Abstract
Studying anxiety in neurogenetic syndromes may inform the intersection of biological and developmental risks, facilitating effective and targeted interventions. We longitudinally examined stranger fear in infants and toddlers with fragile X syndrome (FXS; n=46) and typical controls (n=33), as well as associations between observed stranger fear and rating scales of anxiety, withdrawal and autism features within FXS. Results indicated atypical facial fear in FXS, although facial fear did not index anxiety, autistic symptoms or social withdrawal. Instead, lower withdrawal was associated with decreased distress vocalizations across age, and higher autistic symptoms were associated with lower intensity escape behaviors. Early stranger fear in FXS reflects both typical and atypical dimensions and may help index emergence of social anxiety in this population.
Keywords: fragile x syndrome, anxiety, autism, longitudinal, behavior, social approach
Anxiety disorders are among the most prevalent and impairing forms of psychopathology disorders in young children (Centers for Disease Control, 2014; Kessler et al., 2005). Identifying prodromal features of anxiety increases the likelihood that children “at risk” will be routed to timely, effective and targeted interventions. Indeed, recent studies have successfully reduced the emergence of anxiety in early childhood by introducing prevention programs in infant and preschool periods (Rapee, Kennedy, Ingram, Edwards, & Sweeney, 2005), providing initial evidence that the negative developmental impact of anxiety may be tempered through early intervention. However, a remaining challenge is the need to identify children most in need of these interventions during infancy and toddlerhood. Addressing this challenge requires first establishing reliable predictors of anxiety that generalize across multiple population subgroups. To effectively signal the need for early intervention, these predictors must also differentiate among multiple outcomes, rather than globally predicting a variety of later problem behaviors. Establishing generalizable and specific predictors of anxiety and other forms of psychopathology will lay the foundation for earlier and more efficient risk detection protocols, reducing both individual burden and public health costs associated with these disorders.
The present study examines the emergence of behavioral precursors of social anxiety symptoms in a longitudinal sample of young children with fragile X syndrome (FXS). Fragile X syndrome is a single-gene disorder caused by an atypical expansion of CGG trinucleotide repeats on the promotor region of the FMR1 gene. This expansion causes reduced production of fragile X mental retardation protein (FMRP) necessary for healthy neural plasticity (Fernández, Rajan, & Bagni, 2013). Although FXS is a low-incidence disorder, affecting approximately 1: 3,700–8,900 males (Crawford, Acuña, & Sherman, 2001; Hunter et al., 2014), FXS is the leading known heritable cause of intellectual disability and is also highly associated with a number of comorbid conditions including anxiety disorders (Cordeiro, Ballinger, Hagerman, & Hessl, 2011) and autism (40–60% prevalence; Klusek et al., 2013; Rogers, Wehner, Hagerman, & Wehner, 2001). Social anxiety appears to be particularly prevalent and impairing in FXS, with nearly 70% of children and young adults meeting diagnostic criteria (Cordeiro et al., 2011). Differential diagnosis of social anxiety that is co-morbid or independent of ASD in persons with FXS is complicated, however, given overlapping features, a lack of established guidelines, and different developmental trajectories across comorbid disorders. For example, ASD can be reliably diagnosed by three years of age, whereas the diagnosis of social anxiety is typically not established until five to eight years of age (Beesdo et al., 2007). However, although the age of diagnosis for social anxiety is generally later than for ASD, prodromal features and risk factors have been established for community samples much earlier in development, with stable, elevated behavioral inhibition and social fear emerging in children with later social anxiety in the first few years of life (Chronis-Tuscano et al., 2009). Identifying prodromal features and risk factors for social anxiety in the infant and preschool years requires a high level of attunement to developmental issues, which are exacerbated in neurodevelopmental disorders associated with intellectual impairment and multiple co-occurring conditions.
Despite these challenges, studying the emergence of social anxiety in FXS offers a number of benefits that may complement broader population-based studies. First, a subset of individuals with FXS are diagnosed at or before birth, permitting prospective surveillance of anxiety and associated risk factors as they emerge across very early development. Second, given high rates of multiple disorders in FXS, it is possible to efficiently examine specificity of risk factors to specific psychopathology outcomes, such as distinguishing predictors of social anxiety from predictors of autistic features. Base rates of these disorders are lower in non-FXS samples (anxiety 32% Merikangas et al., 2010; ASD 1–2% Centers for Disease Control, 2014), limiting opportunities to examine specificity of risk factors in non-FXS populations. Third, because the genetic basis of FXS is well-characterized, FXS provides a model for examining biobehavioral patterns of risk, potentially informing the etiology of symptom progression. Although it is likely that the emergence of social anxiety may differ in notable ways from other populations – for example, due to genetic features of FXS or the presence of intellectual disability – exploring FXS as a “portal” to understanding risk in a concentrated, relatively homogeneous subgroup of the population may inform hypotheses and pathways that can be further tested in additional groups. Thus in addition to benefitting early detection within FXS by establishing early risk patterns, examining social anxiety symptom emergence in FXS is a compelling model for establishing and testing specific behavioral and biological pathways of risk.
The present study examines emergent social anxiety symptom risk and its disassociation from autistic symptoms in FXS by focusing on well-established predictors of social anxiety in typically developing populations: behavioral inhibition and social fear. Behavioral inhibition is the tendency to respond to novel social or environmental presses with timid or restrained responses (Kagan, Reznick, Clarke, Snidman, & Garcia-Coll, 1984). In typically developing children, fluctuations in behavioral inhibition are expected across periods of social development, with social fear identified as one of the earliest and most enduring feature. For example, fear of strangers typically emerges around age 6 months, increases in intensity from 6–12 months, then stabilizes before increasing again approaching the preschool period (Brooker et al., 2013; Gartstein et al., 2010). Although gradual increases in stranger fear reflects infants’ adaptive, increased attachment to trusted caretakers (Brooker et al., 2013), extreme social fear in early childhood has been repeatedly associated with later social anxiety (Rapee, 2014), particularly among children whose inhibited behaviors are stable and high rather than low or fluctuating (Brooker et al., 2013). Thus, longitudinal patterns of stranger fear – rather than static snapshots during development – are thought to reliably index pediatric anxiety risk, in general, and social anxiety, in particular.
