Abstract
It has long been thought that chaperones are primarily attracted to their clients through the hydrophobic effect. However, in in vitro studies on the interaction between the chaperone Spy and its substrate Im7, we recently showed that long-range electrostatic interactions also play a key role. Spy functions in the periplasm of Gram-negative bacteria, which is surrounded by a permeable outer membrane. The ionic conditions in the periplasm therefore closely mimic those in the media, which allowed us to vary the ionic strength of the in vivo folding environment. Using folding biosensors that link protein folding to antibiotic resistance, we were able to monitor Spy chaperone activity in Escherichia coli in vivo as a function of media salt concentration. The chaperone activity of Spy decreased when the ionic strength of the media was increased, strongly suggesting that electrostatic forces play a vital role in the action of Spy in vivo.
Keywords: chaperone, electrostatic interaction, Spy, folding biosensor, Escherichia coli
Full-Text
Chaperones act by preventing protein aggregation and facilitating protein folding [1]. It is generally thought that chaperones function in part by binding to hydrophobic regions exposed on the surface of protein folding intermediates, thereby inhibiting their aggregation [2]. However, we and other researchers have shown that electrostatic interactions also play an important role in chaperone action in vitro, particularly in the initial binding of chaperones to their substrates [3–5]. Note that electrostatic interactions apply over a much longer range than the hydrophobic effect, which is active over a relatively short range, which may increase the overall impact of electrostatic interactions [6, 7].
Determining the exact role that various forces play in chaperone action is complicated by the large and complex nature of most chaperone-folding machines, which are often dependent on co-chaperones and ATP for their activity, and by the effect of competing aggregation reactions. Given that these considerations confound in vitro experiments, attempting to dissect the importance of fundamental forces in chaperone action in vivo would seem almost impossible. Fortunately, the simple chaperone–client pair Spy-Im7 neatly sidesteps most of these problems. Spy is a 16 kilodalton ATP-independent chaperone that requires no co-chaperones or ATP for its action, and its Im7 substrate has a well-characterized folding pathway. Several Im7 variants exist, including those that mimic folding intermediates [8, 9]. Im7 and its variants are resistant to aggregation both in vitro and in vivo. In vitro, Im7 is initially attracted to Spy through long-range electrostatic interactions; following binding, the Spy-unfolded client complex is primarily stabilized through hydrophobic interactions. Folding of Im7 while it is bound to Spy decreases its affinity to Spy and consequently promotes release of the client protein [4, 10, 11]. Spy acts to stabilize both wild-type (WT) and thermodynamically destabilized variants of Im7 in vivo and in vitro [12]. Tripartite folding biosensors consisting of Im7 variants inserted into the antibiotic resistance marker β-lactamase can be used to link the stability of Im7 variants to antibiotic resistance. These folding biosensors enable the facile readout of Spy’s chaperone activity on Im7 in vivo simply by measuring the antibiotic resistance of strains containing them [12].
