/mL at week 24; P = .003), and by week 48 (90.1 ng/mL; P < .0001) there were no differences compared to uninfected levels (Figure 1A). After 48 weeks of cART, plasma sCD163 levels in participants with chronic HIV infection decreased (to 271.2 ng/mL) but remained elevated as compared to participants in acute HIV infection and uninfected controls (P < .0001 for all comparisons; Figure 1A). Examination of the longitudinal data from the cohorts revealed declines in plasma sCD163 levels during Fiebig stage III and chronic HIV infection after cART (P < .001 for all comparisons). In participants in Fiebig stage I/II, there was a difference between time points (P = .047), but plasma sCD163 levels never differed from uninfected controls. For the longitudinal analyses, Friedman and Wilcoxon signed rank tests were performed, without adjustment for multiple comparisons.
Figure 1.
Plasma (A) and cerebrospinal fluid (CSF; B) soluble CD163 (sCD163) levels during acute human immunodeficiency virus (HIV) infection, broken down by Fiebig stage, and chronic HIV infection, before and 24 and/or 48 weeks after combination antiretroviral therapy (cART) initiation. Data points that are connected represent longitudinal data for 1 participant. Dashed lines indicate uninfected control median levels, and the gray area above and below the dashed line represents the range of the uninfected control values. P values were calculated by Wilcoxon rank sum tests (for comparisons between groups) and Wilcoxon signed rank tests (for comparisons within groups), without adjustment for multiple comparisons. *P < .05, **P < .01, ***P < .001, and ****P < .0001, for comparisons between the HIV groups, and #P < .05, ##P < .01, and ####P < .0001, for comparisons between HIV-infected and uninfected groups.
Before cART, CSF sCD163 levels in participants in Fiebig stage I/II (6.7 ng/mL) were similar to uninfected controls (7.1 ng/mL) and did not change after cART (6.4 ng/mL; Figure 1B). However, pre-cART CSF sCD163 levels were elevated in participants in Fiebig stage III (10.0 ng/mL) and chronic HIV infection (9.53 ng/mL), compared with participants in Fiebig stage I/II (P = .008 and P = .017, respectively) or uninfected controls (P = .008 and P = .014, respectively; Figure 1B). After cART, CSF sCD163 levels in participants in Fiebig stage III (6.5 ng/mL at 24 weeks) and chronic HIV infection (7.9 ng/mL at 48 weeks) decreased from pre-cART levels (P < .001 and P = .003, respectively) and were comparable to levels seen in uninfected controls (Figure 1B). Analysis of the longitudinal data from the cohorts corroborated the aforementioned results. There were no changes in CSF sCD163 levels among participants in Fiebig stage I/II before and after cART, but there were declines in CSF sCD163 levels in participants in Fiebig stage III and chronic HIV infection after cART (P = .001 and P = .003, respectively).
Associations of Circulating sCD163 Levels With CNS Parameters Before cART
Our primary CNS outcome measures consisted of neuropsychological test performance scores (5 outcomes per Fiebig stage in the acute HIV-infected group), and our secondary outcome consisted of the following brain metabolites (5 outcomes in 4 brain regions, with 20 outcomes per Fiebig stage in the acute HIV-infected group): N-acetylaspartate and N-acetylaspartylglutamic acid, markers of neuronal health; myoinositol, a marker of astrocytosis and gliosis; choline, a marker cellular infiltration and inflammation/glial activation; glutamate and glutamine, markers of neuronal cell function; and creatine, a marker of energy metabolism. In Fiebig stage I/II before cART initiation, sCD163 levels correlated with positive CNS outcomes. Specifically, higher plasma sCD163 levels correlated with higher Grooved Pegboard z scores, an indicator of increased psychomotor performance (rho = 0.627; P = .044), and with higher glutamate and glutamine levels in frontal white matter (rho = 0.709; P = .003; Table 2 and Supplementary Figure 2). In Fiebig stage III before cART, sCD163 levels correlated with negative CNS outcomes, specifically higher plasma sCD163 levels correlating with lower N-acetylaspartate and N-acetylaspartylglutamic acid levels in the posterior cingulate gyrus (rho = −0.468; P = .017; Table 2 and Supplementary Figure 2). Multiple linear regression analyses were performed to explore the associations between CNS indices, sCD163 levels, and Fiebig stages. Evidence that associations with sCD163 levels differed between Fiebig stage I/II and III are shown in Table 2 and Supplementary Table 1. The positive association between plasma sCD163 levels and Grooved Pegboard scores before cART in Fiebig stage I/II differed from the association in Fiebig stage III (interaction P = .017), and similarly the positive association between plasma sCD163 levels and glutamate and glutamine levels in frontal white matter before cART in Fiebig stage I/II differed from the association in Fiebig stage III (interaction P = .017).