Importantly, atypical social behaviors in early childhood are also associated with autism spectrum disorder (ASD), a neurodevelopmental disorder characterized by atypical socio-communicative and repetitive behaviors. In efforts to identify and treat ASD early in development, a number of studies have begun successfully identifying “red flags” of ASD in infancy and toddlerhood, including failure to orient to name calls and reduced attention to social events and stimuli (Zwaigenbaum et al., 2015). Indeed, it has been suggested that examining stranger responses in infants at risk for ASD may inform the emergence of symptoms (Ritvo, 2014), in concert with psychophysiological evidence that infants at risk for ASD process novel faces differently than infants at low risk for ASD (Key & Stone, 2012). Although no studies to date have examined stranger response in infants at risk for ASD, stranger responses have been examined in older children. Scherr and colleagues (2017) recently compared stranger responses in preschool-aged children with idiopathic ASD, FXS and high ASD symptoms (“FXS-High”), FXS and low ASD symptoms (“FXS-Low”) and typical development (Scherr, Hogan, Hatton, & Roberts, 2017). Results indicated similarly reduced gaze toward the stranger and reduced facial fear across FXS and ASD groups, relative to the control group, as well as typical patterns of escape behaviors across groups. Within FXS, participants with greater ASD features were more likely to gaze toward their parent during the task, similar to the ASD group, although gaze toward the stranger was similar across ASD and FXS groups. These results suggest that within FXS, escape behaviors and facial fear are relatively unrelated to comorbid ASD features, although parental referencing may be lower in children with more severe symptoms. These findings provide preliminary evidence that although ASD features may be associated with atypical gaze patterns in FXS and idiopathic ASD, behavioral markers such as facial fear and escape behaviors do not discriminate children with FXS with and without high ASD features. Given the dynamic nature of behavioral inhibition across development, however, it remains unclear whether early trajectories of behavioral inhibition during stranger response paradigms may index ASD features in infancy, particularly given differences in how “high risk” infants process novel versus unfamiliar social stimuli (Key & Stone, 2012). Indeed, given overlap in atypical social approach behaviors across older children with social anxiety and ASD, differentiating between precursors of these disorders is an important step in establishing behavioral inhibition, as measured during stranger response, as a specific early marker of social anxiety risk, particularly for children at elevated risk for multiple disorders.
Within FXS, patterns of behavioral inhibition have been established in older children and adults, although studies in infants and young children are sparse (see Tonnsen & Roberts, 2016, for review). During social challenge tasks, children with FXS exhibit higher rates of gaze aversion, task avoidance, behavioral distress, and abnormal vocalizations relative to their unaffected siblings (Hall, Lightbody, Huffman, Lazzeroni, & Reiss, 2009; Hessl, Glaser, Dyer-Friedman, & Reiss, 2006). Similarly, children with FXS display reduced eye contact and less approaching behavioral movements and facial expressions when introduced to a novel examiner (Roberts et al., 2007, 2009; Scherr, Hogan, Hatton & Roberts, 2017), with persistent eye gaze avoidance among children with FXS and co-occurring features of autism (Hall et al., 2009; Roberts et al., 2007; Scherr et al., 2017), similar to children with idiopathic ASD (Scherr et al., 2017). Atypical social approach behaviors also emerge early in development in FXS, with increased facial fear toward strangers observed in a cross-sectional sample of 12–58 month old children with FXS relative to non-FXS controls (Tonnsen, Shinkareva, Deal, Hatton, & Roberts, 2013). Thus, behavioral inhibition and social fear are common and early-emerging features of FXS, although the specific association between stranger fear and clinical outcomes in this population remain unclear.
We previously identified abnormal patterns of behavioral and physiological responses to a stranger in a cross-sectional sample of children with FXS (Tonnsen et al. 2013). However, the developmental implications of these early behaviors – particularly their association with anxiety versus autistic symptoms – is yet to be established. Thus, to examine early behavioral inhibition and social fear as specific risk factors for social anxiety in FXS, the present study examined longitudinal patterns of stranger fear in young children with FXS relative to typically developing controls, as well as the predictive associations between behavioral features of stranger fear and later rating scales of anxiety, social withdrawal and ASD symptoms. We examined the following research questions:
Does the trajectory of stranger fear differ in infants and toddlers with FXS contrasted to typically developing controls? We hypothesized that infants and toddlers with FXS exhibit atypical longitudinal patterns of stranger fear, particularly increased rates of facial fear over time, consistent with previous cross-sectional work (Tonnsen et al. 2013).
Does the trajectory of stranger fear in infants and toddlers with FXS predict the severity of anxiety or autistic symptomology? We hypothesized that increased stranger fear in infants and toddlers with FXS would predict the severity of anxiety and withdrawal, but not autistic features, consistent with findings that comorbid autistic features are relatively unrelated to facial fear and escape behaviors in preschool-aged children with FXS (Scherr et al., 2017).
Methods
Participants
Data were drawn from two interrelated longitudinal studies on infant development in FXS. The present study included 46 participants with FXS and 33 non-FXS control participants assessed longitudinally for a total of 176 assessments (110 FXS, 66 control). All participants completed at least 2 assessments, and 18 participants with FXS completed 3 assessments. Proportion of females were similar across groups (control 18%, n = 6; FXS 26%, n = 12; Fisher’s p = .59). Average assessment age was slightly older in the FXS group (Wilcoxon Z = −2.02, p = .04; FXS M = 38.85, range 10.82–59.93; TD M = 35.08, range 12.07–57.83). Table 1 details participant characteristics.
Table 1.
Primary demographic and behavioral variables at person- and assessment-levels
| FXS | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | n | Mean | SD | Min | Max | n | Mean | SD | Min | Max |
| Person-Level Variables | ||||||||||
| n Assessments | 46 | 2.39 | 0.49 | 2 | 3 | 33 | 2 | 0 | 2 | 2 |
| Baseline Age (months) | 46 | 26.86 | 9.15 | 10.82 | 37.97 | 33 | 27.50 | 8.82 | 12.07 | 38.10 |
| Outcome Age (months) | 46 | 59.02 | 8.24 | 40.51 | 69.77 | 32 | 41.68 | 9.02 | 24.49 | 57.57 |
| Outcome Mental Age (months) | 46 | 31.96 | 11.70 | 13.75 | 67.25 | 33 | 46.53 | 10.08 | 22 | 66 |
| Outcome Cognitive SS | 46 | 54.65 | 11.66 | 47 | 99 | 33 | 111.06 | 15.46 | 82 | 141 |
| Autistic Symptoms | 46 | 27.41 | 6.54 | 16.5 | 40 | 33 | 15.18 | 0.64 | 15 | 18.5 |
| Anxiety Raw | 46 | 4.07 | 2.78 | 0 | 13 | 32 | 1.97 | 1.89 | 0 | 6 |
| Anxiety T | 46 | 55.63 | 6.92 | 50 | 81 | 32 | 51.66 | 2.89 | 50 | 60 |
| Withdrawal Raw | 46 | 4.30 | 2.56 | 0 | 12 | 32 | 0.72 | 1.20 | 0 | 4 |
| Withdrawal T | 46 | 63.96 | 8.51 | 50 | 88 | 32 | 51.84 | 3.90 | 50 | 63 |
| Assessment-Level Variables | ||||||||||
| Chronological Age (months) | 110 | 38.85 | 13.57 | 10.82 | 59.93 | 66 | 35.08 | 11.59 | 12.07 | 57.83 |
| Facial Fear | 108 | 0.06 | 0.12 | 0.00 | 0.67 | 65 | 0.09 | 0.13 | 0.00 | 0.60 |
| Distress Vocalizations | 109 | 0.22 | 0.35 | 0.00 | 1.98 | 66 | 0.15 | 0.47 | 0.00 | 2.76 |
| Escape Behaviors | 110 | 1.10 | 0.61 | 0.00 | 2.63 | 66 | 1.05 | 0.48 | 0.07 | 1.87 |
Note: Person-level variables refer to demographic and behavioral features collected during one observation (e.g. outcome anxiety) or are summarized as one variable (e.g. number assessments), whereas assessment-level variables were collected at each time point (e.g. facial fear at age 10 months). Thus, each participant contributed one observation per person-level variable and multiple observations per assessment-level variable.
