Abstract
Previous studies have shown that gut-microbiome is associated with nonalcoholic fatty liver disease (NAFLD). We aimed to examine if serum metabolites especially those derived from the gut-microbiome have a shared gene-effect with hepatic steatosis and fibrosis. This is a cross-sectional analysis of a prospective discovery cohort including 156 well-characterized Twins and Families with untargeted metabolome profiling assessment. Hepatic steatosis was assessed using magnetic-resonance-imaging proton-density-fat-fraction (MRI-PDFF) and fibrosis using MR-elastography (MRE). A twin additive genetics and unique environment effects (AE) model was used to estimate the shared gene-effect between metabolites and hepatic steatosis and fibrosis. The findings were validated in an independent prospective validation cohort of 156 participants with biopsy-proven NAFLD including shotgun metagenomics sequencing assessment in a subgroup of the cohort.
In the discovery cohort, 56 metabolites including 6 microbial metabolites had a significant shared gene-effect with both hepatic steatosis and fibrosis after adjustment for age, sex and ethnicity. In the validation cohort 6 metabolites were associated with advanced fibrosis. Among them, only one microbial metabolite, 3-(4-hydroxyphenyl)lactate, remained consistent and statistically significantly associated with liver fibrosis in the discovery and validation cohort (fold-change of higher-MRE versus lower-MRE: 1.78, p<0.001 and of advanced versus no advanced fibrosis: 1.26, p=0.037, respectively). The share genetic determination of 3-(4-hydroxyphenyl)lactate with hepatic steatosis was RG: 0.57, 95%CI: 0.27–0.80, p<0.001 and with fibrosis was RG: 0.54, 95%CI: 0.036–1, p=0.036. Pathway reconstruction linked 3-(4-hydroxyphenyl)lactate to several human gut-microbiome species. In the validation cohort, 3-(4-hydroxyphenyl)lactate was significantly correlated with the abundance of several gut-microbiome species, belonging only to Firmicutes, Bacteroidetes and Proteobacteria phyla, previously reported as associated with advanced fibrosis.
Conclusion: This proof of concept study provides evidence of a link between the gut-microbiome and 3-(4-hydroxyphenyl)lactate that shares gene-effect with hepatic steatosis and fibrosis.
Keywords: NASH, liver fibrosis, genetic factors, biomarker, gut microbiome
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is currently recognized as one of the most prevalent etiologies of chronic liver disease worldwide (1, 2). NAFLD is estimated to afflict approximately 80–100 million adults in the United States. NAFLD is broadly classified into nonalcoholic fatty liver (NAFL), the non-progressive form of NAFLD, and nonalcoholic steatohepatitis (NASH), the progressive form of NAFLD. Patients with NASH have a higher risk of progression to cirrhosis and hepatocellular carcinoma and are at an increased risk of liver-related morbidity and mortality (3).
Previous seminal studies have shown that NAFLD is a heritable trait (4, 5). In addition, we have demonstrated that hepatic steatosis is heritable and has a shared gene-effect with metabolic risk factors and liver fibrosis using a twin study design (6, 7). Although genome-wide association studies (GWAS) have identified genetic variants associated with NAFLD located on PNPLA3 (8) and TM6SF2 (9) genes, these common risk alleles do not account for all of the variance observed in NAFLD (10). Given the epidemic increase of NAFLD and its association with an increase of morbidity and mortality, there is a need to better characterize the heritability of NAFLD to understand its pathogenesis, identify potential therapeutic targets and improve the screening of the patients who are at a higher risk of progression to cirrhosis.
Metabolome profiling has provided new insights into the molecular mechanisms of diseases including NAFLD. Several studies have identified specific metabolomics signatures associated with different stage of the disease in NAFLD (11–15). Moreover, evidence of interaction among the gut, liver, and blood metabolites has emerged in the recent years, suggesting that the gut-liver axis is an important component in the development of NAFLD. Indeed, compositional changes in gut microbiota have been proposed to mechanistically contribute to the progression of NAFLD (16–21).
Several studies have shown a familial resemblance of the concentration of serum metabolites using twin and familial cohorts (22–26). Furthermore, when combined within a twin study design, the heritability and shared gene-effect between serum metabolites and features of NAFLD allows further insight into the pathogenesis of NAFLD. If the gene regulation of a serum metabolite and features of NAFLD (e.g hepatic steatosis and fibrosis) significantly overlap, then targeting specific nodal points in the metabolite pathway could have a major therapeutic impact in NAFLD. In addition, the metabolite would also be a useful biomarker for the screening of patients at risk of advanced stage of NAFLD.
The heritability of the serum metabolites associated with NAFLD has not been systematically assessed yet. Utilizing a well-characterized prospective cohort of community-dwelling Twins and Families, we aimed to investigate whether serum metabolites are heritable, whether serum metabolites have a shared gene-effect with hepatic steatosis and fibrosis, and if gut-microbiome derived metabolites mediate this shared gene-effect. We then validated the association between the gut-microbiome derived serum metabolites, and hepatic steatosis and advanced fibrosis in an independent validation cohort of prospectively recruited individuals with biopsy-proven NAFLD.
MATERIAL AND METHODS
Study participants and design of the discovery Twin and Family cohort
This was a cross sectional analysis of a prospective cohort study of patients from the Twin and Family Study (ClinicalTrials.gov: NCT01643512) residing in Southern California. This study included a total of 156 participants, 100 twins (50 twin pairs) including 37 pairs of monozygotic (MZ), 13 pairs of dizygotic (DZ) and 56 participants either siblings or parents-offspring recruited the University of California at San Diego (UCSD) NAFLD Research Center (6, 7, 27, 28) between December 2011 and January 2014. Please see Supplementary Material for detailed visit research, inclusion and exclusion criteria.
Study participants with NAFLD biopsy-proven validation cohort
The validation cohort included 156 participants prospectively recruited between October 2011 and May 2014 at the UCSD NAFLD Research Center (6, 7, 27, 28). All patients with suspected NAFLD with a clinical indication for liver biopsy underwent a careful evaluation for other causes of hepatic steatosis and liver disease. This study was Health Insurance Portability and Accountability Act (HIPAA) compliant and was approved by the UCSD Institutional Review Board. Informed written consent was obtained from each participant before enrolling in the study. Please see Supplementary Material for detailed inclusion and exclusion criteria.
Primary outcome
The primary outcome was the heritability and presence of shared gene-effect between serum metabolites and hepatic steatosis and fibrosis, and whether any of these metabolites were derived from the gut microbiome.
The secondary outcomes were the association between serum metabolites and hepatic steatosis assessed by MRI-PDFF and hepatic fibrosis assessed by MRE in the Twin and Family cohort, and between gut microbiome derived serum metabolites and advanced fibrosis as defined by histologic stage 3 and 4 fibrosis.
We also assessed if microbial metabolite were associated with liver fibrosis in both the discovery and validation cohort (fold-change of higher-MRE versus lower-MRE and of advanced versus no advanced fibrosis, respectively). Pathway reconstruction linking metabolites to human gut-microbiome species was performed. In the validation cohort, we then assessed whether gut microbiome derived metabolites were significantly correlated with the abundance of known gut-microbiome species including species previously reported to be associated with advanced fibrosis (19).
MRI assessment
MRI was performed at the UCSD MR3T Research Laboratory using the 3T research scanner (GE Signa EXCITE HDxt; GE Healthcare, Waukesha, WI). MRI-PDFF was used to measure hepatic steatosis and MRE was used to measure hepatic fibrosis. The details of the MRI protocol have been previously described in references (29, 30) and are detailed in Supplementary material.
