Abstract
Diterpenoids are considered the major active compounds in Tinospora sinensis in virtue of their special structures and activities. Herein, an analytical method was developed for rapid screening and identification of diterpenoids in T. sinensis using high-performmance liquid chromatography coupled with linear ion trap-Orbitrap mass spectrometry (HPLC-LTQ-Orbitrap) in negative ion mode. Two diterpenoid reference standards were first analyzed to obtain their characteristic ESI-MS/MS fragmentation patterns. Then, based on the extracted ion chromatogram (EIC) data-mining method and characteristic fragmentation pathways analysis, diterpenoids in T. sinensis were rapidly screened and identified. After that, an important parameter, Clog P, was adopted to discriminate between the isomers of diterpenoids. As a result, 63 diterpenoids were characterized from the extract of T. sinensis, including 10 diterpenoids and 53 diterpenoid glycosides. Among them, 15 compounds were tentatively identified as new compounds. Finally, target isolation of one diterpenoid glycoside named tinosineside A was performed based on the obtained results, which further confirmed the deduced fragmentation patterns and identified diterpenoid profile in T. sinensis. The results demonstrated that the established method could be a rapid, effective analytical tool for screening and characterization of diterpenoids in the complex systems of natural medicines.
Keywords: HPLC-LTQ-Orbitrap, diterpenoids, characteristic fragmentation pathways, Tinospora sinensis
1. Introduction
Tinospora sinensis (Kuanjinteng in Chinese) is commonly known as ‘Gurch’ and belongs to the family Menispermaceae, which is mainly distributed in the tropical parts of the eastern hemisphere. It has been traditionally used as folk medicine for treating debility, dyspepsia, rheumatism, gonorrhea, fever, inflammation, syphilis, ulcer, bronchitis, jaundice, urinary disease, skin disease and liver disease [1,2,3]. Previous phytochemical investigations have discovered that this species contains diterpenoids, alkaloids, flavonoids, lignans, triterpenes, amino acids, and so on. These constituents were reported to exert anti-inflammatory, immunomodulatory, antidiabetic, antitumor, antiadhesive, antiviral, anti-infective, antioxidant, antimutagenic, hematopoietic activities, etc. [4,5,6,7,8]. To our best knowledge, few reports on the systematic analysis of the diterpenoids in T. sinensis are available until now.
Recently, with the development of various data acquisition methods, high-resolution mass spectrometry (HRMS), especially linear ion trap-Orbitrap mass spectrometer (LTQ-Orbitrap), has exhibited excellent performance in the detection of targeted mixture constituents owing to its high speed and detection sensitivity [9,10]. The hybrid linear ion trap-Orbitrap mass spectrometer (LTQ-Orbitrap MS) combines high trapping capacity and MSn scanning function of a linear ion trap together with accurate mass measurements within 3 ppm. The resolution power (up to 100,000) over a wider dynamic range is superior to that of many other mass spectrometers [11,12,13]. In the meantime, the combined application of tandem mass spectrometry for identifying the complicated constituents in traditional Chinese medicines (TCMs) could produce a large quantity of information data, such as molecular weights, elemental compositions, fragmentation patterns of multiple-stage, etc. [10]. Off-line processing of data, such as extracted ion chromatogram (EIC) data-mining method, plays a vital role in component identification [14,15]. These advantages have made LTQ-Orbitrap MS one of the most powerful approaches for the rapid identification and characterization of the multiple constituents found in TCMs [16]. It is well known that studies on flavonoids and alkaloids in T. sinensis by LC-MS have been widely reported, but little attention has been paid to its diterpenoids, mainly due to the complexity and variety of their skeletons, the diversity of their substituents, and a lack of corresponding reference standards. Hence, the characterization of diterpenoids in T. sinensis is of great significance.
In the present study, an HPLC-LTQ-Orbitrap data-acquisition approach combined with the EIC data-mining technique was established for comprehensive identification of the diterpenoids and their derivatives in T. sinensis.
2. Results and Discussion
Owing to the low content of diterpenoids in T. sinensis, a sensitive and reliable HPLC-LTQ-Orbitrap approach was established for determining their accurate masses and molecular formulas. In addition, the characteristic fragmentation pathways of diterpenoids were deduced from two obtained standards, and then used for the rapid identification and characterization of the other diterpenoids in T. sinensis. Furthermore, diterpenoid isomers were differentiated by an important parameter, Clog P, which is the absolute numerical value of the distribution coefficient of substances in a two-phase lipid-water system. Finally, target isolation of one diterpenoid glycoside named tinosineside A was performed based on the obtained results, which further confirmed the deduced fragmentation patterns. As a result, a total of 63 diterpenoids in T. sinensis were screened and divided into diterpenoid aglycones and diterpenoid glycosides (see supplementary materials and Table 1).
Table 1.
Summary of chemical constituents identified in Tinospora sinensis by HPLC-LTQ-Orbitrap.
| NO. | tR/min | Identification | Empirical Formula | Proposed Ions | Experimental Mass m/z | Theoretical Mass m/z | Mass Error (×10−6) | MS2 Data (Measured) |
|---|---|---|---|---|---|---|---|---|
| 1 | 16.13 | (2,3,4,6)-2-(Acetoxymethyl)-6-(((2,4,6,6,10,10)-2-(furan-3-yl)-9,10-dimethoxy-7-(methoxycarbonyl)-6,10-dimethyl-4-oxododecahydro-1H-benzo[f]isochromen-6-yl)oxy)-tetrahydro-2H-pyran-3,4,5-triyl triacetate * | C37H49O17 | [M − H]− | 765.29578 | 765.29642 | 0.836 | 747(10), 737(48), 721(38), 720(43), 719(100), 697(5), 672(11), 555(18) |