Table 2.
Correlations Between Plasma and Cerebrospinal Fluid (CSF) Soluble CD163 (sCD163) Levels, in acute HIV infection, With Central Nervous System (CNS) Indices
| Source, Time, acute HIV Fiebig Stage | CNS Index | Rhoa | P a | CNS Outcome | P b |
|---|---|---|---|---|---|
| Plasma | |||||
| Before cART | |||||
| I/II | Grooved Pegboard z score Cognitive performance | 0.627 | .044 | + | .017 |
| I/II | Glutamate and glutamine in frontal white matter Neuronal cell function | 0.709 | .003 | + | .017 |
| III | N-acetylaspartate and N-acetylaspartylglutamic acid in posterior cingulate gyrus Neuronal health | −0.468 | .017 | − | .547 |
| After cART | |||||
| III | Plasma neopterin Monocyte activation | 0.399 | .030 | − | .479 |
| CSF | |||||
| Before cART | |||||
| I/II | Myoinositol in basal ganglia Astrocytosis and gliosis | −0.569 | .037 | + | .156 |
| I/II | Choline in basal ganglia Cellular infiltration | 0.472 | .043 | − | .229 |
| III | CSF neopterin Monocyte activation | 0.479 | .025 | − | .913 |
| After cART | |||||
| III | Neuropsychological z-4 score Cognitive performance | −0.522 | .020 | − | .053 |
| III | N-acetylaspartate plus N-acetylaspartylglutamic acid in basal ganglia Neuronal health | −0.500 | .031 | − | .822 |
Abbreviations: cART, combination antiretroviral therapy; −, negative; +, positive.
aBy Spearman correlation analysis.
bTo provide evidence that associations with sCD163 levels differ between Fiebig stages I/II and III, P values from multiple linear regression models examining the interaction between circulating sCD163 levels and Fiebig stages with CNS indices as outcome variables are shown here. For complete multiple linear regression model data, see Supplementary Table 1.
Similar trends were observed with the univariate correlations with regard to sCD163 levels in CSF. In Fiebig stage I/II, before cART, higher CSF sCD163 levels correlated with the positive CNS outcome of lower myoinositol levels in the basal ganglia (rho = −0.569; P = .037; Table 2 and Supplementary Figure 2). In Fiebig stage III, before cART, higher CSF sCD163 levels correlated with negative CNS outcomes, namely higher choline levels in the basal ganglia (rho = 0.472; P = .043) and higher CSF neopterin levels (rho = 0.479; P = .025; Table 2 and Supplementary Figure 2). In chronic HIV infection, both plasma and CSF sCD163 levels predominantly correlated with negative CNS outcomes, with the exception of higher plasma sCD163 levels correlating with lower choline levels in the frontal white matter (rho = −0.346; P = .049; Table 3 and Supplementary Figure 3). Before cART, higher plasma sCD163 levels correlated with lower N-acetylaspartate and N-acetylaspartylglutamic acid levels in the posterior cingulate gyrus (rho = −0.396; P = .026) and with higher plasma neopterin levels (rho = 0.350; P = .047; Table 3 and Supplementary Figure 3). Before cART, higher CSF sCD163 levels correlated in the frontal white matter with higher myoinositol levels (rho = 0.379; P = .019) and higher choline levels (rho = 0.324; P = .047), along with lower N-acetylaspartate and N-acetylaspartylglutamic acid levels in the frontal gray matter (rho = −0.402; P = .011) and higher CSF neopterin levels (rho = 0.356; P = .028; Table 3 and Supplementary Figure 3).
Table 3.
Correlations Between Plasma and Cerebrospinal Fluid (CSF) Soluble CD163 (sCD163) Levels During Chronic Human Immunodeficiency Virus Infection With Central Nervous System (CNS) Indices
| Source, Time, CNS Index | Rhoa | P a | CNS Outcome |
|---|---|---|---|
| Plasma | |||
| Before cART | |||
| Choline in frontal white matter, cellular infiltration | −0.346 | .049 | + |
| Acetylaspartate + N-acetylaspartylglutamic acid in posterior cingulate gyrus, neuronal health | −0.396 | .026 | − |
| Plasma neopterin, monocyte activation | 0.350 | .047 | − |
| After cART | |||
| Plasma neopterin, monocyte activation | 0.410 | .004 | − |
| CSF | |||
| Before cART | |||
| Myoinositol in frontal white matter, astrocytosis and gliosis | 0.379 | .019 | − |
| Choline in frontal white matter, cellular infiltration | 0.324 | .047 | − |
| N-acetylaspartate plus N-acetylaspartylglutamic acid in frontal gray matter, neuronal health | −0.402 | .011 | − |
| CSF neopterin, monocyte activation | 0.356 | .028 | − |
Abbreviations: cART, combination antiretroviral therapy; −, negative; +, positive.
aBy Spearman correlation analysis.