Untargeted Metabolome profiling
Serum metabolite assessment was performed by Metabolon, Inc (Durham, NC, USA). Samples were extracted and split into equal parts for analysis on the GC/MS and LC/MS/MS platforms(31). Software was used to match ions to an in-house library of standards for metabolite identification and for metabolite quantitation by peak area integration(32). For further details regarding serum metabolites data acquisition, please see Supplementary Material.
Gut microbiome sequencing
The metagenomics analysis of the gut microbiome was performed using whole-genome shotgun sequencing of DNA extracted from stool samples in a subgroup of participant from the NAFLD biopsy-proven cohort (n= 57). The detailed protocol for the DNA extraction, library preparation and sequencing has been previously described in reference (19).
Statistical analysis
Data preparation
Among 1181 serum metabolites detected, 411 unnamed serum metabolites were excluded from the analysis. Metabolites for which there were more than 50% missing values, most definitely due to levels below the limits of detection (57 and 69 out of 770 identified metabolites in the discovery and validation cohort, respectively), were also excluded from the analysis. For the remaining serum metabolites with fewer than 50% missing values, values were imputed to half of the observed minimum value for each metabolite assuming that the metabolites was under the limit of detection. In the Twin and Family cohort, if both twins in a pair had a missing value for the same metabolites, the missing values were left blank to avoid bias heritability estimates and the twin pairs were excluded from the heritability analysis. All the data were log-transformed prior to statistical analysis.
Data analysis
Patients’ demographic, anthropometric, clinical, and biochemical characteristics were summarized. Categorical variables were shown as counts and percentages, and associations were tested using a chi-squared test or Fisher’s exact test. Normally distributed continuous variables were shown as mean (± standard deviation), and differences between groups were analyzed using a two-independent samples t test or Wilcoxon-Mann-Whitney test.
Statistical comparison of serum metabolites between NAFLD and non-NAFLD groups and between individuals with the highest MRE measurements >4.17 kPa (corresponding to the 95th percentile) and the individuals with the lowest measurements <1.67 kPa (corresponding to the 95th percentile) were assessed using Welch’s t-tests, and Pearson correlation between serum metabolites and hepatic steatosis assessed by MRI-PDFF and hepatic fibrosis assessed by MRE or liver biopsy were performed followed by pairwise partial correlation conditional on age, sex, Hispanic ethnicity and obesity. To further explore familial effects within the dataset, a sampling permutation median p-value was calculated for each metabolite by running 1,000 random selections of the dataset and calculating the p-value for all compounds, followed by calculating the median p-value for each metabolite from the 1,000 simulations. Statistical analyses were performed using ArrayStudio and the programs R. The False Discovery Rate using the Benjamini-Hochberg method was used to account for multiple testing.
The correlation between serum metabolites and the abundance of the gut microbiome bacterial species in a subgroup of unrelated participants from biopsy-proven NAFLD cohort was assessed using pairwise partial correlation coefficient and p-values based on Pearson method conditional on age, sex calculated using R package ppcor.
Heritability estimates and share gene-effect
AE models were used to estimate the shared genetic determination (rG) and shared environmental determination (rE) between twin pairs in the subgroup of Twins (n=100, 50 pairs) from the Twins and Family cohort. Detailed protocol have been described in previous studies (6, 7), please see supplementary material for detailed information. The analyses were performed using OpenMx, a structural equation modeling software package for genetically informative data (http://openmx.psyc.virginia.edu). Prior to the model fitting, the measures were adjusted for controlling age, gender, and ethnicity. Overall, AE models tended to provide the best fits to the data. Consequently, the genetic effects estimated in these AE models refer to broad-sense heritability, reflecting the proportion of phenotypic variance accounted for by the combined effect of all genetic influences (A +D).
Sample size estimation
Previous studies have reported that the mean heritability estimates of circulating metabolites was 0.53 ranging from 0.21 to 0.77 in a Netherlands Twin cohort(22) and the median heritability estimates of serum metabolites was 0.49 ranging from 0.23 to 0.76 in a UK Twins cohort(24). We have also previously estimated the heritability of hepatic steatosis to be approximately 0.5(7). Therefore we anticipated that the heritability of serum metabolite and hepatic steatosis with one another would be approximately of 0.50. It has been shown that, to detect an additive genetic component of 0.4–0.8 in an ACE model, approximately 36–74 twin pairs are needed to produce a power of 0.95 with an alpha value of 0.05(33). Therefore, the 50 twin pairs included in this study would be adequate to assess the heritability and shared gene-effect of serum metabolites and hepatic steatosis in this cohort.
RESULTS
Baseline characteristics of the discovery Twin and Family cohort
This study included a total of 156 participants, 100 twins (50 twin pairs) including 37 pairs of monozygotic (MZ), 13 pairs of dizygotic (DZ), and 56 participants either siblings or parents-offspring who underwent serum metabolites assessment, clinical evaluation and advanced MRI assessment. The mean (± standard deviation) age and body mass index (BMI) was 46.3 years (±19.8) and 26.6 kg/m2 (±6.0), respectively. The derivation of cohort is shown in Supplemental Figure S1. The prevalence of NAFLD as defined by MRI-PDFF≥5% was 23% (36/156) and the prevalence of hepatic fibrosis as defined by MRE ≥3 kPa was 18% (28/156). Detailed demographic, biochemical, and imaging data of subjects with NAFLD compared to subjects without NAFLD is provided in Table 1.
Table 1.
