Abstract
Estrogens have historically been associated with female reproduction, but work over the last two decades established that estrogens and their main nuclear receptors (ESR1 and ESR2) and G protein-coupled estrogen receptor (GPER) also regulate male reproductive and nonreproductive organs. 17β-Estradiol (E2) is measureable in blood of men and males of other species, but in rete testis fluids, E2 reaches concentrations normally found only in females and in some species nanomolar concentrations of estrone sulfate are found in semen. Aromatase, which converts androgens to estrogens, is expressed in Leydig cells, seminiferous epithelium, and other male organs. Early studies showed E2 binding in numerous male tissues, and ESR1 and ESR2 each show unique distributions and actions in males. Exogenous estrogen treatment produced male reproductive pathologies in laboratory animals and men, especially during development, and studies with transgenic mice with compromised estrogen signaling demonstrated an E2 role in normal male physiology. Efferent ductules and epididymal functions are dependent on estrogen signaling through ESR1, whose loss impaired ion transport and water reabsorption, resulting in abnormal sperm. Loss of ESR1 or aromatase also produces effects on nonreproductive targets such as brain, adipose, skeletal muscle, bone, cardiovascular, and immune tissues. Expression of GPER is extensive in male tracts, suggesting a possible role for E2 signaling through this receptor in male reproduction. Recent evidence also indicates that membrane ESR1 has critical roles in male reproduction. Thus estrogens are important physiological regulators in males, and future studies may reveal additional roles for estrogen signaling in various target tissues.
I. HISTORICAL PERSPECTIVES ON ESTROGEN FUNCTIONS IN MALES
17β-Estradiol (E2) and other estrogens regulate many aspects of female reproductive development and function. Although estrogens were first detected in stallions in the 1930s (769), by the 1960s and 1970s, it became clear that males produced significant quantities of estrogens and men and males of other species had measureable circulating E2 concentrations. Furthermore, estrogen receptors (ER) were present in males during development and adulthood, and exposure to exogenous estrogens, especially developmentally, had deleterious effects on the male reproductive tract. Despite these data, roles for estrogen signaling in the normal male were difficult to determine for years, due both to a lack of good experimental systems to address this question and a paucity of clear end points for estrogen action. Over the last two decades, work using transgenic mouse models revealed that estrogens are critical for normal development and function of male reproductive and nonreproductive organs. This review traces the discovery of estrogen effects in males and provides an overview of current understanding of physiological roles for estrogens with an emphasis on more recent work with transgenic mouse models that have uncovered the complexity, breadth, and importance of estrogen actions in male reproductive tissues, as well as other organs.
Rapid research progress in the latter 20th century that elucidated E2 roles in female reproduction relied heavily on simple and powerful in vivo model systems. Hormonal fluctuations during the female estrous/menstrual cycle make it problematic to study E2 actions in intact animals. This was addressed partly by utilization of ovariectomized rodents (209, 210, 505). Ovariectomy and hormone replacement allows study of hormone actions in controlled and manipulable endocrine environments. In addition, these studies led to identification of E2-regulated biochemical, histological, and functional end points in female reproductive organs, and these robust end points facilitated E2 research. This approach is illustrated by work of Finn and Martin (210), who described key E2 effects in ovariectomized rodents that shaped present understanding of E2 action in females.
Ovaries are the major source of circulating estrogens in females, but in males, testes produce only ~20% of circulating estrogens, with the remainder from local production by adipose, brain, skin, and bone, which convert testosterone (T) to estrogen through aromatase actions (708). Diffuse estrogen production in males meant that there was no simple method of producing estrogen-deficient states comparable to ovariectomized females. This hindered progress in this area. Despite E2 and ER presence in males, and known deleterious effects of perinatal estrogen treatment, there was no definitive evidence that E2/ER signaling was important in normal male reproduction. Similarly, it was unclear whether E2/ER signaling was involved in etiology or progression of naturally occurring male reproductive pathologies. All these factors constrained scientific interest and limited progress in this field.
Effects of estrogen administration on males both during development and adulthood were described before identification of ER or measurement of circulating estrogens in males. Early work showed that estrogens affected male behavior (201, 407). In addition, estrogen treatment altered development/function of the testis, prostate, and seminal vesicles (13, 59, 84, 356, 413, 445, 461, 465, 527). Estrogen effects on growth (413) and nonreproductive targets such as bone (312) and plasma proteins (475) were also described in males, as well as alterations in circulating luteinizing hormone (LH) and T concentrations (217, 671). Finally, early estrogen administration increased male susceptibility to carcinogen-induced liver cancer (730). Thus males responded to E2, but the question of whether E2 was important for normal development and function of reproductive and nonreproductive organs was not answered until development of various knockout mice decades later.
II. ESTROGEN PRODUCTION AND ACTIONS IN MALES
A. Estrogen Sources and Estrogen Concentrations
Although estrogens in males were first reported in the 1930s, when high estrogen concentrations were detected in stallion urine (769), accurate quantitation of estrogens in serum and other fluids was impossible until development of radioimmunoassay methodologies in the 1960s (750). These studies revealed low but measureable blood concentrations of E2 and other estrogens in various species of males, although circulating E2 concentrations in males exceeded those in ovariectomized female rats or rats in diestrus (Table 1). In men, peripheral blood T concentrations of ~20 nM (495) are at least two orders of magnitude greater than E2 concentrations (30–200 pM; Table 1). In boars and stallions, conjugated estrogens such as estrone sulfate are uniquely elevated in both blood and semen, reaching nanomolar concentrations seen for androgens. Elevated E2 concentrations are found in rete testis fluid and in semen of many species (Table 1). These vary with age, with higher concentrations prepubertally and age-related declines due to natural reductions in T, a E2 precursor (137).
Table 1.
Estrogen concentrations in males
| Source | Concentration | Species | Reference Nos. |
|---|---|---|---|
| Peripheral blood | 3.6–91 pg/ml | Human | 98, 112, 181, 207, 523, 762 |
| 29–197 pM | 125, 397, 495, 607 | ||
| 43–464 pM (estrone) | 397, 607 | ||
| 40–145 pg/ml | Monkey | 716 | |
| 2–175 pg/ml | Rat | 58, 147, 168, 184, 340 | |
| ~70 pM | Mouse | 77 | |
| 73.4 pg/ml | Horse | 623 | |
| 64–250 ng/ml (estrone sulfate) | 138, 571 | ||
| 9–180 pg/ml | Bull | 192, 232 | |
| 6.3 pg/ml | Ram | 459 | |
| ~180 pg/ml | Boar | 139, 624 | |
| 0.18 nM (total estrogens) | |||
| 21.5 nM (estrone sulfate) | |||
| 22.1–24.7 pg/ml | Avian | 401 | |
| Testicular vein | 104–200 pg/ml | Monkey | 716 |
| 19.0 pg/ml | Rat | 168 | |
| 450 ng/ml (estrone sulfate) | Horse | 623 | |
| 1.09 nM (total estrogens), 52.4 nM (estrone sulfate) | Boar | 624 | |
| Testicular lymph | 900 ng /ml (estrone sulfate) | Horse | 623 |
| Testicular homogenate | 5–20 ng/g | Man | 112 |
| 39–751 pg/g | Rat | 137, 340 | |
| ~4,500 pg/g (breeding season), ~100 pg/g (nonbreeding) | Avian | 401 | |
| Rete testis | 14–195 pg/ml | Monkey | 716 |
| 249 pg/ml | Rat | 225 | |
| 11.5 pg/ml | Bull | 232 | |
| 0.38 nM (total estrogens), 8.60 nM (estrone sulfate) | Boar | 624 | |
| Semen | 162 pg/ml | Man | 98 |
| 50–73 pg/ml (E2) | Horse | 138, 399 | |
| 0.73–6.3 ng/ml (estrone sulfate) | 138, 399, 571 | ||
| 50–890 pg/ml | Bull | 192, 232, 246 | |
| 430 pg/ml (E2), 860 pg/ml (estrone) | Boar | 139 |
Many of these references, especially before 2010, used immunoassays to measure estrogen concentrations. It is now recommended that liquid chromatography, tandem mass spectroscopy be used when assaying for steroid hormones present in low concentrations. Unless otherwise indicated, measurements are for E2.
In males, E2 production requires aromatase (Cyp19a1), a ubiquitous NADPH cytochrome P450 reductase enzyme (117). The testis was known to be involved in estrogen synthesis for years (769), but early studies focused on various T metabolites (49, 186, 533, 591). Despite descriptions of E2 binding in both testis and epididymis (161–163), well into the 1990s E2 was not considered a major regulator of male reproduction, at least in adults (reviewed in Ref. 292), and estrogen binding activity was considered a remnant of developmental processes influenced by estrogen action (260, 261, 456).
Initial work suggested that FSH-stimulated Sertoli cells are primary sources of estrogen in immature males, while LH-stimulated Leydig cells are the primary source in adult testis, as they express more aromatase than adult Sertoli cells (113, 275, 377, 385, 405, 501, 502, 533, 591, 700). However, in 1993, aromatase expression in adult testicular germ cells was first reported (496). Aromatase was localized in Golgi of round spermatids and throughout the cytoplasm of elongating and late spermatids (Figure 1). Confirmed by Western and Northern analysis, aromatase activity in germ cells was comparable to that in Leydig cells (115, 328, 329, 496). In testis, the proximal promoter II regulates aromatase transcription, but numerous transcription factors drive this expression in a cell-specific manner, with Sertoli and germ cells showing specificity differing from Leydig cells (275).
FIGURE 1.
Aromatase (Cyp19) expression in male mouse reproductive tract. A: testis (T) and epididymis (E) from an adult (71-day-old) Cyp19RFP mouse showing RFP expression that is extensive within the testis, but lower in epididymis. B: adult testis showing immunohistochemical localization of aromatase in Leydig cells (L), round spermatids (Rs), and elongated spermatids (Es). C: adult caput epididymis showing immunohistochemical localization of aromatase in the cytoplasmic droplet (Cd) of sperm (Sp) in the tubular lumen. E, epithelium.
Aromatase is expressed in male germ cells of several species (Table 2), including mouse, rat, brown bear, bank voles, rooster, and human (reviewed in Refs. 115, 116). Aromatase is located in cytoplasmic droplets of sperm tails (Figure 1), but becomes less intense as sperm traverse the epididymis (330). Carreau’s laboratory reported that germ cells contribute ~62% of total testicular aromatase (405). Only a few species (boar, ram, and stallion) have germ cells that are not aromatase-positive (31, 285, 288, 643). It is unclear whether this reflects differences in aromatase antibodies or simply lack of aromatase in some species. Others report aromatase in epididymal epithelium and interstitium (111, 285, 545, 630), which could supply estrogen when sperm are not its primary luminal source. Thus, in humans and most experimental species, testicular germ cells and epididymal sperm serve as unique estrogen sources, which may target abundant ESR1 in efferent ductule and epididymal epithelium (296) (Table 3).
Table 2.
Aromatase presence in adult male reproductive tract tissues
| Species | Tissues | Reference Nos. |
|---|---|---|
| Mouse | Whole testis, Leydig cell, immature germ cell, spermatozoa | 67, 121, 247, 330, 496, 724 |
| Rat | Whole testis, Leydig cell, immature germ cell, spermatozoa, epididymal epithelium | 34a, 78, 85, 86, 110, 238, 239, 282, 328, 329, 377, 388, 404, 405, 528, 533, 545, 551, 591, 630, 681, 686, 687, 688, 692, 699, 734, 735, 736, 744, 760 |
| Dog | Leydig cell, Sertoli cell, immature germ cell | 546, 733 |
| Monkey | Immature germ cell, Leydig cell, immature germ cell | 545, 692 |
| Human | Immature germ cell, spermatozoa, epithelium of efferent ductule, epithelium of proximal epididymis | 22, 24, 54, 93, 94, 111, 114, 117, 323, 382, 383, 384, 572, 600, 639, 692 |
| Bird | Leydig cell, immature germ cell, spermatozoa | 226, 378, 698 |
| Fish | Total testis analysis, Leydig cell, immature germ cell | Dogfish (S. acanthias) (60, 156), European sea bass (Dicentrarchus labrax) (75, 160, 250), rainbow trout (Oncorhynchus mykiss) (358), Nile tilapia (Oreochromis niloticus) (357), sea bream (Acanthopagrus schlegelii) (396) |
| Amphibian | Total testis analysis | 376, 510 |
| Turtle | Total testis analysis | 245, 552 |
| Bear | Leydig cell, Sertoli cell, immature germ cell | 318, 511, 690 |
| Deer | Leydig cell | 277 |
| Boar | Leydig cell | 145, 146, 149, 150, 224, 467, 732 |
| Bull | Total testis analysis | 704 |
| Ram | Total testis analysis, Leydig cell | 570, 615, 704 |
| Stallion | Leydig cell, Sertoli cell, immature germ cell, epididymis | 14, 193, 282, 288, 399, 400, 643 |
| Bat | Leydig cell, Sertoli cell, germ cells | 50 |
| Squirrel | Leydig cell, Sertoli cell, germ cells | 412, 567 |
| Bank vole | Leydig cell, Sertoli cell, immature germ cell | 68, 223, 362, 616 |
Table 3.
Localization of ESR1 in the male reproductive tract: species comparison
| Species | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ | Bird | Fish | Amphioxus | Newt | Turtle | Lizard | Bat | Rat, Hamster, & Squirrel | Mouse & Vole | Dog | Cat | Goat & Ram | Marsupial | Horse | Boar | Monkey | Human |
| Testis | + | + | + | + | + | + | + | +/− | + | + | + | +/− | + | + | + | +/− | +/− |
| Leydig cell | + | + | + | +/− | +/− | + | + | + | − | + | + | + | + | +/− | |||
| Sertoli cell | − | + | + | − | +/− | +/− | +/− | − | + | + | + | + | − | +/− | |||
| Germ cell | −/+ | + | + | − | + | +/− | +/− | +/− | − | − | + | + | + | +/− | +/− | ||
| Myoid cell* | − | − | +/− | + | + | + | − | + | − | +/− | |||||||
| Rete testis | + | + | + | +/− | + | + | − | − | + | ||||||||
| Efferent ducts | + | + | + | + | + | + | + | + | + | + | + | ||||||
| Epididymis | + | + | + | + | +/− | + | − | + | + | − | |||||||
| Vas deferens | +/− | +/− | − | + | + | ||||||||||||
| Reference Nos. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
Most references show data for ESR1 using immunohistochemistry or Western blotting; however, in some cases, mRNA presence was determined by RT-PCR, ribonuclease protection assay, or in situ hybridization. For some tissue, data from different laboratories may be inconsistent. Positive data are indicated by + and negative results by -. Data variation likely depends on quality of tissue preservation, antibody specificity, and variations in laboratory techniques. In cases where there are no data, the square is blank.
Peritubular myoid cell.
Discussions for numerous species are available in previous reviews (115, 295, 296). Reference numbers refer to the following: 1. Bird: (327, 401, 513). 2. Fish: black porgy, Acanthopagrus schlegeli Bleeker, protandrous hermaphrodite fish (279); channel catfish (746); rainbow trout, Oncorhynchus mykis (83); teleost fish, Sparus aurata (654); killifish, mummichog, Fundulus heteroclitus (262); European eel, Anguilla anguilla (468); Asian swamp eel, Monopterus albus (183). 3. Amphioxus, Branchiostoma belcheri (199). 4. Newt, Triturus marmoratus marmoratus (26). 5. Turtle, Trachemys scripta (158, 245). 6. Lizard, Italian wall lizard, Podarcis sicul (707). 7. Bat (50, 520, 521). 8. Rat, hamster, and squirrel: (33, 78, 137, 190, 211, 219, 297, 359, 370-372, 412, 424, 425, 460, 472, 516, 518, 542, 543, 551, 599, 605, 608, 636, 677, 718, 751, 759, 760). 9. Mouse and vole: (12, 68, 77, 154, 260, 311, 314, 333, 414, 436, 494, 632, 673, 751, 763). 10. Dog: (492, 618, 676). 11. Cat: (492, 617). 12. Goat, ram: (256-258, 441, 606). 13. Marsupial, tammar wallaby, Macropus eugenii: (102). 14. Horse: (236, 282, 530, 535). 15. Boar: (398, 476, 573). 16. Monkey: (90, 211, 280, 342, 542, 609, 610). 17. Human: (23, 58, 88, 123, 177, 196, 202, 203, 205, 249, 270, 359, 384, 435, 439, 525, 542, 543, 610, 622, 637).
Estrogens are inactivated through sulfoconjugation, which is catalyzed by estrogen sulfotransferase (EST) that is abundantly expressed in liver and other organs (657), and thus EST can affect estrogen concentrations in male organs. In males, highest EST concentrations and activities are in testis, but it also occurs in epididymis and ductus deferens (227, 302, 429, 584, 683). In testes, it is exclusively in Leydig cells, but in the mouse it is found in epithelia of the epididymis and ductus deferens, as well as in smooth muscle of the ductus deferens (683). However, its expression in prostate or seminal vesicle expression is controversial.
By inactivating estrogens, this enzyme regulates not only local estrogen exposure but also eventual biological effects. Epididymal epithelial EST (227, 304, 400, 477, 584, 683) may protect from excess estrogen (429) arriving through the efferent ducts as a result of CYP19A1 in spermatozoa (115, 496). Within the epididymal lumen, EST may stabilize acrosomal membranes through sulfation of membrane cholesterol (227, 584).
Testicular and epididymal EST is regulated by LH and androgens (683). Differential EST expression may contribute to differences in estrogen sensitivity among different strains of laboratory animals. For example, CD-1 mouse testes have the highest organ-specific activity (658) and are 16-fold less E2-sensitive, with 3.5 times more EST, than B6 mice (660). Testes from EST knockout mice showed Leydig cell hyperplasia and hypertrophy, with decreased testicular and epididymal weights (568). Sperm motility and fertility were also reduced. Expression of EST decreases with age, which may, along with age-related decreases in T production, contribute to increased serum E2 and decreased T/E2 ratios in elderly men.
B. ER Expression in Male Reproductive Organs
In the 1960s, studies by Jack Gorski, Elwood Jensen, Bert O’Malley, and others determined the mechanism of steroid hormone signaling (252, 453, 656). Early ER studies focused on female organs, but ER were demonstrated in males as well. In males, many studies examined brain regions such as hypothalamus (663). Later, ERs were reported in male reproductive as well as nonreproductive (liver, muscle, and kidney) organs (187, 410, 462). To characterize ER distribution, studies used either biochemical approaches (136) or the nascent technique of steroid autoradiography (663), which employed binding of radioactive estrogen tracers to identify ER. Steroid autoradiography was used for years to visualize ER expression, especially in developing organs. Steroid autoradiography was supplanted by immunohistochemistry in the 1980s, and recent data regarding ER distribution in male organs comes primarily from immunohistochemistry. These studies have benefited from constantly improving antibodies as well as methodological advances (e.g., antigen retrieval) that facilitated ER immunohistochemistry. In addition, methodologies continue to be developed, such as the new mouse model that expresses red fluorescent protein (RFP) under the control of ESR1 and ESR2 promoters that we describe here.
Early autoradiographic studies assumed that E2 binding resulted from a single ER (252, 335, 504, 656). As immunohistochemistry for localizing ER gained preeminence in the 1980s, it was initially assumed that ER immunostaining in reproductive tissues (65, 361) was equivalent to autoradiographic ER localization (663). Indeed, agreement between immunohistochemical and autoradiographic ER localization when both techniques were used simultaneously in organs such as uterus (65) supported this idea. However, identification of a second ER (371) indicated that autoradiographic and immunohistochemical data differed. The new ER, now known as estrogen receptor 2 (ESR2) or estrogen receptor beta (ERβ) to differentiate it from the original ER [now known as estrogen receptor 1 (ESR1) or estrogen receptor alpha], was originally identified in rat prostate, but had wide distribution in reproductive and nonreproductive organs (371).
Identification of ESR2 indicated that autoradiographic E2 binding resulted from both ESR1 and ESR2 in target organs, while ESR1 immunostaining identified only ESR1, but presumably not ESR2 (depending on antibody specificity). Even this was oversimplified, as subsequent work revealed another ER, now known as G protein-coupled estrogen receptor (GPER) (204, 577, 678). This protein is associated with cell membranes and endoplasmic reticulum and binds estrogens such as E2, although with less affinity than ESR1/ESR2 (204, 577, 678). More recent studies have revealed that in addition to the 66 kDa ESR1, some cells express two truncated ESR1 isoforms, ERα36 and ERα46 (126, 216, 722). These variants are found both in the nuclear/cytoplasmic as well as membrane compartments. Some evidence indicates that these variant forms of ESR1 may be important in breast cancer (723), but their role in normal female or male physiology is totally unknown. Thus older steroid autoradiography showing ER expression represents binding activity of several molecules (ESR1, ESR2, GPER, ERα36, and ERα46) and must be interpreted carefully.
1. Expression of ER in adult males of various species
Expression of ESR1 (Figure 2, Table 3) and ESR2 (Figure 2) occurs throughout adult male mouse reproductive tracts, although expression patterns for each are unique (763). In testis, most antibodies localized ESR1 only in Leydig and peritubular cells (Figure 2, Table 3). However, ESR1 mRNA and protein in Sertoli cells were reported (265, 414, 422). Conversely, ESR2 is found in Leydig, peritubular, germ, and Sertoli cells. One commonality has been efferent ductules (Figures 2 and 3), where ESR1 expression is threefold higher than uterus (297) and intense across species (296). Both ESR1 and ESR2 are expressed in nonciliated epithelial cells of all species and ciliated cells of most species (Figure 3). ESR2 is also expressed in stroma. Epididymis expresses both ESR1 and ESR2, but as in other organs, expression patterns are unique and regionally variable. Both ESR1 and ESR2 occur in epithelium and stroma of ductus deferens, although again expression of the two receptors is not superimposable.
FIGURE 2.
Expression of ESR1 and ESR2 in male mouse reproductive tract. Representative samples of immunohistochemical staining with 2 different ESR1 and ESR2 antibodies and red fluorescent protein (EsrRFP) mice. In Esr1RFP (63 days of age) and Esr2RFP mice (59 days of age), cell lineages expressing Esr1 or 2 will subsequently show RFP and may not correlate with current immunohistochemical staining. ESR1 staining is primarily nuclear using 6F11 [NCL-ER-6F11 antibody (Novocastra, Newcastle upon Tyne, UK)] and labels many more epididymal cell types than does the anti-ESR1 antibody 06–935 (Millipore, NH). Testis shows ESR1 exclusively in interstitial or Leydig cells (L) with no immunostaining in seminiferous tubules (St), although low fluorescence was seen with RFP. Efferent ductules show strong epithelial nuclear ESR1 staining with both antibodies, but cytoplasmic staining was also seen, especially with 06–935. In epididymis, apical (Ap) and clear cells (Cl) show strong nuclear staining with 6F11, but staining differences were observed in other cell types with the two antibodies. ESR2 staining was more widespread than ESR1. However, the S-40 ESR2 antibody (Dr. Saunders, Univ. of Edinburgh) showed intense nuclear staining, while PA1–311 (Thermo, Waltham, MA) shows considerable or exclusive cytoplasmic staining. The lack of RFP fluorescence in efferent ductules and most epididymal regions may indicate that ESR2 expression in these regions is delayed past day 60. In rats, ESR1 is expressed in efferent ducts and epididymis earlier than ESR2 (605), although in humans the opposite occurs (627). In the pig epididymis, ESR2 does not appear until after puberty (109). E, epithelium; Lu, lumen; Sm, smooth muscle; Ci, cilia. [Images for ESR1 using the 6F11 antibody and for ESR2 using the S-40 antibody were modified from Zhou et al. (763).]
FIGURE 3.
Efferent duct expression of ESR1 in 3 mammalian species. Efferent ductules from mouse (A), marmoset monkey (B), and hamster (C) show intense immunostaining for ESR1. In most species, both ciliated (Ci) and nonciliated (Nc) cells have strong reactions in the nucleus, with some light cytoplasmic staining. However, the monkey ciliated cells were inconsistent, with some staining slightly positive and others being negative. The hamster image shows the efferent duct/initial segment junction, with intense staining of efferent duct epithelium but minimal epididymal staining.
Although most studies utilized rodents or humans, ESR1 has been reported in various mammalian and nonmammalian vertebrates (Table 3). In testis, ESR1 mRNA and immunostaining were not detected in some studies, but were strongly positive in others (Table 3). Other studies suggested that ESR1 mRNA expression changes prepubertally (333). Epididymal studies were also inconsistent, with some studies showing no epididymal Esr1 mRNA (211), while in mice, nearly all epithelial cells are positive (342, 763).
In contrast to ESR1, most reports suggest that ESR2 protein and mRNA are expressed ubiquitously in male reproductive organs of several species (101, 296, 492, 518, 563, 608, 610, 763), but varies with species, age, antibody, and organ. For example, mouse testicular Esr2 mRNA was strongly expressed from postnatal day 1–5, then nearly absent from days 12–26. Mouse epididymis showed essentially no expression prepubertally (333). In rats, ESR1 is expressed much earlier than ESR2 in efferent ducts and epididymis (605), and some studies report no epithelial expression (359). However, in humans, the opposite was found, with Esr1 mRNA present first (627). In pigs, epididymal Esr2 mRNA does not appear until postpubertally (109). Thus species differences and developmental expression patterns must be considered.
While commonalities were noted in terms of ESR1/ESR2 expression in primates versus mice, there were also differences. For example, although ESR2 was expressed throughout primate testis, as in mice, ESR1 expression was minimal in primate testis. Similar to mice, ESR2 was expressed in stroma and epithelium of human efferent ductules, ductus deferens, and epididymis. Conversely, ESR1 was abundant only in nonciliated cells of efferent ductules, but minimal in epididymal epithelium, despite pronounced staining in some mouse epididymal epithelial cell types. Similar variations in other species have resulted in significant controversy (Table 3), with differences in antibodies, species, fixation, and animal ages all contributing.
The EsrRFP mice provide a new method for identification of Esr1 and Esr2 expression in males, with RFP being localized in cytoplasm of cells expressing these receptors (101). This mouse can be used to compare RFP localization with immunohistochemical data (Figure 2). This model allows visualization of ER expression in whole or even groups of reproductive organs. For example, Esr1RFP was more intense in epididymis than testis, the opposite of Esr2RFP (Figures 2 and 4). In mouse testis, this is consistent with the predominately interstitial immunohistochemical ESR1 expression (Figure 2). However, staining with two different ESR1 antibodies differed in some epididymal areas, while efferent ductal epithelium stained intensely with both. Previous studies have shown higher Esr1 mRNA in corpus than other epididymal regions (296), consistent with Esr1RFP data, but not immunostaining.
FIGURE 4.
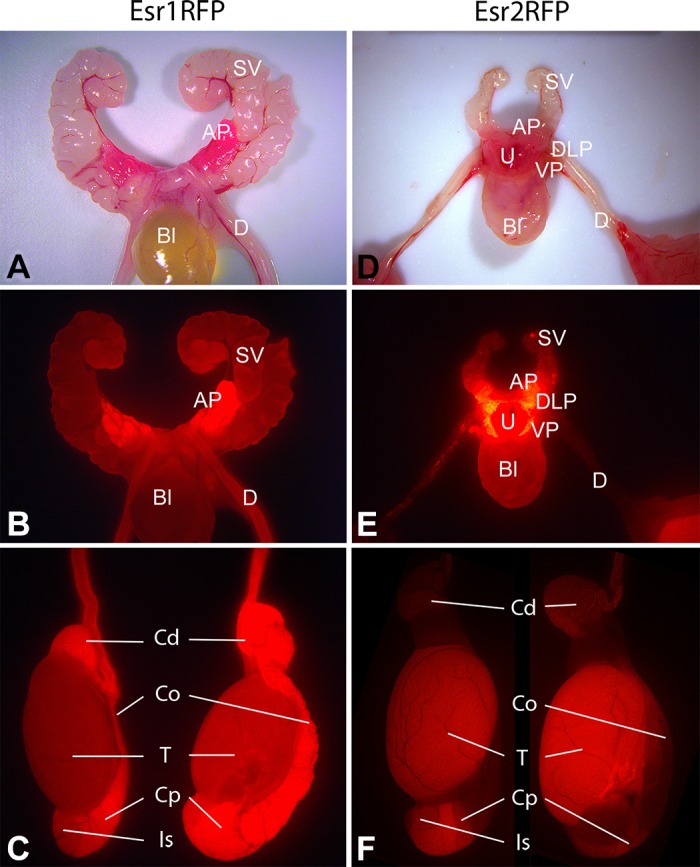
Expression of ESR1 and ESR2 in male mouse reproductive tract visualized using EsrRFP mice. In Esr1RFP (A--C) and Esr2RFP (D--F) mice, red fluorescent protein (RFP) expression is under the control of the respective steroid receptor. A and B: whole mounts of adult (63-day-old) Esr1RFP reproductive tract photographed with normal light (A) or by exposing the tissue to light at 549 nm and then looking at fluorescence emission at 574 nm using a Zeiss HBO 100 illuminating system (B). The anterior prostate (AP; also called the coagulating gland) showed strong ESR1 expression (B) compared with much weaker ESR1 expression in seminal vesicles (SV) and ductus deferens (D), while expression in the bladder (Bl) was at the limit of detection. C: in whole mounts of adult (63-day-old) Esr1RFP testis and associated structures, the testis (T) is lightly positive, but more intense fluorescence is seen in the initial segment (Is), caput (Cp), corpus (Co), and cauda (Cd) regions of the epididymis. In juvenile (22-day-old) Esr2RFP male mice (D and E), ESR2 showed intense expression in ventral and dorsolateral prostate (VP and DLP, respectively), while AP and urethra (U) showed modest expression, Bl and SV showed minimal expression, and the ductus deferens (D) was essentially negative. F: in adult (59-day-old) Esr2RFP male mice, the IS and Cp of the epididymis showed clear signal for ESR2, while ESR2 expression in the Co and Cd regions of the epididymis were basically undetectable. In contrast to the epididymis, where ESR1-RFP expression (C) was more dominant compared with ESR2-RFP (F), in the testis ESR2-RFP expression (F) was stronger than ESR1-RFP expression (C).
High ESR1 expression in anterior prostate (Figure 4) contrasts with much lower expression in neighboring seminal vesicles. Expression of ESR2 in both ventral and dorsolateral prostate is especially intense (Figure 4), contrasting with lower expression in anterior prostate and urethra and minimal seminal vesicle expression.
One limitation of this model is the requirement that normal Esr transcriptional activity involves only one allele, as the other allele is null due to iCre insertion in place of an initiation codon of either Esr1 or Esr2 to drive the universal promoter of the RFP transgene (101). However, RFP will be expressed in a cell lineage beginning with initial Esr expression. Thus cells expressing Esr1 in early development but subsequently losing this expression still show RFP fluorescence, revealing past ESR1 expression even when ESR1 is no longer produced.
Studies have reported increased proliferation of Sertoli cells in response to E2 and weak environmental estrogens during development (55, 122, 237, 411, 425), with rapid estrogen responses in males possibly involving GPER present in Sertoli cells, germ cells, epididymis, and sperm (115, 132, 236, 296, 424). Furthermore, mouse and rat Sertoli cells were reportedly ESR1 negative (518, 551, 763) or positive (137, 414, 422, 425, 718). In Figure 2, Sertoli cells were negative with two different antibodies, although there was weak expression in Esr1RFP mice. However, lack of ESR2 fluorescence in Esr2RFP mice was surprising as ESR2 Sertoli cell immunolocalization has been consistent. This may reflect limitations of this model when expression is low. A Sertoli cell line (SK11) derived from young mice expressed ESR2 but not ESR1, and following transient transfection of a reporter gene with an estrogen response element, these cells showed a dose-response to 5-androstane-3-β,17β-diol, a 5α-reductase metabolite with high ESR2 affinity (652). Interactions of both ERs and GPER may help to explain estrogen actions on testicular cells as well as epididymis and sperm (748, 752, 753). Therefore, future studies are required to understand the presence and activity of the estrogen receptors in male reproductive tracts and the significance of different staining patterns and receptor interactions.
2. Developmental ER expression
Perinatal exposure of males to natural or synthetic estrogens such as diethylstilbestrol (DES) produces long-term changes in male reproductive organs in rodents and other species (reviewed in Ref. 455), suggesting functional ER are present in developing reproductive organs. Early autoradiographic studies showed that ER was present as early as fetal day 13 in mesenchyme of mouse urogenital sinus, the precursor of male prostate and bulbourethral glands, and on day 16 of gestation in mesenchyme of Wolffian ducts (148, 308, 664, 665), which form epididymis, ductus deferens, and seminal vesicles. Efferent ductules were the first male reproductive structure to show nuclear epithelial E2 binding during development (148, 308, 664, 665). These results suggested that fetal male reproductive organs are estrogen targets during their early ambisexual stage, consistent with literature showing early estrogen exposure produces male reproductive abnormalities (455). As organs such as prostate, bulbourethral gland, epididymis, ductus deferens, and seminal vesicles differentiate, they maintain ER expression (148, 308, 614, 664). In addition, ER expression occurs in human fetal testis (110). Thus fetal male reproductive organs and their precursors are targets for endogenous and exogenous estrogens.
C. Deleterious Effects of Early Estrogen Administration in Humans and Animals
1. Developmental DES exposure in humans and animals
In the mid 1970s, McLachlan and colleagues (456, 489, 490) treated mid-gestation pregnant mice with DES, producing adult reproductive abnormalities in offspring, including cryptorchid testes, epididymal cysts, seminal vesicle abnormalities and increased infertility. The window for DES effects extends into postnatal life, and Bern, Iguchi, and colleagues (317) demonstrated that neonatal DES treatment also produced adult male reproductive abnormalities and infertility.
DES-induced developmental defects in efferent ducts and rete testis were similar to those in Esr1KO mice (32, 212, 213). These abnormalities were sometimes accompanied by reduced AR, and this, along with discovery that exogenous estrogens decrease ESR1 (516), illustrates the potential of early estrogen exposure to alter subsequent receptor exposure and the balance between androgen and estrogen signaling in males.
Animal DES studies were driven in part by pioneering studies by Herbst et al. in 1971 (283) showing that young women whose mothers had taken DES during pregnancy were prone to develop a rare cancer, vaginal clear cell carcinoma. At approximately the same time as McLachlan’s animal studies were published, additional studies of male offspring of women given DES during pregnancy indicated that these men had higher incidences of reproductive problems (62, 63) that correlated well with animal models.
2. Environmental estrogens
Environmental estrogens (xenoestrogens) are a heterogeneous class of chemicals, both man-made and natural, with estrogen-mimicking activity. These compounds can be synthetic industrial pollutants or pesticides (e.g., bisphenol A or BPA, methoxychlor, kepone, DDT/DDE, atrazine), pharmaceuticals (e.g., DES, ethinyl estradiol), or naturally occurring phytoestrogens (e.g., genestein, daidzein). Mechanisms of xenoestrogen actions are varied and can include transactivation of nuclear ESR1 and ESR2 as well as activation of membrane-initiated signaling through membrane ESR1, ESR2, and/or GPER, triggering multiple downstream cascades (725). Consequently, xenoestrogen exposure can lead to variable responses between compounds and end points examined. For in-depth discussions of this topic, refer to recent reviews (251, 334, 454). It is notable that due to variable receptor affinities, selectivity for membrane versus nuclear receptors, activation of other steroid and thyroid receptors in addition to ERs, distinctive metabolism and compound-specific pharmacokinetics, xenoestrogens will not necessarily reproduce E2 effects but rather initiate distinct and often nonpredictable and nonmonotonic dose responses that differ between end organs (701). As a result, low doses of xenoestrogens can interfere with natural estrogen actions, even in the presence of higher circulating E2 concentrations.
III. TRANSGENIC ANIMAL MOUSE MODELS FOR STUDYING ESTROGEN PHYSIOLOGY IN MALES
A. Estrogen Receptor 1 Knockout Mice
Testosterone and its metabolite dihydrotestosterone (DHT) facilitate development and continuous release of spermatozoa and act on the epididymis to enable fertilization-competent sperm maturation and storage (501). However, spermatozoa must travel through a complicated region between the rete testis and caput epididymis, which includes the efferent ductules and initial segment of the epididymis (291). This region expresses androgen receptor (AR) but is also uniquely dependent on estrogen and ESR1, specifically in efferent ducts (299, 342), and DHT in the initial segment, where 5α-reductase activity predominates (582). These tubules require luminal delivery of these steroids, as circulating hormones do not fully maintain the epithelium following proximal efferent ductule ligation (200, 583). It is noteworthy that rat efferent duct epithelium expresses more ESR1 than any other male or female tissue (297), and efferent ductule fluid is rich in E2 due to collective activities of Cyp19a in sperm and different testicular cells (115, 330, 496).
Efferent ductules are a series of tubules connecting the rete testis and epididymis. Their epithelium consists of ciliated cells whose beat appears to mix luminal fluid and water-absorbing nonciliated cells whose morphology and physiology resemble kidney proximal tubules (142, 290, 291, 321). Efferent duct epithelium reabsorbs up to 96% of luminal fluid (140–142, 321) and concentrates sperm before their epididymal entry and is responsible in part, along with the caput epididymis, for maintaining an optimal sperm maturation microenvironment (341, 343). As in kidney, physiology of water movement in efferent ducts and epididymis is highly coupled to ion transport (142, 272, 320).
Expression of numerous genes and proteins is altered in male reproductive tracts of Esr1KO mice and rodents treated with ER-specific estrogens or anti-estrogens (248, 296, 341, 375, 544, 629, 754). However, only two physiological roles for estrogen have been demonstrated: ESR1 is required for 1) fluid resorption by efferent ductule epithelium (293) and 2) maintenance of sperm morphology and motility (343). Both of these depend on expression of various ion and water transport proteins (293, 514, 598, 761) to establish luminal environments that maintain optimal pH, osmolality, and sperm concentration.
The original Esr1KO mice had low-level expression of a truncated ESR1 (191, 254, 421), complicating interpretation of their reproductive phenotype. However, key aspects of the Esr1KO phenotype, such as fluid resorption impairment and secondary testicular effects, were replicated in exon 3 Esr1KO mice (21, 129, 189, 254), Esr1KO rats (597), EAAE mice in which Esr1 DNA binding was blocked (6), AF2ERKI mice with mutated AF-2 (activation function domain) (25), and NOER (nuclear-only ESR1) mice (482). ESR1 inactivation also causes Leydig cell hypertrophy and elevated serum T, but these effects are indirect due to increased LH (12, 253) and increased Leydig cell T production capacity (9).
In Esr1KO mice, the major morphological effect was impaired efferent ductule epithelial differentiation, resulting in decreased epithelial height and loss of structures associated with fluid reabsorption in nonciliated cells (Table 4). In addition, there were reductions in several proteins responsible for fluid/ion equilibrium (341–343, 598, 761). These changes caused a more than twofold luminal dilation in Esr1KO efferent ductules compared with WT (Table 4, Figure 5) with fluid accumulation in rete testes and seminiferous tubules (191, 253, 293, 294, 480, 682). Motile cilia numbers were also reduced (Table 4, Figure 5), and those present showed an abnormal beat (294). These changes were observed as early as postnatal day 10 (394) and could have been due to abnormal development. Treatment of adult animals with a potent anti-estrogen, Faslodex (717), confirmed that ESR1 plays a major physiological role in efferent ducts (Figure 5). Adult Faslodex treatment mimicked many Esr1KO phenotypes such as efferent duct and rete testis dilation, but only caused partial seminiferous tubular atrophy without increased testis weight (133, 393). Furthermore, there was a 50-day latency in the response to blocking ESR1 activity. Similar findings were also seen with rats (515, 519).
Table 4.
Key morphological effects of ESR1 disruption in male reproductive tracts
| Morphological Feature | Change |
|---|---|
| Testis | |
| Testis size | Transient increase |
| Seminiferous tubule lumen | Dilation |
| Seminiferous tubule | Atrophy with aging |
| Rete testis | Dilation, glycogen accumulation |
| Leydig cell | No effect |
| Sertoli cell | No effect |
| Germ cells | Decreased number but normal morphology |
| Efferent ducts | |
| Lumen | Dilation |
| Blind-ending tubules | Increased number |
| Epithelium | Decreased height |
| Nucleus | Decreased size |
| Nonciliated cell | Decreased volume |
| Microvilli | Decreased number and size |
| Endocytic apparatus | Decrease |
| Water channels | Decrease |
| SLC9A3 | Decrease |
| CAII | Decrease |
| CFTR | Increase |
| Ciliated cell | Decrease cilia number, abnormal beat |
| Epididymis | |
| Initial segment | Abnormal growth and displacement of epithelium in efferent ducts |
| Apical cells | Abnormal |
| Clear cell | Abnormal |
| Luminal sperm | Decreased concentration |
| Sperm motility | Decrease |
| Sperm morphology | Abnormal |
FIGURE 5.
