 A series of fifteen novel dihydropyrimidinone hybrid compounds were synthesized in good yields via a multicomponent reaction combined with the Huisgen reaction.
A series of fifteen novel dihydropyrimidinone hybrid compounds were synthesized in good yields via a multicomponent reaction combined with the Huisgen reaction.
Abstract
A series of fifteen novel dihydropyrimidinone hybrid compounds were synthesized in good yields via a multicomponent reaction combined with the Huisgen reaction. The antiproliferative activity was investigated against nine tumor cell lines, and four hybrid compounds (TGI < 10 μM) showed promising antiproliferative activity against the tumor cell lines OVCAR-3 (ovarian), UACC-62 (melanoma) and U251 (glioma). Several hybrid compounds assayed have high TGI values (TGI 147.92–507.82) for the human keratinocyte cell line (HaCat), which reveals selectivity to cancer cells.
Introduction
Multifunctional drugs and hybrid compounds
The modern pharmaceutical industry has faced unprecedented challenges regarding the development of new drugs. Although the amount of funds spent in scientific research has doubled since 1991, the approval of new drugs decreased by around 50% over the same period. While most diseases involve multiple pathogenic factors, the decline in new drug development can be attributed in part to the “one drug and one target paradigm”, which defines the development of a specific drug for each target.1 Additionally, many traditional treatments for multifactorial diseases, using a single drug, have proven to be inefficient because of the inability of the drug to act at different and specific sites inside the body.2 Alternatively, the use of drug cocktails represents a breakthrough. However, several drawbacks associated with undesired side effects and low patient compliance prevent their use as a standard protocol.3
To circumvent this problem, a new concept for drugs has been postulated in which the objective is to create a “single chemical entity” with multiple associated biological activities, as exemplified by a pioneer work in the treatment of neurodegenerative disorders.4 In searching for these structures, the goal is to find molecules that can act on various targets or molecular receptors, and this process is called “one-compound-multiple-targets strategy”.5 Molecules that are able to act on more than one molecular target are called “multifunctional compounds” (MFCs) and have brought promising results in terms of improving the therapeutic potential with synergistic effects and minimizing side effects. It is also possible to highlight the lower risk of drug–drug interaction in relation to drug cocktails.6
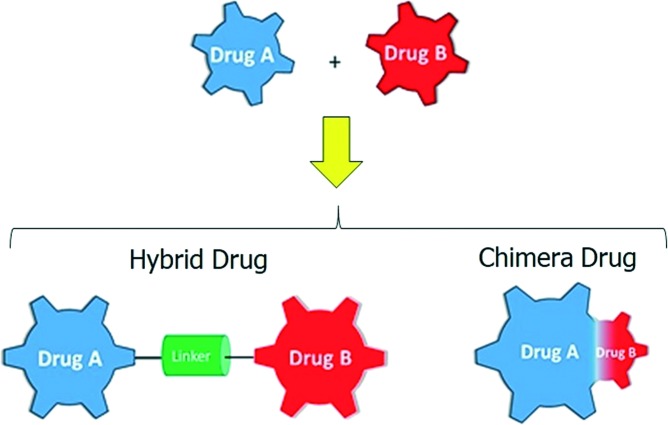
To have access to such compounds, it is necessary to use strategies able to join two structures with different activities in a single chemical entity. The MFCs are classified as hybrid drugs when two or more drugs with different activities are linked through a stable or metabolizable connection (linker). In this case, the chemical structures of the original molecules remain essentially the same. On the other hand, the MCFs are designed as chimeric drugs when the new chemical entity is formed by the fusion of two or more pharmacophores from different molecules, which hold only parts of the original parent structures (Fig. 1).7
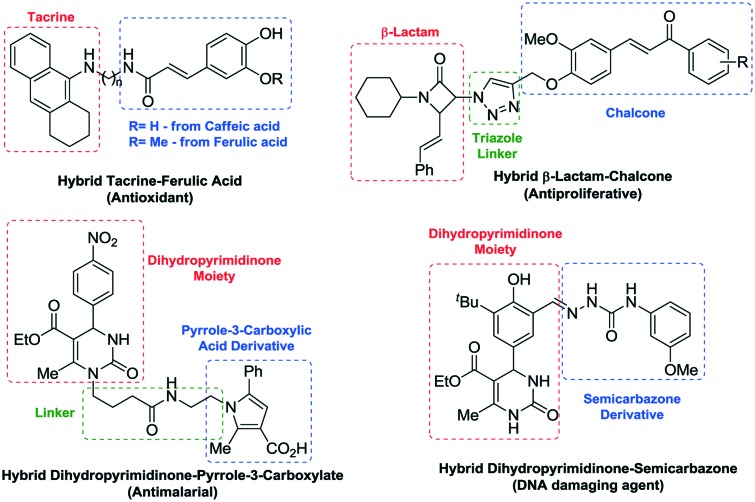
Fig. 1. Picture definition of hybrid drugs and chimera drugs.
Although both types of MFCs (hybrids and chimeras) have their advantages and disadvantages, the central idea of the association of two pharmacophores is to increase the potency of both and/or reduce their dosage.8 Recent reports in the literature have proven the success of hybridization of several drugs, improving the characteristics of each original compound. For example, tacrine was the first drug to combat Alzheimer's disease (AD) by the inhibition of acetylcholinesterase enzyme (AChE). However, poor selectivity and hepatotoxicity were observed as injurious collateral effects due to oxidative stress.9 The hybridization of tacrine with an antioxidant compound such as ferulic or caffeic acid led to new hybrid compounds with an increase in antioxidant properties and a high selectivity for the inhibition of AChE. Additionally, the hybrid compounds have been found to inhibit AChE-induced αβ aggregation inhibitory activity.10
This strategy was also successfully applied in the field of anticancer agents.11 A synergistic effect can be achieved when the two pattern molecules have similar effects. The biologically active β-lactams and chalcones, used to form hybrid compounds via formation of a 1,2,3-triazole linker, showed antiproliferative activity against A-549 (lung), THP-1 (leukemia) and Caco-2 tumor cell lines at low concentrations (Fig. 2).12
Fig. 2. Biologically active hybrid compounds.
The dihydropyrimidinones were also investigated as a component of new hybrid compounds. The hybrid dihydropyrimidinone-pyrrole-3-carboxylate is a promising antimalarial compound due to its high inhibitory activity on the Hsp70 molecular chaperones of Plasmodium falciparum (malaria). The chaperones are a family of proteins that prevent a wrong folding of a protein, helping to maintain its functional structure. Additionally, it induces protein destruction if the correct conformation cannot be achieved.13 Another example is the hybrid dihydropyrimidinone–semicarbazone, which was found to be a highly selective human DNA ligase 1 inhibitor (hLig1) and a promising prototype as a hybrid anticancer compound (see Fig. 2).14
Dihydropyrimidinones, perillyl alcohol and antiproliferative activities
The dihydropyrimidinones (DHPMs) are recognized as effective bioactive agents with a large scope of pharmacological activities, including anticancer properties.15
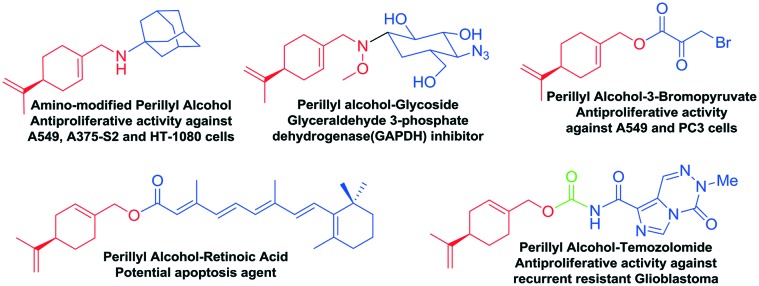
Monastrol,16 dimethylenastron17 and piperastrol18 are three notorious members of this extensive class of bioactive heterocycles and their antiproliferative activity against cancer cell lines has been established (Fig. 3). Their antiproliferative activities were attributed to their ability to inhibit the enzyme Eg5 kinesin, which is responsible for the mobility of organelles during the mitotic cycle of cell division. Inhibition of this enzyme blocks the formation of the bipolar spindle and causes disruption in the cell cycle.19 Armed with this background, we chose the dihydropyrimidinone scaffold as the constitutive half-part of the new hybrid compounds to be synthesized. On the other hand, the other part of the hybrid molecule was chosen based on the antiproliferative activities exhibited by perillyl alcohol.20 Perillyl alcohol is a naturally occurring monocyclic terpene found in the essential oils extracted from lavender, lemongrass, and peppermint, among others. Its antitumoral properties have been extensively studied in the past decades, being an active compound against various cancer cell lines, such as pancreatic,21 liver,22 melanoma,23 colon,24 and leukemia,25 with remarkable activity against gliomas.26 Furthermore, perillyl alcohol has been used clinically in nasal spray applications to minimize the injurious effects of recurrent malignant glioma.27 However, there are few reports of the synthesis and applications of hybrid compounds based on the perillyl moiety (Fig. 4).28
Fig. 3. Antiproliferative dihydropyrimidinones.
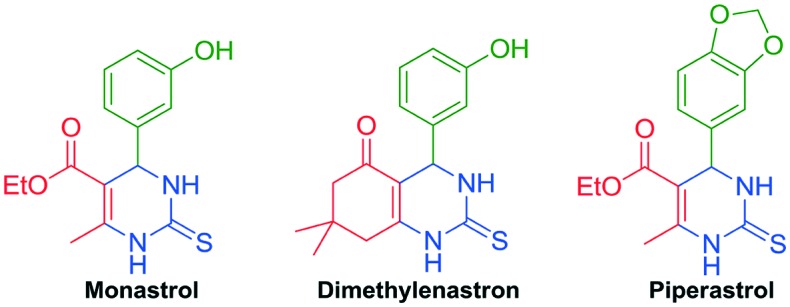
Fig. 4. Hybrid compounds from perillyl alcohol.
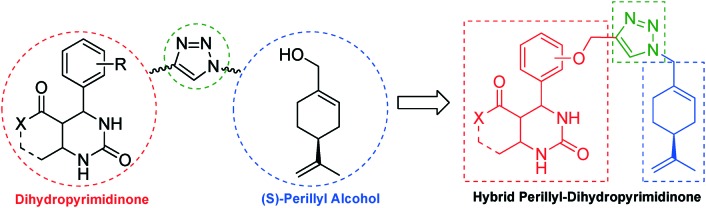
Thus, the present study reports the synthesis and the cytotoxic activities of a series of novel hybrid compounds derived from dihydropyrimidinones and (S)-perillyl alcohol against a set of tumour cell lines. The hybridization process was performed using the Huisgen reaction to create a stable 1,2,3-triazolyl linker (Fig. 5).29
Fig. 5. Strategy to hybridize DHPMs and perillyl alcohol via a 1,2,3-triazolyl linker.
Results and discussion
Synthesis of propargyl dihydropyrimidinones
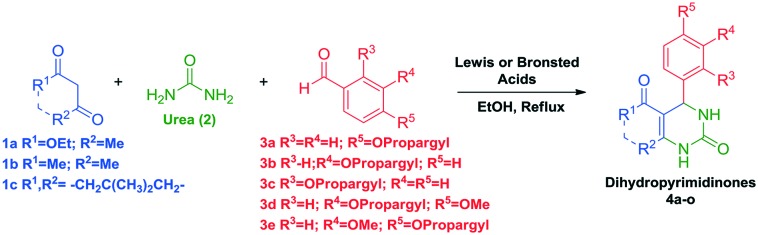
The synthesis of dihydropyrimidinones 4a–o was accomplished via the multicomponent Biginelli reaction through a cyclocondensation of ethyl acetoacetate 1a, acetylacetone 1b or dimedone 1c as the 1,3-dicarbonyl compounds, urea (2), and a series of propargyloxy benzaldehydes 3a–e under Brønsted or Lewis acids catalysis30 (Scheme 1 and Table 1). The aldehydes were easily prepared in good yields by O-alkylation reactions from the corresponding hydroxy-substituted benzaldehydes and propargyl bromide.31
Scheme 1. Synthesis of propargyloxy dihydropyrimidinones.