Baseline Characteristics between Non-NAFLD and NAFLD Individuals in the Twin and Family Cohort
| Characteristics | Overall (n=156) |
NAFLD MRI-PDFF ≥ 5% (n=36) |
Non-NAFLD MRI-PDFF < 5% (n=120) |
P |
|---|---|---|---|---|
| Relationship | 22 Twins; 14 Other relatives (parent, offspring, sibling) |
78 Twins; 42 Other relatives (parent, offspring, sibling) |
||
| Age years | 46.3 (19.8) | 55.2 (15.7) | 43.6 (20.2) | 0.001 |
| Female, n (%) | 114 (73.1) | 20 (55.6) | 94 (78.3) | 0.007 |
| Race | 0.477 | |||
| White, n (%) | 115 (73.7) | 25 (69.4) | 90 (75.0) | |
| Hispanic, n (%) | 26 (16.7) | 7 (19.4) | 19 (15.8) | |
| BMI (kg/m2) | 26.6 (6.0) | 31.8 (5.9) | 25.0 (5.2) | <0.0001 |
| SBP (mm Hg) | 123.7 (21.4) | 137.4 (22.6) | 119.5 (19.3) | <0.001 |
| DBP (mmHg) | 76.6 (12.3) | 81.0 (12.4) | 75.2 (12.0) | 0.13 |
| Waist circumference (cm) | 90.5 (13.4) | 102.4 (13.2) | 86.9 (11.2) | <0.0001 |
| Hip Circumference (cm) | 101.1 (11.9) | 108.8 (13.5) | 98.7 (10.34) | <0.0001 |
| Glucose (mg/dL) | 91.7 (16.7) | 102.5 (27.5) | 88.4 (9.6) | 0.005 |
| Insulin (U/L) | 11.8 (21.8) | 25.5 (42.7) | 7.67 (4.2) | 0.019 |
| HbA1c | 5.8 (0.5) | 6.1 (0.7) | 5.7 (0.4) | 0.001 |
| HOMA-IR | 2.8 (5.9) | 6.6 (11.4) | 1.7 (1.0) | 0.016 |
| AST (U/L) | 23.8 (9.8) | 28.4 (15.8) | 22.4 (6.6) | 0.035 |
| ALT (U/L | 22.7 (14.8) | 31.5 (19.6) | 20.1 (11.9) | 0.002 |
| Alkaline phosphatase (U/L) | 69.4 (22.8) | 72.4 (25.2) | 68.5 (22.1) | 0.372 |
| Total bilirubin (mg/dL) | 0.5 (0.2) | 0.43 (0.20) | 0.46 (0.23) | 0.423 |
| Direct bilirubin (mg/dL) | 0.1 (0.04) | 0.12 (0.04) | 0.12 (0.04) | 0.540 |
| Albumin (g/dL) | 4.5 (0.3) | 4.5 (0.3) | 4.6 (0.3) | 0.170 |
| GGT (U/L) | 25.2 (29.1) | 44.7 (53.0) | 19.3 (10.8) | 0.007 |
| Total cholesterol (mg/dL) | 189.4 (40.3) | 189.2 (39.8) | 189.5 (40.6) | 0.970 |
| HDL-cholesterol (mg/dL) | 63.1 (19.3) | 49.6 (11.5) | 67.2 (19.3) | <0.0001 |
| LDL-cholesterol (mg/dL) | 107.1 (35.0) | 111.1 (35.1) | 105.9 (35.1) | 0.441 |
| Triglycerides (mg/dL) | 98.3 (61.3) | 151.1 (70.3) | 82.2 (48.2) | <0.0001 |
| White blood cell count (× 103/uL) | 6.0 (1.6) | 6.8 (1.5) | 5.8 (1.6) | 0.002 |
| Hemoglobin (g/dL) | 13.8 (2.1) | 14.6 (3.8) | 13.6 (1.1) | 0.116 |
| Hematocrit (%) | 40.5 (3.4) | 41.2 (4.2) | 40.3 (3.1) | 0.232 |
| Platelet count (×103/uL) | 255.4 (51.2) | 249.8 (57.8) | 257.1 (49.1) | 0.454 |
| INR | 1.1 (0.3) | 1.1 (0.3) | 1.1 (0.2) | 0.924 |
| Ferritin (ng/mL) | 91.2 (89.3) | 121.1 (136.5) | 82.4 (67.8) | 0.113 |
| MRI-PDFF (%) | 4.7 (5.4) | 12.7 (6.3) | 2.3 (0.9) | <0.0001 |
| MRE (kPa) | 2.4 (0.8) | 3.2 (1.3) | 2.1 (0.4) | <0.0001 |
Mean values are provided with the standard deviation in parenthesis, unless otherwise noted as n (%). Differences between individuals with and without NAFLD were evaluated with t tests or the Wilcoxon-Mann-Whitney test for continuous variables and the chi squared or Fisher exact test for categorical variables.
Bold indicates significant P-values < 0.05.
Abbreviations: MRI-PDFF, magnetic resonance imaging-protein density fat fraction; MRE, magnetic resonance elastography; HOMA-IR, homeostatic model assessment-insulin resistance
Serum metabolites associated with hepatic steatosis and fibrosis in the Twin and Family cohort
Among the 713 metabolites analyzed, 153 belonging to 8 super pathways: amino-acid, peptide, energy, lipid, carbohydrate, nucleotide, cofactor and vitamins and xenobiotics, were significantly differentially expressed in individuals with NAFLD (MRI-PDFF≥5%) compared to individuals without NAFLD (MRI-PDFF<5%) and 86 belonging to 8 super pathways: amino-acid, peptide, energy, lipid, carbohydrate, nucleotide, cofactor and vitamins and xenobiotics were significantly differentially expressed in individuals with the higher MRE measurements (> 4.17 kPa, 95th percentile) compared to individual with lower MRE measurements (< 1.61 kPa, 5th percentile), after assessment of the familial effect.
Heritability of serum metabolites in the Twin and Family cohort
Among the 713 serum metabolites analyzed in the Twins, 440 serum metabolites were heritable with significant heritability estimates (h2) ranging from 0.28 to 0.91 after adjustment for age, sex and ethnicity Supplemental Figure S2.
Shared gene-effects between serum metabolites and hepatic steatosis and fibrosis
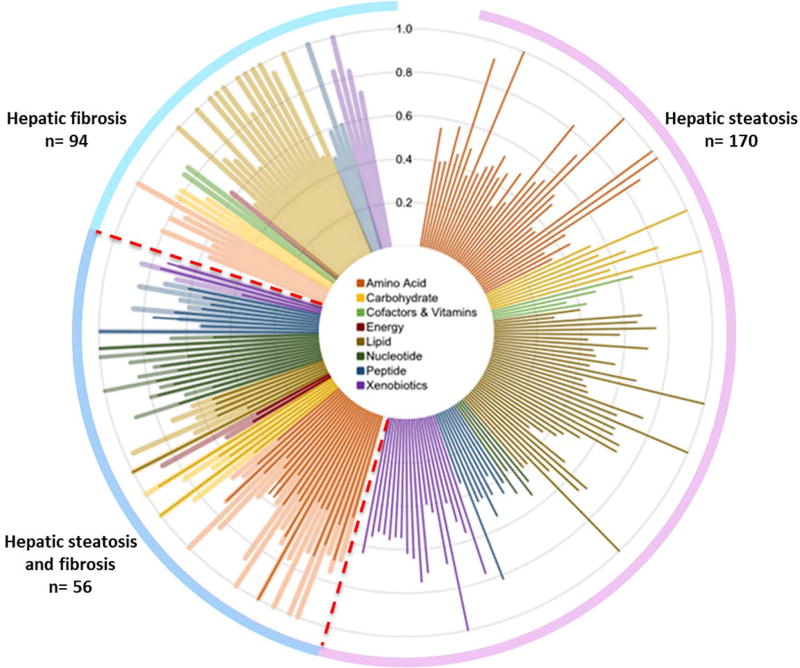
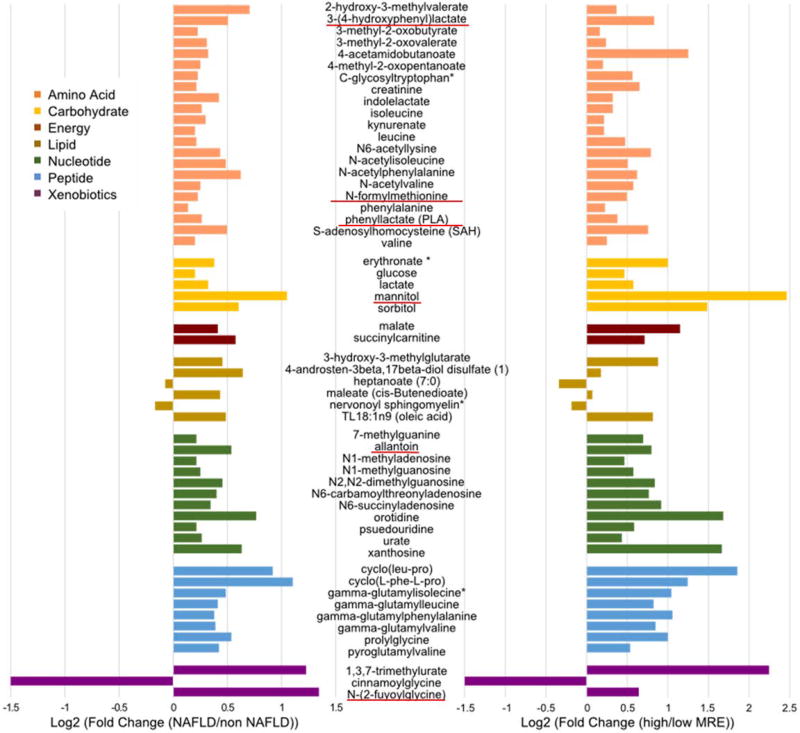
Among the 440 heritable serum metabolites identified, 170 serum metabolites had a significant shared gene-effect with hepatic steatosis, as measured by MRI-PDFF and 94 serum metabolites had a significant shared gene-effect with hepatic fibrosis as measured by MRE. Among them, 56 serum metabolites had a significant shared gene-effect with both hepatic steatosis and fibrosis Figure 1. The fold change of these 56 serum metabolites in individuals with NAFLD versus non-NAFLD and in individuals with higher MRE measurement versus lower MRE measurement are depicted in Figure 2. Interestingly, among these 56 serum metabolites, 6 metabolites had a microbial origin and derive potentially from the gut microbiome: 3-(4-hydroxyphenyl)lactate, N-formylmethionine, phenyllactate, mannitol, allantoine, N-(2-furoyl)glycine based upon the Human Metabolome Database http://www.hmdb.ca/.