Efferent ductule morphology in Esr1KO and anti-estrogen (Faslodex)-treated mice. A and B: light microscopy of adult wild-type (WT) and Esr1KO efferent ductules. WT ducts have a periodic acid (PAS)-positive brush border of microvilli (Mv) on nonciliated cells, which move sodium ions (Na+) and water (H2O) to concentrate luminal sperm that are transported into the epididymis. Long cilia (Ci) project into the lumen. Esr1KO ducts have a dilated lumen and reduced epithelial height. Epithelium is deficient in microvilli, and cilia are fewer and shorter. Sodium transport and water resorption are inhibited, but chloride ion (Cl-) secretion into the lumen is increased, adding to water accumulation. C--F: transmission electron microscopy of wild-type and Esr1KO efferent ductules. WT epithelium is taller than Esr1KO (double-headed red arrows). WT nonciliated cells (Nc) show a well-developed luminal border of microvilli (double-headed black arrows), coated pits (Cp), and apical resorption tubules (At). Esr1KO duct epithelium is short, and microvilli of nonciliated cells are short or absent and coated pits and apical tubules are reduced in apical cytoplasm. G--J: light microscopy of adult control and anti-estrogen (Faslodex)-treated efferent ductules. Control ducts have a smaller lumen but taller epithelium than Faslodex-treated mice. Sodium and water transport are actively moved into the interstitium but inhibited in treated epithelium. Nonciliated cells in controls have a PAS+ brush border of microvilli and ciliated cells support long cilia projecting into the lumen (I), in contrast to Faslodex-treated epithelia (J). K and L: transmission electron microscopy of control Faslodex-treated efferent ductules. Control epithelium is tall (K) compared with Faslodex-treated ducts (L). Control nonciliated cells have a well-developed luminal microvillous border, while treated duct epithelium has short microvilli. Control ciliated cells have numerous basal bodies (red arrowheads) in the apical cytoplasm to support cilia projecting into the lumen, in contrast to reduced cilia in treated cells (L). [The Esr1KO and Faslodex-treated images from Hess et al. (298), with permission from Taylor & Francis Group, LLC; and from Hess (289), with permission from the Brazilian College of Animal Reproduction.]
A model of estrogen production, receptor expression, and action is shown in Figure 6. This model also incorporates the primary E2 effects on efferent ductule epithelial physiology.
FIGURE 6.
Estrogen synthesis and its targets in male reproductive tract. This figure summarizes the variation reported for the localization of estrogen receptors (ESR) in epithelia and stroma of testis, rete testis, efferent ductules, initial segment (seg), caput, cauda epididymis (epi), vas deferens, prostate, and other organs. Only nuclear ESR1 (yellow color) are represented. However, in some tissues, cytoplasmic and membrane ESRs have been documented. Receptor localization varies widely between species and with various antibodies (305). In adult testis, CYP19A1 (red color), the cytochrome P450 aromatase enzyme responsible for converting T to E2, is principally found in spermatids and mature sperm in seminiferous tubules and Leydig cells. These two sources of estrogen in the male reproductive system are directed to separate physiological pathways: 1) E2 from Leydig cells may target the seminiferous epithelium, although Sertoli and germ cells appear to be inconsistent in their ESR1 expression. This minor source of estrogen enters the blood and targets stromal and epithelial tissues not only in the reproductive tract but also all other ER-expression organs. 2) Germ cell production of E2 begins within seminiferous epithelium and continues with the localization of aromatase in the cytoplasmic droplet of spermatozoa transported in the lumen of the reproductive tract. The major target of luminal E2 is efferent ductule epithelium, where ESR1 expression is the highest in the body. The major function of efferent ductules is reabsorption of nearly 90% of the luminal fluid, which increases sperm concentrations entering the initial segment. This major physiological function, under ESR1 regulation, involves kidney-like physiology of the nonciliated cells (outlined in the red box), of which several genes are directly Esr1 regulated (296, 342).
B. Estrogen Receptor 2 Knockout Mice
Initial studies of Esr2 knockout (Esr2KO) mice did not observe the dramatic male or female reproductive changes seen in Esr1KO mice, and the male reproductive phenotype in double-Esr1/Esr2KO mice was similar to Esr1KO males, confirming that ESR1 is the functionally dominant ER in males (189, 368). In some species ESR2 shows considerably less expression compared with ESR1, also suggesting a limited role for ESR2. The original Esr2KO was fertile, but these mice had increased Leydig cell numbers and decreased germ cells due to gonocyte or germ cell apoptosis (175, 253) and hyperplastic prostatic changes with aging (322, 727).
Analysis of these mice was complicated by the presence of alternatively spliced ESR2 transcripts (189, 368), similar to the truncated ESR1 in the original Esr1KO mice (421). Therefore, a true null Esr2KO was developed (19). Mating defects in these mice have been reported (19), although this is controversial, suggesting that lack of ESR2 does not impair sperm production/motility, but may impair mating. The use of selective ER inhibitors in vivo and in vitro, which bypass hypothalamus-pituitary-testicular feedback loop problems (501), have shown that selective ESR2 agonists in rats impair fertility and spermiation, potentially by altering the tubulobulbar complex in seminiferous epithelium, without LH or FSH effects (188, 375). Overall, the data indicate some ESR2 effects on male reproduction.
C. Aromatase Knockout Mice
Aromatase knockout (Cyp19KO) mice, developed to test the hypothesis that estrogen is essential for many physiological systems, had normal efferent ducts and rete testis morphology and normal spermatogenesis until they began to age (339, 502, 585–587, 682). An aromatase inhibitor similarly has no effect on efferent ductules and fluid physiology (248, 534, 693). However, soy-free diets lacking phytoestrogens, which have high ESR2 affinity, accelerated spermatogenic declines in Cyp19KO mice (586), suggesting ESR2 involvement in spermatogenesis.
For years, E2 treatment was the primary approach for demonstrating estrogen roles in male reproduction (36, 271). However, E2 treatment creates interpretational problems due to alterations in hypothalamus-pituitary-testicular feedback (501). Furthermore, in some male tissues, ESR1 shows constitutive expression and possible activity in the absence of luminal estrogen in both Cyp19KO mice and following castration (516, 682). In testis, in vitro E2 treatment of seminiferous tubules increases ESR1 (697), but decreased efferent duct ESR1 in vivo (516), possibly explaining the absence of efferent ductal phenotypes in Cyp19KO mice (682). This suggests that efferent duct morphology and physiology, while ESR1 dependent, may not require direct E2 stimulation.
Other studies have shown that ESR1 may be activated in a ligand-independent manner (450, 503, 554, 642, 729) or non-E2 ESR1 ligands may be active in some male tissues (512, 550). Although Cyp19KO mice showed only a long-term role for direct estrogen actions in testis, other studies are uncovering roles in Sertoli and germ cells, including spermatozoa (124, 422–425; for reviews, see Refs. 115, 296, 342). Therefore, care must be taken when interpreting studies using Cyp19KO mice and estrogen treatment of males. One of the ESR1 target genes in efferent ducts is Slc9A3 (341–343, 598, 761), which not only contains estrogen response elements (ERE), but also androgen response elements (ARE) in its promoter region (685). Thus the potential for dual regulation of efferent ductule physiology, involving an estrogen/androgen balance, would help to explain some of the complexities observed in the male. Future studies must also evaluate the status of ERs and their associated cofactors in a species- and tissue-specific manner.
D. Gper Knockout Mice
In addition to ESR1 and ESR2, GPER, a G protein-coupled receptor originally described as orphan receptor GPR30, also functions as an ER or cooperates in ER activation (204, 577, 678). Activation of GPER results in increased intracellular calcium and phosphatidylinositol 3-kinase (PI3K). Lack of GPER impairs E2 actions in cancer cells, but its effects on normal reproductive development and function are unclear. Several Gper knockouts (GperKO) were developed, but no female reproductive abnormalities were reported (325, 442, 522, 719; for a review, see Ref. 566).
A variety of testicular cell types express GPER, including germ, peritubular, Leydig, and Sertoli cells (28, 58, 131, 203, 236, 296, 406, 444, 602). Expression of GPER has been reported in germ cells during various stages of spermatogenesis in rodents, and direct GPER-mediated effects on germ cells have been suggested (131, 645). Leydig cells express GPER and signaling through GPER has been implicated in both Sertoli cell proliferation and maturation and maintenance of fertility (423, 424, 753). Finally, GPER expression in peritubular cells has been linked to sexual maturation and maintenance of fertility (617). Signaling through GPER may also play a significant role in the epididymis and in expression of GPER in the epididymis (444, 544) and in posttesticular maturation of sperm (236).
Despite documented GPER expression/actions in the male tract, GperKO males are fertile and without reproductive abnormalities, indicating that GPER is dispensable for male reproduction. However, GperKO males are obese with insulin resistance and dyslipidemia (266, 628), phenotypes also seen in mice lacking ESR1 or aromatase. An absence of GPER has metabolic effects in males, as well as effects on cardiovascular, beta cell, and skeletal physiology (566). Therefore, GPER’s overall role in E2 signaling in males remains unclear (235, 406, 431).
E. Transgenic Mice Lacking Membrane ESR1 Signaling
1. Development of mice lacking membrane ESR1
Although ESR1 is predominately cytoplasmic and nuclear, ~5% of this protein localizes to cell membranes (1, 541). This process requires ESR1 palmitoylation at cysteine-451 in mice (538), which was recently used to develop transgenic nuclear-only estrogen receptor 1 (NOER) mice where alanine was substituted for cysteine-451 (C451A) in mouse ESR1. Alanine cannot be palmitoylated, which precludes cell membrane localization of ESR1. Resulting mice expressed normal amounts of fully functional nuclear ESR1 (nESR1), but membrane ESR1 (mESR1) was essentially eliminated in both reproductive and non-reproductive tissues.
Development of NOER mice resulted in identification of critical mERS1 actions in females (3, 538) and suggested that mESR1 might also be important in males. We recently examined male reproductive development and function in transgenic NOER mice (482) and reported that mESR1 is essential for male fertility and that absence of mESR1 causes extensive deleterious male reproductive abnormalities. In adult NOER testes, rete testis (RT) was strikingly enlarged compared with WT and was comparable to that in Esr1KO males (191). Also paralleling Esr1KO males (191, 293), seminiferous tubule luminal diameters were increased in NOER mice, with increased degeneration in NOER seminiferous tubule epithelium.
Decreased sperm production and motility is a hallmark of Esr1KO mice. In 8-mo-old NOER mice, sperm production and motility were reduced by 85 and 60%, respectively, compared with WT. However, many caudal epididymal sperm in NOER mice remain viable, indicating that reduced motility did not simply reflect sperm death. Decreased NOER sperm motility was accompanied by structural abnormalities in over 95% of cauda epididymal sperm. Abnormalities included increases in folded or coiled midpieces and tails, as well as increased numbers of headless sperm.
A critical question for understanding effects of loss of mESR1 on cauda epididymal sperm is when these abnormalities arise. Do structural abnormalities in epididymal sperm originate during development, or following release into the seminiferous tubular lumen? We observed that NOER sperm morphological abnormalities were absent in seminiferous tubules containing late-stage VIII spermatids, when sperm are released into the lumen (Figure 7A). Conversely, in the RT (Figure 7B), extensive tail folding and coiling previously seen in epididymal sperm were observed. Thus NOER sperm abnormalities arise following release from seminiferous epithelium, likely due to altered fluid environments in the rete, efferent ductules and epididymis. These findings are again consistent with Esr1KO and anti-estrogen-treated mice (133, 343, 482).
FIGURE 7.
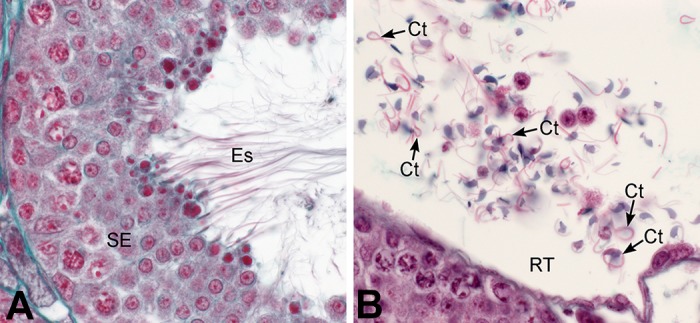
Structural abnormalities of NOER mouse sperm arise in the post-seminiferous tubular environment. Forty-day-old NOER male testes were fixed and stained with Masson’s trichrome. A: seminiferous tubular epithelium at spermiation (stage VIII) shows normal sperm with straight tails. B: rete testis region of NOER mice shows high numbers of abnormal sperm with coiled tails (CT). SE, seminiferous epithelium; Es, elongated spermatids.
In NOER mice, luminal diameters in proximal efferent ductules (adjacent to RT) were increased ~50% in NOER mice, while proximal ductule epithelial height was reduced ~50% in NOER (Figure 8). Previous work has shown that Esr1KO mice show similar changes in these parameters (290, 296).
FIGURE 8.

Efferent ductule epithelium from adult wild-type (WT), Esr1KO, and NOER (nuclear-only ESR1) mice. Periodic acid-Schiff (pink) and hematoxylin (blue) staining. Bar = 20 μm. A: WT epithelium is short columnar with ciliated (Ci) and nonciliated (Nc) cell. Nonciliated cells have a prominent brush border of microvilli (Mv) lining the lumen that contains diluted population of sperm (Sp). B: Esr1KO epithelium is shorter in height than WT, with significant loss of apical cytoplasm and much of the nonciliated microvillus border. Cilia numbers are reduced. C: NOER epithelium is shorter in height than WT, lacks a microvillus border, and shows reduced apical cytoplasm, similar to Esr1KO mice. Cilia also are reduced. Abnormal sperm with coiled tails are seen in the lumen.
Matings of homozygous adult NOER males (5- to 8 mo-old) with fertile WT females never yielded pregnancies. However, when juvenile NOER mice that were ~2 mo of age were placed with proven WT breeders, some of the NOER males sired litters, although litter size was reduced. Thus adult NOER males are infertile, but juvenile NOER males are transiently fertile during development.
Loss of mESR1 in NOER males, even with continued nESR1 presence, leads to extensive reproductive changes culminating in severe structural and functional sperm abnormalities and eventually infertility. These findings identify a previously unknown role for mESR1 in normal E2 signaling in males and indicate that mESR1 expression is necessary for male fertility (482), as it is for female fertility (538).
2. Estrogen mediated effects through membrane ESR1
Despite attenuated E2-induced responses in NOER male and female mice, these mice do not totally lack E2 responsiveness, and establishing the mechanism of this effect is a critical goal. Membrane estrogen receptors activate PI3K and mitogen-activated protein kinase (MAPK) pathways, and have other actions. Protein kinase activation by E2/mESR1 signaling may be crucial for phosphorylation and recruitment of cofactors to nESR1 after E2 binding, regulation of ESR1 synthesis, and degradation and other effects that result in impaired E2 actions.
Extensive evidence indicates mESR1 may also be involved in epigenetic changes arising from early estrogen exposure, and this may be a critical effect mediated through membrane signaling. In the presence of E2, mESR1 interacts with the p85α regulatory subunit of PI3K, leading to activation of protein kinase B/AKT (153). DES or other estrogens can act through mESR1 to increase signaling through the PI3K/AKT pathway in neonatal rodent uteri (89, 259, 745). Critically, increased PI3K/AKT signaling then alters histone methylation. The most critical regulator of epigenetic changes such as histone methylation is the polycomb repressive complex 2 (PRC2) enzyme complex. The PRC2 is a histone methyltransferase that has major effects on gene function by silencing gene activity. The PRC2 functions by adding up to three methyl groups at lysine-27 of histone H3 (H3K27) to form trimethylated histone H3 (H3K27me3). Activity of PRC2 is regulated primarily by expression of enhancer of Zeste homolog 2 (EZH2), the catalytic subunit of the PRC2 complex that provides methyltransferase activity. In response to mESR1 signaling, activated AKT phosphorylates and inactivates EZH2, causing reduced H3K27me3. Since H3K27me3 is a repressive mark, this reduction leads to hyperresponsiveness to estrogen in adulthood (259), resulting in increased tumorigenesis and other reproductive diseases such as leiomyoma in adult rodents, and potentially in women, after early estrogen exposure (705, 757, 768). Estrogen effects mediated through EZH2/H3K27me3 appear to be a main mechanism of epigenetic estrogen effects (89, 259, 745), and EZH2 regulation of H3K27me3 may be involved in prostate cancer, emphasizing its potential role in E2 effects mediated through mESR1 in males (705).
IV. ABNORMALITIES IN ESTROGEN PRODUCTION OR ER EXPRESSION IN HUMANS
Progress in understanding the role of estrogen in men has been made in the past two decades through identification and characterization of human patients with mutations in ESR1 or aromatase. The first man lacking functional ESR1 was reported in 1994 (647) (Table 5), although a woman (569) and three siblings (2 females and 1 male) (57) lacking ESR1 function were recently reported. Shortly thereafter, humans lacking aromatase were identified (176), with 13 reported cases of loss of function mutations in CYP19A1 in men now known (Table 5).
Table 5.
Effects of loss of aromatase or ESR1 in men
| Original and Related Case Reports | Type of Mutation, Subject Age | Reproductive Effects | Skeletal Effects | Metabolic Effects |
|---|---|---|---|---|
| Aromatase mutation | ||||
| Morishima et al. 1995 (469), Bilezikian et al. 1998 (66) | Single point mutation, 27 yr | Virilization of mother; normal sexual and pubertal development; elevated T, LH, and FSH; low E2 levels; macro-orchidism; no semen analysis; heterosexual orientation and behavior | Tall stature, osteopenia, osteoporosis, younger bone age (14 yr), low bone mass and mineral density, unfused epiphyses | Hyperinsulinism, normal glucose, dyslipidemia, BMI 32.5 |
| Carani et al. 1997, 1999 (107, 108), Rochira et al. 2000 (588) | Single point mutation, 0.4% aromatase activity, 31 yr | Normal sexual and pubertal development, normal T, slightly elevated FSH, upper normal LH and undetectable E2, micro-orchidism, infertility, oligospermia with immotile spermatozoa. Hypospermatogenesis and germ cell arrest, heterosexual orientation and behavior | Tall stature and bilateral genu valgum, bone pain, open metacarpal and phalangeal epiphyses, and younger bone age (14.8 yr) | Normal insulin and glucose, dyslipidemia, BMI 27.6 |
| Deladoey et al. 1999 (173) | Base pair deletion in CYP19 gene causing truncated, inactive protein, infant | Normal sexual differentiation; maternal virilization; normal serum-free T and high androstenedione at birth, which decreased by 1 month; normal testes descent | ||
| Herrmann et al. 2002, 2005 (286, 287) | Frameshift mutation resulting in premature stop codon and truncated aromatase protein, 27 yr | Maternal virilization, normal sexual and pubertal development, high T and FSH, normal LH, low E2, normal testicular volume, oligospermia, reduced sperm motility, normal sperm morphology and vitality, heterosexual orientation and behavior | Tall stature, low bone mass and mineral density, increased linear bone growth, genu valgum, kyphoscoliosis, and pectus carniatus | Normal plasma glucose and insulin dyslipidemia, BMI 30.9 |
| Maffei et al. 2004 (433), Carrani et al. 2005 (106), Rochira et al. 2007 (590) | Point mutation resulting in truncated aromatase protein, 29 yr | History of bilateral cryptorchidism, normal sexual and pubertal development, normal LH and T but increased FSH and low E2, micro-orchid testes in inguinal canal, abnormal seminiferous tubules with Sertoli cell-only tubules, atrophy and degenerated epithelium, heterosexual orientation and behavior | Tall stature, persistent linear growth and diffuse bone pain, genu valgum, unfused metacarpal and phalangeal bones and younger bone age (15 yr), osteoporosis, low BMD | Upper normal insulin levels, normal glucose, normal total cholesterol, acanthosis nigricans, BMI 25.4 |
| Bouillon et al. 2004 (82) | Frame-shift mutation causing truncated, inactive enzyme, 17 yr | Congenital hearing problem, high serum T, upper normal range of LH and FSH, undetectable E2 levels, normal testicular volume, sexual and pubertal development | Tall stature, open epiphyses and younger bone age (12 yr), low BMD and bone size | BMI 27.7 |
| Maffei et al. 2007 (434) | Two point mutations, 25 yr | Normal sexual and pubertal development, normal LH and T, FSH slightly elevated, undetectable E2, normal testicular volume, hypospermia, heterosexual orientation and behavior | Tall stature, genu valgum, continuing linear growth, diffuse bone pain, younger bone age (15.3 yr), unfused epiphyses, low BMD, osteoporosis, osteopenia | Obesity, hyperinsulinemia, insulin resistance, dyslipidemia, acanthosis nigricans, nonalcoholic fatty liver disease, hepatomegaly, BMI 35.9 |
| Lanfranco et al. 2008 (386) | Compound heterozygous mutation resulting in truncated, inactive aromatase protein, 26 yr | History of right cryptorchidism, normal sexual and pubertal development, slightly elevated FSH, normal LH and T, undetectable E2, normal testicular volume and sperm concentration with slightly reduced motility, normal sexual behavior, heterosexual orientation | Tall stature, genu valgum, unfused epiphyses, osteopenia, low BMD, younger bone age (15.5 yr) | Increased fasting insulin, insulin resistance, dyslipidemia, fatty liver, impaired liver function, acanthosis nigricans, BMI 29.3 |
| Baykan et al. 2013 (47) | Point mutation, 27 yr proband and younger brother | High LH and FSH, normal testosterone, undetectable E2, ambiguous genitalia, normal testicular volume and sperm count, slightly reduced sperm motility | Tall stature, unfused epiphyses, linear bone growth, bone pain, recurrent bone fractures, osteopenia, osteoporosis, younger bone age (15 yr) | High total cholesterol and triglycerides, low HDL, hepatosteatosis, BMI 25.7 |
| Bouchoucha et al. 2014 (81) | Point mutation resulting in reduced aromatase activity, 1–6 yr | Hypospadias and bilateral cryptorchidism, normal hormonal profiles | ||
| Chen et al. 2015 (130) | Compound heterozygous point mutations resulting in decreased aromatase activity, 24 yr | Normal LH, FSH, and T; undetectable E2; normal sexual and pubertal development, sexual behavior, and orientation; normal testes size, sperm count, and viability | Tall stature, genu valgum, unfused epiphyses, osteopenia, younger bone age (16-18 yr), low BMD | Hyperinsulinemia, impaired glucose tolerance, steatohepatitis, dyslipidemia, acanthosis nigricans, BMI 26.5 |
| Miedlich et al. 2016 (464) | Point mutation resulting in truncated inactive protein, 25 yr | Virilization of mother during pregnancy, normal FSH and LH, high-normal T levels, undetectable E2, normal pubertal development, testis size, sexual behavior and libido | Tall stature, bone abnormalities | Normal insulin, glucose, and lipidemia; moderate acanthosis nigricans |
| Estrogen receptor 1 mutation | ||||
| Smith et al. 1994 (647) | Point mutation resulting in truncated protein, 28 yr | Normal sexual and pubertal development; elevated E2, LH, and FSH; normal T; normal testes size, sperm count, sexual behavior, and orientation; reduced sperm vitality | Tall stature, genu valgum, bone abnormalities, younger bone age (15 yr) | Impaired glucose tolerance and insulin resistance, acanthosis nigricans, BMI 30.5 |
| Bernard et al. 2017 (57) | Point mutation in ligand binding domain with reduced transcriptional activity, 18 yr | Elevated FSH, LH, and E2; low to normal serum T; low serum inhibin B and AMH; unilateral right cryptorchidism; hypoplastic left testis; normal pubertal development | Low bone age (11 yr) | BMI 23.7 |
A. Estrogen Deficiency in Men
Estrogen deficiency due to loss-of-function mutations in CYP19A1 [also known as aromatase deficiency (AD)] in men is characterized by normal male sexual differentiation and pubertal development (47, 82, 107, 130, 287, 386, 433, 434, 464, 469). However, AD cases with birth defects such as hypospadias (81) and cryptorchidism (81, 387, 433) are known. Pregnant mothers carrying male or female fetuses with homozygous aromatase mutations frequently show progressive virilization that resolves after parturition (173, 287, 464, 469). The extent of maternal virilization depends on the specific CYP19A1 mutation, since fetuses with even 1% of normal aromatase activity do not trigger maternal virilization (264).
Postpubertally, AD men and women have sought medical attention for bone pain or continuous linear growth (47, 82, 107, 130, 287, 386, 433, 434, 464, 469). Serum hormone concentrations analysis revealed normal to elevated LH, follicle stimulating hormone (FSH), and T as well as undetectable E2. GnRH stimulation produced robust LH release and subnormal FSH release (287, 434). Similar gonadotropin and T changes were also reported in humans given aromatase inhibitors as young adults (447), further suggesting an E2 role in negative feedback of gonadotropic hormones in men.
Adult men with AD typically show normal testicular size, although macro-orchidism (469) or micro-orchidism (107, 433) have been reported. The role of E2 in human testis function, spermatogenesis, and fertility is unclear, since there were no consistent findings in testes histology or sperm analysis in AD men. Furthermore, due to patient noncompliance for further analysis as well as preexisting conditions such as cryptorochidism and hypospadias, conclusions regarding E2’s role in testis function are difficult. Nonetheless, Sertoli cell-only seminiferous tubules (433), hypospermatogenesis (107, 434), seminiferous epithelial atrophy and degeneration and spermatogenic arrest (107) have been reported. No changes in Leydig cells morphology have been reported. Furthermore, semen analyses revealed a range from normal to severe oligospermia and normal to immotile sperm (107). In AD men, dietary exposure to phytoestrogens or other environmental estrogens may obscure effects of endogenous estrogen deficiency, since phytoestrogen exposure in Cyp19KO mice delays testicular degeneration (586). Fertility of these men is unknown, with the exception of one case (107); however, based on testicular and sperm abnormalities in AD patients, fertility in AD men may be impaired.
Almost all AD men are tall, with eunuchoid body proportions, open epiphyses, genu valgum, osteopenia, osteoporosis, younger than chronological bone age, bone pain, increased bone turnover, and frequent fractures (Table 5) as a result of decreased bone mass and mineral density. Administration of E2 to these patients induced epiphyseal closure, improved bone deposition, and alleviated bone pain (Table 5). Similarly, epidemiological studies show men with low E2 suffer osteopenia and are fracture prone (206, 208, 241, 726), and decreased bone mass is associated with CYP19 gene polymorphisms and decreased aromatase activity in men (349).
Hyperinsulinemia and impaired glucose tolerance occur in most men lacking aromatase (130, 386, 433, 434, 647). Furthermore, these patients have increased body mass index and dyslipidemia (Table 5), consistent with Cyp19KO and Esr1KO mice (189, 281, 368, 509). Most AD men have low growth hormone (GH) concentrations, suggesting E2 control of GH, which was recently validated in knockout mouse models where both ESR1 and ESR2 were shown to regulate GH (34).
B. Lack of ESR1 in Men
The reproductive phenotype of men with mutations in their ESR1 that renders it nonfunctional (57, 647) is almost identical to AD men (Table 5), with normal testes size and sperm count but reduced sperm viability (191, 293). This mutation is accompanied by high gonadotropins, despite large increases in serum E2 (57). Not surprisingly, E2 did not resolve their clinical symptoms, suggesting an essential role for ESR1 that is not compensated by other ERs (647). Although fertility was not evaluated, it may be impaired due to decreased sperm viability. Furthermore, cross-sectional studies have shown that polymorphisms in exon 4 (LBD) of ESR1 are associated with idiopathic azoospermia (373, 669) and male infertility (231, 373). Conversely, mutations and polymorphisms in ESR2 are not associated with infertility (352).
In summary, E2 in men regulates 1) bone growth, 2) glucose and lipid metabolism, and 3) FSH and LH concentrations, while ESR1, but not ESR2, plays an important role in male fertility (reviewed in Refs. 52, 99, 603).
C. Human Cases of Estrogen Excess
Natural cases of excess aromatase activity (EAA) causing estrogen excess in men have been reported. These men have normal male sexual differentiation, pre- or peripubertal gynacomastia, micro-orchidism, accelerated prepubertal growth, advanced bone age, and tall childhood stature. The EAA adults exhibit normal to slightly reduced heights and hypogonadotropic hypogonadism with low to normal LH, FSH, and T and normal to high serum E2 (176, 443). Serum E2/T ratios are elevated (230). However, in contrast to aromatase overexpressing male mice, fertility and spermatogenesis are preserved in EAA men (230, 633).
The EAA condition is transmitted as an autosomal dominant trait (69, 275, 634, 635, 662). Aromatase expression is regulated by its complex tissue-specific promoters and splicing (100). Most men with aromatase overexpression show duplication, inversion, or deletion mutations in CYP19A1 (230), resulting in overexpressed mRNA and protein activity. Furthermore, aromatase overexpression with high E2 and gynecomastia was reported in boys with rare conditions such as fibrolamellar hepatocellular carcinoma (4), human testicular and ovarian sex cord tumors (100, 144), large-cell calcifying Sertoli cell tumors, and Peutz-Jeghers syndrome (255).
V. ROLE OF ESTROGENS IN NORMAL PROSTATIC DEVELOPMENT AND FUNCTION AND IN PROSTATIC PATHOLOGIES
The prostate gland is derived from the endodermal urogenital sinus, in contrast to the mesodermally derived seminal vesicles, vas deferens, and epididymis. This embryonic origin accounts, in part, for the high rates of aberrant growth and cancer observed in aging prostates, whereas diseases of other male accessory sex glands are exceedingly rare (564). Prostatic development, growth, and function are tightly regulated by androgens, in particular DHT, acting through AR, which also play fundamental roles in prostate cancer progression (564, 764). While not essential, it is recognized that estrogens impact prostate growth, homeostasis, and disease throughout life. These effects are mediated through multiple ERs, including ESR1, ESR2, GPER, and estrogen-related receptors (ERR) that are expressed in a cell-specific manner in prostate (Figure 9).
FIGURE 9.
Estrogen signaling pathways within prostatic epithelial cells. E2 and other agonists have multiple receptors and pathways that can be engaged to produce a variety of effects within cells. Both ESR1 and 2 (represented as ER) signal through classic genomic pathways. In addition, both ESR1 and 2 are present at the membrane and activate rapid signaling pathways upon ligand binding, including phosphorylation of Akt and/or the MAPK cascade. Multiple downstream effectors can be activated in a context-specific and perhaps ER-selective manner resulting in histone modifications (H3K4, H3K9, H3K27 trimethylation or demethylation) and direct transcriptional activation through intermediaries that include c-fos, c-jun, SP1, and NFkB as well as phosphorylation of nuclear ERs that amplify their activities. Finally, estrogens can signal through GPER, which activates PKA signaling.
A. Estrogen Actions in Prostate
While low amounts of circulating estrogens are present throughout life in males, during two time periods, in utero development and aging, males are exposed to higher circulating E2, which impacts the prostate gland. During prostate development, estrogens modulate branching morphogenesis and epithelial differentiation through ESR1 and ESR2, respectively (129, 322, 711). However, exposure to elevated endogenous estrogens or variable levels of xenoestrogens (e.g., DES, BPA) can interrupt normal development and predispose to prostatic diseases with aging. Extensive rodent studies involving developmental estrogenization (estrogen imprinting or estrogen reprogramming) have shown that high-dose estrogens during critical developmental windows inhibit prostate growth and drive epithelial and mesenchymal differentiation defects, causing marked structural reorganization (557). Conversely, lower estrogen doses developmentally increase rodent prostate gland bud numbers and adult prostate size, indicating nonmonotonic dose responses (315, 712).
Studies with Esr1KO and Esr2KO mice determined that these prostatic effects are mediated through stromal ESR1, which initiates alterations in prostate steroid receptors and developmental genes (556). Importantly, exposures to natural, synthetic, and environmental estrogens during fetal or neonatal development can lead directly to prostate neoplasia with aging if doses are sufficiently high, and increase susceptibility to hormone-driven carcinogenesis with aging at low doses (560, 565). These life-long changes following brief, early-life estrogenic exposure may result from epigenetic reprogramming of developing prostate cells, leading to altered epigenetic memory at the level of DNA methylation, histone modifications, and noncoding RNAs (301, 674, 720).
Importantly, evidence suggests that similar estrogenic reprogramming occurs in the human prostate (Figure 9). Extensive squamous metaplasia in fetal human prostate epithelium is driven by maternal E2 (770). Furthermore, indicators of high estrogen levels during pregnancy, such as high birth weight and jaundice in newborns, are associated with increased prostate cancer risk, whereas indicators of low estrogen levels, such as preeclampsia, are related to decreased risk (194). Directed differentiation of human embryonic stem cells into prostatic organoids in vitro was perturbed by low-dose exposure to the environmental estrogen BPA (103). Furthermore, exposure of adult human prostate progenitor cells to BPA or E2 activated rapid membrane-initiated signal pathways and modified their transcriptome, including SNORDs, a class of noncoding RNAs, through histone methylation reprogramming (301, 559). When human prostate progenitor cells were grafted in mice to form differentiated prostate-like tissue, brief developmental BPA exposure increased susceptibility to estrogen-initiated carcinogenesis in the human epithelium (559). Taken together, these findings support a developmental origin for prostatic disease following early-life estrogen exposures.
Clear evidence shows that elevated adult estrogens are sufficient to drive prostate carcinogenesis, in both animal models and human prostate epithelium (313, 580). This is noteworthy since men are exposed to relatively higher levels of circulating E2 with age. Bioavailable T declines in aging males due to decreased testicular production and increased sex hormone binding globulin levels that combine to lower free circulating T (350). However, circulating free E2 remains constant or rises in aging males due to age-related increases in adipose tissue, which expresses aromatase (708). In addition, estrogens are produced locally by aromatase in prostatic stroma (195, 446) and induced in prostate cancer cells, with marked increases in metastatic prostate specimens (466). This results in an increased E2-to-T ratio with aging, allowing a shift towards estrogen dominance, which can occur independent of serum E2 concentrations. Thus increased estrogenic stimulation in aging males may lead to reactivation of prostate growth, neoplastic transformation, and tumor progression.
B. Role of Catechol Estrogens in Prostatic Pathologies
Catechol estrogens are metabolites of E2 and other estrogens, and emerging data suggest that these could also be linked to prostatic pathologies. The most studied of the catechol estrogens are 2-hydroxyestradiol-17β (2-OHE2) and 4-hydroxyestradiol-17β (4-OHE2). Catechol estrogen effects do not appear to be entirely mediated through classic ESR1 and ESR2 pathways, although 2-OHE2 and 4-OHE2 both have some affinity for ESR1, and stimulate estrogenic responses in some systems and anti-estrogenic responses in reproductive and nonreproductive tissues (reviewed in Ref. 135).
Das et al. (164) showed that uterine effects typically associated with E2, such as increased lactoferrin production and water imbibition, were still seen in Esr1KO mice. Furthermore, the ER antagonist ICI 182,780 did not block this process, indicating that these effects were not mediated through ESR1 or ESR2. Subsequent work (547) indicated that there may be specific cytoplasmic binding sites for catechol estrogens that are distinct from ESR1 or ESR2, but these have not been definitively characterized. Recent work in zebrafish indicated that 4-OH-E2 can function as a GPER antagonist and block a critical action of E2 that is normally stimulated through E2 actions through GPER (135), further indicating the complexity of catechol estrogen effects.
Although work with catechol estrogens has predominately utilized female systems, these compounds may be involved in prostatic pathologies. Both 2-OHE2 and 4-OHE2 were more potent than E2 in inducing proliferation of a nontransformed prostatic epithelial cell line (BPH-1). In addition, 4-OHE2 was more potent than E2 in terms of neoplastically transforming these cells. Although data on catechol estrogens are limited, a recent report suggested that urinary concentrations of 2-OHE2 were greater in men than in either pre- or postmenopausal women (134). Thus catechol estrogens may play a role in prostate diseases and have other yet undiscovered actions in males.
C. Expression of ER in the Prostate Gland
ESR1 is primarily localized to prostatic stromal cells in humans, monkeys, dogs, and rodents (90, 555, 618, 620). Studies in rodents have shown relatively high stromal ESR1 expression during perinatal morphogenesis, which significantly declines with puberty as androgens rise, suggesting a specific developmental role (555, 680). Indeed, elegant studies with stromal cell-specific deletion of ESR1 in murine prostates demonstrated that fibroblast ESR1 modulates branching morphogenesis whereas smooth muscle ESR1 regulates stromal cell proliferation and ECM deposition (129, 711). In humans, ESR1 is expressed in stromal cells fetally (2, 626). However, while one report restricts ESR1 protein to only stromal cells (2), another identifies it in periurethral prostatic epithelium during mid-to-late gestation (626). Importantly, squamous metaplasia, observed in all developing human prostates during the third trimester, is directly associated with epithelial and stromal ESR1 in peripheral prostatic acini (626). Recent studies have also identified ESR1 in human prostatic epithelial stem and progenitor cells, where they mediate estrogen-induced stimulation of stem cell self-renewal and progenitor cell proliferation (313, 558, 559), implicating an estrogenic role in maintaining prostate homeostasis through stem cell effects. In disease-free adult prostate, ESR1 is mostly restricted to stromal cells but has been noted in basal epithelial cells (392). Stromal proliferation, a hallmark response to estrogen treatment in most species, is mediated through stromal ESR1, and its increased expression is believed to play a role in benign prostatic hyperplasia (BPH) etiology (367).
In adult rodents, periductal stromal cells express ESR1 (555), enabling estrogen-induced paracrine effects on prostate epithelium. Studies with Esr1KO mice demonstrated that developmental estrogen programming of epithelial dysplasia as well as E2-driven squamous metaplasia in adult prostates is mediated through ESR1 (556, 581). While investigations on ESR1 in prostate cancer have produced variable results, possibly a function of disease heterogeneity, ESR1 has been identified in cancerous epithelial cells as well as in cancer-associated fibroblasts (336, 486, 561). Importantly, ample evidence in humans and rodent models identifies a role for estrogens acting through ESR1 to promote prostate cancer growth via stromally mediated factors and recruitment of inflammatory cells, as well as direct actions on tumor cells (see Refs. 366, 486 for review). Of note, ESR1 drives expression of the TMPRSS2-ERG fusion gene in prostate cancer cells, which promotes progression, as well as NEAT1, the most overexpressed lncRNA in human prostate cancer, which in turn epigenetically alters oncogenic target genes (127, 625).
ESR2 was initially cloned from a prostate cDNA library, thus it is not surprising that it plays a role in the prostate, predominantly restraining growth (128, 274, 371). In contrast to ESR1, primarily localized to stromal cells, ESR2 is mainly found in prostatic epithelium (Figure 9) with limited stromal expression in adult prostate. In developing rat prostate, ESR2 expression is low at birth and increases as epithelial cells differentiate postnatally (563). In human fetal prostate, ESR2 is widely expressed in epithelial and stromal cells by week 7 and is maintained throughout gestation and postnatally for several months, suggesting a developmental role (2, 626). Furthermore, ESR2 is found in human prostate stem cells, where it restrains their symmetric self-renewal and promotes progenitor cell differentiation (313, 558). Reports vary on ESR2 localization in adult human prostate, with some finding exclusively basal epithelial cell expression (392, 691) while others report high levels in both basal and luminal epithelium (214, 229).
In addition to promoting prostatic epithelial differentiation (437), prostatic ESR2 has been shown to be pro-apoptotic (180, 457), inhibitory to epithelial-mesenchymal transition (EMT) (80), and immunosuppressive (438, 561) based on in vitro and in vivo studies (see Refs. 366, 486 for reviews). Prostatic epithelium also expresses ligand-independent ESR2 isoform variants (β2–5) which can act as either constitutive activators, transcription enhancers, or dominant negative regulators of estrogen action, further complicating estrogenic action within this gland (274, 403, 487). In this regard, the ESR2 (β1 isoform) is gradually hypomethylated and silenced as prostate cancer develops (766), whereas β2 and β5 isoform expression increases and promotes metastasis (402).
Other molecules that mediate estrogen actions are also found within prostate, although their roles are ill-defined. While both ESR1 and ESR2 can mediate membrane-initiated signaling triggered by estrogens in prostate cells in addition to their nuclear transcriptional activity (559), GPER is only at the membrane, where it activates MAPK pathways in response to E2. GPER has been found in normal human prostatic stroma, where it stimulates proliferation through ERK1/2 (529) and in cancer-associated fibroblasts where it regulates ESR1 actions (336). It is also robustly expressed in prostatic epithelial progenitor cells, although its activation or knock-down has limited effects (313). While GPER was found in approximately half of primary prostate cancers, this increased to 80% in metastasized cancers (381). Importantly, in castration-resistant prostate cancers, but not primary tumors, GPER activation triggered cell cycle arrest through the ERK1/2 pathway (409) and inhibited tumor xenograft growth (381), providing a novel pathway for tumor growth regulation. Constitutively active ERR have also been identified in prostate and prostate cancers, where they can modulate proliferative responses induced by ESR1 (130a).
