Abstract
The resistance among microbes has brought an urgent need for new drugs. Thus, we synthesized a series of Schiff bases derived from the sulfa drug sulfadiazine and various salicylaldehydes. The resulting 4-[(2-hydroxybenzylidene)amino]-N-(pyrimidin-2-yl)benzene-sulfonamides were characterized and evaluated against Gram-positive and Gram-negative bacteria, yeasts, moulds, Mycobacterium tuberculosis, nontuberculous mycobacteria (M. kansasii, M. avium) and their cytotoxicity was determined. Among bacteria, the genus Staphylococcus, including methicillin-resistant S. aureus, showed the highest susceptibility, with minimum inhibitory concentration values from 7.81 µM. The growth of Candida sp. and Trichophyton interdigitale was inhibited at concentrations starting from 1.95 µM. 4-[(2,5-Dihydroxybenzylidene)amino]-N-(pyrimidin-2-yl)-benzenesulfonamide was identified as the most selective Schiff base for these strains with no apparent cytotoxicity and a selectivity index higher than 16. With respect to M. tuberculosis and M. kansasii that were inhibited within the range of 8 to 250 µM, unsubstituted 4-[(2-hydroxy-benzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide meets the selectivity requirement. In general, dihalogenation of the salicylic moiety improved the antibacterial and antifungal activity but also increased the cytotoxicity, especially with an increasing atomic mass. Some derivatives offer more advantageous properties than the parent sulfadiazine, thus constituting promising hits for further antimicrobial drug development.
Keywords: antibacterial activity, antifungal activity, antimycobacterial activity, cytotoxicity, Schiff bases, sulfadiazine, sulfonamides
1. Introduction
Schiff bases are condensation products of primary (aromatic) amines with aldehydes or ketones carrying the azomethine (imino) moiety (-CR=N-). They are considered versatile pharmacophores for various pharmacological activities where the azomethine group has been demonstrated to be critical to the bioactivity. For example, Schiff bases, whether of natural or non-natural origin, have exhibited promising antibacterial, antitubercular, antifungal, antiparasitic, antiviral, antioxidant, anticancer, analgesic, anti-inflammatory properties etc. [1,2,3]. Altogether, they represent very frequently used and useful scaffold in medicinal chemistry.
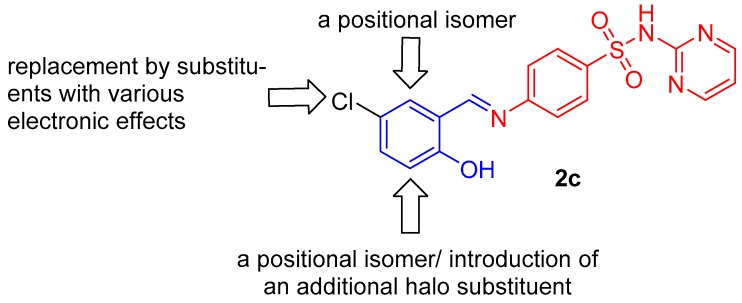
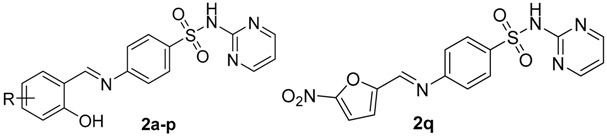
Schiff bases of substituted salicylaldehydes (2-hydroxybenzaldehydes) are well-known antimicrobial agents in “free” form or as ligands in metallic complexes [4,5,6,7]. Similar biological action have been reported for Schiff bases of various sulfonamides [4,8,9]. In addition, cotrimoxazole, sulfamethoxazole and sulfadiazine (1, SDZ, Figure 1) have exhibited activity against Mycobacterium tuberculosis [10] as well as nontuberculous (atypical) mycobacteria (NTM) [11] at clinically achievable concentrations after administration per os. To the best of our knowledge, there is no report about an identical property of sulfathiazole, used now only topically, or other sulfonamides. El-Baradie reported [12] an antibacterial activity of a SDZ-based Schiff base with unsubstituted salicylaldehyde. It exhibited minimum inhibitory concentrations (MICs) of 100–250 µg/mL for both Gram-positive and Gram-negative bacteria. The Schiff base derived from 5-bromosalicylaldehyde exhibited a broad spectrum of antibacterial and antifungal properties [13]. Isosteric 4-(5-chloro-2-hydroxybenzylideneamino)-N-(pyrimidin-2-yl)benzenesulfonamide (2c, Figure 2) showed activity against Staphylococcus aureus, including a methicillin-resistant strain (MRSA), M. tuberculosis and Mycobacterium avium (MIC values within the range of 125–250 µM) whereas it caused a significantly higher inhibition of Mycobacterium kansasii strains (8–32 µM) [4].
Figure 1.
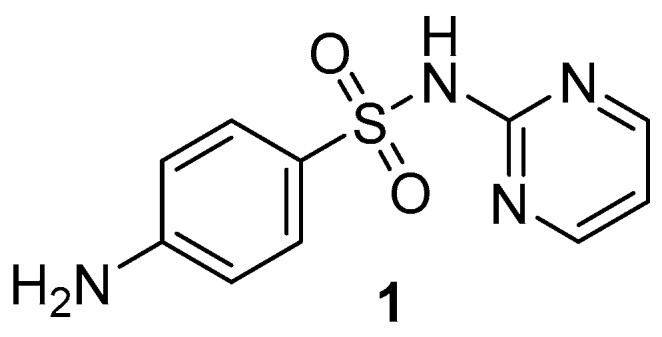
Sulfadiazine 1.
Figure 2.
Rational design of novel Schiff bases combining sulfadiazine (red) and salicylaldehyde (blue) scaffolds based on structural modifications of 2c.
Keeping in mind these facts, we decided to combine two well-established pharmacophores for antimicrobial activity, sulfadiazine and salicylaldehyde, into one molecular Schiff base entity (Figure 2) and to screen systematically their antimicrobial and cytotoxic properties. In our study [4], the activities of sulfadiazine amides and imines based on the salicylic scaffold were comparable against the majority of mycobacterial strains and drug-susceptible Staphylococcus aureus, but further research discovered that the amides are significantly more toxic and less selective for eukaryotic cells. This research is justified also by an increasing resistance of microbes to current antimicrobial drugs.
Based on previous reports about 5-chlorosalicyladehyde-based Schiff bases [4,6], we designed compounds 2a without any substitution of the salicylic scaffold, with chlorine replaced by another halogen (5-F, Br, I; negative inductive, −I, and positive mesomeric, +M, effects), other functional groups with different electronic properties: electron donating groups—alkyls (CH3, tert-butyl), OH and CH3O as well as NO2 as an electron withdrawing group. Additionally, based on our previous report [4], we explored positional isomers of 4-[(5-chloro-2-hydroxybenzylidene)amino]-N-(pyrimidin-2-yl)-benzenesulfonamide (2c, 3-Cl, 6-Cl) and 3,5-dihalogen derivatives (3,5-Cl2, 3,5-I2, 3-Br-5-Cl and 3-I-5-Cl). The drug design based on rational modifications of 2c is depicted in Figure 2. We also synthesized the Schiff base of 5-nitrofuran-2-carbaldehyde (5-nitrofurfural; 2q), a known pharmacophore for antimicrobial activity (e.g., [14,15]). This report is focused on chemical synthesis, characterization and initial biological evaluation (antimicrobial screening, toxicity for eukaryotic cells) to identify the most promising candidates for advanced microbiological and pharmacological tests.
2. Results and Discussion
2.1. Chemistry
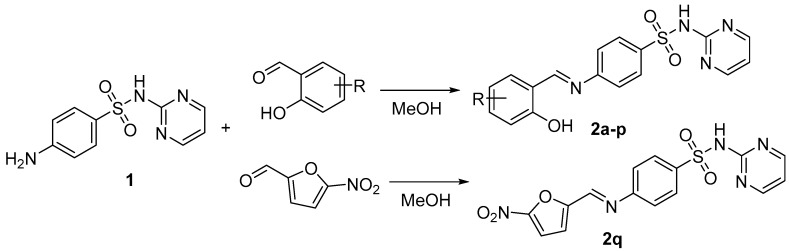
Sulfadiazine-derived Schiff bases 2 were prepared by reactions of SDZ 1 and the corresponding aldehydes (salicylaldehydes, 5-nitrofuran-2-carbaldehyde) in boiling methanol (MeOH) for 5 h (Scheme 1). Aldehydes were added to a hot methanolic suspension of SDZ. The poor solubility of SDZ in MeOH is a reason for an extended reaction time in contrast to previous work [4]. The yields were good, within the range of 85–95%, with 4-[(2-hydroxy-5-nitrobenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2f) being an exception (74 %). In this case, it was beneficial to dissolve 5-nitrosalicylaldehyde in hot MeOH and then add the sulfonamide; the reaction time was extended to 8 h.
Scheme 1.