Table 1. Synthesis of propargyloxy dihydropyrimidinones.
| 1 | 3 | DHPM |
Y (%) | |||||
| R1 | R2 | R3 | R4 | R5 | ||||
| 1a | 3a | 4a | EtO | Me | H | H | OPg a | 90 |
| 1a | 3b | 4b | EtO | Me | H | OPg a | H | 75 |
| 1a | 3c | 4c | EtO | Me | OPg a | H | H | 83 |
| 1a | 3d | 4d | EtO | Me | H | OPg a | OMe | 86 |
| 1a | 3e | 4e | EtO | Me | H | OMe | OPg a | 75 |
| 1b | 3a | 4f | Me | Me | H | H | OPg a | 69 |
| 1b | 3b | 4g | Me | Me | H | OPg a | H | 67 |
| 1b | 3c | 4h | Me | Me | OPg a | H | H | 67 |
| 1b | 3d | 4i | Me | Me | H | OPg a | OMe | 62 |
| 1b | 3e | 4j | Me | Me | H | OMe | OPg a | 64 |
| 1c | 3a | 4k | –CH2C(CH3)2CH2– | H | H | OPg a | 67 | |
| 1c | 3b | 4l | –CH2C(CH3)2CH2– | H | OPg a | H | 74 | |
| 1c | 3c | 4m | –CH2C(CH3)2CH2– | OPg a | H | H | 70 | |
| 1c | 3d | 4n | –CH2C(CH3)2CH2– | H | OPg a | OMe | 72 | |
| 1c | 3e | 4o | –CH2C(CH3)2CH2– | H | OMe | OPg a | 81 | |
aOPg = OCH2CCH.
The propargyloxy dihydropyrimidinones were purified through column chromatography on silica gel (4a–j) or simple crystallization (4k–o). All compounds were fully characterized by conventional spectroscopy methods including HRMS for the novel compounds. The data were in accordance with the proposed structures. The broad singlets at 9.20 ppm and 7.70 ppm assigned to N–H groups are the main 1H NMR spectroscopic evidence of the DHPM formation. The singlet around 5.10 ppm assigned to benzylic hydrogen also corroborates the formation of the DHPM ring.
Synthesis of perillyl–dihydropyrimidinone hybrids
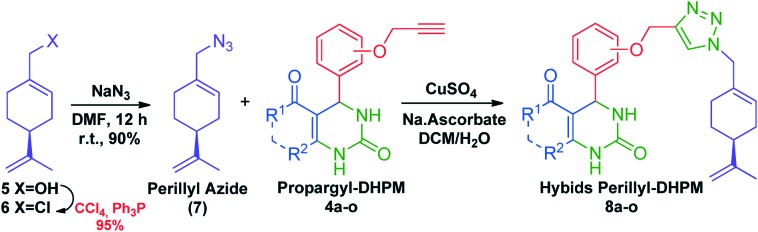
The chosen strategy to hybridize molecules was the regioselective copper(i)-catalyzed [3 + 2] cycloaddition of alkynes and azide compounds.32 Thus, to perform the molecular hybridization with propargyloxy dihydropyrimidinones 4a–o, perillyl azide 7 was required. It was not possible to prepare the azido compound from perillyl alcohol mesylate. Therefore, perillyl alcohol (5) was converted to perillyl chloride (6) via Appel's reaction,33 and then it was transformed into the corresponding perillyl azide (7). The overall yield for these two steps was 85%.
With the propargyloxy dihydropyrimidinones 4a–o and perillyl azide (7) in hand, we proceeded to the synthesis of the hybrids perillyl–dihydropyrimidinone 8a–o through the copper-catalyzed Huisgen reaction. (Scheme 2). The results for the synthesis of the perillyl–DHPM hybrids are shown in Table 2.
Scheme 2. Synthesis of perillyl azide and the Cu(i)-catalyzed Huisgen reaction.
Table 2. Synthesis of hybrids perillyl–dihydropyrimidinones.

| |||||||
| Entry | Perillyl–DHPM hybrids |
Y (%) | |||||
| R1 | R2 | R3 | R4 | R5 | |||
| 1 | 8a | EtO | Me | H | H | Xc* | 90 |
| 2 | 8b | EtO | Me | H | Xc* | H | 79 |
| 3 | 8c | EtO | Me | Xc* | H | H | 85 |
| 4 | 8d | EtO | Me | H | Xc* | OMe | 73 |
| 5 | 8e | EtO | Me | H | OMe | Xc* | 71 |
| 6 | 8f | Me | Me | H | H | Xc* | 72 |
| 7 | 8g | Me | Me | H | Xc* | H | 71 |
| 8 | 8h | Me | Me | Xc* | H | H | 68 |
| 9 | 8i | Me | Me | H | Xc* | OMe | 67 |
| 10 | 8j | Me | Me | H | OMe | Xc* | 76 |
| 11 | 8k | –CH2C(CH3)2CH2– | H | H | Xc* | 84 | |
| 12 | 8l | –CH2C(CH3)2CH2– | H | Xc* | H | 68 | |
| 13 | 8m | –CH2C(CH3)2CH2– | Xc* | H | H | 80 | |
| 14 | 8n | –CH2C(CH3)2CH2– | H | Xc* | OMe | 71 | |
| 15 | 8o | –CH2C(CH3)2CH2– | H | OMe | Xc* | 79 | |
The hybrids perillyl–dihydropyrimidinones 8a–o were purified through column chromatography on silica gel. The yields were calculated after the isolation of the pure products. All compounds were fully characterized by conventional spectroscopic methods including HRMS for the novel compounds and the data were compatible with the proposed structures (Table 2).
The appearance of a low field singlet (7.40–8.20 ppm) assigned to the triazolic hydrogen along with characteristic signals of DHPM and the terpene ring in the 1H NMR spectra corroborate the obtention of the desired compounds. All spectroscopic data were compatible with the proposed structures.
Next, the fifteen synthesized new hybrid molecules were screened for their antiproliferative activities against nine different tumor cell lines using total growth inhibition (TGI) as a parameter of the antiproliferative activity.
In vitro antiproliferative activity of hybrid compounds
In vitro studies of cytotoxicity are widely used in the initial drug screening for anticancer drug development.34 Furthermore, these studies are important to identify whether the tested substance interferes with either cell metabolism or cell survival, and this can be indicative of the mechanism of action by its comparison with known chemotherapeutics.
The antiproliferative activities of hybrid compounds 8a–o were evaluated against the human cancer cell lines UACC-62 (melanoma), U251 (glioma), MCF7 (breast), NCI/ADR-RES (multidrug resistant breast), 786-0 (kidney), NCI-H460 (lung), PC-3 (prostate), OVCAR-3 (ovarian), and HT29 (colon). The TGI values for HaCaT (normal human keratinocytes) were evaluated to determine the action against the non-tumoral cells. Doxorubicin (DOX) was employed as the positive control (Table 3).
Table 3. In vitro antiproliferative activity (TGI) a of hybrid compounds 8a–o.
| Hybrid | UACC-62 | U251 | MCF7 | NCI/ADR-RES | 786-0 | NCI-H460 | PC-3 | OVCAR-3 | HT29 | HaCaT |
| 8a | 19.18 | 12.43 | 43.14 | >100 | 97.08 | >100 | 92.21 | 6.90 | 77.13 | 165.69 |
| 8b | 22.92 | 12.19 | 42.52 | >100 | 39.67 | >100 | >100 | 18.45 | 73.42 | 507.82 |
| 8c | >100 | >100 | >100 | >100 | >100 | >100 | >100 | 81.19 | >100 | 294.37 |
| 8d | 37.75 | 98.09 | >100 | >100 | >100 | >100 | >100 | 13.45 | >100 | 79.75 |
| 8e | 26.73 | 11.34 | 82.50 | >100 | 87.34 | >100 | 54.39 | 53.76 | >100 | 75.76 |
| 8f | 20.50 | 19.99 | 28.97 | >100 | 23.26 | 78.17 | 49.27 | 32.65 | 62.63 | 44.47 |
| 8g | 31.56 | 30.52 | 42.96 | >100 | 44.08 | >100 | 49.76 | 13.33 | 86.22 | 115.66 |
| 8h | 34.23 | 30.21 | 68.70 | >100 | 80.31 | 83.14 | 62.01 | 34.31 | 79.55 | 29.51 |
| 8i | >100 | >100 | >100 | 84.90 | >100 | >100 | >100 | >100 | 95.99 | 486.19 |
| 8j | 3.83 | 77.91 | 53.26 | 80.17 | 88.09 | >100 | 85.69 | 48.03 | 94.95 | 147.92 |
| 8k | 29.94 | 6.61 | >100 | >100 | 53.16 | >100 | 23.98 | 29.34 | >100 | 436.18 |
| 8l | 20.92 | 25.97 | 30.97 | >100 | >100 | 51.61 | 21.87 | >100 | 48.99 | 36.44 |
| 8m | 26.47 | 26.98 | 44.95 | >100 | 26.67 | 38.72 | 21.68 | 11.81 | 28.95 | 78.72 |
| 8n | 62.01 | 8.28 | 44.81 | >100 | >100 | >100 | 79.92 | >100 | >100 | 451.02 |
| 8o | 86.16 | 44.04 | 91.94 | >100 | >100 | >100 | >100 | 94.99 | >100 | 71.34 |
| Dox | 1.66 | 0.09 | 0.99 | 0.78 | 0.02 | 0.46 | 15.69 | 0.04 | 0.88 | 1.47 |
aThe concentration that elicits total growth inhibition (TGI, in μM) was determined from a non-linear regression analysis using ORIGIN 8.0® (OriginLab Corporation). The experiments were conducted in triplicate. Dox: doxorubicin, positive control.
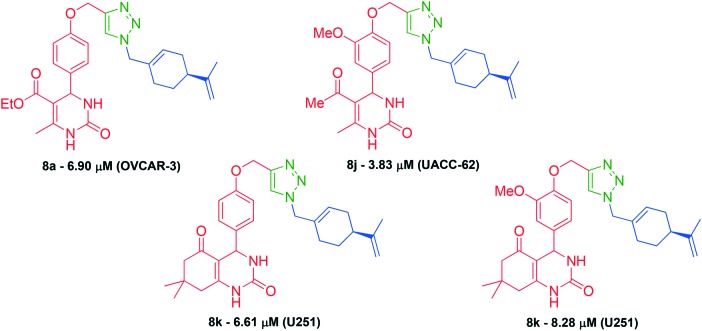
The TGI (concentration that produces total growth inhibition, or cytostatic effect) was determined through a non-linear regression analysis. The values presented in Table 2 were obtained from the tumor cell lines mentioned above. After careful analysis, we were able to identify four compounds with TGI values of less than 10 μM. Compound 8a exhibited a TGI value of 6.90 μM for the OVCAR-3 tumor cell line, while the hybrid 8j showed a TGI value of 3.83 μM for the UACC-62 cell line. Hybrids 8k and 8n displayed TGI values of 6.62 μM and 8.28 μM, respectively, both for the glioblastoma multiforme (U256) tumor cell line (Fig. 6).
Fig. 6. The most potent perillyl–dihydropyrimidinone hybrids.
Additionally, a series of seven hybrid compounds revealed TGI values ranging between 10 and 20 μM, being considered promising compounds: 8a (UACC-62 and U251), 8b (U251 and OVCAR-3), 8d (OVCAR-3), 8e (U251), 8f (U251), 8g (OVCAR-3) and 8m (OVCAR-3). It is interesting to note that these compounds were mainly selective to three tumour cell lines. For UACC-62, hybrid compounds 8a and 8j were the most active, while compounds 8a, 8b, 8e, 8f, 8k, 8n were more active for U251. For the OVCAR cell line, compounds 8a, 8b, 8d, 8g and 8m showed the best activities. Thus, U251 and OVCAR-3 were the tumour cell lines that showed greater sensitivity for most compounds.
