Abstract
Craniofacial secondary cartilages of the mandibular condyle and temporomandibular joint (TMJ) eminence grow in response to the local mechanical environment. The intervening TMJ disc distributes normal loads over the cartilage surfaces and provides lubrication. A better understanding of the mechanical environment and its effects on growth, development, and degeneration of the TMJ may improve treatments aimed at modifying jaw growth and preventing or reversing degenerative joint disease (DJD). This review highlights data recorded in human subjects and from computer modeling that elucidate the role of mechanics in TMJ ontogeny. Presented data provide an approximation of the age-related changes in jaw-loading behaviors and TMJ contact mechanics. The cells of the mandibular condyle, eminence, and disc respond to the mechanical environment associated with behaviors and ultimately determine the TMJ components’ mature morphologies and susceptibility to precocious development of DJD compared to postcranial joints. The TMJ disc may be especially prone to degenerative change due to its avascularity and steep oxygen and glucose gradients consequent to high cell density and rate of nutrient consumption, as well as low solute diffusivities. The combined effects of strain-related hypoxia and limited glucose concentrations dramatically affect synthesis of the extracellular matrix (ECM), which limit repair capabilities. Magnitude and frequency of jaw loading influence this localized in situ environment, including stem and fibrocartilage cell chemistry, as well as the rate of ECM mechanical fatigue. Key in vivo measurements to characterize the mechanical environment include the concentration of work input to articulating tissues, known as energy density, and the percentage of time that muscles are used to load the jaws out of a total recording time, known as duty factor. Combining these measurements into a mechanobehavioral score and linking these to results of computer models of strain-regulated biochemical events may elucidate the mechanisms responsible for growth, maintenance, and deterioration of TMJ tissues.
Keywords: biomechanical phenomena, cartilage, degenerative joint disease, human, growth, TMJ
Introduction
The role of mechanics in the ontogeny of the temporomandibular joint (TMJ) has been the focus of several clinical disciplines. For example, orthodontists have used orthopedic (functional) appliances for more than 100 years, to arrest or enhance growth of the secondary cartilage that caps the mandibular condyles, with the aim of altering maxillomandibular relations. Others within dentistry and medicine change these relations via surgery and manage the tissue destruction and chronic pain associated with degenerative joint disease (DJD) of the TMJ.
Cartilage tissues are sensitive to their mechanical environments (Felsenthal and Zelzer 2017), especially the secondary cartilages of the TMJ (Hinton et al. 2015), which develop later than primary cartilages and which, distinctly, need low oxygen tension and functional loading to sustain their cartilaginous properties. The secondary cartilages found on the mandibular condyles and temporal bone eminences comprise a hypocellular surface layer overlying a prechondroblastic layer that forms the perichondrium that caps the underlying chrondroblastic, hypertrophic, and osteogenic layers (Hinton et al. 2015). The in situ mechanical environment of the TMJ evolves as age-related behaviors develop and change. The cells of the mandibular condyle and eminence respond to changes in loading conditions and ultimately determine the TMJ components’ mature morphologies and, in some individuals, the precocious development of DJD in the second and third decades of life.
This review briefly outlines age-related changes to the mechanical environment of the TMJ and concomitant changes to the secondary cartilages. Data concerning postnatal growth and dentofacial orthopedics will be presented, which suggest that differences in mechanobehavior between diagnostic groups may account, at least in part, for the less than satisfactory success of mandibular functional appliances in approximately 25% of cases (Tulloch et al. 2004). In the United States, estimated direct costs of less than satisfactory results are greater than $500 million annually. In addition, data concerning the relatively precocious affliction and gender bias of TMJ DJD will be presented. Recently developed biphasic (poroelastic) finite element (FE) models have explored the effects of mechanical work per volume of cartilage (energy density, mJ/mm3) and resulting metabolic gradients imposed on the TMJ disc cells. These models elucidate potential mechanisms for early TMJ DJD and the susceptibility for this in women compared to men.
Mechanobehavior and Prenatal TMJ Development
Separate mesenchymal tissue condensations eventually develop into the mandibular condyle, temporal bone, and disc. Secondary TMJ cartilages form in utero in areas of dynamic loading due to fetal jaw movement. The ectomesenchymal cells that differentiate to produce the intramembranous bones of the craniofacial skeleton also produce secondary cartilage from stem cells in the perichondrium of these loaded areas such the mandibular condyle and temporal bone. As few as 16 intermittent movements per day resulted in the development of secondary cartilage in a chick membrane bone model (Hall 1968). Current knowledge regarding the influences on TMJ development in utero comes largely from nonhuman models. These models have marked differences in jaw morphologies and functions (Herring 2003), which limit translation to the human condition. Nevertheless, the molecular and cell biology of TMJ development is likely to be similar between species. A recent review indicates that RUNX2 and Sox 9 are among the genes critical for specification of a chondrogenic path for mandibular cartilage prechondroblastic cells (Hinton et al. 2015). These genes dictate the de novo appearance of the mandibular condylar cartilage anlagen, while downstream mechanosensitive genes such as Indian Hedgehog (Ihh), parathyroid hormone–related protein (Jahan et al. 2014), and the Wnt/Beta-catenin signaling proteins (Brunt et al. 2017) regulate growth. Mechanical stimulation through jaw loading is essential to development of the mandible across species. For example, FE modeling and cell genetic labeling techniques have shown that reduced loading can influence cell differentiation and morphogenesis, where anisotropic microstrain gradients and the corresponding heterogeneity of Wnt signaling within cells were lost when muscles were paralyzed in developing zebrafish (Brunt et al. 2017). Similarly, for the developing mouse mandibular condyle, restriction of jaw loading in utero resulted in reduced cartilage volume and cell numbers in particular, significantly reducing mechanosensitive Ihh-positive cells in the perichondrium layer (Jahan et al. 2014) where stem cells are located (Embree et al. 2016).