Synthesis of sulfadiazine Schiff bases 2 (MeOH = methanol; R = H, 5-F, 5-Cl, 5-Br, 5-I, 5-NO2, 5-Me, 5-MeO, 5-OH, 5-tert-Bu, 3-Cl, 6-Cl, 3,5-Cl2, 3-Br-5-Cl, 3-I-5-Cl, 3,5-I2).
All of the compounds (Table 1) were characterized by 1H-, 13C-NMR, IR spectra and melting points; their purity was checked additionally by elemental analysis. In the 1H-NMR spectra of Schiff bases 2, an azomethine (CH=N) singlet appeared at 9.16–8.89 ppm. The phenolic hydrogen was observed in a comparatively broader range of 13.93–11.68 ppm. IR spectroscopy displayed a typical sharp and strong azomethine (-CH=N-) band observed at 1582–1570 cm−1.
Table 1.
Antibacterial activity of sulfadiazine derivatives 2.
 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | R | MIC (µM) | |||||||||||||||
| SA | MRSA | SE | EF | EC | KP | KP-E | PA | ||||||||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | ||
| 2a | H | 500 | 500 | 250 | 250 | 62.5 | 62.5 | 31.25 | 62.5 | >500 | >500 | >500 | >500 | >500 | >500 | 500 | 500 |
| 2b | 5-F | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 2c [4] | 5-Cl | 250 | 250 | 125 | 125 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 2d | 5-Br | 500 | 500 | 500 | 500 | 250 | 250 | >500 | >500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 |
| 2e | 5-I | 250 | 250 | 250 | 250 | 62.5 | 125 | 250 | 500 | 250 | 250 | 500 | 500 | 500 | 500 | 500 | 500 |
| 2f | 5-NO2 | 250 | 250 | 250 | 250 | 500 | 500 | 500 | 500 | 250 | 250 | >500 | >500 | 500 | 500 | 500 | 500 |
| 2g | 5-CH3 | >250 | >250 | 125 | 125 | 125 | 125 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 2h | 5-CH3O | 500 | 500 | >500 | >500 | 62.5 | 62.5 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | 500 | 500 |
| 2i | 5-OH | 31.25 | 31.25 | 31.25 | 31.25 | 15.62 | 15.62 | 250 | 250 | 500 | 500 | >500 | >500 | >500 | >500 | 500 | 500 |
| 2j | 5-tert-Bu | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 2k | 6-Cl | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 2l | 3-Cl | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 2m | 3,5-Cl2 | 62.5 | 62.5 | 62.5 | 62.5 | 31.25 | 31.25 | >500 | >500 | >500 | >500 | >500 | >500 | 500 | 500 | >500 | >500 |
| 2n | 3-Br-5-Cl | 31.25 | 31.25 | 15.62 | 15.62 | 31.25 | 31.25 | >500 | >500 | 500 | 500 | >500 | >500 | 500 | 500 | >500 | >500 |
| 2o | 3-I-5-Cl | 15.62 | 15.62 | 15.62 | 15.62 | 31.25 | 31.25 | 250 | 250 | 250 | 250 | 500 | 500 | 500 | 500 | 500 | 500 |
| 2p | 3,5-I2 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 500 | 500 |
| 2q | - | 125 | 125 | 125 | 125 | 31.25 | 31.25 | >500 | >500 | >500 | >500 | 500 | 500 | 500 | 500 | 250 | 250 |
| SDZ 1 | - | 500 | >500 | 500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| BAC | - | 7.81 | 15.62 | 15.62 | 15.62 | 15.62 | 31.25 | 15.62 | 62.5 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
BAC = bacitracin; SDZ = sulfadiazine. SA: Staphylococcus aureus CCM 4516/08; MRSA: methicillin-resistant Staphylococcus aureus H 5996/08; SE: Staphylococcus epidermidis H 6966/08; EF: Enterococcus faecalis. J 14365/08. Escherichia coli CCM 4517, Klebsiella pneumoniae D 11750/08; ESBL-positive Klebsiella pneumoniae J 14368/08; PA: Pseudomonas aeruginosa CCM 1961. One or two of the best MIC value(s) for each strain are shown in bold.
2.2. Antimicrobial Activity
The sulfadiazine derivatives 1–2 were screened in vitro for their antimicrobial properties. The panel of pathogens involved Staphylococcus aureus CCM 4516/08, methicillin-resistant Staphylococcus aureus H 5996/08 (MRSA), Staphylococcus epidermidis H 6966/08, Enterococcus faecalis J 14365/08 (Gram-positive bacteria), Escherichia coli CCM 4517, Klebsiella pneumoniae D 11750/08, extended spectrum beta-lactamase (ESBL)-positive Klebsiella pneumoniae J 14368/08, and Pseudomonas aeruginosa CCM 1961 (Gram-negative strains) (Table 1), mycobacteria Mycobacterium tuberculosis 331/88 (H37Rv), Mycobacterium avium 330/88, Mycobacterium kansasii 235/80 and the clinical isolate 6509/96 (Table 2) [16], and fungal species of Candida albicans ATCC 44859, Candida tropicalis 156, Candida krusei E28, Candida glabrata 20/I, Trichosporon asahii 1188, Aspergillus fumigatus 231, Lichtheimia corymbifera 272, and Trichophyton interdigitale 445 (Table 3) [17]. This panel of twenty microbial species covers a wide range of important human pathogens including those with an acquired resistance. It is a useful screening tool for an initial identification of potential antimicrobial activity of novel compounds.
Table 2.
Antimycobacterial activity of sulfadiazine derivatives 2.
| Code | R | MIC (µM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mtb. 331/88 | M. avium 330/88 | M. kansasii 235/80 | M. kansasii 6509/96 | ClogP | ||||||||
| 14 d | 21 d | 14 d | 21 d | 7 d | 14 d | 21 d | 7 d | 14 d | 21 d | |||
| 2a | H | 16 | 32 | 125 | 250 | 8 | 16 | 32 | 8 | 16 | 32 | 2.5 |
| 2b | 5-F | 125 | 125 | 500 | 1000 | 62.5 | 125 | 250 | 125 | 250 | 250 | 2.65 |
| 2c [4] | 5-Cl | 125 | 250 | 125 | 125 | 8 | 16 | 32 | 16 | 32 | 32 | 3.05 |
| 2d | 5-Br | 16 | 32 | 125 | 125 | 32 | 62.5 | 62.5 | 32 | 32 | 62.5 | 3.32 |
| 2e | 5-I | 62.5 | 125 | 125 | 250 | 32 | 62.5 | 125 | 32 | 32 | 62.5 | 3.85 |
| 2f | 5-NO2 | 250 | 250 | 500 | 1000 | 125 | 250 | 500 | 125 | 250 | 250 | 2.15 |
| 2g | 5-CH3 | 125 | 125 | 500 | 1000 | 125 | 125 | 250 | 62.5 | 62.5 | 125 | 2.98 |
| 2h | 5-CH3O | 32 | 62.5 | 125 | 125 | 16 | 32 | 62.5 | 16 | 16 | 32 | 2.37 |
| 2i | 5-OH | 125 | 125 | 500 | 1000 | 62.5 | 125 | 125 | 62.5 | 125 | 125 | 2.11 |
| 2j | 5-tert-Bu | 32 | 62.5 | 125 | 125 | 16 | 32 | 62.5 | 16 | 16 | 32 | 4.2 |
| 2k | 6-Cl | 125 | 125 | 250 | 250 | 62.5 | 125 | 250 | 62.5 | 125 | 125 | 3.05 |
| 2l | 3-Cl | 32 | 62.5 | 125 | 250 | 16 | 32 | 62.5 | 16 | 16 | 32 | 3.05 |
| 2m | 3,5-Cl2 | 125 | 125 | 250 | 500 | 125 | 250 | 500 | 62.5 | 125 | 250 | 3.61 |
| 2n | 3-Br-5-Cl | 125 | 125 | 250 | 500 | 125 | 125 | 250 | 62.5 | 125 | 125 | 3.88 |
| 2o | 3-I-5-Cl | 125 | 125 | 500 | 500 | 125 | 250 | 250 | 62.5 | 125 | 125 | 4.41 |
| 2p | 3,5-I2 | 250 | 250 | 250 | 500 | 125 | 250 | 250 | 62.5 | 125 | 250 | 5.21 |
| 2q | - | 125 | 125 | 500 | 500 | 125 | 250 | 250 | 62.5 | 62.5 | 125 | ND |
| SDZ 1 | - | 32 | 62.5 | 62.5 | 62.5 | 16 | 16 | 32 | 8 | 8 | 8 | 0.21 |
| INH | - | 0.5 | 1 | >250 | >250 | >250 | >250 | >250 | 8 | 8 | 8 | - |
INH = isoniazid; SDZ = sulfadiazine. Mtb. = Mycobacterium tuberculosis. ND = not determined. One or two of the best MIC value(s) for each strain are shown in bold.
Table 3.
