Abstract
Background & Aims
Specific nutritional components are likely to induce intestinal inflammation, which is characterized by increased levels of interleukin 6 (IL6), C-reactive protein (CRP), and TNF receptor superfamily member 1B (TNFRSF1B) in the circulation and promotes colorectal carcinogenesis. The inflammatory effects of a diet can be estimated based on empirical dietary inflammatory pattern (EDIP) score, calculated based on intake of 18 foods associated with plasma levels of IL6, CRP, and TNFRSF1B. An inflammatory environment in the colon (based on increased levels of IL6, CRP, and TNFRSF1B in peripheral blood) contributes to impairment of the mucosal barrier and altered immune cell responses, affecting the composition of the intestinal microbiota. Colonization by Fusobacterium nucleatum has been associated with presence and features of colorectal adenocarcinoma. We investigated the association between diets that promote inflammation (based on EDIP score) and colorectal cancer subtypes classified by level of F nucleatum in the tumor microenvironment.
Methods
We calculated EDIP scores based on answers to questionnaires collected from participants in the Nurses’ Health Study (through June 1, 2012) and the Health Professionals Follow-up Study (through January 31, 2012). Participants in both cohorts reported diagnoses of rectal or colon cancer in biennial questionnaires; deaths from unreported colorectal cancer cases were identified through the National Death Index and next of kin. Colorectal tumor tissues were collected from hospitals where the patients underwent tumor resection and F nucleatum DNA was quantified by a PCR assay. We used multivariable duplication-method Cox proportional hazard regression to assess the associations of EDIP scores with risks of colorectal cancer subclassified by F nucleatum status.
Results
During 28 years of follow up of 124,433 participants, we documented 951 incident cases of colorectal carcinoma with tissue F nucleatum data. Higher EDIP scores associated with increased risk of F nucleatum-positive colorectal tumors (Ptrend=.03); for subjects in the highest vs lowest EDIP score tertiles, the hazard ratio for F nucleatum-positive colorectal tumors was 1.63 (95% CI, 1.03–2.58). EDIP scores did not associate with F nucleatum-negative tumors (Ptrend=.44). High EDIP scores associated with proximal F nucleatum-positive colorectal tumors but not with proximal F nucleatum-negative colorectal tumors (Pheterogeneity=.003).
Conclusion
Diets that promote intestinal inflammation, based on EDIP score, associate with increased risk of F nucleatum-positive colorectal carcinomas, but not carcinomas that do not contain these bacteria. These findings indicate that diet-induced intestinal inflammation alters the gut microbiome to contribute to colorectal carcinogenesis; nutritional interventions might be used in precision medicine and cancer prevention.
Keywords: immunity, microsatellite instability, nutrition, red meat
INTRODUCTION
Chronic inflammation is a well-established etiologic factor for colorectal carcinoma.1,2 We have demonstrated that the inflammatory diets which could induce systemic and intestinal inflammation were associated with higher risk of colorectal cancer.3 Although the underlying mechanisms remain unclear, recent evidence indicates that the cancer-promoting effect of diet-related inflammation can be enhanced by certain bacterial species in the intestinal microbiota.1,2,4 Intestinal inflammation decreases the production of protective mucins and antimicrobial peptides,5 which may facilitate the adherence of bacteria to colonic mucosa. The impaired mucosal barrier function enables bacteria to more readily interact with the epithelium, resulting in colonization of bacteria within colonic mucosa and increased exposures of intestinal cells to bacterial mutagenic metabolites.
Some gut microbiota including Fusobacterium nucleatum (F nucleatum), a potentiator for colorectal cancer, may contribute to carcinogenesis through their influence on expression of transcription factors, oncogenes, and inflammatory genes,1,2,6–8 and recruitment of monocytes and myeloid-derived suppressor cells to generate a inflammatory microenvironment.7,9 Studies have revealed the enrichment of F nucleatum in colorectal tumor tissues compared to adjacent normal tissues.10–13 The presence of detectable F nucleatum in tumor tissues has been associated with proximal tumor location, serrated neoplasia pathway, consensus molecular subtypes, microsatellite instability (MSI), and high-level macrophage and low-level CD3+ T cell infiltrate in tumor.10,14–18 In addition, the existence of F nucleatum within tumor tissues has been reported to contribute to disease progression and chemoresistance in patients with colorectal cancer.19,20 Given the role of F nucleatum in shaping tumor-promoting inflammatory environment and the enrichment of F nucleatum in intestinal carcinomas, we hypothesized that the association of inflammatory diets (diets that promote inflammation) with colorectal cancer risk might be stronger for tumors containing F nucleatum than for tumors without detectable F nucleatum.
To test this hypothesis, we utilized a molecular pathological epidemiology database within two prospective cohort studies [the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS)] with long-term biennial questionnaire data and colorectal tumor tissues available for molecular and microbial analyses. We prospectively examined updated information on inflammatory diet intakes in relation to incidence of colorectal cancer subtypes classified by F nucleatum in tumor tissues.
METHODS
Study population
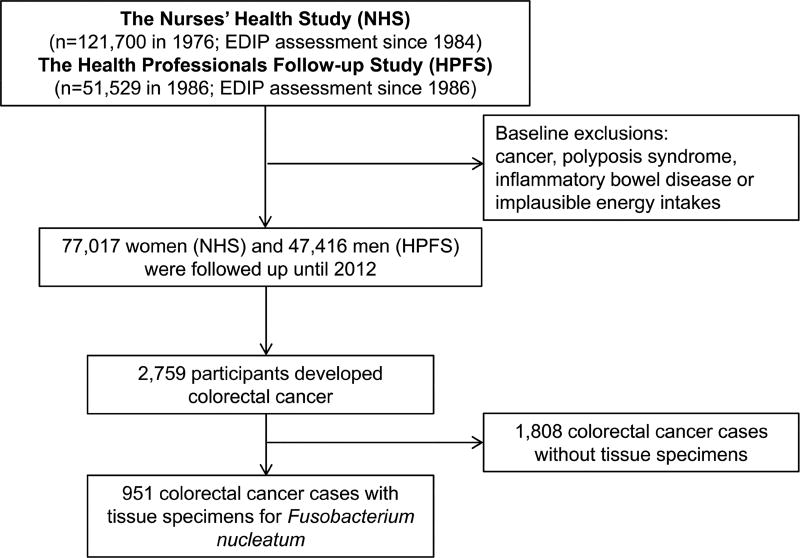
The Nurses’ Health Study (NHS) enrolled 121,700 registered female nurses in the United States of America aged 30 – 55 years at baseline in 1976, and the Health Professionals Follow-up Study (HPFS) recruited 51,529 male health professionals aged 40 – 75 years at baseline in 1986 (Figure 1).21 In both cohorts, follow-up questionnaires were administered at baseline and every two years thereafter to collect and update lifestyle and health-related information. Validated food frequency questionnaires were sent every four years to update dietary information. We followed participants from baseline questionnaire return through June 1, 2012 in the NHS or January 31, 2012 in the HPFS. Written consent was obtained from each participant. This study was approved by Human Subjects Committees at Harvard T.H. Chan School of Public Health and Brigham and Women’s Hospital. This study was reported according to the STROBE statement.22
Figure 1.