Cell transduction of the mechanical environment may be through integrin-dependent signaling cascades and primary cilia, which extend from cell surfaces and attach to the extracellular matrix (ECM). Spatial distribution of primary cilia on hyaline cartilages cells has been reported to be axial, reflecting the primary loading direction of long bones (Farnum and Wilsman 2011), whereas spatial distribution of primary cilia on mandibular secondary cartilage cells appears to be isotropic (Kinumatsu et al. 2011), potentially enhancing sensitivity to more complex strain distributions.
The TMJ disc develops in utero from a condensation of mesenchymal tissues between the newly developed mandibular condyle and temporal bone. The superior and inferior joint spaces that define the disc form consequent to movement of the mandibular condyle through the actions of the mechanosensitive Ihh and WNT/β-catenin protein cascades (Hinton et al. 2015). However, little is currently known about how the nature of the mechanical environment influences the anisotropic organization of the collagen and glycosaminoglycan structural components of the developing TMJ disc.
Mechanobehavior and Postnatal TMJ Growth
The 3-dimensional shapes and relationships of the growing, loaded TMJ surfaces affect and are affected by in situ mechanics. For instance, the position of the mandibular condyle relative to the eminence affects the degree of surface matching or congruity of the bony TMJ components. Specifically, increased congruity, and thus reduced stress concentration, was associated with condylar positions consistent with molar compared to incisor biting (Nickel and McLachlan 1994). Furthermore, dynamic stress concentrations within the TMJ depend on individual joint-specific congruity and movements during jaw functions. Nevertheless, the TMJ eminence reaches morphological maturity earlier than the mandibular condyle, possibly due to different mechanical loading thresholds. At birth, the human TMJ eminence is rudimentary and then quickly develops in association with joint loading during mandibular protrusion, initially for suckling, then use of primary incisors once these have erupted (Nickel et al. 1988). The eminence achieves 50% of its mature size by 3 y of age (Katsavrias 2002) following primary molar eruption and joint loading with the mandible in a retruded position during chewing. Thus, the form of the TMJ eminence reflects the changing mechanical environment and has been shown to develop in a manner consistent with a neuromuscular objective of minimization of joint loads (de Zee et al. 2009). Stem cells within the temporal bone periosteum are the likely source of progenitor cells that produce the chondroid bone of the eminence in response to signals from mechanosensitive gene cascades. Studying these genetic influences remains challenging because current genetic engineering techniques are most conveniently and economically applied to murine models that lack an eminence (Porto et al. 2010).
The mandibular condyle has an asymmetric growth pattern where, between birth and adulthood, the anteroposterior dimension increases by a relatively small amount, whereas the mediolateral dimension approximately doubles (Karlo et al. 2010). This pattern may be explained by differences in the distribution of shear strain across the anteroposterior versus mediolateral dimensions of the cartilage. That is, evidence for mechanical modulation of osteochondroprogenitor cell fates indicates that chondrogenesis is inhibited and osteogenesis promoted across regions of high shear strain (Knothe Tate et al. 2008). Thus, higher shear gradients along the condyle’s anteroposterior compared to mediolateral axis may be responsible for the inhibition of chrondrogenesis and relatively limited growth in this dimension postnatally.
Condylar growth in the vertical dimension between birth and adulthood contributes to increased ramal height, which can vary markedly between individuals (Bjork 1963). Relatively large versus small ramal heights are distinguishing features of the brachyfacial (hypodivergent) versus dolichofacial (hyperdivergent) phenotypes (Appendix Fig.). Mandibular orthopedic appliances aim to arrest or enhance condylar growth; however, the mechanisms to account for differences in effectiveness between appliance types in different phenotypes so far are unknown. Conspicuously missing are foundational data of phenotypic differences in mechanobehavior between these 2 diagnostic groups (Nickel et al. 2017). Mandibular tractional forces associated with orthopedic appliance therapy have been measured and found to be significantly correlated with differences in mandibular phenotypes (Shimazaki et al. 2017). However, forces acting on the mandible can be stabilized by reaction forces of the jaw muscles or TMJs or a combination of both and, hence, because of the mechanical indeterminacy (Nickel et al. 2012), the applied force magnitude may not be directly correlated to the resultant TMJ load magnitude. A way to individualize and solve this mechanical indeterminacy is to use validated numerical modeling (Iwasaki, Liu, et al. 2017). Using this approach, age-dependent differences in ipsilateral and contralateral TMJ loads per unit force on the mandible were found, where TMJ loads were 20% larger at ages 12 and 18 y in dolichofacial compared to brachyfacial children, and larger ramal height was significantly correlated with lower TMJ loads and higher age (Iwasaki, Liu, et al. 2017). Furthermore, the combined effects of mechanics and muscle behavior were measured in dolichofacial and brachyfacial adolescents and showed that 49% of the variance in ramal height was attributable to the amounts of low-level masseter muscle use, measured via duty factor and TMJ loads (Nickel et al. 2017).
Going forward, however, foundational data about TMJ mechanics must reflect the 3-dimensional joint relationships. Specifically, in addition to condylar loads, loading areas must be known. This will allow joint stresses to be estimated to test if there is a mechanical threshold for chondrogenesis and if this is exceeded at an earlier age in dolichofacial compared to brachyfacial individuals and if this can be affected by orthopedic therapies. Evidence for such a threshold, where intermittent compressive stresses greater than 0.05 MPa inhibited chrondrocyte replication and matrix synthesis, has been shown in vitro (Copray et al. 1986). Using this, plus combining age-dependent changes in TMJ loads for the 2 phenotypes (Iwasaki, Liu, et al. 2017) with averaged condylar sizes to approximate loading areas (Karlo et al. 2010), a theoretical model of intermittent TMJ compressive stresses versus age shows that dolichofacial children may reach the inhibitory threshold approximately 3 y earlier than brachyfacial children (Fig. 1). If this type of model can be validated, it could help explain differences in mandibular condylar growth in these phenotypes and be applied to improve mandibular orthopedic appliances.
Figure 1.