Antifungal activity of sulfadiazine derivatives 2.
| Code | R | MIC (µM) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA | CT | CK | CG | TA | AF | LC | TI | ||||||||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 72 h | 120 h | ||
| 2a | H | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | 125 | 125 |
| 2b | 5-F | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 2c [4] | 5-Cl | 125 | 125 | >500 | >500 | >500 | >500 | 125 | 125 | 62.5 | 125 | >500 | >500 | >500 | >500 | >500 | >500 |
| 2d | 5-Br | 125 | 125 | 250 | 250 | 250 | 250 | 62.5 | 62.5 | 62.5 | 62.5 | 250 | 250 | >500 | >500 | 62.5 | 62.5 |
| 2e | 5-I | 62.5 | 125 | 125 | 125 | 62.5 | 125 | 62.5 | 62.5 | 125 | 125 | 250 | 250 | >500 | >500 | 31.25 | 31.25 |
| 2f | 5-NO2 | 500 | 500 | >500 | >500 | >500 | >500 | 250 | 250 | >500 | >500 | >500 | >500 | 500 | 500 | 62.5 | 62.5 |
| 2g | 5-CH3 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 2h | 5-CH3O | 500 | 500 | 500 | 500 | >500 | >500 | 500 | 500 | 500 | 500 | >500 | >500 | >500 | >500 | 62.5 | 62.5 |
| 2i | 5-OH | 15.62 | 15.62 | 15.62 | 15.62 | 31.25 | 31.25 | 15.62 | 15.62 | 15.62 | 15.62 | 125 | 125 | 500 | 500 | 1.95 | 1.95 |
| 2j | 5-tert-Bu | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | 62.5 | 62.5 |
| 2k | 6-Cl | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 2l | 3-Cl | 62.5 | 62.5 | 125 | 125 | 250 | 250 | 125 | 125 | 125 | 125 | 250 | 250 | >500 | >500 | 31.25 | 31.25 |
| 2m | 3,5-Cl2 | 62.5 | 62.5 | 125 | 125 | 62.5 | 62.5 | 31.25 | 31.25 | 62.5 | 62.5 | 250 | 250 | 500 | 500 | 125 | 125 |
| 2n | 3-Br-5-Cl | 31.25 | 31.25 | 62.5 | 62.5 | 62.5 | 62.5 | 31.25 | 31.25 | 62.5 | 62.5 | 250 | 250 | 500 | 500 | 62.5 | 62.5 |
| 2o | 3-I-5-Cl | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 62.5 | 62.5 | 250 | 250 | 1.95 | 1.95 |
| 2p | 3,5-I2 | 3.9 | 3.9 | 7.81 | 7.81 | 7.81 | 7.81 | 3.9 | 3.9 | 3.9 | 3.9 | 62.5 | 62.5 | 62.5 | 62.5 | 15.62 | 15.62 |
| 2q | - | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 500 | 500 | 500 | 500 | >500 | >500 | 250 | 250 |
| FLU | - | 0.24 | 0.24 | >500 | >500 | 125 | 250 | 31.25 | 500 | 250 | 500 | >500 | >500 | >500 | >500 | 7.81 | 125 |
FLU = fluconazole; SDZ = sulfadiazine 1. CA: Candida albicans ATCC 44859; CT: Candida tropicalis; CK: Candida krusei E28; CG: Candida glabrata 20/I; TA: Trichosporon asahii 1188; AF: Aspergillus fumigatus 231; LC: Lichtheimia corymbifera 272; TI: Trichophyton interdigitale 445. One or two of the best MIC value(s) for each strain are shown in bold.
Bacitracin (BAC), fluconazole (FLU) and isoniazid (INH) were employed as the comparative drugs for antibacterial, antifungal and antimycobacterial activity, respectively. The parent sulfadiazine 1 showed only a weak action against both strains of Staphylococcus aureus (Table 1), and no antifungal properties, but it is an antimycobacterial agent (Table 2). Four Schiff bases (compounds 2b, 2j–l) showed no significant antibacterial activity (Table 1). In general, the action against Gram-negative species is only negligible (MICs starting from 125 µM). 4-[(2-Hydroxy-3,5-diiodobenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2p) showed the lowest MIC values, followed by other halogenated Schiff bases 2d, 2e, 2o. Among Gram-positive pathogens, Enterococcus was inhibited by six derivatives (compounds 2a, 2e, 2f, 2i, 2o, 2p) with 4-[(2-hydroxybenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2a) showing superiority (31.25/62.5 µM). Thus, the antibacterial properties are manifested especially for Staphylococci. MICs tend to be even lower for MRSA when compared to the methicillin-susceptible strain (e.g., 2g). For S. aureus, the 3,5-dihalogen substitution is translated into an enhanced activity (MICs of 7.81–62.5 µM) where heavier atoms contribute to more intense action (7.81–62.5 µM). Another substituent decreasing MIC values is a second hydroxyl group (i.e., 2i) in the para-position with respect to the salicylic hydroxyl with a uniform MIC of 31.25 µM.
The rest of sulfadiazines 2 share MICs of 125–500 µM. The analysis of the effect of the chlorine position on the salicylic ring revealed that the activity is conferred by its presence at the position 5 while its shift to another carbon hampers antibacterial activity completely (2c vs. 2k–l). 3,5-Diiodosalicylidene sulfadiazine 2p was also superior for the inhibition of S. epidermidis (7.81 µM), followed by 5-OH (2i, 15.62 µM), other 3,5-dihalogen (2m–o) and 5-nitrofuran 2q (31.25 µM) derivatives. The diiodo derivative 2p was superior to bacitracin against S. epidermidis, and five compounds (2i, 2m–o, 2q) were fully comparable. The activities of BAC and several Schiff bases (compounds 2n–p) are identical against S. aureus. Considering Enterococcus sp., the derivative 2a exhibited comparable MIC values.
All of the compounds 2 were able to inhibit both M. tuberculosis and NTM in vitro with MIC values within the range of 8–1000 µM. Both strains of M. kansasii showed the highest rate of susceptibility (MIC ≥ 8 µM), followed by M. tuberculosis (≥16 µM) and M. avium (MIC values of 125–500 µM). The best results were obtained for the simplest derivative, 4-[(2-hydroxybenzylidene)amino]-N-(pyrimidin-2-yl)benzene-sulfonamide (2a) with MICs of 8–250 µM but for M. tuberculosis and both M. kansasii strains its MICs were ≤32 µM. A previously reported derivative of 5-chlorosalicylaldehyde [4] was efficient in vitro against NTM. Although 5-nitrofuran-2-yl is a well-established scaffold in drug design of potential anti-TB drugs [18], the 5-nitrofurylidene derivative 2q did not meet our expectation of excellent antimycobacterial action (MIC values ≥ 62.5 µM).
Structure-activity relationships for the substitution of the salicylidene fragment are reported in Table 4. In general, the introduction of any substituent does not offer any benefit when compared to unsubstituted Schiff base 2a. Among single halogens, bromine (compound 2d) is favourable, in the case of NTM strains concomitantly with 5- and 3-chloro isomers (compounds 2c, 2l). 5-Methoxy (compound 2h) and tert-butyl (compound 2j) groups contribute to the improved action, too. On the other hand, halo 3,5-disubstitution (compounds 2m–p), and a nitro group (compound 2f) are detrimental for the inhibition of all mycobacteria. For NTM, a fluorine atom (compound 2b), 5-methyl (compound 2g), or 5-nitrofurylidene (compound 2q) abrogate the activity. Although lipophilicity represents an important factor influencing antimycobacterial action, no direct correlation between lipophilicity and MIC values was identified.
Table 4.
SAR for the antimycobacterial activity.
| Strain | Improving Activity | Decreasing Activity |
|---|---|---|
| M. tuberculosis | H, 5-Br, 5-CH3O, 5-t-Bu, 3-Cl | 5-Cl, 5-NO2, 3,5-I2 |
| M. avium | H, 5-Cl, 5-Br, 5-I, 5-CH3O, 5-t-Bu, 3-Cl | 5-F, 5-NO2, 5-CH3, 5-OH, 3-I-5-Cl, 5-NO2-furylidene |
| M. kansasii | H, 5-Cl, 5-Br, 5-CH3O, 5-t-Bu, 3-Cl | 5-F, 5-NO2, 5-CH3, 3,5-X2, 3-X-5-Cl, 5-NO2-furylidene |
Drawing a comparison to INH, none of the Schiff bases 2 exceeded its activity against M. tuberculosis. For the clinical isolate M. kansasii 6509/96, five compounds (2a, 2c, 2h, 2j, and 2l) were comparable after 7 and/or 14 d of incubation (± one dilution). In contrast, eleven derivatives (2a, 2c–e, 2h, 2j–n, 2p) inhibited the growth of M. avium at the concentration of 250 µM that is tolerated in the case of INH. Similarly, all of the derivatives 2 surpassed INH against M. kansasii 235/80.
Unfortunately, the modification of the parent sulfadiazine 1 to the derivatives 2 did not bring any significant improvement of antimycobacterial properties but the most active Schiff bases (2a, 2d, 2h, 2j, and 2l) exhibited activity comparable to that of 1 (± one dilution).