Post-cART Associations of Circulating sCD163 Levels With CNS Measures
Interestingly, no correlations were observed after cART initiation in Fiebig stage I/II. In Fiebig stage III, 24 weeks after cART, sCD163 levels continued to correlate with negative CNS outcomes. Higher CSF sCD163 levels correlated with lower neuropsychological z-4 scores, an indicator of decreased cognitive performance (rho = −0.522; P = .020) and with lower N-acetylaspartate and N-acetylaspartylglutamic acid levels in the basal ganglia (rho = −0.500; P = .031; Table 2 and Supplementary Figure 2). Higher plasma sCD163 levels correlated with higher plasma neopterin levels (rho = 0.399; P = .030; Table 2 and Supplementary Figure 2). Linear regression analyses showed mild evidence that, after cART, the positive association between CSF sCD163 levels and neuropsychological z-4 scores in Fiebig stage I/II differed from the association in Fiebig stage III (interaction P = .053; Table 2 and Supplementary Table 1). Despite the small number of observations, 3 of 9 associations show some evidence that Fiebig stage I/II and III differ in their association with sCD163 levels (Supplementary Table 1). In chronic HIV infection, 48 weeks after cART, sCD163 levels continued to associate with negative CNS outcomes: higher plasma sCD163 levels correlated with higher plasma neopterin levels (rho = 0.410; P = .004; Table 3 and Supplementary Figure 3).
Dynamics of CD163-Expressing Monocytes
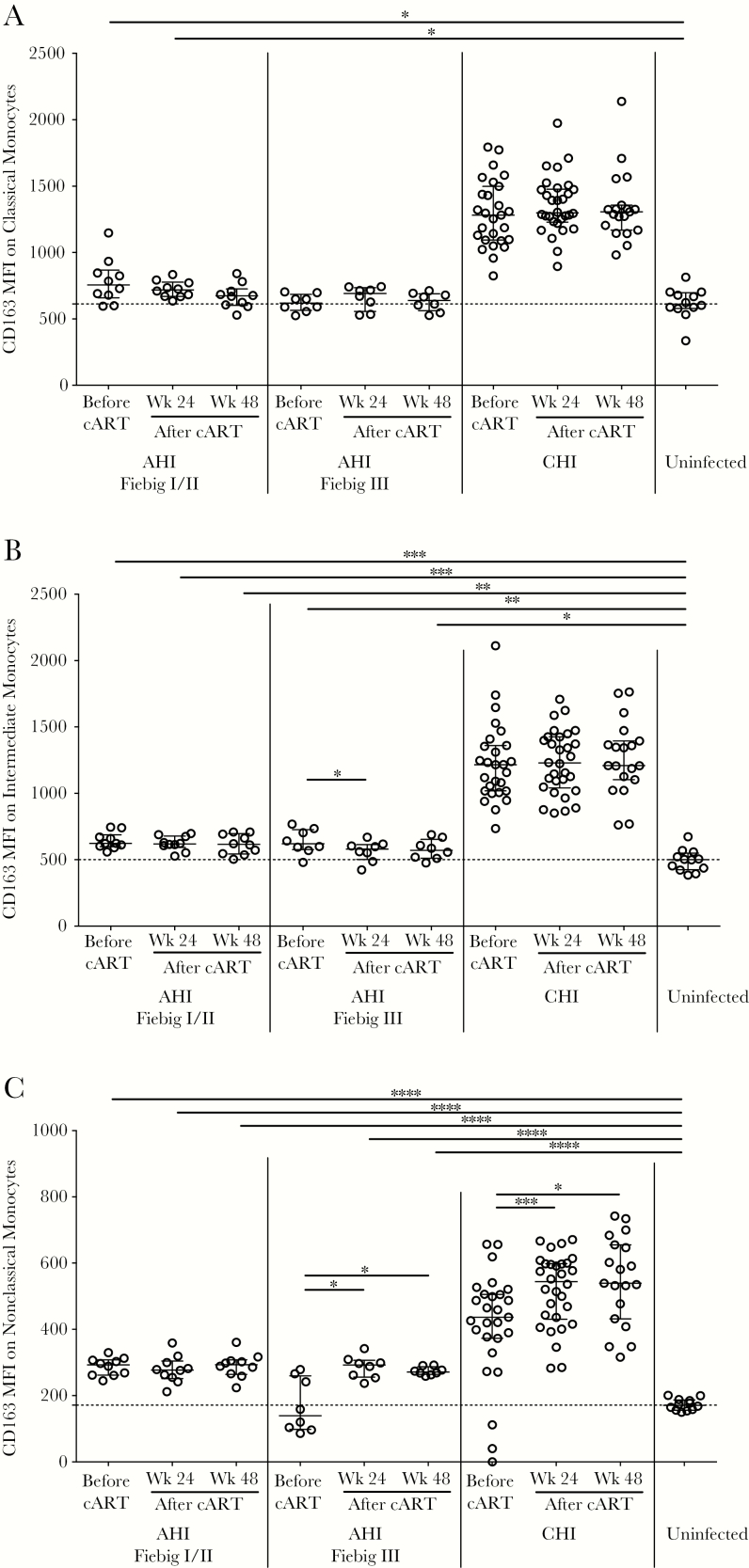
To examine monocytes as a source of sCD163, we assessed cell-surface expression on the monocyte subsets. In participants in Fiebig stage I/II, cell-surface CD163 expression on classical, intermediate, and nonclassical monocytes remained unchanged before and after cART, and with the exception of classical monocytes 48 weeks after cART, surface CD163 levels were higher than in uninfected controls (P < .05 for all comparisons; Figure 2A–C). No differences were noted in cell-surface CD163 expression on the monocyte subsets between Fiebig stage I/II and their pre- or post-cART counterpart in Fiebig stage III (Figure 2A–C).