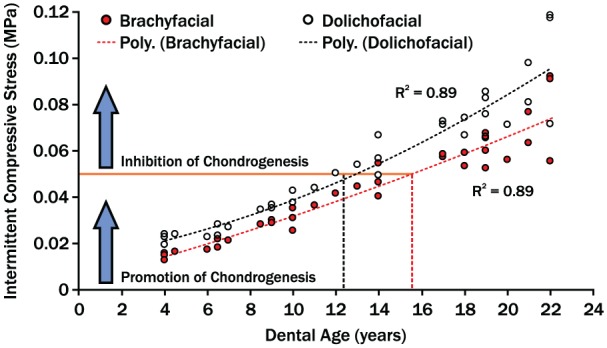
A theoretical model of age-dependent changes in temporomandibular joint (TMJ) compressive stresses. Compressive stresses were estimated by combining age-dependent changes in TMJ loads of growing children (Iwasaki, Liu, et al. 2017) and averaged condyle size to first approximate surface areas (Karlo et al. 2010). The mechanical threshold for inhibition of chondrogenesis was estimated based on in vitro data (Copray et al. 1986). The differences in compressive stress regression trajectories between 2 phenotypic groups suggest that the dolichofacial group reached the inhibitory threshold 3 y earlier than the brachyfacial group. This may account for phenotypic differences in jaw form and responses to mandibular orthopedic therapies.
Mechanobehavior and the Mature TMJ
Temporomandibular disorders (TMDs) are prevalent and debilitating, reported to affect 5% to 12% of Americans (Ahmad and Schiffman 2016), with affliction odds higher for females than males (Slade et al. 2011) and estimated annual costs of $4 billion (Stowell et al. 2007). Osseous changes are late-stage characteristics of DJD (Wiese et al. 2008; Farina et al. 2009) that are found in the TMJs of 20- to 30-y-olds. This is over a decade earlier than in other human joints (Iwasaki et al. 2010). Although TMJ disc displacement leads to DJD in animal models (Sharawy et al. 2003) and in some humans (Kurita et al. 2006), other clinical cases of TMJ disc displacement remain stable or improve with time (Schiffman et al. 2017). Why TMJ tissues prematurely fail compared to postcranial joints is not understood. Plus, the progressive steps to DJD in humans are unknown (Chantaracherd et al. 2015). Early mechanical fatigue of the TMJ disc matrix (Juran et al. 2013; Wright et al. 2013) in combination with the unique physiological susceptibility of TMJ fibrocartilage cells may be contributing factors.
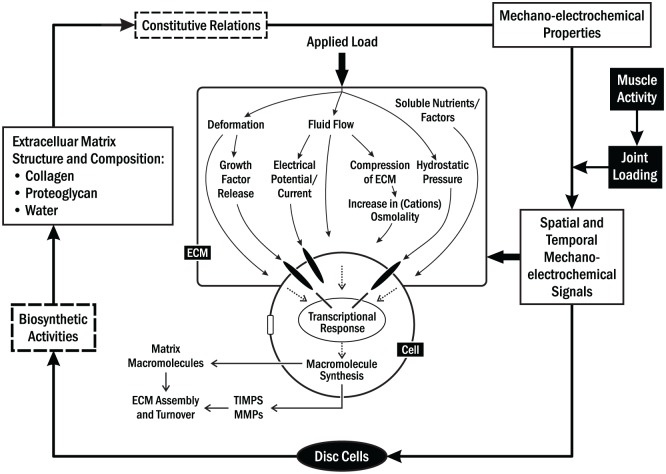
The disc is the primary stress-distributing tissue in the TMJ, providing lubrication and minimizing shear-related tractional forces between moving surfaces. It is a porous viscoelastic material, comprising a fluid phase composed of water with dissolved solutes and mobile ions and a solid phase composed of a densely woven collagen-glycosaminoglycan network (Mow et al. 1999). The disc mechanical environment is complex due to stress-strain interactions on nutrient gradients and metabolism where cells and tissues are sensitive to mechanical loading. To understand it fully, stress-strain physical stimuli, the resulting cellular responses, and their interactions should be studied at the molecular, cellular, and tissue levels (Fig. 2) (Grodzinsky et al. 2000). Alterations to the TMJ disc mechanical environment could be one mechanism associated with, or an early indicator of, degenerative changes (Mow et al. 1999; Yao et al. 2011).
Figure 2.
Illustration of the relationships between extracellular matrix (ECM) composition and structure, mechano-electrochemical signals, and cell biosynthetic activities in temporomandibular joint (TMJ) disc. Mechanocoupling involves the conversion of applied physical forces at the tissue level into detectable physical signals at the cellular level. Mechanical loading induces various signals (e.g., deformation, pressure, electrical, and fluid, solute [e.g., nutrient], and ion flow fields). These signals influence biological activities of TMJ disc cells by acting independently or together.
Mechanical function of the TMJ disc is determined by the composition and structure of its distinct ECM (Detamore et al. 2005). The normal human TMJ disc is largely avascular, so cellular nutrients essential for maintaining a healthy ECM must be supplied by diffusion from the synovial fluid and blood vessels in the disc periphery (Detamore and Athanasiou 2005). As such, the balance between nutrient transport and consumption establishes a concentration gradient across the TMJ disc. This concentration gradient is regulated by mechanical loading, thereby affecting cell viability, energy metabolism, matrix synthesis, and response to inflammatory factors (Cisewski et al. 2015). In contrast to other fibrocartilaginous tissues, the TMJ disc has higher cell density and nutrient consumption rates (Kuo, Shi, et al. 2011) and lower solute diffusivity (Shi et al. 2013; Wright et al. 2013). Hence, the TMJ disc may have uniquely steeper oxygen and glucose gradients and, consequently, increased cellular susceptibility to factors that impede nutrient supply.