Three Schiff bases (2b, 2g, 2k) lacked antifungal activity (Table 3), two others (2a, 2j) inhibited only T. interdigitale at 125 and 62.5 µM, respectively. Three derivatives showed MIC values against several strains starting from 250 µM (2f, 2h, 2q) with T. interdigitale being an exception (MIC of 62.5 µM for 2f and 2h). In general, this strain showed the highest susceptibility from all of the investigated fungi with MIC values of 1.95–250 µM. 3-I-5-Cl (2o), 3,5-I2 (2p), 5-OH (2j) were found as preferred substitution patterns for salicylic ring that are superior to FLU. Additional Schiff bases (2d–f, 2h, 2j, 2l, and 2n) produced lower MICs after 120 h of incubation. On the other hand, L. corymbifera is at least susceptible strain inhibited only by six derivatives (2f, 2i, 2m–p) with MICs ≥ 62.5 µM.
Nine compounds 2d, 2e, 2i, and 2l–q uniformly affected yeasts (Candida sp., T. asahii), three 5-substituted salicylidenesulfadiazines (5-Cl 2c, 5-NO2 2f, and 5-CH3O 2h) were effective in vitro against only some strains. Similar to the antibacterial activity, dihalogen derivatives containing iodine (2o–p, MICs of 3.9–7.81 µM) or the second phenolic group (2i, 15.62–31.25 µM) showed the highest in vitro potency, which was significantly superior to that of the fluconazole; C. albicans represented an exception. Satisfactory results are also conferred by 3,5-dichloro- and 3-bromo-5-iodo-salicylaldehyde scaffolds (compounds 2m, 2n, 31.25–125 µM), followed by a single halogen (Cl, Br, I) substitution pattern and it correlates positively with an increasing atomic mass and consequent lipophilicity. The comparison of isomeric monochlorinated Schiff bases (2c, 2k, 2l) revealed the order: 3-Cl (2l) > 5-Cl (2k) > 6-Cl (inactive 2k).
Seeking insights into the potential mechanism of action, we evaluated the inhibition potency of selected salicylidenesulfonamides against isocitrate lyase, but they were virtually inactive in spite of the substitution pattern (i.e., their inhibition rates were lower than 10% at the single concentration of 100 µM).
2.3. Cytotoxicity and Selectivity
The cytotoxicity of the investigated compounds 1 and 2 was measured using the standard hepatic cell line HepG2 (Table 5) [16]. The used CellTiter 96 assay is based on the reduction of tetrazolium dye in living cells to formazan. This reduction is related to availability of NADH or NADPH. The parameter IC50, i.e., the concentration that reduces the viability of the cells to 50% of the maximal viability, was used as a measure of cytotoxicity. It was not possible to determine the exact IC50 values of two derivatives (compounds 2a, 2b) due to their limited solubility in the cell culture medium used in this experiment.
Table 5.
Cytotoxicity for HepG2 cells and selectivity.
| Code | R | IC50 (µM) | Range of Concentrations Tested | SI for Staphylococci (MRSA/SE) | SI for Candida sp. | SI for TI | SI for Mtb. | SI for M. kansasii |
|---|---|---|---|---|---|---|---|---|
| 2a | H | >500 * | 1–500 | >2/>8 | inactive | >4 | >15.63 | >15.63 |
| 2b | 5-F | >250 * | 1–250 | inactive | inactive | inactive | >2 | >1 |
| 2c | 5-Cl | NT | NT | NT | NT | inactive | NT | NT |
| 2d | 5-Br | 159.1 | 1–500 | 0.32/0.64 | 0.64–2.55 | 2.55 | 4.98–9.94 | 2.55–4.97 |
| 2e | 5-I | 156.0 | 1–500 | 0.62/1.25–2.50 | 1.25–2.50 | 4.99 | 1.25–2.50 | 1.25–4.88 |
| 2f | 5-NO2 | 140.5 | 1–500 | 0.56/0.28 | ≤0.56 | 2.25 | 0.56 | 0.28–1.12 |
| 2g | 5-CH3 | 569.5 ** | 1–500 | 4.56 | inactive | inactive | 4.56 | 2.28–9.11 |
| 2h | 5-CH3O | 351.1 | 1–500 | inactive/5.62 | ≤0.70 | 5.62 | 5.62–11.24 | 5.62–21.94 |
| 2i | 5-OH | >500 | 1–500 | >16/>32 | >16 | >256.41 | >4 | >4 |
| 2j | 5-tert-Bu | 45.6 | 1–500 | inactive | inactive | 0.73 | 0.73–1.46 | 0.73–2.85 |
| 2k | 6-Cl | 102.20 | 1–500 | inactive | inactive | inactive | 0.82 | 0.41–1.64 |
| 2l | 3-Cl | 160.5 | 1–500 | inactive | 0.64–2.57 | 5.14 | 2.57–5.14 | 2.57–10.03 |
| 2m | 3,5-Cl2 | 114.30 | 1–500 | 1.83/3.66 | 0.91–3.66 | 0.91 | 0.91 | 0.23–1.83 |
| 2n | 3-Br-5-Cl | 92.94 | 1–500 | 5.95/2.98 | 1.49–2.97 | 1.49 | 0.74 | 0.37–1.49 |
| 2o | 3-I-5-Cl | 59.23 | 1–500 | 3.79/1.90 | 7.58 | 30.37 | 0.47 | 0.24–0.95 |
| 2p | 3,5-I2 | 38.0 | 1–500 | 4.87 | 4.87–9.73 | 2.43 | 0.15 | 0.15–0.61 |
| 2q | - | 16.3 | 1–500 | 0.13/0.52 | 0.07 | 0.07 | 0.13 | 0.07–0.26 |
| SDZ 1 | - | >1500 | 1–1500 | >3 (24 h) | inactive | inactive | >24 | >46.88 |
* Measurement at higher concentration was not possible due to the precipitation of the compound in the medium. ** an estimated value based on the curve. SI = IC50/MIC. SDZ: sulfadiazine. NT = not tested; MRSA: methicillin-resistant Staphylococcus aureus; SE: Staphylococcus epidermidis; TI: Trichophyton interdigitale. The SI values higher than 10 are shown in bold.
We can divide the investigated Schiff bases 2 into three groups according to their cytotoxicity for HepG2 cells. The first one covers comparatively non-toxic compounds 1, 2a, 2b, and 2g–i (IC50 values > 250 µM). The group of the compounds with an intermediate toxicity and IC50s in the range of 102.2 to 160.5 µM consists of 2d–f, and 2k–m. The last group includes sulfonamides with an increased cytotoxicity (IC50 ≤ 100 µM: 2j, 2n–q). 4-{[(5-Nitrofuran-2-yl)methylene]amino}-N-(pyrimidin-2-yl)benzene-sulfonamide (2q) was found to be the most toxic substance (IC50 = 16.0 µM). Obviously, the replacement of the salicyl ring by 5-nitrofuran-2-yl produced a more pronounced toxicity for mammalian cells. The majority of the modifications of the parent sulfadiazine 1 (IC50 > 1500 µM) increased the cytotoxicity by up to two orders of magnitude for the most toxic compounds. Generally, the enhanced cytotoxicity is observed in the presence of 3,5-halogen disubstitution and a tert-butyl moiety. Among particular halogens, the cytotoxicity correlates positively with an increased atomic mass. The presence of a non-bulky electron-donating group (CH3, CH3O, OH) or hydrogen results in less cytotoxic compounds (i.e., 2a, 2g–i).
We calculated the selectivity indices (SIs) for M. tuberculosis, both strains of M. kansasii, MRSA, S. epidermidis, all of the Candida species and T. interdigitale (Table 5). The SI is defined as the ratio of IC50 to MIC, and values higher than 10 indicate rather acceptable toxicity (based on the analogy of the therapeutic index). Considering mycobacteria, in addition to the parent sulfadiazine 1, two compounds exhibited targeted selectivity: Unsubstituted salicylidene sulfadiazine 2a (SI > 15.63) followed by 5-methoxy derivative 2h after 14 d of the incubation. 3-Chlorosalicylaldehyde-based Schiff base 2l provided for M. kansasii a borderline SI value after 7 and 14 d. 4-[(2,5-Dihydroxybenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2i) showed the best safety profile for Staphylococci and fungi (>16), especially for the filamentous fungus T. interdigitale (>256.41). For this strain, the dihalogenated derivative 2o was selective too (SI = 30.37).
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
All of the reagents and solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Penta Chemicals (Prague, Czech Republic) and were used as received. The reactions and the purity of the products were monitored by thin-layer chromatography (TLC) with a mixture of dichloromethane with n-hexane and methanol (4:3:1, v/v) as the eluent. The plates were coated with 0.2 mm Merck 60 F254 silica gel (Merck Millipore, Darmstadt, Germany) and were visualised by UV irradiation (254 nm). The melting points were determined using a B-540 melting point apparatus (Büchi, Flawil, Switzerland) with open capillaries, and the reported values are uncorrected. The elemental analysis (C, H, N) was performed with an automatic microanalyser instrument CHNS-O CE (Fisons EA 1110, Milano, Italy). The infrared spectra (ATR) were recorded on a Nicolet 6700 FT-IR spectrometer (ThermoFisher Scientific, Waltham, MA, USA) in the range of 600 to 4000 cm−1. The NMR spectra were measured in DMSO-d6 at ambient temperature using a Varian VNMR S500 instrument (500 MHz for 1H and 125 MHz for 13C; Varian Inc., Palo Alto, CA, USA). The chemical shifts, δ, are given in ppm with respect to tetramethylsilane as the internal standard. The coupling constants (J) are reported in Hz. The calculated logP values (ClogP), which are the logarithms of the partition coefficients for octan-1-ol/water, were determined using the CS ChemOffice Ultra version 16.0 program (CambridgeSoft, Cambridge, MA, USA).