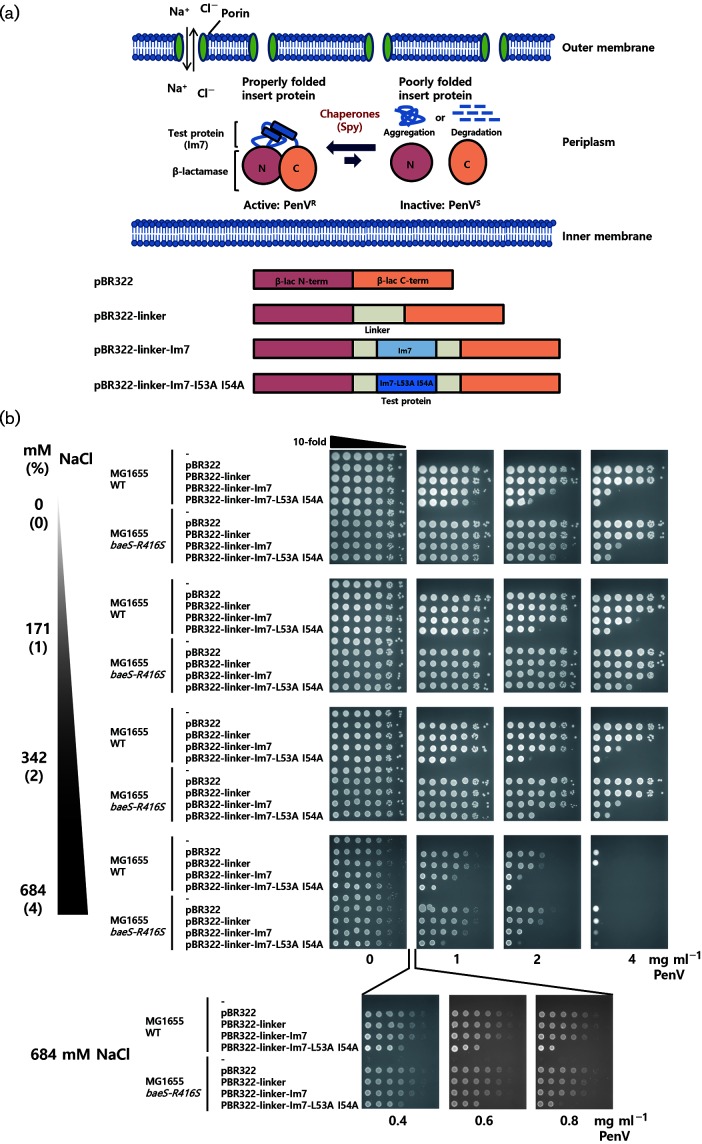
We discovered Spy as a chaperone in an in vivo screen for ‘super folder’ bacteria. Using a folding biosensor to link in vivo protein stability to antibiotic resistance, bacteria were required to stabilize an unstable variant of the model folding protein Im7- L53A I54A in order to survive [12]. The bacteria responded by overproducing Spy. Spy was then shown to both inhibit the aggregation of several proteins including Im7 in vitro and facilitate their folding, demonstrating that it functions directly as a chaperone [12]. In these folding biosensors, an unstable protein is inserted into the middle of a marker that encodes resistance to an antibiotic such as penicillin (PenV). Degradation of the unstable protein acts to separate the N- and C-terminal halves of the selectable marker, decreasing antibiotic resistance. Super-folder bacteria that can stabilize the unstable protein will allow the marker to fold better, keeping the two halves together and increasing the antibiotic resistance of strains containing the biosensor (Fig. 1a) [12, 13].
Fig. 1.
Ionic strength-dependent activity of Spy as monitored by an Im7-fused tripartite reporter system. (a) Our tripartite folding reporter consists of a test protein inserted into a permissive site within an antibiotic resistant protein. If the chaperone assists in the folding or stability of the test protein, cells harbouring this reporter will increase their antibiotic resistance. With decreased chaperone function, the tripartite protein will tend to be misfolded, aggregated, or degraded, and strains containing the reporter will show lower levels of antibiotic resistance. In the illustrated construct, β-lactamase and Im7 were used as the antibiotic-resistant protein and test protein, respectively. Im7 is integrated into a permissive site in β-lactamase using linker sequences [13]. The Im7-fused tripartite reporter system and Spy exist in the periplasm. Porins in the outer membrane allow the free diffusion of compounds smaller than ~600 Da into the periplasm. Therefore, the ionic strength in the periplasm can be adjusted by simply varying the ionic composition of the media. (b) Escherichia coli cells were transformed with pBR322-derived plasmids that contain tripartite fusion folding reporters and PenV resistance was tested by spot titre assay.
Escherichia coli K-12 MG1655 ∆hsdR (SQ765) was used as the WT strain and an isogenic baeS-R416S mutant (SQ1698) was used as a strain that constitutively overexpresses Spy [12]. Cells were transformed with pBR322-derived plasmids that contain tripartite fusion folding reporters [13] and grown in LB medium at 37 °C to reach an optical cell densities at 600 nm (OD600) of 1.0. Cultures were serially diluted in 10-fold increments to 10−6, and 3 µl of these dilutions was spotted onto LB agar media containing NaCl 0–684 mM (0–4 %) and different concentrations of PenV, followed by incubation at 37 °C for 18 h. Because of the instability of Im7-L53A I54A, an E. coli WT strain harbouring the Im7 mutant construct showed lower PenV resistance than the strain containing a WT Im7 construct (Fig. 1b). Previously, we isolated a Spy-overproducing mutant caused by missense mutations in the Spy regulator, baeS. Because Spy stabilizes the Im7-L53A I54A tripartite construct, the resistance to PenV is significantly increased in the baeS mutants compared to the WT strain (Fig. 1b) [12].