Flow diagram of study population. EDIP, empirical dietary inflammatory pattern.
Acquisition of colorectal cancer cases
In both cohorts, participants reported a diagnosis of colon or rectal cancer in biennial questionnaires. Deaths from unreported colorectal cancer cases were identified through the National Death Index and next of kin. All colorectal carcinoma diagnoses were verified through centralized histopathologic examination by the study pathologist (S.O.). We included both colon and rectal carcinomas based on the colorectal continuum model.16,23
Assessment of diets and other covariates
The inflammatory effects of diets were estimated based on empirical dietary inflammatory pattern (EDIP) score, which is the sum of weighted intake scores of 18 (processed meat, red meat, organ meat, fish, vegetables other than green leafy vegetables and dark yellow vegetables, refined grains, high-energy beverages, low-energy beverages, tomatoes, beer, wine, tea, coffee, dark yellow vegetables, green leafy vegetables, snacks, fruit juice, and pizza) constructed to predict plasma levels of IL6 (Interleukin 6), CRP (C-reactive protein), and TNFRSF1B (TNF receptor superfamily member 1B, TNFα-receptor 2).24 The higher scores represent inflammatory diets and lower scores indicate anti-inflammatory diets.24 The EDIP scores were calculated for each participant at each questionnaire cycle. We set 1984 as the study baseline for the NHS, and 1986 for the HPFS. The cumulative average EDIP scores were further computed by averaging all prior EDIP scores up to each questionnaire cycle. Participants were categorized into tertiles using cohort-specific cut-off points of cumulative average of EDIP scores. Information on lifestyles and medication was assessed using biennial questionnaires in both cohorts as previously described.21,25
Analyses of Fusobacterium nucleatum and other tumor characteristics
Archival formalin-fixed paraffin-embedded tumor tissue blocks of confirmed colorectal cancer cases were collected from hospitals where the patients underwent tumor resection. DNA was extracted from colorectal cancer tissue using QIAamp DNA FFPE Tissue Kit (Qiagen). The amount of tissue F nucleatum DNA was measured by a quantitative PCR assay and normalized with the reference gene SLCO2A1 as previously described.13,15 Cases with detectable F nucleatum DNA were categorized as positive, otherwise as negative. Cases with positive F nucleatum were further categorized as low or high relative to the median cut-off point of F nucleatum DNA quantities among F nucleatum-positive cases.26 Microsatellite instability (MSI) and PTGS2 (cyclooxygenase 2) expression in tumors were assessed as previously described.25
Statistical analysis
Participants who died of causes other than colorectal cancer and those who were free of colorectal cancer at the end of follow-up were censored. In addition, colorectal cancer cases with unknown F nucleatum status were censored at the time of diagnosis. For each participant, we calculated follow-up time (in months) from the date of the questionnaire return at the study baseline until the date of death, colorectal cancer diagnosis, or end of follow-up, whichever came first. We used duplication-method Cox proportional cause-specific hazards regression for competing risks data27 to assess the associations between time-varying EDIP scores and risks of colorectal cancer subtypes classified by F nucleatum status in tumors. Testing for trend across tertiles of EDIP scores was performed using the median value of each tertile group in the Cox regression models. To examine the heterogeneity in the associations with various colorectal cancer subtypes, we used the likelihood ratio test by comparing the model in which the association with EDIP was allowed to vary by tumor subtypes to a model in which a common association was assumed across tumor subtypes. The multivariable models were primarily adjusted for smoking status, family history of colorectal cancer, endoscopy status, physical activity levels, total calorie intake, alcohol consumption, current multivitamin use, and regular aspirin use. Considering overweight / obesity may act as a mediator and a confounder,24 body mass index (BMI) was further added into the multivariable models. Given that not all confirmed cases were available for detection of F nucleatum, inverse probability weighting (IPW) was used to reduce bias from potentially varied F nucleatum data availability. This was achieved by calculating the cohort-specific predictive probability of observing F nucleatum data for each case using multivariable logistic regression as previously described.28 SAS 9.4 (SAS Institute Inc, Cary, North Carolina, USA) was used for all statistical analyses. All statistical tests were two-sided.
RESULTS
Characteristics of study participants
The exclusion for baseline diet data, cancer history, polyposis syndrome, inflammatory bowel disease and implausible energy intake led to inclusion of a total of 124,433 participants in the final analysis. During 28 years of follow-up evaluation with 2,998,587 person-years, we documented 951 colorectal cancer cases with available F nucleatum data (Figure 1). Participants reporting high inflammatory diet intake were more likely to have higher BMI and energy intake, but lower amounts of pack-years of cigarette smoking, physical activity, multivitamin intake and alcohol intake (Table 1). We did not observe evidence of a substantial violation of the proportionality of hazards assumption on the basis of interaction terms between empirical dietary inflammatory pattern (EDIP) scores and follow-up time (P = 0.42). Except for the colorectal cancer subtype with negative F nucleatum in tumors (Pheterogeneity = 0.002), we did not observe significant heterogeneity between cohorts for the associations of EDIP scores with risks of other colorectal cancer subtypes. In order to increase statistical power, the NHS and the HPFS were combined to perform pooled analyses stratified by sex (cohort), age in months, and calendar year of the questionnaire cycle.
Table 1.