3.1.2. Synthesis
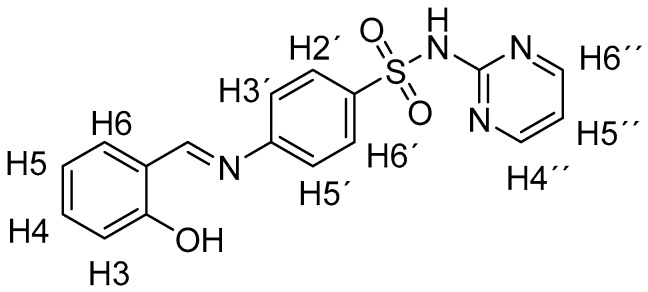
Sulfadiazine 1 (1 mmol, 250.3 mg) was suspended in methanol (MeOH, 10 mL), and 1.1 mmol of the appropriate aldehyde was added in one portion under vigorous stirring. The solution was refluxed for 5 h and then stirred at room temperature overnight. The resulting crystals were filtered off, washed with a small amount of MeOH and then acetonitrile, and dried. The crystals were recrystallized from MeOH or tetrahydrofuran/n-hexane mixture if necessary. The identity of known compounds (i.e., 2a, 2c, 2d, and 2m) was confirmed by NMR (1H and 13C) and IR spectroscopy. All spectroscopic characteristics were in accordance with previously reported data. The purity was checked additionally by melting points measurement and elemental analysis. The numbering of hydrogen atoms in the 1H-NMR spectra is depicted in Figure 3.
Figure 3.
Numbering of H atoms in NMR spectra.
4-[(2-Hydroxybenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2a) [12,19]. Yellow solid; yield 94%; m.p. 246–248.5 °C (lit. [19] 244–245 °C decomp.). IR: 3033, 2937, 2870, 2812, 2740, 1621, 1581 (-HC=N-), 1493, 1446, 1413, 1382, 1339, 1279, 1188, 1165, 1151, 1091, 943, 914, 856, 835, 798, 778, 755, 707, 667 cm−1. 1H-NMR: δ 12.50 (1H, s, OH), 11.86 (1H, s, SO2NH), 8.95 (1H, s, CH=N), 8.51 (2H, d, J = 4.9 Hz, H4′′, H6′′), 8.06–8.02 (2H, m, H2′, H6′), 7.68 (1H, dd, J = 7.7 Hz, J = 1.6 Hz, H6), 7.56–7.52 (2H, m, H3′, H5′), 7.44 (1H, dd, J = 7.8 Hz, J = 1.7 Hz, H4), 7.06 (1H, t, J = 4.9 Hz, H5′′), 7.00–6.96 (2H, m, H3, H5). 13C-NMR: δ 165.49, 160.41, 158.54, 157.03, 152.40, 138.16, 134.17, 132.72, 129.24, 121.88, 119.53, 119.48, 116.88, 115.98. Anal. Calcd. for C17H14N4O3S (354.38): C, 57.62; H, 3.98; N, 15.81. Found: C, 57.71; H, 4.08; N, 15.65.
4-[(5-Fluoro-2-hydroxybenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2b). Yellowish-orange solid; yield 92%; m.p. 273–276 °C (decomp.). IR: 3032, 2939, 2870, 2812, 2736, 1570 (-HC=N-), 1483, 1444, 1414, 1338, 1269, 1251, 1220, 1163, 1144, 1091, 943, 879, 860, 835, 798, 776, 702, 669 cm−1. 1H-NMR: δ 12.07 (1H, s, OH), 11.86 (1H, s, SO2NH), 8.90 (1H, s, CH=N), 8.51 (2H, d, J = 4.8 Hz, H4′′, H6′′), 8.07–8.02 (2H, m, H2′, H6′), 7.55–7.49 (3H, m, H6, H3′, H5′), 7.31 (dd, J = 8.8 Hz, J = 3.2 Hz, H4), 7.06 (1H, t, J = 4.9 Hz, H5′′), 7.01–6.98 (1H, m, H3). 13C-NMR: δ 163.68 (d, J = 2.5 Hz), 158.54, 157.01, 156.54, 155.18 (d, J = 235.1 Hz), 152.55, 138.31, 129.25, 121.84, 121.11 (d, J = 23.6 Hz), 119.96 (d, J = 7.6 Hz), 118.36 (d, J = 7.6 Hz), 116.68 (d, J = 23.4 Hz), 112.28. Anal. Calcd. for C17H13FN4O3S (372.37): C, 54.83; H, 3.52; N, 15.05. Found: C, 54.90; H, 3.78; N, 14.94.
4-[(5-Chloro-2-hydroxybenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2c) [4]
4-[(5-Bromo-2-hydroxybenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2d) [13]. Orange solid; yield 89%; m.p. 273.5–275.5 °C (lit. [13] 246–248 °C). Anal. Calcd. for C17H13BrN4O3S (433.28): C, 47.13; H, 3.02; N, 12.93. Found: C, 46.97; H, 3.21; N, 13.04.
4-[(2-Hydroxy-5-iodobenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2e). Orange solid; yield 92%; m.p. 273–274.5 °C (decomp.). IR: 3038, 2944, 2871, 2812, 2736, 1579 (-HC=N-), 1558, 1493, 1443, 1411, 1340, 1280, 1180, 1163, 1093, 945, 912, 860, 837, 823, 797, 783, 715, 671 cm−1. 1H-NMR: δ 12.42 (1H, s, OH), 11.86 (1H, s, SO2NH), 8.89 (1H, s, CH=N), 8.51 (2H, d, J = 4.9 Hz, H4′′, H6′′), 8.06–8.00 (3H, m, H6, H2′, H6′), 7.70 (1H, dd, J = 8.7 Hz, J = 2.3 Hz, H4), 7.54–7.49 (2H, m, H3′, H5′), 7.05 (1H, t, J = 4.9 Hz, H5′′), 6.83 (1H, d, J = 8.7 Hz, H3). 13C-NMR: δ 163.59, 159.90, 158.53, 157.01, 152.34, 141.93, 139.88, 138.32, 129.24, 122.12, 121.89, 119.64, 112.29, 81.10. Anal. Calcd. for C17H13IN4O3S (480.28): C, 42.51; H, 2.73; N, 11.67. Found: C, 42.33; H, 2.60; N, 11.89.
4-[(2-Hydroxy-5-nitrobenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2f). Yellowish-orange solid; yield 74%; m.p. 277–279 °C (decomp.). IR: 3045, 2733, 1581 (-HC=N-), 1530, 1487, 1444, 1411, 1340, 1338, 1298, 1161, 1093, 946, 840, 808, 785, 753, 733, 673 cm−1. 1H-NMR: δ 13.51 (1H, s, OH), 11.87 (1H, s, SO2NH), 9.10 (1H, s, CH=N), 8.69 (1H, d, J = 2.9 Hz, H6), 8.52 (2H, d, J = 4.9 Hz, H4′′, H6′′), 8.29 (1H, dd, J = 9.1 Hz, J = 2.9 Hz, H4), 8.09–8.03 (2H, m, H2′, H6′), 7.60–7.55 (2H, m, H3′, H5′), 7.16 (1H, d, J = 9.1 Hz, H3), 7.06 (1H, t, J = 4.9 Hz, H5′′). 13C-NMR: δ 189.25, 165.92, 163.00, 158.40, 157.0, 139.76, 138.76, 129.98, 129.29, 127.84, 121.96, 119.43, 118.27, 112.30. Anal. Calcd. for C17H13N5O5S (399.38): C, 51.13; H, 3.28; N, 17.54. Found: C, 51.00; H, 3.11; N, 17.35.
4-[(2-Hydroxy-5-methylbenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2g). Orange solid; yield 90%; m.p. 255–258 °C (decomp.). IR: 3587 (O-H), 3035, 2807, 2737, 1576 (-HC=N-), 1491, 1441, 1409, 1378, 1343, 1284, 1263, 1207, 1185, 1157, 1094, 945, 877, 843, 817, 797, 786, 704, 680, 667 cm−1. 1H-NMR: δ 12.21 (1H, s, OH), 11.82 (1H, s, SO2NH), 8.89 (1H, s, CH=N), 8.51 (2H, d, J = 4.9 Hz, H4′′, H6′′), 8.06–8.01 (2H, m, H2′, H6′), 7.53–7.49 (2H, m, H3′, H5′), 7.47 (1H, d, J = 2.3 Hz, H6), 7.25 (dd, J = 8.4 Hz, J = 2.1 Hz, H4). 7.05 (1H, t, J = 4.9 Hz, H5′′), 6.88 (1H, d, J = 8.4 Hz, H3), 2.26 (3H, s, CH3). 13C-NMR: δ 165.25, 158.39, 158.28, 157.04, 152.58, 138.08, 134.96, 132.32, 129.25, 128.10, 121.83, 119.17, 116.75, 115.98, 20.08. Anal. Calcd. for C18H16N4O3S (368.41): C, 58.68; H, 4.38; N, 15.21. Found: C, 58.84; H, 4.22; N, 15.41.