| 2 | 17.69 | Cordioside | C26H33O12 | [M − H]− | 537.19592 | 537.19665 | 1.359 | 519(4), 491(8), 490(13), 375(100), 357(43), 327(88), 297(4) |
| 3 | 18.55 | (4,6,6,7,9,10,10,10)-2-(Furan-3-yl)-7-hydroxy-10b-methyl-4-oxo-6-(((3,5,6)-3,4,5-trihydroxy-6-(hydroxymethyl)-tetrahydro-2H-pyran-2-yl)-oxy)dodecahydro-1H-benzo-[f]isochromene-9,10-diyl diacetate * | C28H37O14 | [M − H]− | 597.21661 | 597.21778 | 1.959 | 579(3), 551(60), 476(7), 435(10), 389(96), 359(100) |
| 4 | 20.51 | (2,5,6,8,9,10,12)-15,16-Epoxy-2-hydroxy-6-O-{β-d-xylopyranosyl(1→6)-d-glucopyranosyl}-cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester * | C32H43O16 | [M − H]− | 683.25372 | 683.25456 | 1.229 | 665(68), 639(12), 637(16), 615(95), 520(100), 519(79), 370(15), 309(37) |
| 5 | 21.89 | 1-Deacetyltinosposide A | C24H33O12 | [M − H]− | 513.19678 | 513.19665 | −0.253 | 495(3), 351(12), 333(100), 307(11), 305(5), 271(5) |
| 6 | 25.01 | Cordifoliside D | C26H33O12 | [M − H]− | 537.19623 | 537.19665 | 0.782 | 519(38), 492(22), 491(100), 490(71), 469(71), 297(38) |
| 7 | 26.40 | Tinospinoside D | C27H35O13 | [M − H]− | 567.20660 | 567.20721 | 1.075 | 552(1), 521(9), 404(0.5), 359(100), 341(2), 329(2) |
| 8 | 26.55 | (6,6,10,10,10)-Methyl 10-acetoxy-2-(furan-3-yl)-9-hydroxy-10b-methyl-4-oxo-6-(((3,5,6)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-dodecahydro-1H-benzo[f]-isochromene-7-carboxylate * | C28H37O14 | [M − H]− | 597.21741 | 597.21778 | 0.619 | 565(4), 551(21), 550(25), 535(10), 463(12), 435(12), 389(100), 388(12) |
| 9 | 26.98 | Borapetoside B | C27H35O12 | [M − H]− | 551.21204 | 551.21230 | 0.471 | 536(5), 533(2), 507(3), 389(100), 388(15), 371(2), 370(7), 329(2) |
| 10 | 27.65 | Amritoside C | C27H35O13 | [M − H]− | 567.20612 | 567.20721 | 1.921 | 552(6), 529(20), 521(100), 404(10), 341(4) |
| 11 | 29.43 | Tinospinoside B | C27H35O12 | [M − H]− | 551.21298 | 551.21230 | −1.233 | 533(9), 532(31), 515(12), 505(100), 389(8), 344(8) |
| 12 | 29.52 | (2,4,6,6,7,7,8,9,9,9)-2-(furan-3-yl)-6a,9b-dimethyl-6-(((2,3,4,5,6)-3,4,5-trihydroxy-6-(hydroxymethyl)-tetrahydro-2H-pyran-2-yl)oxy)decahydro-1H-9,7-(epoxymethano)-oxireno[2′,3′:4,5]benzo[1,2-f]isochromene-4,11(2H)-dione * | C26H31O12 | [M − H]− | 535.18036 | 535.18100 | 1.196 | 517(0.5), 489(2), 467(2), 373(100), 345(1), 343(2), 313(2) |
| 13 | 30.82 | Tinosposinenside A | C27H35O12 | [M − H]− | 551.21173 | 551.21230 | 1.034 | 536(5), 533(3), 507(3), 389(100), 374(11), 371(16), 359(2), 341(7) |
| 14 | 31.03 | (6,6,7,9,10,10,10)-2-(Furan-3-yl)-7-hydroxy-10b-methyl-4-oxo-6-(((3,5,6)-3,4,5-trihydroxy-6-(hydroxymethyl)-tetrahydro-2H-pyran-2-yl)oxy)dodecahydro-1H-benzo-[f]isochromene-9,10-diyl diacetate * | C28H37O14 | [M − H]− | 597.21692 | 597.21778 | 1.440 | 579(4), 561(11), 551(13), 529(19), 515(5), 477(6), 435(12), 389(82), 359(100) |
| 15 | 31.41 | Rumphioside A | C27H35O13 | [M − H]− | 567.20685 | 567.20721 | 0.635 | 549(14), 548(25), 535(25), 521(34), 405(46), 404(100), 359(56) |
| 16 | 31.97 | Furanoid diterpene glycoside | C26H33O11 | [M − H]− | 521.20099 | 521.20173 | 1.420 | 506(1), 359(100), 345(3), 344(4), 341(3) |
| 17 | 32.03 | Rumphioside F | C27H35O13 | [M − H]− | 567.20642 | 567.20721 | 1.393 | 549(22), 521(47), 491(100), 405(11), 404(18), 387(19) |
| 18 | 33.06 | (2,6,6,7,9,10,10,10)-2-(Furan-3-yl)-6,9,10-trihydroxy-10b-methyl-7-(((2,3,4,5,6)-3,4,5-trihydroxy-6-(hydroxyl-methyl)tetrahydro-2H-pyran-2-yl)oxy)decahydro-1H-benzo[f]isochromen-4(2H)-one * | C24H33O12 | [M − H]− | 513.19635 | 513.19665 | 0.585 | 495(3), 467(1), 351(100), 305(60), 287(7), 161(3) |
| 19 | 34.12 | (2R,5R,6R,8R,9S,10S,12S)-15,16-Epoxy-2-hydroxy-6-O-(β-d-glucopyranosyl)-cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester | C27H35O12 | [M − H]− | 551.21185 | 551.21230 | 0.816 | 533(2), 519(3), 505(4), 482(4), 389(100), 327(67) |
| 20 | 35.07 | Tinosineside B | C28H37O14 | [M − H]− | 597.21716 | 597.21778 | 1.038 | 578(20), 551(100), 529(10), 517(12), 461(25), 179(49) |
| 21 | 35.26 | Palmatoside F | C26H31O12 | [M − H]− | 535.17987 | 535.18100 | 2.111 | 520(4), 517(2), 488(3), 373(93), 329(100) |
| 22 | 35.35 | (2,6,7,9,10,10,10)-2-(Furan-3-yl)-7,10-dihydroxy-9-methoxy-10b-methyl-6-(((2,3,4,5,6)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)deca-hydro-1H-benzo[f]isochromen-4(2H)-one * | C25H35O12 | [M − H]− | 527.21241 | 527.21230 | −0.209 | 511(14), 509(3), 494(14), 481(100), 347(6), 300(7) |
| 23 | 35.80 | Amritoside A | C26H35O13 | [M − H]− | 555.20624 | 555.20721 | 1.747 | 537(5), 513(34), 495(53), 467(19), 393(100), 375(5), 305(74), 287(7) |
| 24 | 36.87 | Rumphioside D | C37H49O17 | [M − H]− | 765.29431 | 765.29642 | 2.757 | 747(19), 734(20), 721(37), 719(41), 718(100), 697(18), 600(26), 418(4), 393(11) |
| 25 | 38.12 | (4,6,6,9,10,10)-Methyl 9-acetoxy-2-(furan-3-yl)-4a- hydroxy-10b-methyl-4-oxo-6-(((3,5,6)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-dodecahydro-1H-benzo[f]isochromene-7-carboxylate * | C28H37O14 | [M − H]− | 597.21619 | 597.21778 | 2.662 | 582(73), 579(9), 556(41), 551(28), 550(72), 434(31), 416(33), 389(75), 329(100) |
| 26 | 38.15 | Sagittatayunnanoside D | C26H35O11 | [M − H]− | 523.21667 | 523.21738 | 1.357 | 508(1), 505(1), 361(100), 347(8), 343(4), 329(4) |
| 27 | 38.26 | Borapetoside H | C33H45O17 | [M − H]− | 713.26588 | 713.26512 | −1.066 | 694(12), 668(18), 667(29), 666(100), 645(23), 551(21), 533(6), 389(6) |
| 28 | 38.38 | (5R,6R,8S,9R,10R,12S)-15,16-Epoxy-2-oxo-6-O-(β-d-glucopyranosyl)–cleroda-3,13(16) 14-trien-17,12-olid-18-oic acid methyl ester | C27H33O12 | [M − H]− | 549.19592 | 549.19665 | 1.329 | 531(0.4), 503(5), 481(3), 387(100), 249(1) |
| 29 | 38.80 | Isocolumbin a | C20H21O6 | [M − H]− | 357.13321 | 357.13326 | 0.140 | 342(9), 339(2), 313(1), 311(13), 151(100), 135(37) |
| 30 | 38.84 | Tinoside | C26H31O11 | [M − H]− | 519.18542 | 519.18608 | 1.271 | 501(0.1), 473(0.3), 358(0.4), 357(100) |
| 31 | 39.44 | Tinosineside A | C26H35O13 | [M − H]− | 555.20581 | 555.20721 | 2.522 | 513(100), 495(69), 393(2), 375(8), 333(28), 315(10), 307(13), 305(1) |
| 32 | 40.03 | Tinocapilactone B | C22H25O8 | [M − H]− | 417.15411 | 417.15439 | 0.671 | 402(42), 371(9), 356(2), 181(100), 166(34), 151(16) |