Figure 1. Shared gene-effect between serum metabolites and hepatic steatosis and fibrosis.
Shared gene-effect of the serum metabolites is presented in a polar plot as genetic determination estimates (RG) adjusted for age, sex and ethnicity colored based on eight metabolomics superpathways (in the central inset).
Figure 2. 56 serum metabolites shared gene-effect with hepatic steatosis and fibrosis.
The variation of the 56 serum metabolites, colored based on eight superpathways, with shared gene-effect with hepatic steatosis and fibrosis is depicts as log2 fold change in participant with NAFLD versus non NAFLD as defined by MRI-PDFF≥5% (left panel) and in participant with higher measurement of MRE (>4.17 kPa) versus lower measurement of MRE (<1.67kPa) (right panel). The 6 serum metabolites derived from the gut microbiome are underlined in red based upon the Human Metabolome Database http://www.hmdb.ca/.
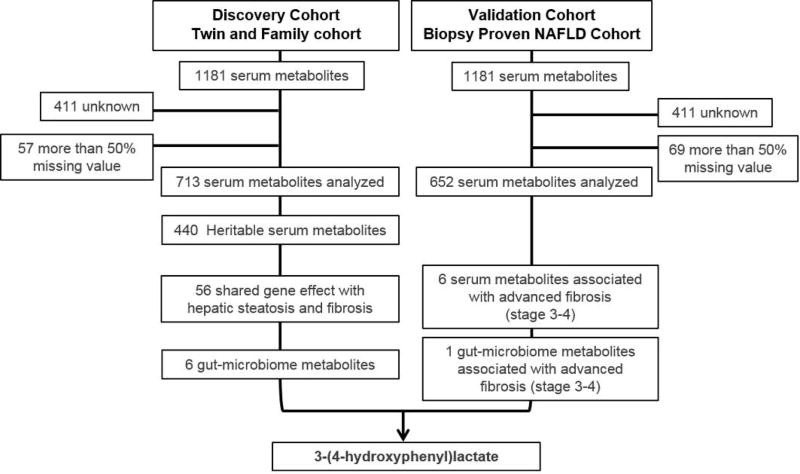
Validation in the biopsy-proven NAFLD cohort
To further assess the statistical and clinical relevance of the 56 serum metabolites identified in the Twins and Family cohort, the association of the serum metabolites with advanced fibrosis was then validated in an independent validation cohort of 156 unrelated participants prospectively recruited with biopsy-proven NAFLD. The mean (±standard deviation) age and BMI was 49.8 years (±14.3) and 32.0 kg/m2 (±6.0) and the prevalence of advanced fibrosis (stage 3–4) was 15% (23/156). Detailed demographic, biochemical, and histological data of subjects with advanced fibrosis compared to subjects without advanced fibrosis is provided in Table 2.
Table 2.
Baseline characteristics of NAFLD Biopsy-proven Cohort
| Characteristics | All (n=156) |
No Advanced Fibrosis (stage 0–2) (n=133) |
Advanced Fibrosis (stage 3–4) (n=23) |
p-value* |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 49.8 ±14.3 | 48.7 ± 14.1 | 56.2 ± 13.9 | 0.023 |
| Male, n (%) | 65 (41.7) | 59 (44.4) | 6 (26.1) | 0.114 |
| White, n (%) | 71 (45.5) | 65 (48.9) | 6 (26.1) | 0.058 |
| Hispanic or Latino, n (%) | 49 (32.7) | 36 (27.1) | 13 (56.5) | 0.007 |
| BMI, kg/m2 | 32.0 ± 6.0 | 31.3 ± 5.0 | 35.9 ± 9.5 | 0.015 |
| Clinical | ||||
| Type 2 Diabetes, n (%) | 41 (26.3) | 32 (24.1) | 9 (39.1) | 0.189 |
| Metformin use | 38 (24.4) | 14 (10.5) | 9 (39.1) | 0.077 |
| Biological data | ||||
| AST (U/L) | 42.2 ± 34.0 | 38.7 ± 31.6 | 62.3 ± 40.7 | 0.001 |
| ALT (U/L) | 59.0 ±57.0 | 56.8 ± 55.0 | 71.7 ± 67.1 | 0.574 |
| Alk P (U/L) | 77.5± 28.3 | 74.2 ± 24.6 | 96.6 ± 39.4 | 0.001 |
| GGT (Ui/L) | 55.5 ±51.0 | 50.6 ± 48.3 | 84.7 ± 57.5 | 0.001 |
| Total Bilirubin (mg/dL) | 1.7 ±15.3 | 0.49 ± 0.24 | 0.64 ± 0.66 | 0.293 |
| Direct Bilirubin (mg/dL) | 0.14± 0.15 | 0.14 ± 0.15 | 0.16 ± 0.15 | 0.539 |
| Albumin (g/dL) | 4.46± 0.44 | 4.49 ± 0.44 | 4.27 ± 0.38 | 0.019 |
| Glucose (mg/dl) | 106.2± 33.1 | 104.0 ± 27.0 | 118.7 ± 56.3 | 0.607 |
| Hemoglobin A1c | 6.0 ±0.9 | 5.97± 0.86 | 6.48 ± 1.11 | 0.048 |
| HOMA-IR | 8.5 ±17.3 | 8.79 ± 18.51 | 6.41 ± 4.96 | 0.229 |
| Insulin (U/ml) | 34.2± 74.9 | 35.0 ± 81.1 | 30.1 ± 20.9 | 0.561 |
| Triglycerides (mg/dL) | 159.0± 83.5 | 161.5 ± 82.8 | 144.7 ± 87.7 | 0.397 |
| Total cholesterol (mg/dL) | 182.8± 44.3 | 184.7 ± 44.9 | 171.8 ± 40.1 | 0.171 |
| HDL-cholesterol (mg/dL) | 48.7± 15.3 | 48.7 ± 15.5 | 48.3 ± 14.7 | 0.888 |
| LDL-cholesterol (mg/dL) | 105.3 ±34.6 | 106.8 ± 34.6 | 96.8 ± 33.7 | 0.212 |
| Platelet count (103/µL) | 244.5 ±782.8 | 255.2 ± 74.6 | 195.4 ± 59.7 | 0.0001 |
| Prothrombin time | 10.5 ± 0.8 | 11.2 ± 1.2 | 0.007 | |
| INR | 1.01± 0.08 | 1.00 ±0.08 | 1.07 ± 0.10 | 0.009 |
| Ferritin (ng/mL) | 190.4 ±171.9 | 200.4 ± 180.9 | 135.3 ± 95.8 | 0.201 |
| Clinical Prediction Rules | ||||
| AST/ALT | 0.81 ± 0.29 | 0.77 ± 0.25 | 1.05 ± 0.38 | <0.001 |
| APRI | 0.57 ± 0.55 | 0.54 ± 0.54 | 0.31 ± 0.16 | <0.001 |
| BARD Median (IQR) | 2 (2) | 2 (2) | 3 (1) | 0.002 |
| FIB-4 | 1.35 ± 1.24 | 1.38 ± 1.28 | 1.16 ± 0.77 | 0.001 |