Participants with FXS were recruited through ongoing research studies and parent support groups, and diagnoses were verified using genetic reports. Controls were recruited from the local community and were born full-term, received developmental scores in the average range, and had no parent-reported developmental concerns at study entry. Parents provided written consent in accordance with approved Institutional Review Board procedures.
A subset of assessments for the present study (n = 32 of 176) was reported in our previous study of cross-sectional behavioral and physiological patterns of approach in FXS (Tonnsen et al. 2013). The current study is distinct in its focus on behavioral data from an expanded mixed-sex longitudinal sample, including 47 additional participants and 144 additional observations, with the goal to characterize longitudinal trajectories and predictive associations between stranger fear and psychopathology in FXS rather than preliminary cross-group, cross-sectional profiles.
Measures
Stranger Fear.
During each visit, participants completed a Stranger Approach episode from the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith & Rothbart, 1996) to elicit behaviors associated with stranger fear. This task has been used to study stranger fear in children with FXS and in typically developing infants and children of similar developmental age to children in our sample (Brooker et al., 2013; Tonnsen, Shinkareva, et al., 2013). The Stranger Approach paradigm is divided into discrete stages that include baseline state of the child (30 s), the stranger knock (30 s), stranger kneel (120 s), and recovery state of the child (30 s). The scripted episode required the infants to be seated in their caregiver’s laps in the examination room where a trained examiner enters with a neutral affect dressed in a standardized outfit (e.g., black hat, sunglasses, oversized sweatshirt, and a long skirt). Prior to entering the room, the examiner knocked loudly, entered the room, and informed the infant they would be coming closer to them. The stranger then knelt in front of the infant for two minutes while maintaining their neutral affect. Once the two minutes had passed, the stranger exited the room. The present study focused on coding three infant behaviors solicited across the stranger paradigm: facial fear, distress vocalizations, and escape behaviors. Behavioral data were coded by trained research assistants using parameters defined in previous studies (Izard, 1979; Scherr et al., 2017; Tonnsen, Shinkareva, et al., 2013), with interrater reliability maintained at K ≥ .80 (facial fear = .81, distress vocalizations = .82, escape behaviors = .80). The primary dependent variables were weighted average intensity of facial fear, distress vocalizations, and escape behaviors (Gagne, Van Hulle, Aksan, Essex, & Goldsmith, 2011). Behavioral anchors for each coding descriptor are outlined in Table 2.
Table 2.
Description of key constructs and behavioral codes
| Construct | Rating | Description |
|---|---|---|
| aDistress Vocalizations | Behavioral Coding | 0 = No Distress |
| 1 = Mild Vocalizations (<1 sec.) | ||
| 2 = Definite whimpering or protest (1–2 secs.) | ||
| 3 = Longer whining, fussing, or low intensity cry (>2 secs.) | ||
| 4 = Definite non-muted crying | ||
| 5 = Full intensity cry/scream | ||
| aFacial Fear | Behavioral Coding | 0 = No facial region shows fear |
| 1 = One facial region shows fear/low intensity fear | ||
| 2 = Two facial regions show fear or one region shows very distinct facial fear | ||
| 3 = Change occurs in all three facial regions; impression of strong facial fear | ||
| aEscape Behavior | Behavioral Coding | 0 = No escape behavior or social referencing |
| 1 = Mild or fleeting escape behavior | ||
| 2 = Moderate escape behavior resulting in significant, but not extreme attempts to get away, including hitting, pushing, slapping, as well as full body movements such as arching back, twisting away, leaning away | ||
| 3 = Vigorous escape behaviors, usually involving linked, intense full body movements (e.g. arching, twisting) | ||
| bWithdrawal Symptoms | Parent Rating | Parent report of child’s symptoms of withdrawal using raw scores from the DSM-Orientated Withdrawal/Depression subscale. This subscale contains a cluster of behavioral features reflective of social anxiety (e.g. displays avoidant eye contact, does not get involved with others or answer when spoken to) |
| bAnxiety Symptoms | Parent Rating | Parent report of child’s symptoms of anxiety using raw scores from the DSM-Orientated Anxiety Problems subscale. This subscale includes symptoms that align with global measures of anxiety (e.g. fear, nervousness, panic) across anxiety disorders in the DSM. |
| cAutistic Symptoms | Examiner Rating | Continuous examiner rating of autism symptom severity, ranging in scores from 15to 60 with scores above 29 indicating mild to severe symptoms. |
Note.
Coding schemes for distress vocalizations, facial fear, and escape behaviors derived from the Stranger Episode in the LocoMotor Version 3.1 Laboratory Temperament Assessment Battery (Goldsmith & Rothbart, 1996)
Child Behavior Checklist (Achenbach, 1991)
Childhood Autism Rating Scale (Schopler et al., 1998)
Anxiety and Withdrawal Symptomology.
Parents completed the Child Behavior Checklist (CBCL/1.5–5; Achenbach & Rescorla, 2000), a rating scale of behavioral and socioemotional functioning in children ages 1.5–5 years. Raw scores, rather than T-scores, from the CBCL DSM-Orientated Anxiety Problems and Withdrawal subscales were used for analyses because T-scores for these scales are truncated at 50 (Achenbach & Rescorla, 2000). Table 2 provides additional information about these constructs. The DSM-Oriented Anxiety Problem scale on the CBCL includes symptoms selected to align with DSM anxiety disorder criteria (e.g. fear, nervousness, panic). The Withdrawal/Depression subscale contains a cluster of behavioral features reflective of social anxiety more specifically (e.g. displays avoidant eye contact, does not get involved with others or answer when spoken to), constructed based on factor analyses. A number of studies have used the CBCL to examine anxiety, withdrawal and other child problem behaviors in same-aged children with FXS (Hatton et al., 2002; Tonnsen, Grefer, Hatton, & Roberts, 2014; Tonnsen, Malone, Hatton, & Roberts, 2013). Although parents completed the CBCL at multiple time points, participants’ final CBCL prior to 60 months was selected to (1) maintain continuity across participants and (2) capture maximal stability of anxiety symptoms, as anxiety is known to emerge and increase in stability across childhood.
Autistic Symptoms.