Compounds 8b, 8i, 8k and 8n showed high TGI values for HaCaT. Noteworthy are compounds 8b, 8k and 8n with antiproliferative activity against tumor cell line U251 and a TGI value for HaCaT of 507.82 μM, 436.18 μM and 451.02 μM, respectively. The values of the HaCaT/U251 ratio for compounds 8b, 8k and 8n are 41.66, 65.98 and 54.47, respectively, and makes evident the high selectivity of these compounds relative to the U251 tumor cell line.
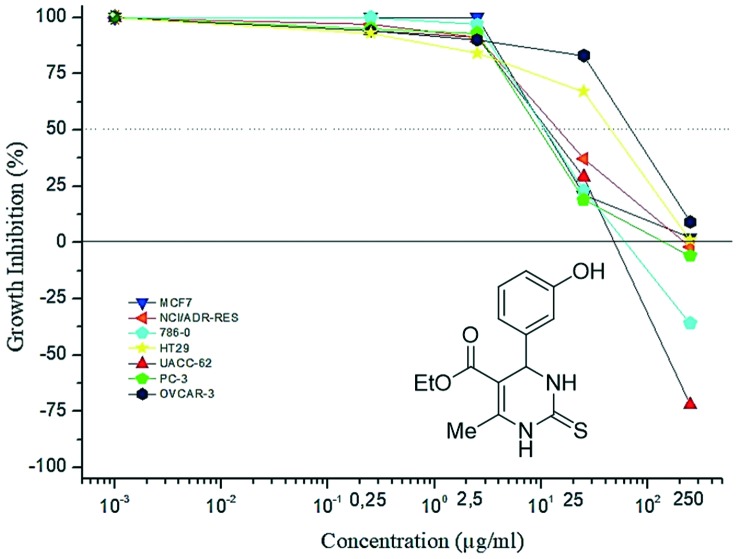
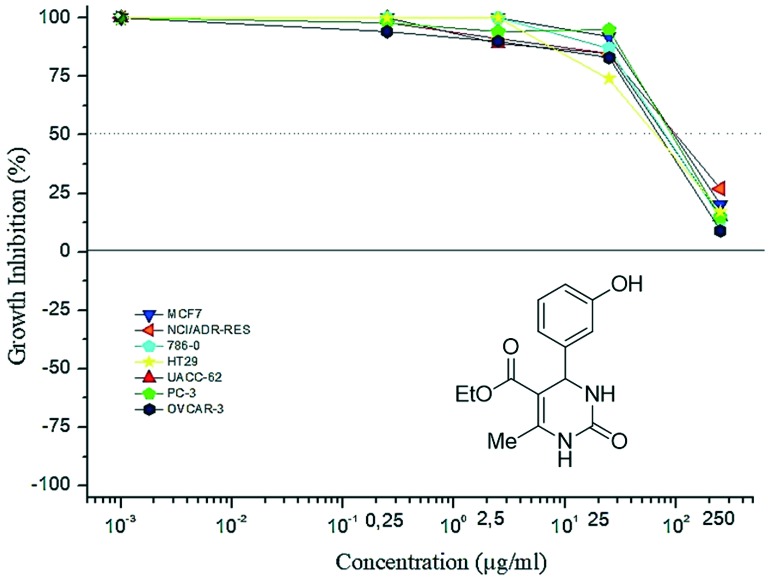
Previous comparative studies of monastrol and oxo-monastrol regarding the antiproliferative activities,18 indicated that thio-derivatives were always cytotoxic while the oxo-analogues were cytostatic. One of the main conclusions from this initial study was the influence of the sulfur atom in monastrol on the potency of antiproliferative activity. Comparison between monastrol and oxo-monastrol showed a significant difference in growth inhibition profiles. For example, monastrol was more potent than oxo-monastrol at a concentration of 25 μM (Fig. 7 and 8, respectively).
Fig. 7. Profile of monastrol against different tumor cell lines.
Fig. 8. Profile of oxo-monastrol against different tumor cell lines.
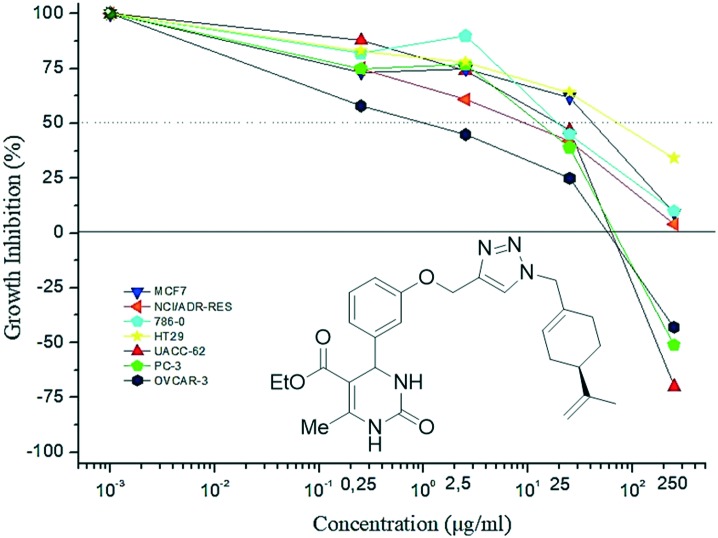
Although the activity of thio-dihydropyrimidinone hybrids were not evaluated in the present study, the results obtained for perillyl–dihydropyrimidinone 8b (an oxo-derivative) showed a growth inhibition profile similar to that of monastrol (thio-derivative) rather than to oxo-monastrol, at the concentration of 25 μM (Fig. 9). Thus, the presence of the perillyl group in the hybrid structures may have brought about an increase in the antiproliferative potencies of oxo-derivative 8b as well as other perillyl–dihydropyrimidinone hybrids described herein.
Fig. 9. Profile of hybrid 8b against different tumor cell lines.
The antiproliferative activity of these DHPM hybrids against glioblastomas was not entirely surprising. Early studies conducted by our research group showed that the DHPMs were active against human U138-MG and rat-C6 tumour cell lines.35 In another study, it was demonstrated that DHPM was able to inhibit ecto-5′-nucleotidase/CD73,36 an ecto-enzyme responsible for hydrolysing the nucleoside monophosphates such as AMP to adenosine.37 More recently, hybrids of monastrol–fatty acids exhibited antiproliferative activity against glioblastomas,38 and the Hantzsch dihydropyridine–fatty acid hybrids showed similar properties.39
Experimental section
Chemistry
General procedure for synthesis of propargyloxy benzaldehydes 3a–e
A mixture of hydroxy aldehydes (5.0 mmol), propargyl bromide (10 mmol), potassium carbonate (10 mmol) and acetone (50 mL) was stirred under reflux for 1–4 h, monitored by TLC. Then, the crude mixture was filtered and the solvent was evaporated under vacuum to yield the propargyloxy benzaldehydes 3a–e with a satisfactory level of purity. The characterization of compound 3a–e was confirmed by comparison with reported data. The compounds 3a–e were used in the next step without further purification.
4-(Prop-2-yn-1-yloxy)benzaldehyde (3a)40
Yield 95%; yellow solid; m.p. 71 °C; 1H NMR (400 MHz, CDCl3): δ 9.90 (s, 1H); 7.86 (d, 2H, J = 8.8 Hz); 7.09 (d, 2H, J = 8.6 Hz); 4.78 (d, 2H, J = 2.5 Hz); 2.58 (t, 1H, J = 2.4 Hz); 13C NMR (75 MHz, CDCl3): δ 190.8; 162.3; 131.9; 130.5; 115.1; 77.5; 76.4; 55.9; IR (νmax cm–1): 3413, 3214, 2834, 2744, 2115, 1686, 1606, 1256, 1160, 1003, 827.
3-(Prop-2-yn-1-yloxy)benzaldehyde (3b)40
Yield 85%; colorless liquid; 1H NMR (400 MHz, CDCl3): δ 9.98 (s, 1H); 7.53–7.46 (m, 3H); 7.23 (ddd, 1H, J = 7.8; 2.8 and 1.5 Hz); 4.76 (d, 2H, J = 2.5 Hz); 2.56 (t, 1H, J = 2.5 Hz); 13C NMR (100 MHz, CDCl3): δ 191.7; 157.9; 137.6; 130.0; 123.9; 121.9; 113.4; 77.8; 76.1; 55.8; IR (νmax cm–1): 3284, 2836, 2740, 2123, 1701, 1588, 1266, 1246, 1038, 791, 681.
2-(Prop-2-yn-1-yloxy)benzaldehyde (3c)40
Yield 95%; yellow solid; m.p. 66 °C; 1H NMR (400 MHz, CDCl3): δ 10.50 (s, 1H); 7.87 (dd, 1H, J = 7.6 and 2.0 Hz); 7.60–7.56 (m, 1H); 7.14–7.08 (m, 2H); 4.85 (d, 2H, J = 2.3 Hz); 2.57 (t, 1H, J = 2.3 Hz); 13C NMR (100 MHz, CDCl3): δ 189.4; 160.0; 135.6; 128.4; 125.4; 121.6; 113.1; 77.6; 76.4; 56.3; IR (νmax cm–1): 3266, 2879, 2119, 1686, 1599, 1478, 1458, 1227, 758.
4-Methoxy-3-(prop-2-yn-1-yloxy)benzaldehyde (3d)41
Yield 97%; yellow solid; m.p. 71 °C; 1H NMR (400 MHz, CDCl3): δ 9.86 (s, 1H); 7.55–7.51 (m, 2H); 7.01 (d, 1H, J = 8.1 Hz); 4.82 (d, 2H, J = 2.3 Hz); 3.96 (s, 3H); 2.55 (t, 1H, J = 2.5 Hz); 13C NMR (100 MHz, CDCl3): δ 190.6; 154.8; 147.2; 129.8; 127.2; 111.9; 110.8; 77.6; 76.4; 56.5; 56.1; IR (νmax cm–1): 3225, 2936, 2836, 2122, 1669, 1588, 1508, 1438, 1257, 1126, 1015, 714, 634.
3-Methoxy-4-(prop-2-yn-1-yloxy)benzaldehyde (3e)40
Yield 96%; yellow solid; m.p. 88 °C; 1H NMR (400 MHz, CDCl3): δ 9.86 (s, 1H); 7.45 (dd, 1H, J = 2.0 and 8.1 Hz); 7.43 (d, 1H, J = 1.8 Hz); 7.14 (d, 1H, J = 8.3 Hz); 4.85 (d, 2H, J = 2.4 Hz); 3.93 (s, 3H); 2.56 (t, 1H, J = 2.4 Hz); 13C NMR (75 MHz, CDCl3): δ 190.8; 152.0; 150.0; 130.8; 126.1; 112.5; 109.4; 77.4; 76.6; 56.5; 55.9; IR (νmax cm–1): 3245, 3006, 2845, 2121, 1685, 1584, 1504, 1263, 1132, 1001, 800, 740.
General procedure for the synthesis of propargyloxy DHPMs 4a–j
Aldehyde (2.0 mmol), urea (2.4 mmol), 1,3-dicarbonyl compound (2.0 mmol), CeCl3·7H2O (20 mol%) and ethanol (2 mL) were stirred under reflux conditions for 3–6 h, monitored by TLC. Then, the solvent was evaporated under vacuum and the crude mixture was purified by chromatography in a column of silica gel using hexane–ethyl acetate as eluent to isolate the desired DHPMs 4a–j in pure form. The characterization of compound 4a was confirmed by comparison with reported data.
Ethyl 6-methyl-2-oxo-4-[4-(prop-2-yn-1-yloxy)phenyl]-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4a)42
Yield 75%; white solid; m.p. 153 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.18 (br s, 1H); 7.69 (br s, 1H); 7.15 (d, 2H, J = 8.8 Hz); 6.92 (d, 2H, J = 8.8 Hz); 5.08 (d, 1H, J = 3.3 Hz); 4.76 (d, 2H, J = 2.3 Hz); 3.98 (q, 2H, J = 7.0 Hz); 3.55 (t, 1H, J = 2.3 Hz); 2.24 (s, 3H); 1.10 (t, 3H, J = 7.0 Hz); 13C NMR (75 MHz, DMSO-d6): δ 165.8; 156.8; 152.6; 148.6; 138.3; 127.8; 115.1; 99.9; 79.8; 78.6; 59.7; 55.8; 53.8; 18.2; 14.6; IR (νmax cm–1): 3276, 3247, 3123, 2978, 2116, 1707, 1655, 1224, 1100, 790, 645; HRMS calc. or [C17H18N2O4 + Na]: 337.1159; found: 337.1152.