Recently, studies have shown that mechanical loading limits nutrient availability within the TMJ disc by altering diffusivity. Electrical conductivity, a material property of biological tissues related to ion diffusivity in soft, hydrated tissues, decreased with increasing mechanical strain (Kuo, Wright, et al. 2011). Decreased electrical conductivity, and therefore TMJ disc diffusivity, was mainly due to reduction in tissue water content (porosity). Correlation between diffusivity and porosity suggested that diffusive transport in the TMJ disc was dependent upon tissue composition. In comparison to other cartilaginous tissues, the TMJ disc has lower tissue porosity (Kuo, Wright, et al. 2011; Wright et al. 2013). Consequently, electrical conductivity and small ion (e.g., Na+ and Cl–) diffusivity were lowest for the TMJ disc compared to hyaline cartilage and the intervertebral disc (IVD) annulus fibrosis (Wright et al. 2013).
Generally, mechanical strain has a greater impact on the diffusivity of larger solutes such as oxygen and glucose compared to small ions. In the TMJ disc, average glucose diffusivity was only about 50% compared to the IVD annulus fibrosus in unloaded conditions and further dropped 28% when compressive strains reached 20% (unpublished data). In the absence of glucose, because of the combined effect of limited diffusion due to tissue composition and impedance due to loading, cell viability significantly decreased. In the presence of glucose, decreased oxygen levels increased TMJ disc cell proliferation, whereas the viability of fibrocartilage cells decreased when glucose levels fell below 0.5 mM (Cisewski et al. 2015). These data support the hypothesis that glucose may be the limiting nutrient for TMJ disc cell survival, rather than oxygen. Proliferating chondrocytes in hyaline cartilage are well adapted to hypoxic conditions within avascular environments and use glycolysis as the main energy source (Rajpurohit et al. 1996). In contrast, TMJ disc cells have a significantly higher oxygen consumption rate (Kuo, Shi, et al. 2011) compared to hyaline cartilage chondrocytes and IVD annulus fibrosis cells. The higher oxygen consumption rate of TMJ disc cells may be related to oxidative phosphorylation and thus demonstrate the Pasteur effect (Lee and Urban 1997). This effect may compromise long-term viability of TMJ disc cells since the rate of glycolysis under hypoxic conditions hastens the depletion of an already limited supply of glucose in avascular tissues (Kuo, Shi, et al. 2011; Cisewski et al. 2015). Moreover, as a result of increased glycolysis, lactic acid production increases in hypoxic regions, producing localized decreases in pH that may further decrease cell viability. Modeling using a mixture theory-based approach should provide new data concerning the effects of mechanobehavior on TMJ disc cell viability.
Another critical factor is the dense TMJ disc cell population, which has higher nutrient consumption rates compared to other articular tissues. Confocal microscopy has been used to determine the in situ volume-based cell densities (Kuo, Shi, et al. 2011), where for the TMJ disc, this was 51.3 × 106 cells/mL tissue and almost 4 times higher than in femoral condyle hyaline cartilage (14.1 × 106 cells/mL tissue) (Stockwell 1979) and more than 50 times higher than in the IVD annulus fibrosis (0.9 × 106 cells/mL tissue) (Huang and Gu 2008). In addition, it has been established that the oxygen consumption rate of TMJ disc cells is at least 5 times that of IVD annulus fibrosis cells and hyaline cartilage chondrocytes (Kuo, Shi, et al. 2011). TMJ disc cells differ in terms of organelle content, because they have a greater number of mitochondria and, thus, likely have higher metabolic activity (Detamore et al. 2005). As such, in the avascular TMJ disc, the oxygen and glucose gradients may be steeper than in hyaline cartilage because of higher cell density and rates of nutrient consumption, as well as lower solute diffusivities (Wright et al. 2013). This combination of factors increases the risk of the TMJ disc to pathology.
Due to technical difficulties of direct in vivo measurements, computational modeling is the only means to determine systematically the effects of mechanobehavior on physicochemical cell signaling within the TMJ disc. Computational modeling provides a multiscale tool to combine physical stimuli, ECM structure, macrocontinuum tissue material properties, and biological responses at the molecular, cellular, and tissue levels. To date, FE computational models have been successfully used to further the understanding of strain/stress distributions inside the disc due to TMJ loading (single phasic models), fluid pressurization (biphasic models), and how loading and fluid pressurization affect the nutrient and charged micro-conditions of the TMJ to predict changes to cellular viability (multiphasic mixture theory).
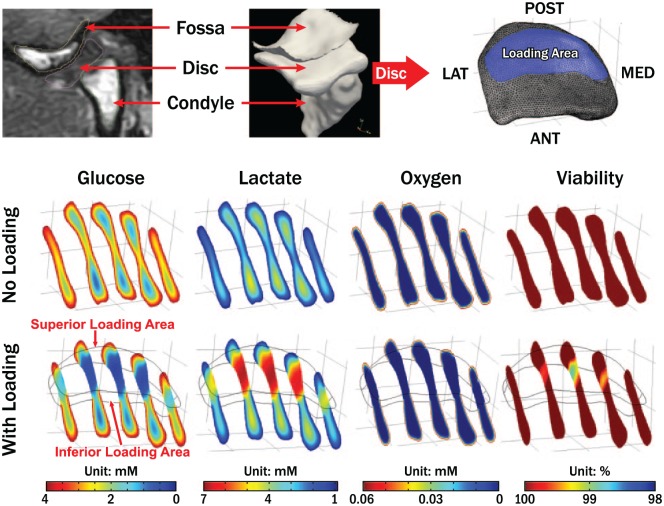
Single-phase FE models suggest that the TMJ disc undergoes significant loading (Koolstra and van Eijden 2005). However, single phasic models have limited ability to describe how loads are transmitted within the solid and fluid components of the cartilage matrix. More recently, electromyography studies of the jaw muscles combined with multibody TMJ models produced data describing jaw-loading behaviors in subjects when engaged in their natural surroundings (Wei et al. 2017). These physiologically meaningful loading conditions have been used in biphasic FE models where applied poroelastic mechanics treat the soft hydrated TMJ disc as a mixed material consisting of 2 continuum and immiscible phases (solid and fluid phases), and fluid transport (hydraulic permeability) in human TMJ discs is considered to describe fully the stress-strain environment. These models have demonstrated that fluid pressurization is a dominating mechanism of load support in the TMJ disc (Appendix Videos 1 and 2) (Wu et al. 2017). The success of these biphasic FE models in describing more completely the TMJ disc loading environment supports the use of mixture theory-based multiphasic FE modeling approaches, which further separate charged ions from the fluid phase as an independent third phase (Lai et al. 1991). Mechano-electro-chemical theory-based multiphasic FE models describing the mechanically coupled transport of multielectrolytes and neutral solutes (e.g., oxygen, glucose) through charged hydrated soft tissues have been developed for the TMJ disc, hyaline cartilage, and IVD (Yao and Gu 2004; 2007). These models can predict deformation behavior and also the electrical response and solute concentration distribution within the tissue during loading (Wright et al. 2018). Model results for the TMJ disc (unpublished data) showed that the physiological ranges of glucose, lactate, and oxygen concentrations were 0.2 to 4.0 mM, 0.9 to 2.3 mM, and 0 to 5.8 kPa without loading. Furthermore, cell viability was predicted by correlating cell death with glucose levels less than 0.5 mM. The results showed that critical zone volumes where these low glucose levels occurred were in the anterior and posterior disc regions and comprised 3.5% of total disc volume without loading but increased to 10.1% of total disc volume with loading (Fig. 3). These mixture theory-based FE models suggested that the TMJ disc had nutrient gradients that were sensitive to loading and vulnerable to conditions that impeded nutrient supply.