Spy functions in the E. coli periplasm, a compartment that is only separated from growth media by the highly permeable outer membrane; porins in this membrane allow the free diffusion of compounds smaller than about 600 Da. (Fig. 1a) [14]. For instance, it has been shown that small-molecule osmolites, such as glycerol, sorbitol and l-proline, can diffuse into periplasm and affect the activity of the β-lactamase tripartite protein folding sensor [5, 15]. We reasoned then that by simply varying the ionic composition of the media and tracking the effect of this on Spy chaperone function in vivo, we could obtain an insight into the role that electrostatic forces play in chaperone activity in vivo. In summary, we found that increasing the ionic strength inhibits Spy activity in vivo, strongly suggesting that electrostatic forces are important for chaperone function in vivo.
E. coli can grow in nutrient-rich media containing NaCl concentrations ranging from 0 to 855 mM (0–5 %) [16]. We used Luria–Bertani (LB) media with various NaCl concentrations (0 to 684 mM) to test Spy activity in vivo. We did this by simply determining the antibiotic resistance of strains containing folding biosensor tripartite fusions that link the in vivo folding of the inserted protein (Im7) to the antibiotic resistance of the strains containing these biosensors. In Fig. 1(b), one can see that the Spy overexpression strain MG1655 baeS-R416S shows significantly enhanced PenV resistance compared to the MG1655 WT strain at most salt concentrations. This is most evident by comparing the efficiency of plating (EOP) in the spot titre serial dilution experiments shown in Fig. 1(b) at 2 mg ml−1 PenV. Consistent with our previous results, Spy overexpression enhances the PenV resistance of the destabilized Im7 mutant Im7-L53A I54A most significantly, but also clearly enhances the stability of WT Im7, which is only a marginally stable protein in the first place. Enhanced resistance is evident at 0 mM added NaCl, is better at 171 mM NaCl, is marginal at 342 mM NaCl, and is marginal or not present at 684 mM NaCl. Cells generally grow more poorly at 684 mM NaCl, making them more sensitive to antibiotics in general. To make sure we can continue to monitor folding in vivo at these high salt concentrations, we also tested their EOP at low doses of PenV (between 0 and 1 mg ml−1) at 684 mM NaCl. We did see that the unstable Im7 mutant showed lower PenV resistance, showing that we can monitor folding, but observed no significant enhancement of PenV resistance by Spy overproduction for any of our constructs in the strain at these salt concentrations. This provides evidence that Spy is unable to facilitate folding at these high salt concentrations.
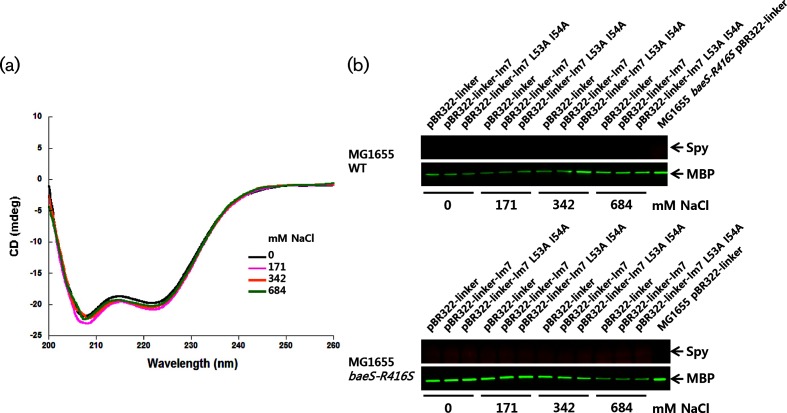
We considered the possibility that the stability of Spy or the β-lactamase portion of the tripartite fusion was directly affected by salt. To assess the effect of salt on the structural stability of Spy, we examined its secondary structure at different NaCl concentrations using circular dichroism (CD) spectroscopy (JASCO, J-1500) (Fig. 2a). We used 5 µM of Spy protein and 20 mM sodium phosphate buffer at pH 7.2 containing NaCl concentrations ranging from 0 to 684 mM. Spy maintained its α-helical secondary structure at all of the tested NaCl concentrations (0 to 684 mM). The same is true for intact β-lactamase; the secondary structure of β-lactamase is unchanged by the addition of KCl or sodium sulfate at concentrations as high as 1.5 M [17]. Thus, both Spy and the β-lactamase portion of the tripartite fusion should maintain their structural integrity at the range of NaCl concentrations used in our experiments.