The Childhood Autism Rating Scale (CARS; Schopler, Reichler, & Renner, 1998) is a widely used measure of autistic symptomology in children. The CARS is an examiner rating scale of behavioral symptoms and characteristics of autism in children across a range of behaviors including activity level, communication, body use, and imitation. Participants’ final CARS score prior to 60 months was included in analyses due to higher stability of ASD symptoms at older ages and to maximize continuity across participants. The CARS was completed following the assessment by two trained examiners who assigned consensus scores based on behavioral observations throughout the assessment. Examiners observed different samples of behavior based on their assigned assessment roles, thus consensus scores were used to capture the greatest sample of behavior. Scores <30 indicate minimal to no autistic symptoms, whereas scores ≥30 indicate mild to severe symptoms. Examiners were trained to ≥ 80% reliability prior to administering the CARS. While the Autism Diagnostic Observation Schedule – 2 (ADOS-2; Lord et al., 2000) used in clinical best estimate procedures is the established standard for diagnoses of ASD, the ADOS-2 was not used in one of the primary studies integrated in our sample and was therefore not used. Notably, the CARS has been reported as a valid and reliable marker of ASD features in non-syndromic ASD (Falkmer, Anderson, Falkmer, & Horlin, 2013) and is strongly associated with the ADOS in FXS samples (r = .90; Scherr et al., 2017).
Mental Age.
The Mullen Scales of Early Learning (MSEL; Mullen, 1995) is a standardized assessment of cognitive and motor ability in children. The MSEL includes five scales that measure the following domains: Gross Motor, Visual Reception, Fine Motor, Expressive Language, and Receptive Language. The MSEL age equivalent score was used due to potential floor effects of standard scores
Procedures.
The stranger approach paradigm was administered alongside a larger standardized protocol. Each participant was assessed by at least two trained examiners during similar times of the day and completed similar activities at each assessment. Trained examiners completed the CARS based on the entire assessment session, and the child’s mother completed the CBCL.
Analyses
Analyses were conducted in SAS 9.4 (Cary, NC) to address our two research questions. The first set of analyses focused on cross-group differences (FXS versus TD) in behavioral features of stranger fear across time. We compared groups’ base rates of behavioral variables, quantified dichotomously as present or absent, using Fisher’s exact tests. To examine group differences in behavioral features across age, we used mixed effect multilevel models in SAS PROC MIXED. Multilevel modeling is an ideal statistical approach for examining these behavioral features across age because models account for nesting of observations within individuals and permit cross-individual differences in the timing and number of assessments. Mixed effects unconditional mean and growth models were constructed for each of the three primary stranger fear variables: facial fear, escape, and distress vocalizations. Intraclass correlations and fit indices were used to determine whether intercept and age should be modeled as a random effect for each variable. We examined effects of group (FXS versus TD) on levels and change of the dependent variables. These conditional models examined whether overall levels (mean) and longitudinal change (slope) in behavioral responses differed across groups.
In the second set of analyses, we examined the association between three outcomes – anxiety, withdrawal and autistic symptom severity – on level and change of stranger fear within the group with FXS. Parallel to group effect models, we constructed conditional growth models that integrated anxiety, withdrawal and autistic symptoms as separate time-invariant covariates for each of the three stranger fear variables. Mental age at outcome was covaried in all within-FXS models.
Behavioral variables were log-transformed due to positive skew. Age was centered at 30 months, and predictors were standardized to minimize multicollinearity and maximize interpretability. Standardized coefficients (β) represent effects in terms of standard deviations (SD), and thus can be interpreted similarly to Cohen’s d (Cohen, 1988; Hedges, 2007; Schmidt & Hunter, 2015), with age effects reported in terms of SD change per year (calculated by multiplying β by 12 months). Alpha was set to .05 (two-tailed, where applicable).
Results
Group Differences in Stranger Fear: Facial Fear, Escape, and Distress Vocalizations
Average assessment age was slightly higher in the FXS than the control group (Wilcoxon Z = −2.02, p = .04) and was incorporated into each mixed effect model. Fit indices suggested that age was best modeled as a fixed effect only for facial fear and escape behaviors. For continuity, this model specification (random intercepts) was used for all three dependent variables.
Covariance parameter estimates for unconditional model random effects suggested facial fear, distress vocalizations, and escape behaviors varied across individuals (facial fear τ00 = .16, p = .05; distress vocalizations τ00 = .14, p = .07; escape behaviors τ00 = .20, p = .02). Fixed effects are listed in Table 3. Unconditional models, which include all observations across participants, indicated escape behaviors increased with age at a rate of 0.17 SD per year, whereas the remaining variables were relatively stable. However, when group was added to the model, we observed less facial fear in the FXS group, who exhibited 0.46 SD lower facial fear intensity than the TD group at 30 months, representing a medium effect. This effect was quantified by a marginal interaction with age. As depicted in Figure 1, average facial fear did not vary across age in the TD group (simple slope β = −0.01, SE = 0.01, p = .31) but marginally increased at a rate of .14 SD per year in FXS (β = 0.01, SE = 0.01, p = 09). Levels and change in the remaining variables did not differ significantly by group. Together, these results suggest that infants and toddlers both with and without FXS exhibit stable levels of distress vocalizations and increased escape behaviors across age. However, the FXS group exhibited lower facial fear overall, with a trend toward increasing fear across age.
Table 3.
Results of Fixed Effects Models for Group Comparisons
| Facial Fear | Distress Vocalizations | Escape Behavior | ||||
|---|---|---|---|---|---|---|
| Model | β (SE) | p | β (SE) | p | β (SE) | p |
| Unconditional Models | ||||||
| Intercept | −0.03 (0.09) | .74 | 0.03 (0.09) | .71 | −0.10 (0.09) | .28 |
| Slope | 0.00 (0.01) | .53 | −0.01 (0.01) | .35 | 0.01 (0.01) | .01 |
| Group Comparison | ||||||
| Intercept | 0.23 (0.14) | .11 | −0.10 (0.14) | .46 | −0.13 (0.14) | .36 |
| Age | −0.01 (0.01) | .31 | −0.02 (0.01) | .10 | 0.03 (0.01) | .02 |
| Group | −0.46 (.19) | .02 | 0.24 (0.18) | .20 | 0.05 (0.19) | .78 |
| Age*Group | 0.02 (0.01) | .07 | 0.02 (0.01) | .22 | −0.01 (0.01) | .23 |
Figure 1. Facial fear intensity across age and group.

Note. Gray lines indicate individual approach trajectories, and black lines indicate average values across each group.
Predictive Models within FXS
The next series of models focused on associations between stranger fear and anxiety, withdrawal and autistic symptom severity within FXS only. To conceptualize the potential effects of mental age on our primary variables, we conducted preliminary partial Spearman correlations, controlling for age of assessment, characterizing the association among outcome variables and mental age. Lower mental age correlated with higher autistic symptoms (partial Spearman σ = −.82, p < .001), higher escape behaviors (σ = −.18, p = .05), and higher distress vocalizations (σ = −.25, p < .01). Mental age was not associated with facial fear (σ = −.04, p = .65) or with anxiety (σ = .17, p = .27). Withdrawal symptoms were positively associated with autistic symptoms (σ = .39, p < .001) and anxiety (σ = .25, p < .01). However, anxiety and autistic symptoms were not related to each other (σ = −.08, p = .38). Given the complex associations between mental age and a subset of predictors and outcomes, all subsequent models were conducted with mental age covaried. Random effect specifications paralleled group-level models.