Figure 3.
Multiphasic finite element analysis of temporomandibular joint (TMJ) disc nutrient environment and cell viability under sustained joint loading. Imaging (computed tomography, magnetic resonance)–based subject-specific TMJ finite element model (top panel). Nutrient (oxygen, glucose, and lactate) profiles and cell viability distributions in the TMJ disc without sustained joint load (middle panel) and with sustained joint load (bottom panel).
Magnitude and frequency of loads imposed on the cartilage surfaces are contributing variables to mechanical fatigue and could explain degenerative changes in the TMJ cartilages. Human jaw-loading behaviors during the day and night have only been estimated or calculated. However, comprehensive data available from sleep-lab (Raphael et al. 2013) and natural surroundings (Iwasaki et al. 2015; Iwasaki et al. 2017a; Wei et al. 2017) show, contrary to expectations, differences in behaviors were limited to low-level jaw muscle activations in subjects with and without myofascial pain. Bruxing at high levels of muscle activations was rare, with no group differences in frequency. Furthermore, the concentration of work done to TMJ tissues during jaw-loading behaviors, known as the energy density, has been measured using dynamic stereometry (Iwasaki et al. 2017b). To investigate the apparent increased susceptibility to TMJ DJD in women compared to men, energy densities per jaw-closing cycle were calculated in subjects with normal TMJ architecture and showed that these were significantly higher in women than men by 38% on the ipsilateral side and 25% on the contralateral side. In addition, energy densities in the contralateral TMJs were 3.6-fold and significantly larger due to significantly larger distances of stress-field translation during asymmetric versus symmetric jaw closing (Gallo et al. 2018). During asymmetric jaw closing, energy densities in the contralateral TMJs were 2-fold larger in women compared to men. This was due to differences in volume of cartilage, which was 1.5-fold and significantly smaller under the stress field in women versus men (Gallo et al. 2018). These findings suggest that for the same jaw-loading task, some individuals have potential for higher concentrations of energy input to TMJ cartilages than others, and this is especially so in women compared to men. Therefore, an important next step is to determine how often jaw-loading behaviors occur. To address this, TMJ energy density has been combined with jaw muscle duty factor to calculate mechanobehavioral scores in women with and without bilateral TMJ disc displacement (Iwasaki et al. 2017a). Subjects were trained to record masseter and temporalis electromyography over 3 days and 3 nights. Average energy densities for contralateral TMJs were significantly larger by 1.4-fold in subjects with, compared to without disc displacement. Average duty factors were significantly larger for those with compared without disc displacement by 1.7-, 2.5-, and 1.9-fold for day, night, and overall, respectively. Daytime mechanobehavioral scores were significantly larger by up to 8.5-fold in those with, compared to without disc displacement (Fig. 4). Future studies need to similarly measure mechanobehavioral scores longitudinally to test the hypothesis that these are predictive of development of and protection from TMJ DJD, respectively.
Figure 4.
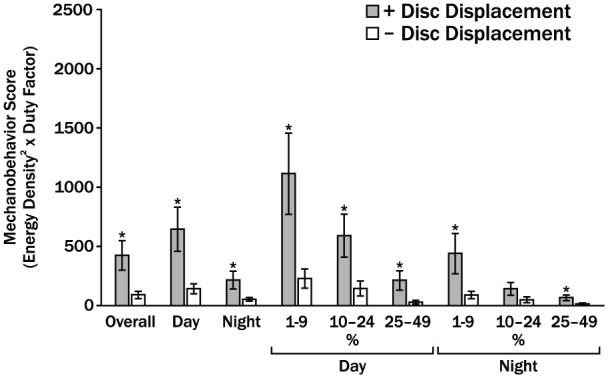
Averaged mechanobehavior scores in women with bilateral temporomandibular joint (TMJ) disc displacement (+DD) and without (Control) are shown for combined ipsilateral and contralateral TMJs (Iwasaki et al. 2017a). Overall indicates results for time (Day, Night) periods and muscle activity thresholds of 1% to 4%, 5% to 9%, and 10% to 24% of muscle activation required to produce a 20-N bite force. Vertical bars indicate standard errors above and below the mean values and * indicates P < 0.05. In the main, women with bilateral disc displacement had significantly higher mechanobehavior scores during the day and night. However, the findings were contrary to the conventional paradigm of the role of high-magnitude bruxism and clenching in TMJ disc failure. The results showed this type of bruxing and clenching was rare and significant differences between the 2 diagnostic groups were limited to muscle activities required to produce low-magnitude mandibular loads of <5 N.