Figure 2.
Geometric mean fluorescence intensity (MFI) of CD163 on classical (A), intermediate (B), and nonclassical (C) monocytes during acute human immunodeficiency (HIV) infection (AHI), by Fiebig stage; during chronic HIV infection (CHI), before and 24 and 48 weeks after combination antiretroviral therapy (cART) initiation; and in HIV- uninfected controls. Data are presented as medians and interquartile ranges. Dashed lines indicate the median level for HIV-uninfected controls. P values were calculated by Wilcoxon rank sum tests (for comparisons between groups) and Wilcoxon signed rank tests (for comparisons within groups), without adjustment for multiple comparisons. *P < .05, **P < .01, ***P < .001, and ****P < .0001.
In Fiebig stage III, cell-surface CD163 expression remained unchanged on classical monocytes (Figure 2A), expression on intermediate monocytes decreased 24 weeks after cART (P = .023), and increased expression on nonclassical monocytes was observed after cART (P = .023 for 24 and 48 weeks; Figure 2B and 2C). The surface CD163 level on intermediate monocytes was higher in participants in Fiebig stage III, compared with uninfected controls (P = .005 before cART and P = .038 48 weeks after cART; Figure 2B). Surface CD163 levels on nonclassical monocytes were higher in participants in Fiebig stage III only after cART, compared with levels in uninfected controls (P < .0001 for 24 and 48 weeks; Figure 2C). There were no differences in surface CD163 levels on classical monocytes between individuals in Fiebig stage III and uninfected controls (Figure 2A). In donors with acute HIV infection (ie, Fiebig stage I–III), cell-surface CD163 levels on all monocyte populations were lower than their chronically HIV-infected counterparts (P < .0001 for all comparisons; Figure 2A–C). Moreover, in participants with chronic HIV infection, cell-surface CD163 levels on all monocyte populations were higher than those in uninfected controls (P < .0001 for all comparisons; Figure 2A–C).
Association Between Monocyte Surface CD163 Expression and HIV-Related CNS Measures
Cell-surface CD163 levels on intermediate monocytes positively correlated with Grooved Pegboard z scores before cART in Fiebig stage I/II (rho = 0.928; P = .002) and with neuropsychological z-4 scores after cART in Fiebig stage III (rho = 0.738; P = .046; Table 4 and Supplementary Figure 4). There were no correlations between cell-surface CD163 expression on intermediate monocytes and MRS brain metabolite or sCD163 levels. Before cART in acute HIV infection (ie, Fiebig stage I–III), lower cell-surface CD163 expression on nonclassical monocytes correlated with higher plasma sCD163 levels (rho = −0.668; P = .008), and lower cell-surface CD163 expression on classical monocytes correlated with higher CSF sCD163 levels (rho = −0.664; P = .009; Supplementary Figure 5). Linear regression analyses demonstrated that the positive association between cell-surface CD163 expression on intermediate monocytes and Grooved Pegboard z scores in Fiebig stage I/II differed from the association in Fiebig stage III (interaction P = .001; Table 4 and Supplementary Table 2). When separating the acutely HIV-infected group into Fiebig stage I/II and Fiebig stage III, no correlations were noted. No correlations were observed between cell-surface CD163 expression on classical and nonclassical monocytes and CNS outcomes or sCD163 levels.
Table 4.