Ethyl 6-methyl-2-oxo-4-[3-(prop-2-yn-1-yloxy)phenyl]-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4b)
Yield 90%; white solid; m.p. 156 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.21 (br s, 1H); 7.75 (br s, 1H); 7.26 (t, 1H, J = 7.8 Hz); 6.90–6.81 (m, 3H); 5.11 (d, 1H, J = 3.3 Hz); 4.75 (d, 2H, J = 2.3 Hz); 4.00 (q, 2H, J = 7.3 Hz); 3.55 (t, 1H, J = 2.5 Hz); 2.24 (s, 3H); 1.11 (t, 3H, J = 7.0 Hz); 13C NMR (100 MHz, DMSO-d6): δ 165.8; 157.7; 152.7; 149.0; 146.8; 130.0; 119.5; 113.8; 113.4; 99.6; 79.6; 78.6; 59.8; 55.8; 54.1; 18.2; 14.6; IR (νmax cm–1): 3279, 3247, 3118, 2977, 2928, 2117, 1717, 1699, 1643, 1225, 1097, 777; HRMS calc. for [C17H18N2O4 + Na]: 337.1159; found: 337.1158.
Ethyl 6-methyl-2-oxo-4-[2-(prop-2-yn-1-yloxy)phenyl]-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4c)
Yield 83%; pale green solid; m.p. 155 °C; 1H NMR (300 MHz, DMSO-d6): δ 9.14 (br s, 1H); 7.21–7.27 (m, 2H); 7.06–7.10 (m, 2H); 6.92 (t, 1H, J = 7.0 Hz); 5.49 (d, 1H, J = 2.2 Hz); 4.85 (dd, 1H, J = 2.3; 16.1 Hz); 4.78 (dd, 1H, J = 16.1 and 2.6 Hz); 3.99–3.84 (m, 2H); 3.58 (t, 1H, J = 2.3 Hz); 2.28 (s, 3H); 1.03 (t, 3H, J = 7.0 Hz); 13C NMR (75 MHz, DMSO-d6): δ 165.3; 154.6; 152.1; 149.0; 132.3; 128.5; 127.4; 121.0; 112.6; 97.6; 79.5; 78.2; 59.0; 55.8; 48.8; 17.8; 14.1; IR (νmax cm–1): 3252, 3115, 2981, 2928, 2123, 1707, 1668, 1637, 1488, 1228, 1088, 759; HRMS calc. for [C17H18N2O4 + Na]: 337.1159; found: 337.1156.
Ethyl 4-[4-methoxy-3-(prop-2-yn-1-yloxy)phenyl]-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4d)
Yield 75%; yellow solid; m.p. 149 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.40 (br s, 1H); 7.04 (d, 1H, J = 1.8 Hz); 6.92 (dd, 1H, J = 8.3 and 1.8 Hz); 6.81 (d, 1H J = 8.3 Hz); 5.97 (br s, 1H); 5.36 (d, 1H, J = 2.5 Hz); 4.73 (d, 2H, J = 2.0 Hz); 4.09 (q, 2H, J = 7.3 Hz); 3.84 (s, 3H); 2.49 (t, 1H, J = 2.4 Hz); 2.34 (s, 3H); 1.18 (t, 3H, J = 7.0 Hz); 13C NMR (100 MHz, DMSO-d6): δ 165.7; 153.6; 149.4; 146.8; 146.3; 136.3; 120.2; 113.3; 111.7; 101.4; 78.5; 76.0; 60.0; 57.0; 55.9; 55.1; 18.6; 14.2; IR (νmax cm–1): 3268, 3237, 2976, 2122, 1701, 1642, 1511, 1219, 1089, 1018, 798; HRMS calc. for [C18H20N2O5 + Na]: 367.1264; found: 367.1259.
Ethyl 4-[3-methoxy-4-(prop-2-yn-1-yloxy)phenyl]-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (4e)
Yield 86%; yellow solid; m.p. 119 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.17 (br s, 1H); 7.70 (br s, 1H); 6.96 (d, 1H, J = 8.3 Hz); 6.87 (d, 1H, J = 1.8 Hz); 6.71 (dd, 1H, J = 8.3 and 1.8 Hz); 5.10 (d, 1H, J = 3.0 Hz); 4.73 (d, 2H, J = 2.0 Hz); 4.00 (q, 2H, J = 7.0 Hz); 3.73 (s, 3H); 3.53 (t, 1H, J = 3.5 Hz); 2.24 (s, 3H); 1.11 (t, 3H, J = 7.0 Hz); 13C NMR (100 MHz, DMSO-d6): δ 165.4; 152.3; 149.0; 148.3; 145.7; 138.6; 117.7; 114.2; 110.8; 99.3; 79.4; 78.2; 59.2; 56.1; 55.5; 53.5; 17.8; 14.2; IR (νmax cm–1): 3236, 3102, 2937, 2122, 1695, 1654, 1510, 1220, 1096, 786, 642; HRMS calc. for [C18H20N2O5 + Na]: 367.1264; found: 367.1269.
5-Acetyl-6-methyl-4-(4-(prop-2-yn-1-yloxy)phenyl)-3,4-dihydropyrimidin-2(1H)-one (4f)
Yield 67%; yellow solid; m.p. 187 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.14 (br s, 1H); 7.75 (br s, 1H); 7.17 (d, 2H, J = 8.6 Hz); 6.93 (d, 2H, J = 8.6 Hz); 5.20 (d, 1H, J = 3.3 Hz); 4.76 (d, 2H, J = 2.3 Hz); 3.53 (t, 1H, J = 2.5 Hz); 2.28 (s, 3H); 2.09 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 194.4; 156.4; 152.1; 147.9; 137.2; 127.6; 114.8; 109.7; 79.3; 78.2; 55.4; 53.3; 30.2; 18.9; IR (νmax cm–1): 3297, 2940, 2117, 1674, 1613, 1238, 1029; HRMS calc. for [C16H16N2O3 + Na]: 307.1053; found: 307.1047.
5-Acetyl-6-methyl-4-(3-(prop-2-yn-1-yloxy)phenyl)-3,4-dihydropyrimidin-2(1H)-one (4g)
Yield 69%; pale yellow solid; m.p. 190 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.17 (br s, 1H); 7.80 (br s, 1H); 7.26 (t, 1H, J = 7.8 Hz); 6.90–6.84 (m, 3H); 5.24 (d, 1H, J = 3.3 Hz); 4.75 (d, 2H, J = 2.0 Hz); 3.54 (t, 1H, J = 2.3 Hz); 2.29 (s, 3H); 2.12 (s, 3H); 13C NMR (75 MHz, DMSO-d6): δ 194.3; 157.3; 152.2; 148.3; 145.8; 129.6; 119.3; 113.6; 112.9; 109.4; 79.2; 78.3; 55.4; 53.6; 30.3; 18.9; IR (νmax cm–1): 3278, 3254, 2959, 2129, 1709, 1680, 1606, 1379, 1225, 1029, 760; HRMS calc. for [C16H16N2O3 + Na]: 307.1053; found: 307.1048.
5-Acetyl-6-methyl-4-(2-(prop-2-yn-1-yloxy)phenyl)-3,4-dihydropyrimidin-2(1H)-one (4h)
Yield 67%; yellow solid; m.p. 182 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.16 (br s, 1H); 7.34 (br s, 1H); 7.27 (td, 1H, J = 7.5 and 1.5 Hz); 7.11 (d, 1H, J = 7.8 Hz); 7.07 (dd, 1H, J = 7.6 and 1.5 Hz) 6.94 (t, 1H, J = 7.3 Hz); 5.56 (d, 1H, J = 3.3 Hz); 4.89 (dd, 2H, J = 16.1 and 2.3 Hz); 4.84 (dd, 2H, J = 16.1 and 2.3 Hz); 3.59 (t, 1H, J = 2.3 Hz); 2.29 (s, 3H); 2.03 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 194.4; 154.3; 152.1; 148.2; 131.7; 128.8; 127.1; 121.3; 112.8; 107.8; 79.4; 78.3; 55.8; 48.7; 29.7; 18.7; IR (νmax cm–1): 3297, 2948, 2117, 1674, 1613, 1225, 1029; HRMS calc. for [C16H16N2O3 + Na]: 307.1053; found: 307.1049.
5-Acetyl-4-(4-methoxy-3-(prop-2-yn-1-yloxy)phenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (4i)
Yield 64%; yellow solid; m.p. 143 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.13 (br s, 1H); 7.73 (br s, 1H); 6.97 (d, 1H, J = 1.8 Hz); 6.93 (d, 1H, J = 8.3 Hz); 6.81 (dd, 1H, J = 8.3 and 1.8 Hz); 5.19 (d, 1H, J = 3.3 Hz); 4.69 (d, 2H, J = 2.3 Hz); 3.73 (s, 1H); 3.51 (t, 1H, J = 2.3 Hz); 2.28 (s, 3H); 2.08 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 194.4; 152.1; 148.7; 148.0; 146.3; 136.5; 119.7; 113.5; 112.2; 109.3; 79.2; 78.4; 56.3; 55.6; 53.2; 30.1; 18.8; IR (νmax cm–1): 3303, 3272, 3117, 2941, 2125, 1691, 1618, 1505, 1226, 1133, 1019; HRMS calc. for [C17H18N2O4 + Na]: 337.1159; found: 337.1167.
5-Acetyl-4-(3-methoxy-4-(prop-2-yn-1-yloxy)phenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (4j)
Yield 62%; yellow solid; m.p. 183 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.15 (br s, 1H); 7.76 (br s, 1H); 6.96 (d, 1H, J = 8.3 Hz); 6.93 (d, 1H, J = 2.0 Hz); 6.70 (dd, 1H, J = 8.3 and 2.0 Hz); 5.21 (d, 1H, J = 3.5 Hz); 4.74 (d, 2H, J = 2.5 Hz); 3.74 (s, 3H); 3.51 (t, 1H, J = 2.4 Hz); 2.29 (s, 3H); 2.10 (s, 3H); 13C MNR (75 MHz, DMSO-d6): δ 194.5; 152.1; 149.2; 148.0; 145.8; 137.8; 117.8; 114.2; 111.1; 109.3; 79.4; 78.2; 56.1; 55.5; 53.5; 30.2; 18.8; IR (νmax cm–1): 3380, 3266, 2947, 2114, 1672, 1590, 1232, 1139, 1015; HRMS calc. for [C17H18N2O4 + Na]: 337.1159; found: 337.1153.
General procedure for the synthesis of propargyloxy DHPMs 4k–o
Aldehyde (3a–e, 2.0 mmol), urea (2.4 mmol), HCl (3 drops) and ethanol (2 mL) were stirred under reflux for 1 h. Next, dimedone (2.0 mmol) was added in 5 portions over a total time of 2.5 h while maintaining the reaction mixture at 60 °C under stirring. The end of the reaction was monitored by TLC and the solvent was retired under vacuum. The crude product was purified through recrystallization from hot ethanol to give the DHPMs in the pure form.
7,7-Dimethyl-4-(4-(prop-2-yn-1-yloxy)phenyl)-4,6,7,8-tetrahydroquinazoline-2,5(1H,3H)-dione (4k)
Yield 74%; pale yellow solid; m.p. 223 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.42 (br s, 1H); 7.69 (br s, 1H); 7.15 (d, 2H, J = 8.8 Hz); 6.91 (d, 2H, J = 8.8 Hz); 5.10 (d, 1H, J = 2.8 Hz); 4.75 (d, 2H, J = 2.5 Hz); 3.52 (t, 1H, J = 2.3 Hz); 2.40 (d, 1H, J = 17.4 Hz); 2.28 (d, 1H, J = 17.1 Hz); 2.18 (d, 1H, J = 16.1 Hz); 2.03 (d, 1H, J = 16.1 Hz); 1.01 (s, 3H); 0.90 (s, 3H); 13C NMR (75 MHz, DMSO-d6): δ 193.4; 156.7; 152.7; 152.3; 130.1; 127.8; 115.0; 108.0; 79.8; 78.6; 55.8; 51.9; 50.3; 39.6; 32.8; 29.2; 27.4; IR (νmax cm–1): 3303, 3241, 3069, 2965, 2872, 2129, 1693, 1625, 1502, 1373, 1250, 1023, 826; HRMS calc. for [C19H20N2O3 + Na]: 347.1366; found: 347.1362.