4-[(2-Hydroxy-5-methoxybenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2h). Orange-yellow solid; yield 86%; m.p. 232–234 °C. IR: 3030, 2734, 1576 (-HC=N-), 1487, 1440, 1410, 1338, 1268, 1215, 1153, 1087, 1035, 939, 876, 868, 852, 831, 820, 797, 754, 716, 694, 668 cm−1. 1H-NMR: δ 12.25 (1H, s, OH), 11.83 (1H, s, SO2NH), 8.91 (1H, s, CH=N), 8.51 (2H, d, J = 4.9 Hz, H4′′, H6′′), 8.06–8.01 (2H, m, H2′, H6′), 7.53–7.47 (2H, m, H3′, H5′), 7.27 (1H, d, J = 3.1 Hz, H6), 7.08–7.04 (2H, m, H4, H5′′), 6.92 (1H, d, J = 9.0 Hz, H3), 3.74 (3H, s, CH3). 13C-NMR: δ 164.73, 158.54, 157.03, 154.51, 152.77, 152.14, 138.05, 129.25, 121.78, 121.54, 119.44, 117.84, 115.98, 114.80, 55.72. Anal. Calcd. for C18H16N4O4S (384.41): C, 56.24; H, 4.20; N, 14.58. Found: C, 56.50; H, 4.31; N, 14.32.
4-[(2,5-Dihydroxybenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2i). Orange-yellow solid; yield 88%; m.p. 247–249 °C (decomp.). IR: 3491 (O-H), 3032, 2939, 2811, 2730, 1573 (-HC=N-), 1480, 1447, 1412, 1359, 1325, 1280, 1248, 1208, 1182, 1142, 1086, 945, 882, 862, 817, 797, 784, 704, 669 cm−1. 1H-NMR: δ 11.84 (1H, s, SO2NH), 11.68 (1H, s, 2-OH), 9.13 (1H, s, CH=N), 8.84 (1H, s, 5-OH), 8.51 (2H, d, J = 4.9 Hz, H4′′, H6′′), 8.06–7.99 (2H, m, H2′, H6′), 7.53–7.45 (2H, m, H3′, H5′), 7.09–7.03 (2H, m, H6, H5′′), 6.89 (1H, dd, J = 8.8 Hz, J = 3.0 Hz, H4), 6.81 (1H, d, J = 8.8 Hz, H3). 13C-NMR: δ 164.96, 158.59, 157.04, 153.30, 152.85, 149.92, 137.95, 129.21, 122.13, 121.82, 119.53, 117.55, 116.72, 115.98. Anal. Calcd. for C17H14N4O4S (370.38): C, 55.13; H, 3.81; N, 15.13. Found: C, 55.02; H, 3.65; N, 15.37.
4-{[5-(Tert-butyl)-2-hydroxybenzylidene]amino}-N-(pyrimidin-2-yl)benzenesulfonamide (2j). Orange-yellow solid; yield 90%; m.p. 267.5–270 °C (decomp.). IR: 3034, 2953, 1620, 1576 (-HC=N-), 1489, 1448, 1411, 1333, 1264, 1181, 1152, 1093, 953, 879, 839, 827, 798, 719, 671 cm−1. 1H-NMR: δ 12.30 (1H, s, OH), 11.83 (1H, s, SO2NH), 8.96 (1H, s, CH=N), 8.51 (2H, d, J = 4.9 Hz, H4′′, H6′′), 8.06–8.00 (2H, m, H2′, H6′), 7.69 (1H, d, J = 2.5 Hz, H6), 7.55–7.51 (2H, m, H3′, H5′), 7.49 (dd, J = 8.7 Hz, J = 2.5 Hz, H4). 7.06 (1H, t, J = 4.9 Hz, H5′′), 6.91 (1H, d, J = 8.7 Hz, H3), 1.27 (9H, s, CH3). 13C-NMR: δ 166.16, 158.86, 158.56, 157.32, 152.85, 141.97, 138.32, 131.83, 129.53, 129.20, 122.12, 119.00, 116.81, 112.58, 34.27, 31.60. Anal. Calcd. for C21H22N4O3S (410.49): C, 61.45; H, 5.40; N, 13.65. Found: C, 61.36; H, 5.28; N, 13.49.
4-[(2-Chloro-6-hydroxybenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2k). Yellow solid; yield 86%; m.p. 248–250 °C (decomp.). IR: 3032, 2943, 2810, 2734, 1580 (-HC=N-), 1489, 1446, 1408, 1339, 1277, 1177, 1158, 1092, 945, 929, 864, 849, 822, 797, 780, 734, 724, 670, 652 cm−1. 1H-NMR: δ 13.86 (1H, s, OH), 11.90 (1H, s, SO2NH), 9.16 (1H, s, CH=N), 8.52 (2H, d, J = 4.9 Hz, H4′′, H6′′), 8.09–8.04 (2H, m, H2′, H6′), 7.65–7.60 (2H, m, H3′, H5′), 7.45 (1H, t, J = 8.2 Hz, H4), 7.10–7.04 (2H, m, H5, H5′′), 6.98 (1H, d, J = 8.4 Hz, H3). 13C-NMR: δ 162.72, 162.55, 158.54, 156.98, 151.00, 138.91, 135.90, 135.11, 129.29, 122.19, 120.50, 116.82, 115.99, 115.64. Anal. Calcd. for C17H13ClN4O3S (388.83): C, 52.51; H, 3.37; N, 14.41. Found: C, 52.39; H, 3.39; N, 14.21.
4-[(3-Chloro-2-hydroxybenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2l). Orange solid; yield 90%; m.p. 265–266.5 °C. IR: 3223, 3041, 2869, 1618, 1576 (-HC=N-), 1489, 1441, 1409, 1360, 1338, 1281, 1202, 1182, 1151, 1115, 1091, 932, 845, 808, 792, 770, 741, 667 cm−1. 1H-NMR: δ 13.77 (1H, s, OH), 11.90 (1H, s, SO2NH), 9.05 (1H, s, CH=N), 8.52 (2H, d, J = 4.9 Hz, H4′′, H6′′), 8.09–8.04 (2H, m, H2′, H6′), 7.66–7.60 (4H, m, H4, H6, H3′, H5′), 7.06 (1H, t, J = 4.9 Hz, H5′′), 7.01 (1H, t, J = 7.8 Hz, H5). 13C-NMR: δ 165.91, 158.55, 156.99, 156.52, 150.86, 138.78, 133.91, 132.28, 129.25, 122.10, 120.55, 120.18, 119.96, 115.97. Anal. Calcd. for C17H13ClN4O3S (388.83): C, 52.51; H, 3.37; N, 14.41. Found: C, 52.60; H, 3.21; N, 14.61.
4-[(3,5-Dichloro-2-hydroxybenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2m) [20]. Orange solid; yield 86%; m.p. 274–276 °C (decomp.; lit. [20] 260 °C from acetone). IR: 3036, 2722, 1582 (-HC=N-), 1494, 1453, 1441, 1410, 1337, 1265, 1199, 1182, 1153, 1092, 947, 890, 869, 843, 798, 742, 727, 673 cm−1. 1H-NMR: δ 13.78 (1H, s, OH), 11.91 (1H, s, SO2NH), 9.01 (1H, s, CH=N), 8.51 (2H, d, J = 4.9 Hz, H4′′, H6′′), 8.09–8.04 (2H, m, H2′, H6′), 7.76 (1H, d, J = 2.5 Hz, H4), 7.73 (1H, d, J = 2.6 Hz, H6), 7.63–7.58 (2H, m, H3′, H5′), 7.06 (1H, t, J = 4.9 Hz, H5′′). 13C-NMR: δ 164.77, 158.51, 156.96, 155.68, 150.47, 139.09, 132.93, 131.07, 129.28, 122.58, 122.11, 121.90, 120.69, 115.91. Anal. Calcd. for C17H12Cl2N4O3S (423.27): C, 48.24; H, 2.86; N, 13.24. Found: C, 48.03; H, 2.74; N, 13.30.