Taken together, evidence shows that there are multiple mechanisms of estrogen action in prostate through several receptors, nuclear and membrane-initiated signaling pathways, and interactions as homo- and heterodimers with both activational and repressive functions. While some pathways trigger growth responses, others restrain growth, drive differentiation, or are pro-apoptotic. Thus estrogen actions in prostate are complex and diverse, which complicates targeting the estrogen axis for control of prostate growth.
VI. NONREPRODUCTIVE FUNCTIONS OF ESTROGENS IN MALES
In addition to male reproductive effects, estrogens also have important actions on many nonreproductive organs, which is a rapidly developing and evolving area of investigation.
A. Adipose Tissue and Metabolism
Many decades of evidence in both humans and laboratory animals show that estrogens are important regulators of white adipose tissue (WAT) in females. Female adipocytes express both ESR1 and ESR2 (155, 537, 714). Ovariectomy of female rodents or other species increases WAT, and estrogen replacement reverses this (reviewed in Ref. 715). Similarly, increased WAT deposition in postmenopausal women is reversed by estrogen replacement (675).
As in females, male WAT expresses ESR1 and ESR2 (155, 536). Other organs associated with satiety, feeding, and body weight regulation, such as hypothalamus and pituitary, also express ESR1/ESR2 in males and females. Males have low but measureable circulating E2 (Table 1), but local adipose E2 concentrations could be higher due to aromatase expression in adipose tissue. Despite the established role of estrogen in female WAT, it was unclear for years whether E2 played a role in male WAT.
Over the last two decades, the generation of Esr1KO, Esr2KO and Cyp19KO mice has provided critical information about heretofore unknown and/or unexpected roles of E2/ESR1 signaling in nonreproductive organs. In 2000, work using Esr1KO (281, 509) and Cyp19KO (338) mice indicated that E2/ESR1 signaling regulated male WAT deposition and various metabolic parameters. Weights of WAT depots in Esr1KO males were 100% greater than wild-type controls by 9–12 mo of age. This reflected both adipocyte hyperplasia and hypertrophy (281) and was accompanied by glucose intolerance and insulin resistance (281, 509). Similar effects were seen in Cyp19KO males (338), which had obesity and metabolic abnormalities paralleling aromatase-deficient men (434, 469) (Table 5). Thus similar increases in WAT and concomitant metabolic changes in both Esr1KO and Cyp19KO male mice and men lacking aromatase emphasized that E2/ESR1 signaling regulated male metabolism and adipose deposition.
1. Use of conditional knockouts to determine tissue-specific roles of ESR1 on adipose tissue
It was clear for many years that E2 effects on WAT involved direct and indirect actions, but development of conditional knockouts lacking ESR1 in specific tissues allowed determination of E2 actions in specific tissues. Ovariectomy suppresses overall activity, and thus caloric expenditure, as well as increasing appetite and food consumption, both of which contribute to obesity in Esr1KO mice. These effects involve E2 actions in brain regions such as the hypothalamus (reviewed in Ref. 448). Conditional ESR1 deletion in brain led to obesity mimicking that of global Esr1KOs (749), indicating a critical role for brain ESR1 in obesity following ESR1 loss.
Male mice with a specific knockdown of ESR1 in adipose tissue showed numerous structural/functional adipose and metabolic changes (166), including increases in adipose markers of inflammation, consistent with global Esr1KOs. They also exhibited increased adipocyte size and impaired glucose tolerance. These results indicate that E2 effects on adipose tissue and metabolism are regulated in part by direct effects on adipocytes.
Both ESR1 and ESR2 are expressed in skeletal muscle of humans (737) and other species (346). Both receptors are ubiquitous in muscle, with expression of both ESR1 and ESR2 in myofibers, endothelial cells, and satellite cells (346, 737, 738). Despite ESR1 and ESR2 coexpression in various muscle cell types, work with knockout mice identified unique effects mediated through either ESR1 or ESR2 (45, 97). Endurance training results in increases in both ESR1 and ESR2 in human skeletal muscle, suggesting that their expression is altered by functional demands on muscle (738).
Estrogens are constituents of steroid regimens administered to castrated male cattle to stimulate muscle growth and improve carcass quality (316, 337), indicating that estrogens have anabolic effects on male muscle mass. Similarly, estrogen replacement appears to facilitate skeletal muscle growth in postmenopausal women (644), possibly through effects on satellite cells (347).
Skeletal muscle is responsible for the majority of glucose uptake following insulin stimulation (171, 172). This, in conjunction with ESR1 expression in muscle and anabolic E2 effects on muscle, suggests that effects on muscle could contribute to metabolic impairments of Esr1KO mice, consistent with a report that glucose metabolism per kilogram of muscle was 45% higher in women than men (756). A recently developed mouse specifically lacking skeletal muscle ESR1 provided a powerful tool to test this (579). Interestingly, loss of E2/ESR1 signaling only in skeletal muscle of female mice led to obesity and impaired glucose metabolism, insulin resistance, and diminished muscle oxidative metabolism (579), paralleling reports that ESR1 expression is reduced in women with metabolic syndrome (579). A similar relationship was seen in mice, where levels of skeletal muscle ESR1 correlated with insulin concentrations and adiposity.
Is male skeletal muscle function also regulated by E2/ESR1? Although this was not directly addressed, strong correlations between adipose and metabolic effects in male and female Esr1KO mice and similar effect in males and females following conditional Esr1 knockout in other tissues suggest this is likely, although further work is needed.
2. Liver and macrophages
Estrogens regulate lipid, glucose, and cholesterol homeostasis in female mouse liver (222, 631), and transcriptional E2 effects in female liver (526) are primarily through ESR1, the primary hepatic ER in both sexes (370). Male mice with a liver-specific Esr1 knockout exhibited liver and whole body insulin resistance when consuming a high-fat diet, with altered liver glycogen metabolism and increased fasting plasma triglycerides (765), indicating that the liver is an important target for E2 effects on adioposity and metabolism. Consistent with this, increased E2 concentrations following partial hepatectomy were reported and E2 supported hepatocyte proliferation and regeneration in male mice (695).
Macrophages express estrogen receptors, predominantly ESR1 (473). In recent years, it has become clear that inflammation plays a role in obesity-associated metabolic changes, such as insulin resistance (169). Ribas et al. (578) recently found that female mice with a conditional knockout of ESR1 in macrophages were obese, glucose intolerant, and insulin resistant (578). Males, not yet examined, may show similar effects based on comparable adipose changes in Esr1KO (281, 509) and Cyp19KO males and females.
3. Adipocyte differentiation
In humans and other species, adult females typically have increased body fat percentages compared with males. This arises pubertally (70), suggesting that despite inhibitory E2 effects on adult WAT, E2/ESR1 can positively regulate developmental adipose differentiation. Recent work from Lapid et al. (389) provides mechanistic insights into this process as well as overall roles of E2/ESR1 signaling in WAT. When ESR1 was knocked out in white adipose progenitors that typically differentiate into subcutaneous fat, these cells preferentially gave rise to alternate brown fat and smooth muscle lineages in males and females. Resultant adults were lean, with improved glucose sensitivity and resistance to weight gain induced by high-fat diets. Thus ESR1 regulates commitment of progenitor cells to WAT in both males and females. These findings, along with earlier findings that estrogen stimulates proliferation of pre-adipocytes (182), may explain the well-known sexual dimorphism in adipose amounts in males and females.
4. Role of ESR2 in adipose tissue
Although ESR2 mRNA is expressed in human and rodent WAT (155), Esr2KO mice lack the obesity/insulin resistance of Esr1KO and Cyp19KO mice (189, 368, 509). Thus ESR1 is most critical for adipose and metabolic effects of E2 in males and females. In addition, mice lacking both ESR1 and ESR2 (189) mimicked adipose/metabolic changes of Esr1KO mice, further suggesting ESR1 as the major regulator of E2 effects on WAT.
Despite evidence that ESR2 is not the major regulator of E2 effects on WAT, some work suggests ESR2 can affect glucose tolerance and insulin resistance (44, 45, 221, 478; reviewed in Ref. 448). Similarly, recent work (166) indicated that in the absence of ESR1, ESR2 plays a protective role in suppressing inflammation in adipocytes.
5. Brown and beige adipose tissue in males
Recent work suggests E2 may have effects on brown adipose tissue (BAT), a specialized adipose tissue with a thermogenic role in neonatal heat production. These cells have extensive mitochondria, accounting for their color, and express uncoupling protein 1 (UCP1), which allows uncoupling of oxidative phosphorylation and extensive heat production. A variant of brown fat, beige fat, are cells that are found in WAT. These cells normally express low amounts of UCP-1, but UCP-1 is inducible in these cells (747), which results in concomitant increases in respiration rates. Although rodents have BAT throughout life, humans were thought to only have BAT neonatally. However, recent work described adult human BAT retaining thermogenic properties of neonatal BAT (485). In addition, methodologies to stimulate differentiation of BAT and beige adipocytes in mice have been developed (345, 415), suggesting similar manipulations might be feasible in humans as a weight loss strategy, and this area has become extremely topical because of this. Recent work has indicated that ESR2 may induce preadipocytes to differentiate into BAT (553). Additionally, an ESR2-specific ligand has been shown to increase expression of BAT-specific genes in epididymal WAT, raising the intriguing possibility that ESR2 signaling may increase beige adipocytes in WAT.
Both ESR1 and ESR2 are expressed in male human fetal BAT (706). Expression of ESR1 was more abundant than ESR2, and there was also a unique distribution pattern of these receptors in BAT. Only mature brown adipocytes expressed ESR2, while ESR1 was expressed in mature brown adipocytes, as well as preadipocytes, mesenchymal cells, and endothelial cells within BAT (706). The critical question of whether E2 has effects on male or female brown and/or beige adipocytes will likely attract extensive future interest.
B. Pancreatic Beta Cells
Estrogens have beneficial effects on pancreatic beta cell function and diabetes mellitus incidence. In humans, diabetes mellitus is more common in men (reviewed in Refs. 391, 419). Similarly, in rodent models involving induction of diabetes, females appear to be protected compared with males (391, 419). This is mediated in part by E2 effects on beta cell apoptosis (391), and E2 also has effects on beta cell insulin content, insulin gene expression, and insulin release (17).
Islet cells contain both ESR1 and ESR2 (17, 40, 419, 679), with ESR1 present in greater quantities (17, 40). GPER is also expressed in islets, where it may play a role in E2 effects (40). A critical question here is whether E2 has beneficial effects on male beta cells; existing evidence suggests this is possible. In males, ESR1 is detectable in beta cells (reviewed in Refs. 419, 679). Importantly, chemicals that damage beta cells cause greater damage in Esr1KO mice (391), indicating that estrogen plays a protective role in male beta cells. Estrogen may alter beta cell development as well, as shown by estrogen stimulation of proliferation in human islet-derived precursor cells (576). As with E2 effects on beta cells, these effects are ESR1 mediated (576).
C. Bone
Although androgens have significant effects on male bone (653), existing literature indicates that estrogens are more important for bone growth and maintenance (440, 589). Bone cells of men and male rodents express ESR1, ESR2, and GPER (reviewed in Ref. 649). Adult men with mutated ESR1 or aromatase have skeletal problems (Table 5), and E2 is essential for normal bone mineralization and mass and bone turnover, but not for linear bone growth, in men (648, 649).
Interventional studies in men using GnRH agonists to suppress endogenous T, aromatase inhibitors, or sex steroid (T and E2) replacement demonstrated that E2 reduced bone remodeling more than T (198). When combined, T and E2 were more effective in maintaining normal baseline bone remodeling than either alone (198). In a recent study, increasing T doses protected from resorptive bone loss and improved bone mineral density (BMD) in androgen-deprived men given a GnRH agonist (208). Importantly, aromatase inhibitors in these men decreased beneficial T effects, suggesting that conversion to E2 is essential for T effects on bone (208). Furthermore, selective estrogen modulators (e.g., raloxifene) reduce bone loss in elderly men (185, 651) or in men undergoing androgen ablation for prostate cancer (650). In addition, aromatase inhibitors are used “off-label” to increase final height in short-stature boys (170, 284, 452). However, long-term health consequences of aromatase inhibitors are not clear. In male rats, peri-pubertal aromatase inhibitor treatment caused decreased bone strength and altered bone geometry in adults (38).
Osteoporotic men have significantly decreased serum T and E2 (18, 354, 458). Aromatase polymorphisms resulting in decreased aromatase activity in vivo are associated with bone pathology in men (240). A recent epidemiological study positively correlated serum E2 and femoral neck BMD in elderly Chinese men (61). Furthermore, low serum E2 in elderly men increases fracture risk (18, 43, 458). Thus serum E2 concentrations better predict osteoporosis, BMD, and fracture risk than serum T concentrations in elderly men (353, 354, 589, 703).
Male Cyp19KO mice have decreased postpubertal femur length growth, lumbar spine BMD, and bone formation (524). Global ESR1 loss decreased bone turnover and cortical bone volume and increased trabecular bone volume in male mice (105, 641, 702). In contrast, loss of ESR2 did not affect bone development or homeostasis (641, 742, 743). Femur length was not affected in either Esr1KO or Esr2KO mice but was reduced in double-knockout male mice, and this was accompanied by decreased growth plate proliferating zone width (641). This suggests that in the absence of one ER, the other might compensate. Interestingly, GperKO male mice show increased BMD, long bones, and growth plate proliferation, suggesting GPER has negative bone growth effects in male mice (218).
It is noteworthy that rodent bone phenotypes do not completely agree with human phenotypes (649). These discrepancies result in part from species differences, expression of other receptors, and changes in sex steroid hormones. Both Cyp19KO and Esr1KO mice, but not humans, show increased serum T (440, 649, 740). In addition, bone expresses more AR in Esr1KO mice (640). To circumvent changes in sex steroid levels, researchers have studied orchidectomy-induced bone loss in global knockout mice and developed conditional knockout mice with bone cell-specific deletion of ERs and/or AR. Orchidectomy (ORX) of Esr1KO and WT mice caused significant trabecular bone loss and decreased cortical bone density (640, 702). Interestingly, T (640, 702) or DHT (471) supplementation completely prevented ORX-induced bone loss in both Esr1KO and WT mice (640, 702). Importantly, E2 administration completely or partially prevented ORX-induced bone loss in WT male mice (471, 640, 702). This bone-sparing effect of E2 is primarily ESR1-mediated since E2 was ineffective in Esr1KO mice (471, 640, 702).
Osteoclast-specific deletion of ESR1 did not induce bone loss in male mice (481, 694). However, E2 induced osteoclast apoptosis in male mice through ESR1 (481), the main mechanism for bone sparing effects of E2 in females. Osteocyte-specific deletion of ESR1 in male mice decreased trabecular bone volume by reducing both osteoblast and osteocytes and bone formation; however, osteoclast number and cortical bone were unaffected (741). In contrast, ESR1 deletion in an osteoblast-specific lineage did not affect trabecular bone in male mice (694). Furthermore, osteoblast-specific deletion of ESR1 had no effect on bone phonotype in young animals, while at 6 mo trabecular bone volume was decreased (430). Finally, osteoblast-specific deletion of ESR1 decreased cortical bone thickness in young, but not older, adult males (15). Together, these results suggest that cell-specific loss of ESR1 has minimal effect on bone phenotype in male mice.
Many reports indicate that bone-sparing E2 effects involve predominately membrane-initiated signaling through mESR1 both in vitro (5, 363) and in vivo (364, 709). Recent progress in designing estrogens that signal through mESR1 but not nESR1 raises the possibility of treatments that mimic beneficial E2 effects on bone without undesirable side effects on reproductive organs (432). In summary, although there are discrepancies between laboratory animals and humans, E2 is clearly required for normal bone growth and maintenance in men (104) and E2 mediates some T effects on bone homeostasis (353).
D. Cardiovascular System
1. Heart
Cardiovascular diseases (CVDs) are less prevalent in premenopausal women compared with age-matched men, but this protection is lost postmenopausally (575). Cardiovascular protection in women is mediated primarily by E2, and similar effects have been demonstrated in laboratory animals (474). In addition, many differences in cardiovascular functions between men and women develop after puberty, suggesting sex steroid involvement (76, 710).
Males express aromatase (263, 273, 332), ESR1 (263, 420), ESR2 (263, 420), and GPER (178) in their cardiovascular system. In mice, ESR1 is predominantly localized to ventricles, while ESR2 expression is more widely distributed (420). Interestingly, ESR1 is primarily localized in sarcolemma while ESR2 is both nuclear and cytoplasmic (420).
Inhibitory effects of E2 on cardiac hypertrophy are well-known in animal models. Males develop more severe cardiac hypertrophy and fibrosis than females after transverse aortic constriction (646), a cardiac hypertrophy model. This sex-specific difference is mediated through ESR2 (215, 539, 540, 646). Interestingly, GperKO (174) and Esr2KO (220) male mice show ventricular hypertrophy by 5 and 6 mo of age, respectively, and E2 attenuates cardiac hypertrophy in male rats (234). Furthermore, GperKO male mice display reduced cardiac function (174). ESR1 and ESR2 are upregulated in left ventricles of men and women with aortic stenosis (499). Cardiac E2 levels and aromatase expression were decreased in male mice subjected to heart failure, while E2 supplementation restored ejection fractions, prevented cardiac hypertrophy and fibrosis, and promoted angiogenesis (324). Other work has suggested E2 effects on regulation of heart weights and incidence of age-related cardiac fibrosis in male mice (300). Loss of Esr1 or Esr2 in mice also did not affect the cardiac-hypertrophic response after pressure overload, although there was reduced hypertrophy in Esr1KO mice (646).
Agonists of ESR1 and ESR2 were effective in protecting heart after ischemia/reperfusion (IR) injury in male rats (713); this may be GPER mediated (344). Furthermore, GPER transcripts were severalfold more abundant than ESR1 and ESR2 mRNA in hearts of male mice (344). The pure GPER agonist G1 protects the heart from IR injury and increases recovery rate in males (79, 178, 532, 728). Aromatase overexpression and increased endogenous E2 in males is associated with improved post-ischemia contractile functions (53) but lower basal systolic functions, similar to females (53). In contrast, aromatase deficiency did not affect basal heart functions in males (267). Interestingly, cardioprotective effects of the anesthetic desflurane after IR injury involves induction of aromatase activity in heart, and aromatase inhibition abolished desflurane protective effects and increased myocardial infarct size (332). It appears that cardioprotective effects of E2 after IR injury occur by decreasing reactive oxygen species (380) and reducing intracellular calcium handling (157).
2. Blood vessels
Vasodilation in response to E2 occurs within minutes through nongenomic mechanisms (463). Aromatase is expressed in aorta and endothelial and vascular smooth muscle cells of male mice (332). In men, aromatase inhibition decreased flow-mediated dilation of brachial artery, an indicator of endothelium-dependent vasorelaxation (408). Similarly, flow-induced vasodilation in the brachial artery was absent in a man lacking ESR1 (596, 666, 667) and in Esr1KO male mice (668). Furthermore, endothelial-derived nitric oxide production and aortic contraction were decreased in Esr1KO males (595). Interestingly, Esr2KO males show increased blood pressure (767). E2 effects on vascular dilation include both endothelial-dependent and -independent mechanisms (355) involving prostacyclin and nitric oxide synthesis by regulation of nitric oxide synthase in an ESR2-dependent manner (767). Increased expression of ESR2 was reported in blood vessels after vascular injury in male rats (417).
Hypertension is less common in premenopausal women than age-matched men. However, postmenopausal women develop hypertension at rates equal to or greater than men (276, 574). Anti-atherogenic E2 effects are well known in females and are ESR1 mediated (303, 497). These E2 effects in males can result from direct and indirect actions on lipid metabolism and adipose deposition (497, 689). E2 acts directly on vascular smooth muscle cells to inhibit proliferation and migration in atherosclerosis (159), effects mediated by both ESR1 and ESR2 in males (306). The importance of this effect is illustrated by the finding that a man lacking ESR1 had accelerated coronary arteriosclerosis (596, 666, 667). Furthermore, CYP19A1 polymorphisms in men are associated with coronary heart disease (51) and hypertension (143). Thus the majority of data in laboratory animal models and estrogen-deficient men suggest that E2 has protective roles in male CVDs.
Some protective cardiovascular T effects are mediated indirectly through E2 (488). Nonetheless, it is unclear from epidemiological studies whether men benefit from increased serum E2 (309, 390, 592). Furthermore, since T is the major source of E2 in men, serum E2 concentrations fluctuate based on T availability and local aromatase and EST activity. Most commonly, lower serum T is more strongly associated with higher risks of death from CVDs in men than serum E2 changes (309, 390, 592). In contrast, higher serum E2 were reported in men with coronary disease (548) and sudden cardiac arrest (483). Thus further research is needed to understand cardiovascular roles of E2 in men.
E. Brain and Behavior
The mammalian brain is a sexually dimorphic organ responsible for gender-specific behaviors. Sex steroids affect behavior by perinatal organizational effects as well as activational effects in adult brains (74, 549, 655). In rodents, fetal Leydig cell T production is essential for male brain differentiation (41). Critically, local T aromatization to E2 is required for masculinization of male brain (41, 451, 479), and thus T effects on brain masculinization are indirect. In the absence of significant T, undifferentiated brains develop as female. In both rats and humans, brain masculinization changes the sizes of several brain structures, including the hypothalamic preoptic area, which is larger in males versus females (326, 670) and controls male-specific sexual behavior in adults (41, 427). Furthermore, sex steroids induce differences in neural circuits between men and women that contribute to gender-specific behaviors (242, 427, 638). Normal male brain differentiation is susceptible to alteration by perinatal exposure to endocrine disruptors (e.g., BPA) (37, 197).
Recently, it has been shown that masculinization of the hypothalamic preoptic area by E2 involves inhibition of DNA methyltransferase (e.g., DNMT3). This decreases the number of methylated CpG sites in masculinizing genes and releases their repression (500). Interestingly, in the same study it was shown that DNA methyltransferase inhibitors were also able to masculinize female brain and induce male sexual behaviors in females (500). This suggests that in the absence of E2 effects on the hypothalamus (as in females), genes that are responsible for masculinization are suppressed due to increased DNA methyltransferase activity. Furthermore, high sex steroid concentrations occur in neonatal male rodent brains even following gonadectomy and adrenalectomy, suggesting de novo steroidogenesis in neonatal brain (360). Thus brain sexual dimorphism is controlled indirectly by T after local conversion to E2, which then masculinizes the brain (29), although other genes on sex chromosomes may affect male brain development (29, 179, 739). Although conclusive evidence is lacking, a similar process may also drive masculinization of the human male brain.
Aromatase, ESR1, ESR2, and GPER are expressed in male brain (325, 379, 661), and locally produced E2 is considered a brain neurosteroid (46). Masculinizing E2 effects in brain might be mediated through both ESR1 and ESR2 (reviewed in Ref. 369), but a GPER role has not been elucidated.
1. Sexual behaviors
In male rodents, E2 regulates various behaviors (e.g., sexual behavior, aggression, vocalization, learning, and cognition) (41, 152, 427) through rapid membrane-initiated signaling as well as nuclear receptor signaling (151). Neonatal castration and subsequent absence of T (precursor of E2) in rodents reduced adult male sexual behavior (e.g., mounting) and induced female sexual behavior (lordosis) (41, 427). Although Esr1KO male mice displayed mounting behavior similar to wild-type controls, intromissions and ejaculations were decreased (507). In addition, Esr1KO male mice were less aggressive (507). Administration of T to gonadectomized Esr1KO male mice was ineffective in restoring male aggression but restored mounting and intromission (508).
Sexual behavior in Esr2KO males is similar to WT controls, although Esr2KO mice showed higher aggression and a delayed first ejaculation age (506). Furthermore, E2 administration to castrated Esr2KO males induced higher levels of aggression compared with wild-type (498). In contrast, a recently developed exon3-deleted Esr2-null male mice showed impaired sexual behaviors (19). However, brain-specific Esr2 deletion does not affect male sexual behaviors or preoptic area dimorphism in males (484), suggesting that ESR2 is not a major regulator of male sexual behavior. Furthermore, Cyp19KO mice showed decreased mounting attempts and intromissions, and no ejaculation in the presence of receptive females (39, 310). This was partially rescued by E2 or DHT (39). Aromatase expression in different parts of the brain controls male aggression (696) and paternal behavior (10). Furthermore, estrogens in female urine stimulate male sexual centers in the brain, relayed through nasal vomeronasal organs (611, 638).
Men lacking aromatase (107, 130, 287, 386, 433, 434, 464, 469) or functional ESR1 (647) reported no change in sexual behavior or orientation, and most had spontaneous erections sufficient for intercourse. However, estrogen influences cannot be completely ruled out since ESR1 are located in brain centers regulating sexual satisfaction and E2 replacement in aromatase-deficient men improves libido and sexual desire in some men (106, 108). Although T supplementation improves libido in hypogonadal men (428), E2 is also beneficial (56, 91, 165), since beneficial T effects on sexual function in men are lost or reduced when T aromatization is inhibited (207, 428). Thus an optimal T/E2 ratio is needed to support normal sexual function in men (613).
2. Nonsexual behaviors
Measurable E2 concentrations are detected in various brain regions (amygdala, hippocampus, cerebral cortex, and cerebellum) in intact adult male rats (119), and gonadectomy reduces brain E2 (42). Administration of E2 to aged male rats improved spatial memory (426). In AR-deficient and WT male rats, E2 increased prefrontal cortex spine growth independent of androgens (e.g., DHT) (269). However, another study showed that E2 decreased dentritic spines in male rat hippocampus (395). In an open-field test measuring anxiety, Esr1KO male and WT female mice showed similar behavior (507), while Esr2KO and WT male mice showed similar behavior in this test (506). Although T has beneficial mood effects in men, higher E2 concentrations are also associated with less depression (16, 118) and might be mediating T effects by modulating serotonin levels/action in brain (228). Local E2 synthesis occurs in the hippocampus (307), and E2 improves memory in an ESR2-dependent manner in male rats (418). Furthermore, higher serum E2 concentrations are associated with improved spatial memory in elderly men (305) and visual memory in young men (348) while another interventional study showed that E2 is negatively associated with working memory in elderly men (758). In summary, E2 effects on cognitive functions in men are still being established, although some evidence suggests positive effects.
3. Neuroprotective functions after brain injury
In both male and female brain, E2 acts as a neurosteroid (46) and facilitates interneuronal communication (27) through neurite growth and establishment of new neuronal connections (27, 48, 638). Neuroprotective E2 effects in males have been demonstrated following traumatic brain injury (167, 659) and ischemia/reperfusion (stroke) brain injury (120, 721). In addition, it has been reported that aromatase is upregulated in reactive astroglial cells after various types of brain injuries (233). However, in uninjured brains, aromatase is restricted to sexually dimorphic areas of brain (233). Finally, local E2 concentrations increase at brain injury sites (601), again suggesting a neuroprotective role for E2 in male brains.
Protective E2 effects on hippocampal neurons of male mice following excitatory neurotoxicity have been shown using Cyp19KO males. Furthermore, aromatase inhibition abolished protective T effects in gonadectomized male mice (35). Treatment with E2 also protects male rat brains after middle cerebral artery occlusion, an experimental stroke model (684). GPER is upregulated in male brain after stroke, and GPER agonists or antagonists worsened or improved, respectively, functional outcomes following stroke (95, 96). Neuroprotective functions of E2 in male brain might be mediated through ESR1, ESR2, GPER1, and other estrogen receptors (reviewed in Ref. 27).
4. Neurodegenerative diseases and learning
Because of its proximity to genes involved in dyslexia (15q21), CYP19A1 has been considered as a candidate gene for cognitive functions and implicated in reading, speech, and language (20). Furthermore, higher serum E2 concentrations at 5 mo of age are positively associated with language performance in 4-yr-old boys and girls (612). There is a gender-specific preponderance in the onset and predisposition to neurodegenerative and psychiatric diseases such as Alzheimer's and Parkinson's disease, and schizophrenia is more common in males (242, 449), suggesting a possible E2 role in these diseases. In addition, E2 is being considered for therapeutic management of schizophrenia in men (374).
5. Circadian rhythms
Circadian rhythms are regulated by clock genes in brain and peripheral tissues (531). The master circadian clock resides in the brain’s suprachiasmatic nucleus, which contains extensive ER in both males and females (531), and E2 has major effects on circadian rhythms and activity in females (11). Recent results suggest estrogens exert similar effects in males (71, 92) through both ESR1 and ESR2 (72, 594). The circadian E2 effects involve both early organizational and adult activational effects (73, 92, 593). Although mechanistic work on circadian clocks has utilized rodents, recent work demonstrated that circadian rhythms and variables regulated by them (e.g., mental function) show marked differences in men and women, suggesting estrogens play critical roles in human circadian rhythms and associated behaviors (604).
F. Effects of Estrogens on the Immune System
Estrogens have major effects on immune system development and function. As for other tissues discussed here, initial studies were on females. However, it has become increasingly obvious that estrogen also regulates male immunity.
1. Estrogen receptor expression in immune organs and cells
Both ESR1 and ESR2 are widely distributed in male and female immune cells (reviewed in Ref. 365), and regulate adaptive and innate immunity. For example, lymphocytes (B cells, CD4+ and CD8+ T cells, NK cells), dendritic cells, and monocytes all express ESR1 and ESR2. In other cell types such as macrophages, hematopoietic stem cells, and myeloid progenitors, ESR1 expression predominates, with ESR2 low or absent (365).
Normal thymic and splenic development depend on ESR1, but not ESR2, in both males and females (416, 755), although thymus expresses ESR1/ESR2 in both sexes (371). Similarly, effects of exogenous administration of estrogen on thymic atrophy in young animals are ESR1 mediated (416).
2. Estrogen function in immune organs and cells
Many major differences in male and female immune function involve estrogen action. Both humoral and cell-mediated immunity are typically more robust in females, but increased immune surveillance in females may predispose to autoimmunity. For example, multiple sclerosis (MS) is three times more common in women than men (7, 8), and disease progression is sexually dimorphic. Despite lower MS incidence, men typically have more rapid MS progression than women following diagnosis (reviewed in Refs. 8, 365). More rapid progression in men may result from neuroprotective E2 effects, which could reduce neurodegenerative processes in females and facilitate tissue repair. The recent study suggesting that disease progression is similar in men and women initially diagnosed at an older age (87) is consistent with the idea of protective estrogen effects only in younger women. Despite apparent protective effects of estrogen in women versus men, it is unclear whether circulating E2 in men modulates rates of overall disease progression or neurodegenerative effects accompanying this disease.
Overall E2 effects on the immune system and its constituent cells are complex and are dependent on estrogen concentration and specific cell types involved, among other factors. In general, E2 regulates a variety of chemokines and cytokines in immune cells such as neutrophils, macrophages, and dendritic cells. For a complete descriptions of E2 effects on immune cells, see Reference 351.
G. Other Estrogen Target Organs and Tissues
1. Urinary system
In male mice, E2 administration following reperfusion injury due to cardiac arrest and cardiopulmonary resuscitation protected kidneys from ischemic injury (319) and E2 protects against age-related kidney changes in male rats (268). Prepubertal castration in male mice decreases kidney weights, while E2 replacement restores kidney size (300). In addition, Cyp19KO male mice have increased age-related renal fibrosis (300), and older Esr2KO males develop epithelial hyperplasia of the urinary bladder wall, suggesting an ESR2 role in bladder function (368).
Treatment of male mice with a regimen of E2 and T that mimicked the increased ratio of E2 to T in older men produced increased prostatic growth, as well as bladder outlet obstruction and dysfunction of bladder voiding (491). Subsequent work indicated that development of the bladder enlargement that accompanies the E2 + T treatment was mediated through ESR1, indicating that ESR1 may play a significant role in the normal bladder as well as in pathologies that involve altered bladder function.
2. Skin
Skin is an E2 target in males (64), and local estrogen synthesis by androgen aromatization in hair of men has been reported (621). In contrast to E2 effects in females, E2 inhibits wound healing in males through ESR1 (243). However, local E2 administration in elderly men improves wound healing (30). Interestingly, wound healing is faster in castrated young male mice compared with intact males, and systemic E2 or T administration inhibits wound healing (244). Furthermore, E2 regulates epidermal thickness in males by increasing keratinocyte proliferation and inhibits hair follicle growth and cycling in gonadectomized male mice through ESR1 (470).
3. Microbiome
Changes in microbial composition in both feces and seminal vesicles of Esr1KO compared with WT mice were recently reported (331). This suggests that microbiomes of male mice, and potentially men, may be regulated by E2.
VII. SUMMARY AND FUTURE DIRECTIONS
The past half-century has witnessed a major paradigm shift in understanding of the role of E2 and other estrogens in the male. Originally considered female hormones, estrogens play critical roles in developing and adult male reproductive organs, especially the efferent ductules where ESR1 is essential for normal fluid reabsorption physiology. In addition, many male nonreproductive tissues express estrogen receptors and are regulated by estrogen. However, many questions remain unanswered, including why the aromatase knockout phenotype does not replicate the Esr1KO male. Future studies are needed to understand how AR and ESR1/ESR2, sometimes all expressed in the same cell, work together to regulate cellular activity, as well as how emerging players such as membrane ESR1 and GPER fit into our continually evolving understanding of estrogen’s role in males.
GRANTS
This work was supported by a New Florida Scholar Boost Award from the State of Florida, a 2015–16 Research Competition Award from the University of Florida, and National Institutes of Health Grant HD087528 (to P. S. Cooke).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Address for reprint requests and other correspondence: P. S. Cooke, Dept. of Physiological Sciences, Univ. of Florida, Gainesville, FL 32610 (e-mail: paulscooke@ufl.edu).