| NAFLD Fibrosis Score | −1.85 ± 1.62 | −1.88 ± 1.60 | −1.55 ± 2.09 | <0.001 |
| Histology | 4.29 ± 1.55 | 4.83 ± 1.34 | 0.090 | |
| Fibrosis n (%) | <0.001 | |||
| 0 | 66 (42.3) | 66 (49.6) | 0 (0.0) | |
| 1 | 51(32.7) | 51 (38.3) | 0 (0.0) | |
| 2 | 16 (10.3) | 16 (12.0) | 0 (0.0) | |
| 3 | 13 (8.3) | 0 (0.0) | 13 (56.5) | |
| 4 | 10 (6.4) | 0 (0.0) | 10 (43.5) | |
| Steatosis n (%) | 0.176 | |||
| 0 | 3 (1.9) | 3 (2.2) | 0 (0) | |
| 1 | 51(32.7) | 39 (29.3) | 12 (52.2) | |
| 2 | 58 (37.2) | 52 (39.1) | 6 (26.1) | |
| 3 | 44 (28.2) | 39 (29.3) | 5 (21.7) | |
| Lobular inflammation n (%) | 0.372 | |||
| 0 | 3 (1.9) | 3 (2.2) | 0 (0.0) | |
| 1 | 76 (49.0) | 68 (51.5) | 8 (34.8) | |
| 2 | 70 (45.2) | 56 (42.1) | 14 (60.9) | |
| 3 | 6 (3.9) | 5 (3.8) | 1 (4.3) | |
| Ballooning n (%) | <0.001 | |||
| 0 | 38 (24.4) | 34 (25.6) | 4 (17.4) | |
| 1 | 87 (55.8) | 82 (61.6) | 5 (21.7) | |
| 2 | 30 (19.2) | 16 (12.0) | 14 (60.9) | |
| 3 | 1 (0.6) | 1 (0.8) | 0 (0.0) | |
| NASH, n (%) | 0.012 | |||
| NAFLD, no NASH | 20 (13.0) | 20 (15.0) | 0 (0.0) | |
| Borderline NASH | 18 (11.7) | 18 (13.5) | 0 (0.0) | |
| Definite NASH | 116 (75.3) | 93 (69.9) | 23 (100.0) | |
| NAS Median (IQR) | 4 (2) | 4 (2) | 5 (2) | 0.602 |
Mean values are provided ± standard deviation, unless otherwise noted as n (%) or median and interquartile range (IQR). APRI: AST to platelet ratio, HOMA: Homeostasis Model Assessment, NAS: NAFLD Activity Score. Differences between individuals with and without advanced fibrosis were evaluated with t tests or the Wilcoxon-Mann-Whitney test for continuous variables and the chi squared or Fisher exact test for categorical variables.
Bold indicates significant P-values <0.05.
3-(4-hydroxyphenyl)lactate gut microbiome-linked metabolites is associated with liver fibrosis
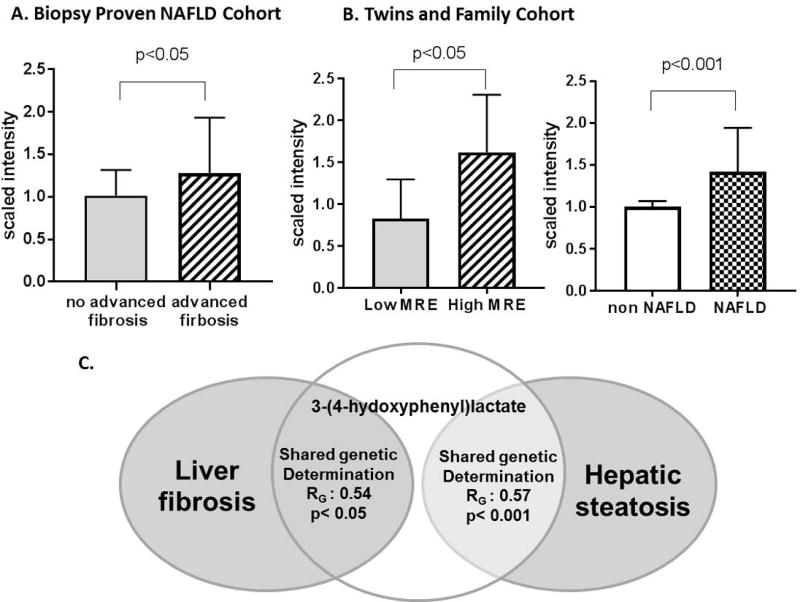
Only 6 out of the 56 serum metabolites identified in the Twin and Family cohort were confirmed and were significantly associated with advanced fibrosis in the validation cohort Supplemental Table S1. Among them, only one metabolite linked to the gut microbiome, 3-(4-hydroxyphenyl)lactate, was significantly associated with hepatic fibrosis in the validation and discovery cohort Figure 3. The level of 3-(4-hydroxyphenyl)lactate was significantly correlated with hepatic fibrosis assessed by MRE in the discovery cohort and by liver biopsy in the validation cohort: r=0.25, p<0.001, q=0.002 and r=0.25, p=0.002, q=0.018, respectively. Significant association between the level of 3-(4-hydroxyphenyl) lactate and gender and obesity was observed in both cohorts whereas no significant association was observed with Hispanic ethnicity Supplemental Table S2. The association between 3-(4-hydroxyphenyl) lactate and hepatic fibrosis remained significant after adjustment for age, sex, obesity and Hispanic ethnicity: r=0.188, p=0.027 and r=0.216, p=0.010 respectively. The mean (±SD) scaled intensity of 3-(4-hydroxyphenyl)lactate was significantly higher in individuals with the higher MRE measurement (> 4.17 kPa, 95th percentile) versus the lower MRE measurement (< 1.61 kPa, 95th percentile) (1.62 ± 0.26 versus 0.91 ±0.09, fold-change:1.78, p=0.0127) in the Twin and Family cohort and was also significantly higher in individuals with advanced fibrosis compared to individuals without advanced fibrosis in the biopsy-proven NAFLD cohort (1.27±0.13 versus 1.01±0.03, fold-change:1.26, p=0.037) Figure 4A and 4B. In addition, the mean (±SD) scaled intensity of 3-(4-hydroxyphenyl)lactate was also significantly higher in individuals with NAFLD compared to individuals without NAFLD in the Twins and Family cohort (1.42 ± 0.09 versus 1.00 ±0.03,fold-change: 1.42 p<0.001) Figure 4B. Finally, 3-(4-hydroxyphenyl)lactate was heritable, h2: 0.67, 95%CI: 0.434–0.814, p<0.001 and had a significant shared gene-effect with both hepatic steatosis (RG: 0.57, 95%CI: 0.27–0.80, p<0.001) and fibrosis (RG: 0.54, 95%CI: 0.036–1, p=0.036) after adjustment for age, sex and ethnicity Figure 4C.