Fig. 2.
The secondary structure of Spy and its expression level was monitored. (a) The secondary structure of the Spy protein was monitored by CD spectroscopy. (b) Total cells were prepared from cells grown on LB agar media with different NaCl concentrations, separated by 4–12 % gradient SDS-PAGE. The Spy protein level was detected by Western blotting using Spy-specific antiserum. Maltose-binding protein (MBP) was also monitored as a reference protein using MBP polyclonal antibody (NEB). Spy and MBP are indicated by arrows.
We also considered that salt-induced changes in Spy expression might affect our results. We therefore checked the Spy levels at different salt concentrations using Western blotting (Fig. 2b). Consistent with previous reports that Spy expression is induced 500-fold by constitutive baeS mutations [12], we found Spy to be undetectable in WT strains but very abundant in the baeS mutant background. Importantly, Spy expression appears to be salt-independent, at least in the MG1655 baeS-R416S mutant strain. Taken together, our observations indicate that high ionic strength does not affect the stability or expression of Spy, but diminishes its ability to function as a chaperone in vivo. There are at least two ways that high ionic strength can diminish Spy’s ability to function. The most straightforward way is by neutralizing the electrostatic interactions that have previously been shown to be important in vitro for the formation of the initial encounter complex that forms between Spy and Im7 [4]. The second way might be by salt favouring a more compact form of Im7, which partially mimics folded Im7, which is known to bind to Spy with lower affinity than either unfolded Im7 or mutants that mimic the intermediate on the folding pathway [4, 10]. To test this idea, we measured the salt dependence of the secondary structure content of the Im7 L53A I54A mutant, which populates the partially folded intermediate state, by CD spectroscopy at several NaCl concentrations (see Fig. S1, available in the online version of this article). The secondary structure of this intermediate mimicking mutant does seem to gain a minor amount of secondary structure as the salt concentration increases, suggesting that the compaction may contribute to the salt dependence of Im7 folding that we see in vivo, although the very slight dependence of the CD on salt concentration suggests that this contribution is likely to be minor in nature.
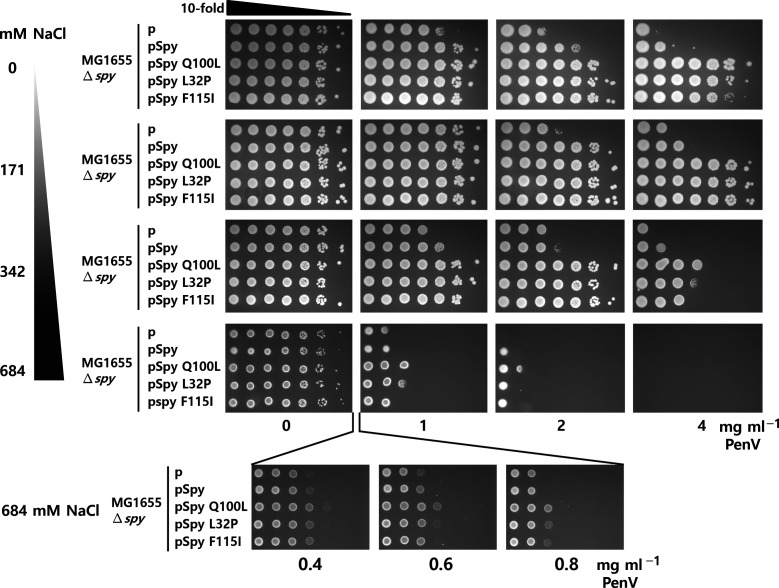
In a previous study, we isolated ‘super spy’ variants with enhanced chaperone activity in vitro and in vivo [18], which we attributed to changes in exposed hydrophobicity or flexibility. To determine whether increased salt concentration also affects the function of super Spy variants in vivo, we expressed the Im7-L53A I54A tripartite reporter system, together with WT Spy or super Spy variants, under an IPTG-inducible promoter. Antibiotic resistance was monitored at a range of NaCl concentrations (Fig. 3). Cells were grown at 37 °C, 0.2 mM IPTG was added to reach an OD600 of 0.3 and cells were incubated for an additional 1 h. We spotted 3 µl of serial dilutions spotted