Results of fixed effects models are detailed in Table 4. Lower mean levels of escape behaviors were associated with higher autistic symptoms, with every 0.46 SD reduction in escape behavior intensity corresponding to 1 SD higher CARS scores (6.54 points in our sample) as estimated at 30 months, constituting a medium effect. In contrast, the association between distress vocalizations and age varied across levels of withdrawal. To probe this interaction, we examined correlations between distress vocalization and age across observations associated with low (raw score < 3; n = 29), medium (3–4, n = 38), and high (5+, n = 42) withdrawal outcomes. Pearson correlations indicated negative associations between distress vocalizations and age for low levels of withdrawal (r = −.38, p = .05) but nonsignificant associations for medium (r = .18, p = .30) and high (r = .06, p = .73) withdrawal, controlling for mental age and age of mental age assessment. These analyses were confirmed by simple slope analyses across withdrawal subgroups, in which distress vocalizations marginally decreased by 0.33 SD per year in the low-withdrawal group (simple slope β = −0.03, SE = 0.01, p = .07), but was more variable in the medium group (β = 0.06, SE = 0.04, p = .13) and stable in the high group (β = 0.006, SE = 0.01, p = .54). Thus, medium and high withdrawal are associated with relatively consistent levels of distress vocalizations across age, whereas low withdrawal is associated with decreased distress vocalizations over time. Stranger fear was not associated with anxiety.
Table 4.
Results of Fixed Effects Models for Predictive Models
| Facial Fear | Distress Vocalizations | Escape Behavior | ||||
|---|---|---|---|---|---|---|
| Model | β (SE) | p | β (SE) | p | β (SE) | p |
| Unconditional Models | ||||||
| Intercept | −0.12 (.12) | .33 | 0.01 (0.13) | .91 | −0.07 (.13) | .57 |
| Slope | 0.01 (0.01) | .09 | −0.00 (0.01) | .75 | 0.01 (0.01) | .14 |
| Autism Model | ||||||
| Intercept | 0.23 (0.46) | .62 | 0.48 (.44) | .28 | −0.70 (.45) | .12 |
| Age | 0.01 (0.01) | .05 | −0.00 (0.01) | .99 | 0.01 (0.01) | .29 |
| Autism | −0.00 (0.22) | .99 | 0.26 (0.20) | .20 | −0.46 (0.20) | .03 |
| Age of Outcome | −0.00 (0.03) | .98 | −0.03 (0.03) | .27 | −0.06 (0.03) | .05 |
| Age*Autism | 0.00 (0.01) | .91 | 0.01 (0.01) | .23 | −0.00 (0.01) | .82 |
| Mental Age (MA) | −0.08 (0.21) | .71 | 0.02 (0.20) | .91 | −0.33 (0.21) | .12 |
| Age of MA Measurement | −0.01 (0.03) | .70 | 0.01 (0.03) | .62 | 0.08 (0.03) | .01 |
| Anxiety Model | ||||||
| Intercept | 0.28 (0.40) | .50 | 0.11 (0.41) | .78 | −0.31 (0.43) | .48 |
| Age | 0.01 (0.01) | .05 | −0.00 (0.01) | .96 | 0.01 (0.01) | .27 |
| Anxiety | 0.11 (0.13) | .42 | −0.19 (0.13) | .15 | −0.16 (0.13) | .24 |
| Age of Outcome | −0.01 (0.03) | .70 | −0.02 (0.03) | .57 | −0.04 (0.03) | .22 |
| Age*Anxiety | −0.00 (0.01) | .96 | 0.01 (0.01) | .41 | 0.00 (0.01) | .91 |
| Mental Age (MA) | −0.09 (0.11) | .40 | −0.25 (0.11) | .03 | 0.13 (0.12) | .27 |
| Age of MA Measurement | −0.00 (0.03) | .88 | 0.01 (0.03) | .67 | 0.04 (0.03) | .14 |
| Withdrawal Model | ||||||
| Intercept | 0.37 (0.40) | .36 | 0.16 (0.42) | .71 | −0.39 (0.43) | .37 |
| Age | 0.02 (0.01) | .04 | 0.00 (0.01) | .97 | 0.01 (0.01) | .30 |
| Withdrawal | 0.14 (0.12) | .28 | −0.24 (0.13) | .07 | −0.23 (0.13) | .09 |
| Age of Outcome | −0.01 (0.03) | .67 | −0.02 (0.03) | .57 | −0.03 (0.03) | .23 |
| Age*Withdrawal | 0.00 (0.01) | .72 | 0.01 (0.01) | .03 | 0.00 (0.01) | .54 |
| Mental Age (MA) | −0.03 (0.11) | .82 | −0.30 (0.12) | .01 | 0.04 (0.12) | .71 |
| Age of MA Measurement | −0.01 (0.03) | .81 | 0.01 (0.03) | .71 | 0.05 (0.03) | .12 |
Summary of Results
Relative to controls, infants and toddlers with FXS displayed similar overall levels of escape behaviors and distress vocalizations, yet lower overall facial fear. Within-FXS models suggest complex associations among distress vocalization and outcome variables, with decreasing levels of distress vocalizations observed among participants with lower withdrawal. Despite atypical longitudinal patterns of facial fear in FXS relative to TD controls, facial fear did not predict autistic symptoms, withdrawal or anxiety symptom severity. Rather, lower intensity of escape behaviors was related to higher autistic symptoms. Thus, stranger fear was not associated with the general index of anxiety in FXS (CBCL Anxiety subscale), but instead was associated with withdrawal features more specifically reflective of social anxiety (CBCL Withdrawal subscale).
Discussion
Anxious, autistic and social withdrawal behaviors are commonly observed and inter-related in FXS. In fact, the high prevalence of ASD in FXS is often attributed to social anxiety as a causative factor rather than as a co-morbid feature, a distinct pattern from idiopathic non-syndromic ASD (Thurman, Mcduffie, Hagerman, & Abbeduto, 2014). However, the degree to which features of anxiety and ASD overlap or are distinct in FXS has yet to be determined, and the early course of symptoms and their behavioral precursors have not been established. The present study integrated longitudinal behavioral surveillance of children with FXS from infancy to the preschool period to test whether stranger fear uniquely predicted anxiety, autistic symptoms or social withdrawal. Two key findings emerged: (1) young children with FXS exhibited distinct patterns of stranger fear from TD controls, and (2) within-group variability in stranger fear predicted the emergence of autistic symptoms and social anxiety in FXS, but not anxiety more generally. Together, these findings suggest that atypical features of stranger fear, which are well-established to predict social anxiety in later childhood and adulthood, emerge in the first years of life in FXS. Furthermore, nuanced patterns of change in these features over time may index prodromal emergence of social anxiety prior to traditional ages of diagnoses. These findings may offer useful clinical information that, with further study in larger samples, may inform early detection and treatment protocols specific to FXS. Our results also support examining prodromal symptoms in FXS as a syndromic model for anxiety surveillance that may provide new insights for non-FXS samples.