7,7-Dimethyl-4-(3-(prop-2-yn-1-yloxy)phenyl)-4,6,7,8-tetrahydroquinazoline-2,5(1H,3H)-dione (4l)
Yield 67%; pale yellow solid; m.p. 203 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.45 (br s, 1H); 7.74 (br s, 1H); 7.24 (t, 1H, J = 7.9 Hz); 6.88–6.81 (m, 3H); 5.12 (d, 1H, J = 2.8 Hz); 4.73 (d, 2H, J = 2.5 Hz); 3.53 (t, 1H, J = 2.3 Hz); 2.40 (d, 1H, J = 17.1 Hz); 2.28 (d, 1H, J = 17.1 Hz); 2.19 (d, 1H, J = 16.1 Hz); 2.05 (d, 1H, J = 16.4 Hz); 1.02 (s, 3H); 0.91 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 193.4; 157.7; 153.1; 152.4; 146.6; 129.9; 119.6; 113.9; 113.2; 107.6; 79.7; 78.7; 55.8; 52.2; 50.3; 39.6; 32.8; 29.2; 27.4; IR (νmax cm–1): 3272, 2952, 2123, 1680, 1607, 1373, 1232, 1035, 760; HRMS calc. for [C19H20N2O3 + Na]: 347.1366; found: 347.1367.
7,7-Dimethyl-4-(2-(prop-2-yn-1-yloxy)phenyl)-4,6,7,8-tetrahydroquinazoline-2,5(1H,3H)-dione (4m)
Yield 70%; pale yellow solid; m.p. 218 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.38 (br s, 1H); 7.25–7.20 (m, 2H); 7.07 (m, 2H); 6.90 (t, 1H, J = 7.3 Hz); 5.39 (d, 1H, J = 1.8 Hz); 4.80 (d, 1H, J = 15.9 Hz); 4.76 (d, 1H, J = 15.9 Hz); 3.56 (s, 1H); 2.40 (d, 1H, J = 17.1 Hz); 2.33 (d, 1H, J = 17.4 Hz); 2.16 (d, 1H, J = 16.1 Hz); 2.01 (d, 1H, J = 16.1 Hz); 1.03 (s, 3H); 0.98 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 192.6; 155.0; 153.1; 151.7; 132.0; 128.5; 128.2; 120.8; 112.8; 105.4; 79.5; 78.2; 55.9; 48.9; 48.5; 39.5; 32.2; 28.7; 27.3; IR (νmax cm–1): 3416, 3290, 3210, 3100, 2960, 2125, 1705, 1613, 1385, 1237, 746; HRMS calc. for [C19H20N2O3 + Na]: 347.1366; found: 347.1362.
7,7-Dimethyl-4-(4-methoxy-3-(prop-2-yn-1-yloxy)phenyl)-4,6,7,8-tetrahydroquinazoline-2,5(1H,3H)-dione (4n)
Yield 81%; white solid; m.p. 236 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.44 (br s, 1H); 7.71 (br s, 1H); 6.95 (d, 1H, J = 1.8 Hz); 6.92 (d, 1H, J = 8.3 Hz); 6.80 (dd, 1H, J = 8.3 and 1.8 Hz); 5.10 (d, 1H, J = 2.8 Hz); 4.68 (d, 2H, J = 2.3 Hz); 3.73 (s, 3H); 3.53 (t, 1H, J = 2.4 Hz); 2.41 (d, 1H, J = 17.1 Hz); 2.28 (d, 1H, J = 17.4 Hz); 2.20 (d, 1H, J = 16.1 Hz); 2.05 (d, 1H, J = 15.9 Hz); 1.02 (s, 3H); 0.94 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 192.9; 152.3; 151.9; 148.6; 146.3; 137.0; 119.6; 113.2; 111.9; 107.4; 79.2; 78.3; 56.4; 55.6; 51.4; 49.9; 39.2; 32.3; 28.8; 27.0; IR (νmax cm–1): 3256, 2974, 2153, 1676, 1603, 1376, 1231, 1138, 1014, 776, 694, 642; HRMS calc. for [C20H22N2O4 + Na]: 377.1472; found: 377.1471.
7,7-Dimethyl-4-(3-methoxy-4-(prop-2-yn-1-yloxy)phenyl)-4,6,7,8-te trahydroquinazoline-2,5(1H,3H)-dione (4o)
Yield 72%; pale green solid; m.p. 215 °C; 1H NMR (300 MHz, DMSO-d6): δ 9.45 (br s, 1H); 7.73 (br s, 1H); 6.95 (d, 1H, J = 8.3 Hz); 6.86 (d, 1H, J = 1.8 Hz); 6.71 (dd, 1H, J = 8.3 and 1.8 Hz); 5.11 (d, 1H, J = 3.0 Hz); 4.73 (d, 2H, J = 2.5 Hz); 3.71 (s, 3H); 3.53 (t, 1H, J = 2.3 Hz); 2.41 (d, 1H, J = 17.1 Hz); 2.28 (d, 1H, J = 17.4 Hz); 2.20 (d, 1H, J = 16.1 Hz); 2.05 (d, 1H, J = 16.1 Hz); 1.02 (s, 3H); 0.94 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 193.0; 152.5; 151.9; 149.0; 145.6; 138.2; 117.8; 114.0; 110.7; 107.3; 79.4; 78.2; 56.1; 55.4; 51.5; 49.9; 39.2; 32.3; 28.8; 26.9; IR (νmax cm–1): 3324, 3272, 2962, 2122, 1676, 1614, 1366, 1222, 1125, 1007, 758; HRMS calc. for [C20H22N2O4 + Na]: 377.1472; found: 377.1469.
Procedure for the synthesis of perillyl azide (7)
To a mixture of (S)-perillyl chloride (5.0 mmol) and dimethylformamide (1.5 mL) was added sodium azide (15.0 mmol) and the reaction was stirred for 12 h at room temperature. The end of the reaction was monitored by TLC and water (5.0 mL) was added in one portion. The aqueous phase (H2O/DMF) was washed with hexane (3 × 15.0 mL). The organic phase was separated and washed with saturated NaCl solution and dried over anhydrous magnesium sulfate. After simple filtration, the filtrate was removed under vacuum (this process should be carefully controlled to avoid the loss of mass) to obtain the crude perillyl azide (7) in 90% yield. The perillyl azide was used in the next step without further purification.
(S)-1-(Azidomethyl)-4-(prop-1-en-2-yl)cyclohex-1-ene (7)
Yield 90%; colorless liquid; 1H NMR (400 MHz, CDCl3): δ 5.76 (br s, 1H); 4.76–4.73 (m, 2H); 3.70 (d, 1H, J = 13.4 Hz); 3.65 (d, 1H, J = 13.1 Hz); 2.19–2.10 (m, 4H); 2.05–1.96 (m, 1H); 1.90–1.86 (m, 1H); 1.75 (s, 3H); 1.55–1.49 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 149.3; 134.1; 126.4; 108.9; 57.4; 40.7; 30.4; 27.3; 27.0; 20.7; IR (νmax cm–1): 3085, 2923, 2096, 1645, 1439, 1242, 891.
General procedure for the synthesis of perillyl–DHPM hybrids 8a–o
DHPM (4a–o) (0.5 mmol), perillyl azide (7, 0.6 mmol), dichloromethane (5.0 mL) and water (5.0 mL) were mixed in a flask and then copper sulfate pentahydrate (10.0 mol%) and sodium ascorbate (10.0 mol%) were added in this order. The reaction was kept under stirring at room temperature for 24 h. The end of the reaction was monitored by TLC. Next, EDTA 0.1 M (10.0 mL) was added and the mixture was stirred for 15 min. The aqueous phase was washed with dichloromethane (3 × 10 mL). The organic phase was separated and washed with saturated NaCl solution. After separation, the organic layer was dried over anhydrous magnesium sulfate and filtered. The filtrate was removed under vacuum and the crude solid was purified by chromatography in a column of silica gel using hexane–ethyl acetate as the eluent to give the perillyl–DHPM hybrid in pure form.
5-Ethoxycarbonyl-6-methyl-4-(4-((1-perillyl-methyl)-1H-1,2,3-triazol-4-yl)-methoxyphenyl)-3,4-dihydropirimidin-2-(1H)-one (8a)
Yield 79%; white solid; m.p. 80 °C; 1H NMR (300 MHz, CDCl3): δ 8.50 (br s, 1H); 7.58 (s, 1H); 7.23 (d, 2H, J = 8.8 Hz); 6.91 (d, 2H, J = 8.8 Hz); 6.02 (br s, 1H); 5.76 (br s, 1H); 5.34 (d, 1H, J = 2.4 Hz); 5.17 (s, 2H); 4.85 (s, 2H); 4.78–4.65 (m, 2H), 4.08 (m, 2H); 2.32 (s, 3H); 2.17–2.10 (m, 2H); 1.94–1.80 (m, 4H); 1.72 (s, 3H); 1.52–1.41 (m, 1H); 1.16 (t, 3H, J = 7.0 Hz); 13C NMR (100 MHz, CDCl3): δ 165.7; 157.9; 153.5; 149.0; 146.2; 144.2; 136.7; 131.8; 127.8; 127.4; 122.4; 114.8; 109.0; 101.4; 62.1; 59.9; 56.5; 55.0; 40.4; 30.4; 27.0; 26.3; 20.7; 18.5; 14.1; IR (νmax cm–1): 3230, 3102, 2936, 1693, 1638, 1600, 1427, 1227, 1082, 777; HRMS calc. for [C27H33N5O4 + H]: 492.2605; found: 492.2607.
5-Ethoxycarbonyl-6-methyl-4-(3-((1-perillyl-methyl)-1H-1,2,3-triazol-4-yl)-methoxyphenyl)-3,4-dihydropirimidin-2-(1H)-one (8b)
Yield 90%; white solid; m.p. 74 °C; 1H NMR (400 MHz, CDCl3): δ 8.41 (br s, 1H); 7.59 (s, 1H); 7.21 (t, 1H, J = 7.8 Hz); 6.95 (s, 1H); 6.91 (d, 1H, J = 7.8 Hz); 6.88 (d, 1H, J = 7.8 Hz); 6.10 (br s, 1H); 5.76 (br s, 1H); 5.36 (s, 1H); 5.17 (s, 2H); 4.84 (s, 2H); 4.73 (s, 1H); 4.69 (s, 1H); 4.06 (q, 2H, J = 7.3 Hz); 2.33 (s, 3H); 2.21–2.11 (m, 2H); 2.04–1.93 (m, 4H); 1.72 (s, 3H); 1.49–1.41 (m, 1H); 1.15 (t, 3H, J = 7.3 Hz); 13C NMR (75 MHz, CDCl3): δ 165.6; 158.5; 153.4; 149.0; 146.7; 145.3; 144.1; 131.8; 129.7; 127.3; 122.5; 119.4; 113.8; 113.4; 109.0; 100.9; 62.1; 59.9; 56.4; 55.4; 40.4; 30.4; 27.0; 26.3; 20.7; 18.6; 14.1; IR (νmax cm–1): 3232, 3101, 2818, 1695, 1634, 1596, 1446, 1224, 1084, 773; HRMS calc. for [C27H33N5O4 + H]: 492.2605; found: 492.2609.