on LB agar media containing 0.2 mM IPTG, NaCl (0–684 mM) and different concentrations of PenV. Plates were incubated at 37 °C for 18 h. Consistent with our previous observations that super Spy variants are superior to WT Spy in stabilizing unstable Im7 variants, the expression of the super Spy variants Q100L, L32P and F115I enhanced PenV resistance more than the expression of WT Spy did. Although overall the resistance was greater, the salt dependence of this resistance roughly paralleled that provided by WT Spy. These results indicate that electrostatic interactions are not only crucial for WT Spy, but also for super Spy variants.
Fig. 3.
Electrostatic interactions are also important for the function of super Spy variants. WT and super Spy variants (Q100L, L32P and F115I) were expressed under the IPTG-inducible promoter from the pCDFTrckanBamHI plasmid in a ∆spy strain (SQ2041) [12, 18]. The tripartite construct with Im7-L53A I54A as a test protein was coexpressed and PenV resistance was monitored by spot titre assay.
In this study, we show that electrostatic interactions play an important role in Spy chaperone function in vivo [4]. Our previous studies revealed that electrostatic interactions are an important driving force for initial binding between Spy and its substrate Im7 in vitro [4]. The results presented here extend this observation to the in vivo environment. We found that the function of the super Spy variant Q100L is also salt-sensitive, despite its increased hydrophobicity, further emphasizing the importance of electrostatic interactions in Spy function. Unlike for the cytoplasm, salt ions in the outside environment can diffuse freely into the E. coli periplasm. Consequently, periplasmic proteins are exposed to changes in ionic strength as E. coli transitions between various environments. Consistent with our results for Spy, electrostatic interaction was also found to accelerate substrate binding for the periplasmic chaperone Skp, at least in vitro [19]. Therefore, it seems that ionic strength may be an important factor that impacts on the function of periplasmic chaperones. Physiological salt concentrations in the human large intestine range from 100 to 165 mM (0.6 to 1 %) [20]. However, enterobacteria such as E. coli not only exist in the gut, but also in various natural and man-made niches [16, 21]. For instance, they survive passage into the sea through the release of untreated sewage, and seawater has a salt content of 598–633 mM (3.5–3.7 %) [21, 22]. Bacterial cells induce a set of genes in response to hyperosmotic stress [23]. Whether any of these act to compensate for the decreased activity of periplasmic chaperones, however, remains an open question.
Funding information
This work was funded by a grant from the National Institutes of Health (R01-GM102829). J. C. A. B. is a Howard Hughes Medical Investigator.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Supplementary Data
Footnotes
Abbreviations: CD, circular dichroism; EOP, efficiency of plating; LB, Luria–Bertani; OD600, optical cell densities at 600 nm; WT, wild-type.
One supplementary figure is available with the online version of this article.