Age-adjusted baseline characteristics of participants across tertiles of the empirical dietary inflammatory pattern scores in the Nurses’ Health Study (women, at 1984) and the Health Professionals Follow-up Study (men, at 1986)
| Characteristic | Tertiles of the empirical dietary inflammatory pattern (EDIP) scores | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Women (NHS) | Men (HPFS) | |||||
|
|
|
|||||
| T1 (lowest) | T2 | T3 (highest) | T1 (lowest) | T2 | T3 (highest) | |
| N of participants | 25,660 | 25,628 | 25,729 | 16,016 | 15,679 | 15,721 |
| Age, years* | 51.15 (6.96) | 51.09 (7.23) | 50.26 (7.25) | 53.74 (9.32) | 54.96 (9.87) | 54.55 (10.00) |
| Race (White), % | 99 | 98 | 97 | 92 | 91 | 89 |
| BMI, kg/m2 | 23.97 (3.84) | 24.86 (4.43) | 26.32 (5.52) | 25.28 (3.03) | 25.38 (3.15) | 25.88 (3.53) |
| Family history of colorectal cancer, % | 8 | 8 | 8 | 9 | 8 | 8 |
| Smoking, pack-years | 14.63 (18.27) | 11.38 (16.92) | 11.15 (17.22) | 15.43 (19.64) | 12.44 (18.24) | 12.34 (18.76) |
| Waist hip ratio | 0.78 (0.08) | 0.78 (0.08) | 0.80 (0.08) | 0.62 (0.44) | 0.61 (0.45) | 0.60 (0.46) |
| Energy intake, kcal/day | 1606 (434) | 1602 (436) | 1769 (476) | 1950 (591) | 1868 (578) | 2141 (655) |
| Total activity, METS-hours/week | 15.60 (23.25) | 13.93 (20.44) | 12.79 (18.82) | 20.26 (27.00) | 18.34 (24.57) | 17.63 (26.45) |
| Current multivitamin use, % | 39 | 37 | 35 | 44 | 42 | 39 |
| History of endoscopy, % | 54 | 55 | 55 | 27 | 26 | 25 |
| Total alcohol intake, g/day | 10.23 (12.47) | 5.43 (8.48) | 4.19 (8.06) | 17.68 (18.83) | 9.18 (12.40) | 6.97 (11.81) |
| Regular aspirin use, % | 39 | 39 | 41 | 31 | 28 | 29 |
| Postmenopausal hormone use, % | 46 | 46 | 45 | - | - | - |
| Components of the empirical dietary inflammatory pattern | ||||||
| Processed meat, servings/week | 1.64 (1.67) | 1.95 (1.89) | 2.92 (3.03) | 1.96 (2.13) | 2.18 (2.39) | 3.44 (3.94) |
| Red meat, servings/week | 3.84 (2.40) | 4.21 (2.55) | 5.31 (3.16) | 3.60 (2.68) | 3.89 (2.88) | 5.30 (3.81) |
| Organ meat, servings/week | 0.16 (0.30) | 0.17 (0.28) | 0.20 (0.36) | 0.11 (0.24) | 0.12 (0.26) | 0.14 (0.28) |
| Other fish, servings/week | 1.83 (1.46) | 1.91 (1.57) | 2.22 (1.99) | 2.04 (1.61) | 2.15 (1.68) | 2.58 (2.44) |
| Other vegetable, servings/week | 5.47 (4.26) | 5.51 (4.16) | 6.41 (5.84) | 5.44 (4.40) | 5.46 (4.31) | 6.50 (5.64) |
| Refined grain, servings/week | 6.66 (5.17) | 8.08 (6.33) | 11.83 (8.85) | 6.52 (5.41) | 7.51 (6.31) | 11.63 (9.82) |
| High energy beverage, servings/week | 1.01 (1.90) | 1.57 (2.61) | 3.67 (6.08) | 1.29 (2.22) | 1.87 (2.88) | 4.12 (6.09) |
| Low energy beverage, servings/week | 2.71 (4.68) | 3.46 (5.36) | 6.78 (10.25) | 2.39 (4.48) | 2.88 (5.04) | 5.26 (9.43) |
| Tomato, servings/week | 3.51 (2.63) | 3.64 (2.65) | 4.56 (3.92) | 3.69 (2.96) | 3.83 (2.91) | 4.99 (4.51) |
| Beer, servings/week | 1.14 (4.03) | 0.43 (1.73) | 0.26 (1.24) | 3.57 (6.75) | 1.40 (2.86) | 0.89 (2.12) |
| Wine, servings/week | 3.65 (5.65) | 1.19 (2.00) | 0.66 (1.36) | 3.48 (5.57) | 1.19 (1.82) | 0.72 (1.37) |
| Tea, servings/week | 5.00 (8.46) | 4.93 (7.93) | 4.63 (7.52) | 3.25 (6.72) | 2.95 (5.78) | 2.84 (5.65) |
| Coffee, servings/day | 3.69 (1.98) | 2.29 (1.66) | 1.48 (1.47) | 3.06 (2.06) | 1.70 (1.57) | 1.13 (1.35) |
| Dark yellow vegetable, servings/week | 2.44 (2.74) | 2.07 (1.93) | 1.92 (1.76) | 2.53 (3.25) | 2.18 (2.17) | 2.09 (2.06) |
| Green leafy vegetable, servings/week | 7.15 (5.82) | 5.46 (3.80) | 4.93 (3.66) | 6.09 (5.38) | 4.81 (3.67) | 4.46 (3.59) |
| Snack, servings/week | 5.57 (8.72) | 3.95 (5.76) | 3.82 (5.26) | 4.36 (6.22) | 3.55 (4.52) | 3.73 (4.46) |
| Fruit juice, servings/week | 5.55 (6.12) | 5.10 (5.01) | 4.81 (4.80) | 6.12 (7.42) | 5.38 (5.27) | 5.03 (5.19) |
| Pizza, servings/week | 0.54 (0.65) | 0.44 (0.45) | 0.41 (0.41) | 0.73 (0.94) | 0.50 (0.54) | 0.44 (0.49) |
BMI, body mass index; HPFS, Health Professionals Follow-up Study; METS, metabolic equivalent task score; NHS, Nurses’ Health Study; T1, tertile 1; T2, tertile 2; T3, tertile 3.
Values are means ± standard deviation (SD) or percentages and are standardized to the age distribution of the study population.
Value is not age adjusted.
Empirical dietary inflammatory pattern (EDIP) scores and colorectal cancer risk by Fusobacterium nucleatum
High EDIP (highest tertile) scores were associated with higher risk of F nucleatum-positive colorectal tumor subtype [Ptrend = 0.03; highest vs lowest EDIP score tertile: multivariable-adjusted HR = 1.63; 95% confidence interval (CI), 1.03–2.58], but not with risk of F nucleatum-negative tumors (Ptrend = 0.44); although the test for heterogeneity did not reach statistical significance (Pheterogeneity = 0.07; Table 2). We conducted an analysis stratified by tumor location since a high amount of F nucleatum in colorectal carcinoma tissues has been associated with proximal tumor location.10,16 Compared to distal colon and rectal cancers, the differential associations of EDIP scores with the tumor subtypes classified by tissue F nucleatum became more pronounced in proximal colon cancer (Pheterogeneity = 0.003; Table 3), where high EDIP scores were associated with higher risk of F nucleatum-positive tumor subtype (Ptrend = 0.003; highest vs lowest EDIP score tertile: multivariable-adjusted HR = 2.61; 95% CI, 1.35–5.05), but not with risk of F nucleatum-negative tumor subtype (Ptrend = 0.84). Sensitivity analyses using Cox proportional cause-specific hazards regression weighted by inverse probability of F nucleatum data availability generated similar results to those of the primary analyses (Supplementary Table 1). Further analyses in each cohort revealed that the associations of EDIP scores with colorectal cancer incidence tended to be stronger for F nucleatum-positive tumor subtype than for F nucleatum-negative tumor subtype in each of NHS and HPFS (Supplementary Table 2).