| 33 | 40.61 | Tinoscorside C | C27H33O12 | [M − H]− | 549.19586 | 549.19665 | 1.438 | 531(7), 513(2), 503(13), 481(10), 417(5), 387(100) |
| 34 | 40.79 | (1,3,10,10)-9-(2-Methoxy-2,5-dihydrofuran-3-yl)-10a-methyl-4,7-dioxo-3-(((2,3,4,5,6)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)tetra-decahydroisobenzofuro[7,1-fg]isochromen-1-yl acetate * | C28H37O14 | [M − H]− | 597.21655 | 597.21778 | 2.060 | 579(51), 551(100), 528(39), 471(12) |
| 35 | 41.60 | Rumphioside I | C27H35O12 | [M − H]− | 551.21210 | 551.21230 | 0.363 | 533(9), 519(11), 505(2), 482(3), 339(100), 324(5) |
| 36 | 42.17 | Borapetoside C | C27H35O11 | [M − H]− | 535.21637 | 535.21738 | 1.887 | 520(1), 517(4), 488(1), 373(100), 359(5), 358(7), 355(2), 341(5) |
| 37 | 43.22 | (1,2,7,8)-1-(2-Ffuran-3-yl)-2-hydroxyethyl)-2-hydroxy-5-(methoxycarbonyl)-1-methyl-7-(((3,4,6)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-1,2,3,4,6,7,8,8-octahydronaphthalene-2-carboxylic acid * | C26H35O13 | [M − H]− | 555.20630 | 555.20721 | 1.639 | 537(0.2), 513(1), 495(100), 393(0.2), 333(1), 297(2), 178(0.2) |
| 38 | 43.99 | Tinosposinenside B | C28H37O13 | [M − H]− | 581.22162 | 581.22286 | 2.133 | 563(8), 535(37), 419(2), 373(33), 343(100), 297(6) |
| 39 | 44.59 | Borapetoside A | C26H33O12 | [M − H]− | 537.19604 | 537.19665 | 1.136 | 519(4), 493(2), 491(62), 375(3), 371(5), 357(1), 341(2), 329(100), 297(1) |
| 40 | 45.22 | 8-Hydroxycolumbin | C20H21O7 | [M − H]− | 373.12759 | 373.12817 | 1.554 | 358(2), 343(45), 325(6), 313(100) |
| 41 | 45.78 | 6-Hydroxycolumbin | C20H21O7 | [M − H]− | 373.12759 | 373.12817 | 1.554 | 358(9), 355(7), 343(79), 329(3), 325(15), 313(100), 305(3), 261(2) |
| 42 | 46.04 | Boropetoside G | C27H37O11 | [M − H]− | 537.23218 | 537.23303 | 1.582 | 518(1), 375(100), 361(9), 360(2), 357(2), 343(2) |
| 43 | 46.82 | (4,6,6,9,10,10)-Methyl 9-acetoxy-2-(furan-3-yl)-4a-hydroxy-10b-methyl-4-oxo-6-(((3,5,6)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)dodec-ahydro-1H-benzo[f]isochromene-7-carboxylate * | C28H37O14 | [M − H]− | 597.2168 | 597.21778 | 1.641 | 579(23), 565(52), 564(45), 551(27), 529(31), 389(10), 329(100) |
| 44 | 46.89 | Tinocrisposide | C27H35O11 | [M − H]− | 535.21600 | 535.21738 | 2.578 | 520(2), 516(5), 488(7), 373(100), 359(8), 358(6), 355(3), 341(2) |
| 45 | 49.44 | Cordifolide A | C28H37O12S | [M − H]− | 597.19922 | 597.20002 | 1.340 | 579(13), 553(33), 550(53), 533(100), 528(25), 467(43), 466(25), 434(18), 372(32), 359(11) |
| 46 | 49.89 | (2R,5R,6R,8S,9S,10S,12S)-15,16-Epoxy-2-hydroxy-6-O-{β-d-glucopyranosyl-(1→6)-α-d-xylopyranosyl}-cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester | C32H43O16 | [M − H]− | 683.25416 | 683.25456 | 0.585 | 665(2), 664(11), 637(2), 615(3), 534(3), 520(100), 502(93), 490(12) |
| 47 | 50.40 | 3-((6,6,7,10,10,10)-7-Hydroxy-6a,10b-dimethyl-4,12-dioxo-6-(((3,5,6)-3,4,5-trihydroxy-6-(hydroxymethyl)-tetrahydro-2H-pyran-2-yl)oxy)-2,4,4,5,6,6,7,10,10,10-decahydro-1H-10,7-(epoxymethano)benzo[f]isochromen-2-yl)tetrahydrofuran-2-yl acetate * | C28H37O14 | [M − H]− | 597.21680 | 597.21778 | 1.641 | 579(1), 561(15), 551(9), 495(6), 487(7), 486(66), 435(4), 433(100), 297(44) |
| 48 | 51.12 | Tinosposinenside C | C26H35O12 | [M − H]− | 539.21155 | 539.21230 | 1.391 | 521(3), 497(100), 479(86), 478(8), 377(2) |
| 49 | 52.20 | (1,3,10,10)-9-(2-Methoxy-2,5-dihydrofuran-3-yl)-10a-methyl-4,7-dioxo-3a-(((2,3,4,5,6)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)tetra-decahydroisobenzofuro[7,1-fg]isochromen-1-yl acetate * | C28H37O14 | [M − H]− | 597.21655 | 597.21778 | 2.060 | 579(0.4), 551(98), 509(7), 491(100), 481(6), 435(2) |
| 50 | 59.05 | 6′-O-Lactoylborapetoside B | C30H39O14 | [M − H]− | 623.23434 | 623.23343 | −1.460 | 513(3), 460(100), 458(7), 446(7), 444(4), 297(1), 283(12) |
| 51 | 59.98 | Columbin a | C20H21O6 | [M − H]− | 357.13297 | 357.13326 | 0.812 | |
| 52 | 60.37 | Tinospinoside E | C26H29O11 | [M − H]− | 517.16986 | 517.17043 | 1.102 | 499(26), 473(10), 471(20), 381(13), 355(6), 341(11), 162(100), 151(6) |
| 53 | 61.73 | Tinosporaside | C25H31O10 | [M − H]− | 491.19087 | 491.19117 | 0.611 | 473(28), 472(100), 460(8), 444(0.3), 327(0.4), 312(2) |
| C26H33O12 | [M − H + HCOOH]− | 537.19697 | 537.19665 | −0.596 | 519(4), 518(11), 491(25), 490(100), 357(3), 343(4), 327(8) | |||
| 54 | 62.14 | Sagittatayunnanoside B | C33H47O17 | [M − H]− | 715.28030 | 715.28077 | 0.657 | 670(2), 628(1), 652(1), 552(100), 551(16) |
| 55 | 63.08 | Tinospinoside C | C27H35O12 | [M − H]− | 551.21155 | 551.21230 | 1.361 | 533(73), 507(90), 505(100), 483(85), 415(84), 389(22), 343(81) |
| 56 | 63.14 | Sagittatayunnanoside C | C32H47O15 | [M − H]− | 671.28912 | 671.29094 | 2.711 | 653(1), 627(2), 509(50), 347(27), 329(100), 301(70), 241(25) |
| 57 | 63.83 | Tinosponone | C19H21O5 | [M − H]− | 329.13791 | 329.13835 | 1.337 | 314(100), 311(24), 285(70), 191(18) |
| 58 | 65.99 | Sagittatayunnanoside A | C26H37O10 | [M − H]− | 509.23792 | 509.23812 | 0.393 | 491(5), 465(5), 347(33), 329(100), 301(89), 257(9), 241(11) |
| 59 | 69.79 | 2-O-Lactoylborapetoside B | C30H39O14 | [M − H]− | 623.23334 | 623.23343 | 0.144 | 608(1), 591(23), 551(7), 486(16), 460(100), 297(34) |
| 60 | 78.98 | Tinotufolin D | C20H25O4 | [M − H]− | 329.17441 | 329.17473 | 0.972 | 311(36), 285(100), 293(7), 267(10), 249(16) |
| 61 | 82.48 | (2aβ,3α,5aβ,6β,7α,8aα)-6-2-(3-Furanyl)ethyl-2a,3,4,5,5a,6,7,8,8a,8b-decahydro-2a,3-dihydroxy-6,7,8b-trimethyl-2H-naphtho1,8-bcfuran-2-one | C20H27O5 | [M − H]− | 347.18491 | 347.18530 | 1.123 | 329(63), 303(3), 301(100), 285(3), 187(1) |
| 62 | 88.91 | (3,4,5,8)-Methyl-5-(2-(furan-3-yl)ethyl)-3-hydroxy-5,8a-dimethyl-3,4,4,5,6,7,8,8a-octahydronaphthalene-1-carboxylate * | C20H27O4 | [M − H]− | 331.19016 | 331.19038 | 0.664 | 313(9), 287(15), 285(100), 283(30), 271(2), 257(5), 243(2), 237(0.4) |
| 63 | 95.27 | Tinotufolin C | C21H31O5 | [M − H]− | 363.21662 | 363.21660 | −0.055 | 345(0.1), 334(0.2), 317(69), 295(100) |
a Comparison with standards. * Tentatively identified as new compounds.