Figure 3. 3-(4-hydroxyphenyl)lactate gut microbiome-linked metabolites is associated with liver fibrosis.
Only one serum metabolites linked to the gut microbiome was significantly associated with liver fibrosis in both the discovery and validation cohort.
Figure 4. 3-(4-hydroxyphenyl)lactate is associated with liver steatosis and fibrosis.
Median scaled intensity and 95% confidence interval of 3-(4-hydroxyphenyl)lactate in A. Biopsy-proven NAFLD cohort in individuals with versus without advanced fibrosis. B. In the Twins and Family cohort in individuals with higher (>4.61 kPa) versus lower MRE measurement (<1.61 kPa) and in individuals with NAFLD (MRI-PDFF≥5%) versus non-NAFLD (MRI-PDFF<5%). Scaled intensity was obtained by re-scaling raw data to have median equal to 1. C. 3-(4-hydroxyphenyl)lactate had significant shared gene-effect with both hepatic steatosis and fibrosis in AE twins model.
3-(4-hydroxyphenyl)lactate level is not associated with metformin use
As metformin is known to be associated with intestinal lactate generation (34), we have further assessed the association between 3-(4-hydroxyphenyl)lactate and metformin use. There were 38 (24.4%) participants under metformin in the biopsy-proven NAFLD cohort and only 5 (3.2%) participants under metformin in the Twins and Family cohort. The level of 3-(4-hydroxyphenyl)lactate was not significantly different in participant under metformin compared to the participant without metformin: mean scaled intensity (±SD) 1.04 (0.30) versus 1.05 (0.41), p=0.823, respectively. In addition, the association between the level of 3-(4-hydroxyphenyl)lactate and hepatic fibrosis remained significant when participants under metformin were excluded from the analysis in the validation and discovery cohort (r=0.174, p=0.045 and r=0.215, p=0.027, respectively).
3-(4-hydroxyphenyl)lactacte is derived from the human gut microbiome
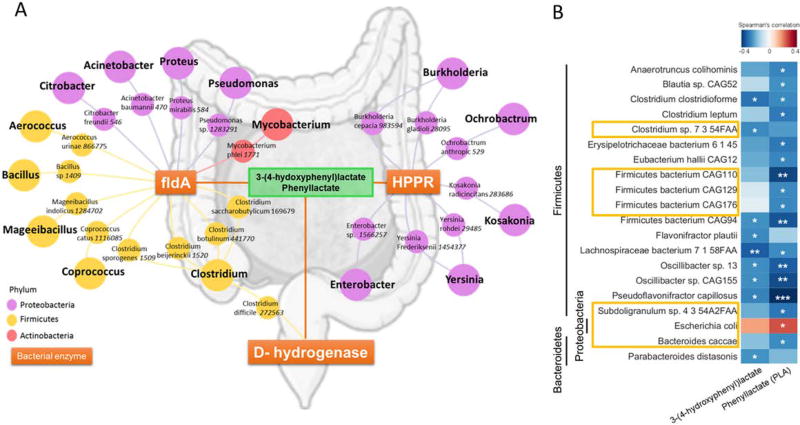
The pathway reconstruction of 3-(4-hydroxyphenyl)lactacte using KEGG PATHWAY Database have identified 3 main bacterial enzymes catalyzing both 3-(4-hydroxyphenyl)lactacte and phenyllactate: hydroxyphenylpyruvate reductase (HPPR), D-hydrogenase and cinnamoyl-CoA: phenyllactate CoA-transferase (FldA) Supplemental Figure S4. In addition, the network reconstruction performed by crossing the bacterial species encoding for the these 3 bacterial enzymes and the bacterial species belonging to the human gut microbiome identified 21 human gut microbiome bacterial species encoding for these 3 main enzymes, mainly Proteobacteria, Firmicutes. Figure 5A. Finally, the gut microbiome was analyzed in a subgroup of individuals from the biopsy-proven NAFLD cohort using shotgun metagenomics sequencing. In these individuals, 3-(4-hydroxyphenyl)lactacte and phenyllactate were significantly correlated with the abundance of several bacterial species belonging only to Firmicutes, Bacteroidetes and Proteobacteria phyla, after adjustment for age and sex. Interestingly, among the bacterial species significantly correlated with phenyllactate and 3-(4-hydroxyphenyl)lactacte, 7 have been demonstrated to be associated with advanced fibrosis in NAFLD in a previously published study (19): Bacteroides caccae, Clostridium sp, Escherichia Coli, Firmicutes bacterium CAG110, Firmicutes bacterium CAG129, Firmicutes bacterium CAG176, Subdoligranulum sp Figure 5B.
Figure 5. 3-(4-hydroxyphenyl)lactate derived from human gut microbiome.
A. Association network of bacterial species from human gut microbiome (identified using the Human microbiome project database (http://www.hmpdacc.org/resources/data_browser.php.) that encodes for the three bacterial enzymes (oranges boxes) catalyzing 3-(4-hydroxyphenyl)lactate and phenyllactate depicted as circles colored by phylum: Proteobacteria in purple, Firmicutes in yellow and Actinobacteria in pink and numbers in italic next to each species name represent unique Taxonomy Database identifiers. Abbreviations: HPPR: hydroxyphenylpyruvate reductase, FldA: cinnamoyl-CoA:phenyllactate CoA-transferase. B. Spearman correlation between 3-(4-hydroxyphenyl)lactate and phenyllactate and the abundance of bacterial species in the human microbiome, only significant correlation are represented, *p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001 after adjustment for age and sex. Framed bacterial species have been previously reported to be significantly associated with the presence of advanced fibrosis (19).
DISCUSSION
Main findings
Utilizing a uniquely, well-characterized, discovery cohort of community-dwelling twins and families, and a validation cohort of patients with biopsy-proven NAFLD, we report the association between a novel gut microbiome-derived serum metabolite, 3-(4-hydroxyphenyl) lactacte that has a statistically and clinically significant shared gene-effect with both hepatic steatosis and fibrosis. These results suggest a common genetic basis underlying the susceptibility towards NAFLD related fibrosis and 3-(4-hydroxyphenyl)lactacte pathway and their joint association with the gut microbiome. The shared gene-effect between NAFLD and this gut microbiome-derived metabolite provide new insights into the emerging linkage between gut microbiome and NAFLD related fibrosis. It is plausible that targeting 3-(4-hydroxyphenyl)lactacte may influence shared genetic or epigenetic pathways associated with the development of NAFLD. However, further larger studies are needed to assess if there is a causal role of these bacterial derived metabolite as modifiers of disease severity rather than sole contributors of disease severity.
In context of published literature
Studies have shown that both NAFLD (4, 5, 35) and serum metabolites are heritable traits (22–26). In addition we have demonstrated significant shared gene-effects between hepatic steatosis and metabolic risk factors (6, 7). We build on the results of these studies to show additional heritability and shared gene-effect between hepatic steatosis and fibrosis and serum metabolites.