Our findings build on a substantial corpus of work characterizing “typical” social approach trajectories in early development. Fear reactivity during the stranger approach paradigm has generally been shown to wax and wane across early development, with higher levels and steeper increases in fear reactivity linked to increased behavioral inhibition later in development (Brooker et al., 2013). We observed that across both FXS and control groups, distress vocalizations were relatively stable, and escape behaviors increased with time. However, facial fear was lower in FXS and marginally increased across age, paralleling previous cross-sectional findings in an overlapping sample (Tonnsen et al. 2013). Importantly, within FXS, facial fear did not predict levels of anxiety or ASD features in later development, suggesting facial fear may be best interpreted as a more universal feature of the FXS early childhood phenotype. Indeed, previous studies have linked withdrawn and anxious features in females with FXS to variability in protein expression on the FMR1 gene, suggesting close associations between the FXS genotype and behavioral inhibition (Hessl et al., 2001). With further replication, translational implications may be that reduced facial fear during social stress is an expected pattern in the majority of young children with FXS rather than a “signal” of other emergent problem behaviors.
It is unclear why infants with FXS exhibited blunted facial fear in early development, especially given facial fear did not correlate with developmental delay and is therefore unlikely to be solely driven by cognitive functioning in FXS. One possibility is that dampened facial expressions in FXS relate to dysregulated autonomic functioning commonly reported in the disorder, particularly vagal function (Klusek, Roberts, & Losh, 2015). In humans, the vagus (tenth cranial nerve) modulates variability in heart activity associated with respiration, often estimated via respiratory sinus arrhythmia (RSA). According to Porges’ polyvagal theory (Porges, 1995), the vagus has also evolved in humans to modulate social functioning by facilitating rapid responses to changing environmental demands, as well as by moderating muscles responsible for expressing emotion via facial expression and vocal intonation. Indeed, infants with FXS exhibit reduced RSA during a variety of tasks, including the stranger approach paradigm (Tonnsen et al. 2013), contributing to theories that atypical vagal functioning may contribute to abnormal social behaviors observed in ASD and FXS (Klusek et al., 2015; Roberts, Tonnsen, Robinson, & Shinkareva, 2012; Tonnsen, Shinkareva, et al., 2013).
It is possible that our finding of increased facial fear in FXS may reflect the intersection of atypical vagal functioning in FXS and additional neurodevelopmental processes. In typically developing infants, facial expressions are posited to be initially maintained by more primitive brainstem functions that are gradually overridden by more complex, coordinated responses across affective, cognitive and motor systems (Brooker et al., 2013; Stifter, 1989). Indeed, the association between vagal functioning and stranger-induced facial fear varies across age, with RSA positively correlating with facial fear in younger infants (e.g. 5–6 months) but not older infants (e.g. 10–12 months) across several studies systems (Brooker et al., 2013; Stifter, 1989). It is possible, therefore, that the dampened facial fear we observed in FXS, which is particularly evident at younger ages, reflects compromised vagal functioning that is gradually overridden by more complex, integrated systems across early childhood. Whereas this maturation typically occurs at younger ages in non-FXS samples, the cognitive and motor delays in FXS (Roberts, Mccary, Shinkareva, & Bailey, 2016) may protract this sequence in infants with FXS, resulting in blunted facial expressions that slowly approach typical levels during the preschool period. Future studies may test this hypothesis by examining dynamic interrelationships among facial expression, developmental abilities, and parasympathetic functioning in FXS, with particular attention toward how associations across these processes shift across early childhood.
In contrast to atypical patterns of facial fear that did not correlate with problem behaviors in FXS, escape behaviors and distress vocalizations were relatively “typical” in FXS at the group level, yet two patterns indexed risk for later-emerging symptoms. First, we observed lower escape behaviors in participants with higher autistic symptoms. One interpretation could be that participants with emergent autistic symptoms experience less stranger fear, signaling blunted emotional responsiveness. However emergent autistic symptoms in FXS may also be associated with difficulty planning and coordinating motor behaviors, consistent with mounting evidence that motor coordination and planning may co-occur with or precede autistic symptomatology, both in FXS (Roberts, Tonnsen, McCary, Caravella, & Shinkareva, 2016) and non-FXS samples (Zwaigenbaum, Bryson, & Garon, 2013). Notably, base rates of autism-associated motor control abnormalities are also generally elevated in FXS, with up to 80% of infants with FXS scoring “atypical” on behavioral motor-related ratings compared to 12% of control infants and 14% of infants with family histories of ASD (Roberts, Tonnsen, et al., 2016). Thus, when considering motor abilities as a marker of autistic symptoms in FXS, it will be important to distinguish the types and rates of behaviors that specifically predict ASD, separating these features from the global atypicalities associated with the FXS phenotype.
Our second major predictive finding was that distress vocalizations within FXS were associated with later withdrawal, with more withdrawn children exhibiting stable distress vocalizations compared to decreased distress vocalizations in participants with few withdrawal symptoms. This finding is consistent with our hypotheses suggesting that distress vocalizations in toddlers with FXS may reflect social fear, perhaps reflecting the child’s attempt to actively change the situation or signal their distress (e.g. obtain support from caretaker, discourage stranger from approaching). In contrast, children who end up less socially withdrawn may exhibit less distress vocalizations across age because they are not experiencing as much social fear. Notably, it is also possible that young children with FXS who are rated as less withdrawn are actually struggling to mobilize the appropriate coping mechanisms to react to fear-inducing contexts, such as the stranger task and other situations that parents may build into their withdrawal ratings. Indeed, in our previous study of physiological stranger response in a cross-sectional FXS sample, lower distress vocalizations were associated with faster heart rate and lower RSA in older participants (51–58 months for heart rate, 47–58 months for RSA) (Tonnsen et al. 2013). Thus, it is possible that a subset of children with FXS may exhibit less withdrawn behaviors – both as rated by parents and as displayed during the stranger approach episode – despite also exhibiting relative hyperarousal, potentially indicating difficulty mobilizing a typical fear response. Future work may integrate autonomic measures to test these competing hypotheses empirically.
Contrary to hypotheses, stranger approach did not predict general anxiety features in FXS, conflicting with prior studies suggesting positive associations between parent-reported fear and anxiety on the CBCL in FXS (Tonnsen, Malone, et al., 2013). The lack of association between stranger response and global anxiety likely reflects specificity between the paradigm used in this study and social anxiety outcomes, rather than more global indices of anxiety. In addition, the previous study examining predictive associations between fear and later global anxiety focused on parent-reported fear on a temperament questionnaire, likely encompassing a broader scope of child fear-related features, which likely differ from the specific social fear solicited in this standardized experimental paradigm.
In summary, our hypotheses regarding early indicators of social fear indexing risk for later emerging anxiety were partially supported. We found that stable vocal distress was a potential predictor for elevated social withdrawal in FXS, potentially indexing emergent social anxiety. However, none of the stranger fear behavioral features were associated with our more global index of generalized anxiety. These differential relationships support the specificity of early features to later symptoms, with social fear mapping onto later social anxiety, but not to later generalized anxiety symptoms. This specificity also converges with the notion that that while anxiety disorders share a number of overlapping features, anxiety represents a number of subconstructs that are associated with differential patterns of early risk. Our data suggest that similar to the general population, early social fear may represent a specific risk factor for social anxiety – rather than for anxiety more broadly – in high-risk children with FXS.