Table 2.
The empirical dietary inflammatory pattern scores and risk of colorectal cancer according to tumor F nucleatum status in the pooled cohorts of the Nurses’ Health Study (women, 1984–2012) and the Health Professionals Follow-up Study (men, 1986–2012)
| Tertiles of the empirical dietary inflammatory pattern (EDIP) scores | Ptrend* | Pheterogeneity† | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| T1 (lowest) | T2 | T3 (highest) | ||||
| Colorectal cancer | Person-years | 1,040,010 | 991,169 | 967,408 | ||
| N of cases (n=951) | 309 | 329 | 313 | |||
| Age-adjusted HR (95% CI) | 1 (reference) | 1.06 (0.91 – 1.24) | 1.08 (0.92 – 1.27) | 0.31 | ||
| Multivariable HR (95% CI)‡ | 1 (reference) | 1.11 (0.94 – 1.30) | 1.12 (0.95 – 1.33) | 0.16 | ||
| Multivariable HR (95% CI)§ | 1 (reference) | 1.09 (0.93 – 1.29) | 1.09 (0.92 – 1.30) | 0.28 | ||
|
| ||||||
| Tumor F nucleatum status | ||||||
| Negative | N of cases (n=836) | 277 | 291 | 268 | 0.07‡ | |
| Age-adjusted HR (95% CI) | 1 (reference) | 1.05 (0.89 – 1.24) | 1.03 (0.87 – 1.22) | 0.73 | ||
| Multivariable HR (95% CI)‡ | 1 (reference) | 1.09 (0.92 – 1.29) | 1.07 (0.89 – 1.28) | 0.44 | ||
| Multivariable HR (95% CI)§ | 1 (reference) | 1.08 (0.91 – 1.28) | 1.04 (0.87 – 1.24) | 0.63 | ||
|
N of cases (n=58) | 20 | 14 | 24 | ||
| Age-adjusted HR (95% CI) | 1 (reference) | 0.68 (0.34 – 1.36) | 1.30 (0.72 – 2.37) | 0.33 | ||
| Multivariable HR (95% CI)‡ | 1 (reference) | 0.70 (0.35 – 1.40) | 1.37 (0.75 – 2.49) | 0.26 | ||
| Multivariable HR (95% CI)§ | 1 (reference) | 0.69 (0.35 – 1.38) | 1.33 (0.73 – 2.42) | 0.30 | ||
| N of cases (n=57) | 12 | 24 | 21 | |||
| Age-adjusted HR (95% CI) | 1 (reference) | 2.04 (1.02 – 4.09) | 1.99 (0.97 – 4.05) | 0.06 | ||
| Multivariable HR (95% CI)‡ | 1 (reference) | 2.16 (1.07 – 4.34) | 2.05 (1.00 – 4.19) | 0.05 | ||
| Multivariable HR (95% CI)§ | 1 (reference) | 2.13 (1.06 – 4.27) | 1.99 (0.97 – 4.07) | 0.07 | ||
| N of cases (n=115) | 32 | 38 | 45 | |||
| Age-adjusted HR (95% CI) | 1 (reference) | 1.19 (0.74 – 1.90) | 1.57 (0.99 – 2.47) | 0.05 | ||
| Multivariable HR (95% CI)‡ | 1 (reference) | 1.24 (0.77 – 1.99) | 1.63 (1.03 – 2.58) | 0.03 | ||
| Multivariable HR (95% CI)§ | 1 (reference) | 1.22 (0.76 – 1.96) | 1.58 (1.00 – 2.50) | 0.04 | ||
CI, confidence interval; F nucleatum, Fusobacterium nucleatum; HR, hazard ratio; T1, tertile 1; T2, tertile 2; T3, tertile 3.
Cox proportional cause-specific hazards regression for competing risks data was used to compute HRs and 95% CIs.
All analyses were stratified by age (in month), year of questionnaire return and sex.
Linear trend test using the median value of each category.
The likelihood ratio test was used for the heterogeneity of the association between the empirical dietary inflammatory pattern scores and colorectal cancer risk according to tumor F nucleatum status (negative vs positive-low vs positive-high).
Multivariable HR was adjusted for pack-years smoked (0 vs 1–19 vs 20–39 vs ≥ 40 pack-years), family history of colorectal cancer (yes vs no), endoscopy status (yes vs no), physical activity level [quintiles of mean metabolic equivalent task score (METS) - hours per week], total calorie intake (quintiles of kcal/day), total alcohol intake (0 vs 1–5 vs 6–15 vs > 15 g/day), current multivitamin use (yes vs no), and regular aspirin use (yes vs no).
Multivariable HR was adjusted for body mass index (< 25 vs 25–29.9 vs ≥ 30 kg/m2), pack-years smoked (0 vs 1–19 vs 20–39 vs ≥ 40 pack-years), family history of colorectal cancer (yes vs no), endoscopy status (yes vs no), physical activity level [quintiles of mean metabolic equivalent task score (METS) - hours per week], total calorie intake (quintiles of kcal/day), total alcohol intake (0 vs 1–5 vs 6–15 vs > 15 g/day), current multivitamin use (yes vs no), and regular aspirin use (yes vs no).
Table 3.