4-[(3-Bromo-5-chloro-2-hydroxybenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2n). Pale orange solid; yield 87%; m.p. 253–256 °C (decomp.). IR: 3587 (O-H), 3032, 2938, 2807, 2729, 1581 (-HC=N-), 1489, 1443, 1411, 1338, 1294, 1279, 1199, 1184, 1159, 1092, 947, 869, 844, 793, 742, 723, 709, 675 cm−1. 1H-NMR: δ 13.93 (1H, s, OH), 11.91 (1H, s, SO2NH), 8.99 (1H, s, CH=N), 8.51 (2H, d, J = 4.9 Hz, H4′′, H6′′), 8.09–8.04 (2H, m, H2′, H6′), 7.88 (1H, d, J = 2.5 Hz, H4), 7.77 (1H, d, J = 2.6 Hz, H6), 7.65–7.59 (2H, m, H3′, H5′), 7.06 (1H, t, J = 4.9 Hz, H5′′). 13C-NMR: δ 164.83, 158.53, 156.96, 156.67, 150.32, 139.10, 135.71, 131.80, 129.28, 122.97, 122.15, 120.41, 115.95, 111.33. Anal. Calcd. for C17H12BrClN4O3S (467.72): C, 43.66; H, 2.59; N, 11.98. Found: C, 44.00; H, 2.61; N, 12.24.
4-[(5-Chloro-2-hydroxy-3-iodobenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2o). Orange solid; yield 92%; m.p. 265–268 °C (decomp.). IR: 3566 (O-H), 3041, 2731, 1580 (-HC=N-), 1493, 1439, 1409, 1340, 1290, 1264, 1182, 1153, 1090, 944, 868, 842, 797, 742, 701, 671 cm−1. 1H-NMR: δ 11.89 (1H, s, SO2NH), 8.93 (1H, s, CH=N), 8.51 (2H, d, J = 4.9 Hz, H4′′, H6′′), 8.10–8.03 (2H, m, H2′, H6′), 7.98 (1H, d, J = 2.5 Hz, H4), 7.77 (1H, d, J = 2.6 Hz, H6), 7.65–7.58 (2H, m, H3′, H5′), 7.06 (1H, t, J = 4.9 Hz, H5′′). 13C-NMR: δ 164.83, 159.14, 158.38, 156.96, 150.30, 141.35, 132.54, 129.96, 129.27, 123.42, 122.17, 119.24, 112.29, 87.44. Anal. Calcd. for C17H12ClIN4O3S (514.72): C, 39.67; H, 2.35; N, 10.89. Found: C, 39.89; H, 2.46; N, 10.68.
4-[(2-Hydroxy-3,5-diiodobenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2p). Red solid; yield 95%; m.p. 266–267.5 °C (decomp.). IR: 3567 (O-H), 3040, 1577 (-HC=N-), 1489, 1456, 1435, 1410, 1339, 1281, 1181, 1152, 1089, 932, 861, 833, 801, 743, 714, 662 cm−1. 1H-NMR: δ 11.90 (1H, s, SO2NH), 8.91 (1H, s, CH=N), 8.51 (2H, d, J = 4.9 Hz, H4′′, H6′′), 8.17 (1H, d, J = 2.1 Hz, H4), 8.08–8.04 (2H, m, H2′, H6′), 7.99 (1H, d, J = 2.2 Hz, H6), 7.63–7.59 (2H, m, H3′, H5′), 7.06 (1H, t, J = 4.9 Hz, H5′′). 13C-NMR: δ 164.68, 159.98, 158.52, 156.96, 150.25, 149.04, 141.51, 138.99, 129.26, 122.15, 120.76, 115.91, 88.42, 81.67. Anal. Calcd. for C17H12I2N4O3S (606.18): C, 33.68; H, 2.00; N, 9.24. Found: C, 33.47; H, 2.14; N, 8.97.
4-{[(5-Nitrofuran-2-yl)methylene]amino}-N-(pyrimidin-2-yl)benzenesulfonamide (2q). Yellowish solid; yield 85%; m.p. 207–209 °C. IR: 3419, 2875, 1600, 1577 (-HC=N-), 1533, 1501, 1489, 1444, 1408, 1354, 1340, 1300, 1240, 1159, 1094, 1022, 970, 935, 831, 814, 803, 740, 722, 673, 659 cm−1. 1H-NMR: δ 11.42 (1H, s, SO2NH), 8.49 (2H, d, J = 4.9 Hz, H4′′, H6′′), 7.78–7.74 (2H, m, H2′, H6′), 7.73–7.69 (2H, m, CH=N, furan H4), 7.02 (1H, t, J = 4.9 Hz, H5′′), 6.95–6.90 (2H, m, H3′, H5′), 6.00 (1H, d, J = 8.5 Hz, furan H3). 13C-NMR: δ 158.46, 158.39, 157.28, 155.29, 151.45, 149.68, 129.65, 128.61, 115.84, 113.75, 112.83, 112.31. Anal. Calcd. for C15H11N5O5S (373.34): C, 48.26; H, 2.97; N, 18.76. Found: C, 48.20; H, 2.83; N, 18.61.
3.2. Antimicrobial Activity
3.2.1. In Vitro Antibacterial Activity
The antibacterial activities were assayed against four Gram-positive and four Gram-negative strains: Staphylococcus aureus CCM 4516/08, methicillin-resistant Staphylococcus aureus H 5996/08 (MRSA), Staphylococcus epidermidis H 6966/08, Enterococcus faecalis J 14365/08; Escherichia coli CCM 4517, Klebsiella pneumoniae D 11750/08, extended-spectrum β-lactamase positive Klebsiella pneumoniae J 14368/08, and Pseudomonas aeruginosa CCM 1961. The microdilution broth method modified according to CLSI standard M07-A10 with Mueller-Hinton broth (HiMedia Laboratories, Mumbai, India) adjusted to pH 7.4 (±0.2) was used. The tested compounds were dissolved in DMSO to final concentrations ranging from 500 to 0.49 µM. Bacitracin (BAC) and sulfadiazine 1 were used as the reference drugs. A bacterial inoculum in sterile water was prepared to reach 0.5 according to McFarland scale (1.5 × 108 CFU/mL). The minimum inhibitory concentrations were assayed as a reduction of growth compared to the control. The results were analysed visually. The MIC values were determined after 24 and 48 h of incubation in the dark at 35 °C (±0.1) in a humid atmosphere [16].
3.2.2. In Vitro Antimycobacterial Activity
The sulfadiazine derivatives 2 were evaluated to determine their in vitro antimycobacterial activity against Mycobacterium tuberculosis 331/88 (H37Rv; the dilution of this strain was 10−3), Mycobacterium avium 330/88 (resistant to INH, rifamycins, ofloxacin, and ethambutol; dilution of 10−5), and two strains of M. kansasii, namely 235/80 (dilution of 10−4) and the clinically isolated strain 6509/96 (dilution of 10−5), using a previously described method [16]. The following concentrations were used: 1000, 500, 250, 125, 62.5, 32, 16, 8, 4, 2, and 1 μM. The MIC (reported in μM) is the lowest concentration at which the complete inhibition of mycobacterial growth was observed. The MICs were determined after incubation at 37 °C for 14 and 21 d, for M. kansasii additionally for 7 d. Isoniazid (INH) and the parent sulfadiazine 1 were chosen as the reference drugs. The inhibition of isocitrate lyase was determined according to [21].
3.2.3. In Vitro Antifungal Activity
The antifungal properties were evaluated against four Candida strains (Candida albicans ATCC 44859, Candida tropicalis 156, Candida krusei E28, and Candida glabrata 20/I), Trichosporon asahii 1188 and three strains of filamentous fungi (Aspergillus fumigatus 231, Lichtheimia corymbifera 272, and Trichophyton interdigitale 445). The microdilution broth method with slight modifications was used according to the CLSI M27-A3 and M38-A2 guidelines in RPMI 1640 with glutamine (KlinLab, Prague, Czech Republic) buffered to pH 7.0 with 0.165 mol of 3-morpholino-propane-1-sulphonic acid (Sigma-Aldrich). DMSO served as a diluent for all the compounds. In yeast, the final size of the inoculum was 5 × 103 ± 0.2 CFU/mL, and in the case of the moulds, the final size of the inoculum was 0.5–5 × 104 CFU/mL. Fluconazole (FLU) was involved as the reference drug. The MIC values were assayed as inhibition of growth compared to the control. The results were analysed visually. The MIC values were determined after 24 and 48 h of incubation in the dark at 35 °C (±0.1) in a humid atmosphere. For T. interdigitale, the final MIC values were determined after 72 and 120 h of incubation [17].
3.3. Cytotoxicity Evaluation
All of the compounds were tested to determine their cytotoxicity in the human hepatocellular liver carcinoma cell line HepG2 (passages 5–6 and 3–8; ECACC, Salisbury, UK) using a standard colorimetric method that involves measuring the reduction of a tetrazolium salt (CellTiter(R) 96 AQueous One Solution Cell Proliferation Assay; Promega, Fichtburg, MA, USA).