2.1. The Fragmentation Patterns of Reference Standards
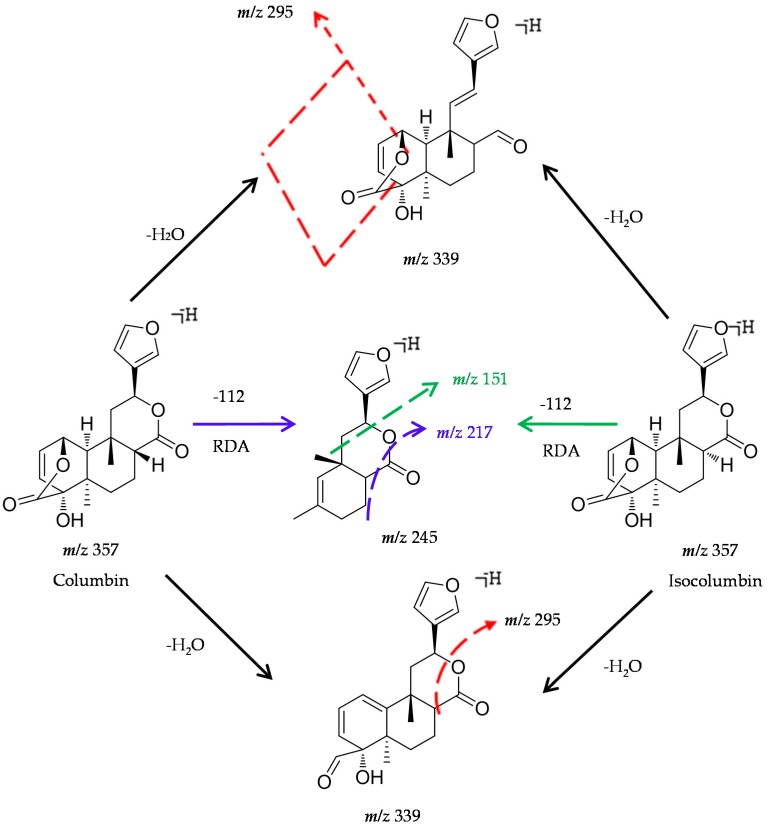
Columbin and isocolumbin yielded their [M − H]− ions at m/z 357.13326 (C20H21O6). Both of their deprotonated molecular ions generated a serial of fragment ions at m/z 342, m/z 339, m/z 313 and m/z 295, corresponding to [M − H − CH3]−, [M − H − H2O]−, [M − H − CO2]− and [M − H − H2O − CO2]−. Columbin produced a fragment ion at m/z 329 through losing carbonyl, and the ion at m/z 329 loss of a CO2 produced its ESI-MS2 base peak ion at m/z 285. The ion at m/z 245 [M − H − 112]− resulted from a Retro-Diels-Alder (RDA) cleavage fragmentation at the 1,4-position of the A-ring. Moreover, the product ion at m/z 245 generated the minor ion at m/z 217 by loss of one molecule of CO.
Meanwhile, isocolumbin produced its ESI-MS2 base peak ion at m/z 339 by neutral loss of H2O. After a RDA cleavage at A-ring and successive fracture of C9–11 (C6H6O, which was D-ring plus –C2H2), the prominent ion at m/z 151 was generated (Figure 1). Therefore, the characteristic fragment ions of reference standards were deduced, such as [M − H − 44]− generated by loss of CO2 from lactone ring, as well as [M − H − 15]−, [M − H − 18]−, [M − H − 44 − 18]−, [M − H − 112]−, etc., According to these characteristic fragmentation patterns, diterpenoids in T. sinensis could be rapidly screened and identified.
Figure 1.
Proposed MS/MS mechanistic pathways for columbin and isocolumbin.
2.2. Identification of Diterpenoids Aglycones
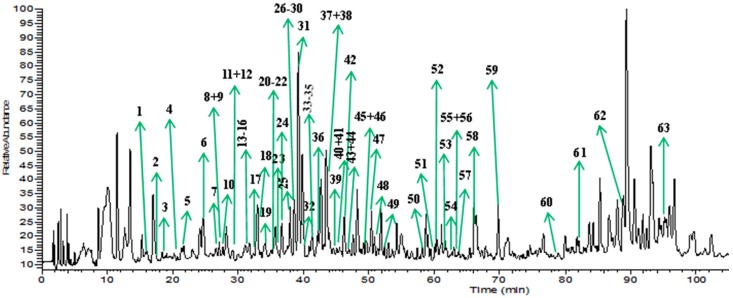
The molecular formulas of diterpenoids were predicted by a HRMS database built in-house. Combined with multi-stage mass spectrometry fragmentation information and bibliographic data, two categories of diterpenoids and its derivatives were screened and determined, including 10 diterpenoid aglycones and 53 diterpenoid glycosides. The typical total ion chromatogram (TIC) of T. sinensis in negative ion mode is presented in Figure 2.
Figure 2.
TIC chromatogram of T. sinensis in negative ion mode.
Compounds 29 and 51 generated their [M − H]− ions at m/z 357.13326 (C20H21O6). They were unambiguously assigned as isocolumbin and columbin, respectively, by comparison of their retention times, literature data and MS fragmentation data with their standards [17,18]. Isocolumbin and columbin constitute a pair of diastereomers, whose retention time and polarity are different under the present chromatographic conditions.
Compound 32 produced its [M − H]− ion at m/z 417.15439 (C22H25O8). In CID mode, it further generated [M − H − CH3]−, [M − H − H2O − CO]− and [M − H − H2O − CO − CH3]− ion at m/z 402, m/z 371 and m/z 356, respectively. The ESI-MS2 base peak ion at m/z 181 (C11H17O2) was generated by RDA cleavage fragmentation from 1,4-position of the A-ring and fracture of C9–11 (C6H4O3). The product ion at m/z 181 loss of a methyl generated the prominent ion at m/z 166. Therefore, compound 32 was tentatively deduced as tinocapilactone B [19].
Compounds 40 and 41 produced their [M − H]− ions at m/z 373.12817 (C20H21O7). Both of their deprotonated molecular ions generated a series of fragment ions at m/z 358 [M − H − CH3]−, m/z 343 [M − H − CO − H2]−, m/z 325 [M − H − 48 amu]− and m/z 313 [M − H − 60 amu]−, respectively. In addition, compound 41 generated two minor ions at m/z 305 and m/z 261. By comparison with the MS fragmentation patterns obtained from the reference standards, compounds 40 and 41 were plausibly characterized as 8-hydroxycolumbin and 6-hydroxycolumbin, respectively.
Compound 57 showed the [M − H]− ion at m/z 329.13835 (C19H21O5). Its ESI-MS2 base peak ion at m/z 314 was generated by loss of a methyl. The major fragment ions at m/z 311 and m/z 285 were yielded by neutral loss of H2O and CO2, respectively. Moreover, the product ion at m/z 285 generated the predominant product ion at m/z 191 due to the overall fracture of C9–11 (C6H6O). According to the literature data, it was tentatively identified as tinosponone [20].
Compounds 60 and 61 produced their respective [M − H]− ion at m/z 329.17473 (C20H25O4) and m/z 347.18530 (C20H27O5). Both of their deprotonated molecular ions generated [M − H − H2O]−, [M − H − CO2]− and [M − H − H2O − CO2]− ions at m/z 311, m/z 329, m/z 285, m/z 303 and m/z 267, m/z 285, respectively. By comparing with the literature data, compounds 60 and 61 were tentatively identified as tinotufolin D and (2aβ,3α,5aβ,6β,7α,8aα)-6-2-(3-furanyl)ethyl-2a,3,4,5,5a,6,7,8,8a,8b- decahydro-2a,3-dihydroxy-6,7,8b-trimethyl-2H-naphtho-1,8-bcfuran-2-one, respectively [21].
Compound 62 gave a [M − H]− ion at m/z 331.19038 (C20H27O4). Its MS2 spectrum produced the fragment ion at m/z 313, which involves the loss of H2O. The molecular ion yielded a series of fragment ions at m/z 287 [M − H − CO2]−, m/z 283 [M − H − H2O − H2CO]− and m/z 271 [M − H − CH4 − CO2]−, suggesting the presence of -COOCH3. The base peak ion at m/z 285 was generated by losing water and carbon monoxide. The [M − H − C6H6O]− ion at m/z 237 was generated by the fracture of C9-11 (C6H6O). Moreover, by comparison with the fragmentation patterns obtained from the two reference standards, compound 62 was tentatively identified as a new compound.
Compound 63 generated a [M − H]− ion at m/z 363.21660 (C21H31O5). It generated a serial of fragment ions at m/z 345 [M − H − H2O]−, m/z 334 [M − H − 29 amu]−, m/z 317 [M − H − H2O − CO]− and m/z 295 [M − H − 2H2O − OCH4]−. By comparison with the literature data, compound 63 was tentatively identified as tinotufolin C [21].
2.3. Identification of Diterpenoids Glycosides
Compounds 1 and 24 produced their [M – H]− ions at m/z 765.29642 (C37H49O17). After the CID cleavage, both of their further fragmentations resulted in a [M − H − CO2]− ion at m/z 721 and a [M − H − H2O − CO]− ion at m/z 719, which were consistent with the characteristic fragmentation pathways of diterpenoids. According to the fragmentation patterns and the values of Clog P, compounds 1 and 24 were plausibly characterized as (2,3,4,6)-2-(acetoxymethyl)-6-(((2,4,6,6,10,10)-2-(furan-3-yl)-9,10-dimethoxy-7-(methoxycarbonyl)-6,10-dimethyl-4-oxododecahydro-1H-benzo[f]isochromen-6-yl)-oxy)tetrahydro- 2H-pyran-3,4,5-triyl triacetate and rumphioside D, respectively.