The empirical dietary inflammatory pattern scores and risk of colorectal cancer according to tumor F nucleatum status in different sub-sites of colorectum in the pooled cohorts of the Nurses’ Health Study (women, 1984–2012) and the Health Professionals Follow-up Study (men, 1986–2012)
| Sub-sites of colorectum | Tumor F nucleatum status |
Tertiles of the empirical dietary inflammatory pattern (EDIP) scores | Ptrend* | Pheterogeneity† | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| T1 (lowest) | T2 | T3 (highest) | |||||
| Proximal colon cancer | |||||||
| Negative | N of cases (n=396) | 136 | 138 | 122 | 0.003 | ||
| Age-adjusted HR (95% CI) | 1 (reference) | 1.00 (0.79 – 1.27) | 0.94 (0.74 – 1.21) | 0.72 | |||
| Multivariable HR (95% CI)‡ | 1 (reference) | 1.01 (0.79 – 1.29) | 0.96 (0.74 – 1.24) | 0.84 | |||
| Positive | N of cases (n=67) | 13 | 24 | 30 | |||
| Age-adjusted HR (95% CI) | 1 (reference) | 1.92 (0.97 – 3.79) | 2.59 (1.35 – 4.98) | 0.003 | |||
| Multivariable HR (95% CI)‡ | 1 (reference) | 1.94 (0.98 – 3.84) | 2.61 (1.35 – 5.05) | 0.003 | |||
| Distal colon cancer | |||||||
| Negative | N of cases (n=253) | 76 | 88 | 89 | 0.35 | ||
| Age-adjusted HR (95% CI) | 1 (reference) | 1.15 (0.85 – 1.57) | 1.24 (0.91 – 1.69) | 0.21 | |||
| Multivariable HR (95% CI)‡ | 1 (reference) | 1.25 (0.91 – 1.72) | 1.32 (0.95 – 1.83) | 0.13 | |||
| Positive | N of cases (n=19) | 8 | 6 | 5 | |||
| Age-adjusted HR (95% CI) | 1 (reference) | 0.72 (0.25 – 2.10) | 0.68 (0.22 – 2.08) | 0.47 | |||
| Multivariable HR (95% CI)‡ | 1 (reference) | 0.81 (0.27 – 2.37) | 0.76 (0.25 – 2.34) | 0.60 | |||
| Rectal cancer | |||||||
| Negative | N of cases (n=178) | 65 | 58 | 55 | 0.49 | ||
| Age-adjusted HR (95% CI) | 1 (reference) | 0.91 (0.64 – 1.31) | 0.92 (0.64 – 1.32) | 0.65 | |||
| Multivariable HR (95% CI)‡ | 1 (reference) | 0.95 (0.66 – 1.37) | 0.94 (0.64 – 1.39) | 0.79 | |||
| Positive | N of cases (n=24) | 9 | 5 | 10 | |||
| Age-adjusted HR (95% CI) | 1 (reference) | 0.53 (0.18 – 1.59) | 1.25 (0.51 – 3.11) | 0.59 | |||
| Multivariable HR (95% CI)‡ | 1 (reference) | 0.56 (0.19 – 1.69) | 1.31 (0.52 – 3.27) | 0.54 | |||
CI, confidence interval; F nucleatum, Fusobacterium nucleatum; HR, hazard ratio; T1, tertile 1; T2, tertile 2; T3, tertile 3.
Cox proportional cause-specific hazards regression for competing risks data was used to compute HRs and 95% CIs.
All analyses were stratified by age (in month), year of questionnaire return and sex.
Linear trend test using the median value of each category.
The likelihood ratio test was used for the heterogeneity of the association between the empirical dietary inflammatory pattern scores and colorectal cancer risk according to tumor F nucleatum status (negative vs positive).
Multivariable HR was adjusted for pack-years smoked (0 vs 1–19 vs 20–39 vs ≥ 40 pack-years), family history of colorectal cancer (yes vs no), endoscopy status (yes vs no), physical activity level [quintiles of mean metabolic equivalent task score (METS) - hours per week], total calorie intake (quintiles of kcal/day), total alcohol intake (0 vs 1–5 vs 6–15 vs > 15 g/day), current multivitamin use (yes vs no), and regular aspirin use (yes vs no).
Because of the reported association of MSI status and PTGS2 (cyclooxygenase 2) expression with F nucleatum in colorectal tumors,10,26,29 we further examined whether the differential association between inflammatory diets and risk of colorectal cancer subtypes classified by tumor F nucleatum status varied according to tumor MSI status or PTGS2 (cyclooxygenase 2) expression levels. We found that the differential association appeared to be generally consistent irrespective of tumor MSI or PTGS2 (cyclooxygenase 2) status, although statistical power was limited in the subset analyses (Table 4).
Table 4.
The empirical dietary inflammatory pattern scores and risk of colorectal cancer according to microsatellite instability, PTGS2 (cyclooxygenase 2) and F nucleatum status in tumor tissues in the pooled cohorts of the Nurses’ Health Study (women, 1984–2012) and the Health Professionals Follow-up Study (men, 1986–2012)
| Molecular characteristic | Tumor F nucleatum status |
Tertiles of the empirical dietary inflammatory pattern (EDIP) scores | Ptrend* | |||
|---|---|---|---|---|---|---|
|
| ||||||
| T1 (lowest) | T2 | T3 (highest) | ||||
| Microsatellite instability (MSI) status | ||||||
| Non-MSI-high | N of cases (n=999) | 330 | 341 | 328 | ||
| Age-adjusted HR (95% CI) | 1 (reference) | 1.02 (0.88 – 1.19) | 1.05 (0.90 – 1.23) | 0.50 | ||
| Multivariable HR (95% CI)† | 1 (reference) | 1.08 (0.92 – 1.26) | 1.12 (0.95 – 1.31) | 0.18 | ||
| Negative | N of cases (n=699) | 225 | 247 | 227 | ||
| Age-adjusted HR (95% CI) | 1 (reference) | 1.10 (0.92 – 1.32) | 1.07 (0.89 – 1.29) | 0.47 | ||
| Multivariable HR (95% CI)† | 1 (reference) | 1.15 (0.95 – 1.38) | 1.12 (0.92 – 1.36) | 0.25 | ||
| Positive | N of cases (n=68) | 22 | 19 | 27 | ||
| Age-adjusted HR (95% CI) | 1 (reference) | 0.84 (0.45 – 1.56) | 1.37 (0.77 – 2.41) | 0.26 | ||
| Multivariable HR (95% CI)† | 1 (reference) | 0.88 (0.47 – 1.63) | 1.44 (0.82 – 2.55) | 0.19 | ||
| MSI-high | N of cases (n=187) | 60 | 73 | 54 | ||
| Age-adjusted HR (95% CI) | 1 (reference) | 1.20 (0.85 – 1.69) | 0.97 (0.67 – 1.40) | 0.92 | ||
| Multivariable HR (95% CI)† | 1 (reference) | 1.26 (0.89 – 1.78) | 1.01 (0.70 – 1.47) | 0.86 | ||
| Negative | N of cases (n=100) | 35 | 36 | 29 | ||
| Age-adjusted HR (95% CI) | 1 (reference) | 1.00 (0.63 – 1.60) | 0.87 (0.53 – 1.42) | 0.65 | ||
| Multivariable HR (95% CI)† | 1 (reference) | 1.04 (0.65 – 1.67) | 0.90 (0.54 – 1.48) | 0.76 | ||
| Positive | N of cases (n=43) | 9 | 18 | 16 | ||
| Age-adjusted HR (95% CI) | 1 (reference) | 2.01 (0.90 – 4.49) | 1.95 (0.86 – 4.43) | 0.11 | ||
| Multivariable HR (95% CI)† | 1 (reference) | 2.10 (0.94 – 4.70) | 2.01 (0.88 – 4.56) | 0.09 | ||
|
| ||||||
| PTGS2 expression status | ||||||
| Negative | N of cases (n=432) | 148 | 154 | 130 | ||
| Age-adjusted HR (95% CI) | 1 (reference) | 1.00 (0.80 – 1.26) | 0.92 (0.73 – 1.17) | 0.53 | ||
| Multivariable HR (95% CI)† | 1 (reference) | 1.05 (0.84 – 1.33) | 0.97 (0.76 – 1.24) | 0.84 | ||
| Negative | N of cases (n=277) | 96 | 99 | 82 | ||
| Age-adjusted HR (95% CI) | 1 (reference) | 1.00 (0.76 – 1.33) | 0.90 (0.67 – 1.21) | 0.53 | ||
| Multivariable HR (95% CI)† | 1 (reference) | 1.05 (0.79 – 1.40) | 0.93 (0.69 – 1.26) | 0.72 | ||
| Positive | N of cases (n=43) | 11 | 16 | 16 | ||