REFERENCES
- 1.Acconcia F, Ascenzi P, Fabozzi G, Visca P, Marino M. S-palmitoylation modulates human estrogen receptor-alpha functions. Biochem Biophys Res Commun 316: 878–883, 2004. doi: 10.1016/j.bbrc.2004.02.129. [DOI] [PubMed] [Google Scholar]
- 2.Adams JY, Leav I, Lau KM, Ho SM, Pflueger SM. Expression of estrogen receptor beta in the fetal, neonatal, and prepubertal human prostate. Prostate 52: 69–81, 2002. doi: 10.1002/pros.10103. [DOI] [PubMed] [Google Scholar]
- 3.Adlanmerini M, Solinhac R, Abot A, Fabre A, Raymond-Letron I, Guihot AL, Boudou F, Sautier L, Vessières E, Kim SH, Lière P, Fontaine C, Krust A, Chambon P, Katzenellenbogen JA, Gourdy P, Shaul PW, Henrion D, Arnal JF, Lenfant F. Mutation of the palmitoylation site of estrogen receptor α in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci USA 111: E283–E290, 2014. doi: 10.1073/pnas.1322057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal VR, Takayama K, Van Wyk JJ, Sasano H, Simpson ER, Bulun SE. Molecular basis of severe gynecomastia associated with aromatase expression in a fibrolamellar hepatocellular carcinoma. J Clin Endocrinol Metab 83: 1797–1800, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre JI, Plotkin LI, Gortazar AR, Millan MM, O’Brien CA, Manolagas SC, Bellido T. A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J Biol Chem 282: 25501–25508, 2007. doi: 10.1074/jbc.M702231200. [DOI] [PubMed] [Google Scholar]
- 6.Ahlbory-Dieker DL, Stride BD, Leder G, Schkoldow J, Trölenberg S, Seidel H, Otto C, Sommer A, Parker MG, Schütz G, Wintermantel TM. DNA binding by estrogen receptor-alpha is essential for the transcriptional response to estrogen in the liver and the uterus. Mol Endocrinol 23: 1544–1555, 2009. doi: 10.1210/me.2009-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahlgren C, Odén A, Lycke J. High nationwide prevalence of multiple sclerosis in Sweden. Mult Scler 17: 901–908, 2011. doi: 10.1177/1352458511403794. [DOI] [PubMed] [Google Scholar]
- 8.Airas L. Hormonal and gender-related immune changes in multiple sclerosis. Acta Neurol Scand 132: 62–70, 2015. doi: 10.1111/ane.12433. [DOI] [PubMed] [Google Scholar]
- 9.Akingbemi BT, Ge R, Rosenfeld CS, Newton LG, Hardy DO, Catterall JF, Lubahn DB, Korach KS, Hardy MP. Estrogen receptor-alpha gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinology 144: 84–93, 2003. doi: 10.1210/en.2002-220292. [DOI] [PubMed] [Google Scholar]
- 10.Akther S, Huang Z, Liang M, Zhong J, Fakhrul AA, Yuhi T, Lopatina O, Salmina AB, Yokoyama S, Higashida C, Tsuji T, Matsuo M, Higashida H. Paternal retrieval behavior regulated by brain estrogen synthetase (aromatase) in mouse sires that engage in communicative interactions with pairmates. Front Neurosci 9: 450, 2015. doi: 10.3389/fnins.2015.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol Regul Integr Comp Physiol 241: R62–R66, 1981. [DOI] [PubMed] [Google Scholar]
- 12.Allan CM, Couse JF, Simanainen U, Spaliviero J, Jimenez M, Rodriguez K, Korach KS, Handelsman DJ. Estradiol induction of spermatogenesis is mediated via an estrogen receptor-alpha mechanism involving neuroendocrine activation of follicle-stimulating hormone secretion. Endocrinology 151: 2800–2810, 2010. doi: 10.1210/en.2009-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen JM. The influence of hormones on cell division. I. Time-response of ear, seminal vesicle, coagulating gland and ventral prostate of castrate male mice to a single injection of estradiol benzoate. Exp Cell Res 10: 523–532, 1956. doi: 10.1016/0014-4827(56)90024-6. [DOI] [PubMed] [Google Scholar]
- 14.Almeida J, Conley AJ, Ball BA. Expression of anti-Müllerian hormone, CDKN1B, connexin 43, androgen receptor and steroidogenic enzymes in the equine cryptorchid testis. Equine Vet J 45: 538–545, 2013. doi: 10.1111/evj.12013. [DOI] [PubMed] [Google Scholar]
- 15.Almeida M, Iyer S, Martin-Millan M, Bartell SM, Han L, Ambrogini E, Onal M, Xiong J, Weinstein RS, Jilka RL, O’Brien CA, Manolagas SC. Estrogen receptor-α signaling in osteoblast progenitors stimulates cortical bone accrual. J Clin Invest 123: 394–404, 2013. doi: 10.1172/JCI65910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology 29: 1071–1081, 2004. doi: 10.1016/j.psyneuen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquié M, Gauthier BR, Nef S, Stefani E, Nadal A. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS One 3: e2069, 2008. doi: 10.1371/journal.pone.0002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amin S, Zhang Y, Felson DT, Sawin CT, Hannan MT, Wilson PW, Kiel DP. Estradiol, testosterone, and the risk for hip fractures in elderly men from the Framingham Study. Am J Med 119: 426–433, 2006. doi: 10.1016/j.amjmed.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 19.Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci USA 105: 2433–2438, 2008. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anthoni H, Sucheston LE, Lewis BA, Tapia-Páez I, Fan X, Zucchelli M, Taipale M, Stein CM, Hokkanen ME, Castrén E, Pennington BF, Smith SD, Olson RK, Tomblin JB, Schulte-Körne G, Nöthen M, Schumacher J, Müller-Myhsok B, Hoffmann P, Gilger JW, Hynd GW, Nopola-Hemmi J, Leppanen PH, Lyytinen H, Schoumans J, Nordenskjöld M, Spencer J, Stanic D, Boon WC, Simpson E, Mäkelä S, Gustafsson JA, Peyrard-Janvid M, Iyengar S, Kere J. The aromatase gene CYP19A1: several genetic and functional lines of evidence supporting a role in reading, speech and language. Behav Genet 42: 509–527, 2012. doi: 10.1007/s10519-012-9532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonson P, Omoto Y, Humire P, Gustafsson JA. Generation of ERα-floxed and knockout mice using the Cre/LoxP system. Biochem Biophys Res Commun 424: 710–716, 2012. doi: 10.1016/j.bbrc.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Aquila S, Sisci D, Gentile M, Carpino A, Middea E, Catalano S, Rago V, Andò S. Towards a physiological role for cytochrome P450 aromatase in ejaculated human sperm. Hum Reprod 18: 1650–1659, 2003. doi: 10.1093/humrep/deg340. [DOI] [PubMed] [Google Scholar]
- 23.Aquila S, Sisci D, Gentile M, Middea E, Catalano S, Carpino A, Rago V, Andò S. Estrogen receptor (ER)alpha and ER beta are both expressed in human ejaculated spermatozoa: evidence of their direct interaction with phosphatidylinositol-3-OH kinase/Akt pathway. J Clin Endocrinol Metab 89: 1443–1451, 2004. doi: 10.1210/jc.2003-031681. [DOI] [PubMed] [Google Scholar]
- 24.Aquila S, Sisci D, Gentile M, Middea E, Siciliano L, Andò S. Human ejaculated spermatozoa contain active P450 aromatase. J Clin Endocrinol Metab 87: 3385–3390, 2002. doi: 10.1210/jcem.87.7.8633. [DOI] [PubMed] [Google Scholar]
- 25.Arao Y, Hamilton KJ, Goulding EH, Janardhan KS, Eddy EM, Korach KS. Transactivating function (AF) 2-mediated AF-1 activity of estrogen receptor α is crucial to maintain male reproductive tract function. Proc Natl Acad Sci USA 109: 21140–21145, 2012. doi: 10.1073/pnas.1216189110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arenas MI, Royuela M, Lobo MV, Alfaro JM, Fraile B, Paniagua R. Androgen receptor (AR), estrogen receptor-alpha (ER-alpha) and estrogen receptor-beta (ER-beta) expression in the testis of the newt, Triturus marmoratus marmoratus during the annual cycle. J Anat 199: 465–472, 2001. doi: 10.1046/j.1469-7580.2001.19940465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arevalo MA, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci 16: 17–29, 2015. doi: 10.1038/nrn3856. [DOI] [PubMed] [Google Scholar]
- 28.Arkoun B, Gautier C, Delalande C, Barrier-Battut I, Guénon I, Goux D, Bouraïma-Lelong H. Stallion spermatozoa: putative target of estrogens; presence of the estrogen receptors ESR1, ESR2 and identification of the estrogen-membrane receptor GPER. Gen Comp Endocrinol 200: 35–43, 2014. doi: 10.1016/j.ygcen.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol 30: 1–9, 2009. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol 155: 1137–1146, 1999. doi: 10.1016/S0002-9440(10)65217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.At-Taras EE, Kim IC, Berger T, Conley A, Roser JF. Reducing endogenous estrogen during development alters hormone production by porcine Leydig cells and seminiferous tubules. Domest Anim Endocrinol 34: 100–108, 2008. doi: 10.1016/j.domaniend.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Atanassova N, McKinnell C, Fisher J, Sharpe RM. Neonatal treatment of rats with diethylstilboestrol (DES) induces stromal-epithelial abnormalities of the vas deferens and cauda epididymis in adulthood following delayed basal cell development. Reproduction 129: 589–601, 2005. doi: 10.1530/rep.1.00546. [DOI] [PubMed] [Google Scholar]
- 33.Atanassova N, McKinnell C, Williams K, Turner KJ, Fisher JS, Saunders PT, Millar MR, Sharpe RM. Age-, cell- and region-specific immunoexpression of estrogen receptor alpha (but not estrogen receptor beta) during postnatal development of the epididymis and vas deferens of the rat and disruption of this pattern by neonatal treatment with diethylstilbestrol. Endocrinology 142: 874–886, 2001. doi: 10.1210/endo.142.2.7978. [DOI] [PubMed] [Google Scholar]
- 34.Avtanski D, Novaira HJ, Wu S, Romero CJ, Kineman R, Luque RM, Wondisford F, Radovick S. Both estrogen receptor α and β stimulate pituitary GH gene expression. Mol Endocrinol 28: 40–52, 2014. doi: 10.1210/me.2013-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a.Awider-Al-Amawi M, Marchlewicz M, Kolasa A, Wenda-Rozewicka L, Wiszniewska B. Rat epididymal epithelial cells and 17beta-estradiol synthesis under hCG stimulation in vitro. Folia Histochem Cytobiol 45: 255–263, 2007. [PubMed] [Google Scholar]
- 35.Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM. Brain aromatase is neuroprotective. J Neurobiol 47: 318–329, 2001. doi: 10.1002/neu.1038. [DOI] [PubMed] [Google Scholar]
- 36.Bacon RL, Kirkman H. The response of the testis of the hamster to chronic treatment with different estrogens. Endocrinology 57: 255–271, 1955. doi: 10.1210/endo-57-3-255. [DOI] [PubMed] [Google Scholar]
- 37.Bai Y, Chang F, Zhou R, Jin PP, Matsumoto H, Sokabe M, Chen L. Increase of anteroventral periventricular kisspeptin neurons and generation of E2-induced LH-surge system in male rats exposed perinatally to environmental dose of bisphenol-A. Endocrinology 152: 1562–1571, 2011. doi: 10.1210/en.2010-1042. [DOI] [PubMed] [Google Scholar]
- 38.Bajpai A, Simm PJ, McPherson SJ, Russo VC, Azar WJ, Wark JD, Risbridger GP, Werther GA. Peripubertal aromatase inhibition in male rats has adverse long-term effects on bone strength and growth and induces prostatic hyperplasia. J Endocrinol 207: 27–34, 2010. doi: 10.1677/JOE-10-0006. [DOI] [PubMed] [Google Scholar]
- 39.Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav 46: 1–10, 2004. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Balhuizen A, Kumar R, Amisten S, Lundquist I, Salehi A. Activation of G protein-coupled receptor 30 modulates hormone secretion and counteracts cytokine-induced apoptosis in pancreatic islets of female mice. Mol Cell Endocrinol 320: 16–24, 2010. doi: 10.1016/j.mce.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 41.Balthazart J. Minireview: hormones and human sexual orientation. Endocrinology 152: 2937–2947, 2011. doi: 10.1210/en.2011-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barker JM, Galea LA. Sex and regional differences in estradiol content in the prefrontal cortex, amygdala and hippocampus of adult male and female rats. Gen Comp Endocrinol 164: 77–84, 2009. doi: 10.1016/j.ygcen.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Barrett-Connor E, Mueller JE, von Mühlen DG, Laughlin GA, Schneider DL, Sartoris DJ. Low levels of estradiol are associated with vertebral fractures in older men, but not women: the Rancho Bernardo Study. J Clin Endocrinol Metab 85: 219–223, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Barros RP, Gabbi C, Morani A, Warner M, Gustafsson JA. Participation of ERalpha and ERbeta in glucose homeostasis in skeletal muscle and white adipose tissue. Am J Physiol Endocrinol Metab 297: E124–E133, 2009. doi: 10.1152/ajpendo.00189.2009. [DOI] [PubMed] [Google Scholar]
- 45.Barros RP, Machado UF, Warner M, Gustafsson JA. Muscle GLUT4 regulation by estrogen receptors ERbeta and ERalpha. Proc Natl Acad Sci USA 103: 1605–1608, 2006. doi: 10.1073/pnas.0510391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology 23: 963–987, 1998. doi: 10.1016/S0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- 47.Baykan EK, Erdoğan M, Özen S, Darcan Ș, Saygılı LF. Aromatase deficiency, a rare syndrome: case report. J Clin Res Pediatr Endocrinol 5: 129–132, 2013. doi: 10.4274/Jcrpe.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bayless DW, Shah NM. Genetic dissection of neural circuits underlying sexually dimorphic social behaviours. Philos Trans R Soc Lond B Biol Sci 371: 20150109, 2016. doi: 10.1098/rstb.2015.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bedrak E, Samuels LT. Steroid biosynthesis by the equine testis. Endocrinology 85: 1186–1195, 1969. doi: 10.1210/endo-85-6-1186. [DOI] [PubMed] [Google Scholar]
- 50.Beguelini MR, Falleiros LR Jr, Góes RM, Rahal P, Morielle-Versute E, Taboga SR. Differential expression of aromatase, estrogen receptor alpha and 17β-HSD associated with the processes of total testicular regression and recrudescence in the bat Myotis nigricans (Chiroptera: Vespertilionidae). Gen Comp Endocrinol 201: 53–64, 2014. doi: 10.1016/j.ygcen.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 51.Beitelshees AL, Johnson JA, Hames ML, Gong Y, Cooper-Dehoff RM, Wu J, Cresci S, Ma CX, Pepine CJ, Province MA, Spertus JA, McLeod HL. Aromatase gene polymorphisms are associated with survival among patients with cardiovascular disease in a sex-specific manner. PLoS One 5: e15180, 2010. doi: 10.1371/journal.pone.0015180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belgorosky A, Guercio G, Pepe C, Saraco N, Rivarola MA. Genetic and clinical spectrum of aromatase deficiency in infancy, childhood and adolescence. Horm Res 72: 321–330, 2009. doi: 10.1159/000249159. [DOI] [PubMed] [Google Scholar]
- 53.Bell JR, Bernasochi GB, Varma U, Boon WC, Ellem SJ, Risbridger GP, Delbridge LM. Aromatase transgenic upregulation modulates basal cardiac performance and the response to ischemic stress in male mice. Am J Physiol Heart Circ Physiol 306: H1265–H1274, 2014. doi: 10.1152/ajpheart.00012.2014. [DOI] [PubMed] [Google Scholar]
- 54.Berensztein EB, Sciara MI, Rivarola MA, Belgorosky A. Apoptosis and proliferation of human testicular somatic and germ cells during prepuberty: high rate of testicular growth in newborns mediated by decreased apoptosis. J Clin Endocrinol Metab 87: 5113–5118, 2002. doi: 10.1210/jc.2002-020032. [DOI] [PubMed] [Google Scholar]
- 55.Berger T, McCarthy M, Pearl CA, At-Taras E, Roser JF, Conley A. Reducing endogenous estrogens during the neonatal and juvenile periods affects reproductive tract development and sperm production in postpuberal boars. Anim Reprod Sci 109: 218–235, 2008. doi: 10.1016/j.anireprosci.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Bergman B, Damber JE, Littbrand B, Sjögren K, Tomić R. Sexual function in prostatic cancer patients treated with radiotherapy, orchiectomy or oestrogens. Br J Urol 56: 64–69, 1984. doi: 10.1111/j.1464-410X.1984.tb07166.x. [DOI] [PubMed] [Google Scholar]
- 57.Bernard V, Kherra S, Francou B, Fagart J, Viengchareun S, Guéchot J, Ladjouze A, Guiochon-Mantel A, Korach KS, Binart N, Lombès M, Christin-Maitre S. Familial multiplicity of estrogen insensitivity associated with a loss-of-function ESR1 mutation. J Clin Endocrinol Metab 102: 93–99, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bernardino RL, Alves MG, Silva J, Barros A, Ferraz L, Sousa M, Sá R, Oliveira PF. Expression of estrogen receptors alpha (ER-α), beta (ER-β), and G protein-coupled receptor 30 (GPR30) in testicular tissue of men with Klinefelter syndrome. Horm Metab Res 48: 413–415, 2016. doi: 10.1055/s-0042-105151. [DOI] [PubMed] [Google Scholar]
- 59.Berthelsen HG. Changes in the urethral smear in the male rabbit following the administration of oestrogens. Acta Endocrinol (Copenh) 29: 375–380, 1958. [DOI] [PubMed] [Google Scholar]
- 60.Betka M, Callard GV. Negative feedback control of the spermatogenic progression by testicular oestrogen synthesis: insights from the shark testis model. APMIS 106: 252–257, 1998. doi: 10.1111/j.1699-0463.1998.tb01344.x. [DOI] [PubMed] [Google Scholar]
- 61.Bian P, Li X, Ying Q, Chen J, Jin X, Yao J, Shou Z. Factors associated with low femoral neck bone mineral density in very elderly Chinese males. Arch Gerontol Geriatr 61: 484–488, 2015. doi: 10.1016/j.archger.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 62.Bibbo M, Al-Naqeeb M, Baccarini I, Gill W, Newton M, Sleeper KM, Sonek RN, Wied GL. Follow-up study of male and female offspring of DES-treated mothers a preliminary report. J Reprod Med 15: 29–32, 1975. [PubMed] [Google Scholar]
- 63.Bibbo M, Ali I, Al-Naqeeb M, Baccarini I, Climaco LA, Gill W, Sonek M, Wied GL. Cytologic findings in female and male offspring of DES treated mothers. Acta Cytol 19: 568–572, 1975. [PubMed] [Google Scholar]
- 64.Bidmon HJ, Pitts JD, Solomon HF, Bondi JV, Stumpf WE. Estradiol distribution and penetration in rat skin after topical application, studied by high resolution autoradiography. Histochemistry 95: 43–54, 1990. doi: 10.1007/BF00737227. [DOI] [PubMed] [Google Scholar]
- 65.Bigsby RM, Aixin L, Luo K, Cunha GR. Strain differences in the ontogeny of estrogen receptors in murine uterine epithelium. Endocrinology 126: 2592–2596, 1990. doi: 10.1210/endo-126-5-2592. [DOI] [PubMed] [Google Scholar]
- 66.Bilezikian JP, Morishima A, Bell J, Grumbach MM. Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N Engl J Med 339: 599–603, 1998. doi: 10.1056/NEJM199808273390905. [DOI] [PubMed] [Google Scholar]
- 67.Bilińska B, Kotula-Balak M, Gancarczyk M, Sadowska J, Tabarowski Z, Wojtusiak A. Androgen aromatization in cryptorchid mouse testis. Acta Histochem 105: 57–65, 2003. doi: 10.1078/0065-1281-00682. [DOI] [PubMed] [Google Scholar]
- 68.Bilińska B, Schmalz-Fraczek B, Kotula M, Carreau S. Photoperiod-dependent capability of androgen aromatization and the role of estrogens in the bank vole testis visualized by means of immunohistochemistry. Mol Cell Endocrinol 178: 189–198, 2001. doi: 10.1016/S0303-7207(01)00427-0. [DOI] [PubMed] [Google Scholar]
- 69.Binder G, Iliev DI, Dufke A, Wabitsch M, Schweizer R, Ranke MB, Schmidt M. Dominant transmission of prepubertal gynecomastia due to serum estrone excess: hormonal, biochemical, and genetic analysis in a large kindred. J Clin Endocrinol Metab 90: 484–492, 2005. doi: 10.1210/jc.2004-1566. [DOI] [PubMed] [Google Scholar]
- 70.Blaak E. Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care 4: 499–502, 2001. doi: 10.1097/00075197-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 71.Blattner MS, Mahoney MM. Changes in estrogen receptor signaling alters the timekeeping system in male mice. Behav Brain Res 294: 43–49, 2015. doi: 10.1016/j.bbr.2015.07.060. [DOI] [PubMed] [Google Scholar]
- 72.Blattner MS, Mahoney MM. Circadian parameters are altered in two strains of mice with transgenic modifications of estrogen receptor subtype 1. Genes Brain Behav 11: 828–836, 2012. doi: 10.1111/j.1601-183X.2012.00831.x. [DOI] [PubMed] [Google Scholar]
- 73.Blattner MS, Mahoney MM. Estrogen receptor 1 modulates circadian rhythms in adult female mice. Chronobiol Int 31: 637–644, 2014. doi: 10.3109/07420528.2014.885528. [DOI] [PubMed] [Google Scholar]
- 74.Blaustein JD, McCarthy MM. Phoenix, Goy, Gerall, and Young, Endocrinology, 1959: 50 years young and going strong. Endocrinology 150: 2501, 2009. doi: 10.1210/en.2009-0414. [DOI] [PubMed] [Google Scholar]
- 75.Blázquez M, González A, Papadaki M, Mylonas C, Piferrer F. Sex-related changes in estrogen receptors and aromatase gene expression and enzymatic activity during early development and sex differentiation in the European sea bass (Dicentrarchus labrax). Gen Comp Endocrinol 158: 95–101, 2008. doi: 10.1016/j.ygcen.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 76.Blenck CL, Harvey PA, Reckelhoff JF, Leinwand LA. The importance of biological sex and estrogen in rodent models of cardiovascular health and disease. Circ Res 118: 1294–1312, 2016. doi: 10.1161/CIRCRESAHA.116.307509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blomberg Jensen M, Lieben L, Nielsen JE, Willems A, Jørgensen A, Juul A, Toppari J, Carmeliet G, Rajpert-De Meyts E. Characterization of the testicular, epididymal and endocrine phenotypes in the Leuven Vdr-deficient mouse model: targeting estrogen signalling. Mol Cell Endocrinol 377: 93–102, 2013. doi: 10.1016/j.mce.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 78.Bois C, Delalande C, Nurmio M, Parvinen M, Zanatta L, Toppari J, Carreau S. Age- and cell-related gene expression of aromatase and estrogen receptors in the rat testis. J Mol Endocrinol 45: 147–159, 2010. doi: 10.1677/JME-10-0041. [DOI] [PubMed] [Google Scholar]
- 79.Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 298: H16–H23, 2010. doi: 10.1152/ajpheart.00588.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bosland MC. The role of estrogens in prostate carcinogenesis: a rationale for chemoprevention. Rev Urol 7, Suppl 3: S4–S10, 2005. [PMC free article] [PubMed] [Google Scholar]
- 81.Bouchoucha N, Samara-Boustani D, Pandey AV, Bony-Trifunovic H, Hofer G, Aigrain Y, Polak M, Flück CE. Characterization of a novel CYP19A1 (aromatase) R192H mutation causing virilization of a 46,XX newborn, undervirilization of the 46,XY brother, but no virilization of the mother during pregnancies. Mol Cell Endocrinol 390: 8–17, 2014. doi: 10.1016/j.mce.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 82.Bouillon R, Bex M, Vanderschueren D, Boonen S. Estrogens are essential for male pubertal periosteal bone expansion. J Clin Endocrinol Metab 89: 6025–6029, 2004. doi: 10.1210/jc.2004-0602. [DOI] [PubMed] [Google Scholar]
- 83.Bouma J, Nagler JJ. Estrogen receptor-alpha protein localization in the testis of the rainbow trout (Oncorhynchus mykiss) during different stages of the reproductive cycle. Biol Reprod 65: 60–65, 2001. doi: 10.1095/biolreprod65.1.60. [DOI] [PubMed] [Google Scholar]
- 84.Bourg R, Van Meensel F. [Mammary glands of the male adult rat after estradiol administration of estradiol]. Ann Endocrinol (Paris) 16: 171–177, 1955. [PubMed] [Google Scholar]
- 85.Bourguiba S, Chater S, Delalande C, Benahmed M, Carreau S. Regulation of aromatase gene expression in purified germ cells of adult male rats: effects of transforming growth factor beta, tumor necrosis factor alpha, and cyclic adenosine 3′,5′-monosphosphate. Biol Reprod 69: 592–601, 2003. doi: 10.1095/biolreprod.102.013961. [DOI] [PubMed] [Google Scholar]
- 86.Bourguiba S, Genissel C, Lambard S, Bouraïma H, Carreau S. Regulation of aromatase gene expression in Leydig cells and germ cells. J Steroid Biochem Mol Biol 86: 335–343, 2003. doi: 10.1016/S0960-0760(03)00343-1. [DOI] [PubMed] [Google Scholar]
- 87.Bove RM, Healy B, Augustine A, Musallam A, Gholipour T, Chitnis T. Effect of gender on late-onset multiple sclerosis. Mult Scler 18: 1472–1479, 2012. doi: 10.1177/1352458512438236. [DOI] [PubMed] [Google Scholar]
- 88.Brand H, Kos M, Denger S, Flouriot G, Gromoll J, Gannon F, Reid G. A novel promoter is involved in the expression of estrogen receptor alpha in human testis and epididymis. Endocrinology 143: 3397–3404, 2002. doi: 10.1210/en.2001-210832. [DOI] [PubMed] [Google Scholar]
- 89.Bredfeldt TG, Greathouse KL, Safe SH, Hung MC, Bedford MT, Walker CL. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol 24: 993–1006, 2010. doi: 10.1210/me.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brenner RM, West NB, McClellan MC. Estrogen and progestin receptors in the reproductive tract of male and female primates. Biol Reprod 42: 11–19, 1990. doi: 10.1095/biolreprod42.1.11. [DOI] [PubMed] [Google Scholar]
- 91.Brett MA, Roberts LF, Johnson TW, Wassersug RJ. Eunuchs in contemporary society: expectations, consequences, and adjustments to castration (part II). J Sex Med 4: 946–955, 2007. doi: 10.1111/j.1743-6109.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- 92.Brockman R, Bunick D, Mahoney MM. Estradiol deficiency during development modulates the expression of circadian and daily rhythms in male and female aromatase knockout mice. Horm Behav 60: 439–447, 2011. doi: 10.1016/j.yhbeh.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 93.Brodie A, Inkster S. Aromatase in the human testis. J Steroid Biochem Mol Biol 44: 549–555, 1993. doi: 10.1016/0960-0760(93)90258-X. [DOI] [PubMed] [Google Scholar]
- 94.Brodie A, Inkster S, Yue W. Aromatase expression in the human male. Mol Cell Endocrinol 178: 23–28, 2001. doi: 10.1016/S0303-7207(01)00444-0. [DOI] [PubMed] [Google Scholar]
- 95.Broughton BR, Brait VH, Guida E, Lee S, Arumugam TV, Gardiner-Mann CV, Miller AA, Tang SC, Drummond GR, Sobey CG. Stroke increases G protein-coupled estrogen receptor expression in the brain of male but not female mice. Neurosignals 21: 229–239, 2013. doi: 10.1159/000338019. [DOI] [PubMed] [Google Scholar]
- 96.Broughton BR, Brait VH, Kim HA, Lee S, Chu HX, Gardiner-Mann CV, Guida E, Evans MA, Miller AA, Arumugam TV, Drummond GR, Sobey CG. Sex-dependent effects of G protein-coupled estrogen receptor activity on outcome after ischemic stroke. Stroke 45: 835–841, 2014. doi: 10.1161/STROKEAHA.113.001499. [DOI] [PubMed] [Google Scholar]
- 97.Brown M, Ning J, Ferreira JA, Bogener JL, Lubahn DB. Estrogen receptor-alpha and -beta and aromatase knockout effects on lower limb muscle mass and contractile function in female mice. Am J Physiol Endocrinol Metab 296: E854–E861, 2009. doi: 10.1152/ajpendo.90696.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bujan L, Mieusset R, Audran F, Lumbroso S, Sultan C. Increased oestradiol level in seminal plasma in infertile men. Hum Reprod 8: 74–77, 1993. doi: 10.1093/oxfordjournals.humrep.a137878. [DOI] [PubMed] [Google Scholar]
- 99.Bulun SE. Aromatase and estrogen receptor α deficiency. Fertil Steril 101: 323–329, 2014. doi: 10.1016/j.fertnstert.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bulun SE, Rosenthal IM, Brodie AM, Inkster SE, Zeller WP, DiGeorge AM, Frasier SD, Kilgore MW, Simpson ER. Use of tissue-specific promoters in the regulation of aromatase cytochrome P450 gene expression in human testicular and ovarian sex cord tumors, as well as in normal fetal and adult gonads. J Clin Endocrinol Metab 77: 1616–1621, 1993. [DOI] [PubMed] [Google Scholar]
- 101.Cacioppo JA, Koo Y, Lin PC, Osmulski SA, Ko CD, Ko C. Generation of an estrogen receptor beta-iCre knock-in mouse. Genesis 54: 38–52, 2016. doi: 10.1002/dvg.22911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Calatayud NE, Pask AJ, Shaw G, Richings NM, Osborn S, Renfree MB. Ontogeny of the oestrogen receptors ESR1 and ESR2 during gonadal development in the tammar wallaby, Macropus eugenii. Reproduction 139: 599–611, 2010. doi: 10.1530/REP-09-0305. [DOI] [PubMed] [Google Scholar]
- 103.Calderon-Gierszal EL, Prins GS. Directed differentiation of human embryonic stem cells into prostate organoids In vitro and its perturbation by low-dose bisphenol A exposure. PLoS One 10: e0133238, 2015. doi: 10.1371/journal.pone.0133238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Callewaert F, Boonen S, Vanderschueren D. Sex steroids and the male skeleton: a tale of two hormones. Trends Endocrinol Metab 21: 89–95, 2010. doi: 10.1016/j.tem.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 105.Callewaert F, Venken K, Ophoff J, De Gendt K, Torcasio A, van Lenthe GH, Van Oosterwyck H, Boonen S, Bouillon R, Verhoeven G, Vanderschueren D. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-alpha. FASEB J 23: 232–240, 2009. doi: 10.1096/fj.08-113456. [DOI] [PubMed] [Google Scholar]
- 106.Carani C, Granata AR, Rochira V, Caffagni G, Aranda C, Antunez P, Maffei LE. Sex steroids and sexual desire in a man with a novel mutation of aromatase gene and hypogonadism. Psychoneuroendocrinology 30: 413–417, 2005. doi: 10.1016/j.psyneuen.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 107.Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 337: 91–95, 1997. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 108.Carani C, Rochira V, Faustini-Fustini M, Balestrieri A, Granata AR. Role of oestrogen in male sexual behaviour: insights from the natural model of aromatase deficiency. Clin Endocrinol (Oxf) 51: 517–524, 1999. doi: 10.1046/j.1365-2265.1999.00849.x. [DOI] [PubMed] [Google Scholar]
- 109.Carpino A, Bilinska B, Siciliano L, Maggiolini M, Rago V. Immunolocalization of estrogen receptor beta in the epididymis of mature and immature pigs. Folia Histochem Cytobiol 42: 13–17, 2004. [PubMed] [Google Scholar]
- 110.Carpino A, Pezzi V, Rago V, Bilinska B, Andò S. Immunolocalization of cytochrome P450 aromatase in rat testis during postnatal development. Tissue Cell 33: 349–353, 2001. doi: 10.1054/tice.2001.0186. [DOI] [PubMed] [Google Scholar]
- 111.Carpino A, Romeo F, Rago V. Aromatase immunolocalization in human ductuli efferentes and proximal ductus epididymis. J Anat 204: 217–220, 2004. doi: 10.1111/j.0021-8782.2004.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carreau S, Bourguiba S, Marie E. Testicular and blood steroid levels in aged men. Reprod Biol 4: 299–304, 2004. [PubMed] [Google Scholar]
- 113.Carreau S, de Vienne C, Galeraud-Denis I. Aromatase and estrogens in man reproduction: a review and latest advances. Adv Med Sci 53: 139–144, 2008. doi: 10.2478/v10039-008-0022-z. [DOI] [PubMed] [Google Scholar]
- 114.Carreau S, Galeraud-Denis I. Transcripts of aromatase and estrogen receptors and significance of other RNAs in human spermatozoa. Arch Androl 53: 249–255, 2007. doi: 10.1080/01485010701569908. [DOI] [PubMed] [Google Scholar]
- 115.Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci 365: 1517–1535, 2010. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carreau S, Lambard S, Delalande C, Denis-Galeraud I, Bilinska B, Bourguiba S. Aromatase expression and role of estrogens in male gonad: a review. Reprod Biol Endocrinol 1: 35, 2003. doi: 10.1186/1477-7827-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carreau S, Wolczynski S, Galeraud-Denis I. Aromatase, oestrogens and human male reproduction. Philos Trans R Soc Lond B Biol Sci 365: 1571–1579, 2010. doi: 10.1098/rstb.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carrier N, Saland SK, Duclot F, He H, Mercer R, Kabbaj M. The anxiolytic and antidepressant-like effects of testosterone and estrogen in gonadectomized male rats. Biol Psychiatry 78: 259–269, 2015. doi: 10.1016/j.biopsych.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Caruso D, Pesaresi M, Abbiati F, Calabrese D, Giatti S, Garcia-Segura LM, Melcangi RC. Comparison of plasma and cerebrospinal fluid levels of neuroactive steroids with their brain, spinal cord and peripheral nerve levels in male and female rats. Psychoneuroendocrinology 38: 2278–2290, 2013. doi: 10.1016/j.psyneuen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 120.Castelló-Ruiz M, Torregrosa G, Burguete MC, Miranda FJ, Centeno JM, López-Morales MA, Gasull T, Alborch E. The selective estrogen receptor modulator, bazedoxifene, reduces ischemic brain damage in male rat. Neurosci Lett 575: 53–57, 2014. doi: 10.1016/j.neulet.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 121.Catalano S, Pezzi V, Chimento A, Giordano C, Carpino A, Young M, McPhaul MJ, Andò S. Triiodothyronine decreases the activity of the proximal promoter (PII) of the aromatase gene in the mouse Sertoli cell line, TM4. Mol Endocrinol 17: 923–934, 2003. doi: 10.1210/me.2002-0102. [DOI] [PubMed] [Google Scholar]
- 122.Catalano S, Rizza P, Gu G, Barone I, Giordano C, Marsico S, Casaburi I, Middea E, Lanzino M, Pellegrino M, Andò S. Fas ligand expression in TM4 Sertoli cells is enhanced by estradiol “in situ” production. J Cell Physiol 211: 448–456, 2007. doi: 10.1002/jcp.20952. [DOI] [PubMed] [Google Scholar]
- 123.Cavaco JE, Laurentino SS, Barros A, Sousa M, Socorro S. Estrogen receptors alpha and beta in human testis: both isoforms are expressed. Syst Biol Reprod Med 55: 137–144, 2009. doi: 10.3109/19396360902855733. [DOI] [PubMed] [Google Scholar]
- 124.Cavalcanti FN, Lucas TF, Lazari MF, Porto CS. Estrogen receptor ESR1 mediates activation of ERK1/2, CREB, and ELK1 in the corpus of the epididymis. J Mol Endocrinol 54: 339–349, 2015. doi: 10.1530/JME-15-0086. [DOI] [PubMed] [Google Scholar]
- 125.Center JR, Nguyen TV, Sambrook PN, Eisman JA. Hormonal and biochemical parameters in the determination of osteoporosis in elderly men. J Clin Endocrinol Metab 84: 3626–3635, 1999. [DOI] [PubMed] [Google Scholar]
- 126.Chakraborty P, Roy SK. Expression of estrogen receptor α 36 (ESR36) in the hamster ovary throughout the estrous cycle: effects of gonadotropins. PLoS One 8: e58291, 2013. doi: 10.1371/journal.pone.0058291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chakravarty D, Sboner A, Nair SS, Giannopoulou E, Li R, Hennig S, Mosquera JM, Pauwels J, Park K, Kossai M, MacDonald TY, Fontugne J, Erho N, Vergara IA, Ghadessi M, Davicioni E, Jenkins RB, Palanisamy N, Chen Z, Nakagawa S, Hirose T, Bander NH, Beltran H, Fox AH, Elemento O, Rubin MA. The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat Commun 5: 5383, 2014. doi: 10.1038/ncomms6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chang WY, Prins GS. Estrogen receptor-β: implications for the prostate gland. Prostate 40: 115–124, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 129.Chen M, Hsu I, Wolfe A, Radovick S, Huang K, Yu S, Chang C, Messing EM, Yeh S. Defects of prostate development and reproductive system in the estrogen receptor-alpha null male mice. Endocrinology 150: 251–259, 2009. doi: 10.1210/en.2008-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen Z, Wang O, Nie M, Elison K, Zhou D, Li M, Jiang Y, Xia W, Meng X, Chen S, Xing X. Aromatase deficiency in a Chinese adult man caused by novel compound heterozygous CYP19A1 mutations: effects of estrogen replacement therapy on the bone, lipid, liver and glucose metabolism. Mol Cell Endocrinol 399: 32–42, 2015. doi: 10.1016/j.mce.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130a.Cheung CP, Yu S, Wong KB, Chan LW, Lai FM, Wang X, Suetsugi M, Chen S, Chan FL. Expression and functional study of estrogen receptor-related receptors in human prostatic cells and tissues. J Clin Endocrinol Metab 90: 1830–1844, 2005. [DOI] [PubMed] [Google Scholar]
- 131.Chimento A, Sirianni R, Casaburi I, Ruggiero C, Maggiolini M, Andò S, Pezzi V. 17β-Estradiol activates GPER- and ESR1-dependent pathways inducing apoptosis in GC-2 cells, a mouse spermatocyte-derived cell line. Mol Cell Endocrinol 355: 49–59, 2012. doi: 10.1016/j.mce.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 132.Chimento A, Sirianni R, Delalande C, Silandre D, Bois C, Andò S, Maggiolini M, Carreau S, Pezzi V. 17 Beta-estradiol activates rapid signaling pathways involved in rat pachytene spermatocytes apoptosis through GPR30 and ER alpha. Mol Cell Endocrinol 320: 136–144, 2010. doi: 10.1016/j.mce.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 133.Cho HW, Nie R, Carnes K, Zhou Q, Sharief NA, Hess RA. The antiestrogen ICI 182,780 induces early effects on the adult male mouse reproductive tract and long-term decreased fertility without testicular atrophy. Reprod Biol Endocrinol 1: 57, 2003. doi: 10.1186/1477-7827-1-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Choi MH, Moon JY, Cho SH, Chung BC, Lee EJ. Metabolic alteration of urinary steroids in pre- and post-menopausal women, and men with papillary thyroid carcinoma. BMC Cancer 11: 342, 2011. doi: 10.1186/1471-2407-11-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chourasia TK, Pang Y, Thomas P. The catecholestrogen, 2-hydroxyestradiol-17beta, acts as a G protein-coupled estrogen receptor 1 (GPER/GPR30) antagonist to promote the resumption of meiosis in zebrafish oocytes. Biol Reprod 92: 69, 2015. doi: 10.1095/biolreprod.114.125674. [DOI] [PubMed] [Google Scholar]
- 136.Clark JH, Campbell PS, Peck EJ Jr. Receptor-estrogen complex in the nuclear fraction of the pituitary and hypothalamus of male and female immature rats. Neuroendocrinology 10: 218–228, 1972. doi: 10.1159/000122091. [DOI] [PubMed] [Google Scholar]
- 137.Clarke M, Pearl CA. Alterations in the estrogen environment of the testis contribute to declining sperm production in aging rats. Syst Biol Reprod Med 60: 89–97, 2014. doi: 10.3109/19396368.2014.885995. [DOI] [PubMed] [Google Scholar]
- 138.Claus R, Dimmick MA, Gimenez T, Hudson LW. Estrogens and prostaglandin F2alpha in the semen and blood plasma of stallions. Theriogenology 38: 687–693, 1992. doi: 10.1016/0093-691X(92)90031-L. [DOI] [PubMed] [Google Scholar]
- 139.Claus R, Schopper D, Hoang-Vu C. Contribution of individual compartments of the genital tract to oestrogen and testosterone concentrations in ejaculates of the boar. Acta Endocrinol (Copenh) 109: 281–288, 1985. [DOI] [PubMed] [Google Scholar]
- 140.Clulow J, Hansen LA, Jones RC. In vivo microperfusion of the ductuli efferentes testis of the rat: flow dependence of fluid reabsorption. Exp Physiol 81: 633–644, 1996. doi: 10.1113/expphysiol.1996.sp003964. [DOI] [PubMed] [Google Scholar]
- 141.Clulow J, Jones RC, Hansen LA. Micropuncture and cannulation studies of fluid composition and transport in the ductuli efferentes testis of the rat: comparisons with the homologous metanephric proximal tubule. Exp Physiol 79: 915–928, 1994. doi: 10.1113/expphysiol.1994.sp003817. [DOI] [PubMed] [Google Scholar]
- 142.Clulow J, Jones RC, Hansen LA, Man SY. Fluid and electrolyte reabsorption in the ductuli efferentes testis. J Reprod Fertil Suppl 53: 1–14, 1998. [PubMed] [Google Scholar]
- 143.Coban N, Onat A, Guclu-Geyik F, Can G, Erginel-Unaltuna N. Sex- and Obesity-specific association of aromatase (CYP19A1) gene variant with apolipoprotein B and hypertension. Arch Med Res 46: 564–571, 2015. doi: 10.1016/j.arcmed.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 144.Coen P, Kulin H, Ballantine T, Zaino R, Frauenhoffer E, Boal D, Inkster S, Brodie A, Santen R. An aromatase-producing sex-cord tumor resulting in prepubertal gynecomastia. N Engl J Med 324: 317–322, 1991. doi: 10.1056/NEJM199101313240507. [DOI] [PubMed] [Google Scholar]