Correlations Between Cell-Surface CD163 Expression on Intermediate Monocytes, by Fiebig Human Immunodeficiency Virus Disease Stage, With Central Nervous System (CNS) Indices
| Time | Fiebig Stage | CNS Index | Rhoa | P a | P b |
|---|---|---|---|---|---|
| Before cART | I/II | Grooved Pegboard z score, cognitive performance | 0.928 | .002 | .001 |
| After cART | III | Neuropsychological z-4 score, cognitive performance | 0.738 | .046 | .875 |
Abbreviations: cART, combination antiretroviral therapy.
aBy Spearman correlation analysis.
bTo provide evidence that associations with cell surface CD163 levels differ between Fiebig stages I/II and III, P values from multiple linear regression models examining the interaction between cell-surface CD163 levels on intermediate monocytes and Fiebig stages with CNS indices as outcome variables are shown here. For complete multiple linear regression model data, see Supplementary Table 1.
DISCUSSION
The RV254/SEARCH010 cohort allows for a unique opportunity to define the earliest CNS and systemic immunological changes that occur during acute HIV infection. In this study, we provide new evidence that cART initiation as early as Fiebig stage I/II may prevent the elevation of circulating sCD163 levels above those in HIV-uninfected individuals. Moreover, although cART administration during Fiebig stage III normalized sCD163 to levels observed in uninfected controls, the transient spike in sCD163 levels during acute infection prior to cART remained associated with detrimental CNS perturbations, with the primary and meaningful neurological outcome of poorer neuropsychological testing performance, a year later. Collectively these results highlight the importance of sCD163 as a crucial marker of neuronal injury that is relevant even as early as approximately 3 weeks after infection, with important implications for long-term neuronal health in this population.
Previous reports examining primary and chronic HIV infection have associated higher plasma sCD163 levels, not higher CSF sCD163 levels, with poor cognitive performance [10, 11, 31]. Here, we extend these findings in the setting of early acute HIV infection. Our data further highlight the association between myeloid activation and CNS perturbations and uncover a paradoxical relationship in Fiebig stage I/II before cART initiation. In this group, early myeloid activation correlated with positive CNS outcomes, suggesting that, during the earliest phase of the HIV insult, CD163 is being shed as a response to restorative tissue repair processes. CD163 shedding is a component of non-pathological biological processes, as evidenced by studies showing that intravenous injection of Escherichia coli endotoxin in healthy humans causes a rapid and transient increase in sCD163 and tumor necrosis factor α levels [8]. The restorative responses observed in Fiebig stage I/II contrast with observations in Fiebig stage III or chronic HIV infection, in which sCD163 may reflect an exacerbated or pathologic response. Although transient activation of myeloid cells is critical for wound healing responses, this activation may be hijacked in the context of HIV infection, leading to the immunological system becoming overwhelmed and the repair becoming unresolved.
Our previous report in chronically HIV-infected Thais found that CCR2+ nonclassical monocytes were an independent index of cognitive impairment [19]. Our current data on cell-surface CD163 expression on nonclassical monocytes also implicate this subset in CNS perturbations during acute infection. Intermediate monocytes have been shown to preferentially cross an in vitro blood brain barrier model, suggesting that this subset may also be important in the pathogenesis of cognitive impairment [32]. Our data linking CD163 expression on intermediate monocytes and cognitive impairment suggest that this subset may be an important cellular source of sCD163. The fact that we noted associations between brain abnormalities and CD163 expression on all monocyte subsets and that there are alternate cellular sources of sCD163 (perivascular macrophages and microglial cells) highlights that the relationship between monocyte subsets, sCD163, and cognitive impairment is complex and warrants further study. Additionally, cART initiation differentially modulated surface CD163 expression on CD16+ monocytes, adding to the complexity of the relationship. Higher cell-surface CD163 expression in chronic as compared to acute HIV infection may be due to immune-related influences. Serum interleukin 10 levels, which are increased during HIV disease progression [33], has the potential to increase CD163 expression on monocytes [34].
sCD163 has been associated with non–CNS-related complications, such as noncalcified coronary plaque [31], liver fibrosis and disease [35, 36], chronic kidney disease [37], and chronic lung disease [36]. Whether early cART initiation during Fiebig stage I/II can limit the development of these aforementioned HIV-associated comorbidities warrants continued investigation in this population. Literature on the biological relevance of sCD163 is very limited. One report has demonstrated that sCD163 has antimicrobial effects by recognizing staphylococci-bound fibronectin [38]. Other studies have shown that sCD163 can inhibit T-lymphocyte activation [39] and proliferation [40], highlighting an antiinflammatory or hyperactivation effect. Additionally, recent studies have revealed that plasma sCD163 can be found in 2 forms, a soluble ectodomain CD163 and an extracellular vesicle–associated CD163, which are components of separate myeloid cell responses in the context of systemic inflammation [37]. A deeper characterization of the composition and the biological role of sCD163 in HIV infection may offer potential prognostic value.