Despite providing one of the first longitudinal characterizations of social approach in very young children with FXS, our study is limited by use of parent-report and examiner rating scales rather than gold-standard diagnostic instruments, with different informants across measures. Although lack of clinical diagnostic information is also a limitation, the use of continuous measures of symptom severity is consistent with our goal to characterize variability in symptoms rather than categorical “disorders,” which are often difficult to quantify in individuals with intellectual disabilities. Diagnostic outcome measures in such a young sample may also be premature, as the median age of anxiety disorder diagnosis in the general population is not until 11 years of age (Kessler et al., 2005). In addition, due to the standardized nature of the stranger approach paradigm, we did not measure parental responses to the child and stranger, nor did we examine the child’s reactions to the stranger as opposed to a more familiar person, such as their parent. Future work may consider modifying the paradigm to examine dyadic factors related to behavioral inhibition, including whether partner familiarity influences behavior similarly across groups and clinical outcomes. Finally, building on the multiple methods we employed in this study, future studies may consider integrating psychophysiological measures to further inform individual differences in behavioral inhibition in FXS. This work is particularly relevant given previous studies documenting atypical autonomic profiles in infants and toddlers with FXS (Roberts et al., 2012; Tonnsen, Shinkareva, et al., 2013).
Together, the present study suggests that behavioral inhibition in FXS begins to diverge from typical development in the first years of life and, with replication from further study, has potential to index degree of risk in this population. Comparisons across multiple co-occurring and overlapping outcomes suggest unique approach-related signatures of withdrawal and autistic symptoms, with unstable vocal distress indexing withdrawal and lower escape behaviors indexing autistic symptoms. These findings may inform earlier detection of emergent co-morbid anxiety and ASD in FXS and identify treatment targets that promote optimal outcomes for children and families.
Figure 2. Differences in distress vocalization trajectories across levels of withdrawal in FXS.
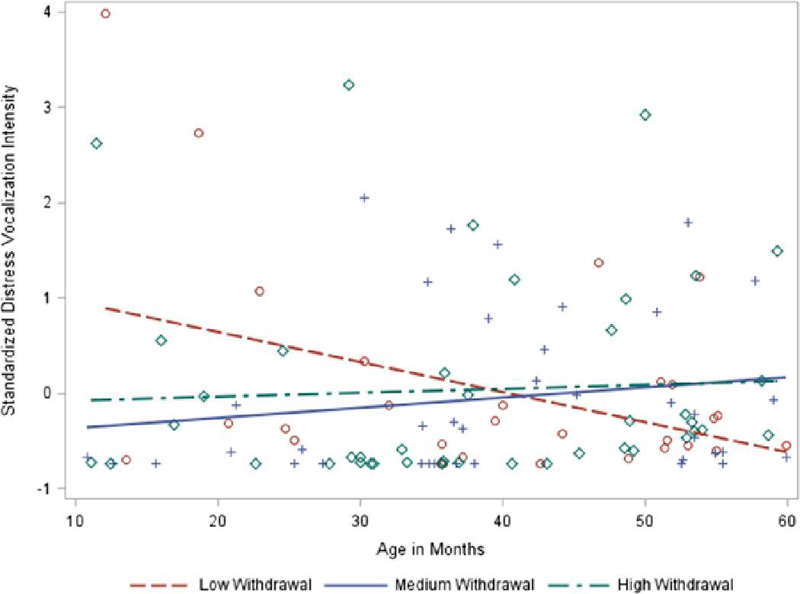
Note. Withdrawal raw scores were categorized as low 0–2; 27%), medium (3–4; 35%) or high (5+; 28%) to roughly divide the sample into thirds for graphical purposes only.
Abbreviations:
- (ASD)
autism spectrum disorder
- (FXS)
fragile X syndrome
References
- Achenbach T. (1991). Manual for the child behavior checklist/4–18 and 1991 profile. Burlington: University of Vermont Department of Psychiatry. [Google Scholar]
- Achenbach T, & Rescorla L. (2000). Manual for the ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Beesdo K, Bittner A, Pine DS, Stein MB, Höfler M, Lieb R, & Wittchen H-U (2007). Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Archives of General Psychiatry, 64(8), 903–912. 10.1001/archpsyc.64.8.903 [DOI] [PubMed] [Google Scholar]
- Brooker RJ, Buss K. a., Lemery-Chalfant K, Aksan N, Davidson RJ, & Goldsmith HH (2013). The development of stranger fear in infancy and toddlerhood: normative development, individual differences, antecedents, and outcomes. Developmental Science, 16(6), 864–78. 10.1111/desc.12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control. (2014). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Netowrk, 11 Sites, United States, 2010. MMWR Surveillance Summaries, 63(2), 1–22. [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson H. a, Diaz Y, … Fox NA (2009). Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry, 48(9), 928–35. 10.1097/CHI.0b013e3181ae09df [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (Erlbaum L, Ed.)Statistical Power Analysis for the Behavioral Sciences (Vol. 2nd). Lawrence Erlbaum Associates; 10.1234/12345678 [DOI] [Google Scholar]
- Cordeiro L, Ballinger E, Hagerman R, & Hessl D (2011). Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: prevalence and characterization. Journal of Neurodevelopmental Disorders, 3(1), 57–67. 10.1007/s11689-010-9067-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Acuña JM, & Sherman SL (2001). FMR1 and the fragile X syndrome: human genome epidemiology review. Genetics in Medicine Official Journal of the American College of Medical Genetics, 3(5), 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández E, Rajan N, & Bagni C (2013). The FMRP regulon: from targets to disease convergence. Frontiers in Neuroscience, 7(October), 191 10.3389/fnins.2013.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JR, Van Hulle C. a, Aksan N, Essex MJ, & Goldsmith HH (2011). Deriving childhood temperament measures from emotion-eliciting behavioral episodes: scale construction and initial validation. Psychological Assessment, 23(2), 337–53. 10.1037/a0021746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein M. a, Bridgett DJ, Rothbart MK, Robertson C, Iddins E, Ramsay K, & Schlect S (2010). A latent growth examination of fear development in infancy: contributions of maternal depression and the risk for toddler anxiety. Developmental Psychology, 46(3), 651–668. 10.1037/a0018898 [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, & Rothbart M. (1996). The laboratory temperament assessment battery. Madison: University of Wisconsin. [Google Scholar]
- Hall SS, Lightbody AA, Huffman LC, Lazzeroni LC, & Reiss AL (2009). Physiological correlates of social avoidance behavior in children and adolescents with fragile x syndrome. Journal of the American Academy of Child & Adolescent Psychiatry, 48(3), 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DD, Hooper SR, Bailey DB, Skinner ML, Sullivan KM, & Wheeler A (2002). Problem behavior in boys with fragile X syndrome. American Journal of Medical Genetics, 108(2), 105–16. [DOI] [PubMed] [Google Scholar]
- Hedges LV (2007). Effect Sizes in Cluster-Randomized Designs. Journal of Educational and Behavioral Statistics, 32(4), 341–370. 10.3102/1076998606298043 [DOI] [Google Scholar]
- Hessl D, Dyer-Friedman J, Glaser B, Wisbeck J, Barajas RG, Taylor A, & Reiss AL (2001). The influence of environmental and genetic factors on behavior problems and autistic symptoms in boys and girls with fragile X syndrome. Pediatrics, 108(5), E88 http://doi.org/108/5/e88 [pii] [DOI] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, & Reiss AL (2006). Social behavior and cortisol reactivity in children with fragile X syndrome. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 47(6), 602–10. 10.1111/j.1469-7610.2005.01556.x [DOI] [PubMed] [Google Scholar]