- 145.Conley A, Mapes S, Corbin CJ, Greger D, Walters K, Trant J, Graham S. A comparative approach to structure-function studies of mammalian aromatases. J Steroid Biochem Mol Biol 79: 289–297, 2001. doi: 10.1016/S0960-0760(01)00145-5. [DOI] [PubMed] [Google Scholar]
- 146.Conley AJ, Corbin CJ, Hinshelwood MM, Liu Z, Simpson ER, Ford JJ, Harada N. Functional aromatase expression in porcine adrenal gland and testis. Biol Reprod 54: 497–505, 1996. doi: 10.1095/biolreprod54.2.497. [DOI] [PubMed] [Google Scholar]
- 147.Cook JC, Johnson L, O’Connor JC, Biegel LB, Krams CH, Frame SR, Hurtt ME. Effects of dietary 17 beta-estradiol exposure on serum hormone concentrations and testicular parameters in male Crl:CD BR rats. Toxicol Sci 44: 155–168, 1998. [DOI] [PubMed] [Google Scholar]
- 148.Cooke PS, Young P, Hess RA, Cunha GR. Estrogen receptor expression in developing epididymis, efferent ductules, and other male reproductive organs. Endocrinology 128: 2874–2879, 1991. doi: 10.1210/endo-128-6-2874. [DOI] [PubMed] [Google Scholar]
- 149.Corbin CJ, Hughes AL, Heffelfinger JR, Berger T, Waltzek TB, Roser JF, Santos TC, Miglino MA, Oliveira MF, Braga FC, Meirelles FV, Conley AJ. Evolution of suiform aromatases: ancestral duplication with conservation of tissue-specific expression in the collared peccary (Pecari tayassu). J Mol Evol 65: 403–412, 2007. doi: 10.1007/s00239-007-9021-0. [DOI] [PubMed] [Google Scholar]
- 150.Corbin CJ, Trant JM, Conley AJ. Porcine gonadal and placental isozymes of aromatase cytochrome P450: sub-cellular distribution and support by NADPH-cytochrome P450 reductase. Mol Cell Endocrinol 172: 115–124, 2001. doi: 10.1016/S0303-7207(00)00373-7. [DOI] [PubMed] [Google Scholar]
- 151.Cornil CA, Ball GF, Balthazart J. The dual action of estrogen hypothesis. Trends Neurosci 38: 408–416, 2015. doi: 10.1016/j.tins.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cornil CA, Ball GF, Balthazart J. Rapid control of male typical behaviors by brain-derived estrogens. Front Neuroendocrinol 33: 425–446, 2012. doi: 10.1016/j.yfrne.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cosentino C, Di Domenico M, Porcellini A, Cuozzo C, De Gregorio G, Santillo MR, Agnese S, Di Stasio R, Feliciello A, Migliaccio A, Avvedimento EV. p85 regulatory subunit of PI3K mediates cAMP-PKA and estrogens biological effects on growth and survival. Oncogene 26: 2095–2103, 2007. doi: 10.1038/sj.onc.1210027. [DOI] [PubMed] [Google Scholar]
- 154.Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology 138: 4613–4621, 1997. 9348186 [DOI] [PubMed] [Google Scholar]
- 155.Crandall DL, Busler DE, Novak TJ, Weber RV, Kral JG. Identification of estrogen receptor beta RNA in human breast and abdominal subcutaneous adipose tissue. Biochem Biophys Res Commun 248: 523–526, 1998. doi: 10.1006/bbrc.1998.8997. [DOI] [PubMed] [Google Scholar]
- 156.Cuevas ME, Callard G. In vitro steroid secretion by staged spermatocysts (Sertoli/germ cell units) of dogfish (Squalus acanthias) testis. Gen Comp Endocrinol 88: 151–165, 1992. doi: 10.1016/0016-6480(92)90204-W. [DOI] [PubMed] [Google Scholar]
- 157.Curl CL, Wendt IR, Kotsanas G. Effects of gender on intracellular. Pflugers Arch 441: 709–716, 2001. doi: 10.1007/s004240000473. [DOI] [PubMed] [Google Scholar]
- 158.Custodia-Lora N, Novillo A, Callard IP. Effect of gonadal steroids on progesterone receptor, estrogen receptor, and vitellogenin expression in male turtles (Chrysemys picta). J Exp Zoolog A Comp Exp Biol 301: 15–25, 2004. doi: 10.1002/jez.a.20004. [DOI] [PubMed] [Google Scholar]
- 159.Dai-Do D, Espinosa E, Liu G, Rabelink TJ, Julmy F, Yang Z, Mahler F, Lüscher TF. 17 Beta-estradiol inhibits proliferation and migration of human vascular smooth muscle cells: similar effects in cells from postmenopausal females and in males. Cardiovasc Res 32: 980–985, 1996. [PubMed] [Google Scholar]
- 160.Dalla Valle L, Lunardi L, Colombo L, Belvedere P. European sea bass (Dicentrarchus labrax L.) cytochrome P450arom: cDNA cloning, expression and genomic organization. J Steroid Biochem Mol Biol 80: 25–34, 2002. doi: 10.1016/S0960-0760(01)00170-4. [DOI] [PubMed] [Google Scholar]
- 161.Danzo BJ, Eller BC. The presence of a cytoplasmic estrogen receptor in sexually mature rabbit epididymides: comparison with the estrogen receptor in immature rabbit epididymal cytosol. Endocrinology 105: 1128–1134, 1979. doi: 10.1210/endo-105-5-1128. [DOI] [PubMed] [Google Scholar]
- 162.Danzo BJ, Eller BC, Judy LA, Trautman JR, Orgebin-Crist MC. Estradiol bending in cytosol from epididymides of immature rabbits. Mol Cell Endocrinol 2: 91–105, 1975. doi: 10.1016/0303-7207(75)90051-9. [DOI] [PubMed] [Google Scholar]
- 163.Danzo BJ, Wolfe MS, Curry JB. The presence of an estradiol binding component in cytosol from immature rat epididymides. Mol Cell Endocrinol 6: 271–279, 1977. doi: 10.1016/0303-7207(77)90101-0. [DOI] [PubMed] [Google Scholar]
- 164.Das SK, Taylor JA, Korach KS, Paria BC, Dey SK, Lubahn DB. Estrogenic responses in estrogen receptor-alpha deficient mice reveal a distinct estrogen signaling pathway. Proc Natl Acad Sci USA 94: 12786–12791, 1997. doi: 10.1073/pnas.94.24.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Davidson JM, Camargo C, Smith ER, Kwan M. Maintenance of sexual function in a castrated man treated with ovarian steroids. Arch Sex Behav 12: 263–274, 1983. doi: 10.1007/BF01542076. [DOI] [PubMed] [Google Scholar]
- 166.Davis KE, Neinast MD, Sun K, Skiles WM, Bills JD, Zehr JA, Zeve D, Hahner LD, Cox DW, Gent LM, Xu Y, Wang ZV, Khan SA, Clegg DJ. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab 2: 227–242, 2013. doi: 10.1016/j.molmet.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Day NL, Floyd CL, D’Alessandro TL, Hubbard WJ, Chaudry IH. 17β-Estradiol confers protection after traumatic brain injury in the rat and involves activation of G protein-coupled estrogen receptor 1. J Neurotrauma 30: 1531–1541, 2013. doi: 10.1089/neu.2013.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.De Jong FH, Hey AH, van der Molen HJ. Effect of gonadotrophins on the secretion of oestradiol- and testosterone by the rat testis. J Endocrinol 57: 277–284, 1973. doi: 10.1677/joe.0.0570277. [DOI] [PubMed] [Google Scholar]
- 169.De Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett 582: 97–105, 2008. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.De Ronde W, de Jong FH. Aromatase inhibitors in men: effects and therapeutic options. Reprod Biol Endocrinol 9: 93, 2011. doi: 10.1186/1477-7827-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30: 1000–1007, 1981. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 172.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 237: E214–E223, 1979. [DOI] [PubMed] [Google Scholar]
- 173.Deladoëy J, Flück C, Bex M, Yoshimura N, Harada N, Mullis PE. Aromatase deficiency caused by a novel P450arom gene mutation: impact of absent estrogen production on serum gonadotropin concentration in a boy. J Clin Endocrinol Metab 84: 4050–4054, 1999. [DOI] [PubMed] [Google Scholar]
- 174.Delbeck M, Golz S, Vonk R, Janssen W, Hucho T, Isensee J, Schäfer S, Otto C. Impaired left-ventricular cardiac function in male GPR30-deficient mice. Mol Med Rep 4: 37–40, 2011. doi: 10.3892/mmr.2010.402. [DOI] [PubMed] [Google Scholar]
- 175.Delbès G, Levacher C, Pairault C, Racine C, Duquenne C, Krust A, Habert R. Estrogen receptor beta-mediated inhibition of male germ cell line development in mice by endogenous estrogens during perinatal life. Endocrinology 145: 3395–3403, 2004. doi: 10.1210/en.2003-1479. [DOI] [PubMed] [Google Scholar]
- 176.Demura M, Martin RM, Shozu M, Sebastian S, Takayama K, Hsu WT, Schultz RA, Neely K, Bryant M, Mendonca BB, Hanaki K, Kanzaki S, Rhoads DB, Misra M, Bulun SE. Regional rearrangements in chromosome 15q21 cause formation of cryptic promoters for the CYP19 (aromatase) gene. Hum Mol Genet 16: 2529–2541, 2007. doi: 10.1093/hmg/ddm145. [DOI] [PubMed] [Google Scholar]
- 177.Denger S, Reid G, Brand H, Kos M, Gannon F. Tissue-specific expression of human ERalpha and ERbeta in the male. Mol Cell Endocrinol 178: 155–160, 2001. doi: 10.1016/S0303-7207(01)00417-8. [DOI] [PubMed] [Google Scholar]
- 178.Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol 297: H1806–H1813, 2009. doi: 10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dewing P, Shi T, Horvath S, Vilain E. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res Mol Brain Res 118: 82–90, 2003. doi: 10.1016/S0169-328X(03)00339-5. [DOI] [PubMed] [Google Scholar]
- 180.Dey P, Ström A, Gustafsson JA. Estrogen receptor β upregulates FOXO3a and causes induction of apoptosis through PUMA in prostate cancer. Oncogene 33: 4213–4225, 2014. doi: 10.1038/onc.2013.384. [DOI] [PubMed] [Google Scholar]
- 181.Dias JP, Melvin D, Simonsick EM, Carlson O, Shardell MD, Ferrucci L, Chia CW, Basaria S, Egan JM. Effects of aromatase inhibition vs. testosterone in older men with low testosterone: randomized-controlled trial. Andrology 4: 33–40, 2016. doi: 10.1111/andr.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dieudonne MN, Pecquery R, Leneveu MC, Giudicelli Y. Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor gamma2. Endocrinology 141: 649–656, 2000. [DOI] [PubMed] [Google Scholar]
- 183.Ding W, Cao L, Cao Z, Bing X, Zhao F. Molecular characterization and expression profile of the estrogen receptor α gene during different reproductive phases in Monopterus albus. Sci Rep 6: 27924, 2016. doi: 10.1038/srep27924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Döhler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology 97: 898–907, 1975. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- 185.Doran PM, Riggs BL, Atkinson EJ, Khosla S. Effects of raloxifene, a selective estrogen receptor modulator, on bone turnover markers and serum sex steroid and lipid levels in elderly men. J Bone Miner Res 16: 2118–2125, 2001. doi: 10.1359/jbmr.2001.16.11.2118. [DOI] [PubMed] [Google Scholar]
- 186.Dorrington JH, Fritz IB, Armstrong DT. Control of testicular estrogen synthesis. Biol Reprod 18: 55–64, 1978. doi: 10.1095/biolreprod18.1.55. [DOI] [PubMed] [Google Scholar]
- 187.Dubé JY, Lesage R, Tremblay RR. Androgen and estrogen binding in rat skeletal and perineal muscles. Can J Biochem 54: 50–55, 1976. doi: 10.1139/o76-008. [DOI] [PubMed] [Google Scholar]
- 188.Dumasia K, Kumar A, Kadam L, Balasinor NH. Effect of estrogen receptor-subtype-specific ligands on fertility in adult male rats. J Endocrinol 225: 169–180, 2015. doi: 10.1530/JOE-15-0045. [DOI] [PubMed] [Google Scholar]
- 189.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 127: 4277–4291, 2000. [DOI] [PubMed] [Google Scholar]
- 190.Echeverría OM, González Maciel A, Traish AM, Wotiz HH, Ubaldo E, Vázquez-Nin GH. Immuno-electron microscopic localization of estradiol receptor in cells of male and female reproductive and non-reproductive organs. Biol Cell 81: 257–265, 1994. doi: 10.1016/0248-4900(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 191.Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology 137: 4796–4805, 1996. [DOI] [PubMed] [Google Scholar]
- 192.Eiler H, Graves CN. Oestrogen content of semen and the effect of exogenous oestradiol-17beta on the oestrogen and androgen concentration in semen and blood plasma of bulls. J Reprod Fertil 50: 17–21, 1977. doi: 10.1530/jrf.0.0500017. [DOI] [PubMed] [Google Scholar]
- 193.Eisenhauer KM, McCue PM, Nayden DK, Osawa Y, Roser JF. Localization of aromatase in equine Leydig cells. Domest Anim Endocrinol 11: 291–298, 1994. doi: 10.1016/0739-7240(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 194.Ekbom A, Wuu J, Adami HO, Lu CM, Lagiou P, Trichopoulos D, Hsieh C. Duration of gestation and prostate cancer risk in offspring. Cancer Epidemiol Biomarkers Prev 9: 221–223, 2000. [PubMed] [Google Scholar]
- 195.Ellem SJ, Schmitt JF, Pedersen JS, Frydenberg M, Risbridger GP. Local aromatase expression in human prostate is altered in malignancy. J Clin Endocrinol Metab 89: 2434–2441, 2004. doi: 10.1210/jc.2003-030933. [DOI] [PubMed] [Google Scholar]
- 196.Ergün S, Ungefroren H, Holstein AF, Davidoff MS. Estrogen and progesterone receptors and estrogen receptor-related antigen (ER-D5) in human epididymis. Mol Reprod Dev 47: 448–455, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 197.Faber KA, Hughes CL Jr. The effect of neonatal exposure to diethylstilbestrol, genistein, and zearalenone on pituitary responsiveness and sexually dimorphic nucleus volume in the castrated adult rat. Biol Reprod 45: 649–653, 1991. doi: 10.1095/biolreprod45.4.649. [DOI] [PubMed] [Google Scholar]
- 198.Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 106: 1553–1560, 2000. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fang YQ, Weng YZ, Huang WQ, Sun L. [Localization of the estrogen receptor alpha and beta-subtype in the nervous system, Hatschek’s pit and gonads of amphioxus, Branchiostoma belcheri]. Shi Yan Sheng Wu Xue Bao 36: 368–374, 2003. [PubMed] [Google Scholar]
- 200.Fawcett DW, Hoffer AP. Failure of exogenous androgen to prevent regression of the initial segments of the rat epididymis after efferent duct ligation or orchidectomy. Biol Reprod 20: 162–181, 1979. doi: 10.1095/biolreprod20.2.162. [DOI] [PubMed] [Google Scholar]
- 201.Feder HH, Whalen RE. Feminine behavior in neonatally castrated and estrogen-treated male rats. Science 147: 306–307, 1965. doi: 10.1126/science.147.3655.306. [DOI] [PubMed] [Google Scholar]
- 202.Fietz D, Bergmann M, Hartmann K. In situ hybridization of estrogen receptors alpha and beta and GPER in the human testis. Methods Mol Biol 1366: 189–205, 2016. doi: 10.1007/978-1-4939-3127-9_15. [DOI] [PubMed] [Google Scholar]
- 203.Fietz D, Ratzenböck C, Hartmann K, Raabe O, Kliesch S, Weidner W, Klug J, Bergmann M. Expression pattern of estrogen receptors α and β and G-protein-coupled estrogen receptor 1 in the human testis. Histochem Cell Biol 142: 421–432, 2014. doi: 10.1007/s00418-014-1216-z. [DOI] [PubMed] [Google Scholar]
- 204.Filardo EJ, Quinn JA, Bland KI, Frackelton AR Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14: 1649–1660, 2000. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 205.Filipiak E, Suliborska D, Laszczynska M, Walczak-Jedrzejowska R, Oszukowska E, Marchlewska K, Kula K, Slowikowska-Hilczer J. Estrogen receptor alpha localization in the testes of men with normal spermatogenesis. Folia Histochem Cytobiol 50: 340–345, 2013. doi: 10.5603/FHC.2012.0046. [DOI] [PubMed] [Google Scholar]
- 206.Fink HA, Ewing SK, Ensrud KE, Barrett-Connor E, Taylor BC, Cauley JA, Orwoll ES. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab 91: 3908–3915, 2006. doi: 10.1210/jc.2006-0173. [DOI] [PubMed] [Google Scholar]
- 207.Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med 369: 1011–1022, 2013. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Finkelstein JS, Lee H, Leder BZ, Burnett-Bowie SA, Goldstein DW, Hahn CW, Hirsch SC, Linker A, Perros N, Servais AB, Taylor AP, Webb ML, Youngner JM, Yu EW. Gonadal steroid-dependent effects on bone turnover and bone mineral density in men. J Clin Invest 126: 1114–1125, 2016. doi: 10.1172/JCI84137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Finn CA, Martin L. Patterns of cell division in the mouse uterus during early pregnancy. J Endocrinol 39: 593–597, 1967. doi: 10.1677/joe.0.0390593. [DOI] [PubMed] [Google Scholar]
- 210.Finn CA, Martin L. The role of the oestrogen secreted before oestrus in the preparation of the uterus for implantation in the mouse. J Endocrinol 47: 431–438, 1970. doi: 10.1677/joe.0.0470431. [DOI] [PubMed] [Google Scholar]
- 211.Fisher JS, Millar MR, Majdic G, Saunders PT, Fraser HM, Sharpe RM. Immunolocalisation of oestrogen receptor-alpha within the testis and excurrent ducts of the rat and marmoset monkey from perinatal life to adulthood. J Endocrinol 153: 485–495, 1997. doi: 10.1677/joe.0.1530485. [DOI] [PubMed] [Google Scholar]
- 212.Fisher JS, Turner KJ, Brown D, Sharpe RM. Effect of neonatal exposure to estrogenic compounds on development of the excurrent ducts of the rat testis through puberty to adulthood. Environ Health Perspect 107: 397–405, 1999. doi: 10.1289/ehp.99107397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fisher JS, Turner KJ, Fraser HM, Saunders PT, Brown D, Sharpe RM. Immunoexpression of aquaporin-1 in the efferent ducts of the rat and marmoset monkey during development, its modulation by estrogens, and its possible role in fluid resorption. Endocrinology 139: 3935–3945, 1998. [DOI] [PubMed] [Google Scholar]
- 214.Fixemer T, Remberger K, Bonkhoff H. Differential expression of the estrogen receptor beta (ERbeta) in human prostate tissue, premalignant changes, and in primary, metastatic, and recurrent prostatic adenocarcinoma. Prostate 54: 79–87, 2003. doi: 10.1002/pros.10171. [DOI] [PubMed] [Google Scholar]
- 215.Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Dworatzek E, Staub E, Martus P, Ruiz Noppinger P, Kintscher U, Gustafsson JA, Regitz-Zagrosek V. Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol Regul Integr Comp Physiol 298: R1597–R1606, 2010. doi: 10.1152/ajpregu.00825.2009. [DOI] [PubMed] [Google Scholar]
- 216.Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, Sonntag-Buck V, Gannon F. Identification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function 1. EMBO J 19: 4688–4700, 2000. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Forchielli E, Rao GS, Sarda IR, Gibree NB, Pochi PE, Strauss JS, Dorfman RI. Effect of ethinyloestradiol on plasma testosterone levels and urinary testosterone excretion in man. Acta Endocrinol (Copenh) 50: 51–54, 1965. [DOI] [PubMed] [Google Scholar]
- 218.Ford J, Hajibeigi A, Long M, Hahner L, Gore C, Hsieh JT, Clegg D, Zerwekh J, Oz OK. GPR30 deficiency causes increased bone mass, mineralization, and growth plate proliferative activity in male mice. J Bone Miner Res 26: 298–307, 2011. doi: 10.1002/jbmr.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ford J Jr, Carnes K, Hess RA. Ductuli efferentes of the male Golden Syrian hamster reproductive tract. Andrology 2: 510–520, 2014. doi: 10.1111/j.2047-2927.2014.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Förster C, Kietz S, Hultenby K, Warner M, Gustafsson JA. Characterization of the ERbeta-/-mouse heart. Proc Natl Acad Sci USA 101: 14234–14239, 2004. doi: 10.1073/pnas.0405571101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A, Gustafsson JA, Unger T, Kintscher U. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet 4: e1000108, 2008. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Foryst-Ludwig A, Kintscher U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J Steroid Biochem Mol Biol 122: 74–81, 2010. doi: 10.1016/j.jsbmb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 223.Fraczek B, Bourguiba S, Carreau S, Bilińska B. Immunolocalization and activity of aromatase in the bank vole testes. Folia Histochem Cytobiol 39: 315–319, 2001. [PubMed] [Google Scholar]
- 224.Fraczek B, Kotula-Balak M, Wojtusiak A, Pierściński A, Bilińska B. Cytochrome P450 aromatase in the testis of immature and mature pigs. Reprod Biol 1: 51–59, 2001. [PubMed] [Google Scholar]
- 225.Free MJ, Jaffe RA. Collection of rete testis fluid from rats without previous efferent duct ligation. Biol Reprod 20: 269–278, 1979. doi: 10.1095/biolreprod20.2.269. [DOI] [PubMed] [Google Scholar]
- 226.Freking F, Nazairians T, Schlinger BA. The expression of the sex steroid-synthesizing enzymes CYP11A1, 3beta-HSD, CYP17, and CYP19 in gonads and adrenals of adult and developing zebra finches. Gen Comp Endocrinol 119: 140–151, 2000. doi: 10.1006/gcen.2000.7503. [DOI] [PubMed] [Google Scholar]
- 227.Frenette G, Leclerc P, D’amours O, Sullivan R. Estrogen sulfotransferase is highly expressed along the bovine epididymis and is secreted into the intraluminal environment. J Androl 30: 580–589, 2009. doi: 10.2164/jandrol.108.006668. [DOI] [PubMed] [Google Scholar]
- 228.Frokjaer VG, Erritzoe D, Juul A, Nielsen FA, Holst K, Svarer C, Madsen J, Paulson OB, Knudsen GM. Endogenous plasma estradiol in healthy men is positively correlated with cerebral cortical serotonin 2A receptor binding. Psychoneuroendocrinology 35: 1311–1320, 2010. doi: 10.1016/j.psyneuen.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 229.Fujimura T, Takahashi S, Urano T, Ogawa S, Ouchi Y, Kitamura T, Muramatsu M, Inoue S. Differential expression of estrogen receptor beta (ERbeta) and its C-terminal truncated splice variant ERbetacx as prognostic predictors in human prostatic cancer. Biochem Biophys Res Commun 289: 692–699, 2001. doi: 10.1006/bbrc.2001.6038. [DOI] [PubMed] [Google Scholar]
- 230.Fukami M, Shozu M, Soneda S, Kato F, Inagaki A, Takagi H, Hanaki K, Kanzaki S, Ohyama K, Sano T, Nishigaki T, Yokoya S, Binder G, Horikawa R, Ogata T. Aromatase excess syndrome: identification of cryptic duplications and deletions leading to gain of function of CYP19A1 and assessment of phenotypic determinants. J Clin Endocrinol Metab 96: E1035–E1043, 2011. doi: 10.1210/jc.2011-0145. [DOI] [PubMed] [Google Scholar]
- 231.Galan JJ, Buch B, Cruz N, Segura A, Moron FJ, Bassas L, Martinez-Pineiro L, Real LM, Ruiz A. Multilocus analyses of estrogen-related genes reveal involvement of the ESR1 gene in male infertility and the polygenic nature of the pathology. Fertil Steril 84: 910–918, 2005. doi: 10.1016/j.fertnstert.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 232.Ganjam VK, Amann RP. Steroids in fluids and sperm entering and leaving the bovine epididymis, epididymal tissue, and accessory sex gland secretions. Endocrinology 99: 1618–1630, 1976. doi: 10.1210/endo-99-6-1618. [DOI] [PubMed] [Google Scholar]
- 233.Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience 89: 567–578, 1999. doi: 10.1016/S0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- 234.Gardner JD, Murray DB, Voloshenyuk TG, Brower GL, Bradley JM, Janicki JS. Estrogen attenuates chronic volume overload induced structural and functional remodeling in male rat hearts. Am J Physiol Heart Circ Physiol 298: H497–H504, 2010. doi: 10.1152/ajpheart.00336.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gaudet HM, Cheng SB, Christensen EM, Filardo EJ. The G-protein coupled estrogen receptor, GPER: The inside and inside-out story. Mol Cell Endocrinol 418: 207–219, 2015. doi: 10.1016/j.mce.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 236.Gautier C, Barrier-Battut I, Guénon I, Goux D, Delalande C, Bouraïma-Lelong H. Implication of the estrogen receptors GPER, ESR1, ESR2 in post-testicular maturations of equine spermatozoa. Gen Comp Endocrinol 233: 100–108, 2016. doi: 10.1016/j.ygcen.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 237.Ge LC, Chen ZJ, Liu HY, Zhang KS, Liu H, Huang HB, Zhang G, Wong CK, Giesy JP, Du J, Wang HS. Involvement of activating ERK1/2 through G protein coupled receptor 30 and estrogen receptor α/β in low doses of bisphenol A promoting growth of Sertoli TM4 cells. Toxicol Lett 226: 81–89, 2014. doi: 10.1016/j.toxlet.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 238.Genissel C, Carreau S. Regulation of the aromatase gene expression in mature rat Leydig cells. Mol Cell Endocrinol 178: 141–146, 2001. doi: 10.1016/S0303-7207(01)00409-9. [DOI] [PubMed] [Google Scholar]
- 239.Genissel C, Levallet J, Carreau S. Regulation of cytochrome P450 aromatase gene expression in adult rat Leydig cells: comparison with estradiol production. J Endocrinol 168: 95–105, 2001. doi: 10.1677/joe.0.1680095. [DOI] [PubMed] [Google Scholar]
- 240.Gennari L, Masi L, Merlotti D, Picariello L, Falchetti A, Tanini A, Mavilia C, Del Monte F, Gonnelli S, Lucani B, Gennari C, Brandi ML. A polymorphic CYP19 TTTA repeat influences aromatase activity and estrogen levels in elderly men: effects on bone metabolism. J Clin Endocrinol Metab 89: 2803–2810, 2004. doi: 10.1210/jc.2003-031342. [DOI] [PubMed] [Google Scholar]
- 241.Gennari L, Merlotti D, Martini G, Gonnelli S, Franci B, Campagna S, Lucani B, Dal Canto N, Valenti R, Gennari C, Nuti R. Longitudinal association between sex hormone levels, bone loss, and bone turnover in elderly men. J Clin Endocrinol Metab 88: 5327–5333, 2003. doi: 10.1210/jc.2003-030736. [DOI] [PubMed] [Google Scholar]
- 242.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev 62: 155–198, 2010. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gilliver SC, Emmerson E, Campbell L, Chambon P, Hardman MJ, Ashcroft GS. 17Beta-estradiol inhibits wound healing in male mice via estrogen receptor-alpha. Am J Pathol 176: 2707–2721, 2010. doi: 10.2353/ajpath.2010.090432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Gilliver SC, Ruckshanthi JP, Hardman MJ, Nakayama T, Ashcroft GS. Sex dimorphism in wound healing: the roles of sex steroids and macrophage migration inhibitory factor. Endocrinology 149: 5747–5757, 2008. doi: 10.1210/en.2008-0355. [DOI] [PubMed] [Google Scholar]
- 245.Gist DH, Bradshaw S, Morrow CM, Congdon JD, Hess RA. Estrogen response system in the reproductive tract of the male turtle: an immunocytochemical study. Gen Comp Endocrinol 151: 27–33, 2007. doi: 10.1016/j.ygcen.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 246.Godfrey RW, Randel RD, Forrest DW, Senger PL. The concentration of estradiol-17 beta in bovine semen. J Anim Sci 60: 760–765, 1985. doi: 10.2527/jas1985.603760x. [DOI] [PubMed] [Google Scholar]
- 247.Golovine K, Schwerin M, Vanselow J. Three different promoters control expression of the aromatase cytochrome p450 gene (cyp19) in mouse gonads and brain. Biol Reprod 68: 978–984, 2003. doi: 10.1095/biolreprod.102.008037. [DOI] [PubMed] [Google Scholar]
- 248.Gomes GR, Yasuhara F, Siu ER, Fernandes SA, Avellar MC, Lazari MF, Porto CS. In vivo treatments with fulvestrant and anastrozole differentially affect gene expression in the rat efferent ductules. Biol Reprod 84: 52–61, 2011. doi: 10.1095/biolreprod.110.085340. [DOI] [PubMed] [Google Scholar]
- 249.Gonzalez-Unzaga M, Téllez J, Calzada L. Clinical significance of nuclear matrix-estradiol receptor complex in human sperm. Arch Androl 49: 77–81, 2003. doi: 10.1080/01485010390129205. [DOI] [PubMed] [Google Scholar]
- 250.González A, Piferrer F. Aromatase activity in the European sea bass (Dicentrarchus labrax L.) brain. Distribution and changes in relation to age, sex, and the annual reproductive cycle. Gen Comp Endocrinol 132: 223–230, 2003. doi: 10.1016/S0016-6480(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 251.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev 36: E1–E150, 2015. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gorski J, Toft D, Shyamala G, Smith D, Notides A. Hormone receptors: studies on the interaction of estrogen with the uterus. Recent Prog Horm Res 24: 45–80, 1968. [DOI] [PubMed] [Google Scholar]
- 253.Gould ML, Hurst PR, Nicholson HD. The effects of oestrogen receptors alpha and beta on testicular cell number and steroidogenesis in mice. Reproduction 134: 271–279, 2007. doi: 10.1530/REP-07-0025. [DOI] [PubMed] [Google Scholar]
- 254.Goulding EH, Hewitt SC, Nakamura N, Hamilton K, Korach KS, Eddy EM. Ex3αERKO male infertility phenotype recapitulates the αERKO male phenotype. J Endocrinol 207: 281–288, 2010. doi: 10.1677/JOE-10-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Gourgari E, Saloustros E, Stratakis CA. Large-cell calcifying Sertoli cell tumors of the testes in pediatrics. Curr Opin Pediatr 24: 518–522, 2012. doi: 10.1097/MOP.0b013e328355a279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Goyal HO, Bartol FF, Wiley AA, Khalil MK, Chiu J, Vig MM. Immunolocalization of androgen receptor and estrogen receptor in the developing testis and excurrent ducts of goats. Anat Rec 249: 54–62, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 257.Goyal HO, Bartol FF, Wiley AA, Khalil MK, Williams CS, Vig MM. Regulation of androgen and estrogen receptors in male excurrent ducts of the goat: an immunohistochemical study. Anat Rec 250: 164–171, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 258.Goyal HO, Bartol FF, Wiley AA, Neff CW. Immunolocalization of receptors for androgen and estrogen in male caprine reproductive tissues: unique distribution of estrogen receptors in efferent ductule epithelium. Biol Reprod 56: 90–101, 1997. doi: 10.1095/biolreprod56.1.90. [DOI] [PubMed] [Google Scholar]
- 259.Greathouse KL, Bredfeldt T, Everitt JI, Lin K, Berry T, Kannan K, Mittelstadt ML, Ho SM, Walker CL. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Mol Cancer Res 10: 546–557, 2012. doi: 10.1158/1541-7786.MCR-11-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Greco TL, Duello TM, Gorski J. Estrogen receptors, estradiol, and diethylstilbestrol in early development: the mouse as a model for the study of estrogen receptors and estrogen sensitivity in embryonic development of male and female reproductive tracts. Endocr Rev 14: 59–71, 1993. [DOI] [PubMed] [Google Scholar]
- 261.Greco TL, Furlow JD, Duello TM, Gorski J. Immunodetection of estrogen receptors in fetal and neonatal female mouse reproductive tracts. Endocrinology 129: 1326–1332, 1991. doi: 10.1210/endo-129-3-1326. [DOI] [PubMed] [Google Scholar]
- 262.Greytak SR, Callard GV. Cloning of three estrogen receptors (ER) from killifish (Fundulus heteroclitus): differences in populations from polluted and reference environments. Gen Comp Endocrinol 150: 174–188, 2007. doi: 10.1016/j.ygcen.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 263.Grohé C, Kahlert S, Löbbert K, Vetter H. Expression of oestrogen receptor alpha and beta in rat heart: role of local oestrogen synthesis. J Endocrinol 156: R1–R7, 1998. doi: 10.1677/joe.0.156R001. [DOI] [PubMed] [Google Scholar]
- 264.Grumbach MM, Auchus RJ. Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab 84: 4677–4694, 1999. [DOI] [PubMed] [Google Scholar]
- 265.Gunawan A, Kaewmala K, Uddin MJ, Cinar MU, Tesfaye D, Phatsara C, Tholen E, Looft C, Schellander K. Association study and expression analysis of porcine ESR1 as a candidate gene for boar fertility and sperm quality. Anim Reprod Sci 128: 11–21, 2011. doi: 10.1016/j.anireprosci.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 266.Haas E, Bhattacharya I, Brailoiu E, Damjanović M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res 104: 288–291, 2009. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Haines CD, Harvey PA, Leinwand LA. Estrogens mediate cardiac hypertrophy in a stimulus-dependent manner. Endocrinology 153: 4480–4490, 2012. doi: 10.1210/en.2012-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hajdu A, Rona G. The protective effect of estrogens against spontaneous pancratic islet and renal changes in aging male rats. Experientia 27: 956–957, 1971. doi: 10.1007/BF02135771. [DOI] [PubMed] [Google Scholar]
- 269.Hajszan T, MacLusky NJ, Johansen JA, Jordan CL, Leranth C. Effects of androgens and estradiol on spine synapse formation in the prefrontal cortex of normal and testicular feminization mutant male rats. Endocrinology 148: 1963–1967, 2007. doi: 10.1210/en.2006-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Han Y, Feng HL, Sandlow JI, Haines CJ. Comparing expression of progesterone and estrogen receptors in testicular tissue from men with obstructive and nonobstructive azoospermia. J Androl 30: 127–133, 2009. doi: 10.2164/jandrol.108.005157. [DOI] [PubMed] [Google Scholar]
- 271.Hansen LA, Clulow J, Jones RC. Perturbation of fluid reabsorption in the efferent ducts of the rat by testosterone propionate, 17beta-oestradiol 3-benzoate, flutamide and tamoxifen. Int J Androl 20: 265–273, 1997. doi: 10.1046/j.1365-2605.1997.00069.x. [DOI] [PubMed] [Google Scholar]
- 272.Hansen LA, Clulow J, Jones RC. The role of Na+-H+ exchange in fluid and solute transport in the rat efferent ducts. Exp Physiol 84: 521–527, 1999. [PubMed] [Google Scholar]
- 273.Harada N, Sasano H, Murakami H, Ohkuma T, Nagura H, Takagi Y. Localized expression of aromatase in human vascular tissues. Circ Res 84: 1285–1291, 1999. doi: 10.1161/01.RES.84.11.1285. [DOI] [PubMed] [Google Scholar]
- 274.Hartman J, Ström A, Gustafsson JA. Current concepts and significance of estrogen receptor β in prostate cancer. Steroids 77: 1262–1266, 2012. doi: 10.1016/j.steroids.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 275.Haverfield JT, Ham S, Brown KA, Simpson ER, Meachem SJ. Teasing out the role of aromatase in the healthy and diseased testis. Spermatogenesis 1: 240–249, 2011. doi: 10.4161/spmg.1.3.18037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hay M. Sex, the brain and hypertension: brain oestrogen receptors and high blood pressure risk factors. Clin Sci (Lond) 130: 9–18, 2016. doi: 10.1042/CS20150654. [DOI] [PubMed] [Google Scholar]
- 277.Hayakawa D, Sasaki M, Akabane C, Kitamura N, Tsubota T, Suzuki M, Yamada J. Immunohistochemical localization of steroidogenic enzymes in the testis of Hokkaido Sika deer (Cervus nippon yesoensis). J Vet Med Sci 66: 1463–1466, 2004. doi: 10.1292/jvms.66.1463. [DOI] [PubMed] [Google Scholar]
- 279.He CL, Du JL, Lee YH, Huang YS, Nagahama Y, Chang CF. Differential messenger RNA transcription of androgen receptor and estrogen receptor in gonad in relation to the sex change in protandrous black porgy, Acanthopagrus schlegeli. Biol Reprod 69: 455–461, 2003. doi: 10.1095/biolreprod.102.015040. [DOI] [PubMed] [Google Scholar]
- 280.Heikinheimo O, Mahony MC, Gordon K, Hsiu JG, Hodgen GD, Gibbons WE. Estrogen and progesterone receptor mRNA are expressed in distinct pattern in male primate reproductive organs. J Assist Reprod Genet 12: 198–204, 1995. doi: 10.1007/BF02211799. [DOI] [PubMed] [Google Scholar]
- 281.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA 97: 12729–12734, 2000. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Hejmej A, Gorazd M, Kosiniak-Kamysz K, Wiszniewska B, Sadowska J, Bilińska B. Expression of aromatase and oestrogen receptors in reproductive tissues of the stallion and a single cryptorchid visualised by means of immunohistochemistry. Domest Anim Endocrinol 29: 534–547, 2005. doi: 10.1016/j.domaniend.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 283.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med 284: 878–881, 1971. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 284.Hero M, Norjavaara E, Dunkel L. Inhibition of estrogen biosynthesis with a potent aromatase inhibitor increases predicted adult height in boys with idiopathic short stature: a randomized controlled trial. J Clin Endocrinol Metab 90: 6396–6402, 2005. doi: 10.1210/jc.2005-1392. [DOI] [PubMed] [Google Scholar]
- 285.Herrera-Luna CV, Scarlet D, Walter I, Aurich C. Effect of stallion age on the expression of LH and FSH receptors and aromatase P450 in equine male reproductive tissues. Reprod Fertil Dev 28: 2016–2026, 2015. doi: 10.1071/RD15027. [DOI] [PubMed] [Google Scholar]
- 286.Herrmann BL, Janssen OE, Hahn S, Broecker-Preuss M, Mann K. Effects of estrogen replacement therapy on bone and glucose metabolism in a male with congenital aromatase deficiency. Horm Metab Res 37: 178–183, 2005. doi: 10.1055/s-2005-861292. [DOI] [PubMed] [Google Scholar]
- 287.Herrmann BL, Saller B, Janssen OE, Gocke P, Bockisch A, Sperling H, Mann K, Broecker M. Impact of estrogen replacement therapy in a male with congenital aromatase deficiency caused by a novel mutation in the CYP19 gene. J Clin Endocrinol Metab 87: 5476–5484, 2002. doi: 10.1210/jc.2002-020498. [DOI] [PubMed] [Google Scholar]
- 288.Hess MF, Roser JF. Immunocytochemical localization of cytochrome P450 aromatase in the testis of prepubertal, pubertal, and postpubertal horses. Theriogenology 61: 293–299, 2004. doi: 10.1016/S0093-691X(03)00237-1. [DOI] [PubMed] [Google Scholar]
- 289.Hess RA. Small tubules, surprising discoveries: from efferent ductules in the turkey to the discovery that estrogen receptor alpha is essential for fertility in the male. Anim Reprod 12: 7–23, 2015. [PMC free article] [PubMed] [Google Scholar]
- 290.Hess RA. Disruption of estrogen receptor signaling and similar pathways in the efferent ductules and initial segment of the epididymis. Spermatogenesis 4: e979103, 2014. doi: 10.4161/21565562.2014.979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Hess RA. The efferent ductules: structure and functions. In: The Epididymis: From Molecules to Clinical Practice, edited by Robaire B, Hinton B. New York: Kluwer Academic/Plenum Publishers, 2002, p. 49–80. doi: 10.1007/978-1-4615-0679-9_4. [DOI] [Google Scholar]
- 292.Hess RA. Estrogen in the adult male: from a curiosity to absolute necessity. Annu Rev Biomed Sci 6: 1–12, 2004. doi: 10.1186/1477-7827-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB. A role for oestrogens in the male reproductive system. Nature 390: 509–512, 1997. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Hess RA, Bunick D, Lubahn DB, Zhou Q, Bouma J. Morphologic changes in efferent ductules and epididymis in estrogen receptor-alpha knockout mice. J Androl 21: 107–121, 2000. doi: 10.1002/j.1939-4640.2000.tb03282.x. [DOI] [PubMed] [Google Scholar]
- 295.Hess RA, Carnes K. The role of estrogen in testis and the male reproductive tract: a review and species comparison. Anim Reprod 1: 5–30, 2004. [Google Scholar]
- 296.Hess RA, Fernandes SA, Gomes GR, Oliveira CA, Lazari MF, Porto CS. Estrogen and its receptors in efferent ductules and epididymis. J Androl 32: 600–613, 2011. doi: 10.2164/jandrol.110.012872. [DOI] [PubMed] [Google Scholar]
- 297.Hess RA, Gist DH, Bunick D, Lubahn DB, Farrell A, Bahr J, Cooke PS, Greene GL. Estrogen receptor (alpha and beta) expression in the excurrent ducts of the adult male rat reproductive tract. J Androl 18: 602–611, 1997. [PubMed] [Google Scholar]
- 298.Hess RA, Hermo L, Robaire B. Lessons learned in andrology: Yves Clermont, an interview by Lonnie D. Russell. Andrology 3: 1015–1021, 2015. doi: 10.1111/andr.12115. [DOI] [PubMed] [Google Scholar]
- 299.Hess RA, Zhou Q, Nie R. The role of estrogens in the endocrine and paracrine regulation of the efferent ductules, epididymis and vas deferens. In: The Epididymis: From Molecules to Clinical Practice, edited by Robaire B, Hinton BT. New York: Kluwer Academic/Plenum Publishers, 2002, p. 317–338. doi: 10.1007/978-1-4615-0679-9_18. [DOI] [Google Scholar]