Our data argue for the initiation of cART early in Fiebig stage I/II to limit CD163 shedding that, in Fiebig stage III, appears to be associated with neuroinflammation and CNS injury. However, identifying HIV infection and starting treatment during the Fiebig I/II stages may prove challenging. For persons who are past the Fiebig stage I/II window, targeted strategies lowering myeloid activation may prove beneficial. Maraviroc, a C-C chemokine receptor type 5 (CCR5) antagonist, has been shown to decrease sCD163 levels while improving neuropsychological test results in chronically infected individuals with cART-suppressed viral loads [41]. While intensified therapy containing maraviroc (ie, the cART+ regimen) in our acute HIV infection cohort did not significantly lower sCD163 levels as compared to participants receiving cART only (data not shown), antagonists that target multiple chemokine receptors, such as cenicriviroc (a dual CCR2 and CCR5 antagonist), may be beneficial. In a 48-week randomized study evaluating cenicriviroc versus efavirenz therapy in treatment-naive HIV-infected adults (clinical trials registration NCT01338883), levels of the monocyte activation marker sCD14 decreased with cenicriviroc and not efavirenz therapy [42].
Our study was in part exploratory, given our access to an unprecedented number of measures of inflammation and CNS measures and our desire to uncover unknown relationships to sCD163. A consequence, however, of our analysis is the generation of a large number of comparisons, potentially clouding data interpretation. With the generated P values, we do not wish to make inferences but rather highlight novel potential signals. We acknowledge that this is a limitation of the work and that further studies will be required to confirm the biological relevance of the discovered relationships. Other limitations of our study include the relatively small sample size and the significantly different ages and sex ratios across the cohorts. The lack of group matching for sex may not have had a significant impact on our study, as we did not note any significant differences when comparing sCD163 levels between sexes, and removing the female participants from the analysis did not appreciably alter the results (data not shown).
The characterization of biomarkers affords an opportunity for insight into acute HIV infection. Here, we show that early initiation of cART in Fiebig stage I/II appears to limit CD163 shedding, which may halt a neuroinflammatory cascade associated with brain abnormalities.
STUDY GROUP MEMBERS
The study group members include the following: from SEARCH/TRCARC/HIV-NAT,
Nipat Teeratakulpisarn, James L. K. Fletcher, Carlo Sacdalan, Nitiya Chomchey, Duanghathai Sutthichom, Somprartthana Rattanamanee, Peeriya Prueksakaew, Sasiwimol Ubolyam, Pacharin Eamyoung, Suwanna Puttamaswin, Putthachard Karnsomlap, Tassanee Luekasemsuk, Jintana Intasan, Khunthalee Benjapornpong, and Nisakorn Ratnaratorn; from AFRIMS, Robert J. O’Connell, Rapee Trichavaroj, Siriwat Akapirat, Yuwadee Phuang-Ngern, Suchada Sukhumvittaya, Chayada Sajjaweerawan, Surat Jongrakthaitae, Putita Saetun, Nipattra Tragonlugsana, Bessara Nuntapinit, Nantana Tantibul, and Hathairat Savadsuk; from the US Military HIV Research Program, Nelson Michael, Lydie Trautmann, Sodsai Tovanabutra, Madelaine Ouellette, Oratai Butterworth, Trevor Crowell, Ellen Turk, Leigh Ann Eller, and Mike Milazzo; and from the University of Hawai’I, Ivo Sah Bandar, Bruce Shiramizu, and Cecilia Shikuma.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank our SEARCH010, SEARCH011 and SEARCH013 study participants and staff from the Thai Red Cross AIDS Research Center, Chulalongkorn University, and AFRIMS, for their valuable contributions to this study; the Government Pharmaceutical Organization of Thailand, ViiV Healthcare, Gilead, and Merck, for providing the antiretrovirals for this study; and John Chen, for his input regarding the statistical analysis.
Disclaimer. The views expressed are those of the authors and should not be construed to represent the positions of the US Army, the US Department of Defense, or the National Institutes of Health.
Financial support. This work was supported by the National Institute of Mental Health (awards R01MH104141 [to L. C. N.] and R01MH095613 [to V. V.]), the National Institute of Neurological Disorders and Stroke (awards R01NS061696 [to V. V.] and 1R01NS084911 [to S. S. and J. A.]), and the National Institute on Minority Health and Health Disparities (awards U54MD007584, G12MD007601, P20GM103466, and U54MD007601 to the University of Hawai’i Biostatistics and Bioinformatics Cores), National Institutes of Health. The RV254 and RV304 studies were also funded by the US Military HIV Research Program, Walter Reed Army Institute of Research (Rockville, Maryland), under a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine and the US Department of Defense.
Potential conflicts of interest. J. A. has received honorarium for participating in advisory meetings for ViiV Healthcare and Merck. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 13–16 February 2017. Abstract 205.