5-Ethoxycarbonyl-6-methyl-4-(2-((1-perillyl-methyl)-1H-1,2,3-triazol-4-yl)-methoxyphenyl)-3,4-dihydropirimidin-2-(1H)-one (8c)
Yield 85%; white solid; m.p. 182 °C; 1H NMR (400 MHz, CDCl3): δ 8.34 (br s, 1H); 7.62 (s, 1H); 7.22 (td, 1H, J = 7.8 and 1.8 Hz); 7.06 (dd, 1H, J = 7.6 and 1.8 Hz); 7.01 (d, 1H, J = 7.8 Hz); 6.90 (t, 1H, J = 7.6 Hz); 5.99 (br s, 1H); 5.74 (br s, 2H); 5.28 (s, 2H); 4.87 (d, 1H, J = 16.1 Hz); 4.83 (d, 1H, J = 16.1 Hz); 4.73 (s, 1H); 4.69 (s, 1H); 4.04 (q, 2H, J = 7.0 Hz); 2.39 (s, 3H); 2.21–2.10 (m, 2H); 1.98–1.79 (m, 4H); 1.72 (s, 3H); 1.50–1.40 (m, 1H); 1.09 (t, 3H, J = 7.0 Hz); 13C NMR (100 MHz, CDCl3): δ 165.8; 155.5; 153.7; 149.0; 148.2; 143.9; 131.8; 130.4; 129.0; 127.3; 127.0; 122.5; 121.2; 112.2; 109.0; 98.2; 62.3; 59.8; 56.5; 50.1; 40.4; 30.4; 27.0; 26.3; 20.7; 18.5; 14.1; IR (νmax cm–1): 3397, 3113, 2980, 1724, 1694, 1639, 1592, 1446, 1221, 1080, 753; HRMS calc. for [C27H33N5O4 + H]: 492.2605; found: 492.2605.
5-Ethoxycarbonyl-6-methyl-4-(4-methoxy-3-((1-perillyl-methyl)-1H-1,2,3-triazol-4-yl)-methoxyphenyl)-3,4-dihydropirimidin-2-(1H)-one (8d)
Yield 71%; yellow solid; m.p. 182 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.17 (br s, 1H); 8.10 (s, 1H); 7.70 (br s, 1H); 7.01 (d, 1H, J = 1.8 Hz); 6.91 (d, 1H, J = 8.6 Hz); 6.76 (dd, 1H, J = 8.3 and 1.8 Hz); 5.66 (br s, 1H); 5.10 (d, 1H, J = 3.0 Hz); 5.07 (d, 1H, J = 11.8 Hz); 5.04 (d, 1H, J = 11.8 Hz); 4.91 (s, 2H); 4.69 (s, 2H); 3.99 (q, 2H, J = 7.3 Hz); 3.71 (s, 3H); 2.25 (s, 3H); 2.14–2.04 (m, 2H); 1.94–1.86 (m, 3H); 1.75–1.72 (m, 1H); 1.69 (s, 3H); 1.39–1.33 (m, 1H); 1.10 (t, 3H, J = 7.0 Hz); 13C NMR (100 MHz, DMSO-d6): δ 165.4; 152.3; 148.9; 148.5; 148.3; 147.1; 142.6; 137.3; 132.5; 125.5; 124.7; 118.8; 112.6; 112.0; 109.1; 99.3; 61.9; 59.2; 55.5; 55.1; 53.4; 40.0; 29.9; 26.8; 26.0; 20.6; 17.8; 14.2; IR (νmax cm–1): 3260, 3115, 2929, 1692, 1511, 1454, 1256, 1225, 1084, 1001, 754; HRMS calc. for [C28H35N5O5 + Na]: 544.2530; found: 544.2527.
5-Ethoxycarbonyl-6-methyl-4-(3-methoxy-4-((1-perillyl-methyl)-1H-1,2,3-triazol-4-yl)-methoxyphenyl)-3,4-dihydropirimidin-2-(1H)-one (8e)
Yield 73%; yellow solid; m.p. 78 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.16 (br s, 1H); 8.13 (s, 1H); 7.68 (br s, 1H); 7.07 (d, 1H, J = 8.3 Hz); 6.87 (d, 1H, J = 2.0 Hz); 6.73 (dd, 1H, J = 8.3 and 2.0 Hz); 5.67 (br s, 1H); 5.12 (d, 1H, J = 3.3 Hz); 5.08 (s, 2H); 4.91 (s, 2H); 4.71 (s, 2H); 4.01 (q, 2H, J = 7.0 Hz); 3.72 (s, 3H); 2.25 (s, 3H); 2.17–2.05 (m, 2H); 1.93–1.89 (m, 3H); 1.77–1.73 (m, 1H); 1.70 (s, 3H); 1.42–1.32 (m, 1H); 1.12 (t, 3H, J = 7.0 Hz); 13C NMR (75 MHz, DMSO-d6): δ 165.4; 152.2; 148.9; 148.8; 148.2; 146.6; 142.7; 138.0; 132.5; 125.4; 124.6; 117.8; 113.7; 110.7; 109.0; 99.3; 61.8; 59.2; 55.4; 55.0; 53.5; 40.0; 29.8; 26.7; 26.0; 20.6; 17.7; 14.1; IR (νmax cm–1): 3232, 3105, 2929, 1698, 1640, 1515, 1449, 1220, 1082, 774; HRMS calc. for [C28H35N5O5 + Na]: 544.2530; found: 544.2533.
5-Acetyl-6-methyl-4-(4-((1-perillyl-methyl)-1H-1,2,3-triazol-4-yl)-methoxyphenyl)-3,4-dihydropirimidin-2-(1H)-one (8f)
Yield 71%; pale green solid; m.p. 92 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.14 (br s, 1H); 8.14 (s, 1H); 7.76 (br s, 1H); 7.17 (d, 2H, J = 8.6 Hz); 6.98 (d, 2H, J = 8.8 Hz); 5.65 (br s, 1H); 5.21 (d, 1H, J = 3.3 Hz); 5.11 (s, 2H); 4.90 (s, 2H); 4.69 (s, 2H); 2.28 (s, 3H); 2.14–2.03 (m, 5H); 1.94–1.84 (m, 3H); 1.75–1.72 (m, 1H); 1.69 (s, 3H); 1.41–1.31 (m, 1H); 13C NMR (75 MHz, DMSO-d6): δ 194.3; 157.3; 152.0; 148.9; 147.8; 142.7; 136.7; 132.5; 127.6; 125.4; 124.5; 114.6; 109.6; 109.0; 61.1; 55.0; 53.3; 40.0; 30.2; 29.8; 26.7; 25.9; 20.5; 18.8; IR (νmax cm–1): 3242, 3112, 2922, 1699, 1609, 1509, 1429, 1227, 1168, 1006, 781; HRMS calc. for [C26H31N5O3 + Na]: 484.2319; found: 484.2317.
5-Acetyl-6-methyl-4-(3-((1-perillyl-methyl)-1H-1,2,3-triazol-4-yl)-methoxyphenyl)-3,4-dihydropirimidin-2-(1H)-one (8g)
Yield 72%; white solid; m.p. 145 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.17 (br s, 1H); 8.14 (s, 1H); 7.81 (br s, 1H); 7.25 (t, 1H, J = 7.8 Hz); 6.96 (dd, 1H, J = 8.1 and 2.3 Hz); 6.87–6.84 (m, 2H); 5.66 (br s, 1H); 5.24 (d, 1H, J = 3.5 Hz); 5.11 (s, 2H); 4.90 (s, 2H); 4.69 (s, 2H); 2.29 (s, 3H); 2.14–2.04 (m, 5H); 1.95–1.88 (m, 3H); 1.76–1.69 (m, 4H); 1.41–1.31 (m, 1H); 13C NMR (75 MHz, DMSO-d6): δ 194.2; 158.1; 152.1; 148.9; 148.2; 145.8; 142.6; 132.4; 129.6; 125.4; 124.6; 118.9; 113.4; 113.0; 109.4; 109.0; 61.0; 55.0; 53.6; 40.0; 30.2; 29.8; 26.7; 26.0; 20.5; 18.9; IR (νmax cm–1): 3270, 3110, 2928, 1690, 1599, 1446, 1240, 1013, 806, 776; HRMS calc. for [C26H31N5O3 + Na]: 484.2319; found: 484.2310.
5-Acetyl-6-methyl-4-(2-((1-perillyl-methyl)-1H-1,2,3-triazol-4-yl)-methoxyphenyl)-3,4-dihydropirimidin-2-(1H)-one (8h)
Yield 68%; white solid; m.p. 139 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.13 (br s, 1H); 8.17 (s, 1H); 7.27–7.25 (m, 2H); 7.20 (dd, 1H, J = 8.3 and 1.1 Hz); 7.07 (dd, 1H, J = 7.6 and 1.8 Hz); 6.92 (td, 1H, J = 7.3 and 1.0 Hz); 5.66 (br s, 1H); 5.60 (d, 1H, J = 3.3 Hz); 5.29 (d, 1H, J = 12.6 Hz); 5.24 (d, 1H, J = 12.6 Hz); 4.91 (s, 2H); 4.69 (s, 2H); 2.28 (s, 3H); 2.13–2.03 (m, 2H); 1.96–1.87 (m, 6H); 1.74–1.68 (m, 4H); 1.40–1.30 (m, 1H); 13C NMR (100 MHz, DMSO-d6): δ 194.5; 154.8; 152.1; 148.9; 148.2; 143.1; 132.5; 131.7; 128.8; 127.0; 125.4; 124.3; 121.0; 113.0; 109.0; 107.7; 61.8; 55.1; 48.6; 40.0; 29.8; 29.6; 26.7; 25.9; 20.5; 18.6; IR (νmax cm–1): 3413, 3207, 3080, 2934, 1685, 1636, 1597, 1430, 1232, 764, 583; HRMS calc. for [C26H31N5O3 + Na]: 484.2319; found: 484,2320.
5-Acetyl-6-methyl-4-(4-methoxy-3-((1-perillyl-methyl)-1H-1,2,3-triazol-4-yl)-methoxyphenyl)-3,4-dihydropirimidin-2-(1H)-one (8i)
Yield 76%; yellow solid; m.p. 154 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.14 (br s, 1H); 8.09 (s, 1H); 7.75 (br s, 1H); 7.04 (d, 1H, J = 1.6 Hz); 6.90 (d, 2H, J = 8.3 Hz); 6.76 (dd, 1H, J = 8.3 and 1.6 Hz); 5.66 (br s, 1H); 5.20 (d, 1H, J = 3.0 Hz); 5.08 (s, 2H); 4.90 (s, 2H); 4.69 (s, 2H); 3.71 (s, 1H); 2.29 (s, 3H); 2.13–2.04 (m, 5H); 1.94–1.88 (m, 3H); 1.75–1.69 (m, 4H); 1.41–1.31 (m, 1H); 13C NMR (100 MHz, DMSO-d6): δ 194.5; 152.2; 149.0; 148.5; 148.1; 147.2; 142.6; 136.6; 132.5; 125.5; 124.8; 119.0; 112.8; 112.1; 109.3; 109.1; 61.8; 55.6; 55.1; 53.6; 40.0; 30.2; 29.9; 26.8; 26.0; 20.6; 18.9; IR (νmax cm–1): 3232, 3125, 2929, 1695, 1598, 1510, 1422, 1223, 1128, 757; HRMS calc. for [C27H33N5O4 + Na]: 514.2425; found: 514.2422.
5-Acetyl-6-methyl-4-(3-methoxy-4-((1-perillyl-methyl)-1H-1,2,3-triazol-4-yl)-methoxyphenyl)-3,4-dihydropirimidin-2-(1H)-one (8j)
Yield 67%; pale green solid; m.p. 93 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.15 (br s, 1H); 8.13 (s, 1H); 7.77 (br s, 1H); 7.07 (d, 1H, J = 8.3 Hz); 6.93 (d, 2H, J = 2.0 Hz); 6.71 (dd, 1H, J = 8.3 and 2.0 Hz); 5.67 (br s, 1H); 5.23 (d, 1H, J = 3.3 Hz); 5.09 (s, 2H); 4.91 (s, 2H); 4.70 (s, 2H); 3.73 (s, 1H); 2.30 (s, 3H); 2.14–2.05 (m, 5H); 1.95–1.89 (m, 3H); 1.76–1.70 (m, 4H); 1.42–1.32 (m, 1H); 13C NMR (75 MHz, DMSO-d6): δ 194.4; 152.1; 149.0; 148.9; 148.0; 146.8; 142.8; 137.2; 132.5; 125.4; 124.6; 118.0; 113.7; 111.0; 109.2; 109.0; 61.8; 55.4; 55.0; 53.6; 40.0; 30.1; 29.8; 26.7; 26.0; 20.6; 18.8; IR (νmax cm–1): 3232, 3134, 2929, 1701, 1600, 1514, 1417, 1226, 1131, 754; HRMS calc. for [C27H33N5O4 + Na]: 514.2425; found: 514.2431.