- 300.Hewitson TD, Zhao C, Wigg B, Lee SW, Simpson ER, Boon WC, Samuel CS. Relaxin and castration in male mice protect from, but testosterone exacerbates, age-related cardiac and renal fibrosis, whereas estrogens are an independent determinant of organ size. Endocrinology 153: 188–199, 2012. doi: 10.1210/en.2011-1311. [DOI] [PubMed] [Google Scholar]
- 301.Ho SM, Cheong A, Lam HM, Hu WY, Shi GB, Zhu X, Chen J, Zhang X, Medvedovic M, Leung YK, Prins GS. Exposure of human prostaspheres to bisphenol A epigenetically regulates SNORD family noncoding RNAs via histone modification. Endocrinology 156: 3984–3995, 2015. doi: 10.1210/en.2015-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Hobkirk R, Glasier MA. Estrogen sulfotransferase distribution in tissues of mouse and guinea pig: steroidal inhibition of the guinea pig enzyme. Biochem Cell Biol 70: 712–715, 1992. doi: 10.1139/o92-108. [DOI] [PubMed] [Google Scholar]
- 303.Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N. Estrogen receptor alpha is a major mediator of 17beta-estradiol’s atheroprotective effects on lesion size in Apoe-/- mice. J Clin Invest 107: 333–340, 2001. doi: 10.1172/JCI11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Hoffmann B, Rostalski A, Mutembei HM, Goericke-Pesch S. Testicular steroid hormone secretion in the boar and expression of testicular and epididymal steroid sulphatase and estrogen sulphotransferase activity. Exp Clin Endocrinol Diabetes 118: 274–280, 2010. doi: 10.1055/s-0029-1231082. [DOI] [PubMed] [Google Scholar]
- 305.Hogervorst E, De Jager C, Budge M, Smith AD. Serum levels of estradiol and testosterone and performance in different cognitive domains in healthy elderly men and women. Psychoneuroendocrinology 29: 405–421, 2004. doi: 10.1016/S0306-4530(03)00053-2. [DOI] [PubMed] [Google Scholar]
- 306.Hogg ME, Vavra AK, Banerjee MN, Martinez J, Jiang Q, Keefer LK, Chambon P, Kibbe MR. The role of estrogen receptor α and β in regulating vascular smooth muscle cell proliferation is based on sex. J Surg Res 173: e1–e10, 2012. doi: 10.1016/j.jss.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci USA 101: 865–870, 2004. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Holderegger C, Keefer D. The ontogeny of the mouse estrogen receptor: the pelvic region. Am J Anat 177: 285–297, 1986. doi: 10.1002/aja.1001770211. [DOI] [PubMed] [Google Scholar]
- 309.Holmboe SA, Vradi E, Jensen TK, Linneberg A, Husemoen LL, Scheike T, Skakkebæk NE, Juul A, Andersson AM. The association of reproductive hormone levels and all-cause, cancer, and cardiovascular disease mortality in men. J Clin Endocrinol Metab 100: 4472–4480, 2015. doi: 10.1210/jc.2015-2460. [DOI] [PubMed] [Google Scholar]
- 310.Honda S, Harada N, Ito S, Takagi Y, Maeda S. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem Biophys Res Commun 252: 445–449, 1998. doi: 10.1006/bbrc.1998.9672. [DOI] [PubMed] [Google Scholar]
- 311.Hoshii T, Takeo T, Nakagata N, Takeya M, Araki K, Yamamura K. LGR4 regulates the postnatal development and integrity of male reproductive tracts in mice. Biol Reprod 76: 303–313, 2007. doi: 10.1095/biolreprod.106.054619. [DOI] [PubMed] [Google Scholar]
- 312.Howard E. Effects of 17-beta-estradiol on bone maturation in mice. Steroids 7: 375–380, 1966. doi: 10.1016/0039-128X(66)90108-5. [DOI] [PubMed] [Google Scholar]
- 313.Hu WY, Shi GB, Lam HM, Hu DP, Ho SM, Madueke IC, Kajdacsy-Balla A, Prins GS. Estrogen-initiated transformation of prostate epithelium derived from normal human prostate stem-progenitor cells. Endocrinology 152: 2150–2163, 2011. doi: 10.1210/en.2010-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Huang B, Butler R, Miao Y, Dai Y, Wu W, Su W, Fujii-Kuriyama Y, Warner M, Gustafsson JA. Dysregulation of Notch and ERα signaling in AhR-/- male mice. Proc Natl Acad Sci USA 113: 11883–11888, 2016. doi: 10.1073/pnas.1613269113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Huang L, Pu Y, Alam S, Birch L, Prins GS. Estrogenic regulation of signaling pathways and homeobox genes during rat prostate development. J Androl 25: 330–337, 2004. doi: 10.1002/j.1939-4640.2004.tb02796.x. [DOI] [PubMed] [Google Scholar]
- 316.Hunt DW, Henricks DM, Skelley GC, Grimes LW. Use of trenbolone acetate and estradiol in intact and castrate male cattle: effects on growth, serum hormones, and carcass characteristics. J Anim Sci 69: 2452–2462, 1991. doi: 10.2527/1991.6962452x. [DOI] [PubMed] [Google Scholar]
- 317.Iguchi T, Ostrander PL, Mills KT, Bern HA. Vaginal abnormalities in ovariectomized BALB/cCrgl mice after neonatal exposure to different doses of diethylstilbestrol. Cancer Lett 43: 207–214, 1988. doi: 10.1016/0304-3835(88)90172-3. [DOI] [PubMed] [Google Scholar]
- 318.Iibuchi R, Shimozuru M, Kamine A, Nio-Kobayashi J, Iwanaga T, Tsubota T. Localization of five steroidogenic enzyme mRNAs in Japanese black bear (Ursus thibetanus japonicus) testes during the mating season by in situ hybridization. J Reprod Dev 56: 236–242, 2010. doi: 10.1262/jrd.09-188N. [DOI] [PubMed] [Google Scholar]
- 319.Ikeda M, Swide T, Vayl A, Lahm T, Anderson S, Hutchens MP. Estrogen administered after cardiac arrest and cardiopulmonary resuscitation ameliorates acute kidney injury in a sex- and age-specific manner. Crit Care 19: 332, 2015. doi: 10.1186/s13054-015-1049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Ilio KY, Hess RA. Localization and activity of Na+,K+-ATPase in the ductuli efferentes of the rat. Anat Rec 234: 190–200, 1992. doi: 10.1002/ar.1092340206. [DOI] [PubMed] [Google Scholar]
- 321.Ilio KY, Hess RA. Structure and function of the ductuli efferentes: a review. Microsc Res Tech 29: 432–467, 1994. doi: 10.1002/jemt.1070290604. [DOI] [PubMed] [Google Scholar]
- 322.Imamov O, Morani A, Shim GJ, Omoto Y, Thulin-Andersson C, Warner M, Gustafsson JA. Estrogen receptor beta regulates epithelial cellular differentiation in the mouse ventral prostate. Proc Natl Acad Sci USA 101: 9375–9380, 2004. doi: 10.1073/pnas.0403041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Inkster S, Yue W, Brodie A. Human testicular aromatase: immunocytochemical and biochemical studies. J Clin Endocrinol Metab 80: 1941–1947, 1995. [DOI] [PubMed] [Google Scholar]
- 324.Iorga A, Li J, Sharma S, Umar S, Bopassa JC, Nadadur RD, Centala A, Ren S, Saito T, Toro L, Wang Y, Stefani E, Eghbali M. Rescue of pressure overload-induced heart failure by estrogen therapy. J Am Heart Assoc 5: e002482, 2016. doi: 10.1161/JAHA.115.002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology 150: 1722–1730, 2009. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- 326.Jacobson CD, Csernus VJ, Shryne JE, Gorski RA. The influence of gonadectomy, androgen exposure, or a gonadal graft in the neonatal rat on the volume of the sexually dimorphic nucleus of the preoptic area. J Neurosci 1: 1142–1147, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Janssen SJ, Bunick D, Finnigan-Bunick C, Chen YC, Hess R, Bahr JM. Morphology and function of rooster efferent ductule epithelial cells in culture. Tissue Cell 30: 554–561, 1998. doi: 10.1016/S0040-8166(98)80036-0. [DOI] [PubMed] [Google Scholar]
- 328.Janulis L, Bahr JM, Hess RA, Bunick D. P450 aromatase messenger ribonucleic acid expression in male rat germ cells: detection by reverse transcription-polymerase chain reaction amplification. J Androl 17: 651–658, 1996. [PubMed] [Google Scholar]
- 329.Janulis L, Bahr JM, Hess RA, Janssen S, Osawa Y, Bunick D. Rat testicular germ cells and epididymal sperm contain active P450 aromatase. J Androl 19: 65–71, 1998. [PubMed] [Google Scholar]
- 330.Janulis L, Hess RA, Bunick D, Nitta H, Janssen S, Asawa Y, Bahr JM. Mouse epididymal sperm contain active P450 aromatase which decreases as sperm traverse the epididymis. J Androl 17: 111–116, 1996. [PubMed] [Google Scholar]
- 331.Javurek AB, Spollen WG, Ali AM, Johnson SA, Lubahn DB, Bivens NJ, Bromert KH, Ellersieck MR, Givan SA, Rosenfeld CS. Discovery of a novel seminal fluid microbiome and influence of estrogen receptor alpha genetic status. Sci Rep 6: 23027, 2016. doi: 10.1038/srep23027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Jazbutyte V, Stumpner J, Redel A, Lorenzen JM, Roewer N, Thum T, Kehl F. Aromatase inhibition attenuates desflurane-induced preconditioning against acute myocardial infarction in male mouse heart in vivo. PLoS One 7: e42032, 2012. doi: 10.1371/journal.pone.0042032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Jefferson WN, Couse JF, Banks EP, Korach KS, Newbold RR. Expression of estrogen receptor beta is developmentally regulated in reproductive tissues of male and female mice. Biol Reprod 62: 310–317, 2000. doi: 10.1095/biolreprod62.2.310. [DOI] [PubMed] [Google Scholar]
- 334.Jeng HA. Exposure to endocrine disrupting chemicals and male reproductive health. Front Public Health 2: 55, 2014. doi: 10.3389/fpubh.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Jensen EV, Suzuki T, Numata M, Smith S, DeSombre ER. Estrogen-binding substances of target tissues. Steroids 13: 417–427, 1969. doi: 10.1016/0039-128X(69)90053-1. [DOI] [PubMed] [Google Scholar]
- 336.Jia B, Gao Y, Li M, Shi J, Peng Y, Du X, Klocker H, Sampson N, Shen Y, Liu M, Zhang J. GPR30 promotes prostate stromal cell activation via suppression of ERalpha expression and its downstream signaling pathway. Endocrinology 157: 3023–3035, 2016. doi: 10.1210/en.2016-1035. [DOI] [PubMed] [Google Scholar]
- 337.Johnson BJ, Anderson PT, Meiske JC, Dayton WR. Effect of a combined trenbolone acetate and estradiol implant on feedlot performance, carcass characteristics, and carcass composition of feedlot steers. J Anim Sci 74: 363–371, 1996. doi: 10.2527/1996.742363x. [DOI] [PubMed] [Google Scholar]
- 338.Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA 97: 12735–12740, 2000. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Jones ME, Robertson KM, O’Donnell L, Boon W, Simpson ER. The aromatase knockout (ArKO) mouse. Re-evaluating a role for estrogen in male physiology (Abstract). 1st European Congress of Andrology, L'Aquila, Italy, 24-27 March 2000. Int J Androl 23, Suppl: 1–67, 2000. [Google Scholar]
- 340.Jong FH, Uilenbroek J, Molen HJ. Oestradiol-17beta, testosterone and gonadotrophins in oestradiol-17beta-treated intact adult male rats. J Endocrinol 65: 281–282, 1975. doi: 10.1677/joe.0.0650281. [DOI] [PubMed] [Google Scholar]
- 341.Joseph A, Hess RA, Schaeffer DJ, Ko C, Hudgin-Spivey S, Chambon P, Shur BD. Absence of estrogen receptor alpha leads to physiological alterations in the mouse epididymis and consequent defects in sperm function. Biol Reprod 82: 948–957, 2010. doi: 10.1095/biolreprod.109.079889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Joseph A, Shur BD, Hess RA. Estrogen, efferent ductules, and the epididymis. Biol Reprod 84: 207–217, 2011. doi: 10.1095/biolreprod.110.087353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Joseph A, Shur BD, Ko C, Chambon P, Hess RA. Epididymal hypo-osmolality induces abnormal sperm morphology and function in the estrogen receptor alpha knockout mouse. Biol Reprod 82: 958–967, 2010. doi: 10.1095/biolreprod.109.080366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Kabir ME, Singh H, Lu R, Olde B, Leeb-Lundberg LM, Bopassa JC. G protein-coupled estrogen receptor 1 mediates acute estrogen-induced cardioprotection via MEK/ERK/GSK-3beta pathway after ischemia/reperfusion. PLoS One 10: e0135988, 2015. doi: 10.1371/journal.pone.0135988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, Spiegelman BM. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 460: 1154–1158, 2009. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Kalbe C, Mau M, Wollenhaupt K, Rehfeldt C. Evidence for estrogen receptor alpha and beta expression in skeletal muscle of pigs. Histochem Cell Biol 127: 95–107, 2007. doi: 10.1007/s00418-006-0224-z. [DOI] [PubMed] [Google Scholar]
- 347.Kamanga-Sollo E, White ME, Weber WJ, Dayton WR. Role of estrogen receptor-α (ESR1) and the type 1 insulin-like growth factor receptor (IGFR1) in estradiol-stimulated proliferation of cultured bovine satellite cells. Domest Anim Endocrinol 44: 36–45, 2013. doi: 10.1016/j.domaniend.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 348.Kampen DL, Sherwin BB. Estradiol is related to visual memory in healthy young men. Behav Neurosci 110: 613–617, 1996. doi: 10.1037/0735-7044.110.3.613. [DOI] [PubMed] [Google Scholar]
- 349.Kastelan D, Grubic Z, Kraljevic I, Duric K, Kardum I, Dusek T, Stingl K, Giljevic Z, Kerhin-Brkljacic V, Suchanek E, Korsic M. Decreased peak bone mass is associated with a 3-bp deletion/insertion of the CYP19 intron 4 polymorphism: preliminary data from the GOOS study. J Endocrinol Invest 30: 465–469, 2007. doi: 10.1007/BF03346329. [DOI] [PubMed] [Google Scholar]
- 350.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 26: 833–876, 2005. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 351.Khan D, Ansar Ahmed S. The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol 6: 635, 2016. doi: 10.3389/fimmu.2015.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Khattri A, Pandey RK, Gupta NJ, Chakravarty B, Deendayal M, Singh L, Thangaraj K. CA repeat and RsaI polymorphisms in ERbeta gene are not associated with infertility in Indian men. Int J Androl 32: 81–87, 2009. doi: 10.1111/j.1365-2605.2007.00821.x. [DOI] [PubMed] [Google Scholar]
- 353.Khosla S. New insights into androgen and estrogen receptor regulation of the male skeleton. J Bone Miner Res 30: 1134–1137, 2015. doi: 10.1002/jbmr.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev 29: 441–464, 2008. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Kimura M, Sudhir K, Jones M, Simpson E, Jefferis AM, Chin-Dusting JP. Impaired acetylcholine-induced release of nitric oxide in the aorta of male aromatase-knockout mice: regulation of nitric oxide production by endogenous sex hormones in males. Circ Res 93: 1267–1271, 2003. doi: 10.1161/01.RES.0000103172.98986.25. [DOI] [PubMed] [Google Scholar]
- 356.Kincl FA, Pi AF, Maqueo M, Lasso LH, Dorfman RI, Oriol A. Inhibition of sexual development in male and female rats treated with various steroids at the age of five days. Acta Endocrinol (Copenh) 49: 193–206, 1965. [DOI] [PubMed] [Google Scholar]
- 357.Kobayashi T, Kajiura-Kobayashi H, Nagahama Y. Induction of XY sex reversal by estrogen involves altered gene expression in a teleost, tilapia. Cytogenet Genome Res 101: 289–294, 2003. doi: 10.1159/000074351. [DOI] [PubMed] [Google Scholar]
- 358.Kobayashi T, Nakamura M, Kajiura-Kobayashi H, Young G, Nagahama Y. Immunolocalization of steroidogenic enzymes (P450scc, P450c17, P450arom, and 3beta-HSD) in immature and mature testes of rainbow trout (Oncorhynchus mykiss). Cell Tissue Res 292: 573–577, 1998. doi: 10.1007/s004410051086. [DOI] [PubMed] [Google Scholar]
- 359.Kolasa A, Wiszniewska B, Marchlewicz M, Wenda-Rózewicka L. Localisation of oestrogen receptors (ERalpha and ERbeta) in the human and rat epididymides. Folia Morphol (Warsz) 62: 467–469, 2003. [PubMed] [Google Scholar]
- 360.Konkle AT, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology 152: 223–235, 2011. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Korach KS, Horigome T, Tomooka Y, Yamashita S, Newbold RR, McLachlan JA. Immunodetection of estrogen receptor in epithelial and stromal tissues of neonatal mouse uterus. Proc Natl Acad Sci USA 85: 3334–3337, 1988. doi: 10.1073/pnas.85.10.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Kotula-Balak M, Slomczynska M, Fraczek B, Bourguiba S, Tabarowski Z, Carreau S, Bilinska B. Complementary approaches demonstrate that cellular aromatization in the bank vole testis is related to photoperiod. Eur J Histochem 47: 55–62, 2003. doi: 10.4081/807. [DOI] [PubMed] [Google Scholar]
- 363.Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104: 719–730, 2001. [PubMed] [Google Scholar]
- 364.Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O’Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science 298: 843–846, 2002. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- 365.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 294: 63–69, 2015. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Kowalska K, Piastowska-Ciesielska AW. Oestrogens and oestrogen receptors in prostate cancer. Springerplus 5: 522, 2016. doi: 10.1186/s40064-016-2185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Kozák I, Bartsch W, Krieg M, Voigt KD. Nuclei of stroma: site of highest estrogen concentration in human benign prostatic hyperplasia. Prostate 3: 433–438, 1982. doi: 10.1002/pros.2990030503. [DOI] [PubMed] [Google Scholar]
- 368.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA 95: 15677–15682, 1998. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience 138: 921–928, 2006. doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 370.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138: 863–870, 1997. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 371.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93: 5925–5930, 1996. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Kuiper GG, Shughrue PJ, Merchenthaler I, Gustafsson JA. The estrogen receptor beta subtype: a novel mediator of estrogen action in neuroendocrine systems. Front Neuroendocrinol 19: 253–286, 1998. doi: 10.1006/frne.1998.0170. [DOI] [PubMed] [Google Scholar]
- 373.Kukuvitis A, Georgiou I, Bouba I, Tsirka A, Giannouli CH, Yapijakis C, Tarlatzis B, Bontis J, Lolis D, Sofikitis N, Papadimas J. Association of oestrogen receptor alpha polymorphisms and androgen receptor CAG trinucleotide repeats with male infertility: a study in 109 Greek infertile men. Int J Androl 25: 149–152, 2002. doi: 10.1046/j.1365-2605.2002.00339.x. [DOI] [PubMed] [Google Scholar]
- 374.Kulkarni J, Gavrilidis E, Worsley R, Hayes E. Role of estrogen treatment in the management of schizophrenia. CNS Drugs 26: 549–557, 2012. doi: 10.2165/11630660-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 375.Kumar A, Dumasia K, Gaonkar R, Sonawane S, Kadam L, Balasinor NH. Estrogen and androgen regulate actin-remodeling and endocytosis-related genes during rat spermiation. Mol Cell Endocrinol 404: 91–101, 2015. doi: 10.1016/j.mce.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 376.Kuntz S, Chardard D, Chesnel A, Ducatez M, Callier M, Flament S. Expression of aromatase and steroidogenic factor 1 in the lung of the urodele amphibian Pleurodeles waltl. Endocrinology 145: 3111–3114, 2004. doi: 10.1210/en.2004-0245. [DOI] [PubMed] [Google Scholar]
- 377.Kurosumi M, Ishimura K, Fujita H, Osawa Y. Immunocytochemical localization of aromatase in rat testis. Histochemistry 83: 401–404, 1985. doi: 10.1007/BF00509199. [DOI] [PubMed] [Google Scholar]
- 378.Kwon S, Hess RA, Bunick D, Nitta H, Janulis L, Osawa Y, Bahr JM. Rooster testicular germ cells and epididymal sperm contain P450 aromatase. Biol Reprod 53: 1259–1264, 1995. doi: 10.1095/biolreprod53.6.1259. [DOI] [PubMed] [Google Scholar]
- 379.Kyi-Tha-Thu C, Okoshi K, Ito H, Matsuda K, Kawata M, Tsukahara S. Sex differences in cells expressing green fluorescent protein under the control of the estrogen receptor-α promoter in the hypothalamus of mice. Neurosci Res 101: 44–52, 2015. doi: 10.1016/j.neures.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 380.Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res 106: 1681–1691, 2010. doi: 10.1161/CIRCRESAHA.109.213645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Lam HM, Ouyang B, Chen J, Ying J, Wang J, Wu CL, Jia L, Medvedovic M, Vessella RL, Ho SM. Targeting GPR30 with G-1: a new therapeutic target for castration-resistant prostate cancer. Endocr Relat Cancer 21: 903–914, 2014. doi: 10.1530/ERC-14-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Lambard S, Carreau S. Aromatase and oestrogens in human male germ cells. Int J Androl 28: 254–259, 2005. doi: 10.1111/j.1365-2605.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 383.Lambard S, Galeraud-Denis I, Bouraïma H, Bourguiba S, Chocat A, Carreau S. Expression of aromatase in human ejaculated spermatozoa: a putative marker of motility. Mol Hum Reprod 9: 117–124, 2003. doi: 10.1093/molehr/gag020. [DOI] [PubMed] [Google Scholar]
- 384.Lambard S, Galeraud-Denis I, Saunders PT, Carreau S. Human immature germ cells and ejaculated spermatozoa contain aromatase and oestrogen receptors. J Mol Endocrinol 32: 279–289, 2004. doi: 10.1677/jme.0.0320279. [DOI] [PubMed] [Google Scholar]
- 385.Lambard S, Silandre D, Delalande C, Denis-Galeraud I, Bourguiba S, Carreau S. Aromatase in testis: expression and role in male reproduction. J Steroid Biochem Mol Biol 95: 63–69, 2005. doi: 10.1016/j.jsbmb.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 386.Lanfranco F, Zirilli L, Baldi M, Pignatti E, Corneli G, Ghigo E, Aimaretti G, Carani C, Rochira V. A novel mutation in the human aromatase gene: insights on the relationship among serum estradiol, longitudinal growth and bone mineral density in an adult man under estrogen replacement treatment. Bone 43: 628–635, 2008. doi: 10.1016/j.bone.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 387.Lanfranco F, Zitzmann M, Simoni M, Nieschlag E. Serum adiponectin levels in hypogonadal males: influence of testosterone replacement therapy. Clin Endocrinol (Oxf) 60: 500–507, 2004. doi: 10.1111/j.1365-2265.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 388.Lanzino M, Catalano S, Genissel C, Ando S, Carreau S, Hamra K, McPhaul MJ. Aromatase messenger RNA is derived from the proximal promoter of the aromatase gene in Leydig, Sertoli, and germ cells of the rat testis. Biol Reprod 64: 1439–1443, 2001. doi: 10.1095/biolreprod64.5.1439. [DOI] [PubMed] [Google Scholar]
- 389.Lapid K, Lim A, Clegg DJ, Zeve D, Graff JM. Oestrogen signalling in white adipose progenitor cells inhibits differentiation into brown adipose and smooth muscle cells. Nat Commun 5: 5196, 2014. doi: 10.1038/ncomms6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 93: 68–75, 2008. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA 103: 9232–9237, 2006. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Leav I, Lau KM, Adams JY, McNeal JE, Taplin ME, Wang J, Singh H, Ho SM. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol 159: 79–92, 2001. doi: 10.1016/S0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Lee KH, Hess RA, Bahr JM, Lubahn DB, Taylor J, Bunick D. Estrogen receptor alpha has a functional role in the mouse rete testis and efferent ductules. Biol Reprod 63: 1873–1880, 2000. doi: 10.1095/biolreprod63.6.1873. [DOI] [PubMed] [Google Scholar]
- 394.Lee KH, Park JH, Bunick D, Lubahn DB, Bahr JM. Morphological comparison of the testis and efferent ductules between wild-type and estrogen receptor alpha knockout mice during postnatal development. J Anat 214: 916–925, 2009. doi: 10.1111/j.1469-7580.2009.01080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Lee SJ, Romeo RD, Svenningsson P, Campomanes CR, Allen PB, Greengard P, McEwen BS. Estradiol affects spinophilin protein differently in gonadectomized males and females. Neuroscience 127: 983–988, 2004. doi: 10.1016/j.neuroscience.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 396.Lee YH, Du JL, Yueh WS, Lin BY, Huang JD, Lee CY, Lee MF, Lau EL, Lee FY, Morrey C, Nagahama Y, Chang CF. Sex change in the protandrous black porgy, Acanthopagrus schlegeli: a review in gonadal development, estradiol, estrogen receptor, aromatase activity and gonadotropin. J Exp Zool 290: 715–726, 2001. doi: 10.1002/jez.1122. [DOI] [PubMed] [Google Scholar]
- 397.Leifke E, Gorenoi V, Wichers C, Von Zur Mühlen A, Von Büren E, Brabant G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin Endocrinol (Oxf) 53: 689–695, 2000. doi: 10.1046/j.1365-2265.2000.01159.x. [DOI] [PubMed] [Google Scholar]
- 398.Lekhkota O, Brehm R, Claus R, Wagner A, Bohle RM, Bergmann M. Cellular localization of estrogen receptor-alpha (ERalpha) and -beta (ERbeta) mRNA in the boar testis. Histochem Cell Biol 125: 259–264, 2006. doi: 10.1007/s00418-005-0008-x. [DOI] [PubMed] [Google Scholar]
- 399.Lemazurier E, Moslemi S, Sourdaine P, Desjardins I, Plainfosse B, Seralini GE. Free and conjugated estrogens and androgens in stallion semen. Gen Comp Endocrinol 125: 272–282, 2002. doi: 10.1006/gcen.2001.7747. [DOI] [PubMed] [Google Scholar]
- 400.Lemazurier E, Séralini GE. Evidence for sulfatase and 17beta-hydroxysteroid dehydrogenase type 1 activities in equine epididymis and uterus. Theriogenology 58: 113–121, 2002. doi: 10.1016/S0093-691X(02)00917-2. [DOI] [PubMed] [Google Scholar]
- 401.Leska A, Kiezun J, Kaminska B, Dusza L. Estradiol concentration and the expression of estrogen receptors in the testes of the domestic goose (Anser anser f. domestica) during the annual reproductive cycle. Domest Anim Endocrinol 51: 96–104, 2015. doi: 10.1016/j.domaniend.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 402.Leung YK, Lam HM, Wu S, Song D, Levin L, Cheng L, Wu CL, Ho SM. Estrogen receptor beta2 and beta5 are associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion. Endocr Relat Cancer 17: 675–689, 2010. doi: 10.1677/ERC-09-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proc Natl Acad Sci USA 103: 13162–13167, 2006. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Levallet J, Carreau S. [In vitro gene expression of aromatase in rat testicular cells]. C R Acad Sci III 320: 123–129, 1997. doi: 10.1016/S0764-4469(97)85003-2. [DOI] [PubMed] [Google Scholar]
- 405.Levallet J, Mittre H, Delarue B, Carreau S. Alternative splicing events in the coding region of the cytochrome P450 aromatase gene in male rat germ cells. J Mol Endocrinol 20: 305–312, 1998. doi: 10.1677/jme.0.0200305. [DOI] [PubMed] [Google Scholar]
- 406.Levin ER. G protein-coupled receptor 30: estrogen receptor or collaborator? Endocrinology 150: 1563–1565, 2009. doi: 10.1210/en.2008-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Levine S, Mullins R Jr. Estrogen administered neonatally affects adult sexual behavior in male and female rats. Science 144: 185–187, 1964. doi: 10.1126/science.144.3615.185. [DOI] [PubMed] [Google Scholar]
- 408.Lew R, Komesaroff P, Williams M, Dawood T, Sudhir K. Endogenous estrogens influence endothelial function in young men. Circ Res 93: 1127–1133, 2003. doi: 10.1161/01.RES.0000103633.57225.BC. [DOI] [PubMed] [Google Scholar]
- 409.Li J, Ying J, Fan Y, Wu L, Ying Y, Chan AT, Srivastava G, Tao Q. WNT5A antagonizes WNT/β-catenin signaling and is frequently silenced by promoter CpG methylation in esophageal squamous cell carcinoma. Cancer Biol Ther 10: 617–624, 2010. doi: 10.4161/cbt.10.6.12609. [DOI] [PubMed] [Google Scholar]
- 410.Li JJ, Talley DJ, Li SA, Villee CA. An estrogen binding protein in the renal cytosol of intact, castrated and estrogenized golden hamsters. Endocrinology 95: 1134–1141, 1974. doi: 10.1210/endo-95-4-1134. [DOI] [PubMed] [Google Scholar]
- 411.Li MW, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol 41: 2302–2314, 2009. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Li Q, Zhang F, Zhang S, Sheng X, Han X, Weng Q, Yuan Z. Seasonal expression of androgen receptor, aromatase, and estrogen receptor alpha and beta in the testis of the wild ground squirrel (Citellus dauricus Brandt). Eur J Histochem 59: 2456, 2015. doi: 10.4081/ejh.2015.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Light AE, Tornaben JA. Effects of prolonged percutaneous administration of methyl testosterone and estradiol on growing male rats. J Nutr 49: 51–63, 1953. [DOI] [PubMed] [Google Scholar]
- 414.Lin J, Zhu J, Li X, Li S, Lan Z, Ko J, Lei Z. Expression of genomic functional estrogen receptor 1 in mouse sertoli cells. Reprod Sci 21: 1411–1422, 2014. doi: 10.1177/1933719114527355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Lin JZ, Martagón AJ, Cimini SL, Gonzalez DD, Tinkey DW, Biter A, Baxter JD, Webb P, Gustafsson JA, Hartig SM, Phillips KJ. Pharmacological activation of thyroid hormone receptors elicits a functional conversion of white to brown fat. Cell Reports 13: 1528–1537, 2015. doi: 10.1016/j.celrep.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Lindberg MK, Weihua Z, Andersson N, Movérare S, Gao H, Vidal O, Erlandsson M, Windahl S, Andersson G, Lubahn DB, Carlsten H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J Endocrinol 174: 167–178, 2002. doi: 10.1677/joe.0.1740167. [DOI] [PubMed] [Google Scholar]
- 417.Lindner V, Kim SK, Karas RH, Kuiper GG, Gustafsson JA, Mendelsohn ME. Increased expression of estrogen receptor-beta mRNA in male blood vessels after vascular injury. Circ Res 83: 224–229, 1998. doi: 10.1161/01.RES.83.2.224. [DOI] [PubMed] [Google Scholar]
- 418.Liu F, Day M, Muñiz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci 11: 334–343, 2008. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 419.Liu S, Mauvais-Jarvis F. Minireview: estrogenic protection of beta-cell failure in metabolic diseases. Endocrinology 151: 859–864, 2010. doi: 10.1210/en.2009-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Lizotte E, Grandy SA, Tremblay A, Allen BG, Fiset C. Expression, distribution and regulation of sex steroid hormone receptors in mouse heart. Cell Physiol Biochem 23: 75–86, 2009. doi: 10.1159/000204096. [DOI] [PubMed] [Google Scholar]
- 421.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90: 11162–11166, 1993. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Lucas TF, Lazari MF, Porto CS. Differential role of the estrogen receptors ESR1 and ESR2 on the regulation of proteins involved with proliferation and differentiation of Sertoli cells from 15-day-old rats. Mol Cell Endocrinol 382: 84–96, 2014. doi: 10.1016/j.mce.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 423.Lucas TF, Pimenta MT, Pisolato R, Lazari MF, Porto CS. 17β-Estradiol signaling and regulation of Sertoli cell function. Spermatogenesis 1: 318–324, 2011. doi: 10.4161/spmg.1.4.18903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Lucas TF, Royer C, Siu ER, Lazari MF, Porto CS. Expression and signaling of G protein-coupled estrogen receptor 1 (GPER) in rat Sertoli cells. Biol Reprod 83: 307–317, 2010. doi: 10.1095/biolreprod.110.084160. [DOI] [PubMed] [Google Scholar]
- 425.Lucas TF, Siu ER, Esteves CA, Monteiro HP, Oliveira CA, Porto CS, Lazari MF. 17Beta-estradiol induces the translocation of the estrogen receptors ESR1 and ESR2 to the cell membrane, MAPK3/1 phosphorylation and proliferation of cultured immature rat Sertoli cells. Biol Reprod 78: 101–114, 2008. doi: 10.1095/biolreprod.107.063909. [DOI] [PubMed] [Google Scholar]
- 426.Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol 62: 230–236, 1994. doi: 10.1016/S0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- 427.Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav 66: 602–618, 2014. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Luisi M, Franchi F. Double-blind group comparative study of testosterone undecanoate and mesterolone in hypogonadal male patients. J Endocrinol Invest 3: 305–308, 1980. doi: 10.1007/BF03348281. [DOI] [PubMed] [Google Scholar]
- 429.Luu-The V, Pelletier G, Labrie F. Quantitative appreciation of steroidogenic gene expression in mouse tissues: new roles for type 2 5alpha-reductase, 20alpha-hydroxysteroid dehydrogenase and estrogen sulfotransferase. J Steroid Biochem Mol Biol 93: 269–276, 2005. doi: 10.1016/j.jsbmb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 430.Määttä JA, Büki KG, Gu G, Alanne MH, Vääräniemi J, Liljenbäck H, Poutanen M, Härkönen P, Väänänen K. Inactivation of estrogen receptor α in bone-forming cells induces bone loss in female mice. FASEB J 27: 478–488, 2013. doi: 10.1096/fj.12-213587. [DOI] [PubMed] [Google Scholar]
- 431.Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol 22: 2116–2127, 2008. doi: 10.1210/me.2008-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Madak-Erdogan Z, Kim SH, Gong P, Zhao YC, Zhang H, Chambliss KL, Carlson KE, Mayne CG, Shaul PW, Korach KS, Katzenellenbogen JA, Katzenellenbogen BS. Design of pathway preferential estrogens that provide beneficial metabolic and vascular effects without stimulating reproductive tissues. Sci Signal 9: ra53, 2016. doi: 10.1126/scisignal.aad8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M, Clyne CD, Davis S, Simpson ER, Carani C. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab 89: 61–70, 2004. doi: 10.1210/jc.2003-030313. [DOI] [PubMed] [Google Scholar]
- 434.Maffei L, Rochira V, Zirilli L, Antunez P, Aranda C, Fabre B, Simone ML, Pignatti E, Simpson ER, Houssami S, Clyne CD, Carani C. A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin Endocrinol (Oxf) 67: 218–224, 2007. doi: 10.1111/j.1365-2265.2007.02864.x. [DOI] [PubMed] [Google Scholar]
- 435.Magers MJ, Udager AM, Chinnaiyan AM, French D, Myers JL, Jentzen JM, McHugh JB, Heider A, Mehra R. Comprehensive immunophenotypic characterization of adult and fetal testes, the excretory duct system, and testicular and epididymal appendages. Appl Immunohistochem Mol Morphol 24: e50–e68, 2016. doi: 10.1097/PAI.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 436.Mahmoud IJ, Selman MO, Shebeb WR. Chronology of estrogen receptor expression in testes of mouse embryos. Turk J Med Sci 45: 526–533, 2015. doi: 10.3906/sag-1403-34. [DOI] [PubMed] [Google Scholar]
- 437.Mak P, Chang C, Pursell B, Mercurio AM. Estrogen receptor β sustains epithelial differentiation by regulating prolyl hydroxylase 2 transcription. Proc Natl Acad Sci USA 110: 4708–4713, 2013. doi: 10.1073/pnas.1221654110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Mak P, Li J, Samanta S, Mercurio AM. ERβ regulation of NF-kB activation in prostate cancer is mediated by HIF-1. Oncotarget 6: 40247–40254, 2015. doi: 10.18632/oncotarget.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Mäkinen S, Mäkelä S, Weihua Z, Warner M, Rosenlund B, Salmi S, Hovatta O, Gustafsson JA. Localization of oestrogen receptors alpha and beta in human testis. Mol Hum Reprod 7: 497–503, 2001. doi: 10.1093/molehr/7.6.497. [DOI] [PubMed] [Google Scholar]
- 440.Manolagas SC, O’Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol 9: 699–712, 2013. doi: 10.1038/nrendo.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Mansour MM, Machen MR, Tarleton BJ, Wiley AA, Wower J, Bartol FF, Goyal HO. Expression and molecular characterization of estrogen receptor alpha messenger RNA in male reproductive organs of adult goats. Biol Reprod 64: 1432–1438, 2001. doi: 10.1095/biolreprod64.5.1432. [DOI] [PubMed] [Google Scholar]
- 442.Mårtensson UE, Salehi SA, Windahl S, Gomez MF, Swärd K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grände PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 150: 687–698, 2009. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 443.Martin RM, Lin CJ, Nishi MY, Billerbeck AE, Latronico AC, Russell DW, Mendonca BB. Familial hyperestrogenism in both sexes: clinical, hormonal, and molecular studies of two siblings. J Clin Endocrinol Metab 88: 3027–3034, 2003. doi: 10.1210/jc.2002-021780. [DOI] [PubMed] [Google Scholar]
- 444.Martínez-Traverso GB, Pearl CA. Immunolocalization of G protein-coupled estrogen receptor in the rat epididymis. Reprod Biol Endocrinol 13: 48, 2015. doi: 10.1186/s12958-015-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Masson G, Selye H. Changes in the accessory sex organs of the male rat after administration of estradiol in combination with progesterone or desoxycorticosterone acetate. Am J Pathol 19: 1–7, 1943. [PMC free article] [PubMed] [Google Scholar]
- 446.Matzkin H, Soloway MS. Immunohistochemical evidence of the existence and localization of aromatase in human prostatic tissues. Prostate 21: 309–314, 1992. doi: 10.1002/pros.2990210407. [DOI] [PubMed] [Google Scholar]
- 447.Mauras N, O’Brien KO, Klein KO, Hayes V. Estrogen suppression in males: metabolic effects. J Clin Endocrinol Metab 85: 2370–2377, 2000. [DOI] [PubMed] [Google Scholar]
- 448.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 34: 309–338, 2013. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.McAllister C, Long J, Bowers A, Walker A, Cao P, Honda S, Harada N, Staufenbiel M, Shen Y, Li R. Genetic targeting aromatase in male amyloid precursor protein transgenic mice down-regulates beta-secretase (BACE1) and prevents Alzheimer-like pathology and cognitive impairment. J Neurosci 30: 7326–7334, 2010. doi: 10.1523/JNEUROSCI.1180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.McDevitt MA, Glidewell-Kenney C, Weiss J, Chambon P, Jameson JL, Levine JE. Estrogen response element-independent estrogen receptor (ER)-alpha signaling does not rescue sexual behavior but restores normal testosterone secretion in male ERalpha knockout mice. Endocrinology 148: 5288–5294, 2007. doi: 10.1210/en.2007-0673. [DOI] [PubMed] [Google Scholar]
- 451.McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav 9: 249–263, 1977. doi: 10.1016/0018-506X(77)90060-5. [DOI] [PubMed] [Google Scholar]
- 452.McGrath N, O’Grady MJ. Aromatase inhibitors for short stature in male children and adolescents. Cochrane Database Syst Rev 10: CD010888, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.McGuire JL, Lisk RD. Estrogen receptors in the intact rat. Proc Natl Acad Sci USA 61: 497–503, 1968. doi: 10.1073/pnas.61.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.McLachlan JA. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocr Rev 22: 319–341, 2001. doi: 10.1210/edrv.22.3.0432. [DOI] [PubMed] [Google Scholar]
- 455.McLachlan JA. Rodent models for perinatal exposure to diethylstilbestrol and their relation to human disease in the male. In: Developmental Effects of Diethylstilbestrol (DES) in Pregnancy, edited by Herbstand AL, Bern HA. New York: Thieme-Stratton, 1981. [Google Scholar]
- 456.McLachlan JA, Newbold RR, Bullock B. Reproductive tract lesions in male mice exposed prenatally to diethylstilbestrol. Science 190: 991–992, 1975. doi: 10.1126/science.242076. [DOI] [PubMed] [Google Scholar]
- 457.McPherson SJ, Hussain S, Balanathan P, Hedwards SL, Niranjan B, Grant M, Chandrasiri UP, Toivanen R, Wang Y, Taylor RA, Risbridger GP. Estrogen receptor-beta activated apoptosis in benign hyperplasia and cancer of the prostate is androgen independent and TNFalpha mediated. Proc Natl Acad Sci USA 107: 3123–3128, 2010. doi: 10.1073/pnas.0905524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Mellström D, Vandenput L, Mallmin H, Holmberg AH, Lorentzon M, Odén A, Johansson H, Orwoll ES, Labrie F, Karlsson MK, Ljunggren O, Ohlsson C. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res 23: 1552–1560, 2008. doi: 10.1359/jbmr.080518. [DOI] [PubMed] [Google Scholar]