| Age-adjusted HR (95% CI) | 1 (reference) | 1.42 (0.65 – 3.07) | 1.55 (0.71 – 3.35) | 0.29 | ||
| Multivariable HR (95% CI)† | 1 (reference) | 1.47 (0.68 – 3.21) | 1.59 (0.73 – 3.44) | 0.26 | ||
| Positive | N of cases (n=692) | 223 | 239 | 230 | ||
| Age-adjusted HR (95% CI) | 1 (reference) | 1.06 (0.89 – 1.28) | 1.10 (0.91 – 1.32) | 0.37 | ||
| Multivariable HR (95% CI)† | 1 (reference) | 1.12 (0.93 – 1.34) | 1.15 (0.95 – 1.39) | 0.18 | ||
| Negative | N of cases (n=444) | 145 | 155 | 144 | ||
| Age-adjusted HR (95% CI) | 1 (reference) | 1.07 (0.85 – 1.34) | 1.05 (0.83 – 1.32) | 0.73 | ||
| Multivariable HR (95% CI)† | 1 (reference) | 1.11 (0.88 – 1.40) | 1.08 (0.85 – 1.37) | 0.56 | ||
| Positive | N of cases (n=51) | 12 | 17 | 22 | ||
| Age-adjusted HR (95% CI) | 1 (reference) | 1.41 (0.67 – 2.97) | 2.15 (1.06 – 4.36) | 0.03 | ||
| Multivariable HR (95% CI)† | 1 (reference) | 1.47 (0.70 – 3.10) | 2.22 (1.09 – 4.51) | 0.02 | ||
CI, confidence interval; F nucleatum, Fusobacterium nucleatum; HR, hazard ratio; MSI, microsatellite instability; T1, tertile 1; T2, tertile 2; T3, tertile 3.
Cox proportional cause-specific hazards regression for competing risks data was used to compute HRs and 95% CIs.
All analyses were stratified by age (in month), year of questionnaire return and sex.
Linear trend test using the median value of each category.
Multivariable HR was adjusted for pack-years smoked (0 vs 1–19 vs 20–39 vs ≥ 40 pack-years), family history of colorectal cancer (yes vs no), endoscopy status (yes vs no), physical activity level [quintiles of mean metabolic equivalent task score (METS) - hours per week], total calorie intake (quintiles of kcal/day), total alcohol intake (0 vs 1–5 vs 6–15 vs > 15 g/day), current multivitamin use (yes vs no), and regular aspirin use (yes vs no).
Considering the protective role of prudent dietary pattern against the F nucleatum-positive colorectal tumor subtype,30 and the very weak negative correlation between EDIP scores and prudent dietary pattern scores (r = −0.04, P < 0.0001), we further tested whether the distinct association of EDIP scores with risk of colorectal cancer subclassified by tumor F nucleatum status differed according to prudent dietary patterns. We found that the differential association maintained in low prudent dietary pattern group, but not in high prudent dietary pattern group (Supplementary Table 3).
DISCUSSION
The current study suggests that diets that promote inflammation (measured by EDIP scores) might be associated with a higher risk of F nucleatum-positive colorectal tumors but not the risk of F nucleatum-negative colorectal tumors. The positive association of EDIP scores with risk of F nucleatum-positive tumors seemed much stronger for proximal colon cancer than for distal colorectal cancer. This is the first population-based study to assess a potential role of intestinal bacteria in mediating the increased colorectal cancer risk associated with diet-induced inflammation. A better understanding of the role of interactions between inflammatory diets and intestinal microbiota in colorectal carcinogenesis can help us design improved dietary prevention strategies against carcinoma.31,32
Inflammation is recognized as a necessary trigger for colorectal cancer, but inflammation alone may be not enough to promote tumorigenesis. Complex interactions among the gut microbiota, inflammation, environmental exposures and host genetics are needed for colorectal carcinogenesis.2 Dietary components and patterns play roles in regulating intestinal homeostasis by altering microbial composition and diversity. Inflammatory diets may contribute to the development of dysbiosis by decreasing the amount of beneficial microorganisms and promoting the growth of harmful bacteria.33
During progression of local intestinal inflammation triggered by inflammatory diets, the epithelial barriers separating the microbiota from immune cells in the lamina propria begin to break down, which facilitates translocation of intestinal microbiota and exposure of immunogenic microbial components to both epithelial cells and antigen-presenting cells.1,4 These immunogenic microbial components, such as bacterial membrane vesicles and enterotoxin, may cause mutations in DNA repair genes and / or tumor suppressor genes, which would likely result in expedited initiation of hyperplasia and polyps.1,2,6 Accumulating evidence indicates that intake of high fat and high sugar could create inflammatory environment in the gut characterized by an overgrowth of inflammatory bacteria and a decrease of beneficial bacteria, and subsequently aggravate tumorigenesis through activating TGFB1 / SMAD3 and NFKB signaling pathways; whereas anti-inflammatory diets could increase the abundance of beneficial bacteria and suppress tumorigenesis through activating chloride channels.34,35 The presence of F nucleatum may represent an immune-compromised intestinal environment.36 F nucleatum adheres to epithelial cells by binding its own adhesin FadA, a virulence factor identified in F nucleatum, to CDH1 (E-cadherin) on epithelial cells. FadA modulates CDH1 (E-cadherin) and activates CTNNB1 (beta-catenin) signaling, leading to increased expression of transcription factors, inflammatory genes, and oncogenes.37 F nucleatum has been reported to be associated with inflammatory microenvironment, which is conducive to colorectal neoplasia progression.9 Furthermore, F nucleatum could accelerate the progression of tumors by inhibiting T cell-mediated immune responses against colorectal tumors.15
The characteristics of the microbiome differ by regions of the gastrointestinal tract given the varying pH, transit time, nutrient availability, exposure to oxygen, host secretions, mucosal surface, and immune system throughout.1,2 Previous evidence has indicated that F nucleatum is often enriched in proximal colon tumors when compared with distal colon and rectal tumors.26 Compared to patients with left-sided colon tumors, patients with right-sided tumors had much higher rates of polymicrobial bacterial biofilms on tumor tissues and tumor-free mucosa far from the tumors. Bacterial biofilms have been correlated with enhanced IL6 and STAT3 activation in epithelial cells, and therefore increased proliferation of these cells.38 This may explain the anatomic difference in associations between inflammatory diets and colorectal cancer risk according to the amount of F nucleatum in tumor tissues.