Compounds 2, 6 and 39 produced their [M − H]− ions at m/z 537.19665 (C26H33O12). They all produced the [M − H − H2O]− and [M − H − H2O − CO]− ions at m/z 519 and m/z 491. In addition, compounds 2 and 39 also yielded the [M − H − 162]− ions at m/z 375 due to the overall fracture of dehydrated glucose, which was the ESI-MS2 base peak of compound 2. As well as we known, [M − H − 162]− was the characteristic ion of glycosides. Furthermore, compounds 2, 6 and 39 generated the ions at m/z 490 [M − 2H − H2O − CO]−, m/z 357 [M − H − Glc]−, m/z 327 [M − H − Glc − 2CH3]−, m/z 297 [M − 2H − CO − Glc − OCH3]−; m/z 492 [M − 2H − CO2]−, m/z 490 [M − 2H − H2O − CO]−, m/z 469 [M − 2H − H2O − CO − OCH3]−, m/z 297 [M − 2H − CO − Glc − OCH3]−; m/z 493 [M − H − CO2]−, m/z 357 [M − H − Glc]−, m/z 341 [M − H − O − Glc]−, m/z 329 [M − H − CO − Glc]−, m/z 297 [M − 2H − CO − Glc −OCH3]−, respectively. By comparison with the literature data, compounds 2, 6 and 39 were tentatively assigned as cordioside, cordifoliside D and borapetoside A, respectively [22,23].
Compounds 3, 8, 14, 20, 25, 34, 43, 47 and 49 were all observed to possess the same [M − H]− ions at m/z 597.21778 (C28H37O14). Firstly, they were divided into two categories based on whether they produced the [M − H − 112]− ion at m/z 486 by the RDA cleavage from A-ring. Compound 47 generated its ESI-MS2 base peak ion at m/z 433 by losing dehydrated glucose. In addition, it also yielded [M − H − 2H2O]− ion at m/z 561, [M − H − H2O − CO]− ion at m/z 551, [M − H − 112]− ion at m/z 486, [M − H − dehydrated Glc]− ion at m/z 435, and so on. As far as we knew, there were no related literatures reported. And thus, compound 47 was finally deduced to be a new compound. Compounds 3, 8, 14, 20, 25, 34, 43 and 49 yielded their [M − H − H2O − CO]− ions at m/z 551, which was the ESI-MS2 base peak of compounds 20 and 34, and it also a high intense characteristic fragment ion of compound 49. Compared with the prominent ions of them, there was no difficulty to deduce that compounds 34 and 49 have two lactone rings, which were different to the other compounds. Furthermore, the fragment ions further validated the above deduction. Finally, according to their Clog P values, they were tentatively identified and differentiated. By comparison with the bibliography and MS fragmentation data, compounds 3, 8, 14, 20, 25, 34, 43 and 49 were tentatively identified (Table 1) [24,25].
Compounds 4 and 46 generated their [M − H]− ions at m/z 683.25456 (C32H43O16). Both of their deprotonated molecular ions yielded [M − H − H2O]− ion at m/z 665, [M − H − H2O − CO]− ion at m/z 637, [M − H − C4H4O]− ion at m/z 615, [M − H − dehydrated Glc]− ion at m/z 520, respectively. Moreover, compound 4 also dissociated into fragment ions [M − H − CO2]− at m/z 639, [M − H − Glc − dehydrated xyl]− at m/z 370 and [M − H − Glc − dehydrated xyl − CO2 − H2O]− at m/z 309. According to the literature, compounds 4 and 46 were plausibly characterized as (2R,5R,6R,8S,9S,10S,12S)-15,16-epoxy-2-hydroxy-6-O-{β-d-xylopyranosyl(1→6)–d-glucopyranosyl}-cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester and (2R,5R,6R,8S,9S,10S,12S)-15,16-epoxy-2-hydroxy-6-O-{β-d-glucopyranosyl-(1→6)-α-d-xylopyran-osyl}-cleroda-3,13(16),14-trien-17,12-olid-18-oic acid methyl ester, respectively [26].
Compounds 5 and 18 produced their [M − H]− ions at m/z 513.19665 (C24H33O12). The dominant characteristic fragment ions were presented at m/z 333 [M – H − 180]− and m/z 351 [M − H − 162]− as the ESI-MS2 base peak corresponding to the cleavage from glucopyranosyl. After the CID cleavage, both of their further fragmentation resulted in [M − H − H2O]− at m/z 495 and [M − H − Glc − CO]− at m/z 305. The product ion at m/z 305 of compound 18 further generated the predominant ion at m/z 287 by losing one molecular of water. By comparison with the literature data and MS fragmentation data, compounds 5 and 18 were tentatively identified as 1-deacetyltinosposide A and (2S,6R,6aR,7R,9S,10R,10aR,10bS)-2-(furan-3-yl)-6,9,10–trihydroxy-10b-methyl-7-(((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)decahydro-1H-benzo[f]isochromen-4(2H)-one, respectively [4].
Compounds 7, 10, 15 and 17 gave their [M − H]− ions at m/z 567.20721 (C27H35O13). They all produced the [M − H − H2O − CO]− and [M − H − Glc]− ions at m/z 521 and m/z 405. Besides, compounds 7 and 10 generated m/z 552 by losing a methyl, and the ion at m/z 341 was generated by losing a series of fragment ions of glucose residue, 2H2O, and CO. Compounds 7 and 15 were also generated [M − H − Glc − CO]− ions at m/z 359, which was the ESI-MS2 base peak of compound 7. Meanwhile, compound 7 generated the minor ion at m/z 329. Compound 17 produced its ESI-MS2 base peak by losing two molecular of methyl, one molecular of water and carbonyl at m/z 491. Combined with bibliography data and fragmentation pathways, these four compounds were tentatively deduced as tinospinoside D, amritoside C, rumphioside A and rumphioside F, respectively [25].
Compounds 9, 11, 13, 19, 35 and 55 all produced their [M − H]− ions at m/z 551.21230 (C27H35O12). Compounds 9, 13 and 19 produced their ESI-MS2 base peak ions at m/z 389 [M − H − dehydrated Glc]−, and further generated a series of fragment ions by losing methyl, water, carbon dioxide units at the mean time. According to the literature and the values of Clog P, compounds 9, 13 and 19 were plausibly characterized as borapetoside B, tinosposinenside A and (2R,5R,6R,8R,9S,10S,12S)-15,16-epoxy-2-hydroxy-6-O-(β-d-glucopyranosyl)-cleroda-3,13(16), 14-trien-17,12-olid-18-oic acid methyl ester, respectively [26]. Compounds 11 and 55 generated their ESI-MS2 base peak ions at m/z 505 by losing water and carbonyl, and their deprotonated molecular ions loss of glucose residue at m/z 389. According to the fragmentation pathways and the values of Clog P, compounds 11 and 55 were tentatively identified as tinospinoside B and tinospinoside C, respectively. According to the above methods, compound 35 was tentatively defined as rumphioside I.
Compounds 12 and 21 yielded their [M − H]− ions at m/z 535.18100 (C26H31O12). Compound 12 produced its ESI-MS2 base peak ion at m/z 373 by loss of a dehydrated glucose and then generated two minor ions at m/z 345 [M − H − dehydrated Glc − CO]− and m/z 313 [M − H − O − dehydrated Glc − CO2]−. While compound 21 generated a series of fragment ions at m/z 520 [M − H − CH3]−, m/z 517 [M − H − H2O]−, m/z 373 [M − H − dehydrated Glc]− and m/z 329 [M − H − dehydrated Glc − CO2]−. According to the fragmentation patterns and the values of Clog P, compounds 12 and 21 were tentatively characterized as (2,4,6,6,7,7,8,9,9,9)-2-(furan-3-yl)-6a,9b-dimethyl-6-(((2,3,4,5,6)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)decahydro-1H-9,7-(epoxymethano) oxireno[2′,3′:4,5]benzo[1,2-f]isochromene-4,11(2H)-dione and palmatoside F, respectively.
Compound 16 produced its [M − H]− ion at m/z 521.20173 (C26H33O11). A high intense characteristic fragment ion was present at m/z 359 [M – H − 162]− as its ESI-MS2 base peak corresponding to the cleavage of the dehydrated glucose. The product ion at m/z 359 successively generated the predominant product ions at m/z 344 and m/z 341 by losing a methyl and one molecular of water. According to the literature data, compound 16 was tentatively identified as furanoid diterpene glycoside [27].
Compound 22 generated its [M − H]− ion at m/z 527.21230 (C25H35O12). Upon CID mode, its further fragmentation resulted in [M − H − H2O]− ion at m/z 509, [M − H − H2O − CH3]− ion at m/z 494, [M – H − H2O − CO]− ion at m/z 481, [M − H − Glc]− ion at m/z 347, and [M − H − Glc − CH2O − CO − CH4]− ion at m/z 273. As far as we knew, there were no related literatures reported. Thus, compound 22 was tentatively deduced to be a new compound.