- 459.Melnyk PM, Sanford LM, Robaire B. Moderate increases in peripheral blood estradiol concentration in the adult ram do not directly inhibit testosterone secretion. Can J Physiol Pharmacol 70: 1384–1391, 1992. doi: 10.1139/y92-194. [DOI] [PubMed] [Google Scholar]
- 460.Menad R, Smaï S, Moudilou E, Khammar F, Exbrayat JM, Gernigon-Spychalowicz T. Immunolocalization of estrogen and androgen receptors in the caput epididymidis of the fat sand rat (Psammomys obesus): effects of seasonal variations, castration and efferent duct ligation. Acta Histochem 116: 559–569, 2014. doi: 10.1016/j.acthis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 461.Merklin RJ. A histochemical study of male mice injected with estradiol dipropionate before puberty. Anat Rec 122: 257–270, 1955. doi: 10.1002/ar.1091220210. [DOI] [PubMed] [Google Scholar]
- 462.Mester J, Baulieu EE. Nuclear estrogen receptor of chick liver. Biochim Biophys Acta 261: 236–244, 1972. doi: 10.1016/0304-4165(72)90334-0. [DOI] [PubMed] [Google Scholar]
- 463.Meyer MR, Haas E, Prossnitz ER, Barton M. Non-genomic regulation of vascular cell function and growth by estrogen. Mol Cell Endocrinol 308: 9–16, 2009. doi: 10.1016/j.mce.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Miedlich SU, Karamooz N, Hammes SR. Aromatase deficiency in a male patient: case report and review of the literature. Bone 93: 181–186, 2016. doi: 10.1016/j.bone.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 465.Moguilevsky JA, Torres HN, Bur GE, Erenfryd AN, Malinow MR. Changes in the concentraton of ascorbic acid induced by estradiol on endocrine glands of male rats. Acta Physiol Lat Am 10: 52–57, 1960. [PubMed] [Google Scholar]
- 466.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 68: 4447–4454, 2008. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Moran FM, Ford JJ, Corbin CJ, Mapes SM, Njar VC, Brodie AM, Conley AJ. Regulation of microsomal P450, redox partner proteins, and steroidogenesis in the developing testes of the neonatal pig. Endocrinology 143: 3361–3369, 2002. doi: 10.1210/en.2002-220329. [DOI] [PubMed] [Google Scholar]
- 468.Morini M, Peñaranda DS, Vílchez MC, Tveiten H, Lafont AG, Dufour S, Pérez L, Asturiano JF. The expression of nuclear and membrane estrogen receptors in the European eel throughout spermatogenesis. Comp Biochem Physiol A Mol Integr Physiol 203: 91–99, 2017. doi: 10.1016/j.cbpa.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 469.Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 80: 3689–3698, 1995. [DOI] [PubMed] [Google Scholar]
- 470.Movérare S, Lindberg MK, Faergemann J, Gustafsson JA, Ohlsson C. Estrogen receptor alpha, but not estrogen receptor beta, is involved in the regulation of the hair follicle cycling as well as the thickness of epidermis in male mice. J Invest Dermatol 119: 1053–1058, 2002. doi: 10.1046/j.1523-1747.2002.00637.x. [DOI] [PubMed] [Google Scholar]
- 471.Movérare S, Venken K, Eriksson AL, Andersson N, Skrtic S, Wergedal J, Mohan S, Salmon P, Bouillon R, Gustafsson JA, Vanderschueren D, Ohlsson C. Differential effects on bone of estrogen receptor alpha and androgen receptor activation in orchidectomized adult male mice. Proc Natl Acad Sci USA 100: 13573–13578, 2003. doi: 10.1073/pnas.2233084100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Mowa CN, Iwanaga T. Expression of estrogen receptor-alpha and -beta mRNAs in the male reproductive system of the rat as revealed by in situ hybridization. J Mol Endocrinol 26: 165–174, 2001. doi: 10.1677/jme.0.0260165. [DOI] [PubMed] [Google Scholar]
- 473.Murphy AJ, Guyre PM, Wira CR, Pioli PA. Estradiol regulates expression of estrogen receptor ERalpha46 in human macrophages. PLoS One 4: e5539, 2009. doi: 10.1371/journal.pone.0005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Murphy E. Estrogen signaling and cardiovascular disease. Circ Res 109: 687–696, 2011. doi: 10.1161/CIRCRESAHA.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Musa BU, Seal US, Doe RP, Lamusga RF, Lewis MD, Glaser R. Elevation of certain plasma proteins in man following estrogen administration: a dose-response relationship. J Clin Endocrinol Metab 25: 1163–1166, 1965. doi: 10.1210/jcem-25-9-1163. [DOI] [PubMed] [Google Scholar]
- 476.Mutembei HM, Pesch S, Schuler G, Hoffmann B. Expression of oestrogen receptors alpha and beta and of aromatase in the testis of immature and mature boars. Reprod Domest Anim 40: 228–236, 2005. doi: 10.1111/j.1439-0531.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 477.Mutembei HM, Kowalewski MP, Ugele B, Schuler G, Hoffmann B. Expression and activity of steroid sulphatase in the boar testis. Reprod Domest Anim 44: 17–23, 2009. doi: 10.1111/j.1439-0531.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- 478.Naaz A, Zakroczymski M, Heine P, Taylor J, Saunders P, Lubahn D, Cooke PS. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ERalpha): a potential role for estrogen receptor beta (ERbeta). Horm Metab Res 34: 758–763, 2002. doi: 10.1055/s-2002-38259. [DOI] [PubMed] [Google Scholar]
- 479.Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by the anterior hypothalamus of adult male and female rats. Endocrinology 90: 295–298, 1972. doi: 10.1210/endo-90-1-295. [DOI] [PubMed] [Google Scholar]
- 480.Nakai M, Bouma J, Nie R, Zhou Q, Carnes K, Lubahn DB, Hess RA. Morphological analysis of endocytosis in efferent ductules of estrogen receptor-alpha knockout male mouse. Anat Rec 263: 10–18, 2001. doi: 10.1002/ar.1071. [DOI] [PubMed] [Google Scholar]
- 481.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130: 811–823, 2007. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 482.Nanjappa MK, Hess RA, Medrano TI, Locker SH, Levin ER, Cooke PS. Membrane-localized estrogen receptor 1 is required for normal male reproductive development and function in mice. Endocrinology 157: 2909–2919, 2016. doi: 10.1210/en.2016-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Narayanan K, Havmoeller R, Reinier K, Jerger K, Teodorescu C, Uy-Evanado A, Navarro J, Huertas-Vazquez A, Gunson K, Jui J, Chugh SS. Sex hormone levels in patients with sudden cardiac arrest. Heart Rhythm 11: 2267–2272, 2014. doi: 10.1016/j.hrthm.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Naulé L, Marie-Luce C, Parmentier C, Martini M, Albac C, Trouillet AC, Keller M, Hardin-Pouzet H, Mhaouty-Kodja S. Revisiting the neural role of estrogen receptor beta in male sexual behavior by conditional mutagenesis. Horm Behav 80: 1–9, 2016. doi: 10.1016/j.yhbeh.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 485.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–E452, 2007. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 486.Nelles JL, Hu WY, Prins GS. Estrogen action and prostate cancer. Expert Rev Endocrinol Metab 6: 437–451, 2011. doi: 10.1586/eem.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Nelson AW, Tilley WD, Neal DE, Carroll JS. Estrogen receptor beta in prostate cancer: friend or foe? Endocr Relat Cancer 21: T219–T234, 2014. doi: 10.1530/ERC-13-0508. [DOI] [PubMed] [Google Scholar]
- 488.Nettleship JE, Jones TH, Channer KS, Jones RD. Physiological testosterone replacement therapy attenuates fatty streak formation and improves high-density lipoprotein cholesterol in the Tfm mouse: an effect that is independent of the classic androgen receptor. Circulation 116: 2427–2434, 2007. doi: 10.1161/CIRCULATIONAHA.107.708768. [DOI] [PubMed] [Google Scholar]
- 489.Newbold RR, Bullock BC, McLachlan JA. Adenocarcinoma of the rete testis. Diethylstilbestrol-induced lesions of the mouse rete testis. Am J Pathol 125: 625–628, 1986. [PMC free article] [PubMed] [Google Scholar]
- 490.Newbold RR, Bullock BC, McLachlan JA. Müllerian remnants of male mice exposed prenatally to diethylstilbestrol. Teratog Carcinog Mutagen 7: 377–389, 1987. doi: 10.1002/tcm.1770070405. [DOI] [PubMed] [Google Scholar]
- 491.Nicholson TM, Moses MA, Uchtmann KS, Keil KP, Bjorling DE, Vezina CM, Wood RW, Ricke WA. Estrogen receptor-α is a key mediator and therapeutic target for bladder complications of benign prostatic hyperplasia. J Urol 193: 722–729, 2015. doi: 10.1016/j.juro.2014.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Nie R, Zhou Q, Jassim E, Saunders PT, Hess RA. Differential expression of estrogen receptors alpha and beta in the reproductive tracts of adult male dogs and cats. Biol Reprod 66: 1161–1168, 2002. doi: 10.1095/biolreprod66.4.1161. [DOI] [PubMed] [Google Scholar]
- 494.Nielsen M, Björnsdóttir S, Høyer PE, Byskov AG. Ontogeny of oestrogen receptor alpha in gonads and sex ducts of fetal and newborn mice. J Reprod Fertil 118: 195–204, 2000. doi: 10.1530/reprod/118.1.195. [DOI] [PubMed] [Google Scholar]
- 495.Nimptsch K, Platz EA, Willett WC, Giovannucci E. Association between plasma 25-OH vitamin D and testosterone levels in men. Clin Endocrinol (Oxf) 77: 106–112, 2012. doi: 10.1111/j.1365-2265.2012.04332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Nitta H, Bunick D, Hess RA, Janulis L, Newton SC, Millette CF, Osawa Y, Shizuta Y, Toda K, Bahr JM. Germ cells of the mouse testis express P450 aromatase. Endocrinology 132: 1396–1401, 1993. [DOI] [PubMed] [Google Scholar]
- 497.Nofer JR. Estrogens and atherosclerosis: insights from animal models and cell systems. J Mol Endocrinol 48: R13–R29, 2012. doi: 10.1530/JME-11-0145. [DOI] [PubMed] [Google Scholar]
- 498.Nomura M, Andersson S, Korach KS, Gustafsson JA, Pfaff DW, Ogawa S. Estrogen receptor-beta gene disruption potentiates estrogen-inducible aggression but not sexual behaviour in male mice. Eur J Neurosci 23: 1860–1868, 2006. doi: 10.1111/j.1460-9568.2006.04703.x. [DOI] [PubMed] [Google Scholar]
- 499.Nordmeyer J, Eder S, Mahmoodzadeh S, Martus P, Fielitz J, Bass J, Bethke N, Zurbrügg HR, Pregla R, Hetzer R, Regitz-Zagrosek V. Upregulation of myocardial estrogen receptors in human aortic stenosis. Circulation 110: 3270–3275, 2004. doi: 10.1161/01.CIR.0000147610.41984.E8. [DOI] [PubMed] [Google Scholar]
- 500.Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci 18: 690–697, 2015. doi: 10.1038/nn.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.O’Donnell L, Meachem SJ, Stanton PG, McLachlan RI. Endocrine regulation of spermatogenesis. In: Kobil and Neill’s Physiology of Reproduction, edited by Neill JD. St. Louis, MO: Elsevier, 2006, p. 1017–1069. doi: 10.1016/B978-012515400-0/50026-9 [DOI] [Google Scholar]
- 502.O’Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr Rev 22: 289–318, 2001. doi: 10.1210/er.22.3.289. [DOI] [PubMed] [Google Scholar]
- 503.O’Malley BW. A life-long search for the molecular pathways of steroid hormone action. Mol Endocrinol 19: 1402–1411, 2005. doi: 10.1210/me.2004-0480. [DOI] [PubMed] [Google Scholar]
- 504.O’Malley BW, Sherman MR, Toft DO. Progesterone “receptors” in the cytoplasm and nucleus of chick oviduct target tissue. Proc Natl Acad Sci USA 67: 501–508, 1970. doi: 10.1073/pnas.67.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Ogasawara Y, Okamoto S, Kitamura Y, Matsumoto K. Proliferative pattern of uterine cells from birth to adulthood in intact, neonatally castrated, and/or adrenalectomized mice, assayed by incorporation of [125I]iododeoxyuridine. Endocrinology 113: 582–587, 1983. doi: 10.1210/endo-113-2-582. [DOI] [PubMed] [Google Scholar]
- 506.Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc Natl Acad Sci USA 96: 12887–12892, 1999. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci USA 94: 1476–1481, 1997. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Ogawa S, Washburn TF, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Modifications of testosterone-dependent behaviors by estrogen receptor-alpha gene disruption in male mice. Endocrinology 139: 5058–5069, 1998. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- 509.Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly-Y M, Rudling M, Lindberg MK, Warner M, Angelin B, Gustafsson JA. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun 278: 640–645, 2000. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- 510.Ohtani H, Miura I, Ichikawa Y. Role of aromatase and androgen receptor expression in gonadal sex differentiation of ZW/ZZ-type frogs, Rana rugosa. Comp Biochem Physiol C Toxicol Pharmacol 134: 215–225, 2003. doi: 10.1016/S1532-0456(02)00252-1. [DOI] [PubMed] [Google Scholar]
- 511.Okano T, Murase T, Tsubota T. Spermatogenesis, serum testosterone levels and immunolocalization of steroidogenic enzymes in the wild male Japanese black bear (Ursus thibetanus japonicus). J Vet Med Sci 65: 1093–1099, 2003. doi: 10.1292/jvms.65.1093. [DOI] [PubMed] [Google Scholar]
- 512.Oliveira AG, Coelho PH, Guedes FD, Mahecha GA, Hess RA, Oliveira CA. 5alpha-Androstane-3beta,17beta-diol (3beta-diol), an estrogenic metabolite of 5alpha-dihydrotestosterone, is a potent modulator of estrogen receptor ERbeta expression in the ventral prostrate of adult rats. Steroids 72: 914–922, 2007. doi: 10.1016/j.steroids.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 513.Oliveira AG, Dornas RA, Praes LC, Hess RA, Mahecha GA, Oliveira CA. Roosters affected by epididymal lithiasis present local alteration in vitamin D3, testosterone and estradiol levels as well as estrogen receptor 2 (beta) expression. Reproduction 142: 439–446, 2011. doi: 10.1530/REP-11-0131. [DOI] [PubMed] [Google Scholar]
- 514.Oliveira CA, Carnes K, França LR, Hermo L, Hess RA. Aquaporin-1 and -9 are differentially regulated by oestrogen in the efferent ductule epithelium and initial segment of the epididymis. Biol Cell 97: 385–395, 2005. doi: 10.1042/BC20040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Oliveira CA, Carnes K, França LR, Hess RA. Infertility and testicular atrophy in the antiestrogen-treated adult male rat. Biol Reprod 65: 913–920, 2001. doi: 10.1095/biolreprod65.3.913. [DOI] [PubMed] [Google Scholar]
- 516.Oliveira CA, Mahecha GA, Carnes K, Prins GS, Saunders PT, França LR, Hess RA. Differential hormonal regulation of estrogen receptors ERalpha and ERbeta and androgen receptor expression in rat efferent ductules. Reproduction 128: 73–86, 2004. doi: 10.1530/rep.1.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Oliveira CA, Nie R, Carnes K, Franca LR, Prins GS, Saunders PT, Hess RA. The antiestrogen ICI 182,780 decreases the expression of estrogen receptor-alpha but has no effect on estrogen receptor-beta and androgen receptor in rat efferent ductules. Reprod Biol Endocrinol 1: 75, 2003. doi: 10.1186/1477-7827-1-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Oliveira CA, Zhou Q, Carnes K, Nie R, Kuehl DE, Jackson GL, Franca LR, Nakai M, Hess RA. ER function in the adult male rat: short- and long-term effects of the antiestrogen ICI 182,780 on the testis and efferent ductules, without changes in testosterone. Endocrinology 143: 2399–2409, 2002. doi: 10.1210/endo.143.6.8873. [DOI] [PubMed] [Google Scholar]
- 520.Oliveira RL, Nogueira JC, Mahecha GA, Oliveira CA. Seasonal variation in estrogen receptor ERα, but not ERβ, androgen receptor and aromatase, in the efferent ductules and epididymis of the big fruit-eating bat Artibeus lituratus. Gen Comp Endocrinol 179: 1–13, 2012. doi: 10.1016/j.ygcen.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 521.Oliveira RL, Oliveira AG, Mahecha GA, Nogueira JC, Oliveira CA. Distribution of estrogen receptors (ERalpha and ERbeta) and androgen receptor in the testis of big fruit-eating bat Artibeus lituratus is cell- and stage-specific and increases during gonadal regression. Gen Comp Endocrinol 161: 283–292, 2009. doi: 10.1016/j.ygcen.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 522.Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod 80: 34–41, 2009. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- 523.Overpeck JG, Colson SH, Hohmann JR, Applestine MS, Reilly JF. Concentrations of circulating steroids in normal prepubertal and adult male and female humans, chimpanzees, rhesus monkeys, rats, mice, and hamsters: a literature survey. J Toxicol Environ Health 4: 785–803, 1978. doi: 10.1080/15287397809529700. [DOI] [PubMed] [Google Scholar]
- 524.Oz OK, Hirasawa G, Lawson J, Nanu L, Constantinescu A, Antich PP, Mason RP, Tsyganov E, Parkey RW, Zerwekh JE, Simpson ER. Bone phenotype of the aromatase deficient mouse. J Steroid Biochem Mol Biol 79: 49–59, 2001. doi: 10.1016/S0960-0760(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 525.Pais V, Leav I, Lau KM, Jiang Z, Ho SM. Estrogen receptor-beta expression in human testicular germ cell tumors. Clin Cancer Res 9: 4475–4482, 2003. [PubMed] [Google Scholar]
- 526.Palierne G, Fabre A, Solinhac R, Le Péron C, Avner S, Lenfant F, Fontaine C, Salbert G, Flouriot G, Arnal JF, Métivier R. Changes in gene expression and estrogen receptor cistrome in mouse liver upon acute E2 treatment. Mol Endocrinol 30: 709–732, 2016. doi: 10.1210/me.2015-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Panda JN, Turner CW. Effect of estrogen on mammary gland growth of immature male rats. Proc Soc Exp Biol Med 121: 803–804, 1966. doi: 10.3181/00379727-121-30892. [DOI] [PubMed] [Google Scholar]
- 528.Papadopoulos V, Carreau S, Szerman-Joly E, Drosdowsky MA, Dehennin L, Scholler R. Rat testis 17 beta-estradiol: identification by gas chromatography-mass spectrometry and age related cellular distribution. J Steroid Biochem 24: 1211–1216, 1986. doi: 10.1016/0022-4731(86)90385-7. [DOI] [PubMed] [Google Scholar]
- 529.Park II, Zhang Q, Liu V, Kozlowski JM, Zhang J, Lee C. 17Beta-estradiol at low concentrations acts through distinct pathways in normal versus benign prostatic hyperplasia-derived prostate stromal cells. Endocrinology 150: 4594–4605, 2009. doi: 10.1210/en.2008-1591. [DOI] [PubMed] [Google Scholar]
- 530.Parlevliet JM, Pearl CA, Hess MF, Famula TR, Roser JF. Immunolocalization of estrogen and androgen receptors and steroid concentrations in the stallion epididymis. Theriogenology 66: 755–765, 2006. doi: 10.1016/j.theriogenology.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 531.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol 24: 90–99, 2014. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Patel VH, Chen J, Ramanjaneya M, Karteris E, Zachariades E, Thomas P, Been M, Randeva HS. G-protein coupled estrogen receptor 1 expression in rat and human heart: protective role during ischaemic stress. Int J Mol Med 26: 193–199, 2010. doi: 10.3892/ijmm_00000452. [DOI] [PubMed] [Google Scholar]
- 533.Payne AH, Perkins LM, Georgiou M, Quinn PG. Intratesticular site of aromatase activity and possible function of testicular estradiol. Steroids 50: 435–448, 1987. doi: 10.1016/0039-128X(87)90030-4. [DOI] [PubMed] [Google Scholar]
- 534.Pearl CA, At-Taras E, Berger T, Roser JF. Reduced endogenous estrogen delays epididymal development but has no effect on efferent duct morphology in boars. Reproduction 134: 593–604, 2007. doi: 10.1530/REP-06-0239. [DOI] [PubMed] [Google Scholar]
- 535.Pearl CA, Mason H, Roser JF. Immunolocalization of estrogen receptor alpha, estrogen receptor beta and androgen receptor in the pre-, peri- and post-pubertal stallion testis. Anim Reprod Sci 125: 103–111, 2011. doi: 10.1016/j.anireprosci.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 536.Pedersen SB, Børglum JD, Eriksen EF, Richelsen B. Nuclear estradiol binding in rat adipocytes. Regional variations and regulatory influences of hormones. Biochim Biophys Acta 1093: 80–86, 1991. doi: 10.1016/0167-4889(91)90141-J. [DOI] [PubMed] [Google Scholar]
- 537.Pedersen SB, Bruun JM, Hube F, Kristensen K, Hauner H, Richelsen B. Demonstration of estrogen receptor subtypes alpha and beta in human adipose tissue: influences of adipose cell differentiation and fat depot localization. Mol Cell Endocrinol 182: 27–37, 2001. doi: 10.1016/S0303-7207(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 538.Pedram A, Razandi M, Lewis M, Hammes S, Levin ER. Membrane-localized estrogen receptor α is required for normal organ development and function. Dev Cell 29: 482–490, 2014. doi: 10.1016/j.devcel.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER. Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-beta to inhibit calcineurin. Endocrinology 149: 3361–3369, 2008. doi: 10.1210/en.2008-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Pedram A, Razandi M, O’Mahony F, Lubahn D, Levin ER. Estrogen receptor-beta prevents cardiac fibrosis. Mol Endocrinol 24: 2152–2165, 2010. doi: 10.1210/me.2010-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem 282: 22278–22288, 2007. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 542.Pelletier G. Localization of androgen and estrogen receptors in rat and primate tissues. Histol Histopathol 15: 1261–1270, 2000. [DOI] [PubMed] [Google Scholar]
- 543.Pelletier G, Labrie C, Labrie F. Localization of oestrogen receptor alpha, oestrogen receptor beta and androgen receptors in the rat reproductive organs. J Endocrinol 165: 359–370, 2000. doi: 10.1677/joe.0.1650359. [DOI] [PubMed] [Google Scholar]
- 544.Pereira MF, Fernandes SA, Nascimento AR, Siu ER, Hess RA, Oliveira CA, Porto CS, Lazari MF. Effects of the oestrogen receptor antagonist Fulvestrant on expression of genes that affect organization of the epididymal epithelium. Andrology 2: 559–571, 2014. doi: 10.1111/j.2047-2927.2014.00219.x. [DOI] [PubMed] [Google Scholar]
- 545.Pereyra-Martinez AC, Roselli CE, Stadelman HL, Resko JA. Cytochrome P450 aromatase in testis and epididymis of male rhesus monkeys. Endocrine 16: 15–19, 2001. doi: 10.1385/ENDO:16:1:15. [DOI] [PubMed] [Google Scholar]
- 546.Peters MA, Mol JA, van Wolferen ME, Oosterlaken-Dijksterhuis MA, Teerds KJ, van Sluijs FJ. Expression of the insulin-like growth factor (IGF) system and steroidogenic enzymes in canine testis tumors. Reprod Biol Endocrinol 1: 22, 2003. doi: 10.1186/1477-7827-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Philips BJ, Ansell PJ, Newton LG, Harada N, Honda S, Ganjam VK, Rottinghaus GE, Welshons WV, Lubahn DB. Estrogen receptor-independent catechol estrogen binding activity: protein binding studies in wild-type, Estrogen receptor-alpha KO, and aromatase KO mice tissues. Biochemistry 43: 6698–6708, 2004. doi: 10.1021/bi036154j. [DOI] [PubMed] [Google Scholar]
- 548.Phillips GB, Castelli WP, Abbott RD, McNamara PM. Association of hyperestrogenemia and coronary heart disease in men in the Framingham cohort. Am J Med 74: 863–869, 1983. doi: 10.1016/0002-9343(83)91078-1. [DOI] [PubMed] [Google Scholar]
- 549.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65: 369–382, 1959. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 550.Picciarelli-Lima P, Oliveira AG, Reis AM, Kalapothakis E, Mahecha GA, Hess RA, Oliveira CA. Effects of 3-beta-diol, an androgen metabolite with intrinsic estrogen-like effects, in modulating the aquaporin-9 expression in the rat efferent ductules. Reprod Biol Endocrinol 4: 51, 2006. doi: 10.1186/1477-7827-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Pilutin A, Misiakiewicz-Has K, Kolasa A, Baranowska-Bosiacka I, Marchlewicz M, Wiszniewska B. The immunoexpression of androgen receptor, estrogen receptors alpha and beta, vanilloid type 1 receptor and cytochrome p450 aromatase in rats testis chronically treated with letrozole, an aromatase inhibitor. Folia Histochem Cytobiol 52: 206–217, 2014. doi: 10.5603/FHC.2014.0024. [DOI] [PubMed] [Google Scholar]
- 552.Place AR, Lang J, Gavasso S, Jeyasuria P. Expression of P450(arom) in Malaclemys terrapin and Chelydra serpentina: a tale of two sites. J Exp Zool 290: 673–690, 2001. doi: 10.1002/jez.1118. [DOI] [PubMed] [Google Scholar]
- 553.Ponnusamy S, Tran QT, Harvey I, Smallwood HS, Thiyagarajan T, Banerjee S, Johnson DL, Dalton JT, Sullivan RD, Miller DD, Bridges D, Narayanan R. Pharmacologic activation of estrogen receptor β increases mitochondrial function, energy expenditure, and brown adipose tissue. FASEB J 31: 266–281, 2017. doi: 10.1096/fj.201600787RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Power RF, Mani SK, Codina J, Conneely OM, O’Malley BW. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science 254: 1636–1639, 1991. doi: 10.1126/science.1749936. [DOI] [PubMed] [Google Scholar]
- 555.Prins GS, Birch L. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology 138: 1801–1809, 1997. [DOI] [PubMed] [Google Scholar]
- 556.Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor α: studies with alphaERKO and betaERKO mice. Cancer Res 61: 6089–6097, 2001. [PubMed] [Google Scholar]
- 557.Prins GS, Birch L, Habermann H, Chang WY, Tebeau C, Putz O, Bieberich C. Influence of neonatal estrogens on rat prostate development. Reprod Fertil Dev 13: 241–252, 2001. doi: 10.1071/RD00107. [DOI] [PubMed] [Google Scholar]
- 558.Prins GS, Calderon-Gierszal EL, Hu WY. Stem cells as hormone targets that lead to increased cancer susceptibility. Endocrinology 156: 3451–3457, 2015. doi: 10.1210/en.2015-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Prins GS, Hu WY, Shi GB, Hu DP, Majumdar S, Li G, Huang K, Nelles JL, Ho SM, Walker CL, Kajdacsy-Balla A, van Breemen RB. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology 155: 805–817, 2014. doi: 10.1210/en.2013-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Prins GS, Huang L, Birch L, Pu Y. The role of estrogens in normal and abnormal development of the prostate gland. Ann NY Acad Sci 1089: 1–13, 2006. doi: 10.1196/annals.1386.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Prins GS, Korach KS. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids 73: 233–244, 2008. doi: 10.1016/j.steroids.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Prins GS, Marmer M, Woodham C, Chang WY, Kuiper G, Gustafsson JA, Birch L. Estrogen receptor-β messenger ribonucleic acid ontogeny in the prostate of normal and neonatally estrogenized rats. Endocrinology 139: 874–883, 1998. doi: 10.1210/endo.139.3.5827. [DOI] [PubMed] [Google Scholar]
- 564.Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation 76: 641–659, 2008. doi: 10.1111/j.1432-0436.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Prins GS, Tang WY, Belmonte J, Ho SM. Perinatal exposure to oestradiol and bisphenol A alters the prostate epigenome and increases susceptibility to carcinogenesis. Basic Clin Pharmacol Toxicol 102: 134–138, 2008. doi: 10.1111/j.1742-7843.2007.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Prossnitz ER, Hathaway HJ. What have we learned about GPER function in physiology and disease from knockout mice? J Steroid Biochem Mol Biol 153: 114–126, 2015. doi: 10.1016/j.jsbmb.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Pudney J, Canick JA, Clifford NM, Knapp JB, Callard GV. Location of enzymes of androgen and estrogen biosynthesis in the testis of the ground squirrel (Citellus lateralis). Biol Reprod 33: 971–980, 1985. doi: 10.1095/biolreprod33.4.971. [DOI] [PubMed] [Google Scholar]
- 568.Qian YM, Sun XJ, Tong MH, Li XP, Richa J, Song WC. Targeted disruption of the mouse estrogen sulfotransferase gene reveals a role of estrogen metabolism in intracrine and paracrine estrogen regulation. Endocrinology 142: 5342–5350, 2001. doi: 10.1210/endo.142.12.8540. [DOI] [PubMed] [Google Scholar]
- 569.Quaynor SD, Stradtman EW Jr, Kim HG, Shen Y, Chorich LP, Schreihofer DA, Layman LC. Delayed puberty and estrogen resistance in a woman with estrogen receptor α variant. N Engl J Med 369: 164–171, 2013. doi: 10.1056/NEJMoa1303611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Quirke LD, Juengel JL, Tisdall DJ, Lun S, Heath DA, McNatty KP. Ontogeny of steroidogenesis in the fetal sheep gonad. Biol Reprod 65: 216–228, 2001. doi: 10.1095/biolreprod65.1.216. [DOI] [PubMed] [Google Scholar]
- 571.Raeside JI, Christie HL. Estrogen concentrations in semen of the stallion. Anim Reprod Sci 48: 293–300, 1997. doi: 10.1016/S0378-4320(97)00045-6. [DOI] [PubMed] [Google Scholar]
- 572.Rago V, Bilińska B, Palma A, Andò S, Carpino A. Evidence of aromatase localization in cytoplasmic droplet of human immature ejaculated spermatozoa. Folia Histochem Cytobiol 41: 23–27, 2003. [PubMed] [Google Scholar]
- 573.Ramesh R, Pearl CA, At-Taras E, Roser JF, Berger T. Ontogeny of androgen and estrogen receptor expression in porcine testis: effect of reducing testicular estrogen synthesis. Anim Reprod Sci 102: 286–299, 2007. doi: 10.1016/j.anireprosci.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 574.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension 37: 1199–1208, 2001. doi: 10.1161/01.HYP.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 575.Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev 97: 1–37, 2017. doi: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 576.Ren Z, Zou C, Ji H, Zhang YA. Oestrogen regulates proliferation and differentiation of human islet-derived precursor cells through oestrogen receptor alpha. Cell Biol Int 34: 523–530, 2010. doi: 10.1042/CBI20090390. [DOI] [PubMed] [Google Scholar]
- 577.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307: 1625–1630, 2005. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 578.Ribas V, Drew BG, Le JA, Soleymani T, Daraei P, Sitz D, Mohammad L, Henstridge DC, Febbraio MA, Hewitt SC, Korach KS, Bensinger SJ, Hevener AL. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proc Natl Acad Sci USA 108: 16457–16462, 2011. doi: 10.1073/pnas.1104533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Ribas V, Drew BG, Zhou Z, Phun J, Kalajian NY, Soleymani T, Daraei P, Widjaja K, Wanagat J, de Aguiar Vallim TQ, Fluitt AH, Bensinger S, Le T, Radu C, Whitelegge JP, Beaven SW, Tontonoz P, Lusis AJ, Parks BW, Vergnes L, Reue K, Singh H, Bopassa JC, Toro L, Stefani E, Watt MJ, Schenk S, Akerstrom T, Kelly M, Pedersen BK, Hewitt SC, Korach KS, Hevener AL. Skeletal muscle action of estrogen receptor α is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci Transl Med 8: 334ra54, 2016. doi: 10.1126/scitranslmed.aad3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Ricke WA, McPherson SJ, Bianco JJ, Cunha GR, Wang Y, Risbridger GP. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB J 22: 1512–1520, 2008. doi: 10.1096/fj.07-9526com. [DOI] [PubMed] [Google Scholar]
- 581.Risbridger G, Wang H, Young P, Kurita T, Wang YZ, Lubahn D, Gustafsson JA, Cunha G. Evidence that epithelial and mesenchymal estrogen receptor-α mediates effects of estrogen on prostatic epithelium. Dev Biol 229: 432–442, 2001. doi: 10.1006/dbio.2000.9994. [DOI] [PubMed] [Google Scholar]
- 582.Robaire B, Hamzeh M. Androgen action in the epididymis. J Androl 32: 592–599, 2011. doi: 10.2164/jandrol.111.014266. [DOI] [PubMed] [Google Scholar]
- 583.Robaire B, Viger RS. Regulation of epididymal epithelial cell functions. Biol Reprod 52: 226–236, 1995. doi: 10.1095/biolreprod52.2.226. [DOI] [PubMed] [Google Scholar]
- 584.Roberts KD. Sterol sulfates in the epididymis; synthesis and possible function in the reproductive process. J Steroid Biochem 27: 337–341, 1987. doi: 10.1016/0022-4731(87)90325-6. [DOI] [PubMed] [Google Scholar]
- 585.Robertson KM, O’Donnell L, Jones ME, Meachem SJ, Boon WC, Fisher CR, Graves KH, McLachlan RI, Simpson ER. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proc Natl Acad Sci USA 96: 7986–7991, 1999. doi: 10.1073/pnas.96.14.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Robertson KM, O’Donnell L, Simpson ER, Jones ME. The phenotype of the aromatase knockout mouse reveals dietary phytoestrogens impact significantly on testis function. Endocrinology 143: 2913–2921, 2002. doi: 10.1210/endo.143.8.8957. [DOI] [PubMed] [Google Scholar]
- 587.Robertson KM, Simpson ER, Lacham-Kaplan O, Jones ME. Characterization of the fertility of male aromatase knockout mice. J Androl 22: 825–830, 2001. [PubMed] [Google Scholar]
- 588.Rochira V, Faustini-Fustini M, Balestrieri A, Carani C. Estrogen replacement therapy in a man with congenital aromatase deficiency: effects of different doses of transdermal estradiol on bone mineral density and hormonal parameters. J Clin Endocrinol Metab 85: 1841–1845, 2000. doi: 10.1210/jcem.85.5.6583. [DOI] [PubMed] [Google Scholar]
- 589.Rochira V, Kara E, Carani C. The endocrine role of estrogens on human male skeleton. Int J Endocrinol 2015: 165215, 2015. doi: 10.1155/2015/165215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Rochira V, Zirilli L, Madeo B, Aranda C, Caffagni G, Fabre B, Montangero VE, Roldan EJ, Maffei L, Carani C. Skeletal effects of long-term estrogen and testosterone replacement treatment in a man with congenital aromatase deficiency: evidences of a priming effect of estrogen for sex steroids action on bone. Bone 40: 1662–1668, 2007. doi: 10.1016/j.bone.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 591.Rommerts FF, Brinkman AO. Modulation of steroidogenic activities in testis Leydig cells. Mol Cell Endocrinol 21: 15–28, 1981. doi: 10.1016/0303-7207(81)90026-5. [DOI] [PubMed] [Google Scholar]
- 592.Rosano GM, Sheiban I, Massaro R, Pagnotta P, Marazzi G, Vitale C, Mercuro G, Volterrani M, Aversa A, Fini M. Low testosterone levels are associated with coronary artery disease in male patients with angina. Int J Impot Res 19: 176–182, 2007. doi: 10.1038/sj.ijir.3901504. [DOI] [PubMed] [Google Scholar]
- 593.Royston SE, Bunick D, Mahoney MM. Oestradiol exposure early in life programs daily and circadian activity rhythms in adult mice. J Neuroendocrinol 28: 12335, 2016. doi: 10.1111/jne.12335. [DOI] [PubMed] [Google Scholar]
- 594.Royston SE, Yasui N, Kondilis AG, Lord SV, Katzenellenbogen JA, Mahoney MM. ESR1 and ESR2 differentially regulate daily and circadian activity rhythms in female mice. Endocrinology 155: 2613–2623, 2014. doi: 10.1210/en.2014-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Rubanyi GM, Freay AD, Kauser K, Sukovich D, Burton G, Lubahn DB, Couse JF, Curtis SW, Korach KS. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Invest 99: 2429–2437, 1997. doi: 10.1172/JCI119426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Rubanyi GM, Kauser K, Johns A. Role of estrogen receptors in the vascular system. Vascul Pharmacol 38: 81–88, 2002. doi: 10.1016/S0306-3623(02)00130-1. [DOI] [PubMed] [Google Scholar]
- 597.Rumi MA, Dhakal P, Kubota K, Chakraborty D, Lei T, Larson MA, Wolfe MW, Roby KF, Vivian JL, Soares MJ. Generation of Esr1-knockout rats using zinc finger nuclease-mediated genome editing. Endocrinology 155: 1991–1999, 2014. doi: 10.1210/en.2013-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Ruz R, Gregory M, Smith CE, Cyr DG, Lubahn DB, Hess RA, Hermo L. Expression of aquaporins in the efferent ductules, sperm counts, and sperm motility in estrogen receptor-alpha deficient mice fed lab chow versus casein. Mol Reprod Dev 73: 226–237, 2006. doi: 10.1002/mrd.20390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Saberwal GS, Sharma MK, Balasinor N, Choudhary J, Juneja HS. Estrogen receptor, calcium mobilization and rat sperm motility. Mol Cell Biochem 237: 11–20, 2002. doi: 10.1023/A:1016549922439. [DOI] [PubMed] [Google Scholar]
- 600.Said L, Saad A, Carreau S. Differential expression of mRNA aromatase in ejaculated spermatozoa from infertile men in relation to either asthenozoospermia or teratozoospermia. Andrologia 46: 136–146, 2014. doi: 10.1111/and.12058. [DOI] [PubMed] [Google Scholar]
- 601.Saldanha CJ, Duncan KA, Walters BJ. Neuroprotective actions of brain aromatase. Front Neuroendocrinol 30: 106–118, 2009. doi: 10.1016/j.yfrne.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Sandner F, Welter H, Schwarzer JU, Köhn FM, Urbanski HF, Mayerhofer A. Expression of the oestrogen receptor GPER by testicular peritubular cells is linked to sexual maturation and male fertility. Andrology 2: 695–701, 2014. doi: 10.1111/j.2047-2927.2014.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev 30: 343–375, 2009. doi: 10.1210/er.2008-0016. [DOI] [PubMed] [Google Scholar]
- 604.Santhi N, Lazar AS, McCabe PJ, Lo JC, Groeger JA, Dijk DJ. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc Natl Acad Sci USA 113: E2730–E2739, 2016. doi: 10.1073/pnas.1521637113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Sar M, Welsch F. Oestrogen receptor alpha and beta in rat prostate and epididymis. Andrologia 32: 295–301, 2000. doi: 10.1046/j.1439-0272.2000.00396.x. [DOI] [PubMed] [Google Scholar]
- 606.Saraswat S, Rout PK, Kharche SD, Jindal SK, Goel AK. Molecular expression of caprine estrogen receptor gene 1 in reproductive and non-reproductive tissues. Reprod Domest Anim 51: 1049–1054, 2016. doi: 10.1111/rda.12774. [DOI] [PubMed] [Google Scholar]
- 607.Sartorius G, Spasevska S, Idan A, Turner L, Forbes E, Zamojska A, Allan CA, Ly LP, Conway AJ, McLachlan RI, Handelsman DJ. Serum testosterone, dihydrotestosterone and estradiol concentrations in older men self-reporting very good health: the healthy man study. Clin Endocrinol (Oxf) 77: 755–763, 2012. doi: 10.1111/j.1365-2265.2012.04432.x. [DOI] [PubMed] [Google Scholar]
- 608.Saunders PT, Fisher JS, Sharpe RM, Millar MR. Expression of oestrogen receptor beta (ER beta) occurs in multiple cell types, including some germ cells, in the rat testis. J Endocrinol 156: R13–R17, 1998. doi: 10.1677/joe.0.156R013. [DOI] [PubMed] [Google Scholar]
- 609.Saunders PT, Millar MR, Macpherson S, Irvine DS, Groome NP, Evans LR, Sharpe RM, Scobie GA. ERbeta1 and the ERbeta2 splice variant (ERbetacx/beta2) are expressed in distinct cell populations in the adult human testis. J Clin Endocrinol Metab 87: 2706–2715, 2002. doi: 10.1210/jcem.87.6.8619. [DOI] [PubMed] [Google Scholar]
- 610.Saunders PT, Sharpe RM, Williams K, Macpherson S, Urquart H, Irvine DS, Millar MR. Differential expression of oestrogen receptor alpha and beta proteins in the testes and male reproductive system of human and non-human primates. Mol Hum Reprod 7: 227–236, 2001. doi: 10.1093/molehr/7.3.227. [DOI] [PubMed] [Google Scholar]
- 611.Savic I, Berglund H, Lindström P. Brain response to putative pheromones in homosexual men. Proc Natl Acad Sci USA 102: 7356–7361, 2005. doi: 10.1073/pnas.0407998102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Schaadt G, Hesse V, Friederici AD. Sex hormones in early infancy seem to predict aspects of later language development. Brain Lang 141: 70–76, 2015. doi: 10.1016/j.bandl.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 613.Schlegel PN. Aromatase inhibitors for male infertility. Fertil Steril 98: 1359–1362, 2012. doi: 10.1016/j.fertnstert.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 614.Schleicher G, Drews U, Stumpf WE, Sar M. Differential distribution of dihydrotestosterone and estradiol binding sites in the epididymis of the mouse. An autoradiographic study. Histochemistry 81: 139–147, 1984. doi: 10.1007/BF00490107. [DOI] [PubMed] [Google Scholar]