Tumor MSI status and PTGS2 (cyclooxygenase-2) expression should be analyzed in the current study of inflammatory diets and risk of colorectal cancer according to the amount of tumor F nucleatum, provided that F nucleatum is enriched in MSI-high tumors26 and that the PTGS2 (cyclooxygenase 2) enzyme produces inflammatory mediators and is implicated in colorectal carcinogenesis.39 In the current study, we found that the differential association between EDIP scores and colorectal cancer risk according to the amount of tumor F nucleatum appeared to be generally consistent in tumors with different MSI or PTGS2 (cyclooxygenase 2) status, further supporting a distinct role of F nucleatum in mediating the association between inflammatory diets and colorectal cancer.
Our current study has limitations. First, despite the large sample size from the two cohorts, the number of cases with detectable tumor F nucleatum was relatively small. Second, EDIP assessments were based on self-reported food frequency questionnaires. Although measurement errors exist, validation studies have shown reasonable validity and reproducibility.24 Third, we could not obtain tumor tissues from every confirmed colorectal cancer case. However, the consistent results from the primary analyses and sensitivity analyses imply the selection bias caused by unavailability of tumor tissues was unlikely substantial. Fourth, more than 90% of participants in our study were non-Hispanic whites; hence, the generalizability of our findings to other population groups remains to be assessed.
There are several advantages of our study. First, the long-term prospective collection of data on dietary intake and other potential confounders enabled us to estimate cumulative averages of EDIP scores and all other quantitative factors with relatively small measurement errors within individuals. Second, our molecular pathological epidemiology database enabled us to estimate the amount of tumor F nucleatum in almost 1000 confirmed colorectal cases, which is rarely achieved in other epidemiological studies. Third, the molecular pathological epidemiology analysis method27 enabled us to assess the differential association of inflammatory diets with incidence of colorectal cancer subtypes classified by F nucleatum in tumor tissues. Hence, we can evaluate the combined role of diet and the microbiome in cancer occurrence.
In summary, our current study has shown that inflammatory diets are associated with a higher risk of F nucleatum-positive colorectal tumors, but not with risk of F nucleatum-negative tumors. This differential association between inflammatory diets and colorectal cancer risk according to the amount of tumor F nucleatum appeared to be stronger in proximal colon cancer than in distal colon and rectal cancer. Our finding suggests potential interactive roles of diet-related inflammation and the gut microbiota in colorectal tumorigenesis. Although further confirmation of our findings is needed, we would like to recommend an overall anti-inflammatory dietary pattern, including high intake of green leafy vegetables, dark-yellow vegetables, coffee, and tea, and low consumption of red meat, processed meat, refined grain, and sugary beverages, to reduce the risk of developing colorectal cancer. Notably, integrated analyses of environment, microbiome, tumor, and immunity are increasingly important.1,2,31,32,39 Further studies are also warranted to determine the potential utility of characterization of F nucleatum in colonic tumor or stool as a biomarker for personalized dietary interventions.
Supplementary Material
Acknowledgments
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Grant support: This work was supported by U.S. National Institutes of Health (NIH) grants [P01 CA87969 to M J Stampfer; UM1 CA186107 to M J Stampfer; P01 CA55075 to W C Willett; UM1 CA167552 to W C Willett; U01 CA167552 to W C Willett and L A Mucci; R01 CA118553 to C S Fuchs; R01 CA169141 to C S Fuchs; P50 CA127003 to C S Fuchs; R01 CA137178 to A T Chan; K24 DK098311 to A T Chan; R01 CA151993 to S Ogino; R35 CA197735 to S Ogino; K07 CA190673 to R Nishihara; K07 CA188126 to X Zhang; and K99 CA207736 to F K Tabung]; Nodal Award (to S Ogino) from the Dana-Farber Harvard Cancer Center; and by grants from The Project P Fund for Colorectal Cancer Research, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance, and American Association for Cancer Research [Stand Up to Cancer Colorectal Cancer Dream Team Translational Research Grant]. L Liu is supported by the grant from National Natural Science Foundation of China No. 81302491, a scholarship grant from Chinese Scholarship Council and a fellowship grant from Huazhong University of Science and Technology. T Hamada is supported by a fellowship grant from the Uehara Memorial Foundation and by a grant from the Mochida Memorial Foundation for Medical and Pharmaceutical Research. K Kosumi is supported by a grant from Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers from Japanese Society for the Promotion of Science. Y Shi is supported by the grants from National Natural Science Foundation of China No. 81402016, Beijing Natural Science Foundation No. 7152140 and Beijing Nova Program XXJH2015B098. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Dr. Fuchs currently serves as a consultant for Genentech/Roche, Lilly, Sanofi, Bayer, Celgene, Merck, Bristol-Myers Squibb, Entrinsic Health, Five Prime Therapeutics, and Agios. This study was not funded by any of these companies.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- EDIP
empirical dietary inflammatory pattern
- HPFS
Health Professionals Follow-up Study
- HR
hazard ratio
- IPW
inverse probability weighting
- MPE
molecular pathological epidemiology
- MSI
microsatellite instability
- NHS
Nurses’ Health Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Use of standardized official symbols: We use HUGO (Human Genome Organization)-approved official symbols (or root symbols) for genes and gene products, including CD3, CDH1, CRP, CTNNB1, IL6, NFKB, PTGS2, SLCO2A1, SMAD3, STAT3, TGFB1, TNF, and TNFRSF1B; all of which are described at www.genenames.org. The official symbols are italicized to differentiate from non-italicized colloquial names that are used along with the official symbols. This format enables readers to familiarize the official symbols for genes and gene products together with common colloquial names.
Disclosures: No other conflict of interest exists. The other authors declare that they have no conflicts of interest.
Guarantor of the article: Drs Liu, Ogino and Giovannucci are accepting full responsibility for the conduct of the study. They have full access to the data and have control of the decision to publish.