Compounds 23, 31 and 37 yielded their [M − H]− ions at m/z 555.20721 (C26H35O13). Compound 31 produced its ESI-MS2 base peak ion at m/z 513 [M − Ac]−, and the m/z 513 ion generated its predominant ions at m/z 495 [M − H − CH2CO − H2O]− and m/z 333 [M − H − CH2CO − dehydrated Glc]− due to the overall fracture of a hydroxy and glycoside bond. The ion at m/z 333 generated two minor ions at m/z 315 and m/z 305 through losing H2O and CO, respectively. Besides, its [M − H]− ion also yielded characteristic fragment ion at m/z 393 [M − H − 162]− by losing a dehydrated glucose. By comparison with the literature data, compound 31 was tentatively identified as tinosineside A [24]. Compounds 23 and 37 produced their fragment ions at m/z 537 [M − H − H2O]−, m/z 513 [M − CH3 − CO]−, m/z 495 [M − H − CH3 − COOH]− and m/z 393 [M − H − dehydrated Glc]−. Furthermore, compound 23 generated the fragment ions at m/z 375 [M − H–H2O–Glc]−, m/z 305 [M − H–dehydrated Glc–CH4 − CO − CO2]− and m/z 297 [M − H − H2O − Glc − CO2 − CH4]− or [M − H − 2H2O − Glc − CH2CO]−. According to the fragment ions and the values of Clog P, compounds 23 and 37 were tentatively identified as amritoside A and (1,2,7,8)-1-(2-(furan-3-yl)-2-hydroxyethyl)-2-hydroxy-5-(methoxycarbonyl)-1-methyl-7-(((3,4,6)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-1,2,3,4,6,7,8,8-octahydronaphthalene-2-carboxylic acid, respectively.
Compound 26 produced its [M − H]− ion at m/z 523.21738 (C26H35O11). Its ESI-MS2 base peak ion at m/z 361 was yielded by loss of a dehydrated glucose. In addition, it generated a series fragment ions, such as m/z 508, m/z 505, m/z 347, m/z 343 and m/z 329, involving the loss of a methyl group, a water molecule, and glucose. By comparing with the fragmentation patterns obtained from two reference standards, compound 26 was tentatively identified as sagittatayunnanoside D. Similarly, based on the literature data, compound 27 was tentatively identified as borapetoside H [28].
Compounds 28 and 33 exhibited their [M − H]− ions at m/z 549.19665 (C27H33O12). Both of their deprotonated molecular ions generated a serial of fragment ions at m/z 531 [M − H − H2O]−, m/z 503 [M − H − H2O − CO]−, m/z 481 [M − H − 2H2O − CH3OH]− and m/z 387 [M − H − dehydrated Glc]−, respectively. Meanwhile, a product ion at m/z 513 [M − H − 2H2O]− was observed in the ESI-MS/MS spectra of compound 33, suggesting there was two hydroxy groups at the skeleton. According to the literature data and the values of Clog P, compounds 28 and 33 were tentatively identified as (5R,6R,8S,9R,10R,12S)-15,16-epoxy-2-oxo-6-O-(β-d-glucopyranosyl)–cleroda-3,13(16),14-trien-17,12 -olid-18-oic acid methyl ester and tinoscorside C, respectively [26].
Compound 30 generated its [M − H]− ion at m/z 519.18608 (C26H31O11). The dominant characteristic fragment ion was present at m/z 357 [M − H − 162]− as its ESI-MS2 base peak, corresponding to the cleavage from the dehydrated glucose. Meanwhile, the product ions [M − H − H2O]− (lose of water) at m/z 501 and [M − H − 46]− (lose of water and carbon monoxide) at m/z 473 were monitored, which were in accordance with the diterpenoids glycosides cracking patterns, suggesting this compound might be tinoside.
Compounds 36 and 44 yielded their [M − H]− ions at m/z 535.21738 (C27H35O11). Both of their deprotonated molecular ions generated a serial of fragment ions at m/z 520 [M − H − CH3]−, m/z 488 [M − 2H − CO − H2O]−, m/z 373 [M − H − dehydrated Glc]−, m/z 359 [M − H − dehydrated Glc − CH2]−, m/z 358 [M − H − dehydrated Glc − CH3]−, m/z 355 [M − H − Glc]− and m/z 341 [M − H − Glc − CH2]−. According to the literature data and the values of Clog P, compounds 36 and 44 were tentatively identified as borapetoside C and tinocrisposide [29].
Compound 38 gave a [M − H]− ion at m/z 581.22286 (C28H37O13). Its major ions in the MS2 spectrum were m/z 563 [M − H − H2O]−, m/z 535 [M − H − H2O − CO]−, m/z 419 [M − H − dehydrated Glc]−, m/z 373 [M − H − Glc − CO]− and m/z 343 [M − Glc − CH2CO2]−, suggesting this compound might contain the glucose and acetyl fragments. Comparison with the parent nucleus and fragmentation patterns, compound 38 was tentatively identified as tinosposinenside B.
Compound 42 exhibited its [M − H]− ion at m/z 537.23303 (C27H37O11). Its ESI-MS2 base peak ion at m/z 375 was produced by losing dehydrated glucose, and the product ion at m/z 375 further generated two minor ions at m/z 360 and m/z 357 by losing CH3 and H2O, respectively. According to the fragmentation pathways, compound 42 was tentatively identified as boropetoside G. Likewise, compound 48 was tentatively defined as tinosposinenside C.
Compound 45 produced its [M − H]− ion at m/z 597.20002 (C28H37O12S), which exhibited the characteristic fragment ion [M − H − C8H14O6S]− at m/z 359 suggesting the overall fracture of side chain. S-diterpenoids glycosides were very rare in Tinospora, and there only two compounds were reported in this family so far [30]. By comparison with the fragmentation pathways, compound 45 was plausibly described as cordifolide A.
Compounds 50 and 59 produced their [M − H]− ions at m/z 623.23343 (C30H39O14). According to the respective fragmentation pathways and the values of Clog P, compounds 50 and 59 were tentatively identified as 6′-O-lactoylborapetoside B and 2-O-lactoylborapetoside B, respectively.
Compounds 52–54 produced their respective [M − H]− ion at m/z 517.17043 (C26H29O11), m/z 491.19117 (C25H31O10) and m/z 715.28077 (C33H47O17). None but compound 53 exhibited the [M − H + HCOOH]− adduct ion at m/z 537.19665 (C26H33O12). Take compound 52 for example, its major ions in ESI-MS2 spectrum were m/z 499 [M − H − H2O]−, m/z 473 [M − H − CO2]−, m/z 471 [M − H − H2O − CO]−, m/z 455 [M − H − CO2 − H2O]−, m/z 355 [M − H − dehydrated Glc]−, m/z 162 −C6H10O5 and m/z 151 –C5H11O5, suggesting this compound might contain glucose and undergo a RDA coverage from the A-ring at the 1,4-position. According to the above analysis, compounds 52–54 were tentatively defined as tinospinoside E, tinosporaside and sagittatayunnanoside B, respectively.
Compounds 56 and 58 generated their respective [M − H]− ion at m/z 671.29094 (C32H47O15) and 509.23812 (C26H37O10). The defference between of these molecules was 162 Da, suggesting that compound 56 had one more glucose unit than compound 58. Both of them generated the same ESI-MS2 base peak ions at m/z 329 due to the overall fracture of glycoside bond. Furthermore, the product ion at m/z 329 generated a predominant ion at m/z 301 by splitting off CO. Comparing with the parent nucleus and respective fragmentation pathways, compounds 56 and 58 were plausibly identified as sagittatayunnanoside C and sagittatayunnanoside A, respectively.
2.4. Target Isolation and Further Verification of Diterpenoids Fragmentation Patterns
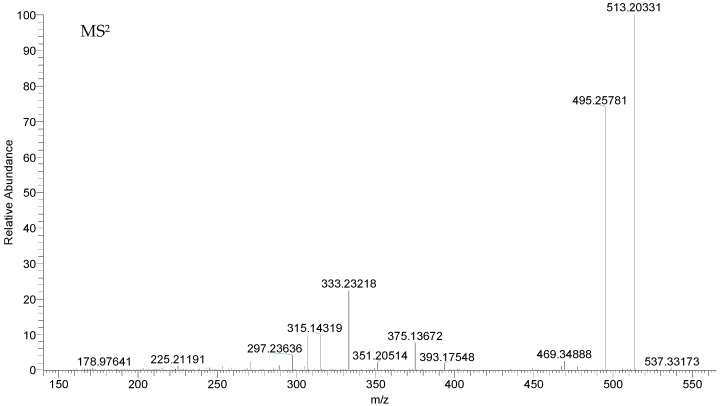
Tinosineside A generated its deprotonated molecular ion [M − H]− at m/z 555.20721 (C26H35O13, mass error within 3 ppm). It firstly produced the ESI-MS2 base peak ion at m/z 513 [M − Ac]− and further generated the ions at m/z 495 and m/z 375 by losing one molecule of water and dehydrated glucose. The ion at m/z 351 generated minor ions at m/z 333, m/z 315 and m/z 307 through losing H2O, 2H2O and CO2, respectively. Besides, its [M − H]− ion also yielded characteristic fragment ion at m/z 393 [M − H − 162]− by losing a dehydrated glucose. The fragmentation pathway was consistent with deduced of compound 31, which further proved the validity of the results (Figure 3).