- 615.Schmalz B, Bilińska B. Immunolocalization of aromatase and estrogen receptors in ram Leydig cells. Ginekol Pol 69: 512–516, 1998. [PubMed] [Google Scholar]
- 616.Schmalz B, Kozieł E, Bilińska B. Immunolocalization of aromatase and estrogen receptors in bank vole Leydig cells. Folia Histochem Cytobiol 37: 89–90, 1999. [PubMed] [Google Scholar]
- 617.Schön J, Neumann S, Wildt DE, Pukazhenthi BS, Jewgenow K. Localization of oestrogen receptors in the epididymis during sexual maturation of the domestic cat. Reprod Domest Anim 44, Suppl 2: 294–301, 2009. doi: 10.1111/j.1439-0531.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- 618.Schulze H, Barrack ER. Immunocytochemical localization of estrogen receptors in the normal male and female canine urinary tract and prostate. Endocrinology 121: 1773–1783, 1987. doi: 10.1210/endo-121-5-1773. [DOI] [PubMed] [Google Scholar]
- 620.Schulze H, Claus S. Histological localization of estrogen receptors in normal and diseased human prostates by immunocytochemistry. Prostate 16: 331–343, 1990. doi: 10.1002/pros.2990160408. [DOI] [PubMed] [Google Scholar]
- 621.Schweikert HU, Milewich L, Wilson JD. Aromatization of androstenedione by isolated human hairs. J Clin Endocrinol Metab 40: 413–417, 1975. doi: 10.1210/jcem-40-3-413. [DOI] [PubMed] [Google Scholar]
- 622.Scobie GA, Macpherson S, Millar MR, Groome NP, Romana PG, Saunders PT. Human oestrogen receptors: differential expression of ER alpha and beta and the identification of ER beta variants. Steroids 67: 985–992, 2002. doi: 10.1016/S0039-128X(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 623.Setchell BP, Cox JE. Secretion of free and conjugated steroids by the horse testis into lymph and venous blood. J Reprod Fertil Suppl 32: 123–127, 1982. [PubMed] [Google Scholar]
- 624.Setchell BP, Laurie MS, Flint AP, Heap RB. Transport of free and conjugated steroids from the boar testis in lymph, venous blood and rete testis fluid. J Endocrinol 96: 127–136, 1983. doi: 10.1677/joe.0.0960127. [DOI] [PubMed] [Google Scholar]
- 625.Setlur SR, Mertz KD, Hoshida Y, Demichelis F, Lupien M, Perner S, Sboner A, Pawitan Y, Andrén O, Johnson LA, Tang J, Adami HO, Calza S, Chinnaiyan AM, Rhodes D, Tomlins S, Fall K, Mucci LA, Kantoff PW, Stampfer MJ, Andersson SO, Varenhorst E, Johansson JE, Brown M, Golub TR, Rubin MA. Estrogen-dependent signaling in a molecularly distinct subclass of aggressive prostate cancer. J Natl Cancer Inst 100: 815–825, 2008. doi: 10.1093/jnci/djn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Shapiro E, Huang H, Masch RJ, McFadden DE, Wilson EL, Wu XR. Immunolocalization of estrogen receptor alpha and beta in human fetal prostate. J Urol 174: 2051–2053, 2005. doi: 10.1097/01.ju.0000176472.90432.5b. [DOI] [PubMed] [Google Scholar]
- 627.Shapiro E, Huang H, Masch RJ, McFadden DE, Wu XR, Ostrer H. Immunolocalization of androgen receptor and estrogen receptors alpha and beta in human fetal testis and epididymis. J Urol 174: 1695–1698, 2005. doi: 10.1097/01.ju.0000179540.28209.de. [DOI] [PubMed] [Google Scholar]
- 628.Sharma G, Hu C, Brigman JL, Zhu G, Hathaway HJ, Prossnitz ER. GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology 154: 4136–4145, 2013. doi: 10.1210/en.2013-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Shayu D, Hardy MP, Rao AJ. Delineating the role of estrogen in regulating epididymal gene expression. Soc Reprod Fertil Suppl 63: 31–43, 2007. [PubMed] [Google Scholar]
- 630.Shayu D, Rao AJ. Expression of functional aromatase in the epididymis: role of androgens and LH in modulation of expression and activity. Mol Cell Endocrinol 249: 40–50, 2006. doi: 10.1016/j.mce.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 631.Shen M, Shi H. Sex hormones and their receptors regulate liver energy homeostasis. Int J Endocrinol 2015: 294278, 2015. doi: 10.1155/2015/294278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Shibayama T, Fukata H, Sakurai K, Adachi T, Komiyama M, Iguchi T, Mori C. Neonatal exposure to genistein reduces expression of estrogen receptor alpha and androgen receptor in testes of adult mice. Endocr J 48: 655–663, 2001. doi: 10.1507/endocrj.48.655. [DOI] [PubMed] [Google Scholar]
- 633.Shihara D, Miyado M, Nakabayashi K, Shozu M, Ogata T, Nagasaki K, Fukami M. Aromatase excess syndrome in a family with upstream deletion of CYP19A1. Clin Endocrinol (Oxf) 81: 314–316, 2014. doi: 10.1111/cen.12329. [DOI] [PubMed] [Google Scholar]
- 634.Shozu M, Fukami M, Ogata T. Understanding the pathological manifestations of aromatase excess syndrome: lessons for clinical diagnosis. Expert Rev Endocrinol Metab 9: 397–409, 2014. doi: 10.1586/17446651.2014.926810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Shozu M, Sebastian S, Takayama K, Hsu WT, Schultz RA, Neely K, Bryant M, Bulun SE. Estrogen excess associated with novel gain-of-function mutations affecting the aromatase gene. N Engl J Med 348: 1855–1865, 2003. doi: 10.1056/NEJMoa021559. [DOI] [PubMed] [Google Scholar]
- 636.Shughrue PJ, Lane MV, Scrimo PJ, Merchenthaler I. Comparative distribution of estrogen receptor-alpha (ER-alpha) and beta (ER-beta) mRNA in the rat pituitary, gonad, and reproductive tract. Steroids 63: 498–504, 1998. doi: 10.1016/S0039-128X(98)00054-3. [DOI] [PubMed] [Google Scholar]
- 637.Sierens JE, Sneddon SF, Collins F, Millar MR, Saunders PT. Estrogens in testis biology. Ann NY Acad Sci 1061: 65–76, 2005. doi: 10.1196/annals.1336.008. [DOI] [PubMed] [Google Scholar]
- 638.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 25: 507–536, 2002. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 639.Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol 86: 225–230, 2003. doi: 10.1016/S0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 640.Sims NA, Clément-Lacroix P, Minet D, Fraslon-Vanhulle C, Gaillard-Kelly M, Resche-Rigon M, Baron R. A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J Clin Invest 111: 1319–1327, 2003. doi: 10.1172/JCI200317246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, Resche-Rigon M, Gaillard-Kelly M, Baron R. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone 30: 18–25, 2002. doi: 10.1016/S8756-3282(01)00643-3. [DOI] [PubMed] [Google Scholar]
- 642.Sinkevicius KW, Laine M, Lotan TL, Woloszyn K, Richburg JH, Greene GL. Estrogen-dependent and -independent estrogen receptor-alpha signaling separately regulate male fertility. Endocrinology 150: 2898–2905, 2009. doi: 10.1210/en.2008-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Sipahutar H, Sourdaine P, Moslemi S, Plainfossé B, Séralini GE. Immunolocalization of aromatase in stallion Leydig cells and seminiferous tubules. J Histochem Cytochem 51: 311–318, 2003. doi: 10.1177/002215540305100306. [DOI] [PubMed] [Google Scholar]
- 644.Sipilä S, Taaffe DR, Cheng S, Puolakka J, Toivanen J, Suominen H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clin Sci (Lond) 101: 147–157, 2001. doi: 10.1042/cs1010147. [DOI] [PubMed] [Google Scholar]
- 645.Sirianni R, Chimento A, Ruggiero C, De Luca A, Lappano R, Andò S, Maggiolini M, Pezzi V. The novel estrogen receptor, G protein-coupled receptor 30, mediates the proliferative effects induced by 17beta-estradiol on mouse spermatogonial GC-1 cell line. Endocrinology 149: 5043–5051, 2008. doi: 10.1210/en.2007-1593. [DOI] [PubMed] [Google Scholar]
- 646.Skavdahl M, Steenbergen C, Clark J, Myers P, Demianenko T, Mao L, Rockman HA, Korach KS, Murphy E. Estrogen receptor-beta mediates male-female differences in the development of pressure overload hypertrophy. Am J Physiol Heart Circ Physiol 288: H469–H476, 2005. doi: 10.1152/ajpheart.00723.2004. [DOI] [PubMed] [Google Scholar]
- 647.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331: 1056–1061, 1994. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 648.Smith EP, Specker B, Bachrach BE, Kimbro KS, Li XJ, Young MF, Fedarko NS, Abuzzahab MJ, Frank GR, Cohen RM, Lubahn DB, Korach KS. Impact on bone of an estrogen receptor-alpha gene loss of function mutation. J Clin Endocrinol Metab 93: 3088–3096, 2008. doi: 10.1210/jc.2007-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Smith EP, Specker B, Korach KS. Recent experimental and clinical findings in the skeleton associated with loss of estrogen hormone or estrogen receptor activity. J Steroid Biochem Mol Biol 118: 264–272, 2010. doi: 10.1016/j.jsbmb.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab 89: 3841–3846, 2004. doi: 10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 651.Smith MR, Malkowicz SB, Brawer MK, Hancock ML, Morton RA, Steiner MS. Toremifene decreases vertebral fractures in men younger than 80 years receiving androgen deprivation therapy for prostate cancer. J Urol 186: 2239–2244, 2011. doi: 10.1016/j.juro.2011.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Sneddon SF, Walther N, Saunders PT. Expression of androgen and estrogen receptors in Sertoli cells: studies using the mouse SK11 cell line. Endocrinology 146: 5304–5312, 2005. doi: 10.1210/en.2005-0914. [DOI] [PubMed] [Google Scholar]
- 653.Sobel V, Schwartz B, Zhu YS, Cordero JJ, Imperato-McGinley J. Bone mineral density in the complete androgen insensitivity and 5alpha-reductase-2 deficiency syndromes. J Clin Endocrinol Metab 91: 3017–3023, 2006. doi: 10.1210/jc.2005-2809. [DOI] [PubMed] [Google Scholar]
- 654.Socorro S, Power DM, Olsson PE, Canario AV. Two estrogen receptors expressed in the teleost fish, Sparus aurata: cDNA cloning, characterization and tissue distribution. J Endocrinol 166: 293–306, 2000. doi: 10.1677/joe.0.1660293. [DOI] [PubMed] [Google Scholar]
- 655.Sodersten P. Steinach and Young, discoverers of the effects of estrogen on male sexual behavior and the “male brain”. eNeuro 2: 1–11, 2015. doi: 10.1523/ENEURO.0058-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Soloff MS, Szego CM. Purification of estradiol receptor from rat uterus and blockade of its estrogen-binding function by specific antibody. Biochem Biophys Res Commun 34: 141–147, 1969. doi: 10.1016/0006-291X(69)90540-3. [DOI] [PubMed] [Google Scholar]
- 657.Song WC. Biochemistry and reproductive endocrinology of estrogen sulfotransferase. Ann NY Acad Sci 948: 43–50, 2001. doi: 10.1111/j.1749-6632.2001.tb03985.x. [DOI] [PubMed] [Google Scholar]
- 658.Song WC, Moore R, McLachlan JA, Negishi M. Molecular characterization of a testis-specific estrogen sulfotransferase and aberrant liver expression in obese and diabetogenic C57BL/KsJ-db/db mice. Endocrinology 136: 2477–2484, 1995. [DOI] [PubMed] [Google Scholar]
- 659.Soustiel JF, Palzur E, Nevo O, Thaler I, Vlodavsky E. Neuroprotective anti-apoptosis effect of estrogens in traumatic brain injury. J Neurotrauma 22: 345–352, 2005. doi: 10.1089/neu.2005.22.345. [DOI] [PubMed] [Google Scholar]
- 660.Spearow JL, O'Henley P, Doemeny P, Sera R, Leffler R, Sofos T, Barkley M. Genetic variation in physiological sensitivity to estrogen in mice. APMIS 109: 356–364, 2001. [DOI] [PubMed] [Google Scholar]
- 661.Stanić D, Dubois S, Chua HK, Tonge B, Rinehart N, Horne MK, Boon WC. Characterization of aromatase expression in the adult male and female mouse brain. I. Coexistence with oestrogen receptors α and β, and androgen receptors. PLoS One 9: e90451, 2014. doi: 10.1371/journal.pone.0090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Stratakis CA. An aroma of complexity: how the unique genetics of aromatase (CYP19A1) explain diverse phenotypes from hens and hyenas to human gynecomastia, and testicular and other tumors. J Clin Endocrinol Metab 98: 4676–4681, 2013. doi: 10.1210/jc.2013-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Stumpf WE. Probable sites for estrogen receptors in brain and pituitary. J Neurovisc Relat 10, Suppl: 51–64, 1971. [DOI] [PubMed] [Google Scholar]
- 664.Stumpf WE, Narbaitz R, Sar M. Estrogen receptors in the fetal mouse. J Steroid Biochem 12: 55–64, 1980. doi: 10.1016/0022-4731(80)90250-2. [DOI] [PubMed] [Google Scholar]
- 665.Stumpf WE, Sar M. Autoradiographic localization of estrogen, androgen, progestin, and glucocorticoid in ‘target tissues” and “non-target” tissues. In: Receptors and Mechanism of Action of Steroid Hormones, edited by Pasqualini J. New York: Dekker, 1976, p. 41–84. [Google Scholar]
- 666.Sudhir K, Chou TM, Chatterjee K, Smith EP, Williams TC, Kane JP, Malloy MJ, Korach KS, Rubanyi GM. Premature coronary artery disease associated with a disruptive mutation in the estrogen receptor gene in a man. Circulation 96: 3774–3777, 1997. doi: 10.1161/01.CIR.96.10.3774. [DOI] [PubMed] [Google Scholar]
- 667.Sudhir K, Chou TM, Messina LM, Hutchison SJ, Korach KS, Chatterjee K, Rubanyi GM. Endothelial dysfunction in a man with disruptive mutation in oestrogen-receptor gene. Lancet 349: 1146–1147, 1997. doi: 10.1016/S0140-6736(05)63022-X. [DOI] [PubMed] [Google Scholar]
- 668.Sun D, Yan C, Jacobson A, Jiang H, Carroll MA, Huang A. Contribution of epoxyeicosatrienoic acids to flow-induced dilation in arteries of male ERalpha knockout mice: role of aromatase. Am J Physiol Regul Integr Comp Physiol 293: R1239–R1246, 2007. doi: 10.1152/ajpregu.00185.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Suzuki Y, Sasagawa I, Itoh K, Ashida J, Muroya K, Ogata T. Estrogen receptor alpha gene polymorphism is associated with idiopathic azoospermia. Fertil Steril 78: 1341–1343, 2002. doi: 10.1016/S0015-0282(02)04267-X. [DOI] [PubMed] [Google Scholar]
- 670.Swaab DF, Hofman MA. Sexual differentiation of the human hypothalamus: ontogeny of the sexually dimorphic nucleus of the preoptic area. Brain Res Dev Brain Res 44: 314–318, 1988. doi: 10.1016/0165-3806(88)90231-3. [DOI] [PubMed] [Google Scholar]
- 671.Swelheim T. The influence of a single high dose of oestradiol benzoate on the Icsh-content in the serum of gonadectomized male and female rats. Acta Endocrinol (Copenh) 49: 231–238, 1965. [DOI] [PubMed] [Google Scholar]
- 673.Takao T, Nanamiya W, Nazarloo HP, Matsumoto R, Asaba K, Hashimoto K. Exposure to the environmental estrogen bisphenol A differentially modulated estrogen receptor-alpha and -beta immunoreactivity and mRNA in male mouse testis. Life Sci 72: 1159–1169, 2003. doi: 10.1016/S0024-3205(02)02364-0. [DOI] [PubMed] [Google Scholar]
- 674.Tang WY, Morey LM, Cheung YY, Birch L, Prins GS, Ho SM. Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs of Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology 153: 42–55, 2012. doi: 10.1210/en.2011-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Tchernof A, Calles-Escandon J, Sites CK, Poehlman ET. Menopause, central body fatness, and insulin resistance: effects of hormone-replacement therapy. Coron Artery Dis 9: 503–511, 1998. doi: 10.1097/00019501-199809080-00006. [DOI] [PubMed] [Google Scholar]
- 676.Telgmann R, Brosens JJ, Käppler-Hanno K, Ivell R, Kirchhoff C. Epididymal epithelium immortalized by simian virus 40 large T antigen: a model to study epididymal gene expression. Mol Hum Reprod 7: 935–945, 2001. doi: 10.1093/molehr/7.10.935. [DOI] [PubMed] [Google Scholar]
- 677.Tena-Sempere M, Navarro J, Pinilla L, González LC, Huhtaniemi I, Aguilar E. Neonatal exposure to estrogen differentially alters estrogen receptor alpha and beta mRNA expression in rat testis during postnatal development. J Endocrinol 165: 345–357, 2000. doi: 10.1677/joe.0.1650345. [DOI] [PubMed] [Google Scholar]
- 678.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146: 624–632, 2005. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 679.Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional β-cell mass in diabetes. Nat Rev Endocrinol 8: 342–351, 2012. doi: 10.1038/nrendo.2011.242. [DOI] [PubMed] [Google Scholar]
- 680.Tilley WD, Horsfall DJ, Skinner JM, Henderson DW, Marshall VR. Effect of pubertal development on estrogen receptor levels and stromal morphology in the guinea pig prostate. Prostate 15: 195–210, 1989. doi: 10.1002/pros.2990150213. [DOI] [PubMed] [Google Scholar]
- 681.Tirado OM, Selva DM, Toràn N, Suárez-Quian CA, Jansen M, McDonnell DP, Reventós J, Munell F. Increased expression of estrogen receptor beta in pachytene spermatocytes after short-term methoxyacetic acid administration. J Androl 25: 84–94, 2004. doi: 10.1002/j.1939-4640.2004.tb02762.x. [DOI] [PubMed] [Google Scholar]
- 682.Toda K, Okada T, Hayashi Y, Saibara T. Preserved tissue structure of efferent ductules in aromatase-deficient mice. J Endocrinol 199: 137–146, 2008. doi: 10.1677/JOE-08-0257. [DOI] [PubMed] [Google Scholar]
- 683.Tong MH, Song WC. Estrogen sulfotransferase: discrete and androgen-dependent expression in the male reproductive tract and demonstration of an in vivo function in the mouse epididymis. Endocrinology 143: 3144–3151, 2002. doi: 10.1210/endo.143.8.8943. [DOI] [PubMed] [Google Scholar]
- 684.Toung TJ, Traystman RJ, Hurn PD, Miller VM. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke 29: 1666–1670, 1998. doi: 10.1161/01.STR.29.8.1666. [DOI] [PubMed] [Google Scholar]
- 685.Trépos-Pouplard M, Lardenois A, Staub C, Guitton N, Dorval-Coiffec I, Pineau C, Primig M, Jégou B. Proteome analysis and genome-wide regulatory motif prediction identify novel potentially sex-hormone regulated proteins in rat efferent ducts. Int J Androl 33: 661–674, 2010. doi: 10.1111/j.1365-2605.2009.01006.x. [DOI] [PubMed] [Google Scholar]
- 686.Tsai-Morris CH, Aquilano DR, Dufau ML. Gonadotropic regulation of aromatase activity in the adult rat testis. Endocrinology 116: 31–37, 1985. doi: 10.1210/endo-116-1-31. [DOI] [PubMed] [Google Scholar]
- 687.Tsai-Morris CH, Knox G, Luna S, Dufau ML. Acquisition of estradiol-mediated regulatory mechanism of steroidogenesis in cultured fetal rat Leydig cells. J Biol Chem 261: 3471–3474, 1986. [PubMed] [Google Scholar]
- 688.Tsai YH, Steinberger A. Effect of sodium molybdate on the binding of androgen-receptor complexes to germ cell and Sertoli cell chromatin. J Steroid Biochem 17: 131–136, 1982. doi: 10.1016/0022-4731(82)90111-X. [DOI] [PubMed] [Google Scholar]
- 689.Tse J, Martin-McNaulty B, Halks-Miller M, Kauser K, DelVecchio V, Vergona R, Sullivan ME, Rubanyi GM. Accelerated atherosclerosis and premature calcified cartilaginous metaplasia in the aorta of diabetic male Apo E knockout mice can be prevented by chronic treatment with 17 beta-estradiol. Atherosclerosis 144: 303–313, 1999. doi: 10.1016/S0021-9150(98)00325-6. [DOI] [PubMed] [Google Scholar]
- 690.Tsubota T, Howell-Skalla L, Nitta H, Osawa Y, Mason JI, Meiers PG, Nelson RA, Bahr JM. Seasonal changes in spermatogenesis and testicular steroidogenesis in the male black bear Ursus americanus. J Reprod Fertil 109: 21–27, 1997. doi: 10.1530/jrf.0.1090021. [DOI] [PubMed] [Google Scholar]
- 691.Tsurusaki T, Aoki D, Kanetake H, Inoue S, Muramatsu M, Hishikawa Y, Koji T. Zone-dependent expression of estrogen receptors alpha and beta in human benign prostatic hyperplasia. J Clin Endocrinol Metab 88: 1333–1340, 2003. doi: 10.1210/jc.2002-021015. [DOI] [PubMed] [Google Scholar]
- 692.Turner KJ, Macpherson S, Millar MR, McNeilly AS, Williams K, Cranfield M, Groome NP, Sharpe RM, Fraser HM, Saunders PT. Development and validation of a new monoclonal antibody to mammalian aromatase. J Endocrinol 172: 21–30, 2002. doi: 10.1677/joe.0.1720021. [DOI] [PubMed] [Google Scholar]
- 693.Turner KJ, Morley M, Atanassova N, Swanston ID, Sharpe RM. Effect of chronic administration of an aromatase inhibitor to adult male rats on pituitary and testicular function and fertility. J Endocrinol 164: 225–238, 2000. doi: 10.1677/joe.0.1640225. [DOI] [PubMed] [Google Scholar]
- 694.Ucer S, Iyer S, Bartell SM, Martin-Millan M, Han L, Kim HN, Weinstein RS, Jilka RL, O’Brien CA, Almeida M, Manolagas SC. The effects of androgens on murine cortical bone do not require AR or ERalpha signaling in osteoblasts and osteoclasts. J Bone Miner Res 30: 1138–1149, 2015. doi: 10.1002/jbmr.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Uebi T, Umeda M, Imai T. Estrogen induces estrogen receptor alpha expression and hepatocyte proliferation in the livers of male mice. Genes Cells 20: 217–223, 2015. doi: 10.1111/gtc.12214. [DOI] [PubMed] [Google Scholar]
- 696.Unger EK, Burke KJ Jr, Yang CF, Bender KJ, Fuller PM, Shah NM. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Reports 10: 453–462, 2015. doi: 10.1016/j.celrep.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Upadhyay RD, Kumar AV, Sonawane S, Gaonkar R, Balasinor NH. Estrogen effects on actin cytoskeletal and endocytic proteins associated with tubulobulbar complex disruption in rat testes. Reprod Sci 20: 1162–1174, 2013. doi: 10.1177/1933719113477491. [DOI] [PubMed] [Google Scholar]
- 698.Vaillant S, Dorizzi M, Pieau C, Richard-Mercier N. Sex reversal and aromatase in chicken. J Exp Zool 290: 727–740, 2001. doi: 10.1002/jez.1123. [DOI] [PubMed] [Google Scholar]
- 699.Valladares LE, Payne AH. Induction of testicular aromatization by luteinizing hormone in mature rats. Endocrinology 105: 431–436, 1979. doi: 10.1210/endo-105-2-431. [DOI] [PubMed] [Google Scholar]
- 700.Van der Molen HJ, Brinkmann AO, de Jong FH, Rommerts FF. Testicular oestrogens. J Endocrinol 89, Suppl: 33P–46P, 1981. [PubMed] [Google Scholar]
- 701.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33: 378–455, 2012. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Vandenput L, Ederveen AG, Erben RG, Stahr K, Swinnen JV, Van Herck E, Verstuyf A, Boonen S, Bouillon R, Vanderschueren D. Testosterone prevents orchidectomy-induced bone loss in estrogen receptor-alpha knockout mice. Biochem Biophys Res Commun 285: 70–76, 2001. doi: 10.1006/bbrc.2001.5101. [DOI] [PubMed] [Google Scholar]
- 703.Vanderschueren D, Laurent MR, Claessens F, Gielen E, Lagerquist MK, Vandenput L, Börjesson AE, Ohlsson C. Sex steroid actions in male bone. Endocr Rev 35: 906–960, 2014. doi: 10.1210/er.2014-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Vanselow J, Fürbass R, Zsolnai A, Kalbe C, Said HM, Schwerin M. Expression of the aromatase cytochrome P450 encoding gene in cattle and sheep. J Steroid Biochem Mol Biol 79: 279–288, 2001. doi: 10.1016/S0960-0760(01)00144-3. [DOI] [PubMed] [Google Scholar]
- 705.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419: 624–629, 2002. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 706.Velickovic K, Cvoro A, Srdic B, Stokic E, Markelic M, Golic I, Otasevic V, Stancic A, Jankovic A, Vucetic M, Buzadzic B, Korac B, Korac A. Expression and subcellular localization of estrogen receptors α and β in human fetal brown adipose tissue. J Clin Endocrinol Metab 99: 151–159, 2014. doi: 10.1210/jc.2013-2017. [DOI] [PubMed] [Google Scholar]
- 707.Verderame M, Angelini F, Limatola E. Expression of estrogen receptor alpha switches off secretory activity in the epididymal channel of the lizard Podarcis sicula. Mol Reprod Dev 79: 107–117, 2012. doi: 10.1002/mrd.22005. [DOI] [PubMed] [Google Scholar]
- 708.Vermeulen A, Kaufman JM, Goemaere S, van Pottelberg I. Estradiol in elderly men. Aging Male 5: 98–102, 2002. doi: 10.1080/tam.5.2.98.102. [DOI] [PubMed] [Google Scholar]
- 709.Vinel A, Hay E, Valera MC, Buscato M, Adlanmerini M, Guillaume M, Cohen-Solal M, Ohlsson C, Lenfant F, Arnal JF, Fontaine C. Role of ERalpha in the effect of estradiol on cancellous and cortical femoral bone in growing female mice. Endocrinology 157: 2533–2544, 2016. doi: 10.1210/en.2015-1994. [DOI] [PubMed] [Google Scholar]
- 710.Vitale C, Mendelsohn ME, Rosano GM. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol 6: 532–542, 2009. doi: 10.1038/nrcardio.2009.105. [DOI] [PubMed] [Google Scholar]
- 711.Vitkus S, Yeh CR, Lin HH, Hsu I, Yu J, Chen M, Yeh S. Distinct function of estrogen receptor α in smooth muscle and fibroblast cells in prostate development. Mol Endocrinol 27: 38–49, 2013. doi: 10.1210/me.2012-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, Dhar MD, Ganjam VK, Parmigiani S, Welshons WV. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci USA 94: 2056–2061, 1997. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Vornehm ND, Wang M, Abarbanell A, Herrmann J, Weil B, Tan J, Wang Y, Kelly M, Meldrum DR. Acute postischemic treatment with estrogen receptor-alpha agonist or estrogen receptor-beta agonist improves myocardial recovery. Surgery 146: 145–154, 2009. doi: 10.1016/j.surg.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Wade GN, Gray JM. Cytoplasmic 17 beta-[3H]estradiol binding in rat adipose tissues. Endocrinology 103: 1695–1701, 1978. doi: 10.1210/endo-103-5-1695. [DOI] [PubMed] [Google Scholar]
- 715.Wade GN, Gray JM, Bartness TJ. Gonadal influences on adiposity. Int J Obes 9, Suppl 1: 83–92, 1985. [PubMed] [Google Scholar]
- 716.Waites GM, Einer-Jensen N. Collection and analysis of rete testis fluid from macaque monkeys. J Reprod Fertil 41: 505–508, 1974. doi: 10.1530/jrf.0.0410505. [DOI] [PubMed] [Google Scholar]
- 717.Wakeling AE, Dukes M, Chester R, Yarwood L, Wakeling AE. Use of pure antioestrogens to elucidate the mode of action of oestrogens. Biochem Pharmacol 49: 1545–1549, 1995. doi: 10.1016/0006-2952(94)00528-T. [DOI] [PubMed] [Google Scholar]
- 718.Wakui S, Shirai M, Motohashi M, Mutou T, Oyama N, Wempe MF, Takahashi H, Inomata T, Ikegami M, Endou H, Asari M. Effects of in utero exposure to di(n-butyl) phthalate for estrogen receptors α, β, and androgen receptor of Leydig cell on rats. Toxicol Pathol 42: 877–887, 2014. doi: 10.1177/0192623313502879. [DOI] [PubMed] [Google Scholar]
- 719.Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, Rosenbaum JS, Vandenbark AA, Offner H. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol 22: 636–648, 2008. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Wang Q, Trevino LS, Wong RL, Medvedovic M, Chen J, Ho SM, Shen J, Foulds CE, Coarfa C, O’Malley BW, Shilatifard A, Walker CL. Reprogramming of the epigenome by MLL1 links early-life environmental exposures to prostate cancer risk. Mol Endocrinol 30: 856–871, 2016. doi: 10.1210/me.2015-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Wang R, Zhang QG, Han D, Xu J, Lü Q, Zhang GY. Inhibition of MLK3-MKK4/7-JNK1/2 pathway by Akt1 in exogenous estrogen-induced neuroprotection against transient global cerebral ischemia by a non-genomic mechanism in male rats. J Neurochem 99: 1543–1554, 2006. doi: 10.1111/j.1471-4159.2006.04201.x. [DOI] [PubMed] [Google Scholar]
- 722.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem Biophys Res Commun 336: 1023–1027, 2005. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- 723.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-alpha, hER-alpha36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci USA 103: 9063–9068, 2006. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Wang ZJ, Jeffs B, Ito M, Achermann JC, Yu RN, Hales DB, Jameson JL. Aromatase (Cyp19) expression is up-regulated by targeted disruption of Dax1. Proc Natl Acad Sci USA 98: 7988–7993, 2001. doi: 10.1073/pnas.141543298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.Watson CS, Jeng YJ, Kochukov MY. Nongenomic signaling pathways of estrogen toxicity. Toxicol Sci 115: 1–11, 2010. doi: 10.1093/toxsci/kfp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726.Weber TJ. Battle of the sex steroids in the male skeleton: and the winner is..... J Clin Invest 126: 829–832, 2016. doi: 10.1172/JCI85006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Weihua Z, Makela S, Andersson LC, Salmi S, Saji S, Webster JI, Jensen EV, Nilsson S, Warner M, Gustafsson JA. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc Natl Acad Sci USA 98: 6330–6335, 2001. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Weil BR, Manukyan MC, Herrmann JL, Wang Y, Abarbanell AM, Poynter JA, Meldrum DR. Signaling via GPR30 protects the myocardium from ischemia/reperfusion injury. Surgery 148: 436–443, 2010. doi: 10.1016/j.surg.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 729.Weis KE, Ekena K, Thomas JA, Lazennec G, Katzenellenbogen BS. Constitutively active human estrogen receptors containing amino acid substitutions for tyrosine 537 in the receptor protein. Mol Endocrinol 10: 1388–1398, 1996. [DOI] [PubMed] [Google Scholar]
- 730.Weisburger JH, Yamamoto RS, Korzis J, Weisburger EK. Liver cancer: neonatal estrogen enhances induction by a carcinogen. Science 154: 673–674, 1966. doi: 10.1126/science.154.3749.673. [DOI] [PubMed] [Google Scholar]
- 731.Weiss J, Bernhardt ML, Laronda MM, Hurley LA, Glidewell-Kenney C, Pillai S, Tong M, Korach KS, Jameson JL. Estrogen actions in the male reproductive system involve estrogen response element-independent pathways. Endocrinology 149: 6198–6206, 2008. doi: 10.1210/en.2008-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Weng Q, Medan MS, Watanabe G, Tsubota T, Tanioka Y, Taya K. Immunolocalization of steroidogenic enzymes P450scc, 3betaHSD, P450c17, and P450arom in Göttingen miniature pig testes. J Reprod Dev 51: 299–304, 2005. doi: 10.1262/jrd.16077. [DOI] [PubMed] [Google Scholar]
- 733.Weng Q, Tsubota T, Dai M, Weng J, Tian Y, Xu M, Watanabe G, Taya K. Immunolocalization of steroidogenic enzymes and their expression during the breeding season in the testes of wild raccoon dogs (Nyctereutes procyonoides). Anim Sci J 83: 535–542, 2012. doi: 10.1111/j.1740-0929.2011.00990.x. [DOI] [PubMed] [Google Scholar]
- 734.Weniger J-P, Zeis A. [Induction of estrogen production in the embryonic chicken testicle by dihydrotestosterone]. Biochimie 55: 1163–1164, 1973. doi: 10.1016/S0300-9084(73)80456-0. [DOI] [PubMed] [Google Scholar]
- 735.Weniger JP, Zeis A. Oestrogen synthesis by the foetal rat testis in organ culture. J Steroid Biochem 28: 307–310, 1987. doi: 10.1016/0022-4731(87)91023-5. [DOI] [PubMed] [Google Scholar]
- 736.Weniger JP, Zeis A. Stimulation of aromatase activity in the fetal rat testis by cyclic AMP and FSH. J Endocrinol 118: 485–489, 1988. doi: 10.1677/joe.0.1180485. [DOI] [PubMed] [Google Scholar]
- 737.Wiik A, Ekman M, Johansson O, Jansson E, Esbjörnsson M. Expression of both oestrogen receptor alpha and beta in human skeletal muscle tissue. Histochem Cell Biol 131: 181–189, 2009. doi: 10.1007/s00418-008-0512-x. [DOI] [PubMed] [Google Scholar]
- 738.Wiik A, Gustafsson T, Esbjörnsson M, Johansson O, Ekman M, Sundberg CJ, Jansson E. Expression of oestrogen receptor alpha and beta is higher in skeletal muscle of highly endurance-trained than of moderately active men. Acta Physiol Scand 184: 105–112, 2005. doi: 10.1111/j.1365-201X.2005.01433.x. [DOI] [PubMed] [Google Scholar]
- 739.Wilhelm D, Koopman P. The makings of maleness: towards an integrated view of male sexual development. Nat Rev Genet 7: 620–631, 2006. doi: 10.1038/nrg1903. [DOI] [PubMed] [Google Scholar]
- 740.Windahl SH, Andersson G, Gustafsson JA. Elucidation of estrogen receptor function in bone with the use of mouse models. Trends Endocrinol Metab 13: 195–200, 2002. doi: 10.1016/S1043-2760(02)00594-5. [DOI] [PubMed] [Google Scholar]
- 741.Windahl SH, Börjesson AE, Farman HH, Engdahl C, Movérare-Skrtic S, Sjögren K, Lagerquist MK, Kindblom JM, Koskela A, Tuukkanen J, Divieti Pajevic P, Feng JQ, Dahlman-Wright K, Antonson P, Gustafsson JA, Ohlsson C. Estrogen receptor-α in osteocytes is important for trabecular bone formation in male mice. Proc Natl Acad Sci USA 110: 2294–2299, 2013. doi: 10.1073/pnas.1220811110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Windahl SH, Hollberg K, Vidal O, Gustafsson JA, Ohlsson C, Andersson G. Female estrogen receptor beta-/- mice are partially protected against age-related trabecular bone loss. J Bone Miner Res 16: 1388–1398, 2001. doi: 10.1359/jbmr.2001.16.8.1388. [DOI] [PubMed] [Google Scholar]
- 743.Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(-/-) mice. J Clin Invest 104: 895–901, 1999. doi: 10.1172/JCI6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Wiszniewska B. Primary culture of the rat epididymal epithelial cells as a source of oestrogen. Andrologia 34: 180–187, 2002. doi: 10.1046/j.1439-0272.2002.00495.x. [DOI] [PubMed] [Google Scholar]
- 745.Wong RL, Walker CL. Molecular pathways: environmental estrogens activate nongenomic signaling to developmentally reprogram the epigenome. Clin Cancer Res 19: 3732–3737, 2013. doi: 10.1158/1078-0432.CCR-13-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Wu C, Patiño R, Davis KB, Chang X. Localization of estrogen receptor alpha and beta RNA in germinal and nongerminal epithelia of the channel catfish testis. Gen Comp Endocrinol 124: 12–20, 2001. doi: 10.1006/gcen.2001.7668. [DOI] [PubMed] [Google Scholar]
- 747.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366–376, 2012. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Wu L, Dong H, Zhao J, Wang Y, Yang Q, Jia C, Ma J. Diosgenin stimulates rat TM4 cell proliferation through activating plasma membrane translocation and transcriptional activity of estrogen receptors. Biol Reprod 92: 24, 2015. doi: 10.1095/biolreprod.114.124206. [DOI] [PubMed] [Google Scholar]
- 749.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 14: 453–465, 2011. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750.Yalow RS, Berson SA. Immunoassay of endogenous plasma insulin in man. J Clin Invest 39: 1157–1175, 1960. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751.Yamashita S. Localization of estrogen and androgen receptors in male reproductive tissues of mice and rats. Anat Rec A Discov Mol Cell Evol Biol 279: 768–778, 2004. doi: 10.1002/ar.a.20061. [DOI] [PubMed] [Google Scholar]
- 752.Yang WR, Wang Y, Wang Y, Zhang JJ, Zhang JH, Lu C, Wang XZ. mTOR is involved in 17β-estradiol-induced, cultured immature boar Sertoli cell proliferation via regulating the expression of SKP2, CCND1, and CCNE1. Mol Reprod Dev 82: 305–314, 2015. doi: 10.1002/mrd.22473. [DOI] [PubMed] [Google Scholar]
- 753.Yang WR, Zhu FW, Zhang JJ, Wang Y, Zhang JH, Lu C, Wang XZ. PI3K/Akt activated by GPR30 and Src regulates 17beta-estradiol-induced cultured immature boar Sertoli cells proliferation. Reprod Sci 24: 57–66, 2016. doi: 10.1177/1933719116649696. [DOI] [PubMed] [Google Scholar]
- 754.Yasuhara F, Gomes GR, Siu ER, Suenaga CI, Maróstica E, Porto CS, Lazari MF. Effects of the antiestrogen fulvestrant (ICI 182,780) on gene expression of the rat efferent ductules. Biol Reprod 79: 432–441, 2008. doi: 10.1095/biolreprod.107.067413. [DOI] [PubMed] [Google Scholar]
- 755.Yellayi S, Teuscher C, Woods JA, Welsh TH Jr, Tung KS, Nakai M, Rosenfeld CS, Lubahn DB, Cooke PS. Normal development of thymus in male and female mice requires estrogen/estrogen receptor-alpha signaling pathway. Endocrine 12: 207–213, 2000. doi: 10.1385/ENDO:12:3:207. [DOI] [PubMed] [Google Scholar]
- 756.Yki-Järvinen H. Sex and insulin sensitivity. Metabolism 33: 1011–1015, 1984. doi: 10.1016/0026-0495(84)90229-4. [DOI] [PubMed] [Google Scholar]
- 757.Yoo KH, Hennighausen L. EZH2 methyltransferase and H3K27 methylation in breast cancer. Int J Biol Sci 8: 59–65, 2012. doi: 10.7150/ijbs.8.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Young LA, Neiss MB, Samuels MH, Roselli CE, Janowsky JS. Cognition is not modified by large but temporary changes in sex hormones in men. J Clin Endocrinol Metab 95: 280–288, 2010. doi: 10.1210/jc.2009-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759.Zaya R, Hennick C, Pearl CA. In vitro expression of androgen and estrogen receptors in prepubertal and adult rat epididymis. Gen Comp Endocrinol 178: 573–586, 2012. doi: 10.1016/j.ygcen.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 760.Zhai J, Lanclos KD, Abney TO. Estrogen receptor messenger ribonucleic acid changes during Leydig cell development. Biol Reprod 55: 782–788, 1996. doi: 10.1095/biolreprod55.4.782. [DOI] [PubMed] [Google Scholar]
- 761.Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, Verkman A, Lubahn D, Fisher JS, Katzenellenbogen BS, Hess RA. Estrogen action and male fertility: roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc Natl Acad Sci USA 98: 14132–14137, 2001. doi: 10.1073/pnas.241245898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Zhou Q, Miao M, Ran M, Ding L, Bai L, Wu T, Yuan W, Gao E, Wang J, Li G, Li DK. Serum bisphenol-A concentration and sex hormone levels in men. Fertil Steril 100: 478–482, 2013. doi: 10.1016/j.fertnstert.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 763.Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl 23: 870–881, 2002. doi: 10.1002/j.1939-4640.2002.tb02345.x. [DOI] [PubMed] [Google Scholar]
- 764.Zhou Y, Bolton EC, Jones JO. Androgens and androgen receptor signaling in prostate tumorigenesis. J Mol Endocrinol 54: R15–R29, 2015. doi: 10.1530/JME-14-0203. [DOI] [PubMed] [Google Scholar]
- 765.Zhu L, Martinez MN, Emfinger CH, Palmisano BT, Stafford JM. Estrogen signaling prevents diet-induced hepatic insulin resistance in male mice with obesity. Am J Physiol Endocrinol Metab 306: E1188–E1197, 2014. doi: 10.1152/ajpendo.00579.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Zhu X, Leav I, Leung YK, Wu M, Liu Q, Gao Y, McNeal JE, Ho SM. Dynamic regulation of estrogen receptor-beta expression by DNA methylation during prostate cancer development and metastasis. Am J Pathol 164: 2003–2012, 2004. doi: 10.1016/S0002-9440(10)63760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 295: 505–508, 2002. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- 768.Zingg D, Debbache J, Schaefer SM, Tuncer E, Frommel SC, Cheng P, Arenas-Ramirez N, Haeusel J, Zhang Y, Bonalli M, McCabe MT, Creasy CL, Levesque MP, Boyman O, Santoro R, Shakhova O, Dummer R, Sommer L. The epigenetic modifier EZH2 controls melanoma growth and metastasis through silencing of distinct tumour suppressors. Nat Commun 6: 6051, 2015. doi: 10.1038/ncomms7051. [DOI] [PubMed] [Google Scholar]
- 769.Zondek B. Mass excretion of oestrogenic hormone in the urine of the stallion. Nature 133: 209–210, 1934. doi: 10.1038/133209a0. [DOI] [Google Scholar]
- 770.Zondek T, Zondek LH. The fetal and neonatal prostate. In: Normal and Abnormal Growth of the Prostate, edited by Goland M. Springfield, IL: Thomas, 1975, p. 5–28. [Google Scholar]