7,7-Dimethyl-4-(4-((1-perillyl-methyl)-1H-1,2,3-triazol-4-yl)-methoxyphenyl)-4,6,7,8-tetrahydroquinazoline-2,5(1H,3H)-dione (8k)
Yield 68%; white solid; m.p. 223 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.42 (br s, 1H); 8.13 (s, 1H); 7.70 (br s, 1H); 7.15 (d, 2H, J = 8.6 Hz); 6.96 (d, 2H, J = 8.6 Hz); 5.65 (s, 1H); 5.09 (br s, 3H); 4.89 (s, 2H); 4.69 (s, 2H); 2.40 (d, 1H, J = 17.4 Hz); 2.27 (d, 1H, J = 17.1 Hz); 2.20–2.00 (m, 4H); 1.94–1.88 (m, 3H); 1.76–1.69 (m, 4H); 1.39–1.33 (m, 1H); 1.01 (s, 3H); 0.90 (s, 3H); 13C NMR (75 MHz, DMSO-d6): δ 192.8; 157.1; 152.1; 151.9; 148.9; 142.7; 137.3; 132.5; 127.3; 125.4; 124.5; 114.4; 109.1; 107.6; 61.1; 55.0; 51.3; 49.8; 40.0; 32.3 (×2); 29.8; 28.8; 26.9; 26.7; 26.0; 20.6; IR (νmax cm–1): 3310, 3099, 2938, 1681, 1641, 1606, 1453, 1372, 1244, 1044, 818, 778, 557; HRMS calc. for [C29H35N5O3 + H]: 502.2813; found: 502.2813.
7,7-Dimethyl-4-(3-((1-perillyl-methyl)-1H-1,2,3-triazol-4-yl)-methoxyphenyl)-4,6,7,8-tetrahydroquinazoline-2,5(1H,3H)-dione (8l)
Yield 84%; white solid; m.p. 192 °C; NMR 1H (400 MHz, DMSO-d6): δ 9.45 (br s, 1H); 8.14 (s, 1H); 7.75 (br s, 1H); 7.23 (t, 1H, J = 8.1 Hz); 6.93 (dd, 2H, J = 8.1 and 2.0 Hz); 6.85–6.82 (m, 2H); 5.66 (s, 1H); 5.14 (d, 1H, J = 2.8 Hz); 5.09 (s, 2H); 4.90 (s, 2H); 4.70 (s, 2H); 2.40 (d, 1H, J = 17.1 Hz); 2.30 (d, 1H, J = 17.4 Hz); 2.20–2.04 (m, 4H); 1.95–1.85 (m, 3H); 1.76–1.72 (m, 1H); 1.69 (s, 3H); 1.41–1.31 (m, 1H); 1.01 (s, 3H); 0.90 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 192.9; 158.0; 152.5; 151.9; 148.9; 146.1; 142.6; 132.5; 129.4; 125.4; 124.6; 118.7; 113.2; 112.8; 109.0; 107.2; 61.0; 55.0; 51.7; 49.8; 40.0; 32.3 (×2); 29.8; 28.6; 27.0; 26.7; 26.0; 20.6; IR (νmax cm–1): 3251, 2911, 1692, 1610, 1378, 1234, 1009, 764; HRMS calc. for [C29H35N5O3 + H]: 502.2813; found: 502.2816.
7,7-Dimethyl-4-(2-((1-perillyl-methyl)-1H-1,2,3-triazol-4-yl)-methoxyphenyl)-4,6,7,8-tetrahydroquinazoline-2,5(1H,3H)-dione (8m)
Yield 80%; white solid; m.p. 198 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.34 (br s, 1H); 8.14 (s, 1H); 7.22–7.14 (m, 3H); 7.04 (dd, 1H, J = 7.6 and 1.5 Hz); 6.88 (t, 1H, J = 7.3 Hz); 5.67 (br s, 1H); 5.48 (d, 1H, J = 2.3 Hz); 5.24 (d, 1H, J = 12.3 Hz); 5.20 (d, 1H, J = 12.6 Hz); 4.91 (s, 2H); 4.69 (s, 2H); 2.38 (d, 1H, J = 17.4 Hz); 2.32 (d, 1H, J = 17.1 Hz); 2.16–1.84 (m, 7H); 1.74–1.68 (m, 4H); 1.41–1.30 (m, 1H); 1.02 (s, 3H); 0.98 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 192.6; 155.3; 153.0; 151.7; 148.9; 143.3; 132.4; 132.0; 128.5; 127.5; 125.4; 124.2; 120.7; 113.0; 109.0; 105.6; 62.0; 55.1; 49.9; 47.5; 40.0; 39.2; 32.3; 29.8; 28.5; 27.4; 26.7; 25.9; 20.6; IR (νmax cm–1): 3355, 3225, 2913, 1695, 1645, 1459, 1371, 1240, 1054, 796, 747, 558; HRMS calc. for [C29H35N5O3 + Na]: 524.2632; found: 524.2626.
7,7-Dimethyl-4-(4-methoxy-3-((1-perillyl-methyl)-1H-1,2,3-triazol-4-yl)-methoxyphenyl)-4,6,7,8-tetrahydroquinazoline-2,5(1H,3H)-dione (8n)
Yield 79%; white solid; m.p. 200 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.41 (br s, 1H); 8.11 (s, 1H); 7.70 (br s, 1H); 7.00 (d, 1H, J = 1.8 Hz); 6.90 (d, 1H, J = 8.3 Hz); 6.76 (dd, 1H, J = 8.3 and 1.8 Hz); 5.67 (br s, 1H); 5.11 (d, 1H, J = 2.8 Hz); 5.06 (d, 1H, J = 11.8 Hz); 5.01 (d, 1H, J = 14.9 Hz); 4.90 (s, 2H); 4.70 (s, 2H); 3.70 (s, 3H); 2.40 (d, 1H, J = 17.4 Hz); 2.32 (d, 1H, J = 17.1 Hz); 2.21–2.04 (m, 4H); 1.94–1.90 (m, 3H); 1.76–1.69 (m, 4H); 1.42–1.31 (m, 1H); 1.02 (s, 3H); 0.94 (s, 3H); 13C NMR (75 MHz, DMSO-d6): δ 193.0; 152.4; 151.9; 148.9; 148.3; 147.0; 142.5; 137.0; 132.5; 125.5; 124.7; 118.8; 112.5; 111.9; 109.0; 107.3; 61.8; 55.5; 55.0; 51.4; 49.9; 40.0; 39.1; 32.3; 29.8; 28.6; 27.1; 26.7; 26.0; 20.6; IR (νmax cm–1): 3265, 3138, 2933, 1692, 1642, 161, 1442, 1374, 1246, 1129, 1002, 770; HRMS calc. for [C30H37N5O4 + H]: 554.2738; found: 554.2735.
7,7-Dimethyl-4-(3-methoxy-4-((1-perillyl-methyl)-1H-1,2,3-triazol-4-yl)-methoxyphenyl)-4,6,7,8-tetrahydroquinazoline-2,5(1H,3H)-dione (8o)
Yield 71%; peach solid; m.p. 188 °C; 1H NMR (400 MHz, DMSO-d6): δ 9.44 (br s, 1H); 8.12 (s, 1H); 7.73 (br s, 1H); 7.05 (d, 1H, J = 8.3 Hz); 6.85 (d, 1H, J = 1.8 Hz); 6.71 (dd, 1H, J = 8.3 and 1.8 Hz); 5.66 (br s, 1H); 5.10–5.06 (m, 3H); 4.90 (s, 2H); 4.69 (s, 2H); 3.68 (s, 3H); 2.42 (d, 1H, J = 17.1 Hz); 2.27 (d, 1H, J = 17.4 Hz); 2.21 (d, 1H, J = 16.1 Hz); 2.14–2.03 (m, 3H); 1.94–1.88 (m, 3H); 1.75–1.69 (m, 4H); 1.41–1.30 (m, 1H); 1.02 (s, 3H); 0.93 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 193.0; 152.4; 152.0; 148.9; 148.8; 146.6; 142.8; 137.7; 132.5; 125.4; 124.6; 117.9; 113.4; 110.5; 109.1; 107.4; 61.7; 55.4; 55.0; 51.4; 49.9; 40.0; 32.3 (×2); 29.8; 28.9; 26.8; 26.7; 26.0; 20.6; IR (νmax cm–1): 3229, 3102, 2936, 1678, 1630, 1517, 1449, 1373, 1246, 1138, 1020, 603; HRMS calc. for [C30H37N5O4 + H]: 554.2738; found: 554.2737.
Biological assays
In vitro antiproliferative assay
The antiproliferative activity of EERP was evaluated in vitro against nine different human cancer cell lines (U251 (glioma), UACC-62 (melanoma), MCF-7 (breast), NCI-ADR/RES (multidrug-resistant ovary carcinoma), OVCAR-3 (ovary), 786-0 (renal), NCI-H460 (non-small cell lung cancer), PC-3 (prostate), and HT-29 (colon)) kindly provided by the Frederick Cancer Research Development Center, National Cancer Institute, Frederick, MA, USA. The antiproliferative activity of EERP was also evaluated in vitro against spontaneously transformed keratinocytes from histologically normal skin (HaCaT cells) provided by Dr. Ricardo Della Coletta (University of Campinas). Doxorubicin was employed as the positive control.
Cells in 96-well plates (100 μL cells per well) were exposed to EERP at 0.25, 2.5, 25 and 250 μg mL–1 in DMSO/RPMI at 37 °C, 5% CO2 in air for 48 h. Doxorubicin was used as the standard (0.025, 0.25, 2.5 and 25 μg mL–1). The final DMSO concentration (0.2%) did not affect cell viability. Before (T0 plate) and after (T1 plates) sample addition, cells were fixed with 50% trichloroacetic acid and cell proliferation was determined by spectrophotometric quantification (540 nm) of cellular protein content using the sulforhodamine B assay. The TGI (concentration that produces total growth inhibition or cytostatic effect) values were determined through non-linear regression analysis using the concentration–response curve for each cell line in ORIGIN 8.0® (OriginLab Corporation).