Conclusions
Within the past decade, there has been a general focus on the genes and proteins associated with TMJ cartilage growth and degeneration without quantification of the mechanical environment that directs cell metabolism. Future research requires focus on mechanobehavior and in situ mechanics that influence TMJ ontogeny. A new theoretical model of the role of mechanobehavior in TMJ growth, homeostasis, and dysregulation is proposed (Fig. 5). The mechanical environment directs the timing of cessation of growth and progression through trajectories to early or late cartilage dysregulation. Testing of this model requires the collection of mechanobehavior data in living humans that are age related and longitudinal in nature. When combined with FE models of mechano-metabolic coupling, it will be possible to quantify more accurately the micro-conditions of cartilage cells for future linkage to biological mechanisms.
Figure 5.
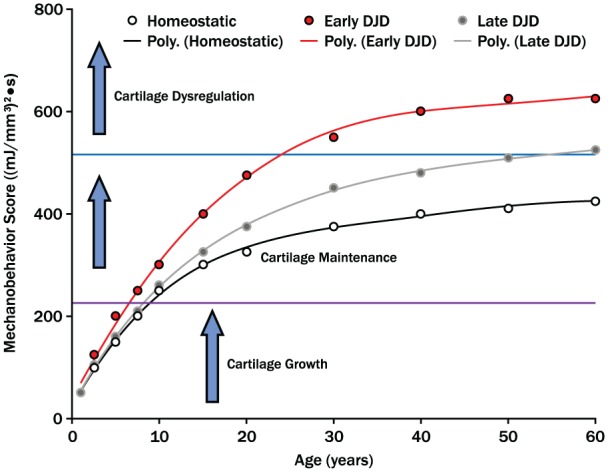
Mechanobehavior and temporomandibular joint (TMJ) ontogeny. This theoretical model proposes that the mechanical environment is defined by mechanobehavior ((mJ/mm3)2•secnormalized) of an individual. The mechanical conditions dictate timing of cessation of TMJ growth, duration of cartilage maintenance or homeostasis, and the likelihood and timing of cartilage dysregulation. Longitudinal data from human subjects are required to test whether or not the trajectories toward early or late cartilage dysregulation and degenerative joint disease can be identified early in life and whether or not the variables that contribute to a trajectory can be manipulated to enhance homeostasis. The identified thresholds for transitioning from growth to maintenance and maintenance to dysregulation were empirically derived from data presented. Research is required to determine, within an individual, the variables that influence the magnitudes of these thresholds.
Author Contributions
J.C. Nickel, contributed to conception, design, and data acquisition, drafted and critically revised the manuscript; L.R. Iwasaki, H. Yao, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; Y.M. Gonzalez, L.M. Gallo, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_002203451878646 for Mechanobehavior and Ontogenesis of the Temporomandibular Joint by J.C. Nickel, L.R. Iwasaki, Y.M. Gonzalez, L.M. Gallo and H. Yao in Journal of Dental Research
Acknowledgments
Thanks to our subjects for their participation and the many graduate students who participated in data collection and analyses. Dr. Hongzeng Liu, Oregon Health & Science University senior research associate, developed the programs for analysis of ambulatory electromyography recordings and further developed the numerical models used to calculate TMJ loads. Dr. Yongren Wu (Clemson University) and Dr. Matthew Coombs (Medical University of South Carolina) developed the multiphasic finite element model of TMJ discs to determine the ECM physiochemical microenvironment and cell viability. Drs. Ying Liu (East Tennessee State University) and JoAnna Scott (University of Missouri Kansas City) helped with statistical analyses.
Footnotes
A supplemental appendix to this article is available online.
Funding, in part, was provided by the National Institutes of Health (National Institute of Dental and Craniofacial Research [NIDCR], R01DE021134, H.Y.; R01DE016417, J.C.N.) and American Association of Orthodontists Foundation (LRI).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ahmad M, Schiffman EL. 2016. Temporomandibular joint disorders and orofacial pain. Dent Clin North Am. 60(1):105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork A. 1963. Variations in the growth pattern of the human mandible: longitudinal radiographic study by the implant method. J Dent Res. 42(1, Pt 2):400–411. [DOI] [PubMed] [Google Scholar]
- Brunt LH, Begg K, Kague E, Cross S, Hammond CL. 2017. Wnt signalling controls the response to mechanical loading during zebrafish joint development. Development. 144(15):2798–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantaracherd P, John MT, Hodges JS, Schiffman EL. 2015. Temporomandibular joint disorders’ impact on pain, function, and disability. J Dent Res. 94(3, Suppl):79S–86S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisewski SE, Zhang L, Kuo J, Wright GJ, Wu Y, Kern MJ, Yao H. 2015. The effects of oxygen level and glucose concentration on the metabolism of porcine TMJ disc cells. Osteoarthritis Cartilage. 23(10):1790–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copray JC, Jansen HW, Duterloo HS. 1986. Growth and growth pressure of mandibular condylar and some primary cartilages of the rat in vitro. Am J Orthod Dentofacial Orthop. 90(1):19–28. [DOI] [PubMed] [Google Scholar]
- de Zee M, Cattaneo PM, Svensson P, Pedersen TK, Melsen B, Rasmussen J, Dalstra M. 2009. Prediction of the articular eminence shape in a patient with unilateral hypoplasia of the right mandibular ramus before and after distraction osteogenesis-a simulation study. J Biomech. 42(8):1049–1053. [DOI] [PubMed] [Google Scholar]
- Detamore MS, Orfanos JG, Almarza AJ, French MM, Wong ME, Athanasiou KA. 2005. Quantitative analysis and comparative regional investigation of the extracellular matrix of the porcine temporomandibular joint disc. Matrix Biol. 24(1):45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embree MC, Chen M, Pylawka S, Kong D, Iwaoka GM, Kalajzic I, Yao H, Shi C, Sun D, Sheu TJ, et al. 2016. Exploiting endogenous fibrocartilage stem cells to regenerate cartilage and repair joint injury. Nat Commun. 7:13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina D, Bodin C, Gandolfi S, De Gasperi W, Borghesi A, Maroldi R. 2009. TMJ disorders and pain: assessment by contrast-enhanced mri. Eur J Radiol. 70(1):25–30. [DOI] [PubMed] [Google Scholar]