In this study, we report similar heritability estimates of serum metabolites as previous studies using twins and family model (22–26). In addition, we have identified a novel amino-acid belonging to tyrosine metabolism, 3-(4-hydroxyphenyl)lactacte, significantly associated with both hepatic steatosis and fibrosis. This result is in line with previous studies showing differences in amino-acid pathway associated with features of NAFLD and underlie the potential role of amino-acids in the development and progression of NAFLD (12–15).
We report a significant correlation between the amino-acid 3-(4-hydroxyphenyl)lactacte and the abundance of several gut microbiome species. Likewise, a recent study by Liu et al. has shown a link between intestinal microbiota alterations and circulating amino acids in obesity (36).
In recent years, the gut-liver axis has emerged as an important component in the development of NAFLD. Compositional changes in gut microbiota have been proposed to mechanistically contribute to the progression of NAFLD (16–20). A higher proportion of Gram-negative bacterial species in the gut microbiome has been reported in NAFLD (17) and significant changes in the gut microbiome have been linked to NAFLD, NASH and NASH-related liver fibrosis (37, 38). We report significant correlation between the abundance of several bacterial species in the gut microbiome and the circulating level of 3-(4-hydroxyphenyl)lactacte, including bacterial species associated with advanced fibrosis in a previous study (19). Interestingly, the abundance of E. Coli was positively correlated with the circulating level of 3-(4-hydroxyphenyl)lactacte and phenyllactate in this study. In line with this observation, E. Coli has been reported to produce hydroxyphenyllactate and phenyllactate in vitro (39). In addition, we have previously demonstrated that E. Coli was significantly increased in advanced fibrosis (19). These data suggest an association between gut-microbiome alteration, the circulating level of 3-(4-hydroxyphenyl)lactacte and hepatic fibrosis in NAFLD. Likewise, intestinal dysbiosis has been shown to be associated with changes in serum metabolites potentially involved in NAFLD pathogenesis. Reduced bacterial diversity in NAFLD patients has been associated with disrupted bile acids profiles in serum and feces potentially due to the loss of gut microbiota members involved in the generation of secondary bile acids. (40) In addition, certain species of the gut microbiota can metabolize choline to trimethylamine which is associated with the presence and severity of NAFLD (41). Further studies are needed to determine whether 3-(4-hydroxyphenyl)lactacte plays a causal role in the development of hepatic steatosis and fibrosis. Limited data are available regarding the potential molecular function of 3-(4-hydroxyphenyl)lactacte. Although, a study by Beloborodova and colleagues have suggested that hydroxyphenyllactate may decrease the production of reactive oxygen species in mitochondria and neutrophils in rodents in vitro (39), these data need to be confirmed in vivo and in humans. Finally, further studies are needed to determine the mechanism involved in the cross-talk with gut-microbiome derived metabolites to induce liver injury and to affect disease progression in NAFLD Figure 6.
Figure 6. Gut-microbiome derived metabolites and development of NAFLD: a potential cross talk.
Our results suggest a link between genetics and the gut microbiome-derived metabolite, 3-(4-hydroxyphenyl)lactacte, in the heritability of NAFLD. Previous studies have demonstrated that the gut microbiome is shared among families (42, 43). Moreover, Goodrich et al. have recently demonstrated that features of the gut microbiome are inherited and determined via the host genotype using a genome-wide association analysis in a twin model (43). Furthermore, metabolomics profiling coupled with GWAS has identified several single nucleotide polymorphisms (SNPs) at various loci associated with human blood metabolites accounting for a significant variance of the metabolites traits (23–25, 44). However these studies did not assess the association between the gut microbiome and serum metabolite. It is possible that inherited features of the gut microbiome accounts for the heritability of the gut microbiome derived metabolites. However, further studies are needed to determine the genetic association between gut microbiome alteration, serum metabolites and NAFLD.
Strengths and limitations
There are several notable strengths in this study including the use of two independent and complimentary well-characterized prospective cohorts in which conditions such as excessive alcohol use, steatogenic therapies, viral hepatitis and secondary causes of steatosis were systematically excluded. The Twin and Family cohort allows for the assessment of the heritability estimates and shared gene-effects using accurate and reproducible non-invasive imaging biomarker for the quantification of hepatic steatosis and fibrosis. The biopsy-proven NAFLD cohort allows to validate and confirm the relevance of the findings using the liver biopsy which remains the gold standard for the diagnosis of liver fibrosis.
However, we acknowledge the following limitations, this cross sectional study design only allowed capturing a snapshot of metabolomics across the different features of NAFLD. Therefore it is not possible to determine whether 3-(4-hydroxyphenyl)lactacte is a causal factor in the pathogenesis of NAFLD. In addition, human cells only produce L-form of lactate whereas bacterial species have the enzymatic capacity to produce both isoform (45) and it was not possible to distinguish the 2 stereoisomeric of 3-(4-hydroxyphenyl)lactacte using the standard mass spectroscopy in this study. Finally, although the association between 3-(4-hydroxyphenyl)lactacte and hepatic fibrosis was significant after adjustment for age, sex, obesity and Hispanic ethnicity, potential other confounding factor such as diet could not be excluded.
Further longitudinal studies are needed to determine the role of 3-(4-hydroxyphenyl)lactacte in the development of NAFLD and whether 3-(4-hydroxyphenyl)lactacte is a reliable biomarker associated with longitudinal changes in hepatic steatosis and fibrosis.
Implication for future study
In this proof of concept study, we report the discovery of a novel gut microbiome-linked serum metabolite, 3-(4-hydroxyphenyl)lactacte that has a statistically and clinically significant shared gene-effect with both hepatic steatosis and fibrosis using two independent cohorts. Further studies with larger sample size are needed to determine the precise genetics mechanism underlying the relationship between the gut microbiome, 3-(4-hydroxyphenyl)lactacte and NAFLD. Future studies from independent cohorts are needed to validate these findings and assess if 3-(4-hydroxyphenyl)lactacte could be a useful non-invasive biomarker of gut microbiome involvement in NAFLD related fibrosis and whether it could be used for monitoring the targeted therapeutic response when modulating the microbiome to affect hepatic fibrosis in NAFLD.
Supplementary Material
Acknowledgments
FUNDINGS
RL is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, OM, the Association of Specialty Professors, and the American Gastroenterological Association and grant K23-DK090303. CS and RL serve as co-PIs on the grant R01-DK106419. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
CC is supported by grants from the Société Francophone du Diabète (SFD), the Philippe Foundation and Monahan Foundation under the Fulbright program.
Footnotes
Guarantor(s) of the article: Rohit Loomba
Conflict of interests: Dr. Sirlin consults, advises, and is on the speakers’ bureau for Bayer. He received grants from GE Healthcare. All other authors report no other conflict of interests.
AUTHOR CONTRIBUTIONS
Cyrielle Caussy: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, approved final submission.
Cynthia Hsu: statistical analysis, critical revision of the manuscript, approved final submission
Min-Tzu Lo: statistical analysis, critical revision of the manuscript, approved final submission.
Amy Liu: data collection, statistical analysis, critical revision of the manuscript, approved final submission.
Ricki Bettencourt: statistical analysis, critical revision of the manuscript, approved final submission
Veeral Ajmera: analysis and interpretation of data, critical revision of the manuscript, approved final submission.
Shirin Bassirian: data collection, critical revision of the manuscript, approved final submission.