Contributor Information
RV254/SEARCH010, SEARCH011, and RV304/SEARCH013 Study Groups:
Nipat Teeratakulpisarn, James L K Fletcher, Carlo Sacdalan, Nitiya Chomchey, Duanghathai Sutthichom, Somprartthana Rattanamanee, Peeriya Prueksakaew, Sasiwimol Ubolyam, Pacharin Eamyoung, Suwanna Puttamaswin, Putthachard Karnsomlap, Tassanee Luekasemsuk, Jintana Intasan, Khunthalee Benjapornpong, Nisakorn Ratnaratorn, Robert J O’Connell, Rapee Trichavaroj, Siriwat Akapirat, Yuwadee Phuang-Ngern, Suchada Sukhumvittaya, Chayada Sajjaweerawan, Surat Jongrakthaitae, Putita Saetun, Nipattra Tragonlugsana, Bessara Nuntapinit, Nantana Tantibul, Hathairat Savadsuk, Nelson Michael, Lydie Trautmann, Sodsai Tovanabutra, Madelaine Ouellette, Oratai Butterworth, Trevor Crowell, Ellen Turk, Leigh Ann Eller, Mike Milazzo, Ivo Sah Bandar, Bruce Shiramizu, and Cecilia Shikuma
References
- 1. Tozzi V, Balestra P, Salvatori MF, et al. Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. J Acquir Immune Defic Syndr 2009; 52:56–63. [DOI] [PubMed] [Google Scholar]
- 2. Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cassol E, Misra V, Morgello S, Gabuzda D. Applications and limitations of inflammatory biomarkers for studies on neurocognitive impairment in HIV infection. J Neuroimmune Pharmacol 2013; 8:1087–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agsalda-Garcia MA, Sithinamsuwan P, Valcour VG, et al. Brief report: CD14+ enriched peripheral cells secrete cytokines unique to HIV-associated neurocognitive disorders. J Acquir Immune Defic Syndr 2017; 74:454–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sereti I, Krebs SJ, Phanuphak N, et al. Initiation of antiretroviral therapy in early HIV infection reduces but does not abrogate chronic residual inflammation. Clin Infect Dis 2016. [Google Scholar]
- 6. Peluso MJ, Valcour V, Phanuphak N, et al. Immediate initiation of cART is associated with lower levels of cerebrospinal fluid YKL-40, a marker of microglial activation, in HIV-1 infection. AIDS 2017; 31:247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weaver LK, Hintz-Goldstein KA, Pioli PA, et al. Pivotal advance: activation of cell surface Toll-like receptors causes shedding of the hemoglobin scavenger receptor CD163. J Leukoc Biol 2006; 80:26–35. [DOI] [PubMed] [Google Scholar]
- 8. Etzerodt A, Maniecki MB, Møller K, Møller HJ, Moestrup SK. Tumor necrosis factor α-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163. J Leukoc Biol 2010; 88:1201–5. [DOI] [PubMed] [Google Scholar]
- 9. Knudsen TB, Ertner G, Petersen J, et al. Plasma CD163 independently predicts all-cause mortality from HIV-1 infection. J Infect Dis 2016; 214:1198–204. [DOI] [PubMed] [Google Scholar]
- 10. Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 2013; 27:1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Imp BM, Rubin LH, Tien PC, et al. Monocyte activation is associated with worse cognitive performance in HIV-infected women with virologic suppression. J Infect Dis 2017; 215:114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hagberg L, Cinque P, Gisslen M, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther 2010; 7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Valcour VG, Ananworanich J, Agsalda M, et al. HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PLoS One 2013; 8:e70164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bryant AK, Moore DJ, Burdo TH, et al. Plasma soluble CD163 is associated with postmortem brain pathology in human immunodeficiency virus infection. AIDS 2017; 31:973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ananworanich J, Phanuphak N, de Souza M, et al. Incidence and characterization of acute HIV-1 infection in a high-risk Thai population. J Acquir Immune Defic Syndr 2008; 49:151–5. [DOI] [PubMed] [Google Scholar]
- 16. Ananworanich J, Fletcher JL, Pinyakorn S, et al. A novel acute HIV infection staging system based on 4th generation immunoassay. Retrovirology 2013; 10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ananworanich J, Schuetz A, Vandergeeten C, et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 2012; 7:e33948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schifitto G, Zhang J, Evans SR, et al. ; ACTG A5090 Team A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology 2007; 69:1314–21. [DOI] [PubMed] [Google Scholar]