- Farnum CE, Wilsman NJ. 2011. Orientation of primary cilia of articular chondrocytes in three-dimensional space. Anat Rec (Hoboken). 294(3):533–549. [DOI] [PubMed] [Google Scholar]
- Felsenthal N, Zelzer E. 2017. Mechanical regulation of musculoskeletal system development. Development. 144(23):4271–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo LM, Fankhauser N, Gonzalez YM, Liu H, Liu Y, Nickel JC, Iwasaki LR. 2018. Jaw closing movement and sex differences in temporomandibular joint energy densities. J Oral Rehabil. 45(2):97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinsky AJ, Levenston ME, Jin M, Frank EH. 2000. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2:691–713. [DOI] [PubMed] [Google Scholar]
- Hall BK. 1968. In vitro studies on the mechanical evocation of advenitious cartilage in the chick. J Exp Zool. 168(3):283–305. [DOI] [PubMed] [Google Scholar]
- Herring SW. 2003. TMJ anatomy and animal models. J Musculoskelet Neuronal Interact. 3(4):391–394. [PMC free article] [PubMed] [Google Scholar]
- Hinton RJ, Jing J, Feng JQ. 2015. Genetic influences on temporomandibular joint development and growth. Curr Top Dev Biol. 115:85–109. [DOI] [PubMed] [Google Scholar]
- Huang CY, Gu WY. 2008. Effects of mechanical compression on metabolism and distribution of oxygen and lactate in intervertebral disc. J Biomech. 41(6):1184–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki LR, Crosby MJ, Marx DB, Gonzalez Y, McCall WD, Jr, Ohrbach R, Nickel JC. 2010. Human temporomandibular joint eminence shape and load minimization. J Dent Res. 89(7):722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki LR, Gonzalez YM, Liu H, Marx DB, Gallo LM, Nickel JC. 2015. A pilot study of ambulatory masticatory muscle activities in temporomandibular joint disorders diagnostic groups. Orthod Craniofac Res. 18(Suppl 1):146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki LR, Gonzalez YM, Liu Y, Liu H, Markova M, Gallo LM, Nickel JC. 2017. a. Mechanobehavioral scores in women with and without TMJ disc displacement. J Dent Res. 96(8):895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki LR, Gonzalez YM, Liu Y, Liu H, Markova M, Gallo LM, Nickel JC. 2017. b. TMJ energy densities in healthy men and women. Osteoarthritis Cartilage. 25(6):846–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki LR, Liu Y, Liu H, Nickel JC. 2017. Jaw mechanics in dolichofacial and brachyfacial phenotypes: a longitudinal cephalometric-based study. Orthod Craniofac Res. 20(Suppl 1):145–150. [DOI] [PubMed] [Google Scholar]
- Jahan E, Matsumoto A, Rafiq AM, Hashimoto R, Inoue T, Udagawa J, Sekine J, Otani H. 2014. Fetal jaw movement affects Ihh signaling in mandibular condylar cartilage development: the possible role of Ihh as mechanotransduction mediator. Arch Oral Biol. 59(10):1108–1118. [DOI] [PubMed] [Google Scholar]
- Juran CM, Dolwick MF, McFetridge PS. 2013. Shear mechanics of the TMJ disc: relationship to common clinical observations. J Dent Res. 92(2):193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlo CA, Stolzmann P, Habernig S, Muller L, Saurenmann T, Kellenberger CJ. 2010. Size, shape and age-related changes of the mandibular condyle during childhood. Eur Radiol. 20(10):2512–2517. [DOI] [PubMed] [Google Scholar]
- Katsavrias EG. 2002. Changes in articular eminence inclination during the craniofacial growth period. Angle Orthod. 72(3):258–264. [DOI] [PubMed] [Google Scholar]
- Kinumatsu T, Shibukawa Y, Yasuda T, Nagayama M, Yamada S, Serra R, Pacifici M, Koyama E. 2011. TMJ development and growth require primary cilia function. J Dent Res. 90(8):988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe Tate ML, Falls TD, McBride SH, Atit R, Knothe UR. 2008. Mechanical modulation of osteochondroprogenitor cell fate. Int J Biochem Cell Biol. 40(12):2720–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolstra JH, van Eijden TM. 2005. Combined finite-element and rigid-body analysis of human jaw joint dynamics. J Biomech. 38(12):2431–2439. [DOI] [PubMed] [Google Scholar]
- Kuo J, Shi C, Cisewski S, Zhang L, Kern MJ, Yao H. 2011. Regional cell density distribution and oxygen consumption rates in porcine TMJ discs: an explant study. Osteoarthritis Cartilage. 19(7):911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J, Wright GJ, Bach DE, Slate EH, Yao H. 2011. Effect of mechanical loading on electrical conductivity in porcine TMJ discs. J Dent Res. 90(10):1216–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita H, Uehara S, Yokochi M, Nakatsuka A, Kobayashi H, Kurashina K. 2006. A long-term follow-up study of radiographically evident degenerative changes in the temporomandibular joint with different conditions of disk displacement. Int J Oral Maxillofac Surg. 35(1):49–54. [DOI] [PubMed] [Google Scholar]
- Lai WM, Hou JS, Mow VC. 1991. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng. 113(3):245–258. [DOI] [PubMed] [Google Scholar]
- Lee RB, Urban JP. 1997. Evidence for a negative Pasteur effect in articular cartilage. Biochem J. 321(Pt 1):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mow VC, Wang CC, Hung CT. 1999. The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthritis Cartilage. 7(1):41–58. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Gonzalez YM, McCall WD, Ohrbach R, Marx DB, Liu H, Iwasaki LR. 2012. Muscle organization in individuals with and without pain and joint dysfunction. J Dent Res. 91(6):568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel JC, Iwasaki LR, Beatty MW, Marx DB. 2004. Laboratory stresses and tractional forces on the TMJ disc surface. J Dent Res. 83(8):650–654. [DOI] [PubMed] [Google Scholar]