Jonathan Hooker: data collection, imaging analysis, critical revision of the manuscript, approved final submission
Ethan Sy: data collection, imaging analysis, critical revision of the manuscript, approved final submission
Lisa Richards: patient visits, critical revision of the manuscript, approved final submission
Nicholas Schork: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
Bernd Schnabl: analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
David A. Brenner: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
Claude B. Sirlin: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
Chi-Hua Chen: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, approved final submission.
Rohit Loomba: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 3.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Struben VM, Hespenheide EE, Caldwell SH. Nonalcoholic steatohepatitis and cryptogenic cirrhosis within kindreds. Am J Med. 2000;108:9–13. doi: 10.1016/s0002-9343(99)00315-0. [DOI] [PubMed] [Google Scholar]
- 5.Schwimmer JB, Celedon MA, Lavine JE, Salem R, Campbell N, Schork NJ, Shiehmorteza M, et al. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009;136:1585–1592. doi: 10.1053/j.gastro.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui J, Chen CH, Lo MT, Schork N, Bettencourt R, Gonzalez MP, Bhatt A, et al. Shared genetic effects between hepatic steatosis and fibrosis: A prospective twin study. Hepatology. 2016;64:1547–1558. doi: 10.1002/hep.28674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loomba R, Schork N, Chen CH, Bettencourt R, Bhatt A, Ang B, Nguyen P, et al. Heritability of Hepatic Fibrosis and Steatosis Based on a Prospective Twin Study. Gastroenterology. 2015;149:1784–1793. doi: 10.1053/j.gastro.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 9.Liu YL, Reeves HL, Burt AD, Tiniakos D, McPherson S, Leathart JB, Allison ME, et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, Gudnason V, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso C, Fernandez-Ramos D, Varela-Rey M, Martinez-Arranz I, Navasa N, Van Liempd SM, Lavin Trueba JL, et al. Metabolomic Identification of Subtypes of Nonalcoholic Steatohepatitis. Gastroenterology. 2017;152:1449–1461 e1447. doi: 10.1053/j.gastro.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin R, Banton S, Tran VT, Konomi JV, Li S, Jones DP, Vos MB. Amino Acid Metabolism is Altered in Adolescents with Nonalcoholic Fatty Liver Disease-An Untargeted, High Resolution Metabolomics Study. J Pediatr. 2016;172:14–19 e15. doi: 10.1016/j.jpeds.2016.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mardinoglu A, Agren R, Kampf C, Asplund A, Uhlen M, Nielsen J. Genome-scale metabolic modelling of hepatocytes reveals serine deficiency in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:3083. doi: 10.1038/ncomms4083. [DOI] [PubMed] [Google Scholar]
- 14.Kaikkonen JE, Wurtz P, Suomela E, Lehtovirta M, Kangas AJ, Jula A, Mikkila V, et al. Metabolic profiling of fatty liver in young and middle-aged adults: Cross-sectional and prospective analyses of the Young Finns Study. Hepatology. 2017;65:491–500. doi: 10.1002/hep.28899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sookoian S, Puri P, Castano GO, Scian R, Mirshahi F, Sanyal AJ, Pirola CJ. Nonalcoholic steatohepatitis is associated with a state of betaine-insufficiency. Liver Int. 2017;37:611–619. doi: 10.1111/liv.13249. [DOI] [PubMed] [Google Scholar]
- 16.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 18.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 19.Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, et al. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017;25:1054–1062 e1055. doi: 10.1016/j.cmet.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Chierico F, Nobili V, Vernocchi P, Russo A, Stefanis C, Gnani D, Furlanello C, et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology. 2017;65:451–464. doi: 10.1002/hep.28572. [DOI] [PubMed] [Google Scholar]
- 22.Draisma HH, Beekman M, Pool R, van Ommen GJ, Adamski J, Prehn C, Vaarhorst AA, et al. Familial resemblance for serum metabolite concentrations. Twin Res Hum Genet. 2013;16:948–961. doi: 10.1017/thg.2013.59. [DOI] [PubMed] [Google Scholar]
- 23.Kettunen J, Tukiainen T, Sarin AP, Ortega-Alonso A, Tikkanen E, Lyytikainen LP, Kangas AJ, et al. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet. 2012;44:269–276. doi: 10.1038/ng.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long T, Hicks M, Yu HC, Biggs WH, Kirkness EF, Menni C, Zierer J, et al. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat Genet. 2017;49:568–578. doi: 10.1038/ng.3809. [DOI] [PubMed] [Google Scholar]
- 25.Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, Arnold M, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. 2014;46:543–550. doi: 10.1038/ng.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholson G, Rantalainen M, Maher AD, Li JV, Malmodin D, Ahmadi KR, Faber JH, et al. Human metabolic profiles are stably controlled by genetic and environmental variation. Mol Syst Biol. 2011;7:525. doi: 10.1038/msb.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarrinpar A, Gupta S, Maurya MR, Subramaniam S, Loomba R. Serum microRNAs explain discordance of non-alcoholic fatty liver disease in monozygotic and dizygotic twins: a prospective study. Gut. 2016;65:1546–1554. doi: 10.1136/gutjnl-2015-309456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caussy C, Soni M, Cui J, Bettencourt R, Schork N, Chen CH, Ikhwan MA, et al. Nonalcoholic fatty liver disease with cirrhosis increases familial risk for advanced fibrosis. J Clin Invest. 2017;127:2697–2704. doi: 10.1172/JCI93465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, Loomba R. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22–29. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel NS, Peterson MR, Brenner DA, Heba E, Sirlin C, Loomba R. Association between novel MRI-estimated pancreatic fat and liver histology-determined steatosis and fibrosis in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;37:630–639. doi: 10.1111/apt.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009;81:6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 32.Dehaven CD, Evans AM, Dai H, Lawton KA. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform. 2010;2:9. doi: 10.1186/1758-2946-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Visscher PM. Power of the classical twin design revisited. Twin Res. 2004;7:505–512. doi: 10.1375/1369052042335250. [DOI] [PubMed] [Google Scholar]
- 34.Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Manneras-Holm L, Stahlman M, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 35.Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E, Bass NM, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943–951. doi: 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, Shi J, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23:859–868. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- 37.Betrapally NS, Gillevet PM, Bajaj JS. Changes in the Intestinal Microbiome and Alcoholic and Nonalcoholic Liver Diseases: Causes or Effects? Gastroenterology. 2016;150:1745–1755 e1743. doi: 10.1053/j.gastro.2016.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beloborodova N, Bairamov I, Olenin A, Shubina V, Teplova V, Fedotcheva N. Effect of phenolic acids of microbial origin on production of reactive oxygen species in mitochondria and neutrophils. J Biomed Sci. 2012;19:89. doi: 10.1186/1423-0127-19-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949–955. doi: 10.1016/j.jhep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen YM, Liu Y, Zhou RF, Chen XL, Wang C, Tan XY, Wang LJ, et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep. 2016;6:19076. doi: 10.1038/srep19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodrich JK, Davenport ER, Beaumont M, Jackson MA, Knight R, Ober C, Spector TD, et al. Genetic Determinants of the Gut Microbiome in UK Twins. Cell Host Microbe. 2016;19:731–743. doi: 10.1016/j.chom.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicholson G, Rantalainen M, Li JV, Maher AD, Malmodin D, Ahmadi KR, Faber JH, et al. A genome-wide metabolic QTL analysis in Europeans implicates two loci shaped by recent positive selection. PLoS Genet. 2011;7:e1002270. doi: 10.1371/journal.pgen.1002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70:5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.