- 19. Ndhlovu LC, D’Antoni ML, Ananworanich J, et al. ; SEARCH 011 study group Loss of CCR2 expressing non-classical monocytes are associated with cognitive impairment in antiretroviral therapy-naïve HIV-infected Thais. J Neuroimmunol 2015; 288:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sailasuta N, Ross W, Ananworanich J, et al. ; RV254/SEARCH 010 protocol teams Change in brain magnetic resonance spectroscopy after treatment during acute HIV infection. PLoS One 2012; 7:e49272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Valcour V, Chalermchai T, Sailasuta N, et al. ; RV254/SEARCH 010 Study Group Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 2012; 206:275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mohamed MA, Lentz MR, Lee V, et al. Factor analysis of proton MR spectroscopic imaging data in HIV infection: metabolite-derived factors help identify infection and dementia. Radiology 2010; 254:577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lentz MR, Kim WK, Kim H, et al. Alterations in brain metabolism during the first year of HIV infection. J Neurovirol 2011; 17:220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang L, Lee PL, Yiannoutsos CT, et al. ; HIV MRS Consortium A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage 2004; 23:1336–47. [DOI] [PubMed] [Google Scholar]
- 25. Ratai EM, Annamalai L, Burdo T, et al. Brain creatine elevation and N-acetylaspartate reduction indicates neuronal dysfunction in the setting of enhanced glial energy metabolism in a macaque model of neuroAIDS. Magn Reson Med 2011; 66:625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naïve HIV patients. Neuroimage 2002; 17:1638–48. [DOI] [PubMed] [Google Scholar]
- 27. Sailasuta N, Shriner K, Ross B. Evidence of reduced glutamate in the frontal lobe of HIV-seropositive patients. NMR Biomed 2009; 22:326–31. [DOI] [PubMed] [Google Scholar]
- 28. Barker PB, Lee RR, McArthur JC. AIDS dementia complex: evaluation with proton MR spectroscopic imaging. Radiology 1995; 195:58–64. [DOI] [PubMed] [Google Scholar]
- 29. Lentz MR, Kim WK, Lee V, et al. Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology 2009; 72:1465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krebs SJ, Slike BM, Sithinamsuwan P, et al. ; SEARCH 011 study team Sex differences in soluble markers vary before and after the initiation of antiretroviral therapy in chronically HIV-infected individuals. AIDS 2016; 30:1533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Williams DW, Calderon TM, Lopez L, et al. Mechanisms of HIV entry into the CNS: increased sensitivity of HIV infected CD14+CD16+ monocytes to CCL2 and key roles of CCR2, JAM-A, and ALCAM in diapedesis. PLoS One 2013; 8:e69270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stylianou E, Aukrust P, Kvale D, Müller F, Frøland SS. IL-10 in HIV infection: increasing serum IL-10 levels with disease progression–down-regulatory effect of potent anti-retroviral therapy. Clin Exp Immunol 1999; 116:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sulahian TH, Högger P, Wahner AE, et al. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine 2000; 12:1312–21. [DOI] [PubMed] [Google Scholar]
- 35. Kooij KW, Wit FW, van Zoest RA, et al. ; AGEhIV Cohort Study Group Liver fibrosis in HIV-infected individuals on long-term antiretroviral therapy: associated with immune activation, immunodeficiency and prior use of didanosine. AIDS 2016; 30:1771–80. [DOI] [PubMed] [Google Scholar]
- 36. Kirkegaard-Klitbo DM, Mejer N, Knudsen TB, et al. Soluble CD163 predicts incident chronic lung, kidney and liver disease in HIV infection. AIDS 2017; 31:981–8. [DOI] [PubMed] [Google Scholar]
- 37. Etzerodt A, Berg RM, Plovsing RR, et al. Soluble ectodomain CD163 and extracellular vesicle-associated CD163 are two differently regulated forms of ‘soluble CD163’ in plasma. Sci Rep 2017; 7:40286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kneidl J, Löffler B, Erat MC, et al. Soluble CD163 promotes recognition, phagocytosis and killing of Staphylococcus aureus via binding of specific fibronectin peptides. Cell Microbiol 2012; 14:914–36. [DOI] [PubMed] [Google Scholar]
- 39. Högger P, Sorg C. Soluble CD163 inhibits phorbol ester-induced lymphocyte proliferation. Biochem Biophys Res Commun 2001; 288:841–3. [DOI] [PubMed] [Google Scholar]
- 40. O’Connell GC, Tennant CS, Lucke-Wold N, et al. Monocyte-lymphocyte cross-communication via soluble CD163 directly links innate immune system activation and adaptive immune system suppression following ischemic stroke. Sci Rep 2017; 7:12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ndhlovu LC, Umaki T, Chew GM, et al. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND). J Neurovirol 2014; 20:571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thompson M, Saag M, DeJesus E, et al. A 48-week randomized phase 2b study evaluating cenicriviroc versus efavirenz in treatment-naive HIV-infected adults with C-C chemokine receptor type 5-tropic virus. AIDS 2016; 30:869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.