- Nickel JC, McLachlan KR. 1994. An analysis of surface congruity in the growing human temporomandibular joint. Arch Oral Biol. 39(4):315–321. [DOI] [PubMed] [Google Scholar]
- Nickel JC, McLachlan KR, Smith DM. 1988. A theoretical model of loading and eminence development of the postnatal human temporomandibular joint. J Dent Res. 67(6):903–910. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Weber AL, Covington Riddle P, Liu Y, Liu H, Iwasaki LR. 2017. Mechanobehaviour in dolichofacial and brachyfacial adolescents. Orthod Craniofac Res. 20(Suppl 1):139–144. [DOI] [PubMed] [Google Scholar]
- Porto GG, Vasconcelos BC, Andrade ES, Silva-Junior VA. 2010. Comparison between human and rat TMJ: anatomic and histopathologic features. Acta Cir Bras. 25(3):290–293. [DOI] [PubMed] [Google Scholar]
- Rajpurohit R, Koch CJ, Tao Z, Teixeira CM, Shapiro IM. 1996. Adaptation of chondrocytes to low oxygen tension: relationship between hypoxia and cellular metabolism. J Cell Physiol. 168(2):424–432. [DOI] [PubMed] [Google Scholar]
- Raphael KG, Janal MN, Sirois DA, Dubrovsky B, Wigren PE, Klausner JJ, Krieger AC, Lavigne GJ. 2013. Masticatory muscle sleep background electromyographic activity is elevated in myofascial temporomandibular disorder patients. J Oral Rehabil. 40(12):883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman EL, Ahmad M, Hollender L, Kartha K, Ohrbach R, Truelove EL, Zhang L, Hodges JS, Sommers E, Anderson GC, et al. 2017. Longitudinal stability of common TMJ structural disorders. J Dent Res. 96(3):270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharawy M, Ali AM, Choi WS. 2003. Experimental induction of anterior disk displacement of the rabbit craniomandibular joint: an immuno-electron microscopic study of collagen and proteoglycan occurrence in the condylar cartilage. J Oral Pathol Med. 32(3):176–184. [DOI] [PubMed] [Google Scholar]
- Shi C, Wright GJ, Ex-Lubeskie CL, Bradshaw AD, Yao H. 2013. Relationship between anisotropic diffusion properties and tissue morphology in porcine TMJ disc. Osteoarthritis Cartilage. 21(4):625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki A, Kimura H, Inou N, Maki K. 2017. Development of a measurement system for the mechanical load of functional appliances. J Biomech. 63:197–202. [DOI] [PubMed] [Google Scholar]
- Slade GD, Bair E, By K, Mulkey F, Baraian C, Rothwell R, Reynolds M, Miller V, Gonzalez Y, Gordon S, et al. 2011. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the oppera study. J Pain. 12(11, Suppl):T12–T26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell RA. 1979. Biology of cartilage cells. Cambridge (UK): Cambridge University Press. [Google Scholar]
- Stowell AW, Gatchel RJ, Wildenstein L. 2007. Cost-effectiveness of treatments for temporomandibular disorders: biopsychosocial intervention versus treatment as usual. J Am Dent Assoc. 138(2):202–208. [DOI] [PubMed] [Google Scholar]
- Tulloch JF, Proffit WR, Phillips C. 2004. Outcomes in a 2-phase randomized clinical trial of early class II treatment. Am J Orthod Dentofacial Orthop. 125(6):657–667. [DOI] [PubMed] [Google Scholar]
- Wei F, Van Horn MH, Coombs MC, She X, Gonzales TS, Gonzalez YM, Scott JM, Iwasaki LR, Nickel JC, Yao H. 2017. A pilot study of nocturnal temporalis muscle activity in TMD diagnostic groups of women. J Oral Rehabil. 44(7):517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese M, Wenzel A, Hintze H, Petersson A, Knutsson K, Bakke M, List T, Svensson P. 2008. Osseous changes and condyle position in TMJ tomograms: impact of RDC/TMD clinical diagnoses on agreement between expected and actual findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 106(2):e52–e63. [DOI] [PubMed] [Google Scholar]
- Wright GJ, Coombs MC, Wu Y, Damon BJ, Bacro TH, Kern MJ, Chen X, Yao H. 2018. Electrical conductivity method to determine sexual dimorphisms in human temporomandibular disc fixed charge density. Ann Biomed Eng. 46(2):310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GJ, Kuo J, Shi C, Bacro TR, Slate EH, Yao H. 2013. Effect of mechanical strain on solute diffusion in human TMJ discs: an electrical conductivity study. Ann Biomed Eng. 41(11):2349–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Cisewski SE, Wei F, She X, Gonzales TS, Iwasaki LR, Nickel JC, Yao H. 2017. Fluid pressurization and tractional forces during TMJ disc loading: a biphasic finite element analysis. Orthod Craniofac Res. 20(Suppl 1):151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Gu WY. 2004. Physical signals and solute transport in cartilage under dynamic unconfined compression: finite element analysis. Ann Biomed Eng. 32(3):380–390. [DOI] [PubMed] [Google Scholar]
- Yao H, Gu WY. 2007. Three-dimensional inhomogeneous triphasic finite-element analysis of physical signals and solute transport in human intervertebral disc under axial compression. J Biomech. 40(9):2071–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Wu YR, Lu XL. 2011. Cartilage mechanobiology. In: Nagatomi J, editor. Mechanobiology handbook. Boca Raton (FL): Taylor and Francis; p. 229–244. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_002203451878646 for Mechanobehavior and Ontogenesis of the Temporomandibular Joint by J.C. Nickel, L.R. Iwasaki, Y.M. Gonzalez, L.M. Gallo and H. Yao in Journal of Dental Research