Author contributions: Study concept and design: all authors
Acquisition of data: all authors
Analysis and interpretation of data: all authors
Drafting of the manuscript: LL, TT, RN, SO, ELG
Critical revision of the manuscript for important intellectual content: all authors
Statistical analysis: LL, RN, FKT, DN, MW
Obtained funding: CSF, SO, ATC, RN, FKT, XZ
Administrative, technical, or material support: SO, ELG, RN, CSF, ATC, FKT, XZ, JAN, ZRQ, TH, DN, KW, SB, WSG, CH, KM, KK, ADK, MG
Study supervision: SO, ELG, RN, ATC, CSF
Final approval of the version to be published: all authors.
References
- 1.Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol. 2017;18:843–850. doi: 10.1038/ni.3754. [DOI] [PubMed] [Google Scholar]
- 2.O'Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13:691–706. doi: 10.1038/nrgastro.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabung FK, Liu L, Wang W, et al. Association of Dietary Inflammatory Potential With Colorectal Cancer Risk in Men and Women. JAMA Oncol. 2018 Jan 18; doi: 10.1001/jamaoncol.2017.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan CA, Garrett WS. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu Rev Microbiol. 2016;70:395–411. doi: 10.1146/annurev-micro-102215-095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inaba Y, Ashida T, Ito T, et al. Expression of the antimicrobial peptide alpha-defensin/cryptdins in intestinal crypts decreases at the initial phase of intestinal inflammation in a model of inflammatory bowel disease, IL-10-deficient mice. Inflamm Bowel Dis. 2010;16:1488–1495. doi: 10.1002/ibd.21253. [DOI] [PubMed] [Google Scholar]
- 6.Arthur JC, Perez-Chanona E, Muhlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye X, Wang R, Bhattacharya R, et al. Fusobacterium Nucleatum Subspecies Animalis Influences Proinflammatory Cytokine Expression and Monocyte Activation in Human Colorectal Tumors. Cancer Prev Res (Phila) 2017;10:398–409. doi: 10.1158/1940-6207.CAPR-16-0178. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Weng W, Peng J, et al. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-kappaB, and Up-regulating Expression of MicroRNA-21. Gastroenterology. 2017;152:851–866.e24. doi: 10.1053/j.gastro.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tahara T, Yamamoto E, Suzuki H, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 2014;74:1311–1318. doi: 10.1158/0008-5472.CAN-13-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakatsu G, Li X, Zhou H, et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015;6:8727. doi: 10.1038/ncomms9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nosho K, Sukawa Y, Adachi Y, et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol. 2016;22:557–566. doi: 10.3748/wjg.v22.i2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mima K, Sukawa Y, Nishihara R, et al. Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol. 2015;1:653–661. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mima K, Cao Y, Chan AT, et al. Fusobacterium nucleatum in Colorectal Carcinoma Tissue According to Tumor Location. Clin Transl Gastroenterol. 2016;7:e200. doi: 10.1038/ctg.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park HE, Kim JH, Cho NY, et al. Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma. Virchows Arch. 2017 Jun 8; doi: 10.1007/s00428-017-2171-6. [DOI] [PubMed] [Google Scholar]
- 18.Park CH, Han DS, Oh YH, et al. Role of Fusobacteria in the serrated pathway of colorectal carcinogenesis. Sci Rep. 2016;6:25271. doi: 10.1038/srep25271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell. 2017;170:548–563.e16. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geller LT, Barzily-Rokni M, Danino T, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–1105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847–854. doi: 10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabung FK, Smith-Warner SA, Chavarro JE, et al. Development and Validation of an Empirical Dietary Inflammatory Index. J Nutr. 2016;146:1560–1570. doi: 10.3945/jn.115.228718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao X, Lochhead P, Nishihara R, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–1606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mima K, Nishihara R, Qian ZR, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65:1973–1980. doi: 10.1136/gutjnl-2015-310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Spiegelman D, Kuchiba A, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35:782–800. doi: 10.1002/sim.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Nishihara R, Qian ZR, et al. Association Between Inflammatory Diet Pattern and Risk of Colorectal Carcinoma Subtypes Classified by Immune Responses to Tumor. Gastroenterology. 2017;153:1517–1530. doi: 10.1053/j.gastro.2017.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nogueira AV, Nokhbehsaim M, Eick S, et al. Biomechanical loading modulates proinflammatory and bone resorptive mediators in bacterial-stimulated PDL cells. Mediators Inflamm. 2014;2014:425421. doi: 10.1155/2014/425421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta RS, Nishihara R, Cao Y, et al. Association of Dietary Patterns With Risk of Colorectal Cancer Subtypes Classified by Fusobacterium nucleatum in Tumor Tissue. JAMA Oncol. 2017;3:921–927. doi: 10.1001/jamaoncol.2016.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajpoot M, Sharma AK, Sharma A, et al. Understanding the Microbiome: Emerging Biomarkers for Exploiting the Microbiota for Personalized Medicine against Cancer. Semin Cancer Biol. 2018 Feb 6; doi: 10.1016/j.semcancer.2018.02.003. pii: S1044-579X(17)30146-3. doi:10.1016/j.semcancer.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Rescigno T, Micolucci L, Tecce MF, et al. Bioactive Nutrients and Nutrigenomics in Age-Related Diseases. Molecules. 2017;22 doi: 10.3390/molecules22010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Keefe SJ, Li JV, Lahti L, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6:6342. doi: 10.1038/ncomms7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agus A, Denizot J, Thévenot J, et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci Rep. 2016;6:19032. doi: 10.1038/srep19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Kang C, Wang XL, et al. Dietary Factors Modulate Colonic Tumorigenesis Through the Interaction of Gut Microbiota and Host Chloride Channels. Mol Nutr Food Res. 2018 Jan 13; doi: 10.1002/mnfr.201700554. [DOI] [PubMed] [Google Scholar]
- 36.Flanagan L, Schmid J, Ebert M, et al. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur J Clin Microbiol Infect Dis. 2014;33:1381–1390. doi: 10.1007/s10096-014-2081-3. [DOI] [PubMed] [Google Scholar]
- 37.Rubinstein MR, Wang X, Liu W, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dejea CM, Wick EC, Hechenbleikner EM, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci U S A. 2014;111:18321–18326. doi: 10.1073/pnas.1406199111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogino S, Nowak JA, Hamada T, et al. Integrative analysis of exogenous, endogenous, tumour and immune factors for precision medicine. Gut. 2018 Feb 6; doi: 10.1136/gutjnl-2017-315537. pii: gutjnl-2017-315537. doi:10.1136/gutjnl-2017-315537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.