Figure 3.
Spectra of ion fragments in MSn analysis of tinosineside A in negative ion mode.
3. Materials and Methods
3.1. Chemicals
The reference standards of columbin and isocolumbin (purity over 98%) were procured from Tauto Biotech (Shanghai, China).The structures of these standards were presented in Figure 1. HPLC grade formic acid, acetonitrile and methanol were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Ultrapure water was purchased from Hangzhou Wahaha Group Co., Ltd. (Hangzhou, Zhejiang, China). Material of T. sinensis was purchased from Anguo Linshi Medicinal Materials Co., Ltd. (Anguo, China) and then authenticated by Professor Chun-sheng Liu in Beijing University of Chinese Medicine.
3.2. Sample Preparation
3.2.1. Standard Solutions
The standard solutions of columbin and isocolumbin were prepared in methanol at appropriate concentrations.
3.2.2. Sample Solutions
Powdered dried alcoholic extracts of T. sinensis were weighed accurately (0.13 g) and refluxed with a tenfold excess of ethanol/water (70:30, v/v) three times, and placed in 20 mL of methanol/water (70:30, v/v). The mixture was then extracted in an ultrasonic bath at room temperature for 0.5 h, and the same solvent was added to compensate for the weight lost during the extraction. The extract was filtered and evaporated to near dryness, then placed on a C18 Solid Phase Extraction (SPE) column (J.T.Baker, Phillipsburg, NJ, USA), which was washed with 4 mL distilled water and 4 mL methanol. The methanol eluent was filtered through a 0.22 µm membrane for analysis. All of the solutions were stored at 4 °C and brought to room temperature before analysis.
3.3. Instrumentation and Condition
HPLC analysis was carried out on a DIONEX Ultimate 3000 UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA) with a binary pump and an autosampler. The samples were separated on a Sunfire C18 column (250 mm × 4.6 mm i.d., 5 μm, Waters Corporation, Milford, MA, USA) at room temperature. The mobile phase consisted of acetonitrile (B) and 0.1% (v/v) formic acid in water (A) with the elution gradient set as follows: 0–5 min, 8–12% B; 5–25 min, 12–16% B, 25–45 min, 16–25% B; 45–75 min, 25–46% B; 75–80 min, 46–58% B; 80–95 min, 58–65% B; 95–105 min. The flow rate was set as 1.0 mL/min.
A LTQ-Orbitrap XL mass spectrometer (Thermo Scientific, Bremen, Germany) was connected to the HPLC system via an electrospray ionization (ESI) interface. The effluent was introduced into the ESI source in a post-column splitting ratio of 1:4. Full scan data acquisition was performed from m/z 100 to 1500 in negative ion mode. The important ESI parameters were set as follows: capillary temperature, 350 °C; sheath gas (nitrogen) flow, 30 arb.; auxiliary gas (nitrogen) flow, 10 arb.; electrospray voltage, 3.0 kV; capillary voltage, −35 V; tube lens voltage, −110 V. The resolution of Orbitrap analyzer was set at 30,000 with data-dependent ESI-MS2 analysis triggered by the three most abundant ions from one-stage mass spectrometry scanning. Collision-induced dissociation (CID) was performed in LTQ with an activation q of 0.25 and activation time of 30 ms. The isolation width was 2 amu, and the normalized collision energy was set to 35%.
3.4. Peak Selections and Data Processing
A Thermo Xcalibur 2.1 workstation was used for the data acquisition and processing, In order to obtain as many fragment ions of the diterpenoids as possible, the peaks detected with intensity over 30,000 were selected for identification. The chemical formulas for all parent ions of the selected peaks were calculated from the accurate mass using a formula predictor by setting the parameters as follows: C (0–50), H (0–100), O (0–30), S (0–2), N (0–2). Other elements were not considered because they are rarely present in diterpenoids. Furthermore, diterpenoids isomers were differentiated by an important Clog P parameter obtained from Chemdraw to distinguish their polarity. And the exact mass error of all determined compounds was within 3 ppm.
3.5. Extraction and Isolation of Tinosineside A
The air-dried stems (10.0 kg) were extracted three times with tenfold excess of 70% EtOH under reflux for 2 h each at 80 °C. The combined extract was evaporated under reduced pressure to obtain a crude residue. This residue was further dispersed in H2O, and then successively extracted with CHCl3, EtOAc, n-BuOH and MeOH. The n-BuOH extract was passed through an AB-8 macroporous resin column and then washed with H2O, 30% EtOH, 50% EtOH, 70% EtOH and 95% EtOH. The 30% EtOH fraction was further purified by silica gel column chromatography with elution by CHCl3–MeOH (15:1→4:1, v/v) to give the diterpenoid glycoside named tinosineside A as a white powder.
4. Conclusions
In this study, a sensitive HPLC-LTQ-Orbitrap coupled with EIC data-mining method was established for the rapid characterization of diterpenoids in T. sinensis. According to the characteristic fragmentation pathways of two reference standards, all diterpenoids were divided into two categories, namely diterpenoid aglycones and diterpenoid glycosides. Furthermore, an important parameter Clog P was used to differentiate the isomers of diterpenoids. As a result, 63 diterpenoids were preliminarily identified, including 48 known compounds and 15 new compounds. These compounds included 10 diterpenoid aglycones, 53 diterpenoid glycosides. Finally, target isolation of one diterpenoid glycoside named tinosineside A was performed based on the obtained results, which further confirmed the deduced fragmentation patterns and identified profile of diterpenoids in T. sinensis. This represents the first systematic report of diterpenoids in T. sinensis. The results indicated that the established method could be employed as a rapid, effective technique to screen and identify diterpenoids in T. sinensis. The study also provided significant guidance for the analysis of other herbal medicines or preparations.
Acknowledgments
This work was financially supported by Beijing Nova Program (Z171100001117029) and the graduate independent subject of Beijing University of Chinese Medicine (2017-JYB-XS-079).
Supplementary Materials
The supplementary files are available online.