References
- 1.Balchin D, Hayer-Hartl M, Hartl FU. In vivo aspects of protein folding and quality control. Science. 2016;353:aac4354. doi: 10.1126/science.aac4354. [DOI] [PubMed] [Google Scholar]
- 2.Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323–355. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- 3.Koldewey P, Horowitz S, Bardwell JCA. Chaperone-client interactions: non-specificity engenders multifunctionality. J Biol Chem. 2017;292:12010–12017. doi: 10.1074/jbc.R117.796862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koldewey P, Stull F, Horowitz S, Martin R, Bardwell JCA. Forces driving chaperone action. Cell. 2016;166:369–379. doi: 10.1016/j.cell.2016.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coyle JE, Jaeger J, Gross M, Robinson CV, Radford SE. Structural and mechanistic consequences of polypeptide binding by GroEL. Fold Des. 1997;2:R93–R104. doi: 10.1016/S1359-0278(97)00046-1. [DOI] [PubMed] [Google Scholar]
- 6.Schreiber G, Haran G, Zhou HX. Fundamental aspects of protein-protein association kinetics. Chem Rev. 2009;109:839–860. doi: 10.1021/cr800373w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selzer T, Schreiber G. Predicting the rate enhancement of protein complex formation from the electrostatic energy of interaction. J Mol Biol. 1999;287:409–419. doi: 10.1006/jmbi.1999.2615. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson N, Capaldi AP, James R, Kleanthous C, Radford SE. Rapid folding with and without populated intermediates in the homologous four-helix proteins Im7 and Im9. J Mol Biol. 1999;286:1597–1608. doi: 10.1006/jmbi.1998.2548. [DOI] [PubMed] [Google Scholar]
- 9.Capaldi AP, Kleanthous C, Radford SE. Im7 folding mechanism: misfolding on a path to the native state. Nat Struct Biol. 2002;9:209–216. doi: 10.1038/nsb757. [DOI] [PubMed] [Google Scholar]
- 10.Stull F, Koldewey P, Humes JR, Radford SE, Bardwell JCA. Substrate protein folds while it is bound to the ATP-independent chaperone Spy. Nat Struct Mol Biol. 2016;23:53–58. doi: 10.1038/nsmb.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horowitz S, Salmon L, Koldewey P, Ahlstrom LS, Martin R, et al. Visualizing chaperone-assisted protein folding. Nat Struct Mol Biol. 2016;23:691–697. doi: 10.1038/nsmb.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quan S, Koldewey P, Tapley T, Kirsch N, Ruane KM, et al. Genetic selection designed to stabilize proteins uncovers a chaperone called Spy. Nat Struct Mol Biol. 2011;18:262–269. doi: 10.1038/nsmb.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foit L, Morgan GJ, Kern MJ, Steimer LR, von Hacht AA, et al. Optimizing protein stability in vivo. Mol Cell. 2009;36:861–871. doi: 10.1016/j.molcel.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SH, Kukushkin Y, Gupta R, Chen T, Konagai A, et al. PolyQ proteins interfere with nuclear degradation of cytosolic proteins by sequestering the Sis1p chaperone. Cell. 2013;154:134–145. doi: 10.1016/j.cell.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Hrenovic J, Ivankovic T. Survival of Escherichia coli and Acinetobacter junii at various concentrations of sodium chloride. EurAsia J BioSci. 2009;3:144–151. doi: 10.5053/ejobios.2009.3.0.18. [DOI] [Google Scholar]
- 17.Goto Y, Fink AL. Conformational states of beta-lactamase: molten-globule states at acidic and alkaline pH with high salt. Biochemistry. 1989;28:945–952. doi: 10.1021/bi00429a004. [DOI] [PubMed] [Google Scholar]
- 18.Quan S, Wang L, Petrotchenko EV, Makepeace KA, Horowitz S, et al. Super Spy variants implicate flexibility in chaperone action. Elife. 2014;3:e01584. doi: 10.7554/eLife.01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu J, Mayer C, Behrens S, Holst O, Kleinschmidt JH. The trimeric periplasmic chaperone Skp of Escherichia coli forms 1:1 complexes with outer membrane proteins via hydrophobic and electrostatic interactions. J Mol Biol. 2007;374:91–105. doi: 10.1016/j.jmb.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16:351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 21.Cabral JP. Water microbiology. Bacterial pathogens and water. Int J Environ Res Public Health. 2010;7:3657–3703. doi: 10.3390/ijerph7103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rozen Y, Belkin S. Survival of enteric bacteria in seawater. FEMS Microbiol Rev. 2001;25:513–529. doi: 10.1111/j.1574-6976.2001.tb00589.x. [DOI] [PubMed] [Google Scholar]
- 23.Sleator RD, Hill C. Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiol Rev. 2002;26:49–71. doi: 10.1111/j.1574-6976.2002.tb00598.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.