The cells were cultured in Minimum Essentials Eagle Medium (Sigma-Aldrich) supplemented with 10% foetal bovine serum (PAA), 1% L-glutamine solution (Sigma-Aldrich) and a non-essential amino acid solution (Sigma-Aldrich) in a humidified atmosphere containing 5% CO2 at 37 °C. The investigated compounds were dissolved in DMSO and a small volume was added to the cell culture. The tested compounds were prepared in triplicates at concentrations ranging from 1 to 1500 µM. Simultaneously, the following types of controls were also included in triplicates: determination of 100% viability and 0% viability (the cells were treated with 10% DMSO), no cell control and vehiculum controls. After 24 h incubation in a humidified atmosphere containing 5% CO2 at 37 °C, the reagent from the kit CellTiter 96 was added. After 2 h incubation at 37 °C, absorbance of samples was recorded at 490 nm (Infinita M200, TECAN, Grödig, Austria). A standard toxicological parameter IC50 was calculated by nonlinear regression from a semilogarithmic plot of incubation concentration versus percentage of absorbance relative to untreated controls using GraphPad Prism 6 software (GraphPad Software Inc., San Diego, CA, USA). IC50 is the inhibitory concentration that reduces the cell viability to 50% of the maximal (control) viability.
4. Conclusions
In conclusion, we identified several interesting structure-activity relationships in some novel sulfadiazine Schiff bases. Our findings suggested that several were selective for microbial pathogens (M. tuberculosis, M. kansasii, Staphylococcus spp. including one MRSA strain, yeasts and Trichophyton interdigitale). 4-[(2-Hydroxybenzylidene)amino]-N-(pyrimidin-2-yl)benzenesulfonamide (2a) is active in the inhibition of tuberculous and atypical mycobacteria. The 2,5-dihydroxybenzaldehyde-derived Schiff base 2i exhibited the highest selectivity for staphylococci and fungi. For these molecules, the desirable action is separated from an unwanted toxicity, thus constituting a promising hit for further structure optimization and development of potential antimicrobial agents. Further studies are required to gain a deeper understanding of their properties (especially mechanism of action and interactions with eukaryotic cells).
Acknowledgments
This work was supported by the Czech Science Foundation project No. 17-27514Y and the Charles University (Project SVV 260 414). The authors also thank the COST action CA15135 (Multitarget Paradigm for Innovative Ligand Identification in the Drug Discovery Process MuTaLig) for support. We would like to thank Ida Dufková for the excellent performance of antibiotic susceptibility tests, the staff of the Department of Organic and Bioorganic Chemistry, Faculty of Pharmacy, for the technical assistance and J. Urbanová, M.A., for the language help provided.
Author Contributions
This study was performed under the guidance of M.K. and J.V., they designed experiments. M.K. and M.D. synthesized and characterized the presented compounds. K.K. and J.S. were responsible for the antimicrobial evaluation, J.J. and F.T. for the cytotoxicity determination. M.K. and J.V. wrote the whole article, made discussion and conclusions.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1, 2a–q are available from the authors.
References
- 1.Da Silva C.M., da Silva D.L., Modolo L.V., Alves R.B., de Resende M.A., Martins C.V.B., de Fátima A. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011;2:1–8. doi: 10.1016/j.jare.2010.05.004. [DOI] [Google Scholar]
- 2.Qin W., Long S., Panunzio M., Biondi S. Schiff Bases: A short survey on an evergreen chemistry tool. Molecules. 2013;18:12264–12289. doi: 10.3390/molecules181012264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kajal A., Bala S., Kamboj S., Sharma N., Saini V. Schiff bases: A versatile pharmacophore. J. Catal. 2013;2013 doi: 10.1155/2013/893512. [DOI] [Google Scholar]
- 4.Krátký M., Vinšová J., Volková M., Buchta V., Trejtnar F., Stolaříková J. Antimicrobial activity of sulfonamides containing 5-chloro-2-hydroxybenzaldehyde and 5-chloro-2-hydroxybenzoic acid scaffold. Eur. J. Med. Chem. 2012;50:433–440. doi: 10.1016/j.ejmech.2012.01.060. [DOI] [PubMed] [Google Scholar]
- 5.Yıldız M., Tan E., Demir N., Yıldırım N., Ünver H., Kiraz A., Mestav B. Synthesis and spectral, antimicrobial, anion sensing, and DNA binding properties of Schiff base podands and their metal complexes. Russ. J. Gen. Chem. 2015;85:2149–2162. doi: 10.1134/S1070363215090200. [DOI] [Google Scholar]
- 6.Shi L., Ge M.M., Tan S.H., Li H.Q., Song Y.C., Zhu H.L., Tan R.X. Synthesis and antimicrobial activities of Schiff bases derived from 5-chloro-salicylaldehyde. Eur. J. Med. Chem. 2007;42:558–564. doi: 10.1016/j.ejmech.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Govindaraj V., Ramanathan S. Synthesis, spectral characterisation, electrochemical, and fluorescence studies of biologically active novel Schiff base complexes derived from E-4-(2-hydroxy-3-methoxybenzlideneamino)-N-(pyrimidin-2-yl)benzenesulfonamide. Turk. J. Chem. 2014;38:521–530. doi: 10.3906/kim-1301-83. [DOI] [Google Scholar]
- 8.Mondal S., Mandal S.M., Mondal T.K., Sinha C. Spectroscopic characterization, antimicrobial activity, DFT computation and docking studies of sulfonamide Schiff bases. J. Mol. Struct. 2017;1127:557–567. doi: 10.1016/j.molstruc.2016.08.011. [DOI] [Google Scholar]
- 9.Chohan Z.H., Youssoufi M.H., Jarrahpour A., Hadda T.B. Identification of antibacterial and antifungal pharmacophore sites for potent bacteria and fungi inhibition: Indolenyl sulfonamide derivatives. Eur. J. Med. Chem. 2010;45:1189–1199. doi: 10.1016/j.ejmech.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 10.Ameen S.M., Drancourt M. In Vitro susceptibility of Mycobacterium tuberculosis to trimethoprim and sulfonamides in France. Antimicrob. Agents Chemother. 2013;57:6370–6371. doi: 10.1128/AAC.01683-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ameen S.M., Drancourt M. In vitro susceptibility of Mycobacterium avium complex mycobacteria to trimethoprim and sulfonamides. Int. J. Antimicrob. Agents. 2013;42:281–288. doi: 10.1016/j.ijantimicag.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 12.El-Baradie K.Y. Preparation and characterization of sulfadiazine Schiff base complexes of Co(II), Ni(II), Cu(II), and Mn(II) Monatshefte Chem. 2005;136:1139–1155. doi: 10.1007/s00706-004-0257-8. [DOI] [Google Scholar]
- 13.Chohan Z.H., Shad H.A., Youssoufi M.H., Hadda T.B. Some new biologically active metal-based sulphonamide. Eur. J. Med. Chem. 2010;45:2893–2901. doi: 10.1016/j.ejmech.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Aal W.S., Hassan H.Y., Aboul-Fadl T., Youssef A.F. Pharmacophoric model building for antitubercular activity of the individual Schiff bases of small combinatorial library. Eur. J. Med. Chem. 2010;45:1098–1106. doi: 10.1016/j.ejmech.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Zorzi R.R., Jorge S.D., Palace-Berl F., Pasqualoto K.F.M., de Sa Bortolozzo L., de Castro Siqueira A.M., Tavares L.C. Exploring 5-nitrofuran derivatives against nosocomial pathogens: Synthesis, antimicrobial activity and chemometric analysis. Bioorg. Med. Chem. 2014;22:2844–2854. doi: 10.1016/j.bmc.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 16.Krátký M., Vinšová J., Novotná E., Mandíková J., Trejtnar F., Stolaříková J. Antibacterial activity of salicylanilide 4-(Trifluoromethyl)benzoates. Molecules. 2013;18:3674–3688. doi: 10.3390/molecules18043674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krátký M., Vinšová J. Antifungal activity of salicylanilides and their esters with 4-(Trifluoromethyl)benzoic acid. Molecules. 2012;17:9426–9442. doi: 10.3390/molecules17089426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sriram D., Yogeeswari P., Dhakla P., Senthilkumar P., Banerjee D., Manjashetty T.H. 5-Nitrofuran-2-yl derivatives: Synthesis and inhibitory activities against growing and dormant mycobacterium species. Bioorg. Med. Chem. Lett. 2009;19:1152–1154. doi: 10.1016/j.bmcl.2008.12.088. [DOI] [PubMed] [Google Scholar]
- 19.Castle R.N., Witt N.F., Poe C.F. The optical crystallographic properties of some sulfonamides and their derivatives. J. Am. Chem. Soc. 1949;71:228–231. doi: 10.1021/ja01169a059. [DOI] [PubMed] [Google Scholar]
- 20.Tsukamoto T., Yuhi K. Studies on halogenosalicylaldehyde. I halogenosalicylaldehyde as reagent for primary amines. Yakugaku Zasshi. 1958;78:706–709. doi: 10.1248/yakushi1947.78.7_706. [DOI] [Google Scholar]
- 21.Krátký M., Vinšová J., Novotná E., Wsól V., Ulmann V., Stolaříková J., Fernandes S., Bhat S., Liu J.O. Salicylanilide derivatives block Mycobacterium tuberculosis through inhibition of isocitrate lyase and methionine aminopeptidase. Tuberculosis (Edinb.) 2012;92:434–439. doi: 10.1016/j.tube.2012.06.001. [DOI] [PubMed] [Google Scholar]