Conclusions
The synthesis of novel perillyl–dihydropyrimidinone hybrid molecules was accomplished through the multicomponent Biginelli reaction combined with the Huisgen triazole ring formation reaction and gave reasonable to good yields. A series of four compounds showed promising antitumoral activity against UACC-62, U251 and OVCAR-3, with TGI values lower than 10 μM (8a, 8j, 8k, 8n), while a series of seven compounds showed antiproliferative activity against UACC-62, U251 and OVCAR-3, with TGI values between 10 and 20 μM (8a, 8b, 8d, 8e, 8f, 8g, 8m). Additionally, four compounds showed high TGI values between 436.18 and 507.82 μM for the non-tumoral cell line HaCaT. These preliminary results are promising and indicate the need for subsequent studies to determine the mechanism of action, which is under current investigation in our laboratory.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for Grant Universal Proc. 455791/2014-7. D. R. acknowledges the CNPq-Produtividade Proc. 302952/2014-4. V.V. acknowledges the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the fellowship.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c8md00270c
References
- Zhang H. Y. FEBS Lett. 2005;579:5260. doi: 10.1016/j.febslet.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Maggiora G. M. J. J. Comput.-Aided Mol. Des. 2011;25:699. doi: 10.1007/s10822-011-9447-8. [DOI] [PubMed] [Google Scholar]
- Bansal Y., Silakari O. Eur. J. Med. Chem. 2014;76:31. doi: 10.1016/j.ejmech.2014.01.060. [DOI] [PubMed] [Google Scholar]
- Youdim M. B. H., Buccafusco J. J. Trends Pharmacol. Sci. 2005;26:27. doi: 10.1016/j.tips.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Keith C. T., Borisy A. A., Stockwell B. R., Medina-Franco J. L., Giulianotti M. A., Welmaker G. S., Houghten R. A. Nat. Rev. Drug Discovery. Drug Discovery Today. 2005;2013;418:71. 495. [Google Scholar]
- Morphy R., Rankovic Z. J. J. Med. Chem. 2005;48:6523. doi: 10.1021/jm058225d. [DOI] [PubMed] [Google Scholar]
- Karoli T., Mamidyala S. K., Zuegg J., Fry S. R., Tee E. H. L., Bradford T. A., Madala P. K., Huang J. X., Ramu S., Butler M. S., Cooper M. A., Muregi F. W., Ishih A. Bioorg. Med. Chem. Lett. Drug Dev. Res. 2012;2010;2271:2428. 20. doi: 10.1016/j.bmcl.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Liu C., Gu X., Zhu Y. Z. Bioorg. Med. Chem. Lett. 2010;20:6942. doi: 10.1016/j.bmcl.2010.09.135. [DOI] [PubMed] [Google Scholar]
- Fernandez-Bachiller M. I., Perez C., Gonzalez-Munoz G. C., Conde S., Lopez M. G., Villarroya M., Garcıa A. G., Rodrıguez-Franco M. I. J. J. Med. Chem. 2010;53:4927. doi: 10.1021/jm100329q. [DOI] [PubMed] [Google Scholar]
- Pi R., Mao X., Chao X., Cheng Z., Liu M., Duan M., Ye M., Chen M., Mei Z., Liu P., Li W., Han Y., Chao X., He X., Yang Y., Zhou X., Jin M., Liu S., Cheng Z., Liu P., Wang P., Yu L., Tan Y., Huang Y., Qin J., Rapposelli S., Pi R. PLoS One. Bioorg. Med. Chem. Lett. 2012;2012;722:31921. 6498. doi: 10.1371/journal.pone.0031921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin S., Beérubé G. Expert Opin. Drug Discovery. 2013;8:1029. doi: 10.1517/17460441.2013.798296. [DOI] [PubMed] [Google Scholar]
- Singh P., Raj R., Kumar V., Mahajan M. P., Bedi P. M. S., Kaur T., Saxena A. K. Eur. J. Med. Chem. 2012;47:594. doi: 10.1016/j.ejmech.2011.10.033. [DOI] [PubMed] [Google Scholar]
- Chiang A. N., Valderramos J.-C., Balachandran R., Chovatiya R. J., Mead B. P., Schneider C., Bell S. L., Klein M. G., Huryn D. N., Chen X. S., Day B. W., Fidock D. A., Wipf P., Brodsky J. L. Bioorg. Med. Chem. 2009;17:1527. doi: 10.1016/j.bmc.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sashidhara K. V., Singh L. R., Shameem M., Shakya S., Kumar A., Laxman T. S., Krishna S., Siddiqi M. I., Bhattac R. S., Banerjee D. Med. Chem. Commun. 2016;7:2349. [Google Scholar]
- For recent Reviews see: Mohammadi B., Behbahani F. K., Mol. Diversity, 2018, 22 , 405 Matos L. H. S., Masson F. T., Simeoni L. A., Homem-de-Mello M., Eur. J. Med. Chem., 2018, 143 , 1779, Kaur R., Chaudhary S., Kumar K., Gupta M. K., Rawal R. K., Eur. J. Med. Chem., 2017, 132 , 108, de Fatima A., Braga T. C., Neto L. S., Terra B. S., Oliveira B. G. F., Silva D. L., Modolo L. V. J., RSC Adv., 2015, 6 , 363 . [Google Scholar]
- Mayer T. U., Kapoor T. M., Haggarty S. J., King R. W., Schreiber S. L., Mitchison T. J. Science. 1999;286:971. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- Gartner M., Sunder-Plassmann N., Seiler J., Utz M., Vernos I., Surrey T., Giannis A. ChemBioChem. 2005;6:1173. doi: 10.1002/cbic.200500005. [DOI] [PubMed] [Google Scholar]
- Russowsky D., Canto R. F. S., Sanches S. A. A., D'Oca M. G. M., de Fatima A., Pilli R. A., Kohn L. K., Antonio M. A., de Carvalho J. E. Bioorg. Chem. 2006;34:173. doi: 10.1016/j.bioorg.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Sarli V., Giannis A., Guido B. C., Ramos L. M., Nolasco D. O., Nobrega C. C., Andrade B. Y. G., Pic-Taylor A., Neto B. A. D., Corrêa J. R. Clin. Cancer Res. BMC Cancer. 2008;2015;1415:7583. 283. [Google Scholar]
- Chen T. C., Da Fonseca C. O., Schönthal A. H. Am. J. Cancer Res. 2015;5:1580. [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Azab B., Quinn B. A., Shen X., Dent P., Klibanov A. L., Emdad L., Das S. K., Sarkar D., Fisher P. B. Curr. Mol. Med. 2014;14:125. doi: 10.2174/1566524013666131118110827. [DOI] [PubMed] [Google Scholar]
- Sultana S., Nafees S., Khan A. Q. Hum. Exp. Toxicol. 2013;32:1179. doi: 10.1177/0960327112474834. [DOI] [PubMed] [Google Scholar]
- Lebedeva I. V., Su Z.-Z., Vozhilla N., Chatman J., Sarkar D., Dent P., Athar M., Fisher P. B. Cancer Res. 2008;68:7439. doi: 10.1158/0008-5472.CAN-08-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardona S., Foussard V., Fournel S., Loubat A. Cancer Lett. 2002;181:187. doi: 10.1016/s0304-3835(02)00047-2. [DOI] [PubMed] [Google Scholar]
- Sahin M. B., Perman S. M., Jenkins G., Clark S. S. Leukemia. 1999;13:1581. doi: 10.1038/sj.leu.2401536. [DOI] [PubMed] [Google Scholar]
- Cho H.-Y., Wang W., Jhaveri N., Torres S., Tseng J., Leong M. N., Lee D. J., Goldkorn A., Xu T., Petasis N. A., Louie S. G., Schonthal A. H., Hofman F. M., Chen T. C., Fernandes J., Da Fonseca C. O., Teixeira A., Gattass C. R., Afshordel S., Kern B., Clasohm J., König H., Priester M., Weissenberger J., Kögel D., Eckerta G. P. Mol. Cancer Ther. Oncol. Rep. Pharmacol. Res. 2012;2005;2015;111391:2462. 943, 69. doi: 10.1158/1535-7163.MCT-12-0321. [DOI] [PubMed] [Google Scholar]
- Da Fonseca C. O., Teixeira R. M., Silva J. C. T., Fischer J. S. G., Meirelles O. C., Landeiro J. A., Quirico-Santos T., Da Fonseca C. O., Linden R., Futuro D., Gattass C. R., Quirico-Santos T., Da Fonseca C. O., Soares I. P., Clemençon D. S., Rochlin S., Cardeman L., Quirico-Santos T. Anticancer Res. Arch. Immunol. Ther. Exp. Histol. Histopathol. 2013;2008;2015;33562:5625. 267, 12. doi: 10.7243/2055-091X-2-12. [DOI] [PubMed] [Google Scholar]
- Hui Z., Zhang M., Cong L., Xia M., Dong J., Nandurkar N. S., Zhang J., Ye Q., Ponomareva L. V., She Q.-B., Thorson J. S., Chen T. C., Yu J., Nigjeh E. N., Wang W., Myint P. T., Zandi E., Hofman F. M., Schönthal A. H., Das B. C., Mahalingam S. M., Panda L., Wang B., Campbell P. D., Evans T., Chen T. C., Da Fonseca C. O., Schönthal A. H. Molecules. J. Med. Chem. Cancer Lett. Tetrahedron Lett. Int. J. Mol. Sci. 2014;2014;2017;2010;2016;19574005117:6671. 7478, 161, 1462, 1463. [Google Scholar]
- Dheer D., Singh V., Shankar R. Bioorg. Chem. 2017;71:30. doi: 10.1016/j.bioorg.2017.01.010. [DOI] [PubMed] [Google Scholar]
- (a) Russowsky D., Lopes F. A., Silva V. S. S., Canto K. F. S., D'Oca M. G. M., Godoi M. N. J. Braz. Chem. Soc. 2004;15:165. [Google Scholar]; (b) de Souza V. P., Vendrusculo V., Moraás A. M., Steffens L., Santos F. S., Moura D. J., Rodembusch F. S., Russowsky D., Silva G. C. O., Correa J. R., Rodrigues M. O., Alvim H. G. O., Guido B. C., Gatto C. C., Wanderley K. A., Fioramonte M., Gozzo F. C., de Souza R. O. M. A., Neto B. A. D. New J. Chem. RSC Adv. 2017;2015;415:15305. 48506. [Google Scholar]
- Giguère J. B., Thibeault D., Cronier F., Marois J. S., Auger M., Morin J. F. Tetrahedron Lett. 2009;50:5497. [Google Scholar]
- Moro V., Ferreira P. C., Migowski P., Rodembusch F. S., Dupont J., Lüdtke D. S. Tetrahedron. 2013;69:201. [Google Scholar]
- Appel R. Angew. Chem., Int. Ed. Engl. 1975;14:801. [Google Scholar]
- Zaharevitz D. W., Holbeck S. L., Bowerman C., Svetlik P. A. J. Mol. Graphics Modell. 2002;20:297. doi: 10.1016/s1093-3263(01)00126-7. [DOI] [PubMed] [Google Scholar]
- Canto R. F. S., Bernardi A., Battastini A. M. O., Russowsky D., Eifler-Lima V. L., Stuepp C. S., Figueiró F., Mendes F. B., Braganhol E., Bernardi A., Frozza R. L., Salbego C. G., Canto R. F. S., Russowsky D., Eifler-Lima V. L., Battastini A. M. O. J. Braz. Chem. Soc. Anticancer Res. 2011;2013;2233:1379. 4463. [Google Scholar]
- Figueiró F., Mendes F. B., Corbelini P. F., Janarelli F., Jandrey E. H. F., Russowsky D., Eifler-Lima V. L., Battastini A. M. O. Anticancer Res. 2014;34:1837. [PubMed] [Google Scholar]
- Zimmermann H., Zebisch M., Strater N. Purinergic Signalling. 2012;8:437. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treptow T. G. M., Figueiró F., Jandrey E. H. F., Battastini A. M. O., Salbego C. G., Hoppe J. B., Taborda P. S., Rosa S. B., Piovesan L. A., D'Oca C. R. M., Russowsky D., D'Oca M. G. M. Eur.Eur. J. Med. Chem.J. Med. Chem. 2015;95:552. doi: 10.1016/j.ejmech.2015.03.062. [DOI] [PubMed] [Google Scholar]
- Cabrera D. C., Rosa S. B., Oliveira F. S., Marinho M. A. G., D'Oca C. R. M., Russowsky D., Horn A. P., D'Oca M. G. M. Med. Chem. Commun. 2016;7:2167. [Google Scholar]
- Hans R. H., Guantai E. M., Lategan C., Smith P. J., Wan B., Franzblau S. G., Gut J., Rosenthal P. J., Chibale K. Bioorg. Med. Chem. Lett. 2010;20:942. doi: 10.1016/j.bmcl.2009.12.062. [DOI] [PubMed] [Google Scholar]
- Zammit S. C., Cox A. J., Gow R. M., Zhang Y., Gilbert R. E., Krum H., Kelly D. J., Williams S. J. Bioorg. Med. Chem. Lett. 2009;19:7003. doi: 10.1016/j.bmcl.2009.09.120. [DOI] [PubMed] [Google Scholar]
- González-Olvera R., Román-Rodríguez V., Negrón-Silva G., Espinoza-Vázquez A., Rodríguez-Gómez F., Santillan R. Molecules. 2016;21:250. doi: 10.3390/molecules21020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.