Author Contributions
Bin Liu and Jia-Yu Zhang designed the experiments; Feng-Xia Guo and Yan-Yan Jiang contributed to the isolation and identification of Tinosineside A; Zi-Jian Wang, Lu-Lu Xu and Jia-Yu Zhang contribute to the data collection and analysis; Sen-Sen Chi, Jia-Yu Zhang and Bin Liu contributed reagents/materials/analysis tools; Lu-Lu Xu, Jia-Yu Zhang and Bin Liu wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Maurya R., Gupta P., Chand K., Kumar M., Dixit P., Singh N., Dube N. Constituents of Tinospora sinensis and their antileishmanial activity against Leishmania donovani. Nat. Prod. Res. 2009;23:1134–1143. doi: 10.1080/14786410802682239. [DOI] [PubMed] [Google Scholar]
- 2.Gupta P., Sharma U., Gupta P.K., Maurya R. A novel protein tyrosine phosphatase 1B inhibitor from Tinospora sinensis. Chron. Young Sci. 2012;3:199–203. doi: 10.4103/2229-5186.99569. [DOI] [Google Scholar]
- 3.Hegde S., Jayaraj M. A Review of the Medicinal Properties, Phytochemical and Biological Active Compounds of Tinospora sinensis (Lour.) Merr. J. Biol. Act. Prod. Nat. 2016;6:84–94. [Google Scholar]
- 4.Dong L.P., Chen C.X., Ni W., Xie B.B., Li J.Z., Liu H.Y. A new dinorclerone diterpenoid glycoside from Tinospora sinensis. Nat. Prod. Res. 2010;24:13–17. doi: 10.1080/14786410802253197. [DOI] [PubMed] [Google Scholar]
- 5.Li W., Koike K., Liu L., Lin L., Fu X., Chen Y., Nikaido T. New Lignan Glucosides from the Stems of Tinospora sinensis. Chem. Pharm. Bull. 2004;52:638–640. doi: 10.1248/cpb.52.638. [DOI] [PubMed] [Google Scholar]
- 6.Kumar V., Nagar S., Tripathi Y.C. Do assorted approaches aid in estimation of uronic acids? Case studies on Tinospora sinensis polysaccharides. Int. J. Biol. Macromol. 2014;70:360–363. doi: 10.1016/j.ijbiomac.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad W., Jantan I., Bukhari S.N. Tinospora crispa (L.) Hook. f. & Thomson: A review of its ethnobotanical, phytochemical and pharmacological aspects. Front. Pharmacol. 2016;7:1–19. doi: 10.3389/fphar.2016.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma R., Amin H., Galib, Prajapati P.K. Antidiabetic claims of Tinospora cordifolia (Willd.) Miers: Critical appraisal and role in therapy. Asian Pac. J. Trop. Biomed. 2015;5:68–78. doi: 10.1016/S2221-1691(15)30173-8. [DOI] [Google Scholar]
- 9.Zhang Q.Q., Dong X., Liu X.G., Gao W., Li P., Yang H. Rapid separation and identification of multiple constituents in Danhong Injection by ultra-high performance liquid chromatography coupled to electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Chin. J. Nat. Med. 2016;14:147–160. doi: 10.1016/S1875-5364(16)60008-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J.Y., Wang F., Zhang H., Lu J.Q., Qiao Y.J. Rapid Identification of polymethoxylated flavonoids in Traditional Chinese Medicines with practical strategy of stepwise mass defect filtering coupled to diagnostic product ions analysis based on a hybrid LTQ-Orbitrap mass spectrometer. Phytochem. Anal. 2014;25:405–414. doi: 10.1002/pca.2508. [DOI] [PubMed] [Google Scholar]
- 11.Li Y., Liu Y., Liu R.R., Liu S.Y., Zhang X.P., Wang Z.J., Zhang J.Y., Lu J.Q. HPLC-LTQ-Orbitrap MSn profiling method to comprehensively characterize multiple chemical constituents in xiao-er-qing-jie granules. Anal. Methods. 2015;7:7511–7526. doi: 10.1039/C5AY00420A. [DOI] [Google Scholar]
- 12.Chen L.W., Wang Q., Qin K.M., Wang X.L., Wang B., Chen D.N., Cai B.C., Cai T. Chemical profiling of Qixue Shuangbu Tincture by ultra-performance liquid chromatography with electrospray ionization quadrupole-time-of-flight high-definition mass spectrometry (UPLC-QTOF/MS) Chin. J. Nat. Med. 2016;14:141–146. doi: 10.1016/S1875-5364(16)60007-9. [DOI] [PubMed] [Google Scholar]
- 13.Wang F., Zhang J.Y., Yin P.H., Wang Z.J., Dong L.Y., Lu J.Q. Rapid identification of polyphenols in Kudiezi injection with a practical technique of mass defect filter based on high-performance liquid chromatography coupled with linear ion trap/orbitrap mass spectrometry. Anal. Methods. 2014;6:3515–3523. doi: 10.1039/c4ay00213j. [DOI] [Google Scholar]
- 14.Zhang J.Y., Li N., Che Y.Y., Zhang Y., Liang S.X., Zhao M.B., Jiang Y., Tu P.F. Characterization of seventy polymethoxylated flavonoids (PMFs) in the leaves of Murraya paniculata by on-line high-performance liquid chromatography coupled to photodiode array detection and electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011;56:950–961. doi: 10.1016/j.jpba.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J.Y., Lu J.Q., Wang F., Cao G.S., Tu P.F., Qiao Y.J. A strategy of EIC-MS coupled with diagnostic product ions analysis for efficient discovery of new hydroxylated polymethoxyflavonoid glycosides from the leaves of Murraya paniculata L. using HPLC-DAD-MS/MS. Anal. Methods. 2013;5:2880–2891. doi: 10.1039/c3ay26475k. [DOI] [Google Scholar]
- 16.Abhishek N., Sandhya M., Alok L., Devindra V.A., Upadhyay R.S., Nautiyal C.S. Identification and Quantification of Heterologous Compounds Parthenin and Organic Acids in Parthenium Hysterophorus L. Using HPLC-PDA-MS-MS. Anal. Lett. 2013;46:48–59. [Google Scholar]
- 17.Li H.B., Hu J., Chen J.C., Qiu M.H. Chemical Constituents of Tinospora craveniana. Nat. Prod. Res. Dev. 2005;17:125–127. doi: 10.1080/14786410410001704723. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y.F., Shi Q.R., Shi P.Y., Zhang W.D., Cheng Y.Y. Characterization of isoquinoline alkaloids, diterpenoids and steroids in the Chinese herb Jin-Guo-Lan (Tinospora sagittata and Tinospora capillipes) by high-performance liquid chromatography/electrospray ionization with multistage mass spectrometry. Rapid Commun. Mass Spectrom. 2006;20:2328–2342. doi: 10.1002/rcm.2593. [DOI] [PubMed] [Google Scholar]
- 19.Zhan Z.J., Zhang X.Y., Hou X.R., Li C.P., Shan W.G. New Diterpenoids from Tinospora capillipes. Helv. Chim. Acta. 2009;92:790–794. doi: 10.1002/hlca.200800369. [DOI] [Google Scholar]
- 20.Maurya R., Wazir V., Tyagi A., Kapil R.S. Clerodane diterpenoids from Tinospora cordifolia. Phytochemistry. 1995;38:659–661. doi: 10.1016/0031-9422(94)00686-N. [DOI] [Google Scholar]
- 21.Fukuda N., Yonemitsus M., Takeatsu K., Isobe R., Komori T. Studies on the Constituents of the Leaves of Tinospora tuberculata, II, Isolation and Structure Elucidation of Four New Furanoid Diterpenes, Tinotufolin C-F. Liebigs Ann. Chem. 1994;1994:755–757. doi: 10.1002/jlac.199419940719. [DOI] [Google Scholar]
- 22.Wazir V., Maurya R., Kapil R.S. Cordioside, a clerodane furano diterpene glucoside from Tinospora cordifolia. Phytochemistry. 1995;38:447–449. doi: 10.1016/0031-9422(94)00601-O. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda N., Yonemitsu M., Kimura T., Hachiyama T., Miyahara K., Kawasaki T. Studies on the constituents of the stems of Tinospora tubereulata BEUMEE. II.1 new diterpenoids, borapetoside A and borapetol A. Chem. Pharm. Bull. 1985;33:4438–4444. doi: 10.1248/cpb.33.4438. [DOI] [Google Scholar]
- 24.Yonemitsu M., Fukuda N., Kimura T., Isobe R., Komori T. Studies on the constituents of the stems of Tinospora sinensis, II. Isolation and structure elucidation of two new dinorditerpene glucosides, tinosineside A and B. Liebigs Ann. 1995;1995:437–439. doi: 10.1002/jlac.199519950255. [DOI] [Google Scholar]
- 25.Cong P.Z., Li S.Y. Natural Organic Mass Spectrometry. China Medical Science Press; Beijing, China: 2001. pp. 963–1019. [Google Scholar]
- 26.Choudhary M.I., Ismail M., Shaari K., Shaari K., Abbaskhan A., Sattar S.A., Lajis N.H., Rahman A. Cis-clerodanetype furanoditerpenoids from Tinospora crispa. J. Nat. Prod. 2010;73:541–547. doi: 10.1021/np900551u. [DOI] [PubMed] [Google Scholar]
- 27.Bhatt R.K., Sabata B.K. A furanoid diterpene glucoside from Tinospora cordifolia. Phytochemistry. 1989;28:2419–2422. doi: 10.1016/S0031-9422(00)97996-2. [DOI] [Google Scholar]
- 28.Fukuda N., Yonemitsu M., Kimura T. Isolation and Structure Elucidation of the New Furanoid Diterpene Glucoside Borapetoside H. Liebigs Ann. 1995;1995:1689–1691. doi: 10.1002/jlac.1995199509234. [DOI] [Google Scholar]
- 29.Fukuda N., Yonemitsu M., Kimura T. Isolation and structure elucidation of the five new furanoid diterpene glycosides borapetoside C-G. Liebigs Ann. Chem. 1993;1993:491–495. doi: 10.1002/jlac.199319930181. [DOI] [Google Scholar]
- 30.Chi S.S., She G.M., Han D., Wang W.H., Liu Z., Liu B. Genus Tinospora: Ethnopharmacology, Phytochemistry, and Pharmacology. Evid. Based Complement. Alternat. Med. 2016;2016:9232593. doi: 10.1155/2016/9232593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.