- Hunter J, Rivero-Arias O, Angelov A, Kim E, Fotheringham I, & Leal J (2014). Epidemiology of fragile X syndrome: a systematic review and meta-analysis. American Journal of Medical Genetics. Part A, 164A(7), 1648–58. 10.1002/ajmg.a.36511 [DOI] [PubMed] [Google Scholar]
- Izard CE (1979). The Maximally Discriminative Facial Movement Coding System (MAX). Newark, DE: University of Deleware Instructional Resource Center. [Google Scholar]
- Kagan J, Reznick S, Clarke C, Snidman N, & Garcia-Coll C (1984). Behavioral Inhibition to the Unfamiliar. Child Development, 55, 2212–2225. [Google Scholar]
- Kaufmann WE, Cortell R, Kau ASM, Bukelis I, Tierney E, Gray RM, … Stanard P (2004). Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. American Journal of Medical Genetics Part A, 129A(3), 225–234. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Key APF, & Stone WL (2012). Same but different: 9-month-old infants at average and high risk for autism look at the same facial features but process them using different brain mechanisms. Autism Research, 5(4), 1–14. 10.1002/aur.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Roberts JR, & Losh M (2015). Cardiac Autonomic Regulation in Autism and Fragile X Syndrome: A Review. Psychological Bulletin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, … Rutter M (2000). The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–23. [PubMed] [Google Scholar]
- Merikangas KR, He J-P, Burstein M, Swanson SA, Avenevoli S, Cui L, … Swendsen J (2010). Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). Journal of the American Academy of Child & Adolescent Psychiatry, 49(10), 980–9. 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen Scales of Early Learning. San Antonio, TX: Pearson. [Google Scholar]
- Porges SW (1995). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology, 32, 301–318. [DOI] [PubMed] [Google Scholar]
- Rapee RM (2014). Preschool environment and temperament as predictors of social and nonsocial anxiety disorders in middle adolescence. Journal of the American Academy of Child and Adolescent Psychiatry, 53(3), 320–328. 10.1016/j.jaac.2013.11.014 [DOI] [PubMed] [Google Scholar]
- Rapee RM, Kennedy S, Ingram M, Edwards S, & Sweeney L (2005). Prevention and early intervention of anxiety disorders in inhibited preschool children. Journal of Consulting and Clinical Psychology, 73(3), 488–97. 10.1037/0022-006X.73.3.488 [DOI] [PubMed] [Google Scholar]
- Ritvo ER (2014). Smiling response, stranger anxiety, and autistic disorder. Journal of Autism and Developmental Disorders, 44(2), 484 10.1007/s10803-013-1889-5 [DOI] [PubMed] [Google Scholar]
- Roberts JE, Clarke M, Alcorn K, Carter JC, Long ACJ, & Kaufmann WE (2009). Autistic behavior in boys with fragile X syndrome: social approach and HPA-axis dysfunction. Journal of Neurodevelopmental Disorders, 1(4), 283–91. 10.1007/s11689-009-9028-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Clarke MA, Alcorn K, Carter JC, Long ACJ, & Kaufmann WE (2007). Social approach and autistic behavior in children with fragile X syndrome. Journal of Autism and Developmental Disorders, 1(4), 1748–1760. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Mccary LM, Shinkareva SV, & Bailey DB (2016). Infant Development in Fragile X Syndrome : Cross-Syndrome Comparisons. Journal of Autism and Developmental Disorders, 46(6), 2088–2099. 10.1007/s10803-016-2737-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen BL, McCary LM, Caravella KE, & Shinkareva SV (2016). Brief Report: Autism Symptoms in Infants with Fragile X Syndrome. Journal of Autism and Developmental Disorders, 46(12), 3830–3837. 10.1007/s10803-016-2903-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen BL, Robinson A, & Shinkareva SV (2012). Heart activity and autistic behavior in infants and toddlers with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities, 117(2), 90–102. 10.1352/1944-7558-117.2.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJSJ, Wehner DE, Hagerman R, & Wehner E (2001). The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental. Journal of Developmental & Behavioral …, 22(6), 410–417. [DOI] [PubMed] [Google Scholar]
- Scherr JF, Hogan AL, Hatton D, & Roberts JE (2017). Stranger Fear and Early Risk for Social Anxiety in Preschoolers with Fragile X Syndrome Contrasted to Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 10.1007/s10803-017-3059-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt FL, & Hunter JE (2015). Methods of Meta-Analysis: Correcting Error and Bias in Research Findings. [Google Scholar]
- Schopler E, Reichler R, & Renner B (1998). The Childhood Autism Rating Scale. Los Angeles: Western Psychological Services. [Google Scholar]
- Stifter CA (1989). Facial Expressivity and Vagal Tone in 5 and IO-Month-Old Infants. Infant Behavior Development, 12, 127–137. [Google Scholar]
- Thurman AJ, Mcduffie A, Hagerman R, & Abbeduto L (2014). Psychiatric symptoms in boys with fragile X syndrome : A comparison with nonsyndromic autism spectrum disorder. Research in Developmental Disabilities, 35(5), 1072–1086. 10.1016/j.ridd.2014.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnsen BL, Grefer ML, Hatton DD, & Roberts JE (2014). Developmental trajectories of attentional control in preschool males with fragile X syndrome. Research in Developmental Disabilities, 36C, 62–71. 10.1016/j.ridd.2014.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnsen BL, Malone PS, Hatton DD, & Roberts JE (2013). Early negative affect predicts anxiety, not autism, in preschool boys with fragile X syndrome. Journal of Abnormal Child Psychology, 41(2), 267–80. 10.1007/s10802-012-9671-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnsen BL, & Roberts JE (2016). Characterizing Emergent Anxiety Through the Lens of Fragile X In Hodapp RM & Fidler DJ (Eds.), International Review of Research in Developmental Disabilities (Vol. 51, pp. 41–83). Elsevier Inc. [Google Scholar]
- Tonnsen BL, Shinkareva SV, Deal SC, Hatton DD, & Roberts JE (2013). Biobehavioral indicators of social fear in young children with fragile x syndrome. American Journal on Intellectual and Developmental Disabilities, 118(6), 447–59. 10.1352/1944-7558-118.6.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bauman ML, Stone WL, Yirmiya N, Estes A, Hansen RL, … Wetherby A. (2015). Early Identification of Autism Spectrum Disorder: Recommendations for Practice and Research. Pediatrics, 136 Suppl1, S10–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, & Garon N. (2013). Early identification of autism spectrum disorders. Behavioural Brain Research, 1–14. 10.1016/j.bbr.2013.04.004 [DOI] [PubMed] [Google Scholar]


